-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Metal Hyperaccumulation Armors Plants against Disease
Metal hyperaccumulation, in which plants store exceptional concentrations of metals in their shoots, is an unusual trait whose evolutionary and ecological significance has prompted extensive debate. Hyperaccumulator plants are usually found on metalliferous soils, and it has been proposed that hyperaccumulation provides a defense against herbivores and pathogens, an idea termed the ‘elemental defense’ hypothesis. We have investigated this hypothesis using the crucifer Thlaspi caerulescens, a hyperaccumulator of zinc, nickel, and cadmium, and the bacterial pathogen Pseudomonas syringae pv. maculicola (Psm). Using leaf inoculation assays, we have shown that hyperaccumulation of any of the three metals inhibits growth of Psm in planta. Metal concentrations in the bulk leaf and in the apoplast, through which the pathogen invades the leaf, were shown to be sufficient to account for the defensive effect by comparison with in vitro dose–response curves. Further, mutants of Psm with increased and decreased zinc tolerance created by transposon insertion had either enhanced or reduced ability, respectively, to grow in high-zinc plants, indicating that the metal affects the pathogen directly. Finally, we have shown that bacteria naturally colonizing T. caerulescens leaves at the site of a former lead–zinc mine have high zinc tolerance compared with bacteria isolated from non-accumulating plants, suggesting local adaptation to high metal. These results demonstrate that the disease resistance observed in metal-exposed T. caerulescens can be attributed to a direct effect of metal hyperaccumulation, which may thus be functionally analogous to the resistance conferred by antimicrobial metabolites in non-accumulating plants.
Published in the journal: Metal Hyperaccumulation Armors Plants against Disease. PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001093
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001093Summary
Metal hyperaccumulation, in which plants store exceptional concentrations of metals in their shoots, is an unusual trait whose evolutionary and ecological significance has prompted extensive debate. Hyperaccumulator plants are usually found on metalliferous soils, and it has been proposed that hyperaccumulation provides a defense against herbivores and pathogens, an idea termed the ‘elemental defense’ hypothesis. We have investigated this hypothesis using the crucifer Thlaspi caerulescens, a hyperaccumulator of zinc, nickel, and cadmium, and the bacterial pathogen Pseudomonas syringae pv. maculicola (Psm). Using leaf inoculation assays, we have shown that hyperaccumulation of any of the three metals inhibits growth of Psm in planta. Metal concentrations in the bulk leaf and in the apoplast, through which the pathogen invades the leaf, were shown to be sufficient to account for the defensive effect by comparison with in vitro dose–response curves. Further, mutants of Psm with increased and decreased zinc tolerance created by transposon insertion had either enhanced or reduced ability, respectively, to grow in high-zinc plants, indicating that the metal affects the pathogen directly. Finally, we have shown that bacteria naturally colonizing T. caerulescens leaves at the site of a former lead–zinc mine have high zinc tolerance compared with bacteria isolated from non-accumulating plants, suggesting local adaptation to high metal. These results demonstrate that the disease resistance observed in metal-exposed T. caerulescens can be attributed to a direct effect of metal hyperaccumulation, which may thus be functionally analogous to the resistance conferred by antimicrobial metabolites in non-accumulating plants.
Introduction
Metal hyperaccumulation is described as the accumulation of exceptionally high concentrations of metallic elements in the aerial parts of a plant [1], [2]. The phenomenon has evolved in around 450 plant species, distributed across several families [2], [3], [4], and most hyperaccumulator species are endemic to metalliferous soils, either natural or anthropogenic in origin [5]. This unusual characteristic has attracted considerable interest, and a number of hypotheses have been proposed to explain the evolution of the hyperaccumulation phenotype [6], [7]. The possibility that accumulated metal provides a defense against herbivores or pathogens, termed the ‘elemental defense hypothesis’, has received most attention and support [7], [8], [9].
A number of studies have reported findings consistent with a defensive effect of hyperaccumulated metals against herbivores [10], [11], [12], but the interpretation of these results remains controversial, as other studies have failed to support the defense hypothesis. For instance, Noret et al. [13], [14] were unable to find a defensive effect attributable to metal hyperaccumulation in the field, despite that reported in laboratory trials. There is also some evidence that the importance of metal-based defense is dependent upon the mode of herbivory [15]. In the case of defense against pathogens, considerably fewer tests have been carried out [e.g. [16]] and only one study [17] has explicitly tested the defense hypothesis with regard to bacterial pathogens. So far, although some evidence has been provided that plants exposed to high metal concentrations have reduced susceptibility to various pathogens, no study has demonstrated that the metal itself was directly responsible for this effect. It is therefore not possible, at present, to state that metal hyperaccumulation trait evolved as an antimicrobial defense.
Here, we test the possibility of a direct elemental defense against bacterial pathogens in the crucifer Thlaspi caerulescens, a hyperaccumulator plant characteristically associated with metalliferous soils [18]. In its natural habitats, this species can hyperaccumulate three different metals: zinc, nickel, and cadmium [19], [20], and it has been widely used in studies of the hyperaccumulation trait [21], [22], [23], [24], [25].
We have tested the elemental defense hypothesis via a three-pronged approach by means of studies in vitro, in planta, and in the field. First, we have monitored the growth of Pseudomonas syringae pv. maculicola M4, a model pathogen of Arabidopsis thaliana [26], in T. caerulescens plants treated with different metal concentrations. This pathogen grows in the apoplastic spaces between plant cells, so we have investigated metal concentrations in the apoplastic phase specifically to determine whether they are likely to be inhibitory to pathogen growth. A number of studies attempting to determine the location of hyperaccumulated metals within the leaf have found the majority to be accumulated in the epidermal vacuoles [27], [28], [29], but apoplastic metal concentrations have not been examined in previous studies. Further, we have assessed the growth of Psm mutants with altered zinc sensitivity in T. caerulescens plants grown on different zinc regimes. This has allowed us to test whether zinc tolerance is important for bacterial growth in planta, as expected if metals are directly involved in plant defense. Finally, we have tested the zinc tolerance of endophytic bacteria found in the leaves of T. caerulescens growing on a metal-rich soil at the site of a former lead–zinc mine to determine whether there is evidence that this form of defense may be effective under natural field conditions.
Results
Pseudomonas syringae pv. maculicola M4 is a pathogen of Thlaspi caerulescens
When Thlaspi caerulescens plants were grown in the glasshouse, it was observed that occasional outbreaks of powdery mildew affected only those plants growing on low metal treatments (Figure 1A and B). This observation prompted us to consider the importance of metals in defending T. caerulescens against pathogens. As powdery mildews are obligate biotrophs and therefore unculturable, we sought a more tractable model system for further study of this phenomenon. Bacterial plant pathogens are suitable for such investigations because they are easily cultured in vitro and are relatively straightforward subjects for genetic manipulations such as mutagenesis.
Fig. 1. High zinc concentrations suppress disease symptoms in Thlaspi caerulescens. 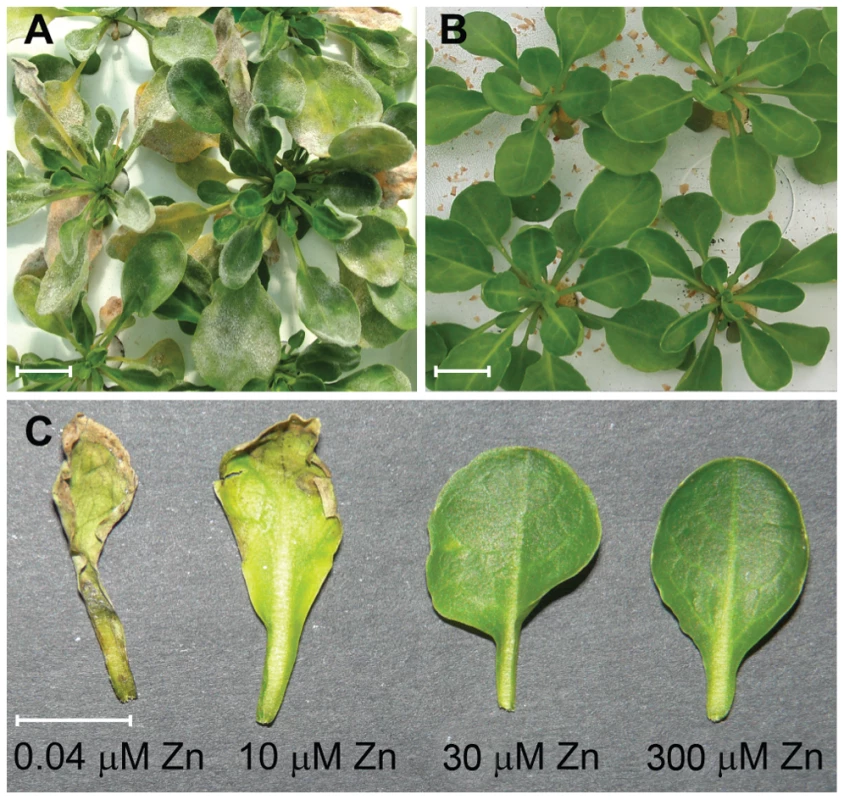
A. T. caerulescens plants growing on 10 µM zinc during an outbreak of mildew (Erysiphe sp.) in the glasshouse. B. T. caerulescens plants growing on 300 µM zinc during the same outbreak of mildew in the glasshouse. C. T. caerulescens plants were grown for 10 weeks on nutrient solution containing 0.04, 10, 30, or 300 µM ZnSO4. Leaves were infiltrated with P. syringae pv. maculicola M4 suspended in 10 mM MgCl2 at 108 cfu/ml and photographed 96 hours after inoculation. Scale bars represent 10 mm. Of sixteen plant pathogenic bacteria tested, eight were found to cause necrotic symptoms in T. caerulescens within 2 to 4 days after inoculation [see Supporting Information Table S1]. Pseudomonas syringae pv. maculicola M4 (Psm), a rifampicin-resistant derivative of Psm 4326 [26], caused necrosis more rapidly and to a greater extent than any other tested strain. To confirm that Psm is pathogenic on T. caerulescens, we assessed the ability of Psm, Psm ES4326 (a streptomycin resistant derivative of Psm 4326) [30] and two type III secretion system (T3SS) mutants of ES4326 (gift of K. Schreiber and D. Desveaux) to grow in T. caerulescens. Over 5 days post-inoculation, Psm M4 and Psm ES4326 both multiplied two to three logs in the leaves of T. caerulescens plants grown on minimal (0.04 µM) zinc. However, neither T3SS mutant was able to grow in T. caerulescens at any of the zinc concentrations tested (Figure 2). Both T3SS mutants grew similarly to wild-type bacteria in vitro (Figure S1). This confirms that Psm is pathogenic towards T. caerulescens and shows that the ability of Psm to grow in T. caerulescens is T3SS-dependent.
