-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
Harnessing genetic differences between cancerous and noncancerous cells offers a strategy for the development of new therapies. Extrapolating from yeast genetic interaction data, we used cultured human cells and siRNA to construct and evaluate a synthetic lethal interaction network comprised of chromosome instability (CIN) genes that are frequently mutated in colorectal cancer. A small number of genes in this network were found to have synthetic lethal interactions with a large number of cancer CIN genes; these genes are thus attractive targets for anticancer therapeutic development. The protein product of one highly connected gene, the flap endonuclease FEN1, was used as a target for small-molecule inhibitor screening using a newly developed fluorescence-based assay for enzyme activity. Thirteen initial hits identified through in vitro biochemical screening were tested in cells, and it was found that two compounds could selectively inhibit the proliferation of cultured cancer cells carrying inactivating mutations in CDC4, a gene frequently mutated in a variety of cancers. Inhibition of flap endonuclease activity was also found to recapitulate a genetic interaction between FEN1 and MRE11A, another gene frequently mutated in colorectal cancers, and to lead to increased endogenous DNA damage. These chemical-genetic interactions in mammalian cells validate evolutionarily conserved synthetic lethal interactions and demonstrate that a cross-species candidate gene approach is successful in identifying small-molecule inhibitors that prove effective in a cell-based cancer model.
Published in the journal: An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development. PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003254
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003254Summary
Harnessing genetic differences between cancerous and noncancerous cells offers a strategy for the development of new therapies. Extrapolating from yeast genetic interaction data, we used cultured human cells and siRNA to construct and evaluate a synthetic lethal interaction network comprised of chromosome instability (CIN) genes that are frequently mutated in colorectal cancer. A small number of genes in this network were found to have synthetic lethal interactions with a large number of cancer CIN genes; these genes are thus attractive targets for anticancer therapeutic development. The protein product of one highly connected gene, the flap endonuclease FEN1, was used as a target for small-molecule inhibitor screening using a newly developed fluorescence-based assay for enzyme activity. Thirteen initial hits identified through in vitro biochemical screening were tested in cells, and it was found that two compounds could selectively inhibit the proliferation of cultured cancer cells carrying inactivating mutations in CDC4, a gene frequently mutated in a variety of cancers. Inhibition of flap endonuclease activity was also found to recapitulate a genetic interaction between FEN1 and MRE11A, another gene frequently mutated in colorectal cancers, and to lead to increased endogenous DNA damage. These chemical-genetic interactions in mammalian cells validate evolutionarily conserved synthetic lethal interactions and demonstrate that a cross-species candidate gene approach is successful in identifying small-molecule inhibitors that prove effective in a cell-based cancer model.
Introduction
Cancerous cells carry somatic mutations that genotypically distinguish them from surrounding noncancerous cells, and this provides an opportunity that can be exploited for therapeutic development. One strategy for the specific targeting of cancer genotypes relative to nonmutated somatic cells is to exploit synthetic lethal interactions [1]. For example, breast cancer cells with mutations in BRCA1 or BRCA2 are extremely susceptible to knockdown or chemical inhibition of PARP1, which encodes poly(ADP)ribose polymerase (PARP) [2], [3].
While exploiting synthetic lethality has the potential to be an effective approach to treating tumors, a major challenge is the identification of clinically relevant small-molecule inhibitors. One approach, pioneered by the National Cancer Institute, is to screen many thousands of unknown potential therapeutics on cancer cell lines [4]. Compounds generate a “fingerprint” of activity against certain cell lines, which can then be deconvolved, usually by mutation sequencing, to yield novel gene-drug interactions, in a so-called “bottom-up” approach. Alternatively, a “top-down” approach applies compounds of known target or mode of action to known genotypes, again to identify new gene-drug interactions. Recently, two groups used such an approach to screen more than 100 compounds against hundreds of cancer cell lines whose mutational status was known [5], [6], observing that gene-drug interactions tended to be more significant for targeted therapies, such as compounds targeting the BCR-ABL fusion protein, than for generally cytotoxic drugs, such as DNA damaging agents or antimitotics [6]. Thus, screening for compounds targeting a specific genetic lesion is preferable to developing new cytotoxic agents. Such targeted compounds can then be deployed as first-line anticancer therapeutics either singly or in a combination regime that would lessen the likelihood of drug-resistant clones developing within the tumor cell population [7], [8].
Many different cancer mutations lead to a limited repertoire of cancer phenotypes, such as chromosome instability, checkpoint dysfunction, and hyperplasia [9]. It is possible to identify a gene target that results in synthetic lethality with a large number of unlinked gene mutations by screening for targets that result in synthetic lethality with a common tumor phenotype. For example, chromosome instability (CIN), an increase in the rate of gain or loss of whole or parts of chromosomes, is observed in the form of aneuploidy in more than 90% of solid tumors and over 75% of blood cancers [10]. As the maintenance of genomic stability is an essential cellular process, CIN represents a phenotype that could potentially be leveraged towards selective killing of cancerous cells relative to normal cells. A gene that is synthetic lethal with a large number of cancer-related CIN genes would be an attractive therapeutic target in a large fraction of tumors.
Genetically tractable model organisms, such as the budding yeast Saccharomyces cerevisiae, facilitate the identification of human CIN genes, via identification and sequencing of their human orthologs. For example, identification of yeast CIN genes [11]) led to the sequencing of the human homologs of 200 yeast CIN genes in human colorectal cancers, and it was discovered that human homologs of the yeast CIN genes SMC1, SCC2, BUB1, PDS1, MRE11, and CDC4 collectively account for approximately 25% of the mutational spectrum of colorectal cancer [12]–[15]. Thus, if a common synthetic lethal interacting partner could be identified for all of these genes, and a highly potent and specific inhibitor of its activity could be developed, inhibition of this target would offer a potentially broad means of targeting CIN cancers. In yeast, technologies exist to screen for genome-wide synthetic lethal interactions with relative ease [16], and identification of the synthetic lethal interaction network of the yeast orthologs of cancer-mutated genes has in previous cases revealed a small number of “hub” genes having synthetic lethal interactions with many yeast cancer-orthologs [17]. Previous studies have found a high degree of conservation between yeast and metazoan genetic interactions [18], [19], suggesting hub gene identification based on a yeast CIN gene synthetic lethal interaction network should yield broad-spectrum, second-site target genes applicable to human cancers.
Here we present and validate a cross-species candidate-based approach to the identification of anticancer targets and the discovery of anticancer therapeutics. We show that a genetic interaction network comprised of colorectal cancer CIN genes is largely conserved between S. cerevisiae and a human cancer cell line. We develop an in vitro assay for the activity of the protein encoded by one such highly connected gene, FEN1, and use this assay to screen for small-molecule inhibitors. Finally, we show that flap endonuclease inhibitors recapitulate conserved genetic interactions. These data demonstrate the effectiveness of a cross-species synthetic lethal approach to the discovery of potential anticancer therapeutics.
Results
A cross-species approach reveals conserved genetic interaction partners of cancer genes
The human genes SMC1, SMC3, NIPBL, STAG3, RNF20, FBXW7/CDC4, MRE11A, RAD54B and BLM have been found to be mutated in colorectal cancer, and together account for approximately 25% of the CIN mutational spectrum of this disease [13]–[15], [20]–[22]. Protein BLAST was used to identify the budding yeast orthologs of these human genes (Table 1) and we constructed a synthetic lethal interaction network (Figure 1A), using literature and publicly available genetic interaction data (BioGrid and the Saccharomyces Genome Database) [18], [23].
