-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Uganda
A clinical trial set in Uganda shows that short-term supplementation with ready-to-use food in children following a bout of acute illness can prevent malnutrition. This short term measure has longer term effects in reducing morbidity in a vulnerable population.
Published in the journal: Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Uganda. PLoS Med 13(2): e32767. doi:10.1371/journal.pmed.1001951
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001951Summary
A clinical trial set in Uganda shows that short-term supplementation with ready-to-use food in children following a bout of acute illness can prevent malnutrition. This short term measure has longer term effects in reducing morbidity in a vulnerable population.
Introduction
Treatment of malnutrition is an important part of the medical programmes of Médecins Sans Frontières (MSF). In 2011, the year the research presented here was conducted, MSF treated 348,017 severely malnourished children and 59,984 moderately malnourished children worldwide. It is imperative that MSF explores appropriate ways to prevent malnutrition, of which an effective strategy might be providing a nutritional supplementation to ill children.
The cause of malnutrition in most tropical countries is multifactorial, involving not only an inadequate diet but also recurrent infections [1,2]. When inadequate nutrition limits recovery, the risk of a permanently lowered nutritional status is increased [3], as is the risk of mortality due to illness [4–6]. There is extensively documented evidence of growth faltering related to diarrhoea [7–9]. Furthermore, malaria episodes have been associated with malnutrition, and malnutrition weakens the immune system and is thus associated with a more severe outcome of a malaria infection [10–13]. Lower respiratory tract infections (LRTIs) have also shown a negative effect on growth [2,8].
Acute weight loss during episodes of acute infection can be mitigated by good nutrition. Studies of supplementation for patients with diarrhoea [14,15] have shown that nutritional supplementation immediately results in lower weight loss. Supplementation for patients with respiratory tract infection and malaria was also studied, and these studies concluded that dietary supplementation would increase weight gain during convalescence [16,17].
A research in the Democratic Republic of Congo showed that children with malaria gained weight faster during the period of supplementation when given 14 d of ready-to-use therapeutic food (RUTF), though this difference was not seen at the 4 wk follow-up [17].
Most studies that show a positive effect of nutritional supplementation in convalescence used a fortified food such as enriched porridges or lipid-based fortified foods such as RUTF [14,17]. Micronutrients also reduce the convalescence time [18–23], although some studies did not find an effect of multi-micronutrients on growth during convalescence of ill children [24,25]. As multi-micronutrients are cheaper than a porridge or a fortified food, it is necessary to investigate whether fortified food or micronutrients alone could promote an improved nutritional status during convalescence.
The World Health Organization (WHO) recommends that caretakers give ill children additional nutritious meal daily during recovery up to 2 wk after the onset of illness [26–28]. In resource-poor settings, the typical context of MSF’s programmes, this strategy is likely to be ineffective, as caregivers often lack healthy ingredients and other resources to implement this recommendation. A more effective strategy to reduce disease-related malnutrition in resource-poor areas may be to provide an ill child with a nutritional supplement at the point of care, the outpatient health clinic. Therefore, MSF investigated whether a supplementation of either a lipid-based fortified food or micronutrients for 2 wk after an illness is effective in reducing the incidence of malnutrition over a period of 6 mo. Two trials, one in Nigeria and one in Uganda, were conducted; here, we report on the trial that took place in Uganda, while the trial in Nigeria is reported elsewhere [29].
Methods
Approval
The trial was registered at clinicaltrials.gov number NCT01497236. The full protocol and the statistical analysis plan (SAP) can be accessed in the supporting information files (S1 Text and S2 Text). Ethical approval was obtained from the Uganda National Council of Science and Technology (UNCST) on 20th December 2010 and an extension for the study granted on 11th November 2011. The MSF Ethical Review Board granted approval for the study on 12th December, 2010.
Setting
Kaabong is a remote and scarcely populated district in the Karamoja region of Uganda. Socioeconomic infrastructure, health services, and facilities—such as schools, health centres, and potable water—are weak, and the region has faced long periods of conflict and insecurity. This makes Karamoja one of the least developed parts of the country.
Karamoja is semiarid and has only one rainy season from early April to May. The agropastoral population of Kaabong grow sorghum, maize, millet, groundnuts, sunflower, cowpeas, and beans. They keep goats and sheep—better-off groups tend to own cattle. Nearly half the households depend on borrowing and exchange and food assistance [30,31]. Food security is fragile as a result of poverty and cycles of drought and floods. In 2009, 58.4% of the Kaabong population was considered to be food insecure [31]. The food insecurity was exacerbated by ongoing conflict that ended during the implementation of the study in 2011. During the study period, overall household food security showed improvement in the entire region.
As a result, during the study period, the prevalence of malnutrition was generally not high. The nutrition surveillance system gave a prevalence of acute malnutrition of 8.5% in May 2011, 8.4% in December 2011, and 11.5% in May 2012; of this, 2% to 3% was severe acute malnutrition [32].
In an analysis of 2014, the UN World Food Program concluded that the poor nutritional status among children in Karamoja was the result of a combination of high incidence of fever, malaria, and diarrhoea, poor sanitation facilities, lack of vitamin A supplementation, and a poor dietary diversity, rather than a lack of food per se [33].
The involvement of MSF in Kaabong started in 2007 with support to mother and child care, including a feeding programme to respond to a nutritional crisis caused by a severe drought. Because of major improvements in the health situation, MSF withdrew its support to the health activities completely in December 2012, and only this study was continued until completion.
During the study, many households (37.3%) received income through food aid cash for work programmes. A targeted food distribution programme to extremely vulnerable households became operational in March 2012. In addition, a supplementary feeding programme for treatment of moderately malnourished children (supported by World Vision International) and a therapeutic feeding program for treatment of severely malnourished children (supported by Action Contre le Faim) were present.
Study Objectives and Endpoints
The aim of the three-armed, partially-blinded, randomised controlled trial was to investigate the effectiveness of 14 d nutritional supplementation with RUTF or a multi-micronutrient powder (MNP) given to non-malnourished children aged 6–59 mo and diagnosed with and treated for malaria, diarrhoea, and/or LRTIs in reducing the incidence of acute malnutrition during a follow-up time of 6 months. Given the complexity of malnutrition in the trial’s study population, a study-specific primary endpoint event was compiled. This is known as a negative nutritional outcome (NNO) and defined as weight-for-height z-score <−2, mid-upper arm circumference (MUAC) <115 mm, or nutritional oedema, whichever came first. Secondary outcomes included changes in anthropometric indicators, morbidity, and mortality.
Study Population and Randomisation
Non-malnourished children (weight-for-height z-score >−2, MUAC >125 mm, and absence of nutritional oedema) aged 6–59 mo diagnosed at the regular outpatient clinic with one or more of the three study diseases (malaria, diarrhoea, LRTI) were included in the study when living within approximately 60 min walking distance from the clinic and intending to stay in the area during the 6-mo follow-up.
Children who met the criteria for severe acute malnutrition (defined as weight-for-height z-score <−3, MUAC <115 mm, or nutritional oedema) or moderate acute malnutrition (defined as weight-for-height z-score between −3 and −2, or MUAC 115–125 mm), were exclusively breastfed, had a severe disease (for example, severe malaria, severe pneumonia, or severe anaemia), needed admission to hospital, had a sibling enrolled in the study, or were offspring of staff of the study were excluded.
Children were randomised in a 1 : 1:1 ratio to one of three intervention groups. During the 6-mo follow-up, children in the RUTF and MNP groups received nutritional supplements for 14 d whenever they were diagnosed with at least one of the three study diseases, with a maximum of 14 d of supplementation in any 28-d period. The MNP (MixMe, DSM Ltd, Switzerland) contained only micronutrients according the UN formulation [34]. Children in the MNP group received two doses per day, to ensure a micronutrient composition comparable to one sachet of RUTF (Plumpynut, Nutriset, France) (Table 1). The caretakers were instructed to mix MNP in the meal (for example, porridge) just before consumption. All groups (including the control group) received health education, including the message that following an illness, a child should eat one extra, healthy meal per day for 2 wk.
