-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
The phenotypic outcome of a mutation cannot be simply mapped onto the underlying DNA variant. Instead, the phenotype is a function of the allele, the genetic background in which it occurs and the environment where the mutational effects are expressed. While the influence of genetic background on the expressivity of individual mutations is recognized, its consequences on the interactions between genes, or the genetic network they form, is largely unknown. The description of genetic networks is essential for much of biology; yet if, and how, the topologies of such networks are influenced by background is unknown. Furthermore, a comprehensive examination of the background dependent nature of genetic interactions may lead to identification of novel modifiers of biological processes. Previous work in Drosophila melanogaster demonstrated that wild-type genetic background influences the effects of an allele of scalloped (sd), with respect to both its principal consequence on wing development and its interactions with a mutation in optomotor blind. In this study we address whether the background dependence of mutational interactions is a general property of genetic systems by performing a genome wide dominant modifier screen of the sdE3 allele in two wild-type genetic backgrounds using molecularly defined deletions. We demonstrate that ∼74% of all modifiers of the sdE3 phenotype are background-dependent due in part to differential sensitivity to genetic perturbation. These background dependent interactions include some with qualitative differences in the phenotypic outcome, as well as instances of sign epistasis. This suggests that genetic interactions are often contingent on genetic background, with flexibility in genetic networks due to segregating variation in populations. Such background dependent effects can substantially alter conclusions about how genes influence biological processes, the potential for genetic screens in alternative wild-type backgrounds identifying new loci that contribute to trait expression, and the inferences of the topology of genetic networks.
Published in the journal: The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen. PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003661
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003661Summary
The phenotypic outcome of a mutation cannot be simply mapped onto the underlying DNA variant. Instead, the phenotype is a function of the allele, the genetic background in which it occurs and the environment where the mutational effects are expressed. While the influence of genetic background on the expressivity of individual mutations is recognized, its consequences on the interactions between genes, or the genetic network they form, is largely unknown. The description of genetic networks is essential for much of biology; yet if, and how, the topologies of such networks are influenced by background is unknown. Furthermore, a comprehensive examination of the background dependent nature of genetic interactions may lead to identification of novel modifiers of biological processes. Previous work in Drosophila melanogaster demonstrated that wild-type genetic background influences the effects of an allele of scalloped (sd), with respect to both its principal consequence on wing development and its interactions with a mutation in optomotor blind. In this study we address whether the background dependence of mutational interactions is a general property of genetic systems by performing a genome wide dominant modifier screen of the sdE3 allele in two wild-type genetic backgrounds using molecularly defined deletions. We demonstrate that ∼74% of all modifiers of the sdE3 phenotype are background-dependent due in part to differential sensitivity to genetic perturbation. These background dependent interactions include some with qualitative differences in the phenotypic outcome, as well as instances of sign epistasis. This suggests that genetic interactions are often contingent on genetic background, with flexibility in genetic networks due to segregating variation in populations. Such background dependent effects can substantially alter conclusions about how genes influence biological processes, the potential for genetic screens in alternative wild-type backgrounds identifying new loci that contribute to trait expression, and the inferences of the topology of genetic networks.
Introduction
Fundamental to the logic of genetic analysis is that the observed variation in a phenotype for a genetically mediated trait is causally linked to one or more DNA lesions/variants. However, it is well known that the phenotypic effects of many individual mutant alleles are context dependent, with respect to environmental influences, as well as the “wild-type” genetic background in which the mutation is observed. Indeed, genetic background has long been known to influence observed phenotypic expression across traits, organisms, and a range of allelic effects, including hypomorphs, amorphs/nulls and neomorphs [1]–[9]. These results make it clear that the phenotypic effects of a mutation (i.e. penetrance and expressivity) are themselves “complex traits”, subject to environmental and polygenic influences [1]. Far beyond being a minor curiosity in genetics, the background dependent effects of a number of mutations have been at the heart of debates over the conclusions and the ability to replicate key findings from several studies, including the genetics of life span [10]–[14], stress tolerance [15]–[17] and pigmentation [18]–[20].
Although the basic influence of genetic background on the expressivity of mutations is well documented, the wider consequences of such influences are poorly understood [21]. In particular, the extent to which wild-type background influences the magnitude and sign of genetic interactions remains unclear. Research to date addressing this question [4], [22], [23], has largely focused on a small set of mutations, and defined genetic backgrounds. Recent work has demonstrated that the magnitude of genetic interactions can be influenced by environmental factors [24], and even ploidy level [25]. Yet the generality of such findings remains uncertain. Thus this remains an essential, but poorly explored area of fundamental genetics, as our understanding of epistasis, and our inferences of the topology of genetic networks are often derived from studies of genetic interactions [26]–[33]. In addition, modifier screens have been extremely important, and have identified large numbers of genes that interact to influence the visible expression of the phenotype of the focal mutation, even when the modifier may not have a visible phenotype by itself [34], [35]. We have previously shown that the phenotypic effects of an allele of the scalloped gene (sdE3) in Drosophila melanogaster is profoundly influenced by wild-type genetic background (Figure 1B), with effects extending to wing disc transcriptional profiles [36]. One gene that was transcriptionally regulated in a background-dependent matter, optomotor blind/bifid (omb/bi), was then examined in a double mutant combination with sdE3. We demonstrated that the phenotypic consequence of the interaction between these mutations was markedly influenced by wild-type genetic background. In one wild-type background the double mutant combination resembled the individual sdE3 phenotype, while in the other wild-type background, the omb mutation behaved as a strong synthetic enhancer of sd [36].
Fig. 1. Genetic background effects influence sdE3, and are used for a dominant modifier screen. 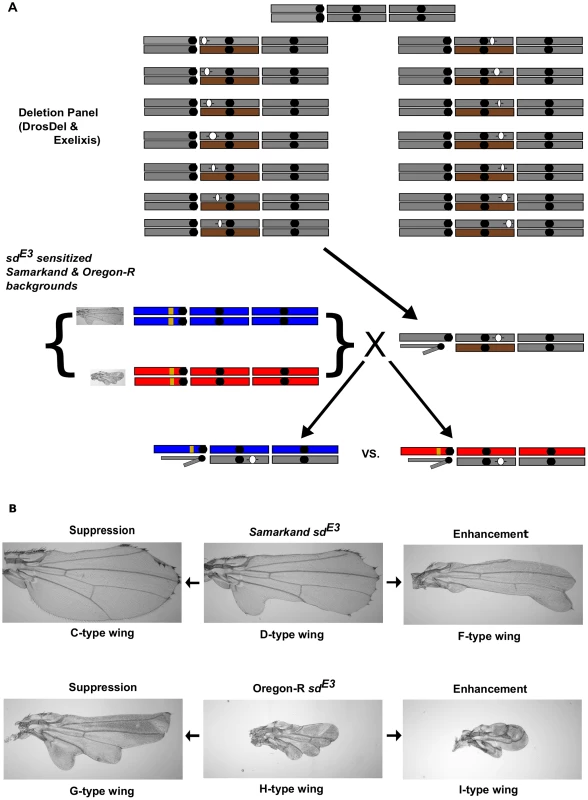
A) Outline of the modifier screen employed in this study (illustrated for 2nd chromosome deletions). Using the DrosDel and Exelixis Deletion collections, male deletion–bearing (denoted with -()- ) flies were crossed to females homozygous for the sdE3 mutation from each wild-type genetic background, Samarkand (blue) and Oregon-R (red). Male offspring that were hemizygous for the sdE3 allele and heterozygous at all other loci, including the deletion, were compared between the two genetic backgrounds. Thus we were scoring male flies hemizygous for sdE3, and heterozygous for the deletion. The co-isogenic progenitor wild-type strains was used for control crosses. Each grey rectangle represents a chromosome (X, 2 & 3 from left to right), with centromeres (black dots), and balancer chromosomes (brown rectangles). Yellow represents the sdE3 mutation and closely linked genomic region on the X chromosome. B) The effect of genetic background on the phenotypic expression of the sdE3 allele, and examples of suppression and enhancement of this allele in each background. Letters beside each image represent the semi-quantitative scores assigned to wings (all figures taken at 40× magnification). Our findings clearly demonstrate the influence of wild-type genetic background on this genetic interaction, but an important challenge is to determine whether such context dependent effects are widespread. To address this question we performed a genome wide-screen for dominant modifiers of sdE3 using two wild-type genetic backgrounds. Our results suggest that the majority (∼74%) of all modifiers are background-dependent. The background-dependence of the modifier alleles are in part due to the wild-type strains differing in overall sensitivity to mutational perturbations. Using a subset of the deletions spanning the range of phenotypic effects of modifiers, we observed that the interaction effects were consistent using an additional allele, sdETX4. Furthermore, we show that the deletion effects are a result of the interaction with mutations at the sd locus, and not a simple consequence of haplo-insufficiency in the genomic region of the deletion. We also demonstrate that the background-dependent interactions of modifiers with sdE3 are linked to the same genomic regions that contribute to the background-dependent effects of the allele itself. We argue that the phenotypic expressivity of mutations can be considered a quantitative trait, and a more comprehensive, context-dependent view of the effects of mutations needs to emerge.
Results
The majority of dominant modifiers of sdE3 are dependent upon wild-type genetic background
Genetic modifier screens are powerful tools to both identify interacting factors that contribute to signaling networks, as well as to infer their topology. This approach has shaped our understanding of the genetic basis of many traits, across numerous organisms. However little is known about how wild-type genetic background influences genetic interactions. We previously demonstrated that the genetic interaction between mutations in two genes, sd and omb, is dependent on genetic background [36]. To determine if such an effect is a general phenomenon we performed an analysis of genome-wide genetic interactions between the sdE3 mutation and deletions generated in otherwise isogenic backgrounds spanning the autosomes of Drosophila.
