-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
Borrelia burgdorferi, the Lyme disease spirochete, dramatically alters its transcriptome and proteome as it cycles between the arthropod vector and mammalian host. During this enzootic cycle, a novel regulatory network, the Rrp2-RpoN-RpoS pathway (also known as the σ54–σS sigma factor cascade), plays a central role in modulating the differential expression of more than 10% of all B. burgdorferi genes, including the major virulence genes ospA and ospC. However, the mechanism(s) by which the upstream activator and response regulator Rrp2 is activated remains unclear. Here, we show that none of the histidine kinases present in the B. burgdorferi genome are required for the activation of Rrp2. Instead, we present biochemical and genetic evidence that supports the hypothesis that activation of the Rrp2-RpoN-RpoS pathway occurs via the small, high-energy, phosphoryl-donor acetyl phosphate (acetyl∼P), the intermediate of the Ack-Pta (acetate kinase-phosphate acetyltransferase) pathway that converts acetate to acetyl-CoA. Supplementation of the growth medium with acetate induced activation of the Rrp2-RpoN-RpoS pathway in a dose-dependent manner. Conversely, the overexpression of Pta virtually abolished acetate-induced activation of this pathway, suggesting that acetate works through acetyl∼P. Overexpression of Pta also greatly inhibited temperature and cell density-induced activation of RpoS and OspC, suggesting that these environmental cues affect the Rrp2-RpoN-RpoS pathway by influencing acetyl∼P. Finally, overexpression of Pta partially reduced infectivity of B. burgdorferi in mice. Taken together, these findings suggest that acetyl∼P is one of the key activating molecule for the activation of the Rrp2-RpoN-RpoS pathway and support the emerging concept that acetyl∼P can serve as a global signal in bacterial pathogenesis.
Published in the journal: Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in. PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001104
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001104Summary
Borrelia burgdorferi, the Lyme disease spirochete, dramatically alters its transcriptome and proteome as it cycles between the arthropod vector and mammalian host. During this enzootic cycle, a novel regulatory network, the Rrp2-RpoN-RpoS pathway (also known as the σ54–σS sigma factor cascade), plays a central role in modulating the differential expression of more than 10% of all B. burgdorferi genes, including the major virulence genes ospA and ospC. However, the mechanism(s) by which the upstream activator and response regulator Rrp2 is activated remains unclear. Here, we show that none of the histidine kinases present in the B. burgdorferi genome are required for the activation of Rrp2. Instead, we present biochemical and genetic evidence that supports the hypothesis that activation of the Rrp2-RpoN-RpoS pathway occurs via the small, high-energy, phosphoryl-donor acetyl phosphate (acetyl∼P), the intermediate of the Ack-Pta (acetate kinase-phosphate acetyltransferase) pathway that converts acetate to acetyl-CoA. Supplementation of the growth medium with acetate induced activation of the Rrp2-RpoN-RpoS pathway in a dose-dependent manner. Conversely, the overexpression of Pta virtually abolished acetate-induced activation of this pathway, suggesting that acetate works through acetyl∼P. Overexpression of Pta also greatly inhibited temperature and cell density-induced activation of RpoS and OspC, suggesting that these environmental cues affect the Rrp2-RpoN-RpoS pathway by influencing acetyl∼P. Finally, overexpression of Pta partially reduced infectivity of B. burgdorferi in mice. Taken together, these findings suggest that acetyl∼P is one of the key activating molecule for the activation of the Rrp2-RpoN-RpoS pathway and support the emerging concept that acetyl∼P can serve as a global signal in bacterial pathogenesis.
Introduction
The enzootic life-cycle of Borrrelia burgdorferi is complex and typically involves transmission between an arthropod vector (Ixodes ticks) and a mammalian host (e.g., Peromyscus rodents) [1]. Accumulated evidence have shown that the alternative sigma factor RpoS plays a central role in this complex natural cycle of B. burgdorferi [2]–[8]. RpoS functions as a global regulator and governs differential expression of more than 10% of all B. burgdorferi genes, including the two major virulence genes ospA and ospC [9]–[13]. One unique feature about rpoS of B. burgdorferi is that its expression is directly controlled by the alternative second sigma factor RpoN (σ54) at a −24/−12 σ54-type promoter. Mutation within this promoter region or inactivation of rpoN that encodes the second alternative sigma factor RpoN (σ54) abolishes expression of rpoS and RpoS-dependent genes such as ospC [6], [8], [14]. This RpoN-dependent transcriptional activation appears to play a major role in modulating RpoS level in B. burgdorferi [3], [5]–[8], [14], [15]. In addition, a small RNA dsrA also has been shown to be involved in post-transcriptional regulation of RpoS [7].
RpoN(σ54)-dependent activation of transcription requires a highly conserved transcriptional activator, the so-called enhancer-binding proteins (EBPs) [16]. B. burgdorferi has a single EBP, Rrp2, a homolog of NtrC family [17], [18]. Members of NtrC family contain three putative functional domains: an N-terminal response regulator receiver domain, a central RpoN-activation domain, and a C-terminal helix-turn-helix (HTH) DNA-binding domain [19]. The central domain becomes activated upon phosphorylation at a conserved aspartic acid residue (corresponding to D52 in Rrp2) within the N-terminal receiver domain. The activated central domain then contacts the Eσ54-holoenzyme through DNA looping, hydrolyzes ATP, and promotes open promoter complex formation for transcriptional initiation. Although direct biochemical evidence remains lacking, genetic data indicates that Rrp2 is the activator for the σ54–σS cascade of B. burgdorferi. First, a single point mutation of glycine (G) residue 239 to cysteine (C) within one of the ATP-binding motifs in the central activation domain of Rrp2 abolishes expression of rpoS and RpoS-dependent genes [4], [18], [20]. Second, when a rpoS promoter-cat reporter and an inducible rrp2 gene were cloned into a surrogate E. coli system, the reporter was activated only upon induction of rrp2 [6]. Thus, Rrp2, RpoN, and RpoS appear to constitute a Rrp2-RpoN-RpoS pathway. Consistent with this notion, recent microarray analyses reveal that genes influenced by Rrp2, RpoN, or RpoS largely overlap [2]–[4], [20].
Given the importance of the Rrp2-RpoN-RpoS pathway to the infectious cycle of B. burgdorferi [3]–[5], [20], it is striking how little we know about the upstream event(s) that lead to its activation. Since Rrp2 is the upstream activator for the pathway, an understanding of the activation of Rrp2 is key to understand the mechanism of activation of this pathway. It is postulated that activation of Rrp2 is through a phosphorylation event by a cognate histidine kinase [21]–[23]. Because of the co-localization of rrp2 and hk2 in the genome (15) and because of the ability of Hk2 to phosphorylate Rrp2 in vitro [6], Hk2 is predicted to be the cognate histidine kinase for Rrp2. A recent study by Burtnick et al. [6], however, showed that an hk2 mutant remains capable of activating Rrp2 under in vitro cultivation conditions, indicating that the molecular mechanism activating the Rrp2-RpoN-RpoS pathway is more complex than previously envisioned. In addition, the contribution of Hk2 during the infectious cycle of B. burgdorferi remains unknown because the previous hk2 mutant lost an important endogenous plasmid (lp36) for mammalian infection [6].
Response regulators can be activated by factors other than their cognate histidine kinases. The best studied mechanisms are phosphorylation by non-cognate histidine kinases (a phenomenon called “cross-talk”) [24]–[28] and phosphorylation by small molecular weight high-energy donors, such as acetyl phosphate (acetyl∼P) or carbamoyl phosphate (carbamoyl∼P) [29]–[31]. While cross-talk appears to be quite rare (48), emerging evidence indicates that acetyl∼P can function in vivo as a global signal by donating its phosphoryl group to certain response regulators [32], [33]. B. burgdorferi possesses four predicted histidine kinases (Hk1, Hk2, CheA1, and CheA2) [17], [34] as well as pathways for the synthesis and degradation of both acetyl∼P and carbamoyl∼P [17]. Burtnick et al. [6] proposed that Hk2-independent activation of Rrp2 could be activated by receiving a phosphoryl group from a non-cognate histidine kinase or a small phosphorylated compound. However, this hypothesis has not been tested experimentally. In this study, we generated an hk2 mutant suitable for in vivo study and showed that Hk2 was not required for the activation of the Rrp2-RpoN-RpoS pathway under in vitro growth conditions or during murine infection. We further showed that cross-talk among two-component systems is not likely to account for Rrp2 activation. Rather, the results obtained support the hypothesis that acetyl∼P functions as an important phosphoryl donor for Rrp2, making this small molecule a key modulator of the activation of the Rrp2-RpoN-RpoS pathway in B. burgdorferi.
Results
Hk2 is not required for the activation of Rrp2-RpoN-RpoS pathway in mammalian host-adapted spirochetes or during murine infection
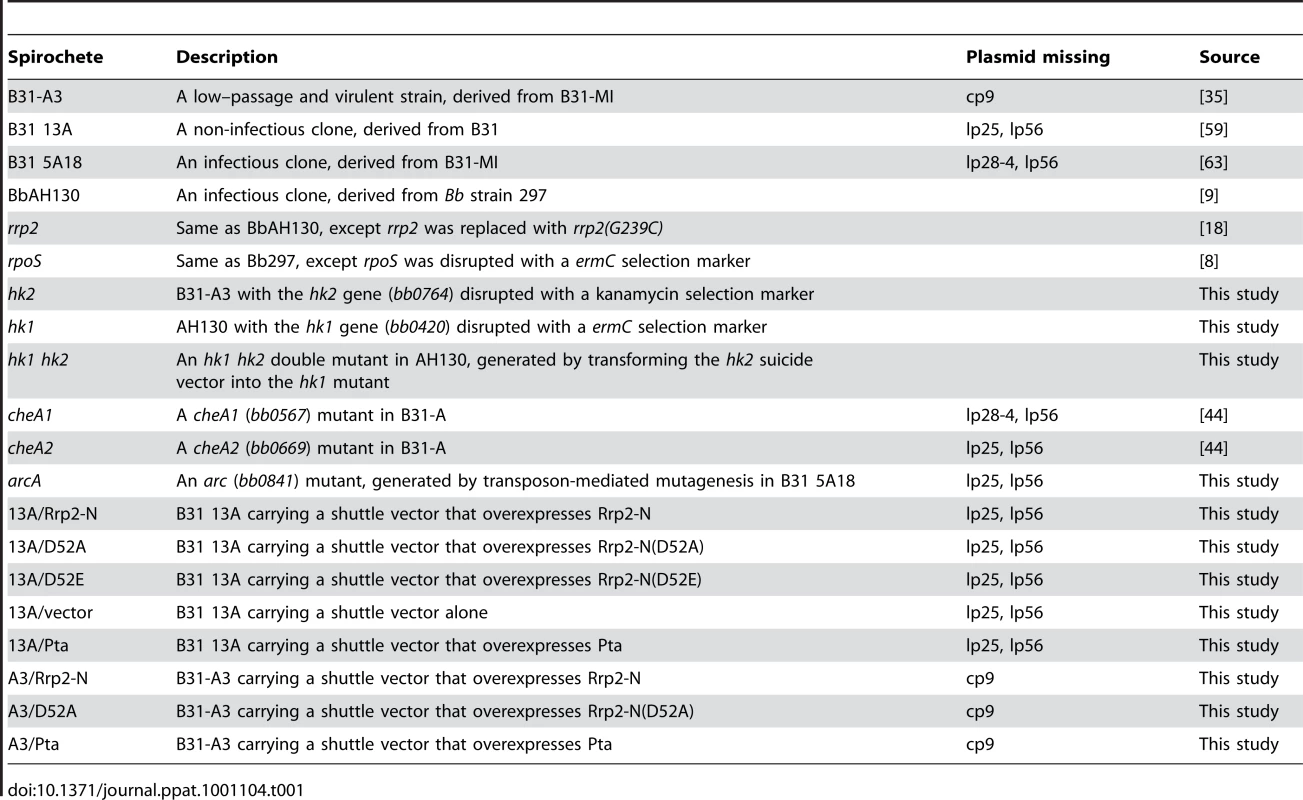
To study the mechanism of activation of the Rrp2-RpoN-RpoS pathway, we focused on the upstream activator Rrp2, a putative response regulator. Burtnick et al. [6] recently reported that inactivation of hk2, which encodes the putative cognate histidine kinase for Rrp2, did not affect activation of the Rrp2-RpoN-RpoS pathway when spirochetes were cultivated in vitro. However, this hk2 mutant was not phenotypically characterized in vivo [6]. Thus, we sought to generate an hk2 mutant suitable for in vivo study. A suicide vector harboring a disrupted hk2 region was transformed into the infectious B. burgdorferi strain B31-A3 (Fig. 1A) [35]. Disruption of hk2 in the transformants was confirmed by PCR (Fig. 1B) and the absence of Hk2 expression was verified by immunoblot analyses (Fig. 1C). Of note, inactivation of hk2 by the KanR cassette did not substantially affect expression of the protein encoded by the downstream gene, rrp2 (Fig. 1C). Three transformed clones were further subjected to plasmid profile analyses (data not shown). Two clones had a plasmid profile identical to that of parental wild-type B31-A3; one of these was designated hk2 and chosen for further study (Table 1).
Fig. 1. Construction and characterization of the hk2 mutant. 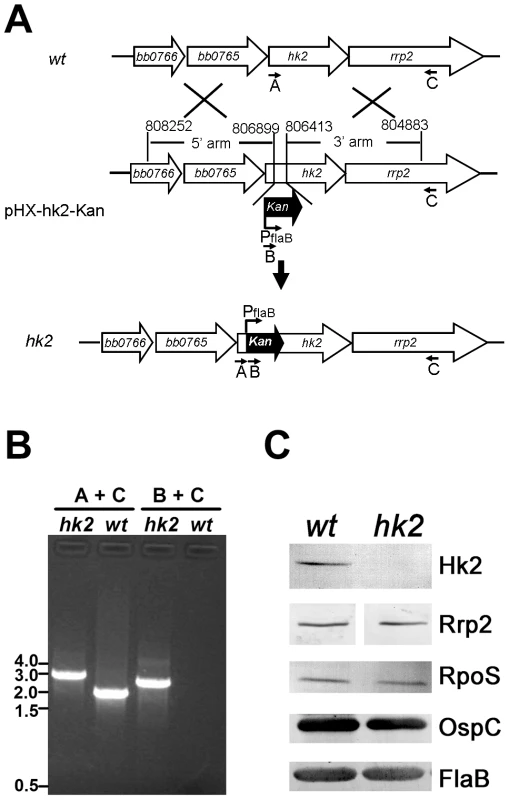
(A) Strategy for insertional inactivation of hk2. wt, genomic structure of hk2 and the surrounding region in wild-type B. burgdorferi. pHX-hk2-Kan, the suicide vector used for generating the hk2 mutant. Only the relevant portion of the plasmid is shown. hk2, the diagram showing the genomic structure of the hk2 mutant. Small labeled arrows denote positions of oligonucleotide primers used for PCR analyses. (B) Confirmation of the hk2 mutant by PCR analyses. Letter combinations denote primer pairs used for PCR. kb: kilobase DNA ladder, wt, wild type strain; hk2, hk2 mutant. (C) Immunoblot analyses of the hk2 mutant. Cultures were grown at 35°C to late logarithmic phase (5×107 spirochetes/ml) and subjected to immunoblotting with monoclonal antibodies against Hk2, Rrp2, RpoS, OspC or FlaB (loading control). Under in vitro growth conditions, a combination of elevated temperature and increased cell density activates the Rrp2-RpoN-RpoS pathway, leading to the production of RpoS and RpoS-controlled proteins such as OspC [2], [5], [6], [8], [18], [36]–[39]. To determine if Hk2 affects temperature and cell density-dependent activation of the Rrp2-RpoN-RpoS pathway, wild-type B. burgdorferi and isogenic hk2 mutant spirochetes were cultivated at elevated temperature (35°C) and harvested at the late-exponential stage of growth (5×107 spirochetes per ml), conditions under which the Rrp2-RpoN-RpoS pathway is known to be activated. The hk2 mutant and its parental strain expressed similar levels of RpoS and OspC (Fig. 1C). Under “non-inducing” conditions (i.e., low cell density or lower culture temperature), neither the hk2 mutant nor the parent strain expressed OspC (data not shown). Thus, consistent with studies by Burtnick et al. [6], the Rrp2-RpoN-RpoS pathway can be activated in vitro in an Hk2-independent manner.
In vitro growth conditions only partially mimic the B. burgdorferi gene expression patterns observed during tick feeding and mammalian infection. For example, spirochetes grown under elevated temperature and high cell density conditions upregulate ospC but do not downregulate ospA [2], [40]–[42]. Therefore, we next examined the phenotype of the hk2 mutant grown in mammalian host-adapted conditions by cultivating spirochetes in dialysis membrane chambers (DMCs) implanted in the peritoneal cavities of rats [2], [40]–[42]. As shown in Fig. 2, wild-type spirochetes cultivated in DMCs produced large amounts of OspC and undetectable amounts of OspA. An rpoS mutant exhibited the opposite phenotype, as previously reported [41]. In contrast, the DMC-cultivated hk2 mutant behaved much like its wild-type parent, indicating that Hk2 was not required for Rrp2 activation within this mammalian host environment.
Fig. 2. hk2 mutant spirochetes cultivated in DMCs are capable of activating the Rrp2-RpoN-RpoS pathway. 
Wild-type (wt), hk2 mutant (hk2), or rpoS mutant (rpoS) spirochetes were cultivated in DMCs, after which whole cell lysates were separated by SDS-PAGE and visualized with silver stain. The bands corresponding to OspA and OspC are indicated by arrowheads on the right. Molecular mass markers in kilodaltons are shown on the left. To further determine whether Hk2 is required for murine infection, groups of C3H/HeN mice were inoculated intradermally with various doses of either wild-type B. burgdorferi B31-A3 or its isogenic hk2 mutant. As shown in Table 2, the infectivity of the hk2 mutant was similar to that of the parental strain. This result suggests that unlike Rrp2, RpoN and RpoS [3]–[5], [20], Hk2 was not required for infection of mice by B. burgdorferi.
Tab. 2. Mouse infectivity of the <i>hk2</i> mutant. 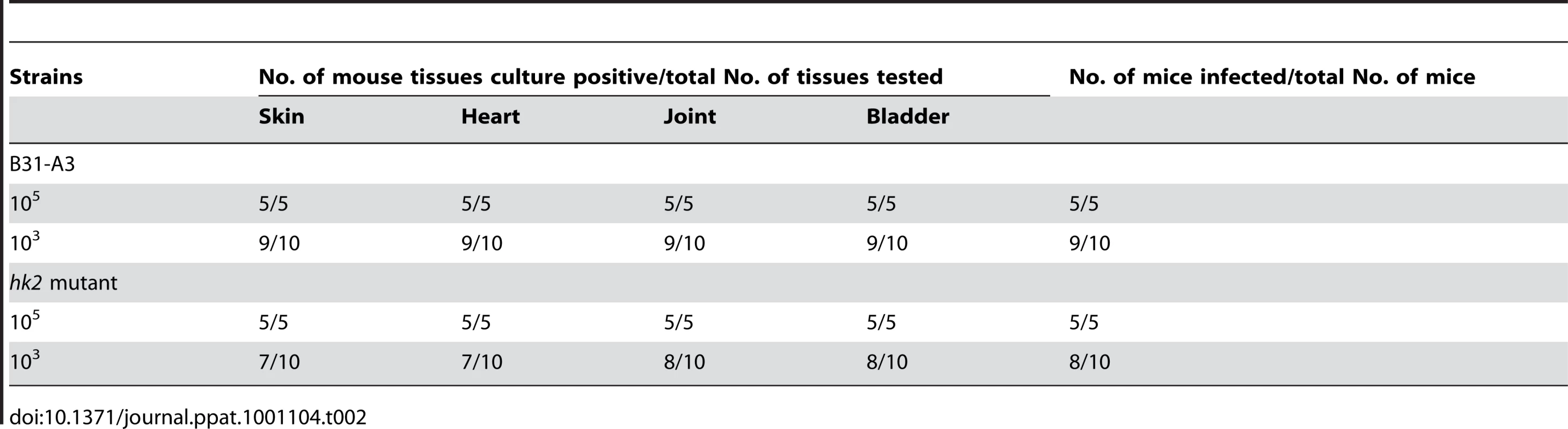
Other histidine kinases are not involved in Rrp2 activation
The results described above indicate that Rrp2 could be activated by an Hk2-independent mechanism. To test the possibility that cross-talk may contribute to Rrp2 activation, we assessed the involvement of the other three B. burgdorferi histidine kinases identified to date [17]. We first constructed an hk1 mutant (hk1) in B. burgdorferi 297 using a strategy similar to that described for generating the hk2 mutant (Fig. 3A). The resulting mutant was verified using RT-PCR to test for the absence of hk1 expression and the lack of polarity on the downstream gene rrp1 (Fig. 3B). Spirochetes were cultivated at elevated temperature and harvested at the late-exponential stage of growth. Unlike the rrp2(G239C) mutant, which failed to express OspC, the hk1 mutant produced levels of OspC that were comparable to those of its wild-type parent, indicating that Hk1 is dispensable for Rrp2 activation (Fig. 3C).
