-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial
Lisa Lindesmith and colleagues assess the potential of a candidate virus-like particle (VLP) vaccine to induce antibody responses to antigenically divergent norovirus strains.
Published in the journal: Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial. PLoS Med 12(3): e32767. doi:10.1371/journal.pmed.1001807
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001807Summary
Lisa Lindesmith and colleagues assess the potential of a candidate virus-like particle (VLP) vaccine to induce antibody responses to antigenically divergent norovirus strains.
Introduction
Diarrheal disease is a significant global health problem with an estimated 1.7 billion annual cases. Symptoms from infection range from dehydration to malnutrition and even to early death, particularly among children less than 5 y old and older adults [1]. Norovirus (NoV) strains are responsible for about 50% of global outbreaks of gastroenteritis and cause an estimated 21 million infections per year in the United States alone [2]. Although most NoV infections result in illness of modest severity, infection in children, older adults, immune-compromised individuals, and individuals with underlying medical conditions such as malnutrition can have severe and even fatal consequences [3–13]. In addition to benefitting these particularly vulnerable populations, an effective NoV vaccine would also benefit members of the military, travelers, and childcare, healthcare, and food industry workers.
NoVs are positive-sense, single-stranded RNA viruses in the Caliciviridae family, with genogroups GI and GII causing almost all human infections. Although new approaches to classification have been suggested [14], NoV genotyping is based primarily on the amino acid sequence of the major capsid protein (VP1) encoded by ORF 2 [15]. The genogroups are further subdivided into a total of 30 different known genotypes. The GII.4 NoV strains cause 70%–80% of documented NoV outbreaks and account for the largest percentage of long-term-care-facility - and vacation-related (e.g., cruise ship) outbreaks [2]. GII strains other than GII.4 cause more food-service - and community-related outbreaks than the less frequent GI strains [2], though both GI and GII strains are routinely detected in outbreak investigations.
NoVs are non-enveloped, small, round, structured viruses. Expression of the ORF 2 sequence results in abundant capsid protein production. These proteins self-assemble into icosahedral virus-like particles (VLPs) that are morphologically and antigenically indistinguishable from native virions [16,17]. The NoV major capsid protein is divided into the shell and protruding domains. The surface-exposed P2 subdomain (residues 279–405) [17] interacts with histoblood group antigens (HBGAs) expressed on gut epithelial cells, facilitating virus binding and entry resulting in infection [18]. Gut HBGA expression is largely dependent upon a functional secretor enzyme (secretor-positive phenotype). Individuals lacking a functional secretor enzyme are secretor-negative and at reduced risk of NoV infection. In the absence of a robust cell culture or small animal model, NoV VLPs serve as surrogate viruses in immunological and biochemical assays, including an in vitro assay of antibody (Ab) blockade of VLP–HBGA interaction. The blockade assay is useful in identifying Abs that functionally block binding of NoV capsid protein to carbohydrate ligands; these Abs are a subset of serum Abs that bind to the VLPs as identified by enzyme immunoassay (EIA) [19,20]. The blockade assay has been investigated for clinical relevance and holds promise as a surrogate neutralization assay, as shown in both infected chimpanzees [21] and Norwalk-virus-challenged human participants as a component of protective immunity [22,23].
The ORF 2 gene of GII.4 strains is evolving via epochal evolution, a pattern of change characterized by circulating strain replacement with a newly emergent strain, followed by a period of relative stasis [19,24]. Resulting new predominant GII.4 NoV strains (see Fig. 1) can have altered antigenicity and potentially altered ligand binding profiles [19]. Within the major capsid protein gene of GII.4 strains, residues of the P2 subdomain are under selective pressure by the host immune response; this pressure is one potential mechanism that drives viral evolution, resulting in strain antigenic drift and escape from herd immunity [19,25–28]. We have identified three GII.4-specific, evolving blockade Ab epitopes. Epitope A is hypervariable, and loss of epitope A blockade Abs correlates with viral escape from herd immunity [20,25,27,29]. Epitope D is less variable and, importantly, also modulates the ligand binding properties of GII.4 strains, explaining altered HBGA binding patterns in evolving strains [19,25,30]. Epitope E is a confirmed Farmington Hills–specific blockade epitope [31]. Epitope F is conserved across all GII.4 strains measured to date. The amino acid coordinates of epitope F are not yet known, but we have both human and mouse monoclonal antibodies (mAbs) that recognize this epitope and have identified a domain (the NERK motif) within the capsid that regulates Ab access to epitope F [25,32]. Additional factors, including inter-strain recombination [33] and polymerase fidelity [34,35], may also contribute to GII.4 predominance. Unfortunately, the lack of a well-established virus propagation method excludes evaluation of the effect of these factors on viral fitness. In contrast to the GII.4 strains, the other NoV genotypes do not appear to be evolving via epochal evolution.
Fig. 1. Temporal relationship between epidemiologically important GII.4 strains, relative to the virus-like particles and sera used in this study. 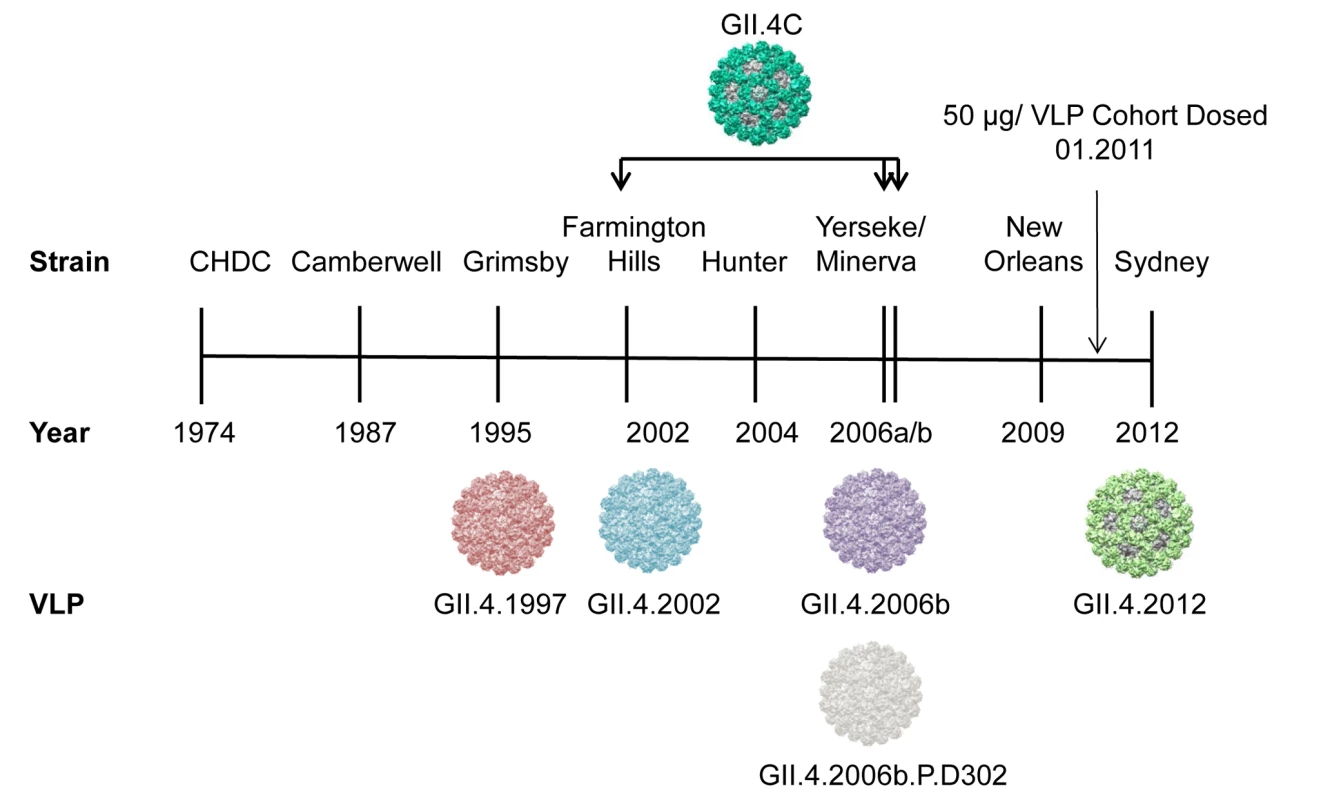
The vaccine GII.4 component (GII.4C) is a consensus VLP composed of GII.4.2002, GII.4.2006a, and GII.4.2006b sequences. GII.4.2006b.P.D302 represents a strain that evolved in vivo and was isolated from an immune-compromised person. The most significant obstacles to development of an effective NoV vaccine are the large number of antigenic variants, an incomplete understanding of the components of protective immunity, and the unknown effect of a lifetime of NoV pre-exposure history on vaccine response and, consequently, the unknown effect of vaccination on response to circulating NoVs. Further, the effect of host genetic susceptibility to NoV infection on vaccine performance is also undefined. A monovalent NoV GI.1 vaccine based on Norwalk virus VLPs was demonstrated to be well tolerated and effective at limiting the risk of Norwalk virus illness and infection upon oral challenge [23,36]. Two doses of this VLP candidate vaccine reduced the rate of symptomatic infection by 47% and the overall rate of infection by 26%. Serum blockade Ab titers above 200 were associated with a 72% reduction in frequency of illness and a 57% reduction in infection, providing evidence that pre-challenge blockade Ab titers correlated to protection following vaccination and challenge in human volunteers [23]. Despite these positive results, a threshold for protection using serum blockade Ab titer has not been established, and because the assay format is not yet standardized, it remains difficult to compare blockade titers from study to study.
These initial findings in monovalent VLP vaccination studies were expanded upon in a follow-up age and dose escalation study of a multivalent VLP candidate vaccine composed of GI.1 and a GII.4 consensus VLP (GII.4C). GII.4C is an engineered VLP composed of the consensus sequence of three NoV GII.4 strains that circulated in 2002 and 2006 (see Fig. 1). The multivalent vaccine was observed to be well tolerated and to elicit Ab responses in adults. Four dosages (5/5, 15/15, 50/50, and 150/150 μg GI.1/GII.4C VLP) of the multivalent vaccine were compared in a group of individuals aged 18–49 y, and the three highest dosages elicited similar Ab responses and reactogenicity [37]. Although secretor-negative participants had lower baseline titers against the vaccine antigens, they responded similarly to secretor-positive participants in titer and geometric mean fold rise (GMFR) [37], supporting in vitro [38] and in vivo [39–41] evidence that some GI and GII NoV strains bind to secretor-negative carbohydrates and infect the secretor-negative population. The 50/50-μg (GI.1/GII.4C) VLP dose was chosen for further study in a human challenge model with a GII.4.2002 strain [42]. Peak Ab titers to both vaccine components were observed after a single dose of vaccine, suggesting that vaccine-induced Ab responses may not rise above a maximum level. Despite high Ab responses, vaccinated individuals were not significantly protected from illness or infection compared to the placebo group. However, disease severity was significantly reduced. Interpretation of these results is complicated by a lower than expected infection rate and the absence of reporting of blockade Ab titers.
In this study we evaluated the potential cross-strain immunity induced by the multivalent candidate vaccine by measuring cross-reactive IgG and blockade Ab titers against the vaccine components, two additional GI VLPs, 2 GII VLPs, and five GII.4 VLPs not included in the vaccine formulation.
Materials and Methods
Ethics Statement
This follow-up study used de-identified human samples under University of North Carolina institutional review board exemption approval #11-0883. Original samples were collected by Takeda Vaccines under approval by institutional review boards at Saint Louis University School of Medicine, Saint Louis, Missouri; University of Rochester School of Medicine and Dentistry, Rochester, New York; and the Navy Medical Research Center, Silver Springs, Maryland, and participants provided written informed consent.
Serum Samples
Serum samples were collected from participants in a NoV vaccine dose–response study conducted by Takeda Vaccines (Deerfield, Illinois) [37]. The results of that study guided selection of the 50-μg/VLP dose as the formulation to test in a human challenge trial (Table 1 and [42]). The 50-μg/VLP dose group of Cohort A (group A3) included ten adults aged 18–49 y vaccinated in January 2011 with an intramuscular injection of 50 μg of both GI.1 and GII.4C VLPs adjuvanted with 3-O-deacylated monophosphoryl lipid A (GlaxoSmithKline) and aluminum hydroxide (Brenntag Biosector, Denmark) on day 0 (dose 1), followed by an identical booster vaccination on day 28 (dose 2). All serum samples collected from the ten participants on day 0, 7, 21, 35, and 180 after dose 1 were evaluated. S1 and S2 Figs. list the total number of samples collected and evaluated for each VLP response by individual and assay. As a group, the Cohort A individuals receiving placebo (PBS without adjuvant) were sero-positive at baseline but did not mount serum responses to the vaccine antigens [37], therefore sera from this group were not included in further analyses of cross-reactive Ab responses. Serum samples were provided blinded of all participant identifiers. A set volume of each serum sample was provided for these exploratory studies.
Tab. 1. Study protocol for the original age and dose escalation study [37]. ![Study protocol for the original age and dose escalation study [<em class="ref">37</em>].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/47082dd75a658a015f7afc8b7d5085b5.png)
Group A3 was further evaluated in the current study. Virus-Like Particles
A diverse panel of VLPs was used in this study (Fig. 2). Except for GII.4C VLPs, ORF 2 genes were inserted directly into the VEE replicon vector for the production of virus replicon particles, as previously described [27,31]. VLPs were expressed in BHK cells and purified by velocity sedimentation in sucrose followed by simultaneous concentration and dialysis into PBS using 100-kDa MWCO centrifugal filter units (Millipore), if needed. GII.4C VLPs were provided by Takeda Vaccines and produced in the baculovirus system. VLP protein concentrations were determined by the BCA Protein Assay (Pierce). All of the VLPs used in this study bind to ligands found in porcine gastric mucin (PGM) type III, with the exception of GII.2. Although bioinformatics analyses do not suggest why, GII.2 does not bind to any identified HBGA sources except human type A and B saliva [43]; therefore, blockade assays were not performed for this VLP.
