-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
Mucosal transmission of HIV is inefficient. The virus must breach physical barriers before it infects mucosal CD4+ T cells. Low-level viral replication occurs initially in mucosal CD4+ T cells, but within days high-level replication occurs in Peyer's patches, the gut lamina propria and mesenteric lymph nodes. Understanding the early events in HIV transmission may provide valuable information relevant to the development of an HIV vaccine. The viral quasispecies in a donor contracts through a genetic bottleneck in the recipient, such that, in low-risk settings, infection is frequently established by a single founder virus. Early-transmitting viruses in subtypes A and C mucosal transmission tend to encode gp120s with reduced numbers of N-linked glycosylation sites at specific positions throughout the V1-V4 domains, relative to typical chronically replicating isolates in the donor quasispecies. The transmission advantage gained by the absence of these N-linked glycosylation sites is unknown. Using primary α4β7+/CD4+ T cells and a flow-cytometry based steady-state binding assay we show that the removal of transmission-associated N-linked glycosylation sites results in large increases in the specific reactivity of gp120 for integrin - α4β7. High-affinity for integrin α4β7, although not found in many gp120s, was observed in early-transmitting gp120s that we analyzed. Increased α4β7 affinity is mediated by sequences encoded in gp120 V1/V2. α4β7-reactivity was also influenced by N-linked glycosylation sites located in C3/V4. These results suggest that the genetic bottleneck that occurs after transmission may frequently involve a relative requirement for the productive infection of α4β7+/CD4+ T cells. Early-transmitting gp120s were further distinguished by their dependence on avidity-effects to interact with CD4, suggesting that these gp120s bear unusual structural features not present in many well-characterized gp120s derived from chronically replicating viruses. Understanding the structural features that characterize early-transmitting gp120s may aid in the design of an effective gp120-based subunit vaccine.
Published in the journal: The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission. PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001301
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001301Summary
Mucosal transmission of HIV is inefficient. The virus must breach physical barriers before it infects mucosal CD4+ T cells. Low-level viral replication occurs initially in mucosal CD4+ T cells, but within days high-level replication occurs in Peyer's patches, the gut lamina propria and mesenteric lymph nodes. Understanding the early events in HIV transmission may provide valuable information relevant to the development of an HIV vaccine. The viral quasispecies in a donor contracts through a genetic bottleneck in the recipient, such that, in low-risk settings, infection is frequently established by a single founder virus. Early-transmitting viruses in subtypes A and C mucosal transmission tend to encode gp120s with reduced numbers of N-linked glycosylation sites at specific positions throughout the V1-V4 domains, relative to typical chronically replicating isolates in the donor quasispecies. The transmission advantage gained by the absence of these N-linked glycosylation sites is unknown. Using primary α4β7+/CD4+ T cells and a flow-cytometry based steady-state binding assay we show that the removal of transmission-associated N-linked glycosylation sites results in large increases in the specific reactivity of gp120 for integrin - α4β7. High-affinity for integrin α4β7, although not found in many gp120s, was observed in early-transmitting gp120s that we analyzed. Increased α4β7 affinity is mediated by sequences encoded in gp120 V1/V2. α4β7-reactivity was also influenced by N-linked glycosylation sites located in C3/V4. These results suggest that the genetic bottleneck that occurs after transmission may frequently involve a relative requirement for the productive infection of α4β7+/CD4+ T cells. Early-transmitting gp120s were further distinguished by their dependence on avidity-effects to interact with CD4, suggesting that these gp120s bear unusual structural features not present in many well-characterized gp120s derived from chronically replicating viruses. Understanding the structural features that characterize early-transmitting gp120s may aid in the design of an effective gp120-based subunit vaccine.
Introduction
Despite widely available prevention modalities against HIV transmission, 2.6 million individuals are newly infected with HIV every year. Thus, there exists an urgent need for an effective HIV vaccine. A number of studies that have focused on the earliest events in HIV transmission raise the possibility that new strategies for an effective vaccine immunogen can be developed.
HIV transmission following mucosal exposure is inefficient[1]. The virus must first breach physical barriers in the mucosa, and then infect suitable target cells. In one study of heterosexual couples discordant for HIV infection, the frequency of transmissions per coital act averaged ∼0.01 [2]. One can therefore infer that, following deposition on the mucosal surface of the genital tract, HIV very frequently fails to establish infection. Both human and an SIV/macaque model studies indicate that during the first days of infection, termed the “eclipse phase”, low levels of viral replication occur, primarily in suboptimally activated memory CD4+ T cells in the genital mucosa[3], [4], [5], [6], [7], [8]. Although these cells are metabolically active, they do not express classical activation markers[4], [7]. Subsequently, HIV-1 infects fully activated memory CD4+ T cells. These events represent a critical point in transmission because they lead to high-level replication and the migration of virus into draining lymphoid tissue and ultimately gut-associated lymphoid tissue (GALT) where activated CD4+ T cells are plentiful, viral replication amplifies and the high level viremia that is associated with acute infection is established[9]. The best opportunity to prevent or abort establishment of HIV infection is likely during the eclipse phase following transmission, before HIV-1 migrates into the GALT.
A striking feature of sexual transmission of HIV is the extreme restriction in the genetic diversity of the viral quasispecies shortly after infection. The genetically diverse viral swarm replicating in an infected transmitting partner constricts through a genetic bottleneck in the course of sexual transmission such that transmission is usually the result of a single infectious event; the productive infection that follows, in most instances, reflects an expansion from a single founder virus [10], [11], [12], [13], [14], [15]. Importantly, this restriction occurs in the recipient, rather than in the transmitting partner. The early progeny of the transmitted founder virus show relative uniformity until adaptive immune responses drive the founder to diversify into a quasispecies. An instructive exception to this pattern occurs in recipients harboring certain sexually transmitted diseases (STDs). Inflammation in the genital mucosa, mediated by STDs, can promote transmission of multiple founder viruses due predominantly to the ready availability of activated CD4+ T cell targets [10], [12], [16], [17], [18]. This underscores the crucial role that metabolically activated CD4+ T-cells likely play in transmission[19].
Considering that the early stages of HIV infection represent a possible window of opportunity for preventing infection, the structural, functional and immunogenic characteristics of founder/early-transmitting gp120s are highly relevant to the design of a preventive HIV vaccine. These viruses invariably utilize CCR5, which is a key phenotype of early-transmitting gut-tropic isolates[20], [21]. The genotype of early-transmitting viruses has also been a subject of great interest. Although a genotypic signature of early-transmitting viruses has proved difficult to identify, two key features have emerged. In studies of both heterosexual and mother to child transmission, early-transmitting gp120s have been found to be shorter in length, and encode fewer potential N-linked glycosylation sites (PNGs) than typical chronically replicating isolates[11], [22], [23]. These features have thus far only been found in the context of infection with HIV subtypes A and C. Length shortening has been observed in the V1/V2 region, as well as V4 and flanking regions of gp120. PNGs absent from early-transmitting isolates (PNGΔs) appear in somewhat specific positions around the N - and C-terminal stems of V1/V2[11], [22] and in C3/V4[24]. These characteristics apparently provide early-transmitting isolates with increased transmission fitness[22], [24]; however, the nature of this fitness-advantage is unknown. The antigenic properties of early-transmitting isolates are the subject of ongoing studies [25], [26]. V1/V2, along with V4 and flanking regions are frequently an early target of autologous neutralizing antibodies (Nabs) [23], [27], [28]. The viral quasispecies escapes neutralization through amino acid substitutions, insertions/deletions (INDELs), and also by adding/shifting PNGs. In this way the bulk of the quasispecies drifts away from the genotypic features that distinguish early-transmitting isolates.
The V1/V2 domain of HIV-1 gp120 mediates binding to integrin α4β7 (α4β7) on CD4+ T-cells[29]. α4β7 has been termed the gut homing integrin[30]. It is upregulated on lymphocytes in Peyer's patches and mesenteric lymph nodes, and then mediates, in concert with chemokine receptors, the homing of these lymphocytes into GALT through interactions with its natural ligands, MadCAM and VCAM, which appear on gut endothelial cells[31]. α4β7 appears in close association with the CD4 receptor on mucosal CD4+ T cells[32]. The biochemical characteristics of the interactions between α4β7 and gp120 closely mimic those of α4β7 with MadCAM and VCAM. There is a strong dependence of this interaction on divalent cations, and the α4β7 binding-site in V1/V2 shares close sequence homology with the binding sites of α4β7's natural ligands. This type of structural mimicry suggests that the specific affinity of gp120 for α4β7 provides increased fitness to HIV. However, unlike CD4 and CCR5, α4β7 is not required for viral entry or replication in vitro.
α4β7+/CCR5+/CD4+ memory T cells appear in the rectal and vaginal mucosa[32], [33]. In this way, this subset of CD4+ T cells links the portal of entry during sexual transmission and the inductive and effector sites of the gut that provide a permissive environment for near-exponential replication. We have proposed that HIV evolved a specific affinity for α4β7 as a means of insuring that productive target cells with gut-homing potential will be infected shortly after transmission. We noted, however, that the α4β7-reactivity of gp120s varied widely among isolates we analyzed [29], suggesting to us that strong α4β7-reactivity might provide increased transmission fitness over those isolates with lower α4β7 –reactivity in the context of mucosal transmission.
In the present study we provide evidence that the apparent selection at the time of mucosal transmission for viral envelopes exhibiting transmission-linked PNGΔs coincides with dramatic increases in α4β7-reactivity. We conclude that α4β7-reactivity is one of the phenotypes that can contribute to the genetic restriction that occurs during mucosal transmission.
Results
Transmission linked PNGΔs in gp120 increase affinity for α4β7
Longitudinal studies of cohorts of couples discordant for HIV infection have been used to isolate and characterize genotypic, phenotypic and immunogenic properties of early-transmitting gp120s. In studies involving heterosexual transmission of subtype A and C viruses, early-transmitting viruses tend to encode more compact variable loops of gp120 with reduced numbers of PNGs relative to isolates replicating during the chronic phase of infection[11], [22], [23]. This pattern represents a bias rather than a rule, and although it is most frequently associated with the V1/V2 domain of gp120, the V4 and flanking regions of early replicating viruses can also display these characteristics. In the V1/V2 domain, transmission-linked PNGΔs are clustered at the N - and C-termini of V1 and V2 respectively, while two central PNGs are well conserved (Figure 1A). The apparent advantage that these characteristics confer upon early-transmitting viruses is not known. The V2 PNGs lie close to a tripeptide (LDV/I – Figure 1A) that mediates gp120 binding to α4β7 [29]. We determined whether removing PNGs in V1/V2 altered reactivity with α4β7. Two recombinant gp120s, 92Ug037 (subtype A), and 93MW959, (subtype C), were employed. Both envelopes were derived from viruses obtained from asymptomatic females who contracted HIV-1 through heterosexual transmission. 93MW959 was isolated ∼12 months post-seroconversion, while the time between seroconversion and isolation of 92Ug037 is unknown. PNGs were removed by site-directed mutagenesis in which asparagines were replaced with glutamines (Figure 1A). α4β7-reactivity was measured using a steady-state binding assay that employs primary α4β7+/CD4+ T-cells (Figure S1). Each PNG mutant was compared to its wild type (w.t.) parent. For 92Ug037, PNGΔs near the N-terminus of V1 or the C-terminus of V2 mediated increases in α4β7-reactivity of up to 20-fold (Figure 1B). For 93MW959, a single PNGΔ at the C-terminus of V2 mediated a ∼3.5-fold increase in α4β7-reactivity, while PNGΔs near the N-terminus of V1 had little effect on α4β7-reactivity (Figure 1C).
Fig. 1. Effect of V1/V2 PNGΔs on the α4β7-reactivities of a subtype A and a subtype C gp120. 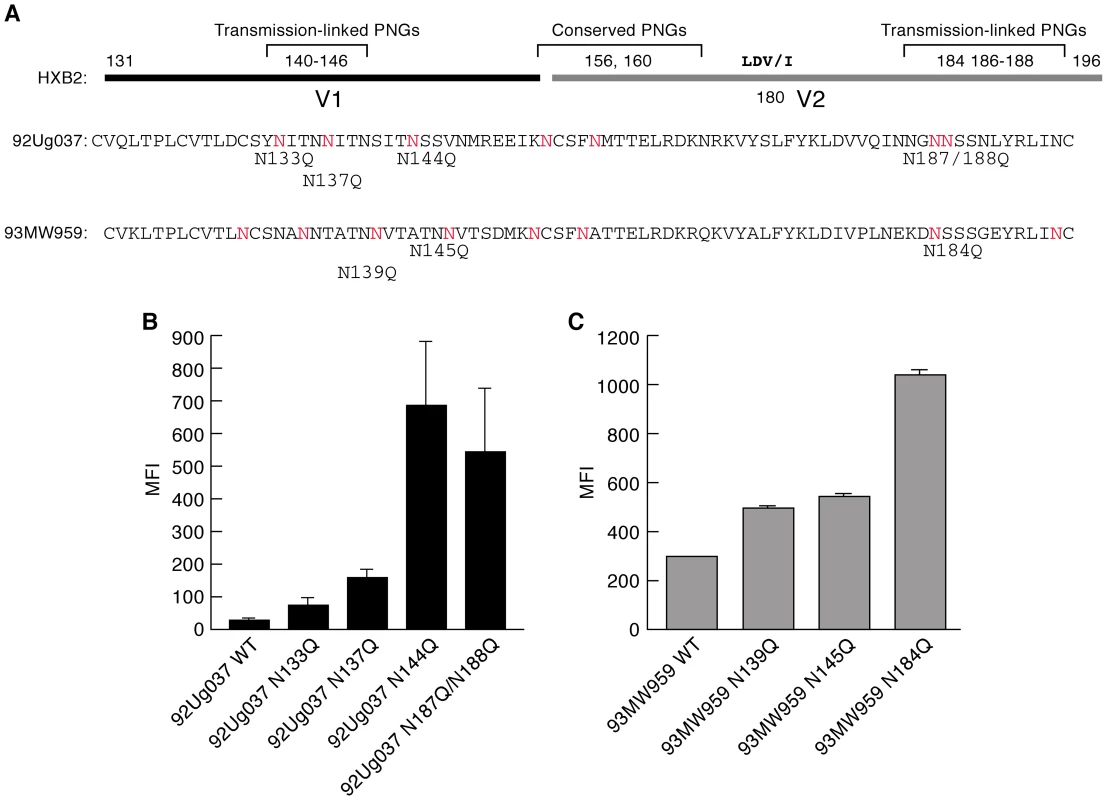
A) The V1/V2 regions of 92Ug037 (subtype A) and 93MW959 (subtype C) aligned with HXBII V1/V2. PNGΔs associated with transmission are designated using HXBII numbering. PNGs that appear in 92Ug037 and 93MW959 are highlighted in red. PNGΔs introduced into each protein by N/Q substitution appear directly below. B) α4β7-reactivities of 92Ug037 w.t. gp120 and PNGΔ mutants are reported as mean fluorescence intensity (MFI). C) α4β7-reactivities of 93MW959 w.t. gp120 and PNGΔ mutants. Error bars report the standard deviation between two replicates, and results are representative of three independent experiments using independent donor CD4+ T cells. Length-shortening in V1/V2 is also associated with early transmitting gp120s. However, because those deletions often result in PNGΔs, it is difficult to determine whether short V1/V2s are favored independently of PNGΔs [34]. We examined the influence of V1/V2 length shortening on α4β7-reactivity by taking advantage of a previously characterized pair of subtype A envelopes isolated from an individual at ∼1-month and ∼41-months post-infection[23]. The month 1 V1/V2 (QA203M1) encodes 63 amino acids and 5 PNGs, while the month 41 V1/V2 (QA203M41) encodes 70 amino acids and 8 PNGs such that the 41 month envelope encodes two additional PNGs near the N-terminus of V1 and one additional PNG near the C-terminus of V2 (Figure 2A). Both V1/V2s were grafted into a subtype A gp120 backbone isolated ∼1year post-infection from a second patient. QA203M1 gp120 displayed ∼20× greater α4β7-reactivity than did w.t. QA203M41 (Figure 2B). To distinguish the influence of V1/V2 length from the influence of the number of PNGs on α4β7 reactivity we constructed a variant of the month-41 V1/V2 (QA203M41variant1) lacking the two V1 PNGs that were missing in QA203M1 without changing its length (Figure 2A). QA203M41 variant 1, exhibited relatively strong binding to α4β7 that was nearly identical to that of QA203M1 gp120. These results indicate that increased α4β7-reactivity mediated by the early-transmitting QA203M1 relative to QA203M41 was due to PNGΔs rather than to a shorter V1/V2. We extended this analysis by removing additional PNGs from QA203M41. QA203M41 variant 2 in which PNGs were removed from the C-terminal region of V2 mediated a small increase in α4β7-reactivity, while QA203M41 variant 3, which combines variant 1 and variant 2 PNGΔs mediated an increase in α4β7-reactivity that was intermediate between variant 1 and variant 2 (Figure 2A, C). These results demonstrate that PNGΔs do not necessarily enhance α4β7-reactivity in an additive manner.
Fig. 2. A comparison of the α4β7-reactivities mediated by the V1/V2 regions of a month-1 gp120 and month 41 gp120 isolated from a single patient. 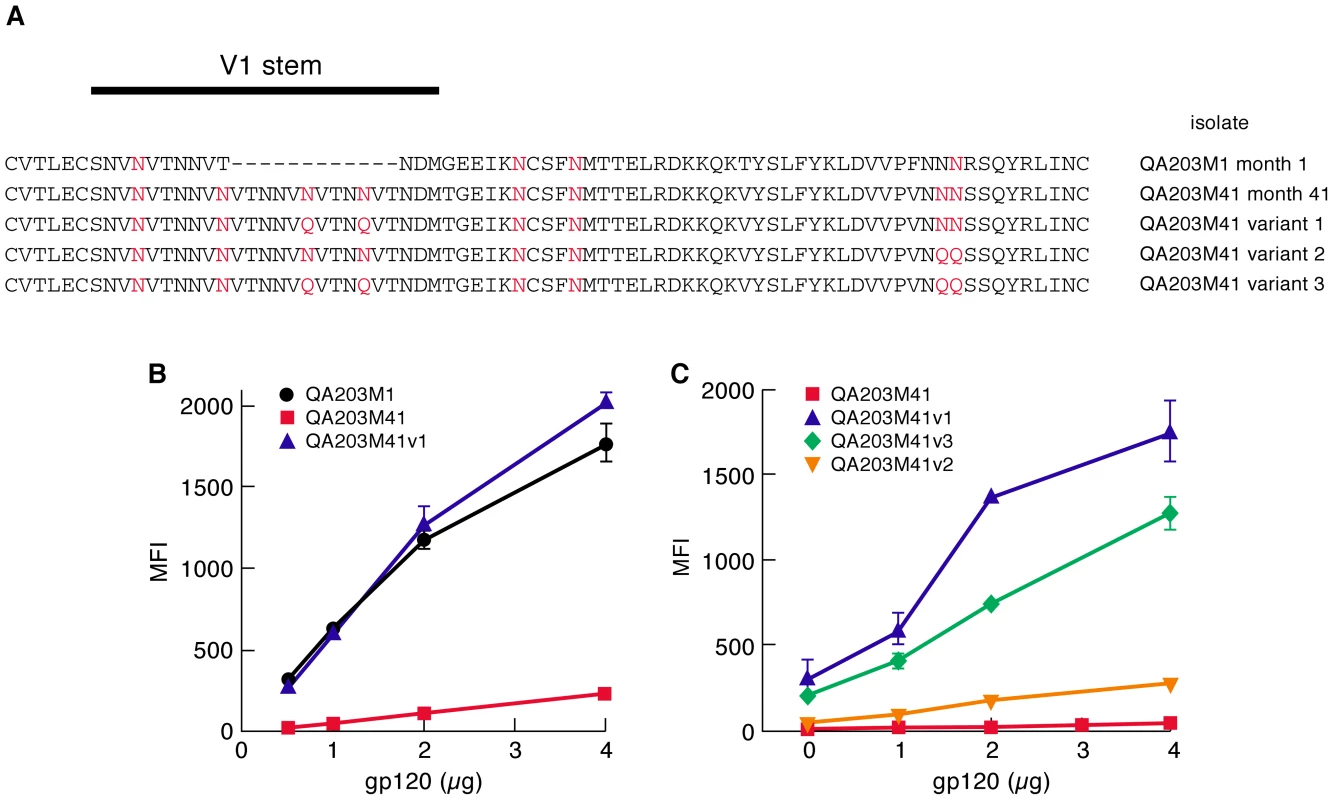
A) The gp120 V1/V2 sequences from patient QA203 obtained at month 1 and month 41 are listed. PNGs are highlighted in red. Variants, 1, 2 and 3 of QA203M41 are included with the N/Q substitutions highlighted in red. B) α4β7-reactivities of chimeric gp120s encoding the V1/V2 regions of QA203M1, QA203M41 and QA203M41 variant 1 are reported as mean fluorescence intensity. C) A comparison of the α4β7-reactivities of variants 1, 2 and 3 of QA203M41. QA203M41 is included for reference. Error bars represent the standard deviation of two replicates, and results are representative of three independent experiments using independent donor CD4+ T cells. Taken together, analysis of all of the PNGΔs described above leads us to conclude that the removal of transmission-linked V1/V2 PNGs can mediate large increases in the α4β7-reactivity of both subtypes A and C gp120s. The pattern in which these increases were mediated is complex such that no single PNGΔ at a given position in V1/V2 mediated increased α4β7-reactivity in all three gp120s. PNGΔs near both the N - and C-termini of the V1/V2 of 92Ug037 increased α4β7-reactivity, while only one PNGΔ near the C-terminus of the V2 of 93MW959 increased α4β7-reactivity. For QA203M41, the opposite was the case, removal of V1 PNGs, but not V2 PNGs mediated a large increase in α4β7-reactivity. These data suggest that increased α4β7-reactivity mediated by PNGΔs is due to changes in V1/V2 conformation rather than through steric occlusion, a subject that will be addressed below.