Tab. 1. Nutritional supplements’ composition per serving. 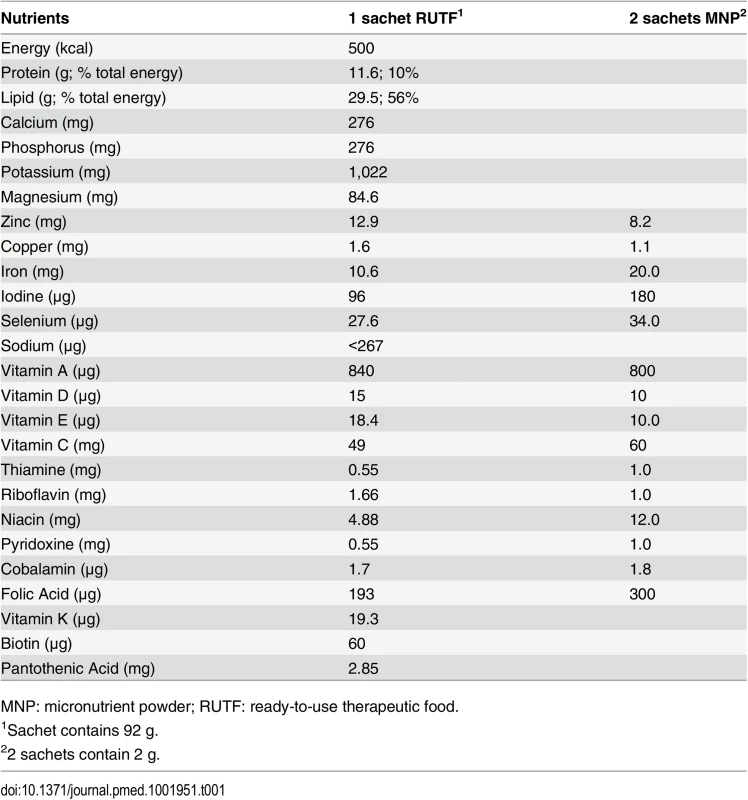
MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. The sample size was based on the assumption of a baseline/control group incidence rate of first NNO event of 0.20 within 6 mo. A 30% reduction, considered a clinically and operationally relevant improvement, would result in a targeted incidence rate of 0.14 events in 6 mo in each of the two treatment groups. Using a Poisson regression model, 80% power at a 0.05 significance level, and an assumed drop-out rate of approximately 10%, a sample size of 734 children was needed in each group (2,202 in total for all three groups).
Simple randomisation was based on a computer-generated randomisation list made by an expert independent of the study. Participants entering the study received a study number, and only the staff members physically giving the supplement were able to connect the study number to the treatment group after all other procedures, including collection of baseline data, were finalised. All other study staff (including clinicians diagnosing illnesses and deciding on giving an allocation) were blinded to the allocation treatment throughout the study. The statistician was unblinded after the completion of the analysis.
Procedures
Patients were screened in the regular outpatient clinic on eligibility, and potential participants were referred to the adjacent study clinic for further inclusion procedures. Activities at inclusion included provision of information and obtaining written consent of caretakers; in case the caretaker was illiterate, the consent form was to be read out loud, and consent was given by thumbprint in the presence of a witness. The enrolled participants were followed after the first 14 d and then monthly during 6 mo. If a child was ill, they were invited to come whenever they felt that was needed. At each visit, the health and nutritional status of the participant was assessed.
Anthropometric measurements—weight, height, oedema, and MUAC—were taken at every visit to the study clinic. Two types of electronic weighing scales were used to measure weight: SECA model 354, with a precision of 10–20 g, and SECA 869, a mother/baby scale with a precision of 100 g. Length or height (change of measuring position at 85 cm of height) was measured using a precision height board (infant–child–adult measuring board ICAM, aluminium, precision 1 mm, Promes). MUAC was measured using a standard MUAC tape (MSF, precision 2 mm).
Malaria was diagnosed by conducting a malaria rapid diagnostic test (RDT; SD-bioline Combo Standard Diagnostic, Korea). All new participants were tested on enrollment; during the study, participants would have a malaria test on indication (fever or history of fever).
Diarrhoea was defined as three or more loose stools (bloody or nonbloody) per 24 h and was diagnosed by mothers’ report. LRTI was diagnosed by the following algorithm: children with cough or having difficulty breathing, an increased respiratory rate (>50 breaths/min for children aged 6–11 mo old and >40 breaths/minute for children aged 12–59 mo), and absence of chest in-drawing were diagnosed as nonsevere LRTI; chest in-drawing was considered a sign of severe LRTI, needing referral to the hospital.
All children received standard care and treatment according to current national medical protocols. Uncomplicated malaria was treated for 3 d with a six-dose regimen of Artemether–Lumefantrine (Coartem). Acute watery diarrhoea was treated using oral rehydration therapy with low osmolarity oral rehydration salts and zinc according to WHO guidelines, regardless of the supplement received. LRTI was treated with amoxicillin 75 mg/kg/d for 5–7 d. The first treatment was given under medical supervision, and the use of and need for the drug were explained to the mother. At every visit, health education was provided, including prevention and treatment of malaria and diarrhoea and the advice to give an ill child an extra nutritious meal for 14 d. Participants presenting with other nonsevere diseases were treated in the study clinic according to the national protocol.
Home visitors supported the study by reminding the caretakers to come to the appointments, urging absentees to return, and reporting on deaths that occurred at home. Any death was immediately reported and reviewed by a national doctor, principal investigator, epidemiologist, and medical director of MSF.
Compliance was measured by questionnaires, asking how many times and for how many days the participant consumed the supplement. In addition, the caretaker was asked to return full or empty sachets of each distribution. In March, June, and September 2012, three focus group discussions were held with caretakers who had completed the trial to discuss the supplements and medication, compliance, and any barriers regarding use of the supplements.
Data Analysis
The primary outcome (rates of first NNO) among the treatment groups was analysed by a Poisson regression model including the three intervention groups with contrasts for each two-group comparison. As there were three interventional groups to be compared, a hierarchical test procedure was used to account for multiplicity. If a significant result (significance level 0.05 two-sided) was observed in the RUTF group compared with control, MNP was then compared with control; if this was also statistically significant at 0.05 two-sided, RUTF was subsequently compared with MNP in a noninferiority approach with a noninferiority margin of 2% (that is, allowing a slightly worse result in the MNP compared with the RUTF group, statistically significant at 0.025 one-sided). If no significant result was observed in the first or second step, all other comparisons were considered exploratory.
The incidence rate of malnutrition (NNO) is expressed in the first event per 365 observation d (events/y) and is extrapolated from the study period of 168 d (24 wk).
A priori defined subgroups were analysed by including interaction terms in the main model. Secondary outcomes were analysed using logistic regression or analysis of covariance, with treatment group and baseline values as covariates.
Any participant attending at least the first and the last visit was counted as having completed the study. A participant developing acute malnutrition (weight-for-height z-score <−2, MUAC <115, or nutritional oedema) was categorised as an NNO and referred to a feeding programme. Participants who were developing a NNO, seriously ill participants, patients needing admission to hospital, and participants with measles were withdrawn and their data up to the moment of withdrawal was used.
When a serious protocol violation occurred or the participant was erroneously included, the participant was withdrawn and the data was not used. The primary outcomes were also analysed for a per protocol population that excluded participants if they had abandoned the study, had been admitted to hospital, had a low compliance, or had measles.
Results
Participant Flow
Recruitment took place from 3rd March 2011 until 4th April 2012. The last follow-up was completed on 18th September 2012. 11,008 patients younger than 5 y visiting the outpatient clinic were screened on age illness and living area; of these 2.6% were also excluded because of severe malnutrition and 8.3% for moderate malnutrition. A total of 2,202 children, aged between 6 and 59 mo, were included; 17 (0.8%) participants were excluded from analysis because of inclusion error, and 148 (6.7%) participants were lost to follow-up (abandoned the study or admitted to hospital) (Fig 1). The average number of days participants were in the study were 161.4, 161.2, and 162.9 d for RUTF, MNP, and control group, respectively.
Fig. 1. Flow diagram of participants’ supplementation study in Kaabong. 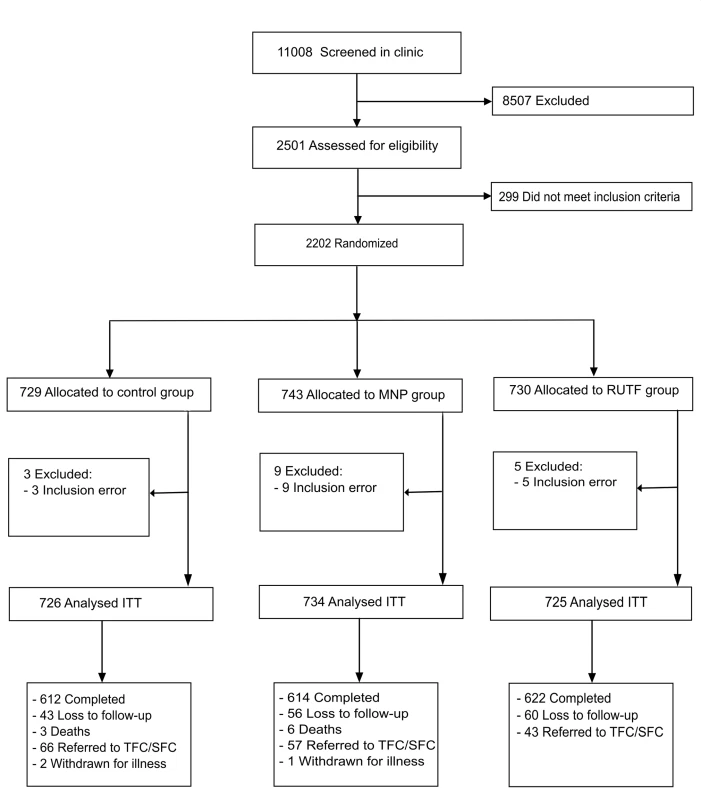
ITT, intention to treat; MNP, micronutrient powder; RUTF, ready-to-use therapeutic food; TFC/SFC, therapeutic/supplementary feeding centre. Baseline Characteristics
Most caretakers (93.4%) were married, and 80.4% had no formal schooling. One-quarter (24.3%) of the heads of households made a living in agriculture or livestock of their own resources. More than half of the heads of households (51.7%) were dependent on daily or seasonal labour. Only 26.4% of the households owned livestock; 7.2% owned cows, which is a sign of wealth; 9.8% of the households owned a bicycle; 8.2% had three or more meals the day before enrollment (Table 2).
Tab. 2. Baseline characteristics. 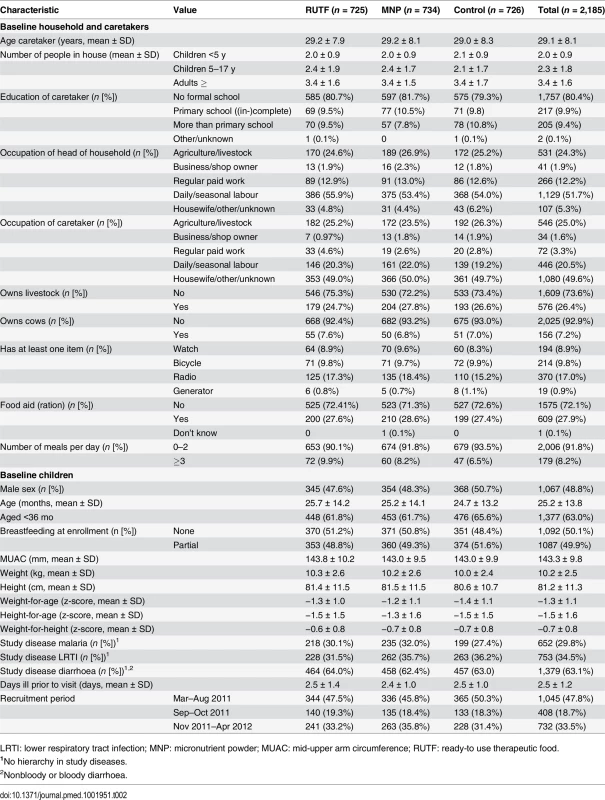
LRTI: lower respiratory tract infection; MNP: micronutrient powder; MUAC: mid-upper arm circumference; RUTF: ready-to use therapeutic food. Most (63.0%) participants were aged 6–36 mo, and about half (49.9%) were partially breastfed. Most (63.1%) of the participants had diarrhoea on enrollment, 29.8% had malaria, and 34.5% had LRTI (more than one disease could be reported). After the onset of illness, caretakers waited for an average of 2.5 d before seeking help at the clinic. Almost half (47.8%) of the participants were enrolled in March–August 2011 and were participating in the study partly during the rainy season; 33.5% enrolled in November 2011–April 2012, and they were also partly participating in the rainy season; 18.7% were enrolled in September–October 2011 and were never participating during the rainy season. There was no clear difference between the supplementation groups in regards to these baseline characteristics (Table 2).
Incidence of Malnutrition
The incidence of first NNO event was 0.143, 0.185, and 0.213 events per year for the RUTF, MNP, and control groups, respectively, during the 6 mo of follow-up (Table 3). The RUTF group showed a significant reduction in the incidence of NNO of 0.070 events/year or a reduction of 33.3% (p = 0.037) compared with the control group. The RUTF group showed a nonsignificant reduction of 0.042 events/y (22.7%) in incidence compared with the MNP group (p = 0.200), and the reduction in incidence in the MNP group compared with the control group was 0.028 events/y (13.8%) (p = 0.413).
Tab. 3. Incidence of first NNO per y. 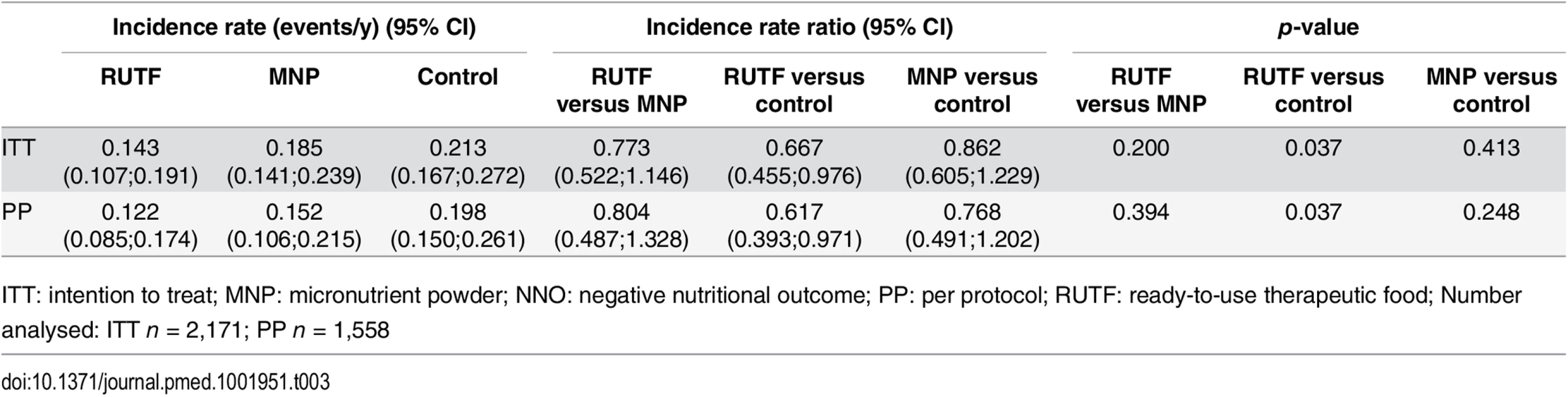
ITT: intention to treat; MNP: micronutrient powder; NNO: negative nutritional outcome; PP: per protocol; RUTF: ready-to-use therapeutic food; Number analysed: ITT n = 2,171; PP n = 1,558 Analysis of the per protocol dataset (n = 1,558) did not show a major difference from the intention to treat dataset. The incidence of NNO was 0.122 events/y for the RUTF group, 0.152 events/y for the MNP group, and 0.198 events/y for the control group during the 6 mo of follow-up. The incidence in the RUTF group was 39.3% lower compared with the control group (p = 0.037) and 19.6% lower compared with the MNP group (p = 0.394), and the incidence in the MNP group was 23.8% lower than the control group (p = 0.248) (Table 3).
A total of 166 participants developed moderate malnutrition (weight-for-height z-score between −2 and −3): 44 (6.09%) in the RUTF group, 56 (7.70%) in the MNP group, and 66 (9.14%) in the control group. Sixteen participants developed severe malnutrition (weight-for-height z-score <−3, MUAC <115 mm, or oedema): 4 (0.55%) in the RUTF group, 9 (1.24%) in the MNP group, and 3 (0.42%) in the control group. None of the participants developed oedema.
Subgroup analyses suggested that the effect of supplementation on the incidence of NNO was not modified by socioeconomic characteristics, season of enrollment, age of the participant, breastfeeding status, or study disease at enrollment (Table 4).
Tab. 4. Incidence rate of first NNO per y by subgroups. 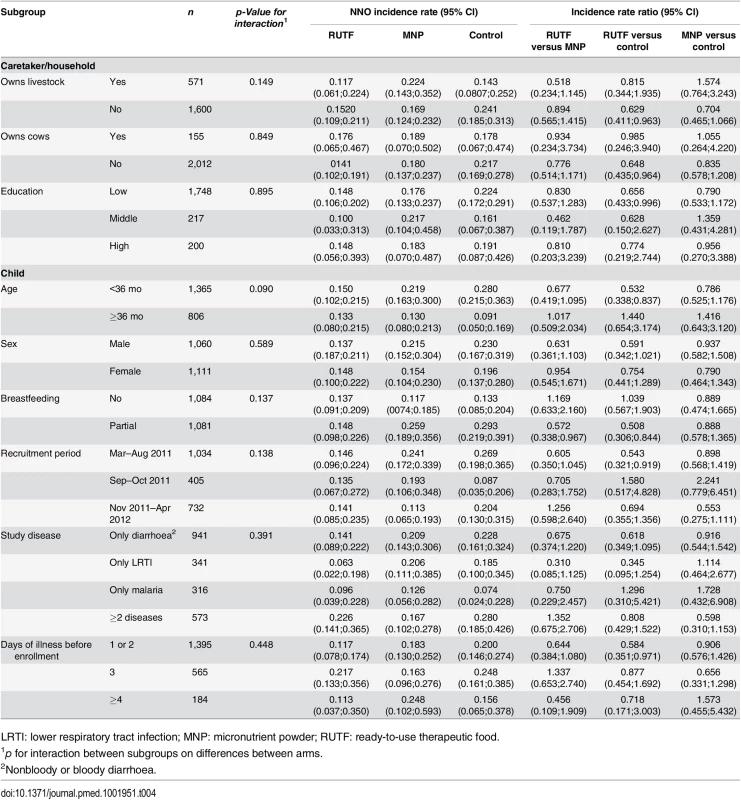
LRTI: lower respiratory tract infection; MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. This is despite the fact that in the control group, some subgroups (e.g., partially breastfed, children younger than 36 months, those enrolled in the wet seasons) did appear to be at higher risk of developing a NNO. (Table 4).
Whereas MNP did not show a significant effect on the incidence of NNO, a positive effect of MNP was seen for the changes in individual anthropometric indicators of participants who completed the study (Table 5).
Tab. 5. Anthropometric indicators: change from baseline to day 14 and day 168. 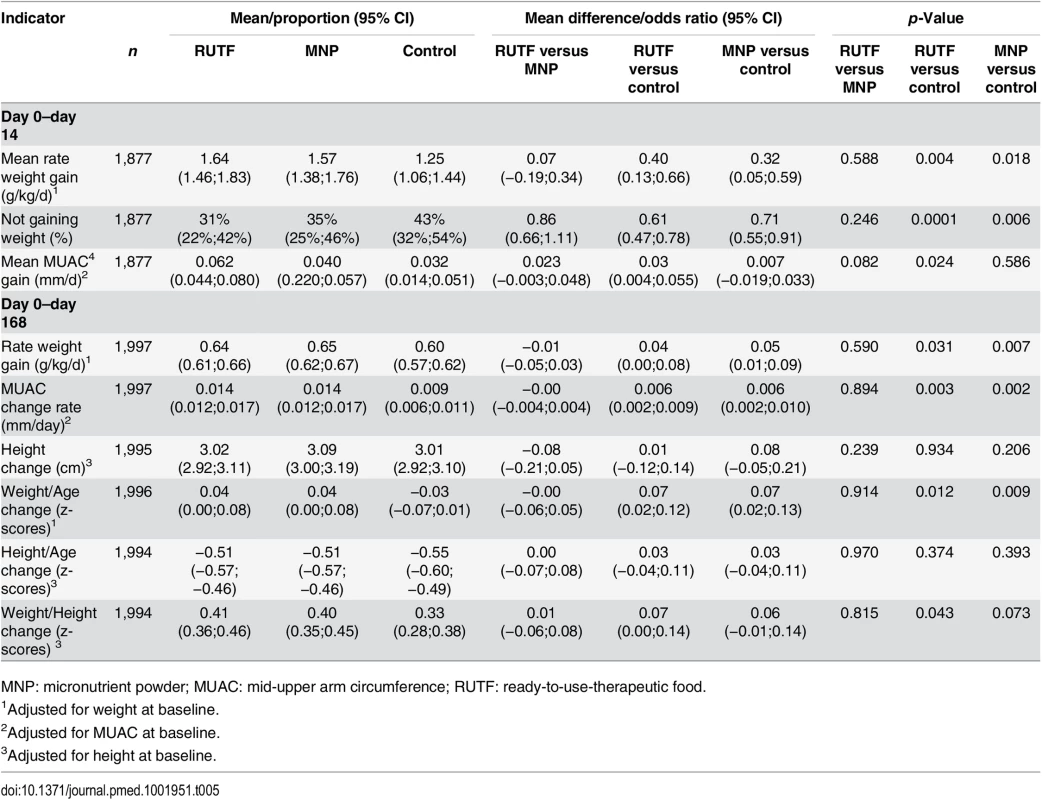
MNP: micronutrient powder; MUAC: mid-upper arm circumference; RUTF: ready-to-use-therapeutic food. The weight gain rate at day 168 was significantly higher for the supplementation groups compared with the control group: 0.64, 0.65, and 0.60 g/kg/d for RUTF, MNP, and control groups, respectively (RUTF versus control, p = 0.031; MNP versus control, p = 0.007). The MUAC gain rate at day 168 was also higher for the supplementation groups compared with the control group: 0.014, 0.014, and 0.009 mm/d for RUTF, MNP, and control groups, respectively (RUTF versus control, p = 0.003; MNP versus control, p = 0.002) (Table 5).
Similarly, the change in weight-for-age index was higher for the supplementation groups than for the control group: z-scores of 0.04, 0.04, and −0.03 for RUTF, MNP, and control groups, respectively (RUTF versus control, p = 0.012; MNP versus control, p = 0.009). Finally, the change in weight-for-height index was also higher for the supplementation groups than the control group: z-scores of 0.41, 0.40, and 0.33 for RUTF, MNP, and control groups, respectively (RUTF versus control, p = 0.043; MNP versus control, p = 0.073). The change in height and the change in height-for-age index were not different between the supplementation arms (Table 5).
In the first 14 d, directly after the first supplementation, the RUTF and MNP groups showed a significantly higher weight gain rate compared with the control group: 1.64, 1.57, and 1.25 g/kg/d for RUTF, MNP, and control groups, respectively (RUTF versus control, p = 0.004; MNP versus control, p = 0.018). Similarly, a lower proportion of participants did not gain weight in the supplementation groups. The proportion of children not gaining weight was 31%, 35%, and 43% for RUTF, MNP, and control groups, respectively (RUTF versus control, p = 0.0001; MNP versus control, p = 0.006) (Table 5).
Disease and Mortality
The proportion of children experiencing one or more new episodes of malaria during the follow-up was 47%, 43%, and 51% for the RUTF, MNP, and control groups, respectively (Table 6). The MNP group showed a lower proportion of children having a new malaria episode compared with the control group (p = 0.001). The proportion of children experiencing one or more new diarrhoea episodes was 44%, 44%, and 46% in the RUTF, MNP, and control groups, respectively; for LRTI, the proportions were 43%, 44%, and 46%. The average number of malaria episodes was 0.79, 0.70, and 0.80 for the RUTF, MNP, and control groups, respectively (MNP versus control, p = 0.075); the average number of diarrhoea episodes was 0.78, 0.73, and 0.80, and the average number of LRTI episodes was 0.71, 0.68, and 0.72. The mean number of all diagnosed study diseases was 2.29, 2.12, and 2.31, for the RUTF, MNP, and control groups, respectively (Table 6).
Tab. 6. Mean number of diagnosed events and proportion of children with at least one newly diagnosed study disease episode1. 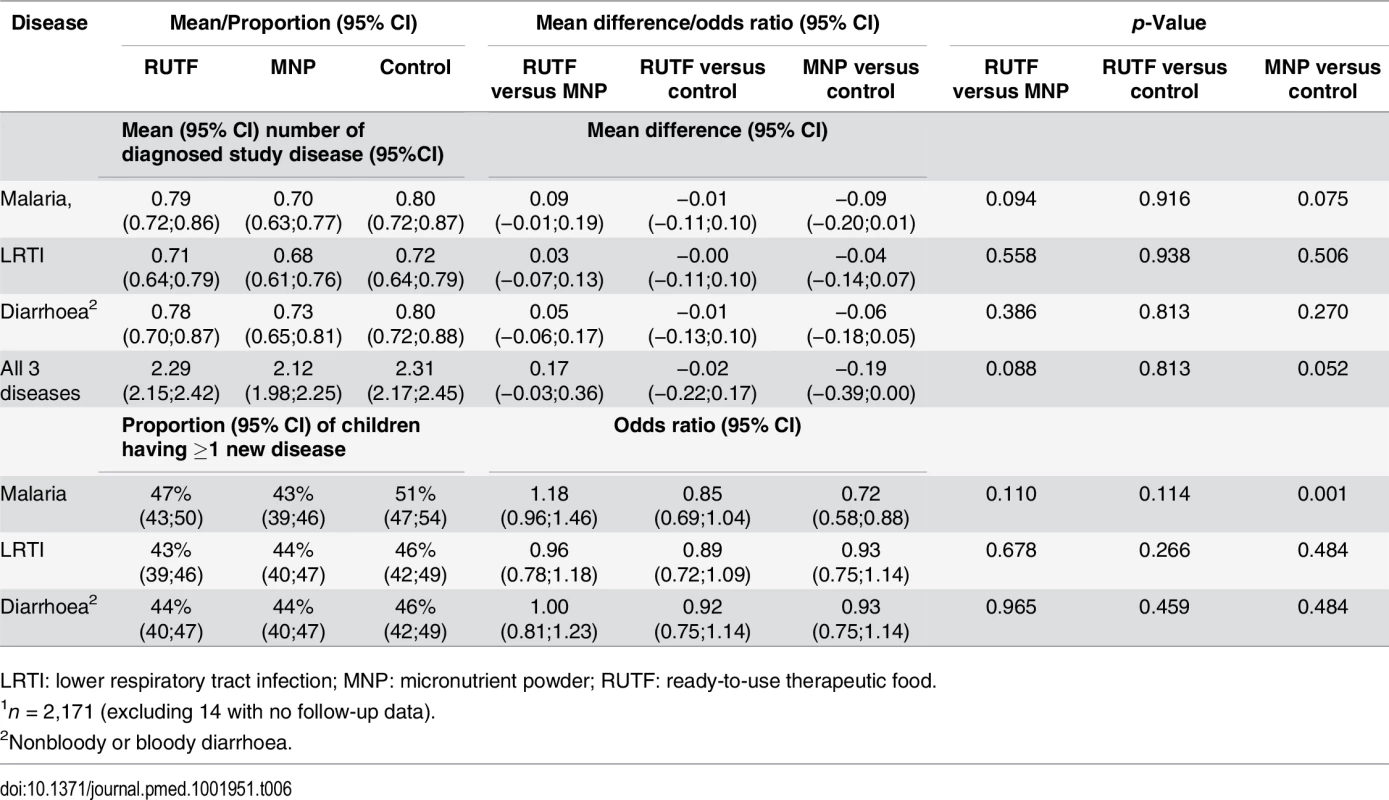
LRTI: lower respiratory tract infection; MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. A total of nine children died during the study duration: none in the RUTF group, six in the MNP group, and three in the control group. Among the nine deaths, three occurred at the hospital (Table 7). The causes of deaths in the hospital were severe malaria and severe anaemia in two patients and bloody diarrhoea in one patient (all in the control group). The other six patients died at home, and the caretakers did not know the cause of death. A total of 14 participants were admitted to the hospital (not deceased): six in the RUTF group, two in the MNP group, and six in the control group. In total, 23 participants were admitted to the hospital or died: six in the RUTF group, eight in the MNP group, and nine in the control group (Table 7).
Tab. 7. Overall mortality and hospital admission by intervention group 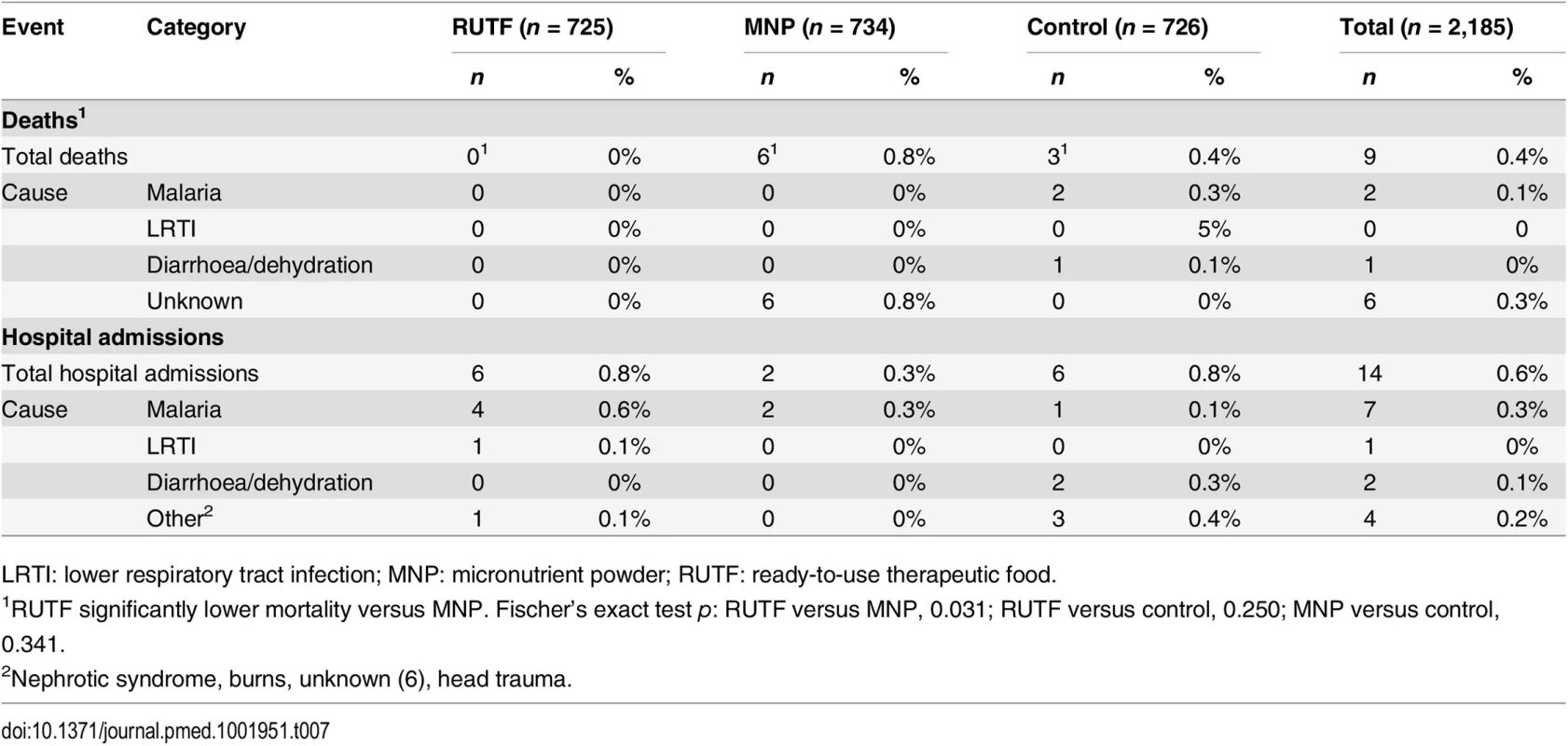
LRTI: lower respiratory tract infection; MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. Consumption and Compliance
Caregivers of children taking RUTF or MNP were asked about the consumption of sachets during the 2 wk following allocation. Reported consumption over the first 14-d period directly after enrollment showed that participants in the RUTF group consumed on average 13.7 (out of 14) sachets, and participants in the MNP group consumed on average 24.3 (out of 28) sachets (Table 8). Reported consumption over the entire study period and all allocations showed an average of 13.8 (out of 14) sachets consumed per participant per allocation in the RUTF group and 24.7 (out of 28) sachets consumed per participant per allocation in the MNP group.
Tab. 8. Allocation of and compliance with supplements in intervention group. 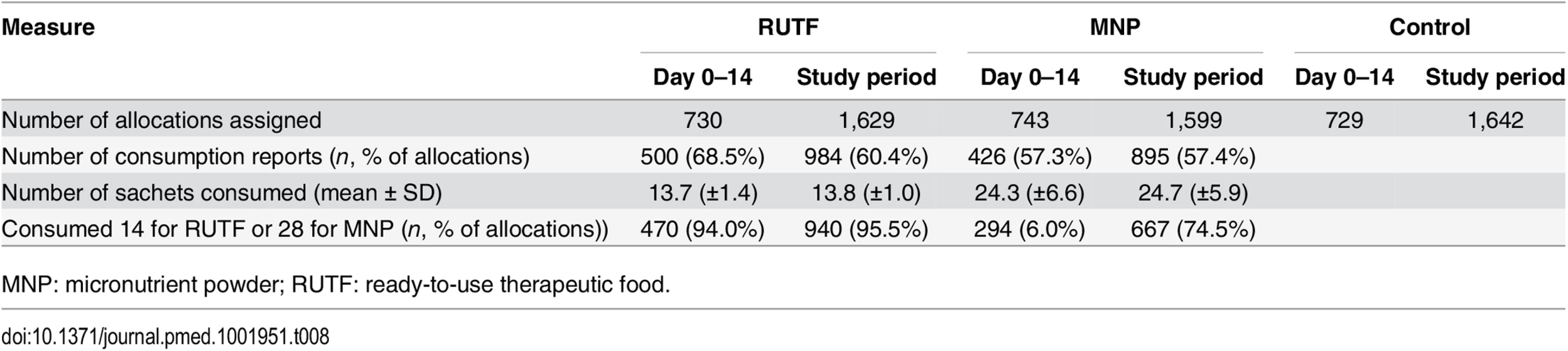
MNP: micronutrient powder; RUTF: ready-to-use therapeutic food. Focus group discussions showed that all mothers enjoyed giving RUTF or MNP to their children. About RUTF, they said the "children gained weight and improved faster than they usually did.” The children liked the RUTF so much that some even tried “grabbing it” from the mothers. All caregivers reported that they did not give any of the supplements to a nonstudy child because it had been prescribed as “medicine” for the sick child. They all denied having sold or shared any of the supplements. All the caregivers said that it was easy to use the MNP and they felt that the supplement was very important for the child to recover from their illness.
Discussion
Incidence of Malnutrition
This study and its companion trial in Goronyo (Nigeria) are the first large randomised controlled trials of the use of high quality supplements (RUTF and MNP) for the prevention of malnutrition in children with a nonsevere illness. In our site in Kaabong, Uganda, we showed that 14 d of supplementing ill children with RUTF was effective in lowering the incidence of malnutrition. The RUTF supplementation group showed a significant reduction in the incidence of NNO of 33% compared with the control group. Supplementation with MNP showed a nonsignificant reduction of 13.8%. Both RUTF and MNP supplementation had a significant positive effect on anthropometric indicators such as rate of weight gain and gain in MUAC. There were fewer deaths observed in the RUTF group than in the control and MNP groups, but the numbers were too low to draw clear conclusions.
In contrast, in the companion study in Nigeria, there was no reduction in incidence of malnutrition with short-term supplementation of either RUTF or MNP compared with a control group, and there was no impact on any of the anthropometric indices. It is postulated that the high morbidity in Nigeria necessitates a higher dose or a longer duration of supplementation for effectiveness. In addition, the supplements were not effective among the moderately malnourished children included in the study in Nigeria. Children with moderate acute malnutrition (MAM), while at higher risk of progressing to severe acute malnutrition (SAM), generally require 4 wk or more of treatment to fully recover their nutritional status so it may be that the 14 d period of supplementation was insufficient. Similar to our findings in this study, there was a trend to fewer deaths in the RUTF group compared with the control or MNP group in the Nigerian study [29].
The findings in Kaabong support a study on the effect of supplementing all children (not necessarily ill) in Niger that also showed a reduction in malnutrition. A supplementation of one sachet of RUTF daily for a period of 3 mo during the hunger season in children led to a significantly lower incidence of malnutrition (weight-for-height z-scores) in the supplemented group after a follow-up period of 8 mo. The incidences in the RUTF and control groups were, respectively, 0.17 and 0.26 per child per year; a reduction in incidence of malnutrition of 35% in the RUTF group [35].
The overall incidence of malnutrition among ill children included in our study was remarkably low in Kaabong. The incidence of a first event of malnutrition in the control group was 0.21 events/y, whereas we had anticipated an incidence of first time events of 0.44 events/y in the control group. In comparison, in the companion study in Nigeria, the incidence in the control group was 0.57 events/y and 0.59 events/y when excluding moderate malnourished [29]. A study among sick and nonsick children in Niger during the hunger gap also showed an incidence of 0.26 events/y [35].
The lower incidence of malnutrition in Kaabong in our study participants could be due to the changed context of a reduced intensity of the conflict and improved security. At the time the study was being planned, security in Karamoja was hampered owing to clashes between tribes; when the study was implemented, the region had stabilised.
The difference in incidence rate between the RUTF and control groups over the observation period of 6 mo was 0.032; the number needed to treat (NNT) was 31. This implies that 31 non-malnourished, ill children need to be supplemented after an illness during 6 mo to prevent 1 case of NNO in this group. In the situation of Kaabong, with a relatively low incidence of malnutrition, it is more efficient to target specific groups within the group of ill children with a higher incidence of malnutrition (but a similar Incidence Rate Ratio [IRR]), to reduce the NNT—for example, partially breastfed children younger than 3 y who have an overall higher incidence of malnutrition, or supplementation during the rainy season.
The MNP supplementation did not show a significant effect on the incidence of malnutrition among ill children. However, both RUTF and MNP seem to have a significant effect on several anthropometric indicators: higher mean weight gain rate, MUAC gain, weight-for-height gain, and weight-for-age gain at the end of the study. A very direct effect was shown by the weight gain at day 14 immediately after supplementation. Both MNP and RUTF groups showed a higher weight gain and also a lower percentage of participants who did not gain weight at all after 14 d of follow-up. These data show that both RUTF and MNP improved the nutritional status, such as weight and MUAC gain, but that RUTF was more effective in preventing children falling below the threshold of malnutrition. A general improvement in nutritional status is beneficial; however, from a clinical point of view, reduction in the incidence of malnutrition is crucial to reduce mortality among under-5s.
The promising results with micronutrients alone (MNP), and the clear effect of a high quality food with 500 kcal (RUTF), suggest that a supplement with a lower energy content might also be effective in reducing malnutrition. After this study was designed, more types of fortified foods became available—these are generally called lipid-based supplements and contain varying quantities of fat, carbohydrates, and proteins and a varying micronutrient composition. Compared with RUTF, these foods are more adapted to the needs of very young non-malnourished or moderately acutely malnourished children. In a study in Burkina Faso, children aged 9 mo (from the general population) receiving a low quantity lipid-based supplement and relevant medical care for 12 mo showed a significant reduction in wasting [36]. However, a study in Chad using the same supplement for 4 mo in children from the general population alongside general food distribution failed to show any effect of the supplementation in children aged 6–36 mo [37]. Unlike our study and the study in Burkina Faso, no specific morbidity surveillance and care was given in Chad, which might be a crucial element in preventing malnutrition for these age groups. Thus, from a cost-effectiveness point of view, it seems important to investigate whether supplementation with products containing less energy (lipid-based supplements, medium quantity) provided alongside morbidity surveillance and treatment is also effective in reducing the incidence of acute malnutrition in ill children.
Morbidity and Mortality
The effect of supplementation on further morbidity in ill children was mixed. The average number of study diseases diagnosed was 2.29, 2.12, and 2.31 study diseases for RUTF, MNP, and control groups, respectively. The proportion of children having a newly diagnosed episode of diarrhoea or LRTI during the study was similar among the study groups. However, the MNP group had a lower average number of malaria episodes than the control group, as well as a lower proportion of children having at least one new episode of malaria. These findings are curious, as a malaria infection is unlikely to be influenced by supplementation. Possibly, the clinical expression is slightly different (for example, less fever), which might influence the decision to perform a rapid malaria test and thus result in a lower incidence of malaria episodes in the MNP group.
Only 14 children required admission to hospital (of which nine were due to malaria), and the MNP group had the lowest number of hospital admissions. However, the RUTF group had no mortality; three children died in the control group, and the MNP group showed the highest mortality with six cases. The mortality data are difficult to interpret because of the low numbers and lack of data on the cause of death for the participants who died at home. It is unlikely that the study underestimated mortality, as all participants who stopped attending the study were followed up, and information from the family and their neighbours was obtained on the whereabouts of the child. When taken together, the RUTF group showed a tendency to have a lower risk of hospital admission and death. A similar trend was noted in the companion study in Goronyo.
Risks
A study in Pakistan found an increased risk of severe diarrhoea and severe LRTI in the MNP group [38], but this was not confirmed by our study.
Some literature suggests that supplements containing iron might worsen the outcome of malaria episodes [39,40]. Systematic reviews did not confirm this concern [41,42]. In our study, we used two doses of micronutrients resulting in twice the amount of recommended iron of 20 mg instead of 10 mg per day. Although the number of deaths were highest in the MNP group (6 versus 0 in the RUTF and 3 in the control group), the causes were unknown. On the other hand, the MNP group had the lowest number of the hospitalized patients related to malaria. These data neither confirm nor reject the concern that malaria has a more severe outcome in the MNP group.
The data does not show any clear negative effect of the supplement on severe morbidity or mortality. It also does not confirm concerns that supplements might increase the risk on severe outcome related to diarrhoea or malaria.
Finally, the compliance and focus group results demonstrated that RUTF and MNP are both acceptable in terms of correct use, likeability, organoleptic properties (such as colour and taste), side effects, and compliance.
Limitations
Despite the robust study protocols and strict implementation, this study has some limitations. First of all, the participants were not blinded for the RUTF or MNP supplements. As the population had possibly a prior positive attitude towards RUTF, this could introduce a bias to more positive reporting and compliance compared with MNP. However, blinding is difficult to achieve, and our efforts to produce a placebo for MNP failed. Nevertheless, because MNP was new to the population, we did not anticipate any positive or negative feelings about the product.
Because of the presence of feeding programmes of other organisations for treatment of severe and moderate malnourished children, we did not enroll moderate or severe malnourished children in the research but referred them instead. Therefore, the results cannot be generalised to the overall group of ill children presenting at a clinic, as these usually include acutely malnourished children. Caution should therefore be used in comparing the results with other trials, such as the study in Goronyo that did include moderate malnourished children. Also, the results cannot be extrapolated to the general population of all children (ill and not ill), and comparison with research implemented in the general population of children should be approached with caution.
Finally, although the number of lost-to-follow-up was lower than anticipated, this still might have influenced the outcome. As their outcome is unknown, some may have been malnourished and may represent missed endpoints, giving an underestimation of NNO.
Conclusions
A 2-wk nutrition supplementation programme with RUTF as part of routine primary medical care to non-malnourished children with malaria, LRTI, or diarrhoea was effective in preventing malnutrition. The low incidence of malnutrition in this population may warrant a more targeted intervention to improve cost-effectiveness, such as ill children younger than 3 y or during the hunger season.
While supplementation with MNP showed a positive trend for several nutritional indicators, it seems that a certain level of macronutrients is needed to prevent malnutrition. Therefore, further research should focus on an appropriate balance between macro - and micronutrients to optimise the cost effectiveness of supplementation with lipid-based fortified foods in preventing malnutrition in ill children.
Supporting Information
Zdroje
1. Goulet O, Lebenthal E, Branski D, Martin A, Antoine JM, Jones PJH. Nutritional solutions to major health problems of preschool children: how to optimise growth en development. J Pediatr Gastroenterol Nutr. 2006;43(Suppl. 3):1–3.
2. Rowland MG, Rowland SG, Cole TJ. Impact of infection on the growth of children from 0 to 2 years in an urban West African community. Am J Clin Nutr. 1988; 47(1):134–8. 3337031
3. Tomkins AM. Protein–energy malnutrition and risk of infection. Proceedings of the Nutrition Society. Cambridge Univ Press 1986; 289–304. 3099300
4. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382 : 427–51. doi: 10.1016/S0140-6736(13)60937-X 23746772
5. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008; 371 : 243–60. doi: 10.1016/S0140-6736(07)61690-0 18207566
6. Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004; 80 : 193–8. 15213048
7. Chen LC. Interactions of diarrhea and malnutrition: mechanisms and interventions. In: Chen LC, Scrimshaw NS, editors. Diarrhea and malnutrition: interactions, mechanisms and interventions. Plenum Publishing Corporation, New York 1983; 3–19.
8. Assis AMO, Barreto ML, Santos LMP, Fiaccone R, da Silva Gomes GS. Growth faltering in childhood related to diarrhea: a longitudinal community based study. Eur J Clin Nutr 2005; 59 : 1317–23. 16077743
9. Weisz A, Meuli G, Thakwalakwa C, Trehan I, Maleta K, Manary M. The duration of diarrhea and fever is associated with growth faltering in rural Malawian children aged 6–18 months. Nutr J. 2011; 10 : 25. doi: 10.1186/1475-2891-10-25 21418600
10. Williams T, Maitland K, Phelps L, Bennett S, Viji J, Clegg JB, et al. Plasmodium vivax : a cause of malnutrition in young children. QJMed. 1997; 90 : 751–757.
11. Fillol F, Sarr JB, Boulanger D, Cisse B, Sokhna C, Riveau G, et al. Impact of child malnutrition on the specific anti-Plasmodium falciparum antibody response. Malar J. 2009; 8 : 116. doi: 10.1186/1475-2875-8-116 19490641
12. Shiff C, Checkley W, Winch P, Premji Z, Minjas J, Lubega P. Changes in weight gain and anaemia atttributable to malaria in Tanzanian children living under holoendemic conditions. Trans R Soc Trop Med Hyg. 1996; 90 : 262–65. 8758071
13. Sowunmi A, Gbotosho GO, Adedeji AA, Fateye BA, Sabitu MF, Happi CT, et al. Effects of acute Plasmodium falciparum malaria on body weight in children in an endemic area. Parasitol Res. 2007; 101 : 343–49. 17323138
14. Hoare S, Poppitt SD, Prentice AM, Weaver LT. Dietary supplementation and rapid catch-up growth after acute diarrhoea in childhood. Br J Nutr. 1996; 76 : 479–90. 8942357
15. Valentiner-Branth P, Steinsland H, Santos G, Perch M, Begtrup K, Bhan MK, et al. Community-based controlled trial of dietary management of children with persistent diarrhea: sustained beneficial effect on ponderal and linear growth. Am J Clin Nutr. 2001; 73 : 968–974. 11333852
16. Bahwere P, Donnen P, Dramaix-Wilmet M, Butzler JP, Hennart P, Levy J. Improvements in nutritional management as a determinant of reduced mortality from community-acquired lower respiratory tract infection in hospitalized children from rural central Africa. Pediatr Infect Dis J. 2004; 23 : 739–47. 15295224
17. Van der Kam S, Swarthout T, Niragira O, Froud A, Sompwe ME, Mills C, et al. Ready-to-use therapeutic food for catch-up growth in children after an episode of Plasmodium falciparum malaria: an open randomised controlled trial. PLoS ONE. 2012; 7(4): e35006 doi: 10.1371/journal.pone.0035006 22558108
18. Mahalanabis D, Bhan MK. Micronutrients as adjunct therapy of acute illness in children : impact on the episode outcome and policy implications of current findings. Br J Nut. 2001; 85 (Suppl):S151–8.
19. Chen K, Zhang X, Li T, Chen L, Wei X, Qu P, et al. Effect of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on infectious morbidity of preschool children. Nutrition 2011; 27 : 428–34. doi: 10.1016/j.nut.2010.04.004 20605698
20. Fischer Walker CL, Black RE. Micronutrients and diarrheal disease. Clin Infect Dis. 2007; 45 (Suppl):S73–7.
21. Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics 2007; 119 : 1120–1130. 17545379
22. Haider B, Bhutta Z. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutr Bull. 2009; 30 (Suppl):S41–59.
23. Mahalanabis D, Lahiri M, Paul D, Gupta S, Gupta A, Wahed MA, et al. Efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr. 2004; 79 : 430–6. 14985218
24. Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lönnerdal B, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. Am J Clin Nutr. 2004; 79 : 457–65. 14985222
25. Richard S, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg. 2006; 75 : 126–32. 16837718
26. WHO. Food, Water and Family Health : A manual for community educators. Geneva: WHO, UNDP; 1994; p.19, 27. http://apps.who.int/iris/bitstream/10665/62963/1/WHO_HEP_94.2.pdf?ua=1
27. Dewey KG. Guiding principles for complementary feeding of the breastfed child. WHO; 2003:p.26. http://whqlibdoc.who.int/paho/2003/a85622.pdf?ua=1
28. Dewey KG. Guiding principles for feeding non-breastfed children 6–24 months of age. WHO; 2005: p.23. http://apps.who.int/iris/bitstream/10665/43281/1/9241593431.pdf?ua=1&ua=1
29. Van der Kam S, Salse N, Swarthout T, Roll S, Jiya NM, Shanks L. Effect of short-term supplementation with ready-to-use therapeutic food or micronutrients for children after Illness for prevention of malnutrition: a randomised controlled trial in Nigeria. In press PLoS Med 201613(1). doi: 10.1371/journal.pmed.1001951
30. FEWSNET. Uganda-Karamoja region livelihood zones and descriptions. 2013. http://www.fews.net/sites/default/files/documents/reports/KaramojaFinal_LHZ Report.pdf
31. McKinney P. Comprehensive food security & vulnerability analysis. VAM 2009; 57–80. http://home.wfp.org/stellent/groups/public/documents/ena/wfp202495.pdf
32. ACF Action Against Hunger. Nutrition surveillance Karamoja Region, Uganda. Round 8, May 2012. 2012. http://www.actionagainsthunger.org/sites/default/files/publications/DHO-ACF_Karamoja_Nutrition_Surveillance_Round_8_-_Final_Report_2012.05.pdf
33. WFP (AME unit). Food security and nutrition assessment. 2014. http://documents.wfp.org/stellent/groups/public/documents/ena/wfp266721.pdf
34. WHO; WFP; UNICEF. Preventing and controlling micronutrient deficiencies in populations affected by an emergency. 2007. http://www.who.int/nutrition/publications/WHO_WFP_UNICEFstatement.pdf
35. Isanaka S, Nombela N, Djibo A, Poupard M, Van Beckhoven D, Gaboulaud V, et al. Effect of preventive supplementation with ready-to-use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 2009; 301 : 277–85. doi: 10.1001/jama.2008.1018 19155454
36. Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti S a, Ouédraogo ZP, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young burkinabe children: a cluster-randomized trial. PLoS ONE 2015; 10(3):e0122242 doi: 10.1371/journal.pone.0122242 25816354
37. Huybregts L, Houngbé F, Salpéteur C, Brown R, Roberfroid D, Ait-Aissa M, et al. The effect of adding ready-to-use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster-randomized controlled trial. PLOoS Med. 2012;9(9): e1001313.
38. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 2013; 382 : 29–40. doi: 10.1016/S0140-6736(13)60437-7 23602230
39. Prentice AM. Iron metabolism, malaria and other infections: what Is all the fuss about? J Nutr. 2008; 138 : 2537–41. doi: 10.3945/jn.108.098806 19022986
40. Veenemans J, Milligan P, Prentice AM, Schouten LR a, Inja N, van der Heijden AC, et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011; 8(11):e1001125. doi: 10.1371/journal.pmed.1001125 22131908
41. Ojukwu JU, Okebe JU, Yahav D, Paul M. Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Syst Rev. 2009; (4).
42. Okebe J, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2011; (10).
Štítky
Interné lekárstvo
Článek 2015 Reviewer Thank You
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Statinová intolerance
- Genetický podklad a screening familiární hypercholesterolémie
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
- DESATORO PRE PRAX: Aktuálne odporúčanie ESPEN pre nutričný manažment u pacientov s COVID-19
-
Všetky články tohto čísla
- 2015 Reviewer Thank You
- The Future of Diabetes Prevention: A Call for Papers
- The Case for Reforming Drug Naming: Should Brand Name Trademark Protections Expire upon Generic Entry?
- The Health Care Consequences Of Australian Immigration Policies
- Microenvironmental Heterogeneity Parallels Breast Cancer Progression: A Histology–Genomic Integration Analysis
- Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination
- Estimated Effects of Different Alcohol Taxation and Price Policies on Health Inequalities: A Mathematical Modelling Study
- Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Uganda
- Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Nigeria
- When Children Become Adults: Should Biobanks Re-Contact?
- Transforming Living Kidney Donation with a Comprehensive Strategy
- A Time for Global Action: Addressing Girls’ Menstrual Hygiene Management Needs in Schools
- The Rise of Consumer Health Wearables: Promises and Barriers
- Risk of Injurious Fall and Hip Fracture up to 26 y before the Diagnosis of Parkinson Disease: Nested Case–Control Studies in a Nationwide Cohort
- Mortality, Morbidity, and Developmental Outcomes in Infants Born to Women Who Received Either Mefloquine or Sulfadoxine-Pyrimethamine as Intermittent Preventive Treatment of Malaria in Pregnancy: A Cohort Study
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Hand, Foot, and Mouth Disease in China: Modeling Epidemic Dynamics of Enterovirus Serotypes and Implications for Vaccination
- A Time for Global Action: Addressing Girls’ Menstrual Hygiene Management Needs in Schools
- Transforming Living Kidney Donation with a Comprehensive Strategy
- The Rise of Consumer Health Wearables: Promises and Barriers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



