-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells
The kinetochore (centromeric DNA and associated proteins) is a key determinant for high fidelity chromosome transmission. Evolutionarily conserved Scm3p is an essential component of centromeric chromatin and is required for assembly and function of kinetochores in humans, fission yeast, and budding yeast. Overexpression of HJURP, the mammalian homolog of budding yeast Scm3p, has been observed in lung and breast cancers and is associated with poor prognosis; however, the physiological relevance of these observations is not well understood. We overexpressed SCM3 and HJURP in Saccharomyces cerevisiae and HJURP in human cells and defined domains within Scm3p that mediate its chromosome loss phenotype. Our results showed that the overexpression of SCM3 (GALSCM3) or HJURP (GALHJURP) caused chromosome loss in a wild-type yeast strain, and overexpression of HJURP led to mitotic defects in human cells. GALSCM3 resulted in reduced viability in kinetochore mutants, premature separation of sister chromatids, and reduction in Cse4p and histone H4 at centromeres. Overexpression of CSE4 or histone H4 suppressed chromosome loss and restored levels of Cse4p at centromeres in GALSCM3 strains. Using mutant alleles of scm3, we identified a domain in the N-terminus of Scm3p that mediates its interaction with CEN DNA and determined that the chromosome loss phenotype of GALSCM3 is due to centromeric association of Scm3p devoid of Cse4p/H4. Furthermore, we determined that similar to other systems the centromeric association of Scm3p is cell cycle regulated. Our results show that altered stoichiometry of Scm3p/HJURP, Cse4p, and histone H4 lead to defects in chromosome segregation. We conclude that stringent regulation of HJURP and SCM3 expression are critical for genome stability.
Published in the journal: Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells. PLoS Genet 7(9): e32767. doi:10.1371/journal.pgen.1002303
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002303Summary
The kinetochore (centromeric DNA and associated proteins) is a key determinant for high fidelity chromosome transmission. Evolutionarily conserved Scm3p is an essential component of centromeric chromatin and is required for assembly and function of kinetochores in humans, fission yeast, and budding yeast. Overexpression of HJURP, the mammalian homolog of budding yeast Scm3p, has been observed in lung and breast cancers and is associated with poor prognosis; however, the physiological relevance of these observations is not well understood. We overexpressed SCM3 and HJURP in Saccharomyces cerevisiae and HJURP in human cells and defined domains within Scm3p that mediate its chromosome loss phenotype. Our results showed that the overexpression of SCM3 (GALSCM3) or HJURP (GALHJURP) caused chromosome loss in a wild-type yeast strain, and overexpression of HJURP led to mitotic defects in human cells. GALSCM3 resulted in reduced viability in kinetochore mutants, premature separation of sister chromatids, and reduction in Cse4p and histone H4 at centromeres. Overexpression of CSE4 or histone H4 suppressed chromosome loss and restored levels of Cse4p at centromeres in GALSCM3 strains. Using mutant alleles of scm3, we identified a domain in the N-terminus of Scm3p that mediates its interaction with CEN DNA and determined that the chromosome loss phenotype of GALSCM3 is due to centromeric association of Scm3p devoid of Cse4p/H4. Furthermore, we determined that similar to other systems the centromeric association of Scm3p is cell cycle regulated. Our results show that altered stoichiometry of Scm3p/HJURP, Cse4p, and histone H4 lead to defects in chromosome segregation. We conclude that stringent regulation of HJURP and SCM3 expression are critical for genome stability.
Introduction
Proper chromosome segregation is essential for normal cell proliferation. Errors in this process lead to birth defects, developmental disorders, aneuploidy and possibly cancer [1]. The kinetochore (centromeric DNA and associated proteins), microtubules, spindle pole bodies, condensins, and telomeres, as well as regulatory components that establish checkpoints [2] are essential for faithful chromosome segregation. The centromere (CEN) is the cis-acting DNA locus that specifies the site of kinetochore assembly, participates in the attachment of chromosomes to the mitotic and meiotic spindles and maintains cohesion between sister chromatids [3], [4]. CEN DNA sequences are highly variable among eukaryotes. Budding yeast contains “point” centromeres, whereas other eukaryotes, for example fission yeast, fruit fly, plants and mammals have “regional” centromeres. The point centromeres are small (∼125 bp in size) and consist of three conserved DNA elements (CDEI, CDEII, and CDEIII), whereas regional centromeres are relatively large in size (40–4000 kb) and contain species-specific arrays of repeated DNA [5]–[7]. Despite CEN DNA sequence variation, replacement of histone H3 in centromeric chromatin by the centromere-specific histone H3 variant CenH3 is universally conserved [6]. CenH3 homologs (Cse4p in budding yeast, Cnp1 in fission yeast, CID in fruit fly, HTR12 in Arabidopsis and CENP-A in humans) function as an epigenetic mark in all organisms and is essential for determining centromere identity and proper kinetochore function [8]–[14]. In budding yeast, kinetochore sub-complexes Ctf3p (Ctf3p, Mcm16p and Mcm22p) and COMA (Ame1p, Ctf19p, Mcm21p and Okp1p) exhibit genetic and physical interactions with Cse4p [15], [16].
It has been shown that the point centromeres of Saccharomyces cerevisiae consist of a single Cse4p nucleosome [17], and a novel inner kinetochore protein Scm3p (Suppressor of Chromosome Missegregation) is required for the centromeric deposition of Cse4p [18]–[23]. Scm3p is evolutionarily conserved with homologs identified in a range of species including fission yeast (Sp-Scm3p), fungi, humans (HJURP, Holliday Junction Recognition Protein), and other vertebrates [24], [25]. HJURP and Scm3p share a common ancestry and both proteins have an evolutionarily conserved N-terminal domain [24]. This evolutionary conserved domain of HJURP directly interacts with the centromere targeting domain (CATD) of CENP-A [25]–[27]. In budding yeast, depletion of Scm3p leads to cell cycle arrest and chromosome segregation defects [18]–[20], [28], [29]. Studies from fission yeast have shown that Sp-Scm3p functions as a Cnp1p receptor and facilitates its assembly into centromeric chromatin [30], [31]. Sp-Scm3p shows cell cycle dependent centromeric enrichment, and it dissociates from centromeres in early mitosis [30], [31]. Similarly, studies with human cells have shown that centromeric association of HJURP is cell cycle regulated and HJURP promotes the deposition and maintenance of CENP-A at centromeres [26], [32], [33].
Previous studies have shown that balanced stoichiometry of histones and CenH3 is important for chromosome transmission fidelity. For example, maintenance of a balanced ratio between Cse4p/Cnp1, histone H4 or histone H3 affects centromere function in fission and budding yeast, and altered dosage of these proteins results in chromosome missegregation [34], [35]. In other systems such as, fruit fly, CID overexpression results in its mislocalization, formation of ectopic kinetochores and aneuploidy [36]. Altered expression and mis-localization of CENP-A has been observed in colorectal tumors [37]. Also, altered dosage and mis-localization of kinetochore proteins, such as CENP-H, Aurora-B and INCENP has been observed in various types of tumor cells [37]–[39]. As observed for CENP-A, the expression of its chaperone, HJURP is also tightly regulated and this is critical for high fidelity chromosome transmission, because perturbation of its expression leads to mitotic defects [32], [38]. Overexpression and mis-localization of HJURP has been observed in lung cancer cell lines and these observations were linked with chromosomal instability and immortality of cancer cells [38]. Notably, overexpression of HJURP has also been observed in breast cancer cells, where patients with elevated HJURP mRNA levels showed decreased survival rate and were more sensitive to radiotherapy [39]. However, the physiological basis for these observations is not well understood, nor is it known if HJURP overexpression plays a direct role in cancer initiation or progression. Considering the structural and functional homology between SCM3 and HJURP, we used budding yeast to investigate the physiological consequences of SCM3/HJURP overexpression and understand how missregulation of these proteins affects chromosome segregation.
Here, we show that overexpression of SCM3 leads to defects in chromosome transmission fidelity in a wild-type strain, reduced viability in kinetochore mutants, premature separation of sister chromatids, perturbation of its centromeric enrichment pattern and reduced levels of Cse4p at centromeres. Overexpression of CSE4 (GALCSE4) or histone H4 (GALH4) suppressed the GALSCM3-induced chromosome loss and restored levels of Cse4p at centromeres. Our results revealed that the N-terminus of Scm3p (first 100 amino acids) mediates its centromeric interaction and established that Scm3p can associate with centromeres independent of Cse4p. Using mutant alleles of scm3, we determined that the kinetochore integrity defects of GALSCM3 strains are due to centromeric association of Scm3p devoid of Cse4p. Our results show that overexpression of HJURP in budding yeast, and in human cells leads to chromosome loss.
Results
Misregulation of SCM3 leads to defects in chromosome transmission fidelity (ctf) in wild-type strains and reduced levels of Cse4p at CEN DNA
To examine the physiological consequence of misregulation of SCM3, we assayed the chromosome loss phenotype of wild-type strains overexpressing SCM3 from a GAL1 promoter on a multi-copy plasmid. Western blot analysis confirmed the galactose-induced overexpression of GALSCM3HA (Figure 1A). The loss of a non-essential reporter chromosome fragment (CF) in this strain background results in red sectors in an otherwise white colony. We determined the chromosome loss rate by counting the number of colonies that were at least half red on galactose media reflecting the loss of CF in the first cell division (Figure 1B, see arrows). Overexpression of SCM3 results in a 10-fold higher chromosome loss rate compared to strains expressing vector alone (Figure 1C). The chromosome loss phenotype of the control vector alone strain is similar to that previously reported for wild-type strains on galactose media [35]. Strains expressing untagged GALSCM3 or GALSCM3HA integrated into the genome had chromosome loss rates similar to that of plasmid-borne GALSCM3HA strains (Figure S1). We previously reported that overexpression of histone H3 (GALH3) alters the stoichiometry between Cse4p and histone H3 leading to defective kinetochores and a chromosome loss phenotype in wild type yeast strains [35]. The chromosome loss phenotype of strains co-expressing GALSCM3 and GALH3 is higher but not statistically different than those expressing GALSCM3 or GALH3 alone (Figure 1C).
Fig. 1. Overexpression of SCM3 causes chromosome instability and reduction in CEN-bound Cse4p and histone H4. 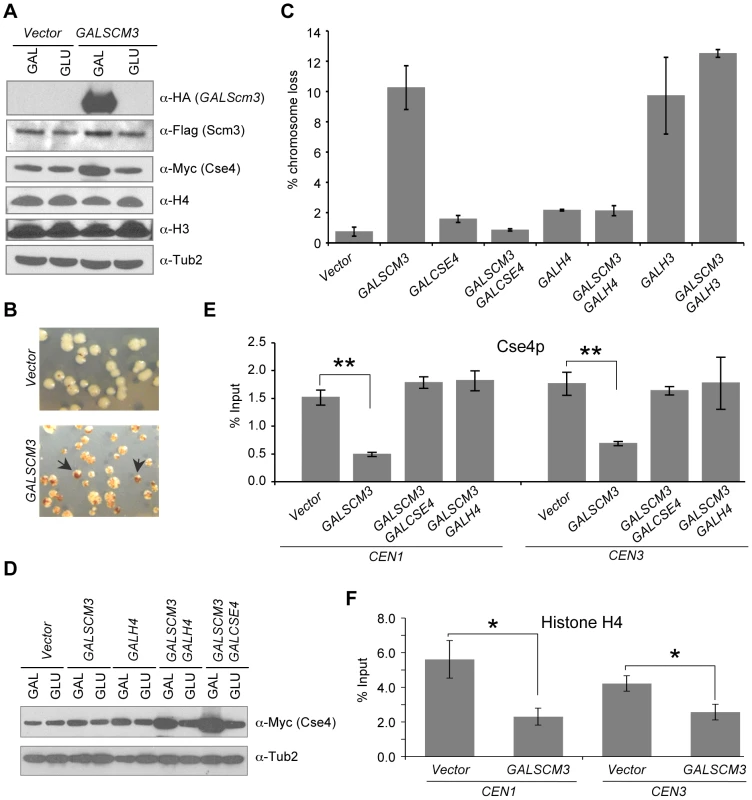
(A) Western blot analysis was done using whole cell protein extracts prepared from wild type strains (RC154 or JG595) transformed with GALSCM3HA (pMB1306), or vector (pRS426 GAL1). Strains were grown to logarithmic phase of growth in synthetic media and gene expression induced in the presence of galactose (2%) at 30°C for 12 hours. Blots were probed with anti-HA for expression of GALSCM3HA, anti-FLAG for expression of FLAG tagged Scm3p expressed from its own promoter, anti-Myc for expression of Cse4p, anti-H3 (abcam # ab1791-100) for expression of histone H3, anti-H4 (Millipore # 62-141-13) for expression of histone H4 and α-Tub2p served as a loading control. (B) Wild type strains with reporter chromosome fragment (YPH1018) expressing SCM3 from GAL1 promoter (pMB1306) or vector (pRS426 GAL1) were plated on SC-URA with limiting adenine and galactose (2%). Representative images showing red sectors in white colonies represent the loss of the non-essential chromosome fragment (CF). Arrows indicate colonies that show a half red-half white phenotype which represent CF loss in the first cell division. (C) GALSCM3-induces chromosome missegregation, which is suppressed by GALCSE4 and GALH4. Quantification of chromosome loss by half-sector analysis. Reporter strains with chromosome fragment (YPH1018) transformed with vectors (pRS425 GAL1, pRS426 GAL1), GALSCM3HA (pMB1306), GALCSE4 (pMB1147), GALSCM3HA, GALCSE4 (pMB1306, pMB1147), GALH4 (pMB1158), GALSCM3HA, GALH4 (pMB1306, pMB1158), GALH3 (pMB1159), and GALSCM3HA, GALH3 (pMB1306, pMB1159) were assayed for chromosome loss. At least 3000 colonies were counted and values represent the average ± standard error for three independent transformants normalized to the value of 100. (D) Co-overexpression of histone H4 and SCM3 increases the levels of Cse4p. Western blot analysis was done using whole cell protein extracts prepared from a wild type strain (JG595) transformed with vector (pRS425 GAL1), GALSCM3 (pMB1193), GALH4 (pMB1158), GALSCM3, GALH4 (pMB1193, pMB1158), and GALSCM3, GALCSE4 (pMB1193, pMB976). Strains were grown to logarithmic phase of growth in media selective for the plasmid and gene expression induced in the presence of galactose (2%) at 30°C for 12 hours. Blots were probed with anti-Myc for expression of Myc tagged Cse4p expressed from its own promoter and α-Tub2p served as a loading control. (E) GALSCM3 strains show reduced levels of CEN-associated Cse4p. ChIP experiments were done using wild type strain expressing Cse4p-Myc from its endogenous promoter (YMB6094) with vectors (pRS425 GAL1, pRS426 GAL1), GALSCM3HA (pMB1306), GALSCM3HA, GALCSE4 (pMB1306, pMB976), and GALSCM3HA, GALH4 (pMB1306, pMB1158) grown in galactose (2%) media for 12 hours and immunoprecipitation were done with α-Myc, and α-GST (mock) antibodies. Enrichment of Cse4p to CEN1 and CEN3 DNA was determined by qPCR and is shown as % input. Average from at least three independent experiments ± standard error is shown. *p value <0.05, **p value <0.01, Student's t test. (F) GALSCM3 strains show reduced levels of CEN-associated histone H4. ChIP experiments were done using wild type strain (RC154) with vectors (pRS426 GAL1), or GALSCM3HA (pMB1306) grown in galactose (2%) media for 12 hours and immunoprecipitation were done with α-H4, and α-GST (mock) antibodies. Enrichment of histone H4 to CEN1 and CEN3 DNA was determined by qPCR and is shown as % input. Average from at least three independent experiments ± standard error is shown. *p value <0.05, **p value <0.01, Student's t test. Scm3p directly binds and forms a stoichiometric complex with Cse4p and histone H4, and is required for their assembly into centromeric chromatin [18]–[20]. Similarly, HJURP interacts stoichiometrically with CENP-A/H4 [26], [27]. We reasoned that the chromosome loss phenotype of GALSCM3 strains may be due to imbalanced stoichiometry between Scm3p and Cse4p/H4. Hence, we determined the effect of overexpression of CSE4 in GALSCM3 strains. Our results showed that GALCSE4 suppressed the chromosome loss phenotype of GALSCM3 strains (Figure 1C). Previous studies have shown that overexpression of histone H4 (GALH4) suppresses certain cse4 alleles [40]. Consistent with these observations, we found that GALH4 (GALHHF1) suppressed the chromosome loss phenotype of GALSCM3 strains (Figure 1C). We performed western blot analysis to determine if overexpression of Scm3p affects the levels of Cse4p, Histone H3 or Histone H4. We found that GALSCM3 strains show slightly higher levels of Cse4p, whereas levels of histone H3 or H4 are not affected in these strains (Figure 1A). We also observed higher levels of Cse4p in strains co-expressing GALSCM3 with either GALH4 or GALCSE4 when compared to strains expressing GALSCM3 or GALH4 alone (Figure 1D). The increased levels of Cse4p in GALSCM3 strains are consistent with a recent report describing a role for Scm3p in protecting Cse4p from ubiquitin-mediated degradation [41]. The increased levels of Cse4p in GALH4 strains can be explained by previous observations for genetic interactions between Cse4p and histone H4 [40].
Based on the suppression of GALSCM3-induced chromosome loss phenotype by GALCSE4 and GALH4, we hypothesized that excess Scm3p devoid of Cse4p/H4 may associate with CEN DNA. Hence, we performed chromatin immunoprecipitation (ChIP) experiments to measure the levels of CEN-bound Cse4p in SCM3 overexpressing (GALSCM3) and vector strains. Consistent with our hypothesis, we observed that GALSCM3 strains showed about 3-fold reduction in the levels of Cse4p at both CEN1 (0.54% of input) and CEN3 (0.44%) compared to vector alone (1.50% at CEN1, and 1.48% at CEN3) (Figure 1E). CEN-bound H4 was also reduced in strains overexpressing SCM3. ChIP results showed a 2 - to 3-fold reduction in CEN-bound histone H4 in GALSCM3 (2.30% at CEN1, and 2.57% at CEN3) than vector alone (5.61% at CEN1, and 4.22% at CEN3) strains (Figure 1F). Western blot analysis showed that the reduction in CEN-bound Cse4p or histone H4 in GALSCM3 strains was not due to reduced expression of Cse4p or histone H4 (Figure 1A). Furthermore, CEN-associated Cse4p in the GALSCM3 strain was restored to wild-type levels in the presence of GALCSE4 or GALH4 (Figure 1E). These results indicate that centromeric association of excess Scm3p devoid of Cse4p/H4 contributes to the chromosome loss phenotype in GALSCM3 strains.
In order to determine if the chromosome loss phenotype of GALSCM3 strains may be due to mislocalization of Scm3p and/or Cse4p to non-centromeric regions, we performed ChIP experiments using strains expressing SCM3 from its native promoter (control) or a GAL1 promoter. We examined the association of Scm3p and Cse4p at CEN DNA, AT rich intergenic regions (IR1, IR2, IR3, and IR4), and transcribed regions (AGP1, CWH43, ACT1, and YGL036W). The choice of the intergenic regions was based on the rationale that CDEII of CEN DNA is also AT rich and we have previously shown association of a mutant form of Cse4p (Cse4K16R) when overexpressed at two of these regions (IR1, IR2) [35]. Our results showed higher levels of GALSCM3 at the CEN3 (∼1.5-fold) compared to SCM3 expressed from its own promoter (Figure S2A). Also, consistent with earlier results (Figure 1E), we observed lower levels of Cse4p at CEN3 in GALSCM3 strains (Figure S2B). We did not see association of either Scm3p or Cse4p at the intergenic (IR1, IR2, IR3, and IR4) or transcribed (AGP1, CWH43, ACT1, and YGL036W) regions in the control or GALSCM3 strains (Figure S2A, S2B). Based on these results, we conclude that the chromosome loss phenotype of GALSCM3 strains is not due to non-centromeric association of Scm3p and Cse4p.
Overexpression of SCM3 alters its cell cycle–dependent centromeric association pattern
Fission yeast Scm3 (Sp-Scm3p) is cell cycle regulated, with Sp-Scm3p dissociating from centromeres in early mitosis [30], [31]. In humans, HJURP also exhibits cell cycle-regulated CEN localization, showing strong enrichment in G1 and depletion during G2 and mitosis [26], [32]. However, in budding yeast, centromeric localization pattern of Scm3p through the cell cycle has not been determined. Hence, we used ChIP to determine the centromeric localization pattern of FLAG-Scm3p expressed from its native promoter in three independent cell cycle synchronization experiments. In the first experiment, we did ChIP analysis using chromatin from cultures grown in yeast extract-peptone medium with 2% glucose (YPD), synchronized in G1 with alpha factor and released into pheromone free media (Figure 2). Based on nuclear and cell morphology, cells were categorized as G1, S, M (metaphase), A (anaphase) and T (telophase) as described in Materials and Methods [42]. Our results showed that centromeric association of Scm3p is lower in cells released from alpha factor arrest (15 min), maximal in S phase cells (45–60 min), reduced in early mitotic cells (90 min), and enriched in anaphase cells (105 min) (Figure 2). We did not detect Scm3p enrichment at ACT1 used as a background control (Figure 2C). These results show that centromeric association of Scm3p is cell cycle regulated. We validated these results in two additional experiments using cells arrested with: a) alpha factor and released into medium containing nocodazole (Figure S3A), or b) nocodazole and released into medium containing alpha factor (Figure S3B). Similar to results in Figure 2, we observed a cell cycle regulated pattern of CEN association of Scm3p with lower levels in cells released from alpha factor (15–30 min, Figure S3A), maximal in S phase cells (45 min, Figure S3A), reduction in early mitotic cells (75 to 105 min, Figure S3A; and 0 min, Figure S3B) followed by enrichment in anaphase cells (20–30 min, Figure S3B). Western blot analysis showed that expression of Scm3p is not cell cycle regulated (Figure S4). We conclude that the mitotic depletion of centromeric Scm3p in budding yeast is largely similar to that of fission yeast where Sp-Scm3p and to humans where HJURP show a transient depletion at the CEN during mitosis [26], [30]–[32].
Fig. 2. Centromeric association of Scm3p is cell cycle regulated. 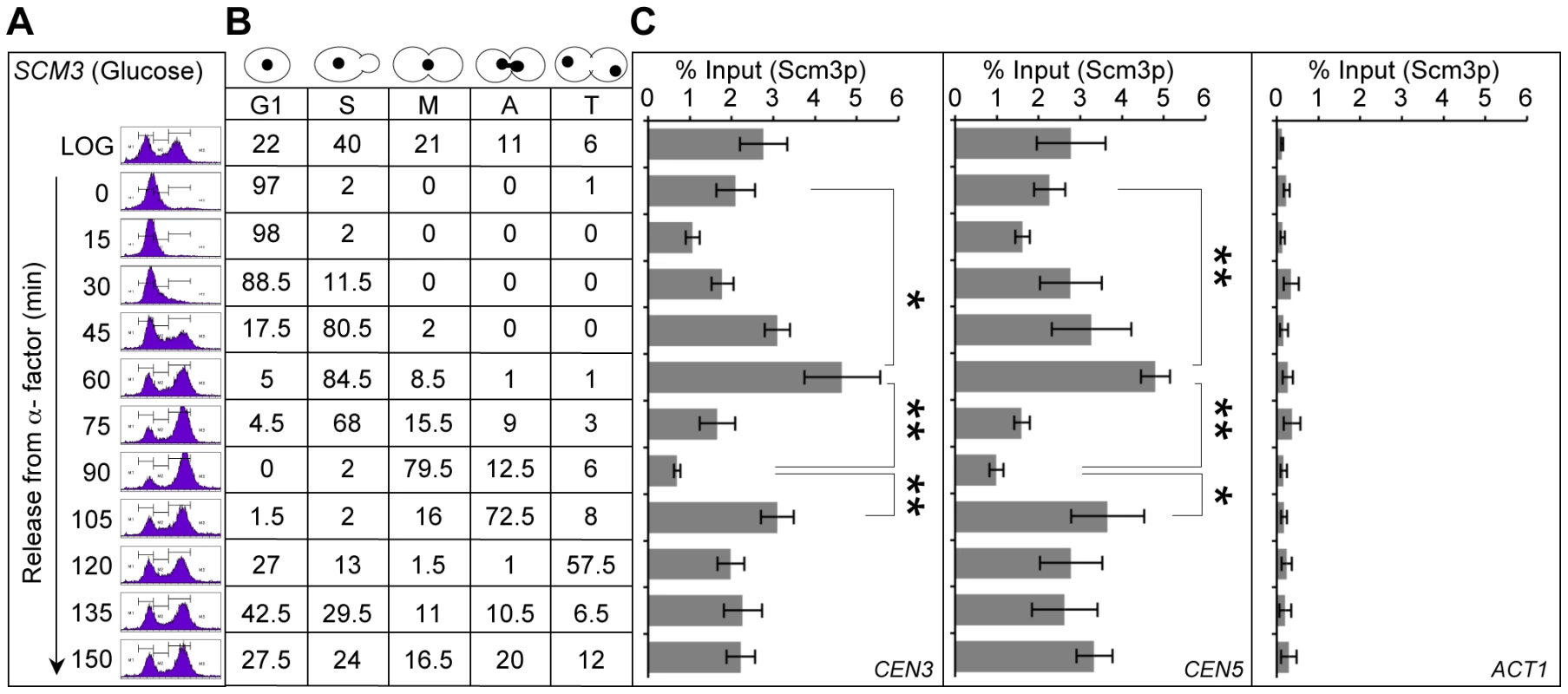
A wild-type strain (RC154) with FLAG tagged Scm3p expressed from its own endogenous promoter was grown in YPD, synchronized in G1 with α-factor, washed, and released into pheromone-free YPD medium. Samples were taken at time points (min) after release from G1. (A) DNA content was determined by FACS. (B) Cell cycle stages were determined based on cell morphology and nuclear position by microscopic examination of 200 cells for each time point as described in Materials and Methods. (C) Enrichment levels of Scm3p at CEN3, CEN5 and ACT1 (background control). ChIP experiments were done using α-FLAG (Scm3p), and α-GST (mock) antibodies. The enrichment of Scm3p at CEN3, CEN5, and ACT1 was determined by qPCR and is shown as % input. Average from at least three independent experiments ± standard error is shown. *p value <0.05, **p value <0.01 derived from the Student's t test. In order to rule out the possibility that our observations for Scm3p may be due to a general of effect of the cell cycle on association of kinetochore proteins to the centromeres, we examined the association of Cse4p at CEN DNA through the cell cycle (Figure S5). We observed lower levels of Cse4p in cells released from alpha factor (15 min), higher levels in S phase cells (45 min) and unlike Scm3p, the levels of Cse4p at CEN DNA did not show transient depletion in early mitotic cells (75–90 min). The centromeric enrichment of Cse4p in S phase cells is consistent with fluorescence microscopy results demonstrating more intense Cse4-GFP foci in S phase cells [43]. The reduced levels of CEN-bound Cse4p at 60 min time point may be due to cells in late S phase and those exiting S phase (Figure S5).
Next, we determined if overexpression of Scm3p affects its cell cycle regulated centromeric association pattern. Thus, we compared the centromeric association pattern of FLAG-Scm3p expressed from its native promoter (control strain SCM3, Figure 3A) to the HA-Scm3p expressed from a GAL1 promoter (GALSCM3, Figure 3B). Cultures were grown in synthetic media with 2% galactose, synchronized in G1 with alpha factor and released into pheromone free media. Centromeric enrichment pattern of Scm3p in S phase (90 min) and anaphase (340 min) is similar in strains expressing SCM3 either from its native (control, Figure 3A) or GAL1 (GALSCM3, Figure 3B) promoter and the difference observed for these time points is not statistically significant (p-value >0.05, Figure 3C). However, we observed a statistically significant difference (p-value = 0.012, Figure 3C) in the level of centromeric Scm3p in early mitotic cells (300 minute, Figure 3A, 3B) between the control (SCM3) and GALSCM3 strains. We confirmed the latter observations in an additional cell cycle experiment in which GALSCM3 strain was synchronized in G2/M with nocodazole and released into alpha factor (Figure S6). We did not observe a depletion of Scm3p in GALSCM3 strains at CEN DNA in G2/M cells (0 min, Figure S6). We conclude that the mitotic depletion of Scm3p at CEN is affected in GALSCM3 strains.
Fig. 3. Overexpression of SCM3 alters its cell cycle–regulated centromeric association pattern. 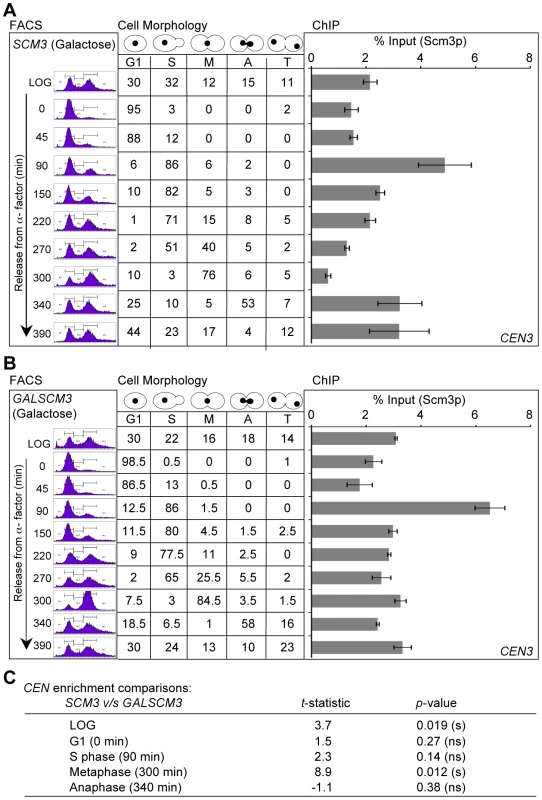
(A) Wild-type strain expressing FLAG-tagged Scm3p (SCM3) from its own endogenous promoter (RC154) were grown in minimal media with galactose (2%) to logarithmic phase, treated with α-factor for G1 arrest, washed, and released into pheromone-free media. Samples were taken at time points (min) after release from G1 arrest. DNA content (FACS), Cell cycle stages based on cell shape and nuclear position (cell morphology), and levels of Scm3p at CEN DNA (ChIP) were determined as described in Figure 2. (B) Wild-type strain (RC100) overexpressing HA-tagged Scm3p from GAL1 promoter (GALSCM3) was grown in minimal media with galactose (2%), synchronized in G1 with α-factor, washed, and released into pheromone-free media. Samples were taken at time points (min) after release from G1. DNA content (FACS), cell cycle stages (cell morphology) and the levels of Scm3p at CEN DNA (ChIP) were determined as described in (A) above. (C) Statistical comparisons of CEN-bound Scm3p levels at different cell cycle stages between strains expressing Scm3p from endogenous (panel A above) or GAL1 promoter (panel B above). Significance was determined using Student's t-test. The t-statistic and p-values are shown: significant (s), not significant (ns). Overexpression of SCM3 (GALSCM3) reduces viability in a subset of kinetochore mutants and leads to premature separation of sister chromatids
The chromosome loss phenotype of GALSCM3 strains may be due to compromised kinetochore function and hence, we examined genetic interactions between GALSCM3 and genes encoding other kinetochore components, specifically subunits of the COMA and Ctf3p complexes. These proteins interact with Cse4p and are important for maintenance of kinetochore integrity [15], [16]. GALSCM3 showed reduced viability in a subset of the kinetochore mutants, with the most severe phenotype in the mcm21Δ strain (Figure 4A). Deletion of MCM21 is known to cause defects in pericentromeric cohesion, leading to precocious separation of sister chromatids and premature initiation of anaphase [44]. Since GALSCM3 showed the most severe phenotype in mcm21Δ strains, we examined if GALSCM3 exacerbated the sister chromatid cohesion defect of mcm21Δ strains. We monitored the segregation of sister chromatids in metaphase cells by assaying the binding of GFP-LacI to operator sequences inserted 12-kb from the centromere (pericentromere) on Chromosome IV [44]. Wild-type strains arrested in metaphase show a predominance of cells with a single GFP-LacI focus marking two closely associated sister chromatids, whereas premature separation of sister chromatids results in the appearance of two GFP-LacI foci [44]. Consistent with previous findings, we observed a low incidence of two GFP-LacI foci in wild-type cells (5%) compared to that in mcm21Δ cells (26%) (Figure 4B, 4C). Strains overexpressing SCM3 showed a higher incidence of two GFP-LacI foci in wild-type (16%) as well as mcm21Δ strains (39%) (Figure 4B, 4C) suggesting that overexpression of SCM3 enhances premature separation of sister chromatids. The higher incidence of two GFP-LacI foci was observed in mitotic cells but not in G1-arrested cells (Figure 4B). mcm21Δ cells depend on the IPL1 biorientation checkpoint for viability [44]. Similarly, we found that GALSCM3 leads to growth defects in the ipl1-321 strain but not in wild-type strains (Figure 4D). Together, these results show that overexpression of SCM3 enhances premature separation of sister chromatids.
Fig. 4. Overexpression of SCM3 causes reduced viability in a subset of kinetochore mutants and premature separation of sister chromatids. 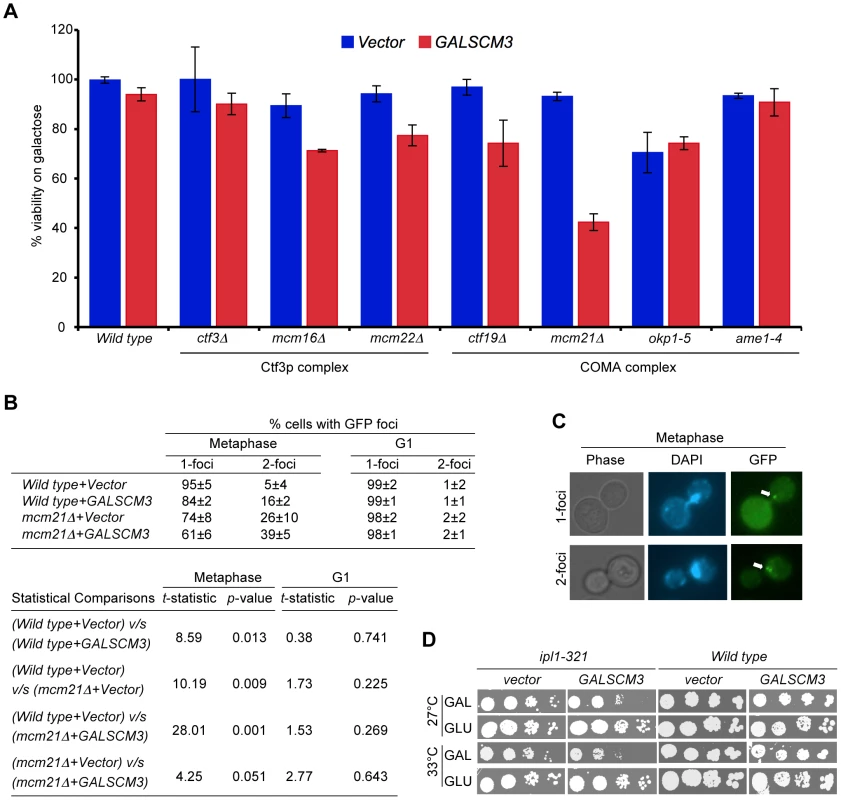
(A) GALSCM3 causes reduced viability in kinetochore mutants. A wild-type strain (Y5563) and kinetochore mutants ctf3Δ (YPH1712), mcm16Δ (YPH1714), mcm22Δ (YPH1716), ctf19Δ (YPH1713), mcm21Δ (YPH1715), okp1-5 (YPH1678), and ame1-4 (YPH1676) were transformed with GALSCM3HA (pMB1306) or vector (pRS426 GAL1). Equal number of cells from three independent transformants for each strain were plated on SC-URA with either glucose (2%) or galactose (2%). At least 2500 colonies were counted and % viability is expressed as the ratio of the number of colonies on galactose over the glucose media. (B) Overexpression of SCM3 leads to premature separation of sister chromatids in metaphase. Sister chromatid separation was monitored in nocodazole-arrested (metaphase) and alpha factor arrested (G1) cells by counting the number of GFP-LacI foci at the marked pericentromere of ChrIV in a wild-type (SBY818) and mcm21Δ (SBY1897) strains overexpressing SCM3 (pMB1306) or a vector (pRS426 GAL1). At least 300 cells were analyzed for each strain. Pair-wise comparisons using Student's t-test were done to determine the statistical significance between samples. (C) Representative images of metaphase cells showing 1- and 2-GFP LacI foci (see, white arrows) are shown. (D) Overexpression of SCM3 causes lethality in ipl1-321 strain. Serial dilutions (5-fold) of ipl1-321 (SBY630) and wild type (SBY3) strains containing GALSCM3HA (pMB1306) or a vector (pRS426 GAL1) were plated on SC-URA with glucose (2%) or galactose (2%) plates and grown at 27°C and 33°C for 5 days. Scm3p can associate with CEN DNA independently of Cse4p
Our observation that GALSCM3 causes reduction in CEN-bound Cse4p led us to postulate that the chromosome loss phenotype of GALSCM3 may be due to centromeric association of Scm3p devoid of Cse4p. Hence, we determined if CEN-association of Scm3p requires bound Cse4p. Previous studies of Scm3p have defined two essential domains, a nuclear export signal (NES) that presumably mediates export of Scm3p from the nucleus, and a coiled-coil heptad repeat (HR) domain that mediates interaction with Cse4p [19], [20]. Since deletion of NES and HR domains from endogenous SCM3 causes lethality [20], we used galactose inducible forms of mutant scm3 alleles for our analysis. We constructed plasmids expressing scm3nesΔ (amino acid residues 13–24 deleted) and scm3hrΔ (amino acids 101–139 deleted) from GAL1 promoter (Figure 5A). Our results showed that GALscm3nesΔ and GALscm3hrΔ strains exhibit a 6 - and 8-fold higher chromosome loss than GALSCM3 strains (Figure 5B). We reasoned that the chromosome loss phenotype of strains overexpressing scm3nesΔ, which can interact with Cse4p, but not scm3hrΔ, which lacks the Cse4p interacting domain, would be suppressed by GALCSE4 [20]. Indeed, GALCSE4 suppresses the chromosome loss phenotype of GALscm3nesΔ strains but not GALscm3hrΔ strains (Figure 5B). ChIP results showed that both GALscm3nesΔ and GALscm3hrΔ can associate with CEN DNA (Figure 5C). Co-immunoprecipitation experiments verified previous observations that a deletion of the HR domain of Scm3p but not the NES abolishes interaction with Cse4p (Figure 5D) [20]. Since GALscm3hrΔ does not interact with Cse4p and can still bind CEN DNA, we conclude that Scm3p can associate with CEN DNA independent of its interaction with Cse4p.
Fig. 5. Scm3p can associate with CEN DNA independently of Cse4p, and the N-terminus of Scm3p mediates its centromeric association. 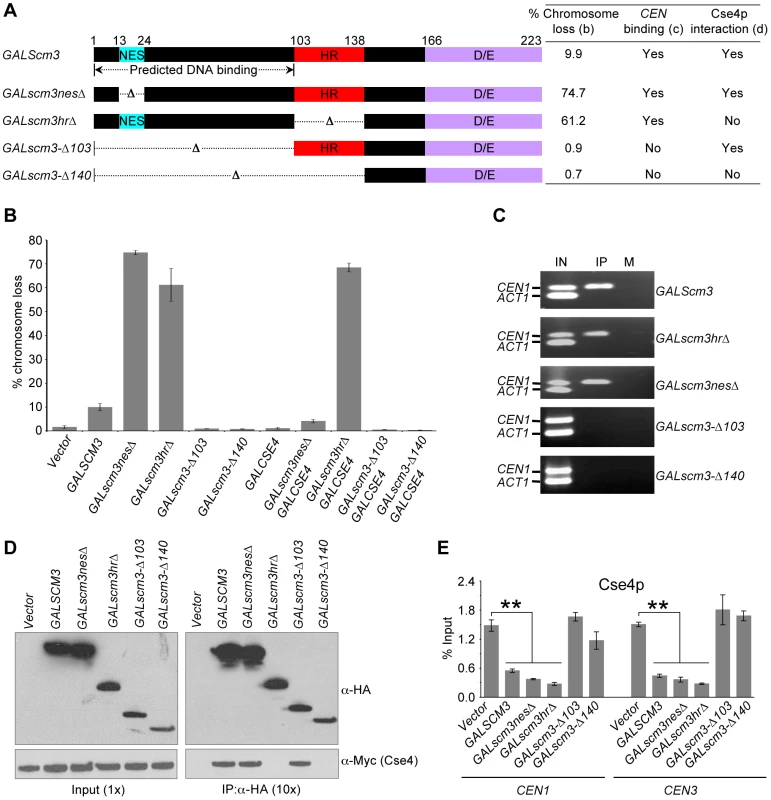
(A) Schematic of full-length Scm3p and its allelic mutant proteins constructed using gene deletion approach. NES: nuclear export signal; HR: heptad repeat; and D/E: C-terminus acidic domain. Results from chromosome loss (B), CEN-binding (C) and Cse4p interactions (D) are summarized in the table. (B) Overexpression of N-terminal mutants (scm3-Δ103HA, scm3-Δ140HA) does not result in increased chromosome loss. Reporter strains with chromosome fragment (YPH1018) transformed with vector (pRS426 GAL1), GALSCM3HA (pMB1306), GALscm3nesΔHA (pMB1393), GALscm3hrΔHA (pMB1455), GALscm3-Δ103HA (pMB1520), GALscm3-Δ140HA (pMB1521), GALCSE4 (pMB1147), GALscm3nesΔHA, GALCSE4 (pMB1393, pMB1147), GALscm3hrΔHA, GALCSE4 (pMB1455, pMB1147), GALscm3-Δ103HA, GALCSE4 (pMB1520, pMB976), GALscm3-Δ140HA, GALCSE4 (pMB1521, pMB976) were assayed for chromosome loss as described in Figure 1 (C). (C) N-terminal mutants of Scm3 (scm3-Δ103HA, scm3-Δ140HA) do not bind CEN DNA. ChIP experiments were done using wild type strain (YMB6398 or JG595) with GALSCM3HA (pMB1306), GALscm3nesΔHA (pMB1393), GALscm3hrΔHA (pMB1455), GALscm3-Δ103HA (pMB1520), GALscm3-Δ140HA (pMB1521) grown in minimal media with galactose (2%) for 12 hours at 30°C and immunoprecipitated with α-HA, and α-GST (mock) antibodies. Enrichment of Scm3p and its allelic mutant forms at CEN1, and ACT1 (internal control) is determined by PCR. Lanes: IN (input), IP (DNA from chromatin immunoprecipitation using α-HA antibodies), and M (DNA from chromatin immunoprecipitation using α-GST antibodies). (D) Western blots of proteins copurifying with Scm3p or its allelic mutant forms. Extracts from cells coexpressing Myc-tagged Cse4p from its native promoter (Cse4-12Myc) and HA-tagged Scm3p or scm3 mutant proteins expressed from GAL1 promoter were used in immunoprecipitation (IP) experiments using anti-HA conjugated agarose beads. Eluted proteins were analyzed by western blotting with anti-HA and anti-Myc antibodies. Ten-fold more protein was loaded for IP samples. (E) Overexpression of Scm3p and its mutant alleles (GALscm3nesΔHA and GALscm3hrΔHA) cause reduction in Cse4p at centromeres. ChIP experiments were done using wild type strain expressing Cse4p-Myc from its endogenous promoter (YMB6398 or JG595) with vector (pRS426 GAL1), GALSCM3HA (pMB1306), GALscm3nesΔHA (pMB1393), GALscm3hrΔHA (pMB1455), GALscm3-Δ103HA (pMB1520), GALscm3-Δ140HA (pMB1521) grown in minimal media with galactose (2%) for 12 hours at 30°C and immunoprecipitated with α-Myc, and α-GST (mock) antibodies. Enrichment of Cse4p to CEN1 and CEN3 DNA was determined by qPCR and is shown as % input. Average from at least three independent experiments ± standard error is shown. The N-terminus of Scm3p is required for centromeric association of Scm3p
If the GALSCM3-induced chromosome loss phenotype is due to centromeric association of Scm3p devoid of Cse4p, overexpression of scm3 alleles that do not associate with CEN DNA should not affect chromosome segregation. To identify the putative DNA interacting domain of Scm3p, we used computational analysis using BindN-RF [45] and MEME [46] software. These analyses suggested that the first 100 amino acids of N-terminus of Scm3p exhibit properties of DNA binding sequences (Figure S7); therefore, we constructed Scm3p variants with a deletion of the putative CEN DNA interacting motif (amino acid residues 1–103) either alone or in combination with the HR domain (amino acid residues 1–140) (Figure 5A). Consistent with our hypothesis, GALscm3-Δ103 or GALscm3-Δ140 strains do not exhibit a chromosome loss phenotype (Figure 5B) nor do the mutant proteins associate with CEN DNA (Figure 5C). As expected, results of co-immunoprecipitation showed that Scm3-Δ103p interacts with Cse4p, while Scm3-Δ140p does not (Figure 5D). Consistent with the lack of chromosome loss phenotype, overexpression of scm3-Δ103 and scm3-Δ140 alleles do not affect the level of Cse4p at CEN DNA, whereas the overexpressed scm3nesΔ, scm3hrΔ alleles showed about 3 - to 5-fold reduction in the levels of Cse4p at CEN1 and CEN3 relative to that of the vector control strain (Figure 5E). Our results show that the N-terminus of Scm3p mediates its association with CEN DNA and overexpression of N-terminal scm3 mutants (scm3-Δ103 and scm3-Δ140) that fail to associate with centromeric chromatin do not result in increased chromosome loss.
Overexpression of HJURP causes chromosome loss in budding yeast and mitotic defects in human cell lines
Overexpression of HJURP has been observed in mammalian cancer cell lines, such as lung and breast cancers [38], [39]. Based on our results with GALSCM3, we tested if overexpression of HJURP leads to mitotic defects in yeast. We cloned HJURP into a yeast expression vector allowing regulated expression of the gene from a GAL1 promoter. A wild-type reporter strain overexpressing HJURP exhibits a chromosome loss phenotype (7.3±0.3%) similar to that observed for GALSCM3 (8.9±0.8%) (Figure 6A). GALCSE4 suppresses the chromosome loss phenotype of the GALHJURP strain to the same extent as that observed for strains co-expressing GALSCM3 and GALCSE4 (Figure 6A). We confirmed galactose-induced expression of GALHJURP by western blotting (Figure 6B).
Fig. 6. Overexpression of HJURP causes chromosome missegregation in budding yeast. 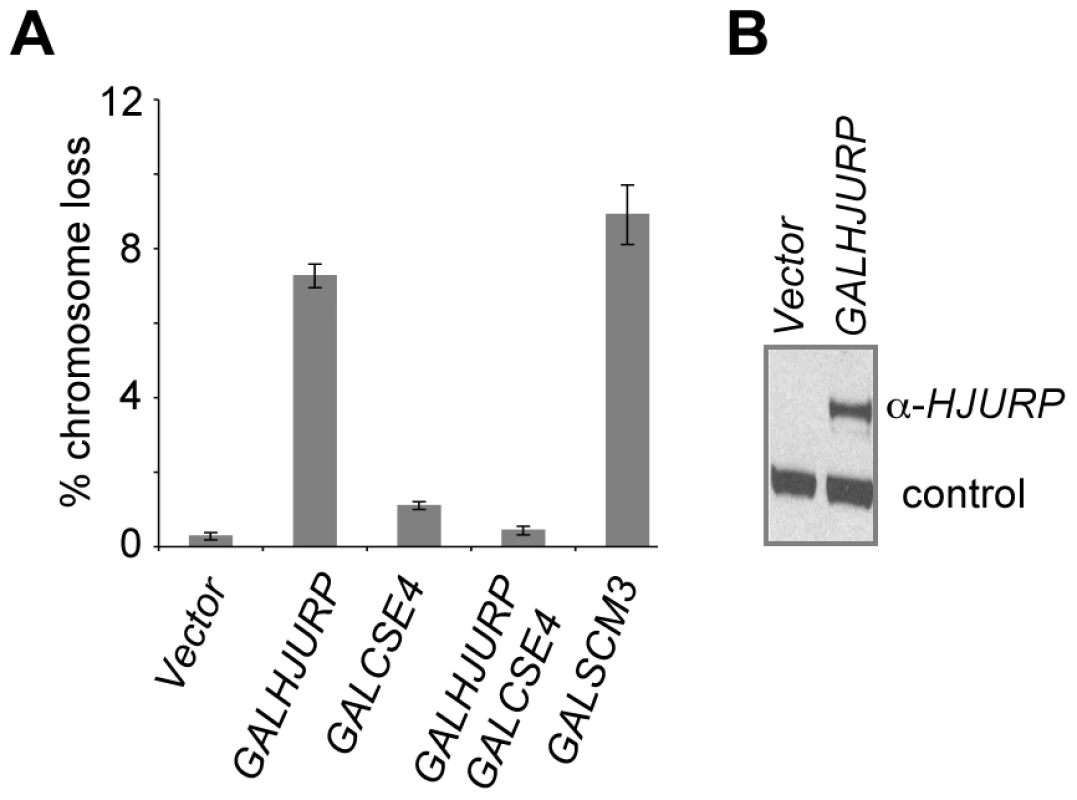
(A) Overexpression of HJURP leads to chromosome loss in budding yeast. Reporter strains with chromosome fragment (YPH1018) transformed with a vector (pRS426 GAL1), GALHJURP (pMB1490), GALCSE4 (pMB1147), GALHJURP, GALCSE4 (pMB1490, pMB1147), and GALSCM3HA (pMB1306) were assayed for chromosome loss as described in Figure 1 (C). (B) Western blot analysis showing galactose induced expression of HJURP. Whole cell protein extracts prepared from wild type strains (YPH1018) transformed with GALHJURP (pMB1490), or vector (pRS426 GAL1). Strains were grown to logarithmic phase of growth in synthetic media and gene expression induced in the presence of galactose (2%) at 30°C for 12 hours. Blots were probed with anti-HJURP. A non-specific band served as a loading control. The chromosome loss phenotypes observed upon Scm3p and HJURP overexpression in S. cerevisiae led us to ask if increased levels of HJURP in human cells would also result in chromosome segregation errors. HeLa cells were transfected with a construct to express GFP-tagged HJURP. The population was enriched for cells that had recently undergone chromosome segregation by single thymidine block followed by a 12 hr release. The number of micronuclei and lagging or bridged chromosomes (Figure 7A) were assessed as a measure of chromosome missegregation. Ninety percent of control cells (±7%) transfected with GFP vector alone showed no indication of abnormal chromosome segregation; however, when HJURP was overexpressed, only 56% (±19%) of nuclei appeared normal. Cells overexpressing HJURP had an increased number of recently divided nuclei pairs that contained micronuclei (29±8%) or lagging chromosomes (15±11%) relative to controls (Figure 7B).
Fig. 7. Chromosome segregation errors in human cells over-expressing of HJURP and CENP-A. 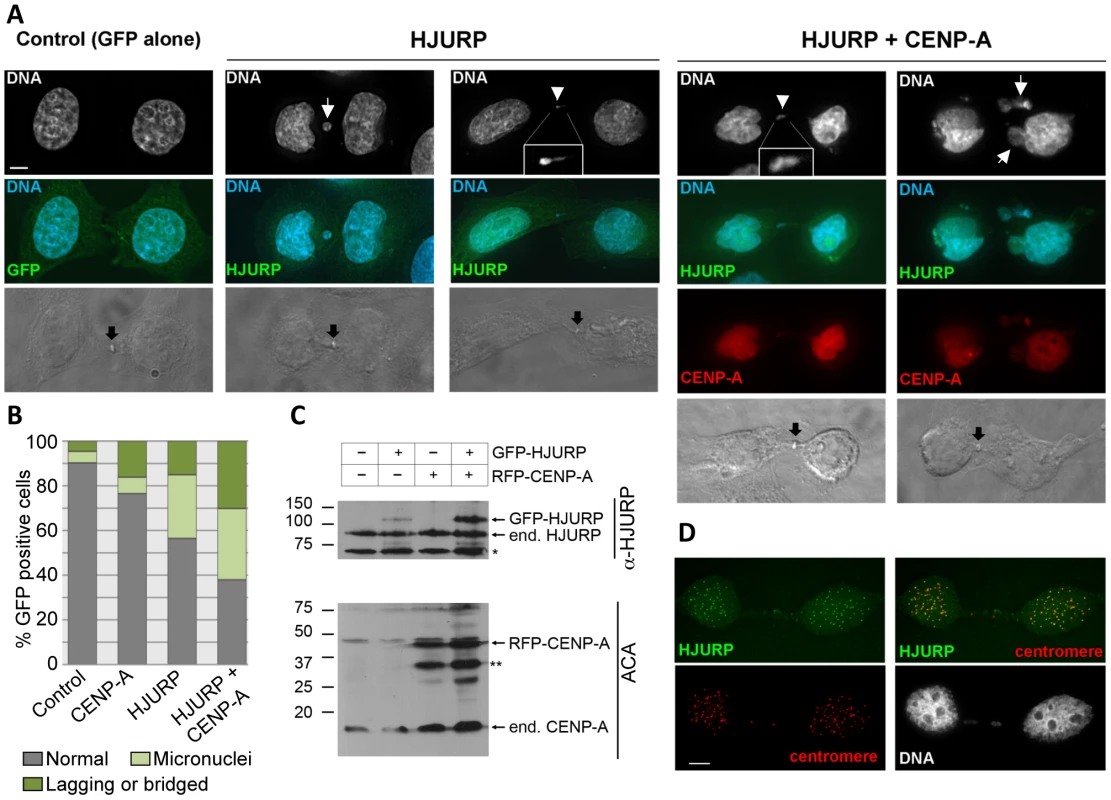
(A) HeLa cells were transient transfected with GFP-HJURP and/or RFP-CENP-A. Cells were blocked in thymidine for 20 hours and released for 12 hours to enrich for cells that have recently undergone mitosis (early G1). Cells in early G1 were identified by the presence of a midbody (black arrow). DNA was stained with DAPI. Micronuclei (white arrow), chromosome bridges or lagging chromosomes (white arrowhead) were evident in cells over-expressing HJURP, and both HJURP and CENP-A. (B) The degree of micronuclei and lagging or bridged chromosomes were quantified in cell transiently over-expressing RFP-CENP-A, GFP-HJURP or RFP-CENP-A and GFP-HJURP. While individual over-expression of CENP-A or HJURP yielded rates of chromosome abnormalities greater than controls, the highest degree of micronuclei and lagging or bridged chromosomes was observed with co-overexpression of both CENP-A and HJURP. Values are the average from two independent experiments, >100 cells per experiment. (C) Western blot showing expression of GFP-HJURP and RFP-CENP-A in HeLa cells. Asterisk represents non-specific bands. (D) Cells over-expressing GFP-HJURP were pre-extracted in 0.1% Triton prior to fixation. Centromeres were identified using anticentromere autosera. GFP-HJURP colocalizes with centromeres demonstrating that over-expressed HJURP is able to localize similar endogenous HJURP. Scale bar equals 5 micron. Studies with human breast cancer cell lines and primary breast tumors have shown that HJURP mRNA levels were significantly correlated with CENP-A mRNA levels [39]; hence, we tested whether co-overexpression of CENP-A and HJURP exacerbated the chromosome missegregation observed with HJURP overexpression alone. Cells overexpressing CENP-A exhibited an increased incidence of lagging chromosomes (16±3%) compared with controls, not as severe as the effect of HJURP overexpression. However, when CENP-A and HJURP were co-overexpressed in the same cell, the incidence of micronuclei (32±18%) and lagging chromosomes (30±17%) was increased relative to CENP-A or HJURP overexpression alone (Figure 7B). Increased rates of chromosome loss with overexpression of both CENP-A and HJURP are consistent with observations in breast cancer where there is a correlation between increased levels of HJURP and CENP-A [39]. Overexpression of both CENP-A and HJURP may result in stabilization of HJURP (Figure 7C). Alternatively, overexpression of CENP-A and HJURP may independently lead to a phenotype that is more susceptible to acquiring a tumorigenic potential.
Discussion
Here we report that misregulation of Scm3p/HJURP causes defects in kinetochore function and results in chromosome instability in yeast and human cells. We used budding yeast to show that overexpression of SCM3 exhibits reduced viability in kinetochore mutants, premature separation of sister chromatids and lower levels of Cse4p and histone H4 at centromeres. To understand the molecular basis of the chromosome loss phenotype of GALSCM3 strains, we created strains overexpressing mutant alleles of SCM3, and these studies have shown that: a) Scm3p can associate with centromeres independently of its association with Cse4p, b) the N-terminal domain of Scm3p mediates its association with centromeric chromatin, and c) centromeric association of Scm3p devoid of Cse4p is the likely cause of the chromosome loss phenotype of GALSCM3 strains. Our results establish that balanced stoichiometry of Scm3p and its human homolog HJURP are critical for maintenance of genome stability.
Since mutations affecting the HR, NES, and N terminus of Scm3p are lethal, limiting their usefulness, we used overexpression alleles to gain insight into Scm3p function. Overexpression studies have been conducted in other systems to understand the molecular mechanisms of chromosome segregation and kinetochore assembly. For example, overexpression of wild-type or mutant forms of CENP-A or its homologs in different organisms such as flies, budding and fission yeasts, leads to chromosome segregation defects [34]–[36]. In flies, excess CENP-A promotes the formation of ectopic kinetochores [36]. In budding yeast, excess Cse4p is degraded by ubiquitin mediated proteolysis [41], [47], however a mutant form of Cse4p (Cse4K16R) can be stably overexpressed [48], and such overexpression results in Cse4p mis-localization and chromosome loss [35]. Our results showed that the increased chromosome loss of GALSCM3 strains does not appear to be due to spreading of Scm3p to non-centromeric regions, as we found no evidence for mis-localization of overexpressed Scm3p to non-CEN regions.
In-vitro studies in budding yeast have shown that Scm3p directly binds and forms a stoichiometric complex with Cse4p and histone H4 [19]. Similarly, HJURP in humans also interacts at a stoichiometric ratio with CENP-A/H4 tetramers [27]. Our results here have shown that overexpression of SCM3 adversely affects the incorporation of Cse4p/H4 into centromeric chromatin as is evident from the reduction in the levels of Cse4p and H4 at the CEN DNA and suppression of GALSCM3-induced chromosome loss phenotype by GALCSE4 or GALH4 (Figure 1C, 1E, 1F). We propose that GALSCM3 leads to defects in kinetochore integrity and that this contributes to reduced viability in kinetochore mutants such as mcm21Δ. Previous reports have shown that mcm21 genetically interacts with cse4 and ipl1 mutants and mcm21Δ strains exhibit precociously separation of sister chromatids in metaphase [44]. It is possible that the lower levels of centromeric Cse4p in GALSCM3 strains contribute to altered pericentromeric cohesion, which in turn results in premature separation of sister chromatids in the GALSCM3 strains. Defect in pericentromeric cohesion has been previously reported for cse4 mutants [49].
We propose a model whereby Scm3p overexpression leads to increased levels of unbound Scm3p, which localizes to CEN DNA and interferes with the productive association of Cse4p/H4-Scm3p complexes, resulting in decreased Cse4p incorporation, defects in kinetochore function, and, ultimately, chromosome loss (Figure 8). The model posits that Scm3p devoid of Cse4p can associate with CEN DNA, and it correctly predicts that mutant alleles of scm3 that fail to associate with CEN DNA should not affect chromosome segregation (Figure 5B). Mutant alleles of scm3 deleted of the N terminus (GALscm3-Δ103) either alone or in combination with the HR domain (GALscm3-Δ140) do not associate with CEN DNA (Figure 5C). Our results are consistent with in vitro studies demonstrating a DNA-binding domain located within the N-terminal 113 of Scm3p (Carl Wu, personal communication). That GALscm3hrΔ, which fails to bind Cse4p (Figure 5D), is still found associated with CEN DNA (Figure 5C) indicates that CEN DNA association of Scm3p is not dependent on Cse4p interaction even though centromeric localization of Cse4p is dependent on Scm3p [18]–[20], [29]. The higher chromosome loss phenotype of GALscm3hrΔ, and GALscm3nesΔ strains compared to that of GALSCM3 strains suggests that these mutant alleles exhibit a dominant interfering phenotype and this may be due to additional roles of the HR and NES domains. For example, Shivaraju et al. have reported that expression of scm3nesΔ from its native promoter and simultaneous depletion of Scm3p results in checkpoint activation, and G2/M arrest [29]. Future studies should help us better understand the additional roles of different Scm3p domains.
Fig. 8. Schematic model for GALSCM3-induced chromosome instability in budding yeast. 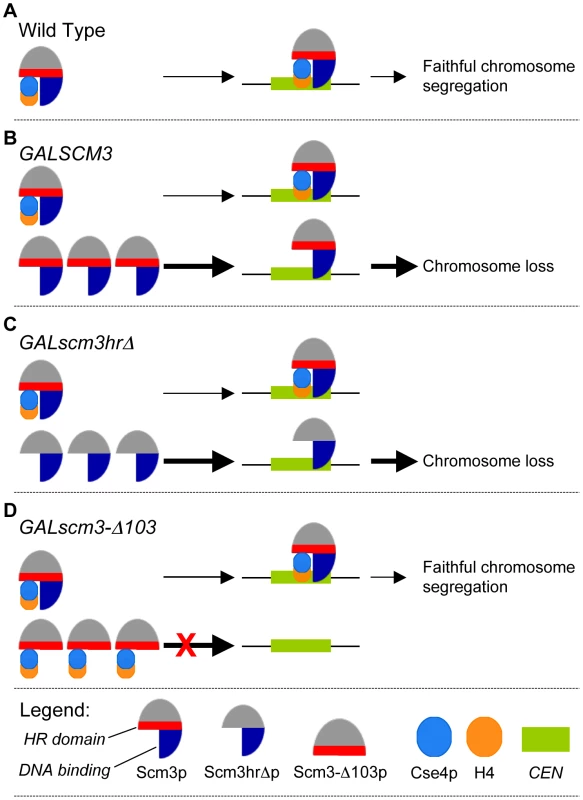
(A) In wild type cells, Scm3p forms a stoichiometric complex with Cse4p/H4 tetramers and mediates the assembly of Cse4p at the CEN DNA resulting in faithful chromosome segregation. (B) Centromeric association of Scm3p devoid of Cse4p contributes to the chromosome loss phenotye of GALSCM3 strains. In GALSCM3 expressing cells, there is an excess of Scm3p compared to the available pools of Cse4p and H4. Centromeric association of Scm3p devoid of Cse4/H4 leads to chromosome loss. Support for this is based on chromosome loss, ChIP experiments and suppression analysis from Figure 1. (C) Scm3p devoid of Cse4p can associate with centromeric DNA. GALscm3hrΔ that cannot interact with Cse4p can bind CEN DNA and association of Scm3p devoid of Cse4p leads to the chromosome loss phenotype of GALscm3hrΔ strains (Figure 5). (D) The N-terminus of Scm3p defines the centromeric association domain of Scm3p. Overexpression of scm3 mutants (GALscm3-Δ103) that can interact with Cse4p but do not associate with CEN DNA do not exhibit increased chromosome loss (Figure 5). Thick arrows represent excess Scm3p or its allelic mutant forms. Taken together our data support the model that centromeric association of Scm3p devoid of Cse4p contributes to the chromosome loss phenotype of GALSCM3 strains. Our results for the cell cycle regulated enrichment of Scm3p in S phase cells correlates with a similar enrichment of Cse4p in S phase cells and is consistent with the role of Scm3p as a Cse4p assembly factor [18]–[20], [29]–[31]. Studies with budding yeast have shown that replication of CEN DNA occurs in early S phase [50], and the centromeres transiently detach from the microtubules in order for the replication machinery to pass through the centromeric region [51]. The S phase co-enrichment of Scm3p and Cse4p could likely reflect the CEN DNA replication dependent centromeric assembly of Cse4p. Scm3p homologs in fission yeast also exhibits cell cycle dependent centromeric localization pattern and an enrichment in S phase that correlates with the centromeric assembly of Cnp1 [30], [31]. Our results for depletion of Scm3p in early mitotic cells and enrichment in anaphase cells is similar to findings from fission yeast, wherein Scm3p dissociates from CEN in metaphase cells and reappears in anaphase [30], [31]. Despite the difference in centromere structure of fission and budding yeast, the overall cell cycle regulated centromeric localization pattern of Scm3p seems to be evolutionarily conserved in these two systems. However, in humans no HJURP signals were detected at centromeres in anaphase and telophase cells [26]. The mechanism and physiological relevance of the mitotic depletion of Scm3p or its homologs has not been investigated in fission yeast or human cells. It is possible that the lack of mitotic depletion of Scm3p in GALSCM3 strains (300 min, Figure 3B) may contribute to the chromosome loss phenotype. Future studies using mutant alleles of SCM3, which fail to show mitotic depletion should give us insights into the role of the cell cycle regulated centromeric association of Scm3p.
We have shown that overexpression of SCM3 or HJURP causes chromosome loss in a wild-type yeast strain, and overexpression of HJURP leads to mitotic defects in human cells. Unlike our observations in budding yeast where overexpression of CSE4 suppresses GALSCM3 induced chromosome loss, overexpression of CENP-A does not suppress, but exacerbates the chromosome loss phenotype of human cells overexpressing HJURP. We propose that these differences may be due to the composition of centromeric chromatin, timing of incorporation of CenH3 or additional roles of HJURP in humans. For example, in contrast to the point centromeres of budding yeast, human centromeres contain a large number of CENP-A nucleosomes that are stably propagated through S phase [52], whereas the single budding yeast Cse4p nucleosome is replaced during S phase [43]. Furthermore, HJURP occupies the centromere during a limited time in the cell cycle [26]. Therefore, overexpression of HJURP would not be expected to deplete stable CENP-A nucleosomes. Recently, HJURP mediated deposition of CENP-A has been shown to be sufficient to recruit centromeric proteins to an ectopic site within the chromatin [53]. Co-overexpression of CENP-A and HJURP in human cells may result in the increased incorporation of CENP-A at non-centromeric loci. As a result, limiting amounts of critical centromere proteins may be titrated away from endogenous centromeres under these conditions leading to chromosome missegregation. Alternatively, the non-centromeric roles of HJURP may contribute to chromosome missegregation [38] and this affect may be increased when CENP-A is present because of the increased stability of HJURP (Figure 7C) [39]. Unlike HJURP overexpression, which causes its mislocalization to different genomic regions [38], overexpression of Scm3p does not result in mis-localization to non-centromeric regions (Figure S2A).
Overall, our results show that proper regulation of SCM3 and HJURP contribute to genome stability. The importance of our results with SCM3/HJURP is further reinforced by the fact that HJURP overexpression has been observed in human cancer cells and is associated with chromosomal aberrations and aneuploidy [38], [39]. Also, altered dosage and mis-localization of kinetochore proteins, such as CENP-A, CENP-H, Aurora-B and INCENP has been observed in various types of tumor cells [37]–[39], [54]. The elucidation of mechanisms underlying regulation of SCM3/HJURP in humans may help us identify and develop therapeutic targets for cancer therapy.
Materials and Methods
Media, strains, plasmids, and growth conditions
All yeast strains and plasmids used in this study are listed in Table 1 and Table 2, respectively. Yeast growth media, and protocols are as described previously [35]. Plasmid pMB1193 (2 µm LEU2 GAL1/10-SCM3) was derived by inserting a PCR-amplified SCM3 fragment between the BamHI and XhoI sites of pRS425 GAL1. The NES domain of SCM3 was deleted from pMB1306 using QuickChange Site Directed Mutagenesis Kit (Stratagene) resulting in pMB1393 (2 µm URA3 GAL1/10-scm3nesΔ-HA). pMB1455 (2 µm LEU2 GAL1/10-scm3hrΔ-HA) was constructed by cloning the PCR-amplified SCM3hrΔ-HA fragment from pRB923 between SpeI and XhoI sites of pRS425 GAL1, whereas pMB1490 (2 µm URA3 GAL1/10-HJURP) was derived from subcloning the EcoRI -XhoI fragment of HJURP (clone id 2820741, Open Biosystem), into pRS426 GAL1. Plasmids pMB1520 (2 µm LEU2 GAL1/10-scm3-Δ103HA) and pMB1521 (2 µm LEU2 GAL1/10-scm3-Δ140HA) were constructed by cloning the PCR-amplified SCM3-Δ103HA, and SCM3-Δ140HA fragments from pMB1306 between SpeI and XhoI sites of pRS425 GAL1. Strains were grown in YPD (1% yeast extract, 2% Bacto-peptone, 2% glucose) or in synthetic medium containing either 2% glucose or 2% galactose and supplements to allow selection of plasmids being tested.
Tab. 1. <i>S. cerevisiae</i> strains used in this study. 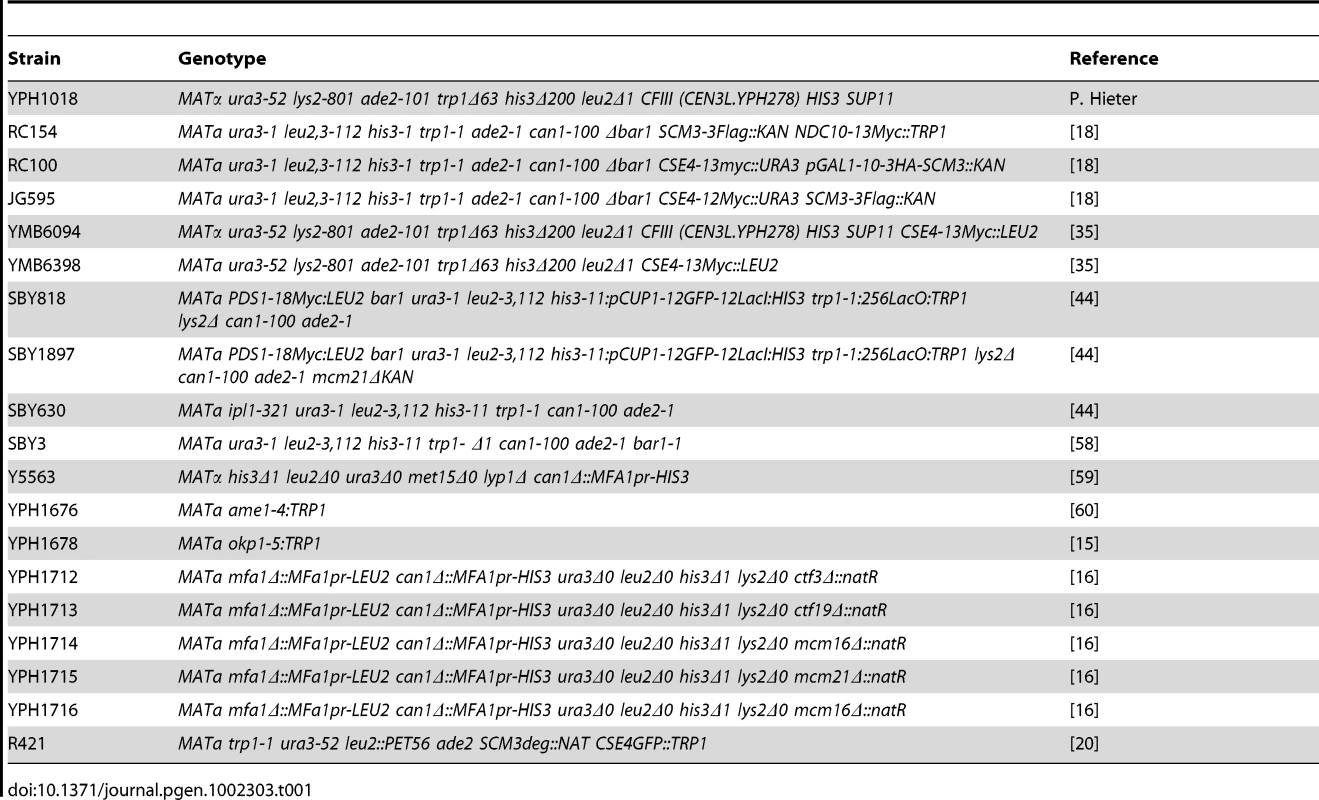
Tab. 2. List of plasmids used in this study. 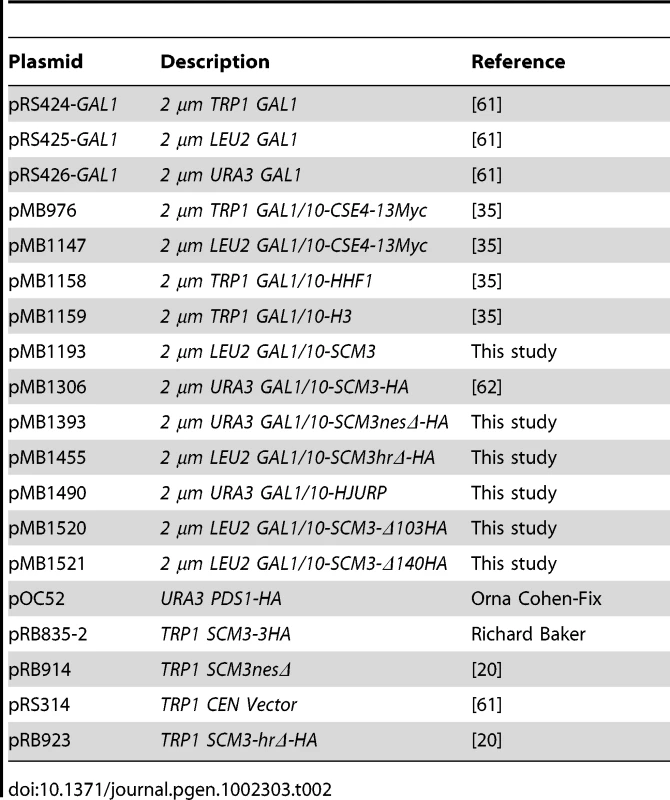
Chromosome transmission fidelity (ctf) and viability assays
We used an assay developed previously [55] to measure the loss of a non-essential reporter chromosome fragment (CF). Reporter strains were grown to logarithmic phase in synthetic media to maintain the CF and plasmids to be assayed. Cultures were then diluted and plated on synthetic medium to maintain plasmid selection with limiting adenine. The loss of non-essential CF leads to red sectors in an otherwise a white colony. Chromosome loss was measured by counting the percentage of colonies that were at least half red, which represents the loss of reporter chromosome during the first cell division. A minimum of 3000 colonies were assayed from three individual transformants for each strain.
Viability assays for Ctf3p and COMA complex strains containing GALSCM3HA (pMB1306) or vector (pRS426 GAL1) were carried out by plating equal numbers of cells from three independent transformants for each strain on synthetic media with either glucose (2%) or galactose (2%) and grown at 30°C for 5–7 days. At least 2500 colonies were counted for each strain. Percent viability is expressed as the ratio of the number of colonies obtained on galactose media over the number of colonies obtained on glucose plates and normalized to the value of 100.
Chromatin immunoprecipitation (ChIP) and quantitative PCR
All ChIP experiments were carried out (three biological replicates) as described [35] with minor modifications. Cultures were cross-linked with formaldehyde (1% final concentration) at room temperature for 15 min and excess formaldehyde was quenched with 125 mM glycine for 5 min. Cell pellets were collected by centrifugation and spheroplasts prepared using Zymolyase 100T, followed by sequential washes with PBS (150 mM NaCl, 10 mM sodium phosphate, pH 7.4), and FA buffer (50 mM Na-Hepes pH 7.6, 1 mM EDTA, 1% Triton X-100, 150 mM NaCl, 0.1% Na-deoxycholate). Spheroplasts were resuspended in FA buffer with protease inhibitors (1 mM PMSF, 1 mM benzamidine, 10 µg/ml leupeptin, 10 µg/ml aprotinin) and sonicated on ice at setting 3, 100% duty cycle, for four 12 sec bursts applied 2 min apart to obtain an average fragment size of 400 bp. The resulting soluble fraction was denoted as input and used for chromatin immunoprecipitation. Protein A magnetic beads (Invitrogen Inc.) coated with antibodies were used in immunoprecipitation performed at 4°C for 12 hours. Antibodies used were anti-FLAG (A2220, Sigma), anti-HA (A2095, Sigma), anti-Myc (sc-789 Santa Cruz Inc), anti-histone H4 (clone 62-141-13, Millipore), anti-HJURP [26] or anti-GST (negative control). The immunoprecipitated nucleic acid-protein complexes were washed sequentially at room temperature for 5 min each wash in FA buffer (thrice), FA-HS buffer (50 mM Na-Hepes pH 7.6, 1 mM EDTA, 1% Triton X-100, 500 mM NaCl, 0.1% Na-deoxycholate) (twice), RIPA buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA, 250 mM LiCl, 0.5% Nonidet P40, 0.5% Na-deoxycholate) (twice), 1 x TE pH 8.0 (twice), and finally resuspended in elution buffer (25 mM Tris-HCl pH 7.6, 10 mM EDTA, 0.5% SDS). Immunoprecipitated DNA was recovered after cross-link reversal at 65°C for 16 hours and RNase A/proteinase K treatment, purified by phenol/chloroform/isoamyl alcohol (25∶24∶1) extractions, and ethanol precipitated (−20°C for 12 hours). Enriched DNA was recovered by centrifugation and dissolved in TE (10 mM Tris, 1 mM EDTA, pH 8.0).
Quantitative real time ChIP-PCR was performed using Fast SYBR Green Master Mix in 7500 Fast real Time PCR System (Applied Biosystem Inc.) following manufacturer's instructions. PCR conditions were: denaturation at 95°C for 20 sec, followed by 40 cycles of 95°C for 3 sec, 60°C for 30 sec. The amplification readings were recorded and threshold cycle (Ct) was determined by 7500 Fast System Software v1.4.0 (Applied Biosystem Inc.). The enrichment values were calculated using the ΔΔCt method [56] and are presented as % input.
ChIP-PCR was done using Hotstar-Taq polymerase Master Mix (Qiagen Inc.), with initial denaturation at 94°C for 15 min, followed by 25 cycles of 94°C for 1 min annealing at 54°C for 1 min and extension at 68°C for 10 sec. The linear amplification conditions were determined by various cycles of amplification using serial dilutions of the input DNA. PCR products were separated on 2% agarose gel, stained with ethidium bromide and photographed. The intensity of PCR amplicons was determined using the software ImageJ 1.43u (http://rsb.info.nih.gov/ij). Sequences of primers used in this study are listed in Table 3.
Tab. 3. List of primers used in this study. 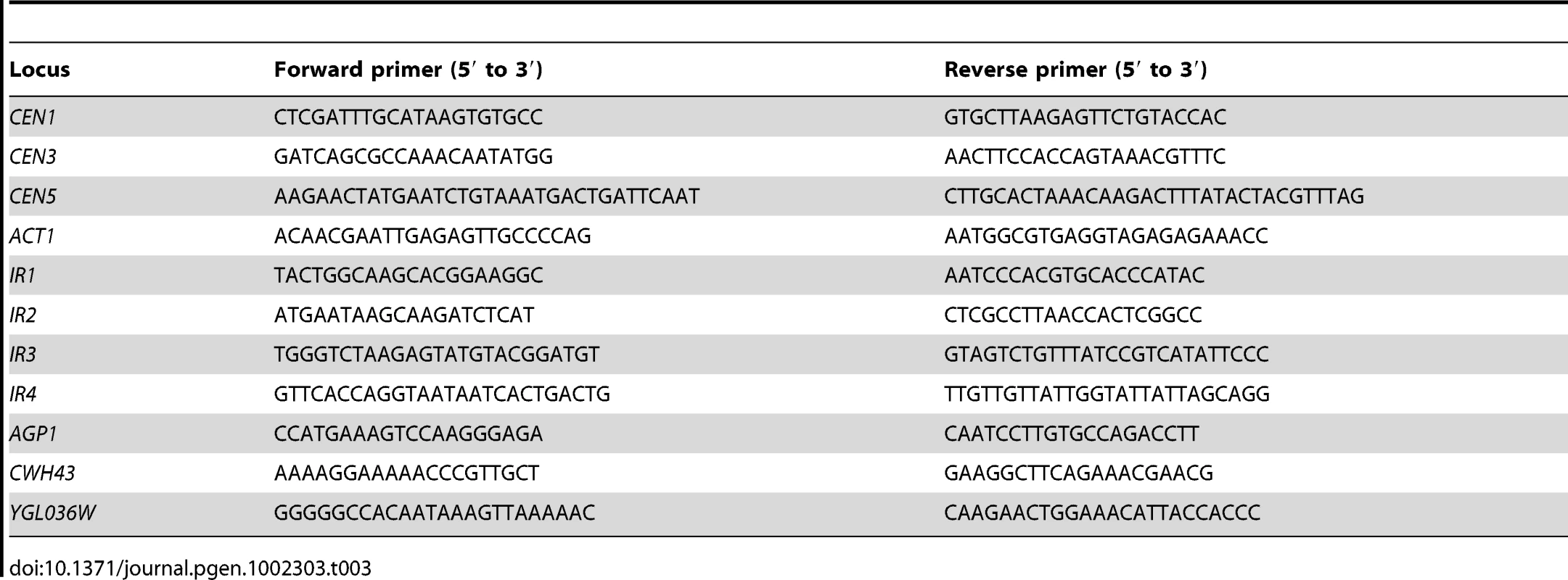
Immunoprecipitation and Western blot analysis
Immunoprecipitation experiments were performed as described previously [18]. Protein samples for western blot analysis were prepared using the TCA procedure as described previously [57], and protein concentration was measured using the Bio-Rad DC protein assay (Bio-Rad, CA). Equal amounts of protein for each sample were size separated on 4–12% gradient polyacrylamide gels and transferred to nitrocellulose membrane. Primary antibodies used were anti-HA (clone 12CA5, Roche), anti-Myc (Z-5, sc-789, Santa Cruz Inc), anti-histone H3 (ab1791-100, Abcam), anti-histone H4 (clone 62-141-13, Millipore), anti-Flag (A-6457, Molecular Probes) or anti-HJURP [26]. Secondary antibodies used were HRP-conjugated sheep anti-mouse IgG (NA931V–Amersham) and HRP-conjugated sheep anti-rabbit IgG (NA934V–Amersham). Rabbit polyclonal antibodies against Tub2p were custom made by Covance, Inc.
Cell cycle arrest and release experiments
For determining the CEN enrichment of Scm3p through the cell cycle, wild-type strain (RC154) with FLAG-tagged Scm3p expressed from its endogenous promoter was used. A wild type strain (JG595) with Myc-tagged Cse4p expressed from its endogenous promoter was used to determine the CEN enrichment of Cse4p through the cell cycle. Cells were grown in YPD to logarithmic (LOG) phase at 30°C, treated with 3 µM α-factor (T-6901, Sigma, St. Louis) for 90 minutes to arrest cells in G1, washed, and released into pheromone-free YPD medium. Samples were taken at 15 min time points after release from G1 arrest and used for FACS, protein and ChIP analysis. Additional synchronizations were carried out to confirm the results: cells were arrested in G1 with α-factor as described above and released into YPD medium containing 15 µg/ml nocodazole (M1404, Sigma); cells were arrested in G2/M with nocodazole for 2 hours and released into YPD medium containing 3 µM α-factor (T-6901, Sigma).
To examine the CEN enrichment of overexpressed Scm3p (GALSCM3) through the cell cycle, strain RC100 with HA tagged Scm3p integrated at its endogenous locus (only copy in the genome) and expressed from GAL1 promoter was used. Cells were grown in SC media with 2% galactose to logarithmic (LOG) phase at 30°C, treated with 3 µM α-factor (T-6901, Sigma, St. Louis) for 90 minutes for G1 arrest, washed, and released into pheromone-free SC media with 2% galactose medium. Samples were taken at different time points after release from G1 arrest and were used in FACS and ChIP analysis.
FACS and nuclear morphology analyses
FACS assays was performed to confirm the cell cycle arrest and release. Cells were fixed in 70% ethanol, washed in 0.2 M Tris buffer, treated with RNase A, Proteinase K, stained with propidium iodide, and analyzed using a Becton-Dickinson FACSort flow cytometer and Cell Quest software (BD Biosciences, Boston, MA). Cell cycle stages were determined by examining cell morphology and nuclear position in propidium iodide-stained cells under the Zeiss Axioskop 2 microscope (Carl Zeiss Inc., Thornwood, USA) as described previously [42]. Cell cycle stages were defined as follows: G1, single cells with undivided nuclei; S-phase, cells showing a small bud with undivided nuclei; metaphase, large budded cells with nucleus at the neck; anaphase, large budded cells with elongated/separated nuclei; and telophase, large budded cells with nuclei separated between mother and daughter cells (see Figure 2).
Human cell culture
HeLa cells were plated 24 hours prior to transfection to poly-lysine coated 12mm square coverslips in a 6-well plate. Cells were incubated with DNA and Effectene transfection reagent (Qiagen) according the manufacturer instructions in Optimem (Invitrogen) for 8 hours and at which time the media was replaced with normal growth media (DMEM, 10% FBS, supplemented with Penicillin and Streptomycin). Beginning 24 hours after transfection, cells were incubated with 2mM thymidine for 20 hours, then released from thymidine by washing cells in PBS and incubating cells in normal growth media supplemented with 20mM deoxycytidine for 12 hours prior to fixation. Cells were fixed after in 4% formaldehyde and blocked in PBS with 2% FBS, 2% BSA and 0.1% TritonX-100. Antibody incubates were conducted for 1 hour at room temperature in blocking buffer. Centromeres were detected using anti-centromere autosera (Antibodies incorporated) and Cy5 conjugated goat-anti-human secondary antibodies (Jackson Labs). DNA was stained using 2 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) and cells were mounted in Prolong Antifade (Invitrogen). Z-stacked images were collected on a Deltavision microscope (Applied Precision Instruments) using a 60X objective. Images were deconvolved and are presented as maximum projections.
Supporting Information
Zdroje
1. LengauerCKinzlerKWVogelsteinB 1998 Genetic instabilities in human cancers. Nature 396 643 649
2. SkibbensRVHieterP 1998 Kinetochores and the checkpoint mechanism that monitors for defects in the chromosome segregation machinery. Annu Rev Genet 32 307 337
3. EkwallK 2007 Epigenetic control of centromere behavior. Annu Rev Genet 41 63 81
4. BuscainoAAllshireRPidouxA 2010 Building centromeres: home sweet home or a nomadic existence? Curr Opin Genet Dev 20 118 126
5. CheesemanIMDrubinDGBarnesG 2002 Simple centromere, complex kinetochore: linking spindle microtubules and centromeric DNA in budding yeast. J Cell Biol 157 199 203
6. ClevelandDWMaoYSullivanKF 2003 Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112 407 421
7. HymanAASorgerPK 1995 Structure and function of kinetochores in budding yeast. Annu Rev Cell Dev Biol 11 471 495
8. BlowerMDKarpenGH 2001 The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat Cell Biol 3 730 739
9. BuchwitzBJAhmadKMooreLLRothMBHenikoffS 1999 A histone-H3-like protein in C. elegans. Nature 401 547 548
10. ChenESSaitohSYanagidaMTakahashiK 2003 A cell cycle-regulated GATA factor promotes centromeric localization of CENP-A in fission yeast. Mol Cell 11 175 187
11. MeluhPBYangPGlowczewskiLKoshlandDSmithMM 1998 Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell 94 607 613
12. SmithMMYangPSantistebanMSBoonePWGoldsteinAT 1996 A novel histone H4 mutant defective in nuclear division and mitotic chromosome transmission. Mol Cell Biol 16 1017 1026
13. StolerSKeithKCCurnickKEFitzgerald-HayesM 1995 A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev 9 573 586
14. KarpenGHAllshireRC 1997 The case for epigenetic effects on centromere identity and function. Trends Genet 13 489 496
15. OrtizJStemmannORankSLechnerJ 1999 A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev 13 1140 1155
16. MeasdayVHaileyDWPotIGivanSAHylandKM 2002 Ctf3p, the Mis6 budding yeast homolog, interacts with Mcm22p and Mcm16p at the yeast outer kinetochore. Genes Dev 16 101 113
17. FuruyamaSBigginsS 2007 Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc Natl Acad Sci U S A 104 14706 14711
18. CamahortRLiBFlorensLSwansonSKWashburnMP 2007 Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol Cell 26 853 865
19. MizuguchiGXiaoHWisniewskiJSmithMMWuC 2007 Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell 129 1153 1164
20. StolerSRogersKWeitzeSMoreyLFitzgerald-HayesM 2007 Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci U S A 104 10571 10576
21. ZhangWMelloneBGKarpenGH 2007 A specialized nucleosome has a "point" to make. Cell 129 1047 1049
22. ZhouZFengHZhouBRGhirlandoRHuK 2011 Structural basis for recognition of centromere histone variant CenH3 by the chaperone Scm3. Nature 472 234 237
23. ChoUSHarrisonSC 2011 Recognition of the centromere-specific histone Cse4 by the chaperone Scm3. Proc Natl Acad Sci U S A
24. Sanchez-PulidoLPidouxALPontingCPAllshireRC 2009 Common ancestry of the CENP-A chaperones Scm3 and HJURP. Cell 137 1173 1174
25. HuHLiuYWangMFangJHuangH 2011 Structure of a CENP-A-histone H4 heterodimer in complex with chaperone HJURP. Genes Dev 25 901 906
26. FoltzDRJansenLEBaileyAOYatesJR3rdBassettEA 2009 Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell 137 472 484
27. ShuaibMOuararhniKDimitrovSHamicheA 2010 HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc Natl Acad Sci U S A 107 1349 1354
28. CamahortRShivarajuMMattinglyMLiBNakanishiS 2009 Cse4 is part of an octameric nucleosome in budding yeast. Mol Cell 35 794 805
29. ShivarajuMCamahortRMattinglyMGertonJL 2011 Scm3 is a centromeric nucleosome assembly factor. J Biol Chem 286 12016 12023
30. PidouxALChoiESAbbottJKLiuXKaganskyA 2009 Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell 33 299 311
31. WilliamsJSHayashiTYanagidaMRussellP 2009 Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell 33 287 298
32. DunleavyEMRocheDTagamiHLacosteNRay-GalletD 2009 HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell 137 485 497
33. BernadRSanchezPRiveraTRodriguez-CorsinoMBoyarchukE 2011 Xenopus HJURP and condensin II are required for CENP-A assembly. J Cell Biol 192 569 582
34. CastilloAGMelloneBGPartridgeJFRichardsonWHamiltonGL 2007 Plasticity of fission yeast CENP-A chromatin driven by relative levels of histone H3 and H4. PLoS Genet 3 e121 doi:10.1371/journal.pgen.0030121
35. AuWCCrispMJDeLucaSZRandoOJBasraiMA 2008 Altered dosage and mislocalization of histone H3 and Cse4p lead to chromosome loss in Saccharomyces cerevisiae. Genetics 179 263 275
36. HeunPErhardtSBlowerMDWeissSSkoraAD 2006 Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell 10 303 315
37. TomonagaTMatsushitaKYamaguchiSOohashiTShimadaH 2003 Overexpression and mistargeting of centromere protein-A in human primary colorectal cancer. Cancer Res 63 3511 3516
38. KatoTSatoNHayamaSYamabukiTItoT 2007 Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res 67 8544 8553
39. HuZHuangGSadanandamAGuSLenburgME 2010 The expression level of HJURP has an independent prognostic impact and predicts the sensitivity to radiotherapy in breast cancer. Breast Cancer Res 12 R18
40. GlowczewskiLYangPKalashnikovaTSantistebanMSSmithMM 2000 Histone-histone interactions and centromere function. Mol Cell Biol 20 5700 5711
41. HewawasamGShivarajuMMattinglyMVenkateshSMartin-BrownS 2010 Psh1 is an E3 ubiquitin ligase that targets the centromeric histone variant Cse4. Mol Cell 40 444 454
42. CalvertMELanniganJ 2010 Yeast cell cycle analysis: combining DNA staining with cell and nuclear morphology. Curr Protoc Cytom Chapter 9 Unit 9 32 31–16
43. PearsonCGYehEGardnerMOddeDSalmonED 2004 Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr Biol 14 1962 1967
44. NgTMWaplesWGLavoieBDBigginsS 2009 Pericentromeric sister chromatid cohesion promotes kinetochore biorientation. Mol Biol Cell 20 3818 3827
45. WangLYangMQYangJY 2009 Prediction of DNA-binding residues from protein sequence information using random forests. BMC Genomics 10 Suppl 1 S1
46. BaileyTLElkanC 1994 Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2 28 36
47. RanjitkarPPressMOYiXBakerRMacCossMJ 2010 An E3 ubiquitin ligase prevents ectopic localization of the centromeric histone H3 variant via the centromere targeting domain. Mol Cell 40 455 464
48. CollinsKAFuruyamaSBigginsS 2004 Proteolysis contributes to the exclusive centromere localization of the yeast Cse4/CENP-A histone H3 variant. Curr Biol 14 1968 1972
49. EckertCAGravdahlDJMegeePC 2007 The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev 21 278 291
50. McCarrollRMFangmanWL 1988 Time of replication of yeast centromeres and telomeres. Cell 54 505 513
51. KitamuraETanakaKKitamuraYTanakaTU 2007 Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae. Genes Dev 21 3319 3330
52. JansenLEBlackBEFoltzDRClevelandDW 2007 Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol 176 795 805
53. BarnhartMCKuichPHStellfoxMEWardJABassettEA 2011 HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol 194 229 243
54. AmatoASchillaciTLentiniLDi LeonardoA 2009 CENPA overexpression promotes genome instability in pRb-depleted human cells. Mol Cancer 8 119
55. SpencerFGerringSLConnellyCHieterP 1990 Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics 124 237 249
56. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
57. KastenmayerJPLeeMSHongALSpencerFABasraiMA 2005 The C-terminal half of Saccharomyces cerevisiae Mad1p mediates spindle checkpoint function, chromosome transmission fidelity and CEN association. Genetics 170 509 517
58. CollinsKACamahortRSeidelCGertonJLBigginsS 2007 The overexpression of a Saccharomyces cerevisiae centromeric histone H3 variant mutant protein leads to a defect in kinetochore biorientation. Genetics 175 513 525
59. TongAHBooneC 2006 Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 313 171 192
60. PotIKnocklebyJAneliunasVNguyenTAh-KyeS 2005 Spindle checkpoint maintenance requires Ame1 and Okp1. Cell Cycle 4 1448 1456
61. MumbergDMullerRFunkM 1994 Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res 22 5767 5768
62. GelperinDMWhiteMAWilkinsonMLKonYKungLA 2005 Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev 19 2816 2826
Štítky
Genetika Reprodukčná medicína
Článek Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and BrainČlánek Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-StateČlánek A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated LociČlánek Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional PolymorphismsČlánek Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of ChromosomesČlánek The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and LearningČlánek PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian CellsČlánek Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Temporal Trends in Results Availability from Genome-Wide Association Studies
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
- Genetic Variants at Chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 Influence the Risk of Breast Cancer in Men
- Large-Scale Gene-Centric Analysis Identifies Novel Variants for Coronary Artery Disease
- Genetic Association for Renal Traits among Participants of African Ancestry Reveals New Loci for Renal Function
- Transcriptome Kinetics Is Governed by a Genome-Wide Coupling of mRNA Production and Degradation: A Role for RNA Pol II
- Conserved Regulation of p53 Network Dosage by MicroRNA–125b Occurs through Evolving miRNA–Target Gene Pairs
- Heterozygous Mutations of Are Associated with an Increased Risk of Isolated Metopic Craniosynostosis in Humans and Mice
- Study of FoxA Pioneer Factor at Silent Genes Reveals Rfx-Repressed Enhancer at and a Potential Indicator of Esophageal Adenocarcinoma Development
- Cholesterol Metabolism Is Required for Intracellular Hedgehog Signal Transduction
- Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and Brain
- Age-Dependent Recombination Rates in Human Pedigrees
- Sequence Conservation and Functional Constraint on Intergenic Spacers in Reduced Genomes of the Obligate Symbiont
- Sex Chromosome Mosaicism and Hybrid Speciation among Tiger Swallowtail Butterflies
- A Negative Feedback Loop That Limits the Ectopic Activation of a Cell Type–Specific Sporulation Sigma Factor of
- Phased Whole-Genome Genetic Risk in a Family Quartet Using a Major Allele Reference Sequence
- Mutations in or near the Transmembrane Domain Alter PMEL Amyloid Formation from Functional to Pathogenic
- Inactivation of Alters Melanosome Shape But Has Only a Subtle Effect on Visible Pigmentation
- Novel Interactions between Actin and the Proteasome Revealed by Complex Haploinsufficiency
- Germline Genetic Variants Disturbing the /LIN28 Double-Negative Feedback Loop Alter Breast Cancer Susceptibility
- Separation of Recombination and SOS Response in RecA Suggests LexA Interaction Sites
- Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-State
- Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells
- A Noncoding Point Mutation of Causes Multiple Developmental Malformations and Obesity in Twirler Mice
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection
- Bacterial Communities of Diverse Species: Ecological Context of a Host–Microbe Model System
- A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated Loci
- Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification
- Elevated Proteasome Capacity Extends Replicative Lifespan in
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- MicroRNA Predictors of Longevity in
- An Iterative Genetic and Dynamical Modelling Approach Identifies Novel Features of the Gene Regulatory Network Underlying Melanocyte Development
- Atypical AT Skew in Firmicute Genomes Results from Selection and Not from Mutation
- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- Genomic Analysis of QTLs and Genes Altering Natural Variation in Stochastic Noise
- The Abnormal Phenotypes of Cartilage and Bone in Calcium-Sensing Receptor Deficient Mice Are Dependent on the Actions of Calcium, Phosphorus, and PTH
- Cell Type–Specific Transcriptome Analysis Reveals a Major Role for and miR-200b in Mouse Inner Ear Morphogenesis
- Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of Chromosomes
- IAP1-Mediated Ubiquitylation Controls Activation of the Initiator Caspase DRONC Independent of Protein Degradation
- VANG-1 and PRKL-1 Cooperate to Negatively Regulate Neurite Formation in
- The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and Learning
- Comparative and Functional Genomics of PD630 for Biofuels Development
- Identification of Type 1 Diabetes–Associated DNA Methylation Variable Positions That Precede Disease Diagnosis
- PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian Cells
- Genetic Effects at Pleiotropic Loci Are Context-Dependent with Consequences for the Maintenance of Genetic Variation in Populations
- Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
- Bmp and Nodal Independently Regulate Expression to Maintain Unilateral Nodal Activity during Left-Right Axis Specification in Zebrafish
- Inter-Allelic Prion Propagation Reveals Conformational Relationships among a Multitude of [] Strains
- Emergence and Modular Evolution of a Novel Motility Machinery in Bacteria
- Histone Methyltransferase MET-2 Shields the Male X Chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



