-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
Phagocytosis of bacteria by innate immune cells is a primary method of bacterial clearance during infection. However, the mechanisms by which the host cell recognizes bacteria and consequentially initiates phagocytosis are largely unclear. Previous studies of the bacterium Pseudomonas aeruginosa have indicated that bacterial flagella and flagellar motility play an important role in colonization of the host and, importantly, that loss of flagellar motility enables phagocytic evasion. Here we use molecular, cellular, and genetic methods to provide the first formal evidence that phagocytic cells recognize bacterial motility rather than flagella and initiate phagocytosis in response to this motility. We demonstrate that deletion of genes coding for the flagellar stator complex, which results in non-swimming bacteria that retain an initial flagellar structure, confers resistance to phagocytic binding and ingestion in several species of the gamma proteobacterial group of Gram-negative bacteria, indicative of a shared strategy for phagocytic evasion. Furthermore, we show for the first time that susceptibility to phagocytosis in swimming bacteria is proportional to mot gene function and, consequently, flagellar rotation since complementary genetically - and biochemically-modulated incremental decreases in flagellar motility result in corresponding and proportional phagocytic evasion. These findings identify that phagocytic cells respond to flagellar movement, which represents a novel mechanism for non-opsonized phagocytic recognition of pathogenic bacteria.
Published in the journal: Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion. PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002253
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002253Summary
Phagocytosis of bacteria by innate immune cells is a primary method of bacterial clearance during infection. However, the mechanisms by which the host cell recognizes bacteria and consequentially initiates phagocytosis are largely unclear. Previous studies of the bacterium Pseudomonas aeruginosa have indicated that bacterial flagella and flagellar motility play an important role in colonization of the host and, importantly, that loss of flagellar motility enables phagocytic evasion. Here we use molecular, cellular, and genetic methods to provide the first formal evidence that phagocytic cells recognize bacterial motility rather than flagella and initiate phagocytosis in response to this motility. We demonstrate that deletion of genes coding for the flagellar stator complex, which results in non-swimming bacteria that retain an initial flagellar structure, confers resistance to phagocytic binding and ingestion in several species of the gamma proteobacterial group of Gram-negative bacteria, indicative of a shared strategy for phagocytic evasion. Furthermore, we show for the first time that susceptibility to phagocytosis in swimming bacteria is proportional to mot gene function and, consequently, flagellar rotation since complementary genetically - and biochemically-modulated incremental decreases in flagellar motility result in corresponding and proportional phagocytic evasion. These findings identify that phagocytic cells respond to flagellar movement, which represents a novel mechanism for non-opsonized phagocytic recognition of pathogenic bacteria.
Introduction
Pathogen recognition by the innate immune system is one of the first lines of defense in cellular immunity to infection [1]. However, how bacteria establish chronic infections, as observed in patients with cystic fibrosis (CF), and the reasons that these infective agents cannot be eliminated by the immune system are still largely unclear [2], [3]. A relevant example of this is Pseudomonas aeruginosa, a Gram-negative opportunistic pathogen which establishes infection in the lung tissue of CF patients and effectively evades immune clearance [2], [3]; CF disease severity correlates with chronic infection of the pulmonary compartment by P. aeruginosa [2], [3]. One contributing factor that enables immune evasion is the loss of bacterial flagellar motility during colonization [4]–[8]. P. aeruginosa has a single, polar, monotrichous flagellum which provides force for swimming locomotion in aqueous environments [9]. Multiple studies have found that the majority of P. aeruginosa isolates taken from chronically infected CF patients have down-regulated flagellar gene expression and are phenotypically deficient in the ability to swim [6], [7]. The previous paradigm suggested that the loss of flagellin as a phagocytic ligand facilitates evasion of innate immune cells and results in increased bacterial burden in the CF lung [5], [8]. Recently, with the use of flagellated and non-flagellated swimming-defective P. aeruginosa genetic mutants, we demonstrated that it is not the loss of the flagellum itself, but rather the loss of flagellar-based swimming motility that allows P. aeruginosa to avoid phagocytic clearance [4]. However, it is currently unclear how the loss of bacterial swimming motility enables phagocytic evasion from innate immune cells and, to date, no published reports have examined in detail the dynamics of non-opsonized P. aeruginosa-phagocyte association and subsequent fate as a function of bacterial swimming motility.
In order to delineate how bacterial swimming contributes to phagocytic recognition and uptake, we take advantage of isogenic bacterial mutations that affect flagellar swimming motility and we identify the individual components that comprise the phagocytic process as it relates to swimming and non-swimming bacteria. Swimming motility in Gram-negative bacteria is powered by generation of an ion gradient to turn a flagellar rotor against a stationary stator complex [10]. The resultant force provides the necessary torque to turn the flagellar filament and thus propel the bacteria [10]. In these studies we utilize genetic mutants which lack structural and functional flagella due to mutations in either the flagellin monomer or the flagellar hook protein and are therefore non-swimming, and also mutants which do not produce all or part of the flagellar stator complex. These mot stator mutants all have fully assembled flagella, since loss of the Mot stator proteins does not impede construction of the flagellar filament, and are instead partially or fully defective in the ability to rotate the flagellum depending on which stator components are omitted [9], [11], [12]. Our previous work with these mutants found that the phagocytic response to P. aeruginosa infection depends on flagellar motility, but does not depend on the flagellum itself as an activating ligand [4].
Since loss of flagellar motility confers phagocytic resistance, these data suggest that innate immune cells have the ability to recognize bacterial movement and that swimming bacteria provide an important sensory input for phagocytic engulfment [4]. However, an alternative explanation is that bacteria change the expression of unknown secreted and/or cell-surface ligands in response to the loss of swimming motility and therefore alter their phagocytic recognition and uptake. Here we test these hypotheses and provide the first evidence that phagocytic cells utilize bacterial swimming motility as a global mechanism for bacterial recognition. Significantly, we show that alterations in swimming motility allow multiple bacterial species to evade phagocytic recognition. This is not due to measurable changes in the expression of common outer membrane proteins (OMPs) or known regulators of pathogen-associated molecular patterns (PAMPs). Rather, we provide evidence that phagocytic cells are able to respond to bacterial swimming as a function of flagellar rotation after initial contact and, importantly, that phagocytosis is directly proportional to the flagellar torque of the bacteria. We therefore propose a model in which the step-wise loss of flagellar function confers a progressive increase in the ability of the bacteria to evade the phagocytic response of the innate immune system, which promotes an environmentally beneficial niche during infection. This selective pressure provides an explanation for the down-regulation of motility genes and phenotypic loss of swimming that is observed in isolates procured from chronic infections [4]–[8].
Results
Loss of flagellar motility is a widespread mechanism amongst Gram-negative bacteria for resistance to phagocytic uptake
To determine whether phagocytic evasion through loss of swimming motility is specific to P. aeruginosa or is a mechanism shared amongst flagellated Gram-negative pathogens, we used genetically modified motility mutants in multiple bacterial backgrounds (Table 1). P. aeruginosa PA14 is a non-mucoid clinical isolate and is considered the wild-type (WT) in this study. All P. aeruginosa genetic mutants used in this study are on the PA14 background. All Vibrio cholerae mutants are constructed using the classical biotype O395 strain and all Escherichia coli mutants are in the K12 background. All non-flagellated strains (which lack swimming motility) have a mutation in either the flagellar hook gene (flgK), or in the gene coding for the flagellin monomer (flaA and fliC for V. cholerae and E. coli, respectively) [9], [12], [13].
Tab. 1. Bacterial strains used in this study. 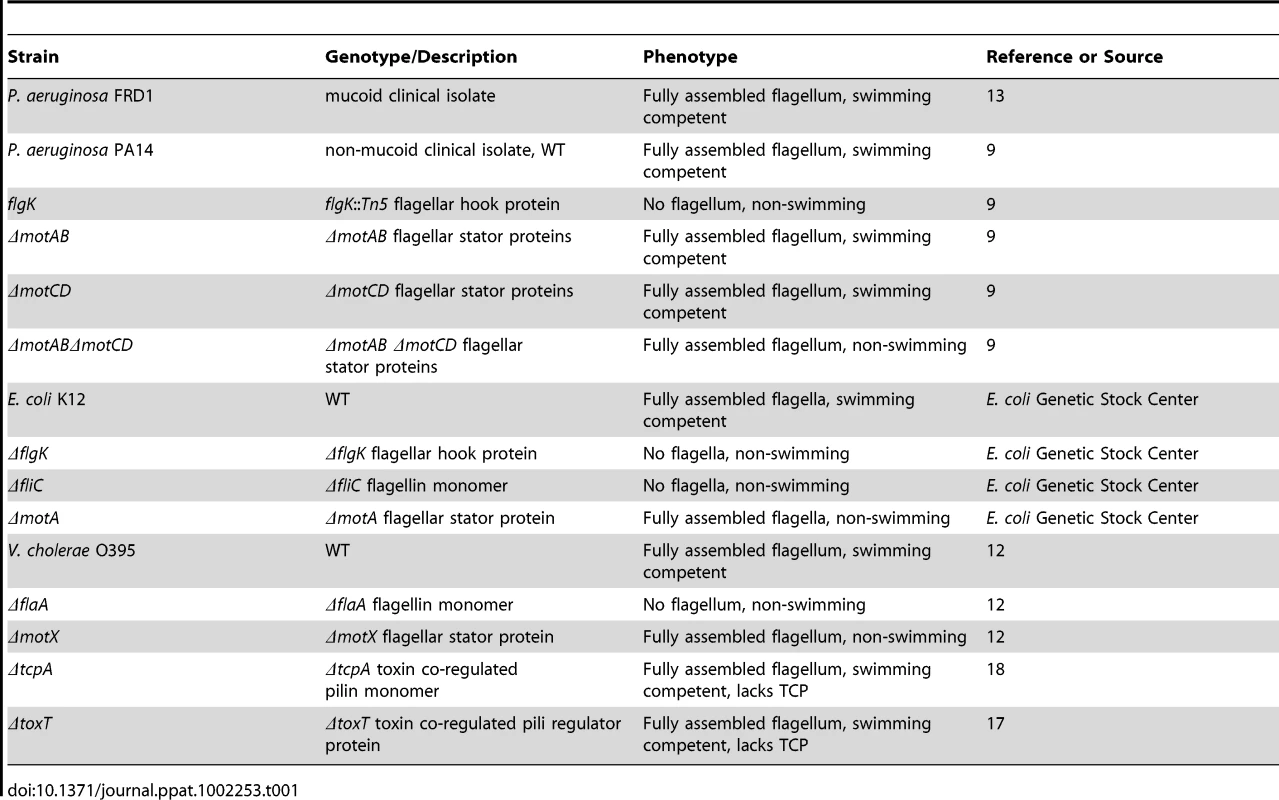
The two stator complexes (MotAB and MotCD) in P. aeruginosa are each composed of two proteins and are functionally partially-redundant. Importantly, deletion of all four genes (motABmotCD) inhibits flagellar rotation, but not flagellar assembly, resulting in a mutant that is flagellated but incapable of swimming [9]. The motAB mutant, and to a lesser extent the motCD mutant, are swimming competent, though not to the same degree as the parental WT [9], [14]. The stator complexes in V. cholerae and E. coli are analogous to those of P. aeruginosa, though not identical in composition. The stator of V. cholerae is also composed of at least four proteins, termed PomA, PomB, MotX, and MotY [15]. The contribution of each protein to stator functionality in V. cholerae is still unclear, however loss of the motX gene results in a flagellated, but non-swimming mutant that is phenotypically similar to the P. aeruginosa motABmotCD mutant [12], [15]. In E. coli, the stator is composed of only two proteins, MotA and MotB [13]. Loss of either gene product (MotA in this study) results in a similar flagellated, but non-swimming mutant [13]. We previously reported that the genetic loss of the stator complexes in P. aeruginosa PA14 confers resistance to phagocytosis in vitro and in vivo in comparison to the swimming-competent parental strain [4]. Phagocytic evasion is not dependent on flagellar assembly, as both flagellated and non-flagellated mutants were equally capable of avoiding phagocytic ingestion [4]. In order to better understand the dynamics of phagocytic resistance by strains incapable of swimming motility, we first verified that strains competent in swimming motility were as equally susceptible to gentamicin as non-swimming strains and remained equally viable during incubation (Figure S1 and data not shown), and then performed gentamicin protection assays with bone marrow-derived dendritic cells (BMDCs) and increasing concentrations of non-swimming P. aeruginosa relative to the WT concentration. We were not able to identify a resistance threshold in either the flgK or the motABmotCD mutants where phagocytic susceptibility approximated WT levels (Figure 1A). In assays where the concentration of non-swimming bacteria was increased to 100-times that of WT, we observed only a ∼30% increase in recovery relative to WT (Figure 1A), indicating that the mechanism facilitating phagocytic resistance of non-swimming P. aeruginosa can only partially be overcome even in the presence of increased non-swimming bacterial concentrations. This degree of phagocytic resistance conferred by loss of bacterial motility is highlighted by the comparison to other phenotypes that have been reported to alter bacterial clearance. For example, alginate production (mucoidy) by P. aeruginosa has been reported to alter bacterial phagocytic susceptibility [16], however the swimming mucoid P. aeruginosa strain FRD1 [17] exhibited only a ∼2-fold change in phagocytosis compared to non-mucoid PA14 WT (Figure 1A).
Fig. 1. Non-swimming gram-negative bacteria are resistant to phagocytosis. 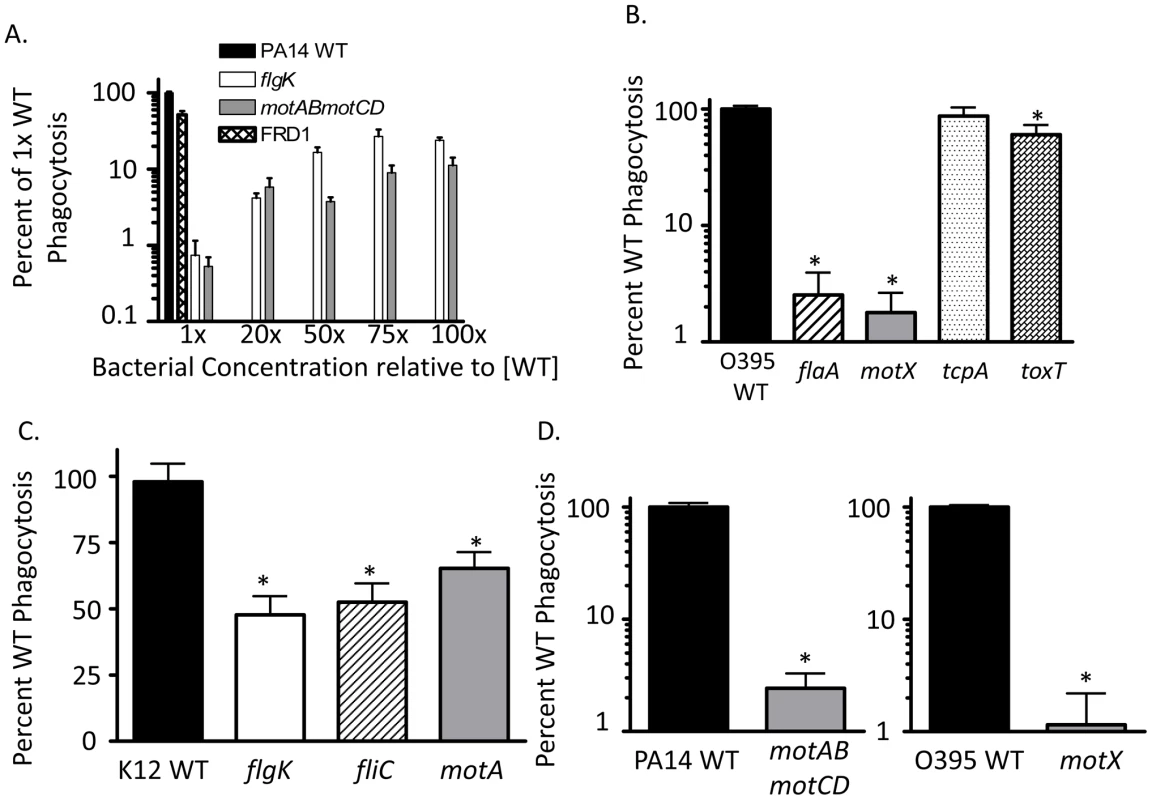
Gentamicin protection assays were used to assess: (A) C57BL/6 BMDC phagocytosis of WT P. aeruginosa strain PA14, the independent mucoid clinical isolate FRD1, and increasing concentrations of non-flagellated mutant flgK and flagellated but non-swimming mutant motABmotCD (in PA14 background). (B) Murine BMDC phagocytosis of WT V. cholerae strain O395, and the flaA, motX, tcpA, and toxT mutants. (C) Murine BMDC phagocytosis of WT E. coli strain K12, and the flgK, fliC, and motA mutants. (D) Human THP-1 leukocyte phagocytosis of P. aeruginosa WT, flgK, and motABmotCD (left); or V. cholerae WT, flaA, and motX (right). Where indicated throughout this and the other figures, phagocytosis of WT strains (PA14, K12 and 0395 in this figure) has been normalized to 100% and the relative phagocytosis of the mutant strains shown as the percent of WT. N≥6, *p<0.05 compared to WT. To test whether motility-based phagocytic recognition is specific to P. aeruginosa, or if this mechanism extends to other bacterial pathogens as well, we performed similar assays using flagellated and non-flagellated V. cholerae and E. coli genetic mutants that contain analogous mutations to the P. aeruginosa mutants described previously. In assays using V. cholerae, both the non-flagellated flaA mutant and the flagellated but non-swimming motX mutant were ∼100-fold more resistant to phagocytosis than the isogenic WT (Figure 1B). In comparison, the swimming-competent tcpA and toxT mutants, which instead lack toxin co-regulated pili (TCP) which facilitate attachment [18]–[20], were ingested to a similar degree as WT V. cholerae (Figure 1B). In experiments using E. coli, the non-flagellated flgK and fliC strains and the flagellated but non-swimming motA strain were all significantly more resistant to phagocytosis compared to the swimming WT, although to a lesser degree than observed with P. aeruginosa and V. cholerae (Figure 1C). To test if these findings also applied to human phagocytes, we tested human THP-1 cells for their preferential ability to phagocytose swimming bacteria. The human THP-1 phagocytic cell line recapitulated our observations using murine BMDCs (Figure 1D) which supports a general mechanism by which non-opsonized Gram-negative bacterial recognition by phagocytic cells is swimming motility-dependent and is not species-specific.
P. aeruginosa lacking swimming motility have decreased overall association with innate immune cells independent of flagellar assembly
In order to visualize the host-pathogen interactions that occur between P. aeruginosa and innate immune cells, and to confirm the assays presented in Figure 1, murine peritoneal macrophages were incubated at 37°C with equal numbers of either GFP-transformed P. aeruginosa PA14 WT or motABmotCD, or V. cholerae O395 WT or motX bacteria and the non-adherent bacteria were washed away prior to counter-staining exposed cell-surfaces with Alexa-647-labeled wheat germ agglutinin (WGA). Multiple images per co-incubation were generated by randomly choosing a viewing field and counting the internalized bacteria along the Z-axis of all visible cells. Representative images of co-incubations using O395 WT (Figure 2A, left) or motX (Figure 2A, right) demonstrate that bacteria with swimming motility associate with macrophages to a much higher extent than do non-swimming bacteria. In co-incubations using O395 WT or PA14 WT bacteria (as in Figure 2A) the quantified internalization, as assessed by bacteria within the phagocytes that do not co-localize with the WGA, is increased >10-fold over motX or motABmotCD, respectively (Figure 2B). These data both further support our gentamicin protection assays and the hypothesis that loss of flagellar motility inhibits the ability of phagocytic cells to engulf bacteria.
Fig. 2. Fluorescence microscopy of phagocytic interactions with GFP-expressing bacteria. 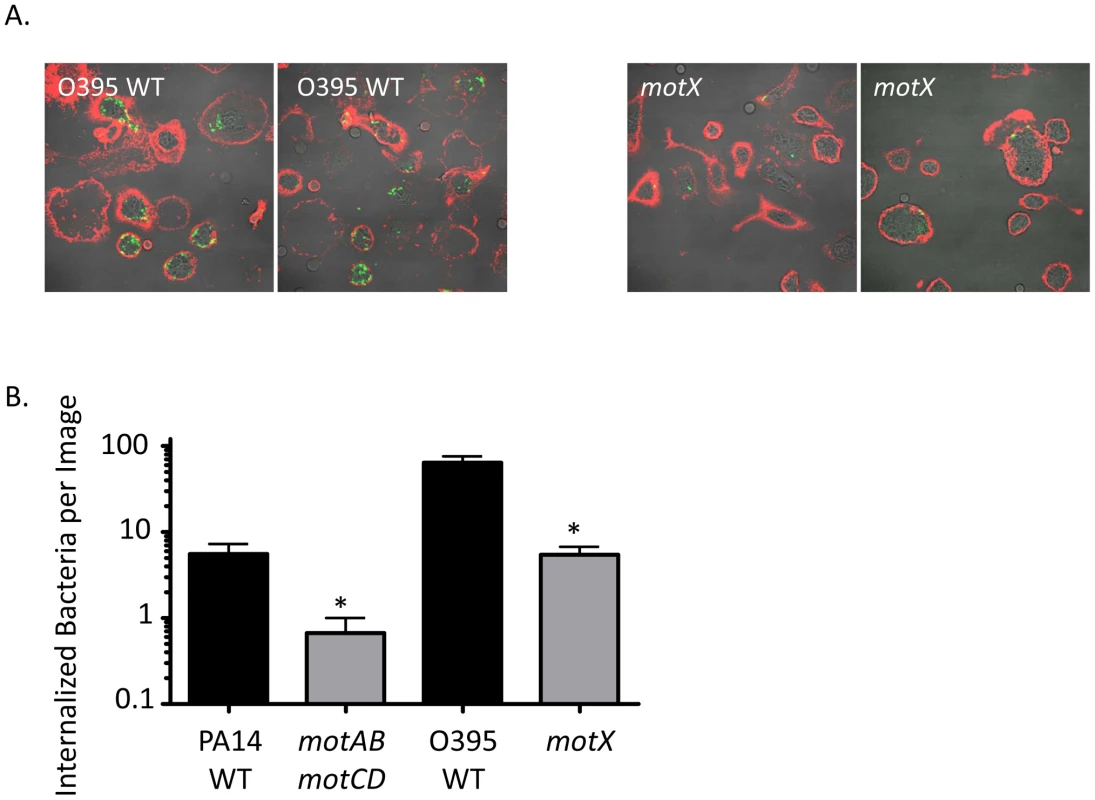
(A) Confocal fluorescence microscopy of untreated murine peritoneal macrophages co-incubated at 37°C for 45 minutes with GFP-transformed V. cholerae O395 WT (left) or motX (right), washed, and subsequently stained on ice with Alexa647-conjugated wheat germ agglutinin (WGA). (B) Internalized bacteria, as in (A), were quantified on the basis of being within a contiguous WGA-decorated phagocyte plasma membrane and not co-localizing with WGA (co-localization seen as yellow, as at the plasma membrane or being external to a phagocytic cell). N≥6 images, *p<0.05. Increased phagocytic resistance by P. aeruginosa motABmotCD is not due to compensatory changes in bacterial secretions, extracellular protein expression, or PAMP presentation
One possible explanation for our current observations is that motility or loss of motility elicits the release of an unknown soluble factor, and that this hypothetical ligand is acting to either induce phagocytosis (if elicited in the motile bacteria) or to impair phagocytosis (if elicited in the non-motile bacteria) by affecting either the neighboring bacteria or the phagocyte itself. In either scenario, we hypothesized that one bacterial strain may affect the phagocytosis of the other strain in trans. We tested this hypothesis with mixed cultures of PA14 WT and motABmotCD. Carbinicillin-resistant (Carbr) WT or the motABmotCD mutant were mixed in equal numbers with the Carbinicillin-sensitive (Carbs) version of the other strain and introduced to murine BMDCs in a standard gentamicin protection assay, after which lysates were plated on Carbinicillin-selective plates. The number of recovered Carbr-motABmotCD CFUs after co-incubation with Carbs-WT and BMDCs was not significantly different than when motABmotCD alone was incubated with BMDCs (Figure 3A). Likewise, recovered CFUs of Carbr-WT when mixed with Carbs-motABmotCD did not change from what is observed when WT alone is assayed by gentamicin protection assay (Figure 3A). This indicates that a swimming competent strain is not able to confer phagocytic susceptibility to a non-swimming strain, nor can a non-swimming mutant confer resistance to a swimming WT. Therefore, differences in phagocytic response as elicited by swimming verses non-swimming P. aeruginosa are not due to any soluble factor being secreted into the extracellular environment or altering the phagocytic activity of the BMDCs.
Fig. 3. The phagocytic resistance by P. aeruginosa motABmotCD is not due to resultant changes in bacterial secretions, extracellular protein expression, or PAMP regulation. 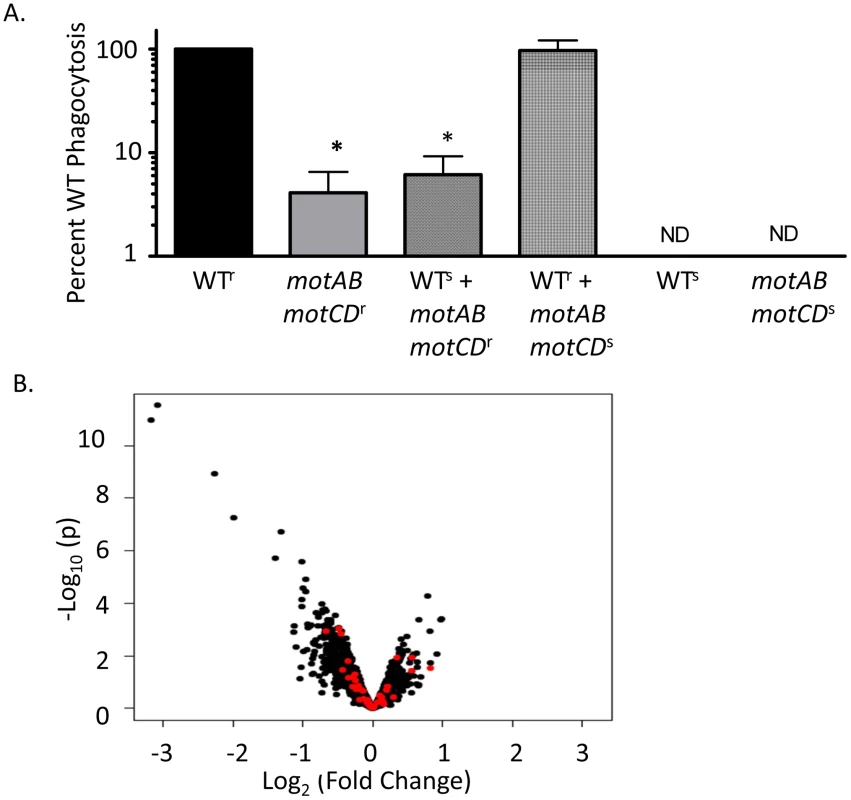
(A) BMDCs were co-incubated with a mixture of equal numbers of carbinicillin-resistant PA14 WT and carb-sensitive motABmotCD or, conversely, carb-resistant motABmotCD and carb-sensitive WT. Phagocytic susceptibility was assayed by gentamicin protection assay and plating on carbinicillin-treated LB agar. (B) Volcano plot of WT gene expression versus motABmotCD mutant gene expression. Red points indicate genes corresponding to likely immunogenic molecules (see Table S1). N≥7, *p<0.05. Many of the regulatory pathways controlling synthesis of outer membrane proteins and peripheral structures on P. aeruginosa are still being elucidated; however phagocytosis assays with P. aeruginosa swarming mutants, type-III secretion mutants, and mucoid strains did not result in significantly increased phagocytic resistance relative to controls (data not shown). Nonetheless, it is still possible that flagellar rotation is co-regulated with gain or loss of expression of an unknown extracellular PAMP or ligand that is recognized by innate immune cells. To identify if deletion of the mot genes correlates with changes in peripheral gene expression levels, we performed genome-wide microarray analysis of the WT and motABmotCD strains. Comparison of gene expression levels between WT and motABmotCD showed no significant change in any recognizable PAMP regulators, OMP genes, lipopolysaccharide synthesis elements, or known immune activating factors (Figure 3B and Table S1). Genes which did change expression more than 2-fold with loss of the mot operons are listed in Table 2. However, swimming motility assays and preliminary phagocytic assays with PA14 strains containing transposon insertions in each of those genes identified in Table 2 did not recapitulate the phenotypes observed with motABmotCD (data not shown). These data support the hypothesis that phagocytic cells are able to directly respond to swimming motility by bacteria.
Tab. 2. Change in gene expression ≥2-fold with loss of <i>motABmotCD</i>. 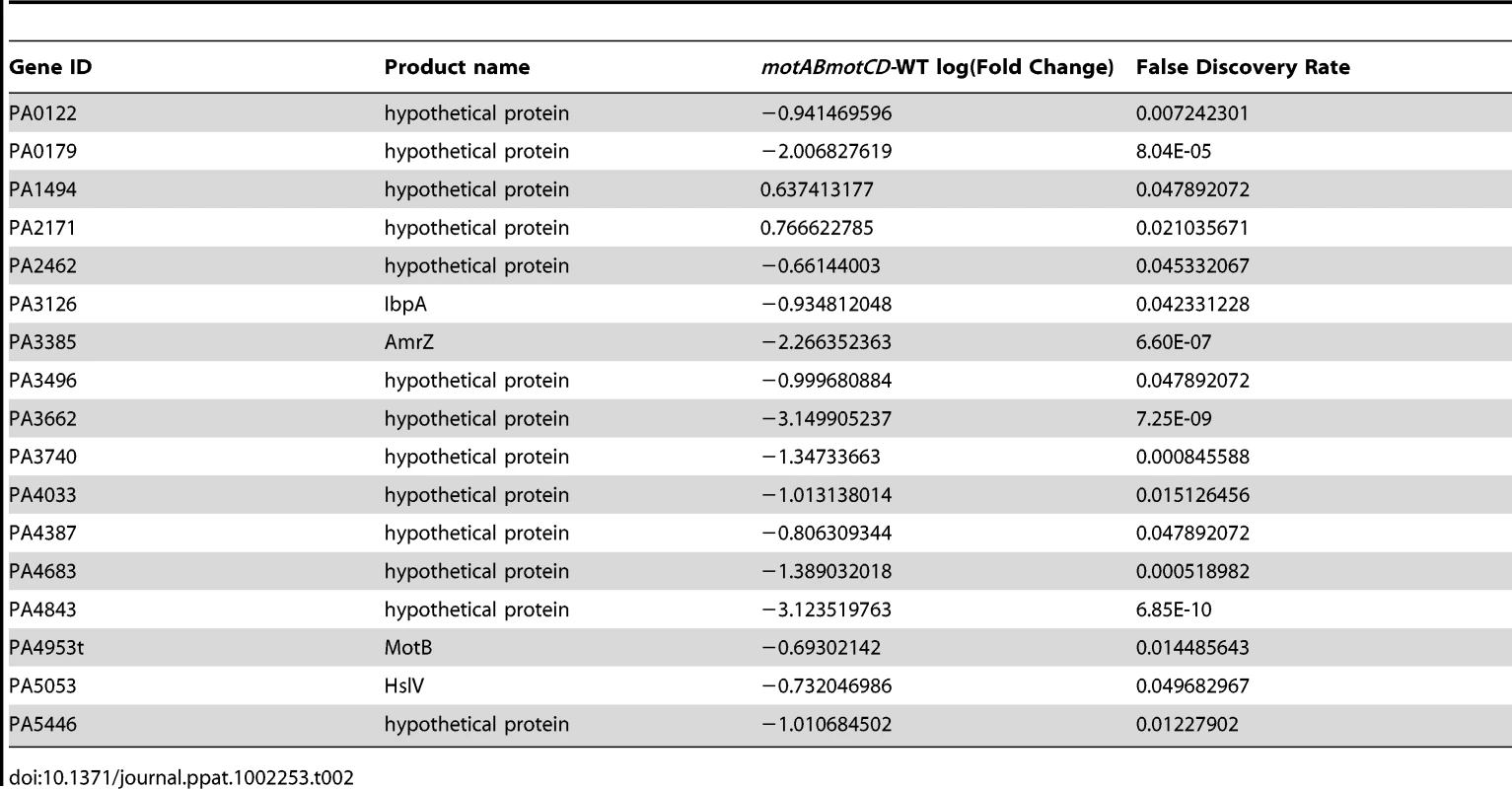
Inherent microbiocidal activity and limited bacteria-cell contact does not provide for phagocytic resistance in non-swimming bacteria
An alternative hypothesis to the cellular sensing of bacterial motility is that instead of non-swimming strains evading phagocytic uptake, the loss of flagellar motility renders the bacteria more susceptible to killing within the phagolysosome. While there is no prior evidence of this, we rigorously tested relative bacterial association and recovery over time by co-incubating WT or motABmotCD with adherent macrophages and then separating the cell-unassociated bacteria in the media from the macrophage-associated bacteria and plating both fractions to quantitatively assess relative CFUs in each. At all time points tested, greater CFU recovery was observed in the unassociated fraction when using motABmotCD, while in the associated fraction, significantly higher CFUs were recovered with WT (Figure 4A). If intracellular killing were increased for motABmotCD, extracellular CFUs would decrease below that of WT as bacteria were removed from the system at a higher rate. We therefore conclude that microbiocidal vulnerability and bacterial death does not measurably account for the differences observed between swimming and non-swimming strains. These data support previous observations that intracellular killing of non-opsonized P. aeruginosa is <5% of available bacteria within a 45-min co-incubation time period [4]. Another alternative explanation for the current observations is that non-swimming bacterial mutants do not come into contact with phagocytes to the same degree as swimming-capable WT. To test this hypothesis we performed multiple, complementary assays. First, we performed gentamicin protection assays with WT or motABmotCD in the presence of surfactant in order to decrease surface tension that may inhibit contact between bacteria and phagocytes. In co-incubations performed with either the non-ionic detergents Tween80 or beta-octyl glucoside (used as a biofilm inhibitor [21]), or the artificial lung surfactant Survanta, we did not observe any increase in motABmotCD uptake (Figure 4B). Secondly, we tested whether forced contact between bacteria and phagocytes would overcome the phagocytic deficit of the non-swimming bacteria. To do so, we centrifuged bacteria onto BMDCs or macrophages and then subsequently assayed for phagocytosis. The degree of initial contact of WT or motABmotCD bacteria with the phagocytes following centrifugation was analyzed by FACS and was not different between strains (Figure 4C, inset). We observed a slight increase in CFU recovery of the non-swimming P. aeruginosa flgK and motABmotCD mutants (Figure 4C) as well as the non-swimming V. cholerae flaA and motX mutants (Figure 4D) relative to the respective swimming bacterial strains when contact was artificially initiated. However, the increased internalization did not recapitulate WT levels of phagocytosis, since non-swimming strains were still at least 10-fold more resistant to uptake as compared to their respective parental strains. These data demonstrate that phagocytic recognition is not solely dependent on contact between bacteria and phagocyte and supports a role for flagellar motion in pathogen recognition and ingestion.
Fig. 4. Microbiocidal activity and limited bacteria-cell contact does not provide for decreased phagocytic clearance of non-swimming bacteria. 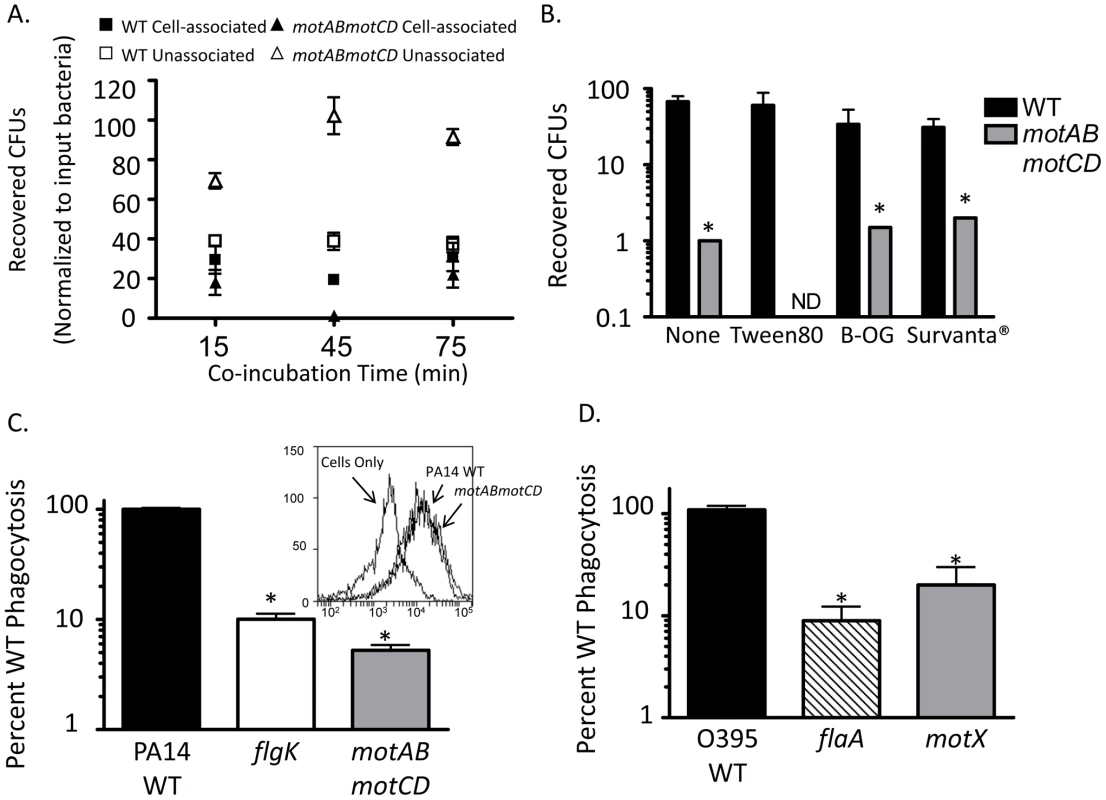
(A) Adherent murine peritoneal macrophages were co-incubated with PA14 WT or motABmotCD and cellular associated bacteria and non-associated bacteria were quantitatively assessed at the indicated time points. (B) BMDCs were co-incubated with WT or motABmotCD in the presence or absence of 0.01% Tween80, 0.01% beta-octyl glucoside, or 2% Survanta, and assayed by gentamicin protection assay for relative bacterial phagocytosis. (C, inset) GFP-expressing PA14 WT or motABmotCD were centrifuged onto BMDCs and immediately fixed and analyzed by FACs for cellular association. Phagocytic cells in the absence of bacteria are shown as background. (C) P. aeruginosa PA14 WT, flgK, or motABmotCD, or (D) V. cholerae O395 WT, flaA, or motX were centrifuged onto BMDCs or peritoneal macrophages, respectively, and assayed by gentamicin protection assay. N≥5, *p<0.05 as compared to WT. Flagellar motility enhances both the association and the uptake of bacteria by phagocytes
The relative contributions of binding verses phagocytic uptake and engulfment are not well understood in non-opsonized phagocytosis. To further elucidate the individual components that promote the phagocytosis of swimming bacteria, we quantitatively assessed bacterial association with macrophages under 3 sequential conditions. We first co-incubated swimming or non-swimming P. aeruginosa with adherent murine macrophages at 4°C, which is permissive for binding but prevents both bacterial motility and phagocytic uptake, and then washed away non-associated bacteria and plated the cellular lysates. In parallel, we warmed cells and bacteria to 37° after the initial binding and washing at 4°C, thus initiating both bacterial movement and phagocytosis of bound bacteria, and then plated lysates directly, or treated with gentamicin and then plated. In co-incubations held at 4°C, recovered CFUs between WT, flgK, and motABmotCD were similar, as was expected since all bacteria were immobilized (Figure 5A). Of note, this also supports that it is not an unknown bacterial cell-surface ligand, with expression altered by changes in motility, that affects bacterial binding to phagocytes. However, the difference in relative bacterial association with macrophages increased dramatically when bound-bacteria and cells were warmed to 37°C, demonstrating that binding of bacteria is a necessary but insufficient component to the differential phagocytic recognition (Figure 5A). Even once associated with phagocytic cells, non-swimming P. aeruginosa evade uptake and, as evidenced by the progressively decreasing number of CFUs recovered after successive washes (Figure 5A left), disassociate at a higher efficiency than WT bacteria. Treatment with gentamicin demonstrated that the remaining associated bacteria, after washing, are further differentially ingested dependent on swimming-capability (Figure 5A). However, it is possible that co-incubation at 4°C distorts initial receptor-ligand interactions that nominally occur at physiological temperature. To confirm that non-swimming P. aeruginosa is impaired in its ability to bind innate immune cells, we pre-treated macrophages with cytochalasin D to inhibit phagocytic uptake and subsequently incubated WT or non-swimming mutants with these macrophages at 37o. We then washed and plated cellular lysates to quantitatively assess the bacteria that bound to the outside of the cells. In support of the previous assays, we recovered significantly fewer flgK and motABmotCD CFUs than WT (Figure 5B). Visualization of these co-incubations using GFP-expressing strains and Alexa-647-stained macrophages confirmed that bacterial association is decreased in non-swimming P. aeruginosa strains (Figure 5C).
Fig. 5. Flagellar motility enhances both the association and the uptake of bacteria by phagocytes. 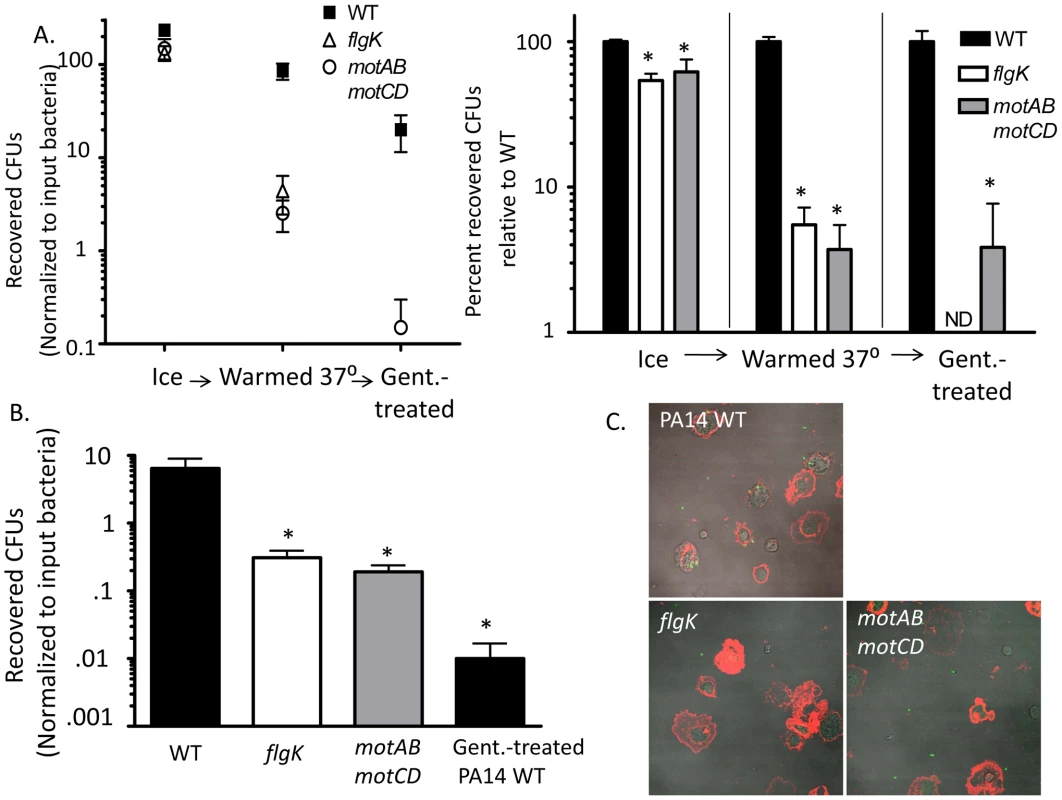
(A) P. aeruginosa PA14 WT, flgK, or motABmotCD that were incubated in parallel with adherent macrophages at 4°C exhibited similar binding (assessed by CFUs following washing and lysis of the macrophages) (left). However, the difference in relative bacterial association with macrophages dramatically changed when, following washing, the bound bacteria and cells were warmed to 37°C (middle); and this differential was even more substantial when assessed on the basis of phagocytosed (gentamicin-resistant) bacteria (right). Plots show total mean recovered CFUs accounting for input bacteria (left panel) and the number of recovered CFUs plotted relative to WT (right panel). (B) Murine peritoneal macrophages were treated with cytochalasin D prior to co-incubation with PA14 WT, flgK, or motABmotCD and were assayed for bacterial association and protection from gentamicin. (C) Fluorescence microscopy of cytochalasin D-treated macrophages co-incubated with GFP-expressing PA14 WT, GFP-expressing flgK, or GFP-expressing motABmotCD and subsequently stained with wheat germ agglutinin-conjugated Alexa647. 65x magnification. N≥5, *p<0.05. Live cell imaging of P. aeruginosa interactions with murine peritoneal macrophages
In order to better understand and visualize how phagocytic cells bind swimming verses non-swimming bacteria we performed live cell microscopy of adherent macrophages interacting with P. aeruginosa. Equal concentrations of either GFP-expressing WT or GFP-expressing motABmotCD were flowed across adherent macrophages at a constant rate and visualized under fluorescence and DIC. WT readily accumulated on macrophage cell surfaces with prolonged associations and visible and substantial adherence events (Figure 6 top, Video S1). The motABmotCD mutant displayed little or no accumulation on the cells, visually flowing past macrophages with appreciably shorter adherent associations (Figure 6 bottom, Video S2). These images support the previous data which show that phagocytic evasion by non-swimming bacteria is achieved through multi-faceted resistance to binding accompanied by phagocytic unresponsiveness even with contact.
Fig. 6. Live imaging of murine peritoneal macrophage interactions with PA14 WT or motABmotCD bacteria in vitro. 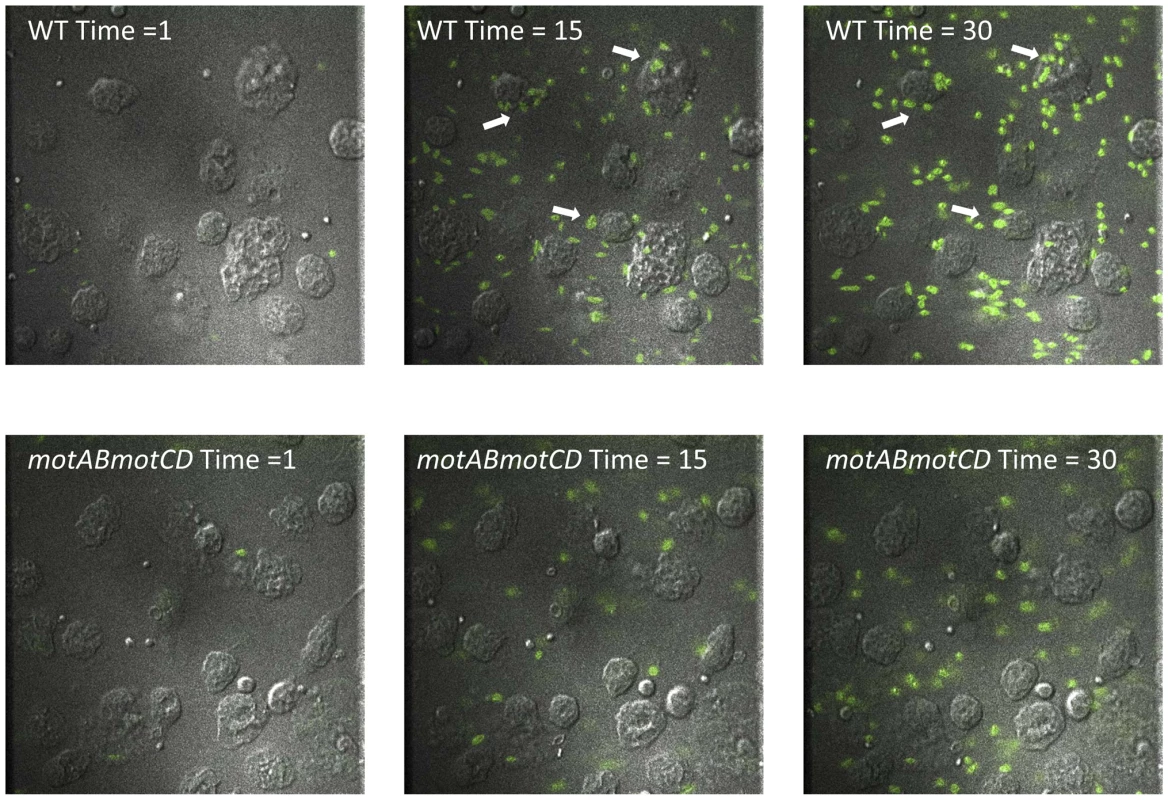
Representative images of adherent macrophages treated with liquid culture of GFP-expressing PA14 WT (top) or GFP-expressing motABmotCD (bottom) under constant flow at Time = 1 min, Time = 15 min, and Time = 30 min. Bacterial concentrations were equalized prior to imaging for comparative visualization of bacterial accumulation and retention (arrows) on phagocytes. Macrophages viewed by DIC, bacteria by fluorescence. See Videos S1 and S2. Step-wise loss of flagellar torsion progressively increases phagocytic resistance
Our data indicate that flagellar rotation confers phagocytic recognition by innate immune cells. As a formal test of this, we hypothesized that bacterial flagellar motility would be proportional to phagocytic uptake. Motility studies with P. aeruginosa grown in media of increasing viscosity have shown that successive genetic deletions of the partially-redundant mot flagellar stator complexes result in decreases in swimming capability [9], [14]. Specifically, swimming and flagellar-based motility in P. aeruginosa is tied to the degree of flagellar stator function, since loss of rotation from deleting motAB decreases flagellar-based motility below that of WT, while loss of motCD further decreases flagellar-based motility below that of the motAB mutant [9], [14], and loss of all four mot genes (both complexes) renders P. aeruginosa completely unable to swim or swarm (maximal expansion of colonies of WT, motAB, motCD and motABmotD in 0.6% agar were previously assessed as 29.5, 21.9, 7.3, and 6.3 mm, respectively [9], [14]). Therefore, we used isogenic mot mutants to test if decreases in swimming ability confer proportional increases in phagocytic evasion. Total bacterial association between GFP-expressing motAB and BMDCs was significantly decreased as compared to GFP-expressing WT as measured by fluorescence-activated cell sorting (FACS), while association was further decreased in GFP-expressing motCD and GFP-expressing motABmotCD (Figure 7A). To more rigorously and quantitatively assess relative phagocytosis of these mutants we returned to the gentamicin protection assay. Phagocytosis of motAB was slightly but significantly decreased compared to WT (Figure 7B). Further phagocytic resistance was observed in motCD, with non-swimming motABmotCD mutant being the most resistant (Figure 7B). This was not due to measurable differences in binding between the mot mutants, since these all bound to cytochalasin D-treated BMDCs similarly, though binding was impaired relative to GFP-expressing WT and better than GFP-expressing flgK (Figure 7C). Importantly, microarray analysis comparing gene expression profiles between WT, motAB, motCD and motABmotCD did not reveal any genetic changes that progressively correlate amongst these four strains with motility and therefore there were also no changes amongst the bacterial strains that correlated with phagocytosis (Table S2). Using methodology similar to that in Figure 5A, we next used the mot mutants to compare relative swimming ability with phagocytosis by adherent macrophages. Assessment of retained association and subsequent engulfment after initial binding revealed that all 3 mot mutants were slightly, but comparably, deficient in binding to adherent macrophages at 4°C (Figure 7D). However, upon warming of cells and bound bacteria to 37°C, followed by treatment with gentamicin, a progressive loss of association relative to WT was observed where association and engulfment of WT > motAB > motCD > motABmotCD (Figure 7D). This is the first evidence that the MotAB and MotCD proteins regulate phagocytic susceptibility in P. aeruginosa and that sequential loss of the Mot complexes confers increasing phagocytic evasion.
Fig. 7. Successive loss of flagellar functionality enables stepwise increases in phagocytic resistance. 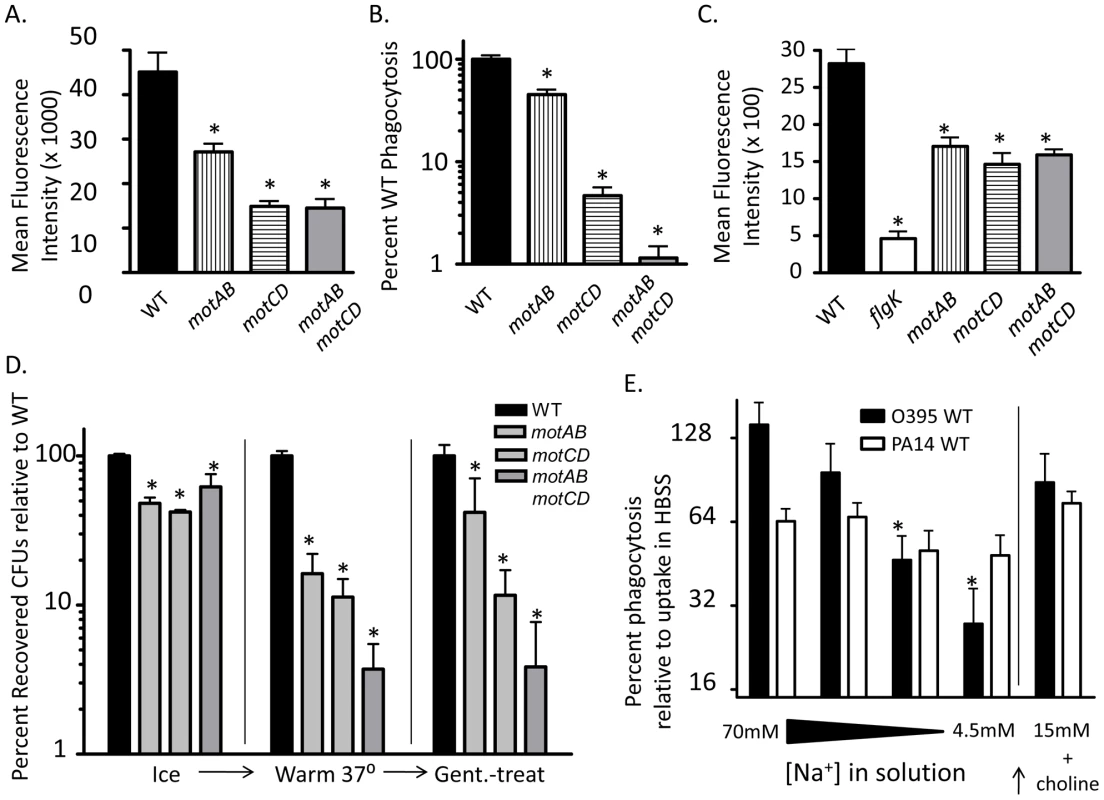
(A) FACS analysis and (B) gentamicin protection assay of BMDCs co-incubated with PA14 WT, motAB, motCD, or motABmotCD. (C) FACS analysis of cytochalasin D treated BMDCs co-incubated with GFP-expressing PA14 WT, flgK, motAB, motCD, or motABmotCD. (D) Adherent macrophage assay of cells co-incubated with PA14 WT, motAB, motCD, or motABmotCD bacteria at 4°C, warmed to 37 °C, or treated with gentamicin after warming to 37 °C. (E) Gentamicin protection assay of murine peritoneal macrophages co-incubated as indicated with P. aeruginosa PA14 WT or V. cholerae O395 WT in 70 mM, 18 mM, 9 mM, or 4.5 mM NaCl buffers, or in 15 mM NaCl buffer with 135 mM choline chloride. Recovered CFUs are normalized against recovery in HBSS (138 mM NaCl). N≥5, *p<0.05. Since we observed increasingly dramatic phagocytic evasion phenotypes through genetic manipulation of the bacterial stator complexes, we turned to V. cholerae for biochemical proof-of-principle in support of our genetic evidence. Flagellar torque in Pseudomonas is believed to be generated through active transport of protons across the outer and inner membranes [14], [22]. In V. cholerae, however, flagellar torque is generated through transport of sodium ions [15], [23]. Progressively limiting the concentration of sodium in the media leads to proportional inhibition of V. cholerae flagellar rotation, and thus its ability to swim [23]. We performed gentamicin protection assays in serum-free buffers containing successively titrated concentrations of NaCl, while maintaining a constant osmolarity by substituting choline chloride. As expected, decreasing the availability of sodium did not significantly change the degree of uptake of WT P. aeruginosa, which does not depend on sodium for flagellar motility (Figure 7E). However, loss of sodium availability correlated with increased phagocytic resistance by WT V. cholerae (Figure 7E). Treatment of V. cholerae or P. aeruginosa alone with sodium-limited buffers did not significantly decrease recovery of colony forming units (data not shown). Importantly, reconstitution of the lowest sodium-containing buffer with 15mM NaCl, while maintaining the choline chloride concentration constant, elicited a recovery in phagocytic susceptibility of V. cholerae, thereby confirming that choline chloride itself is not responsible for increased phagocytic resistance or for death of the V. cholerae (Figure 7E). Taken together, these data indicate that the step-wise loss of flagellar torsion and swimming ability, whether through genetic deletion of the stator complex or limiting ion motive force, provides for an increasing ability to evade recognition and phagocytosis by innate immune cells.
Discussion
In this work, we define the steps that comprise phagocytic recognition of non-opsonized bacterial pathogens and identify that changes in flagellar swimming motility can titrate the phagocytic clearance of bacteria. In support of previous results, phagocytosis is independent of flagellar assembly, since both flagellated and non-flagellated non-swimming strains are equally resistant to uptake [4]. While the majority of these studies focus on P. aeruginosa, we show that this immune resistance phenotype in swimming-defective strains is not specific to a single species, but represents a potentially widespread mechanism of immune evasion by Gram-negative bacteria. This phenotype is not due to motility-regulated secreted factors or compensatory changes in expression of bacterial genes. Likewise, phagocytic evasion is not due to avoidance of contact with phagocytic cells. Instead, as shown with P. aeruginosa, non-swimming strains avoid phagocytic recognition by disassociation after initial contact and remain resistant to phagocytosis even after being bound at the cell surface.
In comparing resistance across multiple bacterial species, it is interesting that non-swimming V. cholerae mimicked P. aeruginosa in terms of scope and magnitude of phagocytic resistance, while the degree of non-swimming E. coli resistance was much less dramatic. Since loss of motility in the V. cholerae and E. coli isogenic mutants was confirmed by swimming motility assays in 0.3% agar (data not shown), it is not immediately clear why swimming deficiency in E. coli conferred resistance to a lesser degree. Of note, both P. aeruginosa and V. cholerae have a single, polar, monotrichous flagellum under standard conditions, whereas E. coli swim via multiple peritrichous flagella [9], [12], [13]. However, non-flagellated flgK and fliC strains of E. coli were not significantly different than the flagellated, non-swimming motA mutant in terms of phagocytic uptake. It is therefore unlikely that multiple flagella are causative of the discrepancy; more plausible is that the recognition of alternative structures, specific to E. coli, are able to partially compensate for the resistance phenotype of non-swimming strains. The small decrease in uptake observed with a mucoid strain of swimming-capable P. aeruginosa, as well as the trending decrease in recovered V. cholerae CFUs when TCP is eliminated, suggest that such compensatory mechanisms are feasible. Even so, the significant drop in phagocytic susceptibility when E. coli loses flagellar motility, independent of flagellar assembly, supports our hypothesis of a widespread mechanism utilized by innate immune cells for phagocytosis of motile, non-opsonized pathogens. Moreover, we demonstrate that this response to motility is shared amongst phagocytic cell types, anatomical locations, and cells of human or mouse origin (Figure 1 and [4]).
To delineate the phagocytic process, we examined bacterial engulfment in a step-wise manner. Our data indicate that for an uptake event to occur, contact must be made between the pathogen and the phagocyte, followed by adhesion and recognition, which culminates in a stimulus to ingest. In assessing P. aeruginosa binding and association with cytochalasin D-treated BMDCs, both the non-flagellated flgK mutant and the flagellated mot mutants were modestly but significantly decreased in binding to BMDCs compared to WT, though the degree of flgK association was well below that of all three mot mutants, which were not different from each other. This is of interest since multiple reports have identified bacterial flagella as potent adhesins in physiological systems [24]–[26]. Taken together, these data point to the flagellum in P. aeruginosa as indeed having adhesive properties, but calls into question the role of flagella as direct ligands for phagocytosis since all non-swimming mutants, regardless of flagellar expression, were indistinguishable in phagocytic assays. Furthermore, artificial enforcement of contact between non-swimming P. aeruginosa or V. cholerae and phagocytes did not recapitulate WT levels of uptake. In the experiments where pathogen-host cell contact was induced, and therefore initially equal between strains, we recovered significantly fewer CFUs using non-swimming strains relative to controls and the presence of the flagellum did not change phagocytic uptake among strains that were non-swimming. Therefore, the presence of a non-rotating flagellum can only partially recover WT levels of binding to phagocytic cells, and contact alone, regardless of flagellar assembly, is necessary but insufficient for full phagocytic activation. Importantly, among those bacteria that bound to the cell-surface, we discovered that their subsequent phagocytic fate is dependent on their swimming capability. Overall dissociation after contact, and therefore also evasion of engulfment, was increased in non-swimming P. aeruginosa compared to WT. These data support an overall model where loss of flagellar rotation enables the evasion of both phagocyte binding and, importantly, recognition and response after initial contact.
The evidence that the flagellum itself is not contributing to phagocytic susceptibility, but that flagellar rotation is, raises the question of how the cells preferentially recognize swimming bacteria. We previously demonstrated that loss of the MyD88 adaptor protein did not alter phagocytic uptake of P. aeruginosa [4]. Therefore, none of the MyD88-dependent toll-like receptors (TLRs), specifically TLR5 which recognizes bacterial flagellin, are required for phagocytosis of P. aeruginosa. The two likely possibilities, not mutually exclusive, are that bacterial motility alters the expression of an unknown bacterially-produced factor or ligand that alters the ability of the phagocyte to recognize or ingest the bacteria; or that the phagocyte can sense the motility and that this drives the phagocytic event. Our data demonstrate that it is unlikely that phagocytic cells are sensing a motility co-regulated secreted molecule or extracellular ligand, since the mixed-culture assay did not indicate that WT could confer phagocytic susceptibility to the motABmotCD mutant in trans, nor could motABmotCD confer phagocytic resistance to WT. This inability to physiologically complement the phagocytic phenotype suggests that the extracellular environment of cells co-incubated with WT is not different than when cells are co-incubated with motABmotCD. In support of the complementation data, microarray analysis of bacterial gene expression when the mot complexes were successively deleted from WT indicate that no known immunogenic effectors are significantly altered. Most importantly, there was no significant change in gene expression pattern that correlated with successive deletion of the mot complexes and therefore no compensatory bacterial genetic changes that correlated with phagocytic susceptibility. Those genes that do change expression more than 2-fold in response to deletion of mot genes are likely bystander effects, as we could not identify phagocytic or motility phenotypes in corresponding transposon mutants. Thus, we believe it is unlikely that phagocytic evasion by motABmotCD is due to indirect effects of mot gene deletion. The alternative explanation is that leukocytes possess a mechanism that recognizes and responds to flagellar torsion as a phagocytic initiation signal. We hypothesized that, if this were the case, then step-wise decreases in flagellar torsion would result in proportional increases in phagocytic resistance. Deleting MotAB from the stator complex of P. aeruginosa results in decreased swimming speed, as it partially contributes to flagellar torque generation [9], [14]. Our data show that this decrease in swimming capability confers a small but significant degree of resistance to phagocytosis. Further loss of flagellar motility, due to deletion of MotCD, which is a larger contributor to stator functionality than MotAB [9], [14], conferred a greater degree of phagocytic resistance. Complete loss of flagellar function, the phenotypic result of deletion of all four mot genes [9], [14], conferred the greatest degree of phagocytic resistance, equal to that of non-flagellated mutants. Once bacteria are cell-bound, subsequent dissociation and phagocytic evasion follow the same pattern, with resistance in motABmotCD > motCD > motAB > WT. This is the first demonstration of titrated phagocytic resistance in P. aeruginosa being regulated through mot gene function, and fits an infection model where the sensory mechanisms of the innate immune system provide a selective pressure for P. aeruginosa to down-regulate flagellar motility. Since the partial loss of the stator does not completely inhibit swimming capability, selective pressure to lose stator functionality would not necessarily impede P. aeruginosa colonization, but provide increasing degrees of resistance to phagocytic recognition. Indeed, the partial redundancy in the P. aeruginosa stator proteins may have evolved to provide such an advantage during infection.
While a functional stator complex is required for flagellar rotation, additional requirements, such as the electro-chemical gradient that provides rotational force, are necessary for full flagellar motility [23]. Limiting the availability of ions required for flagellar rotation can selectively impede flagellar motility [23]. We found that progressively decreasing sodium availability to V. cholerae, which depends specifically on sodium ions for flagellar rotation, conferred step-wise increases in phagocytic resistance, analogous to our observations with the P. aeruginosa genetic mutants. Limiting sodium availability to P. aeruginosa did not alter phagocytic susceptibility, which fits with P. aeruginosa use of a proton motive force and not sodium for flagellar motility [14], [22]. These data are in agreement with our results using genetically modified bacteria, indicating that loss of flagellar motility, regardless of the means, confers resistance to phagocytic uptake.
While the down-regulation of flagellar motility in P. aeruginosa isolates from persistent infections has been previously documented [5]–[7], these results provide an explanation for the observed loss of motility in clinical strains recovered from CF patients over the course of chronic infection, but which is not limited to just P. aeruginosa. Consistent with a pleiotropic mechano-sensory system, both non-swimming V. cholerae and E. coli also demonstrate phagocytic resistance. We believe it is therefore likely that innate immune cells are able sense bacterial motility, possibly through membrane depression or activation of an unknown tension receptor(s), and that this mechanical perturbation, analogous to a “fish on a hook”, provides the necessary sensory stimulant for the cell to “set the hook” and initiate phagocytic uptake. Examples of cellular mechano-sensory systems exist in other physiological systems, such as cellular stretch detection in muscle sarcoma cells [27] and shear-enhanced adhesive catch bonds in rolling leukocytes [28], but to date no reports have identified such a mechanism contributing to pathogen recognition. Since flagellar motility is a necessary virulence factor for many pathogens to effectively colonize a host [29]–[32], it makes evolutionary sense that the innate immune system, as a first line of defense, would develop strategies to exploit this phenotype. Concomitantly, loss of flagellar motility in isolates taken from established infections corresponds to selective pressure to bypass this immune strategy.
In conclusion, in this work we demonstrate that bacterial flagellar rotation is recognized as a phagocytic activator by innate immune cells. We show that this mechanism responds to at least three different species of bacteria, P. aeruginosa, V. cholerae, and E. coli, and thus likely represents a common and widespread immune strategy for bacterial recognition by direct sensing of flagellar torsion. In the P. aeruginosa model, swimming-deficient strains avoid phagocytic uptake through a combinatorial strategy of limiting prolonged association after initial contact with phagocytic cells and not eliciting uptake when bound to the cell surface. We show for the first time that phagocytic recognition is directly proportional to mot gene function as it relates to phenotypic flagellar torsion. These results provide a basis for the reported observations of non-motility in clinical strains isolated from established infections, and provide evidence of a novel strategy utilized by the innate immune system to fight bacterial infection.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Dartmouth IACUC Committee (Permit Number: A3259-01). No surgery was performed, and all efforts were made to minimize suffering.
Mice and cells
Bone marrow-derived dendritic cells (BMDCs) were cultured from C57BL/6 WT mice obtained from NCI using a modification of Inaba et al. as previously described [33]. For isolation of murine macrophages, naïve C57BL/6 mice were injected i.p. with 1 ml of 4% thioglycolate and subsequently sacrificed 4 days later. The peritoneal cavity was lavaged with 6 ml of serum-free Hank's Balanced Salt Solution (HBSS). The lavage fluid was centrifuged and pelleted cells were washed twice in serum-free HBSS before being resuspended in 2 ml serum-free HBSS. For these studies, the Pseudomonas aeruginosa strain FRD1 is a mucoid clinical isolate, while the non-mucoid clinical isolate PA14, Vibrio cholerae strain O395 (generously provided by Dr. Ron Taylor, Dartmouth), and Escherichia coli strain K12 (obtained from Yale CGSC) are the parental bacterial strains and wild type controls for all of the respective mutants studied.
Bacterial binding assays and FACS-based bacterial association
Bacterial strains expressing green-fluorescence protein (GFP) were generated by transformation of the indicated strains with a multi-copy plasmid (pSMC21 Ampr Kanr Carbr GFP+) that constitutively expresses GFP under control of a derivative of the Ptac promoter [34], [35]. FACS-based bacterial association was assayed as a modified version previous protocols [4]. Briefly, 2.5×105 C57BL/6 BMDCs or macrophages were incubated with the indicated non-transformed or GFP-expressing bacterial strains at an MOI of ∼10 in serum-free HBSS for 45 minutes at 37°C or 4°C as indicated in the text. Cells were washed in phosphate-buffered saline (PBS) and mean fluorescence intensity of the phagocyte populations were assessed and graphed to obtain relative efficiency of cellular association with the indicated bacterial strains. For bacterial binding assays, BMDCs or macrophages were pre-incubated in 10 uM cytochalasin D (Sigma) in serum-free HBSS for 60 minutes at 37°C. Co-incubation between phagocytes and the indicated bacterial strains took place in the presence of 10 uM cytochalasin D in serum-free HBSS or in HBSS alone for 45 minutes at 37°C. Cells were subsequently washed in serum-free HBSS or PBS and then analyzed by plating cellular lysates and counting recovered CFUs, or by FACS for the acquisition of fluorescence as a function of GFP+ bacterial association.
Gentamicin protection assays
Phagocytosis of live bacteria was performed as a modified version of published protocols [36] and as previously described [4]. Briefly, overnight cultures of P. aeruginosa, V. cholerae, or E. coli were washed and resuspended in serum-free HBSS or the indicated buffer and bacteria concentrations were determined. 2.5×105 BMDCs or the indicated cell type were incubated with bacteria at an MOI of ∼10 for 45 minutes at 37°C, followed by incubation in 100 µg/ml gentamicin for 20 minutes at 37°C. Recovered CFUs are normalized to input bacteria to account for variability in initial strain concentration. Where indicated, recovered CFUs are presented as a percent of the isogenic WT to compare relative degrees of phagocytosis. In experiments utilizing sodium-limited buffers, solutions were made with 0.9 mM CaCl, 4 mM KCl, 0.5 mM MgCl, 5 mM HEPES and the indicated amount of NaCl and reconstituted with choline chloride for a combined concentration of 140 mM (pH 7.5). For phagocytic threshold experiments, the concentration of non-swimming P. aeruginosa was successively increased 10-fold relative to the concentration of WT. For forced-contact experiments, BMDCs or macrophages were centrifuged for 5 min at 400 g. Bacteria were then layered onto pelleted cells followed by centrifugation at 4°C for 10 min at 715 g. An equal degree of cell-to-bacteria contact after centrifugation in swimming verses non-swimming strains was verified by immediately fixing cells and GFP-transformed bacteria with 4% paraformeldahyde and measuring the accumulated cellular GFP signal via FACS. For bacteria-phagocyte cell surface tension experiments, gentamicin protection assays were performed in the presence of the indicated concentrations of surfactant.
Microarray
RNA from P. aeruginosa strains was prepared with TRI Reagent (Sigma) followed by the RNeasy kit (Qiagen), following manufacturer's instructions. Microarray analysis was performed on a Pseudomonas aeruginosa PA01 gene chip using raw oligonucleotide probes generated from wild-type PA14, the motAB mutant, the motCD mutant, or the motABmotCD mutant. Each sample was analyzed in triplicate (N = 3), and summarized in one probe intensity by the Vermont Genetics Network Microarray Facility using Affymetrix GCOS software. Data analysis was performed using R [37] / BioConductor tools [38], [39]. Probe set sample matrix expression statistics were calculated using the Robust Multichip Average (RMA) method of Speed and coworkers [40], [41], implemented in the aroma.affymetrix package of Bengtsson [42]. Quality statistics were calculated using the Simpleaffy [43] and AffyQCReport packages [44]. The linear mixed effects model was fit using the lme4 package [45].
Microscopy
For static imaging, BMDCs were washed twice in 400 uL of serum-free HBSS prior to a 10 minute cytospin onto glass slides at 89.5 g. Alternatively, primary macrophages were allowed to adhere to glass slides for 1 hour at 37°C. Cells were co-incubated with GFP-expressing P. aeruginosa or GFP-expressing V. cholerae strains as indicated for 45 minutes at 37°C at a MOI∼10. Cells were stained with Alexa647-labeled wheat germ agglutinin (Molecular Probes) to delineate the cell surfaces. Cells were visualized via fluorescence microscopy on a Zeiss LSM510 Meta microscope using a 40X or 63X lens, followed by image analysis with LSM5 Image Browser software. For live cell imaging, GFP-expressing P. aeruginosa in phosphate buffer saline (PBS) were flowed over a monolayer of adherent macrophages at 50 mL/h for 20 min at an MOI∼10, followed by fresh media for 10 min. Bacterial accumulation was monitored at 5 sec intervals at 60X magnification using fluorescence and DIC. Imaging was performed using a Nikon TE2000 swept field confocal microscope with 0.17 mm Delta TPG dishes and analysis was performed with NIS-Elements viewing software.
Adherent macrophage assays
Macrophages were allowed to adhere to the bottom of 24 - or 48-well plates in serum-free HBSS for 60 min at 37°C. Adherent cells were washed twice in serum-free HBSS followed by co-incubation with P. aeruginosa strains at 4°C for 30 min. Non-associated bacteria were removed by washing with serum-free HBSS. Cell-associated bacteria were quantified by lysing cells in 0.1% Triton-X 100, plating lysate on LB media for >12 hours at 37°C, and counting recovered CFUs. Alternatively, following co-incubation at 4°C and removal of non-associated bacteria, cells and associated P. aeruginosa were warmed to 37°C for 30 min and quantified as above or warmed for 30 min, washed with serum-free HBSS, treated with 100 ug/mL gentamicin, and then quantified as above.
Bacterial viability assays
P. aeruginosa or V. cholerae cultures were grown to mid-log phase in LB broth and diluted to O.D. 600<0.1 in serum-free HBSS. To measure susceptibility to gentamicin, samples were treated with 100 ug/mL for 15 minutes at 37°C and then directly plated on LB agar or, alternatively, untreated samples were further diluted 1∶200 (P.a.) or 1∶555 (V.c.) and plated on LB agar and resultant CFUs were counted. To measure bacterial replication and death in HBSS, bacterial cultures were prepared as above and then plated directly, or incubated at 37°C for 60 min and quantified as above.
Supporting Information
Zdroje
1. AkiraSUematsuSTakeuchiO 2006 Pathogen recognition and innate immunity. Cell 124 783 801
2. LyczakJBCannonCLPierGB 2000 Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2 1051 1060
3. LyczakJBCannonCLPierGB 2002 Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15 194 222
4. AmielELovewellRRO'TooleGAHoganDABerwinB 2010 Pseudomonas aeruginosa evasion of phagocytosis is mediated by loss of swimming motility and is independent of flagellum expression. Infect Immun 78 2937 2945
5. BalloyVVermaAKuraviSSi-TaharMChignardM 2007 The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis 196 289 296
6. LuzarMAThomassenMJMontieTC 1985 Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun 50 577 582
7. MahenthiralingamECampbellMESpeertDP 1994 Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 62 596 605
8. MahenthiralingamESpeertDP 1995 Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun 63 4519 4523
9. ToutainCMZegansMEO'TooleGA 2005 Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J. Bacteriol 187 771 777
10. TerashimaHKojimaSHommaM 2008 Flagellar motility in bacteria structure and function of flagellar motor. Int Rev Cell Mol Biol 270 39 85
11. DeRosierDJ 1998 The turn of the screw: the bacterial flagellar motor. Cell 93 17 20
12. MartinezRMDharmasenaMNKirnTJTaylorRK 2009 Characterization of two outer membrane proteins, FlgO and FlgP, that influence vibrio cholerae motility. J Bacteriol 191 5669 5679
13. BraunTFPSGullyJBEmpeyJCVan WaySPutnamA 1999 Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli. J Bacteriol 181 3542 3551
14. DoyleTBHawkinsACMcCarterLL 2004 The complex flagellar torque generator of Pseudomonas aeruginosa. J Bacteriol 186 6341 6350
15. GosinkKKHaseCC 2000 Requirements for conversion of the Na(+)-driven flagellar motor of Vibrio cholerae to the H(+)-driven motor of Escherichia coli. J Bacteriol 182 4234 4240
16. PrittBO'BrienLWinnW 2007 Mucoid Pseudomonas in cystic fibrosis. Am J Clin Pathol 128 32 34
17. OhmanDEChakrabartyAM 1981 Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun 33 142 148
18. HulbertRRTaylorRK 2002 Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J Bacteriol 184 5533 5544
19. StonehouseEKovacikovaGTaylorRKSkorupskiK 2008 Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J Bacteriol 190 4736 4748
20. SunDXMekalanosJJTaylorRK 1990 Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J Infect Dis 161 1231 1236
21. SantosLRodriguesDLiraMOliveiraRReal OliveiraME 2007 The effect of octylglucoside and sodium cholate in Staphylococcus epidermidis and Pseudomonas aeruginosa adhesion to soft contact lenses. Optom Vis Sci 84 429 434
22. KandaETatsutaTSuzukiTTaguchiFNaitoK 2010 Two flagellar stators and their roles in motility and virulence in Pseudomonas syringae pv. tabaci 6605. Mol Genet Genomics
23. KojimaSYamamotoKKawagishiIHommaM 1999 The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J Bacteriol 181 1927 1930
24. AroraSKRitchingsBWAlmiraECLorySRamphalR 1998 The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun 66 1000 1007
25. AttridgeSRRowleyD 1983 The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis 147 864 872
26. GironJATorresAGFreerEKaperJB 2002 The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol 44 361 379
27. BirukovKGShirinskyVPStepanovaOVTkachukVAHahnAW 1995 Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol Cell Biochem 144 131 139
28. FingerEBPuriKDAlonRLawrenceMBvon AndrianUH 1996 Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 379 266 269
29. DrakeDMontieTC 1988 Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J Gen Microbiol 134 43 52
30. FreterRJonesGW 1976 Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun 14 246 256
31. PierGBMeluleniGGoldbergJB 1995 Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun 63 2818 2825
32. SiitonenANurminenM 1992 Bacterial motility is a colonization factor in experimental urinary tract infection. Infect Immun 60 3918 3920
33. InabaKInabaMDeguchiMHagiKYasumizuR 1993 Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A 90 3038 3042
34. BloembergGVO'TooleGALugtenbergBJJKolterR 1997 Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol 63 4543 4551
35. MacFerrinKDTerranovaMPSchreiberSLVerdineGL 1990 Overproduction and dissection of proteins by the expression-cassette polymerase chain reaction. Proc Natl Acad Sci U S A 87 1937 1941
36. DuncanMJLiGShinJSCarsonJLAbrahamSN 2004 Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem 279 18944 18951
37. R Development Core Team 2009 R: A Language and Environment for Statistical Computing. http://www.R-project.org
38. GentlemanR 2005 Bioinformatics and computational biology solutions using R and Bioconductor, in Statistics for biology and health. Vol. xix New york Springer Science+Business Media 473
39. GentlemanRCCareyVJBatesDMBolstadBDettlingM 2004 Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5 R80
40. BolstadBMIrizarryRAAstrandMSpeedTP 2003 A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 185 193
41. IrizarryRABolstadBMCollinFCopeLMBHobbs 2003 Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31 e15
42. BengtssonHSimpsonKBullardJHansenK 2008 aroma.affymetrix: A generic framework in R for analyzing small to very large Affymetrix data sets in bounded memory, in Technical Report #745, Department of Statistics, University of California, Berkeley
43. WilsonCLMillerCJ 2005 Simpleaffy: a BioConductor package for Affymetrix Quality Control and data analysis. Bioinformatics 21 3683 3685
44. ParmanCHallingCGentlemanR 2005 affyQCReport: QC Report Generation for affyBatch objects
45. BatesDMoechlerMDaiB 2008 lme4: Linear mixed-effects models using S4 classes
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



