-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Genomic Evidence of Rapid and Stable Adaptive Oscillations over Seasonal Time Scales in Drosophila
Herein, we investigate the genomic basis of rapid adaptive evolution in response to seasonal fluctuations in the environment. We identify hundreds of polymorphisms (seasonal SNPs) that undergo dramatic shifts in allele frequency – on average between 40 and 60% – and oscillate between seasons repeatedly over multiple years, likely inducing high levels of genome-wide genetic differentiation. We provide evidence that seasonal SNPs are functional, being both sensitive to an acute frost event and associated with two stress tolerance traits. Finally, we show that some seasonal SNPs are possibly ancient balanced polymorphisms. Taken together, our results suggest that environmental heterogeneity can promote the long-term persistence of functional polymorphisms within populations that fuels fast directional adaptive response at any one time.
Published in the journal: Genomic Evidence of Rapid and Stable Adaptive Oscillations over Seasonal Time Scales in Drosophila. PLoS Genet 10(11): e32767. doi:10.1371/journal.pgen.1004775
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004775Summary
Herein, we investigate the genomic basis of rapid adaptive evolution in response to seasonal fluctuations in the environment. We identify hundreds of polymorphisms (seasonal SNPs) that undergo dramatic shifts in allele frequency – on average between 40 and 60% – and oscillate between seasons repeatedly over multiple years, likely inducing high levels of genome-wide genetic differentiation. We provide evidence that seasonal SNPs are functional, being both sensitive to an acute frost event and associated with two stress tolerance traits. Finally, we show that some seasonal SNPs are possibly ancient balanced polymorphisms. Taken together, our results suggest that environmental heterogeneity can promote the long-term persistence of functional polymorphisms within populations that fuels fast directional adaptive response at any one time.
Introduction
All organisms live in environments that vary through time and such environmental heterogeneity can impose highly variable selection pressures on populations. In this situation, an allele may be beneficial during one environmental regime and subsequently deleterious during another. Such an allele would be subject to short bursts of directional selection, alternately being favored and disfavored. When this situation occurs in diploids, the heterozygote can have a higher geometric mean fitness than either homozygote and allelic variation at this locus could be maintained for long periods despite being subject to directional selection at any given time [1]–[8]. This situation is referred to as marginal overdominance and is a form of balancing selection.
There is substantial evidence for the maintenance of phenotypic and genetic variation by temporally variable selection in a variety of organisms. For instance, evolutionary response to rapid changes in selection pressures has been demonstrated for morphological and life-history traits in mammals [9], [10], birds [11]–[13], plants [14], invertebrates [15]–[24], and others (reviewed in [25], [26]). Chromosomal inversions and allozyme alleles in a variety of drosophilids vary among seasons [27]–[33] suggesting that these polymorphisms confer differential fitness in alternating seasons. Further, in some species of drosophilids, life-history [34], [35], morphological [36], [37] and stress tolerance traits [38], [39] also fluctuate seasonally suggesting that these traits respond to seasonal shifts in selection pressures.
Although theoretical models suggest that temporal variation in selection pressures can maintain fitness-related genetic variation in populations [1]–[8] and empirical evidence from a variety of species [9]–[39] demonstrates that variation in selection pressures over short time periods does alter phenotypes and allele frequencies, we still lack a basic understanding of many fundamental questions about the genetics and evolutionary history of alleles that undergo rapid adaptation in response to temporal variation in selection pressures. Specifically, we do not know how many loci respond to temporally variable selection within a population, the strength of selection at each locus, nor the effects of such strong selection on neutral genetic differentiation through time. We do not know whether adaptation at loci that respond to temporally variable selection is predictable nor do we know the relationship between loci that respond to temporally variable selection and spatially varying selection. Finally, it is unclear whether rapid adaptation to temporally variable selection pressures is primarily fueled by young alleles that constantly enter the population but cannot be maintained for long periods of time or, rather, by old alleles that have possibly been maintained by variable selection associated with environmental heterogeneity despite short bursts of strong directional selection.
To address these questions, we estimated allele frequencies genome-wide from samples of D. melanogaster individuals collected along a broad latitudinal cline in North America and in the spring and fall over three consecutive years in a single temperate orchard. We demonstrate that samples of flies collected in a single Pennsylvania orchard over the course of several years are as differentiated as populations separated by 5–10° latitude. We identify hundreds of polymorphisms that are subject to strong, temporally varying selection and argue that genetic draft [40] in the wake of rapid, multilocus adaptation is sufficient to explain the high degree of genetic turnover that we observe in this population over several years. We examine the genome-wide relationship between spatial and temporal variation in allele frequencies and find that spatial genetic differentiation, but not clinality per se, in allele frequency is a good predictor of temporal variation in allele frequency. Moreover, at SNPs subject to seasonal fluctuations in selection pressures, northern populations are more similar to spring populations than southern ones are. Next, we show that allele frequencies at SNPs subject to seasonal fluctuations in selection pressures become more ‘spring-like’ (i.e., they move towards the average spring frequency) immediately following a hard frost event and that seasonally variably SNPs tend to be associated with two seasonally variable phenotypes, chill coma recovery time and starvation tolerance. Finally, we demonstrate that some of the loci that respond to temporal variation in selection pressures are likely ancient, balanced polymorphisms that predate the split of D. melanogaster from its sister species, D. simulans. Taken together, our results are consistent with a model in which temporally variable selection maintains fitness-related genetic variation at hundreds of loci throughout the genome for millions of generations if not millions of years.
Results/Discussion
Genomic differentiation through time and space
To test for the genomic signatures of balancing selection caused by seasonal fluctuations in selection pressures, we performed whole genome, pooled resequencing of samples of male flies collected in the spring and fall over three consecutive years (2009–2011) in a temperate, Pennsylvanian orchard. We contrast changes in allele frequencies through time with estimates of allele frequencies we made from five additional populations spanning Florida to Maine along the east coast of North America over a number of years (2003–2010) largely during periods of peak abundance of D. melanogaster (Fig. 1A, Table S1). From each population and time point, we sampled approximately 50–100 flies and resequenced each sample to average read depth of 20–200× coverage (Table S1, and see Text S1). Estimates of allele frequency using this sampling design have been shown to be highly accurate [40].
Fig. 1. Experimental design and genomic turnover through time and space. 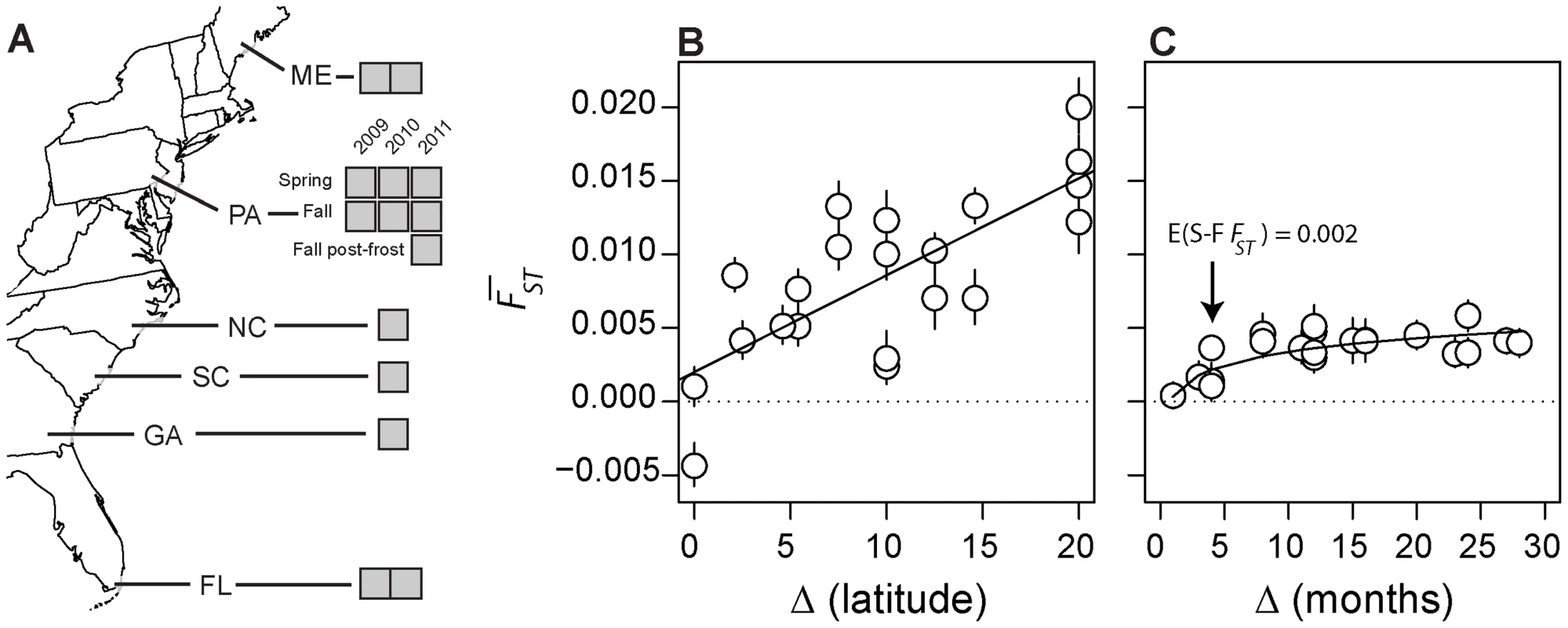
(A) Map of sampling locations in North America used in this study. Grey boxes represent individual samples from each locale. Genome-wide differentiation among spatially (B) and temporally (C) separated samples, measured as genome-wide average FST (y-axis). Lines represent the predicted value of FST based on the linear (A; y = a+bx) and non-linear (B; y = abX) regression. Note: Pennsylvanian samples are not represented in (B) and the negative FST in (B) results from the conservative correction of heterozygosity [102], [103]. In addition, please note that there are four estimates of pairwise FST between the two replicate Maine and Florida samples (corresponding to a difference in latitude of 20°) and that there are two estimates of FST between each of the remaining clinal populations and each Maine and Florida replicate sample. Error bars represent 95% confidence intervals based on 500 blocked bootstrap samples of ∼2000 SNPs. As a point of departure and to provide context for understanding the magnitude of genetic variation through the seasons, we first examined genetic differentiation along the cline (Fig. 1B, Fig. S1A). We calculated genome-wide average FST among pairs of populations (excluding Pennsylvanian populations; hereafter ‘spatial FST’) as well as the proportion of SNPs where average spatial FST between a pair of populations is greater than expected by chance conditional on our sampling design and assuming panmixia using allele frequency estimates of 500,000 common polymorphisms (Table S1). Genome-wide average spatial FST (Fig. 1B) as well as the proportion of SNPs where spatial FST is greater than expected by chance (Fig. S1A) is positively correlated with geographic distance (r = 0.75; p = 7e-5), a pattern consistent with isolation by distance [41]. Pooled resequencing did identify polymorphisms in or near genes previously shown to be clinal in North American populations (see Text S1) demonstrating that clines are stable over multiple years. This suggests that populations sampled along the cline represent resident populations, and further confirms that our pooled resequencing design gives accurate estimates of allele frequencies [42].
Next, we calculated genome-wide average FST between samples collected through time in the Pennsylvanian population (‘temporal FST’) as well as the proportion of SNPs where average temporal FST is greater than expected by chance given our sampling design and assuming no allele frequency change through time (Fig. 1C, Fig. S1B). Genome-wide average temporal FST (Fig. 1C) as well as the proportion of SNPs where the observed temporal FST is greater than expected by chance (Fig. S1B) increases with the difference in time between samples. The temporal FST increases non-linearly with duration of time between samples (slopelog-log = 0.59, plog-log slope = 1 = 0.0004, df = 19). Genome-wide average temporal FST appears to asymptote by ∼7 months, corresponding to the duration of time between fall samples and the subsequent spring sample. Remarkably, samples of the Pennsylvanian population collected one to three years apart are as differentiated as populations separated by 5–10° latitude, demonstrating high genetic turnover through time.
Identification and genomic features of seasonal SNPs
We sought to identify alleles whose frequency consistently and repeatedly oscillated between spring and fall over three years with the assumption that these polymorphisms would be the most likely to be adaptively responding to selection pressures that oscillate between the seasons. We identified seasonally variable polymorphisms that had a large and recurrent deviation from spring to fall around the average frequency using a generalized linear model (GLM) of allele frequency change as a function of season (spring or fall) that took into account read depth and the number of sampled chromosomes (see Materials and Methods for details).
Of the ∼500,000 common SNPs tested, we identified approximately 1750 sites that cycle approximately 20% in frequency between spring and fall at FDR less than 0.3 (hereafter ‘seasonal SNPs’; Fig. 2A, Fig. S2A). Statistically significant changes in allele frequency of this magnitude at seasonal SNPs correspond to selection coefficients of 5–50% per locus per generation (Fig. 2B, see Materials and Methods), assuming 10 generations per summer or 1–2 generations per winter. Given the statistical power of our experiment (Fig. 2B), we estimate there may be as many as 10 times as many sites that could cycle either directly in response to seasonally varying selection or could be linked to seasonal SNPs.
Fig. 2. Genomic features of seasonal SNPs. 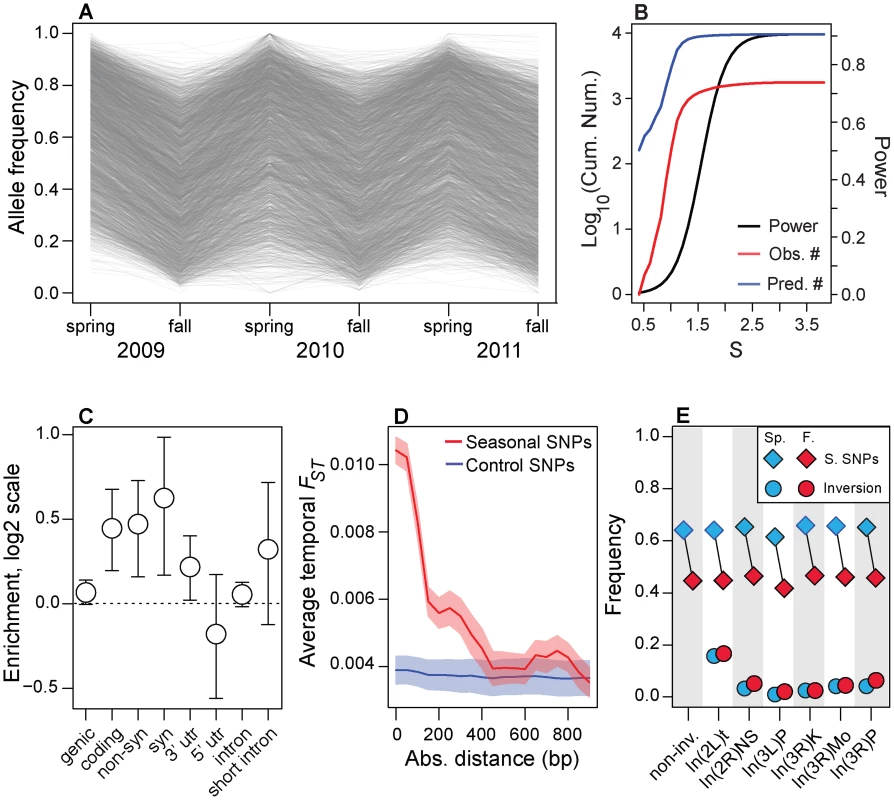
(A) Allele frequency change at each of the ∼1750 seasonal SNPs. Allele frequencies are polarized so that spring allele frequencies are higher than fall allele frequencies. (B) Power to detect seasonal SNPs (black line) is limited and we estimate that we have only identified ∼10% (red line) of all SNPs that repeatedly change in frequency through time (black line). The units of the x-axis (S) are the cumulative selection coefficient. See the Materials and Methods for the definition of S. (C) Enrichment (log2 odds ratio) of seasonal SNPs that are annotated for each class of genetic element relative to control polymorphisms. (D) Seasonal FST surrounding seasonal SNPs decays to background levels by ∼500 bp. (E) Allele frequency estimates at seasonal SNPs outside any large, cosmopolitan inversion (non-inv) or within the cosmopolitian inversions (diamonds) during the spring (blue) or fall (red). Allele frequency estimates at SNPs perfectly linked to the inversion during the spring and fall are denoted by circles. Error bars (C) and confidence bands (D) represent 95% confidence intervals based on blocked bootstrap resampling. Our rationale for focusing on the1750 seasonal SNPs at the FDR of 0.3 is that we are seeking to assess general molecular and evolutionary features of polymorphisms that may underlie rapid adaptive evolution in response to seasonal fluctuations in selection pressure. To assess these general features and enrichments, we require a sufficient number of true positive SNPs while maintaining as low a false positive rate as possible. Reducing FDR rates to lower values yielded an insufficient number of polymorphisms to assess enrichments with adequate precision (FDR of 10% yields 11 SNPs; FDR cutoff of 20% yields 200 SNPs).
We note that our estimation of ∼1750 seasonal SNPs and their associated FDR should only be taken as a rough estimate of the number of seasonally varying SNPs: variance in linkage disequilibrium through the genome, heterscedasticity due to possible demographic events, limited statistical, unbalanced sampling of flies and variance in read-depth among samples, and modeling assumptions will affect our ability to infer the exact number of seasonally varying SNPs. One way to address some of these issues (e.g., heteroscedasticity) is to model allele frequency change through time with generalized linear mixed-effect (GLMM) or general estimation equation (GEE) models that account, to varying degrees, for the structured, time-series nature of our data. Seasonal SNPs inferred with these models are highly congruent with seasonal SNPs inferred using a simple GLM (Fig. S2D,E) and q-q plots of the distribution of p-values from GLM, GLMM and GEE models suggest that GLM and GLMM modeling strategies fit the bulk of the genome well, with GEE models appearing to be anti-conservative (Fig. S2B,C). However, the identification of a statistical excess of seasonally oscillating SNPs by any modeling strategy will be subject to a number of assumptions that will almost certainly be violated in some way or another and such violations could possibly lead to an increased false-positive rate.
Because the false positive and false negative rates are inherently difficult to estimate, we adopt an empirical strategy to demonstrate that the seasonal SNPs identified though a simple GLM are not a random sample of SNPs but rather are enriched for true positive SNPs that directly underlie the adaptive response to seasonal fluctuations selection pressure. The identified seasonal SNPs are enriched for many signatures consistent with natural selection relative to control SNPs that are matched for several biologically and experimentally relevant parameters such as chromosome, recombination rate, allele frequency, and SNP quality coupled with a rigorous blocked-bootstrap procedure that accounts for the spatial distribution of seasonal SNPs along the chromosome (see Materials and Methods and Table S3). We now proceed to demonstrate these enrichments.
Seasonal SNPs are enriched among functional genetic elements. These polymorphisms are likely to be in genic (i.e., 3′ and 5′ UTR, synonymous and non-synonymous, and long-intron SNPs; p = 0.054) and coding regions (synonymous and non-synonymous; p<0.002) and are enriched among synonymous (p<0.002), non-synonymous (p = 0.002) and 3′ UTR (p = 0.024, Fig. 2C) relative to control, putatively neutral polymorphisms in short-introns [43]. The p-values of the enrichment tests were calculated after controlling for the spatial distribution of seasonal SNPs along the chromosome using a block bootstrap procedure coupled with the identification of paired control SNPs matched for several key genomic features (Table S3), such as recombination rate, average allele frequency in the Pennsylvanian orchard, chromosome, and SNP quality (see ‘Block Bootstrap’ section in Materials and Methods). Enrichment of adaptively oscillating polymorphisms among these genetic elements, including synonymous sites, suggests that these SNPs may affect organismal form and function through modification of protein function, translation rates, or mRNA expression and stability [43], [44].
Next, we show that rapid shifts in allele frequency at seasonal SNPs perturb allele frequencies at nearby SNPs. Adaptively oscillating polymorphisms are in regions of elevated temporal FST (Fig. 2D) and the elevation of temporal FST decays, on average, by ∼500 bp, consistent with patterns of linkage disequilibrium in D. melanogaster [45]. Elevation of temporal FST within 500 bp of seasonal SNPs could contribute to high levels of genome-wide average FST through time (Fig. 1C). However, excluding SNPs within 500 bp of seasonal SNPs did not change patterns of genome-wide differentiation through time suggesting that genome-wide patterns of FST through time are not driven by the seasonal SNPs themselves nor the SNPs in their immediate vicinity (Fig. S3).
Seasonal SNPs are spread throughout the genome (Fig. 3A) and there is a 95% chance of finding at least one seasonal SNP per megabase of the euchromatic genome. This result suggests that seasonal SNPs are not exclusively concentrated in any single region (such as an inversion) nor distributed among a small number of regions (such as a limited number of genes). Although seasonal SNPs are distributed throughout the genome, their distribution is over-dispersed. To assess this, we calculated the number of seasonal SNPs per 1000 SNPs under investigation in non-overlapping windows of 1000 SNPs. If seasonal SNPs are homogeneously distributed throughout the genome, the rate of seasonal SNPs/1000 SNPs should follow a Poisson distribution with mean equal to the variance. After accounting for heterogeneity in recombination rate throughout the genome (see Materials and Methods), we find that the variance in the rate of seasonal SNPs is ∼2.3 times greater than expected under a Poisson distribution (p<10−10) implying that some regions have an excess of seasonal SNPs and some have a deficit of seasonal SNPs. The overdispersion of seasonal SNPs throughout the genome could be caused by several factors including variation in the density of functional elements, multiple functional and clustered seasonal SNPs, variance in the age of seasonal SNPs, or inversion status.
Fig. 3. Spatial and temporal variation in allele frequencies. 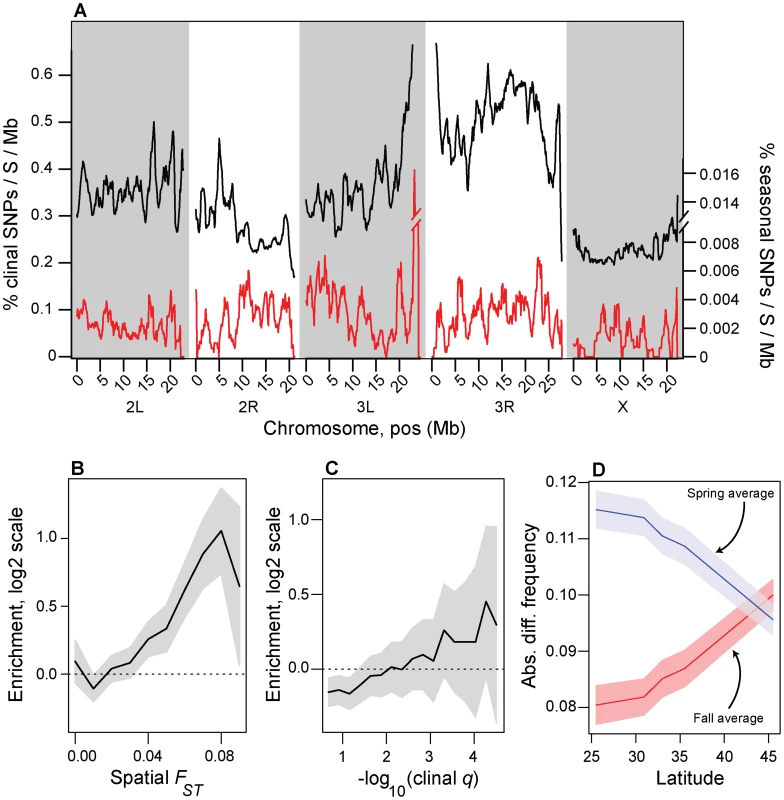
(A) Genomic distribution of clinal (black line) and seasonal SNPs (red line) per megabase per common polymorphism used in this study (Table S1). (B). Enrichment (log2 odds ratio) of seasonal SNPs with spatial FST greater than or equal to value on x-axis relative to control SNPs. (C) Enrichment (log2 odds ratio) of seasonal SNPs with –log10(spatial q-value) greater than or equal to value on x-axis relative to control SNPs. (D) Absolute difference between average spring (blue) and fall (red) frequencies in the Pennsylvanian population and frequency estimates along the cline. Confidence bands represent 95% confidence intervals based on blocked bootstrap resampling. In general, we find no evidence that seasonal SNPs are enriched among large, cosmopolitan inversions segregating in North American populations (p>0.05, Fig. S4), with only one inversion, In3R(Mo), marginally enriched for seasonal SNPs (p = 0.02, with p = 0.18 after Bonferroni correction for multiple testing). In addition, seasonal SNPs are significantly more common in the Pennsylvanian orchard population than polymorphisms perfectly linked [46] to large cosmopolitan inversions (Fig. 2E) and polymorphisms linked to inversions do not vary between seasons (Fig. 2E, p>0.05), including those linked to In3R(Mo). Therefore, enrichment of seasonal SNPs within In3R(Mo), if present, is most likely due to increased linkage disequilibrium caused by decreased recombination surrounding this inversion [47]. Taken together, these results indicate that the inversions themselves do not cycle seasonally in the Pennsylvanian population in any appreciable manner (Fig. 2E) and suggests that adaptive evolution to seasonal variation in selection pressures may be highly polygenic.
Relationship between spatial and temporal variation in allele frequencies
To test the hypothesis that spatially varying selection pressures along the latitudinal cline reflect seasonally varying selection pressures in the Pennsylvanian population, we examined the relationship between temporal and spatial variation in allele frequencies. To quantify spatial variation in allele frequency, we calculated two statistics. First, we estimated average pairwise FST among all populations for each SNP (‘spatial FST’). Second, we estimated clinality for each SNP by calculating the per-SNP false discovery rate (FDR) of the relationship between allele frequency and latitude using a generalized linear model that takes into account read depth and the number of sampled chromosomes (hereafter ‘clinal q-value’). Spatial FST and clinal q-value are highly correlated (r = 0.63, p<1e-10; Fig. S5) demonstrating that most, but not all, spatial variation along the latitudinal cline is represented by monotonic changes in allele frequency between northern and southern populations.
We calculated the number of clinally varying polymorphisms (clinal q-value<0.1) and the number of adaptively oscillating polymorphisms per common segregating SNP (average, North American MAF>0.15) per megabase of the genome (Fig. 3A). Approximately one out of every three common polymorphisms varies with latitude with FDR<0.1 (i.e., clinal q-value<0.1) whereas only one out of every three thousand polymorphisms varies predictably between seasons with seasonal FDR<0.3 (Fig. 3A). Although our ability to detect clinal SNPs at FDR<0.1 is greater than our ability to detect seasonal SNPs at FDR<0.3 (cf. Fig. 2B, Fig. S6), differences in power cannot explain the three order of magnitude difference in the number of detected clinal and seasonal SNPs (cf. Fig. 2B, Fig. S6).
Next, we formally tested whether seasonal SNPs are enriched among spatially varying SNPs. Spatially varying SNPs, as defined by spatial FST, are more likely to be seasonal SNPs than expected by chance (Fig. 3B), and the odds of this enrichment increases with increasing spatial differentiation. In contrast, we cannot reject the null hypothesis of no enrichment of seasonal SNPs among clinal SNPs as defined by clinal q-value (Fig. 3C).
The observed differences in the enrichment of seasonal SNPs among SNPs with high spatial FST and low clinal q-value may reflect aspects of our sampling design and differences in the evolutionary forces that shape allele frequencies through time and space. We sampled flies along the East Coast during different years and at different points of time relative to the progression of the growing season in each population (Table S1). Thus, in each sampled clinal population, seasonal SNPs would be at different points in their adaptive trajectory. Consequently, seasonal SNPs would not likely have exceedingly low clinal q-values, a statistic which reflects the deviation of observed allele frequencies from the predicted value as estimated by a GLM. Rather, seasonal SNPs would likely be highly differentiated along the cline (i.e., have a large spatial FST). SNPs with low clinal q-values, therefore, represent those SNPs that do not change in frequency between seasons and possibly reflect long-term demographic processes or adaptation to selection pressures that vary clinally, but not seasonally.
Because of the relationship between spatial differentiation and seasonal variation in allele frequencies (Fig. 3B) and because of parallels between spatial and seasonal variation in climate, we hypothesized that northern populations should be more ‘spring-like’ and southern populations should be more ‘fall-like’ in allele frequencies at the seasonal SNPs. To test this hypothesis, we calculated the absolute difference in allele frequencies for each population sampled along the cline with the average spring and fall allele frequency estimates for the Pennsylvanian population for all seasonal SNPs. Indeed, allele frequency estimates at seasonal SNPs from high latitude populations are more similar to spring Pennsylvanian populations and those from low latitude are more similar to fall populations (Fig. 3D) demonstrating that latitudinally varying selection pressures at least partially reflect seasonally varying selection pressures.
Immediate adaptive response to an acute frost event
In the late fall of 2011, about two weeks after our 2011 fall sample was collected, a hard frost occurred in the Pennsylvanian orchard (Fig. 4A). We were able to obtain a sample of D. melanogaster approximately one week after the frost and we estimated allele frequencies genome-wide from this sample. We hypothesized that allele frequencies at seasonal SNPs would predictably change following the frost event and would become more ‘spring-like.’ To test this hypothesis, we calculated the probability that post-frost allele frequencies at seasonal SNPs overshoot the long-term average allele frequency (i.e., become more ‘spring-like’). We also estimated this probability for control polymorphisms, matched to adaptively oscillating polymorphisms by several characteristics (Table S3) including, importantly, difference in allele frequency between the long-term average and the pre-frost allele frequency. This later control is essential given that some shift in the ‘spring-like’ direction is expected here simply by chance due to regression to the mean. The probability that seasonal SNPs overshoot the long-term average allele frequency is ∼43%, whereas only ∼35% of control polymorphisms overshoot the long-term average. This significant excess of adaptively oscillating polymorphisms that become more ‘spring-like’ following the frost event (Fig. 4B; log2(OR) = 0.48, p<0.002) suggests that these SNPs respond to acute changes in climate and that cold temperatures associated with winter is one selective force acting on this population shaping allele frequencies between seasons.
Fig. 4. Adaptive evolution to frost. 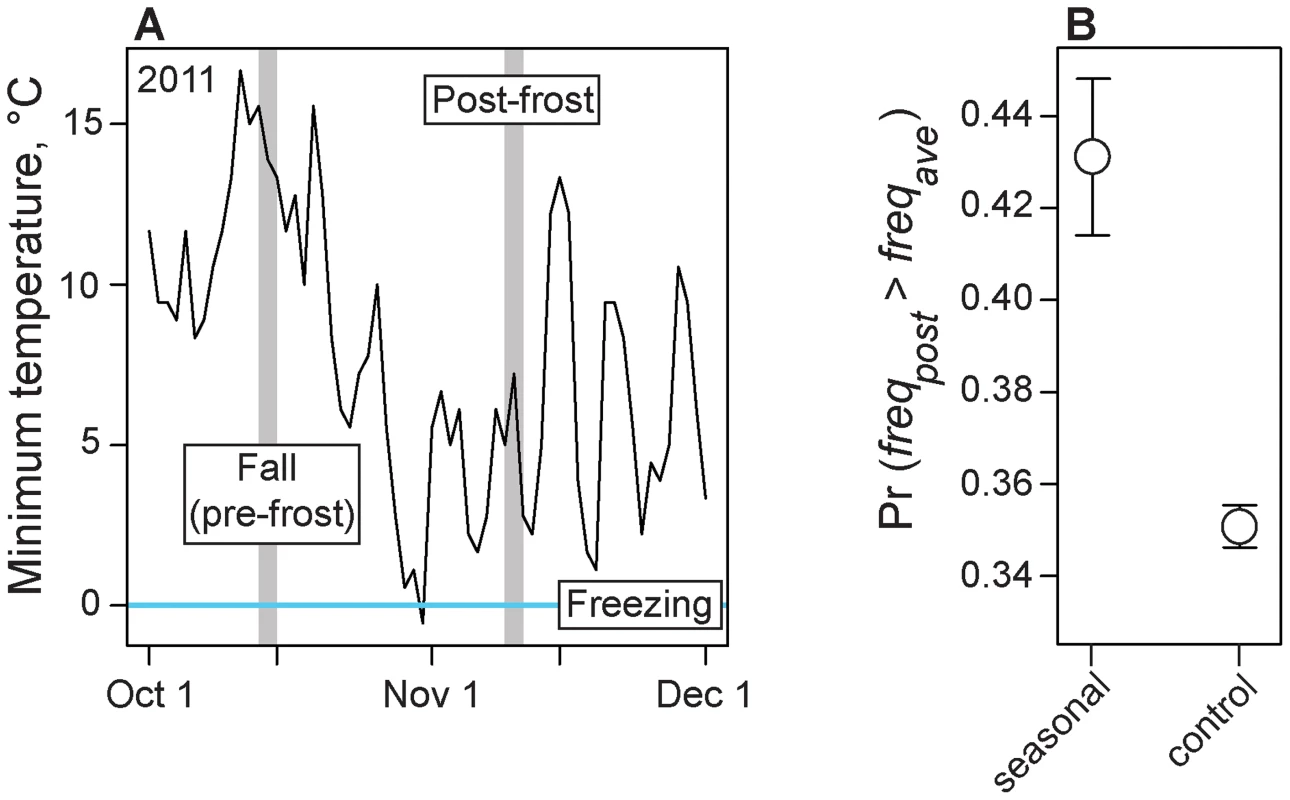
(A) Temperature records at a weather station close to the focal orchard. Grey lines indicate collection dates for pre- and post-frost samples. (B) Probability that post-frost allele frequencies at seasonal and control SNPs overshoot the long-term average (based on 2009 and 2010 estimates) allele frequency at each site. Confidence intervals based on blocked bootstrap resampling. Association with seasonally variable phenotypes
Chill-coma recovery time and starvation tolerance are two phenotypes that vary seasonally in drosophilid populations [48]–[53]. Accordingly, we hypothesized that the winter-favored allele at seasonal SNPs would be associated with decreased chill-coma recovery time and increased starvation tolerance. To test this hypothesis, we used allele frequency data from previously published tail-based mapping of chill-coma recovery time and starvation tolerance [54]. We show that the winter favored allele at seasonal SNPs is more likely to be associated with fast chill coma recovery time than expected by chance across a range of GWAS p-values (Fig. 5A). A similar analysis of starvation tolerance was equivocal but the general pattern is that the winter-adaptive allele is associated with increased starvation tolerance (Fig. 5B).
Fig. 5. Association with seasonally variable phenotypes. 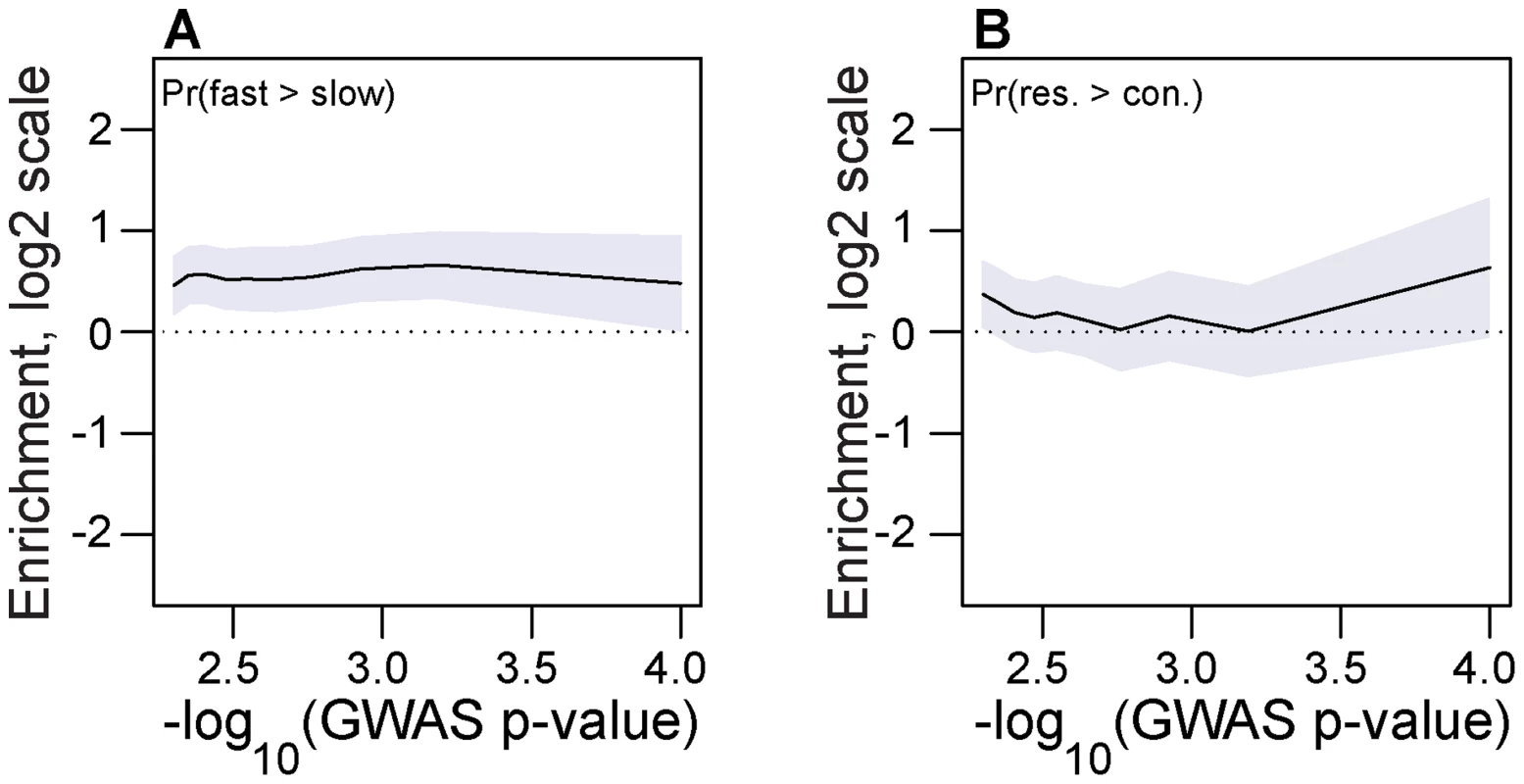
Enrichment (log2 odds ratio) of seasonal SNPs that change in frequency in the expected direction at SNPs associated with chill coma recovery time (A) and starvation tolerance (B) relative to contronl SNPs. The x-axis represents the threshold -log10(GWAS p-value), i.e. values along the x-axis represent the minimum -log10(GWAS p-value) for SNPs under consideration. Error bars represent 95% confidence intervals based on blocked bootstrap resampling. Long-term balancing selection
Balancing selection caused by variation in selection pressures through time can in principle maintain allelic variation at adaptively oscillating loci and elevate levels of neutral diversity surrounding these balanced polymorphisms. Thus, if seasonal variation in selection pressures promotes balanced polymorphisms we hypothesized that seasonal SNPs would be old and present in regions of elevated polymorphism.
We tested the hypothesis that seasonal SNPs are old by first examining their allele frequencies in a broad survey of African D. melanogaster populations [55]. Approximately 5% of seasonal SNPs are rare in Africa (MAF<0.01), however these SNPs are not more likely to be rare in Africa than control polymorphisms (log2(odds ratio) = 0.96; p = 0.328). Interestingly, for seasonal SNPs where one allele is rare in Africa, the summer favored alleles are more likely to be rare in Africa than winter favored alleles (log2(odds ratio) = 0.475; p = 0.018). Because the vast majority of seasonal SNPs segregate in Africa, it appears that adaptation to temperate environments, and particularly winter conditions, relies primarily on old, standing genetic variation.
Balancing selection acts to maintain alleles at intermediate frequencies for long periods of time and, in some instances, can maintain polymorphism across species boundaries [56], [57]. We examined whether seasonal SNPs showed signatures of long-term balancing selection by examining patterns of polymorphism surrounding orthologous regions in D. simulans, the sister species to D. melanogaster. We note that the following analyses are conservative because we underestimate D. simulans diversity given the small number (<6) of D. simulans haplotypes used.
First, we demonstrate that seasonal SNPs are approximately 1.5 times more likely to be polymorphic and share the same two alleles identical by state in both species relative to control SNPs. This pattern is observed for all seasonal SNPs (Fig. 6, p<0.002) and for seasonal SNPs residing in genes (Fig. 6, p<0.002). The increased probability of shared polymorphism between D. melanogaster and D. simulans at seasonal SNPs could, in principle, be driven by an over-representation of synonymous, genic SNPs (Fig. 2C). Unless synonymous SNPs are in four-fold degenerate positions, certain mutations may cause them to be non-synonymous thereby limiting the number of possible neutral allelic states and increasing the probability of shared polymorphism between species. However, adaptively oscillating SNPs that do not reside in synonymous sites are also more likely than expected by chance to be polymorphic and share the same two alleles by state in D. melanogaster and D. simulans (Fig. 6, p = 0.014).
Fig. 6. Long term balancing selection. 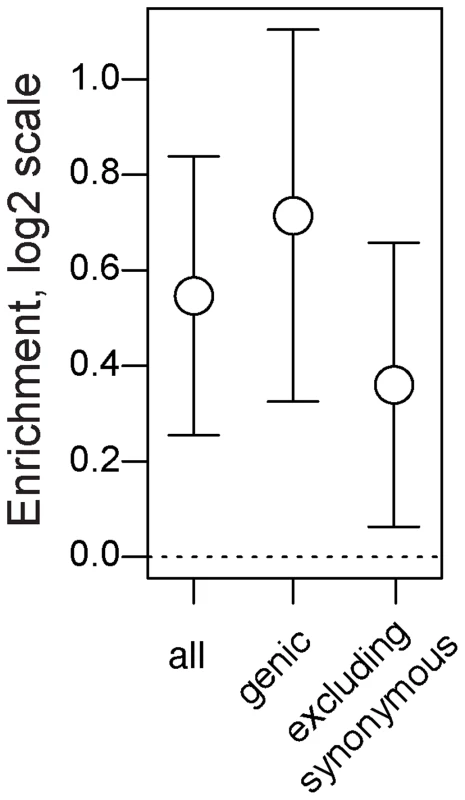
Enrichment (log2 odds ratio) of seasonal SNPs among SNPs that polymorphic and identical by state among 6 lineages of D. simulans relative to control SNPs. Error bars represent 95% confidence intervals based on blocked bootstrap resampling. The co-occurrence of shared polymorphism between D. melanogaster and D. simulans could result from three evolutionary mechanisms. First, trans-specific polymorphisms could result from adaptive introgression. This scenario seems implausible given the high degree of pre - and post-zygotic isolating mechanisms between these two species [58], [59]. Furthermore, if trans-specific polymorphisms resulted from recent adaptive introgression we would expect average pairwise divergence between D. melanogaster and D. simulans surrounding seasonal SNPs to be smaller than at control SNPs. However, there is no significant difference in estimates of divergence between seasonal and control SNPs (p = 0.7 for windows ±250 bp). Second, trans-specific polymorphisms could result from convergent adaptive evolution. Finally, trans-specific polymorphisms could be millions of years old [60], predating the divergence of D. melanogaster from D. simulans. While we cannot differentiate these latter two mechanisms, we postulate that the most parsimonious explanation is that trans-specific seasonal SNPs predate the divergence of these two sister species.
Seasonally variable selection is required to generate genome-wide patterns of allele frequency change through time
Despite empirical support for the conclusion that seasonal SNPs show many signatures consistent with adaptive response to seasonally variable selection, drift, caused by cyclic population booms and busts, or migration from neighboring demes are alternative mechanisms that could drastically perturb allele frequencies in the Pennsylvanian population and could generate some of the genome-wide patterns we observe. We address these possibilities here and conclude that neither cyclic changes in population size nor seasonal migration can plausibly explain the extent of genome-wide genetic differentiation through time, the observed number of seasonal SNPs, nor the enrichment of seasonal SNPs among many distinct genomic features (e.g., Figs. 2–6). At the same time, we also show through several simulation approaches that rapid adaptive evolution in response to seasonal fluctuations in selection pressure is sufficient to explain patterns of allele frequency change through time. Furthermore, we discuss how large-scale migration is internally inconsistent with certain aspects of our data. Taken together, we conclude that rapid adaptive evolution to seasonally variable selection is required to explain the patterns of allele frequency change through time at seasonal SNPs and at linked neutral loci that we observe in our dataset.
First, we assessed the possibility that extensive drift caused by population contraction every winter [31], [61], [62] could generate genome-wide patterns of genetic differentiation through time observed in our data. To do so, we conducted forward genetic simulations that model biologically plausible variation in population size and included loci that cycle in frequency due to variable selection pressures [63]. For these simulations, we modeled a 20 Mb chromosome with constant recombination rate of 2 cM/Mb, representing the genome-wide average recombination rate in D. melanogaster [64]. We simulated population contraction to one of various minimum, ‘overwintering’ population sizes followed by exponential growth over 10 generations in the ‘summer’ to a fixed maximum population size. In these models, we included various numbers of loci that respond to seasonally varying selection. Selection coefficients for each locus were set such that allele frequencies at selected sites oscillated by ∼20%, between 60 and 40%, representing the average change in allele frequency we actually see between spring and fall at seasonal SNPs. Finally, we placed 500 neutral loci randomly along the simulated chromosome and measured FST at these neutral loci between three ‘spring’ (i.e., first generation of population expansion) and ‘fall’ (last generation of population expansion) samples. See Materials and Methods for more details these models.
In the absence of seasonal selection, these forward simulations suggest that overwintering Ne would have to be exceedingly low (∼20; Fig. 7A) to generate levels of FST between spring and fall as high as we observe in our data (arrow in Fig. 1C). However, with overwintering Ne of 200 and 5–10 seasonally adaptive SNPs per chromosome arm, simulated FST at neutral loci is on the order of 0.002 (Fig. 7A), which we observe in our data (arrow in Fig. 1C). While we do not know overwintering population size, we speculate it could be on the order of 200 flies or likely substantially larger [61], [62] and conclude that at least 25–50 (5–10 per main chromosome arm) loci are sufficient to generate patterns of differentiation we observe through time. Note that increasing the overwintering population size requires concomitant increase in number of seasonally selected loci.
Fig. 7. Demographic models. 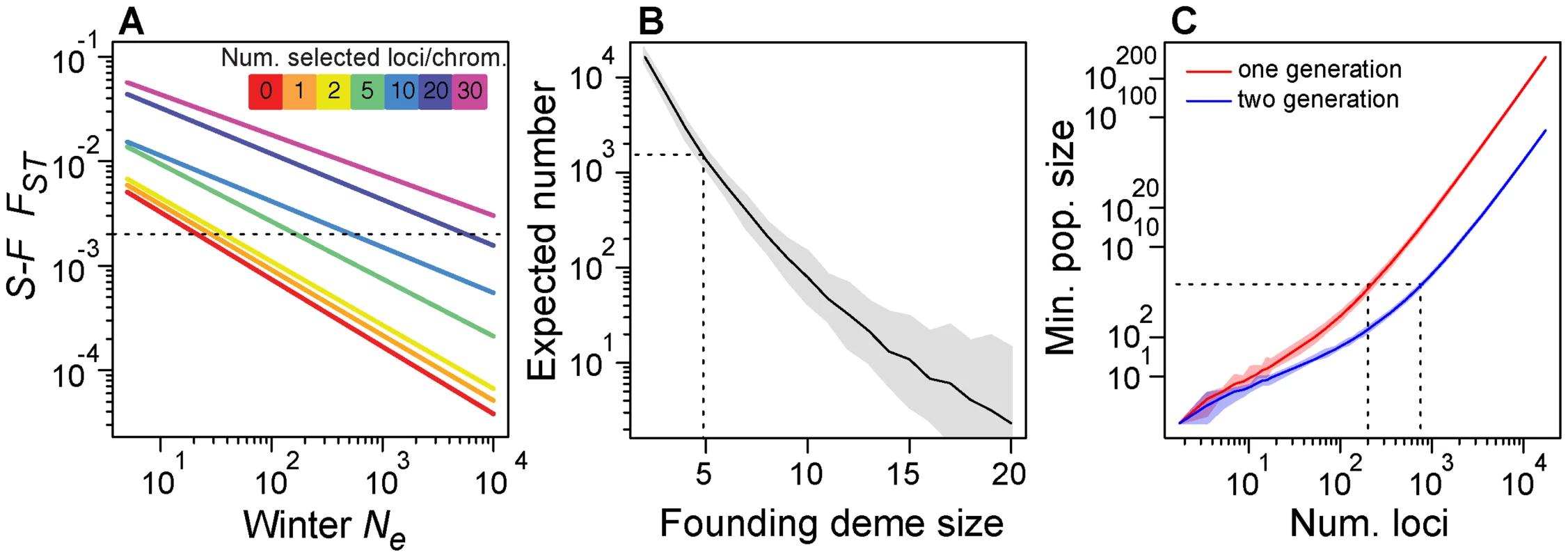
(A) Expected value of FST between simulated spring and fall samples (y-axis), conditional on overwintering effective population size and the number of seasonally adaptive alleles (color key). Dotted line represents observed average, genome-wide after FST between spring and fall samples from the Pennsylvanian population. (B) Expected number of SNPs that would vary repeatedly between seasons three times in a row conditional on founding deme size for a simple model of recolonization of the orchard population. Dotted line represents the observed number of seasonal SNPs and the corresponding founding deme size required, in this case 5 flies. (C) Minimum population size (y-axis) for the required for varying number of seasonally selected loci (x-axis) under a truncation selection model assuming independent response to selection at each locus. Dotted line represents our best guess of fall population size and corresponding number of loci that could independently respond to truncation selection. Confidence bands based on resampling of observed allele frequency change at seasonal SNPs. We regard overwintering population sizes of ∼20 flies to be inconsistent with certain aspects of our data and also implausible given what we know about the biology of the species. First, such a severe population contraction would result in reduction of genetic diversity, particularly for low frequency alleles. However, the observed allele frequency spectrum between fall and the following spring samples is similar and spring samples do not exhibit the expected loss of low frequency polymorphisms that would result from a population contraction to 20 individuals (Fig. S7). Second, population contraction to 20 individuals would often lead to population extirpation in the Pennsylvanian orchard and would certainly lead to extirpation at localities further north that experience more severe winters. However, D. melanogaster are routinely collected in Northern orchards very early in the season [65] and are routinely found in populations at as far north as 45° (Schmidt pers. obs). Furthermore, certain rare alleles have persisted in northern D. melanogaster populations for upwards of 30 years [66] cf. [67] and allele frequency clines are relatively stable over decadal scales [68] demonstrating that high latitude populations are not frequently extirpated and that overwintering bottlenecks cannot be so severe as our neutral simulations would require.
In our forward simulations, seasonally variable selection is sufficient to generate high levels of genome-wide genetic differentiation through time. In addition, our forward simulations are consistent with the increase of genome-wide average FST through time excluding polymorphisms that are within 500 bp of seasonal SNPs (Fig. S3). In our simulations, 500 neutral loci were placed randomly along a 20 Mb chromosome and were initially completely unlinked to selected loci. Therefore, the high levels of simulated FST are a consequence of genetic draft acting over long physical distances with low to moderate linkage disequilibrium between neutral and selected polymorphisms. Our observation that genome-wide average FST (excluding polymorphisms near seasonal SNPs, Fig. S3) increases with time resembles our simulations suggesting that draft can perturb allele frequencies over long genetic distances.
We also note that long-range genetic draft, caused by rapid frequency shifts of ancient balanced alleles to seasonally variable selection would likely cause an asymptotic change in genome-wide temporal FST, whereas a purely drift-based model would likely cause a linear increase in genome-wide FST through time. Seasonal SNPs tend to be old and are therefore likely found on a diverse array of haplotypes. Therefore, the exact composition of haplotypes that rise and fall every seasonal cycle will be somewhat stochastic giving rise to a high genome-wide FST over a duration of time less than ∼7 months (the duration of time between fall and the following spring). Among years, genome-wide average FST would possibly plateau if local Ne were large (as we suspect it is, see Results and Discussion: The plausibility…), coupled with the effects of recombination, gene conversion, and low-level migration from neighboring demes or populations. Finally, we note that because seasonal SNPs likely exist on a diverse array of haplotypes we do not expect genome-wide average FST to oscillate with a period corresponding to approximately 6–7 months. For such oscillations to occur, a large (i.e., much larger than we identify) number of loci would have to be repeatedly shifting between seasons.
Next, we explore the possibility that migration could drastically alter allele frequencies in the Pennsylvanian population and generate the large number of loci that vary repeatedly among seasons. First, we examined a simple but general demographic model where the Pennsylvanian orchard population becomes extirpated every year and recolonized from a refugium such as a southern population or a large, local site such as a compost pile. Either situation is plausible given the purportedly high rates of migration in North American D. melanogaster populations [67], [69] and what little is known about the overwintering biology of high latitude D. melanogaster [66]. In our model, we envisioned a resident, refugial population with stable allele frequencies across years that colonizes the orchard population. In this model, the orchard would be colonized early in the season with a random subsample of flies from the refugium and would therefore have aberrant allele frequencies. As more migrants arrived to the orchard from the refugium, allele frequencies at the orchard would stabilize to that of the source population. In such a scenario, allele frequencies in spring samples could vary considerably and a small fraction of SNPs might, by chance, have the same aberrant allele frequencies year after year and would appear to cycle seasonally.
We calculated the expected number of SNPs that would cycle by chance alone as a function of the number of initial migrants (Fig. 7B). For instance, if five migrants arrived at the orchard prior to our spring sample every year, approximately 1300 SNPs would cycle seasonally producing similar patterns to the observed change in allele frequency through time as at ‘seasonal SNPs’ (Fig. 2A). However, if four migrants arrived at the orchard prior to our sampling, ∼2600 SNPs would vary repeatedly but if six migrants arrived, only ∼700 would. Although the expected number of sites that oscillate under this migration model with 5 migrants is approximately the number we observe, we note that the expected number is highly dependent on the exact number of migrants. It seems unlikely that exactly five flies would migrate from the refugium to the orchard before our first spring sample three times in a row. Therefore, the extreme sensitivity of the expected number of sites to the number of migrants makes this general demographic scenario implausible. We are therefore led to conclude that the simple migration model presented here is likely to be insufficient to explain changes in allele frequency through time in the Pennsylvanian orchard.
In addition to our conclusion that a simple model of recolonization of the orchard is insufficient to explain the number of seasonally variable loci we observe, our data are also inconsistent with large-scale migrations from adjacent populations. For instance, if a large-scale migration from the South to resident northern populations were to occur, we would expect that clinally varying SNPs should also vary seasonally. Such a pattern would be expected both if a large-scale migration occurred randomly or were genotype dependent. However, seasonal SNPs are apparently not enriched among clinally varying polymorphisms (Fig. 3C). A similar logic would apply for an early season migration from the North followed by a subsequent, late season migration from the South. We also note that this dual migration model is biologically implausible. The relationship between latitude and the onset of spring would suggest that far northern populations would be quite small in the early part of the growing season and the subsequent probability of emigration to southern locales would be low. Therefore, we conclude that large-scale migration does not play a major role shaping seasonal variation in allele frequencies in the Pennsylvanian orchard. Furthermore, even if seasonal SNPs were enriched among clinally varying polymorphisms (which they do not appear to be), adaptation to seasonally variable selection would need to be invoked in order to explain the yearly shift in allele frequencies every winter.
Taken together, the models presented here demonstrate that seasonal boom-bust or migration-based scenarios are insufficient to explain allele frequency change through time in the Pennsylvanian population. While temperate populations of D. melanogaster clearly undergo cyclic population booms and busts due to changes in climate associated with the season, the extent of these population contractions necessary to generate the patterns of genetic variation through time that we observe would be too extreme to allow for stable population persistence. Similarly, the Pennsylvanian population certainly exists as a part of a complex metapopulation and experiences immigration and emigration. However, analysis of a simple demographic model of population recolonization during the spring is also insufficient to explain the patterns of allele frequency change through time that we observe and our data are internally inconsistent with a model of large-scale migration from neighboring populations.
Finally, we point out that the boom-bust and recolonization models we presented here undoubtedly are oversimplifications and that there are other, more complex demographic models that we have not explored. Nonetheless, any stochastic demographic event would affect SNPs throughout the genome with equal probability. Many aspects of our data clearly show that seasonal SNPs are not a random set of common SNPs but rather show signatures consistent with both functional effect and long-term balancing selection such as enrichment in specific classes of genetic elements, association with seasonally variable phenotypes and predictable and virtually instantaneous shifts in allele frequency in response to frost. Therefore, while we cannot conclusively rule out the possibility that demographic events affect the temporal dynamics of allele frequencies at seasonal - and non-seasonal SNPs in the Pennsylvanian population, these demographic events are most likely coupled with adaptive evolution in response to temporally varying selection pressures.
The plausibility of seasonally variable selection
We have previously argued that adaptive response to seasonally fluctuating selection at no less than 25–50 loci is necessary to generate the high levels of genome-wide genetic differentiation through time observed in the Pennsylvanian population. Next, we considered the plausibility of such strong selection and estimated the upper bound of the number of loci that could independently respond to seasonally variable selection. To do so, we modeled independent selection at 1–10,000 simulated seasonal SNPs whose allele frequency change was drawn from the observed allele frequency change at seasonal SNPs. Using a simple Poisson model (see Materials and Methods), we estimated the minimum fall census size required for that number of loci to shift in allele frequency during one or two rounds of truncation selection. Using these models, we sought to estimate the most likely number of seasonal SNPs that could independently respond to seasonally variable selection by contrasting model-based estimates of population size with our best estimates of population size in the field.
Although fall census size of D. melanogaster in the focal Pennsylvanian population is unknown, some estimates of drosophilid population size have been made. Global population size of D. melanogaster is likely to be extremely large, greater than 108 [70]. However, estimates of local population size made from mark-release-recapture methods report census sizes on the order of 104 to 105 [71]–[73]), with considerable variation among seasons, years and locales. D. melanogaster samples from orchards and vineyards often exceed 104 flies [74], [75] and thousands of flies can easily be collected over large compost piles (Bergland pers. obs.). Therefore, we speculate that census size of temperate D. melanogaster populations at any locale is a function of the local ecology (e.g., amount of windfall fruit, number and size of compost piles, humidity) and given the favorable conditions in the focal Pennsylvanian orchard (Schmidt pers. obs.), large census sizes of more than 105 are conceivable. If fall census size in the Pennsylvanian population is on the order of 105, our truncation selection model suggests that no more than several hundred (200–700, Fig. 7C) seasonal SNPs could respond to seasonally varying selection independently. We note that increasing the number of generations of winter-like selection pressures or the fall census size would lead to a concomitant increase in the number of seasonally selected loci that could independently respond to seasonally varying selection pressures.
Our survey of temporal changes in allele frequency identified 1750 seasonal SNPs that cycle significantly by ∼20% between seasons at FDR of 0.3. Unless local census size in the Pennsylvanian population were unrealistically large – on the order of 1010 or 1020 – it is unlikely that all of these loci respond to selection independently. Our model suggests, however, that a large fraction, on the order of 200–700 could vary independently in every cycle. One explanation for cycling in the remaining SNPs is linkage with loci responding to seasonally variable selection. It is possible that this linkage is generated either stochastically and neutrally or, alternatively, by selective processes such as assortative mating [76] or epistatic selection [77], [78]. For instance, if winter adapted flies were more likely to mate with other winter adapted flies during the summer, winter adapted alleles may become coupled and linkage disequilibrium between these alleles could increase. Similarly, certain forms of epistatic interactions could also generate linkage disequilibrium between seasonal SNPs if, for instance, couplings of winter and summer favored alleles at multiple loci were particularly deleterious relative to winter-winter or summer-summer combinations. The net effect of selective mechanisms that promote positive linkage disequilibrium between seasonal SNPs is that the effective number of ‘independently’ seasonally selected loci decreases. If seasonal SNPs are in linkage disequilibrium due to selective processes, it would imply that more than 200–700 seasonal SNPs contribute to organismal form and function and modify fitness during the summer and winter.
Conclusions – Summary
Herein, we present results from population based resequencing of samples of flies collected along a latitudinal cline in North America and over three years during the spring and fall in a Pennsylvanian orchard. We identify repeatable and dramatic changes in allele frequencies through time at hundreds of polymorphisms spread throughout the genome. Response to strong selection at these seasonal SNPs likely drives genetic differentiation through time at linked, neutral polymorphisms. This process leads to genome-wide differentiation between samples collected several years apart comparable to populations separated by 5–10° latitude. Seasonal SNPs are likely to be functional as they show enrichment at functional sites, vary predictably among populations sampled along the cline, respond immediately to a hard frost event, and are associated with phenotypes previously shown to vary seasonally in temperate D. melanogaster populations. Finally, our results suggest that some adaptively oscillating SNPs are possibly millions of years old, predating the split of D. melanogaster from its sister species D. simulans. Taken together, our results provide the first genomic picture of balancing selection caused by temporal fluctuations in selection pressures and provide novel insight into the biology of marginal overdominance.
Conclusions – Functional properties of adaptively oscillating polymorphisms
Temperate populations of D. melanogaster are exposed to high levels of environmental heterogeneity among seasons due to changes in various aspects of the environment including temperature, humidity, and nutritional quality and quantity. These shifts in the environment are primary determinants of cyclic population booms and busts [66], [71], [72] and impose strong temporally and spatially variable selection. Intuition, theoretical models [79], laboratory experimentation [35], and inference from patterns of clinal variation [80]–[82] and seasonal variation in morphological, behavioral and life-history traits suggest that alternate seasons favor differing life-history strategies. In general, populations exposed to more harsh conditions such as those from Northern locales or those collected early in the season are larger [83], [84], more stress tolerant [49]–[51], [82], longer lived [81], and are less fecund [81], [85] than those collected in Southern locales or during the fall. The general picture that emerges, therefore, is that in temperate populations winter conditions select for hardier but less fecund individuals whereas summer selects for high reproductive output at the cost of somatic maintenance. Nonetheless, there is surprisingly little evidence directly linking adaptive differentiation between seasonally favored genetic polymorphisms, phenotypes and environmental perturbations (but see [35]). Herein we present several key results that link seasonal and spatial patterns of genotypic and phenotypic variation with environmental perturbations.
First, our data suggest that that acute bouts of cold temperature elicit adaptive response at seasonally oscillating polymorphisms (Fig. 4). Heretofore, the specific environmental factors altering allele frequencies through time and space among dipteran species has generally remained elusive largely stemming from the fact that many aspects of the environment co-vary over temporal and spatial scales. Here we show that acute exposure to sub-freezing temperatures in the field shifts allele frequencies in a spring like direction at seasonal SNPs but not at control polymorphisms, thereby suggesting that sharp modulation of temperature can act as a selective force in the field. While post-frost allele frequencies at seasonal SNPs move in a ‘spring-like’ direction, they do not reach average spring allele frequencies. This suggests that multiple frost events, long-term exposure to cold temperatures or other selective factors linked to winter conditions such as starvation also impose strong selection in temperate populations.
Next, we demonstrate that environmental differences among populations predict, to a certain extent, changes in allele frequency at seasonal SNPs. Environmental factors that vary over seasonal time scales also vary with latitude. This fact has facilitated studies that substitute space for time and has led to a paradigm in many aspects of contemporary research in drosophilid evolutionary ecology of examining phenotypic and genetic differentiation along latitudinal (and altitudinal) clines as a proxy for studying adaptation to temperate environments [e.g., 86]. Using allele frequency estimates that we made from populations sampled along the North American latitudinal cline, we demonstrate that southern populations are more ‘fall-like’ at seasonal SNPs whereas northern populations are more ‘spring-like’ (Fig. 3D). Northern populations experience more severe winters and have shorter growing seasons; therefore, we speculate that the changes in allele frequency at adaptively oscillating polymorphisms along the cline is because (1) the summer favored allele would be at lower frequency due to stronger selection during the winter and (2) the summer favored allele would not rise in frequency as much during the summer because of the shorter growing season. The converse would be the case for Southern populations.
Finally, we relate seasonally variable SNPs with ecologically relevant phenotypic variation. Previous studies have demonstrated that two important stress tolerance traits, chill coma recovery time and starvation resistance vary in predictable ways among temperate populations of D. melanogaster. Northern populations tend to have fast chill coma recovery time [87]–[89] recapitulating deeper phylogenetic patterns among drosophilids originating from temperate and tropical locales [48]. Evidence for latitudinal variation in starvation tolerance is more equivocal with low latitude populations of D. melanogaster being more starvation tolerant in some studies but not significantly so in others [49], [91] and closely related species showing equally ambiguous patterns [52], [90], [91]. However, diapause-competent genotypes that are at high frequency in Northern populations and in the spring show increased starvation tolerance [52] suggesting that spatial and temporal differentiation in starvation tolerance may be parallel in the context of specific polymorphisms. Nonetheless, because selection pressures along latitudinal clines are generally parallel with seasonal selection pressures (e.g., Fig. 3D) we reasoned that winter adapted alleles at seasonal SNPs would be associated with fast chill coma recovery time and increased starvation tolerance.
We show that winter adapted alleles at seasonal SNPs are likely to be associated with fast chill coma recovery time and, to a lesser extent, starvation tolerance (Fig. 5). The strength of the relationship between seasonal SNPs with these two phenotypes likely differs for many reasons, including intrinsic differences in the statistical power and the complex genetic architecture of these traits. Nonetheless, the fact that seasonal SNPs are associated with chill coma recovery and starvation tolerance in the predicted direction given our prior knowledge of seasonal variation in these two traits strongly suggests that seasonal SNPs are functional and affect seasonally dependent fitness via stress tolerance traits. In addition, the concordance between seasonal SNPs and SNPs moderately associated with chill coma recovery time and starvation tolerance suggests that the intermediate frequency SNPs that we are investigating here have small effects on phenotype but nonetheless have large effects on average population fitness.
Taken together, our analysis has linked adaptive oscillations at hundreds of polymorphisms in D. melanogaster to specific and persistent differences in climate and to phenotypes known to be under diversifying selection through time and space. Our results support the hypothesis that stress tolerance traits are favored during the winter and disfavored during the summer. Stress tolerance traits such as chill coma recovery time and starvation tolerance often have negative genetic correlations with reproductive output [52], [92] or development time [93], two phenotypes that would be favored during exponential growth during the summer. Therefore, it is likely that a subset of seasonal SNPs directly contribute to a tradeoff between stress tolerance and reproductive output.
Because D. melanogaster originated in sub-Saharan Africa and colonized the world in the wake of human migration 200–10,000 years ago [94] it has been hypothesized [95] that phenotypes favored during the winter are derived whereas those favored during the summer are ancestral with respect to tropical, African populations. Although we show that the vast majority of seasonal SNPs are common in Africa, a small set (∼5%) are rare, segregating at less than 1%. Somewhat surprisingly, summer favored alleles are more likely to be rare in Africa than winter favored alleles (see Results and Discussion: Long term…) suggesting that some environmental aspects of summer in temperate orchards are new for D. melanogaster. Consistent with the observation that flies sampled at low latitudes are likely subject to intense intra - and inter-specific competition [83], we speculate that the cornucopia of rotten fruit during the summer in mid - to high-latitude locales coupled with decreased inter-specific competition is a novel environment for D. melanogaster that has allowed formerly rare alleles associated with increased reproductive output to flourish.
Conclusions – Long-term, polygenic balancing selection, and ecological generality
Herein, we present several lines of evidence demonstrating that hundreds of loci adaptively respond to seasonal fluctuations in the environment. Despite (or because of) the fact that these loci promote rapid adaptive evolution, many have remained polymorphic for millions of generations within D. melanogaster and some possibly predate the divergence of D. melanogaster and D. simulans ∼5 million years ago. Taken together, these observations suggest that alleles at these loci have may have been maintained by environmental heterogeneity for exceptionally long periods of time. Long-term balancing selection is typically regarded as an evolutionary oddity, found predominantly in the genetic systems regulating host-pathogen interactions, self-incompatibility, and sex-determination [56], [96]. Herein, we provide evidence that environmental heterogeneity might promote long-term balanced polymorphisms at hundreds of loci that affect quantitative, stress tolerance traits.
Theory predicts that temporal variation in selection coefficients can maintain adaptive genetic variation for long periods of time when certain genetic and ecological conditions are met. Classic models suggest that the adaptive variation can be maintained in populations because of temporal shifts in selection pressure only when the heterozygote has a higher geometric mean fitness than either homozygote [1]. Such conditions are necessary for both finite and infinite populations and, moreover, in finite populations the persistence time of adaptive polymorphisms may be shorter than for neutral ones [8]. However, alternative models have demonstrated that overlapping generations [97], the combination of spatial and temporal variation in selection pressures [4], habitat fidelity [98], [99], and multiple liked loci subject to temporally variable selection [3] will increase the persistence time of balanced polymorphisms maintained by environmental heterogeneity.
Each of these conditions are met in for D. melanogaster. First, flies are highly fecund [100], iteroparous insects with generation time a fraction of lifespan [80], [81]. Therefore natural populations are likely to be highly age structured which will prevent the loss of balanced alleles during alternate seasons. Second, spatial selection pressures vary on the order of meters to kilometers [101], [102], all well within the dispersal radius of flies [72]. In addition, flies often return to the substrate they were collected on [103], [104] and flies collected within a locale show signatures of population structure on the order of tens of meters [105], [106]. Therefore, low to moderate levels of migration between demes separated by various distances [67], [69], [72] and environmental heterogeneity over small spatial scales may help mitigate the loss of balanced polymorphisms in any one orchard. Finally, our study identified hundreds of adaptively oscillating polymorphisms. Although the vast majority of these polymorphisms are unlinked due to the large physical distance between them, there is evidence of heterogeneity in the abundance of seasonal SNPs throughout the genome suggesting that some might be in partial linkage disequilibrium. Some models [3] have suggested that linkage between polymorphisms subject to temporally variable selection can allow for long-term persistence of both alleles at multiple sites. Taken together, we suspect D. melanogaster satisfies several key features required for the long-term maintenance of balanced polymorphisms due to temporal (and spatial) variation in selection pressures. Nonetheless, how do we account for the observation that these polymorphisms have been possibly maintained across different continents with clear differences in climate and between species with different ecologies [107]?
The long-term persistence of these adaptively oscillating polymorphisms across populations, continents, and species suggests that these polymorphisms contribute to short-term and local adaptation in response to very generalized environmental conditions. This is in contrast to the hypothesis [108] that adaptation to temperate environments in D. melanogaster was largely in response to novel environments, exclusively associated with life in northern, temperate locales. Rather, we speculate that the selective pressures associated with seasons in temperate environments are merely manifestations of general selective pressures resulting from cyclic population booms and busts. That is, during times of plenty, such as during the summer in temperate locales, populations rapidly expand and alleles that confer increased reproductive output or faster time to sexual maturity are strongly favored. Likewise, when population size contracts due to biotic and abiotic stressors such as those experienced during winter, alleles that confer increased stress resistance are favored.
Cyclic population booms and busts are almost certainly a perennial feature of D. melanogaster populations, are a likely common occurrence in highly fecund species that exploit ephemeral resources, and may be an inherent property of most species in general [108]. If true, we speculate that such species may harbor alleles that promote reproductive fitness during population growth (at the cost of somatic maintenance) and increase stress tolerance (at the cost of reproductive growth) during population contraction. Such balanced polymorphisms may be particularly common for species whose population cycles are decoupled from predictable environmental cues (e.g., photoperiod) but are rather linked to stochastic changes in resource abundance. For species such as these, including many microorganisms and invertebrates, balanced polymorphisms maintained by environmental heterogeneity through time and space may be the norm rather than the exception.
Materials and Methods
Fly collections
We resequenced samples of D. melanogaster from populations sampled over several years (2003–2010) largely during periods of peak abundance along a broad latitudinal cline in North America and during multiple time points over three consecutive years (2009 to 2011) at the Linvilla Orchard in Media, PA (39.9°N, 75.4°W). From each locality and sampling period, we collected ∼50–200 D. melanogaster largely by aspiration from individual fruits or baiting at strawberry fields and apple and peach orchards, established isofemale lines and collected male progeny at generation 1–5 for sequencing. One male progeny per isofemale line per population was pooled together to generate template DNA for high throughput sequencing (Table S1). The only two exceptions are the second replicate sample from Maine which was derived from wild-caught males and the sample from North Carolina which was sampled from the Drosophila Genetic Reference Panel (DGRP) inbred lines. For the DGRP population, we resequenced a pooled sample consisting of one male from each of 92 DGRP strains and used allele frequency estimates from pooled samples when estimating clinality (see [41] for more information on this sample and [45] for more information on this population). Note, there is evidence that two samples (Florida replicate 2 and post-frost Pennsylvania) show low levels of contamination with the sister species D. simulans (i.e., ∼ one wild caught D. simulans was accidentally included in our pooled sample). However, we have no evidence that the low level of contamination in two samples affects our results in any way (see Text S1).
Sample preparation, sequencing, and bioinformatics of pooled samples
DNA libraries were prepared for sequencing on the Illumina HiSeq2000 platform. To generate these libraries, we homogenized whole, male flies in 200 µL lysis buffer (100 mM Tris-Cl, 100 mM EDTA, 100 mM NaCL, 0.5% SDS) using a motorized pestle grinder. An additional 200 µL of lysis buffer was added to each sample and the homogenate was incubated at 65°C for 30 minutes. After lysis, we added 800 µL of 2 parts 5M potassium acetate, 5 parts 6M lithium chloride solution and incubated on ice for 15 minutes to precipitate proteins. The homogenate was centrifuged for at 12 K rotations per minute (RPM) for 15 minutes at room temperature, 1 mL supernatant was transferred to a new tube, and the sample was centrifuged again at 12K RPM for 15 minutes at room temperature. To precipitate DNA, we added 800 µL of isopropanol and centrifuged the sample at 12K RPM for 15 minutes. The supernatant was discarded and the DNA pellet was washed with 70% ethanol and centrifuged at 14K RPM for 10 minutes, washed with ethanol again and centrifuged once more. The ethanol was removed and the pellet was allowed to dry at room temperature. We resuspended the pellet in 100 µL TE buffer.
DNA was prepared for Illumina sequencing by shearing, end-repair and ligation. To do so, 50 µL of DNA was mixed with an additional 50 µL of TE and this DNA was sheared to ∼500 bp using a Covaris machine. DNA was eluted to 30 µL using a QIAGEN PCR-purification kit (product number 28104). We performed end repair by incubating each sample of DNA with 5 µL T4 DNA ligation buffer (New England Biolabs [NEB] product number B0202S), 4 µL of 10 mM dNTPs, 2.5 µL T4 DNA polymerase (NEB product number M0203S), 0.5 µL Klenow large fragment (NEB product number M0210S), 2.5 µL T4 PNK (NEB product number M0201S), and 5.5 µL nuclease free water for 30 minutes at 20°C. Following incubation, DNA was purified using a QIAGEN PCR-clean up kit. Next, we performed dATP addition by incubating 32 µL of DNA with 5 µL 10× NEBuffer 2 (NEB product number B7002S), 1 µL 10 mM dATP, 3 µL Klenow Exo-minus (NEB product number M0212S), and 9 µL nuclease free water at 37°C for 30 minutes. Following incubation, DNA was purified using a QIAGEN MinElute kit (product number 28004) to a final volume of 11 µL. Sequencing adapters (custom synthesized by IDT) were ligated to DNA using T/A ligation by incubating 10 µL DNA with 2 µL T4 DNA ligation buffer, 1 µL T4 ligase (NEB product number M020S), 40 µL of 40 µM pre-annealed adapter mix and 6 µL nuclease free water for 15 minutes at 20°C followed by 65°C at 10 minutes to deactivate the DNA ligase.
Finally, we performed size-selection and PCR amplification as a final step to prepare DNA sequencing libraries. Immediately following ligation, DNA was loaded into a 2%, pre-cast SizeSelect E-Gel (Life Technologies product number G661002) and run along side a 100 bp ladder. DNA at ∼500 bp was removed from the gel into a volume of ∼15 µL nuclease free water. To amplify ligated DNA, we performed two replicate PCR reactions for each sample where we used 7.5 µL template DNA, 0.25 µL of 100 µM forward and reverse primers (custom synthesized by IDT), 0.5 µL 10 mM dNTPs, 4 µL 5× High-Fidelity buffer (NEB product number B0518S), 0.5 µL Phusion High-Fidelity DNA polymerase (NEB product number M0530S), and 5 µL nuclease free water. Note, the use of two replicate PCR reactions and a high volume of template DNA was meant to prevent PCR-jackpotting. PCR was performed by 30 sections of initial denaturation at 98°C followed by 11 rounds of 10 seconds denaturation (98°C), 30 seconds annealing (65°C), 30 seconds elongation (72°C), followed by a final elongation at 72°C for 5 minutes. DNA was purified using a QIAGEN PCR-cleanup kit.
Following PCR, DNA was quantified on a Life Technologies Qubit spectrophotometer as well as with a Agilent Bioanalyzer. Libraries were diluted to the appropriate concentration and sent to the Sequencing Service Center at the Stanford Center Genomics and Personalized Medicine for sequencing on the HiSeq 2000 platform.
Raw, paired-end 100 bp sequence reads were mapped to the D. melanogaster reference genome version 5.39 using bwa version 0.5.9-r16 [109] allowing for a maximum insert size of 800 bp and no more than 10 mismatches per 100 bp. PCR duplicates (∼5% per library) were removed using samtools version 0.1.18 [110] and local realignment around indels was performed using GATK version 1.4–25 [111]. We mapped SNPs and short indels (i.e., those occurring within the sequence reads) using CRISP [112], excluding reads with base or mapping quality below 10. SNPs mapping to repetitive regions such as microsatellites and transposable elements, identified in the standard RepeatMasker library for D. melanogaster (obtained from http://genome.ucsc.edu) were excluded from analysis as were SNPs within 5 bp of polymorphic indels. SNPs with average minor allele frequency across all populations less than 15%, with minimum per-population coverage less than 10× or maximum per-population coverage greater than 400× were removed from analysis. Finally, to ensure that the examined SNPs were not artifacts of our pooled resequencing, we removed any SNP not present in the SNP tables provided by freeze 2 of the DGRP [45] (http://www.hgsc.bcm.tmc.edu/projects/dgrp/). The inclusion of reads with read and mapping qualities greater than 10 (rather than greater than 20) is justified because we are restricting our analysis to common SNPs that have been previously identified in the DGRP. Of the 1,500,000 SNPs initially identified, ∼500,000 SNPs remained after applying these filters (Table S2). SNPs were annotated using SNPeff version 2.0.5 [113]. Short intron annotations were taken from [43]. An annotated VCF file with allele frequency calls, genic annotations, and seaonsal/clinal p - and q-values is avaible on DataDryad (doi:10.5061/dryad.v883p). Raw sequence data are available from NCBI SRA (BioProject accession PRJNA256231, and see Table S1 for accession numbers of individual libraries).
FST estimates
To estimate average differentiation between populations or between samples collected trough time, we calculated genome-wide average (mean) FST between pairs of populations. FST was calculated as,
where Htotal is the expected heterozygosity between two populations under panmixia and Hwith is the heterozygosity averaged between the two populations. Estimates of heterozygosity were corrected for read depth and number of sampled chromosomes by the factor, where, and where Nchr is the number of sampled chromosomes and Nrd is the number of reads at any site [114]–[116].We performed a parametric permutation analysis to calculate the expected, genome-wide average FST between pairs of populations under the null hypothesis of panmixia (spatial) or no allele frequency change through time (temporal) conditional on our experimental sampling design. To do so, we calculated the average allele frequency between any two pairs of populations or samples and randomly generated two estimates of allele frequency conditional on the average allele frequency, the number of reads at that site and the number of chromosomes sampled.
To calculate the proportion of SNPs where observed FST is greater than expected by chance, we generated 500 block bootstrap samples of ∼2300 SNPs, where one SNP was drawn per 50 kb interval. The proportion of SNPs where the observed FST distribution is greater than expected by chance is thus,
with standard deviation, where i refers to the ith SNP from jth block bootstrap sample.Identification of seasonally and clinally varying polymorphisms
To identify clinally varying and seasonally oscillating polymorphisms, we used generalized linear models implemented in R 2.10 [117] with binomial error structure and weights proportional to the number of reads sampled at a site and the number of chromosomes sampled (see above, Neff). To identify clinal polymorphisms, we regressed allele frequency at each site (excluding all Pennsylvanian samples) on latitude (Table S1) according to the form,
where yi is the observed allele frequencies of the ith SNP and εi is the binomial error given the number of effective reads (see above) at the ith SNP. To identify seasonally oscillating polymorphisms, we regressed allele frequency for the three sets of spring and fall samples on a binary variable corresponding to spring or fall according to the form, In addition, we modeled allele frequency change through time using generalized linear mixed models (GLMM) implemented in the lme4 R package [118] and generalized estimation equations (GEE) implemented in the geepack R package [119]. We fit GLMMs with the model, where (1|populationi) corresponds to the random effect of population j and εi corresponds to the binomial error. We fit GEEs with the model, where populationj corresponds to the population level strata and εi corresponds to the binomial error fit with an autoregressive order one correlation structure. q-q plots (Fig. S2) demonstrate that these models (clinal and seasonal) fit the bulk of the data adequately, with the exception of the seasonal GEE model which appears to be exceedingly anti-conservative. The false discovery rate was estimated using the Benjamini & Hochberg procedure [120].For seasonal SNPs, we estimated the cumulative selection coefficient as,
where fSp is the average allele frequency at seasonal SNPs in the spring and fFall is the average allele frequency at seasonal SNPs in the fall. This estimation of S is derived from a basic model of logistic growth of a beneficial allele [121], namely, Because we do not know the specific values of heterozygosity (h) nor the number of generations of selections during each season (t), we calculate S as the product of s, h, and t.Modeling the distribution of seasonal SNPs throughout the genome
We sought to test whether seasonal SNPs were homogeneously distributed throughout the genome. To do so, we grouped the genome into bins of 1000 non-overlapping SNPs (utilizing the ∼500,000 SNPs under investigation). For each window, we calculated the number of seasonal SNPs. The number of seasonal SNPs is Poisson distributed and we examined whether the observed distribution is over-dispersed after correcting for variation in rates of recombination within chromosomes and between the autosomes and X-chromsome. To do so, we fit the generalized linear model,
where n is the count of seasonal SNPs per 1000 SNPs, chrType is the binary classification of autosome or X-chromosome, and rec is the average recombination rate estimated in [64], and ε is the Poisson distributed error. To explicitly test if the number of seasonal SNPs is overdispersed, we used the dispersiontest function in the R package AER [122].Control polymorphisms and the block bootstrap
Throughout our analysis, we contrasted seasonal SNPs with control polymorphisms (Figs. 2–6). For these analyses, we identified 500 sets of control polymorphisms matched to each seasonal SNP. For each test described in the results, control polymorphisms were identified based on different sets of characteristics that have been shown, or could plausibly, influence the parameter we sought to investigate. In general, we matched seasonal SNPs to control SNPs by chromosome, recombination rate, and allele frequency in either Pennsylvania, North Carolina, North America, and/or Africa. The choice of which population to match allele frequencies was determined by the specific test. These three parameters (chromosome, recombination rate, allele frequency) correspond with many important evolutionary processes as well as genetic patterns (e.g., [123]) and therefore control SNPs will be matched to seasonal SNPs with respect to long-term evolutionary history, gene-density, and background levels of genetic variation. In general, we used as many parameters as possible while still identifying a sufficient number of control SNPs for each test and a full list of the matched characters for each test are listed in Table S3. For continuous characters, such as allele frequency, we typically rounded values so that a sufficient number of unique control sites could be identified. If no matched control SNPs were identified for a seasonal SNP, that seasonal SNP was removed from subsequent analyses.
In addition, we implemented a block-bootstrap procedure to ameliorate positive dependence of our test-statistics due to linkage disequilbrium between seasonal SNPs. We generated 500 sets of seasonal SNPs where one seasonal SNP was sampled from each 50 kb consecutive interval of the genome. This block-bootstrap yielded ∼850 SNPs that were spaced approximately every 50 Kb.
Estimates of expected values (E) of test statistics [e.g. log2-odds-ratios (Fig. 2C, 3B–C, 6A), FST (Fig. 2D), probability (Fig. 4B)] and standard deviations (SD) about those expected values were calculated as,
where i refers to control bootstrap set i and j refers to block bootstrap set j of any test-statistic, TS.Power calculations
To calculate statistical power of our experiment and to estimate the expected number of SNPs that are likely to vary repeatedly between seasons and along the cline we used Monte Carlo simulations based on the observed changes in allele frequency between spring and fall at seasonal SNPs or Maine and Florida at clinal SNPs. We calculated statistical power to detect seasonal SNPs as the probability of rejecting the null hypothesis of no repeatable change in allele frequency between spring and fall over three years given our sampling effort (e.g., number of chromosomes from nature and distribution of read depths in our Pennsylvanian samples) at α<∼1e-5, corresponding to observed seasonal q-value of 0.3, conditional on S, the cumulative change in allele frequency between seasons calculated from the logistic function. Similarly, we calculated statistical power to detect clinal SNPs as the probability of rejecting the null hypothesis of no change in allele frequency with latitude given our sampling effort at α<0.02, corresponding to the observed clinal q-value of 0.1, conditional on beta, the slope of the relationship between allele frequency and latitude. The expected number of seasonally (clinally) varying SNPs is then, the number of observed seasonal (clinal) SNPs at a particular value of S (beta) divided by the power to detect a seasonal (clinal) SNP at a selection coefficient S (beta).
Comparison with D. simulans
To estimate the extent of trans-specific polymorphism between D. melanogaster and D. simulans, we used D. simulans haplotype data available from the DPGP [124] (http://www.dpgp.org/). First, we remapped raw shot-gun sequences of each D. simulans strain (GenBank accessions AASS00000000 - AASW00000000) to the latest release of the D. simulans reference genome [125] with bwa version 0.5.9-r16 using the bwa-sw method.
To convert the genomic coordinate system of the new D. simulans genome to the D. melanogaster genome, we generated a lift-over file using lastz [126] and components of the UCSC genome-browser toolkit [127]. Gap parameters corresponded to those used to generate the lift-over file between the first generation D. simulans genome and the D. melanogaster genome (http://hgdownload.soe.ucsc.edu/goldenPath/dm3/vsDroSim1/). The lift-over file to translate the coordinate system of the second generation D. simulans genome to the D. melanogaster version 5 genome is available on Data Dryad (doi:10.5061/dryad.v883p).
We calculated average pairwise distance between D. melanogaster and D. simulans haplotypes at seasonal SNPs that were polymorphic in both species and shared the same two alleles by state. We calculated average pairwise distance at two windows surrounding seasonal SNPs, ±1–250 bp. Note, we excluded the focal, seasonal SNP. Pairwise distance calculations were performed using the ape [128] package in R.
Forward genetic simulations
To simulate genome-wide allele frequency change due to cyclic changes in population size and selection at seasonally adaptive polymorphisms, we used a modified version of the forward genetic simulation software SLiM [63]. Source code for the modified version of SLiM is available upon request. In these simulations, we modeled a 20 Mb chromosome with constant recombination rate of 2 cM/Mb. For all simulations, we seeded the chromosome with 500 neutral mutations randomly placed along the chromosome all starting at 50% initial allele frequency and in complete linkage equilibrium. The number of loci under selection varied between 0 and 30 and loci under temporally heterogeneous selection were placed equidistantly along the chromosome. Selection coefficients for each selected locus were set to produce adaptive oscillations between 40 and 60% frequency every 2 (simulated ‘winter’) and 10 (simulated ‘summer’) generations. Genotypic state was assigned randomly to each simulated diploid genome at each selected locus. Population size varied over the course of each simulation. Populations grew exponentially each ‘summer’ to a maximum population size of 105 over 10 generations. Population size instantaneously crashed at the start of winter to between 5 and 104 individuals and was held constant for two generations. Simulations were run for 100 generations and FST was estimated from the last three summer-winter cycles.
Truncation selection model
To estimate the upper bound of the number of loci that could plausibly respond to seasonally variable selection, we modeled a simple truncation selection scenario. For these models we calculated the expected number of winter adaptive alleles in the fall and the spring as the sum of average allele frequencies of the winter alleles in our fall and spring samples. If the oscillating alleles segregate independently, the variance in the number of winter alleles at any given time follows a Poisson distribution with mean and variance equal to the expected number of winter alleles. Therefore, the proportion of the population in the selected tail over winter is the probability of sampling the expected number of winter alleles in the spring from a Poisson distribution with mean equal to the number of winter alleles in the fall. To vary the number of independently oscillating polymorphisms in the spring and fall, we sub-sampled the number of oscillating polymorphisms 500 times for a range of values.
Supporting Information
Zdroje
1. GillespieJH (1973) Polymorphism in Random Environments. Theoretical Population Biology 4 : 193–195.
2. EllnerS, SasakiA (1996) Patterns of genetic polymorphism maintained by fluctuating selection with overlapping generations. Theor Popul Biol 50 : 31–65.
3. KorolAB, KirzhnerVM, RoninYI, NevoE (1996) Cyclical environmental changes as a factor maintaining genetic polymorphism. 2. Diploid selection for an additive trait. Evolution 50 : 1432–1441.
4. EwingEP (1979) Genetic-Variation in a Heterogeneous Environment. 7. Temporal and Spatial Heterogeneity in Infinite Populations. American Naturalist 114 : 197–212.
5. GillespieJH, LangleyCH (1974) General Model to Account for Enzyme Variation in Natural-Populations. Genetics 76 : 837–884.
6. HoekstraRF (1978) Sufficient Conditions for Polymorphism with Cyclical Selection in a Subdivided Population. Genetical Research 31 : 67–73.
7. HaldaneJBS, JayakarSD (1962) Polymorphism Due to Selection of Varying Direction. Journal of Genetics 58 : 237–242.
8. HedrickPW (1976) Genetic-Variation in a Heterogeneous Environment. 2. Temporal Heterogeneity and Directional Selection. Genetics 84 : 145–157.
9. GershensonS (1945) Evolutionary Studies on the Distribution and Dynamics of Melanism in the Hamster (Cricetus cricetus L). 1. Distribution of Black Hamsters in the Ukrainian and Bashkirian Soviet Socialist Republics (USSR). Genetics 30 : 207–232.
10. GrantPR, GrantBR (2002) Unpredictable evolution in a 30-year study of Darwin's finches. Science 296 : 707–711.
11. TarwaterCE, BeissingerSR (2013) Opposing selection and environmental variation modify optimal timing of breeding. Proc Natl Acad Sci U S A 110 : 15365–15370.
12. BrownCR, BrownMB, RocheEA (2013) Fluctuating viability selection on morphology of cliff swallows is driven by climate. J Evol Biol 26 : 1129–1142.
13. WallS, CarterMA, ClarkeB (1980) Temporal Changes of Gene-Frequencies in Cepaea hortensis. Biological Journal of the Linnean Society 14 : 303–317.
14. BrakefieldPM (1985) Differential Winter Mortality and Seasonal Selection in the Polymorphic Ladybird Adalia bipunctata (L) in the Netherlands. Biological Journal of the Linnean Society 24 : 189–206.
15. HairstonNG, DillonTA (1990) Fluctuating Selection and Response in a Population of Fresh-Water Copepods. Evolution 44 : 1796–1805.
16. BradshawWE (1973) Homeostasis and Polymorphism in Vernal Development of Chaoborus americanus. Ecology 54 : 1247–1259.
17. VavrekMC, McGrawJB, YangHS (1996) Within-population variation in demography of Taraxacum officinale: Maintenance of genetic diversity. Ecology 77 : 2098–2107.
18. AxenovichTI, ZorkoltsevaIV, AkberdinIR, BeketovSV, KashtanovSN, et al. (2007) Inheritance of litter size at birth in farmed arctic foxes (Alopex lagopus, Canidae, Carnivora). Heredity 98 : 99–105.
19. VorburgerC (2006) Temporal dynamics of genotypic diversity reveal strong clonal selection in the aphid Myzus persicae. Journal of Evolutionary Biology 19 : 97–107.
20. NiklassonM, TomiukJ, ParkerED (2004) Maintenance of clonal diversity in Dipsa bifurcata (Fallen, 1810) (Diptera: Lonchopteridae). I. Fluctuating seasonal selection moulds long-term coexistence. Heredity 93 : 62–71.
21. TomiukJ, NiklassonM, ParkerED (2004) Maintenance of clonal diversity in Dipsa bifurcata (Fallen, 1810) (Diptera: Lonchopteridae). II. Diapause stabilizes clonal coexistence. Heredity 93 : 72–77.
22. Templeton A, Johnston JS (1982) Life history evolution under pleiotropy and K-selection in a natural population of Drosophila mercatorum. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. New York: Academic Press. pp. 225–239.
23. Hart-SchmidtRA (2012) Geographically patterned variation in diapause and its relationship to other climate-associated phenotypes and genotypes of Drosophila americana: University of Iowa.
24. Rodriguez-TrellesF, TarrioR, SantosM (2013) Genome-wide evolutionary response to a heat wave in Drosophila. Biol Lett 9 : 20130228.
25. BellG (2010) Fluctuating selection: the perpetual renewal of adaptation in variable environments. Philos Trans R Soc Lond B Biol Sci 365 : 87–97.
26. SiepielskiAM, DiBattistaJD, CarlsonSM (2009) It's about time: the temporal dynamics of phenotypic selection in the wild. Ecol Lett 12 : 1261–1276.
27. DobzhanskyT (1943) Genetics of Natural Populations. IX. Temporal Changes in the Composition of Populations of Drosophila pseudoobscura. Genetics 28 : 162–186.
28. DobzhanskyT, AyalaFJ (1973) Temporal Frequency Changes of Enzyme and Chromosomal Polymorphisms in Natural Populations of Drosophila. Proceedings of the National Academy of Sciences of the United States of America 70 : 680–683.
29. MuellerLD, BarrLG, AyalaFJ (1985) Natural-Selection vs Random Drift - Evidence from Temporal Variation in Allele Frequencies in Nature. Genetics 111 : 517–554.
30. NielsenKM, HoffmannAA, MckechnieSW (1985) Population-Genetics of the Metabolically Related Adh, Gpdh and Tpi Polymorphisms in Drosophila melanogaster. 2. Temporal and Spatial Variation in an Orchard Population. Genetics Selection Evolution 17 : 41–58.
31. KnibbWR (1986) Temporal Variation of Drosophila melanogaster Adh Allele Frequencies, Inversion Frequencies, and Population Sizes. Genetica 71 : 175–190.
32. Rodriguez-TrellesF, AlvarezG, ZapataC (1996) Time-series analysis of seasonal changes of the O inversion polymorphism of Drosophila subobscura. Genetics 142 : 179–187.
33. AnaninaG, PeixotoAA, Bitner-MatheBC, SouzaWN, da SilvaLB, et al. (2004) Chromosomal inversion polymorphism in Drosophila mediopunctata: seasonal, altitudinal, and latitudinal variation. Genetics and Molecular Biology 27 : 61–69.
34. BouletreaumerleJ, FouilletP, TerrierO (1992) Clinal and Seasonal-Variations in Initial Retention Capacity of Virgin Drosophila melanogaster Females as a Strategy for Fitness. Evolutionary Ecology 6 : 223–242.
35. SchmidtPS, CondeDR (2006) Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila melanogaster. Evolution 60 : 1602–1611.
36. StalkerHD, CarsonHL (1949) Seasonal Variation in the Morphology of Drosophila robusta Sturtevant. Evolution 3 : 330–343.
37. TantawyAO (1964) Studies on Natural-Populations of Drosophila. 3. Morphological and Genetic-Differences of Wing Length in Drosophila melanogaster and Drosophila simulans in Relation to Season. Evolution 18 : 560–570.
38. MiyoT, AkaiS, OgumaY (2000) Seasonal fluctuation in susceptibility to insecticides within natural populations of Drosophila melanogaster: empirical observations of fitness costs of insecticide resistance. Genes & Genetic Systems 75 : 97–104.
39. DevK, ChahalJ, ParkashR, KatariaSK (2013) Correlated Changes in Body Melanisation and Mating Traits of Drosophila melanogaster: A Seasonal Analysis. Evolutionary Biology 40 : 366–376.
40. GillespieGH (2000) Genetic Drift in an Infinite Population: The Pseudohitchhiking Model. Genetics 155 : 909–919.
41. WrightS (1943) Isolation by Distance. Genetics 28 : 114–138.
42. ZhuY, BerglandAO, GonzalezJ, PetrovDA (2012) Empirical Validation of Pooled Whole Genome Population Re-Sequencing in Drosophila melanogaster. PLoS One 7: e41901.
43. LawrieDS, MesserPW, HershbergR, PetrovDA (2013) Strong Purifying Selection at Synonymous Sites in Drosophila melanogaster. PLoS Genetics 9(5): e1003527.
44. PechmannS, FrydmanJ (2013) Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat Struct Mol Biol 20 : 237–243.
45. MackayTF, RichardsS, StoneEA, BarbadillaA, AyrolesJF, et al. (2012) The Drosophila melanogaster Genetic Reference Panel. Nature 482 : 173–178.
46. KapunM, van SchalkwykH, McAllisterB, FlattT, SchlottererC (2014) Inference of chromosomal inversion dynamics from Pool-Seq data in natural and laboratory populations of Drosophila melanogaster. Molecular Ecology 23 : 1813–1827.
47. Corbett-DetigRB, HartlDL (2012) Population genomics of inversion polymorphisms in Drosophila melanogaster. PLoS Genet 8: e1003056.
48. GibertP, MoreteauB, PetavyG, KaranD, DavidJR (2001) Chill-coma tolerance, a major climatic adaptation among Drosophila species. Evolution 55 : 1063–1068.
49. KaranD, DahiyaN, MunjalAK, GibertP, MoreteauB, et al. (1998) Desiccation and starvation tolerance of adult Drosophila: Opposite latitudinal clines in natural populations of three different species. Evolution 52 : 825–831.
50. KennyMC, WiltonA, BallardJWO (2008) Seasonal trade-off between starvation resistance and cold resistance in temperate wild-caught Drosophila simulans. Australian Journal of Entomology 47 : 20–23.
51. ParkashR, SharmaV, KalraB (2009) Impact of body melanisation on desiccation resistance in montane populations of D. melanogaster: Analysis of seasonal variation. Journal of Insect Physiology 55 : 898–908.
52. SchmidtPS, PaabyAB, HeschelMS (2005) Genetic variance for diapause expression and associated life histories in Drosophila melanogaster. Evolution 59 : 2616–2625.
53. SisodiaS, SinghBN (2010) Resistance to environmental stress in Drosophila ananassae: latitudinal variation and adaptation among populations. Journal of Evolutionary Biology 23 : 1979–1988.
54. HuangW, RichardsS, CarboneMA, ZhuDH, AnholtRRH, et al. (2012) Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proceedings of the National Academy of Sciences of the United States of America 109 : 15553–15559.
55. PoolJE, Corbett-DetigRB, SuginoRP, StevensKA, CardenoCM, et al. (2012) Population Genomics of Sub-Saharan Drosophila melanogaster: African Diversity and Non-African Admixture. PLoS Genet 8: e1003080.
56. KleinJ, SatoA, NaglS, O'hUiginC (1998) Molecular trans-species polymorphism. Annual Review of Ecology and Systematics 29 : 1–21.
57. LefflerEM, GaoZ, PfeiferS, SegurelL, AutonA, et al. (2013) Multiple instances of ancient balancing selection shared between humans and chimpanzees. Science 339 : 1578–1582.
58. WelbergenP, van DijkenFR, ScharlooW, KohlerW (1992) The genetic basis of sexual isolation between Drosophila melanogaster and D. simulans. Evolution 46 : 1385–1398.
59. SturtevantAH (1920) Genetic studies on Drosophila simulans. I. Introduction. Hybrids with Drosophila melanogaster. Genetics 5 : 488–500.
60. TamuraK, SubramanianS, KumarS (2004) Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol 21 : 36–44.
61. BandHT, IvesPT (1963) Genetic-Structure of Populations. 1. Nature of Genetic Load in South Amherst Population of Drosophila melanogaster. Evolution 17 : 198–215.
62. IvesPT, BandHT (1986) Continuing Studies on the South Amherst Drosophila melanogaster Natural Population during the 1970s and 1980s. Evolution 40 : 1289–1302.
63. MesserPW (2013) SLiM: Simulating Evolution with Selection and Linkage. Genetics 194 : 1037–1039.
64. ComeronJM, RatnappanR, BailinS (2012) The Many Landscapes of Recombination in Drosophila melanogaster. PLoS Genetics 8(10): e1002905.
65. SchmidtPS, CondeDR (2006) Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila melanogaster. Evolution 60 : 1602–1611.
66. IvesPT (1970) Futher genetic studies on the South Amherst population of Drosophila melanogaster. Evolution 24 : 507–518.
67. CoyneJA, MilsteadB (1987) Long-Distance Migration of Drosophila. 3. Dispersal of Drosophila melanogaster Alleles from a Maryland Orchard. American Naturalist 130 : 70–82.
68. UminaPA (2005) A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Nature 308 : 691–693.
69. BerryA, KreitmanM (1993) Molecular analysis of an allozyme cline: alcohol dehydrogenase in Drosophila melanogaster on the east coast of North America. Genetics 134 : 869–893.
70. KarasovT, MesserPW, PetrovDA (2010) Evidence that adaptation in Drosophila is not limited by mutation at single sites. PLoS Genet 6: e1000924.
71. MckenzieJA (1980) An Ecological Study of the Alcohol-Dehydrogenase (Adh) Polymorphism of Drosophila melanogaster. Australian Journal of Zoology 28 : 709–716.
72. McInnisDO, SchafferHE, MettlerLE (1982) Field Dispersal and Population Sizes of Native Drosophila from North-Carolina. American Naturalist 119 : 319–330.
73. Powell J (1997) Progress and prospects in evolutionary biology: The Drosophila Model. New York: Oxford University Press.
74. GravotE, HuetM, VeuilleM (2004) Effect of breeding structure on population genetic parameters in Drosophila. Genetics 166 : 779–788.
75. BastideH, BetancourtA, NolteV, ToblerR, StobeP, et al. (2013) A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in Drosophila melanogaster. PLoS Genetics 9: e1003534.
76. KirkpatrickM (2000) Reinforcement and divergence under assortative mating. Proceedings of the Royal Society B-Biological Sciences 267 : 1649–1655.
77. LewontinRC (1964) The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics 49 : 49–67.
78. GieselJT (1977) A model of functional epistasis and linkage disequilibrium in populations with overlapping generations. Genetics 86 : 679–686.
79. Lewontin RC (1965) Selection for colonizing ability. In: Baker HG, Stebbins GL, editors. The Genetics of Colonizing Species First International Union of Biological Sciences Symposia on General Biology, Aslimoar, Calif, 1964. New York: Academic Press. pp. 77–94.
80. SchmidtPS, PaabyAB (2008) Reproductive diapause and life-history clines in North American populations of Drosophila melanogaster. Evolution 62 : 1204–1215.
81. SchmidtPS, MatzkinL, IppolitoM, EanesWF (2005) Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution 59 : 1721–1732.
82. ArthurAL, WeeksAR, SgroCM (2008) Investigating latitudinal clines for life history and stress resistance traits in Drosophila simulans from eastern Australia. Journal of Evolutionary Biology 21 : 1470–1479.
83. JamesAC, AzevedoRB, PartridgeL (1997) Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics 146 : 881–890.
84. RobinsonSJ, ZwaanB, PartridgeL (2000) Starvation resistance and adult body composition in a latitudinal cline of Drosophila melanogaster. Evolution 54 : 1819–1824.
85. LazzaroBP, FloresHA, LoriganJG, YourthCP (2008) Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathogens 4: e1000025.
86. SinghRS, LongAD (1992) Geographic variation in Drosophila: From molecules to morphology and back. Trends Ecol Evol 7 : 340–345.
87. AyrinhacA, DebatV, GibertP, KisterAG, LegoutH, et al. (2004) Cold adaptation in geographical populations of Drosophila melanogaster: phenotypic plasticity is more important than genetic variability. Functional Ecology 18 : 700–706.
88. HoffmannAA, AndersonA, HallasR (2002) Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecology Letters 5 : 614–618.
89. OvergaardJ, KristensenTN, MitchellKA, HoffmannAA (2011) Thermal Tolerance in Widespread and Tropical Drosophila Species: Does Phenotypic Plasticity Increase with Latitude? American Naturalist 178: S80–S96.
90. HoffmannAA, HarshmanLG (1999) Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity 83 : 637–643.
91. GilchristGW, JeffersLM, WestB, FolkDG, SuessJ, et al. (2008) Clinal patterns of desiccation and starvation resistance in ancestral and invading populations of Drosophila subobscura. Evolutionary Applications 1 : 513–523.
92. AyrolesJF, CarboneMA, StoneEA, JordanKW, LymanRF, et al. (2009) Systems genetics of complex traits in Drosophila melanogaster. Nature Genetics 41 : 299–307.
93. Reynolds LA (2013) The effects of starvation selection on Drosophila melanogaster life history and development. PhD dissertation, UNLV.
94. DavidJR, CapyP (1988) Genetic-Variation of Drosophila melanogaster Natural-Populations. Trends in Genetics 4 : 106–111.
95. SezginE, DuvernellDD, MatzkinLM, DuanY, ZhuCT, et al. (2004) Single-locus latitudinal clines and their relationship to temperate adaptation in metabolic genes and derived alleles in Drosophila melanogaster. Genetics 168 : 923–931.
96. LangleyCH, StevensK, CardenoC, LeeYC, SchriderDR, et al. (2012) Genomic variation in natural populations of Drosophila melanogaster. Genetics 192 : 533–598.
97. EllnerS, HairstonN (1994) Role of overlapping generations in maintaining genetic variation in a fluctuating environment. The American Naturalist 143 : 403–417.
98. Garcia-DoradoA (1986) The effect of niche preference on polymorphism protection in a hetergeneous environment. Evolution 40 : 936–945.
99. Hedrick PW (1990) Theoretical analysis of habitat selection and the maintenance of genetic variation. In: Barker JSF, Starmer WT, MacIntyre RJ, editors. Ecological and Evolutionary Genetics of Drosophila. US: Plenum Press. pp. 209–227.
100. BerglandAO, ChaeH-s, KimY-J, TatarM (2012) Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin. PLoS Genet 8: e1002631.
101. McKechnieSW, GeerBW (1993) Micro-evolution in a wine cellar: an historical perspecitve. Genetica 90 : 201–215.
102. RashkovetskyE, IliadiK, MichalakP, LupuA, NevoE, FederME, KorolA (2006) Adaptive differentiation of thermotolerance in Drosophila along a microclimatic gradient. Heredity 96 : 353–359.
103. TurelliM, CoyneJA, ProutT (1984) Resource choice in orchard populations of Drosophila. Biol J Linn Soc 22 : 95–106.
104. Hoffmann A, O'Donnell S (1990) Heritable variation in resource use in Drosophila in the field. In: Barker JSF, Starmer WT, MacIntyre RJ, editors. Ecological and Evolutionary Genetics of Drosophila. US: Plenum Press. pp. 177–193.
105. WallaceB (1966) Distance and the allelism of lethals in a tropical population of Drosophila melanogaster. The American Naturalist 100 : 565–578.
106. OshimaC (1970) Frequencies of intra - and interpopulation allelism of lethals in the several natural populations and the relationship between the dispersal of flies and the frequency of allelism of lethals in natural populations. Drosophila Information Service 45 : 167–168.
107. Capy P, Gibert P, Boussy I (1992) Drosophila melanogaster, Drosophila simulans: so similar, so different. USA: Kluwer Academic Publishers. 292 p.
108. Ginzburg L, Colyvan M (2003) Ecological orbits: How planets move and populations grow. New York: Oxford University Press.
109. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
110. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
111. McKennaA, HannaM, BanksE, SivachenkoA, CibulskisK, et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20 : 1297–1303.
112. BansalV (2010) A statistical method for the detection of variants from next-generation resequencing of DNA pools. Bioinformatics 26: i318–324.
113. CingolaniP, PlattsA, Wang leL, CoonM, NguyenT, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6 : 80–92.
114. Nei M (1987) Molecular Evolutionary Genetics. New York: Columbia University Press.
115. KolaczkowskiB, KernAD, HollowayAK, BegunDJ (2010) Genomic Differentiation Between Temperate and Tropical Australian Populations of Drosophila melanogaster. Genetics 187 : 245–260.
116. FederAF, PetrovDA, BerglandAO (2012) LDx: Estimation of Linkage Disequilibrium from High-Throughput Pooled Resequencing Data. PLoS One 7: e48588.
117. R Core Development Team (2009) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
118. Bates D, Maechler M, Bolker B (2011) lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42. http://CRAN.R-project.org/package=lme4
119. HøjsgaardS, HalekohU, YanJ (2006) The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software 15 : 1–11.
120. BenjaminiY, HochbergY (1995) Controlling the false discovery rate: a practical and powerful approach ot multiple testing. Journal of the Royal Statistical Society B 57 : 289–300.
121. Ewens WJ (2004) Mathematical Population Genetics. I. Theoretical introduction. Springer-Verlag, New York, NY.
122. Kleiber C, Zeileis A (2008) Applied econometrics with R. Springer-Verlag, New York, NY.
123. BegunDJ, AquadroCF (1992) Levels of naturally occurring DNA polymorphism correlate with recombinatin rates in D. melanogaster. Nature 356 : 519–520.
124. BegunDJ, HollowayAK, StevensK, HillierLW, PohYP, et al. (2007) Population genomics: whole-genome analysis of polymorphism and divergence in Drosophila simulans. PLoS Biol 5: e310.
125. HuTT, EisenMB, ThorntonKR, AndolfattoP (2013) A second-generation assembly of the Drosophila simulans genome provides new insights into patterns of lineage-specific divergence. Genome Research 23 : 89–98.
126. HarrisRS (2007) Improved pairwise alignment of genomic DNA: The Pennsylvania State University.
127. KentWJ, SugnetCW, FureyTS, RoskinKM, PringleTH, et al. (2002) The human genome browser at UCSC. Genome Research 12 : 996–1006.
128. ParadisE, ClaudeJ, StrimmerK (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20 : 289–290.
Štítky
Genetika Reprodukčná medicína
Článek The COP9 Signalosome Converts Temporal Hormone Signaling to Spatial Restriction on Neural CompetenceČlánek Coordinate Regulation of Stem Cell Competition by Slit-Robo and JAK-STAT Signaling in the TestisČlánek The CSN/COP9 Signalosome Regulates Synaptonemal Complex Assembly during Meiotic Prophase I ofČlánek GPA: A Statistical Approach to Prioritizing GWAS Results by Integrating Pleiotropy and AnnotationČlánek Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant BiomassČlánek Heat-Induced Release of Epigenetic Silencing Reveals the Concealed Role of an Imprinted Plant GeneČlánek p53- and ERK7-Dependent Ribosome Surveillance Response Regulates Insulin-Like Peptide SecretionČlánek The Complex I Subunit Selectively Rescues Mutants through a Mechanism Independent of MitophagyČlánek Rad59-Facilitated Acquisition of Y′ Elements by Short Telomeres Delays the Onset of Senescence
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 11- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Establishing a Multidisciplinary Context for Modeling 3D Facial Shape from DNA
- RNA Processing Factors Swd2.2 and Sen1 Antagonize RNA Pol III-Dependent Transcription and the Localization of Condensin at Pol III Genes
- Inversion of the Chromosomal Region between Two Mating Type Loci Switches the Mating Type in
- A Thermolabile Aldolase A Mutant Causes Fever-Induced Recurrent Rhabdomyolysis without Hemolytic Anemia
- The Role of Regulatory Evolution in Maize Domestication
- Stress Granule-Defective Mutants Deregulate Stress Responsive Transcripts
- 24-Hour Rhythms of DNA Methylation and Their Relation with Rhythms of RNA Expression in the Human Dorsolateral Prefrontal Cortex
- Pseudoautosomal Region 1 Length Polymorphism in the Human Population
- Fungal Communication Requires the MAK-2 Pathway Elements STE-20 and RAS-2, the NRC-1 Adapter STE-50 and the MAP Kinase Scaffold HAM-5
- The COP9 Signalosome Converts Temporal Hormone Signaling to Spatial Restriction on Neural Competence
- The Protein -glucosyltransferase Rumi Modifies Eyes Shut to Promote Rhabdomere Separation in
- The Talin Head Domain Reinforces Integrin-Mediated Adhesion by Promoting Adhesion Complex Stability and Clustering
- Quantitative Genetics of CTCF Binding Reveal Local Sequence Effects and Different Modes of X-Chromosome Association
- Coordinate Regulation of Stem Cell Competition by Slit-Robo and JAK-STAT Signaling in the Testis
- Genetic Analysis of a Novel Tubulin Mutation That Redirects Synaptic Vesicle Targeting and Causes Neurite Degeneration in
- A Systems Genetics Approach Identifies , , and as Novel Aggressive Prostate Cancer Susceptibility Genes
- Three RNA Binding Proteins Form a Complex to Promote Differentiation of Germline Stem Cell Lineage in
- Approximation to the Distribution of Fitness Effects across Functional Categories in Human Segregating Polymorphisms
- The CSN/COP9 Signalosome Regulates Synaptonemal Complex Assembly during Meiotic Prophase I of
- SAS-1 Is a C2 Domain Protein Critical for Centriole Integrity in
- An RNA-Seq Screen of the Antenna Identifies a Transporter Necessary for Ammonia Detection
- GPA: A Statistical Approach to Prioritizing GWAS Results by Integrating Pleiotropy and Annotation
- Let's Face It—Complex Traits Are Just Not That Simple
- Glutamate Receptor Gene , Coffee, and Parkinson Disease
- The Red Queen Model of Recombination Hotspots Evolution in the Light of Archaic and Modern Human Genomes
- The Ethics of Our Inquiry: An Interview with Hank Greely
- Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant Biomass
- Regularized Machine Learning in the Genetic Prediction of Complex Traits
- Phylogenetically Driven Sequencing of Extremely Halophilic Archaea Reveals Strategies for Static and Dynamic Osmo-response
- Lack of Replication of the -by-Coffee Interaction in Parkinson Disease
- Natural Polymorphisms in Human APOBEC3H and HIV-1 Vif Combine in Primary T Lymphocytes to Affect Viral G-to-A Mutation Levels and Infectivity
- A Germline Polymorphism of Thymine DNA Glycosylase Induces Genomic Instability and Cellular Transformation
- Heat-Induced Release of Epigenetic Silencing Reveals the Concealed Role of an Imprinted Plant Gene
- ATPase-Independent Type-III Protein Secretion in
- p53- and ERK7-Dependent Ribosome Surveillance Response Regulates Insulin-Like Peptide Secretion
- The Complex I Subunit Selectively Rescues Mutants through a Mechanism Independent of Mitophagy
- Evolution of DNA Methylation Patterns in the Brassicaceae is Driven by Differences in Genome Organization
- Regulation of mRNA Abundance by Polypyrimidine Tract-Binding Protein-Controlled Alternate 5′ Splice Site Choice
- Systematic Comparison of the Effects of Alpha-synuclein Mutations on Its Oligomerization and Aggregation
- Rad59-Facilitated Acquisition of Y′ Elements by Short Telomeres Delays the Onset of Senescence
- A Functional Portrait of Med7 and the Mediator Complex in
- Systematic Analysis of the Role of RNA-Binding Proteins in the Regulation of RNA Stability
- ARTIST: High-Resolution Genome-Wide Assessment of Fitness Using Transposon-Insertion Sequencing
- Genomic Evidence of Rapid and Stable Adaptive Oscillations over Seasonal Time Scales in Drosophila
- Genome-Wide Associations between Genetic and Epigenetic Variation Influence mRNA Expression and Insulin Secretion in Human Pancreatic Islets
- HAM-5 Functions As a MAP Kinase Scaffold during Cell Fusion in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- An RNA-Seq Screen of the Antenna Identifies a Transporter Necessary for Ammonia Detection
- Systematic Comparison of the Effects of Alpha-synuclein Mutations on Its Oligomerization and Aggregation
- Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant Biomass
- Regularized Machine Learning in the Genetic Prediction of Complex Traits
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy

















