-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
Invasion of erythrocytes by Plasmodium falciparum involves a complex cascade of protein-protein interactions between parasite ligands and host receptors. The reticulocyte binding-like homologue (PfRh) protein family is involved in binding to and initiating entry of the invasive merozoite into erythrocytes. An important member of this family is PfRh5. Using ion-exchange chromatography, immunoprecipitation and mass spectroscopy, we have identified a novel cysteine-rich protein we have called P. falciparum Rh5 interacting protein (PfRipr) (PFC1045c), which forms a complex with PfRh5 in merozoites. Mature PfRipr has a molecular weight of 123 kDa with 10 epidermal growth factor-like domains and 87 cysteine residues distributed along the protein. In mature schizont stages this protein is processed into two polypeptides that associate and form a complex with PfRh5. The PfRipr protein localises to the apical end of the merozoites in micronemes whilst PfRh5 is contained within rhoptries and both are released during invasion when they form a complex that is shed into the culture supernatant. Antibodies to PfRipr1 potently inhibit merozoite attachment and invasion into human red blood cells consistent with this complex playing an essential role in this process.
Published in the journal: An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by. PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002199
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002199Summary
Invasion of erythrocytes by Plasmodium falciparum involves a complex cascade of protein-protein interactions between parasite ligands and host receptors. The reticulocyte binding-like homologue (PfRh) protein family is involved in binding to and initiating entry of the invasive merozoite into erythrocytes. An important member of this family is PfRh5. Using ion-exchange chromatography, immunoprecipitation and mass spectroscopy, we have identified a novel cysteine-rich protein we have called P. falciparum Rh5 interacting protein (PfRipr) (PFC1045c), which forms a complex with PfRh5 in merozoites. Mature PfRipr has a molecular weight of 123 kDa with 10 epidermal growth factor-like domains and 87 cysteine residues distributed along the protein. In mature schizont stages this protein is processed into two polypeptides that associate and form a complex with PfRh5. The PfRipr protein localises to the apical end of the merozoites in micronemes whilst PfRh5 is contained within rhoptries and both are released during invasion when they form a complex that is shed into the culture supernatant. Antibodies to PfRipr1 potently inhibit merozoite attachment and invasion into human red blood cells consistent with this complex playing an essential role in this process.
Introduction
Malaria is caused by parasites from the genus Plasmodium, of which Plasmodium falciparum is associated with the most severe form of the disease in humans. Sporozoite forms of these parasites are injected into humans during mosquito feeding and they migrate to the liver where they invade hepatocytes and develop into merozoites, which are released to invade erythrocytes in the blood stream. The blood stage cycle of P. falciparum is responsible for all of the clinical symptoms associated with malaria [1]. Once a merozoite has invaded an erythrocyte it develops, within this protected intracellular niche, to form around 16 new merozoites that are released and then bind and invade other red blood cells. Invasion of merozoites into the host erythrocyte is a rapid process involving multiple steps in a cascade of protein-protein interactions (see for review [2]).
The reticulocyte binding-like homologues (PfRh or PfRBP) and erythrocyte binding-like (EBL) proteins play important roles in merozoite invasion [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The PfRh family consists of PfRh1 (PFD0110w), PfRh2a (PF13_0198), PfRh2b (MAL13P1.176), PfRh3 (PFL2520w), PfRh4 (PFD1150c) and PfRh5 (PFD1145c) [4], [5], [7], [9], [16], [17], [18], [19], [20]. PfRh3 is a transcribed psuedogene in all the P. falciparum strains that have been analysed [21]. PfRh1, PfRh2b, PfRh2a, PfRh4 and PfRh5 bind to erythrocytes and antibodies to them can inhibit merozoite invasion thus showing they play a role in this process [11], [13], [18], [19], [20], [22], [23], [24].
Polymorphisms in the PfRh5 protein have been linked to differential virulence in infection of Aotus monkeys suggesting that amino acid changes in its binding domain can switch receptor recognition [19]. PfRh5 has been shown to bind red blood cells but its putative receptor has not been identified [18], [19], [20]. In contrast to other members of the PfRh protein family, PfRh5 is considerably smaller and lacks a transmembrane region, which combined with its role as an invasion ligand, suggests it may be part of a functional complex. It has not been possible to genetically disrupt the gene encoding PfRh5 and antibodies to it can partially inhibit merozoite invasion, pointing to an essential role of this protein in the invasion process [20].
The EBL family of proteins includes EBA-175 (MAL7P1.176) [3], [26], EBA-181 (also known as JESEBL) (PFA0125c) [27], [28], EBA-140 (also known as BAEBL) (MAL13P1.60) [6], [29], [30], [31] and EBL-1 [32]. Whilst these parasite ligands function in merozoite invasion by binding to specific receptors on the erythrocyte, they appear to have a central role in activation of the invasion process. For example, it has been shown that binding of EBA-175 to its receptor, glycophorin A restores the basal cytosolic calcium levels after interaction of the merozoite with the erythrocyte and triggers release of rhoptry proteins and it is likely that the PfRh protein family plays a similar role [37].
The PfRh and EBL protein families are responsible for a mechanism of phenotypic variation that allows different strains of P. falciparum to invade erythrocytes using different patterns of host receptors [7], [28], [38]. This is important for evasion of host immune responses and also provides a means to circumvent the polymorphic nature of the erythrocyte surface in the human population [39]. Indeed, analysis of immune responses from individuals in malaria endemic areas has suggested that the PfRh proteins are targets of human invasion inhibitory antibodies and therefore a critical component of acquired protective immunity [39], [40], [41].
In order to understand the function of PfRh5 in merozoite invasion, we purified it from parasite culture supernatant and show this protein exists as a complex with a cysteine-rich protein containing 10 epidermal growth factor-like domains (PFC1045c). This novel protein functions with PfRh5 to play an essential role in invasion of merozoites into human erythrocytes.
Results
Purification and identification of a PfRh5 complex
PfRh5 binds to erythrocytes and is functionally important in merozoite invasion of erythrocytes [18], [19], [20]. Whilst other members of the PfRh family also bind erythrocytes, they have a transmembrane region, a feature that PfRh5 lacks suggesting that it may exist as a complex. In order to determine if PfRh5 was shed as a complex into culture supernatant during merozoite invasion we set out to purify it using ion-exchange chromatography. PfRh5, that had been partially purified from culture supernatant by ion-exchange chromatography, migrated on SDS-PAGE gels as an approximately 45 kDa fragment as detected using 2F1, a anti-PfRh5 specific monoclonal antibody (data not shown), consistent with our previous results showing that the 63 kDa mature PfRh5 was processed in late schizonts and shed into culture supernatant during merozoite invasion [20]. However, analysis of PfRh5 by gel-filtration chromatography revealed that the 45 kDa polypeptide was eluted in the peak (# 19–24) (Fig. 1A) which is immediately before the peak of 158 kDa bovine Υ-globulin (#21–25) (Fig. S1), suggesting PfRh5 is indeed in a complex with a molecular weight of approximately 150–200 kDa.
Fig. 1. The processed 45 kDa PfRh5 C-terminal domain exists as a larger complex. 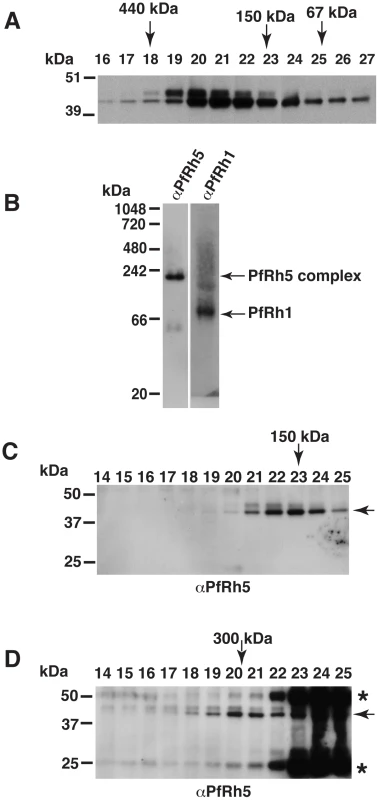
(A) PfRh5 partially purified from parasite culture supernatant was analysed by size exclusion chromatography on a Superdex 200 analytical column. PfRh5 was eluted from the column as a ∼150–200 kDa species. Molecular weight of eluted protein fractions are indicated by arrows as shown using standard proteins of known size. PfRh5 was detected using 2F1, a specific monoclonal antibody to the protein. (B) Blue native gel electrophoresis confirmed that PfRh5 migrates as a ∼150–200 kDa species. The processed PfRh1 fragment of 110 kDa is included as a control. (C) 300 µl of the PfRh5-containing fraction, isolated from culture supernatant, was loaded onto a Superdex 200 analytical column and eluted with PBS. (D) An identical 300 µl sample was pre-incubated with 25 µg of monoclonal anti-PfRh5 antibody before loading onto the same Superdex 200 column. The peak of the eluted PfRh5 protein lies at two preceding fractions as compared to panel C, corresponding to an increase in molecular weight of ∼150 kDa indicating that one antibody molecule was bound to the PfRh5-containing species. The monoclonal antibody-bound PfRh5 is indicated by an arrow. PfRh5 and the immunoglobulin heavy and light chain of antibody seen in fraction #22–25 are the free PfRh5 complex and the excess antibody. The asterisks refer to cross-hybridising bands corresponding to the immunoglobulin heavy and light chain. To confirm PfRh5 exists as a higher molecular weight species, blue native gel electrophoresis was performed and showed the PfRh5 migrated at an approximate molecular weight of 150–200 kDa (Fig. 1 B). This was in contrast with the processed PfRh1 fragment that migrated at the expected size of 110 kDa (Fig. 1B) [12]. This suggested that PfRh5 either forms a homo-oligomer or was in complex with other molecule/s. To distinguish between these possibilities, gel-filtration chromatography was used to identify the size increment of PfRh5-containing species after it was incubated with a monoclonal anti-PfRh5 antibody [20]. It was found that pre-incubation with anti-PfRh5 antibody caused the PfRh5 protein peak to appear two fractions earlier, which correspond to an increase in size of the PfRh5-containing species by 150 kDa, indicating that one antibody molecule was associated with the complex (Fig. 1C and D). The free PfRh5 co-eluted with the excess antibody in the protein peak comprising fraction 22 to 25 (Fig. 1D), further indicating PfRh5 is in a complex of approximate 150 kDa. Therefore we proposed that the processed 45 kDa PfRh5 species formed a complex with another molecule/s rather than existing as a homo-oligomer.
