-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
Tandem direct duplications are a common feature of the genomes of eukaryotes ranging from yeast to human, where they comprise a significant fraction of copy number variations. The prevailing model for the formation of tandem direct duplications is non-allelic homologous recombination (NAHR). Here we report the isolation of a series of duplications and reciprocal deletions isolated de novo from a maize allele containing two Class II Ac/Ds transposons. The duplication/deletion structures suggest that they were generated by alternative transposition reactions involving the termini of two nearby transposable elements. The deletion/duplication breakpoint junctions contain 8 bp target site duplications characteristic of Ac/Ds transposition events, confirming their formation directly by an alternative transposition mechanism. Tandem direct duplications and reciprocal deletions were generated at a relatively high frequency (∼0.5 to 1%) in the materials examined here in which transposons are positioned nearby each other in appropriate orientation; frequencies would likely be much lower in other genotypes. To test whether this mechanism may have contributed to maize genome evolution, we analyzed sequences flanking Ac/Ds and other hAT family transposons and identified three small tandem direct duplications with the structural features predicted by the alternative transposition mechanism. Together these results show that some class II transposons are capable of directly inducing tandem sequence duplications, and that this activity has contributed to the evolution of the maize genome.
Published in the journal: Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements. PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003691
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003691Summary
Tandem direct duplications are a common feature of the genomes of eukaryotes ranging from yeast to human, where they comprise a significant fraction of copy number variations. The prevailing model for the formation of tandem direct duplications is non-allelic homologous recombination (NAHR). Here we report the isolation of a series of duplications and reciprocal deletions isolated de novo from a maize allele containing two Class II Ac/Ds transposons. The duplication/deletion structures suggest that they were generated by alternative transposition reactions involving the termini of two nearby transposable elements. The deletion/duplication breakpoint junctions contain 8 bp target site duplications characteristic of Ac/Ds transposition events, confirming their formation directly by an alternative transposition mechanism. Tandem direct duplications and reciprocal deletions were generated at a relatively high frequency (∼0.5 to 1%) in the materials examined here in which transposons are positioned nearby each other in appropriate orientation; frequencies would likely be much lower in other genotypes. To test whether this mechanism may have contributed to maize genome evolution, we analyzed sequences flanking Ac/Ds and other hAT family transposons and identified three small tandem direct duplications with the structural features predicted by the alternative transposition mechanism. Together these results show that some class II transposons are capable of directly inducing tandem sequence duplications, and that this activity has contributed to the evolution of the maize genome.
Introduction
In addition to generating additional copies of coding sequences that can be used as substrates for gene evolution [1], gene duplication may also cause immediate phenotypic impacts such as human disease [2]. Segmental duplications (SD)–two or more chromosomal segments with high homology–are common in higher plant and animal genomes. In humans and mice, ∼5% of the genome is composed of segmental duplications (≥90% in identity and ≥1 kb in length); tandem duplications (direct and inverted) account for 35.2% and 21.6% of the total duplications in the mice and human genomes, respectively [3], [4]. Many plants contain an even higher percentage of duplicated sequences. In rice, segmental duplications comprise 15–62% of the genome, depending on the sequences compared and classification criteria employed [5]–[8]. Moreover, ca. 29% of rice genes are arranged in tandem repeats [9]. Recent studies by others have also confirmed the presence of numerous duplicated sequences in the maize genome [10]–[14]. Comparison of genome sequences from different individuals of the same species revealed that copy number variation (CNV) is widespread, and that tandem duplications account for a significant proportion of the observed CNV. In Arabidopsis and maize, more than 50% of CNV segments contain tandem duplications [15]–[17]. In cattle and mice, copy number “gain” CNVs are predominantly associated with tandem local duplications, rather than interspersed duplications [18]. These observations indicate that CNVs and associated tandem duplications are contributing to rapid genome evolution.
There are several mechanisms proposed to generate tandem duplications, including 1) non-allelic homologous recombination (NAHR) between short repeats flanking a DNA segment [19], [20]; 2) break-induced replication (BIR) [21], [22] which can be mediated by short microhomology regions [19], [23]; and 3) fork stalling and template switching (FoSTeS) [24]. Here, we investigated the potential role of Class II transposable elements in directly generating tandem sequence duplications via aberrant transposition reactions.
The standard model for transposition of DNA elements involves excision of the termini of a single transposon from a donor locus and reinsertion into a target site; the net effect is the movement of the element, without any other changes to the genome. In contrast, Alternative Transposition (AT) events involve the termini of two separate, usually nearby elements. AT reactions can generate a variety of genome rearrangements; for example, the Drosophila P element system can undergo Hybrid Element Insertion (HEI) events that produce a wide array of flanking rearrangements [25]–[27] In maize, the Ac/Ds transposable element system is known to undergo at least two types of AT events that lead to genome rearrangements. First, Sister Chromatid Transposition (SCT) involves the directly-oriented 5′ and 3′ termini of closely-linked elements located on sister chromatids. Depending on the location of the transposition target site, SCT can generate chromatid bridges and breaks [28], [29], as well as flanking inverted duplications and deletions [30]. Second, Reversed Ends Transposition (RET) involves the reversely-oriented 5′ and 3′ termini of two elements located nearby each other on the same chromatid. In addition to bridges and breaks [29], RET can generate flanking inversions, deletions, permutations, and reciprocal translocations [31], [32]. An additional type of AT event termed Single Chromatid Transposition (SLCT) which involves the directly-oriented 5′ and 3′ termini of nearby elements on the same chromatid has been observed in transgenic rice containing maize Ac/Ds elements, but this reaction was not detected in maize [33].
We predicted that RET may also generate tandem direct duplications. Here we show that a single pair of reversed Ac termini induced a series of nine flanking tandem duplications ranging in size from 8157 bp to ∼5.3 Mbp. The structures of these tandem duplications and their associated deletions strongly indicate that they were indeed generated by reversed Ac ends transposition. Moreover, we identified three tandem duplications flanking other hAT transposons with the features predicted by RET in the maize B73 reference genome sequence.
Results
Identification of duplication candidates from maize twin sectors
To detect newly-formed duplications, we screened maize materials that contain elements of the Ac/Ds transposon system inserted into the p1 gene that controls kernel pericarp (seed coat) pigmentation. We initiated the screen with the progenitor allele P1-ovov454, which carries a pair of reversely-oriented Ac termini in the p1 gene intron 2 (Figure 1A). If transposition of the reversed Ac ends occurs during DNA replication and the excised termini insert into the sister chromatid, two unequal chromatids can be generated: one chromatid contains a tandem direct duplication, and the other contains a corresponding deletion (Figure 1D, lower and upper chromatids, respectively; for animated version please see Movie S1). These two chromatids will segregate into two adjacent daughter cells at mitosis; further mitotic divisions could generate a visible twinned sector. The new mutant chromosomes can be transmitted through meiosis to the kernels within the sectors and subsequently propagated as heritable alleles. Because the P1-ovov454 allele specifies orange variegated pericarp and orange variegated cob, both gains and losses of p1 expression can be recognized. The sector containing the deletion chromosome (white twin, p1-ww-Twin) would have white (colorless) pericarp due to loss of p1 gene exons 1 and 2, while the sector with the duplication chromosome (red twin, P1-rr-Twin) would contain two copies of Ac and exhibit fewer red and white stripes due to the negative Ac dosage effect [34], [35] (see Methods for details). We screened ∼2000 P1-ovov454/p1-ww ears and identified six ears with this type of twinned sector. Two such ears which gave rise to duplication alleles P1-rr-T1 and P1-rr-T481 are shown in Figure 2; the remaining four twin sector ears gave rise to more complex rearrangements which are still under investigation.
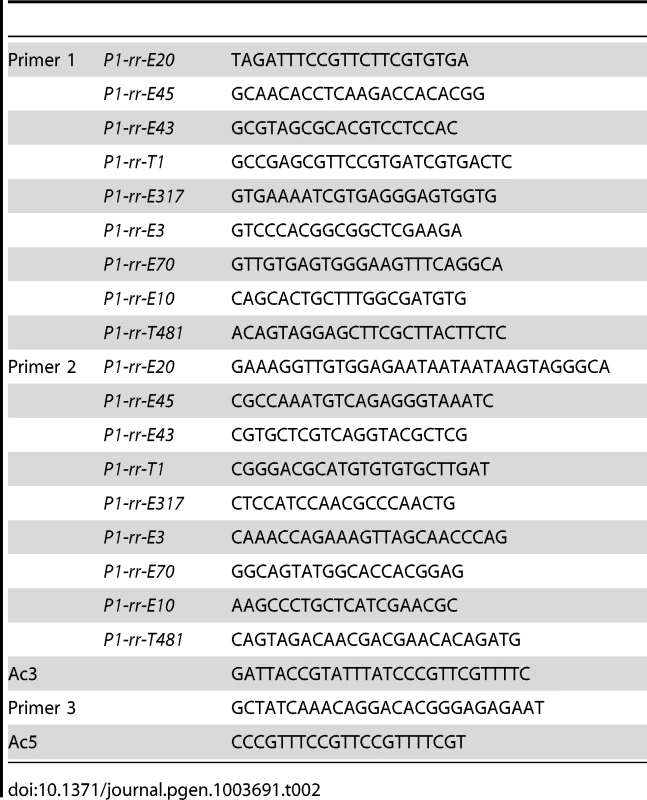
Fig. 1. Reversed Ac ends transposition generates direct duplication. 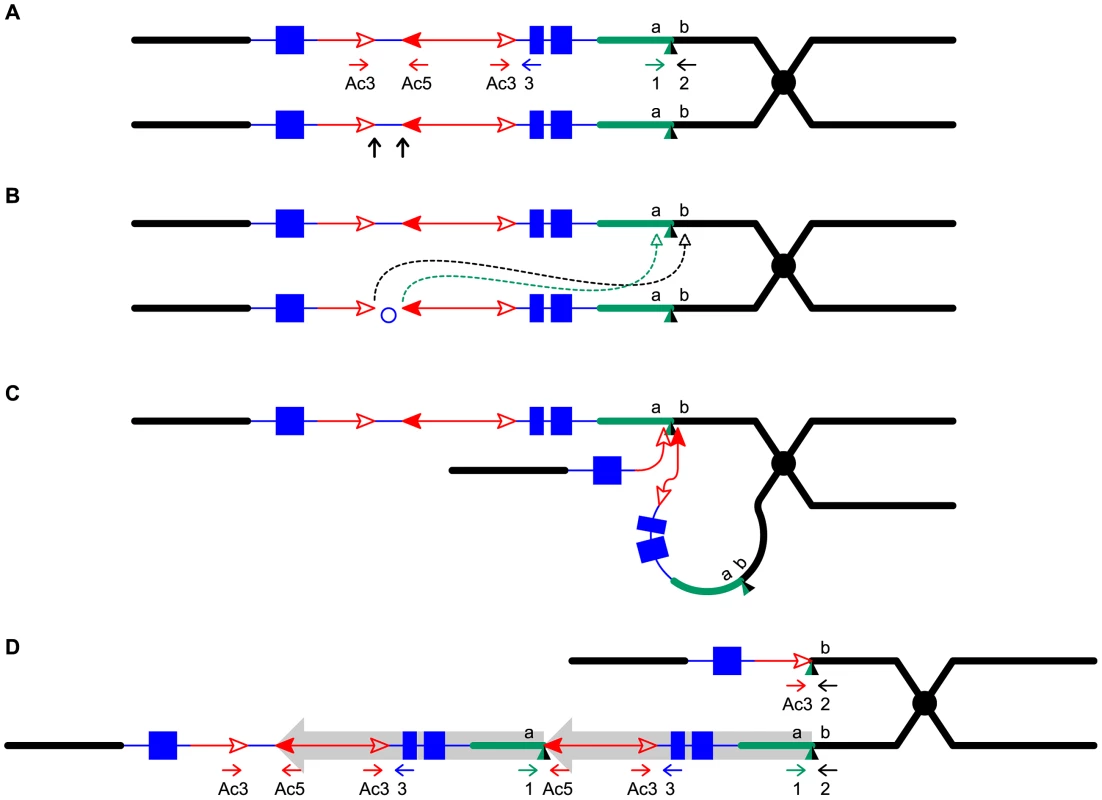
The two lines indicate sister chromatids of maize chromosome 1, joined at the centromere (black). The blue boxes are exons 3, 2, and 1 (left to right) of the p1 gene. Red lines with arrowhead(s) indicate Ac/fAc insertions, and the open and solid arrowheads indicate the 3′ and 5′ ends, respectively, of Ac/fAc. The short horizontal arrows show the orientations and approximate positions of PCR primers, and the numbers below are the primer names. The green/black triangles indicate the transposon target site sequences and target site duplications. (A) Ac transposase cleaves the lower chromatid at the 3′ end of fAc and the 5′ end of Ac (arrows). (B) Following transposase cleavage, the internal p1 genomic sequences are joined to form a circle. Dotted lines indicate the insertion of the fAc and Ac termini into the a/b site on the sister chromatid. (C) Transposon ends insert into the upper sister chromatid at a proximal site. (D) The Ac 5′ end joins to the distal side (green) of the target site and the fAc 3′ end joins to the proximal side (black) of the target site to generate a proximal deletion (upper chromatid) and a direct duplication (lower chromatid). The shaded arrows encompass the duplicated segments. For animation, please see Movie S1. Fig. 2. Ears with twinned sectors T1 (left) and T481 (right). 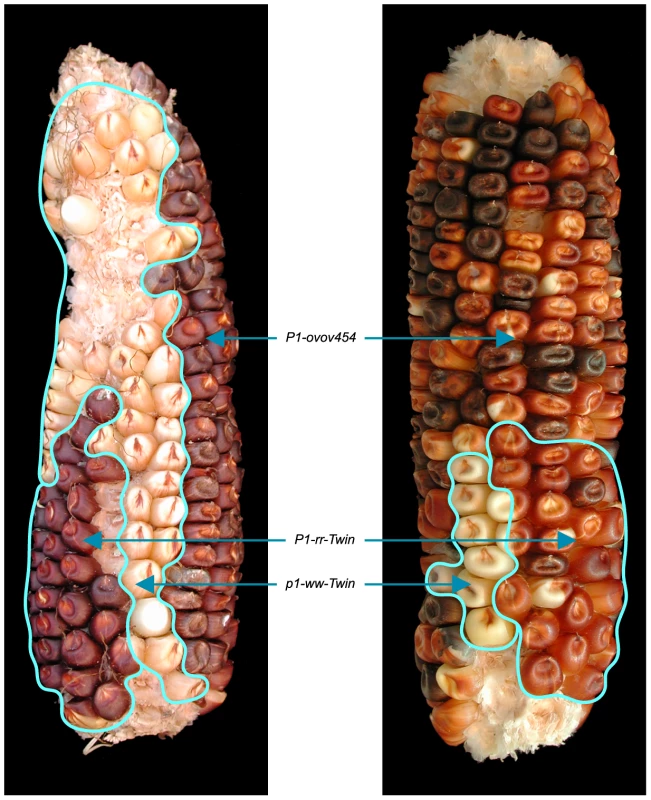
The white and red phenotypic twinned sectors are outlined. The remainder of the ear has the orange-variegated phenotype specified by the progenitor P1-ovov454 allele. The red twin carries a tandem direct duplication
The RET model (Figure 1) predicts that the breakpoints of the duplication alleles (sequence a in Figure 1D) should be adjacent to Ac and p1 sequences. Therefore we used Ac casting [36], [37] and inverse PCR to isolate the sequences at the junction of the two duplication segments (Text S1). Comparison with the maize B73 genome sequence (Release 5b.60) [14] indicates that the breakpoints in P1-rr-T1 and P1-rr-T481 are located ∼460 kb and ∼5.3 Mb proximal to p1, respectively. For each allele we designed two new primers (1 and 2, Figure 1) flanking the predicted insertion sites and used these in PCR together with Ac-specific primer Ac5. Primers 1+2 amplified products containing the intact insertion sites, and primers 1+Ac5 amplified the duplication junctions of sequence a with 5′ Ac (Figure 3); the results indicate that the breakpoint sequence is duplicated in both P1-rr-T1 and P1-rr-T481. Previous semi-quantitative PCR analysis indicated that the p1 sequence proximal to Ac is duplicated; hence these alleles carry duplications. To determine the relative orientations of the duplicated segments, we performed PCR with primers 1+3 which flank the duplication junction of each allele. As shown in Figure 1D, primers 1 and 3 are separated by a 4565 bp Ac element at the duplication. By use of short PCR cycle times we could preferentially amplify products derived from somatic excision of Ac. PCR bands with sizes expected from Ac excision were amplified from both P1-rr-T1 and P1-rr-T481; sequencing of the PCR products shows that the sequence a of each breakpoint allele is linked to p1 gene sequences via a short footprint sequence typical of an Ac excision (Figure S1), and that the duplicated segments are in direct orientation as shown in Figure 1D. Together these results confirm the conclusion that P1-rr-T1 and P1-rr-T481 each carry a large segmental duplication of the sequence proximal to p1, in direct orientation.
