-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
Even a single mutation can cause a marked change in a protein's properties. When the mutant protein functions within a network, complex phenotypes may emerge that are not intrinsic properties of the protein itself. Network architectures that enable such dramatic changes in function from a few mutations remain relatively uncharacterized. We describe a remarkable example of this versatility in the well-studied PhoQ/PhoP bacterial signaling network, which has an architecture found in many two-component systems. We found that a single point mutation that abolishes the phosphatase activity of the sensor kinase PhoQ results in a striking change in phenotype. The mutant responds to stimulus in a bistable manner, as opposed to the wild-type, which has a graded response. Mutant cells in on and off states have different morphologies, and their state is inherited over many generations. Interestingly, external conditions that repress signaling in the wild-type drive the mutant to the on state. Mathematical modeling and experiments suggest that the bistability depends on positive autoregulation of the two key proteins in the circuit, PhoP and PhoQ. The qualitatively different characteristics of the mutant come at a substantial fitness cost. Relative to the off state, the on state has a lower fitness in stationary phase cultures in rich medium (LB). However, due to the high inheritance of the on state, a population of on cells can be epigenetically trapped in a low-fitness state. Our results demonstrate the remarkable versatility of the prototypical two-component signaling architecture and highlight the tradeoffs in the particular case of the PhoQ/PhoP system.
Published in the journal: The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties. PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003706
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003706Summary
Even a single mutation can cause a marked change in a protein's properties. When the mutant protein functions within a network, complex phenotypes may emerge that are not intrinsic properties of the protein itself. Network architectures that enable such dramatic changes in function from a few mutations remain relatively uncharacterized. We describe a remarkable example of this versatility in the well-studied PhoQ/PhoP bacterial signaling network, which has an architecture found in many two-component systems. We found that a single point mutation that abolishes the phosphatase activity of the sensor kinase PhoQ results in a striking change in phenotype. The mutant responds to stimulus in a bistable manner, as opposed to the wild-type, which has a graded response. Mutant cells in on and off states have different morphologies, and their state is inherited over many generations. Interestingly, external conditions that repress signaling in the wild-type drive the mutant to the on state. Mathematical modeling and experiments suggest that the bistability depends on positive autoregulation of the two key proteins in the circuit, PhoP and PhoQ. The qualitatively different characteristics of the mutant come at a substantial fitness cost. Relative to the off state, the on state has a lower fitness in stationary phase cultures in rich medium (LB). However, due to the high inheritance of the on state, a population of on cells can be epigenetically trapped in a low-fitness state. Our results demonstrate the remarkable versatility of the prototypical two-component signaling architecture and highlight the tradeoffs in the particular case of the PhoQ/PhoP system.
Introduction
A few mutations can lead to significant changes in a protein's functional properties. Examples include mutations that change the absorption and emission spectra of a fluorescent protein [1], the substrate specificity of an enzyme [2], or the allosteric control of a transcription factor [3]. In all of these examples, the change in phenotype can be directly traced to modifications in intrinsic properties of the protein. However, networks of interacting proteins can have system-level characteristics that bear a complex relationship to the intrinsic properties of the component molecules [4]. This complexity makes some network architectures inherently versatile, with different networks that share the same architecture exhibiting qualitatively different system-level behavior [5]. It remains a challenge to identify aspects of network architectures that promote versatility and permit novel properties to emerge by a few mutations to network components.
In this study, we demonstrate the versatility of the E. coli PhoQ/PhoP system. We show that a single point mutation in the histidine kinase PhoQ produces a striking change in the properties of the circuit. The PhoQ/PhoP system, which has an architecture found in many bacterial two-component signaling systems [6], responds to a variety of environmental conditions such as low Mg2+ [7], low pH [8], and the presence of cationic antimicrobial peptides [9], and controls transcription of a large set of genes [10]. The histidine kinase PhoQ senses these signals and modulates the phosphorylation level of the response regulator PhoP (PhoP-P), which functions as a transcription factor. PhoQ autophosphorylates and then transfers the phosphoryl group to PhoP, but also acts as a phosphatase, catalyzing PhoP-P dephosphorylation [11]. This bifunctional design, which is shared among many two-component systems, affects various properties of the system, including buffering the input-output relationship of the system to changes in histidine kinase and response regulator concentrations, and suppression of cross-talk [12]–[15]. The PhoQ/PhoP system is also autoregulated, that is, transcription of the phoPphoQ operon is activated by PhoP-P. Autoregulation is another common feature of many two-component systems [6] and is a mechanism for ultrasensitive response to stimulus without the need for cooperativity [16] as well as “learning” behaviors where prior exposure to stimulus improves response times to subsequent stimulating conditions [17], [18]. In the case of the PhoQ/PhoP system, autoregulation improves the dynamic range of the network output at high stimulus [13] and also gives rise to a surge in transcription upon activation [19].
The wild-type PhoQ/PhoP system responds to external stimulus in a graded rather than an all-or-none manner, with increasing stimulus (lower Mg2+ concentration) resulting in a higher mean response [13]. Moreover, the response of a population of cells is unimodal, with the PhoP-P levels of individual cells (inferred from transcriptional reporters) clustered around the population mean [13]. In this study, we show that a single point mutation that abolishes phosphatase activity in PhoQ produces a dramatic change in phenotype. The mutation, which produces a T281R substitution in PhoQ, results in bistability with mutants persisting in morphologically distinct OFF and ON states for many generations. We find that the architectural features of the network that allow the mutant to exhibit bistability are shared among many two-component systems. For PhoQ (T281R) mutants, however, the bistable phenotype comes at a fitness cost. We find that a population of cells can be epigenetically trapped in a low-fitness state.
Results
Bimodal Phenotype and Phenotypic Hysteresis of phoQ (T281R)
A previous study reported that the phoQ (T281R) mutation results in a broad distribution of PhoP-regulated transcription in a population of E. coli cells [13]. To explore the origin of this heterogeneity, we engineered a phoQ (T281R) strain with the mutation at the native phoPphoQ locus. The strain also contained a PhoP-P responsive promoter controlling yfp transcription and a constitutive promoter controlling cfp, allowing the use of YFP fluorescence to infer PhoP-P levels (Figure 1A and Methods). Two fluorescent colony phenotypes could be discerned on agar plates: YFP-dim, which we designated phoQ (T281R) OFF, and YFP-bright, which we designated phoQ (T281R) ON (Figure 1B). Considering that each colony is composed of hundreds of millions of cells that originated from a single cell, the appearance of two distinct colony phenotypes suggests that individual cells have two phenotypic states and that these states are heritable.
Fig. 1. Phenotypic bimodality and hysteresis in the phoQ (T281R) mutant. 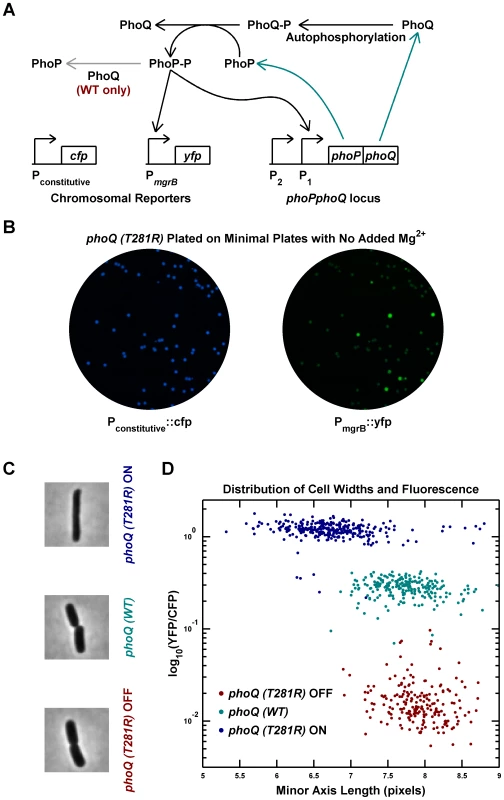
(A) Schematic of the PhoQ/PhoP circuit. The phoPQ operon in transcribed and translated to produce PhoP and PhoQ proteins (cyan arrows). Post-tranlational interactions between PhoQ and PhoP generate PhoP-P, which upregulates the expression of the phoPQ operon and the yfp reporter (solid black arrows). The PhoQ phosphatase activity, which is absent in PhoQ (T281R), is depicted with a gray arrow. (B) Bimodal behavior of YFP colony fluorescence. Panel shows CFP and YFP channel images of a minimal medium plate on which an LB overnight culture of the phoQ (T281R) strain was spread. (C and D) Phenotypic hysteresis. Overnight cultures of phoQ (WT), phoQ (T281R) OFF, and phoQ (T281R) ON strains in LB were diluted 1000-fold into Minimal A medium with 100 µM Mg2, grown to mid-exponential phase and imaged by fluorescence microscopy (Methods). The PhoP-P state of the cell was determined by measuring YFP expression driven by the PhoP-P responsive mgrB promoter and normalized to CFP expression driven by a constitutive promoter. Cell widths were quantified by fitting phase image masks to ellipses and computing the minor axis length. Representative phase images of the three strains are shown in panel C and reveal morphological differences between OFF and ON cells. Fluorescence and cell width values for each cell are plotted in panel D with indicated colors distinguishing the three strains. Note that phoQ (T281R) OFF cells (maroon) have much lower fluorescence and are wider than phoQ (T281R) ON cells (blue). To determine whether single cells showed a bimodal response, overnight cultures in LB medium were inoculated with OFF and ON colonies, and individual cells were imaged after dilution and growth to mid-exponential phase in minimal medium with 100 µM Mg2+ (Figure 1C–D). As with colonies, individual cells could be classified as OFF (low PhoP-P, YFP-dim) or ON (high PhoP-P, YFP-bright) with a ∼60-fold difference in YFP fluorescence between the two states (Figure 1D). The wild-type phoQ strain (denoted phoQ (WT)) cultured in the same manner showed an intermediate fluorescence, roughly 12-fold higher than the OFF state of the mutant. In addition to the bimodal distribution of phenotypes for the phoQ (T281R) strain, we also observed phenotypic hysteresis, i.e., an OFF colony yielded mostly OFF cells, and an ON colony gave rise to mostly ON cells, even though both populations of cells were cultured under the same conditions (Figure 1D). In these experiments, it is likely that the mgrB promoter driving yfp is near saturation in the ON state and the true change in PhoP-P levels between the OFF and ON states may be higher than 60-fold. Furthermore, since the YFP protein used in this study is stable, the switching of YFP-state from ON to OFF is limited by dilution of YFP due to growth. Consequently, one can see cells with an intermediate YFP-state occasionally even though the PhoP-P levels may actually be low.
Interestingly, the single-cell experiments also revealed a morphological difference between OFF and ON state cells (Figure 1C–D). ON cells have, on average, lower cell-widths (as quantified by the minor axis of the best-fit ellipse) than OFF cells, and the latter are similar to wild-type cells. This is likely an indirect effect of high PhoP-P levels, but we do not know the mechanism.
Characterization of the OFF state of phoQ (T281R)
Why is the phosphatase-deficient mutant not constitutively ON? To gain insights into this question, we formulated a simple mathematical model of the PhoQ/PhoP network that consisted of only three species, viz., PhoP, PhoQ, and PhoP-P and ignored PhoQ-P and intermediate complexes (Text S1). In this model, the kinase rate is a proxy for any factor that can influence the production of PhoP-P from PhoP. Analysis of this model revealed that at high kinase rates, a phosphatase-deficient mutant would indeed be constitutively ON and the network would be monostable (Figure S1A). However, at low kinase rates, the phosphatase-deficient PhoQ/PhoP network could exhibit bistability (exist in OFF and ON states). The bistability results from positive feedback (transcriptional autoregulation, see Figure 1A) and the presence of two non-linearities: (a) the kinase and phosphatase reactions each depend on the product of two concentrations, and (b) the non-linear dependence of phoPQ operon transcription on PhoP-P concentration (Text S1). In vitro experiments have demonstrated that PhoQ (T281R) is a poorer kinase compared to PhoQ (WT) [13]. The model thus suggests it is both the low kinase activity and the absence of phosphatase activity of the PhoQ (T281R) mutant that facilitates the emergence of bistability. One of the assumptions in our model is that growth-mediated dilution is the dominant mechanism for reduction in concentrations of the stable proteins PhoP, PhoQ and PhoP-P (in the absence of a specific phosphatase). Consequently, growth rate is another parameter that influences the response of the network, with low growth rates leading to slower dilution and a constitutively ON phenotype.
To experimentally validate the insights from the simple model, we reasoned that changing [Mg2+] could be used to modulate the PhoP→PhoP-P flux (the kinase rate equivalent). Accordingly, we grew cultures of mostly OFF cells in minimal media with different [Mg2+] and maintained them exclusively in the exponential phase by serial dilution (growth rates were similar over the [Mg2+] range tested). Surprisingly, we found that high [Mg2+] (10 mM) drove all OFF cells to ON, whereas low [Mg2+] (100 µM or 1 mM) preserved the OFF state (EXP lineages, Figure 2A–B). This, in effect, represents a reversal of sensitivity to Mg2+ in the phoQ (T281R) strain as the wild-type strain is repressed (produces lower PhoP-P) by high Mg2+. If a portion of the same batch of OFF cells that was maintained in the exponential phase in the EXP lineages was instead passaged through stationary phase (effectively, a slow growth phase) and subsequently diluted and grown to mid-exponential phase, then even the low [Mg2+] lineages turned mostly ON (STA lineages, Figure 2A–B). Note that the different outcomes of STA and EXP lineages at low [Mg2+] represent yet another manifestation of hysteresis, since both lineages have identical starting points (same culture of OFF cells) and similar end points (mid-exponential cultures with the same [Mg2+]), but different history between start and end.
Fig. 2. Passage through stationary phase and High Mg2+ cause OFF cells to prime to ON state. 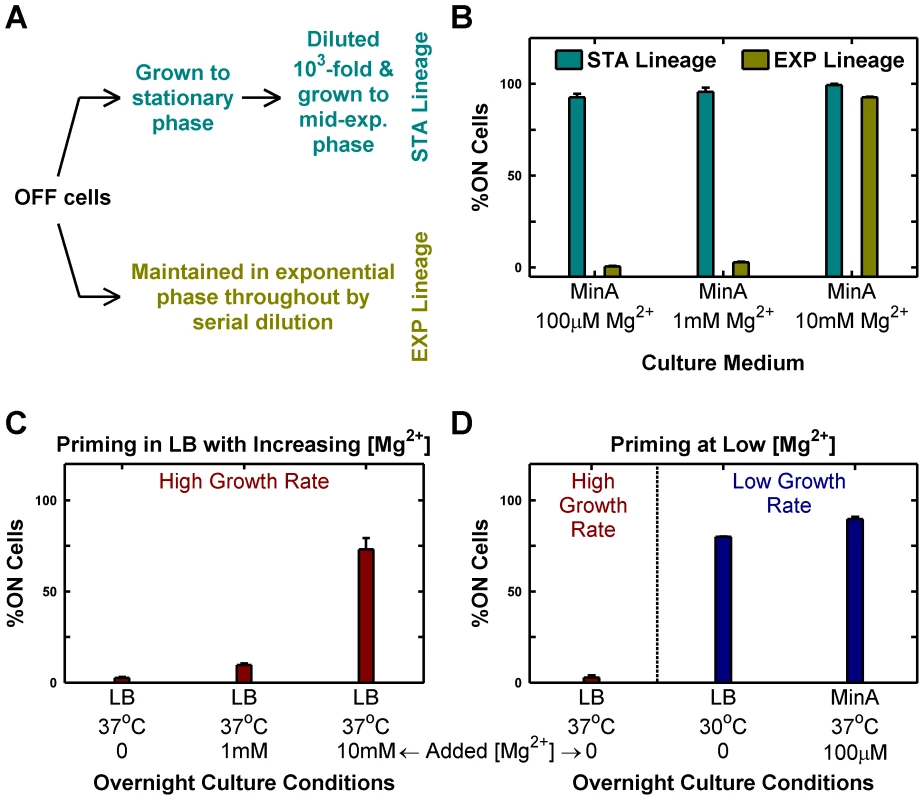
Priming is defined as the deterministic conversion of OFF cells to the ON state. (A and B) Passage through stationary phase primes OFF cells in minimal medium. Starting from OFF cells, two lineages (STA and EXP) were established in minimal medium with various magnesium concentrations as shown schematically in panel A and in detail in Figure S2. Cells from these lineages were imaged under a microscope as described in Methods and the percentage of ON cells in the mid-exponential culture obtained at the end was plotted (panel B). (C and D) Overnight culture at slow growth rates and high Mg2+ results in priming. Overnight cultures inoculated with OFF colonies were set up in indicated conditions, diluted 1000-fold into Minimal A medium with 100 µM Mg2+, grown to mid-exponential phase at 37°C (even for 30°C overnight cultures) and imaged under a microscope (Methods). The percentage of ON cells present in the images of mid-exponential cultures is shown. Panel C shows the effect of increasing [Mg2+] in LB, while panel D documents the effect of changing growth rate in low [Mg2+] media. In panels B, C and D, error bars indicate half the range of two independent experiments. The range is less than 0.5% in the instances where error bars are not visible. In the present context, the phenotypic hysteresis of phoQ (T281R) OFF shown in Figure 1D (i.e. the persistence of these cells in the OFF state) seems anomalous since the OFF cultures were passaged through stationary phase in LB. LB is a rich medium with low (but undetermined) [Mg2+]. Consistent with the reversal of Mg2+ sensitivity in phoQ (T281R), we find that supplementing LB with high [Mg2+] increases conversion of OFF cells to the ON state substantially (Figure 2C). In addition, when we grew LB overnight cultures at 30°C instead of 37°C, we were able to observe OFF→ON conversion at levels comparable to minimal medium with low [Mg2+] at 37°C, suggesting that the higher growth rate in LB plays a major role in the absence of OFF→ON conversion at 37°C (Figure 2D). However, we also note that the apparent low OFF→ON conversion in LB at 37°C (Figure 2C) may reflect the competitive advantage of OFF cells over ON ones in LB stationary phase cultures (see below), which would suppress our ability to detect the number of cells that had turned ON.
Taken together, the above results indicate that slow growth histories or high [Mg2+] are sufficient for en masse conversion of OFF cells to ON (Figure 3A). We call this deterministic conversion from OFF to ON state “priming”.
Fig. 3. A conceptual framework for priming. 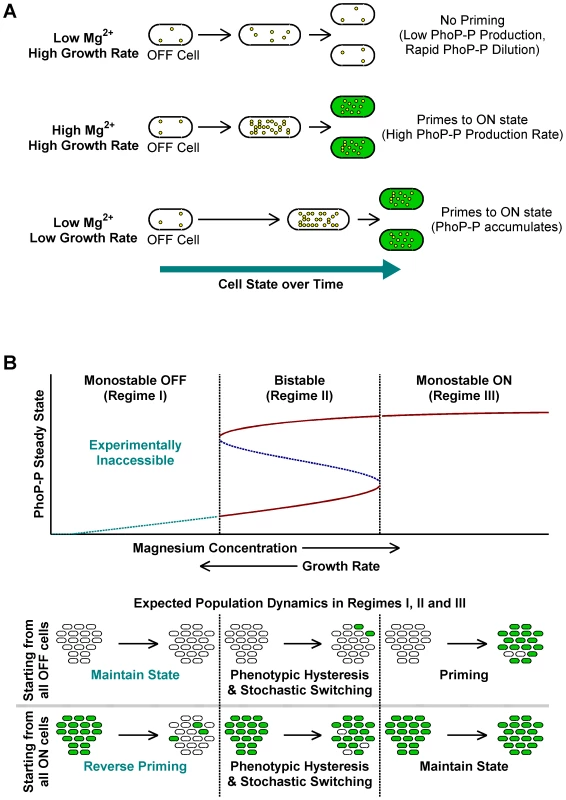
(A) Typical fates of OFF cells under different growth conditions. Yellow circles within cells represent PhoP-P molecules. High Mg2+ and slow growth rates lead to higher PhoP-P concentrations, which result in conversion of OFF cells to the ON state. (B) Stochastic switching and priming. Typical plot obtained by varying system parameters such as kinase rate, maximal expression rate or growth rate in a mathematical model of the phoQ (T281R) network (Text S1) is shown. Steady state PhoP-P values are depicted as a function of the system parameter being varied and the plot can be categorized into three distinct regimes as indicated. Stable OFF and ON state PhoP-P values are plotted in solid maroon. Dashed, blue line represents the unstable intermediate state in the bistable regime. Experimentally inaccessible monostable OFF steady states are shown with a dashed, cyan line. The empirically observed effect of changing magnesium concentrations and growth rate is indicated below the x-axis of the plot. Expected fates of pure OFF and pure ON populations in the three regimes are also illustrated (bottom). The bistable regime is characterized by phenotypic hysteresis and stochastic state switching, whereas priming would be seen in the monostable ON regime. Conceptual Framework for the phoQ (T281R) Network
To further explore the mechanisms responsible for the phenomena described above, we developed a more detailed mathematical model of the PhoQ/PhoP network consisting of six species (PhoP, PhoP-P, PhoQ, PhoQ-P, and two intermediate protein complexes). While bistability provides an explanation for the observed all-or-none behavior based on deterministic steady-state analysis, it is also possible for a monostable network to exhibit bimodal behavior because of slow kinetics and positive feedback, as has been seen in stochastic models [16], [20], [21]. To compare and contrast these mechanisms, we examined the detailed model using both deterministic steady-state analysis and stochastic simulations (Text S1).
Qualitatively, the steady state behavior of the detailed model is similar to the simpler 3-species model indicating that the simpler model captures the essential features of the network required for bistability. In the model, there are three important kinetic parameters influencing bistability – the kinase rate of PhoQ (T281R), the maximal expression rate of the phoPQ operon, and the growth rate of the organism (Text S1). When one of these parameters is varied while keeping all others constant, the system can transition from a monostable OFF regime to a bistable regime and thence to a monostable ON regime (Figure 3B). Note that our model does not incorporate the role of [Mg2+] explicitly, but we posit that for PhoQ (T281R), raising [Mg2+] leads to an increase in the kinase rate or in the maximal operon expression rate.
Stochastic simulations also largely agree with the deterministic analysis, except that noise-induced bimodality can be observed with parameters in the monostable ON regime close to the bistable regime (Text S1). While noise-induced bimodality and deterministic bistability are different mechanisms, operationally it is difficult to distinguish them without detailed measurement of kinetic parameters in vivo. In either case, one would see inheritance of OFF and ON states over several generations.
Within our modeling framework, exponential phase in minimal medium with low [Mg2+] can be considered a condition in, or close to, the bistable regime. In this regime, cells retain their state except for rare, stochastic switching events (Figure 3B, Regime II). Slow growth rates or high [Mg2+] can independently drive the system towards the monostable ON regime, whereupon OFF cells proceed deterministically towards the ON state (Figure 3B, Regime III). Note that the rate of priming may be kinetically limited, but eventually all cells will turn ON. When these ON cells are sub-cultured in low [Mg2+] minimal medium, a bistable (or near-bistable) regime is established again, but hysteresis ensures that cells remain in the ON state as seen in STA lineages in Figure 2B.
Characteristics of the ON State of phoQ (T281R)
Given that a majority of OFF cells can be turned ON by increasing [Mg2+] or by passaging through stationary phase, we asked whether there were culture conditions in which an ON population could be deterministically transformed to the OFF state. In other words, we were interested in establishing a monostable OFF regime (Figure 3B, Regime I). According to our model, this could in principle be achieved with low [Mg2+] and high growth rates. However, we were unable to observe a monostable OFF regime for exponential growth in minimal medium with 100 µM Mg2+ (data not shown) or for growth in LB (Figure 1D). We could not use significantly lower [Mg2+] levels without affecting growth rate. These results suggest that it may not be possible to experimentally realize the monostable OFF regime, i.e., the ON state may always be stable. A bistable system with this property is termed irreversible [22]. Note that irreversibility of the system only means that a population of ON cells cannot be deterministically turned OFF. Individual ON cells can still transition to the OFF state in the bistable regime because of stochastic fluctuations (Figure 3B, Regime II). Stochastic switching from the ON to OFF state and its implications are examined later in this study.
The high PhoP-P level in the ON state has pleiotropic effects on the cell. As presented in Figure 1C–D, cell morphology is affected in the ON state. We also determined that the ON state has a lower fitness in stationary phase in LB. To explore fitness differences between ON and OFF cells, we performed competitions using chloramphenicol resistant (CmR) and sensitive (CmS) phoQ(T281R) strains prepared in the ON and OFF states (Figure 4, S3 and Methods). When ON and OFF cells were competed for 10 hours in stationary phase in LB, the ON fraction in the population showed a significant decrease (competitive ratio, Figure 4, columns 2 and 4). In contrast, competitions between CmS and CmR OFF cells or between CmS and CmR ON cells yielded a near-neutral competitive ratio (Figure 4, columns 1 and 3 respectively).
Fig. 4. ON cells have a competitive disadvantage in stationary phase in LB. 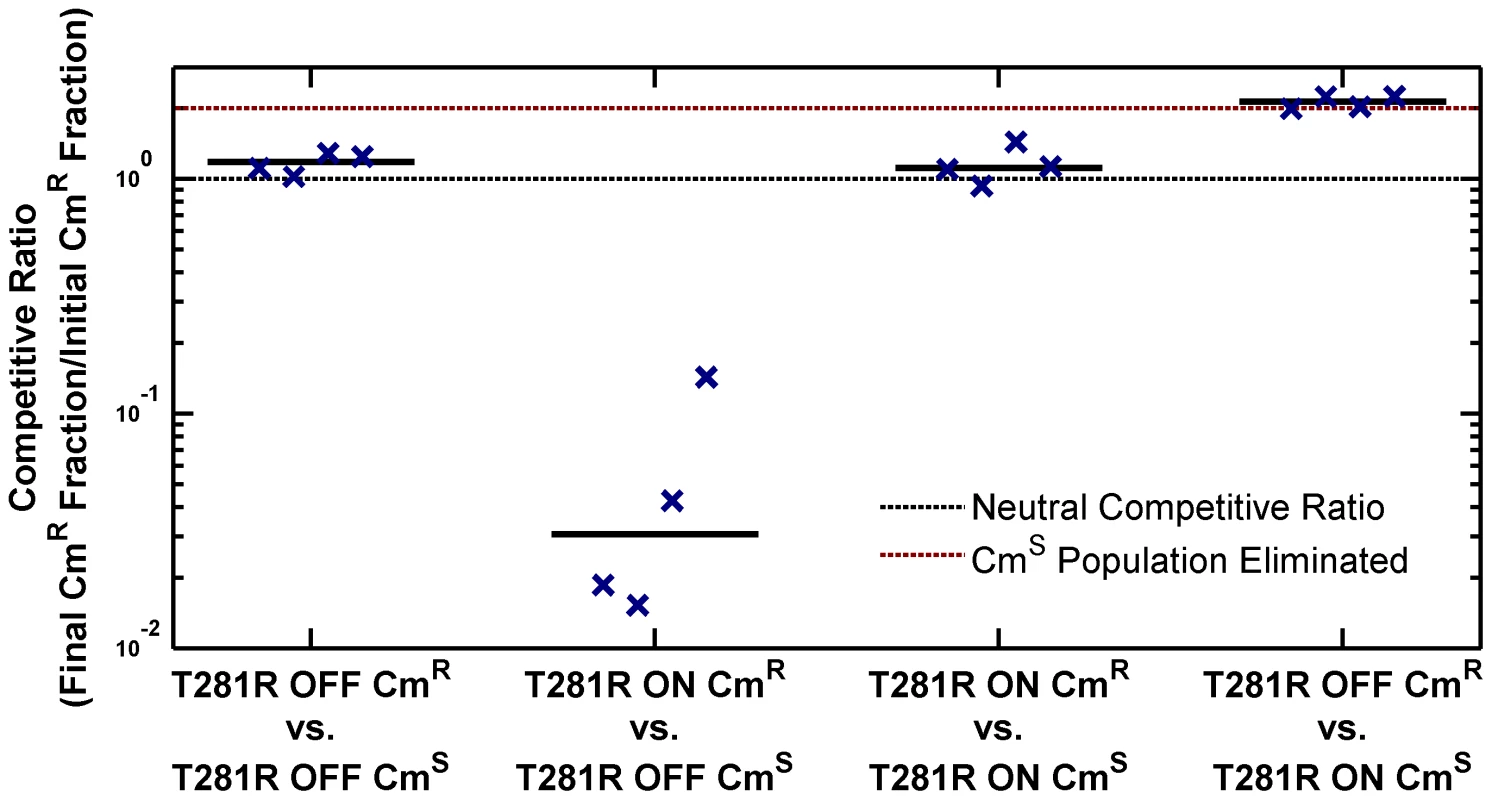
Overnight cultures of chloramphenicol-sensitive (CmS) and chloramphenicol-resistant (CmR) variants of phoQ (T281R) OFF and phoQ (T281R) ON were set up independently in LB. For each competition experiment, 1 ml of CmS and CmR overnight cultures were mixed and co-cultured for an additional 10 hours in stationary phase. Initial and final total and CmR populations were quantified by plating appropriate dilutions on LB and LB+chloramphenicol plates (see Figure S3 for a detailed protocol). Colony counts were used to compute a competitive ratio (CR), defined as CR = (C(10)L(0))/(C(0)L(10)), where C(T) and L(T) denote the counts on chloramphenicol and LB plates at time T respectively. Note that CR is different from the competitive index (CI) that is frequently used to quantify the outcome of competition experiments (CI is the quotient of final and initial ratios of the population of the two competing strains). In our experimental design, CR has an upper bound of ∼2, whereas CI is, in principle, an unbounded quantity. In competitions between CmS ON and CmR OFF cells, the final CmS population is below the detection limit and CI cannot be computed. Symbols indicate CR values obtained from 4 independent competition experiments. The solid, black line represents the median. Dashed, black line indicates a neutral competitive ratio of 1. Note that a CR of 2 (dashed, maroon line) corresponds to a near-elimination of the CmS population. Metastability of the ON State and Epigenetic Trapping
To examine stochastic ON→OFF transitions more closely, we performed long-term culture experiments in LB. As mentioned above, a low [Mg2+], high growth rate medium such as LB is ideal for observing ON→OFF switching since that growth condition is likely to be close to the theoretical monostable OFF regime. Furthermore, we reasoned that the competitive advantage of OFF cells in stationary phase in LB could be used to amplify the effects of switching and enhance our ability to detect switching events. We established independent lineages by inoculating LB cultures with either phoQ (T281R) OFF or phoQ (T281R) ON colonies and maintained them through million-fold dilution once per day. The state of the population was assayed by spreading overnight cultures on minimal media plates on alternate days and measuring the fraction of ON (YFP-bright) colonies (Figure S4). This protocol subjected populations to ∼20 generations of exponential growth per day but the majority of time (>14 hours/day) was spent in stationary phase. Furthermore, at least ∼2000 cells were transferred from one day to the next, which meant that even if the fraction of OFF cells in the saturated previous day culture of an ON lineage was as low as 0.1%, there was a ∼90% chance that an OFF cell would be present in the inoculum for the next day culture based on the statistics of binomial sampling.
As expected, lineages inoculated with phoQ (T281R) OFF yielded mostly OFF colonies on the assay plates (Figure 5). Lineages inoculated with ON colonies, however, showed a different pattern. These remained close to 100% ON for several days (Figure 5, see Day 3 time point), but on Day 5, an appreciable fraction of YFP-dim colonies could be seen in 6 out of 7 lineages. Furthermore, the fraction of YFP-dim colonies obtained in the different lineages was not the same. This divergence highlights both the stochastic nature and the low probability of ON→OFF switching. Since all the ON lineages are likely to converge to a mostly OFF state eventually, the ON lineage in LB is metastable – a long-lived, but not truly stable state due to the competitive advantage of the OFF state. We note that the ON→OFF transitions seen in the ON lineages are unlikely to be the result of mutational events since the YFP-dim colonies obtained in these lineages could be primed ON by overnight growth in minimal medium with 10 mM Mg2+ (1 dim colony was tested per lineage, data not shown).
Fig. 5. Long-term culturing demonstrates the metastability of the ON state in LB. 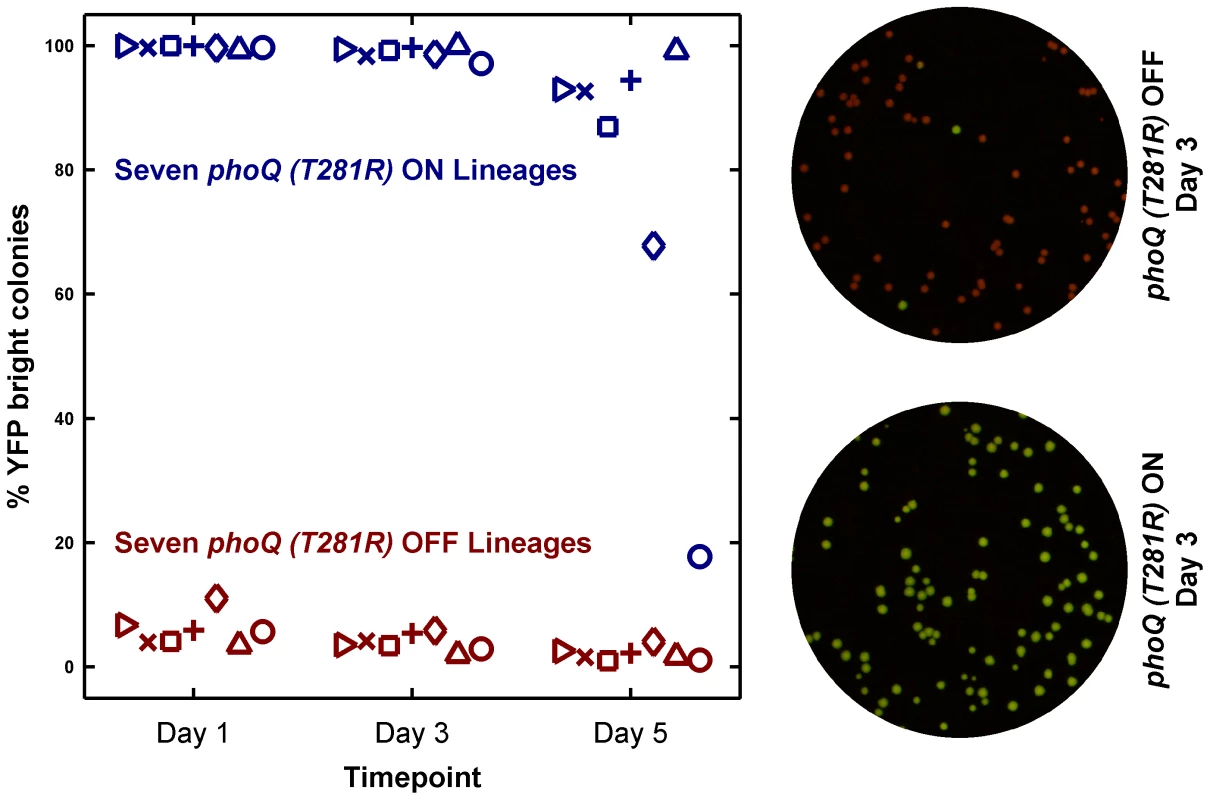
Seven independent lineages of phoQ (T281R) OFF and phoQ (T281R) ON were established and maintained as depicted in detail in Figure S4. OFF and ON colonies were inoculated in LB and grown for 24 hours to generate a Day 1 culture. For each lineage, the Day 1 culture was diluted 106-fold to generate a corresponding Day 2 culture, and this procedure was repeated for a total of 5 days. Day 1, 3, and 5 cultures were also diluted and plated in duplicate on minimal medium plates. The mean percentage of YFP-bright colonies on these plates was plotted in maroon (phoQ (T281R) OFF) or blue (phoQ (T281R) ON) with different symbols representing independent lineages. Representative merged images of Day 3 plates are shown on the right. These were constructed by merging the background-subtracted CFP image with its corresponding background-subtracted YFP image as the red and green channels respectively. These results show that strong epigenetic inheritance can effectively trap a population in a low-fitness state under some circumstances. Irreversibility enhances this phenomenon by rendering rare, stochastic transitions between states as the fastest means for “escape” from the epigenetic trap.
phoQ (T281R) Properties Influenced by Network Architecture
Having examined the unique characteristics of the phoQ (T281R) strain, we asked whether there were any essential features in the PhoQ/PhoP network architecture that enabled a single point mutation to produce such remarkable behavior. Our modeling suggested that bistability (or near-bistability for the stochastic model) in the network was strongly dependent on PhoP-P regulation of both phoP and phoQ transcription (Text S1). We found that an altered PhoQ/PhoP network where phoP transcription is autoregulated but phoQ (T281R) transcription is independent of PhoP-P was likely to be monostable, especially if the phosphatase defect in PhoQ (T281R) stemmed from a high dissociation constant of the complex between PhoP-P and PhoQ (an intermediate in the phosphatase reaction) (Text S1 and Figure S1D).
To test whether elimination of PhoP-P dependent transcription of phoQ (T281R) abrogated bistability, we constructed a strain in which phoQ was deleted from its native locus and phoQ (T281R) was inserted at a phage attachment site under the control of the IPTG-inducible (and PhoP-P insensitive) Ptrc promoter (Figure 6A). We measured YFP/CFP in individual cells after 10 hours of growth (∼15 generations) at various IPTG concentrations starting from uninduced and fully induced populations of cells (Figure 6B). We did not observe any evidence of hysteresis in this strain: cultures started with both fully induced and uninduced cells converge to similar distributions for all IPTG concentrations tested (Figure 6B). Furthermore, distributions spanning intermediate values of YFP/CFP could be observed even after 15 generations of culture (i.e. populations did not converge to distributions with low and high modes). Taken together, these observations suggest that there is no bistability in the decoupled strain. The wide distributions seen at 12.5 µM and 25 µM IPTG can be attributed to a combination of noise in IPTG induction [23], [24] and the sensitivity of the system to induction level in this range (Figure 6B inset) and are also seen in our stochastic simulations (Figure S5D). The slightly lower median of samples derived from the uninduced culture compared with the induced culture could be a result of a stochastic effect that slows down induction kinetics in autoregulated systems [16]. We also determined that a strain similar to the one depicted in Figure 6A, but with phoQ (T281R) under the control of its native phoPQ promoter instead of Ptrc exhibited bistability (data not shown).
Fig. 6. Architectural features of the PhoQ/PhoP network that govern bistability and priming. 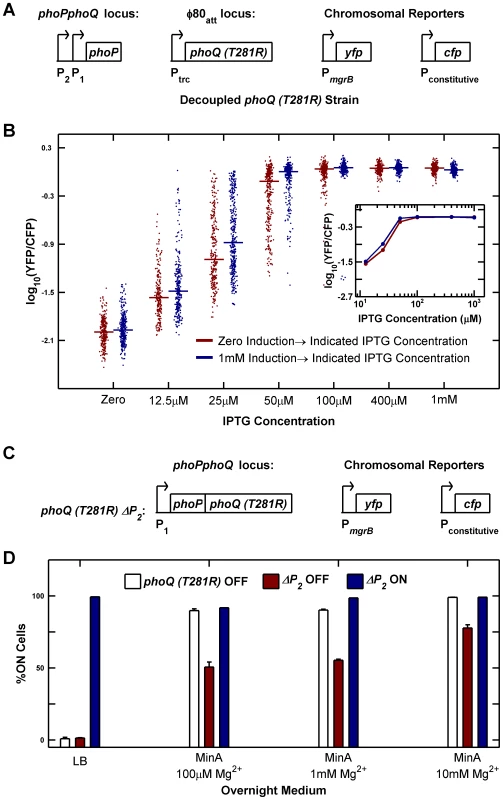
(A and B) Decoupling of phoQ (T281R) expression from PhoP-P control results in loss of bistability. Panel A is a schematic of the decoupled phoQ (T281R) strain. phoP is driven by its native promoters: the constitutive P2 and the PhoP-P responsive P1 promoters, whereas expression of phoQ (T281R) is governed by the PhoP-P independent, IPTG-inducible Ptrc promoter. Uninduced and fully induced cultures of the decoupled strain were washed twice in minimal medium without IPTG, diluted 105-fold into Minimal A medium containing 100 µM Mg2+ with indicated IPTG concentrations, grown to mid-exponential phase and imaged under a microscope (Methods). YFP/CFP distributions at various induction levels are shown in panel B. For each sample, the median value of YFP/CFP is represented as a horizontal line. Samples derived from uninduced and fully induced cultures are plotted in maroon and blue respectively. Inset shows the median YFP/CFP value as a function of IPTG concentration for samples derived from the induced (blue) and uninduced (maroon) cultures. (C and D) Deletion of the constitutive P2 promoter of the phoPQ operon reduces priming. Panel C is a schematic of the ΔP2 strain in which the constitutive P2 promoter of the phoPQ operon had been deleted. Overnight cultures of phoQ (T281R) OFF, ΔP2 OFF, and ΔP2 ON strains in indicated medium at 37°C were diluted 1000-fold into Minimal A medium with 100 µM Mg2+, grown to mid-exponential phase and imaged under a microscope (Methods). The percentage of ON cells in the images is plotted in panel D. Error bars indicate half the range of two independent experiments. The range is less than 0.5% in the instances where error bars are not visible. As documented in Figure 2, one of the defects in the phoQ (T281R) strain is that OFF cells transition to the ON state both in response to specific signals such as high [Mg2+] and to extraneous factors such as growth rate reduction in stationary phase. We therefore explored whether the phoQ (T281R) network could be modified to reduce priming in stationary phase whilst minimally affecting other characteristics of the strain. Based on our model, we predicted that a reduction in the basal level of phoPQ transcription (PhoP-P independent transcription) would extend the range of growth rates for which the OFF state was stable, thereby reducing conversion to the ON state at lower growth rates (Text S1 and Figure S1C). To test this prediction, we took advantage of the fact that phoPphoQ transcription is driven by two promoters: one is activated by PhoP-P and the other is constitutive [25]. We constructed a variant of the phoQ (T281R) strain with the −35 and upstream region of the constitutive P2 promoter deleted (Figure 6C). As with the parent strain, we obtained YFP-dim and YFP-bright colonies and designated these ΔP2 OFF and ΔP2 ON respectively. Consistent with the model predictions, only half of ΔP2 OFF cells prime in minimal medium with low [Mg2+] compared to ∼90% priming in OFF cells of the parent strain in these conditions (Figure 6D, compare maroon and white bars). The primed fraction in ΔP2 OFF also decreases in minimal medium with high [Mg2+], but only to ∼80% indicating that sensitivity to high [Mg2+] is mostly retained (Figure 6D, last column). As expected, neither ΔP2 OFF cells nor OFF cells of the parent strain show any priming in LB overnight cultures (Figure 6D, first column). In contrast to the OFF cells, ΔP2 ON cells remain ON in all four culture media indicating that the irreversibility of the system is not affected (Figure 6D, blue bars). We also verified that the deletion of the P2 promoter did not adversely affect the PhoP-P responsiveness of the P1 promoter by comparing YFP reporters driven by the native phoPQ promoter and P1 promoter alone and determining that the two reporters behave similarly at high PhoP-P levels (Figure S6).
Discussion
We have demonstrated that a single point mutation, causing a T281R substitution in the histidine kinase PhoQ, results in a remarkable change in phenotype, with cells exhibiting bistability/bimodality and an inverted response to [Mg2+]. Previous modeling work indicates that two-component systems have the potential to exhibit bimodal behavior as a result of stochastic fluctuations in molecular components [16], [20], [21] as well as bistability [26], [27]. Bistability has been observed experimentally in the MprB/MprA two-component system [28], although additional feedback loops not present in simple two-component signaling architectures may be responsible for the bistability in that system [29]. Beyond two-component systems, the response of the lac operon to certain gratuitous inducers is another setting where bistability and strong epigenetic inheritance has been observed in bacteria [30], [31]. Like the lac operon, we find the OFF and ON states in PhoQ (T281R) can be inherited over many generations. However, we have not been able to find conditions where ON cells can be deterministically converted to an OFF state. Such irreversibility has been seen in the development of Xenopus oocytes [32] and is more akin to transient or terminal differentiation seen during bacterial competence or sporulation. Both competence and sporulation are highly regulated phenomena [33] and neither is, strictly speaking, a manifestation of a simple positive-feedback based bistable switch [34], [35]. In this sense, irreversibile bistability is a distinctive feature of the PhoQ (T281R) network among characterized two-component signaling systems.
While bistability and noise-induced bimodality are alternate explanations for the observed behavior of the PhoQ (T281R) network, it may be difficult to differentiate between these mechanisms experimentally. First, in our simulations, we observe noise-induced bimodality only for monostable parameters close to the true bistable regime (Text S1). Thus, distinguishing between these mechanisms would likely require careful measurement of kinetic parameters in vivo, which can be challenging even for simple networks. Second, for the mechanism based on noise-induced biomodality, if the timescale for conversion from OFF to ON state in the bimodal regime is tens of generations, then any small fitness difference between the two states can become relevant and even lead to the emergence of bistability, as has been shown in a synthetic circuit [36].
What are the architectural features of the PhoQ/PhoP circuit that enable the T281R mutation to produce such unusual properties? The modeling presented here reveals that the mutant network can readily show bistable behavior when both the kinase and phosphatase activities of the histidine kinase are low (Text S1). A point mutation that simultaneously affects both functions of the histidine kinase can produce a bistable response, as is the case with the T281R mutation, where the kinase activity is low and the phosphatase activity is not detectable. Modeling also indicated that autoregulation of both the response regulator and histidine kinase is required for bistability (Figure 5B). Thus, since phoP and phoQ are organized as an operon that is driven by a PhoP-P responsive promoter, the architecture of the PhoQ/PhoP network (Figure 1A) is poised to show bistability upon introduction of the T281R mutation in PhoQ. Given that bifunctional histidine kinases and autoregulation are common themes in two-component signaling [6], mutations decreasing phosphatase activity in other histidine kinases may similarly lead to bistable behavior.
Since our model does not explicitly incorporate the role of [Mg2+] as an input signal, we can only speculate on the potential mechanism for the inversion of [Mg2+]-sensitivity in the mutant. In terms of our model, the inversion can be explained if higher [Mg2+] increases the kinase rate of PhoQ (T281R) or increases the maximal expression rate of the phoPphoQ operon (potentially through a PhoP-P independent mechanism). Both of these effects could be masked in the wild-type network. For example, an increase in kinase rate at high [Mg2+] could be accompanied by a much larger increase in phosphatase rate in PhoQ (WT) resulting in the observed repression of PhoP-P levels under these conditions. Any increase in transcriptional activity is likely to be unnoticed in the wildtype as the output for the wild-type circuit is relatively insensitive to PhoP and PhoQ protein levels for most ranges of magnesium concentration [13]. We should also note that the reversal of input sensitivity may be an idiosyncrasy of the PhoQ/PhoP system and not a general feature of phosphatase mutants of bifunctional histidine kinases.
The phoQ (T281R) mutant strain exhibits phenotypic hysteresis, that is, the state of a population depends strongly on the culture history (Figure 1D and 2B). OFF cells retain their state in exponential phase, but prime to the ON state upon passage through stationary phase in minimal medium with low [Mg2+] (Figure 2B). In the lac operon, slow growth rate has been shown to increase the transition probability from the uninduced to induced state through the accumulation of inducer and the permease protein LacY [37]. A reduction in growth rate of the phoQ (T281R) strain can similarly cause PhoP and PhoQ to accumulate because of residual expression of the phoPphoQ operon. In addition, PhoP-P would be expected to accumulate from PhoQ (T281R) kinase activity and from the lack of a specific phosphatase. Either mechanism can account for the phenomenon of priming.
Is it possible for strains with wild-type phoQ to show bistability? The wild-type PhoQ/PhoP system responds to lowering Mg2+ concentrations in a unimodal, graded manner [13]. As long as the loss of PhoP-P due to the phosphatase activity of PhoQ (WT) dominates over the reduction in PhoP-P concentration due to growth-mediated dilution, the network is monostable (Text S1 and ref. [13]). It is conceivable that under highly activating conditions, the phosphatase activity of PhoQ (WT) is sufficiently low, in effect, phenocopying the phosphatase defect in phoQ (T281R). Under these circumstances, our analysis (Text S1) suggests that bistability is possible provided that (1) enough PhoP-P can be produced to begin to saturate the PhoP-P mediated feedback expression of the phoPQ operon and (2) the constitutive expression of the operon is sufficiently low (Figure S1B). However, at a Mg2+ concentration of 100 µM, which is an activating stimulus for PhoQ (WT), condition (1) is not satisfied in the wild-type network [38]. It is, therefore, possible that the wild-type circuit has evolved to always maintain a significant level of phosphatase activity to avoid bistability.
The phenotypic heterogeneity associated with bistability can improve fitness in fluctuating or unpredictable environments [39], [40]. Bistability also provides a mechanism for storing information about past environmental conditions, and the persistence of this information can be influenced by the network architecture [41]. In the case of phoQ (T281R), however, bistability comes at a fitness cost because of its irreversible nature. Long-term culture experiments in LB showed that a population of ON cells could be trapped in this low-fitness state. In principle, epigenetic trapping can also occur in a reversible system, but in that case there would be environmental conditions in which the population can deterministically transition to the high-fitness state. In an irreversible system, rare stochastic transitions followed by enrichment due to selection are the only means of escaping from the trap.
Interactions between multiple levels of organization in biological systems can result in unexpected or counter-intuitive phenomena. For example, noise at the molecular level can produce bimodal outcomes in a genetic network that is monostable when analyzed as a deterministic system [42]. Likewise, bistability can emerge in a monostable network because of the influence of the output of the network on the growth rate of the organism [36]. In the case of phoQ (T281R), we find that a bistable genetic network gives rise to a stable and a metastable mode since one of the stable states of the network is inherited efficiently, but has a fitness disadvantage. The phoQ (T281R) mutation also perfectly illustrates a recent suggestion by Kitano that biological networks may be more sensitive to ‘fail-on’ failures where components function in unexpected ways than to ‘fail-off’ failures where components do not function at all or are removed [43]. While a phoQ deletion would be unresponsive to Mg2+ levels, it would not show bistability or epigenetic trapping. In contrast, a single point mutation in the robust PhoQ/PhoP signaling module results in a phosphatase-deficient PhoQ protein that gives rise to bistability and an ON state with pleiotropic alterations in cell physiology.
Methods
Bacterial Strains and Growth Conditions
Strains and plasmids used in this study are listed in Tables S1 and S2 respectively, and details of their construction are included in Text S2. Table S3 lists primers used for strain construction. A figure-wise list of strains used to collect data is included as Table S4. Freshly streaked E. coli strains grown on Miller LB Agar (Fisher BioReagents, Pittsburgh, PA) plates at 37°C were inoculated either in Miller LB broth (Difco - BD, Franklin Lakes, NJ) or in Minimal A medium (MinA, [44]) supplemented with 0.2% glucose, 0.1% casamino acids (Difco - BD, Franklin Lakes, NJ) and with 100 µM, 1 mM or 10 mM MgSO4 as indicated. The phenotype of the colony (YFP-dim or YFP-bright) was always verified prior to inoculation. Overnight cultures were grown at 37°C with aeration in a roller drum and for ∼22.5 hours unless otherwise indicated. These were typically diluted 1000-fold into the indicated medium and grown until most of the cultures reached an OD600 of 0.2–0.3 for microscopy. Some ON state cultures did not reach an OD600 of 0.2 within 4.5 hours of dilution and these were concentrated by gentle centrifugation and resuspension in a smaller volume prior to imaging.
IPTG-induction experiments with the decoupled phoQ (T281R) strain (Figure 6A–B) were performed by overnight culture of the strain in MinA/100 µM Mg2+ for 12 hours at 37°C and diluting the overnight 1000-fold into two MinA/100 µM Mg2+ cultures. After 2.2 hours, one of these cultures was fully induced by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Both the induced and uninduced cultures were grown for an additional 2 hours and then washed twice in MinA/100 µM Mg2+ by gentle centrifugation and resuspension. Resuspended cultures were then diluted 105-fold into MinA/100 µM Mg2+ with indicated IPTG concentrations, grown at 37°C for 10 hours and imaged as described below. For comparing the phoPQ reporter and ΔP2 reporter strains (Figure S6), overnight cultures of these strains were grown in MinA/1 mM Mg2+ or MinA/10 mM Mg2+ for ∼22.5 hours at 37°C, diluted 1000-fold into MinA/1 mM Mg2+ or MinA/10 mM Mg2+ with indicated IPTG concentrations and imaged as described below.
Single-Cell Microscopy
Mid-exponential cultures were cooled rapidly in ice-water slurry and streptomycin was added to a final concentration of 250 µg/ml to inhibit further protein synthesis. Imaging was performed on a motorized inverted microscope (Olympus IX81) essentially as described previously [13], [45]. CFP and YFP fluorescence were quantified in single-cells using custom macros written for ImageJ [46]. At least 150 cells were imaged per condition. For quantifying cell-widths, phase images were thresholded and used to segment individual cells. The minor axis of the best-fit ellipse for each identified cell was used as a measure of cell-width.
Fluorescence Imaging of Plates
Overnight cultures were diluted 106-fold in MinA supplemented with 0.2% glucose and 0.1% casamino acids. 100 µl of the diluted culture was spread on 1.5% agar plates composed of MinA with 0.2% glucose, 0.1% casamino acids, and no added MgSO4. These plates were incubated at 37°C for 20 hours and imaged with a home-built fluorescence illuminator as described previously [15].
Competition Experiments
Freshly streaked colonies of indicated chloramphenicol-sensitive (CmS) and chloramphenicol-resistant (CmR) strains were inoculated in LB medium and grown at 37°C for 20 hours with aeration in a roller drum. At the beginning of the competition, these saturated cultures were mixed 1∶1 by volume and combined cultures were grown for another 10 hours at 37°C. Total and CmR colony forming units (cfus) at the beginning and end of the competition were determined by spreading dilutions on LB Agar plates without antibiotic and with 15 µg/ml chloramphenicol respectively.
Modeling of the phoQ (T281R) Network
The phoQ (T281R) network was mathematically modeled as described in Text S1. Symbolic manipulations and numerical computations were performed using custom code written in MATLAB (Mathworks, Natick, MA) or C programming language. The region of bistability seen in deterministic models is shown in Figures S7, S8, S9, and representative results from stochastic simulations are shown in Figure S5. Parameters and reactions used in stochastic simulations are presented in Tables S5 and S6.
Supporting Information
Zdroje
1. ShanerNC, CampbellRE, SteinbachPA, GiepmansBN, PalmerAE, et al. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22 : 1567–1572.
2. TracewellCA, ArnoldFH (2009) Directed enzyme evolution: climbing fitness peaks one amino acid at a time. Curr Opin Chem Biol 13 : 3–9.
3. PoelwijkFJ, de VosMG, TansSJ (2011) Tradeoffs and optimality in the evolution of gene regulation. Cell 146 : 462–470.
4. BhallaUS, IyengarR (1999) Emergent properties of networks of biological signaling pathways. Science 283 : 381–387.
5. GuetCC, ElowitzMB, HsingW, LeiblerS (2002) Combinatorial synthesis of genetic networks. Science 296 : 1466–1470.
6. GoulianM (2010) Two-component signaling circuit structure and properties. Current Opinion in Microbiology 13 : 184–189.
7. Garcia VescoviE, SonciniFC, GroismanEA (1996) Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84 : 165–174.
8. ProstLR, DaleyME, Le SageV, BaderMW, Le MoualH, et al. (2007) Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell 26 : 165–174.
9. BaderMW, SanowarS, DaleyME, SchneiderAR, ChoU, et al. (2005) Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122 : 461–472.
10. GroismanEA (2001) The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 183 : 1835–1842.
11. CastelliME, Garcia VescoviE, SonciniFC (2000) The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem 275 : 22948–22954.
12. BatchelorE, GoulianM (2003) Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proceedings of the National Academy of Sciences of the United States of America 100 : 691–696.
13. MiyashiroT, GoulianM (2008) High stimulus unmasks positive feedback in an autoregulated bacterial signaling circuit. Proceedings of the National Academy of Sciences of the United States of America 105 : 17457–17462.
14. ShinarG, MiloR, MartinezMR, AlonU (2007) Input-output robustness in simple bacterial signaling systems. Proceedings of the National Academy of Sciences of the United States of America 104 : 19931–19935.
15. SiryapornA, GoulianM (2008) Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Mol Microbiol 70 : 494–506.
16. HermsenR, EricksonDW, HwaT (2011) Speed, Sensitivity, and Bistability in Auto-activating Signaling Circuits. Plos Computational Biology 7: e1002265.
17. HofferSM, WesterhoffHV, HellingwerfKJ, PostmaPW, TommassenJ (2001) Autoamplification of a two-component regulatory system results in “learning” behavior. Journal of Bacteriology 183 : 4914–4917.
18. RayJCJ, IgoshinOA (2010) Adaptable functionality of transcriptional feedback in bacterial two-component systems. Plos Computational Biology 6: e1000676.
19. ShinD, LeeEJ, HuangH, GroismanEA (2006) A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science 314 : 1607–1609.
20. HoyleRB, AvitabileD, KierzekAM (2012) Equation-free analysis of two-component system signalling model reveals the emergence of co-existing phenotypes in the absence of multistationarity. PLoS Comput Biol 8: e1002396.
21. KierzekAM, ZhouL, WannerBL (2010) Stochastic kinetic model of two component system signalling reveals all-or-none, graded and mixed mode stochastic switching responses. Mol Biosyst 6 : 531–542.
22. FerrellJEJr (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14 : 140–148.
23. ElowitzMB, LevineAJ, SiggiaED, SwainPS (2002) Stochastic gene expression in a single cell. Science 297 : 1183–1186.
24. OzbudakEM, ThattaiM, KurtserI, GrossmanAD, van OudenaardenA (2002) Regulation of noise in the expression of a single gene. Nature Genetics 31 : 69–73.
25. KatoA, TanabeH, UtsumiR (1999) Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol 181 : 5516–5520.
26. IgoshinOA, AlvesR, SavageauMA (2008) Hysteretic and graded responses in bacterial two-component signal transduction. Molecular Microbiology 68 : 1196–1215.
27. TiwariA, RayJC, NarulaJ, IgoshinOA (2011) Bistable responses in bacterial genetic networks: designs and dynamical consequences. Math Biosci 231 : 76–89.
28. SurekaK, GhoshB, DasguptaA, BasuJ, KunduM, et al. (2008) Positive feedback and noise activate the stringent response regulator rel in mycobacteria. PLoS One 3: e1771.
29. TiwariA, BalazsiG, GennaroML, IgoshinOA (2010) The interplay of multiple feedback loops with post-translational kinetics results in bistability of mycobacterial stress response. Phys Biol 7 : 036005.
30. NovickA, WeinerM (1957) Enzyme Induction as an All-or-None Phenomenon. Proc Natl Acad Sci U S A 43 : 553–566.
31. OzbudakEM, ThattaiM, LimHN, ShraimanBI, Van OudenaardenA (2004) Multistability in the lactose utilization network of Escherichia coli. Nature 427 : 737–740.
32. XiongW, FerrellJEJr (2003) A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature 426 : 460–465.
33. DubnauD, LosickR (2006) Bistability in bacteria. Mol Microbiol 61 : 564–572.
34. LevineJH, FontesME, DworkinJ, ElowitzMB (2012) Pulsed feedback defers cellular differentiation. PLoS Biol 10: e1001252.
35. SuelGM, Garcia-OjalvoJ, LibermanLM, ElowitzMB (2006) An excitable gene regulatory circuit induces transient cellular differentiation. Nature 440 : 545–550.
36. TanC, MarguetP, YouL (2009) Emergent bistability by a growth-modulating positive feedback circuit. Nat Chem Biol 5 : 842–848.
37. RobertL, PaulG, ChenY, TaddeiF, BaiglD, et al. (2010) Pre-dispositions and epigenetic inheritance in the Escherichia coli lactose operon bistable switch. Molecular Systems Biology 6 doi: 10.1038/msb.2010.12
38. MiyashiroT, GoulianM (2007) Stimulus-dependent differential regulation in the Escherichia coli PhoQ-PhoP system. Proceedings of the National Academy of Sciences of the United States of America 104 : 16305–16310.
39. Davidson CJ, Surette MG (2008) Individuality in Bacteria. Annual Review of Genetics. pp. 253–268.
40. KussellE, LeiblerS (2005) Phenotypic diversity, population growth, and information in fluctuating environments. Science 309 : 2075–2078.
41. AcarM, BecskeiA, van OudenaardenA (2005) Enhancement of cellular memory by reducing stochastic transitions. Nature 435 : 228–232.
42. ArtyomovMN, DasJ, KardarM, ChakrabortyAK (2007) Purely stochastic binary decisions in cell signaling models without underlying deterministic bistabilities. Proc Natl Acad Sci U S A 104 : 18958–18963.
43. KitanoH (2007) A robustness-based approach to systems-oriented drug design. Nat Rev Drug Discov 6 : 202–210.
44. Miller JH (1992) A short course in bacterial genetics : a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y.: Cold Spring Harbor Laboratory Press. 456 p.
45. MiyashiroT, GoulianM (2007) Single-cell analysis of gene expression by fluorescence microscopy. Methods Enzymol 423 : 458–475.
46. Rasband WS (1997–2012) ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, Available: http://imagej.nih.gov/ij/
Štítky
Genetika Reprodukčná medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