Fig. 2. Characteristics of virus-like particles. 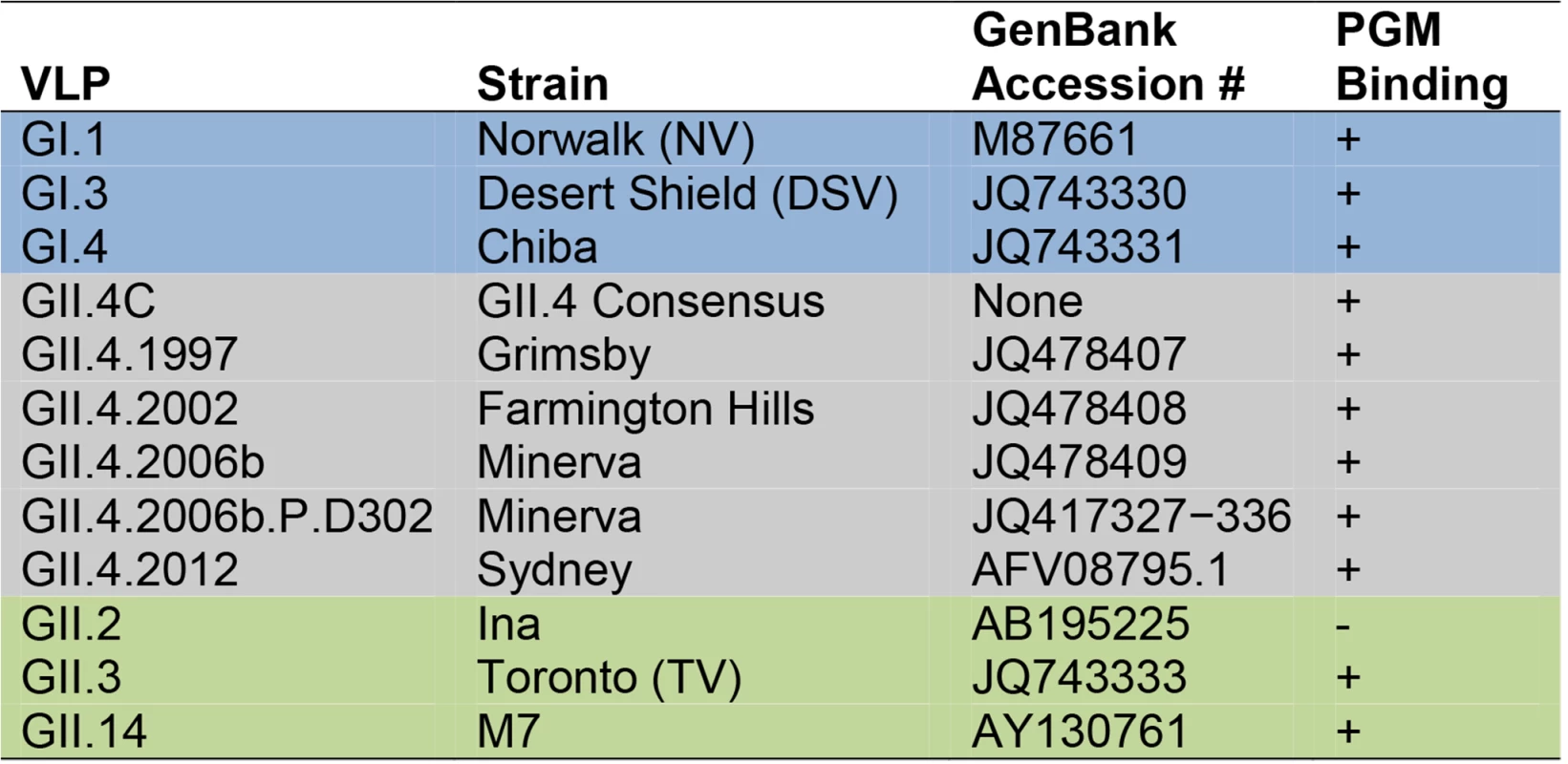
Ab responses to a diverse panel of GI (blue), GII.4 (grey), and non-GII.4 GII (green) VLPs were compared in this study. Virus-Like-Particle-Specific Enzyme Immunoassay
EIA plates were coated with 0.25 μg/ml VLP in PBS before adding 2-fold serial dilutions of serum. The primary Ab incubation was followed by anti-human-IgG-HRP secondary Ab (GE Healthcare), and positive wells were color-developed with Ultra TMB substrate (Thermo Scientific). Plates were washed with PBS/0.05% Tween 20 (Thermo Scientific) between steps, and Ab dilutions were done in 5% Blotto/PBS/0.05% Tween 20. All incubations were done at room temperature. The percent maximum binding was calculated, sigmoidal dose–response curves fit to the data, and EC50 values determined using GraphPad Prism version 6.02 for Windows (GraphPad Software). All samples were tested in a minimum of two independent assays. EC50 titers below the lower limit of detection (100) were assigned a titer of 50.
Carbohydrate-Binding Blockade Assay
As described previously [20,25], EIA plates were coated with 10 μg/ml PGM type III (Sigma Aldrich) diluted in PBS and blocked with 5% Blotto in PBS/0.05% Tween 20. VLPs (0.5 μg/ml) were pretreated with decreasing concentrations of serum for 1 h before being added to the carbohydrate-ligand-coated plates for 1 h. Ligand-bound VLP was detected with rabbit anti-GI or -GII or -GII.4 VLP hyperimmune serum followed by anti-rabbit-IgG-HRP (GE Healthcare). All tested VLPs, except for GII.4.2012 and GII.4.2006b.P.D302, were components of the VLP cocktails used to immunize rabbits. Multivalent GII.4 VLP immunization resulted in broadly reactive serum that recognizes GII.4.2012 and GII.4.2006b.P.D302 [44]. Wash steps, Ab dilutions, and color development were completed as described above. All incubations were done at room temperature. The percent control binding was defined as the binding level in the presence of Ab pretreatment divided by the binding level in the absence of Ab pretreatment multiplied by 100. Blockade data were fit using sigmoidal dose–response analysis of nonlinear data in GraphPad Prism 6.02. EC50 values were calculated for sera that demonstrated blockade of at least 50% at the dilution series (40–20480) tested. Sera that did not block 50% of binding at the highest concentration tested were assigned an EC50 of 20 for statistical comparison.
Cartography
As described previously [45], we utilized multi-dimensional scaling (MDS) to analyze and visualize the relationships between Ab titers against VLPs across the serum samples from this study. This approach allows for data of large dimensions (e.g., multiple serological responses across a range of individuals) to be “shrunk” down to a more manageable number of dimensions for analysis, while maintaining accurate relationships between data points. Briefly, the IgG EC50 titers of each serum against a panel of VLPs (Fig. 2) were normalized to the maximum response of each serum, as well as to the maximum overall titers across sera (normalization method 1 [46]; the same normalization was used for the blockade Ab EC50 titers). We calculated Euclidean distances, D, between each pair of Ab titers against VLPs. We utilized MDS to then identify XYZ coordinates that maintain the underlying Euclidean distances. We used Matlab 8.1’s (MathWorks) cmdscale function for the MDS. We used R (http://www.r-project.org) and the rgl package for 3-D visualization of our data, as well as for statistical analysis of the antigenic cartography data. Because of the unique nature of the D values (that they belong to pairs of data points instead of to singular data points), in most cases we focus our statistical analyses on contrasting D values within specific groups of interest with D values between specific groups of interest (e.g., D values within either GI or GII VLPs versus D values between GI and GII VLPs). Unless noted otherwise, these tests take the form of a set of t-tests within a time point/assay combination, and we report as significant only those tests that pass an ad hoc Bonferroni correction (p = 0.05/number of tests done within a time point/assay combination).
Blocking of Binding Assay
EIA plates were coated with 0.25 μg/ml GII.4.1997 or GII.4.2006b VLP, and serum samples were added to VLP-coated plates at serial 2-fold dilutions. After 1 h, mouse mAbs to epitope A (GII.4.1987.G1 [26] or GII.4.2006.G2 [26]) or epitope F (GII.4.2002.G5 [32]; MAB227P from Maine Biotechnology Services) were added and incubated at room temperature for 1 h. Bound mouse mAb was detected with sheep anti-mouse-IgG-HRP (GE Healthcare). Plates were washed, the color developed, and the amount of serum (1/serum dilution) needed to block 50% of the mAb binding (EC50) determined as described above for EIAs.
Statistical Analysis
Statistical analyses were done using GraphPad Prism 6.02 and SAS (version 9.3) unless otherwise noted. For serum IgG and blockade Ab titers, geometric mean titer (GMT), GMFR from baseline, and the seroresponse frequency were calculated for each group. The changes in GMFR from baseline were tested using a paired t-test of the log values. Seroresponse was defined as a ≥4-fold increase above baseline titer. Generalized estimating equations (GEEs) were used to estimate the relative risk of (1) a 4-fold increase in blockade Ab titers at day 7 compared to day 0 given baseline titers above or below the assay limit of detection, for any VLP and (2) day 7 titers above or below the assay limit of detection given baseline titers above or below the assay limit of detection, for any VLP, along with corresponding chi-squared p-values. GEEs were used to account for the correlated data points (specimens collected at different follow-up time points from the same participant are correlated), as opposed to linear regression, which assumes independent data points.
Results
Vaccination with the Multivalent Virus-Like Particle Candidate Vaccine Results in Cross-Strain-Reactive IgG Responses
Based on results from a dose escalation study in adults aged 18–49 y (Table 1 and [37]), the 50-μg GI.1/GII.4C VLP vaccine formulation was chosen for further study. Complete demographic information for the group in the dose escalation study that received this dose (Group A3) compared to the other dose groups is provided in Table 2. As the vaccine target population will not be pre-screened for secretor status and some NoV strains infect secretor-negative individuals [39–41], secretor-negative participants were included in the larger dose escalation cohort and Group A3 (four out ten participants). Complete data comparing IgG and blockade Ab responses by secretor status, day, and VLP are provided in S1 and S2 Figs. As reported for the full study [37], Ab responses between the secretor-negative and secretor-positive participants in Group A3 were generally not significantly different in GMT or GMFR, beyond the exceptions discussed below.
Tab. 2. Demographics of the Cohort A dose groups in the dose escalation study. 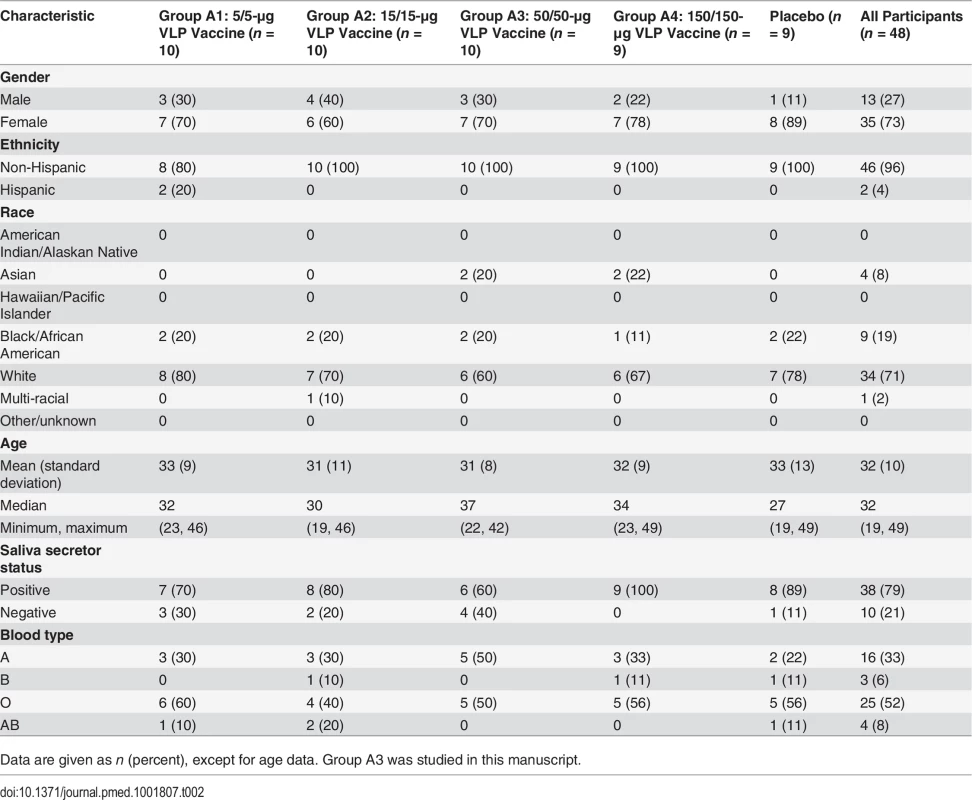
Data are given as n (percent), except for age data. Group A3 was studied in this manuscript. To measure the potential cross-strain protection provided by the multivalent NoV VLP vaccine, we retrospectively analyzed serum samples from the ten participants in the 50-μg dose group from the dose escalation study for cross-reactive IgG and blockade Ab titer to VLPs representing three different GI genotypes; five time-ordered, antigenically distinct GII.4 strains; and two additional non-GII.4 GII genotypes (Fig. 2). Participants received a dose of adjuvanted vaccine on day 0 and day 28. Sera collected on day 0 (before vaccine dose 1), day 7 after vaccine dose 1, day 35 (7 d after vaccine dose 2), and day 180 (6 mo after vaccine dose 1) were evaluated from each of the ten participants. One participant failed to return for the day 180 sample collection.
As a group (Fig. 3), the baseline IgG GMTs to GI types were relatively low, with significant increases in GMFRs at day 7, 35, and 180 after dose 1. Titers peaked at day 7 post-vaccination, rising 10.3-fold for GI.1, 7.4-fold for GI.3, and 7.6-fold for GI.4. Despite a booster vaccination at day 28, day 35 IgG titers were generally lower than day 7 titers, with GMFRs of 12-, 4-. and 4.4-fold for GI.1, GI.3, and GI.4, respectively. At day 180, GMFRs were 4.1-fold for GI.1, 2.3-fold for GI.3, and 2.1-fold for GI.4. Secretor-negative participants had significantly less titer to GI.4 at day 0 and to GI.1 at day 0 and day 180, possibly reflecting less infection history with these strains (S1 Fig.). At day 7, the IgG seroresponse rate was 8/10 for GI.1 and GI.3 and 7/10 for GI.4 (Figs. 3 and S3). At day 35, 10/10 participants seroresponded for GI.1, and 5/10 for the other GI VLPs, and these responses persisted at day 180 in 5/9 participants for GI.1, 2/9 for GI.3, and 1/9 for GI.4.
Fig. 3. Mean EC50 IgG titer in vaccinated participants. 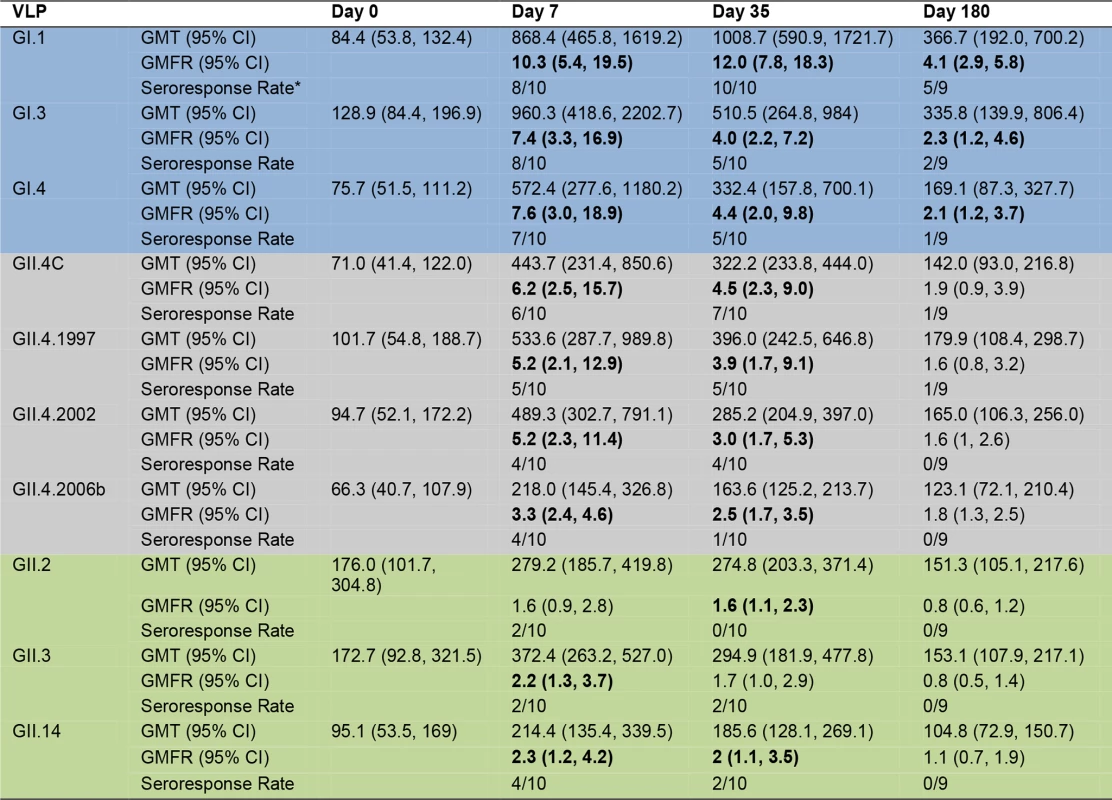
Serum samples collected from participants who received the 50/50-μg VLP dose were assayed for IgG reactivity to a panel of GI (blue), GII.4 (grey), and non-GII.4 GII (green) VLPs. The seroresponse rate is the ratio of the number of participants with a ≥4-fold titer increase above day 0 titer compared to the total number of samples tested at day 0 for each VLP. Bolded values denote significant increases in GMFR above baseline. The IgG response among different strains of GII.4 VLPs was relatively consistent (Fig. 3), with no significant differences in GMFR between GII.4 VLPs at any time point. As a group, GII.4 baseline GMTs were relatively low and in the same range as the GI titers, with significant increases in GMFR at day 7 and day 35 after dose 1 for all GII.4 VLPs. Titers generally peaked at day 7 post-vaccination, rising 6.2-fold for GII.4C, 5.2-fold for GII.4.1997, 5.2-fold for GII.4.2002, and 3.3-fold for GII.4.2006b. As demonstrated for GI VLPs, despite a booster vaccination at day 28, day 35 titers were lower than day 7 titers, with GMFRs of 4.5 for GII.4C, 3.9 for GII.4.1997, 3.0 for 2002, and 2.5 for GII.4.2006b. At day 180, IgG titers were less than 2-fold above baseline for all of the GII.4 VLPs. Secretor-negative and -positive participants responded similarly to GII.4 VLPs (S1 Fig.). As individuals (S3 Fig.), at day 7, 6/10 participants seroresponded to GII.4C, 5/10 to GII.4.1997, and 4/10 to GII.4.2002 and GII.4.2006b VLPs. At day 35, the response rates were 7/10, 5/10, 4/10, and 1/10, respectively. At day 180, 1/9 participants maintained a seroresponse to GII.4C and GII.4.1997.
Increases in cross-reactive IgG were detected to GII.2, GII.3, and GII.14 VLPs after vaccination. As a group (Fig. 3), a ≥4-fold increase in titer was not detected for any of the non-GII.4 GII VLPs at any time point. Some individuals did serorespond to each of the non-GII.4 GII VLPs, although none of the responses persisted to day 180 (S3 Fig.). GMTs were not significantly different for any non-GII.4 GII VLPs at any time point. Despite this observation, secretor status significantly impacted the GMFRs to non-GII.4 GII VLPs. Secretor-negative participants had significantly higher GMFRs on day 7 and 35 to GII.2, GII.3, and GII.14 (S1 Fig.). Together, these data indicate that the multivalent vaccine induces production of IgG that reacts with not only the immunizing strain VLPs but also VLPs from strains and genotypes not included in the vaccine. Maximum serum IgG levels were seen after a single dose of vaccine, and serum IgG was not equally reactive with all NoV genotypes.
Vaccination with the Multivalent Virus-Like Particle Candidate Vaccine Results in Cross-Strain Blockade Antibody Responses
The inherent cross-strain reactivity of serum IgG demonstrated above and elsewhere [19,41] has confounded interpretation of the association between IgG and risk of NoV infection. However, the specificity of measuring blockade Abs demonstrated a clear correlation between titer and protection both from GI.1 symptomatic infection and reinfection with homotypic virus in challenge studies [23]. Therefore, we tested the serum samples for blockade Ab titers against the panel of GI, GII.4, and non-GII.4 GII VLPs as a predictor of cross-strain protection after vaccination. As a group, there were no significant differences in blockade Ab titer between any VLP pair at day 0. Day 0 geometric mean blockade Ab titers to GI VLPs were low, with significant increases in GMFRs at day 7 and 35 for all three GI VLPS (Fig. 4). Titers peaked at day 7 post-vaccination, rising 30.7-fold above baseline for GI.1, 7.4-fold for GI.3, and 4.1-fold for GI.4. At day 35, GMFRs were 12.3 for GI.1, 3.1 for GI.3, and 1.5 for GI.4. Blockade Ab titers returned to baseline levels at day 180. GI blockade Ab GMFRs were not dependent on secretor status (S2 Fig.). Individually (S4 Fig.), at day 7, 9/10 participants responded with a ≥4-fold increase in blockade Ab titer to GI.1, 6/10 to GI.3, and 4/8 to GI.4. At day 35, elevated titers were maintained in 8/10 participants to GI.1, 4/10 to GI.3, and 1/10 to GI.4. At day 180, 2/9 participants maintained a ≥4-fold increase in blockade Ab titer to GI.1, and 1/9 to GI.3.
Fig. 4. Mean EC50 blockade antibody titer in vaccinated participants. 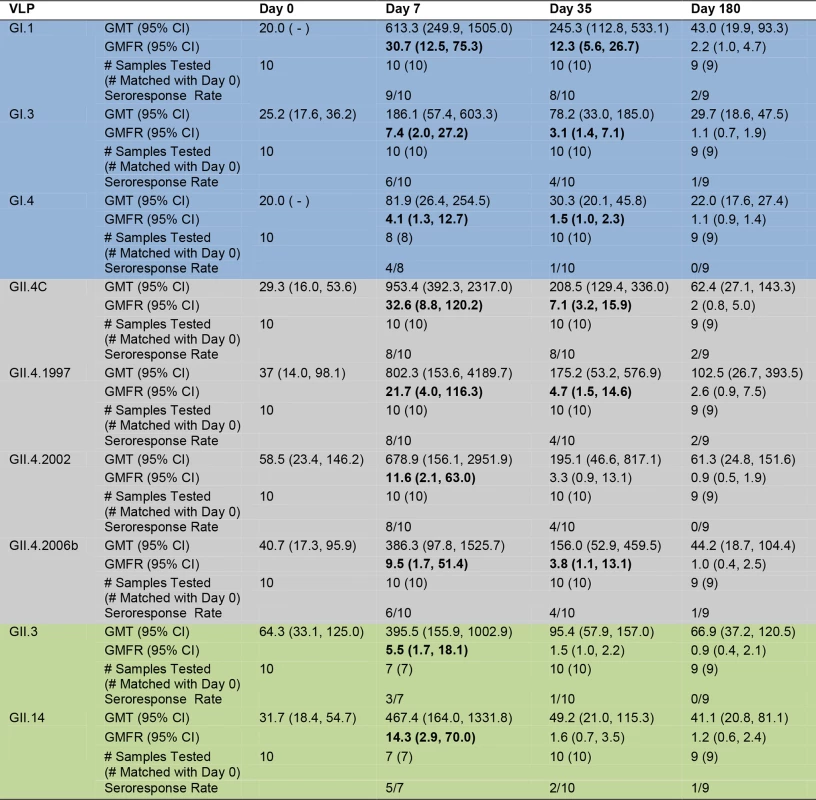
Serum samples collected from participants who received the 50/50-μg VLP dose were assayed for blockade Ab to a panel of GI (blue), GII.4 (grey), and non-GII.4 GII (green) VLPs. The seroresponse rate is the ratio of the number of participants with a ≥4-fold titer increase above day 0 titer compared to the total number of samples tested at day 0 for each VLP. Bolded values denote significant increases in GMFR above baseline. The nearly 100% NoV seropositivity in adults indicates wide NoV exposure across the population [38]. Because Ab matures through somatic hypermutation (SHM) after each additional antigen encounter [47], we evaluated the ratio of blockade Ab to reactive IgG for each VLP as a potential marker of the specificity and maturity of the vaccine-induced Ab response amongst the panel of VLPs. A ratio ≥ 1 suggests that serum may have more blockade activity per IgG activity and thus may be more mature and the result of a repeat exposure. The ratio of blockade Ab to IgG increased ∼3-fold above baseline for GI.1 at day 7, while remaining unchanged for GI.3 and GI.4 (S5 Fig.). These data suggest that vaccination may primarily induce production of Abs with enhanced potency for GI.1 blockade, likely because of SHM of preexisting cross-GI blockade Abs that were affinity-matured against GI.1 post-vaccine exposure, while maintaining some fraction of cross-strain blockade Abs.
For GII.4 VLPs (Fig. 4), the day 0 geometric mean blockade Ab titers were relatively low, with significant increases in GMFRs at day 7 for all GII.4 VLPs and at day 35 for GII.4C, GII.4.1997, and GII.4.2006b. Blockade titers were similar across the panel of different GII.4 strains, with no significant differences in GMFR at any time point. Titers peaked at day 7 post-vaccination, rising 32.6-fold for GII.4C, 21.7-fold for GII.4.1997, 11.6-fold for GII.4.2002, and 9.5-fold for GII.4.2006b. Day 35 GMFRs for blockade Ab titers were 7.1 for GII.4C, 4.7 for GII.4.1997, 3.3 for GII.4.2002, and 3.8 for GII.4.2006b. At day 180, blockade Ab titers approached baseline (1.1 - to 2.6-fold) for all of the GII.4 VLPs. Secretor-negative participants responded similarly to secretor-positive participants across the GII.4 panel (S2 Fig.). Individually (S4 Fig.), 8/10 participants responded with a ≥4-fold increase in blockade Ab titer to GII.4C at day 7 and day 35. At day 180, ≥4-fold titers were maintained in 2/9 of the participants. Similar high levels of fold increase above baseline blockade Ab titer extended across the GII.4 panel at day 7 (GII.4.1997 and GII.4.2002, 8/10; GII.4.2006b, 6/10). By day 35, 4/10 of participants maintained titers ≥4-fold above baseline for GII.4.1997, GII.4.2002, and GII.4.2006b. At day 180, 2/9 participants sustained ≥4-fold titers to GII.4.1997, and 1/9 participants to GII.4.2006b. Notably, the ratio of blockade Ab to IgG at day 7 compared to the baseline ratio increased for all GII.4 VLPs (fold increase: GII.4C, 5.2; GII.4.1997, 4.1; GII.4.2002, 2.2; GII.4.2006b, 2.9) (S5 Fig.), suggesting that vaccination induces a broad GII.4-reactive response despite strain evolution over the 15-y period represented in the panel.
Baseline blockade Ab titers to GII.3 and GII.14 non-vaccine types were low and had significant increases in GMFR only on day 7. As a group (Fig. 4), titers peaked at day 7 at 5.5-fold (GII.3) and 14.3-fold (GII.14) above baseline. Elevated blockade Ab titers were not boosted or maintained at day 35 or 180, although some individuals did maintain ≥4-fold increases at these later time points (S4 Fig.). Contrary to IgG findings, secretor-negative status did not associate with higher GMFR in blockade Ab titer post-vaccination (S2 Fig.). The ratio of blockade Ab to IgG increased for both VLPs at day 7 compared to day 0 (fold increase: GII.3, 3.1; GII.14, 6.8) (S5 Fig.) before returning to baseline ratios, supporting GII.4 VLP data indicating that vaccination induces a rapid, broad GII Ab response.
Elevated Preexisting Blockade Antibody Titers against Any Norovirus Virus-Like Particles Do Not Prevent a Vaccine Response
Preexisting blockade Ab titers have been shown to correlate with protection from GI.1 infection and illness in a NoV challenge study [23]; however, the effect of preexisting Ab on vaccine response is unknown. The absence of a titer boost after the second vaccine dose suggests that preexisting Ab titers may have a limiting effect on the magnitude of subsequent vaccine responses or that vaccine-induced Ab titers plateau [37,42]. To determine whether elevated blockade Ab titer in the 50-μg dose group may have impacted the multivalent VLP vaccine response, we evaluated the association between day 0 blockade Ab titers to any of the NoV VLPs and the likelihood of developing a ≥4-fold increase in blockade Ab titer. The lack of preexisting blockade Ab to any NoV VLP tested significantly correlated with increased vaccine response by day 7 (p < 0.001, chi-squared test) (Fig. 5A). Independent of VLP tested, 51 out of 57 (89.5%) samples with a ≥4-fold increase in blockade Ab titer post-vaccination had no detectable VLP-specific blockade Ab titer at day 0. Sera with baseline blockade Ab titer below the limit of detection were 2.6 (excluding vaccine VLP responses for the analysis) or 3.1 times (including all VLPs) more likely to have a ≥4-fold increase in blockade Ab titer to the same VLP at day 7 post-vaccination compared to participants with higher blockade Ab titers. However, further analyses of the relationship between day 0 blockade Ab titers and day 7 titers revealed no significant association (Fig. 5B), regardless of inclusion of vaccine VLP responses, as participants with higher baseline titers also responded with an increase in titer of ≥4-fold at day 7. Together these data show that the presence of preexisting blockade Ab does not prevent a NoV blockade Ab response to vaccination, but instead that an Ab titer plateau can be reached regardless of baseline titer.
Fig. 5. Day 0 blockade antibody titers below the assay limit of detection for any norovirus virus-like particle are predictive of a ≥4-fold increase, but not overall blockade Ab titer, at day 7. 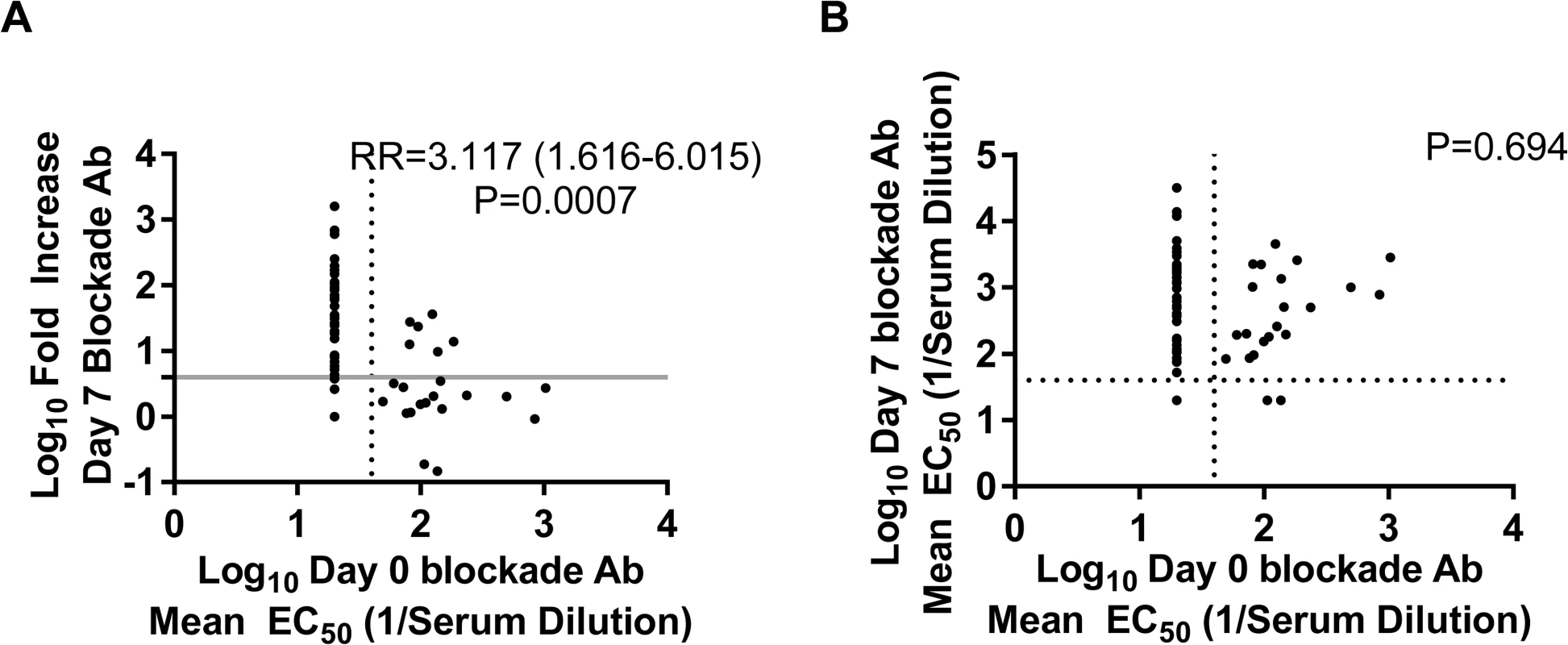
Day 0 blockade Ab titers for all of the NoV VLPs studied were compared to the corresponding day 7 fold increase (A) and titer of blockade Ab (B) using GEEs (n = 10 participants, 82 samples). The likelihood of responding to vaccination with a ≥4-fold increase in blockade Ab titer to any NoV VLP was 3-fold greater if the day 0 titer was below the assay limit of detection. Day 0 blockade Ab titer did not correspond with day 7 titer. The dotted lines mark the lower limit of detection of the blockade Ab assay. The solid grey line marks a 4-fold increase in blockade titer at day 7. RR, relative risk. Vaccination with the Multivalent Virus-Like Particle Candidate Vaccine Induces Antibody That Blocks Novel GII.4 Norovirus Strain Virus-Like Particles
The data described above clearly indicate that vaccination induces a boost in blockade Ab across GII.4 VLPs that circulated before the vaccination program. To evaluate Ab activity against a newly emergent GII.4 strain for which preexisting immunity is unlikely, we tested the serum samples for reactive IgG and blockade Ab titer against two novel GII.4 strain VLPs (Figs. 1 and 6–8). GII.4.2012 began circulating globally in 2012, more than a year after the trial sample collection was completed. GII.4.2006b.P.D302 VLP represents a unique GII.4 strain sequenced from an immune-compromised patient with symptomatic NoV infection for over 1 y [45,48]. Importantly, GII.4.2006b.P.D302 is divergent from GII.4.2006b at all three evolving blockade epitopes, resulting in loss of Ab blocking activity of mouse and human polyclonal sera and mAbs (Fig. 8 and [45]). As the extensive cross-reactivity studies described above had depleted the finite volume of each serum sample that had been set aside for these exploratory studies, we included day 21 serum samples from the same participants in the novel GII.4 VLP studies. Consequently, responses to the novel GII.4 VLPs are likely impacted by sample number loss due to volume depletion (Figs. 6, 7, and S1–S4). Similar to the titers for the other GII.4 strains, baseline reactive IgG GMTs to GII.4.2012 and GII.4.2006b.P.D302 were relatively low, with significant increases in GMFRs at day 7, 21, 35, and 180 to GII.4.2012 (Fig. 6). GII.4.2012 titers peaked at 4.1-fold on day 7, followed by 2.8-fold at day 21, 2.3-fold at day 35, and 1.5-fold at day 180 after dose 1. IgG GMFRs to both novel GII.4 VLPs were similar between secretor-negative and -positive participants (S1 Fig.) Individually, GII.4.2012 seroresponse rates were 3/7 at day 7, 1/9 at day 21, 2/9 at day 35, and 0/7 at day 180 (S3 Fig.). IgG GMFRs to GII.4.2006b.P.D302 were significant on day 7, 21, and 35, rising 2.2-, 2.2-, and 2.0-fold, respectively. Unlike for the other VLPs tested, GII.4.2006b.P.D302 IgG titers did not change ≥4-fold with vaccination at any time point (Figs. 6 and S3).
Fig. 6. Mean EC50 IgG titers to novel GII.4 strain virus-like particles. 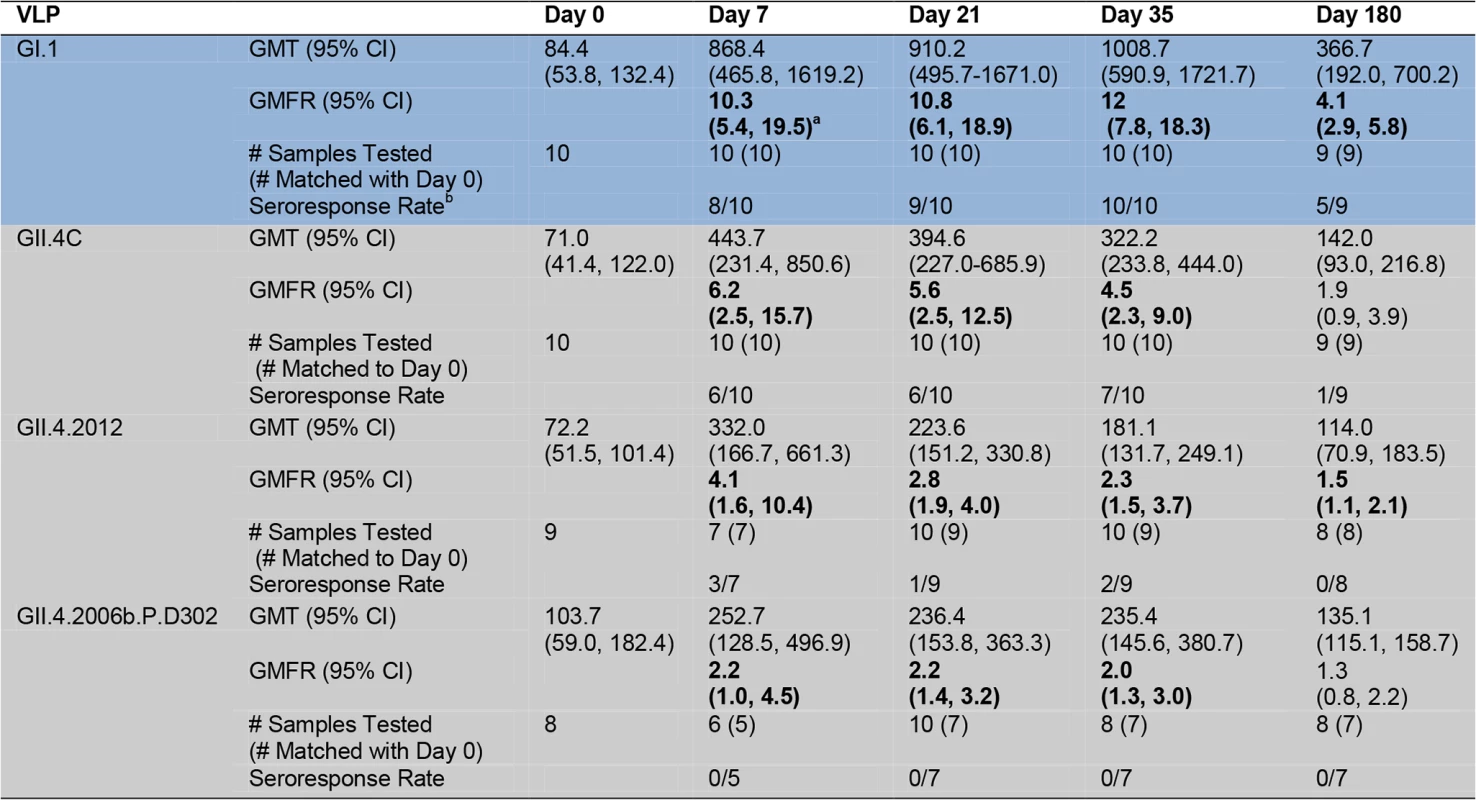
Serum samples collected from participants who received the 50/50-μg VLP dose were assayed for IgG reactivity to the vaccine components and to two novel GII.4 VLPs. GI VLP is shaded blue; GII.4 VLPs are shaded grey. The seroresponse rate is the ratio of the number of participants with a ≥4-fold titer increase above day 0 titer compared to the total number of samples tested at day 0 for each VLP. Bolded values denote significant increases in GMFR above baseline. Fig. 7. Mean EC50 blockade antibody titers to novel GII.4 strain virus-like particles. 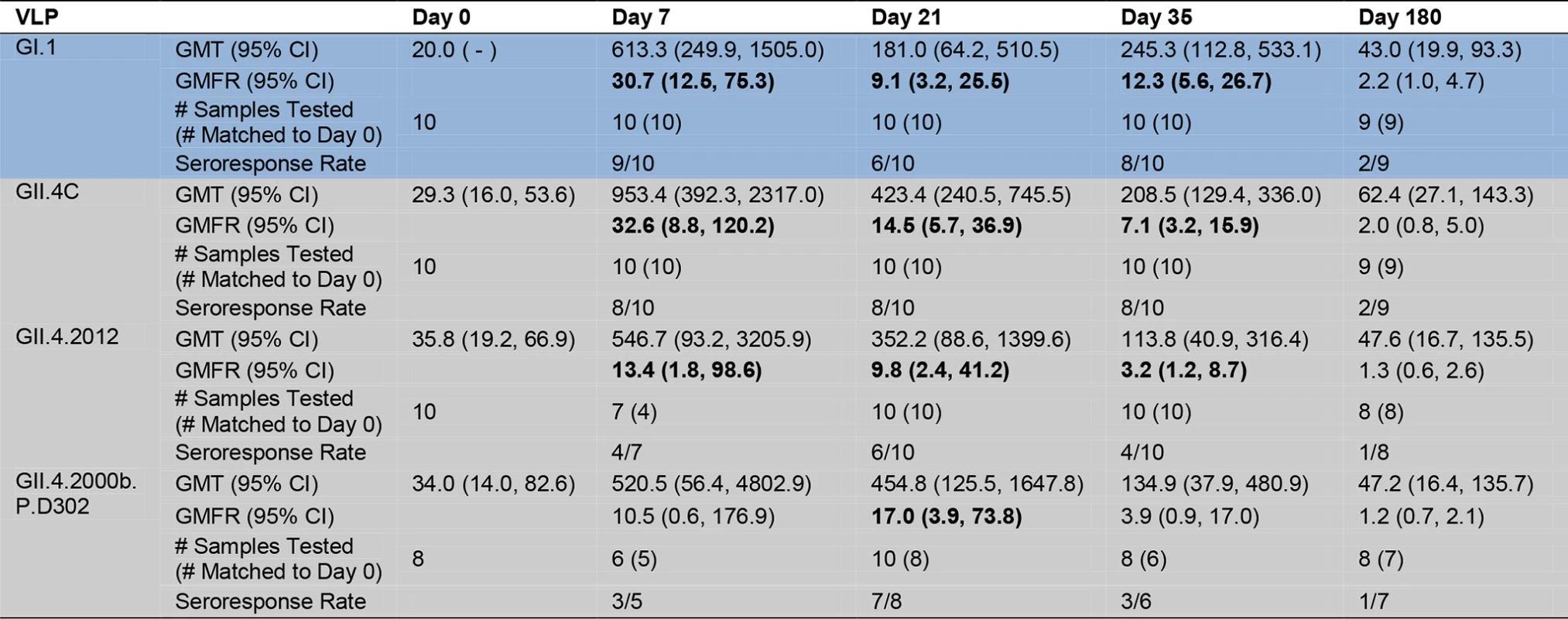
Serum samples collected from participants who received the 50/50-μg VLP dose were assayed for blockade Ab to the vaccine components and to two novel GII.4 VLPs. GI VLP is shaded blue; GII.4 VLPs are shaded grey. The seroresponse rate is the ratio of the number of participants with a ≥4-fold titer increase above day 0 titer compared to the total number of samples tested at day 0 for each VLP. Bolded values denote significant increases in GMFR above baseline. Fig. 8. Amino acid sequence of identified GII.4 blockade antibody epitopes (A, D, and E) and the regulating domain of epitope F (NERK motif) in GII.4 virus-like particles relevant to this study. 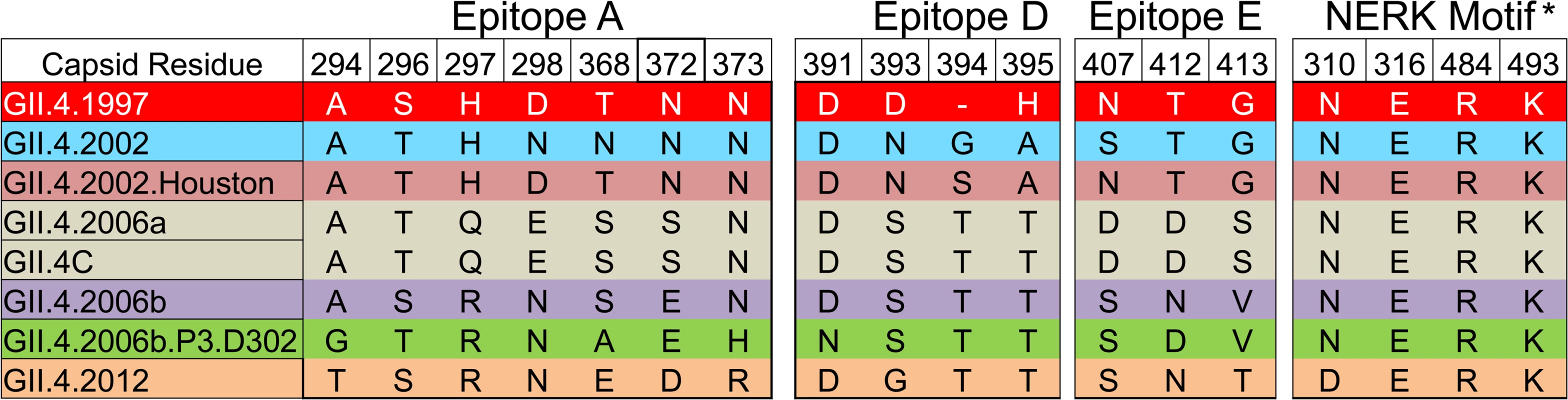