Fig. 3. PCR analysis of the twinned alleles with primers 1+2+Ac5. 
Lane 1: DNA ladder; lanes 2 and 5: water (negative control); lanes 3 and 4: P1-ovov454, P1-rr-T1; lanes 6 and 7: P1-ovov454, P1-rr-T481. Note that primers 1 and 2 are specific for each allele. The white twin p1-ww-T1 carries a reciprocal deletion
Another prediction of the RET model (Figure 1) is that the white twin alleles (p1-ww-T1 and p1-ww-T481) should each carry a deletion as the reciprocal product of their corresponding red duplication twins. To test this, PCR analysis was performed with primer pairs 2+Ac3 and 1+Ac5 which are specific for the predicted deletion and duplication junctions, respectively (Figure 4). Products of the expected sizes were amplified from p1-ww-T1 and P1-RR-T1 (Figure 4B). Importantly, sequencing of the PCR products showed that the 8 bp sequences immediately flanking the fAc 3′ end in p1-ww-T1 and the Ac 5′ end in P1-rr-T1 are identical, indicating their origin as a target site duplication (Figure 4C), the hallmark of Ac/Ds transposition. This result confirms that the twinned duplication/deletion alleles P1-rr-T1 and p1-ww-T1 originated as reciprocal products of a single reversed Ac ends transposition event.
Fig. 4. Breakpoint sequences of reciprocal duplication/deletion alleles P1-rr-T1 and p1-ww-T1 generated by Reversed Ends Transposition. 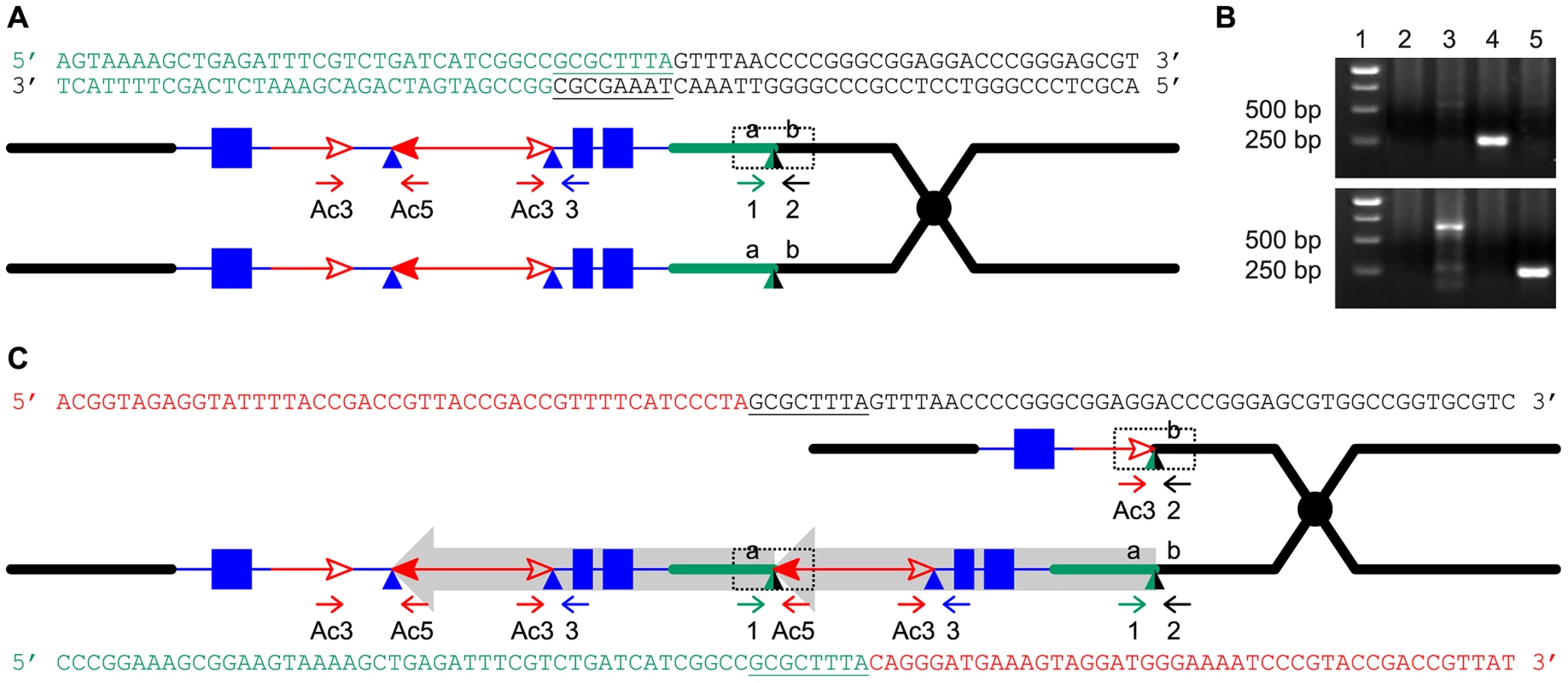
(A) Diagram shows the structure of the progenitor P1-ovov454 allele prior to RET. Two sister chromatids are shown, with symbols as in Figure 1. The dotted box shows the a/b target site region, whose sequence is indicated above. The color of the letters in the sequences matches the chromatid line color. (B) PCR amplification of the deletion/duplication breakpoints in p1-ww-T1 and P1-rr-T1 with primers 2+Ac3 (upper panel) or 1+Ac5 (lower panel). Lane 1: DNA ladder, lane 2: p1-ww[4Co63], lane 3: P1-ovov454/p1-ww[4Co63], lane 4: p1-ww-T1/p1-ww[4Co63], lane 5: P1-rr-T1/p1-ww[4Co63]. (C) Sister chromatid structures of p1-ww-T1 (upper) and P1-rr-T1 (lower). Sequences of the deletion and duplication breakpoints (dotted boxes) are shown in color matching the chromatid line color. Note that each breakpoint has a copy of the 8 bp TSD GCGCTTTA which is present in a single copy at the a/b target site in the progenitor allele. We attempted to isolate the p1-ww-T481 allele, but none of the plants grown from the seven kernels within the white twin sector carried the expected deletion; all carried a standard p1-ww allele derived from the normal homologous chromosome. Because the duplication in the corresponding red twin is 5.3 Mb, and a deletion of this size is most likely gametophyte lethal, we suspect that female gametophytes that received the deletion chromosome in meiosis had aborted and thus were not represented in the mature sector. This idea is consistent with the fact that the white sector contained fewer kernels than its red co-twin (P1-rr-T481; Figure 2)
DNA gel blotting was conducted to further test the structures of the candidate duplication alleles (Figure 5). Genomic DNAs were digested with SalI, and the blot was hybridized with p1-specific probe 15. The progenitor allele P1-ovov454 shows three probe 15-hybridizing bands: a 5451 bp band containing fAc, a 2693 bp band located proximal to Ac, and a 1269 bp band which is present on both sides flanking p1 and hence has a two-fold intensity on the blot. In the P1-rr-T1 and P1-rr-T481 samples, the 2693 bp band is twice the intensity of the 5451 bp band, consistent with a duplication of this proximal segment. In the p1-ww-T1 lane the 2693 band is deleted, and the 5451 bp band is absent and has shifted to a new band of ∼12 kb due to the deletion. An additional band of 1075 bp present in the P1-ovov454 and p1-ww-T1 lanes is derived from the p1-ww allele that is present in heterozygous condition in these samples (Figure 5).
Fig. 5. DNA gel blot analysis of the twinned alleles. 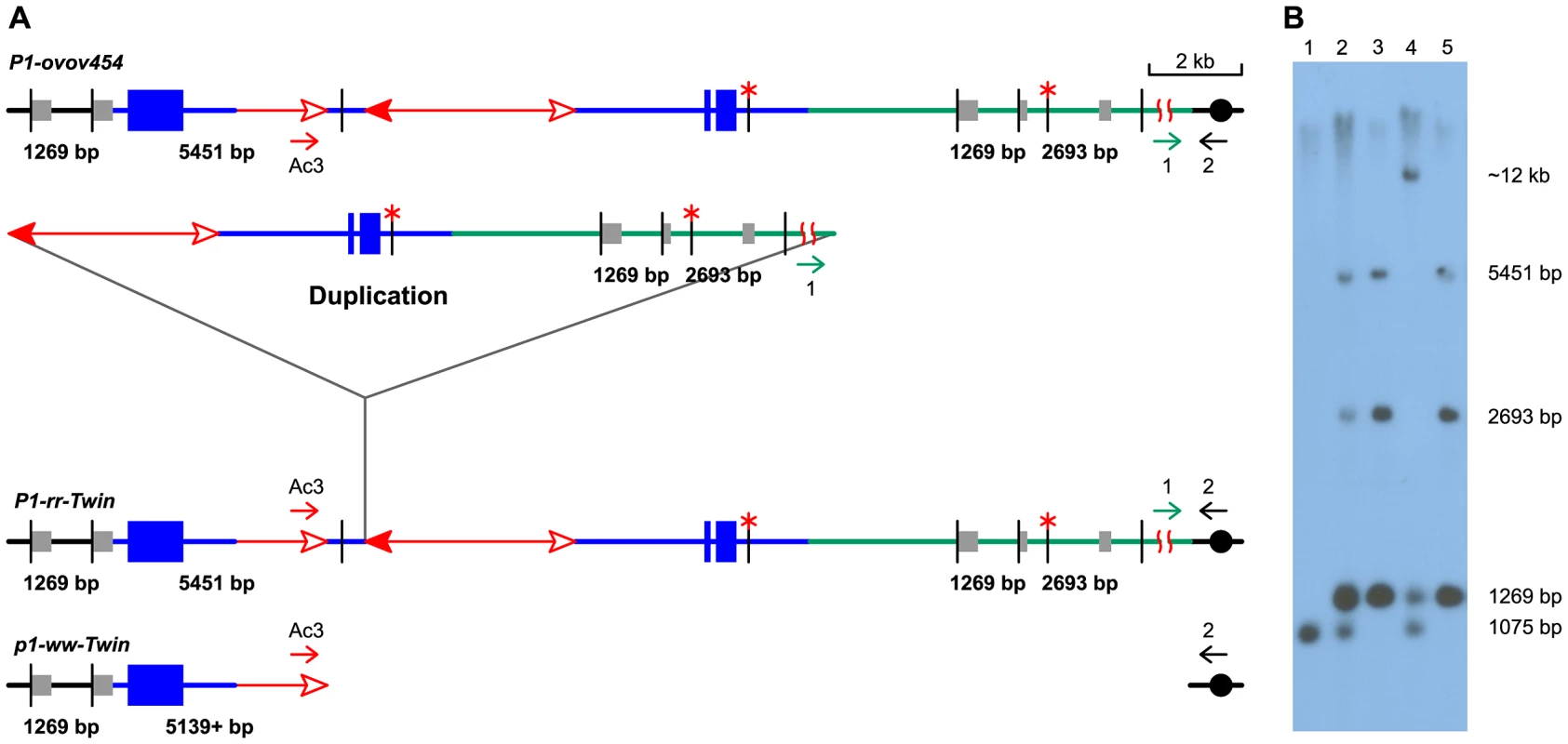
(A) Structure of progenitor P1-ovov454 allele (upper), and predicted structures of the P1-rr-Twin (duplication) and p1-ww-Twin (deletion) alleles (lower) generated via reversed Ac ends transposition. Blue lines and boxes indicate p1 sequences, green lines indicate sequences proximal to p1, and gray boxes indicate sequences homologous to probe 15. The short vertical black lines indicate SalI sites, and red asterisks (*) mark methylated SalI sites. The other symbols have the same meaning as in Figure 1. (B) DNA gel blot. Genomic DNA was digested with SalI and hybridized with p1 genomic probe 15 (gray boxes in Figure 5A). Lane 1: p1-ww[4Co63], Lane 2: P1-ovov454/p1-ww[4Co63], Lane 3: P1-rr-T1, Lane 4: p1-ww-T1/p1-ww[4Co63], Lane 5: P1-rr-T481. Isolation of additional duplication alleles
As described above, the P1-rr-T1 and P1-rr-T481 duplication alleles were isolated from twin sectors with a pericarp phenotype distinct from the parental allele. Multikernel twin sectors are produced by transpositions that occur during a narrow window of early ear development and thus are relatively rare. Therefore we asked whether additional duplication alleles could be isolated from whole ears that exhibited a similar phenotype as that of the red co-twins (i.e. less red/white pericarp variegation). These whole-ear cases could have originated from reversed-ends transposition events that occurred either earlier in embryo development (such that the red twin sector encompassed the entire ear), or as pre-meiotic events. Approximately ∼80 ears of this type were identified among the ∼2000 p1-ovov454/p1-ww ears screened. Plants grown from these whole-ear cases were analyzed by semi-quantitative PCR (Figure S2) to detect changes in copy number of the p1-proximal sequences. In this way we identified 13 additional candidate duplication alleles. The breakpoints of 11 duplication candidates were cloned via Ac casting or inverse PCR (iPCR); sequencing the PCR products revealed that the breakpoints were located at various sites up to 3.3 Mb proximal to the p1 gene on chromosome 1 (Text S1). Based on the breakpoint sequences and the maize genome sequences, new primers 1 and 2 specific for each candidate allele were designed and used in PCR together with Ac primer Ac5. The results of PCR using primers 1+2+Ac5 (Figure S3) confirmed that seven of the 11 candidates carried tandem direct duplications ranging in size from 8157 bp to 3.3 Mb (Table 1). PCR using primers 1+3 flanking the presumed duplication breakpoint confirm that all of the seven alleles derived from whole ears contain tandem direct duplications. The structures of the other four alleles are more complex and are under further investigation.
Tab. 1. The lengths of the duplications. 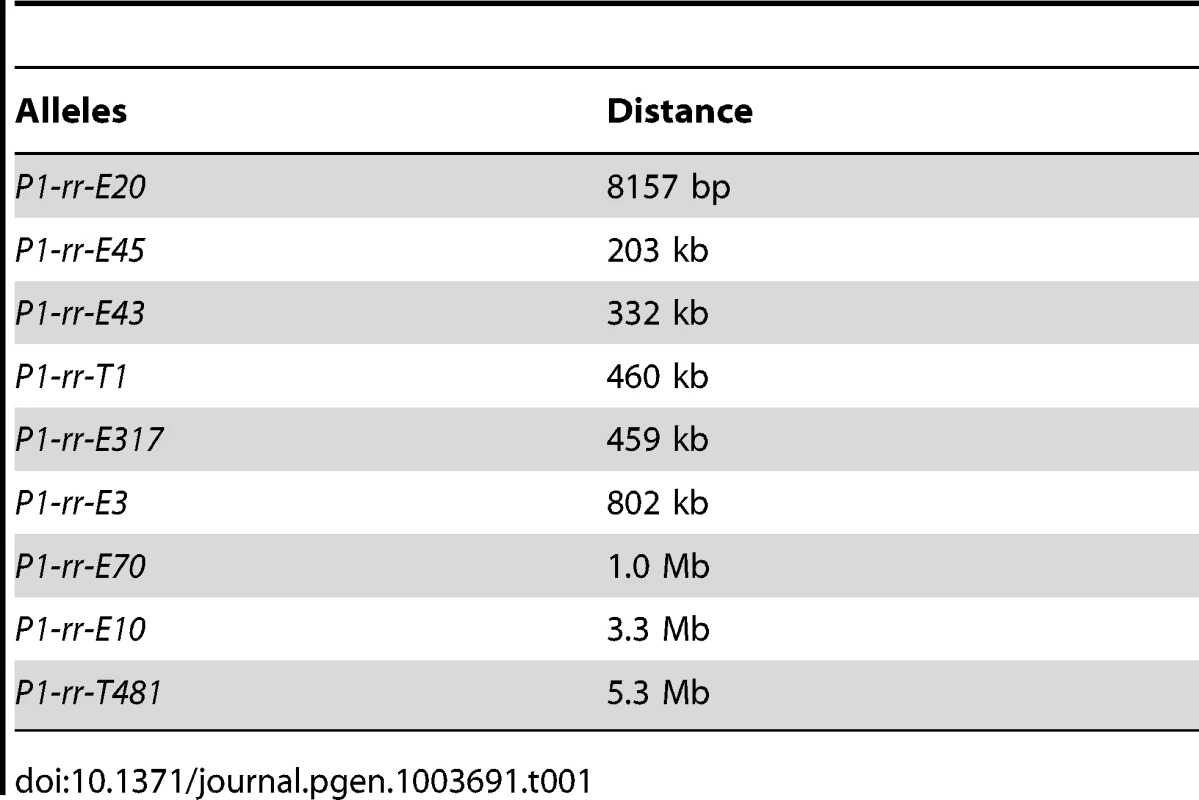
These seven candidate duplication alleles were also subject to DNA gel blot analysis (Figure S4); the results show a higher relative intensity of the 2693 bp fragment in all of the candidate alleles except for P1-rr-E20, whose 8157 bp duplication does not extend into the 2693 bp fragment detected by the probe. Together the DNA gel blot results confirm the allele structures predicted from the duplication breakpoint sequences. The DNA gel blot results and semi-quantitative PCR indicated that P1-rr-E301 and P1-rr-E336 also contain duplications, but their breakpoints are not yet cloned.
Identification of transposon-induced duplications in the maize genome
The experiments described above identified nine tandem direct duplication alleles apparently generated de novo by RET of Ac/Ds elements. If this mechanism has contributed to genome evolution, one would expect to find evidence of transposon-induced duplications in the maize genome sequence. Therefore we conducted a bioinformatics search of the maize B73 reference genome for duplications with the structural features predicted by the RET model. First we identified sequences flanking known hAT family transposons and compared the flanking sequences to detect duplications; we then analyzed these candidate duplications for the sequence features predicted by the RET model. In total, 26 known maize hAT family transposons, including Ac/Ds element and 25 dhAT family elements identified in the lab of Dr. Jinsheng Lai, China Agricultural University (personal communication), were used to search for associated duplications in maize B73 reference genome (ZmB73_RefGen_V2). In this way, we identified three small duplicated segments (Figure 6) that have the sequence features predicted by the RET model (Figure 1). These three tandem duplications are associated with 3 different dhAT family elements, dhAT-Zm1, dhAT-Zm13 and dhAT-Zm24. The first duplication is located on chromosome 1 and contains two tandem direct repeats of 147 bp and 148 bp that are 93% identical. The duplicated segments are initiated by two dhAT-Zm1 elements with 95% sequence identity (Figure 6). The second duplication is located on chromosome 7 and contains two tandem direct repeats of 1262 bp and 1257 bp that are 96% identical. The duplicated segments are initiated by two dhAT-Zm13 elements with 95% sequence identity; one is intact (568 bp) and the other has a deletion of 12 bp from the 5′ TIR sequence (Figure 6). In both duplications, the first dhAT element is flanked by 8 bp direct repeats that represent the Target Site Duplications (TSDs) generated by hAT element insertion. Whereas, the second hAT element is flanked on one side by the same TSD as the first element, but the other terminus does not have a matching TSD. This is exactly the structure predicted by the RET model (Figure 1) and observed in the Ac-induced duplications (Figure 4): the first transposon has TSDs derived from the original insertion of the transposon (pre-duplication); the second transposon copy has the same TSD on one end, but the other end has a non-matching flanking sequence because it represents the subsequent RET event that generated the duplication. The third duplication (on chromosome 6) has a somewhat different structure, but is still consistent with the predictions of the RET model. This case contains direct repeats of 116 bp and 118 bp that are 99% identical; these repeats are initiated by two fractured dhAT-Zm24 elements with 96% identity. The intact dhAT-Zm24 element is 904 bp long, whereas these fractured elements contain only 288 bp and 289 bp from the 3′ end. A duplication with these structural features could also be formed by a mechanism of RET as shown in Figure S5 (Movie S2).
Fig. 6. Tandem direct duplications in maize generated by Reversed-Ends Transposition. 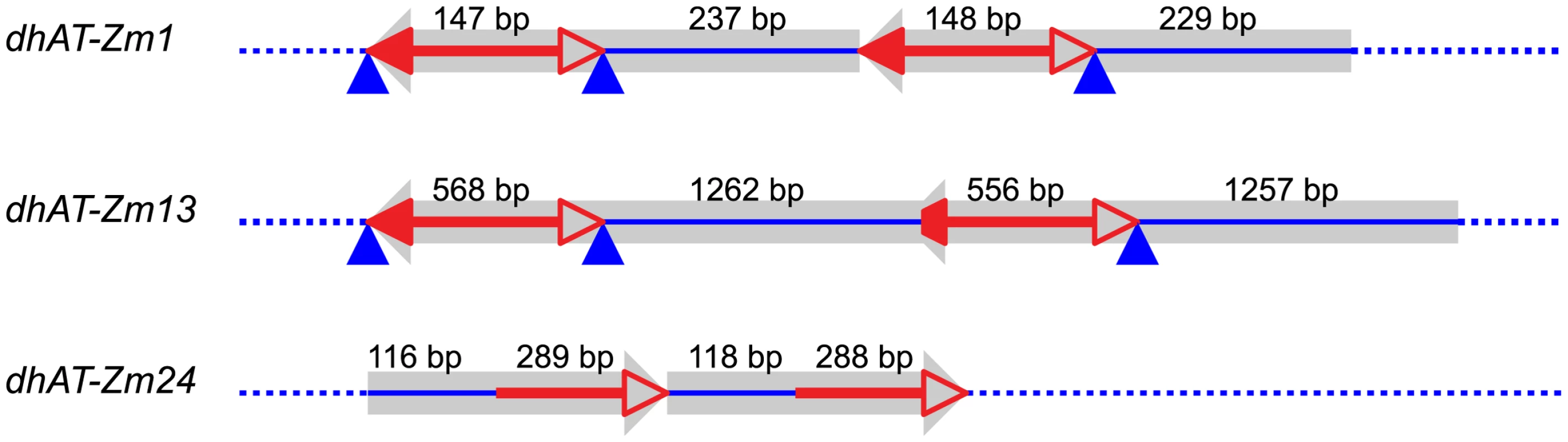
Red lines with arrowhead(s) indicate the dhAT family elements associated with each duplication; solid and open arrowheads indicate the transposon 5′ and 3′ ends, respectively. The truncated solid arrowhead in dhAT-Zm13 indicates a deletion of 12 bp from the 5′ TIR. The blue lines represent duplicated segments. The blue triangles indicate the transposon target site duplications. Numbers above each line indicate the length of that segment. Sequences and genomic positions are shown in Text S2. Discussion
By taking advantage of a visual screen to identify chromosome rearrangements associated with Ac transposition events, we have isolated and characterized nine tandem duplications that arose de novo from a single progenitor allele. The endpoints of all nine duplications coincide precisely with Ac termini. Two duplications were isolated from phenotypic twinned sectors, and in one case we were able to recover and characterize a complementary deletion allele. Importantly, the endpoints of the twinned duplication/deletion alleles share a matching 8 bp TSD which is a hallmark of Ac transposition. These results indicate that the duplications originated through reversed Ac ends transpositions (RET) that occurred during or shortly after DNA replication; the excised Ac/fAc ends inserted into sites in the sister chromatid, resulting in reciprocal chromatids, one containing a tandem direct duplication, and the other bearing a corresponding deletion (Figure 1). These structures are not consistent with origin via other mechanisms. BIR and FoSTeS generally do not produce a deletion and a reciprocal duplication in the same event [19]. NAHR can generate a deletion and a reciprocal duplication. However, if these duplications were generated by NAHR between non-allelic Ac elements, then they should contain three copies of Ac (one Ac flanking the proximal and distal duplication endpoints, and one between the duplicated segments). All of the duplications we isolated lack an Ac element at one breakpoint. Although it is formally possible that one Ac element excised after the formation of the duplication, this can be excluded because the sequences at the junctions do not contain any evidence of an Ac excision footprint. Moreover, duplications generated via NAHR are recurrent; independent NAHR events between the same repeats generate duplications of the same size. However, our duplications share only one breakpoint in intron 2 of the p1 gene; the second breakpoint is different for each of the duplications, resulting in a set of nine overlapping duplications ranging in size from 8157 bp to ∼5.3 Mb.
The Drosophila P element transposon can undergo various types of alternative transposition events that can produce a multitude of rearrangement structures, depending on which transposon termini are involved in the transposition reactions, and the location of the target site (see [25] for review). In the case of the maize Ac/Ds system, fewer types of alternative transposition can occur because the transposition competence of each Ac/Ds end is dependent on strand-specific hemi-methylation of the transposon TIR. The tandem duplications described here are entirely consistent with the RET model shown in Figure 1, and with the known restriction on transposition competence of Ac/Ds elements [38], [39].
NAHR is reported to occur at a frequency of 10−5 to 10−6 in human [40]–[42]; in Arabidopsis, a frequency of 10−4 to 10−6 was observed for NAHR between two ∼1.2 kb repeats separated by ∼4.0 kb unique DNA sequence [43]. Rates of NAHR have not, to our knowledge, been reported for maize. Our results indicate that transposition-induced duplications can occur at a relatively high frequency, depending on the presence of an active transposon system with appropriately positioned elements. From a population of approximately 2000 plants, we identified seven whole ears and two twinned-sector ears with transposition-generated tandem direct duplications. DNA gel blotting and semi-quantitative PCR results indicate that two additional cases (P1-rr-E301 and P1-rr-E336; Figure S4) also carry duplications, although we could not clone their breakpoints. The calculated duplication frequency (∼0.5%) is very likely an underestimate for two reasons. First, the visual phenotype used to detect duplications (darker red pericarp and fewer purple aleurone spots) is somewhat subtle and some events may have been overlooked. Second, the screen would not have detected distal duplications because these would not alter the p1 gene or Ac dose. Distal duplications would result from insertion of the excised Ac/fAc termini into a site between the p1 gene and telomere (Figure S5; Movie S2), and these would be expected to occur as frequently as proximal duplications. Thus the real frequency of duplications derived from the P1-ovov454 allele may be closer to 1%. Given this high frequency, we asked whether Ac/Ds-induced tandem duplications could be detected in the maize B73 genome, which contains ∼50 Ac/Ds elements [44]. However, we failed to find any Ac/Ds copies adjacent to a tandem duplication, possibly because the Ac/Ds elements in the B73 genome are widely separated, and efficient reversed-ends Ac/Ds transposition requires two elements in close proximity and correct orientation [29].
