-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
The endosymbiotic bacterium Wolbachia is being investigated as a potential control agent in several important vector insect species. Recent studies have shown that Wolbachia can protect the insect host against a wide variety of pathogens, resulting in reduced transmission of parasites and viruses. It has been proposed that compromised vector competence of Wolbachia-infected insects is due to up-regulation of the host innate immune system or metabolic competition. Anopheles mosquitoes, which transmit human malaria parasites, have never been found to harbor Wolbachia in nature. While transient somatic infections can be established in Anopheles, no stable artificially-transinfected Anopheles line has been developed despite numerous attempts. However, cultured Anopheles cells can be stably infected with multiple Wolbachia strains such as wAlbB from Aedes albopictus, wRi from Drosophila simulans and wMelPop from Drosophila melanogaster. Infected cell lines provide an amenable system to investigate Wolbachia-Anopheles interactions in the absence of an infected mosquito strain. We used Affymetrix GeneChip microarrays to investigate the effect of wAlbB and wRi infection on the transcriptome of cultured Anopheles Sua5B cells, and for a subset of genes used quantitative PCR to validate results in somatically-infected Anopheles mosquitoes. Wolbachia infection had a dramatic strain-specific effect on gene expression in this cell line, with almost 700 genes in total regulated representing a diverse array of functional classes. Very strikingly, infection resulted in a significant down-regulation of many immune, stress and detoxification-related transcripts. This is in stark contrast to the induction of immune genes observed in other insect hosts. We also identified genes that may be potentially involved in Wolbachia-induced reproductive and pathogenic phenotypes. Somatically-infected mosquitoes had similar responses to cultured cells. The data show that Wolbachia has a profound and unique effect on Anopheles gene expression in cultured cells, and has important implications for mechanistic understanding of Wolbachia-induced phenotypes and potential novel strategies to control malaria.
Published in the journal: Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction. PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001296
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001296Summary
The endosymbiotic bacterium Wolbachia is being investigated as a potential control agent in several important vector insect species. Recent studies have shown that Wolbachia can protect the insect host against a wide variety of pathogens, resulting in reduced transmission of parasites and viruses. It has been proposed that compromised vector competence of Wolbachia-infected insects is due to up-regulation of the host innate immune system or metabolic competition. Anopheles mosquitoes, which transmit human malaria parasites, have never been found to harbor Wolbachia in nature. While transient somatic infections can be established in Anopheles, no stable artificially-transinfected Anopheles line has been developed despite numerous attempts. However, cultured Anopheles cells can be stably infected with multiple Wolbachia strains such as wAlbB from Aedes albopictus, wRi from Drosophila simulans and wMelPop from Drosophila melanogaster. Infected cell lines provide an amenable system to investigate Wolbachia-Anopheles interactions in the absence of an infected mosquito strain. We used Affymetrix GeneChip microarrays to investigate the effect of wAlbB and wRi infection on the transcriptome of cultured Anopheles Sua5B cells, and for a subset of genes used quantitative PCR to validate results in somatically-infected Anopheles mosquitoes. Wolbachia infection had a dramatic strain-specific effect on gene expression in this cell line, with almost 700 genes in total regulated representing a diverse array of functional classes. Very strikingly, infection resulted in a significant down-regulation of many immune, stress and detoxification-related transcripts. This is in stark contrast to the induction of immune genes observed in other insect hosts. We also identified genes that may be potentially involved in Wolbachia-induced reproductive and pathogenic phenotypes. Somatically-infected mosquitoes had similar responses to cultured cells. The data show that Wolbachia has a profound and unique effect on Anopheles gene expression in cultured cells, and has important implications for mechanistic understanding of Wolbachia-induced phenotypes and potential novel strategies to control malaria.
Introduction
Wolbachia are alpha-proteobacteria that infect a range of arthropods and nematodes, and are possibly the most common endosymbiotic bacteria on the planet. In their arthropod hosts, Wolbachia induce a variety of reproductive manipulations that enhance the fitness of infected females compared to their uninfected counterparts [1]. Wolbachia have recently been shown to interfere with pathogen infection and transmission in both naturally-infected and artificially-transinfected insects [2], [3], [4], [5], [6], [7]. These phenotypes make Wolbachia-based control strategies an attractive option to minimize the impact of arthropod-borne diseases and insect pests [8], [9].
Anopheles mosquitoes transmit human malaria, a devastating disease that kills approximately 2 million people per year, and are naturally uninfected with Wolbachia [10], [11], [12]. Transfer of Wolbachia into cultured Anopheles gambiae cells and transient somatic infection of adult female mosquitoes demonstrates that the bacteria can survive in this species, suggesting that the Anopheles genus may be amenable to stable infection [13], [14]. Although several novel Wolbachia-mosquito associations have been created using a variety of transinfection techniques, no stable Wolbachia-infected Anopheles line has been developed [15], [16], [17], [18], [19], [20]. The development of such a strain may open the possibility for Wolbachia-based control strategies for malaria. Indeed somatic infections of the wMelPop strain reduce oocyst levels in the murine malaria model, Plasmodium berghei [7]. However the global effects of Wolbachia on Anopheles and the interplay within the tripartite association of the human malaria Plasmodium parasites and the mosquito host are currently unknown.
Novel phenotypes are sometimes observed upon transinfection of Wolbachia into novel insect hosts [15], [21], [22]. In the artificially infected wMelPop-Aedes aegypti strain (wMelPop CLA), Wolbachia limits infection by a broad range of pathogens including dengue virus, filarial nematodes and Plasmodium [2], [3]. The mode of action for pathogen resistance is uncertain, however two mechanisms have been postulated; immune activation of the host by Wolbachia and/or metabolic competition between the bacteria and the pathogen. Evidence for both hypotheses was observed with a range of immune genes up-regulated in wMelPop-infected Ae. aegypti [2], [3] and the finding that dengue virus only persisted in Wolbachia-uninfected cells of the insect [3]. A similar phenotype was observed in some infected Drosophila strains where Wolbachia infection induced refractoriness to multiple RNA viruses [4], [5]. Interestingly, a previous study using naturally infected hosts found that Wolbachia seems to be able to evade the host immune response in Drosophila and Aedes albopictus [23], suggesting Wolbachia-induced immune activation may be more likely in novel rather than co-evolved Wolbachia-host associations.
Within Anopheles mosquitoes, there is a conserved immune response towards foreign bacteria and Plasmodium [24]. By using multiple methods such as co-feeding, injection or removal of microflora, bacteria have been seen to mediate Plasmodium infection levels in the Anopheles host [25], [26], [27], [28], which is thought to be due to the bacteria priming the host immune response. Interestingly, Gram-negative bacteria elicit a greater response compared to Gram positive, although there are species-specific differences [25], [27]. If Wolbachia (a Gram-negative bacterium) evokes a similar response and up-regulates the basal immunity in infected Anopheles, infection may confer an anti-Plasmodium phenotype. Some evidence for this has been shown in somatically-infected mosquitoes infected with rodent malaria [7].
The generation of Wolbachia-infected Anopheles cell lines allows the investigation of Wolbachia-Anopheles interactions in the absence of a stably-infected mosquito strain [14]. Cell lines provide a platform whereby Wolbachia host lineages can be generated with relative ease, and allow the exploration of both natural and artificial Wolbachia host interactions [29], [30], [31]. To investigate the effect of Wolbachia infection on global patterns of Anopheles gene expression we performed microarray analysis on both wAlbB (from Ae. albopictus) and wRi (from Drosophila simulans) infected Anopheles gambiae Sua5B cells compared to uninfected cells. We validated microarray results in vitro, and in vivo for a subset of differentially expressed genes in somatically-infected adult female mosquitoes.
Results/Discussion
Wolbachia infection of Anopheles cells resulted in the regulation of 690 genes relative to uninfected Sua5B cells (False discovery rate (FDR) P<0.05, ≥ 2.0 fold-change (FC)) (Table S1). When comparing Wolbachia strains, 255 genes were uniquely regulated by wAlbB infection, while 331 were regulated specifically by wRi infection (Figure 1A). Of the 104 genes regulated by both strains, the majority (74 genes) were down-regulated, 11 were similarly up-regulated and the remainder had alternating regulation patterns between the two Wolbachia strains (Figure 1B). Interestingly, we observed a greater number of genes regulated by wRi compared to wAlbB even though the cell infection density of wRi was much less than wAlbB (wRi∼10% cells infected, wAlbB >90% of cells infected) [14]. It is possible that since wRi was purified from live flies, it has a greater impact that wAlbB which was purified from another cell line [14]. Of the regulated genes, a diverse range of functional classes was represented with a large proportion being genes of unknown or diverse function, which was consistent for both Wolbachia strains. Among the genes assigned to specific known functional classes, immune-, transport - and metabolism-related transcripts were the most abundant categories regulated by Wolbachia (Figure 1C). Strikingly, over 75% of the immune related transcripts were down-regulated, which was consistent between both strains. Overall, down-regulation was a common theme, with only redox/stess/mitochondrial (RSM) and replication/transcription/translation (RTT) classes not down-regulated in wRi infected cells and RTT in wAlbB. Microarray data is available at gene expression omnibus (accession number GSE23215) [32].
Fig. 1. Anopheles gambiae gene regulation in response to Wolbachia infection. 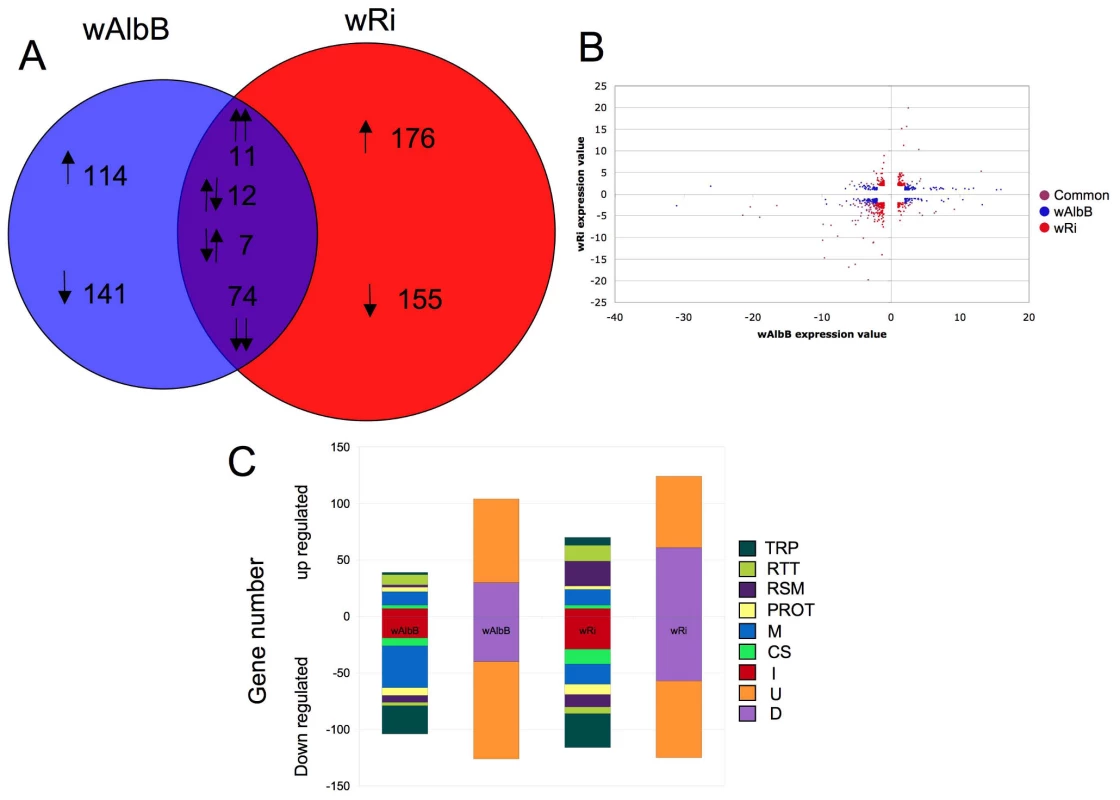
A. Venn diagram of 690 Anopheles transcripts which display differential expression due to wAlbB or wRi infection. 104 transcripts were common to both strains, while 389 were down regulated and 320 up regulated due to Wolbachia infection. B. Scatter plot of regulated significant genes (>2 fold regulation; False discovery rate P value <0.05). Blue dots represent significant genes regulated by wRi only, red regulated by wAlbB only and purple, genes commonly regulated. C. Number of genes in each functional classes class up or down regulated in response to either wAlbB or wRi infection. Genes were classified into groups; transport (TRP), replication, transcription and translation (RTT), redox, stress and mitochondrial (RSM) proteolysis and digestion (PROT), metabolism (M) cytoskeletal and structural (CS) and immune (I) depicted in the first column, and diverse (D) and unknown (U), in the second column. qPCR validation of microarray genes and comparison to whole mosquitoes
To gauge the accuracy of the microarray data, we selected a subset of genes to validate by quantitative real-time PCR from cell culture. Eight genes, (HSP20, HSP90, HSPDnaJ, cold-shock protein, cecropin, Serpin11, Filamin, TEP3) with varying expression profiles, regulated by both Wolbachia strains were evaluated. These genes spanned a variety of functional classes including defensive and immune genes that may be relevant to Plasmodium infection and potential Wolbachia-mediated reproductive phenotypes. qPCR results corroborated the array data and had a positive linear correlation (R2 = 0.9595) when comparing the log2 values using both gene expression techniques (Figure 2A).
Fig. 2. Validation of microarray data in cell culture and whole mosquitoes. 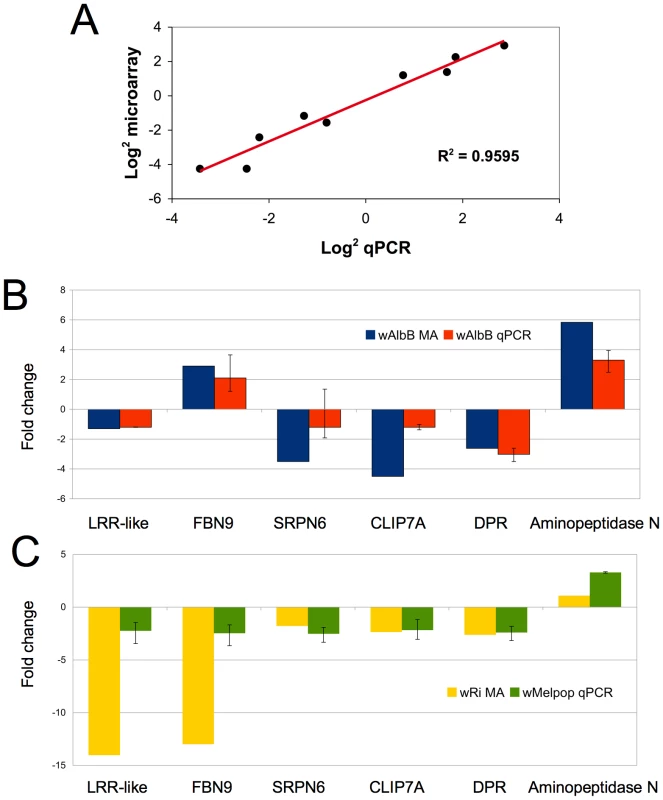
A. Log2 fold change for selected An. gambiae genes (HSP20, HSP90, HSPDnaJ, cold-shock protein, cecropin, Serpin6, Filamin, TEP3) comparing microarray and QPCR methods. B. Comparison of Anopheles gene expression in response to Wolbachia in cell culture and whole mosquitoes. Expression of 6 genes from wAlbB in Sua5B cells analyzed using microarrays (MA) compared to wAlbB somatically-infected whole mosquitoes 15 days post injection (N = 5 mosquitoes/treatment). C. Microarray data from wRi infected Sua5B cells compared to wMelpop somatically-infected whole mosquitoes 15 days post injection (N = 5 mosquitoes/treatment). qPCR gene expression is a ratio of Wolbachia infected (wAlbB or wRi) to Schneider's injected control. Error bars represent maximum and minimum range of expression. Wolbachia has been shown to persist, disseminate, and replicate in injected adult Anopheles mosquitoes [13]. We injected live female mosquitoes with Wolbachia to determine if the effect of infection on gene regulation in vivo was consistent with results observed from infected cell cultures. Several immune related transcripts and other genes, which potentially convey interesting phenotypes and had varying expression profiles identified in cell culture, were assessed. When comparing wAlbB regulation in cells and mosquitoes, the direction of regulated expression was similar (Figure 2B), although, not surprisingly, the intensity of expression varied leading to a lack of significant correlation (data not shown). The loss of the wRi-infected cell line prevented a direct comparison to somatically infected mosquitoes, however, this array data was compared to wMelPop-infected mosquitoes. wMelpop and wRi both infect Drosophila and are classed in supergroup A. When making this comparison, again we observed that the direction of gene regulation was similar (Figure 2C), but the intensity of expression varied. Notably, the intensity of two genes, the LRR-like transcript and FBN9 is greater in the cells compared to the whole mosquito. This may be explained by the hemocyte-like character of the cell line or Wolbachia strain-specific variation. Nevertheless, the similarity in the direction of gene regulation in vivo and in vitro suggests that the effect of Wolbachia in the cell line may be applicable to whole mosquitoes.
Comparison to other systems
We compared Wolbachia-regulated Anopheles transcripts identified in this study to genes regulated by wMelPop in Aedes aegypti [2] and by bacterial infection in A. gambiae [26]. Fourteen A. aegypti homologues were identified from differentially expressed Anopheles transcripts in response to Wolbachia infection, with five having an immune related function (Table S2). When comparing these results, 75% of both wRi and wAlbB regulated homologs displayed a similar direction of expression. Similarly, when comparing Wolbachia-regulated transcripts to those of regulated by bacterial infection in A. gambiae, 15 homologs were regulated by Wolbachia. (Table S3). Most of these homologs were of unknown function.
In comparison to other studies using Drosophila cell culture systems to examine the influence of Wolbachia on host gene expression, we find a dramatically elevated number of identified regulated Anopheles genes compared to Drosophila. In Drosophila S2 cells, 263 genes had a 1.2 fold change due to Wolbachia infection, however when the more common ≥2 fold criteria was used, very few regulated Drosophila genes were identified [33]. At the proteomic level, only four proteins, all host antioxidant proteins, were elevated in Wolbachia infected Ae. albopictus Aa23 cells [34]. A lower Wolbachia titer may account for the subtle gene regulation in wRi infected Drosophila cells [33], although the infection density of wRi in infected Anopheles was similarly sparse [14]. Alternatively, the mild effect of Wolbachia on gene regulation in Drosophila and protein expression in Ae. albopictus could be due to previous co-evolution between the Wolbachia strains and their naturally infected hosts.
Effect of Wolbachia on transcription of Anopheles genes potentially affecting pathogen transmission
Stress-response
The most striking effect observed for both wRi and wAlbB infections was the general suppression of heat shock protein transcripts (HSP20, HSP70, HSP90, HSP-DNAJ). Cells infected with wAlbB had a dramatic suppression of these genes with 5 out of the top 6 most down-regulated genes (FC −31 to −16). Similarly, these genes were down-regulated by wRi, albeit to a lesser extent (to −5.3). Presenting a similar pattern of regulation, multiple HSPs were down-regulated by wRi infected Drosophila S2 cells [33]. In vivo, it has been shown that Wolbachia-infected flies have altered expression of HSP, which in turn affects Wolbachia-induced reproductive phenotypes [35]. HSPs have also been implicated in Anopheles-pathogen interactions. Elevated levels of HSP20 were identified in An. gambiae heads after infection with P. berghei [36]. If this protein assists transmission, either directly or indirectly, the antagonistic actions of may potentially reduce P. berghei sporozoite infection. Additionally, knockdown of a heat shock proteins (HSC70B) via injection of dsRNAi in conjunction with O'nyong nyong virus (ONNV) significantly reduced the lifespan of adult mosquitoes as compared with the control [37]. We speculate that if this expression pattern translates to in vivo Anopheles infections, Wolbachia-induced down-regulation of HSPs may modulate vector competence of ONNV or shorten mosquito lifespan.
Metabolic and other genes
Wolbachia regulates a suite of genes involved in Anopheles metabolism, with most of these transcripts being down-regulated by infection. Although the heterotrophic needs of Plasmodium and mosquito growth factors required for parasite development are not well understood in the insect, changes in transcription of metabolism genes which alter the mosquito environment may affect Plasmodium growth. Infection of Sua5B cells with wAlbB drastically reduces phosphoenolpyruvate carboxykinase (PEPCK) transcripts 26 fold. In response to P. falciparum, PEPCK is up-regulated in the mosquito [38], [39]. Carbonic anhydrase, which catalyses the reversible hydration of carbon dioxide to bicarbonate, is down-regulated in wAlbB-infected cells by 2.6 fold. In many mosquitoes, inhibition of this enzyme results in a reduction in pH of the mosquito midgut [40]. Moreover, carbonic anhydrase inhibitors in P. falciparum reduced parasite survival in the human blood stages and have been suggested as targets of anti-malarial drug design [41], [42]. The effect of these host derived enzymes on parasite development is unknown, however changes in regulation between mosquito and Plasmodium suggest that further examination of these genes is warranted to determine their affects on parasite development. Although not strictly metabolism related, laminin and collagen are components of the basal lamina, which are interrelated with parasite invasion [43], [44], [45]. Both laminin (FC −2.1, −3.8) and collagen (FC −4.4) are down-regulated by wRI infection. RNAi knock down of laminin lead to a substantial reduction of oocysts in mosquito midguts [44] possibly due to laminin inhibiting the melanotic encapsulation of oocysts [46].
Immunity-related transcripts
Many Anopheles genes associated with arthropod immunity were regulated by Wolbachia infection. Genes within all the broad categories of immunity (pathogen recognition receptors, signaling amplification cascades, immune signaling pathways, and effector molecules) were regulated. Immune genes up-regulated by both infections included CLIPs and antimicrobial peptides (AMP), while serpins (SRPN), and a leucine rich repeat (LRR) were induced by wRi and fibrinogens (FBN) and thioester-containing protein (TEP) were induced by wAlbB (Figure 3). More striking were those immune genes down-regulated by infection. wRi significantly suppressed expression of class C scavenger receptors, Gram-negative binding proteins (GNBP), FBN, CLIP, SRPN, LRR-containing genes, a TEP, effector proteins involved in phagocytosis and a lysozyme (Figure 3). The wAlbB strain down-regulated genes of similar functions, however in the class of effector molecules, this strain had more of an influence on peroxidases rather than AMPs (Figure 3).
Fig. 3. Wolbachia strain-specific regulation of Anopheles gambiae immune pathways. 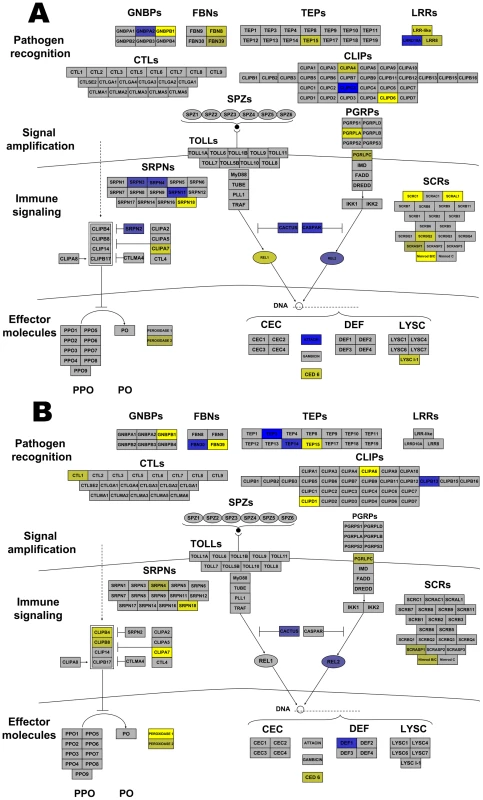
Anopheles immune networks regulated by wRi (A) and wAlbB (B). Pathways are models of the IMD and Toll pathways [81] and components of the melanization regulatory module [51] divided into the 4 broad categories of immune molecules. Blue color represents induction, while yellow color represents suppression. The intensity of coloring is proportional to the intensity of expression. Regulation is depicted to a maximum fold change of ±4. Some transcripts were greater than ±4 regulated. Abbreviations: LLR leucine rich repeats; FBNs fibrinogens; TEPs thioester containing proteins; GNBPs Gram-negative binding proteins; CTLs C type lectins; CLIPs clip-domain serine protease; PGRPs peptidoglycan recognition proteins; SRPNs serpins; CEC cecropins; Def defensins; PPO Prophenoloxidase; PO phenoloxidase; LYS lysozmyes. In addition, other immune-associated apoptosis and detoxification transcripts were regulated by infection. Brennan et al. [34] identified Wolbachia-induced host antioxidant proteins in cell culture. In contrast to the enrichment of these genes at the protein level, a peroxiredoxin transcript was down-regulated 2.1 times by wAlbB and 11 times by wRi. Likewise, superoxide dismutase was down-regulated in wRi-infected cells (FC −2). Additionally, eight glutathione S transferases were regulated. Two of these were co-regulated by both strains, while 3 were induced and 3 suppressed in wRi. The level of regulation for these genes was approximately 2–3 fold, however one transcript was suppressed 19-fold by wRi compared to uninfected cells. Taken in total, these data suggest that Wolbachia can significantly affect cellular defense, detoxification and immunity in An. gambiae cells, and that expression of many of these defensive genes is suppressed rather than induced. These results contrast with observations of up-regulation of the majority of immune-related transcripts in stably-infected Ae. aegypti mosquitoes, which have reduced capacity to transmit pathogens [2], [3]. Gene expression of a small subset of immune genes were characterized in response to wMelPop infection of a different An. gambiae cell line (Mos55), where they were up-regulated, suggesting a potential difference between Anopheles cell lines or Wolbachia strain-specific variation [7].
Although pathogen interference occurs in naturally infected hosts, there is evidence that the transfer of Wolbachia to a new host is a catalyst for pathogen interference, illustrated by wAlbB inducing dengue resistance in a novel host, Ae. aegypti, yet not conferring interference in it's native host, Ae. albopictus [6]. The effects of tripartite relationship of Wolbachia-Anopheles-Plasmodium are relatively unknown, however, recently wMelPop somatically infected into Anopheles was seen to decrease P. berghei oocyst levels, with evidence that TEP1 may involved in the process [7]. Many of the regulated defensive genes we identified have been shown to directly or indirectly affect Plasmodium infection in Anopheles, either positively or negatively. TEP3 was dramatically up-regulated (FC 7.6) in response to wAlbB. Similar up-regulation is observed when mosquitoes are fed a blood meal, either uninfected or infected (P. berghei), or challenged with bacteria [47], [48]. TEP1, a protein similar to TEP3, has been shown to be an important molecule involved in the melanization and anti-Plasmodium response across the Anopheles genus [49], [50]. Looking at genes involved in the immune signaling cascade, CLIP7A, a suppressor of melanization, was suppressed by both wAlbB (FC −5.2) and wRi (FC −2.6), which may confer an anti-Plasmodium phenotype as seen in knock-down experiments of this gene [51]. In contrast, the gene galectin, which is up-regulated in response to P. berghei infection and immune challenge by Micrococcus luteus, had conflicting strain-specific responses: up-regulated by wAlbB (FC 9.1) but down-regulated by wRi (FC −3.5) [52].
In contrast to genes that may abate Plasmodium infection, a suite of genes were also regulated in ways that may elevate parasite levels in infected mosquitoes. For example we observed down-regulation of many CLIPs. Reverse genetic techniques have shown that both CLIPB4 and CLIPB8 are involved in the melanization process, where knock-down of these genes ablates melanization [53]. In double knock-down (KD) experiments, reducing transcripts of both CLIPB4 and CLIPB8 in tandem with CTL4 partially interferes with P. berghei ookinete melanization [51]. Using over-expression, up-regulation of cecropin was shown to decrease Plasmodium levels in Anopheles [46]. Expression of both SRPN18 (FC wAlbB −3.2, wRi −3.6) and TEP15 (FC wAlbB −3.5, wRi −2.1) is suppressed by both Wolbachia strains and although the specific function of these molecules has not been identified, these classes of molecules are associated with immunity [48], [54]. In Ae. aegypti, TEP15 is one of the most strongly induced genes in response to KD of Cactus, the negative regulator of the Toll pathway [55]. In addition, GNBPB1, which was also down-regulated by both strains (FC wAlbB −5.2, wRi −6.0), is strongly induced by parasite invasion of the midgut and bacterial challenge [52], [56], [57]. In contrast to our study, GNBP was induced in Aedes mosquitoes infected with wAlbB and wMelpop [2], [6].
In terms of a general response to bacterial infection, we see the regulatory transcriptional factor for the Toll pathway (Rel1) down-regulated 2.3 times by wRi infection. We observed an up-regulation of caspar (FC 2.2), the negative regulator of the IMD pathway in response to wRi. PGRP-LA expression was suppressed 3.2 times by wRi. In Drosophila, PGRP-LA is likely to be a hemocyte transmembrane protein [58], while other PGRPs activate negative feedback loops in the IMD pathway [59], [60]. A similar long transcript PGRP (PGRP-LC) in An. gambiae controlled proliferation of gut microbiota, which subsequently influenced Plasmodium infection [61]. When all three PGRP-LC isoforms were silenced simultaneously, mosquitoes challenged with Staphylococcus aureus had induced expression of cecropin and defensin. In Drosophila, silencing of PGRP-LC by RNAi induced expression of diptericin, cecropin A1, and attacin A, but these effector molecules were not regulated due to depletion of PGRP-LA [62]. Here we see similar independent regulation of attacin which was up-regulated 3.3 times in wRi infected cells, while defensin is also up-regulated by wAlbB (FC 2.3). Interestingly, attacin was found to inhibit the outer membrane synthesis of Escherichia coli in the giant silk moth, Hyalophora cecropia [63]. Thus, we may be observing an active defensive response from Anopheles to prevent Wolbachia infection.
The general pattern of immune gene down-regulation appears to be a Wolbachia-specific phenomenon in this cell line. In addition to Wolbachia, Sua5B cells can support infection of additional intracellular bacteria such as Rickettsia [64]. We used qPCR to test selected immune-related genes (cecropin1, defensin1, gambicin and immune-responsive serpin-related protein [IserpF1]) in Sua5B cells that had been infected with a taxonomically and phenotypically diverse array of Rickettsia species: R. typhi (typhus group), R. felis (transitional group), R. montenensis and R. peakockii (both in the spotted fever group). R. typhi and R. felis are human pathogens, while R. montenensis is non-pathogenic. R. peakockii is a non-pathogenic vertically-transmitted tick endosymbiont. While there was variation between bacterial species and the gene tested, all four Rickettsia induced expression of most tested immune genes (up to 12-fold induction), including the endosymbiont R. peakockii (Figure S1). These results suggest that the natural response of Sua5B cells to intracellular bacterial infection is immune up-regulation, and that Wolbachia is suppressing this response. It should be noted however that Wolbachia exist in a potentially protective host vacuole, while Rickettsia are free in the cytoplasm.
Wolbachia influence on reproduction-related genes
Wolbachia-induced CI expression is associated with abnormal decondensation of the paternal pronucleus during fertilization, epigenetic factors, and/or problems during embryogenesis. Xi et al. [33] observed that in wRi-infected Drosophila cells, the gene angiotensin converting enzyme (Ance), which is involved in spermatogenesis, was up-regulated by Wolbachia infection in cells and flies, and was potentially involved in the CI phenotype. In our study, the six Anopheles homologues of Ance on the microarray were not affected by Wolbachia infection. We screened our data for other significantly regulated genes associated with cytoskeleton formation/function, epigenetic modification, gametogenesis or embryonic development. Multiple cytoskeleton-associated genes, genes associated with chromatin formation and remodeling and genes associated with embryogenesis and cell division were regulated by both infections.
We identified multiple genes that may be linked to the CI phenotype. Transcription of a Kazal-like serpin was enhanced dramatically due to Wolbachia infection (FC wAlbB 13.1, wRi 5.3). Kazal domain-containing proteins identified in animals have a diverse array of functions. A Kazal-like serpin was found to inhibit both gelatinolytic activity of sperm and the proteolytic activity of sperm extracts to vitelline coat in prawns [65], while in mice, a serine protease inhibitor Kazal-type-like protein bound to sperm, enhancing motility and suppressing sperm capacitation [66]. Although in these two species the function of the Kazel-like serpin is varied, it has the commonality that it interferes with sperm–oocyte interactions. Up-regulated (FC 2.3) in wRi-infected cells, crooked neck (crn) transcripts are involved in embryogenesis. In it's recessive form, crn is lethal to embryos, while heterozygotes display a crooked phenotype [67]. In both Drosophila and humans, crn has been implicated in the mRNA splicing process and is thought to be a pre-mRNA splicing factor [68], [69]. Another gene induced by wRi (FC 3.1), otefin, codes for a nuclear laminin which is essential for germ cell maintenance in Drosophila [70]. A further candidate protein, Dumpy-30 (Dpy-30) is expressed in spermatids in Drosophila, and mutations or knockout of the male-specific dpy-30L2 gene results in male sterility as mutant sperm have impaired motility and fail to accumulate in sperm storage organs of females [71]. In Anopheles cells, wAlbB up-regulates (FC 2.0; significant at unadjusted P<0.05) Dpy-30, and although the effect of over-expression is unknown, this could potentially have a role in the CI phenotype. Serine active site containing (Serac1) mediates sterility in mice [69] and is up regulated (FC 2.4) by wRi infection. TEP15, suppressed by both strains (FC wAlbB −3.5, wRi −2.2), may influence reproduction. TEP15 is a male accessory glands protein and is transferred to female in the mating plug [72]. It would be interesting to determine if Wolbachia-induced regulation of these transcripts is Anopheles specific or common to other insect species infected with Wolbachia.
In addition to these genes, heat shock proteins were dramatically down regulated by both bacterial strains, but the effect was most dramatic by wAlbB. HSPs have been associated with sperm production and are inferred to be involved in CI [35], [73]. A range of chaperone proteins were also up-regulated by wRi, including a cold shock protein (FC 4.8) multiple DNAJ heat shock proteins (FC 3.3, 2.1), GrpE protein (FC 2.7), and a ubiquilin-1 gene (FC 2.3).
Pathogen related phenotypes
Other identified regulated genes may have behavioral implications for infected Anopheles. It has been reported that some older wMelPop-infected Ae. aegypti mosquitoes have “bendy” and “shaky” phenotypes [74], [75]. The proboscis of “bendy” individuals is flexible and unable to penetrate the skin [74]. Mosquitoes with the “shaky” phenotype have a jittering action of the insect body [75]. Here, we have identified genes that may elucidate these phenotypes at the molecular level. Both Wolbachia strains suppress the defective proboscis extension response (dpr) gene (wAlbB −3.3, wRi −2.6). Moreover, this gene is also down regulated in Wolbachia-injected mosquitoes (wAlbB −3.0, wMelPop −2.4; Figure 2B & 2C). In Drosophila, dpr is part of a gene family encoding predicted cell adhesion molecules that contain two Ig domains [76]. It is possible that a reduction in cell adhesion causes plasticity in the proboscis leading to the “bendy” phenotype. In addition to reduced dpr transcripts, Wolbachia down-regulated numerous other cell adhesion genes. Interestingly, dpr also has been shown to be required for the proper timing of male courtship [76], and given that Anopheles have elaborate swarming courtship behaviors in the wild, Wolbachia infection may have the potential to alter reproductive success.
Sestrins (Sesn), a family of conserved proteins, accumulate in cells in response to stress and are inhibitors of target of rampamycin (TOR) that prevent age-related pathologies [77], [78]. In wAlbB-infected cells, we see a down regulation of Sesn (FC −3.5). In Drosophila dSesn-null mutants, age related degeneration of muscle was observed in the form of cardiac malfunction and abnormal skeletal muscle [78]. Possibly, suppression of Sesn in wMelpop infected Ae. aegypti is related to the “shaky” phenotype [75]. Moreover, it would be interesting to correlate Sesn levels in both Drosophila and Ae. aegypti infected with wMelpop, which display life shortening and age related pathologies [17], [79], to determine if Sesn plays a role in life shortening from this strain of Wolbachia. The “shaky” and “bendy” phenotypes are more prevalent in older Wolbachia infected Aedes mosquitoes [75]. If the genes identified here confer the “bendy” and “shaky phenotypes in a Wolbachia-infected Anopheles mosquito, these effects could be more influential on malaria transmission compared to direct pathogen interference.
Conclusion
Wolbachia-infected mosquito cells provide a tractable platform to characterize Wolbachia-Anopheles transcriptomic interactions in the absence of a stably-infected mosquito strain. Using this system, we identified a suite of Anopheles genes regulated by two divergent Wolbachia strains. As a general theme, Wolbachia have a profound effect on transcription of many host defensive genes, possibly to facilitate and maintain intracellular infection. These data may give insights into the transfer of Wolbachia into novel hosts, Anopheles-Wolbachia interplay, interaction with pathogens transmitted by Anopheles and other Wolbachia-induced phenotypes such as reproductive manipulations.
Materials and Methods
Cell culture
Wolbachia-infected (wRi and wAlbB) and uninfected Sua5B cells were generated and cultured as previously described [14]. Both cell lines were >30 passages post-infection at the time of experiments. Cell line transcriptome expression was assessed using the Affymetrix Anopheles/Plasmodium GeneChip. Processing of samples for microarray analysis was performed by the Johns Hopkins Malaria Research Institute Gene Array Core Facility (JHMRI-GACF), using standard Core protocols as described below.
RNA extraction
Cells were harvested, washed, resuspended in PBS, flash frozen in liquid nitrogen, and stored at −80°C. Homogenization and lysis of cells was performed with Lysing Matrix D (Qbiogene) in Trizol LS reagent (Invitrogen) by rapid agitation in a FastPrep 120 Instrument (Qbiogene) for 15 seconds at speed setting 5. Homogenates were subsequently processed according to the manufacturer's (Invitrogen) protocol with the following minor modifications. Two microliters of 5 mg/ml glycogen was used as a carrier for overnight isopropanol precipitation, and all centrifugation times were increased to 15 minutes. RNA pellets were resuspended in Nuclease-free water. Further purification was performed using the Qiagen RNeasy Mini kit, according to manufacturer's recommended protocol. Quantitation of RNA was performed using a NanoDrop spectrophotometer, and quality assessment determined by RNA Nano LabChip analysis on an Agilent BioAnalyzer 2100.
Affymetrix GeneChip protocols
Processing of templates for GeneChip Analysis was in accordance with methods described in the Affymetrix GeneChip Expression Analysis Technical Manual, Revision 5. Double stranded cDNA was synthesized from 5 micrograms of total RNA using the GeneChip Expression 3′ amplification reagents one-cycle cDNA synthesis kit (Affymetrix), and subsequently column-purified using the GeneChip Sample Cleanup Module. Biotinylated cRNA was synthesized from the double-stranded cDNA by in vitro transcription (IVT) using the GeneChip Expression 3′ amplification reagents for IVT labeling (Affymetrix), according to the manufacturer's recommended protocol. Resultant cRNAs were purified by column purification with the GeneChip Sample Cleanup Module (Affymetrix), and quantified. 15 micrograms of cRNA were fragmented by metal-induced hydrolysis in fragmentation buffer (250 mM Tris acetate pH 8.1, 150 mM MgOAc, 500 mM KOAc) at 94°C for 35 minutes. Quality of pre - and post-fragmentation cRNAs was assessed by RNA Nano LabChip analysis on an Agilent Bioanalyzer 2100. Hybridization cocktails were prepared as recommended for arrays of “Standard” format including incubation at 94°C for 5 minutes and 45°C for 5 minutes, and centrifugation at maximum speed for 5 minutes prior to pipetting into the GeneChips (Affymetrix Plasmodium/Anopheles). Hybridization was performed at 45°C for 16 hours at 60 rpm in the Affymetrix rotisserie hybridization oven. The signal amplification protocol for washing and staining of eukaryotic targets was performed in an automated fluidics station (Affymetrix FS450). Arrays were scanned in a GeneChip 3000 7G laser scanner with autoloader (Affymetrix) at an emission wavelength of 570 nm and 2.5 µm resolution. Intensity of hybridization for each probe pair was computed by GCOS software.
Data analysis
Detailed analysis was performed with Genomics Suite Software, version 6.4 (Partek). GC-RMA algorithm defaults were used for background correction (GC-RMA), normalization (Quantile), and summarization (median polish) of probesets. Analysis of variance (ANOVA) was performed with linear contrasts for each Wolbachia treatment (strain) vs. control. Gene lists were developed based on 2 fold or greater gene expression and a False Discovery rate P<0.05 criteria. Lists were annotated manually. Immune gene networks were developed using Pathvisio2 [69].
qPCR verification of expression analysis
Using qPCR, microarray data were validated using infected cell cultures and also somatically-infected mosquitoes. Live female mosquitoes (2 days post emergence) were immobilized on ice and transferred to an electronic cold plate. Mosquitoes were injected with Wolbachia (wMelpop or wAlbB) or Schneider's medium as described previously [13]. Although a standard protocol was followed for Wolbachia preparations, titers were not explicitly standardized. Injected mosquitoes were incubated at 19°C for 2 days before transfer to 28°C (80% humidity) insectary and were provided with access to a 10% sucrose solution through a cotton wick. After 15 days, mosquitoes were collected and RNA was extracted using TriReagent (Ambion) following manufactures guidelines. For verification of microarray data, total RNA was extracted from Sua5B cell lines (uninfected, wAlbB-infected, or wRi-infected) using the RiboPureTM kit (Ambion) following the manufacturer's instructions. RNA from cells or mosquitoes was DNase treated (Ambion) and cDNA synthesized using superscript III (Invitrogen) following manufactures guidelines. qPCR was performed in triplicate on an AB 7300 Sequence Detection System using the QuantiTect SYBR Green PCR Kit (Qiagen). Analysis was performed using Sequence Detection Software v.1.3 (ABI). Relative quantitation was completed by normalizing gene of interest to the ribosomal protein S7 gene (primers listed in Table 1) and data analyzed using the comparative Ct method (ΔΔCt method) [80].
Tab. 1. List of primers for qPCR. 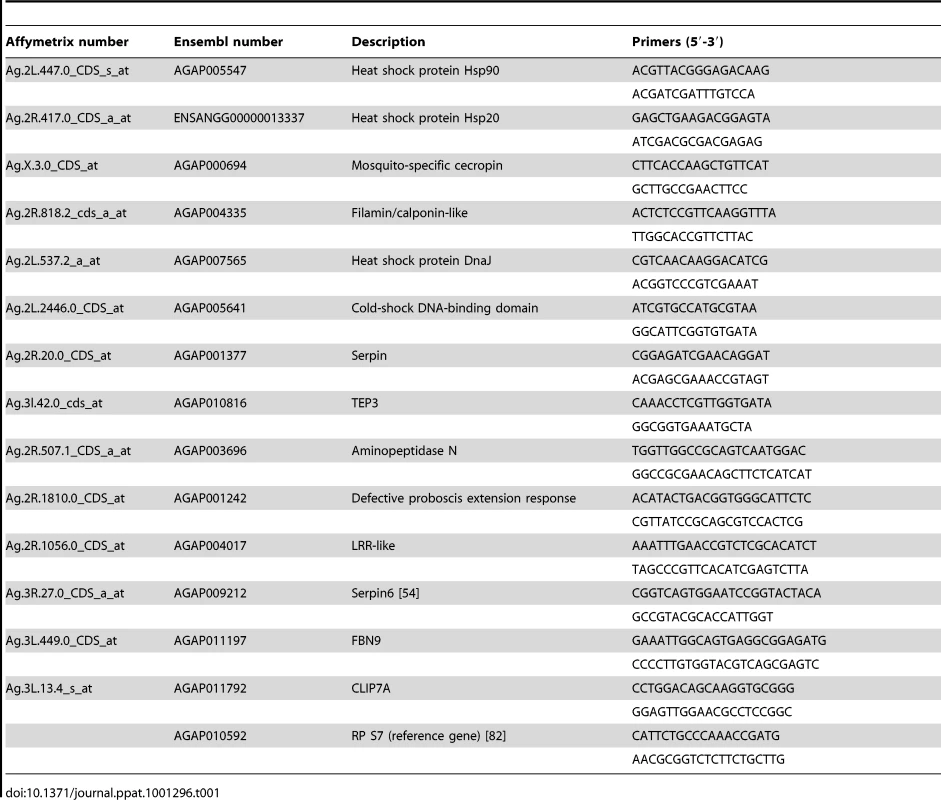
Accesion numbers
The following is a list of genes and their ENSEMBL or affymetrix accession numbers which are listed in the text: HSP20 AGAP005547, HSP90 Ag.2R.417.0_CDS_a_at, HSPDnaJ AGAP007565 AGAP001810, Cold-shock protein AGAP005641, Cecropin3 AGAP000694, SRPN11 AGAP001377, Filamin, AGAP004335, TEP3 AGAP010816, LRR-like AGAP004017, FBN9 AGAP011197, HSP70 AY137766.1_s_at, PEPCK AGAP003350, Carbonic anhydrase AGAP010052, Laminin AGAP001381 AGAP004993, Collagen AGAP009201, Peroxiredoxin AGAP011824, Superoxide dismutase AGAP010517, glutathione S transferases AGAP004164 AGAP004163 AGAP000761 AGAP009194 AGAP009193 AGAP004173 AGAP000165, CLIP7A AGAP011792, Galectin AGAP012529, CLIPB4 AGAP003250, CLIPB8 AGAP003057, Cecropin1 AGAP000693, TEP15 AGAP008364, GNBPB1 AGAP004455, Caspar AGAP006473, PGRP-LA AGAP005205, Attacin AGAP005620 ANCE AGAP009751 AGAP009756 AGAP009757 AGAP004563 AGAP007622 AGAP004563 AGAP007982, Kazal-like serpin AGAP011482, Crooked neck AGAP001879, Otefin AGAP007603, Dpy-30 AGAP007884, Serac1 AGAP011044, GrpE AGAP011150, ubiquilin AGAP004294, Defective proboscis extension response AGAP001242, Sestrin AGAP007169.
Supporting Information
Zdroje
1. WerrenJH
BaldoL
ClarkME
2008 Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 741 751
2. KambrisZ
CookPE
PhucHK
SinkinsSP
2009 Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326 134 136
3. MoreiraLA
Iturbe-OrmaetxeI
JefferyJA
LuG
PykeAT
2009 A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139 1268 1278
4. HedgesLM
BrownlieJC
O'NeillSL
JohnsonKN
2008 Wolbachia and virus protection in insects. Science 322 702 702
5. TeixeiraL
FerreiraA
AshburnerM
2008 The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PloS Biol 6 e2
6. BianG
XuY
LuP
XieY
XiZ
2010 The endosymbiotic bacterium Wolbachia induces resistance to Dengue virus in Aedes aegypti. PLoS Pathog 6 e1000833
7. KambrisZ
BlagboroughAM
PintoSB
BlagroveMSC
GodfrayHCJ
2010 Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog 6 e1001143
8. SinkinsSP
O'NeillSL
2000 Wolbachia as a vehicle to modify insect populations.
HandlerAF
JamesAA
Insect transgenesis: methods and applications New York CRC Press 271 287
9. SinkinsSP
CurtisC
O'NeillSL
1997 The potential application of inherited symbiont systems to pest control.
O'NeillSL
HoffmannAA
WerrenJH
Influential passengers Oxford Oxford University Press 155 175
10. KittayapongP
BaisleyKJ
BaimaiV
O'NeillSL
2000 Distribution and diversity of Wolbachia infections in southeast Asian mosquitoes (Diptera: Culicidea). J Med Entomol 37 340 345
11. RasgonJL
ScottTW
2004 An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J Med Entomol 41 255 257
12. RicciI
CancriniG
GabrielliS
D'AmelioS
FaviG
2002 Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J Med Entomol 39 562 567
13. JinC
RenX
RasgonJL
2009 The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Appl Environ Microbiol 75 3373 3376
14. RasgonJL
RenX
PetridisM
2006 Can Anopheles gambiae be infected with Wolbachia pipientis? Insights from an in vitro system. Appl Environ Microbiol 72 7718 7722
15. SuhE
MercerD
FuY
DobsonS
2009 Life-shortening Wolbachia infection from Drosophila melanogaster transferred into Aedes albopictus is pathogenic. Appl Environ Microbiol 75 7783 7788
16. XiZ
KhooC
DobsonSL
2006 Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc R Soc Lond B 273 1317 1322
17. McMenimanCJ
LaneAM
CassBN
FongAWC
SidhuM
2009 Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323 141 144
18. XiZ
DeanJ
KhooC
DobsonSL
2005 Generation of artificial Wolbachia infections in Aedes mosquito and manipulation of population with cytoplasmic incompatibilty. Am J Trop Med Hyg 73 349 349
19. XiZ
DeanJ
KhooC
DobsonSL
2005 Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Mol Biol 35 903 910
20. XiZ
KhooC
DobsonSL
2005 Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310 326 328
21. McGrawEA
MerrittDJ
DrollerJN
O'NeillSL
2001 Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc R Soc Lond B 268 2565 2570
22. McGrawEA
MerrittDJ
DrollerJN
O'NeillSL
2002 Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci USA 99 2918 2923
23. BourtzisK
PettigrewMM
O'NeillSL
2000 Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol 9 635 639
24. DongY
AguilarR
XiZ
WarrE
MonginE
2006 Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2 e52
25. Gonzalez-CeronL
SantillanF
RodriguezMH
MendezD
Hernandez-AvilaJE
2003 Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol 40 371 374
26. DongY
ManfrediniF
DimopoulosG
2009 Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5 e1000423
27. PumpuniCB
BeierMS
NataroJP
GuersLD
DavisJR
1993 Plasmodium falciparum: inhibition of sporogonic development in Anopheles stephensi by Gram-negative bacteria. Exp Parasitol 77 195 199
28. PumpuniCB
DemaioJ
KentM
DavisJR
BeierJ
1996 Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg 54 214 218
29. NodaH
MiyoshiT
KoizumiY
2002 In vitro cultivation of Wolbachia in insect and mammalian cell lines. In Vitro Cell Dev Biol Anim 38 423 427
30. O'NeillSL
PettigrewMM
SinkinsSP
BraigHR
AndreadisTG
1997 In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol 6 33 39
31. FurukawaS
TanakaK
FukatsuT
SasakiT
2008 In vitro infection of Wolbachia in insect cell lines. Appl Entomol and Zool 43 519 525
32. BarrettT
TroupDB
WilhiteSE
LedouxP
RudnevD
2009 NCBI GEO: archive for high-throughput functional genomic data. Nucl Acids Res 37 D885 890
33. XiZ
GavotteL
XieY
DobsonSL
2008 Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics 9 1
34. BrennanLJ
KeddieBA
BraigHR
HarrisHL
2008 The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS ONE 3 e2083
35. FederME
KarrTL
YangFW
HoekstraJ
JamesDC
1999 Interaction of Drosophila and its endosymbiont Wolbachia: natural heat shock and the overcoming of sexual incompatibility. Amer Zool 39 363 373
36. LefevreT
ThomasF
SchwartzA
LevashinaE
BlandinS
2007 Malaria Plasmodium agent induces alteration in the head proteome of their Anopheles mosquito host. Proteomics 7 1908 1915
37. SimC
HongYS
TsetsarkinKA
VanlandinghamDL
HiggsS
2007 Anopheles gambiae heat shock protein cognate 70B impedes O'nyong-nyong virus replication. BMC Genomics 8 231
38. HaywardR
2000 Plasmodium falciparum phosphoenolpyruvate carboxykinase is developmentally regulated in gametocytes. Mol Biochem Parasitol 107 227 240
39. LasonderE
JanseCJ
van GemertG-J
MairGR
VermuntAMW
2008 Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog 4 e1000195
40. del Pilar CorenaM
VanEkerisL
SalazarMI
BowersD
FiedlerMM
2005 Carbonic anhydrase in the adult mosquito midgut. J Exp Biol 208 3263 3273
41. ReungprapavutS
KrungkraiSR
KrungkraiJ
2004 Plasmodium falciparum carbonic anhydrase is a possible target for malaria chemotherapy. J Enzyme Inhib Med Chem 19 249 256
42. KrungkraiSR
SuraveratumN
RochanakijS
KrungkraiJ
2001 Characterisation of carbonic anhydrase in Plasmodium falciparum. Int J Parasitol 31 661 668
43. AdiniA
WarburgA
1999 Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology 119 331 336
44. ArrighiRBG
LycettG
MahairakiV
Siden-KiamosI
LouisC
2005 Laminin and the malaria parasite's journey through the mosquito midgut. J Exp Biol 208 2497 2502
45. GareDC
PiertneySB
BillingsleyPF
2003 Anopheles gambiae collagen IV genes: cloning, phylogeny and midgut expression associated with blood feeding and Plasmodium infection. Int J Parasitol 33 681 690
46. WarburgA
ShternA
CohenN
DahanN
2007 Laminin and a Plasmodium ookinete surface protein inhibit melanotic encapsulation of Sephadex beads in the hemocoel of mosquitoes. Microb Infect 9 192 199
47. KumarS
ChristophidesGK
CanteraR
CharlesB
HanYS
2003 The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci USA 100 14139 14144
48. BlandinSA
LevashinaEA
2004 Thioester-containing proteins and insect immunity. Mol Immunol 40 903 908
49. BlandinSA
ShiaoS-H
MoitaLF
JanseCJ
WatersAP
2004 Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 661 670
50. LevashinaEA
MoitaLF
BlandinSA
VriendG
LagueuxM
2001 Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104 709 718
51. VolzJ
MuellerH
ZdanowiczA
KafatosFC
OstaMA
2006 A genetic module regulates the melanization response of Anopheles to Plasmodium. Cell Microbiol 8 1392 1405
52. DimopoulosG
RichmanA
MüllerHM
KafatosFC
1997 Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA 94 11508 11513
53. PaskewitzSM
AndreevO
ShiL
2006 Gene silencing of serine proteases affects melanization of Sephadex beads in Anopheles gambiae. Insect Biochem Mol Biol 36 701 711
54. AbrahamEG
PintoSB
GhoshA
VanlandinghamDL
BuddA
2005 An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci USA 102 16327 16332
55. ZouZ
ShinSW
AlvarezKS
BianG
KokozaV
2008 Mosquito RUNX4 in the immune regulation of PPO gene expression and its effect on avian malaria parasite infection. Proc Natl Acad Sci USA 105 18454 18459
56. RichmanAM
DimopoulosG
SeeleyD
KafatosFC
1997 Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J 16 6114 6119
57. WarrE
DasS
DongY
DimopoulosG
2008 The Gram-negative bacteria-binding protein gene family: its role in the innate immune system of Anopheles gambiae and in anti-Plasmodium defence. Insect Mol Biol 17 39 51
58. WernerT
LiuG
KangD
EkengrenS
SteinerH
2000 A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA 97 13772 13777
59. PerssonC
OldenviS
SteinerH
2007 Peptidoglycan recognition protein LF: a negative regulator of Drosophila immunity. Insect Biochem Mol Biol 37 1309 1316
60. Zaidman-RémyA
HervéM
PoidevinM
Pili-FlouryS
KimM-S
2006 The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 24 463 473
61. MeisterS
AgianianB
TurlureF
RelógioA
MorlaisI
2009 Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog 5 e1000542
62. ChoeK-M
WernerT
StövenS
HultmarkD
AndersonKV
2002 Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296 359 362
63. CarlssonA
EngströmP
PalvaET
BennichH
1991 Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infect Immun 59 3040 3045
64. SakamotoJM
AzadAF
2007 Propagation of arthropod-borne Rickettsia spp. in two mosquito cell lines. Appl Environ Microbiol 73 6637 6643
65. LiY
MaW-M
DaiJ-Q
FengC-Z
YangF
2008 Inhibition of a novel sperm gelatinase in prawn sperm by the male reproduction-related Kazal-type peptidase inhibitor. Mol Reprod Dev 75 1327 1337
66. LinM-H
LeeRK-K
HwuY-M
LuC-H
ChuS-L
2008 SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction 136 559 571
67. PerrimonN
EngstromL
MahowaldAP
1984 Developmental genetics of the 2E-F: a regions of the Drosophila X chromosome: a region rich in “developmentally important” genes. Genetics 108 559 572
68. BurnetteJM
HattonAR
LopezAJ
1999 Trans-acting factors required for inclusion of regulated exons in the Ultrabithorax mRNAs of Drosophila melanogaster. Genetics 151 1517 1529
69. ChungS
ZhouZ
HuddlestonKA
HarrisonDA
ReedR
2002 Crooked neck is a component of the human spliceosome and implicated in the splicing process. Biochim Biophys Acta 1576 287 297
70. JiangX
XiaL
ChenD
YangY
HuangH
2008 Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Dev Cell 14 494 506
71. VardanyanA
AtanesyanL
EgliD
RajaSJ
Steinmann-ZwickyM
2008 Dumpy-30 family members as determinants of male fertility and interaction partners of metal-responsive transcription factor 1 (MTF-1) in Drosophila. BMC Dev Biol 8 68
72. DottoriniT
NicolaidesL
RansonH
RogersDW
CrisantiA
2007 A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA 104 16215 16220
73. SnookRR
ClelandSY
WolfnerMF
KarrTL
2000 Offsetting effects of Wolbachia infection and heat shock on sperm production in Drosophila simulans: analyses of fecundity, fertility and accessory gland proteins. Genetics 155 167 178
74. TurleyAP
MoreiraLA
O'NeillSL
McGrawEA
2009 Wolbachia Infection Reduces Blood-Feeding Success in the Dengue Fever Mosquito, Aedes aegypti. PloS Negl Trop Dis 3 e516
75. MoreiraLA
SaigE
TurleyAP
RibeiroJMC
O'NeillSL
2009 Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PloS Negl Trop Dis 3 e568
76. GoldmanTD
ArbeitmanMN
2007 Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet 3 e216
77. BudanovAV
SablinaAA
FeinsteinE
KooninEV
ChumakovPM
2004 Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304 596 600
78. LeeJH
BudanovAV
ParkEJ
BirseR
KimTE
2010 Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327 1223 1228
79. MinKT
BenzerS
1997 Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA 94 10792 10796
80. PfafflMW
2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29 e45
81. WaterhouseRM
KriventsevaEV
MeisterS
XiZ
AlvarezKS
2007 Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316 1738 1743
82. DanaAN
HongYS
KernMK
HillenmeyerME
HarkerBW
2005 Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genomics 6 5
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



