-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
The infectious and diagnostic stage of Giardia lamblia (also known as G. intestinalis or G. duodenalis) is the cyst. The Giardia cyst wall contains fibrils of a unique β-1,3-linked N-acetylgalactosamine (GalNAc) homopolymer and at least three cyst wall proteins (CWPs) composed of Leu-rich repeats (CWPLRR) and a C-terminal conserved Cys-rich region (CWPCRR). Our goals were to dissect the structure of the cyst wall and determine how it is disrupted during excystation. The intact Giardia cyst wall is thin (∼400 nm), easily fractured by sonication, and impermeable to small molecules. Curled fibrils of the GalNAc homopolymer are restricted to a narrow plane and are coated with linear arrays of oval-shaped protein complex. In contrast, cyst walls of Giardia treated with hot alkali to deproteinate fibrils of the GalNAc homopolymer are thick (∼1.2 µm), resistant to sonication, and permeable. The deproteinated GalNAc homopolymer, which forms a loose lattice of curled fibrils, is bound by native CWP1 and CWP2, as well as by maltose-binding protein (MBP)-fusions containing the full-length CWP1 or CWP1LRR. In contrast, neither MBP alone nor MBP fused to CWP1CRR bind to the GalNAc homopolymer. Recombinant CWP1 binds to the GalNAc homopolymer within secretory vesicles of Giardia encysting in vitro. Fibrils of the GalNAc homopolymer are exposed during excystation or by treatment of heat-killed cysts with chymotrypsin, while deproteinated fibrils of the GalNAc homopolymer are degraded by extracts of Giardia cysts but not trophozoites. These results show the Leu-rich repeat domain of CWP1 is a lectin that binds to curled fibrils of the GalNAc homopolymer. During excystation, host and Giardia proteases appear to degrade bound CWPs, exposing fibrils of the GalNAc homopolymer that are digested by a stage-specific glycohydrolase.
Published in the journal: Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer. PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001059
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001059Summary
The infectious and diagnostic stage of Giardia lamblia (also known as G. intestinalis or G. duodenalis) is the cyst. The Giardia cyst wall contains fibrils of a unique β-1,3-linked N-acetylgalactosamine (GalNAc) homopolymer and at least three cyst wall proteins (CWPs) composed of Leu-rich repeats (CWPLRR) and a C-terminal conserved Cys-rich region (CWPCRR). Our goals were to dissect the structure of the cyst wall and determine how it is disrupted during excystation. The intact Giardia cyst wall is thin (∼400 nm), easily fractured by sonication, and impermeable to small molecules. Curled fibrils of the GalNAc homopolymer are restricted to a narrow plane and are coated with linear arrays of oval-shaped protein complex. In contrast, cyst walls of Giardia treated with hot alkali to deproteinate fibrils of the GalNAc homopolymer are thick (∼1.2 µm), resistant to sonication, and permeable. The deproteinated GalNAc homopolymer, which forms a loose lattice of curled fibrils, is bound by native CWP1 and CWP2, as well as by maltose-binding protein (MBP)-fusions containing the full-length CWP1 or CWP1LRR. In contrast, neither MBP alone nor MBP fused to CWP1CRR bind to the GalNAc homopolymer. Recombinant CWP1 binds to the GalNAc homopolymer within secretory vesicles of Giardia encysting in vitro. Fibrils of the GalNAc homopolymer are exposed during excystation or by treatment of heat-killed cysts with chymotrypsin, while deproteinated fibrils of the GalNAc homopolymer are degraded by extracts of Giardia cysts but not trophozoites. These results show the Leu-rich repeat domain of CWP1 is a lectin that binds to curled fibrils of the GalNAc homopolymer. During excystation, host and Giardia proteases appear to degrade bound CWPs, exposing fibrils of the GalNAc homopolymer that are digested by a stage-specific glycohydrolase.
Introduction
Giardia lamblia, which is a deeply divergent protist, causes diarrhea in the developing world where hygiene is inadequate to block its transmission by the fecal-oral route [1]–[3]. In addition, some two million Americans are infected each year with Giardia, which is present in streams and lakes or is transmitted in day-care centers [4]. Giardia is then an important pathogen in both developing and developed countries.
The infectious and diagnostic stage of Giardia is the quadranucleate cyst [5]. Three abundant cyst wall proteins (CWP1, CWP2, and CWP3) have N-terminal Leu-rich repeats (LRRs) and a C-terminal Cys-rich region (CRR) [6]–[9]. Cys-rich CWP1 is the target for diagnostic monoclonal antibodies to Giardia in clinical specimens, and anti-CWP1 antibodies reduce excystation of Giardia in vitro [10], [11]. CWP2, which has an additional positively charged domain at its C-terminus, has been used to immunize mice and reduce cyst formation by Giardia [12]. The three Giardia CWPs have relatively few sites for N-linked glycosylation, so that wheat germ agglutinin (WGA), which binds to the very short N-glycan of Giardia (GlcNAc2), predominantly stains membranes closely apposed to the cyst wall rather than the wall itself [13]–[15]. A fourth cyst wall protein (HCNCp) is part of a new family of Cys-rich, non-VSP proteins of Giardia [16]. In addition, a family of proteins referred to as EGF-like cyst proteins (EGFCPs), which contain a series of Cys-rich repeats, are targeted to Giardia cyst walls [17].
CWPs are present in encystation-specific secretory vesicles (ESVs), which are part of a Golgi-like compartment that lacks membrane stacks and luminal glycosyltransferases but is sensitive to Brefeldin A [18]. The positively charged domain at the C-terminus of CWP2 is important for biogenesis of ESVs [19]. Selective condensation drives portioning and sequential secretion of cyst wall proteins, so that CWP1 and the major portion of CWP2 are added first to the cyst wall followed by CWP3 [20].
Giardia cysteine proteinases are necessary for encystation and excystation, while host proteases (trypsin and/or chymotrypsin) are required for excystation [21]–[23]. ESVs and cyst wall formation are interrupted by dithiothreitol (DTT) that blocks disulfide formation within Cys-rich C-terminal domains of CWPs and blocks polymerization of CWPs [24]. Cyst wall formation is also dependent upon isopeptide bonds formed in CWPs by a novel transglutaminase activity [25]. Finally, protein phosphatases are involved in cyst wall formation [26], [27].
Pioneering studies of Edward Jarroll and colleagues have shown that the sugar homopolymer in Giardia cyst walls is composed of β-1,3-linked N-acetylgalactosamine (GalNAc) rather than chitin (β-1,4-linked GlcNAc), as previously suggested [28]. Electron microscopic studies demonstrate the deposition of fibrils of the GalNAc homopolymer onto the surface of encysting Giardia, as well as within intracellular vesicles [29], [30]. However, the GalNAc homopolymer has not been obtained free of protein contamination, so that its structure in the absence of protein has not been visualized. In addition, no reagents (lectins or antibodies) have been identified for labeling the GalNAc homopolymer, which does not stain with GalNAc-binding plant lectins [13]. The GalNAc homopolymer is made from cytosolic UDP-GalNAc by a synthase [31].
The walls of fungi, plants, and Dictyostelium contain multiple layers, more than one sugar polymer, and many proteins, and so these walls likely do not represent a good model for the relatively simple cyst wall of Giardia [32]–[34]. In contrast, the cyst wall of Entamoeba histolytica, the protist that causes amebic dysentery and liver abscess, is relatively simple [35], [36]. The amebic wall is composed of chitin and three unique chitin-binding lectins, which degrade chitin (chitinase), cross-link fibrils (multivalent Jacob lectins), and self-aggregate to make the amebic wall impermeable to small molecules (Jessie lectins) [37]–[39].
In an effort to better understand how the Giardia cyst wall is assembled during encystation, we asked the following questions:
-
Can we use methods used to isolate chitin and glucans from fungal walls (strong alkali and high temperatures) to deproteinate cyst walls of Giardia and isolate fibrils of the GalNAc homopolymer [40]? If so, what do the fibrils look like?
-
Do native CWPs, which are released with non-ionic detergent from encysting Giardia, bind to deproteinated fibrils of the GalNAc homopolymer? If so, can we use recombinant maltose-binding fusion-proteins (MBP) to determine whether the lectin domain of CWP1 is present in the N-terminal Leu-rich repeats (CWP1LRR) or in the C-terminal Cys-rich region (CWP1CRR) [39], [41]?
-
Can we use recombinant CWP1 to determine whether the GalNAc homopolymer is made at the plasma membrane, as described for fungal chitin, or within intracellular vesicles as described for chitin of Entamoeba [39], [42]?
-
Can we provide evidence that an endogenous Giardia glycohydrolase (comparable to chitinases of Entamoebae) degrades fibrils of the GalNAc homopolymer during excystation (37)?
Results
Intact Giardia cyst walls are thin, brittle, and impermeable to small molecules
Giardia cyst walls were visualized well with an anti-CWP1 monoclonal antibody (Fig. 1A) [7], [10]. Giardia cyst walls are impermeable, so we froze and thawed cysts multiple times in order to label nuclei with DAPI and label N-linked glycans that are present for the most part in membranes closely apposed to the cyst wall with WGA (Fig. 1A) [15]. Giardia cyst walls are brittle and so fracture into multiple fragments or shards with sharp edges when sonicated (Fig. 1B). Giardia cyst walls, which stain well with the poly-cationic dye ruthenium red, are 0.3 to 0.6 µm thick (depending upon the compactness of the fibrils) when viewed on cross-section with the transmission electron microscope (TEM) (Fig. 1C) [43]. Fibrils of the GalNAc homopolymer are covered with linear arrays of oval-shaped protein complexes that have a uniform appearance (Fig. 1C). These oval-shaped protein complexes, which are too big to represent a single CWP, are lost when cyst walls are deproteinated with NaOH (see next section).
