-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
In the present study, we analyzed the functional profile of CD8+ T-cell responses directed against autologous transmitted/founder HIV-1 isolates during acute and early infection, and examined whether multifunctionality is required for selection of virus escape mutations. Seven anti-retroviral therapy-naïve subjects were studied in detail between 1 and 87 weeks following onset of symptoms of acute HIV-1 infection. Synthetic peptides representing the autologous transmitted/founder HIV-1 sequences were used in multiparameter flow cytometry assays to determine the functionality of HIV-1-specific CD8+ T memory cells. In all seven patients, the earliest T cell responses were predominantly oligofunctional, although the relative contribution of multifunctional cell responses increased significantly with time from infection. Interestingly, only the magnitude of the total and not of the poly-functional T-cell responses was significantly associated with the selection of escape mutants. However, the high contribution of MIP-1β-producing CD8+ T-cells to the total response suggests that mechanisms not limited to cytotoxicity could be exerting immune pressure during acute infection. Lastly, we show that epitope entropy, reflecting the capacity of the epitope to tolerate mutational change and defined as the diversity of epitope sequences at the population level, was also correlated with rate of emergence of escape mutants.
Published in the journal: Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection. PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001273
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001273Summary
In the present study, we analyzed the functional profile of CD8+ T-cell responses directed against autologous transmitted/founder HIV-1 isolates during acute and early infection, and examined whether multifunctionality is required for selection of virus escape mutations. Seven anti-retroviral therapy-naïve subjects were studied in detail between 1 and 87 weeks following onset of symptoms of acute HIV-1 infection. Synthetic peptides representing the autologous transmitted/founder HIV-1 sequences were used in multiparameter flow cytometry assays to determine the functionality of HIV-1-specific CD8+ T memory cells. In all seven patients, the earliest T cell responses were predominantly oligofunctional, although the relative contribution of multifunctional cell responses increased significantly with time from infection. Interestingly, only the magnitude of the total and not of the poly-functional T-cell responses was significantly associated with the selection of escape mutants. However, the high contribution of MIP-1β-producing CD8+ T-cells to the total response suggests that mechanisms not limited to cytotoxicity could be exerting immune pressure during acute infection. Lastly, we show that epitope entropy, reflecting the capacity of the epitope to tolerate mutational change and defined as the diversity of epitope sequences at the population level, was also correlated with rate of emergence of escape mutants.
Introduction
The development of vaccines capable of controlling infections by intracellular pathogens, including HIV-1, poses major challenges, since correlates of protection remain elusive [1], [2]. The initial observation that the appearance of HIV-specific CD8+ T cell responses is temporally associated with the resolution of peak viremia suggested that they may represent a critical component of initial protective immunity in humans [3], [4], [5]. Modeling based on the dynamics of the immune response and epitope escape data from very early in infection provides further support for the key role of CD8+ T cells responses in containing the virus during acute and early infection [6].
CD8+ T cell responses are undoubtedly able to place substantial pressure upon the virus, as indicated by the rapid appearance of escape mutations following HIV infection [7], [8], [9], [10]. In some cases, the appearance of these escape mutants is associated with the loss of virologic control, resulting in disease progression in HIV-1 [11], [12] and SIV infection [13], [14], [15]. However, CD8+ escape mutations do not always precede disease progression, and a number have been shown to result in a fitness cost that can lessen the replicative capacity of HIV [16], [17], [18], [19] and SIV [16], [20], [21].
Previous observations in chronic infection have shown that CD8+ T cell multifunctionality is associated, with slow HIV disease progression [22], [23]. CD8+ T cell responses exert significant immune pressure on HIV-1 in acute infection that results in rapid immune escape implying that they are important in early control of the virus [6], [24]. In the present report, we characterized the functional profile of CD8+ T cell responses directed against the transmitted/founder HIV-1 isolates arising during acute and early infection in order to determine the relationship between the functionality of epitope-specific CD8+ T cells and the selection of transmitted/founder virus escape mutants.
Results
Clinical parameters and course of viremia
The seven study participants were males between 23 and 56 years old at the time of diagnosis and all were infected as consequence of homosexual intercourse (MSM) (Table 1). The participants were enrolled within 3 weeks following onset of symptoms (FOSx) of acute HIV-1 infection. At the time of screening, the three patients enrolled in CHAVI 001 were classified as Fiebig stage 2, whereas the four patients enrolled at the Mortimer Market Center were stage 3. None elected to receive anti-retroviral therapy following diagnosis of acute HIV-1 infection and for the duration of the study. The analysis of the CD4 slopes (Figure 1A) and plasma virus load (pVL) (Figure 1B) were conducted according to previous publications [25] and revealed: 1) a sustained loss of CD4 cells in 4 patients (MM33, MM39, MM42, MM43), and 2) pVL set point at 40 weeks was above 2,000 copies/ml in all but patient CH058. In our study, a controller was classified as having a pVL of less than 2,000 RNA copies/ml at three consecutive time points during the first 12-month period (CH058, Figure 1A). Accordingly we identified one controller (CH058) and six non-controllers that each had greater than 2,000 copies/ml at multiple time points (Figure 1A).
Fig. 1. CD4 cell counts and virus load. 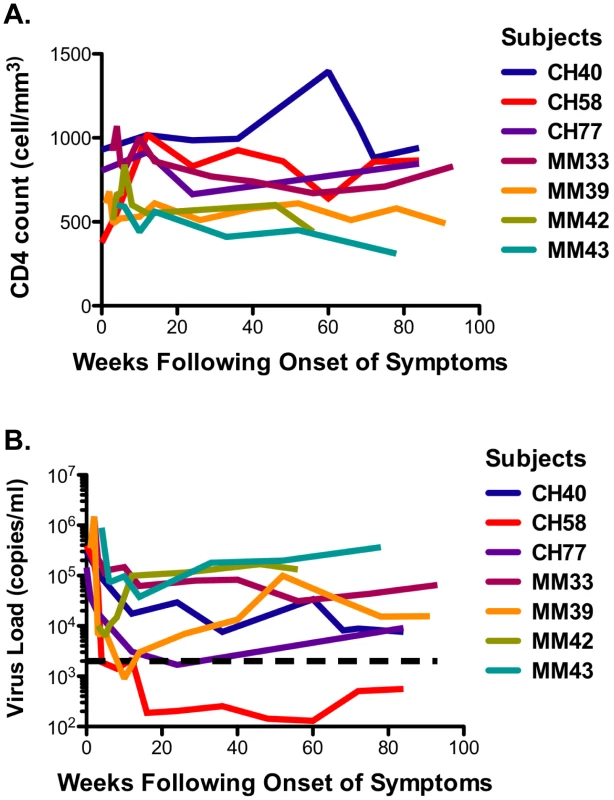
A. The CD4 counts are reported in cell/mm3 at each time point following the onset of symptoms. B. The virus load in each subject is reported on a logarithmic scale as RNA copies/ml at each time point following the onset of the symptoms. The black dotted line represents the limits of 2,000 copies/ml used to identify controllers based on the CHAVI definitions of less than 2,000 copies/ml at 3 consecutive time points in 12 months. Tab. 1. Patient demographic, CD4 slope, and plasma virus load set point, and class I HLA. 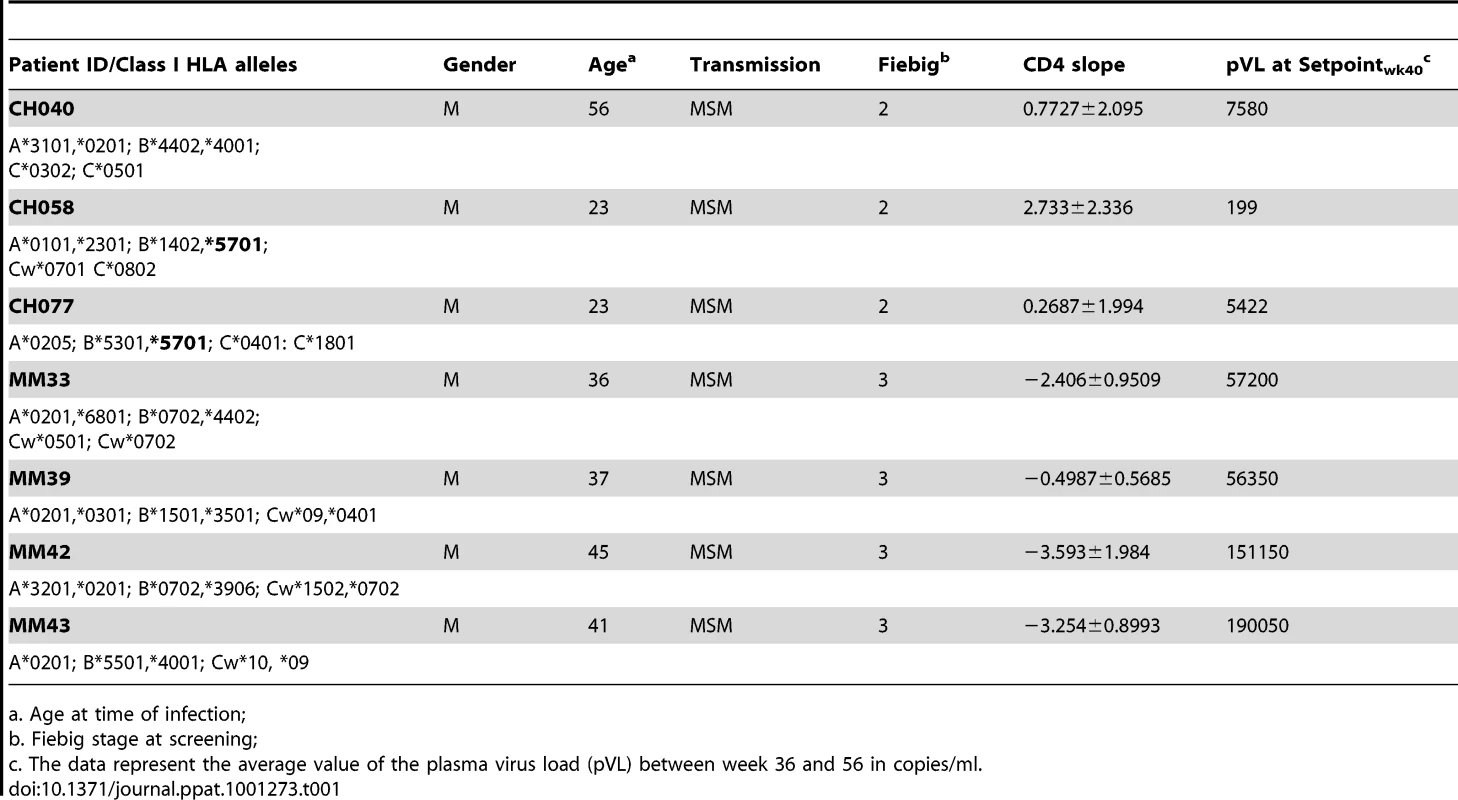
a. Age at time of infection; Functional profile of Gag-specific CD8+ memory T cell responses
We initially analyzed the magnitude and kinetics of the total autologous Gag-specific CD8+ memory T cell response in each subject between 1 and 87 weeks (WFOSx) of acute HIV-1 infection along with associated multifunctional attributes of responding cells. Peripheral blood mononuclear cells (PBMC) cryopreserved at sequential time points FOSx were stimulated with a pool of overlapping peptides corresponding to the entire Gag sequence of the autologous transmitted/founder virus. The responses were evaluated by multiparameter flow cytometry, measuring the ability of CD8+ memory T cell subsets to degranulate (CD107a expression) and/or produce IFN-γ, IL-2, MIP-1β, and TNF-α (Figure S1). In 5 patients, Gag-specific CD8+ T cell responses were detected within 2–3 WFOSx and in all patients by 8 weeks WFOSx. The frequency of responding memory CD8+ T cells is reported within each pie chart (average ± standard deviation) in Figure 2. Analysis of the absolute magnitude of the responses did not reveal a reduction in the frequency of responses over time in each patient. The proportion of each responding population found to exhibit 1, 2, 3, 4 and 5 functions at each time point is presented in the pie charts (Figure 2A), while the relative frequencies of cells exhibiting each number of functions are reported in the graphs (Figure 2B). The analysis of the polyfunctional profiles among the seven patients indicated that Gag-specific CD8+ subsets mediating ≥3 functions contributed less than 20% of the total response in each subject at the two earliest time points (2–3 and 4–8 WFOSx) (Figure 2A and 2B). However there was some increase in the functionality of Gag-specific responses with time from infection. The comparison of contributions of each functional family to the overall response at different time points revealed that the families with 3 and 4 functions were significantly higher after 78 WFOSx compared to at earlier time points (p = 0.021 and 0.032, respectively, according to the LME model analysis), but remained below 20% of the total response in all but one instance. We also observed an absence (<0.005%) of the highly multifunctional IFN-γ+IL-2+MIP-1β+TNF-α+CD107+ subset at all time points tested. This was attributable to an absence of IL-2-producing CD8+ T cells (data not shown). The profile of the Gag-specific response in the controller CH058 was no different from those detected in the other six non-controller patients at any time points, and therefore it is not presented as segregated data.
Fig. 2. Functionality of Gag-specific responses. 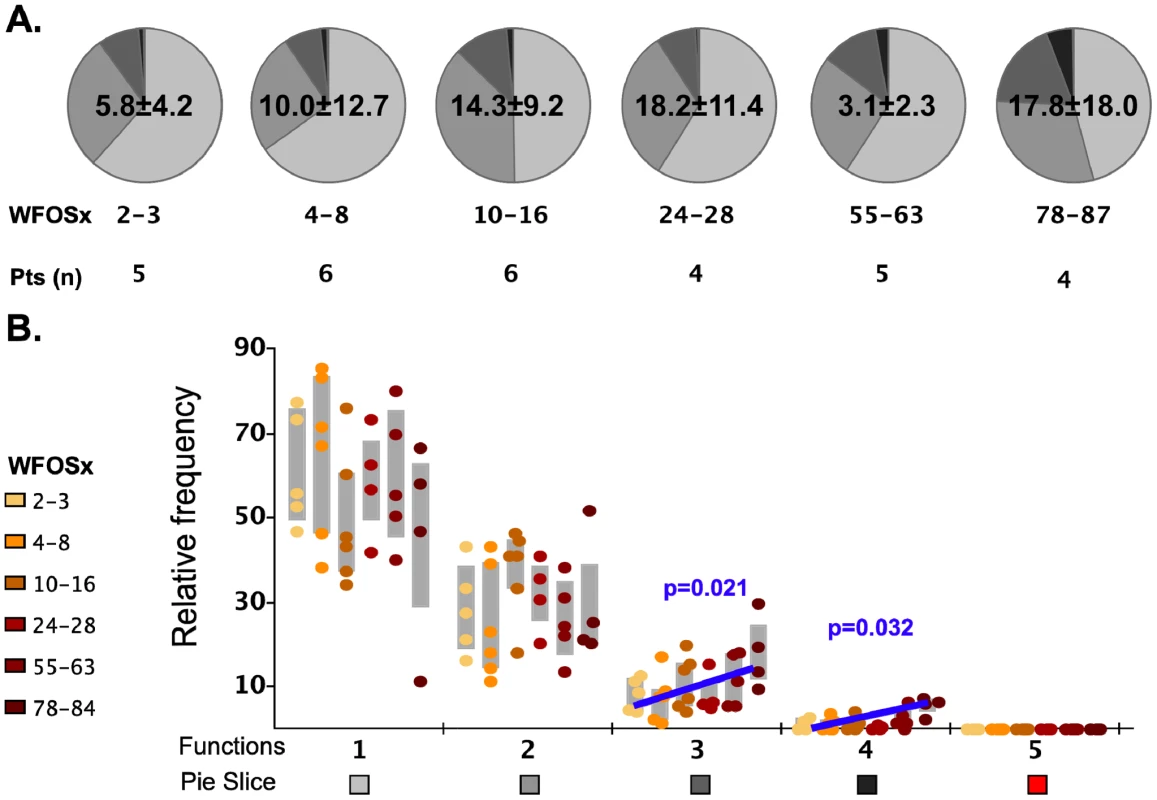
The total frequency of Gag-specific CD8+ memory T cells within the memory CD8+ T cell pool is reported within each pie as mean ± standard deviation. A) The pie charts represent the relative contributions of each functional family to the total response at each time point for the seven HIV-1 infected subjects. The color code for each number of functions is reported below the x-axis of the graph. The number of subjects studied at each time point is reported under each pie chart. B) In the graph each point represents one subject, and they are color coded according to the legend in the figure to represent the different weeks following onset of symptoms (WFOSx). Each grey box represents the inter-quartile distribution of the responses. The graphs represent the relative contribution to the total response made by each functional family at each time point. The trend line and p values for the statistically significant increase in subsets with 3 and 4 functions are also reported in the graph. Polyclonal stimulation induces highly multifunctional CD8+ responses
We next determined whether this initial absence of highly multifunctional responses was due to a general functional impairment of the memory CD8+ T cell population. We stimulated PBMC from each HIV-infected and non-infected control subject with the superantigen Staphylococcus aureus enterotoxin B (SEB) in order to detect the presence of the different functional subsets in response to a polyclonal stimulus. The frequency of cells in the total CD8+ memory T cell population responding to SEB in the non-infected individuals was not significantly different (p>0.05) to that observed in the HIV-infected subjects within the first 8 WFOSx as reported in Figure 3. We detected every functional family including IFN-γ+IL-2+MIP-1β+TNF-α+CD107+ cells with a frequency greater than 0.05% in both HIV seronegative and seropositive individuals. This suggests that the limited contribution of cells with ≥3 functions may be confined to antigen-specific populations.
Fig. 3. Functionality of SEB responses. 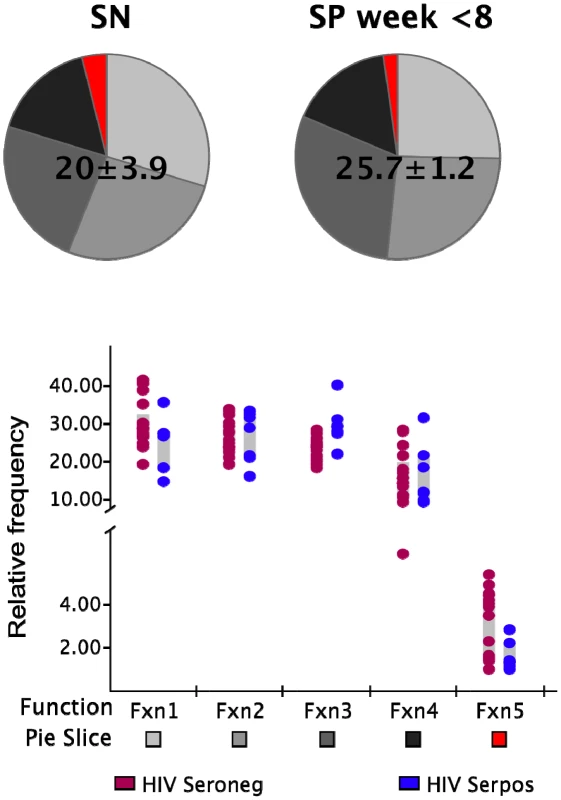
The frequency of SEB-responsive CD8+ T cells (irrespective of their functional profile) within the total memory CD8+ T cell pool is shown above each pie. The pie charts represent the relative contribution of each functional family to the total response for the non-HIV-infected (SN; left pie charts) and infected donors (SP; right pie charts). The responses in the latter group represent those detected within the first 8 weeks post-onset of symptoms. The color code for each functional family is reported below the x-axis of the graph. Each dot represents one individual in the graph and color codes for the two groups are reported at the bottom of the figure (brown SN; blue SP). Each grey box represents the inter-quartile distribution of the responses. The graph represents the frequency of each functional family within the total SEB-responsive population for the subjects in each group. Functional profiles of responses to mutating and non-mutating epitopes are similar
As the second aim of our study, we characterized the functional profile of CD8 responses directed against 23 individual epitopes previously mapped in the 7 subjects within HIV gene products not limited to Gag, as detailed in Table 2. The epitopic sequences are reported as those of the 18-mer peptides used to test the responses. 18-mer peptides were chosen for analysis of responses because we did not want to preclude characterization of responses that were not directed against previously described optimal epitopes (which was the case for 5 of the 23 epitopes reported in Table 2). In a side-by-side comparison, stimulation with representative 18-mers compared to optimal length peptides showed no differences in the functionality of the stimulated CD8+ T cells (data not presented), and we did not observe CD4+ T cell responses recognizing overlapping epitopes within the 18-mer peptides. The epitopes studied all elicited early T cell responses, previously mapped by ELISpot assay [6], [26], and could be detected by ICS within the first 6 WFOSx, except for epitopes c, e, and f in CH040, responses to which were first detected at week 12 (c and e) and 24 (f). As shown in Figure 4, we performed longitudinal sequence analysis of the viral quasispecies in each individual to reveal mutations within the epitope and/or 9 aa of the C - and N-flanking regions (all epitope variants are included in the Figure S1) that were either hypothesized or subsequently demonstrated to confer escape from the epitope-specific T cell response [6], [26]. Based on this definition, we detected the presence of escape mutations in twelve epitopes within 0–55 WFOSx and in seven additional epitopes between weeks 56 and 87. According to the time of detection of escape, the two groups are referred to as early (e-ME) or late (l-ME) mutating epitopes (ME) in Table 2 and Figure 4, respectively. There was no evidence for mutation or escape in the other four epitopes within the time frame of this study; these are referred to as non-mutating epitopes (nME). The dynamics of appearance of escape and the loss of transmitted virus sequences are illustrated as the shaded gray bars above each epitope. According to the appearance of mutations, the epitopes are ranked within each patient both in Table 2 and Figure 4 (lower case letter in the “Epitope” column and row, respectively). The time of the earliest observed and the highest recorded frequency (HRF) of CD8 T cell responses for each epitope are also reported in Figure 4. Importantly, T cell responses were detected prior to the emergence of the epitope variants in 16 out of 17 escaping epitopes. The HRF occurred prior to the loss of transmitted virus in all epitopes but two, epitope “c” (Env 830–847) and “b” (Gag 236–235) in patients CH040 and CH077, respectively.
Fig. 4. Timing of escape of each epitope relative to the first CD8+ T-cell responses. 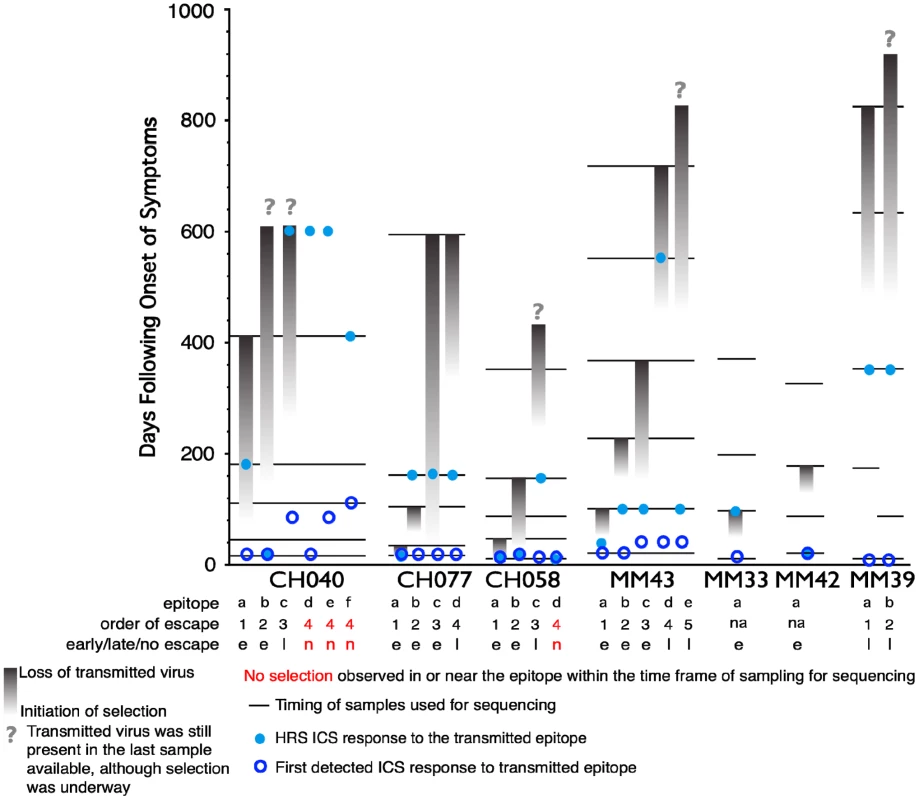
In studies of this kind, the timing of escape mutations and rate of loss of the transmitted form of epitope are based on limited temporal sampling of viral RNA from the plasma. Therefore, the time that immune escape was initiated can only be resolved to a period between the sample where only the transmitted epitope was present, and the sample where the first evidence of the accumulation of escape mutations appears. Similarly, the loss of the transmitted form can be prolonged or rapid, and in some cases was still ongoing at the last time point sampled. To illustrate and rank the order of escape while conveying the uncertainty of the rate and timing due to inevitable limitations in sampling, we used gradations of a grey bar, fading in during the period between the two samples when selection first is initiated, and ending at the sample where the transmitted form was no longer evident; the transmitted form diminishes through this period, and escape forms accumulate, indicated by the darkening gray. If the transmitted form was still present in the last available sample, we ended with a question mark to indicate selection was still ongoing. Four epitopes that elicited ICS responses that showed no evidence for escape during the course of the study are highlighted in red. Open blue circles indicate the timing of the sample where the first CD8+ T cell response was observed against the epitope, the light blue dots indicate time with the highest response to the epitope (HRS). The rank order of escape (reported for each epitope listed in Table 2 within each patient by the lower case letter as ”epitope”) provides the values used for the correlation tests comparing order of escape to epitope entropy and the fraction of CD8+ T cell subtypes. Tab. 2. Analysis of Entropy of sequences and epitope-specific functional responses based on the order of time of appearance of epitope mutations within each individual. 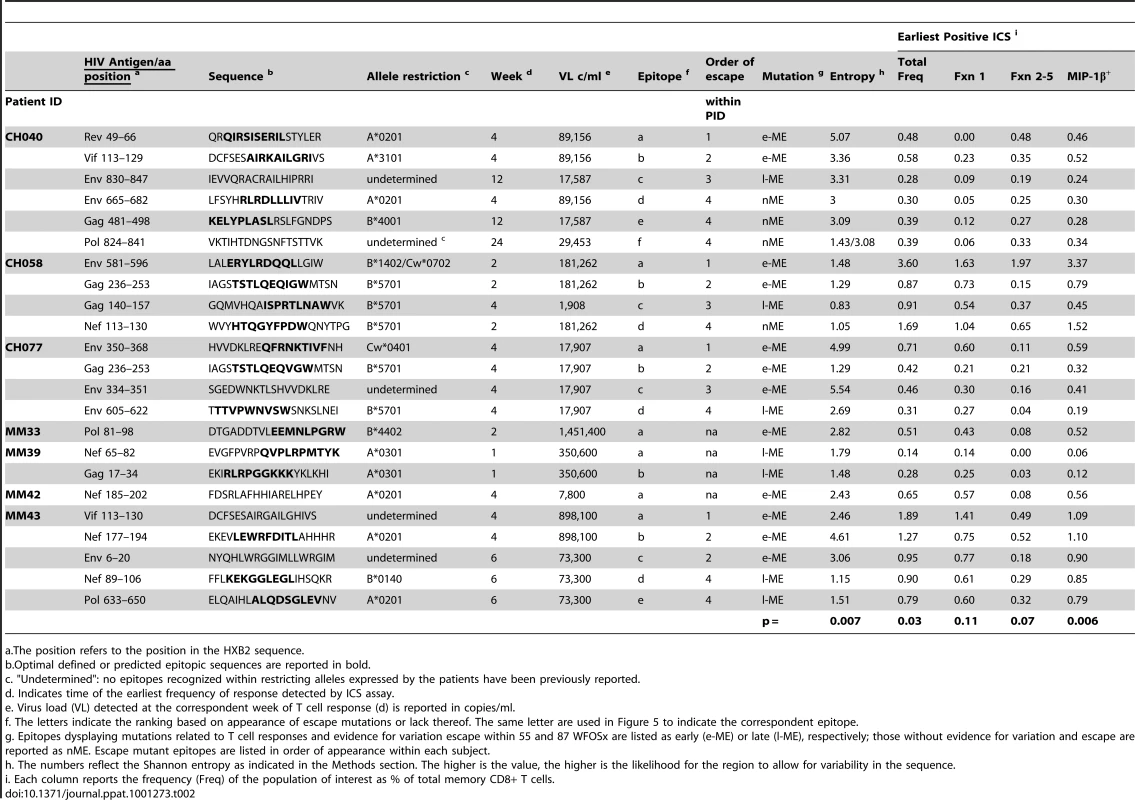
a.The position refers to the position in the HXB2 sequence. We further analyzed the responses according to the profile of the functional families at the earliest detectable time point, and at the time of HRF of the response. At the level of group analysis within the first 24 WFOSx (Figure 5A), we observed greater representation of the monofunctional subset in the responses against the early and late mutating epitopes than those against the non-mutating epitopes, whereas cells mediating 2 and 3 functions were relatively more abundant in the non-mutating-epitope-specific responses (>30%). These profiles changed slightly at the time of HRF, which for 12 out 23 (52%) epitope-specific responses was after 24 WFOSx. There was some overall increase in functionality with the 2 - and 3-functional subsets contributing more to the responses for each group of epitopes, in line with the data reported in Figure 2. Interestingly, the 4-functional subset contributed more to responses to the non-mutating epitopes (Figure 5B). We confirmed that the IFN-γ+IL-2+MIP-1β+TNF-α+CD107+ subset made a negligible contribution to the overall response, with frequencies <0.005% at all time points analyzed.
Fig. 5. Analysis of functional profile of responses to mutating and non-mutating epitopes. 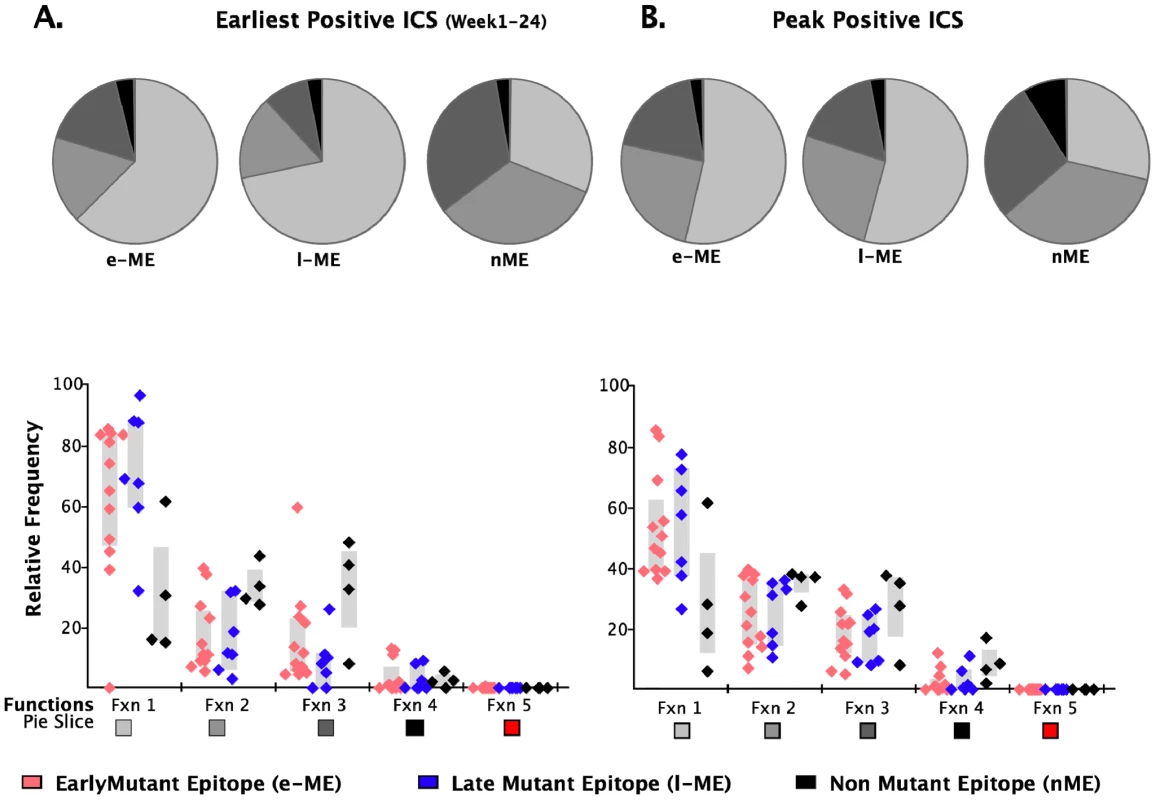
The relative contributions of each functional family to the total CD8 T cell response are reported within each pie chart for epitopes that mutated within 55 (early-mutating: e-ME) and 56–87 WFOSx (late-mutating: l-ME) and for those that did not mutate (non-mutating: nME) epitopes. A) Profile of responses within 12 weeks FOSx. B) Profile of responses at the time of detection of the highest response by ICS (peak). The color code for each functional family is reported below the x-axis of the graph. In the graph each dot represents a single epitope. The color code for each group of epitopes is reported at the bottom of the figure. The grey bar represents the inter-quartile distribution of relative frequency of each functional family for the e-ME-, l-ME, and nME responses. Epitope sequence entropy and total frequency of functional CD8+ T cell responses are both associated with the temporal appearance of escape mutants
Given no clear correlation between functionality and virus escape, we hypothesized that rapid selection for CD8+ T cell escape mutants might be a function of both the intrinsic ability of the targeted epitope to tolerate change as well as the patient's immune pressure exerted by the epitope-specific CD8+ T cell responses whether detected at the earliest or HRF time point. To provide a relative measure of how readily the epitopes included in this study tolerate change, we calculated the Shannon entropy of each epitope based the full collection of B clade sequences in the Los Alamos database. Highly conserved epitopes will have a low entropy score, and variable epitopes that frequently are altered and clearly tolerate many patterns of substitution at the population level will have a high entropy (see Methods for details). We indicate in Figure 4 when the first evidence for selection and the last traces of the transmitted epitope were observed. In some cases the population completely shifted from transmitted to escape variants between 2 sampling time points. In other cases selection manifested gradually, with escape mutations accruing slowly over many months. In 4 cases, escape mutations in recognized epitopes did not arise within the time frame of the study. We ranked the order of escape for each epitope within each subject based on the temporal appearance of mutations (Table 2 and Figure 4). By using a Spearman's ρ statistic as described in the methods to assess correlations, we observed a significant association of the temporal appearance of mutation with both the entropy of the epitope (p = 0.007) and the total frequency of CD8+ T cell responses detectable at the earliest time point (p = 0.03) as reported in Table 2.
Relative contribution of T cells producing MIP-1β to the overall response is significantly greater for early-ME-specific responses
As analysis of the functional families at the individual patient level revealed an association of total responses and ability to select for escape mutations, we investigated whether this association may reside at the level of any single or combination of the five functional parameters. We noticed that CD8+IL2+ T cells did not contribute to any of the three groups of epitope-specific responses and that the majority of CD8+TNF-α+ were also CD8+IFN-γ+ T cells, therefore, we analyzed the remaining response subsets without regard to production of IL2 and TNF-α. We observed that the earliest recorded frequency of the CD8+ T cells producing MIP-1β+ alone and in any combination with any other parameter was also significantly associated with the appearance of escape responses within subjects (Table 2, p = 0.006). A complication in interpreting the role of MIP-1β+CD8+ T cells on immune pressure is that their frequency is highly correlated with the total ICS memory fraction at the earliest time point, making it difficult to resolve which parameter, the total response or MIP-1β+, is driving the pace of immune escape. The median relative contribution of total CD8+MIP-1β+ T cells was >80% to all ME-specific responses at early time points, as shown in Figure 6A, and declined with time for the l-ME and nME groups as shown in Figure 6C. We further analyzed whether degranulation or IFN-γ production were also associated with the significant predominant contribution of CD8+MIP-1β+ cells to responses to e-ME. As shown in Figure 6B and 6D, among the possible CD8+MIP-1β+ subsets with more than one function we could identify that both CD8+MIP-1β+CD107+ and CD8+MIP-1β+CD107+IFN-γ+ subsets contributed to the e-ME-specific responses but also made a similar contribution to the l-ME and nME responses. Moreover, segregate analysis of both total CD8+IFN-γ+ (p = 0.99) and total CD8+IFN-γ+ MIP-1β+ (p = 0.27) subsets did not reveal any significant association with the appearance of escape mutants. Thus, we observed that the MIP-1β-producing CD8+T cells represent the dominant proportion of cells detectable in the early stage of infection prior to virus escape, and that this was strongly associated with immune pressure resulting in selection of the early escape mutants.
Fig. 6. Analysis of individual functions and polyfunctional subsets. 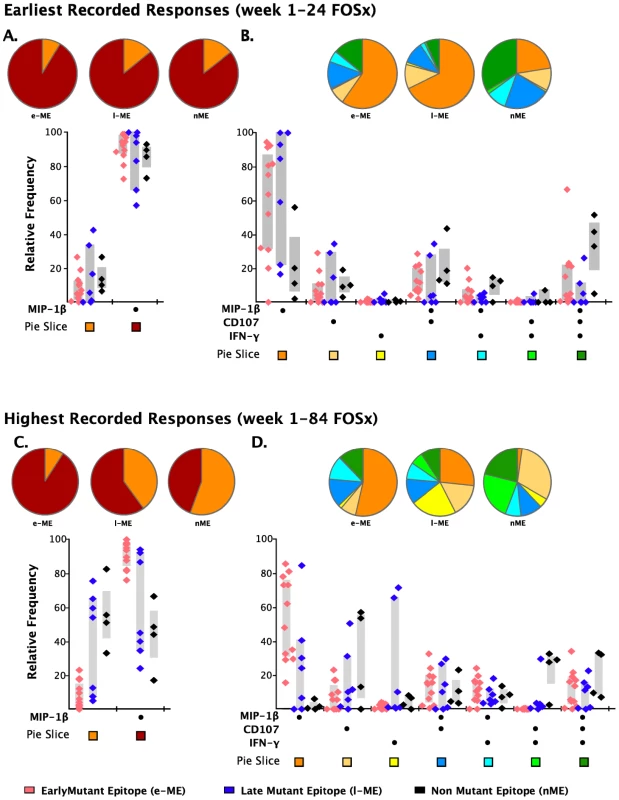
The analysis of the responses at the earliest (panels A and B) and highest recorded (panels C and D) time points are shown. The pie charts represent the relative contribution to the total response of cells exhibiting each functional parameter or combination of functional parameters. The color code for each parameter (pie slice) is reported below the x-axis of the graph. In the graph, each triangle represents the early (e-ME), late (l-ME), and non-mutating (nME) epitope-specific CD8+ T cell response detected at each time point by ICS. The color code for each group of epitopes is reported at the bottom of the figure. The grey bars represent the inter-quartile distribution of the relative frequency of each functional parameter. The dots under the x-axis represent the positive responses for each parameter. (A and C) Total MIP-1β+ responses are compared to the total responses detected as any combination of the other parameters excluding MIP-1β. (B and D) Analysis of the single or combined contribution of degranulation (CD107), IFN-γ and MIP-1β production is represented by the dots under the x-axis. Discussion
The results of both the STEP and RV144 clinical trials suggest that CD8+ T cell responses alone might neither prevent HIV-1 infection nor lower virus set-point after infection [27], [28]. However, given the relatively weak and narrow T-lymphocyte responses were induced by the vaccine protocol used the in the STEP trial [29], and encouraging non-human primate vaccine studies [30], [31], [32] it is still important to continue this approach. Thus, features of HIV-specific CD8+ T cells that determine their in vivo efficacy need to be defined. It has been widely documented that CD8 T cell responses are elicited early following HIV-1 infection and that they are associated with control of viremia as well as with selecting for escape mutants that may impact virus fitness [33]. As their most important impact may take place during acute infection, we have focused our study on characterization of the functionality of HIV-specific CD8 T cell responses at this time, concentrating on epitope-specific responses which did or did not drive selection for escape mutations of the transmitted virus.
We evaluated CD8+ T cell responses using 18-mer peptides corresponding to the autologous transmitted/founder viral sequence of seven individuals recruited within 45 days from the onset of acute HIV-1 infection [6], [26]. We first studied the total response to the gag gene product, because of its importance in vaccine design, and then extended the study to epitopes that we had previously characterized for escape mutants because of their potential impact on virus set point [6], [26]. We observed that: 1) more than 80% of the epitope specific T cells in acute infection were oligofunctional; 2) MIP-1β-producing T cells were associated with epitopes that escaped; 3) transmitted/founder virus escape mutants in acute infection occur in epitopes that have high entropy at the population level.
Previously in chronically infected patients, multifunctionality of HIV-specific CD8+ T cell responses in the peripheral blood has been shown to be associated with long-term non-progression [22], [23], and it has been related to the CD4 count and inversely related to the virus load at the rectal mucosal level [34]. In our study, we characterized the earliest functional responses and observed that the early CD8 T cell response was predominantly mono - or bi-functional. The contribution of T cells with 3-5 functions was small but increased with time from infection and we cannot exclude that they become a major subset as the infection progresses, particularly in those who control the infection well, as previously described. Although we cannot exclude the possibility that the presence of early poly-functional CD8+T cell responses may predict control of viremia, the observation that the only controller in our cohort displayed a profile of functional responses similar to those who did not control virus replication would suggest otherwise. Our observations are largely in agreement with those made by Streeck and collaborators, who reported an increase in poly-functionality with the appearance of escape mutants and a subsequent decrease in functionality over time [35]. In fact, we likewise observed an increase in functionality of epitope-specific CD8+ T cell responses from the early to the HRF time point, although, we showed that only the total CD8+ T cell responses were significantly associated with the appearance of escape mutations. This could be related to the fact that, in our study, responses were detected in most cases before full escape occurred when virus load had not yet reached the virus set-point. The initially low level of multifunctionality might be related to high levels of virus replication that would in turn favor upregulation of inhibitory receptors such as PD1. Such upregulation would have a negative impact on the functionality of the CD8+ T cells – an impact that is rescued in chronically-infected individuals upon control of VL following initiation of therapy [36], [37]. By comparing our data to those obtained from individuals immunized with MVA to protect against smallpox virus infection [38], we also hypothesize that HIV-specific CD8 responses may be substantially delayed in acquiring multifunctionality because of impairments in CD4+ T cell populations, which are otherwise preserved and functional in MVA vaccine recipients. It is, therefore, possible that maturation of HIV-1 specific multifunctional CD8+ T cell responses might be affected by both the impact of high levels of virus replication and the early loss of CD4+ T helper function.
In the present study we also sought to characterize whether the functionality of the CD8+ T cell responses generated during acute/early HIV-1 infection may be related to the immune pressure exerted on the transmitted virus. For the 19 ME epitope-specific CD8+ T cell responses studied, the total magnitude of functional CD8+ T cell response at early time points was significantly associated with the early appearance of escape mutations, when responses were mainly oligofunctional. Multifunctional T cells were a minor component of the response. The observation of the overall impact of the magnitude of functional T cell responses on the appearance of escape mutations is in agreement with previous results obtained by the analysis of IFN-γ+CD8+ T cell responses, although, in this cohort of patients the IFN-γ+ responses alone did not show significant association with the appearance of escape mutants [26], [39]. Other characteristics of the T cell responses to e-ME may also have influenced the rate at which they were escaped. For example, e-ME may generally elicit clonal CD8+ T cell populations with higher avidity [40], [41], [42] and/or a better selection of a T cell receptor (TcR) repertoire, as suggested by both human and NHP studies [42], [43]. These questions are being addressed separately.
Further analysis of the proportion of epitope-specific T cells producing individual cytokines revealed for the first time that MIP-1β+-producing CD8+ T cells constituted greater than 80% of the cells participating in the early responses and, therefore, were associated with the appearance of escape mutants. The contribution of MIP-1β+-producing CD8+ T cells to the overall response declined with time for the l-ME and nME responses, suggesting that persistent antigenic stimulation might also be an important factor in selecting for clones whose functional profile is dominated more by IFN-γ+ production as part of the maturation process [44]. Among the CD8+MIP-1β+ responding cells, we observed that CD8+MIP-1β+CD107+ and CD8+MIP-1β+CD107+IFN-γ+ T cells were the two multifunctional subsets most highly contributing to the early responses. We have recently demonstrated that CD8+ T cell mediated antiviral function in virus controllers and vaccinees is associated with Clade B Env and Gag-specific MIP-1β and CD107a expression as monofunctional and dual-functional populations, suggesting the importance of both cytolytic and non-cytolytic mechanisms in control of HIV replication [45]. CD8+ T cells can suppress HIV-1 replication noncytolytically through the secretion of a number of soluble factors, including the B-chemokines, MIP1α, MIP1β and RANTES, which can potently inhibit CCR5 tropic virus infection [46]. We have found that the secretion of MIP-1α, MIP-1β, IP-10, MIG, IL-1, and interferon gamma correlated most strongly with soluble noncytolytic suppression [47]. Using the ability of histone hyperacetylation to alter the expression of immune-related genes, we identified that MIP-1α and IP-10 were also among the genes that were down-regulated by histone hyperacetylation; again supporting the idea that they contributed to soluble CD8+ T cell mediated suppression [47], [48]. Taken together, these observations suggest that T cells involved in selecting for escape mutants during acute HIV-1 infection may possess a combination of activities that include both killing and inhibitory activity. This possibility has also been suggested in the SIV model where loss of HIV-infected CD4+ T cells was not prevented by administration of antiretroviral therapy in CD8-depleted animals, suggesting that CD8+ T cell functions other than cytotoxic activity might contribute to early control of virus replication and recovery of CD4 counts [49], [50].
Neither the magnitude, the phenotype, nor the functionality of CD8+ T cell responses at time of HRF showed any association with the appearance of escape mutants. These lack of associations could be related to several factors: 1) our inability to detect the true peak of responses; 2) the fact that the maturation of responding cells may be impaired by high levels of virus replication; and 3) rapid appearance of escape mutants.
Our data indicate that high entropy at the population level is significantly associated with the appearance of escape within the first six months post-infection, extending observations made in previous studies of escape in acute infection [51], [52]. Epitopes that exhibited delayed escape despite an early response were associated with low entropy at the population level, hence higher fitness costs may be associated with change in these epitopes. Our finding is in line with results from earlier studies that have demonstrated the role of fitness costs of sequence changes in/around CD8+ T cell epitopes in constraining escape from epitope-specific responses [6], [16], [26]. Delayed escape was also associated with less vigorous CD8+ T cell responses as revealed by lower total response magnitudes assessed by ICS.
Overall, our data show that fully multifunctional (5+) Gag-specific CD8+ T cell responses are not detectable during the initial weeks following HIV-1 infection in the response to the transmitted virus. Moreover, the relative contribution of 3 - and 4-multifunctional subsets to the total response is small, although it increases with time from initial infection. Importantly, the magnitude of epitope-specific CD8+ T cell responses including the frequency of epitope-specific MIP-1β -producing cells is associated with the order of appearance of escape mutants. The order in which different epitopes escaped CD8 responses is also associated with the population diversity (entropy) of the epitopes. These findings underscore the important role that the magnitude of the CD8+ T cell responses plays in exerting pressure on replication of transmitted/founder HIV-1 in the early stages of infection, and illustrate the importance of considering both epitope entropy and functional responses beyond the cytotoxic properties of epitope-specific CD8+ T cells for vaccine design.
Methods
Subjects
The seven individuals enrolled in this study were identified as acutely HIV-1-infected by the presence of detectable HIV-1 viral RNA in their plasma, but either negative or discordant HIV-1 serology at the screening visit. Three infected patients (CH040, CH058 and CH077) were enrolled in the CHAVI 001 study at the Duke University Medical Center and Chapel Hill UNC-Hospital (USA). Four additional patients (MM33, MM39, MM42 and MM43) were recruited at the Mortimer Market Center (London, UK). CD4 cell counts and viral loads were determined by local accredited clinical laboratories. The Fiebig stage was determined as previously reported [53]. All seven individuals remained HAART naïve for the duration of the study.
PBMC were isolated by standard density gradient centrifugation from anti-coagulated blood samples, within 8 hours of collection. PBMC were viably cryopreserved in fetal calf serum supplemented with 10% DMSO and stored in vapor phase liquid nitrogen.
PBMC samples were also obtained from 16 HIV-1 seronegative individuals as a control group.
Each participant enrolled in the study provided written informed consent in conjunction with protocols approved by the institutional review boards of Duke University Medical Center and the Mortimer Market Center for Sexual Health and HIV research.
Sequence analysis of transmitted virus isolates
Sequences of the transmitted virus were derived from plasma obtained at either the screening or enrollment visit. RNA extraction, complementary DNA synthesis, and sequence analysis were performed as previously described [26], [54]. GeneBank accession numbers for sequences are as follows: HM586186-HM586209 correspond to 24 bulk sequences from MM33, MM39, MM2, and MM43; HM153284-HM153412 correspond to 129 SGA sequences from MM39 and MM43; and FJ495804-FJ496071, FJ496086-FJ496144, and FJ919955-FJ919967 include 340 SGA sequences derived from CH40, CH58, and CH77. Alternatively, longitudinal alignments from subjects CH40, CH58 and CH77 can be found at http://www.hiv.lanl.gov/content/sequence/HIV/USER_ALIGNMENTS/Salazar.html as previously published [54].
Peptides
Eighteen amino acid peptides, overlapping by 10 amino acid residues, were synthesized based on the autologous transmitted HIV-1 sequences for each infected study subject as previously described [6], [26].
T cell epitope mapping
The viral peptides recognized by the primary HIV-specific T cell response in each patient were identified by ex vivo interferon-gamma (IFN-γ) ELISpot assay as previously reported [6], [26]. Briefly, PBMC were thawed and, after overnight (CH040/058/077–2 hr resting) resting, stimulated with peptide pools at a final concentration of 2 µg/ml for 20–24 hr at 37°C in 5% CO2. For each assay, six negative control wells (no antigen) and triplicate wells of at least 1 positive control (PHA at 10 µg/ml; Sigma-Aldrich) were included. Each epitope-specific response identified from the screening was confirmed in a secondary assay, and, for some responses, by testing the recognition of shorter peptides A criteria of positivity of ≥50 Spot Forming Cells (SFCs)/106 cells and >3x background was established.
Multi-parameter intracellular cytokine staining assay
Cryopreserved PBMC were thawed and rested overnight. PBMC were stimulated with individual or pools of peptides representing the sequence of the autologous transmitted HIV-1 isolate. For all subjects, a polyclonal stimulation with the superantigen Staphylococcus aureus enterotoxin B (SEB) was also used as positive control. Each antibody was titered to determine the saturating concentration used for the final staining. The stimulations were conducted in the presence of 1 µg/ml anti-CD28 mAb (clone L293; BD), 1 µg/ml anti-CD49d mAb (clone L25; BD), anti-CD107a Alexa Fluor 680 (clone H4A3; BD), 5 µg/ml brefeldin A (Sigma-Aldrich) and 1 µg/ml of Golgi Stop (BD) for 5.5 h at 37°C in 5% CO2. After washing, the cells were surface stained with anti–CD4-Cy5.5-PE (clone M-T477; Biolegend), anti–CD8-QD705 (clone RPA-T8; InVitroGen), anti–CD27-Cy5-PE (clone M-T271, BD Bioscience), anti–CD57-QD565 (clone NK-1, AbD; conjugated in M. Betts Lab), and anti–CD45RO-PE–Texas Red (clone UCHL1, Beckman Coulter) for 20 min at room temperature. Admixtures of anti–CD14–Cascade blue (clone M5E2, Biolegend), anti–CD19–Cascade blue (clone HIB 19, Biolegend), and a vital dye (ViVid; InVitroGen) were also used to exclude monocytes/macrophages, B cells, and non-viable cells from the analysis.
PBMC were subsequently fixed and permeabilized with Cytofix/Cytoperm and Perm/Wash buffer (Pharmingen, San Diego, CA) for 20 minutes, washed twice, and stained with anti–CD3-Cy7-APC (clone SK7, BD Bioscience), anti–IFN-γ–FITC (B27), anti–IL-2–APC (clone MQ1-17H12), anti–TNF-α–Cy7-PE (clone MAb11), and anti–MIP-1β-PE (clone D21-1351; all obtained from BD) for 1 h at 4°C. After washing and fixation, samples were acquired on a custom made LSRII (BD Bioscience, San Jose, CA) within the next 24 hours. A minimum of 300,000 total viable events was acquired for each test.
Gating strategy
Gates were set to include singlet events, live CD3+ cells, lymphocytes, and CD4+ and CD8+ subsets (Figure 1). From the total CD8+ population, the naive subset was identified as CD45RO−CD27+. This subset was excluded from the subsequent analysis, and only the memory population was included, indicated by the red gate in Figure S1.I. Within the memory population, the central memory CD45RO+CD27+ (CM; RO+27+), effector CD45RO+CD57+ (E; RO+57+), and the terminal effector CD45RO−CD57+ (TE; RO−57+) populations were identified.
Antigen-specific populations were identified within the memory population as single-function cells shown in the sequential single cytokine/chemokine/degranulation gates. Thirty-two poly-functional subsets were identified using a Boolean gating strategy that included both the functions and memory phenotype of the cells. Based on the initial 32 functional subsets, we calculated the frequency of the total CD8+ memory response as well as of the total frequency of families of subsets having the same numbers of functional parameters as shown in Figure S1.II.
Data analysis
Data analysis was performed using FlowJo 8.8.4 software (TreeStar), Pestle for background subtraction, and SPICE for frequency analysis. Pestle and SPICE were kindly provided by Dr. M. Roederer Vaccine Research Center, NIH, Bethesda, MD.
Based on the results obtained with the sixteen seronegative subjects (Figure S2), responses were considered positive if the percentage of antigen-specific cells was 2-fold above the background and greater than 0.05% after background subtraction for at least two of the functional parameters.
Assigning the rank order of timing of escape mutations
Sequence analysis of epitope-containing regions was performed at multiple time points over the first 87 weeks of infection. For some samples and epitopes, many single gene amplification (SGA) sequences were obtained spanning the epitope, and for others, particularly samples that were sequenced in the context of previous studies [26], only a single direct population sequence was available (see supporting information for a complete listing of epitope sequences at each time point studied). Direct sequences readily detect mixed bases at positions if they are present in roughly 25% of the population. The times at which the first evidence for selection of sequence changes in/around epitopes and the last traces of the transmitted epitope were observed were determined.
Evaluation of the entropy of each epitopic region
To test the hypothesis that the pace of early escape may correlate with the capacity of the epitope to readily tolerate mutations, reflected in diversity of the epitope at the population level, we used Shannon entropy as a measure of epitope diversity. Shannon entropy ( where p is the probability of the ith form of the epitope), is an information theory measure of the uncertainty in a variable [55]. The Shannon entropy can be used to compare the variability in positions or regions in protein alignments [56], [57]. Here we extracted each epitope region studied from the B subtype of Los Alamos database web alignments using the database tool QuickAlign (www.hiv.lanl.gov), and used the observed frequency of each epitope variant to estimate the probability of a given variant and to calculate the entropy. The database alignments that we used contained a single randomly selected sequence per infected person, to be representative of the circulating population, and included between 500–1000 sequences, depending on what was available for a given protein. Entropy is thus reported as a measure of epitope variation at the population level; the higher the value the more variable the epitope, both in terms of the number of variant forms and the distribution of their frequencies. If the defined epitope was 9 amino acids long, we used the precise epitope fragment; if it was longer or not precisely defined within the reactive peptide, we used the 9 amino acids centered on the escape mutations observed in the subject, so each epitope region studied was exactly 9 amino acids long and thus we could compare relative entropy values between epitopes.
Statistical analysis
A statistical test for linear trend in mean values over the six time points was computed to analyze each degree of polyfunctionality for the Gag-specific responses. A linear mixed effects (LME) model was fit to the Gag-specific longitudinal data to account for correlations amongst the measurements over time.
A generalized linear mixed model assuming a normal distribution was used to analyze the contribution of T cell responses and epitope entropy to predicting the rank order of escape across all subjects using the statistical package R (http://www.R-project.org). To determine if these variables were correlated to the within-subject rank-order of escape, we calculated the non-parametric Spearman's ρ statistic comparing either the ranks of the epitope entropy or the T cell response level with the sequential timing of escape for each subject. Spearman's ρ was calculated for each the 5 subjects with multiple epitopes studied, and the ρ's were summed. We then randomized within-subject data 100,000 times, and a recalculated the sum of the ρ's for each of the randomized data sets. This provided a Monte Carlo framework to test against the null that within-subject ranks were not correlated.
Supporting Information
Zdroje
1. DouekDC
KwongPD
NabelGJ
2006
The rational design of an AIDS vaccine.
Cell
124
677
681
2. AppayV
DouekD
PriceD
2008
CD8+ T cell efficacy in vaccination and disease.
Nat Med
3. BorrowP
LewickiH
HahnBH
ShawGM
OldstoneMBA
1994
Virus-specific CD8+ cytotoxic T lymphocyte activity associated with control viremia in primary human immunodeficiency virus type 1 infection.
J Virol
68
6103
6110
4. KoupRA
SafritJF
CaoY
AndrewsCA
McLeodG
1994
Temporal association of cellular immune response with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome.
J Virol
68
4650
4655
5. SafritJT
AndrewsCA
ZhuT
HoDD
KoupRA
1994
Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope.
J Exp Med
179
463
472
6. GoonetillekeN
LiuMKP
Salazar-GonzalezJF
FerrariG
GiorgiE
2009
The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection.
J Exp Med
206
1253
1272
7. PhillipsRE
Rowland-JonesS
NixonDF
GotchFM
EdwardsJP
1991
Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition.
Nature
354
453
459
8. BorrowP
LewickiH
WeiX
HorwitzMS
PefferN
1997
Antiviral pressure exerted by HIV-1-specific cytotoxic T limphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus.
Nat Med
3
205
217
9. PriceDA
GoulderPJ
KlenermanP
SewellAK
EasterbrookPJ
1997
Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection.
Proc Natl Acad Sci USA
94
1890
1895
10. GoulderPJ
WatkinsDI
2004
HIV and SIV CTL escape: implications for vaccine design.
Nat Rev Immunol
4
630
640
11. GoulderPJ
PhillipsRE
ColbertRA
McAdamS
OggG
1997
Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS.
Nat Med
3
212
217
12. FeeneyME
TangY
RooseveltKA
LeslieAJ
McIntoshK
2004
Immune Escape Precedes Breakthrough Human Immunodeficiency Virus Type 1 Viremia and Broadening of the Cytotoxic T-Lymphocyte Response in an HLA-B27-Positive Long-Term-Nonprogressing Child.
J Virol
78
8927
8930
13. AllenTM
O'ConnorDH
JingP
DzurisJL
MotheBR
2000
Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia [see comments].
Nature
407
386
390
14. O'ConnorDH
AllenTM
VogelTU
JingP
DeSouzaIP
2002
Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection.
Nat Med
8
493
499
15. BarouchDH
KunstmanJ
KurodaMJ
SchmitzJE
SantraS
2002
Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes.
Nature
415
335
339
16. Martinez-PicadoJ
PradoJG
FryEE
PfafferottK
LeslieA
2006
Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1.
J Virol
80
3617
3623
17. LiB
GladdenAD
AltfeldM
KaldorJM
CooperDA
2007
Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution.
J Virol
81
193
201
18. Sanchez-MerinoV
FarrowMA
BrewsterF
SomasundaranM
LuzuriagaK
2008
Identification and characterization of HIV-1 CD8+ T cell escape variants with impaired fitness.
J Infect Dis
197
300
308
19. BoutwellCL
RowleyCF
EssexM
2009
Reduced viral replication capacity of human immunodeficiency virus type 1 subtype C caused by cytotoxic-T-lymphocyte escape mutations in HLA-B57 epitopes of capsid protein.
J Virol
83
2460
2468
20. GanusovV
de BoerR
2006
Estimating costs and benefits of CTL escape mutations in SIV/HIV infection.
PLoS Comput Biol
21. LohL
BattenCJ
PetravicJ
DavenportMP
KentSJ
2007
In vivo fitness costs of different Gag CD8 T-cell escape mutant simian-human immunodeficiency viruses for macaques.
J Virol
81
5418
5422
22. ZimmerliSC
HarariA
CelleraiC
VallelianF
BartPA
2005
HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells.
Proc Natl Acad Sci U S A
102
7239
7244
23. BettsMR
NasonMC
WestSM
De RosaSC
MiguelesSA
2006
HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells.
Blood
107
4781
4789
24. FisherW
GanusovV
GiorgiE
HraberP
KeeleBF
2010
Transmission of Single HIV-1 Genomes and Dynamics of Early Immune Escape Revealed by Ultra-deep Sequencing.
PLoS One
25. BurgersWA
RiouC
MlotshwaM
MaenetjeP
de Assis RosaD
2009
Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point.
J Immunol
182
4751
4761
26. TurnbullEL
WongM
WangS
WeiX
JonesNA
2009
Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection.
J Immunol
182
7131
7145
27. McElrathMJ
De RosaSC
MoodieZ
DubeyS
KiersteadL
2008
HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis.
Lancet
372
1894
1905
28. Rerks-NgarmS
PitisuttithumP
NitayaphanS
KaewkungwalJ
ChiuJ
2009
Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand.
N Engl J Med
361
2209
2220
29. CoreyL
McElrathMJ
KublinJG
2009
Post-step modifications for research on HIV vaccines.
AIDS
23
3
8
30. LiuJ
O'BrienKL
LynchDM
SimmonsNL
La PorteA
2009
Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys.
Nature
457
87
91
31. ReynoldsMR
WeilerAM
WeisgrauKL
PiaskowskiSM
FurlottJR
2008
Macaques vaccinated with live-attenuated SIV control replication of heterologous virus.
J Exp Med
205
2537
2550
32. HansenSG
VievilleC
WhizinN
Coyne-JohnsonL
SiessDC
2009
Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge.
Nat Med
15
293
299
33. McMichaelAJ
BorrowP
TomarasGD
GoonetillekeN
HaynesBF
2010
The immune response during acute HIV-1 infection: clues for vaccine development.
Nat Rev Immunol
10
11
23
34. CritchfieldJW
YoungDH
HayesTL
BraunJV
GarciaJC
2008
Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status.
PLoS ONE
3
e3577
35. StreeckH
BrummeZL
AnastarioM
CohenKW
JolinJS
2008
Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells.
PLoS Med
5
e100
36. TrautmannL
JanbazianL
ChomontN
SaidE
2006
Upregulation of PD-1 expression on HIV-specific CD8 T cells leads to reversible immune dysfunction.
Nat Med
37. PetrovasC
CasazzaJP
BrenchleyJM
PriceDA
GostickE
2006
PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection.
J Exp Med
203
2281
2292
38. PrecopioML
BettsMR
ParrinoJ
PriceDA
GostickE
2007
Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses.
J Exp Med
204
1405
1416
39. BrummeZL
BrummeCJ
CarlsonJ
StreeckH
JohnM
2008
Marked epitope - and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection.
J Virol
82
9216
9227
40. GallimoreA
DumreseT
HengartnerH
ZinkernagelRM
RammenseeHG
1998
Protective immunity does not correlate with the hierarchy of virus - specific cytotoxic T cell responses to naturally processed peptides.
J Exp Med
187
1647
1657
41. AlmeidaJR
PriceDA
PapagnoL
ArkoubZA
SauceD
2007
Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover.
J Exp Med
204
2473
2485
42. AlmeidaJR
SauceD
PriceDA
PapagnoL
ShinSY
2009
Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity.
Blood
113
6351
6360
43. PriceDA
AsherTE
WilsonNA
NasonMC
BrenchleyJM
2009
Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection.
J Exp Med
206
923
936
44. AppayV
van LierR
SallustoF
RoedererM
2008
Phenotype and function of human T lymphocyte subsets: Consensus and issues.
Cytometry Part B: Clinical Cytometry
45. FreelSA
LamoreauxL
ChattopadhyayPK
SaundersK
ZarkowskyD
2010
Phenotypic and Functional Profile of HIV-inhibitory CD8 T cells Elicited by Natural Infection and Heterologous Prime/boost Vaccination.
J Virol
46. CocchiF
DeVicoAL
Garzino-DemoA
AryaSK
GalloRC
1995
Identification of RANTES, MIP-1αand MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells.
Science
270
1811
1815
47. SaundersKO
Ward-CavinessC
SchutteRJ
FreelSA
OvermanRG
2010
Secretion of MIP-1β and MIP-1α by CD8(+) T-lymphocytes correlates with HIV-1 inhibition independent of coreceptor usage.
Cellular immunology
48. SaundersKO
FreelSA
OvermanRG
CunninghamCK
TomarasGD
2010
Epigenetic regulation of CD8(+) T-lymphocyte mediated suppression of HIV-1 replication.
Virology
405
234
242
49. WongJK
StrainMC
PorrataR
ReayE
Sankaran-WaltersS
2010
In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells.
PLoS Pathog
6
e1000748
50. KlattNR
ShudoE
OrtizAM
EngramJC
PaiardiniM
2010
CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells.
PLoS Pathog
6
e1000747
51. BansalA
GoughE
SabbajS
RitterD
YusimK
2005
CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides.
AIDS
19
241
250
52. JonesNA
WeiX
FlowerDR
WongM
MichorF
2004
Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response.
J Exp Med
200
1243
1256
53. FiebigEW
WrightDJ
RawalBD
GarrettPE
SchumacherRT
2003
Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection.
AIDS
17
1871
1879
54. Salazar-GonzalezJF
SalazarMG
KeeleBF
LearnGH
GiorgiEE
2009
Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection.
J Exp Med
206
1273
1289
55. ShannonCE
1948
A mathematical Theory of Communication".
Bell System Technical Journal
27
379
423, 623-656
56. Fontaine CostaAI
RaoX
LechenadecE
van BaarleD
KeşmirC
2010
HLA-B molecules target more conserved regions of the HIV-1 proteome.
AIDS
24
211
215
57. KorberBT
KunstmanKJ
PattersonBK
FurtadoM
McEvillyMM
1994
Genetic differences between blood - and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences.
J Virol
68
7467
7481
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



