-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
Clostridium difficile, a Gram positive, anaerobic, spore-forming bacterium is an emergent pathogen and the most common cause of nosocomial diarrhea. Although transmission of C. difficile is mediated by contamination of the gut by spores, the regulatory cascade controlling spore formation remains poorly characterized. During Bacillus subtilis sporulation, a cascade of four sigma factors, σF and σG in the forespore and σE and σK in the mother cell governs compartment-specific gene expression. In this work, we combined genome wide transcriptional analyses and promoter mapping to define the C. difficile σF, σE, σG and σK regulons. We identified about 225 genes under the control of these sigma factors: 25 in the σF regulon, 97 σE-dependent genes, 50 σG-governed genes and 56 genes under σK control. A significant fraction of genes in each regulon is of unknown function but new candidates for spore coat proteins could be proposed as being synthesized under σE or σK control and detected in a previously published spore proteome. SpoIIID of C. difficile also plays a pivotal role in the mother cell line of expression repressing the transcription of many members of the σE regulon and activating sigK expression. Global analysis of developmental gene expression under the control of these sigma factors revealed deviations from the B. subtilis model regarding the communication between mother cell and forespore in C. difficile. We showed that the expression of the σE regulon in the mother cell was not strictly under the control of σF despite the fact that the forespore product SpoIIR was required for the processing of pro-σE. In addition, the σK regulon was not controlled by σG in C. difficile in agreement with the lack of pro-σK processing. This work is one key step to obtain new insights about the diversity and evolution of the sporulation process among Firmicutes.
Published in the journal: Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in. PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003756
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003756Summary
Clostridium difficile, a Gram positive, anaerobic, spore-forming bacterium is an emergent pathogen and the most common cause of nosocomial diarrhea. Although transmission of C. difficile is mediated by contamination of the gut by spores, the regulatory cascade controlling spore formation remains poorly characterized. During Bacillus subtilis sporulation, a cascade of four sigma factors, σF and σG in the forespore and σE and σK in the mother cell governs compartment-specific gene expression. In this work, we combined genome wide transcriptional analyses and promoter mapping to define the C. difficile σF, σE, σG and σK regulons. We identified about 225 genes under the control of these sigma factors: 25 in the σF regulon, 97 σE-dependent genes, 50 σG-governed genes and 56 genes under σK control. A significant fraction of genes in each regulon is of unknown function but new candidates for spore coat proteins could be proposed as being synthesized under σE or σK control and detected in a previously published spore proteome. SpoIIID of C. difficile also plays a pivotal role in the mother cell line of expression repressing the transcription of many members of the σE regulon and activating sigK expression. Global analysis of developmental gene expression under the control of these sigma factors revealed deviations from the B. subtilis model regarding the communication between mother cell and forespore in C. difficile. We showed that the expression of the σE regulon in the mother cell was not strictly under the control of σF despite the fact that the forespore product SpoIIR was required for the processing of pro-σE. In addition, the σK regulon was not controlled by σG in C. difficile in agreement with the lack of pro-σK processing. This work is one key step to obtain new insights about the diversity and evolution of the sporulation process among Firmicutes.
Introduction
Clostridium difficile, a Gram positive, anaerobic, spore-forming bacterium is a major cause of nosocomial infections associated with antibiotic therapy and is a major burden to health care services. This enteropathogen can lead to antibiotic-associated diarrhea and pseudo-membranous colitis, a potentially lethal disease. Two large toxins, the enterotoxin TcdA and the cytotoxin TcdB are the main virulence factors required for the development of symptoms of C. difficile infection (CDI). C. difficile produces highly resistant spores that facilitate the persistence of this bacterium in the environment in particular in aerobic conditions and contaminate hospital environments contributing to the establishment of a reservoir. Transmission of C. difficile is further mediated by contamination of the gut by spores as demonstrated recently using a murine model for CDI [1], [2]. The disruption of the colonic microflora by antimicrobial therapy precipitates CDI and colonization of the intestinal tract. An early event towards this colonization is the germination process that converts dormant spores into vegetative cells that multiply leading to the production of toxins [3]. Glycine and bile salts like sodium cholate or sodium taurocholate are co-germinants required to induce C. difficile spores germination [4]. However, the molecular mechanisms involved in sporulation and germination are still poorly studied in C. difficile and our current knowledge on these processes is based mainly on data derived from the studies on Bacillus subtilis.
At the onset of sporulation in B. subtilis, sporulating cells undergo an asymmetric division which partitions the sporangial cell into a larger mother cell and a smaller forespore (the future spore). The forespore is next wholly engulfed by the mother cell and later the dormant spore is released from the mother cell by lysis [5]. The developmental program of sporulation is mainly governed by the sequential activation of four sigma factors: σF, σE, σG and σK. Their activity is confined to the forespore for σF and σG and to the mother cell for σE and σK. Compartmentalization of gene expression is coupled to morphogenesis with σF and σE becoming active after asymmetric division and σG and σK after completion of engulfment of the forespore by the mother cell [5], [6], [7].
In B. subtilis, coordinated changes in gene expression underlie morphological differentiation in both the predivisional sporangium and later in the two compartments with the existence of communication between the mother cell and the forespore. The response regulator, Spo0A, and a phosphorelay involving five kinases, intermediary phosphorylated proteins and phosphatases control sporulation initiation. The alternative sigma factor, σH, which transcribes spo0A and sigF also controls early sporulation steps. When Spo0A-P level reaches a critical threshold, Spo0A-P activates sporulation genes including spoIIE as well as both the spoIIAA-spoIIAB-sigF and the spoIIGA-sigE operons encoding σF and σE, respectively [8]. After its synthesis, σF is held inactive by the anti-sigma factor SpoIIAB until the phosphatase SpoIIE dephosphorylates the anti-anti sigma factor SpoIIAA leading to the release of an active σF from SpoIIAB after completion of asymmetric cell division [5]. σF then transcribes about 50 genes in the forespore [9], [10] including spoIIR encoding a secretory protein required for the processing of pro-σE into active σE in the mother cell [11]. σE regulates in turn the expression of mother cell specific genes [9], [12], [13] and activates sigK expression with the combined activity of the SpoIIID regulator [14]. In the forespore, σF also controls sigG transcription. However, σG becomes active coincidently with the completion of forespore engulfment by the mother cell. Following engulfment completion, the σE-controlled SpoIIIA proteins, together with the forespore-specific SpoIIQ protein, are required to maintain the potential for macromolecular synthesis in the forespore [6]. The RNA polymerase σG holoenzyme transcribed 95 genes in the forespore including proteins like SpoIVB or CtpB involved in the processing and activation of σK the last factor sigma of sporulation [5], [6].
The four sporulation specific sigma factors are conserved in Clostridia and are present in C. difficile [15], [16], [17], [18], [19], [20]. This is also the case for key genes involved in the spore morphogenesis [16], [17], [20]. In Clostridium acetobutylicum and Clostridium perfringens, the role of sporulation sigma factors in spore morphogenesis has been analyzed [21], [22], [23], [24]. No resistant spores are formed by the sigF and sigG mutants of both Clostridia and by the sigE mutant of C. acetobutylicum whereas the sigE and sigK mutants of C. perfringens are severely defective in their ability to sporulate. Interestingly, sigE and sigF mutants of C. acetobutylicum fail to form the asymmetric division septum [22], [24] while a sigK mutant is blocked earlier in sporulation than a sigE mutant in C. perfringens [21]. A detailed study of the morphological changes during the C. difficile spore differentiation process has recently been performed [19]. The C. difficile sigF and sigE mutants are arrested at the asymmetric stage while the sigG mutant is blocked after the completion of engulfment but unlike in B. subtilis, shows deposition of electrodense coat material around the forespore. These mutants are unable to sporulate [19]. A sigK mutant of C. difficile forms four orders of magnitude fewer heat resistant spores than the isogenic wild-type strain. While showing no signs of coat deposition around the developing spore, this mutant shows accumulation of at least some cortex material unlike the case for B. subtilis [19]. The characterization of the sig sporulation mutants in several Clostridia suggests deviations from the B. subtilis paradigm regarding the function of the sporulation sigma factors [19], [20], [21], [22], [24], [25].
The regulatory cascade controlling spore formation is also still poorly characterized in Clostridia compared to B. subtilis. The global analysis of the C. acetobutylicum transcriptional program during sporulation has given the dynamic orchestration of expression of the sporulation sigma factors and of key sporulation genes in this Clostridia [26] but the definition of the four-sigma factors regulons remains to be determined. In C. difficile, recent data have been obtained on sporulation initiation. Both Spo0A and σH are present and required for efficient C. difficile sporulation [27], [28]. By contrast, the phosphorelay is absent and the sporulation initiation pathway remains in C. difficile and other Clostridia as a two-component system with Spo0A and associated kinases [18], [28], [29]. Both σH and Spo0A directly control the expression of the spoIIAA-spoIIAB-sigF operon while Spo0A binds in vitro to the promoter region of spoIIAA, spoIIE and spoIIGA (in operon with sigE) [27], [30]. By contrast, little is known about the molecular mechanisms controlling later steps in the sporulation regulatory cascade in C. difficile. In this work, we combined transcriptome, genome-wide transcriptional start site (TSS) mapping and in silico identification of promoters to define the σF, σE, σG and σK regulons. This helped us to propose candidates for new spore coat proteins. Finally, we also identified interesting differences in the regulatory cascade of C. difficile compared to the B. subtilis model. We showed that activation of the σE regulon was partially independent of σF and that the σK regulon was not controlled by σG.
Results and Discussion
I. Analysis of the sporulation regulatory network by global approaches
Overview of the transcriptome data
The C. difficile mutants inactivated for σF, σE, σG or σK have been recently constructed [19]. The sigF and sigE mutants are blocked at stage II of sporulation. The sigG mutant is blocked after the completion of engulfment and the sigK mutant form spores lacking coat material [19]. Thus, the sequential appearance of events seems to be similar to that observed in B. subtilis: σF and σE control early stages of development and are likely replaced by σG and σK later during sporulation. These studies have also shown the confinement of the activities of σF and σG to the forespore, and those of σE and σK to the mother cell [19]. To investigate more in detail the sporulation regulatory cascade in C. difficile and to define the regulons controlled by the four cell type-specific RNA polymerase sigma factors, we compared the expression profiles of the wild-type 630Δerm strain and of the sigF, sigE, sigG or sigK mutant. We performed preliminary tests to determine conditions allowing detection of differential expression between the 630Δerm strain and each mutant inactivated for a sigma factor as described in Materials and Methods. The cells were harvested 14 h after inoculation for the wild-type strain 630Δerm and the sigF or the sigE mutant, after 19 h of growth for strain 630Δerm and the sigG mutant or after 24 h of growth for strain 630Δerm and the sigK mutant. We found 111 genes and 141 genes differentially expressed with a p value <0.05 between the 630Δerm strain and the sigF mutant or the sigE mutant, respectively (Table S1 and S2). While 92 and 19 genes were down and upregulated in the sigF mutant compared to the 630Δerm strain, all the 141 genes were downregulated in the sigE mutant. In addition, 51 and 66 genes were differentially expressed with a p value <0.05 between the 630Δerm strain and the sigG or the sigK mutant in transcriptome, respectively (Table S3 and S4). All of the presumptive sigG-controlled genes were downregulated in the mutant compared to the 630Δerm strain. Finally, 58 and 8 genes were down and upregulated in the sigK mutant relative to the 630Δerm strain. To confirm the results obtained in the microarrays experiments, we performed qRT-PCR on a subset of 10 to 15 genes representative of various cell functions and regulated by each of the cell type-specific sigma factors. The qRT-PCR results confirmed the microarrays data for all of the tested genes (Table S5).
Mapping of transcriptional start sites of genes controlled by sporulation sigma factors
We recently performed a genome wide determination of transcriptional start sites (TSS) for the 630Δerm strain using RNA-seq [31]. We searched in our data for TSS of all the genes found to be controlled by σF, σE, σG or σK in the transcriptome analysis. By this global approach, we mapped about 111 TSS upstream of genes positively controlled by at least one of the four sporulation-specific sigma factors (Table S6 and S7). We analyzed these data by an iterative in silico strategy as described in Materials and Methods. We further manually analyzed all promoters not found by this in silico procedure (<30%). These strategies allowed us to identify promoters for each sigma factor (Table S6, S7 and S8). We identified about 40 promoters likely to be utilized by RNA polymerase associated to one of the forespore sigma factors (σF or σG) (Table S6). The consensus elements recognized by σF in C. difficile were determined by using 10 mapped promoters of genes specifically controlled by σF in transcriptome (downregulated in a sigF mutant but not in the sigE, sigG or sigK mutant) (Table S6). The conserved motifs for σG-dependent promoters were defined from a dataset of 30 promoters located upstream of genes specifically controlled by σG in transcriptome (downregulated in a sigG mutant but not in a sigK mutant) (). The in silico analysis identified 9 of 10 σF promoters and 23 of 30 σG promoters. The consensus sequences for C. difficile σF - and σG-controlled promoters (Fig. 1A) are very similar to those of B. subtilis [10], [16] and to each other in their −10 and −35 elements as noted for B. subtilis [10]. However, they differ in that a G is conserved on the upstream side of the −10 promoter element in σF-dependent promoters (position 23 in Fig. 1A) whereas for σG-dependent promoters a A is conserved on the downstream side of the −10 element (position 32 in Fig. 1A).
