-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
article has not abstract
Published in the journal: Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis. PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002623
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002623Summary
article has not abstract
Cyclin E, together with cyclin-dependent kinase 2 (CDK2), functions as a gatekeeper to promote G1/S transitions and the initiation of DNA replication. In normal cells, cyclin E–associated kinase activity is exquisitely regulated, with activity being limited to a brief time interval between late G1 and early S phase. Human cancers frequently exhibit deregulated cyclin E–associated kinase activity resulting from overexpression of cyclin E and loss of cyclin-dependent kinase inhibition (via p53 mutations) promoting genetic instability and cell proliferation [1]. Increased levels of cyclin E correlate with tumorigenesis and are a poor prognostic indicator independent of proliferation rate, suggesting that cyclin E's role in tumorigenesis is not limited to promoting increased cell proliferation [2], [3]. By eliminating regulatory constraints using p53 null cells, we and others have shown that overexpression or endogenous expression of stabilizing mutant forms of cyclin E can lead to hyperproliferation, genetic instability, and malignancy in cell culture and murine models [4], [5]. Normal cells suppress the effects of excess/stabilized cyclin E via the G1/S checkpoint involving the p53/p21 pathway.
Five isoforms of cyclin E, ranging in size from 33 to 44 kDa, have been identified in tumors over-expressing cyclin E. These low molecular weight forms of cyclin E (LMW-E) are generated through post-translational cleavage of full-length cyclin E by the elastase family of serine proteases in tumor cells [6], [7]. In comparison to full-length cyclin E (50 kDa), LMW-E forms are uniquely expressed in tumor cells, exhibit enhanced CDK2-associated kinase activity, have increased affinity for CDK2 [7]–[9], and exhibit decreased inhibition by CDK2 inhibitors, p21 and p27 (Figure 1) [10], [11]. Ectopic expression of LMW-E isoforms promotes cell proliferation, genetic instability, centrosome amplification, and malignancy [12], [13]. In addition, clinical studies have shown that high LMW-E is strongly associated with poor survival in breast cancer [2], colorectal cancer [14], [15], ovarian cancer [16], and melanomas [17]. Given its unique properties and distinct function in human cancers, targeting LMW-E could have important therapeutic implications.
Fig. 1. Low molecular weight cyclin E promotes tumorigenesis. 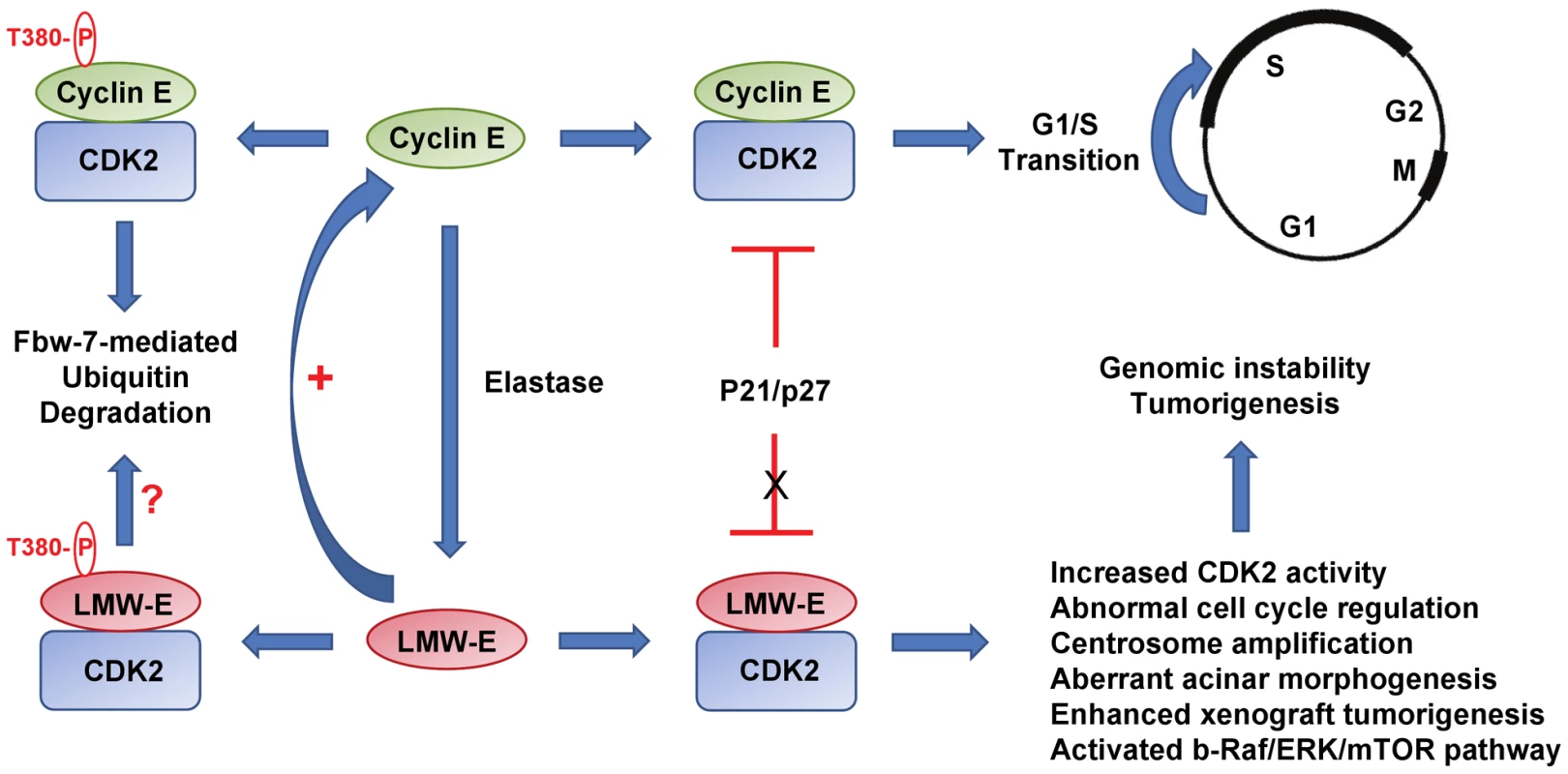
In normal cells, cyclin E/CDK2 is tightly regulated and triggers the onset of S phase. In tumors, cyclin E undergoes proteolytic processing generating low molecular weight species that exhibit increased kinase activity and resistance to inhibition by cyclin kinase inhibitors p21/p27. The expression of LMW-E promotes aberrant acinar morphogenesis, centrosome amplification, and tumors associated with activation of the bRaf/ERK/mTOR pathway. LMW-E, low molecular weight cyclin E. The study by Duong et al. in this issue of PLoS Genetics [18] convincingly uncovers the tumorigenic potential of LMW-E. The authors used three different model systems—3D acinar cultures, xenograft transplantation, and transgenic mice—to show that overexpression of LMW-E is sufficient to induce aberrant acinar morphogenesis in culture and mammary tumors in mice (Figure 1). When grown on Matrigel, immortalized human mammary epithelial cells (hMECs) expressing LMW-E exhibit large misshapen multiacinar structures resulting from defective growth arrest and apoptosis that mimic morphologic features of breast carcinomas. Further, ectopic expression of LMW-E in immortalized hMECs promotes tumorigenesis in xenografts and transgenic mice to a much greater extent than full-length cyclin E. Consistent with the reports by Akli et al. and Nanos-Webb et al., tumorigenesis associated with LMW-E is dependent on CDK2 [19], [20]. Furthermore, in vivo passaging of tumor cells increases the expression of LMW-E, suggesting that LMW-E provides a selective growth advantage to the tumor. Duong et al. also took advantage of a proteomic analysis termed reverse-phase protein array assay (RPPA) to examine protein expression patterns in cultured tumor cells and in breast tumors expressing high LMW-E levels. Their analyses revealed that multiple components of the b-RAF-ERK1/2-mTOR pathway are elevated in these cells. Activation of the b-RAF-ERK1/2-mTOR pathway normally promotes cell division and cell survival. Consistent with this, the authors observed that endogenous cyclin E levels are also increased in cells expressing high LMW-E, indicative of the existence of a positive feedback loop that promotes cell proliferation. Both high LMW-E levels and up-regulation of the b-RAF-ERK1/2-mTOR signaling pathway are associated with poor survival, suggesting functional correlation of these events in aggressive tumors. Importantly, the authors demonstrated that combination therapy targeting LMW-E/CDK2 and the b-RAF-ERK1/2-mTOR pathway has a synergistic effect in abrogating the tumorigenic effect of LMW-E. Thus, the identification of these downstream regulators may provide novel biomarkers and/or potential therapeutic targets for LMW-E–expressing tumors.
The report that LMW-E potentiates tumorigenesis in three independent model systems associated with activation of the b-RAF-ERK1/2-mTOR pathway is intriguing. However, there are many important questions about the role of LMW-E in tumorigenesis that need to be addressed. 1) What is the functional relationship between LMW-E and full-length cyclin E? In each tumor model reported by Duong et al., the effect was examined by over-expressing LMW-E in a background of endogenous full-length cyclin E. Further, the authors show that ectopic expression of LMW-E in transplanted xenografts triggers tumor evolution and results in increased levels of endogenous cyclin E. Thus, the contribution of endogenous full-length cyclin E in tumorigenesis cannot be excluded. In addition, Spruck et al. reported that the level of LMW-E correlates with full-length cyclin E, suggesting that LMW-E reflects the total cyclin E protein in primary breast tumors, cell lines, and even normal breast tissue [21]. To examine the effect of LMW-E in the absence of over-expression, and in the absence of full-length cyclin E, it will be important to use a knock-in model in which expression of LMW-E is driven from the endogenous cyclin E promoter. 2) What is the relationship between LMW-E and the b-Raf-ERK1/2-mTOR signaling pathway? The authors demonstrated that the b-Raf-ERK1/2-mTOR signaling pathway is activated in tumors expressing high levels of LMW-E. The b-Raf-ERK1/2-mTOR pathway may be a downstream signaling pathway deregulated by LMW-E, or it could be a parallel survival pathway selected in LMW-E–expressing tumors. In particular, the fact that only combinational therapy targeting both cyclin E–associated kinase activity and the b-Raf-ERK1/2-mTOR pathway generates an anti-tumor effect argues against a direct cause–effect relationship and is suggestive of a parallel pathway. 3) Is LMW-E expression required for tumor growth and does down-regulation of LMW-E alter tumor growth, invasion, or metastasis? The authors have generated an inducible model that should facilitate these studies. 4) Is the tumor-promoting activity of LMW-E due to enhanced deregulated kinase activity or to alternative target specificity? The LMW-E construct used in these studies has an N-terminal deletion (40 amino acids) that eliminates the proposed nuclear localization signal (NLS) and potentially affects the intracellular localization and substrate specificity [22], [23]. 5) How is LMW-E generated in tumors, and is it tumor-type specific? It has been proposed and demonstrated by Caruso et al. that many tumors have elevated protease activity and decreased levels of protease inhibitors such as elafin [24] that may contribute to the generation of LMW-E. Further characterization of the proteolytic pathways that target cyclin E in tumors may provide alternative therapeutic targets.
Zdroje
1. HwangHCClurmanBE 2005 Cyclin E in normal and neoplastic cell cycles. Oncogene 24 2776 2786
2. KeyomarsiKTuckerSLBuchholzTACallisterMDingY 2002 Cyclin E and survival in patients with breast cancer. N Engl J Med 347 1566 1575
3. PorterPLMaloneKEHeagertyPJAlexanderGMGattiLA 1997 Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 3 222 225
4. LoebKRKostnerHFirpoENorwoodTTsuchiyaKD 2005 A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell 8 35 47
5. MinellaACSwangerJBryantEWelckerMHwangH 2002 p53 and p21 form an inducible barrier that protects cells against cyclin E-cdk2 deregulation. Curr Biol 12 1817 1827
6. HarwellRMPorterDCDanesCKeyomarsiK 2000 Processing of cyclin E differs between normal and tumor breast cells. Cancer Res 60 481 489
7. PorterDCZhangNDanesCMcGahrenMJHarwellRM 2001 Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol 21 6254 6269
8. HarwellRMMullBBPorterDCKeyomarsiK 2004 Activation of cyclin-dependent kinase 2 by full length and low molecular weight forms of cyclin E in breast cancer cells. J Biol Chem 279 12695 12705
9. WingateHPuskasADuongMBuiTRichardsonD 2009 Low molecular weight cyclin E is specific in breast cancer and is associated with mechanisms of tumor progression. Cell Cycle 8 1062 1068
10. AkliSZhengPJMultaniASWingateHFPathakS 2004 Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res 64 3198 3208
11. WingateHZhangNMcGarhenMJBedrosianIHarperJW 2005 The tumor-specific hyperactive forms of cyclin E are resistant to inhibition by p21 and p27. J Biol Chem 280 15148 15157
12. AkliSVan PeltCSBuiTMultaniASChangS 2007 Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res 67 7212 7222
13. Bagheri-YarmandRBiernackaAHuntKKKeyomarsiK 2010 Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer Res 70 5074 5084
14. ZhouYJXieYTGuJYanLGuanGX 2011 Overexpression of cyclin E isoforms correlates with poor prognosis in rectal cancer. Eur J Surg Oncol 37 1078 1084
15. CorinIDi GiacomoMCLastellaPBagnuloRGuantiG 2006 Tumor-specific hyperactive low-molecular-weight cyclin E isoforms detection and characterization in non-metastatic colorectal tumors. Cancer Biol Ther 5 198 203
16. DavidsonBSkredeMSilinsIShih IeMTropeCG 2007 Low-molecular weight forms of cyclin E differentiate ovarian carcinoma from cells of mesothelial origin and are associated with poor survival in ovarian carcinoma. Cancer 110 1264 1271
17. BalesEMillsLMilamNMcGahren-MurrayMBandyopadhyayD 2005 The low molecular weight cyclin E isoforms augment angiogenesis and metastasis of human melanoma cells in vivo. Cancer Res 65 692 697
18. DuongMTAkliSWeiCWingateHFLiuW 2012 LMW-E/CDK2 deregulates acinar morphogenesis, induces tumorigenesis and associates with the activated b-Raf-ERK1/2-mTOR pathway in breast cancer patients. PLoS Genet 8 e1002538 doi:10.1371/journal.pgen.1002538
19. AkliSVan PeltCSBuiTMeijerLKeyomarsiK 2011 Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res 71 3377 3386
20. Nanos-WebbAJabbourNAMultaniASWingateHOumataN 2011 Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast Cancer Res Treat E-pub ahead of print 22 June 2011
21. SpruckCSunDFieglHMarthCMueller-HolznerE 2006 Detection of low molecular weight derivatives of cyclin E1 is a function of cyclin E1 protein levels in breast cancer. Cancer Res 66 7355 7360
22. DelkNAHuntKKKeyomarsiK 2009 Altered subcellular localization of tumor-specific cyclin E isoforms affects cyclin-dependent kinase 2 complex formation and proteasomal regulation. Cancer Res 69 2817 2825
23. JackmanMKubotaYden ElzenNHagtingAPinesJ 2002 Cyclin A - and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol Biol Cell 13 1030 1045
24. CarusoJAHuntKKKeyomarsiK 2010 The neutrophil elastase inhibitor elafin triggers rb-mediated growth arrest and caspase-dependent apoptosis in breast cancer. Cancer Res 70 7125 7136
Štítky
Genetika Reprodukčná medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