Color indicates antigenic groupings based on epitope A sequence. *The amino acid coordinates of epitope F are unknown. The NERK motif is a temperature-sensitive regulator of Ab access to epitope F [32]. Similar to IgG titers, as a group, day 0 geometric mean blockade Ab titers against both novel GII.4 VLPs were low, with significant increases in GMFR on day 7, 21, and 35 for GII.4.2012 (Fig. 7). GII.4.2012 titers peaked at day 7 post-vaccination, rising 13.4-fold, followed by 9.8-fold on day 21 and 3.2-fold on day 35, before returning to baseline on day 180. Blockade Ab GMFRs to both novel GII.4 VLPs were similar between secretor-negative and -positive participants (S2 Fig.). Individually (S4 Fig.), 4/7 participants had a ≥4-fold increase in blockade Ab titer to GII.4.2012 at day 7, 6/10 at day 21, 4/10 at day 35, and 1/8 at day 180. GII.4.2006b.P.D302 titers peaked at day 21 at 17.0-fold above baseline. Day 35 titers were 3.9-fold above baseline. The lack of significant response at day 7 and day 35 is likely impacted by additional sample depletion (Figs. 7, S2, and S4). At day 180, blockade Ab titers had returned to near baseline. Individually (S4 Fig.), 3/5 participants had a ≥4-fold increase in blockade Ab titer to GII.4.2006b.P.D302 at day 7, 7/8 at day 21, 3/6 at day 35, and 1/7 at day 180. The ratio of blockade Ab to IgG increased for both VLPs at day 7 compared to day 0 (S5 Fig.) before returning to baseline ratios. As identified for the other GII.4 VLPs, the ratio of blockade Ab to IgG was greater than one through day 35, indicating that the GII.4.2012 and GII.4.2006b.P.D302 blockade Ab may be mature Ab derived from previous NoV exposures. Importantly, these data demonstrate that NoV multivalent VLP vaccination can induce a blockade Ab response to a GII.4 strain unknown at the time of vaccination, suggesting that broad-based NoV immunity by vaccination is an achievable goal.
Multivalent Virus-Like Particle Vaccine-Induced Antibody Responses Associate with Antibody Responses to Early (1997–2002) GII.4 Strains
To test for any relationship between the vaccine components and other NoV VLPs that may be indicative of shared epitopes, we first compared the antigenic relationship of the panel of NoV VLPs based on IgG reactivity and blockade Ab titer using MDS computational and statistical approaches. These approaches are a powerful tool to help visualize and analyze similarities between samples across a range of variables, in this case, analyzing the similarities between different VLPs (samples) based on their likelihood of an immune response being raised across a range of individuals (variables). In order to assess the overall similarities that pairs of VLPs exhibited across sera from vaccinated individuals, we calculated Euclidean distances, D (see S1 Table), between each VLP pair at each time point. Euclidean distances are calculated such that two VLPs possessing identical EC50 values for each tested serum will have a D of 0; with increasing dissimilarity in the responses these VLPs share within any specific sera, D will increase. In our analysis of IgG similarities, each D unit equaled roughly a 3.4-fold difference in overall serum response. We utilized MDS to identify XYZ coordinates for each VLP that accurately capture the D values between VLPs, as described previously [46,49]. This approach allowed us to visualize and analyze the cross-reactivity of the IgG response over the time course of this study (Fig. 9).
Fig. 9. Evolving IgG responses to virus-like particles throughout the time course of the study. 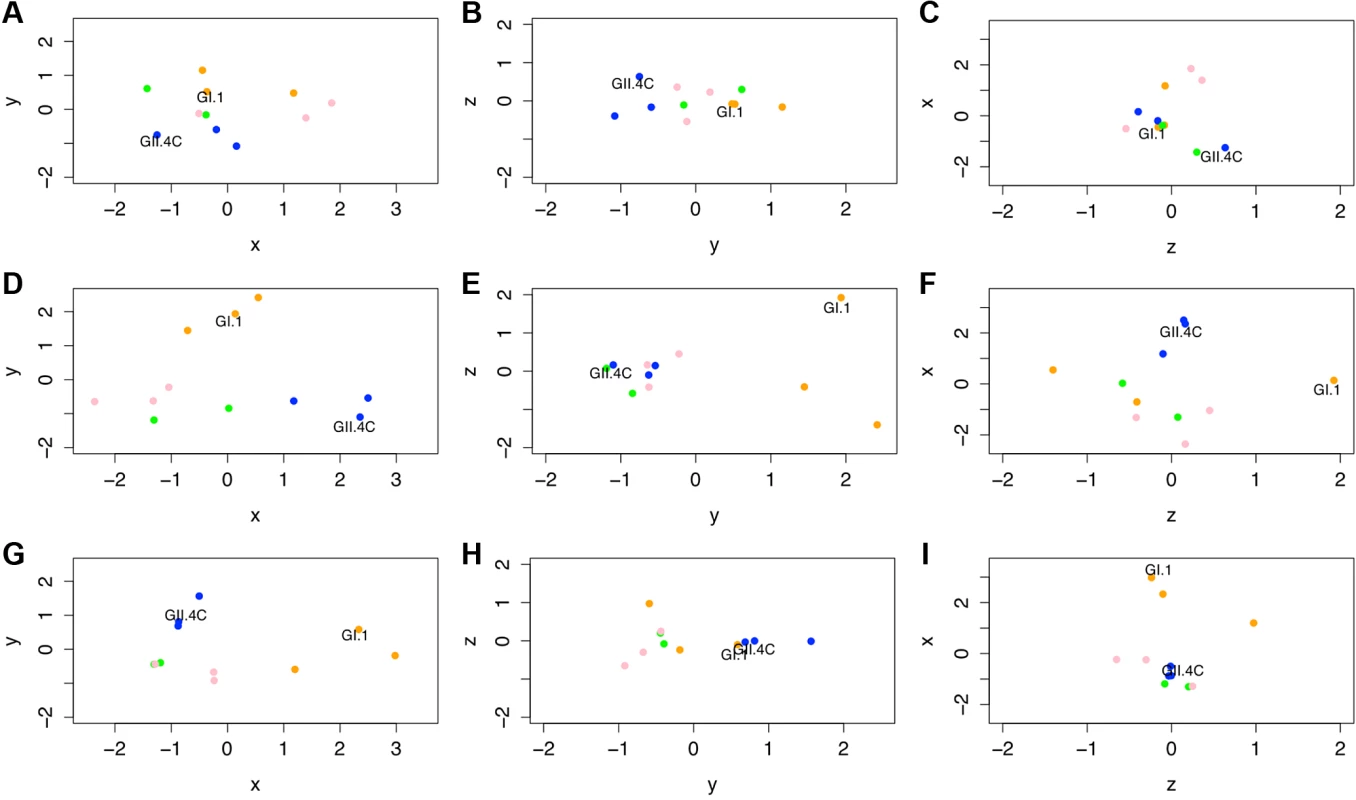
Individual points represent VLPs, and the distances between points represent the overall differences in the magnitude of IgG responses from all ten participants against these NoV VLPs on day 0 (A–C), day 7 (D–F), and day 180 (G–I). Specifically, the distance between VLPs (with each unit in any dimension [D] relating to a 3.4-fold difference in total IgG responses) shows how similar total IgG responses across the ten participants were to each pair of VLPs tested within this study and how vaccine components (noted in each panel) cluster with other NoV strain VLPs. Despite similarities across these responses, we are able to show that GI VLPs (orange) cluster with the GI.1 vaccine component, early GII.4 VLPs (blue) cluster with the GII.4C vaccine component, and late GII.4 VLPs (green) and the other GII VLPs (pink) cluster together and away from the other VLPs. For each time point, the x-axis is that showing the most variation between all VLPs, then the y-axis, then the z-axis. Therefore, down each column, we can see how immune responses change and track through the time course of the study. Of note are the clusterings of each virus subtype through these responses. At day 0 before vaccination, the NoV VLPs were generally clustered together and did not sort by subtypes (GI versus early GII.4 [1997 and 2002] versus late GII.4 [2006b and 2012] versus other GII genotype VLPs; Fig. 9A–C), with the average D between VLPs being 1.79 (range 0.55–3.34). At day 7, the peak of vaccine response (Fig. 9D–F), the distance between VLPs expanded, with an average D of 3.07 (range 1.12–4.89), illustrating the fact that individual vaccine recipients mounted responses to aspects of the vaccine, but that these responses were not universal across VLPs (if this were the case, all VLPs would still remain closely clustered), specifically when examining VLPs not included in the vaccine formulation. As could be seen when examining the primary IgG response to GI.1, there was a distinct response to this vaccine component. Utilization of antigenic cartography allowed us to show that the responses to this VLP were quite distinct from those raised against other VLPs. In particular, the D values between GI.1 and all GII viruses (average D = 3.79, range 3.0–4.24) show that the response raised against the GI.1 component of the vaccine was fairly independent of the responses raised against GII VLPs. While not attaining multiple-test-correcting significance (t25 = 4.795, p = 0.038), the effect of cross-reactivity between GI.1 and the other GI VLPs was apparent in our analysis. These VLPs are individually fairly distant from GI.1 (D of 3.4 and 2.7), but as a trio they segregate away from the GII VLPs (average D = 3.59, range 2.24–4.56). This result illustrates our ability to detect even weak secondary responses against these dissimilar GI VLPs by utilizing antigenic cartographic approaches.
As described above, GII VLPs were distinct from the GI VLPs at 7 d post-vaccination. Interestingly, the GII.4C VLP (representing the vaccine component) associated much more closely with the early GII.4 VLPs than with either the late GII.4 VLPs or the GII VLPs from other genotypes (ANOVA: F2,4 = 14.74, p = 0.0143), showing that the IgG response to GII.4C vaccination is highly cross-reactive with the early GII.4 VLPs but not the antigenically distinct contemporary GII.4 VLPs or other GII VLPs. Even as late as 180 d post-vaccination (Fig. 9G–I), clustering by genogroup (or by subclades within genogroups) remained (t-test: t53 = 23.96, p < 0.001), although overall distances (dissimilarities) in IgG responses differentiating between VLPs had decreased compared to day 7. Specifically, the GI viruses clustered relative to the other virus strains (t25 = 11.32, p = 0.0024), GII.4C continued to cluster with GII.4.1997 and GII.4.2002 (t25 = 8.973, p = 0.0061), and the contemporary GII.4 VLPs remained tightly clustered with the other GII genotypes (t38 = 19.24, p < 0.001). Interestingly, and echoing our findings above, GII.4.1997 remained somewhat distinct from GII.4C and GII.4.2002 (Fig. 9G–I) because of the elevated levels of IgG against GII.4.1997 VLP still found in several of the vaccine recipients 6 mo post-vaccination.
Similar analyses using blockade Ab titers rather than IgG titers supported several of these findings, with the D values calculated for blockade titers corresponding to roughly a 3.2-fold change in blockade levels. In contrast to the tight clustering of VLPs in the EIA-reactive IgG before vaccination, the spread of the distances between VLPs was greater (average D = 2.88, range 0–4.52), indicative of the strain specificity of blockade Ab as well as the individual-to-individual variation in pre-immunization blockade levels (Fig. 10). There was some limited cross-reactivity even at day 0 (Fig. 10A–C), with subtypes being significantly more related than genotypes (t43 = 11.99, p = 0.001; within-subtype average D = 1.77, range 0–2.79; between-subtype average D = 3.13, range 0.77–4.52).
Fig. 10. Evolving blockade antibody responses to virus-like particles throughout the time course of the study. 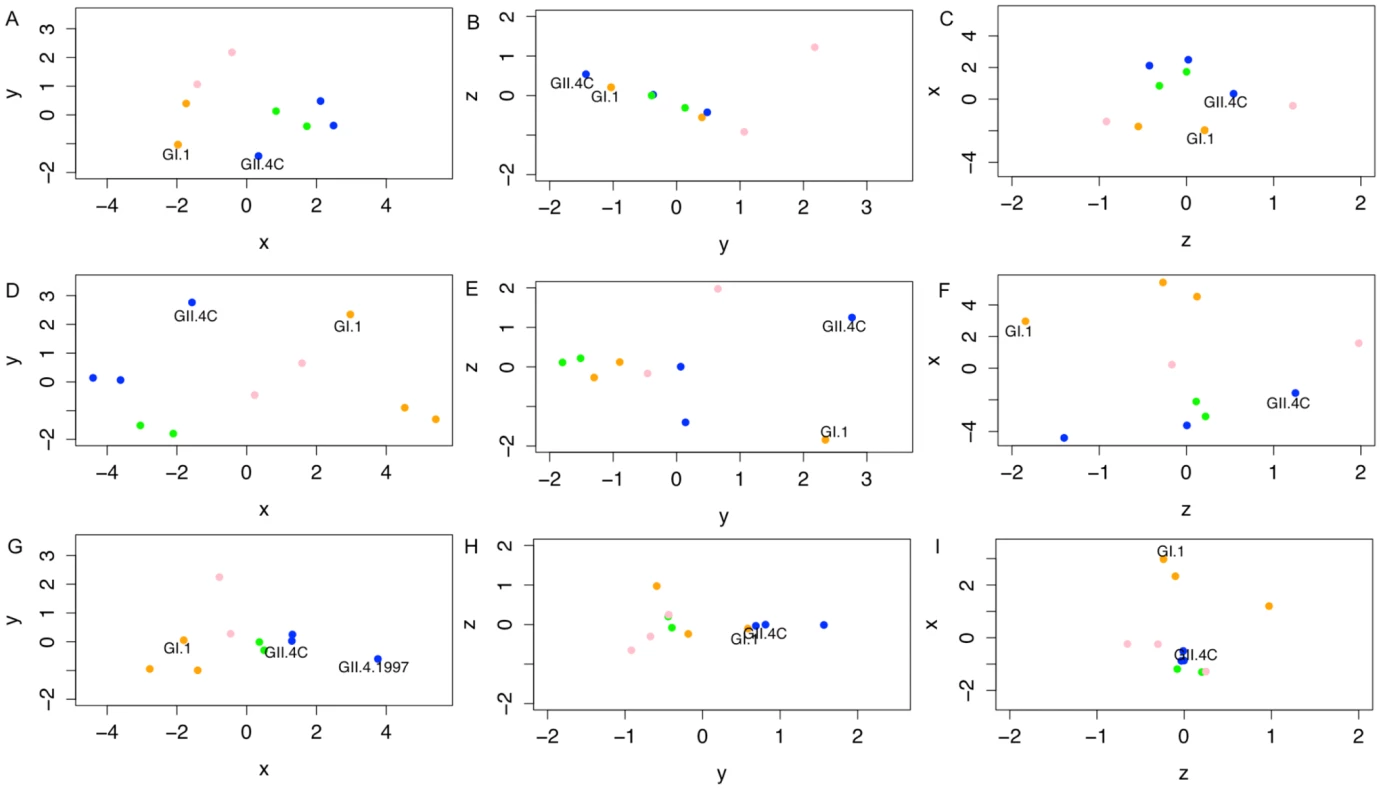
Individual points represent VLPs, and the distances between points represent the overall differences in the magnitude of blockade Ab responses from all ten participants against these NoV VLPs on day 0 (A–C), day 7 (D–F), and day 180 (G–I). Specifically, the distance between VLPs (with each unit in any dimension [D] relating to a 3.2-fold difference in blockade Ab, or EC50 response) shows how similar total blockade Ab responses across the ten participants were between each pair of VLPs tested within this study, and how vaccine components (noted in each panel) cluster with other NoV strain VLPs. Across these responses, we are able to show that GI VLPs (orange) cluster with the GI.1 vaccine component, early GII.4 VLPs (blue) cluster with the GII.4C vaccine component, and the late GII.4 VLPs (green) and the other GII VLPs (pink) cluster together and away from the other VLPs. For each time point, the x-axis is that showing the most variation between all VLPs, then the y-axis, then the z-axis. Therefore, down each column, we can see how immune responses change and track through the time course of the study. Of note are the clusterings of each virus subtype through time, although with clear distinction of the vaccine components at day 7. At day 180, as titers have fallen, the blockade Ab distinctions between VLPs are diminished across the panels (G–I), with the exception of responses to GII.4.1997, suggesting that a memory Ab response to this strain may be driving the GII.4-reactive vaccine response. At 7 d post-vaccination (Fig. 10D–F), there was a wider range of D values (average D = 5.43, range 1.86–10.10). There was still a significant relationship between subtypes (t43 = 9.327, p = 0.0039), although the strong participant-specific responses to some VLPs obscured some of these groupings. Genotype grouping could not be definitively assigned to any given vaccine responses, as the GI VLPs remained only moderately clustered together (t22 = 7.517, p = 0.011; multiple-test cutoff is p = 0.0166), and while the GII VLPs clustered together (t40 = 40.11, p < 0.001), the GII.4C VLP did not cluster with any given GII subgroup. Consistent with our other observations above, while we cannot assign statistical confidence to this observation (as there is only a single D value to contrast), the two vaccine components (GI.1 and GII.4C) appeared to slightly co-segregate within the three-dimensional space (Fig. 10D–F), corresponding to the strong vaccine responses driven across vaccine recipients.
At day 180 (Fig. 10G–I), the distance between VLP clusters decreased (average D = 3.07, range 1.19–6.57), but was still elevated relative to pre-vaccine levels. In contrast to other time points, there was only a very moderate subgroup-based clustering (t43 = 4.277, p = 0.045), which could not be attributed to any given subgroup. Most of the variation within these responses is due to the maintenance of blockade Ab to GII.4.1997 VLP (t43 = 29.8, p < 0.001). These data support our premise that the GI.1 response can be characterized as an immature Ab response while the GII.4C response can be characterized as a memory Ab response driven by Abs that react primarily with epitopes present in GII.4.1997. We arrive at this conclusion specifically because blockade Ab responses are much stronger for GII.4.1997 than even for the vaccine component GII.4C.
Vaccination with the Multivalent Virus-Like Particle Candidate Vaccine Induces Blockade Antibody Responses to Multiple Epitopes Cross-Reactive between Antigenically Divergent GII.4 Virus-Like Particles
To expand on the antigenic cartography findings identifying Abs to GII.4.1997 as key driving components of the vaccine-induced Ab response, we then asked whether the GII.4 Ab response generated after vaccination could be explained by activation of Abs to known GII.4 blockade epitopes A or F. We evaluated the ability of day 0, 7, 35, and 180 serum samples to block binding of mAbs to highly variable, surface-exposed blockade epitope A and sub-surface, conserved blockade epitope F using the blocking of binding (BOB) assay and GII.4.1997 and GII.4.2006b VLPs. GII.4.1997 and GII.4.2006b are antigenically distinct at evolving blockade epitope A (Fig. 8). Mouse mAb GII.4.1987.G1 and GII.4.2006.G2 [27] recognize epitope A of GII.4.1997 and GII.4.2006b, respectively. Mouse mAb GII.4.2002.G5 [32] recognizes the epitope F conserved among the GII.4 types. All serum samples remaining were tested for BOB. Pre-vaccination sera (n = 9) did not block binding of mAbs to epitope A or to epitope F to either VLP (Fig. 11). At day 7 (n = 7), significant broad BOB of epitope A and F mAbs was observed for both GII.4.1997 and GII.4.2006b, with significantly higher levels of inhibition noted against the GII.4.1997 strain for both epitopes (epitope A, 7-fold higher; epitope F, 5-fold higher). By day 35 (n = 8), significantly higher BOB titers to only epitope A of both strains remained. At day 180, titers (n = 8) to GII.4.1997 epitope A persisted but at levels not significantly different from baseline titers. These findings support the antigenic cartography analyses indicating that Ab to structures present in GII.4.1997 may be more persistent than Ab to other structures. Further, it suggests that the broad cross-reactive GII.4 Ab response targets residues either within, near, or regulating access to multiple epitopes, including epitopes A and F.
Fig. 11. Vaccination results in a rapid but transient increase in antibody titer to multiple blockade epitopes in multiple GII.4 strains. 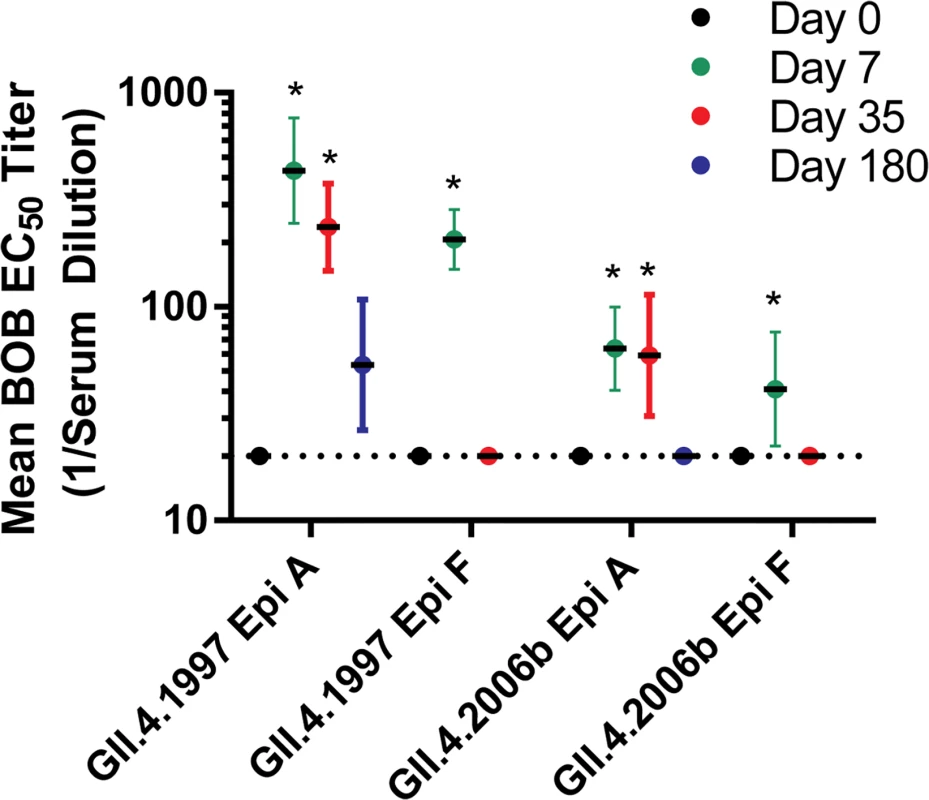
Serum samples were evaluated for ability to block binding of mouse mAbs to epitope (Epi) A or F in GII.4.1997 and GII.4.2006b using a BOB assay. Sigmoidal curves were fit to the mean percent control binding (percent of mouse mAb bound to VLP in the presence of serum pretreatment compared to the amount of mouse mAb bound in the absence of serum pretreatment), and the mean EC50 titer (1/serum dilution) for BOB calculated. Dotted line marks 0.5 times the assay limit of detection. Error bars represent 95% confidence intervals. An asterisk indicates that the EC50 titer is significantly different from that of day 0. Discussion
The success of the rotavirus vaccination campaign has elevated NoV to the main cause of acute viral gastroenteritis in infants and young children in the US and elsewhere, and, subsequently, has focused attention on the development of a NoV vaccine (reviewed in [36,50]). Here we provide evidence that a NoV VLP-based vaccine induced broadly reactive IgG and blockade Ab responses among antigenically diverse NoV VLPs, as has been shown for mice immunized with a multivalent NoV VLP vaccine [44] and humans infected with GI.1 [51]. Ab cross-reactivity included two GII.4 VLPs representing viral strains that did not circulate within the population before the study sample collection was completed, indicating that vaccination may protect against emergent GII.4 strains that are antigenically distinct from the vaccine components.
As NoV strains share common Ab epitopes, the Ab detection assays utilized here were optimized for specificity by limiting the amount of antigen. This approach allows detection of subtle differences in Ab affinities between closely related strains but decreases assay sensitivity, resulting in more samples registering below the limit of detection. Similarly, the high valency of PGM facilitates binding of many diverse NoV VLPs to a single, readily available substrate but also translates to increased serum titers needed for Ab blockade of VLP–ligand binding compared to the less valent synthetic carbohydrates frequently used [25,29]. Consequently, the magnitude and duration of Ab responses (GMTs and GMFRs) in these analyses were lower than those reported by other vaccine investigations, which used ∼10× more antigen in Ab detection assays and synthetic carbohydrates for blockade Ab assays [37]. The limiting amount of antigen likely also caused underestimation of the baseline differences between participants of secretor-negative and -positive phenotypes. Further, the VLPs used here, except GII.4C, were produced at 37°C from mammalian cell expression vectors, whereas the GII.4C VLPs and the vaccine VLPs were produced at a lower temperature in insect cells. How these differences in VLP production might affect particle structure and Ab binding is unclear, but antigenic differences have been described between NoV VLPs manufactured in the two different expression systems [32].
Pre-exposure history likely shaped the breadth and magnitude of the vaccine-induced blockade Ab cross-reactivity. Although a secretor-negative phenotype is associated with decreased risk of infection with some NoV strains [18,40], secretor status was not a primary driver of vaccine response, as secretor-negative participants responded similarly to secretor-positive participants for blockade Ab to the entire panel of VLPs, in concordance with in vitro [38] and in vivo [39–41] data indicating that some GI and GII NoV strains bind to secretor-negative carbohydrates and infect secretor-negative individuals. Further, as GII.4 strains commonly circulate, it is likely that participants in the study have a recent exposure history to GII.4, and this may have influenced the characteristics of the vaccine response. The increased ratio of blockade Ab to EIA-reactive IgG across GII.4 VLPs suggests that, likely as a result of previous exposure to GII.4 strains through natural infection, the GII.4C vaccine component response may be driven by activation of preexisting, cross-reactive memory B cells, as has been described for broadly neutralizing influenza Ab [52,53], while the GI.1 response may be driven by SHM and expansion of more strain-specific B cells (Fig. 12). Higher preexisting blockade Ab titers did not interfere with additional Ab production, but individuals with higher baseline titers tended to have lower fold increases, which may be indicative of a ceiling for maximum responses, as described for measles virus vector and some influenza A vaccinations [54,55]. Similarly, lack of an additional titer boost after a second vaccination <1 mo later is documented for influenza vaccinations, where the vaccine regimen achieves high levels of seroresponse and seroprotection (hemagglutination inhibition > 40) after only one dose [56,57], indicating that higher Ab titers correlate with a lower magnitude of response but not necessarily less protection from infection. Of note, in the influenza studies cited above, children and older adults had better Ab responses after two vaccine doses. Given that both of these demographics would be key candidates for a NoV vaccine, follow-up studies of different age cohorts will be necessary and are ongoing. Such studies will specifically address the kinetics and breadth of the blockade Ab response in children and may clarify the relationship between pre-exposure history and vaccination response.
Fig. 12. Proposed mechanisms for antibody responses induced by GI.1/GII.4C multivalent vaccine. 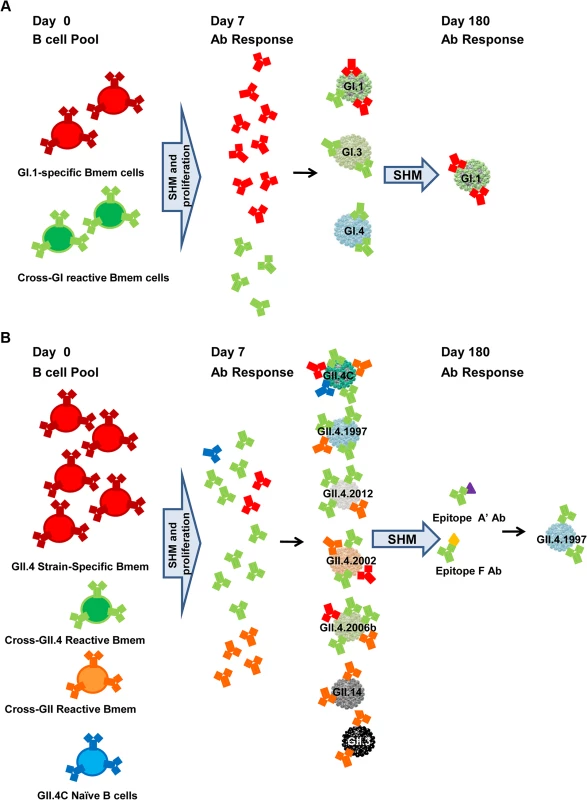
At the time of vaccination, adult participants have a lifetime of NoV exposure history and a pool of NoV-reactive memory B cells (Bmem cells), both strain-specific and strain cross-reactive clones. Vaccination activates memory B cells to undergo SHM of the variable region of the Ab gene, to proliferate, and, for some cells, to differentiate into plasma cells secreting high-affinity Ab by day 7 post-vaccination. The GI.1 vaccine component elicits activation of both GI.1-specific memory B cells and memory B cells with specificity for shared GI epitopes, resulting in increased Ab to the panel of GI VLPs, but the strongest response to the homotypic GI.1 VLP because more blockade epitopes are unique to GI.1 than are shared across the GI VLPs. By day 180, low levels of GI.1-specific Ab persist (A). In comparison, the GII.4C vaccine component does not elicit a strong strain-specific response but could in theory activate memory B cells with GII.4.2002, GII.4.2006a, or GII.4.2006b specificity. However, the uniformity in the GII.4 VLP response across an antigenically diverse panel suggests that GII.4C preferentially activates memory B cells for conserved GII.4 epitopes and a smaller subset of memory B cells for a conserved GII epitope, resulting in more potent GII.4 and less potent GII blockade Ab production at day 7. The GII.4C Ab response continues to track with GII.4.2002 and GII.4.1997 through day 35, but by day 180, only GII.4.1997 Ab responses remain distinct, suggesting that the common GII.4 blockade epitopes recognized by the vaccine-induced Abs are most similar to sequences found in GII.4.1997, possibly because of extensive long-term immune focusing for this strain. Ab responses to at least two epitopes are maintained. Epitope F is a conserved GII.4 blockade epitope located sub-surface on the particle, and proposed epitope A′ is likely a surface-exposed blockade epitope physically near, overlapping, or within epitope A (B). At day 7 post-vaccination, Ab titers peak in magnitude and breadth of reactivity, corresponding with the timing of an expanded B cell clonal population [58]. This broad reactivity may be the result of the multivalency of the VLP vaccine, epitope presentation on a VLP compared to natural virus, or the difference in dose or route of antigen delivered by vaccination versus infection. Alternatively, it is possible that a broad blockade Ab response also occurs after natural infection at day 7, as this has not been tested. Although vaccination induced homotypic serum Ab responses at levels similar to or higher than natural infection in a challenge study [42], titers did not boost after a second immunization, agreeing with our findings. These data suggest one vaccine dose may induce maximum responses. Although, mechanistically, it is unclear why this would be the case in participants with lower preexisting titers, these findings may indicate that for the age group 18–49 y (characterized by extensive previous exposure history), more time may be needed between the first and second vaccination to allow titers to return to baseline and the Ab pool to mature and develop higher affinities. Alternately, serum titers may be decreasing because ASCs may be relocating to the mucosal induction sites (e.g., Peyer’s patches in the intestine), with a resulting boost in a responder B cell population at the site of future exposure to NoV. B cell and ASC migration to the gut has been shown to be critical for conferring protection against intestinal pathogens [59], and memory B cells from beta-7-integrin-deficient mice have been shown to exhibit a decreased capacity to protect against rotavirus infections, implying that gut homing plays an important role in protection against intestinal viruses [60]. To understand NoV immunity and protection from infection, direct measurement of mucosal Ab responses is needed, as mucosal IgA responses have been shown to correlate with protection in GI.1 virus challenge [18]. Challenge studies in vaccinated and non-vaccinated participants need to be done to address these fundamental questions.
The technique of antigenic cartography has been implemented by the World Health Organization to track influenza [49], as the power of cartography in sorting antigenic variants has been demonstrated for influenza [61], enterovirus [62], and NoV [63]. This technique has typically been used to assess the general relationships between variant viruses, using hyperimmune sera to assess antigenic similarities. Here we have slightly modified this approach to utilize variant viruses as probes to disentangle the complex immune responses following vaccination, while maintaining a well-developed analytical framework. This approach recapitulated many of the findings observed by our more traditional analyses of immune responses, and also allowed us to identify several more subtle differences in immune responses across this population of vaccine recipients. Here, IgG and blockade Ab responses to GI.1 are less distant from those to other GI VLPs compared to those to non-GI VLPs, but remain clearly separate from responses to GI.3 and GI.4. In contrast, IgG and blockade Ab responses cluster GII.4C close to the other GII.4 VLPs, while the distance between GII.4C and the other GII VLPs is greater. The distance, and thus antigenic divergence, between GII.4C and GII.4.1997 and GII.4.2002 is relatively close at day 7. By day 180, GII.4C and GII.4.2002 associate very closely with each other and cluster more closely to the other VLPs, and only GII.4.1997 remains distinct.
The strength of the blockade Ab response to GII.4.1997 and degree of cross-blockade among GII.4 VLPs is contrary to findings with mAbs and polyclonal serum collected from outbreaks and immunized mice. Pandemic GII.4 strains can be considered successive immune escape variants. The GII.4 VLPs studied here represent the antigenic diversity of the GII.4 outbreak strains that have circulated worldwide from 1995 to the present. Using human outbreak and immunized-mouse polyclonal sera, GII.4.1997 and GII.4.2002 have some cross-blockade activity between them and are each distinct from GII.4C at the identified GII.4 evolving blockade epitopes A, D, and E. However, GII.4.1997, GII.4.2002, and GII.4C are all blocked by mAbs that recognize epitope F, a conserved GII.4 blockade epitope [25,32], indicating a possible mechanism for cross-GII.4 blockade Ab activity, despite antigenic drift within the GII.4 strains.
Vaccination did lead to increased titers of Ab that interfered with binding of epitope F mAb to GII.4.1997 and GII.4.2006b, as measured by the BOB assay, supporting activation of cross-GII.4 memory B cells with vaccination. Epitope F is conserved but not identical within the GII.4 strains [25], explaining the difference in epitope F BOB potency between GII.4.1997 and GII.4.2006b. Epitope A is highly divergent between pandemic GII.4 strains; thus, it was an unlikely candidate to explain the broad GII.4 blockade Ab response identified here. However, minimizing steric hindrance as the explanation for these results, binding of mAb to epitope F or epitope A did not interfere with subsequent binding of the reciprocal epitope mAb to the immobilized VLP, indicating that Ab binding to one epitope does not necessarily prevent Ab binding at a second epitope [32,64].
Alternatively, the serum BOB to epitope A could be mediated by a rare class of cross-reactive epitope A Abs that are expressed post-vaccination because of the novel antigen that GII.4C represents. Because epitope A is the immunodominant epitope, NoV memory B cells should be numerically dominated by epitope A clones. If, as a chimeric-designed VLP, GII.4C has a unique epitope A, then vaccination could preferentially select for clones that recognize a conserved set of residues that are either part of epitope A or structurally near epitope A but are as yet undefined. Despite significant change within epitope A between GII.4 strains, epitope A does have a linear, conserved stretch of amino acids with demonstrated cross-GII.4 EIA binding with select mAbs [20,27], providing mechanistic support for the possibility of a broadly reactive GII.4 epitope A Ab response. Additional conserved epitope A residues may be identified as more mAbs are developed. The high degree of cross-GII.4 reactivity prevented us from using epitope-exchanged VLPs to specifically map GII.4 epitopes, as we have successfully done with mAbs and sera from infected individuals [25,27].
Both epitope mapping and antigenic cartography identified persistent responses to GII.4.1997, the first known pandemic NoV strain, after vaccination. As all study participants were at least 18 y old, it is highly likely that they were exposed to this first pandemic strain and retained memory B cells from that exposure. The preferential recall Ab response to GII.4.1997 after vaccination with an antigenically distinct VLP, coupled with lower Ab titers to the most recent pandemic strain, GII.4.2006b, all in the context of a broad cross-GII.4 blockade Ab response, suggests that the vaccine targets memory B cells specific for conserved epitopes that originally derived from naïve B cells specific for GII.4.1997 and were subsequently refined by SHM following repeated NoV exposure (Fig. 12). This response pattern, termed “antigenic seniority” has also been identified in influenza vaccinated participants [65]. Like original antigenic sin (OAS), the theory of antigenic seniority predicts that the highest affinity Ab responses will be to the strain primarily circulating during participants’ childhoods and that this first encounter will shape all immune responses to viral variants going forward. With each subsequent virus exposure, memory B cells undergo further maturation. Unlike OAS, antigenic seniority doesn’t predict that responses to the current virus will be negated by the response to the first virus, but instead that the Ab response will be lower in affinity for the most recent variant. Alternatively, vaccination, as opposed to infection, may produce Ab to conserved epitopes that either have a greater affinity for the epitope as it is presented in GII.4.1997 or have better access to the epitope in GII.4.1997. If this is true, titers to GII.4.1997 would predominate and may define the Ab response maximum described. It remains to be seen whether these same Ab response patterns develop with virus challenge. Sequencing and cloning of human Ab repertoires at pre-vaccination and early and late post-vaccination time points, coupled with investigation of the structures of Ab-bound VLPs, would directly address how the Ab response evolves. We further note that we did not see preferred recall of non-vaccine-strain responses to the rarely circulating GI NoVs. While this may be due to the overall similarities within the GI or the GII clusters, our expectation is that completely naïve immune responses should most match the GI.1 or GII.4C vaccine components, and not a non-vaccine-component VLP.
The primary limitation of this study is the incomplete understanding of the relationship between the in vitro blockade Ab assay and protection from infection and illness. In a vaccine challenge study with a GII.4.2002 challenge virus, vaccination did not significantly protect from infection, although participant-reported gastroenteritis symptoms were reduced and symptom severity was decreased. It is difficult to compare these findings to our prediction of potential cross-protection based on blockade Ab response because blockade Ab titers were not reported for those participants [42]. Further, the limited-antigen design of our experiments complicates prediction of protection from infection and understanding of the implications of the lack of titer boost after the second dose and rapid waning of the Ab titer. The diversity of the VLP panel studied is not all-inclusive, as new NoV strains are regularly identified from outbreak investigations. The VLPs used here were selected for their epidemiological importance, antigenic divergence, and availability. Although we cannot discount that Ab responses to additional genotypes may have been identified with a larger VLP panel, to our knowledge, this study characterizes blockade Ab responses to the most antigenically diverse panel of VLPs to date. Furthermore, our work here highlights in many ways the complex interactions between prior exposure, each participant’s ability to respond to variable vaccine components, and the relationships between tested VLPs. Future work assessing the impact of all of these variables will assist in the rational design of vaccines. Despite these limitations, we were able to utilize our molecular, immunological, and computational approaches to identify several important responses to NoV vaccination that allowed for cross-reactivity to other VLPs.
To be effective, any NoV vaccine must induce a protective immune response to novel, epitope-evolved GII.4 viruses. The data presented here suggest that the GII.4C vaccine component Ab response is likely driven by activation of preexisting cross-reactive memory B cells, resulting in cross-blockade Ab production and potential protection from new GII.4 strains not included in the vaccine. Given the considerable disease burden globally, these results provide sufficient optimism for continued human studies and vaccine development.
Supporting Information
Zdroje
1. World Health Organization (2013) Diarrhoeal disease. Fact sheet No. 330. Geneva: World Health Organization.
2. Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (2011) Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep 60 : 1–18. 22108587
3. Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, et al. (2013) Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control 41 : 654–657. doi: 10.1016/j.ajic.2012.08.002 23266383
4. Bok K, Green KY (2012) Norovirus gastroenteritis in immunocompromised patients. N Engl J Med 367 : 2126–2132. doi: 10.1056/NEJMra1207742 23190223
5. Trivedi TK, DeSalvo T, Lee L, Palumbo A, Moll M, et al. (2012) Hospitalizations and mortality associated with norovirus outbreaks in nursing homes, 2009–2010. JAMA 308 : 1668–1675. 23079758
6. Hutson AM, Atmar RL, Estes MK (2004) Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol 12 : 279–287. 15165606
7. Estes MK, Prasad BV, Atmar RL (2006) Noroviruses everywhere: has something changed? Curr Opin Infect Dis 19 : 467–474. 16940871
8. Koopmans M, Vinje J, de Wit M, Leenen I, van der Poel W, et al. (2000) Molecular epidemiology of human enteric caliciviruses in The Netherlands. J Infect Dis 181 (Suppl 2): S262–S269. 10804135
9. Centers for Disease Control and Prevention (2007) Norovirus activity—United States, 2006–2007. MMWR Morb Mortal Wekly Rep 56 : 842–846. 17717513
10. Okada M, Tanaka T, Oseto M, Takeda N, Shinozaki K (2006) Genetic analysis of noroviruses associated with fatalities in healthcare facilities. Arch Virol 151 : 1635–1641. 16521046
11. Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA (2008) Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis 14 : 1546–1552. doi: 10.3201/eid1410.080188 18826817
12. Schorn R, Hohne M, Meerbach A, Bossart W, Wuthrich RP, et al. (2010) Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin Infect Dis 51 : 307–314. doi: 10.1086/653939 20575662
13. Hall AJ, Eisenbart VG, Etingue AL, Gould LH, Lopman BA, et al. (2012) Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg Infect Dis 18 : 1566–1573. doi: 10.3201/eid1810.120833 23017158
14. Kroneman A, Harris J, Vennema H, Duizer E, van Duynhoven Y, et al. (2008) Data quality of 5 years of central norovirus outbreak reporting in the European Network for food-borne viruses. J Public Health (Oxf) 30 : 82–90.
15. Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, et al. (2006) Norovirus classification and proposed strain nomenclature. Virology 346 : 312–323. 16343580
16. Green KY, Lew JF, Jiang X, Kapikian AZ, Estes MK (1993) Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J Clin Microbiol 31 : 2185–2191. 8396590
17. Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, et al. (1999) X-ray crystallographic structure of the Norwalk virus capsid. Science 286 : 287–290. 10514371
18. Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, et al. (2003) Human susceptibility and resistance to Norwalk virus infection. Nat Med 9 : 548–553. 12692541
19. Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, et al. (2008) Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med 5: e0050031. doi: 10.1371/journal.pmed.0050031 18271619
20. Lindesmith LC, Costantini V, Swanstrom J, Debbink K, Donaldson EF, et al. (2013) Emergence of a norovirus GII.4 strain correlates with changes in evolving blockade epitopes. J Virol 87 : 2803–2813. doi: 10.1128/JVI.03106-12 23269783
21. Bok K, Parra GI, Mitra T, Abente E, Shaver CK, et al. (2011) Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A 108 : 325–330. doi: 10.1073/pnas.1014577107 21173246
22. Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, et al. (2010) Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 202 : 1212–1218. doi: 10.1086/656364 20815703
23. Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, et al. (2011) Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med 365 : 2178–2187. doi: 10.1056/NEJMoa1101245 22150036
24. Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, et al. (2007) Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol 81 : 9932–9941. 17609280
25. Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, et al. (2012) Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog 8: e1002705. doi: 10.1371/journal.ppat.1002705 22615565
26. Lindesmith LC, Donaldson EF, Baric RS (2011) Norovirus GII.4 strain antigenic variation. J Virol 85 : 231–242. doi: 10.1128/JVI.01364-10 20980508
27. Debbink K, Donaldson EF, Lindesmith LC, Baric RS (2012) Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity. J Virol 86 : 1214–1226. doi: 10.1128/JVI.06189-11 22090110
28. Cannon JL, Lindesmith LC, Donaldson EF, Saxe L, Baric RS, et al. (2009) Herd immunity to GII.4 noroviruses is supported by outbreak patient sera. J Virol 83 : 5363–5374. doi: 10.1128/JVI.02518-08 19297483
29. Debbink K, Lindesmith LC, Donaldson EF, Costantini V, Beltramello M, et al. (2013) Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis 208 : 1877–1887. doi: 10.1093/infdis/jit370 23908476
30. Donaldson EF, Lindesmith LC, Lobue AD, Baric RS (2008) Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev 225 : 190–211. doi: 10.1111/j.1600-065X.2008.00680.x 18837783
31. Lindesmith LC, Debbink K, Swanstrom J, Vinje J, Costantini V, et al. (2012) Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J Virol 86 : 873–883. doi: 10.1128/JVI.06200-11 22090098
32. Lindesmith LC, Donaldson EF, Beltramello M, Pintus S, Corti D, et al. (2014) Particle conformation regulates antibody access to a conserved GII.4 norovirus blockade epitope. J Virol 88 : 8826–8842. doi: 10.1128/JVI.01192-14 24872579
33. Eden JS, Tanaka MM, Boni MF, Rawlinson WD, White PA (2013) Recombination within the pandemic norovirus GII.4 lineage. J Virol 87 : 6270–6282. doi: 10.1128/JVI.03464-12 23536665
34. Bull RA, Eden JS, Rawlinson WD, White PA (2010) Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog 6: e1000831. doi: 10.1371/journal.ppat.1000831 20360972
35. Siebenga JJ, Lemey P, Kosakovsky Pond SL, Rambaut A, Vennema H, et al. (2010) Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog 6: e1000884. doi: 10.1371/journal.ppat.1000884 20463813
36. Richardson C, Bargatze RF, Goodwin R, Mendelman PM (2013) Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev Vaccines 12 : 155–167. doi: 10.1586/erv.12.145 23414407
37. Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, et al. (2014) A novel intramuscular bivalent norovirus virus-like particle vaccine candidate-reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis 210 : 1763–1771. doi: 10.1093/infdis/jiu337 24951828
38. Donaldson EF, Lindesmith LC, Lobue AD, Baric RS (2010) Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol 8 : 231–241. doi: 10.1038/nrmicro2296 20125087
39. Nordgren J, Kindberg E, Lindgren PE, Matussek A, Svensson L (2010) Norovirus gastroenteritis outbreak with a secretor-independent susceptibility pattern, Sweden. Emerg Infect Dis 16 : 81–87. doi: 10.3201/eid1601.090633 20031047
40. Frenck R, Bernstein DI, Xia M, Huang P, Zhong W, et al. (2012) Predicting susceptibility to norovirus GII.4 by use of a challenge model involving humans. J Infect Dis 206 : 1386–1393. doi: 10.1093/infdis/jis514 22927452
41. Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, et al. (2005) Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 79 : 2900–2909. 15709009
42. Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, et al. (2014) Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis. E-pub ahead of print. doi: 10.1093/infdis/jiu497
43. Swanstrom J, Lindesmith LC, Donaldson EF, Yount B, Baric RS (2014) Characterization of blockade antibody responses in GII.2.1976 Snow Mountain virus-infected subjects. J Virol 88 : 829–837. doi: 10.1128/JVI.02793-13 24173225
44. LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, et al. (2006) Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 24 : 5220–5234. 16650512
45. Debbink K, Lindesmith LC, Ferris MT, Swanstrom J, Beltramello M, et al. (2014) Within-host evolution results in antigenically distinct GII.4 noroviruses. J Virol 88 : 7244–7255. doi: 10.1128/JVI.00203-14 24648459
46. Cai Z, Zhang T, Wan XF (2010) A computational framework for influenza antigenic cartography. PLoS Comput Biol 6: e1000949. doi: 10.1371/journal.pcbi.1000949 20949097
47. Wang F, Sen S, Zhang Y, Ahmad I, Zhu X, et al. (2013) Somatic hypermutation maintains antibody thermodynamic stability during affinity maturation. Proc Natl Acad Sci U S A 110 : 4261–4266. doi: 10.1073/pnas.1301810110 23440204
48. Hoffmann D, Hutzenthaler M, Seebach J, Panning M, Umgelter A, et al. (2012) Norovirus GII.4 and GII.7 capsid sequences undergo positive selection in chronically infected patients. Infect Genet Evol 12 : 461–466. doi: 10.1016/j.meegid.2012.01.020 22310302
49. Barnett JL, Yang J, Cai Z, Zhang T, Wan XF (2012) AntigenMap 3D: an online antigenic cartography resource. Bioinformatics 28 : 1292–1293. doi: 10.1093/bioinformatics/bts105 22399675
50. Debbink K, Lindesmith LC, Donaldson EF, Baric RS (2012) Norovirus immunity and the great escape. PLoS Pathog 8: e1002921. doi: 10.1371/journal.ppat.1002921 23093932
51. Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, et al. (2010) Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 84 : 1800–1815. doi: 10.1128/JVI.02179-09 20007270
52. Schmidt AG, Xu H, Khan AR, O’Donnell T, Khurana S, et al. (2013) Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc Natl Acad Sci U S A 110 : 264–269. doi: 10.1073/pnas.1218256109 23175789
53. Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, et al. (2012) Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 109 : 9047–9052. doi: 10.1073/pnas.1118979109 22615367
54. Kobayashi M, Ohfuji S, Fukushima W, Maeda A, Maeda K, et al. (2012) Immunogenicity and reactogenicity of a monovalent inactivated 2009 influenza A vaccine in adolescents: with special reference to pre-existing antibody. J Pediatr 160 : 632–637. doi: 10.1016/j.jpeds.2011.09.055 22094234
55. Knuchel MC, Marty RR, Morin TN, Ilter O, Zuniga A, et al. (2013) Relevance of a pre-existing measles immunity prior immunization with a recombinant measles virus vector. Hum Vaccin Immunother 9 : 599–606. 23324399
56. Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, et al. (2010) Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375 : 56–66. doi: 10.1016/S0140-6736(09)62003-1 20018364
57. Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, et al. (2009) A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 361 : 2414–2423. doi: 10.1056/NEJMoa0908535 19846844
58. Wrammert J, Smith K, Miller J, Langley WA, Kokko K, et al. (2008) Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453 : 667–671. doi: 10.1038/nature06890 18449194
59. Mora JR (2008) Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis 14 : 275–289. 17924560
60. Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, et al. (2001) Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol 166 : 1894–1902. 11160237
61. Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, et al. (2004) Mapping the antigenic and genetic evolution of influenza virus. Science 305 : 371–376. 15218094
62. Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, et al. (2009) Reemergence of enterovirus 71 in 2008 in taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol 47 : 3653–3662. doi: 10.1128/JCM.00630-09 19776232
63. Debbink K, Lindesmith LC, Donaldson EF, Swanstrom J, Baric RS (2014) Chimeric GII.4 norovirus virus-like-particle-based vaccines induce broadly blocking immune responses. J Virol 88 : 7256–7266. doi: 10.1128/JVI.00785-14 24741081
64. Corti D, Suguitan AL Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, et al. (2010) Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest 120 : 1663–1673. doi: 10.1172/JCI41902 20389023
65. Lessler J, Riley S, Read JM, Wang S, Zhu H, et al. (2012) Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog 8: e1002802. doi: 10.1371/journal.ppat.1002802 22829765
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2015 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Testing and Treating the Missing Millions with Tuberculosis
- UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age
- Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study
- Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial
- Strengthening the Detection of and Early Response to Public Health Emergencies: Lessons from the West African Ebola Epidemic
- HIV Treatment-As-Prevention Research: Authors’ Reply
- Role of Acute HIV Infection in Driving HIV Transmission: Implications for HIV Treatment as Prevention
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- HIV Treatment-As-Prevention Research: Taking the Right Road at the Crossroads
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
- A Public Health Approach to Hepatitis C Control in Low- and Middle-Income Countries
- Development and Validation of a Risk Score for Chronic Kidney Disease in HIV Infection Using Prospective Cohort Data from the D:A:D Study
- Reassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts
- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