Fig. 1. Consensus sequences of σF, σE, σG or σK-dependent promoters in C. difficile. 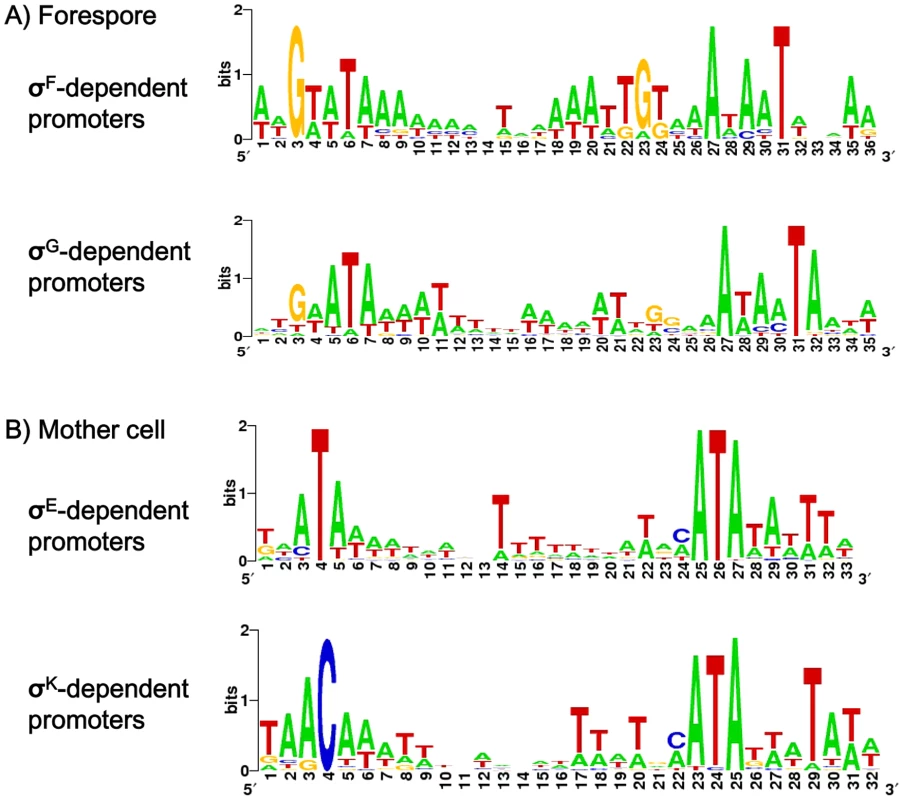
Consensus promoter sequences for σF- and σG-controlled promoters (A) or σE- and σK-controlled promoters (B) in C. difficile. The sequence logo was created on the WebLogo website (http://weblogo.berkeley.edu) using promoters mapped in this study and listed in Tables S6 and Table S7: compilation of 10 common motifs upstream of σF-regulated genes, of 30 common elements upstream of σG-regulated genes, of 47 conserved sequences upstream of σE-regulated genes or 24 conserved elements σK-regulated genes. The height of the letters is proportional to their frequency. Upstream of genes specifically controlled by σE (downregulated in a sigE mutant but neither in a sigK mutant nor in a sigG mutant), we identified 47 promoters, 25 of which were seen in the in silico analysis (Table S7 and S8). Moreover, we mapped 24 TSS corresponding to promoters recognized by σK upstream of σK-controlled genes in transcriptome, 19 of which were found in silico. The C. difficile consensus elements specific for σE - and σK-controlled promoters are very similar to those determined in B. subtilis [12], [14], [16]. The motif recognized by σE and σK shared very similar −10 sequences while the −35 elements differed with an ATA motif for σE and AC for σK (Fig. 1B). In B. subtilis, the specificity of interaction of these sigma factors with the −35 region sequences is associated with the presence of a glutamine at position 217 of σE and an arginine in σK. Indeed, the replacement of glutamine 217 in σE by an arginine allows σE to recognize σK-controlled genes [32]. Interestingly, σE and σK of C. difficile contain a glutamine and an arginine at position 218, respectively (Fig. S1). This observation supports our inference that a conserved T or C is an important feature of the −35 element herein identified for C. difficile σE and σK promoters.
To date, less than 10 promoters of sporulation-regulated genes have been characterized in Clostridia, mainly in C. acetobutylicum (spoIIGA, sigG, sigK) and C. perfringens (spoIIGA, cpe, sigK) [33], [34]. This work greatly increased the number of characterized promoters for clostridial sporulation genes. The probability to observe orthologs with high-scoring candidate promoters in many clostridial genomes (Table S8) is highest for genes regulated both in C. difficile and B. subtilis [9], [10], [12], [14], as these genes likely form the regulon cores.
II. An overview of the four compartment-specific σF, σE, σG and σK regulons
We combined transcriptome data and promoter identifications to define the σF, σE, σG and σK regulons and to determine the pool of genes probably under the direct control of each sigma factor among genes positively controlled by these four sigma factors (Table 1 and 2).
Tab. 1. The forespore line of expression with the identification of the σF and σG regulon. 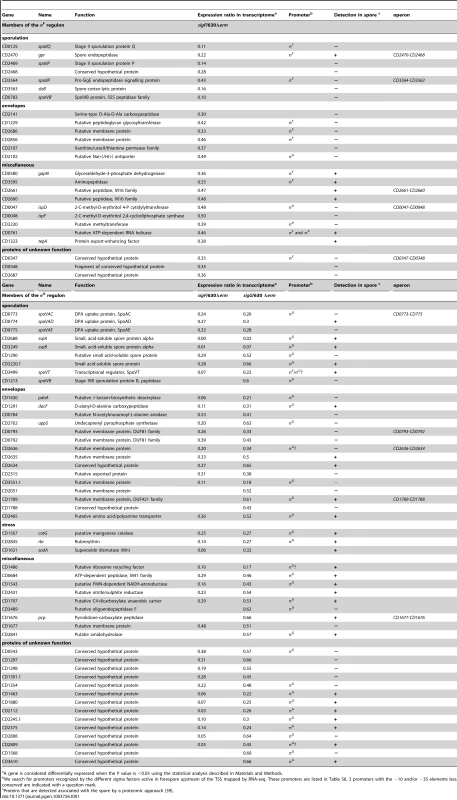
A gene is considered differentially expressed when the P value is <0.05 using the statistical analysis described in Materials and Methods. Tab. 2. The mother cell line expression with the identification of the σE and σK regulons and the regulation by SpoIIID of σE-controlled genes. 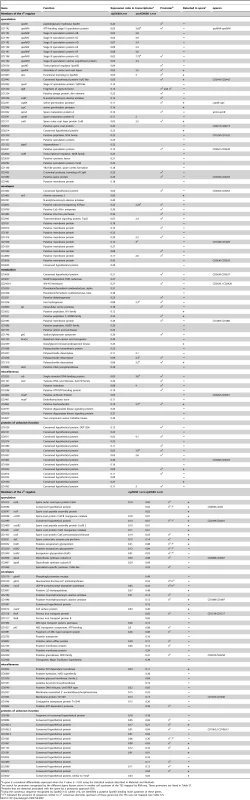
A gene is considered differentially expressed when the P value is <0.05 using the statistical analysis described in Materials and Methods. The σF regulon
Among the 111 genes controlled by σF in transcriptome, 25 were downregulated in the sigF mutant but not in the sigE, sigG or sigK mutant (Table 1). We identified 10 σF-dependent promoters controlling the expression of 14 genes. The σF regulon is the smallest of the four-compartment specific regulons as observed in B. subtilis 9,10. The orthologs of B. subtilis genes involved in sporulation regulatory functions, in spore morphogenesis or in germination [5], [16], [17], [20] were downregulated in the sigF mutant compared to strain 630Δerm. σF positively controlled the expression of spoIIR, encoding a signaling protein that in B. subtilis triggers the activation of the membrane bound SpoIIGA protease that in turn cleaves the pro-σE protein and leads to the production of an active σE factor [5]. The spoIIQ and spoIIP genes, the expression of which was also downregulated in the sigF mutant, are involved in the engulfment process. SpoIIP is a component of a molecular machine involved in dissolution of the septal cell wall during engulfment in B. subtilis [35], [36]. The B. subtilis SpoIIQ protein interacts with a mother cell-specific protein across the intermembrane space, an interaction that helps driving engulfment and that is also central to the formation of a channel linking the mother cell to the forespore [6], [37], [38]. In addition, σF was required to transcribe the gpr gene encoding a protease that is important for the degradation of small acid-soluble spore proteins (SASPs) during germination. The orthologs of these genes are members of the σF regulon in B. subtilis [9], [10], [16]. On the contrary, gpr, spoIIR and spoIIP are not controlled by σF in C. acetobutylicum [22]. σF was also required for the expression of genes involved in cell wall metabolism: sleB, CD2141 and CD1229 encoding a spore cortex hydrolysing protein, a DAla-DAla carboxypeptidase and a peptidoglycan (PG) glycosyltransferase, respectively. Moreover, σF positively controlled the expression of 5 genes encoding proteins previously detected in the spore proteome [39]: a glyceraldehyde-3P-dehydrogenase (CD0580), three peptidases (CD2660, CD2661 CD3595), and an RNA helicase (CD0761) (Table 1). Finally, 3 genes encoding proteins of unknown function and 2 genes encoding putative membrane proteins (CD2686 and CD2856) were downregulated in the sigF mutant.
The σE regulon
We found 97 genes to be downregulated in a sigE mutant but not in a sigG mutant or in a sigK mutant (Table 2). TSS mapping allowed us to identify 47 σE-dependent promoters controlling the expression of 63 genes (Table S7). σE controls the largest of the four cell type-specific sporulation regulons as also found for B. subtilis [9], [12]. Several σE target genes in C. difficile are orthologs of genes known to control engulfment, cortex formation, initiation of spore coat assembly, preparation of the late phase of sporulation or germination in B. subtilis (Table 2). This is consistent with the morphological block imposed by a sigE insertional mutation, which arrests development at the asymmetric division stage [19]. spoIID, which encodes a PG hydrolase required for the engulfment of the forespore by the mother cell was controlled by σE. In B. subtilis, SpoIID is associated to SpoIIP and SpoIIM to form the DMP machine necessary for engulfment [35], [36]. The spoIIP gene was only controlled by σF in the transcriptome analysis. However, in qRT-PCR experiments, we showed that spoIIP expression decreased 10-fold in a sigE mutant and 800-fold in a sigF mutant. This suggested that this gene was under the control of both sigma factors as observed in B. subtilis 10,12. Also, even though a membrane protein (CD1221) sharing weak similarity to the N-terminal part of B. subtilis SpoIIM is present in the C. difficile genome, its gene was not differentially expressed between the 630Δerm strain and the sigE or sigF mutant in our conditions. In addition, σE activated the expression of the spoIIIAA octacistronic operon encoding an ATPase and membrane proteins that localize to the outer forespore membrane in B. subtilis, and integrate with SpoIIQ a novel type of secretion system [6], [37], [38].
In B. subtilis, the synthesis of the protective envelopes that encase the spore, the coat and the cortex PG, is initiated under σE control [12]. In C. difficile, the expression of spoIVA and sipL encoding an ortholog of the SpoIVA morphogenetic ATPase and a functional homolog to B. subtilis SpoVID was downregulated in the sigE mutant. These proteins are required for proper spore coat localization around the forespore in C. difficile [40]. The expression of cotB encoding a recently identified coat protein [41] was also specifically regulated by σE. Accordingly, a σE-dependent promoter was mapped upstream of spoIVA, sipL and cotB (Table S7). Moreover, an ortholog of the cwlD gene, encoding a N-acetylmuramoyl-L-alanine amidase required for the synthesis of muramic ∂-lactam, a specific cortex PG compound [42], was controlled by σE. On the contrary, the second enzyme of this pathway, the PdaA deacetylase, was produced in the forespore (see below). The synthesis of other polysaccharide deacetylases (CD3257, CD3248, CD1319), of a polysaccharide biosynthetic enzyme (CD1068), of an alanine racemase (Alr2) and of a N-acetylmuramoyl-L-alanine amidase (CD2761) was also under σE control. CD1168 encoding an YlbJ-like protein, and the CD2443-CD2442 operon encoding YqfC and YqfD-like proteins were controlled by σE. The inactivation of yqfC, yqfD and ylbJ in B. subtilis reduces heat resistance of the spore and prevents the development of refractility, both phenotypes attributed to defects in cortex formation [12], [13]. In addition, the expression of the spmA-spmB operon involved in spore core dehydratation and heat resistance in B. subtilis and C. perfringens [43] was reduced in the sigE mutant. We observed a positive control by σE of the synthesis of CD2865, an homolog of a bacterioferritin involved in iron storage and oxidative stress protection in the anaerobe Desulfovibrio vulgaris [44].
Several genes under σE control in B. subtilis are required for spore germination because they are involved in modifications of the cortex or in coat assembly. In C. difficile, σE was required for the transcription of the csp operon encoding serine proteases. In C. perfringens and C. difficile, CspB is necessary for pro-SleC maturation to form the spore cortex lytic enzyme SleC during germination [45], [46], [47] while CspC is a germinant receptor for taurocholate [48]. The promoter of the C. difficile csp operon matched the consensus for σE-dependent promoters while sleC was transcribed from a σK-dependent promoter (see below). We also observed a positive control by σE of three other proteases (CD2000, CD3455, CD1320). CD3455 shares 38% identity with CtpB, a protease required for σK activation in B. subtilis [6] and the Isp ortholog of CD2000, is involved in sporulation in Bacillus thuringiensis [49]. Presumably, these proteases also participate in the spore formation process in C. difficile.
Another major function under σE control in B. subtilis is the maintenance of a sufficient level of metabolic activity to enable the completion of sporulation [9], [12]. σE also controlled the expression of several metabolic genes in C. difficile (Table 2). Among them are genes encoding proteins likely to be required for oxidoreduction pathways and energy metabolism: an oxido-reductase (CD2429.1-CD2429-CD2428), a FMN reductase (CD3637), a dehydrogenase (CD3251) and an iron hydrogenase (CD3258). In addition, σE activated the expression of genes encoding peptidases (CD3652, CD1086, CD3521) or amino acids permeases (CD1746, CD1555 and CD1259). Moreover, the CD2833 and CD0760 genes encoding a Ca2+-transporting ATPase and a Ca2+/Na+ antiporter, respectively, were downregulated in the sigE mutant. These proteins are probably important for the import or export of Ca2+, a key element of the sporulation and germination processes in endospore forming Firmicutes [47].
Other C. difficile σE controlled genes do not belong to any of the categories above. Of particular interest, σE controlled the expression of the mazF-mazE operon, encoding the unique toxin-antitoxin (TA) system of C. difficile [50]. In B. subtilis, a different TA system, SpoIISA-SpoIISB synthesized in the mother cell, is involved in sporulation [51]. MazF cleaves mRNA while the SpoIISA toxin is targeted to the cell membrane. The role of MazEF in the sporulation process remains to be characterized. Finally, 18 genes encoding proteins of unknown function were downregulated in the sigE mutant compared to the 630Δerm strain. Only 15 members of the σE regulon are detected in the spore proteome [39]. This included the SpoIVA and SipL proteins, the CotB coat protein, the Csp-like proteases, the YqfC like protein and three proteins of unknown function (CD0214, CD3522 and CD1930).
The σG regulon
From the transcriptome experiments, we identified 50 genes controlled by σG but not by σK (Table 1). The TSS mapping performed by RNA-seq allowed us to identify 30 σG-dependent promoters controlling the expression of 33 genes. Several σG-controlled genes encode proteins sharing similarities with proteins involved in spore resistance in B. subtilis [5], [16], [52]. Dipicolinic acid (DPA) is a major spore component important for spore resistance. The expression of the spoVA operon whose products are required for the import of DPA into the forespore from the mother cell [53], decreased in a sigG mutant (Table 1). A C. perfringens spoVA mutation prevents the accumulation of DPA and reduces spore viability [47]. As observed in B. subtilis, we also showed the requirement for σG for the expression of all the genes encoding SASPs; these proteins bind to the forespore chromosome protecting the DNA from damage [52]. Indeed, the sspA (CD2688) and sspB (CD3249) genes encoding alpha/beta-type SASP and two other genes annotated as SASPs (CD1290 and CD3220.1) were expressed under the direct σG control (Table 1). Moreover, CD0684 encoding an ATP-dependent protease sharing similarity with FtsH, a protein involved in protein quality control and stress resistance in eubacteria, was downregulated in a sigG mutant. In addition, genes encoding proteins presumably involved in mitigating oxidative stress were also controlled by σG. CD1567 (cotG), CD1631 (sodA) and CD2845 encode a catalase, a superoxide dismutase and a rubrerythrin, respectively. Interestingly, SodA and CotG has recently been detected at the C. difficile spore surface [54]. These results suggested that proteins synthesized under the control of σG are located to the spore surface in C. difficile.
Expression of several genes required for cell wall synthesis and cortex formation was controlled by σG. Thus, expression of CD1430 (pdaA) encoding a N-acetylmuramic acid deacetylase involved in the formation of muramic ∂-lactam in the spore cortex PG and indirectly required for efficient spore germination [42] was downregulated in a sigG mutant. In addition, dacF, encoding a D-alanyl-D-alanine carboxypeptidase regulating the degree of cross-linking of the spore PG as well as CD0784 and uppS coding for an amidase and an undecaprenyl pyrophosphate synthetase, respectively were also positively controlled by σG.
Finally, the transcriptome analysis revealed 16 σG-controlled genes coding for proteins of unknown function. One of the most important aspects of spore biology is the mechanism by which dormant spores sense a suitable environment for germination and trigger the process through the specific recognition of germinants by germination receptors. These receptors are located in the spore inner membrane and are synthesized in the forespore under σG control in B. subtilis [9], [10]. No known homologs of the B. subtilis gerA, gerB and gerK operons as well as of the gerA, gerKB, gerKA and gerKC genes of C. perfringens are found in the C. difficile genome [55], [56]. However, a bile acid germinant receptor CspC has been recently identified [48]. Nine genes encoding probable membrane proteins (CD0792, CD0793, CD1677, CD1789, CD2051, CD2465, CD2635, CD2636, CD3551.1) were downregulated in the sigG mutant and these proteins may be involved in germination. 55% of our σG-controlled genes encode proteins associated to the spore in proteome [39]. This is in agreement with the crucial role of σG in the synthesis of key components protecting the spore or in preparation for germination.
The σK regulon
In transcriptome analysis, we found 56 genes positively controlled by σK (Table 2). The TSS mapping allowed us to identify 24 σK-dependent promoters controlling the expression of 29 genes. In B. subtilis, σK plays a crucial role in the last steps of the spore coat assembly [5], [57]. While the B. subtilis spore coat comprises over 70 different proteins, only few of them are conserved in C. difficile [57]. Recent studies have identified eight components of the C. difficile spore coat, CotA, CotB, CotCB, CotD, CotE, CotF, CotG and SodA [41], [54]. CotCB and CotD are catalases while CotE is a bifunctional protein with peroxiredoxin and chitinase activities [41], [54]. Interestingly, the expression of the cotA and cotE genes as well as the CD0596-cotF-cotCB and CD2399-cotJB2-cotD operons was downregulated in a sigK mutant compared to strain 630Δerm. We mapped a σK-dependent promoter upstream of cotA and cotE while σK consensus elements were found upstream of CD0596 and CD2399 (Table S7). The expression of CD3569 encoding a YabG-like protease involved in post-translational modification of spore surface proteins in B. subtilis [57] was also positively controlled by σK. Three orthologs of the B. anthracis bclA gene (bclA1, bclA2 and bclA3) are present in C. difficile. BclA of B. anthracis is a collagen-like protein that forms the hair-like nap of the exosporium, and is the immunodominant antigen of the spore surface [58]. The bclA1, bclA2 and bclA3 genes were positively controlled by σK and sequences similar to promoters recognized by σK are found upstream of these genes (Table S7).
An important conserved function of the mother cell is DPA production. The expression of dpaA and dpaB encoding the dipicolinate synthase was controlled by σK. This operon was transcribed from a σK-dependent promoter as observed in B. subtilis [9]. DPA is then transported into the spore by the SpoVA transporter, which is synthesized in the forespore. Mother cell lysis is a late developmental event that in B. subtilis involves the σK-controlled production of the CwlC and CwlH N-acetylmuramic acid L-alanine amidases [14], [59]. Two genes encoding N-acetylmuramic acid L-alanine amidases (CD1898 and CD2184) were downregulated in a sigK mutant and are possibly involved in mother cell lysis. We also found that sleC encoding the major spore cortex lytic enzyme that is essential for C. difficile germination [60] was a member of the σK regulon.
Other genes controlled by σK have no counterparts in B. subtilis. CD0564 encodes a putative Lon-type protease. The late production of CD0564 from a σK promoter (Table 2) may play a role in the degradation of early mother cell-specific proteins such as σE or the MazE antitoxin whose ortholog in E. coli is degraded by ClpXP and Lon proteases [61]. If so, proteolysis of MazE may lead to the accumulation of active MazF, which may possibly participate in mother cell death. Finally, the transcriptome analysis showed that genes encoding 17 proteins of unknown function were downregulated in a sigK mutant. Interestingly, nine of these proteins are associated to the spore [39] and we mapped a σK-dependent promoter upstream five of their encoding genes (CD1063.1, CD1067, CD1133, CD3580 and CD3613). These proteins are most likely spore coat proteins but further work would be necessary to characterize their localization and their function in C. difficile. In conclusion, 21 out of the 57 genes controlled by σK were found to encode components of the spore proteome [39]. The overall composition of the σK regulon is in agreement with the crucial role of σK in the assembly of the spore surface layers, spore maturation and mother cell lysis in C. difficile as well as in other endospore forming Firmicutes [19], [57].
III. The forespore line of gene expression
Our global approaches allowed to obtain new insights into the forespore line of gene expression, which is governed by the RNA polymerase sigma factors, σF and σG, and the DNA-binding transcriptional regulator, SpoVT.
Control of sigF and sigG expression
The spoIIAA-spoIIAB-sigFoperon, which is responsible for the synthesis and the activation/inactivation of σF, is transcribed by the RNA polymerase σH holoenzyme and is positively controlled by Spo0A [27]. Like in B. subtilis and other spore formers, the spoIIGA, sigE and sigG genes are clustered in C. difficile [16], [20]. The B. subtilis sigG gene is transcribed from two promoters, one located upstream of spoIIGA (σA) and the second located upstream of sigG transcribed first by σF and later by σG after engulfment [5]. In both B. subtilis and C. acetobutylicum, three transcripts are detected for the spoIIGA-sigE-sigG locus, corresponding to a spoIIGA-sigE-sigG, a spoIIGA-sigE and a sigG transcript [5], [33], [62]. In C. difficile, a σA-dependent promoter (GTGACA and TATAAT boxes) was mapped by RNA-seq upstream of spoIIGA (TSS at position 3052270 in the genome of strain 630). A promoter was also mapped upstream of sigG both by RNA-seq (Table S6) and by 5′-RACE [27]. The associated consensus motifs clearly correspond to those found for the forespore sigma factors (Fig. 1A) but appear closer to that found for σF promoters as the −10 element contains a G at position 23 and lacks the A conserved at position 32 for σG-dependent promoters (Fig. 1A). However, sigG expression was not downregulated in the sigF mutant in the transcriptome analysis as well as by qRT-PCR, under our conditions. In B. subtilis, sigG expression also appears not to be regulated in transcriptome analyses comparing sigF+ and sigF− strains [9], [10]. By contrast, sigG expression is downregulated in a sigF mutant in C. acetobutylicum [22] suggesting some differences in the control of sigG expression. In C. difficile, the absence of control of sigG expression by σF strongly suggests that sigG is transcribed from at least two promoters, one in front of spoIIGA recognized by σA, and one just upstream of sigG recognized by σF and/or by σG. Studies with a PsigG-SNAPCd transcriptional fusion indicate that this second promoter responsible for the forespore-specific transcription of sigG is dependent on σF [19]. A more complete analysis of the fine tuning of spoIIGA-sigE-sigG locus expression will require further investigations.
It is worth noting that the expression of many sigG-controlled genes (82% of σG regulon members) including those for key sporulation proteins like SpoVA, SspA, SspB and PdaA was strongly reduced in a sigF mutant in transcriptome (Table 1). The absence of detection of the complete σG regulon in the sigF mutant might be due to the timing of sampling (14 h) that is probably not optimal for sigG targets. Moreover, inactivation of sigF also eliminated fluorescence from a PsspA-SNAPCd fusion [19]. Thus, σF directly or indirectly controls expression of the σG regulon. One possibility is that synthesis of the σG protein is abolished in a sigF mutant as observed in C. acetobutylicum and C. perfringens [22], [23]. Alternatively, the positive regulation of σG target genes by σF could be mediated through modulation of σG activity. In B. subtilis, for instance, two anti-sigma factors, SpoIIAB and CsfB (Gin) negatively regulate σG activity. CsfB, in particular, plays an important role in σG regulation in the forespore, and is produced under σF control [63], [64]. No CsfB ortholog is present in the C. difficile genome and the phenotype expected if σF would be required for the synthesis of an anti-σG factor would be the opposite. However, we cannot exclude that proteins synthesized under σF control might be necessary for σG activity. Yet another possibility is that both σF and σG were involved in the transcription of most σG-target genes. In B. subtilis, σF and σG have overlapping promoter specificities and some genes are under the dual control of these sigma factors [9], [10]. This could be also the case in C. difficile. Finally, σF might control σG targets through an as yet unknown regulatory factor.
The role of SpoVT in sporulation
In B. subtilis, two transcriptional regulators participate in the forespore regulatory network, RfsA and SpoVT. SpoVT controls the synthesis of about half of the members of the σG regulon [10]. RfsA is absent from the genome of C. difficile and several Clostridia while an ortholog of SpoVT (55% identity with SpoVT of B. subtilis) is present. Interestingly, we found that spoVT expression was controlled by both σF and σG in transcriptome (Table 1). We confirmed by qRT-PCR a 280 - and a 14-fold decrease of spoVT transcription in a sigF or in a sigG mutant compared to strain 630Δerm, respectively (Table 3). We also showed that the expression of spoVT was restored to the wild-type level in the sigG mutant complemented by sigG while its expression was 19-fold higher in the sigF mutant complemented by sigF compared to the wild-type strain (Table S9). Upstream of the spoVT TSS, we identified a promoter resembling both the σF and σG consensus elements with the presence of a G at position 23 and an A at position 32 (Table S6). So, spoVT might be a direct target of both σF and σG because contrary to other σG-controlled genes, σF inactivation caused a much more important decrease of spoVT expression than σG inactivation.
Tab. 3. Control of σG or σK target genes by both σF and σE. 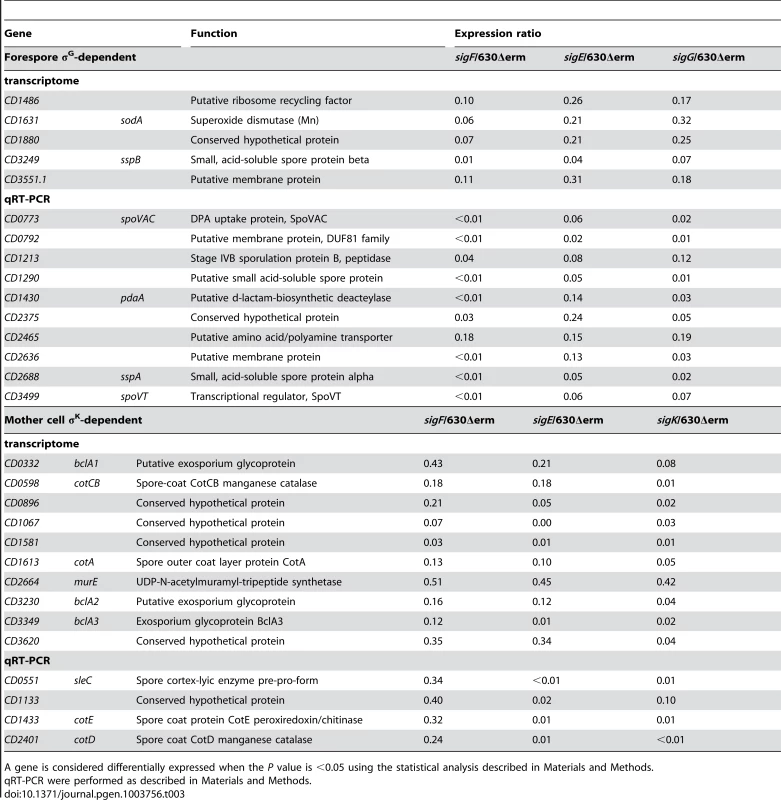
A gene is considered differentially expressed when the P value is <0.05 using the statistical analysis described in Materials and Methods. To test the possible role of SpoVT in sporulation in C. difficile, we constructed a spoVT mutant (Fig. S2). A complemented strain, CDIP263 carrying a multicopy allele of spoVT under the control of its native promoter was also obtained. To determine the impact of SpoVT inactivation on spore morphogenesis, samples of the strain 630Δerm, of the spoVT mutant and of the complemented strain CDIP263 (spoVT::erm, pMTL84121-spoVT) were collected and labeled with the DNA stain DAPI and the lipophilic membrane dye FM4-64. Phase contrast and fluorescence microscopy experiments showed that the spoVT mutant was able to complete the engulfment process (Fig. 2). However, this mutant formed phase dark immature spores, which were not released from the sporangial cells, clearly suggesting that the cortex is absent or highly reduced. The wild-type phenotype was restored in the complemented strain CDIP263. We further tested the ability of this mutant to sporulate. After 72 h of growth in SM medium, the 630Δerm strain or the spoVT mutant containing the plasmid pMTL84121-spoVT produced 4×106 and 107 heat-resistant spores/ml, respectively. In contrast, no clones were obtained after an heat treatment for the spoVT mutant. This was probably due to the lack of production of heat-resistant spores as suggested by the phase dark spores phenotype observed for this mutant (Fig. 2). In conclusion, SpoVT is required for mature spore formation in C. difficile but the phenotype of the spoVT mutant of C. difficile differs from that of the same mutant of B. subtilis. Indeed, the spoVT mutant of C. difficile formed phase dark spores instead of phase bright spores [65] and the spoVT mutant of B. subtilis has a reduced ability to sporulate [65] while we were unable to detect heat resistant spores for the mutant of C. difficile.
Fig. 2. Morphological characterization of the spoIIID and spoVT mutants. 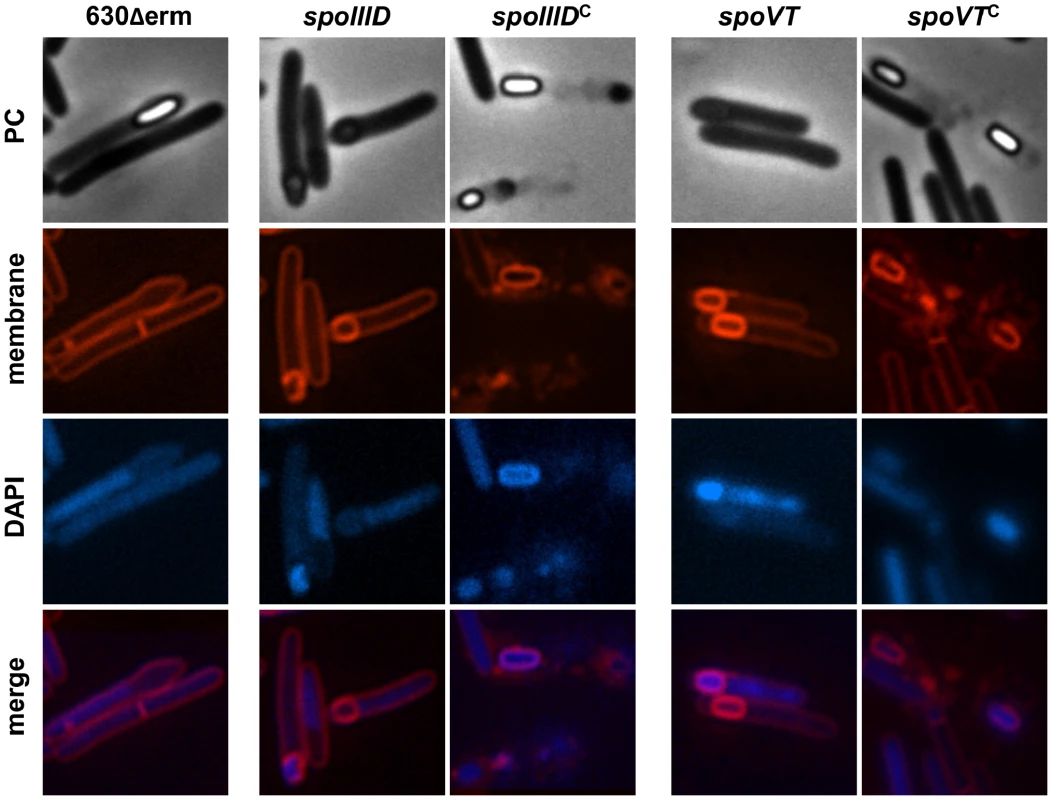
Cells of the C. difficile 630Δerm strain, the spoIIID or spoVT mutant and the complemented strains, CDIP262, carrying a multicopy spoIIID gene controlled by its native promoter or CDIP263, carrying a multicopy spoVT gene controlled by its native promoter were collected after 24 h of growth in SM broth, stained with the DNA stain DAPI and the membrane dye FM4-64 and examined by phase contrast and fluorescence microscopy. Then, we used qRT-PCR to measure the impact of SpoVT inactivation on the expression of selected σG targets after 20 h of growth. We showed that the expression of sspA and sspB decreased 70 - and 12-fold, respectively in the spoVT mutant compared to strain 630Δerm (Table S11). A positive effect of SpoVT on sspA and sspB expression is also observed in B. subtilis [65], [66]. The decreased expression of the sspA and sspB genes is probably not sufficient to explain the phenotypes observed for the spoVT mutant but we did not see a reproducible differential expression for other tested genes in our conditions. However, the expression of spoIIR and gpr, two members of the σF regulon, was upregulated 4 - and 9-fold in a spoVT mutant compared to strain 630Δerm earlier during sporulation (15 h) (Table S11). This negative effect might be due either to a direct control by SpoVT playing the role of RfsA in the negative control of σF targets or to an indirect effect associated with the blocked sporulation in the spoVT mutant.
No other genes encoding transcriptional regulators or proteins with DNA binding motifs were regulated by σF or σG in our transcriptome study (Table 1). Even though we cannot exclude that other regulatory proteins are at play, SpoVT is probably the key, if not the only, ancillary regulator of forespore gene expression. Further work will be required to analyse more precisely the morphology of the spoVT mutant and to understand at the molecular level the complex role of SpoVT in the regulatory network controlling sporulation.
IV. The mother cell line of gene expression
As discussed above, sigE transcription is probably initiated at the σA-dependent promoter located upstream of spoIIGA, which forms an operon with sigE and maybe also with sigG. In B. subtilis, σE positively controls the expression of spoIIID and gerR encoding regulatory proteins. GerR acts as a repressor of certain early σE-controlled genes and as an activator of some σK-dependent genes [67]. SpoIIID is an ambivalent regulator, acting as a repressor as well as an activator of a late class of σE-controlled genes [14]. SpoIIID and σE jointly activate the expression of sigK, and σK in turn drives production of another ancillary regulator, GerE [14]. In C. difficile, the SpoIIID regulator is present while GerR and GerE are absent. The SpoIIID protein of C. difficile shares 64% identity with SpoIIID from B. subtilis with conservation of the HTH motif and of the basic DNA binding motif located near the C-ter of the protein (Fig. S3) [68].
Characterization of the SpoIIID regulon
The expression of spoIIID was strongly curtailed in a sigE mutant (25 - and 800-fold in transcriptome and qRT-PCR) whereas the spoIIID expression was restored when a plasmid pMTL84121 containing the sigE gene with its promoter was introduced into the sigE mutant (Table S9). A TSS corresponding to a σE-controlled promoter (ATA-N16-CATATATA) was mapped upstream of spoIIID as observed in B. subtilis. It is interesting to note that in C. perfringens the expression of spoIIID is not controlled by σE [21].
In B. subtilis, SpoIIID positively or negatively regulates almost half of the σE target genes [14]. To test the possible role of SpoIIID in the mother cell regulatory network in C. difficile, we constructed a spoIIID mutant (Fig. S2) and compared the expression profiles of the 630Δerm strain and of the spoIIID mutant after 15 h of growth in SM medium. We found 96 genes differentially expressed with a p value <0.05 between the 630Δerm strain and the spoIIID mutant (Table S10). 12 and 84 genes were down and upregulated in the spoIIID mutant, respectively. We then performed qRT-PCR on a subset of genes regulated by SpoIIID in our transcriptome. The qRT-PCR results confirmed the microarrays data for the tested genes (Table S5). First, 47 genes that are not under the control of sporulation sigma factors in our transcriptome analyses were regulated by SpoIIID either positivelly (10 genes) or negativelly (37 genes) (Table S10). Most of these genes encode proteins involved in metabolism. Among the genes under the negative control of spoIIID, 28 were bona fide members of the σE-regulon (Table 2). Therefore, a principal function of SpoIIID is to inhibit the transcription of about 30% of the genes transcribed by σE as observed in B. subtilis [14]. SpoIIID repressed the expression of the spoIIIA operon and of the spoIVA, sipL, cotB and spm genes. In B. subtilis, spoIIIAA and spoIVA are direct SpoIIID targets [14]. By contrast, the spoIID gene, which is a direct SpoIIID target in B. subtilis, was not regulated by SpoIIID in transcriptome and qRT-PCR experiments in our conditions. In addition, the expression of genes encoding a transmembrane signaling protein (CD2445), an iron hydrogenase (CD3258), a bacterioferritin (CD2865), three polysaccharide deacetylases (CD3257, CD3248 and CD1319) and of several genes of unknown function was positively controlled by σE and negativelly regulated by SpoIIID (Table 2). By using the consensus sequence recognized by SpoIIID in B. subtilis [14], we identified a putative SpoIIID binding motif upstream of 11 of these genes (Table 2) suggesting that they might be direct SpoIIID targets in C. difficile. However, the characterization of direct and indirect SpoIIID targets and the experimental identification of the DNA binding motif of SpoIIID will deserve further investigations.
Interestingly, SpoIIID was also an activator of the expression of two genes belonging to the σK regulon, bclA3 and CD1067 in our transcriptome experiment (Table S10). To determine if sigK itself and other members of the σK regulon were under SpoIIID control, we performed qRT-PCR experiments (Table S11) using RNA extracted after 15 h of growth or after 24 h, a time where the σK targets are more highly expressed. We found that sigK expression was 25-fold and 250-fold downregulated in the spoIIID mutant after 15 h and 24 h of growth, respectively. This clearly indicated that the expression of sigK was positively controlled by SpoIIID in C. difficile as observed in B. subtilis [14]. In B. subtilis, SpoIIID is also required for the expression of spoIVCA encoding the site-specific recombinase involved in the excision of the skin element that creates a functional sigK gene [69]. In contrast, we did not observe a control of CD1231 encoding the specific recombinase of the skin element by SpoIIID but also by SigE both in transcriptome and qRT-PCR experiments. This result strongly suggests a different mechanism of control for the skin excision in C. difficile compared to B. subtilis. We also showed that eleven bona fide σK targets such as cotA, cotCB, cotD, cotE, sleC, bclA1, bclA2, bclA3, CD3580, CD1067 and CD1133 were downregulated 30 - to 1000-fold in the spoIIID mutant as compared to the parental strain after 24 h of growth (Table S11). The positive regulation of members of the σK regulon by SpoIIID might be due either to a direct binding of this regulator to their promoter regions or to an indirect effect mediated through the control of sigK transcription by SpoIIID.
To investigate the role of SpoIIID in sporulation, we examined the morphology of the spoIIID mutant. Samples of the 630Δerm strain, of the spoIIID mutant and of the complemented strain CDIP262 carrying a multicopy allele of spoIIID under the control of its native promoter (spoIIID::erm, pMTL84121-spoIIID) were collected and labeled with the DNA stain DAPI and the lipophilic membrane dye FM4-64. Phase contrast and fluorescence microscopy experiments showed that the spoIIID mutant completed engulfment of the forespore by the mother cell and formed partially refractile spores with irregular shapes and positioning (Fig. 2). The wild-type phenotype with the production of free spores was restored in the strain CDIP262 containing a copy of spoIIID on a plasmid (Fig. 2). We also tested the ability of the spoIIID mutant to sporulate. After 72 h of growth in SM medium, the 630Δerm strain produced 9×105 heat-resistant spores/ml while a titer of 3×102 heat-resistant spores/ml was obtained for the spoIIID mutant. When we complemented the spoIIID mutant with a pMTL84121-spoIIID plasmid, the titer of heat resistant spores increased to 5×105/ml. Interestingly, both the morphology and the sporulation efficiency of the spoIIID mutant is reminiscent of the oligosporogenous phenotype obtained for the sigK mutant of C. difficile [19] in agreement with the dependency on SpoIIID of the transcription of sigK. The morphology and the sporulation efficiency of the spoIIID mutant of C. difficile are similar to those of the B. subtilis mutant [70], [71]. As demonstrated in B. subtilis [14], SpoIIID in C. difficile plays a pivotal role in the mother cell line of gene expression switching off the transcription of many members of the σE regulon and switching on the expression of sigK and of members of the σK regulon.
Control of sigK transcription
In addition to the positive control of sigK expression by SpoIIID, its expression also decreased 8 - and 600-fold in a sigE mutant, in transcriptome and qRT-PCR experiments, respectively. The introduction of a plasmid pMTL84121 containing the sigE gene with its promoter into the sigE mutant restored sigK expression (Table S9). Interestingly, we mapped two TSS, 67 nt (P1) and 26 nt (P2) upstream of the translational start site of sigK (Table S7). A canonical −10 box (CATATTAT) for mother cell sigma factors is located upstream of the TSS corresponding to P1 while either a TTA sequence 15 bp upstream from the −10 box or a TTT motif with a more classical 16–18 bp spacing between the −10 and −35 elements could be proposed for a −35 motif. So, this promoter resembles a σE-dependent promoter (Fig. 1B and Table S7). Upstream of the second TSS (P2), we found a consensus for σK-dependent promoters with a −10 box (CATATAAT) and a −35 box (AC) (Table S7). We note that in B. subtilis, following the initial transcription of sigK under the command of σE and SpoIIID, an autoregulatory loop is established, which is responsible for about 60% of sigK transcription [72]. In C. difficile, sigK is likely first transcribed by σE-associated RNA polymerase at P1 and then by σK-associated RNA polymerase at P2 probably later during sporulation. In any event, lending support to the idea that σE has a crucial role in sigK transcription, 65% of the σK-controlled genes were positively controlled by σE including genes encoding key components of the spore surface layers (cotCB, cotA, cotE, cotD, sleC, bclA1, bclA2, bclA3). Most of these genes were much more downregulated in the sigK mutant than in the sigE mutant (Table 2) suggesting that the timing of sampling of the sigE mutant (14 h) is probably not optimal for σK targets. This might explain the absence of detection of the complete σK regulon in the sigE mutant. Importantly, with the exception of nrdR, we did not identify other genes for putative transcriptional regulators that were downregulated in sigE or sigK mutant (Table 2). Overall, our data indicates that in C. difficile, the mother cell line of gene expression is deployed according to a hierarchical regulatory cascade simpler than in B. subtilis [14].
The regulation of mother cell sigma factors synthesis in C. difficile also differs from other Clostridia. In C. perfringens, a biphasic pattern of sigK expression (early and late in sporulation) is observed and σE and σK coregulate the expression of each other [21]. In C. botulinum, sigK is expressed at the onset of stationary phase and σK positively controls the expression of sigF and spo0A [73]. In C. difficile, we did not observe a control of spo0A, sigF or bona fide members of the σE regulon by σK (Table 2). In both C. perfringens and C. botulinum, sporulation is arrested at an early stage in a sigK mutant and not at a late stage as observed in B. subtilis and C. difficile [5], [19]. It is also worth noting that the sigK gene is disrupted by a skin element in B. subtilis and C. difficile but not in C. acetobutylicum, C. perfringens and C. botulinum, suggesting that excision of this element may be an important factor in controlling the timing of σK activity in C. difficile.
V. Communication between the forespore and the mother cell
A hallmark of sporulation in B. subtilis is the existence of cell-cell signaling pathways that link the forespore and mother cell-specific lines of gene expression. Because these pathways operate at critical morphological stages of sporulation, the result is the coordinated deployment of the two lines of gene expression, in close register with the course of morphogenesis [5], [6]. Indeed, σF is required for the activation of pro-σE into σE in the mother cell and σE in turn is necessary to activate σG in the forespore. Finally, σG is required for the activation of pro-σK into σK in the mother cell [5], [6]. We obtained new information on the intercompartment signaling pathway in C. difficile.
Absence of a strict control of the σE regulon by σF
In B. subtilis, the σE regulon is indirectly controlled by σF, which is required for the proteolytical activation of pro-σE. Surprisingly, we did not find any global control of the σE regulon by σF in C. difficile (Table S1 and Table S2). We confirmed by qRT-PCR that the expression of bona fide σE targets such as spoIIIAA, spoIVA, spoIIID or CD2864 did not significantly decrease in a sigF mutant (Fig. S4). This is a major difference relative to the B. subtilis sporulation regulatory network [74] and indicates that σF is not strictly required for σE functionality in C. difficile. One possibility is that the SpoIIGA mediated proteolytical activation of pro-σE is not fully dependent on σF. To test this hypothesis, we detected in several strains (630Δerm, spo0A, sigF and sigE strains) the σF, σE and pro-σE polypeptides by Western-blotting using antibodies raised against either σF or σE (Fig. 3). In strain 630Δerm, we observed σF production while two forms corresponding to σE and pro-σE were detected with an anti-σE antibody. In a spo0A mutant, neither σE, pro-σE nor σF were detected (Fig. 3). This result is in agreement with the strong decrease of expression of the spoIIAA operon in a spo0A mutant [27]. As shown by qRT-PCR, spo0A inactivation also led to a 240-fold decrease of sigE expression indicating that this regulator controls pro-σE synthesis. It has also been shown that Spo0A directly binds with low affinity to the spoIIGA and spoIIAA promoter regions [30]. In a sigE mutant, we did not detect σE or pro-σE with an anti-σE antibody while we specifically detected σF with an anti-σF antibody. Interestingly, in a sigF mutant in which σF was absent, we detected pro-σE but also the processed σE form in a reduced quantity compared to the situation in a wild-type strain (Fig. 3). The expression of the σE regulon in the sigF mutant shows that even reduced the level of active σE in this mutant is sufficient to allow the transcription of σE-controlled genes. This clearly demonstrates that an active σE protein can be produced in the absence of σF in C. difficile contrary to B. subtilis [75]. In C. perfringens and C. acetobutylicum, neither pro-σE nor σE protein are produced in a sigF mutant [22], [23] suggesting a diversity in the mode of forespore control of σE activity among spore forming Firmicutes.
Fig. 3. The involvement of σF and SpoIIR in the control of formation of the processed σE protein. 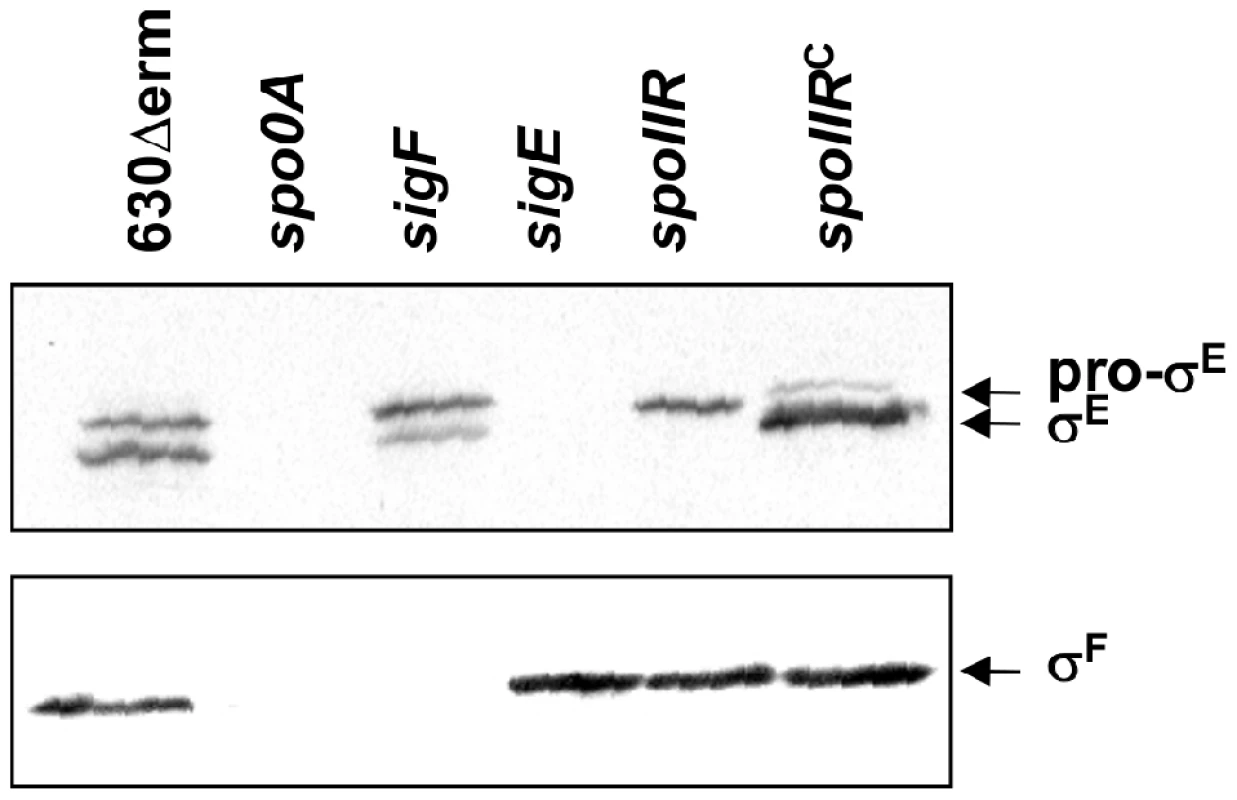
15 µg of total protein extracted from 630Δerm strain, the spo0A, sigF, sigE and spoIIR mutants and a complemented strain, CDIP246, a spoIIR mutant carrying a multicopy spoIIR gene controlled by its native promoter were loaded on a SDS-PAGE (12%). The various samples were analysed by immunoblotting with polyclonal anti-σE and anti-σF antibodies as described in Materials and Methods. The role of SpoIIR in the regulatory cascade
In B. subtilis, σF drives production of the signaling protein SpoIIR, which is secreted across the forespore inner membrane into the intermembrane space, where it stimulates the SpoIIGA-dependent pro-σE processing in the mother cell [6], [11], [76]. The results exposed above raised the possibility that SpoIIR is dispensable for pro-σE processing in C. difficile, or that expression of the spoIIR gene is partially independent on σF. To determine the role of SpoIIR in C. difficile, we inactivated spoIIR. A complemented strain, CDIP246, carrying a multicopy allele of spoIIR under the control of its native promoter was also constructed. We first examined the morphology of the spoIIR mutant. Phase contrast and fluorescence microscopy experiments showed that the spoIIR mutant was blocked at the asymmetric septation stage and accumulated disporic cells (Fig. 4) as shown for the spoIIR mutant in B. subtilis [11]. The wild-type phenotype was restored in the complemented strain CDIP246. The phenotype caused by the spoIIR mutation in C. difficile phenocopied that imposed by a sigE mutation [19] strongly suggesting that σE is inactive in this mutant. In B. subtilis, loss of σE or interference with its activation leads to disporic forms [35], [77]. We also measured the sporulation efficiency of these three strains after a heat treatment. After 72 h of growth in SM medium, the strain 630Δerm produced 2×106 heat-resistant spores/ml. In contrast, no heat resistant spores were detected for the spoIIR mutant. When we complemented the spoIIR mutant using a pMTL84121-spoIIR plasmid, the titer of heat resistant spores was of 9×105/ml. So, inactivation of the spoIIR gene resulted in a complete inability of C. difficile to sporulate.
Fig. 4. Morphological characterization of a spoIIR mutant. 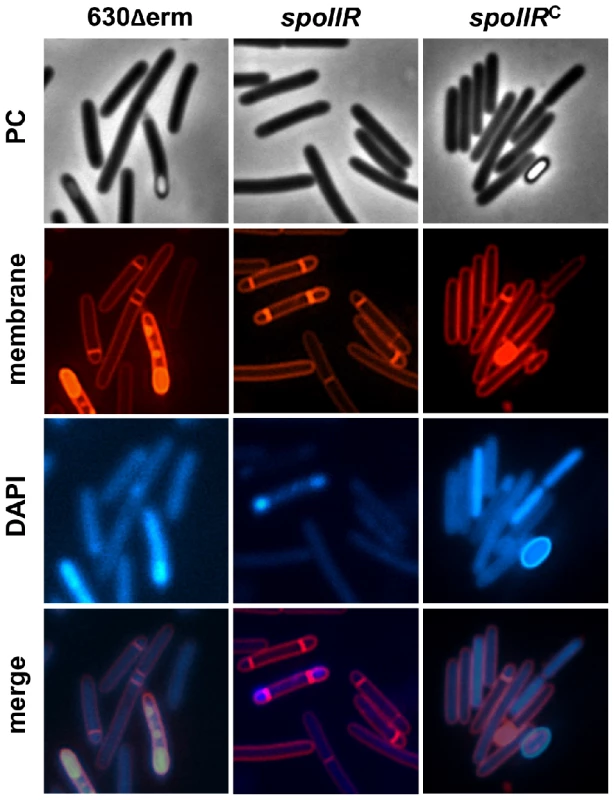
Cells of the C. difficile 630Δerm strain, the spoIIR mutant and the complemented strain, CDIP246, carrying a multicopy spoIIR gene controlled by its native promoter were collected after 24 h of growth in SM broth, stained with the DNA stain DAPI and the membrane dye FM4-64 and examined by phase contrast and fluorescence microscopy. To test the prediction that σE is inactive in the spoIIR mutant, we compared the accumulation of σF, σE and pro-σE polypeptides in strain 630Δerm and in the spoIIR mutant by immunoblotting. σF and pro-σE but not σE accumulated in the spoIIR mutant (Fig. 3). To independently test the impact of the spoIIR mutation on the activity of σE, the expression of selected σE target genes was analyzed by qRT-PCR. The expression of spoIIIAA, spoIVA, spoIIID and sigK genes decreased 10-, 8-, 16 - and 23-fold in a spoIIR mutant compared to strain 630Δerm (Table S11) while the expression of sigE itself was not reduced in this mutant. When the pMTL84121-spoIIR plasmid was introduced into the spoIIR mutant, the expression of spoIIIAA, spoIVA, and spoIIID increased 10-, 12 - and 7-fold compared to strain 630Δerm while the expression of the sigK gene was restored to the wild-type level. Therefore, both the immunoblot and the qRT-PCR studies support the idea that spoIIR, but not σF, is strictly required for the activation of σE (Fig. 3, Table S11).
It follows that the expression of spoIIR has to occur in part independently of σF. We first showed that the expression of spoIIR decreased 2.5 - and 38-fold in a sigF mutant in transcriptome and by qRT-PCR (Table 1 and Table S5) while the introduction of pMTL84121-sigF into the sigF mutant increased spoIIR expression 9-fold compared to strain 630Δerm (Table S9). So, the spoIIR transcription is under σF control. We also constructed a transcriptional fusion between the spoIIR promoter and the SNAP tag [19]. This PspoIIR-SNAPCd fusion was introduced by conjugation into strain 630Δerm and a spo0A, sigF or sigE mutant. Labeling with the fluorescent substrate TMR allowed localization of PspoIIR-SNAPCd expression. When examined by fluorescence microscopy, most of the cells of strain 630Δerm showed fluorescence in the forespore as expected for a gene under σF control (Fig. 5). spoIIR expression was not controlled by σE since a fluorescence signal was detected in the small compartments of disporic cells in a sigE mutant. Interestingly, a fluorescence of the spoIIR-SNAPCd fusion was still observed in the sigF mutant suggesting that some expression of spoIIR occurs in the absence of σF. In a spo0A mutant, the fluorescence completely disappeared and the spoIIR expression strongly decreased by qRT-PCR. So, the residual expression observed in the sigF mutant is likely Spo0A-dependent. Presumably, the expression of spoIIR in the sigF mutant allows the production of sufficient SpoIIR to trigger pro-σE processing. It is worth noting that spoIIR is not downregulated in a sigF mutant in C. acetobutylicum and only partially under σF control in C. difficile (Fig. 5). Also interestingly, in B. subtilis, the forced expression of spoIIR in pre-divisional cells, still allows pro-σE processing to occur, even in the absence of σF [11], [78]. It is possible that the activation of σE in ancestral spore formers occurred independently of the forespore. The expression of spoIIR under the exclusive control of σF may have appeared later and would allow a better coordination between the forespore and mother cell lines of gene expression.
Fig. 5. Fluorescence of a PspoIIR-SNAP fusion in strain 630Δerm and in a spo0A, sigF or sigE mutant. 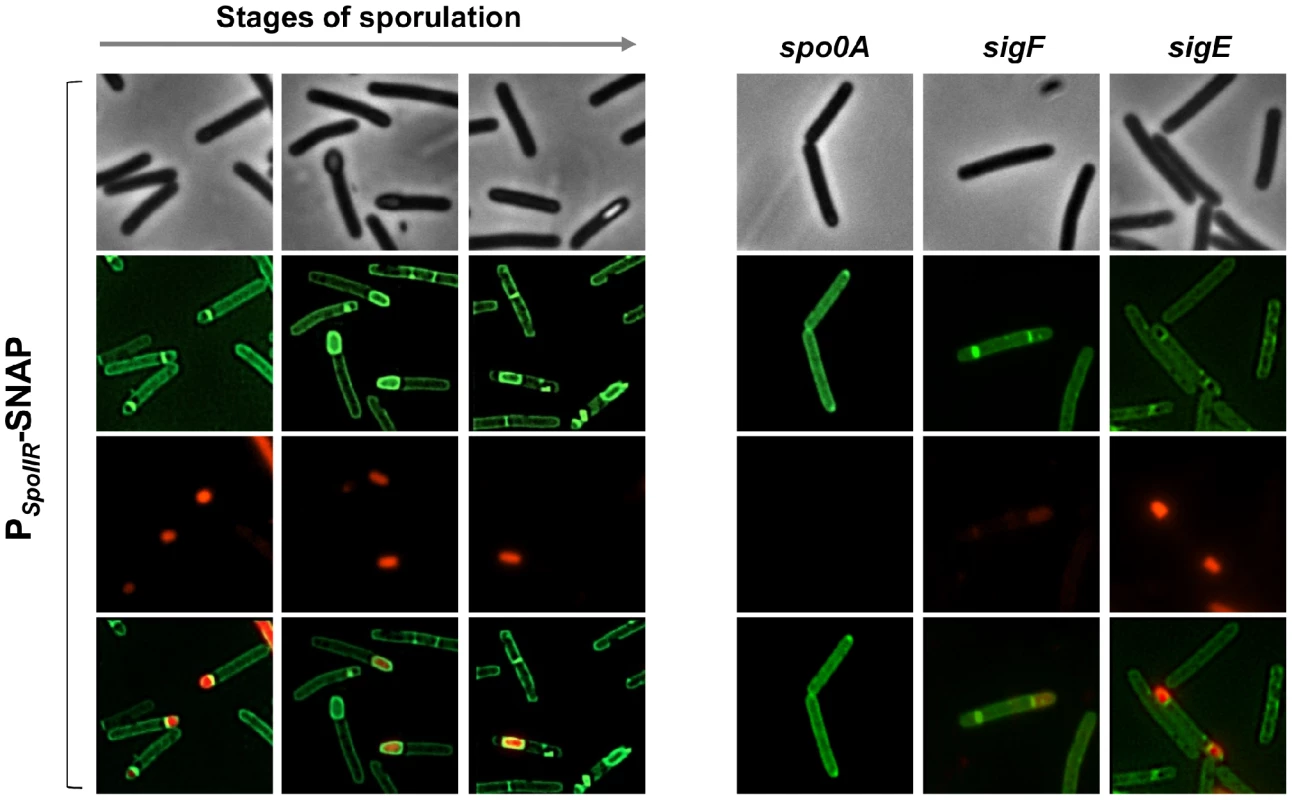
Cells of the C. difficile 630Δerm strain, and of the spo0A, sigF and sigE mutants carrying a PspoIIR-SNAPCd transcriptional fusion in a multicopy plasmid were collected 24 h of following inocculation in SM broth. Cells were labelled with the fluorescent substrate TMR to allow localization of SNAPCd production driven by the spoIIR promoter, stained with the DNA marker DAPI and the membrane dye MTG and examined by phase contrast and fluorescence microscopy. The regulation of σG-dependent genes by σE
In B. subtilis, most of the σG activity occurs after engulfment completion. In addition, the expression of sigG target genes in the engulfed forespore depends upon σE activation in the mother cell at least in part through synthesis of the SpoIIIA proteins [5], [6], [37], [38]. In the transcriptome analysis, we observed that the expression of 6 genes belonging to the σG regulon (sspB, CD1486, CD1631, CD1880, CD2465 and CD3551.1) was downregulated in the sigE mutant (Table 3). We also examined by qRT-PCR if a sigE mutation affected the expression of several other σG targets. The expression of 9 additional σG-controlled genes decreased in a sigE mutant (Table 3). Interestingly, SpoIIID also repressed the expression of 12 members of the σG regulon (sspA, sspB, spoVT, CD1631, CD1463, CD2112, CD2245.1, CD2375, CD2808, CD2809, CD3489, CD3610) in transcriptome (Table S10). This clearly indicated that both σE and SpoIIID participated in the control of σG-target genes in C. difficile. In B. subtilis, σG activity is dependent on the SpoIIIA-SpoIIQ channel [6], [37], [38]. The SpoIIIA proteins are encoded by the octacistronic spoIIIAA-AH operon, which is expressed in the mother cell under the direct control of σE and SpoIIID [9], [12], [14]. As noted above, the spoIIIAA operon is a member of the σE and spoIIID regulons also in C. difficile (Table 2 and Table S10) and is transcribed from two promoters recognized by σE located upstream of the spoIIIAA and spoIIIAG cistrons (Table S7), as observed in B. subtilis [79]. It thus seems possible that control of forespore-specific gene expression by σE and SpoIIID involves similar mechanisms in C. difficile. However, we note that fluorescence from a PsspA-SNAPCd fusion is detected in the forespore compartment of sigE mutant cells [19]. This indicates that a strict requirement for σE is not observed for the expression of C. difficile σG-targets contrary to what is shown in B. subtilis suggesting a less tight control of σE on gene expression in the forespore.
The absence of control of the σK regulon by σG
In B. subtilis, sigG regulates the expression of the σK regulon in the mother cell through the control of pro-σK processing [5], [6]. A sigG mutant is blocked just after engulfment completion, and does not show any signs of assembly of the surface layers around the forespore. Surprisingly, no σK target genes were downregulated in a sigG mutant in the transcriptome analysis and this lack of effect of the sigG mutation was confirmed by qRT-PCR for eight σK-controlled genes (cotA, cotCB, cotD, bclA1, bclA2, bclA3, CD1067, CD3620). Thus, synthesis of the spore coat proteins belonging to the σK regulon (CotA, CotCB, CotD, CotE, CotF) is σG-independent. This result is in agreement with the phenotype of the C. difficile sigG mutant that shows deposition of some coat material around the engulfed forespore [19]. Interestingly, mutations that bypass the need for σG for pro-σK processing, or a pro-less allele of the sigK gene in B. subtilis allow expression of σK targets [80]. In C. difficile, the pro-sequence of σK is absent and no homologs of SpoIVFB, the membrane-embedded protease that processes pro-σK in B. subtilis and of two other membrane proteins (SpoIVFA and BofA) that form a complex with and control the localization and activity of SpoIVFB, can be found [15], [17], [25], [81]. Thus, σK activity is not controlled through processing of a pre-protein in C. difficile and this may be related to the absence of σG control. However, both the synthesis and the processing of pro-σK are also σG independent in C. perfringens [23]. Therefore, the absence of control of σK by σG is not restricted to Clostridia lacking a pro-σK protein.
Intriguingly, C. difficile codes for two homologs of the B. subtilis signaling protein SpoIVB. CD1213 and CD0783 share 37% and 36% identity with SpoIVB, respectively. CD0783 and CD1213 belong to the σF and σG regulons, respectively (Table 1). In B. subtilis, SpoIVB is a protease synthesized under the control of σF and σG. Similarly, spoIIP is transcribed by σF and σE in B. subtilis, whereas two spoIIP genes are present in B. anthracis and transcribed by σF and σE, respectively [82]. SpoIVB is secreted to the intermembrane space where it cleaves SpoIVFA releasing SpoIVFB and allowing pro-σK processing [5], [6]. However, B. subtilis SpoIVB has two distinct developmental functions: pro-σK processing control and another role essential for spore formation probably corresponding to the cleavage of SpoIIQ [83], [84]. It is possible that the C. difficile SpoIVB-like proteins retained the second role, which might correspond to an ancestral function.
Unexpectedly, 14 σK target genes were downregulated in a sigF mutant compared to strain 630Δerm in transcriptome (cotA, cotCB, bclA1, bclA2, bclA3, cwpV, CD0896 CD1067, CD1581, CD1891, CD2184, CD2537, CD2664, CD3620). We further showed by qRT-PCR that 4 additional σK target genes (sleC, cotD, cotE and CD1133) were positively controlled by σF (Table 3) suggesting a more global regulation of σK targets by σF. This effect might be mediated through an indirect control by σF of the synthesis or the activity of σK itself or of another regulator of members of the σK regulon. Another hypothesis might be that the σK activity requires engulfment completion a process blocked in a sigF mutant but not in a sigG mutant. Interestingly, the synthesis of pro-σK and σK in C. perfringens is also abolished in a sigF mutant but not in a sigG mutant [23] suggesting a switch in the forespore control of σK-activity from σG to σF. However, a sigK mutant of C. perfringens is blocked early in sporulation, at the asymmetric division stage [21]. The molecular mechanisms involved in the σF-dependent control of late mother cell-specific gene expression remain to be determined.
Conclusion
Global approaches combining transcriptome and TSS mapping allow us to have a view of the expression pattern during the early and late stages of sporulation in both forespore and mother cell and to define the four compartment-specific sigma regulons in C. difficile. We identified 25, 97, 50 and 56 members for the σF, σE, σG and σK regulons, respectively and in each regulon, we found key representatives of the homologous regulons of B. subtilis as proposed previously [16]. However, while larger than the evolutionary conserved core machinery for endosporulation, of about 145 genes [15], [17], this set of C. difficile sporulation genes (around 225 genes) corresponds to about half the number of genes under the control of cell type-specific sigma factors of B. subtilis [9], [10], [12]. A more dynamic study with a detailed kinetic analysis would provide additional members for each regulon but it is also probable that the presumably more ancestral type of sporulation process proposed for Clostridia [15], [17], [18], [20] involves a smaller collection of proteins. This has already been observed for the initiation of sporulation where the complex signaling transduction pathway involving in B. subtilis a phosphorelay that modulates Spo0A activity is replaced by a simple two-component system [18], [20], [29]. In general, the most significant variations between the B. subtilis and C. difficile sporulation process are observed at the interface with their environment: the signal transduction pathway triggering sporulation initiation, composition of the coat shell and the germination-activating pathways [16], [18], [20], [47]. Indeed, the germination signals and germination receptors differ among spore forming Firmicutes and only a few spore coat layer proteins are conserved in Bacilli and Clostridia [47], [57]. In each regulon, a significant fraction of genes encodes proteins with unknown function but our work offer new insights about the role of some of them. Some σG-controlled membrane proteins might be involved in germination, which remain poorly characterized in C. difficile [48], [55]. Three proteins of unknown function belonging to the σE regulon (CD0214, CD1930, CD3522) and several proteins synthesized under σK control (CD1063.1, CD1067, CD1133, CD3580 and CD3613) that are detected in the spore proteome [39] are probably new spore coat components. Finally, we found several oxygen detoxification proteins that might help in the long-term survival of clostridial spores as recently proposed by Galperin et al [17]. This probably favors the dissemination of spores of strictly anaerobic Clostridia in aerobic environment, a crucial step for persistence and transmission of pathogenic Clostridia [1], [2].
In this work, we also exposed important deviations from the B. subtilis paradigm in C. difficile. Both the global analysis of the program of gene expression under the control of the four cell type-specific sigma factors and the morphological characterization of the corresponding mutants (this work, [19]) indicate that coupling between gene expression and morphogenesis is less tight in C. difficile than in B. subtilis (Fig. 6). First, the σE regulon in the C. difficile mother cell is not strictly under σF control despite the fact that the forespore product SpoIIR is required for pro-σE processing. The residual spoIIR expression in the sigF mutant might be responsible for this less strict connection. Second, the tight coordination between σG and σK activities observed in B. subtilis, is absent in C. difficile since σK activity does not depend on σG as clearly shown by the morphology of a sigK mutant [19]. In the absence of a σK precursor, the rearrangement of the sigK gene associated to excision of the skin element may be the only way to control the timing of σK activity in C. difficile [16], [20], [25] and this event does not appear to be under σG control. However, a control of the forespore on σK target genes seems to be maintained through a σF-dependent regulation.
Fig. 6. Model of the regulatory network controlling sporulation in C. difficile. 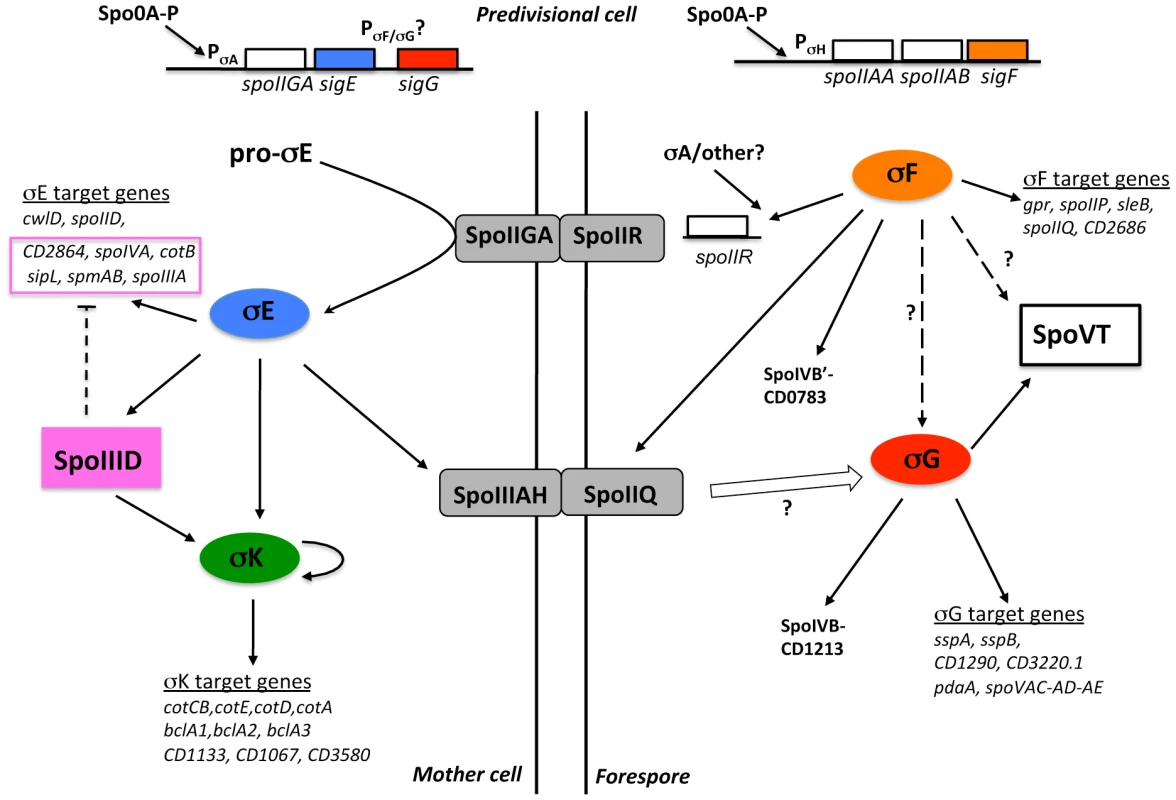
The upper part of the figure represents the cell before asymmetric division. The parallel vertical lines represent the two membranes separating the forespore (right) and the mother cell (left). The four sigma factors of sporulation are encircled (by oval boxes). Precursor protein of σE is indicated as pro-σE. Square boxes correspond to transcriptional regulators. Proteins associated with the membrane or located into the intermembrane space are illustrated as embedded in the parallel vertical lines. Black solid arrows and white arrow indicate activation at the transcriptional level or at the level of protein activity, respectively. Broken arrows with a question mark represent mechanisms that are not yet fully understood. Dotted arrows indicate a possible direct transcriptional activation or repression. The σE target genes under the negative control of SpoIIID are inside a pink box. Recent data discussed above also suggest differences in the regulatory network controlling sporulation among Clostridia. This is especially the case for the impact of each sigma factor inactivation on the synthesis of the others and for the role of σK [21], [23], [73], [85]. The timing of sigK expression, the phenotype of sigK mutants and/or some σK targets differ. In addition, with the exception of C. difficile, σK activity is controlled through processing in Clostridia while the insertion of a skin element in the sigK gene is found only in C. difficile strains [25]. The rather low probability to observe orthologs in clostridial genomes for many C. difficile regulon members also suggests a moderate conservation of the sporulation sigma-factor regulons among Clostridia. This work gives new insights about the diversity and evolution of the sporulation process. The sporulation in C. difficile might reflect a more ancestral way of sporulation while a more sophisticated system of developmental control would have been gradually introduced during evolution.
Materials and Methods
Bacterial strains, growth conditions and sporulation assay
C. difficile strains and plasmids used in this study are presented in Table 4. C. difficile strains were grown anaerobically (10% H2, 10% CO2, and 80% N2) in TY or in Brain Heart Infusion (BHI, Difco), which was used for conjugation. Sporulation medium (SM) [86] was used for sporulation assays. SM medium (pH at 7.4) contained per liter : 90 g Bacto tryptone, 5 g Bacto peptone, 1 g (NH4)2SO4, 1.5 g Tris Base. When necessary, cefoxitin (Cfx; 25 µg/ml), thiamphenicol (Tm; 15 µg/ml) and erythromycin (Erm; 2.5 µg/ml) were added to C. difficile cultures. E. coli strains were grown in Luria-Bertani (LB) broth. When indicated, ampicillin (100 µg/ml) or chloramphenicol (15 µg/ml) was added to the culture medium.
Tab. 4. Strains and plasmids used in this study. 
Sporulation assay were performed as follows. After 72 h of growth in SM medium, 1 ml of culture was divided into two samples. To determine the total number of cells, the first sample was serially diluted and plated on BHI with 0.1% taurocholate (Sigma-Aldrich). Taurocholate is required for the germination of C. difficile spores [4]. To determine the number of spores, the vegetative bacteria of the second sample were heat killed by incubation for 30 min at 65°C prior to plating on BHI with 0.1% taurocholate. The percentage of sporulation was determined as the ratio of the number of spores/ml and the total number of bacteria/ml (×100).
Construction of C. difficile strains
The ClosTron gene knockout system [87] was used to inactivate the spoIIID (CD0126), spoVT (CD3499) and spoIIR (CD3564) genes giving strains CDIP224 (630Δerm CD0126::erm), CDIP227 (630Δerm CD3499::erm) and CDIP238 (630Δerm CD3564::erm). Primers to retarget the group II intron of pMTL007 to these genes (Table S12) were designed by the Targetron design software (http://www.sigmaaldrich.com). The PCR primer sets were used with the EBS universal primer and intron template DNA to generate by overlap extension PCR, a 353-bp product that would facilitate intron retargeting to CD0126, CD3499 or CD3564. The PCR products were cloned between the HindIII and BsrGI sites of pMTL007 to yield pDIA6123 (pMTL007::Cdi-CD0216-39s), pDIA6120 (pMTL007::Cdi-CD3499-157a) and pMS459 (pMTL007::Cdi-CD3564-38a). The presence and orientation of the insert in pMS459 was verified by DNA sequencing using the pMTL007-specific primers pMTL007-F and pMTL007-R. pDIA6120, pDIA6123 and pMS459 were introduced into E. coli HB101 (RP4) and the resulting strains subsequently mated with C. difficile 630Δerm [88]. C. difficile transconjugants were selected by sub-culturing on BHI agar containing Tm and Cfx and then plated on BHI agar containing Erm. Chromosomal DNA of transconjugants was isolated as previously described. PCR using the ErmRAM primers (ErmF and ErmR) confirmed that the Erm resistant phenotype was due to the splicing of the group I intron from the group II intron following integration. In order to verify the integration of the Ll.LtrB intron into the right gene targets, we performed PCR with two primers flanking the insertion site in CD0126 (LS184-LS185), CD3499 (LS186-LS187) or CD3564 (IMV649-LS113) and in one hand with the intron primer EBSu and in other hand with a primer in CD0126 (LS184), CD3499 (LS187) or CD3564 (LS113).
To complement the spoIIR mutant, the spoIIR gene with its promoter (−169 to +791 from the translational start site), was amplified by PCR using oligonucleotides IMV642 and IMV641 (Table S12). To complement the spoIIID and the spoVT mutants, the spoIIID gene with its promoter (−117 to +320 from the translational start site) and the spoVT gene with its promoter (−165 to +731 from the translational start site), were amplified by PCR using oligonucleotides LS283 and IMV647 or IMV644 and IMV648 (Table S12). The PCR fragments were cloned into the XhoI and BamHI sites of pMTL84121 [89] to produce plasmids pDIA6132 (spoVT), pDIA6133 (spoIIID) and pDIA6135 (spoIIR). Using the E. coli HB101 (RP4) strain containing pDIA6135 as donor, this plasmid was transferred by conjugation into the C. difficile spoIIR mutant giving strain CDIP246 (Table 4). Similarly the plasmids pDIA6132 and pDIA6133 were transferred by conjugation into the spoVT or spoIIID mutant giving strains CDIP263 and CDIP262.
To construct a PspoIIR-SNAP fusion reporter strain, a 470 bp fragment encompassing the region upstream of the spoIIR gene was amplified by PCR using primers PspoIIR-SNAP EcoRIFw and PspoIIR-SNAP XhoIRev (Table S12). The PCR product was inserted between the EcoRI and XhoI sites of pFT47 [19] to create pMS462. pMS462 was introduced into E. coli HB101 (RP4) and then transferred to C. difficile 630Δerm, spo0A::erm, sigF::erm or sigE::erm strains by conjugation. Transconjugants were selected on BHI agar plates containing Tm and Cfx followed by plating on BHI agar containing Tm.
RNA extraction, quantitative real-time PCR and transcriptional start site mapping
For RNA-seq experiment allowing TSS mapping, total RNA was isolated from C. difficile 630 strain grown in TY medium after 4 h and 10 h of growth or under starvation conditions that correspond to a 1 h resuspension of exponentially growing cells (6 h of growth) into phosphate-buffered saline (PBS: 137 mM NaCl, 10 mM Phosphate, 2.7 mM KCl, pH 7.4) [31]. The Tobacco Acid Pyrophosphatase (TAP)+/ − library construction and high-throughput sequencing was realized on a mixed sample combining RNAs extracted from these three different growth conditions as previously described [31]. After Illumina sequencing, the reads were mapped to the C. difficile genome using Bowtie [90] then converted into BAM files with the Samtools [91]. The data were visualized at a strand-specific manner using COV2HTML (http://mmonot.eu/COV2HTML/). All TSS detected by RNA-seq were inspected manually. Deep sequencing data are available at https://mmonot.eu/COV2HTML/visualisation.php?str_id=-14.
To perform transcriptional analysis for each sigma factor, we first tested the impact of their inactivation on the expression of two target genes at different times to optimize conditions. For this purpose, we used bona fide targets of these sigma factors in B. subtilis [16]: gpr and spoIIR for σF, spoIIIAA and spoIVA for σE and sspA and sspB for σG. For σK, we used cotCD and cotA encoding recently described C. difficile spore coat proteins [41]. This preliminary test allowed us to define a time for maximal differential expression for these targets between wild-type and mutant strains for each sigma factor. To study σE - or σF-dependent control, we harvested cells of strain 630Δerm, the sigE and the sigF mutants after 14 h of growth in SM medium. The strain 630Δerm and the sigG or the sigK mutant were harvested after 19 h (630Δerm, sigG mutant) and 24 h (630Δerm, sigK mutant) of growth in SM medium. For the spoIIR and spoIIID mutants, we harvested cells after 14 h and 15 h of growth in SM medium, respectively. The culture pellets were resuspended in RNApro solution (MP Biomedicals) and RNA extracted using the FastRNA Pro Blue Kit, according to the manufacturer's instructions. The RNA quality was determined using RNA 6000 Nano Reagents (Agilent). For the spo0A mutant and the 630Δerm strain, RNA previously obtained were used to test the impact of Spo0A inactivation on gene expression [27].
Quantitative real-time PCR (qRT-PCR) analysis was performed as previously described [27]. The primers used for each marker are listed in Table S12. In each sample, the quantity of cDNAs of a gene was normalized to the quantity of cDNAs of the DNApolIII gene. The relative change in gene expression was recorded as the ratio of normalized target concentrations (ΔΔCt) [92].
Microarray design, DNA-array hybridization and transcriptome analysis
The microarray of C. difficile 630 genome (GEO database accession number GPL10556) was designed as previously described [27]. Transcriptome was performed using for each condition four (630Δerm compared to the mutant inactivated for each sigma factor) or two different RNA preparations (630Δerm compared to spoIIID). Labeled DNA hybridization to microarrays and array scanning were done as previously described [27]. The slides were analyzed using R and limma software (Linear Model for Microarray Data) from Bioconductor (www.bioconductor.org). We corrected background with the ‘normexp’ method, resulting in strictly positive values and reducing variability in the log ratios for genes with low levels of hybridization signal. Then, we normalized each slide with the ‘loess’ method [93]. To test for differential expression, we used the bayesian adjusted t-statistics and we performed a multiple testing correction of Benjamini and Hochberg based on the false discovery rate (FDR) [94]. A gene was considered as differentially expressed when the p-value is <0.05. The complete data set was deposited in the GEO database with a series record accession number GSE43202.
In silico analysis of promoters
We analyzed the TSS data by the following in silico iterative strategy. For σF, σE, σG and σK, we first worked with a reduced training set corresponding mainly to promoters located upstream of genes having an orthologous gene controlled by the same sigma factor in B. subtilis [16], of genes encoding experimentally characterized spore coat proteins for σK [41] or of genes encoding proteins associated with the spore [39] (Table S8). Half-sites (boxes) were then manually re-aligned to maximize similarity and to construct recognition profiles for each sigma factor. The score of a candidate promoter was defined as the sum of positional nucleotide weights W(bi,i), with an additional term V(k) to account for preferred interbox spacer length: S(b1…bmx1…xkbm+1bm+n) = σi = 1…m+n W(bi,i)+V(k), where b1…bm, bm+1bm+n are nucleotides in the promoter boxes, x denotes any nucleotide, m and n are the distal and proximal box lengths, respectively, k is the spacer length. Positional nucleotide weights were defined as W(b,i) = log (N(b,i)+0.5)−0.25xσd = A,T,G,C log (N(d,i)+0.5), where N(b,i) is the count of nucleotide b at alignment position i, and box length weight was defined as V(k) = log (M(k)+0.5)−(1/(kmax−kmin+1))xσj = kmin…kmax log (M(j)+0.5), kmax and kmin are the maximal and minimal observed spacer length, respectively, and M(k) is the count of spacer length k. The necessary procedures were implemented in SignalX [95]. These profiles determined with the training set were further used to score all identified TSS. Candidate promoters scoring higher than the lowest-scoring promoter in the training set were retained for further analysis (Table S8). A powerful approach, as for transcription factors, is to apply comparative techniques based on the assumptions that functional regulatory sites should be conserved in related species. Genome sequences from C. difficile six closest relatives from the Clostridium genus (C. saccharolyticum, C. thermocellum, C. tetani, C. cellulolyticum, C. ljungdahlii, C. kluyveri) and B. subtilis 168 were downloaded from GenBank. We searched the intergenic regions of the six Clostridia using the profiles defined for C. difficile (Table S8). As the exact TSS positions in these species are unknown, we analyzed regions (−100 +10) relative to annotated start codons. The data on B. subtilis promoters were obtained from DBTBS [96]. Finally, for each candidate promoter, we calculated the number of genomes also having a candidate promoter upstream of an orthologous gene. Orthologs were defined using the bidirectional best hit criterion implemented in GenomeExplorer [95].
Purification of σF-His6 and σE-His6 for antibody production
The coding sequences of sigF and sigE were amplified by PCR using primer pairs CDsigF-pET28aFw/CDsigF-pET28aRev and CDsigE-pET28aFw/CDsigE-pET28aRev, respectively. The resulting fragments were introduced between the NcoI and XhoI sites of pET28a to produce pFT35 (σF-His6) and pFT34 (σE-His6). BL21(DE3) derivatives carrying pFT34 or pFT35 were grown in LB to an OD600 nm of 0.5 and induced with 1 mM IPTG for 4 h. The cells were then harvested by centrifugation at 4000 g, ressuspended in 20 mM phosphate, 1 mM phenylmethyl-sulfonyl fluoride (PMSF), 10 mM imidazole, and lysed using a French pressure cell (18000 lb/in2). After centrifugation, the supernatant (or, for the case of σE-His6, the sediment after solubilization with 8M Urea for 30 min) was loaded onto a 1 ml Histrap column (Amersham Phamarcia Biotech). The bound proteins were eluted with a discontinuous imidazole gradient. The purified proteins were used for the production of rabbit polyclonal antibodies (Davids Biotechnologie GmbH).
Whole cell lysates and immunoblot analysis
To prepare C. difficile whole cell extracts, 10 ml samples were withdrawn from SM cultures at the desired times following inoculation and the cells collected by centrifugation (4000 g, 10 min, at 4°C). Cells were lysed in 1 ml buffer (10 mM Tris pH 8.0, 10 mM MgCl2, 0.5 mM EDTA, 0.2 mM NaCl, 10% glycerol, 1 mM PMSF) using a French pressure cell. Samples (15 µg of protein) were resolved by 12% SDS-PAGE, transferred to a nitrocellulose membrane (BioRad), and subjected to immunoblot analysis as described previously [97]. Antibodies against σF and σE were used at a 1∶2000 dilution. A rabbit secondary antibody conjugated to horseradish peroxidase (Sigma) was used at a 1∶5000 dilution. The immunoblots were developed with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Microscopy and image analysis
1 ml samples were withdrawn from SM cultures at the desired times following inoculation, and the cells collected by centrifugation (4000 g, 10 min, at 4°C). The cells were washed with 1 ml of PBS and ressuspended in 0.1 ml of PBS supplemented with the membrane dye FM4-64 (10 µg.ml−1) and the DNA stain DAPI (4′,6-diamidino-2-phenylindole; 50 µg.ml−1) (Molecular Probes, Invitrogen). For SNAP staining, 1 ml samples were stained for 30 min with 50 nM SNAP-Cell TMR-Star (New England Biolabs) as described [19]. Cells were washed four times by centrifugation (4000 g, 5 min) and ressupended in 1 ml of PBS. Following washing, the cells were ressuspended in 1 ml of PBS supplemented with the membrane dye Mitotracker Green (0.5 µg.ml−1) (Molecular Probes, Invitrogen). Cells were mounted on 1.7% agarose coated glass slides and imaged in a Leica DM6000B microscope as previously described [64]. Fluorescent signals were visualized with a phase contrast Uplan F1 100× objective and captured with a CCD Andor IxonEM camera (Andor Technologies). Images were acquired and analyzed using the Metamorph software suite version 5.8 (Universal Imaging).
Supporting Information
Zdroje
1. DeakinLJ, ClareS, FaganRP, DawsonLF, PickardDJ, et al. (2012) The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun 80 : 2704–2711.
2. LawleyTD, ClareS, DeakinLJ, GouldingD, YenJL, et al. (2010) Use of purified Clostridium difficile spores to facilitate evaluation of health care disinfection regimens. Appl Environ Microbiol 76 : 6895–6900.
3. SarkerMR, Paredes-SabjaD (2012) Molecular basis of early stages of Clostridium difficile infection: germination and colonization. Future Microbiol 7 : 933–943.
4. SorgJA, SonensheinAL (2008) Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190 : 2505–2512.
5. HilbertDW, PiggotPJ (2004) Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol Rev 68 : 234–262.
6. HigginsD, DworkinJ (2012) Recent progress in Bacillus subtilis sporulation. FEMS Microbiol Rev 36 : 131–148.
7. StragierP, LosickR (1996) Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet 30 : 297–341.
8. MolleV, FujitaM, JensenST, EichenbergerP, Gonzalez-PastorJE, et al. (2003) The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50 : 1683–1701.
9. SteilL, SerranoM, HenriquesAO, VolkerU (2005) Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology 151 : 399–420.
10. WangST, SetlowB, ConlonEM, LyonJL, ImamuraD, et al. (2006) The forespore line of gene expression in Bacillus subtilis. J Mol Biol 358 : 16–37.
11. Londono-VallejoJA, StragierP (1995) Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev 9 : 503–508.
12. EichenbergerP, JensenST, ConlonEM, van OoijC, SilvaggiJ, et al. (2003) The sigmaE regulon and the identification of additional sporulation genes in Bacillus subtilis. J Mol Biol 327 : 945–972.
13. FeuchtA, EvansL, ErringtonJ (2003) Identification of sporulation genes by genome-wide analysis of the sigmaE regulon of Bacillus subtilis. Microbiology 149 : 3023–3034.
14. EichenbergerP, FujitaM, JensenST, ConlonEM, RudnerDZ, et al. (2004) The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol 2: e328.
15. AbecassisA, SerranoM, AlvesR, QuintaisL, Pereira-LealJB, et al. (2013) A genomic signature and the identification of new endosporulation genes. J Bacteriol 195 : 2101–2115.
16. de HoonMJ, EichenbergerP, VitkupD (2010) Hierarchical evolution of the bacterial sporulation network. Curr Biol 20: R735–745.
17. GalperinMY, MekhedovSL, PuigboP, SmirnovS, WolfYI, et al. (2012) Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ Microbiol 14 : 2870–2890.
18. ParedesCJ, AlsakerKV, PapoutsakisET (2005) A comparative genomic view of clostridial sporulation and physiology. Nat Rev Microbiol 3 : 969–978.
19. PereiraFC, SaujetL, ToméAR, SerranoM, MonotM, et al. (2013) The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet 9: e1003782.
20. Stragier P (2002) A gene odyssey: exploring the genomes of endospore-forming bacteria; In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis: from cells to genes and from genes to cells. Washington, D. C.: ASM Press.
21. HarryKH, ZhouR, KroosL, MelvilleSB (2009) Sporulation and enterotoxin (CPE) synthesis are controlled by the sporulation-specific sigma factors SigE and SigK in Clostridium perfringens. J Bacteriol 191 : 2728–2742.
22. JonesSW, TracyBP, GaidaSM, PapoutsakisET (2011) Inactivation of sigmaF in Clostridium acetobutylicum ATCC 824 blocks sporulation prior to asymmetric division and abolishes sigmaE and sigmaG protein expression but does not block solvent formation. J Bacteriol 193 : 2429–2440.
23. LiJ, McClaneBA (2010) Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect Immun 78 : 4286–4293.
24. TracyBP, JonesSW, PapoutsakisET (2011) Inactivation of sigmaE and sigmaG in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J Bacteriol 193 : 1414–1426.
25. HaraldsenJD, SonensheinAL (2003) Efficient sporulation in Clostridium difficile requires disruption of the sigmaK gene. Mol Microbiol 48 : 811–821.
26. JonesSW, ParedesCJ, TracyB, ChengN, SillersR, et al. (2008) The transcriptional program underlying the physiology of clostridial sporulation. Genome Biol 9: R114.
27. SaujetL, MonotM, DupuyB, SoutourinaO, Martin-VerstraeteI (2011) The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. J Bacteriol 193 : 3186–3196.
28. UnderwoodS, GuanS, VijayasubhashV, BainesSD, GrahamL, et al. (2009) Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J Bacteriol 191 : 7296–7305.
29. SteinerE, DagoAE, YoungDI, HeapJT, MintonNP, et al. (2011) Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Mol Microbiol 80 : 641–654.
30. RosenbuschKE, BakkerD, KuijperEJ, SmitsWK (2012) C. difficile 630Deltaerm Spo0A Regulates Sporulation, but Does Not Contribute to Toxin Production, by Direct High-Affinity Binding to Target DNA. PLoS One 7: e48608.
31. SoutourinaO, MonotM, BoudryP, SaujetL, PichonC, et al. (2013) Genome-wide identification of regulatory RNAs in the human pathogen Clostridium difficile. PLoS Genet 9: e1003493.
32. TattiKM, ShulerMF, MoranCPJr (1995) Sequence-specific interactions between promoter DNA and the RNA polymerase sigma factor E. J Mol Biol 253 : 8–16.
33. SantangeloJD, KuhnA, Treuner-LangeA, DurreP (1998) Sporulation and time course expression of sigma-factor homologous genes in Clostridium acetobutylicum. FEMS Microbiol Lett 161 : 157–164.
34. ZhaoY, MelvilleSB (1998) Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J Bacteriol 180 : 136–142.
35. EichenbergerP, FawcettP, LosickR (2001) A three-protein inhibitor of polar septation during sporulation in Bacillus subtilis. Mol Microbiol 42 : 1147–1162.
36. MorlotC, UeharaT, MarquisKA, BernhardtTG, RudnerDZ (2010) A highly coordinated cell wall degradation machine governs spore morphogenesis in Bacillus subtilis. Genes Dev 24 : 411–422.
37. CampAH, LosickR (2008) A novel pathway of intercellular signalling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol Microbiol 69 : 402–417.
38. MeisnerJ, MaehigashiT, AndreI, DunhamCM, MoranCPJr (2012) Structure of the basal components of a bacterial transporter. Proc Natl Acad Sci U S A 109 : 5446–5451.
39. LawleyTD, CroucherNJ, YuL, ClareS, SebaihiaM, et al. (2009) Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J Bacteriol 191 : 5377–5386.
40. PutmanE, NockA, LawleyT, ShenA (2013) SpoIVA and SipL are Clostridium difficile spore morphogenic proteins. J Bacteriol 195 : 1214–1225.
41. PermpoonpattanaP, TollsEH, NademR, TanS, BrissonA, et al. (2011) Surface layers of Clostridium difficile endospores. J Bacteriol 193 : 6461–6470.
42. GilmoreM, BandyopadhyayD, DeanAM, LinnstaedtSD, DLP (2004) Production of muramic delta-lactam in Bacillus subtilis spore peptidoglycan. J Bacteriol 186 : 80–89.
43. Paredes-SabjaD, SarkerN, SetlowB, SetlowP, SarkerMR (2008) Roles of DacB and spm proteins in Clostridium perfringens spore resistance to moist heat, chemicals, and UV radiation. Appl Environ Microbiol 74 : 3730–3738.
44. FigueiredoMC, LoboSA, CaritaJN, NobreLS, SaraivaLM (2012) Bacterioferritin protects the anaerobe Desulfovibrio vulgaris Hildenborough against oxygen. Anaerobe 18 : 454–458.
45. AdamsCM, EckenrothBE, PutnamEE, DoublieS, ShenA (2013) Structural and Functional Analysis of the CspB Protease Required for Clostridium Spore Germination. PLoS Pathog 9: e1003165.
46. CartmanST, MintonNP (2010) A mariner-Based Transposon System for In Vivo Random Mutagenesis of Clostridium difficile. Appl Environ Microbiol 76 : 1103–1109.
47. Paredes-SabjaD, SetlowP, SarkerMR (2011) Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19 : 85–94.
48. FrancisMB, AllenCA, ShresthaR, SorgJA (2013) Bile Acid Recognition by the Clostridium difficile Germinant Receptor, CspC, Is Important for Establishing Infection. PLoS Pathog 9: e1003356.
49. ChenFC, ShenLF, TsaiMC, ChakKF (2003) The IspA protease's involvement in the regulation of the sporulation process of Bacillus thuringiensis is revealed by proteomic analysis. Biochem Biophys Res Commun 312 : 708–715.
50. RothenbacherFP, SuzukiM, HurleyJM, MontvilleTJ, KirnTJ, et al. (2012) Clostridium difficile MazF toxin exhibits selective, not global, mRNA cleavage. J Bacteriol 194 : 3464–3474.
51. AdlerE, BarakI, StragierP (2001) Bacillus subtilis locus encoding a killer protein and its antidote. J Bacteriol 183 : 3574–3581.
52. SetlowP (2007) I will survive: DNA protection in bacterial spores. Trends Microbiol 15 : 172–180.
53. Tovar-RojoF, ChanderM, SetlowB, SetlowP (2002) The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 184 : 584–587.
54. PermpoonpattanaP, PhetcharaburaninJ, MikelsoneA, DembekM, TanS, et al. (2013) Functional characterization of Clostridium difficile spore coat proteins. J Bacteriol 195 : 1492–1503.
55. BurnsDA, HeapJT, MintonNP (2010) Clostridium difficile spore germination: an update. Res Microbiol 161 : 730–734.
56. XiaoY, FranckeC, AbeeT, Wells-BennikMH (2011) Clostridial spore germination versus bacilli: genome mining and current insights. Food Microbiol 28 : 266–274.
57. HenriquesAO, MoranCPJr (2007) Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61 : 555–588.
58. SteichenCT, KearneyJF, TurnboughCLJr (2007) Non-uniform assembly of the Bacillus anthracis exosporium and a bottle cap model for spore germination and outgrowth. Mol Microbiol 64 : 359–367.
59. NugrohoFA, YamamotoH, KobayashiY, SekiguchiJ (1999) Characterization of a new sigma-K-dependent peptidoglycan hydrolase gene that plays a role in Bacillus subtilis mother cell lysis. J Bacteriol 181 : 6230–6237.
60. BurnsDA, HeapJT, MintonNP (2010) SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J Bacteriol 192 : 657–664.
61. GerdesK, ChristensenSK, Lobner-OlesenA (2005) Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol 3 : 371–382.
62. HarrisLM, WelkerNE, PapoutsakisET (2002) Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J Bacteriol 184 : 3586–3597.
63. RhayatL, DuperrierS, Carballido-LopezR, PellegriniO, StragierP (2009) Genetic dissection of an inhibitor of the sporulation sigma factor sigma(G). J Mol Biol 390 : 835–844.
64. SerranoM, RealG, SantosJ, CarneiroJ, MoranCPJr, et al. (2011) A negative feedback loop that limits the ectopic activation of a cell type-specific sporulation sigma factor of Bacillus subtilis. PLoS Genet 7: e1002220.
65. BagyanI, HobotJ, CuttingS (1996) A compartmentalized regulator of developmental gene expression in Bacillus subtilis. J Bacteriol 178 : 4500–4507.
66. Ramirez-PeraltaA, StewartKA, ThomasSK, SetlowB, ChenZ, et al. (2012) Effects of the SpoVT regulatory protein on the germination and germination protein levels of spores of Bacillus subtilis. J Bacteriol 194 : 3417–3425.
67. CangianoG, MazzoneA, BaccigalupiL, IsticatoR, EichenbergerP, et al. (2010) Direct and indirect control of late sporulation genes by GerR of Bacillus subtilis. J Bacteriol 192 : 3406–3413.
68. HimesP, McBryantSJ, KroosL (2010) Two regions of Bacillus subtilis transcription factor SpoIIID allow a monomer to bind DNA. J Bacteriol 192 : 1596–1606.
69. KunkelB, LosickR, StragierP (1990) The Bacillus subtilis gene for the development transcription factor sigma K is generated by excision of a dispensable DNA element containing a sporulation recombinase gene. Genes Dev 4 : 525–535.
70. ErringtonJ (1993) Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev 57 : 1–33.
71. YoshisueH, IharaK, NishimotoT, SakaiH, KomanoT (1995) Cloning and characterization of a Bacillus thuringiensis homolog of the spoIIID gene from Bacillus subtilis. Gene 154 : 23–29.
72. OkeV, LosickR (1993) Multilevel regulation of the sporulation transcription factor sigma K in Bacillus subtilis. J Bacteriol 175 : 7341–7347.
73. KirkDG, DahlstenE, ZhangZ, KorkealaH, LindstromM (2012) Involvement of Clostridium botulinum ATCC 3502 sigma factor K in early-stage sporulation. Appl Environ Microbiol 78 : 4590–4606.
74. FawcettP, EichenbergerP, LosickR, YoungmanP (2000) The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A 97 : 8063–8068.
75. JonasRM, HaldenwangWG (1989) Influence of spo mutations on sigma E synthesis in Bacillus subtilis. J Bacteriol 171 : 5226–5228.
76. KarowML, GlaserP, PiggotPJ (1995) Identification of a gene, spoIIR, that links the activation of sigma E to the transcriptional activity of sigma F during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A 92 : 2012–2016.
77. EldarA, CharyVK, XenopoulosP, FontesME, LosonOC, et al. (2009) Partial penetrance facilitates developmental evolution in bacteria. Nature 460 : 510–514.
78. ZhangL, HigginsML, PiggotPJ, KarowML (1996) Analysis of the role of prespore gene expression in the compartmentalization of mother cell-specific gene expression during sporulation of Bacillus subtilis. J Bacteriol 178 : 2813–2817.
79. GuillotC, MoranCPJr (2007) Essential internal promoter in the spoIIIA locus of Bacillus subtilis. J Bacteriol 189 : 7181–7189.
80. CuttingS, OkeV, DriksA, LosickR, LuS, et al. (1990) A forespore checkpoint for mother cell gene expression during development in B. subtilis. Cell 62 : 239–250.
81. TraagB, PuglieseA, EisenJ, LosickR (2013) Gene Conservation among Endospore-Forming Bacteria Reveals Additional Sporulation Genes in Bacillus subtilis. J Bacteriol 195 : 253–260.
82. DworkinJ, LosickR (2005) Developmental commitment in a bacterium. Cell 121 : 401–409.
83. JiangX, RubioA, ChibaS, PoglianoK (2005) Engulfment-regulated proteolysis of SpoIIQ: evidence that dual checkpoints control sigma activity. Mol Microbiol 58 : 102–115.
84. OkeV, ShchepetovM, CuttingS (1997) SpoIVB has two distinct functions during spore formation in Bacillus subtilis. Mol Microbiol 23 : 223–230.
85. WangY, LiX, MaoY, BlaschekHP (2012) Genome-wide dynamic transcriptional profiling in Clostridium beijerinckii NCIMB 8052 using single-nucleotide resolution RNA-Seq. BMC Genomics 13 : 102.
86. WilsonKH, KennedyMJ, FeketyFR (1982) Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 15 : 443–446.
87. HeapJT, PenningtonOJ, CartmanST, CarterGP, MintonNP (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70 : 452–464.
88. HussainHA, RobertsAP, MullanyP (2005) Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Deltaerm) and demonstration that the conjugative transposon Tn916DeltaE enters the genome of this strain at multiple sites. J Med Microbiol 54 : 137–141.
89. HeapJT, PenningtonOJ, CartmanST, MintonNP (2009) A modular system for Clostridium shuttle plasmids. J Microbiol Methods 78 : 79–85.
90. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
91. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
92. LivakKJ, SchmittgenTD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25 : 402–408.
93. SmythGK, SpeedT (2003) Normalization of cDNA microarray data. Methods 31 : 265–273.
94. BenjaminiY, HochbergY (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser 289–300.
95. MironovA, VinokurovaN, GelfandM (2000) GenomeExplorer: software for analysis of complete bacterial genomes. Mol Biol (Mosk) 34 : 253–262.
96. SierroN, MakitaY, de HoonM, NakaiK (2008) DBTBS: a database of transcriptional regulation in Bacillus subtilis containing upstream intergenic conservation information. Nucleic Acids Res 36: D93–96.
97. SerranoM, ZilhaoR, RiccaE, OzinAJ, MoranCPJr, et al. (1999) A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J Bacteriol 181 : 3632–3643.
Štítky
Genetika Reprodukčná medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



