-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
Diabetic kidney disease, or diabetic nephropathy (DN), is a major complication of diabetes and the leading cause of end-stage renal disease (ESRD) that requires dialysis treatment or kidney transplantation. In addition to the decrease in the quality of life, DN accounts for a large proportion of the excess mortality associated with type 1 diabetes (T1D). Whereas the degree of glycemia plays a pivotal role in DN, a subset of individuals with poorly controlled T1D do not develop DN. Furthermore, strong familial aggregation supports genetic susceptibility to DN. However, the genes and the molecular mechanisms behind the disease remain poorly understood, and current therapeutic strategies rarely result in reversal of DN. In the GEnetics of Nephropathy: an International Effort (GENIE) consortium, we have undertaken a meta-analysis of genome-wide association studies (GWAS) of T1D DN comprising ∼2.4 million single nucleotide polymorphisms (SNPs) imputed in 6,691 individuals. After additional genotyping of 41 top ranked SNPs representing 24 independent signals in 5,873 individuals, combined meta-analysis revealed association of two SNPs with ESRD: rs7583877 in the AFF3 gene (P = 1.2×10−8) and an intergenic SNP on chromosome 15q26 between the genes RGMA and MCTP2, rs12437854 (P = 2.0×10−9). Functional data suggest that AFF3 influences renal tubule fibrosis via the transforming growth factor-beta (TGF-β1) pathway. The strongest association with DN as a primary phenotype was seen for an intronic SNP in the ERBB4 gene (rs7588550, P = 2.1×10−7), a gene with type 2 diabetes DN differential expression and in the same intron as a variant with cis-eQTL expression of ERBB4. All these detected associations represent new signals in the pathogenesis of DN.
Published in the journal: New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes. PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002921
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002921Summary
Diabetic kidney disease, or diabetic nephropathy (DN), is a major complication of diabetes and the leading cause of end-stage renal disease (ESRD) that requires dialysis treatment or kidney transplantation. In addition to the decrease in the quality of life, DN accounts for a large proportion of the excess mortality associated with type 1 diabetes (T1D). Whereas the degree of glycemia plays a pivotal role in DN, a subset of individuals with poorly controlled T1D do not develop DN. Furthermore, strong familial aggregation supports genetic susceptibility to DN. However, the genes and the molecular mechanisms behind the disease remain poorly understood, and current therapeutic strategies rarely result in reversal of DN. In the GEnetics of Nephropathy: an International Effort (GENIE) consortium, we have undertaken a meta-analysis of genome-wide association studies (GWAS) of T1D DN comprising ∼2.4 million single nucleotide polymorphisms (SNPs) imputed in 6,691 individuals. After additional genotyping of 41 top ranked SNPs representing 24 independent signals in 5,873 individuals, combined meta-analysis revealed association of two SNPs with ESRD: rs7583877 in the AFF3 gene (P = 1.2×10−8) and an intergenic SNP on chromosome 15q26 between the genes RGMA and MCTP2, rs12437854 (P = 2.0×10−9). Functional data suggest that AFF3 influences renal tubule fibrosis via the transforming growth factor-beta (TGF-β1) pathway. The strongest association with DN as a primary phenotype was seen for an intronic SNP in the ERBB4 gene (rs7588550, P = 2.1×10−7), a gene with type 2 diabetes DN differential expression and in the same intron as a variant with cis-eQTL expression of ERBB4. All these detected associations represent new signals in the pathogenesis of DN.
Introduction
Diabetic kidney disease, or diabetic nephropathy (DN), is the leading cause of end-stage renal disease (ESRD) worldwide [1]. It affects approximately 30% of patients with long-standing type 1 and type 2 diabetes [2], [3], and confers added risks of cardiovascular disease and mortality. DN is a progressive disorder that is characterized by proteinuria (abnormal loss of protein from the blood compartment into the urine) and gradual loss of kidney function. Early in its course, the kidneys are hypertrophic, and glomerular filtration is increased. However, with progression over several years, proteinuria and decline in kidney function set in, and may result in fibrosis and terminal kidney failure, necessitating costly renal replacement therapies, such as dialysis and renal transplantation. While current treatments that decrease proteinuria will moderately abate DN progression, recent studies show that even with delivery of optimal care, high risks of cardiovascular disease, ESRD and mortality persist [4], [5]. Therefore, discovery of genetic factors that influence development and susceptibility to DN is a critical step towards the identification of novel pathophysiologic mechanisms that may be targeted for interventions to improve the adverse clinical outcomes in diabetic patients.
Whereas the degree of glycemia plays a pivotal role in DN, a subset of individuals with poorly controlled type 1 diabetes (T1D) do not develop DN. Furthermore, strong familial aggregation supports genetic susceptibility to DN. The sibling risk of DN has been estimated to be 2.3-fold [6]. While prior studies of individuals with T1D have reported on the possible existence of genetic associations for DN, results have been inconclusive. In GENIE, we leveraged three existing collections for T1D nephropathy (All Ireland Warren 3 Genetics of Kidneys in Diabetes UK Collection [UK-ROI], Finnish Diabetic Nephropathy Study [FinnDiane], and Genetics of Kidneys in Diabetes US Study [GoKinD US]) comprising 6,691 individuals to perform the most comprehensive and well powered DN susceptibility genome-wide association study (GWAS) and meta-analysis to date, with the aim to identify genetic markers associated with DN by meta-analyzing independent GWAS, imputed to HapMap CEU II (Table 1, Figure 1). As a result, we here present two new loci associated with ESRD and a locus suggestively associated with DN.