Fig. 2. T3SS mutants of Pseudomonas syringae pv. maculicola are unable to colonise Thlaspi caerulescens. 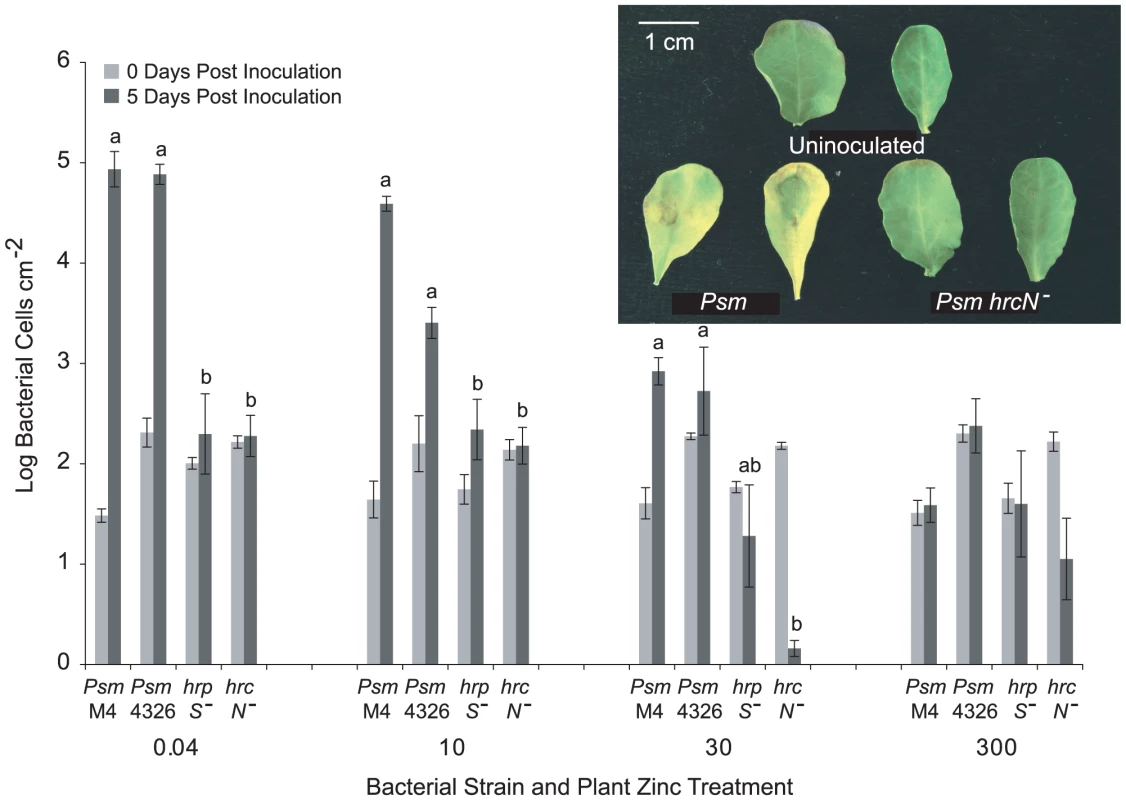
P. syringae pv. maculicola M4 (Psm M4), P. syringae pv. maculicola ES4326 (Psm 4326) and two T3SS mutants of Psm (hrpS− and hrcN−) were inoculated into fully expanded leaves of T. caerulescens at 106 cfu/ml. Three samples were taken for each strain, zinc treatment and time point, each consisting of three leaf discs pooled together. Values are means±SE (n = 3). The mean growth of the four strains at each zinc treatment over 5 days post-inoculation was compared in ANOVAs; growth of the four strains was found to differ significantly in all treatments except in the 300 µM zinc treatment, where no strain was able to grow (P<0.0005 for 0.04 and 10 µM Zn; P = 0.001 for 30 µM Zn; P = 0.169 for 300 µM Zn). Within each zinc treatment, Bonferroni simultaneous comparisons were used to determine which means differed significantly at 5 days post-inoculation, and these are marked with different letters. The inset shows symptoms observed in leaves from T. caerulescens plants grown on 0.04 µM zinc 72 hours after inoculation with wild-type Psm ES4326 and the Psm hrcN− mutant at 106 cfu/ml compared with uninoculated control leaves. Hyperaccumulation of zinc, nickel, or cadmium inhibits the growth of Pseudomonas syringae pv. maculicola M4 in Thlaspi caerulescens
On inoculation of Psm into T. caerulescens plants treated with progressively higher concentrations of zinc, both symptom development and Psm growth were significantly reduced as the concentration of the zinc treatment increased (Figure 1C and D; Figure 2).
T. caerulescens is able to accumulate three metals, zinc, nickel and cadmium, in its shoots. To determine whether nickel and cadmium also conferred increased resistance to infection, bacterial growth was quantified in leaves of T. caerulescens plants grown on nutrient solution supplemented with different concentrations of zinc, nickel, or cadmium. For all three metals, increasing metal concentration resulted in reduced bacterial growth. Zinc treatments at concentrations ≥30 µM, and nickel and cadmium treatments at concentrations ≥10 µM, caused significant inhibition of bacterial growth at 2 and 5 days post-inoculation (Figure 3). Accumulation of any of these metals therefore inhibits bacterial growth in planta and defends T. caerulescens against disease.
Fig. 3. High metal concentrations inhibit bacterial growth in Thlaspi caerulescens. 
T. caerulescens plants were treated with a range of zinc (A), nickel (B), or cadmium (C) concentrations. Nine leaves of each of six plants were infiltrated with P. syringae pv. maculicola M4 suspended in 10 mM MgCl2 at 106 cfu/ml and leaves sampled at 0, 2 and 5 days after inoculation. Six samples were taken per time point and treatment, each sample consisting of three leaves pooled from one plant. Plant zinc, nickel and cadmium treatments were significant predictors of Psm growth at both day 2 and day 5 (ANOVAs; P<0.0005). Bonferroni simultaneous comparisons were carried out; means that were not significantly different are marked with the same letter. Values are means ± SE (n = 6). The experiment was repeated twice with similar results. Studies of plants that are not normally exposed to high concentrations of metals have shown that metal treatment can induce a range of stress responses, including up-regulation of genes associated with local plant defense responses and systemic acquired resistance (SAR) such as PR-1 [31]. In order to determine whether some of the protection conferred by high zinc was a consequence of the effect of high zinc concentrations on defense-associated gene expression, we compared the expression of PR-1 in leaves of T. caerulescens plants grown on 0.04 and 300 µM zinc by quantitative real-time PCR. Normalized PR-1 expression in the two metal treatments was similar to PR-1 expression in healthy A. thaliana leaves (relative expression levels in 0.04 µM, 1.423±0.299; 300 µM Zn, 1.942±0.520; untreated A. thaliana, 2.102±1.435), and there was no significant increase in expression in response to increased zinc (Figure S2). This indicates that the inhibitory effect of increasing metal treatments on Psm growth is not due to up-regulation of SAR.
Apoplast extracts from Thlaspi caerulescens plants grown on high metal concentrations show decreased ability to support bacterial growth
Psm infects the apoplastic phase between plant cells. To test whether metal accumulation reduces the suitability of this environment for bacterial growth, Psm was cultivated in vitro in apoplastic fluid extracted from plants treated with supplementary metals. Apoplast extracts from zinc - and nickel-treated plants supported significantly less bacterial growth than those from plants grown without supplementary metal (Figure 4A and B). Apoplast extracts from plants treated with higher cadmium concentrations also inhibited bacterial growth for up to 18 hours, although this inhibition was eventually released (Figure 4C). As such, it is clear that metal hyperaccumulation by T. caerulescens makes the apoplast a more hostile environment for the growth of Psm.
