-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
Generation of reactive oxygen species and reactive nitrogen species in phagocytes is an important innate immune response mechanism to eliminate microbial pathogens. It is known that deoxynucleotides (dNTPs), the precursor nucleotides to DNA synthesis, are one group of the significant targets for these oxidants and incorporation of oxidized dNTPs into genomic DNA may cause mutations and even cell death. Here we show that the mycobacterial dNTP pyrophosphohydrolase MazG safeguards the bacilli genome by degrading 5-OH-dCTP, thereby, preventing it from incorporation into DNA. Deletion of the (d)NTP pyrophosphohydrolase-encoding mazG in mycobacteria leads to a mutator phenotype both under oxidative stress and in the stationary phase of growth, resulting in increased CG to TA mutations. Biochemical analyses demonstrate that mycobacterial MazG can efficiently hydrolyze 5-OH-dCTP, an oxidized nucleotide that induces CG to TA mutation upon incorporation by polymerase. Moreover, chemical genetic analyses show that direct incorporation of 5-OH-dCTP into mazG-null mutant strain of Mycobacterium smegmatis (Msm) leads to a dose-dependent mutagenesis phenotype, indicating that 5-OH-dCTP is a natural substrate of mycobacterial MazG. Furthermore, deletion of mazG in Mycobacterium tuberculosis (Mtb) leads to reduced survival in activated macrophages and in the spleen of infected mice. This study not only characterizes the mycobacterial MazG as a novel pyrimidine-specific housecleaning enzyme that prevents CG to TA mutation by degrading 5-OH-dCTP but also reveals a genome-safeguarding mechanism for survival of Mtb in vivo.
Published in the journal: Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP. PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003814
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003814Summary
Generation of reactive oxygen species and reactive nitrogen species in phagocytes is an important innate immune response mechanism to eliminate microbial pathogens. It is known that deoxynucleotides (dNTPs), the precursor nucleotides to DNA synthesis, are one group of the significant targets for these oxidants and incorporation of oxidized dNTPs into genomic DNA may cause mutations and even cell death. Here we show that the mycobacterial dNTP pyrophosphohydrolase MazG safeguards the bacilli genome by degrading 5-OH-dCTP, thereby, preventing it from incorporation into DNA. Deletion of the (d)NTP pyrophosphohydrolase-encoding mazG in mycobacteria leads to a mutator phenotype both under oxidative stress and in the stationary phase of growth, resulting in increased CG to TA mutations. Biochemical analyses demonstrate that mycobacterial MazG can efficiently hydrolyze 5-OH-dCTP, an oxidized nucleotide that induces CG to TA mutation upon incorporation by polymerase. Moreover, chemical genetic analyses show that direct incorporation of 5-OH-dCTP into mazG-null mutant strain of Mycobacterium smegmatis (Msm) leads to a dose-dependent mutagenesis phenotype, indicating that 5-OH-dCTP is a natural substrate of mycobacterial MazG. Furthermore, deletion of mazG in Mycobacterium tuberculosis (Mtb) leads to reduced survival in activated macrophages and in the spleen of infected mice. This study not only characterizes the mycobacterial MazG as a novel pyrimidine-specific housecleaning enzyme that prevents CG to TA mutation by degrading 5-OH-dCTP but also reveals a genome-safeguarding mechanism for survival of Mtb in vivo.
Introduction
Oxidative damage to DNA and the DNA precursors, deoxynucleotides (dNTPs) is an inevitable mutagenic challenge occurring in normal aerobic metabolism, generating a large amount of reactive oxygen species (ROS) as by-products during respiration or oxidation-reduction reaction [1]-[3]. Oxidative DNA damage is also an important innate immune response mechanism implemented by phagocytes, which produce large amount of ROS and reactive nitrogen species (RNS) as a bactericidal strategy to eliminate microbial pathogens [4], [5]. Increasing evidence shows that the nucleotide pool is a significant target for oxidative modification via ROS and substantial portion of the oxidative damage to genomic DNA is caused by incorporation of oxidized dNTPs from the nucleotide pool [3], [6], [7]. Due to their ambiguous conformation (anti/syn) compared to that of the canonical dNTPs, incorporation of oxidized dNTPs into DNA is known to cause mispairing and mutation, and may be related to carcinogenesis, aging and neurodegeneration [6], [8]-[10]. Recent studies also established that incorporation of oxidized dNTPs into DNA is a major causative mechanism for bacterial cell death induced by bactericidal antibiotics [11], [12]. Therefore, like the DNA repair enzymes, elimination of the oxidatively damaged dNTPs from the nucleotide pool is an important defense line for cells to maintain genetic stability.
Cells have evolved a group of non-canonical nucleotide-specific bio-degradation enzymes, named housecleaning enzyme, to eliminate the oxidized non-canonical dNTPs from the nucleotide pool and thus prevent their incorporation into DNA [13], [14]. These proteins belong to four structural superfamilies: 1) dUTPase, 2) ITPase, 3) Nudix (nucleoside diphosphate linked to an X moiety, or MutT-like) hydrolase, and 4) all-α NTP pyrophosphohydrolase (MazG NTP-PPase) [14]. The dUTPase and ITPase are NTP phosphatases that target dUTP, an intermediate during dTTP synthesis, and ITP/XTP, the deamination products of purine nucleotides, respectively. MutT is the best-studied Nudix hydrolase specific for oxidatively damaged nucleotides [14], [15]. Escherichia coli MutT is the first characterized Nudix enzyme with 8-oxo-dGTP and 8-oxo-GTP as its natural substrates. Deletion of mutT in E. coli results in increased AT to CG mutation in both DNA and mRNA [8], [16]. MTH1, the MutT-like protein in humans, is active against 8-oxo-dGTP, 8-oxo-dATP and 2-OH-dATP [17]. Depletion of MTH1 in mice leads to a higher incidence of spontaneous tumorigenesis [18], while in human cells, MTH1 is involved in maintenance of genome stability and suppression of degenerative disorders such as neurodegeneration and carcinogenesis [6], [7], [19]. However, all the natural substrates for the MutT-like proteins that have been characterized in various organisms so far have been the oxidized purine nucleotides [15].
Oxidized pyrimidine nucleotides likely have a mutagenic effect similar to that of oxidized purine nucleotides. First, dCTP and dTTP can be oxidatively modified by ROS to form 5-OH-dCTP and 5-CHO-dUTP, respectively [20], [21]. Second, direct incorporation of 5-OH-dCTP or 5-CHO-dUTP into E. coli cells may cause an increase in mutation frequency, and both of these oxidized nucleotides may be mispaired with adenine rather than guanine leading to CG to TA mutation [10], [22]. Furthermore, 5-OH-dCTP is known to be incorporated into DNA more efficiently than 8-oxo-dGTP catalyzed by the exonuclease-free Klenow fragment [10]. Finally, it was found that the amount of 5-OH-dC in normal or oxidized cellular DNA is comparable to that of 8-oxo-dG [1], [23]. In addition to their role in mutagenesis, oxidized pyrimidine nucleotides also show a highly lethal effect on E. coli, indicating that these non-canonical nucleotides may disturb normal DNA replication and nucleotide metabolism [22]. Therefore, it is reasonable to conclude that cells have evolved housecleaning enzymes to eliminate oxidized pyrimidine nucleotides [10], [22]. However, although various enzymes responsible for the removal of oxidized pyrimidine in DNA molecules have been identified [24]–[26], the long-awaited housecleaning enzyme specific for elimination of oxidized pyrimidine nucleotides has yet to be characterized.
MazG-like proteins are widespread in all three domains of life and have been biochemically characterized as NTP-PPase while structurally categorized into the all-α NTP pyrophosphohydrolases superfamily unrelated to the MutT-like housecleaning enzymes [14], [27], [28]. It was found that E. coli MazG can regulate cellular (p)ppGpp levels and thus, may control programmed cell death under starvation conditions [29]. However, the mechanism whereby MazG regulates the cellular (p)ppGpp levels is still unclear. Structure-based modeling study of MazG from sulfolobus solfataricus suggested that 2-OH-dATP might be its most likely substrate and thus proposed, for the first time, a probable role of housecleaning for this enzyme [27]. Recently, it was reported that RS21-C6, a MazG-like enzyme in mice, showed a preference for degrading dCTP and its derivatives, with 5-I-dCTP as the most preferred substrate in vitro [30]. This attempt to identify a pyrimidine-specific housecleaning enzyme was echoed by structure-based analysis, which found that RS21-C6 binds to 5-methyl dCTP [31]. However, the natural substrate of the MazG family proteins remained unclear because none of the suggested functions involving regulation of pyrimidine synthesis [30], prevention of inappropriate DNA methylation [31], or antimutagenesis by degrading abnormal dCTP [27], [30] have ever been verified in vivo.
Although mycobacterial MazG has been characterized as a potent NTP pyrophosphohydrolase capable of hydrolyzing all canonical (d)NTPs in vitro, MazG can also hydrolyze dUTP and 8-oxo-dGTP, with their affinity to these substrates being similar to their affinity to the canonical dNTPs (Km∼1 mM). Therefore, it is unlikely that these non-canonical nucleotides are the natural substrate of the mycobacterial MazG in vivo [28]. In this study, we demonstrate that 5-OH-dCTP is a natural substrate of mycobacterial MazG by means of enzymatic and chemical genetic analyses. In addition to confirming the antimutator function of MazG, we show that deletion of mazG in the virulent Mtb strain H37Rv results in reduced survival in activated macrophage and mice. Our results reveal that mycobacterial MazG is a novel housecleaning enzyme involved in a pathway preventing the CG to TA mutation and ensuring the survival of Mtb in vivo.
Results
Mycobacterial mazG is an antimutator
Previously, we demonstrated that lack of the MazG NTP-PPase activity in Msm strain mc2 155 rendered the bacilli more susceptible to killing by hydrogen peroxide (H2O2) [28]. In order to test whether the oxidative stress resistant effect of the mycobacterial MazG is truly attributable to its potential housecleaning function in degrading certain oxidatively damaged dNTP(s), the spontaneous rifampicin-resistance mutation frequencies in wild-type and mazG-null (ΔmazG::hyg) Msm (bacterial strains used in this study are list in Table S1) were measured under different physiological conditions. We showed that the rifampicin-resistance mutation frequency in the mazG-null Msm increased 8.7-fold when treated with H2O2 (known to generate hydroxyl radicals which damage the dNTP pool [11], [32]), in contrast to merely 2.5 times increase in the wild-type Msm (Figure 1A). It was also found that, under the oxidative stress conditions, the expression level of recA and dnaE2, which is known to be elevated by damaged DNA (SOS response) [33], was 2-fold and 3-fold higher in the mazG-null Msm than that in the wild-type Msm, respectively (Figure S1). This suggests that, under oxidative stress, the mazG-null Msm suffers more genetic assaults than does the wild type. On the other hand, during the exponential phase of growth, the rifampicin-resistance mutation frequency of mazG-null Msm is comparable to that of the wild-type Msm (Figure 1A).
Fig. 1. The antimutator role of MazG in Msm (A) and Mtb (B). 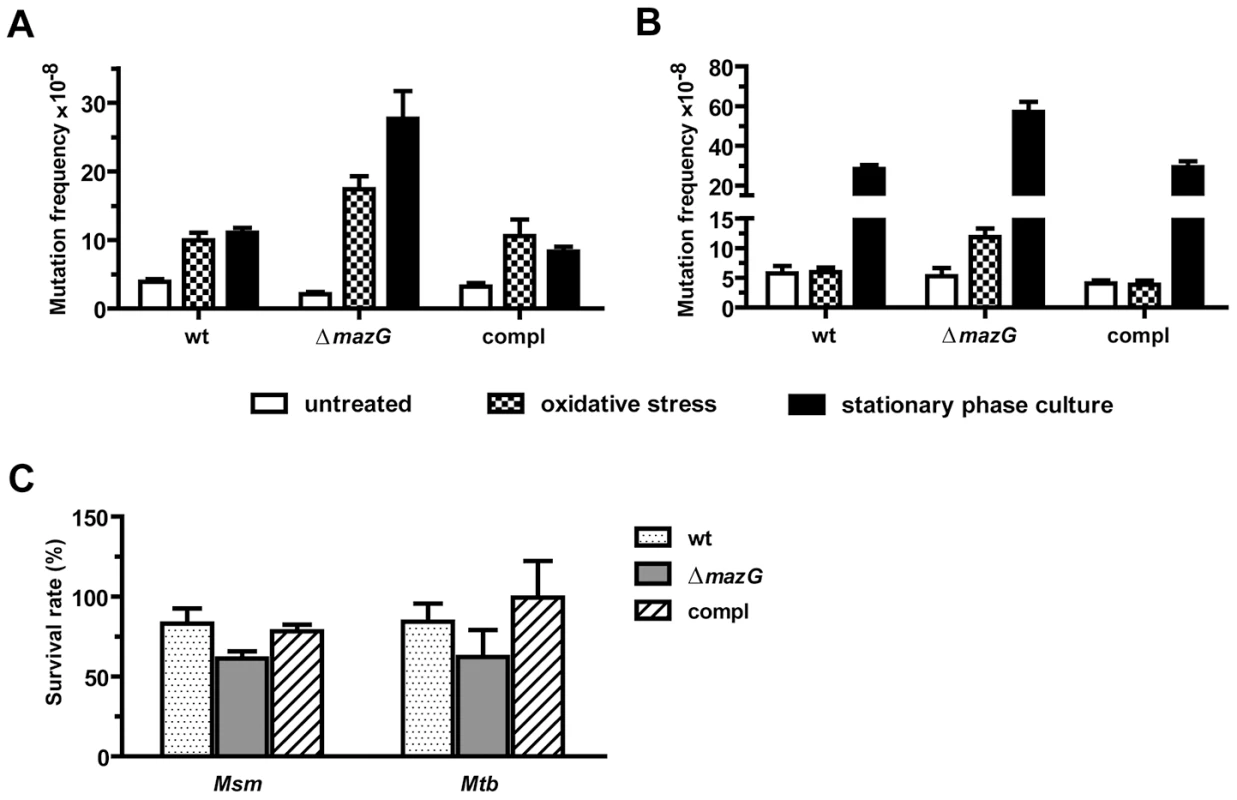
Both the bacterial culture conditions and the methods for determination of mutation frequencies were illustrated in Materials and Methods in detail. The frequencies conferring resistance to rifampicin in wild-type (wt), mazG-null (ΔmazG) and the complemented mutant (compl) strains were determined in exponential phase (OD600∼0.5) with or without oxidative stress and in the stationary growth phase. Oxidative stress was induced by treating exponential phase cultures with 10 mM H2O2 for 5 h (Msm) or 24 h (Mtb). Stationary phase was at the 5th-day or 28th-day of culture for Msm or Mtb, respectively. (C) Survival rate of Msm and Mtb strains after exposure to H2O2. The numbers shown are mean ± S.E. of 3 independent experiments totaling 15 cultures of Msm and 6 of Mtb. We also measured the rifampicin-resistance mutation frequency in the stationary phase of growth, a stage known to accumulate metabolic byproducts and mutations [34]. It was found that the rifampicin-resistance mutation frequency in 5-day-old mazG-null Msm cultures was 2.5 times greater than that in wild type (Figure 1A), suggesting a mutator phenotype of mazG-null Msm during the stationary phase of growth. A similar result was observed with 8-day-old cultures (data not shown), indicating prolonged incubation during the stationary phase does not further increase the mutation frequency in mazG-null Msm. To test whether mazG plays the same function in Mtb, we constructed a ΔmazG::hyg null mutation in the virulent Mtb strain H37Rv by means of allelic exchange and the deletion of this gene was confirmed by Southern blot (Figure S2). The mazG-null Mtb exhibited the same mutator phenotype as that of the mazG-null Msm (Figure 1B), showing a 2.5-fold increase in rifampicin-resistance mutation frequency compared to that of the wild type under oxidative stress or the stationary phase of growth.
In order to test the cytotoxic effects of H2O2 upon the bacteria studied, we measured the survival rates of the mazG-null strains versus the wild-type strains of Msm and Mtb under the same H2O2 treatment conditions as that for mutation analysis. The survival rate of the mazG-null Msm decreased only slightly after 5 hours of H2O2 treatment compared to that of the wild type, while in the mazG-null Mtb, no significant effect was observed (Figure 1C). These data suggest that other than the change of mutation frequencies, H2O2 treatment in this study did not induce other major physiological change affecting the survival of the bacilli. Because the wild-type mazG gene complements all of the defective phenotypes of the mazG-null mutants (Figure 1), the antimutator role of mycobacterial MazG is genetically established.
Mycobacterial MazG prevents CG to TA mutation
It has been shown that incorporation of different oxidized dNTPs into DNA preferentially induces a specific spectrum of mutation, e.g., 8-oxo-dGTP leads to AT to CG mutation [35], [36] while 5-OH-dCTP induces GC to AT mutation [9], [10], [22]. Therefore, we compared the mutation spectra between the mazG-null Msm and its parental strains to infer the probable substrate of mycobacterial MazG.
We sequenced the cluster I region of the rpoB gene [37] from randomly isolated rifampicin-resistant colonies. All of the sequences contained single nonsynonymous nucleotide variations. Of these, >99% were located within the cluster I region (the remaining mutations occurred outside of the cluster I region) and caused mutations in the well characterized rifampicin-resistance mutation hot spots (Table S2 and S3). Of the mutations detected, the frequency of CG to TA mutation exhibited a significant difference between the wild type and the mazG-null mutant (Table 1). Among the rifampicin-resistant mutants derived from the exponential-phase cells, the CG to TA mutation frequency in wild-type Msm increased from 2.2×10−8 in the untreated samples to 6.8×10−8 in the H2O2 treated cultures (∼3-fold increase), while in the mazG-null Msm, the frequency of this type of mutations increased significantly from 0.8×10−8 to 14.3×10−8 (∼18-fold increase). Meanwhile, the rifampicin-resistant mutants of the wild-type Msm exhibited a CG to TA mutation frequency in the stationary-phase cells similar to that in the exponential-phase cells (1.9×10−8 and 2.2×10−8, respectively), suggesting that the CG to TA mutation rate is likely constant during replication in the wild-type Msm. However, the CG to TA mutation in the mazG-null Msm increased 26-fold, from 0.8×10−8 in the exponential-phase cells to 20.5×10−8 in the stationary-phase cells (Table 1). These results clearly suggest that mycobacterial MazG is involved in safeguarding genomic DNA by preventing CG to TA mutation under adverse growth conditions.
Tab. 1. mazG-null Msm exhibited elevated CG to TA mutation under oxidative stress conditions and in the stationary phase of growth. 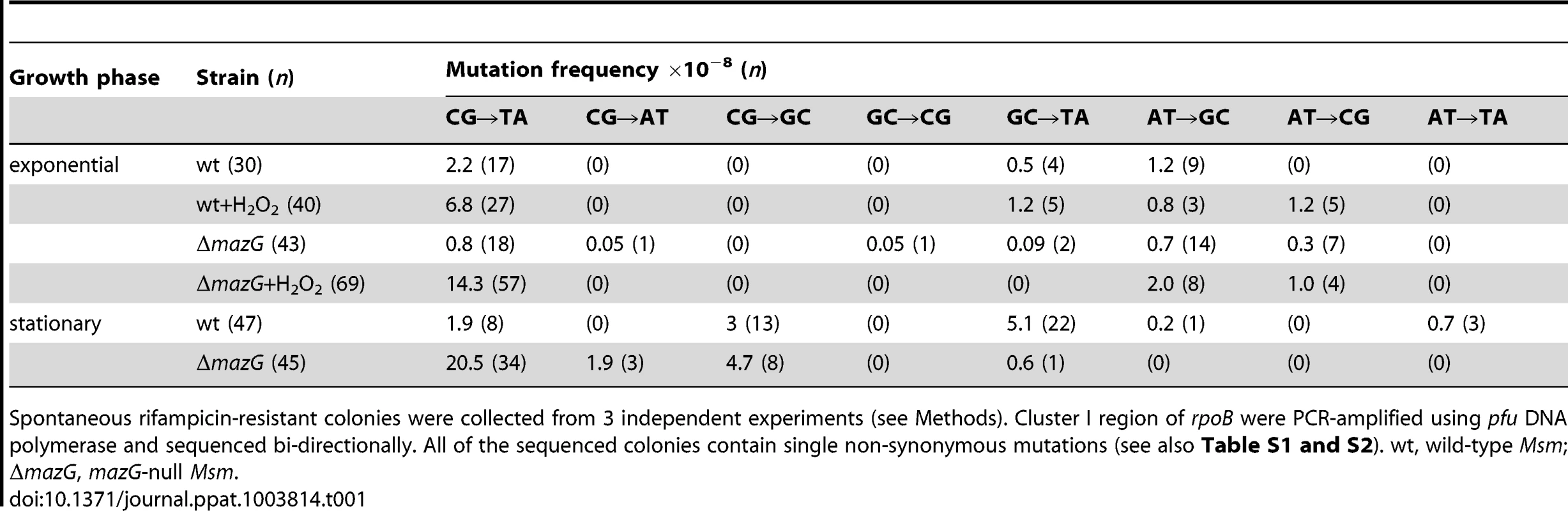
Spontaneous rifampicin-resistant colonies were collected from 3 independent experiments (see Methods). Cluster I region of rpoB were PCR-amplified using pfu DNA polymerase and sequenced bi-directionally. All of the sequenced colonies contain single non-synonymous mutations (see also Table S1 and S2). wt, wild-type Msm; ΔmazG, mazG-null Msm. 5-OH-dCTP is a preferred substrate for mycobacterial MazG
CG to TA transition, the most common base substitution occurring in aerobic organisms [38], [39], can be induced by incorporation of oxidatively damaged nucleotides into DNA, especially oxidized pyrimidine nucleotides [9], [10], [22]. We measured the MazG NTP-PPase activity towards 5-OH-dCTP, 5-CHO-dUTP and 2-OH-dATP, all of which are known to induce CG to TA mutation when incorporated into DNA [9], [22], [40].
Each substrate was mixed with mycobacterial MazG proteins of different origin and was incubated at 37°C for 10 minutes. The hydrolyzed product, pyrophosphate, was quantified by an enzyme coupled colorimetric method [28]. It was found that all of these substrates were hydrolyzed into monophosphate derivatives and pyrophosphate in a time - and enzyme concentration-dependent manner (Figure 2A). Of the nucleotides examined, 5-OH-dCTP and 2-OH-dATP were the most preferred substrates for the Mtb MazG, with Km values of 1.9 and 2.4 µM, respectively, approximately 26 times lower than that of their canonical nucleotides (Table 2 and Figure 2B). It appears that 5-CHO-dUTP is unlikely to be the natural substrate of Mtb MazG, shown by its extremely high Km value (∼500 µΜ, Table 2). The Msm MazG exhibited similar kinetic constants compared to its Mtb counterpart, except for 2-OH-dATP, which showed a Km of 311 µΜ (Table S4), much higher than that of the Mtb MazG (Table 2). Based on the kinetic constants and the same antimutator role of MazG in Msm and Mtb, we conclude that 5-OH-dCTP is the most likely natural substrate of mycobacterial MazG.
Fig. 2. The NTP-PPase activity of mycobacterial MazG against 5-OH-dCTP. 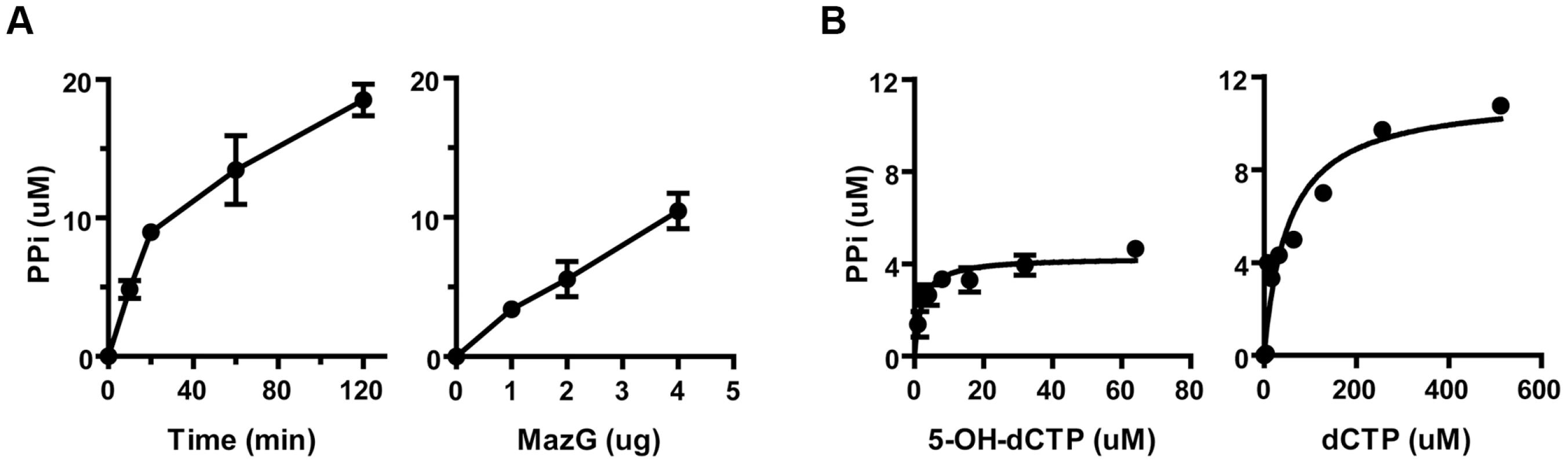
(A) Time- and enzyme concentration-dependent hydrolysis of 5-OH-dCTP. 5-OH-dCTP (200 µM) was incubated with 1 µg or varied amounts (from 1 µg to 4 µg) of heterogeneously expressed MazG purified to nearly SDS-PAGE homogeneity. The reaction was carried out at 37°C and terminated after 10 min or at the time points as indicated. PPi, pyrophosphate. Shown are mean ± S.E. of 3 repeats. (B) Michaelis-Menten curves of MazG with 5-OH-dCTP or dCTP as substrate. The hydrolytic product PPi is shown as µM/10 min. Data shown are mean ± S.E. of 3 independent experiments. Tab. 2. Kinetic constants of <i>Mtb</i> MazG. 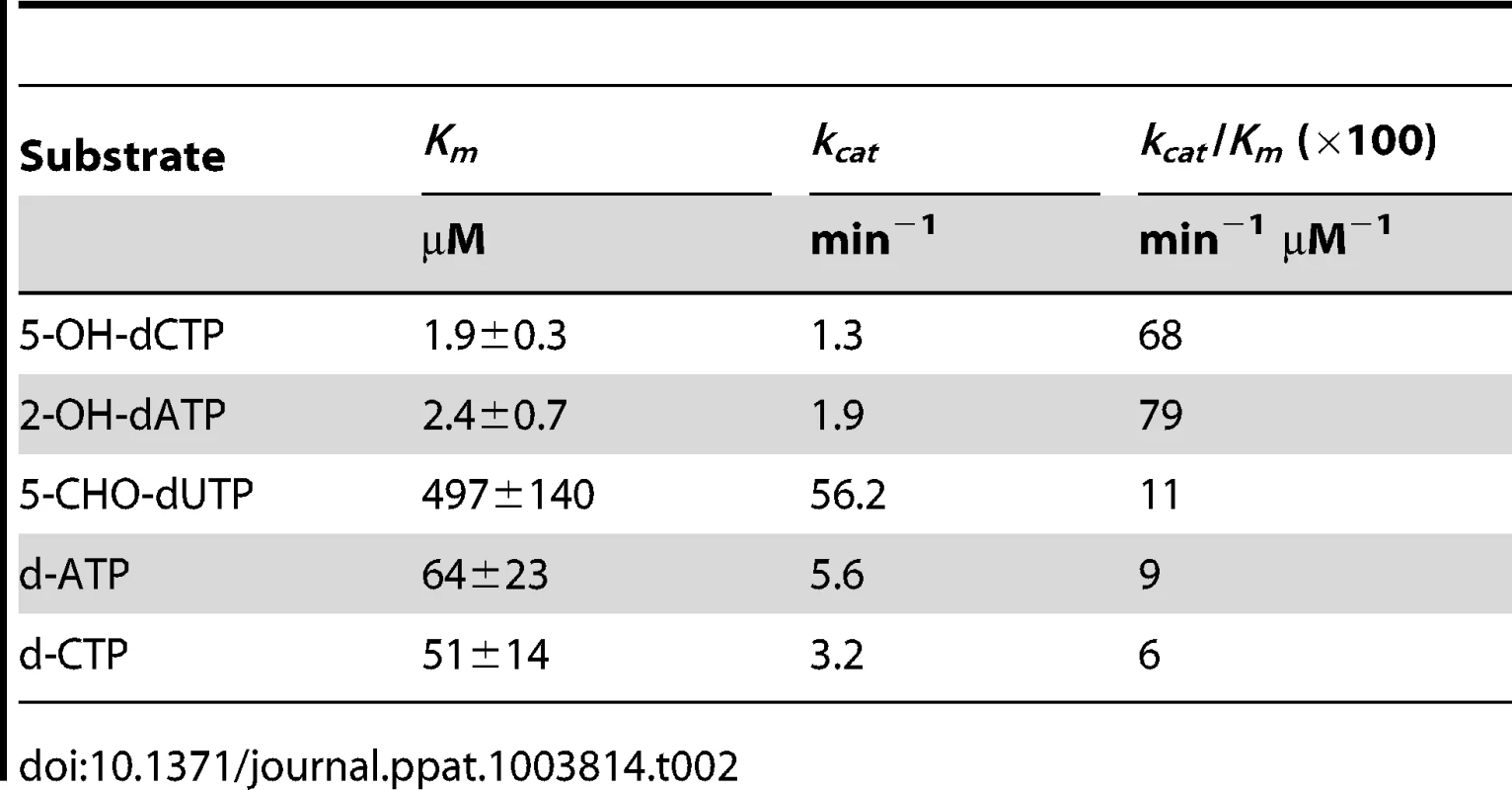
5-OH-dCTP is an in vivo substrate of mycobacterial MazG
To further characterize the natural substrate of mycobacterial MazG under cellular physiological conditions, we compared the in vivo mutagenic effects of these oxidized nucleotides in wild-type and mazG-null Msm strains using an established in vivo incorporation assay [22], [36].
Of the nucleotides tested, only 5-OH-dCTP exhibited a mutagenic effect upon the mazG-null Msm in a dose dependent manner (Figure 3A–C). When treated with 100 µM 5-OH-dCTP, the mazG-null mutant showed a ∼2 fold increase (P<0.01) in rifampicin-resistance mutation frequency compared to that of the wild-type Msm (Figure 3B). The increased mutation frequency of the mazG-null mutant can be restored to normal by complementation with a single copy of the wild-type mazG from either Msm or Mtb, indicating that MazG plays the same role in these two mycobacteria species (Figure 3B). Therefore, the antimutator role of mycobacterial MazG, particularly related to 5-OH-dCTP induced mutagenesis, is inferred. Furthermore, expression of the loss-of-function A219E MazG variant [28], [41] in mazG-null Msm failed to restore the mutator phenotype (Figure 3B), implying that the in vivo antimutator role of MazG requires NTP-PPase activity. We also found that the mazG-null Msm was susceptible to killing by 5-OH-dCTP treatment (Figure 3D), suggesting that mycobacterial MazG was involved in the defense against both the cytotoxic and the mutagenic effects of 5-OH-dCTP. Based on these biochemical and chemical genetic results, we conclude that 5-OH-dCTP is one of the in vivo substrates of mycobacterial MazG. However, we cannot exclude the possibility that other substrates may also exist.
Fig. 3. 5-OH-dCTP is an in vivo substrate of mycobacterial MazG proved by chemical genetic analysis. 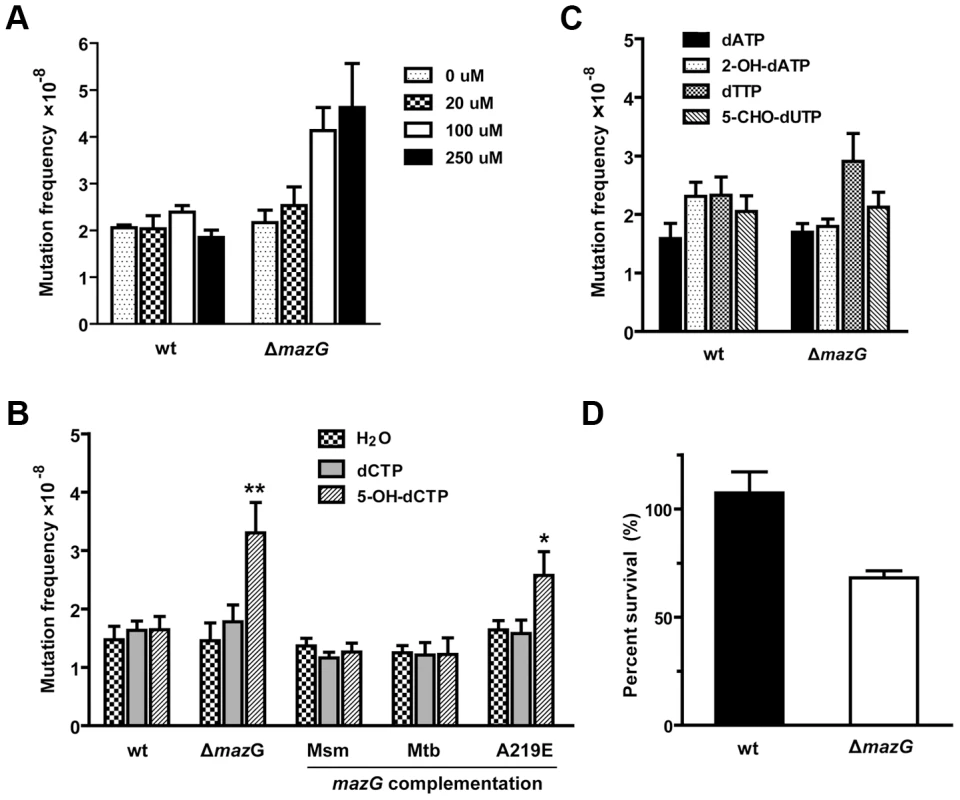
The Msm competent cells were prepared as described in Materials and Methods, the nucleotides were incorporated by transformation. (A) mazG-null Msm (ΔmazG) exhibited a dose-dependent 5-OH-dCTP induced mutagenesis. wt, wild-type Msm. The data shown are mean ± S.E. of four repeats. (B) The rifampicin-resistance mutation frequencies of wild-type Msm, the mazG-null mutant and the complemented mutant treated with 5-OH-dCTP and dCTP. Nucleotides were added at 100 µM final concentration. Mean ± S.E of 12 independent transformations. **p<0.01 vs wt, * p<0.05 vs wt. (C) The rifampicin-resistance mutation frequencies of wild-type and mazG-null Msm treated with 100 µM 2-OH-dATP, 5-CHO-dUTP and normal dNTPs. Mean ± S.E of 8 independent transformations. (D) mazG-null Msm is susceptible to killing by 5-OH-dCTP. Strains were treated with 100 µM 5-OH-dCTP for 5 hours at 37°C. Shown is percent survival compared to an untreated control (100%). The data shown are mean ± S.E. of four repeats. The mazG-null Mtb is hypersensitive to RNS
During intracellular infection, Mtb is exposed to genetic assaults elicited by both ROS and RNS produced by host macrophages [5], [42]. We tested whether the MazG housecleaning function is involved in Mtb resistance to ROS and RNS. The mazG-null Mtb was found to be more susceptible to killing by acidified nitrite treatment in vitro than the wild-type Mtb, showing a 0.8-log10 lower CFU. The reduced survival ability of the mazG mutant can be fully restored by expression of a single copy of the wild-type mazG in the mutant (Figure 4A). Accordingly, transcription of the mazG gene in the wild-type Mtb was upregulated 3∼5 fold by the treatment of acid nitrite or DETA/NO (2,2-(hydroxynitrosohydrazino)-bisethanamine), which liberates nitric oxide (Figure 4B), indicating that mazG is involved in the genetic response to RNS. However, unlike the mazG-null Msm [28], the mazG-null Mtb was just as susceptible to H2O2 as the wild type (Figure 1C), a property shared by other Mtb mutants which are more sensitive to RNS in vitro [43], [44].
Fig. 4. Mtb MazG is required for resistance to RNS shown in vitro and ex vivo. 
(A) mazG-null Mtb is susceptible to killing by acid nitrite. Exponential phase cultures (OD600∼0.5) were suspended in 7H9-OADC pH4.5 with or without 2.5 mM NaNO2 and treated for 20 hours. wt, wild-type Mtb; ΔmazG, mazG-null Mtb; compl, the complemented mutant. Data shown are mean ± S.E. of triplicates. * p<0.05 vs wt. (B) Quantitative real-time PCR analyses of mazG and dosR from Mtb treated with DETA/NO or acid nitrite. All data were normalized to the levels of sigA. The dosR gene was used as a positive control [70]. Results are expressed as the changes in expression levels compared to untreated samples. Mean ± S.E of three independent repeats. C to E, Survival of wild-type Mtb, the mazG-null mutant and the complemented mutant in resting (C), activated (D) or NMMA treated (E) cells of murine macrophage cell line RAW264.7. Shown is one representative result of two independent experiments. We further compared the intracellular survival ability between wild-type Mtb and the mazG-null mutant. No difference was observed between the growth of these two strains in resting macrophages (Figure 4C). However, when infected with activated macrophages, the titer of the mazG-null Mtb declined from 2 days post-infection and onward (Figure 4D), showing 1 log10 lower CFU than that of wild-type Mtb by 5 days post-infection. This suggests that MazG is required for Mtb resistance to intracellular RNS. Consistent with this finding, the attenuated survival of mazG-null Mtb in activated macrophages was partially rescued by addition of NMMA (NG-Methyl-L-arginine acetate salt), a specific inhibitor for macrophage inducible NO synthase (Figure 4E) [45]. Introduction of a wild-type mazG into the mutant strain restored the attenuated phenotype of the mazG-null Mtb (Figure 4D–E).
MazG is required for Mtb survival in vivo
Our results demonstrate that Mtb MazG is required for maintenance of genetic stability and resistance to RNS both in vitro and ex vivo, indicating that MazG may function as a virulence factor during Mtb infection. To investigate whether mazG is involved in survival of Mtb in vivo, immune-competent mice were infected by a low-dose aerosol challenge with Mtb strains. Compared to wild-type Mtb, the mazG-null Mtb exhibited 1.1-log10 lower CFU in mice spleens by 4 weeks post-infection, and 0.7-log10 lower CFU by 8 and 12 weeks post-infection, indicating an attenuation at the stage of persistent infection (Figure 5A). No significant difference between the growth of wild-type and the mazG-null Mtb strains was observed in mice lung (Figure 5B). However, histological stained sections of the infected lung tissue (8 weeks after infection) showed that the mazG-null Mtb caused minimal pneumonitis, with a 3-fold reduction in lung inflammation compared to wild-type Mtb (Figure 5C). The attenuated phenotype of the mazG-null mutant can be fully restored by complementing the mutant with a single copy of the wild-type mazG (Figure 5). Taken together, these results suggest that the housecleaning role of MazG is required for Mtb survival and pathogenesis in vivo.
Fig. 5. MazG is required for Mtb survival in vivo and the corresponding lung pathogenesis. 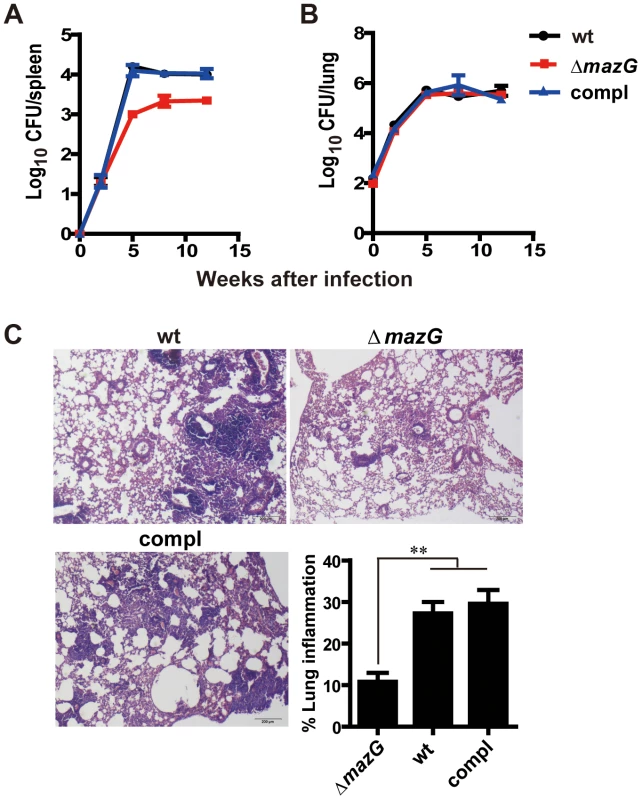
A to B, Bacterial loads in spleen (A) and lung (B) of mice infected with wild-type Mtb (wt), the mazG-null mutant (ΔmazG) and the complemented mutant (compl). Data shown are mean ± S.E from 4 mice per group. (C) Lung sections taken from mice at 8-wk after infection and stained with hematoxylin and eosin. Inserted column shows mean ± S.E of lung inflammation of each group. Discussion
Growing evidence suggests that elimination of oxidized nucleotides from the cellular dNTP pool is an important safeguarding mechanism for maintenance of genetic stability [2], [3], [6], [7]. However, most of the knowledge about this housecleaning role has focused on oxidized purine nucleotides and the MutT-like NTP-PPase. Due to lack of knowledge of pyrimidine specific housecleaning NTP-PPase, the contribution of oxidized pyrimidine nucleotides to DNA mutagenesis and its related mechanism remains unclear. Here we characterized a NTP-PPase that specifically degrades 5-OH-dCTP in vivo and prevents CG to TA mutation. To our knowledge, MazG is the first oxidized pyrimidine-specific housecleaning enzyme to which an antimutator function can be assigned.
Our previous study suggested that 8-oxo-dGTP and dUTP are unlikely to be the natural substrates of mycobacterial MazG, as the Km values for these nucleotides are substantially high [28]. In this study, based on the observation that deletion of mazG leads to the increase of CG to TA mutation frequency, we considered 5-OH-dCTP, 5-CHO-dUTP and 2-OH-dATP as putative substrates of MazG, and all of these oxidized nucleotides are known to induce CG to TA mutations upon incorporated into DNA by polymerase [9], [22], [40]. Among the substrates tested, mycobacterial MazG showed high affinity towards 5-OH-dCTP (Km = 1.9 µM) and 2-OH-dATP (Km = 2.4 µΜ), values comparable to that of E. coli MutT towards its natural substrate 8-oxo-dGTP (Km∼0.5 µM) [8]. Through direct incorporation of these oxidized nucleotides into Msm cells, we observed a dose-dependent 5-OH-dCTP-specific mutagenic effect in mazG-null Msm, indicating 5-OH-dCTP is an in vivo substrate of mycobacterial MazG (Figure 3). Moreover, we also found that the mycobacterial MazG was involved in the defense against the cytotoxic effect of 5-OH-dCTP (Figure 3D). This cytotoxic effect is likely to be caused by lethal DNA strand breaks or replication block [11], [19] induced by incorporation of 5-OH-dCTP into DNA. Taken together, these data demonstrate that the mycobacterial MazG is a 5-OH-dCTP-specific housecleaning enzyme involved in preventing CG to TA mutation.
In mazG-null Msm with H2O2 treatment, we did not observe an increase of GC-TA mutations that should be induced by incorporation of 2-OH-dATP [46]. On the other hand, MazG does exhibit a high affinity to 2-OH-dATP, similar to its affinity to 5-OH-dCTP (Table 2). It is still unclear whether MazG is the only 2-OH-dATPase existing in mycobacteria. Therefore, whether 2-OH-dATP is a natural substrate of mycobacterial MazG remains unclear. It is worth noting that Mtb encodes four MutT proteins (MutT1 to 4) [47]. A recent study showed that the MutT1 carried out the physiological role of MutT (8-oxo-dGTPase) in Mtb [48], while the MutT2 did not function as an 8-oxo-dGTPase [49]. Therefore, to date, the natural substrate of MutT2-4 is still unidentified.
CG to TA transition can be induced by oxidative deamination of cytosines on DNA [50], misincorporation of oxidized pyrimidine nucleotides into DNA by DNA polymerase [9], [10], [22] and mismatch induced by keto-enol transitions of guanine [51]. Based on the biochemical and chemical genetic results described above, and the fact that MazG is unlikely to perform a DNA repair role, as this protein family does not contain any DNA binding/repair signatures [27], [28], we conclude that the increased CG to TA mutation in mazG-null Msm is mainly due to incorporation of oxidized nucleotide 5-OH-dCTP.
Deletion of mazG in mycobacteria did not lead to a mutator phenotype under the exponential phase of growth. However, lack of MazG activity in mycobacteria resulted in higher CG to TA mutation under both oxidative stress and the stationary growth phase, compared to that of the parental strains (Table 1). The likely mechanism underlying this stress-related mutagenesis is that under stress conditions, mycobacterial cells may accumulate 5-OH-dCTP and lacking MazG, more 5-OH-dCTP is misincorporated into DNA. Moreover, down-regulated DNA repair activity under these stress conditions [34], [52] may also contribute to the stress-related mutagenesis observed in the mazG-null strains. Nonetheless, although the related molecular mechanism of 5-OH-dCTP induced stress-related mutagenesis remains to be determined, as the host environment for Mtb parasitism is always adverse, 5-OH-dCTP induced mutagenesis may be hypothesized to play an important role in the microevolution process of the infected Mtb under stress conditions, i.e. emergence of drug resistant mutations during bacterial infection. In this connection, it is worth noting that CG to TA transition is a dominant mutation in Mtb isolated from either macaques with latent/reactivated infection or humans [53], [54].
It is still unclear why the lack of the 5-OH-dCTP sanitization function in Mtb results in hypersusceptibility to RNS (Figure 4). RNS is a group of radicals derived from nitric oxide (NO•) which are produced by macrophage as antimicrobial effector molecules [5]. An important antimicrobial action of RNS is inhibition of DNA replication and repair. It was found that NO• can inhibit DNA synthesis by zinc mobilization from DNA-binding metalloproteins [55]. RNS can also inhibit ribonucleotide reductase [56], and thus, limit the availability of precursors for the synthesis and repair of DNA. Based on the fact that lack of MazG activity leads to increased incorporation of 5-OH-dCTP into DNA (Table 1), a possible explanation for the hypersusceptibility of mazG-null Mtb to RNS is that inhibition of DNA repair activities and lack of DNA precursors caused by RNS mediated enzyme inactivation result in higher levels of genetic instability (such as DNA strand breakage) in mazG-null Mtb than that in the wild-type Mtb.
During infection, Mtb is exposed to an oxidative environment of host macrophages rich in DNA-damaging ROS and RNS. Therefore, safeguarding of the genetic information is essential for mycobacterial survival, especially during the non-replicating dormancy stage, as slow or non-replicated genomic DNA and diminished DNA repair activities are likely lead to more genetic assaults than that during fast growing phase [52], [57]. Our results demonstrated that deletion of mazG leads to attenuated survival of Mtb in mice spleen during the persistent infection phase, suggesting that oxidative damage to nucleotides and the subsequent genetic assault is one of the bactericidal effects of the adaptive immune response (corresponding to the bacterial persistent infection stage). This is consistent with the data indicating that genes involved in removal of oxidized pyrimidines are essential for Mtb survival during primates' infection [58]. Although the difference between the lung and spleen microenvironments exposed to Mtb is unclear, it is conceivable that the immune responses and metabolic constraints are different between the two tissues. Interestingly, tissue specific attenuation have been demonstrated for several Mtb mutants, including the dosR, fadD26, mptpB and narG mutants [59]–[62].
Recent studies have proven that bactericidal antibiotics-induced ROS production within bacterial cells is a common mechanism for cell death [12], [63]–[65], predominantly elicited by incorporation of 8-oxo-dGTP into DNA [11]. Therefore, it is not surprising that 5-OH-dCTP and other oxidized nucleotides have a similar bactericidal effect, as shown in our results (Figure 3D and Figure 5A). These findings suggest that clinical treatment of tuberculosis with specific inhibitors of housecleaning enzymes might facilitate Mtb elimination, especially when combined with bactericidal antibiotics which are known to induce oxidative stress.
Materials and Methods
Ethics statement
Six-to-eight week old female C57BL/6 mice were purchased from the Shanghai SLAC Laboratory Animal Company. The mice were housed and cared for in a specific pathogen-free (SPF) biosafety level 3 facility at Shanghai Public Health Clinical Center. Mice were provided food and water ad libitum as well as appropriate monitoring and clinical care. Animal experiments were carried out in strict accordance with the regulations in the Guidance Suggestions for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People's Republic of China. The protocol was approved by the Chinese Science Academy Committee on Care and Use of Laboratory Animals and the Laboratory Animal Ethical Board of Shanghai Public Health Clinical Center (Permit Number: 2012A002).
Bacterial strains and culture conditions
Bacterial strains used in this study are list in Table S1. Bacterial culturing was performed as described [66]. Msm strains were grown at 37°C in 7H9 broth (BD Difco), or on Luria-Bertani agar supplemented with 0.5% glycerol (LBG agar). Mtb strains were grown at 37°C in 7H9 broth supplemented with 10% OADC (7H9-OADC), or on 7H11 plates supplemented with 10% OADC (7H11-OADC). When required, the following antibiotics were used at the specified concentrations: kanamycin (15 µg/ml), hygromycin B (150 µg/ml for Msm and 50 µg/ml for Mtb) and rifampicin (250 µg/ml for Msm and 10 µg/ml for Mtb). For treatment with acid NO, Mtb stains grown to OD600∼0.5 were pelleted and re-suspended in 7H9-OADC pH 4.5 (adjusted by 1 M citrate) with or without 2.5 mM NaNO2 [45]. After 20 h treatment, bacteria were plated on 7H11-OADC, CFUs were counted after 3∼4 weeks culture at 37°C
Generation of mazG mutants and complemented strains
The mazG-null mutant was generated by the phage transduction method [66]. mazG-null Msm and the complemented strains were generated as described [28]. To construct a transducing phage for Mtb mazG knockout, the left homologue arm was PCR amplified using primers KOP1 and KOP2 (primers used in this study are listed in Table S5). The right homologue arm was PCR amplified using primers KOP3 and KOP4. The PCR products were ligated into the AflII/XbaI and HindIII/XhoI sites of pYUB854. The recombinant transducing phage was used to construct the mazG-null Mtb as described [28]. The mazG-null mutant was verified by southern blot and PCR (Figure S2). The probe for Southern blot was PCR amplified using primers SB1and SB2. A dUTP-biotin labeled probe (Fermentas) was used for Southern blot analysis of the PstI/KpnI digested chromosomal DNA on the Hybond-N+ nylon membrane (GE Amersham), according to the standard method [67]. Primers used for genotyping PCR were P1, P2 and P3. The complementation plasmid for mazG-null Mtb was generated by ligating the PCR product amplified using primers C1 and C2 into the BamHI and HindIII sites of pMV306. Expression of Mtb mazG was controlled by its own promoter (1142671–1143646).
Determination of rifampicin-resistance mutation frequency
Single colonies of various Msm strains from the 7H11 agar plate were inoculated in 5 ml media and cultured at 37°C for 48 h (2 weeks for Mtb strains). For determination of rifampicin-resistance mutation frequency, the cultures were inoculated with 1% of primary culture in 20 ml 7H9 media (in a 100-ml flask) without antibiotics and grown at 37°C with rolling (150 rpm) to exponential phase (OD600∼0.5). Then 10 ml of the cultures were treated with 10 mM H2O2, and another 10 ml cultures were untreated. After incubation at 37°C, 150 rpm, for 5 h, CFU per ml was determined by plating; the cell pellet from 3 ml culture was plated on LBG agar (3 plates of each sample) containing 250 µg/ml rifampicin (Sigma-Aldrich). The CFU and rifampicin-resistant colonies were counted after culturing at 37°C for 4 days (28 days for Mtb strains). The rifampicin-resistance mutant frequency was calculated by dividing the number of rifampicin-resistant colonies on each plate by the counts of the total viable cells plated. Rifampicin-resistance mutation frequencies of Mtb strains were determined by the same method, except that the oxidative stress was elicited by resuspending the exponential-phase cell pellet in 7H9 media containing 10 mM H2O2, followed with incubating at 37°C for 24 h. Mtb strains were plated on 7H11-OADC with or without 10 µg/ml rifampicin. For determination of rifampicin-resistance mutation frequency of the stationary phase cultures, cells were cultured in liquid media for 5 days (for Msm) or 28 days (for Mtb) and plated as described above. Three independent experiments were performed with totaling 15 cultures of each Msm strains and 6 of Mtb.
Analysis of mutation spectra
Rifampicin-resistant colonies were collected from three independent experiments. The isolated colonies were grown in 1 ml 7H9 at 37°C for 1 week. Cells were pelleted and suspended in 50 µl TE buffer (10 mM Tris–HCl, pH 8.0, and 1 mM EDTA) and incubated at 95°C for 10 min to extract the genome DNA [37]. The lysate was centrifuged at 12000 g for 5 min. The supernatant was used as template to amplify (using pfu DNA polymerase) the fragment containing the cluster I region of rpoB using primers Rpo1 and Rpo2. All PCR products were sequenced by bi-directionally. Mutation spectra of the sequenced region were analyzed by BioEdit software.
NTP-PPase assay
Protein expression and purification was performed as described [28]. Protein was purified to nearly SDS-PAGE homogeneity. Protein concentration was determined by the bicinchoninic acid (BCA) method [68]. The oxidized nucleotides used as substrates for MazG were purchased from TriLink Biotechnologies Inc. (5-OH-dCTP) or Hongene Biotechnologies Inc. (2-OH-dATP and 5-CHO-dUTP). The NTP-PPase activity of MazG was assayed as described [28]. The NTP-PPase assay was carried out in 20 µl reaction buffer (20 mM Tris-HCl, pH 7.5, 5 mM MgSO4, 100 mM NaCl) containing 1 µg mycobacterial MazG and substrate nucleoside triphosphates at 37°C for 10∼20 min. The reaction was stopped by heating at 65°C for 5 min, and 10-20 µl products were applied for pyrophosphate assay (Molecular Probes) according to the manufacturer's instructions. Reactions with heat inactivated (95°C for 20 min) MazG protein were set up as a background controls. GraphPad Prism 5.0 (GraphPad Software, Inc.) was used for enzyme kinetic constants analysis.
Incorporation of nucleotides into Msm competent cells
The Msm competent cells were prepared from 400-ml cultures (OD600 = 0.8∼1.0) as previously described [69]. Incorporation of nucleotides into Msm competent cells was performed as described [22], [36]. Briefly, Nucleotide solution (100 µM final concentration) was added to 150 µl competent cells suspension and the mixture was placed on ice for 10 min. After heat shock treatment (42°C for 90 sec and then on ice for 30 min), 2 ml 7H9 was added and the cells were incubated at 37°C with rolling (150 rpm) for 5 h. After treatment, 2 ml of culture was centrifuged at 4000 g for 5 min and plated on LBG agar containing 250 µg/ml rifampicin. The remaining culture was diluted and plated onto LBG agar for CFU determination. Rifampicin-resistance mutation frequencies were calculated as described above.
RNA extraction and quantitative real-time PCR
Wild-type Mtb (OD600∼0.5) was treated with acid NO or 2.5 mM DETA/NO for 1 h. Total RNA was extracted with TRIzol-Reagent (Invitrogen) and further purified with RiboPure-Bacteria kit (Ambion). Briefly, cell pellet was resuspended in 1 ml TRIzol reagent, mixed with 400 µl 0.1 mm Zirconia Beads (BioSpec Products) and lysed in a mini-beadbeater (Biospec) for three cycles (40 s at maximal speed) with cooling on ice for 1 min between pulses. RNA was extracted according to the protocol of TRIzol-Reagent. The extracted RNA was further purified using the RiboPure-Bacteria kit followed by DNase I treatment to eliminate DNA contamination. cDNA was synthesized using the SuperScript III First Strand kit (Invitrogen) with random hexamer primer. Target gene transcript levels were measured by real-time PCR using SYBR® Premix Ex Taq GC (TaKaRa) on Mastercycler ep realplex thermal cyclers: 95°C 60 sec, 40 cycles of 95°C 5 sec, 62°C 8 sec and 72°C 20 sec, followed by melting curve analysis. Data were normalized to sigA and expressed as fold change compared to the untreated samples. PCR primers for sigA, mazG, dosR, recA and dnaE2 are listed in Table S5.
Macrophage infection
The murine macrophage cell line RAW264.7 was grown in DMEM medium (GIBCO) supplemented with 10% fetal calf serum (FCS) and incubated at 37°C with 5% CO2. For Mtb infection, cells were plated at a density of 2.0×105 cells per well in 24-well plates without antibiotics and activated with 200 U/ml murine IFN-γ (Peprotech) for 16 h [45]. Cells were primed with 1 µg/ml lipopolysaccharides (LPS, Sigma) for 1 h and then infected at a multiplicity of infection (MOI) of 2∶1 (bacteria∶cells). After 4 h incubation at 37°C with 5% CO2, cells were washed three times with DMEM to remove extracellular bacteria and cultured with complete DMEM medium. To inhibit macrophage NO production during the infection cause, NMMA (Sigma) was added to the culture medium at a final concentration of 400 µM. At indicated time points, bacteria were released with PBS solution containing 0.05% Tween-80 and 0.025% SDS, and plated onto 7H11-OADC plates. CFUs were counted after 3∼4 weeks culture at 37°C.
Mice infection
Mice were infected with wild-type Mtb, mazG-null mutant or complemented strain at an inhaled aerosol dose of 100-200 CFU per lung by an inhalation exposure system (Glas-Col, Terre Haute, IN). At indicated time points, mice were sacrificed and lung and spleen homogenates (four mice per group) were plated onto 7H11-OADC followed by incubation at 37°C for 4 weeks.
Histopathology
Lung sections stained with hematoxylin and eosin were photographed using a Nikon Optiphot 2 microscope fitted with a camera which was connected to a computer. The Image Pro Plus program (Media Cybernetics) was utilized to objectively assess the level of inflammation present in each image. To quantify the percent area inflamed, we determined the mean percent inflamed area from three to five lung sections of each mouse.
Statistical analysis
Statistical significance was determined with the unpaired two-tailed Student's t test at P<0.05 level of significance using GraphPad Prism 5.0 software.
Supporting Information
Zdroje
1. WagnerJR, HuCC, AmesBN (1992) Endogenous oxidative damage of deoxycytidine in DNA. Proc Natl Acad Sci U S A 89 : 3380–3384.
2. SekiguchiM, TsuzukiT (2002) Oxidative nucleotide damage: consequences and prevention. Oncogene 21 : 8895–8904.
3. HaghdoostS, SjolanderL, CzeneS, Harms-RingdahlM (2006) The nucleotide pool is a significant target for oxidative stress. Free Radic Biol Med 41 : 620–626.
4. NathanC, ShilohMU (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97 : 8841–8848.
5. FangFC (2004) Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2 : 820–832.
6. RaiP (2010) Oxidation in the nucleotide pool, the DNA damage response and cellular senescence: Defective bricks build a defective house. Mutat Res 703 : 71–81.
7. VenturaI, RussoMT, De LucaG, BignamiM (2010) Oxidized purine nucleotides, genome instability and neurodegeneration. Mutat Res 703 : 59–65.
8. MakiH, SekiguchiM (1992) MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355 : 273–275.
9. FeigDI, SowersLC, LoebLA (1994) Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc Natl Acad Sci U S A 91 : 6609–6613.
10. PurmalAA, KowYW, WallaceSS (1994) 5-Hydroxypyrimidine deoxynucleoside triphosphates are more efficiently incorporated into DNA by exonuclease-free Klenow fragment than 8-oxopurine deoxynucleoside triphosphates. Nucleic Acids Res 22 : 3930–3935.
11. FotiJJ, DevadossB, WinklerJA, CollinsJJ, WalkerGC (2012) Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336 : 315–319.
12. GutierrezA, LauretiL, CrussardS, AbidaH, Rodriguez-RojasA, et al. (2013) beta-lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat Commun 4 : 1610.
13. BessmanMJ, FrickDN, O'HandleySF (1996) The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem 271 : 25059–25062.
14. GalperinMY, MorozOV, WilsonKS, MurzinAG (2006) House cleaning, a part of good housekeeping. Mol Microbiol 59 : 5–19.
15. McLennanAG (2006) The Nudix hydrolase superfamily. Cell Mol Life Sci 63 : 123–143.
16. TaddeiF, HayakawaH, BoutonM, CirinesiA, MaticI, et al. (1997) Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science 278 : 128–130.
17. FujikawaK, KamiyaH, YakushijiH, FujiiY, NakabeppuY, et al. (1999) The oxidized forms of dATP are substrates for the human MutT homologue, the hMTH1 protein. J Biol Chem 274 : 18201–18205.
18. TsuzukiT, EgashiraA, IgarashiH, IwakumaT, NakatsuruY, et al. (2001) Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase. Proc Natl Acad Sci U S A 98 : 11456–11461.
19. NakabeppuY, OkaS, ShengZ, TsuchimotoD, SakumiK (2010) Programmed cell death triggered by nucleotide pool damage and its prevention by MutT homolog-1 (MTH1) with oxidized purine nucleoside triphosphatase. Mutat Res 703 : 51–58.
20. JarugaP, DizdarogluM (1996) Repair of products of oxidative DNA base damage in human cells. Nucleic Acids Res 24 : 1389–1394.
21. Murata-KamiyaN, KamiyaH, MuraokaM, KajiH, KasaiH (1997) Comparison of oxidation products from DNA components by gamma-irradiation and Fenton-type reactions. J Radiat Res 38 : 121–131.
22. FujikawaK, KamiyaH, KasaiH (1998) The mutations induced by oxidatively damaged nucleotides, 5-formyl-dUTP and 5-hydroxy-dCTP,in Escherichia coli. Nucleic Acids Res 26 : 4582–4587.
23. FragaCG, ShigenagaMK, ParkJW, DeganP, AmesBN (1990) Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A 87 : 4533–4537.
24. HatahetZ, KowYW, PurmalAA, CunninghamRP, WallaceSS (1994) New substrates for old enzymes. 5-Hydroxy-2′-deoxycytidine and 5-hydroxy-2′-deoxyuridine are substrates for Escherichia coli endonuclease III and formamidopyrimidine DNA N-glycosylase, while 5-hydroxy-2′-deoxyuridine is a substrate for uracil DNA N-glycosylase. J Biol Chem 269 : 18814–18820.
25. PurmalAA, LampmanGW, BondJP, HatahetZ, WallaceSS (1998) Enzymatic processing of uracil glycol, a major oxidative product of DNA cytosine. J Biol Chem 273 : 10026–10035.
26. D'HamC, RomieuA, JaquinodM, GasparuttoD, CadetJ (1999) Excision of 5,6-dihydroxy-5,6-dihydrothymine, 5,6-dihydrothymine, and 5-hydroxycytosine from defined sequence oligonucleotides by Escherichia coli endonuclease III and Fpg proteins: kinetic and mechanistic aspects. Biochemistry 38 : 3335–3344.
27. MorozOV, MurzinAG, MakarovaKS, KooninEV, WilsonKS, et al. (2005) Dimeric dUTPases, HisE, and MazG belong to a new superfamily of all-alpha NTP pyrophosphohydrolases with potential “house-cleaning” functions. J Mol Biol 347 : 243–255.
28. LuLD, SunQ, FanXY, ZhongY, YaoYF, et al. (2010) Mycobacterial MazG is a novel NTP pyrophosphohydrolase involved in oxidative stress response. J Biol Chem 285 : 28076–28085.
29. GrossM, MarianovskyI, GlaserG (2006) MazG — a regulator of programmed cell death in Escherichia coli. Mol Microbiol 59 : 590–601.
30. NonakaM, TsuchimotoD, SakumiK, NakabeppuY (2009) Mouse RS21-C6 is a mammalian 2′-deoxycytidine 5′-triphosphate pyrophosphohydrolase that prefers 5-iodocytosine. Febs J 276 : 1654–1666.
31. WuB, LiuY, ZhaoQ, LiaoS, ZhangJ, et al. (2007) Crystal structure of RS21-C6, involved in nucleoside triphosphate pyrophosphohydrolysis. J Mol Biol 367 : 1405–1412.
32. ImlayJA, ChinSM, LinnS (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240 : 640–642.
33. BoshoffHI, ReedMB, BarryCE3rd, MizrahiV (2003) DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113 : 183–193.
34. Saint-RufC, PesutJ, SoptaM, MaticI (2007) Causes and consequences of DNA repair activity modulation during stationary phase in Escherichia coli. Crit Rev Biochem Mol Biol 42 : 259–270.
35. MichaelsML, CruzC, GrollmanAP, MillerJH (1992) Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc Natl Acad Sci U S A 89 : 7022–7025.
36. InoueM, KamiyaH, FujikawaK, OotsuyamaY, Murata-KamiyaN, et al. (1998) Induction of chromosomal gene mutations in Escherichia coli by direct incorporation of oxidatively damaged nucleotides. New evaluation method for mutagenesis by damaged DNA precursors in vivo. J Biol Chem 273 : 11069–11074.
37. HeepM, BrandstatterB, RiegerU, LehnN, RichterE, et al. (2001) Frequency of rpoB mutations inside and outside the cluster I region in rifampin-resistant clinical Mycobacterium tuberculosis isolates. J Clin Microbiol 39 : 107–110.
38. SchaaperRM, DanforthBN, GlickmanBW (1986) Mechanisms of spontaneous mutagenesis: an analysis of the spectrum of spontaneous mutation in the Escherichia coli lacI gene. J Mol Biol 189 : 273–284.
39. SchaaperRM, DunnRL (1991) Spontaneous mutation in the Escherichia coli lacI gene. Genetics 129 : 317–326.
40. SatouK, HarashimaH, KamiyaH (2003) Mutagenic effects of 2-hydroxy-dATP on replication in a HeLa extract: induction of substitution and deletion mutations. Nucleic Acids Res 31 : 2570–2575.
41. ZhengH, LuL, WangB, PuS, ZhangX, et al. (2008) Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One 3: e2375.
42. DarwinKH, NathanCF (2005) Role for nucleotide excision repair in virulence of Mycobacterium tuberculosis. Infect Immun 73 : 4581–4587.
43. DarwinKH, EhrtS, Gutierrez-RamosJC, WeichN, NathanCF (2003) The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302 : 1963–1966.
44. VenugopalA, BrykR, ShiS, RheeK, RathP, et al. (2011) Virulence of Mycobacterium tuberculosis depends on lipoamide dehydrogenase, a member of three multienzyme complexes. Cell Host Microbe 9 : 21–31.
45. ChanJ, XingY, MagliozzoRS, BloomBR (1992) Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med 175 : 1111–1122.
46. KamiyaH (2003) Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary. Nucleic Acids Res 31 : 517–531.
47. Dos VultosT, BlazquezJ, RauzierJ, MaticI, GicquelB (2006) Identification of Nudix hydrolase family members with an antimutator role in Mycobacterium tuberculosis and Mycobacterium smegmatis. J Bacteriol 188 : 3159–3161.
48. PatilAG, SangPB, GovindanA, VarshneyU (2013) Mycobacterium tuberculosis MutT1 (Rv2985) and ADPRase (Rv1700) proteins constitute a two-stage mechanism of 8-oxo-dGTP and 8-oxo-GTP detoxification and adenosine to cytidine mutation avoidance. J Biol Chem 288 : 11252–11262.
49. SangPB, VarshneyU (2013) Biochemical properties of MutT2 proteins from Mycobacterium tuberculosis and M. smegmatis and their contrasting antimutator roles in Escherichia coli. J Bacteriol 195 : 1552–1560.
50. KreutzerDA, EssigmannJM (1998) Oxidized, deaminated cytosines are a source of C —> T transitions in vivo. Proc Natl Acad Sci U S A 95 : 3578–3582.
51. BebenekK, PedersenLC, KunkelTA (2011) Replication infidelity via a mismatch with Watson-Crick geometry. Proc Natl Acad Sci U S A 108 : 1862–1867.
52. BjedovI, TenaillonO, GerardB, SouzaV, DenamurE, et al. (2003) Stress-induced mutagenesis in bacteria. Science 300 : 1404–1409.
53. IoergerTR, KooS, NoEG, ChenX, LarsenMH, et al. (2009) Genome analysis of multi - and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS One 4: e7778.
54. FordCB, LinPL, ChaseMR, ShahRR, IartchoukO, et al. (2011) Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet 43 : 482–486.
55. SchapiroJM, LibbySJ, FangFC (2003) Inhibition of bacterial DNA replication by zinc mobilization during nitrosative stress. Proc Natl Acad Sci U S A 100 : 8496–8501.
56. LepoivreM, FieschiF, CovesJ, ThelanderL, FontecaveM (1991) Inactivation of ribonucleotide reductase by nitric oxide. Biochem Biophys Res Commun 179 : 442–448.
57. BarryCE3rd, BoshoffHI, DartoisV, DickT, EhrtS, et al. (2009) The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7 : 845–855.
58. DuttaNK, MehraS, DidierPJ, RoyCJ, DoyleLA, et al. (2010) Genetic requirements for the survival of tubercle bacilli in primates. J Infect Dis 201 : 1743–1752.
59. FritzC, MaassS, KreftA, BangeFC (2002) Dependence of Mycobacterium bovis BCG on anaerobic nitrate reductase for persistence is tissue specific. Infect Immun 70 : 286–291.
60. SinghR, RaoV, ShakilaH, GuptaR, KheraA, et al. (2003) Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol Microbiol 50 : 751–762.
61. MalhotraV, SharmaD, RamanathanVD, ShakilaH, SainiDK, et al. (2004) Disruption of response regulator gene, devR, leads to attenuation in virulence of Mycobacterium tuberculosis. FEMS Microbiol Lett 231 : 237–245.
62. DharN, McKinneyJD (2010) Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc Natl Acad Sci U S A 107 : 12275–12280.
63. KohanskiMA, DwyerDJ, HayeteB, LawrenceCA, CollinsJJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130 : 797–810.
64. WangX, ZhaoX (2009) Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother 53 : 1395–1402.
65. GrantSS, KaufmannBB, ChandNS, HaseleyN, HungDT (2012) Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci U S A 109 : 12147–12152.
66. BardarovS, Bardarov JrSJr, Pavelka JrMSJr, SambandamurthyV, LarsenM, et al. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148 : 3007–3017.
67. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed. , Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
68. WalkerJM (1994) The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol 32 : 5–8.
69. Van KesselJC, HatfullGF (2008) Mycobacterial recombineering. Methods Mol Biol 435 : 203–215.
70. VoskuilMI, SchnappingerD, ViscontiKC, HarrellMI, DolganovGM, et al. (2003) Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198 : 705–713.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2013 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



