-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
Using a phenome-wide association study (PheWAS) approach, we comprehensively tested genetic variants for association with phenotypes available for 70,061 study participants in the Population Architecture using Genomics and Epidemiology (PAGE) network. Our aim was to better characterize the genetic architecture of complex traits and identify novel pleiotropic relationships. This PheWAS drew on five population-based studies representing four major racial/ethnic groups (European Americans (EA), African Americans (AA), Hispanics/Mexican-Americans, and Asian/Pacific Islanders) in PAGE, each site with measurements for multiple traits, associated laboratory measures, and intermediate biomarkers. A total of 83 single nucleotide polymorphisms (SNPs) identified by genome-wide association studies (GWAS) were genotyped across two or more PAGE study sites. Comprehensive tests of association, stratified by race/ethnicity, were performed, encompassing 4,706 phenotypes mapped to 105 phenotype-classes, and association results were compared across study sites. A total of 111 PheWAS results had significant associations for two or more PAGE study sites with consistent direction of effect with a significance threshold of p<0.01 for the same racial/ethnic group, SNP, and phenotype-class. Among results identified for SNPs previously associated with phenotypes such as lipid traits, type 2 diabetes, and body mass index, 52 replicated previously published genotype–phenotype associations, 26 represented phenotypes closely related to previously known genotype–phenotype associations, and 33 represented potentially novel genotype–phenotype associations with pleiotropic effects. The majority of the potentially novel results were for single PheWAS phenotype-classes, for example, for CDKN2A/B rs1333049 (previously associated with type 2 diabetes in EA) a PheWAS association was identified for hemoglobin levels in AA. Of note, however, GALNT2 rs2144300 (previously associated with high-density lipoprotein cholesterol levels in EA) had multiple potentially novel PheWAS associations, with hypertension related phenotypes in AA and with serum calcium levels and coronary artery disease phenotypes in EA. PheWAS identifies associations for hypothesis generation and exploration of the genetic architecture of complex traits.
Published in the journal: Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network. PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003087
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003087Summary
Using a phenome-wide association study (PheWAS) approach, we comprehensively tested genetic variants for association with phenotypes available for 70,061 study participants in the Population Architecture using Genomics and Epidemiology (PAGE) network. Our aim was to better characterize the genetic architecture of complex traits and identify novel pleiotropic relationships. This PheWAS drew on five population-based studies representing four major racial/ethnic groups (European Americans (EA), African Americans (AA), Hispanics/Mexican-Americans, and Asian/Pacific Islanders) in PAGE, each site with measurements for multiple traits, associated laboratory measures, and intermediate biomarkers. A total of 83 single nucleotide polymorphisms (SNPs) identified by genome-wide association studies (GWAS) were genotyped across two or more PAGE study sites. Comprehensive tests of association, stratified by race/ethnicity, were performed, encompassing 4,706 phenotypes mapped to 105 phenotype-classes, and association results were compared across study sites. A total of 111 PheWAS results had significant associations for two or more PAGE study sites with consistent direction of effect with a significance threshold of p<0.01 for the same racial/ethnic group, SNP, and phenotype-class. Among results identified for SNPs previously associated with phenotypes such as lipid traits, type 2 diabetes, and body mass index, 52 replicated previously published genotype–phenotype associations, 26 represented phenotypes closely related to previously known genotype–phenotype associations, and 33 represented potentially novel genotype–phenotype associations with pleiotropic effects. The majority of the potentially novel results were for single PheWAS phenotype-classes, for example, for CDKN2A/B rs1333049 (previously associated with type 2 diabetes in EA) a PheWAS association was identified for hemoglobin levels in AA. Of note, however, GALNT2 rs2144300 (previously associated with high-density lipoprotein cholesterol levels in EA) had multiple potentially novel PheWAS associations, with hypertension related phenotypes in AA and with serum calcium levels and coronary artery disease phenotypes in EA. PheWAS identifies associations for hypothesis generation and exploration of the genetic architecture of complex traits.
Introduction
Phenomic approaches are complementary to the more prevalent paradigm of genome-wide association studies (GWAS), which have provided some information about the contribution of genetic variation to a wide range of diseases and phenotypes [1]. While a typical GWAS evaluates the association between the variation of hundreds of thousands, to over a million, genotyped single nucleotide polymorphisms (SNPs) and one or a few phenotypes, a common limitation of GWAS is the focus on a pre-defined and limited phenotypic domain. An alternate approach is that of PheWAS, which utilizes all available phenotypic information and all genetic variants in the estimation of associations between genotype and phenotype [1]. By investigating the association between SNPs and a diverse range of phenotypes, a broader picture of the relationship between genetic variation and networks of phenotypes is possible.
A challenge for PheWAS is the availability of large studies with genotypic data that are also linked to a wide array of high quality phenotypic measurements and traits for study. Biorepositories linked to electronic medical records (EMR) have been an initial resource for PheWAS, but these EMR-based studies are often limited to phenotypes and traits commonly collected for clinical use and may represent sets of limited racial/ethnic diversity [2], [3]. While there is no U.S. national, population-based cohort [4], several diverse, population-based studies exist with tens of thousands of samples linked to detailed survey, laboratory, and medical data. These large population-based studies have limitations [5], but collectively [6] they offer an opportunity to perform a PheWAS of unprecedented size and diversity.
To capitalize on the potential for collaborative discovery among some of the large population-based studies of the U.S., the National Human Genome Research Institute (NHGRI) funded the Population Architecture using Genomics and Epidemiology (PAGE) network. PAGE includes eight extensively characterized, large population-based epidemiologic studies where data were collected across multiple racial/ethnic groups, supported by a coordinating center [7], providing an exceptional opportunity to pursue PheWAS with a large number of SNPs, and thousands of phenotypic measurements including a wide range of common diseases, risk factors, intermediate biomarkers and quantitative traits in diverse populations. Herein, we illustrate the feasibility and utility of the PheWAS approach for large population-based studies and demonstrate that PheWAS provides information on, and exposes the complexity of, the relationship between genetic variation and interrelated and independent phenotypes. We have found PheWAS results that replicate previously identified genotype-phenotype associations with the exact phenotype in previous associations or closely related phenotypes, as well as a series of novel genotype-phenotype associations. This data exploration method exposes a more complete picture of the relationship between genetic variation and phenotypic outcome. PheWAS provides the unbiased, high throughput design achieved by GWAS in the genome and phenotype domains simultaneously. This approach changes the paradigm of phenotypic characterization and allows for exploratory research in both genomics and phenomics.
Results
Data from five PAGE study sites were available for this PheWAS: Epidemiologic Architecture for Genes Linked to Environment (EAGLE) using data from the National Health and Nutrition Examination Surveys (NHANES); the Multiethnic Cohort Study (MEC); the Women's Health Initiative (WHI); and two studies of the Causal Variants Across the Life Course (CALiCo) group: the Cardiovascular Health Study (CHS) and Atherosclerosis Risk in Communities (ARIC). Text S1 provides full information on study design, phenotype measurement, and genotyping for each study. These studies collectively include four major racial/ethnic groups: European Americans (EA), African Americans (AA), Hispanics/Mexican Americans (H), and Asian/Pacific Islanders (API). All PAGE study sites included both males and females, except for WHI (which includes only women). Table 1 provides an overview of the sample sizes by PAGE study site as well as the number of SNPs and phenotypes available for this PheWAS. Sample size and the number of phenotypes varied across studies, and the sample size for various phenotypes within each study varied dependent on the number of individuals for which a given phenotype was measured. The number of phenotypes available for this PheWAS ranged within studies from 63 (MEC) to 3,363 (WHI). Study sites also had differing numbers of genotyped SNPs, and Table S1 contains the list of all SNPs available for two or more sites in this study, arranged by previously associated phenotypes. The PAGE network has focused on characterization of well-replicated variants across multiple race/ethnicities, so each study independently genotyped a set of SNPs with previously reported associations with phenotypes such as body mass index, C-reactive protein, and lipid levels.
Tab. 1. Study Descriptions. 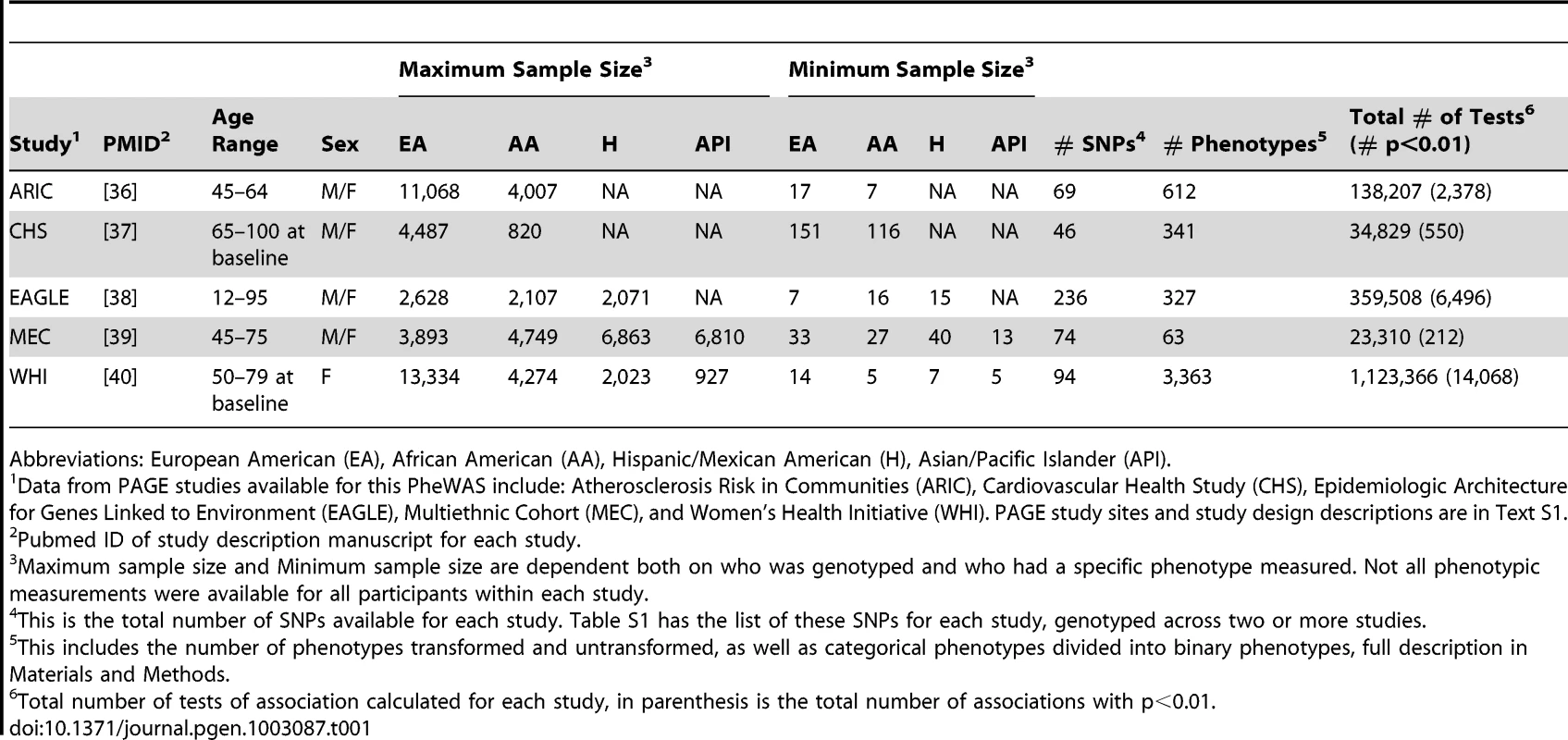
Abbreviations: European American (EA), African American (AA), Hispanic/Mexican American (H), Asian/Pacific Islander (API). Tests of association assuming an additive genetic model were performed independently by each PAGE study site for each SNP and each phenotype, stratified by race/ethnicity. The last column of Table 1 presents the total number of comprehensive associations with and without a p-value cutoff of 0.01, showing the proportion of significant results for this many tests of association. The total number of tests of association ranged from >20,000 (MEC) to >1 million (WHI) reflecting the variability in both the number of phenotypes available for study as well as the number of SNPs genotyped by each PAGE study site. As expected, the total number of significant tests of association (p<0.01) represented a fraction of the total number of tests performed.
Results from these tests of association were then compared across study sites to identify overlapping significant associations, as these results most likely represent robust findings. To facilitate determining overlapping significant associations, similar phenotypes that existed across more than one study were binned into 105 distinct phenotype-classes. For some phenotypes, the specific phenotype existed across more than one PAGE study, such as for the phenotype “Hemoglobin”, where hemoglobin measurements were available for ARIC, CHS, EAGLE, and WHI. Other groups of phenotypes binned within phenotype-classes were within similar phenotypic domains but were not represented in exact same form across studies. Table S2 contains a list of the study level phenotypes, the study from which the phenotype is available, and the phenotype-class for each phenotype that overlapped with another study.
The same or similar phenotypes may or may not have been collected by each PAGE study. Thus, the number of studies that were available for comparison of results across studies varied from one phenotype-class to another phenotype-class. Table 2 presents the number of results where at least two of five independent studies had SNP-phenotype associations with p<0.01 for single phenotype-class and single race/ethnicity group, compared to the total number of SNP-phenotype association tests performed. For example, >8,500 tests of association for the same SNP and same phenotype were available from two PAGE study sites whereas only 906 and 58 tests of association were available from four and five PAGE study sites, respectively. There were 3 results where two or more of the groups had a SNP–phenotype association p<0.01 for a single phenotype class across 5 groups represented.
Tab. 2. The number of SNP–Phenotype tests of association for phenotype-classes varies by PAGE study site genotype and phenotype overlap. 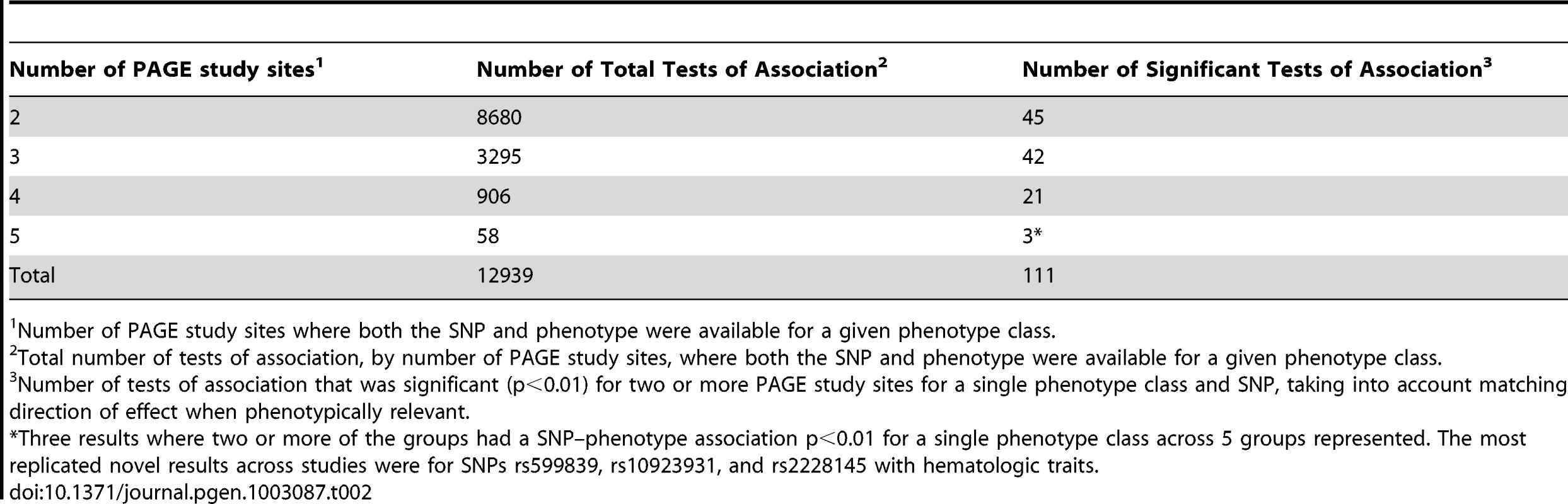
Number of PAGE study sites where both the SNP and phenotype were available for a given phenotype class. For this PAGE-wide PheWAS, tests of association were considered significant across PAGE study sites where two or more phenotypes in the same phenotype-class in the same racial/ethnic group passed a significance threshold of p<0.01 with a consistent direction of genetic effect. Based on these criteria, a total of 111 PheWAS associations were identified (Table S3). Overall, among the 111 significant PheWAS associations identified, 52 PheWAS results replicated previously published genotype-phenotype associations (Table S4), 26 represented phenotype-classes closely related to previously known genotype-phenotype associations (Table 3), and 33 represented novel genotype-phenotype associations (Table 4).
10.1371/journal.pgen.1003087.t003 Table 3PheWAS Tests of Association: Related Associations.
Nearest Gene SNP ID CA1, (CAF2) Phenotypes3 Associated Phenotype Class4 Ethnicity5 P-Values6 Beta(SE)7 Sample Size8 Substudies9 Substudy Count10 Previously Associated Phenotype11 References12 CELSR2, PSRC1 RS599839 A (0.8) Ever hospitalized for chest pain? (Y/N) ANGINA EA 2.57e-3 0.55(0.18) 568 ARIC 3 Coronary Artery Disease, LDL cholesterol, Total cholesterol 18262040, 18193043, 18179892, 17634449, 18193044 Angina status at baseline (Y/N) 8.79e-3 0.21(0.08) 4482 CHS Angina (Y/N) 2.10e-4 0.25(0.07) 13308 WHI Ever had pain/discomfort in your chest (Y/N) 1.06e-4 0.21(0.05) 4477 CHS CDKN2A, CDKN2B RS1333049 C (0.5) Chest pain or discomfort? (Y/N) ANGINA EA 4.69e-3 0.08(0.03) 9338 ARIC 3 Coronary Artery Disease, Type 2 Diabetes, Hypertension 17554300, 17634449 See a doctor because of chest pain? (Y/N) 8.80e-3 0.32(0.12) 572 ARIC Ever had pain/discomfort in your chest (Y/N) 9.72e-3 0.11(0.04) 4472 CHS Angina (Y/N) 7.75e-3 0.14(0.05) 13331 WHI LPL RS6586891 A (0.6) LN1 Total triglycerides in mmol/l Serum Triglycerides EA 7.76e-7 −0.02(4.75e-3) 9326 ARIC 3 HDL Cholesterol 18193043 Triglyceride (mg/dl) 3.45e-3 −5.18(1.77) 4469 CHS LN1 core triglyceride (mg/dl) 6.68e-3 −0.06(0.02) 922 WHI Total Triglycerides (mmol/l) 3.50e-4 −0.06(0.02) 9326 ARIC LN1 Triglycerides (mg/dl) 2.45e-3 −0.03(9.65e-3) 4469 CHS CDKN2A, CDKN2B RS10757278 A (0.5) Ever had pain/discomfort in your chest (Y/N) ANGINA EA 5.96e-3 −0.12(0.04) 4474 CHS 3 Myocardial infarction 17478679 Angina (Y/N) 6.59e-3 −0.14(0.05) 13313 WHI Chest pain or discomfort? (Y/N) 9.73e-3 −0.07(0.03) 11047 ARIC See a doc because of chest pain? (Y/N) 4.44e-3 −0.31(0.11), 722 ARIC Rose angina (Y/N) 8.17e-3 0.16(0.06) 11040 ARIC LDLR RS6511720 G (0.9) Total cholesterol (mmol/l) Serum Cholesterol EA 6.84e-16 0.18(0.02) 10891 ARIC 2 LDL Cholesterol 18193043 Total cholesterol (mg/dl) 2.64e-5 12.05(2.85) 924 WHI LN1 Total cholesterol (mmol/l) 4.15e-17 0.03(3.30e-3) 10891 ARIC High cholesterol requiring pills ever (Y/N) 10.00e-12 0.42(0.06) 12431 WHI LN1 Total cholesterol (mg/dl) 1.87e-5 0.05(0.01) 924 WHI PCSK9 RS11591147 G (0.99) Total cholesterol (mmol/l) Serum Cholesterol EA 1.13e-16 0.46(0.06) 10833 ARIC 2 LDL Cholesterol, lipids 18193044 Total cholesterol (mg/dl) 8.47e-3 18.62(7.06) 924 WHI LN1 total cholesterol (mg/dl) 4.67e-3 0.09(0.03) 924 WHI High cholesterol requiring pills ever (Y/N) 4.72e-6 0.92(0.20) 12419 WHI LN1 total cholesterol (mmol/l) 1.59e-18 0.07(8.39e-3) 10833 ARIC APOE, APOC1, APOC4, APOC2 RS4420638 A (0.8) High cholesterol requiring pills ever (Y/N) Serum Cholesterol EA 1.18e-6 −0.22(0.04) 12430 WHI 2 LDL Cholesterol, lipids, Alzheimer's disease, Coronary Artery Disease, C-reactive protein, Sporadic late onset Alzheimer's disease, ApoB, Triglycerides, Total cholesterol 17474819, 17463246, 18193043, 18802019, 17998437, 19197348, 19567438 Total cholesterol (mmol/l) 2.31e-12 −0.14(0.02) 9323 ARIC LN1 Total cholesterol (mmol/l) 1.96e-13 −0.02(3.00e-3) 9323 ARIC High cholesterol requiring pills ever (Y/N) 1.18e-6 −0.22(0.04) 12430 WHI Total cholesterol (mmol/l) 2.31e-12 −0.14(0.02) 9323 ARIC LN1 Total cholesterol (mmol/l) 1.96e-13 −0.02(3.00e-3) 9323 ARIC TMEM18 RS6548238 C (0.8) Hip circumference cm Hip Circumference EA 5.99e-3 0.62(0.22) 4456 CHS 2 Body Mass Index 19079261 Hip circumference cm 7.12e-3 0.59(0.22) 13272 WHI LN1 hip circumference cm 6.85e-3 5.84e-3(2.16e-3) 4456 CHS LN1 hip circumference cm 3.93e-3 5.60e-3(1.94e-3) 13272 WHI TMEM18 RS6548238 C (0.9) High blood pressure ever diagnosed? Hypertension AA 7.69e-3 0.19(0.07) 3946 ARIC 2 Body Mass Index 19079261 Pills for hypertension now (Y/N) 7.17e-3 0.18(0.07) 4122 WHI Hypertension category: never hypertensive (Y/N) 1.03e-3 −0.22(0.07) 4076 WHI Hypertension ever (Y/N) 5.85e-4 0.23(0.07) 4206 WHI Pills for hypertension ever (Y/N) 6.30e-3 0.19(0.07) 4186 WHI Hypertension category: treated hypertensive (Y/N) 6.54e-3 0.19(0.07) 4076 WHI FTO RS3751812 T (0.4) Waist circumference (cm) WaistSize EA 3.88e-3 1.33(0.46) 2360 EAGLEIII 2 Body Mass Index 17658951 LN1 waist circumference cm 3.68e-8 0.01(1.99e-3) 13282 WHI LN1 waist circumference (cm) 2.73e-3 0.01(4.99e-3) 2360 EAGLEIII Waist circumference cm 2.32e-8 1.03(0.18) 13282 WHI CELSR2, PSRC1 RS599839 A (0.3) Re-calibrated HDL(3) cholesterol in mg/dl HDL AA 3.07e-3 −1.00(0.34) 3023 ARIC 2 Coronary Artery Disease, LDL cholesterol, Total cholesterol 18262040, 18193043, 18179892, 17634449, 18193044 LN1 high-density lipoprotein-3 (mg/dl) 2.55e-3 −0.03(9.54e-3) 974 WHI LN1 HDL Cholesterol in mg/dl 4.60e-3 −0.02(8.74e-3) 3024 ARIC High-density lipoprotein-2 (mg/dl) 2.94e-3 −1.09(0.36) 974 WHI LN1 re-calibrated HDL(3) cholesterol in mg/dl 2.06e-3 −0.03(9.10e-3) 3023 ARIC HDL cholesterol (mg/dl) 1.51e-3 −2.20(0.69) 982 WHI LN1 high-density lipoprotein-2 (mg/dl) 2.43e-3 −0.06(0.02) 974 WHI LN1 HDL cholesterol (mg/dl) 1.23e-3 −0.04(0.01) 982 WHI High-density lipoprotein-3 (mg/dl) 2.70e-3 −1.20(0.40) 974 WHI CDKN2A, CDKN2 RS1333049 C (0.5) Percutaneous transluminal coronary angioplasty (Y/N) Artery Treatment EA 2.93e-6 0.17(0.04) 13331 WHI 2 Coronary Artery Disease, Type 2 Diabetes, Hypertension 17554300, 17634449 Coronary bypass surgery (Y/N) 6.61e-3 0.28(0.10) 4446 CHS CDKN2A, CDKN2B RS1333049 C (0.5) Myocardial infarction status at baseline (Y/N) Cardiac EA 8.24e-3 0.17(0.06) 4477 CHS 2 Coronary Artery Disease, Type 2 Diabetes, Hypertension 17554300, 17634449 Myocardial infarction (Incident or Prevalent)) 2.03e-3 0.22(0.07) 4477 CHS Myocardial infarction (Y/N) 8.78e-5 0.11(0.03) 13331 WHI APOA1, APOC3, APOA4, APOA5 RS964184 G (0.3) LN1 serum cholesterol (mg/dl) Serum Cholesterol MA 2.18e-3 0.02(7.61e-3), 2034 EAGLEIII 2 HDL Cholesterol, lipids, Triglycerides, Triglyceride levels 18193043 LN1 serum cholesterol (mmol/l) 2.91e-3 0.02(6.36e-3) 2040 EAGLEIII Serum cholesterol (mmol/l) 2.50e-3 0.12(0.04) 2034 EAGLEIII High cholesterol requiring pills ever (Y/N) 3.39e-3 0.29(0.10) 1859 WHI Serum cholesterol (mmol/l) 3.44e-3 0.12(0.04) 2040 EAGLEIII LN1 serum cholesterol (mg/dl) 2.98e-3 0.02(7.64e-3) 2040 EAGLEIII Serum cholesterol (mg/dl) 3.42e-3 4.50(1.53) 2040 EAGLEIII LN1 serum cholesterol: si (mmol/l) 2.10e-3 0.02(6.35e-3) 2034 EAGLEIII Serum cholesterol (mg/dl) 2.50e-3 4.64(1.53) 2034 EAGLEIII APOA1, APOC3, APOA4, APOA5 RS964184 G (0.1) LN1 total cholesterol (mg/dl) Serum Cholesterol EA 1.53e-3 0.03(0.01) 924 WHI 2 HDL Cholesterol, lipids, Triglycerides, Triglyceride levels 18193043 Total cholesterol (mg/dl) 3.02e-3 7.46(2.51) 924 WHI Serum cholesterol (mmol/l) 8.29e-3 0.13(0.05) 2592 EAGLEIII High cholesterol requiring pills ever (Y/N) 2.06e-14 0.35(0.05) 12433 WHI Serum cholesterol (mg/dl) 8.28e-3 4.89(1.85) 2592 EAGLEIII TIMD4, HAVCR1 RS1501908 C (0.6) LN1 serum cholesterol (mmol/l) Serum Cholesterol EA 9.63e-3 0.01(5.48e-3) 2582 EAGLEIII 2 LDL Cholesterol 19060906 LN1 total cholesterol in mmol/l 4.04e-4 8.64e-3(2.44e-3) 9323 ARIC Serum cholesterol (mmol/l) 9.35e-3 0.09(0.03) 2584 EAGLEIII Serum cholesterol (mg/dl) 9.40e-3 3.45(1.33) 2584 EAGLEIII Total cholesterol in mmol/l 7.27e-4 0.05(0.02) 9323 ARIC LN1 serum cholesterol (mmol/l) 8.93e-3 0.01(5.43e-3) 2584 EAGLEIII LN1 serum cholesterol (mg/dl) 9.83e-3 0.02(6.52e-3) 2582 EAGLEIII LN1 serum cholesterol (mg/dl) 9.17e-3 0.02(6.49e-3) 2584 EAGLEIII LDLR RS6511720 G (0.9) Total cholesterol in mmol/l Serum Cholesterol AA 1.50e-10 0.25(0.04) 3786 ARIC 2 LDL Cholesterol 18193043 LN1 total cholesterol (mg/dl) 8.37e-4 0.04(0.01) 1009 WHI High cholesterol requiring pills ever (Y/N) 2.21e-3 0.31(0.10) 4041 WHI Total cholesterol (mg/dl) 1.36e-3 8.31(2.59) 1009 WHI LN1 total cholesterol in mmol/l 6.45e-11 0.04(5.85e-3) 3786 ARIC ABCG8 RS6544713 C (0.7) High cholesterol requiring pills ever (Y/N) Serum Cholesterol EA 7.94e-5 −0.14(0.04) 12438 WHI 2 LDL Cholesterol 19060906 Total cholesterol in mmol/l 2.23e-9 −0.09(0.02) 10826 ARIC LN1 total cholesterol in mmol/l 2.13e-9 −0.01(2.34e-3) 10826 ARIC APOB RS754523 T (0.7) LN1 total cholesterol in mmol/l Serum Cholesterol EA 3.01e-10 −0.02(2.49e-3) 9314 ARIC 2 LDL Cholesterol 19750184 High cholesterol requiring pills ever (Y/N) 4.76e-4 −0.13(0.04) 12427 WHI Total cholesterol in mmol/l 5.52e-10 −0.10(0.02) 9314 ARIC APOE, APOC1, APOC4, APOC2 RS4420638 A (0.8) LN1 dietary cholesterol (mg) (dietary consumption) Cholesterol MG EA 1.48e-3 0.03(9.34e-3) 9194 ARIC 2 LDL Cholesterol, lipids, Alzheimer's disease, Coronary Artery Disease, C-reactive protein, Sporadic late onset Alzheimer's disease, ApoB, Triglycerides, Total cholesterol 17474819, 17463246, 18193043, 18802019, 17998437, 19197348, 19567438 Dietary cholesterol (mg) 9.48e-3 5.84(2.25) 13291 WHI LN1 dietary cholesterol (mg) 4.35e-3 0.03(9.66e-3) 13291 WHI Dietary cholesterol (mg) 3.28e-3 6.94(2.36) 9194 ARIC CDKN2A, CDKN2B RS10757278 A (0.5) Coronary bypass surgery (Y/N) Artery Treatment EA 9.60e-3 −0.26(0.10) 4448 CHS 2 Myocardial infarction 17478679 Percutaneous transluminal coronary angioplasty (Y/N) 2.86e-6 −0.17(0.04) 13313 WHI FTO RS8050136 A (0.4) LN1 hip circumference cm Hip Circumference EA 3.11e-7 7.67e-3(1.50e-3) 13272 WHI 2 Obesity, Type 2 diabetes 17554300, 19079260, 17463249, 17463248, 18372903, 18159244, 19056611, 17658951 LN1 hip girth to nearest cm 1.26e-8 7.35e-3(1.29e-3) 9334 ARIC Hip girth to nearest cm 7.26e-9 0.81(0.14) 9334 ARIC Hip circumference cm 6.06e-7 0.84(0.17) 13272 WHI FTO RS8050136 A (0.4) LN1 waist hip ratio Waist Hip Ratio EA 7.15e-3 1.46e-3(5.43e-4) 13269 WHI 2 Obesity, Type 2 diabetes 17554300, 19079260, 17463249, 17463248, 18372903, 18159244, 19056611, 17658951 Waist-to-hip ratio 1.71e-7 6.11e-3(1.17e-3) 9333 ARIC LN1 waist-to-hip ratio 1.75e-7 3.21e-3(6.10e-4) 9333 ARIC FTO RS8050136 A (0.4) LN1 waist circumference cm Waist Size EA 2.88e-8 0.01(1.99e-3) 13284 WHI 2 Obesity, Type 2 diabetes 17554300, 19079260, 17463249, 17463248, 18159244, 19056611, 17658951 LN1 waist girth to nearest cm 3.63e-12 0.01(2.01e-3) 9334,9334 ARIC Waist girth to nearest cm 1.29e-12 1.40(0.20) 13284 ARIC Waist circumference cm 1.91e-8 1.03(0.18) WHI IGF2BP2 RS4402960 G (0.7) LN1 circulating glucose value in mg/dl Plasma Serum Glucose EA 7.38e-3 −8.10e-3(3.02e-3) 9339 ARIC 2 Type 2 Diabetes 17463249, 17463248, 17463246, 19401414 Baseline glucose (mg/dl) 4.80e-3 −2.07(0.73) 4468 CHS LN1 baseline glucose (mg/dl) 2.28e-3 −0.01(4.84e-3) 4468 CHS TCF7L2 RS7903146 C (0.7) LN1 circulating glucose (mg/dl) Plasma Serum Glucose EA 8.39e-3 −0.02(8.95e-3) 918 WHI 2 Type 2 Diabetes 19734900, 17668382, 17463246, 17463248, 18372903, 17293876, 17460697, 19056611, 19401414 LN1 circulating glucose value in mg/dl 2.09e-7 −0.02(2.99e-3) 10739 ARIC Circulating glucose value in mg/dl 1.73e-5 −2.04(0.48) 10739 ARICRelated associations that met the criteria for PheWAS significance are given here, sorted by the number of PAGE site replications for a given phenotype-class. Related associations were defined as SNPs significantly associated in this PheWAS with phenotype-classes closely related to phenotypes among known associations. Significance was defined as a test of association with p<0.01 observed in two or more PAGE studies for the same SNP, phenotype-class, and race/ethnicity and consistent direction of effect when relevant. For each, the nearest gene(s), the SNP rs number, coded allele (CA) and frequency (CAF), associated phenotypes, phenotype-class, race/ethnicity, p-values, genetic effect/beta values (standard error; SE), sample sizes, substudies, number of substudies with results passing our p-value cutoff, the previously associated phenotype for that SNP, and references for the previously associated phenotypes are given.
1Coded Allele.
2Coded allele frequency.
3Associated phenotypes for individual results.
4Phenotype-class.
5Race/ethnicity for association, abbreviations: African American (AA), European American (EA), Mexican American/Hispanic (H).
6P-Values of results that passed p = 0.01 threshold in order of the associated phenotypes.
7Beta and standard error in order of the associated phenotypes.
8Sample size in order of the associated phenotypes.
9Studies with the significant result, in order of the associated phenotypes.
10Total number of studies with at least one result passing p-value threshold for specific phenotype-class and SNP.
11Previously reported associated phenotypes for SNP.
12Pubmed ID's for previously associated phenotypes.
10.1371/journal.pgen.1003087.t004 Table 4PheWAS Tests of Association: Novel Associations.
Nearest Gene SNP ID CA1 (CAF2) Phenotypes8 Associated Phenotype Class3 Ethnicity5 P-Values6 Beta (SE)7 Sample Size9 Substudies10 Substudy Count11 Previously Associated Phenotype4 References12 CELSR2,PSRC1 RS599839 A (0.3) White blood cell (kcell/ml) White Blood Count AA 2.14e-3 0.88(0.29) 4147 WHI 3 Coronary Artery Disease, LDL cholesterol, Total cholesterol 18262040, 18193043, 18179892, 17634449, 18193044 White blood count 3.69e-7 0.29(0.06) 3049 ARIC LN1 white blood count (×1,000/cubic mm) 5.62e-4 0.05(0.01) 777 CHS LN1 white blood cell (kcell/ml) 9.99e-15 0.05(6.03e-3) 4147 WHI LN1 white blood count 1.28e-8 0.04(7.65e-3) 3049 ARIC NOTCH2 RS10923931 G (0.7) LN1 white blood cell count White Blood Count AA 9.59e-4 0.03(8.51e-3) 2083 EAGLEIII 3 Type 2 Diabetes, Type I Diabetes 18372903 LN1 white blood cell count 9.59e-4 0.03(8.51e-3) 2083 EAGLEIII White blood count 8.74e-4 0.18(0.05) 3051 ARIC White blood cell count 2.75e-4 0.25(0.07) 2083 EAGLEIII LN1 white blood cell (kcell/ml) 1.36e-10 0.04(6.14e-3) 4215 WHI White blood cell count 2.75e-4 0.25(0.07) 2083 EAGLEIII LN1 white blood count 1.25e-3 0.02(7.14e-3) 3051 ARIC IL6R RS2228145 A (0.9) White blood count White Blood Count AA 4.17e-8 −0.36(0.06) 3806 ARIC 3 C-reactive Protein 20186139 White blood cell count 2.83e-4 −0.35(0.10) 2038 EAGLEIII LN1 white blood count (×1,000/cubic mm) 5.61e-4 −0.06(0.02) 783 CHS White blood cell count 2.83e-4 −0.35(0.10) 2038 EAGLEIII LN1 white blood count 8.42e-10 −0.05(8.70e-3) 3806 ARIC LN1 white blood cell count 3.57e-4 −0.04(0.01) 2038 EAGLEIII LN1 white blood cell count 3.57e-4 −0.04(0.01) 2038 EAGLEIII NEGR1 RS2815752 T (0.08) Ever smoked cigarettes (Y/N) Ever Smoked EA 7.57e-3 0.08(0.03) 9339 ARIC,WHI 2 Body Mass Index 19079261 Smoked at least 100 cigarettes ever (Y/N) 7.48e-4 0.09(0.03) 13222 IL6 RS1800795 C (0.06) LN1 beta carotene mcg (dietary consumption) Carotene AA 5.43e-3 0.12(0.04) 3071 ARIC 2 C-reactive Protein 15820616, 16544245, 16644865, 17003362, 17416766, 17623760, 17694420, 17916900, 17996468, 18041006, 18239642, 18257935, 18276608, 18321738, 18449426, 18458677, 18752089, 18992263, 19056105, 19106168, 19140096, 19267250, 19280716, 19330901, 19377912, 19387461, 19435922, 19452524, 19542902, 19592000, 19671870, 19833146, 19853505, 19876004, 20043205, 20044998, 20132806, 20149313, 20175976, 20176930, 20333461, 20361391, 20436380, 20459474, 20592333, 20622166 LN1 carotenes (dietary consumption) 9.44e-3 0.24(0.09) 1964 EAGLEIII LN1 alpha carotene mcg (dietary consumption) 2.74e-3 0.26(0.09) 3071 ARIC IL6R RS2228145 A (0.9) LN1 lymphocytes Lymphocytes AA 5.12e-10 0.06(0.01) 3769 ARIC 2 C-reactive Protein 20186139 Lymphocytes (percent of 100 cells) 5.39e-5 2.96(0.73) 949 EAGLEIII Lymphocytes 6.63e-10 2.35(0.38) 3769 ARIC LN1 lymphocytes (percent of 100 cells) 3.88e-5 0.08(0.02) 949 EAGLEIII IL6R RS2228145 A (0.9) LN1 neutrophils Neutrophils AA 1.54e-8 −0.06(9.81e-3) 3769 ARIC 2 C-reactive Protein 20186139 LN1 Segmented neutrophils 2.44e-4 −0.06(0.02) 949 EAGLEIII Neutrophils 4.66e-10 −2.61(0.42) 3769 ARIC Segmented neutrophils 7.74e-5 −3.12(0.79) 949 EAGLEIII CELSR2,PSRC1 RS599839 A (0.8) Currently of on a special diet? (Y/N) Dieting EA 9.48e-3 0.12(0.05) 9328 ARIC 2 Coronary Artery Disease, LDL cholesterol, Total cholesterol 18262040, 18193043, 18179892, 17634449, 18193044 Low-fat or low cholesterol diet (Y/N) 5.87e-3 0.08(0.03) 13068 WHI GALNT2 RS2144300 C (0.8) LN1 FEV(3) over FEV(6) (Lung function) FEV3 AA 4.90e-4 −0.11(0.03) 3090 ARIC 2 Coronary Heart Disease, HDL cholesterol, Triglycerides 18193043 FEV(3) (liters) (Lung function) 1.13e-3 −0.10(0.03) 3090 ARIC LN1 FEV3 at 3.0 seconds Largest value (Lung Function) 8.82e-3 −0.03(0.01) 1953 ARIC FEV(3) over FEV(6) (Lung Function) 7.93e-4 −2.26(0.67) 3090 EAGLEIII LN1 FEV(3) (liters) (Lung Function) 8.43e-4 −0.03(7.92e-3) 3090 ARIC GALNT2 RS2144300 C (0.8) Pills for hypertension ever (Y/N) Hypertension AA 8.27e-3 0.15(0.06) 4180 WHI 2 Coronary Heart Disease, HDL cholesterol, Triglycerides 18193043 High blood pressure ever diagnosed? (Y/N) 1.61e-3 0.24(0.08) 3142 ARIC GALNT2 RS2144300 C (0.4) LN1 serum total calcium: (mmol/l) Serum Calcium EA 8.10e-3 −2.36e-3(8.92e-4) 2587 EAGLEIII 2 Coronary Heart Disease, HDL cholesterol, Triglycerides 18193043 LN1 serum calcium (mg-dl) 1.47e-3 −1.84e-3(5.80e-4) 9098 ARIC Serum total calcium 5.95e-3 0.03(0.01) 2585 EAGLEIII Serum total calcium (mmol/l) 7.47e-3 −7.97e-3(2.98e-3) 2587 EAGLEIII LN1 serum total calcium (mmol/l) 6.28e-3 −2.39e-3(8.75e-4) 2585 EAGLEIII LN1 serum total calcium 6.40e-3 −3.09e-3(1.13e-3) 2585 EAGLEIII Serum calcium (mg-dl) 1.71e-3 −0.02(6.24e-3) 9098 ARIC Serum total calcium (mmol/l) 5.95e-3 −7.98e-3(2.90e-3) 2585 EAGLEIII GALNT2 RS2144300 C (0.8) LN1 white blood cell (kcell/ml) White Blood Count AA 3.32e-6 −0.04(7.53e-3) 4210 WHI 2 Coronary Heart Disease, HDL cholesterol, Triglycerides 18193043 LN1 white blood count (×1,000/cubic mm) 7.96e-3 −0.05(0.02) 785 CHS GALNT2 RS2144300 C (0.4) Coronary artery bypass graft (cabg) (Y/N) Artery Treatment EA 2.46e-3 0.24(0.08) 13152 WHI 2 Coronary Heart Disease, HDL cholesterol, Triglycerides 18193043 Aortic Aneurysm repair (Y/N) 5.49e-3 0.57(0.20) 4250 CHS LIPG RS2156552 A (0.2) Doctor ever told you had goiter? (Y/N) Thyroid Goiter EA 8.60e-3 −1.91(0.25) 2273 EAGLEIII 2 HDL Cholesterol 18193043 Age told had goiter/thyroid disease 7.57e-3 −2.97(1.11) 763 ARIC CETP RS3764261 T (0.3) Age at first period category: 11 years Menstruation EA 4.30e-3 −0.11(0.04) 13276 WHI 2 HDL Cholesterol, LDL cholesterol, Waist circumference 18193043, 19359809 Age when menstruation began 2.04e-3 0.11(0.03) 5705 WHI LN1 age when menstruation began 5.43e-4 9.19e-3(2.66e-3) 5705 ARIC Age at first period category: 10 years 6.81e-3 −0.17(0.06) 13276 ARIC FADS1, FADS2, FADS3 RS174547 C (0.3) Platelet count Platelet Count EA 7.37e-3 3.26(1.22) 9174 ARIC 2 HDL Cholesterol, Triglycerides, Total cholesterol, LDL cholesterol 20037589, 19060906 LN1 platelet count (kcell/ml) 2.28e-3 0.01(3.30e-3) 13140 WHI LN1 platelet count 1.68e-3 0.01(3.90e-3) 9174 ARIC LDLR RS6511720 G (0.9) Max % arterial stenosis (1–24%) (Arterial measurement) Artery EA 4.19e-3 −0.22(0.08) 4460 CHS 2 LDL Cholesterol 18193043 Arterial plaque in any site (Y/N) 9.19e-3 0.13(0.05) 8269 ARIC Max % arterial stenosis (25–49%) (Arterial measurement) 4.91e-3 0.21(0.07) 4460 CHS PCSK9 RS11591147 G (0.996) Coronary artery bypass graft (Y/N) Artery Treatment AA 2.11e-4 −2.23(0.60) 4274 WHI 2 LDL Cholesterol, lipids 18193044 Heart or arterial surgery? (Y/N) 1.53e-3 −1.97(0.62) 3909 ARIC APOE, APOC1, APOC4, APOC2 RS4420638 A (0.8) Circulating glucose value in mg/dl Plasma Serum Glucose EA 8.86e-4 1.89(0.57) 9336 ARIC 2 LDL Cholesterol, lipids, Alzheimer's disease, Coronary Artery Disease, C-reactive protein, Sporadic late onset Alzheimer's disease, ApoB, Triglycerides, Total cholesterol 17474819, 17463246, 18193043, 18802019, 17998437, 19197348, 19567438 Baseline glucose (mg/dl) 4.65e-3 2.67(0.94) 4470 CHS LN1 circulating baseline glucose (mg/dl) 5.67e-3 0.02(6.23e-3) 4470 CHS LN1 circulating glucose value in mg/dl 6.81e-4 0.01(3.62e-3) 9336 ARIC CELSR2, PSRC1,SORT1 RS646776 G (imputed) Are you currently on a special diet (Y/N) Dieting EA 4.20e-3 −0.14(0.05) 9331 ARIC 2 LDL Cholesterol, Myocardial infarction (early onset) 18193044, 18262040 Low-fat or low cholesterol diet (Y/N) 8.78e-3 −0.08(0.03) 13051 WHI APOB RS562338 T (0.6) Are you following a special diet (Y/N) Dieting AA 4.82e-3 −0.34(0.12) 817 CHS 2 LDL Cholesterol, Total cholesterol, Type 2 Diabetes 18193043 Currently of diet (Y/N) 5.07e-3 −0.19(0.07) 3149 ARIC CDKN2A,CDKN2B RS10757278 A (0.8) LN1 white blood count White Blood Count AA 5.05e-4 −0.03(7.32e-3) 3877 ARIC 2 Myocardial infarction 17478679 White blood count 3.24e-3 −0.16(0.05) 3877 ARIC LN1 white blood cell (kcell/ml) 3.80e-3 −0.02(6.96e-3) 4214 WHI CDKN2A,CDKN2B RS2383207 A (imputed) LN1 dietary vitamin b12 (mcg) VitaminB12 EA 4.34e-3 0.02(5.69e-3) 13254 WHI 2 Myocardial infarction 17478679 Dietary vitamin b12 (mcg) 3.35e-3 0.14(0.05) 13254 WHI LN1 vitamin b12 (micrograms) 3.95e-3 0.02(6.98e-3) 9197 ARIC ANGPTL3 RS1748195 G (0.6) LN1 hemoglobin (g/dl) Hemoglobin AA 7.66e-3 −9.61e-3(3.60e-3) 2085 EAGLEIII 2 Triglycerides, lipids 18193043 LN1 hemoglobin (gm/dl) 9.57e-3 −4.64e-3(1.79e-3) 4195 WHI Hemoglobin (g/dl) 6.28e-3 −0.14(0.05) 2085 EAGLEIII ADAMTS9 RS4607103 C (0.8) LN1 min lumen diameter (arterial measurement) Artery EA 7.00e-3 0.07(0.03) 120 ARIC 2 Type 2 Diabetes 18372903 LN1 average near and far wall max common carotid artery (mm) (Arterial measurement) 6.22e-3 6.32e-3(2.31e-3) 4460 CHS Ave near and far wall max common carotid artery (mm) (Arterial measurement) 7.91e-3 0.01(5.02e-3) 4460 CHS ADAMTS9 RS4607103 C (0.8) Smoked at least 100 cigarettes ever? (Y/N) Ever Smoked EA 1.38e-3 −0.09(0.03) 13214 WHI 2 Type 2 Diabetes 18372903 Ever smoked? (Y/N) 4.11e-3 −0.14(0.05) 4477 CHS IGF2BP2 RS4402960 G (0.7) Currently of diet? (Y/N) Dieting EA 3.14e-4 −0.15(0.04) 9331 ARIC 2 Type 2 Diabetes 17463249, 17463248, 17463246, 19401414 Diabetic or ADA diet? (Y/N) 9.63e-4 −0.23(0.07) 12958 WHI TCF7L2 RS7901695 C (0.5) LN1 white blood count, white blood count White Blood Count AA 2.75e-3 −0.02(6.77e-3) 3053 ARIC 2 Type 2 Diabetes 17554300, 17463249, 17463246, 17668382 LN1 white blood cell (kcell/ml) 7.03e-3 −0.14(0.05) 3053 ARIC 7.73e-3 −0.02(5.76e-3) 4182 WHI TSPAN8/LGR5 RS7961581 C (0.3) Pulse rate (beats/min) (age 5+ years) HeartRate EA 8.38e-3 −1.33(0.50) 2570 EAGLEIII 2 Type 2 Diabetes 18372903 LN1 heart rate per minute 8.17e-3 −6.20e-3(2.34e-3) 9314 ARIC NOTCH2 RS10923931 G (0.7) Hypertension ever (Y/N) Hypertension AA 3.07e-3 −0.14(0.05) 4206 WHI 2 Type 2 Diabetes, Type I Diabetes 18372903 History of high blood pressure from baseline questionnaire (Y/N) 2.00e-3 −0.14(0.04) 4736 MEC History of high blood pressure (Y/N) 3.50e-3 −0.14(0.05) 4362 MEC, Hypertension interested category: never Hypertensive (Y/N) 2.33e-3 −0.15(0.05) 4076 WHI Hypertension interested category: treated Hypertensive (Y/N) 2.92e-3 −0.14(0.05) 4076 WHI Pills for hypertension ever (Y/N) 8.18e-4 −0.16(0.05) 4186 WHI Pills for hypertension now (Y/N) 2.82e-3 −0.14(0.05) 4122 WHI FTO RS8050136 A (0.4) Age at first period interested category: 14 years Menstruation EA 5.26e-3 −0.10(0.04) 13275 WHI 2 Obesity, Type 2 diabetes 17554300, 19079260, 17463249, 17463248, 18159244, 19056611, 17658951 Age when menstruation began 1.18e-4 −0.14(0.04) 4917 ARIC LN1 age when Menstruation began 6.09e-5 −0.01(2.74e-3) 4917 ARIC CELSR2, PSRC1, SORT1 RS646776 G (imputed) Hospitalized for chest pain? (Y/N) ANGINA EA 3.05e-3 −0.55(0.18) 568 ARIC 2 LDL Cholesterol 19060911, 19198609, 18193044, 18262040, 19060910 Angina (Y/N) 3.17e-4 −0.25(0.07) 13289 WHI CDKN2A,CDKN2B RS1333049 C (0.2) Hemoglobin (g/dl) Hemoglobin AA 5.40e-3 0.15(0.05) 2092 EAGLEIII 2 Coronary Artery Disease, Type 2 Diabetes, Hypertension 17554300, 17634449 Hemoglobin (g/dl) 8.21e-3 0.26(0.10) 786 CHS LN1 Hemoglobin (g/dl) 4.07e-3 0.01(3.95e-3) 2092 EAGLEIIINovel associations that met the criteria for PheWAS significance are given here, sorted by the most to least number of PAGE study sites available. Related associations were defined as SNPs significantly associated in this PheWAS with phenotype-classes closely related to phenotypes among known associations. Significance was defined as a test of association with p<0.01 observed in two or more PAGE studies for the same SNP, phenotype class, and race/ethnicity and consistent direction of effect when relevant. For each, the nearest gene(s), the SNP rs number, coded allele (CA) and frequency (CAF), associated phenotypes, phenotype-class, race/ethnicity, p-values, genetic effect/beta values (standard error; SE), sample sizes, substudies, number of substudies with results passing our p-value cutoff, the previously associated phenotype for that SNP, and references for the previously associated phenotypes are given.
1Coded Allele.
2Coded allele frequency.
3Associated phenotypes.
4Phenotype-class.
5Race/ethnicity for association, abbreviations: African American (AA), European American (EA), Mexican American/Hispanic (H).
6P-Values of results that passed p = 0.01 threshold in order of the associated phenotypes.
7Beta and standard error in order of the associated phenotypes.
8Sample size in order of the associated phenotypes.
9Studies with the significant result, in order of the associated phenotypes.
10Total number of studies with at least one result passing p-value threshold for specific phenotype class and SNP.
11Previously reported associated phenotypes for the SNP.
12Pubmed ID's for previously associated phenotypes.
Known Associations—Validating the PheWAS Approach
Almost half of the PAGE PheWAS results (52/111; 48%) replicated previously known genotype-phenotype associations. These replicated results serve as positive controls and demonstrate that the high-throughput PheWAS approach is feasible and valid. As an example, low-density lipoprotein cholesterol (LDL-C) has previously been associated with rs4420638 near APOE/APOC1/C1P1/C2/C4 in European Americans [8], [9]. In the PAGE PheWAS, a significant association between the same SNP and LDL-C phenotypes of the “LDL-C” phenotype-class in European Americans as reported in the literature [8], [9] was observed in two PAGE study sites, with the same direction of effect (β) as well as a third PAGE site with near significant results: ARIC (p = 1.27×10−15, β = −5.75), CHS (p = 7.89×10−12, β = −7.06), and WHI (p = 0.06, β = −4.15). Figure 1 shows the significant PheWAS LDL-C results, as well as other associations considered significant for rs4420638 across PAGE study sites for other phenotype-classes in a similar racial/ethnic group passed a significance threshold of p<0.01 with a consistent direction of genetic effect.
10.1371/journal.pgen.1003087.g001 Figure 1PheWAS associations for rs4420638 near APOC1.
SNP rs4420638 has previously been associated with LDL cholesterol levels, triglycerides, Alzheimer's disease, coronary artery disease, and sporadic late onset Alzheimer's. The length of the lines correspond to –log10(p-value), and the lines are plotted clockwise starting at top for the association with the smallest p-value. Lines are labeled with the study-specific phenotype, the PAGE study, racial/ethnic group, and direction of effect (+ or −). Red lines represent associations at p<0.01. “LN1” indicates the phenotype had 1 added to the variable, and then the variable was natural log transformed. The PheWAS phenotypes significantly associated with this SNP varied, with known associations for LDL cholesterol levels, as well as the related phenotypes “Total cholesterol (mmol/l)” and “Dietary cholesterol (mg)”, and novel phenotypes such as “Baseline glucose (mg/dl)”.
Related Associations
Approximately one-fourth of the PAGE PheWAS results (26/111; 23%) represented SNP-phenotype associations in phenotype-classes closely related to previously known genotype-phenotype associations. For example, rs10757278 near CDKN2A/CDKN2B has been robustly associated with myocardial infarction (MI) [10], [11]. In this PheWAS, rs10757278 was associated with the “Cardiac” phenotype-class, but also with the related phenotype-classes of “Artery Treatment” and “Angina”. Specifically, rs10757278 was associated with phenotypes in the Artery Treatment phenotype-class, such as “percutaneous transluminal coronary angioplasty” (WHI, p = 2.86×10−6, β = −0.17, EA), and “coronary bypass surgery” (CHS, p = 9.60×10−3, β = −0.26, EA). The SNP rs10757278 was also associated with phenotypes in the Angina phenotype-class, such as presence or absence of angina (WHI, p = 6.59×10−3, β = −0.14, EA) and the phenotype “Ever see a doctor because of chest pain?” (ARIC, p = 4.44×10−3, β = −0.31, EA). Replication of association of this SNP with previously known phenotypes were also found with the phenotype-class “Cardiac”, with phenotypes such as “MI (Y/N)” (WHI, p = 1.39×10−4, β = −0.11, EA), and “MI status at baseline (Y/N)” (CHS, p = 6.35×10−3, β = −0.18, EA). Significant PheWAS associations at p<0.01 for rs10757278 are plotted by phenotype in Figure 2, as well as additional results at p<0.05.
10.1371/journal.pgen.1003087.g002 Figure 2PheWAS associations for rs10757278 near CDKN2A/CDKN2B.
SNP rs10757278 was previously associated with myocardial infarction (MI). Associations are plotted clockwise starting at top for the association with the smallest p-value and the length of the line corresponds to –log10(p-value). Lines are labeled with the study-specific phenotype, the PAGE study, racial/ethnic group, and direction of effect (+ or −). Red lines represent associations at p<0.01, and results with p<0.05 are also plotted in grey to show trends for additional phenotypes. “LN1” indicates the phenotype had 1 added to the variable, and then the variable was natural log transformed. The PheWAS phenotypes significantly associated with this SNP varied, from MI (known), to coronary artery disease and MI related phenotypes such as presence or absence of “percutaneous transluminal coronary angioplasty”, “angina”, and “coronary bypass surgery”.
Another example of PheWAS associations for phenotype-classes closely related to known genotype-phenotype associations existed for rs599839 near the CELSR2/PSRC1/SORT1 gene cluster. The SNP rs599839 has been associated with serum LDL cholesterol levels [8], [12]–[14], and coronary artery disease [13], [15]. In our PheWAS, associations were found for the “LDL-C” phenotype-class, as well the coronary artery disease related “Angina” and lipid related “HDL-C” phenotype-classes, including specific phenotypes such as “angina, presence or absence of” (WHI, p = 2.10×10−4, β = 0.25, EA), and “HDL-C” (WHI, p = 1.23×10−3, β = −0.04, AA). As expected, a significant association was also identified for the LDL-C level related phenotype “LDL-C (mg/dl)” (ARIC, p = 5.25×10−22, β = 6.42 EA). Significant PheWAS associations at p<0.01 for rs599839 are plotted by phenotype in Figure 3, as well as additional results at p<0.05.
10.1371/journal.pgen.1003087.g003 Figure 3PheWAS associations for rs599839 near CELSR2/PSRC1.
This SNP has previously published associations with serum LDL cholesterol levels, total cholesterol, and coronary artery disease. Genotype-phenotype associations are plotted clockwise starting at top for the association with the smallest p-value. The length of the line corresponds to –log10(p-value), the longer the line the more significant the result. The study race/ethnicity/and phenotype for each tests of association are listed. Red lines represent associations at p<0.01, and results with p<0.05 are also plotted in grey to show trends for additional phenotypes. “LN1” indicates the phenotype had 1 added to the variable, and then the variable was natural log transformed. The PheWAS phenotypes significantly associated with this SNP varied, from LDL cholesterol levels (previously published), to lipid level-related phenotypes such as “High cholesterol requiring pills ever”. In the case of coronary artery disease, phenotypes with significant results that were related to coronary artery disease included “Ever had pain/discomfort in your chest”, and “Hospitalized for chest pain”.
Potentially Novel Associations
PheWAS results were considered novel, if the significant phenotype-class associations varied substantially from the previously reported GWAS and candidate gene studies. Approximately one-third of the PAGE PheWAS results (33/111; 30%) represented novel genotype-phenotype-class associations. Further research will be required to determine the further validity of these exploratory results.
The most statistically significant of the novel phenotype-class associations identified by this PheWAS include multiple associations involving phenotype-classes for hematologic traits in African Americans (Figure 4). SNPs rs599839 (CELSR2/PSRC1), rs10923931 (NOTCH2), rs2228145 (IL6R), rs2144300 (GALNT2), rs10757278 (CDKN2A,CDKN2B), and rs7901695 (TCF7L2) were each associated with white blood cell count phenotypes among AA (significant p-values ranging 7.96×10−3 to 9.99×10−15). IL6R rs2228145 was also associated with neutrophils and lymphocyte numbers in AA with p-values ranging from 2.44×10−4 to 4.66×10−10. These SNPs were previously associated with LDL-C, total cholesterol levels, and coronary artery disease (rs599839) [8], [12]–[14]; type 2 diabetes (rs10923931) [16]; C-reactive protein (rs2228145) [17]; coronary heart disease, HDL-C and triglycerides (rs2144300) [13]; MI (rs10757278) [11]; and type 2 diabetes (rs7901695) in EA [18]–[20]. It is likely that the majority of the significant findings for three of the SNPs on chromosome 1 [rs599839 (CELSR2/PSRC1), rs10923931 (NOTCH2), rs2228145 (IL6R)] are not truly novel given that these variants are likely in linkage disequilibrium with the white blood cell count-associated Duffy null allele (DARC rs2814778) [21], [22] in African Americans. Of note is GALNT2 rs2144300 (p = 3.32×10−6 in WHI and 7.96×10−3 in CHS), located outside the 90 Mb region known to be associated with white blood cell counts in African Americans [21] and possibly representing a novel genotype-phenotype association for this trait. Also for chromosome 1, novel associations were identified in African Americans at p<0.01 for the phenotype-class “Hemoglobin” and ANGPTL3 rs1748195, previously associated with triglycerides in European-descent populations [13], [19].
10.1371/journal.pgen.1003087.g004 Figure 4PheWAS results for blood cell counts and hemoglobin levels.
Eleven novel genotype-phenotype-class associations were identified for white blood cell counts and hemoglobin levels collectively. The top track indicates the chromosomal location of each SNP, below that track is a SNP/Phenotype identification track containing the SNP ID, as well as the phenotype, phenotype transformation if present (LN1 = ln(1+variable)), and the race-ethnicity for the test population (AA or EA). The next track is a “presence/absence” track, box presence indicates if the SNP was present for ARIC (blue), CHS (red), WHI (orange), or EAGLE (purple). The next tracks are as follows: –log10(p-value), where the each p-value is plotted, the direction of the triangle indicates the direction of effect (triangle pointed up is positive, triangle pointed down is negative), base of the triangle corresponds to the location of the p-value, solid red line is positioned at p-value = 0.01; The next track is magnitude of effect (beta) dotted grey line is positioned at the null; Next are coded allele frequencies (CAF) for each study; Final track is sample size for each test of association.
Of the remaining hematologic trait associations identified that were not on chromosome 1, rs10757278 near CDKN2A/B on chromosome 9 and TCF7L2 rs7901695 on chromosome 10 were both associated with white blood cell count, neither of which were previously reported in GWAS for this trait [21], [22]. For CDKN2A/B rs1333049, a SNP previously associated with type 2 diabetes, coronary artery disease, and hypertension in European-descent populations [15], [23] p<0.01 associations were identified for the phenotype-class of Hemoglobin. Finally, a novel association in European Americans was noted between FADS1 rs174547, a SNP previously associated with LDL-C [13], [19], and the phenotype-class of “Platelet Count” at p<0.01.
Aside from hematologic traits, the most significant novel association identified in this PheWAS was identified for phenotypes in the phenotype-class “Forced Expiratory Volume in 3 Seconds (FEV3)” and GALNT2 rs2144300 in African Americans (p-values ranging from 8.82×10−3 to 4.90×10−4). GALNT2 rs2144300, previously associated with HDL-C in European Americans and African Americans [13], [24], has not previously been associated with lung function or asthma quantitative traits. Interestingly, GALNT2 rs2144300 was also associated with phenotypes in the “Hypertension” phenotype-class among African Americans in this PheWAS Specifically the phenotypes were “High blood pressure ever diagnosed?” (ARIC, p = 1.61×10−3, β = 0.24) and “Pills for hypertension ever?” (WHI, 8.27×10−3, β = 0.15). Indeed, GALNT2 rs2144300 displayed the most suggestion of pleiotropy among all the SNPs tested in this study. In addition to the associations identified in African Americans, rs2144300 was associated with phenotypes in the phenotype-classes “Serum Calcium” (p-values ranging from 1.47×10−4 to 8.10×10−3) and “Artery Treatment”, specifically the phenotypes “Coronary artery bypass graft (CABG)” (WHI, p = 2.46×10−3, β = 0.24) and “Aortic aneurysm repair” (CHS, 5.49×10−3, β = 0.57) in European Americans. Significant PheWAS associations at p<0.01 for rs2144300 are plotted by phenotype in Figure 5, as well as additional results at p<0.05.
10.1371/journal.pgen.1003087.g005 Figure 5PheWAS associations for rs2144300 within GALNT2.
The previously published associations for this SNP were with triglyceride and HDL cholesterol levels. Genotype-phenotype associations are plotted clockwise starting at top for the association with the smallest p-value. The length of the line corresponds to –log10(p-value), the longer the line the more significant the result. The study race/ethnicity/and phenotype for each tests of association are listed. Red lines represent associations at p<0.01, and results with p<0.05 are also plotted in grey to show trends for additional phenotypes. The novel PheWAS phenotypes significantly associated with this SNP varied, including white blood cell counts, forced vital capacity at three seconds (FEV3), and serum calcium levels.
The remaining significant novel PheWAS results have identified potentially pleiotropic effects for SNPs previously associated with lipid traits, type 2 diabetes, inflammation, myocardial infarction, and body mass index. The lipid trait-associated SNPs were associated with the “Menstruation” phenotype-class (specifically age at menarche) in European Americans (CETP rs3764261), the “Dieting” phenotype-class (APOB rs562338 in African Americans and CELSR2/PSRC1/SORT1 rs599839 and rs646776 in European Americans), “Thyroid Goiter” in European Americans (LIPG rs2156552), “Artery Measurements” in European Americans (LDLR rs6511720) and “Artery Treatment” in African Americans (PCSK9 rs11591147), “Plasma Serum Glucose Levels” (APOE/APOC1/APOC4/APOC2/APOC3 rs4420638) in European Americans, and the “Angina” phenotype-class in European Americans (CELSR2/PSRC1/SORT1 rs646776). For the type 2 diabetes-associated SNPs, the PheWAS-identified associations were observed for the phenotype-classes of “Dieting” (IGFBP2 rs4402960) in European Americans, “Artery” and “Ever Smoked” (ADAMTS9 rs4607103) in European Americans, “Hypertension” (NOTCH2 rs10923931) in African Americans, “Heart Rate” (LGR5 rs7961581) in European Americans, and “Menstruation” (specifically age at menarche) in European Americans (FTO rs8050136). Like type 2 diabetes-associated ADAMTS9 rs4607103, BMI-associated NEGR1 rs2815752 was associated with the phenotype-class of “Ever Smoked” in European Americans. The final two PheWAS-identified significant associations involved nutrient based phenotype-classes: MI-associated CDKN2A/B rs2383207 was associated with the phenotype-classes of “Vitamin B12” in European Americans, and inflammation-associated IL6 rs1800795 was associated with the phenotype-class of “Carotene” in African Americans.
Discussion
The PheWAS results herein present the result of tests of association between a large number of SNPs and an extensive range of phenotypes and traits available within five studies of the PAGE network. For this first PAGE PheWAS analysis we have emphasized associations that replicated across two or more independent PAGE studies for the same phenotype class and same race/ethnicity. Most of the robust findings reported here represent previously known genotype-phenotype relationships, but a tantalizing few also represent potentially novel pleiotropic relationships.
The 33 novel results presented here are intriguing, but it is important to emphasize that these first-pass analyses are considered hypothesis-generating, exploratory, and require additional scrutiny before the findings are further considered for follow-up, unlike the directed a priori hypothesis-testing analyses within PAGE that involve SNPs hypothesized to be associated with specific phenotypes. Further analysis of PheWAS results will be on an individual result basis and will include careful phenotype harmonization for traits and outcomes that cross two or more PAGE studies, as well as considerable investigation of the possible effect of covariates such as age, sex, and environmental exposure(s) on the association between genetic variation and phenotypic outcome.
One of the many challenges for the interpretation of PheWAS results is dissecting the genetic effect observed among correlated phenotypes. In some cases, the relationship is likely attributable to a common biological process with known genetic contribution (e.g., body mass index and waist circumference). In other cases, the networks that exist between intermediary and/or outcome related phenotypes add complexity to interpreting association results. For instance, genetic variation may impact the variation of a single phenotype, but variation in that phenotype could then result in changes in other downstream phenotypes indirectly. Examples of added complexity include obesity leading to impaired immune function [28], and metabolic syndrome where there is a spectrum of risk factors that are all associated with increased risk of cardiovascular disease and type 2 diabetes [25]. As a result, significant associations between a genetic variant and many phenotypes could represent a network or cascade of events. This is a potential interpretation of results found for SNP rs10923931 (NOTCH2) in AA, where type 2 diabetes was the previously reported association for this SNP and the novel result was found for hypertension, and type 2 diabetes and hypertension are often a co-occurrence. Further analysis of individual PheWAS results is necessary to conclusively establish the impact of the relationship between phenotypes on significant SNP-phenotype associations.
With the large number of phenotype-genotype associations calculated, there will be an increase in type 1 error due to multiple testing. A Bonferroni correction could be used within each individual study to choose a cutoff for significance that controls for multiple hypothesis testing. However, this would not take into account the correlations that exist between the phenotypes in these studies that impact the assumption of independence between tests as well as the correlations between the genotypes.
For our first PAGE PheWAS analysis, we chose to seek replication of results across studies and required the same direction of effect as one approach to reduce the false discovery rate. Significant results can still be found by chance across more than one study. Multiple challenges arise when attempting to get a metric of the type 1 error rate across multiple studies. First, as with individual studies, correlations between phenotypes and previous associations for the SNPs are still present. Also, there are varying type 1 error rates depending on the number of studies available for seeking replication. Quantification of how many results were found with a p-value cutoff, and without a p-value cutoff, depending on the number of studies where replication could be sought (2, 3, 4, or 5) provides some information about the number of significant results we found, in Table 2. Table 1 has the total number of results with and without p-value cutoff for individual studies. It is important to note that in cases where replication could be sought in more than two studies, there were cases where the result replicated in 3 or more studies, further increasing our confidence in the result.
A potential limitation of this study is the granularity of phenotypes within our phenotype classes. The phenotypes within some phenotype classes are the same or extremely similar, such as white blood cell count measurements across studies. However, the phenotype class “Artery Treatment” is broad in terms of the types of phenotypes included, such as presence/absence aortic aneurysm repair and presence/absence of angioplasty of the coronary arteries. For some classes, the replicated results encompass more variation in the phenotypes captured, compared to other results. As a result, significant associations between a genetic variant and all phenotypes in a network may be present. PheWAS is an exploratory and hypothesis generating exercise, thus the choice was made to have a broader match for some groups of phenotypes in order to allow for those phenotypes to be part of the exploration of the data. In addition, misclassification of phenotypes when matching is possible, and thus can limit identification of significant associations across studies. Other potential limitations include sample size/power, study heterogeneity, and the SNPs selected for study. As shown in Table 1, there is much variability across independent PAGE studies. While each PAGE study is sizeable, individual tests of association may be underpowered depending on the availability of the genetic variant, phenotype class, and race/ethnicity. Tests of association that failed to reach statistical significance may represent underpowered genotype-phenotype relationships and will require larger epidemiologic or clinic-based samples to identify. In regards to the potential impact of heterogeneity, we have some cases where replication existed in only two or three studies out of those where replication could be sought. In some instances this may be due to power, but this also may reflect the heterogeneity between studies, such as how various phenotypes are measured in individual studies and variation in mean age across the different studies. Finally, SNPs were originally selected for this study to replicate known genotype-phenotype associations and to generalize them to diverse populations. A comprehensive set of genome-wide“agnostic” SNPs may uncover additional pleiotropic or novel genotype-phentoype relationships not tested here.
Despite the the limitations present for this PheWAS, there are multiple strengths within our study. We have had the opportunity to perform a PheWAS of substantial size with an unprecedented diversity of high quality phenotypic measurements and traits, across multiple races/ethnicities. In addition, because of this PheWAS was conducted across multiple independent studies, we were able to identify the most robust genotype-phenotype relationships across studies
Conclusion
This initial PheWAS within PAGE has presented challenges in terms of generating high-throughput tests of association across large epidemiologic studies as well as the synthesis of the resulting data and its eventual interpretation. Even with these limitations, this PheWAS demonstrates the utility of investigating the relationship between genetic variation and an extensive range of phenotypes by validating known genotype-phenotype associations as well as identifying novel genotype-phenotype associations, revealing complex phenotypic relationships and perhaps actual pleiotropy. The utility of this hypothesis-generating approach will continue to improve over time as more samples, variants, and phenotypes/traits across diverse populations are available for study in PAGE and other genomic resources. Larger, richer datasets coupled with methods development promise to more fully reveal the complex nature of genetic variation and its relationship with human diseases and traits.
Methods
Study Populations
All studies were approved by Institutional Review Boards at their respective sites (details are given in Text S1). The Population Architecture using Genomics and Epidemiology (PAGE) study includes the following epidemiologic collections: Atherosclerosis Risk in Communities (ARIC), Coronary Artery Risk in Young Adults (CARIDA), Cardiovascular Health Study (CHS), the Multiethnic Cohort (MEC), the National Health and Nutrition Examination Surveys (NHANES), Strong Heart Study (SHS), and Women's Health Initiative (WHI). For this PheWAS, data were available from ARIC, CHS, MEC, NHANES III, NHANES 1999–2002, and WHI (Table 1). The PAGE study design is described in Matise et al [26] and the PAGE PheWAS study design is described in Pendergrass et al [1].
SNP Selection and Genotyping
All SNPs considered for genotyping in PAGE were candidate gene or GWAS-identified variants for phenotypes and traits available in the epidemiologic collections accessed by PAGE study sites. Cohorts and surveys were genotyped using either commercially available genotyping arrays (Affymetrix 6.0, Illumina 370CNV BeadChip), and/or custom mid - and low-throughput assays (TaqMan, Sequenom, Illumina GoldenGate or BeadXpress). Quality control was implemented at each PAGE study site independently. Study specific genotyping details are described in Text S1.
In this PheWAS, data were available for SNPs previously associated with HDL-C, LDL-C, and triglycerides [27], body mass index, obesity [28], type 2 diabetes, glucose, insulin [29], and measures of inflammation (C-reactive protein), among other diseases/traits. A total of 83 SNPs overlapped across at least PAGE study sites: ten were specifically selected for body mass index traits replication, three for C-reactive protein, six for coronary/cardiac traits, three for gout/kidney, 41 for lipids, and 20 for type 2 diabetes. Table S1 lists these SNPs, along with references reporting phenotypic associations from the NHGRI GWAS catalog [30] and the open access database of GWAS results of Johnson et al. 2009 [31]. The NHGRI GWAS catalog was most recently accessed in October, 2011. If no references were available from either of those two sources, a PubMed search was performed to retrieve relevant citations.
Statistical Methods
All tests of association were performed independently by each PAGE study site using the following analysis protocol: Linear or logistic regressions were performed for continuous or categorical dependent variables, respectively, assuming an additive genetic model (0, 1, or 2 copies of the coded allele). For variables with multiple categories, binning was used to create new variables of the form “A versus not A” for each category, and logistic regression was used to model the new binary variable. Linear regressions were repeated following a y to log (y+1) transformation of the response variable with +1 added to all continuous measurements before transformation to prevent variables recorded as zero from being omitted from analysis. All analyses were stratified by race-ethnicity.
Test of association were calculated for the number of SNPs and phenotypes listed in Table 1. The software used to calculate the associations for each study was as follows: ARIC (StatSoftware), CHS (R [32]), MEC (SAS), MEC (SAS v9.2), WHI (R), EAGLE (SAS v9.2 using the Analytic Data Research by Email (ANDRE) portal of the CDC Research Data Center in Hyattsville, MD).
All association results from the tests of association were reported in standardized templates designed by the PAGE coordinating center to facilitate data sharing. All results were then imported into a relational database (MySQL). The database was also used to match previously reported GWAS data with the SNPs analyzed in this study.
Plotting Significant Results
The software PheWAS-View was developed for data visualization of the PheWAS results as well as for plotting “Sun Plots” [33]. Synthesis-View [34], [35] was also used to present results within this manuscript. Both software packages are freely available software for academic users: http://ritchielab.psu.edu/ritchielab/software, and can be used with a web interface at: http://visualization.ritchielab.psu.edu/.
Matching Phenotypes
A total of 105 phenotype-classes were developed to manually match related phenotypes across studies. To bin related phenotypes into classes the following steps were used as visualized in Figure 6: First, using a MySQL database, the data from EAGLE, MEC, CHS, ARIC, and WHI were independently filtered for any tests of association results at p<0.01, and then lists of the unique phenotypes for each individual PAGE study were generated. The number of phenotypes that passed this significance threshold for each of the four groups was 604 (ARIC), 331 (CHS), 63 (MEC), 324 (EAGLE), 1,342 (WHI). Resulting phenotypes were then manually matched up between ARIC, CHS, MEC, EAGLE and WHI using knowledge about the phenotypes and the known focus of specific PAGE study survey questions (such as bone fracture questions used primarily for collecting information about osteoporosis). For some phenotypes, the specific phenotype existed clearly across more than one PAGE study, such as for the phenotype “Hemoglobin”, where hemoglobin measurements were present for ARIC, CHS, EAGLE, and WHI. Other groups of phenotypes that fell within similar phenotypic domains but were not represented in the same form across studies were also collected into phenotype classes. One example is the phenotypes grouped together for the phenotype class of “Allergy”. EAGLE collected specific quantitative data from allergy skin testing and had survey questions about the presence of allergies in participants. ARIC and MEC did not have skin allergy testing, but did have survey questions about the presence of allergies. Thus these allergy phenotypes were grouped together. Finally, phenotypes from all studies, regardless of significance from genotype-phenotype tests of association, were matched to the already-defined phenotype classes using the criteria described above. A phenotype that matched a phenotype class but was not associated with a SNP at the significance threshold of p<0.01 for a single study would still be included in the phenotype-class list. Using these criteria, a second curator reviewed the resultant phenotypes and phenotype classes for consistency and accuracy. To provide examples of the phenotype-classes, and which subphenotypes were matched with phenotype-classes, we show three phenotype-class examples in Table 5, and Table S2 contains the matched phenotypes across studies within the phenotype-classes for all phenotype-classes used within this study.
10.1371/journal.pgen.1003087.g006 Figure 6Workflow for phenotype matching, to develop the 105 phenotype classes.
A MySQL database was used to filter the data from five studies for any results with p<0.01 to generate lists of the unique phenotypes for each individual PAGE study. The number of phenotypes that passed this significance threshold for each of the four groups was 604 (ARIC), 331 (CHS), 63 (MEC), 324 (EAGLE), 1,342 (WHI). Note that during the binning process, a smaller number of phenotypes are listed in Figure 6 than the total number of phenotypes referred to in the manuscript for the actual associations, in the phenotype matching process we only took into account distinct phenotypes regardless of whether or not they were transformed or untransformed or if they were categorical phenotypes binned into case/control phenotypes. Next, resulting phenotypes were then manually matched up between ARIC, CHS, MEC, EAGLE and WHI using and knowledge about the phenotypes and the known focus of specific PAGE study questions (such as arterial measurements including degree of arterial stenosis). In the last step, phenotypes from all studies, regardless of significance from genotype-phenotype tests of association, were matched to the already-defined phenotype classes using the criteria described above.
10.1371/journal.pgen.1003087.t005 Table 5Example phenotype-classes and binned subphenotypes within phenotype-classes.
Phenotype Class Study Sub-phenotype binned within the phenotype-class Asthma ARIC Asthma ever diagnosed? Asthma ARIC Chest wheeze, whistle alot? Asthma ARIC Chest wheeze, whistle, otherwise? Asthma ARIC Age at 1st wheezing attack Asthma ARIC Age asthma started Asthma ARIC Age asthma stopped Asthma ARIC Short of breath wheezing attack? Asthma CHS Inhaled steroids for asthma Asthma CHS Asthma confirmed by doctor Asthma CHS Current asthma diagnosis by doctor Asthma EAGLE Doctor ever told you had: asthma Asthma MEC Asthma: History of Asthma, Hayfever, Skin Allergy, Food Allergy or Any Other Allergy from Baseline Questionnaire CRP CHS C-reactive protein, adjusted original values (mg/l) CRP CHS C-reactive protein, original values (mg/l) CRP EAGLE Serum C-reactive protein (mg/dL) Hemoglobin ARIC Hemoglobin Hemoglobin CHS hemoglobin (g/dl) Hemoglobin EAGLE Hemoglobin (g/dL) Hemoglobin WHI Hemoglobin (gm/dl) Hemoglobin EAGLE Mean cell hemoglobin concentration Hemoglobin EAGLE Mean cell hemoglobin: SI (pg)Presented below are examples of phenotypes binned into three phenotype classes, “Asthma”, “BMI”, and “CRP”. Table S2 contains the complete list of matched phenotypes across studies within phenotype-classes, for all phenotype-classes used within this study.
It is important to note resources that can be used for further investigation of the phenotypes listed in Table S2, as well as in the results presented in this paper. The following study websites contain additional information about all collected study information, including how those phenotypes were collected:
-
CHS http://www.chs-nhlbi.org/CHSData.htm, https://biolincc.nhlbi.nih.gov/static/studies/chs/Other_Documents.htm
-
EAGLE http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm/
-
MEC http://www.crch.org/multiethniccohort/mec_questionnaires.htm
Criteria for Significance of Association
After creating phenotype-classes, significant PheWAS tests of association for single genotype-phenotype associations across PAGE studies were identified using a database query. Our criteria for considering a PheWAS test of association significant included a threshold of p<0.01 observed in two or more PAGE studies for the same SNP, phenotype class, and race/ethnicity and consistent direction of effect.
A total of 111 PheWAS tests of association met our criteria for significance (Table S3). Significant results were then binned based on class of association: known, related, and novel. In this PheWAS, Known Associations are positive controls and represent previously reported genotype-phenotype associations. Related Associations are SNPs significantly associated in this PheWAS with phenotypes judged to be closely related to phenotypes among Known Associations found here and the literature. Novel Associations are significant PheWAS results where 1) the association does not match a known association and 2) the phenotype for the PheWAS association is not within a similar phenotypic domain as the phenotype of known association.
Ethics Statement
All participating studies were approved by their respective IRBs, and all study participants signed informed consent forms.
Supporting Information
Zdroje
1. PendergrassSA, Brown-GentryK, DudekSM, TorstensonES, AmbiteJL, et al. (2011) The use of phenome-wide association studies (PheWAS) for exploration of novel genotype-phenotype relationships and pleiotropy discovery. Genetic epidemiology 35 : 410–422.
2. McCartyCA, ChisholmRL, ChuteCG, KulloIJ, JarvikGP, et al. (2011) The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC medical genomics 4 : 13.
3. DennyJC, RitchieMD, BasfordMA, PulleyJM, BastaracheL, et al. (2010) PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics 26 : 1205–1210.
4. CollinsFS (2004) The case for a US prospective cohort study of genes and environment. Nature 429 : 475–477.
5. CollinsFS, ManolioTA (2007) Merging and emerging cohorts: necessary but not sufficient. Nature 445 : 259.
6. WillettWC, BlotWJ, ColditzGA, FolsomAR, HendersonBE, et al. (2007) Merging and emerging cohorts: not worth the wait. Nature 445 : 257–258.
7. MatiseTC, AmbiteJL, BuyskeS, CarlsonCS, ColeSA, et al. (2011) The Next PAGE in Understanding Complex Traits: Design for the Analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. American journal of epidemiology 174 : 849–859.
8. KathiresanS, MelanderO, GuiducciC, SurtiA, BurttNP, et al. (2008) Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nature genetics 40 : 189–197.
9. TeslovichTM, MusunuruK, SmithAV, EdmondsonAC, StylianouIM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 : 707–713.
10. PatelRS, SuS, NeelandIJ, AhujaA, VeledarE, et al. (2010) The chromosome 9p21 risk locus is associated with angiographic severity and progression of coronary artery disease. Eur Heart J 31 : 3017–3023.
11. HelgadottirA, ThorleifssonG, ManolescuA, GretarsdottirS, BlondalT, et al. (2007) A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316 : 1491–1493.
12. SandhuMS, WaterworthDM, DebenhamSL, WheelerE, PapadakisK, et al. (2008) LDL-cholesterol concentrations: a genome-wide association study. Lancet 371 : 483–491.
13. WillerCJ, SannaS, JacksonAU, ScuteriA, BonnycastleLL, et al. (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nature genetics 40 : 161–169.
14. WallaceC, NewhouseSJ, BraundP, ZhangF, TobinM, et al. (2008) Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. American journal of human genetics 82 : 139–149.
15. SamaniNJ, ErdmannJ, HallAS, HengstenbergC, ManginoM, et al. (2007) Genomewide association analysis of coronary artery disease. N Engl J Med 357 : 443–453.
16. ZegginiE, ScottLJ, SaxenaR, VoightBF, MarchiniJL, et al. (2008) Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nature genetics 40 : 638–645.
17. JiangCQ, LamTH, LiuB, LinJM, YueXJ, et al. (2010) Interleukin-6 receptor gene polymorphism modulates interleukin-6 levels and the metabolic syndrome: GBCS-CVD. Obesity (Silver Spring) 18 : 1969–1974.
18. ZegginiE, WeedonMN, LindgrenCM, FraylingTM, ElliottKS, et al. (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316 : 1336–1341.
19. SaxenaR, VoightBF, LyssenkoV, BurttNP, de BakkerPI, et al. (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 : 1331–1336.
20. SalonenJT, UimariP, AaltoJM, PirskanenM, KaikkonenJ, et al. (2007) Type 2 diabetes whole-genome association study in four populations: the DiaGen consortium. American journal of human genetics 81 : 338–345.
21. ReinerAP, LettreG, NallsMA, GaneshSK, MathiasR, et al. (2011) Genome-wide association study of white blood cell count in 16,388 African Americans: the continental origins and genetic epidemiology network (COGENT). PLoS Genet 7: e1002108 doi:10.1371/journal.pgen.1002108.
22. CrosslinDR, McDavidA, WestonN, NelsonSC, ZhengX, et al. (2011) Genetic variants associated with the white blood cell count in 13,923 subjects in the eMERGE Network. Hum Genet
23. Wellcome Trust Case Control Consortium (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 : 661–678.
24. LettreG, PalmerCD, YoungT, EjebeKG, AllayeeH, et al. (2011) Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS Genet 7: e1001300 doi:10.1371/journal.pgen.1001300.
25. IsomaaB, AlmgrenP, TuomiT, ForsenB, LahtiK, et al. (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes care 24 : 683–689.
26. MatiseTC, AmbiteJL, BuyskeS, CarlsonCS, ColeSA, et al. (2011) The Next PAGE in Understanding Complex Traits: Design for the Analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. American journal of epidemiology 174 : 849–859.
27. DumitrescuL, CartyCL, TaylorK, SchumacherFR, HindorffLA, et al. (2011) Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study. PLoS Genet 7: e1002138 doi:10.1371/journal.pgen.1002138.
28. FesinmeyerMD, NorthKE, RitchieMD, LimU, FranceschiniN, et al. (2012) Genetic Risk Factors for BMI and Obesity in an Ethnically Diverse Population: Results From the Population Architecture Using Genomics and Epidemiology (PAGE) Study. Obesity (Silver Spring).
29. HaimanCA, FesinmeyerMD, SpencerKL, BuzkovaP, VorugantiVS, et al. (2012) Consistent Directions of Effect for Established Type 2 Diabetes Risk Variants Across Populations: The Population Architecture using Genomics and Epidemiology (PAGE) Consortium. Diabetes 61 : 1642–1647.
30. HindorffLA, SethupathyP, JunkinsHA, RamosEM, MehtaJP, et al. (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106 : 9362–9367.
31. JohnsonAD, O'DonnellCJ (2009) An open access database of genome-wide association results. BMC Med Genet 10 : 6.
32. Team TRDC (2009) R: A Language and Environment for Statistical Computing.
33. PendergrassSA, DudekS, CrawfordDC, RitchieMD (2012) Visually integrating and exploring high throughput Phenome-Wide Association (PheWAS) results using PheWAS-View. BioData Min 5 : 5.
34. PendergrassSA, DudekSM, CrawfordDC, RitchieMD (2010) Synthesis-View: visualization and interpretation of SNP association results for multi-cohort, multi-phenotype data and meta-analysis. BioData Min 3 : 10.
35. PendergrassS, DudekSM, RodenDM, CrawfordDC, RitchieMD (2011) Visual integration of results from a large DNA biobank (biovu) using synthesis-view. Pac Symp Biocomput 265–275.
36. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. American journal of epidemiology 129 : 687–702.
37. FriedLP, BorhaniNO, EnrightP, FurbergCD, GardinJM, et al. (1991) The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1 : 263–276.
38. Centers for Disease Control and Prevention NCfHS (1994) Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1 : 1–407.
39. KolonelLN, HendersonBE, HankinJH, NomuraAM, WilkensLR, et al. (2000) A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. American journal of epidemiology 151 : 346–357.
40. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials 19 : 61–109.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