Fig. 3. The hk1 mutant remains capable of activating the Rrp2-RpoN-RpoS pathway. 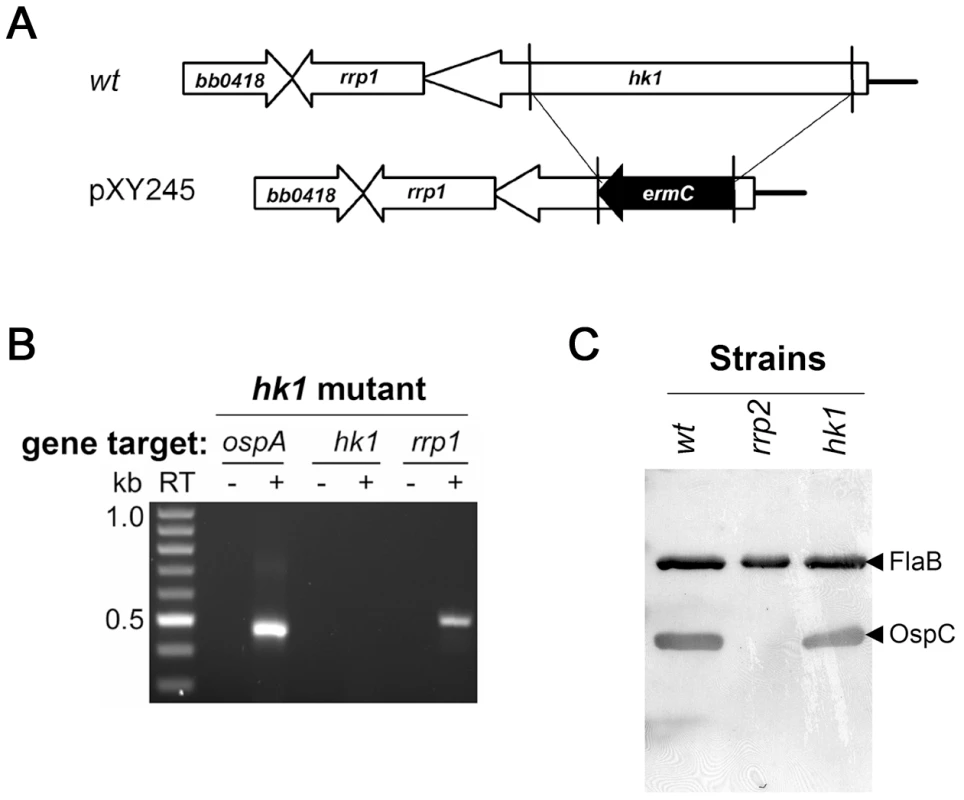
(A) Strategy for insertional inactivation of hk1. wt, genomic structure of hk1 in wild-type B. burgdorferi. pXY245, the suicide vector used for generating the hk1 mutant. Only the relevant portion of the plasmid is shown. (B) Confirmation of the hk1 mutant by RT-PCR analyses. RT-PCR was performed using primers specific for ospA, hk1, or rrp1 (labeled on the top). kb: the kilobase DNA ladder. RT indicates the absence (-) or presence (+) of reverse transcriptase in the reaction. (C) Production of OspC by the hk1 mutant. Various strains of spirochetes (labeled on the top) were grown at 35°C and harvested in the late logarithmic phase (5×107 spirochetes/ml) and subjected to immunoblot analysis using a mixture of monoclonal antibodies specific for OspC and FlaB, respectively. A strain harboring a G239C point mutation within Rrp2 [18], serves as a negative control for OspC expression. The bands corresponding to OspC or FlaB are indicated by the arrowhead on the right. It remained possible that Hk1 and Hk2 are involved in Rrp2 activation but that they may compensate for each other in a single knockout mutant. To rule out this possibility, we generated an hk1 hk2 double mutant in B. burgdorferi 297 by transforming the hk1 mutant with the suicide vector used for generating the hk2 mutant. Immunoblot analysis of the double mutant confirmed the absence of Hk2 in the hk1 hk2 mutant, and, more importantly, demonstrated that temperature and cell density-induced expression of OspC was unaffected despite the loss of both histidine kinases (Fig. 4A). These results indicate that during in vitro growth, Hk1 is not responsible for Rrp2 activation in the absence of Hk2.
Fig. 4. The hk1 hk2 double mutant and the cheA1 or cheA2 mutant have normal level of Rrp2 activation. 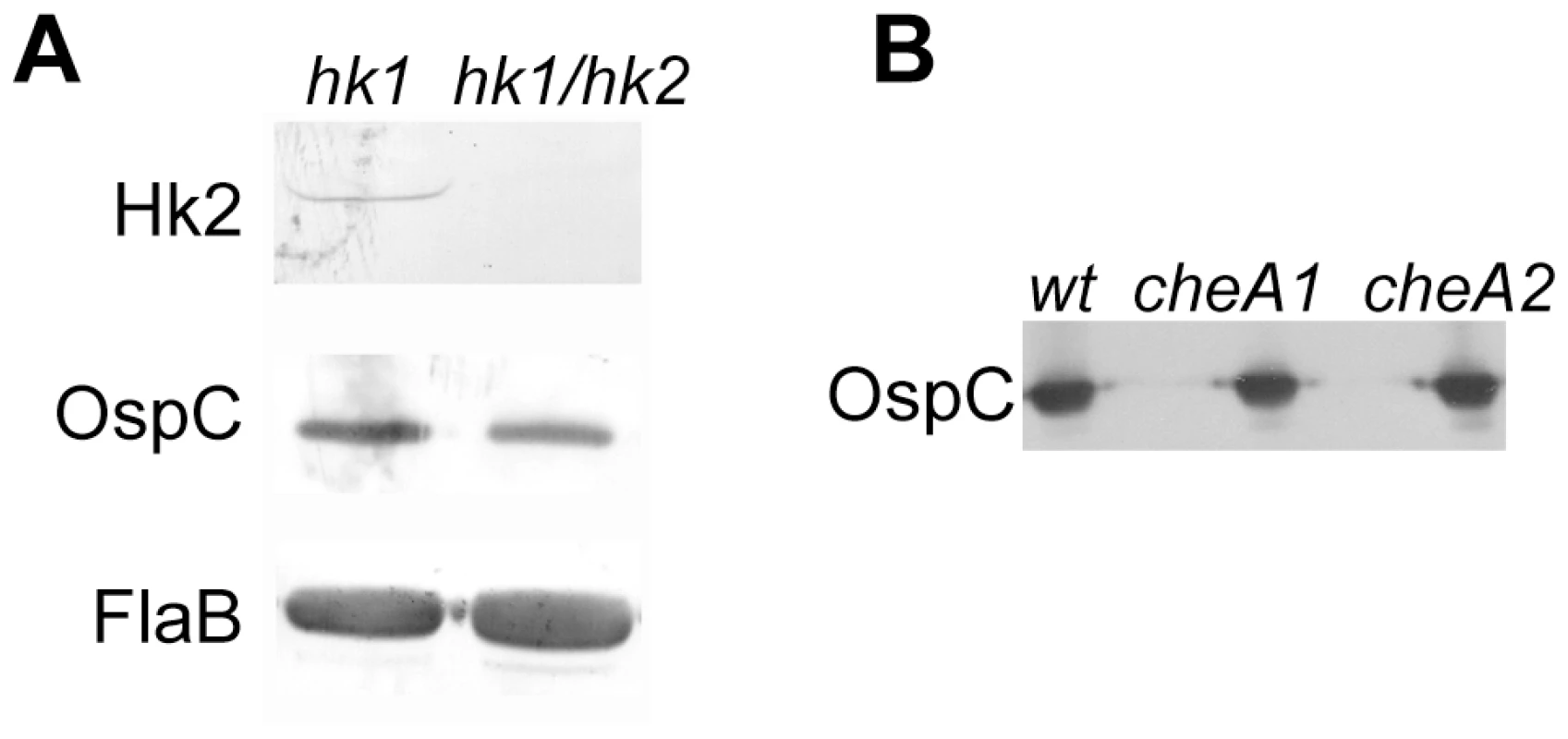
Various strains of spirochetes (labeled on the top) were grown at 35°C, harvested in the late logarithmic phase (5×107 spirochetes/ml), and subjected to immunoblot analysis using monoclonal antibodies specific against Hk2, OspC or FlaB as indicated. In addition to Hk1 and Hk2, B. burgdorferi expresses two other histidine kinases, CheA1 and CheA2, both of which are involved in chemotaxis [43], [44]. To determine whether CheA1 or CheA2 participate in Rrp2 activation, we examined the ability of cheA1 and cheA2 mutants to produce OspC. As shown in Fig. 4B, both cheA mutants expressed normal levels of OspC, indicating that neither CheA1 nor CheA2 is required for Rrp2 activation under in vitro growth conditions.
Rrp2 activation requires the conserved phosphorylation site D52
As a putative two-component response regulator, it is predicted that Rrp2 becomes activated upon phosphorylation of a conserved aspartate residue (D52) located within its N-terminal receiver domain [6], [18] (Fig. 5A). Since deletion of each histidine kinase gene exerted no effect on the activation of the Rrp2-RpoN-RpoS pathway, we asked whether Rrp2 activation actually requires phosphorylation. Repeated attempts to replace the wild-type rrp2 with a mutated allele containing a D52A mutation were unsuccessful. As an alternative strategy, we reasoned that, if phosphorylation is important for Rrp2 activation, overexpression of a wild-type N-terminal Rrp2 fragment (Rrp2-N) (phosphorylatable but not active) would interfere with phosphorylation of endogenous full-length Rrp2 and therefore affect activation of the Rrp2-RpoN-RpoS pathway. Conversely, overexpression of a non-phosphorylatable mutant version of the Rrp2 N-terminus should have no effect. Accordingly, we constructed a series of shuttle vectors that carried the wild-type allele rrp2-N or the mutant alleles rrp2-N(D52A) or rrp2-N(D52E) under control of the constitutive flaB promoter (Fig. 5A). Each constructed vector then was transformed into a non-infectious but highly transformable strain, B31 13A. The resulting transformants were verified by immunoblot analysis showing that each produced native full-length Rrp2 and the overexpressed Rrp2-N fragment (Fig. 5B). We then evaluated the ability of these transformants to express OspC. Overexpression of wild-type Rrp2-N almost completely abolished expression of ospC (Fig. 5B and 5C). These results were consistent with the expectation that the Rrp2-N fragment can successfully compete with native full-length Rrp2 for phosphorylation and, thus, interfere with Rrp2 and RpoN (σ54)-dependent transcription of rpoS [14], [15]. In contrast, cells expressing non-phosphorylatable Rrp2-N(D52A) or Rrp2-N(D52E) behaved like the vector control (Fig. 5B and 5C), as would be expected if Rrp2 activation requires phosphorylation of D52.