Fig. 1. Flow chart summarizing study design. 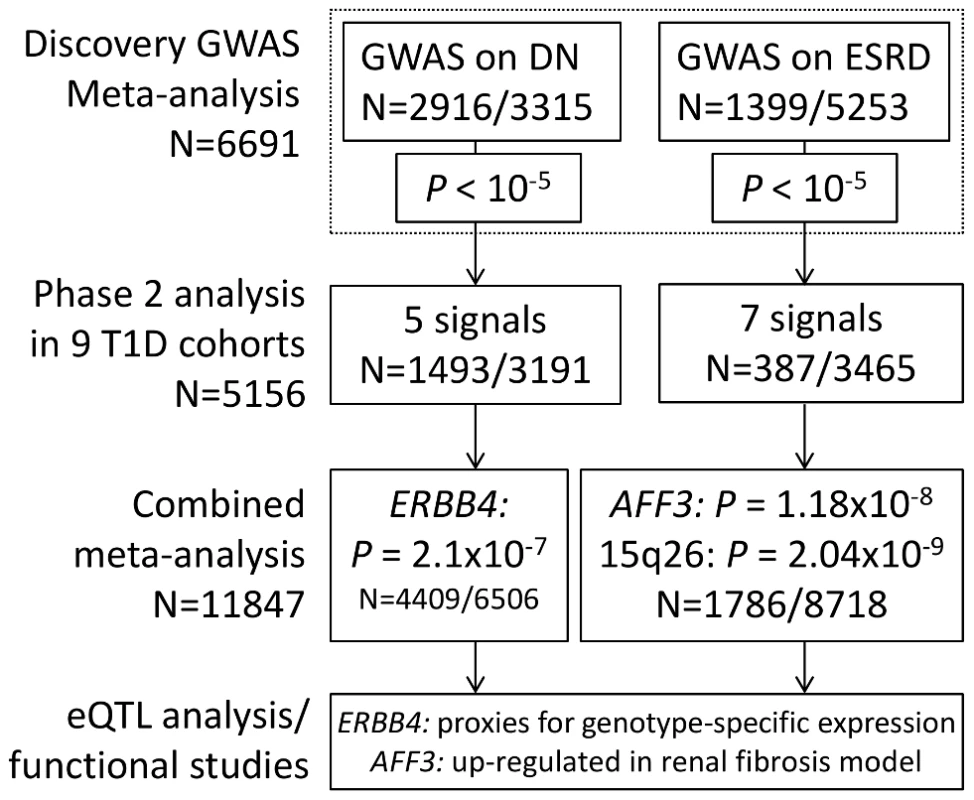
We applied a two stage study design, where the top signals from the meta-analysis of three GENIE studies (UK-ROI, FinnDiane and GoKinD US) were followed up in phase two analysis, consisting of nine T1D cohorts. After combined meta-analysis, two signals reached genome-wide significance in the analysis of ESRD (P<5×10−8). For DN phenotype no loci reached this threshold, but the strongest association was observed for ERBB4. These signals were followed up with eQTL studies and functional analysis. The number of patients (N) refers to the number of samples after genotype quality control; either the total number of samples or divided into cases/controls. Tab. 1. Characteristics of samples successfully analyzed in each discovery collection and the meta-analyses. 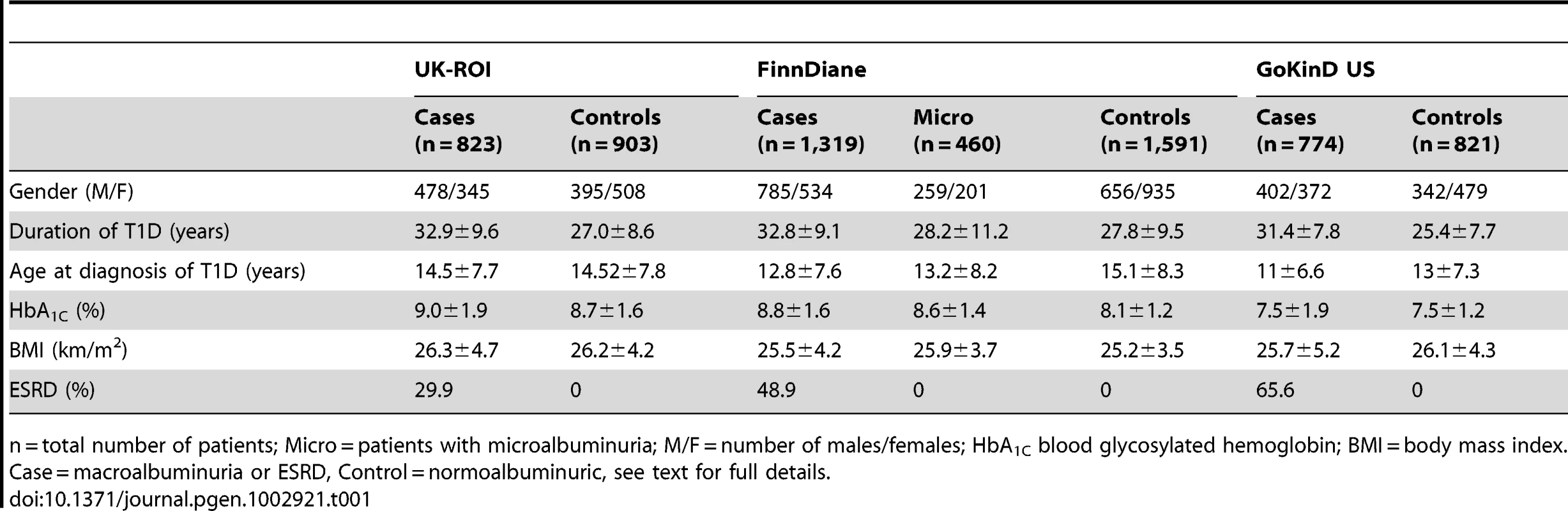
n = total number of patients; Micro = patients with microalbuminuria; M/F = number of males/females; HbA1C blood glycosylated hemoglobin; BMI = body mass index. Case = macroalbuminuria or ESRD, Control = normoalbuminuric, see text for full details. Results/Discussion
The primary phenotype of interest was DN, defined by the presence of persistent macroalbuminuria or ESRD in individuals aged over 18 who had T1D for at least 10-year duration. Controls were defined as individuals with T1D for at least 15 years but without any clinical evidence of kidney disease (see Methods for more detailed definitions). Meta-analysis of the DN results from each cohort resulted in five independent signals with P<10−5 (Table S1, Figure S1A). In a parallel analysis of ESRD versus non-ESRD (n cases = 1,399, n controls = 5,253; referred to as “ESRD” analysis throughout the manuscript, unless otherwise stated), SNP rs7583877 on chromosome 2q11.2-q12 achieved genome-wide significance (P = 4.8×10−9), primarily driven by FinnDiane and the UK-ROI samples, along with six other independent signals reaching P<10−5 (Figure 2A, Table S1, Figure S1C).
Fig. 2. Regional association plots for top ranked SNPs with associated gene expression data. 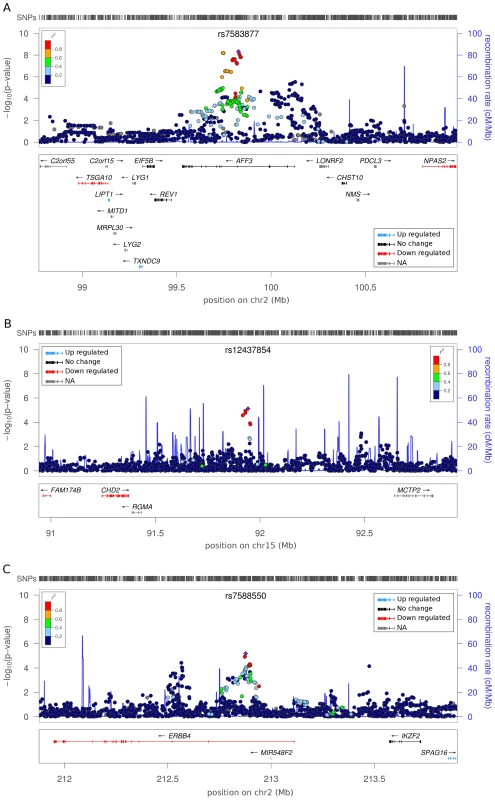
Panels represent independent signals for the primary DN and ESRD analysis. The color of the SNP symbol indicates the linkage disequilibrium (r2) with the index SNP which is colored purple. Blue and red gene colors in the lower part of each figure panel indicate up and down regulation in tubulointerstitial or glomerular DN kidney biopsies, respectively. Genes with no change in expression are indicated with black; no data on gene expression with gray color. (A) Association of rs7583877 with ESRD. (B) Association of rs12437854 with ESRD. (C) Association of rs7588550 with DN. We invited investigators responsible for available collections with similar phenotypes to participate in the secondary genotyping phase of the top ranked SNPs (n = 41 including proxies, representing 24 independent signals) from the initial meta-analysis. Nine independent cohorts contributed 5,873 individuals with comparable phenotypic inclusion criteria (Table S2). After the combined meta-analysis of the first and second phase cohorts, the association of the intronic SNP rs7583877 in AFF3 with ESRD retained genome-wide significance (odds ratio [OR] = 1.29, 95% confidence interval [CI]: 1.18–1.40, P = 1.2×10−8; Figure 3A), with the bulk of the association evidence still provided by the FinnDiane and UK-ROI cohorts. The population attributable risk [PAR] for the causal variant underlying the observed association at rs7583877 was estimated to be 3.5%–10.5%. AFF3 belongs to the AFF (AF4/FMR2) family and encodes a transcriptional activator, with DNA-binding activity, initially found to be fused with MLL in some acute lymphoblastic leukemia patients [7], [8]. Recent evidence points to a role for AFF3 as an RNA-binding protein, with overexpression affecting organization of nuclear speckles and splice machinery integrity [9]. Variants near AFF3 have been associated with acute lymphoblastic leukemia [10], rheumatoid arthritis [11], [12] and recently T1D [13], [14]. Another locus between the RGMA (RGM domain family, member A) and MCTP2 (multiple C2 domains, transmembrane 2) genes on chromosome 15q26 also reached genome-wide significance for association with ESRD (rs12437854, OR 1.80, 95% CI: 1.48–2.17, P = 2.0×10−9; Table 2, Figure 3B). PAR estimates for this locus varied from 0.5% to 4.1%. For the primary DN phenotype, an intronic SNP in the ERBB4 gene demonstrated consistent protective effects in the replication samples and was the top associated SNP identified from the combined discovery and second stage analysis; however, this did not reach genome-wide statistical significance (rs7588550, OR 0.66, 95% CI: 0.56–0.77, P = 2.1×10−7, PAR 28.3%–32.5% for removal of the major risk allele; Table 2, Figure 3C). ERBB4 encodes an epidermal growth factor receptor subfamily member, and has been implicated in cardiac, mammary gland and neural development [15], [16]. Mutations in ERBB4 have previously been reported in cancer [17]. Several studies using Madin-Darby canine kidney (MDCK) cells and conditional ERBB4 overexpression/knock-out mice, suggest a crucial role for ERBB4 in renal development and tubulogenesis [18], [19].
Fig. 3. Forest plots for significant hits incorporating discovery and replication plots. 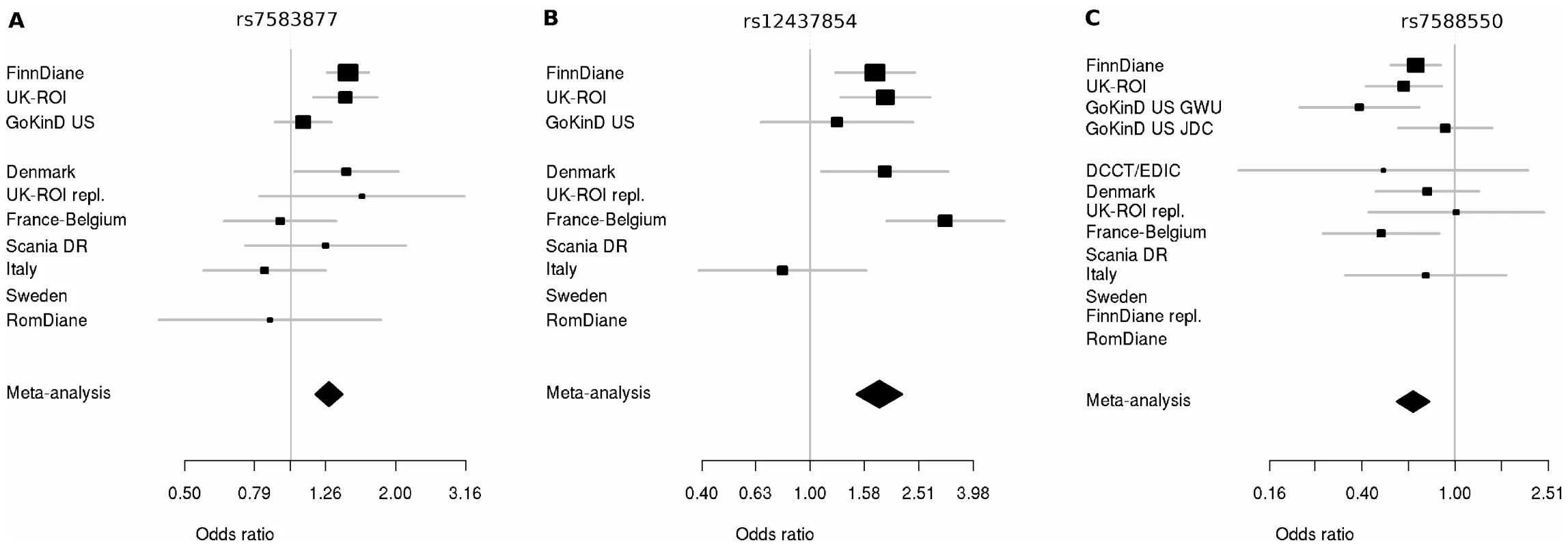
Plots show the study-specific association estimates (OR) and 95% confidence intervals for the discovery and second phase studies. (A) Association of rs7583877 with ESRD; heterogeneity P = 0.037. (B) Association of rs12437854 with ESRD; heterogeneity P = 0.046. (C) Association of rs7588550 with DN; heterogeneity P = 0.467. The association estimate and confidence interval for the meta-analysis combining the discovery and second-stage results are denoted by the diamond. Tab. 2. Results from discovery, second stage, and combined meta-analysis for supported markers. 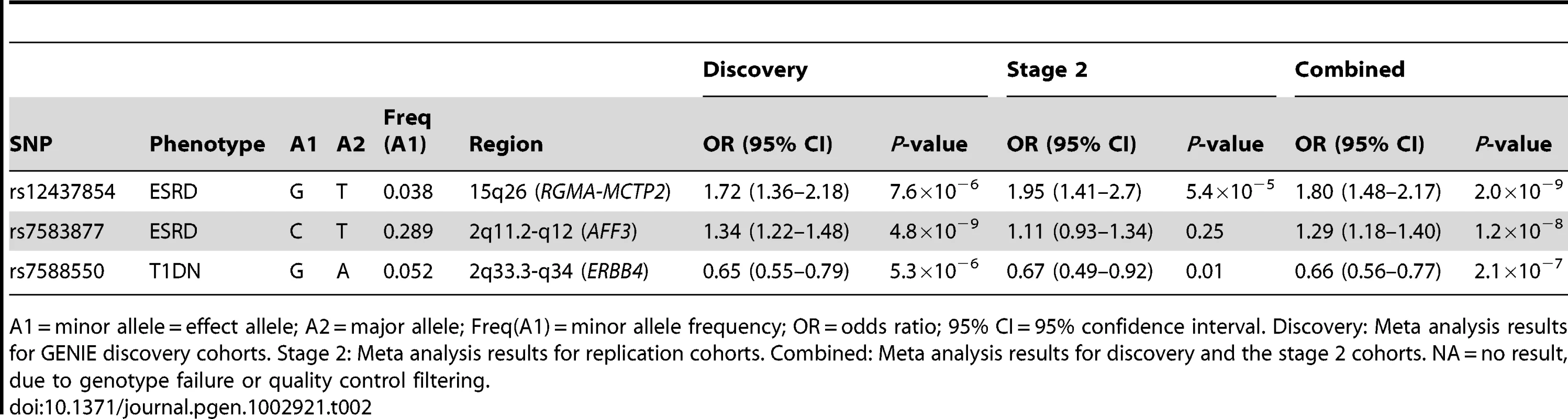
A1 = minor allele = effect allele; A2 = major allele; Freq(A1) = minor allele frequency; OR = odds ratio; 95% CI = 95% confidence interval. Discovery: Meta analysis results for GENIE discovery cohorts. Stage 2: Meta analysis results for replication cohorts. Combined: Meta analysis results for discovery and the stage 2 cohorts. NA = no result, due to genotype failure or quality control filtering. It is possible that our observed signal is in linkage disequilibrium with an untyped SNP, or exerts functional effects over an extended genomic region. To explore a putative biological signature we identified, for the top three SNPs, all genes within a 2 Mb window (1 Mb upstream and downstream). Gene ontology analysis revealed no significant enrichment of biological terms or pathways within this subset of flanking genes (Table S3). We determined whether any of these genes were differentially expressed in microarray data derived from tubulointerstitial (n = 49) or glomerular (n = 70) human early DN renal biopsy material versus pre-transplant renal biopsies from living kidney donors (n = 32) [20]. Around rs7583877 (AFF3), we noted upregulation of LIPT1 and TXNDC9, while TSGA10 was downregulated in both tubulointerstitial and glomerular enriched kidney biopsies (Figure 2 and Table S4). NPAS2, which flanks rs7583877 (AFF3), and FAM174B and CHD2, which flank rs12437854 (15q26), were downregulated in glomerular enriched biopsies of DN patients versus control, but remained unchanged in tubulointerstitial biopsies (Figure 2 and Table S4). NPAS2 (neuronal PAS domain protein 2), has been implicated in circadian rhythms in the distal nephron segments, acting as a regulator of kidney function [21]. Interestingly, mutations in chromodomain helicase DNA binding protein 2 (CHD2), encoding a chromatin-remodeling enzyme, result in impaired glomerular function in mice [22]. Furthermore, at the rs7588550 (ERBB4) locus expression of ERBB4 was down, and SPAG16 upregulated in tubulointerstitial enriched kidney biopsy tissue of DN versus control subjects (Figure 2 and Table S4).
We also examined whether any of the top three SNPs modulated expression of neighboring genes in cis in a dataset of glomerular and tubulointerstitial kidney biopsies of Pima Indians with type 2 diabetes and DN who had been genotyped on the Affymetrix 6.0 array [23]. In Pima Indians, no adequate proxies (haplotype-based D′≥0.8) for the Affymetrix 6.0 SNPs that were strongly correlated with GWAS findings (r2≥0.8) could be found for rs12437854, and expression of AFF3 was below detectable thresholds in this dataset; however, two SNPs in the same intron of ERBB4 as rs7588550 (rs17418640 and rs17418814) were associated with genotype-specific expression of ERBB4 in tubulointerstitial but not in glomerular tissue in the Pima cohort (P<0.05; Figure S2). Follow-up work is required to investigate the DN associated and eQTL signals in this ERBB4 intron.
To explore the potential functional role of these ERBB4 SNPs, we looked for other genes whose expression is correlated with that of ERBB4. A total of 388 ERBB4-correlated genes were found in the Pima population (Benjamini-Hochberg Q-value<0.1). Pathway analysis of these genes indicates coexpression of ERBB4 with collagen-related genes, which have been implicated in renal fibrosis [24], [25] (Genomatix Pathway System; Table S5).
Because the low expression level of AFF3 limited exploration of this gene using expression data, we pursued additional functional experiments in an in vitro model of renal fibrosis, namely human tubular epithelia exposed to transforming growth factor-β1 (TGF-β1). Low-level basal expression of the AFF3 mouse homologue (LAF4) has been reported in kidney tubules during embryonic development [26] suggesting proximal renal tubule epithelial cells may be suitable for detection and functional interrogation of AFF3. TGF-β1 is implicated in the development of diabetic glomerulosclerosis, and there is recent appreciation of its role as a key driver of tubulointerstitial fibrosis. TGF-β1 induces epithelial cell de-differentiation into a more mesenchymal-like phenotype, characterized by a switch in predominant cadherins from E-cadherin (epithelial) to N-cadherin (mesenchymal), and increased vimentin, α-smooth muscle actin, connective tissue growth factor (CTGF) and Jagged 1 [27], [28]. TGF-β1-mediated loss of E-cadherin in renal epithelia, is believed to be mediated through loss of miR-192 expression [29]. We and others have previously shown that Jagged 1, a ligand for multiple Notch receptors, is up-regulated in human diabetic kidney disease [30], [31], with the Notch signaling pathway implicated in driving renal fibrosis [32], [33]. CTGF is a member of the CCN protein family, with biological roles in differentiation and tissue repair. CTGF is induced by TGF-β1 and enhances expression of multiple extracellular matrix proteins observed in DN, including collagens and fibronectin, and CTGF expression is elevated in the glomeruli of STZ (streptozotocin) - treated rats, an in vivo model of T1D [34]. Basal AFF3 expression was detectable in HK-2 cells, and expression levels were upregulated upon stimulation with TGF-β1 (5 ng/ml; 48 h), as measured at protein and RNA level (Figure 4A–4B). Inhibition of AFF3 by siRNA attenuated the expression of TGF-β1-driven markers of fibrosis - CTGF and N-cadherin (Figure 4C–4E). Taken together, these data suggest that AFF3 may play a role in TGF-β1-induced fibrotic responses of renal epithelial cells.
Fig. 4. AFF3 is upregulated in renal epithelial cells (HK-2) stimulated with pro-fibrotic TGF-β1. 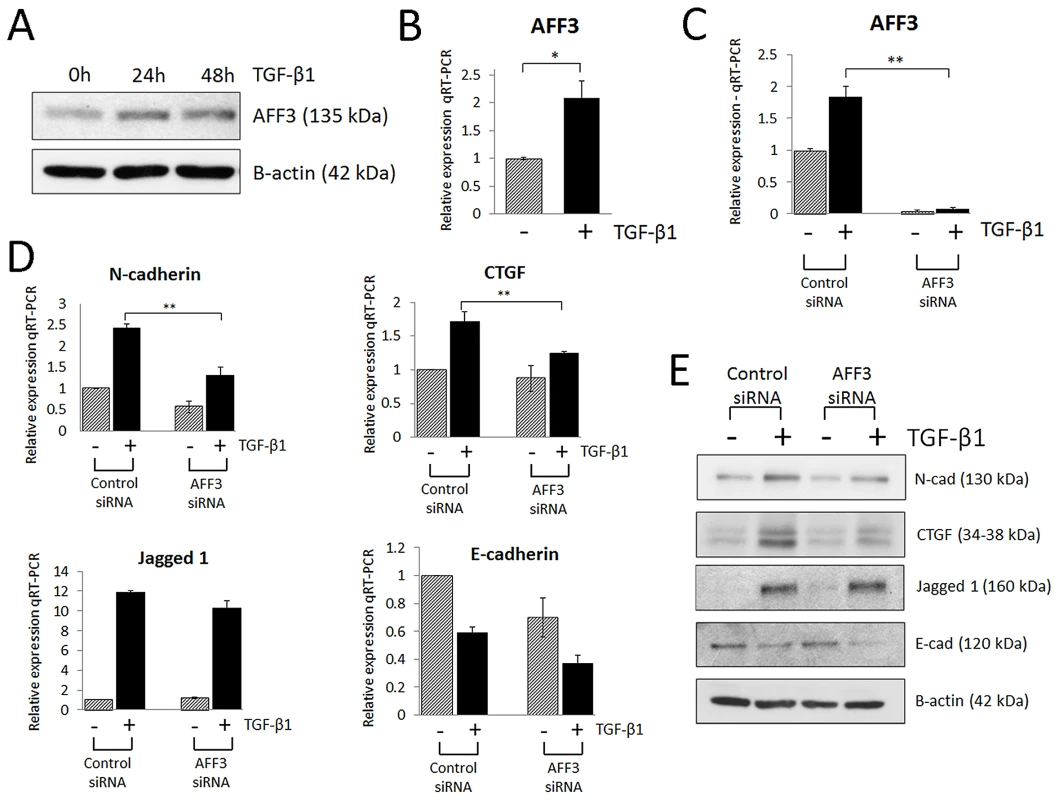
(A) Western blot of AFF3 protein expression in HK-2 cells stimulated with TGF-β1 (5 ng/ml; 24–48 h). (B) TaqMan quantitative PCR analysis of AFF3 mRNA expression in HK-2 cells stimulated with TGF-β1 (5 ng/ml; 48 h) and (C) AFF3 mRNA expression in HK-2 cells transfected with AFF3 siRNA in the presence (black bar)/absence (grey bar) of TGF-β1 (5 ng/ml; 48 h). (D) TaqMan quantitative PCR analysis of N-cadherin, CTGF, Jagged1 and E-cadherin expression in HK-2 cells transfected with AFF3 siRNA in the presence (black bar)/absence (grey bar) of TGF-β1 (5 ng/ml; 48 h). (E) Representative Western blot of N-cadherin, CTGF, Jagged1 and E-cadherin protein responses in HK-2 cells transfected with AFF3 siRNA in the presence/absence of TGF-β1 (5 ng/ml; 48 h). HK-2 cells transfected with control siRNA were selected as a control. For TaqMan PCR, expression was normalized to GAPDH. Data are plotted as mean ± SE (n = 3; *P<0.05, **P<0.01). Traditionally, DN has been viewed as a continuous trait with onset at microalbuminuria, progression to macroalbuminuria, loss of GFR, and culmination in ESRD. Recent studies have called this paradigm into question, suggesting that the syndrome may perhaps be composed of varying phenotypes [35], [36]. Association of rs7583877 (AFF3) and rs12437854 (RGMA – MCTP2) with the different stages of DN was tested on a time-to-event analysis of relevant endpoints using longitudinal data for participants in the FinnDiane discovery collection. Consistent with our case-control GWAS analyses, the strongest association for rs7583877 was observed for the time from T1D diagnosis to development of ESRD (hazard ratio [HR] 1.33, 95% CI: 1.18–1.49, P = 1.9×10−6), but also the time from T1D diagnosis to development of macroalbuminuria (HR 1.15, 95% CI: 1.04–1.27, P = 0.006) and the time from macroalbuminuria to ESRD (HR 1.16, 95% CI: 1.01–1.36, P = 0.04) reached nominal significance. Similarly, rs12437854 was associated with time from T1D diagnosis to development of macroalbuminuria (HR 1.31, 95% CI: 1.03–1.67, P = 0.03) and ESRD (HR 1.35, 95% CI: 1.02–1.77, P = 0.03) (Text S1, Table S6, Figure S3). When we studied these SNPs and their association with various DN-related phenotypes in the case-control setting of the discovery cohorts, similar observations were made supporting the role of these SNPs in the development of ESRD: Whereas we found evidence of association between rs7583877 (AFF3) and all the examined phenotypes with ESRD as the case definition, only moderate association was observed for the DN phenotype (OR = 1.14, P = 0.002) and no association when patients with macroalbuminuria were compared to controls with normoalbuminuria (OR = 1.00, P = 0.95). rs12437854 (RGMA – MCTP2) had the strongest association with the original ESRD phenotype (controls defined as all non-ESRD subjects) and with the ESRD vs. normoalbuminuria phenotype, and moderate association with the DN phenotype and comparison of ESRD vs. macroalbuminuric patients (Table S7).
An alternative explanation for our ESRD findings may be that the associated variants in AFF3 gene and on chromosome 15q26 might be markers of survival. Mortality rates are extremely high in patients with kidney disease and macroalbuminuria, with at least 25% of macroalbuminuric patients dying before they reach ESRD [37]. Thus, the selection of patients with ESRD may be biased towards selection of severe kidney disease survival. To address this question, we used the time until death as the final end point in the longitudinal analysis. Neither of the loci associated with ESRD was also associated with mortality (Text S1, Table S6, Figure S3), suggesting that these loci are associated with ESRD per se.
To explore whether these SNPs contribute to DN via related intermediate phenotypes, such as adiposity, fasting lipid levels, or blood pressure we performed in silico searching of publicly available GWAS datasets for our top SNPs [38]–[41]. We found nominal, directionally consistent associations of rs12437854 with fasting glucose (P = 0.03) [42] and of rs7583877 with waist-hip ratio (P = 0.04) [43] (Table S8). We also considered if previously published T1D and CKD SNP associations were associated with DN or ESRD in our GWAS meta analyses. Eight of 80 SNPs at T1D-associated loci showed nominal significance with DN or ESRD (including three at AFF3 that are in weak LD [r2 0.030–0.046 in CEU] with the SNPs described here), while no CKD SNPs were nominally significant (Table S9) [44]–[47]. The lack of association with DN for CKD-associated SNPs suggests that the genetic risk factors for DN may differ from the genetic risk factors for CKD in a nondiabetic population.
Finally, to generate further biological hypotheses based on our GWAS results, we employed MAGENTA [48] gene set enrichment analysis software integrating Gene Ontology (GO) terms, KEGG and Ingenuity pathways and PANTHER database entries (Table S10). In the analysis of DN as a case phenotype, enriched gene sets included “sugar binding” (P = 0.0006), “double stranded DNA binding” (P = 0.001) and “nucleic acid binding” (P = 0.004). In the analysis of ESRD significantly enriched gene sets (P<0.01) included an enrichment of terms associated with DNA binding, including “sequence-specific DNA binding” (P = 0.003), “positive regulation of transcription” (P = 0.003), and “homeobox transcription factor” (P = 0.004). Taken together, the principal biological signal found within GWAS data suggests an enrichment of transcriptional regulators.
In this largest meta-analysis to date of DN from individuals with T1D, we found two genome-wide significant associations with ESRD. Variants in AFF3 have been shown to be associated with juvenile idiopathic rheumatoid arthritis, Graves' disease, celiac disease and T1D, indicating this may be a pan-autoimmune disease gene. It is possible that the AFF3 signal represents an association with T1D and/or is a false positive finding, as it was not seen in the follow-up cohorts. However, we note the following: 1) both FinnDiane and UK-ROI yielded very similar association results, 2) the number of ESRD cases in the replication cohorts is small (n = 363), indicating that statistical power to replicate the original association is limiting, 3) the association result in the second stage, while non-significant, trends in a consistent direction (OR 1.11), 4) after evaluating >12,000 individuals the AFF3 signal remained genome-wide significant (P = 1.2×10−8), and 5) we have provided supportive functional evidence that suggests AFF3 may be a relevant contributor to renal disease. Although survival bias is a possibility in the analyses of ESRD, longitudinal analysis revealed the association of the AFF3 and chromosome 15q26 loci with renal end-points and not with death. Experimental models provide independent evidence of AFF3 involvement in renal fibrosis and support an association of this locus with a renal phenotype. Importantly, despite our large sample size, we did not achieve genome-wide statistical significance for DN using a combined proteinuria/ESRD phenotype, suggesting that this phenotype may have been too heterogeneous to detect significant associations with a sample of this size. For example, lifelong glycemic control, a known risk factor for DN, is not well captured in most existing cohorts. Nevertheless, this study is the largest, well powered GWAS on DN to date. We demonstrated a suggestive signal of association at ERBB4 that is supported by experimental data showing haplotype specific mRNA expression in DN biopsies. Our findings reinforce the need for additional studies of patients with T1D and a homogeneous renal phenotype, in whom additional GWAS, fine-mapping and sequencing to uncover rare variants could be performed. Integration of our findings with ongoing GWAS in both type 1 and type 2 diabetes DN may also lead to discovery of additional genetic determinants of DN. The traditional phenotypic definition of DN for individuals with type 2 diabetes may be even more challenging for genetic studies given the heterogeneity of vascular complications and differential renal diagnoses. Several larger-scale GWAS have now been conducted for renal phenotypes [49]–[56], however in most cases the true disease-causing variant and functional impact for specific phenotypes remains to be established. Encouraging reports include the association of uromodulin with CKD [57], MYH9/APOL1 with non-diabetic ESRD [58], [59], and PLA2R1 with membranous nephropathy, where anti-PLA2R antibodies appear to predict activity of the disease as well as response to therapy [60].
Our findings point to two transcriptional networks centered around AFF3 and ERBB4 that may be operational in the pathogenesis of kidney disease in diabetes.
Methods
Ethics statement
All human research was approved by the relevant institutional review boards, and conducted according to the Declaration of Helsinki.
Study populations
We implemented a two stage analysis, in which a GWAS was performed using a set of three discovery cohorts in the GENIE consortium, and top signals for the DN and ESRD analyses were analyzed further in the second phase in a set of nine independent cohorts (described below) with 5,873 patients in total. The patient numbers in the individual studies are given in Table S11. Additional details are provided in the online material Text S1.
All Ireland, Warren 3, Genetics of Kidneys in Diabetes UK (UK-ROI) Collection [61]
Inclusion criteria included white individuals with T1D, diagnosed before 31 years of age, whose parents and grandparents were born in the UK and Ireland. The case group comprised 903 individuals with persistent proteinuria (>500 mg/24 h) developing more than 10 years after the diagnosis of diabetes, hypertension (>135/85 mmHg and/or treatment with antihypertensive medication), and retinopathy; ESRD (27.2%) was defined as individuals requiring renal replacement therapy or having received a kidney transplant. Absence of DN was defined as persistent normal urine albumin excretion rate (AER; 2 out of 3 urine albumin to creatinine ratio [ACR] measurements <20 µg of albumin/mg of creatinine) despite duration of T1D for at least 15 years, while not taking an antihypertensive medication, and having no history of treatment with ACE inhibitors; 1,001 individuals formed the control group. After exclusion of patients with low quality DNA samples, 914 DN/ESRD cases and 956 controls remained for the GWAS.
Finnish Diabetic Nephropathy Study (FinnDiane) [62]
The FinnDiane study is a Finnish cohort of more than 4,800 adult ethnic Finns with T1D, recruited from across Finland, diagnosed prior to age 35 and insulin treatment begun within 1 year. This study comprises 1,721 patients with normal AER, 516 with microalbuminuria, 733 with macroalbuminuria and 682 with ESRD. The disease status was defined by urine AER or urine ACR in at least two out of three consecutive urine collections at local centers: Microalbuminuria was defined as AER≥20<200 µg min−1 or ≥30<300 mg/24 h or an ACR of 2.5–25 mg mmol−1 for men and 3.5–35 mg mmol−1 for women in overnight, 24-hour or spot urine collections, respectively. Similarly, the limit for macroalbuminuria was AER≥200 µg min−1 or ≥300 mg/24 h or ACR≥25 mg mmol−1 for men and ≥35 mg mmol−1 for women. ESRD was defined as ongoing dialysis treatment or transplanted kidney. Control patients with normal AER were required to have T1D duration of at least 15 years. 558 of these patients were included from an independent Finnish cohort collected by the National Institute of Health and Welfare. These patients met the FinnDiane diagnosis and selection criteria, and were analyzed together with the FinnDiane cohort.
Genetics of Kidneys in Diabetes US Study (GoKinD US) [63]
The GoKinD US study consists of a DN case-control cohort of individuals diagnosed with T1D prior to 31 years of age who began insulin treatment within 1 year of T1D diagnosis. Controls were 18–59 years of age, with T1D for at least 15 years but without DN, n = 889. DN definition includes individuals with ESRD, dialysis or kidney transplant and persistent macroalbuminuria (at least 2 out of 3 tests positive for albuminuria by dipstick ≥1+, or ACR>300 µg albumin/mg of urine creatinine). Cases were defined as people 18–54 years of age, with T1D for at least 10 years and DN, n = 903. Individuals recruited to the control group employed the same inclusion criteria as UK-ROI. Individuals were recruited at two study centers, George Washington University (GWU) and the Joslin Diabetes Centre (JDC) using differing methods of ascertainment and recruitment [64]. Analysis of the GoKinD US cohort was limited to individuals whose primary ethnicity was Caucasian.
Collections genotyped in Phase 2
DNA was sought from worldwide case-control collections of individuals with T1D and known renal status. A total of 5,873 individuals from nine independent collections were genotyped or imputed for the top-ranked SNPs (n = 41 including 17 proxies), with the exception of the DCCT/EDIC cohort where GWAS data was imputed. All the patients included in the phase two analysis were adults of European descent and had T1D diagnosed before 35 years of age. Controls with normal AER had duration of T1D at least 15 years, and cases with DN had minimum T1D duration of 10 years. If a collection included patients with microalbuminuria, they were excluded from the primary analysis of DN, but included as controls in the analysis of ESRD versus non-ESRD. The main clinical characteristics of all the replication cohorts are shown in the Table S2 and the cohorts are described in Text S1.
Phenotype definitions
The primary phenotype of interest was DN, defined as individuals aged over 18, with T1D for at least 10 years and diabetic kidney disease. DN includes ESRD or persistent macroalbuminuria as defined in the cohort descriptions above. Controls were defined as individuals with T1D for at least 15 years but without any clinical evidence of kidney disease. Individuals with microalbuminuria were excluded from the primary DN analysis in all cohorts. Disease status definitions were consistent across all the study cohorts. Details of clinical characteristics for each cohort are defined in Table 1 and Table S2. We evaluated a second phenotype to gain further insights into the genetic basis of the most severe form of DN (leading to ESRD), and compared ESRD cases to all those without ESRD. This phenotype is referred to as the “ESRD” or “ESRD vs. non-ESRD” phenotype throughout the manuscript. We also considered individuals with ESRD compared to T1D controls with no clinical evidence of DN. Results for this comparison are given in the online supporting material (Tables S1, S6, S7, S9, S10), where this contrast is called “ESRD vs. normoalbuminuria” or “ESRD vs. normo”.
Genotyping
DNA from individuals in the UK-ROI collection were genotyped using the Omni1-Quad array (Illumina, San Diego, CA, USA) while FinnDiane samples employed Illumina's BeadArray 610-Quad array. Samples in UK-ROI and FinnDiane were excluded if they had insufficient DNA quality, quantity or poor genotype concordance with previous genotypes during the fingerprint evaluation stage. Existing genotype data for the GoKinD US genotype data was downloaded from dbGAP (phs000018.v2.p1, retrieved June 2010), containing updated genotype data from Affymetrix 500 K set (Affymetrix, Santa Clara, CA, USA).
Genotype quality control
Samples for UK-ROI and FinnDiane were excluded for insufficient DNA quality, quantity or poor genotype concordance with previous genotypes during a fingerprint evaluation stage. In the UK-ROI sample, 1,830 unique case (n = 872) and control (n = 958) individuals were submitted for genotyping on the Omni1-Quad. For FinnDiane, 3,651 individuals (cases, n = 1,934; controls n = 1,721) were submitted for genotyping on the 610-Quad. For all three discovery datasets (UK-ROI, FinnDiane, GoKinD US), uniform and extensive genotype quality control procedures were applied: SNPs were filtered for those with call rates greater than 90%, minor allele frequency (MAF) exceeding 1%, and concordance with Hardy Weinberg Equilibrium (HWE, P<10−7). Sample filters included individual call rates greater than 95%, no extreme heterozygosity and cryptic relatedness as determined using identity by descent (first degree relatives, estimated identity by descent >0.4), and admixture assessment using principal components (plotted with HapMap reference panel, Figure S4). Additional quality control measures included test of missing by haplotype (P<10−8), missing by phenotype (P>10−8) and plate effects (P<10−7). These quality control steps were performed using PLINK [65] with custom Perl and R analysis scripts. Known copy number variation and mitochondrial SNPs were excluded from analyses. Detailed results of each QC step are reported in Table S12 for each study population.
A HapMap control sample was included on all genotyping plates for UK-ROI; average call rate was 99.9% with HapMap concordance equaling 99.7%. The average sample call rate was 99.5% in UK-ROI with sample heterozygosity 22.1%. Concordance with internal control for FinnDiane was 99.996% with an average sample call rate of 99.8%.
Principal Component Analysis (PCA) was performed separately for each of the three studies with the EIGENSTRAT program [66] in order to detect genetic outliers and to adjust the analyses for population structure. Genetic outliers were defined as more than six standard deviations away from the center of distribution along any of the ten first principal components and the procedure was repeated until no outliers were detected. After filtering, PCA were calculated for each study cohort combined with unrelated individuals from three original HapMap populations (www.hapmap.org), and plotted to identify additional admixed individuals. The first ten principal components were employed to adjust the association analysis for any residual population structure from the cleaned datasets.
In total, directly genotyped results for 823 cases and 903 controls in 791,687 SNPs passed QC procedure in UK-ROI. Similarly, 549,530 SNPs with average genotyping rate of 99.9% passed the QC filters in 1,319 cases, 1,591 controls and 460 individuals with microalbuminuria for FinnDiane. 360,899 SNPs in 774 cases and 821 controls for GoKinD US passed quality control and were included in the analysis.
Imputation
Imputation was performed after the quality control employing MACH 1.0 software (http://www.sph.umich.edu/csg/abecasis/MACH) with HapMap phase II CEU population as a reference, resulting in ∼2.4 million SNPs for each cohort. The cross-over and error rates were estimated with 50 iteration rounds in roughly 300 randomly selected samples. The imputation was run with the greedy algorithm and the maximum likelihood method in order to obtain expected allele dosages rather than integer allele counts. SNPs with low imputation quality (r2<0.6) are not reported.
Statistical analysis
PLINK v1.07 [67] was employed to conduct association tests for the allele dosage data with logistic regression adjusted for sex, age, the duration of diabetes and the ten first components of the study specific principal component analysis. UK-ROI and GoKinD US were adjusted for study center, but in the primary DN phenotype the two GoKinD US centers; GWU and JDC, were analyzed separately. Results from individual studies were adjusted for study specific genomic inflation factor and then combined by fixed effect meta-analysis model using METAL [68], to estimate the combined effect sizes and significances from beta values and standard error. Regional association plots were generated using hg18 in LocusZoom [69]. Quantile-Quantile plots were generated to evaluate the number and magnitude of observed associations compared with those expected under the null hypothesis (Figure S1).
Second-phase SNP selection and genotyping
All SNPs observed with P<10−5 were selected for further analysis. These SNPs were reviewed and a top SNP (with a proxy) was selected for each independent signal (SNPs more than 500 kb distant or LD r2<0.3 in HapMap II CEU) using the LD-based clumping procedure implemented in PLINK. De novo genotyping was performed for all phase two cohorts except for DCCT/EDIC using identical designs of Sequenom IPLEX assays (Sequenom Inc, San Diego, US). The DCCT/EDIC samples were imputed from their GWAS results that had undergone their respective quality control procedure. The statistical analysis was similar to the discovery cohorts with the difference that the models were not adjusted for principal components. All results were then combined by meta-analysis using METAL software as previously described.
Longitudinal analysis
Time to event analyses were performed on longitudinal data from the FinnDiane discovery cohort using Kaplan-Meier and Cox proportional hazards regression with the aim to evaluate the genetic association of rs7583877 and rs12437854 with time from the diagnosis of T1D to the onset of the following end points: microalbuminuria, macroalbuminuria or ESRD. Additionally, we analyzed time from onset of macroalbuminuria to development of ESRD. The most recent kidney status data were utilized for each patient. We also examined if the two main association loci, rs7583877 and rs12437854, were associated with mortality using data from the Finnish Death Registry (as per 30.9.2010). As DN (defined as macroalbuminuria or ESRD) is strongly associated with mortality, the time to death was separately analyzed for patients without DN (time from T1D onset to death; patients who developed DN were censored at the time of the onset of DN) and for those with DN (time from onset of DN to death and time from onset of ESRD to death). Analyses were performed using the ‘survival’ package in R software (version 2.36-10, http://cran.r-project.org/web/packages/survival). (See Text S1.)
Additional analyses
SNPs were annotated with associated genes and function using dbSNP build 132, human build 37.1. Cytogenetic locations for genes were sourced from Entrez gene; locations for SNPs that were not associated with genes were recorded from NCBI MapView. In silico analyses included gene set enrichment using MAGENTA [48]. To explore functional implications of AFF3, human kidney epithelial cells (HK-2) were cultured and evaluated (Figure 4).
Renal biopsy populations
Gene expression was measured in renal tissue compartments micro-dissected from renal biopsies from Pima Indians with type 2 diabetes and early stage DN (n = 77), as well as from Caucasian living kidney transplant donors (n = 20). Pima Indian subjects are 25–68 in age, with measured ACR in the range 5.23–7162, and GFR in the range 40.45–274.80.
Renal expression
Renal biopsies were micro-dissected into glomeruli and either tubulointerstitial or cortical compartments, and gene expression measured using the Affymetrix HGU-133A and HGU-133 Plus 2 platforms [70]. Background adjustment, quantile normalization and probe-set summarization were performed with in a GenePattern (www.genepattern.org) pipeline using Robust Multichip Analysis [71] with batch correction using Combat [72]. The differential expression data sets were processed with the Entrez Gene Custom CDF v.10, and the eQTL data sets were processed with the RefSeq Custom CDF v.12 [73] for probe-sets common to both expression platforms.
eQTL association
The Affymetrix 6.0 genotyping platform was used to genotype Pima Indians with glomerular expression (n = 65), a subset of which (n = 54) also had tubulointerstitial/cortical expression. The cis region of each gene was defined as 150 kb upstream of the transcript start site and 50 kb downstream of the transcription end site.
Supporting Information
Zdroje
1. U S Renal Data System (1-12-0011) USRDS 2011 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2011.
2. RitzE, ZengXX, RychlikI (2011) Clinical manifestation and natural history of diabetic nephropathy. Contrib Nephrol 170 : 19–27.
3. KrolewskiAS, WarramJH, ChristliebAR, BusickEJ, KahnCR (1985) The changing natural history of nephropathy in type I diabetes. Am J Med 78 : 785–794.
4. de BoerIH, RueTC, HallYN, HeagertyPJ, WeissNS, et al. (2011) Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305 : 2532–2539.
5. RosolowskyET, SkupienJ, SmilesAM, NiewczasM, RoshanB, et al. (2011) Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22 : 545–553.
6. HarjutsaloV, KatohS, SartiC, TajimaN, TuomilehtoJ (2004) Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes 53 : 2449–2454.
7. von BerghAR, BeverlooHB, RomboutP, van WeringER, van WeelMH, et al. (2002) LAF4, an AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia. Genes Chromosomes Cancer 35 : 92–96.
8. MaC, StaudtLM (1996) LAF-4 encodes a lymphoid nuclear protein with transactivation potential that is homologous to AF-4, the gene fused to MLL in t(4;11) leukemias. Blood 87 : 734–745.
9. MelkoM, DouguetD, BensaidM, ZongaroS, VerheggenC, et al. (2011) Functional characterization of the AFF (AF4/FMR2) family of RNA-binding proteins: insights into the molecular pathology of FRAXE intellectual disability. Hum Mol Genet 20 : 1873–1885.
10. von BerghAR, BeverlooHB, RomboutP, van WeringER, van WeelMH, et al. (2002) LAF4, an AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia. Genes Chromosomes Cancer 35 : 92–96.
11. BartonA, EyreS, KeX, HinksA, BowesJ, et al. (2009) Identification of AF4/FMR2 family, member 3 (AFF3) as a novel rheumatoid arthritis susceptibility locus and confirmation of two further pan-autoimmune susceptibility genes. Hum Mol Genet 18 : 2518–2522.
12. PlantD, FlynnE, MbarekH, DieudeP, CornelisF, et al. (2010) Investigation of potential non-HLA rheumatoid arthritis susceptibility loci in a European cohort increases the evidence for nine markers. Ann Rheum Dis 69 : 1548–1553.
13. BarrettJC, ClaytonDG, ConcannonP, AkolkarB, CooperJD, et al. (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41 : 703–707.
14. WallaceC, RotivalM, CooperJD, RiceCM, YangJH, et al. (2012) Statistical colocalization of monocyte gene expression and genetic risk variants for type 1 diabetes. Hum Mol Genet
15. GassmannM, CasagrandaF, OrioliD, SimonH, LaiC, et al. (1995) Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378 : 390–394.
16. TidcombeH, Jackson-FisherA, MathersK, SternDF, GassmannM, et al. (2003) Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A 100 : 8281–8286.
17. PrickettTD, AgrawalNS, WeiX, YatesKE, LinJC, et al. (2009) Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet 41 : 1127–1132.
18. VeikkolainenV, NaillatF, RailoA, ChiL, ManninenA, et al. (2012) ErbB4 modulates tubular cell polarity and lumen diameter during kidney development. J Am Soc Nephrol 23 : 112–122.
19. ZengF, ZhangMZ, SinghAB, ZentR, HarrisRC (2007) ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol Cell 18 : 4446–4456.
20. BerthierCC, ZhangH, SchinM, HengerA, NelsonRG, et al. (2009) Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58 : 469–477.
21. ZuberAM, CentenoG, PradervandS, NikolaevaS, MaquelinL, et al. (2009) Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 106 : 16523–16528.
22. MarfellaCG, HenningerN, LeBlancSE, KrishnanN, GarlickDS, et al. (2008) A mutation in the mouse Chd2 chromatin remodeling enzyme results in a complex renal phenotype. Kidney Blood Press Res 31 : 421–432.
23. MalhotraA, KobesS, KnowlerWC, BaierLJ, BogardusC, et al. (2011) A genome-wide association study of BMI in American Indians. Obesity (Silver Spring) 19 : 2102–2106.
24. KimY, KleppelMM, ButkowskiR, MauerSM, WieslanderJ, et al. (1991) Differential expression of basement membrane collagen chains in diabetic nephropathy. Am J Pathol 138 : 413–420.
25. YagameM, KimY, ZhuD, SuzukiD, EguchiK, et al. (1995) Differential distribution of type IV collagen chains in patients with diabetic nephropathy in non-insulin-dependent diabetes mellitus. Nephron 70 : 42–48.
26. BritanovaO, LukyanovS, GrussP, TarabykinV (2002) The mouse Laf4 gene: exon/intron organization, cDNA sequence, alternative splicing, and expression during central nervous system development. Genomics 80 : 31–37.
27. BrennanEP, MorineMJ, WalshDW, RoxburghSA, LindenmeyerMT, et al. (2012) Next-generation sequencing identifies TGF-beta1-associated gene expression profiles in renal epithelial cells reiterated in human diabetic nephropathy. Biochim Biophys Acta 1822 : 589–599.
28. KalluriR, WeinbergRA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119 : 1420–1428.
29. KrupaA, JenkinsR, LuoDD, LewisA, PhillipsA, et al. (2010) Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 21 : 438–447.
30. MorrisseyJ, GuoG, MoridairaK, FitzgeraldM, McCrackenR, et al. (2002) Transforming growth factor-beta induces renal epithelial jagged-1 expression in fibrotic disease. J Am Soc Nephrol 13 : 1499–1508.
31. WalshDW, RoxburghSA, McGettiganP, BerthierCC, HigginsDG, et al. (2008) Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim Biophys Acta 1782 : 10–21.
32. BieleszB, SirinY, SiH, NiranjanT, GruenwaldA, et al. (2010) Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120 : 4040–4054.
33. DresslerGR (2008) Another niche for Notch. Kidney Int 73 : 1207–1209.
34. MurphyM, GodsonC, CannonS, KatoS, MackenzieHS, et al. (1999) Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem 274 : 5830–5834.
35. KramerHJ, NguyenQD, CurhanG, HsuCY (2003) Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289 : 3273–3277.
36. PerkinsBA, NelsonRG, OstranderBE, BlouchKL, KrolewskiAS, et al. (2005) Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 16 : 1404–1412.
37. ForsblomC, HarjutsaloV, ThornLM, WadenJ, TolonenN, et al. (2011) Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 22 : 537–544.
38. DupuisJ, LangenbergC, ProkopenkoI, SaxenaR, SoranzoN, et al. (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 : 105–116.
39. EhretGB, MunroePB, RiceKM, BochudM, JohnsonAD, et al. (2011) Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 : 103–109.
40. HeidIM, JacksonAU, RandallJC, WinklerTW, QiL, et al. (2010) Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42 : 949–960.
41. WillerCJ, SannaS, JacksonAU, ScuteriA, BonnycastleLL, et al. (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40 : 161–169.
42. DupuisJ, LangenbergC, ProkopenkoI, SaxenaR, SoranzoN, et al. (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 : 105–116.
43. HeidIM, JacksonAU, RandallJC, WinklerTW, QiL, et al. (2010) Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42 : 949–960.
44. KottgenA, GlazerNL, DehghanA, HwangSJ, KatzR, et al. (2009) Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41 : 712–717.
45. ChambersJC, ZhangW, LordGM, van derHP, LawlorDA, et al. (2010) Genetic loci influencing kidney function and chronic kidney disease. Nat Genet 42 : 373–375.
46. BurrenOS, AdlemEC, AchuthanP, ChristensenM, CoulsonRM, et al. (2011) T1DBase: update 2011, organization and presentation of large-scale data sets for type 1 diabetes research. Nucleic Acids Res 39: D997–1001.
47. KottgenA, PattaroC, BogerCA, FuchsbergerC, OldenM, et al. (2010) New loci associated with kidney function and chronic kidney disease. Nat Genet 42 : 376–384.
48. SegreAV, GroopL, MoothaVK, DalyMJ, AltshulerD (2010) Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet 6 doi:10.1371/journal.pgen.1001058.
49. ChambersJC, ZhangW, LordGM, van derHP, LawlorDA, et al. (2010) Genetic loci influencing kidney function and chronic kidney disease. Nat Genet 42 : 373–375.
50. FeehallyJ, FarrallM, BolandA, GaleDP, GutI, et al. (2010) HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 21 : 1791–1797.
51. RampoldiL, ScolariF, AmorosoA, GhiggeriG, DevuystO (2011) The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80 : 338–347.
52. KaoWH, KlagMJ, MeoniLA, ReichD, Berthier-SchaadY, et al. (2008) MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40 : 1185–1192.
53. RossetS, TzurS, BeharDM, WasserWG, SkoreckiK (2011) The population genetics of chronic kidney disease: insights from the MYH9-APOL1 locus. Nat Rev Nephrol 7 : 313–326.
54. StanescuHC, Arcos-BurgosM, MedlarA, BockenhauerD, KottgenA, et al. (2011) Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364 : 616–626.
55. KottgenA, GlazerNL, DehghanA, HwangSJ, KatzR, et al. (2009) Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41 : 712–717.
56. KottgenA, PattaroC, BogerCA, FuchsbergerC, OldenM, et al. (2010) New loci associated with kidney function and chronic kidney disease. Nat Genet 42 : 376–384.
57. RampoldiL, ScolariF, AmorosoA, GhiggeriG, DevuystO (2011) The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80 : 338–347.
58. KaoWH, KlagMJ, MeoniLA, ReichD, Berthier-SchaadY, et al. (2008) MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40 : 1185–1192.
59. RossetS, TzurS, BeharDM, WasserWG, SkoreckiK (2011) The population genetics of chronic kidney disease: insights from the MYH9-APOL1 locus. Nat Rev Nephrol 7 : 313–326.
60. StanescuHC, Arcos-BurgosM, MedlarA, BockenhauerD, KottgenA, et al. (2011) Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364 : 616–626.
61. McKnightAJ, PattersonCC, PettigrewKA, SavageDA, KilnerJ, et al. (2010) A GREM1 gene variant associates with diabetic nephropathy. J Am Soc Nephrol 21 : 773–781.
62. SyreeniA, El OstaA, ForsblomC, SandholmN, ParkkonenM, et al. (2011) Genetic examination of SETD7 and SUV39H1/H2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes 60 : 3073–3080.
63. PezzolesiMG, PoznikGD, MychaleckyjJC, PatersonAD, BaratiMT, et al. (2009) Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58 : 1403–1410.
64. PezzolesiMG, PoznikGD, MychaleckyjJC, PatersonAD, BaratiMT, et al. (2009) Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58 : 1403–1410.
65. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
66. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909.
67. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
68. WillerCJ, LiY, AbecasisGR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 : 2190–2191.
69. PruimRJ, WelchRP, SannaS, TeslovichTM, ChinesPS, et al. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26 : 2336–2337.
70. CohenCD, KretzlerM (2002) Gene expression analysis in microdissected renal tissue. Current challenges and strategies. Nephron 92 : 522–528.
71. IrizarryRA, BolstadBM, CollinF, CopeLM, HobbsB, et al. (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15.
72. JohnsonWE, LiC, RabinovicA (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8 : 118–127.
73. DaiM, WangP, BoydAD, KostovG, AtheyB, et al. (2005) Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 33: e175.
Štítky
Genetika Reprodukčná medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