Fig. 4. Apoplast extracts from Thlaspi caerulescens plants grown in high metal concentrations inhibit bacterial growth. 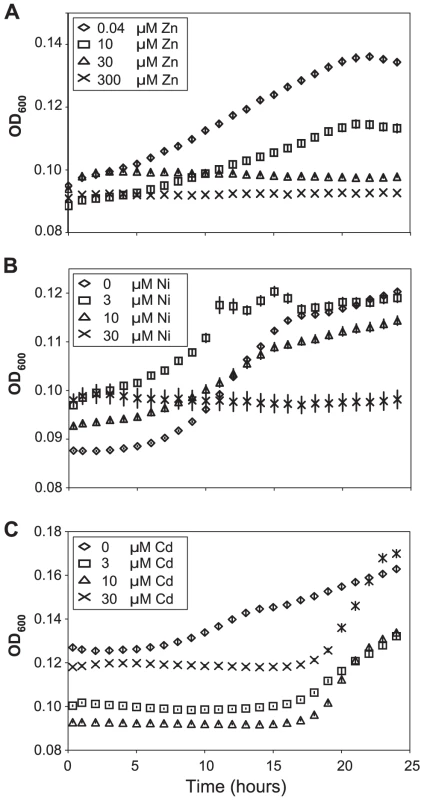
Apoplast extracts obtained from T. caerulescens plants treated with the same zinc (A), nickel (B), or cadmium (C) concentrations used in the bacterial colonization assays shown in Figure 3 were used as a growth medium for P. syringae pv. maculicola M4. Six samples of 100 µl of apoplast were used for each treatment. Values are means ± SE (n = 6). The experiment was performed three times with similar results, and the results shown are from one experiment representative of the three. Concentrations of all three metals were significant predictors of growth at 18 and 24 hours (ANOVAs: P<0.0005 in all cases except P = 0.015 for cadmium at 24 h). Bonferroni simultaneous comparisons (α = 1%) show that all zinc and nickel treatments resulted in significantly less growth than the control at 18 h and 24 h, as did all cadmium treatments up to 18 h. Metal concentrations in Thlaspi caerulescens leaves and extracted apoplast are sufficient to explain the high disease resistance of metal-treated plants
To determine whether hyperaccumulated metal could be responsible for the observed reductions in bacterial growth in leaves, the ability of Psm to tolerate each metal was tested in a range of synthetic media and in extracted apoplast (Figure S3) and compared with the metal concentrations of apoplast extracts and whole-leaf tissue samples (Figure 5; Table 1). For all metals tested, concentrations in whole-leaf tissue of T. caerulescens plants grown at the two highest treatments were sufficient (i.e. higher than the IC50 values for the respective metals) to explain the observed reduction in bacterial growth in planta. Apoplastic zinc and nickel concentrations from the highest treatments were also sufficient to explain bacterial growth reduction. Apoplastic cadmium concentrations were considerably lower, reaching around one-third of the IC50 concentration for Psm in vitro in higher treatments, perhaps explaining the ability of Psm eventually to overcome inhibition by cadmium.
Fig. 5. Metal concentrations in zinc-, nickel- and cadmium-treated plants. 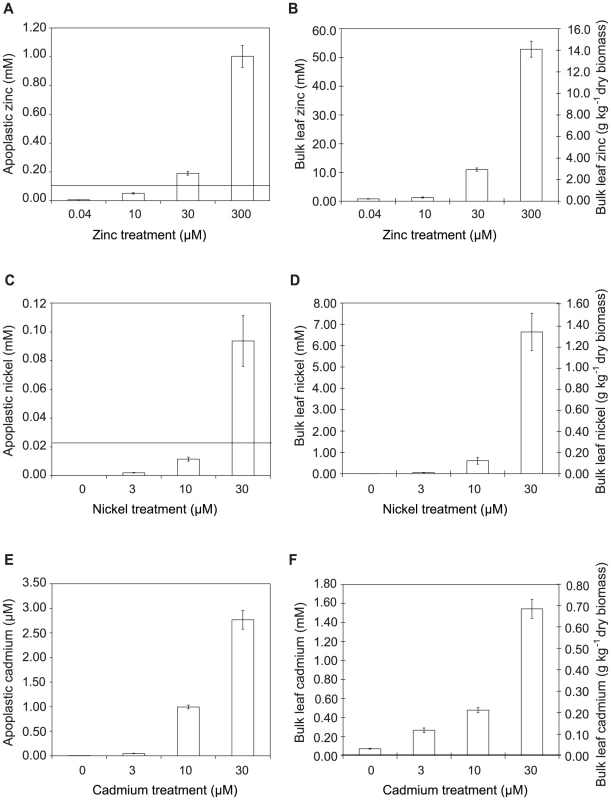
Data represent average values from three independent sets of plants. Metal content was determined by atomic absorption spectrophotometry. A, C, E: Apoplastic concentrations of zinc, nickel and cadmium, respectively. B, D, F: Bulk-leaf concentrations of zinc, nickel and cadmium, respectively, expressed both as molar concentration calculated on the basis of leaf fresh biomass, and as mass concentration relative to leaf dry biomass. Values are means ± SE (n = 6). Horizontal bars in A, C and F indicate metal concentrations representing the respective IC50 values for P. syringae pv. maculicola M4 determined experimentally in extracted apoplast (i.e. Zn = 0.12 mM; Ni = 0.025 mM; Cd = 0.01 mM); IC50 values measured in LB were higher (Zn = 0.63 mM; Ni = 0.78 mM; Cd = 0.22 mM). Tab. 1. Summary of bacterial growth across treatments in relation to metal concentration and metal-dependent growth inhibition of P. syringae pv. maculicola in vitro. 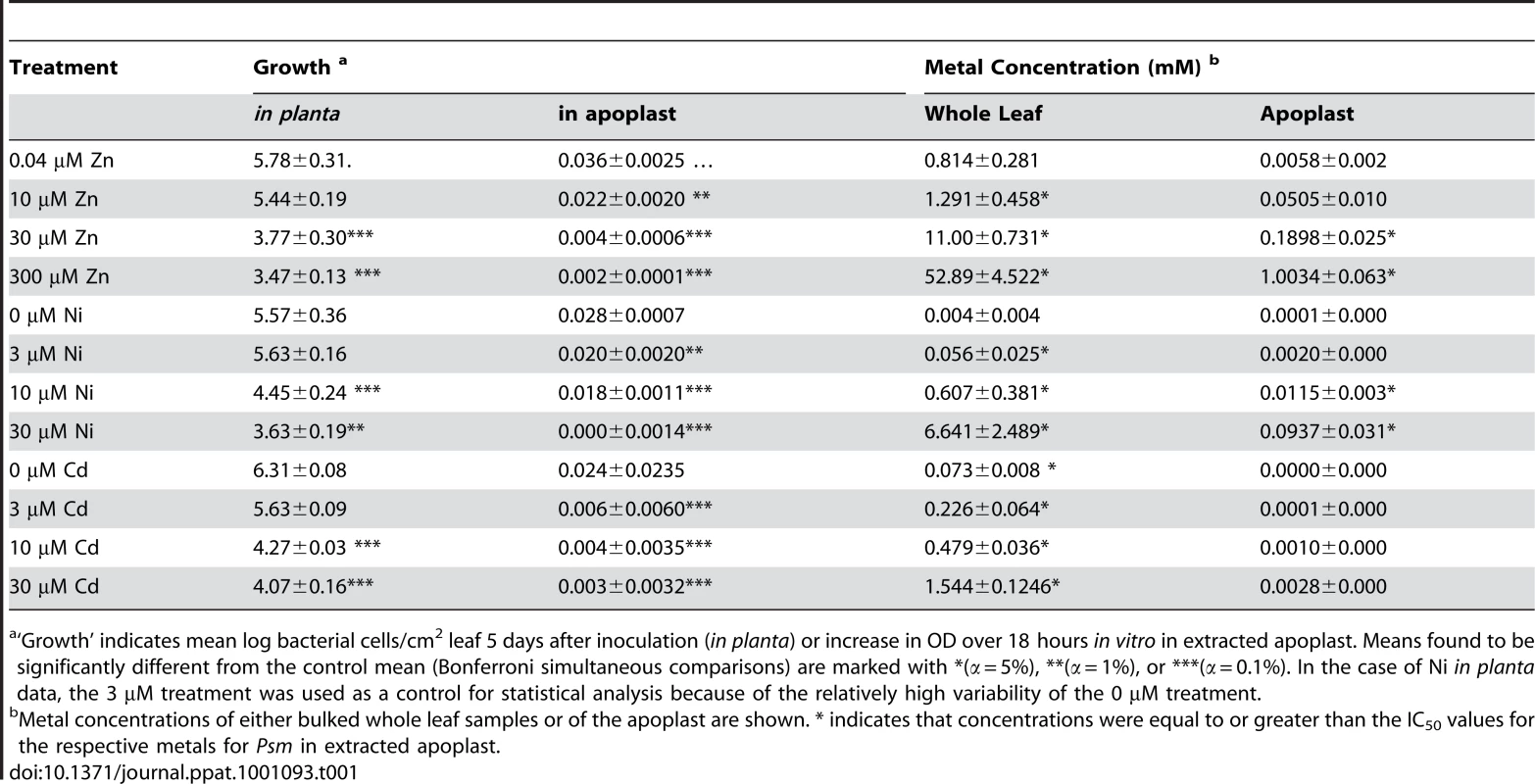
‘Growth’ indicates mean log bacterial cells/cm2 leaf 5 days after inoculation (in planta) or increase in OD over 18 hours in vitro in extracted apoplast. Means found to be significantly different from the control mean (Bonferroni simultaneous comparisons) are marked with *(α = 5%), **(α = 1%), or ***(α = 0.1%). In the case of Ni in planta data, the 3 µM treatment was used as a control for statistical analysis because of the relatively high variability of the 0 µM treatment. Pseudomonas syringae pv. maculicola zinc tolerance mutants show differential growth in Thlaspi caerulescens
The importance of metal tolerance for bacteria growing in metal-hyperaccumulating T. caerulescens was assessed by screening a transposon mutant library of Psm to identify mutants with increased or decreased zinc tolerance relative to wild-type Psm. The performance of four representative mutants in planta was then compared to that of the wild-type strain under four zinc regimes. Two mutants with increased zinc tolerance (9A6 and 9A3) and two with reduced zinc tolerance (10C1 and 7C11) were used (Figure 6). These four mutants grew similarly to wild-type Psm in A. thaliana (Figure S4) and were able to cause similar symptoms to wild-type Psm in A. thaliana and pak choi, Brassica rapa ssp. chinensis (data not shown). Mutant 7C11 showed slightly reduced growth relative to wild-type Psm during late log phase and stationary phase in in vitro growth assays in KB broth (Figure S5), but the in vitro growth kinetics of the other three mutants were not significantly different from wild-type Psm.
