Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles
Background:
The extent to which baseline couple characteristics affect the probability of live birth and adverse perinatal outcomes after assisted conception is unknown.
Methods and Findings:
We utilised the Human Fertilisation and Embryology Authority database to examine the predictors of live birth in all in vitro fertilisation (IVF) cycles undertaken in the UK between 2003 and 2007 (n = 144,018). We examined the potential clinical utility of a validated model that pre-dated the introduction of intracytoplasmic sperm injection (ICSI) as compared to a novel model. For those treatment cycles that resulted in a live singleton birth (n = 24,226), we determined the associates of potential risk factors with preterm birth, low birth weight, and macrosomia. The overall rate of at least one live birth was 23.4 per 100 cycles (95% confidence interval [CI] 23.2–23.7). In multivariable models the odds of at least one live birth decreased with increasing maternal age, increasing duration of infertility, a greater number of previously unsuccessful IVF treatments, use of own oocytes, necessity for a second or third treatment cycle, or if it was not unexplained infertility. The association of own versus donor oocyte with reduced odds of live birth strengthened with increasing age of the mother. A previous IVF live birth increased the odds of future success (OR 1.58, 95% CI 1.46–1.71) more than that of a previous spontaneous live birth (OR 1.19, 95% CI 0.99–1.24); p-value for difference in estimate <0.001. Use of ICSI increased the odds of live birth, and male causes of infertility were associated with reduced odds of live birth only in couples who had not received ICSI. Prediction of live birth was feasible with moderate discrimination and excellent calibration; calibration was markedly improved in the novel compared to the established model. Preterm birth and low birth weight were increased if oocyte donation was required and ICSI was not used. Risk of macrosomia increased with advancing maternal age and a history of previous live births. Infertility due to cervical problems was associated with increased odds of all three outcomes—preterm birth, low birth weight, and macrosomia.
Conclusions:
Pending external validation, our results show that couple- and treatment-specific factors can be used to provide infertile couples with an accurate assessment of whether they have low or high risk of a successful outcome following IVF.
: Please see later in the article for the Editors' Summary
Published in the journal:
Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles. PLoS Med 8(1): e32767. doi:10.1371/journal.pmed.1000386
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pmed.1000386
Summary
Background:
The extent to which baseline couple characteristics affect the probability of live birth and adverse perinatal outcomes after assisted conception is unknown.
Methods and Findings:
We utilised the Human Fertilisation and Embryology Authority database to examine the predictors of live birth in all in vitro fertilisation (IVF) cycles undertaken in the UK between 2003 and 2007 (n = 144,018). We examined the potential clinical utility of a validated model that pre-dated the introduction of intracytoplasmic sperm injection (ICSI) as compared to a novel model. For those treatment cycles that resulted in a live singleton birth (n = 24,226), we determined the associates of potential risk factors with preterm birth, low birth weight, and macrosomia. The overall rate of at least one live birth was 23.4 per 100 cycles (95% confidence interval [CI] 23.2–23.7). In multivariable models the odds of at least one live birth decreased with increasing maternal age, increasing duration of infertility, a greater number of previously unsuccessful IVF treatments, use of own oocytes, necessity for a second or third treatment cycle, or if it was not unexplained infertility. The association of own versus donor oocyte with reduced odds of live birth strengthened with increasing age of the mother. A previous IVF live birth increased the odds of future success (OR 1.58, 95% CI 1.46–1.71) more than that of a previous spontaneous live birth (OR 1.19, 95% CI 0.99–1.24); p-value for difference in estimate <0.001. Use of ICSI increased the odds of live birth, and male causes of infertility were associated with reduced odds of live birth only in couples who had not received ICSI. Prediction of live birth was feasible with moderate discrimination and excellent calibration; calibration was markedly improved in the novel compared to the established model. Preterm birth and low birth weight were increased if oocyte donation was required and ICSI was not used. Risk of macrosomia increased with advancing maternal age and a history of previous live births. Infertility due to cervical problems was associated with increased odds of all three outcomes—preterm birth, low birth weight, and macrosomia.
Conclusions:
Pending external validation, our results show that couple- and treatment-specific factors can be used to provide infertile couples with an accurate assessment of whether they have low or high risk of a successful outcome following IVF.
: Please see later in the article for the Editors' Summary
Introduction
In-vitro fertilisation (IVF) is now widely used for the treatment of infertility, and validated age-stratified national success rates and outcomes are published annually [1],[2],[3]. To facilitate patient counselling, clinical decision-making, and access to health care provision, prediction models for live birth after IVF have been constructed [4]. However, these studies have been limited by their sample size, development before the introduction of intracytoplasmic sperm injection (ICSI), or lack of validation in external populations [5],[6],[7],[8],[9]. Established multivariable prediction models may therefore not be applicable to contemporary couples seeking treatment. Consequently, clinicians and regulatory bodies have not adopted prediction models and predominantly quote age-related success rates [1],[2],[3].
Given the known complications with multiple gestations and prematurity, the focus has moved to defining the most appropriate IVF outcome variable as a singleton term live birth [10],[11],[12]. Low birth weight and macrosomia are also known to be associated with immediate and long-term risk to offspring heath [13], and IVF singletons are at increased risk of these complications [14],[15]. It is now recognised that factors leading to infertility may be responsible for adverse perinatal outcome rather than the process itself [16],[17],[18],[19]; however, which parental characteristics of infertile couples contribute to adverse perinatal outcomes in IVF singletons and can thereby be targeted for intervention remain unknown.
In this prospective cohort study of 144,018 treatment cycles we assessed the extent to which baseline characteristics can be used to predict live birth after IVF-assisted conception, and for those cycles in which a singleton pregnancy was achieved we identified which factors were associated with preterm delivery, low birth weight, and macrosomia.
Methods
Source of Data
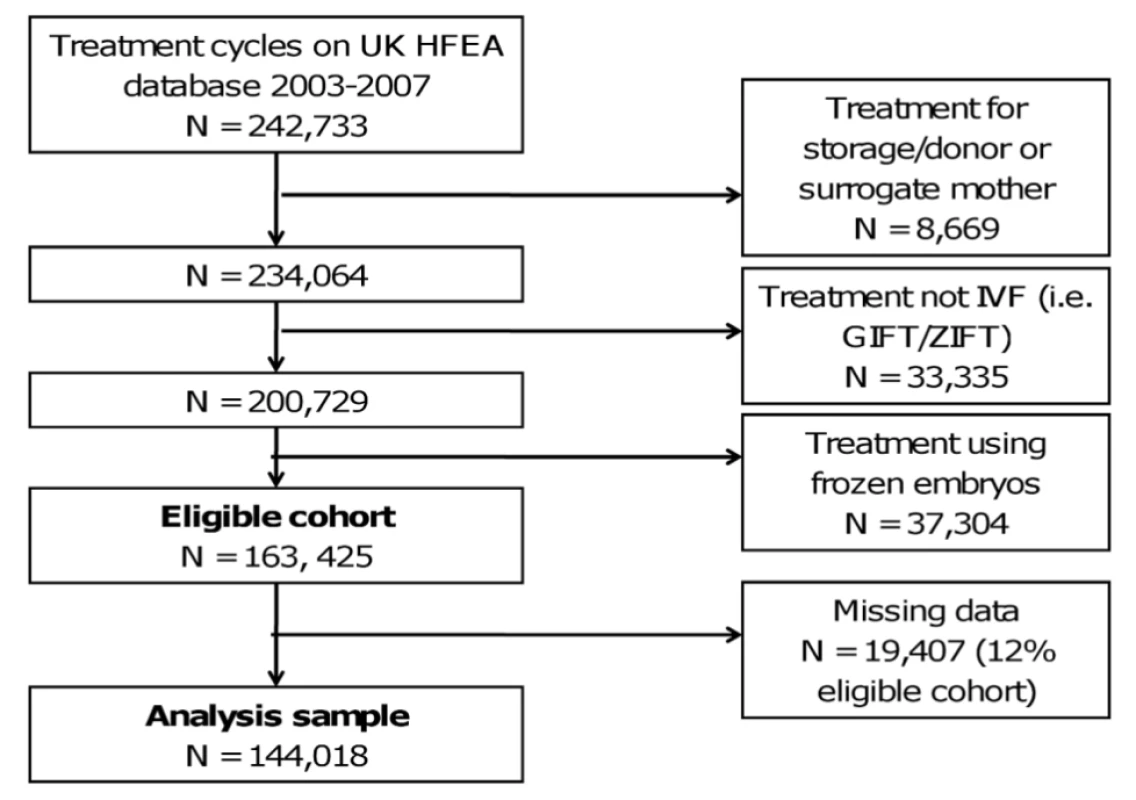
The UK Human Fertilisation and Embryology Authority (HFEA), which is responsible for the regulation of assisted conception treatment in the UK, has had a Parliamentary statutory obligation to prospectively collect baseline information and birth outcomes on all licensed fertility treatment cycles performed in the UK since 1991 [7]. All treatment cycles and outcomes registered on the HFEA database between January 2003 and December 2007 were used in our study, with the final analysis cohort details and exclusion criteria provided in Figure 1 [20]. Treatment cycles that were for storage or donation of gametes, were not IVF, or were frozen embryo transfers were excluded. Although there is a move to greater use of frozen embryo cycles we excluded these from our analyses to be consistent with previous publications, including that by Templeton et al. [7] in which the established model was developed. Furthermore, during the time studied very few elective single embryo transfers were performed (<0.05% of all cycles). HFEA data relating to treatments between April 1999 and March 2002 were not verified by licensed treatment centres and are therefore deemed less accurate. Furthermore, few treatment cycles had treatment with ICSI before and during this period. Whilst data have been collected beyond 2007, validation checks on the computerised data undertaken by HFEA are currently complete only to December 2007. Anonymised data were provided by the HFEA per cycle of treatment rather than for individual women, so our outcomes are expressed as rates and/or odds per cycle of treatment (rather than per individual woman). Ethical approval of the study was provided by the HFEA.
Measurements
Maternal age, duration and cause of infertility, previous number of IVF attempts, number of previous spontaneous and IVF live births, source of gametes, and cycle number were recorded at the time of treatment. Duration of fertility, number of previous IVF attempts, number of previous pregnancies, number of previous IVF pregnancies, and total number of previous live births were all categorised in accordance with the previous analysis by Templeton [7]. Cycle number was collapsed, with more than three cycles as one category, because of small numbers. Live birth was defined as a baby born alive after 24 wk gestation. Our main outcome was at least one live birth, which was defined as any birth event in which at least one baby was born alive and survived for more than 1 mo. This outcome is consistent with previous publications, including that by Templeton et al. [7] used to define the established prediction model. In a sensitivity analysis we repeated associations with this main outcome after exclusion of multiple pregnancies, defined as those in which two or more fetal heartbeats were noted at 8 wk gestation.
For assessment of perinatal outcomes in cycles with a singleton live birth, gestational age at birth was defined as completed weeks of gestation. For the main outcome of preterm we examined multivariable associations with preterm birth, defined as ≤36 completed weeks; we also examined associations with extreme preterm (<33 wk). Birth-weight outcomes were supplied in 500 g increments and categorised as low birth weight (LBW <2.5 kg), normal (≥2.5 to <4.0 kg) or macrosomic (≥4 kg). In these analyses we included only cycles in which there was one heartbeat at 8 wk gestation and one live birth (i.e. these were singleton live births).
Statistical Methods
We performed univariable and multivariable logistic regression to assess associations with at least one live birth. Given that the historical multivariable Templeton model [7] had been externally validated we first tested its predictive ability. We used the reported characteristics that were associated with live birth and their respective regression coefficients from that model to generate the probability of live birth in our cohort (see Text S1 for full details of these calculations) [7]. The predictive ability of the model was assessed by determining the discrimination, using the area under the curve of receiver operator characteristics (AUROC), and its calibration. Calibration was assessed by ranking participants into tenths based on their predicted risk for the Templeton prediction model, and then within each tenth comparing the predicted mean rate to the observed rate of live birth.
The multivariable logistic regression model formed the basis of our novel prediction model of live birth. In the novel prediction model we used the same characteristics as those used in the Templeton model but included all causes of infertility (Templeton includes only tubal versus all other causes), and allowed the coefficients for this and all other variables to be newly derived, and included four additional characteristics—the source of the egg (donor or patient's own), type of hormonal preparation used (antioestrogen, gonadotrophin, or hormone replacement therapy), whether or not ICSI was used, and the number of the treatment cycle (1, 2 or ≥3). We tested all two-way interactions between pairs of predictors included in our multivariable analyses and used a Bonferroni-corrected (for multiple testing) p-value threshold of 0.05 to define statistical evidence of an interaction. The discrimination and calibration of this novel model was assessed as described above. The AUROC between the Templeton model and our model was compared using the ROCCOMP command in Stata [21]. When we repeated the multivariable analyses using 1,000 bootstrap replications, the estimates and their standard errors were essentially the same and results are presented here without bootstrapping.
Lastly, we examined model reclassification by determining the integrated discrimination improvement (IDI) of the novel prediction model compared to the original Templeton model [7]. The IDI is a summary measure of the extent to which a new prediction model increases risk prediction in individuals who ultimately have the outcome of interest [22], and reduces risk prediction in those who remain healthy in comparison to the established risk prediction model (in this case the Templeton model [7]).
To explore risk factors for adverse perinatal outcomes (preterm, extreme preterm, low birth weight, and macrosomia) we used logistic regression to examine the univariable and independent multivariable associations of all risk factors assessed in the multivariable analyses of at least one live birth, as described above. The selection of these potential risk factors for adverse perinatal outcomes was based on previous studies and the plausibility that risk factors that influence odds of live birth are also likely to affect gestational age and birth weight. For associations with preterm as the outcome we additionally adjusted for mean birth weight, and for outcomes with low birth weight and macrosomia we adjusted for mean gestational age. These analyses were conducted only for cycles in which there was only one heartbeat at 8 wk gestation and at least one live birth.
All statistical analyses were performed using Stata version 11 (StataCorp LP).
Dealing with Missing Data
For the vast majority of variables there was no missing data; 3.9% of cycles had missing data on method of hormonal preparation used and 8.4% had missing data on duration of infertility; overall 12% of the eligible cohort had some missing data (Figure 1 and Table S1). Univariable associations were very similar when maximum numbers for each variable were used (Table S2) and when only those with complete data were used (Table 1), suggesting that missing data did not result in bias.
Results
Figure 1 shows how we established the eligible cohort of IVF treatment cycles (N = 163,425) and the sample used for the main multivariable analyses (i.e. without any missing data N = 144,018; 88% of eligible). Table S1 shows the study characteristics. Amongst the 163,425 eligible cohort, the overall rate of at least one live birth was 23.4 per 100 cycles (95% CI 23.2–23.7). Rates of successful live birth increased linearly over time from 22.7 per 100 cycles in 2003 to 24.9 per 100 cycles in 2007 (p<0.001 for linear trend) (Figure S1).
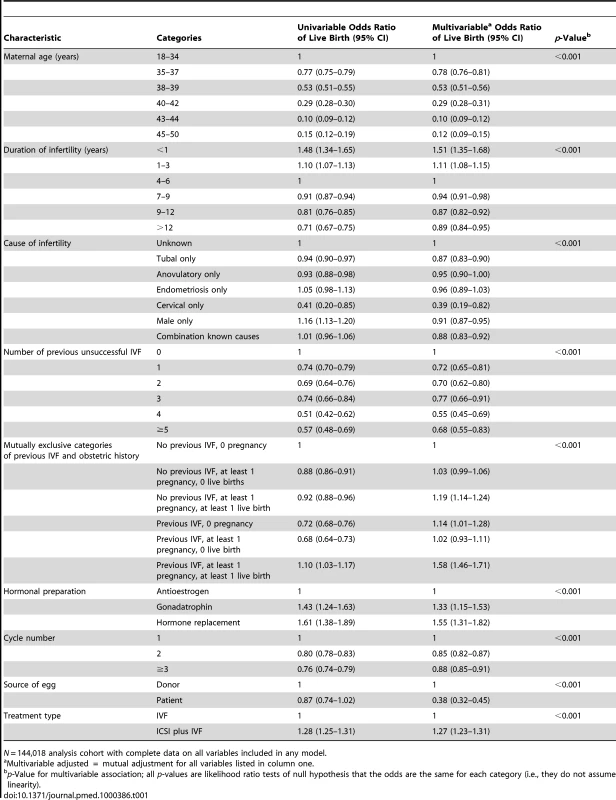
Table 1 shows univariable and multivariable associations of live birth. The odds of successful live birth decreased with increasing maternal age, increasing duration of infertility, greater number of previously unsuccessful IVF treatments, when the woman's own egg (as opposed to donor) was used, and when this was the second or third (as opposed to first) treatment cycle. Odds of successful live birth were lower when the cause of infertility was tubal, anovulatory, or cervical disease or when it was due to a male cause. Women who had at least one previous live birth (either natural or with IVF) had increased odds of a successful live birth with this cycle, as did those in whom gonadotrophin or hormone replacement (as opposed to antioestrogens) were used and ICSI was used with IVF. A previous IVF live birth increased the odds of future success (OR 1.58, 95% CI 1.46–1.71) more than previous spontaneous live birth (OR 1.19, 95% CI 0.99–1.24); p-value for difference in estimate <0.001 (estimated using 1,000 bootstrap replications to estimate standard errors of differences between the log odds between the two regression coefficients).
There was statistical evidence for four interactions, and stratified analyses reflecting these interactions are shown in Table S3 (for interactions with age) and Table S4 (for interactions with ICSI). The increased odds of success in cycles in which the duration of infertility was less than one year increased with increasing maternal age, though only a very small proportion of all cycles were in the category of less than one year duration of infertility. The reduced odds of successful outcome amongst own versus donor oocytes strengthened with increasing age. In couples who had not used ICSI all three causes—male infertility, infertility due to cervical disorders, and infertility due to a combination of causes—were associated with reduced odds of live birth, whereas there were no such associations in those using ICSI. Requiring three or more treatment cycles was associated with reduced odds of live birth in those in which ICSI was used, but not where it was not used. These four interactions were included in our novel prediction model, which is described in Text S2.
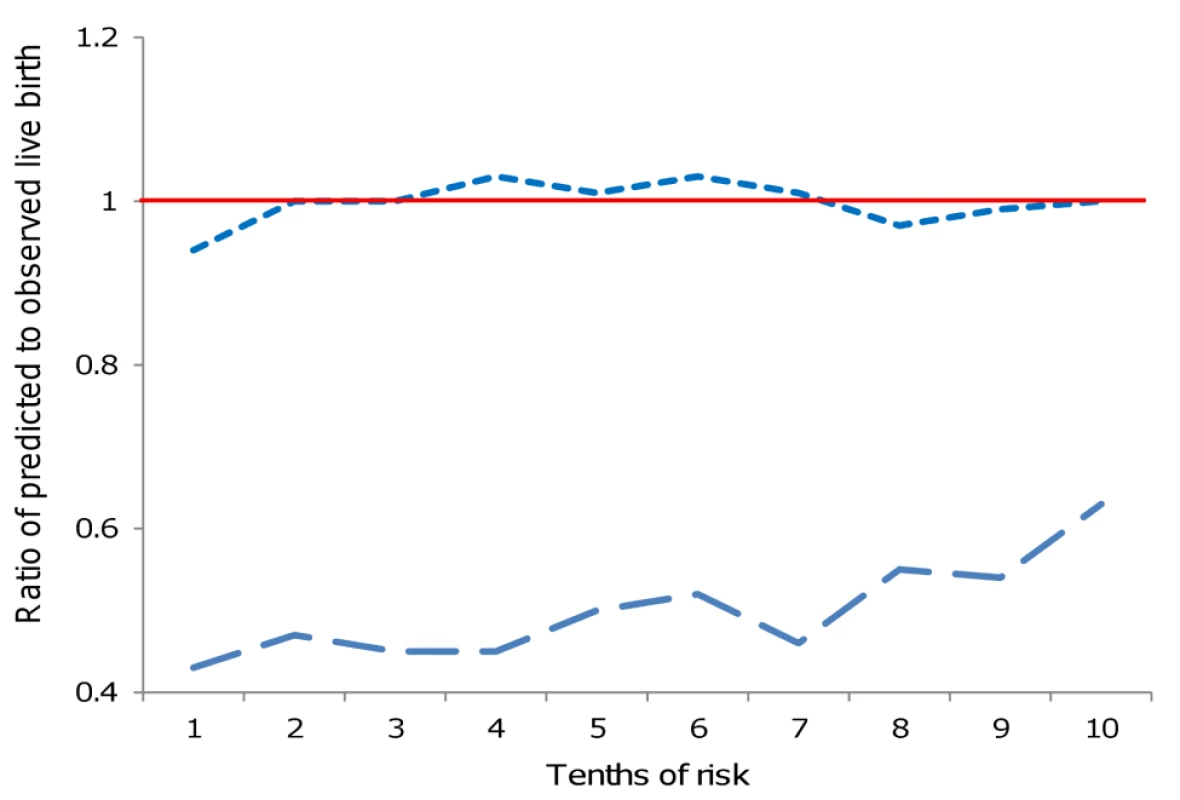
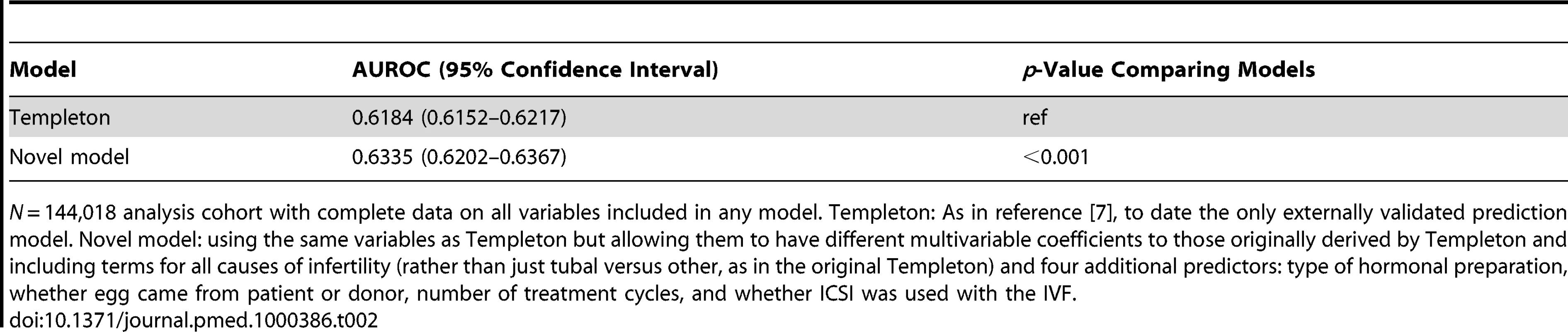
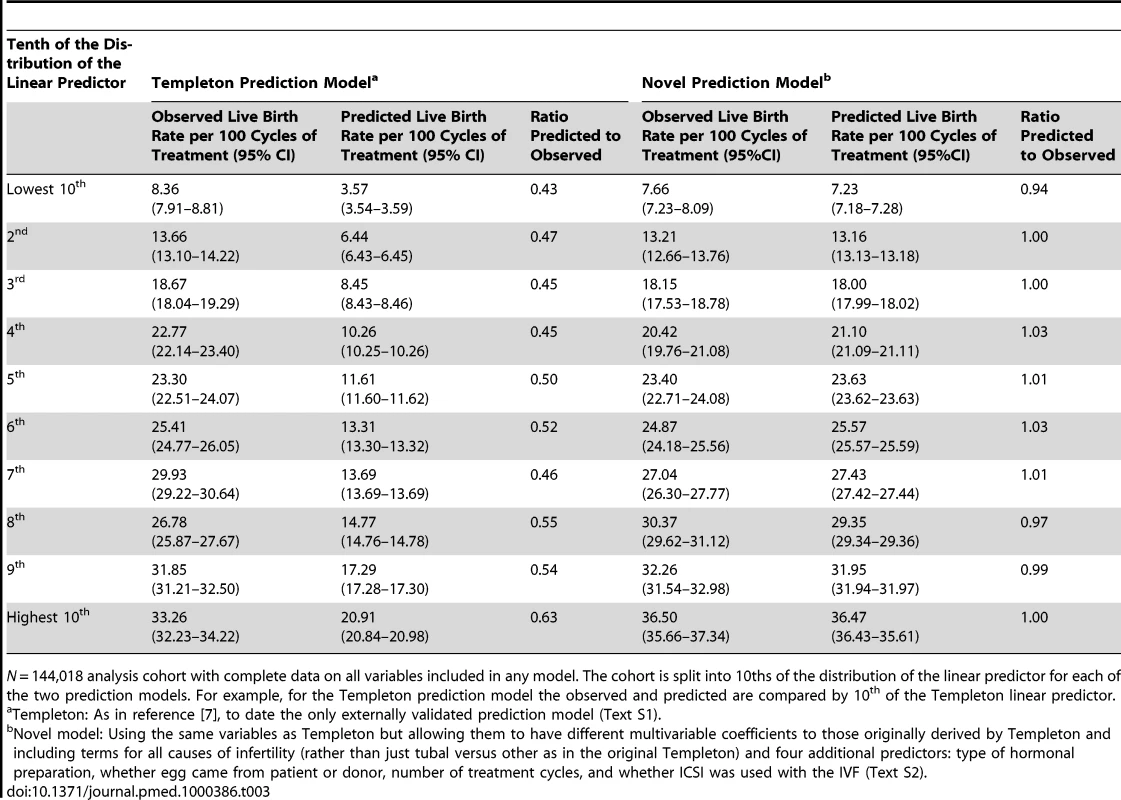
Table 2 shows the AUROC for each of the Templeton and our new prediction models, with statistically significant improvement in discrimination for our novel model. Figure 2 and Table 3 show the observed to predicted rate of successful live birth by tenths of the distribution of the linear prediction models for each of the models. Calibration was poor with the Templeton model, which markedly underestimated the likelihood of successful live birth across the entire distribution of risk, particularly in those at lowest risk. By contrast the novel model had excellent calibration and reclassified cycle probability of a live birth in a way that improved upon the original Templeton model (IDI = 2.1%, p<0.001 comparing the novel model to the Templeton [7]).
Of the 144,018 cycles 9931 (7%) were multiple pregnancies (i.e. had two or more fetal heart beats noted at 8 wk gestation). Of these, 1,264 (13%) resulted in one live birth, 7,925 (80%) in two live births, and 109 (1%) in three live births; 633 (6%) did not result in a live birth. When we removed these 9,931 cycles from our analyses results were essentially unchanged from those presented here. For example, Table S5 shows the univariable and multivariable associations of potential predictors with live birth after these exclusions (i.e., the equivalent of Table 1 in this paper). The AUROC, observed to predicted ratios and IDI were the same as those presented in Tables 2 and 3 with these exclusions.
Table 4 provides examples of how our novel prediction model could be used in clinical practice to give an estimate of a couple's probability of achieving a live birth in a given cycle of treatment. This illustrates not only the clinical use of this model (which we have developed into a freely available computer programme, http://www.IVFpredict.com, and iPhone/Android application, IVFpredict) but also how both couple characteristics and treatment choice influence prognosis.

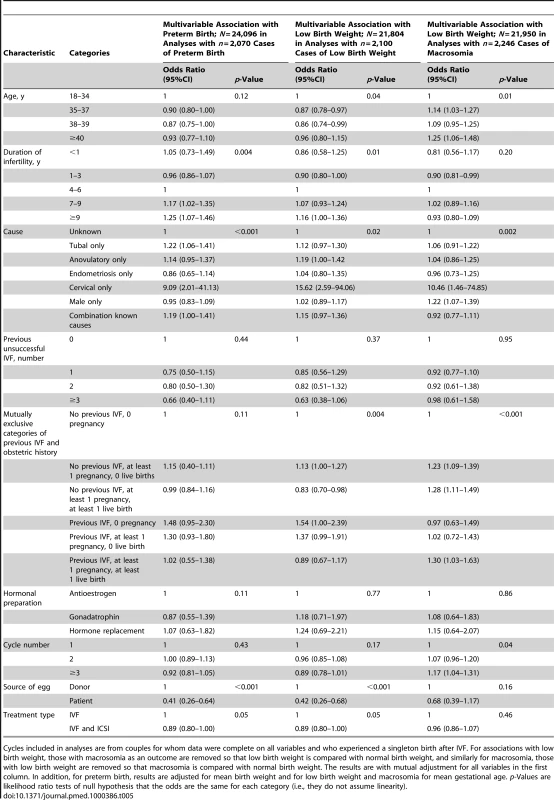
Of the 144,018 cycles included in our main analyses for prediction of successful live birth, there were 24,226 live singleton births; 24,096 (99.5%) of these had gestational age data and 24,050 (99.3%) had birth weight data. Mean (SD) gestational age was 38.98 (2.12) completed weeks, with 472 (2.0%) being less than 33 wk, 1,598 (6.6%) between 33–36 weeks and 22,026 (91.4%) 37 or greater weeks. Mean (SD) birth weight was 3.277 (0.629) kg, with 2,100 (8.7%) low birth weight (<2.5 kg), 19,704 (81.9%) a healthy birth weight, and 2,246 (9.3%) macrosomic (≥4.0 kg). Table S6 shows the univariable associations of risk factors preterm (<37 completed weeks), low birth weight (<2.5 kg) and macrosomia (≥4.0 kg). Table 5 shows the multivariable associations of these risk factors with preterm birth, low birth weight, and macrosomia. In multivariable analyses the odds of both preterm birth and low birth weight in singleton IVF live births were reduced when the woman's (rather than donor) egg was used and when ICSI was used. The odds of low birth weight were also reduced with increasing maternal age and with a history of previous pregnancy (either spontaneous or following IVF). Odds of macrosomia increased with increasing maternal age and in cycles in which there was history of a previous pregnancy (either spontaneous or IVF). Odds of all three factors—preterm birth, low birth weight, and macrosomia—were increased when infertility was due to a cervical disorder. Tubal causes of infertility were associated with increased odds of preterm birth, anovulatory causes with low birth weight, and male causes of infertility with macrosomia. Table S7 shows univariable and multivariable associations with extreme preterm birth (<33 wk; n = 472). Characteristics that were associated with preterm birth in general were also associated with extreme preterm birth. In addition, extreme preterm birth was lower in 38- to 39-year-olds compared to all other ages and was increased in those who had a previous history of IVF.
Discussion
In this study we identify precise estimates of the strength and independence of the factors affecting the odds of IVF success and their association with adverse perinatal outcome. To date, successful prediction of live birth after assisted conception has been limited, with a recent systematic review [4] finding that models were limited by their sample size, incorporating fewer than 3,100 cycles or couples and their lack of external validation. The notable exception was the model of Templeton et al., which analysed 36,961 treatment cycles undertaken in the UK between 1991 and 1994 and was validated in a population of 1,253 couples receiving IVF treatment in The Netherlands between 1991 and 1999 [7],[23]. Since then, ICSI for male factor infertility has been widely adopted, and consequently we demonstrate that this previously validated model, although showing reasonable discrimination, is poorly calibrated and of limited use in contemporary populations. We have developed a new model, which encompasses a series of new measures including use of donor oocytes, ICSI, cycle number, and whether there had been a previous spontaneous or IVF-related live birth or fetal loss. Using this novel model we can statistically significantly improve the overall prediction of live birth as assessed by area under the curve and attain excellent calibration with accurate identification of couples with a poor, moderate, or good prognosis. We also find that maternal characteristics, in particular maternal age, source of the oocyte and cervical causes of infertility are strongly associated with the risk of low birth weight and preterm delivery in singleton live births resulting from IVF. Notably, some of these associations were in the opposite direction to those seen for successful live birth. Thus, in women who successfully have a singleton live birth with IVF, the risk of low birth weight is reduced in older compared with younger women and both low birth weight and preterm are reduced when the woman's own embryo has been used.
The use of assisted conception has increased dramatically over recent years, with concomitant increases in success rates, in part driven by the widespread uptake of ICSI for male factor infertility [24]. The importance of ICSI in general and in particular causes of infertility is demonstrated in our study by its association with increased odds of successful live birth and by the fact that couples with male causes of infertility, cervical causes, or combined causes have reduced odds of success if ICSI has not been used, but are unrelated to success if ICSI has been used. Recent technical advances have, however, failed to overcome the reduction in success rates associated with increasing duration of infertility, necessity for repeated IVF attempts, or increasing maternal age, all of which are independently associated with reduced odds of live birth. The detrimental impact of prolonged infertility suggests that early recourse to treatment is appropriate and that extended treatment waiting times, for example whilst trying lifestyle interventions, might militate against eventual success. The marked reduction in the success of the second cycle but then a relative plateau is in contrast to previous reports, which suggested a subtle decline with increasing cycle number [7]. This difference may indicate that previous declines in success rates with increasing cycle number principally reflected increasing maternal age, which we have adjusted for.
In keeping with all previous reports, live birth rates decline with increasing maternal age [2],[4],[7],[23]. By contrast, ours is the first study that we are aware of to find that, in women with successful IVF delivery of a singleton live birth, younger maternal age is associated with increased risk of low birth weight. This latter finding is however, in keeping with the recent observation that maternal age is positively associated with first trimester growth [25], which if impaired is an important determinant of later adverse perinatal outcome [26],[27]. For older women, the use of donor oocytes is a successful strategy for the attainment of a live birth, however, we identify that donor oocyte recipients have a marked increase in the risk of delivering a preterm or low birth weight infant. This may reflect the primary relationship between ovarian senescence and vascular function. Premature and natural menopause have both been associated with widespread vascular dysfunction, dyslipidaemia, a proinflammatory phenotype, and an increased risk of cardiovascular events [28],[29],[30]. These same factors have been implicated in the aetiology of fetal growth restriction and preeclampsia, the major determinants of preterm birth [31]. Furthermore, increased incidence of these complications have been reported in young and old donated oocyte recipients [32],[33]. With respect to macrosomia the associations with older maternal age may reflect higher maternal socioeconomic class due to deferred child bearing or increased maternal obesity, both of which would be contribute to improved fetal nutrition. Similarly, a previous successful pregnancy would be associated with potential maternal weight retention and thereby increased fetal weight in subsequent pregnancies [34].
We examined the associates of preterm birth (<37 weeks the established definition of preterm). Although it is possible that obstetricians may consider IVF pregnancies as precious and have a lower threshold for iatrogenic preterm birth, we think this is unlikely because of the established associations of prematurity with neonatal respiratory complications [35],[36], and our finding that similar associations were also found for extreme preterm birth support this assumption. In the UK since 2005 only two embryos are allowed to be replaced under the age of 40 to reduce the risks of preterm birth and low birth weight, which are associated with multiple pregnancies. We have restricted the analysis of perinatal outcomes to delivery of a singleton pregnancy only because of the relevance of understanding risk factors associated with these outcomes in couples requiring IVF even when there is a singleton pregnancy. Few previous studies have examined the relationship of couple and treatment characteristics with gestational age and birth weight after live singleton IVF birth; our findings highlight important areas for further research aimed at maximising the success of IVF in terms of a healthy-weight, term live birth.
We demonstrate that a previous live birth as a consequence of IVF has an even greater effect on the prospect of successful assisted conception therapy than does previous spontaneous conception. Although many couples undergoing assisted conception feel encouraged by achieving a pregnancy, even if it subsequently results in fetal loss, we found no beneficial or negative effect of a history of a nonviable pregnancy on live birth. This suggests that embryonic chromosomal errors, rather than a defective maternal environment, may be primarily responsible.
Our work has a number of strengths. We have considered a range of anamnestic couple characteristics simultaneously with respect to validated live birth and perinatal outcomes rather than one or two in isolation. As a result, our data give a better overall reflection of predictive abilities, or lack thereof, for many factors. The size of our study was extremely large compared to other such studies in the literature. Finally, we considered a relevant multivariable historical model for consistency of findings before developing and assessing a novel prognostic model.
We acknowledge, however, a number of limitations. Data were not complete on 12% of the eligible cohort; however, univariable analysis was similar in the whole cohort, and multivariable multiple imputation did not alter the overall conclusions (results available from authors on request). Treatment cycles rather than individual patients were identified because of concerns regarding confidentiality and breach of the terms of the HFEA Act, and therefore it was not possible to examine the effect of multiple cycles within one patient or to use robust standard errors that take account of clustering of women. However, the previous HFEA analysis could account for clustering and did not show a significant effect as compared with per treatment cycle [7]. Maternal age was supplied in categories because of recent concerns over confidentiality; however, our findings of a decline in live birth rates with increasing age are in keeping with the previous analysis of the HFEA database and population reports [1],[7],[37]. We accept that the cause of infertility may have been underinvestigated or misreported [38], although for male factors this was cross-validated with the use of ICSI, and for tubal disease our observed decrease in success rate is consistent with the control arm of randomised controlled trials of salpingectomy prior to IVF [39], suggesting that these data are accurate.
For the main analyses with successful birth as the outcome we included both single and multiple pregnancy, i.e. our outcome was at least one live birth irrespective of whether there was one or more heart beats at 8 wk. Our reasons for doing this were, first, that this is a relevant outcome for infertile couples and, second, this was the outcome used in the study that developed the established prediction model, and therefore we wanted to test this model with the same outcome. Note that restriction of the data to pregnancies in which only one fetal sac was evident at 8 wk gestation produced similar results.
Finally, we acknowledge lack of external validation of our model. Nonetheless, we believe that this model will improve the ability to stratify contemporary couples seeking IVF on the basis of low, moderate, or high likelihood of success. This is of particular relevance to couples willing to consider all therapeutic options, including use of donor oocytes, as there is a 5-fold difference in live birth between the lowest and highest decile of our prediction model. To facilitate validation of the model we are currently generating a free web-based prediction tool (http://www.IVFpredict.com) and iPhone/Android application (IVFpredict) for widespread use of our new prediction tool. These will acknowledge the current lack of external validation and will request provision of anonymised data (all variables included in the prediction model, country of treatment, and outcome) that in the coming years we will use as a means of external validation of this model. We have included full model details in Text S2 thereby facilitating model validation by other research groups.
In conclusion, we show that baseline couple and treatment characteristics can provide a basis for counselling and informing couples of their likely prognosis in terms of low, moderate, or high odds of success (see Table 4).
Supporting Information
Zdroje
1. Nyboe AndersenA
GoossensV
BhattacharyaS
FerrarettiAP
KupkaMS
2009 Assisted reproductive technology and intrauterine inseminations in Europe, 2005: results generated from European registers by ESHRE: ESHRE. The European IVF Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 24 1267 1287
2. Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology 2008 2006 Assisted reproductive technology success rates: National summary and fertility clinic reports. Atlanta: CDC. Available: http://www.cdc.gov/art/art2006/index.htm. Accessed: 30 November 2010
3. Australian Government Department of Health and Ageing 2006 Report of the independent review of assisted reproductive technologies. Available: http://www.health.gov.au/internet/main/publishing.nsf/Content/ART-Report. Accessed: 30 November 2010
4. LeushuisE
van der SteegJW
SteuresP
BossuytPMM
EijkemansMJC
2009 Prediction models in reproductive medicine: A critical appraisal. Hum Reprod Update 15 537 552
5. StolwijkAM
ZielhuisGA
HamiltonCJCM
StraatmanH
HollandersJMG
1996 Pregnancy: Prognostic models for the probability of achieving an ongoing pregnancy after in-vitro fertilization and the importance of testing their predictive value. Hum Reprod 11 2298 2303
6. StolwijkAM
StraatmanH
ZielhuisGA
JansenCA
BraatDD
1998 External validation of prognostic models for ongoing pregnancy after in- vitro fertilization. Hum Reprod 13 3542 3549
7. TempletonA
MorrisJK
ParslowW
1996 Factors that affect outcome of in-vitro fertilisation treatment. Lancet 348 1402 1406
8. HunaultCC
EijkemansMJ
PietersMH
te VeldeER
HabbemaJD
2002 A prediction model for selecting patients undergoing in vitro fertilization for elective single embryo transfer. Fertil Steril 77 725 732
9. HunaultCC
te VeldeER
WeimaSM
MacklonNS
EijkemansMJ
2007 A case study of the applicability of a prediction model for the selection of patients undergoing in vitro fertilization for single embryo transfer in another center. Fertil Steril 87 1314 1321
10. EversJL
2002 Female subfertility. Lancet 360 151 159
11. WHO 2002 Current practices and controversies in assisted reproduction: Report of a WHO Meeting.
VayenaE
RowePJ
GriffinPD
Medical, Ethical and Social Aspects of Assisted Reproduction Geneva WHO Publications 381 396
12. MinJK
BrehenySA
MacLachlanV
HealyDL
2004 What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: The BESST endpoint for assisted reproduction. Hum Reprod 19 3 7
13. BarkerDJ
Fetal and Infant origins of Adult Disease. London 1992 Publisher British Medical JournalISBN 978-0-7279-0743-1
14. JacksonRA
GibsonKA
WuYW
CroughanMS
2004 Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obstet Gynecol 103 551 563
15. AllenVM
WilsonRD
CheungA
2006 Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can 28 220 250
16. DraperES
KurinczukJJ
AbramsKR
ClarkeM
1999 Assessment of separate contributions to perinatal mortality of infertility history and treatment: A case-control analysis. Lancet 353 1746 1749
17. RomundstadLB
RomundstadPR
SundeA
von DuringV
SkjaervenR
2008 Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: A population-based cohort study. Lancet 372 737 743
18. BassoO
BairdDD
2003 Infertility and preterm delivery, birthweight, and caesarean section: A study within the Danish National Birth Cohort. Hum Reprod 18 2478 2484
19. NygrenKG
FinnstromO
KallenB
OlaussonPO
2007 Population-based Swedish studies of outcomes after in vitro fertilisation. Acta Obstet Gynecol Scand 86 774 782
20. von ElmE
AltmanDG
EggerM
PocockSJ
GøtzschePC
2007 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370 1453 1457
21. DeLongER
DeLongDM
Clarke-PearsonDL
1988 Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44 837 845
22. PencinaMJ
D'AgostinoRBSr
D'AgostinoRBJr
VasanRS
2008 Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27 157 172
23. SmeenkJMJ
StolwijkAM
KremerJAM
BraatDDM
2000 External validation of the Templeton model for predicting success after IVF. Hum Reprod 15 1065 1068
24. PalermoG
JorisH
DevroeyP
Van SteirteghemAC
1992 Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340 17 18
25. Mook-KanamoriDO
SteegersEA
EilersPH
RaatH
HofmanA
2010 Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 303 527 534
26. SmithGC
SmithMF
McNayMB
FlemingJE
1998 First-trimester growth and the risk of low birth weight. N Engl J Med 339 1817 1822
27. BukowskiR
SmithGCS
MaloneFD
BallRH
NybergDA
2007 Fetal growth in early pregnancy and risk of delivering low birth weight infant: Prospective cohort study. BMJ 334 836-
28. ColditzGA
WillettWC
StampferMJ
RosnerB
SpeizerFE
1987 Menopause and the risk of coronary heart disease in women. N Engl J Med 316 1105 1110
29. van der SchouwYT
van der GraafY
SteyerbergEW
EijkemansJC
BangaJD
1996 Age at menopause as a risk factor for cardiovascular mortality. Lancet 347 714 718
30. CooleyM
BakalovV
BondyCA
2003 Lipid profiles in women with 45,X vs 46,XX primary ovarian failure. JAMA 290 2127 2128
31. RedmanCW
SargentIL
2003 Pre-eclampsia, the placenta and the maternal systemic inflammatory response–A review. Placenta 24 Suppl A S21 27
32. BodriD
VernaeveV
FiguerasF
VidalR
GuillenJJ
2006 Oocyte donation in patients with Turner's syndrome: A successful technique but with an accompanying high risk of hypertensive disorders during pregnancy. Hum Reprod 21 829 832
33. PaulsonRJ
BoostanfarR
SaadatP
MorE
TourgemanDE
2002 Pregnancy in the sixth decade of life: Obstetric outcomes in women of advanced reproductive age. JAMA 288 2320 2323
34. VillamorE
CnattingiusCnattingius
Interpregnancy weight change and risk of adverse pregnancy outcomes: A population-based study. Lancet 368 1164 1170
35. MorrisonJJ
RennieJM
MiltonPJ
1995 Neonatal respiratory morbidity and mode of delivery at term: Influence of timing of elective caesarean section. Br J Obstet Gynaecol 102 101 106
36. StutchfieldP
WhitakerR
RussellI
2005 Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: Pragmatic randomised trial. BMJ 331 662
37. Nyboe AndersenA
GoossensV
FerrarettiAP
BhattacharyaS
FelberbaumR
2008 Assisted reproductive technology in Europe, 2004: Results generated from European registers by ESHRE. Hum Reprod 23 756 771
38. CraftI
FormanR
1997 Analysis of IVF data. Lancet 349 284
39. JohnsonN
van VoorstS
SowterMC
StrandellA
MolBW
2010 Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev CD002125
Štítky
Interné lekárstvoČlánok vyšiel v časopise
PLOS Medicine
2011 Číslo 1
- Statinová intolerance
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Metamizol v liečbe pooperačnej bolesti u detí do 6 rokov veku
- Co dělat při intoleranci statinů?
Najčítanejšie v tomto čísle
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