Fig. 5. Influence of overexpression of wild-type or mutated version of the Rrp2 N-terminal receiver domain on Rrp2 activation. 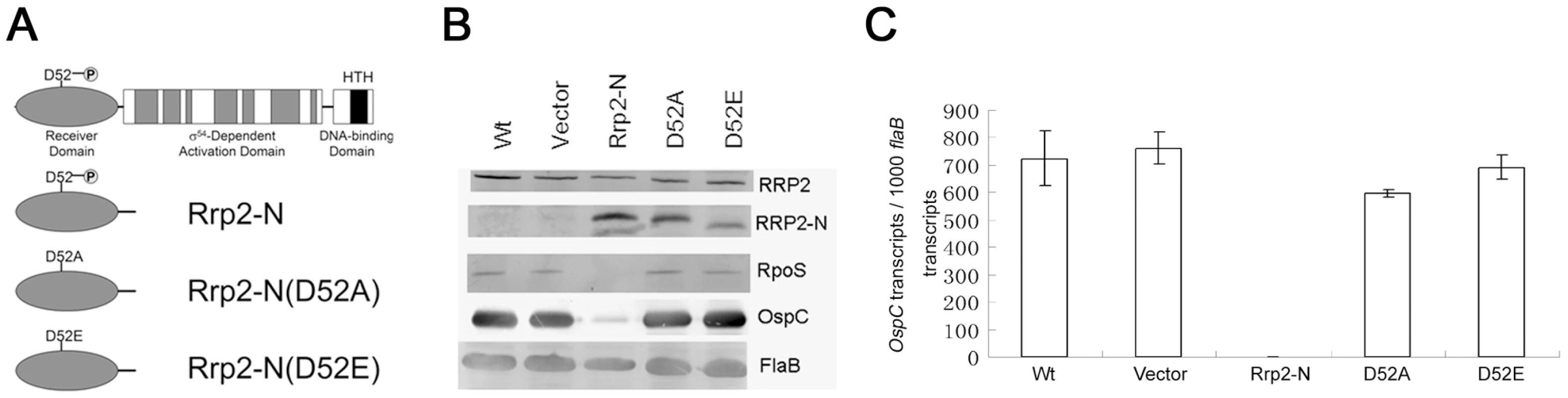
(A) Schematic diagram of predicted Rrp2 domain structure and various versions of overexpressed N-terminal receiver domains. D52 is the putative phosphorylation site. (B) Immunoblot of wild-type strain (lane 1), the strain carrying the shuttle vector only (lane 2), the strain with overexpression of Rrp2-N (lane 3), the strain with overexpression of Rrp2-N(D52A) (lane 4), and the strain with overexpression of Rrp2-N(D52E) (lane 5). Cultures were grown to late logarithmic phase at 35°C. Pooled antibodies/antisera against Rrp2, FlaB, and OspC were used. Bands corresponding to each protein were labeled on the right. (C) qRT-PCR analysis of ospC expression in various strains shown in (B). Levels of ospC transcript were normalized per 1000 copies of flaB in each sample. Given that the Rrp2-RpoN-RpoS pathway is essential for mammalian infection, we hypothesized that overexpression of Rrp2-N, but not Rrp2-N(D52A) would affect the spirochete's ability to infect mice. To test this hypothesis, we re-transformed the corresponding shuttle vectors into the infectious strain B31-A3. Positive transformants that had endogenous plasmid profiles identical to that of B31-A3 were then needle-inoculated into groups of C3H/HeN mice. As shown in Table 3, although the strain overexpressing wild-type Rrp2-N was capable of infecting mice with a high dose of inoculation (1×105 spirochetes per mouse), its infectivity was greatly reduced; only 1 out of 5 mice was infected at the dose of 1×103 spirochetes (Table 3). In contrast, overexpression of Rrp2-N(D52A) exerted no such effect. Thus, overexpression of Rrp2-N impaired the activation of the Rrp2-RpoN-RpoS pathway both in vitro and in vivo, further supporting the hypothesis that phosphorylation of Rrp2 is likely required for the activation of the Rrp2-RpoN-RpoS pathway.
Tab. 3. Mouse infectivity of Borrelia burgdorferi with overproduction of Rrp2-N or Pta. 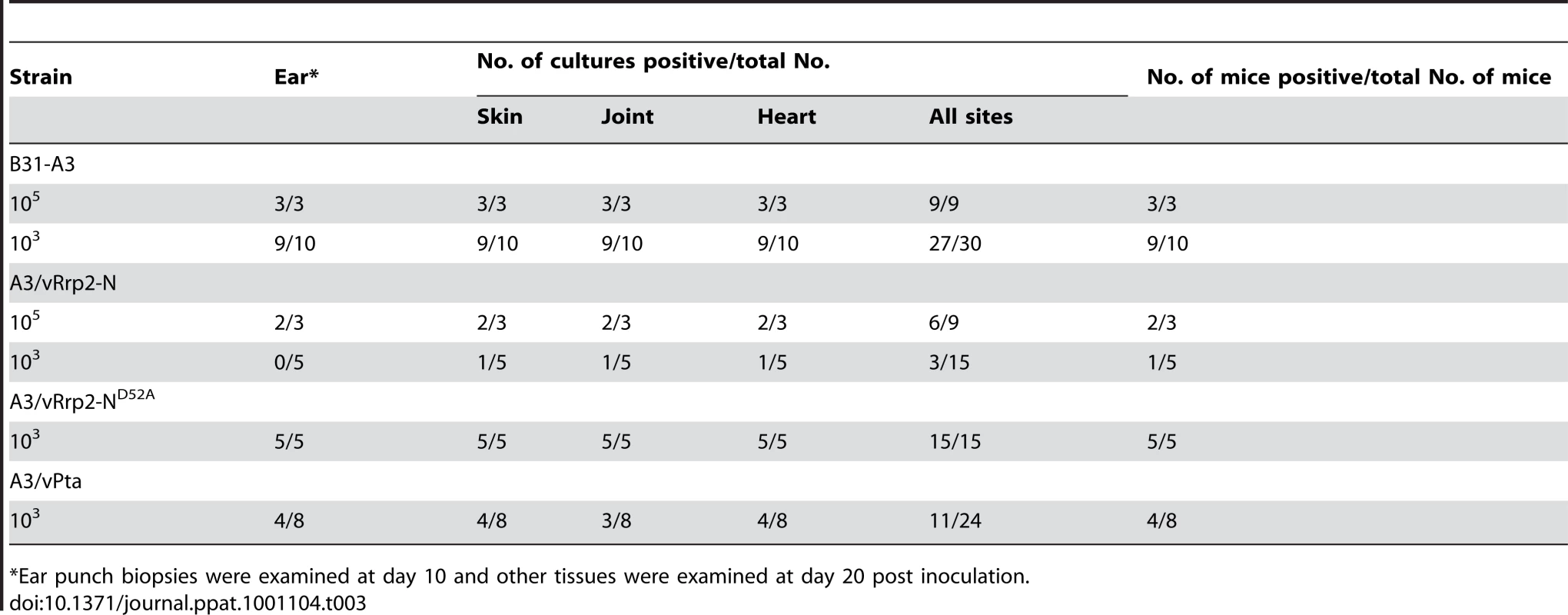
*Ear punch biopsies were examined at day 10 and other tissues were examined at day 20 post inoculation. Carbamoyl phosphate does not contribute to Rrp2 activation
Since Rrp2 activation appears to require D52, but not the B. burgdorferi histidine kinases, we reasoned that small metabolic intermediates (e.g., carbamoyl∼P or acetyl∼P) might be responsible for phosphorylation of D52. The B. burgdorferi genome is predicted to encode a single pathway that can produce carbamoyl-P, the so-called arginine fermentation or ArcA-ArcB pathway, in which the enzyme arginine deaminase (ArcA) converts arginine to citrulline, which is then converted to ornithine and carbamoyl∼P by the enzyme ornithine carbamoyltransferase (ArcB) (Fig. 6A). To assess the ability of carbamoyl∼P to influence Rrp2 activation, we used transposon mutagenesis to construct an arcA (bb0841) mutant (see Materials and Methods). The arcA mutant had no growth defect in vitro (data not shown) and produced levels of OspC similar to those of the wild-type parent strain (Fig. 6B). Moreover, wild-type spirochetes cultivated in growth medium supplemented with an excess of arginine or ornithine showed no change in OspC expression (data not shown). Collectively, these results argue that carbamoyl∼P does not donate its phosphoryl group to activate Rrp2, at least under in vitro cultivation conditions.
Fig. 6. Inactivation of the carbamoyl-P biosynthesis pathway does not affect Rrp2 activation. 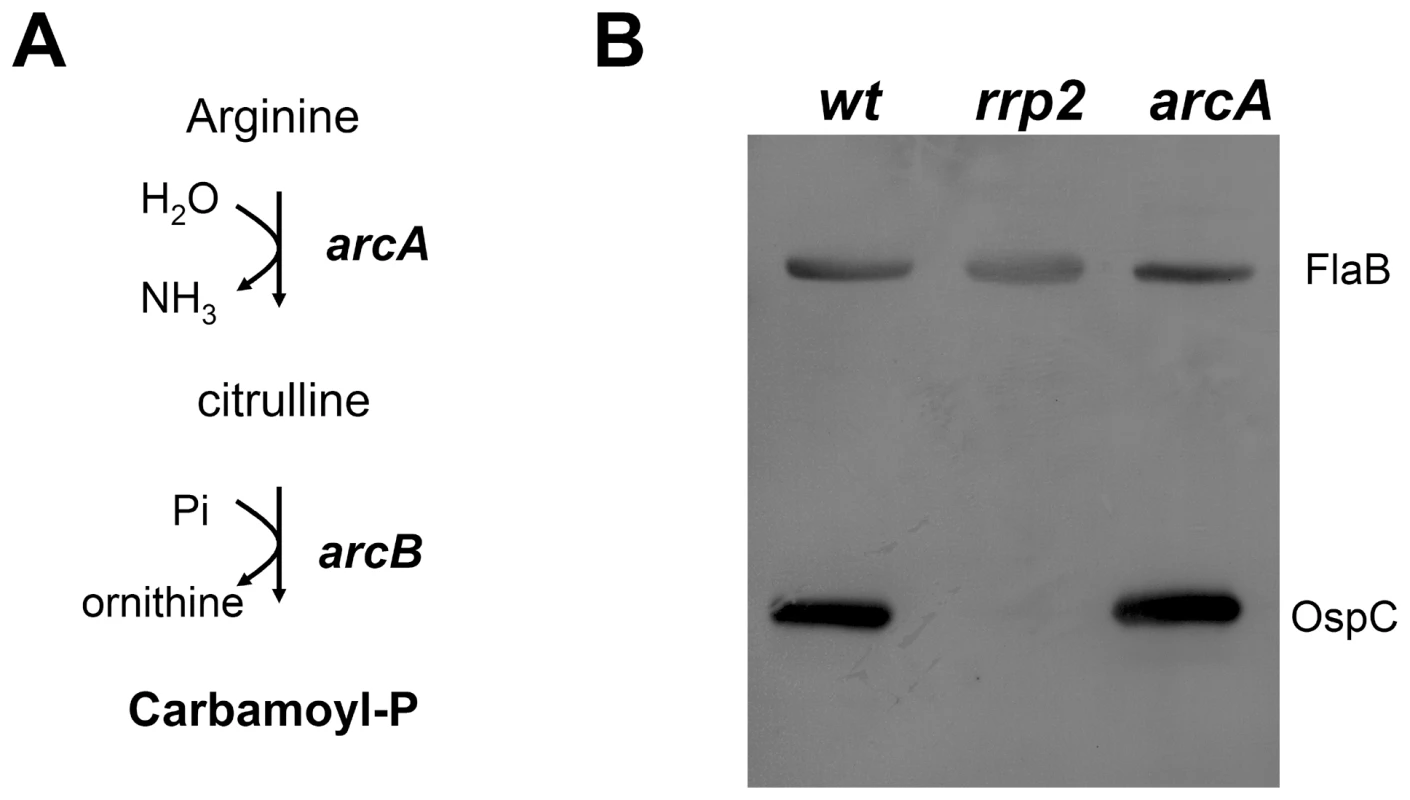
(A) Diagram of the arginine fermentation pathway in B. burgdorferi. The arcA (bb0841) and arcB (bb0842) genes are predicted to encode arginine deaminase and ornithine carbamoyltransferase, respectively. (B) Immunoblot analysis of whole cell lysates of wild-type (wt), the rrp2 mutant [rrp2(G239C)], and the arcA mutant (arcA) with a mixture of antibodies against OspC and FlaB. Spirochetes were cultured at 35°C and harvested at late logarithmic growth. The bands corresponding to FlaB and OspC are indicated on the right. Acetate induces activation of the Rrp2-RpoN-RpoS pathway
Acetyl∼P is the intermediate in the acetate kinase (Ack) – phosphate acetyltransferase (Pta) pathway. B. burgdorferi possesses genes predicted to encode both Ack (BB0622) and Pta (BB0589) [17] (Fig. 7A). However, the B. burgdorferi genome encodes neither an AMP-ACS pathway that converts acetate to acetyl-coA nor other known pathways that produce acetyl-CoA. It also lacks the TCA cycle which utilizes acetyl-CoA for energy production. The genome does have a mevalonate pathway (BB0683-BB0688) that requires acetyl-CoA for cell wall synthesis. Therefore, the Ack-Pta pathway appears to be the sole pathway for biosynthesis of acetyl-CoA required for cell wall synthesis
Fig. 7. Acetyl∼P plays an important role in Rrp2 activation under in vitro cultivation conditions. 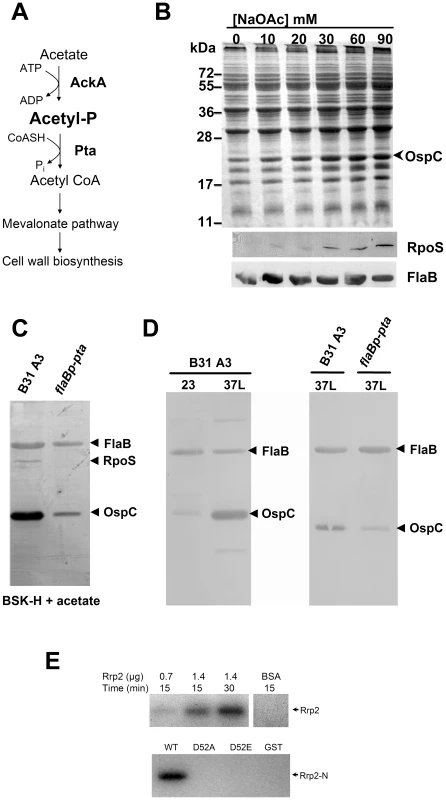
(A) Diagram of the ACK-PTA pathway in B. burgdorferi. ack (bb0622) encodes acetate kinase (Ack), which converts acetate to the intermediate acetyl∼P, while pta (bb0589) encodes phosphate acetyltransferase (Pta), which synthesizes acetyl-CoA from acetyl∼P and CoASH [17]. In B. burgdorferi, the Ack-Pta pathway appears to be the sole pathway for biosynthesis of acetyl-CoA, a molecule required for cell membrane biosynthesis (see Results and Discussion for details). (B) Acetate induces activation of the Rrp2-RpoN-RpoS pathway. Wild-type B. burgdorferi strain B31-A3 was cultivated in the BSK-H medium supplemented with 0–90 mM NaOAc with a final media pH value of 7.0. Cells were harvested at the early-logarithmic phase (5×106 spirochetes/ml). Cell lysates were subjected to SDS-PAGE (top panel) or immunoblot (bottom panels) analysis. The bands corresponding to OspC, RpoS and FlaB were labeled on the right. (C) Overexpression of Pta reduces acetate-induced Rrp2 activation. Wild-type B. burgdorferi strain B31 13A (-) or the strain carrying flaBp-pta (+) were cultivated in the BSK-H medium supplemented with 15 mM NaOAc at pH 7. Cells were harvested at the cell density of 5×106 and then subjected to immunoblot analyses with a mixture of antibodies against RpoS, OspC, or FlaB (internal control). The bands corresponding to each protein are indicated on the right. (D) Overexpression of Pta reduces temperature and cell density-induced activation of the Rrp2-RpoN-RpoS pathway. Wild-type B. burgdorferi strain B31 13A (-) or the strain carrying flaBp-pta (+) were cultivated either at 23 or 35°C in the standard BSK-H medium. Cells were harvested at the late-logarithmic growth phase (5×107 spirochetes/ml) and then subjected to immunoblot analyses. (E) In vitro phosphorylation of recombinant Rrp2 by acetyl∼P. Different quantities of purified recombinant Rrp2 or various versions of Rrp2-N were incubated with [32P]acetyl phosphate and the reactions were terminated at 15 or 30 min. The reaction mixtures were separated by SDS-PAGE followed by exposure on Kodak X-ray film. As a short-chain fatty acid, acetate can diffuse into cells under neutral or acidic conditions [32]. Then the enzyme Ack can convert acetate to acetyl∼P, which in turn is converted to acetyl-CoA by the enzyme Pta. Thus, increasing concentrations of exogenous acetate can elevate intracellular levels of acetyl∼P [32]. To assess whether acetyl∼P plays a role in Rrp2 activation, wild-type B. burgdorferi B31-A3 were cultivated in BSK-H medium supplemented with increasing concentrations of sodium acetate (NaOAc) with the final medium pH adjusted to 7.0. In order to detect the effect of acetate, cells were harvested at low density (5×106 spirochetes/ml) when activation of the Rrp2-RpoN-RpoS pathway (monitored by RpoS and OspC expression) is low [37], [38]. As shown in Fig. 7B, supplementation of NaOAc to the growth media dramatically increased the expression of OspC and RpoS in a dose-dependent fashion. This increase was not due to an elevated salt concentration (or to osmotic shock) since supplementation of the medium with as much as 150 mM NaCl did not reproduce this effect (data not shown).
Overexpression of Pta inhibits the activation of the Rrp2-RpoN-RpoS pathway
To determine whether acetate-induced RpoS and OspC expression occurs via the Ack-Pta pathway, we attempted to generate ack and pta mutants but were unsuccessful. We reasoned that the Ack-Pta pathway may be indispensable for borrelial growth (see discussion). As an alternative approach, we overexpressed Pta in wild-type spirochetes. We reasoned that if acetate-induced Rrp2 activation results from accumulation of acetyl∼P, then overexpression of Pta would reduce the level of acetyl∼P and abolish the acetate effect. A shuttle vector carrying the pta gene under the control of the flaB promoter was introduced into strain B31 13A. The resulting transformants were cultivated in the presence of 15 mM NaOAc at pH 7.0 and harvested at low cell density (5×106 spirochetes/ml). As shown in Fig. 7C, overexpression of Pta dramatically reduced acetate-induced Rrp2 activation as assessed by expression of OspC. These results are consistent with the hypothesis that acetate activates Rrp2 via accumulation of acetyl∼P.
A combination of elevated culture temperature and increased cell density or lowered pH (pH 6.8–7.0) induces RpoS and OspC expression [5], [37], [38], [45], yet the underlying mechanism remains unclear. Since temperature, cell density, and pH are capable of influencing intracellular level of acetyl∼P in other organisms, such as E. coli [32], we sought to determine if overexpression of Pta also affects temperature and cell density-induced Rrp2 activation. Thus, spirochetes were cultivated at 23 or 35°C in standard BSK-H and harvested during late exponential growth (∼5×107 spirochetes/ml). Consistent with previous observation, elevated temperature and cell density induced OspC expression in wild-type spirochetes (Fig. 7D, the left panel). However, overexpression of Pta dramatically inhibited such effect (Fig. 7D, the right panel). These results suggest that the effect of environmental cues such as temperature - and cell density on RpoS and OspC expression might be through the small molecule acetyl∼P.
To determine whether overexpression of Pta would affect mammalian infection by B. burgdorferi, we re-constructed a Pta-overexpressing strain in the infectious strain B31-A3. One of the transformed clones harboring flaBp-pta had an endogenous plasmid profile identical to that of B31-A3, and was chosen for subsequent infection study. As shown in Table 3, overexpression of Pta resulted in a moderate reduction of infectivity; half of the mice (4 out of 8) were infected at the dose of 1×103 spirochetes. This result suggests that the AckA-Pta pathway contributes to mammalian infection, likely by synthesizing acetyl∼P, which can donate its phorphoryl group to Rrp2.
Acetyl∼P phosphorylates Rrp2 in vitro
To determine whether Rrp2 can be directly phosphorylated by acetyl∼P, we performed an in vitro phosphorylation assay. Different amounts of purified recombinant Rrp2, Rrp2-N, Rrp2-N(D52A), or Rrp2-N(D52E) were incubated with 32P-labeled acetyl∼P in the reaction buffer at 37°C for 15 or 30 min. As shown in Fig. 7E, phosphorylated Rrp2 was readily detected in a time - and dose-dependent manner. Furthermore, phosphorylation of Rrp2 requires D52, since wild-type Rrp2-N, but not Rrp2-N(D52A) or Rrp2-N(D52E) could be phosphorylated by acetyl∼P (Fig. 7E). These results indicate that acetyl∼P can directly donate its phosphoryl group to Rrp2 in a histidine kinase-independent manner.
Discussion
The discovery of the central regulatory network, the Rrp2-RpoN-RpoS pathway, was a significant advance in B. burgdorferi gene regulation. However, the dearth of knowledge regarding the mechanism underlying the activation of this pathway has been a major gap in our understanding of Borrelia host adaptation. In this study, we showed that temperature - and cell density-induced Rrp2-RpoN-RpoS activation occurs via a histidine kinase-independent mechanism. We further provided evidence suggesting the hypothesis that the high-energy metabolic intermediate acetyl∼P plays a key role in Rrp2 phosphorylation and, consequently, the activation of the Rrp2-RpoN-RpoS pathway.
In this study we first extended the recent finding by Burtnick et al. [6] that Hk2 was not essential for Rrp2 activation under in vitro cultivation conditions, by further showing that the hk2 mutant was capable of activating the Rrp2-RpoN-RpoS pathway in a mammalian host-adapted model and establishing infection in mice. The fact that the hk2 mutant remained capable of upregulation of OspC and downregulation of OspA in the DMC model (Fig. 2) indicates that this sensor kinase and its PAS sensing domain does not play a major in sensing mammalian host-specific signals for RpoS activation. We next tested the hypothesis that Hk1, the only other B. burgdorferi histidine kinase with no assigned function, could be responsible for activation of the Rrp2 pathway. We found that the hk1 and hk1 hk2 mutants exhibited normal levels of temperature-induced Rrp2-dependent OspC expression. We further found that spirochetes lacking other histidine kinases identified in the B. burgdorferi genome, the chemotaxis histidine kinases CheA1 or CheA2, also exhibited normal OspC expression. One caveat is that we have not tested cheA1 hk2 and cheA2 hk2 double mutants and thus cannot formally rule out a possible compensatory effect between Hk2 and CheA1 or CheA2.
Several groups have reported the existence of atypical response regulators in other bacteria whose activities do not require phosphorylation of their receiver domains [46]–[48]. These atypical response regulators either do not possess the conserved aspartate residue shown to function as the phosphorylation site (e.g., HP1021 and HP1043 in Helicobacter pylori) [46], or lack conserved residues for Mg++ chelation, which is essential for phosphorylation (e.g., FrzS in Myxococcus or NblR in Synechococcus) [47], [48]. However, Rrp2 retains all the conserved residues for phosphorylation (D52), Mg++ binding (D8, D9), and signal transduction (T80, F99, K102). Thus, it is unlikely that Rrp2 is an atypical response regulator. Indeed, in this study, we showed that Rrp2 can autophosphorylate using acetyl∼P as its sole phosphoryl donor. Furthermore, overexpression of the phosphorylatable receiver domain of Rrp2 (Rrp2-N), but not variants of Rrp2-N that carry the D52A or D52E mutations, interfered with endogenous Rrp2 activity. This result is consistent with the assumption that Rrp2 activation requires phosphorylation of D52. Another evidence supporting phosphorylation-dependent Rrp2 activation is our previous observation that the ATPase activity of Rrp2, an activity that is essential for its transcriptional activation function, also is dependent on phosphorylation of Rrp2 [15]. Of note, overproduction of a protein from a strong constitutive promoter (e.g., flaB) could have pleiotropic effects. An ideal approach to study the function of Rrp2 phosphorylation would be to replace the endogenous copy of rrp2 with the D52A mutant allele. Despite multiple efforts, however, we failed to generate the desired strain. This lack of success is consistent with previous reports that inactivation of rrp2 may be lethal [6], [18]. We hypothesize that phosphorylated Rrp2 may be important for cell growth. Consistent with this hypothesis, overexpression of Rrp2 exhibited a moderate growth defect (data not shown).
The finding that activation of RpoS and OspC requires phosphorylation of Rrp2 but does not require any of the four histidine kinases led us to hypothesize that the phosphoryl donor might be a high-energy central metabolic intermediate [29], [31], [32]. Indeed, bioinformatic analysis of the B. burgdorferi genome revealed one pathway capable of producing carbamoyl-P (ArcA-ArcB) and one pathway that can synthesize acetyl∼P (Ack-Pta). Loss of ArcA, which should result in the inability to synthesize carbamoyl-P, had no effect upon Rrp2-dependent expression, suggesting that carbamoyl-P does not serve as the phosphoryl donor to Rrp2.
Acetyl∼P is the intermediate of the Ack-Pta pathway. The Ack-Pta pathway functions in acetogenesis through the conversion of acetyl-CoA obtained from pyruvate into acetate; operation of this pathway in the opposite direction enables other bacteria to use acetate as a carbon source by activating acetate to acetyl-CoA, which subsequently enters the tricarboxylic acid (TCA) cycle. In some organisms, such as E. coli, the pathway is reversible and thus can function in both acetogenesis and acetate activation [32]. The relatively small genome of B. burgdorferi, an obligate parasite, does not encode any enzyme known to convert pyruvate to acetyl-CoA, nor does it encode the enzymes of the TCA cycle. Instead, B. burgdorferi performs lactogenesis, converting pyruvate to lactate [17] (Xu H. and Yang, X.F., unpublished result). As such, the main function of the Ack-Pta pathway of B. burgdorferi is likely not for converting acetyl-CoA to acetate, but for generating acetyl-CoA from acetate. This acetyl-CoA could then be used for cell wall synthesis (via the mevalonate pathway [BB0683-BB0688]) and possibly for other metabolic pathways (Fig. 7A). Furthermore, B. burgdorferi seems to lack other acetyl-CoA synthetic pathways (e.g., the AMP-ACS pathway, β-oxidation of fatty acids, and several amino acid degradation pathways). Thus, the Ack-Pta pathway appears to be the sole pathway for biosynthesis of acetyl-CoA. If so, one would predict that the Ack-Pta pathway is essential for spirochetal growth. This notion is consistent with the fact that we failed to generate either an ack or a pta mutant by either targeted mutagenesis or random transposon mutagenesis (data not shown). What's the source of acetate for B. burgdorferi? Our measurement showed that acetate concentration in mouse blood and the midgut of fed ticks is ∼1.0 M and ∼1.8 mM, respectively (Xu H. and Yang, XF, unpublished data). One of the ingredients of the BSK-H medium, CMRL, also contains 0.61 mM acetate (other ingredients of this complex medium, such as rabbit serum, also may contribute to the overall levels of acetate). Through diffusion or an unknown transport system, B. burgdorferi may obtain sufficient acetate from these environments for acetyl-CoA production.
Acetyl∼P has drawn attention as a global regulator of gene expression via its ability to donate its phosphoryl group to a subset of response regulators under certain environmental conditions [32]. In E. coli, the intracellular acetyl∼P concentration can reach levels sufficient to phosphorylate a subset of response regulators [49] and thus influence the biological processes controlled by those proteins [32]. Although we have not yet measured the intracellular acetyl∼P levels to determine if this is also the case in B. burgdorferi, we were able to provide three lines of evidence to support the conclusion that acetyl∼P plays an important role in Rrp2 activation: (i) the activation of the Rrp2-RpoN-RpoS pathway can be induced by increasing concentration of exogenous acetate (Fig. 7B); (ii) overexpression of Pta reduced acetate-induced activation of the Rrp2-RpoN-RpoS pathway (Fig. 7C); and (iii) acetyl∼P served as a phosphoryl donor to Rrp2 in vitro (Fig. 7E). Note that overexpression of Pta did not completely abolish OspC production, suggesting that a low level of Rrp2 activation still occurs. This might be due to the presence of low levels of acetyl∼P, as overexpression of Pta does not abolish the production of acetyl∼P. Alternatively, Hk2 may contribute to Rrp2 activation. We are currently in the process of testing this possibility by overexpressing Pta in the hk2 mutant. Nevertheless, this partial inhibition of RpoS and OspC expression by overexpression of Pta is consistent with the in vivo phenotype that overexpression of Pta resulted in a moderate reduction of spirochetal infectivity in mice (Table 3).
It is well established that the Rrp2 pathway can be activated by many environmental cues such as temperature, pH, cell density, oxygen, and CO2 levels [37]–[39], [45], [50], [51]. However, the underlying mechanism for these phenomena has not been elucidated. In this regard, it is striking that virtually all the environmental cues that activate the Rrp2 pathway also have been shown to influence the acetyl∼P pool in E. coli [32]. This observation is consistent with our hypothesis that acetyl∼P serves as a signaling molecule that responds to environmental cues and in response activates the Rrp2 pathway. Indeed, we showed that overexpression of pta greatly inhibited both temperature - and cell density-induced activation of Rrp2 (Fig. 7D), suggesting that elevated temperature and increased cell density activate the Rrp2-RpoN-RpoS pathway in an acetyl∼P-dependent manner. Elevated temperature may increase acetyl∼P levels by enhancing diffusion of acetate into the cells and/or from increased transport efficiency via an unidentified transport system for acetate. Elevated temperature also increases cell growth rates that likely lead to increased levels of acetyl∼P [32], [52]. The effect of increased cell density on acetyl∼P levels, on the other hand, can result simply by a change in extracellular pH. As cell density increases, the culture pH diminishes from 7.5 to 7.0 or lower [38], which favors the passive diffusion of acetate into the cells [32].
One caveat of this study is that we used expression of RpoS and OspC as the readout for Rrp2 phosphorylation. An ideal approach for such study would be directly to detect the phosphorylated form of Rrp2. Unfortunately this approach is not technically feasible since most forms of the Asp-phosphorylation are unstable and there is no antibody available for detecting Asp-phosphorylation. Thus, a common approach for studying phosphorylation of response regulators is to monitor the output product as a result of phosphorylation of a response regulator. In the case of Rrp2, the only direct target gene identified thus far is rpoS and therefore, expression of rpoS faithfully reflects the activation of Rrp2 modulated by phosphorylation. One concern for this approach is whether the effect on RpoS expression observed in this study is through another transcriptional activator, BB647 (BosR). BB647 is a fur homologue and was recently shown that inactivation of this gene significantly reduced rpoS and ospC expression [53]–[56]. Although it remains unclear how BosR fits into the Rrp2-RpoN-RpoS pathway, we found that neither overexpression of Rrp2-N nor overexpression of Pta affected the level of BosR (data not shown), suggesting that the effects of Rrp2-N or Pta overexpression on RpoS and OspC was not through BosR, rather through Rrp2.
In summary, we have shown that temperature - and cell density-induced the activation of the Rrp2-RpoN-RpoS pathway proceeds independently of histidine kinases and carbamoyl-P. In contrast, biochemical and genetic manipulation of the acetyl∼P-producing Ack-Pta pathway dramatically impacts activation of the Rrp2-RpoN-RpoS pathway, providing strong evidence that acetyl∼P plays an important role in Rrp2 activation under in vitro growth conditions. We also provide evidence showing that, during mammalian infection, the Rrp2-RpoN-RpoS pathway is also activated via an Hk2-independent mechanism and that acetyl∼P plays an important role in this process. Then, what is the function of Hk2? One possibility is that Hk2 may play a role in sensing host signals and activating Rrp2 during the process of tick feeding. In this regard, we have examined the phenotype of the hk2 mutant in ticks and found that the hk2 mutant indeed has reduced infectivity via the route of tick infestation. Unfortunately, we have not been able to construct an infectious complemented strain and, thus, have been unable to show restoration of this defect, which prevents us from drawing a definitive conclusion on Hk2 function in the enzootic cycle of B. burgdorferi. Nevertheless, this preliminary finding suggests that Hk2 may contribute to Rrp2 activation during the process of tick feeding. In addition, spirochetes likely have increased levels of intracellular acetyl∼P in feeding ticks, as they encounter increased temperature [39], as well as a massive influx of nutrients that leads to a dramatic increase of growth rates during this process [57], [58]. Thus, we postulate that while acetyl∼P plays an important in activating the Rrp2-RpoN-RpoS pathway during mammalian infection, both acetyl∼P and Hk2 are likely involved in integrating complex environmental and host signals to modulate the Rrp2-RpoN-RpoS pathway during the process of spirochetal transmission from ticks to mammals.
Materials and Methods
Ethics statement
All animal experimentation was conducted following the NIH guidelines for housing and care of laboratory animals and performed in accordance with Indiana University Institutional regulation after review and approval by the institutional Animal Care and Use Committee at Indiana University.
Bacterial strains and plasmids
Low–passage, virulent B. burgdorferi strain B31-A3 was kindly provided by Dr. P. Rosa (Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health) [35]. Strain B31 13A that lacks lp25 was kindly provided by Dr. F. T. Liang (Louisiana State University) [59]. The rrp2 mutant was described previously [9] [20]. The cheA1 and cheA2 mutants were kindly provided by Dr. Li (New York medical college, NY) [44]. Borreliae were cultivated in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma, St. Louis, MO) supplemented with 6% normal rabbit serum (Pel Freez Biologicals, Rogers, AR) at 35°C unless indicated otherwise. A shuttle vector pBSV2 (a gift from Dr. P. Rosa) was maintained in E. coli strain TOP10. Relevant antibiotics were added to the cultures in the following final concentrations: 300 µg/ml for kanamycin and 50 ng/ml for erythromycin.
Construction of the hk2 mutant
To generate an hk2 mutant in strain B31-A3, a 2.5 kb fragment containing hk2 and its surrounding region was amplified with primers hk2-delF and hk2-delR (Supplemental Table S1) and cloned into the cloning vector pCR-XL-TOPO (Invitrogen). The plasmid was digested with Hind III (19 bp downstream of the 5' end of hk2) and ClaI (637 bp upstream of the 3' end of hk2), and a kanamycin-resistance cassette driven by the flaB promoter was then inserted into the disrupted hk2 gene (Fig. 1A). The suicide vector was confirmed by sequencing, and the plasmid DNA was transformed into B. burgdorferi strain B31-A3 as previously described [9], [60]. Whole cell lysates from positive clones were analyzed by PCR and Western immunoblot analysis using a monoclonal antibody against Hk2 to confirm marker insertion and inactivation of hk2. The plasmid profiles of the hk2 mutant clones were determined by PCR analyses with twenty-one pairs of primers specific for each of the endogenous plasmids [61]–[63]. Two of the three randomly picked clones had plasmid profiles that were identical to the parental strain B31-A3 [35], and one of these was chosen for further study.
Cultivation of B. burgdorferi B31 within dialysis membrane chambers (DMCs)
Dialysis membrane chambers (DMCs) containing 1×103 organisms diluted from a mid-logarithmic growth culture at 33°C in vitro, were implanted into the peritoneal cavities of female Sprague-Dawley rats as previously described [40], [42]. The DMCs were explanted 192 h after implantation; the spirochetes then were harvested, washed with 1x PBS buffer, and then examined by SDS-PAGE and silver staining.
Construction of the hk1 mutant and the hk1 hk2 double mutant strain
To construct a suicide vector for inactivation of hk1, regions of DNA corresponding to 1.3 kb upstream and 1.3 kb downstream of hk1 regions were PCR amplified from B31-A3 genomic DNA. The resulting DNA fragments were then cloned upstream and downstream of an erythromycin-resistant marker (ermR) within the pCR-XL-TOPO cloning vector, resulting in suicide vector pXY245. The inserts of pXY245 were confirmed by sequencing. The plasmid DNA was transformed into B. burgdorferi 297 strain BbAH130 as previously described [9], [60], resulting in a mutant with 3.4 kb deletion within hk1 (except the 460 bp to the 5' end and 385 bp to the 3' end of hk1) and an insertion of the ermR marker. Loss of hk1 expression was confirmed by RT-PCR analysis.
To construct the hk1 hk2 double mutant, the suicide vector pHX-hk2-kan DNA was transformed into the hk1 mutant. Kanamycin and erythromycin-resistant clones were selected and the loss of hk2 was confirmed by Western immunoblot analysis using an anti-Hk2 monoclonal antibody.
Construction of shuttle vectors for overexpression of wild type and mutant Rrp2 N-terminal domains and for overexpression of Pta
To constitutively express the wild-type Rrp2 N-terminal domain, the DNA fragment corresponding to the Rrp2-N terminal region was PCR-amplified from B. burgdorferi B31-A3 genomic DNA using primers rrp2-N-F and rrp2-N-R (Supplemental Table S1). Two restriction sites, NdeI and PstI, were incorporated into the designated primers and used for insertion of the digested PCR fragment into the pBSV2-derived shuttle vector pJD55 [4] harboring a flaB promoter. Thus, expression of Rrp2-N was placed under the control of the flaB promoter, flaBp-Rrp2-N. The resulting shuttle vector, pJD55/rrp2-N, was verified by sequencing and then transformed into B31 13A and B31-A3.
To introduce a single amino acid substitution (D52A or D52E) into the Rrp2-N terminal domain on pJD55/rrp2-N, site-directed mutagenesis was carried out by using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) with the mutagenic PAGE-purified primers D52A-F/D52A-R and D52E-F/D52E-R (Supplemental Table S1) as described by the manufacturer. Briefly, PCR was carried out as follows: 95°C for 50 seconds, 60°C for 50 seconds, 68°C for 10 minutes and 18 cycles. The resulting shuttle vectors with point mutations in Rrp2-N were verified by sequencing and designated pJD55-Rrp2-N(D52A) and pJD55-Rrp2-N(D52E), respectively.
To overexpress Pta, the DNA fragment corresponding to pta (bb0589) was PCR amplified from B. burgdorferi B31-A3 genomic DNA using primers Bb589F and Bb589R (Supplemental Table S1) and then subsequently cloned into pJD55, which places pta under the control of the flaB promoter. The resulting shuttle vector was verified by sequencing and then transformed into B31 13A and B31-A3.
Construction of the arcA (bb0841) mutant by transposon mutagenesis
The arcA mutant was generated by transposon-mediated mutagenesis as part of an on-going transposon signature tagged mutagenesis (STM) study. Briefly, twelve independent mutant libraries, each having a unique 7 bp sequence tag, were created using modified versions of the suicide plasmid pMarGentKan derived from pMarGent [64] (kindly provided by Dr. P. E. Stewart, Rocky Mountain Laboratories, National Institutes of Health, Hamilton, MN). The resulting plasmids were transformed into B. burgdorferi B31 5A18; transformants were selected on solid BSK-II media containing 200 µg/ml of kanamycin and 40 µg/ml of gentamicin as described previously [65]. Transposon insertion sites were determined by restriction digestion of the Borrelia genomic DNA, plasmid rescue in E. coli, and sequencing, as described previously [1].
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
SDS-PAGE and immunoblot analyses were performed as previously described [66]. Monoclonal antibodies against OspC, RpoS, and FlaB were described previously [20], [38]. Monoclonal antibodies against Rrp2 and HK2 were produced using a previously described method [66]. Rrp2-N fragments were detected using a previously reported polyclonal rat antiserum specific against full length Rrp2 [18].
Mouse infection via needle inoculation
Three or four week-old C3H/HeN mice (Harlan, Indianapolis, IN) were subcutaneously inoculated with spirochetes at a dose of 105 spirochetes per mouse. Ear punch biopsy and tissue samples (skin, heart, spleen and joint) were collected at the time points indicated for each experiment and cultured in BSK-H medium supplemented with 1× Borrelia antibiotic mixture (Sigma, Saint Louis, MO). A single growth-positive culture was used as the criterion for infection of each mouse. All animal protocols were approved by the Institutional Animal Care and Use Committee at Indiana University.
Quantitative RT-PCR (qRT-PCR)
RNA samples were extracted from B. burgdorferi cultures using the RNeasy® mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. Three independent culture samples were used for each strain. Digestion of contaminating genomic DNA in the RNA samples was performed using RNase-free DNase I (Promega, Madison, WI), and removal of DNA was confirmed by PCR amplification using primers specific for the B. burgdorferi flaB gene [67]. The cDNA was synthesized using the SuperScript III reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). To quantify the transcript levels of ospC, an absolute quantitation method was used by creating a standard curve in qPCR assay by following the manufacture's protocol (Strategene, La Jolla, CA). Briefly, a cloning vector containing the ospC gene serves as standard template. A series of ten-fold dilution (100 to 107 copies/µl) of the standard template was prepared and qPCR was performed to generate a standard curve by plotting the initial template quantity against the Ct values for the standards. The quantity of the ospC and flaB in cDNA samples were calculated by comparing their Ct values of the Standard Curve plot. Both standards and samples were performed in triplicate on an ABI 7000 Sequence Detection System using GREEN PCR Master Mix (ABI, Pleasanton, CA). Levels of ospC transcript were reported as per 1000 copies of flaB transcripts.
Expression and purification of recombinant Rrp2-N, Rrp2-N/D52A and Rrp2-N/D52E
Purification of recombinant Rrp2 protein was described previously [15]. The PCR fragments encoding Rrp2-N, Rrp2-N/D52A and Rrp2-N/D52E were cloned into the expression vector pGEX4t-2 with a glutathione S-transferase (GST) at the N-terminus. Fusion proteins GST-Rrp2, GST/Rrp2-N, GST/Rrp2-N/D52A and GST/Rrp2-N/D52E were expressed in E. coli under inducible condition of 1 mM IPTG at 37°C for 6 hours. Proteins were purified from cell lysates using GST SpinTrap (GE Healthcare, Piscataway, NJ) according to the manufacturer's manual.
In vitro phosphorylation assay
[32P]acetyl phosphate was synthesized as described by Quon et al. [68]. Briefly, the reaction mixture includes 0.3 U E. coli acetate kinase (Sigma), 10 µCi of [32P]ATP (6000 Ci/mmol, PerkinElmer) in AKP buffer (25 mM Tris-HCl [pH 7.4], 60 mM KOAc, 10 mM MgCl2; final pH 7.6) and was incubated at room temperature for 20 min. [32P]acetyl phosphate was used either without further treatment or with further purification by filtering through a 30 kDa cut-off membrane to remove acetate kinase (Amicon ultra with 30 kDa cut-off, Millipore). [32P]acetyl phosphate was mixed with recombinant Rrp2 (2.5 µl, 0.7 or 1.4 µg), Rrp2-N (2 µg), Rrp2-N/D52A (2 µg), Rrp2-N/D52E (2 µg) for 15 min or 30 min at 37°C. The reaction was terminated by addition of SDS-PAGE loading buffer and then loaded to 12% SDS-PAGE without boiling. The gel was then exposed to a Kodak X-ray film.
Supporting Information
Zdroje
1. LaneRS
PiesmanJ
BurgdorferW
1991 Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol 36 587 609
2. CaimanoMJ
IyerR
EggersCH
GonzalezC
MortonEA
2007 Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol 65 1193 1217
3. FisherMA
GrimmD
HenionAK
EliasAF
StewartPE
2005 Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A 102 5162 5167
4. OuyangZ
BlevinsJS
NorgardMV
2008 Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154 2641 2658
5. CaimanoMJ
EggersCH
HazlettKRO
RadolfJD
2004 RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun 72 6433 6445
6. BurtnickMN
DowneyJS
BrettPJ
BoylanJA
FryeJG
2007 Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol Microbiol 65 277 293
7. LybeckerMC
SamuelsDS
2007 Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol Microbiol 64 1075 1089
8. HübnerA
YangX
NolenDM
PopovaTG
CabelloFC
2001 Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A 98 12724 12729
9. YangXF
PalU
AlaniSM
FikrigE
NorgardMV
2004 Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med 199 641 648
10. GrimmD
TillyK
ByramR
StewartPE
KrumJG
2004 Outer-surface protein C of the Lyme disease spirochete: A protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101 3142 3147
11. PalU
YangX
ChenM
BockenstedtLK
AndersonJF
2004 OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest 113 220 230
12. TillyK
KrumJG
BestorA
JewettMW
GrimmD
2006 Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74 3554 3564
13. BattistiJM
BonoJL
RosaPA
SchrumpfME
SchwanTG
2008 Outer Surface Protein A Protects Lyme Disease Spirochetes from Acquired Host Immunity in the Tick Vector. Infect Immun 76 5228 5237
14. SmithAH
BlevinsJS
BachlaniGN
YangXF
NorgardMV
2007 Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN). J Bacteriol 189 2139 2144
15. BlevinsJS
XuH
HeM
NorgardMV
ReitzerL
2009 Rrp2, a σ54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J Bacteriol 191 2902 2905
16. StudholmeDJ
BuckM
2000 The biology of enhancer-dependent transcriptional regulation in bacteria: insights from genome sequences. FEMS Microbiol Letts 186 1 9
17. FraserCM
CasjensS
HuangWM
SuttonGG
ClaytonR
1997 Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390 580 586
18. YangXF
AlaniSM
NorgardMV
2003 The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci U S A 100 11001 11006
19. NorthAK
KloseKE
StedmanKM
KustuS
1993 Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J Bacteriol 175 4267 4273
20. BoardmanBK
HeM
OuyangZ
XuH
PangX
2008 Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun 76 3844 3853
21. HochJA
2000 Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3 165 170
22. MascherT
HelmannJD
UndenG
2006 Stimulus Perception in Bacterial Signal-Transducing Histidine Kinases. Microbiol Mol Biol Rev 70 910 938
23. StockAM
RobinsonVL
GoudreauPN
2000 Two component signal transduction. Annu Rev Biochem 69 183 215
24. YamamotoK
HiraoK
OshimaT
AibaH
UtsumiR
2005 Functional characterization in vitro of all twocomponent signal transduction systems from Escherichia coli. J Biol Chem 280 1448
25. NinfaAJ
NinfaEG
LupasAN
StockA
MagasanikB
1988 Crosstalk between bacterial chemotaxis signal transduction proteins and regulators of transcription of the Ntr regulon: evidence that nitrogen assimilation and chemotaxis are controlled by a common phosphotransfer mechanism. Proc Natl Acad Sci U S A 85 5492 5496
26. BijlsmaJE
GroismanEA
2003 Making informed decisions: regulatory interactions between two-component systems. Trends Micro 11 359 366
27. LaubMT
GoulianM
2007 Specificity in two-component signal transduction pathways. Annu Rev Genet 41 121 145
28. WannerBL
1992 Is cross regulation by phosphorylation of two-component response regulator proteins important in bacteria? J Bacteriol 174 2053 2058
29. McClearyWR
StockJB
NinfaAJ
1993 Is acetyl phosphate a global signal in Escherichia coli? J Bacteriol 175 2793 2798
30. LukatGS
McClearyWR
StockAM
StockJB
1992 Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci U S A 89 718 722
31. WannerBL
1993 Gene regulation by phosphate in enteric bacteria. J Cell Biochem 51 47 51
32. WolfeAJ
2005 The acetate switch. Microbiol Mol Biol Rev 69 12 50
33. WolfeAJ
2010 Physiologically relevant small phosphodonors link metabolism to signal transduction. Current Opinion in Microbiology 13 204 209
34. RogersEA
TerekhovaD
ZhangH
HovisKM
SchwartzI
2009 Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol
35. EliasAF
StewartPE
GrimmD
CaimanoMJ
EggersCH
2002 Clonal Polymorphism of Borrelia burgdorferi Strain B31 MI: Implications for Mutagenesis in an Infectious Strain Background. Infect Immun 70 2139 2150
36. GilbertMA
MortonEA
BundleSF
SamuelsDS
2007 Artificial regulation of ospC expression in Borrelia burgdorferi Mol Microbiol 63 1259 1273
37. IndestKJ
RamamoorthyR
SoleM
GilmoreRD
JohnsonBJB
1997 Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun 65 1165 1171
38. YangX
GoldbergMS
PopovaTG
SchoelerGB
WikelSK
2000 Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol 37 1470 1479
39. SchwanTG
PiesmanJ
GoldeWT
DolanMC
RosaPA
1995 Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 92 2909 2913
40. AkinsDR
BourellKW
CaimanoMJ
NorgardMV
RadolfJD
1998 A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest 101 2240 2250
41. CaimanoMJ
EggersCH
GonzalezCA
RadolfJD
2005 Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J Bacteriol 187 7845 7852
42. CaimanoM
2005 Cultivation of Borrelia burgdorferi in dialysis membrane chambers in rat peritonea. Curr Protoc Microbiol 12. Unit 12C.3
43. CharonNW
GoldsteinSF
2002 Genetics of motility and chemotaxis of a fascinating gruop of bacteria: the spirochetes. Annu Rev Genet 36 47 73
44. LiC
BakkerRG
MotalebMA
SartakovaML
CabelloFC
CharonNW
2002 Asymetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc Natl Acad Sci USA 99 6169 6174
45. CarrollJA
GaronCF
SchwanTG
1999 Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun 67 3181 3187
46. ScharJ
SickmannA
BeierD
2005 Phosphorylation-Independent Activity of Atypical Response Regulators of Helicobacter pylori. J Bacteriol 187 3100 3109
47. RuizD
SalinasP
Lopez-RedondoML
CayuelaML
MarinaA
ContrerasA
2008 Phosphorylation-independent activation of the atypical response regulator NblR. Microbiology 154 3002 3015
48. FraserJS
MerlieJPJ
EcholsN
WeisfieldSR
MignotT
WemmerDE
ZusmanDR
AlberT
2007 An atypical receiver domain controls the dynamic polar localization of the Myxococcus xanthus social motility protein FrzS. Mol Microbiol 65 319 332
49. KleinAH
ShullaA
ReimannA
KeatingDH
WolfeAJ
2007 The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol 189 5574 5581
50. StevensonB
SchwanTG
RosaPA
1995 Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 63 4535 4539
51. HydeJA
TrzeciakowskiJP
SkareJT
2007 Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol 189 437 445
52. PrussBM
WolfeAJ
1994 Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol Microbiol 12 973 984
53. HydeJA
ShawDK
SmithR
III
TrzeciakowskiJP
SkareJT
2010 Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect Immun 78 265 74
54. OuyangZ
KumarM
KariuT
HaqS
GoldbergM
2009 BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol 74 1331 1343
55. HydeJA
ShawDK
SmithLR
TrzeciakowskiJP
SkareJT
2009 The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol 74 1344 1355
56. SamuelsDS
RadolfJD
2009 Who is the BosR around here anyway? Mol Microbiol 74 1295 1299
57. de SilvaAM
FikrigE
1995 Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg 53 397 404
58. PiesmanJ
SchneiderBS
ZeidnerNS
2001 Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J Clin Microbiol 39 4145 4148
59. XuQ
McShanK
LiangFT
2007 Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol Microbiol 64 220 231
60. SamuelsDS
1995 Electrotransformation of the spirochete Borrelia burgdorferi.
NickoloffJA
Methods in molecular biology. 45 ed 253-259 Totowa, NJ Humana Press
61. Labandeira-ReyM
SkareJT
2001 Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun 69 446 455
62. McDowellJV
SungSY
Labandeira-ReyM
SkareJT
MarconiRT
2001 Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect Immun 69 3670 3677
63. PurserJE
NorrisSJ
2000 Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97 13865 13870
64. StewartPE
HoffJ
FischerE
KrumJG
RosaPA
2004 Genome-Wide Transposon Mutagenesis of Borrelia burgdorferi for Identification of Phenotypic Mutants. Appl Environ Microbiol 70 5973 5979
65. NorrisSJ
HowellJK
GarzaSA
FerdowsMS
BarbourAG
1995 High - and low-infectivity phenotypes of clonal populations of in vitro - cultured Borrelia burgdorferi. Infect Immun 63 2206 2212
66. YangX
PopovaTG
HagmanKE
WikelSK
SchoelerGB
1999 Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun 67 6008 6018
67. XuH
HeM
PangX
XuZC
PiesmanJ
YangXF
2009 Characterization of the highly regulated antigen BBA05 in the enzootic cycle of Borrelia burgdorferi. Infect Immun 15 1872 1887
68. QuonKC
MarczynskiGT
ShapiroL
1996 Cell Cycle Control by an Essential Bacterial Two-Component Signal Transduction Protein. Cell 84 83 93
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy