-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Targeted Disruption of : Invasion of
Erythrocytes by Using an Alternative Py235
Erythrocyte Binding Protein
Plasmodium yoelii YM asexual blood stage parasites express
multiple members of the py235 gene family, part of the
super-family of genes including those coding for Plasmodium
vivax reticulocyte binding proteins and Plasmodium
falciparum RH proteins. We previously identified a Py235
erythrocyte binding protein (Py235EBP-1, encoded by the PY01365 gene) that is
recognized by protective mAb 25.77. Proteins recognized by a second protective
mAb 25.37 have been identified by mass spectrometry and are encoded by two
genes, PY01185 and PY05995/PY03534. We deleted the PY01365 gene and examined the
phenotype. The expression of the members of the py235 family in
both the WT and gene deletion parasites was measured by quantitative RT-PCR and
RNA-Seq. py235ebp-1 expression was undetectable in the knockout
parasite, but transcription of other members of the family was essentially
unaffected. The knockout parasites continued to react with mAb 25.77; and the
25.77-binding proteins in these parasites were the PY01185 and PY05995/PY03534
products. The PY01185 product was also identified as erythrocyte binding. There
was no clear change in erythrocyte invasion profile suggesting that the PY01185
gene product (designated PY235EBP-2) is able to fulfill the role of EBP-1 by
serving as an invasion ligand although the molecular details of its interaction
with erythrocytes have not been examined. The PY01365, PY01185, and
PY05995/PY03534 genes are part of a distinct subset of the py235 family. In
P. falciparum, the RH protein genes are under epigenetic
control and expression correlates with binding to distinct erythrocyte receptors
and specific invasion pathways, whereas in P. yoelii YM all the
genes are expressed and deletion of one does not result in upregulation of
another. We propose that simultaneous expression of multiple Py235 ligands
enables invasion of a wide range of host erythrocytes even in the presence of
antibodies to one or more of the proteins and that this functional redundancy at
the protein level gives the parasite phenotypic plasticity in the absence of
differences in gene expression.
Published in the journal: Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein. PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001288
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001288Summary
Plasmodium yoelii YM asexual blood stage parasites express
multiple members of the py235 gene family, part of the
super-family of genes including those coding for Plasmodium
vivax reticulocyte binding proteins and Plasmodium
falciparum RH proteins. We previously identified a Py235
erythrocyte binding protein (Py235EBP-1, encoded by the PY01365 gene) that is
recognized by protective mAb 25.77. Proteins recognized by a second protective
mAb 25.37 have been identified by mass spectrometry and are encoded by two
genes, PY01185 and PY05995/PY03534. We deleted the PY01365 gene and examined the
phenotype. The expression of the members of the py235 family in
both the WT and gene deletion parasites was measured by quantitative RT-PCR and
RNA-Seq. py235ebp-1 expression was undetectable in the knockout
parasite, but transcription of other members of the family was essentially
unaffected. The knockout parasites continued to react with mAb 25.77; and the
25.77-binding proteins in these parasites were the PY01185 and PY05995/PY03534
products. The PY01185 product was also identified as erythrocyte binding. There
was no clear change in erythrocyte invasion profile suggesting that the PY01185
gene product (designated PY235EBP-2) is able to fulfill the role of EBP-1 by
serving as an invasion ligand although the molecular details of its interaction
with erythrocytes have not been examined. The PY01365, PY01185, and
PY05995/PY03534 genes are part of a distinct subset of the py235 family. In
P. falciparum, the RH protein genes are under epigenetic
control and expression correlates with binding to distinct erythrocyte receptors
and specific invasion pathways, whereas in P. yoelii YM all the
genes are expressed and deletion of one does not result in upregulation of
another. We propose that simultaneous expression of multiple Py235 ligands
enables invasion of a wide range of host erythrocytes even in the presence of
antibodies to one or more of the proteins and that this functional redundancy at
the protein level gives the parasite phenotypic plasticity in the absence of
differences in gene expression.Introduction
Despite the recent renewed onslaught to tackle a disease that infects 300-660 million people and kills one million each year worldwide [1], the malaria parasite remains an elusive target. During the asexual blood stage, which is responsible for the disease, the parasite invades and develops within erythrocytes, but the precise molecular mechanisms employed to gain entry into the erythrocyte are still being worked out. A number of parasite adhesion proteins have been identified as important in the selection and invasion of host cells and have been grouped according to structural and sequence homology rather than host molecular specificity or cellular phenotype (reviewed in [2], [3], [4], [5]). The role of the actin-myosin motor complex in the invasion of erythrocytes is also being elucidated [6], [7]. Together, merozoite surface proteins, the adhesion ligands and the motor complex add up to a multifaceted molecular interaction that results in the successful selection and invasion of host cells [3], [4], [5]. Understanding the role played in the invasion cascade by adhesion proteins with homologues in both human and rodent Plasmodium is of paramount importance in the quest to design intervention tools that will inhibit invasion pathways and so kill the parasite and prevent disease.
Of the Plasmodium adhesion ligand families identified to date, one of the most studied is the erythrocyte binding ligand family (EBL), which includes P. falciparum erythrocyte binding antigen (EBA)-175 and the Duffy binding protein (DBP) of P. vivax and P. knowlesi (reviewed in [3], [4]) located in the apical organelles of the merozoite. A second group of high molecular mass adhesion proteins, which was first described in the rodent malaria parasite Plasmodium yoelii as Py235 [8], [9], is the reticulocyte binding-like (RBL) super family, so named because of sequence homology with the reticulocyte binding protein (RBP)-1 and RBP-2, of Plasmodium vivax. In P. vivax, these proteins are thought to be involved in erythrocyte selection as they bind to reticulocytes but not mature erythrocytes thereby restricting P. vivax to the invasion of reticulocytes [10]. P. falciparum contains a small group of genes coding for proteins with similarities to Py235 and PvRBP, the PfRH family [11], [12], [13]. In contrast to the PvRBP and PfRH gene families, which are small, the Py235 multigene family contains at least 11 members [9], [14], [15], [16], [17], [18]. Analysis of the sequences on fifteen contigs identified in the P. yoelii genome database [15], which represent members of the Py235 gene family (and some of which are incomplete), show they have overall conserved structural elements [16], [19], [20].
The Py235 proteins have been implicated in the selection, recognition and invasion of erythrocytes. For example, passive immunization of mice with monoclonal antibodies (mAbs) 25.77 and 25.37 specific for Py235, or immunization of mice with mAb 25.77-affinity purified protein restricts the growth of the virulent YM line of P. yoelii [8], [21]. In these experiments the invasion profile was switched from invasion of erythrocytes of all ages to invasion of only reticulocytes, suggesting that the antibodies prevent parasite recognition and invasion of mature erythrocytes. This restriction in cell specificity resulted in a non-lethal infection similar to that of the avirulent 17X line, in contrast to the normal lethal phenotype of the YM parasite. Of note is the finding that a combination of both protective mAbs together conferred greater protection [22], suggesting that the epitope recognized by each of the mAbs is not identical and may or may not be on distinct members of the family.
Py235 proteins are released in soluble form from parasitized cells maintained in vitro, and two of these are recognized by mAb 25.77 [23], [24], [25]. However, only one of these forms was detected binding specifically and preferentially to the surface of mature mouse erythrocytes [24]. Binding was to neuraminidase-resistant, chymotrypsin - and trypsin-sensitive erythrocyte receptors, and the binding was abolished by incubation with Py235-specific antibodies [25].
Several invasion pathways coexist in a single parasite as exemplified by P. vivax that requires selection of reticulocytes (using the RBPs [26]) that are Duffy blood group antigen positive, (using the DBP [27], [28]) to successfully gain entry into the host cell. In P. falciparum the Dd2 clone can switch from being dependent on sialic acid for entry into the erythrocyte, allowing it to invade neuraminidase-treated erythrocytes [29]. This change of phenotype has been found to be due to the up-regulation of PfRH4 [30], [31]. Polymorphism due to amino acid substitutions in the binding domain of PfRH5 leads to recognition of different erythrocyte surface receptors, [32]. That several pathways are available to a single parasite is further demonstrated by the observation that invasion into an enzyme-treated cell is not all or nothing even though enzymatic treatment goes to completion, (in P. falciparum [11], [33], and in P. yoelii [25]). Therefore, the invasion pathway of a parasite depends not only on the set of ligands expressed or silenced, some of which are coded by genes under epigenetic control [34], but also on a molecular hierarchy that determines which of the expressed ligands are used [2], [3]. This variant expression of adhesion-/invasion-related proteins is thought to be primarily driven by immune evasion although it may also help to increase the range of erythrocytes that can be invaded [35], [36].
Populations of P. yoelii asexual blood stage parasites express multiple members of the py235 gene family [17], [36], [37]. Multiple gene products were detected in individual schizonts although only single products were identified in single merozoites, leading to the suggestion that the presence of the family allowed clonal phenotypic variation [38]. On the other hand, all merozoites within schizonts reacted with mAb 25.77 [17], suggesting that they either share the protein recognized by this antibody or the epitope is present on multiple members of the family. We have previously identified a specific erythrocyte binding member of the Py235 family (Py235EBP-1), which is recognized by mAb 25.77, and its corresponding gene (py235ebp-1[PY01365]) [20].
Here, we describe the effect on parasite growth in vivo of deleting the gene that encodes the Py235EBP-1 expressed in asexual blood stages of the virulent P. yoelii YM line in order to better understand the role of the Py235 protein family in erythrocyte recognition, binding, and merozoite invasion. We also examine the expression of other family members in this py235ebp-1 knock out (KO) parasite line. Furthermore, we identify the proteins recognised by the other protective mAb 25.37 to obtain an understanding of the relationship in the invasion process between the two protective mAbs and the Py235 proteins they recognize. Whilst there is no difference in the level of expression of other genes in the family, other proteins compensate for the loss of the erythrocyte binding protein, highlighting the importance of functional redundancy to provide plasticity in interaction with the host.
Results
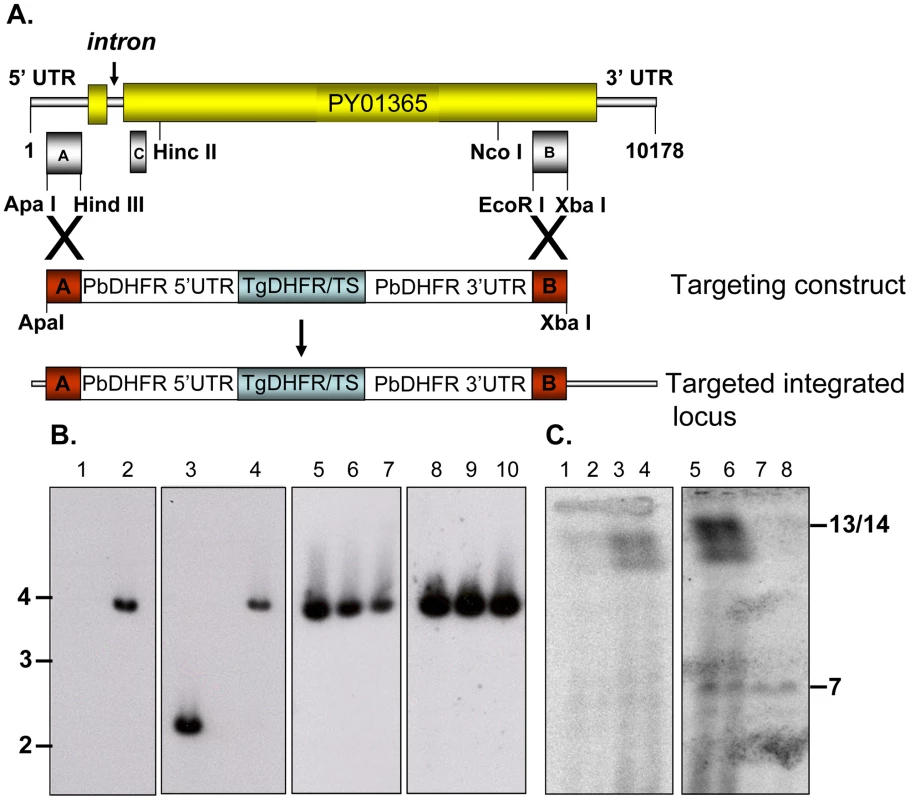
py235ebp-1 (PY01365) can be deleted from the genome of the virulent P. yoelii YM line
Disruption of the py235ebp-1 (PY01365) by insertion of the DHFR cassette by double homologous recombination (Figure 1A) was carried out. Southern blot analysis of digested gDNA from transfected parasites selected with pyrimethamine identified a single band of the expected size in this population, when the filter was probed with a fragment of DHFR/TS (Figure 1B, lane 2). In contrast, hybridization with the probe that binds to the 3′ coding region of py235ebp-1 (Fragment B), detected DNA in both the wild type (WT) (2.3Kb) and the KO (3.8Kb) parasite lines as expected (Figure 1B, lanes 3 and 4). Four individual clones (1 to 3 shown) derived from the population of parasites gave a similar result with both fragment B (Figure 1B, lanes 5 to 7) and the DHFR/TS probe (Figure 1B, lanes 8 to 10), clearly showing that the PY01365 gene had been disrupted. Further evidence that the PY01365 gene had been deleted from the P. yoelii genome was obtained by chromosome analysis (Figure 1C). Hybridization with the probe that binds to a 5′ coding sequence of PY01365 (Fragment C), detected a signal only in the WT parasite lanes, (Figure 1C, lanes 3 and 4). Hybridization with a probe that binds the 3′ UTR of DHFR/TS detected both the modified py235ebp-1 locus (chromosome 13/14) in the KO parasite line (Figure 1C, lanes 5 and 6) and the endogenous dhfr locus (chromosome 7) in both KO and WT parasites (Figure 1C, lanes 5 to 8). Hybridization of a chromosome blot with the Fragment B probe identified a band in all the lanes as expected (data not shown).
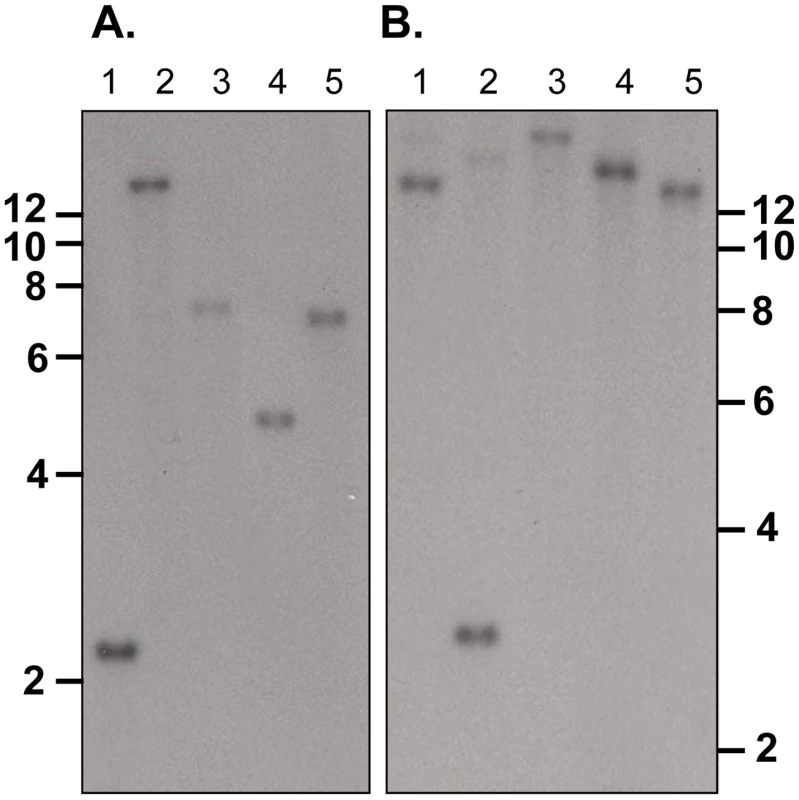
py235ebp-1 (PY01365) is a single copy gene in the P. yoelii YM genome
Southern blot analysis of ten sets of double restriction enzyme digested gDNA from WT P. yoelii YM-parasitized erythrocytes probed with either Fragment B or C gave single bands under low stringency washes (Figure 2). These data suggest that PY01365 is a single copy gene in the line of P. yoelii YM parasites used in this study. This conclusion is also supported by the absence of any RNA-Seq reads mapping to any part of PY01365 from the PY01365-KO parasite.
Merozoites in both WT and PY01365-KO parasitized erythrocytes express proteins with epitopes recognized by both mAb 25.77 and 25.37
The mAb 25.77 had previously been used to identify Py235EBP-1, the product of the PY01365 gene. By IFA, this mAb gives a punctuate pattern of fluorescence in the WT parasite line (Figure 3A). Surprisingly, a similar pattern was also observed for the PY01365-KO parasite line, even though Py235ebp-1 is no longer being expressed. The pattern of reactivity (Figure 3B) was similar but not identical to that of antibodies specific for the micronemal protein, Apical Membrane Antigen 1 (AMA1) [39], the erythrocyte binding ligand protein (EBL), which has a dense granule location in this parasite line [40], and rhoptry neck protein 4 (RON 4) [41]. Furthermore, when proteins released into in vitro culture supernatant from radiolabeled WT and PY01365-KO parasitized erythrocytes were immunoprecipitated using mAbs 25.77 and 25.37 (Figure 3C), or bound to erythrocytes, eluted and then immunoprecipitated (Figure 3D), both mAbs recognized proteins of approximately 235 kDa showing that Py235 proteins were expressed by both WT and PY01365-KO parasite lines. Clearly the Py235 proteins now expressed by the KO parasite line, although at least in part different to those being expressed by the WT parasite, share common epitopes bound by the antibodies.
Fig. 3. Detection of Py235 protein expression. 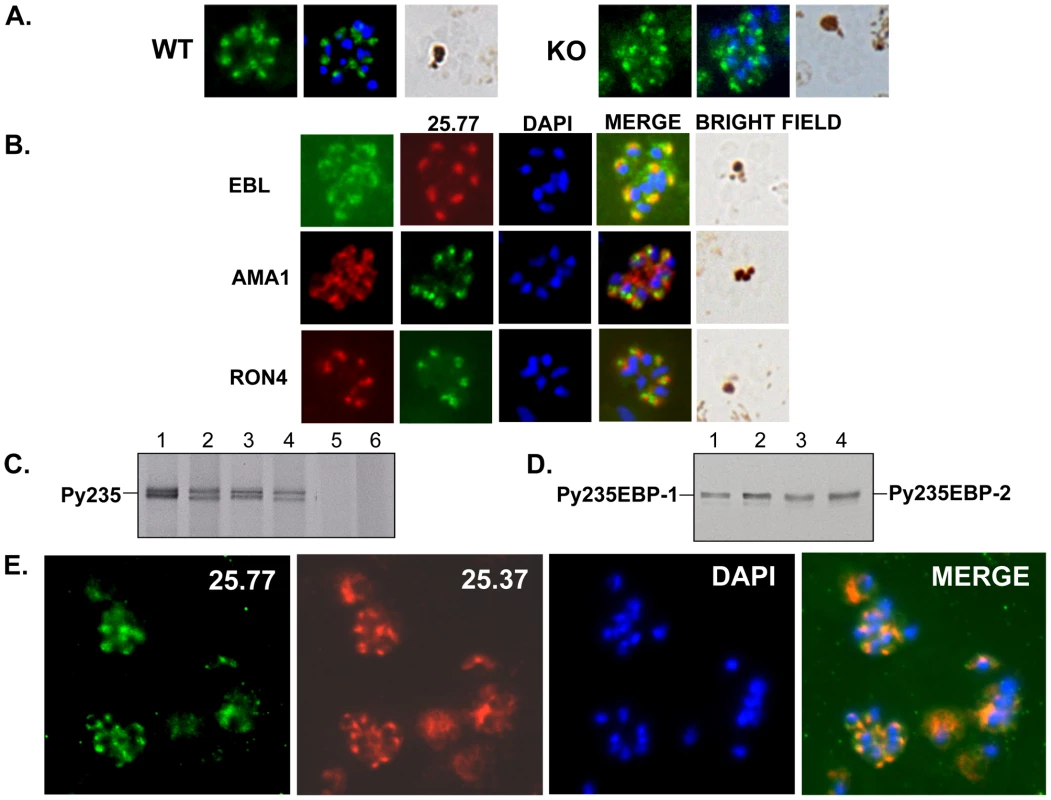
Further confirmation that merozoites express epitopes recognized by both mAb 25.77 and 25.37 was obtained using WT parasitized erythrocytes in a dual labeling fluorescent assay. Alexa Fluor 488-conjugated mAb 25.77 and Alexa Fluor 594-conjugated mAb 25.37 were used to probe the same thin blood smears of mixed stage WT P. yoelii YM parasites (Figure 3E). Overlay of the individual images showed clearly that both antibodies recognized the same parasites.
Two genes comprised of three different contigs encode the Py235 proteins recognized by mAb 25.37
The gene encoding the protein recognized by mAb 25.77 and expressed in WT parasites has previously been identified. A second protective mAb 25.37 also recognizes Py235 proteins and we wished to identify the protein(s) to which it binds. To identify the corresponding genes, peptide mass fingerprinting was carried out on the Py235 proteins affinity purified using mAb 25.37 from both schizonts (Figure 4A) and from culture supernatant of WT parasites maintained in vitro (Figure 4B) and fractionated by SDS-PAGE on a 5% gel. The peptides detected were derived from three contigs in the genome database; one contained a full length (8172bp) py235 gene sequence, PY01185, and the remaining two contained partial gene sequences (Table 1). Contig PY05995 is 2685bp in length and contains sequence that aligned with the 5′ end of other Py235 gene family members, while PY03534 (5478bp) aligned with the 3′ of other Py235 genes. To establish whether or not these two contigs are part of the same gene, primers designed to the 3′-sequence of PY05995 and to the 5′-sequence of PY03534 were used to amplify sequence from gDNA, and gave a single PCR product of the expected size, 392bp (Figure 5A). Sequence analysis and alignment with the gene sequences from the database showed perfect alignment (Figure 5B) and confirmed that the contigs were part of the same single full length Py235 gene, PY05995/PY03534. This conclusion was further confirmed by read pairs of the RNA-Seq data. 153 mates mapped to the end of PY03534 and the beginning of PY05995, and the entire gene could be assembled from the mapping reads (Figure 5C).
Comparison of the sequences of the proteins bound by mAbs 25.37 and 25.77
Sequence data for the Py235 family was aligned and examined for structural features. Partial or full length sequences were compiled from the literature [15], [16], [17], [19], [42], [43] resulting in eleven essentially full length protein sequences, one almost full length but lacking the C-terminus and two others, one representing the N - and the other the C-terminal sequence of one or two further genes. The PY01365, PY01185, and PY05995/PY03534 sequences form a discrete subset of the family with a degree of similarity in pairwise alignment of greater than 80% at the amino acid sequence level (Table 2). None has the Asp-Ile-Asn (DIN) repeats close to the C-terminus of the protein found in some members of the family. A further subgroup contains genes 11, 10, PY03184_E3, PY02104_E5, and PY04438_PY0618_E8.
Tab. 2. Protein sequence identity and similarity. 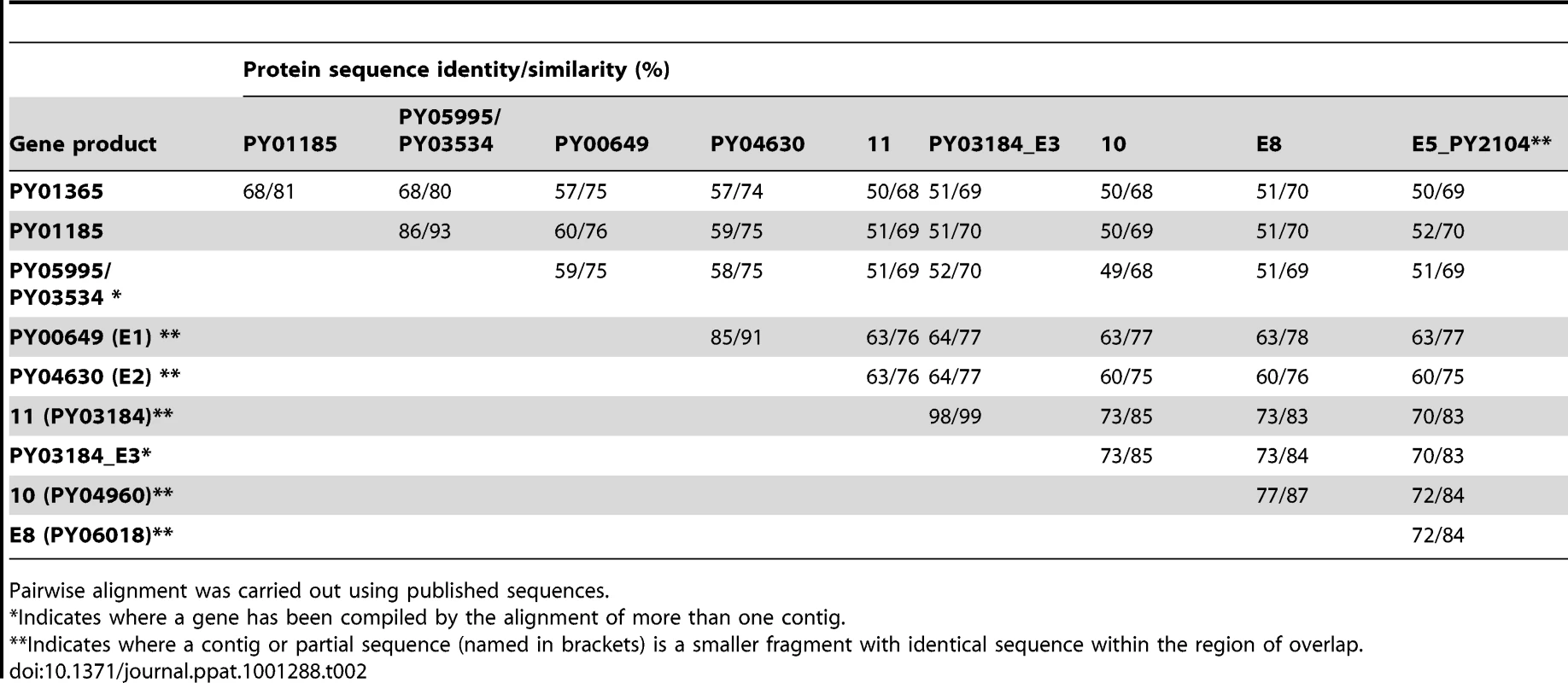
Pairwise alignment was carried out using published sequences. Expression of proteins encoded by the Py235 gene family in the PY01365-KO parasite and their recognition by mAb 25.77
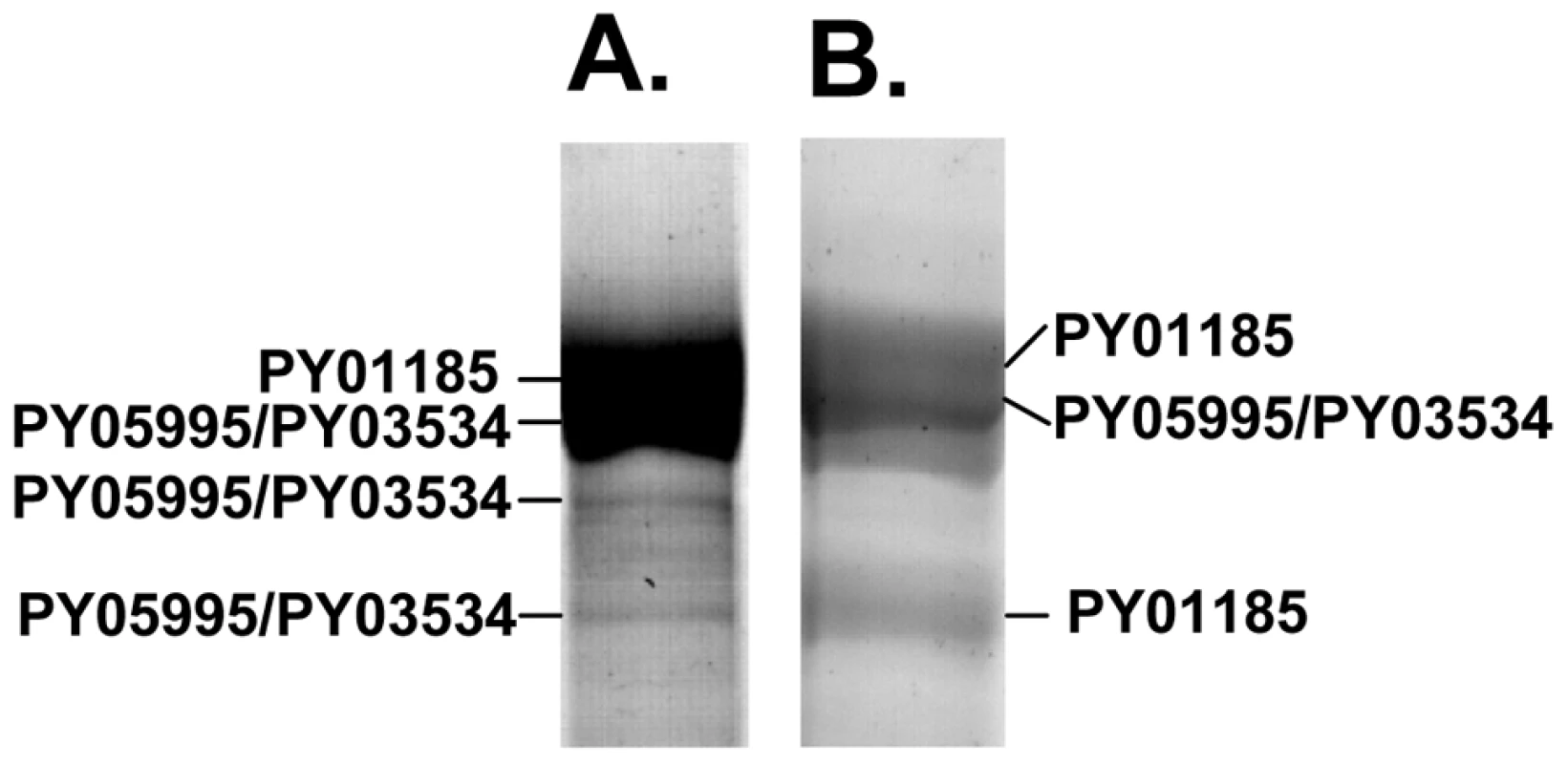
We were interested to identify the Py235 proteins expressed by the PY01365-KO parasite line that were recognised by mAb 25.77 in the absence of Py235EBP-1. Py235 proteins affinity purified using mAb 25.77 from both detergent-solubilised parasite preparations (Figure 6A) and culture supernatant (Figure 6B) from the PY01365-KO parasite line were fractionated on a 5% SDS-PAGE gel and processed for mass spectrometry analysis. MASCOT searches using the peptides and the NCBI database gave significant matches to three Py235 contigs, PY01185, PY05995 and PY03534 (Table 2), mirroring the results obtained with mAb 25.37 and WT parasites. The results suggest that mAb 25.77 has a higher affinity for PY01365, than PY01185 and PY05995/PY03534 protein products, since in the absence of PY01365 mAb 25.77 was able now to detect the other proteins. While mass spectrometry analysis of proteins affinity purified from WT parasites using mAb 25.77 routinely clearly identified PY01365 (Py235EBP-1) (Table 2), peptides derived from PY01185, PY05995 and PY03534 were also present in small amounts (data not shown). Due to the high number of unique peptides required for positive identification of these large proteins, the few unique peptides obtained for PY01185, PY05995 and PY03534 was insufficient.
Identification of a second Py235 protein that binds to the erythrocyte surface and is recognized by mAb 25.37 in extracts of WT parasites and by mAb 25.77 in extracts of the PY01365-KO parasite line
Radiolabeled proteins from WT and PY01365-KO parasites that had been released into the supernatant of in vitro cultures were used in erythrocyte binding assays. Proteins bound to and eluted from the erythrocyte surface were immunoprecipitated using mAbs 25.77 and 25.37. These mAbs recognized single protein bands of approximately 235 kDa in this fraction (Figure 3C and D). We have shown previously that of the several biosynthetically-labeled Py235 proteins released into the supernatant of parasites incubated in vitro, only one binds to the surface of erythrocytes and is recognized by mAb 25.77 (Py235EBP-1). Similarly, we now show that mAb 25.37 also recognizes two Py235 proteins released into culture supernatants (Figure 3C) and that only one of them, the upper of the two bands (Figure 3D), binds to erythrocytes. This upper band has been identified as the protein encoded by the gene, PY01185 (Figure 4A and B), identifying another Py235 protein that binds to the surface of erythrocytes and is recognized by the protective mAb 25.37. This protein has been designated Py235 erythrocyte binding protein-2 (Py235EBP-2), as the second known erythrocyte binding protein from this family and encoded by the PY01185 gene. Our result suggests that in WT parasites there are at least two erythrocyte binding proteins, Py235EBP-1, encoded by PY01365 and Py235EBP-2 encoded by PY01185. In the PY01365-KO parasite line, the erythrocyte binding protein is Py235EBP-2.
By western blotting, similar amounts of Py235 protein were detected by mAbs 25.37 and 25.77 in extracts of both WT and PY01365-KO parasites (Figure S1) indicating that there has been no compensatory change in protein levels such as upregulation of Py235EBP-2.
Expression of Py235 genes in the WT and PY01365-KO parasite lines
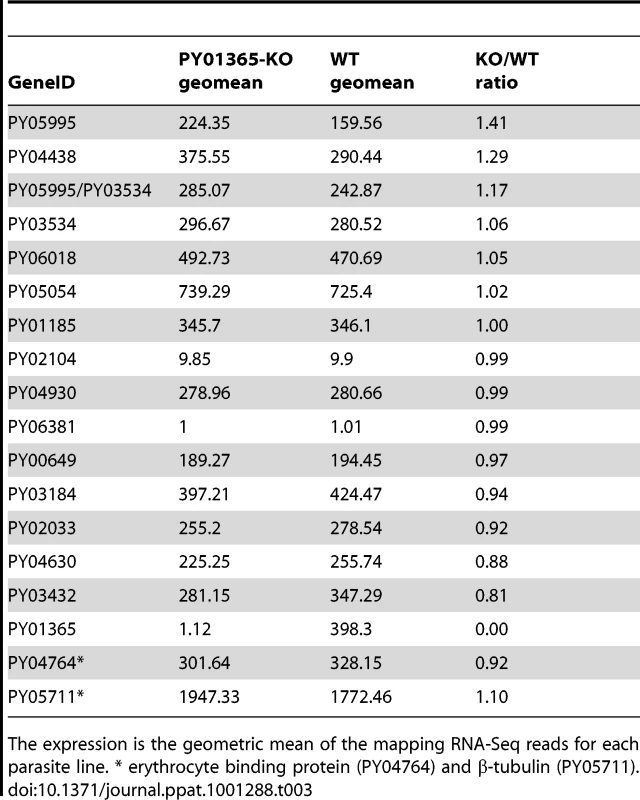
To test the hypothesis that there had been a switch, for example the up-regulation of other members of the Py235 family expressed in the PY01365-KO parasite line, two methods were used: quantitative RT-PCR (qPCR) for some specific members and RNA-Seq for all known members of the family.
qPCR was carried out using primers specific to the genes of interest and to reference genes coding for PyEBL (PY04764), which is expressed at the same developmental stage as Py235 proteins, and the gene for the constitutively expressed protein Pyβ-tubulin (PY05711) (Table 2). Of the 3 genes in the Py235 family that were examined, PY01365 had the lowest transcription level followed by PY05995/PY03534, with PY01185 having the highest transcription level in the WT parasite line (Figure 7a).
Fig. 7. Analysis of Py235 gene family expression. 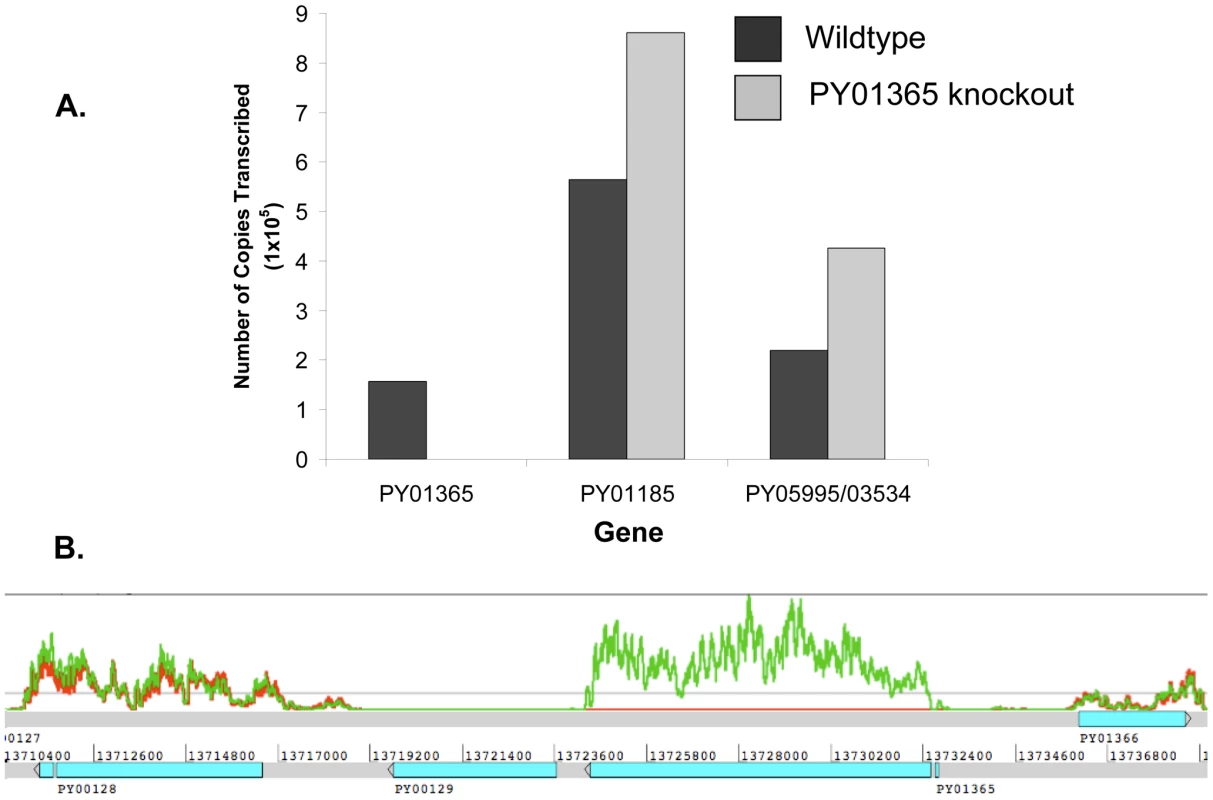
Two reference genes, PyEBL and Pyβ-tubulin had similar amounts relative to each other in both parasite lines. The quantification cycle (Cq) value obtained for PY01365 was similar to those for the negative controls (-RT or no template control), confirming that PY01365 had been deleted from the genome of P. yoelii YM in the PY01365-KO line. Fold change transcriptional calculations between the KO and WT lines were made. There was a fold change increase of 1.5 in the transcription level of PY01185 in the KO line, and for PY05995/PY03534 the fold change increase was 1.9. The measured fold change decrease of PY01365 in the KO line was 350, showing its absence in the PY01365-KO line.
In the RNA-Seq data, the Pearson correlation of all expressed genes between both parasite lines is nearly 0.99 (0.9899136), taking the log of the geometric mean [44] (Figure S2). Also the ratio of expression between the two reference genes of the qPCR (PY04764 and PY05711) was between 0.9 and 1.1 (Table 3). The data show that within the family, in the WT parasite, two genes are very poorly expressed (PY02104 and PY06381). Preliminary analysis of P. yoelii YM genomic DNA suggests that PY06381 is absent from this genome (data not shown). Of the remaining genes all are expressed (geometric means 159.56 to 725.4) with the PY05054 transcript being most abundant. Comparing the WT and PY01365-KO lines, as expected PY01365 is not expressed in the PY01365-KO parasite line but is clearly present and expressed in the WT, confirming the deletion of the PY01365 gene (Figure 7b). For the other members of the family the average ratio of expression in KO versus WT lines was 1.022 and for PY05995 and PY01185 it was 1.406 and 0.999, respectively. This indicates that there was no compensatory significant upregulation of expression of any of the other Py235 genes in the KO parasite.
Similar in vivo growth kinetics are observed for both WT and PY01365-KO parasite lines
To evaluate the phenotypic effect of deleting py235ebp-1 from the genome of the virulent P. yoelii YM line, for example on the age of the host cell invaded or on the course of infection, groups of 5 mice were injected with parasitized erythrocytes. P. yoelii parasites of the KO line showed no changed preference for a particular host cell type relative to WT parasites. All host cell types, both mature erythrocytes and reticulocytes, were invaded, indicating that deletion of the PY01365 gene (py235ebp-1) did not restrict the parasites to invasion of reticulocytes. In mice made reticulocytemic there was no difference in cell preference or growth rate between the two parasite lines (data not shown). Parasite growth kinetics for all groups of mice were very similar and there was no clear difference in the parasite multiplication rate (Figure 8). Only in the group injected with a thousand parasitized erythrocytes was there a significant reduction in parasite growth, when comparing the KO and WT parasite lines (P<0.05). Disruption of the PY01365 gene was not lethal to the parasite and no phenotype was detectable with respect to the age of host cell invaded.
Fig. 8. Parasite growth <i>in vivo.</i> 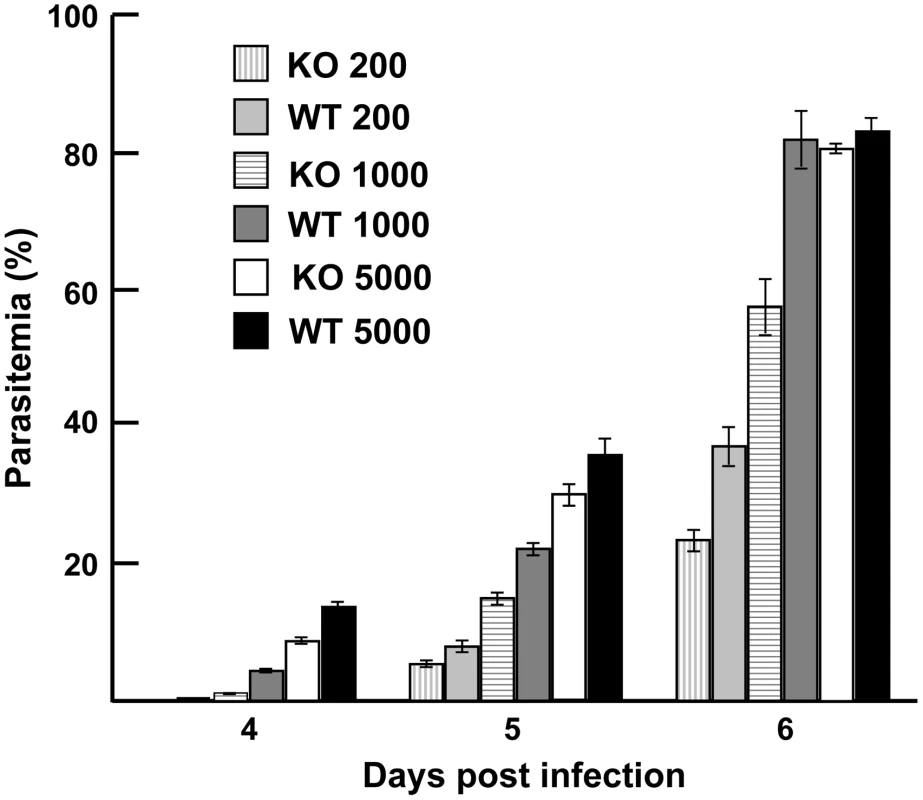
Discussion
We were interested to examine the effect on growth in vivo of deleting the gene that encodes the Py235EBP-1 protein expressed in asexual blood stages of the virulent P. yoelii YM line. Additionally, we wished to examine the expression of other family members in this py235ebp-1-KO parasite line and identify the proteins recognised by the other protective mAb 25.37 in WT parasites.
We have previously shown that although there are several biosynthetically-labeled soluble Py235 proteins released into the supernatant of parasites incubated in vitro, only one of these proteins binds to the surface of erythrocytes and is recognized by the protective mAb 25.77 [24]. We show here that the second protective mAb 25.37 also recognizes two Py235 proteins in the in vitro culture supernatant, namely the products of PY01185 and PY05995/PY03534. We have obtained peptide mass and sequence information from the Py235 proteins either purified from parasitized erythrocytes or from the in vitro culture supernatant, which identifies the corresponding genes as members of the Py235 family. As in the case of the proteins recognized by mAb 25.77, only one of the two Py235 proteins, the upper of the two protein bands, PY01185, binds to erythrocytes. Therefore PY01185 is the gene identified as coding for the erythrocyte binding protein recognized by 25.37, which has been designated Py235 erythrocyte binding protein-2 (Py235EBP-2).
We sought to identify the proteins being expressed by the py235ebp1-KO parasite line that could still be recognized by mAb 25.77. Interestingly, proteins affinity purified from both detergent solubilized parasites and culture supernatant using mAb 25.77 were shown to be Py235EBP-2 (PY01185) and PY05995/PY03534 - the same gene products recognized by mAb 25.37 in WT parasites. Our results clearly show that in WT parasites there are two erythrocyte binding proteins, namely, Py235EBP-1, recognized by the protective mAb 25.77 and encoded by PY01365 and Py235EBP-2 recognized by the protective mAb 25.37 and encoded by PY01185. In the PY01365-KO parasite line, the erythrocyte binding protein recognized by mAb 25.77 is Py235EBP-2. Immunofluorescence studies indicate that each merozoite within a schizont expresses proteins recognized by both protective mAb 25.77 and 25.37 and that proteins recognized by the protective mAbs are expressed by all merozoites; this confirms and extends the conclusions of Narum et al [17]. The location of the proteins still needs to be resolved: in the immunofluorescence studies there was only partial overlap of mAb 25.77 staining and that of other antibodies specific for microneme, dense granule and rhoptry neck proteins.
It has been reported that some P. yoelii lines contain two copies of the PY01365 gene [45]. For the YM line we have analysed, the data indicate that only one copy of the gene is present. This conclusion is based on the Southern blot analysis of separated chromosomes, and of digested gDNA, and is supported by the qPCR and RNA-Seq analyses. However, other members of the gene family with a significant homology to PY01365 may be detected with certain probes at low stringency. This is in agreement with work carried out by Iyer et al [36]. The absence of the PY06381 gene in the YM genome is consistent with an extra gene being detected on chromosome blots of 17X parasites [18].
We examined gene transcription in both the WT and PY01365 parasites by qPCR and as expected, there was no difference in the mRNA levels of PyEBP and Pyβ-tubulin between the WT and KO parasite lines. Of the 3 genes in the py235 family whose transcriptional level was examined, PY01365 had the lowest transcription level followed by PY05995/PY03534 and then PY01185. This result is in contrast to that of Iyer et al [36] who reported that PY01365 was the most highly expressed Py235 family member. This difference may be due to the use of different P. yoelii YM parasite lines with different gene copy numbers. There was a small difference in the level of transcription of PY01185 and PY05995/PY03534 between the WT and KO parasite lines. A more detailed analysis of the Py235 family using RNA-Seq indicated that most of the Py235 gene family is transcribed in these asexual blood stage parasites and there is no change following the deletion of PY01365 other than in the absence of products from this gene. Thus the redundancy in the function of this family must occur at the protein level rather than being reliant on genes being up-regulated; PY01185 protein probably takes over the function of PY01365, although the level and role of other Py235 proteins cannot be addressed. This result is in contrast to the picture in P. falciparum where individual RH protein genes are under epigenetic control and change in expression can lead to change in receptor recognition and host cell invasion pathway. It is possible that the expression of most of the Py235 genes at the same time could contribute to the noted virulence of the YM parasite [46].
Targeted disruption of py235ebp-1 (PY01365) did not lead to a change in the invasion phenotype. Although there was a significant difference in the course of infection in groups of mice injected with 1000 parasitized erythrocytes (P<0.05), there was no significant difference in the groups of mice that received either 200 or 5000 WT and KO parasites. It will be of interest to delete both PY01365 and PY01185, since this double deletion might be expected to result in a much more severe phenotype.
The most puzzling result was that we were unable to detect at a significant level by affinity purification the PY01185 and PY05995/PY03534 proteins in extracts of WT parasites using mAb 25.77 even though the transcripts were present in the parasite at similar or higher levels than that of PY01365. In contrast these proteins were clearly detectable with mAb 25.77 in extracts from the KO parasite line using exactly the same methodology and could be purified from the extract of WT parasites using the second mAb, 25.37. One limitation of MALDI-TOF fingerprinting is that large proteins require a relatively large number of matched peptides to generate a significant MASCOT score. The few peptides unique to PY05995/PY03534 identified on detailed analysis of the peptide mass finger print data were insufficient to establish the presence of PY01185 and PY05995/PY03534 in the proteins extracted from WT parasite using mAb 25.77 and so these proteins were below the level of detection by mass spectrometry. Even if the level of transcription as judged by qPCR and RNA-Seq did not correlate with the level of protein expression, this does not explain the discordant results obtained with the two antibodies. It is possible that the two mAbs may recognize common binding domains in the Py235 proteins but with different affinities, dependent upon the precise amino acid sequence of the antigens. It is conceivable that in the absence of Py235EBP-1 (which may have a higher affinity for mAb 25.77) other members of the family, such as Py235EBP-2, and PY05995/PY03534, can now bind to mAb 25.77.
In the absence of the gene coding for the Py235EBP-1, other family member proteins carry out the same function; this redundancy facilitates binding and erythrocyte invasion. We show that the removal of the erythrocyte ligand expressed by PY01365, in the py235ebp1-KO line allows other expressed members of the py235 gene family to be used. In this instance, there was no change in phenotype with respect to the age of host cell invaded and mediated by the new set of parasite ligands. The invasion phenotype/pathway of a parasite depends not only on the set of ligands expressed or silenced, but also on a molecular/functional hierarchy that determines which of the expressed ligands are used [3], reviewed by Cortes [2]. Several pathways probably coexist in a single parasite so that invasion into different cells such as those in different mammalian hosts or experimentally generated, such as enzyme-treated cells is not all or nothing even though enzymatic treatment goes to completion, (in P. falciparum, [11], [33] and in P. yoelii [25]). Our data would also fit in with the ‘limited space hypothesis’ proposed for the P. falciparum (PfRH family) whereby the position of a particular PfRH ligand at the apex of the merozoite determines which ligand is used for invasion [47], [48]. In this current study, perhaps the absence of Py235EBP-1 allows space for the binding of another member of the Py235 family member, such as Py235EBP-2 (PY01185) to initiate erythrocyte invasion.
Different levels of protein expression in Plasmodium that are not matched by the level of transcription as seen by qPCR or RNA-Seq may arise through post-transcriptional controls of these proteins [34]. The sub-telomeric location of invasion-associated multigene families, including the Py235 family [18], may be important for variant expression, alternatively, new forms of protein regulation at the level of translation [49] may occur. However, none of these mechanisms appears to contribute to our findings because the level of proteins seems to be unchanged.
Analysis of the protein sequences we have identified showed that they are coded by a subset of the Py235 family. For example, they all lack the short repetitive sequence based on the tripeptide, Asp-Ile-Asn (DIN), which is located just N-terminal to the transmembrane domain. The significance of this is obscure but the observation does cast doubt on the validity of using this repeat sequence as a diagnostic marker for the expression of all genes in the Py235 family [37], [38], [50].
In this study we have shown by targeted disruption that the py235ebp-1 (PY01365) gene is not essential to the parasite and the KO did not result in a change in the invasion phenotype with respect to the age of mouse cell invaded or the parasite growth rate. However, deletion of py235ebp-1 did seem to result in an alteration in the level or accessibility of other Py235 protein family members such that they became able to bind to mAb 25.77; the proteins coded by PY01185 and PY05995/PY03534 appeared to compensate for the absence of py235ebp-1 (PY01365). The basis for this new accessibility is obscure, but it is possible that these proteins form complexes either with each other or other proteins, which could make the antibody binding site cryptic. Our result suggests that in WT parasites there are at least two Py235 erythrocyte binding proteins, Py235EBP-1, (recognized by mAb 25.77 and encoded by PY01365) and Py235EBP-2 (recognized by mAb 25.37 and encoded by PY01185). In the PY01365-KO parasite line, the erythrocyte binding protein is changed to Py235EBP-2 recognized by both mAb 25.77 and 25.37 in the absence of Py235EBP-1. In conclusion, in the absence of Py235EBP-1, invasion of erythrocytes by P. yoelii takes place using Py235EBP-2, an alternative Py235 erythrocyte binding protein; modulation of erythrocyte binding appears to occur at the level of the proteins without significant changes in gene expression.
Materials and Methods
Ethics statement
All animal work protocols were reviewed and approved by the Ethical Review Panel of the MRC National Institute for Medical Research and approved and licensed by the UK Home Office as governed by law under the Animals (Scientific Procedures) Act 1986 (Project license 80/1832, Malaria parasite-host interactions). Animals were handled in strict accordance with the “Code of Practice Part 1 for the housing and care of animals (21/03/05)” available at http://www.homeoffice.gov.uk/science-research/animal-research/. The numbers of animals used was the minimum consistent with obtaining scientifically valid data. The experimental procedures were designed to minimize the extent and duration of any harm and included predefined clinical and parasitological endpoints to avoid unnecessary suffering.
Animals and parasites
Female BALB/c mice, with an average weight of 18 to 22 g and 6 to 8 weeks old were obtained from the specific pathogen-free unit at the MRC National Institute for Medical Research. The cloned virulent YM line of P. yoelii [46], [51], was obtained from Dr. David Walliker, University of Edinburgh. Parasites were passaged no more than five times in the same mouse strain, before returning to a fresh stabilate.
Alignment of the py235 gene and protein family sequences
All full-length and partial py235 gene sequences identified in the database (www.PlasmoDb.org) were retrieved and Clustal X 1.81 [52] was used to align them. Areas of sequence similarity and difference were identified at both the amino acid and nucleotide levels and analysed using Bioedit [53]. The sequence information was then used to design gene-specific reagents, and compare features of the individual sequences.
Cloning of targeting construct
PY01365 (py235ebp-1) gene sequences were amplified from P. yoelii YM line genomic DNA using gene specific primers. A 500bp fragment from the 5′UTR of PY01365 (Fragment A) was amplified with forward primer, (restriction sites are underlined), 5′-gccgggggcccACTATAACACTAATTATTTATTATAAAACG-3′ and reverse primer, 5′-gccggaagcttATGTATGTATCTATGTATGCATGCATG-3′. A region from the 3′ coding sequence of PY01365 (Fragment B) was amplified with forward primer, 5′-gccgggaattcACGAACTCACTCGAATACAAAGTCGTTTAG-3′ and reverse primer, 5′-ggcggtctagaATAATTTTTATATTTTGCATCATCATTATTATTATGG-3′. Fragment A PCR product was digested with ApaI and Hind III and Fragment B PCR product was digested with EcoRI and XbaI. The targeting construct was made by the cloning of fragments A and B sequentially into the plasmid vector pBSDHFR, in which the Toxoplasma gondii dihydrofolate reductase/thymidylate synthase gene (DHFR/TS) is flanked by the upstream and downstream control elements from P. berghei DHFR/TS. First, Fragment A was cloned into pBSDHFR that had been digested with Apa1 and Hind III and the inserted DNA sequence was verified by sequencing. This construct was digested with EcoRI and XbaI, and then Fragment B cloned into it, and its sequence verified. The final targeting construct was digested with the enzymes ApaI and XbaI and inserted by double homologous recombination into the PY01365 gene following transfection of the virulent YM line of P. yoelii.
Transfection of parasites
Transfection of parasites was carried out essentially as described previously [54], [55]. Briefly, erythrocytes containing late stage parasites, were harvested at 20 to 25% parasitaemia and schizonts were purified by centrifugation for 25 min at 600 g at room temperature (RT) on a 55% Nycodenz (Nycoprep) cushion (NYCOMED Pharma AS). 5×107 schizonts were mixed with 90 µl AMAXA nucleofactor T-cell solution (plus supplements) and 5 µg of targeting construct DNA was added. These parasites were transfected using AMAXA Nucleofector programme U33. Immediately, 100 µl of RPMI 1640 medium containing 20% foetal calf serum (FCS) was added to the transfected parasites and the suspension injected intravenously (i.v.) into the tail vein of a single mouse. Electroporation of parasites with targeting construct and injection into individual mice was carried out twice independently using the above conditions.
Twenty seven hours post injection, day (D)1, and on D3 and D4 mice were treated with 1 mg kg −1 pyrimethamine, intraperitoneally (i.p.). From day 2 post injection, pyrimethamine was administered continuously in the drinking water at a final concentration of 70 µg/ml. Three sets of control mice were set up. One set was injected with transfected schizonts as above but did not receive any drug treatment, a second set was injected with schizonts in T-cell solution (without DNA or electroporation) and was drug treated as above, and the third set of controls was as the second set but without subsequent drug treatment. The parasitaemia of each set of mice was monitored daily. Stabilates of drug resistant parasites were made and stored in liquid nitrogen, additionally the parasites were used to infect naïve mice (2×107–5×107 parasitized erythrocytes, administered i.v.) for a second round of drug pressure. These mice were given pyrimethamine continuously in their drinking water as above. Parasites were allowed to grow sufficiently for samples to be taken for analysis and for stabilates to be made. After verification of the pyrimethamine-resistant parasites by PCR and Southern blot analysis, the transgenic parasite line was cloned by limiting dilution using 10 mice injected i.v so that each inoculum contained a maximum of one parasite. Four clones were obtained and genotyped.
Southern blot analysis
Genomic DNA (gDNA) was isolated from leukocyte-depleted, Percoll-purified late stage WT and PY01365-KO parasitized erythrocytes lysed in a buffer containing SDS. The DNA was phenol chloroform extracted and precipitated with ethanol. Various restriction enzymes were used to digest the gDNA and samples were resolved by electrophoresis on a 0.8% agarose gel and transferred onto Hybond N+ in 7.5 mM NaOH overnight. The filter was neutralized in 2 x SSC and UV cross-linked prior to hybridisation [13]. DNA probes used were: Fragment B (see above), and a 739 bp TgDHFR DNA sequence PCR amplified from plasmid DNA using the forward primer: 5′-GCCGGGATCCCATCATTCGACCCTGATATATATAACGA-3′ and the reverse primer: 5′-GCCGGGAATTCATTCTAAAAATTCATAGTAATAAGGTG-3′.
For an estimation of the number of copies of PY01365 in the P. yoelii genome, WT gDNA was digested with various restriction enzymes in double digest reactions and the samples transferred onto nylon filters as above. DNA probes used were Fragment B, (as above) and Fragment C derived from the 5′ coding sequence of PY01365, amplified using the forward primer: 5′ - ATCATCTGCACCATCATTCGAC-3′ and the reverse primer: 5′ - CAATATGGAATCTAATAGACG-3′. DNA probes were labelled using DECAprime II labelling system (Ambion) and hybridized to the filters.
Pulse field gel electrophoresis
Chromosomes from leukocyte–depleted, Percoll-purified late stage WT and PY01365-KO parasites were fractionated by contour-clamped homogeneous electric field (CHEF) electrophoresis as described [18]. The gel was blotted and hybridized sequentially with three different probes. First a probe that binds to the 5′ coding region of py235ebp-1 (Fragment C); second, a probe that binds to the 3′ coding region of py235ebp-1 (Fragment B); and finally, a probe that binds to the 3′ UTR of DHFR/TS.
Protein purification, mass spectrometry analysis and bioinformatics
Erythrocytes were harvested from BALB/c mice infected with P. yoelii YM WT or PY01365-KO lines and depleted of leukocytes [23]. Py235 protein from both supernatant and detergent solubilized parasite preparations was purified on separate columns by affinity chromatography using mAb 25.77 as described previously [20]. Affinity chromatography using mAb 25.37 was also carried out as above but only using WT parasites.
Proteins eluted from the affinity columns were subjected to SDS-PAGE under reducing conditions on a 5% polyacrylamide gel and visualised using colloidal blue stain (Novex). Bands were excised, reduced, alkylated and digested with trypsin [56]. Peptide mass fingerprinting was carried out using a Reflex III MALDI-ToF mass spectrometer (Bruker Daltonik, Germany). The peptide mass fingerprints were used to query sequences in both the rodent malaria database [15] and the general non-redundant database at the National Centre for Biotechnology Information, (NCBI; http://www.ncbi.nlm.nih.gov). The gene accession numbers identified were used to carry out further searches of the NCBI database to obtain full gene sequence information.
PCR amplification of a py235 gene to link two contigs
Two of the three py235 sequences identified by mass spectrometry analysis using peptides from 25.37-affinity purified protein, did not correspond to full-length genes, instead they corresponded to two contigs, PY05995 and PY03534. A gene specific forward primer was designed based on DNA sequences from the last 200 nucleotides of the 3′ coding sequence of PY05995 (Forward primer 5′-GAAATGAAACGTACAAAAGATGACATC-3′), and a reverse primer was designed based on the first 200 nucleotides of the 5′ coding sequence of PY03534 (Reverse primer 5′-CTGTATATGATTGTTCTATTAAATTAC-3′). Using WT gDNA, PCR amplification was carried out using Pfu ultra DNA polymerase (Strategen). The PCR product was directly sequenced (Cogenics), analysed, aligned and assembled with the PY05995 and PY03534 contigs to create a single py235 gene, using Bioedit [53].
Quantitative real time RT-PCR (qPCR)
Total RNA was prepared [57] from leukocyte-depleted, Percoll-purified late stage WT and PY01365-KO parasitized erythrocytes using Trizol (Invitrogen Life Technologies). RNA samples were first treated with RNase-Free DNase I (Quiagen) and cleaned up using RNeasy MiniElute to remove contaminating gDNA. First strand cDNA was synthesized using 1 µg RNA, AMV reverse transcriptase (RT) and random primers according to the manufacturer's instructions (Promega). RT-PCR amplification using the synthesized cDNA was carried out and samples amplified without the addition of RT were included as controls.
Gene specific primers were designed for the Py235 genes of interest PY01365, PY01885, and PY05995/PY03534 and the reference genes, P. yoelii erythrocyte binding protein (PyEBL) and Pyβ-tubulin (Table S1). Short regions of the genes (150bp–193bp) were amplified using gDNA extracted from purified late stage WT P. yoelii YM parasites. cDNA was used as a template to PCR amplify Pyβ-tubulin. PCR products were cloned into TA vector, and clones containing inserts were identified by PCR and the insert DNA verified by sequencing.
qPCR was carried according to the MIQE guidelines [58]. qPCR reactions (25 µl) were set up in triplicates in Absolute SYBR Green mix (containing Thermo-Start, DNA polymerase and ROX Dye) (Abgene), 0.2 µM each primer and 1 µl cDNA and amplified in an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Cycle conditions were 50°C, 2 min; 95°C, 15 min; 40 cycles of 95°C, 15 s; 60°C, 1 min. gDNA was used to check that amplification efficiencies of primers were comparable and plasmids used to generate standard curves were included in each assay. Transcript levels for each gene in the WT and PY01365-KO parasite lines were quantified and normalized with Pyβ-tubulin and PyEBL. For analysis, cDNA prepared from two independent RNA samples was used.
RNA-Seq analysis
Parasites were purified using a MACS type-D depletion column with a SuperMACS II magnetic separator (Miltenyi Biotec GmbH) [59]. RNA from the purified WT and PY01365-KO parasite lines, prepared as described above, was sequenced and analyzed as described [44]. Briefly, the RNA of both samples was depleted of ribosomal RNAs with exonuclease and sequenced on an Illumina GA II platform using the Illumina RNA-seq protocol. Of the approximately 61 million 76-base pair paired-end reads per run, around 95% percent mapped with SSAHA2 [60] against the P. yoelii 17XNL genome sequence (GeneDB: ftp: ftp://ftp.sanger.ac.uk/pub/pathogens/P_yoelii/June_2010/). From the coverage of the uniquely mapped Illumina reads, a perl script was used to calculate the geometric mean for expression of each predicted gene, representing the level of messenger RNA. We compared the expression in both samples by obtaining the ratio of expression values for each gene.
Immunofluorescent antibody (IFA) assay
IFA assay of Py235 protein expression in WT and PY01365-KO parasites was carried out using mAb 25.77 on formaldehyde fixed parasitized erythrocytes, followed by Alexa Fluor 488-conjugated affinity purified goat anti-mouse IgG (Molecular Probes). In colocalization studies EBL was detected using rabbit antibodies provided by Dr Osamu Kaneko. Alternatively the slide was first probed with mAbs specific for either RON4 (48F8) or for AMA1 (45B1) [39] followed by Alexa Fluor594 congugated secondary antibody. After washing, this was followed by incubation with mAb 25.77 directly congugated to Alexa Fluor488. In a separate assay, Alexa Fluor 488-conjugated 25.77 mAb was used to probe thin blood smears of mixed stage WT P. yoelii YM parasites, followed by Alexa Fluor 594-conjugated 25.37 mAb in a dual labeling experiment [61]. mAbs were labeled with Alexa Fluor (Molecular Probes) succinimidyl esters according to the manufacturer's instructions. The labeled antibodies were separated from excess labelling reagent by gel filtration on PD-10 columns (Amersham Pharmacia), eluted using PBS/1% BSA. All slides were examined and images captured on an Axioplan 2 imaging system (Zeiss).
Western blotting
Percoll-purified late stage parasites were solubilized under reducing conditions in a buffer containing DTT, resolved by SDS-PAGE on a 5% Bis-Tris polyacrylamide gel and transferred onto nitrocellulose membrane. Primary antibodies (at 10 µg/ml) were used to immunostain the membrane and were detected by incubation with HRP-congugated goat anti-mouse IgG (H+ L) antibody (Bio-Rad) and the ECL Western Blotting detection reagent (GE Healthcare/Amersham). Protein bands were visualized on a Kodak BioMax MR film. The blots were stripped with Restore PLUS according to the manufacturer's instructions and then probed with mAb 48F8 to detect PyRON4 [41] as a control for protein loading.
Erythrocyte binding assay and immunoprecipitation
[35S]methionine/cysteine (Promix, GE Healthcare, Little Chalfont, UK)) radiolabeled proteins from P. yoelii YM (WT and PY01365-KO) either released into culture supernatant, extracted in a buffer containing 0.5% (w/v) sodium deoxycholate, or eluted from erythrocytes were immunoprecipitated using mAbs 25.77 and 25.37, hyperimmune serum (HIS) and normal mouse serum (NMS). The erythrocyte binding assay and immunoprecipitations were carried out as described previously [24].
Course of parasite infection in vivo
Groups of 5 Balb/c mice were infected i.v. on day 0 with either 200, 1000, or 5000 WT or PY01365-KO parasitized erythrocytes [62]. Blood smears from each mouse were made daily from D3, stained with Giemsa's reagent and infected cells counted to monitor the course of infection.
Supporting Information
Zdroje
1. World Health Organization
2009
World Malaria Report 2009. Available: http://wwwwho.int/malaria/publications/atoz/9789241563901/en/indexhtml
2. Cortes
A
2008
Switching Plasmodium falciparum genes on and off
for erythrocyte invasion.
Trends Parasitol
24
517
524
3. Baum
J
Maier
AG
Good
RT
Simpson
KM
Cowman
AF
2005
Invasion by P. falciparum merozoites suggests a
hierarchy of molecular interactions.
PLoS Pathog
1
e37
4. Gaur
D
Mayer
DC
Miller
LH
2004
Parasite ligand-host receptor interactions during invasion of
erythrocytes by Plasmodium merozoites.
Int J Parasitol
34
1413
1429
5. Iyer
J
Gruner
AC
Renia
L
Snounou
G
Preiser
PR
2007
Invasion of host cells by malaria parasites: a tale of two
protein families.
Mol Microbiol
65
231
249
6. Baum
J
Papenfuss
AT
Baum
B
Speed
TP
Cowman
AF
2006
Regulation of apicomplexan actin-based motility.
Nat Rev Microbiol
4
621
628
7. Kappe
SH
Buscaglia
CA
Bergman
LW
Coppens
I
Nussenzweig
V
2004
Apicomplexan gliding motility and host cell invasion: overhauling
the motor model.
Trends Parasitol
20
13
16
8. Holder
AA
Freeman
RR
1981
Immunization against blood-stage rodent malaria using purified
parasite antigens.
Nature
294
361
364
9. Keen
J
Holder
A
Playfair
J
Lockyer
M
Lewis
A
1990
Identification of the gene for a Plasmodium
yoelii rhoptry protein. Multiple copies in the parasite
genome.
Mol Biochem Parasitol
42
241
246
10. Galinski
MR
Medina
CC
Ingravallo
P
Barnwell
JW
1992
A reticulocyte-binding protein complex of Plasmodium
vivax merozoites.
Cell
69
1213
1226
11. Rayner
JC
Vargas-Serrato
E
Huber
CS
Galinski
MR
Barnwell
JW
2001
A Plasmodium falciparum homologue of
Plasmodium vivax reticulocyte binding protein (PvRBP1)
defines a trypsin-resistant erythrocyte invasion pathway.
J Exp Med
194
1571
1581
12. Taylor
HM
Grainger
M
Holder
AA
2002
Variation in the expression of a Plasmodium
falciparum protein family implicated in erythrocyte
invasion.
Infect Immun
70
5779
5789
13. Taylor
HM
Triglia
T
Thompson
J
Sajid
M
Fowler
R
2001
Plasmodium falciparum homologue of the genes for
Plasmodium vivax and Plasmodium yoelii
adhesive proteins, which is transcribed but not translated.
Infect Immun
69
3635
3645
14. Borre
MB
Owen
CA
Keen
JK
Sinha
KA
Holder
AA
1995
Multiple genes code for high-molecular-mass rhoptry proteins of
Plasmodium yoelii.
Mol Biochem Parasitol
70
149
155
15. Carlton
JM
Angiuoli
SV
Suh
BB
Kooij
TW
Pertea
M
2002
Genome sequence and comparative analysis of the model rodent
malaria parasite Plasmodium yoelii yoelii.
Nature
419
512
519
16. Khan
SM
Jarra
W
Bayele
H
Preiser
PR
2001
Distribution and characterisation of the 235 kDa rhoptry
multigene family within the genomes of virulent and avirulent lines of
Plasmodium yoelii.
Mol Biochem Parasitol
114
197
208
17. Narum
DL
Green
JL
Ogun
SA
Holder
AA
2001
Sequence diversity and antigenic polymorphism in the
Plasmodium yoelii p235 high molecular mass rhoptry
proteins and their genes.
Mol Biochem Parasitol
112
193
200
18. Owen
CA
Sinha
KA
Keen
JK
Ogun
SA
Holder
AA
1999
Chromosomal organisation of a gene family encoding rhoptry
proteins in Plasmodium yoelii.
Mol Biochem Parasitol
99
183
192
19. Keen
JK
Sinha
KA
Brown
KN
Holder
AA
1994
A gene coding for a high-molecular mass rhoptry protein of
Plasmodium yoelii.
Mol Biochem Parasitol
65
171
177
20. Ogun
SA
Howell
SA
Taylor
HM
Holder
AA
2006
A member of the py235 gene family of Plasmodium
yoelii encodes an erythrocyte binding protein recognised by a
protective monoclonal antibody.
Mol Biochem Parasitol
147
140
143
21. Freeman
RR
Trejdosiewicz
AJ
Cross
GA
1980
Protective monoclonal antibodies recognising stage-specific
merozoite antigens of a rodent malaria parasite.
Nature
284
366
368
22. Freeman
RR
Holder
AA
Trejdosiewicz
AJ
Cross
GAM
1980
Monoclonal antibodies against the rodent malaria parasite,
Plasmodium yoelii.
Bossche
Hvd
Biochemistry of Parasites and Host-parasite Relationships:Host-Invader
Interplay; 3rd International Symposium Proceedings
Amsterdam
Elsevier/North Holland
121
124
23. Ogun
SA
Holder
AA
1994
Plasmodium yoelii: brefeldin A-sensitive
processing of proteins targeted to the rhoptries.
Exp Parasitol
79
270
278
24. Ogun
SA
Holder
AA
1996
A high molecular mass Plasmodium yoelii rhoptry
protein binds to erythrocytes.
Mol Biochem Parasitol
76
321
324
25. Ogun
SA
Scott-Finnigan
TJ
Narum
DL
Holder
AA
2000
Plasmodium yoelii: effects of red blood cell
modification and antibodies on the binding characteristics of the 235-kDa
rhoptry protein.
Exp Parasitol
95
187
195
26. Galinski
MR
Barnwell
JW
1996
Plasmodium vivax: Merozoites, invasion of
reticulocytes and considerations for malaria vaccine
development.
Parasitol Today
12
20
29
27. Chitnis
CE
Miller
LH
1994
Identification of the erythrocyte binding domains of
Plasmodium vivax and Plasmodium
knowlesi proteins involved in erythrocyte
invasion.
J Exp Med
180
497
506
28. Horuk
R
Chitnis
CE
Darbonne
WC
Colby
TJ
Rybicki
A
1993
A receptor for the malarial parasite Plasmodium
vivax: the erythrocyte chemokine receptor.
Science
261
1182
1184
29. Dolan
SA
Miller
LH
Wellems
TE
1990
Evidence for a switching mechanism in the invasion of
erythrocytes by Plasmodium falciparum.
J Clin Invest
86
618
624
30. Gaur
D
Furuya
T
Mu
J
Jiang
LB
Su
XZ
2006
Upregulation of expression of the reticulocyte homology gene 4 in
the Plasmodium falciparum clone Dd2 is associated with a
switch in the erythrocyte invasion pathway.
Mol Biochem Parasitol
145
205
215
31. Stubbs
J
Simpson
KM
Triglia
T
Plouffe
D
Tonkin
CJ
2005
Molecular mechanism for switching of P. falciparum
invasion pathways into human erythrocytes.
Science
309
1384
1387
32. Hayton
K
Gaur
D
Liu
A
Takahashi
J
Henschen
B
2008
Erythrocyte binding protein PfRH5 polymorphisms determine
species-specific pathways of Plasmodium falciparum
invasion.
Cell Host Microbe
4
40
51
33. Dolan
SA
Proctor
JL
Alling
DW
Okubo
Y
Wellems
TE
1994
Glycophorin B as an EBA-175 independent Plasmodium
falciparum receptor of human erythrocytes.
Mol Biochem Parasitol
64
55
63
34. Cortes
A
Carret
C
Kaneko
O
Yim Lim
BY
Ivens
A
2007
Epigenetic silencing of Plasmodium falciparum
genes linked to erythrocyte invasion.
PLoS Pathog
3
e107
35. Gao
X
Yeo
KP
Aw
SS
Kuss
C
Iyer
JK
2008
Antibodies targeting the PfRH1 binding domain inhibit invasion of
Plasmodium falciparum merozoites.
PLoS Pathog
4
e1000104
36. Iyer
JK
Amaladoss
A
Genesan
S
Preiser
PR
2007
Variable expression of the 235 kDa rhoptry protein of
Plasmodium yoelii mediate host cell adaptation and
immune evasion.
Mol Microbiol
65
333
346
37. Preiser
PR
Jarra
W
1998
Plasmodium yoelii: differences in the
transcription of the 235-kDa rhoptry protein multigene family in lethal and
nonlethal lines.
Exp Parasitol
89
50
57
38. Preiser
PR
Jarra
W
Capiod
T
Snounou
G
1999
A rhoptry-protein-associated mechanism of clonal phenotypic
variation in rodent malaria.
Nature
398
618
622
39. Narum
DL
Ogun
SA
Thomas
AW
Holder
AA
2000
Immunization with parasite-derived apical membrane antigen 1 or
passive immunization with a specific monoclonal antibody protects BALB/c
mice against lethal Plasmodium yoelii yoelii YM blood-stage
infection.
Infect Immun
68
2899
2906
40. Otsuki
H
Kaneko
O
Thongkukiatkul
A
Tachibana
M
Iriko
H
2009
Single amino acid substitution in Plasmodium
yoelii erythrocyte ligand determines its localization and
controls parasite virulence.
Proc Natl Acad Sci U S A
106
7167
7172
41. Narum
DL
Nguyen
V
Zhang
Y
Glen
J
Shimp
RL
2008
Identification and characterization of the Plasmodium
yoelii PyP140/RON4 protein, an orthologue of Toxoplasma
gondii RON4, whose cysteine-rich domain does not protect
against lethal parasite challenge infection.
Infect Immun
76
4876
4882
42. Green
JL
Holder
AA
2000
Structure of the E8 gene encoding a high molecular mass rhoptry
protein of Plasmodium yoelii.
Mol Biochem Parasitol
110
167
169
43. Sinha
KA
Keen
JK
Ogun
SA
Holder
AA
1996
Comparison of two members of a multigene family coding for
high-molecular mass rhoptry proteins of Plasmodium
yoelii.
Mol Biochem Parasitol
76
329
332
44. Otto
TD
Wilinski
D
Assefa
S
Keane
TM
Sarry
LR
2010
New insights into the blood-stage transcriptome of
Plasmodium falciparum using RNA-Seq.
Mol Microbiol
76
12
24
45. Iyer
JK
Fuller
K
Preiser
PR
2006
Differences in the copy number of the py235 gene family in
virulent and avirulent lines of Plasmodium
yoelii.
Mol Biochem Parasitol
150
186
191
46. Yoeli
M
Hargreaves
B
Carter
R
Walliker
D
1975
Sudden increase in virulence in a strain of Plasmodium
berghei yoelii.
Ann Trop Med Parasitol
69
173
178
47. Duraisingh
MT
Triglia
T
Ralph
SA
Rayner
JC
Barnwell
JW
2003
Phenotypic variation of Plasmodium falciparum
merozoite proteins directs receptor targeting for invasion of human
erythrocytes.
EMBO J
22
1047
1057
48. Triglia
T
Duraisingh
MT
Good
RT
Cowman
AF
2005
Reticulocyte-binding protein homologue 1 is required for sialic
acid-dependent invasion into human erythrocytes by Plasmodium
falciparum.
Mol Microbiol
55
162
174
49. Amulic
B
Salanti
A
Lavstsen
T
Nielsen
MA
Deitsch
KW
2009
An upstream open reading frame controls translation of var2csa, a
gene implicated in placental malaria.
PLoS Pathog
5
e1000256
50. Preiser
PR
Khan
S
Costa
FT
Jarra
W
Belnoue
E
2002
Stage-specific transcription of distinct repertoires of a
multigene family during Plasmodium life cycle.
Science
295
342
345
51. Walliker
D
Sanderson
A
Yoeli
M
Hargreaves
BJ
1976
A genetic investigation of virulence in a rodent malaria
parasite.
Parasitology
72
183
194
52. Chenna
R
Sugawara
H
Koike
T
Lopez
R
Gibson
TJ
2003
Multiple sequence alignment with the Clustal series of
programs.
Nucleic Acids Res
31
3497
3500
53. Hall
TA
1999
BioEdit: a user-friendly biological sequence alignment editor and
analysis program for Windows 95/98/NT.
Nucleic Acids Symp Ser
41
95
98
54. Jongco
AM
Ting
LM
Thathy
V
Mota
MM
Kim
K
2006
Improved transfection and new selectable markers for the rodent
malaria parasite Plasmodium yoelii.
Mol Biochem Parasitol
146
242
250
55. Janse
CJ
Ramesar
J
Waters
AP
2006
High-efficiency transfection and drug selection of genetically
transformed blood stages of the rodent malaria parasite Plasmodium
berghei.
Nat Protoc
1
346
356
56. Shevchenko
A
Jensen
ON
Podtelejnikov
AV
Sagliocco
F
Wilm
M
1996
Linking genome and proteome by mass spectrometry: large-scale
identification of yeast proteins from two dimensional gels.
Proc Natl Acad Sci U S A
93
14440
14445
57. Kyes
S
Pinches
R
Newbold
C
2000
A simple RNA analysis method shows var and rif multigene family
expression patterns in Plasmodium
falciparum.
Mol Biochem Parasitol
105
311
315
58. Bustin
SA
Benes
V
Garson
JA
Hellemans
J
Huggett
J
2009
The MIQE guidelines: minimum information for publication of
quantitative real-time PCR experiments.
Clin Chem
55
611
622
59. Le Roch
KG
Johnson
JR
Florens
L
Zhou
Y
Santrosyan
A
2004
Global analysis of transcript and protein levels across the
Plasmodium falciparum life cycle.
Genome Res
14
2308
2318
60. Ning
Z
Cox
AJ
Mullikin
JC
2001
SSAHA: a fast search method for large DNA
databases.
Genome Res
11
1725
1729
61. Dluzewski
AR
Ling
IT
Hopkins
JM
Grainger
M
Margos
G
2008
Formation of the food vacuole in Plasmodium
falciparum: a potential role for the 19 kDa fragment of
merozoite surface protein 1 (MSP119).
PLoS One
3
e3085
62. Ling
IT
Ogun
SA
Holder
AA
1994
Immunization against malaria with a recombinant
protein.
Parasite Immunol
16
63
67
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy