-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
Polycomb Repressive Complex (PRC) 1 and PRC2 regulate genes involved in differentiation and development. However, the mechanism for how PRC1 and PRC2 are recruited to genes in mammalian cells is unclear. Here we present evidence for an interaction between the transcription factor REST, PRC1, and PRC2 and show that RNF2 and REST co-regulate a number of neuronal genes in human teratocarcinoma cells (NT2-D1). Using NT2-D1 cells as a model of neuronal differentiation, we furthermore showed that retinoic-acid stimulation led to displacement of PRC1 at REST binding sites, reduced H3K27Me3, and increased gene expression. Genome-wide analysis of Polycomb binding in Rest−/− and Eed−/− mouse embryonic stem (mES) cells showed that Rest was required for PRC1 recruitment to a subset of Polycomb regulated neuronal genes. Furthermore, we found that PRC1 can be recruited to Rest binding sites independently of CpG islands and the H3K27Me3 mark. Surprisingly, PRC2 was frequently increased around Rest binding sites located in CpG-rich regions in the Rest−/− mES cells, indicating a more complex interplay where Rest also can limit PRC2 recruitment. Therefore, we propose that Rest has context-dependent functions for PRC1 - and PRC2 - recruitment, which allows this transcription factor to act both as a recruiter of Polycomb as well as a limiting factor for PRC2 recruitment at CpG islands.
Published in the journal: REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells. PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002494
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002494Summary
Polycomb Repressive Complex (PRC) 1 and PRC2 regulate genes involved in differentiation and development. However, the mechanism for how PRC1 and PRC2 are recruited to genes in mammalian cells is unclear. Here we present evidence for an interaction between the transcription factor REST, PRC1, and PRC2 and show that RNF2 and REST co-regulate a number of neuronal genes in human teratocarcinoma cells (NT2-D1). Using NT2-D1 cells as a model of neuronal differentiation, we furthermore showed that retinoic-acid stimulation led to displacement of PRC1 at REST binding sites, reduced H3K27Me3, and increased gene expression. Genome-wide analysis of Polycomb binding in Rest−/− and Eed−/− mouse embryonic stem (mES) cells showed that Rest was required for PRC1 recruitment to a subset of Polycomb regulated neuronal genes. Furthermore, we found that PRC1 can be recruited to Rest binding sites independently of CpG islands and the H3K27Me3 mark. Surprisingly, PRC2 was frequently increased around Rest binding sites located in CpG-rich regions in the Rest−/− mES cells, indicating a more complex interplay where Rest also can limit PRC2 recruitment. Therefore, we propose that Rest has context-dependent functions for PRC1 - and PRC2 - recruitment, which allows this transcription factor to act both as a recruiter of Polycomb as well as a limiting factor for PRC2 recruitment at CpG islands.
Introduction
Polycomb group (PcG) proteins are epigenetic regulators of gene expression and play an essential role during embryonic development [1]. The Polycomb repressive complex 2 (PRC2) is the only known enzyme that mediates di - and tri-methylation of histone H3 on lysine 27 (H3K27Me2/3), modifications believed to be required for PcG-mediated gene repression [2], [3], [4], [5]. PRC2 consist of three core components, Ezh2, Suz12 and Eed, which are all required for early mouse development [6], [7], [8]. H3K27Me3 can function as an epigenetic mark for the recruitment of PRC1, a large heterogenous complex [9], which among others include the Cbx - and Rnf2 (Ring1B) proteins. Rnf2 catalyzes the ubiquitination of histone H2A on lysine 119 (H2AK119Ubi) [10], [11] and as for the members of the PRC2 complex, disruption of the Rnf2 gene in mouse causes a similar developmental phenotype with arrest at gastrulation [12]. Furthermore, Rnf2 has recently been shown to be part of at least two additional gene regulatory complexes, the E2F6.com-1 complex [13] and the Fbxl10-BcoR complex [14].
The importance of PcG protein complexes in stem cell maintenance and differentiation has been extensively studied in mouse embryonic stem (mES) cells. Previous work have shown that genetic elimination of either PRC1 or PRC2 function, by knockout of Rnf2 or Eed [12], [15], [16], [17], [18] leads to derepression of several lineage-specific genes, that tend to destabilize mES cells, although they still preserve their ability to self-renew and differentiate. Interestingly, the Wutz laboratory showed, that the simultanous loss of both the PRC1 and PRC2 complexes in mES cells abrogates differentiation [19]. In the same study it was suggested that the PRC1 - and PRC2-complexes can function independently to repress a common set of genes important for stem cell maintenance.
There are several models explaining how the PcG complexes are recruited to specific target genes. In mammalian cells, it is a long-standing dogma that the PRC2 complex is recruited to target gene promoters by so far unidentified transcription factors (TFs), through the recognition of the underlying DNA sequences. Subsequently, this leads to H3K27 methylation and recruitment of PRC1 through the chromodomain-containing CBX proteins [20], [21]. Several recent publications suggest that Jarid2 can function as a PRC2 recruitment factor [22], [23], [24], [25], [26]. Furthermore, non-coding RNAs (ncRNA) interact with both PRC1 and PRC2 and seem to be working in parallel or in combination with TFs in the ability to recruit the PcG complexes to genomic loci [27], [28], [29]. Interestingly, recent data have implicated ncRNAs as being important for the recruitment of PRC2 to CpG islands and suggested that these genomic entities are sufficient for PRC2 recruitment [30], [31]. In Drosophila, several TFs are involved in recruiting the PcG complexes to DNA elements called Polycomb Response Elements (PREs) [32], [33], [34]. However, among these transcription factors only an ortholog of Pho, YY1, is preserved in mammals and has been found to interact with PRC1 - [35] and PRC2-subunits [36]. Based on the data from Drosophila, it is therefore likely that several TFs beside YY1 are involved in the recruitment of PcG complexes in mammalian cells and identification of such factors is needed in order to define mammalian PREs.
We now present evidence for an interaction between the TF REST (also called Neuron-Restrictive Silencing Factor, NRSF) and the PRC1 - and PRC2-complexes, which is independent of RNA. By shRNA mediated knockdown, DNA microarray expression analysis and ChIP analysis, we show that a number of genes are co-regulated by REST and RNF2 in human teratocarcinoma NT2-D1 cells. Genome-wide analysis by ChIP-sequencing (ChIP-seq) in mES cells reveal the co-existence of PRC1 and PRC2 on Rest binding sites. Combining our biochemical interaction analysis of Rest-PcG complexes and the genome-wide analysis of Rest-PcG binding sites, suggest that Rest is required for the recruitment of PRC1 and PRC2 to a subset of its target genes in mES cells. Interestingly, the recruitment of Rnf2 to Rest binding sites can occur independently of both CpG islands and PRC2 activity. Surprisingly, PRC2 was frequently increased around Rest binding sites located in CpG rich regions in the Rest−/− mES cells, which suggest that other Rest-associated activities can limit PRC2 recruitment. Based on these observations we propose that Rest has context-dependent functions for PRC1 - and PRC2-recruitment to target genes in mammalian cells and that PRC1 is a co-repressor for Rest.
Results
REST and the PRC1 – and PRC2–complexes interact in mammalian cells
We were interested to examine whether the transcription factor REST and the PRC1 complex would interact in vivo, encouraged by previous observations, where we identified REST in a double-tag purification of the CBX8 interacting protein, HAN11 (WDR68) (Figure S1; see the information in Procedure S1). We performed size-exclusion chromatography of nuclear extracts from the human teratocarcinoma cells, NT2-D1 (Figure 1A, left part) and HEK 293 cells (Figure S1E) and performed immunoprecipitation of REST from pools of different fractions as indicated (Figure 1A, right part). The data showed, that the PRC1 core subunits, RNF2, BMI1, NSPC1 and CBX8 co-immunoprecipitated with REST from high-molecular weight fractions (F7–9, labelled pooled fractions 1). In addition to CBX8, we also detected CBX7 when performing the REST immunoprecipitation on the pool of fractions F11–13 (labelled pooled fractions 2). Importantly, we did not detect E2F6, BCOR or HP1γ in the REST immunoprecipitations from NT2-D1 cells, showing that the complex that we found associated to REST, contains members of the canonical PRC1 complex and differs from the previously described E2F6.com-1 [13] and Fbxl10-BcoR complexes [14], [37]. For simplicity we will refer to this REST associated PcG complex as PRC1 throughout the remaining part of this work. Importantly, beside core components of the PRC1 complex, we furthermore found that the PRC2 subunits EZH2 and SUZ12 co-immunoprecipitated with REST from pool 1 and 2.
Fig. 1. REST and Polycomb Repressor Complex 1 (PRC1) and PRC2 interact in vivo. 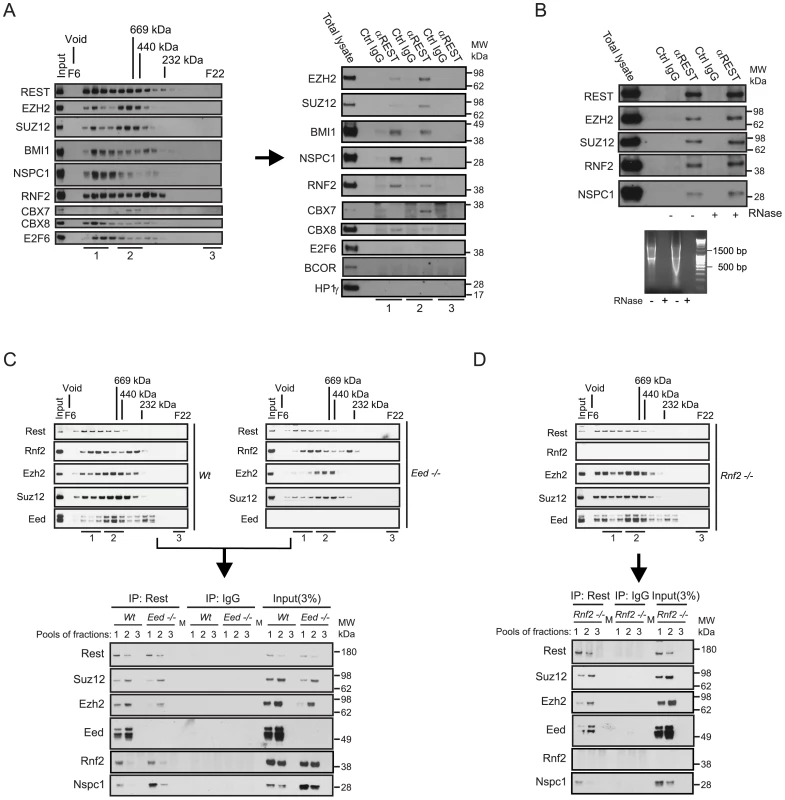
(A) Nuclear extracts from NT2-D1 cells were processed for size-exclusion chromatography followed by Western blotting to reveal the profiles of Polycomb proteins, the transcription factors REST and E2F6. Pooled fractions (1: Fractions F7–9; 2: F11–13; 3: F21–22) were used for immunoprecipitation (IP) with anti-REST or control IgG and processed for Western blotting with antibodies as indicated (total lysate: 12 µg of protein). (B) IPs using REST or control IgG on total nuclear extract (NT2-D1 cells; 500 µg per IP). After IPs the samples were either treated with a combination of RNase V1 and RNase A or left without RNase followed by repeated washes. Eluted proteins were processed for Western blotting using antibodies as indicated. Lower panel: Control experiment for the efficiency of RNase treatment using either 2 µg (left part) or 4 µg (right part) of RNA. Samples were incubated under the conditions used for REST IPs. (C–D) Nuclear protein extracts from mouse embryonic stem cells (mES) of different genetic background (Wt, Eed−/− or Rnf2−/−) were separated by size-exclusion chromatography (C–D: upper panels) and pooled fractions (1: F8–10; 2: F11–13; 3: F22) were processed for IPs (C–D: lower panels) using antibodies for Rest or control IgG. Western blots were processed with antibodies as indicated. Input corresponds to 3% of the material used for each IP. (C) Represents IPs comparing Wt and Eed−/− mES cells and (D) represents IPs in the Rnf2−/− mES cells. The samples were processed for Western blotting with antibodies as indicated. Lanes marked “M” represents loading of a pre-stained molecular weight marker. Since it has recently been shown that the long non-coding RNA HOTAIR can recruit CoREST/REST/LSD1 - and PRC2 complexes through the 5′ and 3′ends respectively [27], we checked whether RNase treatment of our immunoprecipitates would dissociate PcG complexes from REST. As seen in Figure 1B, degradation of single - and double-stranded RNA had no effect on the interactions between endogenous REST, PRC1 and PRC2. Furthermore, we found that the interactions between REST and the Polycomb complexes were not due to DNA bridging, as these were not eliminated by ethidium bromide treatment of immunoprecipitated complexes (Figure S1D). To confirm the interaction between Rest and the PRC1 and PRC2 complexes in mouse embryonic stem (mES) cells, we performed immunoprecipitations of Rest on pooled fractions obtained from size-exclusion chromatography of nuclear extracts from Wt, Eed−/− and Rnf2−/− mES cells (Figure 1C and 1D). Similarly to the results obtained in NT2-D1 cells, we found that Rest interacts with core subunits of the PRC1 and the PRC2 complexes in mES cells. When comparing the relative efficiency of co-immunoprecipitation in Wt and Eed−/− mES cells, we furthermore found that the interaction between Rest and the PRC2 subunits, Ezh2 and Suz12, did not require Eed. As expected, the total level of the other two core subunits of PRC2, Suz12 and Ezh2, were somewhat reduced in the Eed−/− cells (due to destabilization), which was reflected in diminished amounts of these two subunits in the Rest IPs from the Eed−/ − mES cells. Furthermore, there was a clear change in size distribution of the PRC2 complex in the Eed−/− mES compared to the Wt cells, showing reduced amounts of the most high-molecular weight forms (Pooled fractions 1, Figure 1C, upper panels). This was reflected in the co-immunoprecipitation of Ezh2 and Suz12 by Rest, where the binding of these two subunits to Rest, in the absence of Eed, was evident in the IP from pool 2, but very reduced in the IP from pool 1 (Figure 1C, lower panel). Moreover, the absence of Eed did not affect the efficiency of co-immunoprecipitation between Rest and the PRC1 subunits, Rnf2 and Nspc1 (Figure 1C). Interestingly, the Nspc1 subunit was more abundant in the Eed−/− mES cells (see input lysates), which was also reflected in the Rest IPs when comparing Wt and Eed−/− mES cells (Figure 1C, lower panel).
Immunoprecipitations performed on fractions from Rnf2−/− mES cells furthermore revealed that Rnf2 is not required for the interaction between Rest and the PRC1 subunit Nspc1. This suggested that the interaction between Rest and PRC1 was not mediated directly through Rnf2.
In conclusion, our biochemical data clearly showed that REST interacts with PRC1 and PRC2 protein complexes in mammalian cells and that these REST-PcG complexes are independent of non-coding RNAs.
REST and PRC1 co-regulate the expression of genes in NT2-D1 cells
The interaction between REST and PRC1 suggested that the PRC1 complex might function as a co-repressor for REST. To investigate this, we performed a DNA microarray expression analysis to compare the effect of shRNA mediated knockdown of REST with the knockdown of RNF2 (Figure 2A and 2B). We decided to use the human NT2-D1 cells, since these can easily be induced to differentiate along the neuronal path by retinoic acid (RA) stimulation [38]. As summarized in the cluster analysis and the Venn diagram, a highly significant number of genes were co-regulated by REST and RNF2 (Figure 2B) (see the information on data handling in Procedure S1). While REST knockdown lead to significantly increased expression of 1,862 genes (>2 fold), RNF2 knockdown lead to increase in the expression of 775 genes (>2 fold) (Figure 2B and Table S1). Among these genes, 258 were found to be co-regulated, corresponding to more than 30% of all the genes up-regulated in response to RNF2 knockdown. Although, the majority of the co-regulated genes were up-regulated, we also found that a considerable number of genes were co-down-regulated, suggesting that we not only observe direct effects, but also indirect effects on expression. In agreement with previous reports on REST - and PcG targets, co-up-regulated genes enrich for developmental functions, while co-down-regulated genes showed no significant enrichment for any particular biological function (Table S9). This suggests that among the co-up-regulated genes, there was enrichment for genes that were directly targeted by the REST-PRC1 complex.
Fig. 2. REST is required for PRC1 binding and the maintenance of H3K27Me3. 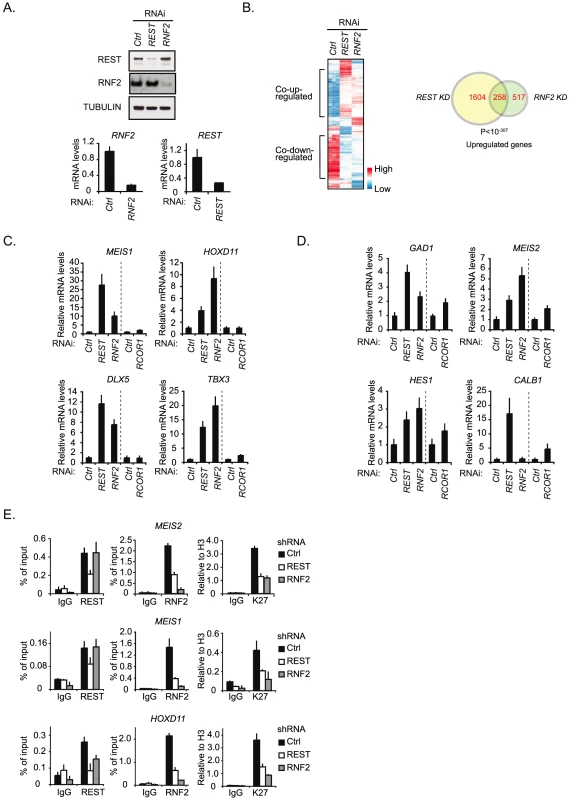
(A) shRNA mediated knockdown of REST and RNF2 in NT2-D1 cells. Samples were processed for Western blot analysis (upper panel) and quantitative real-time RT-PCR (lower panel). (B) Expression array analysis of RNA isolated from NT2-D1 cells treated with a control shRNA or a shRNA against either REST or RNF2. Left panel: Cluster analysis of regulated genes displayed as a heat-map. Right panel: Venn diagram for genes up-regulated more than 2 fold and a P-value<0.05. In C and D relative mRNA levels in NT2-D1 cells treated with shRNA against REST, RNF2, or RCOR1 are shown. Vertical punctured line separate two independent experiments (knockdown of REST and RNF2 were done in parallel and knockdown of RCOR1 was done separately). (C) Group of genes repressed by REST and RNF2. (D) Group of genes repressed by REST, RNF2 and/or CoREST. (E) ChIP analysis on NT2-D1 cells treated with shRNA against REST and RNF2 using antibodies as indicated (K27 = H3K27Me3). Error bars represent standard deviations calculated from triplicate qPCR reactions. To compare the influence of RNF2 to a well-described REST-interacting co-repressor complex, the CoREST complex [39], we decided to perform a new shRNA mediated knockdown experiment using constructs for REST, RNF2 and RCOR1 (gene coding for the CoREST protein) (Figure 2C and 2D). Using QPCR, we analyzed the change in expression of 7 different genes from the group of 258 co-regulated genes identified in the microarray (Figure 2B). Interestingly, the data suggest that the genes can be divided into two groups. The first group contains genes (MEIS1, HOXD11, DLX5 and TBX3) that were co-regulated by REST and RNF2 but only slightly affected by RCOR1 knockdown (Figure 2C). The second group includes genes (GAD1, MEIS2 and HES1) that were co-regulated by REST and RNF2, but furthermore showed an increase in expression upon RCOR1 knockdown (Figure 2D). We also found an example of a gene (CALB1) that was co-regulated by REST and CoREST, but unaffected by RNF2 knockdown (Figure 2D).
To confirm the interaction between REST and RNF2 on co-regulated target genes we performed ChIP for two of the genes in the first group (MEIS1 and HOXD11) and one gene in the second group (MEIS2) in NT2-D1 cells treated with shRNAs for REST, RNF2 or empty control shRNA (Figure 2E). As expected, REST knockdown reduced the level of REST at all three target genes. Importantly, REST knockdown also lead to a reduced amount of RNF2 at these genes (Figure 2E). In contrast, while a shRNA against RNF2 almost completely eliminated RNF2 binding to the MEIS1 and MEIS2 genes, it had no effect on REST binding, which is in agreement with REST being a DNA binding factor recruiting RNF2. At the HOXD11 locus there was a small, but significant, reduction of REST binding in response to RNF2 knockdown. Interestingly, H3K27Me3 was significantly reduced in both REST- and RNF2 knockdowns, indicating that the PRC2 complex interacted with both REST and RNF2 containing complexes on the MEIS1, MEIS2 and HOXD11 genes.
Altogether we found that REST was required for the recruitment of RNF2 and the maintenance of H3K27Me3 on a selected number of neuronal genes and that REST and RNF2 co-regulate a highly significant number of genes in NT2-D1 cells.
Rest is required for the recruitment of PRC1 and PRC2 to specific genes in mES cells
To test if Rest is required for the recruitment of PRC1 and PRC2 to their target genes in mouse embryonic stem (mES) cells, we performed our analyses in the previously published Wt and Rest−/ − mES cells [40], [41]. We observed that the overall levels of Suz12, Ezh2, Rnf2 and Oct4 proteins were unchanged in the Rest−/− mES cells as compared to the Wt mES cells (Figure 3A). Although, the global level of the H3K27Me3 was unchanged between the Wt and Rest−/ − mES cells (Figure 3A), ChIP-sequencing (ChIP-seq) (see information on data handling in Procedure S1) for H3K27Me3, Suz12 and Rnf2 revealed clear local differences for these factors. This is illustrated in Figure 3B by ChIP-seq profiles for three genes (Brunol6, Best2 and Prrxl1) showing co-localization between Rest, Rnf2 and Suz12 in the Wt mES cells. Notably, for two of the genes (Brunol6 and Best2) there was an almost complete loss of Rnf2 binding at the center of the Rest peaks in the Rest−/ − mES cells. For the Prrxl1 gene, there was a surprising increase in Rnf2 binding, suggesting that Rest can also counteract PRC1 recruitment at certain loci. Moreover, the increase in Rnf2 was accompanied by an increase in PRC1 subunit Cbx7 on the Prrxl1 gene (Figure S2C). The effects on Suz12 binding were similar, but less pronounced, with reduced binding at Brunol6 and Best2 and somewhat increased binding at the Prrxl1 gene. For the distribution of H3K27Me3, we observed more widespread effects and the signals were reduced throughout the gene body at Brunol6 and Best2, while at Prrxl1 the signal was essentially unchanged. Moreover, the non-Rest target gene, Gjb2, did not show changes in Rnf2 and Suz12 binding as well as H3K27Me3 levels in the Rest−/− mES cells (Figure 3B). Importantly, the mRNA levels for Brunol6 and Best2 were up-regulated in Rest−/ − mES cells while unchanged for Prrxl1 (Figure 3D), showing a functional link between the loss of PcG proteins, the reduced H3K27Me3 mark and gene activity in the absence of Rest. We confirmed the effects observed in the ChIP-seq profiles by direct ChIP (Figure 3C) using primers at the Rest peak position (as indicated by arrow heads in Figure 3B).
Fig. 3. Rest regulates recruitment of PRC1 and PRC2 at specific genomic loci. 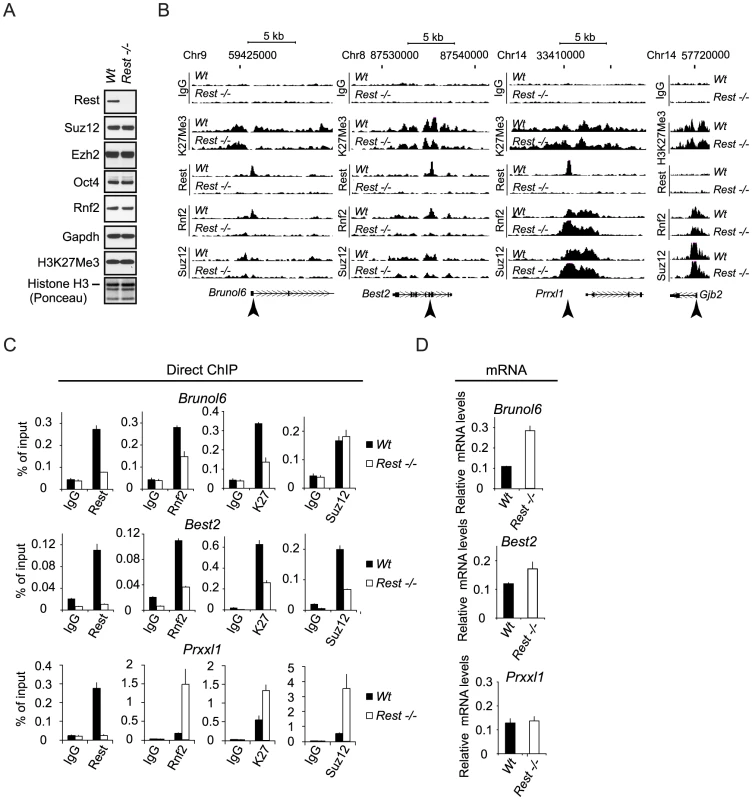
(A) Western blot analysis of Wt and Rest−/− mES cell lysates. Histones were extracted using LSB. (B) ChIP-seq profiles for Rnf2, Suz12 and H3K27Me3 levels in Wt and Rest−/− mES cells for three Rest target genes (Brunol6, Best2 and Prrxl1) and as control, one Polycomb target gene with no Rest binding site (Gjb2). Direct ChIP for the three Rest target genes shown in (B) comparing Wt and Rest−/− mES cells (primer positions are indicated by arrow heads in B and K27 = H3K27Me3) and mRNA levels (C). Error bars represent standard deviations calculated from triplicate qPCR reactions. Global analysis of Rest and PcG occupancy in Wt and Rest−/− mES cells
To obtain a global picture of how important Rest is for the recruitment of PRC1 and PRC2 in mES cells, we plotted the average distributions of Rnf2 (PRC1) and Suz12 (PRC2) signals in Wt and Rest−/ − mES cells from our ChIP seq analysis relative to 3,378 identified Rest binding sites in the Wt cells (Figure 4A and Table S2). We included Jarid2 in this analysis, since this protein has recently been shown to be important for PRC2 recruitment in mES cells [24], [25], [22], [23], [26]. These results showed that in the Wt mES cells Rnf2, Suz12 and Jarid2 all enrich at Rest binding sites. Interestingly, Rnf2 and Jarid2 displayed focused localization at the Rest binding sites, whereas Suz12 had a broader distribution. When comparing Rest−/ − to Wt mES cells we observed that the overall Rnf2 signal was reduced at the center of the Rest peaks, but was slightly increased in the flanking regions (Figure 4A). Surprisingly, the overall signal for Suz12 was clearly increased in the Rest−/ − mES cells, suggesting that Rest can function to limit PRC2 binding in certain contexts. Moreover, when looking at these average distributions, we observed a similar increase in the Jarid2 signal flanking the Rest binding sites in the Rest−/ − mES cells (Figure 4A), which is in agreement with Jarid2 being part of the PRC2 complex. As we observed reduced Rnf2 and Suz12 levels at specific Rest binding sites in the Rest−/− mES cells (Figure 3B and 3C), we wanted to investigate how frequently Rest target loci had lost or gained PcG-binding. For this we generated heat-maps based on ChIP-seq tracks for Rnf2, Suz12 and Jarid2 around individual Rest binding sites scored positive for PcG proteins either in Wt or Rest−/− mES cells (Figure 4B and Table S3). These heat-maps showed that Rnf2, Suz12 and Jarid2 all displayed a focused signal centered at a large fraction of the Rest binding sites in Wt mES cells, whereas this focused signal was absent in the Rest−/ − mES cells. To test if the PcG binding was specific for Rest binding sites, we generated matched control regions for all 3,378 Rest binding sites identified in Wt mES cells. Matched control regions were randomly chosen, but matched to the distribution of the Rest binding sites relative to TSS [42]. In agreement with the conclusion that Rnf2, Suz12 and Jarid2 enrich at Rest binding sites, we found roughly four to five times as many Rest binding sites scored positive for PcG proteins compared to the matched control regions (Figure 4D and 4E). Moreover, at the 395 Rest binding sites shown for Rnf2, the signal was reduced 1.5 fold or more at 172 sites (Ratio-image: blue) and increased 1.5 fold or more at 45 sites (Ratio-image: red) in the Rest−/ − mES cells compared to the Wt mES cells (Figure 4B; upper part). Examples of ChIP-seq tracks for target genes from upper and lower parts of the heat-maps are shown in Figure 4C (numbers 1–6 refers to the position in the individual heat-maps). For Suz12, we found that out of 292 Rest binding sites 45 and 105 sites had a signal that was reduced or increased 1.5 fold or more, respectively (Figure 4B; middle part). For Jarid2, we found that out of 300 Rest binding sites, the signal was reduced 1.5 fold or more at 85 sites and increased 1.5 fold or more at 152 sites (Figure 4B; lower part). Interestingly, heat-maps furthermore showed that the loss of binding in the Rest−/ − mES cells, for all three proteins (Rnf2, Suz12 and Jarid2) was focused at the Rest binding site. On the other hand, increased binding was more widely distributed and not confined to the actual Rest binding site (Figure 4B). This explains why we observed increased signals for PcG proteins in the regions flanking the Rest binding sites in Figure 4A. When the changes in PcG binding observed at Rest binding sites were compared to the changes at the matched control regions, we found that both the loss and the gain of signal observed at Rnf2 - and Jarid2-positive Rest binding sites, as well as the gain of signal at Suz12-positive Rest binding sites, occurred much more frequently than would be expected from Rnf2-, Jarid2-, and Suz12-positive matched control regions (Figure 4B and 4E).
Fig. 4. Occupancy of PRC1 and PRC2 at Rest peaks in Wt and Rest−/− mES cells. 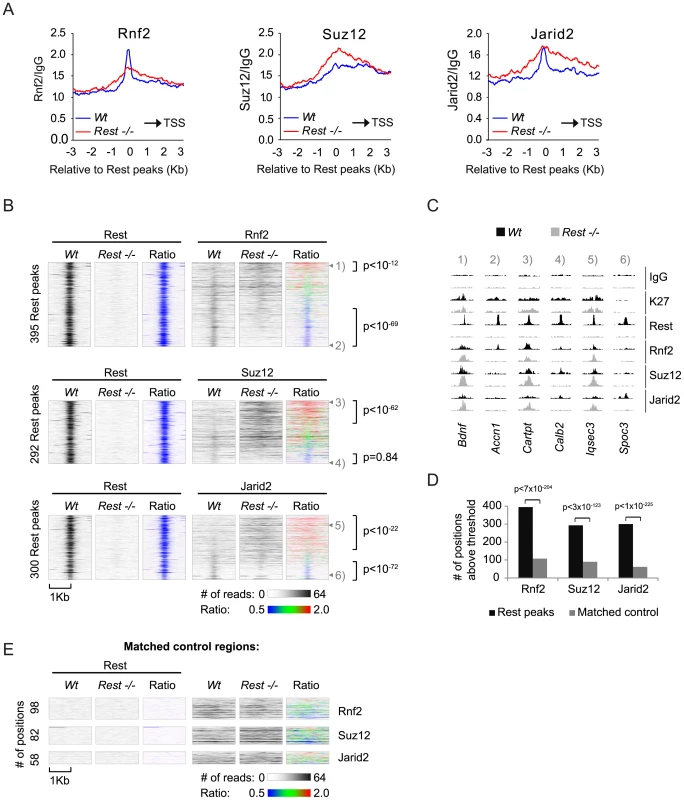
(A) Average ChIP-seq read counts in Wt control and Rest−/− mES cells, normalized to IgG controls for Rnf2, Suz12 and Jarid2 within 3 Kb of the 3,378 peaks identified for Rest in the Wt control cells. Black arrow indicates the direction of the nearest TSS. (B) Heat-maps showing Rnf2, Suz12 and Jarid2 occupancy in a 2 Kb window centered around individual Rest peaks identified in Wt control mES cells. The ratiometric heat-maps depict the part of the profiles that were increased, unaffected, or decreased in Rest−/− mES cells as red, green and blue, respectively. Out of the 3,378 Rest peak positions identified in the Wt control mES cells, positions with very low levels or no PcG (Rnf2, Suz12 and Jarid2) ChIP-seq signal in either Wt control or Rest−/− mES cells were filtered out (for thresholds and genomic peak positions see Table S3). The regions were sorted according to the fold change in PcG (Rnf2, Suz12 and Jarid2) signal between the Wt control and Rest−/− mES cells, within the Rest peaks. P-values were calculated by comparing the number of Rest binding sites with 1.5 fold increase or decrease to those expected from a population with similar distribution as the matched control using chi2-tests (C) ChIP-seq profiles for the positions marked 1 to 6 in Figure 4B as examples. Data from Wt and Rest−/− mES cells are shown in black and grey, respectively (K27 = H3K27Me3). (D) Diagram showing the number of Rest peaks and matched control regions (no Rest peaks) scored positive for Rnf2, Suz12 or Jarid2 signal. The filtering process described in the legend for Figure 4B. P-values compares binding sites scoring positive for PcG binding to those that scored positive by chance in the matched control regions using chi2-tests (E) Heat-maps of the matched control regions shown in 4D. In conclusion, we found that Rest was required for the specific recruitment of Rnf2 to a considerable number of Rest binding sites, whereas Suz12 and Jarid2 showed dependency on a smaller number of loci. In addition, many loci in the Rest−/ − mES cells, which bound Rest in the Wt mES cells, had increased levels of Suz12 and Jarid2. This was also observed for Rnf2, but less frequently, suggesting that Rest can also limit PcG protein recruitment and that other factors control how Rest affects the level of PRC1 and PRC2 at specific genomic loci.
Rest recruits PRC1 independently of CpG islands
Given the biochemical interaction between Rest and Rnf2 and the enrichment of Rnf2 at Rest binding sites, it was surprising to see that Rest binding sites in Rest−/ − mES cells, not always lost Rnf2 binding, but that some Rest binding sites even gained Rnf2 signal. To study this enigma further, we correlated the changes in Rnf2 binding between Wt and Rest−/− mES cells to several different parameters, such as PRC2 levels and distance to the nearest CpG island (Figure 5A). This analysis showed a strong correlation between changes in Rnf2 binding and the levels of Suz12 at Rest binding sites in both Wt and Rest−/− mES cells (Figure 5A). Changes in Rnf2 binding also correlated well with the distance to TSS and CpG islands, but not to the overall composition (% CpG or %GC) and size of the nearest CpG island (Figure 5A).
Fig. 5. Effects of different parameters on Rnf2 binding. 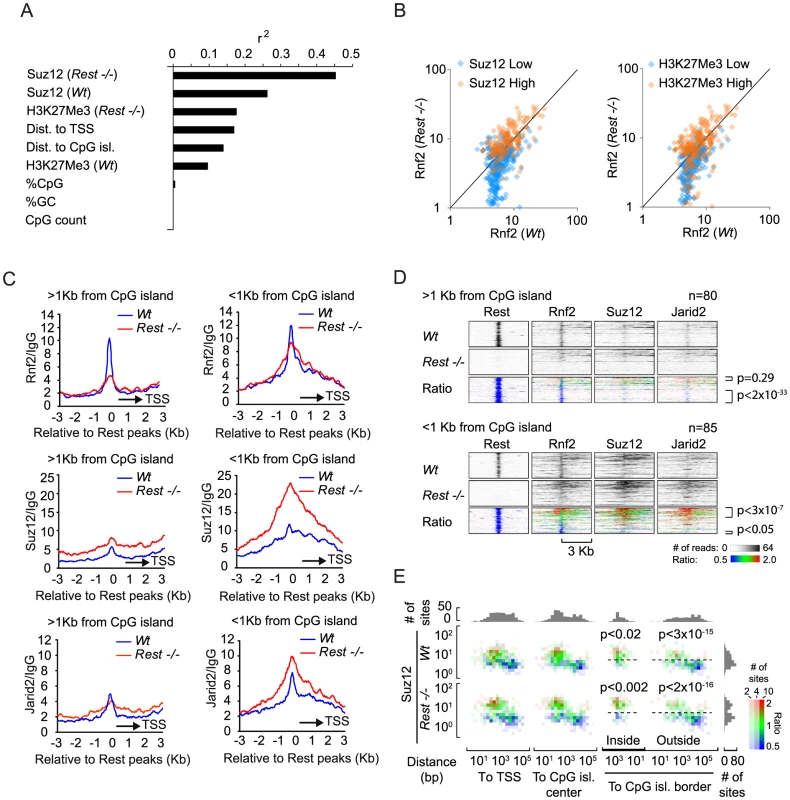
(A) Graph showing the Pearson's correlation coefficients (r2) of the change in Rnf2 at Rest peaks correlated to different parameters as indicated (the data were log-transformed before the correlation was done). (B) Scatter diagram showing the Rnf2 signal for the Rest peaks with either relative low (blue color) or high (red color) levels of Suz12 (left panel) or H3K27Me3 (right panel) in the Wt versus Rest−/− mES cells. The Rnf2 positive Rest peaks shown in Figure 4B were separated into two groups having either relative low (blue color: <5 reads on average within 1 Kb of the Rest peak) or relative high (red color: >5 reads on average within 1 Kb of the Rest peak) ChIP-seq signals for Suz12 and H3K27Me3. (C) Average ChIP-seq read counts for Rnf2, Suz12 and Jarid2 in Wt and Rest−/− mES cells normalized to IgG controls. The regions were subdivided into two groups, depending on whether or not a CpG island was located within 1 Kb of the borders of the Rest peaks. Only regions with a direct overlap between Rest and Rnf2 peaks in the Wt control mES cells were included (Table S4). (D) Heat-maps of Rnf2, Suz12 and Jarid2 for the individual peak positions analysed in C. The regions were sorted according to the fold change in PcG (Rnf2, Suz12 and Jarid2) signal between the Wt control and Rest−/− mES cells, within the Rest peaks. The ratiometric region map depicts the part of the profiles that were increased, unaffected, or decreased in Rest−/− mES cells as red, green and blue, respectively. (E) Color-coded density plots showing the difference in Rnf2 ChIP-seq signal in Wt and Rest−/− mES cells relative to the distance to TSS, distance to the nearest CpG-island, and the Suz12-signal at the 395 Rest peak positions that were scored Rnf2-positive. Densities, within each bin, depend on the number of peaks, whereas the color-coding corresponds to the average Rnf2Rest−/−/Rnf2Wt ratio with red, green and blue illustrating increased, unaffected, or decreased signal, respectively. Peaks were binned in 1.5 fold bins for Suz12 and 2 fold bins for the TSS or CpG position. The flanking histograms in the top and right part of the figure illustrate the number of peaks in each bin. P-values were calculated using heteroschedastic Student's t-tests. To analyze the PRC2 dependency for PRC1 recruitment further, we separated Rnf2-positive Rest binding sites into two groups, which either had relatively high or low Suz12 or H3K27Me3 signals in the Wt mES cells. The signal for Rnf2 in Wt versus Rest−/− mES cells was plotted in a scatter diagram (Figure 5B). The results showed that Rnf2 binding was more likely to be Rest-dependent at sites with low Suz12 and low H3K27Me3 levels (Figure 5B; blue color) compared to sites with high signals for Suz12 and H3K27Me3 in Wt mES cells (Figure 5B; orange color). In contrast, sites with high levels of Suz12 and H3K27Me3 were more likely to maintain or even gain Rnf2 binding in the absence of Rest. Furthermore, we observed that sites with increased PRC2 binding in general had increased Rnf2 levels in Rest−/− mES cells (Figure S3).
To study the effect of CpG islands on changes in Rnf2 binding in the absence of Rest, we separated a subset of Rest binding sites with the strongest Rnf2 binding (Wt mES) into two categories: 1) sites with an annotated CpG island within 1 Kb (CpG island-positive) and 2) sites more than 1 Kb away from any annotated CpG island (CpG island-negative) (UCSC browser). Using this approach we found that out of 165 Rest binding sites overlapping with Rnf2 sites app. 50% are within 1 Kb of a CpG island (Figure 5C, 5D and Table S4). Interestingly, when we plotted the average distribution of Rnf2 at CpG island-negative Rest binding sites (>1 Kb from a CpG island), we observed that the Rnf2 enrichment was almost completely lost at Rest binding sites in the Rest−/ − mES cells. In contrast, at CpG islands-positive Rest binding sites (<1 Kb from a CpG island), we observed only a minor decrease in Rnf2 binding (Figure 5C and 5D). For Suz12, the data showed that the levels of Suz12 were clearly higher at CpG islands-positive Rest binding sites, and that the absence of Rest resulted in a marked increase in Suz12 at these sites (Figure 5C and 5D). We found similar effects for Jarid2 (Figure 5C and 5D), suggesting that CpG islands could be part of a mechanism responsible for the increased recruitment of PRC2 in the absence of the transcription factor Rest in the mES Rest−/− mES cells.
Finally, we wanted to distinguish, whether the effect of CpG islands on Rnf2 binding was due to the presence of PRC2 at the CpG islands, or if CpG islands represented an independent entity responsible for Rnf2 recruitment. Therefore, we plotted the Rnf2Rest−/−/Rnf2Wt ratio of Rnf2-positive Rest binding sites relative to the position of the nearest TSS or the nearest CpG island, as well as to the level of Suz12 in either Wt or Rest−/− mES cells. As previously described, we observed that a larger fraction of Rnf2 positive Rest binding sites had increased Rnf2Rest−/−/Rnf2Wt ratio at positions close to TSS and CpG islands (Figure 5E, red coloring), compared to those that were located far from TSS or CpG islands (Figure 5E, blue coloring). Importantly, the key determinant for the change in Rnf2Rest−/−/Rnf2Wt ratios appeared to be the level of the PRC2 subunit Suz12. Rest binding sites with high Suz12 levels and located at or close to CpG islands, had significantly increased Rnf2Rest−/−/Rnf2Wt ratios, whereas those with low Suz12 levels had a lower Rnf2Rest−/−/Rnf2Wt ratio (Figure 5E). Also, a minor group of Rnf2-positive Rest binding sites, far from annotated CpG islands, had a high level of Suz12 and these had a correspondently increased Rnf2Rest−/−/Rnf2Wt ratio compared to sites with similar position and lower Suz12 levels. In summary, it appears from this analysis that 1) Rest was the dominant recruitment factor for PRC1 at Rest binding sites with low PRC2 levels, and 2) that the increased recruitment of Rnf2 observed at CpG islands in Rest−/− mES cells was an indirect effect of increased PRC2 recruitment to CpG islands and TSS-proximal Rest binding sites in the Rest−/− mES cells.
Recruitment of PRC1 to Rest binding sites, independent of Eed and the H3K27Me3 mark
The general view that PRC1 is recruited to chromatin exclusively by PRC2 activity [43] has recently been challenged, by the observation that mES cells lacking PRC2 activity still maintained H2AUbi marked histones catalyzed by the PRC1 complex [19]. Since we observed that Rest was needed for PRC1 recruitment at Rest binding sites with low levels of Suz12 and H3K27Me3 (Figure 5B), we wanted to test if Rest could recruit PRC1 to Rest binding sites in cells deficient for PRC2 activity. We performed ChIP-seq for Rest and Rnf2 in E14 Wt control and Eed−/− mES cells and found that app. 90% of the Rnf2 binding sites identified in the Wt cells were lost in Eed−/− cells (Table S6). In line with PRC1 being recruited to CpG islands via PRC2, we observed that the loss of Rnf2 binding sites were most pronounced for peaks positioned at or close to CpG islands (Figure 6A, upper part), while the majority of binding sites positioned distant from CpG islands were in general maintained in the Eed−/− mES cells (Figure 6A, lower part). Nonetheless, the average Rnf2 signal at Rest binding sites was only marginally reduced (Figure 6B, upper part) when analyzing all Rest binding sites both distant from CpG islands and at CpG island proximal regions. Notably in the Wt mES cells, we observed increased Rnf2 binding from the Rest binding sites towards the TSS, which was virtually lost in the absence of Eed (Figure 6B, upper part). Furthermore, when comparing the distribution of Rest peaks in Wt versus Eed−/ − mES cells, we only observed a minor effect indicating, that the overall binding of Rest to DNA was independent of PRC2 (Figure 6B, lower part).
Fig. 6. The recruitment of PRC1 at a number of Rest binding sites is independent of PRC2 activity in mES cells. 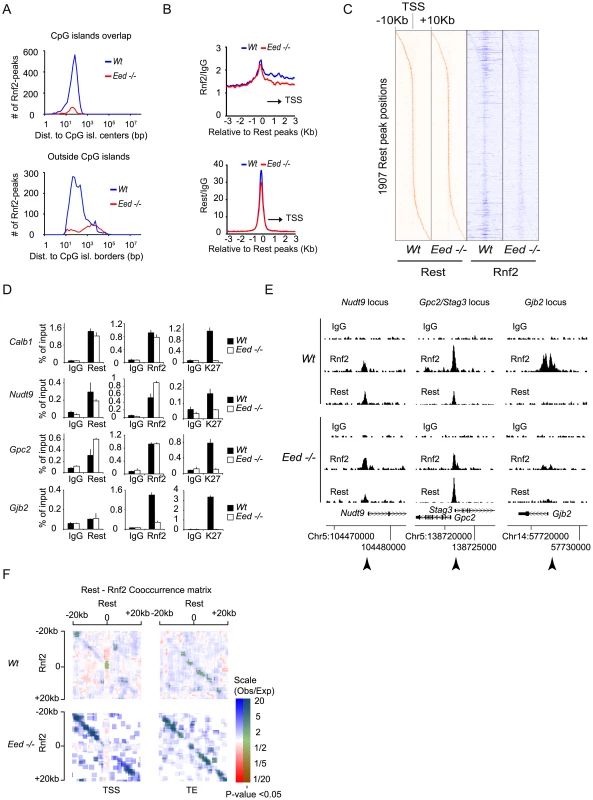
(A) Histograms showing the number of Rnf2 peaks in Wt and Eed−/− mES cells at positions relative to CpG islands. Upper panel: Rnf2 peaks that overlap with CpG-islands relative to the distance between the center of the peak and the center of the CpG-island. Lower panel: Rnf2 peaks, not overlapping with annotated CpG-islands, plotted relative to the distance between the borders of the Rnf2 peak and the border of the CpG-island. (B) Average ChIP-seq reads in E14 Wt and Eed−/− for Rnf2 (upper panel) and Rest (lower panel) within 3 Kb of the 5,097 Rest peaks identified in E14 Wt mES cells (Table S5). Read counts were normalized to the IgG control. The black arrow indicates the direction of the nearest TSS. (C) Heat-maps of Rest and Rnf2 in Wt and Eed−/− mES cells at 1,907 Rest peaks within 10 Kb of a TSS. (D) Direct ChIP in E14 Wt and Eed−/− mES cells for Calb1, Nudt9, Gpc2/Stag3 and Gjb2 (positions of primers used are indicated by arrow heads in E). Error bars represent standard deviations calculated from triplicate qPCR reactions. (E) ChIP-seq profiles for the Nudt9, Gpc2/Stag3 and Gjb2 loci using antibodies for Rnf2, Rest and control IgG. (F) Co-occurrence matrix of Rest and Rnf2 peaks from Wt and Eed−/− mES cells. The maps are based on scans, with a 2,500 bp window, along different positions near the transcription start site (TSS) or transcription end points (TE) annotated in the mouse genome (Mouse genome: mm9). For each scan the number of observed peaks were calculated and normalized to the number of expected peaks based on the frequency of Rest and Rnf2 peaks in that particular position, relative to the TSS or TE. Scans with a higher or lower frequency than expected from random localization, were colored blue or red, respectively. Scans with a P-value below 0.05 (Bonferroni corrected for multiple sampling), are overlaid with transparent green color. In order to visualize the data summarized by the average distributions in Figure 6B, for individual genes, we generated heat-maps based on ChIP-seq tracks for individual Rest binding sites within 10 Kb of a TSS (Figure 6C). In agreement with the data in Figure 6B, the wide-spread Rnf2 signals in the Wt was virtually lost in the Eed−/ − mES cells, but the signal was clearly maintained at the Rest binding sites (Figure 6C).
To confirm the results obtained by ChIP-seq, we performed direct ChIP on four genes. We compared the Rnf2 binding at Rest binding sites in Wt and Eed−/ − cells and also included a ChIP for the H3K27Me3 mark. As shown in Figure 6D, the four genes behaved differently. Gjb2, a PcG target gene [44] showed very low levels of Rest binding in the Wt mES cells, which was not affected in the Eed−/ − mES cells, while H3K27Me3 was absent and Rnf2 strongly reduced. For the Calb1 gene, a classical Rest target [45], we confirmed a strong Rest enrichment, which correlated with enrichment for Rnf2 in the Wt mES cells (Figure 6D). Even though H3K27Me3 was absent at the Calb1 locus in the Eed−/ − mES cells the binding of Rnf2 was preserved (Figure 6D), demonstrating PRC2 - and H3K27Me3-independent recruitment of Rest-PRC1. Nudt9 and Gpc2, two other Rest targets in mES cells showed either a small decrease or increase, respectively, in Rest binding in the Eed−/− mES cells as compared to the Wt mES cells, while Rnf2 was not reduced. Similarly, we detected Cbx7 binding on two non-Rest PcG targets (Gjb2 and Neurog1) in Wt mES cells that was lost in Eed−/− mES cells, while Cbx7 was still bound to the Stag3 gene in Eed−/− mES cells (Figure S2). In Figure 6E we present ChIP-seq profiles for the Nudt9, Gpc2/Stag3, and Gjb2 loci, which reflects the results obtained by direct ChIP (ChIP followed by QPCR analysis; arrow heads indicate the position of primers used in Figure 6D). It is noteworthy that not all Rest binding sites were preserved between the two Wt mES cell lines (compare Figure 3B and Figure 6E to Figure S4).
Since both Rnf2 and Rest binding sites in E14 Wt mES cells frequently appeared in close proximity of the TSS of genes, we asked whether Rest and Rnf2 co-localized more frequently than expected by chance in Wt and Eed−/ − mES cells. We calculated the frequencies of Rest binding sites alone, Rnf2 sites alone or in combination with Rest, 20 Kb up - and down-stream of all annotated TSS positions and transcription ends (TE) in the mouse genome (mm9). We plotted ratios between the observed frequencies versus the frequencies expected by chance in a co-occurrence matrix (Figure 6F). The observed co-occurences of Rest and Rnf2 at the TSS in Wt mES cells were actually significantly below what could be expected by chance (red), whereas upstream and downstream of TSS and at TE, we observed significantly more co-occurrence than expected by chance (blue) (Figure 6F). Remarkably and in agreement with Rnf2 binding being preserved at Rest binding sites in the Eed−/ − mES cells, we observed much higher co-occurrence than expected by chance both up - and down-stream of the TSS and TE in the absence of PRC2 activity.
Taken together these results substantiate the relation between PRC1 and Rest in mES cells and suggest that Rest-PRC1 can be recruited to neuronal genes independently of the PRC2 complex.
Neuronal differentiation of NT2-D1 cells
Finally, we wanted to study the interplay between REST and the PcG complexes at genes that are induced during differentiation. In order to do this we took advantage of the fact that NT2-D1 cells can be induced to differentiate along the neuronal path by retinoic acid (RA) - stimulation, and used these cells to study the dynamics of REST and RNF2 binding on a number of selected target genes that were co-regulated by REST and RNF2 (Figure 2B). RA-stimulation for 3 days led to an almost complete loss of the pluripotency factor OCT4 (Figure 7A), while the protein levels of EZH2, RNF2 and REST were only marginally affected. Furthermore, OCT4 and another pluripotency factor NANOG were almost completely silenced transcriptionally (Figure 7B), while the REST transcript was unaffected and HOXA1 was strongly induced (Figure 7B). The genes we selected for ChIP analysis were all induced by RA-stimulation (Figure 7D and 7F), but can be divided into two groups, based on the events taking place at the REST binding sites. The first group is represented by MEIS1 and TBX3, which both have reduced REST binding to the gene after 3 days of RA-stimulation (Figure 7C). These genes lost RNF2 binding and had reduced H3K27Me3 after RA treatment. For the second group of genes represented by MEIS2 and DLX5, REST stayed on the genes after 3 days of RA-stimulation, while RNF2 was lost (Figure 7E). These genes also lost H3K27Me3 (MEIS2) or had significantly reduced H3K27Me3 after RA-stimulation (DLX5). The data suggested, that gene induction in response to RA-stimulation does not necessary require displacement of REST, but correlated with the displacement of PRC1 (RNF2) and reduction in H3K27Me3. The determining factor(s) for how REST-PcG complexes are regulated at specific target genes is still an open question. We imagine that post-translational modifications of REST and/or PcG proteins could be part of a mechanism regulating the interaction(s) between these factors and the affinity of REST for DNA. Furthermore, other REST associated factors, beside the PcG proteins, could affect the affinity of REST complexes for chromatin on individual genomic loci.
Fig. 7. Neuronal differentiation in NT2-D1 cells. 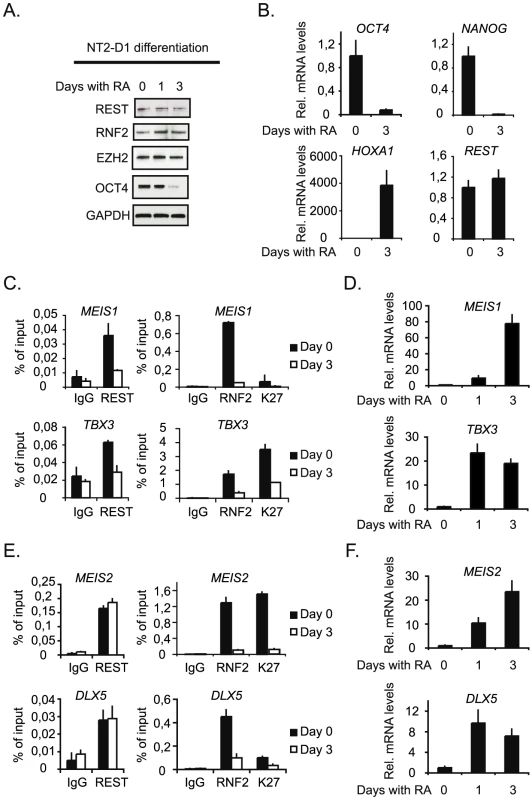
NT2-D1 cells were treated with Retinoic Acid (RA) for 1 and 3 days or left untreated (day 0). Protein-, RNA- and ChIP-samples were harvested. (A) Western blot analysis of protein samples taken at day 0, 1 and 3. (B) Relative mRNA levels at day 0 and 3 of REST, OCT4 and NANOG and HOXA1. (C) Group of genes, which loose binding of both REST and PcG proteins during differentiation. ChIP analysis was performed for day 0 and 3 of RA treatment. (D) Relative mRNA levels of the genes displayed in (C), for untreated cells and cells treated with RA for 3 days. (E) Group of genes, which maintain REST binding but loose PcG proteins during differentiation. ChIP analysis at day 0 and 3 after RA treatment. (F) Relative mRNA levels of the genes displayed in (E) after 0 and 3 days of RA treatment. Error bars represent standard deviations calculated from triplicate qPCR reactions. Discussion
In Drosophila there are at least 5 different transcription factors shown to be important for PcG recruitment [32], however, among these only the ortholog of Pho called YY1 is preserved in mammals. Even though YY1 and a second transcription factor, E2F6, have been found to interact with PRC2 [36], [46] and PRC1 in mammals [13], [14], [35], [47], there have been only two studies presenting evidence to support the existence of mammalian PREs. The first potential mammalian PRE identified was an element shown to regulate the expression of a hindbrain segmentation gene MafB (Kreisler). This putative PRE was shown to recruit PRC1 and PRC2, thereby mediating silencing of an ectopically introduced transgene in both flies and mice [48]. In a second study Woo et al. found a 1.8 Kb element between the HOXD11 and HOXD12 genes that was regulated by PcG proteins and H3K27Me3 [49]. Interestingly, this locus contained several YY1 binding sites and YY1 binding to the locus was coincident with PcG enrichment. Loss of YY1 binding sites had however only modest effect on repression.
Based on previous studies showing H3K27Me3 enrichment around REST binding sites (RE1 elements) in human T-cells [50] and the fact that we identified REST in the purification of the CBX8 interacting protein HAN11 ([44] and Figure S1), we decided to investigate the functional relationship between REST and the PRC1 - and PRC2-complexes.
We found that REST was in complex with core members of both the PRC1 - and PRC2-complexes in the human NT2-D1, HEK293 cells and mouse embryonic stem cells. For PRC2 we found that REST interacted with the three essential core members: EZH2, SUZ12 and EED, showing that REST was associated to a functional PRC2 complex. Due to the existence of both PRC1 and other RNF2 containing complexes, we performed a more detailed analysis of the REST-PRC1 complex. This analysis revealed that REST form complex with RNF2 and other selected core members of the PRC1 complex (BMI1, NSPC1, CBX7 and CBX8). Importantly, we did not detect E2F6, HP1γ or BCOR in the REST IPs, proteins that have previously been described in RNF2 containing E2F6.com-1 (E2F6 and HP1γ) [13] and Fbxl10-BcoR (BcoR and HP1γ) [14], [37] complexes. These observations suggest that the REST-PRC1 complex that we have identified is distinct from the previously described Rnf2-containing complexes.
Recently, an interesting work related to PRC2 and Rest was published by Tsai et al. [27]. They found that the previously described long non-coding RNA, HOTAIR [51], [52] interacted not only with PRC2 through its 5′end, but even bound a LSD1/CoREST/REST-complex through the 3′end and in this fashion function as a modular bifunctional RNA. To understand if HOTAIR or other ncRNAs could be involved in bridging PRC1 and PRC2 to REST in the complexes that we identified, we treated our REST immunoprecipitates from NT2-D1 cells with RNases, but did not observe changes in the relative amounts of PRC1 and PRC2 subunits bound to REST. Thus, we conclude that the REST-PcG complexes that we have identified are stable in the absence of ncRNAs such as HOTAIR. Further studies are needed to determine if PRC1 and PRC2 are part of one and the same REST-PcG complex or are separate entities.
In support of a functional interaction between REST and PRC1, we found a significant number of genes that were co-regulated in the NT2-D1 cells. In line with REST and PRC1 being repressors of gene activity, the majority of co-regulated genes were up-regulated (258) and gene-ontology analysis revealed an enrichment of genes involved in development and neuronal function (Table S9). This is in agreement with previous publications showing that REST and PRC1 directly target genes involved in developmental processes and neuronal function. In contrast, the group of co-down-regulated genes did not enrich for any particular biological function suggesting that this group of genes were not primary targets of REST and PRC1.
In our genome-wide analysis of Rest and PcG occupancy in mES cells, we furthermore found that both PRC1 (Rnf2) and PRC2 (Suz12, Jarid2) subunits specifically enriched at Rest binding sites. Similarly to the co-up-regulated genes identified in NT2-D1 cells, there was enrichment for genes involved in developmental and neuronal function among the genes targeted by both Rest and the Polycomb complexes (Figure S5 and Table S7) suggesting a similar set of target genes in these different cell types of human (NT2-D1 cells) and mouse origin (mES cells). However, the loss of REST in NT2-D1 and mES cells have different outcome with respect to transcription. Whereas, loss of REST in NT2-D1 cells leads to up-regulation of a large group of genes (1862) the effect in mES cells was less than expected and did not compare to the number of target genes [41] (Tables S1 and S2). This suggest that genes bound by REST in NT2-D1 and mES cells are differently regulated and that in mES cells, Rest target genes are regulated by additional repressive mechanisms, which ensure that developmental genes are kept in an “off-state”.
The genome-wide analyses for PcG occupancy in mES cells, demonstrated a specific enrichment of the average Rnf2, Suz12 and Jarid2 signals at the positions corresponding to Rest binding sites (Figure 4A and Figure 6B). To improve the validity of these observations, we included a range of controls and extra analyses. Bona fide signals may co-exist at specific genes or gene features in a manner that seems significant due to congregations at groups of genes or gene features. Therefore, we used a set of randomly chosen control positions that matched the Rest-binding sites in terms of TSS-proximity. In comparison, Rest binding sites scored positive for PcG proteins (Rnf2, Suz12 and Jarid2) 4–5 times as frequent as the matched control regions (Figure 4D). Co-occurrence analysis of Rest and Rnf2 furthermore allowed us to rule out that Rest and Rnf2 co-enriched by chance because both proteins were frequently found at TSS (Figure 6F). With these tests, we are confident that the co-localization of Rest and PcG complexes in the genome was unlikely to be a mere coincidence. In support of this we furthermore found that Rest was required for PcG protein binding to a highly significant number of Rest binding sites in mES cells as well as for the binding to a selected number of neuronal target genes in NT2-D1 cells. Taken together our observations, strongly support a role for Rest as a DNA specific PcG recruitment factor.
To understand if PRC2 was required for Rest-mediated PRC1 binding, we took advantage of the Eed−/− mES cells, which lack PRC2 activity and therefore the H3K27Me3 mark [53]. Accordingly, PRC1, which is recruited to H3K27Me3 marked chromatin regions through the chromodomain-containing CBX proteins, was expected to be displaced from chromatin in cells lacking Eed. Nonetheless, when Eed−/− mES cells were compared to Wt mES cells, the Rnf2 signal strength was only marginally affected at Rest binding sites, although roughly 9 out of 10 Rnf2-binding sites were lost in Eed−/− mES cells (Figure 6A and 6B). This interesting finding demonstrated that Rest-dependent recruitment of PRC1 to chromatin occured independently of PRC2 activity and the H3K27Me3 mark, and offers a mechanism that, in part, could explain why Wt and Eed−/− mES cells have similar levels of the PRC1 catalyzed H2AUbi mark [19].
Given the aforesaid recruitment of PcGs to Rest binding sites and our biochemical data showing interaction between Rest, PRC2 and PRC1, we were highly puzzled by the observation that the absence of Rest, at a subpopulation of Rest binding sites, resulted in an increased binding of both PRC1 and PRC2 members in the Rest−/− mES cells. Interestingly, comparisons with matched control regions, revealed that both gain and loss of PcG binding in the Rest−/− mES cells were highly significant (except Suz12 loss) and specific to Rest binding sites. This suggested that the changes in PcG binding between Wt and Rest−/− mES cells indeed was a result of the absence of Rest and that Rest recruited PcG proteins to some genomic loci, while limiting the binding to others. In agreement with the view that PRC1 is recruited downstream of PRC2, the increase in Rnf2 binding at some Rest binding sites were linked to a similar increase in Suz12 binding at these sites (Figure S3). Moreover, when the influence of the position relative to TSS or CpG-islands was compared to that of PRC2 levels, it was clear that the gain in Rnf2 binding observed at Rest binding sites, close to TSS and CpG-islands, were indirect consequences of increased PRC2 recruitment to these entities (Figure 5E). Based on this, and the genome-wide analyses of Rnf2 in Eed−/− mES cells, we propose that PRC2 is dispensable for the majority of PRC1 recruitment far from CpG-islands, whereas PRC2 is the prime mechanism responsible for CpG-island proximal PRC1-recruitment, and only a minority of the CpG-island proximal Rnf2 binding sites is instead depending on alternative mechanisms, such as Rest. The observed gain of PRC2-signal at many Rest binding sites in the Rest−/− mES cells, suggest that Rest not only serves as a factor recruiting PcG complexes, but can directly or indirectly limit the amounts of PcG proteins on chromatin near some of its binding sites. Indeed, Rest is known to interact with other co-repressors (HDACs, CoRest/Lsd1 and G9a), which may affect chromatin structure in a way that limit PRC2 binding. It is tempting to speculate that this is part of a mechanism, which has evolved to increase robustness in gene regulation by compensating for the loss of one repressive complex, and thereby ensure redundancy and fidelity in the silencing of lineage specific genes. This might also be the reason for the relatively modest effects of Rest knock-out or knock-down on gene expression in mES cells [41] compared to the more pronounced changes observed in the NT2-D1 cells (Figure 2B). In line with this, using the expression analyses data from Færk et al. [41], looking at genes with either gain or loss of Rnf2 or Suz12 from our ChIP-seq analysis, we found no significant effects on gene expression correlating with the change in PcG binding in mES cells (Figure S6), although these genes are regulated by PcG according to Leeb et al. (Figure S7).
During the final preparation of our work a study from the Kerppola laboratory was published [54], which supports our findings of an interaction between PRC1 and Rest. Based on a selected number of genes, divided into either promoter proximal - or promoter distal-Rest binding sites, they concluded that Rest inhibits PRC1 recruitment at proximal binding sites, while recruiting PRC1 at distal binding sites. Since promoter proximal elements are rich in CpG islands this conclusion is in agreement with our genome-wide analysis of PcG protein binding and annotated CpG islands, which showed that Rest is required for PRC1 binding in the absence of CpG islands. However, it should be noted that whereas the Ren et al. study, on the basis of relatively few selected target genes, gives the impression that all Rest binding sites co-localize with PRC1, our genome wide analysis show direct overlap of Rest and PRC1 on 165 Rest binding sites out of a total of 3,378 Rest binding sites identified in our ChIP-sequencing analysis in mES Wt cells. Of these 165 co-localizing sites, 80 were found outside promoter proximal CpG islands and 85 on CpG islands. Furthermore, our study revealed that the increase of PRC1 (Rnf2) at promoter proximal Rest binding sites correlated with an increased recruitment of PRC2 to CpG islands at these locations. The increase in PRC2 was not directly translated into increased H3K27Me3 as one might expect, which suggest that H3K27Me3 in those regions were already quite high. Therefore, it is possible that the recruitment of PRC2 to these CpG rich regions is controlled by other histone modifications mediated by Rest complexes, which prevent efficient binding of PRC2 to the H3K27Me3 marked regions [55]. Furthermore, related to the fact that only about 5% of the Rest binding sites in mES cells showed co-localization with Rnf2, although PRC1 complexes are very abundant, suggest that other factors have influence on the recruitment of the Rest-PRC1 complex. Even though, we found that the Rest-PcG protein complexes were biochemically stable in the absence of ncRNAs (RNase treatment) it is still possible that ncRNAs such as HOTAIR could play a significant role in stabilizing Rest-PcG complexes on chromatin and thereby influence target gene specificity. Considering that Rest binding sites might be part of mammalian PRE elements, it is furthermore likely that other transcription factors, binding in the vicinity of Rest and with affinity for PcG complexes, influence whether or not PRC1 is stably interacting with REST on a particular binding site. Since our HAN11 double-tag affinity purification revealed a number of other transcription factors beside REST, future studies will aim at investigating if any of those can co-operate with REST in targeting PcG complexes to specific genomic loci.
In conclusion, we have shown that the transcription factor REST interacts with PRC1 - and PRC2-complexes, interactions that we found to be independent of ncRNAs. Importantly, our data furthermore showed that the PRC1 complex can be recruited to a number of Rest binding sites independently of PRC2 activity and CpG islands. Surprisingly, we also found that there exist a CpG-island-associated increased recruitment of PRC2 in the absence of Rest, at a number of genes. We propose that this up-regulation of PRC2 binding functions to prevent unscheduled activation of differentiation specific genes and contribute to the robustness of mES cells. To understand the details regarding this compensatory mechanism and whether genomic regions, recruiting REST-PRC1 independently of PRC2 activity, are part of mammalian Polycomb Responsive Elements (PREs) will be the focus of future experiments.
Materials and Methods
Cell culture
Mouse embryonic stem (mES) cells were cultured on 0.1% (w/v) gelatin-coated plates in Glasgow medium (Sigma) supplemented with glutamax-1 (Gibco), non-essential amino acids (Gibco), 50 µM 2-mercaptoethanol–PBS, 15% ES-cell-qualified FBS (Gibco) in the presence of 1,000 U/ml of LIF (Millipore), and 1% (v/v) pen/strep. In addition, the Rest−/ − and Wt control mES cells were cultured in the presence of feeder cells (Mitomycin C treated primary mouse embryonic fibroblasts). The mES Eed−/ − and Rnf2−/ − mES cells were provided by Dr. Anton Wutz. The Rest−/ − and Wt control mES cells were provided by Dr. Zhou-Feng Chen and Dr. Helle Færk Jørgensen. HEK293FT and NT2-D1 cells were grown in DMEM (4.5 g/l D-glucose, Gibco) supplemented with 10% (v/v) FCS and 1% (v/v) pen/strep.
To induce neuronal differentiation of NT2-D1 cells, exponentially growing cells were seeded at 30% confluency and 24 hours later retinoic acid (RA) was added to a final concentration of 10 µM. Medium was changed every second day.
Size-exclusion chromatography and immunoprecipitation
Cells or nuclear preparations were lysed in high-salt (HS) lysis buffer (50 mM Tris, pH 7.2, 300 mM NaCl, 0.5% (w/v) Igepal CA-630, Leupeptin (1 µg/ml), Aprotinin (1 µg/ml), 1 mM PMSF, 1 mM EDTA (pH 7.4)). After a short 2 second ultrasonication pulse (Branson Sonifier; 20% max amplitude) the lysates were left on ice for 30 min. After centrifugation at 20,000 g (4°C) for 15 min and 100,000 g for 30 min the lysates were passed through a 0.45 µm low protein binding filter (Ultrafree MC spinfilter, Millipore) followed by a 0.22 µm filter before the protein concentration was determined by Bradford assay (BioRad). Between 5 and 10 mg of protein was loaded on a Superose 6 HR 10/300 (24 ml) equilibrated in GF buffer (25 mM Tris, pH 7.2, 150 mM NaCl, 0.2% (w/v) Igepal CA-630, 1 mM EDTA (pH 8.0), 1% (w/v) glycerol) in 1 ml (flowrate 0.3 ml/min). One ml fractions were collected (4°C) and characterized by Western blotting and afterwards processed for immunoprecipitation by pooling peak fractions of interest. Typically 100–200 µl (pooled fractions) was used for each immunoprecipitation using anti-REST or control IgG. For direct Western blotting, to visualize the elution profile of individual proteins, an equal volume of each fraction was mixed with 2X LSB and heated at 95°C for 5 min. Ten µl was loaded per lane for SDS-PAGE and Western blotting. All SDS-PAGE gels used were precast 4–12% gradient gels or 10% homogenous gels (Invitrogen) using the MES buffer system.
Anti-REST and general IgG (rabbit; DAKO) were pre-coupled to proteinA-Sepharose beads (0.5 µg antibody per 40 µl 1∶1 slurry) and cross-linked using DMP (dimethyl pimelimidate dihydrochloride, Fluka) at pH 9.0 in 200 mM borate buffer according to standard procedures.
For samples treated with RNase V1 and RNase A the immunoprecipitates were washed twice in HS buffer followed by two washes in HS buffer adjusted to 500 mM NaCl and ones in HS buffer before adding 0.1 Unit of RNase V1 (Ambion) and 0.1 Unit of RNase A (Roche) in HS buffer. Samples were incubated at 10°C for 30 min followed by one wash in HS buffer, one wash in 500 mM HS buffer and one final wash in HS buffer. Samples were eluted in 2X LSB and heated to 95°C for 5 min before SDS-PAGE and immunoblotting.
Quantification of mRNA levels by qPCR
RNA was purified using RNeasy Plus Mini kit (Qiagen) and cDNA was generated by RT–PCR. Quantifications were done using the Fast SYBR Green Master Mix (Applied Biosystems) and an ABI Step One Plus. Beta-Actin was used for normalization. The sequences of the primers used can be found in Table S8.
