-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
The high-risk HPV E6 and E7 proteins cooperate to immortalize primary human cervical cells and the E7 protein can independently transform fibroblasts in vitro, primarily due to its ability to associate with and degrade the retinoblastoma tumor suppressor protein, pRb. The binding of E7 to pRb is mediated by a conserved Leu-X-Cys-X-Glu (LXCXE) motif in the conserved region 2 (CR2) of E7 and this domain is both necessary and sufficient for E7/pRb association. In the current study, we report that the E7 protein of the malignancy-associated canine papillomavirus type 2 encodes an E7 protein that has serine substituted for cysteine in the LXCXE motif. In HPV, this substitution in E7 abrogates pRb binding and degradation. However, despite variation at this critical site, the canine papillomavirus E7 protein still bound and degraded pRb. Even complete deletion of the LXSXE domain of canine E7 failed to interfere with binding to pRb in vitro and in vivo. Rather, the dominant binding site for pRb mapped to the C-terminal domain of canine E7. Finally, while the CR1 and CR2 domains of HPV E7 are sufficient for degradation of pRb, the C-terminal region of canine E7 was also required for pRb degradation. Screening of HPV genome sequences revealed that the LXSXE motif of the canine E7 protein was also present in the gamma HPVs and we demonstrate that the gamma HPV-4 E7 protein also binds pRb in a similar way. It appears, therefore, that the type 2 canine PV and gamma-type HPVs not only share similar properties with respect to tissue specificity and association with immunosuppression, but also the mechanism by which their E7 proteins interact with pRb.
Published in the journal: The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein. PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001089
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001089Summary
The high-risk HPV E6 and E7 proteins cooperate to immortalize primary human cervical cells and the E7 protein can independently transform fibroblasts in vitro, primarily due to its ability to associate with and degrade the retinoblastoma tumor suppressor protein, pRb. The binding of E7 to pRb is mediated by a conserved Leu-X-Cys-X-Glu (LXCXE) motif in the conserved region 2 (CR2) of E7 and this domain is both necessary and sufficient for E7/pRb association. In the current study, we report that the E7 protein of the malignancy-associated canine papillomavirus type 2 encodes an E7 protein that has serine substituted for cysteine in the LXCXE motif. In HPV, this substitution in E7 abrogates pRb binding and degradation. However, despite variation at this critical site, the canine papillomavirus E7 protein still bound and degraded pRb. Even complete deletion of the LXSXE domain of canine E7 failed to interfere with binding to pRb in vitro and in vivo. Rather, the dominant binding site for pRb mapped to the C-terminal domain of canine E7. Finally, while the CR1 and CR2 domains of HPV E7 are sufficient for degradation of pRb, the C-terminal region of canine E7 was also required for pRb degradation. Screening of HPV genome sequences revealed that the LXSXE motif of the canine E7 protein was also present in the gamma HPVs and we demonstrate that the gamma HPV-4 E7 protein also binds pRb in a similar way. It appears, therefore, that the type 2 canine PV and gamma-type HPVs not only share similar properties with respect to tissue specificity and association with immunosuppression, but also the mechanism by which their E7 proteins interact with pRb.
Introduction
Human papillomaviruses (HPVs) mediate the initiation and maintenance of cervical cancer [1], [2]. Based upon DNA sequence homology, there are more than 150 different HPV genotypes, 40 of which infect anogenital and oral mucosa [3]. In addition to genotyping, HPVs can also be classified as low-risk and high-risk based on the clinical prognosis of their associated lesions. Low-risk HPVs cause benign epithelial hyperplasias while high-risk HPVs cause lesions that can progress to malignancy. Integration of the HPV genome into a host cell chromosome is a frequent event during malignant progression and it may play a significant role in dysregulated expression of the HPV E6 and E7 proteins [4]. The high-risk HPV E6 binds to several cell targets, including p53, Myc, E6AP, PDZ proteins and other cellular proteins to alter apoptotic/growth regulatory pathways and induce cellular telomerase activity [5]. The E7 protein binds and sequesters pRb and directs its ubiquitin-mediated proteolysis [6], thereby altering E2F-regulated genes and allowing cells to enter the S phase of the cell cycle.
The E7 oncoprotein is approximately 100 amino acids in length and contains two highly conserved regions (CRs), the amino-terminal CR1 and CR2 domains [7]. The E7 CR1 and CR2 domains share strikingly high homology with the CR1 and CR2 regions of adenovirus (Ad) E1A and related sequences in simian vacuolating virus 40 (SV40) large tumor antigen (T Ag) [4], [8]. For each of these viruses, the CRs contribute significantly to cell transformation [9], [10], [11], [12]. A conserved Leu-X-Cys-X-Glu (LXCXE) motif in the CR2 domain of HPV E7, as well the ones in Adenovirus E1A and SV40 LT, are necessary and sufficient for association with pRb [13]. The crystal structure of pRb bound to an E7 peptide was resolved, and revealed that LXCXE of HPV E7 binds entirely through the B domain of pRb [14]. For high risk HPV, the LXCXE motif is also required for pRb degradation[15], [16]. The carboxyl-terminal domain of E7 consists of a metal binding domain composed of two CXXC motifs separated from each other by 29 amino acids [14]. This zinc-binding region is important for E7 dimerization and intracellular stabilization [10], [17]. The carboxyl-terminal domain also contributes to E7 association with chromatin-modifying enzymes, particularly histone deacetylases and histone acetyl transferases [18]. Although the carboxyl-terminus of high-risk HPV E7 does not appear to have a direct role in the binding and degradation of pRb [15], [19], it has been proposed to be important for releasing E2F from pRb [20], [21].
Papillomavirus can be isolated from a wide range of vertebrates, ranging from birds to manatees [22], [23] and infection by these viruses is, in general, species-specific. The canine papillomavirus model has been used successfully for vaccine [24], [25] and therapeutics studies [26]. Recently, our lab isolated and sequenced canine papillomavirus type 2 (CPV-2, previously named CfPV2), which we showed to be an epidermotropic virus that occurred frequently in immunosuppressed animals and induced tumors that progressed to aggressive cancers [27], [28]. The E7 gene of CPV-2 appeared unique in that it lacked the conserved LXCXE motif. In this study we show that this variant E7 protein is still able to bind and degrade pRb and that the primary domain for binding pRb is in its carboxyl-terminus. Interestingly, upon searching the HPV genome database, we observed that the gamma HPVs also contained the variant LXSXE E7 domain and, similar to the canine E7 protein, could still bind and degrade pRb. In addition to this similarity, we also noted that both the type 2 canine PV and gamma HPVs both exhibited a tropism for skin and for immunocompromised hosts.
Materials and Methods
Plasmids
Wild-type CPV-2 E6 and E7 were generated by PCR using CPV-2 genome as template [27] and subcloned into retrovirus vector pLXSN (Clontech) at the sites EcoR I and BamH I. The CPV-2 E7 mutants CPV-2 E7 ΔLXSXE, S26C, and S26G were generated by using the QuikChange Site-Directed Mutagenesis kit (Stratagene), and CR1, CR2, CR1CR2 and CT were generated by PCR using pLXSN.CPV-2E7 as template. All the wild type E7 and mutants were cloned into the sites EcoR I and Not I of the pGEX4T-2 (GE Healthcare) for GST fusion protein expression. CPV-2 E7 and mutants with a hemagglutinin (HA) epitope tag at their amino terminal or carboxyl terminal were generated by PCR. PCR products were then subcloned into the mammalian expression vector pJS55 [29] at the sites EcoR I and BamH I. All plasmids were sequenced to confirm the presence of corresponding mutations. All primer sequences used in subcloning and site-directed mutagenesis please see Supporting Information S1. All the wild type and mutants of HPV-4 E7 DNA (GenBank NC_001457.1) were synthesized (Celtek Bioscience), and cloned into pGEX4T-2 (GE Healthcare) for GST fusion protein expression.
Cell lines and culture
U2OS cells, Hela cells and SD3443 cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen) supplemented with 10% Fetal Bovine Serum (FBS). Primary human keratinocytes were derived from neonatal foreskins as described previously [30] and were grown in Keratinocyte-SFM medium (Invitrogen).
Transfection and retrovirus Infection
U2OS cells were co-transfected with RcCMV-Rb and pJs55, pJS55-HA.HPV16 E7, pJS55-HA.CPV-2 E7ΔLXSXE, pJS55-HA.CPV-2 E7CR1CR2, pJS55-HA.CPV-2 E7C-RT or pJS55-HA.CPV-2 E7 using Lipofectamine 2000 (Invitrogen) as specified by the manufacturer. Hela cells were transfected with pJs55, pJS55-HA.HPV16 E7 or pJS55-HA.CPV-2 E7 using Lipofectamine 2000 (Invitrogen) as specified by the manufacturer. Cell were harvested and lysed by RIPA buffer (25 mM Tris•HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) 24 hours post transfection.
To prepare retrovirus stocks, SD3443 cells were transfected with E7 retrovirus constructs using Fugen (Roche applied science, US.) as specified by the manufacturer. Culture supernatants containing retrovirus were collected 48 h post-transfection. Viral titers of the supernatants were determined using 3T3 cells. The primary HFK cells (passage 0) were infected at a multiplicity of 10 PFU/cell with retrovirus expressing wild type E6, E7 or E7 mutants. Retrovirus-infected cells were selected in G418 (50 ng/ml) for 2 days.
GST fusion protein expression and purification
GST and GSTE7 fusion proteins were expressed in BL21pLysS cells (Invitrogen). The cells were induced with 100 µM isopropyl-β-D-thiogalactopyranoside (IPTG) 6 hours at 25°C once the optical density at 600 nm reached 0.8–1.0. Recombinant CPV-2 E7 and mutants were purified from the supernatant of disrupted cells by glutathione-Sepharose chromatography as previously described [24].
Preparation of cell extracts and Western blot analysis
Proteins were extracted from cells and measured concentration as previously described[31]. Proteins were separated on a 4 to 20% Tris-glycine gradient gel (Novex) and then were electrophoretically transferred to an Immobilon-P polyvinylidane difluorid (PVDF) membrane (Millipore). The primary antibody was used at a dilution of 1∶1,000 or 1∶3,000. The secondary antibodies, alkaline phosphatase-conjugated goat anti-mouse IgG and anti-rabbit IgG (Tropix) antibodies, were used at a dilution of 1∶2,000. Western blots were visualized by using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). The following commercial antibodies were used: for pRb (1∶1000 dilution), Rb (4H1) Mouse mAb (Cell Signaling technology); for glutathione S-transferase (1∶3000 dilution), catalog no. 3818-1 (Clontech); for HA (1∶1000 dilution), HA.11 clone 16B12 (Covance).
Analysis of protein interactions
For GST pull-down assays, Jurkat cell or CPEK cell nuclear extract (50 µg) was incubated with 5 µg of GST or GST fusion protein in binding buffer [20 mM Hepes/150 mM KCl/4 mM MgCl2/1 mM EDTA/0.02% Nonidet P-40/10% glycerol/0.035% 2-mercaptoethanol/1% (vol/vol) Sigma protease inhibitor mixture] and rocked for 1 h at 4°C. Glutathione-Sepharose beads (Amersham Pharmacia Biosciences) were added to each reaction and rocked for another 1 h at 4°C. The beads were then washed with 1 ml of washing buffer (125 mM Tris, 150 mM NaCl, pH 8.0) four times and boiled with 2× SDS sample buffer, and the proteins were separated by SDS/PAGE. Western blots were used to measure the level of pRb and GSTE7 proteins. The bands of pRb and GSTE7 proteins were quantified by densitometry using Quantity One (BioRad). The relative binding activities were calculated using pRb bound by wild type CPV-2 E7 GST fusion protein as 100%, and normalized with GSTE7 bands.
For co-immunoprecipitation assays, N-terminal HA-tagged CPV-2 E7 proteins were immunoprecipitated with a polyclonal anti-pRb antibody (Santa cruz). C-terminal HA-tagged CPV-2 or HPV16 E7 proteins were immunoprecipitated with a polyclonal anti-HA antibody (Santa Cruz). Bead washing buffer was 25 mM Tris•HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1% Sigma protease inhibitor mixture. The pulled down complexes were resolved on a 4 to 20% gradient gel and then analyzed by Western blotting using either anti-pRb antibody, anti-HA antibody or anti-cullin2 antibody (Invitrogen).
Reverse transcription PCR
Cells in a 3.5 cm diameter dish were lysed with 1 ml TRIZOL (Invitrogen). Total RNA was isolated according to manufacturer's protocol. Reverse transcription PCR was performed using ONE STEP RT-PCR KIT (QIAGEN) as specified by the manufacturer. All primer sequences and condition please see Supporting Information S1.
Protein sequence analysis
Multiple sequence alignments of E7 were prepared using Clustal W. The phyllogenetic analysis was conducted using the Mega version 4.0 [32].
Results
Despite lacking the conserved LXCXE motif, CPV-2 E7 can still degrade pRb in cells
Several studies have demonstrated that the conserved LXCXE motif in the HPV E7 CR2 domain is necessary and sufficient for binding pRb [4]). Studies have also revealed that the substitution of C or E or complete deletion mutation of the LXCXE motif destroys pRb binding. Without binding to the pRb, E7 is unable to degrade pRb [4]. Our laboratory recently isolated CPV-2 from footpad and interdigital papillomas of immunosuppressed dogs. Sequencing revealed that the CPV-2 E7 protein contained the typical two C-X-X-C motifs within its carboxyl terminal half but lacked the conserved pRb binding site (LXCXE) which is present in COPV (CPV-1) E7 and most HPVs (Figure 1A). CPV-2 E7 has a serine (amino acid 26) in the position of cysteine in the LXCXE motif. In order to test whether CPV-2 E7 could degrade pRb, canine E7 was transduced into human keratinocytes (HFKs), canine kidney cells (MDCKs) or canine keratinocytes (CPEKs) by using retrovirus infection. Cell lysates were collected, and pRb levels were measured by western blots. Surprisingly, despite lacking the conserved LXCXE motif, CPV-2 E7 was still able to degrade pRb in HFKs, MDCKs and CPEKs (Figure 1B, C and D). To test whether the lower level of pRb was due to a change at the transcriptional level, RT-PCR was performed to measure the level of pRb mRNA. There was no significant difference between the amount of pRb mRNA in control cells and cells with CPV-2 E7 (Figure 1E). In addition, treatment of the E7 expressing cells with the proteasome inhibitor, MG132, restored the level of pRb (Figure 1F). These data suggest that the reduction of pRb by CPV-2 E7 occurs at the protein level rather than mRNA level, and that degradation is most likely responsible.