Fig. 1. Evolutionary conservation of synthetic lethal interactions in HCT116 cells. 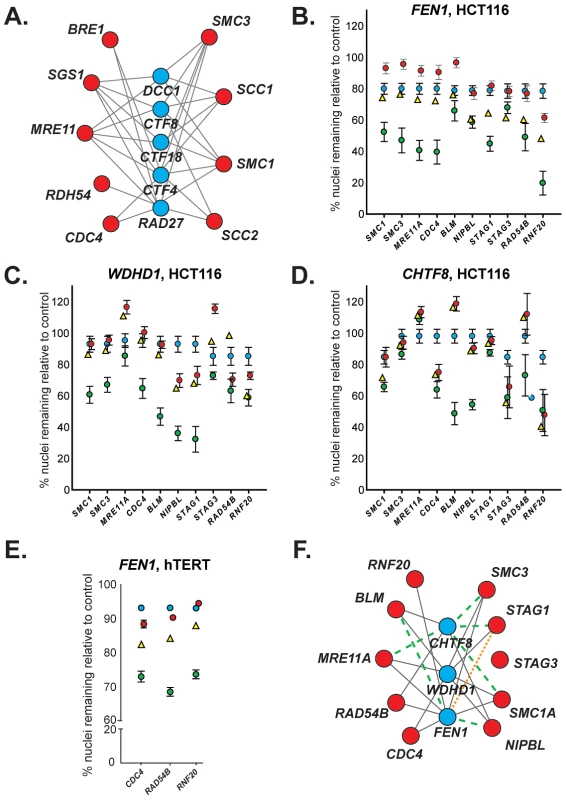
(A) A yeast cancer-ortholog synthetic lethal network. Lines indicate synthetic lethal genetic interactions. Yeast genes and human orthologs are presented in Table 1. Red circles represent S. cerevisiae orthologs of genes mutated in cancer; blue circles indicate common interacting partners, which are referred to as “central” genes. (B–D) Representative data depicting mean percentage of remaining HCT116 cells (± SEM) treated with pooled siRNAs targeting central genes (top) and cancer genes (along x-axis) relative to GAPDH-silenced controls. Blue circles, siRNA targeting central gene alone. Red circles, siRNA targeting cancer gene alone. Yellow triangles, predicted viability of double siRNA treatment. Green circles, observed viability of double siRNA treatment. For raw data, please refer to . GAPDH siRNA does not significantly reduce viability relative to a non-silencing siRNA (Figure S2A). (E) Representative data depicting mean percentage viability of hTERT cells (± SEM) treated with pooled siRNAs targeting FEN1 and cancer genes (along x-axis) relative to GAPDH-silenced controls. Symbols are as in B. (F) Mammalian genetic interaction network. Solid grey line, interaction observed in both S. cerevisiae and HCT116 cells; green dashed line, interaction observed only in S. cerevisiae; orange dotted line, interaction observed only in HCT116 cells. Tab. 1. Yeast and human gene orthologs.A 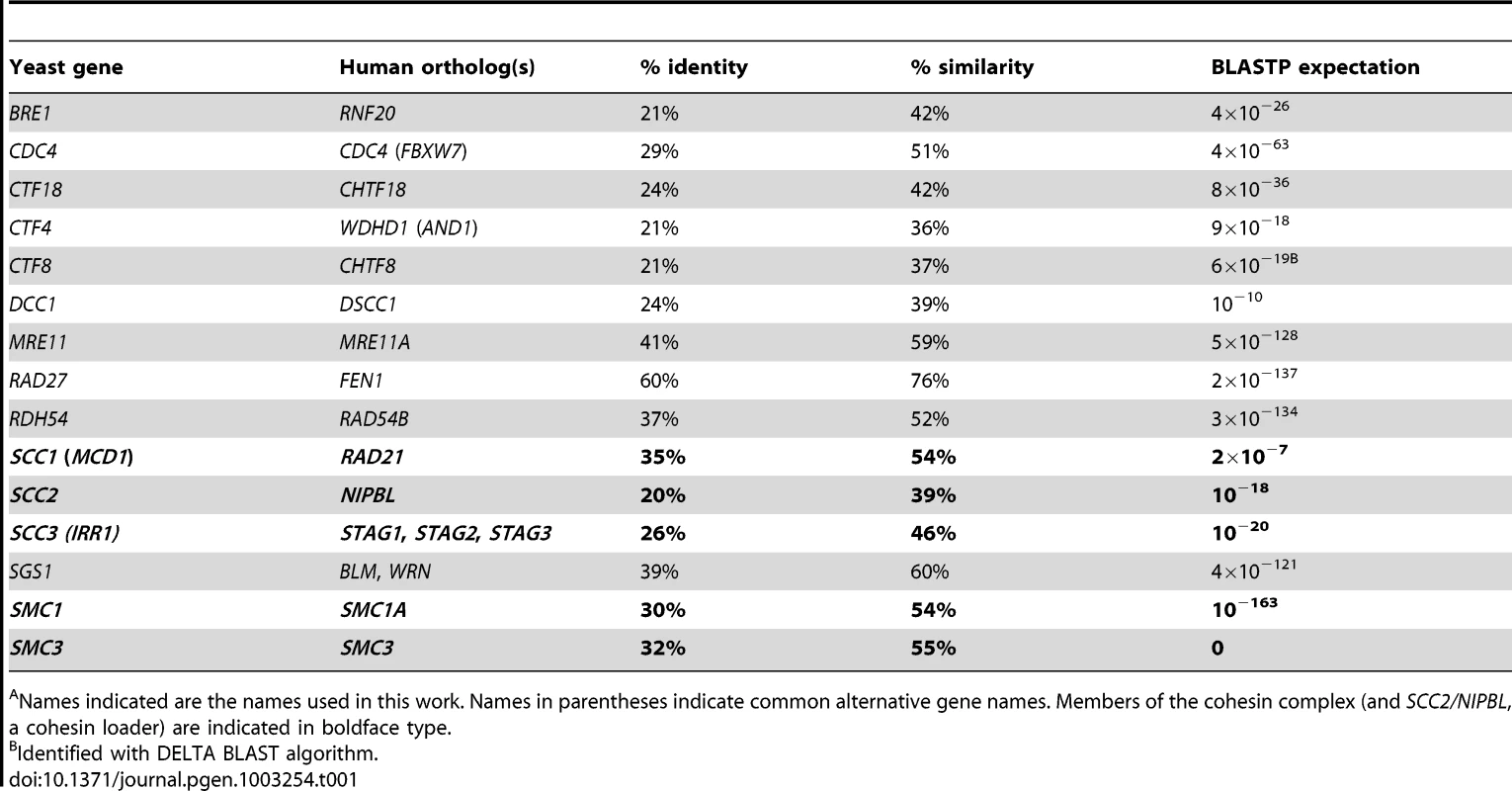
Names indicated are the names used in this work. Names in parentheses indicate common alternative gene names. Members of the cohesin complex (and SCC2/NIPBL, a cohesin loader) are indicated in boldface type. To investigate the conservation of this network between yeast and a human cell line, we used siRNA-mediated knockdown of potential synthetic lethal gene pairs in the cell line HCT116. Knockdown efficiencies were evaluated by Western blots (Figure S1A). All pair-wise combinations between the three “central” synthetic lethal partner genes, WDHD1, FEN1, and CHTF8, and the ten outer cancer-mutated CIN genes were evaluated for synthetic lethality (Figure 1B, 1C, 1D). (CHTF8 was selected as a representative of the alternative RFCCHTF18, comprised of Dcc1, Ctf8, and Ctf18 in S. cerevisiae).
Of the 30 possible synthetic lethal interactions among the genes tested, 22 have been reported in yeast [18], [23]. We found 16 of the predicted interactions (73%) were conserved between yeast and human cells, and 6 predicted interactions did not appear conserved in our assay (27%). Furthermore, one interaction, between FEN1 and STAG1, was not predicted based on yeast data; however, we detected a genetic interaction between these genes (Figure 1F and Table S1). No interactions were observed with STAG3, which functions primarily in human meiosis [24]. As in yeast, all three central genes – WDHD1, FEN1, and CHTF8 – were highly connected to sister chromatid cohesion genes (e.g. cohesin and/or cohesin loaders) (Figure 1F).