Fig. 6. Zinc tolerance of P. syringae pv. maculicola M4 and four mutants. 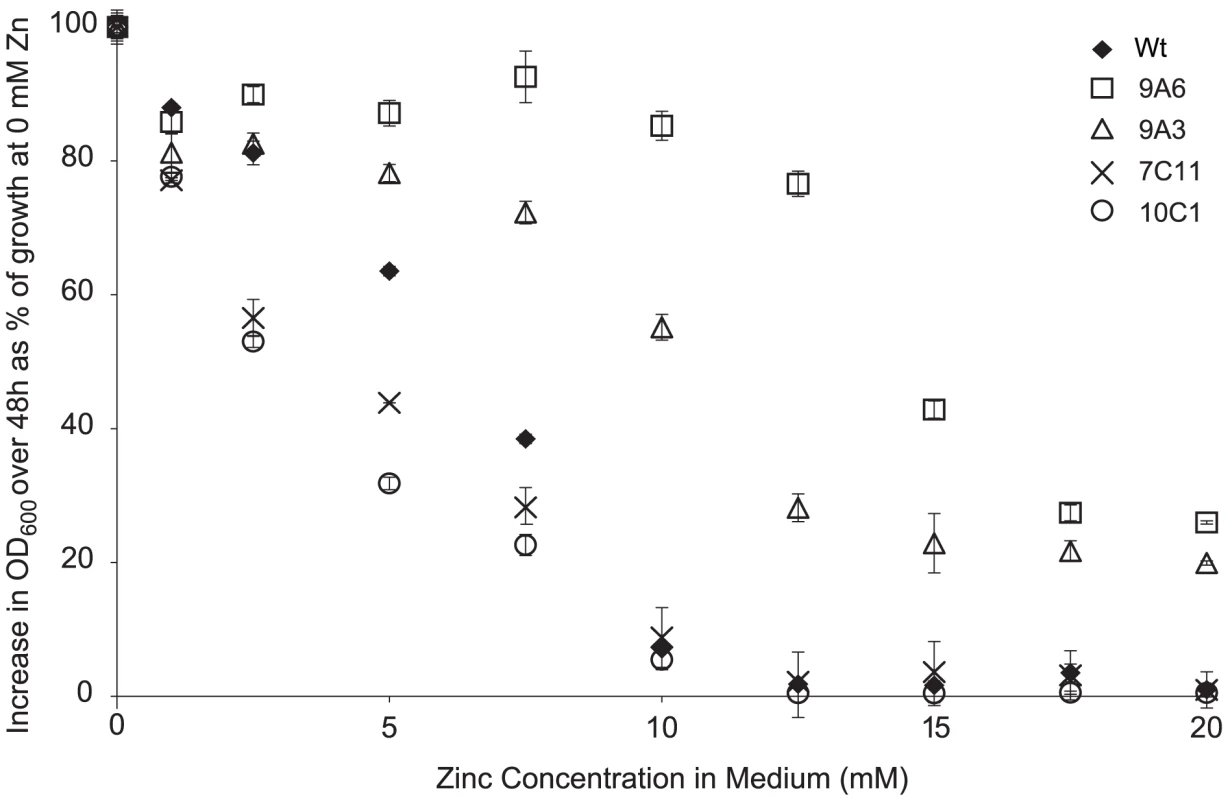
Five µl of bacterial suspension at an OD600 of 0.2 were inoculated into 200 µl of KB broth supplemented with zinc at 0 to 20 mM. The graph shows the percentage increase in OD600 48 hours after inoculation, relative to the increase in OD600 over 48 hours observed for the same strain in the absence of zinc. At least four samples were analysed for each treatment. Values are means ± SE (n = 8). The disrupted genes were sequenced using an inverse PCR method and their predicted functions are described in Table 2. Interestingly, mutant 7C11 was found to contain an insertion in a TonB-dependent siderophore receptor (PSPTO_2152), which suggests that the slight growth defect observed in KB broth for this strain may be linked to impaired iron acquisition. The other mutation giving rise to reduced zinc tolerance, in mutant 10C1, was located in an NAD-dependent DNA ligase gene (PSPTO_0382); this does not have an obvious role in metal transport or metal tolerance, but is located close to two operons predicted to encode a heavy-metal-sensing two component regulatory system and components of a cobalt-zinc-cadmium (Czc) cation efflux system (PSPTO_0375 – PSPTO_0379) in the genome of P. syringae pv. tomato DC3000. The two mutations giving rise to increased zinc tolerance were located in pslF (PSPTO_3533; 9A3), a gene within the psl operon, involved in exopolysaccharide synthesis and biofilm formation in Pseudomonas aeruginosa [32], and in a proline iminopeptidase (pip) gene (PSPTO_5164; 9A6). The pip gene of Xanthomonas campestris pv. campestris has been shown to be induced during plant colonisation and to be essential for pathogenesis on cabbage [33]. However, our results indicate that the pip gene of Psm is not required for pathogenesis in A. thaliana. Pip belongs to a family of metalloproteases and its enzymatic activity may be dependent on a metal cofactor such as zinc or cobalt. In the genome of P. syringae pv. tomato DC3000, pip is located upstream of, and in a putative operon with, a predicted D-Tyr-tRNAtyr deacylase, which may have a role in protecting cells against D-tyrosine toxicity. In E. coli, zinc and D-tyrosine have been shown to have opposite effects on the phosphatase activity of the aromatic amino acid biosynthesis regulator TyrR, which is stimulated by zinc and suppressed by L-tyrosine and D-tyrosine [34].
Tab. 2. <i>P. syringae</i> pv. maculicola M4 mutants with altered zinc tolerance. 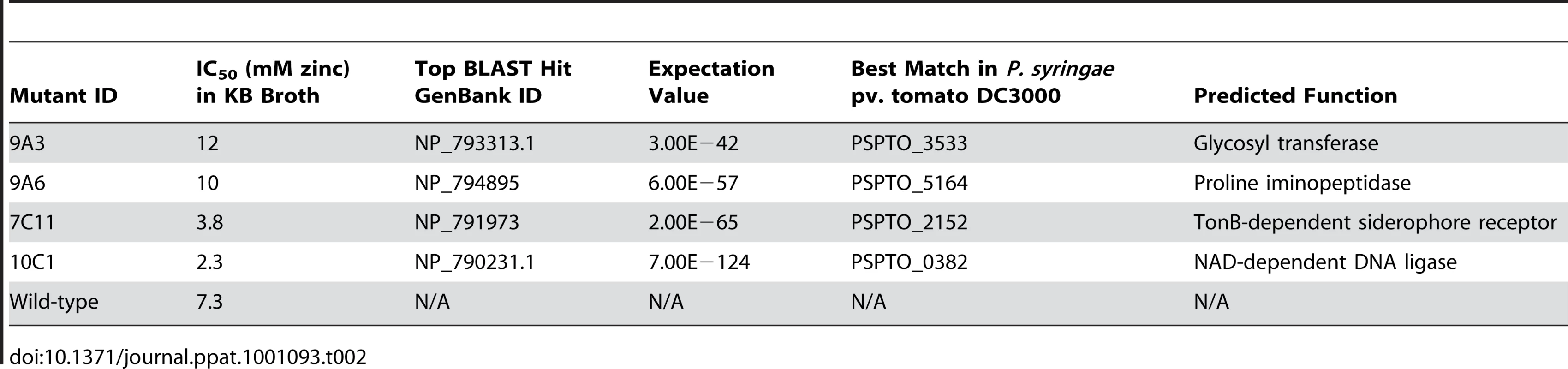
The ability of the four Psm mutants to grow in T. caerulescens plants cultivated on 0.04, 10, 30, or 300 µM zinc was found to vary according to the zinc tolerance of the inoculated strain (Figure 7). Thus, while the wild-type showed significant growth reduction with plant zinc treatments of 30 µM or higher, the two mutants with increased zinc tolerance, 9A6 and 9A3, were capable of growth in plants treated with 300 µM zinc. The two mutants with decreased zinc tolerance, 7C11 and 10C1, were unable to grow even in plants treated with 10 µM zinc, at which concentration the wild-type grew well.
Fig. 7. P. syringae pv. maculicola M4 mutants with altered zinc tolerance show differential growth in Thlaspi caerulescens. 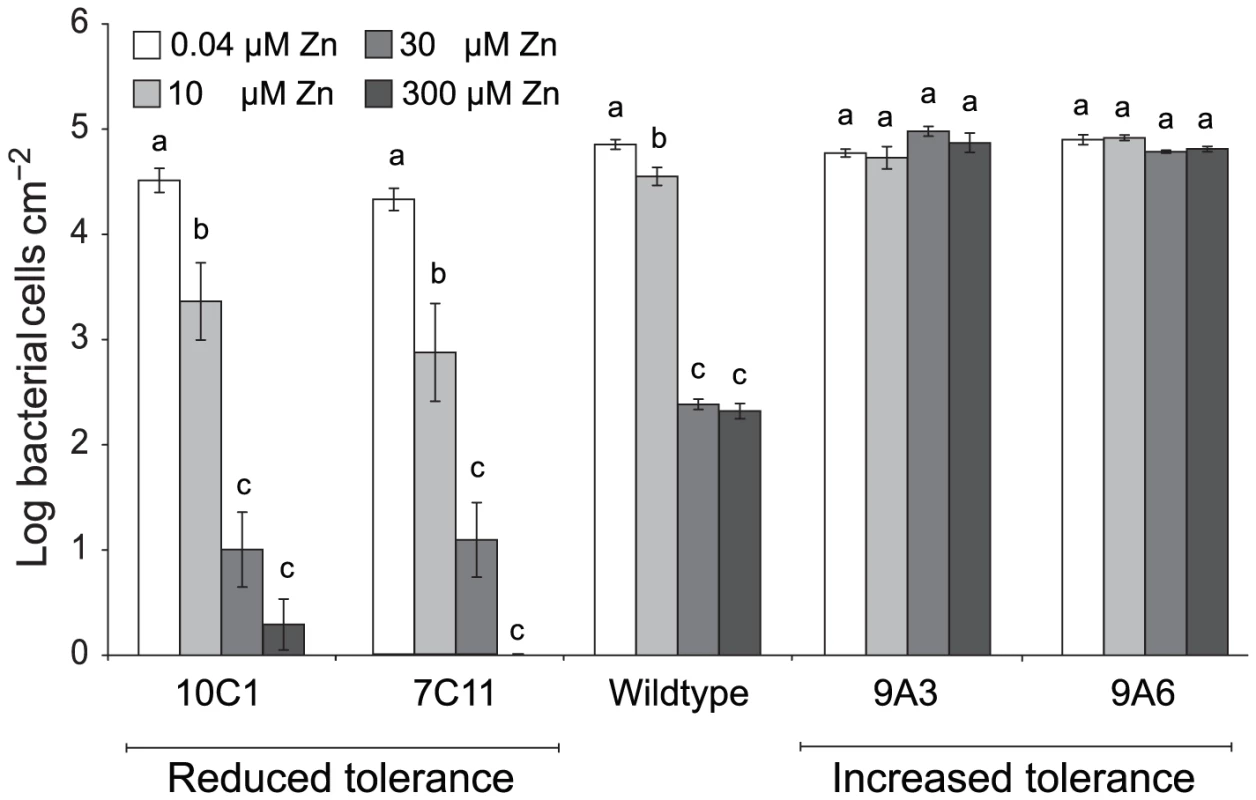
T. caerulescens plants were treated with 0.04, 10, 30, or 300 µM zinc. Nine leaves of three plants were infiltrated with either P. syringae pv. maculicola M4 or one of four zinc-tolerance mutants (9A6, 9A3, 7C11, or 10C1) suspended in 10 mM MgCl2 at 106 cfu/ml. Leaves were sampled for bacterial counts at 0 and 5 days after inoculation. Each replicate consisted of three leaves from one plant, giving a total of three replicates per time point and treatment. Values are means ± SE (n = 3). The experiment was performed three times with similar results, and the results shown are from one experiment representative of the three. ANOVAs were used to test for a significant effect of plant zinc treatment on the growth of each bacterial strain. No effect was detected for the high tolerance mutant 9A3 (P = 0.13). For the other strains, growth was found to be dependent on zinc (9A6: P = 0.01; wild-type, 7C11 and 10C1: P<0.0005 in each case). Where a significant effect was found, Bonferroni simultaneous comparisons (α = 5%) were carried out. Within each strain, means marked with the same letter were not significantly different. Because increased zinc tolerance could be correlated with reduced zinc uptake, we tested the ability of all four mutants to grow at low zinc concentrations. Wild-type Psm grew equally well in M9 minimal medium and in M9 supplemented with 1 to 10 µM Zn. Of the four mutants, only 9A6 had an increased zinc requirement relative to wild-type bacteria, showing optimal growth at concentrations ranging from 1 to 5 µM zinc.
Naturally occurring endophytes of Thlaspi caerulescens show high zinc tolerance
To test further the hypothesis that high metal tolerance is a prerequisite for bacterial growth in T. caerulescens, endophytic bacteria were collected from leaves of a natural population of T. caerulescens plants at Hafna Mine, a former lead–zinc mine in North Wales, UK [35], and their zinc tolerance assessed. The mean zinc content of the leaves of these plants was 16.2±1.39 g Zn per kg leaf dry mass (± s.e.m., n = 23 leaves), slightly greater (p<0.05) than that of plants treated with the highest concentration of zinc (300 µM) used in the laboratory experiment (13.7±0.71 g Zn per kg dry mass (± s.e.m., n = 18 leaves)). The average IC50 for zinc of these naturally occurring endophytes was 9.4 mM (n = 86) in KB medium. In contrast, a set of plant pathogenic bacteria isolated from non-hyperaccumulating plants were found to have a significantly (p<0.001) lower mean IC50 for zinc of 5.4 mM in KB (n = 7; Figure 8). When the zinc IC50 values for individual strains isolated from the Hafna mine plants were compared to the zinc IC50 of Psm, the most zinc tolerant of the plant pathogens used in this study, 65% were found to have a significantly higher zinc tolerance (p≤0.05), while only a single strain out of 85 had a significantly lower zinc tolerance. Therefore, in the field, as in the laboratory, only metal-tolerant bacteria can colonize T. caerulescens.
Fig. 8. Bacterial endophytes isolated from a natural population of Thlaspi caerulescens exhibit high zinc tolerance. 
To determine IC50 values for zinc, 5 µl of bacterial suspension at an OD600 of 0.2 were inoculated into 200 µl of KB broth supplemented with zinc at 0 to 20 mM. OD600 was measured after incubation, with continuous shaking, at 28°C for 48 hours, and IC50 values were calculated from the resulting dose–response curves. Values are means ± SE (n = 3). Diamonds = Hafna mine endophytes; triangles = plant pathogenic bacteria isolated from non-metal-accumulating crop plants; squares = mutants of P. syringae pv. maculicola generated in the present work. Abbreviations: DC3000, Pseudomonas syringae pv. tomato DC3000; X.c.c., Xanthomonas campestris pv. campestris 8004; P. cic 3109, Pseudomonas cichorii NCPPB3109; P. cic 907, P. cichorii NCPPB907; P. cic 943, P. cichorii NCPPB943; Ea286, Erwinia amylovora Ea286; B728a, P. syringae pv. syringae B728a. Discussion
In this work, we have developed a model system to study the elemental defense hypothesis for plant metal hyperaccumulation using the bacterial pathogen Pseudomonas syringae pv. maculicola M4. We have shown that Psm displays T3SS-dependent pathogenesis in Thlaspi caerulescens plants grown in low metal concentrations and that metal hyperaccumulation by T. caerulescens at higher metal concentrations provides an effective defense against Psm. Further, we have demonstrated that zinc tolerance is essential for bacterial colonization of zinc-hyperaccumulating plants. Thus, our work is consistent with the hypothesis that hyperaccumulation benefits plants by increasing their resistance to pathogens.
Although studies have found that zinc, nickel and cadmium are mainly stored in the leaf cell vacuoles of Thlaspi species, particularly in epidermal cells [27], [28], [36], [37], [38], metals are transported into the leaf through the extracellular spaces of the apoplast by means of the transpiration stream. By measuring metal concentrations in the apoplastic fluid, we were able to provide an approximation of the conditions experienced by invading pathogens in the leaves of T. caerulescens. Comparison of the metal concentrations in planta with the IC50 values measured for Psm in vitro in extracted apoplastic fluid allowed us to demonstrate that direct inhibition of bacterial growth by hyperaccumulated zinc or nickel is a realistic possibility. T. caerulescens, when grown on at least 10 µM Zn or 30 µM Ni, accumulated sufficient metal in apoplastic fluid to provide an elemental defense against Psm. This result is particularly striking considering that, when grown at 10 µM Zn, T. caerulescens accumulated only an average of 0.3 g Zn per kg dry mass, while the threshold for designation as a zinc hyperaccumulating plant is 10 000 mg per kg [1]. This indicates that T. caerulescens could be protected against pathogens by zinc accumulation even when growing on relatively low-zinc soils. However, although T. caerulescens accumulates sufficiently high metal concentrations to account for its metal-dependent resistance to Psm, we cannot rule out the possibility that additional metal-dependent factors act in conjunction with metals to limit the growth of Psm in T. caerulescens. Accumulation of metals requires mechanisms by which the plant may tolerate elevated intracellular metal concentrations, which have not been fully elucidated, but which have been shown to involve redox-related compounds such as glutathione [39], enzymes such as superoxide dismutase [40], and metal-binding ligands such as organic acids and amino acids [27], [41], as well as proteins (e.g. metallothioneins [42]). Such changes may affect the quality of the plant environment for growth of Psm or zinc tolerance in Psm, without having any expressly defensive function.
One case in which additional factors seem likely to contribute to metal-dependent defenses is in plants treated with cadmium. Cadmium was able to defend T. caerulescens against Psm in planta when plants were treated with ≥10 µM cadmium. Bacterial growth was also inhibited for around 18 hours in apoplastic fluid extracted from these plants. After this time, inhibition was released, possibly as a result of changes in bacterial gene expression or in the composition of the apoplast during this time in vitro, which may include alterations in cadmium bioavailability. However, the cadmium concentrations detected in apoplast extracts from cadmium-treated plants were lower than those required for inhibition of bacterial growth in apoplast extracts from plants grown in the absence of cadmium, so it is possible that cadmium concentrations are correlated with another defensive factor which may be unstable (such as ROS) or volatile.
