-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Phylogenetically Driven Sequencing of Extremely Halophilic Archaea Reveals Strategies for Static and Dynamic Osmo-response
The ability to adjust to changing osmotic conditions (osmoadaptation) is crucial to the survival of organisms across the tree of life. However, significant gaps still exist in our understanding of this important phenomenon. To help fill some of these gaps, we have produced high-quality draft genomes for 59 osmoadaptation “experts” (extreme halophiles of the euryarchaeal family Halobacteriaceae). We describe the dispersal of osmoadaptive protein families across the haloarchaeal evolutionary tree. We use this data to suggest a generalized model for haloarchaeal ion transport in response to changing osmotic conditions, including proposed new mechanisms for magnesium and chloride accumulation. We describe the evolutionary expansion and differentiation of haloarchaeal general transcription factor families and discuss their potential for enabling rapid adaptation to environmental fluxes. Lastly, we challenge a recent high-profile proposal regarding the evolutionary origins of the haloarchaea by showing that inclusion of additional genomes significantly reduces support for a proposed large-scale horizontal gene transfer into the ancestral haloarchaeon from the bacterial domain. This result highlights the power of our dataset for making evolutionary inferences, a feature which will make it useful to the broader evolutionary community. We distribute our genomic dataset through a user-friendly graphical interface.
Published in the journal: Phylogenetically Driven Sequencing of Extremely Halophilic Archaea Reveals Strategies for Static and Dynamic Osmo-response. PLoS Genet 10(11): e32767. doi:10.1371/journal.pgen.1004784
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004784Summary
The ability to adjust to changing osmotic conditions (osmoadaptation) is crucial to the survival of organisms across the tree of life. However, significant gaps still exist in our understanding of this important phenomenon. To help fill some of these gaps, we have produced high-quality draft genomes for 59 osmoadaptation “experts” (extreme halophiles of the euryarchaeal family Halobacteriaceae). We describe the dispersal of osmoadaptive protein families across the haloarchaeal evolutionary tree. We use this data to suggest a generalized model for haloarchaeal ion transport in response to changing osmotic conditions, including proposed new mechanisms for magnesium and chloride accumulation. We describe the evolutionary expansion and differentiation of haloarchaeal general transcription factor families and discuss their potential for enabling rapid adaptation to environmental fluxes. Lastly, we challenge a recent high-profile proposal regarding the evolutionary origins of the haloarchaea by showing that inclusion of additional genomes significantly reduces support for a proposed large-scale horizontal gene transfer into the ancestral haloarchaeon from the bacterial domain. This result highlights the power of our dataset for making evolutionary inferences, a feature which will make it useful to the broader evolutionary community. We distribute our genomic dataset through a user-friendly graphical interface.
Introduction
Organisms across the tree of life routinely experience changes in osmotic conditions. The ability to adjust physiological responses to these osmotic fluxes plays a role in processes ranging from desiccation tolerance and virulence of pathogenic bacteria [1], [2], to drought resistance in food crops [3], to mammalian reproduction [4]. In humans, osmotic response is essential for proper functioning of the heart [5], kidneys [6] and nervous system [7], and defects in osmo-response are implicated in a variety of chronic disorders [8]. Although there exists a large body of work on osmoadaptation, there remain a number of gaps in our knowledge. For example, how are different osmoadaptation strategies dispersed across phylogenetic space? Does there exist a strict delimitation between obligate halophiles and halotolerant organisms, or do these designations obscure a more nuanced biological reality? How do organisms with a wide range of salinity tolerances regulate the large physiological changes required to rapidly adapt to fluctuations in environmental salinity? Is there a fitness trade-off between static adaptation to constant level of high-salinity and the ability to adapt to changing salinity levels? How did the halophilic phenotype arise in evolutionary history? Here we use comparative genomics of a large number of extreme halophiles to begin to fill in some of the gaps in our current understanding of osmoadaptation.
The haloarchaea (a family of microorganisms belonging to the domain Archaea) have mastered the art of osmoadaptation. Members of this family thrive in extremely saline environments (up to NaCl saturation), and must constantly adapt to large shifts in salinity due to rainfall and evaporation. Although united by their ability to live in hypersaline environments (salinity greater than that of ocean water), the haloarchaea exhibit a diverse set of metabolic capabilities and span a broad range of environmental phenotypes [9], [10], including psychrotolerance (growth below 10°C), thermotolerance (growth above 45°C), and alkaliphilicity (maximum growth in basic environments). This diversity, along with the presence of well-developed genetic and biochemical toolkits [11], [12], makes this clade an excellent target of study for expanding our understanding of osmo-response.
To investigate the genetic potential for osmo-response in this clade, we have sequenced high-quality draft genomes for 59 species of haloarchaea isolated from 20 countries across six continents and environments ranging from fermented fish sauce to Permian age salt deposits (see Table S1). Combined with 21 previously sequenced haloarchaeal genomes, this dataset provides a rich opportunity for insight into osmoadaptation. Here we present an analysis highlighting adaptations to high salt at the gene and protein levels as well as analysis of ion transport capabilities and transcriptional machinery likely to play a role in mediating responses to changing osmotic conditions.
This genomic dataset will also be of use to the broader genomic and archaeal research communities. Although the archaea play major roles in global element cycling and ecosystem stability, this domain has been understudied. Our sequencing project nearly quintuples the number of available genomes for the haloarchaea and increases by ∼30% the number of sequenced archaea. We demonstrate several utilities of this dataset for defining community structure in metagenomic studies, improving automated genome annotation, and inferring the timing of evolutionary events along the haloarchaeal tree.
To facilitate large-scale use of this dataset, we have made sequence and annotation information available through an SQL database as well as the NCBI genome repository (for accession numbers, see Table S2). We provide gene calls and annotations derived using two independent automated annotation pipelines - the Rapid Annotation using Subsystems Technology (RAST) server [13] and NCBI's Prokaryotic Genome Annotation Pipeline (PGAAP) [14]. Using a combination of BLAST [15] and TRIBE-MCL [16], we have generated clusters of homologous proteins representing distinct protein families. We have made genome data and homology clusters for all 80 sequenced haloarchaea available through the genome context visualization tool JContextExplorer [17] (see Text S1 for access instructions). We believe that this genomic data will provide a rich source of information for the archaeal, genomics, evolutionary biology, and systems biology research communities for many years to come.
Results/Discussion
Sequencing, assembly and annotation
Fifty-nine haloarchaeal isolates from 17 genera were sequenced on Illumina GAII and HiSeq platforms using a combination of paired-end (85 nt reads), mate-pair (6 Kbp fragments), PCR-free paired-end, and unbarcoded “SOUP” libraries. SOUP libraries were prepared by pooling unbarcoded libraries for several species, which were phylogenetically distant enough to enable unambiguous read mapping. For library preparation details, see Materials and Methods; for information on methods used for each genome, see Table S2. Mean per base sequencing depth ranged from 13x to 188x, with a mean coverage of 90x (Figure 1). Sequence reads were assembled into contigs using the a5 pipeline [18], with a cross-species mean of 75 and median of 66 contigs. Genome assemblies ranged from 3.06 to 4.94 Mbp in size and all demonstrated high G+C content (mean of 62%) and high coding density (mean of 82%), as expected. Contig assemblies have been deposited in the NCBI genomic database along with annotations derived from the Prokaryotic Genome Annotation Pipeline (PGAAP) [14]. This annotation pipeline called between 2,945 and 4,645 putative protein coding regions, depending on the species.
Fig. 1. Sequencing depth of newly sequenced haloarchaeal genomes. 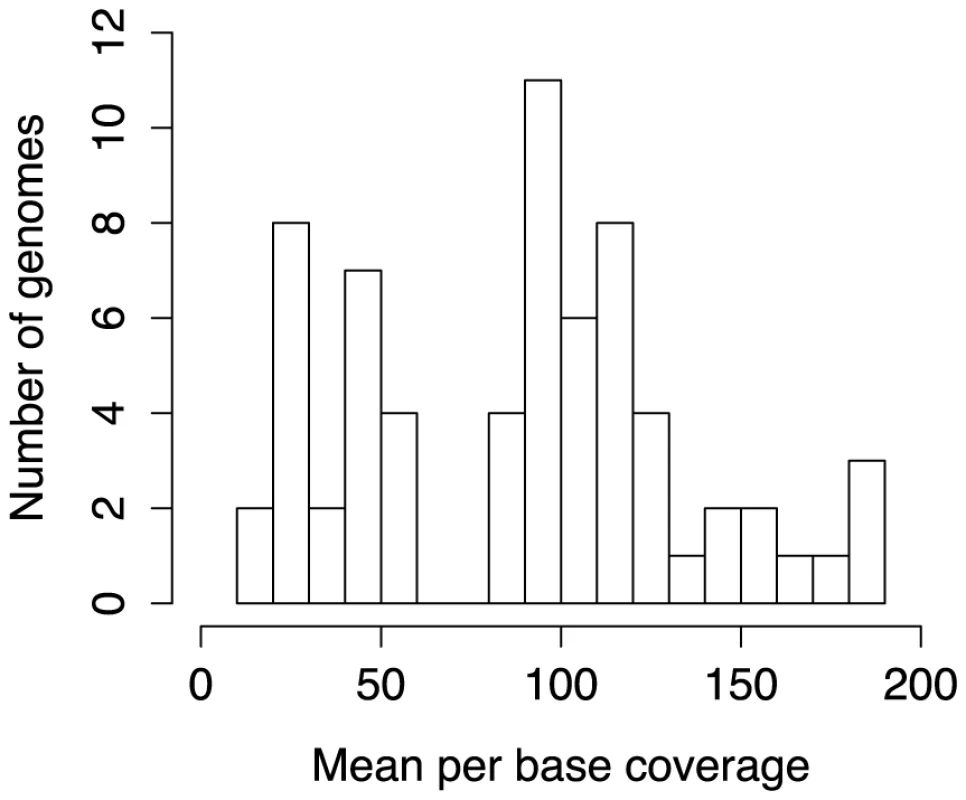
Histogram showing distribution of mean per base coverage for the 59 newly-sequenced and seven previously published draft genomes [109] included in this study. A large fraction of proteins (∼41%) were annotated as hypothetical or of unknown function, likely a consequence of low experimental coverage of the archaeal domain. As sequencing projects study organisms of increasingly distant relationship to experimentally characterized model organisms, our ability to accurately analogize functions based on homology to previously characterized proteins declines. As such, these 124,149 unannotated haloarchaeal proteins represent a rich set of potential experimental targets for uncovering mechanisms of salt adaptation and other aspects of archaeal biology. Progress in understanding these mechanisms will benefit from an experimental focus on highly-conserved haloarchaeal proteins, as these are most likely to be involved in physiological processes integral to haloarchaeal biology. A significant fraction of these proteins are widely distributed, with 44% present in at least 10 of the 23 haloarchaeal genera with sequenced members, and 34% present in at least half of the included 80 genomes. To facilitate informed selection of targets for experimental work, we provide the distribution of these proteins in Dataset S1. Incorporation of our dataset into existing curated databases and automated workflows will facilitate downstream extrapolation of functional information learned from experimental approaches.
Since the beginning of our study, genome data from independently conducted sequencing projects have been released for several species included in the present study. For a comparison of sequencing statistics for these independently sequenced genomes see Table S3.
An updated haloarchaeal phylogeny
Previous phylogenetic studies have described two major haloarchaeal clades and several smaller groups with poorly defined relationships to these clades [19]. Here we update this previous work based on a phylogeny constructed using a concatenated set of 40 highly conserved genes (Figure 2) [20]. We expand the previously defined clades as follows: we consider a species to belong to a clade if a member of that genus was previously assigned to that clade and the genus is not paraphyletic or polyphyletic, and we also include any species which group with that clade with at least 75% support. Using this process for determining clade relationship, the increased resolution in the multi-marker phylogeny allows us to assign Halovivax to Clade 1. On the basis of the same phylogeny, we also propose designation of a third haloarchaeal clade, including the Halobacterium, Natronomonas, Halorhabdus, Halosimplex, Halomicrobium, and Haloarcula genera.
Fig. 2. Updated haloarchaeal phylogeny. 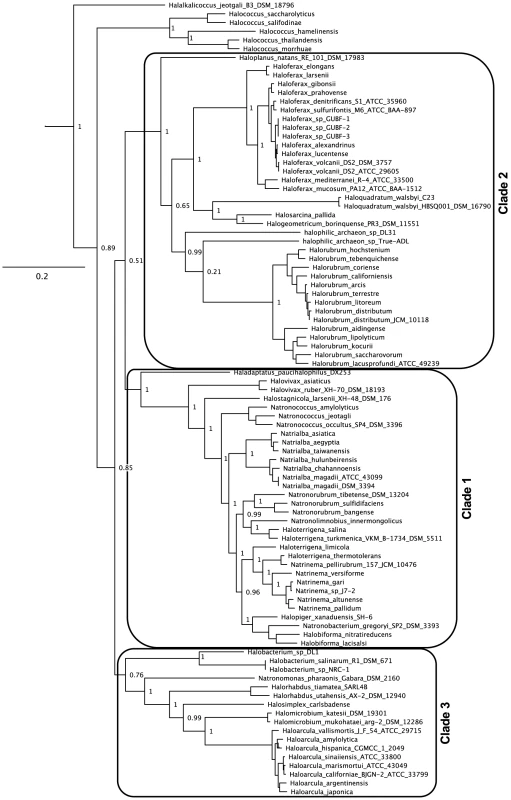
Multi-marker concatenated phylogeny of the 80 genomes included in this study and other haloarchaeal genomes gathered from IMG. The unrooted tree was built from a concatenated alignment of 40 PhyEco markers using PHYML. Branch support values for top-level branches have been removed for ease of visualization. The full tree file can be accessed through Dataset S19. Grey boxes represent haloarchaeal clades as described in [19], expanded as described in text. Tree roots in the Methanocella (not shown). Previous studies have commented on the poorly resolved relationship between the Haloterrigena and Natrinema genera, which were originally designated based on lipid composition and DNA-DNA hybridization patterns [21]. Although Tindall [21] suggests that difficulties in genera-level assignment of some Haloterrigena and Natrinema species are simply the result of experimental error (including faulty DNA-DNA hybridization data), our results suggests that these genera, as currently defined, are actually polyphyletic. Species within these genera should therefore be reassigned using modern phylogenetic metrics. The multi-marker phylogeny was also instrumental in resolving other apparent genera-level paraphylies and polyphylies. These include the Natronorubrum and Halobiforma genera, which appear to be non-monophyletic when only rpoB' DNA or protein sequence similarity is considered [22].
Breadth of the haloarchaeal pangenome
To estimate the fraction of haloarchaeal phylogenetic diversity represented by this set of 80 haloarchaea, we performed rarefaction analysis, plotting the number of unique protein families against the number of randomly drawn genomes (Figure 3). Accurately grouping proteins into families is a non-trivial problem that has sparked the development of a large number of protein clustering algorithms [23]. As there is no experimental data for the vast majority of haloarchaeal proteins, clustering must rely on computational sequence similarity metrics. We therefore selected three methods to define protein families and generated rarefaction curves for each. The methods were as follows: (1) COG orthology groups [24], (2) in-house homology clusters defined using the clustering algorithm TRIBE-MCL [16] (see Materials and Methods, Dataset S2, Figures S1 & S2)), and (3) TRIBE-MCL defined homology clusters excluding those with only a single member (singletons).
Fig. 3. Rarefaction analysis of sampled haloarchaeal protein space. 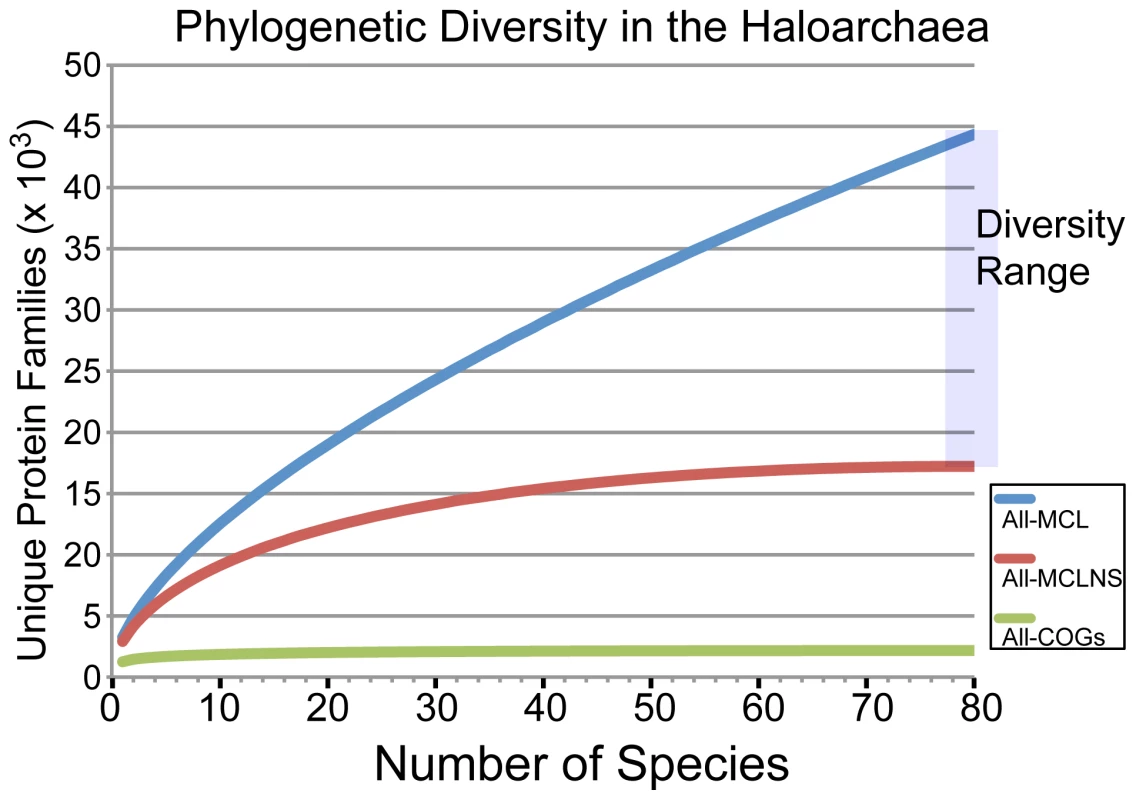
A rarefaction curve of protein diversity was created for the 80 haloarchaea included in this study using three alternative methods to define protein families: COG number (green), TRIBE-MCL clusters removing singletons (red), and TRIBE-MCL clusters without removing singletons (blue). The COG database is expected to represent an under-estimate of the true diversity. Similarly, using TRIBE-MCL clusters with all singleton genes excluded underestimates true diversity. The true diversity of the set is likely located between the blue and red curves. As the COG database is limited to proteins with putative orthologs in at least three major phylogenetic groups, and only two archaeal lineages are included in the genomic dataset used to build the COGs (Euryarchaeota and Crenarchaeota), protein families unique to the archaea are not present in this database. Thus, only protein families that existed prior to the eukaryotic-archaeal split or were subsequently exchanged by horizontal gene transfer between eukaryotes or bacteria and archaea are included. As such, the rarefaction curve of COG proteins in the haloarchaea saturates very quickly, with only 2,172 COGs being present in the haloarchaea, and only 17 genomes being required to discover 90% of these families.
In contrast, using the TRIBE-MCL derived homology clusters, which do not exclude proteins specific to the archaea, the number of unique protein families in the sequenced set of haloarchaeal genomes was 17,223. Forty-two genomes were required to discover the first 90% of these families, when singleton genes were excluded. Including singletons, however, it is apparent that much more haloarchaeal diversity remains to be discovered, as more than 300 new protein families are added with each new genome. Taking into account the likelihood that many singleton gene families are the result of spurious gene calls, the true sampled phylogenetic diversity of the haloarchaea lies between the singleton-excluded and the singleton-included cases. By revealing the diversity of the haloarchaeal pangenome, this analysis highlights the importance of deep sequencing for phylogenetically informed selection of experimental targets.
The haloarchaea did not originate via mass-acquisition of 1,000 bacterial genes by a methanogen
A recent paper analyzing 10 haloarchaeal genomes posited that the evolution of the haloarchaeal phenotype was the result of a single mass horizontal transfer of ∼1,000 bacterial genes into an ancestral archaeal methanogen [25]. This conclusion was based on 1) the finding that a large number of gene trees (1,089), built from a set of protein families with both archaeal and bacterial members (1,479), placed haloarchaeal genes in a monophyletic group with bacterial rather than archaeal homologs and 2) diverse phylogenetic evidence supporting the Methanomicrobia as sister group to the Haloarchaea [26]–[28] (see also Dataset S3). Further supporting this hypothesis, several of these transferred genes were associated with functions required for the proposed physiological transformation of an obligately anaerobic, autotrophic methanogen to a heterotrophic, facultatively aerobic haloarchaeon [25].
Accuracy of inferred evolutionary events is strongly influenced by the selection of representative species on which the inference is based. We tested whether the conclusions made by Nelson-Sathi, et al. [25] from an analysis of 10 haloarchaeal genomes were robust to a reanalysis against our more phylogenetically diverse dataset. In our analysis we also observed a large amount of horizontal gene transfer from the bacteria, however, we found that this exchange of genetic material was not limited to a single acquisition event at the haloarchaeal root, but rather occurred in many different transfer events throughout haloarchaeal evolution.
For each of the protein families in the original set reported by Nelson-Sathi, et al. [25], we added homologs from 65 additional haloarchaeal genomes and rebuilt gene trees using the same software tools and parameters (obtained via correspondence with the authors). More than two-thirds (67.2%) of the protein families originally designated as basal acquisitions no longer retained this characteristic after incorporating the additional haloarchaeal homologs (Table 1). Analysis of the re-computed gene trees revealed that, not only did most transfers not happen near the base of the haloarchaeal clade, but, for many protein families, multiple independent transfer events from bacteria to the haloarchaea have occurred. Depending on the gene, these additional transfers either predate (Figure S3) or follow (Figure S4) the acquisition discovered by Nelson-Sathi, et al. Both cases are inconsistent with a single, basal transfer scenario: rather, our results are consistent with previous findings that horizontal gene transfer is rampant among bacteria and archaea [29]. This interpretation is further supported by the fact that the putatively transferred genes do not appear to have been transferred from a common bacterial phylum, as indicated by the phylogenetic affiliation of the most closely related bacterial homolog for each protein family (see Table S2 in [25]).
Tab. 1. Number of inferred basal bacterial imports decreases with added genomes. 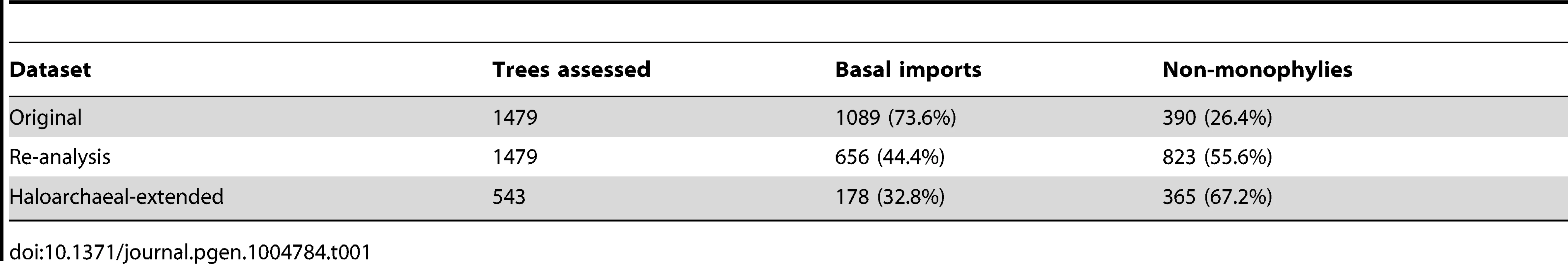
The simplest explanation for the difference in findings between the original study and our reanalysis is rooted in the nature of the two genomic datasets investigated. Due to the limited number of genomes available at the time, Nelson-Sathi, et al. worked under the assumption that the 10 haloarchaeal genomes they sampled reasonably represented haloarchaeal diversity. This lead to the identification of genes as bacterial transfers to the last common ancestor of the haloarchaea on the basis of as few as two haloarchaeal homologs. Based on the distribution of genomes used (two from Clade 1, two from Clade 2, and six from Clade 3, see Figure 2), a gene present in only two haloarchaea will often represent a transfer to a single clade, rather than to the haloarchaeal root. By contrast, our genomic dataset, representing a more even phylogenetic sampling of the haloarchaea, revealed multiple, clade specific transfer events indicative of a complicated history of gene transfer between the haloarchaea and bacteria. This analysis highlights the value of large, phylogenetically informed genomic data sets for increasing the accuracy with which we can make evolutionary inferences, and reveals the dangers in making wide-reaching evolutionary claims based on limited genomic data.
Insights into salt adaptation from the haloarchaeal core genome
Despite their metabolic and physiological diversity [9], [10], all members of the haloarchaeal clade share an obligately halophilic lifestyle. To understand the common mechanisms underlying this lifestyle, we investigated the haloarchaeal core genome. A total of 304 of the in-house defined protein families (see Materials and Methods) were present in all of the 80 investigated genomes (Table S4). Of these 304 core proteins, 55 (18.1%) were predicted to be involved in translation, transcription, or regulation thereof. In addition to the expected ribosomal proteins, RNA polymerase subunits, and known general transcription factors, the core genome included a number of predicted transcription factors whose functional importance in regulating haloarchaeal gene expression is underexplored. These include a ArsR-family transcription factor involved in alleviation of heavy metal toxicity, an AsnC-family member involved in feast/famine response, a CBS domain-containing protein of unknown function, and a PadR-family protein, possibly involved in regulation of phenolic acid metabolism [30]. As the level of functional specificity provided by domain-level matches is limited (for example, PadR-family proteins have also been shown to be involved in regulation of multidrug pumps [31]) the contributions of these conserved transcriptional regulators to haloarchaeal biology will need to be experimentally determined. However, their wide distribution across 23 haloarchaeal genera suggests that these proteins likely play important roles in regulating physiological responses to shifting environmental parameters routinely experienced in hypersaline environments, including changes in oxygen availability, salinity, and concentrations of heavy metals.
The core genome includes 10 protein families predicted to be involved in stress response, including both cold shock and heat shock members as well as stress response proteins with no predicted specific function. Previous studies of haloarchaea have shown upregulation of heat and cold shock genes in response to salinity changes, indicating that these chaperones may play a wider role in mediating stress responses than previously believed [32], [33]. As such, these 10 core haloarchaeal stress response proteins are candidates for experimental work to study the complex interplay among different stress response mechanisms.
DNA mismatch (MutSL), homologous recombination (RadAB) and base excision repair mechanisms are also universally conserved in this clade. Conspicuously lacking from the haloarchaeal core genome is a photolyase, responsible for correcting UV induced thymidine-dimers. Seven species were missing an annotated photolyase, all of which were Clade 1 haloarchaea (see Text S1 and Figure 2). If these genes are indeed absent – not hiding in unassembled regions of the genome - their absence would be surprising, given that many haloarchaeal species are routinely exposed to high levels of UV radiation due to evaporation of shallow hypersaline lagoons. Only two of these species (Natrialba taiwanensis and Natrialba aegyptia) encode genes annotated as belonging to the UVR system of UV damage repair, with each encoding only one of the five proteins in this repair system. Although haloarchaeal high G+C content has been proposed as an adaptation for avoiding UV-induced dimerization [34], potentially reducing the need for a photolyase repair system, the link between G+C content and UV damage in this clade is far from certain and alternate explanations for high G+C content have been proposed [35].
A number of transport-related protein families were also universally conserved, including several ABC transporters with peptides, amino acids and/or metals as predicted substrates. ABC transporters were extremely abundant, making up six of only eleven protein families with greater than 400 members. Although precautions were taken to filter out spurious domain-level matches (see Materials and Methods), each of these ABC transporter families may be composed of many members with divergent substrate specificities. Four protein families related to phosphate transport were also conserved, including two low-affinity phosphate transporters and two regulators of phosphate transport.
Not surprisingly, a number of proteins involved in biosynthesis of isoprenoid lipids were conserved across the haloarchaea. Isoprenoids are characteristic of haloarchaeal cell membranes [36], and are known to reduce membrane permeability to Na+ and Cl− ions [37], a necessary prerequisite for regulating ionic composition at high salinities. The committed step in isoprenoid synthesis may be upregulated under high salt conditions [38], further suggesting their importance to cell maintenance in hypersaline environments. However, as isoprenoids serve as precursors for a number of other compounds, alternative hypotheses must also be considered.
Fifty-five of the 304 conserved haloarchaeal proteins (∼20%) had either no assigned functional annotation or only a domain-level match to a previously characterized protein. Based on their high conservation, these proteins likely play important roles in the haloarchaeal biology, including adaptation to hypersaline environments. These 55 protein families, therefore, represent a manageable set of targets for exploring the genetic mechanisms of halophilicity.
A generalized model for haloarchaeal osmoadaptation and ion transport
Haloarchaea are generally considered “salt-in” strategists – actively accumulating potassium and chloride ions to prevent water efflux in hypersaline environments. In contrast, the “salt-out” strategy entails accumulation or synthesis of organic compatible solutes to increase internal osmolarity without increasing cytoplasmic salinity. Although recent work has shown that some haloarchaea may utilize compatible solutes in some situations [39]–[41], and many halotolerant organisms transiently accumulate moderate levels of intracellular K+ ions in the initial stage of osmoadaptation [42], [43], distinguishing between salt-in and salt-out strategists remains useful for differentiating between obligate and facultative halophiles.
Due to the dynamic nature of hypersaline environments, the haloarchaea possess a range of ion transporters for accommodating fluctuating salinity levels. We investigated the phylogenetic distribution of a number of ion transporter genes potentially involved in osmoadaptation to hyper-osmotic or hypo-osmotic shock, as well as compatible solute import and biosynthesis genes (Figure S5). This analysis enabled us to propose a generalized haloarchaeal strategy for dynamic osmoadaptation (Figure 4A).
Fig. 4. Haloarchaeal osmoadaptation. 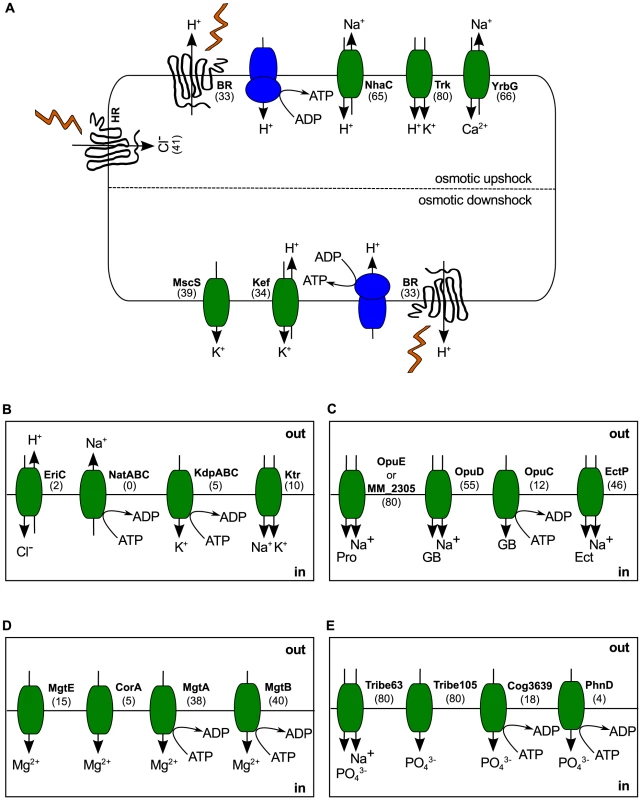
(A) Generalized model for osmoadaptation in the haloarchaea. During osmotic upshock, potassium is imported through Trk H+/K+ symporters and Na+ is expelled using a combination of NhaC H+/Na+ antiporters and/or YrbG Ca2+/Na+ antiporters. During osmotic downshock, excess potassium is removed through a combination of Kef H+/K+ antiporters and the non-specific mechanosensitive channel MscS. (B) Ion transport strategies not generally encoded by the haloarchaea. Sodium export and potassium import using ABC transporters (NatABC and KdpABC) may be less energetically efficient than secondary transport systems. Use of Ktr K+/Na+ symporters for potassium uptake would result in over-accumulation of sodium. (C) Compatible solute transport systems. Na+/proline symporters (OpuE and MM_2305) are ubiquitous in the haloarchaea. Glycine betaine uptake is mediated by OpuD through symport with sodium or, rarely, through the ABC transporter OpuC. The ectoine/sodium symporter EctP is also widespread. For transport systems with multiple substrates, a representative compound is shown. (D) Magnesium uptake is mediated by primary active transport (MgtA/B) or, rarely, by facilitated diffusion (MgtE/CorA). (E) Potassium accumulation is possible in all 80 haloarchaea via both secondary active transport (Tribe63) and facilitated diffusion (Tribe105). Some species also possess ATP-dependent potassium transporters (Cog3639/PhnD). Numbers in parenthesis represent the number of haloarchaeal species possessing the transporter gene. BR – bacteriorhodopsin, HR – halorhodopsin, GB – glycine betaine, Pro - proline, Ect – ectoine. Kef, Ktr and Trk each represent a class of transporters, rather than a single homolog. Tribe63 and Tribe105 refer to protein families defined in this study. During osmotic upshock, potassium import and sodium extrusion are mediated by secondary transport, using the proton gradient generated either by direct light-activated proton translocation through bacteriorhodopsin (BR) or through respiration. All 80 haloarchaea investigated possess a H+/K+ symporter of the Trk family for potassium uptake, while few (10 species) also possess the closely related Na+/K+ symporter of the Ktr family (Figure 4B). This observation is consistent with Corratgé-Faillie et al.'s. prediction that use of the Trk system may be evolutionarily advantageous in hypersaline environments by avoiding Na+ uptake [44]. Many haloarchaea (66 species) may power sodium extrusion through YrbG Na+/Ca2+ antiporters, although this strategy would be limited to environments with high calcium concentration. During osmotic downshock, it is vital for organisms using a salt-in strategy to rapidly rid the cytoplasm of excess salts to avoid hypertonic cell lysis. The haloarchaea have the genetic potential to export excess potassium ions through a combination of secondary transport with Kef-like H+/K+ antiporters and non-specific ion loss through the mechanosensitive channel MscS, which has been shown to play an important role in potassium efflux during osmotic downshock in E. coli [45].
In contrast to the prevalence of these predicted secondary transporters, only a small number of genomes encode ATP-dependent transporters for osmoadaptation (Figure 4B). Prominently lacking in most haloarchaeal genomes are the outwardly rectifying Na+ pump NatABC and the inwardly rectifying K+ pump KdpABC, both of which depend on ATP hydrolysis to power ion transport. Previous researchers have calculated a large difference in energetic cost between salt-in and salt-out strategies, with production of compatible solutes being more costly than establishment of ionic gradients [46]. We propose that, in situations where large amounts of material must be transported, even a small difference in energetic efficiency between secondary and primary transport would result in a bias towards secondary transport systems, which require fewer steps. If true, this would explain the observed bias of haloarchaeal genomes for secondary transport systems for K+ accumulation and Na+ extrusion. However, experimental work will be required to calculate the ion exchange stoichiometry of these transporters and to determine the relative efficiency of secondary versus primary transport in this system.
Haloarchaeal strategies for uptake of chloride are difficult to decipher from the genomic data, as metabolism of this important counterion is not well understood [47]. The most recent review of the topic states that chloride is imported through “cotransport with sodium ions and/or using the light-driven primary chloride pump halorhodopsin” [47]. However, although experimental work has suggested the presence of a light-independent chloride uptake system in Halobacterium sp. NRC-1 [48], neither the energy source for this system, nor a genetic mechanism for its implementation have been identified. As only 41 of the organisms analyzed here possess a halorhodopsin homolog, some alternate strategy for chloride import must exist. We screened for homologs to archaeal and bacterial chloride transport proteins, including a predicted (Na+/K+)/Cl− symporter from Methanosarcina acetivorans [49], a predicted bacterial cation chloride transporter from Aminomonas paucivorans, and EriC, a H+/Cl− antiporter [50] involved in acidic shock tolerance in Escherichia coli [51]. Of these, we discovered only two EriC homologs, belonging to the alkalitolerant Natrialba aegyptia [52] and the alkaliphilic Natrialba magadii [53]. Based on these observations we propose a possible role for EriC homologs in Cl− uptake in alkaliphilic environments where export of protons down their concentration gradient would enable accumulation of a high intracellular level of chloride ions.
Due to recent interest in haloarchaeal use of compatible solutes (as part of a broader osmoadaptation strategy which also includes potassium accumulation) [39]–[41], we analyzed the phylogenetic distribution of many genes involved in compatible solute transport and biosynthesis (Dataset S4). This analysis reveals prevalent uptake mechanisms for the compatible solutes glycine betaine, ectoine, and proline (Figure 4C), commonly used as osmoprotectants by facultative halophiles. Chemotaxis towards, and accumulation of the trimethylammonium compounds glycine betaine, carnitine, and choline, has been demonstrated in a model haloarchaeaon [39]. We find that, although putative homologs to the binding and transducer proteins for this system (CosB and CosT) are widely distributed, only 10 species possess both members (Figure S5). Due to high levels of sequence similarity between CosB and the trimethylammonium compound transporters OpuCC/OpuBC [39], we cannot confidently assert presence of a trimethylammonium compatible solute chemotaxis system in these species, and caution that experimental work must be done to validate these results. The abundance of compatible solute transporters within haloarchaeal genomes does not, however, necessarily indicate utilization of a salt-out strategy. During periods of decreased environmental salinity haloarchaea often coexist with halotolerant microorganisms, which are generally salt-out strategists. As evaporation increases salinity, these organisms lyse and the released compatible solutes may then be used as carbon and nitrogen sources by extreme halophiles [54]. Compatible solutes have also been shown to have thermoprotective effects in both mesophilic bacteria [55] and hyperthermophilic archaea [56]. Experimental evidence of compatible solute accumulation suggests that some haloarchaea may utilize a salt-out strategy under certain conditions [39]–[41].
Recently, biosynthesis of the compatible solute trehalose was found to be widespread in the haloarchaea [41]. We interrogated our genomes for genes associated with compatible solute biosynthesis, including the ProJH pathway for proline synthesis during osmotic upshock [57], the Ne-acetyl-β-lysine and cyclic 2,3-bisphosphoglycerate (cBPG) synthesis pathways [43], [58], and the BetAB/GbsBA pathways for oxidation of choline to glycine betaine [59], [60] (Figure S5). We found that, in contrast to the widespread mechanisms for compatible solute uptake, pathways for biosynthesis of these compatible solutes were rare. Complete pathways for osmotically regulated proline synthesis and Ne-acetyl-β-lysine production were absent in all 80 genomes. Although putative homologs to ProH were present in six species of Halorubrum, their function is unclear in the absence of ProJ, which catalyzes initial transformation of glutamate for proline biosynthesis [57]. One species (Natronorubrum tibetense) was found to encode an intact pathway for biosynthesis of cBPG, previously thought to be restricted to methanogens [58]. Only nine species were found to possess homologs for the three components required for glycine betaine synthesis from extracellular choline: 1) a choline transporter, 2) a choline dehydrogenase, and 3) a glycine betaine aldehyde dehydrogenase. In eight of these species, the preferred strategy appears to be choline uptake via OpuB, oxidation to glycine betaine aldehyde using GbsB, and final oxidation to glycine betaine via GbsA/BetB. The ninth species (Halococcus saccharolyticus) appears to utilize the flavin adenine dinucleotide-bound choline dehydrogenase BetA rather than the type III alcohol dehydrogenase GbsB [58] for initial choline oxidation.
We also investigated uptake mechanisms for the biologically important magnesium and phosphate ions (Figure 4D & E). For phosphate accumulation, all 80 sequenced haloarchaea encode two members of the PHO4 superfamily (PF01384), annotated as an anion permease (TRIBE-MCL cluster 105; Tribe105), and a sodium dependent phosphate transporter (Tribe63). This second annotation potentially enables active phosphate uptake at the expense of the sodium gradient in hypersaline environments. As discussed above in the context of potassium and sodium transport, ATP-dependent phosphate uptake appears not to be favored, with only 22 homologs of ABC-type phosphate transporters encoded in this genome set. The opposite was found to be the case with magnesium transport, with the ATP-dependent transporters MgtA and MgtB being far more common than the inwardly rectifying Mg2+ channels MgtE and CorA. However, with only 52 of 80 sequenced haloarchaea encoding at least one of these Mg2+ transport mechanisms, it is clear that alternate strategies for magnesium uptake remain to be discovered. As magnesium concentrations have been shown to play important roles in stabilizing halophilic enzymes [61], discovery of these alternative magnesium uptake strategies is vital to understanding the nature of halophilic proteins.
Our investigations into haloarchaeal ion transport reveal both a core set of highly conserved strategies (eg. Trk-based K+ uptake, Na+-mediated phosphate accumulation) as well as more sparsely distributed abilities (eg. alkaliphilic chloride import via EriC). We have observed that secondary transport seems to be preferred to ATP-dependent primary transport for maintenance of large ion gradients in hypersaline environments, and have identified important gaps in our understanding of chloride and magnesium accumulation. The generalized nature of our model of dynamic osmoadaptation in haloarchaea, based on a broad and deep sampling scheme, contrasts with the specificity of previous models, which were largely limited to single model systems widely spaced across the phylogenetic tree. By highlighting strategies conserved across the haloarchaea, we hope to help build a general understanding of osmoadaptation across the tree of life.
Proteome acidification and exceptions to the rule
In addition to the ability to rapidly adapt to changing environmental salinities, haloarchaeal adaptations to high salt also include intrinsic physiological features present under all environmental conditions. These adaptations include an acidified proteome and high genomic G+C content [35], [62], [63], discussed here and in the subsequent section, respectively. Proteome acidification may be beneficial for salt-in strategists by prevention of protein aggregation via the ability of acidic amino acid residues to reorganize protein-solvent interactions [61], [64], [65], although alternative explanations have been proposed [66]. As expected, all 59 organisms whose draft genome we report here have both high genomic G+C content (ranging from 59–69%) and a highly acidified proteome. Histograms of predicted isoelectric points of haloarchaeal proteomes revealed an asymmetric bimodal distribution, with a dramatically larger major mode around pH 4.5, a minor mode around pH 10.0, and a consistent overall shape across haloarchaeal species (Figure 5 and Data Dryad package [67]). Corroborating previous work, this major mode was shifted towards lower pI values compared to non-haloarchaea [68].
Fig. 5. Proteome-wide isoelectric point distributions. 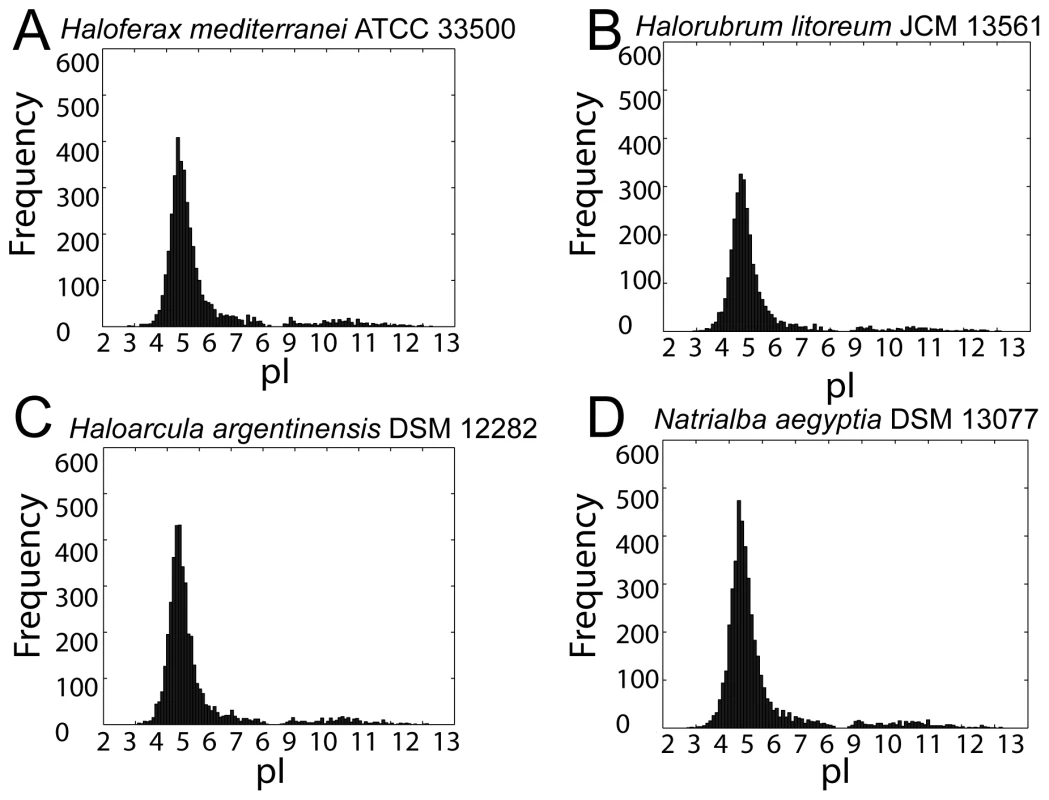
Histograms of computed pI values for (A) Haloferax mediterranei, (B) Halorubrum litoreum, (C) Haloarcula argentinensis, and (D) Natrialba aegyptia. Each of these four species is a representative from the four most populous genera in the set, which collectively contain 66% of the organisms in the study. For all sequenced haloarchaea, the pI histograms exhibited a bimodal distribution with a major mode at about 4.5 and much smaller minor mode around 10. For all isoelectric point plots see Data Dryad package [67]. Certain haloarchaeal proteins were not acidified, including many ribosomal subunits, membrane proteins, and DNA-binding proteins (Table S5). Potential reasons for non-acidification include 1) shielding from the hypersaline cytoplasm (eg. internal ribosome subunits, membrane proteins), and 2) presence of a selective force against acidification (eg. DNA-binding proteins). Structural mapping of the ribosome demonstrates that subunits exposed to the hypersaline cytosol tend to be acidified, while shielded internal subunits have high pI (Figure 6). Large regions of transporters and other membrane–associated proteins are likewise shielded from the saline cytoplasm by the cell membrane, mitigating selective pressure to acidify. DNA-binding proteins must retain positively charged residues to interact efficiently with negatively charged DNA, and so also tend not to acidify (shown for the general transcription factor TATA-binding protein (TBP), Figure S6, and ribosome elongation factor α-1, Figure S7). A large number (11,087) of non-acidified proteins were unannotated. Based on functional consistency we identified among other high pI proteins, we propose these proteins as candidates for exploratory research seeking novel DNA-binding or membrane associated proteins such as transcription factors, transporters, and chemotaxis/sensory receptors.
Fig. 6. Ribosomal subunit isoelectric points. 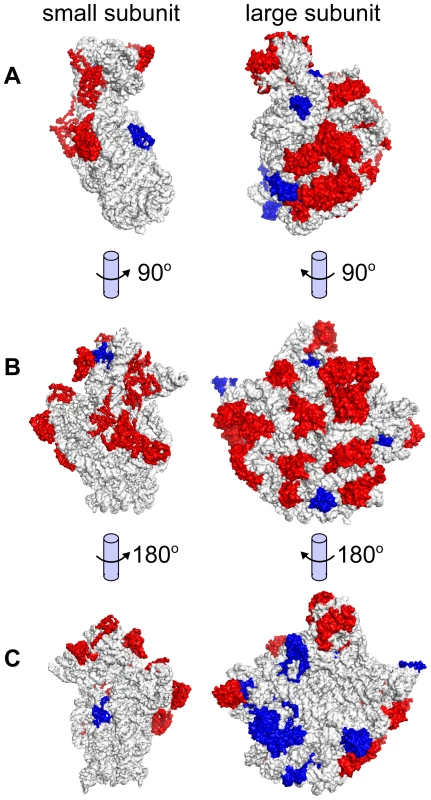
Structural mappings of the large and small ribosomal subunits, showing protein monomers with predicted low (red) and high (blue) pI. Subunits are oriented according to (A) complex formation, (B) cytoplasmic view of each subunit, and (C) internal view of each subunit. Subunits exposed to the cytoplasm tend to be acidified (red subunits, visible in B), while subunits buried within the ribosome tend to have an alkaline isoelectric point (blue subunits, visible in C). Structural models used were those for Thermus thermophilus (1FKA) [97] and Haloarcula marismortui (1QVG) [96]. Local variation in genomic G+C as a proxy for horizontal gene transfer events
In addition to their acidified proteomes, the highly G+C biased genomes of the haloarchaea are also predicted to be an adaptation for life in high salt. Although the mechanism for this adaptive benefit is unknown, several possibilities have been proposed, including decreased risk of thymine dimers resulting from high UV exposure in shallow brine pools [34], or selective pressure driven by A+T bias of insertion sequence elements [35]. Notably, the only known haloarchaeon lacking a G+C biased genome – Haloquadratum walsbyi (48%) – possesses a large number of photolyase genes, postulated to enable it to mitigate the effects of UV induced pyrimidine dimerization [69]. The Nanohaloarchaea, an uncultured clade of halophilic archaea proposed as a sister group to the Haloarchaea, also have low G+C content (43 and 56% for the two members of this clade with draft genomes), although they inhabit the same hypersaline environments as the high G+C Haloarchaea [70]. The evolutionary rationale behind this difference is unknown.
Regardless of the mechanism for its maintenance, genome-wide G+C bias offers a method for identification of candidates for horizontal gene transfer from organisms with G+C content differing from the recipient species, as horizontally transferred genes are often A+T shifted relative to the host genome [71]. We examined the G+C content of the 80 haloarchaeal genomes, using a sliding 100 bp window, and conducted change-point analysis to extract regions with local G+C content differing from the genome average. It is important to note here that haloarchaeal plasmids, including minichromosomes and megaplasmids, are known to have decreased G+C content compared with primary replicons (“chromosomes”) [63]. As the mechanisms for maintaining decreased G+C content in smaller replicons are unknown, and in order to accommodate draft genomes where the identity of the primary replicons are unknown, we have chosen to be replicon size neutral. Some regions of the genome are also expected to have low local G+C content due to selective pressure for maintaining a higher A+T percentage (eg. origin of replication sites). In addition, we have been neutral as to the direction of divergence from genome-wide G+C average, in order to allow detection of regions of unusually high as well as unusually low G+C (Figure 7, Figure S8, Datasets S6 & S7).
Fig. 7. Sliding-window G+C content analysis. 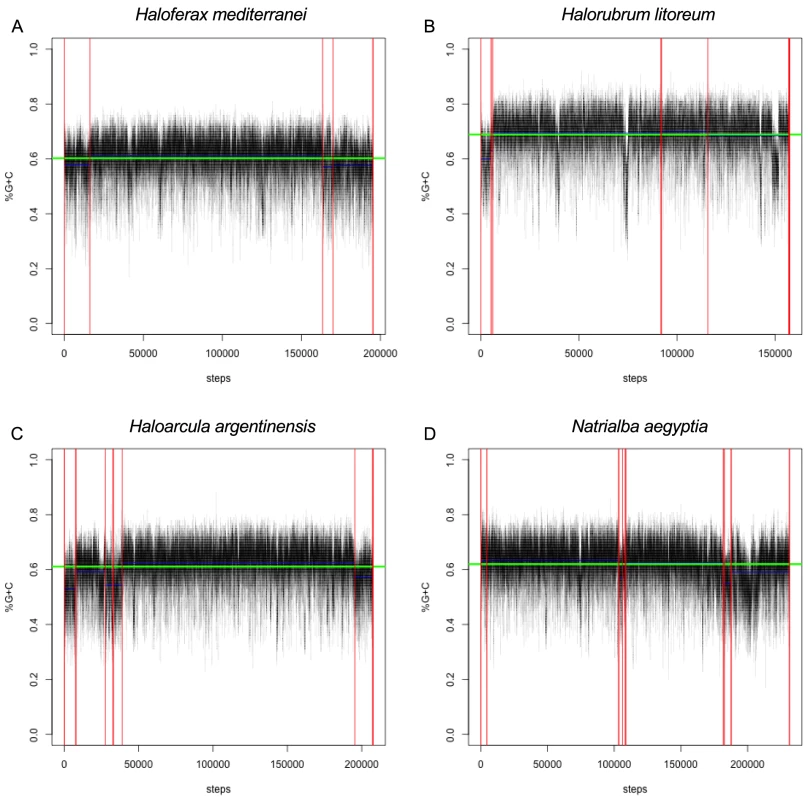
Representative G+C content plots for (A) Haloferax mediterranei, (B) Halorubrum litoreum, (C) Haloarcula argentinensis, and (D) Natrialba aegyptia. Each of these four species is a representative from the four most populous genera in the set, which collectively contain 66% of the organisms in the study. Black line represents calculated G+C percent for each 100 bp window. Contig boundaries are represented as vertical red lines, contig mean G+C as horizontal blue lines, and genome mean G+C as a horizontal green line. The horizontal axis displays the number of 20 bp steps taken along the genome. For all G+C plots see Data Dryad package [67]. We found these regions to be highly enriched in protein families involved in DNA metabolism and transcriptional regulation, transmembrane transport (and other membrane proteins), and horizontal transfer of genetic information. Specifically, of the seventy-nine functionally annotated protein families with at least five members which were enriched at least eight-fold in the divergent G+C regions, thirty-six (45%) were annotated with DNA/RNA-binding capabilities, eleven (14%) were associated with horizontal gene transfer mechanisms, and seven (9%) were associated with the cell membrane or cell surface (Table S6). These results are consistent with our investigations into non-acidified haloarchaeal proteins, in that both analyses identified nucleic acid binding and transmembrane proteins as potentially shielded from selective pressure to acidify and accumulate high G+C content. However, the specific proteins identified by these analyses were not identical and previous work has indicated that G+C bias and acidification are not correlated for individual proteins [35]. We speculate that many of the 138 unannotated protein families enriched in these regions of abnormal G+C content may be involved in DNA or RNA binding. We provide these regions in our Data Dryad package [67], as a rich source of data for identification of novel nucleic acid binding proteins and investigation of functionally important horizontal gene transfer events into the haloarchaea.
In addition to local variation in G+C content, we also investigated variation at the genus level. We found that, although some genera display little variability in genomic G+C content (eg. Halorubrum, Haloarcula), others exhibit a wide range (eg. Haloferax, Halococcus) (Figure 8C). This wide deviation in G+C content cannot be attributed to tolerance of a wide range of salinities, as the known NaCl tolerance range of Haloarcula and Halococcus species are very similar (3.2 M and 3.5 M respectively), as are those for Haloferax and Halorubrum species (4.1 M and 4.2 M respectively) [72]. Thus, the link between high G+C content and salinity tolerance in the haloarchaea appears to be more complex than previously appreciated.
Fig. 8. Genera-level comparisons of genomic features. 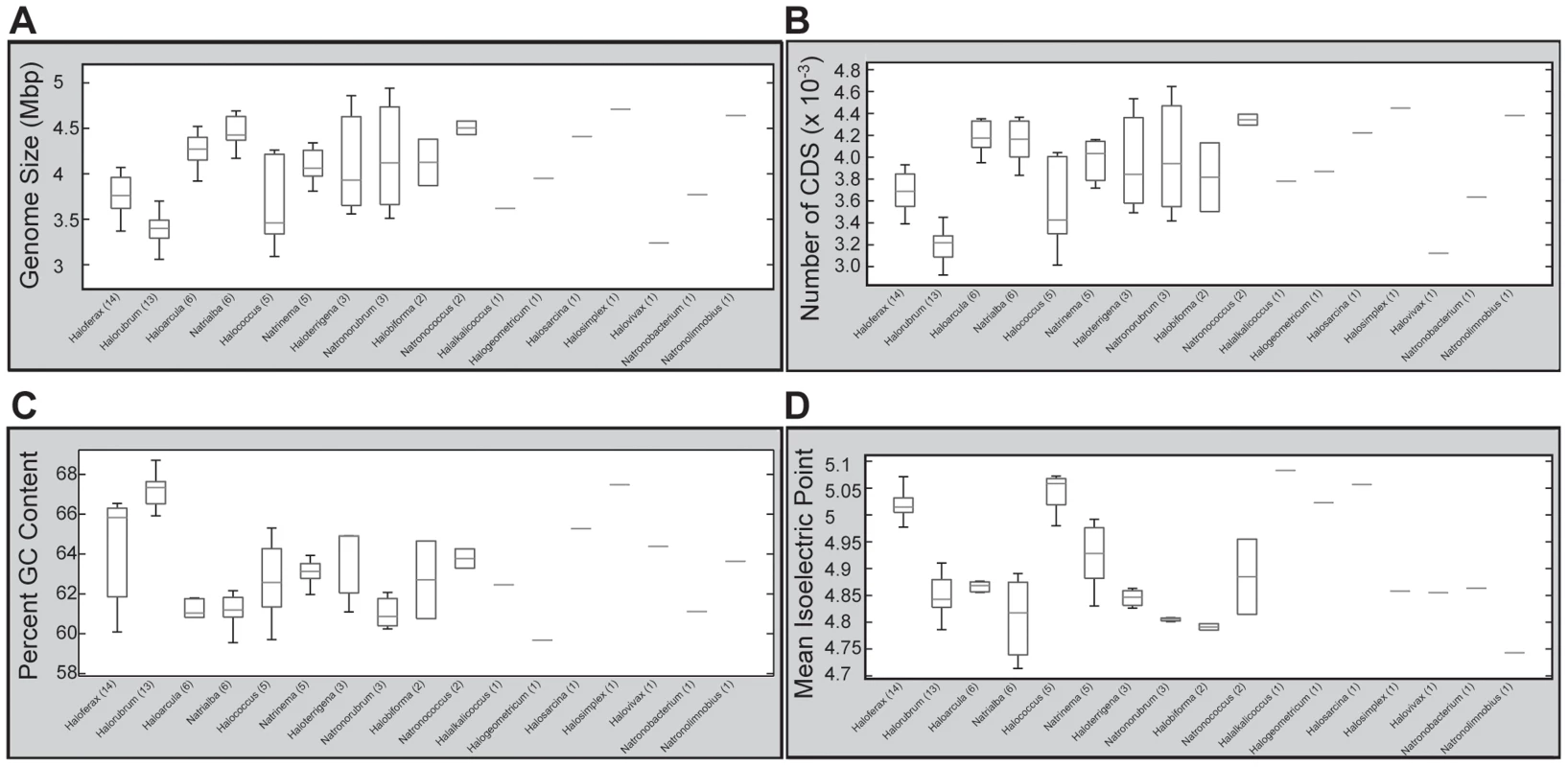
Assembled genome size (Mbp) (A), number of predicted protein coding sequences (B), %G+C (C), and mean protein isoelectric point (D) were extracted from each genome, organized by genus, and boxplots calculated using MATLAB's Statistics toolbox. Boxplots were generated using 25th and 75th percentile as box edges, with median demarcated with horizontal line within box. Genera are ordered by descending number of species sequenced, with the number of species shown in parentheses. Genera with only a single sequenced member are shown as horizontal lines. Multiple general transcription factors provide potential for rapid adaptation to environmental fluxes
Recent work has uncovered surprising roles for eukaryotic and archaeal general transcription factors in mediating differential gene regulation during cellular differentiation and environmental response [73]–[75]. In the haloarchaea, both the TATA-binding protein (TBP) and transcription factor B (TFB, known as transcription factor IIB in eukaryotes) families have undergone extensive expansion [75], [76]. TFB paralogs of Halobacterium sp. NRC-1 have been shown to differentially contribute to fitness under stresses commonly encountered in hypersaline environments, including variations in salinity and heavy metal concentration [75]. Multiple TBP and TFB paralogs may enable haloarchaeal species to quickly and efficiently modify transcriptional response to these environmental fluxes.
We examined the evolutionary history of haloarchaeal TBP and TFB homologs in order to understand their potential impact on environmental response. Phylogenetic distribution of paralog classes suggest that expansions of the TFB family are ancient, with several duplications occurring prior to haloarchaeal diversification (Figure 9, Dataset S5, Figure S9, Dataset S6). Homologs of five of the seven TFB paralogs from the model haloarchaeon Halobacterium sp. NRC-1 were present in at least 79 of 80 sequenced isolates, while another (tfbA) was present in 74 isolates. The remaining paralog, tfbE, was found in only 39 of the 80 genomes sequenced, suggesting either that this paralog emerged from a relatively late gene duplication event, or has been lost from a large number of genomes.
Fig. 9. Haloarchaeal transcription factor B phylogeny. 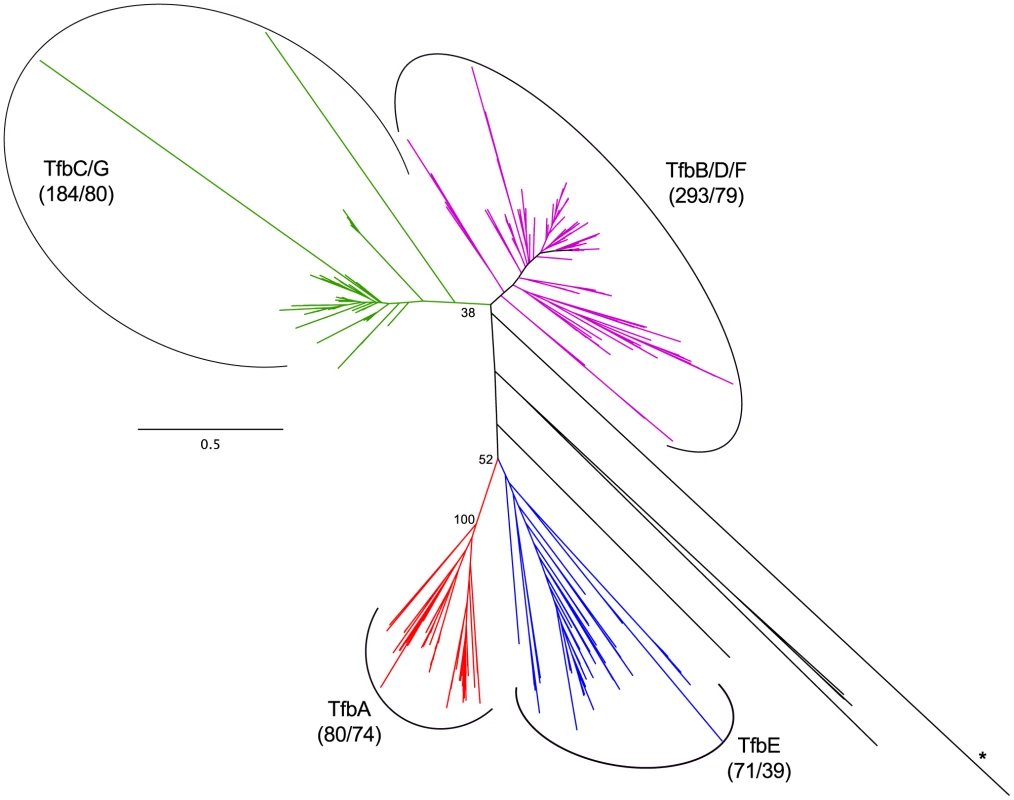
Unrooted phylogenetic tree of haloarchaeal transcription factor B (TFB) homologs. Green - TfbC/G, magenta - TfbB/D/F, blue - TfbE, red - TfbA, black - unassigned. Number of sequences and species represented in each clade are shown in parenthesis. Bootstrap support values over 30% are shown for major clades. Branch marked with asterisk is truncated. See Dataset S5 for tree file. Evolutionary expansion of the TBP family appears to be a more recent phenomenon, with three haloarchaeal lineages showing distinct patterns of duplication and divergence (Figure 10, Dataset S7, Figure S10, Dataset S8). Phylogenetic distribution of TBP paralogs suggests that the ancestral haloarchaeal TBP was most similar to tbpE of Hbt. sp. NRC-1, with only one species (Natrinema pallidum) apparently lacking this homolog. We speculate that this gene may be present at a contig boundary in the assembly for this organism (which consists of 116 contigs), and may later be uncovered by additional sequencing. The ancestral nature of the tbpE homolog is also supported by it being the only TBP in the natural TBP knockout strain Halobacterium salinarum PHH4 [77].
Fig. 10. Haloarchaeal TATA-binding protein phylogeny. 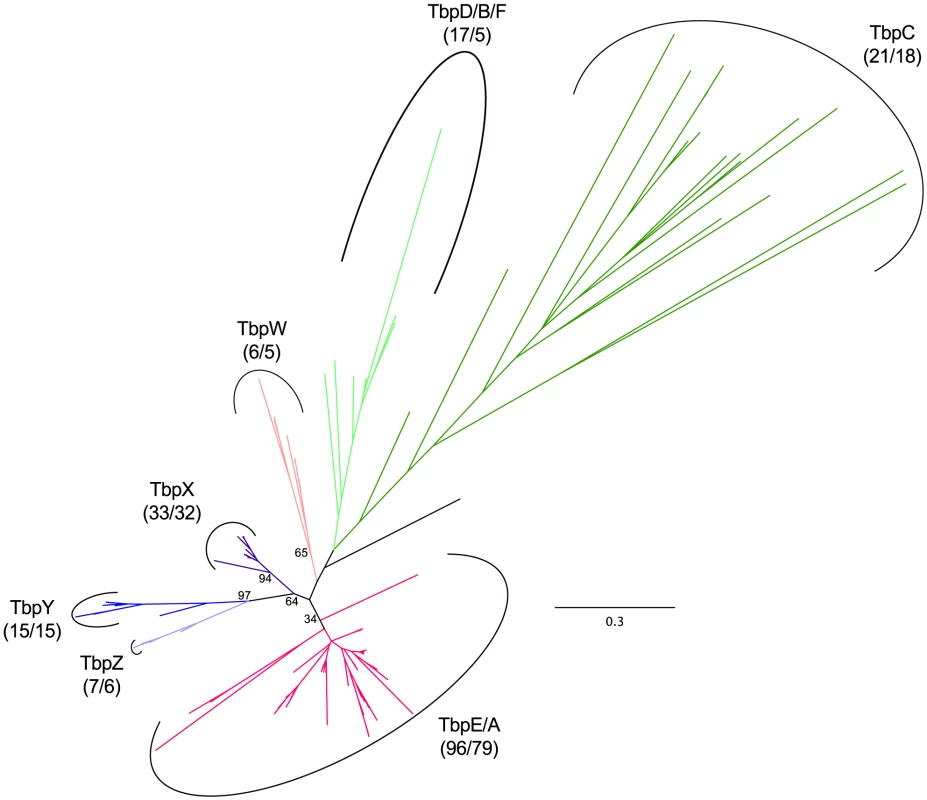
Unrooted phylogenetic tree of haloarchaeal TATA-binding protein (TBP) homologs. Dark green - TbpC, light green - TbpD/B/F, magenta - TbpE/A, salmon - TbpW, dark purple - TbpX, blue – TbpY, light purple – TbpZ, black - unassigned. Number of sequences and species represented in each clade is shown in parenthesis. Bootstrap support values over 30% are shown for major clades. See Dataset S7 for tree file. The previously recognized tbpD/B/F expansion appears to be limited to Halobacterium species and three haloalkalitolerant Clade 1 haloarchaea (Natrialba aegyptia, Natrialba taiwanensis, and Natrinema pellirubrum). In addition to this well-known expansion in the Halobacterium clade, we have uncovered two additional clade-specific diversifications. The smaller of these two expansions appears to be the result of a single gene duplication event (giving rise to tbpW) at the base of the Halococcus genus. An additional expansion has occurred near the base of the Clade 2 haloarchaea, with each species possessing at least one (tbpX) and in the Haloferax genus, up to three (tbpX, tbpY, and tbpZ) TBP homologs derived from this duplication event.
Finally, our analysis of haloarchaeal TBPs revealed a large number of tbpC-like homologs. For eukaryotes and archaea, TATA-binding protein normally consists of two domains derived from a duplication event. In each domain, DNA-binding is dependent upon a pair of intercalating phenylalanines [78]. In Halobacterium spp., the tbpC gene has lost the N-terminal phenylalanine pair, while retaining the C-terminal pair. The tbpC gene is easily knocked-out in Hbt. sp. NRC-1 [76] and was not detected at the transcriptional level under any growth condition tested in Hbt. salinarum PHH1 [77]. These data suggest that TbpC may be either nonfunctional or may play a very specialized role in transcriptional regulation under non-laboratory conditions. It is unclear whether defunctionalization may be a result of loss of DNA-binding ability by the N-terminal TBP domain or if loss of the intercalating phenylalanines may be part of an overall loss of function resulting from relaxed selective pressure. Our phylogenetic analysis grouped 21 TBP homologs from 18 species with Halobacterium spp. tbpC, of which 18 sequences were missing the N-terminal phenylalanine pair, one was missing a single phenylalanine from the N-terminal pair, and two possessed all four phenylalanine residues. Interestingly, sequences missing only the C-terminal phenylalanines, and several sequences missing the N-terminal pair, were not grouped with the tbpC homologs, suggesting formation of this clade is not merely an artifact of long-branch attraction. Collectively, this evidence suggests multiple losses of DNA-binding ability in either the N-terminal or C-terminal TBP domain (presumably, sequences having lost both pairs of DNA-intercalating phenylalanines have lost transcription factor function).
Specialization of haloarchaeal general transcription factor paralogs has been implicated in regulating response to a number of environmental perturbations, including variations in temperature, salinity, pH and concentration of heavy metals [75]. Understanding the complicated history of haloarchaeal TBP and TFB diversification will facilitate design of evolutionarily informed experiments for investigating the contribution of general transcription factor paralogs to fitness in the dynamic environments in which these species live.
Bioinformatic applications of this dataset
In addition to the applications we have already discussed (primarily focused on learning about osmoadaptation), our dataset has the power to address diverse problems in genomics, metagenomics, and other areas of bioinformatics. Here we illustrate some examples highlighting the diverse applicability of our dataset.
Genera-level metagenomic profiling of saline environments
The first step in understanding a complex community is often determining what phylogenetic groups are present. This task may be facilitated by use of molecular markers – genes unique to and universal within a specific clade (following the example of [20]). We have developed a set of molecular markers for three deeply sequenced haloarchaeal genera (the Haloarcula, Halorubrum and Haloferax), and provide a list of these protein families as well as the protein sequences, alignments and hidden Markov models for each proposed molecular marker in our Data Dryad package [67]. These markers may be integrated into metagenomics platforms such as Phylosift [79], for mining of metagenomic datasets from hypersaline environments. In addition, we provide a more extensive list of genera-specific protein families for 23 haloarchaeal genera (Dataset S9). This list includes any protein family for which members are found only within a specific genus, regardless of copy number or universality within the genus. Ranging from 1,456 unique protein families (Haloferax) to only two (Halopiger), this list provides a rich resource for exploring genera-specific haloarchaeal biology.
Improvements to automated gene calling algorithms
Automated genome annotation programs inevitably result in both spurious and missed gene calls [80]. The two annotation programs used in our analysis, NCBI's PGAAP [14] and the GLIMMER gene caller used in the RAST annotation pipeline [13] have been shown to exhibit this behavior [80]. As previously reported [80], we found that analyzing gene neighborhoods from closely related species can be helpful in detecting gene call errors. We identified an example of a highly conserved gene neighborhood where three of the fifteen sequenced Haloferax genomes had a gap in a location where a gene call was expected based on the other Haloferax genomes (Figure S11). Manual investigation revealed a possible protein-coding region for each of these three species, which aligned at the protein level with the twelve called genes (Figure S12). We were also able to identify, in the Haloarcula genus, regions with possible missing gene calls, erroneous start and stop sites, and a short hypothetical protein which may represent a spurious gene call (Figure S13). These examples highlight the potential for deep phylogenetic sampling in conjunction with gene neighborhood analysis, to aid automated gene calling programs. Genomic neighborhood analysis of closely related species has already been implemented in the genome annotation modification tool GenePrimp [80].
Improvements to automated gene annotation algorithms
Deep phylogenetic sampling also has the potential to improve gene annotation. By investigating patterns of protein family presence and absence across the haloarchaea, we were able to form testable hypotheses about the function of otherwise unannotated genes. We grouped protein families with similar phylogenetic distributions using hierarchical clustering. In cases where several protein families in a cluster were annotated as being involved in a particular process, unannotated members of that cluster were hypothesized to also be involved in this process. Two examples are discussed here, with additional examples in Dataset S10. First, a group of nine unannotated proteins were found to have similar phylogenetic distribution to six annotated redox proteins, including two menaquinol cytochrome-c reductases, two ferredoxins, a ubiquinol cytochrome-c reductase and a sulfite oxidase. Due to this redundancy, we speculate that this cluster represents more than one respiratory chain, one possibly using a sulfite as an electron donor (Figure S14). We hypothesize that several of the nine unannotated protein families in this cluster are also electron transport chain components. Secondly, using this method, we have identified two potentially novel members of the cobalamin biosynthesis pathway (Figure S15). A protein family cluster was identified in which 15 of 16 annotated members are involved in cobalamin biosynthesis. We therefore hypothesize that the two unannotated members of this cluster are also involved in this process. We present these cases as specific examples of the potential utility of phylogenetic profiling in improving automatically generated gene annotations. Additional examples can be found in Dataset S10 and Figures S16–S22.
In this section we have illustrated a few of the ways in which deep sequencing projects such as ours can benefit the genomics and metagenomics communities. These examples should not be taken as a complete set of problems to which our dataset may be applied, but rather as an indication of its broad utility and an invitation to the community to explore and utilize this data. To facilitate its widespread use, we have made the data available not only through the NCBI genome repository, but also through an SQL database and as a loadable ”popular genome set” within the visualization tool JContextExplorer [17]. We believe that lessons gleaned from explorations of this dataset will continue to enrich the genomics, halophile, archaeal, evolutionary and broader biology research communities for years to come.
Materials and Methods
Strain growth and DNA isolation
Strains were acquired as desiccated cells from the American Type Culture Collection (ATCC) in Manassas, Virginia, USA; the Leibniz Institute DSMZ German Collection of Microorganisms and Cell Cultures (DSM) in Braunschweig, Germany; and the Japan Collection of Microorganisms (JCM) in Ibaraki, Japan, as indicated in Table S2. Cells were rehydrated in recommended media according to culture collection center protocols and grown to stationary phase at 37°C in liquid culture. Genomic DNA was harvested with Wizard Genomic DNA purification kit (Promega).
Sequencing, assembly, and annotation
Sequence libraries were constructed using a combination of standard fragmentation (200–500 bp), mate-pair fragmentation (6 Kbp), and PCR-free transposon-mediated insertion of sequencing primers (Epicentre Nextera) [81]. Transposase was purified from E. coli BL21 (DE3) containing the cloning vector pWH1891. For mate-pair libraries, 6 Kb pair-end libraries were constructed and the terminal 50 bases of each end were sequenced, according to standard protocols. Additional libraries were constructed by combining DNA fragments from haloarchaeal species distantly enough related to enable unambiguous assignment of non-barcoded reads (“SOUP”). All sequencing was performed on Illumina HiSeq and GAII platforms. The paired-end information and trimming information were specified using annotation strings on the description line of the reads. Reads were assembled using the a5 pipeline [18]. Following assembly, genomic DNA contamination arising from transpose-mediated library preparation was removed by searching assembled reads against a local BLAST database consisting of E. coli BL21 (DE3) genomic DNA and the cloning vector pWH1891. BLAST hits with an E-value ≤ 10−20 were considered to be significant matches and candidates for contamination. Contigs with matches covering ≥80% of the contig length and contigs ≤1 Kbp with matches covering any portion of the contig were treated as contamination and discarded from further analysis. Long contigs for which only a small portion of the contig matched to the local BLAST database were also discarded if there were either no annotated features, or if the annotated features were E. coli genes. These criteria resulted in a total of 497 contigs equaling 265.57 Kbp being removed from the final assemblies.
A dual annotation pipeline was implemented in order to take advantage of the strengths of different existing automated annotation tools. Assembled genomes were first submitted to the Rapid Annotation using Subsystem Technology (RAST) server at the National Microbial Pathogen Data Resource. RAST-based gene calls and annotations were used for building of protein families, core genome and pan genome analyses, phylogenetic reconstruction, analysis of general transcription factor expansions, GC-bias analysis, building of molecular marker sets and phylogenetically informed re-annotation. These annotations can be accessed as a loadable “popular genome set” through the genome context viewer JContextExplorer [17] as well as a custom MySQL database (see Text S1 for instructions). The RAST annotation system was particularly useful in enabling comparison of our genomes with previously sequenced haloarchaea, by allowing standardization via rapid reannotation of existing genomes.
In addition, the newly sequenced genomes were annotated using NCBI's Prokaryotic Genome Annotation Pipeline (PGAAP) [14]. PGAAP gene calls and annotations were used for COG analysis, proteome acidification calculations, and genera-based genomic feature comparisons. These annotations can be accessed through the NCBI website using the accession numbers listed in Table S2 as well as through our custom MySQL database.
Phylogeny
A phylogeny was constructed for all archaeal genomes available through the Integrated Microbial Genomes database along with our sequenced haloarchaea using a concatenated set of 40 conserved marker genes [20]. Peptide sequences were downloaded for all archaeal genomes from the IMG 4.0 database on January 4, 2013. HMM profiles of 40 bacterial and archaeal PhyEco markers were searched against these peptide sequences. We excluded genomes with less than 35 of these markers, as well as duplicate genomes which were included in both our analysis and the IMG database. A total of 151 IMG archaeal genomes and all 80 haloarchaeal genomes were included in the phylogenetic tree building. For each PhyEco marker family, only single-copy members from the genomes were included. Independent alignments were built using MUSCLE [82] for each gene families and then concatenated. A phylogenetic tree was built from the concatenated alignment using PHYML 3.0 [83] with the LG substitution model. Tree topology and branch lengths were optimized by the program and aLRT SH-like statistics was used for branch support estimation. The R package ape was used to remove duplicate genomes [84] and the final tree was visualized using FigTree [85]. The tree files are available as Dataset S3 (all archaea) and Dataset S11 (haloarchaea only).
Protein families
Homologous protein families were constructed using a Markov clustering-based approach. First, an all-vs-all BLASTp search of all protein coding genes called by RAST in the 80 haloarchaeal genomes was conducted using an E-value cutoff of 10−5, soft masking, and the Smith-Waterman alignment algorithm. These options have been shown to improve homolog detection over other BLAST methods [86]. To enable detection of large homology groups, the number of returned matches was set at 100,000. To remove spurious, domain-level matches, BLAST results were post-filtered and matches retained only if bi-directional query-match coverage was ≥80%, and each sequence was ≥75% of the length of the other. Matches with E-values>10−10 were also excluded at this stage (Text S2).
BLAST results were then clustered into homology groups using the clustering algorithm TRIBE-MCL [16], which utilizes the protein-protein similarity network implicit in the BLAST scores. This method has the benefit of building inclusive homology families including orthologs, xenologs and both in - and out-paralogs, unlike reciprocal best BLAST methods which often overlook complex gene relationships in the search for one-to-one ortholog mapping. To determine an appropriate inflation parameter for TRIBE-MCL clustering, homology clusters derived from inflation parameters ranging from 1.4 to 8.0 were compared and benchmarked using manually curated protein families for haloarchaeal TATA-binding proteins and opsins. The inflation parameter I = 2.5 was selected, as it most closely recapitulated known ortholog/paralog relationships for the selected protein families. A total of 17,591 homology clusters (protein families) were obtained with this method, comprising 276,364 of 303,129 total haloarchaeal proteins. The remaining proteins were singletons, lacking significant sequence similarity to other sequences in the dataset. These sequences may represent species-specific innovation, recent gene influx from horizontal gene transfer, or simply a lack of sequencing depth along some sampled haloarchaeal lineages.
Rarefaction curves
Random subsets of haloarchaeal genomes (ranging from zero to eight genomes) were selected from the set of RAST-annotated haloarchaea, using the Java Samplers package [87]. For each selection the following quantities were tabulated: (1) the number of unique COGs, (2) the number of unique TRIBE-MCL determined homology clusters, and (3) the number of unique non-singleton TRIBE-MCL determined homology clusters. Ten thousand random selections were performed for each sample size and the average number of unique protein families obtained for each sample were plotted in Microsoft Excel (Figure 3).
Reanalysis of bacterial gene mass acquisition study
The 1,479 protein families used in the analysis described in [25] were obtained from the authors. All genes were renamed with an “E”, “A”, or “H” tag prepended to their original NCBI gene ID number, according to their taxonomic classification as, respectively, (eu)bacteria, archaea, or haloarchaea. Proteins from 65 haloarchaeal species not included in the original study were assembled into a set of “additional haloarchaeal proteins”, and named with an “H” tag and unique ID number. Five haloarchaeal genomes in our set were either different strains of a species included in the original study, or a re-sequencing of the same strain, and so were excluded from re-analysis. Gene trees were re-constructed for all 1,479 of the original protein families, using the alignment program MAFFT [88], with command line options –legacygappenalty, –anysymbol, and –quiet; the protein model determination tool ProtTest [89], using command line options –all-matrices, all-distributions, -S 2 –F –t1; and the gene tree creation tool PhyML [83]. All command line options were obtained from the authors of [25]). Gene trees were determined to be single transfers from the bacteria only if (1) all haloarchaeal homologs formed a monophyletic group and (2) this monophyletic group rooted with bacterial rather than archaeal homologs. For gene trees matching these criteria, corresponding protein families were extended to include homologs predicted from the set of “additional haloarchaeal proteins”, using BLAST with an E-value cutoff of 10−10 and a minimum percent identity of 30%. Gene trees were generated for these extended protein families using the parameters described above. Extended gene trees were re-evaluated according to the criteria described above to determine whether they could still be classified as single bacterial transfers. Some (17.2%) of the extended protein families contained a sufficiently large number of members such that determination of gene trees was infeasible, and so were excluded from re-analysis. Gene tree classification results are provided in Table 1. Phylogenetic trees visualized in Figures S4 and S3 were made using Phyfi [90].
Phylogenetic distribution of osmoadaptation genes
A list of 74 genes involved in ion transport and compatible solute transport or synthesis was compiled. Representative protein sequences for each of the selected genes were obtained by searching the NCBI protein database by annotation (Dataset S12). When available, sequences from the model organisms Escherichia coli and Bacillus subtilis were selected. Genes with “MM” prefix derive from Methanosarcina mazei Go1. BLASTp searches for these query sequences were conducted against a local BLAST database containing 80 haloarchaeal genomes. In order to obtain all potential homologs, the maximum target sequences parameter was set to 100,000 and an E-value cutoff of 1 was used. Resulting matches were then filtered using custom perl scripts to remove spurious domain-level matches by requiring a bidirectional query-target coverage of ≥80%, and a bidirectional query-target length of ≥75%. To reduce the number of non-specific family-level matches (for example, to differentiate between the closely related Trk H+/K+ and Ktr Na+/K+ symporters), sequences were also filtered to remove matches with an E-value of>1e−20. A presence/absence matrix was constructed from these filtered BLAST results using custom bash and R scripts, and visualized in iTOL [91], [92]. Genes were grouped into functional categories based on substrate specificity and directionality for ion transporters, substrate imported for compatible solute transporters, or product for compatible solute biosynthesis proteins. As 20 of the query genes were not detected in the set of investigated species, only 54 columns appear in Figure S5. Gene presence/absence data was scaffold on the multi-marker concatenated haloarchaeal phylogeny (Dataset S11).
Genera-based comparisons
Whole-genome feature data for the 59 newly sequenced, PGAAP-annotated haloarchaea were retrieved from an in-house MySQL database using custom scripts [67]. For each organism the genome size, number of contigs, percent G+C content, percent coding, number of coding regions, and number of insertion sequence elements were extracted. Genomes were organized into genera and box plots for each of the above traits were drawn for each genus using MATLAB's Statistics toolbox [93]. Images were exported using the supplementary export_fig function [94]. See Figure S23.
Isoelectric point analysis
All isoelectric point analyses were carried out exclusively on the PGAAP gene call set. The pI of every protein coding sequence was computationally predicted using the protein sequence isoelectric point prediction method included in the BioJava framework [95]. pI histograms for each genome were computed using MATLAB's statistics toolbox [93], sorting proteins into 100 equally spaced bins ranging from a pI of 2.0 to 13.0. All proteins with a pI of 7.5 or greater were collected and a list of all represented gene annotations was procured. For each gene annotation in this list, all instances were tabulated, including the number of instances with a pI greater than or equal to 7.5. A table showing these results, sorted by frequency of instances with high pI can be found in Table S5. All proteins annotated as ribosomal subunits were collected, and all subunits which could confidently be mapped to experimentally characterized structures for the large (1QVG) [96] and small (1FKA) [97] ribosomal subunits were mapped. Subunits with a pI>7.5 in 60% or more of cases were colored red, and subunits with a pI<7.5 in 60% or more of cases were colored blue. Any subunits which could not be confidently mapped or which had variable pI across species were left the default color of green. All mappings were done using the PyMOL protein visualization program [98].
Comparative acidification visualizations of TBP and ribosome elongation factor α-1 proteins for haloarchaea and non-halophilic organisms were generated using the SWISS-MODEL web interface [99] and 1D3U [100] and 3VMF [101] as the structural templates, respectively.
GC bias analysis
To investigate localized variations in G+C content across each genome, custom scripts were created (Text S3 & S4). First, %G+C was calculated for 100 bp windows across the genome, with a 20 bp step size. The terminal <100 bps of each contig were not included in calculation, so as not to artificially inflate the final G+C calculation for each contig. Windows with>10% ambiguous nucleotides were assumed to have %G+C equal to the contig mean. Plots were generated showing a) the overall mean G+C percent for the entire genome as a horizontal green line, b) the contig boundaries as vertical red lines, c) the mean G+C percent for each contig as horizontal blue lines, and d) all G+C percentages for individual 100 bp segments as a black line [67]. Steps where the mean %G+C inflects away from the local mean were calculated using the R changepoint package [102], and these changepoints were plotted over the stepwise G+C percentages as vertical green lines [67]. After manual curation of changepoints for each species, annotated features between changepoints were retrieved from each species' general feature format (GFF) file. Ten species were excluded from this analysis, either due to having a very large number of contigs, or having no observable inflections in local mean G+C content. For a list of excluded organisms and reasons, see Text S1. The set of features extracted from regions of abnormal G+C content were analyzed for enrichment of homologous protein families (TRIBE-MCL clusters). The frequency of each protein family in the abnormal G+C feature set was compared to its frequency in the entire genome set, and families with at least eight-fold enrichment were investigated further. To avoid including families which were enriched artificially by having a small number of members which are all present in abnormal G+C regions, families with fewer than five members were excluded from enrichment analysis.
General transcription factors
A local BLAST database was constructed using RAST-derived gene calls for the 80 haloarchaea. This database, and the NCBI non-redundant protein database (as of October 30th, 2012), were queried for homologs to the archaeal and eukaryotic general transcription factors TATA-binding protein (TBP) and transcription factor B/IIB (TFB) using a query set of 19 curated TBP homologs and 27 curated TFB homologs from six archaeal and one eukaryotic species (Datasets S18 & S19). A BLASTp search was conducted (BLAST+2.2.27) using a maximum expect value of 10−5 and maximum target sequence of 100,000. For each set of homologs, a multi-sequence alignment was constructed using MUSCLE v3.8.31 [82] and manually curated to remove poorly aligning regions. Highly divergent and fragmentary sequences were manually removed. One thousand bootstrapped alignments were created by resampling the curated alignment using Seqboot in the Phylip toolkit (v3.69) [103]. Phylogenetic trees were created for each bootstrapped alignment using FastTree v2.1.5 SSE3 [104] and a consensus tree was produced by comparing these trees to a guide tree, also constructed using FastTree, using the tree comparison tool CompareToBootstrap.pl [105]. For all clades in the guide tree, the fraction of bootstrapped trees in which that clade appeared were tabulated and recorded as the bootstrap support values for that clade. Because this approach is limited in that only clades appearing in the initial guide tree are considered, we also constructed a consensus tree with the Consense program in the Phylip package [105] using the extended majority rule option. Each clade in the resulting consensus tree represents the most frequent grouping of those species in the 1000 bootstrap replicate trees, independent of any guide tree. For Consense tree files, see Datasets S20–S23. Branch lengths and node labels in Consense tree represent the number of bootstrapped tree replicates for which each clade was observed, rather than sequence divergence. Trees were visualized in FigTree [85].
Genera-specific protein families
Marker genes were identified by the following procedure: Homology clusters were identified that were (1) universal to the genus of interest, (2) not found in any other haloarchaeal species, and (3) single copy. Homologs were aligned using MUSCLE [82] with default parameters, and a hidden Markov model was generated from each alignment using HMMer [106] with default parameters. Additionally, a list of marker homology clusters was generated, as were the genomic coordinates for each marker gene [67]. This list may be loaded into JContextExplorer [17] as a custom context set.
Improvements to automated gene calls
Putative missed gene calls were identified using JContextExplorer [17], with the Haloarchaea genome set loaded. To retrieve the context surrounding Tribe4688 (with gene calls in 12 Haloferax species) a “Between” context set was created with a 5 Kbp limit, and the query “966; 458” was carried out under “Cluster Search”. Regions were visualized by selecting all instances and pushing the “View Contexts” button. This produced a set of either two or three gene groupings, in the order Tribe458 (always) – Tribe4688 (sometimes) – Tribe966 (always). The sequence between instances of Tribe458 and Tribe966 in the three Haloferax species that did not contain an instance of Tribe4688 was extracted, and every contiguous region of 117 nucleotides was translated into a protein using MATLAB's bioinformatics toolbox [107]. Each match was manually compared to the gene members of protein sequences of Tribe4688, and three matches were identified, one in each of the three organisms lacking an instance of Tribe4688 (Haloferax alexandrinus, Hfx. elongans, and Hfx. sulfurifontis). The sequence alignments were visualized, with amino acids colored according to the properties of their side chains. To retrieve the region of poor gene call consistency in Haloarcula, the same between context set was used with a 5 Kbp limit, with the query “254; 369” under “Cluster Search”. Genomic segments were visualized by selecting all instances and pushing the “View Contexts” button.
Improvements to automated gene annotations
A presence/absence matrix was constructed for each TRIBE-MCL protein family across the 80 haloarchaea and transformed into a distance matrix using Euclidean distances. Hierarchical clustering of this distance matrix was performed using Mev [108], and the resulting matrix was visualized. Groups of protein families which clustered together have a similar phylogenetic dispersal pattern, and may therefore perform functionally related tasks. This distribution was manually interrogated to find groups of clustered protein families with functionally related annotations. Unannotated protein families in that cluster were then hypothesized to also be involved in that task.
Supporting Information
Zdroje
1. VogelBF, HansenLT, MordhorstH, GramL (2010) The survival of Listeria monocytogenes during long term desiccation is facilitated by sodium chloride and organic material. Int J Food Microbiol 140 : 192–200 doi:10.1016/j.ijfoodmicro.2010.03.035
2. FinnS, HändlerK, CondellO, ColganA, CooneyS, et al. (2013) ProP is required for the survival of desiccated Salmonella enterica serovar typhimurium cells on a stainless steel surface. Appl Environ Microbiol 79 : 4376–4384 doi:10.1128/AEM.00515-13
3. JogaiahS, GovindSR, TranL-SP (2013) Systems biology-based approaches toward understanding drought tolerance in food crops. Crit Rev Biotechnol 33 : 23–39 doi:10.3109/07388551.2012.659174
4. ChenQ, DuanE (2011) Aquaporins in sperm osmoadaptation: an emerging role for volume regulation. Acta Pharmacol Sin 32 : 721–724 doi:10.1038/aps.2011.35
5. BettariL, FiuzatM, FelkerGM, O'ConnorCM (2012) Significance of hyponatremia in heart failure. Heart Fail Rev 17 : 17–26 doi:10.1007/s10741-010-9193-3
6. LevillainO, SchmolkeM, GuderW (2001) Influence of dehydratation on glycerophosphorylcholine and choline distribution along the rat nephron. Pflugers Arch - Eur J Physiol 442 : 218–222 doi:10.1007/s004240100534
7. MaallemS, WierinckxA, LachuerJ, KwonMH, TappazML (2008) Gene expression profiling in brain following acute systemic hypertonicity: novel genes possibly involved in osmoadaptation. J Neurochem 105 : 1198–1211 doi:10.1111/j.1471-4159.2008.05222.x
8. VerbalisJG (2003) Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 17 : 471–503 doi:10.1016/S1521-690X(03)00049-6
9. FalbM, MüllerK, KönigsmaierL, OberwinklerT, HornP, et al. (2008) Metabolism of halophilic archaea. Extremophiles 12 : 177–196 doi:10.1007/s00792-008-0138-x
10. BowersKJ, WiegelJ (2011) Temperature and pH optima of extremely halophilic archaea: a mini-review. Extremophiles 15 : 119–128 doi:10.1007/s00792-010-0347-y
11. SoppaJ (2006) From genomes to function: haloarchaea as model organisms. Microbiology 152 : 585–590 doi:10.1099/mic.0.28504-0
12. SoppaJ (2011) Functional genomic and advanced genetic studies reveal novel insights into the metabolism, regulation, and biology of Haloferax volcanii. Archaea 2011 : 602408 doi:10.1155/2011/602408
13. AzizRK, BartelsD, BestAA, DeJonghM, DiszT, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9 : 75 doi:10.1186/1471-2164-9-75
14. NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP) (n.d.). Available: http://www.ncbi.nlm.nih.gov/genomes/static/Pipeline.html. Accessed 17 April 2013.
15. AltschulS (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402 doi:10.1093/nar/25.17.3389
16. EnrightAJ, Van DongenS, OuzounisCA (2002) An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res 30 : 1575–1584.
17. SeitzerP, HuynhTA, FacciottiMT (2013) JContextExplorer: a tree-based approach to facilitate cross-species genomic context comparison. BMC Bioinformatics 14 : 18 doi:10.1186/1471-2105-14-18
18. TrittA, EisenJA, FacciottiMT, DarlingAE (2012) An integrated pipeline for de novo assembly of microbial genomes. PLoS One 7: e42304 doi:10.1371/journal.pone.0042304
19. WalshDA, BaptesteE, KamekuraM, DoolittleWF (2004) Evolution of the RNA polymerase B' subunit gene (rpoB') in Halobacteriales: a complementary molecular marker to the SSU rRNA gene. Mol Biol Evol 21 : 2340–2351 doi:10.1093/molbev/msh248
20. WuD, JospinG, EisenJA (2013) Systematic identification of gene families for use as “markers” for phylogenetic and phylogeny-driven ecological studies of bacteria and archaea and their major subgroups. PLoS One 8: e77033 doi:10.1371/journal.pone.0077033
21. TindallBJ (2003) Taxonomic problems arising in the genera Haloterrigena and Natrinema. Int J Syst Evol Microbiol 53 : 1697–1698 doi:10.1099/ijs.0.02529-0
22. MinegishiH, KamekuraM, ItohT, EchigoA, UsamiR, et al. (2010) Further refinement of the phylogeny of the Halobacteriaceae based on the full-length RNA polymerase subunit B' (rpoB') gene. Int J Syst Evol Microbiol 60 : 2398–2408 doi:10.1099/ijs.0.017160-0
23. KuzniarA, van HamRCHJ, PongorS, LeunissenJAM (2008) The quest for orthologs: finding the corresponding gene across genomes. Trends Genet 24 : 539–551 doi:10.1016/j.tig.2008.08.009
24. TatusovRL, GalperinMY, NataleDA, Koonin EV (2000) The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res 28 : 33–36.
25. Nelson-Sathi S, Dagan T, Landan G, Janssen A., Steel M, et al.. (2012) Acquisition of 1,000 eubacterial genes physiologically transformed a methanogen at the origin of Haloarchaea. Proc Natl Acad Sci 109. doi: 10.1073/pnas.1209119109.
26. Brochier-ArmanetC, BoussauB, GribaldoS, ForterreP (2008) Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6 : 245–252 doi:10.1038/nrmicro1852
27. KellyS, WicksteadB, GullK (2011) Archaeal phylogenomics provides evidence in support of a methanogenic origin of the Archaea and a thaumarchaeal origin for the eukaryotes. Proc Biol Sci 278 : 1009–1018 doi:10.1098/rspb.2010.1427
28. MakarovaKS, YutinN, BellSD, KooninEV (2010) Evolution of diverse cell division and vesicle formation systems in Archaea. Nat Rev Microbiol 8 : 731–741 doi:10.1038/nrmicro2406
29. GogartenJP, TownsendJP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 3 : 679–687 doi:10.1038/nrmicro1204
30. Barthelmebs L, Lecomte B, Divies C (2000) Inducible Metabolism of Phenolic Acids inPediococcus pentosaceus Is Encoded by an Autoregulated Operon Which Involves a New Class of Negative Transcriptional Regulator Inducible Metabolism of Phenolic Acids in Pediococcus pentosaceus Is Encoded by an Auto. doi: 10.1128/JB.182.23.6724–6731.2000.Updated.
31. HuilletE, VelgeP, VallaeysT, PardonP (2006) LadR, a new PadR-related transcriptional regulator from Listeria monocytogenes, negatively regulates the expression of the multidrug efflux pump MdrL. FEMS Microbiol Lett 254 : 87–94 doi:10.1111/j.1574-6968.2005.00014.x
32. DanielsCJ, McKee aH, DoolittleWF (1984) Archaebacterial heat-shock proteins. EMBO J 3 : 745–749.
33. CokerJA, DasSarmaS (2007) Genetic and transcriptomic analysis of transcription factor genes in the model halophilic Archaeon: coordinate action of TbpD and TfbA. BMC Genet 8 : 61 doi:10.1186/1471-2156-8-61
34. DuttaC, PaulS (2012) Microbial lifestyle and genome signatures. Curr Genomics 13 : 153–162 doi:10.2174/138920212799860698
35. HartmanAL, NoraisC, BadgerJH, DelmasS, HaldenbyS, et al. (2010) The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS One 5: e9605 doi:10.1371/journal.pone.0009605
36. De RosaM, GambacortaA (1988) The lipids of archaebacteria. Prog Lipid Res 27 : 153–175.
37. YamauchiK, DoiK, YoshidaY, KinoshitaM (1993) Archaebacterial lipids: highly proton-impermeable membranes from 1,2-diphytanyl-sn-glycero-3-phosphocholine. Biochim Biophys Acta 1146 : 178–182.
38. BidleKA, HansonTE, HowellK, NannenJ (2007) HMG-CoA reductase is regulated by salinity at the level of transcription in Haloferax volcanii. Extremophiles 11 : 49–55 doi:10.1007/s00792-006-0008-3
39. Kokoeva MV, Storch K, Klein C, Oesterhelt D (2002) A novel mode of sensory transduction in archaea: binding protein-mediated chemotaxis towards osmoprotectants and amino acids. 21.
40. GohF, JeonYJ, BarrowK, NeilanBA, BurnsBP (2011) Osmoadaptive strategies of the archaeon Halococcus hamelinensis isolated from a hypersaline stromatolite environment. Astrobiology 11 : 529–536 doi:10.1089/ast.2010.0591
41. YoussefNH, Savage-AshlockKN, McCullyAL, LuedtkeB, ShawEI, et al. (2014) Trehalose/2-sulfotrehalose biosynthesis and glycine-betaine uptake are widely spread mechanisms for osmoadaptation in the Halobacteriales. ISME J 8 : 636–649 doi:10.1038/ismej.2013.165
42. KempfB, BremerE (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170 : 319–330.
43. SpanheimerR, MüllerV (2008) The molecular basis of salt adaptation in Methanosarcina mazei Gö1. Arch Microbiol 190 : 271–279 doi:10.1007/s00203-008-0363-9
44. Corratgé-FaillieC, JabnouneM, ZimmermannS, VéryA-A, FizamesC, et al. (2010) Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell Mol Life Sci 67 : 2511–2532 doi:10.1007/s00018-010-0317-7
45. LevinaN, TötemeyerS, StokesNR, LouisP, JonesMA, et al. (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18 : 1730–1737 doi:10.1093/emboj/18.7.1730
46. OrenA (1999) Bioenergetic Aspects of Halophilism. Microbiol Mol Biol Rev 63 : 334.
47. MüllerV, OrenA (2003) Metabolism of chloride in halophilic prokaryotes. Extremophiles 7 : 261–266 doi:10.1007/s00792-003-0332-9
48. DuschlA, WagnerG (1986) Primary and secondary chloride transport in Halobacterium halobium. J Bacteriol 168 : 548–552.
49. WarmuthS, ZimmermannI, DutzlerR (2009) X-ray structure of the C-terminal domain of a prokaryotic cation-chloride cotransporter. Structure 17 : 538–546 doi:10.1016/j.str.2009.02.009
50. AccardiA, MillerC (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl - channels. Nature 427 : 803–807 doi:10.1038/nature02314
51. IyerR, IversonTM, AccardiA, MillerC (2002) A biological role for prokaryotic ClC chloride channels. 419 : 7–9 doi:10.1038/nature01003.1
52. HezayenFF, RehmBH, TindallBJ, SteinbüchelA (2001) Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and description of Natrialba aegyptiaca sp. nov., a novel extremely halophilic, aerobic, non-pigmented member of the Archaea from Egypt that produces extracellular poly (glutamic. Int J Syst Evol Microbiol 51 : 1133–1142.
53. SiddaramappaS, ChallacombeJF, DecastroRE, PfeifferF, SastreDE, et al. (2012) A comparative genomics perspective on the genetic content of the alkaliphilic haloarchaeon Natrialba magadii ATCC 43099T. BMC Genomics 13 : 165 doi:10.1186/1471-2164-13-165
54. OrellanaMV, PangWL, DurandPM, WhiteheadK, BaligaNS (2013) A role for programmed cell death in the microbial loop. PLoS One 8: e62595 doi:10.1371/journal.pone.0062595
55. Holtmann G, Bremer E (2004) Thermoprotection of Bacillus subtilis by Exogenously Provided Glycine Betaine and Structurally Related Compatible Solutes: Involvement of Opu Transporters Thermoprotection of Bacillus subtilis by Exogenously Provided Glycine Betaine and Structurally Rela. doi: 10.1128/JB.186.6.1683.
56. TschapekB, PittelkowM, Sohn-BösserL, HoltmannG, SmitsSHJ, et al. (2011) Arg149 is involved in switching the low affinity, open state of the binding protein AfProX into its high affinity, closed state. J Mol Biol 411 : 36–52 doi:10.1016/j.jmb.2011.05.039
57. WoodJM, BremerE, CsonkaLN, KraemerR, PoolmanB, et al. (2001) Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol A Mol Integr Physiol 130 : 437–460.
58. SantosH, CostaMS (2002) Minireview Compatible solutes of organisms that live in hot saline environments. 4 : 501–509.
59. LamarkT, KaasenI, EshooMW, FalkenbergP, McDougallJ, et al. (1991) DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol Microbiol 5 : 1049–1064.
60. Boch J, Kempf B, Schmid R, Bremer E, Boch J, et al.. (1996) Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB genes. 178.
61. MadernD, EbelC, ZaccaiG (2000) Halophilic adaptation of enzymes. Extremophiles 4 : 91–98.
62. NgWV, KennedySP, MahairasGG, BerquistB, PanM, et al. (2000) Genome sequence of Halobacterium species NRC-1. Proc Natl Acad Sci U S A 97 : 12176–12181 doi:10.1073/pnas.190337797
63. SoppaJ, BaumannA, BrenneisM, DambeckM, HeringO, et al. (2008) Genomics and functional genomics with haloarchaea. Arch Microbiol 190 : 197–215 doi:10.1007/s00203-008-0376-4
64. ZaccaiG (2013) Hydration shells with a pinch of salt. Biopolymers 99 : 233–238 doi:10.1002/bip.22154
65. TalonR, CoquelleN, MadernD, GirardE (2014) An experimental point of view on hydration/solvation in halophilic proteins. Front Microbiol 5 : 66 doi:10.3389/fmicb.2014.00066
66. DeoleR, ChallacombeJ, RaifordDW, HoffWD (2013) An extremely halophilic proteobacterium combines a highly acidic proteome with a low cytoplasmic potassium content. J Biol Chem 288 : 581–588 doi:10.1074/jbc.M112.420505
67. Becker EA, Seitzer PM, Tritt A, Larsen D, Krusor M, et al.. (2014) Data Dryad Package. Data Dryad. doi: 10.5061/dryad.1546n.
68. SchwartzR, TingCS, KingJ (2001) Whole proteome pI values correlate with subcellular localizations of proteins for organisms within the three domains of life. Genome Res 11 : 703–709 doi:10.1101/gr.158701
69. BolhuisH, PalmP, WendeA, FalbM, RamppM, et al. (2006) The genome of the square archaeon Haloquadratum walsbyi: life at the limits of water activity. BMC Genomics 7 : 169 doi:10.1186/1471-2164-7-169
70. NarasingaraoP, PodellS, UgaldeJA, Brochier-ArmanetC, EmersonJB, et al. (2012) De novo metagenomic assembly reveals abundant novel major lineage of Archaea in hypersaline microbial communities. ISME J 6 : 81–93 doi:10.1038/ismej.2011.78
71. DaubinV, LeratE, PerrièreG (2003) The source of laterally transferred genes in bacterial genomes. Genome Biol 4: R57 doi:10.1186/gb-2003-4-9-r57
72. OrenA, ArahalDR, VentosaA (2009) Emended descriptions of genera of the family Halobacteriaceae. Int J Syst Evol Microbiol 59 : 637–642 doi:10.1099/ijs.0.008904-0
73. GoodrichJA, TjianR (2010) Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat Rev Genet 11 : 549–558 doi:10.1038/nrg2847
74. ReichlenMJ, MurakamiKS, FerryJG (2010) Functional analysis of the three TATA binding protein homologs in Methanosarcina acetivorans. J Bacteriol 192 : 1511–1517 doi:10.1128/JB.01165-09
75. TurkarslanS, ReissDJ, GibbinsG, SuWL, PanM, et al. (2011) Niche adaptation by expansion and reprogramming of general transcription factors. Mol Syst Biol 7 : 554 doi:10.1038/msb.2011.87
76. FacciottiMT, ReissDJ, PanM, KaurA, VuthooriM, et al. (2007) General transcription factor specified global gene regulation in archaea. Proc Natl Acad Sci U S A 104 : 4630–4635 doi:10.1073/pnas.0611663104
77. TeufelK, BleiholderA, GriesbachT, PfeiferF (2008) Variations in the multiple tbp genes in different Halobacterium salinarum strains and their expression during growth. Arch Microbiol 190 : 309–318 doi:10.1007/s00203-008-0383-5
78. SoppaJ (1999) Transcription initiation in Archaea: facts, factors and future aspects. Mol Microbiol 31 : 1295–1305.
79. DarlingAE, JospinG, LoweE, MatsenFA, BikHM, et al. (2014) PhyloSift: phylogenetic analysis of genomes and metagenomes. PeerJ 2: e243 doi:10.7717/peerj.243
80. PatiA, IvanovaNN, MikhailovaN, OvchinnikovaG, HooperSD, et al. (2010) GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 7 : 455–457 doi:10.1038/nmeth.1457
81. AdeyA, MorrisonHG, Asan, XunX, KitzmanJO, et al. (2010) Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol 11: R119 doi:10.1186/gb-2010-11-12-r119
82. EdgarRC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5 : 113 doi:10.1186/1471-2105-5-113
83. GuindonS, DufayardJ-F, LefortV, AnisimovaM, HordijkW, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59 : 307–321 doi:10.1093/sysbio/syq010
84. ParadisE, ClaudeJ, StrimmerK (2004) APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20 : 289–290 doi:10.1093/bioinformatics/btg412
85. Rambaut A (2012) FigTree v12.05: http://tree.bio.ed.ac.uk/software/figtree/.
86. Moreno-HagelsiebG, LatimerK (2008) Choosing BLAST options for better detection of orthologs as reciprocal best hits. Bioinformatics 24 : 319–324 doi:10.1093/bioinformatics/btm585
87. Heinrich (2012) org.knowceans.util.Samplers. Available: http://www.arbylon.net/projects/knowceans-tools/doc/org/knowceans/util/Samplers.html. Accessed 2 September 2012.
88. KatohK, MisawaK, KumaK, MiyataT (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30 : 3059–3066.
89. DarribaD, TaboadaGL, DoalloR, PosadaD (2011) ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27 : 1164–1165 doi:10.1093/bioinformatics/btr088
90. FredslundJ (2006) PHY.FI: fast and easy online creation and manipulation of phylogeny color figures. BMC Bioinformatics 7 : 315 doi:10.1186/1471-2105-7-315
91. LetunicI, BorkP (2011) Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 39: W475–8 doi:10.1093/nar/gkr201
92. LetunicI, BorkP (2007) Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23 : 127–128 doi:10.1093/bioinformatics/btl529
93. MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States. (n.d.).
94. Woodward O (2009) export_fig.
95. HollandRCG, DownTA, PocockM, PrlićA, HuenD, et al. (2008) BioJava: an open-source framework for bioinformatics. Bioinformatics 24 : 2096–2097 doi:10.1093/bioinformatics/btn397
96. Schmeing TM, Moore PB, Steitz TA (2003) Structures of deacylated tRNA mimics bound to the E site of the large ribosomal subunit Structures of deacylated tRNA mimics bound to the E site of the large ribosomal subunit: 1345–1352. doi: 10.1261/rna.5120503.The.
97. SchluenzenF, TociljA, ZarivachR, HarmsJ, GluehmannM, et al. (2000) Small Ribosomal Subunit ° Resolution at 3. 3 A. 102 : 615–623.
98. The PyMOL Molecular Graphics System, Version 1.5.04, Schrödinger, LLC. (n.d.).
99. ArnoldK, BordoliL, KoppJ, SchwedeT (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22 : 195–201 doi:10.1093/bioinformatics/bti770
100. LittlefieldO, KorkhinY, SiglerPB (1999) The structural basis for the oriented assembly of a TBP/TFB/promoter complex. Proc Natl Acad Sci U S A 96 : 13668–13673.
101. KobayashiK, SaitoK, IshitaniR, ItoK, NurekiO (2012) Structural basis for translation termination by archaeal RF1 and GTP-bound EF1α complex. Nucleic Acids Res 40 : 9319–9328 doi:10.1093/nar/gks660
102. Killick R, Eckley I (2012) Changepoint: an R package for changepoint analysis. R package version 0.6.1.
103. Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.69. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle.
104. PriceMN, DehalPS, ArkinAP (2009) FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26 : 1641–1650 doi:10.1093/molbev/msp077
105. Price M (n.d.) Fast Tree-Comparison Tools. Distributed by the author. Department of Computational and Theoretical Biology, Lawrence Berkeley National Laboratory.
106. EddyS (2009) A new generation of homology search tools based on probabilistic inference. Genome Inform 23 : 205–211.
107. MATLAB and Bioinformatics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States. (n.d.).
108. EisenM, SpellmanP, BrownP, BotsteinD (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95 : 14863–14868.
109. LynchEA, LangilleMGI, DarlingA, WilbanksEG, HaltinerC, et al. (2012) Sequencing of seven haloarchaeal genomes reveals patterns of genomic flux. PLoS One 7: e41389 doi:10.1371/journal.pone.0041389
110. RohSW, NamY-D, NamS-H, ChoiS-H, ParkH-S, et al. (2010) Complete genome sequence of Halalkalicoccus jeotgali B3(T), an extremely halophilic archaeon. J Bacteriol 192 : 4528–4529 doi:10.1128/JB.00663-10
111. JiangX, WangS, ChengH, HuoY, ZhangX, et al. (2011) Genome sequence of Halobiforma lacisalsi AJ5, an extremely halophilic archaeon which harbors a bop gene. J Bacteriol 193 : 7023–7024 doi:10.1128/JB.06282-11
112. BurnsBP, GudhkaRK, Neilan Ba (2012) Genome sequence of the halophilic archaeon Halococcus hamelinensis. J Bacteriol 194 : 2100–2101 doi:10.1128/JB.06599-11
113. HanJ, ZhangF, HouJ, LiuX, LiM, et al. (2012) Complete genome sequence of the metabolically versatile halophilic archaeon Haloferax mediterranei, a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) producer. J Bacteriol 194 : 4463–4464 doi:10.1128/JB.00880-12
114. Malfatti S, Tindall BJ, Schneider S, Fähnrich R, Lapidus A (2009) Complete genome sequence of Halogeometricum borinquense type strain (PR3 T): 150–158. doi: 10.4056/23264.
115. SiddaramappaS, ChallacombeJF, DecastroRE, PfeifferF, SastreDE, et al. (2012) A comparative genomics perspective on the genetic content of the alkaliphilic haloarchaeon Natrialba magadii ATCC 43099T. BMC Genomics 13 : 165 doi:10.1186/1471-2164-13-165
Štítky
Genetika Reprodukčná medicína
Článek The COP9 Signalosome Converts Temporal Hormone Signaling to Spatial Restriction on Neural CompetenceČlánek Coordinate Regulation of Stem Cell Competition by Slit-Robo and JAK-STAT Signaling in the TestisČlánek The CSN/COP9 Signalosome Regulates Synaptonemal Complex Assembly during Meiotic Prophase I ofČlánek GPA: A Statistical Approach to Prioritizing GWAS Results by Integrating Pleiotropy and AnnotationČlánek Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant BiomassČlánek Heat-Induced Release of Epigenetic Silencing Reveals the Concealed Role of an Imprinted Plant GeneČlánek p53- and ERK7-Dependent Ribosome Surveillance Response Regulates Insulin-Like Peptide SecretionČlánek The Complex I Subunit Selectively Rescues Mutants through a Mechanism Independent of MitophagyČlánek Rad59-Facilitated Acquisition of Y′ Elements by Short Telomeres Delays the Onset of SenescenceČlánek ARTIST: High-Resolution Genome-Wide Assessment of Fitness Using Transposon-Insertion Sequencing
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 11- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Establishing a Multidisciplinary Context for Modeling 3D Facial Shape from DNA
- RNA Processing Factors Swd2.2 and Sen1 Antagonize RNA Pol III-Dependent Transcription and the Localization of Condensin at Pol III Genes
- Inversion of the Chromosomal Region between Two Mating Type Loci Switches the Mating Type in
- A Thermolabile Aldolase A Mutant Causes Fever-Induced Recurrent Rhabdomyolysis without Hemolytic Anemia
- The Role of Regulatory Evolution in Maize Domestication
- Stress Granule-Defective Mutants Deregulate Stress Responsive Transcripts
- 24-Hour Rhythms of DNA Methylation and Their Relation with Rhythms of RNA Expression in the Human Dorsolateral Prefrontal Cortex
- Pseudoautosomal Region 1 Length Polymorphism in the Human Population
- Fungal Communication Requires the MAK-2 Pathway Elements STE-20 and RAS-2, the NRC-1 Adapter STE-50 and the MAP Kinase Scaffold HAM-5
- The COP9 Signalosome Converts Temporal Hormone Signaling to Spatial Restriction on Neural Competence
- The Protein -glucosyltransferase Rumi Modifies Eyes Shut to Promote Rhabdomere Separation in
- The Talin Head Domain Reinforces Integrin-Mediated Adhesion by Promoting Adhesion Complex Stability and Clustering
- Quantitative Genetics of CTCF Binding Reveal Local Sequence Effects and Different Modes of X-Chromosome Association
- Coordinate Regulation of Stem Cell Competition by Slit-Robo and JAK-STAT Signaling in the Testis
- Genetic Analysis of a Novel Tubulin Mutation That Redirects Synaptic Vesicle Targeting and Causes Neurite Degeneration in
- A Systems Genetics Approach Identifies , , and as Novel Aggressive Prostate Cancer Susceptibility Genes
- Three RNA Binding Proteins Form a Complex to Promote Differentiation of Germline Stem Cell Lineage in
- Approximation to the Distribution of Fitness Effects across Functional Categories in Human Segregating Polymorphisms
- The CSN/COP9 Signalosome Regulates Synaptonemal Complex Assembly during Meiotic Prophase I of
- SAS-1 Is a C2 Domain Protein Critical for Centriole Integrity in
- An RNA-Seq Screen of the Antenna Identifies a Transporter Necessary for Ammonia Detection
- GPA: A Statistical Approach to Prioritizing GWAS Results by Integrating Pleiotropy and Annotation
- Let's Face It—Complex Traits Are Just Not That Simple
- Glutamate Receptor Gene , Coffee, and Parkinson Disease
- The Red Queen Model of Recombination Hotspots Evolution in the Light of Archaic and Modern Human Genomes
- The Ethics of Our Inquiry: An Interview with Hank Greely
- Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant Biomass
- Regularized Machine Learning in the Genetic Prediction of Complex Traits
- Phylogenetically Driven Sequencing of Extremely Halophilic Archaea Reveals Strategies for Static and Dynamic Osmo-response
- Lack of Replication of the -by-Coffee Interaction in Parkinson Disease
- Natural Polymorphisms in Human APOBEC3H and HIV-1 Vif Combine in Primary T Lymphocytes to Affect Viral G-to-A Mutation Levels and Infectivity
- A Germline Polymorphism of Thymine DNA Glycosylase Induces Genomic Instability and Cellular Transformation
- Heat-Induced Release of Epigenetic Silencing Reveals the Concealed Role of an Imprinted Plant Gene
- ATPase-Independent Type-III Protein Secretion in
- p53- and ERK7-Dependent Ribosome Surveillance Response Regulates Insulin-Like Peptide Secretion
- The Complex I Subunit Selectively Rescues Mutants through a Mechanism Independent of Mitophagy
- Evolution of DNA Methylation Patterns in the Brassicaceae is Driven by Differences in Genome Organization
- Regulation of mRNA Abundance by Polypyrimidine Tract-Binding Protein-Controlled Alternate 5′ Splice Site Choice
- Systematic Comparison of the Effects of Alpha-synuclein Mutations on Its Oligomerization and Aggregation
- Rad59-Facilitated Acquisition of Y′ Elements by Short Telomeres Delays the Onset of Senescence
- A Functional Portrait of Med7 and the Mediator Complex in
- Systematic Analysis of the Role of RNA-Binding Proteins in the Regulation of RNA Stability
- ARTIST: High-Resolution Genome-Wide Assessment of Fitness Using Transposon-Insertion Sequencing
- Genomic Evidence of Rapid and Stable Adaptive Oscillations over Seasonal Time Scales in Drosophila
- Genome-Wide Associations between Genetic and Epigenetic Variation Influence mRNA Expression and Insulin Secretion in Human Pancreatic Islets
- HAM-5 Functions As a MAP Kinase Scaffold during Cell Fusion in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- An RNA-Seq Screen of the Antenna Identifies a Transporter Necessary for Ammonia Detection
- Systematic Comparison of the Effects of Alpha-synuclein Mutations on Its Oligomerization and Aggregation
- Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant Biomass
- Regularized Machine Learning in the Genetic Prediction of Complex Traits
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



