-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Fis Protein Insulates the Gene from Uncontrolled Transcription
The Escherichia coli curved DNA binding protein A (CbpA) is a poorly characterised nucleoid associated factor and co-chaperone. It is expressed at high levels as cells enter stationary phase. Using genetics, biochemistry, and genomics, we have examined regulation of, and DNA binding by, CbpA. We show that Fis, the dominant growth-phase nucleoid protein, prevents CbpA expression in growing cells. Regulation by Fis involves an unusual “insulation” mechanism. Thus, Fis protects cbpA from the effects of a distal promoter, located in an adjacent gene. In stationary phase, when Fis levels are low, CbpA binds the E. coli chromosome with a preference for the intrinsically curved Ter macrodomain. Disruption of the cbpA gene prompts dramatic changes in DNA topology. Thus, our work identifies a novel role for Fis and incorporates CbpA into the growing network of factors that mediate bacterial chromosome structure.
Published in the journal: Fis Protein Insulates the Gene from Uncontrolled Transcription. PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003152
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003152Summary
The Escherichia coli curved DNA binding protein A (CbpA) is a poorly characterised nucleoid associated factor and co-chaperone. It is expressed at high levels as cells enter stationary phase. Using genetics, biochemistry, and genomics, we have examined regulation of, and DNA binding by, CbpA. We show that Fis, the dominant growth-phase nucleoid protein, prevents CbpA expression in growing cells. Regulation by Fis involves an unusual “insulation” mechanism. Thus, Fis protects cbpA from the effects of a distal promoter, located in an adjacent gene. In stationary phase, when Fis levels are low, CbpA binds the E. coli chromosome with a preference for the intrinsically curved Ter macrodomain. Disruption of the cbpA gene prompts dramatic changes in DNA topology. Thus, our work identifies a novel role for Fis and incorporates CbpA into the growing network of factors that mediate bacterial chromosome structure.
Introduction
Bacterial chromosomes are organised into a nucleoid by an integrated network of supercoiling, transcription and nucleoid associated DNA binding proteins [1]. This network is highly dynamic and responsive to changes in the extracellular environment. In Escherichia coli, a particularly notable change in nucleoid structure occurs when cells are starved. Namely, the nucleoid adopts a super compact conformation that is believed to protect the genome. These changes in nucleoid structure coincide with changes in supercoiling [2], transcription [3] and the available pool of nucleoid proteins [4]. Strikingly, only two nucleoid proteins are specifically up-regulated as cells approach stationary phase; the DNA binding protein from starved cells (Dps) and Curved DNA binding protein A (CbpA) [4]. The Dps protein has been studied for decades and has well characterised DNA binding, compaction and protection properties [5]–[7]. In growing cells expression of Dps is blocked by Fis, the major growth phase nucleoid protein [8]. In sharp contrast to Dps, the regulation and DNA binding properties of CbpA have hardly been studied.
The CbpA protein was first isolated as a factor present in crude E. coli cell extracts that preferentially bound curved DNA in vitro [9]. The affinity of CbpA for DNA is similar to that observed for other nucleoid associated proteins [10]. CbpA consists of an N-terminal J-domain separated from two C-terminal domains (CTDI and II) by a flexible linker [11]. The J-domain interacts with the modulator protein CbpM, which inhibits CbpA co-chaperone activity and DNA recognition [12]–[14]. DNA binding activity locates to the linker-CTDI region and CTDII mediates dimerisation [11], [15]. Transcription of cbpA initiates from overlapping promoters referred to as P1 and P2 [16]. Most cbpA transcription is driven by the σ38 dependent P2 promoter with σ70 dependent P1 making only a small contribution [16]. Consistent with this, CbpA accumulates in starved E. coli, reaching 15,000 copies per cell after two days [4]. In contrast to Dps, which is uniformly distributed within the nucleoid, CbpA forms nucleoid associated foci [17]. Nothing is known about the function of CbpA in starved E. coli cells.
In this work we sought to better understand the regulation and function of CbpA. We show that Fis plays a crucial role in preventing CbpA expression in growing cells. Hence, Fis binds to DNA target sites in the cbpA regulatory region. When bound to these sites Fis prevents cbpA being transcribed from an aberrant promoter, located within the coding sequence of an adjacent gene. In starved cells, when cbpA transcription is induced by σ38, CbpA binds pervasively across the E. coli genome with a bias to the intrinsically curved Ter macrodomain. Disruption of the cbpA gene prompts dramatic changes in DNA supercoiling.
Results
CbpA expression is uncoupled from growth-phase in a fis mutant
As E. coli cells approach stationary phase they induce expression of two nucleoid proteins, Dps and CbpA. Previously, we found that Dps expression in growing E. coli cells is repressed by Fis [8]. Thus, we wondered if Fis may also control production of CbpA. In an initial experiment we cloned a 302 base pair DNA fragment, containing the entire cbpA regulatory region and part of the adjacent yccE gene, upstream of lacZ (illustrated schematically in Figure 1Ai). We then measured LacZ activity in WT JCB387 cells, carrying this fusion, throughout growth. We observed basal levels of LacZ activity until the onset of stationary phase at which point LacZ activity increased (green line in Figure 1Aii). Strikingly, in JCB3871Δfis cells, LacZ activity was high throughout the time course (red line in Figure 1Aii). In complementary western blotting experiments we measured Fis levels in E. coli cells at different stages of growth (Figure 1Aiii). Fis levels were inversely correlated with cbpA induction. Intrigued by this observation we measured Fis binding to the cbpA regulatory DNA using Electrophoretic Mobility Shift Assays (EMSA). For comparison, we also tested DNA fragments that drive expression of other nucleoid proteins and the nirB promoter, which has a high affinity for Fis [18]. The raw EMSA data are shown in Figure S1 and a quantification of the experiment is shown in Figure 1B. The data show that Fis binds particularly tightly to the cbpA regulatory region. Note that Fis forms three distinct complexes with the cbpA regulatory DNA, suggesting three separate Fis binding sites (Figure S1).
Fig. 1. CbpA expression is uncoupled from growth-phase in a fis mutant. 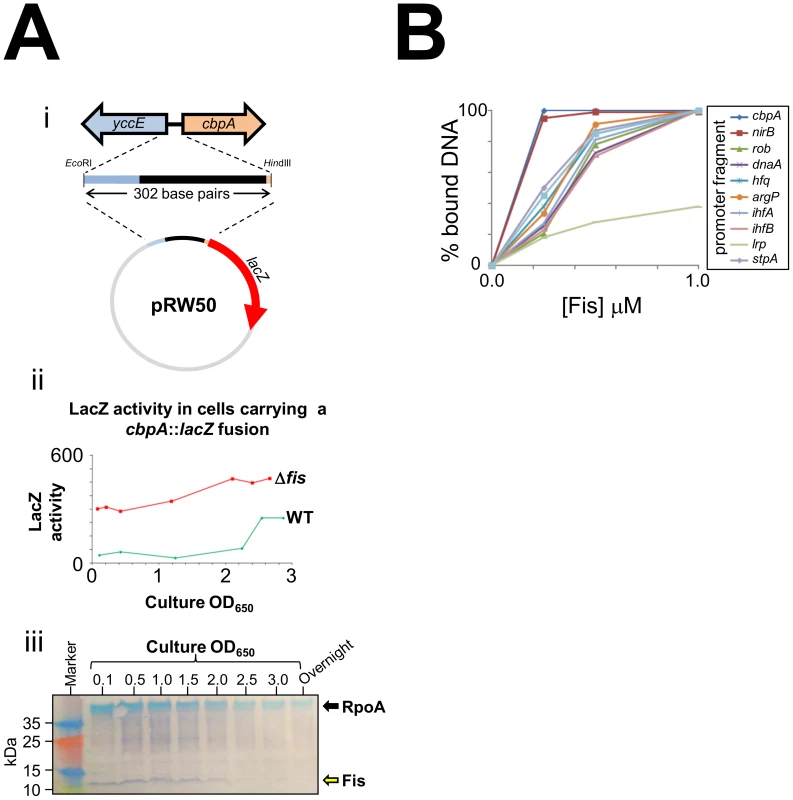
(A) Activity of a cbpA::lacZ fusion in different growth-phases. i) Schematic representation of the cbpA::lacZ fusion. A 302 base pair DNA fragment, encompassing the cbpA start codon, the entire cbpA-yccE intergenic region, and a portion of the yccE gene, was cloned upstream of lacZ in the low copy number lacZ reporter plasmid pRW50. ii) The graph shows LacZ activity calculated for either wild type or Δfis cells carrying the plasmid construct shown in panel i). iii) Western blot showing Fis levels in E. coli K-12 throughout growth and in overnight cultures. The blots were also probed with antibodies against RpoA as a control. (B) Fis has a high affinity for the cbpA regulatory region. The graph illustrates the binding of Fis to different DNA fragments (see key). The raw EMSA data are shown in Figure S1. Location of Fis binding sites at the cbpA regulatory region
Fis binds to a 15-base-pair AT-rich DNA target that is highly degenerate. A common feature of many Fis sites is a G at position 1 and a C at position 15; however even these features are not universally conserved [19]–[21]. Thus, DNAse I footprinting was used to locate Fis bound at the cbpA regulatory region. The full sequence of the 302 base pair DNA fragment is shown in Figure S2. The previously characterised cbpA P1 and P2 promoters are highlighted. Throughout this work, all numbering is with respect to the P1 promoter. The results show that Fis binds a DNA element between 90 and 145 base pairs upstream of the cbpA P1 transcript start (Figure 2A). Since Fis binds to a 15 base pair recognition sequence the large 55 base pair footprint most likely contains three Fis binding sites, as observed in our EMSA analysis (Figure S1). Scrutiny of the DNA sequence corresponding to the region bound by Fis identified one match to the canonical Fis binding sequence (i.e. containing a G at position 1 and a C at position 15). This site is centred 101 base pairs upstream of the P1 transcription start (Figure S2) and can be disrupted by altering the key positions in the Fis recognition sequence. Thus, the cbpA-108C-94G DNA fragment has a greatly reduced affinity for Fis in EMSA assays (Figure S3). We presume that adjacent DNA sites for Fis in this region must have an atypical sequence. This is not exceptional and similar observations have been made for Fis binding sites at the rrn promoters [20].
Fig. 2. Location of Fis, RNA polymerase, and transcription start sites at the cbpA regulatory region. 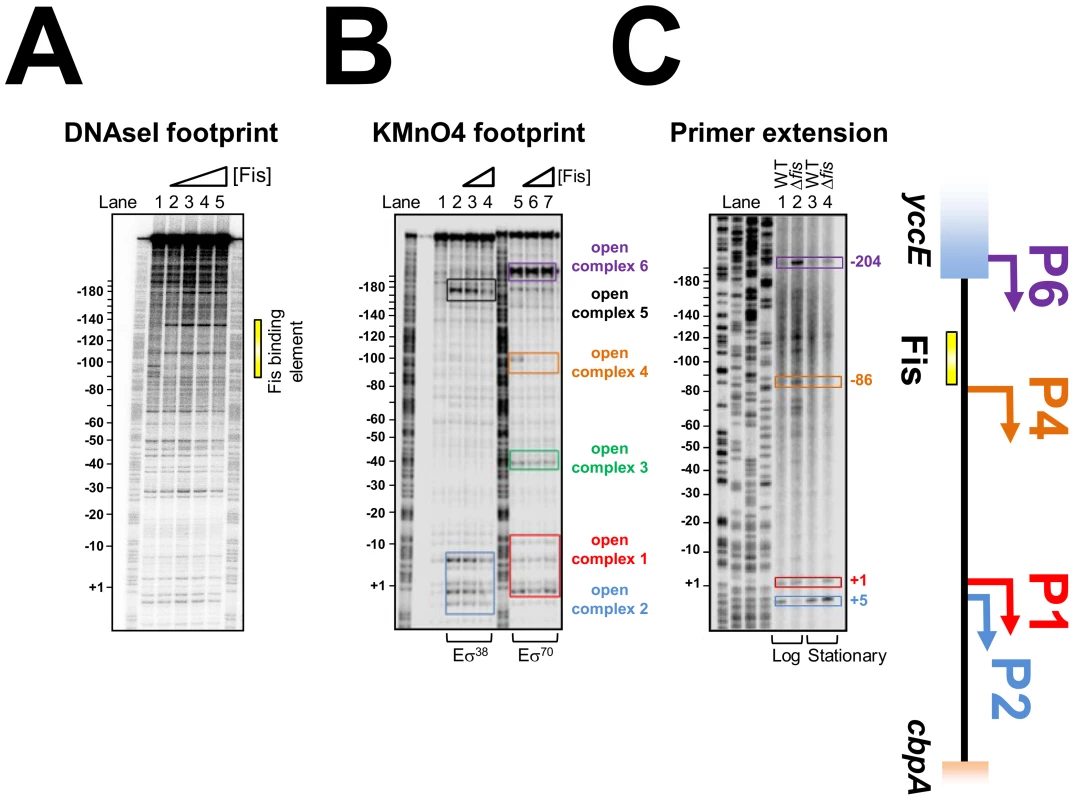
(A) Fis binds to a 55 base pair DNA region upstream of cbpA. DNAseI footprinting analysis of Fis binding to the cbpA regulatory region. The image shows a scan of a dried polyacrylamide sequencing gel on which DNase I digestion of the 302 base pair cbpA fragment (∼20 nM) was compared in the presence and absence of 250 nM, 500 nM or 1 µM Fis. The 55 base pair element bound by Fis is highlighted by a yellow bar. (B) Location of RNA polymerase binding sites in vitro. Results of a KMnO4 footprinting experiment to detect open complex formation by either σ38 or σ70 associated RNA polymerase (400 nM) in the presence and absence of Fis (250 or 500 nM). Open complexes are highlighted by coloured boxes and are numbered. (C) Location of cbpA transcription start sites in vivo. The gel shows a cbpA mRNA primer extension analysis. The location of transcription start sites are given with respect to the cbpA P1 promoter (+1). Bands corresponding to transcripts that initiate from the P1 and P2 promoters are highlighted in red and blue respectively. Two further transcript start sites, observed only in growing JCB3871Δfis cells, are highlighted in orange and purple. A schematic of the cbpA regulatory region is shown to the right of the figure. Location of RNA polymerase binding sites at the cbpA regulatory region
As a first step to understanding control of cbpA by Fis we utilised KMnO4 footprinting that detects DNA melting around transcription start sites. Thus we were able to measure RNA polymerase binding in vitro, to the cbpA regulatory region, in the presence and absence of Fis. Recall that cbpA transcription can be stimulated by σ70 or σ38 associated RNA polymerase. The results in the absence of Fis, illustrated in Figure 2B, show different patterns of σ38 (lane 2) and σ70 (lane 5) dependent DNA opening. As expected, our analysis identified DNA melting at the known P1 and P2 promoters (highlighted by red and blue boxes in Figure 2B). Surprisingly, we also observed σ70 dependent DNA opening at three further locations (highlighted by green, orange and purple boxes in Figure 2B) and one additional σ38 dependent DNA opening event (highlighted by a black box in Figure 2B). Given the large effect of Fis on cbpA expression in vivo (Figure 1Aii), we were surprised that addition of Fis to the KMnO4 footprinting reaction had minor effects (lanes 3–4 and 6–7). Thus, Fis only inhibited DNA untwisting at position −100 (open complex 4) which overlaps the Fis binding element.
Identification of cbpA transcription start sites in vivo
To aid interpretation of the in vitro KMnO4 footprinting analysis we conducted in vivo mRNA primer extension experiments. This enabled us to identify cbpA transcript start sites, used in the presence and absence of Fis, in growing and stationary phase cells. The results of this analysis are shown in Figure 2C. In WT cells we observed only two cbpA mRNA primer extension products. As expected these correspond to the P1 and P2 promoters (see lanes 1 and 3 in Figure 2C). The Δfis mutation had little effect on transcription start site selection in stationary phase cells (see lane 4 in Figure 2C). However, the Δfis mutation had a dramatic effect in growing cells (see lane 2 in Figure 2C). Thus, in growing Δfis cells, the most abundant cbpA transcript did not originate from either the P1 or P2 promoter. Rather, it initiated form a site located 204 base pairs upstream of the P1 promoter in the yccE gene (highlighted in purple in Figure 2C). This transcription start site, labelled P6 in the schematic, aligns precisely with open complex 6 in the KMnO4 footprinting experiments (compare Figure 2B and 2C). An additional transcription start site, which we refer to as P4, aligns with open complex 4 (see orange highlighting in Figure 2B and 2C). Note that additional primer extension products in lane 2 of Figure 2C, which are also close to open complex 4, are likely due to degradation of longer transcripts. To confirm that we correctly identified the P4 promoter, we created a P4::lacZ fusion and investigated the effect of mutating the proposed −10 element (Figure S4A). The data show that the P4 promoter drives LacZ expression and that transcription from the P4 promoter increases when the proposed −10 element is improved (Figure S4B). However, overall P4 makes only a small contribution to cbpA transcription.
Fis represses transcription from the cbpA P4 and P6 promoters in vitro
To investigate further the effect of Fis on each cbpA promoter we used in vitro transcription assays. The 302 base pair cbpA regulatory DNA fragment was cloned upstream of the factor-independent λoop transcription terminator in plasmid pSR. The different mRNA products expected to be produced using this DNA template are illustrated in Figure 3Ai. We also created two derivatives of this construct lacking either the P6 promoter or a combination of the P6, P2 and P1 promoters. The different promoters were disrupted by making mutations in the promoter −10 element. Thus, the −217G–216G mutation negates the P6 promoter, the −11G mutation disrupts the P2 promoter and the −7G–6G mutation abolishes transcription from P1 (Figure 3Ai). Figure 3Aii shows the results of in vitro transcription assays with the different DNA templates. As expected, using the wild type DNA template, a mixture of σ38 and σ70 associated RNA polymerase stimulated production of transcripts corresponding to the P1, P2, P4 and P6 promoters (Figure 3Aii lane 1). The −216G–217G mutation abolished transcription from the P6 promoter (Figure 3Aii lane 2). Similarly, mutations −6G,−7G and −11G, which disrupt the P1 and P2–10 hexamers respectively, abolish transcription from the P1 and P2 promoters (Figure 3Aii lane 3). Note that, the P1 and P2 transcripts differ in length by only 5 nucleotides and cannot be resolved in this experiment. Lanes 4 and 5 of Figure 3Aii show the pattern of transcripts generated in the absence and presence of Fis respectively. As expected, Fis greatly reduced transcription from the P6 and P4 promoters. Conversely, Fis had only a minor effect on the P1 and P2 promoters. Note that the small RNAI transcript, from the pSR replication origin, acts as an internal control.
Fig. 3. Repression of transcription from the P4 and P6 promoters by Fis. 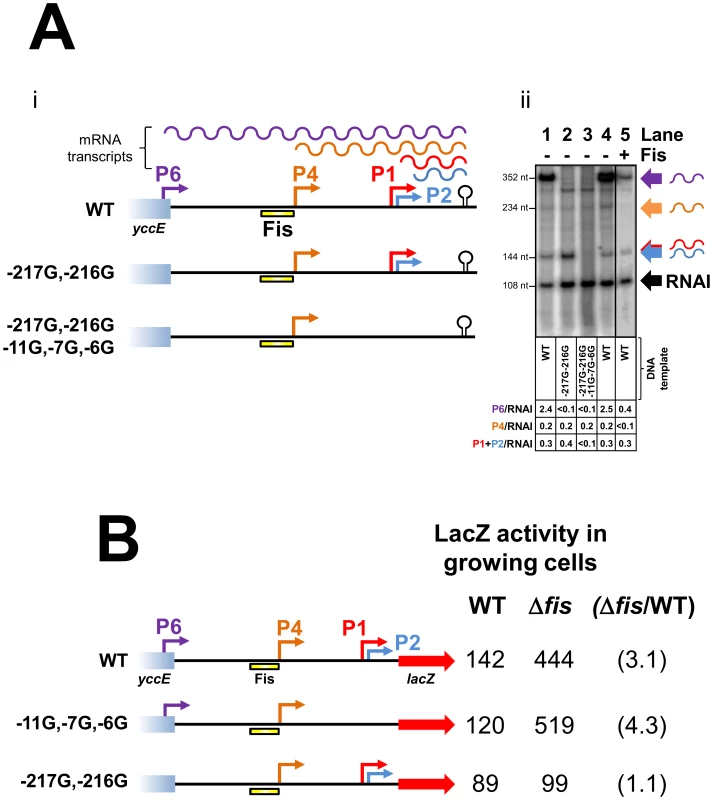
(A) Repression by Fis in vitro. i) Schematic of the cbpA regulatory region DNA used for in vitro transcription assays. Promoters are shown by coloured arrows and the λoop transcription terminator is shown by a black “lollipop”. The Fis binding element is shown as a yellow bar. Different mRNA transcripts are shown by coloured wavy lines. ii) mRNA transcripts generated by a combination of σ70 and σ38 associated RNA polymerase (400 nM each) in the presence and absence of Fis (500 nM). Transcripts are labelled according to the scheme in panel i). (B) Repression by Fis in vivo requires P6 and the Fis binding element. Different cbpA::lacZ fusions are illustrated. Promoters are shown by coloured arrows. The Fis binding element is shown as a yellow bar. LacZ activity values from growing JCB387 and JCB3871Δfis cells are given adjacent to each promoter::lacZ fusion. The fold repression by Fis is shown in parenthesis. Fis has no effect on cbpA expression in the absence of P6
Taken together our data suggest that Fis prevents CbpA expression in growing cells primarily by silencing the strong P6 promoter. To confirm this we designed cbpA::lacZ fusions lacking either P1 and P2, or P6, due to mutations in the promoter −10 hexamer. Our hypothesis was that disruption of the P6 promoter would negate the effect of Fis whereas disruption of P1 and P2 would not. The different DNA fragments, fused to lacZ, are illustrated in Figure 3B alongside LacZ activity data from growing JCB387 or JCB3871Δfis cells. As expected, LacZ expression from the wild type cbpA::lacZ fusion increased in JCB3871Δfis cells. An almost identical result was obtained using the −11G,−7G,−6G fragment lacking P1 and P2. Hence, Fis does not exert its effect via the P1 and P2 promoters. The DNA fragment lacking the P6 promoter, due to the −217G and −216G mutations, stimulated low levels of LacZ expression that did not increase in JCB3871Δfis cells.
Deletion of the Fis binding region leads to de-repression of P6
To confirm that the Fis binding region was responsible for mediating repression of the P6 promoter we generated a series of P6::lacZ fusions containing nested deletions downstream of the P6 transcription start site. The different DNA fragments, fused to lacZ, are illustrated in Figure 4 alongside LacZ activity data from growing JCB387 or JCB3871Δfis cells. The starting P6::lacZ fusion, containing the P6 promoter and full Fis binding region, stimulated lacZ expression that increased in cells lacking fis (Figure 4A). Deletions of between 10 and 30 base pairs (Δ10, Δ20 and Δ30) sequentially place the Fis binding element closer to the P6 promoter. These deletions had little effect on either LacZ expression or repression by Fis (Figure 4Bi). Conversely, larger 60, 80 and 100 base pair deletions (Δ60, Δ80, Δ100), which sequentially degrade the Fis binding element, lead to stepwise increases in LacZ expression and a reduction in repression by Fis (Figure 4Bii). Thus, our data are consistent with Fis “protecting” cbpA from the effects of the P6 promoter by interacting with the Fis binding region.
Fig. 4. Deletion analysis of the Fis binding region. 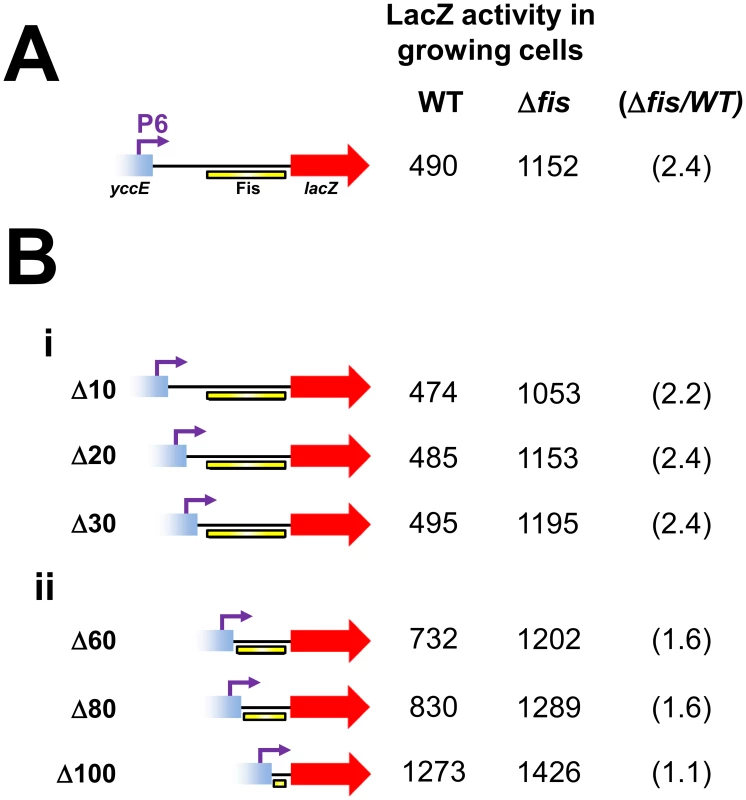
(A) The P6::lacZ fusion. The P6 promoter is shown as a purple arrow. The Fis binding element is shown as a yellow bar. LacZ activity values from growing JCB387 and JCB3871Δfis cells are given adjacent to the illustrated promoter::lacZ fusion. The fold repression by Fis is shown in parenthesis. (B) Deletion analysis of the P6::lacZ fusion. Panel i) shows deletions (Δ) of 10, 20 or 30 base pairs introduced downstream of the cbpA P6 transcription start site. These deletions do not disrupt the region bound by Fis. Rather, they move the region bound by Fis closer to the P6 promoter. LacZ activity values from growing JCB387 and JCB3871Δfis cells are given adjacent to each promoter::lacZ fusion. The fold repression by Fis is shown in parenthesis. Panel ii) shows deletions of (Δ) of 60, 800 or 100 base pairs introduced downstream of the cbpA P6 transcription start site. The Δ60, Δ80 and Δ100 deletions sequentially degrade the region bound by Fis. LacZ activity values from growing JCB387 and JCB3871Δfis cells are given adjacent to each promoter::lacZ fusion. The fold repression by Fis is shown in parenthesis. Binding of Fis and RNA polymerase to the cbpA chromosomal locus in vivo
We next sought support for our model by re-evaluating published data from three independent chromatin immunoprecipitation (ChIP) studies of Fis binding across the E. coli chromosome [22]–[24]. We also scrutinised our own unpublished mRNA deep sequencing (RNA-seq) data from E. coli cells at different stages of growth. Inspection of the ChIP data revealed that DNA upstream of cbpA regulatory region was a target for Fis in all three ChIP studies. Strikingly, the single nucleotide resolution mapping of Fis binding performed by Kahramanoglou et al [24] identified exactly the Fis binding site defined by our in vitro footprinting analysis (Figure S5). Examination of RNA-seq data revealed that no transcripts originate from the cbpA P6 promoter during rapid growth when Fis levels are high (Figure S5). Conversely, in starved E. coli cells that contain low levels of Fis, transcription from the P6 promoter was evident (Figure S5).
The cbpA Fis binding region reduces LacZ expression from non-canonical promoters
We reasoned that we should be able to “transplant” the repressive effect of the cbpA Fis binding region to unrelated promoters. Furthermore, we expected that the Fis binding region would only inhibit promoters active during periods of growth, when Fis levels are high. Thus, we selected four promoter regions with different regulatory properties. Three of the promoters that we selected (galP1, ynfE and yeaR) are σ70 dependent promoters active in growing cells. Transcription from galP1 is stimulated by CRP, ynfE is FNR dependent and yeaR transcription requires NarL [25]–[27]. The fourth promoter that we selected (aer) is σ28 dependent and active during periods of starvation [28]. We compared LacZ expression from each promoter either in the presence or absence of the cbpA Fis binding element. When present the Fis binding region was placed between the promoter transcription start site and the lacZ start codon. The data show that the Fis binding element from the cbpA regulatory region repressed LacZ expression from all three “growth phase” promoters. Conversely, the aer promoter, which is active during periods of starvation, was not repressed (Table 1).
Tab. 1. LacZ expression from different promoter::lacZ fusions in the presence and the absence of the cbpA Fis binding region. 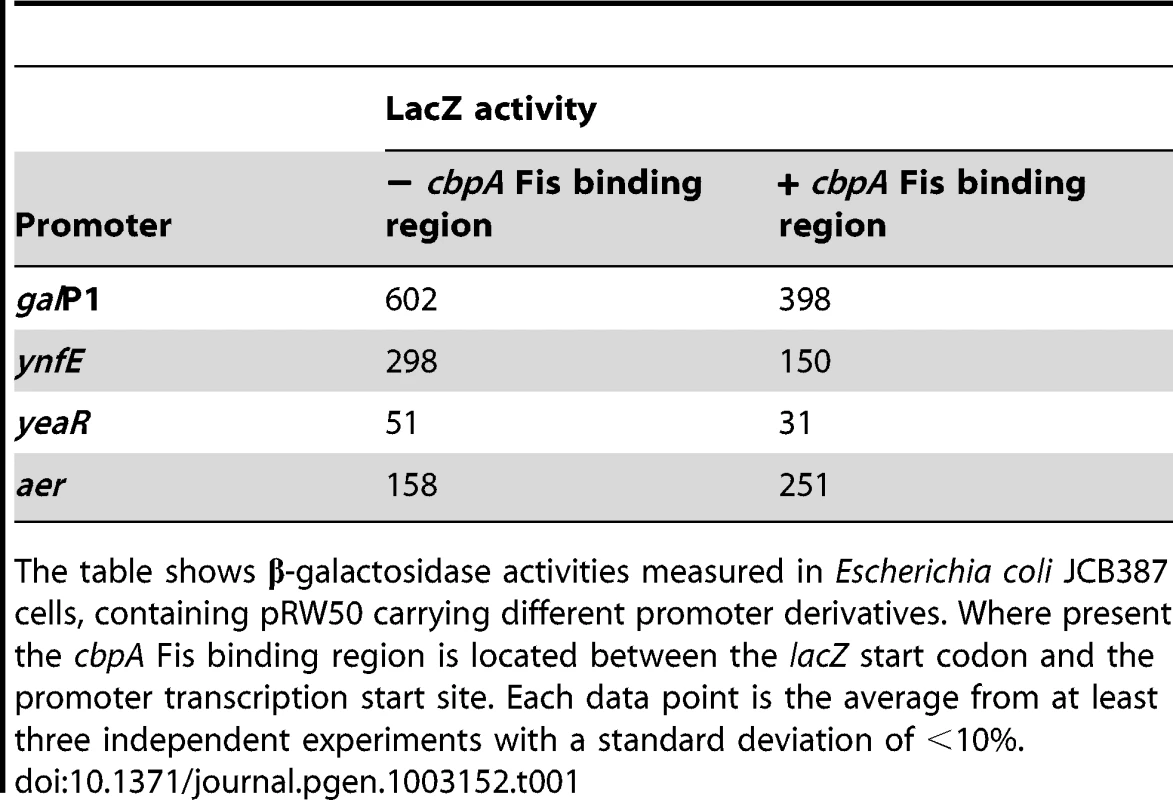
The table shows β-galactosidase activities measured in Escherichia coli JCB387 cells, containing pRW50 carrying different promoter derivatives. Where present the cbpA Fis binding region is located between the lacZ start codon and the promoter transcription start site. Each data point is the average from at least three independent experiments with a standard deviation of <10%. CbpA binding across the chromosome of starved E. coli
We next turned our attention to the DNA binding properties of CbpA in starved E. coli. To investigate DNA binding in vivo we utilised chromatin immunoprecipitation and DNA microarrays (ChIP-chip). In a preliminary analysis we compared CbpA binding in wild type E. coli BW27784 and the cbpM derivative MC108. The data show similar patterns of CbpA binding across the E. coli chromosome in both cases (Figure S6). Recall that CbpM is known to interfere with DNA binding by CbpA. Hence, in vivo sequence requirements for CbpA-DNA interactions were determined using ChIP-chip data generated in the absence of CbpM. Because CbpA was isolated on the basis of its propensity to bind curved DNA in vitro we aligned the CbpA ChIP-chip profile with profiles of predicted DNA curvature [29] and DNA GC content. Figure 5Ai gives an overview of the alignments for the whole genome. Figure 5Aii provides a more detailed view for a smaller segment of the chromosome. Two characteristics of CbpA binding are apparent. First, CbpA binding is biased towards the Ter macrodomain (Figure 5Ai). Second, there is a positive correlation between CbpA binding and DNA curvature (Figure 5Ai and 5Aii). This pattern of DNA binding was also confirmed in vitro for selected targets (Figure S7). To better ascertain the relationship between DNA sequence and CbpA binding we grouped all probes on the DNA microarray according to their percentage GC content. For each group of probes we then calculated the mean CbpA binding signal. The results of this analysis confirm that CbpA binding is greatly reduced at GC-rich DNA sequences (Figure 5B). Optimal CbpA binding was observed at DNA with a GC content of 43%. The average GC content of the E. coli genome is 51%. We note that, whilst CbpA seldom binds to regions that are not intrinsically curved, not all regions of predicted curvature are bound by CbpA (Figure 5Aii). This is likely due to competition with other proteins that recognise curved DNA. Similarly, the in silico DNA curvature predictions may not hold true at all locations in vivo.
Fig. 5. Chromosome-wide distribution of CbpA in starved E. coli. 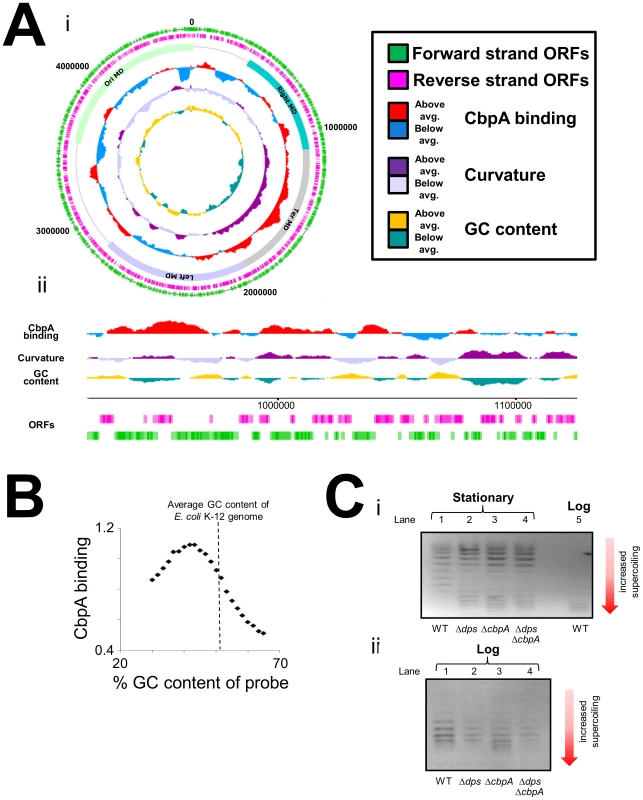
(A) Distribution of CbpA across the E. coli chromosome. i) Genome-wide view of CbpA binding in starved MC108 cells. The figure shows ChIP-chip data for CbpA binding plotted against features of the E. coli genome in the form of a genome atlas. The data have been averaged across a 100,000 base pair window. The four chromosomal macrodomains (MD) are labelled ii) CbpA ChIP-chip data for a small section of the E. coli chromosome. The data have been averaged across a 10,000 base pair window. (B) Relationship between CbpA binding and DNA GC content. The graph shows the average CbpA binding signal plotted against the GC content of probes on the DNA microarray. The average GC content of the E. coli K-12 chromosome is shown by a dashed line. Very GC rich and GC poor probes were excluded during microarray design and are thus absent. (C) Effect of CbpA on DNA supercoiling in vivo. Panel i) shows an image of a 1% (v/v) agarose gel containing 2.5 µg/ml chloroquine. Plasmids were isolated from different genetic backgrounds and different stages of growth as indicated. Panel ii) also shows an image of a 1% (v/v) agarose gel containing 2.5 µg/ml chloroquine. Plasmids were isolated from rapidly dividing cells. CbpA influences DNA topology in stationary phase E. coli cells
Numerous studies have shown that entry to stationary phase results in a marked decrease in DNA supercoiling [2], [30], [31]. Thus, we investigated the effect of CbpA, and as a control Dps, on DNA topology in vivo using a reporter plasmid. After extraction from three day old cultures plasmid topoisomers were separated on a 1% agarose gel containing 2.5 µg/ml chloroquine. A sample of plasmid DNA isolated from log phase cells was also analysed as a control. As expected, the plasmid isolated from growing cells was considerably more supercoiled than the plasmid isolated from starved cells (Figure 5Ci, compare lanes 1 and 5). Remarkably, starved cells lacking dps or cbpA yielded a split distribution of plasmid topoisomers. Some plasmids were highly supercoiled with similar topology to plasmids isolated from growing cells (compare lanes 2, 3 and 5). Other plasmids in the sample were relaxed, as seen for starved cells (compare lanes 1–3). Plasmids isolated from the ΔdpsΔcbpA strain had similar topology to the plasmids isolated from the individual gene knockout strains (compare lanes 2–4). These effects were specific to plasmids isolated from starved cells; plasmid topoisomers obtained from growing cultures of each strain did not significantly differ (Figure 5Cii).
Discussion
Mechanism of cbpA repression by Fis during rapid growth
In wild type E. coli cells cbpA transcription initiates from the P1 and P2 promoters with the σ38 dependent P2 promoter being dominant [16], [32] (Figure 2C). However, during rapid growth, Fis is required to prevent uncontrolled transcription of cbpA (Figure 1Aii). This uncontrolled transcription is primarily driven by the P6 promoter, which is located in the yccE gene, more than 250 base pairs upstream of cbpA. Whilst Fis prevents transcription from P6 in vivo (Figure 2C, Figure 3B and Figure 4) and in vitro (Figure 3A) Fis appears unable to prevent RNA polymerase binding at the P6 promoter (Figure 2B). This is not surprising since the DNA element bound by Fis is located ∼60 base pairs downstream of the P6 transcription start. We conclude that Fis must either act as a “road block”, to prevent transcription elongation, or prevent RNA polymerase escape from the P6 promoter. We currently favour the latter model since we were unable to detect “road blocked” transcripts in our in vitro transcription assay (Figure 3A). Note that Fis also inhibits transcription from the weak P4 promoter (Figure 2C, Figure 3A and Figure S4). In this case the P4 −10 element is embedded within the primary Fis binding sequence (Figure S4). Hence, Fis binding to this site prevents RNA polymerase association with the P4 promoter (Figure 2B lanes 5–7). Our model for Fis regulation of cbpA is outlined in Figure 6A. This regulatory mechanism is salient since it is becoming increasingly apparent that bacterial chromosomes contain many intragenic promoters [33]–[35]. Thus, mechanisms must exist to ensure that these promoters do not adversely affect transcription of neighbouring operons. We speculate that Fis, and other nucleoid proteins, may serve such a purpose.
Fig. 6. The nucleoid protein response to starvation in E. coli. 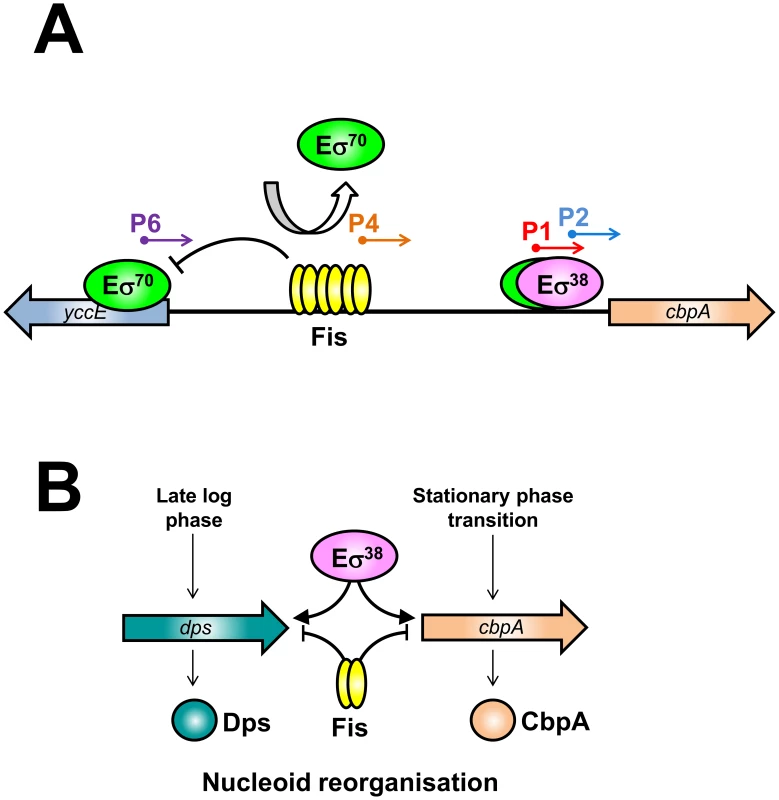
(A) Model of cbpA regulation by Fis. (B) Model for staged induction of cbpA and dps. Role of Fis in controlling nucleoid re-organisation on transition to stationary phase
Taken together with previous work our results provide a detailed molecular explanation for the phenomenon of nucleoid reorganisation that occurs in starved E. coli cells [36]. We propose that regulatory crosstalk between nucleoid proteins plays a pivotal role. In our model, Fis sits at the fulcrum of the regulatory process by binding low affinity elements overlapping the dps promoter [8] (to inhibit σ70 dependent transcription) and by binding high affinity sites in the cbpA regulatory region (Figure 1) (to insulate cbpA from σ70 dependent transcription). As cells divide, and Fis levels decrease, the different affinity of Fis for the dps and cbpA regulatory regions contributes to staged induction of dps and cbpA (Figure 6B). Interestingly, despite their similar DNA binding properties, CbpA and Dps both have distinct stress response functions. Thus, CbpA can function as a co-chaperone, by virtue of its N-terminal J-domain, whilst Dps has a bacterioferritin like fold and can sequester Fe2+ ions [7], [9]. We speculate that, to fully understand the function of CbpA and Dps in stationary phase, the relationship between their different activities will need to be unravelled.
Materials and Methods
Strains, plasmids, and oligonucleotides
Bacterial strains, plasmids and oligonucleotide sequences are listed in Table S1. All cbpA regulatory region sequences are numbered with respect to the P1 transcription start point (+1) and with upstream and downstream locations denoted by ‘−’ and ‘+’ prefixes respectively.
β-galactosidase assays
Activities are shown in Miller units [37] and are the average of three or more independent experiments with a standard deviation of <10%. Background LacZ activity values, generated from cells carrying a “promoterless” pRW50, were subtracted. Cells were grown aerobically, at 37°C, in LB media. Assays were performed using either JCB387 or the derivative JCB3871Δfis.
mRNA primer extension assays
Transcript start sites were mapped by primer extension, as described in Lloyd et al. [38], using RNA purified from strains carrying the 302 base pair cbpA regulatory DNA fragment cloned in pRW50. The 5′ end-labelled primer D49724, which anneals downstream of the HindIII site in pRW50 was used in all experiments. Primer extension products were analysed on denaturing 6% polyacrylamide gels, calibrated with arbitrary sequencing reactions, and visualized using a Fuji phosphor screen and Bio-Rad Molecular Imager FX.
Protein purification, in vitro DNA binding, and in vitro transcription assays
CbpA and derivatives were all purified as described [15]. Fis and RNA polymerase were prepared as described previously [8]. EMSA, DNAseI and KMnO4 footprinting with Fis and/or RNA polymerase are described by Grainger et al. [8]. The in vitro transcription experiments were performed as described [8] using the system of Kolb et al. [39]. Protein and DNA concentrations used for all in vitro experiments are provided in the figure legends.
Chromatin immunoprecipitation and DNA microarray analysis
Chromatin Immunoprecipitation was done exactly as described previously [40]. Formaldehyde crosslinked nucleoprotein obtained from stationary phase BW27784 or MC108 cells was fragmented by sonication and CbpA-DNA complexes were precipitated using a rabbit polyclonal antibody against CbpA. A control mock immunoprecipitation (from which anti-CbpA was omitted) was done in parallel. The “plus and minus antibody” DNA samples were then labelled with Cy5 and Cy3 respectively before being mixed and hybridised to a 43,450 feature DNA microarray (Oxford Gene Technology). After hybridisation, washing and scanning the Cy5 and Cy3 signal was calculated for each probe on the array (Table S2). The MC108 experiment was done in duplicate, and an average Cy5/Cy3 ratio was used for further analysis (referred to as the CbpA binding signal). The images shown in Figure 4 were generated using DNA plotter software [41]. To facilitate detailed inspection of the CbpA ChIP-chip data a file that can be loaded into DNA plotter or the Artemis genome browser is provided in the supplementary material (Table S3). These data should be loaded into the software as a graph after first installing the E. coli K-12 genome sequence (provided as a genbank file in Table S4).
Analysis of DNA curvature and GC content
The DNA curvature analysis of the E. coli chromosome was done using the CURVATURE software package [42] exactly as described previously [29]. The DNA GC profile was calculated using the internal graph function in DNA plotter. We note that low GC content is not an absolute indicator of increased DNA curvature on a local scale of a few base pairs. However, for the large segments of DNA considered here, there is a clear inverse correlation between GC content and DNA curvature.
Chloroquine gel electrophoresis
We monitored superhelicity of plasmid pJ204 in strain BW27784 and the Δdps, ΔcbpA or ΔdpsΔcbpA derivatives. Transformants were grown in LB medium at 37°C for three days. Plasmid DNA samples were prepared using a QIAprep Spin Miniprep Kit (Qiagen). Topoisomers were separated on a 1% agarose gel containing 2.5 µg/ml chloroquine. Gels were run for 60 hours at 40 V in the dark. After washing with water for at least 2 hours the gel was stained with ethidium bromide for 2 hours and photographed under UV illumination.
Western blotting
Overnight cultures of E. coli BW25113 were diluted 1∶100 into fresh LB media and grown at 37°C with aeration. Samples (1 ml) were taken at the indicated OD650 values and the cells harvested by centrifugation. Cells were re-suspended in Laemmli buffer so that the number of cells in each sample was equivalent. After boiling for 10 min cytoplasmic proteins were separated by SDS-PAGE and Fis was detected using Western immunoblotting as previously described [43]. The blots were also probed using antibodies against RpoA (Neoclone) as a control.
Supporting Information
Zdroje
1. DillonSC, DormanCJ (2010) Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8 : 185–195.
2. DormanCJ, BarrGC, Ni BhriainN, HigginsCF (1988) DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol 6 : 2816–2826.
3. SobetzkoP, TraversA, MuskhelishviliG (2012) Gene order and chromosome dynamics coordinate spatiotemporal gene expression during the bacterial growth cycle. Proc Natl Acad Sci 109: E42–E50.
4. AzamTA, IwataA, NishimuraA, UedaS, IshihamaA (1999) Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol 181 : 6361–6370.
5. AlmirónM, LinkAJ, FurlongD, KolterR (1992) A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev 6 : 2646–2654.
6. WolfSG, FrenkielD, AradT, FinkelSE, KolterR, et al. (1999) DNA protection by stress-induced biocrystallization. Nature 400 : 83–85.
7. CalhounLN, KwonYM (2011) Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: a review. J Appl Microbiol 110 : 375–386.
8. GraingerDC, GoldbergMD, LeeDJ, BusbySJ (2008) Selective repression by Fis and H-NS at the Escherichia coli dps promoter. Mol Microbiol 68 : 1366–1377.
9. UeguchiC, KakedaM, YamadaH, MizunoT (1994) An analogue of the DnaJ molecular chaperone in Escherichia coli. Proc Natl Acad Sci 91 : 1054–1058.
10. AzamTA, IshihamaA (1999) Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J Biol Chem 274 : 33105–33113.
11. BirdJG, SharmaS, RoshwalbSC, HoskinsJR, WicknerS (2006) Functional analysis of CbpA, a DnaJ homolog and nucleoid-associated DNA-binding protein. J Biol Chem 281 : 34349–34356.
12. ChintakayalaK, GraingerDC (2011) A conserved acidic amino acid mediates the interaction between modulators and co-chaperones in enterobacteria. J Mol Biol 411 : 313–320.
13. ChaeC, SharmaS, HoskinsJR, WicknerS (2004) CbpA, a DnaJ homolog, is a DnaK co-chaperone, and its activity is modulated by CbpM. J Biol Chem 279 : 33147–33153.
14. SarrafNS, BaardsnesJ, ChengJ, O'Connor-McCourtM, CyglerM, et al. (2010) Structural basis of the regulation of the CbpA co-chaperone by its specific modulator CbpM. J Mol Biol 398 : 111–121.
15. CosgriffS, ChintakayalaK, ChimYT, ChenX, AllenS, et al. (2010) Dimerization and DNA-dependent aggregation of the Escherichia coli nucleoid protein and chaperone CbpA. Mol Microbiol 77 : 1289–1300.
16. SinghSS, TypasA, HenggeR, GraingerDC (2011) Escherichia coli σ70 senses sequence and conformation of the promoter spacer region. Nucleic Acids Res 39 : 5109–5118.
17. AzamTA, HiragaS, IshihamaA (2000) Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells 5 : 613–626.
18. BrowningDF, ColeJA, BusbySJ (2004) Transcription activation by remodelling of a nucleoprotein assembly: the role of NarL at the FNR-dependent Escherichia coli nir promoter. Mol Microbiol 53 : 203–215.
19. HengenPN, BartramSL, StewartLE, SchneiderTD (1997) Information analysis of Fis binding sites. Nucleic Acids Res 25 : 4994–5002.
20. HirvonenCA, RossW, WozniakCE, MarascoE, AnthonyJR, et al. (2001) Contributions of UP elements and the transcription factor FIS to expression from the seven rrn P1 promoters in Escherichia coli. J Bacteriol 183 : 6305–6314.
21. ShaoY, Feldman-CohenLS, OsunaR (2008) Functional characterisation of the Escherichia coli Fis-DNA binding sequence. J Mol Biol 376 : 771–785.
22. GraingerDC, HurdD, GoldbergMD, BusbySJ (2006) Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res 34 : 4642–4652.
23. ChoBK, KnightEM, BarrettCL, PalssonBØ (2008) Genome-wide analysis of Fis binding in Escherichia coli indicates a causative role for A-/AT-tracts. Genome Res 18 : 900–910.
24. KahramanoglouC, SeshasayeeAS, PrietoAI, IbbersonD, SchmidtS, et al. (2011) Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res 39 : 2073–2091.
25. AtteyA, BelyaevaT, SaveryN, HoggettJ, FujitaN, et al. (1994) Interactions between the cyclic AMP receptor protein and the alpha subunit of RNA polymerase at the Escherichia coli galactose operon P1 promoter. Nucleic Acids Res 22 : 4375–4380.
26. XuM, BusbySJ, BrowningDF (2009) Activation and repression at the Escherichia coli ynfEFGHI operon promoter. J Bacteriol 191 : 3172–3176.
27. SquireDJ, XuM, ColeJA, BusbySJ, BrowningDF (2009) Competition between NarL-dependent activation and Fis-dependent repression controls expression from the Escherichia coli yeaR and ogt promoters. Biochem J 420 : 249–257.
28. HollandsK, LeeDJ, LloydGS, BusbySJ (2010) Activation of sigma 28-dependent transcription in Escherichia coli by the cyclic AMP receptor protein requires an unusual promoter organization. Mol Microbiol 75 : 1098–1111.
29. PedersenAG, JensenLJ, BrunakS, StaerfeldtHH, UsseryDW (2000) A DNA structural atlas for Escherichia coli. J Mol Biol 299 : 907–930.
30. ConterA (2003) Plasmid DNA supercoiling and survival in long-term cultures of Escherichia coli: role of NaCl. J Bacteriol 185 : 5324–5327.
31. ConterA, MenchonC, GutierrezC (1997) Role of DNA supercoiling and rpoS sigma factor in the osmotic and growth phase-dependent induction of the gene osmE of Escherichia coli K12. J Mol Biol 273 : 75–83.
32. YamashinoT, KakedaM, UeguchiC, MizunoT (1994) An analogue of the DnaJ molecular chaperone whose expression is controlled by σS during the stationary phase and phosphate starvation in Escherichia coli. Mol Microbiol 13 : 475–483.
33. DornenburgJE, DevitaAM, PalumboMJ, WadeJT (2010) Widespread antisense transcription in Escherichia coli. mBio 1(1): e00024–10 doi:10.1128/mBio.00024-10.
34. SharmaCM, HoffmannS, DarfeuilleF, ReignierJ, FindeissS, et al. (2010) The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464 : 250–255.
35. KawanoM, StorzG, RaoBS, RosnerJL, MartinRG (2005) Detection of low-level promoter activity within open reading frame sequences of Escherichia coli. Nucleic Acids Res 33 : 6268–6276.
36. OhniwaRL, MorikawaK, KimJ, OhtaT, IshihamaA, et al. (2006) Dynamic state of DNA topology is essential for genome condensation in bacteria. EMBO J 25 : 5591–5602.
37. Miller J (1972) Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
38. LloydGS, HollandsK, GodfreyRE, BusbySJ (2008) Transcription initiation in the Escherichia coli K-12 malI-malX intergenic region and the role of the cyclic AMP receptor protein. FEMS Microbiol Lett 288 : 250–7.
39. KolbA, KotlarzD, KusanoS, IshihamaA (1995) Selectivity of the Escherichia coli RNA polymerase E sigma 38 for overlapping promoters and ability to support CRP activation. Nucleic Acids Res 23 : 819–826.
40. GraingerDC, OvertonTW, ReppasN, WadeJT, TamaiE, et al. (2004) Genomic studies with Escherichia coli MelR protein: applications of chromatin immunoprecipitation and microarrays. J Bacteriol 186 : 6938–6943.
41. CarverT, ThomsonN, BleasbyA, BerrimanM, ParkhillJ (2009) DNAPlotter: circular and linear interactive genome visualization. Bioinformatics 25 : 119–120.
42. ShpigelmanES, TrifonovEN, BolshoyA (1993) CURVATURE: software for the analysis of curved DNA. Comput Appl Biosci 9 : 435–440.
43. BallCA, OsunaR, FergusonKC, JohnsonRC (1992) Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli.. J Bacteriol 174 : 8043–8056.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



