-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
UNC93B1 Mediates Host Resistance to Infection with
UNC93B1 associates with Toll-Like Receptor (TLR) 3, TLR7 and TLR9, mediating their translocation from the endoplasmic reticulum to the endolysosome, hence allowing proper activation by nucleic acid ligands. We found that the triple deficient ‘3d’ mice, which lack functional UNC93B1, are hyper-susceptible to infection with Toxoplasma gondii. We established that while mounting a normal systemic pro-inflammatory response, i.e. producing abundant MCP-1, IL-6, TNFα and IFNγ, the 3d mice were unable to control parasite replication. Nevertheless, infection of reciprocal bone marrow chimeras between wild-type and 3d mice with T. gondii demonstrated a primary role of hemopoietic cell lineages in the enhanced susceptibility of UNC93B1 mutant mice. The protective role mediated by UNC93B1 to T. gondii infection was associated with impaired IL-12 responses and delayed IFNγ by spleen cells. Notably, in macrophages infected with T. gondii, UNC93B1 accumulates on the parasitophorous vacuole. Furthermore, upon in vitro infection the rate of tachyzoite replication was enhanced in non-activated macrophages carrying mutant UNC93B1 as compared to wild type gene. Strikingly, the role of UNC93B1 on intracellular parasite growth appears to be independent of TLR function. Altogether, our results reveal a critical role for UNC93B1 on induction of IL-12/IFNγ production as well as autonomous control of Toxoplasma replication by macrophages.
Published in the journal: UNC93B1 Mediates Host Resistance to Infection with. PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001071
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001071Summary
UNC93B1 associates with Toll-Like Receptor (TLR) 3, TLR7 and TLR9, mediating their translocation from the endoplasmic reticulum to the endolysosome, hence allowing proper activation by nucleic acid ligands. We found that the triple deficient ‘3d’ mice, which lack functional UNC93B1, are hyper-susceptible to infection with Toxoplasma gondii. We established that while mounting a normal systemic pro-inflammatory response, i.e. producing abundant MCP-1, IL-6, TNFα and IFNγ, the 3d mice were unable to control parasite replication. Nevertheless, infection of reciprocal bone marrow chimeras between wild-type and 3d mice with T. gondii demonstrated a primary role of hemopoietic cell lineages in the enhanced susceptibility of UNC93B1 mutant mice. The protective role mediated by UNC93B1 to T. gondii infection was associated with impaired IL-12 responses and delayed IFNγ by spleen cells. Notably, in macrophages infected with T. gondii, UNC93B1 accumulates on the parasitophorous vacuole. Furthermore, upon in vitro infection the rate of tachyzoite replication was enhanced in non-activated macrophages carrying mutant UNC93B1 as compared to wild type gene. Strikingly, the role of UNC93B1 on intracellular parasite growth appears to be independent of TLR function. Altogether, our results reveal a critical role for UNC93B1 on induction of IL-12/IFNγ production as well as autonomous control of Toxoplasma replication by macrophages.
Introduction
Toxoplasma gondii is a widespread obligate intracellular protozoan parasite, which establishes itself in the brain and muscle tissues, persisting for life in humans and other vertebrate hosts [1]. One of the most distinctive aspects of T. gondii life cycle is the establishment of an often benign chronic infection, which is dependent on the parasite's ability to elicit a strong and persistent cell-mediated immunity [1]. Severe forms of toxoplasmosis are often seen in humans with an immature or suppressed immune system. In particular, cytokines that activate macrophage effector functions, such as IFNγ and TNFα, are critical in mediating host resistance to T. gondii infection [2].
The mammalian Toll-like receptors (TLRs) sense conserved molecules from all classes of microorganisms [3], including those from protozoan parasites [4]. Studies employing MyD88−/− mice, which are deficient in the function of most TLRs (except for TLR3), suggest that TLRs are critical in many aspects of host:protozoan parasite interaction, including the initiation of the pro-inflammatory cytokine response and the expression of co-stimulatory molecules [5], [6], [7]. The initial activation of the innate immune system leads to the immediate activation of anti-microbial effector mechanisms. In addition, innate immune activation gives way, over time, to the development of Th1 lymphocytes and host resistance to protozoa, including T. gondii [4]. TLR2, TLR4, TLR9, and TLR11 have been shown to be important cognate innate immune receptors involved in the recognition of T. gondii derived components, such as glycosilphosphatidylinositol (GPI) anchors [8], CpG DNA [9] and profilin [10], [11]. However, the deficiency of each of these TLRs, and even the loss of two TLRs, as is the case with TLR2/TLR4 double knockout mice, leads to a relatively minor phenotype after T. gondii infection, as compared to the results obtained with infected MyD88−/− mice [6].
The “endosomal TLRs”, TLR3, TLR7, TLR8 and TLR9, recognize microbial RNA and DNA [12], [13], [14], [15]. In addition to its well-described function in the recognition of CpG motifs, TLR9 has been shown to play an important role in the recognition of parasite DNA and host resistance to infection by several different protozoan parasites [16], [17], [18]. However, the combined role of nucleotide-sensing TLRs in host resistance to T. gondii has not been explored. Tabeta and colleagues [19] identified a mutant mouse line by forward genetic screening that they named “3d”, so called because of its deficiency in response to TLR3, TLR7 and TLR9 ligands (mouse TLR8 do not respond to single stranded RNA). The 3d mouse was shown to have altered UNC93B1 function, an endoplasmic reticulum protein with distant homology to an ion transporter in worms. UNC93B1 is now known to be essential for signaling through mouse TLR3, TLR7, and TLR9, and the consequent production of pro-inflammatory cytokines [19], [20]. The combined deficiency of nucleic acid-sensing TLRs results in altered host resistance to microbial infections [3], [19], [20]. Specifically, UNC93B1 associates and mediates the translocation of the nucleotide-sensing TLRs from the endoplasmic reticulum (ER) to the endolysosomal compartment, allowing their proper activation by microbial RNA and DNA [21], [22].
Here, we show that although mounting a normal systemic pro-inflammatory response, the 3d mice are extremely susceptible to infection with T. gondii. Nevertheless, we provide evidence of a critical role of UNC93B1 in mediating IL-12 as well as early IFNγ production during acute infection with T. gondii. Its well known that active host cell invasion by T. gondii leads to formation of a high pH-parasitophorous vacuole [23], parasite replication and parasitism, whereas passive internalization of the parasite by phagocytosis results in parasite elimination in the lysosomes [24], [25]. We also found that in macrophages infected with T. gondii, UNC93B1 translocates to the parasitophorous vacuole, rather than to the predicted phagolysosomes. Finally, our results demonstrate that the lack of functional UNC93B1 results in enhanced tachyzoite replication in macrophages. Altogether, our experiments reveal a role for UNC93B1 on IL-12 production induced by Toxoplasma infection, as well as an unprecedented TLR-independent role for UNC93B1 on host cell control of T. gondii replication, which combined are of central importance for the in vivo resistance to infection with this intracellular protozoan parasite.
Results
Extreme susceptibility of 3d mice to acute infection with T. gondii is associated with enhanced parasite replication and unimpaired systemic production of pro-inflammatory cytokines
We found that the 3d mice are highly susceptible to infection with T. gondii, suggesting the possibility that the combined action of TLR3, TLR7 and TLR9 is critical for host resistance to infection with T. gondii. However, we used as a control the double MyD88/TRIF-null mice, which are deficient in all TLR responses. While very susceptible, we found that upon infection with T. gondii the double MyD88/TRIF deficient mice were somewhat more resistant than the 3d mice (Fig. 1A). While this difference was not dramatic, it was consistent, suggesting that UNC93B1 may also mediate host resistance to this parasitic infection by a novel mechanism, independent of TLR function.
Fig. 1. 3d mice are highly susceptible to infection with T. gondii and succumb due to excessive parasite burden. 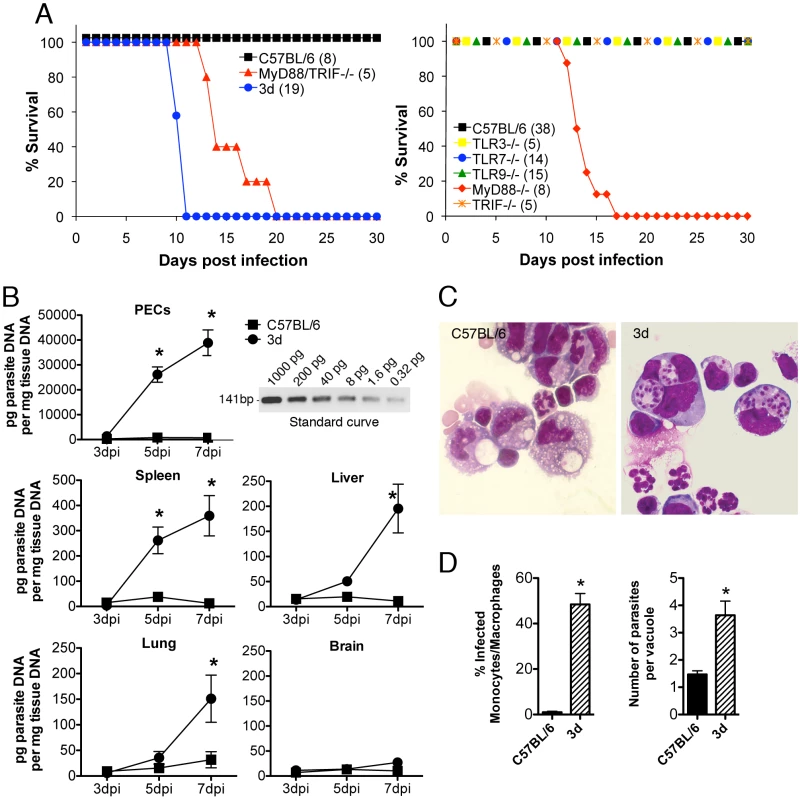
(A) Mortality after intraperitoneal injection of 25 cysts of the ME-49 strain of T. gondii. Data are expressed as percentage of cumulative survival during the experiment. The total numbers of tested animals in each group are indicated within parentheses. p<0.0001 when comparing WT versus 3d mice, and p = 0.0004 when comparing MyD88KO or MyD88/TRIFdKO versus 3d mice. (B) Quantitative real-time PCR analysis was performed on the indicated tissues collected from animals infected for 3, 5 or 7 days with T. gondii cysts. Relative quantification was performed using standard curve analysis of purified parasite DNA. Inset shows an agarose gel electrophoresis of the RT-PCR amplification product obtained using different amounts of purified parasite DNA as template. Results were expressed as pg of parasite DNA per mg of total tissue DNA. Asterisk indicate that p<0.05 when comparing WT and 3d mice (error bars, s.e.m.). (C) Photomicrographs of peritoneal exudates stained with Giemsa, collected from C57BL/6 (top panel) or 3d (bottom panel) mice at 8 days after infection with T. gondii. (D) Percentage of infected monocyte/macrophage-like cells as well as number of parasites per vacuoles present in the peritoneal exudate cells showed in figure 1C. Asterisk indicate that p<0.05 when comparing WT and 3d mice (error bars, s.e.m.). As measured by real-time PCR, enhanced parasite replication was observed in different peripheral organs (i.e. spleen, liver, and lungs) (Fig. 1B), despite of unimpaired systemic IFNγ and TNFα production from 3d mice (Figs. 2A and 2B). This is remarkable in view of the long established role for these cytokines in triggering macrophage effector functions and mediating host resistance to T. gondii. In agreement with the real-time PCR data, analysis of the cells collected from the peritoneal cavity of infected animals showed significant higher numbers of infected cells in 3d animals (Figs. 1C and 1D). Phenotypic analysis revealed that 55–75% of the cells recruited to the peritoneal cavity of 3d animals expressed the surface marker CD11b. Of these, 75% were neutrophils (CD11b+, Ly6G+, F4/80−) and the remaining 25% were inflammatory monocytes (CD11b+, Ly6Chigh, Ly6G−, F4/80low). Although infected neutrophils were observed, they rarely contained more than two parasites, whereas four or more tachyzoites were typically observed in monocyte/machophage-like cells. Thus, enhanced susceptibility to T. gondii infection was associated with uncontrolled parasite replication within monocyte/macrophage-like cells in peritoneal cavity of 3d mice.
Fig. 2. Unimpaired production of pro-inflammatory cytokines in 3d mice infected with T. gondii. 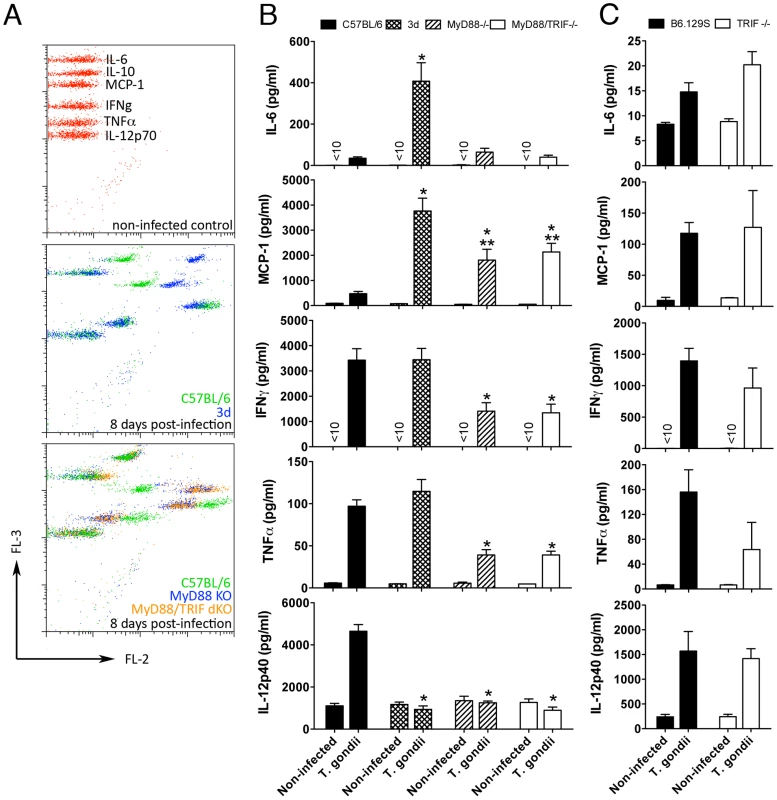
(A–C) Levels of IL-6, MCP-1, IFNγ, and TNFα measured in sera of mice at 0 and 8 days after infection, employing the BD Cytometric Bead Assay (CBA) Mouse Inflammation Kit. IL-12p40 levels in sera of mice were measured by ELISA. A panel with results from representative individual wild-type, 3d, MyD88-deficient and MyD88/TRIF double deficient animals is shown in A. One asterisk indicates that p<0.05 when comparing results from 3d or MyD88-deficient mice to WT animals infected with T. gondii. Two asterisks indicate p<0.05 when comparing the results from 3d mice with MyD88-deficient animals infected with T. gondii. Data are representative of experiments done three (A–B) or two (C) times using at least 4 animals per group (error bars, s.e.m.). In order to further investigate the role of nucleotide-sensing TLRs in resistance to T. gondii, we infected the TLR3−/−, TLR7−/− and TLR9−/− mice, as well as the single knockout mice deficient in each of the two main adaptors required for TLR function, TRIF and MyD88. Our results showed that except for the MyD88−/− mice, which were very susceptible to infection, all of the other mice lineages had a similar survival curve and cyst numbers, in comparison to wild-type mice (Fig. 1A and data not shown).
We anticipated that the lack of endosomal TLR function would result in impaired cytokine production during infection. However, to our surprise, we found that upon infection with T. gondii, except for impaired IL-12p40 production, 3d mice showed high levels of IL-6, MCP-1, IFNγ, and TNFα in their sera (Fig. 2, A–B). Similarly, splenocytes from 3d mice infected with T. gondii demonstrated unimpaired ex-vivo cytokine production (data not shown). While infected MyD88−/− and MyD88/TRIF double deficient mice had decreased serum levels and ex-vivo production of several cytokines (Fig. 2B and data not shown), unimpaired cytokine production of IL-12p40, IFNγ, TNFα, IL-6 and MCP-1 were confirmed in the sera from TRIF−/− (Fig. 2C), TLR3−/−, TLR7−/−, and TLR9−/− mice (Fig. S1) infected with T. gondii. Together, these initial results suggest that the extreme susceptibility of the 3d mouse to T. gondii infection is due to an additional function of UNC93B1 that is not related to the regulation of endosomal TLRs.
Enhanced susceptibility of 3d mice to T. gondii infection is mediated by cells from hemopoietic lineage
T. gondii is a promiscuous parasite that infect any nucleated host cells of both hemopoietic and non-hemopoietic origin. Thus, its replication could be controlled by metabolites secreted by activated macrophages or, alternatively, directly by cytokine-induced microbicidal mechanisms triggered within infected non-phagocytic cells. To distinguish between these two basic mechanisms of cell-mediated immunity, reciprocal bone marrow chimeras were constructed between wild-type and 3d mice and their survival assessed following challenge with T. gondii. The reverse chimeras were generated employing wild type (B6.SJL) and 3d mice, which hemopoietic cells express CD45.1 and CD45.2 respectively (Fig. S2A). Notably, transplanted mice, which possess hemopoietic cells from 3d mice, became non-responsive to any of the TLR7 and TLR9 agonists, but sustained cytokine response (TNFα and RANTES) to LPS and Concanavalin A (Fig. S2B). Finally, infectious challenge of reciprocal chimeras demonstrated that expression of wild type, functional UNC93B1, in the hemopoietic, but not in the non-hemopoietic compartment was necessary for host resistance to infection with T. gondii (Fig. 3). These findings are consistent with data indicating the primary expression of UNC93B1 in murine cells from myeloid origin (http://biogps.gnf.org/#goto=genereport&id=81622).
Fig. 3. Cells from hemopoietic compartment bearing the UNC93B1 mutation are responsible for enhanced susceptibility of 3d mice to T. gondii infection. 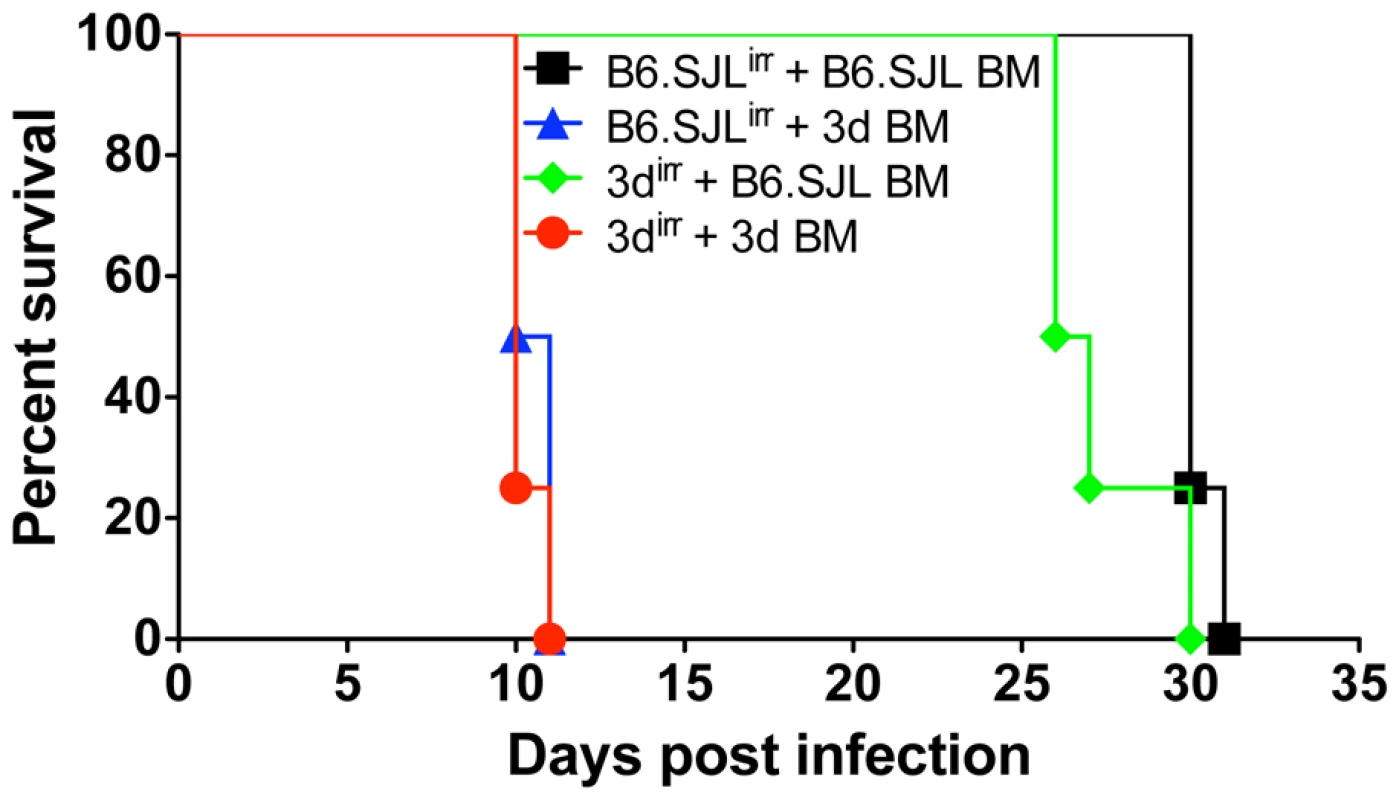
B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) and 3d (CD45.2+) mice were used as partners for chimera construction. 6–9 weeks after reconstitution, chimeric mice were infected intraperitoneally with 25 cysts of T. gondii, and their survival monitored daily. Data presented are representative of experiments done twice with at least 4 mice per group. Partial impairment on activation of antigen presenting cells and T lymphocytes from 3d mice
Based on the results obtained with the reverse chimeras, we decided to focus our attention to evaluate the function of lymphoid/myeloid cells from UN93B1 mutant mice. Because UNC93B1 has also been suggested to be involved on antigen presentation and T cell responses [19], we evaluated the expansion and expression of activation markers on antigen presenting cells (i.e., CD11b+ and CD11c+), as well as CD4+ T and CD8+ T cells from mice infected with T. gondii. CD11b+ as well as CD11c+ cells from 3d mice expressed significant amounts of activation markers, e.g. MHC class I and II, CD40, CD80 and CD86, which were intermediary between cells from wild type and MyD88−/− mice infected with T. gondii (Fig. 4). In addition, spleens of 3d mice infected with T. gondii contained 20–40% less CD4+ and CD8+ T cells expressing CD25, CD69 and CD154 (Fig. 5A). While these differences in wild-type vs. infected 3d mice were statistically significant, the impairment in activation of cells was far more pronounced in MyD88−/− mice (Fig. 4 and Fig. 5A), whose splenocytes showed no signs of expansion after T. gondii infection (data not shown). The percentage of CD4+T as well as CD8+T lymphocytes producing IFNγ was similar when comparing wild type and 3d mice (Fig. 5B), whereas the total number of IFNγ -producing T cells was significantly smaller in infected 3d as compared to wild type mice (Fig. 5C).
Fig. 4. Antigen presenting cells from infected 3d mice express significant amounts of activation markers. 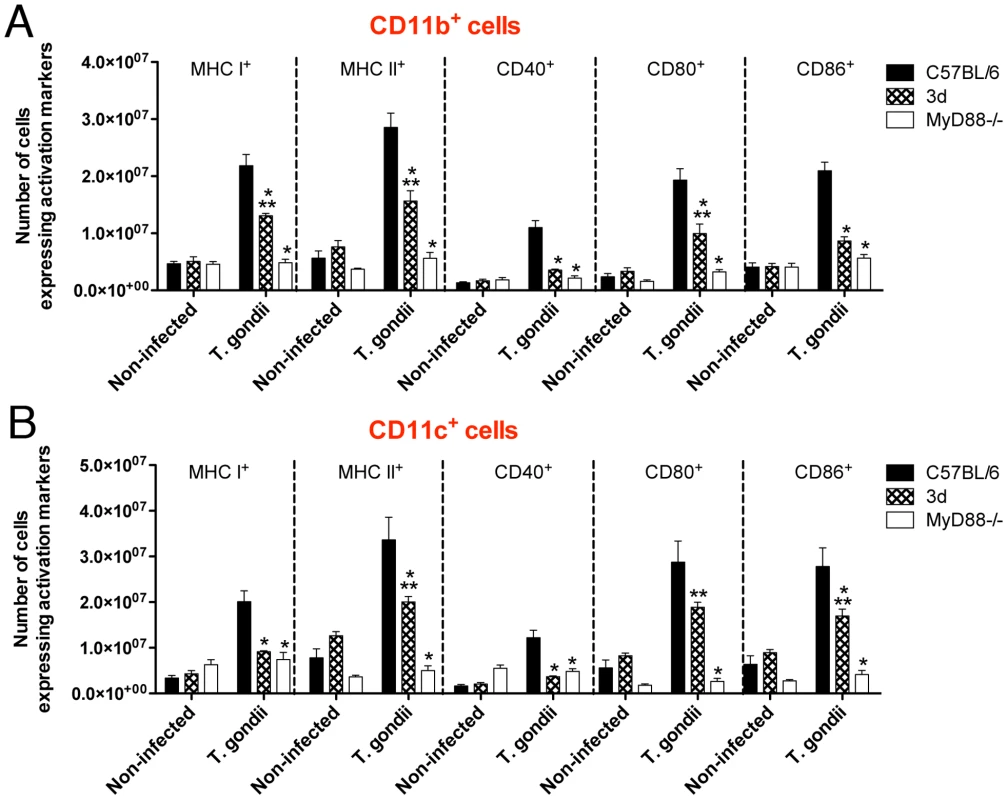
(A–B) Flow cytometry analysis of splenocytes isolated from non-infected animals or 8 days after infection with T. gondii. Cells were stained with fluorescent antibodies anti-CD11b, anti-CD11c, anti-MHC I, anti-MHC II, anti-CD40, anti-CD80 and anti-CD86. One asterisk indicate that p<0.05 when comparing results from 3d or MyD88-deficient mice to WT animals infected with T. gondii. Two asterisks indicate p<0.05 when comparing the results from 3d mice with MyD88-deficient animals infected with T. gondii. Data are representative of experiments done two times, using at least four mice per group (error bars, s.e.m.). Fig. 5. Upon infection with T. gondii, 3d mice display a significant number of T cells expressing activation markers in the surface. 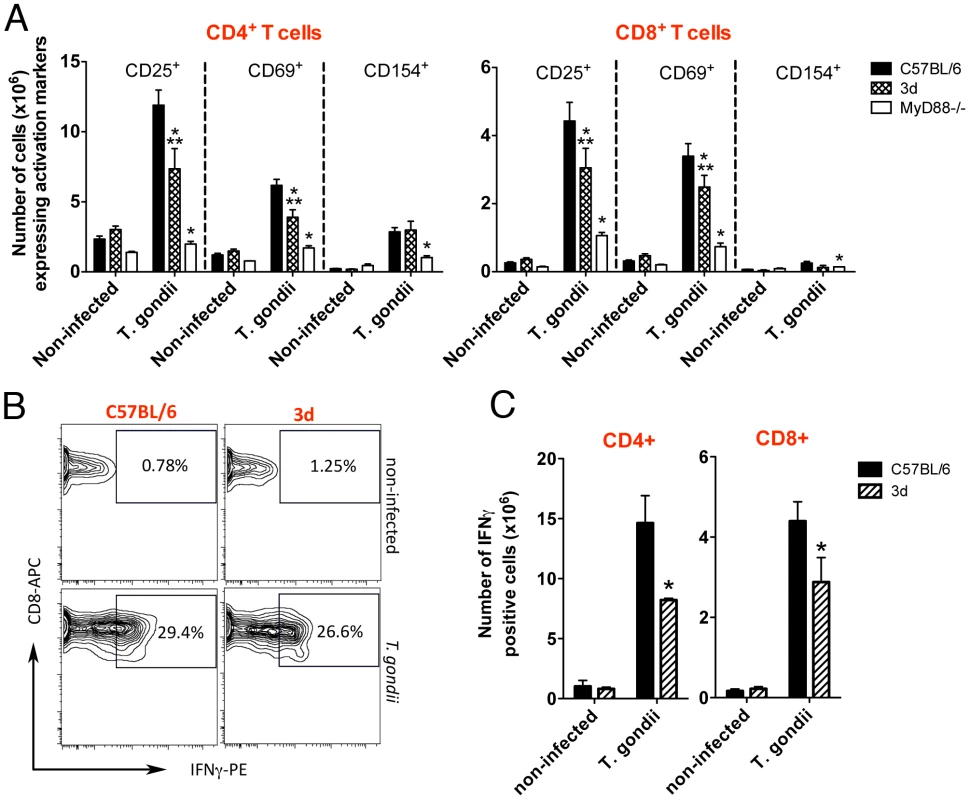
(A) Flow cytometry analysis of splenocytes isolated from non-infected animals or at 8 days after infection with T. gondii. Cells were stained with fluorescent antibodies anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-CD69 and anti-CD154. (B–C) IFNγ-producing CD4+ and CD8+-T cells present in splenocytes of C57BL/6 and 3d mice non-infected or at 8 days post-infection, as measured by flow cytometry. A panel with results for IFNγ -positive CD8+-T cells from representative individual wild-type and 3d mice is shown in B. One asterisk indicates that p<0.05 when comparing results from 3d or MyD88-deficient mice to WT animals infected with T. gondii. Two asterisks indicate that p<0.05 when comparing the results from 3d mice with MyD88-deficient animals infected with T. gondii. Data are representative of experiments done two times, using four mice per group (error bars, s.e.m.). Normal IFNγ responsiveness in 3d mice infected with T. gondii
Consistent with the low serum levels of IL-12 (Fig. 2) and partial impairment on activation of antigen presenting cells (CD11b+ or CD11c+ cells) (Fig. 4), we observed a largely impaired IL-12 production by spleen cells from infected 3d mice (Fig. 6A, top panel). Production of IL-12 was also severely impaired at the infection site (Fig. 6B, top panel). Importantly, bone-marrow derived macrophages from 3d animals exposed to T. gondii tachyzoites in vitro also produce 50% less IL-12 than WT cells (Fig. 6C). Consistent with the delayed production of IL-12, we also observed a late IFNγ response by spleen or peritoneal exudate cells from 3d mice, which was lower on days three and five, but not on days seven or eight post-infection (Figs. 6A and 6B, bottom panels).
Fig. 6. UNC93B1 mutation affects IL-12 and early IFNγ production. 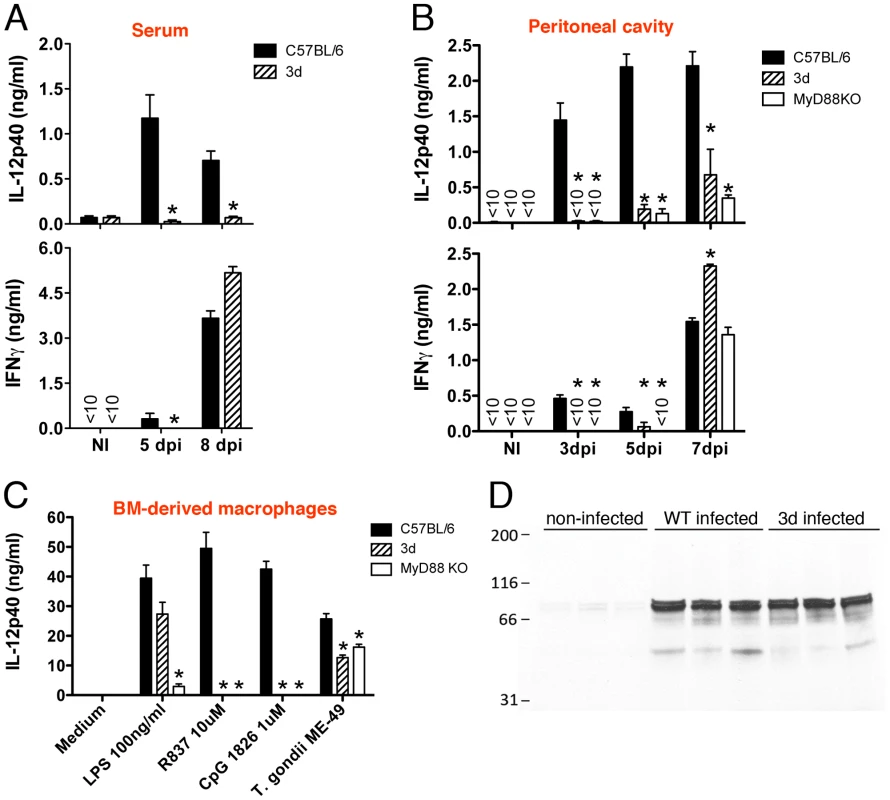
(A) Levels of IL-12p40 and IFNγ produced by splenocytes collected from non-infected controls, as well as mice at 5 and 8 days after infection with T. gondii. Spleen cells were cultured for 24 h in absence of exogenous stimuli. (B) Levels of cytokines present in the peritoneal cavity exudate of non-infected controls, as well as mice at 3, 5 or 7 days after intraperitoneal infection with T. gondii cysts. (C) Wild-type, 3d and MyD88 knockout bone marrow-derived macrophages were stimulated overnight with various TLR agonists or infected with T. gondii (MOI 5∶1), and cytokine levels in supernatants quantified by ELISA. (D) Immunoblot analysis of STAT-1 phosphorylation in splenocytes collected from animals either non-infected or at 8 days after infection. Asterisk indicates that p<0.05 when comparing results from 3d or MyD88−/− to WT mice/cells. Data are representative of three (A–C) or two (D) experiments yielding similar results (error bars, s.e.m.). IFNγ is thought to be the most critical cytokine in controlling T. gondii replication [2], and mediates the IL-12 role on host resistance to T. gondii [26], [27]. Despite the fact that MyD88 knockout and MyD88/TRIF double deficient animals displayed a similar impairment on IL-12 production as the 3d mice (Figs. 2B and 6B–C), and an even more dramatic defect on IFNγ production (Fig. 2B, and 6B–C), the 3d mice consistently showed a more pronounced susceptibility to T. gondii infection (Fig. 1A). Therefore, we believe that the delay of IFNγ production could not be solely responsible for the profound susceptibility phenotype observed on 3d mice. Thus, we sought to determine if cells from 3d mice were properly responding to IFNγ. We observed normal STAT1 phosphorylation, an essential component for IFNγ signaling, in 3d mice infected with T. gondii (Fig. 6D). Furthermore, 3d macrophages responded normally to IFNγ by producing high levels of TNFα, IL-12, and IL-6 when stimulated in vitro in combination with LPS (Fig. S3). Similarly, activation with IFNγ resulted in the enhanced expression of MHC I, MHC II and CD40 in macrophages from 3d mice (Fig. S3). Thus, we found no evidence that the response to IFNγ is affected in mice lacking functional UNC93B1.
UNC93B1 accumulates around the parasitophorous vacuole in host cells infected with T. gondii
Since we found large amounts of parasite within the peritoneal cavity of 3d mice, despite of the high levels of IFNγ, we decided to investigate the ability of macrophages expressing UNC93B1 to control tachyzoite growth in vitro. T. gondii can actively invade host cells or it can be actively internalized by professional phagocytic cells. The fate of the parasite inside the host cell depends on the way the parasite is internalized [24], [28]. Active invasion normally results in generation of a unique organelle known as the parasitophorous vacuole, which is incompetent to fuse with lysosomes. The parasitophorous vacuole consequently has a high pH and is not stained by LysoTracker [23]. In contrast to active invasion by the parasite, phagocytosis directs the parasite to the phagolysosome, which has a low pH, and leads to elimination of the parasite.
We next evaluated the association of UNC93B1 with T. gondii parasites in infected host cells. We began by generating immortalized macrophage cell lines from the UNC93B1 mouse. These lines were then genetically engineered to express either YFP-tagged wild-type UNC93B1 or YFP-tagged UNC93B1H412R (the non-functional mutant expressed by the 3d mouse). Both cell lines expressed high levels of UNC93B1 (Fig. 7A). Macrophage cell lines expressing the wild-type form, but not the mutated UNC93B1H412R, recovered cytokine responses to agonists for TLR9 and TLR7, respectively (Fig. 7B). In non-activated cells, UNC93B1 was observed as a resident ER protein, and did not co-localize to LysoTracker positive acidic compartments (Fig. S4). Confocal microscopy revealed that after infection, there was an enrichment of UNC93B1 around the internalized parasites in cell lines that expressed the wild-type, but not the mutated/non-functional protein (Fig. 7C).
Fig. 7. UNC93B1 accumulates around T. gondii parasitophorous vacuole. 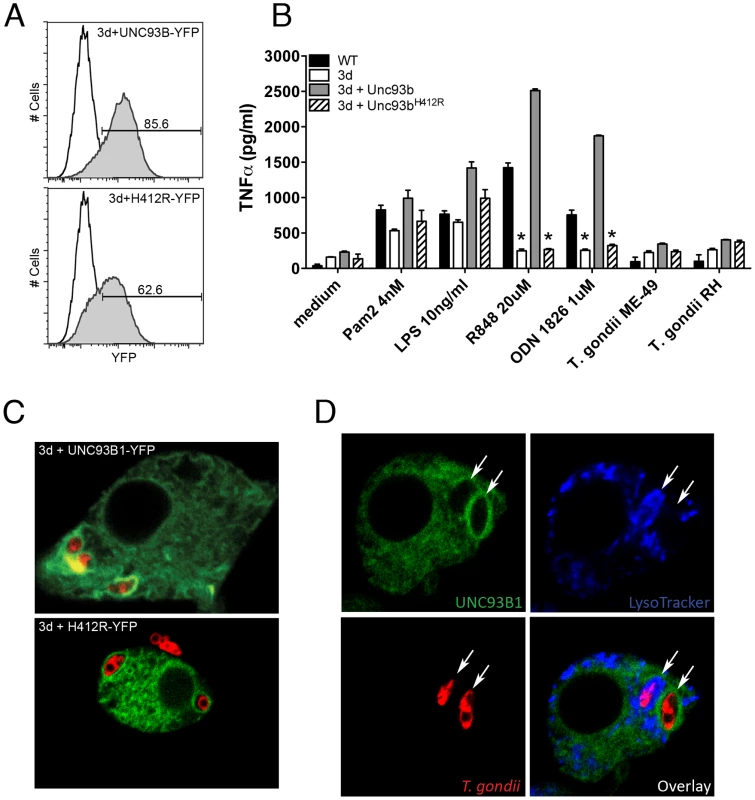
(A) Flow cytometry analysis of immortalized macrophages from 3d mice transduced with YFP-tagged wilde-type or mutant UNC93B1. Black line and shaded histograms show the results from non-transduced controls and cells transduced with UNC93B1-YFP, respectively. (B) Wild-type, 3d immortalized macrophages as well as 3d macrophages stably expressing wild-type or mutated (H421R) form of UNC93B1 were stimulated overnight with various TLR agonists or infected with T. gondii (MOI 5∶1). TNFα production was assessed by ELISA in cell culture supernatants. Asterisk indicates that p<0.05 when comparing results to WT cells. (C–D) Confocal microscopy of immortalized macrophages from 3d mice stably expressing wild type or mutant UNC93B1-YFP and infected with CMTPX-stained T. gondii. Live cells were imaged 2 h after infection. Acidic compartments were stained with LysoTracker Blue. 92% of tachyzoites present in LysoTracker negative compartments were surrounded by a membrane enriched with YFP-tagged UNC93B1. Arrows indicate internalized parasites. Data are from one representative experiment of two (A–B) or four (C–D). The results presented in Fig. 7D show a macrophage cell containing two intracellular parasites. One of these parasites was seen in the acidic LysoTracker positive phagolysosome, and was not surrounded by UNC93B1. Conversely, the parasite found in the parasitophorous vacuole was LysoTracker negative and was surrounded by an intense green ring, indicating a high concentration of UNC93B1-YFP around the parasitophorous vacuole, but not in the predicted phagolysosomes [22]. Immunofluorescence analysis of the parasitophorous vacuoles showed that 92% of internalized tachyzoites present in LysoTracker negative compartments, also positive for the parasite-specific protein GRA7 [29], were surrounded by a membrane enriched with UNC93B1. Such enrichment was never observed in acidic organelles containing phagocytosed parasites.
UNC93B1 promotes host cell resistance to infection with T. gondii parasites
We also tested the ability of bone marrow immortalized macrophages derived from wild type and 3d mice to control parasite replication in vitro. Our results show that upon activation IFNγ (10 or 100 U/ml) macrophages from 3d mice were as efficient as the ones derived from wild type mice to control tachyzoite replication as detected by 3H-uracil uptake (Fig. 8A). Notably, the levels of reactive nitrogen intermediates release as measured by nitrite levels (Fig. S5A), induction of Irga6, Irgm1 and Irgm3 (Fig. S5B), and translocation of Irga6 and Igrb6 to the parasitophorous vacuole (Fig. S5C), all involved on tachyzoite control by IFNγ activated macrophages, were normal in cells from 3d mice. Nevertheless, we consistently observed and enhanced replication of tachyzoites in non-activated macrophages from 3d mice as compared to the same cells derived from wild type mice. Thus, we further explore the possibility that non-activated macrophages from 3d mice are more permissive to tachyzoite growth.
Fig. 8. UNC93B1 mediates host cell resistance to infection with T. gondii through an IFNγ–independent mechanism. 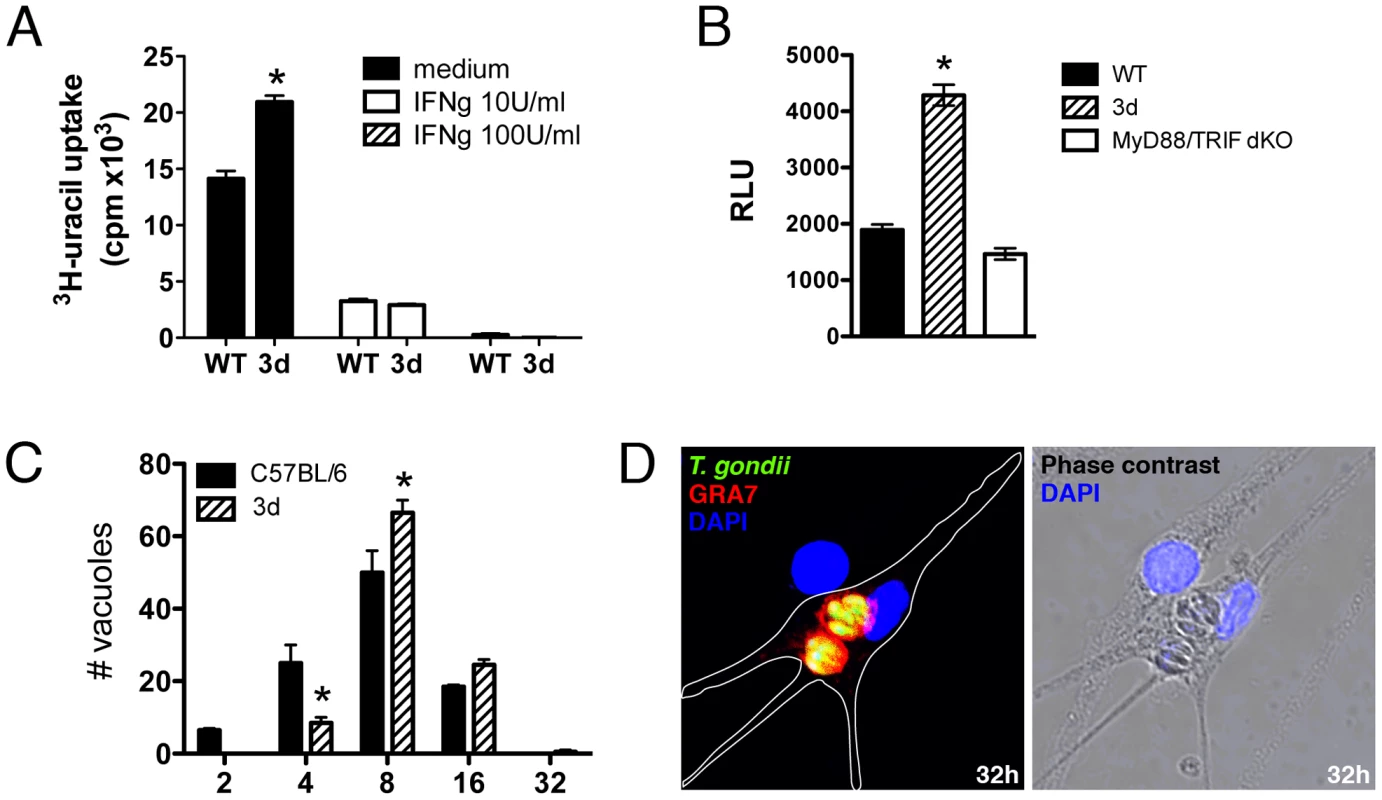
(A) Immortalized macrophages were treated overnight with indicated concentrations of IFNγ, and subsequently infected with ME-49 tachyzoites in a multiplicity of infection of 1. The growth of intracellular parasites was monitored by uracil incorporation assay. (B) Immortalized macrophages from WT, 3d and MyD88/TRIF double deficient mice were infected for 48 h with a 1∶1 ratio of luciferase expressing tachyzoites per cell and the relative luciferase units (RLU) were calculated by normalizing the raw luminescence values to the background (error bars, s.e.m.). Asterisk indicates that p<0.05 when comparing results from 3d to WT or MyD88/TRIF−/− cells. (C–D) BMDMs isolated from WT or 3d mice were infected with GFP-expressing parasites at a multiplicity of infection of 1, and the number of parasites per vacuole was quantified 32 hours post infection. To differentiate parasites in phagosomes from parasites in the PV only vacuoles positive for GRA7 were counted. Absolute numbers are shown. Representative images of infected 3d BMDMs is shown in D. Asterisk indicates that p<0.05 when comparing results to WT cells. Data are representative experiment of three. Unprimed immortalized macrophages from wild type, 3d and double MyD88/TRIF knockout mice were infected with T. gondii (ME-49 strain) expressing luciferase reporter gene at a ratio of one parasite per cell, and parasite growth evaluated 48 hs later, by measuring luciferase activity (Fig. 8B). Our experiments demonstrated that parasite replication was more pronounced in cells from 3d mice (Fig. 8B). In contrast, when we compared parasite replication in macrophages from wild type versus MyD88/TRIF double knockout mice, no difference was observed in parasite growth (Fig. 8B). Finally, we performed a detailed analysis of parasite growth in bone marrow-derived macrophages from wild type and 3d mice, evaluating the numbers of vacuoles per cells and tachyzoites per parasitophorous vacuole (Figs. 8C and 8D). The results of this experiment demonstrate a lower number of parasitophorous vacuoles containing 2 or 4 tachyzoites (p<0.05), and a significantly higher number of vacuoles containing 8 (p<0.05) or 16 parasites when comparing macrophages from wild type and 3d mice. The number of parasitophorous vacuoles per infected cell was similar (1.72±0.22) in both cell types. Altogether, these results indicate that UNC93B1 mediates host cell resistance to T. gondii through a mechanism that controls parasite replication in the parasitophorous vacuole and is independent from both TLR activation and the anti-parasitic effects of IFNγ.
Discussion
UNC93B1 is a critical mediator of the translocation of nucleotide-sensing TLR3, TLR7 and TLR9 from the ER to endolysosomes [22]. Here, we tested the 3d mouse, which has a non-functional UNC93B1 [19], to evaluate the combined role of nucleotide sensing TLRs in controlling initial activation of innate immunity and host resistance to infection with T. gondii. Despite the fact that none of single TLR3, TLR7 or TLR9 knockout yield an altered phenotype on cytokine response or enhanced susceptibility, we found that 3d mice are extremely susceptible to infection with T. gondii. Therefore, our results raise the possibility that combined action of nucleotide sensing TLRs is critical for host resistance to T. gondii.
Much to our surprise, the MyD88/TRIF null mice, which are devoid of all TLR functions and show impaired production of pro-inflammatory cytokines when infected with T. gondii, were consistently more resistant to infection than the 3d mice. Furthermore, except for IL-12, 3d mice infected with T. gondii mounted a normal systemic pro-inflammatory response, while the MyD88/TRIF double knockouts did not, indicating that the immunological response to infection was fundamentally different. Nevertheless, animals bearing UNC93B1 mutation succumbed to infection as a result of unchecked tachyzoite replication, similar to IFNγ−/− mice [30]. Thus, while we cannot exclude that the combined action of intracellular TLR 3, 7 and 9 contributes to host resistance against T. gondii, our hypothesis is that UNC93B1 also mediates host resistance against T. gondii through an additional mechanism, which is TLR-independent.
Considering the UNC93B1 involvement on antigen presentation [19], we first investigated whether antigen presenting cells (APCs), CD4+ T, and CD8+ T lymphocytes were properly activated in 3d mice. Our results show that production of IL-12 as well as expression of activation markers by APCs was significantly impaired in 3d mice infected with T. gondii. Intriguingly, the IFNγ levels were similar in the sera, splenocyte cultures and peritoneal cavity, when comparing 3d and WT mice at day 8 post-infection with T. gondii. While the percentage of T cells producing IFNγ in splenocytes from infected 3d was comparable to infected C57BL/6 mice, the total numbers of IFNγ producing-CD4+ T as well as -CD8+ T cells were lower in de 3d mice. Regardless, mice deficient in CD8+ T cells or in the so-called transporter associated with antigen processing (TAP-1) protein, while more susceptible to infection with T. gondii, often survive 30–40 days post-infection [31], [32]. Thus, our data suggest that defective antigen cross-presentation and CD8+ T cell activation are not the primary events accounting for the extreme susceptibility of 3d mice to T. gondii infection.
Importantly, our in vivo experiments suggest that UNC93B1 is an important mediator of IL-12 production during T. gondii infection. We also addressed this question in vitro and observed that exposure of macrophages from 3d or MyD88−/− mice to live ME49 tachyzoites resulted in impaired production of IL-12, as compared to macrophages from WT mice. Since IL-12 is a key mediator of IFNγ production during T. gondii infection [26], [27], [33], [34], it is surprising that IFNγ responses, as discussed above, were close to normal in the infected 3d mice. Therefore, we performed experiments at earlier time points and observed that production of IL-12 and IFNγ was significantly impaired in the peritoneal cavity and peritoneal cavity/spleens from 3d mice at 3 and 5 days post-infection, respectively. These findings could be explained as a result of combined deficiency of nucleotide sensing-TLRs, since no phenotype is observed in each of the single TLR3, TLR7 or TLR9 knockout mice.
Consistently, experiments performed in our laboratory and elsewhere [35] demonstrate that despite of severe impairment IL-12 production in MyD88−/− mice, IFNγ is still produced at 8 days post-infection, and yet, mice are highly susceptible to ME-49 infection. Similarly, different studies demonstrate that except for IL-12, infection with the highly virulent RH strain of T. gondii elicits elevated levels of pro-inflammatory cytokines, including IFNγ [36], [37]. However due to the inherent ability of RH parasites to rapidly replicate and disseminate, infected animals are still unable to control parasite burden and die during the acute phase of infection [36], [37].
Even though the observed defect on IL-12/IFNγ axis seems to be sufficient to render animals more susceptible to T. gondii, our results clearly indicate that an additional TLR independent-function mediated by UNC93B1 contributes for the extreme susceptibility of 3d mice, when compared to MyD88−/− or MyD88/TRIF double knockouts infected with T. gondii. Notably, from in vitro and in vivo experiments we had no evidence that cells from 3d mice have a defect in responding to IFNγ. Thus, we next investigated the ability of host cells from UNC93B1 mutant mice to control parasite replication. The fate of T. gondii inside the host cell relies upon the mechanism of entry. Passive internalization by phagocytosis directs parasites to the lysosomal compartment, leading to tachyzoite elimination [24], [25]. Upon active invasion, T. gondii establishes itself in the parasitophorous vacuole [38], [39], [40], which avoids fusion with lysosomes, allowing parasite survival. Interestingly, we found that UNC93B1 is recruited from the ER to the parasitophorous vacuole (PV), rather than to the expected endolysosomal compartment [22].
In spite of being considered a non-fusogenic compartment, few host cell proteins are found at the membrane of parasitophorous vacuole containing tachyzoites. Specific proteins appear to be selectively recruited from the host cell plasma membrane [41], or after host cell activation [42]. In addition, the ER is known to be in close contact with the parasitophorous vacuole membrane [43], and fusion between the ER and parasitophorous vacuole containing live parasites has been demonstrated [44]. Notably, UNC93B1 was shown to translocate from ER to the endolysosomal compartment upon cell stimulation with TLR agonists [22]. Notwithstanding, here we show that UNC93B1 is recruited from the ER to the parasitophorous vacuoles. It is likely that the recruitment of UNC93B1 to the parasitophorous vacuole membrane occurs during the process of ER fusion, given that UNC93B1 is an ER resident protein [19], [21]. The transfer of ER proteins to the parasitophorous vacuole membrane seems to be selective, since neither the mutant form of UNC93B1 nor TLR9 (not shown) were found to be enriched around the parasitophorous vacuole. Dissociation of the intracellular traffic of UNC93B1 and TLR9 has also been suggested by Ewald and co-workers [45], as they observed that forced expression of UNC93B1 at the plasma membrane was not accompanied by relocation of TLR9. Certainly, the process that involves selection or exclusion of specific host proteins, including UNC93B1 and nucleotide sensing TLRs, to the parasitophorous vacuole membrane, is likely to be a key event in the successful establishment of parasitism, and remains to be elucidated.
Most of the mechanisms involved in the control of intracellular replication of T. gondii have been studied in IFNγ-activated macrophages. For example the downstream effects of GTPases [46], [47], production of reactive nitrogen intermediates [48], tryptophan degradation in human cells [49] or autophagy [50], [51] are all IFNγ-inducible mechanisms involved in controlling and/or killing of T. gondii replication. Markedly, we found that in vitro, macrophages from 3d mice present a normal response and are perfectly able to control tachyzoite replication when activated with IFNγ. Consistently, the production of reactive nitrogen intermediates as well as and expression and translocation of IFNγ-inducible GTPases (i.e. Irga6, Irgb6, Irgm1 and Irgm3) or formation of autophagic vacuoles (data not shown) are not impaired in macrophages from 3d mice activated with IFNγ.
Remarkably, our results demonstrate an uncontrolled parasite replication in macrophages from 3d mice infected in vivo with T. gondii, despite an unimpaired IFNγ response. Further, we found that in vitro the 3d mutation renders non-activated macrophages more susceptible to intracellular tachyzoite replication. Even though the difference in parasite numbers is modest, when comparing macrophages from WT and 3d mice, it may reflect large differences in vivo, where multiple rounds of parasite replication during a long period of time will result in exponential parasite growth. To support our interpretation, other studies also show that small but significant differences in parasite replication in vitro, reflects dramatic differences in parasite growth and virulence in vivo [52], [53]. It is difficult to imagine how this phenomenon could be related to the effects of the UNC93B1 mutation on TLR signaling, since the rates of parasite replication in non-activated macrophages from MyD88/TRIF null mice were similar to that observed in the same cells derived from wild-type mice. Thus, UNC93B1 effects on parasite control appear to be independent of what are thought important immune mediators of host resistance to T. gondii, such as TLRs, IFNγ and TNFα.
To establish itself inside a host cell T. gondii has to acquire metabolites from intracellular stores. Indeed, UNC93B1 is a distant ortholog to an ion transporter from Caenorhabditis elegans [54], [55], [56]. Despite this homology, a similar function has not been described for UNC93B1 in mammals [19]. Alternatively, nutrient can be acquired from channels present at the parasitophorous vacuole membrane, which allow free diffusion of small metabolites up to 1300 Da [57]; lipids may be acquired through the closely apposed mitochondrial and ER membranes [43]; and parasite seems to exploit the host endolysosomal system via sequestration of host organelles into invaginations present at the parasitophorous vacuole membrane [58]. Therefore, it is also possible that UNC93B1 regulates metabolite/nutrient acquisition by T. gondii tachyzoites, and hence interferes with parasite replication.
In conclusion, our study reveals a critical anti-parasitic role for UNC93B1. This role appears to involved in at least two steps: (i) control of IL-12 and early IFNγ response, which may be a result of combined TLR3/TLR7/TLR9 deficiency; and (ii) UNC93B1 enrichment in the membranes surrounding the parasitophorous vacuole containing T. gondii tachyzoites, which mediates control of parasite growth in a TLR - and IFNγ-independent manner. Altogether our results indicate that UNC93B1 plays a critical role on innate immune response and host resistance to T. gondii infection.
Methods
Ethics statement
All experiments involving animals were in accordance with guidelines set forth by the American Association for Laboratory Animal Science (AALAS). All protocols developed for this work were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Massachusetts Medical School.
Reagents
All cell culture reagents were obtained from Mediatech, unless otherwise indicated. LPS derived from Escherichia coli strain 0111:B4 was purchased from Sigma and re-extracted by phenol chloroform to remove lipopeptides as described [59]. Pam2CysSer(Lys)4 was obtained from EMC Microcollections. R848, a synthetic small molecule agonist for TLR7 was provided by 3 M Pharmaceuticals. Phosphorothioate-stabilized unmethylated DNA oligonucleotide-bearing CpG (ODN 1826, 5′-TCCATGACGTTCCTGACGTT-3′) and qPCR primers were obtained from Integrated DNA Technologies. Interferon - γ was purchased from R&D Systems.
Mice
C57BL/6J (CD45.2+), B6.129SF2/J (CD45.2+) and B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) mice were obtained from The Jackson Laboratory. UNC93B1 mutant (3d) mice were generated by Dr. Bruce Beutler at The Scripps Research Institute, La Jolla, California [19]. TLR3, TLR7, TLR9, MyD88 and TRIF deficient mice were provided by Dr. Shizuo Akira (Department of Host Defense, Osaka University, Osaka, Japan). Mice deficient in both MyD88 and TRIF were generated by interbreeding single knockout animals. Except for the TRIF−/− mice, which are F5, all mice used were backcrossed to C57BL/6 background at least for 8 generations. Age (5–8 weeks old) and sex matched groups of wild-type (WT) and knockout mice were used in all experiments. Mice were bred and housed under specific pathogen-free conditions at the University of Massachusetts Medical School animal facilities.
Parasites
ME-49 tachyzoites were initially obtained by inoculating brain homogenate containing cysts from C57BL/6 mice infected one month earlier onto fibroblast monolayers. After the emergence of tachyzoites, parasites were maintained in human foreskin fibroblast monolayers by weekly passages as described [60]. Luciferase and GFP expressing ME-49 and RH parasites [61] were a kind gift from Dr. Jeroen P. J. Saeij (MIT, Cambridge, MA).
In vivo experimental infections
Animals were inoculated intraperitoneally (i.p.) with 25 cysts obtained from brain homogenates of 6–8 weeks infected mice. Mice were monitored for survival or sacrificed at 0, 5 and 8 days post infection in order to collect peritoneal exudate cells and fluid, blood and spleens. Samples from each mouse were individually processed and analyzed.
Quantitative real-time PCR
Peritoneal exudate cells, spleen, liver, lung and brain from mice at days 0, 3, 5 or 7 after infection were collected and frozen in liquid nitrogen. Total DNA was extracted using the DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer's instructions, and quantified using a NanoDrop Spectrophotometer (Thermo Scientific). Primers used for amplification of Toxoplasma gondii B1 gene (Forward: 5′ - CTGGCAAATACAGGTGAAATG-3′; Reverse: 5′ - GTGTACTGCGAAAATGAATCC-3′) were designed using the PrimerSelect application (Lasergene software suite, DNASTAR). PCR reactions were setup in a final 20 µl volume using 5 ng of total tissue DNA, 200 ng of each primer and 1x of the iQ SYBR Green Supermix (BioRad). Quantitative RT-PCR analysis was performed on a DNA Engine Opticon 2 Real-Time Cycler (MJ Research). Specificity of amplification was assessed for each sample by melting curve analysis. Non-infected samples gave no signal. Relative quantification was performed using standard curve analysis of purified parasite DNA, and results were expressed as pg of parasite DNA per mg of total tissue DNA.
Flow cytometry
Cells were stained for 30 min with conjugated antibodies against the surface markers CD3, CD4, CD8, CD11b, CD25, CD40, CD69, CD80, CD86, CD154, MHC I or MHC II (eBioscience). For intracellular measurement of cytokines splenocytes were cultivated for 4 h in presence of GolgiPlug (BD Bioscience), surface stained, permeabilized and incubated with Phycoerythrin-anti - IFNγ or TNFα for 30 min. Subsequently cells were washed and analyzed by flow cytometry in an LSRII cytometer (BD Bioscience). Fluorescent cell lines were analyzed without staining. Data were acquired with DIVA software (BD Bioscience) and analyzed with FlowJo (Tree Star).
Bone marrow transplantation
Recipient B6.SJL-PtprcaPepcb/BoyJ and 3d mice were given lethal total body irradiation (900 rads) and reconstituted intravenously with 5–10 million bone marrow cells within 4 h. Marrow cell suspensions were prepared from donor tibial and femoral bones by flushing with phosphate-buffered saline using a 30-gauge needle syringe. Irradiated and reconstituted mice were given 150 mg/ml Sulfamethoxazole and 30 mg/ml N-Trimethoprim in their drinking water for 6 weeks. Thereafter, they were switched to sterile drinking water, thus ensuring that the antibiotic treatment would not affect the ensuing experimental infection with T. gondii. Mice were used for experimental infection or for analysis of chimerism 7–9 weeks after transplant. Animals showed full reconstitution of lymphoid and myeloid cell populations as determined by flow cytometric analysis (not shown).
Spleen cell culture
Spleens were homogenized, RBC lysed (Red Blood Cell Lysis buffer, Sigma), and splenocytes were re-suspended in complete RPMI medium. Cells were cultured at 5×106/well in 24-well tissue culture plates in absence of exogenous stimuli, and supernatants were collected 24 h later.
Cytokine and NO measurement
Splenocyte, macrophage culture supernatants or peritoneal exudates were assayed for pro-inflammatory cytokines with DuoSet ELISA kits from R&D Systems according to the manufacturer's instructions. Reactive nitrogen intermediates were measured by the Griess reaction of nitrites accumulated in the supernatants [62] with chemicals from Sigma. Cytokines present in mouse serum were assayed using the BD Cytometric Bead Assay (CBA) Mouse Inflammation kit according to the manufacturer's instructions.
Immunoblot analysis
Splenocytes collected from either non-infected animals or from mice infected for 5 or 8 days were lysed, the extract re-suspended in Laemmli sample buffer [63] and boiled for 5 min at 95°C. Samples were separated by 10% SDS-PAGE and were transferred onto nitrocellulose membranes [64]. Blots were incubated with mouse monoclonal antibodies against STAT-1 (C-terminus) or STAT-1P (BD Biosciences) and subsequently incubated with HRP-conjugated anti-mouse IgG (Bio-Rad). Membranes were then incubated with HRP substrate (enhanced chemiluminescence substrate; Amersham Biosciences) and developed by exposure to film (Hyperfilm; Amersham Biosciences).
Cell culture
Bone marrow-derived macrophages (BMDMs) were isolated as described [65], and were cultured in RPMI medium supplemented with 25 mM Hepes, 10 mM L-glutamine, 100 U/ml Penicillin-Streptomycin, 50 µM 2-mercaptoethanol (Sigma) and 10% FCS (Hyclone). Immortalized macrophage cell lines were generated as described [66], [67]. Briefly, primary bone marrow cells were incubated in L929 mouse fibroblast-conditioned medium for 3–4 days for the induction of macrophage differentiation. Subsequently, cells were infected with J2 recombinant retrovirus carrying v-myc and v-raf(mil) oncogenes [68]. Growth factors were removed from the culture medium and cells were maintained until they were growing in the absence of conditioning medium. Macrophages phenotype was verified by surface expression of the markers CD11b (M1/70, BD Pharmigen) and F4/80 (BM8, eBioscience) as well as a range of functional parameters, including responsiveness to Toll-like ligands. Macrophage cell lines were generated from wild-type (C57BL/6), 3d and TLR9-deficent mice. Cells were treated with the indicated stimuli or infected in a multiplicity of infection (MOI) of 5, and supernatants were collected 24 h later.
Fluorescent DNA constructs
Murine UNC93B1 (BC018388) was C-terminally fused with YFP and cloned into pcDNA3 (Invitrogen). The point mutant UNC93B1 (H412R) was generated by sequential PCR with primers carrying the point mutation CAC (His) to CGC (Arg). YFP-tagged UNC93B1 wild type and H412R were then cloned into a retroviral vector that was modified from the original pCLXSN backbone from Imgenex (Fig. S6). All of the constructs were verified by sequencing.
Viral transduction of immortalized macrophages
Recombinant retroviruses were produced as described [69]. Briefly, human embryonic kidney (HEK293T) cells were co-transfected with the vectors encoding YFP-tagged UNC93B1 and plasmids carrying the retroviral gag-pol genes and the envelope protein VSV-G using the GeneJuice transfection reagent (Novagen) according to the manufacturer's instructions. The virus containing supernatants were filtered and used to infect immortalized 3d or TLR9-deficient cells.
In vitro infection
ME-49 tachyzoites were stained with the Cell Tracker Red CMTPX (Invitrogen) according to the manufacturer's instruction. Immortalized macrophages were infected in a multiplicity of infection (MOI) of 3. After 2 h, unbound parasites were washed off and live cells were imaged by confocal microscopy at 37°C. Acidic intracellular compartments were stained with the acidophilic lysomotropic dye LysoTracker Blue (Invitrogen). To study the kinetics of parasite growth, BMDMs were infected with GFP-expressing ME-49 tachyzoites in a MOI of 1. After 32 hours cells were fixed and processed for immunofluorescent staining as described below. Number of GRA7-positive vacuoles per cell, and of parasites per vacuole, were evaluated in 10 randomly selected microscopic fields, and at least 100 vacuoles were counted per sample. In order to quantify number of vacuoles positive for interferon-induced GTPases (IRGs), immortalized macrophages were induced overnight with 200 U/ml IFNγ before infection. Cells were fixed and processed for immunofluorescence. Intracellular parasites were identified by GRA7 staining, and the percentage of IRG-positive vacuoles was determined after analysis of 200 vacuoles.
Cytotoxicity assays
Murine immortalized macrophages were stimulated with IFNγ (BD Pharmigen) at 10–100 U/ml as indicated for 24 h prior to infection while control cultures were left untreated. Cells were then infected with ME-49 tachyzoites at a multiplicity of infection of 1. After 24 h of incubation, cultures were labeled with 1 µCi/well [3H]-uracil for additional 24 h. The amount of incorporated uracil was determined by liquid scintillation counting [70]. Alternatively, immortalized macrophages were infected with luciferase-expressing parasites. After 48 h samples were lysed, cell lysates were mixed with a Luciferase reporter assay system substrate (Promega) and luciferase units calculated by normalizing the raw luminescence values to the background from non-infected cells.
Immunofluorescence
Cells were fixed with paraformaldehyde 4% for 15 min at room temperature, washed with PBS, permeabilized with saponin 0.1% in PBS for 10 min and incubated with primary antibodies for 1 h. Subsequently cells were washed and incubated with Alexa conjugated secondary antibodies for an additional 1 h. Host and parasite DNA was stained with DAPI. Slides were mounted with Gel-mount anti-fading reagent (EMS) and analyzed by confocal microscopy. Primary antibodies used: α-GRA7 antiserum (a kind gift from Dr. George Yap, New Jersey Medical School, Newark, NJ), rabbit α-Irga6 antiserum 165°3 [71], rabbit anti-Irgb6 antiserum 141/1 (raised against bacterial purified full length protein, unpublished), α-Irgm1 (Santa Cruz Biotechnology), and α-Irgm3 (BD Pharmigen). Secondary antibodies: Alexa 633 goat α-rabbit, Alexa 488 donkey α-goat and Alexa 488 goat α-mouse conjugated antibodies (Invitrogen).
Confocal microscopy
We used an inverted Axiovert 100-M microscope equipped with a Zeiss LSM 510 META scanning unit and a 1.4 NA 63x plan apochromat objective (Zeiss), and an inverted Leica LSM TSC SP2 AOBS. Cells were cultured on glass-bottom 35-mm tissue-culture dishes (Matek). Dual or triple color images were acquired by consecutive scanning with only one laser line active per scan to avoid cross-excitation.
Statistics
The statistical significance of the differences in the means of experimental groups was determined by one-way or two-way ANOVA analysis and Bonferroni post-test using GraphPad Prism 5.0a Software.
Supporting Information
Zdroje
1. HillD
DubeyJP
2002 Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 8 634 640
2. DenkersEY
GazzinelliRT
1998 Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11 569 588
3. AkiraS
UematsuS
TakeuchiO
2006 Pathogen recognition and innate immunity. Cell 124 783 801
4. GazzinelliRT
DenkersEY
2006 Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 6 895 906
5. CamposMA
CloselM
ValenteEP
CardosoJE
AkiraS
2004 Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol 172 1711 1718
6. ScangaCA
AlibertiJ
JankovicD
TilloyF
BennounaS
2002 Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168 5997 6001
7. MurailleE
De TrezC
BraitM
De BaetselierP
LeoO
2003 Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol 170 4237 4241
8. Debierre-GrockiegoF
CamposMA
AzzouzN
SchmidtJ
BiekerU
2007 Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol 179 1129 1137
9. MinnsLA
MenardLC
FoureauDM
DarcheS
RonetC
2006 TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol 176 7589 7597
10. PlattnerF
YarovinskyF
RomeroS
DidryD
CarlierM
2008 Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3 77 87
11. YarovinskyF
ZhangD
AndersenJF
BannenbergGL
SerhanCN
2005 TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308 1626 1629
12. AlexopoulouL
HoltA
MedzhitovR
FlavellR
2001 Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413 732 738
13. DieboldS
KaishoT
HemmiH
AkiraS
Reis e SousaC
2004 Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303 1529 1531
14. HeilF
HemmiH
HochreinH
AmpenbergerF
KirschningC
2004 Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303 1526 1529
15. HemmiH
TakeuchiO
KawaiT
KaishoT
SatoS
2000 A Toll-like receptor recognizes bacterial DNA. Nature 408 740 745
16. DrennanM
StijlemansB
Van den AbbeeleJ
QuesniauxV
BarkhuizenM
2005 The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J Immunol 175 2501 2509
17. BartholomeuD
RopertC
MeloM
ParrocheP
JunqueiraC
2008 Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J Immunol 181 1333 1344
18. ParrocheP
LauwF
GoutagnyN
LatzE
MonksB
2007 Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 104 1919 1924
19. TabetaK
HoebeK
JanssenEM
DuX
GeorgelP
2006 The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol 7 156 164
20. CasrougeA
ZhangS-Y
EidenschenkC
JouanguyE
PuelA
2006 Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314 308 312
21. BrinkmannMM
SpoonerE
HoebeK
BeutlerB
PloeghHL
2007 The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. The Journal of Cell Biology 177 265 275
22. KimYM
BrinkmannMM
PaquetME
PloeghHL
2008 UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452 234 238
23. SibleyL
WeidnerE
KrahenbuhlJ
1985 Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature 315 416 419
24. MordueD
SibleyL
1997 Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol 159 4452 4459
25. JoinerK
FuhrmanS
MiettinenH
KasperL
MellmanI
1990 Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249 641 646
26. GazzinelliRT
HienyS
WynnTA
WolfS
SherA
1993 Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA 90 6115 6119
27. GazzinelliRT
WysockaM
HayashiS
DenkersEY
HienyS
1994 Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153 2533 2543
28. MorisakiJH
HeuserJE
SibleyLD
1995 Invasion of Toxoplasma gondii occurs by active penetration of the host cell. Journal of Cell Science 108 ( Pt 6) 2457 2464
29. JacobsD
DubremetzJF
LoyensA
BosmanF
SamanE
1998 Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol Biochem Parasitol 91 237 249
30. Scharton-KerstenTM
WynnTA
DenkersEY
BalaS
GrunvaldE
1996 In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol 157 4045 4054
31. BrownCR
McLeodR
1990 Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol 145 3438 3441
32. GoldszmidRS
BaficaA
JankovicD
FengCG
CasparP
2007 TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med 204 2591 2602
33. Reis e SousaC
HienyS
Scharton-KerstenT
JankovicD
CharestH
1997 In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med 186 1819 1829
34. MordueDG
SibleyLD
2003 A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. Journal of Leukocyte Biology 74 1015 1025
35. SukhumavasiW
EganCE
WarrenAL
TaylorGA
FoxBA
2008 TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol 181 3464 3473
36. MordueDG
MonroyF
La ReginaM
DinarelloCA
SibleyLD
2001 Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167 4574 4584
37. GavrilescuLC
DenkersEY
2001 IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol 167 902 909
38. JonesTC
YehS
HirschJG
1972 The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med 136 1157 1172
39. JonesTC
HirschJG
1972 The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med 136 1173 1194
40. MordueDG
HåkanssonS
NiesmanI
SibleyLD
1999 Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol 92 87 99
41. CharronAJ
SibleyLD
2004 Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic 5 855 867
42. MartensS
ParvanovaI
ZerrahnJ
GriffithsG
SchellG
2005 Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog 1 e24
43. SinaiAP
WebsterP
JoinerKA
1997 Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. Journal of Cell Science 110 ( Pt 17) 2117 2128
44. GoldszmidRS
CoppensI
LevA
CasparP
MellmanI
2009 Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med - Suppl 206 399 410
45. EwaldSE
LeeBL
LauL
WickliffeKE
ShiG-P
2008 The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456 658 662
46. ZhaoYO
KhaminetsA
HunnJP
HowardJC
2009 Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog 5 e1000288
47. ZhaoY
FergusonDJP
WilsonDC
HowardJC
SibleyLD
2009 Virulent Toxoplasma gondii evade immunity-related GTPase-mediated parasite vacuole disruption within primed macrophages. J Immunol 182 3775 3781
48. Scharton-KerstenTM
YapG
MagramJ
SherA
1997 Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185 1261 1273
49. PfefferkornER
1984 Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA 81 908 912
50. AndradeRM
WessendarpM
GubbelsM-J
StriepenB
SubausteCS
2006 CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest 116 2366 2377
51. LingYM
ShawMH
AyalaC
CoppensI
TaylorGA
2006 Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 203 2063 2071
52. El HajjH
LebrunM
AroldST
VialH
LabesseG
2007 ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog 3 e14
53. TaylorS
BarraganA
SuC
FuxB
FentressSJ
2006 A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314 1776 1780
54. de la CruzIP
LevinJZ
CumminsC
AndersonP
HorvitzHR
2003 sup-9, sup-10, and unc-93 may encode components of a two-pore K+ channel that coordinates muscle contraction in Caenorhabditis elegans. J Neurosci 23 9133 9145
55. GreenwaldIS
HorvitzHR
1980 unc-93(e1500): A behavioral mutant of Caenorhabditis elegans that defines a gene with a wild-type null phenotype. Genetics 96 147 164
56. LevinJZ
HorvitzHR
1992 The Caenorhabditis elegans unc-93 gene encodes a putative transmembrane protein that regulates muscle contraction. The Journal of Cell Biology 117 143 155
57. SchwabJC
BeckersCJ
JoinerKA
1994 The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA 91 509 513
58. CoppensI
DunnJD
RomanoJD
PypaertM
ZhangH
2006 Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125 261 274
59. HirschfeldM
MaY
WeisJ
VogelS
WeisJ
2000 Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 165 618 622
60. LockJ
1953 Cultivation of Toxoplasma gondii in tissue culture in mammalian cells. Lancet 1 324 325
61. SaeijJPJ
BoyleJP
GriggME
ArrizabalagaG
BoothroydJC
2005 Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infection and Immunity 73 695 702
62. GreenL
WagnerD
GlogowskiJ
SkipperP
WishnokJ
1982 Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126 131 138
63. LaemmliU
1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680 685
64. TowbinH
StaehelinT
GordonJ
1979 Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76 4350 4354
65. AustinP
McCullochE
TillJ
1971 Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J Cell Physiol 77 121 134
66. HalleA
HornungV
PetzoldGC
StewartCR
MonksBG
2008 The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9 857 865
67. HornungV
BauernfeindF
HalleA
SamstadEO
KonoH
2008 Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9 847 856
68. RobersonS
WalkerW
1988 Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell Immunol 116 341 351
69. MannR
MulliganR
BaltimoreD
1983 Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 33 153 159
70. PfefferkornER
PfefferkornLC
1977 Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool 24 449 453
71. MartensS
SabelK
LangeR
UthaiahR
WolfE
2004 Mechanisms regulating the positioning of mouse p47 resistance GTPases LRG-47 and IIGP1 on cellular membranes: retargeting to plasma membrane induced by phagocytosis. J Immunol 173 2594 2606
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