In addition to the Drosophila P element and Ac/Ds systems, the Antirhinnum Tam3 element, a founding member of the hAT transposon superfamily, is known to induce flanking genome rearrangements [45]–[47], possibly via alternative transposition mechanism(s). This suggested that other transposons, in particular hAT family elements, may be capable of undergoing alternative transposition to mediate genomic rearrangements. Therefore we extended our bioinformatics searches for transposon-associated tandem duplications to a set of 25 other hAT family elements previously identified in the maize B73 reference genome (personal communication). These searches returned a total of 7611 hAT element insertions, and among these we identified three tandem direct duplications with the key structural features predicted by the RET model: First, they have exactly two repeated copies, and each repeat is initiated precisely by the transposon. Moreover, in two of the duplications the first hAT element is flanked by 8 bp TSDs, while the second (middle) element is flanked by only one of these 8 bp sequences. These features are not expected from other duplication mechanisms such as NAHR, BIR and FoSTeS, but they are perfectly predicted by the RET model. Although the duplications observed are relatively short and their frequency is low, it is possible that some examples may not have been detected for various reasons. First, the maize B73 reference genome sequence still has numerous gaps and uncertainties in the order and orientations of many sequence contigs, and these ambiguities will interfere with the identification of duplications, especially larger ones. Second, those more recent and therefore nearly identical duplications may be under-represented in the reference sequence due to collapse during sequence assembly [48], [49]. Third, those duplications in which either one of the TEs excised after formation of the duplication would not be detected by our search criteria. Nevertheless, we conclude from these results that RET-induced tandem duplication has occurred in maize evolutionary history. Given the high frequency and diversity of Class II transposons present in many eukaryotic species [50], [51], the impact of this mechanism in eukaryotic genome evolution may be significant. The RET model described here provides the conceptual basis for additional bioinformatics searches that will be necessary to assess the actual impact of this mechanism in different species.
Methods
Genetic stocks
The maize p1 gene encodes a Myb-like transcription factor controlling the pigmentation of floral tissues, including kernel pericarp (seed coat) and cob. The suffix of a p1 allele indicates its expression pattern in pericarp and cob, e.g., P1-rr specifies red pericarp and red cob, p1-ww specifies white (colorless) pericarp white (colorless) cob, and P1-ovov specifies orange variegated pericarp (seed coat) and orange variegated cob. The numeral following the suffix indicates the origin of the allele; alleles with the same phenotype but different numeral may have different structures. The P1-ovov454 allele conditions a high frequency of colorless sectors, presumably resulting from alternative transposition events which interrupt or delete the p1 gene [52]. The p1-ww-[4Co63] allele is from the maize inbred line 4Co63 [53]; heterozygous plants of genotype P1-ovov454/p1-ww-[4Co63] were fertilized with pollen from plants of genotype C1, r1-m3::Ds [4Co63]. Ac induces excision of Ds from r1-m3::Ds, resulting in restoration of r1 gene function and purple aleurone sectors. Ac/Ds transposition is subject to the negative Ac dosage effect [34], [35], in which increases in Ac copy number result in a developmental delay in Ac/Ds transposition. If reversed Ac ends transposition occurs as shown in Figure 1, two different sister chromatids would be produced: one carrying a tandem direct duplication, and the other a reciprocal deletion (Figure 1D). These chromatids will separate into two adjacent daughter cells at mitosis, forming a twinned sector after successive rounds of cell division. The sector with the deletion chromosome has lost Ac and exons 1 and 2 of the p1 gene, and thus should have colorless pericarp with no purple aleurone sectors. The sector with the duplication chromosome retains a functional P1-ovov454 gene and two copies of Ac, and thus should exhibit fewer colorless pericarp sectors, and smaller kernel aleurone sectors.
Genomic DNA extractions, Southern blot hybridization
Total genomic DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) extraction protocol [54]. Agarose gel electrophoresis and Southern hybridizations were performed according to Sambrook et al [55] , except hybridization buffers contained 250 mM NaHPO4, pH 7.2, 7% SDS, and wash buffers contained 20 mM NaHPO4, pH 7.2, 1% SDS.
PCR amplifications
Sequences of oligonucleotide primers used in PCR reactions are given in Table 2; note that primers 1 and 2 are specific to each allele. PCR was performed using HotMaster Taq polymerase from 5 PRIME (Hamburg, Germany). Reactions were heated at 94°C for 2 min, and then cycled 35 times at 94°C for 20 s, 60°C for 10 s, and 65°C for 1 min per 1 kb length of expected PCR product, then 65°C for 8 min. For difficult templates, 0.5–1 M betaine and 4%–8% DMSO were added. The band amplified was purified from an agarose gel and sequenced directly. Sequencing was done by the DNA Synthesis and Sequencing Facility, Iowa State University, Ames, Iowa, United States. Ac casting and inverse PCR were performed as described previously [36].
Bioinformatics
The sequences of 26 hAT family transposable elements were used as queries to search for homologous elements in the maize B73 reference genome (ZmB73_RefGen_V2) via local BLASTN with default parameters. Two types of homologous sequences were identified: 1) intact elements, which contained both 5′ and 3′ termini; 2) fractured elements, which contained one terminal end (either 5′ or 3′) but having lengths greater than 100 bp. A PERL script was developed to extract two 100 bp segments flanking each transposon, one 5′ adjacent and one 3′ adjacent. Pair-wise comparisons were performed among the segments flanking the same terminal end within each individual hAT family. Two hAT family members with the same orientation, less than 100 kb apart, and with homologous sequences flanking one terminal end but not the other end were retained for further structural analysis. Such cases were examined manually for the following features: 1) the duplication comprises the complete sequence between the two hAT elements, and 2) the duplication is initiated by the transposable element insertion. Sequences that met the above criteria were considered as putative duplications generated by alternative transposition and were examined further for the presence of TSDs as described in the text.
Supporting Information
Zdroje
1. Ohno S (1970) Evolution by Gene Duplication: Springer-Verlag.
2. ZhangF, GuW, HurlesME, LupskiJR (2009) Copy Number Variation in Human Health, Disease, and Evolution. Annual Review of Genomics and Human Genetics 10 : 451–481.
3. BaileyJA, GuZ, ClarkRA, ReinertK, SamonteRV, et al. (2002) Recent segmental duplications in the human genome. Science (New York, NY) 297 : 1003–1007.
4. SheX, ChengZ, ZollnerS, ChurchDM, EichlerEE (2008) Mouse segmental duplication and copy number variation. Nat Genet 40 : 909–914.
5. LinH, ZhuW, SilvaJC, GuX, BuellCR (2006) Intron gain and loss in segmentally duplicated genes in rice. Genome Biol 7: R41.
6. VandepoeleK, SimillionC, Van de PeerY (2003) Evidence that rice and other cereals are ancient aneuploids. Plant Cell 15 : 2192–2202.
7. WangX, ShiX, HaoB, GeS, LuoJ (2005) Duplication and DNA segmental loss in the rice genome: implications for diploidization. New Phytol 165 : 937–946.
8. PatersonAH, BowersJE, ChapmanBA (2004) Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci U S A 101 : 9903–9908.
9. International-Rice-Genome-Sequencing-Project (2005) The map-based sequence of the rice genome. Nature 436 : 793–800.
10. GautBS (2001) Patterns of chromosomal duplication in maize and their implications for comparative maps of the grasses. Genome Res 11 : 55–66.