Fig. 1. In intact Giardia cyst walls, curled fibrils of the GalNAc homopolymer form a protein-coated lattice that is restricted to a narrow plane. 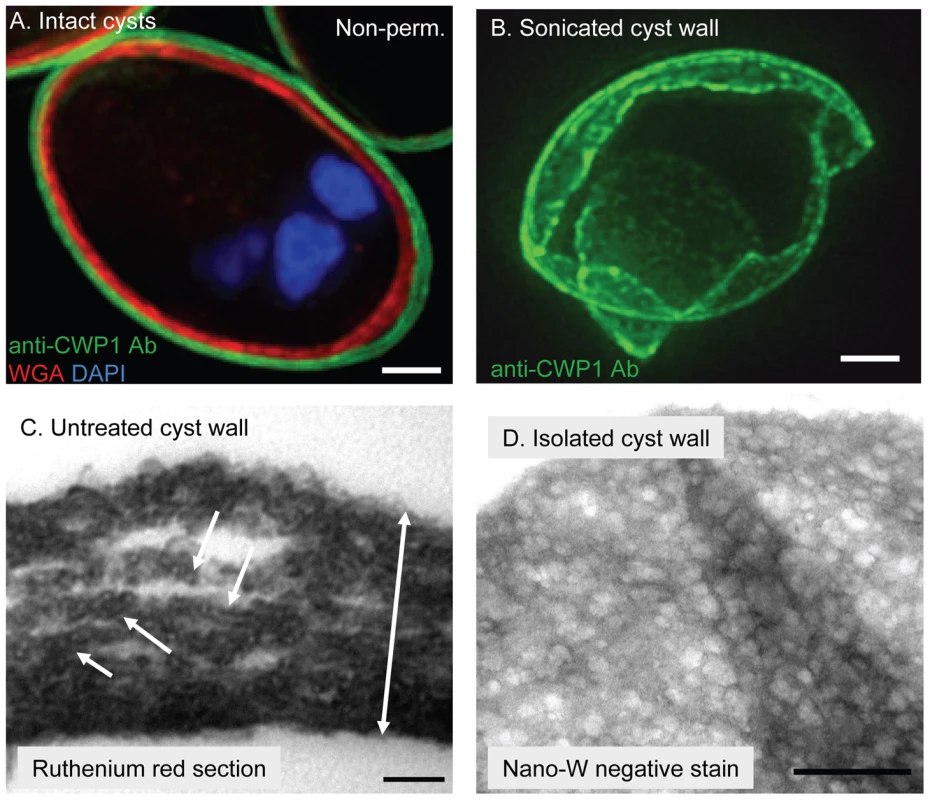
A. Cross-section by deconvolving microscopy shows intact cysts label well with anti-CWP1 monoclonal antibody (green). Unless cysts were frozen and thawed, WGA (red), which intensely labels N-glycans present in membrane glycoproteins closely apposed to the cyst wall, does not penetrate (for example, cyst labeled non-perm. in upper right corner of A). Nuclei are stained blue with DAPI. B. Three-dimensional reconstruction by deconvolving microscopy shows that a cyst wall broken by sonication and then labeled with anti-CWP1 antibodies (green) is thin and shows sharp lines of fracture. C. TEM of a thin section of ruthenium red-stained Giardia cysts shows the intact cyst wall is thin (two-headed arrow is 0.4 microns long). Fibers of the GalNAc homopolymer are coated with a linear array of oval-shaped protein complexes (arrows). D. TEMs of a Giardia cyst wall isolated on a sucrose gradient and then negatively stained with NANO-W shows a two-dimensional honey-comb appearance, in which curled fibrils of the GalNAc homopolymer are held in a narrow plane by adherent CWPs. Note folds in the sheet-like cyst wall. Bars (A and B) are 1 micron. Bar (C) is 100 nm. Bar (D) is 500 nm. Fibrils of the GalNAc homopolymer were also visualized when Giardia cyst walls were isolated on sucrose gradients, negative-stained with NANO-W (an organo-tungstate compound that is weakly penetrating and so highlights superficial structures), and viewed with TEM (Fig. 1D) [44]. In these cyst wall preparations, curled fibrils of the GalNAc homopolymer form a lattice that is pressed into thin sheets and has the appearance of a two-dimensional honey comb. Curled fibrils of the GalNAc homopolymer are also present in cyst walls deproteinated by NaOH-treatment, but the curled fibrils no longer form a thin sheet (see next section).
Treatment of Giardia cyst walls with hot alkali removes cyst wall proteins (CWPs)
Because preparations of the GalNAc homopolymer from cyst walls of Giardia that have been treated with proteases and detergents still contain substantial quantities of contaminating protein [28], we chose hot alkali treatment that is frequently used to isolate chitin and β-1,3-glucans from deproteinated fungal walls [40]. We isolated fibrils of the GalNAc homopolymer, which generally maintains the hollow spherical shape of cyst walls, by boiling whole Giardia cysts in 0.75 to 1 N NaOH for 1 to 2 hrs (Fig. 2). The purity of the deproteinated fibrils of the GalNAc homopolymer (synonym for NaOH-treated cyst walls) was demonstrated in three ways:
Fig. 2. The deproteinated GalNAc homopolymer (NaOH-treated cyst wall) is a loose, thick-walled lattice of curled fibrils that maintains a hollow, spherical shape. 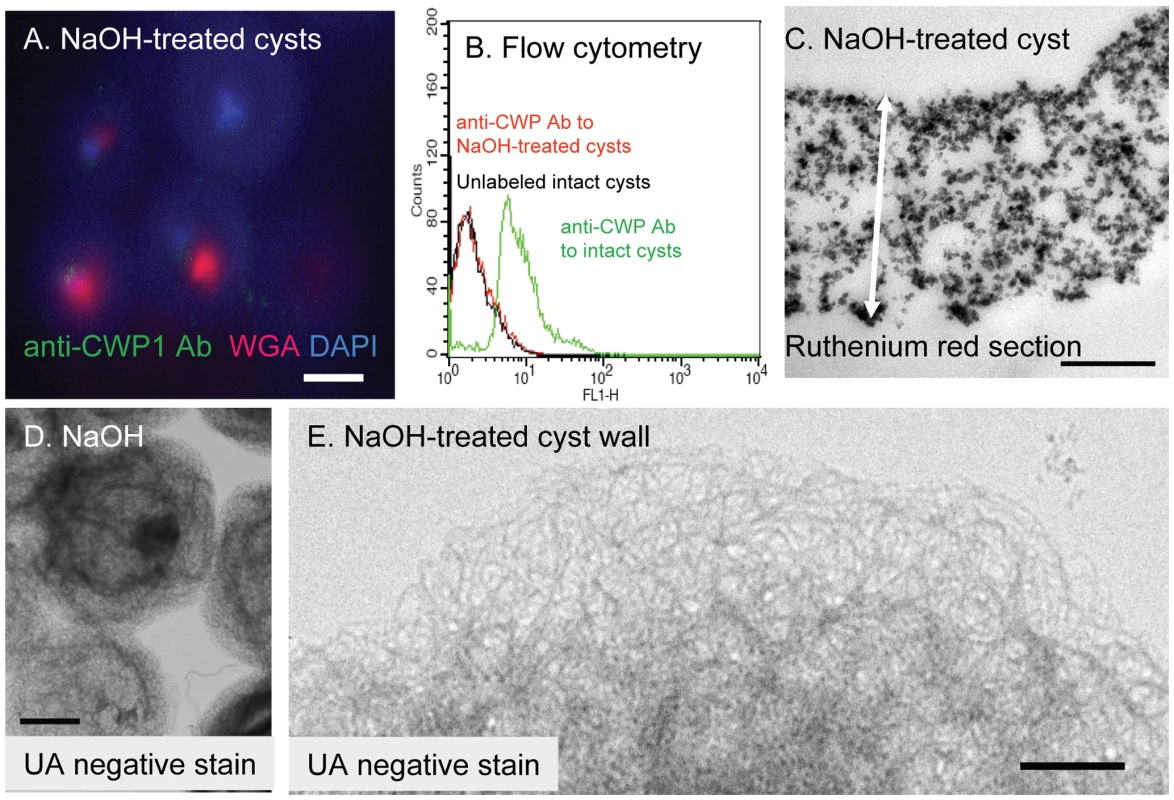
A. Deconvolving microscopy shows NaOH-treatment removes cyst wall proteins, so that there is no longer any binding of anti-CWP1 antibodies (green). WGA (red) labels some denatured parasite glycoproteins that are not part of the cyst walls, while DAPI (blue) stains residual nuclei acid. B. Flow cytometry shows that binding of anti-CWP1 antibodies to intact cyst walls (green) is completely removed by NaOH treatment (red). A control with unlabeled intact cysts is shown in black. C. A TEM of a thin section of ruthenium red-stained cyst wall after treatment with NaOH shows fibrils of the GalNAc homopolymer are no longer coated with oval-shaped protein complexes, while the cyst wall is markedly thickened (two-headed arrow is 1.2 microns long). D and E. TEM and negative staining with uranyl acetate (UA) shows NaOH-treated cyst walls of Giardia are composed of a loose lattice of 5 nm thick, curled fibrils that generally maintain the hollow spherical shape of the cyst wall. NaOH-treated cyst walls do not stain with NANO-W (data not shown). Bar (A) is 1 micron. Bars (C and E) are 100 nm. Bar (D) is 5 microns. -
While both intact cysts and shards of the cyst wall label strongly with an anti-CWP1 monoclonal antibody (Figs. 1A and 1B), NaOH-treated cyst walls do not label with the anti-CWP1 antibody (Fig. 2A). The results with deconvolving microscopy were confirmed by flow cytometry (Fig. 2B). The opposite results occur when intact and NaOH-treated cyst walls are labeled with recombinant CWP1, which binds to fibrils of the GalNAc homopolymer (see below).
-
Only GalN and GalNAc are released by acid hydrolysis of NaOH-treated Giardia cyst walls. These sugars were shown by gas chromatography and mass spectrometry (GC-MS) and by high performance anion exchange chromatography (HPAEC) (Fig. S1 in the Supplemental materials). These results are in agreement with release of monomers, dimers, and short oligosaccharides of β-1,3-linked GalNAc by partial acid hydrolysis of Giardia cyst walls that were deproteinated with SDS and proteases [21].
-
NaOH-treated cyst walls do not contain protein, as judged by a BCA assay and by SDS-PAGE followed by silver-staining or Western blotting with anti-CWP1 antibody (lane 1 in Figs. 3A and 3B). Positive controls for the BCA-assays, silver stains, and Western blots were made by examining NaOH-treated cyst walls that had been incubated with native Giardia CWPs or with MBP fusion-proteins containing Giardia CWP1 (lanes 2 and 3, respectively, in Figs. 3A and 3B and see below).
-
We cannot rule out, however, the possibility that treatment with strong base may cleave certain covalent linkages between chains of GalNAc homopolymers and/or remove other attached sugars.
Fig. 3. Binding of CWP1 and CWP2 to deproteinated fibrils of the GalNAc homopolymer. 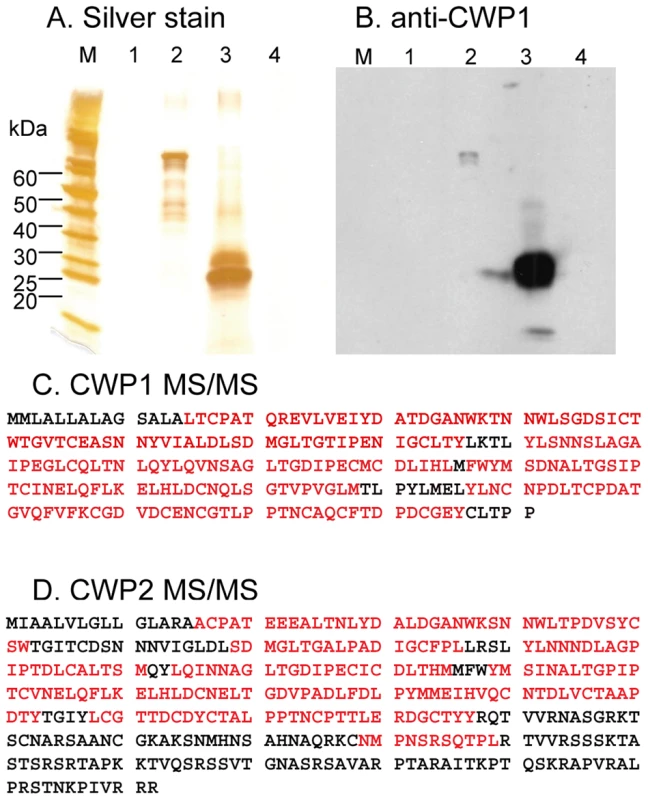
Silver stain (A) and Western blot with anti-CWP1 antibody (B) show that cyst walls treated with NaOH (lane 1 in each figure) contain no detectable protein (see Fig. 2 for the appearance of deproteinated fibrils of the GalNAc homopolymer). In contrast, recombinant MBP-CWP1FL made in bacteria (lane 2 in A and B) binds well to deproteinated fibrils of the GalNAc homopolymer. Native CWP1, which is present in extracts of encysting Giardia (lane 3 in A and B), also binds to deproteinated fibrils of the GalNAc homopolymer. In contrast, no trophozoite proteins bind to deproteinated cyst walls (lane 4 in A and B). C. Mass spectrometry shows that CWP1 present in extracts of encysting Giardia but minus the N-terminal signal peptide binds to deproteinated fibrils of the GalNAc homopolymer (peptide coverage is 86%). D. Mass spectrometry shows that CWP2 present in extracts of encysting Giardia but minus the N-terminal signal peptide and the C-terminal positively charged tail also binds to deproteinated fibrils of the GalNAc homopolymer (peptide coverage is 56%). These results show that NaOH removes proteins from Giardia cyst walls and show that the fibrils of the GalNAc homopolymer bind native and recombinant CWP1, as well as native CWP2. The deproteinated GalNAc homopolymer is a loose, thick-walled lattice of curled fibrils
NaOH-treated Giardia cysts stick to one another, most likely by interlocking fibrils of the GalNAc homopolymer that were exposed by removal of cyst wall proteins (Figs. 2D and 2E). The deproteinated fibrils of the GalNAc homopolymer, which are five nm thick and curled, are no longer coated by oval-shaped protein complexes. The fibrils of the deproteinated GalNAc polymer form a loose lattice in the general shape of untreated cyst walls, but they are no longer pressed into a narrow two-dimensional sheet. Instead NaOH-treated cyst walls are substantially thicker than untreated cyst walls (compare Fig. 1C to Fig. 2C and compare Fig. 2B to Fig. 4D). In addition, NaOH-treated cysts are permeable to WGA and DAPI that bind to remnant coagulated protein and DNA, respectively, in poorly washed preparations (Fig. 2A). NaOH-treated cysts are no longer brittle and so cannot be broken by sonication (data not shown). The fragment of the cyst wall shown in Fig. 4D was prepared by sonicating intact cysts that were then treated with hot alkali prior to labeling with recombinant CWP1 (see description in next section).
Fig. 4. N-terminal Leu-rich repeats of Giardia CWP1 (CWP1LRR) form a lectin domain that binds to deproteinated fibrils of the GalNAc homopolymer. 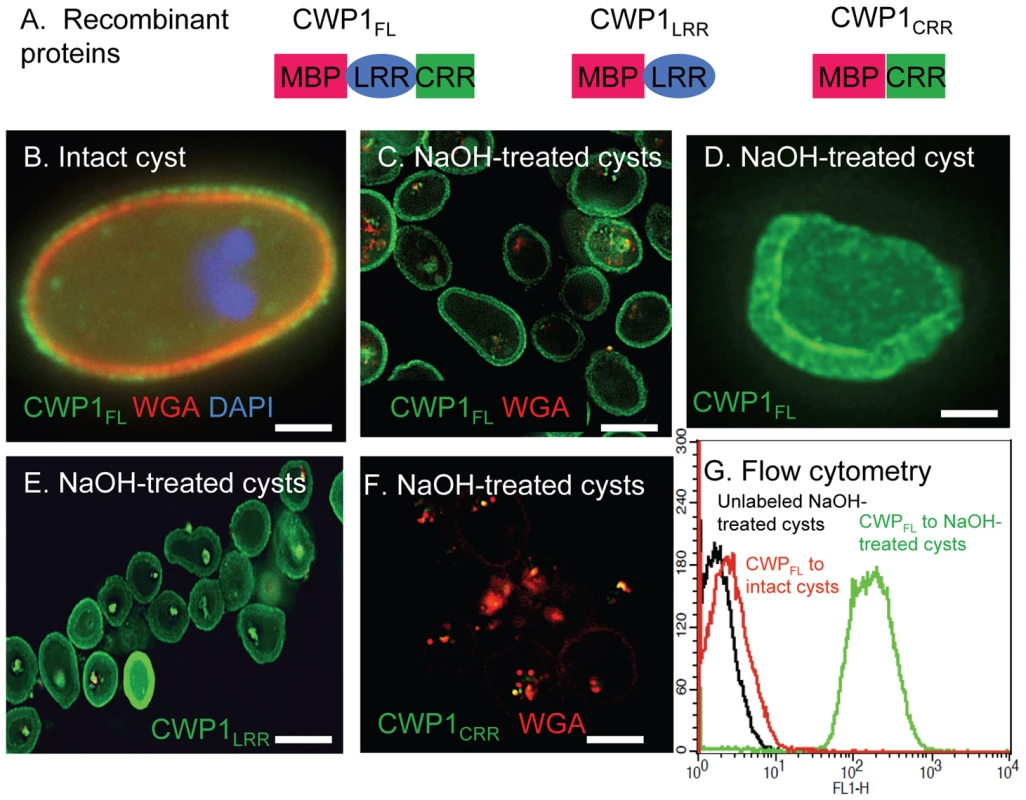
A. Each recombinant protein used to identify the lectin domain of CWP1 has MBP at the N-terminus. At the C-terminus, CWP1LRR contains the Leu-rich repeats of CWP1; CWP1CRR contains the Cys-rich region; while full-length CWP1 (CWP1FL) contains both domains. B. A representative deconvolving micrograph shows CWP1FL (green) binds in a punctate pattern to the wall of an intact cyst, while WGA (red) and DAPI (blue) appear as in Fig. 1A. C. CWP1FL (green) strongly labels NaOH-treated cyst walls (deproteinated GalNAc homopolymer), while there is minimal labeling of residual glycoproteins with WGA. D. A shard of a Giardia cyst wall made by sonication, treatment with NaOH, and then incubation with CWP1FL (green) is >1 µm thick. E. CWP1LRR (green) also labels deproteinated fibrils of the GalNAc homopolymer. F. In contrast, there is no binding of CWP1CRR (green) to NaOH-treated cyst walls. Again the only staining is with WGA that binds to glycoproteins that are not part of the cyst wall. There is no binding of unfused MBP to Giardia deproteinated fibrils of the GalNAc homopolymer (data not shown). G. Flow cytometry shows that binding of CWP1FL to intact cyst walls (black) is much less than the binding of the same probe to NaOH treated walls (green). A control with unlabeled NaOH-treated cysts is shown in red. All micrographs are cross-sections with the exception of C, which is a three-dimensional reconstruction. Bars (B and D) are 2 microns. Bar (C, E, and F) are 10 microns. Together these results suggest that Giardia cyst wall proteins, which bind in linear arrays of oval-shaped protein complexes to fibrils of the GalNAc homopolymer, contribute to the thinness, brittleness, and impermeability of the cyst wall. However, deproteinated curled fibrils of the GalNAc homopolymer are capable of maintaining the hollow spherical shape of the cyst wall.
Native CWP1 and CWP2 from encysting Giardia bind to deproteinated fibrils of the GalNAc homopolymer
We used the deproteinated fibrils of the GalNAc homopolymer to pull down proteins of encysting Giardia that have lectin (carbohydrate-binding) activity. Native CWP1, which was demonstrated by silver staining of SDS-PAGE, Western blotting with anti-CWP1 antibodies, and mass spectrometry of peptides released by chymotrypsin, bound strongly to fibrils of the GalNAc homopolymer (Figs. 3A to 3C). In particular, there was 96% peptide coverage of CWP1 (less the signal peptide) in mass spectrograms of encystation-specific proteins of Giardia binding to deproteinated fibrils of the GalNAc homopolymer. As a negative control, no trophozoite proteins bound to the deproteinated fibrils of the GalNAc homopolymer (lane 4 in Figs. 3A and 3B).
Native CWP2 (less the signal peptide), also demonstrated by mass spectrometry, bound strongly to fibrils of the GalNAc homopolymer (Fig. 3D). There was much less peptide coverage of the C-terminus of CWP2, consistent with previous observations that ∼60 amino acids are cleaved from the C-terminus by an endogenous protease [20], [21]. The peptide coverage of CWP3 bound to deproteinated fibrils of the GalNAc homopolymer (12%) was much less than those for CWP1 (86%) and CWP2 (56%). This result suggests CWP3 is much less abundant in lysates of encysting Giardia and/or binds much less well to fibrils of the GalNAc homopolymer. Other proteins identified by mass spectrometry showed many fewer peptides, included cytoskeletal proteins, fermentation enzymes, and chaperones. All of these proteins are cytosolic in origin and are likely contaminants.
The Leu-rich repeats of Giardia cyst wall protein 1 (CWP1LRR) form a lectin domain that binds fibrils of the GalNAc homopolymer
To determine which domain of CWP1 contains the lectin activity that binds to fibrils of the GalNAc homopolymer, we made MBP fusion-proteins containing at the C-terminus either the full-length CWP1 less the signal peptide (CWP1FL), the N-terminal Leu-rich repeats of CWP1 less the signal peptide (CWP1LRR), or the C-terminal Cys-rich regions of CWP1 (CWP1CRR) (Fig. 4A) [41]. CWP1FL, which weakly labels intact cyst walls of Giardia (Fig. 5B), binds strongly to deproteinated fibrils of the GalNAc homopolymer (Figs. 4C and 4D). The CWP1FL-labeled fibrils of the GalNAc homopolymer are thick-walled, and the edges of the walls are mostly densely labeled, producing a “two-layered appearance.” This two-layered appearance was not reproduced in transmission micrographs of sections of deproteinated walls (Fig. 2C) or negative stains of the deproteinated walls (Figs. 2D and 2E), suggesting the two layers may explained by an increased density of curled fibrils at the outer and inner surfaces of the deproteinated walls. These deconvolving microscopy results were confirmed by flow cytometry (Fig. 4G) and by SDS-PAGE and Western blotting (lane 2 in Figs. 3A and 3B). While CWP1LRR labels NaOH-treated cyst walls as strongly as does CWP1FL (Fig. 4E), CWP1CRR does not bind at all to deproteinated fibrils of the GalNAc homopolymer (Fig. 4F).
Fig. 5. The GalNAc homopolymer is made within small vesicles of encysting Giardia and is added early to the cyst wall. 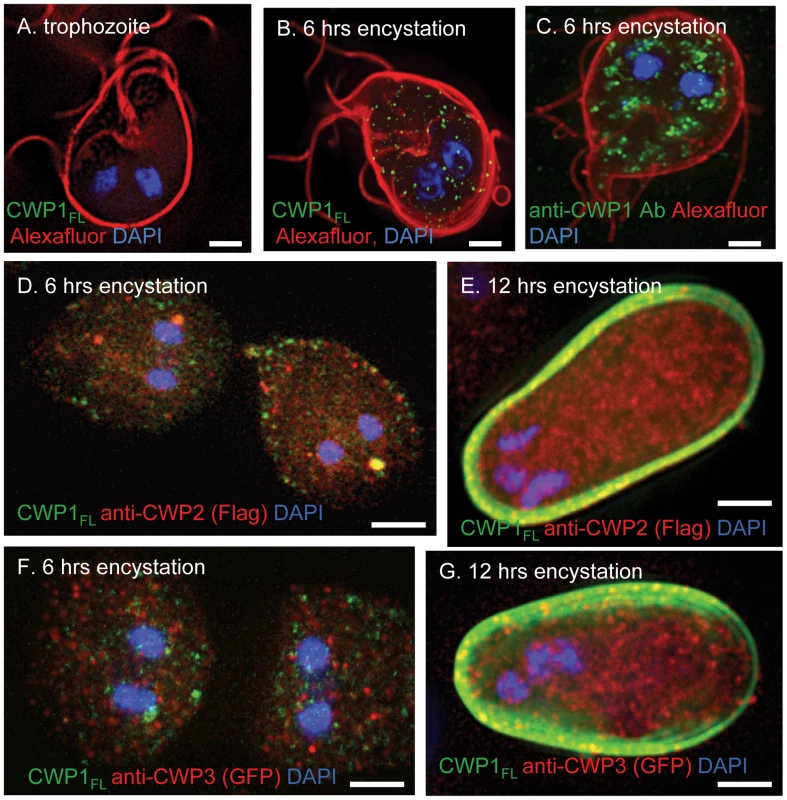
A. A representative deconvolving micrograph shows there is no synthesis in Giardia trophozoites of the GalNAc homopolymer that is detected with an MBP-CWP1 full-length fusion-protein (CWP1FL) (green). The plasma membrane is labeled red with Alexafluor. B. Giardia encysting for 6 hrs shows an extensive sets of vesicles labeled with CWP1FL (green), which binds to the GalNAc homopolymer. C. These vesicles are smaller and less clumped than those labeled green with anti-CWP1 antibodies in a parallel set of Giardia encysting for 6 hrs. D. Double-labeling experiment of a transformed Giardia expressing epitope-tagged CWP2 shows the GalNAc homopolymer (labeled green with recombinant CWP1FL) is present in vesicles of parasites encysting for 6 hrs that are for the most part distinct from ESVs containing CWP2 (labeled red with an antibody to the FLAG-tag). E. After 12 hrs encystation, the GalNAc homopolymer is present in the cyst wall, while the bulk of CWP2 remains in ESVs. F. Double-labeling experiment of a transformed Giardia expressing epitope-tagged CWP3 shows the GalNAc homopolymer is present in vesicles of parasites encysting for 6 hrs that are for the most part distinct from ESVs containing CWP3 (labeled red with an antibody to GFP). G. After 12 hrs, the GalNAc homopolymer is present in the cyst wall, while the bulk of CWP3 remains in ESVs. All figures are three-dimensional micrographs. Bars (A to G) are 2 microns. These results show that the CWP1LRR contains a lectin domain that binds to deproteinated fibrils of the GalNAc homopolymer and suggest the use of recombinant CWP1FL to visualize the GalNAc homopolymer during encystation (see next section). Recombinant CWP1FL is an important new tool for localizing fibrils of the GalNAc homopolymer, as GalNAc-binding plant lectins (e.g. Maclura pomifera agglutinin, also known as MPA) fail to bind to the deproteinated fibrils of the GalNAc homopolymer (data not shown) [13]. Conversely, recombinant CWP1FL does not bind to oocyst walls of Cryptosporidia that label strongly with MPA (data not shown) [45].
Recombinant MBP-CWP2 and MBP-CWP3 each failed to bind to the GalNAc homopolymer (data not shown). Because we cannot rule out the possibilities that MBP-CWP1CRR, MBP-CWP2, and MBP-CWP3 fusion-proteins are not well-folded, it is possible that these experiments failed for technical reasons and do not reflect the situation in vivo (see pull down of native CWP2 by the GalNAc homopolymer in Fig. 3D). Because native CWP1 is also present in extract of encysting Giardia, it is possible that native CWP2 is binding to CWP1 rather than to the GalNAc homopolymer. In particular, intermolecular disulfide bonds have been shown between CWP1 and CWP2 [24].
The GalNAc homopolymer is synthesized within small vesicles in encysting Giardia and is an early component of the cyst wall
These experiments used recombinant CWP1FL to identify the GalNAc homopolymer during encystation of Giardia in vitro. Giardia trophozoites, the surfaces of which were labeled with an Alexafluor dye, do not stain with CWP1FL (Fig. 5A) [46]. The GalNAc homopolymer, which was detected with recombinant CWP1FL, is present in small vesicles throughout the cytosol of Giardia encysting for 6 hrs (Fig. 5B). CWP1, which was detected by a monoclonal antibody (Fig. 5C), is present in ESVs [5], [6], [19], [20]. ESVs tend to be larger and located closer to the nucleus than vesicles that contain the GalNAc homopolymer. These results confirm TEM visualization of fibrils of the GalNAc homopolymer within secretory vesicles of encysting Giardia that are then deposited on the surface of encysting organisms [29], [30].
Because we used recombinant CWP1 FL to visualize the GalNAc homopolymer, we could not use a double-label with the anti-CWP1 antibody. However, we performed double-labels with recombinant CWP1 FL and antibodies that bound to epitope-tagged CWP2 and CWP3 in transformed Giardia that were encysting in vitro (Figs. 5D to 5G) [20]. For the most part, vesicles labeled with recombinant CWPFL do not also label with antibodies that bind to CWP1 and CWP2. Further recombinant CWP1 labels cyst walls when antibodies to CWP2 and CWP3 predominantly labeled ESVs in the interior of Giardia. These results suggest that the GalNAc homopolymer is made in vesicles distinct from ESVs and is secreted early onto the surface of encysting Giardia, as has also been shown by the scanning electron microscope [29].
During excystation in vitro, CWPs appear to be digested by proteases, uncovering fibrils of the GalNAc homopolymer that appear to be degraded by endogenous glycohydrolases
We chose to use Giardia cysts isolated from gerbil infections to study excystation, because the in vivo cysts excyst much more efficiently than do cysts made in vitro [22], [23]. Excystation in vitro has been shown to be dependent upon treatment with trypsin or chymotrypsin, a result that we repeated here (data not shown) [23]. Four changes in the Giardia cyst wall occur during excystation:
-
Compared to walls of intact cysts stained with anti-CWP1 antibodies, excysted walls are thicker and “fuzzier” in their appearance (compare Fig. 1B to Fig. 6A).
-
Excysted walls appear softer and more deformable than intact or sonicated cyst walls (see arrow in Fig. 6A).
-
During excystation, fibrils of the GalNAc homopolymer, which can be stained with recombinant CWP1FL, become much more accessible (compare Fig. 4B to Fig. 6B).
-
Large portions of the walls of excysted organisms disappear, using either the anti-CWP1 antibodies that detects protein (Fig. 6A) or recombinant CWP1FL that detects fibrils of the GalNAc homopolymers (Fig. 6B).
