-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
Upon contact with human plasma, bacteria are rapidly recognized by the complement system that labels their surface for uptake and clearance by phagocytic cells. Staphylococcus aureus secretes the 16 kD Extracellular fibrinogen binding protein (Efb) that binds two different plasma proteins using separate domains: the Efb N-terminus binds to fibrinogen, while the C-terminus binds complement C3. In this study, we show that Efb blocks phagocytosis of S. aureus by human neutrophils. In vitro, we demonstrate that Efb blocks phagocytosis in plasma and in human whole blood. Using a mouse peritonitis model we show that Efb effectively blocks phagocytosis in vivo, either as a purified protein or when produced endogenously by S. aureus. Mutational analysis revealed that Efb requires both its fibrinogen and complement binding residues for phagocytic escape. Using confocal and transmission electron microscopy we show that Efb attracts fibrinogen to the surface of complement-labeled S. aureus generating a ‘capsule’-like shield. This thick layer of fibrinogen shields both surface-bound C3b and antibodies from recognition by phagocytic receptors. This information is critical for future vaccination attempts, since opsonizing antibodies may not function in the presence of Efb. Altogether we discover that Efb from S. aureus uniquely escapes phagocytosis by forming a bridge between a complement and coagulation protein.
Published in the journal: Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface. PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003816
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003816Summary
Upon contact with human plasma, bacteria are rapidly recognized by the complement system that labels their surface for uptake and clearance by phagocytic cells. Staphylococcus aureus secretes the 16 kD Extracellular fibrinogen binding protein (Efb) that binds two different plasma proteins using separate domains: the Efb N-terminus binds to fibrinogen, while the C-terminus binds complement C3. In this study, we show that Efb blocks phagocytosis of S. aureus by human neutrophils. In vitro, we demonstrate that Efb blocks phagocytosis in plasma and in human whole blood. Using a mouse peritonitis model we show that Efb effectively blocks phagocytosis in vivo, either as a purified protein or when produced endogenously by S. aureus. Mutational analysis revealed that Efb requires both its fibrinogen and complement binding residues for phagocytic escape. Using confocal and transmission electron microscopy we show that Efb attracts fibrinogen to the surface of complement-labeled S. aureus generating a ‘capsule’-like shield. This thick layer of fibrinogen shields both surface-bound C3b and antibodies from recognition by phagocytic receptors. This information is critical for future vaccination attempts, since opsonizing antibodies may not function in the presence of Efb. Altogether we discover that Efb from S. aureus uniquely escapes phagocytosis by forming a bridge between a complement and coagulation protein.
Introduction
Phagocytosis by neutrophils is crucial to the host innate defense against invading bacteria since it leads to intracellular destruction of bacteria by production of oxygen radicals and proteolytic enzymes [1]. Bacterial engulfment by neutrophils is strongly enhanced by the labeling or ‘opsonization’ of bacteria with plasma factors such as antibodies and complement activation products (C3b, iC3b) [2]. Complement activation takes place at the bacterial surface and is initiated by recognition molecules (C1q, Mannose Binding Lectin (MBL)) that interact with bacterial surface structures like sugars or proteins [3]. Complement activation occurs through three different pathways (classical, lectin and alternative) that converge in the formation of C3 convertase enzymes that cleave the central complement protein C3 [4]. This cleavage step leads to massive decoration of the bacterial surface with covalently deposited C3b and iC3b molecules, which are recognized by complement receptor 1 and 3 (CR1 and CR3) on neutrophils. Complement activation proceeds by formation of C5 convertase enzymes that cleave C5 to release the potent chemoattractant C5a and C5b, which initiates formation of the membrane attack complex [5].
Staphylococcus aureus is an important human pathogen notorious for its ability to cause both community - and hospital-acquired diseases, ranging from mild skin infections to bacteremia, sepsis and endocarditis [6]. Although Methicillin-resistant S. aureus (MRSA) was previously considered as an opportunistic pathogen causing hospital-acquired infections in immune-compromised patients, the emergence of the highly virulent community-associated MRSA showed that this bacterium could also cause serious infections in otherwise healthy persons [7]. Due to the rapid emergence of antibiotic resistance strains, alternative therapy options are now being explored [8]. Vaccination has not been successful so far and an important reason may be the bacteria's elaborate immune evasion repertoire. Therefore, immune evasion proteins are now considered as important vaccination targets [9]. One proposed vaccine candidate is Extracellular fibrinogen binding protein (Efb), a 16-kD secreted protein with a presumable role in disease pathogenesis [10], [11], which is found in 85% of S. aureus strains [12]. Efb consists of two functionally distinct domains: a disordered 9 kD N-terminus (Efb-N) that harbors two binding sites for fibrinogen (Fg) [13] and a folded 7 kD C-terminus (Efb-C) that binds to the C3d domain of complement C3 (which is also present in C3b and iC3b) [14], [15]. Although previous papers described various functions for the isolated N - and C-terminal domains of Efb [12]–[19], it is currently not understood why the full-length Efb protein harbors both a Fg and C3d binding site. In this study we demonstrate that Efb potently blocks phagocytosis of bacteria via a novel mechanism linking the complement and coagulation proteins.
Results
Full-length Efb inhibits phagocytosis in the presence of plasma
To study a potential role for full-length Efb in phagocyte escape, we mixed fluorescently labeled S. aureus with purified human neutrophils, Efb (0.5 µM) and human serum or plasma as a source for complement and analyzed bacterial uptake by flow cytometry. In the presence of serum, Efb did not affect bacterial uptake by neutrophils (Fig. 1A). However when we used human plasma as a complement source, we found that Efb strongly prevented phagocytosis (Fig. 1A,B) and subsequent bacterial killing by neutrophils (Supplemental Fig. S1). Phagocytosis inhibition in plasma occurred in a dose-dependent fashion with a calculated IC50 of 0.08 µM (Fig. 1C). Since the main difference between plasma and serum lies in the presence of coagulation proteins, we investigated whether the observed differences in phagocytosis inhibition were caused by the fact that serum lacks Fg. Indeed, we observed that supplementation of serum with physiological concentrations of Fg led to phagocytosis inhibition by Efb (Fig. 1D). Fg is a large (340 kD) dimeric protein that comprises one central E-fragment and two lateral D-fragments. Since Efb binds to the D-fragment of Fg [13], we examined if supplementing serum with Fg-D would also lead to phagocytosis inhibition by Efb. Interestingly, we found that Efb could not block phagocytosis in the presence of Fg-D (Fig. 1E) indicating that full-length Fg is required for phagocytosis inhibition by Efb. Since Fg is a ligand for CR3 (or Mac-1 [20]) on neutrophils, we studied whether the binding of Fg to this receptor is important for the anti-phagocytic effect of Efb. Therefore, we purified Fg from wild-type mice or Fgγ390–396A (ΔMac-1 Fg) mice that express a mutated form of Fg lacking the Mac-1 binding site but retaining clotting function [21]. Fig. 1F shows that supplementation of human serum with both forms of mouse Fg led to inhibition by Efb, indicating that Fg binding to Mac-1 is not important for inhibition. In conclusion, Efb interferes with phagocytosis in a plasma environment and the presence of full-length Fg is required for this inhibition.
Fig. 1. Full-length Efb inhibits phagocytosis of S. aureus in human plasma. 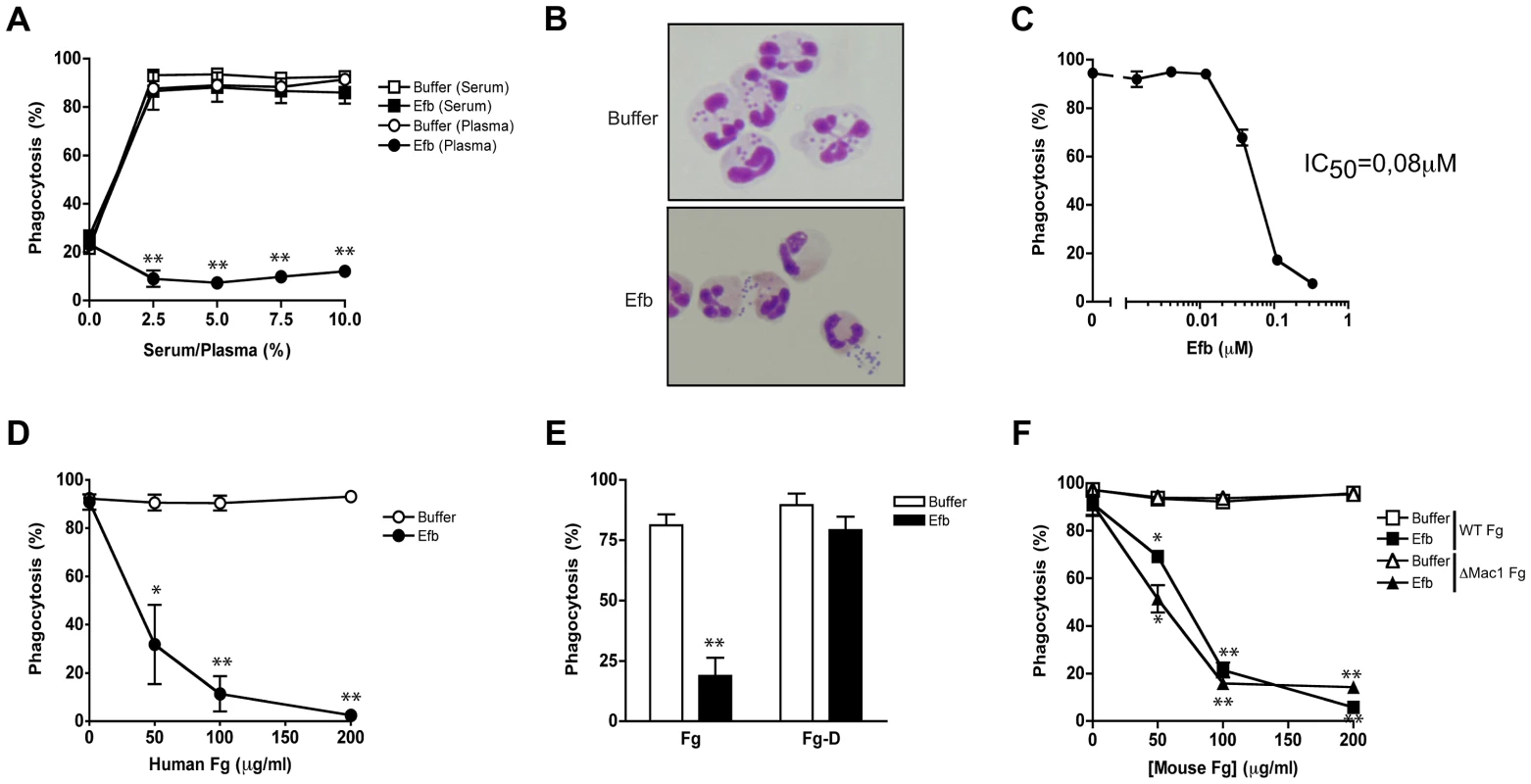
A. Phagocytosis of fluorescently labeled S. aureus by purified human neutrophils in the presence of human serum or plasma and Efb (0.5 µM). B. Histology image of human neutrophils incubated with S. aureus and 2.5% plasma in the presence or absence of Efb (0.5 µM). Cells were stained using Diff-Quick. C. Dose-dependent phagocytosis inhibition by Efb in the presence of 2.5% human plasma. IC50 was calculated using non-linear regression analysis, R2 = 0.95. D–F. Phagocytosis in the presence of 5% human serum supplemented with either full-length human Fg (Fig. 1D), the D domain of human Fg (1 µM or 86 µg/ml) (Fig. 1E) or mouse Fg (WT or lacking the Mac-1 binding site) (Fig. 1F). A, C–F are mean ± se of three independent experiments. B is a representative image. *P<0.05, **P<0.005 for Efb versus buffer (two-tailed Student's t-test). Simultaneous binding to Fg and C3 is essential for phagocytosis inhibition by Efb
To get more insight into the mechanism of inhibition, we constructed a panel of Efb mutants (Fig. 2A). First we observed that the individual N - or C-termini of Efb could not block phagocytosis in plasma (Fig. 2B). In addition, mixing the N - and C-terminal fragments of Efb did not markedly affect phagocytosis, indicating that full-length Efb is required. Second, we generated mutants of full-length Efb lacking the previously characterized binding sites for Fg and C3 (Fig. 2A [13], [14]). We created three different Fg-binding mutants: EfbΔFg1 lacking residues 30–45, EfbΔFg2 lacking residues 68–76 and EfbΔFg1+2 lacking both these Fg binding sites. Furthermore we created EfbΔC3 in which the C3d-binding residues R131 and N138 were each replaced with a glutamic acid (E) (also known as Efb-RENE [14]). Using direct binding ELISAs we verified that EfbΔFg1+2 could no longer bind Fg, while the single EfbΔFg1 and EfbΔFg2 mutants and EfbΔC3 still bound Fg (Supplemental Fig. S2A). As expected, all mutants except EfbΔC3 bound to C3b (Supplemental Fig. S2B). Next, we compared these mutants in the neutrophil phagocytosis assay in the presence of human plasma. We show that EfbΔFg1+2 and EfbΔC3 could no longer block phagocytosis (Fig. 2C), indicating that a simultaneous interaction with both Fg and complement C3 (products) is essential for the anti-phagocytic action of Efb. The finding that EfbΔFg1 and EfbΔFg2 were still active indicates that Efb requires only one of its two Fg binding sites to block phagocytosis.
Fig. 2. Simultaneous binding to Fg and C3 is essential for phagocytosis inhibition by Efb. 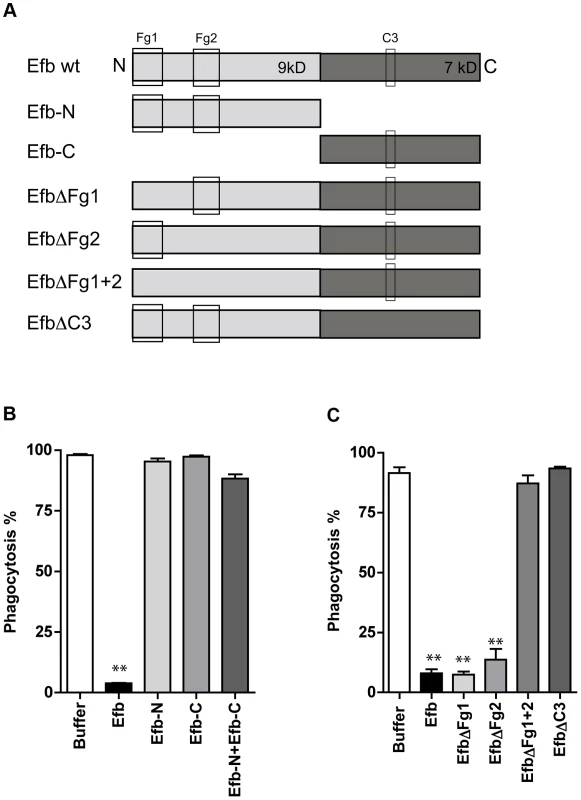
A. Schematic overview of Efb mutants generated in this study. Efb is depicted in its secreted form (30–165) lacking the signal peptide (1–29). Bounding boxes indicate Fg- and C3-binding domains. The N-terminus of Efb (light grey, 9 kD) harbors two Fg binding sites named Fg1 (residues 30–67) and Fg2 (residues 68–98). The C-terminus of Efb (dark grey, 7 kD) harbors the C3 binding site (residues R131 and N138). EfbΔFg1 has deletion of residues 30–45, resulting in non-functional binding Fg1; whereas EfbΔFg2 has deletion of residues 68–76, resulting in non-functional binding Fg2. B–C. Phagocytosis of fluorescent S. aureus by human neutrophils in the presence of 5% human plasma and Efb fragments (B) or Efb mutants (C) (all at 1 µM). B,C are mean ± se of three independent experiments. **P<0.005 for Efb versus buffer (two-tailed Student's t-test). Efb blocks phagocytosis ex vivo and in vivo
To study whether Efb can also block phagocytosis in a natural environment, we tested its activity in ex vivo and in vivo phagocytosis models. In an ex vivo human whole blood model, we incubated fluorescent S. aureus with 50% human whole blood and Efb. After 25 minutes, neutrophil phagocytosis was analyzed by flow cytometry. We observed that full-length Efb potently blocked phagocytosis by human neutrophils in whole blood (Fig. 3A) and that this inhibition depends on the interaction of Efb with both Fg and C3. Next, we studied phagocytosis of S. aureus in an in vivo mouse peritonitis model. To this end, mice were treated with carrageenan intraperitoneally (i.p.) to induce neutrophil infiltration into the peritoneal cavity and subsequently challenged with 108 heat-inactivated fluorescent S. aureus in the presence or absence of Efb (1 µM). One hour later, mice were sacrificed and the peritoneum was lavaged with sterile PBS. Neutrophils were stained and phagocytosis of fluorescent bacteria was analyzed by flow cytometry. We observed that Efb blocked phagocytosis in the peritoneum (Fig. 3B,C). Efb mutants showed that inhibition of phagocytosis in vivo also depends on the Fg and C3 binding domains of Efb.
Fig. 3. Purified Efb blocks phagocytosis ex vivo and in vivo. 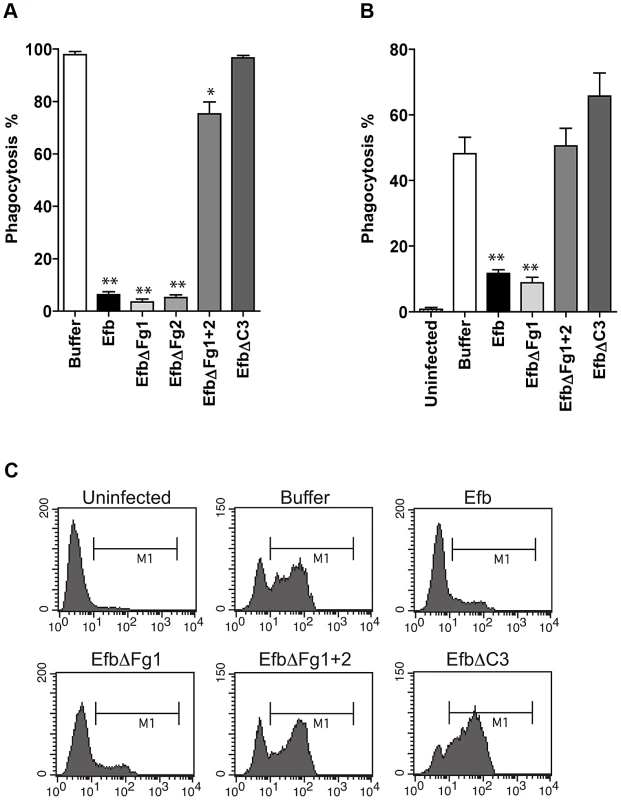
A. Ex vivo phagocytosis of fluorescent S. aureus incubated with 50% human whole blood and Efb (1 µM). Neutrophils were gated based on forward and side scatter properties. B. In vivo phagocytosis of fluorescent S. aureus by human neutrophils in the mouse peritoneum. Neutrophils were attracted to the peritoneal cavity using carrageenan (i.p.) and subsequently challenged with 108 heat-inactivated fluorescent S. aureus and Efb (1 µM) for 1 hour. The peritoneal lavage was collected and neutrophil phagocytosis was analyzed by flow cytometry. Neutrophils were gated based on Gr-1 expression. The mouse experiments were carried out three times. In each experiment, we used 3 mice per group and the cells of these 3 mice were pooled for phagocytosis analysis. C. Representative histograms of B. A,B are mean ± se of three independent experiments. *P<0.05, **P<0.005 for Efb versus buffer (two-tailed Student's t-test). Phagocytosis inhibition by Efb is independent of complement inhibition
Experiments shown above indicate that Efb requires an interaction with both complement and Fg to block phagocytosis. To study whether Efb also interacts with S. aureus specifically, we analyzed whether purified Efb can block phagocytosis of other bacteria as well. Fluorescent S. epidermidis or E. coli were mixed with human plasma and phagocytosis by neutrophils was evaluated. Efb potently inhibits the uptake of these bacteria as well, indicating that Efb can block phagocytosis independently of S. aureus (Fig. 4A). Previously, we observed that the C-terminal domain of Efb is a complement inhibitor that inactivates C5 convertases to prevent cleavage of C5 [12]. Efb-C did not affect C3b labeling of bacteria in conditions where all complement pathways are active. However, since the effects of Efb on complement inhibition were performed with serum instead of plasma, we wondered whether full-length Efb might affect C3b labeling of bacteria in a plasma environment. Therefore, we incubated S. aureus with human plasma and Efb and quantified surface-bound C3b using immunoblotting. As a control, we added EDTA to prevent activation of all complement routes (which are calcium and magnesium dependent). Similar amounts of C3b were found on the bacterial surface in the presence of Efb compared to buffer (Fig. 4B), indicating that Efb does not interfere with C3b labeling in plasma. Subsequently, we re-analyzed the inhibition of C5 convertases by Efb (mutants) in plasma using an alternative pathway hemolytic assay. Rabbit erythrocytes were incubated with human plasma and C5 cleavage was measured by means of C5b-9 dependent lysis of erythrocytes. In conjunction with previous results in serum, we found that all Efb mutants except for EfbΔC3 inhibited C5 cleavage in plasma (Fig. 4C). Since this inhibition exclusively depends on the C-terminal domain (all Fg binding mutants of Efb could still block C5 cleavage), this proves that interference with C5 cleavage is at least not sufficient for phagocytosis inhibition by Efb. To further show that the effects of Efb on complement activation are dispensable for phagocytosis inhibition, we introduced a washing step in our phagocytosis assay. Bacteria were first incubated with serum (in the absence of Efb) to deposit C3b. After washing away unbound serum proteins (including C5a), these pre-opsonized bacteria were incubated with Fg and neutrophils. In this assay, Efb could potently block phagocytosis (Fig. 4D). In conclusion, these results indicate that the anti-phagocytic activity of Efb is not related to its complement-inhibitory effect.
Fig. 4. Phagocytosis inhibition by Efb is independent of complement inhibition. 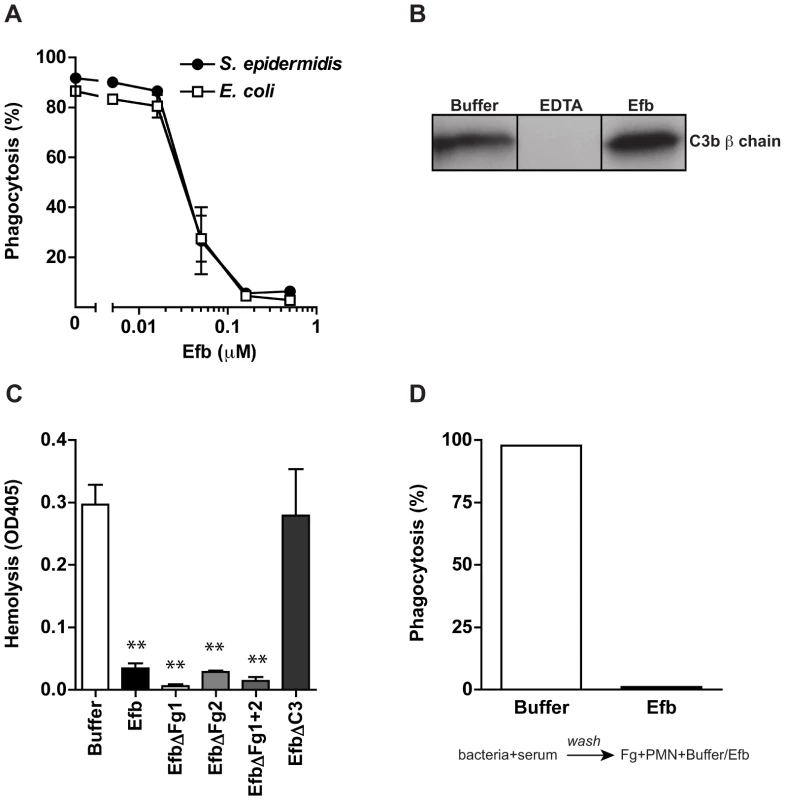
A. Phagocytosis of fluorescently labeled S. epidermidis and E. coli by purified human neutrophils in the presence of human plasma (5%) and Efb. B. Immunoblot detecting surface-bound C3b after incubation of S. aureus with 5% human plasma in the presence of 5 mM EDTA or 0.5 µM Efb. Blot is a representative of 3 independent experiments. C. Alternative pathway hemolysis of rabbit erythrocytes in 5% human plasma and Efb (mutants) (1 µM). Bars are the mean ± se of three independent experiments. **P<0.005 for Efb versus buffer (two-tailed Student's t-test). D. Phagocytosis with a washing step. Fluorescent S. aureus was first incubated with 5% serum to deposit complement. Bacteria were washed and subsequently mixed with neutrophils and Fg in the presence or absence of Efb (0.5 µM). Graph is a representative of three independent experiments. Efb covers S. aureus with a shield of Fg
We wondered whether Efb might bind to C3b-labeled bacteria and then attract Fg to the surface. First, we studied whether full-length Efb can bind to Fg and C3b at the same time. C3b-coated microtiter plates were incubated with Efb and, after a washing step, treated with Fg. Fig. 5A shows that Efb is able to form a complex with C3b and Fg. Also, the EfbΔFg1 and EfbΔFg2 mutants could still form Fg-C3b complexes. In contrast, complex formation was not detected for the mutants that lack either both Fg (EfbΔFg1+2) or the C3 binding domains (EfbΔC3) (Fig. 5A). Then, we investigated whether Efb could attract Fg to pre-opsonized bacteria. Therefore, S. aureus was pre-opsonized with human serum to deposit complement and subsequently incubated with Efb. After washing, bacteria were incubated with Alexa-488 conjugated Fg. Using both flow cytometry and confocal microscopy we observed that Efb mediates Fg binding to pre-opsonized bacteria (Fig. 5B,C). Consistent with the ELISA data for complex formation, no Fg binding was detected in the presence of EfbΔFg1+2 or EfbΔC3. Confocal analyses indicated that Efb covers the complete bacterial surface with Fg (Fig. 5C). Using Transmission Electron Microscopy we analyzed this Fg layer created by Efb in more detail. After incubation of S. aureus with plasma and Efb, we observed a diffuse outer layer formed around the bacteria (Fig. 5D). Altogether these experiments show that Efb binds to C3b on the bacterial surface and subsequently attracts Fg forming a shield around the bacterial surface.
Fig. 5. Efb attracts Fg to the bacterial surface. 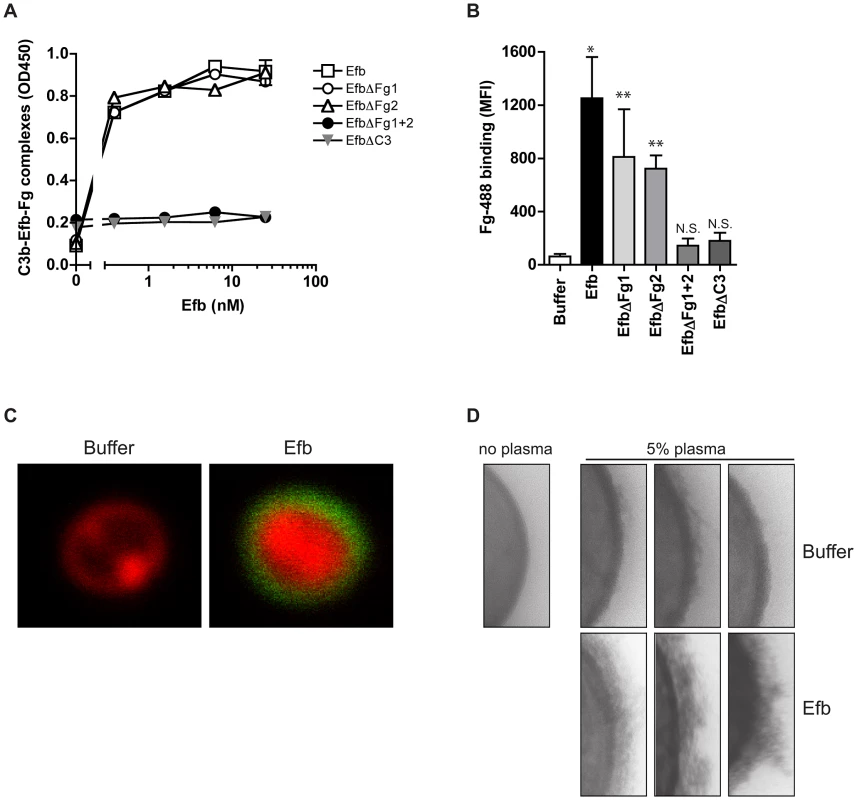
A. ELISA showing that Efb can bind Fg and C3b at the same time. C3b-coated microtiter wells were incubated with Efb (mutants) and, after washing, incubated with 50 nM Fg that was detected with a peroxidase-conjugated anti-Fg antibody (Abcam). Graph is a representative of two independent experiments performed in duplicate. B. Binding of Alexa488-labeled Fg (60 µg/ml) to serum-opsonized S. aureus in the presence of Efb (mutants) (0.5 µM). Graph represents mean ± se of three independent experiments. *P<0.05, **P<0.005 for Efb versus buffer (two-tailed Student's t-test). N.S. is not significant. C. Confocal analysis of samples generated in B (representative images). D. TEM pictures of S. aureus incubated with 5% human plasma in the absence or presence of Efb (0.5 µM). Three representative images are shown. Efb blocks recognition of C3b and IgG on the surface
Since Efb covers bacteria with a shield of Fg, we hypothesized that this would frustrate the binding of phagocytic receptors to their ligands on the bacterial surface Using flow cytometry, we first analyzed whether C3b-labeled bacteria were still recognized by CR1. Pre-opsonized S. aureus was incubated with soluble CR1 in the presence of Fg and Efb. Clearly, binding of CR1 to pre-opsonized bacteria was blocked by the presence of both Fg and Efb (Fig. 6A). Addition of Fg or Efb alone did not affect CR1 binding. Next, we investigated whether the Fg shield specifically blocks C3b-CR1 interactions or whether it also disturbs the binding of neutrophil Fc receptors to opsonic antibodies. To analyze this, we determined whether the Fc part of bacterium-bound IgG could still be recognized by specific antibodies. We found that incubation of pre-opsonized bacteria with Efb and Fg disturbs recognition of the antibody Fc domain on the surface (Fig. 6B), suggesting that Fc receptors can no longer recognize their target. This information is crucial for future vaccine development since opsonic antibodies against S. aureus may not function when Efb hides these antibodies underneath an Fg shield. To further prove that Efb functionally blocks opsonization, we analyzed phagocytosis of an encapsulated S. aureus strain in the presence or absence of anti-capsular antibodies. The encapsulated S. aureus strain Reynolds was grown for 24 hours in Columbia agar supplemented with 2% NaCl (for optimal capsule expression [22]) and subsequently labeled with FITC. We verified capsule expression after FITC-labeling using specific antibodies (Supplemental Fig. S3). In low plasma concentrations (0–1%), we observed that anti-capsular antibodies caused a 6-fold increase in phagocytic uptake of encapsulated S. aureus (Fig. 6C). At these plasma concentrations, Efb could not block phagocytosis. However at higher plasma concentrations (3% and more), Efb potently impeded phagocytosis in the presence of anti-capsule antibody (Fig. 6C). These data support our idea that the Fg shield created by Efb prevents recognition of important opsonins like C3b and IgG, also in the context of a capsule-expressing strain that is targeted by specific antibodies.
Fig. 6. Efb prevents recognition of opsonic C3b and IgG. 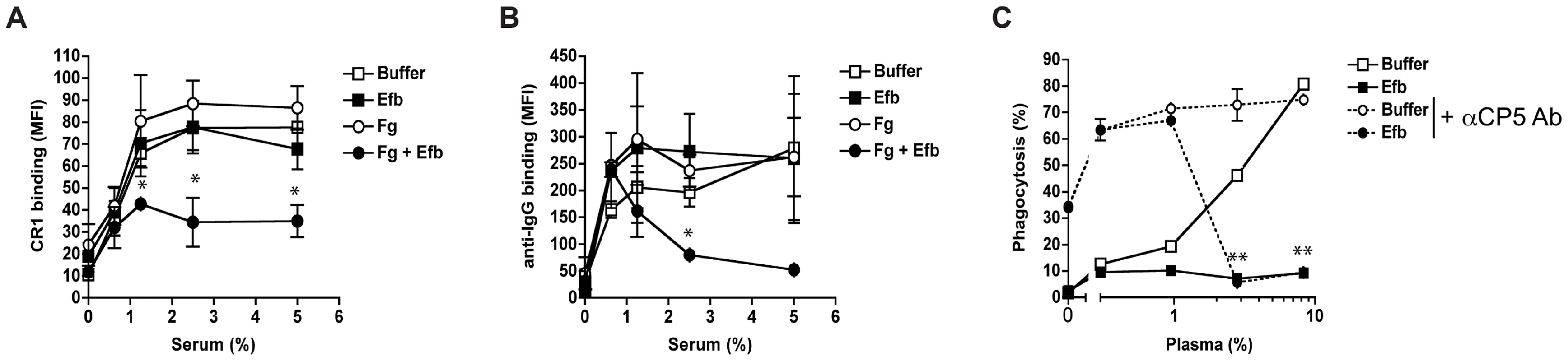
A–B. Flow cytometry assay detecting binding of soluble CR1 (A) or anti-IgG antibody (B) to pre-opsonized S. aureus in the presence of buffer, Efb (0.5 µM) and/or Fg (200 µg/ml). C. Efb inhibits phagocytosis of encapsulated S. aureus by human neutrophils. FITC-labeled S. aureus strain Reynolds (high capsule CP5 expressing strain) was incubated with human plasma and/or Efb (0.5 µM) in the presence (dotted line) or absence (solid line) of polyclonal rabbit anti-CP5 antibody. All figures represent the mean ± se of three separate experiments. *P<0.05, **P<0.005 for Efb+Fg versus buffer (A,B) or Efb versus buffer (for dotted lines) (two-tailed Student's t-test). Endogenous Efb blocks phagocytosis in vitro and in vivo
To study whether endogenous expression of Efb leads to impaired phagocytosis of S. aureus via complex formation, we extended our analyses with (supernatants of) an isogenic Efb-deletion mutant in S. aureus Newman (previously described in [23]). First we performed Immunoblotting to semi-quantify the production levels of Efb in liquid bacterial culture supernatants. Supernatants of wild-type (WT) S. aureus Newman were subjected to Immunoblotting and developed using polyclonal anti-Efb antibodies (Fig. 7A). Efb expression in the supernatant was quantified using ImageJ software and compared with fixed concentrations of purified (His-tagged) Efb using linear regression analysis (R2 = 0.986). Efb levels in 4 h and 20 h supernatants contained 1,1 µM and 0,9 µM Efb respectively. Although the Efb levels in strain Newman are suspected to be higher than in other S. aureus strains (up to 10-fold [24], due to a point mutation in the SaeR/S regulatory system that drives expression of immune evasion genes [25]), the fact that these levels are >10 times higher than the calculated IC50 needed for phagocytosis inhibition (0.08 µM, Fig. 1C), suggests that Efb concentrations required for phagocytosis inhibition can be reached in vivo. In a separate Immunoblot, we checked for the presence of Efb in 4 h supernatants of the WT, Efb-deficient (ΔEfb) and the complemented strain (ΔEfb+pEfb) confirming the lack of Efb expression in the mutant (Fig. 7A). Next we used these supernatants to study whether endogenous Efb can mediate C3b-Fg complex formation on the bacterial surface. S. aureus was first incubated with serum to deposit C3b, then mixed with bacterial supernatants and subsequently incubated with fluorescently labeled Fg. Whereas WT supernatants attracted Fg to the surface of pre-opsonized bacteria, Efb-deficient supernatants did not mediate complex formation (Fig. 7B). This phenotype was restored in the complemented strain. Then we studied whether endogenous Efb could inhibit phagocytosis by neutrophils in vitro. Therefore we repeated the latter experiment (but using fluorescent bacteria and unlabeled Fg) and subsequently mixed the bacteria with human neutrophils. We found that supernatants of WT and complemented strains inhibited phagocytosis, while Efb-deficient supernatants did not influence this process (Fig. 7B). To mimic bacterial phagocytosis during a natural infection, carrageenan-treated mice were injected i.p. with GFP-expressing WT S. aureus or the Efb-deficient mutant in their original broth culture and sacrificed 1 h thereafter. Mice were subjected to peritoneal lavage and the percentage of neutrophils with internalized staphylococci was determined by flow cytometry. As depicted in Fig. 7D, the Efb-deficient S. aureus strain was phagocytosed by neutrophils to a significantly higher extent than the WT strain despite of the fact that the amount of inoculated bacteria was comparable in both groups (app. 2×107). These observations demonstrate that the levels of Efb produced by S. aureus are sufficient for preventing phagocytosis in vivo.
Fig. 7. Endogenously produced Efb blocks phagocytosis via complex formation. 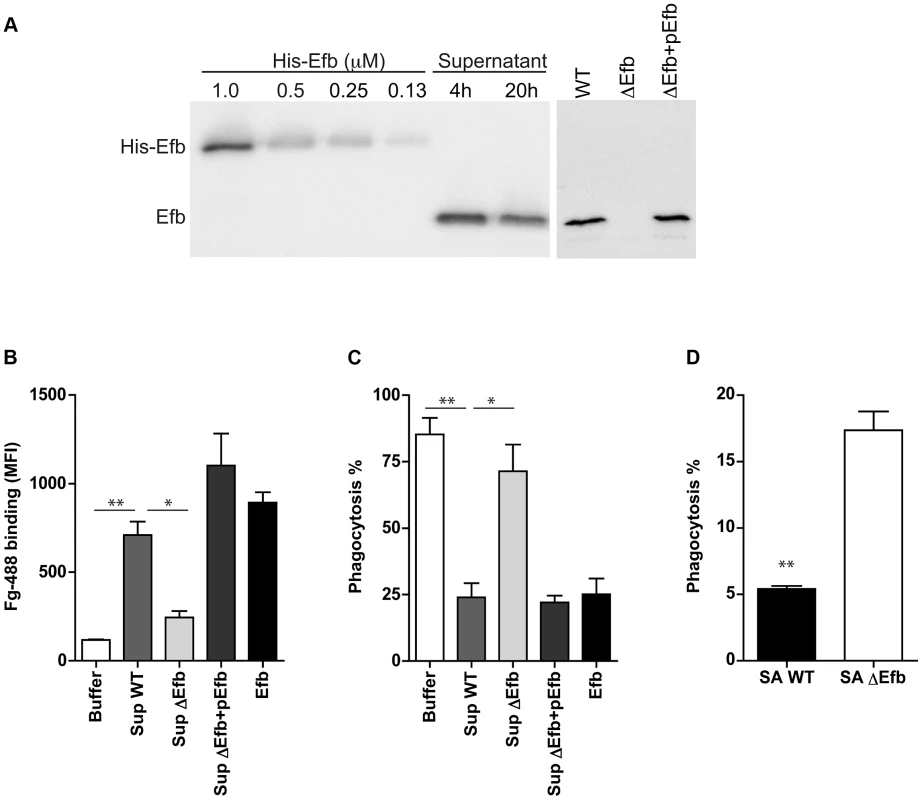
A. Left. Immunoblot detecting Efb in 4 h and 20 h culture supernatants of S. aureus Newman; fixed concentrations of His-tagged Efb were loaded as controls. Right. Immunoblot of 4 h culture supernatants of S. aureus Newman (WT), an isogenic Efb deletion mutant (ΔEfb) and its complemented strain (ΔEfb+pEfb). Blots were developed using polyclonal sheep anti-Efb and Peroxidase-labeled donkey anti-sheep antibodies. Blot is a representative of two independent experiments. B. Flow cytometry analysis of the binding of Alexa488-labeled Fg to pre-opsonized S. aureus in the presence of 4 h culture supernatants (2-fold diluted) or purified Efb (250 nM). C. In vitro phagocytosis of fluorescently labeled S. aureus by purified human neutrophils. Pre-opsonized S. aureus was first incubated with 4 h culture supernatants (2-fold diluted) or purified Efb (250 nM) and subsequently mixed with Fg and neutrophils. D. In vivo phagocytosis of GFP-expressing wild-type or Efb-deficient S. aureus strains by neutrophils in the mouse peritoneal cavity. Neutrophils were attracted to the peritoneal cavity using carrageenan (i.p.) and subsequently injected with 300 µl of GFP-expressing wild-type (SA WT) or Efb-deficient (SAΔEfb) S. aureus strains during the exponential phase of growth. The peritoneal lavage was collected 1 h thereafter and neutrophil phagocytosis was analyzed by flow cytometry. Neutrophils were gated based on Gr-1 expression. Graphs in B–D represent mean ± se of three independent experiments. *P<0.05, **P<0.005 for WT versus Buffer or ΔEfb (two-tailed Student's t-test). Discussion
The coagulation system has a dual role in the host defense against bacterial infections. On one hand, coagulation supports innate defenses by entrapment and killing of invading bacteria inside clots [26] or via the formation of small antibacterial and pro-inflammatory peptides [27], [28]. On the other hand, bacterial pathogens can utilize coagulation proteins to protect themselves from immune defenses. In this study, we find that S. aureus effectively protects itself from immune recognition by secreting Efb that specifically attracts Fg from the solution to the bacterial surface creating a capsule-like shield (Figure 8). To accomplish this, Efb forms a multi-molecular complex of soluble Fg and surface-bound C3b. The fact that the levels of C3b at the bacterial surface are high [29] and that Fg is an abundant plasma protein (1.5–4.0 g/L) makes this a very efficient anti-phagocytic mechanism. The Fg shield created by Efb effectively protects S. aureus from recognition by phagocyte receptors. We found that the attracted Fg does not only block the binding of C3b to its receptor, but also hides the important opsonin IgG underneath the Fg shield. We think that this information is critical for future vaccine development against S. aureus. Generation of protective ‘opsonizing’ antibodies recognizing S. aureus surface structures is considered to be an important goal of vaccination. However, these antibodies will not function if they are protected underneath a layer of Fg. We anticipate that including Efb in future vaccines might be beneficial as it could prevent formation of this anti-phagocytic shield and enhance the function of opsonizing antibodies. The fact that Efb is conserved among S. aureus strains may make it a suitable vaccine candidate [30].
Fig. 8. Proposed mechanism for phagocytosis inhibition by Efb. 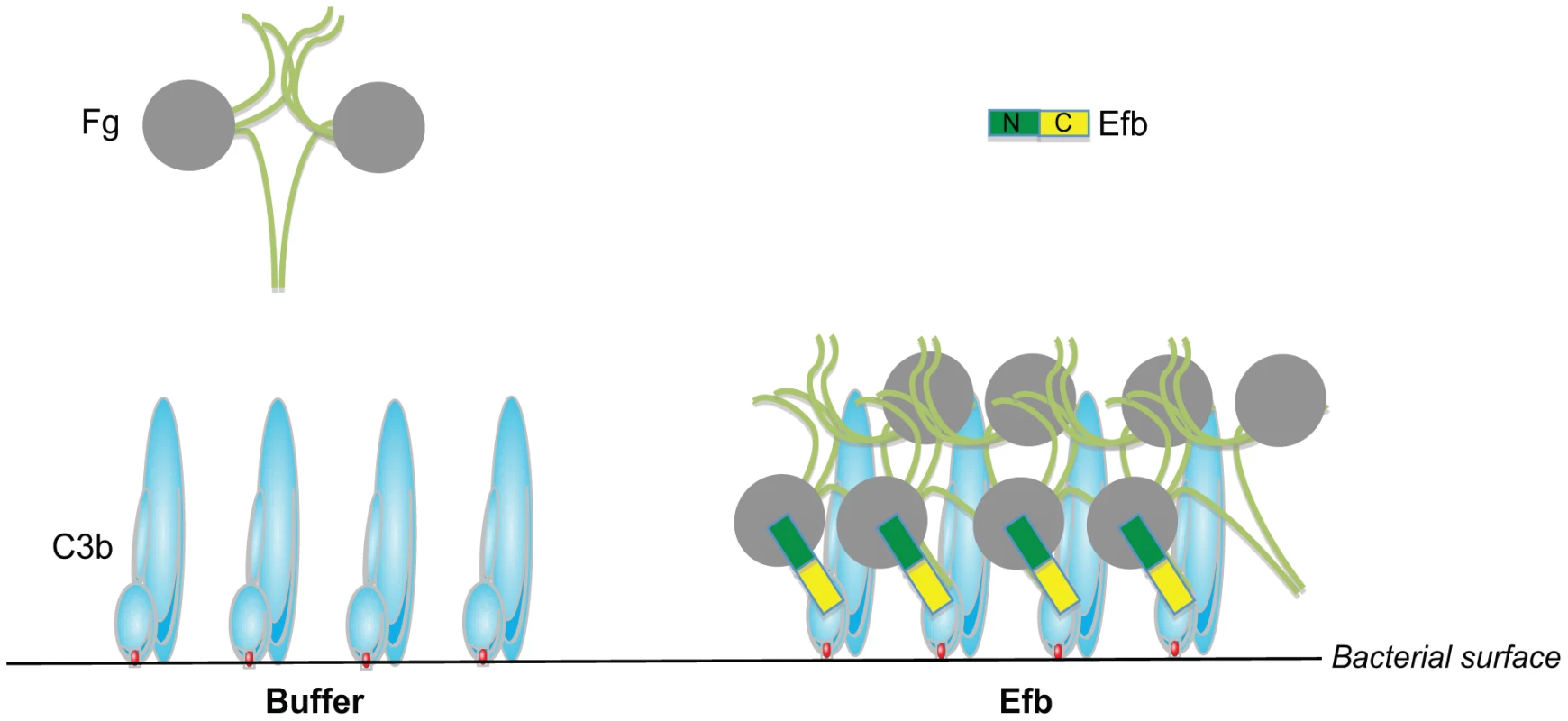
Schematic picture of the proposed phagocytosis escape mechanism by Efb. Left, Complement activation on the bacterial surface results in massive labeling of S. aureus with C3b molecules while Fg stays in solution. Right, S. aureus secretes Efb, which binds to surface-bound C3b via its C-terminal domain (colored yellow). Using its N-terminus (green), Efb attracts Fg to the bacterial surface. This way, S. aureus is covered with a shield of Fg that prevents binding of phagocytic receptors to important opsonins like C3b and IgG. Next to Efb, S. aureus secretes two other proteins that specifically interact with the coagulation system: the S. aureus ‘coagulases’ named Coagulase and Von Willebrand factor binding protein are secreted proteins that activate prothrombin in a nonproteolytic manner and subsequently convert Fg into fibrin [31]. Thereby, coagulases embed bacteria within a network of fibrin, protecting them from immune recognition and facilitate formation of S. aureus abscesses and persistence in host tissues [32]. Coagulase and Efb are expressed at the same time during infection since they are both regulated by the SaeR/S regulator for secreted (immune evasion) proteins [25], [33]. Based on our study, we hypothesize that Efb may be highly important for proper functioning of Coagulase since Efb can attract Fg to the bacterial surface. This way, Efb may aid Coagulase-dependent fibrin formation to occur close to the bacterial surface instead of in solution. Nevertheless our studies also indicate that Efb can block phagocytosis in the absence of prothrombin and Coagulase. However, in a more complex environment the anti-phagocytic mechanisms of Efb and S. aureus Coagulase might work synergistically. Furthermore, it seems tempting to speculate that the ability of Efb to attract Fg to the bacterial surface is also beneficial in other infection processes like adhesion. Since Fg is an important constituent of the extracellular matrix (ECM), Efb might also facilitate binding of C3b-opsonized bacteria to the ECM. In fact, Efb was previously classified as an adhesion molecule belonging to the group of SERAMs (secreted expanded repertoire adhesive molecules) [34]. However, as a secreted protein, Efb cannot facilitate bacterial adhesion if it solely binds to Fg in the ECM without interacting with the bacterial surface. Binding to C3b-labeled bacteria via the Efb C-terminus might therefore be crucial for effective bacterial adhesion to Fg.
The pathogenic potential of S. aureus is a result of its versatile interactions with multiple host factors, evidenced by the fact that it can survive at multiple sites of the body causing a wide range of infections. At most body sites, S. aureus has to deal with cellular and humoral components of the immune system. However, increasing evidence now suggests that S. aureus protects itself from immune defense by forming abscess communities surrounded by capsule-like structures that prevent neutrophil invasion [35], [36]. Our study implicates that Efb might be crucial in the formation of these capsules. Furthermore, our whole blood assays shows that Efb may also play an important role in S. aureus survival in the blood allowing it to spread to other sites of the body. Previous studies using animal models have highlighted the critical role of Efb in S. aureus pathogenesis. For instance, Efb delays wound healing in a rat wound infection model [10] and is important for S. aureus pneumonia and abscess formation in kidneys [23]. Our in vivo studies corroborate the in vitro findings and suggest that complex formation can occur under physiological conditions in vivo. However, the available mouse models do not closely mimic this process during clinical infections in humans. Efb is produced in later stages of bacterial growth, thus the bacteria need time to produce Efb before they come into contact with neutrophils. Since neutrophils need to be recruited from the blood to the site of the infection, there normally is time for Efb production and complex formation, especially in the human host where an infection starts with a low number of bacteria. In contrast, in available mouse models the timing is much different as a high inoculum (up to 108 bacteria) is required to establish an infection and these high numbers of bacteria trigger a strong inflammatory response resulting in that the bacteria are already phagocytized before Efb is produced. For this reason, we have mixed the bacteria with their supernatants to ensure the presence of endogenous Efb during the course of the experiments and have chosen a model in which neutrophils are already attracted to the infection site to focus on the anti-phagocytic activity of the molecule. Future studies are needed to design and execute appropriate animal studies that overcome the limitations of current models and better reflect the clinical situation.
In summary, we describe that full-length Efb can inhibit phagocytosis in a unique way through its dual interaction with complement and Fg. Our study indicates that Efb is a highly effective immune escape molecule that blocks phagocytosis of S. aureus in vivo.
Materials and Methods
Ethics statement
Study participants provided written informed consent in accordance with the Declaration of Helsinki. Approval was obtained from the medical ethics committee of the UMC Utrecht. Animal experiments were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV-SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). All experiments were approved by the ethical board Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Oldenburg in Germany (Permit No. 33.9-42502-04-10/0296).
Bacterial strains, fluorescent labeling and supernatants
In this study we used the laboratory S. aureus strains Newman, SH1000, Reynolds and Wood 46 (with low expression of Protein A). The S. aureus strain KV27 and the S. epidermidis and E. coli strains were clinical isolates obtained within the UMCU. Targeted deletion (and complementation) of Efb in S. aureus Newman was described previously in [23]. All strains were cultured overnight on Tryptic Soy Blood Agar (BD) or Todd Hewitt Agar (with appropriate antibiotics) at 37°C. The capsule-expressing S. aureus strain Reynolds and its isogenic CP5-deficient mutant were a kind gift from Jean Lee (Harvard Medical School, Boston, USA) [22]. To optimize capsule expression, strain Reynolds was grown on Columbia Agar supplemented with 2% NaCl (CSA) for 24 h at 37°C. For fluorescent labeling of strains, bacteria were resuspended in PBS and incubated with 0.5 mg/ml FITC (Sigma) for 30 minutes on ice. Bacteria were washed twice with PBS, resuspended in RPMI medium with HSA and stored at −20°C until further use. For in vivo experiments, S. aureus Newman and the Efb mutant were transformed with the pCM29 plasmid (kindly provided by Alexander Horswill, University of Iowa) allowing constitutive expression of the superfolder green fluorescent protein (sGFP) via the sarAP1 promoter [37]. To isolate bacterial supernatants, WT and mutant strains were cultured overnight in Todd Hewitt Broth (THB) without antibiotics and subsequently sub cultured in fresh THB for 4 h or 20 h. Cultures were centrifuged at 13,000 rpm and collected supernatants were stored at −20°C until further use.
Protein expression and purification
Recombinant Efb proteins were generated in E. coli as described previously [12], [13]. Briefly, (parts of) the efb gene from S. aureus strain Newman (without the signal peptide) were amplified by PCR and ligated into either the pGEX-5x-1 vector (GE healthcare) or the pRSETB vector (Invitrogen) for N-terminal fusions with glutathione S-transferase (GST) or polyhistidine respectively. Mutations of the Fg and C3 binding domains were introduced in pGEX plasmids containing full-length GST-Efb as described previously [13]. Recombinant proteins were expressed and purified according to the manufacturer's manual. In all experiments where wild-type Efb was compared with mutants, we used GST-tagged Efb. Otherwise His-tagged Efb was used.
ELISA
Microtiter plates were coated with human C3b [38] or Fg, blocked with 3% BSA-PBS, and incubated with 6 nM Efb for one hour at room temperature. Efb binding was detected using peroxidase-conjugated rabbit anti-GST polyclonal antibodies (Abcam) and quantified using 0-phenylenediamine dihydrochloride (Sigma). To study formation of C3b-Efb-Fg complexes, C3b-coated plates were incubated with Efb for one hour at room temperature. After washing, human Fg (50 nM) was added and detected through incubation with peroxidase-conjugated anti-Fg antibodies (Abcam).
Preparation of Fg-D fragments
D fragments of Fg were generated by digestion of human Fg (Enzyme research) with plasmin (Enzyme research, 10 µg/15 mg Fg) in TBS containing 10 mM CaCl2 for 4 hours at 37°C as described earlier [39] with modifications. D fragments (85 kD) were purified by gel filtration on Sephacryl S-200 (GE Healthcare) and analyzed by SDS-PAGE.
Purification of human blood products
For preparation of plasma, venous blood from 10 healthy volunteers was collected in glass vacutainers (BD) containing the anticoagulant lepirudin (50 µg/ml) [40]. To prepare serum, blood was collected in glass vacutainers (BD) without anticoagulant and allowed to clot for 15 min at room temperature. Plasma and serum were collected after centrifugation for 10 minutes at 4000 rpm at 4°C, pooled and subsequently stored at −80°C. Complement-inactivated serum was prepared by incubation of serum for 30 min at 56°C. Human neutrophils were isolated freshly from heparinized blood using the Ficoll-Histopaque gradient method [41] and used on the same day.
Mice
C57BL/6 female mice were purchased from Harlan-Winkelmann (Borchen, Germany) and used in experiments when they were between 8 and 10 weeks of age. They were housed in micro isolator cages and given food and water ad libitum.
Phagocytosis assays
Whole blood phagocytosis
FITC-labeled S. aureus KV27 (1×108/ml) was incubated with freshly isolated human lepirudin blood (50%) and buffer or Efb (0.5 µM) in RPMI-0.05% HSA for 25 min at 37°C. The reaction was stopped using FACS lysing solution (BD Biosciences); samples were washed with RPMI-0.05% HSA and analyzed by flow cytometry using a FACSCalibur (BD). Gating of cells occurred on basis of forward and side scatter; for each sample we measured the fluorescence intensity of 10,000 gated neutrophils. Phagocytosis was expressed as the percentage of neutrophils that became fluorescent.
Phagocytosis with purified neutrophils and plasma/serum
FITC-labeled bacteria (5×107/ml) were mixed with human serum or plasma for 2 min at 37°C in the presence or absence of Efb. Freshly isolated neutrophils (5×106/ml) were added and phagocytosis was allowed for 15 min at 37°C. The reaction was stopped by formaldehyde fixation and analyzed by flow cytometry. Alternatively, phagocytosis mixtures were cytospinned on glass slides and stained using Giemsa-based Diff-Quick solution. To analyze killing, phagocytosis mixtures were not fixed but incubated for an additional 90 minutes before they were diluted into ice-cold water (pH 11) and incubated for 15 min on ice to enable neutrophil lysis. Viable bacteria were quantified by colony enumeration. For Fg supplementation, 5% serum was supplemented with 50–200 µg/ml human or mouse Fg (kindly provided by Dr. Jay L. Degen; purified from plasma of wild type and Fgγ390–396A mice [21]). To analyze the influence of bacterial supernatants on phagocytosis, FITC-labeled S. aureus KV27 (2.5×107 cfu) was pre-incubated with human serum for 30 min at 37°C in Veronal Buffered Saline containing Ca2+ and Mg2+ (VBS++). After washing in VBS++-0.5% BSA, bacteria were incubated with (2-fold) diluted culture supernatants or purified Efb (250 nM) for 1 hour at 37°C. After washing, bacteria were incubated with purified Fg (60 µg/ml, Invitrogen) in RPMI-HSA for 1 hour at 37°C and subsequently, neutrophils were added (7.5×105 cells) and phagocytosis was allowed for 30 min at 37°C.
In vivo phagocytosis
S. aureus strain SH1000 [42] was grown to mid-log phase, heat-inactivated for 60 min at 90°C, and fluorescently labeled with carboxyfluorescein (Molecular Probes, Göttingen, Germany). To induce infiltration of neutrophils within the peritoneal cavity, mice were treated i.p. with 1 mg of carrageenan (Type IV1, Sigma) 4 and 2 days prior to bacterial challenge. Subsequently, mice were injected i.p. with 200 µl of a solution containing 108 heat-inactivated carboxyfluorescein-labeled S. aureus SH1000 and Efb (1 µM). To compare WT and ΔEfb strains, mice were directly inoculated in the peritoneal cavity with 300 µl of GFP-expressing WT or ΔEfb S. aureus cultures grown to a late exponential phase. Mice were sacrificed 1 h thereafter, and their peritoneum was lavaged with sterile PBS. Lavage samples were centrifuged and pelleted cells were incubated with purified anti-CD32 antibodies to block the FcR, followed by PE-conjugated anti-mouse Gr-1 antibodies. Cells were washed and quenched with trypan blue (2 mg/ml). Samples were immediately subjected to flow-cytometric analysis using a FACScan (Becton Dickinson, San Jose, California). Neutrophils were gated according to their expression of Gr-1 antigen (FL2). Phagocytosis was expressed as the percentage of neutrophils that became fluorescent.
Alternative pathway hemolysis assay
Human serum (5%) was incubated with buffer or Efb proteins (1 µM) in HEPES-MgEGTA (20 mM HEPES, 5 mM MgCl2, 10 mM EGTA) for 15 min at RT. Rabbit erythrocytes (Biotrading Benelux B.V.) were added and incubated for 60 min at 37°C. Mixtures were centrifuged and hemolysis was determined by measuring the absorbance of supernatants at 405 nm.
Immunoblotting
To analyze C3b deposition on the bacterial surface, S. aureus strain Wood46 (3×108/ml) was incubated with 5% human plasma in the presence of Efb (0.5 µM), EDTA (5 mM) or buffer (HEPES++; 20 nM HEPES, 5 mM CaCl2, 2.5 mM MgCl2, pH 7.4) for 30 min at 37°C shaking at 1100 rpm. Bacteria were washed twice with PBS-0.1% BSA and boiled in Laemmli sample buffer containing Dithiothreitol. Samples were subjected to SDS-PAGE and subsequently transferred to a nitrocellulose membrane (Millipore). C3b was detected using a peroxidase-labeled polyclonal anti-human C3 antibody (Protos Immunoresearch, Burlingame, USA) and developed using Enhanced Chemiluminescence (ECL, GE). To quantify Efb in bacterial supernatants, His-Efb and supernatants were run together on an SDS-PAGE gel. After transfer, blots were developed using a polyclonal sheep anti-Efb antibody (kindly provided by Prof JI Flock), peroxidase-labeled donkey anti-sheep antibodies (Fluka Analytical) and ECL. Bands were quantified using ImageJ software and linear regression analysis was performed using GraphPad software.
Flow cytometry assays with S. aureus
S. aureus strain Wood46 (3×108/ml) was pre-incubated with human serum for 30 min at 37°C in VBS++ buffer, washed with VBS++-0.5% BSA and incubated with Efb (0.5 µM) or 2-fold diluted culture supernatants for 1 hour at 37°C shaking. After another washing step, bacteria were incubated with Alexa-488 conjugated Fg (60 µg/ml, Invitrogen) for 1 hour at 37°C shaking. Washed bacteria were analyzed by flow cytometry using a FACSCalibur (BD). Bacteria were gated on the basis of forward and side scatter properties and fluorescence of 10,000 bacteria was analyzed. Alternatively, we incubated pre-opsonized bacteria with Efb (0.5 µM) and/or unlabeled Fg (200 µg/ml) for 1 hour at 37°C shaking. Washed bacteria were incubated with soluble rCR1 (10 µg/ml, kindly provided by Prof. Atkinson, Washington University, St. Louis, MO), FITC-labeled F(ab′)2 anti-human C3 antibody (Protos Immunoresearch) or anti-human IgG antibody for 30 min at 37°C. CR1 was detected using PE-labeled anti-CD35 antibodies (BD Pharmingen); the IgG antibody was detected using goat-anti-mouse PE antibodies (BD Pharmingen). Capsule expression on strain Reynolds was analyzed by incubating bacteria with polyclonal anti-CP5 rabbit serum and Phycoerythrin (PE)-conjugated goat anti-rabbit antibody.
Confocal microscopy
Samples were transferred to glass slides and air-dried. Membrane dye FM 5-95 (Invitrogen) was added and slides were covered with a coverslip. Confocal images were obtained using a Leica TCS SP5 inverted microscope equipped with a HCX PL APO 406/0.85 objective (Leica Microsystems, The Netherlands).
Transmission electron microscopy
S. aureus strain Wood 46 (3×108) was incubated with human plasma (10%) in the presence or absence of Efb (0.5 µM) in HEPES++ for 30 min at 37°C, washed once with PBS-1% BSA and adsorbed to 100 mesh hexagonal Formvar-carbon coated copper grids (Stork-Veco, Zoetermeer, NL). Samples were contrasted with 0.4% uranyl acetate (pH 4.0) and 1.8% methylcellulose [43] and analyzed in a JEOL 1010 transmission electron microscope (JEOL Europe, Nieuw Vennep, the Netherlands) at 80 kV.
Statistics
Statistical analyses were performed using GraphPad Prism 4.0 package and the differences between groups were analyzed for significance using the two-tailed Student's t-test.
Accession number
The accession number for Efb in S. aureus Newman is YP_001332103, locus NWMN_1069.
Supporting Information
Zdroje
1. NathanC (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6 : 173–182.
2. GasqueP (2004) Complement: a unique innate immune sensor for danger signals. Mol Immunol 41 : 1089–1098.
3. RicklinD, HajishengallisG, YangK, LambrisJD (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11 : 785–797.
4. GrosP, MilderFJ, JanssenBJC (2008) Complement driven by conformational changes. Nat Rev Immunol 8 : 48–58.
5. WalportMJ (2001) Complement. First of two parts. N Engl J Med 344 : 1058–1066.
6. LowyF (1998) Staphylococcus aureus infections. N Engl J Med 339 (8) 520–32.
7. LiM, DiepBA, VillaruzAE, BraughtonKR, JiangX, et al. (2009) Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proceedings of the National Academy of Sciences 106 : 5883–5888.
8. SchafferAC, LeeJC (2008) Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents 32 Suppl 1: S71–S78.
9. DeDentA, KimHK, MissiakasD, SchneewindO (2012) Exploring Staphylococcus aureus pathways to disease for vaccine development. Semin Immunopathol 34 : 317–333.
10. PalmaM, NozohoorS, SchenningsT, HeimdahlA, FlockJI (1996) Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infection and Immunity 64 : 5284–5289.
11. SchenningsT, FarneboF, SzekelyL, FlockJ-I (2012) Protective immunization against Staphylococcus aureus infection in a novel experimental wound model in mice. APMIS 120 : 786–793.
12. JongeriusI, KöhlJ, PandeyMK, RuykenM, van KesselKPM, et al. (2007) Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med 204 : 2461–2471.
13. KoY-P, LiangX, SmithCW, DegenJL, HöökM (2011) Binding of Efb from Staphylococcus aureus to fibrinogen blocks neutrophil adherence. Journal of Biological Chemistry 286 : 9865–9874.
14. HammelM, SfyroeraG, RicklinD, MagottiP, LambrisJD, et al. (2007) A structural basis for complement inhibition by Staphylococcus aureus. Nat Immunol 8 : 430–437.
15. LeeLYL, HöökM, HavilandD, WetselRA, YonterEO, et al. (2004) Inhibition of complement activation by a secreted Staphylococcus aureus protein. J Infect Dis 190 : 571–579.
16. PalmaM, ShannonO, QuezadaHC, BergA, FlockJ-I (2001) Extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus blocks platelet aggregation due to its binding to the alpha-chain. J Biol Chem 276 : 31691–31697.
17. ShannonO, FlockJ-I (2004) Extracellular fibrinogen binding protein, Efb, from Staphylococcus aureus binds to platelets and inhibits platelet aggregation. Thromb Haemost 91 (4) 779–89.
18. KochTK, ReuterM, BarthelD, BöhmS, van den ElsenJ, et al. (2012) Staphylococcus aureus proteins Sbi and Efb recruit human plasmin to degrade complement C3 and C3b. PLoS ONE 7 (10) e47638.
19. PalmaM, WadeD, FlockM, FlockJ-I (1998) Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J Biol Chem 273 : 13177–13181.
20. PlowEF, HaasTA, ZhangL, LoftusJ, SmithJW (2000) Ligand binding to integrins. J Biol Chem 275 : 21785–21788.
21. FlickMJ, DuX, WitteDP, JirouskováM, SolovievDA, et al. (2004) Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest 113 : 1596–1606.
22. ThakkerM, ParkJS, CareyV, LeeJC (1998) Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infection and Immunity 66 : 5183–5189.
23. JongeriusI, Köckritz-Blickwede vonM, HorsburghMJ, RuykenM, NizetV, et al. (2012) Staphylococcus aureus Virulence Is Enhanced by Secreted Factors That Block Innate Immune Defenses. Journal of Innate Immunity 4 (3) 301–311.
24. PantrangiM, SinghVK, WolzC, ShuklaSK (2010) Staphylococcal superantigen-like genes, ssl5 and ssl8, are positively regulated by Sae and negatively by Agr in the Newman strain. FEMS Microbiology Letters 308 : 175–184.
25. VoyichJM, VuongC, (null), NygaardTK, (null), et al. (2009) The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 199 : 1698–1706.
26. LoofTG, MorgelinM, JohanssonL, OehmckeS, OlinAI, et al. (2011) Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood 118 : 2589–2598.
27. FrickI-M, AkessonP, HerwaldH, MörgelinM, MalmstenM, et al. (2006) The contact system–a novel branch of innate immunity generating antibacterial peptides. EMBO J 25 : 5569–5578.
28. MattssonE, HerwaldH, CramerH, PerssonK, SjöbringU, et al. (2001) Staphylococcus aureus induces release of bradykinin in human plasma. Infection and Immunity 69 : 3877–3882.
29. NordenfeltP, WaldemarsonS, LinderA, MorgelinM, KarlssonC, et al. (2012) Antibody orientation at bacterial surfaces is related to invasive infection. Journal of Experimental Medicine 209 (13) 2367–81.
30. McCarthyAJ, LindsayJA (2010) Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol 10 : 173.
31. McAdowM, MissiakasDM, SchneewindO (2012) Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections. Journal of Innate Immunity 4 : 141–148.
32. ChengAG, McAdowM, KimHK, BaeT, MissiakasDM, et al. (2010) Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog 6: e1001036.
33. NygaardTK, PallisterKB, RuzevichP, GriffithS, VuongC, et al. (2010) SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis 201 : 241–254.
34. ChavakisT, WiechmannK, PreissnerKT, HerrmannM (2005) Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb Haemost 94 : 278–285.
35. ChengAG, KimHK, BurtsML, KrauszT, SchneewindO, et al. (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. The FASEB Journal 23 : 3393–3404.
36. GuggenbergerC, WolzC, MorrisseyJA, HeesemannJ (2012) Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLoS Pathog 8: e1002434.
37. PangYY, SchwartzJ, ThoendelM, AckermannLW, HorswillAR, et al. (2010) agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. Journal of Innate Immunity 2 : 546–559.
38. RooijakkersSHM, WuJ, RuykenM, van DomselaarR, PlankenKL, et al. (2009) Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol 10 : 721–727.
39. EverseSJ, PelletierH, DoolittleRF (1995) Crystallization of fragment D from human fibrinogen. Protein Sci 4 : 1013–1016.
40. MollnesTE, BrekkeO-L, FungM, FureH, ChristiansenD, et al. (2002) Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100 : 1869–1877.
41. BestebroerJ, PoppelierMJJG, UlfmanLH, LentingPJ, DenisCV, et al. (2007) Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109 : 2936–2943.
42. HorsburghMJ, AishJL, WhiteIJ, ShawL, LithgowJK, et al. (2002) sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184 : 5457–5467.
43. SlotJW, GeuzeHJ (2007) Cryosectioning and immunolabeling. Nat Protoc 2 : 2480–2491.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2013 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