11. AhnS, AndersonJA, SorrellsME, TanksleySD (1993) Homoeologous relationships of rice, wheat and maize chromosomes. Mol Gen Genet 241 : 483–490.
12. OdlandW, BaumgartenA, PhillipsR (2006) Ancestral Rice Blocks Define Multiple Related Regions in the Maize Genome. Crop Science 46: S-41–S-48.
13. MooreG, DevosKM, WangZ, GaleMD (1995) Cereal genome evolution. Grasses, line up and form a circle. Curr Biol 5 : 737–739.
14. SchnablePS, WareD, FultonRS, SteinJC, WeiF, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326 : 1112–1115.
15. SpringerNM, YingK, FuY, JiT, YehC-T, et al. (2009) Maize Inbreds Exhibit High Levels of Copy Number Variation (CNV) and Presence/Absence Variation (PAV) in Genome Content. PLoS Genet 5: e1000734.
16. DeBoltS (2010) Copy number variation shapes genome diversity in Arabidopsis over immediate family generational scales. Genome Biol Evol 2 : 441–453.
17. CannonS, MitraA, BaumgartenA, YoungN, MayG (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biology 4 : 10.
18. HouY, LiuGE, BickhartDM, CardoneM, WangK, et al. (2011) Genomic characteristics of cattle copy number variations. BMC Genomics 12 : 127.
19. HastingsPJ, LupskiJR, RosenbergSM, IraG (2009) Mechanisms of change in gene copy number. Nat Rev Genet 10 : 551–564.
20. ZhangF, GuW, HurlesME, LupskiJR (2009) Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet 10 : 451–481.
21. KrausE, LeungWY, HaberJE (2001) Break-induced replication: a review and an example in budding yeast. Proc Natl Acad Sci U S A 98 : 8255–8262.
22. MorrowDM, ConnellyC, HieterP (1997) “Break Copy” Duplication: A Model for Chromosome Fragment Formation in Saccharomyces cerevisiae. Genetics 147 : 371–382.
23. HastingsPJ, IraG, LupskiJR (2009) A Microhomology-Mediated Break-Induced Replication Model for the Origin of Human Copy Number Variation. PLoS Genet 5: e1000327.
24. LeeJA, CarvalhoCM, LupskiJR (2007) A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131 : 1235–1247.
25. GrayYHM (2000) It takes two transposons to tango: transposable-element-mediated chromosomal rearrangements. Trends in Genetics 16 : 461–468.
26. GrayYH, TanakaMM, SvedJA (1996) P-element-induced recombination in Drosophila melanogaster: hybrid element insertion. Genetics 144 : 1601–1610.
27. PrestonCR, SvedJA, EngelsWR (1996) Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics 144 : 1623–1638.
28. WeilCF, WesslerSR (1993) Molecular evidence that chromosome breakage by Ds elements is caused by aberrant transposition. Plant Cell 5 : 515–522.
29. YuC, ZhangJ, PulletikurtiV, WeberDF, PetersonT (2010) Spatial configuration of transposable element Ac termini affects their ability to induce chromosomal breakage in maize. Plant Cell 22 : 744–754.
30. ZhangJ, PetersonT (1999) Genome rearrangements by nonlinear transposons in maize. Genetics 153 : 1403–1410.
31. ZhangJ, PetersonT (2004) Transposition of reversed Ac element ends generates chromosome rearrangements in maize. Genetics 167 : 1929–1937.
32. HuangJT, DoonerHK (2008) Macrotransposition and other complex chromosomal restructuring in maize by closely linked transposons in direct orientation. Plant Cell 20 : 2019–2032.
33. XuanYH, PiaoHL, JeBI, ParkSJ, ParkSH, et al. (2011) Transposon Ac/Ds-induced chromosomal rearrangements at the rice OsRLG5 locus. Nucleic Acids Res 39: e149.
34. McClintockB (1948) Mutable loci in maize. Carnegie Inst Wash Year Book 47 : 155–169.
35. McClintockB (1951) Chromosome organization and genic expression. Cold Spring Harb Symp Quant Biol 16 : 13–47.
36. ZhangJ, YuC, PulletikurtiV, LambJ, DanilovaT, et al. (2009) Alternative Ac/Ds transposition induces major chromosomal rearrangements in maize. Genes Dev 23 : 755–765.
37. SinghM, LewisPE, HardemanK, BaiL, RoseJK, et al. (2003) Activator mutagenesis of the pink scutellum1/viviparous7 locus of maize. Plant Cell 15 : 874–884.
38. RosF, KunzeR (2001) Regulation of activator/dissociation transposition by replication and DNA methylation. Genetics 157 : 1723–1733.
39. Peterson T, Zhang J (2013) The Mechanism of Ac/Ds Transposition. In: Fedoroff NV, editor. Plant Transposons and Genome Dynamics in Evolution. Oxford, UK: Wiley-Blackwell. pp. 41–59.
40. LamK-WG, JeffreysAJ (2006) Processes of copy-number change in human DNA: The dynamics of α-globin gene deletion. Proceedings of the National Academy of Sciences 103 : 8921–8927.
41. LamK-WG, JeffreysAJ (2007) Processes of de novo duplication of human α-globin genes. Proceedings of the National Academy of Sciences 104 : 10950–10955.
42. TurnerDJ, MirettiM, RajanD, FieglerH, CarterNP, et al. (2008) Germline rates of de novo meiotic deletions and duplications causing several genomic disorders. Nat Genet 40 : 90–95.
43. MolinierJ, RiesG, BonhoefferS, HohnB (2004) Interchromatid and interhomolog recombination in Arabidopsis thaliana. Plant Cell 16 : 342–352.
44. DuC, HoffmanA, HeL, CaronnaJ, DoonerHK (2011) The complete Ac/Ds transposon family of maize. BMC Genomics 12 : 588.
45. MartinC, MackayS, CarpenterR (1988) Large-scale chromosomal restructuring is induced by the transposable element tam3 at the nivea locus of antirrhinum majus. Genetics 119 : 171–184.
46. MartinC, ListerC (1989) Genome juggling by transposons: Tam3-induced rearrangements in Antirrhinum majus. Dev Genet 10 : 438–451.
47. ListerC, JacksonD, MartinC (1993) Transposon-induced inversion in Antirrhinum modifies nivea gene expression to give a novel flower color pattern under the control of cycloidearadialis. Plant Cell 5 : 1541–1553.
48. EmrichSJ, BarbazukWB, LiL, SchnablePS (2007) Gene discovery and annotation using LCM-454 transcriptome sequencing. Genome Res 17 : 69–73.
49. PhillippyAM, SchatzMC, PopM (2008) Genome assembly forensics: finding the elusive mis-assembly. Genome Biol 9: R55.
50. PrithamEJ, PutliwalaT, FeschotteC (2007) Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene 390 : 3–17.
51. FeschotteC, PrithamEJ (2007) DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 41 : 331–368.
52. YuC, ZhangJ, PetersonT (2011) Genome rearrangements in maize induced by alternative transposition of reversed Ac/Ds termini. Genetics 188 : 59–67.
53. GoettelW, MessingJ (2010) Divergence of gene regulation through chromosomal rearrangements. BMC Genomics 11 : 678.
54. PorebskiS, BaileyL, BaumB (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter 15 : 8–15.
55. Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual New York: Cold Spring Harbor Laboratory Press.
Štítky
Genetika Reprodukčná medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy