-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
In mammalian females, genes on one X are largely silenced by X-chromosome inactivation (XCI), although some “escape” XCI and are expressed from both Xs. Escapees can closely juxtapose X-inactivated genes and provide a tractable model for assessing boundary function at epigenetically regulated loci. To delimit sequences at an XCI boundary, we examined female mouse embryonic stem cells carrying X-linked BAC transgenes derived from an endogenous escape locus. Previously we determined that large BACs carrying escapee Kdm5c and flanking X-inactivated transcripts are properly regulated. Here we identify two lines with truncated BACs that partially and completely delete the distal Kdm5c XCI boundary. This boundary is not required for escape, since despite integrating into regions that are normally X inactivated, transgenic Kdm5c escapes XCI, as determined by RNA FISH and by structurally adopting an active conformation that facilitates long-range preferential association with other escapees. Yet, XCI regulation is disrupted in the transgene fully lacking the distal boundary; integration site genes up to 350 kb downstream of the transgene now inappropriately escape XCI. Altogether, these results reveal two genetically separable XCI regulatory activities at Kdm5c. XCI escape is driven by a dominant element(s) retained in the shortest transgene that therefore lies within or upstream of the Kdm5c locus. Additionally, the distal XCI boundary normally plays an essential role in preventing nearby genes from escaping XCI.
Published in the journal: Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing. PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003952
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003952Summary
In mammalian females, genes on one X are largely silenced by X-chromosome inactivation (XCI), although some “escape” XCI and are expressed from both Xs. Escapees can closely juxtapose X-inactivated genes and provide a tractable model for assessing boundary function at epigenetically regulated loci. To delimit sequences at an XCI boundary, we examined female mouse embryonic stem cells carrying X-linked BAC transgenes derived from an endogenous escape locus. Previously we determined that large BACs carrying escapee Kdm5c and flanking X-inactivated transcripts are properly regulated. Here we identify two lines with truncated BACs that partially and completely delete the distal Kdm5c XCI boundary. This boundary is not required for escape, since despite integrating into regions that are normally X inactivated, transgenic Kdm5c escapes XCI, as determined by RNA FISH and by structurally adopting an active conformation that facilitates long-range preferential association with other escapees. Yet, XCI regulation is disrupted in the transgene fully lacking the distal boundary; integration site genes up to 350 kb downstream of the transgene now inappropriately escape XCI. Altogether, these results reveal two genetically separable XCI regulatory activities at Kdm5c. XCI escape is driven by a dominant element(s) retained in the shortest transgene that therefore lies within or upstream of the Kdm5c locus. Additionally, the distal XCI boundary normally plays an essential role in preventing nearby genes from escaping XCI.
Introduction
Recent annotation of the human and mouse genomes has revealed chromosome domains that are distinguished by sequence and gene content, regulatory-factor binding, replication dynamics, chromatin composition, or nuclear location. Many of these domains overlap and can functionally segregate active and inactive transcripts [1]–[4]. What regulates such extensive genome compartmentalization is not fully understood. Intriguingly, many boundaries share common features including opposing chromatin marks, active transcription, or binding by the CCCTC binding factor, CTCF [1], [4]–[6]. Whether these elements are essential for segregating domains has not been thoroughly examined, yet boundary deletion can lead to misregulation (e.g. [7]).
An interesting example of partitioned, closely juxtaposed, active and inactive transcripts is found on one X chromosome in female mammals. This X is largely silenced during early embryonic development in order to balance dosage between the sexes. X-chromosome inactivation (XCI) is mediated by the cis-limited action of Xist, a structural RNA that coats the X chromosome and recruits inactive chromatin modifiers [8]. Nevertheless, XCI is not chromosome-wide, as some genes “escape” inactivation [9]. Current understanding of how genes escape XCI on an otherwise silenced chromosome is incomplete, but the answer may reveal novel insights about regulatory sequences not only at XCI boundaries but also at other expression transitions throughout the genome.
Escape and X-inactivated genes are epigenetically and structurally distinct [9]. Escape genes are depleted in Xist RNA and promoters are marked by active histone modifications and lack silent epigenetic marks associated with X-inactivated transcripts (e.g. [10]–[12]). However, long-range regulation is likely involved, as many escape genes, particularly in humans, are physically clustered [13], [14]. Further supporting this idea, unique sequence composition distinguishes these domains relative to the rest of the X [15], [16]. Distant escapees also frequently interact on the inactive X [17] and can be spatially separated from silent inactive X regions [18].
To functionally delimit sequences sufficient to confer XCI escape, we previously developed a transgene approach in female mouse embryonic stem (ES) cells, a well established ex vivo XCI model [19]. X-linked BAC transgene lines were isolated that carry the escapee Kdm5c (previously Jarid1c) that encodes a histone H3K4 demethylase [20], [21]. The BACs also included an adjacent long non-coding RNA (lncRNA) AK148627 that escapes XCI [14], [22] and flanking X-inactivated genes [16], [19], [23]. Endogenous expression patterns examined were maintained including transgenic Kdm5c (Kdm5c-tg) escape at four ectopic X-chromosome locations. Therefore, these BACs must include sequences necessary for Kdm5c to escape XCI. What features at this locus direct XCI escape? Plausible candidates include CTCF and the AK148627 lncRNA, as both CTCF and lncRNAs are found at a number of XCI boundaries [14], [22], [24]. Further, such elements are enriched at other boundaries throughout the genome (e.g. [1], [4], [5]), and can function to regulate adjacent genes in cis [25], [26]. Intriguing associations notwithstanding, both candidates lack functional validation. To better understand the role of boundary sequences in inactive X regulation we now extend our analysis of Kdm5c BAC transgenes. We further narrow sequences necessary for XCI escape and identify a novel role for XCI boundary sequences in regulating inactive X expression.
Results
An X-linked transgene deletion series
Previous studies focused on four full-length BAC transgenes that were derived from two overlapping BACs [19] (Figure 1). However, by PCR analysis of BAC-backbone sequences, six additional female ES lines carry X-linked integrants of the BAC RP23-391D18 with partial deletions. We turned to these truncated transgenes to further delimit sequences that dictate XCI states at the Kdm5c locus.
Fig. 1. Mapping transgene deletion breakpoints. 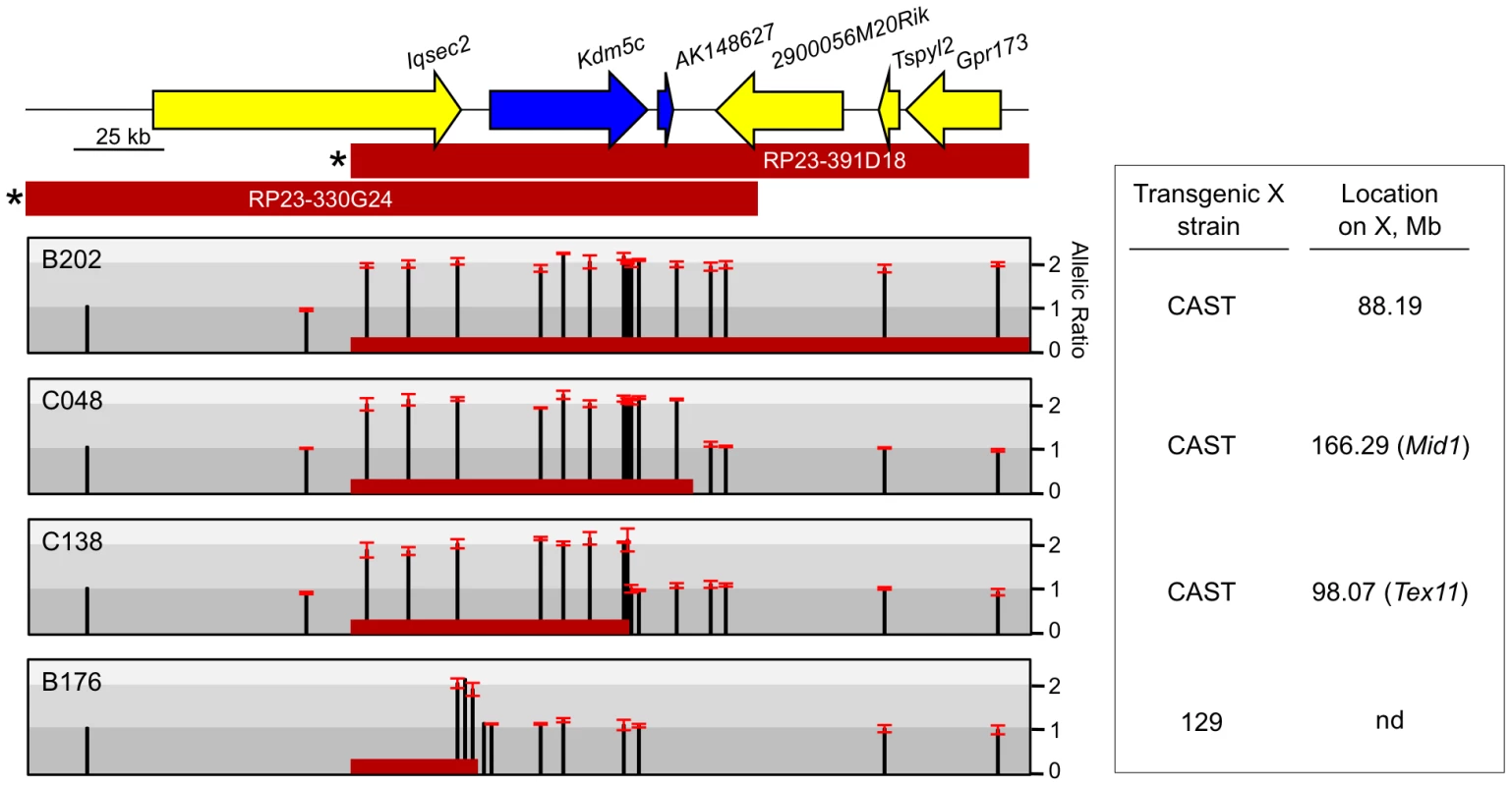
Endogenous Kdm5c locus at chrX:148,557,326-148,807,402 (mm9). Escapees are blue. BACs used to generate transgenes are indicated with selectable marker denoted (*) [19]. Relative allele ratios of SNPs in ES lines carrying derivatives of BAC RP23-391D18 are represented as (129 + BAC)/CAST. Error bars indicate standard deviation of technical triplicates. Based on SNP ratios, red bar indicates transgene content in each line with the 3′ end positioned at the midpoint of the breakpoint interval. nd: not determined. To determine transgene content and copy number, we exploited allele differences between the 129 and M.m. castaneous (CAST) X chromosomes in the ES cell line and assayed for the presence or absence of an additional BAC-transgene allele. Allele ratios for up to 18 SNPs across the region were measured using a quantitative primer-extension assay, qSNaPshot [13], [19], with primers that abut each SNP. The approach was validated with allele ratios of 2∶1 ((129+BAC)/CAST) for all SNPs mapping within full-length, single-copy BACs (e.g. B202) [19] (Figure 1, Figure S1). Similar analysis excluded three lines with multi-copy inserts (Figure S1). Further, the transgene in the B176 line is severely truncated and deletes the entire Kdm5c-tg.
Breakpoint analysis for two other transgene lines revealed deletions of distal XCI boundary sequences. ES lines C048 and C138 carry single-copy inserts that retain all or most of Kdm5c-tg (Figure 1). The C048 transgene contains the AK148627 lncRNA but deletes a large portion of non-transcribed XCI boundary sequence. The transgene in C138 is more extensively deleted as all sequences downstream of Kdm5c are removed including the lncRNA. Additional SNPs narrowed the C138 transgene breakpoint to a small ∼900 bp window and indicate that at least 90% of the Kdm5c genomic locus remains intact. Further, by RNA fluorescence in situ hybridization (FISH) a stable nascent Kdm5c-tg transcript is detected in pre-XCI undifferentiated ES cells (not shown). Therefore, the C048 and C138 transgenes lack all or part of the intervening region between the 3′ end of escapee Kdm5c and the closest X-inactivated gene and allow the role of sequences within an escape domain and at an XCI boundary to be evaluated.
Transgenes integrated into normally X-inactivated regions
Prior to examining transgene expression we surveyed the local chromosomal environment flanking the C048 and C138 BAC transgenes. By inverse PCR and subsequent analysis of an adjacent SNP, the C048 transgene inserted on the CAST X, upstream of the first coding exon of the Mid1 gene (166,290,616 bp, mm9). Importantly, Mid1 is normally X inactivated on the CAST X [19]. Additionally, FISH and SNP screening indicate that this transgene insertion was accompanied by a large and likely terminal deletion that removes the entire pseudoautosomal region (Figure S2A).
Similar characterization of the C138 transgene revealed that the BAC integrated on the CAST X at 98,065,555 bp (mm9) (Figure S2B). DNA FISH and SNP analysis near the C138 transgene integration site ensured that the BAC insertion was not accompanied by a larger chromosomal rearrangement or deletion (Figure S2B). This places Kdm5c-tg in an intron near the 3′ end of Tex11, a gene that functions in male meiosis [27], [28]. Although predominantly expressed in testis [29], we detected a low level of Tex11 expression in somatic tissues by RT-PCR; monoallelic expression of a transcribed polymorphism in female fibroblasts with non-random XCI confirms that Tex11 is normally X inactivated (Figure S2C). Therefore, both transgenes integrated into regions that are normally silenced by XCI, enabling direct testing of BAC sequence influences on Kdm5c-tg expression.
Transgenic Kdm5c escapes XCI
Will Kdm5c-tg still escape XCI in the absence of distal boundary sequences? Expression was examined by sequential RNA and DNA FISH upon ES cell differentiation and concomitant XCI. Non-denatured cells were hybridized with a Kdm5c BAC probe to detect nascent transcripts from the endogenous and transgenic loci. Following probe fixation, cells were denatured and hybridized for DNA FISH to demarcate all Kdm5c loci. In C138 and C048, three expressed foci were detected in most cells (Figure 2). Importantly for each line, nuclei with two RNA signals colocalizing with Xist RNA demonstrate that both endogenous and transgenic loci are expressed on the inactive X. Additional FISH for C138 directly confirmed Kdm5c-tg escape, as one inactive X transcript colocalizes with a DNA signal from a probe at the integration site (Figure S3A). RNA FISH using a smaller Kdm5c-specific probe ensured results reflect Kdm5c expression (Figure S3B). Because of genetic background differences in the ES cells, XCI is skewed and the transgene is on the inactive CAST X in ∼25% of cells [19], [30]. For both C138 and C048, the proportion of cells with two expressed Kdm5c foci from the inactive X closely mirrors the frequency that cells inactive the CAST X chromosome (Figure 2B, Figure S3). Therefore, these data indicate Kdm5c-tg escapes XCI at a frequency similar to the non-transgenic locus.
Fig. 2. XCI status of Kdm5c-tg by sequential RNA and DNA FISH. 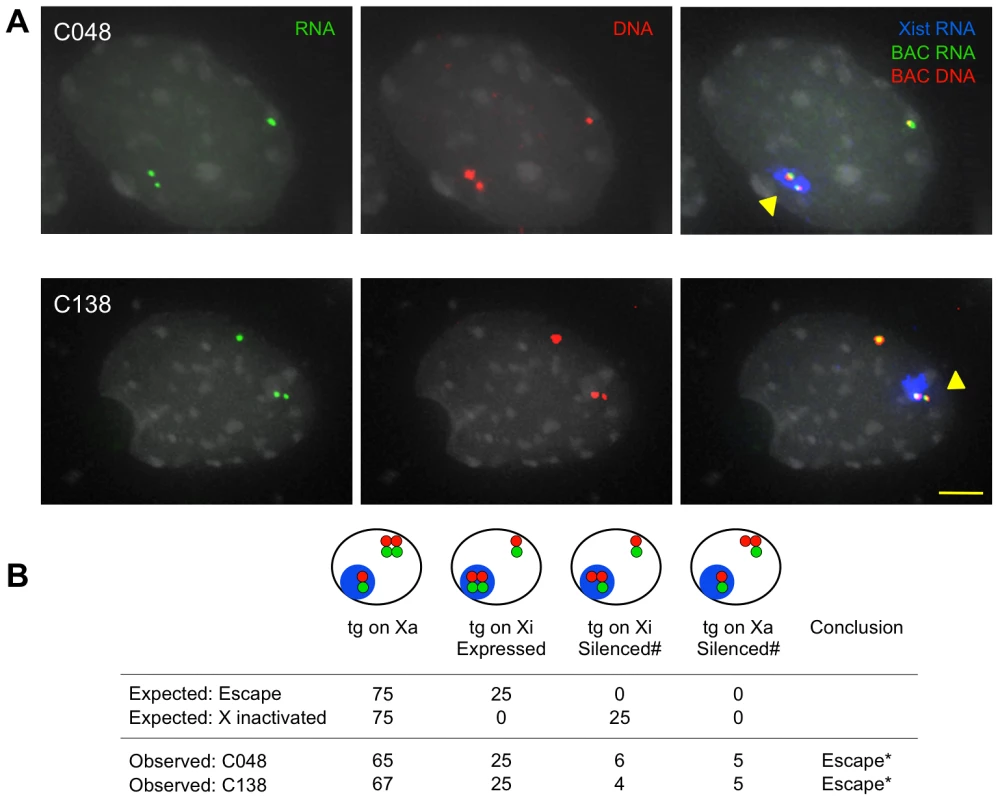
(A) Representative nuclei showing Kdm5c-tg escapes XCI. Kdm5c BAC probes detect nascent transcripts (green) and DNA loci (red). Xist RNA (pseudocolored blue) identifies the inactive X (Xi). (B) Observed results were compared to expected values for the transgene to either escape or be X inactivated as influenced by XCI skewing [19]. Cartoons depict hybridization patterns scored, with colors as in A. (#) indicates that an absent transcript signal from the transgenic X (Xa or Xi) could reflect the endogenous or transgene locus, but cells were scored as silencing Kdm5c-tg. n = 100. *p<0.001. To better estimate the level of Kdm5c-tg escape in C138, we isolated a clonal line that carries the transgene on the inactive X chromosome. Allelic expression, measured by qSNaPshot, is consistent with Kdm5c-tg and the non-transgenic locus each partially escaping XCI, at levels that are ∼34% of active X expression (see methods). Such levels are in good agreement with previous reports of partial escape for the endogenous locus [18], [19], [31], [32]. These data indicate that despite BAC truncation, Kdm5c-tg is expressed from the inactive X chromosome. Altogether, we conclude that Kdm5c escape does not require distal sequences.
Transgene induces an active structural conformation
Previous studies of Kdm5c indicate that escape genes preferentially assume an exterior location on the Xist-coated inactive X in interphase nuclei [18]. This positioning likely facilitates more frequent long-range associations with other escape genes than with X-inactivated genes [17]. To further confirm the active state of Kdm5c-tg, we asked if transgenes establish similar interactions with distant escapees. Interactions were evaluated in differentiated post-XCI cells by FISH using three-dimensional deconvolution microscopy (Figure 3A). Inactive X distances were initially measured between the escapee Ddx3x and a probe detecting either escapee Kdm5c or an X-inactivated gene (Figure 3A,B). For each comparison, cumulative frequency plots indicate the proportion of nuclei in which two loci are closer than a given nuclear distance (normalized for area) (Figure 3B). This approach was first validated in a non-transgenic line and confirmed that profiles differ for the active and inactive X [17]; distant loci are more frequently in close proximity on the inactive X relative to their distance on the active X (Figure S4A). Further, inactive X escapee associations are also consistent with previous observations [17]. A higher proportion of nuclei have two escape loci in close proximity as the cumulative frequency plot of nuclear distances between escapees Ddx3x and Kdm5c is significantly shifted to the left relative to profiles comparing Ddx3x and either X-inactivated gene, Tex11 or Mecp2 (Figure 3B, Figure S5A). All differences were readily apparent regardless of whether or not probe distances were normalized to nuclear area (Figure S4B).
Fig. 3. Transgenes assume frequent long-range escape-gene interactions. 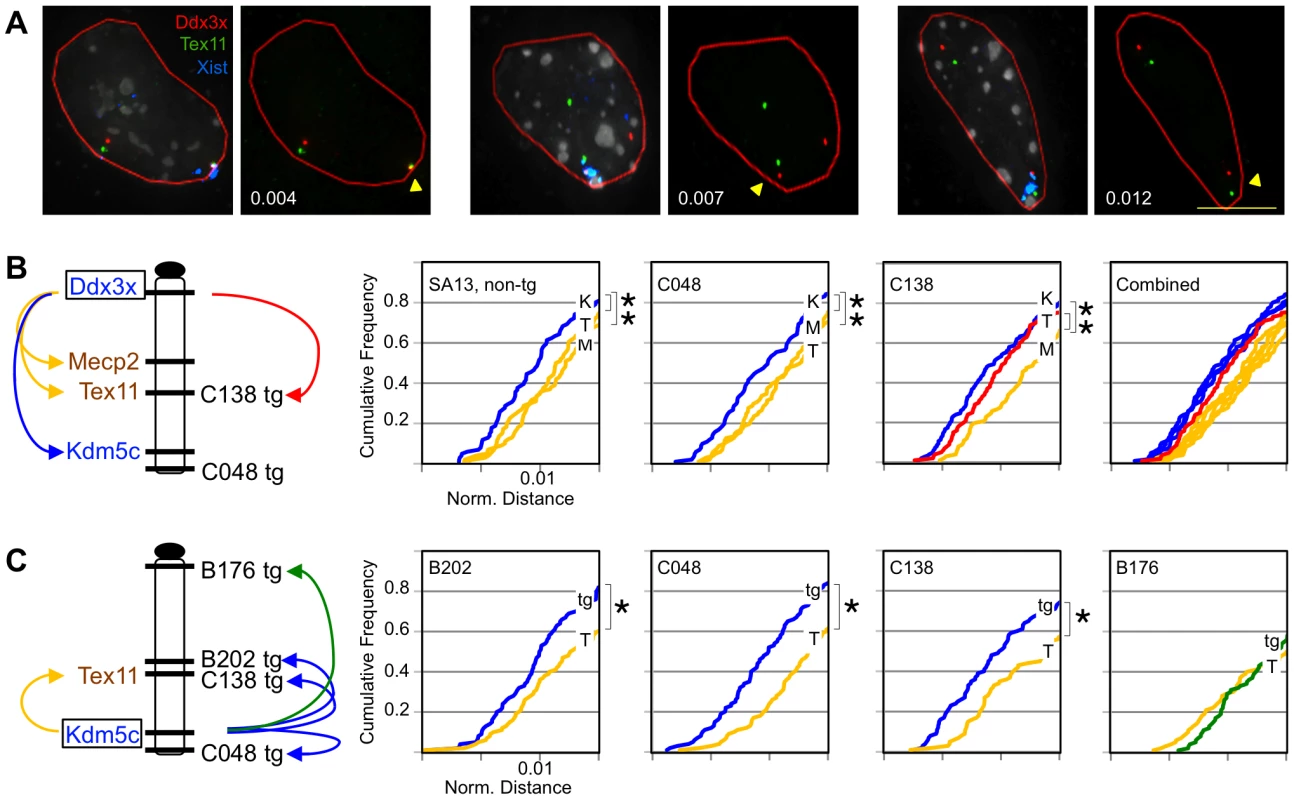
(A) Representative C138 nuclei compressed from 3D-image stacks hybridized to detect BAC DNA and Xist RNA. Arrowhead denotes inactive X. Normalized distances indicated (µm/µm2) reflect inactive X, 3D-probe distances (0.32, 0.89, and 1.33 µm) normalized to nuclear area (red outline). (B,C) Chromosome location of transgenes and BAC probes. BACs tested include X-inactivated genes Mecp2 (M) and Tex11 (T) (yellow/brown) or escapee Kdm5c (K) (blue). (B) Cumulative frequency curves show loci scored relative to Ddx3x. The shifted Ddx3x-Tex11 curve in C138 (red) reflects transgene association in the subset of cells that inactivate the transgenic X. (C) Inactive X distances between Kdm5c and Kdm5c-tg (tg) are compared to endogenous Kdm5c and Tex11 (T) on the non-transgenic X. B176 (green) lacks an expressed Kdm5c-tg. All plots include the 60–80% of cells with closest probe distances (complete data in Figure S5). Each probe comparison includes 100–150 nuclei. *p<0.02. Similar probe comparisons were performed in the transgene lines. All profiles in line C048, with the Mid1-integrated transgene, were indistinguishable from the non-transgenic line (Figure 3B) indicating that a transgene at a location unrelated to the genes tested is insufficient to alter gene localization and interaction. In contrast, while C138 cumulative frequency curves comparing Ddx3x to active and inactive non-transgenic loci mirrored the other lines tested, comparison to the Tex11 BAC revealed a significant left shift (Figure 3B, Figure S5A). Tex11 lies at the C138 transgene integration site and proxies for the transgene in cells that inactivate the transgenic X. Indeed, the Tex11 BAC is frequently located near Ddx3x on the inactive X, with a profile that is more similar to plots comparing two escapees than to curves for genes with differing XCI states, e.g. Ddx3x and Mecp2. These data suggest that a transgene can reconfigure associations on the inactive X.
To more directly visualize transgene interactions we specifically scored transgenic inactive X associations between Kdm5c-tg and the endogenous Kdm5c locus. Compared to interactions with X-inactivated Tex11 (measured on non-transgenic inactive Xs), Kdm5c more frequently lies in close proximity to the transgene in C048, C138, and the full-length B202 transgene (Figure 3C, Figure S5B). In contrast, profiles for the severely truncated transgene in B176 resemble those with X-inactivated locus Tex11 (Figure 3C, Figure S5B). Such a profile likely reflects the absence of Kdm5c-tg transcript in this line and indicates that the partial proximal boundary sequences retained in B176 are insufficient to direct interactions with escape loci. Importantly, these studies demonstrate that Kdm5c-tg in C138 and C048 structurally interacts in a manner similar to the endogenous locus, further confirming the active state of the transgenes on the inactive X. Therefore, despite truncating the endogenous escape domain, retained sequences are sufficient to induce an altered inactive X conformation even when inserted at a different chromosomal location.
A role for distal boundary sequences in silencing adjacent genes
We previously established that the full-length BAC transgenes retain intact XCI boundaries as Kdm5c-tg is expressed, but adjacent transgenic Tspyl2 or Iqsec2 properly undergo XCI [19]. Therefore, we next sought to determine if transcripts near the integration site would remain silent despite the absence of distal boundary sequences (Figure 4A). Given the orientation and close proximity of the C048 transgene to the pseudoautosomal boundary (Figure S2A) we focused on the C138 line. C138 proximal transgene sequences and XCI expression boundary are intact and therefore, adjacent genes are predicted to remain X inactivated. Consistent with this expectation, robust mono-allelic expression from the active X was detected by RNA FISH in both C048 (used to control for a non-transgenic Tex11 locus) and C138 (Figure 4B). These data further establish that transcripts in this region are normally X inactivated and are not altered upon transgene integration.
Fig. 4. Disruption of distal XCI regulation. 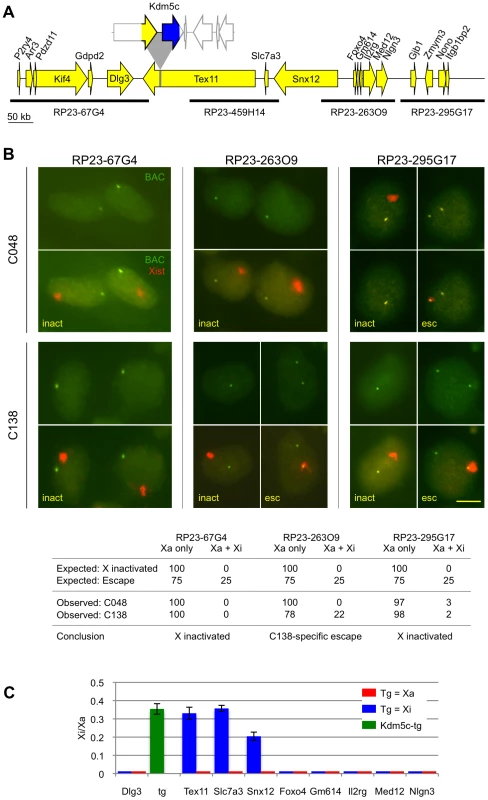
(A) Tex11 locus with the C138 transgene drawn to scale. (B) RNA FISH for BAC probes flanking the C138 transgene insertion are compared to C048 (non-transgenic at the Tex11 locus). Scale bar = 10 µm. Expected values used to conclude aberrant escape (p<0.001) consider the proportion of cells that inactivate the transgenic X due to XCI skewing. 100 cells were scored and the percentage with each expression pattern is indicated. (C) XCI status of clonal C138 cell lines that carry the transgene on the active or inactive X. Inactive X expression is indicated as a proportion of active X expression. Kdm5c-tg escape was estimated as described (see methods). No inactive X expression was detected for most genes tested, indicating that levels are at or below assay sensitivity of ∼1% of active X expression. All reactions were performed at least twice, with error bars indicating the standard deviation of technical triplicates. To examine effects at the C138 distal boundary we queried transcripts included in BAC RP23-263O9 because low Tex11 expression was undetectable on either X by RNA FISH (Figure S6). Monoallelic expression from only the active X in C048 confirms that RP23-263O9 transcripts are normally X inactivated (Figure 4B). However, a heterogeneous pattern was seen in C138, with inactive X expression in 22% of cells. This proportion closely approximates the percentage of cells that inactivate the transgenic CAST X (Figure 4B), and argues that distal genes on the transgenic X escape XCI at a high frequency. Aberrant XCI regulation does not extend further, as adjacent transcripts detected by BAC RP23-295G17 are properly X inactivated (Figure 4B).
To confirm and extend these results, we determined the XCI status of proximal and distal transcripts in differentiated clonal lines that carry the C138 transgene only on the active X or only on the inactive X chromosome. First, allele-specific expression of cDNA from the C138-derived clonal lines confirmed that the proximal gene Dlg3 is X inactivated (Figure 4C). Next, Tex11 at the integration site was tested. While Tex11 is X inactivated in the clonal line that carries the transgene on the active X (Figure 4C), the gene now escapes XCI when interrupted by Kdm5c-tg. To determine the extent of XCI misregulation, we queried additional genes downstream of Tex11. Two additional transcripts, Slc7a3 and Snx12, aberrantly escape XCI on the transgenic X (Figure 4C). By qSNaPshot, the level of inactive X escape relative to active X expression is quite similar for all three genes. However, it is unlikely that absolute inactive X expression is equivalent given that RNA FISH suggests significantly higher Snx12 transcription on both Xs (Figure 4B, Figure S6). Altogether these results argue that absence of the distal XCI boundary results in 350 kb expansion of an escape domain.
Discussion
Recent genome-wide studies have made tremendous strides in uncovering long-range organization and predicting functional domains [33]. Direct annotation of the inactive X is more challenging, in part because it is masked by its active X counterpart. Despite recent efforts to catalogue allele-specific epigenetic features (e.g. [10], [11], [34]), current understanding of the pivotal sequences and modifications that regulate how a gene responds to XCI remains incomplete. While inactive X profiling has identified intriguing candidates, functional dissection can reveal unexpected regulatory modes, such as uncovered here at Kdm5c.
These studies have expanded our understanding of the Kdm5c locus. Because our BAC transgenes carry large inserts encompassing X-chromosome genes that normally are influenced by XCI, effects are expected to recapitulate endogenous regulation and identify candidate sequences that are highly likely to be relevant. Our previous full-length BAC transgene studies allowed us to conclude that an element(s) within the BAC is sufficient to initiate Kdm5c-tg escape [19]. Such a regulatory element could also explain XCI escape of a human autosomal transgene [35]. For the Kdm5c locus, this activity was mapped to a 112 kb region defined by BAC overlap (Figure 1) [19]. Here we examine additional transgenes that further narrow this interval, as Kdm5c-tg still escapes XCI from BAC transgenes lacking distal boundary sequences (Figure 2). Because the truncated BACs integrated into X-inactivated regions, we conclude that the remaining transgene sequences must include a dominant element(s) sufficient to initiate Kdm5c escape and to structurally remodel the X in a manner that allows preferential association with escape genes (Figure 3). Further, our studies of the C138 transgene reveal an additional role for distal XCI boundary sequences, since in contrast to the full-length BACs [19] XCI regulation of adjacent X-inactivated genes was disrupted (Figure 4).
What sequences are necessary for XCI escape and do these elements also facilitate long-range escapee interactions? Sequences orchestrating these activities must map within the C138 transgene and likely reside within the proximal XCI boundary (Figure 5A). Therefore, the complete escape domain, including the escapee lncRNA, cannot be necessary for directing inactive X expression. Retained BAC sequences include the Kdm5c promoter and CTCF-binding sites that are proposed to delimit this proximal XCI boundary [24] (Figure 5A). Nevertheless, CTCF binding alone is not sufficient to confer XCI escape [36]. Further, whether specific promoter elements alone can drive escape is untested, but large-scale transgenesis likely excludes promoter strength as a sole property [35]. Sequences within C138 also enable long-distance association with other escape genes. Yet, the region may be further narrowed as the short B176 transgene, lacking Kdm5c-tg and its promoter, fails to preferentially interact.
Fig. 5. Two genetically separable regulatory activities at the Kdm5c locus. 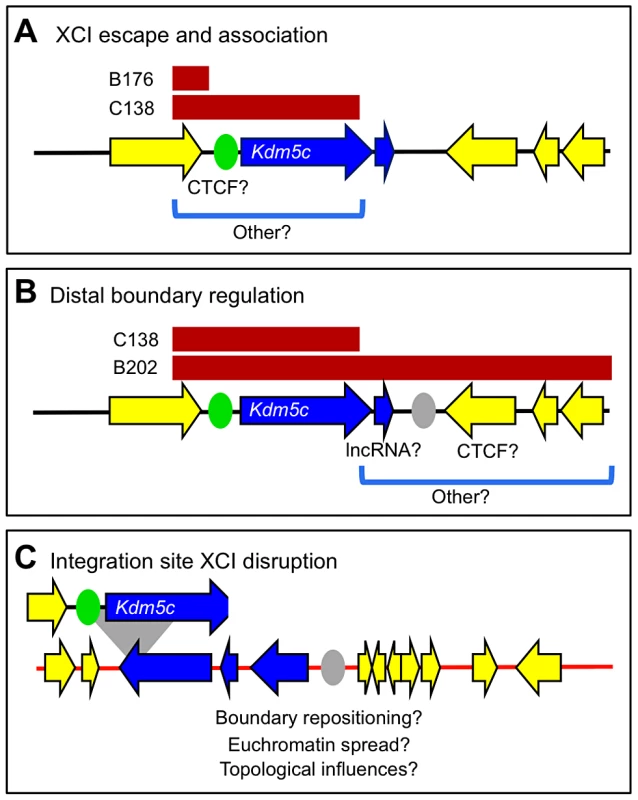
(A,B) Endogenous Kdm5c locus with transcripts ordered and XCI states annotated as in Figure 1 (not to scale). (A) An escape element that maps within the C138 BAC sequences (bracketed) is sufficient to direct Kdm5c-tg escape and long-distance escapee interactions. A previously described CTCF site is proposed to demarcate proximal boundary (green oval) [24]. (B) Regulation at the distal XCI boundary. While sequences sufficient to establish a distal XCI boundary (gray oval), are present in full length B202 BAC, they are absent in the short C138 transgene. (C) C138 transgene integration into the Tex11 locus (not to scale) results in aberrant inactive X expression of three distal genes (blue). Deletion of distal transgene sequences in C138 reveals additional regulation at Kdm5c. In the absence of an XCI boundary, three normally X-inactivated genes near the BAC integration site now escape XCI (Figure 4). We asked whether aberrant distal expression is due to permissive chromatin propagated by read-though transcription from the truncated Kdm5c-tg. This possibility seems unlikely, as transcription does not extend across the entire escape domain (Figure S6). Further any read-through is at most minimal, as no transcription across the Tex11 locus is seen by RNA FISH, even when the transgene is on the active X. Nevertheless, strand-specific RT-PCR within Tex11 detects low-level sense and antisense transcripts from both non-transgenic and transgenic undifferentiated ES cells (Figure S6). That these transcripts are not unique to the Kdm5c-tg locus argues that low levels of transcription alone cannot enable escape. Therefore, while the extent that XCI is disrupted is likely dependent on integration site characteristics, the C138 transgene must lack a regulatory element that normally has an essential role in establishing an XCI boundary at the endogenous Kdm5c locus (Figure 5B). How this element functions is not clear, but could actively prevent heterochromatin encroachment into active domains or instead block escapee regulators from influencing adjacent silenced genes. Consistent with the former, a chromatin barrier could act as a boundary if upon deletion other distal elements reposition the XCI boundary (Figure 5C). CTCF could perform such a function, as sites are found near the distal Kdm5c boundary and are normally present at locations that could delimit the expanded escape domain (Figure S7). Moreover, CTCF frequently binds at chromatin boundaries throughout the genome [37], and can organize and reorganize chromatin loops [38]–[40]. This would suggest plasticity at XCI boundaries and could explain tissue differences in some escape genes [11], [17], [41].
Sequences at the distal XCI boundary could instead actively block adjacent genes from escape in a manner that is directional and in cis (Figure 5C). Deletion of such a boundary could appear as euchromatin spreading, although, to our knowledge, similar effects have not been described elsewhere in the genome. Yet, elements at other loci could explain this observation. CTCF functioning as an enhancer-blocking element fits this model [42]–[44], particularly since deletion at other epigenetically regulated loci can induce gene reactivation [45]. Alternatively, transcripts near escape genes may require additional elements to be properly X inactivated [46]. In this role, the lncRNA could silence by transcriptional interference [47], although effects extending such distances are not reported. Further, lncRNAs can recruit chromatin-modifying enzymes in cis (e.g. [47], [48]). Supporting recruitment, it is intriguing the AK148627 lncRNA is amongst transcripts immunoprecipitated by the PRC2 polycomb-complex component EZH2 [49].
Finally, we considered the role that inactive X topological structure plays in determining XCI states. Distant escapee contacts are maintained for Kdm5c-tg at all three ectopic locations tested. Therefore, long-distance interaction is another inherent property of an escape locus, yet its mechanistic relationship to active transcription remains undefined. Transgenic loci are likely repositioned at the exterior of the Xist compartment, similar to endogenous Kdm5c [18]. Such rearrangement would also impact genes adjacent to the transgenes. While positioning on the inactive X could influence distal gene escape in C138, it cannot be sufficient since proximal genes remain X inactivated. Additional factors must be necessary to direct XCI fates.
Epigenomic features may refine the XCI boundary and localize key regulatory sequences. Using available data sets, H3K27me3 profiles in non-transgenic female lines mirror inactive X expression, with depletion clearly characterizing the expressed Kdm5c locus (Figure S7A). Intriguingly, while the proximal H3K27me3 transition is quite distinct, the distal boundary appears more diffuse (Figure S7A). Both H3K27me3 patterns occur at domain boundaries throughout the genome [50] and the distal profile may be indicative of an expression transition [9]. That this moderate H3K27me3 region contains critical regulatory sequences is supported by our current studies, since the shortest transgene breakpoint directly abuts this region. Nevertheless, the nature of the boundary makes regulatory element localization more difficult. If boundary repositioning expands the escape domain, it is intriguing that the novel boundary appears demarcated by H3K27me3 even on non-transgenic chromosomes (Figure S7B). However, further conclusions will require chromatin profiling on transgenic chromosomes. We next turned to DNaseI hypersensitivity that demarcates many regulatory elements [51]. At both the endogenous Kdm5c locus and C138 transgene integration site available data only identify hypersensitive sites at gene promoters and CTCF-binding sites (Figure S7). Perhaps this strengthens CTCF as a candidate. A caveat is that such a function may be developmentally regulated and no female lines have been profiled upon the onset of XCI.
Altogether, work here has defined two separable functions at the Kdm5c locus. We narrowed sequences required for directing escape and for the first time have assigned a function to an XCI boundary in actively delimiting expression domains. By defining and demarcating regions responsible for each activity, future experiments can be directed to examine specific candidate elements.
Methods
Transgenic cell lines
The parental ES line SA13 was derived from a (129×CAST)F1 female [19]. ES cell lines carrying X-linked BAC transgene RP23-391D18 were described previously [19]. All cells were cultured using established conditions and were maintained in the absence of drug selection [19]. For post-XCI experiments, cells were differentiated for ten days following LIF removal.
Clonal C138 lines were isolated by first differentiating ES lines for 10 days. Cells were replated using conditions that further enrich for differentiated cells [19] and after two days were infected with SV40-VA4554 [52]. Cells were passaged as required and after >20 days plated at very low cell density and allowed to clonally expand. Monoallelic expression of SNPs within Hprt and/or Pctk1 [32] confirmed clonality.
Mapping transgene breakpoints
Due to the location of the selectable marker within the RP23-391D18 BAC vector [19], truncated transgenic lines surviving initial drug selection lack genomic sequences at the distal XCI boundary. Informative SNPs to delimit these transgene breakpoints were identified (http://cgd.jax.org/cgdsnpdb) and are listed in Table S1. Allelic ratios were evaluated using a quantitative primer extension assay, qSNaPshot [13], [19]. Samples were run on an ABI 3130XL sequencer and peak heights measured using GeneMapper 4.0 software with SNaPshot default settings. Allele ratios in transgenic lines were normalized by comparison with the non-transgenic ES line. Results were further adjusted as allele ratios for a non-transgenic SNP rs29296320 deviated slightly from an expected ratio of 1.0 (ranging from 0.84 to 1.07), likely reflecting loss of an X in a small proportion of cells.
Identification of transgene integration sites
Precise transgene integration sites were determined by inverse PCR [53]. For C048 and C138, genomic DNA was digested with XbaI or PstI respectively. Purified DNA was self-ligated in dilute conditions and used as template for PCR with BAC-derived primers. PCR products were cloned and sequenced. Similar efforts for B176 failed to isolate integration sequences, consistent with a more complex vector rearrangement upon insertion. To determine if C138 and C048 transgene integrations resulted in large-scale deletions, genomic SNPs distal to the integration site were analyzed by qSNAPshot (Table S1 for primers and SNPs).
To identify the strain origin of the transgenic Xs in C138 and C048, SNP alleles were assayed from transgenic X specific PCR products that were generated by anchoring one primer to the BAC backbone. For C048, the closest informative SNP was >6 kb away and required initial amplification from a self-ligated template, similar to inverse PCR (Table S1 for SNP and primer information). Strain origin of the transgenic X in additional lines was inferred by determining the frequency that the BAC is on the inactive X since XCI skewing results in inactivation of the CAST X in 25% of cells [19].
Inactive X expression analysis
The normal XCI status of transcripts at the transgene integration site was assayed using qSNaPshot to measure allelic expression in the non-randomly X-inactivated mouse fibroblast lines B120 or B119 [13], [14]. Mid1 was tested previously in a similar manner [19]. Mid1 has a unique gene organization and XCI pattern; it straddles the pseudoautosomal (PAR) boundary in some strains, but is X-specific in others [54], [55] (Figure S2A). Mid1 escapes XCI in domestic mouse strains [10], [54], but is X inactivated on the CAST X [19].
Allelic expression was similarly assayed in the C138 clonal lines. For genes flanking the transgene, inactive X expression was measured relative to the active X allele and normalized to DNA. Kdm5c, with three expressed loci in C138, required the expression ratio to be normalized to non-transgenic DNA (to account for dye incorporation differences) and additionally to DNA from the clonal line (to account for loss of an X in a small subset of cells). However, both Kdm5c-tg and the endogenous locus on the active X are derived from domestic strains and are not distinguishable. Therefore, levels of Kdm5c-tg escape were estimated from the normalized allele ratios as if equivalent to the endogenous CAST inactive X allele. This estimate appears justified since both inactive X alleles (CAST and Kdm5c-tg) are predicted to partially escape at levels similar to those previously reported [18], [19], [31], [32]. Further, given the measured allele ratios, estimates of lower Kdm5c-tg escape require concomitant reduction in the endogenous CAST allele to levels below that been previously seen.
Fluorescent in situ hybridization
FISH probes included Xist (7.2 kb of exon 1) [19], Kdm5c (19 kb spanning exons 5–12 [19]), DXWas70, an X-specific repeat [56], and BACs RP23-391D18 (includes Kdm5c), RP23-330G24 (Kdm5c), RP23-67G4, RP23-459H14 (Tex11), RP23-263O9, RP24-255O24, RP23-295G17, RP23-459P19 (Ddx3x), and RP23-378I14 (Mecp2). Probes were directly labeled with Alexa Fluors 488, 594, or 647 by nick translation using either ARES DNA labeling kits (Invitrogen) or ChromaTide Alexa Fluor dUTPs (Invitrogen) as indicated by the manufacturer.
Slides were prepared and FISH performed for each specific experiment as follows. For DNA FISH studies, metaphase spreads were prepared and FISH performed as previously described [19], [57]. For all other studies, embryoid bodies were plated on slides at day 3 of differentiation and cultured to day 10. RNA FISH was performed on non-denatured slides as described [18], [58]. For sequential RNA and DNA FISH, slides were initially processed as for RNA FISH. Subsequently, signals were fixed in 4% paraformaldehyde in PBS prior to denaturing (75°C for 5 minutes) and processing for DNA FISH [19]. For association studies, cells were fixed in 4% paraformaldehyde before permeablization to preserve nuclear morphology [17]. Slides were denatured at 85°C for 4′ or 75°C for 7′, which allowed sufficient retention of Xist RNA to identify the inactive X chromosome.
Slides were imaged on Nikon TE2000-U microscope with Roper Scientific CCD camera and NIS elements software equipped with a 60× objective. Alternatively, a DeltaVision Elite microscope was used that is equipped with 60× or 100× objective and CoolSnap HQ2 Photometrics camera. Deltavision images were acquired across 0.2 µm Z stacks, deconvolved, and analyzed using softWoRx software version 5.5.5. In all cases, wavelengths were captured separately and merged and pseudocolored in Adobe Photoshop. Image manipulation was restricted to overall fluorescent level adjustment applied uniformly across the image.
RNA FISH analysis
To ensure optimal hybridization, we adopted specific scoring criteria for each experiment. For all FISH expression studies, we required hybridization patterns for scored cells to at least reflect known endogenous XCI expression. That is, for a gene that normally escapes XCI (Kdm5c), all cells included had at least one active X and one inactive X signal; for normally X-inactivated genes, only cells with at least one robust active X signal were scored. Additional RNA signals then reflect transgene expression (Kdm5c escape) or aberrant escape (for normally X-inactivated genes). Multiple planes were examined to ensure that out-of-focus signals were not excluded. Assignment of Kdm5c signals was facilitated by colocalization with BAC DNA FISH signals to pinpoint the Kdm5c locus or the integration site locus. Unless noted, each experiment scored at least 100 nuclei that met criteria, with each scored cell selected from an independent field of vision. Statistical significance was evaluated by Chi square analysis.
Structural association analysis
To evaluate probe association slides were viewed on a DeltaVision Elite microscope (100× objective). X,Y,Z coordinates were recorded for each signal and the 3D distance between probes calculated [17]. Nuclear area was calculated by averaging polygon areas (demarcating the nucleus) across all in focus Z sections and was used to normalize for differences in nuclear size and morphology. Analysis was limited to ∼95% of cells with nuclear area <175 µm2 to ensure overlapping distributions across all cell lines. For each probe set 100–150 nuclei were scored per cell line. Significance was assessed using a Kolmogorov-Smirnov two-sample statistic [59].
Supporting Information
Zdroje
1. DixonJR, SelvarajS, YueF, KimA, LiY, et al. (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485 : 376–380.
2. RybaT, HirataniI, LuJ, ItohM, KulikM, et al. (2010) Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res 20 : 761–770.
3. Lieberman-AidenE, van BerkumNL, WilliamsL, ImakaevM, RagoczyT, et al. (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326 : 289–293.
4. GuelenL, PagieL, BrassetE, MeulemanW, FazaMB, et al. (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453 : 948–951.
5. WenB, WuH, ShinkaiY, IrizarryRA, FeinbergAP (2009) Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat Genet 41 : 246–250.
6. PaulerFM, SloaneMA, HuangR, ReghaK, KoernerMV, et al. (2009) H3K27me3 forms BLOCs over silent genes and intergenic regions and specifies a histone banding pattern on a mouse autosomal chromosome. Genome Res 19 : 221–233.
7. NoraEP, LajoieBR, SchulzEG, GiorgettiL, OkamotoI, et al. (2012) Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485 : 381–385.
8. WutzA (2011) Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat Rev Genet 12 : 542–553.
9. BerletchJB, YangF, XuJ, CarrelL, DistecheCM (2011) Genes that escape from X inactivation. Hum Genet 130 : 237–245.
10. PinterSF, SadreyevRI, YildirimE, JeonY, OhsumiTK, et al. (2012) Spreading of X chromosome inactivation via a hierarchy of defined Polycomb stations. Genome Res 22 : 1864–76.
11. CalabreseJM, SunW, SongL, MugfordJW, WilliamsL, et al. (2012) Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell 151 : 951–963.
12. EngreitzJM, Pandya-JonesA, McDonelP, ShishkinA, SirokmanK, et al. (2013) The Xist lncRNA Exploits Three-Dimensional Genome Architecture to Spread Across the X Chromosome. Science 341 : 1237973.
13. CarrelL, WillardHF (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434 : 400–404.
14. LopesAM, Arnold-CroopSE, AmorimA, CarrelL (2011) Clustered transcripts that escape X inactivation at mouse XqD. Mamm Genome 22 : 572–582.
15. CarrelL, ParkC, TyekuchevaS, DunnJ, ChiaromonteF, et al. (2006) Genomic environment predicts expression patterns on the human inactive X chromosome. PLoS Genet 2: e151.
16. TsuchiyaKD, GreallyJM, YiY, NoelKP, TruongJP, et al. (2004) Comparative sequence and X-inactivation analyses of a domain of escape in human Xp11.2 and the conserved segment in mouse. Genome Res 14 : 1275–1284.
17. SplinterE, de WitE, NoraEP, KlousP, van de WerkenHJ, et al. (2011) The inactive X chromosome adopts a unique three-dimensional conformation that is dependent on Xist RNA. Genes Dev 25 : 1371–1383.
18. ChaumeilJ, Le BacconP, WutzA, HeardE (2006) A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev 20 : 2223–2237.
19. LiN, CarrelL (2008) Escape from X chromosome inactivation is an intrinsic property of the Jarid1c locus. Proc Natl Acad Sci U S A 105 : 17055–17060.
20. AgulnikAI, MitchellMJ, MatteiMG, BorsaniG, AvnerPA, et al. (1994) A novel X gene with a widely transcribed Y-linked homologue escapes X-inactivation in mouse and human. Hum Mol Genet 3 : 879–884.
21. IwaseS, LanF, BaylissP, de la Torre-UbietaL, HuarteM, et al. (2007) The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell 128 : 1077–1088.
22. ReiniusB, ShiC, HengshuoL, SandhuKS, RadomskaKJ, et al. (2010) Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse. BMC Genomics 11 : 614.
23. TsuchiyaKD, WillardHF (2000) Chromosomal domains and escape from X inactivation: comparative X inactivation analysis in mouse and human. Mamm Genome 11 : 849–854.
24. FilippovaGN, ChengMK, MooreJM, TruongJP, HuYJ, et al. (2005) Boundaries between chromosomal domains of X inactivation and escape bind CTCF and lack CpG methylation during early development. Dev Cell 8 : 31–42.
25. PhillipsJE, CorcesVG (2009) CTCF: master weaver of the genome. Cell 137 : 1194–1211.
26. LeeJT (2012) Epigenetic regulation by long noncoding RNAs. Science 338 : 1435–1439.
27. YangF, GellK, van der HeijdenGW, EckardtS, LeuNA, et al. (2008) Meiotic failure in male mice lacking an X-linked factor. Genes Dev 22 : 682–691.
28. AdelmanCA, PetriniJH (2008) ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over. PLoS Genet 4: e1000042.
29. WangPJ, McCarreyJR, YangF, PageDC (2001) An abundance of X-linked genes expressed in spermatogonia. Nat Genet 27 : 422–426.
30. LeeJT, LuN (1999) Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99 : 47–57.
31. SheardownS, NorrisD, FisherA, BrockdorffN (1996) The mouse Smcx gene exhibits developmental and tissue specific variation in degree of escape from X inactivation. Hum Mol Genet 5 : 1355–1360.
32. CarrelL, HuntPA, WillardHF (1996) Tissue and lineage-specific variation in inactive X chromosome expression of the murine Smcx gene. Hum Mol Genet 5 : 1361–1366.
33. DunhamI, KundajeA, AldredSF, CollinsPJ, DavisCA, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74.
34. SadreyevRI, YildirimE, PinterSF, LeeJT (2013) Bimodal quantitative relationships between histone modifications for X-linked and autosomal loci. Proc Natl Acad Sci U S A 110 : 6949–6954.
35. YangC, McLeodAJ, CottonAM, de LeeuwCN, LapriseS, et al. (2012) Targeting of over 1.5 Mb of human DNA into the mouse X chromosome reveals presence of cis-acting regulators of epigenetic silencing. Genetics 192 : 1281–1293.
36. CiavattaD, KalantryS, MagnusonT, SmithiesO (2006) A DNA insulator prevents repression of a targeted X-linked transgene but not its random or imprinted X inactivation. Proc Natl Acad Sci U S A 103 : 9958–9963.
37. CuddapahS, JothiR, SchonesDE, RohTY, CuiK, et al. (2009) Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains. Genome Res 19 : 24–32.
38. YusufzaiTM, TagamiH, NakataniY, FelsenfeldG (2004) CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol Cell 13 : 291–298.
39. HouC, ZhaoH, TanimotoK, DeanA (2008) CTCF-dependent enhancer-blocking by alternative chromatin loop formation. Proc Natl Acad Sci U S A 105 : 20398–20403.
40. MurrellA, HeesonS, ReikW (2004) Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat Genet 36 : 889–893.
41. ReiniusB, JohanssonMM, RadomskaKJ, MorrowEH, PandeyGK, et al. (2012) Abundance of female-biased and paucity of male-biased somatically expressed genes on the mouse X-chromosome. BMC Genomics 13 : 607.
42. BellAC, WestAG, FelsenfeldG (1999) The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98 : 387–396.
43. SaitohN, BellAC, Recillas-TargaF, WestAG, SimpsonM, et al. (2000) Structural and functional conservation at the boundaries of the chicken beta-globin domain. EMBO J 19 : 2315–2322.
44. HarkAT, SchoenherrCJ, KatzDJ, IngramRS, LevorseJM, et al. (2000) CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405 : 486–489.
45. BrentonJD, DrewellRA, VivilleS, HiltonKJ, BartonSC, et al. (1999) A silencer element identified in Drosophila is required for imprinting of H19 reporter transgenes in mice. Proc Natl Acad Sci U S A 96 : 9242–9247.
46. ChowJC, CiaudoC, FazzariMJ, MiseN, ServantN, et al. (2010) LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141 : 956–969.
47. NaganoT, MitchellJA, SanzLA, PaulerFM, Ferguson-SmithAC, et al. (2008) The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322 : 1717–1720.
48. PandeyRR, MondalT, MohammadF, EnrothS, RedrupL, et al. (2008) Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 32 : 232–246.
49. ZhaoJ, OhsumiTK, KungJT, OgawaY, GrauDJ, et al. (2010) Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell 40 : 939–953.
50. BarkessG, WestAG (2012) Chromatin insulator elements: establishing barriers to set heterochromatin boundaries. Epigenomics 4 : 67–80.
51. SongL, ZhangZ, GrasfederLL, BoyleAP, GiresiPG, et al. (2011) Open chromatin defined by DNaseI and FAIRE identifies regulatory elements that shape cell-type identity. Genome Res 21 : 1757–1767.
52. TevethiaMJ, OzerHL (2001) SV40-mediated immortalization. Methods Mol Biol 165 : 185–199.
53. LiZH, LiuDP, LiangCC (1999) Modified inverse PCR method for cloning the flanking sequences from human cell pools. Biotechniques 27 : 660–662.
54. Dal ZottoL, QuaderiNA, ElliottR, LingerfelterPA, CarrelL, et al. (1998) The mouse Mid1 gene: implications for the pathogenesis of Opitz syndrome and the evolution of the mammalian pseudoautosomal region. Hum Mol Genet 7 : 489–499.
55. PerryJ, PalmerS, GabrielA, AshworthA (2001) A short pseudoautosomal region in laboratory mice. Genome Res 11 : 1826–1832.
56. DistecheCM, GandySL, AdlerDA (1987) Translocation and amplification of an X-chromosome DNA repeat in inbred strains of mice. Nucleic Acids Res 15 : 4393–4401.
57. MillerAP, GustashawK, WolffDJ, RiderSH, MonacoAP, et al. (1995) Three genes that escape X chromosome inactivation are clustered within a 6 Mb YAC contig and STS map in Xp11.21-p11.22. Hum Mol Genet 4 : 731–739.
58. ChaumeilJ, AuguiS, ChowJC, HeardE (2008) Combined immunofluorescence, RNA fluorescent in situ hybridization, and DNA fluorescent in situ hybridization to study chromatin changes, transcriptional activity, nuclear organization, and X-chromosome inactivation. Methods Mol Biol 463 : 297–308.
59. XuN, TsaiCL, LeeJT (2006) Transient homologous chromosome pairing marks the onset of X inactivation. Science 311 : 1149–1152.
60. YangF, BabakT, ShendureJ, DistecheCM (2010) Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res 20 : 614–622.
Štítky
Genetika Reprodukčná medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 11- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



