-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
The Caenorhabditis elegans dosage compensation complex (DCC) equalizes X-chromosome gene dosage between XO males and XX hermaphrodites by two-fold repression of X-linked gene expression in hermaphrodites. The DCC localizes to the X chromosomes in hermaphrodites but not in males, and some subunits form a complex homologous to condensin. The mechanism by which the DCC downregulates gene expression remains unclear. Here we show that the DCC controls the methylation state of lysine 20 of histone H4, leading to higher H4K20me1 and lower H4K20me3 levels on the X chromosomes of XX hermaphrodites relative to autosomes. We identify the PR-SET7 ortholog SET-1 and the Suv4-20 ortholog SET-4 as the major histone methyltransferases for monomethylation and di/trimethylation of H4K20, respectively, and provide evidence that X-chromosome enrichment of H4K20me1 involves inhibition of SET-4 activity on the X. RNAi knockdown of set-1 results in synthetic lethality with dosage compensation mutants and upregulation of X-linked gene expression, supporting a model whereby H4K20me1 functions with the condensin-like C. elegans DCC to repress transcription of X-linked genes. H4K20me1 is important for mitotic chromosome condensation in mammals, suggesting that increased H4K20me1 on the X may restrict access of the transcription machinery to X-linked genes via chromatin compaction.
Published in the journal: H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation. PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002933
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002933Summary
The Caenorhabditis elegans dosage compensation complex (DCC) equalizes X-chromosome gene dosage between XO males and XX hermaphrodites by two-fold repression of X-linked gene expression in hermaphrodites. The DCC localizes to the X chromosomes in hermaphrodites but not in males, and some subunits form a complex homologous to condensin. The mechanism by which the DCC downregulates gene expression remains unclear. Here we show that the DCC controls the methylation state of lysine 20 of histone H4, leading to higher H4K20me1 and lower H4K20me3 levels on the X chromosomes of XX hermaphrodites relative to autosomes. We identify the PR-SET7 ortholog SET-1 and the Suv4-20 ortholog SET-4 as the major histone methyltransferases for monomethylation and di/trimethylation of H4K20, respectively, and provide evidence that X-chromosome enrichment of H4K20me1 involves inhibition of SET-4 activity on the X. RNAi knockdown of set-1 results in synthetic lethality with dosage compensation mutants and upregulation of X-linked gene expression, supporting a model whereby H4K20me1 functions with the condensin-like C. elegans DCC to repress transcription of X-linked genes. H4K20me1 is important for mitotic chromosome condensation in mammals, suggesting that increased H4K20me1 on the X may restrict access of the transcription machinery to X-linked genes via chromatin compaction.
Introduction
In many animals, males and females have a different number of X chromosomes. Dosage compensation is a chromosome-wide process of gene regulation that equalizes gene expression between the sexes despite the difference in X-linked gene dosage, and is achieved by a variety of mechanisms [1], [2]. In humans, one X chromosome is inactivated in females. In Drosophila, expression from the single X chromosome in males is upregulated two-fold to match expression from the two X chromosomes in females. In C. elegans, the two X chromosomes in hermaphrodites are downregulated two-fold to match expression from the single X chromosome in males. In each of these cases, regulation of gene expression involves the targeting of specialized protein complexes specifically to the X chromosome. Studies of different dosage compensation mechanisms have uncovered chromatin-mediated mechanisms of gene regulation.
In C. elegans, dosage compensation is achieved by the dosage compensation complex (DCC) (reviewed in [3]). The core of the DCC consists of a five-subunit condensin complex named condensin IDC. Condensin complexes mediate chromosome condensation and resolution in mitosis and meiosis, and participate in crossover control during meiosis [4]. Four subunits (MIX-1, DPY-26, DPY-28, and CAPG-1) are shared with canonical condensin I, while DPY-27 is specific to condensin IDC [5]. The central role of a condensin-like complex in dosage compensation suggests that the mechanism of dosage compensation involves regulation of chromatin structure. Five other components of the DCC (SDC-1, SDC-2, SDC-3, DPY-21 and DPY-30) physically interact with different subunits of condensin IDC, and all examined components of the DCC are enriched on hermaphrodite X chromosomes relative to autosomes [3].
The DCC is targeted to the X chromosome through specific sequence elements, called rex (recruitment elements on X) sites (reviewed in [3]). After recruitment, the DCC spreads to dox (dependent on X) sites, which consist mostly of active promoters. The zinc finger protein SDC-2 is the primary X-chromosome recruitment factor for the DCC. The DCC also binds to some autosomal sites at lower levels, but the functional significance of autosomal binding is not yet known [6], [7]. SDC-3 requires SDC-2 for X-chromosome binding, and all of the other DCC components require SDC-2 and SDC-3 for recruitment. Loss of DCC proteins impairs dosage compensation, resulting in upregulation of X-linked genes and death of XX animals. DPY-21 and SDC-1 null mutants have milder dosage compensation defects and are viable, with apparently normal DCC protein localization on X [8], [9], [10], [11], [12].
A current model is that SDC proteins recruit condensin IDC to the X chromosome, leading to changes in chromatin structure that result in reduction of gene expression. In XX animals, not all genes on X are dosage compensated, and DCC association with genes correlates with gene expression level but not with the degree of repression [7]. The mechanism of repression is not understood, but DCC mutants have increased levels of RNA polymerase II on X-linked genes, indicating regulation at the level of transcription [13].
Here we show that the DCC is required for enrichment of the histone modification H4K20me1 on the X chromosomes of hermaphrodites and that the responsible histone methyltransferase SET-1 is important for downregulation of dosage-compensated genes. Our study implicates the histone modification H4K20me1 in the process of dosage compensation.
Results
The localization of dosage compensation proteins to the X chromosome become apparent at around the 30-cell stage of embryogenesis [3], [14]. Our previous experiments mapping the genome-wide patterns of H4K20me1 suggested that this histone modification might function in dosage compensation: H4K20me1 is weakly enriched on the X chromosome in early embryo populations that span initiation of dosage compensation (1–300-cell stages with 76%<100-cell) and strong enrichment at the third larval (L3) stage, when dosage compensation is fully active (Figure 1A) [15]. We performed additional ChIP experiments to better define the timing of H4K20me1 enrichment on X. We found that late embryos (99% 100-cell to 3-fold stage), which have activated dosage compensation, display high H4K20me1 on the X, similar to the pattern in L3 larvae (Figure 1A). Moreover, enrichment on the X chromosome is maintained in adult hermaphrodites (Figure 1A). Therefore, H4K20me1 is enriched on the X chromosome at developmental stages when dosage compensation is active.
Fig. 1. H4K20me1 becomes enriched on the X chromosome in wild type but not in a mutant defective in dosage compensation. 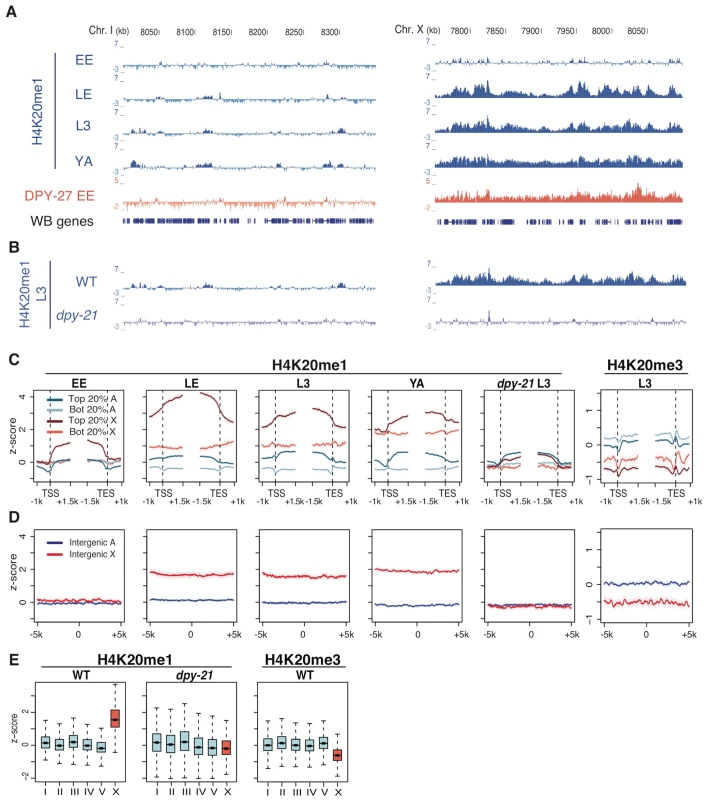
(A) Genome browser tracks of indicated ChIP signals across representative regions of chromosome I and chromosome X in wild-type early embryos (EE), late embryos (LE), L3 larvae (L3), and fem-2 female young adults (YA). For each track, the average of two independent biological replicates is shown as z-scores (standardized log2 ratios of ChIP/Input signals). High H4K20me1 enrichment on X begins in late embryos. A track of DPY-27 (a DCC component) is shown for reference. (B) Enrichment of H4K20me1 on the X is lost in dpy-21 mutants. (C) Plots of H4K20me1 and H4K20me3 signal across the TSS (transcript start site) and TES (transcript end site) of genes on X (red) and autosomes (blue). Genes in the top 20% of expression (dark shades) and bottom 20% (light shades) are plotted separately. (D) Plots of H4K20me1 and H4K20me3 signal centered at intergenic regions at least 5 kb from any annotated feature. (E) Box plots of overall H4K20me1 and H4K20me3 signals on each chromosome. Each box shows the median and extends from the 25th to the 75th percentile of the z-scores in the set; whiskers extending from the box indicate the 2.5th and 97.5th percentiles. H4K20me1 is enriched in both coding and non-coding regions along the X chromosome in dosage-compensating XX hermaphrodites
In C. elegans, dosage compensation downregulates genes on the X chromosome. We therefore examined the pattern of H4K20me1 with respect to gene features. As seen previously in early embryos and L3 larvae [15], H4K20me1 is enriched across active gene regions at all developmental stages examined, with high enrichment on X-linked genes and lower enrichment on autosomal genes (Figure 1C). H4K20me1 levels are positively correlated with transcript levels for both X-linked and autosomal genes (rs = 0.66 and rs = 0.56, respectively). Notably, inactive X-linked genes have higher levels of H4K20me1 than active autosomal genes (Figure 1C). On autosomes H4K20me1 is primarily confined to transcribed regions, while on the X, H4K20me1 is elevated across the chromosome, including regions that are more than 5 kb from any annotated gene feature (Figure 1D). This widespread distribution of H4K20me1 on the X chromosome suggests a role in chromosome-wide regulation of gene expression.
H4K20me1 is enriched on the X after the onset of dosage compensation
Our ChIP experiments from early and late stage embryos, L3s, and young adults showed that the timing of H4K20me1 enrichment on the X chromosome is consistent with a role in dosage compensation. To determine more precisely when H4K20me1 becomes enriched on the X chromosome, we performed immunofluorescence experiments using two different antibodies specific for H4K20me1. In embryos prior to the 30-cell stage, neither DPY-27 nor H4K20me1 was concentrated in any subnuclear region (Figure 2A). However, we observed that nuclear staining of H4K20me1 increased dramatically during mitosis (Figure 2A and Figure S1). This is consistent with reports showing that in other organisms, H4K20me1 is present at high levels in mitosis and has a role in chromosome condensation [16]. At around the 30-cell stage, DPY-27 became localized to subregions of each nucleus that have been shown to be the X chromosomes [14] (Figure 2B, 2F). At this time, H4K20me1 was still distributed uniformly in the nucleus (Figure 2B, 2F). In embryos beginning the differentiation stage, H4K20me1 staining began to concentrate on the X chromosomes, as evidenced by colocalization with DPY-27, and this pattern became widespread in somatic cells throughout hermaphrodite development (Figure 2D, 2E, 2G; Figure S2A). Therefore, localization of the DCC to the X chromosome precedes enrichment of H4K20me1.
Fig. 2. H4K20me1 becomes concentrated on the X chromosome after the DCC. 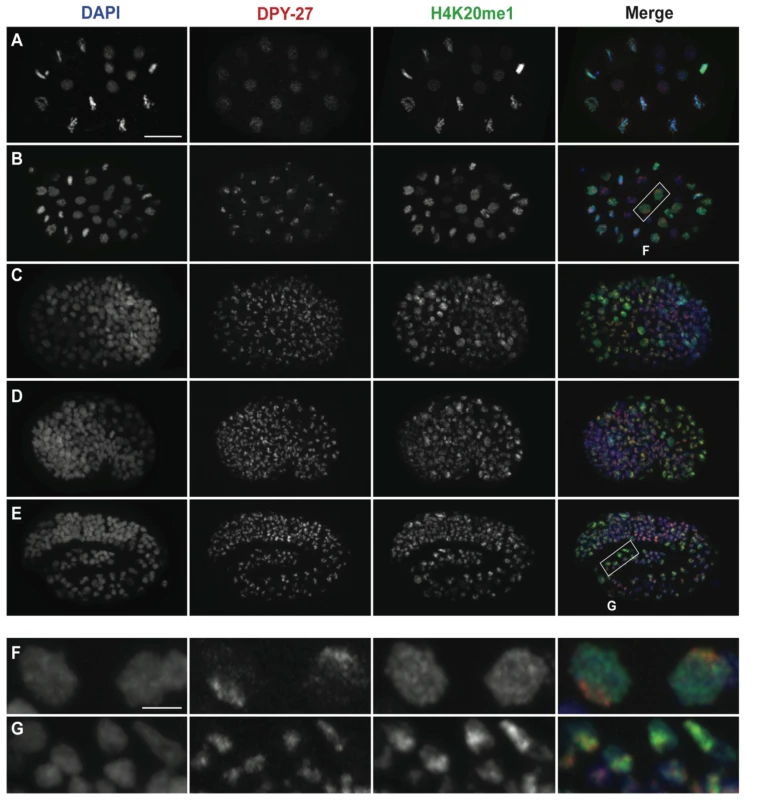
Wild-type embryos at (A) 20-cell, (B) 40-cell, (C) bean, (D) comma, and (E) three-fold stages stained for DNA (DAPI, blue, left column), DPY-27 (red, second column), and H4K20me1 (green, third column). Boxed regions in B and E are enlarged in F and G, respectively. In very early embryos (A) DPY-27 exhibits weak and diffuse nuclear staining while H4K20me1 is diffuse in interphase nuclei and associated with condensed chromatin during mitosis. By the ∼40-cell stage (B, F) DPY-27 is enriched on the X chromosome, while H4K20me1 continues to show a uniform nuclear distribution. Comma-stage embryos (D) contain some nuclei with H4K20me1 enrichment on the X. This enrichment becomes more apparent as embryogenesis progresses, and by the 3-fold stage (E, G) H4K20me1 is concentrated in bright foci that co-localize with DPY-27 on the X. Scale bars represent 10 um (A–E) and 2 um (F, G). Monoclonal antibody15F11 was used to detect H4K20me1. H4K20me1 is reduced on the X in the germ line during meiotic silencing
In the germ line, the DCC does not localize to the X chromosome [10]. The X chromosome is largely silent, and the silent state is mediated by a mechanism independent from somatic dosage compensation [10], [17], [18], [19]. We observed a distinct and dynamic pattern of H4K20me1 staining in the adult germ line. H4K20me1 levels were highest in the distal mitotic region, and decreased as nuclei entered meiosis (Figure S3A). Early meiotic nuclei entering the transition zone showed similar H4K20me1 levels on X and autosomes (Figure S3B). As nuclei progressed through the transition zone into early pachytene, H4K20me1 levels were much lower on the X chromosome than on autosomes (Figure S3B). The X chromosome is silent during early and mid-pachytene [18]. Later in meiotic prophase, H4K20me1 levels increased on the X, coincident with activation of X-linked gene expression (Figure S3B; [18]). In contrast to the enrichment of H4K20me1 on the X chromosome in somatic nuclei, levels of H4K20me1 in late meiotic prophase were similar on the X chromosome and autosomes (Figure S3B). To summarize, H4K20me1 shows a dynamic localization pattern on germline chromatin, with two characteristics of localization shared with somatic chromatin. First, enrichment of H4K20me1 is observed on mitotic chromosomes. Second, in meiotic nuclei, H4K20me1 is associated with chromatin that has active gene expression.
H4K20me1 enrichment on the X depends on DCC function
To test whether H4K20me1 enrichment on the X chromosome depends on the DCC, we carried out H4K20me1 ChIP-chip experiments using extracts from dpy-21 mutant L3 larvae, which are deficient in dosage compensation [12]. Strikingly, H4K20me1 enrichment on the X relative to autosomes was abolished in dpy-21 mutants (Figure 1B–1F), indicating that the DCC facilitates enrichment of H4K20me1 on the X. To further investigate this link, we asked whether the DCC could increase H4K20me1 levels at ectopic sites. Previous studies showed that DCC proteins bind recruiting elements on the X and then spread to neighboring regions that lack recruiting sites [6], [20]. Spreading is observed in chromosomal fusions between the X chromosome (which contains recruiting sites) and an autosome (which lacks recruiting sites; Figure 3). Using this assay, we observed spreading of H4K20me1 into the autosomal region flanking the site of the fusion, similar to that seen for the DCC component DPY-27 (Figure 3). This is consistent with a direct role for the DCC in generating enrichment of H4K20me1 on the X.
Fig. 3. H4K20me1 ectopically spreads onto the autosomal region of an X;II fusion chromosome. 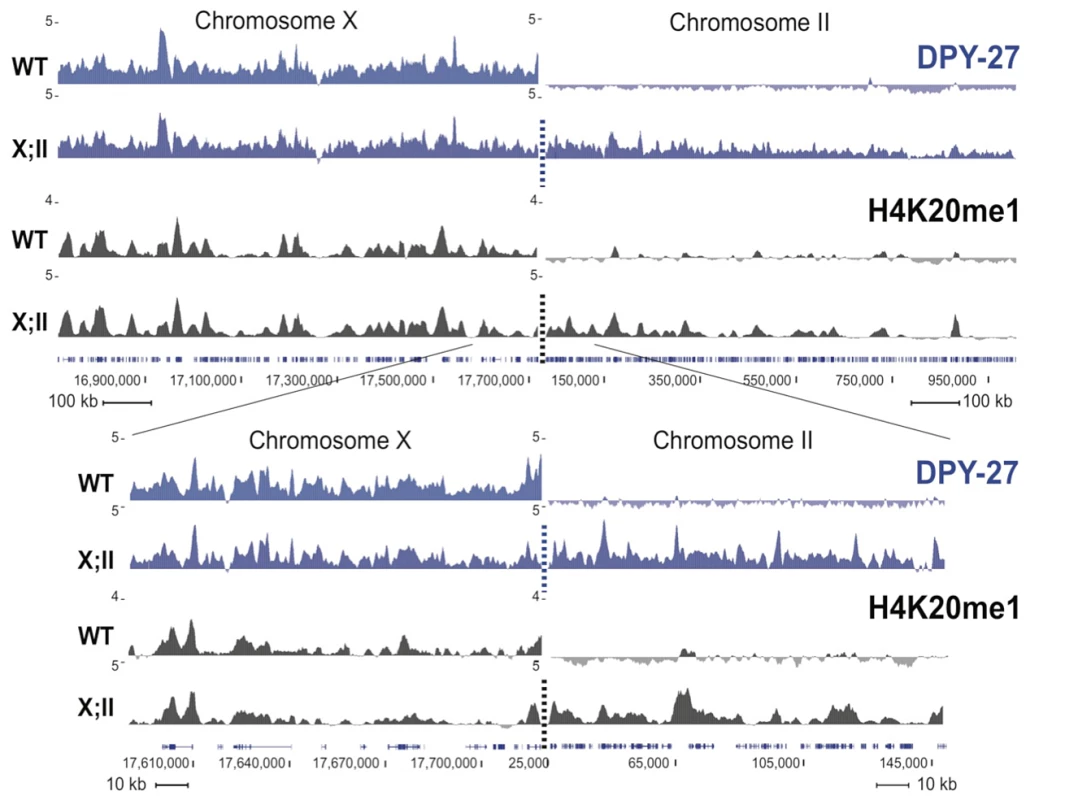
H4K20me1 ChIPs were performed in wild-type (WT) and YPT41 mixed stage embryos. YPT41 carries a fusion of the right end of X to the left end of chromosome II. The fusion site is indicated with a dashed line. DPY-27 ChIP-chip and H4K20me1 ChIP-seq enrichment are shown in blue and black, respectively. For each track, the average of two independent biological replicates is shown as z-scores (standardized log2 ratios of ChIP/Input signals). Genome browser views of ∼2 Mb and ∼200 kb spanning the fusion site are shown on the top and below, respectively. To better define the timing and requirement for dosage compensation in H4K20me1 enrichment on the X, we performed immunofluorescence experiments on dosage compensation mutant embryos. H4K20me1 was not enriched on the X chromosome in any dosage compensation mutant tested (dpy-21, dpy-26, dpy-28, and dpy-30; Figure 4 and Figure S4). Instead, H4K20me1 was evenly distributed on all chromosomes. We next analyzed XO males, which do not undergo dosage compensation and found no H4K20me1 enrichment on the single X of XO embryos or XO adult somatic nuclei (Figure 4B, Figures S2B and S4B). We also observed no H4K20me1 enrichment to X in adult gut nuclei of DCC mutants, similar to [21] (Figure S2). These results indicate that the DCC is required for the X-chromosome enrichment of H4K20me1.
Fig. 4. H4K20me1 enrichment to the X chromosome depends on dosage compensation. 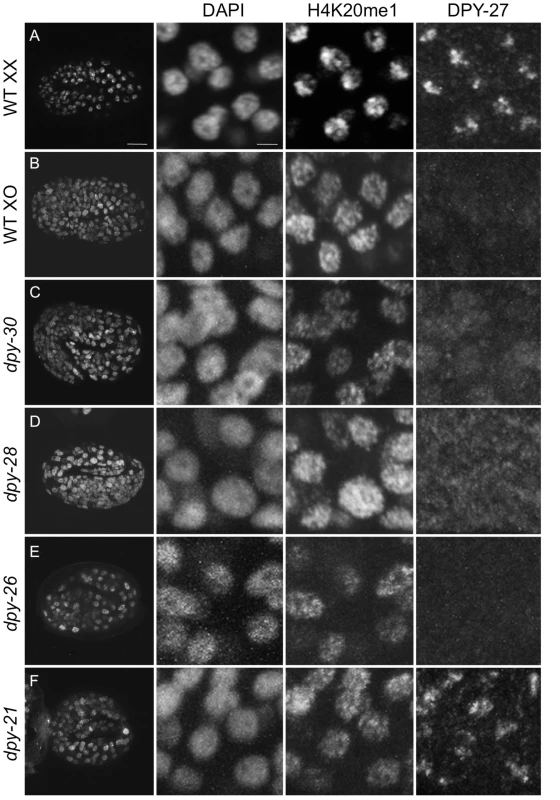
Three-fold embryos were stained with DAPI and antibodies to DPY-27 and H4K20me1. (A) Wild-type (WT) XX hermaphrodite, (B) him-8 XO male, (C) dpy-30(y228) (D) dpy-28(y1), (E) dpy-26(n199), and (F) dpy-21(e428) hermaphrodites. Left panel shows whole embryos, right panels show enlargements of nuclei. Foci of H4K20me1 X-chromosome enrichment are absent in males and dosage compensation mutants. Scale bars represent 10 um (left column) and 2 um (enlargements). Monoclonal antibody 15F11 antibody was used to detect H4K20me1. SET-1 and SET-4 enzymes generate H4K20me1 and H4K20me2/3, respectively
To determine whether H4K20me1 functions in dosage compensation, we sought to identify the enzymes responsible for methylation of H4K20. In other organisms, PR-Set7/SETD8 catalyzes monomethylation of H4K20 and Suv4-20 catalyzes di - and trimethylation of H4K20 [22], [23], [24]. The C. elegans orthologs of these proteins are SET-1 (PR-Set7/SETD8) and SET-4 (Suv4-20). Deletion mutants for both genes are available: set-1(tm1821) homozygotes develop into sterile adults (S. Mitani, pers. comm.), whereas set-4(n4600) homozygotes are viable and fertile [25].
Among embryos from fertile set-1/balancer mothers, heterozygous set-1/balancer embryos contain robust H4K20me1 while set-1 homozygous mutant embryos lack detectable H4K20me1 staining (Figure 5A). Furthermore, by western blot analysis, set-1 mutant adult extracts lack detectable H4K20me1, as well as H4K20me2 and H4K20me3 (Figure 5B). We conclude that SET-1 is the major histone methyltransferase required for generation of H4K20me1.
Fig. 5. The H4K20me1 methyltransferase SET-1 genetically interacts with dosage compensation mutants and is required for repression of X-linked gene expression. 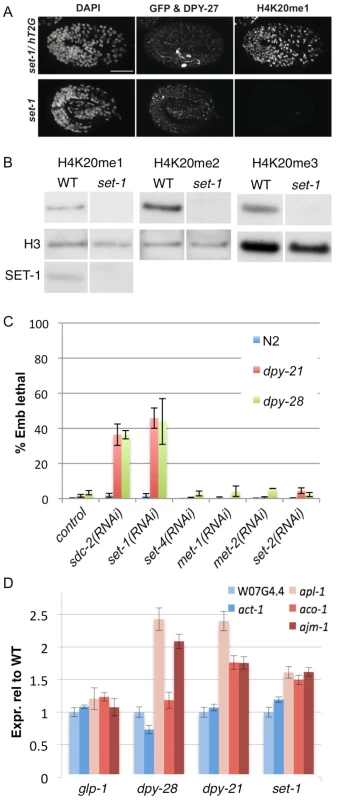
(A) Embryos were stained with DAPI and antibodies to DPY-27 and H4K20me1. In set-1/hT2G embryos (identified by pharyngeal GFP signal from the hT2G balancer chromosome), DPY-27 and H4K20me1 are enriched on the X chromosome. In homozygous set-1 mutant embryos, DPY-27 is enriched on the X, but H4K20me1 is undetectable. Scale bar represents 10 um. (B) Western blots of H4K20me1, H4K20me2, and H4K20me3 in wild-type (WT) and set-1(tm1821) mutant adults. The loading control is histone H3. SET-1 protein is not detected in set-1 mutant extract. (C) Tests of genetic interactions with dosage compensation mutants. Shown is % embryonic lethality of wild type (WT), dpy-21(e428) and dpy-28(y1ts) mutants after RNAi of the indicated gene. RNAi was performed by feeding as described in Methods. (D) Expression of autosomal genes in blue (W07G4.4 and act-1) and X-linked genes in red (aco-1, ajm-1, and apl-1) in the indicated mutants relative to wild-type. Expression levels are normalized to autosomal gene W07G4.4. Error bars show 95% confidence intervals. C. elegans SET-4 appears to be the major histone methyltransferase for converting H4K20me1 to H4K20me2 and H4K20me3. In set-4(n4600) homozygotes, we observed strongly reduced levels of H4K20me2 and H4K20me3 and increased levels of H4K20me1 compared to wild type, both by western blot and immunofluorescence analyses (Figure 6). In summary, our results show that SET-1 generates H4K20me1 and SET-4 converts H4K20me1 to H4K20me2 and H4K20me3, consistent with a recent report [21]. We note that DPY-27 localization appears normal in set-1 and set-4 mutants (Figure 5A and Figure 6B), suggesting that H4K20 methylations are not important for DCC recruitment.
Fig. 6. The SET-4 histone methyltransferase is necessary to generate H4K20me2 and H4K20me3. 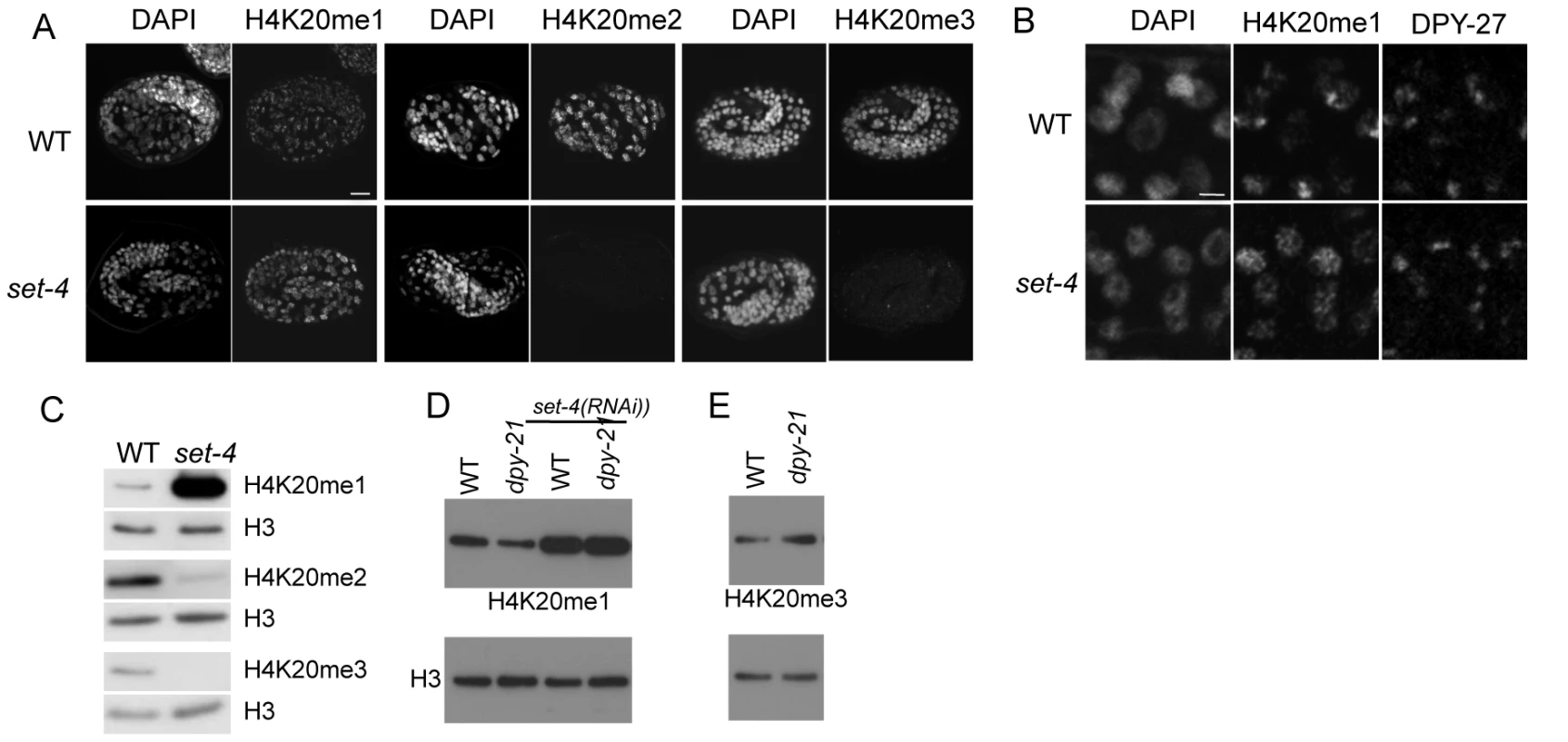
(A) Wild-type (WT) and set-4(n4600) mutant embryos were stained for DNA (DAPI), and H4K20me1, H4K20me2, or H4K20me3. H420me1 levels are higher and H4K20me2 and H4K20me3 levels strongly reduced in set-4 mutant embryos. Scale bar, 10 um. (B) Enlargements of nuclei in wild-type and set-4(n4600) mutant embryos stained for DNA (DAPI), H4K20me1, and DPY-27. In set-4 embryos, H4K20me1 does not show enrichment in DPY-27 positive regions. Confocal settings for H4K20me1 in set-4 were reduced to allow comparison with wild-type images. Scale bar, 2 um. (C) Western blots of extracts made from wild-type and set-4 adults, probed for H4K20me1, H420me2, or H4K20me3. Histone H3 is a loading control. Compared to wild type, set-4 mutants have increased H4K20me1 levels, and reduced H4K20me2 and H4K20me3 levels. (D, E) Western blots of H4K20me1 (D) and H4K20me3 (E) in wild-type and dpy-21 L3 mutant extracts. Lower panels show H3 loading controls. (D) H4K20me1 abundance is lower in dpy-21 than in wild type. (E) H4K20me3 abundance is higher in dpy-21 mutant L3 extract than in wild type. In (D) RNAi of set-4 was additionally performed. H4K20me1 abundance is elevated in both wild-type and dpy-21 after depletion of set-4. The following primary antibodies were used: H4K20me1 (Diagenode SN-147), H420me2 (Kimura 2E2), H4K20me3 (Abcam ab78517). The X chromosome has lower levels of H4K20me2/3 than autosomes
In contrast to H4K20me1, the nuclear distributions of H4K20me2 and H4K20me3 are relatively uniform. To examine the levels of H4K20me2 and H4K20me3 more closely, we. co-stained late stage embryos for DPY-27 to mark the X chromosome. We observed that H4K20me2 and H4K20me3 were depleted on the X chromosome relative to autosomes (Figure S5). These differences were not observed in the DCC mutant dpy-21 (Figure S5). We investigated the distribution of H4K20me3 at higher resolution using ChIP. Similar to other organisms (e.g. [26]), H4K20me3 is found at higher levels on inactive genes (bottom 20%) than on active genes (top 20%) (Figure 1C). However, overall levels are lower on the X chromosome compared to the autosomes, consistent with immunofluorescence results (Figure 1C–1E and Figure S5). We conclude that in hermaphrodites, the X chromosome has higher levels of H4K20me1 and lower levels of H4K20me2 and H4K20me3 relative to autosomes.
Regulation of the SET-4 H4K20me2/3 methyltransferase contributes to X-chromosome enrichment of H4K20me1
In principle, these patterns could be generated through differential activity of SET-1 or SET-4 on X versus autosomes. For example, SET-1 might be more active in generating H4K20me1 on the X chromosome compared to autosomes, or SET-4 could be more active in converting H4K20me1 to H4K20me2 or H4K20me3 on autosomes compared to the X. To distinguish between these possibilities, we investigated H4K20me1 patterns in set-4 mutant embryos. We observed that H4K20me1 levels in set-4 mutant embryos were elevated and uniformly distributed in all nuclear regions, with no detectable X-chromosome enrichment (Figure 6B, 6C; similar to [21]). Similarly, ChIP experiments show that H4K20me1 is present at similar levels on the X chromosome and autosomes in set-4 L3 mutant extracts (Figure S6). We conclude that the higher level of H4K20me1 on the X chromosome compared to the autosomes in wild type is achieved at least in part through higher SET-4 directed conversion of H4K20me1 to H4K20me2/3 on the autosomes.
If dosage compensation inhibits SET-4 from acting on the X chromosome, then dosage compensation mutants would be expected to have increased activity of SET-4 on the X, and as a result, reduced H4H20me1 and increased H4K20me3 levels compared to wild-type. To test this hypothesis, we carried out western blot analyses using dosage compensation mutant dpy-21 and sdc-1 L3 extracts. We found that the level of H4K20me1 is reduced in these mutants compared to wild type (Figure 6D and data not shown). Reduced H4K20me1 is due to inappropriate SET-4 activity, because levels are greatly increased in dpy-21; set-4(RNAi) or sdc-1; set-4(RNAi) animals (Figure 6D and data not shown). Furthermore, we found that the reduction in H4K20me1 in dpy-21 mutants is accompanied by an increase in the level of H4K20me3, as expected if SET-4 has increased activity (Figure 6E). These results support the hypothesis that dosage compensation inhibits SET-4 activity on the X chromosome, leading to relatively high H4K20me1 levels and relatively low H4K20me3 levels on X.
The SET-1 H4K20me1 methyltransferase is important for dosage compensation
We reasoned that if H4K20me1 is important for dosage compensation, then reducing H4K20me1 levels should impair this process. To test this hypothesis, we depleted SET-1 in two mutant backgrounds partially compromised for dosage compensation: null dpy-21(e428) mutants, which are viable, and a weak temperature-sensitive allele of dpy-28(y1ts), which shows low embryonic lethality at the permissive temperature [12], [27]. A complete lack of dosage compensation in XX hermaphrodites results in 100% embryonic lethality [28]. Therefore, further perturbing the dosage compensation process in the sensitized backgrounds should cause an increase in embryonic lethality. We first validated the assay by testing whether knockdown of three different DCC subunits causes synthetic lethality in the two dosage compensation mutant backgrounds described above. As expected, RNAi of sdc-2, dpy-27, or dpy-30 resulted in greatly increased lethality in dpy-21 and dpy-28(ts) mutant backgrounds compared to wild type (Figure 5C and data not shown). Using this assay we found that RNAi of set-1 induced synthetic lethality in dpy-21 and dpy-28(ts) mutant backgrounds, elevating embryonic lethality from 2% in a wild-type background to 46% and 44% in dpy-21 and dpy-28(ts), respectively (Figure 5C). RNAi of set-4 or of three other histone methyltransferases (met-1, met-2, set-2) had no effect (Figure 5C). The specific synthetic genetic interactions between set-1 and dosage compensation mutants support the view that H4K20me1 is important for dosage compensation.
In C. elegans, the DCC represses dosage-compensated genes on the X by approximately 2-fold. To test directly the role of H4K20me1 in this repression, we compared the expression of X-linked dosage-compensated genes to non-compensated autosomal genes in wild-type and set-1 mutant animals. Because set-1 homozygous mutants are sterile and the X chromosome is not dosage compensated in the germ line, we focused on genes expressed in the soma. In order to control for possible germline effects, we performed analyses in glp-1 mutant animals, which lack a germ line. Finally, as some X-linked genes are not dosage compensated, we chose X-linked genes that are upregulated in dosage compensation mutants dpy-21 or dpy-28. As expected, we found that the expression of three X-linked genes (aco-1, ajm-1, and apl-1) relative to two autosomal genes (W07G4.4 and act-1) is unchanged in glp-1 mutants and significantly increased in dpy-21 and/or dpy-28 mutants (Figure 5D). Similar to the DCC mutants, we observed a significant increase in the expression of dosage compensated X-linked genes relative to autosomal genes in the set-1 mutant (Figure 5D). These results show that H4K20 monomethylation mediated by SET-1 has a role in repression of dosage-compensated X-linked genes.
Discussion
Downregulation of X-linked gene expression during C. elegans dosage compensation allows study of gene expression mechanisms that act over large chromosomal regions. Previous studies have identified a condensin-like complex and other chromatin-associated proteins required for this process, but the mechanism by which these proteins lower X-linked gene transcription is not known. Here we show that the DCC generates X-linked enrichment of the post-transcriptional histone modification H4K20me1 and that this modification is important for dosage compensation.
As we observed here for C. elegans, H4K20me1 in other organisms is enriched on gene regions and its level is positively correlated with gene expression [29]. Although H4K20me1 levels are highest on actively transcribed genes, functional experiments in vertebrates and Drosophila point to a repressive role in gene expression. Knockdown of the H4K20me1 methyltransferase Pr-Set7 in human cells caused a two-fold upregulation of genes normally harbouring H4K20me1 [29], and mutation of Pr-Set7 in Drosophila leads to position effect variegation, a hallmark of genes required for heterochromatic gene repression [30]. H4K20me1 is also associated with the inactive X chromosome during X inactivation in vertebrates [31]. Therefore, the high levels of H4K20me1 we observed on the C. elegans X chromosome are consistent with a role in dosage compensation-mediated repression of gene expression.
What is the mechanism that leads to higher H4K20me1 levels and lower H4K20me3 levels on the hermaphrodite X chromosomes relative to autosomes? Our results suggest that this is achieved at least in part through DCC inhibition of SET-4 activity on X. Lower conversion of H4K20me1 to H4K20me2/3 by SET-4 on the X chromosome would lead to relatively higher H4K20me1 levels and lower H4K20me2/3 levels there. Several observations support this model. First, dosage compensation mutants show lower overall H4K20me1 levels and higher H4K20me3 levels compared to wild type. Second, the lower overall H4K20me1 level in DCC mutants is due to inappropriate SET-4 activity, supporting the idea that active dosage compensation inhibits SET-4 activity. Third, the difference in H4K20me1 levels on the X versus the autosomes is abolished in set-4 mutants, indicating a role for SET-4 in generating the asymmetry. We propose that a component of the DCC prevents SET-4 from acting on the X chromosome, leading to maintenance of H4K20me1 on X, whereas H4K20me1 is preferentially converted to H4K20me2/3 on the autosomes. Because H4K20me1 levels are similar on X and autosomes in set-4 mutants, SET-1 might be equally active in generating H4K20me1 on all chromosomes.
Our results suggest that of the three H4K20 methylation states, H4K20me1 is the key modification for dosage compensation. Whereas loss of dosage compensation leads to lethality of XX embryos, set-4 null mutants, which have strongly reduced levels of H4K20me2 and H4K20me3, are viable, and RNAi of set-4 does not enhance lethality of DCC mutants. This suggests that these modifications are not necessary for dosage compensation. In contrast, RNAi depletion of maternal and zygotic set-1 leads to loss of H4K20me1 and embryonic lethality, set-1 genetically interacts with dosage compensation mutants, and set-1 mutants show upregulation of X-linked gene expression.
When does H4K20me1 function in dosage compensation? The DCC is recruited to the X chromosome around the 30-cell stage whereas X-chromosome enrichment of H4K20me1 occurs several hours later. This difference in timing suggests that there might be two separable aspects of dosage compensation during embryogenesis, for example initiation and maintenance. Although the DCC is recruited to the X in early embryogenesis, it is not yet known when repression of X-linked gene expression is initiated. The DCC might be active immediately after recruitment or might become active later in embryogenesis. Furthermore, although H4K20me1 becomes highly enriched on X in late embryogenesis, it is possible that a basal level on the X chromosome is functional earlier. Because the DCC component DPY-27 shows apparently normal localization to the X chromosome in the absence of SET-1 or SET-4, H4K20me1 does not appear to be a recruitment signal for the DCC. Instead, H4K20me1 may be important for the function of the DCC in downregulating gene expression. Key future questions to address are when during embryogenesis X-linked gene expression is initially downregulated, and when H4K20me1 function is necessary.
Regulation of histone modification levels also occurs during dosage compensation in other organisms. For example, in Drosophila, where gene expression on the single X chromosome in males is upregulated two-fold to match that of the two X chromosomes in females, dosage compensation acts to increase H4K16ac levels on the single male X. In addition, the inactive X chromosome of female mammals displays high levels of several histone modifications, including H4K20me1 [31]. H4K20me1 enrichment correlates with Xist expression, is independent of transcriptional silencing, and marks the early steps of X inactivation [31].
In addition to the strong enrichment of H4K20me1 on the X chromosome in C. elegans, Liu et al. showed that several marks of gene activity, including H4K16ac, were lower on X linked genes than on autosomal genes [15]. Using immunofluorescence assays on gut nuclei, a recent report by Wells et al. showed that the X/A difference in H4K16ac levels depends on dosage compensation and on sir-2.1, a putative H4K16 deacetylase [21]. It is not clear if H4K16Ac plays a role in dosage compensation as depletion of sir-2.1 did not genetically interact with a DC mutant. The enzyme that generates H4K16Ac is not yet known.
Using immunofluorescence assays, Wells et al. also observed that H4K20me1 enrichment on X is dependent on dosage compensation, and on SET-1 and SET-4 [21]. Our immunofluorescence results are broadly similar, and our ChIP experiments give a higher resolution view, strengthening these conclusions. In support of a role for methylation of H4K20 in dosage compensation, Wells et al. observed that simultaneous reduction of set-1 and set-4 by RNAi could rescue mutant males that normally die due to active dosage compensation. However, the H4K20 methylation state was not determined after simultaneous depletion of set-1 and set-4, so the specific alteration of methylation of H4K20 that caused rescue is not known. The reported X/A differences in H4K16ac levels also depended on set-1 and set-4, suggesting that H4K16Ac might be regulated by H4K20 methylation state. Although the exact mechanisms of dosage compensation vary, studies in different organisms suggest that global regulation of H4K16ac and H4K20me1 levels might be a conserved feature of these chromosome-wide gene regulation mechanisms.
Our results indicate that H4K20me1 is important for repression of X-linked gene expression. How might H4K20me1 function in transcriptional repression? Several links in the literature suggest roles for H4K20me1 in chromatin compaction. For example, the Malignant-Brain-Tumor (MBT) domains of human L3MBTL1 compact nucleosomal arrays by recognizing mono and dimethylation of H4K20 and H1bK26 [32]. Furthermore, L3MBTL1 has transcription repressor activity that is enhanced by Pr-Set7, and its chromatin association depends on H4K20me1 [33], [34]. It is not yet known whether C. elegans MBT repeat proteins LIN-61 or MBTR-1 are involved in dosage compensation or bind H4K20me1.
H4K20me1 has also been shown to be important during mitosis. H4K20me1 levels are high on mitotic chromatin ([35], [36] and this study), and in mammalian cells inhibition of Pr-Set7 leads to defects in cell cycle progression [36], [37], [38], [39]. Although the function of H420me1 in cell cycle progression is not yet understood, a key aspect of the loss of function phenotype is reduced chromosome compaction. Furthermore, a recent study demonstrated that two components of condensin II, N-CAPD3 and N-CAPG2, can directly bind H4K20me1 [35]. This raises the exciting possibility that condensin IDC might function to compact chromatin through binding H4K20me1. Increased compaction of the X chromosome relative to the autosomes might reduce access by RNA polymerase, leading to lower X-linked gene expression. Consistent with this idea, DCC mutants were recently shown to have increased RNA polymerase II levels on the X [13]. We propose that condensin complexes and H4K20me1 might be intimately linked in diverse chromatin-regulating events.
Methods
Worm culture and strains
The following strains were used and cultured using standard methods (Brenner, 1974): TY0621 [yDp1(IV,V:f); dpy-26(n199) unc-30(e191) IV], DR1410 [dpy-27(y56)/qC1 III], TY1621 [unc-49(e382) dpy-28(y1ts) III], TY148 [dpy-28(y1ts) III], CB428 [dpy-21(e428) V], KK0423 [dpy-21(e428) par-4(it57ts) V], TY1936 [dpy-30(y228) V/nT1[unc-?(n754) let-?](IV;V)], SS1075 [set-1(tm1821)/hT2G(qIs48) I;III], JA1574 [set-1(tm1821)/hT2G(qIs48) I;III], MT14911 [set-4(n4600], YPT41 [X;II].
Chromatin immunoprecipitation analyses
Late embryo (LE) extracts were prepared by growing wild-type N2 adult worms from synchronized L1s in standard S-basal medium with shaking. Worms were fed HB101 E. coli and grown at 20°C until they were gravid, approximately 70 hours. Embryos were obtained by dissolving adult worms with bleach, and then the embryos were aged by incubating in M9 media for 3.5 hrs at 20°C with gentle rocking. The embryos were washed once with PBS and flash frozen in liquid nitrogen, then processed for ChIP as in [40]. Wild-type, dpy-21(e428), and set-4(n4600) L3 and fem-2 YA animals were grown and ChIPs performed as in [41] except that DNA was sonicated to a size range of 200–400 bp. EE and L3 in Figure 1 are from [15] (Abcam ab9051; lot 104513). H4K20me1 ChIP conditions were: LE (1 mg extract and 5 ul Diagenode SN-147), fem-2 young adults (1.25 mg extract and 3 ug Abcam ab9051 lot 104513), dpy-21 L3s (500 ug extract and 2 ug Abcam ab9051 lot 104513), WT and set-4 L3s in Figure S6 (500 ug extract and 2 ug Abcam ab9051 lot 602259), WT L3 in Figure S7 (500 ug extract and 5 ul Diagenode SN-147). The Diagenode SN-147 and Abcam ab9051 antibodies give concordant patterns by ChIP in L3 extracts (Figure S7A, S7B). H4K20me3 ChIP conditions: 500 ug L3 extract and XX Abcam 78517 lot 827718. Antibodies used for ChIP, western blot, or immunofluorescence were tested for specificity to histone peptide tails using dot blots ([42] and http://compbio.med.harvard.edu/antibodies/).
Early Embryo, Late Embryo, L3, and dpy-21 L3 H4K20me1 ChIPs and L3 H4K20me3 ChIPs were hybridized to a C. elegans full-genome tiled microarray (NimbleGen 2.1, Roche). For H4K20me1 ChIPs, log2 ratios of IP/Input were obtained and standardized so the autosomal signal had mean 0 and standard deviation 1. The H4K20me3 L3 ChIP dataset was processed similarly except that all chromosome regions were used. fem-2 young adult (Figure 1) and WT and set-4 L3 H4k20me1 ChIPs in Figure S6 were sequenced on the Illumina platform, aligned using BWA with default settings [43], normalized using BEADS [44], then converted to log2 ratios of BEADS scores (enrichment relative to input) and standardized so the mean of the autosomal signal was 0 and the standard deviation 1. Biological ChIP-chip and ChIP-seq replicates were averaged after standardization. Gene profile plots (Figure 1 and Figure S6) were generated by aligning genes at their TSS and TES (WS190/ce6). The genomic regions 1 kb upstream to 1 kb downstream from TSS and 1.5 kb upstream to 1 kb downstream from TES were divided into 50-bp bins. Genes were grouped into top 20% and bottom 20% expressed and autosomal and X-linked genes. Mean signals for each group of genes and each bin were plotted as well as the 95% confidence intervals of the mean (as error bars). Profile plots of intergenic regions were obtained by first identifying regions of at least 10 kb length without any annotation (WS190/ce6) (491 such regions on autosomes, 193 on chromosome X). The regions were aligned at their center and the genomic regions from 5 kb upstream to 5 kb downstream from the center were divided into 50 bp bins. Mean signals for the autosomal and X intergenic regions were plotted as well as the 95% confidence intervals of the means.
Boxplots (Figure 1 and Figure S7) were obtained after 1 kb median smoothing along the chromosomes of the respective standardized log2 ratios. Each box indicates the median with the center line and extends from the 25th to the 75th percentile of the standardized log2 ratios; whiskers extending from the box indicate the 2.5th and 97.5th percentiles.
Transcript data for the EE, LE, L3 and YA stages were obtained from the modENCODE DCC (http://intermine.modencode.org). The platform was a single color 4-plex Nimblegen expression array with 72,000 probes (three 60-mer oligo probes per gene). Quantile normalization [45] and the Robust Multichip Average (RMA) algorithm [46] were used to normalize and summarize the multiple probe values per gene to obtain one expression value per gene and sample.
Western blotting
Sample buffer was added directly to worms, samples heated to 65°C for 10 minutes, sonicated for 15 minutes (30 sec in/30 sec out), incubated at 65°C for 5 min and finally boiled at 95°C for 5 min. Proteins were separated on 4–12% NuPage SDS pre-cast gels (Invitrogen). The following antibodies were used: anti-H4K20me1 (Abcam ab9051 at 1∶2000), anti-H4K20me2 (H. Kimura monoclonal antibody 2E2 at 1∶20,000), anti-H4K20me3 (Abcam ab78517 at 1∶500), anti-H3 (Active Motif 39163 at 1∶8000), and anti-SET-1 (SDI SDQ3895). JA1574 was used to obtain set-1 homozygotes in Figure 5B.
Immunostaining
Immunostaining of embryos and dissected intestines was done using methanol/acetone fixation as in [47] (Figure 2; Figure 5; Figure S1, S2, and S4) or using methanol fixation as in [48] (Figure 4, Figure 6, Figure S5). Germline immunofluorescence experiments (Figure S3) were carried out after dissecting worms in egg buffer containing 0.1% Tween 20 and fixation in 1% formaldehyde in egg buffer [49]. SS1075 was used for the IF experiment in Figure 5A. Primary antibodies used are indicated in the figure legends. Antibody concentrations used were: anti-H4K20me1 Kimura 1F11 (1∶40,000), anti-H4K20me1 Diagenode SN-147 (1∶100,000), anti-H4K20me1 Abcam ab9051 (1∶400 for Figure S1; 1∶5000 for Figure S3), anti-H4K20me2 Kimura 2E2 (1∶25,000), anti-H4K20me3 ab78517 (1∶200), anti-DPY-27 SDI SDQ3995 (1∶4000), anti-GFP Abcam ab290 (1∶6000), anti-H3S10p Kimura 10H12 (1∶1000), anti-HIM-8 (1∶250). Secondary antibodies were purchased from Jackson ImmunoResearch or Molecular Probes.
qPCR quantification of gene expression
Total RNA was extracted from N2 (wild type), dpy-21(e428), dpy-28(y1), or set-1(tm1821) L3 worms grown at 25°C using TriPure (Roche). RNA was further purified using an RNeasy column (Qiagen). Reverse transcription was carried out using the Invitrogen SuperScript III First-Strand Synthesis System. Quantitative PCR was performed using primers specific to the target genes:
Chromosome V:
W07G4.4 F: GCAATCGCTCCAGCCGTTAACAAT; R: TCGTCCAGATGGAACGACAGATGA
act-1 F: TGCAGAAGGAAATCACCGCTCTTG; R: AAGCACTTGCGGTGAACGATGGAT
Chromosome X:
apl-1 F: ACGACGACGATGAGGATGATGCTT; R: TGAACTTCTCGGCTCCCTTTGGAT
aco-1 F: CAAGATCAACCCAGTATGCCCAGT; R: ACCTGATGGACGATTCCAGATCCT
ajm-1 F: TCGTCTTGATGAGATGGAACGCGA; R: AAGTTCTGCGTTACGTTGGGCTTG
Gene expression levels in each strain were normalized to the levels of the autosomal gene W07G4.4. Expression of each gene in wild-type N2 was then set to 1 and mutant expression levels expressed relative to N2.
Genetic interaction tests
RNAi by feeding was performed on N2 (wild type), dpy-21(e428) and dpy-28(y1) animals as follows: 3–5 L3 larvae were placed on RNAi bacteria for three days at 15°C, transferred to new RNAi plates for 24 hours, transferred again after 24 hours, then removed. The progeny on the latter two plates were scored for embryonic lethality. We note that RNAi of set-1 leads to embryonic lethality in the N2 (wild-type) background in embryos produced in the next 24 hours of RNAi feeding, or if RNAi is performed by injection (not shown). RNAi plates were prepared as in [50]. The following RNAi clones were used from [51], [52]: sdc-2 (C35C5.1), set-1 (T26A5.7), set-2 (C26E6.9), met-1 (C43E11.3), and met-2 (R05D3.11). The set-4 RNAi clone was made by PCR amplifying and cloning a portion of the set-4 gene into RNAi feeding vector L4440. The primers used were: atacgaattcacaggtcggc and tgctactacgcttgtcgtcg. RNAi plasmids were in the HT115(DE3) bacterial strain, which was used as the control. All RNAi clones were verified by sequencing.
Assaying ectopic spreading
H4K20me1 ChIPs were performed in mixed stage embryos from wild-type N2 normal karyotype strain (2 replicates) and in YPT41 X;II fusion strain (2 replicates) using methods described previously [53]. 1 mg of total embryo extract and 2 ug of H4K20me1 antibody (Abcam ab9051) were used per ChIP. The ChIP DNA was prepared for Illumina multiplex sequencing with slight modifications to the manufacturers protocol [54]. Briefly, sequencing libraries were prepared from half of the ChIP DNA and 10 ng of corresponding input DNA. NEB Klenow, T4 DNA polymerase and T4 PNK were used to repair ends at 20°C for 30 min. Exo(-) Klenow fragment and dATP was used to add adenosine at the 3′ ends for 1 hour at 37°C. DNA was ligated to multiplex adaptors (Illumina) and amplified by PCR, introducing the following indices: N2 H4K20me1 ChIP index #6 (GCCAAT), N2 Input index #12 (CTTGTA), YTP41 H4K20me1 ChIP replicate 1 index #2 (CGATGT), input index #3 (TTAGGC), and H4K20me1 ChIP replicate 2 index #1 (ATCACG), input index #5 (ACAGTG). DNA between 200–500 bp in size was gel purified. Multiplexed single-end sequencing was performed by GAIIx at the UNC high-throughput sequencing facility. The sequencing reads, obtained from the Illumina pipeline in fastq format, were aligned to the ce6 (WS190) version of the C. elegans genome with Bowtie [55], using default parameters. Each sequence read was extended to calculate coverage per base pair using MACS [56]. The coverage from the input data was subtracted from that of the ChIP data, and ChIP enrichment was standardized by z score transformation.
Datasets
Accession numbers for datasets generated in this paper are listed in Table S1.
Supporting Information
Zdroje
1. LeeJT (2011) Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control. Nat Rev Mol Cell Biol 12 : 815–826.
2. StraubT, BeckerPB (2011) Transcription modulation chromosome-wide: universal features and principles of dosage compensation in worms and flies. Curr Opin Genet Dev 21 : 147–153.
3. MeyerBJ (2010) Targeting X chromosomes for repression. Curr Opin Genet Dev 20 : 179–189.
4. WoodAJ, SeversonAF, MeyerBJ (2010) Condensin and cohesin complexity: the expanding repertoire of functions. Nat Rev Genet 11 : 391–404.
5. CsankovszkiG, ColletteK, SpahlK, CareyJ, SnyderM, et al. (2009) Three distinct condensin complexes control C. elegans chromosome dynamics. Curr Biol 19 : 9–19.
6. ErcanS, DickLL, LiebJD (2009) The C. elegans dosage compensation complex propagates dynamically and independently of X chromosome sequence. Curr Biol 19 : 1777–1787.
7. JansJ, GladdenJM, RalstonEJ, PickleCS, MichelAH, et al. (2009) A condensin-like dosage compensation complex acts at a distance to control expression throughout the genome. Genes Dev 23 : 602–618.
8. ChuangPT, LiebJD, MeyerBJ (1996) Sex-specific assembly of a dosage compensation complex on the nematode X chromosome. Science 274 : 1736–1739.
9. DavisTL, MeyerBJ (1997) SDC-3 coordinates the assembly of a dosage compensation complex on the nematode X chromosome. Development 124 : 1019–1031.
10. LiebJD, CapowskiEE, MeneelyP, MeyerBJ (1996) DPY-26, a link between dosage compensation and meiotic chromosome segregation in the nematode. Science 274 : 1732–1736.
11. NonetML, MeyerBJ (1991) Early aspects of Caenorhabditis elegans sex determination and dosage compensation are regulated by a zinc-finger protein. Nature 351 : 65–68.
12. YonkerSA, MeyerBJ (2003) Recruitment of C. elegans dosage compensation proteins for gene-specific versus chromosome-wide repression. Development 130 : 6519–6532.
13. PferdehirtRR, KruesiWS, MeyerBJ (2011) An MLL/COMPASS subunit functions in the C. elegans dosage compensation complex to target X chromosomes for transcriptional regulation of gene expression. Genes Dev 25 : 499–515.
14. ChuangPT, AlbertsonDG, MeyerBJ (1994) DPY-27: a chromosome condensation protein homolog that regulates C. elegans dosage compensation through association with the X chromosome. Cell 79 : 459–474.
15. LiuT, RechtsteinerA, EgelhoferTA, VielleA, LatorreI, et al. (2011) Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res 21 : 227–236.
16. BeckDB, OdaH, ShenSS, ReinbergD (2012) PR-Set7 and H4K20me1: at the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev 26 : 325–337.
17. FongY, BenderL, WangW, StromeS (2002) Chromatin States of Autosomes and X Chromosomes in the Germ Line of C. elegans. Science 296 : 2235–2238.
18. KellyWG, SchanerCE, DernburgAF, LeeMH, KimSK, et al. (2002) X-chromosome silencing in the germline of C. elegans. Development 129 : 479–492.
19. ReinkeV, SmithHE, NanceJ, WangJ, van DorenC, et al. (2000) A global profile of germline gene expression in C. elegans.. Mol Cell 6 : 605–616.
20. LiebJD, de SolorzanoCO, RodriguezEG, JonesA, AngeloM, et al. (2000) The Caenorhabditis elegans dosage compensation machinery is recruited to X chromosome DNA attached to an autosome. Genetics 156 : 1603–1621.
21. WellsMB, SnyderMJ, CusterLM, CsankovszkiG (2012) C. elegans Dosage Compensation Regulates Histone H4 Chromatin State on X. Mol Cell Biol in press.
22. NishiokaK, RiceJC, SarmaK, Erdjument-BromageH, WernerJ, et al. (2002) PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell 9 : 1201–1213.
23. SchottaG, LachnerM, SarmaK, EbertA, SenguptaR, et al. (2004) A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev 18 : 1251–1262.
24. YangH, PesaventoJJ, StarnesTW, CrydermanDE, WallrathLL, et al. (2008) Preferential dimethylation of histone H4 lysine 20 by Suv4-20. J Biol Chem 283 : 12085–12092.
25. AndersenEC, HorvitzHR (2007) Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development 134 : 2991–2999.
26. BarskiA, CuddapahS, CuiK, RohTY, SchonesDE, et al. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129 : 823–837.
27. PlenefischJD, DeLongL, MeyerBJ (1989) Genes that implement the hermaphrodite mode of dosage compensation in Caenorhabditis elegans. Genetics 121 : 57–76.
28. MeyerBJ (2005) X-Chromosome dosage compensation. WormBook 1–14.
29. YangH, MizzenCA (2009) The multiple facets of histone H4-lysine 20 methylation. Biochem Cell Biol 87 : 151–161.
30. KarachentsevD, SarmaK, ReinbergD, StewardR (2005) PR-Set7-dependent methylation of histone H4 Lys 20 functions in repression of gene expression and is essential for mitosis. Genes Dev 19 : 431–435.
31. KohlmaierA, SavareseF, LachnerM, MartensJ, JenuweinT, et al. (2004) A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol 2: e171 doi:10.1371/journal.pbio.0020171.
32. TrojerP, LiG, SimsRJ3rd, VaqueroA, KalakondaN, et al. (2007) L3MBTL1, a histone-methylation-dependent chromatin lock. Cell 129 : 915–928.
33. BoccuniP, MacGroganD, ScanduraJM, NimerSD (2003) The human L(3)MBT polycomb group protein is a transcriptional repressor and interacts physically and functionally with TEL (ETV6). J Biol Chem 278 : 15412–15420.
34. KalakondaN, FischleW, BoccuniP, GurvichN, Hoya-AriasR, et al. (2008) Histone H4 lysine 20 monomethylation promotes transcriptional repression by L3MBTL1. Oncogene 27 : 4293–4304.
35. LiuW, TanasaB, TyurinaOV, ZhouTY, GassmannR, et al. (2010) PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature 466 : 508–512.
36. OdaH, OkamotoI, MurphyN, ChuJ, PriceSM, et al. (2009) Monomethylation of histone H4-lysine 20 is involved in chromosome structure and stability and is essential for mouse development. Mol Cell Biol 29 : 2278–2295.
37. HoustonSI, McManusKJ, AdamsMM, SimsJK, CarpenterPB, et al. (2008) Catalytic function of the PR-Set7 histone H4 lysine 20 monomethyltransferase is essential for mitotic entry and genomic stability. J Biol Chem 283 : 19478–19488.
38. JorgensenS, ElversI, TrelleMB, MenzelT, EskildsenM, et al. (2007) The histone methyltransferase SET8 is required for S-phase progression. J Cell Biol 179 : 1337–1345.
39. TardatM, MurrR, HercegZ, SardetC, JulienE (2007) PR-Set7-dependent lysine methylation ensures genome replication and stability through S phase. J Cell Biol 179 : 1413–1426.
40. RechtsteinerA, ErcanS, TakasakiT, PhippenTM, EgelhoferTA, et al. (2010) The histone H3K36 methyltransferase MES-4 acts epigenetically to transmit the memory of germline gene expression to progeny. PLoS Genet 6 doi:10.1371/journal.pgen.1001091.
41. Kolasinska-ZwierzP, DownT, LatorreI, LiuT, LiuXS, et al. (2009) Differential chromatin marking of introns and expressed exons by H3K36me3. Nat Genet 41 : 376–381.
42. EgelhoferTA, MinodaA, KlugmanS, LeeK, Kolasinska-ZwierzP, et al. (2010) An assessment of histone-modification antibody quality. Nat Struct Mol Biol 18 : 91–93.
43. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
44. CheungMS, DownTA, LatorreI, AhringerJ (2011) Systematic bias in high-throughput sequencing data and its correction by BEADS. Nucleic Acids Res 39: e103.
45. BolstadBM, IrizarryRA, AstrandM, SpeedTP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19.
46. IrizarryRA, ale (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15.
47. StromeS, WoodWB (1983) Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35 : 15–25.
48. AndrewsR, AhringerJ (2007) Asymmetry of early endosome distribution in C. elegans embryos. PLoS ONE 2: e493 doi:10.1371/journal.pone.0000493.
49. DernburgAF, McDonaldK, MoulderG, BarsteadR, DresserM, et al. (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94 : 387–398.
50. Ahringer (ed.) J Reverse genetics. In: Community TCeR, editor. Wormbook: Wormbook.
51. FraserAG, KamathRS, ZipperlenP, Martinez-CamposM, SohrmannM, et al. (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 : 325–330.
52. KamathRS, FraserAG, DongY, PoulinG, DurbinR, et al. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 : 231–237.
53. ErcanS, GiresiPG, WhittleCM, ZhangX, GreenRD, et al. (2007) X chromosome repression by localization of the C. elegans dosage compensation machinery to sites of transcription initiation. Nat Genet 39 : 403–408.
54. ErcanS, LublingY, SegalE, LiebJD (2010) High nucleosome occupancy is encoded at X-linked gene promoters in C. elegans. Genome Res 21 : 237–244.
55. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
56. ZhangY, LiuT, MeyerCA, EeckhouteJ, JohnsonDS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137.
Štítky
Genetika Reprodukčná medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