To identify the proteins present within the PfRh5 complex, we performed a large-scale purification using culture supernatant from the parasite line 3D7-Rh5HA in which the PfRh5 gene had been tagged with haemagglutinin epitopes [20]. The PfRh5 protein was enriched and partially purified using ion-exchange chromatography followed by further purification on an anti-HA affinity matrix. The bound proteins were eluted and subjected to trypsin digestion followed by analysis of the resulting peptides by mass spectrometry (LC-MS/MS) [42]. Since powerful anion ion-exchange chromatography was employed to enrich the complex and remove the majority of contaminants before it was subjected to anti-HA antibody affinity pull-down), the mass spectrometry analysis yielded a very clean result (Table S1). Database analysis identified parasite protein peptides that were predominantly derived from two proteins, which included PfRh5 and a conserved hypothetical protein (PFC1045c), designated here as P. falciparum PfRh5 interacting protein (PfRipr) (Table 1 and Table S1). The most N-terminal peptide found for PfRh5 was amino acid 187–197, consistent with it being shed into culture supernatant as an approximately 45 kDa fragment produced by cleavage between amino acid 125 and 135 of the mature protein. In the case of PfRipr, we identified peptides from both N-terminal and C-terminal regions of the protein suggesting that the full-length protein was present within the complex and this was confirmed by co-immunoprecipitation experiments with specific antibodies (see below section). The mature form of PfRipr was 123 kDa and this together with the 45 kDa PfRh5 fragment would produce a complex with an overall molecular weight of approximately 170 kDa in agreement with the size observed by gel-filtration chromatography and blue native gel electrophoresis (Fig. 1A and B). The PfRh5/PfRipr complex was stably purified by ion-exchange chromatography and 350 mM NaCl in the elution buffer did not disrupt their association, suggesting the tight interaction of two proteins. Such specific high affinity protein-protein interaction implies the biological significance of the complex.
Tab. 1. Peptides identified by Mass spectrometry from purified PfRh5. 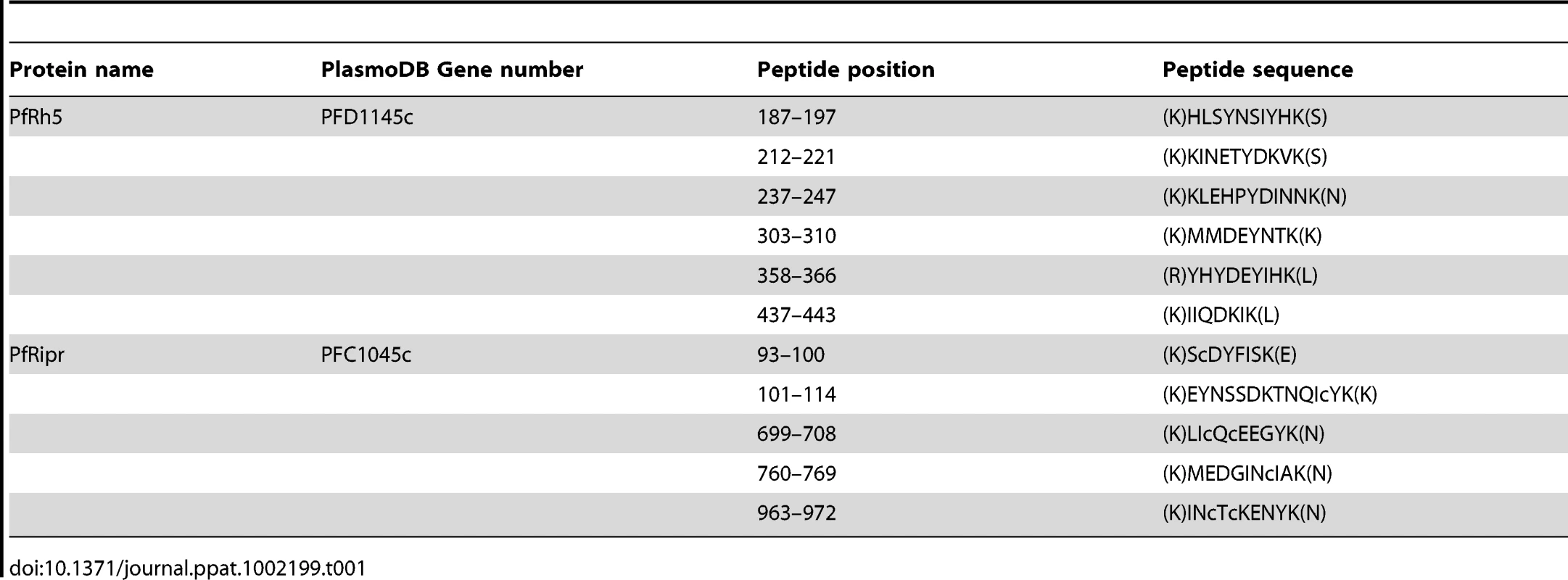
PfRh5 and PfRipr form a complex in P. falciparum
In order to confirm that PfRh5 and PfRipr form a complex, we inserted a plasmid by single cross-over homologous recombination at the 3′ end of the Pfripr gene to fuse three HA epitopes and derived the parasite line 3D7RiprHA (Fig. 2A) [20]. Successful tagging was confirmed by immunoblot experiments using anti-HA antibodies on both saponin lysed schizont pellet preparations and proteins from culture supernatants (Fig. 2B). In parasite schizont pellets a minor band of approximately 125 kDa and a major band of approximately 65 kDa were detected whereas in culture supernatants only the smaller protein band was predominantly observed. The 125 kDa protein presumably represents the full-length mature protein in schizonts that was processed and shed into the culture supernatant. Since only one processed fragment of 65 kDa was detected in culture supernatants using anti-HA antibody, we propose that PfRipr was processed into at least two fragments of approximately the same size. Mobility of the 65 kDa fragment remains similar under the reducing and non-reducing condition (Fig. 2C) demonstrating that the processed fragments are not linked by a disulphide bond. Both 65 and 125 kDa fragments migrated slightly faster on SDS-PAGE gels under non-reducing conditions consistent with the presence of multiple intramolecular disulfide linkages creating tightly folded domains (Fig. 2C).
Fig. 2. PfRh5 and PfRipr form a complex. 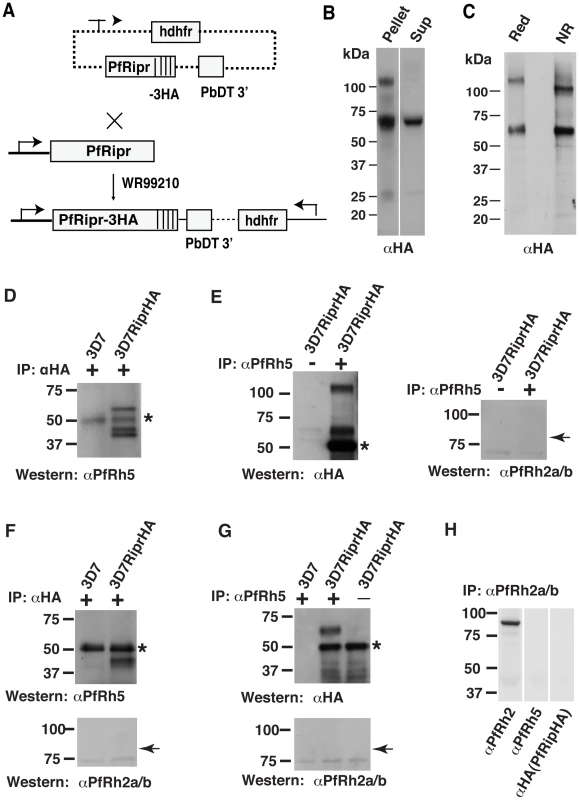
(A) A triple Haemaglutinin (HA) tag was added to the C-terminus of PfRipr by 3′ single homologous crossover recombination. (B) HA-tagged PfRiprHA was detected in the parasite membrane pellet and in the culture supernatant of 3D7PfRiprHA line, confirming successful tagging. (C) PfRiprHA was analysed by SDS-PAGE under reducing and non-reducing conditions. Similar mobility, of the 65 kDa C-terminal fragment under both conditions, indicates that the N-terminal and C-terminal of PfRipr was not linked by disulphide bonds after processing. (D) Co-immunoprecitation of PfRh5 with PfRiprHA was performed using proteins solubilized from saponin pellets of 3D7-PfRiprHA schizont-infected erythrocytes. Immunoprecipitation of HA-tagged PfRipr by anti-HA antibody co-immunoprecipitated PfRh5. (E) Immunoprecitation with anti-PfRh5 antibody using the same schizont materials co-immunoprecipited PfRiprHA but not PfRh2a/b. (F) Co-immnuoprecipitation of PfRh5 with PfRiprHA from culture supernatants using anti-HA-Sepharose bead. Detection of PfRh5 but not PfRh2a or PfRh2b in the anti-HA bead bound material confirmed that PfRh5 was specifically co-immunoprecipitated with PfRiprHA. (G) Co-immunoprecipitation of PfRiprHA with PfRh5 from culture supernatants using monoclonal anti-PfRh5 antibody coupled to Mini-beads. Probing of the bound material with anti-HA antibody detected PfRiprHA only from the 3D7-PfRiprHA parasites and no PfRh2a/b was detected in the bound materials, indicating that PfRiprHA was specifically co-immunoprecipitated with PfRh5. (H) Immnunoprecipitation of culture supernatant from PfRiprHA parasites with rabbit anti-PfRh2a/b antibodies that recognize 85 kDa processed PfRh2a/b fragment did not pull-down PfRh5 or PfRipr. In all cases, the asterisk indicates a cross-hybridizing band corresponding to the heavy chain of IgG eluted from the antibody affinity beads. The arrows in Figure 2E, F and G indicate where 85 kDa PfRh2a/b band should appear. To further confirm that PfRh5 and PfRipr form a complex, we performed co-immunoprecipitation experiments with anti-PfRh5 and anti-HA antibodies using proteins from the parasite line 3D7RiprHA (Fig. 2D to G). Immunoprecipitation with anti-HA antibodies, to pull-down tagged PfRipr from schizont-infected erythrocytes, also brought down PfRh5 as shown by immunoblots with a specific monoclonal anti-PfRh5 antibody (Fig. 2D). The full-length mature protein (approximately 63 kDa) as well as two processed fragments of similar molecular weight (approximately 45 kDa) was observed (Fig. 2D). The reciprocal experiment in which we immunoprecipitated PfRh5, using the monoclonal anti-PfRh5 antibody, also pulled down PfRipr (Fig. 2E, left panel) but not PfRh2a or PfRh2b, two other members of PfRh protein family (Fig. 2E, right panel). Using culture supernatants, a similar immunoprecipitation experiment with the anti-HA antibodies to pull down PfRiprHA also co-precipitated the 45 kDa doublet of PfRh5 but not PfRh2a or PfRh2b (Fig. 2F). The reciprocal experiment using culture supernatants in which anti-PfRh5 antibody was used for immunoprecipitation detected HA-tagged PfRipr but not PfRh2a or PfRh2b in the pull-down sample (Fig. 2G). As an additional control, immunoprecipitaion of the culture supernatant with rabbit anti-PfRh2a/b antibody recognizing the 85 kDa fragment of both PfRh2a and PfRh2b was performed [24]. The anti-PfRh2a/b antibodies immunoprecipitated the 85 kDa PfRh2a/b but did not pull-down PfRh5 or PfRipr, further confirming that the PfRh5/PfRipr complex was specific. It was not possible to determine with these experiments whether the PfRipr/PfRh5 proteins associate in schizont stage parasites or at a later stage. However, since PfRh5 and PfRipr appear to be located in different compartments within the schizont (see below section), it is likely that they form a complex during detergent extraction as has been shown for the AMA1 and RON complex [43], [44], [45].
PfRipr is a cysteine-rich protein that contains epidermal growth factor-like domains
PfRipr is highly conserved in Plasmodium spp. and the gene is syntenic with other genes, all of which are annotated as hypothetical (http://plasmodb.org). The full-length PfRipr protein consists of 1,086 amino acids with a molecular weight of 126 kDa. It has a putative hydrophobic signal sequence at the N-terminus consistent with it being secreted and the rest of the protein contains 87 cysteine-residues, many of which are clustered in epidermal growth factor (EGF)-like domains (Fig. 3A and B). There are ten EGF-like domains in the protein with two in the N-terminal region and eight clustered towards the C-terminus (Fig. 3A). An EGF domain has 6 cysteine residues and the position of each is relatively conserved in the ten EGF-like domains of PfRipr (Fig. 3B) [46], [47]. Processing of PfRipr occurs in the area between the second and third EGF-like domain (Fig. 3A); however, the processed N - and C-terminal regions remain associated as peptides from both regions were obtained in the mass spectrometry analysis of the immuno-precipitated PfRh5/PfRipr complex (Table 1) and the two regions were co-immunoprecipitated (see below).
Fig. 3. PfRipr protein has ten EGF-like domains. 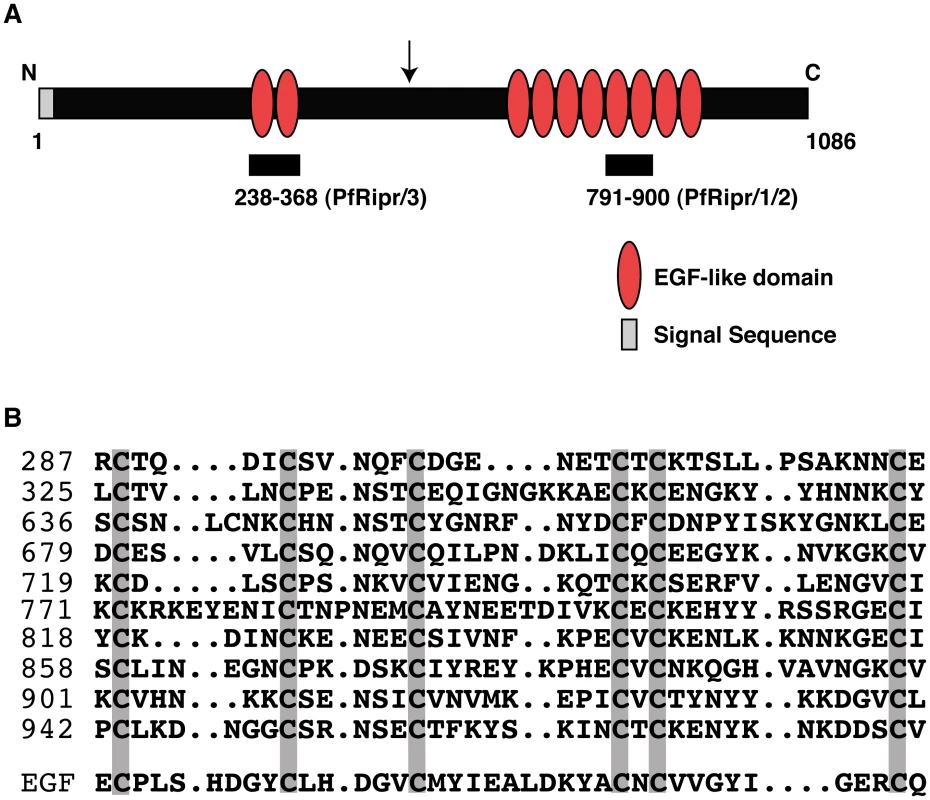
(A) Diagram shows the characteristics of PfRipr and the region used to generate the recombinant proteins. The arrow corresponds to the approximate position of the PfRipr cleavage site. (B) Alignment of the ten EGF-like domains within PfRipr. The N - and C-terminal domains of PfRipr form a complex
To provide additional tools to probe the function of PfRh5/PfRipr complex, we expressed amino acids 238–368 of PfRipr that encompass the two EGF-like domains in the N-terminus as well as amino acids 791–900 of PfRir that encompass two EGF-like domains in the C-terminus (Fig. 3A), in E. coli as a recombinant protein tagged at the N-terminus with six histidines. The recombinant proteins of approximately 17 kDa were purified (Fig. 4A) and used to immunise rabbits. The anti-PfRipr/1 and -PfRipr/2 IgG antibodies, raised in two rabbits, were tested by western blot against solubilised pellets from schizont stages and a protein band of approximately 65 kDa was observed (Fig. 4B). Specificity of the antibodies was confirmed with HA-tagged PfRipr immunoprecipitated from culture supernatant with anti-HA antibodies and in both cases a 65 kDa protein was detected (Fig. 4B). This was the molecular weight expected for the processed C-terminal region of PfRipr; however, the mature full-length protein of 123 kDa was not easily observed as it was mostly processed at late schizont stage and in the supernatant. The anti-PfRipr/3 antibody has low reactivity to the denature protein but recognizes native PfRipr well.
Fig. 4. The N-terminal and C-terminal cleaved polypeptides remain associated after cleavage. 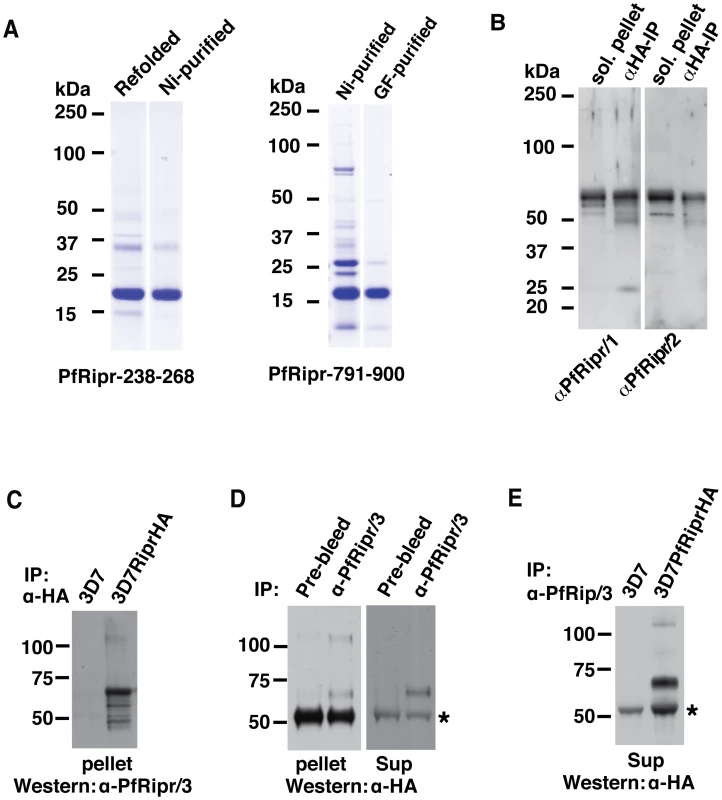
(A) Amino acids 238-368 and 791-900 of PfRipr that encompassed the N-terminal first two EGF-like domains and C-terminal two EGF-like domains respectively were expressed as recombinant proteins in E. coli and purified for immunizing rabbits. The proteins were stained with Coomassie blue. (B) Rabbit polyclonal antibodies (anti-PfRipr/1 and anti-PfRipr/2) raised against the recombinant protein recognize native PfRipr. (C) Rat anti-HA antibody affinity beads were used to specifically immuno-precipitate proteins solubilized from schizont stages parasites of 3D7 and 3D7RiprHA lines and the bound materials immunoblotted and probed with rabbit anti-PfRipr/3 antibodies. (D) Rabbit anti-PfRipr/3 antibodies, in conjunction with protein G Sepharose, was used to specifically immuno-precipitate the proteins solubilized from schizont stages of 3D7 (panel 1) or culture supernatants (panel 2) and then the bound material was immunoblotted and probed with mouse anti-HA antibodies. The IgG from the pre-bleed serum of the same rabbit was used as a control. (E) Rabbit anti-PfRipr/3 antibodies, in conjunction with protein G Sepharose, was used to specifically immuno-precipitate the proteins from culture supernatant of 3D7 and 3D7RiprHA parasites and the bound material immunoblotted and probed with mouse anti-HA antibodies. The protein marked with a * in the panel D and E is protein G eluted from the beads that cross-reacts with the anti-HA antibodies. The anti-PfRipr/3 was therefore used in co-immunoprecipitation experiments to confirm that the cleaved N-terminus and C-terminus of PfRipr remain associated. Immunoprecipitation of the HA-tagged C-terminus of PfRipr from 3D7RiprHA parasites using anti-HA antibodies co-precipitated a fragment of approximately 60 kDa recognized by the anti-PfRipr/3 (Fig. 4C). The size of the fragment is expected for the N-terminal domain of the processed PfRipr. The full-length mature protein of approximately 125 kDa was also observed as expected since it should react with both the anti-PfRipr/3 and anti-HA antibodies. The reciprocal experiment to immunoprecipitate the N-terminal domain with schizont material using anti-PfRipr/3 co-precipitated the 65 kDa C-terminal fragment and the 125 kDa full length protein (Fig. 4D, left panel). An identical experiment using culture supernatant co-precipitated the 65 kDa C-terminal domain (Fig. 4D, right panel); the full-length protein was not detected in this case, presumably because it was processed. Immunoprecipitation using anti-PfRipr/3 and immunoblots with anti-HA antibodies detected the expected bands of the 65 kDa C-terminal domain and the 125 kDa full-length protein in 3D7RiprHA but not from the parental parasite line 3D7 (Fig. 4E), further confirming the specificity of the antibodies. Together these experiments show that PfRipr is processed into two fragments of approximately 65 kDa each and they remain associated with each other to form a complex with PfRh5, consistent with mass spectrometry results (Table 1). Their association does not involve intermolecular disulfide linkages (Fig. 2C).
The anti-PfRipr/1 was used in western blot to probe the PfRipr separated on a blue native gel (Fig. 5A). Indeed anti-PfRipr/1 recognizes the same protein complex detected by anti-PfRh5, with the size of the complex (approximately 200 kDa) consistent with that seen in Fig. 1B.
Fig. 5. Protein expression patterns of PfRh5 and PfRipr are consistent with the complex formation. 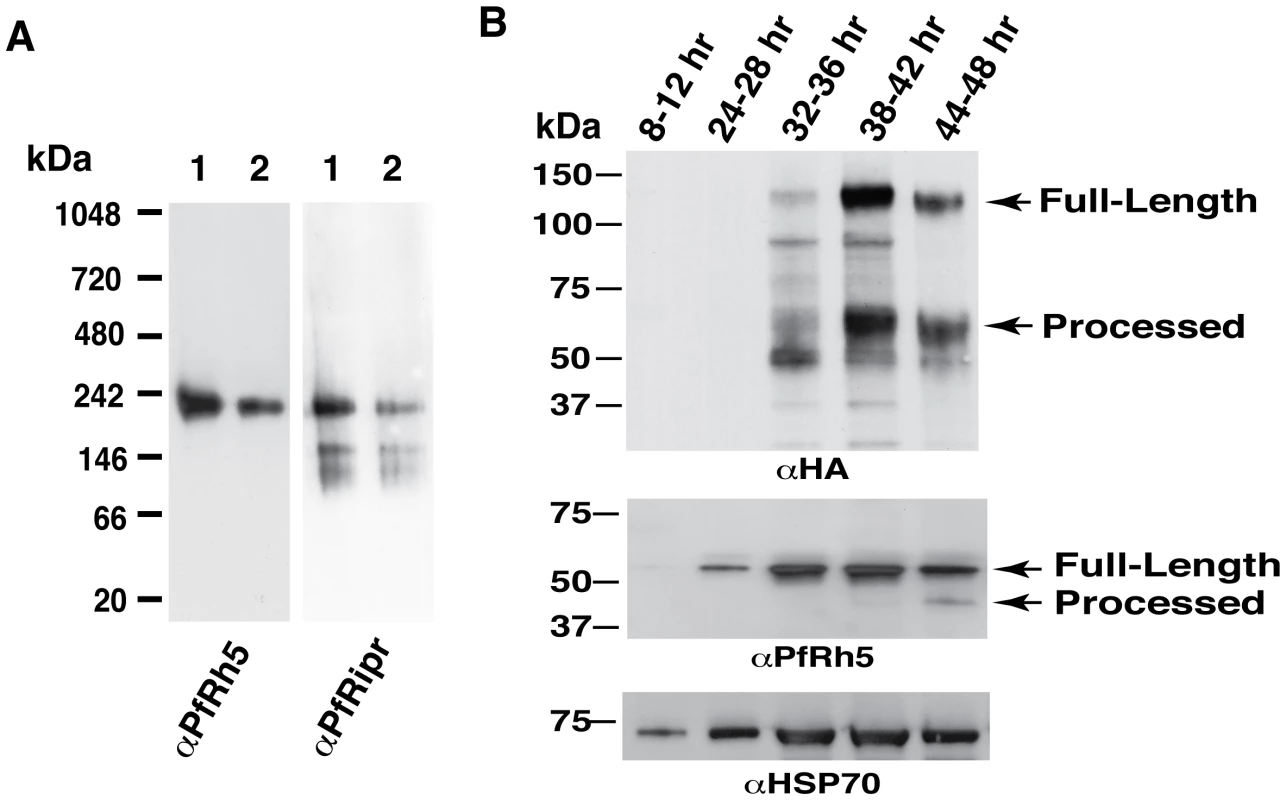
(A) Both anti-PfRh5 and anti-PfRipr antibodies recognize the same complex resolved by blue native gel electrophoresis. (B) Both PfRh5 and PfRipr are expressed late in the blood stage life cycle of parasite development. Synchronized PfRiprHA parasites were distributed into six 5 ml cultures. One was harvested immediately after synchronization (8 – 12 hr ring stage). The second was harvested 16 hr post synchronization, and the third 8 hr later. The rest of the time points were harvested every 6 hr until the end of growth cycle. Proteins were extracted from saponin pellets, separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was probed with monoclonal anti-HA antibody (top panel) to detect PfRiprHA and then stripped and re-probed to detect PfRh5 (middle panel) and PfHsp70 (bottom panel). Both PfRh5 and PfRipr proteins are expressed primarily in late schizonts. Expression of PfRipr in the asexual blood stage life cycle was determined using synchronised ring stages parasites from 3D7RiprHA. Saponin pellets were prepared from the samples taken during the parasite life cycle as indicated (Fig. 5B). Anti-HA antibody detected both the 125 kDa full-length and 65 kDa fragment of PfRipr and expression was evident in late trophozoites to schizonts, the expected pattern for proteins that play a role in merozoite invasion [20]. The 65 kDa processed form appeared late in parasite blood stage development indicating that this processing event occurs in the schizont stage before merozoite egress. The same samples were probed for PfRh5, with which PfRipr forms a complex, and it showed a similar timing of expression but processing of this protein appears to occur late in schizogony, as observed earlier [20]. The similar pattern of PfRipr and PfRh5 expression late in the asexual blood stage life cycle of P. falciparum was consistent with formation of a complex between them either in the schizont stage or during merozoite egress before interaction of the parasite with the host cell.
The PfRipr/PfRh5 complex is tightly associated with the membrane of P. falciparum
Whilst both PfRipr and PfRh5 have a signal sequence at the N-terminus for entry into the ER, neither has a hydrophobic domain that would provide the means for anchoring in the membrane. As PfRh5 binds to the host erythrocyte [18], [19], [20] it would be expected that the PfRh5/PfRipr complex would be bound to the membrane of the merozoite to provide a junction and perhaps transmit an appropriate signal [37]. To determine if this complex was associated with a membrane, we used differential solubilisation of the parasite to determine the properties of the PfRipr/PfRh5 complex (Fig. 6). P. falciparum parasites were hypotonically lysed in water and centrifuged to obtain a pellet and supernatant fraction. Treatment of the pellet with 10 mM Tris showed that both PfRipr and PfRh5 remained predominantly in the pellet fraction suggesting they were associated with a membrane (Fig. 6A). However, treatment of the pellet fraction with Na2CO3 showed that both proteins moved to the soluble fraction suggesting they were extrinsically associated as peripheral membrane proteins of the parasite. PfRipr was mainly insoluble in Triton X-100 but the smaller processed form of the protein was more soluble in CHAPs detergent. In contrast, PfRh5 was more soluble in Triton X-100 and CHAPS implying either PfRipr/PfRh5 complex involves hydrophobic interactions that can be disrupted by non-ionic detergents [48], or PfRipr and PfRh5 have different membrane association properties. Similar solubility characteristics were obtained from saponin treated parasites isolated with saponin treatment (Fig. 6B). In summary, these results showed that the PfRh5/PfRipr complex was peripherally associated with the parasite membrane and it is possible that another protein(s) may be involved in the membrane attachment of the PfRh5/PfRipr complex.
Fig. 6. PfRipr and PfRh5 are peripherally associated with parasite membrane. 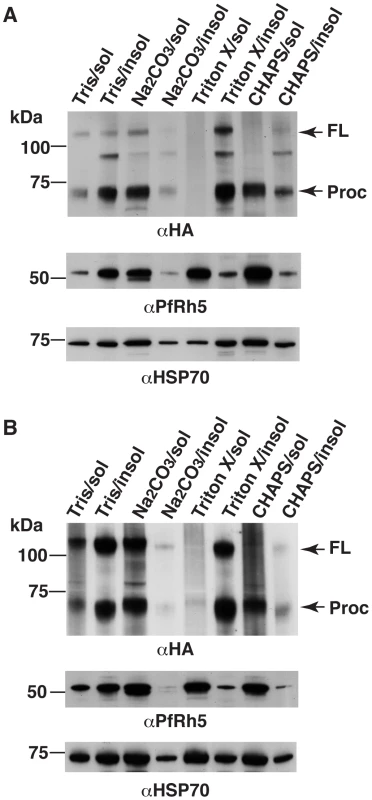
(A) Differential solubilization of proteins from pellet prepared by hypotonic lysis of red blood cells that had been infected by the late schitzont stage 3D7-RiprHA parasite. (B) Differential solubilization of proteins from saponin pellets of late schitzont stage 3D7RiprHA parasites. PfRipr is located in the apical organelles of the merozoite
With anti-PfRipr antibodies, we performed immuno-fluorescence assays to determine the subcellular localization of PfRipr with respect to known markers of compartments such as micronemes and rhoptries, organelles that are important for erythrocyte invasion. We firstly confirmed that the HA-tagged PfRipr protein showed the same localisation when detected using both anti-HA and anti-PfRipr/1 antibodies, with both showing a punctate pattern in schizonts (Fig. 7, panel a). Co-labelling of schizonts and merozoites with PfRipr and the rhoptry neck marker RON4 (Fig. 7, panel b and c) or the rhoptry body marker, RAP1 (Fig. 7, panel d and e), both showed close alignment but no significant overlap of signal. These results suggest that PfRipr localises to the apical tip of the merozoite in an area distinct from the very apical tip and the bulb of the rhoptries. Comparison of the subcellular localisation of PfRh5 and PfRipr in schizont stages showed that whilst there appeared to be some overlap, there were substantial areas of the punctate pattern that were clearly in a separate part of the cell (Fig. 7, panel f). In contrast, PfRh5 and PfRipr showed substantial co-localisation in merozoites suggesting that the two proteins may form a complex in late schitzonts or merozoits (Fig. 7, panel g). Furthermore, the substantial overlap of PfRipr and the micronemal protein EBA-175 in the schizont stage suggest that PfRipr may be predominately present within this organelle at this stage (Fig. 7, panel h). However, in merozoites they clearly did not co-localise although there was some overlap (Fig. 7, panel i). Taken together, these data are consistent with PfRipr localising to micronemes and being released in merozoites.
Fig. 7. PfRipr localizes to the apical end of merozoites. 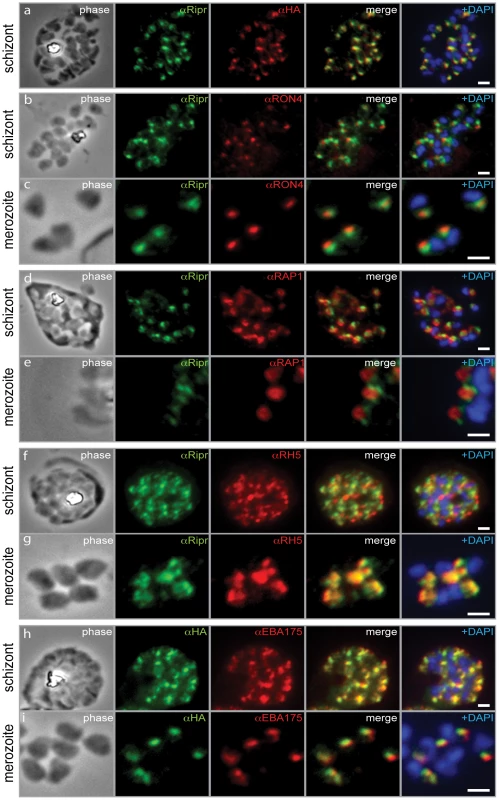
a) Rabbit polyclonal anti-PfRip antibody (anti-PfRipr/1) recognizes HA-tagged PfRiprHA. b) PfRipr does not co-localize with the rhoptry neck protein RON4 in schizonts. c) PfRipr does not co-localize with the rhoptry neck protein RON4 in merozoites. d) PfRipr does not co-localize with the rhoptry bulb protein, RAP1 in schizonts. e) PfRipr does not co-localize with the rhoptry bulb protein, RAP1 in merozoites. f) PfRipr partially co-localizes with PfRh5 in the schizonts. g) PfRipr mainly co-localizes with PfRh5 in purified merozoites. h) PfRipr co-localizes with the micronemal marker, EBA175, in schizonts. i) PfRipr does not co-localize with the micronemal marker, EBA175, in merozoites. To more finely determine the subcellular localisation of PfRipr, immuno-electron microscopy was performed on 3D7RiprHA merozoites (Fig 8A to D and Fig. S2). HA-tagged PfRiprHA was observed towards the apical end of merozoites in more electron dense structures, suggesting localisation in micronemes (Fig. 8A). In merozoites fixed during erythrocyte invasion we observed a concentration of PfRipr reactivity at the leading edge of the tight junction, consistent with the protein being shed and released into the culture supernatant during invasion (Fig. 8B). This suggests that PfRipr was shed from the merozoite surface as the tight junction moves across its surface so that it concentrates at the posterior end before sealing of the parasitophorous vacuole membrane. Whilst there were clearly concentrations of PfRipr in structures at the apical end (Fig. 8C) we also observed considerable surface localisation on free merozoites suggesting it is released prior to invasion (Fig. 8D). In comparison PfRh5 seems to localise to rhoptries (Fig. 8E, F) as we have observed previously [20]; however, there was also some labelling at the apical surface, suggesting this protein was also being released prior to invasion (Fig. 8 E, F). These results are consistent with that observed by immuno-fluorescent microscopy (Fig. 7). From these localization data, we suggest that PfRipr and PfRh5 localise to separate subcellular compartments and thus do not form a complex in schizont stage parasites. Presumably following fusion, or secretion, of these distinct compartments, they then come together in merozoite or late schizont stages. Once complexed and at the apical end of the merozoite, they interact with a receptor on the erythrocyte surface. Such an interaction between proteins originating in separate organelles is not unprecedented, with one prominent example being the interaction between AMA1 and RON4 to form the invasion tight junction across apicomplexan species [43], [44], [45].
Fig. 8. Subcellular localization of PfRipr and PfRh5 by immuno-electron microscopy. 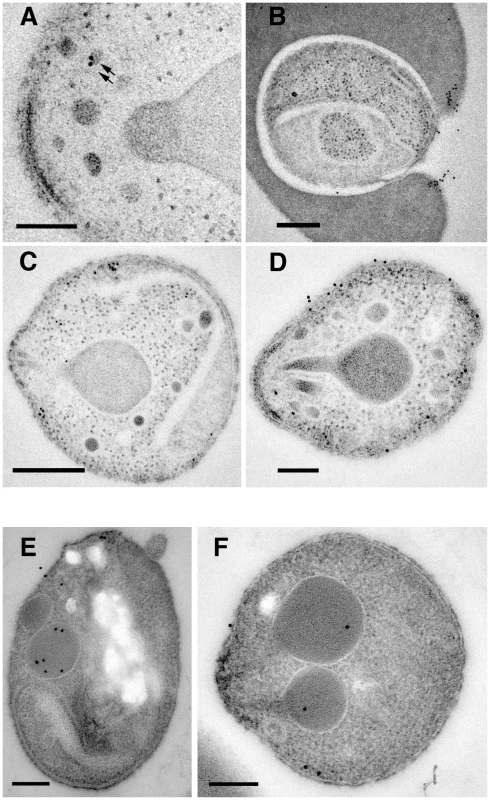
(A) Localisation of HA-tagged PfRiprHA using anti-HA antibodies. The protein localizes to electron dense structures at the apical end of the merozoite that resemble micronemes (arrows). Mn, micronemes: Rh, rhoptries. (B) PfRiprHA can be detected at the leading edge of the tight junction formed between the erythrocyte and merozoite in a merozoite in the process of invading a red blood cell. Nu, nucleus: TJ, tight junction. (C) PfRiprHA can be observed concentrating in structures at the apical end at the periphery of the parasite. Rh, rhoptries. (D) Some merozoites show labeling of PfRipHA on the merozoite surface. (E) and (F) Localisation of PfRh5 using monoclonal anti-PfRh5 antibody. PfRh5 localises to rhoptries but was also observed at the apical surface, suggesting it was being released in free merozoites. Rh, Rhoptries. Scale bar is 200 nm. Antibodies to PfRipr inhibit attachment and invasion of P. falciparum merozoites
To determine if PfRipr was required for development of P. falciparum, we tested the ability of anti-PfRipr/1 and/2 antibodies (Fig. 3) to block parasite growth (growth inhibition assays, GIA [7]) using the P. falciparum strains FCR3, W2mef, T994, CSL2, E8B, MCAMP, 7G8, D10, HB3, and 3D7 (Fig. 9A). The antibodies inhibited parasite growth of all strains tested and significantly, FCR3 was inhibited to 80% whilst in comparison 3D7 was inhibited to 35% with αPfRipr/1 at 2 mg/ml (Fig. 9A). The inhibition observed for 3D7 was comparable to that observed for other antibodies raised to regions of the PfRh or EBL protein families [7], [12]. Similar results were observed for 3D7 using the αPfRipr/2 (data not shown). As for the anti-PfRipr/1 and/2 antibodies, rabbit polyclonal to the N-terminal EGF-like domains (anti-PfRipr/3) also inhibited parasite growth for all strains tested (Fig. S3). The αPfRipr/1 antibody was titrated in GIAs in comparison with IgG from normal serum for both FCR3 and 3D7 parasite strains (Fig. 9B and C). Growth of FCR3, a parasite that invades preferentially by sialic acid-dependent pathways, was almost completely abolished at 3 mg/ml and significant inhibition still remained at 1 mg/ml (40%) (Fig. 9B). In comparison, the 3D7 parasite strain, which can efficiently use sialic acid-independent invasion pathways primarily by using the ligand PfRh4 and complement receptor 1 [49], was inhibited at significantly lower levels of 50% at 3 mg/ml and this decreased to 20% at 1 mg/ml of antibody (Fig. 9C). This suggests that the PfRipr/PfRh5 complex may be more functionally important in P. falciparum strains that efficiently use sialic acid-dependent invasion pathways, which is supported by our finding (Fig. S4) that anti-PfRipr/1 inhibits W2mef, a sialic acid-dependent strain, more effectively than W2mefΔ175, a sialic acid-independent strain [8]. Among other P. falciparum strains tested, the αPfRipr/1 antibody also exhibited significantly higher inhibitory activity for those that invade erythrocytes preferentially using sialic acid-dependent receptors (ie. glycophorins), which includes T994, CSL2 and E8B (Fig. 9A) [9].
Fig. 9. Antibodies to PfRipr inhibit attachment of merozoites to erythrocytes and parasite growth. 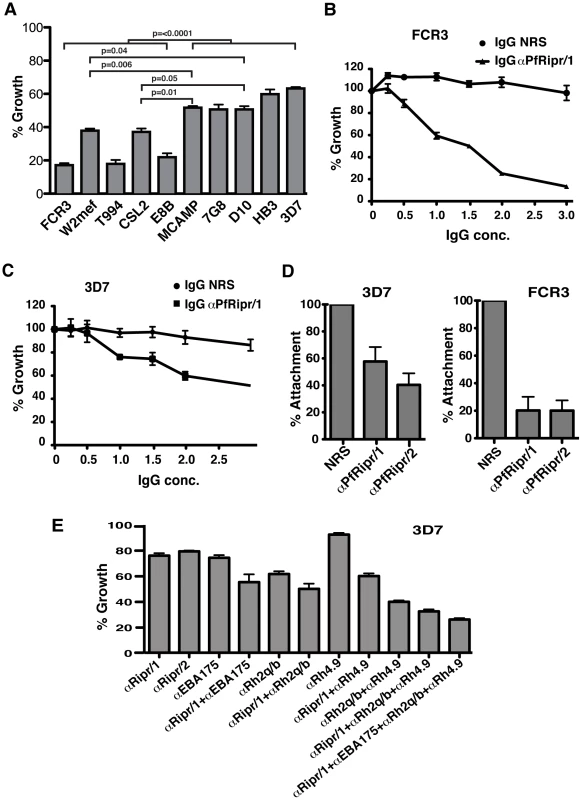
(A) Anti-PfRipr/1 antibodies inhibit invasion of P. falciparum strains into erythrocytes. Shown are growth inhibition assays of the parasite strains FCR3, W2mef, T994, CSL2, E8B, MCAMP, 7G8, D10, HB3 and 3D7. The final antibody concentration is 2 mg/ml. (B) Titration of anti-PfRipr/1 antibodies in growth inhibition assays of the FCR3 strain. (C) Titration of anti-PfRipr/1 antibodies in growth inhibition assays of the 3D7 strain. (D) Pre-incubation of purified merozoites from 3D7 strain (left panel) and FCR3 strain (right panel) with protein-A purified antibodies raised against recombinant PfRipr inhibited merozoite attachment to red blood cells. Protein-A purified antibodies from normal serum were used as a negative control. The final antibody concentration is 2 mg/ml. (E) Various combinations of anti-PfRipr/1, EBA-175, PfRh2a/b and PfRh4 antibodies increase inhibitory activity for the 3D7 strain of P. falciparum. The final concentration of each antibody is 1 mg/ml. In all the cases, each graph represents three independent experiments done in triplicate with each normalised to the negative control (Protein A purified IgG from normal rabbit serum). The error bars represent standard error of the mean of the three independent experiments. To investigate the mechanism of inhibition by anti-PfRipr antibodies, we performed merozoite attachment assays (Fig. 9D). Viable merozoites from 3D7 and FCR3 parasites were purified [45], [50] and mixed with erythrocytes in the presence of αPfRipr/1 or αPfRipr/2 antibodies. Both antibodies inhibited merozoite attachment for 3D7 to a significant level (45% and 60% respectively) (Fig. 9D, first panel). In contrast, the same antibodies inhibited attachment of FCR3 merozoites to a considerably greater extent (80% for both antibodies) (Fig. 9D, second panel). The level of inhibitory activity observed with the αPfRipr/1 and αPfRipr/2 antibodies for 3D7 and FCR3 parasites was similar to that observed in the growth inhibition assays (Fig. 9A, B and C) demonstrating that inhibition was occurring at merozoite invasion rather than during growth of the parasite. This inhibition was likely due to interference of these antibodies with the function of the PfRipr/PfRh5 complex since we could not detect PfRipr binding to erythrocytes while consistently observing PfRh5 binding (data not shown) [20]. It was possible that the PfRipr/PfRh5 complex disassociated on binding of PfRh5 to red blood cells. To test this possibility we purified the PfRh5/PfRipr complex using ion-exchange chromatography (Fig. S5A) and tested its binding to red blood cells (Fig. S5B). This showed that PfRh5 bound to red blood cells but PfRipr was not detected suggesting that the complex was disrupted on binding.
The region of PfRipr to which the αPfRipr/1, 2 antibodies were raised was from the 3D7 strain of P. falciparum; however, this domain does not show any polymorphisms compared to other strains so far sequenced (http://plasmodb.org/and http://www.broadinstitute.org/) (Fig. S6). Also we did not observe any cross-reactivity of the antibodies with other proteins that contain EGF-like domains such as MSP1 (data not shown). This was not surprising as the only conserved amino acids are the six cysteine residues that define each EGF-like domain (Fig. 3). Therefore the differences in inhibition observed in GIA with the various strains was unlikely to be due to cross reactivity with other proteins containing EGF-like domains or polymorphisms within this region of PfRipr. It more likely reflects the reliance of these strains on the PfRh5/PfRipr complex to mediate a specific invasion pathway in comparison to the function of other members of the PfRh and EBA protein families [7], [51]. To test this we used a combination of antibodies raised to PfRipr, EBA-175 [51], PfRh4 [13], [49], PfRh2a and PfRh2b [7] to determine if they increased the level of inhibition in GIAs for 3D7 parasites (Fig. 9E). Both αPfRipr/1 and αPfRipr/2 antibodies at 1 mg/ml inhibited 3D7 parasite growth to 24 and 21% respectively (Fig. 9E), similar to our previous experiments (Fig. 9C). Anti-EBA-175 antibodies on its own also inhibited 3D7 parasite growth to 25%. Anti-PfRh2a/b and PfRh4 antibodies inhibited the 3D7 parasite growth at 39% and 8% respectively. The combination of αPfRipr/1 with αEBA-175 antibodies showed an additive inhibition of 45%. This was a similar result to that observed for the combination of αPfRipr/1 with αPfRh2a/b or αPfRh4 antibodies (50% and 40% respectively). Significantly, a combination of αPfRipr/1, αPfRh2a/b and αPfRh4 as well as αPfRipr/1, αEBA-175, αPfRh2a/b and αPfRh4 showed a much higher level of inhibition (67% and 74% respectively). This additive effect was consistent with parasites using multiple invasion pathways to gain entry to the erythrocyte. A similar additive inhibitory effect was obtained with FCR3 parasites (Fig. S7).
Our inability to disrupt the PfRipr gene (Fig. S8 and S9) suggests the function of PfRipr and thus the PfRipr/PfRh5 complex is essential for parasite invasion and survival. Therefore PfRipr represents a novel protein that plays a critical role in merozoite invasion and thus it is a new candidate for a combination vaccine with other PfRh and EBL proteins to produce antibody responses to block a broad array of invasion pathways.
Discussion
Invasion of P. falciparum into host erythrocytes requires specific ligand-receptor interactions that identify the appropriate host cell followed by activation of the parasite actomyosin motor for entry (see for review [2]). The PfRh family of proteins are important ligands that bind directly to specific receptors on the red blood cell during invasion [4], [5], [7], [9], [11], [13], [16], [18], [19], [20], [22], [49]. Differential expression and activation of these ligands provide a mechanism of phenotypic variation to evade host immune responses and to circumvent the polymorphic nature of the red blood cell surface in the human population [7], [8], [39]. PfRh5 is an important member of the PfRh family that appears to be essential in P. falciparum [19], [20]. This ligand binds to an unknown receptor and plays a key role during merozoite invasion; however, in contrast to all other PfRh family members, it lacks an identifiable transmembrane region, suggesting that it may function in a complex with another protein(s) [20]. We have shown here that PfRh5 exists in a membrane-associated complex with a novel cysteine-rich protein that we have called PfRipr.
The ability of PfRipr antibodies to inhibit merozoite attachment and invasion and our inability to genetically disrupt the corresponding gene implies that this protein plays an important role in these processes. Before invasion, the merozoite undergoes a step of irreversible attachment to the host red blood cell and current evidence suggests that the PfRh and EBL protein families play a key role in this process [37], [45]. Attachment triggers downstream events presumably through signalling from the cytoplasmic domain of each molecule [37], [45]. Merozoite attachment can be inhibited with anti-PfRipr antibodies consistent with the PfRh5/PfRipr complex playing a role in the irreversible binding to erythrocytes and commitment to invasion. The ability to inhibit merozoite invasion was not due to cross reactivity of the antibodies with other proteins such as MSP-1, that also have EGF-like domains. Whilst neither PfRh5 nor PfRipr has a transmembrane region they are tightly associated with the membrane, suggesting they most likely complex with another protein(s) that would hold the complex on the parasite surface after their release from the apical organelles. This would link the parasite to the erythrocyte by binding of PfRh5 to its specific receptor during merozoite invasion.
The subcellular localisation of PfRh5 and PfRipr appears to be different in schizont stages of the parasite suggesting that they may not form a complex until egress of the merozoite and activation of erythrocyte invasion. PfRh5 was present within the rhoptries whilst PfRipr appears to be in micronemes at the apical end and these proteins would be released during apical interaction where they could associate and form a complex. This is similar to the formation of the AMA-1 and RON complex in both T. gondii and P. falciparum where the former protein is located in micronemes and the latter in the neck of the rhoptries [43], [44], [52], [53]. Components of the RON complex translocate onto and under the erythrocyte membrane forming a bridge across the host cell to the invading merozoite through AMA-1 and this is associated with the tight junction. It is possible that PfRh5/PfRipr interacts with a membrane associated protein and our immuno-electron microscopy analysis has suggested that the complex is at the leading edge of the tight junction (i.e. basal to the merozoite) and shed into the culture supernatant, or onto the exterior of the red blood cell, before membrane fusion to form the enclosing parasitophorous vacuole membrane at the posterior end of the invading merozoite.
The PfRh and EBL proteins may have overlapping functions as loss of EBA-175 can be compensated by increased function of PfRh4 [8], [51], [54]. Furthermore, different parasite lines can differentially express these proteins and in some cases polymorphisms affect their function or potentially alter receptor selectivity [7], [8], [19], [28], [38]. For example, both PfRh2a and PfRh2b are not expressed in FCR3 line [7] and additionally, both EBA140 and EBA-181 encode polymorphisms that greatly reduce their binding to the red blood cell surface and render them non-functional when tested in growth inhibition assays with specific antibodies [55]. Similarly, in the W2mef parasite line PfRh4 is not expressed and polymorphisms of EBA-140 and EBA-181 also weaken their binding abilities and function in invasion [55]. Since both FCR3 and W2mef have a reduced complement of functional PfRh and EBL proteins, one would predict that these parasites would have greater reliance on the PfRh5/PfRipr complex for their invasion. That is what we have observed in GIAs with the anti-PfRipr antibodies that efficiently inhibit merozoite invasion of these strains. In addition, the additive effect observed with anti-PfRipr antibody in combination with antibodies raised against other PfRh and/or EBA in GIAs would be consistent with the PfRh5/PfRip complex having a similar function to other members of the PfRh and EBL protein families [54]. However, in contrast to the other PfRh and EBL proteins, both PfRh5 and PfRipr could not be genetically disrupted, pointing to the possibility that the complex may also play a broader role that is essential to the parasites.
The PfRh1, PfRh2a and PfRh2b proteins undergo a complex series of proteolytic cleavage events in the schizont when trafficked to the neck of the rhoptries as well as during merozoite invasion and these events are important in their function [9], [12], [24]. In the case of PfRh2a and PfRh2b, the proteins are processed to produce the N-terminal binding domain that remains associated with the C-terminal domain [24]. It makes sense for N-terminal binding domain and C-terminal domain with a transmembrane region to associate together after processing for the parasites to attach to the erythrocytes. PfRh5 is also located in the neck of the merozoite rhoptries and is processed to a 45 kDa binding region [20]. Here we showed that the 45 kDa binding region forms a complex with PfRipr. Since neither of them contain a transmembrane region, it is likely that the complex binds another protein(s) to link it to the merozoite surface for PfRh5 to bind erythrocyte during invasion. Our results that the PfRipr/PfRh5 complex is peripherally attached to the parasite membrane are consistent with this possibility.
Epidermal growth factor-like domains are found widely throughout eukaryotes and are defined by six cysteine residues that link to form a tightly folded structure [46], [47]. In Plasmodium spp. this domain is contained in a large number of proteins in different stages of the parasite lifecycle. The merozoite surface antigen 1 (MSP-1) has two EGF-like domains and it is localised to the merozoite surface where antibodies can access it and inhibit invasion, which has suggested it is involved in interacting with the erythrocyte surface [56], [57]. Other proteins with EGF-like domains include the P28 family that protect the parasite from the harsh proteolytic environment in the mosquito gut [58]. PfRipr would not play such a protective role as it is released only during invasion. Instead, it may act as a scaffold on which PfRh5 can be mounted so that it is displayed for binding to its erythrocyte receptor. As the N-terminal and C-terminal of PfRipr remains associated after processing, it is likely that the EGF-like domains are responsible for their association as well as interaction with PfRh5. PfRipr has previously been predicted to act as a parasite adhesin by a bioinformatic method based on protein physiochemical properties [59]. However, we could not detect direct binding of PfRipr to erythrocytes and our results suggest that the PfRipr/PfRh5 complex dissociates upon PfRh5 binding to red blood cells. This may be similar to the processed N-terminus of PfRh2a and PfRh2b that form a complex with the C-terminus of the same proteins. However, only the free processed N-terminus can be detected in erythrocyte binding assays suggesting that this complex also disassociates [24].
The PfRh and EBL protein families function in binding the erythrocyte and activating the invasion process [54], [55]. It has been proposed that a combination of these ligands may be a useful blood stage vaccine against P. falciparum malaria. Indeed it has been shown in immunisation studies that vaccination with combined portions of EBA-175, PfRh2a/b and PfRh4 proteins can raise antibodies that efficiently block merozoite invasion [54]. The functional importance of PfRh5 has also led to suggestion that it has potential to be included in such a combination vaccine. However, this has not been tested due to the difficulty of producing functional protein. Identification of the PfRh5/PfRipr complex and the ability of anti-PfRipr antibodies to block merozoite invasion open the possibility for its inclusion in a combination vaccine. Indeed, the efficient inhibition of invasion was observed when a combination of antibodies to PfRipr, PfRh2a/b and PfRh4 was used. This further supports PfRipr as a new vaccine target, especially in a combination approach to efficiently block merozoite invasion.
Materials and Methods
Ethics statement
Antibodies were raised in mice and rabbits under the guidelines of the National Health and Medical Research Committee and the PHS Policy on Humane Care and Use of Laboratory Animals. The specific details of our protocol were approved by the Royal Melbourne Hospital Animal Welfare Committee.
Parasite culture
P. falciparum asexual parasites were maintained in human erythrocytes (blood group O+) at a hematocrit of 4% with 10%(w/v) AlbumaxTM (Invitrogen) [60]. 3D7 is a cloned line derived from NF54 from David Walliker at Edinburgh University. FCR3 is a cloned line. Cultures were synchronised as previously described [61]. Culture supernatants enriched in parasite proteins were prepared by growing synchronized parasite cultures to high parasitemia, typically to 5% and allowing schizonts to rupture. Harvested culture supernatant was spun at 1200 rpm to remove residual erythrocytes and then 10,000 rpm to remove insoluble materials before use for experiments. Total proteins from schizont stage parasites were obtained by saponin lysis of infected erythrocytes.
To prepare culture supernatants for affinity purification of the PfRh5 complex, the PfRh5HA parasite line was synchronized, grown to late schizont stage in normal culture conditions and then culture medium was replaced by RPMI without Albumax to allow the schizonts to rupture [5]. This culture supernatant has a minimal amount of BSA to facilitate purification of the PfRh5 complex.
Purification and identification of PfRh5 complex
Culture supernatant (900 ml), prepared as described above, was dialysed against 12.5 mM Tris.Cl pH 7.2 overnight at 4°C and loaded onto a 15 ml Q-Sepharose column (GE Heathcare) equilibrated with 12.5 mM Tris.Cl, pH 7.2. The bound proteins were eluted with NaCl in 12.5 mM Tris.Cl pH 7.2. PfRh5HA was eluted with 350 mM NaCl. The eluted PfRh5HA-containing fractions were further purified using anti-HA affinity matrix (Roche Apply Science) and the bound proteins eluted with 0.1 M glycine, pH 2.6. The eluted proteins were then subjected to trypsin digestion and analyzed by mass spectrometry (LC-MS/MS) and proteins identified by database searches [42].
Gel filtration chromatography and blue native gel electrophoresis
Gel-filtration chromatography was performed on an analytical Superdex 200 column (24 ml, GE Healthcare) and proteins were eluted with PBS or Tris buffer. Blue Native Gel Electrophoresis was conducted using the company's protocols (Invitrogen). NativePAGETM Novex 4–16% or 3–12% Bis-Tris gels were used to resolve the proteins and the NativeMarkTM Unstained Protein Standard used as molecular weight markers.
Generation of P. falciparum expressing HA-tagged PfRipr
To attach a triple HA tag (3xHA) to the 3′ end of the Pfripr gene, an 844 bp fragment of Pfripr was amplified from 3D7 genomic DNA using the primers 5′-ATCCCGCGGTGAATGTATATTAAATGATTATTG-3′ and 5′-TTATCTCGAGATTCTGATTACTATAATAAAATACATTTTC-3′ (Sac II and Xho I restriction sites underlined). The DNA fragment was digested with Sac II and Xho I, and cloned into pHAST, a derivative of pGEM-3Z containing a 3xHA tag and single Strep II tag in tandem. Parasites were transfected as described previously [62], [63]. Successful integration of the 3xHA tag was determined by Southern and Western blot analysis using a mouse monoclonal anti-HA antibody.
Immunoprecipitation and immunoblotting
Immunoprecipitation of PfRh5 from culture supernatant was performed using anti-PfRh5 monoclonal antibody (clone 2F1) coupled to Minileak resin (KEM-En-Tec). Briefly, 1.5 ml culture supernatants from both 3D7 and 3D7-PfRiprHA parasite lines were incubated with 20 µl anti-PfRh5-Minileak resins at 4°C for 4 hr. Also 1.5 ml culture supernatant of 3D7-PfRiprHA parasites was incubated with just 20 µl Mini-bead as an additional control. After incubation, the samples were spun to remove the supernatant and the resin washed three times with PBS containing 0.1% Tween-20. Bound proteins were eluted with SDS sample buffer and separated by SDS-PAGE, transferred to nitrocellulose membrane and probed with a monoclonal anti-HA antibody (12CA5). The membrane was then stripped and re-probed with rabbit anti-Rh2a/b polyclonal antibodies recognizing 85 kDa domain. Immunoprecipitation of HA-tagged PfRipr from culture supernatant of 3D7-PfRiprHA was performed using rat anti-HA affinity matrix (Roche Applied Science). The culture supernatants from 3D7 parasites were used as a control. The bound material was analysed by western blot to probe for PfRh5 using monoclonal anti-PfRh5 antibody (2F1) or to probe for PfRipr using polyclonal antibodies raised against N-terminal fragment (α-PfRip/3). The material was also probed with anti-Rh2a/b polyclonal antibodies as a control. Immunoprecipitation of PfRipr from culture supernatant of 3D7-PfRipHA with α-PfRip/3 was performed using protein G. The IgG from a pre-bleed of the same rabbit was used as a control. Immunoprecipitation of PfRipr from solubilised saponin pellets of the 3D7RiprHA parasite line was performed similarly. The proteins were extracted from the saponin pellet using 2% n-Dodecyl-N, N-Dimethylamine-N-Oxide in PBS. Immunoprecipitation of culture supernatant from 3D7-PfRipHA with rabbit anti-Rh2a/b polyclonal antibodies was also performed with protein G. The bound material was probed for PfRh2a, PfRh2b, PfRh5 and PfRipr using corresponding antibodies raised in mice. The proteins were detected by enhanced chemiluminescence (ECL, Amersham Biosciences).
Analyses of protein expression
Synchronized 3D7RiprHA early ring stage parasites were harvested and the red blood cells lysed using saponin. A second aliquot was harvested 16 hr later, the third aliquot another 8 hr later and subsequent samples every 6 hr later until the end of schizogony. Proteins were extracted from the saponin pellets using SDS-PAGE sample buffer, separated by 4-12% SDS-PAGE gels (Novagen) and transferred to a nitrocellulose membrane. The membrane was firstly probed with monoclonal anti-HA antibody for PfRiprHA and then stripped to probe for PfRh5 and PfHsp70 [64].
Generation of recombinant PfRipr and antibody production
To produce recombinant C-terminal fragment of PfRipr comprise of amino acid 791-900, a 345 bp DNA fragment was amplified from genomic DNA prepared from 3D7 parasites using oligonucleotides (5′ CGCTAGCCATATGAATGAAGAAACAGATATTGTAAAATG 3′ and 5′ CGAGGATCCCTAATCTTCTAAAACACATTTTCC 3′). The resulting PCR fragment was cloned into pET14b vector (Novagen) with Nde I and Bam HI, transformed into BL21 RIL E. coli strain to express the recombinant PfRipr-791-900 as a hexa-His-tagged protein (Fig. 3 A). The His-tagged protein was purified from soluble lysate of bacteria cells by affinity purification on Ni-NTA agarose resin (Qiagen) followed by gel-filtration chromatography on SuperdexTM 75 column. To produce recombinant N-terminal fragment of PfRipr consisting of amino acid 238–368, a codon-optimised gene was synthesized and cloned into pET28a vector (Novagen) with Nhe I and BamHI sites, transformed into BL21 RIL E. coli strain for expressing recombinant PfRip-238-368 as a hexa-His-tagged protein. The His-tagged protein was expressed in E. coli as inclusion body. Protein isolated from inclusion bodies was refolded in vitro and purified on Ni-NTA agarose resin (Qiagen) under native conditions. Both purified PfRip-791-900 and PfRip-238-368 proteins were used to immunise a rabbit. Rabbit immunoglobulins were purified on Protein A or G-Sepharose and buffer exchanged to PBS for subsequent experiments. The antibody production was done by the WEHI antibody production facility.
Immunofluorescence assays
Immunofluorescence assays (IFA) were performed as described [46], using primary antibodies as follows: rabbit anti-Ripr [1∶1000]; rat anti-HA (monoclonal CF10, Roche) [1∶50]; rabbit anti-RON4 [44] [1∶250]; mouse anti-RAP1 [65] [1∶500]; mouse anti-Rh5 2F1 [1∶200]; rabbit anti-EBA175 [66] [1∶300]. Images were captured on a Zeiss Axiovert 200 m microscope (Zeiss) with a 100x/1.40NA PlanApochromat phase contrast oil immersion objective lens (Zeiss), an Axiocam MRm camera (Zeiss) and running Axiovision version 4.8 software (Zeiss).
Differential solubilization of membrane proteins
PfRiprHA parasite (late schitzont stage)-infected red blood cells were hypotonically lysed with water, centrifuged and the pellet fraction washed with PBS. The pellet was then divided into four eppendorf tubes and incubated on ice for 2 hr with 10 mM Tris/pH 8.0; 100 mM sodium carbonate/pH 11.5; 2% Triton X100 and 2% CHAPS in 50 mM Tris/pH8.0 containing 1 mM EDTA and 100 mM sodium chloride respectively. The samples were then centrifuged to separate soluble and insoluble fractions. The insoluble fraction was washed twice with PBS and analyzed by Western blot together with the soluble fraction. Saponin pellet, prepared from the late schizont stage PfRiprHA parasites, were subjected to the same analyses as described above.
Growth inhibition assay (GIA)
GIA were performed as described [67]. Briefly, late trophozoite stage parasites were added to erythrocytes to give a parasitemia of 0.2% and haematocrit of 2% in 45 µl of 0.5% Albumax II (Gibco, Auckland, New Zealand) in 96 well round bottom microtiter plates (Becton Dickinson, Fanklin Lakes, NJ, U.S.A.). 5 µl of purified rabbit IgG was added to a final concentration of 2 mg/ml. For the titration experiments, the antibodies were added to a final concentration of 0.125, 0.25, 0.5, 1, 2.0 and 3.0 mg/ml. Cultures were incubated for 72 hr (2 cycles) of growth and parasitaemia of each well was counted by flow cytometry, a FACSCalibur with a plate reader (Becton Dickinson, Fanklin Lakes, NJ, U.S.A.) after ethidium bromide (10 µg/ml, Biorad, Hercules, CA, U.S.A) staining trophozoite stage parasites. For each well, more than 50,000 cells were counted, and all samples were tested in triplicate. Growth was expressed as a percentage of the parasitaemia obtained from the pre-immunization IgG control. Three independent assays were performed.
Merozoite attachment assay
Merozoite attachment was performed using viable cells purified as described [45], [50]. Briefly, late shizonts of 3D7 and FCR3 (40–46 hr post invasion) were purified from culture by magnet separation (Macs Miltenyi Biotec) to >95% purity. The purified schizonts were incubated with 10 µM E64 (Sigma) for 7–8 hr, then pelleted at 1900g for 5 min. Resulting parasitophorous vacuole membrane enclosed merozoites (PEMS) [68], [69] were resuspended in a small volume of incomplete culture medium (containing no protein) and filtered through a 1.2 µm, 32 mm syringe filter (Sartorius Stedim biotech, France). Antibodies were added to filtered merozoites at a final concentration of 2 mg/ml and incubated for 2 min after which uninfected erythrocytes were added and incubated for 10 min. Following fixation at room temperature for 30 min using 0.0075% glutaraldehyde/4% paraformaldehyde (ProSciTech, Australia) in PBS [70] the merozoite-bound erythrocytes were washed and stained with 0.1 ng/µL 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Imaging was performed using a either Plan-Apochromat 100x/1.40NA or 40x/1.3NA oil immersion Phase contrast lens (Zeiss) on an AxioVert 200 M microscope (Zeiss) equipped with an AxioCam Mrm camera (Zeiss). The MosaiX application from the Axiovision release 4.8 software (Zeiss) was used for counting. For each condition, at least 2000 red blood cells with bound merozoites were scored.
Immuno-Electron microscopy
Merozoites from 3D7RiprHA parsites were purified according to a previously described method [50] and mixed with uninfected human erythrocytes. After a 2 min incubation, invading merozoites were fixed in 1% glutaraldehyde (Pro SciTech, Australia) in RPMI Hepes for 30 min on ice and prepared for Transmission electron microscopy as described [71]. Briefly, samples were dehydrated with increasing ethanol concentrations and embedded in LR Gold resin (Pro SciTech, Australia). The resin was polymerised using benzoyl peroxide (0.5%) (SPI-Chem, USA) and the preparation was sectioned on a Leica Ultracut R ultramicrotome (Wetzlar). The sections were blocked with 5% BSA and 0.1% Tween-20 in PBS for 30 min. Immunolabeling was performed with mouse anti-Rh5 clone 6H2 (dilution 1∶100) and mouse anti-HA clone 12AC5 (dilution 1∶100). Samples were incubated with 18 nm colloidal gold-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch, Baltimore, USA). Post-staining was done with 2% aqueous uranyl acetate and 5% triple lead and observed at 120 kV on a Philips CM120 BioTWIN Transmission Electron Microscope.
Supporting Information
Zdroje
1. MillerLHGreenwoodB 2002 Malaria–a shadow over Africa. Science 298 121 122
2. CowmanAFCrabbBS 2006 Invasion of red blood cells by malaria parasites. Cell 124 755 766
3. SimBKLOrlandiPAHaynesJDKlotzFWCarterJM 1990 Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol 111 1877 1884
4. RaynerJCVargas-SerratoEHuberCSGalinskiMRBarnwellJW 2001 A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med 194 1571 1581
5. TrigliaTThompsonJCaruanaSRDelorenziMSpeedT 2001 Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun 69 1084 1092
6. MaierAGDuraisinghMTReederJCPatelSSKazuraJW 2003 Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med 9 87 92
7. DuraisinghMTTrigliaTRalphSARaynerJCBarnwellJW 2003 Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J 22 1047 1057
8. StubbsJSimpsonKMTrigliaTPlouffeDTonkinCJ 2005 Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 309 1384 1387
9. TrigliaTDuraisinghMTGoodRTCowmanAF 2005 Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol 55 162 174
10. GaurDFuruyaTMuJJiangLBSuXZ 2006 Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway. Mol Biochem Parasitol 145 205 215
11. GaoXYeoKPAwSSKussCIyerJK 2008 Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog 4 e1000104
12. TrigliaTThamWHHodderACowmanAF 2009 Reticulocyte binding protein homologues are key adhesins during erythrocyte invasion by Plasmodium falciparum. Cell Microbiol 11 1671 1687
13. ThamWHWilsonDWReilingLChenLBeesonJG 2009 Antibodies to reticulocyte binding protein-like homologue 4 inhibit invasion of Plasmodium falciparum into human erythrocytes. Infect Immun 77 2427 2435
14. DeSimoneTMBeiAKJenningsCVDuraisinghMT 2009 Genetic analysis of the cytoplasmic domain of the PfRh2b merozoite invasion protein of Plasmodium falciparum. Int J Parasitol 39 399 405
15. DesimoneTMJenningsCVBeiAKComeauxCColemanBI 2009 Cooperativity between Plasmodium falciparum adhesive proteins for invasion into erythrocytes. Mol Microbiol 72 578 589
16. RaynerJCGalinskiMRIngravalloPBarnwellJW 2000 Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci USA 97 9648 9653
17. KanekoOMuJTsuboiTSuXToriiM 2002 Gene structure and expression of a Plasmodium falciparum 220-kDa protein homologous to the Plasmodium vivax reticulocyte binding proteins. Mol Biochem Parasitol 121 275 278
18. RodriguezMLustigmanSMonteroEOksovYLoboCA 2008 PfRH5: a novel reticulocyte-binding family homolog of Plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PLoS ONE 3 e3300
19. HaytonKGaurDLiuATakahashiJHenschenB 2008 Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe 4 40 51
20. BaumJChenLHealerJLopatickiSBoyleM 2009 Reticulocyte-binding protein homologue 5-an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol 39 371 380
21. TaylorHMTrigliaTThompsonJSajidMFowlerR 2001 Plasmodium falciparum homologue of the genes for Plasmodium vivax and Plasmodium yoelii adhesive proteins, which is transcribed but not translated. Infect Immun 69 3635 3645
22. GaurDSinghSSinghSJiangLDioufA 2007 Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc Natl Acad Sci U S A 104 17789 17794
23. SaharTReddyKSBharadwajMPandeyAKSinghS 2011 Plasmodium falciparum Reticulocyte Binding-Like Homologue Protein 2 (PfRH2) Is a Key Adhesive Molecule Involved in Erythrocyte Invasion. PLoS ONE 6 e17102
24. TrigliaTChenLLopatickiSDekiwadiaCRiglarDT 2011 Plasmodium falciparum merozoite invasion is inhibited by antibodies that target the PfRh2a and b binding domains. Plos Pathogen In Press
25. SpadaforaCAwandareGAKopydlowskiKMCzegeJMochJK 2010 Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog 6 e1000968
26. OrlandiPASimKLChulayJDHaynesJD 1990 Characterization of the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum. Mol Biochem Parasitol 40 285 294
27. GilbergerTWThompsonJKTrigliaTGoodRTDuraisinghMT 2003 A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem 278 14480 14486
28. MayerDCMuJBKanekoODuanJSuXZ 2004 Polymorphism in the Plasmodium falciparum erythrocyte-binding ligand JESEBL/EBA-181 alters its receptor specificity. Proc Natl Acad Sci U S A 101 2518 2523
29. ThompsonJKTrigliaTReedMBCowmanAF 2001 A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol Micro 41 47 58
30. MayerDCKanekoOHudson-TaylorDEReidMEMillerLH 2001 Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A 98 5222 5227
31. NarumDLFuhrmannSRLuuTSimBK 2002 A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol Biochem Parasitol 119 159 168
32. MayerDCCofieJJiangLHartlDLTracyE 2009 Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc Natl Acad Sci USA 106 5348 5352
33. OrlandiPAKlotzFWHaynesJD 1992 A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha2-3)Gal - sequences of glycophorin A. J Cell Biol 116 901 909
34. AdamsJHSimBKLDolanSAFangXKaslowDC 1992 A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA 89 7085 7089
35. SimBKLChitnisCEWasniowskaKHadleyTJMillerLH 1994 Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264 1941 1944
36. LoboCARodriguezMReidMLustigmanS 2003 Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 6 6
37. SinghSAlamMMPal-BhowmickIBrzostowskiJAChitnisCE 2010 Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog 6 e1000746
38. MayerDCMuJBFengXSuXZMillerLH 2002 Polymorphism in a Plasmodium falciparum erythrocyte-binding ligand changes its receptor specificity. J Exp Med 196 1523 1528
39. PerssonKEMcCallumFJReilingLListerNAStubbsJ 2008 Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest 118 342 351
40. ReilingLRichardsJSFowkesFJBarryAETrigliaT 2010 Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol 185 6157 6167
41. RichardsJSStanisicDIFowkesFJTavulLDabodE 2011 Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51 e50 60
42. BoddeyJAMoritzRLSimpsonRJCowmanAF 2009 Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic 10 285 299
43. AlexanderDLArastu-KapurSDubremetzJFBoothroydJC 2006 Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii. Eukaryot Cell 5 1169 1173
44. RichardDMacRaildCARiglarDTChanJAFoleyM 2010 Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem 285 14815 14822
45. RiglarDTRichardDWilsonDWBoyleMJDekiwadiaC 2011 Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9 9 20
46. SavageCRJrInagamiTCohenS 1972 The primary structure of epidermal growth factor. J Biol Chem 247 7612 7621
47. SavageCRJrHashJHCohenS 1973 Epidermal growth factor. Location of disulfide bonds. J Biol Chem 248 7669 7672
48. HaradaYLiHWallJSLennarzWJ 2011 Structural studies and the assembly of the heptameric post-translational translocon complex. J Biol Chem 286 2956 2965
49. ThamWHWilsonDWLopatickiSSchmidtCQTetteh-QuarcooPB 2010 Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Natl Acad Sci U S A 107 17327 17332
50. BoyleMJWilsonDWRichardsJSRiglarDTTettehKK 2010 Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci U S A 107 14378 14383
51. BaumJMaierAGGoodRTSimpsonKMCowmanAF 2005 Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS Pathog 1 e37
52. AlexanderDLMitalJWardGEBradleyPBoothroydJC 2005 Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog 1 e17
53. BesteiroSMichelinAPoncetJDubremetzJFLebrunM 2009 Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5 e1000309
54. LopatickiSMaierAGThompsonJWilsonDWThamWH 2011 Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect Immun 79 1107 1117
55. MaierAGBaumJSmithBConwayDJCowmanAF 2009 Polymorphisms in erythrocyte binding antigens 140 and 181 affect function and binding but not receptor specificity in Plasmodium falciparum. Infect Immun 77 1689 1699
56. HolderAA 1988 The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy 41 72 97
57. ChitarraVHolmIBentleyGPetresSLongacreS 1999 The crystal structure of the C-terminal merozoite protein 1 at 1.8A resolution, a highly protective malaria vaccine candidate. Mol Cell 3 457 464
58. TomasAMMargosGDimopoulosGvan LinLHde Koning-WardTF 2001 P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J 20 3975 3983
59. AnsariFAKumarNBala SubramanyamMGnanamaniMRamachandranS 2008 MAAP: malarial adhesins and adhesin-like proteins predictor. Proteins 70 659 666
60. TragerWJensenJB 1976 Human malaria parasites in continuous culture. Science 193 673 675
61. LambrosCVanderbergJP 1979 Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65 418 420
62. CrabbBSCookeBMReederJCWallerRFCaruanaSR 1997 Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89 287 296
63. VossTSHealerJMartyAJDuffyMFThompsonJK 2006 A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature 439 1004 1008
64. BiancoAEFavaloroJMBurkotTRCulvenorJGCrewtherPE 1986 A repetitive antigen of Plasmodium falciparum that is homologous to heat shock protein 70 of Drosophila melanogaster. Proc Natl Acad Sci USA 83 8713 8717
65. SchofieldLBushellGRCooperJASaulAJUpcroftJA 1986 A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol Biochem Parasitol 18 183 195
66. ReedMBCaruanaSRBatchelorAHThompsonJKCrabbBS 2000 Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid independent pathway of invasion. Proc Natl Acad Sci USA 97 7509 7514
67. PerssonKELeeCTMarshKBeesonJG 2006 Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J Clin Microbiol 44 1665 1673
68. SalmonBLOksmanAGoldbergDE 2001 Malaria parasite exit from the host erythrocyte: A two - step process requiring extraerythrocytic proteolysis. Proc Natl Acad Sci U S A 98 271 276
69. WickhamMECulvenorJGCowmanAF 2003 Selective inhibition of a two-step egress of malaria parasites from the host erythrocyte. J Biol Chem 278 37658 37663
70. TonkinCJvan DoorenGGSpurckTPStruckNSGoodRT 2004 Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol 137 13 21
71. HealerJCrawfordSRalphSMcFaddenGCowmanAF 2002 Independent translocation of two micronemal proteins in developing Plasmodium falciparum merozoites. Infect Immun 70 5751 5758
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



