-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
article has not abstract
Published in the journal: GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?. PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002989
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002989Summary
article has not abstract
Diabetic nephropathy (DN) is associated with excess morbidity and mortality, in both type 1 (T1D) and type 2 (T2D) diabetic patients. Despite intensification of treatment, DN remains a growing problem worldwide [1]–[6]. In 2009, treatment of diabetic end stage renal disease patients accounted for approximately 40% of the US$43 billion expended for dialysis treatment in the United States
New management and treatment approaches are desperately needed and defining the genetic architecture regulating DN would accelerate their development. The landmark study by Seaquist et al. in 1989 [7] showed strong familial aggregation of DN and spurred the search for genetic risk variants associated with DN. However, family-based linkage and candidate gene analyses as well as the initial genome-wide association studies (GWAS), performed in single studies with limited power, showed inconsistent results in both T1D and T2D patients [8].
In this issue of PLOS Genetics, the GENIE consortium presents results of the largest DN GWAS meta-analysis performed to date. The discovery phase included 6,691 T1D patients from three cohorts, and SNPs with p<10−5 were moved forward into a replication analysis that included an additional 5,156 T1D patients in nine cohorts ascertained for nephropathy phenotypes [9]. Generally accepted phenotype definitions were used to identify DN cases (macroalbuminuria or end stage renal disease [ESRD] due to DN) and diabetic control individuals without nephropathy (diabetes duration of at least 10 years with normal albumin excretion). The combined metaanalysis for DN showed, disappointingly, no genome-wide signals, although an intronic SNP in ERBB4 (chromosome 2) showed a consistent protective effect across cohorts (OR 0.66, p = 2.1×10−7). Intriguingly, ERBB4 encodes a member of the EGF receptor tyrosine kinase family and modulates kidney tubule proliferation and polarity during nephrogenesis [10].
However, the DN definition essentially mixes two traits, each with distinct underlying pathomechanisms: ESRD as the extreme form of reduced kidney function (glomerular filtration rate, GFR), and macroalbuminuria reflecting severe glomerular filtration barrier dysfunction. Since these two traits have distinct genetic underpinnings [11]–[16], the authors refined their DN case definition to include only diabetic ESRD patients, which were contrasted with all other diabetic individuals regardless of albumin excretion level. Using these phenotypic criteria, the combined meta-analysis of discovery and replication cohorts identified genome-wide significant signals in an intron in the AFF3 gene, and an intergenic locus between RGMA and MCTP2 on chromosome 15. However, as the authors correctly point out, enthusiasm for AFF3, a transcriptional activator, should be tempered. This locus appears driven by two cohorts and technically did not replicate (p = 0.25 in stage 2 replication), although the effect direction was consistent across studies. The authors argue that power of the replication sample was limited for the alternative case definition due to the low number of ESRD cases (n = 363 versus n = 3,465 controls). The authors further support the association of AFF3 with diabetic ESRD by providing experimental evidence that AFF3 expression levels mediate TGF-β-1–driven fibrosis in an epithelial cell culture model. TGF-β-1 has consistently been implicated in the pathogenesis of fibrosis in DN, and these data provide a plausible function for AFF3 in profibrotic pathways that characterize progressive diabetic kidney disease. However, the lack of significant association in replication analysis calls for independent confirmation of this locus in other studies before its implications for DN mechanisms can be drawn.
So—does this publication really let the “GENIE” for DN gene discovery out of the bottle, discovering at last the definitive “DN gene(s)”—or is this merely wishful thinking? It is sobering that this largest and long-awaited GWAS of T1D DN fails to provide unassailable statistical genetic evidence for associated variants, especially when compared to the success of GWAS in identifying convincing loci associated with other kidney diseases such as idiopathic membranous and IgA nephropathy, ANCA-associated nephropathy, or nondiabetic ESRD in African Americans [17]–[22].
We believe that the definition of DN may lie at the crux of the overall disappointing reproducibility of genetic DN studies. In contrast to kidney diseases where diagnosis is based on a kidney biopsy (e.g., IgA nephropathy, membranous nephropathy) or imaging studies (e.g., ADPKD), the diagnosis of DN is almost always made using clinical criteria and not by histology. The clinical diagnosis uses phenotypic parameters derived from the typical course of DN: after many years of diabetes duration with normal GFR and absent albuminuria, DN onset is marked by mildly elevated albuminuria (also termed microalbuminuria), frequently with increased GFR (Figure 1). Subsequently, DN is characterised by overlapping stages of declining GFR and progressive proteinuria [23], finally leading to ESRD, with mortality as a competing risk. However, we have learned that proteinuria is more variable in DN than initially thought. In early stages, regression to normoalbuminuria is frequently observed [24]. Further, severe albuminuria (also termed macroalbuminuria) is not an invariate antecedent for profound kidney damage. Indeed, studies indicate that chronic kidney disease in diabetes may evolve in the absence of considerable proteinuria and progress to ESRD [25], justifying GENIE's analytic design contrasting diabetic patients with ESRD to all other diabetic subjects.
Fig. 1. Schematic presentation of variable clinical courses of diabetic nephropathy. 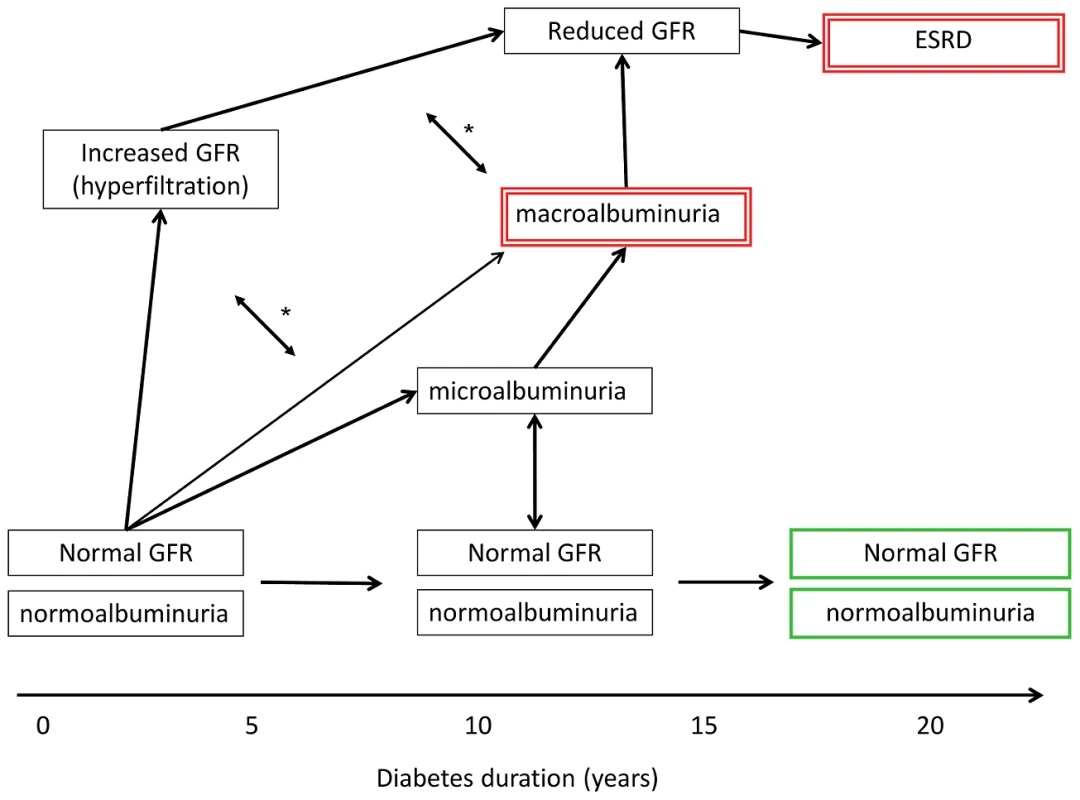
The case and control definitions used in the primary GWAS in Sandholm et al. [9] are indicated by green bold (controls) and red double-lined boxes (cases). GFR, glomerular filtration rate; ESRD, end stage renal disease. Microalbuminuria, urinary albumin excretion rate of 30–300 mg per day; macroalbuminuria, urinary albumin excretion rate of >300 mg per day. *, GFR and albuminuria may progress independently of each other, i.e., patients may have micro- or macroalbuminuria even though their GFR is normal or even slightly elevated. However, macroalbuminuria is usually associated with reduced GFR, and is a strong risk factor for progressive loss of eGFR and ESRD. The present study consists primarily of cross-sectional studies and cannot capture the definitive DN outcome on an individual level. Using the most severe forms of DN to define cases reduces some potential misclassification but definitely does not overcome the critical inaccuracy of case definition not based on histology (many other kidney diseases can cause macroalbuminuria and ESRD). Further, the diabetic patient is exposed to many nonspecific kidney-damaging events in the course of disease (e.g., contrast agent imaging, nephrotoxic drugs, prerenal phases in infection and cardiovascular events), which in their sum also contribute to progression to ESRD. Overall, the sum of potential misclassification involved in using an exclusively clinical DN definition in cross-sectional studies reduces statistical power to detect underlying genetic variants.
With this meta-analysis of DN in T1D patients, Sandholm et al. have taken an important step towards defining the genetic architecture of DN. Strengths of the study include its large sample size, consideration of alternative DN phenotypes based on reproducible epidemiological and genetic data reported by other groups studying kidney diseases, and experimental support for the associated loci. Now the challenge will be building on these results. Two consortia, FIND and SUMMIT, should be reporting GWAS results for type 2 DN, and it will be interesting to see if common or unique genetic loci are identified of DN in T1D and T2D patients. Similarities in the clinical phenotypes and treatment responses of patients with T1D and T2D DN suggest a shared pathogenesis. Finally, additional meta-analyses of T1D and T2D DN cohorts with larger sample sizes, application of sequencing technologies, and use of more precise DN phenotypes from longitudinal studies should further define the genetic architecture of this most common cause of chronic kidney disease. These data will provide the foundation needed to advance understanding of diabetic nephropathy and impact patient outcomes.
Zdroje
1. Ismail-BeigiF, CravenT, BanerjiMA, BasileJ, CallesJ, et al. (2010) Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376 : 419–430.
2. GersteinHC, MillerME, ByingtonRP, GoffDCJr, BiggerJT, et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358 : 2545–2559.
3. PatelA, MacMahonS, ChalmersJ, NealB, BillotL, et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358 : 2560–2572.
4. HallerH, ItoS, IzzoJLJr, JanuszewiczA, KatayamaS, et al. (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364 : 907–917.
5. ReavenPD, MoritzTE, SchwenkeDC, AndersonRJ, CriquiM, et al. (2009) Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 58 : 2642–2648.
6. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2003) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290 : 2159–2167.
7. SeaquistER, GoetzFC, RichS, BarbosaJ (1989) Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320 : 1161–1165.
8. BögerC, HeidI (2011) Chronic kidney disease: novel insights from genome wide association studies. Kidney Blood Press Res 34 : 27–36.
9. SandholmN, SalemRM, McKnightAJ, BrennanEP, ForsblomC, et al. (2012) New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 8: e1002921 doi:10.1371/journal.pgen.1002921.
10. VeikkolainenV, NaillatF, RailoA, ChiL, ManninenA, et al. (2012) ErbB4 modulates tubular cell polarity and lumen diameter during kidney development. J Am Soc Nephrol 23 : 112–122.
11. EllisJW, ChenMH, FosterMC, LiuCT, LarsonMG, et al. (2012) Validated SNPs for eGFR and their associations with albuminuria. Hum Mol Genet 21 : 3293–3298.
12. PlachaG, CananiLH, WarramJH, KrolewskiAS (2005) Evidence for different susceptibility genes for proteinuria and ESRD in type 2 diabetes. Adv Chronic Kidney Dis 12 : 155–169.
13. BögerC, ChenMH, TinA, OldenM, KöttgenA, de BoerIH, et al. (2011) CUBN is a Gene Locus for Albuminuria. J Am Soc Nephrol 22 : 555–570.
14. KöttgenA, PattaroC, BögerCA, FuchsbergerC, OldenM, et al. (2010) New loci associated with kidney function and chronic kidney disease. Nat Genet 42 : 376–384.
15. PattaroC, KottgenA, TeumerA, GarnaasM, BogerCA, et al. (2012) Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 8: e1002584 doi:10.1371/journal.pgen.1002584.
16. BögerC, GorskiM, LiM, HoffmannM, HuangC, et al. (2011) Association of eGFR-related loci identified by GWAS with incident CKD and ESRD. PLoS Genet 7: e1002292 doi:10.1371/journal.pgen.1002292.
17. KaoWH, KlagMJ, MeoniLA, ReichD, Berthier-SchaadY, et al. (2008) MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40 : 1185–1192.
18. KoppJB, SmithMW, NelsonGW, JohnsonRC, FreedmanBI, et al. (2008) MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40 : 1175–1184.
19. StanescuHC, Arcos-BurgosM, MedlarA, BockenhauerD, KöttgenA, et al. (2011) Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364 : 616–626.
20. GharaviAG, KirylukK, ChoiM, LiY, HouP, et al. (2011) Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43 : 321–327.
21. LyonsPA, RaynerTF, TrivediS, HolleJU, WattsRA, et al. (2012) Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 367 : 214–223.
22. GenoveseG, FriedmanDJ, RossMD, LecordierL, UzureauP, et al. (2010) Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329 : 841–845.
23. RemuzziG, BenigniA, RemuzziA (2006) Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116 : 288–296.
24. SteinkeJM, SinaikoAR, KramerMS, SuissaS, ChaversBM, et al. (2005) The early natural history of nephropathy in type 1 diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54 : 2164–2171.
25. PerkinsBA, FicocielloLH, RoshanB, WarramJH, KrolewskiAS (2010) In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 77 : 57–64.
Štítky
Genetika Reprodukčná medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