A study of early-transmitting viruses following mother to child transmission reported that PNGs in the C3/V4 region of gp120 are also underrepresented in early replicating isolates in a manner similar to that described above for V1/V2 PNGs [24]. Overbaugh and colleagues found that PNGs at specific positions throughout C3/V4 were underrepresented in early-transmitting isolates of subtype A viruses isolated from infants shortly after birth. Although this region of gp120 is far removed from the known α4β7-binding site in V1/V2, we determined whether removing these PNGs would also increase α4β7-reactivity. The C3/V4 region of 92Ug037 was aligned with subtype A sequences from the study noted above and five transmission-linked 92Ug037 PNGΔ gp120s were analyzed (Figure 3A). 92Ug037N333Q, 92Ug037N362Q, and 92Ug037N393Q gp120s mediated increases in α4β7-reactivity of ∼18 to 21-fold. Interestingly, Ug037N355Q mediated a ∼27-fold increase in α4β7-reactivity. This increase was greater than that mediated by Ug037N144Q, which among the 92Ug037 V1/V2 PNGΔs mediated the largest increase in α4β7-reactivity. 92Ug037N385Q mediated an ∼8-fold increase in α4β7-reactivity (Figure 3B). We conclude that, as with V1/V2, PNGΔs in C3/V4 can mediate large increases in α4β7-reactivity. The increased α4β7-reactivity achieved by PNGΔs in C3/V4 supports the proposition that the manner in which glycans inhibit gp120 binding to α4β7 involves conformational changes in gp120 rather than simple steric occlusion. Data supporting this interpretation will be presented below.
Fig. 3. Effect of PNGΔs in the C3/V4 region on the α4β7-reactivity of 92Ug037 gp120. 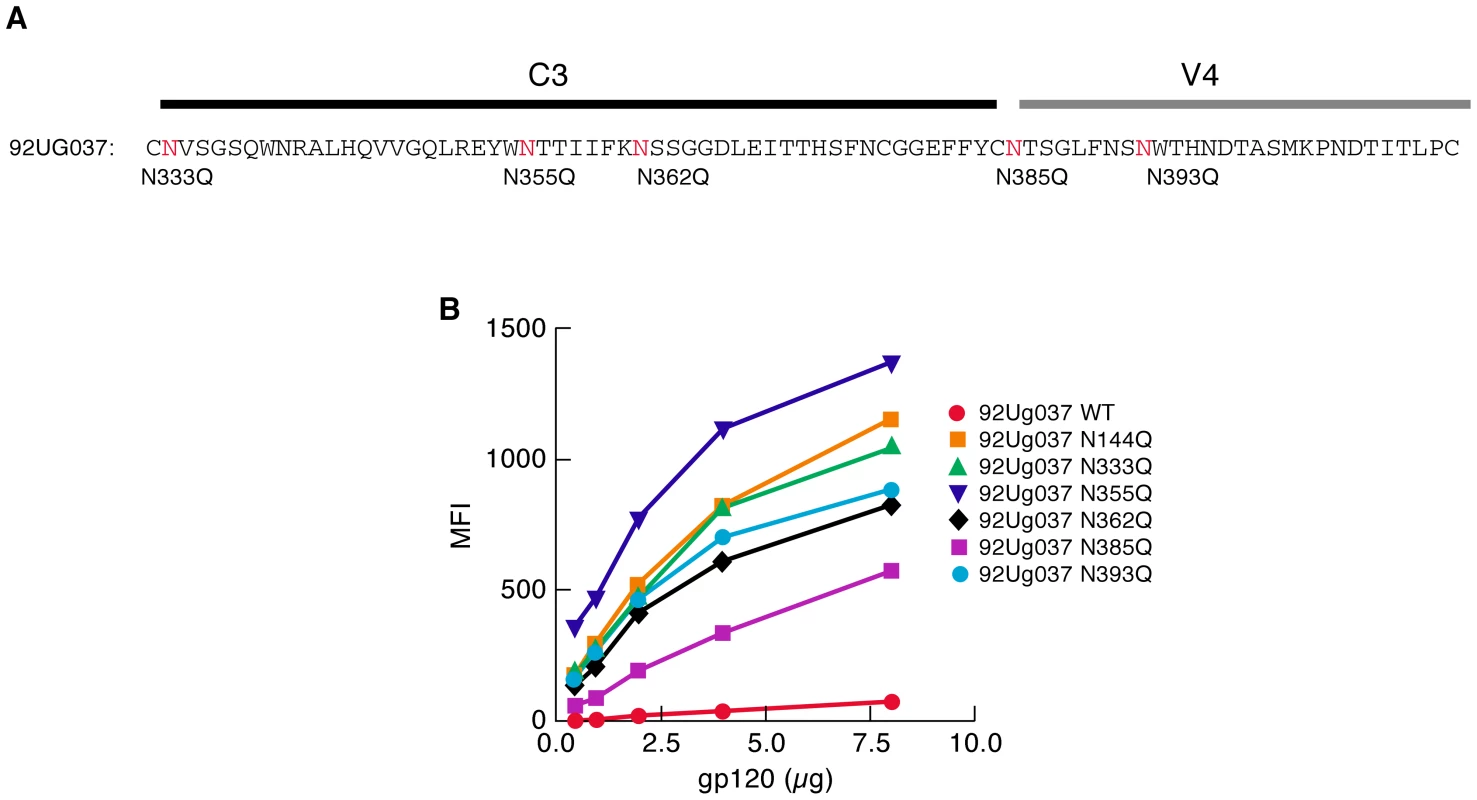
A) The positions of transmission-linked PNGs in the C3/V4 region of subtype A gp120s are highlighted in red in the C3/V4 region of 92Ug037 and N/Q substitutions are listed directly below. B) α4β7-reactivity of each 92Ug037 PNGΔ mutant was compared to w.t 92Ug037 gp120 and reported as mean fluorescence intensity (MFI) 92Ug037N144Q was included for reference. Results are representative of three independent experiments using independent donor CD4+ T cells. Glycan type impacts gp120 - α4β7 interactions
To better understand the role of glycan deletion in gp120-α4β7 interactions we compared the α4β7-reactivity of AN1 w.t. gp120, a subtype B ancestral/consensus gp120[35], expressed in three different cell lines known to glycosylate gp120 in different ways. AN1 w.t. was expressed in CHO S cells (a nonadherent subclone of CHO K1), the same cell line in which all of the gp120s described in the present study thus far were produced. gp120s expressed in CHO K1 cells present a heterogeneous pattern of oligo-mannose and complex carbohydrate type glycans [36]. Complex carbohydrates tend to appear on the solvent-exposed loops, including V1/V2 of recombinant gp120 proteins [37]. We also expressed AN1 gp120 in CHO lec1 cells, a CHO derivative that lacks N-acetylglucosamine (GlcNAc) glycosyl transferase activity, so that N-linked carbohydrate trimming is blocked at the Man5-GlcNAc2-Asn intermediate (where Man is Mannose)[38]. gp120s produced in CHO lec1 cells are devoid of complex carbohydrate, and are instead enriched with oligo-mannose type glycans. Finally, we expressed AN1 w.t. gp120 in 293F cells (a nonadherent subclone of HEK 293T cells), which differ from both CHO cell lines in the manner in which it modifies complex carbohydrate. 293T derived cells sialylate the terminal galactose moieties of complex carbohydrates through both α-2,3 and α-2,6 linkages. CHO cells, which lack α-2, 6-sialyltransferase, establish these linkages only at the α-2,3 position [39]. CHO S expressed AN1 w.t. gp120 reacted with α4β7 at an intermediate-low level (Figure 4A), similar in magnitude to many of the gp120s that we have previously reported[29]. CHO lec1-derived AN1 w.t. gp120 reacted ∼100× more efficiently with α4β7 than did CHO S AN1 w.t. gp120 (Figure 4A). It is likely that this increase in α4β7-reactivity results from the substitution of complex carbohydrates in V1/V2 with oligo-mannose type glycans. In contrast, 293F expressed AN1 w.t. gp120 showed no detectable α4β7-reactivity. We subsequently analyzed six additional 293F and T derived gp120s, including CAP881m.c17, a gp120 that exhibits very strong α4β7-reactivity when derived in CHO S cells, and without exception they all exhibited low or undetectable α4β7-reactivity (Figure S2 and data not shown). Considering the differential sialylation mediated by CHO S and 293 cells, we digested 293F AN1 w.t. gp120 with neuraminidase, which catalyzes the hydrolysis of terminal sialic acid residues; however, this failed to rescue α4β7-reactivity (data not shown). We next expressed AN1 w.t. gp120 in 293F cells cultured in the presence of kifunensine, a mannosidase I inhibitor that restricts the processing of N-linked glycosylation beyond the Man9GlcNac2 intermediate and swainsonine, a mannosidase II inhibitor that restricts the processing of N-linked glycosylation beyond hybrid glycan intermediates [40]. Expressing gp120 in 293F cells in the presence of these drugs should, to some degree approximate the complex carbohydrate deficient glycan characteristics of CHOlec1 derived gp120. Neither drug rescued α4β7-reactivity (Figure 4A and data not shown). Although we do not know what the post-translational defect is in 293F and 293T-expressed gp120s, we conclude that utilizing gp120s produced in this manner may not be ideal for studies involving α4β7.
Fig. 4. Effect of glycan content on the α4β7-reactivity. 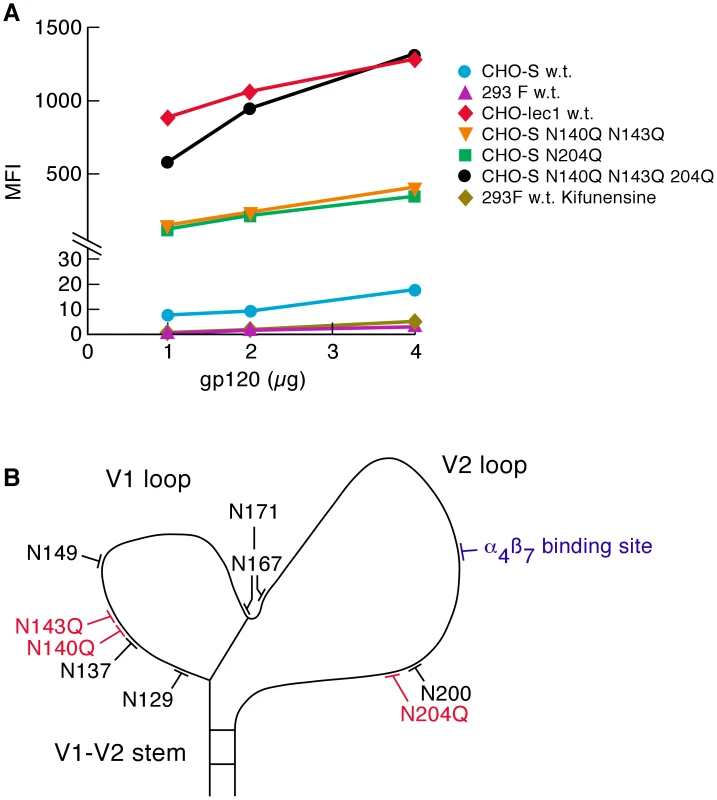
A) α4β7-reactivity of the ancestral AN1 gp120 (subtype B) produced in CHO-S cells, 293F cells, CHO lec1 cells and kifunensine treated 293F cells, were compared and reported as mean fluorescence intensity (MFI). AN1 V1/V2 PNGΔs were also included. Results are representative of three independent experiments using independent donor CD4+ T cells. B) Positions of all PNGs and the α4β7 binding site in the V1/V2 loop of AN1 gp120 are illustrated. We next determined what effect restricting the glycans on AN1 w.t. gp120 to the oligo-mannose type, as occurs in CHO lec1 cells, would have compared to the effect of the deletion of transmission-linked glycans in AN1 derived from CHO K1 cells. Three AN1 PNGΔ derivatives: AN1 NN140/143QQ, AN1 N204Q, and the combined mutant AN1 NN140/143QQ,N204Q, were expressed in CHO-S cells (Figure 4B). These PNGs appear near the N - and C-termini of V1 and V2 and their positions correspond to transmission-linked PNGs. Compared to AN1 w.t. expressed in CHO-S cells, AN1 NN140/143QQ increased α4β7-reactivity by ∼25-fold (Figure 4A). AN1 N204Q increased α4β7-reactivity by ∼23-fold. The combined mutant NN140/143QQ,N204Q increased α4β7-reactivity by ∼96-fold, and resulted in a CHO-S derived gp120 with α4β7-reactivity ∼equivalent to CHO lec1 gp120. To formally demonstrate that increased α4β7-reactivity can result from reduced N-linked glycosylation we carried out a brief digestions of CHOlec1 derived AN1 gp120 with endoglycosidase H (Endo-β-N-acetylglucosaminidase H) and determined that the enzymatic removal of PNGs from AN1 gp120 leads to increased α4β7-reactivity (Figure S3). Overall these results demonstrate that different glycosylation patterns mediated by different cells can exert a strong influence on α4β7-reactivity. In the case of AN1 gp120, replacing complex carbohydrate with oligo-mannose type glycans mediates stronger α4β7-reactivity, but this same strong binding can be achieved by deleting a small number of PNGs in V1/V2.
α4β7 –Reactivity and escape from autologous neutralizing antibodies
A pseudotyped virus encoding the month 1 QA203M1 V1/V2, described above (Figure 2), was efficiently neutralized by autologous serum taken at month 40, while a pseudotyped month 41 QA203M41 virus escapes neutralization from this same serum[23], indicating that neutralization-escape in the 40 month isolate was mediated by V1/V2. This pattern of escape is somewhat typical of early - vs. chronic-replicating HIV-1 viruses and reflects the fact that in subtype A and C viruses V1/V2 is frequently a direct target of early autologous neutralizing antibodies[23], [27], [28]. Additionally, V1/V2 can mediate a second type of neutralization-escape that affects epitopes throughout gp120. Mutations in V1/V2 can enhance a structural property of gp120 that has been termed conformational masking[41], [42], [43], [44]. Both mechanisms of escape are mediated by a combination of sequence variation, INDELS, and the addition and/or position-shifting of PNGs. We questioned whether changes in gp120 leading to neutralization-escape would disrupt α4β7-reactivity. Two recent studies [27], [28], in which quasispecies evolution and autologous neutralizing Ab responses were followed longitudinally, beginning shortly after transmission, provide perhaps the best-defined examples of V1/V2-mediated escape. Rong, Derdeyn and colleagues[28], demonstrated that the antibodies present in an HIV-1 subtype C infected female patient (patient 205F) at ∼38 months post-infection neutralized the early-replicating founder virus isolated within the first month following sexual transmission. Importantly, virtually all of the neutralizing activity in the 38-month sera targeted V1/V2 dependent epitopes. In a second study Moore, Morris and colleagues describe a more complex pattern of neutralization-escape[27], in which the early-transmitting month 1 virus isolated from a female patient, CAP88 (subtype C), was sensitive to autologous serum taken after 13 months, while viruses replicating at month 12 were not. Month 12 isolates were able to escape the month 13 sera via INDELS and PNG additions in both V1/V2 and C3.
We produced the neutralization sensitive 205F 0-month founder gp120 (Z205F.ENV1.1), and four escape mutants. The 0 - and 8 - month escape viruses are highly sensitive to neutralization by 38-month plasma and this escape was mediated by sequence changes that lie entirely within V1/V2 (Figure S4). The two 38-month viruses are partially resistant to neutralization by 38-month autologous plasma. The 205FENV1.1 0-month founder exhibited ∼8-fold greater α4β7–reactivity than did the 0-month escape, and at least 17-fold greater than did the 8 - and 38-month escape gp120s (Figure 5A). The level of α4β7-reactivity exhibited by the 205F.ENV1.1 0-month founder appeared to be substantially greater than many of the w.t. gp120s we previously reported[29]. We conclude that the high level of α4β7 reactivity in the 0-month founder was lost concomitant with sequence changes that mediated neutralization-escape.
Fig. 5. α4β7-reactivity of early-transmitting gp120s. 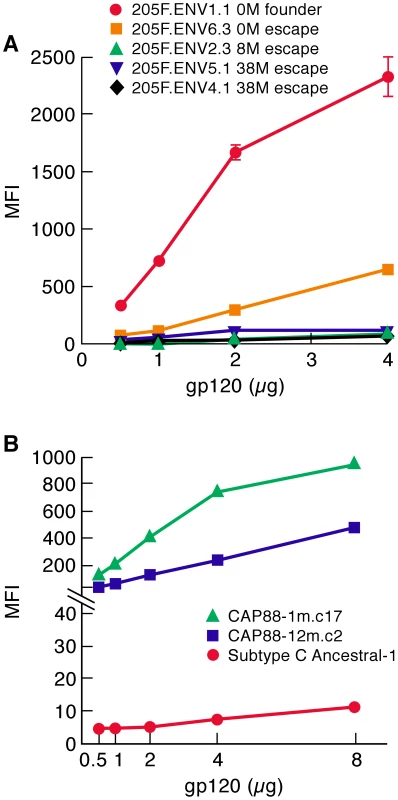
A) gp120s corresponding to patient 205F 0 month founder, 0 month escape, 8 month escape and two 38 month escape viruses were assayed for α4β7-reactivity and reported as mean fluorescence intensity (MFI). B) gp120s corresponding to a 1 month virus and a 12 month virus isolated from patient CAP88 were assayed for α4β7-reactivity and reported as mean fluorescence intensity (MFI). A consensus/ancestor subtype C gp120 was included for reference. Error bars represent the standard deviation of two replicates, and results are representative of three independent experiments using independent donor CD4+ T cells. We next compared the α4β7-reactivity of CAP88 gp120s isolated at 1 month and 12 months post infection. We chose CAP88.1m.c17, which reflects the predominant circulating isolate early in the first month post-infection. We also expressed the 12 month isolate CAP88.12m.c2 that diverged from CAP88.1m.c17 by adding PNGs in both V1/V2 and C3 at positions that correspond to transmission-linked glycans (Figure S4). The addition of these PNGs was shown to contribute to the resistance of CAP88.12m.c2 to autologous serum taken after month 13[27]. Like the 205F 0 month founder, CAP88.1m.c17 exhibited strong α4β7 reactivity (Figure 5B). Surprisingly this high level of reactivity was maintained in CAP88.12m.c2 despite the fact that PNGs were added at transmission-linked PNG positions in both V1/V2 and C3. This result demonstrates that viruses with relatively strong α4β7-reactivity can escape from autologous neutralizing antibodies without losing their α4β7-reactivity. Considering the complex pattern of changes in α4β7-reactivity we observed with the various N/Q substitutions described in Figures 1, 2 and 4, in which no single PNGΔ generated the same effect on each of the proteins analyzed, we conclude that the glycans that can mediate neutralization-escape may impact α4β7 reactivity (e.g. 205F), but not in all gp120s, and not in a way that is easily predictable.
