Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
Previous studies in narcolepsy, an autoimmune disorder affecting hypocretin (orexin) neurons and recently associated with H1N1 influenza, have demonstrated significant associations with five loci. Using a well-characterized Chinese cohort, we refined known associations in TRA@ and P2RY11-DNMT1 and identified new associations in the TCR beta (TRB@; rs9648789 max P = 3.7×10−9 OR 0.77), ZNF365 (rs10995245 max P = 1.2×10−11 OR 1.23), and IL10RB-IFNAR1 loci (rs2252931 max P = 2.2×10−9 OR 0.75). Variants in the Human Leukocyte Antigen (HLA)- DQ region were associated with age of onset (rs7744020 P = 7.9×10−9 beta −1.9 years) and varied significantly among cases with onset after the 2009 H1N1 influenza pandemic compared to previous years (rs9271117 P = 7.8×10−10 OR 0.57). These reflected an association of DQB1*03:01 with earlier onset and decreased DQB1*06:02 homozygosity following 2009. Our results illustrate how genetic association can change in the presence of new environmental challenges and suggest that the monitoring of genetic architecture over time may help reveal the appearance of novel triggers for autoimmune diseases.
Published in the journal:
Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic. PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003880
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pgen.1003880
Summary
Previous studies in narcolepsy, an autoimmune disorder affecting hypocretin (orexin) neurons and recently associated with H1N1 influenza, have demonstrated significant associations with five loci. Using a well-characterized Chinese cohort, we refined known associations in TRA@ and P2RY11-DNMT1 and identified new associations in the TCR beta (TRB@; rs9648789 max P = 3.7×10−9 OR 0.77), ZNF365 (rs10995245 max P = 1.2×10−11 OR 1.23), and IL10RB-IFNAR1 loci (rs2252931 max P = 2.2×10−9 OR 0.75). Variants in the Human Leukocyte Antigen (HLA)- DQ region were associated with age of onset (rs7744020 P = 7.9×10−9 beta −1.9 years) and varied significantly among cases with onset after the 2009 H1N1 influenza pandemic compared to previous years (rs9271117 P = 7.8×10−10 OR 0.57). These reflected an association of DQB1*03:01 with earlier onset and decreased DQB1*06:02 homozygosity following 2009. Our results illustrate how genetic association can change in the presence of new environmental challenges and suggest that the monitoring of genetic architecture over time may help reveal the appearance of novel triggers for autoimmune diseases.
Introduction
A remarkable feature of narcolepsy is its strong HLA association, with similar effects across different ethnicities and countries [1]–[4]. Almost all (98%) cases carry the HLA DQA1*01:02-DQB1*06:02 haplotype, expressing a functional DQα/DQβ heterodimer denoted as DQ0602. Susceptibility is further increased in DQB1*06:02 homozygotes [5], and DQB1*06:02/DQB1*03:01 heterozygotes [1]–[3]. It is also lower in subjects with HLA DQA1*01:02-DQB1*06:02 and other, non-DQA1*01:02 and DQB1*06:02 DQ1 alleles [1]–[4], an effect likely due to trans-dimerization and reduction of DQ0602 availability [3]. Genome wide association studies (GWAs) of individuals of European ancestry have identified TRA@, P2RY11-DNMT1, CTSH and TNFSF4 loci as additional susceptibility genes [6]–[8].
Recently, a strong link between upper airway winter infections and narcolepsy has emerged. Yearly patterns of narcolepsy onset in China revealed a ∼6 fold increase in spring and summer versus winter [9]. Associations between group A Streptococcus Pyogenes and narcolepsy have been found in several studies [10]–[12]. Following a 2009 pandemic H1N1 (pH1N1) vaccination campaign in Europe, increased risk linked to Pandemrix exposure, an ASO3 adjuvanted vaccine formulation, was reported in multiple countries [13]–[17], raising alarm. Incidence in China sharply increased 4 months after the 2009 H1N1 influenza pandemic peak, returning to previous rates following the pandemic [9], [18]. All these cases are HLA DQB1*06:02 positive, and have hypocretin deficiency when documented [12], [19]. The fact pH1N1 was practically unknown to humans prior to late 2009 [20] offers a unique opportunity to understand how pathogens are involved in triggering autoimmune diseases.
To identify novel narcolepsy susceptibility loci potentially missed in previous studies focused on European ancestry, we studied 1,189 Chinese narcolepsy cases primarily characterized at a single clinical center (Beijing University) [9], [18], [21] and 1,997 Chinese controls genotyped on the Affymetrix Axiom CHB array. All cases had documented hypocretin deficiency or had clear-cut cataplexy and HLA DQB1*06:02, ensuring etiological homogeneity and meeting ICSD3 criteria for type 1 narcolepsy. We tested allelic association at 603,382 non-HLA, autosomal SNPs, correcting for stratification using a mixed model method (inflation statistic, lambda = 1.001).
Results and Discussion
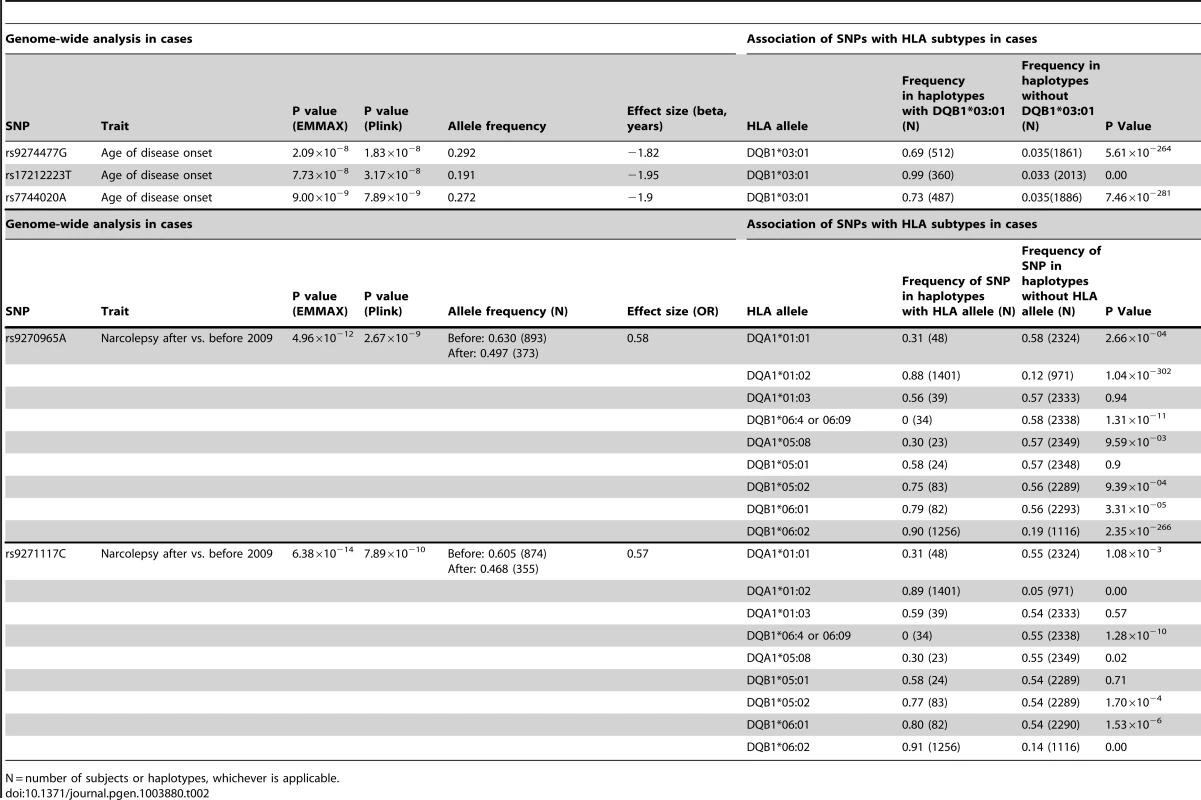
Genome wide significant association signal (GWAS, p≤5×10−8) was seen for 9 SNPs in the TRA@ locus (Figure S1). We selected the top 80 nominally significant SNP loci for replication testing or combined analysis (see methods) in narcoleptics from two European cohorts typed on the Illumina ImmunoChip (1886 cases, 10,421 controls) and Affymetrix 6.0 arrays (807 cases, 1074 controls) [6], [8], (Table S1). These cohorts had partially overlapping cases, thus we first attempted replication in the ImmunoChip dataset, which has a larger sample size but covers only selected immune-related loci; when this was not possible, we used the smaller Affymetrix 6.0 dataset. Two of the 5 SNPs that were testable in the ImmunoChip dataset were significant after Bonferroni correction: rs1154155 at TRA@ (pChinese = 6.32×10−20,pEur = 8.87×10−30), and rs10995245 at ZNF365 (pChinese = 2.34×10−4,pEur = 3.24×10−7). Thirty-eight additional variants were testable using the Affymetrix 6.0 data set, with confirmation of the PPAN-P2RY11-DNMT1 region (rs1551570, pChinese = 1.88×10−6,pEur = 5.10×10−8), and new associations identified at rs2834188 on Chromosome 21 at the IL10RB-IFNAR1 locus (pChinese = 1.78×10−4,pEur = 5.92×10−5, and rs2853536 on Chromosome 7 at the T cell receptor beta locus (TRB@, pChinese = 7.20×10−5,pEur = 9.44×10−4), both strong biological candidates (Table 1). To further characterize these associations, we imputed genotypes surrounding these five loci in Chinese and Europeans, and performed combined regional associations. The combined association studies yielded genome-wide significant values at each of the 5 loci (Table 1).
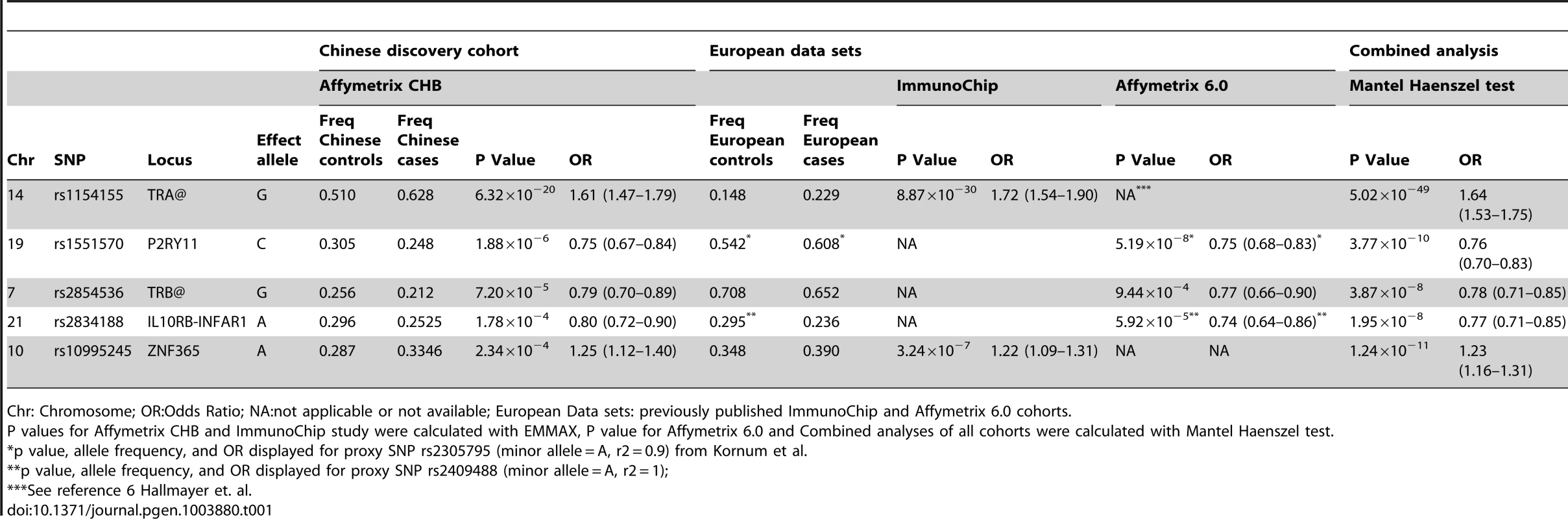
Consistent with previous reports [6], [8], [22], the large TRA@ locus encoding the α-chain of the T-cell receptor, showed the strongest association with narcolepsy. Combining the present sample with the large sample typed on the ImmunoChip yielded a p value of 5.0×10−49 at rs1154155G (OR 1.6), but low coverage on that array prevented fine mapping. This variant is also the most significant (P = 2.3×10−31, OR 1.7) among 35 SNPs when the Chinese and European Affymetrix 6.0 data sets are combined. The most highly significant variants cluster within a 22 kb region encompassing the TRAJ segments J8 through J28. (Figure 1A). Of special interest as a candidate causal SNP is rs1483979, located within the J24 segment and projected to change an aminoacid (F8L) within the CDR3 peptide-binding site of any TCR carrying J24. Linkage Disequilibrium (LD) between rs1483979C and rs1154155G is high in both populations (r2≥0.8).
Complementing the TRA@ association, we found associations at three variants within the T cell receptor beta locus (TRB@), which encodes the β-chain of the T-cell receptor. A first association at rs2854536 (3.87×10−8, OR 0.78), near the pseudogene TRBV8-1, reached genome wide significance, extending on a tentative finding in a prior study [6]. In addition, we found an even higher independent association at 2 tightly linked SNPs, rs9648789T and rs3020837A (both 3.7×10−9, OR 0.77 pairwise r2 = 1) that are unlinked with rs2854536 (r2 = 0.21) and encompass an area of approximately 14 kb containing the TRBV7-1 and TRBV4-2 segments (Figure 1B). Inspection of 1000genomes did not reveal candidate coding SNPs for these segments, suggesting more complex effects, for example on recombination and Vβ usage. Like antibody genes, T cell receptor loci undergo somatic recombination, thus most of the diversity is not encoded at the genomic level. While the TRA@ locus contains only V, J and C segments, the TRB@ locus also includes two D segments, dramatically increasing repertoire diversity, and likely obscuring genomic associations in smaller, less powerful samples. The fact that associations with these loci are found in narcolepsy but not other HLA-associated diseases may reflect a relative oligoclonality of the TCR subtypes involved in the autoimmune process, itself tightly associated with a single DQα/β heterodimer.
Our study also extends on a previously reported association within the purinergic receptor P2RY11 gene [7], [22] at rs2010353G (P = 2.8×10−10, OR 0.76) in intron 1 (Figure 1C). Four SNPs with tight LD in both Chinese and European populations are representative of this association, which does not extend to the DNMT1 locus, where the association signal is low. This supports our previous results implicating this gene, which mediates survival of T lymphocytes and NK cells in response to ATP-induced cell death, in the pathogenesis of narcolepsy ([7]). Of interest, rare mutations in the neighboring DNMT1 locus cause narcolepsy together with ataxia, deafness and dementia [23]. The present results do not support a hypothesis that the P2RY11 association reflects the action of a causative variant within the DNMT1 locus, although this conclusion is limited by available SNPs present on the two chip arrays and their ability to tag any such putative variants. Further, elements located within the P2RY11 may regulate DNMT1 expression. The P2RY11-DNMT1 gene segment is conserved in zebrafish, suggesting co-evolutionary pressures.
ZNF365, a gene associated with multiple phenotypes including breast cancer, sudden cardiac death from coronary disease, Crohn's disease, and atopic dermatitis [24]–[27] already had suggestive association in the ImmunoChip study of narcolepsy in European ancestry [8] and was strongly replicated in the Chinese cohort, reaching GWA-significance at rs10995245A in the combined sample (P = 1.2×10−11, OR 1.23) (Figure 1D). Two additional variants in moderate LD in both populations also surpassed this threshold. The gene has high levels of expression in the brain and spans nearly 300 kb, encoding 10 transcripts and several isoforms encoding distinct proteins with different expression patterns and functions. The narcolepsy variant is not linked to coding SNP rs7076156 (Ala62Thr) implicated in Crohn's disease [28] (r2 = 0.166 in Europeans).
A last locus, encompassing the IL10RB-IFNAR1 genes, also replicated strongly and reached GWAS significance in the combined sample. SNPs rs2409488A and rs2834190T, located between IL10RB and IFNAR1, were most significant (both P = 1.2×10−8 OR 0.76) although a total of 13 variants reached GWA significance (Figure 1E). The two loci are located within a cluster of class II cytokine receptor genes on chr21q22 and are separated by 27 kb. The segment is within a region of high LD covering most of IL10RB gene through the 3′ end of the IFNAR1 gene. Both genes are strong candidates for narcolepsy. The IL10RB gene encodes a chain shared by several receptors of the IL10 cytokine family (an anti-inflammatory cytokine), and is associated with Crohn's disease both in GWA studies and in rare multiplex families with early onset Inflammatory Bowel Disease [29], [30]. IFNAR1 encodes the α-chain of the IFN-α/β receptor, and is another appealing functional candidate, as IFN-α/β signaling is not only an early response to viral infection, but IFNAR1 null mice demonstrated more severe autoimmune disease of the central nervous system in a model of experimental autoimmune encephalomyelitis [31].
We also examined variants previously reported to be associated with narcolepsy. It was not possible to replicate associations at the TNFSF4 and CTSH loci [8] in the Chinese cohort due to a lack of markers in high LD with previously associated variants (rs7553711 at TNFSF4, or rs3843303 and rs34593439 at CTSH) on the array. There was no association around CPT1B-CHKB (rs5770911 proxy r2 = 1 with rs5770917, P = 0.66, OR 1.02), consistent with previous findings [22]. Overall, this study brings the number of GWA significant loci for narcolepsy to 8, further implicating the immune system notably antigen presentation by HLA-DQ to the TCR as the primary cause for the disorder. None of the explored loci showed significant pairwise interactions, including those involving HLA and the T-Cell receptor (data not shown).
We next examined genetic associations with specific clinical characteristics within the cohort, starting with previously associated loci and extending to genome-wide analyses. One of the unique features of the Chinese cohort is the large sample size with phenotype data consistently collected using the same procedure for over 10 years. The majority of these cases are children and diagnostic delay was short (5 years) compared to Europe (15 years) [32], likely improving recollection (see methods). Clinical characteristics that were available on ≥1000 cases were examined, including age of onset (cataplexy, sleepiness or the earliest of either) and sleep test results. Because narcolepsy incidence increased 6 fold following the 2009 influenza pandemic, we also compared cases with onset before versus after September 2009.
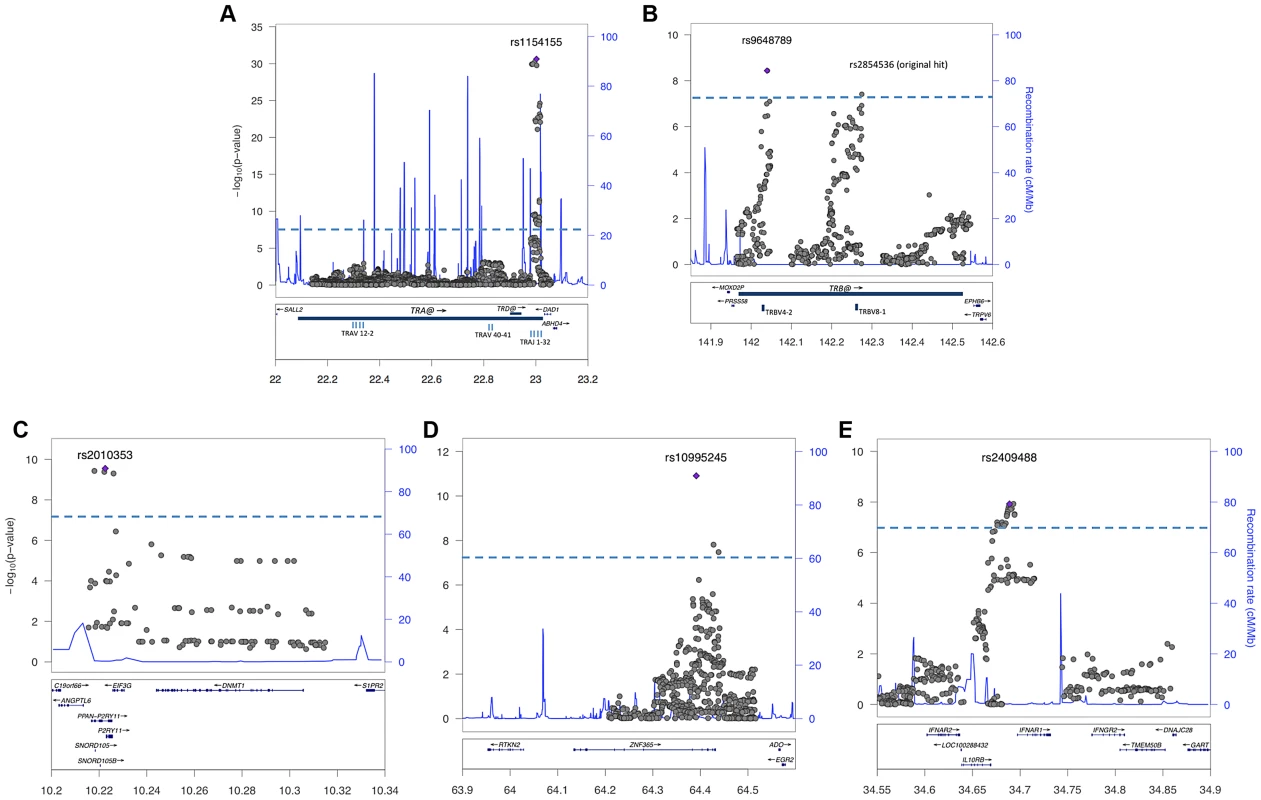
Genome-wide significant effects were observed for multiple SNPs in the HLA-DQ region for both age of onset (rs7744020 P = 9.0×10−9 Beta −1.9 years, SE 0.33), and among cases with onset after the 2009 H1N1 pandemic versus prior years (rs9271117 P = 6.7×10−14 OR 0.57) (Figure 2, Table 2). The association was not due to population stratification as cases pre and post 2009 did not differ in their geographic distribution or principal components (Figure S2 and S3). Among 685 cases with onset ≤10 years, rs7744020A had a frequency of 0.24, compared to 0.14 in 155 cases with onset ≥15 (P = 0.0003, OR 1.88). No other significant associations were observed with other characteristics, including for rs12322530 and cataplexy onset (1069 individuals cataplexy onset age 2–55; P = 0.99, beta 0.01, SE 0.69), a proxy for rs12425451 (r2 = 0.94) that was nominally associated with cataplexy onset in a European cohort [32]. None of the identified GWA significant loci showed significant interactions with clinical variables.
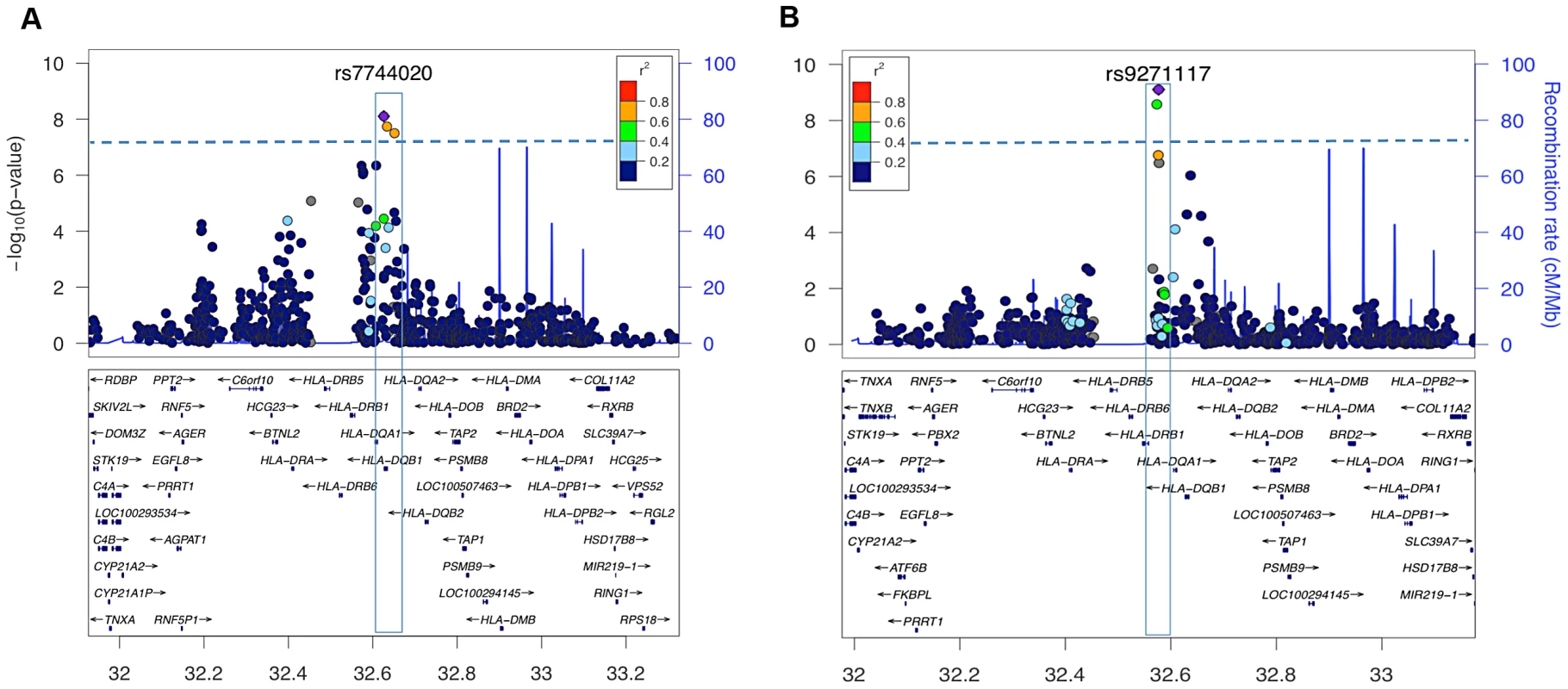
SNP associations within the HLA are difficult to interpret, as specific SNPs may be associated with multiple haplotypes, creating inflated or deflated p values that may not have a simple interpretation. We therefore examined the effects of specific HLA alleles carried by our cases on the clinical presentation. In narcolepsy, HLA-DR and DQ associations are established, and the fact nearly all patients carry DQA1*01:02-DQB1*06:02 on at least one chromosome facilitates interpretation and imputation. To study HLA allele effects, we imputed HLA-DQ in our sample using a reference set of 239 cases and 14 controls (partially described in [3]). All reference individuals carried DQB1*06:02 and were fully HLA-DQA1 and DQB1 typed. Further analyses on the effects of specific HLA alleles in our sample were then performed only on individuals carrying least one copy of DQB1*06:02 (1183 cases and 438 controls) to avoid issues pertaining to differential imputation quality in cases versus controls (who carry a more diverse array of HLA alleles). Using this method, DR and DQ allele frequencies in cases versus controls were compiled, and showed expected effects (Table 3, Table S3). We next categorized the sample into 5 subgroups based on established relative risk categories. Consistent with prior reports, DQB1*06:02 homozygotes carried the highest risk, followed by DQB1*06:02/DQB1*03:01 heterozygotes (Table 3). In contrast, individuals carrying non-DQA1*01:02, non-DQB1*06:02-DQ1 alleles were rather protected as predicted by the allele competition model [3]. These results establish validity of our imputed HLA data.
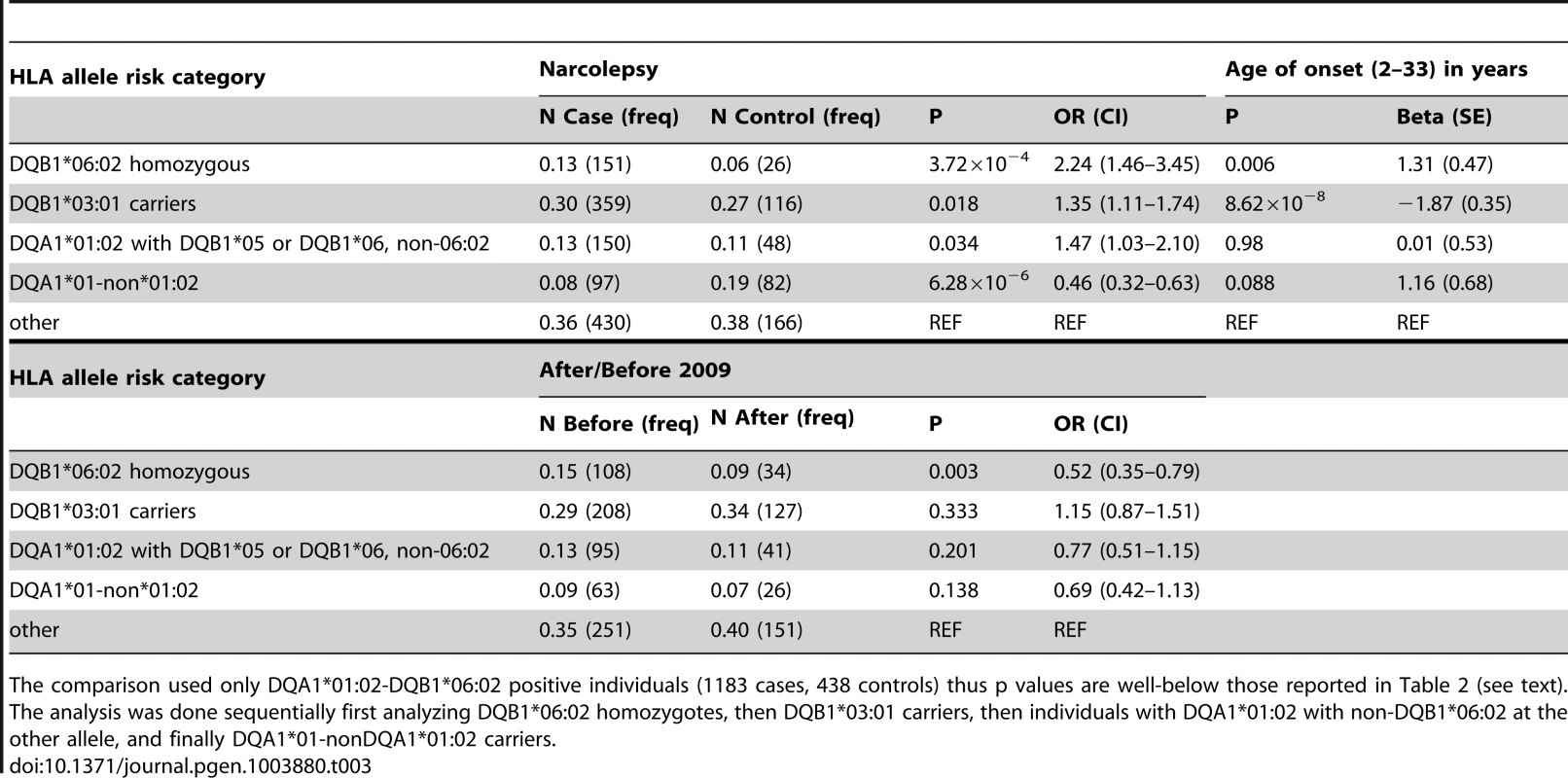
As suggested by the rs7744020 association with this allele (Table 2, Table S2), DQB1*03:01 had a strong effect on earlier age of onset (P = 8.62×10−8 Beta −1.87 years; Table 3). This finding is of particular interest, as the predisposing mechanism of DQB1*03:01 is not explained by the allelic competition model, and is independent of DQA1 [1], [2]. The divergent effects of DQB1*03:01 and DQB1*06:02 on age of onset further support a different mechanism of action for this allele, perhaps an effect of T-Cell receptor repertoire. A prior study also found no effect of DQB1*06:02 homozygosity on disease onset in Caucasians [33]. Interestingly, DQB1*03:01 frequency is high in China, and variable across Europe, possibly explaining why an unusually large number of cases with childhood onset are reported in China versus US and Europe.
Table 3 also describes overall HLA association across HLA genotype risk categories for cases with onset after versus prior to the 2009 H1N1 pandemic. Consistent with the genome wide significant effect of rs9271117, a marker located between DQA1 and DRB1 and mostly linked with DQA1*01:02 (Table 2, Table S2), fewer DQB1*06:02 homozygotes were found in subjects with disease onset following the influenza H1N1 pandemic in China (P = 0.003 OR 0.52; Table 3, Table S3). In contrast, DQB1*03:01 had no effect (Table 3). Although these results suggest HLA differences in subjects with onset prior versus after 2009, the effect of rs9271117 (Table 2) was stronger than the effects of DQB1*06:02 homozygocity (Table 3) and of all other individual HLA effects we could impute (Table S3). It was also independent of DQB1*03:01 and of age of onset differences, remaining genome wide significant after controlling for these factors (data not shown). This finding is unique as, to our knowledge it is the first time a GWA significant signal has been shown to vary with calendar time, a variable rarely considered in existing GWAS of autoimmune diseases. The 2009 H1N1 pandemic was a remarkable event, as although sporadic cases of swine to human infections were reported with similar swine flu strains as early as 1998, only in 2009 did this new variant cross the species barrier [20], transmitting rapidly first in children and young adults in the winter of 2009–2010 [34]. Perhaps post H1N1 cases involve presentation of new epitopes to HLA alleles not identified in prior studies, explaining the differential HLA region association, an hypothesis that will only be answered through full HLA typing, notably of additional DRB genes. Alternatively, a linked variant could have regulatory effects. We hypothesize that GWA analysis of other diseases across time, notably those with an autoimmune component, may help decipher the timing and nature of environmental factors linked to specific disease pathophysiology. This may prove powerful as sample size for these diseases increases, as identification of environmental factors for most diseases has been more resistant to investigation than genetic analysis.
Materials and Methods
Subjects
Our sample included 1189 narcolepsy cases, 1136 of whom were seen at the sleep laboratory of People's Hospital, Peking University, Beijing, a unit in the pulmonary medicine department evaluating patients with sleep disorders and receiving referrals from all over China. In addition, 51 samples came from Taiwan (Dr. Huang, National Taiwan University) and two from Stanford. All patients had either documented hypocretin deficiency (CSF hypocretin-1≤110 pg/ml, n = 119), or clear-cut cataplexy and HLA-DQB1*06:02 [6]. Cases were mostly Han descent (87%) and from North China (85%). The majority of our cases were male (67%). A majority of cases are children (70%) [21]; mean age was 11.2±0.2 years (11.6±0.3 in males versus 10.55±0.39 for females). Clinical data included age of disease onset (earliest onset of cataplexy or sleepiness), presence or absence of cataplexy, sleepiness, sleep paralysis, hypnogogic hallucinations, and disturbed nocturnal sleep, and Multiple Sleep Latency Testing data (mean sleep latency and number of sleep onset REM sleep periods). Delay between disease onset and diagnosis was also noted [9] [18]. Mean ± SEM are reported for age of onset, and diagnostic delay. Control genotypes from China came from university employees and students (41% male), and shared controls from GWAS studies underway for colon cancer and Sjogren's syndrome.
Ethics statement
Informed consent in accordance with governing institutions was obtained from all subjects. The research protocols were approved by IRB Panels on Medical Human Subjects at both Stanford University and the Beijing University People's Hospital.
Genotyping, quality control and SNP selection
DNA samples were genotyped on the Affymetrix Axiom CHB array. Genotypes were called using Affymetrix Genotyping Console. Individuals with call rate <95%, or that were outliers following principal components analysis (n = 47), or related (n = 53), were removed, leaving 1189 cases and 1997 controls. For the main association study, we selected SNP variants with MAF ≥1%, call rate ≥90%, and HWE p value ≥0.001 in controls. Because of the near requirement for DQB1*06:02 in narcolepsy, an extended HLA region from rs1419229 to rs9368865 (Chr 6:24112537-35363736) was excluded from the primary association, leaving 603,382 autosomal, non-HLA SNP variants. The extended HLA region contained 3,000 SNPs and was analyzed separately for imputation of HLA haplotypes and effects on clinical presentation.
Association statistics
The majority of the analysis (including quality control, LD calculations, quantitative trait association, and interactions) was performed using the Plink suite of software (v 1.07) [35]. To control for population stratification, the association was performed using a variance component model implemented in EMMAX [36]. Cluster quality of top ranking SNP markers was verified by visual examination of clusters. As EMMAX does not return OR or MAF data, these data were gathered and reported from corresponding analyses in Plink. Inflation statistics were estimated, and QQ plots were generated using estlambda (GenABEL package (v1.7-4) in R (2.15.3) [37] [38]. In addition, Principal Components was performed, and Manhattan plot generated using SVS v7 (GoldenHelix).
Replication
The top ranked 150 SNPs from associations performed in EMMAX and Plink (a total of 188 SNPs, p≤0.001 for EMMAX, ≤0.003 for Plink) were selected for follow-up. After visual check and exclusion of 16 poorly clustered SNPs, we selected the top-ranked SNP from each locus (defined as 2 SNPs within 100 kb) resulting in 80 variants for replication in our previously published European cohorts (Affymetrix 6.0 array study: 807 narcolepsy, 1071 controls; Illumina Immunochip array study 1886 cases, 10,421 controls). The Affymetrix and Illumina Immunochip samples were partially overlapping and thus the replication was first done in the larger Illumina immunochip sample. If a variant was not present or tagged by variants on that array, then replication was next attempted in the smaller Affymetrix 6.0 data set. When a selected CHB variant was not on the corresponding array, an analysis of LD in a 10–20 kb window was performed in Chinese and Europeans (Ensembl 1000genomes browser LD data) to identify potential proxies with an r2 ≥0.8 available on the ImmunoChip or Affymetrix 6.0 array. In the case of rs1551570, the variant was not tagged on either array, but shows strong LD with rs2305795, a SNP found to be associated with narcolepsy following fine mapping [7] where corresponding p values were extracted from the previous analysis. A Bonferroni correction was applied to determine significance.
Clinical associations
Multiple Sleep Latency Testing data (mean sleep latency and number of sleep onset REM sleep periods) and age of onset were studied using genome wide linear regressions as quantitative traits (Plink). Genome-wide significant values obtained by Plink were then re-tested using EMMAX, with EMMAX P values reported in Table 2. Presence or absence of cataplexy, sleep paralysis, hypnogogic hallucinations, as well as whether or not onset was prior to September 2009, were studied as binary phenotypes (Plink).
Imputation and combined association analysis of Chinese and Caucasian data
We imputed genotypes in windows that fully surrounded each of the 5 replicated loci. Imputation was performed separately in the Chinese Axiom CHB, and European ImmunoChip, Affymetrix 6.0 using Beagle v3.3 [39] against the CHB reference Chinese population, or 4 European populations (286 individuals from CEU, TSI, GBR, IBS) in the 1000 genomes integrated data set (phase 1 release v3). Imputation was performed in the Chinese cohort for all five replicated loci (TRA@, TRB@, ZNF365, PPAN-DNMT-1, IL10RB-IFNAR1 region). These loci were also imputed in European ancestry narcolepsy samples depending on regional SNP coverage on the corresponding genotype array (TRA@ in Affymetrix 6.0, with rs1154155 also in Immunochip; TRB@ in Affymetrix 6.0, not covered in ImmunoChip; ZNF365 in ImmunoChip, not covered on Affymetrix 6.0; PPAN-DNMT1 in Affymetrix 6.0, not covered in ImmunoChip, IL10RB-IFNAR1 Affymetrix 6.0- no SNPs in LD with rs2834188 on ImmunoChip). Imputed genotypes were combined (Chinese+ ImmunoChip, or Chinese+Affymetrix 6.0) and associations were performed using a Mantel Haenszel test (Plink). SNP markers with poor imputation quality scores in either Chinese or Europeans (r2<0.8) were excluded from further analysis. Plots of association statistics were made using LocusZoom [40].
Imputation of HLA haplotypes and alleles in DQB1*06:02 positive subjects
High resolution HLA typing had been performed on a subset of 239 narcolepsy cases and 14 controls for HLA DRB1, DQA1 and DQB1 genes [3], all positive for DQB1*06:02. These individuals were also genotyped on the Axiom CHB array (see above). In order to impute the HLA genotypes for the rest of the data set, the HLA types, together with array-genotyped SNPs in a 500 kb window surrounding HLA DRB1, DQA1 and DQB1 were submitted as a training set to HIBAG package in R. HIBAG is an HLA imputation tool that uses attribute bootstrap aggregation of several classifiers (SNPs) to select groups of SNPS that predict HLA type [41]. The resulting sets of haplotype predictive SNPs were then used to impute HLA type in the remaining samples (cases and controls). Allele frequencies of DRB1, DQA1 and DQB1 alleles obtained after imputation was consistent with population data (r2 = 0.96) [42]. Imputation was acceptable in both DQB1*06:02 positive and negative controls, but in consideration of our training set, we only used data from DQB1*06:02 positive individuals, which was imputed with better quality (85.0% in DQB1*06:02 positive individuals and 77.5% in DQB1*06:02 negative individuals). Imputation of DQB1*03:01, DQB1*06:02 and DQB1*01:02, the alleles of principal interest in this study was highly accurate, (DQB1*03:01: 92.8% in controls 97.6% in cases, DQB1*06:02: 90.5% in controls, 97.4% in cases, DQA1*01:02: 92.2% in controls, 97.5% in cases) in the data set. The imputation quality may be overestimated in cases since the narcolepsy population is more homogenous for their HLA haplotype.
Sub analyses of DQB1*06:02 positive individuals
The imputed HLA haplotype data was first used to study association between rs7744020, rs9274477, rs17212223 (age of onset associated), rs9271117, rs9270965 (associated with onset prior versus after September 2009) and various HLA genotypes (Table 2). To verify whether the HLA association in narcolepsy versus controls was consistent with previous reports in other ethnic groups [1], including Chinese subjects [3] (N = 1183 DQB1*06:02 positive cases and N = 438 DQB1*06:02 positive controls), we next categorized subjects into five risk groups based on our prior model of allelic competition [3]: 1) DQB1*06:02 homozygous (highest risk); 2) DQB1*03:01 carriers (second highest risk); 3) DQA1*01:02-DQB1*05 or DQB1*06 non-06:02 (intermediate) 4) DQA1*01 non-DQA1*01:02 (protective) 5) alleles with no predisposition or protection effects for narcolepsy. These categories were then compared using χ2 square tests between cases versus controls and cases after versus before September 2009. χ2 analyses were performed sequentially, starting with DQB1*06:02 homozygotes, then moving on to DQB1*03:01 carriers, then to DQA1*01:02-DQB1*05 or DQB1*06 non-06:02 carriers, and finally to DQA1*01 non-DQA1*01:02 carriers, as previously reported [3], a technique similar to relative predisposition effect statistics [43].
Interaction analysis
Potential interactions between narcolepsy risk SNPs and HLA genotypes were analyzed with R version 2.15.3, and with an epistasis model Y ∼b0 + b1.A + b2.B + b3.AB +e implemented in Plink. The interaction was performed with narcolepsy, onset after/before 2009 and with age of onset.
URLs
http://faculty.washington.edu/browning/beagle/beagle.html
http://bochet.gcc.biostat.washington.edu/beagle/1000_Genomes.phase1_release_v3/
http://csg.sph.umich.edu/locuszoom/
Supporting Information
Zdroje
1. MignotE, LinL, RogersW, HondaY, QiuX, et al. (2001) Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. American journal of human genetics 68: 686–699.
2. HongSC, LinL, LoB, JeongJH, ShinYK, et al. (2007) DQB1*0301 and DQB1*0601 modulate narcolepsy susceptibility in Koreans. Human immunology 68: 59–68.
3. HanF, LinL, LiJ, DongSX, AnP, et al. (2012) HLA-DQ association and allele competition in Chinese narcolepsy. Tissue Antigens 80: 328–335.
4. HorH, KutalikZ, DauvilliersY, ValsesiaA, LammersGJ, et al. (2010) Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nature genetics 42: 786–789.
5. PelinZ, GuilleminaultC, RischN, GrumetFC, MignotE (1998) HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 51: 96–100.
6. HallmayerJ, FaracoJ, LinL, HesselsonS, WinkelmannJ, et al. (2009) Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet 41: 708–711.
7. KornumBR, KawashimaM, FaracoJ, LinL, RicoTJ, et al. (2011) Common variants in P2RY11 are associated with narcolepsy. Nat Genet 43: 66–71.
8. FaracoJ, LinL, KornumBR, KennyEE, TrynkaG, et al. (2013) ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet 9: e1003270.
9. HanF, LinL, WarbySC, FaracoJ, LiJ, et al. (2011) Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 70: 410–417.
10. AranA, LinL, NevsimalovaS, PlazziG, HongSC, et al. (2009) Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep 32: 979–983.
11. LongstrethWTJr, TonTG, KoepsellTD (2009) Narcolepsy and streptococcal infections. Sleep 32: 1548.
12. DauvilliersY, MontplaisirJ, CochenV, DesautelsA, EinenM, et al. (2010) Post-H1N1 narcolepsy-cataplexy. Sleep 33: 1428–1430.
13. MeeyaiA, CooperBS, CokerR (2013) Analysis of 2009 pandemic influenza A/H1N1 outcomes in 19 European countries: association with completeness of national strategic plans. BMJ open 3: e002253.
14. NohynekH, JokinenJ, PartinenM, VaaralaO, KirjavainenT, et al. (2012) AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One 7: e33536.
15. MillerE, AndrewsN, StellitanoL, StoweJ, WinstoneAM, et al. (2013) Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ 346: f794.
16. SzakacsA, DarinN, HallbookT (2013) Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology 80: 1315–1321.
17. WijnansL, LecomteC, de VriesC, WeibelD, SammonC, et al. (2013) The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine 31: 1246–1254.
18. HanF, LinL, LiJ, DongXS, MignotE (2012) Decreased incidence of childhood narcolepsy 2 years after the 2009 H1N1 winter flu pandemic. Ann Neurol E-pub ahead of print. doi: 10.1002/ana.23799
19. PartinenM, Saarenpaa-HeikkilaO, IlveskoskiI, HublinC, LinnaM, et al. (2012) Increased Incidence and Clinical Picture of Childhood Narcolepsy following the 2009 H1N1 Pandemic Vaccination Campaign in Finland. PLoS One 7: e33723.
20. LanL, LuW, LiS, YinJ, XieJ, et al. (2011) Evolutionary characteristics of swine-origin H1N1 influenza virus that infected humans from sporadic to pandemic. Journal of Public Health and Epidemiology 3: 254–270.
21. HanF, LinL, LiJ, AranA, DongSX, et al. (2011) Presentations of primary hypersomnia in Chinese children. Sleep 34: 627–632.
22. HanF, LinL, LiJ, AranA, DongSX, et al. (2012) TCRA, P2RY11, and CPT1B/CHKB associations in Chinese narcolepsy. Sleep Med 13: 269–272.
23. WinkelmannJ, LinL, SchormairB, KornumBR, FaracoJ, et al. (2012) Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum Mol Genet 21: 2205–2210.
24. HirotaT, TakahashiA, KuboM, TsunodaT, TomitaK, et al. (2012) Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet 44: 1222–1226.
25. WatermanM, XuW, StempakJM, MilgromR, BernsteinCN, et al. (2011) Distinct and overlapping genetic loci in Crohn's disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis 17: 1936–1942.
26. Huertas-VazquezA, NelsonCP, GuoX, ReinierK, Uy-EvanadoA, et al. (2013) Novel Loci associated with increased risk of sudden cardiac death in the context of coronary artery disease. PLoS One 8: e59905.
27. CouchFJ, GaudetMM, AntoniouAC, RamusSJ, KuchenbaeckerKB, et al. (2012) Common variants at the 19p13.1 and ZNF365 loci are associated with ER subtypes of breast cancer and ovarian cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev 21: 645–657.
28. HarituniansT, JonesMR, McGovernDP, ShihDQ, BarrettRJ, et al. (2011) Variants in ZNF365 isoform D are associated with Crohn's disease. Gut 60: 1060–1067.
29. BegueB, VerdierJ, Rieux-LaucatF, GouletO, MoraliA, et al. (2011) Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol 106: 1544–1555.
30. MaoH, YangW, LeePP, HoMH, YangJ, et al. (2012) Exome sequencing identifies novel compound heterozygous mutations of IL-10 receptor 1 in neonatal-onset Crohn's disease. Genes Immun 13: 437–442.
31. KalinkeU, PrinzM (2012) Endogenous, or therapeutically induced, type I interferon responses differentially modulate Th1/Th17-mediated autoimmunity in the CNS. Immunol Cell Biol 90: 505–509.
32. LucaG, Haba-RubioJ, DauvilliersY, LammersGJ, OvereemS, et al. (2013) Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res 22: 482–495.
33. PelinZ, BozluolcayM, KaynakD, KaynakH (2002) Childhood onset of narcolepsy-cataplexy syndrome in Turkey: clinical and genetic study. Turk J Pediatr 44: 321–325.
34. FraserC, DonnellyCA, CauchemezS, HanageWP, Van KerkhoveMD, et al. (2009) Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324: 1557–1561.
35. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 81: 559–575.
36. KangHM, SulJH, ServiceSK, ZaitlenNA, KongSY, et al. (2010) Variance component model to account for sample structure in genome-wide association studies. Nature genetics 42: 348–354.
37. AulchenkoYS, RipkeS, IsaacsA, van DuijnCM (2007) GenABEL: an R library for genome-wide association analysis. Bioinformatics 23: 1294–1296.
38. Team RDC (2008) R: A language and environment for statistical computing. Computing RFfS, editor. Vienna, Austria.
39. BrowningSR, BrowningBL (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. American journal of human genetics 81: 1084–1097.
40. PruimRJ, WelchRP, SannaS, TeslovichTM, ChinesPS, et al. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26: 2336–2337.
41. ZhengX, ShenJ, CoxC, WakefieldJC, EhmMG, et al. (2013) HIBAG-HLA genotype imputation with attribute bagging. Pharmacogenomics J E-pub ahead of print. doi: 10.1038/tpj.2013.18
42. HeiAL, LiW, DengZH, HeJ, JinWM, et al. (2009) Analysis of high-resolution HLA-A, -B, -Cw, -DRB1, and -DQB1 alleles and haplotypes in 718 Chinese marrow donors based on donor-recipient confirmatory typings. International journal of immunogenetics 36: 275–282.
43. HollenbachJA, MackSJ, ThomsonG, GourraudPA (2012) Analytical methods for disease association studies with immunogenetic data. Methods in molecular biology 882: 245–266.
Štítky
Genetika Reprodukčná medicínaČlánok vyšiel v časopise
PLOS Genetics
2013 Číslo 10
- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
Najčítanejšie v tomto čísle
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