Fig. 1. Despite the lack of a conserved LXCXE motif, CPV-2 E7 still degrades pRb. 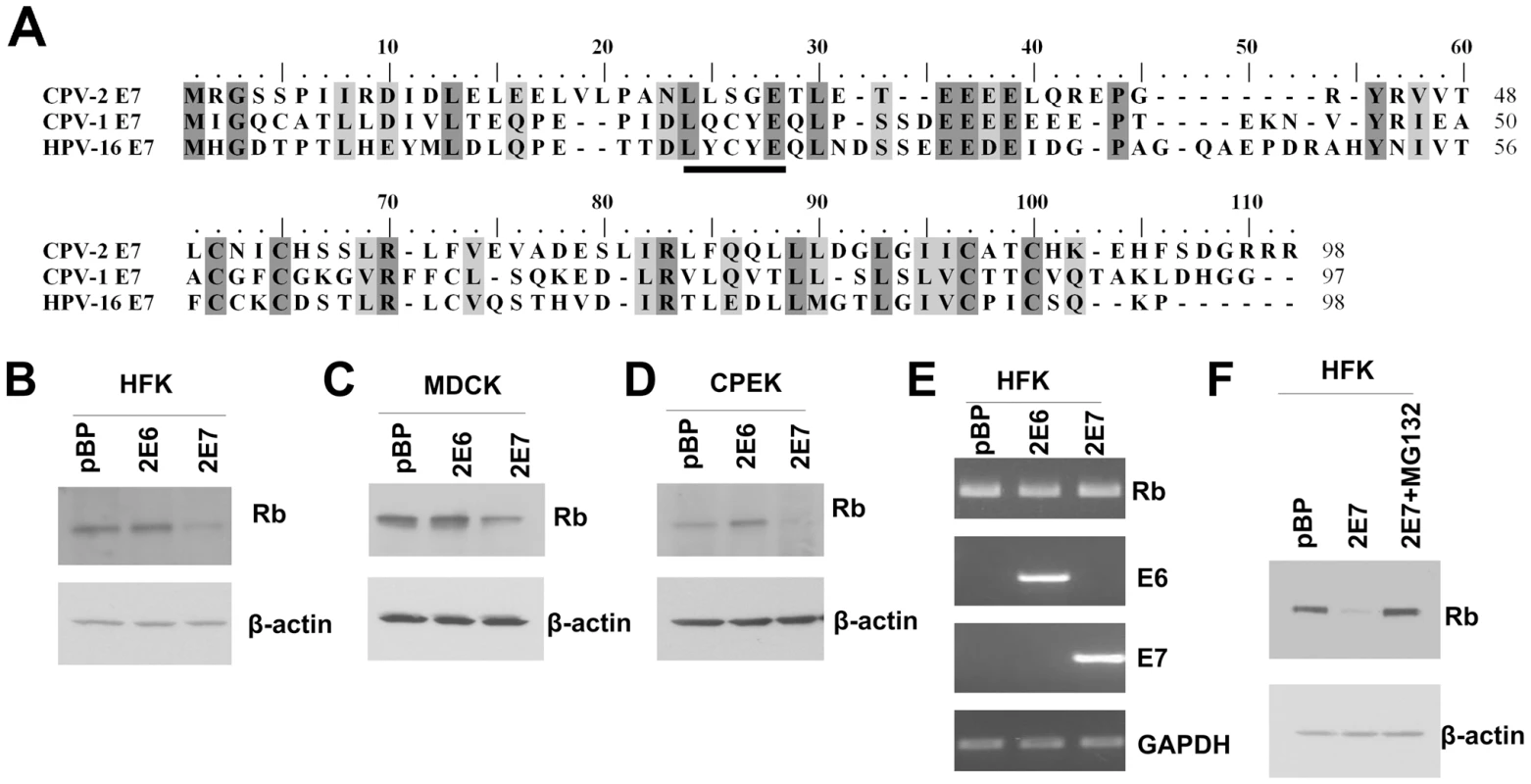
(A) Alignment of CPV-2 E7, CPV-1E7 and HPV16 E7 protein sequences. The underlined sequence is the conserved LXCXE domain. (B-D) Human foreskin keratinocytes (HFKs), canine MDCK cells or canine keratinocytes (CPEKs) were infected with control retrovirus or retrovirus encoding for CPV-2 E6 (2E6) or E7 (2E7). Cell lysates were prepared from indicated cells. Proteins were separated on a 4 to 20% gradient gel and then analyzed by Western blotting using anti-pRb antibody. (E) RNA was isolated from indicated cells, and RT-PCR was used to measure the level of RNA transcripts. (F) Cell lysates were prepared from cells treated with control DMSO or 40 um of MG132 for 4 hours. The level of pRb was measured by western blot. In this figure and all following figures, the described experiments were repeated at least three times, with representative results shown. The LXSXE motif is not required for binding of pRb by CPV-2 E7
The degradation of pRb by HPV16 E7 requires high affinity binding [19]. Since CPV-2 E7 lacks the conserved pRb binding motif, LXCXE, there could be two possibilities for the high affinity binding of pRb by CPV-2 E7. It could be either that the LXSXE motif has the same binding properties as LXCXE, or that CPV-2 E7 has an alternative dominant binding site. We generated E7 mutants (Figure 2A) with mutations within the LXSXE domain to investigate whether the LXSXE motif exhibits similar binding to pRb as LXCXE. The GST E7 wild type and mutant fusion proteins were purified from bacteria (Figure 2B), and tested for their binding to pRb. As demonstrated in Figure 2C, wild type CPV-2 E7 binds well to pRb. Substitution of serine26 to cysteine in CPV-2 E7 significantly increased the interaction between E7 and pRb. This is in agreement with mutagenesis studies showing that mutation of HPV16 E7 cysteine24 to serine substantially decreased the binding activity [13], [33]. In the studies of HPV16 E7, the substitution of cysteine in LXCXE for glycine abolished the binding between E7 and pRb [14], [33], [34], [35]. However, for CPV-2 E7, the pRb binding activity of the glycine mutant protein was still retained (Figure 2C). More importantly, the deletion of the entire LXSXE motif did not significantly affect the ability of CPV-2 E7 to bind pRb. This is very surprising since the deletion of the LXCXE motif in HPV 16 E7 totally abolished pRb binding [14], [33], [34], [35]. Thus, while the CPV-2 LXSXE motif exhibits lower pRb binding than the conserved LXCXE motif, it is clear that there is an alternative pRb binding site in the CPV-2 E7 protein.