Fig. 6. Evidence for the roles of proteases and glycohydrolases in disruption of Giardia cyst walls during excystation. 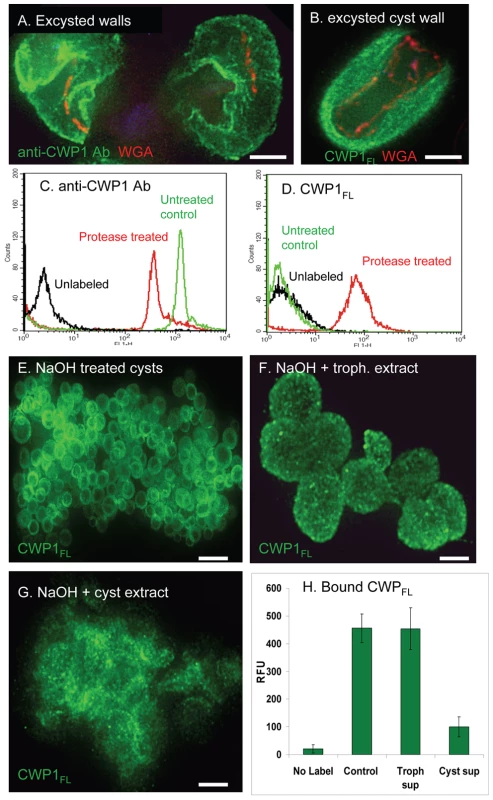
A. A representative deconvolving micrograph shows excysted walls of Giardia, which were labeled green with anti-CWP1 antibodies, are thickened, have irregular breaks, and appear soft (arrow) and moth-eaten when compared with a sonicated cyst wall (Fig. 1B). B. An excysted wall is extensively labeled green with CWP1FL, suggesting the fibrils of the GalNAc homopolymer are exposed by removal of cyst wall proteins by proteases. C and D. Flow cytometry shows that treatment of heat-killed Giardia cysts with chymotrypsin decreases the binding of anti-CWP1 antibody (C) but increases the binding of Alexafluor-conjugated CWP1FL. E. Representative deconvolving micrograph shows spherical NaOH-treated cyst walls (deproteinated fibrils of the GalNAc homopolymer) labeled green with Alexafluor-conjugated CWP1FL. F. These deproteinated cyst walls maintain their spherical shape after incubation with extracts of trophozoites. G. In contrast, NaOH-treated cyst walls, which were incubated with extracts of Giardia cysts, lose their spherical shape and form amorphous aggregates. H. These results were confirmed using fluorimetry of NaOH-treated cysts that were incubated with trophozoite and cyst extracts and then labeled with Alexafluor-conjugated CWP1FL. All figures are three-dimensional micrographs. Bars (A and B) are 3 microns. Bar (E) is 20 microns. Bars (F and G) are 5 microns. These results suggest that during excystation proteases degrade CWPs and then glycohydrolases degrade fibrils of the GalNAc homopolymer. This two step model for excystation was tested in the following two sections.
Chymotrypsin removes CWP1 from heat-killed cysts and exposes fibrils of the GalNAc homopolymer
The goal here was to determine whether exogenous chymotrypsin degrades CWPs and exposes fibrils of the GalNAc homopolymer. To test this idea, gerbil-derived cysts were heat-killed by treatment at 56°C for 20 min and then incubated for in 1 mg/ml chymotrypsin at 37°C for 30 min (the same treatment for excystation) prior to washing and labeling with anti-CWP1 antibody (protein label) or recombinant CWP1FL (GalNAc homopolymer label). A negative control was heat-killed cysts that were not treated with chymotrypsin. Flow cytometry showed there is a substantial decrease in labeling with anti-CWP1 antibody after chymotrypsin treatment and a concomitant marked increase in labeling with recombinant CWP1FL (Figs. 6C and 6D). These results echo the effect of NaOH treatment on Giardia cyst walls, in which anti-CWP1 antibody binding is eliminated (Fig. 2B) and binding of recombinant CWP1FL is dramatically increased (Fig. 4G).
Deproteinated fibrils of the GalNAc homopolymer are hydrolyzed by extracts of encysting Giardia
To further explore the possibility that Giardia contain endogenous glycohydrolases capable of degrading fibrils of the GalNAc homopolymer, we incubated deproteinated fibrils of the GalNAc homopolymer with extracts of trophozoites or with extracts of encysting Giardia (Figs. 6E to 6H). Deproteinated fibrils of the GalNAc homopolymer incubated with trophozoite extracts maintain their spherical shape when labeled with recombinant CWP1FL (Figs. 6E and 6F). In contrast, extracts of encysting Giardia degrade deproteinated fibrils of the GalNAc homopolymer, so that they form amorphous aggregates that are no longer composed of distinct spheres (Fig. 6G). In addition, the pellet of NaOH-treated cyst walls is much smaller after incubation with the cyst extracts. These results with the deconvolving microscope, which demonstrate a stage-specific glycohydrolase capable of degrading deproteinated fibrils of the GalNAc homopolymer, were confirmed using a fluorimeter (Fig. 6H). In addition, we used GC-MS to demonstrate the release of GalNAc from NaOH-treated walls incubated with cyst extracts (Supplemental Fig. S1C).
Discussion
Incorporation of the new findings into a two-component (curled fibril and lectin) model of the Giardia cyst wall
The intact Giardia cyst wall, which is thin, brittle, and impermeable, is composed of fibrils of the GalNAc homopolymer and CWPs (Figs. 2 and 7A). Components of Giardia cyst walls studied here (CWPs and fibers of the GalNAc homopolymer) were identified by previous investigators [5]–[9], [24], [25], [27]. What is new is the use of ruthenium red to stain thin sections of intact Giardia cyst walls for TEM and use of NANO-W to negatively stain fibrils of the GalNAc homopolymer in sonicated cyst walls (Fig. 1) [43], [44]. Ruthenium red stain demonstrates linear arrays of oval-shaped protein complexes that coat fibrils of the GalNAc homopolymer (Fig. 1C). NANO-W stain demonstrates curled fibrils of the GalNAc homopolymer, which are compressed into a narrow plane by bound CWPs (Figs. 1D and 7A). To our knowledge, there is no other wall (fungal or parasite), which is made on the principle of curled, interlocking fibrils that are pressed into a narrow plane by adherent proteins. While we cannot rule out the possibility that there are cross-links between fibrils of the GalNAc homopolymer, as described from glucans and chitin in fungal walls [33], [42], our images do not show evidence for branching of the deproteinated fibrils.
Fig. 7. New models for the structure of the Giardia cyst wall and for the mechanism of its disruption during excystation based upon the data presented here. 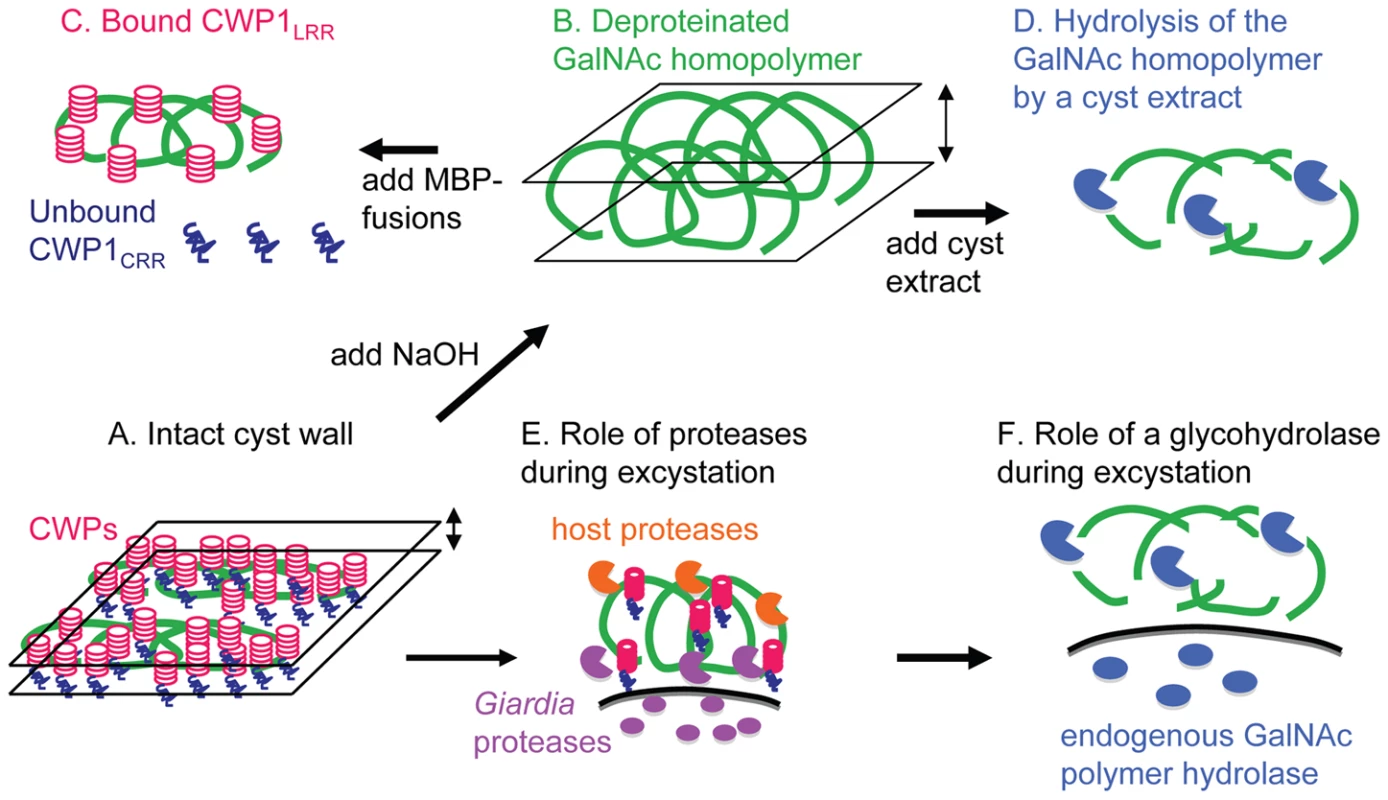
A. In the intact Giardia cyst wall, curled fibrils of the GalNAc homopolymer are compressed into a narrow plane by bound CWPs (see also Fig. 1). B. When CWPs are removed by hot alkali, curled fibrils of the GalNAc homopolymer form a markedly thickened wall (see also Fig. 2). C. Giardia CWPs bind via the N-terminal Leu-rich repeats (CWPLRR) to fibrils of the GalNAc homopolymer (see also Figs. 3 and4 ). In contrast, the C-terminal Cys-rich regions of CWPs (CWPCRR) do not bind to fibrils of the GalNAc homoplymer. D. Deproteinated fibrils of the GalNAc homopolymer are degraded by putative glycohydrolases present in cyst extracts (see also Fig. 6). Disruption of the cyst wall during excystation appears to occur by at least two mechanisms. E. Host and Giardia proteases degrade CWPs, which increases the thickness and softens the cyst wall (see also Fig. 6A) [23]. F. Fibrils of the GalNAc homopolymer, which are exposed by proteolysis of CWPs (Figs. 6B to 6D), are degraded by a cyst-specific glycohydrolase (see also Figs. 6E to 6H). As noted in the Introduction and the Discussion, other Cys-rich proteins may be present in the Giardia cyst wall [16], [17], and CWPs may be cross-linked by disulfides and be modified by a novel transglutaminase and protein phosphatases [24] to [26]. Treatment with strong base that has previously been used to deproteinate fungal walls effectively removes proteins from cyst walls of Giardia (Figs. 2, 3, and 7B) [40]. This is an important improvement over treatment of Giardia cyst walls with detergents and proteases, which is time consuming and ineffective [28]. Fibrils of the GalNAc homopolymer prepared by NaOH-treatment of Giardia cysts have a uniform diameter of 5 nm and form a loose lattice that has the same hollow, spherical shape as untreated cyst walls. While fibrils of the GalNAc homopolymer of Giardia are thinner than the ∼10 nm diameter chitin fibrils present in the cyst wall of Entamoeba [35], they are thicker than the 3 to 5 nm diameter microfibrils made by cellulose synthases of corn [47]. These microfibrils, which are composed of 36 parallel chains of β-1,3-linked glucose, coalesce into macrofibrils of cellulose that are 50 to 250 nm in diameter. While the fibrils of the Giardia GalNAc homopolymer do not appear to coalesce into macrofibrils, we assume they are also built upon parallel chains of the β-1,3-linked GalNAc that that are closely bound to each other by hydrogen bonds or covalent cross-links. These results are then in agreement with the structural studies of the GalNAc homopolymer [28], which concluded that “the highly insoluble nature of the cyst wall does not stem from the conformational properties of a single GalNAc chain, but must be due to strong interchain interactions.”
How the 10 nm diameter fibrils of the GalNAc homopolymer form such long curled fibrils in the Giardia cyst wall is not known, but it may depend upon properties of the GalNAc homopolymer synthase that has not yet been molecularly identified but has been partially purified [31]. Recent genetic analyses of Candida albicans chitin synthases show that short microcrystalline rodlets that compose the bulk of the cell wall are made by Chs3p, while long-chitin microfibrils are made by Chs8p [48].
Deproteinated Giardia cyst walls are much thicker than untreated walls and are no longer brittle and impermeable to small molecules. These results show the thinness, brittleness, and impermeability of the Giardia cyst wall depend upon the presence of CWPs. These qualities of the cyst wall likely also depend upon formation of disulfide bonds, isopeptide bonds, and dephosphorylation of CWPs, as suggested by previous studies of encystation by Giardia [24 to 27].
The demonstration here that native CWP1 and MBP-fusion proteins containing the full length CWP1 (CWP1FL) or the Leu-rich repeat domain of CWP1 (CWP1LRR) bind to deproteinated fibrils of the GalNAc homopolymer strongly suggests CWP1 is a novel lectin that binds to fibrils of the unique GalNAc homopolymer (Figs. 3A to 3C, 4B to 4D, 6B, 6D to 6H, and 7C). There is mass spectrometry evidence that native CWP2 also binds to the deproteinated fibrils of the GalNAc homopolymer (Fig. 3D). Mass spectrometry results failed to make a compelling case that CWP3, which is added late to the cyst wall [20], binds to deproteinated fibrils of the GalNAc homopolymer. In addition, we did not have evidence for binding of Cys-rich Giardia cyst wall proteins (HCNCp and EGFCPs) to deproteinated fibrils of the GalNAc homopolymer [16], [17].
The Cys-rich domain of CWP1 does not appear to contain the lectin activity that binds to the deproteinated fibrils of the GalNAc homopolymer (Fig. 4F). It is not known whether the Cys-rich domains of other Giardia cyst wall proteins (HCNCp and EGFCPs) have lectin activity for the GalNAc homopolymer [16], [17]. In contrast, Cys-rich domains have lectin activities in Entamoeba cyst wall proteins and in plant lectins such as WGA [35], [36], [38], [39], [49]. In addition, Leu-rich repeats present in Toll-like receptors and in cytosolic NOD-like receptors recognize peptides, lipids, or nucleic acids rather than carbohydrates [50], [51].
Using recombinant CWP1FL as a novel probe for fibrils of the GalNAc homopolymer, we showed that synthesis of the GalNAc homopolymer occurs within numerous small vesicles in encysting Giardia (Fig. 5). This result, which is consistent with TEM observations of fibrils within vesicles of encysting Giardia, suggests that the GalNAc homopolymer synthase is not restricted to the plasma membrane, as has been shown for fungal chitin synthases [30], [42]. Using similar methods, we found that Entamoeba also synthesizes chitin in vesicles [39], while single-cell algae also synthesize cellulose within intracellular vesicles [52]. Double-labeling experiments here suggest that the fibrils of the GalNAc homopolymer are not made in ESVs that contain CWP2 and CWP3 and are present early on the surface of encysting Giardia when epitope-tagged CWP2 and CWP3 are for the most part still present in ESVs (Figs. 5D to 5G). These experiments complement the recent demonstration that CWP1 and the major portion of CWP2 are added first to the cyst wall followed by CWP3 [20].
A two-component (protease and glycohydrolase) model for excystation
Our experiments suggest that breaking the cyst wall of Giardia during excystation likely occurs in at least two conceptual steps. First, exogenous serine proteases (e.g. trypsin and chymotrypsin) degrade CWPs that thickens and softens the cyst wall (Figs. 6A, 6C, and 7E) [23]. As shown previously by inhibition studies, endogenous cysteine proteases of Giardia are also involved in excystation [22]. Second, fibrils of the GalNAc homopolymer, which are exposed by proteolysis of CWPs (Figs. 6B, 6D, and 7F), are degraded by an endogenous glycohydrolase. Evidence for the endogenous glycohydrolase of Giardia includes disappearance of large portions of the cyst wall (detected by anti-CWP1 antibodies and by CWP1FL) during excystation (Figs. 6A and 6B) and demonstration that extracts of Giardia cysts are capable of degrading deproteinated fibrils of the GalNAc homopolymer (Figs. 6E to 6H and 7D). The role of stomach acids and protist motility during excystation are not addressed in this model [23].
Summary and unanswered questions
The major discoveries here are the following: 1) In intact cyst walls of Giardia, fibrils of the unique GalNAc homopolymer are curled and form a lattice that is compressed into a narrow plane by bound protein. 2) Leu-rich repeats of CWP1 form a novel lectin domain that is specific for fibrils of the GalNAc homopolymer, which can be isolated by methods used to deproteinate fungal walls [40]. 3) A cyst-specific glycohydrolase is able to degrade deproteinated fibrils of the GalNAc homopolymer.
We understand that our models for Giardia cyst wall structure and mechanism of disruption during excystation are oversimplified and incompletely demonstrated. Follow up studies will include identification of proteins present in oval-shaped aggregates that form linear arrays on fibrils of the GalNAc homopolymer. We plan to determine whether and how fibrils of the GalNAc homopolymer interact with CWPs in intracellular vesicles prior to release onto the surface of encysting Giardia. In addition, the Giardia enzymes that synthesize and degrade the unique GalNAc homopolymer need to be molecularly characterized, and their function tested by knock-in or knock-down methods.
Materials and Methods
Ethics statement
Culture and manipulation of Giardia, including production of cysts in vitro and handling of cysts from gerbils, has been has been approved by the Boston University Institutional Biosafety Committee (BU IBC). Similarly, recombinant expression of Giardia proteins in bacteria has been approved by the BU IBC. Monoclonal anti-CWP1 antibodies and cysts of the H3 strain of Giardia, which had been passaged through gerbils, are catalog items from a commercial vendor (Waterborne Incorporated, New Orleans, LA).
Parasites examined
Trophozoites of the WB strain of Giardia lamblia (the first genome project strain) were grown axenically in TYI-S-33 medium supplemented with 0.1% bovine bile for 48 hrs at 37°C [2]. Trophozoites were encysted by standard methods [5]. Encystation was induced by exchanging trophozoite medium with TYI-S-33 medium supplemented with 10 mg/ml bile salts, pH 7.8. Non-adherent water-resistant cysts were isolated and washed twice with deionized water. Contaminating fecal bacteria were removed from cysts in fecal material by sucrose and percoll density gradient centrifugation.
Isolation and biochemical characterization of the GalNAc homopolymer
Giardia cysts obtained in vitro (∼40 million) were treated with 1 N NaOH for 1 hr at 100°C, insoluble cyst wall material was recovered by centrifugation at 2000 rpm at room temperature for 10 min. This material was then suspended in 0.75 N NaOH and heated to 75°C for 2 hrs. The suspension was then allowed to cool to room temperature and extraction was continued for 16 hrs. The insoluble material was recovered by centrifugation at 2000 rpm at room temperature for 10 min and then washed ten times with deionized water [40].
Proteins associated with the NaOH-treated cyst walls were determined using a micro BCA protein assay kit (Pierce Biotechnology). Alternatively, NaOH-treated cysts were boiled in 1% SDS, and extracted proteins were separated on SDS-PAGE containing 4–20% polyacrylamide. Gels were fixed and stained with silver, or unfixed proteins were transferred to PVDF membranes and probed with anti-CWP1 monoclonal antibody [7]. The latter was detected with anti-mouse secondary antibody conjugated to horse-radish peroxidase and developed with a chemiluminescent substrate. Positive controls for the BCA assay, silver stains, and anti-CWP1 blot were NaOH-treated cysts, which were labeled with recombinant MBP-CWP1 fusion-proteins (see below).
Monosaccharides in the NaOH-treated cyst walls were released with 4 N H2SO4 at 100°C for 4 hrs; H2SO4 was neutralized with saturated Ba(OH)2 solution; and sugars were concentrated using a rotavap. Released monosaccharides were analyzed on a high performance anion exchange chromatography (HPAEC) with a pulsed amperometric detector in a Dionex LC20 instrument using ED40 gold electrode and an analytical Carbopac MAI column (250×4 mm) equilibrated in 100 mM NaOH. The flow rate was 0.4 ml/min with 100 mM to 800 mM sodium hydroxide gradient. GalNAc, GlcNAc, GalN, GlcN, and Glc were used as standards. Alternatively, released monosaccharides were analyzed by GC/MS (next section).
GC-MS analysis of carbohydrates in deproteinated cyst walls
NaOH-extracted Giardia cyst walls were dissolved in 500 µl of 1 M methanolic-HCl at 90°C for 16 h. The re-N-acetylation of the monosaccharides mixture was performed by adding 500 µl of methanol, 10 µl of pyridine and 50 µl of acetic anhydride at room temperature for 15 min. Sugars were trimethylsilylated in 200 µl of N,O-bis-(trimethylsilyl)-acetamide (TMSA) at 90°C for 15 min. The sample was dried down under nitrogen, dissolved in 50 µl of hexane and centrifuged to remove the excess of solid reagents. The hexane supernatant (1/60) was used for the GC-MS analysis.
GC-MS analyses were performed on a Hewlett-Packard 5890 instrument, in the following conditions: ZB-5 capillary column (Phenomenex, 30 m×0.25 mm i.d., flow rate 0.8 ml/min, He as carrier gas). The injection temperature was 250°C. For sugar analyses the oven temperature was increased from 25°C to 90°C in 1 min and held at 90°C for 1 min before increasing to 140°C at a rate of 25°C/min, to 200°C at 5°C/min and finally to 300°C at 10°C/min. Electron ionisation (EI) mass spectra were recorded by continuous quadrupole scanning at 70 eV ionisation energy.
Transmission electron microscopy (TEM) of Giardia cyst walls
Giardia cysts were treated with NaOH, and deproteinated cyst walls were isolated by centrifugation and washed in PBS. Alternatively, Giardia cysts were treated with 200 pulses from a probe sonicator in PBS on ice, and cyst walls were purified by centrifugation through a 60% sucrose cushion, using methods similar to those used to prepare Entamoeba cyst walls [35], [38]. For negative-staining, NaOH-treated cyst walls or cyst walls isolated on sucrose gradients were washed and resuspended in sterile deionized water and applied to carbon-coated, copper grids. The grids were stained with 1% uranyl acetate for 15 sec and visualized on a Phillips CM-12 microscope at 80 KV, 3800 to 17,000 times magnification. Alternatively, grids were stained with 2% Nano-W solution in water at pH 6.8 for 30 sec [44]. Images were recorded on Kodak 50–63 film and digitized on a Nikon 9000 scanner at 2000 dpi.
For conventional transmission microscopy, cyst walls were fixed in 2% paraformaldehyde and 1% gluteraldehyde buffered by 0.1 M sodium cacodylate, pH 7.3, for 12+ hrs at 4°C for conventional TEM. Cyst walls were post-fixed for 12+ hrs at 4°C in 1% osmium tetroxide +/ − 0.5 mg/ml ruthenium red in cacodylate buffer [43]. Water was removed from pellets in graded ethanols and propylene oxide, and organisms were embedded in Epon at the Harvard Medical School Electron Microscopy Facility. Ultrathin sections (∼60–80 nm) were cut on a Reichert Ultracut-S microtome, picked up onto copper grids, stained with 1% ruthenium red and osmium tetroxide and examined in a “Tecnai G2 Spirit BioTWIN” transmission electron microscope. Images were taken with a 2k AMT CCD camera.
Recombinant expression of Giardia cyst wall proteins in transformed bacteria
The full length Giardia CWP1 gene (CWP1FL) minus the 5′ end that encodes the N-terminal signal sequence was PCR amplified using a sense primer (GGAATTCCTCACTTGCCCGGCTACTC) and an anti-sense primer (GCTCTAGAAGGCGGGGTGAGGCAGTA). The N-terminal Leu-rich repeat of CWP1 (CWP1LRR) was amplified using the same sense primer and a new anti-sense primer (GCTCTAGAGTTGAGATAGAGCTCCATAAGGTAGG). The C-terminal Cy-rich region of CWP1 (CWP1CRR) was amplified using a new sense primer (GGAATTCCTCTATCTCAACTGCAACCCTGA) and anti-sense primer for CWP1FL. The three Giardia CWP1 PCR products were cloned into pMAL-p2E vector (New England Biolabs (NEB), Beverley, MA), which makes an IPTG-inducible, periplasm-targeted fusion-protein with MBP at the N-terminus and CWP1 at the C-terminus [41]. Bl21-DE3 cells from Invitrogen were transformed with the pMAL-p2E - constructs, and recombinant proteins were induced with 0.1 mM IPTG. E. coli expressing MBP fusion proteins in the periplasm were suspended in 20% sucrose in PBS for 10 min in ice. After removing sucrose solution, periplasmic proteins were released by hypotonic shock in phosphate-buffer (PB) and 4 mM PMSF on ice. Fusion-proteins were purified by amylose resin (NEB) using 50 mM maltose as an eluent. Enriched MBP-CWP1 fusion-proteins were checked for purity on SDS-PAGE stained with Coomassie brilliant blue and by Western blot using horse-radish peroxidase-tagged anti-MBP antibody from NEB.
Deconvolving microscopy
For labeling the surface of Giardia red, trophozoites and encysting organisms were incubated for 60 min at 4°C in 200 mM carbonate-bicarbonate buffer, pH 9.3, with 20 mg/ml of the amine-reactive probe Alexa Fluor 610-X, succinimidyl ester, bis(triethylammonium salt) 6-isomer (Molecular Probes, Invitrogen) [46]. Alexafluor-labeled trophozoites were washed three times with PBS to remove excess fluorochrome, and parasites were fixed and in 2% paraformaldehyde for 5 min at 4°C and permeabilized with 0.1% Triton X-100 in PB for 1 min at 4°C temp. Alternatively, N-glycans on glycoproteins of fixed and permeabilized Giardia were labeled red using WGA conjugated with TRITC (EY laboratories), using methods described [16]. NaOH-treated cyst walls, which were used without fixation, were also labeled with WGA-TRITC to detect residual glycoproteins.
Recombinant MBP-CWP1 fusion-proteins were labeled green with Alexafluor 488 carboxylic acid, succinimidyl ester (Molecular Probes, Invitrogen) in 200 mM carbonate-bicarbonate buffer, pH 9.3, and unreacted fluorochromes were removed by dialysis versus PBS. Fixed and permeabilized Giardia or NaOH-treated cyst walls were incubated with 0.05 to 0.1 mg/ml of each fusion-protein for 1hr at room temperature in PBS and washed 3 times in PBS. Prior to deconvolving microscopy, nuclei were stained blue with 0.1 mg/ml DAPI.
Mouse anti-CWP1 monoclonal antibodies, which were labeled with fluorescein, were purchased from Waterborne Incorporated [7], [11]. Antibodies were diluted 1 : 200 and incubated with fixed and permeabilized Giardia or NaOH-treated walls for 1 hr at RT in PBS. One percent bovine serum albumin (BSA) in PBS was used as a blocking reagent. Preparations were washed three times in PBS.
Double-labeling experiments were performed with transformed Giardia using vectors designed to express epitope-tagged CWP2 or CWP3 during encystation that were a generous gift of Adrian Hehl [20]. Briefly, these vectors were linearized, and Giardia were transformed by electroporation and selected with neomycin or puromycin. CWP2 had a FLAG-tag at the N-terminus that was detected with an anti-FLAG antibody conjugated to Alexafluor-594. CWP3 had a GFP-tag at the C-terminus that was detected with an anti-GFP antibody conjugated to Alexafluor-594. Recombinant CWPFL conjugated to Alexafluor-488 was used to detect the GalNAc homopolymer. Double-labeling experiments were performed on Giardia trophozoites and organisms encysting for 6 to 12 hrs.
Slides were examined by three-dimensional multiple wavelength fluorescence microscopy using an Olympus IX70 microscope equipped for Deltavision deconvolution (Applied Precision). This system employs restorative as well as deconvolution techniques to provide resolutions up to four times greater than conventional light microscopes [53]. Images were collected at 0.2 mm optical sections for the indicated wavelengths and were subsequently deconvolved using SoftWoRx (Applied Precision). Data were examined as either optical sections or as a projection of the entire stack.
Flow cytometry
Intact gerbil-derived cysts were labeled with Alexafluor-488-conjugated to anti-CWP1 antibody or to MBP-CWP1FL and then examined with an FCCF FACSCaliber flow cytometer using BD Cellquest Pro v5.2 software (Becton Dickinson). NaOH-treated cyst walls (also referred to a deproteinated fibrils of the GalNAc homopolymer were also labeled with anti-CWP1 antibody or MBP-CWP1FL and then examined with the flow cytometer. A third labeling experiment used gerbil-derived cysts that were heat-killed by treatment at 56°C for 20 min and then incubated for in 1 mg/ml chymotrypsin at 37°C for 30 min prior to washing and labeling with anti-CWP antibody or recombinant CWP1FL.
Identification of a GalNAc homopolymer hydrolase activity in extracts of Giardia trophozoites and cysts
Protein extracts from Giardia trophozoites and from Giardia, which had encysted for 24 to 48 hrs, were prepared by lysing Giardia in PBS with 0.1% Triton X -100 and 10 µm E64 to inhibit Cys-proteases. Fibrils of the GalNAc homopolymer, which had been deproteinated with NaOH, were incubated extracts from trophozoites or cysts for 18 hrs at 37°C. Hydrolysis of the GalNAc homopolymer by the protein extracts was determined by three methods. First, residual fibrils of the GalNAc homopolymer were labeled with Alexafluor-conjugated CWP1FL and examined with the deconvolving microscope. Second, the relative amount of Alexafluor-conjugated CWP1FL bound to fibrils of the GalNAc homopolymer treated with each protein extract was compared to that bound to untreated fibrils of the GalNAc homopolymer using a Tecan Spectrafluor plus fluorimeter. Third, release of GalNAc from fibrils of the GalNAc homopolymer treated with the cyst extract into the supernatant was determined by GC-MS essentially as described above for analysis of monosaccharides released from deproteinated fibrils of the GalNAc homopolymer by treatment with strong acid.
Supporting Information
Zdroje
1. AdamRD
2001 Biology of Giardia lamblia. Clin Microbiol Rev 14 447 475
2. MorrisonHG
McArthurAG
GillinFD
AleySB
AdamRD
2007 Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317 1921 1926
3. FranzénO
Jerlström-HultqvistJ
CastroE
SherwoodE
AnkarklevJ
2009 Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species? PLoS Pathog 5 e1000560
4. SteinerTS
ThielmanNM
GuerrantRL
1997 Protozoal agents: what are the dangers for the public water supply? Annu Rev Med 48 329 340
5. LauwaetT
DavidsBJ
ReinerDS
GillinFD
2007 Encystation of Giardia lamblia: a model for other parasites. Curr Opin Microbiol 210 554 559
6. LujanHD
MowattMR
ConradJT
BowersB
NashTE
1995 Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J Biol Chem 270 29307 29313
7. MowattMR
LujanHD
CottenDB
BowersB
YeeJ
1995 Developmentally regulated expression of a Giardia lamblia cyst wall protein gene. Mol Microbiol 15 955 963
8. HehlAB
MartiM
KohlerP
2000 Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol Biol Cell 11 1789 1800
9. SunCH
McCafferyJM
ReinerDS
GillinFD
2003 Mining the Giardia lamblia genome for new cyst wall proteins. J Biol Chem 278 21701 21708
10. MengTC
HetskoML
GillinFD
1996 Inhibition of Giardia lamblia excystation by antibodies against cyst walls and by wheat germ agglutinin. Infect Immun 64 2151 2157
11. BooneJH
WilkinsTD
NashTE
BrandonJE
MaciasEA
1999 TechLab and alexon Giardia enzyme-linked immunosorbent assay kits detect cyst wall protein 1. J Clin Microbiol 37 611 614
12. LeeP
Abdul-WahidA
FaubertGM
2009 Comparison of the local immune response against Giardia lamblia cyst wall protein 2 induced by recombinant Lactococcus lactis and Streptococcus gordonii. Microbes Infect 11 20 28
13. Ortega-BarriaE
WardHD
EvansJE
PereiraME
1990 N-acetyl-D-glucosamine is present in cysts and trophozoites of Giardia lamblia and serves as receptor for wheatgerm agglutinin. Mol Biochem Parasitol 43 151 165
14. SamuelsonJ
BanerjeeS
MagnelliP
CuiJ
KelleherDJ
2005 The diversity of protist and fungal dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc Natl Acad Sci USA 102 1548 1553
15. RatnerDM
CuiJ
SteffenM
MooreLL
RobbinsPW
2008 Changes in the N-glycome, glycoproteins with Asn linked glycans, of Giardia lamblia with differentiation from trophozoites to cysts. Eukaryot Cell 7 1930 1940
16. DavidsBJ
ReinerDS
BirkelandSR
PreheimSP
CiprianoMJ
2006 A new family of giardial cysteine-rich non-VSP protein genes and a novel cyst protein. PLoS ONE 1 e44
17. ChiuPW
HuangYC
PanYJ
WangCH
SunCH
2010 A novel family of cyst proteins with epidermal growth factor repeats in Giardia lamblia. PLoS Negl Trop Dis 45 e677
18. StefanicS
MorfL
KulangaraC
RegosA
SondaS
2009 Neogenesis and maturation of transient Golgi-like cisternae in a simple eukaryote. J Cell Sci 122 2846 2856
19. GottigN
EliasEV
QuirogaR
NoresMJ
SolariAJ
2006 Active and passive mechanisms drive secretory granule biogenesis during differentiation of the intestinal parasite Giardia lamblia. J Biol Chem 281 18156 18166
20. KonradC
SpycherC
HehlAB
2010 Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia. PLoS Pathog 6 e1000835
21. TouzMC
NoresMJ
SlavinI
CarmonaC
ConradJT
2002 The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J Biol Chem 277 8474 8481
22. WardW
AlvaradoL
RawlingsND
EngelJC
FranklinC
1997 A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell 89 437 444
23. BoucherSE
GillinFD
1990 Excystation of in vitro-derived Giardia lamblia cysts. Infect Immun 58 3516 3522
24. ReinerDS
McCafferyJM
GillinFD
2001 Reversible interruption of Giardia lamblia cyst wall protein transport in a novel regulated secretory pathway. Cell Microbiol 3 459 472
25. DavidsBJ
MehtaK
FesusL
McCafferyJM
GillinFD
2004 Dependence of Giardia lamblia encystation on novel transglutaminase activity. Mol Biochem Parasitol 136 173 180
26. SlavinI
SauraA
CarranzaPG
TouzMC
NoresMJ
2002 Dephosphorylation of cyst wall proteins by a secreted lysosomal acid phosphatase is essential for excystation of Giardia lamblia. Mol Biochem Parasitol 122 95 98
27. LauwaetT
DavidsBJ
Torres-EscobarA
BirkelandSR
CiprianoMJ
2007 Protein phosphatase 2A plays a crucial role in Giardia lamblia differentiation. Mol Biochem Parasitol 152 80 89
28. GerwigGJ
van KuikJA
LeeflangBR
KamerlingJP
VliegenthartJF
2002 The Giardia intestinalis filamentous cyst wall contains a novel beta(1-3)-N-acetyl-D-galactosamine polymer: a structural and conformational study. Glycobiology 12 499 505
29. ErlandsenSL
MacechkoPT
van KeulenH
JarrollEL
1996 Formation of the Giardia cyst wall: studies on extracellular assembly using immunogold labeling and high resolution field emission SEM. J Eukaryot Microbiol 43 416 429
30. Chávez-MunguíaB
Cedillo-RiveraR
Martínez-PalomoA
2004 The ultrastructure of the cyst wall of Giardia lamblia. J Eukaryot Microbiol 51 220 226
31. KarrCD
JarrollEL
2004 Cyst wall synthase: N-acetylgalactosaminyltransferase activity is induced to form the novel N-acetylgalactosamine polysaccharide in the Giardia cyst wall. Microbiology 150 1237 1243
32. WestCM
2003 Comparative analysis of spore coat formation, structure, and function in Dictyostelium. Int Rev Cytol 222 237 293
33. LesageG
BusseyH
2006 Cell wall assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 70 317 343
34. JametE
AlbenneC
BoudartG
IrshadM
CanutH
2008 Recent advances in plant cell wall proteomics. Proteomics 8 893 908
35. FrisardiM
GhoshSK
FieldJ
Van DellenK
RogersR
2000 The most abundant glycoprotein of amebic cyst walls (Jacob) is a lectin with five Cys-rich, chitin-binding domains. Infect Immun 68 4217 4224
36. Van DellenK
GhoshSK
RobbinsPW
LoftusB
SamuelsonJ
2002 Entamoeba histolytica lectins contain unique 6-Cys or 8-Cys chitin-binding domains. Infect Immun 70 3259 3263
37. de la VegaH
SpechtCA
SeminoCE
RobbinsPW
EichingerD
1997 Cloning and expression of chitinases of Entamoebae. Mol Biochem Parasitol 85 139 147
38. Van DellenKL
ChatterjeeA
RatnerDM
MagnelliPE
CipolloJF
2006 Unique posttranslational modifications of chitin-binding lectins of Entamoeba invadens cyst walls. Eukaryot Cell 5 836 848
39. ChatterjeeA
GhoshSK
JangK
BullittE
MooreL
2009 Evidence for a “wattle and daub” model of the cyst wall of entamoeba. PLoS Pathog 5 e1000498
40. MannersDJ
MeyerMT
1977 The molecular structures of some glucans from the cell walls of Schizosaccharomyces pombe. Carbohydr Res 57 189 203
41. NallamsettyS
WaughDS
2007 A generic protocol for the expression and purification of recombinant proteins in Escherichia coli using a combinatorial His6-maltose binding protein fusion tag. Nat Protoc 2 383 391
42. CabibE
RohDH
SchmidtM
CrottiLB
VarmaA
2001 The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J Biol Chem 276 19679 19682
43. NanduriJ
WilliamsS
AjiT
FlaniganTP
1999 Characterization of an immunogenic glycocalyx on the surfaces of Cryptosporidium parvum oocysts and sporozoites. Infect Immun 67 2022 2024
44. OliverRM
1973 Negative stain electron microscopy of protein macromolecules. Methods Enzymol 27 616 762
45. ChatterjeeA
BanerjeeS
SteffenM
O'ConnorRM
WardHD
2010 Evidence for mucin-like glycoproteins that tether sporozoites of Cryptosporidium parvum to the inner surface of the oocyst wall. Eukaryot Cell 9 84 96
46. GhoshS
FrisardiM
RogersR
SamuelsonJ
2001 How Giardia swim and divide. Infect Immun 69 7866 7872
47. DingSY
HimmelME
2006 The maize primary cell wall microfibril: a new model derived from direct visualization. J Agric Food Chem 54 597 606
48. LenardonMD
WhittonRK
MunroCA
MarshallD
GowNA
2007 Individual chitin synthase enzymes synthesize microfibrils of differing structure at specific locations in the Candida albicans cell wall. Mol Microbiol 66 1164 1173
49. WrightHT
SandrasegaramG
WrightCS
1991 Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin. J Mol Evol 33 283 294
50. JinMS
LeeJO
2008 Structures of the toll-like receptor family and its ligand complexes. Immunity 29 182 191
51. ShawMH
ReimerT
KimYG
NuñezG
2008 NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol 20 377 382
52. CabibE
ShematuckEM
1981 Structural polysaccharides of plants and fungi: comparative and morphogenetic aspects.
GinsburgV
RobbinsP
Biology of Carbohydrates vol1 New York John Wiley & Sons 51 90
53. CarringtonWA
LynchRM
MooreED
IsenbergG
FogartyKE
1995 Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science 268 1483 1487
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



