Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
The herpesviruses, like most other DNA viruses, replicate in the host cell nucleus. Subnuclear domains known as promyelocytic leukemia protein nuclear bodies (PML-NBs), or ND10 bodies, have been implicated in restricting early herpesviral gene expression. These viruses have evolved countermeasures to disperse PML-NBs, as shown in cells infected in vitro, but information about the fate of PML-NBs and their functions in herpesvirus infected cells in vivo is limited. Varicella-zoster virus (VZV) is an alphaherpesvirus with tropism for skin, lymphocytes and sensory ganglia, where it establishes latency. Here, we identify large PML-NBs that sequester newly assembled nucleocapsids (NC) in neurons and satellite cells of human dorsal root ganglia (DRG) and skin cells infected with VZV in vivo. Quantitative immuno-electron microscopy revealed that these distinctive nuclear bodies consisted of PML fibers forming spherical cages that enclosed mature and immature VZV NCs. Of six PML isoforms, only PML IV promoted the sequestration of NCs. PML IV significantly inhibited viral infection and interacted with the ORF23 capsid surface protein, which was identified as a target for PML-mediated NC sequestration. The unique PML IV C-terminal domain was required for both capsid entrapment and antiviral activity. Similar large PML-NBs, termed clastosomes, sequester aberrant polyglutamine (polyQ) proteins, such as Huntingtin (Htt), in several neurodegenerative disorders. We found that PML IV cages co-sequester HttQ72 and ORF23 protein in VZV infected cells. Our data show that PML cages contribute to the intrinsic antiviral defense by sensing and entrapping VZV nucleocapsids, thereby preventing their nuclear egress and inhibiting formation of infectious virus particles. The efficient sequestration of virion capsids in PML cages appears to be the outcome of a basic cytoprotective function of this distinctive category of PML-NBs in sensing and safely containing nuclear aggregates of aberrant proteins.
Published in the journal:
Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus. PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001266
Category:
Research Article
doi:
https://doi.org/10.1371/journal.ppat.1001266
Summary
The herpesviruses, like most other DNA viruses, replicate in the host cell nucleus. Subnuclear domains known as promyelocytic leukemia protein nuclear bodies (PML-NBs), or ND10 bodies, have been implicated in restricting early herpesviral gene expression. These viruses have evolved countermeasures to disperse PML-NBs, as shown in cells infected in vitro, but information about the fate of PML-NBs and their functions in herpesvirus infected cells in vivo is limited. Varicella-zoster virus (VZV) is an alphaherpesvirus with tropism for skin, lymphocytes and sensory ganglia, where it establishes latency. Here, we identify large PML-NBs that sequester newly assembled nucleocapsids (NC) in neurons and satellite cells of human dorsal root ganglia (DRG) and skin cells infected with VZV in vivo. Quantitative immuno-electron microscopy revealed that these distinctive nuclear bodies consisted of PML fibers forming spherical cages that enclosed mature and immature VZV NCs. Of six PML isoforms, only PML IV promoted the sequestration of NCs. PML IV significantly inhibited viral infection and interacted with the ORF23 capsid surface protein, which was identified as a target for PML-mediated NC sequestration. The unique PML IV C-terminal domain was required for both capsid entrapment and antiviral activity. Similar large PML-NBs, termed clastosomes, sequester aberrant polyglutamine (polyQ) proteins, such as Huntingtin (Htt), in several neurodegenerative disorders. We found that PML IV cages co-sequester HttQ72 and ORF23 protein in VZV infected cells. Our data show that PML cages contribute to the intrinsic antiviral defense by sensing and entrapping VZV nucleocapsids, thereby preventing their nuclear egress and inhibiting formation of infectious virus particles. The efficient sequestration of virion capsids in PML cages appears to be the outcome of a basic cytoprotective function of this distinctive category of PML-NBs in sensing and safely containing nuclear aggregates of aberrant proteins.
Introduction
Promyelocytic leukemia protein (PML) is a major organizing component of structures that are referred to as PML nuclear bodies (PML-NBs) or nuclear domain 10 (ND10) bodies [1]–[3]. These nuclear bodies are heterogenous in size, shape and molecular composition [4]–[6], are prominent in most mammalian cell types and participate in many basic cellular functions, including transcriptional regulation [7], DNA repair [8] and apoptosis [9]–[12]. Human PML, located on chromosome 15, has nine exons and alternative splicing of PML transcripts produces at least 11 isoforms [13], [14]. PML isoforms share a conserved N-terminus, which has the characteristic RBCC/TRIM motif, including a RING finger domain, B boxes and a coiled coil domain. The PML N-terminus is important for PML heterodimer formation and oligomeriztion but each isoform has a unique C-terminal domain [14], [15]. The PML isoforms create PML-NBs with varying morphologies but the functional implications of these differences are not well understood [16]–[18]. While little is known about the tissue or cell specific patterns of expression of individual PML isoforms, these non-conserved PML regions are of interest for their likely involvement in isoform-dependent functions within particular cell types and in the response to changing intracellular or extracellular conditions [17].
Since their discovery, PML protein and PML-NBs have been investigated for their role in the virus-host cell interactions of DNA viruses that must replicate in the mammalian cell nucleus [19], [20]. These viruses include the very extensive Herpesviridae family as well as adenoviruses, papillomaviruses, polyomaviruses and other pathogens. During herpesvirus infection, genome copies are synthesized in nuclear replication compartments and genomic DNA is packaged into icosahedral nucleocapsids (NCs) formed by the major capsid protein and smaller capsid surface proteins. After assembly, NCs egress across the nuclear membrane for secondary envelopment in the cytoplasm and release as infectious virus particles [21], [22]. PML-NBs have been implicated in controlling the replication of herpes simplex virus (HSV) 1 and human cytomegalovirus shortly after virus entry by mechanisms that limit early viral gene transcription [19], [20], [23], [24]. To overcome these antiviral effects, HSV-1 targets PML for immediate proteosome-mediated degradation through functions of the viral ICP0 ubiquitin ligase protein [25]–[28]. PML is also an interferon (IFN)-inducible protein and must be degraded to prevent IFN-mediated inhibition of HSV-1 [29]. Like HSV-1, varicella-zoster virus (VZV) is a common human alphaherpesvirus [30]. In contrast to HSV-1 infection, PML protein and some PML-NBs persist in VZV-infected cells [31] although not in association with early nuclear replication compartments [32]. Depleting PML enhances VZV replication, suggesting a possible role for PML in the host cell defense [31].
VZV, which causes varicella (chickenpox) and herpes zoster (shingles), is a highly human-restricted pathogen [30], [33]. However, VZV pathogenesis can be investigated in vivo using xenografts of human dorsal root ganglia (DRG) and skin in a severe combined immunodeficiency (SCID) mouse model [34]–[36]. This model allows the analysis of viral and cellular mechanisms that facilitate or inhibit VZV when differentiated neural and skin cells are infected within their usual tissue microenvironments in vivo and in the absence of antiviral effects mediated by the adaptive immune response [37], [38]. The fundamental importance of intrinsic and innate cellular responses in achieving the usual balance between VZV and its human host is evident from the observations that VZV undergoes a transition to persistence in neurons within DRG xenografts without requiring VZV-specific adaptive immunity [36] and that VZV skin infection is highly regulated by type-I interferon produced by epidermal cells [39]. In these experiments, we have used the SCID mouse model to define VZV interactions with PML-NBs in human neural and epidermal cells infected with this nuclear replicating DNA virus in vivo.
The characteristics and functions of PML-NBs in human neural cells are of particular interest because VZV is a neurotropic herpesvirus [30]. Aberrant proteins generated in several neurodegenerative diseases, including Huntington's disease and spinocerebellar ataxias, accumulate in PML-NBs within neural cells and the usual nuclear distribution of PML is altered by their expression [40]–[45]. These diseases, classified as polyglutamine (polyQ) disorders, are associated with an unstable CAG repeat expansion resulting in the elongation of a polyglutamine (polyQ) tract in the abnormal gene product [46]. The mis-folded, oligomeric polyQ proteins are neurotoxic and form intranuclear aggregates, often with PML and proteins of the ubiquitin-proteasome system [47], [48]. Similar structures have been referred to as clastosomes [44], [49] and in some cases appear to protect the cell by confining the abnormal proteins to an intranuclear “safehouse” where they may be degraded [44], [50]–[52]. Here we report that endogenous PML forms distinctive, large PML-NBs consisting of spherical cages in the nuclei of neurons and satellite cells in human DRG and in skin cells during VZV pathogenesis. These nuclear organelles efficiently entrap newly assembled VZV capsids in vivo as well as in cultured cells in vitro. The PML-NBs closely resemble those that retain polyQ proteins in neurodegenerative diseases. Exogenous PML IV was the only isoform that sequestered VZV NCs and interacted with the ORF23 capsid surface protein, functions that required its unique C-terminus. PML IV-NBs also retained the Huntington's disease protein, Htt, and could simultaneously sequester NCs. Importantly, the nuclear entrapment of viral capsids in PML IV-NBs inhibited the production of infectious VZV progeny. These observations suggest that PML-NBs can retain nascent virion capsids in the infected cell nucleus, resulting in an intrinsic antiviral defense at later stages of viral replication.
Results
PML-NBs Colocalize with the ORF23 Capsid Protein in VZV-infected Cells
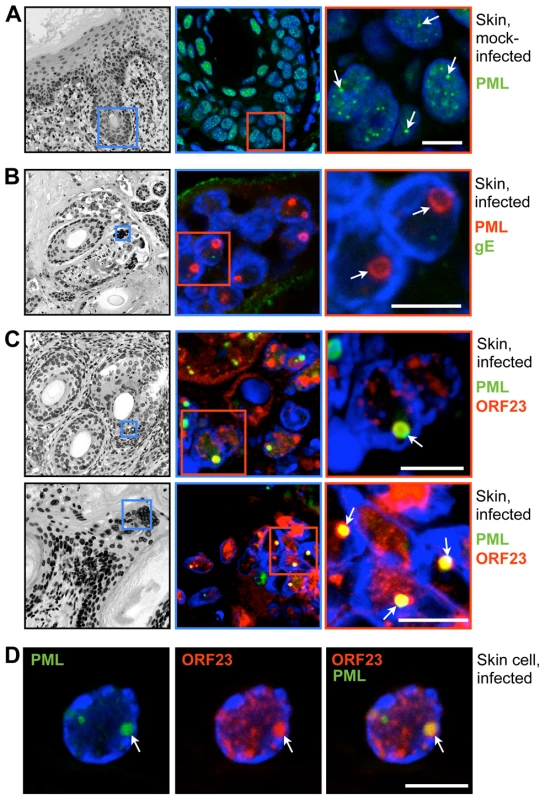
Since PML protein and some PML-NBs persist in VZV infected cells [31], we first investigated the intracellular localization of VZV proteins that are found in nuclear viral replication compartments, including ORF29, the single stranded DNA binding protein and IE62, the major viral transactivating factor, as well as the ORF23 capsid protein, which is present in later stages of VZV infection when progeny virion assembly occurs [32]. As expected, analysis of uninfected human embryonic lung fibroblasts (HELF) by confocal microscopy showed numerous PML-NBs that contained both PML and SP100 protein, which are known components of PML-NBs [53](Figure 1A). In infected HELF, PML-NBs did not colocalize with viral DNA replication compartments, which were identified by ORF29 and IE62 expression [32], at this late time at 24 hr after infection (Figure S1A and B).
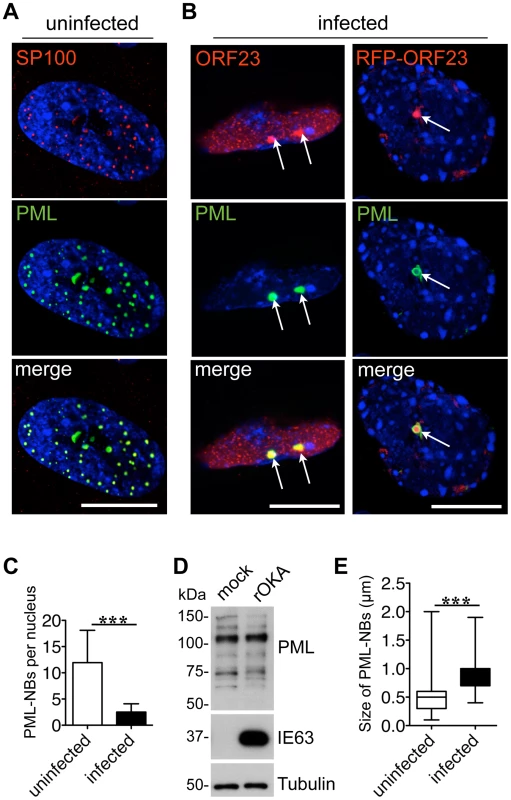
In marked contrast, the newly synthesized ORF23 capsid protein was present in almost all PML-NBs (94%, N = 228) at this stage of VZV infection (Figure 1B, left panel) using confocal imaging conditions that were optimized to identify bright PML-NBs associated with NCs. Based on its homology to the HSV-1 VP26 small capsid protein, ORF23 protein is predicted to be exposed on VZV NCs [54]. ORF23 protein was similarly enriched in PML-NBs in HELF infected with a VZV recombinant virus that expresses ORF23 protein tagged with the red fluorescent protein (RFP-ORF23) (Figure 1B, right panels) as well as in melanoma cells (Figure S2A). The number of PML-NBs decreased by about five-fold in both HELF and melanoma cells at 48 hr after infection compared to uninfected HELF and melanoma cells (Figure 1C and Figure S2B). Since melanoma cells have fewer PML-NBs than HELF before infection, the majority of infected melanoma cells had no PML-NBs left (Figure S2C). However, as previously reported [31], immunoblot analysis with a polyclonal anti-PML antibody showed that PML protein levels were not decreased in infected HELF or melanoma cells (Figures 1D and Figure S2D). The PML-specific bands were of varying sizes and may represent different PML isoforms or post-translational modifications of one or more of the PML isoforms. PML-NBs that colocalized with ORF23 protein in infected cells were also significantly larger than those in uninfected cells (Figure 1E). These results suggested that PML-NBs present at later stages of VZV infection sequestered ORF23 protein or possibly newly assembled NCs with surface ORF23 protein and might differ from the PML-NBs that modulate virus-cell interactions shortly after herpesvirus entry [55].
VZV Nucleocapsids Are Sequestered in Endogenous Nuclear PML Cages
We next used cryoimmuno-electron microscopy (cryoimmuno-EM) to determine with ultrastructural precision whether the ORF23 protein that was associated with PML-NBs represented assembled NCs retained within these nuclear bodies or consisted of ORF23-PML protein co-aggregates. This question was of interest because PML has been observed to be associated with aberrant protein aggregates, termed nuclear aggresomes [56] or clastosomes [44] and with capsid proteins of other nuclear replicating viruses, including papillomavirus [57], [58], the neurotropic JC virus [59] and HSV-1 [60]. Except for the HSV-1 study, these analyses showed association of PML and capsid proteins by confocal light microscopy which does not optically resolve individual virion capsids; however some NC-like structures appeared to be associated with PML gold labeling in HSV-1 infected cells by immuno-EM [60].
Our experiments to detect both PML and ORF23 protein at ultrastructural resolution were first done with the Tokuyasu method which allows single and double-immunogold labeling of ultrathin (50–80 nm) cryosections with exquisite sensitivity [61]–[63]. These results were then correlated with patterns of PML and ORF23 protein localization observed by confocal microscopy (Figure 2). By cryoimmuno-EM, endogenous PML-NBs in uninfected cells were electron-dense subnuclear domains that exhibited extensive PML gold labeling, consistent with the punctate PML pattern seen by confocal microcopy (Figure 2A). In VZV infected cells, cryoimmuno-EM revealed VZV NCs of about 100 nm diameter (Figure 2B, arrow) that were abundantly labeled with antibody to ORF23 protein, confirming its predicted location on the capsid shell [54], [64], [65]. The viral NCs most likely corresponded to the tiny ORF23-expressing punctae seen in the nuclei of infected cells by confocal microcopy (Figure 2B, left panel). Furthermore, cryoimmuno-EM revealed clusters of VZV capsids that were surrounded by PML gold labeling (Figure 2C, right panel) and double-immunogold labeling proved the spatial association of PML protein (15 nm gold labeling) with individual NCs and clusters of VZV NCs detected with antibody to ORF23 protein (10 nm gold labeling) (Figure 2D). Therefore, the co-localization of PML-NBs and ORF23 protein detected by confocal microscopy (Figure 1 and Figure 2C, left panel) resulted from the sequestration of newly assembled VZV capsids in PML-NBs rather than from the formation of PML-ORF23 protein aggregates.
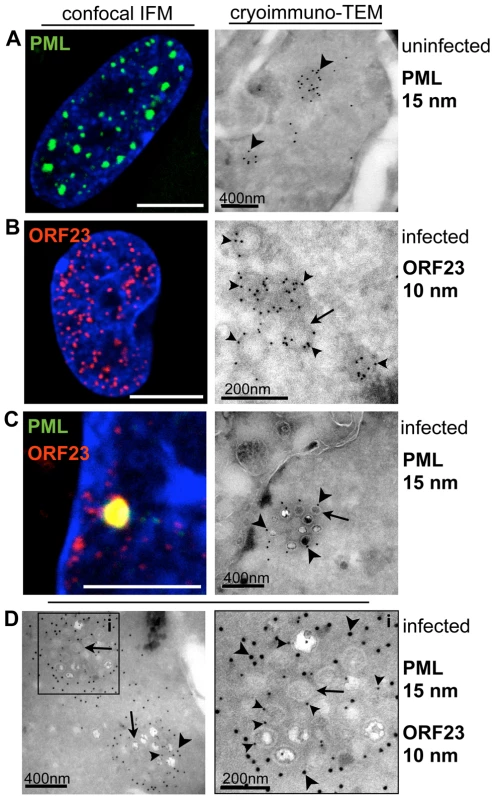
To define the ultrastructure of PML subnuclear domains harboring VZV NCs even more precisely, high-pressure freezing and freeze-substitution was used to optimally preserve both cell morphology and proteins [66] in uninfected and VZV infected HELF (Figure 3 and Figure S3). Again, endogenous PML-NBs in uninfected cell nuclei showed PML-specific labeling and were electron-dense (Figure 3A). In VZV infected cells, individual mature NC, which were identified by their dark electron-dense centers, as well as immature NCs, were associated with varying amounts of PML-positive fibrous material (Figure 3B and Figure S3). Furthermore, clusters of NCs were observed within cage-like structures that had dense PML-specific labeling (Figure 3C and Figure S3). These PML cages consisted of concentrically arranged protein fibers that tightly enclosed mature and immature NCs. Thus, the sequestration of virion capsids within PML subnuclear domains was confirmed with a second ultrastructural method. Furthermore, the presence of fibrous PML cages that entrapped both mature and immature herpesvirus capsids was demonstrated for the first time because of better contrast and superior ultrastructural preservation achieved with high pressure freezing and freeze-substitution.
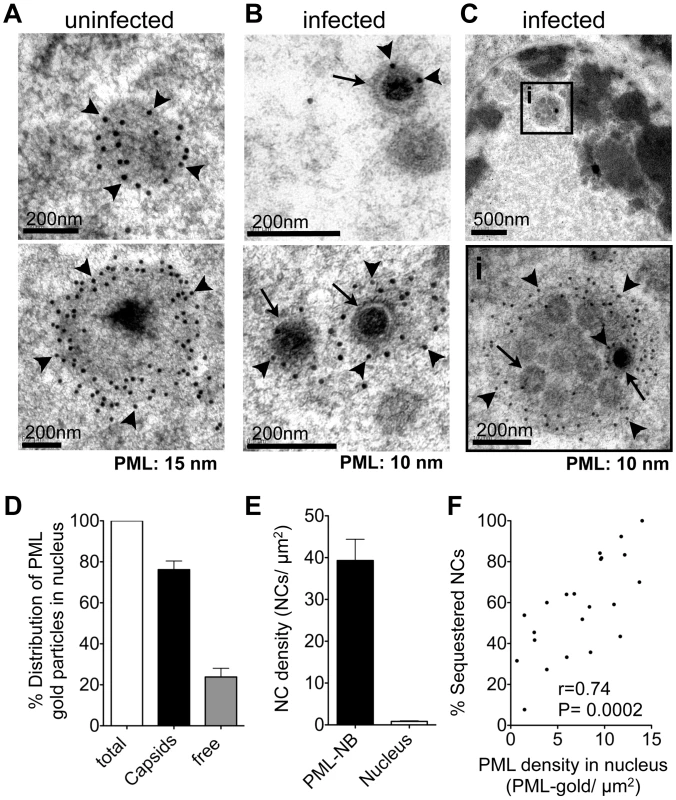
Validating observations from EM studies requires quantitative analysis [67]. Examination of 30 VZV infected nuclei revealed that about 80% (N = 1,611) of all PML-specific gold particles were associated with viral NCs (Figure 3D). The concentration of NCs (number of NCs/µm2) within PML subnuclear domains was nearly 40 times higher than in other nuclear areas (Figure 3E), proving that PML and VZV capsids were highly co-enriched within PML-NBs and free PML protein was rare elsewhere in infected nuclei. In infected nuclei that contained endogenous PML-NBs an average of 62%±5 SEM (N = 30 nuclear profiles) of VZV NCs was sequestered in PML-NBs. The proportion of sequestered NCs (among all NCs present in an infected nucleus) was higher when more PML protein was assembled to PML cages and correlated positively with the amount of endogenous PML protein present in the infected cell nucleus, measured as the PML protein density (PML-gold/µm2) (Figure 3F). Importantly, the clusters of VZV NCs in these PML structures differed from the paracrystalline arrays of NCs that form nuclear inclusions in HSV-1 infected cells (compare Figure 3 and Figure S3 with Figure S4). Thus, the ultrastructural analysis unequivocally identified the association of both mature and immature virion capsids with PML protein in the nucleus and NCs were usually confined within endogenous cages consisting of PML-positive protein fibers in cells infected with VZV in vitro. Furthermore, these results demonstrated that immunogold EM is the method of choice to show the distribution of PML in infected nuclei unequivocally, including demonstrating the presence of PML on some individual NCs, that may be difficult to image by standard confocal microscopy. Immunogold EM has the significant advantage that individual gold particles constitute a stable, discrete and quantifiable signal that allows a very wide range of labeling densities within the same section to be imaged and quantified accurately.
PML Cages Sequester Nucleocapsids During Infection of Human DRG and Skin Xenografts In Vivo
Since mature herpesvirus NCs must egress from the nucleus and undergo secondary envelopment in the cytoplasm before infectious virus release from the host cell [22], the sequestration of VZV NCs by endogenous PML cages suggested a novel mechanism by which PML might restrict viral replication and spread. However, this observation might reflect a phenomenon unique to cultured cells because VZV is known for its poor replication in vitro. Therefore, we next explored whether this cellular response also occurred in the differentiated neurons and satellite cells of sensory ganglia that are targeted during acute VZV infection in vivo [30] and when VZV reactivates from latency in neurons [68]–[70]. For these experiments, human DRG xenografts in SCID mice were infected for 14 days (acute phase of VZV infection [36]) and then harvested and processed for semithin (500 nm) cryosectioning and confocal microscopy or cryoimmuno-EM.
Numerous small PML-NBs (<1 µm) were detected in the nuclei of neurons and satellite cells in uninfected DRG by confocal microscopy (Figure 4A). In contrast, in infected neural cells, PML-NBs were either dispersed or reorganized such that only one or two enlarged structures were present; the mean diameter was (1.9 µm±0.8 SD; N = 62). These structures were often ring-shaped and exhibited intense enrichment of ORF23 protein in their interior (Figure 4B–D). ORF23 protein was present in a majority but not all (87%, N = 116) of the ring-shaped PML-NBs in VZV-infected neural cells. Ultrastructural studies revealed that these large PML-NBs consisted of spherical PML cages containing numerous sequestered viral NCs, as demonstrated by cryoimmuno-EM with PML-specific labeling (Figure 4E).
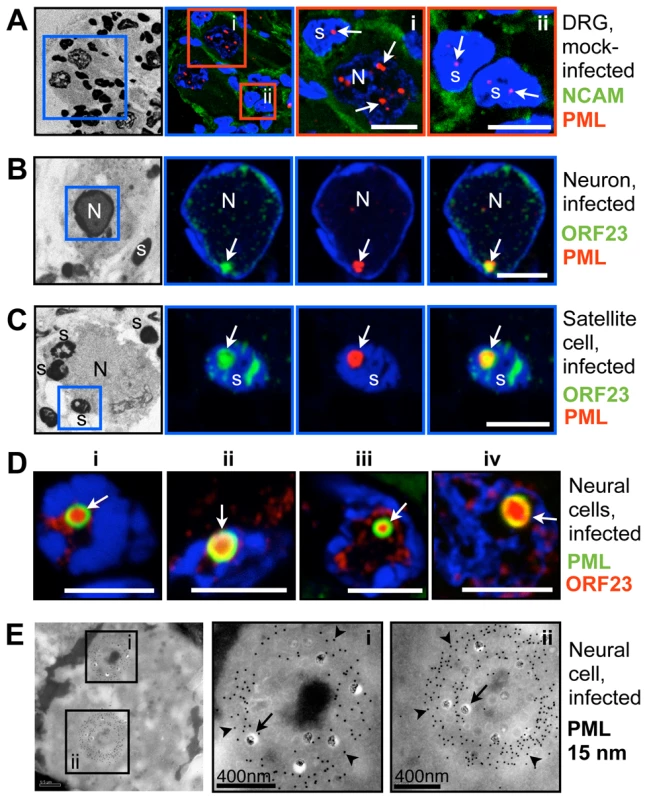
VZV also targets skin for replication, creating cutaneous lesions from which the virus is transmitted during varicella or herpes zoster [30]. To determine whether NC sequestration by PML-NBs also occurred in skin cells, skin xenografts were infected for 21 days, harvested and processed for paraffin-sectioning and confocal microscopy. Like uninfected neural cells, uninfected skin cells contained numerous small (<1 µm) PML-NBs (Figure 5A). However, very large ring-like PML-NBs were observed in the nuclei of infected cells that formed the typical VZV polykaryons and expressed the VZV glycoprotein gE on plasma membranes (Figure 5B); their mean diameter was 1.5 µm±0.3 SD (N = 81). Importantly, as observed in VZV infected human DRG cells, the ORF23 capsid protein was enriched in many of these large PML-NBs in infected skin cells (Figure 5C and D).
These experiments established that endogenous PML formed spherical nuclear cages containing VZV NCs not only in cultured cells in vitro, but also in differentiated human cells infected in vivo. Since neural and skin cells are targeted during VZV pathogenesis, we hypothesized that PML cages might play a role in the intrinsic host defense against VZV. These findings provided a rationale to further investigate the molecular mechanisms of this cellular response and to assess whether it could interfere with production of infectious virus progeny.
Only the PML IV Isoform Promotes the Sequestration of VZV Capsids in Nuclear PML Cages
PML protein exists in several isoforms that have unique C-terminal domains resulting from alternative splicing of the PML gene [14]. To define the process of NC sequestration in PML cages during VZV infection and to ask if this process might function as an intrinsic antiviral host defense, we evaluated six major PML isoforms, PML I, II, III, IV, V and VI. These experiments investigated whether exogenous expression of a particular PML isoform specifically altered the intranuclear distribution of ORF23 protein and sequestered VZV capsids. PML-NBs were formed by the exogenously expressed isoforms over the background of endogenous PML in uninfected and infected cells (Figure S5). In control cells that were not transfected but were infected with VZV, the ORF23 capsid protein showed no redistribution if endogenous PML-NBs were completely dispersed as happens in the majority of infected melanoma cells (Figure 6A, upper panel; see also Figure S2A). When melanoma cells were transfected with the PML isoforms and infected with VZV, ORF23 protein was redistributed and colocalized with more than 95% (N = 550) of PML-NBs only in cells that expressed PML IV or EGFP-PML IV (Figure 6A). None of the other five PML isoforms recruited ORF23 protein to PML-NBs (Figure 6B and Figure S5).
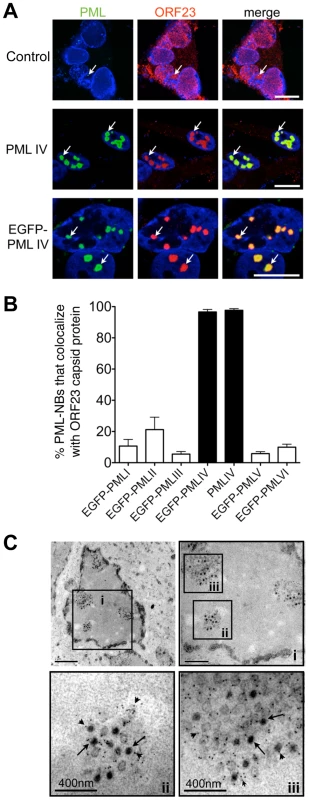
Photo-bleaching experiments with cells that expressed EGFP-tagged PML IV and that were infected with recombinant VZV expressing RFP-tagged ORF23 protein, revealed the striking immobilization of ORF23 protein within EGFP-PML IV-NBs. Even 45 min after photo-bleaching, RFP-ORF23 remained confined to PML IV-NBs (Figure S6), indicating that PML IV may form a physical barrier that constrains the mobility of ORF23 capsid protein and possibly of assembled capsids in the nucleoplasm. Therefore we next analyzed VZV infected cells expressing EGFP-tagged PML IV protein by immuno-EM to determine whether VZV capsids were confined in PML cages (Figure 6C). A quantitative analysis of 100 infected cell nuclei showed that more than 90% of VZV NCs (N = 4,900) were sequestered (Figure 6C) whereas other nuclear areas were essentially devoid of NCs, demonstrating a highly efficient retention of NCs in PML IV-NBs.
Furthermore, like endogenous PML cages, the exogenous PML IV cages (PML IV cages that formed in cells with endogenous PML and over-expressed PML IV) in VZV infected cells did not colocalize with the viral DNA replication compartments identified by IE62 and ORF29 protein expression or in situ hybridization for viral genomic DNA (Figure S7A–C). These experiments also demonstrated that ORF23 protein recruitment was specific, as compared to IE62 and ORF29 proteins, which showed no redistribution by PML IV.
ORF23 Capsid Protein Interacts with PML IV in the Absence of Other Viral Proteins
Having shown that PML IV reorganized the nuclear distribution of ORF23 protein and sequestered NCs into large PML-NBs during VZV infection, we next investigated whether ORF23 protein was a molecular target of PML IV. ORF23 protein was considered a likely candidate for recognition by PML IV because, like the related HSV-1 VP26 small capsid protein [64], [65], ORF23 protein may decorate the capsid surface on hexons formed by the major capsid protein and is therefore likely to be accessible on NC surfaces. This prediction was supported by the dense ORF23-specific gold-labeling observed at the outer edges of VZV NCs (Figure 2B).
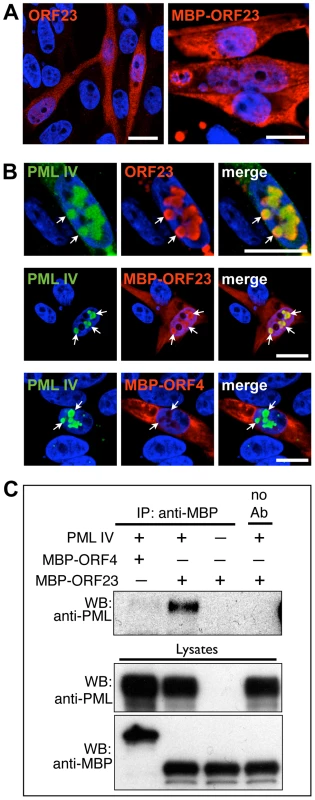
When expressed in melanoma cells, untagged ORF23 protein or ORF23 protein tagged with maltose-binding protein (MBP) protein was found to be distributed diffusely in both the cytoplasm and nucleus (Figure 7A). However, when cells were transfected with plasmids expressing PML IV, both ORF23 protein and MBP-tagged ORF23 protein were highly enriched within PML IV-NBs (Figure 7B). ORF4 protein, which is a VZV regulatory/tegument protein [71], [72], was used as an MBP-tagged control; it was not recognized by PML IV and did not colocalize with PML IV-NBs (Figure 7B, lower panels). The specific interaction of PML IV with MBP-ORF23 protein but not with MBP-ORF4 protein was confirmed by coimmunoprecipitation (Figure 7C). These data showed that ORF23 protein was recruited to PML IV-NBs in the absence of other VZV proteins. Therefore ORF23 protein is a potential molecular target on NC surfaces that may physically interact with PML IV during infection.
The Unique C-Terminal Domain of PML IV Is Necessary for PML-mediated Sequestration of VZV Nucleocapsids
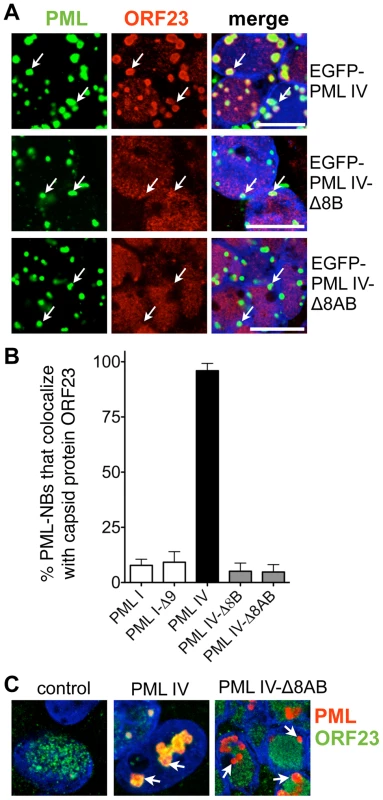
The unique C-terminal domain of PML IV, which is encoded by exons 8a and 8b of the PML gene, differentiates this isoform from the other five PML isoforms that failed to sequester NCs [14]. We therefore asked whether expression of truncated PML IV protein that had a deletion of exon 8b (PML IV-Δ8B) or of both exons 8a and 8b (PML IV-Δ8AB) would eliminate the redistribution of ORF23 protein or capsids to PML cages. EGFP-tagged PML IV-Δ8B or PML IV-Δ8AB continued to form PML-NBs when exogenously expressed in VZV infected cells (Figure 8A). However ORF23 protein no longer colocalized with these mutant PML-NBs (Figure 8A and B), suggesting that the truncated PML IV proteins could not promote NC sequestration. PML I and the truncated PML I-Δ9 protein, lacking the unique PML I C-terminus encoded by exon 9, were included as controls and also failed to cause redistribution of ORF23 protein (Figure 8B). We next investigated whether these results could be reproduced using stable melanoma cells lines that avoided EGFP-tagging and expressed untagged PML IV or PML IV-Δ8AB protein under the control of a doxycycline-inducible promoter (Figure 8C and Figure S8). These recombinant cell lines expressed similar amounts of PML IV and PML IV-Δ8AB protein at 24 hr after induction with 5 µg/ml doxycycline and formed PML-NBs that were similar in size (Figure S8A and B) and fewer than 0.5% cells exhibited apoptosis based on Annexin V staining (Figure S8C). When the induced cell lines were infected with VZV, ORF23 protein colocalized completely with PML IV at 24 hr after infection whereas ORF23 protein was not redistributed to PML-NBs in cells that expressed the truncated PML IV-Δ8AB (Figure 8C), again demonstrating that PML-NBs composed of truncated PML IV were deficient in the sequestration of ORF23 protein, presumed to be on VZV capsids.
To test this assumption, quantitative immuno-EM was used to show patterns of NC distribution in cells induced to express PML IV or PML IV-Δ8AB. For this purpose, samples were processed with high-pressure freezing and freeze-substitution to optimally preserve the ultrastructure of PML cages and virion capsids and to achieve sensitive detection of PML protein by immunogold labeling. Large spherical PML cages that harbored VZV NCs were readily observed in cells expressing PML IV (Figure 9A, left panel, and Figure 9B) and all PML IV cages entrapped both mature and immature NCs (Figure 9A, left panel; Figure 9B and Figure S9A–C). In contrast, PML IV-Δ8AB-NBs rarely sequestered any NCs even though numerous NCs were present in the infected cell nucleus (Figure 9A; right panel). Infected nuclei without any PML cages usually contained numerous randomly scattered NCs (Figure S9A; compare left and right panels).
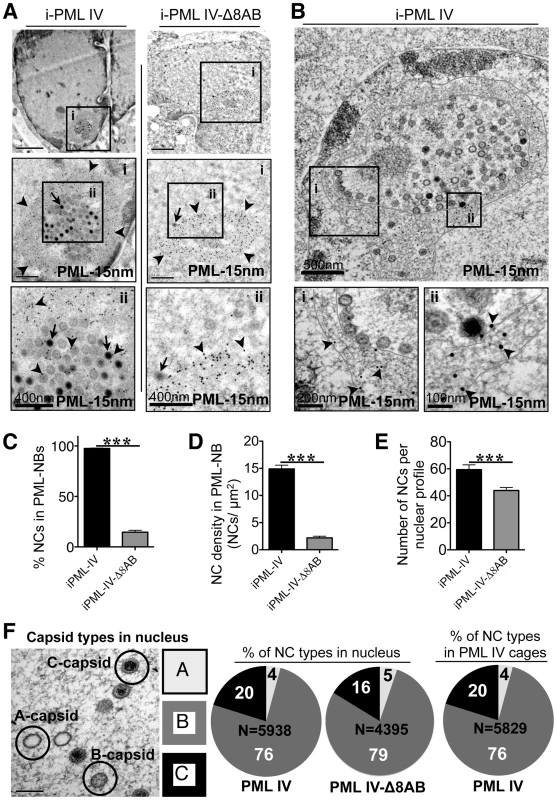
The mean size of PML cages in infected cells induced to express PML IV was 1.6 µm ±0.5 SD (N = 100), which was similar to the sizes of PML cages in neural cells (1.9 µm ±0.8 SD; N = 62) and skin cells (1.5 µm ±0.3 SD; N = 81) in human tissue xenografts that were infected with VZV (Figures 4 and 5). However, PML IV-Δ8AB structures were significantly smaller than PML IV-NBs (1.2 µm ±0.4 SD vs. 1.6 µm ±0.5 SD; p<0.0001; N = 100) and had a more compact appearance than PML IV-NBs, presumably because fewer capsids were sequestered in these mutant PML-NBs. However, large PML IV cages that were only partly filled with sequestered NCs were also readily observed (Figure 9B) indicating that size and architecture of PML IV cages is not only determined by the sequestered cargo, but also by the assembly properties of PML-positive fibers itself. Thus, truncation of the unique C-terminal domain of PML IV influenced sequestration of NCs and the overall architecture of PML-NBs.
Analysis of PML IV cages by TEM at high magnification revealed a shell structure consisting of PML-positive fibers that appeared to form a physical barrier entrapping VZV capsids (Figure 9B); NCs were often aligned along the inner side of the fibrous shell. PML IV cages were morphologically similar to the endogenous PML cages found in VZV infected HELF in vitro (Figure 2 and Figure 3) and in neural cells in vivo (Figure 4E). Importantly, the thin PML-positive fibers exhibited rather weak electron density (Figure 10B) and could only be identified unequivocally by high magnification and after PML-specific immunogold-labeling (Figure S9B and C). Therefore, despite their size, these PML structures would be easily be missed in virus-infected cells analyzed by morphological criteria only.
Quantitative analysis of NC distribution confirmed that PML IV cages in infected inducible cell lines were highly efficient in sequestration with more than 95% of NCs (N = 5,938) being retained in these structures (Figure 9C). In marked contrast, only 15% of NCs (N = 4,395) were found within PML-NBs in cell lines induced to express PML IV-Δ8AB (Figure 9C). The density of the packing of NCs within individual PML cages was also significantly higher in PML IV-expressing cells than in cells expressing PML IV-Δ8AB, suggesting that PML IV cages had more capacity to sequester NCs than those formed by truncated PML IV (Figure 9D). Furthermore, the total number of NCs was significantly higher (approximately 20%) in cells with PML IV cages compared to those with PML IV-Δ8AB-NBs (p<0.0001), indicating that NCs accumulated in PML IV-NBs (Figure 9E).
Herpesvirus capsids may be abortive (A type), intermediate (B type) or mature (C type) containing viral DNA genomes [73]. To determine whether NC assembly or packaging was effected by PML IV and if NC sequestration by PML IV cages was selective for a specific type of capsids, the relative proportion of the three major capsid types was quantified in nuclei expressing PML IV or PML IV-Δ8AB-NBs, as well as in PML IV cages (Figure 9F). Analysis of 5,938 NCs in 100 nuclei of cells expressing PML IV, of 4,395 NCs in 100 PML IV-Δ8AB expressing cells and of 5,829 NCs within PML IV cages, respectively, revealed similar proportions of all NC types; 16–20% of all NCs were mature, 76–79% were intermediate and 4–5% were abortive in each cell line and in PML cages (Figure 9F). This finding suggests that expression of PML IV did not increase the proportion of abortive or intermediate NCs compared to PML IV-Δ8AB expressing cells and that PML IV cages may participate in the antiviral host cell response against VZV by targeting all three types of NCs that are made in the infected cell nucleus for entrapment.
PML IV Has Antiviral Activity against VZV that Requires the Unique PML IV C-Terminal Domain
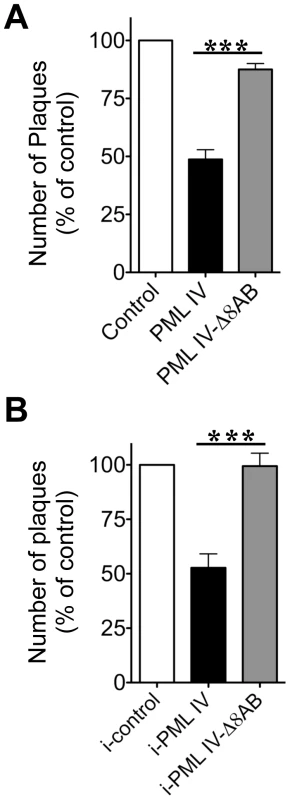
Since PML IV restricted NCs to PML cages, we hypothesized that PML IV would impair the production of infectious virus. Conversely, we predicted that infectious virus yields would be unaffected in cells expressing the truncated PML IV-Δ8AB mutant, which does not recruit VZV capsids (Figures 8 and 9). Since VZV is highly cell-associated in vitro [74], viral replication is measured by plaque assays using infected cells as the inoculum. Given the critical importance of cell-cell spread for VZV infection, our objective was to assess the infectivity of VZV infected cells with and without PML-IV cages based on the transfer of virus from these cell populations to a permissive cell monolayer. We focused our analysis on the 24 hr time point after VZV infection of PML IV and PML IV mutant-expressing cells in order to avoid any potential effect of PML expression on the induction of apoptosis which could also reduce viral titers and because 24 hr is a late stage of VZV replication in vitro when NCs are present. We used two plaque assay methods to assess the antiviral activity of PML IV. In the first method, cells were transfected with an EGFP control, EGFP-tagged PML IV or PML IV-Δ8AB constructs, infected with recombinant VZV expressing RFP-tagged ORF23 protein for 24 hr and separated into subpopulations by fluorescent activated cell sorting (FACS). Only cells that emitted both green (EGFP, indicating transfection) and red (RFP, indicating infection) fluorescence were recovered and equal numbers of infected cells that expressed EGFP, EGFP-PML IV or EGFP-PML IV-Δ8AB were added to permissive cell monolayers and observed for plaque formation (Figure 10A–C). Plaques generated from inoculum cells that expressed PML IV were significantly reduced (p<0.0001) in five independent experiments; plaque numbers were determined in triplicate in each independent experiment. Plaque numbers were about 50% lower when compared to infected cells expressing PML IV-Δ8AB or the EGFP control (Figure 10A). Furthermore, the mean diameter of plaques that were created by infected cells expressing PML IV (0.95 mm2 ±0.06 SEM; N = 70) was significantly smaller than plaques produced by PML IV-Δ8AB expressing cells (1.46 mm2 ±0.09 SEM; N = 70) (p<0.0001). In contrast, the number and size of plaques from cells expressing PML IV-Δ8AB was very similar to the EGFP control (Figure 10A).
In the second method, the antiviral activity of PML IV was tested using the stable cell lines expressing inducible PML IV or PML IV-Δ8AB. These conditions avoided variables that might be associated with EGFP tagging of PML or infection with the recombinant virus in which ORF23 capsid protein was expressed as an RFP fusion protein. Inoculum cells that expressed induced PML IV and that were infected for 24 hr were added to permissive cell monolayers and observed for plaque formation (Figure 10B). Plaques generated from inoculum cells that expressed induced PML IV were significantly reduced (p<0.0018, N = 4) by about 50% when compared to infected cells expressing PML IV-Δ8AB or to control cells.
Thus, PML IV exhibited antiviral activity against VZV in transfected cells as well as in an inducible cell line and its antiviral activity was confirmed to require the unique PML IV C-terminal domain. The N-terminal region, which contains the RBCC/TRIM domain and is common to all PML isoforms and is present in the truncated PML IV mutant, was not sufficient for antiviral activity against VZV. Impaired virus production in PML IV expressing cells when compared to PML IV-Δ8AB and control cells correlated with the capacity of PML IV cages to sequester NCs as observed by immuno-EM. Conversely, the failure of the truncated PML IV-Δ8AB protein to exhibit antiviral activity correlated with impaired nuclear retention of NCs in VZV infected cells. These data together with the quantitative EM data that showed that more than 90% of NCs were present within PML cages at 48 hr suggest that NC are stably and not transiently contained in the PML IV cages.
Individual PML IV Cages Co-sequester Capsid Protein and Huntington's Disease Protein in VZV Infected Cells
Ring-shaped PML-NBs similar to the endogenous PML cages in VZV infected neurons and satellite cells in human DRG and to PML IV cages in vitro have been observed in neural cell nuclei in neurodegenerative disorders associated with expanded CAG repeats encoding polyglutamine (polyQ) tracts in the abnormal gene product [41], [43]. Of particular interest, PML IV, which promotes VZV capsid sequestration has been reported to retain and degrade mutant polyQ-expanded Ataxin7 and the Huntington's disease protein (huntingtin or Htt) within ring-shaped PML clastosomes in cortical neurons [44]. We therefore asked whether the PML IV cages formed in our inducible melanoma cell lines were functionally like PML clastosomes as determined by their capacity to sequester GFP-tagged Htt [75]. We used the HttQ72 construct, which expresses the aberrant Htt protein with a 72 amino acid polyQ tract [75]. When PML IV cells were transfected without induction, HttQ72-GFP was primarily distributed diffusely in the cytoplasm (Figure 11A); it was also detectable in nuclei at higher levels of expression. This pattern changed dramatically when the cells were induced to make PML IV; almost all HttQ72-GFP was redistributed into nuclear PML IV-NBs (Figure 11B). This data confirmed observations about polyQ protein interactions with PML IV [44] and showed that PML IV-NBs in melanoma cells can sequester polyQ-tract proteins, as occurs in neuronal PML clastosomes.
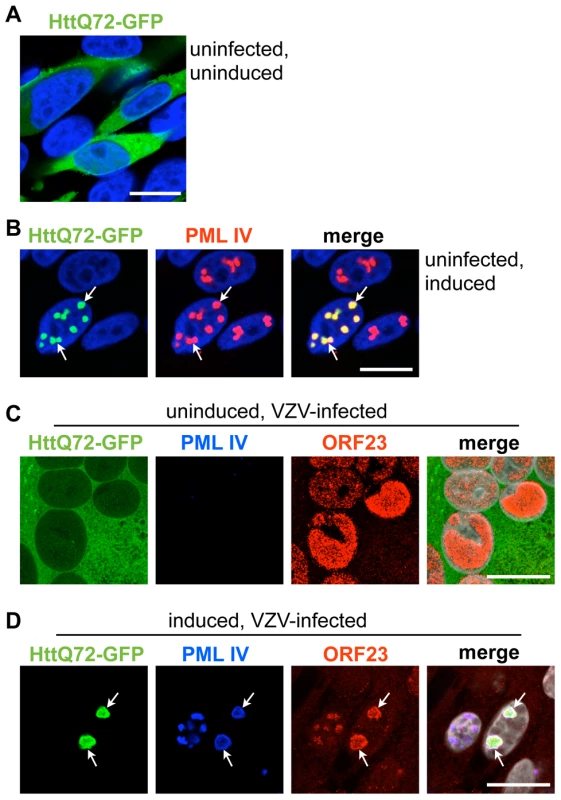
These experiments led us to consider whether PML IV cages could co-sequester the ORF23 capsid protein marker, indicating retention of VZV capsids, together with the mutant Huntington's disease protein. VZV infection of cells that were transfected with HttQ72-GFP but not induced showed no change in the diffuse cytoplasmic pattern of HttQ72-GFP suggesting that VZV infection was not sufficient to redistribute this polyQ-protein (Figure 11C). However, when PML IV expression was induced, HttQ72-GFP was dramatically reorganized and colocalized in PML-NBs together with the ORF23 capsid protein in infected cells (Figure 11D). To our knowledge, these experiments demonstrated for the first time that the same PML isoform has the capacity to sequester both the aberrant poly-Q protein produced in a neurodegenerative disease and a viral capsid protein associated with NC entrapment in PML-NBs and that individual PML cages can simultaneously target and sequester an aberrant polyQ-protein and viral NCs.
Discussion
Diverse types of PML-NBs that are distinguishable by their size, shape and dynamic behavior are found in mammalian cell nuclei [4], [5], [11], [18]. We identify a distinctive class of endogenous PML-NBs that form large spherical PML cages in differentiated human neural and skin cells infected with VZV in vivo. These endogenous PML cages were found to sequester newly assembled mature and immature viral nucleocapsids in the nuclei of infected neurons, satellite cells and epidermal cells, and in cultured cells infected with VZV in vitro.
Similar large PML cages that retained VZV NCs were formed when the PML IV isoform was expressed in cultured cells in vitro but not by PML isoforms I, II, III, V or VI. Importantly, PML IV exhibited antiviral activity against VZV. The antiviral function of PML IV was associated not only with a unique capacity to sequester NCs but also to bind the ORF23 capsid surface protein. All of these functions required the unique C-terminal domain of PML IV. The N-terminal region, which is common to all PML isoforms and contains the RBCC/TRIM domain [14] was not sufficient for antiviral activity or NC retention. The antiviral activity of PML IV was not likely to be explained by inhibition of viral genome synthesis or packaging into capsids by PML IV since the relative proportion of empty capsids was not increased in PML IV expressing cells. PML IV-Δ8AB may lack the capacity to produce PML-NBs with the architecture of those formed by intact PML IV, rendering them ineffective for capsid entrapment. It is also possible that the failure of PML-NB in PML IV-Δ8AB expressing cells to entrap virions reflects in part a dominant negative effect of PML IV-Δ8AB, preventing any endogenous PML IV from interacting with the ORF23 capsid protein.
The correlation between PML-mediated antiviral activity, capsid sequestration and ORF23 protein binding provides strong evidence that PML cages constitute an intrinsic host defense against this common herpesvirus. This role is supported by the finding that human neural and skin cells infected with VZV in vivo contained PML-NBs with the same distinctive morphology. ORF23 capsid protein was recruited to these structures and they consisted of cages formed by concentric rings of PML fibers sequestering NCs. Notably, while VZV infection promoted the dispersal of most PML-NBs, PML protein itself was not degraded, indicating that PML nuclear cages, rather than free PML protein, may be the primary obstacle to VZV replication.
How do NCs become entrapped within PML cages? Our working hypothesis is that individual NCs are recognized by PML protein (PML oligomers or fibers) through interactions with the capsid surface. These initial interactions may result in recruitment of more PML and NCs, thus growing into larger PML-NC assemblies. Alternatively, although they were rare in our immuno-EM studies, some aggregates of NCs may form spontaneously, which are then recognized by PML and enclosed in PML cages. Pre-existing, non-disrupted PML-NBs may also constitute a favorable environment (a platform) for NC aggregation, as has been proposed to account for the accumulation of aberrant cellular proteins in PML-NBs in neurons [45]. These possibilities are not mutually exclusive and a combination of all three may occur in the infected cell nucleus when capsids are produced.
Most PML cages observed in VZV infected neural and skin cells were significantly larger than the small PML-NBs that associate with viral genomes and exert antiviral effects by interfering with the initial transcription of herpesviral genes [76], [77]. PML cages were also more prominent later in infection, at the stage of viral capsid assembly. A significant antiviral effect was predicted because sequestration of abortive, immature and mature virions into fibrous PML cages was highly efficient, leaving very few mature capsids free to egress across the nuclear membrane to form infectious particles in the cytoplasm. Thus, PML cages conferred antiviral activity at a later stage of infection by a mechanism completely different from PML suppression of early viral gene transcription. These two PML-mediated antiviral mechanisms are not mutually exclusive and would be expected to function synergistically. The absence of PML cages may contribute to the increase in VZV replication observed when PML protein was depleted by RNA interference [31].
Our experiments provide the first evidence that a specific PML isoform, PML IV, contributes to the cellular antiviral response by promoting nuclear immobilization of herpesvirus nucleocapsids. PML IV interacted specifically with the ORF23 capsid protein in the absence of other VZV proteins. Since ORF23 protein was expressed on NC surfaces, it offers a likely target for PML IV recognition, as the first step leading to the retention of assembled NCs in PML cages. PML IV, as well as PML III and PML V has been reported to be expressed less abundantly than PML isoforms I/II in some cell lines and primary cells in vitro [17]. However, the pattern of PML isoform expression in vivo in human neural and skin cells for which VZV exhibits tropism or when these cells become infected with VZV is not known. We propose that PML IV may act as a potent modulator of PML-NB architecture, since others have also observed that exogenous PML IV (expressed along with endogenous PML) promotes the formation of large spherical PML-NBs [44]. As noted, PML cages that formed upon overexpression of PML IV together with endogenous PML in vitro best mimicked (both structurally and functionally) those endogenous large PML cages observed in neural and skin cells infected with VZV in vivo and both sequester NCs with ORF23 capsid protein. If PML IV is not abundant in the small PML-NBs of uninfected neurons, satellite cells or epidermal cells, the ratio of PML isoforms may change upon infection, PML IV may become more abundant or specific modifications on PML isoforms may be altered when these differentiated cells are infected or otherwise stressed. It was apparent that PML-NBs exhibited a striking reorganization from multiple small compact structures to a few large ring-shaped cages upon VZV infection in human tissues, which could be consistent with changes in the ratio of PML isoforms and upregulation of PML IV. Of particular interest, PML IV transcription was upregulated when cultured cells were stimulated with type I IFN [44], neurons have been shown to produce type I IFN during viral infection [78] and the IFN pathway is upregulated dramatically in human tissues infected with VZV in vivo [39]. Taken together, our findings point to the need to better understand the patterns and regulation of the tissue specific expression of PML isoforms and their functions in virus-infected cells. For example, different PML isoforms might recognize the outer capsid proteins of other herpesviruses or nuclear replicating DNA viruses and retain NCs, causing antiviral effects when a specific PML isoform is either not degraded efficiently or its production is induced in the infected cell.
Since the intranuclear mobility of herpesvirus NCs via actin filaments may facilitate formation of NC assembly domains and NC transport to the nuclear membrane [79], [80], we suggest that PML-mediated entrapment of NCs is likely to prevent NC interactions with the viral export machinery at the inner nuclear membrane. This hypothesis is supported by our observation that the number of intranuclear NCs was significantly higher in cells with functional PML IV cages as compared to control cells.
An intrinsic defense mechanism in which PML binds to a viral capsid protein and sequesters NCs obviously depends on the persistence or new synthesis of PML protein in the infected cell nucleus at the stage of capsid assembly, which occurs in VZV infected cells but not in HSV infected cells. Since HSV-1 degrades PML protein shortly after virus entry and shuts off host cell protein synthesis [25]–[27], both the early effects of PML on viral gene expression and this later antiviral activity would be expected to be counteracted. Some PML-specific gold labeling associated with NC-like structures, although not in PML cages, has been reported in HSV-1 infected cells at late times [60]. Overexpression of PML as a 69 kDa protein did not inhibit HSV-1 infection and PML cages were not observed but the PML isoform was not unequivocally identified [81]. Of interest, other nuclear replicating DNA viruses, including papillomavirus and JC (polymoma) virus also have limited capacity to degrade PML protein and PML binding to their capsid proteins has been reported [57], [59]. These interactions were proposed to support infection by directing the viral genome to replication compartments or facilitating virion assembly but enhancing or inhibitory effects of specific PML isoforms on viral replication could not be assessed [82], [83]. Nevertheless, particular PML isoforms could have pro-viral functions through some PML-capsid protein interactions and antiviral consequences in others, as we observed here in VZV infected cells.
Interestingly, stimulation of PML expression by type-I interferon and PML-mediated sequestration and immobilization NCs of VZV, a DNA virus, has parallels with the antiviral mechanism of IFN-induced Mx-GTPases against RNA viruses. These proteins also form oligomeric structures and inhibit the replication and assembly of several negative-strand RNA viruses by sequestering and immobilizing viral ribonucleocapsids or nucleocapsid protein [84]. For example, the human MxA GTPase was found to sequester the LaCrosse bunyavirus nucleocapsid protein into fibrillar cytoplasmic complexes thereby inhibiting viral RNA genome replication and the formation of enveloped virion particles on membranes in the Golgi compartment [85], [86]. Thus, these two IFN-induced antiviral responses appear to depend on creating intracellular structures that act as physical barriers during the later viral assembly stage of infection.
Our observations of VZV NC sequestration in PML-NBs in neurons, satellite cells and skin cells in vivo are relevant to a better understanding of the control of VZV infection in the human host. We suggest that the role of NC entrapment by PML cages in skin is important for the modulation of the infection by the innate, interferon-induced response through restriction of cell-cell spread. Diverting a substantial majority of mature capsids to PML cages is likely to regulate lytic VZV infection in vivo. In fact, this process may benefit the virus since it is important for VZV and other herpesviruses to achieve a well-balanced virus-host interaction. Persistence of the virus in the human population requires a relatively mild infection. An incapacitating infection of the host that would limit opportunities for VZV transmission to susceptible contacts and would hinder, rather than support the persistence of the virus in the population as a whole. In addition to limiting skin infection, the consistent finding of large PML cages in neuronal cells of human DRG xenografts infected with VZV suggests the possibility that this intrinsic host cell response might restrict episodes of VZV reactivation from latency so that the episode remains subclinical [33], [87]. We hypothesize that when VZV reactivates from latency in persistently infected neurons, trapping newly formed NCs by PML cages would reduce the possibility for spread of infectious virus particles down the axons to skin and would also limit infection of adjacent satellite cells and neurons in the DRG where reactivation is occurring. This process would result in abortive VZV reactivation [88]. From a clinical perspective, it is notable that patients treated with arsenicals have an extremely high rate of clinically symptomatic herpes zoster, due to VZV reactivation [89], [90] and arsenicals are potent triggers of PML degradation [91], [92].
Since VZV is a human neurotropic virus, it is of interest that ring-like PML-NBs similar in size to endogenous PML cages in VZV infected neurons and satellite cells in DRG in vivo and PML IV cages in vitro are found in neural cell nuclei in several neurodegenerative disorders [45]. Many of these disorders are associated with expanded CAG repeats encoding polyglutamine (polyQ) tracts, including Huntington's disease and several types of spinocerebellar ataxia and PML was associated with these protein inclusions [40]–[43]. Importantly, the same PML isoform, PML IV, which promotes VZV capsid sequestration has been reported by others to form ring-like PML clastosomes that mediate the sequestration and degradation of mutant polyQ-expanded Ataxin7 and Huntington's disease protein (huntingtin or Htt) in cortical neurons [44]. The morphological similarity between PML cages found in VZV infected cells and these ring-like PML clastosomes and the finding that PML IV can co-sequester ORF23 capsid protein together with the Huntington's disease protein in VZV infected cells, suggest that the PML cages described here and PML clastosomes are structurally and functionally related subnuclear domains. Neurons are post-mitotic cells and need to safely contain toxic proteins and/or increase their clearance, whether aberrant host cell proteins or virion structures [46], [50]. Of interest, aberrant polyQ proteins tend to form large aggregates that like NCs may be particularly resistant to the celluar mechanisms for degradation of unwanted proteins and the nucleus may have limited capacity to cope with misfolded proteins compared to the cytoplasm [75]. PML IV may specialize in restricting these degradation-resistant structures to nuclear subdomains and limit their toxicity longer term.
In summary, the efficient sequestration of virion capsids in PML cages that contributes to the intrinsic antiviral host response against VZV appears to be the outcome of a basic cytoprotective function of this distinctive category of PML-NBs in safely containing aggregation-prone aberrant proteins. Both aggregation-prone proteins and virion capsids are therefore potential substrates for sequestration in these PML cages.
Materials and Methods
Ethics Statement
Human fetal tissues for SCID xenograft studies were obtained from Advanced Bioscience Resources, Inc. (Alameda, CA) in compliance with state and federal regulations for tissue acquisition for biomedical research, in accordance with FDA 21 CFR Part 1271 GTP (Good Tissue Practices), UAGA and NOTA. Animal protocols complied with the Animal Welfare Act and were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Cells and Viruses
Human lung embryonic fibroblasts (HELF) and a human melanoma cell line (MeWo, ATCC number: HTB-65) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, nonessential amino acids (100 µM) and antibiotics (penicillin at 100 U/ml and streptomycin at 100 µg/ml). HELF and the melanoma cell line (as obtained from ATCC) were passaged less than 25 times.
VZV used in these experiments was recombinant Oka (rOka) derived from the wild type low passage parent Oka strain (pOka). VZV (rOka) and the recombinant rOka-RFP-ORF23 expressing RFP-tagged ORF23 [54] were propagated in melanoma cells and HELF cells [32]. Transfection was done with Lipofectamin 2000 (Invitrogen) for 14 hr. Viral infection was done with cell-associated VZV at a ratio of 1/20 (infected cells/uninfected cells) for 24 or 48 hr.
Expression Plasmids
Plasmids pEGFP-PML (I-VI) [18] were gifts from Prof. Peter Hemmerich, Leibnitz-Institute of Age Research, Jena, Germany. Plasmid pcDNA3-PML IV [44] was generously provided by Prof. Annie Sittler, Universite Pierre et Marie Curie, Paris, France. Plasmids pCR3-MBP-ORF4, pCR3-MBP-ORF23 and pENTR207-ORF23 vectors have been described [93]. The plasmid pcDNA3-HttQ72-GFP [75] was a kind gift from Prof. Ron Kopito, Stanford University, Stanford, USA. Plasmid pCR3-ORF23 that expresses untagged ORF23 was constructed using the pENTR207-entry vector and the pCR3 destination vector with the LR Clonase II gateway cloning reaction (Invitrogen). PML deletion constructs EGFP-PML I-Δ9, EGFP-PML IV-Δ8AB and EGFP-PML IV-Δ8B were made as follows: exon 9 was removed from PML I by PCR (primers PML IΔ9 5′A/PML IΔ9 5′B and PML IΔ9 3′A/PML IΔ9 3′B). pEGFP-CI PML IV was the template used to delete exon 8b (primers PML IVΔ8B 5′A/PML IΔ9 5′B and PML IV 3′A/PML IV 3′B) and exons 8ab (primers PML IVΔ8AB 5′A/PML IVΔ8AB 5′B and PML IV 3′A/PML IV 3′B). pEGFP-C1 PML I was digested with MluI and a 6.1 kb vector DNA was isolated. pEGFP-C1 PML IV was digested with BbvCI/MluI and a 5.6 kb vector DNA was isolated. Purified, digested PCR products were ligated into the appropriate vectors to generate pEGFP-PML I-Δ9, pEGFP-PML IV-Δ8AB and pEGFP-PML IV-Δ8B. Primer sequences are listed in Table S1.
Construction of Inducible PML Expressing Melanoma Cell Lines
Melanoma cells expressing doxycyline-inducible PML IV or PML IV-Δ8AB were made using the pRetro-X-Tet-On-Advanced vector system and pRetro-X-Tight-Pur plasmid (Clontech Laboratories) with the PML IV or PML IV-Δ8AB sequence derived from the plasmid pcDNA3-PML IV [44]. The stable cells were induced with 5 µg/ml doxycyline for 24 hr before infection with VZV.
Human Dorsal Root Ganglion (DRG) and Skin Cell Xenografts
Human fetal DRG were inserted under the kidney capsule of male CB-17scid/scid mice (Taconic Farms, Germantown, NY), inoculated with rOka-infected HELF and harvested after 14 days [36]. Human fetal skin xenografts were inoculated with VZV rOka-infected HELF and harvested after 21 days [34]. Human tissues were provided by Advanced Bioscience Resources (ABR, Alameda, CA) and were obtained in accordance with state and federal regulations. Animal protocols complied with the Animal Welfare Act and were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Microscopy Analysis of Xenograft Tissue Sections and Cultured Cells
DRG xenografts were prepared for cryosectioning as described previously [88]. Human skin xenografts were embedded in paraffin and 5 µm sections were prepared. For confocal microscopy, sections were deparaffinized with tissue clearing agent (“Safeclear II”, Fisher Scientific), rehydrated and antigen retrieval was performed in citric acid-based antigen retrieval solution for 90 sec at 100°C. Sections were then blocked and immunolabeled as described previously [88]. Cultured cells on glass coverslips were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature or were fixed with methanol at -20°C for 10 min and air-dried. Cells were blocked and immunostained as described previously [32].
Antibodies and Confocal Immunofluorescence Microscopy
Antibodies used for confocal microscopy were: rabbit polyclonal anti-PML and mouse monoclonal anti-PML (PG-M3), rabbit polyclonal anti-Sp100 (all Santa Cruz Biotech), mouse monoclonal anti-N-CAM (Zymed), rabbit polyclonal anti-VZV-ORF23 [54], rabbit polyclonal anti-VZV-IE62 and rabbit polyclonal anti-VZV-ORF29 [32]. Antibodies for secondary detection were Alexa Fluor 488, Alexa Fluor 594 or Alexa Fluor 647 conjugated donkey anti-mouse or donkey anti-rabbit antibodies (Invitrogen). Infected tissues, cultured cells and PML-NBs were imaged and quantified using a Leica TCSSP2 confocal laser scanning microscope (Heidelberg, Germany). Microscope objectives were 40/1.0 (Numerical Aperture, N.A.) or a 63/1.4 (N.A.) plan apochromate objectives. Images were scanned at 1024×1024 pixels with at least four times frame averaging and the pinhole adjusted to one airy unit. Brightness and contrast were adjusted using Photoshop CS3 (Adobe) or iPhoto (Apple). The number of PML-NBs and colocalization of PML-NBs with ORF23 protein were quantified using captured digital images and the analysis and measurement tools in Photoshop CS3 extended version (Adobe).
Electron Microscopy (EM)
Samples were either prepared for cryosectioning or high-pressure frozen (HPF) and freeze substituted (FS) and then embedded in either LR-white or Epoxy resin (Embed812). Preparation and labeling of cryosections was done as described previously [88]. Cells were high-pressure frozen in a Leica EM PACT2. Frozen specimen carriers with cells were placed into frozen cryovials containing acetone with 0.1% glutaraldehyde and 0.1% uranyl acetate (for LR White embedding) or in acetone with 1% osmium tetroxide and 0.1% uranyl acetate (for Epon embedding). The frozen vials were placed into a Leica AFS for the freeze-substitution procedure and then embedded in either epoxy resin (Embed 812) or LR-White resin. Ultrathin (50–70 nm) LR-white or Epon-sections were prepared with a diamond knife (Diatome) using a ultramicrotome (Ultracut, Leica). For immunogold-labeling of Epon-sections, these sections were first etched with 5% hydrogen peroxide for 5 min and then rinsed with distilled water. Sections were pre-blocked in DIG-blocking solution (Roche) for 30 min. Primary antibodies and Protein A-gold particles were diluted in blocking solution and sections were incubated for 1 hr or 30 min, respectively, at RT. Rabbit polyclonal anti-PML antibody (Santa Cruz Biotech) and rabbit polyclonal anti-ORF23 antibody were used at 1∶10 dilution. Finally the sections were stained with 3.5% aqueous uranylacetate for five minutes and with 0.2% lead citrate for three minutes and air-dried. Sections were analyzed using a JEOL 1230 transmission electron microscope (TEM) at 80 kV and digital photographs were taken with a GATAN Multiscan 701 digital camera. Quantification of PML-specific immunogold-labeling and evaluation and counting of virion capsids was done by analyzing captured digital images with Photoshop CS3 (extended edition, Adobe) using the counting tool and the measuring tool to calculate areas of nuclear profiles and PML compartments. PML-labeling density (number of gold particles per µm2) and capsid density (number of capsids per µm2) were calculated with Microsoft Excel (2008). Statistical analysis was performed using GraphPad Prism (version 5.0) statistical software.
VZV Plaque Assays
For plaque assays after fluorescent activated cell sorting (FACS), cells were transfected with pEGFP, pEGFP-PML IV or pEGFP-PML IV-Δ8AB expression plasmids, infected for 24 hr with rOka-RFP-ORF23 and sorted by FACS (Digital Vantage, BD Bioscience). Cells that were both green (transfected) and red (infected) were recovered, equal numbers were seeded onto melanoma cell monolayers in triplicate and plates were incubated for 72 hr. Plaques were visualized by standard methods; five independent FACS experiments were performed. Plaque numbers were counted and plaque sizes were determined from digital photographs by calculating the area of >70 randomly selected plaques per sample using Photoshop CS3 (extended edition, Adobe). Virus plaque assays with inducible PML expressing cell lines (Mel39-control, Mel39-PML IV and Mel39-PML IV-Δ8ab) were done using cells induced overnight with 5 µg/ml doxycycline, infected for 24 hr, harvested, serial diluted and seeded in triplicate onto melanoma cell monolayers. Plaques were counted and numbers expressed as percentages of the control; four independent experiments were performed.
Immunoprecipitation (IP) and Western Blotting
For immunoprecipitation studies, samples were analyzed using the ExactaCruz IP-Western Kit (Santa Cruz Biotechnology, Inc.), according to manufacturer's instructions. MBP-ORF23 or MBP-ORF4 and PML IV transfected cells were grown in 75 cm2 flasks and lysed in 300 µl high salt buffer (20 mM HEPES [pH 7.2], 450 mM NaCl, 1.5 mM MgCl2, 0.5% NP-40, 20% Glycerol) supplemented with EDTA-free protease inhibitor cocktail (Roche). Cell debris was removed by centrifugation. The supernatant was combined with 1,200 µl NaCl-free low salt buffer (20 mM HEPES [pH 7.2], 1.5 mM MgCl2, 0.5% NP-40, 20% Glycerol) and centrifuged again. 4 µg of anti-MBP monoclonal antibody (New England Biolabs) was coupled to 100 µl sepharose beads. Beads were washed in PBS and then incubated with 1,000 µl pre-cleared cell lysate at 4° C for 4 hr. Beads were then washed six times with the binding buffer (20 mM HEPES [pH 7.2], 90 mM NaCl, 1.5 mM MgCl2, 0.5% NP-40, 20% glycerol), boiled with SDS sample buffer, and analyzed by Western blot using rabbit polyclonal anti-PML antibody (1∶2,000), anti-MBP antibody (1∶2,000). For Western Blot analysis of cultured cell lysates, cells grown in 6-well plates were lysed in 150 µl high salt buffer (see above). Whole lysates were then combined with 150 µl SDS sample buffer and boiled for 5 min before loading on a 7.5% SDS PAGE. MBP-tagged constructs of ORF23 were used in these cotransfection and IP experiments because they yielded higher expression levels and showed less cytotoxicity; the monoclonal anti-MBP-antibody was more effective for IP than the polyclonal ORF23 antibody.
Apoptosis Assay
The Vibrant Apoptosis Assay (Invitrogen) with Annexin V conjugated to Alexa Fluor 488 was used to evaluate the percentage of apoptotic cells at 24 hr after doxycycline induction of PML IV and PML IV-Δ8AB expressing cell lines. Approximately 5,500 cells per cell line were evaluated by confocal microscopy.
Statistical Analysis
Student's t tests were performed for all experiments using Graph Pad Prism (version 5.0) statistical software. A p-value of <0.05 was considered statistically significant.
Supporting Information
Zdroje
1. IshovAM
SotnikovAG
NegorevD
VladimirovaOV
NeffN
1999
PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1.
J Cell Biol
147
221
234
2. ZhongS
MullerS
RonchettiS
FreemontPS
DejeanA
2000
Role of SUMO-1-modified PML in nuclear body formation.
Blood
95
2748
2752
3. Lallemand-BreitenbachV
ZhuJ
PuvionF
KokenM
HonoreN
2001
Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation.
J Exp Med
193
1361
1371
4. EskiwCH
DellaireG
MymrykJS
Bazett-JonesDP
2003
Size, position and dynamic behavior of PML nuclear bodies following cell stress as a paradigm for supramolecular trafficking and assembly.
J Cell Sci
116
4455
4466
5. MurataniM
GerlichD
JanickiSM
GebhardM
EilsR
2002
Metabolic-energy-dependent movement of PML bodies within the mammalian cell nucleus.
Nat Cell Biol
4
106
110
6. BrandP
LenserT
HemmerichP
2010
Assembly dynamics of PML nuclear bodies in living cells.
PMC Biophys
3
3
7. ZhongS
SalomoniP
PandolfiPP
2000
The transcriptional role of PML and the nuclear body.
Nat Cell Biol
2
E85
90
8. DellaireG
Bazett-JonesDP
2004
PML nuclear bodies: dynamic sensors of DNA damage and cellular stress.
Bioessays
26
963
977
9. TakahashiY
Lallemand-BreitenbachV
ZhuJ
de TheH
2004
PML nuclear bodies and apoptosis.
Oncogene
23
2819
2824
10. BernardiR
PandolfiPP
2003
Role of PML and the PML-nuclear body in the control of programmed cell death.
Oncogene
22
9048
9057
11. Lallemand-BreitenbachV
de TheH
2010
PML nuclear bodies.
2
5
Cold Spring Harb Perspect Biol
12. GiorgiC
ItoK
LinHK
SantangeloC
WieckowskiMR
2010
PML Regulates Apoptosis at Endoplasmic Reticulum by Modulating Calcium Release.
Science
330
1183
1184
13. FagioliM
AlcalayM
PandolfiPP
VenturiniL
MencarelliA
1992
Alternative splicing of PML transcripts predicts coexpression of several carboxy-terminally different protein isoforms.
Oncogene
7
1083
1091
14. JensenK
ShielsC
FreemontPS
2001
PML protein isoforms and the RBCC/TRIM motif.
Oncogene
20
7223
7233
15. KastnerP
PerezA
LutzY
Rochette-EglyC
GaubMP
1992
Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins.
Embo J
11
629
642
16. BeechSJ
LethbridgeKJ
KillickN
McGlincyN
LeppardKN
2005
Isoforms of the promyelocytic leukemia protein differ in their effects on ND10 organization.
Exp Cell Res
307
109
117
17. CondemineW
TakahashiY
ZhuJ
Puvion-DutilleulF
GueganS
2006
Characterization of endogenous human promyelocytic leukemia isoforms.
Cancer Res
66
6192
6198
18. Weidtkamp-PetersS
LenserT
NegorevD
GerstnerN
HofmannTG
2008
Dynamics of component exchange at PML nuclear bodies.
J Cell Sci
121
2731
2743
19. EverettRD
Chelbi-AlixMK
2007
PML and PML nuclear bodies: implications in antiviral defence.
Biochimie
89
819
830
20. TavalaiN
StammingerT
2008
New insights into the role of the subnuclear structure ND10 for viral infection.
Biochim Biophys Acta
1783
2207
2221
21. PellettPE
RoizmanB
2007
The Family: Herpesviridae, A Brief Introduction.
KnipeDM
HowleyPM
Fields Virology
Philadelphia
Lippincott Williams and Wilkins, a Wolters Kluwer Business
2479
2500
5 ed
22. MettenleiterTC
KluppBG
GranzowH
2009
Herpesvirus assembly: an update.
Virus Res
143
222
234
23. EverettRD
RechterS
PapiorP
TavalaiN
StammingerT
2006
PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0.
J Virol
80
7995
8005
24. TavalaiN
PapiorP
RechterS
LeisM
StammingerT
2006
Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections.
J Virol
80
8006
8018
25. MaulGG
GuldnerHH
SpivackJG
1993
Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0).
J Gen Virol
74
2679
2690
26. EverettRD
MaulGG
1994
HSV-1 IE protein Vmw110 causes redistribution of PML.
Embo J
13
5062
5069
27. Chelbi-AlixMK
de TheH
1999
Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins.
Oncogene
18
935
941
28. BoutellC
SadisS
EverettRD
2002
Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro.
J Virol
76
841
850
29. CheeAV
LopezP
PandolfiPP
RoizmanB
2003
Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects.
J Virol
77
7101
7105
30. CohenJI
StrausSE
ArvinAM
2007
Varicella-Zoster Virus.
KnipeDM
HowleyPM
Fields Virology
Philadelphia
Lippincott Williams and Wilkins, a Wolters Kluwer Business
2773
2818
5 ed
31. KyratsousCA
SilversteinSJ
2009
Components of nuclear domain 10 bodies regulate varicella-zoster virus replication.
J Virol
83
4262
4274
32. ReicheltM
BradyJ
ArvinAM
2009
The replication cycle of varicella-zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level.
J Virol
83
3904
3918
33. MuellerNH
GildenDH
CohrsRJ
MahalingamR
NagelMA
2008
Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency.
Neurol Clin
26
675
697
34. MoffatJF
SteinMD
KaneshimaH
ArvinAM
1995
Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice.
J Virol
69
5236
5242
35. MoffatJF
ZerboniL
SommerMH
HeinemanTC
CohenJI
1998
The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse.
Proc Natl Acad Sci U S A
95
11969
11974
36. ZerboniL
KuCC
JonesCD
ZehnderJL
ArvinAM
2005
Varicella-zoster virus infection of human dorsal root ganglia in vivo.
Proc Natl Acad Sci U S A
102
6490
6495
37. ArvinAM
MoffatJ
SommerM
OliverS
CheX
2010
Varicella-Zoster Virus T Cell Tropism and the Pathogenesis of Skin Infection.
Curr Top Microbiol Immunol
342
189
209
38. ZerboniL
ReicheltM
ArvinA
2010
Varicella-Zoster Virus Neurotropism in SCID Mouse-Human Dorsal Root Ganglia Xenografts.
Curr Top Microbiol Immunol
342
255
276
39. KuCC
ZerboniL
ItoH
GrahamBS
WallaceM
2004
Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epidermal cell interferon-alpha.
J Exp Med
200
917
925
40. SkinnerPJ
KoshyBT
CummingsCJ
KlementIA
HelinK
1997
Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures.
Nature
389
971
974
41. YamadaM
SatoT
ShimohataT
HayashiS
IgarashiS
2001
Interaction between neuronal intranuclear inclusions and promyelocytic leukemia protein nuclear and coiled bodies in CAG repeat diseases.
Am J Pathol
159
1785
1795
42. TakahashiJ
FujigasakiH
ZanderC
El HachimiKH
StevaninG
2002
Two populations of neuronal intranuclear inclusions in SCA7 differ in size and promyelocytic leukaemia protein content.
Brain
125
1534
1543
43. TakahashiJ
FujigasakiH
IwabuchiK
BruniAC
UchiharaT
2003
PML nuclear bodies and neuronal intranuclear inclusion in polyglutamine diseases.
Neurobiol Dis
13
230
237
44. JanerA
MartinE
MurielMP
LatoucheM
FujigasakiH
2006
PML clastosomes prevent nuclear accumulation of mutant ataxin-7 and other polyglutamine proteins.
J Cell Biol
174
65
76
45. WoulfeJ
2008
Nuclear bodies in neurodegenerative disease.
Biochim Biophys Acta
1783
2195
2206
46. WilliamsAJ
PaulsonHL
2008
Polyglutamine neurodegeneration: protein misfolding revisited.
Trends Neurosci
31
521
528
47. YasudaS
InoueK
HirabayashiM
HigashiyamaH
YamamotoY
1999
Triggering of neuronal cell death by accumulation of activated SEK1 on nuclear polyglutamine aggregations in PML bodies.
Genes Cells
4
743
756
48. VillagraNT
NavascuesJ
CasafontI
Val-BernalJF
LafargaM
2006
The PML-nuclear inclusion of human supraoptic neurons: a new compartment with SUMO-1 - and ubiquitin-proteasome-associated domains.
Neurobiol Dis
21
181
193
49. LafargaM
BercianoMT
PenaE
MayoI
CastanoJG
2002
Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome.
Mol Biol Cell
13
2771
2782
50. ArrasateM
MitraS
SchweitzerES
SegalMR
FinkbeinerS
2004
Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death.
Nature
431
805
810
51. QinQ
InatomeR
HottaA
KojimaM
YamamuraH
2006
A novel GTPase, CRAG, mediates promyelocytic leukemia protein-associated nuclear body formation and degradation of expanded polyglutamine protein.
J Cell Biol
172
497
504
52. TorashimaT
KoyamaC
IizukaA
MitsumuraK
TakayamaK
2008
Lentivector-mediated rescue from cerebellar ataxia in a mouse model of spinocerebellar ataxia.
EMBO Rep
9
393
399
53. StuurmanN
de GraafA
FlooreA
JossoA
HumbelB
1992
A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies.
J Cell Sci
101
Pt 4
773
784
54. ChaudhuriV
SommerM
RajamaniJ
ZerboniL
ArvinAM
2008
Functions of Varicella-zoster virus ORF23 capsid protein in viral replication and the pathogenesis of skin infection.
J Virol
82
10231
10246
55. EverettRD
SourvinosG
LeiperC
ClementsJB
OrrA
2004
Formation of nuclear foci of the herpes simplex virus type 1 regulatory protein ICP4 at early times of infection: localization, dynamics, recruitment of ICP27, and evidence for the de novo induction of ND10-like complexes.
J Virol
78
1903
1917
56. WilemanT
2007
Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design?
Annu Rev Microbiol
61
149
167
57. DayPM
RodenRB
LowyDR
SchillerJT
1998
The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains.
J Virol
72
142
150
58. FlorinL
SchaferF
SotlarK
StreeckRE
SappM
2002
Reorganization of nuclear domain 10 induced by papillomavirus capsid protein l2.
Virology
295
97
107
59. Shishido-HaraY
IchinoseS
HiguchiK
HaraY
YasuiK
2004
Major and minor capsid proteins of human polyomavirus JC cooperatively accumulate to nuclear domain 10 for assembly into virions.
J Virol
78
9890
9903
60. Puvion-DutilleulF
VenturiniL
GuilleminMC
de TheH
PuvionE
1995
Sequestration of PML and Sp100 proteins in an intranuclear viral structure during herpes simplex virus type 1 infection.
Exp Cell Res
221
448
461
61. TokuyasuKT
1973
A technique for ultracryotomy of cell suspensions and tissues.
J Cell Biol
57
551
565
62. GriffithsG
SimonsK
WarrenG
TokuyasuKT
1983
Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins.
Methods Enzymol
96
466
485
63. WebsterP
SchwarzH
GriffithsG
2008
Preparation of cells and tissues for immuno EM.
Methods Cell Biol
88
45
58
64. ZhouZH
HeJ
JakanaJ
TatmanJD
RixonFJ
1995
Assembly of VP26 in herpes simplex virus-1 inferred from structures of wild-type and recombinant capsids.
Nat Struct Biol
2
1026
1030
65. ZhouZH
DoughertyM
JakanaJ
HeJ
RixonFJ
2000
Seeing the herpesvirus capsid at 8.5 A.
Science
288
877
880
66. McDonaldKentL
MorphewMary
VerkadePaul
Mueller-ReichertThomas
2007
Recent Advances in High-Pressure Freezing: Equipment and Specimen-Loading Methods.
KuoJ
Methods In Molecular Biology: Electron Microscopy, Methods and Protocols
Totowa, New Jersey
Humana Press
143
173
2 ed
67. RabouilleC
1999
Quantitative aspects of immunogold labeling in embedded and nonembedded sections.
Methods Mol Biol
117
125
144
68. GildenDH
RozenmanY
MurrayR
DevlinM
VafaiA
1987
Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia.
Ann Neurol
22
377
380
69. LevinMJ
CaiGY
ManchakMD
PizerLI
2003
Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia.
J Virol
77
6979
6987
70. WangK
LauTY
MoralesM
MontEK
StrausSE
2005
Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal Ganglia at the single-cell level.
J Virol
79
14079
14087
71. MoriuchiH
MoriuchiM
SmithHA
CohenJI
1994
Varicella-zoster virus open reading frame 4 protein is functionally distinct from and does not complement its herpes simplex virus type 1 homolog, ICP27.
J Virol
68
1987
1992
72. DefechereuxP
DebrusS
BaudouxL
RentierB
PietteJ
1997
Varicella-zoster virus open reading frame 4 encodes an immediate-early protein with posttranscriptional regulatory properties.
J Virol
71
7073
7079
73. HomaFL
BrownJC
1997
Capsid assembly and DNA packaging in herpes simplex virus.
Rev Med Virol
7
107
122
74. ChenJJ
ZhuZ
GershonAA
GershonMD
2004
Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster.
Cell
119
915
926
75. IwataA
ChristiansonJC
BucciM
EllerbyLM
NukinaN
2005
Increased susceptibility of cytoplasmic over nuclear polyglutamine aggregates to autophagic degradation.
Proc Natl Acad Sci U S A
102
13135
13140
76. BurkhamJ
CoenDM
HwangCB
WellerSK
2001
Interactions of herpes simplex virus type 1 with ND10 and recruitment of PML to replication compartments.
J Virol
75
2353
2367
77. EverettRD
MurrayJ
2005
ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection.
J Virol
79
5078
5089
78. DelhayeS
PaulS
BlakqoriG
MinetM
WeberF
2006
Neurons produce type I interferon during viral encephalitis.
Proc Natl Acad Sci U S A
103
7835
7840
79. ForestT
BarnardS
BainesJD
2005
Active intranuclear movement of herpesvirus capsids.
Nat Cell Biol
7
429
431
80. FeierbachB
PiccinottiS
BisherM
DenkW
EnquistLW
2006
Alpha-herpesvirus infection induces the formation of nuclear actin filaments.
PLoS Pathog
2
e85
81. LopezP
JacobRJ
RoizmanB
2002
Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins.
J Virol
76
9355
9367
82. DayPM
BakerCC
LowyDR
SchillerJT
2004
Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression.
Proc Natl Acad Sci U S A
101
14252
14257
83. Shishido-HaraY
HiguchiK
OharaS
DuyckaertsC
HauwJJ
2008
Promyelocytic leukemia nuclear bodies provide a scaffold for human polyomavirus JC replication and are disrupted after development of viral inclusions in progressive multifocal leukoencephalopathy.
J Neuropathol Exp Neurol
67
299
308
84. HallerO
StertzS
KochsG
2007
The Mx GTPase family of interferon-induced antiviral proteins.
Microbes Infect
9
1636
1643
85. KochsG
JanzenC
HohenbergH
HallerO
2002
Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes.
Proc Natl Acad Sci U S A
99
3153
3158
86. ReicheltM
StertzS
Krijnse-LockerJ
HallerO
KochsG
2004
Missorting of LaCrosse virus nucleocapsid protein by the interferon-induced MxA GTPase involves smooth ER membranes.
Traffic
5
772
784
87. SchunemannS
MainkaC
WolffMH
1998
Subclinical reactivation of varicella-zoster virus in immunocompromised and immunocompetent individuals.
Intervirology
41
98
102
88. ReicheltM
ZerboniL
ArvinAM
2008
Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia.
J Virol
82
3971
3983
89. AuWY
KwongYL
2005
Frequent varicella zoster reactivation associated with therapeutic use of arsenic trioxide: portents of an old scourge.
J Am Acad Dermatol
53
890
892
90. NouriK
RicottiCAJr
BouzariN
ChenH
AhnE
2006
The incidence of recurrent herpes simplex and herpes zoster infection during treatment with arsenic trioxide.
J Drugs Dermatol
5
182
185
91. JeanneM
Lallemand-BreitenbachV
FerhiO
KokenM
Le BrasM
2010
PML/RARA oxidation and arsenic binding initiate the antileukemia response of As2O3.
Cancer Cell
18
88
98
92. ZhangXW
YanXJ
ZhouZR
YangFF
WuZY
2010
Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML.
Science
328
240
243
93. Vizoso PintoMG
VillegasJM
PeterJ
HaaseR
HaasJ
2009
LuMPIS-a modified luminescence-based mammalian interactome mapping pull-down assay for the investigation of protein-protein interactions encoded by GC-low ORFs.
Proteomics
9
5303
5308
Štítky
Hygiena a epidemiológia Infekčné lekárstvo LaboratóriumČlánok vyšiel v časopise
PLOS Pathogens
2011 Číslo 2
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
Najčítanejšie v tomto čísle
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