As FEN1 encodes an enzyme, whereas WDHD1 and CHTF8 do not; it may be amenable to biochemical inhibitor screening. Thus, we sought to further validate genetic interactions between FEN1 and other genes in the network. To ensure that these observed interactions were not cell line-dependent, we attempted to recapitulate interactions between FEN1 and each of CDC4, RAD54B, and RNF20 in the karyotypically stable, immortalized fibroblast cell line hTERT. As in HCT116 cells, genetic interactions were observed following knockdown of all three gene pairs (Figure 1E, Table S2, Figure S1C). We found that individual siRNAs could recapitulate the genetic interactions observed with the pooled siRNAs (Table S3). These data validate a subset of genetic interactions identified in the HCT116 cells and thus confirm FEN1 as a strong candidate therapeutic target.
Developing a high-throughput in vitro assay for FEN1 activity
FEN1 (Flap ENdonuclease 1) encodes an enzyme previously shown to be amenable to biochemical assay development in vitro [25] that has been implicated in almost all DNA transactions, including DNA repair and replication [26]). Adapting a previous radiolabel-based in vitro assay, we developed an in vitro assay for FEN1 activity based on fluorescence quenching [25]). In this assay, three oligonucleotides are annealed to generate the synthetic substrate, positioning a fluorophore and fluorescent quencher in close proximity. The flap endonuclease activity of FEN1 cleaves the 5′ flap to which the fluorophore is attached, allowing it to diffuse away from the quencher and fluoresce (Figure 2). Using a potent, previously described in vitro FEN1 inhibitor, compound 16 from Tumey, et al. [27], we observed significant inhibition of flap endonuclease activity (Figure 3, upper left panel). A screen of 30 000 compounds, from libraries containing known and FDA-approved drugs, and the Canadian Chemical Biology Network library, yielded approximately 90 hits, following a counterscreen using a quencherless substrate to eliminate false positives caused by fluorescent compounds and fluorescent quenchers. Ultimately, 13 compounds were selected for further investigation based on structural diversity and having drug-like properties (as described by Lipinski's “Rule of Five”; [28]). These compounds were found to have mid-nanomolar to low micromolar IC50s in vitro (Figure 3, remaining panels).
Fig. 2. Screening for FEN1 inhibitors in vitro. 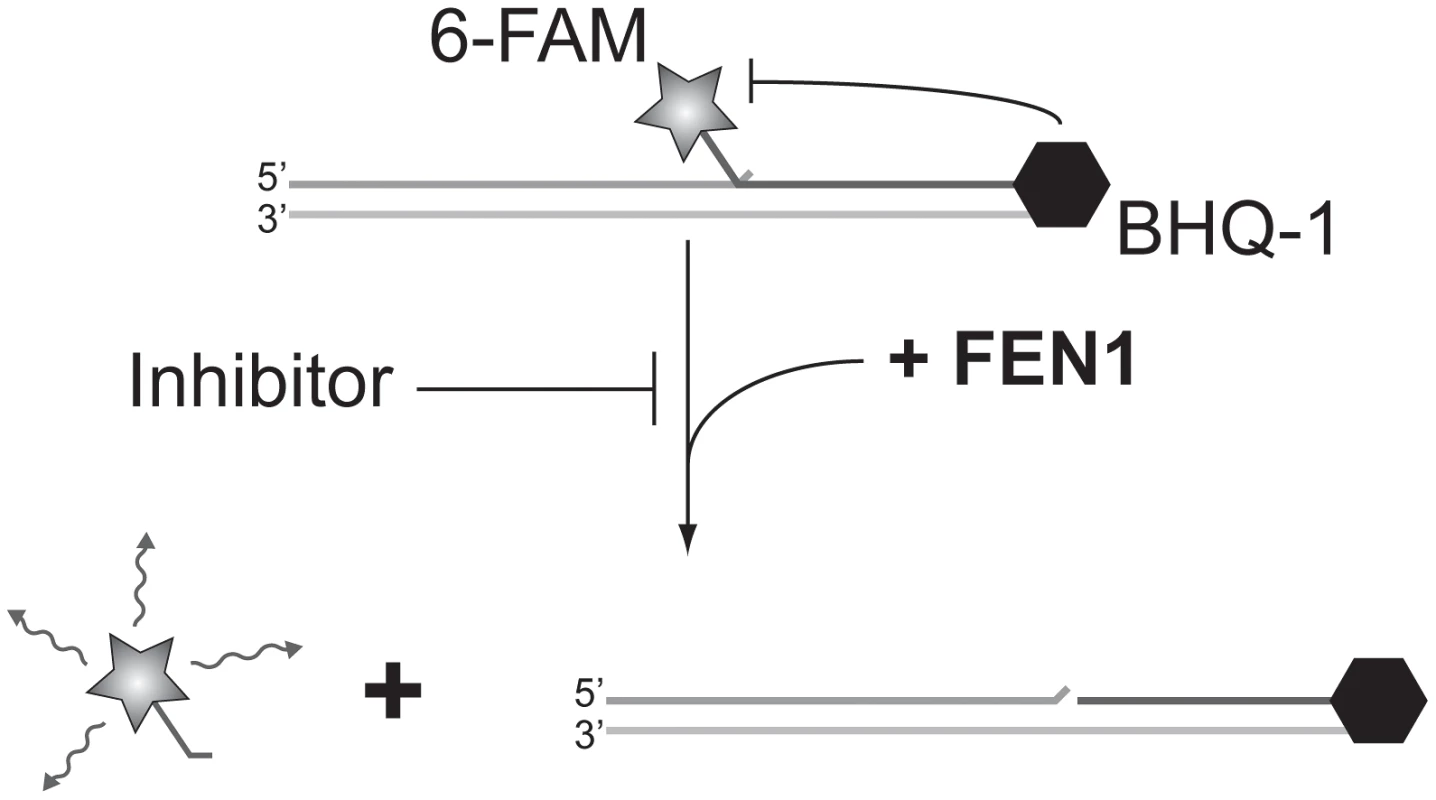
Schematic representation of the fluorescence-based assay employed to identify FEN1 inhibitors. In the absence of inhibitor, FEN1 cleaves the 5′ flap to which the 6-FAM fluorophore is attached, allowing it to diffuse away from the BHQ-1 quencher and fluoresce. Activity is read as increasing fluorescence over time. Fig. 3. IC50 curves of flap endonuclease inhibitors. 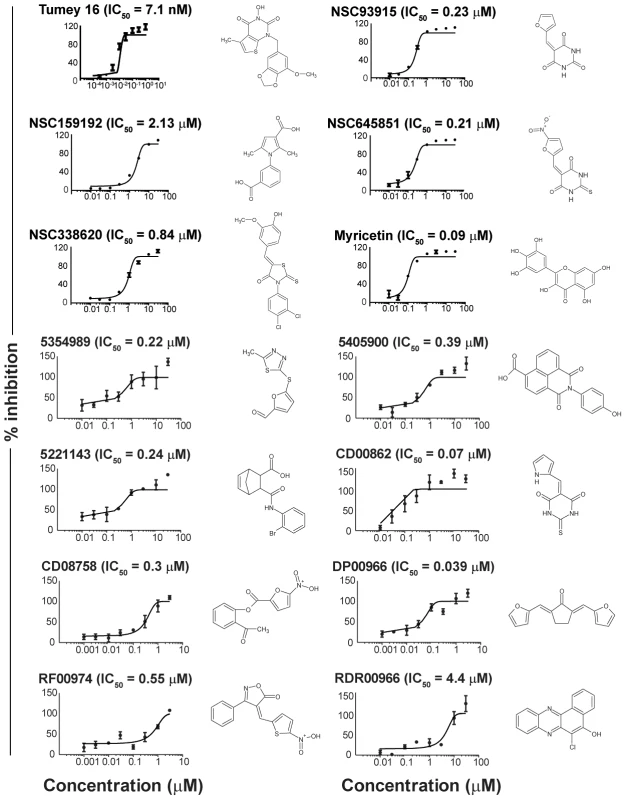
FEN1 assays were carried out as described in Materials and Methods. Compound names are indicated above each graph, and structures are given to the right of each graph. Tumey 16 (top-left panel) was included as a positive control for flap endonuclease inhibition. Flap endonuclease inhibitors recapitulate genetic interactions in cells
We next sought to determine whether the flap endonuclease inhibitors we identified could recapitulate any of the genetic interactions found previously (Figure 1F). We first targeted the interaction between FEN1 and CDC4, owing to the fact that CDC4 has been shown to be a CIN gene mutated in many tumor types [11], [29]–[32]. We took advantage of a matched pair of cell lines in which both copies of CDC4 had been inactivated in HCT116 cells [13]. siRNA-mediated knockdown of FEN1 in this cell pair resulted in selective proliferation inhibition (Figure 4A). We applied the small-molecule hits from the screen to this matched pair of cell lines and found six compounds that selectively inhibited the proliferation of CDC4-knockout HCT116 cells relative to wild type cells (Figure 4B and Figure S2B). To ensure that these results were not cell line-specific, we utilized another matched pair of cell lines with inactivated CDC4, this time in DLD-1 cells. The six compounds showing selective proliferation inhibition of CDC4-knockout HCT116 cells were applied to CDC4-knockout and wild type DLD-1 cells [13], and RF00974 and NSC645851 were found to selectively inhibit the proliferation of CDC4-knockout DLD-1 cells relative to wild type (Figure 4C).
Fig. 4. Cell-based assays for flap endonuclease inhibitor activity reveal two compounds that selectively inhibit the proliferation of cells deficient in CDC4. 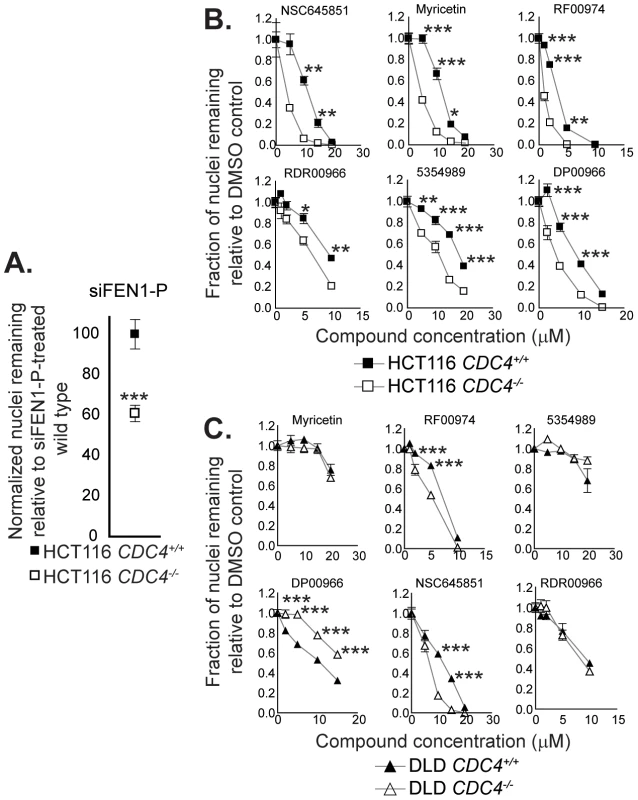
(A) siRNA-mediated knockdown of FEN1 selectively inhibits the proliferation of CDC4-knockout HCT116 cells. siRNA transfections were carried out as described in Materials and Methods. Cells were fixed and imaged four days following siRNA transfection. Data were analyzed by one-way ANOVA followed by a Tukey test. Shown is mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001. (B) Some flap endonuclease inhibitors recapitulate the genetic interaction between FEN1 and CDC4 in HCT116 cells. Cells were incubated with compound at the indicated concentration for 72 hours in optically clear 96-well plates prior to fixation and imaging as described in Materials and Methods. Data were analyzed by one-way ANOVA followed by a Tukey test. Shown is mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001. (C) RF00974 and NSC645851 recapitulate the genetic interaction between FEN1 and CDC4 in DLD-1 cells. Experiments were carried out as in (B). Data were analyzed by one-way ANOVA followed by a Tukey test. Shown is mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001. To further test the idea that CDC4 activity is responsible for the observed effect, cells in which CDC4 had been inactivated in a heterozygous state were also treated with RF00974 and NSC645851. As with homozygous CDC4−/− cells, heterozygous CDC4+/− cells displayed a statistically significant decrease in proliferation relative to wild type CDC4+/+ cells, albeit lesser in magnitude (Figure 5).
Fig. 5. NSC645851 and RF00974 selectively inhibit the proliferation of HCT116 and DLD-1 cells with both homozygous and heterozygous inactivating mutations of CDC4. 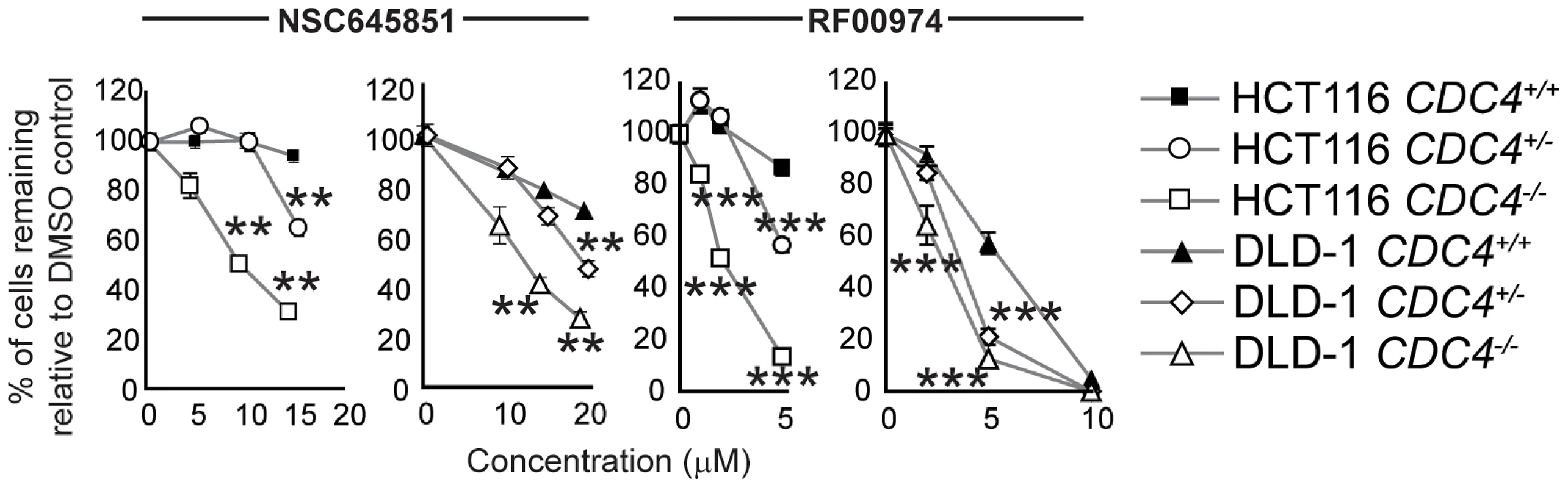
Experiments were carried out as described in Figure 4B. Data were analyzed by one-way ANOVA followed by a Tukey test. Shown is mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001. We next attempted to recapitulate the interaction between FEN1 and MRE11A, as MRE11A has been shown to be mutated at a frequency of 4% in colorectal cancers [15]. We treated cells in which MRE11A had been depleted via siRNA with the more potent of the two flap endonuclease inhibitors described above, RF00974, and found that MRE11A depletion sensitized cells to flap endonuclease inhibitor treatment (Figure 6A). We also found that treatment with a previously-described small-molecule inhibitor of MRE11A, mirin [33], was able to sensitize cells to treatment with RF00974 (Figure 6B). Taken together, these data suggest that inhibition of flap endonuclease activity is sufficient to recapitulate evolutionarily conserved, colorectal cancer-relevant synthetic lethal genetic interactions.
Fig. 6. RF00974 recapitulates the interaction between FEN1 and MRE11A, and leads to increased endogenous DNA damage. 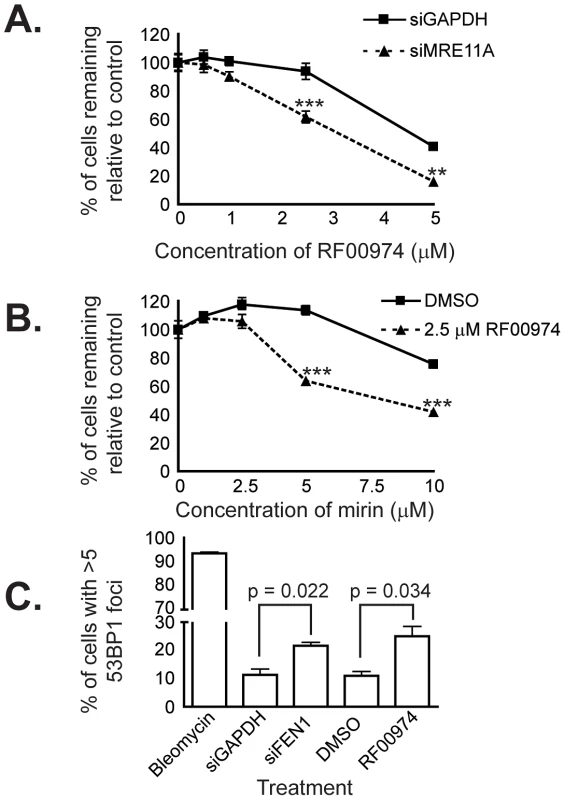
(A) siRNA-mediated knockdown of MRE11A sensitizes HCT116 cells to treatment with RF00974. siRNA transfection experiments were carried out as described in Materials and Methods. Cells were fixed and imaged four days following siRNA transfection, after cells had been incubated in compound for 48 hours. Data were analyzed by one-way ANOVA followed by a Tukey test. Shown is mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001. (B) Chemical inhibition of MRE11A sensitizes cells to RF00974. Cells were incubated in the indicated compounds at the indicated concentrations for three days prior to fixation and imaging as described in Materials and Methods. Data were analyzed by one-way ANOVA followed by a Tukey test. Shown is mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001. (C) Inhibition of flap endonuclease activity with RF00974 mimics siRNA-mediated knockdown of FEN1 by increasing endogenous DNA damage. Data were analyzed by Student's t test. Shown is mean ± SEM. Finally, we wished to characterize the mechanism by which inhibition of flap endonuclease activity may lead to cell death. Given the role of FEN1 in DNA replication and repair, we asked whether endogenous DNA damage increases as a result of FEN1 inhibition. We used HCT116 cells in which 53BP1 had been stably tagged with mCherry to ask whether 53BP1 focus formation, indicative of DNA repair centers [34], [35], increased. We found a statistically significant (p<0.05) increase in the frequency of cells with many 53BP1 foci following siRNA-mediated knockdown of FEN1. Furthermore, we observed a similar increase (p<0.05) following treatment with the flap endonuclease inhibitor RF00974 (Figure 6C). We next measured the level of H2AX phosphorylation (γ-H2AX), an independent indicator of DNA damage [36], in HCT116 CDC4+/+ and CDC4−/− cells in response to RF00974. We found that, similar to increasing 53BP1 focus formation, RF00974 treatment increased H2AX phosphorylation (Figure S3). H2AX phosphorylation was increased even in untreated HCT116 CDC4−/− cells, so no increase in phosphorylation was observed. In order to determine whether RF00974 leads to an increase in apoptosis in CDC4-deficient cells, we asked whether PARP cleavage, a marker of apoptosis [37], is increased following RF00974 treatment. We found that RF00974 treatment did not increase PARP cleavage in either wild type or CDC4-deficient cells. Taken together, these results suggest that loss of FEN1, or inhibition of flap endonuclease activity, lead to an increase in endogenous DNA damage that inhibits the proliferation of CDC4-deficient cells by non-apoptotic means.
Discussion
In this study, we used a cross-species candidate approach to identify new anticancer therapeutic targets for small-molecule inhibition having a potentially broad spectrum of applicability. We found that a yeast CIN synthetic lethal interaction network is largely conserved between S. cerevisiae and a human tumor cell line. Based on this network, we screened for in vitro inhibitors of the highly connected enzyme FEN1. Flap endonuclease inhibitors discovered in this screen recapitulated synthetic lethal interactions between FEN1 and each of CDC4 and MRE11A, demonstrating that evolutionarily conserved genetic interactions in a core cellular process, such as the maintenance of genomic stability, can be exploited as a means to inhibit the proliferation of tumor cells carrying specific and cancer-relevant mutations.
The idea of using the unique genetic profile of tumor cells relative to somatic cells to selectively kill cancer has been applied by various groups, such as in the case of the chemical-genetic interaction between BRCA1/2 and PARP inhibitors [2], [3]. Several studies have focused on DNA damage, usually by identifying inhibitors of DNA damage response proteins that either directly kill tumor cells, or that potentiate the effects of DNA damaging agents [38]–[42]. Recently, two large-scale studies examining chemical-genetic interactions between new or established anti-cancer treatments and cancer cell lines of known genotype demonstrated the promise of such top-down approaches by identifying previously unknown sensitivities of many cancer genotypes, such as between Ewing's sarcomas and PARP inhibitors [5], [6]. An alternative means to construct genetic interaction networks for the discovery of therapeutic targets is to take a cross-species candidate approach in a genetically tractable model organism. In S. cerevisiae, defined genetic changes can be introduced and subsequently screened in a high-throughput manner [16], [43] (though mammalian genome editing technologies are advancing rapidly [44], [45]). The nearly 75% (16/22) conservation of synthetic lethal interactions we found between yeast and human cells is similar to the degree of conservation of genetic interactions between S. cerevisiae and the model metazoan Caenorhabditis elegans in a related network, identified by our group and others [18], [19], [46], and expands upon previous proof-of-principle work by our group [47]. Although we ultimately targeted the highly conserved flap endonuclease FEN1 in the current study, yeast genetic data has the potential to implicate biological processes, as opposed to specific proteins, as therapeutic targets; in this way, targets can be identified that are not conserved in S. cerevisiae. For example, we recently demonstrated that mutation of cohesin genes in yeast was synthetic lethal with mutation of proteins playing a role in replication fork stability. siRNA-mediated knockdown of cohesin genes was found to sensitize human cells to inhibition of PARP, a protein involved in replication fork progression, but without a known ortholog in yeast [19]. Thus, the versatility of yeast synthetic lethal networks to predict therapeutic targets makes our approach complementary to large-scale screening for gene-drug interactions [4]–[6].
Therapeutics that target a specific genotype, such as EGFR family inhibitors in the case of ERBB2 (also known as HER2) amplification, produce more significant gene-drug interactions than more general cytotoxic agents [6]; however, the indications for such agents are limited to a handful of genotypes. FEN1 plays a critical role in nearly all DNA transactions, including DNA replication via Okazaki fragment maturation [48], [49], long-patch base excision repair [50], [51], the prevention of trinucleotide repeat expansions [25], [52], and restart of stalled replication forks [53]. Yeast RAD27 is one of the most highly genetically connected genes in the yeast genome (Tables S5 and S6); many of these interactors are CIN genes [11], and many of the corresponding human orthologs may prove to be mutated and cause CIN in tumours. Given that the majority of the genetic interactions were conserved in the CIN synthetic lethal interaction network interrogated here, FEN1 may be a widely applicable target in cancers harboring mutations in a variety of CIN genes. More generally, DNA repair and replication protein inhibitors are being actively developed as anticancer therapeutics [2], [3], [41], [54] and the process of DNA replication forms a genetic hub in S. cerevisiae [16], [23], [43], [55]. The critical role of FEN1 in DNA transactions is analogous to that of PARP, a protein playing a role in DNA repair and the protection of stalled DNA replication forks [56], [57]. PARP is synthetic lethal with mutations in BRCA1/2 [2], [3], and its therapeutic range has been extended more recently to include cells with mutations in PTEN [38] and cohesins [19]. Thus, like PARP, FEN1 potentially represents a potent, broadly-applicable target for anticancer therapeutic development.
In turn, the ideal anticancer therapeutic would have a broad spectrum, suggesting it would be more advantageous to target a phenotype common in cancer. CIN in the form of aneuploidy is seen in >90% of solid tumors [10] and represents a sub-lethal mutation in an otherwise essential process. Of relevance to the current work, moderate aneuploidy and CIN correlate with poor prognosis in cancer, but extreme aneuploidy correlates with improved patient outcomes [58], [59]. Yeast RAD27 is a CIN gene [17], and FEN1 mutation in various systems leads to CIN and has been associated with cancer [17], [60]; thus, inhibition of FEN1 in cancers that already exhibit CIN could lead to a level of CIN incompatible with viability. In the present study, flap endonuclease inhibitors were found to recapitulate the synthetic lethal interactions between FEN1 and each of CDC4 and MRE11A [18], [23]. We observed that both depletion and inhibition of flap endonuclease activity led to an increase in endogenous DNA damage. Recent reports have shown that γ-H2AX levels are not increased following FEN1 depletion [61]; however, we observed increases in DNA damage using two independent assays following two means of FEN1 inhibition, and attribute these results to cell background differences, such as the mismatch repair deficiency present in HCT116 cells. Furthermore, this increase in DNA damage led to a non-apoptotic inhibition of proliferation. Thus, one explanation for the lethality in combination with inactivation of CDC4 is that the cell is inappropriately driven through the cell cycle, owing to elevated levels of cyclin E [13], when otherwise it would arrest to try to repair DNA damage. Likewise, increased endogenous DNA damage combined with loss of MRE11A, a protein playing a critical role in the first steps of the DNA damage response [62], could lead to a level of DNA damage or mutation that is incompatible with proliferation. CDC4 has been reported to be mutated in a wide variety of tumor types, at frequencies ranging from 6% to >30%, depending on the tumor type [13], [21], [29], [63], [64]. Recently, it has been suggested that reduction of CDC4 activity to some level below that of wild type, but above complete abrogation of function, is optimal for tumor progression [63]. Thus, the fact that two flap endonuclease inhibitors described here were able to selectively inhibit the proliferation of both heterozygous and homozygous CDC4-knockout cell lines suggests that CDC4 loss, whether complete or partial, sensitizes cells to inhibition of flap endonuclease activity. As well, the fact that both genotypes were sensitive to inhibition of flap endonuclease activity adds weight to the suggestion that this response is specific to CDC4 activity, in the same way that changing response following alteration in dosage in biochemical screening is suggestive of target identity [65].
In summary, here we have presented a rational, cross-species approach to the identification of anticancer therapeutic targets by targeting CIN, a common cancer phenotype. The use of conserved synthetic lethal interaction networks to identify highly-connected second-site targets is an accessible alternative to large scale screens: it narrows down the number of synthetic lethal gene pairs to be directly retested from tens of thousands to dozens, and is based on strong synthetic lethal interactions discovered in yeast networks. We have demonstrated the potential of this approach to identify targets and therapeutics, such as FEN1 and the flap endonuclease inhibitors described here, having potentially broad applicability in the treatment of cancer.
Materials and Methods
Cell culture
HCT116 cells were purchased from ATCC. HCT116 derivatives, DLD-1 and DLD-1 derivatives were gifts of Dr. Bert Vogelstein (Johns Hopkins University). (Importantly, we observed that the deleted exon in CDC4 in these cell lines is not exon 5, as previously reported [13], but exon 8. We attribute the difference to changing annotations in public sequence databases between 2004 and the present.) 53BP1-mCherry HCT116 cells were a gift of Dr. Sam Aparicio (UBC). These cells were grown in McCoy's 5A medium with 10% FBS. Immortalized (telomerase) BJ normal human skin fibroblasts, hTERT [66], were generously provided by Dr. C.P. Case (University of Bristol) and were grown in DMEM containing 10% FBS. Mirin was purchased from Sigma-Aldrich. RF00974 was purchased from Maybridge, Ltd.
Western blotting
Western blots were performed as detailed elsewhere [47]. Antibodies used for Western blots are described in Table S4.
RNA interference
Subconfluent and asynchronous cells were transiently transfected with siRNAs. HCT116 cells were transfected with ON-TARGETplus siRNA pools at a total siRNA concentration of 25 nM using DharmaFECT I (Dharmacon). In dual siRNA experiments, the total siRNA concentration was 50 nM. Cultures were replenished with fresh medium 11 hours after transfection. hTERT cells were transfected with ON-TARGETplus siRNA pools, or independent duplexes, at a total siRNA concentration of 100 nM using RNAiMax (Invitrogen). Cultures were replenished with fresh medium 24 hours after transfection.
Synthetic lethal assays, cell imaging, and compound incubation
HCT116 cells were harvested 24 hours after siRNA transfection and re-plated in 96-well optical bottom plates. hTERT cells were transfected directly in 96-well plates. HCT116 cells were fixed four days after transfection, and hTERT cells were fixed seven days after transfection, in 4% paraformaldehyde/PBS. Nuclei were labelled with Hoechst 33342. Stained nuclei were counted using a Cellomics Arrayscan VTI fluorescence imager as described previously [47] or a Zeiss AxioObserver Z1 equipped with an LED Colibri light source, a 20× plan apochromat dry lens (numerical aperture = 0.8) and AxioVision v4.8 software. Images were analyzed using the Physiology Analyzer (Assaybuilder) option within the AxioVision software. Data were normalized to GAPDH-silenced controls and conventional statistics (e.g. column statistics and Student's t-tests) were performed. Experiments were performed twice; indicated numbers are averaged from at least 6 wells.
To determine the presence of a synthetic lethal interaction, the proliferative defect was calculated, and is defined as
where the predicted proliferation was the product of the proliferation of the two individual gene knockdowns, following a multiplicative model of genetic interactions [67]. Synthetic lethal interactions were scored as a proliferative defect of three times the average SEM of the experiment or greater.During compound incubation experiments, cells were incubated in compound of interest in 96-well optical bottom plates for approximately three days prior to fixation and analysis. Data (from six independent wells) were analyzed using a one-way ANOVA followed by a Tukey test.
FEN1 purification
FEN1 was expressed in BL21 E. coli from pET28b(+) (a generous gift from R. Bambara, University of Rochester) using 1 mM IPTG. Bacteria were lysed in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0 containing 2× protease inhibitor) via a French press at 10 000 psi. The lysate was clarified and passed through a 0.22 µM filter before being loaded onto a HisTrap FF column (1 mL, GE Healthcare) in an ÄKTAFPLC P-920 system (GE Healthcare). The column was washed in 10 volumes of wash buffer (lysis buffer+20 mM imidazole), and FEN1 was eluted with 5 volumes of elution buffer (lysis buffer+125 mM imidazole). The lysate was diluted with 9 volumes HI buffer (30 mM HEPES-KOH, 0.5% myo-inositol, pH 7.8) with 30 mM NaH2PO4 and concentrated in a protein concentrator (Amicon). It was then loaded onto a hydroxyapatite resin (HA Ultrogel, Pall Life Sciences). The hydroxyapatite resin was washed with 10 volumes of HI-30 mM PO4, and FEN1 was eluted with 5 volumes of HI-200 mM PO4. The eluate was diluted with 5 volumes HI-30 mM KCl prior to concentration, and then loaded onto a strong cation exchange column (1 mL HiTRAP SP FF FPLC, GE Healthcare Life Sciences). The column was washed with 10 volumes of HI-30 mM KCl, then 10 volumes of HI-200 mM KCl, and FEN1 was eluted with a gradient from HI-200 mM KCl to HI-500 mM KCl over 10 column volumes. Purified FEN1 was concentrated in FEN1 dilution buffer (30 mM HEPES-KOH, 5% glycerol, 0.1 mg/mL BSA, 0.01% NP-40), and aliquots of known concentration were frozen at −80°C.
In vitro FEN1 inhibition assay
Oligonucleotides used were as follows: “template”, 5′-GGTGGACGGGTGGATTGAAATTTAGGCTGGCACGGTCG-3′, “upstream”, 5′-CGACCGTGCCAGCCTAAATTTCAATC-3′, “downstream”, 5′-6-FAM-CCAAGGCCACCCGTCCAC-BHQ-1-3′. (6-FAM is 6-carboxyfluorescein; BHQ-1 is black hole quencher 1.) The three oligonucleotides were annealed at equimolar amounts in annealing buffer (50 mM Tris, 50 mM NaCl, 1 mM DTT, pH 8.0) by heating to 94°C, cooling to 70°C, and gradually cooling to room temperature. FEN1 assays were carried out with 6 pmol FEN1 and 20 nM annealed substrate in FEN1 buffer (50 mM Tris pH 8.0, 30 mM NaCl, 8 mM MgCl2, 0.1 mg/mL BSA, 2 mM DTT). Assays were carried out at room temperature and kinetic reads were taken over approximately ten minutes in a Varioskan plate reader (Thermo Fisher Scientific), using excitation and emission wavelengths of 492 nm and 517 nm, respectively.
Fluorescent imaging
53BP1-mCherry cells were grown on cover slips. Following desired treatment (either two hours of bleomycin treatment at 5 µg/mL, four days following siRNA transfection, or after 24 hours of RF00974 treatment at 10 µM), cells were fixed for five minutes in 4% paraformaldehyde/PBS, mounted in Vectashield mounting medium containing DAPI (500 ng/mL), and imaged on a Zeiss Axioplan microscope with a Coolsnap HQ camera, using appropriate filters and controlled by Metamorph software.
Apoptosis analysis
Cells were treated with RF00974 for 48 hours prior to harvesting of medium and cells in lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton-X-100, pH 7.5). Lysates were sonicated and clarified by centrifugation at 13 000 rpm for 15 minutes at 4°C. As a positive control, HCT116 cells were treated with 1 µM staurosporine prior to harvesting. Lysates were subjected to Western blotting as described above.
Synthetic genetic array
Synthetic genetic array analysis of rad27Δ against a collection of yeast essential DAmP alleles [68] and temperature sensitive alleles [69] was carried out as described previously [11], [19].
Supporting Information
Zdroje
1. HartwellLH, SzankasiP, RobertsCJ, MurrayAW, FriendSH (1997) Integrating genetic approaches into the discovery of anticancer drugs. Science 278 : 1064–1068 10.1126/science.278.5340.1064.
2. FarmerH, McCabeN, LordCJ, TuttAN, JohnsonDA, et al. (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434 : 917–921.
3. BryantHE, SchultzN, ThomasHD, ParkerKM, FlowerD, et al. (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 : 913–917.
4. WeinsteinJN, MyersTG, O'ConnorPM, FriendSH, FornaceAJJr, et al. (1997) An information-intensive approach to the molecular pharmacology of cancer. Science 275 : 343–349.
5. BarretinaJ, CaponigroG, StranskyN, VenkatesanK, MargolinAA, et al. (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483 : 603–607.
6. GarnettMJ, EdelmanEJ, HeidornSJ, GreenmanCD, DasturA, et al. (2012) Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483 : 570–575.
7. GrebienF, HantschelO, WojcikJ, KaupeI, KovacicB, et al. (2011) Targeting the SH2-kinase interface in Bcr-Abl inhibits leukemogenesis. Cell 147 : 306–319.
8. ZhaoC, ChenA, JamiesonCH, FereshtehM, AbrahamssonA, et al. (2009) Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458 : 776–779.
9. HanahanD, WeinbergRA (2011) Hallmarks of cancer: the next generation. Cell 144 : 646–674.
10. WeaverBA, ClevelandDW (2006) Does aneuploidy cause cancer? Curr Opin Cell Biol 18 : 658–667.
11. StirlingPC, BloomMS, Solanki-PatilT, SmithS, SipahimalaniP, et al. (2011) The complete spectrum of yeast chromosome instability genes identifies candidate CIN cancer genes and functional roles for ASTRA complex components. PLoS Genet 7: e1002057 doi:10.1371/journal.pgen.1002057.
12. CahillDP, LengauerC, YuJ, RigginsGJ, WillsonJKV, et al. (1998) Mutations of mitotic checkpoint genes in human cancers. Nature 392 : 300–303 10.1038/32688.
13. RajagopalanH, JallepalliPV, RagoC, VelculescuVE, KinzlerKW, et al. (2004) Inactivation of hCDC4 can cause chromosomal instability. Nature 428 : 77–81 10.1038/nature02313.
14. BarberTD, McManusK, YuenKW, ReisM, ParmigianiG, et al. (2008) Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A 105 : 3443–3448.
15. WangZ, CumminsJM, ShenD, CahillDP, JallepalliPV, et al. (2004) Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res 64 : 2998–3001.
16. TongAH, EvangelistaM, ParsonsAB, XuH, BaderGD, et al. (2001) Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294 : 2364–2368.
17. YuenKW, WarrenCD, ChenO, KwokT, HieterP, et al. (2007) Systematic genome instability screens in yeast and their potential relevance to cancer. Proc Natl Acad Sci U S A 104 : 3925–3930.
18. McLellanJ, O'NeilN, TarailoS, StoepelJ, BryanJ, et al. (2009) Synthetic lethal genetic interactions that decrease somatic cell proliferation in Caenorhabditis elegans identify the alternative RFC CTF18 as a candidate cancer drug target. Mol Biol Cell 20 : 5306–5313.
19. McLellanJL, O'NeilNJ, BarrettI, FerreeE, van PelDM, et al. (2012) Synthetic lethality of cohesins with PARPs and replication fork mediators. PLoS Genet 8: e1002574 doi:10.1371/journal.pgen.1002574.
20. HiramotoT, NakanishiT, SumiyoshiT, FukudaT, MatsuuraS, et al. (1999) Mutations of a novel human RAD54 homologue, RAD54B, in primary cancer. Oncogene 18 : 3422–3426.
21. KempZ, RowanA, ChambersW, WorthamN, HalfordS, et al. (2005) CDC4 mutations occur in a subset of colorectal cancers but are not predicted to cause loss of function and are not associated with chromosomal instability. Cancer Res 65 : 11361–11366.
22. CahillDP, KinzlerKW, VogelsteinB, LengauerC (1999) Genetic instability and darwinian selection in tumours. Trends Cell Biol 9: M57–M60 10.1016/S0962-8924(99)01661-X.
23. CostanzoM, BaryshnikovaA, BellayJ, KimY, SpearED, et al. (2010) The genetic landscape of a cell. Science 327 : 425–431.
24. PrietoI, SujaJA, PezziN, KremerL, MartinezA, et al. (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3 : 761–766.
25. LiuY, BambaraRA (2003) Analysis of human flap endonuclease 1 mutants reveals a mechanism to prevent triplet repeat expansion. J Biol Chem 278 : 13728–13739.
26. ZhengL, JiaJ, FingerLD, GuoZ, ZerC, et al. (2011) Functional regulation of FEN1 nuclease and its link to cancer. Nucleic Acids Res 39 : 781–794.
27. TumeyLN, BomD, HuckB, GleasonE, WangJ, et al. (2005) The identification and optimization of a N-hydroxy urea series of flap endonuclease 1 inhibitors. Bioorg Med Chem Lett 15 : 277–281.
28. LipinskiCA, LombardoF, DominyBW, FeeneyPJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46 : 3–26.
29. AkhoondiS, SunD, von derLN, ApostolidouS, KlotzK, et al. (2007) FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res 67 : 9006–9012.
30. KohJL, DingH, CostanzoM, BaryshnikovaA, ToufighiK, et al. (2010) DRYGIN: a database of quantitative genetic interaction networks in yeast. Nucleic Acids Res 38: D502–D507.
31. MiyakiM, YamaguchiT, IijimaT, TakahashiK, MatsumotoH, et al. (2009) Somatic mutations of the CDC4 (FBXW7) gene in hereditary colorectal tumors. Oncology 76 : 430–434.
32. MilneAN, LeguitR, CorverWE, MorsinkFH, PolakM, et al. (2010) Loss of CDC4/FBXW7 in gastric carcinoma. Cell Oncol 32 : 347–359.
33. DupreA, Boyer-ChatenetL, SattlerRM, ModiAP, LeeJH, et al. (2008) A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol 4 : 119–125.
34. AndersonL, HendersonC, AdachiY (2001) Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol Cell Biol 21 : 1719–1729.
35. RappoldI, IwabuchiK, DateT, ChenJ (2001) Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J Cell Biol 153 : 613–620.
36. MarkovaE, SchultzN, BelyaevIY (2007) Kinetics and dose-response of residual 53BP1/gamma-H2AX foci: co-localization, relationship with DSB repair and clonogenic survival. Int J Radiat Biol 83 : 319–329.
37. LazebnikYA, KaufmannSH, DesnoyersS, PoirierGG, EarnshawWC (1994) Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371 : 346–347.
38. Mendes-PereiraAM, MartinSA, BroughR, McCarthyA, TaylorJR, et al. (2009) Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med 1 : 315–322.
39. ReaperPM, GriffithsMR, LongJM, CharrierJD, MaccormickS, et al. (2011) Selective killing of ATM - or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol
40. LengauerC, KinzlerKW, VogelsteinB (1997) Genetic instability in colorectal cancers. Nature 386 : 623–627 10.1038/386623a0.
41. FengZ, ScottSP, BussenW, SharmaGG, GuoG, et al. (2011) Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc Natl Acad Sci U S A 108 : 686–691.
42. MartinSA, McCabeN, MullarkeyM, CumminsR, BurgessDJ, et al. (2010) DNA polymerases as potential therapeutic targets for cancers deficient in the DNA mismatch repair proteins MSH2 or MLH1. Cancer Cell 17 : 235–248.
43. PanX, YuanDS, XiangD, WangX, Sookhai-MahadeoS, et al. (2004) A robust toolkit for functional profiling of the yeast genome. Mol Cell 16 : 487–496 10.1016/j.molcel.2004.09.035.
44. LePF, LillicoS, PassetB, YoungR, WhitelawB, et al. (2010) Zinc finger nuclease technology heralds a new era in mammalian transgenesis. Trends Biotechnol 28 : 134–141.
45. CermakT, DoyleEL, ChristianM, WangL, ZhangY, et al. (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39: e82.
46. TarailoM, TarailoS, RoseAM (2007) Synthetic lethal interactions identify phenotypic “interologs” of the spindle assembly checkpoint components. Genetics 177 : 2525–2530.
47. McManusKJ, BarrettIJ, NouhiY, HieterP (2009) Specific synthetic lethal killing of RAD54B-deficient human colorectal cancer cells by FEN1 silencing. Proc Natl Acad Sci U S A 106 : 3276–3281.
48. MuranteRS, RumbaughJA, BarnesCJ, NortonJR, BambaraRA (1996) Calf RTH-1 nuclease can remove the initiator RNAs of Okazaki fragments by endonuclease activity. J Biol Chem 271 : 25888–25897.
49. HarringtonJJ, LieberMR (1994) The characterization of a mammalian DNA structure-specific endonuclease. EMBO J 13 : 1235–1246.
50. KimK, BiadeS, MatsumotoY (1998) Involvement of flap endonuclease 1 in base excision DNA repair. J Biol Chem 273 : 8842–8848.
51. KlunglandA, LindahlT (1997) Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1). EMBO J 16 : 3341–3348.
52. SinghP, ZhengL, ChavezV, QiuJ, ShenB (2007) Concerted action of exonuclease and Gap-dependent endonuclease activities of FEN-1 contributes to the resolution of triplet repeat sequences (CTG)n - and (GAA)n-derived secondary structures formed during maturation of Okazaki fragments. J Biol Chem 282 : 3465–3477.
53. ZhengL, ZhouM, ChaiQ, ParrishJ, XueD, et al. (2005) Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep 6 : 83–89.
54. JaiswalAS, BanerjeeS, AnejaR, SarkarFH, OstrovDA, et al. (2011) DNA Polymerase beta as a Novel Target for Chemotherapeutic Intervention of Colorectal Cancer. PLoS ONE 6: e16691 doi:10.1371/journal.pone.0016691.
55. TongAH, LesageG, BaderGD, DingH, XuH, et al. (2004) Global mapping of the yeast genetic interaction network. Science 303 : 808–813 10.1126/science.1091317.
56. StromCE, JohanssonF, UhlenM, SzigyartoCA, ErixonK, et al. (2011) Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res 39 : 3166–3175.
57. YingS, HamdyFC, HelledayT (2012) Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res 72 : 2814–2821.
58. BirkbakNJ, EklundAC, LiQ, McClellandSE, EndesfelderD, et al. (2011) Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res 71 : 3447–3452.
59. LeeAJ, EndesfelderD, RowanAJ, WaltherA, BirkbakNJ, et al. (2011) Chromosomal instability confers intrinsic multidrug resistance. Cancer Res 71 : 1858–1870.
60. ZhengL, DaiH, HegdeML, ZhouM, GuoZ, et al. (2011) Fen1 mutations that specifically disrupt its interaction with PCNA cause aneuploidy-associated cancer. Cell Res
61. DuxinJP, MooreHR, SidorovaJ, KaranjaK, HonakerY, et al. (2012) An Okazaki fragment processing-independent role for human Dna2 during DNA replication. J Biol Chem
62. StrackerTH, PetriniJH (2011) The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 12 : 90–103.
63. DavisH, TomlinsonI (2012) CDC4/FBXW7 and the ‘just enough’ model of tumourigenesis. J Pathol 227 : 131–135.
64. LeGM, O'HaraAJ, RuddML, UrickME, HansenNF, et al. (2012) Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 10.
65. HoonS, SmithAM, WallaceIM, SureshS, MirandaM, et al. (2008) An integrated platform of genomic assays reveals small-molecule bioactivities. Nat Chem Biol 4 : 498–506.
66. CoganN, BairdDM, PhillipsR, CromptonLA, CaldwellMA, et al. (2010) DNA damaging bystander signalling from stem cells, cancer cells and fibroblasts after Cr(VI) exposure and its dependence on telomerase. Mutat Res 683 : 1–8.
67. BaryshnikovaA, CostanzoM, DixonS, VizeacoumarFJ, MyersCL, et al. (2010) Synthetic genetic array (SGA) analysis in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Methods Enzymol 470 : 145–79 Epub;%2010 Mar 1.: 145–179.
68. BreslowDK, CameronDM, CollinsSR, SchuldinerM, Stewart-OrnsteinJ, et al. (2008) A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods 5 : 711–718.
69. LiZ, VizeacoumarFJ, BahrS, LiJ, WarringerJ, et al. (2011) Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat Biotechnol 29 : 361–367.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