ChIP assays
Cells were fixed for 10 min in culture media containing 1% formaldehyde and were processed for ChIP as previously described [44]. Antibodies used: Rabbit IgG (DAKO), rabbit anti-H3K27Me3 (Cell Signalling, C36B11), rabbit anti-H3 (GERA; antigen sequence: CGIQLARRIRGERA), rabbit anti-REST (Millipore, 07-579), rabbit anti-RNF2 (NAST; antigen sequence: NASTHSNQEAGPSNKRTKT), rabbit anti-SUZ12 (Cell signaling), rabbit anti-Jarid2 (Novus Biologicals), anti-CBX7 (“RELF”, [44]), anti-CBX8 (“LAST”, [44]), anti-NSPC1 (XW5).
Western blotting
Western blotting was performed according to standard procedures using the following antibodies: Anti-RNF2 (“NAST”), anti-REST (Millipore, 07-579), anti-EZH2 (BD43-43), anti-OCT4 (ab19857, Abcam), anti-GAPDH (6G5, Biogenesis), anti-TUBULIN (Sigma, T6074), goat anti-SUZ12 (Santa Cruz, sc-46264), anti-BCOR (Novus Biologicals, NB100-87005), anti-CBX7 (“RELF”, [44]), anti-CBX8 (“LAST”, [44]), anti-NSPC1 (XW5), anti-E2F6 (TFE61), anti-BMI1 (DC9), anti-EED (AA19). Blots were developed using HRP-conjugated anti-rabbit, mouse or goat antibodies, depending on the species of the primary antibody, and enhanced chemiluminescence (ECL; Pierce). All exposures were done using Hyperfilm (Amersham).
ShRNA and lenti virus production
VSV-G virus particles were generated by calcium phosphate-mediated co-transfection of PAX8 and VSV-G plasmids together with the pLKO.1 targeting construct in 293FT cells. pLKO.1 targeting constructs: pLKO.1-RNF2 (TRCN0000033697, Sigma), pLKO.1-REST (TRCN0000014783, Sigma) and pLKO.1-RCOR1 (TRCN0000147184). An empty pLKO.1 vector was used as control. NT2-D1 cells were incubated with virus supernatant for 8 hours and selection using 2 µg/ml puromycin started after 24 hrs PI. The NT2-D1 cells were harvested for ChIP, WB, and RNA after 4 days of selection.
Affymetrix microarray
Total RNA was extracted from NT2-D1 cells treated with pLKO.1-Ct or shRNA to knockdown REST and RNF2 mRNA. RNA was prepared from 3 independent experiments. 300 ng of total RNA from each experiments was processed for microarray expression analysis according to Affymetrix standard procedures. Up-regulated genes were identified using Microsoft Excel 2003 by individual probe-sets showing more than a two-fold change compared to control and with p-values below 0.05 in Student's t-test. Cluster analysis was performed using BRB-ArrayTools v3.81 (http://linus.nci.nih.gov/BRB-ArrayTools.html) using standard settings. Before cluster analysis probe sets were first filtered for 1) at least one array with a read above 10 and 2) more than one array deviating at least 1.5 fold from average.
ChIP sequencing
DNA from three parallel ChIPs were pooled and 10 ng was used for making ChIP-seq libraries. Libraries were generated according to Illumina recommendations and sequencing was done on a Genome Analyzer II (Illumina). High quality reads (Chastity score > = 0.6) were aligned to the mouse genome (mm9) using Eland (Illumina) allowing up to two mismatches within the first 32 bases. Reads not aligning uniquely to the mouse genome were removed and profiles were presented using the UCSC Genome Browser (http://genome.ucsc.edu/) [56]. For detailed information about data handling and analyses see Procedure S1.
Supporting Information
Zdroje
1. SchuettengruberBCavalliG 2009 Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development 136 3531 3542
2. CaoRWangLWangHXiaLErdjument-BromageH 2002 Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298 1039 1043
3. CzerminBMelfiRMcCabeDSeitzVImhofA 2002 Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111 185 196
4. KuzmichevANishiokaKErdjument-BromageHTempstPReinbergD 2002 Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev 16 2893 2905
5. MullerJHartCMFrancisNJVargasMLSenguptaA 2002 Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111 197 208
6. FaustCLawsonKASchorkNJThielBMagnusonT 1998 The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development 125 4495 4506
7. O'CarrollDErhardtSPaganiMBartonSCSuraniMA 2001 The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol 21 4330 4336
8. PasiniDBrackenAPJensenMRLazzerini DenchiEHelinK 2004 Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J 23 4061 4071
9. SimonJAKingstonRE 2009 Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol 10 697 708
10. de NapolesMMermoudJEWakaoRTangYAEndohM 2004 Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 7 663 676
11. FangJChenTChadwickBLiEZhangY 2004 Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in initiation of X inactivation. J Biol Chem 279 52812 52815
12. VonckenJWRoelenBARoefsMde VriesSVerhoevenE 2003 Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci U S A 100 2468 2473
13. OgawaHIshiguroKGaubatzSLivingstonDMNakataniY 2002 A complex with chromatin modifiers that occupies E2F - and Myc-responsive genes in G0 cells. Science 296 1132 1136
14. SanchezCSanchezIDemmersJARodriguezPStrouboulisJ 2007 Proteomics analysis of Ring1B/Rnf2 interactors identifies a novel complex with the Fbxl10/Jhdm1B histone demethylase and the Bcl6 interacting corepressor. Mol Cell Proteomics 6 820 834
15. BoyerLAPlathKZeitlingerJBrambrinkTMedeirosLA 2006 Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441 349 353
16. LeebMWutzA 2007 Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells. J Cell Biol 178 219 229
17. EndohMEndoTAEndohTFujimuraYOharaO 2008 Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development 135 1513 1524
18. van der StoopPBoutsmaEAHulsmanDNobackSHeimerikxM 2008 Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLoS ONE 3 e2235 doi:10.1371/journal.pone.0002235
19. LeebMPasiniDNovatchkovaMJaritzMHelinK 2010 Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev 24 265 276
20. FischleWWangYJacobsSAKimYAllisCD 2003 Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev 17 1870 1881
21. BernsteinEDuncanEMMasuiOGilJHeardE 2006 Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol 26 2560 2569
22. LiGMargueronRKuMChambonPBernsteinBE 2010 Jarid2 and PRC2, partners in regulating gene expression. Genes Dev 24 368 380
23. PasiniDCloosPAWalfridssonJOlssonLBukowskiJP 2010 JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 464 306 310
24. PengJCValouevASwigutTZhangJZhaoY 2009 Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139 1290 1302
25. ShenXKimWFujiwaraYSimonMDLiuY 2009 Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell 139 1303 1314
26. LandeiraDSauerSPootRDvorkinaMMazzarellaL 2010 Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nat Cell Biol 12 618 624
27. TsaiMCManorOWanYMosammaparastNWangJK 2010 Long noncoding RNA as modular scaffold of histone modification complexes. Science 329 689 693
28. YapDBChuJBergTSchapiraMChengSW 2010 Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood
29. ZhaoJOhsumiTKKungJTOgawaYGrauDJ 2010 Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell 40 939 953
30. KanhereAViiriKAraujoCCRasaiyaahJBouwmanRD 2010 Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell 38 675 688
31. MendenhallEMKocheRPTruongTZhouVWIssacB 2010 GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet 6 e1001244 doi:10.1371/journal.pgen.1001244
32. MullerJKassisJA 2006 Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr Opin Genet Dev 16 476 484
33. RingroseLParoR 2007 Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development 134 223 232
34. SchwartzYBPirrottaV 2007 Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet 8 9 22
35. LorenteMPerezCSanchezCDonohoeMShiY 2006 Homeotic transformations of the axial skeleton of YY1 mutant mice and genetic interaction with the Polycomb group gene Ring1/Ring1A. Mech Dev 123 312 320
36. SatijnDPHamerKMden BlaauwenJOtteAP 2001 The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol Cell Biol 21 1360 1369
37. GearhartMDCorcoranCMWamstadJABardwellVJ 2006 Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol Cell Biol 26 6880 6889
38. AndrewsPW 1984 Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol 103 285 293
39. AndresMEBurgerCPeral-RubioMJBattaglioliEAndersonME 1999 CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci U S A 96 9873 9878
40. ChenZFPaquetteAJAndersonDJ 1998 NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet 20 136 142
41. JorgensenHFTerryABerettaCPereiraCFLeleuM 2009 REST selectively represses a subset of RE1-containing neuronal genes in mouse embryonic stem cells. Development 136 715 721
42. JiHVokesSAWongWH 2006 A comparative analysis of genome-wide chromatin immunoprecipitation data for mammalian transcription factors. Nucleic Acids Res 34 e146
43. PietersenAMvan LohuizenM 2008 Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol 20 201 207
44. DietrichNBrackenAPTrinhESchjerlingCKKosekiH 2007 Bypass of senescence by the polycomb group protein CBX8 through direct binding to the INK4A-ARF locus. EMBO J 26 1637 1648
45. BallasNGrunseichCLuDDSpehJCMandelG 2005 REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121 645 657
46. AttwoollCOddiSCartwrightPProsperiniEAggerK 2005 A novel repressive E2F6 complex containing the polycomb group protein, EPC1, that interacts with EZH2 in a proliferation-specific manner. J Biol Chem 280 1199 1208
47. TrimarchiJMFairchildBWenJLeesJA 2001 The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc Natl Acad Sci U S A 98 1519 1524
48. SingAPannellDKaraiskakisASturgeonKDjabaliM 2009 A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell 138 885 897
49. WooCJKharchenkoPVDaheronLParkPJKingstonRE 2010 A region of the human HOXD cluster that confers polycomb-group responsiveness. Cell 140 99 110
50. ZhengDZhaoKMehlerMF 2009 Profiling RE1/REST-mediated histone modifications in the human genome. Genome Biol 10 R9
51. RinnJLKerteszMWangJKSquazzoSLXuX 2007 Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129 1311 1323
52. GuptaRAShahNWangKCKimJHorlingsHM 2010 Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464 1071 1076
53. ChamberlainSJYeeDMagnusonT 2008 Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells 26 1496 1505
54. RenXKerppolaTK 2011 REST interacts with Cbx proteins and regulates polycomb repressive complex 1 occupancy at RE1 elements. Mol Cell Biol
55. HansenKHBrackenAPPasiniDDietrichNGehaniSS 2008 A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol 10 1291 1300
56. KentWJSugnetCWFureyTSRoskinKMPringleTH 2002 The human genome browser at UCSC. Genome Res 12 996 1006
Štítky
Genetika Reprodukčná medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