Fig. 2. The canine E7 LXSXE motif is not required for pRb binding. 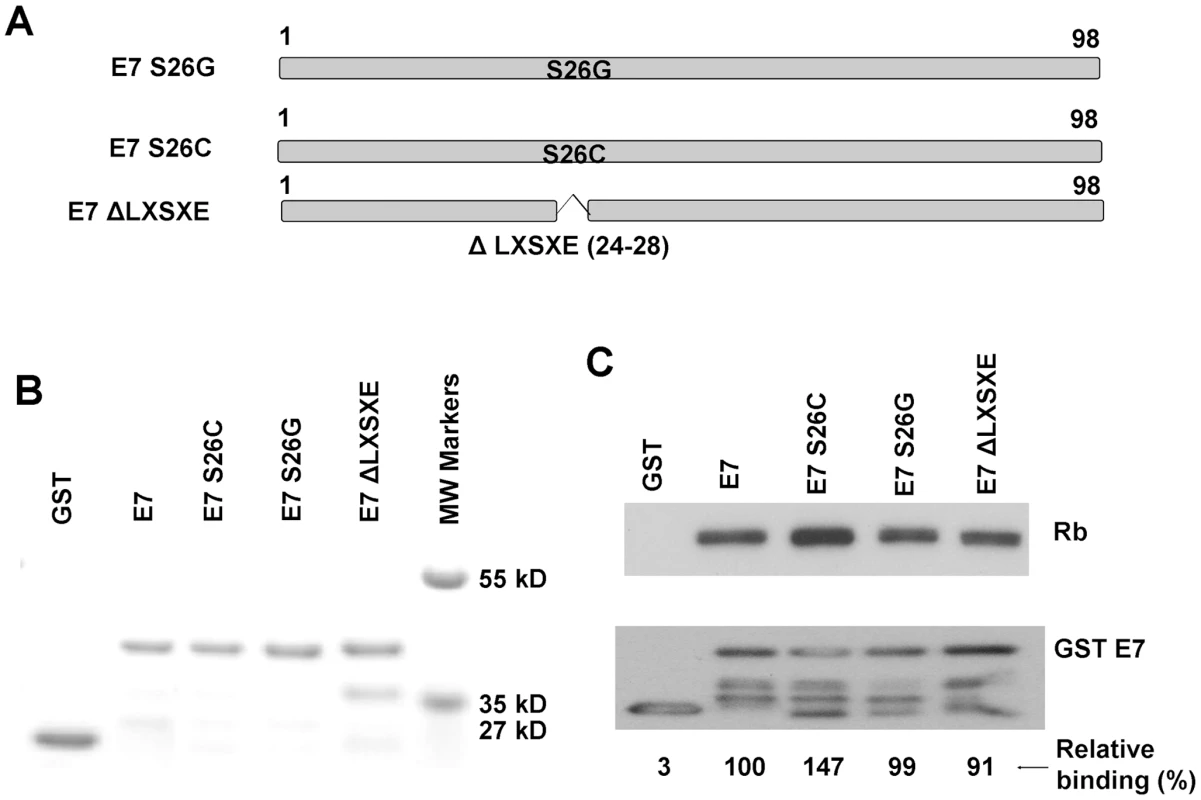
(A) Schematic representation of CPV-2 E7 proteins with mutations. (B) Expression of bacterially expressed GST E7 fusion proteins were confirmed by SDS-PAGE and Coomassie blue staining. (C) GST pull-down experiments were used to analyze the binding of GST E7 constructs to pRb. Complexes were resolved on 4–20% gradient gels and then analyzed by Western blotting using anti-pRb antibody or anti-GST antibody. The pRb and GSTE7 proteins were quantified by densitometry. The relative binding activities were normalized to GSTE7 protein and the wild type CPV-2 E7 GST fusion protein was set to 100%. In vitro, the CPV-2 E7 carboxyl-terminal domain mediates pRb binding
In order to locate potential alternative pRb binding sites in CPV-2 E7, several CPV-2 E7 truncation mutants were generated (Figure 3A). CPV-2 E7 protein was divided into CR1 (amino acids 1 to 15), CR2 (amino acids 16 to 38) and the C terminal domains (amino acids 39 to 98). The GST E7 wild type and mutant fusion proteins were purified from bacteria (Figure 3B). A binding assay with GSTE7 mutants and pRb was performed. In agreement with earlier studies [4], [14], [36], HPV16 E7CR1CR2 bound pRb much more efficiently than the HPV16 E7 carboxyl-terminus domain (Figure 3C), with 6 times more pRb being bound by the combined CR domains. In contrast, the carboxyl-terminus of CPV-2 E7 has much higher binding than the CR1 and CR2 domains, on the average 7 times higher. Importantly, similar binding affinities were also observed between the canine pRb and CPV-2 E7 (Figure 3D), indicating that these unique binding interactions were not due to differences in the species of Rb used for analysis. Thus, CPV-2 E7 uses its carboxyl-terminus instead of the CR1 and CR2 domains to associate with pRb.
Fig. 3. The carboxyl-terminus of canine E7 protein mediates pRb binding in vitro. 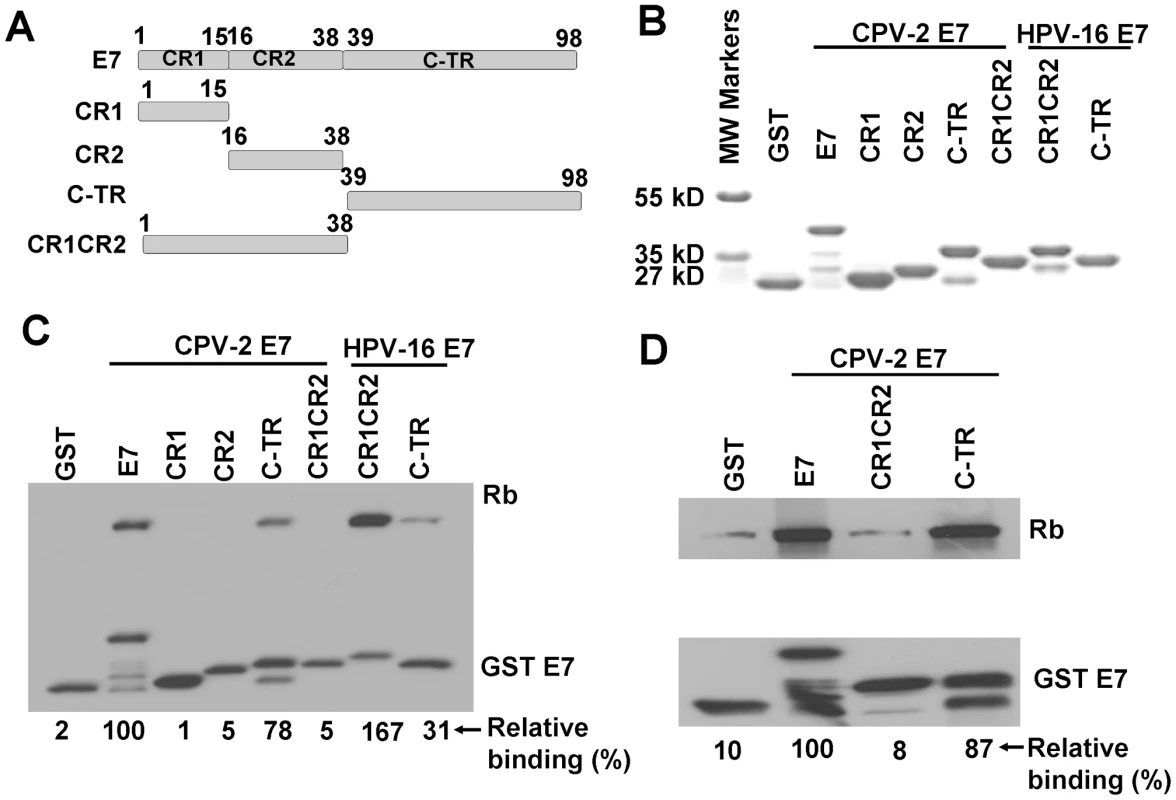
(A) Schematic representation of CPV-2 E7 proteins with mutations. CPV-2 E7 protein was divided into CR1 (amino acids 1 to 15), CR2 (amino acids 16 to 38) and C terminal domains (amino acids 39 to 98). (B) Expression of bacterially expressed GST tagged E7 truncated mutants were confirmed by SDS-PAGE and Coomassie blue staining. GST pull-down experiments were used to analyze the binding of GST E7 constructs to human pRb (C) and canine pRb (D). Complexes were resolved on 4–20% gradient gels and then analyzed by Western blotting using anti-pRb antibody or anti-GST antibody. The bands of pRb and GSTE7 proteins were quantified by densitometry. The relative binding activities were calculated using pRb bound by wild type CPV-2 E7 GST fusion protein as 100%, and normalized with GSTE7 bands. The CPV-2 E7 carboxyl-terminal domain also mediates pRb binding in vivo
To verify that the above in vitro binding studies were relevant to in vivo conditions, U2OS cells transduced by HA-tagged CPV-2 E7 constructs were used to study the in vivo association of CPV-2 E7 protein and pRb. Co-precipitation experiments were performed with the anti-pRb antibody (Figure 4). In contrast to results with HPV 16 E7, the CPV-2 E7 mutant deleted of the LXCXE-like motif bound pRb as well as the wild-type E7 did. Furthermore, the carboxy-terminal domain of CPV-2 alone bound to pRb very well. Thus, both in vitro and in vivo studies indicate that the E7 carboxyl-terminus mediates pRb binding. The level of canine E7 CR1CR2 construct was not detectable in the cell since it appears to be unstable. The doublet band noted for both wild-type E7 and the LXCXE deletion E7 proteins is most likely due to alkylation by protease inhibitors during the IP procedure. In previous studies, we also observed two distinct forms of HPV-16 E7 and showed that they were generated in vitro by the alkylating reagents, TPCK and TLCK [37]. These reagents are used as a component of a protease-inhibitor cocktail during IP studies to prevent protein degradation. We identified cysteine 27 near the amino terminus of HPV-16 E7 as the alkylation target. In our current study, we did not observe this modification of HPV-16 E7, apparently due to interference by the amino-terminal epitope tag. In the published study where we observed alkylated HPV-16 E7, we had used an E7 protein tagged at its C-terminus. We presume that the CPV-2 E7 protein is being modified in a similar fashion, although different sites might be altered than in HPV-16 E7.
Fig. 4. The carboxyl-terminus of canine E7 protein also mediates in vivo binding to pRb. 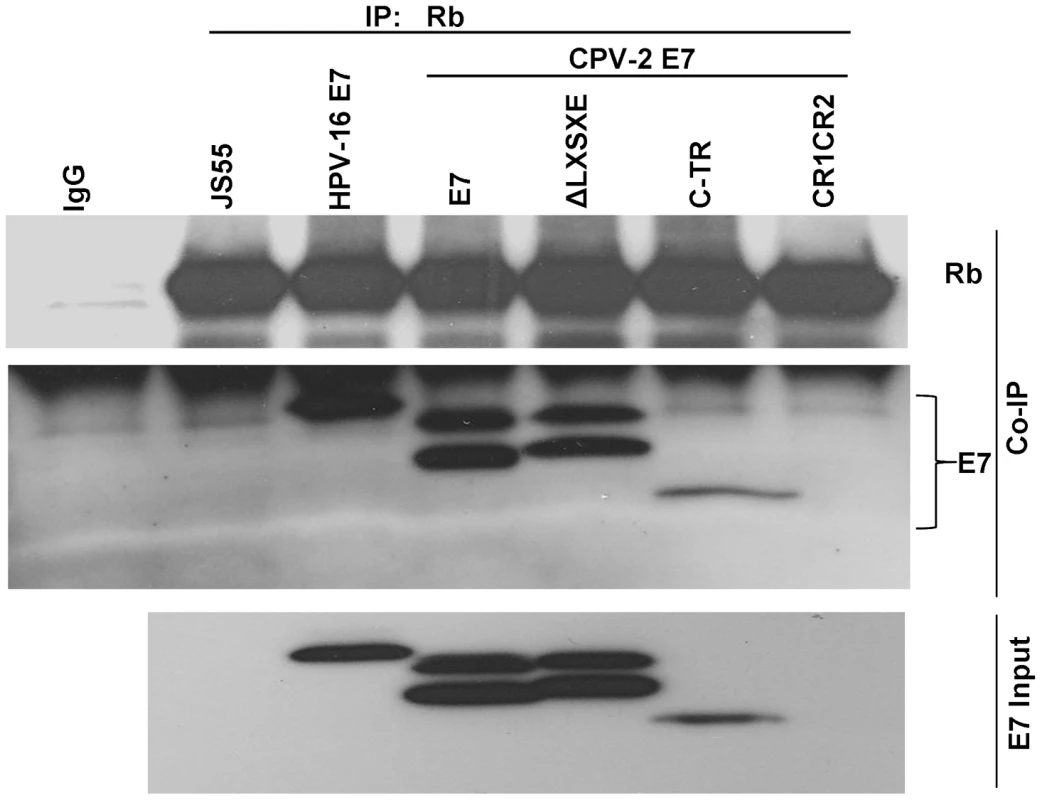
U2OS cells were co-transfected with a series of HA-tagged E7 mutants and Rb expression plasmids. Immuno-precipitation was performed with anti-pRb antibody and the pull-down complexes were resolved on a 4 to 20% gradient gel and then analyzed by Western blotting using either anti-pRb antibody or anti-HA antibody. The total relative level of E7 proteins expressed in the transfected cells is shown in the lower panel (diluted 1∶10). Unlike HPV-16 E7, the CPV-2 E7 carboxyl-terminus domain is critical for destabilization of pRb
Since the mechanism used by CPV-2 E7 to bind pRb is different from that used by HPV E7, it was also possible that the two E7 proteins used alternative methods to degrade pRb. To test this possibility, a series of E7 deletion and single amino acid substitution mutants were generated. Keratinocytes were transduced with retrovirus encoding either wild type E7 or E7 mutants. Cell lysates were collected, and the level of pRb was measured with immunoblots. Surprisingly, the LXSXE-deletion mutant (which retains pRb binding) lost the ability to degrade pRb (Figure 5A). In addition, when the serine at 26 was changed to cysteine (which increases pRb binding), the degradation of pRb was not enhanced (Figure 5A). Thus, although the primary pRb binding of CPV-2 resides in the carboxyl-terminus, we observed that the amino-terminal LXSXE sequence is necessary for the degradation of pRb. Neither the E7 amino - nor carboxyl-terminal domains could independently degrade pRb (Figure 5B). This is in contrast to studies performed on high risk HPV E7 (reviewed by Munger [38]) showing that the sequences important for binding and degradation of pRb localized to CR1 and CR2. Another difference between HPV16 E7 and CPV-2 E7 is highlighted by previous studies showing that HPV16 E7 associates with the cullin 2 ubiquitin ligase complex and that this association contributes to degradation of pRb [4], [14], [36]. This does not appear to be true for CPV-2 E7 protein. Co-immunoprecipitation assays failed to detect any association of Cullin2 with CPV-2 E7 (Figure 5C).
Fig. 5. Although not the primary binding site, the canine E7 amino-terminal domain of CPV-2 E7 is important for destabilization of pRb. 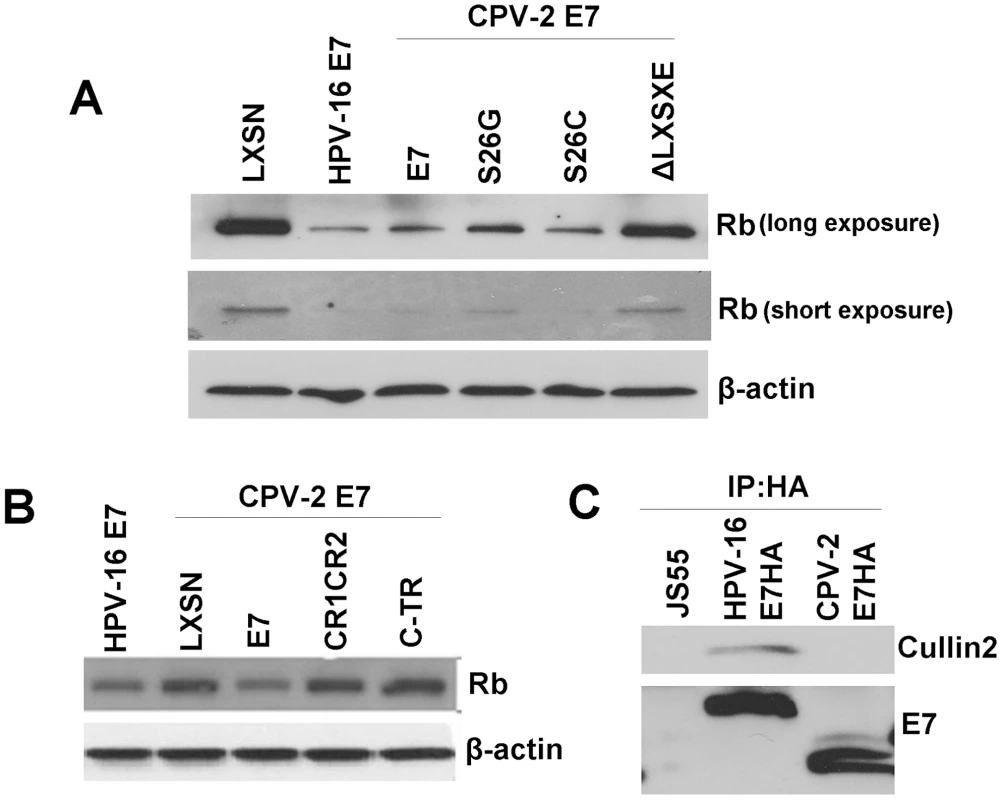
Keratinocytes were infected by retrovirus encoding wild type E7, E7 mutants with mutation on the LXSXE motif (A), or the domain truncation mutants (B). Cell lysates were prepared from indicated cells. Proteins were separated on a 4 to 20% gradient gel and then analyzed by Western blotting using anti-pRb antibody. (C) Cell lysates were prepared from Hela cells transfected with pJs55, pJS55-HA.HPV16 E7 or pJS55-HA.CPV-2 E7. Immuno-precipitation assay was performed with anti-HA antibody. The pulled down complexes were resolved on a 4 to 20% gradient gel and then analyzed by Western blotting using either anti-Cullin2 antibody or anti-HA antibody. A gamma HPV E7 protein also binds pRb via its carboxyl-terminus
Our current results demonstrate that at least one animal papillomavirus uses a different mechanism to bind and degrade pRb. To investigate whether some HPVs might use a similar alternative binding mechanism, we screened a papillomavirus phylogentic tree based upon the E7 protein sequence (Figure 6A). E7 proteins from 33 HPVs and 15 animal papillomaviruses were selected according to their genus [39] and aligned using Clustal W [40] with MEGA version 4.0 [32]. Based on the alignment, a phyllogenetic tree was assembled by using the minimum evolution method with MEGA version 4.0 [32]. CPV-2 E7 was most closely related to the genus gamma-papillomaviruses (HPV-4, 48, 50, 60, 65, 88, 95 and 116) and only distantly related to the genus lambda-papillomaviruses (CPV-1 and Felis domesticus papillomavirus). Interestingly, the alignment of E7 proteins revealed that all the gamma HPVs lack the LXCXE motif (Figure 6B) and, indeed, nearly all these gamma-HPVs contain the same LXSXE sequence found in CPV-2. One of the gamma-HPVs, HPV60, contains alanine rather than serine in the LXSXE sequence. To test the binding of a representative gamma-HPV E7 to pRb, HPV-4 E7 was synthesized and cloned into an expression vector. HPV-4 E7 protein was divided into CR1 (amino acids 1 to 15), CR2 (amino acids 16 to 38) and carboxyl-terminal domains (amino acids 39 to 100) (Figure 6C). Wild type HPV-4 E7 and truncation mutants were expressed as GST fusion proteins (Figure 6D) and tested for their abilities to bind pRb. Similar to CPV-2 E7, the HPV-4 E7 protein contained an LXSXE motif and bound pRb (Figure 6E). More interestingly, as shown in Figure 6E, the carboxyl - terminus of HPV-4 E7 bound pRb more efficiently than the CR1CR2 domains. It appears, therefore, that the gamma-type HPVs and CPV-2 share a mechanism by which their E7 proteins interact with pRb via the carboxyl-terminal domain.
Fig. 6. Similarities of the CPV-2 and gamma HPV E7 proteins. 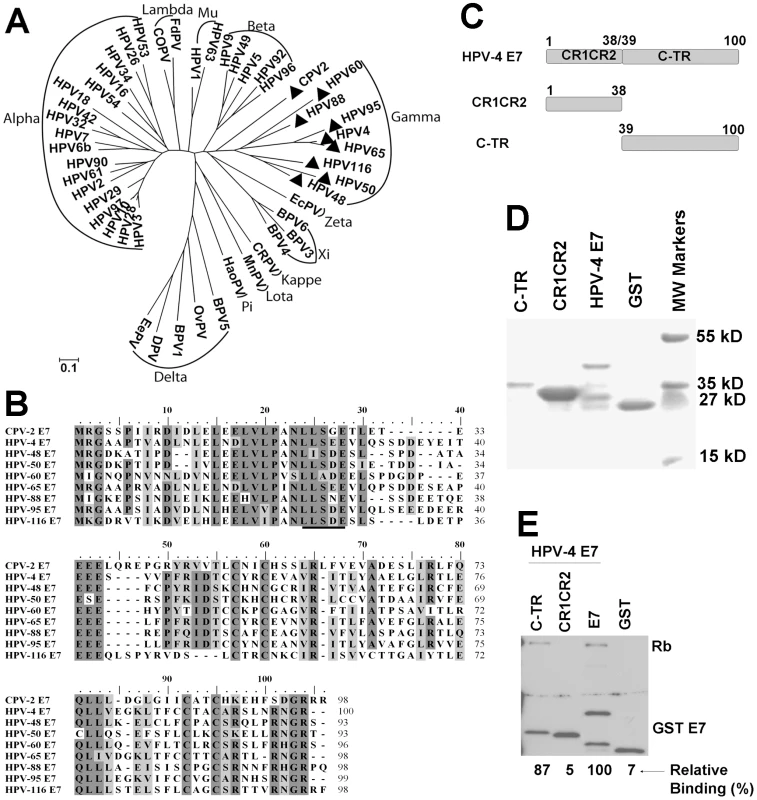
(A) Phylogenetic analysis of papillomavirus E7 gene sequences. The E7 gene of CPV-2 was compared with 33 HPV and 15 animal PV DNA sequences in order to establish its closest relatives. The analyzed papillomavirus genomes included were FcPV (K02019), bovine BPV1 (NC_001522), BPV2 (NC_001521), BPV3 (NC_004197)BPV4 (X05817), canine oral CPV-1 (NC_001619), Canine papillomavirus type 2 CPV-2 (AY722648), cotton rabbit CRPV (NC_001541), deer DPV (NC_001523), Equus caballus EcPV1 (AF498323), European elk EEPV (NC_001524), Felis domesticus FdPV1 (AF480454), Hamster oral HaOPV (E15110), HPV1 (NC_001356), HPV3 (NC_001588), HPV-4 (NC_001457), HPV5 (NC_001531), HPV6b (NC_000904), HPV9 (NC_001596), HPV10 (X74465), HPV16 (NC_001526), HPV18 (NC_001357), HPV26 (X74472), HPV29 (NC_001685), HPV32 (X74475), HPV34 (X74476), HPV-41 (X56147), HPV-42 (M73236), HPV-48 (NC_001690), HPV-49 (NC_001591), HPV50 (NC_001691), HPV53 (NC_001593), HPV54 (U37488), HPV60 (NC_001693), HPV61 (U31793), HPV63 (X70828), HPV90 (AY057438), HPV92 (AF531420), HPV94 (AJ620211), HPV96 (AY382779), Mastomys natalensis MnPV (NC_001605), Ovine OvPV1 (NC_001789), OvPV2 (NC_001790), Psittacus erithacus PePV (AF502599), Phocoena spinipinnis PsPV1 (NC_003348) and rabbit oral ROPV (NC_002232). (B) E7 proteins from CPV-2 and Gamma HPVs papillomaviruses were selected, and aligned using Clustal W with MEGA version 4.0. The conserved LXSXE domain is underlined. (C) Schematic representation of HPV-4 E7 proteins with mutations. HPV-4 E7 protein was divided into CR1 (amino acids 1 to 15), CR2 (amino acids 16 to 38) and C terminus domains (amino acids 39 to 100). (D) Expression and purification of bacterial expressed GST tagged E7 truncated mutants were confirmed by SDS-PAGE and Coomassie blue staining. (E) GST pull down experiments were used to analyze the binding of GST E7 constructs to pRb. Complexes were resolved on a 4 to 20% gradient gel and then analyzed by Western blotting using anti-pRb antibody or anti-GST antibody. Densitometry was used to measure the relative binding activities and normalized with GSTE7 bands. HPV-4 E7 is able to degrade pRb
Due to the similar ability of the CPV-2 and HPV-4 E7 proteins to bind pRb, we also evaluated whether HPV-4 E7 could degrade pRb. In HFKs, HPV-4 E7 reduced the level of pRb in transduced cells, although somewhat less than observed with CPV-2 or HPV16 E7 (Figure 7A). To test whether the lower level of pRb protein might be due to altered gene transcription, we measured pRb mRNA levels by RT-PCR. There was no significant difference in the amount of pRb mRNA in the control cells compared to cells expressing CPV-2 E7 (Figure 7B), indicating that the pRb protein changes were post-translational. More importantly, treatment of the E7 expressing cells with proteasome inhibitor, MG132, restored the level of pRb protein (Figure 7C). These data, similar to that for CPV-2, suggest that the reduction of pRb by HPV-4 E7 is most likely the result of protein degradation.
Fig. 7. HPV-4 E7 also degrades pRb. 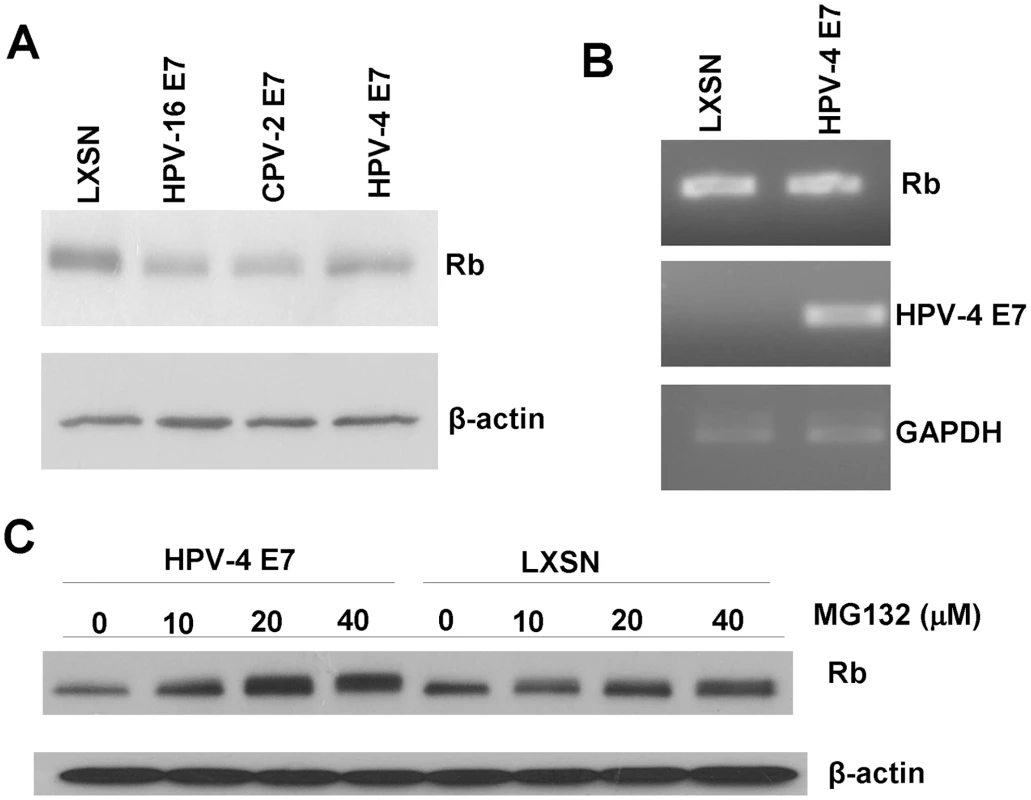
HFKs were infected with control retrovirus or retrovirus encoding for HPV16, CPV-2 or HPV-4 E7. (A) Cell lysates were prepared from indicated cells. Proteins were separated on a 4 to 20% gradient gel and then analyzed by Western blotting using anti-pRb antibody. (B) RNA was prepared from the cells, and the levels of Rb, E7 or GAPDH mRNA were quantified by RT-PCR. (C) Cells were treated for 4 hours with proteasome inhibitor, MG132, and lysates were prepared from the indicated cells. Proteins were separated on a 4 to 20% gradient gel and then analyzed by Western blotting using anti-pRb antibody. Discussion
Small DNA tumor viruses, such as HPV, Adenovirus, and Polyomavirus, produce viral oncoproteins that can interact with pRb and alter its function. Targeting pRb appears important for the ability of these viruses to regulate E2F and cell DNA replication and to complete the virus life cycle. All these oncoproteins, E7, E1A, and LT, use a conserved CR2 domain and LXCXE motif to bind pRb [41]. However, for CPV-2 E7, the LXCXE-like motif is not necessary for association with or degradation of pRb. It appears that CfPV has evolved an alternative mechanism to bind pRb by using the E7 carboxyl-terminal domain.
Although the HPV E7 carboxyl-terminus has been proposed to have an independent, low affinity pRb binding site [14], [36], HPV16 E7 mutants with a deletion of the LXCXE motif in CR2 fail to associate with pRb family members as determined by Western blotting and extensive proteomic analyses of associated cellular protein complexes [4]. In contrast, the carboxyl-terminus of CPV-2 E7 exhibits greater pRb binding than the CR1 and CR2 domains. Even more interesting is the finding that the CPV-2 E7 mutant deleted of the LXSXE domain still can bind to pRb with high efficiency. Furthermore, the carboxyl-terminus of CPV-2 E7 alone can bind pRb in vitro and in vivo. The carboxyl-terminus of HPV E7 has been proposed to be important for releasing E2F from pRb [20], [21]. It will be interesting to map the association site on pRb, and to determine whether the binding induces the release of E2F from pRb.
During preparation of this manuscript, three new canine papillomaviruses were identified [42]. One of the viruses, CPV7, shares high sequence homology with CPV-2 and its predicted E7 ORF encodes a protein with the LXSXE motif. Overall, however, 5 of the 7 identified canine papillomaviruses contain the LXCXE motif in their E7 protein. CPV-1 E7, which has LXCXE motif, degrades pRb in cells as anticipated (data not shown).
As shown in this study, the LXSXE motif is not limited to canine papillomaviruses; the gamma genus of HPVs also have the same motif. This genus consists of eight HPVs, 7 of which contain the E7 LXSXE motif. Interestingly, the gamma HPVs have several other similarities to CPV-2. First, they induce cutaneous rather than mucosal lesions. Second, they most likely persist in the population as subclinical infections and induce visually detectable tumors only under conditions of immunosuppression. Third, their tumor cells are characterized by histologically-distinguishable intracytoplasmic inclusion bodies [39]. The canine model may provide a new approach for studying the biology of this unique category of papillomaviruses and their stringent regulation by the host immune response.
Supporting Information
Zdroje
1. zur HausenH
2002 Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2 342 350
2. StarkA
GregoireL
PilarskiR
ZarboA
GabaA
2008 Human papillomavirus, cervical cancer and women's knowledge. Cancer Detect Prev
3. WoodmanCB
CollinsSI
YoungLS
2007 The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer 7 11 22
4. McLaughlin-DrubinME
MungerK
2009 The human papillomavirus E7 oncoprotein. Virology 384 335 344
5. HowieHL
KatzenellenbogenRA
GallowayDA
2009 Papillomavirus E6 proteins. Virology 384 324 334
6. HuhK
ZhouX
HayakawaH
ChoJY
LibermannTA
2007 Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol 81 9737 9747
7. BouletG
HorvathC
Vanden BroeckD
SahebaliS
BogersJ
2007 Human papillomavirus: E6 and E7 oncogenes. Int J Biochem Cell Biol 39 2006 2011
8. FiggeJ
WebsterT
SmithTF
PauchaE
1988 Prediction of similar transforming regions in simian virus 40 large T, adenovirus E1A, and myc oncoproteins. J Virol 62 1814 1818
9. EdmondsC
VousdenKH
1989 A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol 63 2650 2656
10. PhelpsWC
MungerK
YeeCL
BarnesJA
HowleyPM
1992 Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol 66 2418 2427
11. JewersRJ
HildebrandtP
LudlowJW
KellB
McCanceDJ
1992 Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J Virol 66 1329 1335
12. StoreyA
AlmondN
OsbornK
CrawfordL
1990 Mutations of the human papillomavirus type 16 E7 gene that affect transformation, transactivation and phosphorylation by the E7 protein. J Gen Virol 71 (Pt 4) 965 970
13. MungerK
WernessBA
DysonN
PhelpsWC
HarlowE
1989 Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. Embo J 8 4099 4105
14. LiuX
ClementsA
ZhaoK
MarmorsteinR
2006 Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem 281 578 586
15. HeltAM
GallowayDA
2001 Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J Virol 75 6737 6747
16. DysonN
GuidaP
MungerK
HarlowE
1992 Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol 66 6893 6902
17. ClemensKE
BrentR
GyurisJ
MungerK
1995 Dimerization of the human papillomavirus E7 oncoprotein in vivo. Virology 214 289 293
18. BrehmA
NielsenSJ
MiskaEA
McCanceDJ
ReidJL
1999 The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. Embo J 18 2449 2458
19. MungerK
BasileJR
DuensingS
EichtenA
GonzalezSL
2001 Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20 7888 7898
20. HuangPS
PatrickDR
EdwardsG
GoodhartPJ
HuberHE
1993 Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol Cell Biol 13 953 960
21. WuEW
ClemensKE
HeckDV
MungerK
1993 The human papillomavirus E7 oncoprotein and the cellular transcription factor E2F bind to separate sites on the retinoblastoma tumor suppressor protein. J Virol 67 2402 2407
22. RectorA
BossartGD
GhimSJ
SundbergJP
JensonAB
2004 Characterization of a novel close-to-root papillomavirus from a Florida manatee by using multiply primed rolling-circle amplification: Trichechus manatus latirostris papillomavirus type 1. J Virol 78 12698 12702
23. Moreno-LopezJ
AholaH
StenlundA
OsterhausA
PetterssonU
1984 Genome of an avian papillomavirus. J Virol 51 872 875
24. YuanH
EstesPA
ChenY
NewsomeJ
OlceseVA
2001 Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol 75 7848 7853
25. StanleyMA
MooreRA
NichollsPK
SantosEB
ThomsenL
2001 Intra-epithelial vaccination with COPV L1 DNA by particle-mediated DNA delivery protects against mucosal challenge with infectious COPV in beagle dogs. Vaccine 19 2783 2792
26. DisbrowGL
BaegeAC
KierpiecKA
YuanH
CentenoJA
2005 Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res 65 10854 10861
27. YuanH
GhimS
NewsomeJ
ApolinarioT
OlceseV
2007 An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology 359 28 36
28. GoldschmidtMH
KennedyJS
KennedyDR
YuanH
HoltDE
2006 Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J Virol 80 6621 6628
29. SparkowskiJ
AndersJ
SchlegelR
1994 Mutation of the bovine papillomavirus E5 oncoprotein at amino acid 17 generates both high - and low-transforming variants. J Virol 68 6120 6123
30. SchlegelR
PhelpsWC
ZhangYL
BarbosaM
1988 Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. Embo J 7 3181 3187
31. YuanH
VeldmanT
RundellK
SchlegelR
2002 Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J Virol 76 10685 10691
32. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
33. JonesRE
WegrzynRJ
PatrickDR
BalishinNL
VuocoloGA
1990 Identification of HPV-16 E7 peptides that are potent antagonists of E7 binding to the retinoblastoma suppressor protein. J Biol Chem 265 12782 12785
34. ForngRY
AtreyaCD
1999 Mutations in the retinoblastoma protein-binding LXCXE motif of rubella virus putative replicase affect virus replication. J Gen Virol 80 (Pt 2) 327 332
35. MungerK
HalpernAL
1997 hpv e7 primary structure and biological properties
36. PatrickDR
OliffA
HeimbrookDC
1994 Identification of a novel retinoblastoma gene product binding site on human papillomavirus type 16 E7 protein. J Biol Chem 269 6842 6850
37. StopplerH
StopplerMC
AdduciA
KovalD
SchlegelR
1996 The serine protease inhibitors TLCK and TPCK react with the RB-binding core of HPV-18 E7 protein and abolish its RB-binding capability. Virology 217 542 553
38. MungerK
BaldwinA
EdwardsKM
HayakawaH
NguyenCL
2004 Mechanisms of human papillomavirus-induced oncogenesis. J Virol 78 11451 11460
39. de VilliersE-M
FauquetC
BrokerTR
BernardH-U
zur HausenH
2004 Classification of papillomaviruses. Virology 324 17 27
40. ThompsonJD
HigginsDG
GibsonTJ
1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
41. FelsaniA
MileoAM
PaggiMG
2006 Retinoblastoma family proteins as key targets of the small DNA virus oncoproteins. Oncogene 25 5277 5285
42. LangeCE
ToblerK
AckermannM
PanakovaL
ThodayKL
2009 Three novel canine papillomaviruses support taxonomic clade formation. J Gen Virol
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