In summary, we find that both of the early-replicating gp120s we analyzed showed high levels of α4β7-reactivity. Escape from neutralizing antibodies disrupted this activity in gp120s derived from patient 205F, but the α4β7-reactivity of a gp120 derived from patient CAP88 persisted 12 months post-infection despite sequence changes in V1/V2 and C3 that mediated escape from autologous neutralizing antibodies.
α4β7-Reactivity in relation to CD4 affinity
The affinity of HIV-1 gp120s for CD4 varies over a wide range and these differences can influence the cell-tropism of a viral isolate. For example, high CD4 affinity facilitates replication in macrophages by compensating for the low density of CD4 appearing on the macrophage membrane[45]. Changes in CD4 affinity could theoretically impact the transmissibility of a viral isolate; however, studies of early replicating gp120s have not, to date, found any clear correlation between CD4 affinity and transmission fitness [25]. Numerous studies have, however, shown that both amino acid substitutions and glycan additions/deletions in V1/V2 can influence the conformation of gp120 in a global way [44], [46]. Because V1/V2 plays an important role in α4β7 recognition we determined whether there was any relationship between α4β7-reactivity and CD4-reactivity. We first employed a steady-state binding assay to compare the reactivity of gp120s for α4β7 and CD4. In this assay, retinoic acid-cultured CD4+ T cells were differentially masked with either a CD4 mAb or an α4 mAb, as described in supporting Figure S1. For gp120s derived from early-transmitting viruses we found that α4β7 mediated a greater degree of binding to the cell surface than did CD4. The amount of 205F 0-month founder gp120 bound to α4β7 was 37-fold greater than that bound to CD4, but this differential disappeared in each of the 205F escape gp120s (Figure 6A). Such differences could be mediated entirely by V1/V2, as demonstrated by comparing the CD4 - and α4β7-reactivities of the chimeric QA203M1 and QA203M41 gp120s, which are identical in all domains other than V1/V2. 1-month QA203M1 bound 5-fold more to α4β7 than to CD4 while the 41 month QA203M41 was captured primarily by CD4 (Figure 6B). The deletion of a single transmission-linked PNG was sufficient to achieve a binding profile in which more binding to the cell membrane was mediated by α4β7 than by CD4. For example, while the majority of w.t. 92Ug037 binding to the cell surface was mediated by CD4, PNGΔs at the N terminus of V1/V2 altered this pattern such that more binding was now mediated by α4β7 (Figure 6C). Finally, we compared the two CAP88 gp120s to a panel that included several widely studied gp120s and subtypes B and C ancestral/consensus gp120s[35], [47]. Similar to the 205F 0 month founder and the chimeric QA203M1, both CAP88 proteins showed preferential binding to α4β7 over CD4 (Figure 6D). In contrast to the CAP88 gp120s, all other gp120s in the panel, with one exception, exhibited CD4 binding that was equal to or greater than α4β7 binding. The one exception was SF162, a highly neutralization-sensitive gp120 whose gut tropic characteristics have been well-documented[20], [21]. Because levels of α4β7 expression on the cells we employed were equivalent to, or less than CD4 expression levels (Figure 6 inset and Materials and Methods) we can conclude that the steady-state affinity of gp120s, like the two CAP88 proteins, for Mn++ activated α4β7 is greater than their steady-state affinity for CD4. In addition, this comparison underscores the strong α4β7-reactivity of the early-replicating gp120s we analyzed, relative to a number of well-studied gp120s. In this way, these gp120s appear to be better adapted to interact with CD4+ T cells that express a gut-homing receptor.
Fig. 6. A comparison of steady-state CD4-reactivity and α4β7-reactivity of a panel of gp120s. 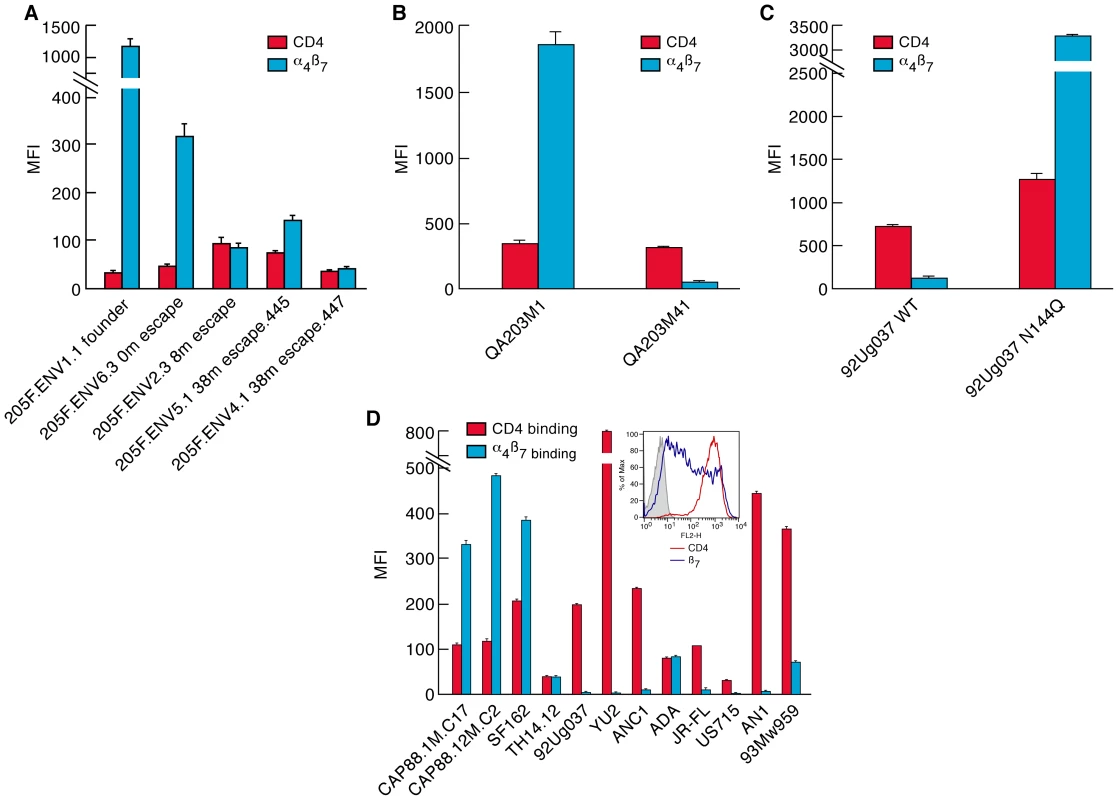
A) Levels of CD4-reactivity (red) and α4β7-reactivity (blue) were assessed for each patient 205F gp120, B) the QA203 month 1 and month 41 chimeric gp120s, C) w.t. and N144Q 92Ug037 gp120s, D) the 1-month and 12 month CAP88 gp120s and a panel of well characterized gp120s including an ancestral B (AN1) and an ancestral C (ANC1) gp120. Inset indicates the staining of the cells employed in this assay with PE conjugated CD4 (red) and β7 (blue) mAbs. Error bars represent the standard deviation of two replicates, and results are representative of three independent experiments using independent donor CD4+ T cells. It is noteworthy that the large changes in steady-state binding to α4β7, mediated by sequence changes in V1/V2, were coupled with relatively small changes in steady-state binding to CD4 (Figure 6). In some HIV-1 isolates, insensitivity to sCD4 and CD4 binding-site antibody neutralization is mediated by V1/V2 [44], [46], a phenomenon termed conformational masking[41], [42], [43]. Of note, this masking-effect can be modulated by the same transmission-linked PNGs that exert a strong influence on α4β7-reactivity [44], [46]. To better understand the relationship between CD4-reactivity, V1/V2 masking, and α4β7-reactivity we employed a surface plasmon resonance-based kinetic CD4 binding assay. gp120s were immobilized on the surface of a biosensor chip and either monomeric sCD4 (D1D2), or D1D2-Ig αtp, a highly oligomerized (dodecameric) CD4-Ig derivative[48], [49], [50], was passed over the surface, allowing us to measure reaction kinetics (Figure 7A). In this format D1D2-Ig αtp can bind >1 gp120 in a near-simultaneous manner, allowing avidity-effects to contribute to interactions between it and gp120. However, in this format, avidity cannot contribute to monomeric sCD4-gp120 interactions. Sensorgrams of nine well-studied gp120s, including JR-FL gp120 (Figure 7B), and eight additional gp120s (Figure S5) are provided for reference. 205FENV1.1 0-month founder gp120 failed to recognize monomeric CD4, but did exhibit high affinity for dodecameric D1D2-Igαtp (Figure 7C). The failure of this gp120 to react with monomeric CD4 distinguishes it from the nine standard gp120s we analyzed (Figure 7B and Figure S5). However, we observed the same phenomenon with the1-month QA203M1 chimeric gp120 (Figure 7D), and CAP88 1m.c17(Figure 7E). In patient 205F and QA203 reactivity with monomeric sCD4 reappeared as the viral quasispecies evolved away from the early transmitting isolate. Both the 38 month 205F.ENV5.1 gp120 (Figure 7C) and 41 month QA203M41(Figure 7D), which bind weakly to α4β7, did bind monomeric sCD4 with high-affinity. In summary, all three early-transmitting gp120s failed to react with monomeric sCD4, which distinguishes them from nine standard gp120s. Because the chimeric QA203M41 and QA203M1 gp120s differ only in V1/V2 we conclude that the failure of QA203M1 to bind monomeric sCD4 was mediated by V1/V2, and it seems likely that this also holds for 205F.ENV1.1 0-month founder, and CAP88 1m.c17. All three of these gp120s did, however, react with dodecameric D1D2-Igαtp (Figure 7 C, D, E), and with CD4 displayed on the surface of a T cell (Figure 6). The failure to interact with monomeric sCD4 in this SPR based assay does not indicate that the viruses from which these gp120s were derived are CD4-independent, nor can one conclude that these viruses would be resistant to sCD4 neutralization. This observation does indicate that the CD4-reactivity of these early-replicating gp120s is more dependent on avidity-effects than is the case for the other gp120s that we analyzed, and further suggests that, in addition to high-level α4β7-reactivity, these gp120s share structural features that distinguish them from many gp120s. Although these distinguishing biochemical features involve gp120 monomers, it is reasonable to suggest that these features will in some manner impact the stability and immunogenicity of trimeric spikes[51].
Fig. 7. CD4 binding characteristics of early-transmitting gp120s. 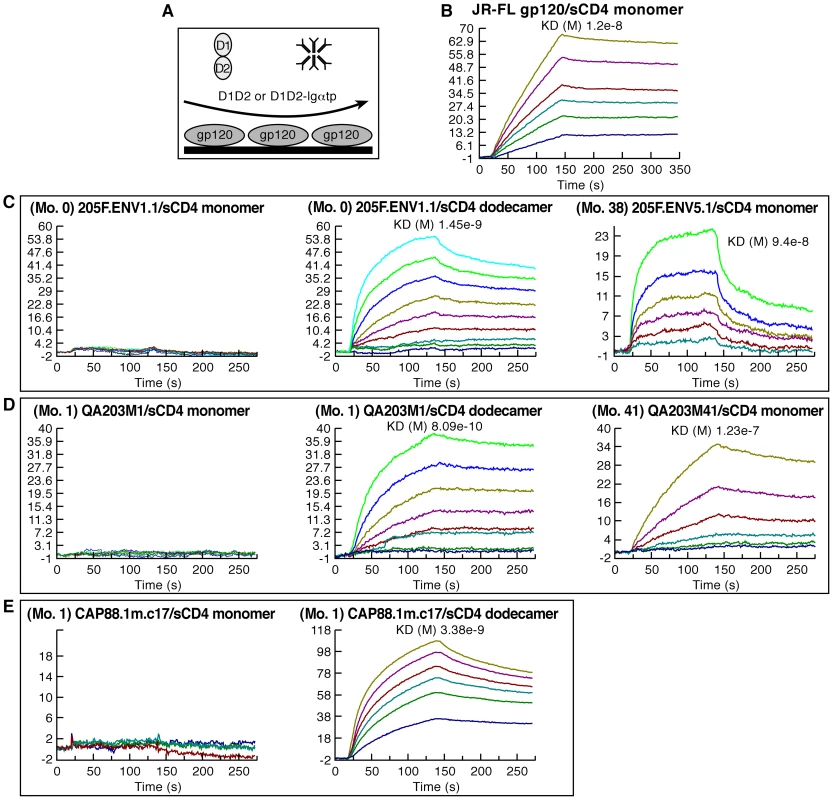
A) Schematic of the Surface Plasmon resonance analysis strategy used to measure the reactivity of monomeric sCD4 (D1D2) and dodecameric sCD4 (D1D2-Igαtp). B–E) Sensorgrams depicting the binding kinetics of increasing concentrations of D1D2 or D1D2-Igαtp reacting with immobilized gp120s, the identities of which are noted in each panel. Each ligand/analyte pair is listed and overall affinity is reported as KD (where applicable). These results are representative of three independent experiments using independently coupled gp120 biosensor surfaces. Discussion
The search for a consistent genotypic signature of early-transmitting viruses has proven difficult. The most consistently observed genotypic marker thus far identified is a more compact V1–V4 with a reduction in the number of PNGs, relative to the length and average number of PNGs in chronically replicating isolates[11], [22], [23]. Early-transmitting viruses bearing this genotype do not appear to use CD4 or CCR5 in any way that distinguishes them clearly from chronically replicating isolates[25], [52]. However, we found that removing transmission-linked PNGs from multiple gp120s consistently resulted in greatly increased α4β7-reactivity. Additionally, the early-transmitting gp120s that we analyzed showed notably higher levels of α4β7-reactivity than many of the chronically replicating isolates that we assayed. These results suggest that increased α4β7-reactivity is likely to be part of the phenotype underlying PNGΔs in some early - transmitting viruses and that this increased reactivity provides, under certain conditions, increased fitness in the process of transmission. Under this scenario, engaging α4β7 could provide an advantage at an early stage of transmission, but may not always be required. We should emphasize that additional analyses, that include greater numbers of early-transmitting gp120s are needed to better estimate the importance of α4β7+/CD4+ T-cells in mucosal transmission. Moreover, we do not yet know the specific point in the process of transmission where α4β7-reactivity may have an impact, nor do we know the relative importance of α4β7-reactivity on transmission fitness relative to other phenotypic features of gp120. However, the fact that HIV has evolved an affinity for α4β7 reflects the important role that α4β7+/CD4+ T-cells likely play in the process of mucosal transmission. This is of potential importance since it follows logically that blocking viruses from infecting these cells will reduce the frequency of successful transmission.
We analyzed only two early-transmitting gp120s and one early-transmitting chimera. Clearly, we cannot generalize from this small number, yet it is noteworthy that all three envelopes bind more efficiently to activated α4β7 than to CD4. Thus, these gp120s are better adapted to interact with α4β7+/CD4+ T cells as opposed to α4β7lo-neg/CD4+ T cells. We previously reported that metabolically activated cells are enriched in the α4β7+ subset of CD4+ T cells in rectal mucosa[32], and a similar observation has been made for cervical α4β7+/CD4+ T cells (R. Kaul, personal communication). CD4 is not a marker of cellular activation or of gut-homing. When the affinity of a gp120 for CD4 is high, e.g. JR-FL gp120, it engages all CD4 cells regardless of their metabolic state or homing potential. CCR5 expression can correlate with metabolic activity; however, prior to CD4-binding it is hidden from HIV. By increasing α4β7-reactivity, which is CD4-independent, we find that the early-transmitting gp120s that we analyzed are better adapted to engage with a specific subset of CD4+ T cells that are highly susceptible to infection by both HIV and SIV[32], [53], and located in anatomical sites relevant to HIV transmission. The context in which the interaction between HIV-1 and α4β7+/CD4+ T cells takes place remains unclear. It may involve cell-free virions, but it might also occur in the context of dendritic cell-CD4+ T cell interactions that are potentially important in mucosal transmission[54]. With one exception (SF162 gp120), none of the commonly studied gp120s that we characterized here or in our previous report exhibited this phenotype[29]. Although these standard gp120s may not necessarily be representative of chronic HIV isolates, it is noteworthy that several showed near-undetectable α4β7-reactivity. This is consistent with our previous observation that α4β7 interactions are not required for viral replication[29] and underscores the fact that α4β7-reactivity can diminish rapidly in a newly infected individual (e.g. patient 205F). We note, however, that the introduction of a small number of transmission-linked PNGΔs into a gp120 derived from a chronically replicating virus (e.g QA203M41 variant 1) can generate a strong α4β7-reactive phenotype such that this gp120 is now better adapted to engage CD4+ T cells that express α4β7. Because these transmission-linked PNGΔs have been found in a somewhat consistent manner in early-transmitting gp120s in multiple studies involving transmission of subtypes A and C viruses [11], [22], [23], [24], [55], it will be important in future studies to determine the extent to which strong α4β7-reactivity is a phenotype that is overrepresented in early-transmitting isolates. It will also be important to determine the frequency with which early-replicating viruses bind more efficiently to activated α4β7 than to CD4.
The frequency and circumstances under which viruses encoding fewer PNGs in V1–V4 are preferentially established in a newly infected individual provides clues concerning the possible role of gp120-α4β7 interactions in facilitating mucosal transmission. We note that transmission-linked PNGΔs have thus far been found only in cohorts of heterosexual couples discordant for HIV infection and a mother to child transmission cohort, all of which involve subtypes A and C, but not B[56]. The failure to observe this genotypic pattern in subtype B cohorts may reflect differences in the predominant modes of transmission i.e. heterosexual transmission versus transmission among men who have sex with men and intravenous drug users. It may also reflect other acquisition related risk factors, such that gp120-α4β7 interactions may play a greater role under conditions in which the risk of acquisition is low, but less of a role when that risk is high. One risk factor that can influence susceptibility to infection is the presence of STDs that cause inflammation of genital tissues in a recipient [10], [12], [16], [17], [18]. To the extent that inflammation increases the availability of activated CD4+ T cells near the site of infection, the selection pressure for a virus with strong α4β7-reactivity may be diminished. However, an opposite dynamic may also be operative under different circumstances. Chlamydia infection (and possibly other STDs) in the female genital tract has been shown to increase the number of antigen-specific α4β7+ T cells migrating through the female genital tract and into GALT[33], [57], [58], [59]. Under these conditions, isolates that can bind α4β7 are more likely to engage activated CD4+ T cells. Further phenotypic characterization of α4β7+ T cells in mucosal tissues, as well as the early-transmitting viruses derived from different types of transmission cohorts, will be necessary to clarify the relationship between acquisition risk factors and α4β7-reactivity.
We found that the type of glycans that decorate gp120 can exert a strong influence on α4β7-reactivity. By producing gp120 in a cell that fails to process oligomannose glycans into larger complex glycans we were able to increase the α4β7-reactivity of AN1 gp120 by >100-fold. In this regard, it is well established that different cell types, and even the same cell type, under different metabolic conditions, glycosylate proteins differently [60], [61]. We know that gp120s produced in macrophages are glycosylated differently than the same gp120s produced in CD4+ T cells[62]. It follows then that α4β7-reactivity, and possibly the transmissibility of a virion, may be influenced by the type of cell in which that virion is produced in vivo. It would be useful if we could tailor our in vitro gp120 expression systems in a way that reflects in vivo glycosylation; however, although a recent study has characterized the glycan content presented on virion-associated spikes generated in primary PBMCs in vitro [63], we do not yet know the true glycan profile of any in vivo derived HIV-1 gp120. In addition to glycosylation, other post-translational modifications may also influence α4β7-reactivity. We found that gp120s produced in 293F and 293T cells showed greatly reduced α4β7-reactivity, which could not be explained entirely by differences in glycosylation. These observations may prove useful in evaluating the limitations of 293-derived gp120 for transmission-related studies. Overall these data indicate that post-translational modifications in gp120 have the potential to influence the transmissibility of HIV-1, and that the type of carbohydrate that appears on an envelope may play a critical role in the selection of early-transmitting isolates.
Although certain PNGs in V1/V2 may sterically interfere with gp120 binding to α4β7, the manner in which transmission-linked PNGΔs throughout V1–V4 increased α4β7-reactivity suggests that increased α4β7-reactivity resulted from a change in the conformational state(s) of gp120. In this regard, it is well established that V1-V4 glycans can influence gp120 conformation globally[64], [65]. In addition, it is noteworthy that the naturally occurring gp120s that we analyzed exhibited either strong or weak α4β7-reactivity, since it suggests that there is a distinct conformational or structural feature associated with some early-transmitting gp120s that allows them to engage α4β7 in an efficient way. It is highly likely that sequence changes independent from PNGs will also influence α4β7-reactivity. In fact, the relatively high level of α4β7-reactivity exhibited by the 205F 0-month founder gp120 when compared to later isolates from this patient cannot be explained by a reduced number of PNGs. This underscores the limitation of using a genotypic feature i.e. the number of PNGs, to predict a phenotype. As more sequence information involving early-replicating isolates becomes available, and additional genotypic signatures are identified, it will be interesting to determine how they influence α4β7-reactivity. The number of gp120s analyzed here is small; therefore, it will be important to analyze the α4β7-reactivity of additional early-transmitting gp120s. Definition of the distinguishing features of early-transmitting gp120s is clearly relevant to subunit-based vaccine design.
The influence that PNGs can exert on gp120 structure and immunogenicity has been well studied[66]. The transmission-linked glycans in V1/V2 can, when they are present on a gp120, promote conformational masking. This term is used to describe the capacity of V1/V2 to increase the resistance of HIV-1 isolates to neutralizing antibodies with specificities throughout gp120[41], [42], [43], [44]. This effect has also been referred to as “entropic masking” or “flickering”, both of which describe the property of envelope spikes to rapidly transition between alternate conformations. Rapidly alternating conformations has the net effect of reducing the apparent affinity of neutralizing gp120 antibodies. We find that PNGs, which have been shown to promote conformational masking [44], [46], are among those that can influence α4β7-reactivity. The manner in which high affinity for α4β7 is related to conformational masking is an important question that requires further investigation. In any case, our demonstration that the early-transmitting gp120s we analyzed (205F.ENV1.1 0 month founder, CAP88.1m and QA203M1) rely on avidity effects to engage sCD4 suggests that there are structural properties that distinguish these gp120s from many commonly studied gp120s.
Finally, apart from the early-transmitting gp120s, strong α4β7-reactivity was infrequent among the panel of gp120s that we analyzed. This raises the question as to how frequently viruses bearing this phenotype appear in the viral quasispecies throughout the course of HIV disease. It may be that they appear spontaneously. This seems to be the case in patient 205F in which only the early-transmitting 0 month founder exhibited strong α4β7-reactivity. It is also possible that viruses bearing this phenotype are compartmentalized in mucosal tissues such that sampling virus from peripheral blood may lead to an underestimation of their frequency. In this regard, it is important to understand how frequently these viruses appear in the donor genital fluids from which virus is transferred to a recipient during sexual transmission.
In conclusion, the transmission-linked PNGΔs that characterize some early-transmitting HIV-1 gp120s mediate a phenotype that includes increased α4β7-reactivity, suggesting that virions that react strongly with α4β7 may possess increased transmission-fitness. Further studies will be required to establish a direct link between α4β7-reactivity and increased transmission across mucosal surfaces. Our observations also suggest that α4β7+/CD4+ T-cells are an important target population in the process of transmission. The gp120s we analyzed that exhibited strong α4β7-reactivity interacted with sCD4 in a way that distinguishes them from many gp120s, and suggests that early-transmitting gp120s bear structural features that might be exploited in the context of a subunit vaccine. Additional genotypic, phenotypic and structural analyses of early-transmitting viral envelopes are essential.
Materials and Methods
Ethics statement
PBMCs were collected from healthy donors through a NIH Department of Transfusion Medicine protocol that was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Informed consent was written and was provided by study participants and/or their legal guardians.
Cells and reagents
Freshly isolated PBMCs were obtained from healthy donors and separated by Ficoll-Hypaque. Purified CD4+ T cells were obtained by negative selection using magnetic beads (StemCell Technologies). Cultured CD4+ T cells were activated with OKT3, IL2 (20 IU/ml) and retinoic acid (10 nM) unless otherwise specified. RA was obtained from Sigma Chemical and discarded 1-month after reconstitution. CHO lec1 cells and HEK293T cells were obtained from ATCC. CHO-S and 293F cells were obtained from Invitrogen. Integrin antibodies were purchased from BD Biosciences, Beckman Coulter and R&D. Leu3A (SK3) and CD45RO were purchased from BD Biosciences.
Recombinant envelope proteins
The genbank accession numbers of all gp120s used in this study are listed below. The QA203M1 and QA203M41 chimeric gp120s were constructed by inserting the V1/V2 sequences of each of these gp120s into a Q23 backbone. QA203M1: VTLECSNVNVTNNVTNDMGEEIKNCSFNMTTELRDKKQKTYSLFYKLDVVPFNNRSQYRLIN.QA203M41: VTLECSNVNVTNNVNVTNNVNVTNNVTNDMTGEIKNCSFNMTTELRDKKQKVYSLFYKLDVVPVNNNSSQYRLIN. All gp120 coding sequences were synthesized and codon-optimized for expression in mammalian cells (DNA2.0). All proteins were produced and purified in an identical manner unless noted otherwise. The mature coding sequences of each envelope protein, from +1 to the gp120-gp41 junction were inserted into a mammalian expression vector downstream of a synthetic leader sequence. Vectors were transiently transfected into either 293F or CHO-S cells (Invitrogen) using FreeStyle MAX Reagent (Invitrogen) per the manufacturers instructions. gp120s expressed in CHOlec1 cells were transfected by CaPO4 and stable cell lines were selected in media containing 1 mg/ml G418. Clonal cell lines were established and subsequently seeded into hollow-fiber cartridges (30 kD MW cutoff) (Fibercell systems, Frederick MD). Protein containing supernatants were harvested daily from the extra-capillary space. Protein containing supernatants were harvested and passed over a column of galanthus nivalis lectin sepharose (Vector Labs) which was diluted 1∶5 with unliganded sepharose 4B to minimize avid binding. gp120 was eluted with 20 mM Glycine-HCl, pH 2.5, 150 mM NaCl, 500 mM α-methyl-manno pyranoside (Sigma), in 5 mL fractions directly into 1 mL M Tris-HCL, pH 8.0. Low pH elution was found to be necessary with certain gp120s which bound too tightly to the lectin to be efficiently eluted using α-methyl-manno pyranoside. Peak fractions were pooled, concentrated with a stirred cell concentrator (Millipore) and dialyzed exhaustively against HEPES, pH 7.4, 150 mM NaCl. Proteins were quantitated by UV adsorption at O.D. λ280 (extinction coefficient 1.1) and values were confirmed by a bicinchoninic acid protein assay (Pierce).
Recombinant gp120 protein labeling
Purified recombinant gp120s were biotinylated using amine-coupling chemistry. Proteins were reacted with a 100-fold molar excess of EZ-Link NHS-Biotin (Pierce) for 30 min, and reactions were quenched by rapid buffer-exchange into HBS. Biotin incorporation was determined by reacting gp120s with 4′-hydroxyazobenzene-2-carboxylic acid-avidin conjugates (HABA) per the manufacturers instructions (Pierce). Protein preparations exhibiting a 1.0–1.2 mol/mole, biotin/gp120 incorporation were used in comparative semi-quantitative flow-cytometric binding assays.
Flow cytometry binding assays
CD4+ T cells were cultured in RA for at least 6 days prior to use, and were stained with fluoresceinated anti β7 mAb FIB27 (ATCC) to insure RA-mediated upregulation of α4β7. Receptor quantitation using anti-mouse IgG coated microbead analysis of CD4 and β7 expression levels of these cells from multiple donors indicates that levels of β7 expressed on the surface of these cells typically lower and less uniform than CD4 expression levels (data not shown). The entire staining procedure, including wash steps was carried out in a 10 mM HEPES, 150 mM NaCl (HBS Buffer) buffer containing 100 µM CaCl2 and 1 mM MnCl2. Cells were pre-blocked with normal mouse IgG and human IgG (5 µg each per 106 cells. 3×105 cells were stained in a volume of 50 µl on ice. Where indicated CD4-gp120 interactions were masked by preincubating cells for 15′ with 5 µg Leu3A (SK3) (Becton Dickinson). Where indicated α4β7-gp120 interactions were masked with 5 µg unlabeled α4 mAb HP2/1 (Beckman Coulter). Masking antibodies were not washed away prior to gp120 staining. Biotin gp120 was added for 25′ on ice, after which cells were washed twice with staining buffer. In some assays CD45RO FITC and CCR5 APC were included, Neutravidin PE (Pierce) was then added, and incubation proceeded for an additional 30′ on ice. Cells were washed three times in staining buffer and then fixed in a 1% paraformaldehyde solution. Data were acquired using a BD FACSCalibur and mean fluorescence intensity measurements were obtained from the CD45RO+/CCR5+ gate.
Surface plasmon resonance analysis
Analysis was performed on a Biacore 3000 instrument (GE Life Sciences) using CM5 sensor chips. The data were evaluated using BIAevaluation 4.1 software (GE Life Sciences). The chip surface was activated by injecting 35 µl of a 1/1 mixture of 0.05 M N-hydroxysuccinimide and 0.2 M N-ethyl-N-(dimethylaminopropyl)carbodiimide at 5 µl/min. Purified gp120 (5 µg/ml in 10 mM NaOAc (pH 5)) was immobilized to a density of approximately 750 resonance units (RU) and blocked with 35 µl of 1 M Tris-HCl (pH 8.0). Human IgG was immobilized to one flow cell and used as a background control. Running buffer was HBS (pH 7.4), 0.005% Tween p20. To evaluate Env: CD4 interactions, increasing concentrations of sCD4 (D1D2) (6.25–400 nM) were sequentially injected over surface-bound gp120 at a flow rate of 25 µl/min. After a 2 min injection of sCD4, the surface was washed for 2 min in running buffer to follow the dissociation of sCD4 from gp120. The surfaces were regenerated by injection of 4.5 M MgCl2 at a flow rate of 50 µl/min.
Genbank accession numbers of gp120s
The following gp120s were employed in these studies: 93MW959 (GenBank accession # U08453, R5-tropic), 92TH14-12 (GenBank accession #U08801, R5-tropic), 93Ug037 (GenBank accession # U51190, R5-tropic), AN1 gp120 [35] (sequence available at http://ubik.mullins.microbiol.washington.edu/HIV/Doria-Rose2005/, R5-tropic), 92Ug21-9 (GenBank accession # U08804, X4-tropic).), ADA (GenBank accession #AF004394, R5-tropic), YU-2 (GenBank accession #M93258, R5-tropic), NL4-3 (GenBank accession # AF003887, X4-tropic), 92Ug21-9 (GenBank accession #AY669753, X4-tropic), and SF162 (GenBank accession # AY669736, R5-tropic). Z205F.ENV1.1 0Mfounder (GenBank accession #GQ485415, R5-tropic) Z205FENV6.3 0Mescape GenBank accession #GQ485419, R5-tropic), Z205FENV2.3 8Mescape (GenBank accession # GQ485425, R5-tropic), Z205FENV5.1 38Mescape GenBank accession #GQ485445, R5-tropic), Z205FENV4.1 38Mescape GenBank accession # GQ485447, R5-tropic) QA203M1 (GenBank accession #DQ136332, R5-tropic) QA203M41 (GenBank accession #DQ136341, R5-tropic), Q23 (GenBank accession # AF004885, R5-tropic).
Supporting Information
Zdroje
1. BoilyMC
BaggaleyRF
WangL
MasseB
WhiteRG
2009 Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis 9 118 129
2. WawerMJ
GrayRH
SewankamboNK
SerwaddaD
LiX
2005 Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 191 1403 1409
3. LiQ
DuanL
EstesJD
MaZM
RourkeT
2005 Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434 1148 1152
4. ZhangZQ
WietgrefeSW
LiQ
ShoreMD
DuanL
2004 Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A 101 5640 5645
5. LiQ
EstesJD
SchlievertPM
DuanL
BrosnahanAJ
2009 Glycerol monolaurate prevents mucosal SIV transmission. Nature 458 1034 1038
6. VeazeyRS
ShattockRJ
PopeM
KirijanJC
JonesJ
2003 Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 9 343 346
7. ZhangZ
SchulerT
ZupancicM
WietgrefeS
StaskusKA
1999 Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286 1353 1357
8. GuptaP
CollinsKB
RatnerD
WatkinsS
NausGJ
2002 Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 76 9868 9876
9. HaaseAT
2005 Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol 5 783 792
10. AbrahamsMR
AndersonJA
GiorgiEE
SeoigheC
MlisanaK
2009 Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol 83 3556 3567
11. DerdeynCA
DeckerJM
Bibollet-RucheF
MokiliJL
MuldoonM
2004 Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303 2019 2022
12. HaalandRE
HawkinsPA
Salazar-GonzalezJ
JohnsonA
TichacekA
2009 Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog 5 e1000274
13. KearneyM
MaldarelliF
ShaoW
MargolickJB
DaarES
2009 Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol 83 2715 2727
14. KeeleBF
GiorgiEE
Salazar-GonzalezJF
DeckerJM
PhamKT
2008 Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105 7552 7557
15. SagarM
LaeyendeckerO
LeeS
GamielJ
WawerMJ
2009 Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis 199 580 589
16. LongEM
MartinHLJr
KreissJK
RainwaterSM
LavreysL
2000 Gender differences in HIV-1 diversity at time of infection. Nat Med 6 71 75
17. RitolaK
PilcherCD
FiscusSA
HoffmanNG
NelsonJA
2004 Multiple V1/V2 env variants are frequently present during primary infection with human immunodeficiency virus type 1. J Virol 78 11208 11218
18. SagarM
LavreysL
BaetenJM
RichardsonBA
MandaliyaK
2004 Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population. Aids 18 615 619
19. HaaseAT
2010 Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464 217 223
20. HarouseJM
GettieA
EshetuT
TanRC
BohmR
2001 Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J Virol 75 1990 1995
21. HarouseJM
GettieA
TanRC
BlanchardJ
Cheng-MayerC
1999 Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284 816 819
22. ChohanB
LangD
SagarM
KorberB
LavreysL
2005 Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol 79 6528 6531
23. SagarM
WuX
LeeS
OverbaughJ
2006 Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol 80 9586 9598
24. WuX
ParastAB
RichardsonBA
NduatiR
John-StewartG
2006 Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol 80 835 844
25. DerdeynCA
HunterE
2008 Viral characteristics of transmitted HIV. Curr Opin HIV AIDS 3 16 21
26. MoorePL
GrayES
MorrisL
2009 Specificity of the autologous neutralizing antibody response. Curr Opin HIV AIDS 4 358 363
27. MoorePL
RanchobeN
LambsonBE
GrayES
CaveE
2009 Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog 5 e1000598
28. RongR
LiB
LynchRM
HaalandRE
MurphyMK
2009 Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 5 e1000594
29. ArthosJ
CicalaC
MartinelliE
MacleodK
Van RykD
2008 HIV-1 envelope protein binds to and signals through integrin alpha(4)beta(7), the gut mucosal homing receptor for peripheral T cells. Nat Immunol 9 3 301 9
30. WagnerN
LohlerJ
KunkelEJ
LeyK
LeungE
1996 Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382 366 370
31. BargatzeRF
JutilaMA
ButcherEC
1995 Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3 99 108
32. CicalaC
MartinelliE
McNallyJP
GoodeDJ
GopaulR
2009 The integrin {alpha}4{beta}7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A 106 49 20877 82
33. KellyKA
WileyD
WiesmeierE
BriskinM
ButchA
2009 The Combination of the Gastrointestinal Integrin (alpha4beta7) and Selectin Ligand Enhances T-Cell Migration to the Reproductive Tract During Infection with Chlamydia trachomatis. Am J Reprod Immunol 6 446 452
34. HuangZ
ChouA
TanguayJ
ShenS
MboudjekaI
2008 Levels of N-linked glycosylation on the V1 loop of HIV-1 Env proteins and their relationship to the antigenicity of Env from primary viral isolates. Curr HIV Res 6 296 305
35. Doria-RoseNA
LearnGH
RodrigoAG
NickleDC
LiF
2005 Human immunodeficiency virus type 1 subtype B ancestral envelope protein is functional and elicits neutralizing antibodies in rabbits similar to those elicited by a circulating subtype B envelope. J Virol 79 11214 11224
36. YehJC
SealsJR
MurphyCI
van HalbeekH
CummingsRD
1993 Site-specific N-glycosylation and oligosaccharide structures of recombinant HIV-1 gp120 derived from a baculovirus expression system. Biochemistry 32 11087 11099
37. BinleyJM
BanYE
CrooksET
EgginkD
OsawaK
2010 Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol 84 5637 5655
38. StanleyP
NarasimhanS
SiminovitchL
SchachterH
1975 Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine—glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A 72 3323 3327
39. LeeEU
RothJ
PaulsonJC
1989 Alteration of terminal glycosylation sequences on N-linked oligosaccharides of Chinese hamster ovary cells by expression of beta-galactoside alpha 2,6-sialyltransferase. J Biol Chem 264 13848 13855
40. ChangVT
CrispinM
AricescuAR
HarveyDJ
NettleshipJE
2007 Glycoprotein structural genomics: solving the glycosylation problem. Structure 15 267 273
41. KrachmarovCP
HonnenWJ
KaymanSC
GornyMK
Zolla-PaznerS
2006 Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol 80 7127 7135
42. KwongPD
DoyleML
CasperDJ
CicalaC
LeavittSA
2002 HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420 678 682
43. WeiX
DeckerJM
WangS
HuiH
KappesJC
2003 Antibody neutralization and escape by HIV-1. Nature 422 307 312
44. LyA
StamatatosL
2000 V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J Virol 74 6769 6776
45. KozakSL
PlattEJ
MadaniN
FerroFEJr
PedenK
1997 CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol 71 873 882
46. PinterA
HonnenWJ
HeY
GornyMK
Zolla-PaznerS
2004 The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J Virol 78 5205 5215
47. KotheDL
LiY
DeckerJM
Bibollet-RucheF
ZammitKP
2006 Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology 352 438 449
48. ArthosJ
CicalaC
SteenbekeTD
ChunTW
Dela CruzC
2002 Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein: implications for therapeutic and vaccine strategies. J Biol Chem 277 11456 11464
49. BennettA
LiuJ
Van RykD
BlissD
ArthosJ
2007 Cryoelectron tomographic analysis of an HIV-neutralizing protein and its complex with native viral gp120. J Biol Chem 282 27754 27759
50. ZhouT
XuL
DeyB
HessellAJ
Van RykD
2007 Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445 732 737
51. CenterRJ
EarlPL
LebowitzJ
SchuckP
MossB
2000 The human immunodeficiency virus type 1 gp120 V2 domain mediates gp41-independent intersubunit contacts. J Virol 74 4448 4455
52. KeeleBF
DerdeynCA
2009 Genetic and antigenic features of the transmitted virus. Curr Opin HIV AIDS 4 352 357
53. MattapallilJJ
DouekDC
HillB
NishimuraY
MartinM
2005 Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434 1093 1097
54. HladikF
HopeTJ
2009 HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep 6 20 28
55. LiB
DeckerJM
JohnsonRW
Bibollet-RucheF
WeiX
2006 Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J Virol 80 5211 5218
56. LiuY
CurlinME
DiemK
ZhaoH
GhoshAK
2008 Env length and N-linked glycosylation following transmission of human immunodeficiency virus Type 1 subtype B viruses. Virology 374 229 233
57. HawkinsRA
RankRG
KellyKA
2000 Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun 68 5587 5594
58. KellyKA
ChanAM
ButchA
DarvilleT
2009 Two Different Homing Pathways Involving Integrin beta7 and E-selectin Significantly Influence Trafficking of CD4 Cells to the Genital Tract Following Chlamydia muridarum Infection. Am J Reprod Immunol 184 885 891
59. KellyKA
NatarajanS
RutherP
WisseA
ChangMH
2001 Chlamydia trachomatis infection induces mucosal addressin cell adhesion molecule-1 and vascular cell adhesion molecule-1, providing an immunologic link between the fallopian tube and other mucosal tissues. J Infect Dis 184 885 891
60. JenkinsN
ParekhRB
JamesDC
1996 Getting the glycosylation right: implications for the biotechnology industry. Nat Biotechnol 14 975 981
61. MarthJD
GrewalPK
2008 Mammalian glycosylation in immunity. Nat Rev Immunol 8 874 887
62. WilleyRL
ShibataR
FreedEO
ChoMW
MartinMA
1996 Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J Virol 70 6431 6436
63. DooresKJ
BonomelliC
HarveyDJ
VasiljevicS
DwekRA
2010 Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci U S A 107 13800 13805
64. PinterA
2007 Roles of HIV-1 Env variable regions in viral neutralization and vaccine development. Curr HIV Res 5 542 553
65. Zolla-PaznerS
CardozoT
2010 Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat Rev Immunol 10 527 535
66. HuSL
StamatatosL
2007 Prospects of HIV Env modification as an approach to HIV vaccine design. Curr HIV Res 5 507 513
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