To investigate further the possibility of a true elemental defense, we tested the importance of zinc tolerance for bacterial growth in planta in zinc-treated T. caerulescens. The results obtained using mutants of Psm provide the clearest evidence to date of a direct role for zinc as an elemental defense against pathogens in T. caerulescens. Mutants with reduced zinc tolerance were unable to multiply in plants grown at 10 µM zinc, in leaves of which their wild-type counterpart was successful; conversely, mutants with increased zinc tolerance grew well in leaves of plants grown at 30 µM or even 300 µM zinc, in which the wild-type could not survive. This clear link between bacterial zinc tolerance and ability to colonize plants hyperaccumulating zinc provides strong support for the concept of an elemental defense by zinc in T. caerulescens.
Finally, we have compared the zinc tolerance of strains used in this work with that of bacteria isolated from the leaves of T. caerulescens plants growing under natural conditions in a zinc-polluted field site. We have shown that bacteria naturally colonizing these plants in the field had a range of zinc tolerances much higher than those of plant pathogenic strains isolated from non-accumulating crop plants, providing evidence for local adaptation of these endophytes to their environment [43], [44], [45]. This is in agreement with previous studies demonstrating that endophytic bacteria isolated from nickel hyperaccumulators exhibit high nickel tolerance [46], [47]. Plant pathogens that have not been subject to this selection may find it difficult to grow and cause disease in T. caerulescens.
Metal-dependent resistance in T. caerulescens may be particularly effective against airborne, foliar pathogens such as P. syringae and powdery mildew, which may be deposited onto the surface of T. caerulescens leaves by rain and wind having previously colonized non-accumulating or metal-excluding plants, with no prior selection for metal tolerance. Thus, hyperaccumulated metals may be functionally equivalent to the diverse array of anti-microbial secondary metabolites used by plants to provide protection against infection. Only pathogens that are able to tolerate or inhibit the chemical defenses present in a specific plant species or genotype can grow in plant tissues. Similarly, in the case of metal-hyperaccumulation, only a small number of organisms – those possessing high metal tolerance – are able to grow in these plants. When the plants are deprived of this form of defense by cultivation on a low-metal growth medium, they may become vulnerable to a wider range of pathogens, explaining the spontaneous outbreaks of mildew infection observed on such plants (Figure 1).
When considering the role of metals in protecting plants against infection in a natural setting it is important to note that metal concentrations in the leaves of hyperaccumulating plants are typically orders of magnitude higher than metal concentrations in the environment, as illustrated in Figure 5. Therefore, even though the environment surrounding these plants may favour the growth of moderately metal tolerant microorganisms, many of these organisms may have insufficient metal tolerance to be able to grow in the tissues of hyperaccumulating plants. In addition, the observation that T3SS mutants of Psm were unable to infect T. caerulescens plants grown on low metal concentrations indicates that the plants possess additional defense mechanisms that act in conjunction with metal hyperaccumulation to protect plants, but which can be suppressed by the action of T3SS effectors. Thus, successful pathogens of T. caerulescens must not only be adapted for growth in a metal-rich environment, but must also possess at least some of the pathogenicity mechanisms known to be required for infection of non-accumulating plants such as Arabidopsis thaliana.
We have demonstrated that hyperaccumulation of any of three metals, zinc, nickel, or cadmium, by T. caerulescens provides the plant with an elemental defense against the hemibiotrophic pathogen Psm. The validity of the defense hypothesis has been challenged by some studies in which no evidence was found that metal hyperaccumulation defended T. caerulescens from herbivory in the field [13], [14]. Our work, however, suggests that the defensive effect of metal hyperaccumulation against pathogens remains relevant in field conditions, with only metal-tolerant bacteria found growing naturally in the leaves of T. caerulescens. Further, we have shown that metal concentrations in the leaves are sufficient to account for this defensive effect without invoking any other factors. For all of the metals hyperaccumulated by T. caerulescens, we have shown that growth is also inhibited in apoplastic fluid, and that both zinc and nickel are found in this specific compartment at concentrations sufficient to account for the defensive effect. Moreover, we have demonstrated that the zinc tolerance of Psm mutants is correlated with their ability to colonize zinc-hyperaccumulating T. caerulescens plants. This result is mirrored by our findings concerning the zinc tolerance of natural endophytes of T. caerulescens from a zinc-rich field site. We therefore believe that metal hyperaccumulation by T. caerulescens can provide an effective form of defense against a wide range of pathogens.
Materials and Methods
Plant material
Seeds of Thlaspi caerulescens J. & C. Presl from Prayon, Belgium (provided by A.J.M. Baker and C. Lefèbvre) were cultured hydroponically on modified 0.1-strength Hoagland solution [20] in a glasshouse. The Prayon population of T. caerulescens was chosen as it is a widely studied, well characterized population showing typical hyperaccumulation behavior and producing relatively large amounts of biomass under the growth conditions described [20], [22], [24], [25]. Natural radiation was supplemented by sodium-vapor lamps for 14 hours per day. Night temperature was maintained at 14°C and day temperature at a minimum of 24°C. Two-week-old plants were transferred to modified 0.1-strength Hoagland solution containing 0.04, 10, 30, or 300 µM ZnSO4, or 0, 3, 10, or 30 µM NiSO4 or CdSO4. The lowest zinc concentration used was 0.04 µM, rather than 0 µM for Ni and Cd. This is because zinc is an essential micronutrient without which the plants do not survive. The Hoagland solution used for all Ni and Cd assays contained 10 µM Zn for this reason; at 0.04 µM Zn, signs of zinc deficiency become apparent. T. caerulescens plants were grown on these metal treatments for a further 8 weeks, the nutrient solution being exchanged fortnightly for the first 6 weeks and weekly for the final 2 weeks. Arabidopsis thaliana (L.) Heynh. (Col-0) were sown on peat-based compost and grown in a glasshouse under the same conditions for 6 weeks.
Bacterial growth conditions, media and antibiotics
Bacterial strains used in pathogenicity assays are listed in Table S1. Psm ES4326, Psm hrpN− and Psm hrpS− were provided by K. Schreiber and D. Desveaux. Psm hrpN− and Psm hrpS− were isolated from a transposon library of Psm ES4326 constructed using a kanamycin-resistant derivative of mini-Tn5 ([48]; D. Desveaux, personal communication). All bacterial strains were streaked onto Luria–Bertani (LB) agar [49] from stocks kept in glycerol at −80°C and incubated at 28°C (37°C for Escherichia coli) for 24 to 48 hours prior to use. Single colonies were transferred to LB broth and incubated at 28°C with shaking for most applications. King's B (KB) agar [50], supplemented with CFC (Cetrimide-Fucidin-Cephalosporin; Oxoid) at half the manufacturer's recommended concentration, was used to selectively culture bacteria isolated from plant tissues for in planta growth studies. LB and KB broths were used in metal-tolerance experiments. For zinc-requirement assays, the minimal medium M9 [49] was used.
Bacterial growth in vitro
To compare the in vitro growth of wild-type and mutant strains of Psm, bacteria were grown overnight on LB agar and resuspended in KB broth to give an OD600 of 0.1. One hundred microliters of this suspension were added to a further 100 µl of media in each of 16 wells of a 96-well microplate. Sixteen wells were inoculated with media alone as a media control. OD was measured every 20 min for the next 48 hours using an Infinite M200 plate reader (Tecan Group Ltd., Männedorf, Switzerland).
Pathogenicity assays and bacterial growth in planta
For assays to examine the ability of bacteria to cause disease symptoms in T. caerulescens, bacteria were grown overnight on LB agar and re-suspended in sterile 10 mM MgCl2 at an optical density at 600 nm (OD600) of 0.35. Bacterial suspensions were infiltrated into at least three fully expanded leaves from 6-week-old T. caerulescens plants grown on nutrient solution (10 µM zinc) or 6-week-old A. thaliana using a blunt 1 ml syringe. Symptoms resulting from bacterial inoculation were monitored over 7 days. For growth assays, bacteria were resuspended in sterile 10 mM MgCl2 at an OD600 of 0.2. This suspension was diluted 100-fold to give a suspension of approximately 106 cfu/ml and infiltrated into fully expanded T. caerulescens or A. thaliana leaves through the abaxial surface using a blunt 1 ml syringe. Nine leaves on each of six plants were inoculated within each metal treatment. Leaf discs of 10-mm diameter were taken from three of the inoculated leaves immediately, and from three further leaves at 2 and 5 days after inoculation. Leaf discs were homogenized in 10 mM MgCl2 and the resulting suspension spread onto agar plates with a minimum of three technical replicates used for each sample. After incubation at 28°C for 48 hours, the number of bacterial colonies was counted and used to estimate the number of bacterial cells per unit area of leaf.
Apoplast extracts
Apoplastic fluid was extracted from T. caerulescens and A. thaliana leaves by a modification of the method described by Rico and Preston [51], using vacuum infiltration of the intercellular spaces with distilled water followed by centrifugation to extract apoplastic fluid. This fluid was centrifuged for a further 10 minutes at 4°C, filter-sterilized, and stored at −80°C. The degree of apoplast dilution was estimated as described by Rico and Preston [51]. For bacterial growth experiments, apoplast extract was freeze-dried and resuspended in an appropriate volume of distilled water to return it to its estimated concentration in planta.
Bacterial growth in apoplast extracts
Apoplast extracts from T. caerulescens plants were used as a substrate for the growth of Psm in vitro. Six 100 µl samples of apoplast extract from each metal treatment were aliquoted into a 96-well microwell plate and inoculated with 5 µl of Psm suspended in 10 mM MgCl2 to give a final OD600 of 0.05. The plate was incubated at 28°C with shaking and the OD600 measured at 20-minute intervals for 24 hours using the plate reader.
Metal content of whole-leaf and apoplast extracts of Thlaspi caerulescens
For determination of whole-leaf metal concentrations, fresh leaf material was oven-dried at 80°C for 48 hours. Subsamples of 50 mg of dried leaf material were digested in 3 ml of concentrated (69%, v/v) nitric acid for 16 hours in glass vials. Samples were then diluted 10-fold with ultrapure water and filtered using Whatman grade 3 filter paper. Metal contents of samples were measured in an air–acetylene flame by atomic absorption spectrophotometry using a double-beam optical system with deuterium arc background correction (AAnalyst 100; Perkin-Elmer, UK). Samples were further diluted as necessary to fall within the linear range of calibration curves prepared using appropriate standard solutions and reagent blanks. The accuracy of the calibration curves was validated using Certified Reference Material LGC7162 (strawberry leaves; LGC Standards, Teddington, UK). Three technical replicates were analysed for each measurement, and two samples from each of three plants were analysed for each metal treatment. Measurements of the fresh and dry biomass of 24 individual T. caerulescens plants were used to provide an average ratio between fresh and dry biomass, which allowed the metal content to be expressed as an approximate molar concentration in the fresh tissue. Apoplast samples were diluted appropriately with ultrapure water and measured without further treatment.
Metal-tolerance assays
The metal tolerance of Psm in vitro was tested in LB. A 5 µl aliquot of an overnight liquid culture was added to 200 µl of media supplemented with zinc, nickel, or cadmium at a range of concentrations. OD600 was read using the plate reader. Metal tolerance was also tested in apoplast extracted from T. caerulescens plants grown in 0.1-strength Hoagland solution (10 µM zinc). For these experiments, 2 µl of an overnight culture of P. syringae pv. maculicola M4 was added to 75 µl of apoplast extract or apoplast extract supplemented with metal.
Transposon mutagenesis of Pseudomonas syringae pv. maculicola M4
Transposon mutagenesis was carried out using the transposon miniTn5::gfp::lux cloned into pGP704 (generous gift of Phil Hill). The transposon was introduced into Psm by triparental mating using the helper plasmid pRK2013 [52]. The resultant bacterial mixture was spread onto LB agar containing 50 µg/ml kanamycin and 50 µg/ml rifampicin. Resulting colonies were inoculated into 150 µl of KB broth in 96-well plates and incubated at 28°C overnight. Thirty microliters of 50% (v/v) glycerol was then added to each well and plates were stored at −80°C as a mutant library.
Screening the Pseudomonas syringae pv. maculicola mutant library for zinc tolerance mutants
Mutants were screened in a four-stage process. In round one, mutants were grown in KB in 96-well plates in which a wild-type was also included. A 5 µl aliquot of overnight culture was transferred to 200 µl of KB in a black, clear-bottomed 96-well plate and incubated at 28°C in the plate reader. Plates were maintained at 28°C with continuous shaking and the OD600 and luminescence from each well was read at hourly intervals. After 3.5 hours, cultures were supplemented with 5 µl of 0.5 mM ZnSO4. OD600 and luminescence were read immediately and then at intervals of 3 to 9 hours for the next 48 hours. Changes in luminescence after the addition of zinc were recorded and growth was compared to wild-type.
A total of 866 strains were selected with markedly increased or decreased tolerance to zinc shock. Each of these mutants was transferred to 200 µl KB in two separate wells of a 96-well plate and growth was monitored with and without 0.5 mM zinc over 48 hours. Of these, 134 mutants whose tolerance differed notably from the wild-type were selected and further screened for growth in 200 µl KB in 96-well plates with zinc concentrations from 0 to 3.5 mM for 48 hours. IC50 values were then calculated and compared to wild-type. These results were validated in a second experiment for ten mutants that showed the largest consistent changes in zinc tolerance compared to wild-type. These ten mutants were then tested for their ability to cause symptoms in T. caerulescens, A. thaliana and pak choi (Brassica rapa spp. chinensis).
Inverse-PCR based sequencing of transposon mutants
Genomic DNA was extracted using a DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer's protocol for Gram negative bacteria. Eight microliters of the subsequent DNA suspension were digested with 1 µl of either the restriction endonuclease SphI or NarI (New England BioLabs) and 1 µl of the appropriate 10× buffer according to the manufacturer's specifications. NarI cleaves the transposon DNA near the 3′ end, while SphI cleaves it towards the 5′ end; both also cleave the Psm genome frequently. Digested DNA was ethanol-precipitated, resuspended in 8 µl of ultrapure water, and self-ligated overnight at 14°C using 1 µl of T4 ligase and 1 µl of 10× buffer (New England BioLabs). One µl of circularized DNA was then used as a template for inverse PCR. Primers designed to the ends of the short fragments of transposon resulting from NarI (AACAATCTAGCGAGGGCTTGGTAAGGTGATCC and CTTGCAGTGGGCTTACATGACGATAGCTAGAC) or SphI (GGAACGCCGCAGGAATG and CAGCAGCTGTTACAAACTCAAGAAG) digestion, and facing outwards, were used to amplify the flanking DNA. This was then sequenced using a primer to the end of the transposon (CGGTTTACAAGCTAAAGCTTGC for NarI-digested DNA and either CTTCTTTAAAATCAATACC or TTCCAGTAGTGCAAATAA for SphI-digested DNA).
Assessment of zinc tolerance of naturally occurring endophytes of Thlaspi caerulescens from a zinc-rich site
Leaves of T. caerulescens were collected in the field at Hafna mine (Snowdonia, North Wales, UK: 53°07′N, 3°49′W). They were then returned to the laboratory and immediately surface-sterilized by immersion in 10% (w/v) sodium hypochlorite for 3 minutes, followed by immersion in 100% ethanol for 3 minutes. Sterile leaves were rinsed in ultrapure water and macerated in 1 ml of 10 mM MgCl2 solution. The resulting suspension was plated onto KB-CFC agar. Plates were incubated at 28°C for 48 hours. Resultant colonies were transferred to 150 µl of KB broth in 96-well plates. After 48 hours of growth at 28°C, 30 µl of 50% (v/v) glycerol was added to each well and the plates stored at −80°C. For zinc tolerance experiments, bacteria were grown in 200 µl KB for 48 hours and replicated into 200 µl KB supplemented with 0, 1, 2.5, 5, 7.5, 10, 12.5, 15, or 20 µM ZnSO4. Bacterial growth at each of these zinc concentrations was determined by measuring OD600 at 0 and 48 hours using the plate reader. Bacterial growth at 48 hours was then plotted against zinc concentration, from which the concentrations giving half-maximal inhibition (IC50) were estimated.
Quantitative RT-PCR of PR-1 gene expression
RNA for qRT-PCR analysis was isolated from leaves of 10-week-old T. caerulescens grown on either 0.04 or 300 µM zinc and from leaves of 6-week-old, non flowering A. thaliana. A. thaliana leaves inoculated with Psm at 106 cfu/ml and incubated for 24 hours under standard plant growth conditions were used as a positive control for PR-1 expression. Leaves were snap frozen in liquid nitrogen and RNA was extracted using the RNeasy kit (Qiagen) according to the manufacturer's instructions. After elution, RNA was precipitated in 100 µl of 8 M LiCl overnight. After two washes in 70% (v/v) ethanol, pellets were resuspended in 30 µl ultrapure water. RNA concentration was measured using a Nanodrop-1000 spectrophotometer (Thermo Scientific) and integrity was checked by electrophoresis on an ethidium bromide gel. cDNA was prepared from 1 µg RNA using the Bioline cDNA synthesis kit with oligo dT primer according to the supplier's instructions. qRT-PCR was performed using SYBR green PCR master mix (Applied Biosystems) in a 7300 Realtime PCR machine (Applied Biosystems) and analysed by calibration to a standard curve of gene expression created from pooled cDNA from all samples under test, using the 7300 SDS system software v1.3.1 (Applied Biosystems). Four control genes were analysed in the same way, and PR-1 gene expression normalized to the geometric mean of the expression of these genes [53]. Gene-specific primers used are listed in Table 3.
Tab. 3. Primers used in qRT-PCR. 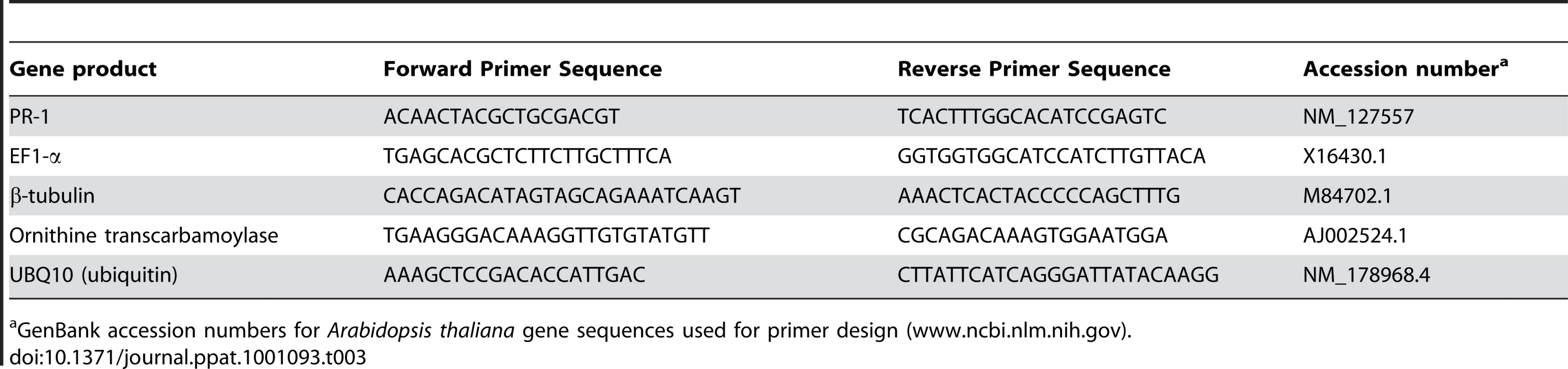
GenBank accession numbers for Arabidopsis thaliana gene sequences used for primer design (www.ncbi.nlm.nih.gov). Accession numbers
The GenBank (http://www.ncbi.nlm.nih.gov) accession numbers for the genes and gene products discussed in this paper are: PSPTO_0382 (NP_790231.1); PSPTO_2153 (NP_791973); PSPTO_3533 (NP_793313.1); PSPTO_5164 (NP_794895).
Acknowledgments
We thank Prof. A.J.M. Baker for providing seeds of Thlaspi caerulescens; Prof. J.L. Dangl for providing Pseudomonas syringae pv. maculicola M4, Dr. K. Schreiber and Dr. D. Desveaux for providing hrp mutants of Pseudomonas syringae pv. maculicola ES4326, Dr. P. Hill for proving the transposon used for mutagenesis, and Prof. J.A. Langdale, Prof. N.P. Harberd and Dr. A. Buckling for helpful discussions.
Supporting Information
Zdroje
1. BakerAJM
BrooksRR
1989 Terrestrial higher plants which hyperaccumulate metallic elements – a review of their distribution, ecology and phytochemistry. Biorecovery 1 81 126
2. ReevesRD
BakerAJM
2000 Metal-accumulating plants.
RaskinI
EnsleyBD
Phytoremediation of Toxic Metals: Using Plants to Clean-up the Environment New York John Wiley & Sons 193 230
3. BrooksRR
1998 Plants that Hyperaccumulate Heavy Metals. Wallingford CAB International 380
4. VerbruggenN
HermansC
SchatH
2009 Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181 759 776
5. PollardAJ
PowellKD
HarperFA
SmithJAC
2002 The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci 21 539 566
6. BoydRS
MartensSN
1992 The raison d'être for metal hyperaccumulation by plants.
BakerAJM
ProctorJ
ReevesRD
The Vegetation of Ultramafic (Serpentine) Soils Andover Intercept Limited 279 289
7. PoschenriederC
TolràR
BarcelóJ
2006 Can metals defend plants against biotic stress? Trends Plant Sci 11 288 295
8. BoydRS
2007 The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant Soil 293 153 176
9. VeskPA
ReichmanS
2009 Hyperaccumulators and herbivores – a Bayesian meta-analysis of feeding choice trials. J Chem Ecol 35 289 296
10. HansonB
LindblemSD
LoefflerML
Pilon-SmithEAH
2004 Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol 162 655 662
11. BehmerST
LloydCM
RaubenheimerD
Stewart-ClarkJ
KnightJ
2005 Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Funct Ecol 19 55 66
12. JiangRF
MaDY
ZhaoFJ
McGrathSP
2005 Cadmium hyperaccumulation protects Thlaspi caerulescens from leaf feeding damage by thrips (Frankliniella occidentalis). New Phytol 167 805 814
13. NoretN
MeertsP
TolràR
PoschenriederC
BarcelóJ
2005 Palatability of Thlaspi caerulescens for snails: influence of zinc and glucosinolates. New Phytol 165 763 772
14. NoretN
MeertsP
VanhaelenM
Dos SantosA
EscarréJ
2007 Do metal-rich plants deter herbivores? A field test of the defence hypothesis. Oecologia 152 92 100
15. JheeEM
BoydRS
EubanksMD
2005 Nickel hyperaccumulation as an elemental defense of Streptanthus polygaloides (Brassicaceae): influence of herbivore feeding mode. New Phytol 168 331 344
16. GhaderianYSM
LyonAJE
BakerAJM
2000 Seedling mortality of metal hyperaccumulator plants resulting from damping off by Pythium spp. New Phytol 146 219 224
17. BoydRS
ShawJJ
MartensSN
1994 Nickel hyperaccumulation defends Streptanthus polygaloides (Brassicaceae) against pathogens. Am J Bot 81 294 300
18. ReevesRD
SchwartzC
MorelJL
EdmondsonJ
2001 Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France. Int J Phytorem 3 145 172
19. BakerAJM
ReevesRD
HajarASM
1994 Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J & C Presl (Brassicaceae). New Phytol 127 61 68
20. RoosensN
VerbruggenN
MeertsP
Ximénez-EmbúnP
SmithJAC
2003 Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ 26 1657 1672
21. HammondJP
BowenHC
WhitePJ
MillsV
PykeKA
2006 A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol 170 239 260
22. AssunçãoAGL
SchatH
AartsMGM
2003 Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159 351 360
23. CobbettC
2003 Heavy metals and plants – model systems and hyperaccumulators. New Phytol 159 289 293
24. PeerWA
MahmoudianM
FreemanJL
LahnerB
RichardsEL
2006 Assessment of plants from the Brassicaceae family as genetic models for the study of nickel and zinc hyperaccumulation. New Phytol 172 248 260
25. MilnerMJ
KochianLV
2008 Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot 102 3 13
26. DebenerT
LehnackersH
ArnoldM
DanglJL
1991 Identification and molecular mapping of a single Arabidopsis thaliana locus determining resistance to a phytopathogenic Pseudomonas syringae isolate. Plant J 289 302
27. KüpperH
ZhaoFJ
McGrathSP
1999 Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119 305 311
28. KüpperH
MijovilovichA
Meyer-KlauckeW
KroneckPMH
2004 Tissue - and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol 134 748 757
29. CosioC
DeSantisL
FreyB
DialloS
KellerC
2005 Distribution of cadmium in leaves of Thlaspi caerulescens. J Exp Bot 56 765 775
30. DongX
MindrinosM
DavisKR
AusubelFM
1991 Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3 61 72
31. ChmielowskaJ
VelosoJ
GutiérrezJ
SilvarC
DíazJ
2009 Cross-protection of pepper plants stressed by copper against a vascular pathogen is accompanied by the induction of a defence response. Plant Sci 178 176 182
32. ByrdMS
SadovskayaI
VinogradovE
LuHP
SprinkleAB
2009 Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol 73 622 638
33. ZhangLL
JiaYT
WangL
FangRX
2007 A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol Microbiol 65 121 136
34. ZhaoS
ZhuQ
SomervilleRL
2000 The σ70 transcription factor TyrR has zinc-stimulated phosphatase activity that is inhibited by ATP and tyrosine. J Bacteriol 182 1053 1061
35. BennettJ
VernonRW
1990 Mines of the Gwydyr Forest: Part 2. The Hafna Mine, Llanrwst and some early ventures in Gwydyr Nant Cuddington, Cheshire, UK Gwydyr Mines Publications
36. KrämerU
PickeringIJ
PrinceRC
RaskinI
SaltDE
2000 Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species. Plant Physiol 122 1343 1354
37. Vogel-MikušK
RegvarM
Mesjasz-PrzybyłowiczJ
PrzybyłowiczWJ
SimčicJ
2008 Spatial distribution of cadmium in leaves of metal hyperaccumulating Thlaspi praecox using micro-PIXE. New Phytol 179 712 721
38. WójcikM
VangronsveldJ
D'HaenJ
TukiendorfA
2005 Cadmium tolerance in Thlaspi caerulescens. II. Localization of cadmium in Thlaspi caerulescens. Env Exp Bot 53 163 171
39. Freeman
JL
PersansMW
NiemanK
AlbrechtC
PeerW
2004 Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 16 2176 2191
40. TuomainenMH
NunanN
LehesrantaSJ
TervahautaAI
HassinenVH
2006 Multivariate analysis of protein profiles of metal hyperaccumulator accessions. Proteomics 6 3696 3706
41. KrämerU
Cotter-HowellsJD
CharnockJM
BakerAJM
SmithJAC
1996 Free histidine as a metal chelator in plants that accumulate nickel. Nature 379 635 638
42. RoosensNH
LeplaeR
BernardC
VerbruggenN
2005 Variations in plant metallothioneins: the heavy metal hyperaccumulator Thlaspi caerulescens as a study case. Planta 222 716 729
43. FrankSA
1992 Models of plant pathogen coevolution. Trends Genet 8 213 219
44. KaweckiTJ
EbertD
2004 Conceptual issues in local adaptation. Ecol Lett 7 1225 1241
45. NuismerSL
GandonS
2008 Moving beyond common-garden and transplant designs: insights into the causes of local adaptation in species interactions. Am Nat 171 658 668
46. IdrisR
TrifonovaR
PuschenreiterM
WenzelWW
SessitschA
2004 Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Env Microbiol 70 2667 2677
47. BarzantiR
OzinoF
BazzicalupoM
GabbrielliR
GalardiF
2007 Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii. Microbial Ecol 53 306 316
48. AlexeyevMF
ShokolenkoIN
CroughanTP
1995 New mini-Tn5 derivatives for insertion mutagenesis and genetic engineering in Gram-negative bacteria. Can. J Microbiol 41 1053 1055
49. SambrookJ
RussellDW
2001 Molecular Cloning. New York Cold Spring Harbor Laboratory Press
50. KingEO
WardMK
RaneyDE
1954 Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 22 301 307
51. RicoA
PrestonGM
2008 Pseudomonas syringae pv. tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol Plant–Microbe Interact 21 269 282
52. FigurskiDH
HelinskiDR
1979 Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76 1648 1652
53. LarionovA
KrauseA
MillerW
2005 A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6 62
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



