-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
Prader-Willi Syndrome is the most common syndromic form of human obesity and is caused by the loss of function of several genes, including MAGEL2. Mice lacking Magel2 display increased weight gain with excess adiposity and other defects suggestive of hypothalamic deficiency. We demonstrate Magel2-null mice are insensitive to the anorexic effect of peripherally administered leptin. Although their excessive adiposity and hyperleptinemia likely contribute to this physiological leptin resistance, we hypothesized that Magel2 may also have an essential role in intracellular leptin responses in hypothalamic neurons. We therefore measured neuronal activation by immunohistochemistry on brain sections from leptin-injected mice and found a reduced number of arcuate nucleus neurons activated after leptin injection in the Magel2-null animals, suggesting that most but not all leptin receptor–expressing neurons retain leptin sensitivity despite hyperleptinemia. Electrophysiological measurements of arcuate nucleus neurons expressing the leptin receptor demonstrated that although neurons exhibiting hyperpolarizing responses to leptin are present in normal numbers, there were no neurons exhibiting depolarizing responses to leptin in the mutant mice. Additional studies demonstrate that arcuate nucleus pro-opiomelanocortin (POMC) expressing neurons are unresponsive to leptin. Interestingly, Magel2-null mice are hypersensitive to the anorexigenic effects of the melanocortin receptor agonist MT-II. In Prader-Willi Syndrome, loss of MAGEL2 may likewise abolish leptin responses in POMC hypothalamic neurons. This neural defect, together with increased fat mass, blunted circadian rhythm, and growth hormone response pathway defects that are also linked to loss of MAGEL2, could contribute to the hyperphagia and obesity that are hallmarks of this disorder.
Published in the journal: Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice. PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003207
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003207Summary
Prader-Willi Syndrome is the most common syndromic form of human obesity and is caused by the loss of function of several genes, including MAGEL2. Mice lacking Magel2 display increased weight gain with excess adiposity and other defects suggestive of hypothalamic deficiency. We demonstrate Magel2-null mice are insensitive to the anorexic effect of peripherally administered leptin. Although their excessive adiposity and hyperleptinemia likely contribute to this physiological leptin resistance, we hypothesized that Magel2 may also have an essential role in intracellular leptin responses in hypothalamic neurons. We therefore measured neuronal activation by immunohistochemistry on brain sections from leptin-injected mice and found a reduced number of arcuate nucleus neurons activated after leptin injection in the Magel2-null animals, suggesting that most but not all leptin receptor–expressing neurons retain leptin sensitivity despite hyperleptinemia. Electrophysiological measurements of arcuate nucleus neurons expressing the leptin receptor demonstrated that although neurons exhibiting hyperpolarizing responses to leptin are present in normal numbers, there were no neurons exhibiting depolarizing responses to leptin in the mutant mice. Additional studies demonstrate that arcuate nucleus pro-opiomelanocortin (POMC) expressing neurons are unresponsive to leptin. Interestingly, Magel2-null mice are hypersensitive to the anorexigenic effects of the melanocortin receptor agonist MT-II. In Prader-Willi Syndrome, loss of MAGEL2 may likewise abolish leptin responses in POMC hypothalamic neurons. This neural defect, together with increased fat mass, blunted circadian rhythm, and growth hormone response pathway defects that are also linked to loss of MAGEL2, could contribute to the hyperphagia and obesity that are hallmarks of this disorder.
Introduction
Energy balance is regulated in part by the coordinated action of specialized neurons within the hypothalamus of the brain, which sense circulating signals of energy stores such as the adipocyte derived hormone, leptin [1]. The arcuate nucleus (ARC) is a key hypothalamic region involved in energy balance regulation, and is a major site for leptin action. Two distinct populations of ARC neurons, expressing either Neuropeptide Y (NPY) and Agouti-related peptide (AgRP) or pro-opiomelanocortin (POMC), have opposing effects on energy balance. NPY and AgRP, via different mechanisms, stimulate food intake and reduce energy expenditure, with the overexpression of either leading to obesity [2]–[4]. In contrast, POMC is processed into several shorter peptides including α-MSH, which reduces food intake and stimulates energy expenditure through melanocortin-responsive neurons in the paraventricular nucleus and elsewhere [5]. Mutations that affect processing or lead to loss of expression of the POMC gene also cause obesity in mice and humans [6]–[8].
Impaired hypothalamic regulation of energy balance is found in numerous genetic forms of human obesity, including congenital deficiency of leptin (MIM 164160) [9], leptin receptor mutations (MIM 601007) [10], MC4R melanocortin receptor mutations (MIM 601665) [11], and Bardet-Biedl Syndrome (MIM 209900) [12]. Impaired energy homeostasis may also contribute to the severe hyperphagia and obesity seen in people with Prader-Willi Syndrome (PWS, MIM 176270), the most common genetic form of syndromic obesity in humans [13]. People with PWS typically have a loss of function of several contiguous genes, including MAGEL2, a member of the melanoma antigen (MAGE) family of proteins [14]. MAGE proteins act in intracellular signaling pathways that modulate protein modification, protein degradation, cytoskeletal rearrangement, and transcription [15]. In mice, Magel2 is predominantly expressed in the central nervous system, with highest expression levels in the hypothalamus [16], [17]. We previously showed that gene-targeted mice lacking Magel2 become overweight with increased adiposity as adults [18], and exhibit delayed puberty, irregular estrous cycles, and early onset infertility [19]. As obesity and infertility are common in animal models with impaired leptin responses [20], we hypothesized that Magel2-null mice may also respond abnormally to leptin. We now report that Magel2-null mice display physiological leptin resistance, that leptin resistance precedes the development of increased adiposity, and that leptin-mediated electrophysiological responses in POMC neurons are conspicuously absent in these animals.
Results
Magel2-Null Mice Lose Less Weight during a Fast, and Eat Less Food and Gain Less Weight after Fasting
Leptin maintains homeostatic control of weight, regulating ingestive behavior and energy expenditure in response to changes in nutritional energy availability. The fall in circulating leptin that occurs with food deprivation normally causes increased feeding when food is reinstated, restoring normal weight and fat mass [1]. However, refeeding-associated weight gain and hyperphagia are dysregulated in mice with diet-induced obesity [21] or mice carrying mutations that selectively ablate POMC neurons [22], [23] or that decrease levels of hypothalamic neuropeptides [24], [25]. To determine if Magel2 is important for compensatory responses after fasting, we subjected mice to a prolonged (48 h) fast. While control mice lost 16% of their body weight after fasting, Magel2-null mice lost significantly less body weight (12% of initial weight), consistent with their previously noted reduced locomotor activity (Figure 1A). We then refed the fasted mice, and measured food intake and body weight over the next 3 days. Body weight returned to baseline within 2 days of refeeding in control mice, but Magel2-null mice remained underweight even after 3 days (Figure 1A). Food intake was similar before fasting (Figure 1B), but Magel2-null mice ate less food during the initial 24 h recovery period (Figure 1C), resulting in a significantly reduced food intake ratio - the ratio of food consumed after fasting to food consumed before fasting - compared to control mice (Figure 1D). These results suggest that the hypothalamic pathways required for compensatory refeeding are defective in Magel2-null mice.
Fig. 1. Magel2-null mice have abnormal weight recovery and compensatory refeeding after fasting. 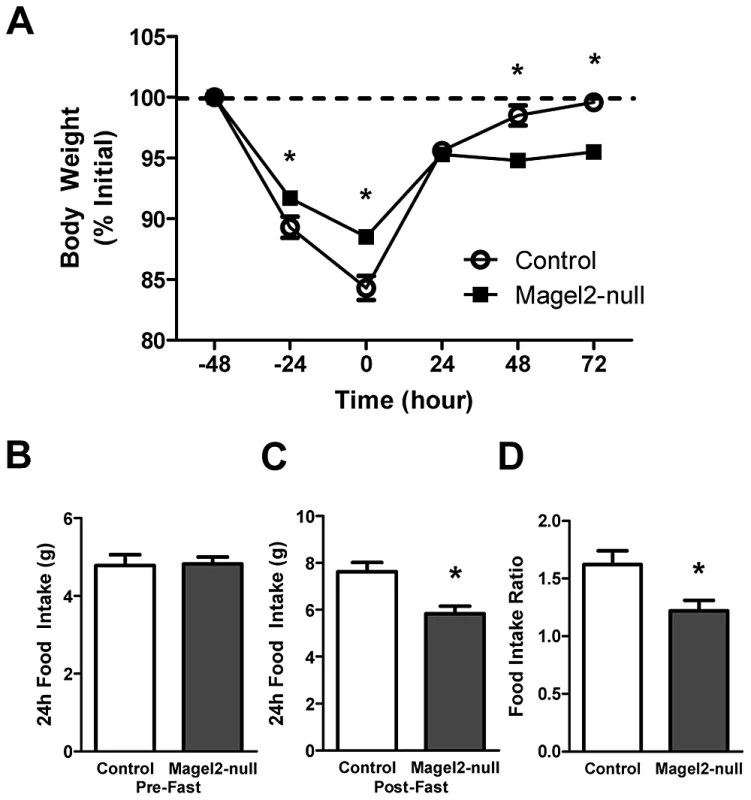
A) Body weights of adult male mice subjected to a 48 h fast and a 72 h refeeding period. Magel2-null mice lost less weight while fasting, and recovered less weight during refeeding (*P<0.05, compared between genotypes by Student's t-test). B) 24 h food intake pre-fast and C) post-fast (*P<0.01). D) Magel2-null mice have a reduced food intake ratio - the ratio of food consumed after fasting to food consumed before fasting - compared to controls (*P<0.05). n = 6 mice of each genotype. Values are means ± SEM. Magel2-Null Mice Lack the Anorexigenic Response to Peripherally Administered Leptin
Magel2-null mice have excess adipose tissue, and high levels of circulating leptin suggesting reduced leptin sensitivity [18]. At 20 weeks of age, Magel2-null mice are 14% heavier than control mice (Figure 2A). To examine whether Magel2-null mice are sensitive to exogenous leptin, we measured food intake in singly housed male mice using a crossover study design in which the same animals received either intraperitoneal (ip) leptin (2.5 mg/kg) or phosphate buffered saline (PBS) approximately 1 week apart. In control leptin-treated mice, food intake was reduced by about 30% in the 24 h following leptin injection, as expected. However, leptin-treated Magel2-null mice showed no reduction in food intake following ip leptin (Figure 2B). Decreased sensitivity to peripherally administered leptin can occur in mice with diet-induced obesity that have very high (e.g. ten-fold elevated) leptin levels even in the absence of a genetic mutation [26], [27]. In contrast, Magel2 mice typically have only two-fold elevated leptin even as older adults. Nonetheless, we tested leptin sensitivity in younger (6-week old) mice, where there is no difference in body weight between Magel2-null and control animals (Figure 2C). Leptin treatment in young control mice again caused a reduction of approximately 35% in 24 h food intake compared to PBS treatment. In contrast, there was no reduction in 24 h food intake in leptin-treated young Magel2-null mice (Figure 2D). These results suggest that Magel2-null mice that are similar in weight to controls are nevertheless insensitive to the anorexigenic effect of peripherally administered leptin.
Fig. 2. Peripherally administered leptin fails to reduce food intake in Magel2-null mice. 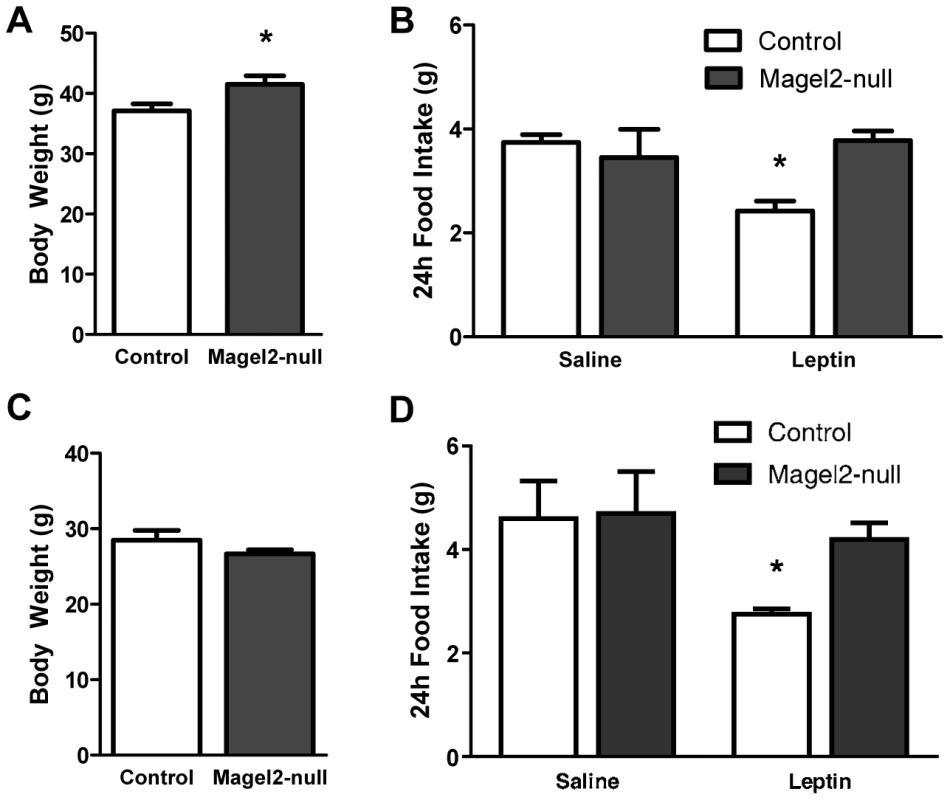
A) Body weights of 20 week old male mice (*P<0.05, compared between genotypes by Student's t-test), and B) 24 h food intake following leptin or PBS (saline) injections. Leptin reduced food intake in control mice (*P<0.005, compared before and after treatment), while leptin-treated Magel2-null mice showed no significant change in food intake. n = 10 of each genotype. C) Body weights of 6 week old male mice and D) 24 h food intake following leptin or PBS injections. Leptin reduced food intake in control (*P<0.05, compared before and after treatment) but not Magel2-null mice. Controls, n = 7, Magel2-null, n = 6. Values are means ± SEM. Magel2 Deficiency Reduces Leptin-Mediated Phosphorylation of STAT3 and Induction of c-Fos Expression in the Arcuate Nucleus
We next examined the activation of the leptin receptor by measuring levels of phosphorylated Signal Transducer and Activator of Transcription 3 (pSTAT3) [28], [29] in the ARC following a single ip leptin (2.5 mg/kg) injection. While few pSTAT3-positive neurons were seen in the ARC following PBS injection in both Magel2-null and control animals (Figure 3A, 3C, 3E), numerous pSTAT3-positive cells were seen in the ARC of both genotypes after leptin injection (Figure 3B, 3D). Nonetheless, detailed cell counts throughout the ARC revealed a 30–35% reduction in pSTAT3-positive cells in leptin-injected Magel2-null mice compared to leptin-injected control (Figure 3E). Next, we measured the induction of c-fos, an immediate early gene marker of neuronal activation that is detected in POMC but not NPY neurons in the ARC after leptin injection [30], [31]. Baseline c-fos immunoreactivity was observed in PBS-injected control animals (Figure 3F, 3H, 3J), and leptin treatment caused a significant increase in c-fos expression in both control and Magel2-null mice (Figure 3G, 3I, 3J), particularly in more posterior regions of the ARC where the majority of leptin-sensitive POMC neurons are located [32]. Interestingly, fewer c-fos positive cells were observed in Magel2-null mice after either PBS or leptin injection compared to similarly treated control mice (Figure 3J).
Fig. 3. pSTAT3 and c-fos expression in ARC neurons in leptin-treated mice. 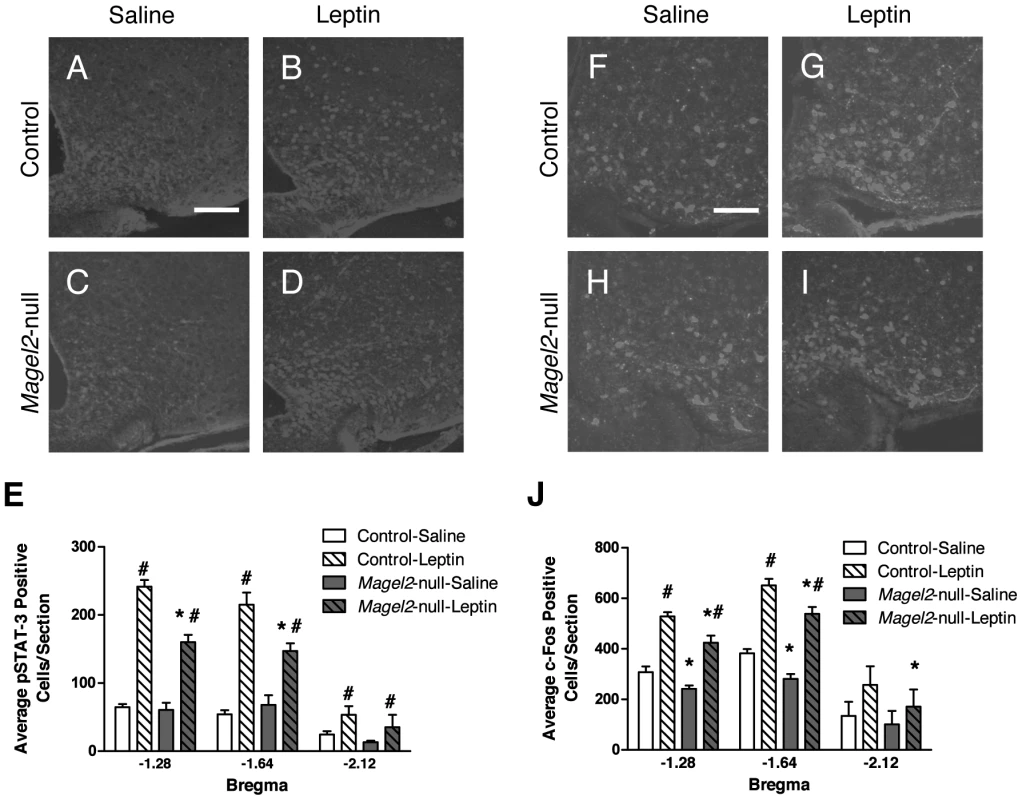
A–D) Representative immunohistochemistry (IHC) images showing pSTAT3 immunoreactivity following ip PBS (saline) or leptin injection. Scale bar, 100 µm. E) Magel2-null mice have fewer pSTAT3 positive cells than control following leptin injection (Bregma −1.28, P<0.001; Bregma −1.64, P<0.005; Bregma −2.12, P>0.5, *compared between genotypes by Student's t-test) but both genotypes have more pSTAT3 positive cells after leptin treatment than after PBS (#, P<0.05, compared between treatments by Student's t-test; n = 8 mice of each genotype). F–I) Representative images of c-fos IHC following ip PBS and leptin injection. Scale bar 100 µm. J) Leptin induced c-fos expression in both Magel2-null and control mice compared to PBS (#P<0.05, compared between treatments by Student's t-test). At both baseline and following leptin treatment, Magel2-null mice had significantly fewer c-fos positive cells compared to controls (Bregma −1.28 ∼18% reduction, Bregma −1.64 ∼20% reduction, Bregma −2.12 ∼35% reduction, *P<0.05, compared between genotypes by Student's t-test). Values in E) and J) are means ± SEM. Magel2-Null Mice Have Fewer ARC POMC Neurons
POMC neurons form an important part of the hypothalamic energy balance neural circuitry, and are activated in response to leptin [33]. Fewer leptin-induced pSTAT3 and c-fos immunoreactive cells were observed in the ARC of Magel2-null mice, particularly in areas previously shown to contain higher levels of leptin-sensitive POMC neurons. We therefore counted POMC/EGFP-positive neurons in the ARC of Magel2×POMCEGFP and control mice, and found on average 39% fewer POMC+ neurons in the Magel2-null mice than in controls (Figure 4). This reduction was most evident in the more posterior region of the ARC, where 52% fewer POMC+ cells were found (Figure 4C). The number of LepRb positive neurons (measured as EGFP positive cells in the ARC of offspring from a Magel2×LepRbEGFP cross) did not differ between mutants and controls. Thus, loss of POMC neurons can partially explain the reduction seen in leptin-induced pSTAT3 and c-fos expression in the ARC of Magel2-null mice. Alternatively, it is possible that these neurons are still present, but that the expression of POMC/EGFP has fallen below the detection limit of this experiment.
Fig. 4. Magel2-null mice have fewer ARC POMC neurons. 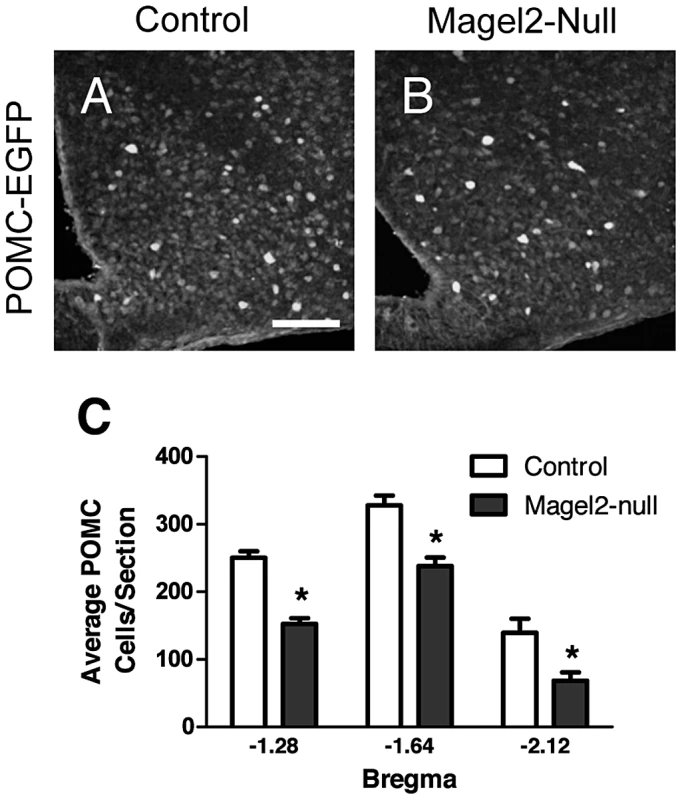
A–B) Representative images of GFP (POMC) IHC in A) control and B) Magel2×POMCEGFP mice. Scale bar 100 µm. C) Magel2×POMCEGFP mice have fewer GFP expressing (POMC) cells at all levels of the ARC (Bregma −1.28, 39% reduction, *P<10−8; Bregma −1.64, 27% reduction, *P<10−5; Bregma −2.12, 52% reduction, *P<0.01), compared between genotypes by Student's t-test. Values are means ± SEM. Leptin Fails to Activate POMC Neurons in the ARC of Magel2-Null Mice
To directly examine leptin responses in ARC neurons, we performed whole-cell visualized-patch recordings of fluorescent neurons in mice expressing enhanced GFP in leptin receptor-positive (LepRb+) neurons (Figure 5A, 5B). First, the resting membrane potential (RMP) of LepRb+ neurons (Figure 5C) and the input resistance (data not shown) were comparable between Magel2×LepRbEGFP and control mice. NPY hyperpolarizes the majority of leptin-responsive ARC cells (Figure 5D) [34]. Application of either 100 nM (data not shown) or 300 nM NPY produced a robust hyperpolarization of virtually all ARC LepRb+ neurons tested in both Magel2-null and control slices, indicating that Magel2 is not required for normal NPY signaling (Figure 5E). We then examined the leptin (100 nM) responses in LepRb+ neurons in the ARC. Leptin normally activates (depolarizes) POMC neurons, and inhibits (hyperpolarizes) NPY neurons [33], [35], so we expected to observe both responses in the mixed neuronal populations represented by LepRb+ cells in the ARC. Leptin induced both hyperpolarizing and depolarizing responses in LepRb+ cells in slices from control mice, with a few unresponsive cells (Figure 5F–5H). All cells tested, including leptin-unresponsive cells, exhibited a normal electrophysiological response to 300 nM NPY. In striking contrast, LepRb+ cells in slices from Magel2-null mice never exhibited depolarizing responses to leptin. In these slices, leptin-mediated hyperpolarizing responses were seen at a frequency comparable to controls, while more leptin-unresponsive cells (which nevertheless showed normal NPY responses) were found (Figure 5H). These results suggest that the inhibitory action of leptin is unaffected at ARC LepRb+ neurons of Magel2-null mice, but that the excitatory effect of leptin, typically observed at POMC neurons, is selectively absent.
Fig. 5. Magel2 is required for the leptin-induced depolarizing response in POMC neurons. 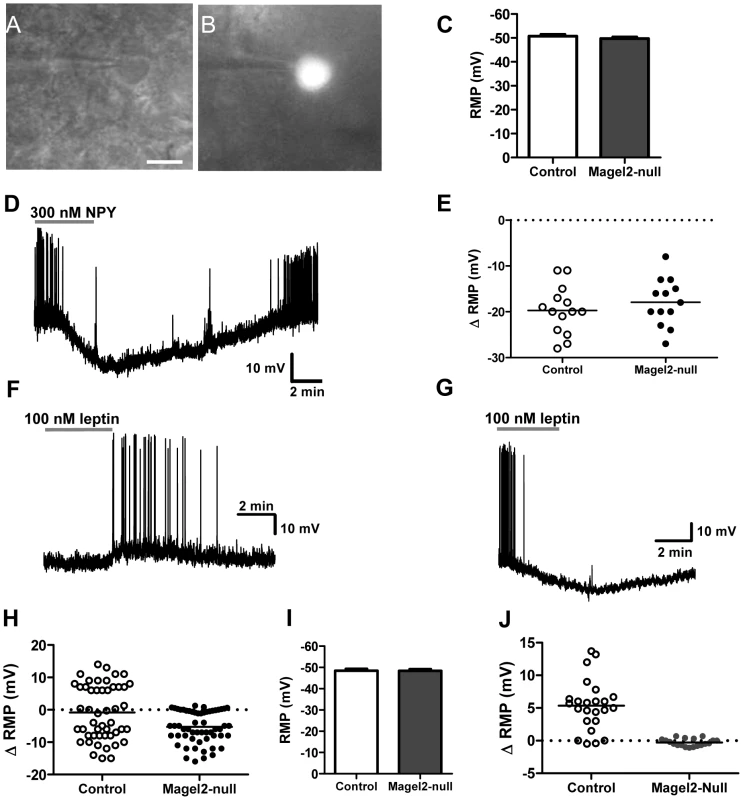
Example of a LepRbEGFP positive neuron identified for electrophysiological recordings using: A) infrared-differential interference contrast imaging (scale bar, 10 µm) and B) epifluorescence. C) Mean RMP of ARC LepRb+ neurons (n>50 of each genotype, values are means ± SEM). D) Current clamp recording of a LepRbEGFP neuron showing the hyperpolarizing effect of 300 nM NPY. E) There was no difference in the magnitude of hyperpolarization between control and Magel2-null neurons treated with 300 nM NPY. F–H) Current clamp recordings of typical responses to 100 nM leptin in F) depolarizing neurons (ΔRMP>2 mV over baseline), and G) hyperpolarizing neurons (ΔRMP>2 mV below baseline). H) Changes in RMP with application of 100 nM leptin to ARC LepRbEGFP neurons. Circles represent individually tested neurons. Depolarizing, hyperpolarizing, and unresponsive neurons were found in control slices, while only hyperpolarizing and unresponsive neurons were found in Magel2-null slices. Difference between genotypes is significant by Fishers Exact Test, P<10−8. I) Mean RMP of ARC POMCEGFP neurons (n>40 of each genotype, means ± SEM). J) Changes in RMP caused by application of 100 nM leptin to ARC POMCEGFP neurons. Depolarizing responses were observed in control but not Magel2-null neurons. Difference between genotypes in the number of depolarizing neurons is significant by Fishers Exact Test, P<10−8. To more directly examine the population of neurons specifically activated by leptin in the ARC, we identified POMC neurons using crosses with mice expressing GFP in POMC cells (Magel2×POMCEGFP and littermate controls). As with LepRb+ neurons in the ARC, POMC+ neurons from control and Magel2-null animals did not differ in their RMP (Figure 5I). We then tested leptin (100 nM) responses in POMC+ cells located in the posterior and medial regions of the ARC, where a large number of POMC neurons are leptin-sensitive [32]. Leptin induced a depolarization in the majority of POMC neurons from control mice, but no depolarizing effects were seen in response to leptin in POMC neurons of Magel2-null mice. This confirms that POMC neurons in these animals are insensitive to the acute administration of leptin (Figure 5J).
In addition to the ARC POMC neurons, many other neurons in the hypothalamus are depolarized by leptin [36], [37]. To determine the specificity of the effect of Magel2 loss on depolarizing actions mediated by the leptin receptor, we studied leptin responses in the ventromedial hypothalamic nucleus, which comprise both depolarizing and hyperpolarizing responses [38]. The serial microscope sections stained for pSTAT3 used in the experiments on ARC above were re-imaged for the VMN using confocal microscopy, and pSTAT3-positive neurons were counted. Leptin treatment caused an increase in numbers of neurons immunopositive for pSTAT3, but in contrast to results for ARC, no significant differences in numbers of pSTAT3 neurons were seen in the Magel2-null animals compared to controls (Figure 6A). Electrophysiological recordings were made from neurons in the dorsomedial and central VMN as described [39] in slices prepared from Magel2-null×LepRbEGFP and LepRbEGFP control animals. In VMN from control LepRbEGFP mice, we observed a mixture of depolarizing and hyperpolarizing responses to 100 nM leptin, along with unresponsive neurons. In contrast to the results in ARC, there were no significant differences in the numbers of neurons depolarized or hyperpolarized by leptin (Figure 6B). Thus, leptin-mediated depolarization of VMN neurons is unaffected by the loss of Magel2.
Fig. 6. Magel2 is not required for leptin-mediated responses in the VMN. 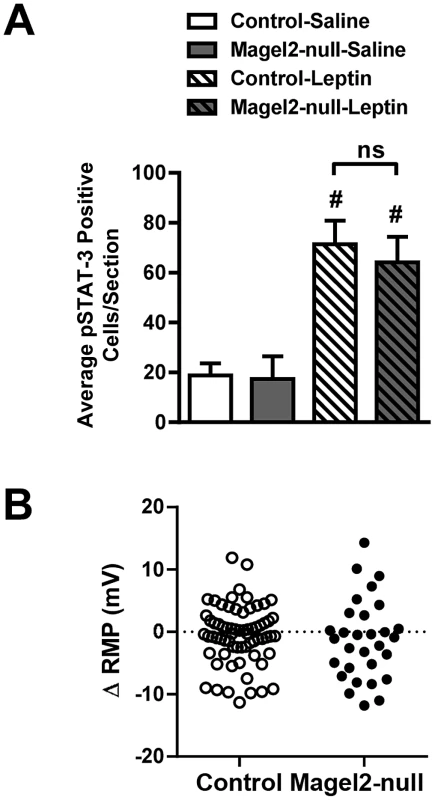
A) While injection of leptin induced a significant increase in pSTAT3 immunoreactivity compared with saline throughout the entire VMN both in control and Magel2-null mice, there was no difference between genotypes in cell numbers demonstrating pSTAT3 immunoreactivity in response to leptin injection. Values are means ± SEM, # P<0.05, compared within genotypes for saline vs. leptin treatment (Student's t-test). B) Changes in RMP with application of 100 nM leptin to VMN LepRbEGFP neurons. Data points represent individually tested neurons (total n = 68 in control, n = 30 in Magel2-null). Depolarizing, hyperpolarizing, and unresponsive neurons were found in both control and Magel2-null slices. There was no significant difference between genotypes in the populations of depolarizing or hyperpolarizing responses to leptin application (Fishers Exact Test, P>0.2). Magel2-Null Mice Are Hypersensitive to the Melanocortin Agonist MT-II
A failure of POMC neurons to depolarize in response to leptin application is predicted to cause loss of α-MSH release. In other animal models of leptin insensitivity, an enhanced response to the direct application of either α-MSH or the synthetic melanocortin agonist MT-II is observed [40]–[42]. We therefore examined the effect of MT-II on food intake in Magel2-null mice. Mice were fasted for 24 h, and then injected with MT-II (2.5 mg/kg ip). Compared to PBS-injected control fasted mice, MT-II-injected control fasted mice consumed 50% less food over the first 2 h of refeeding. After this time, there was no significant difference in food intake between control mice injected with MT-II or PBS. In contrast, Magel2-null mice injected with MT-II had a greater reduction in food intake compared to PBS injection, and this decrease was still evident after 24 h (Figure 7). This result suggests that the melanocortin system is chronically upregulated in Magel2-null mice, likely as a result of the loss of melanocortinergic tone from ARC POMC neurons.
Fig. 7. Effects of intraperitoneal MT-II on food intake. 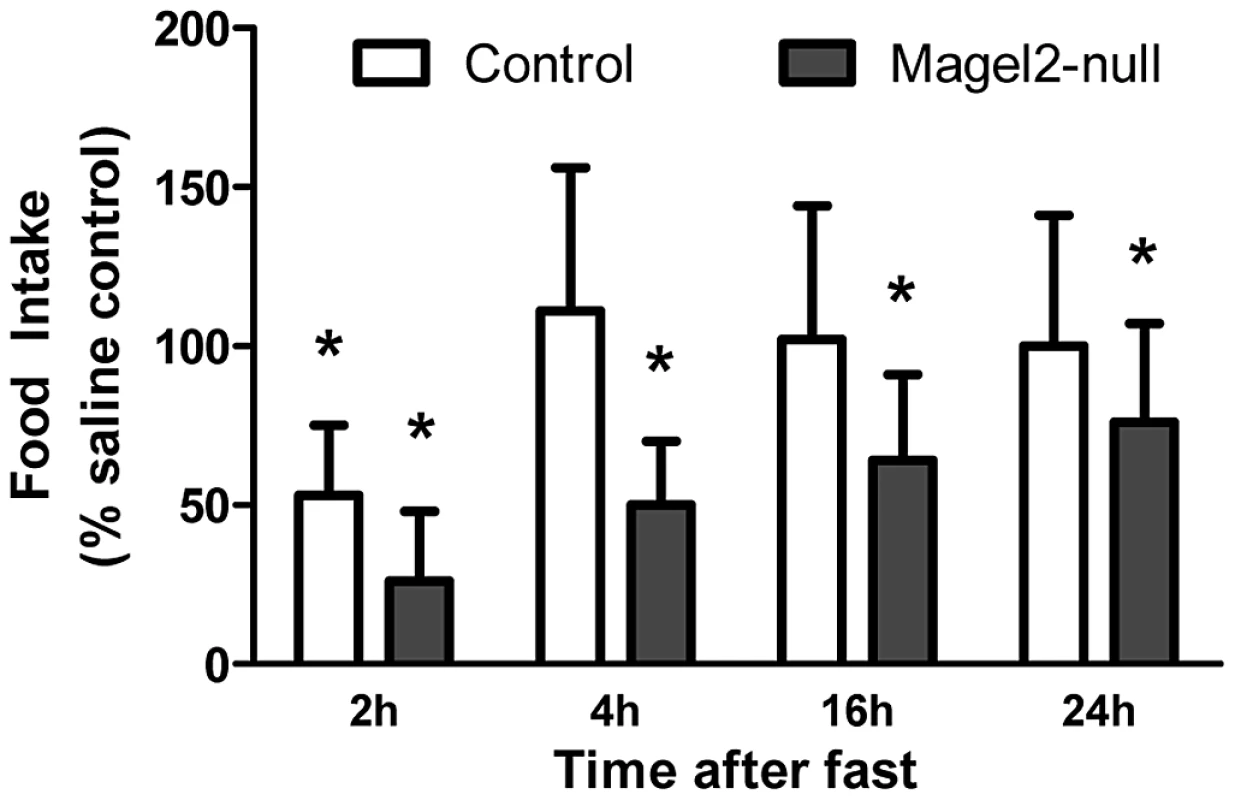
Food intake following 2.5 mg/kg injection of MT-II or saline in 24 h fasted mice. MT-II significantly reduced food intake for the first 2 h of refeeding in control mice, but this effect was no longer present by 4 h. In Magel2-null mice, MT-II reduced food intake to a greater extent and this effect was still evident 24 h after injection. n = 6 mice of each genotype. Values are means ± SEM. *P<0.05, compared to saline-injected (100%) food intake within each genotype by Student's t-test. Discussion
Mice lacking Magel2 have increased adiposity with proportionately increased leptin, suggesting leptin insensitivity [18], [43]. Here, we show that Magel2-null mice are physiologically resistant to the effects of exogenously applied leptin, both before and after the onset of increased adiposity. Further, this leptin resistance is accompanied by a 39% reduction in the number of POMC neurons in the ARC, and by a complete absence of leptin-induced depolarization responses in the remaining POMC neurons. Magel2 is therefore essential for normal leptin signaling in POMC neurons, and for the differentiation, proliferation, or survival of this population of neurons. Interestingly, the effect of Magel2 loss on leptin-mediated depolarization is not universal, even within the hypothalamus, as equivalent numbers of energy balanced-related VMN neurons not only exhibit pSTAT3 immunoreactivity, but also equal numbers are depolarized in the Magel2-null animals. Loss of POMC neuronal activation is accompanied by an exaggerated anorexigenic response to exogenous melanocortins, suggesting a compensatory upregulation of downstream melanocortin response pathways in Magel2-null mice. The role of MAGEL2 in melanocortin-associated neuronal pathways may provide important insights into dysfunctional ingestive behavior and obesity in Prader-Willi syndrome.
Insensitivity to peripheral leptin has been demonstrated in diet-induced and genetic models of obesity [44]–[47]. In principle, a failure to respond to acutely or chronically elevated leptin could be caused by reduced transport across the blood brain barrier, or by an intrinsic defect in leptin-responsive neurons. In the latter case, leptin insensitivity could be caused by failure of leptin either to inhibit the orexigenic drive (at NPY neurons), or to activate the anorexigenic drive (through POMC neurons), or both mechanisms, as in congenital leptin insensitivity in mice carrying an inactive form of the leptin receptor (LepRdb mice). Although the anorexic response to peripherally administered leptin is absent in the Magel2-null mice, the electrophysiology results demonstrate that many arcuate hypothalamic neurons that express the leptin receptor remain leptin-sensitive. Specifically, Magel2-null ARC slices have a similar proportion of neurons displaying inhibitory responses to leptin as do slices from control animals, and these responses are of similar amplitude. Moreover, the remaining POMC neurons retain sensitivity to NPY, so the loss of the leptin-mediated excitatory response is not indicative of a global cellular defect within the ARC. This retention of leptin-mediated inhibitory responses is consistent with the modest level of obesity in Magel2-null mice compared with leptin-deficient Lepob or leptin receptor null Leprdb mice. We did not test the response of VMN neurons to NPY in Magel2-null animals here.
Several mouse strains have been constructed in which leptin signaling pathways are selectively impaired in POMC neurons. Mice engineered without leptin receptor expression only in POMC neurons are mildly obese, with a significant increase in fat mass [48], [49], similar in magnitude to that previously reported in Magel2-null mice [18]. A similar degree of obesity and adiposity is seen in mice with inactivation of STAT3 in POMC neurons [23]. Unlike the Magel2-null mice, the POMC-STAT3 mutants remain sensitive to peripheral leptin, but they display defects in compensatory refeeding following food deprivation leading to reduced weight regain, similar to what we have observed in Magel2-null mice. Though the largely glutamatergic neurons of the VMN [50] remain leptin-responsive in the mutants, the loss of leptin signaling in other hypothalamic neurons in Magel2-null mice could underlie their more severe insensitivity to peripherally administered leptin. Rapid effects of leptin action on ARC leptin receptors have been linked to increased phosphatidyl inositol-3-kinase (PI3K) signaling [51], [52]. Accordingly, pharmacological blockade of PI3K signaling inhibits leptin-induced activation of POMC neurons [53]. Targeted deletion of PI3K signaling in POMC neurons also eliminates leptin-induced activation of POMC neurons, and significantly blunts the reduction in food intake provoked by intracerebroventricular leptin administration [53]. Interestingly, these mice do not appear to have any defects in weight gain or body composition, though a different strategy aimed at the downregulation of PI3K in POMC neurons does lead to a modest obesity phenotype and increased sensitivity to diet-induced obesity [54]. Investigations of a possible role of Magel2 in PI3K signaling are thus warranted.
The complete absence of a physiological response to leptin in Magel2-null mice could have several causes. First, the Magel2-null mice catch up in weight compared to control and start accumulating excessive fat mass after weaning onto a standard chow diet, albeit at a modest rate. The resulting hyperleptinemia could contribute to systemic leptin resistance through a mechanism unrelated to or secondary to defective POMC neuron activation, but in any event caused ultimately by loss of Magel2 function. Secondly, only half the normal number of ARC neurons expressed pSTAT3 in the ARC of Magel2-null mice after peripheral leptin treatment, and fewer Magel2-null neurons were activated by leptin as measured by c-fos expression. Third, Magel2-null mice had fewer POMC ARC neurons, and the remaining POMC neurons were not activated by leptin. The loss of excitatory leptin signaling at POMC neurons and their increased adiposity are consistent with a loss of key actions of leptin at ARC POMC and potentially other neurons in the Magel2-null mice [48], [49]. While our findings demonstrate a crucial role for POMC in the Magel2-null phenotype, the ARC contains a heterogeneous population of leptin-activated neurons it remains possible that the leptin-mediated activation of these neurons is also affected by loss of Magel2 [20].
Intracellular responses to leptin receptor activation are mediated by a complex signaling cascade in POMC neurons [55], and this process is similar but not identical in other leptin-responsive neurons. For example, in leptin-activated neurons in the ventromedial nucleus (VMN), some neurons depolarize in response to leptin, some cells hyperpolarize, and the majority of cells do not respond to leptin administration [39], [56], [57]. The identical rates of leptin responsiveness in VMN of Magel2-null and control mice indicates that Magel2 is required for depolarizing responses in some neuronal subtypes but not in others. Likewise, the relative increase in the number of neurons expressing pSTAT3 in the VMN of leptin-injected compared to saline injected mice did not differ between genotypes.
Fasting in rodents induces a state of negative energy balance that is reflected by dramatic decreases in circulating leptin levels [1], [58], [59] and compensatory hyperphagia on re-feeding. Deficiencies in fasting-induced hyperphagia and compensatory weight gain are found in models of POMC neuron degeneration or in POMC-specific STAT3 mutant mice [22], [23]. Thus, appropriate regulation of POMC neurons in the ARC is critical to normal responses to food deprivation, which are clearly impaired in Magel2-null mice. Other hypothalamic pathways could also contribute to dysfunctional feeding behavior in Magel2-null mice. For example, orexin neurons normally activate NPY and inhibit POMC neurons to stimulate increases in food intake [60], and ablation of orexin neurons in the lateral hypothalamus causes a loss of fasting-induced arousal and defense of body weight during fasting [61]. In fact, Magel2-null mice have fewer orexin neurons and a significant reduction in hypothalamic levels of orexin-A [43], [62], which could contribute to the impaired compensatory hyperphagic responses in the Magel2 null mice. In addition, there may be developmental defects in axonal outgrowth and synaptic contacts with other neurons in the remaining POMC, orexin, and other neuronal subtypes that require Magel2 developmentally, further impairing their leptin-mediated excitability.
Notably, the anorexic response to melanocortins is intact and hyperactivated in Magel2-null mice, suggesting that melanocortin receptors in the paraventricular nucleus and elsewhere in the central nervous system are not impaired by loss of Magel2. Further examination of melanocortin responsiveness in Magel2-null mice could provide compelling evidence for potential therapeutic intervention in PWS. The exact biochemical roles of Magel2 and how it participates in neuronal differentiation and/or survival as well as cellular activation in response to leptin remain to be determined. In summary, our results demonstrate that Magel2 is critical for leptin responses in POMC neurons in the ARC and for energy homeostasis in mice. Further experiments are required to determine whether this defect is degenerative in nature or whether mice lacking Magel2 are congenitally leptin insensitive. It will also be important to address whether loss of MAGEL2 in people with PWS likewise contributes to disrupted ingestive behavior and energy homeostasis in this disorder.
Materials and Methods
Mouse Strains
All animal procedures were approved by the University of Alberta Animal Care and Use Committee in accordance with the guidelines of the Canadian Council on Animal Care. Mice were weaned between 3–4 weeks of age and then group housed 3–5 per cage with food (PicoLab Rodent Diet 5001) and water ad lib., and housed under a 12∶12 light∶dark cycle.
Magel2-null mice were generated [43] and housed [19] as described, and are available from the Jackson Laboratories (C57BL/6-Magel2tm1Stw/J, stock 009062). To identify specific neuronal populations, Magel2−m/+p carrier males were crossed with homozygous LepRbEGFP reporter mice, which express enhanced green fluorescent protein (EGFP) in LepRb+ cells [63], or homozygous POMCEGFP reporter mice, expressing EGFP in POMC+ cells (The Jackson Laboratory stock #009593, Bar Harbor, Maine) [33]. This cross produces Magel2×LepRbEGFP or Magel2×POMCEGFP mice, lacking Magel2 but expressing LepRbEGFP or POMCEGFP, and control littermates expressing wildtype Magel2 and the reporter gene.
Food Withdrawal and Refeeding
Male (12–16 weeks) mice were singly housed for at least one week, then weighed and fasted for 48 h beginning at 1600 h. Body weight was recorded 24 h and 48 h later, and food intake and body weight change were measured during 3 days of refeeding.
Leptin and Melanocortin Sensitivity
Mice were singly housed for at least one week before the start of the experiment. One week before drug injections, mice were injected daily for 3 days with phosphate buffered saline, pH 7.3 (PBS). Body weight and food intake were measured during this time. On experimental day 1, food was removed at 1500 h and mice injected intraperitoneally (ip) with either 2.5 mg/kg mouse recombinant leptin (Dr. A.F. Parlow, National Hormone and Peptide Program, NHPP-NIDDK, Torrance, California), 2.5 mg/kg synthetic melanocortin agonist melanotan-II (MT-II Phoenix Pharmaceuticals, Burlingame, California) [64], or PBS at 1600 h. Food intake was measured 2–24 h later. After a 3 day recovery, the experiment was repeated using a cross-over design.
Leptin Stimulation and Immunohistochemistry
Adult (12–16 weeks) mice were handled daily for 2 weeks, including one week of PBS injections, to minimize injection-related c-fos responses in the brain. Mice were then injected ip with either 2.5 mg/kg leptin or PBS 45 min before terminal pentobarbital anesthesia, paraformaldehyde perfusion, and preparation of coronal 30 µm hypothalamic sections for immunohistochemistry (IHC). For pSTAT3 IHC, sections were pretreated with 1% NaOH and 1% H202 in H20 for 20 min, 0.3% glycine in PBS for 10 min, and 0.03% sodium dodecyl sulfate in PBS for 10 min, blocked for 1 h with 3% normal goat serum in PBS/0.3% Triton X-100, then incubated overnight with anti-pSTAT3 antibody (1∶1000, 9131, Cell Signaling, Danvers, Massachusetts). For c-fos and POMC/EGFP IHC, sections were washed in PBS, blocked for 30 min in 3% normal serum in PBS/0.3% Triton X-100, then incubated for 48 h with primary antibody (c-fos (Ab-5), 1∶2000, PC-38, Millipore, Billerica, Massachusetts; POMC, anti-GFP, 1∶4000, ab13970, AbCam, Cambridge, Massachusetts). After primary incubation, sections were washed with PBS/0.3% Triton X-100 and incubated for 2 h with goat anti-rabbit secondary antibody (Alexa Fluor 594) or goat anti-chicken secondary antibodies (Alexa Fluor 488) (1∶500, Invitrogen, Carlsbad, California), slide-mounted then imaged using a Zeiss LSM510 confocal microscope. For cell counting in the arcuate nucleus, sections were organized in a rostral to caudal manner through the hypothalamus according to the mouse brain atlas (www.mbl.org). Cells were counted using MetaMorph Imaging Suite (Molecular Devices, Sunnyvale, California) for pSTAT3, and Image J (National Institutes of Health, Bethesda, Maryland) for c-fos and GFP.
Brain Slice Preparation and Electrophysiology
Brains from 6–12 week old male and non-estrous female reporter mice were prepared for patch clamp electrophysiology [39]. Slices were incubated for at least 1 h at room temperature in carbogenated artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 3 KCl, 1.3 MgSO4, 1.4 NaH2PO4, 2.5 glucose, 7.5 sucrose, 26 NaHCO3 and 2.5 CaCl2 (300–305 mOsm/L). For electrophysiology, slices were continuously perfused (2–4 ml/min) with warm (32–34°C), carbogenated aCSF. Cells expressing GFP were identified by epifluorescence illumination, then the light source was switched to infrared-differential interference contrast imaging to obtain whole-cell recordings. Visualized-patch whole-cell recordings were obtained using thin-walled glass patch pipettes with resistances of 5–7 MΩ when backfilled with an internal solution containing (in mM): 126 K-gluconate, 4 KCl, 10 HEPES, 5 MgATP, 0.3 NaGTP, 1 EGTA, 0.3 CaCl2 and 0.02% neurobiotin (pH adjusted to 7.25 with KOH, 280 mOsm/L). Stock solutions were prepared in PBS, pH 7.8 (leptin) or HPLC grade water (human NPY, Peptidec Technologies Ltd., Pierrefonds, Quebec, Canada), then diluted into aCSF immediately before use, and gravity-perfused into the recording chamber for at least 3 min. Slices were washed with aCSF for at least 10 min between drugs. A stable and reversible change in membrane potential of at least 2 mV from baseline appearing within minutes after drug application was considered a valid pharmacological response.
Statistical Analysis
Statistical analyses were performed using a Student's unpaired t-test or a Fisher's Exact Test (GraphPad, La Jolla, California), with differences with P<0.05 after correction for multiple t-testing considered significant.
Zdroje
1. AhimaRS, LazarMA (2008) Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22 : 1023–1031.
2. GrahamM, ShutterJR, SarmientoU, SarosiI, StarkKL (1997) Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet 17 : 273–274.
3. OllmannMM, WilsonBD, YangYK, KernsJA, ChenY, et al. (1997) Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278 : 135–138.
4. TiesjemaB, la FleurSE, LuijendijkMC, AdanRA (2009) Sustained NPY overexpression in the PVN results in obesity via temporarily increasing food intake. Obesity 17 : 1448–1450.
5. MortonGJ, CummingsDE, BaskinDG, BarshGS, SchwartzMW (2006) Central nervous system control of food intake and body weight. Nature 443 : 289–295.
6. YaswenL, DiehlN, BrennanMB, HochgeschwenderU (1999) Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5 : 1066–1070.
7. KrudeH, BiebermannH, SchnabelD, TansekMZ, TheunissenP, et al. (2003) Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab 88 : 4633–4640.
8. FarooqiIS, DropS, ClementsA, KeoghJM, BiernackaJ, et al. (2006) Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes 55 : 2549–2553.
9. MontagueCT, FarooqiIS, WhiteheadJP, SoosMA, RauH, et al. (1997) Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387 : 903–908.
10. ClementK, VaisseC, LahlouN, CabrolS, PellouxV, et al. (1998) A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392 : 398–401.
11. FarooqiIS, KeoghJM, YeoGS, LankEJ, CheethamT, et al. (2003) Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348 : 1085–1095.
12. SeoS, GuoDF, BuggeK, MorganDA, RahmouniK, et al. (2009) Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet 18 : 1323–1331.
13. CassidySB, DriscollDJ (2009) Prader-Willi syndrome. Eur J Hum Genet 17 : 3–13.
14. LeeS, KozlovS, HernandezL, ChamberlainSJ, BrannanCI, et al. (2000) Expression and imprinting of MAGEL2 suggest a role in Prader-Willi syndrome and the homologous murine imprinting phenotype. Hum Mol Genet 9 : 1813–1819.
15. DoyleJM, GaoJ, WangJ, YangM, PottsPR (2010) MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell 39 : 963–974.
16. LeeS, WalkerCL, WevrickR (2003) Prader-Willi syndrome transcripts are expressed in phenotypically significant regions of the developing mouse brain. Gene Expr Patterns 3 : 599–609.
17. MercerRE, KwolekEM, BischofJM, van EedeM, HenkelmanRM, et al. (2009) Regionally reduced brain volume, altered serotonin neurochemistry, and abnormal behavior in mice null for the circadian rhythm output gene Magel2. Am J Med Genet B Neuropsychiatr Genet 150B: 1085–1099.
18. BischofJM, StewartCL, WevrickR (2007) Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum Mol Genet 16 : 2713–2719.
19. MercerRE, WevrickR (2009) Loss of Magel2, a candidate gene for features of prader-willi syndrome, impairs reproductive function in mice. PLoS ONE 4: e4291 doi:10.1371/journal.pone.0004291.
20. IsraelD, ChuaSJr (2010) Leptin receptor modulation of adiposity and fertility. Trends Endocrinol. Metab 21 : 10–16.
21. BecskeiC, LutzTA, RiedigerT (2009) Blunted fasting-induced hypothalamic activation and refeeding hyperphagia in late-onset obesity. Neuroendocrinology 90 : 371–382.
22. XuAW, KaelinCB, MortonGJ, OgimotoK, StanhopeK, et al. (2005) Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol 3: e415 doi:10.1371/journal.pbio.0030415.
23. XuAW, Ste-MarieL, KaelinCB, BarshGS (2007) Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148 : 72–80.
24. Segal-LiebermanG, TromblyDJ, JuthaniV, WangX, Maratos-FlierE (2003) NPY ablation in C57BL/6 mice leads to mild obesity and to an impaired refeeding response to fasting. Am J Physiol Endocrinol Metab 284: E1131–1139.
25. PatelHR, QiY, HawkinsEJ, HilemanSM, ElmquistJK, et al. (2006) Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes 55 : 3091–3098.
26. Van HeekM, ComptonDS, FranceCF, TedescoRP, FawziAB, et al. (1997) Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99 : 385–390.
27. MunzbergH, FlierJS, BjorbaekC (2004) Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145 : 4880–4889.
28. MunzbergH, HuoL, NillniEA, HollenbergAN, BjorbaekC (2003) Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144 : 2121–2131.
29. PiperML, UngerEK, MyersMGJr, XuAW (2008) Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 22 : 751–759.
30. DragunowM, FaullR (1989) The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 29 : 261–265.
31. EliasCF, AschkenasiC, LeeC, KellyJ, AhimaRS, et al. (1999) Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23 : 775–786.
32. WilliamsKW, MargathoLO, LeeCE, ChoiM, LeeS, et al. (2010) Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30 : 2472–2479.
33. CowleyMA, SmartJL, RubinsteinM, CerdanMG, DianoS, et al. (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411 : 480–484.
34. Acuna-GoycoleaC, van den PolAN (2005) Peptide YY(3–36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis. J Neurosci 25 : 10510–10519.
35. van den TopM, LeeK, WhymentAD, BlanksAM, SpanswickD (2004) Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7 : 493–494.
36. ElmquistJK, AhimaRS, Maratos-FlierE, FlierJS, SaperCB (1997) Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138 : 839–842.
37. EliasCF, KellyJF, LeeCE, AhimaRS, DruckerDJ, et al. (2000) Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 423 : 261–281.
38. IraniBG, Le FollC, Dunn-MeynellA, LevinBE (2008) Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology 149 : 5146–5154.
39. CheeMJ, PriceCJ, StatnickMA, ColmersWF (2011) Nociceptin/orphanin FQ suppresses the excitability of neurons in the ventromedial nucleus of the hypothalamus. J Physiol 589 : 3103–3114.
40. HansenMJ, BallMJ, MorrisMJ (2001) Enhanced inhibitory feeding response to alpha-melanocyte stimulating hormone in the diet-induced obese rat. Brain research 892 : 130–137.
41. ScarpacePJ, MathenyM, ZolotukhinS, TumerN, ZhangY (2003) Leptin-induced leptin resistant rats exhibit enhanced responses to the melanocortin agonist MT II. Neuropharmacology 45 : 211–219.
42. LiG, ZhangY, WilseyJT, ScarpacePJ (2004) Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol 182 : 123–132.
43. KozlovSV, BogenpohlJW, HowellMP, WevrickR, PandaS, et al. (2007) The imprinted gene Magel2 regulates normal circadian output. Nat Genet 39 : 1266–1272.
44. El-HaschimiK, PierrozDD, HilemanSM, BjorbaekC, FlierJS (2000) Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105 : 1827–1832.
45. RahmouniK, FathMA, SeoS, ThedensDR, BerryCJ, et al. (2008) Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118 : 1458–1467.
46. PrpicV, WatsonPM, FramptonIC, SabolMA, JezekGE, et al. (2003) Differential mechanisms and development of leptin resistance in A/J versus C57BL/6J mice during diet-induced obesity. Endocrinology 144 : 1155–1163.
47. LinS, ThomasTC, StorlienLH, HuangXF (2000) Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 24 : 639–646.
48. van de WallE, LeshanR, XuAW, BalthasarN, CoppariR, et al. (2008) Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149 : 1773–1785.
49. BalthasarN, CoppariR, McMinnJ, LiuSM, LeeCE, et al. (2004) Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42 : 983–991.
50. CollinM, BackbergM, OvesjoML, FisoneG, EdwardsRH, et al. (2003) Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci 18 : 1265–1278.
51. XuAW, KaelinCB, TakedaK, AkiraS, SchwartzMW, et al. (2005) PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115 : 951–958.
52. MorrisonCD, MortonGJ, NiswenderKD, GellingRW, SchwartzMW (2005) Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289: E1051–1057.
53. HillJW, WilliamsKW, YeC, LuoJ, BalthasarN, et al. (2008) Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118 : 1796–1805.
54. HillJW, XuY, PreitnerF, FukudaM, ChoYR, et al. (2009) Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 150 : 4874–4882.
55. QiuJ, FangY, RonnekleivOK, KellyMJ (2010) Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30 : 1560–1565.
56. ShiraishiT, OomuraY, SasakiK, WaynerMJ (2000) Effects of leptin and orexin-A on food intake and feeding related hypothalamic neurons. Physiol Behav 71 : 251–261.
57. DhillonH, ZigmanJM, YeC, LeeCE, McGovernRA, et al. (2006) Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49 : 191–203.
58. AhimaRS, PrabakaranD, MantzorosC, QuD, LowellB, et al. (1996) Role of leptin in the neuroendocrine response to fasting. Nature 382 : 250–252.
59. FrederichRC, LollmannB, HamannA, Napolitano-RosenA, KahnBB, et al. (1995) Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96 : 1658–1663.
60. MuroyaS, FunahashiH, YamanakaA, KohnoD, UramuraK, et al. (2004) Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci 19 : 1524–1534.
61. YamanakaA, BeuckmannCT, WillieJT, HaraJ, TsujinoN, et al. (2003) Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38 : 701–713.
62. SchallerF, WatrinF, SturnyR, MassacrierA, SzepetowskiP, et al. (2010) A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet 19 : 4895–4905.
63. LeshanRL, LouisGW, JoYH, RhodesCJ, MunzbergH, et al. (2009) Direct innervation of GnRH neurons by metabolic - and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29 : 3138–3147.
64. HrubyVJ, LuD, SharmaSD, CastrucciAL, KestersonRA, et al. (1995) Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem 38 : 3454–3461.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