We first verified that deletions spanning a number of putative candidate genes (Dll, wg, vg) previously demonstrated to interact with sd modify the sdE3 phenotype. In each of these instances the deletions confirmed previous expectations for the interaction (Figure S1B). We then screened the autosomes, with two independent sets of genomic deletions, DrosDel [37] and Exelixis/BSC [38], [39], each generated in an independent isogenic progenitor background (Figure 1B). In total 723 deletion-bearing strains (spanning ∼90% of the autosomal genome) were crossed to sdE3 in each wild-type background. F1 males hemizygous for the sdE3 mutation and heterozygous for the deficiencies were scored.
For the 198 deletion strains that consistently modified the sdE3 wing phenotype, ∼74% of the observed effects were dependent on wild-type (Oregon-R vs. Samarkand) genetic background (Table 1). Frequently, the background contingency was a result of severe effects in one wild-type genetic background, with modest or no effects in the other (Figure 1A and 2, Figure 3A). A complete list of modifier regions, and putative candidate genes can be found in Table S1. An example of the physical location and contribution of these effects is illustrated using the left arm of chromosome 3 (Figure 3, Figure S4), where background-independent and -dependent effects are illustrated, including some deletions with opposing effects in terms of modifying the sdE3 phenotype.
Fig. 2. The majority of autosomal modifiers of sdE3 are background-dependent. 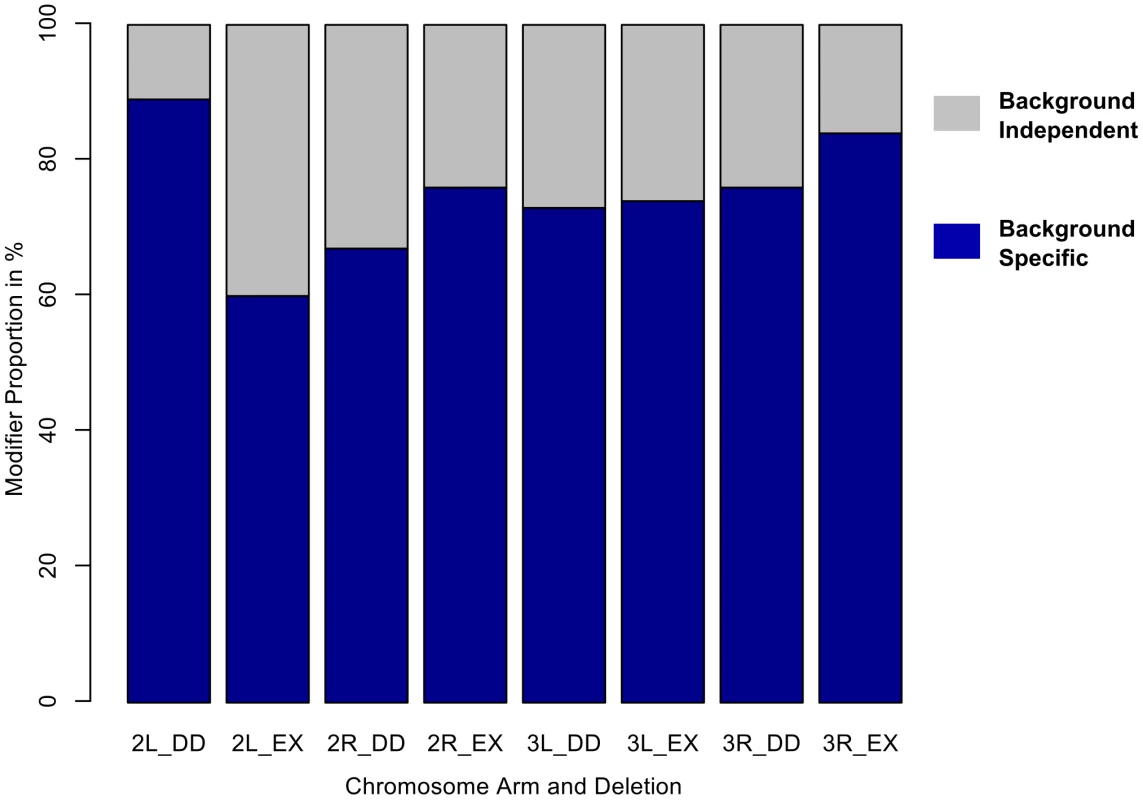
A) Proportion of deletions that modify the sdE3 phenotype in a background-dependent or -independent manner, by chromosome arm and deletion collection. DD = DrosDel collection. EX = Exelixis collection. Numbers at the bottom of each bar indicate whether the effects are in autosomal chromosome two or three, while the letters L and R represent whether the effects are found on the left or right chromosome arms, respectively. Fig. 3. Genomic distribution of background-dependent and independent modifiers of sdE3. 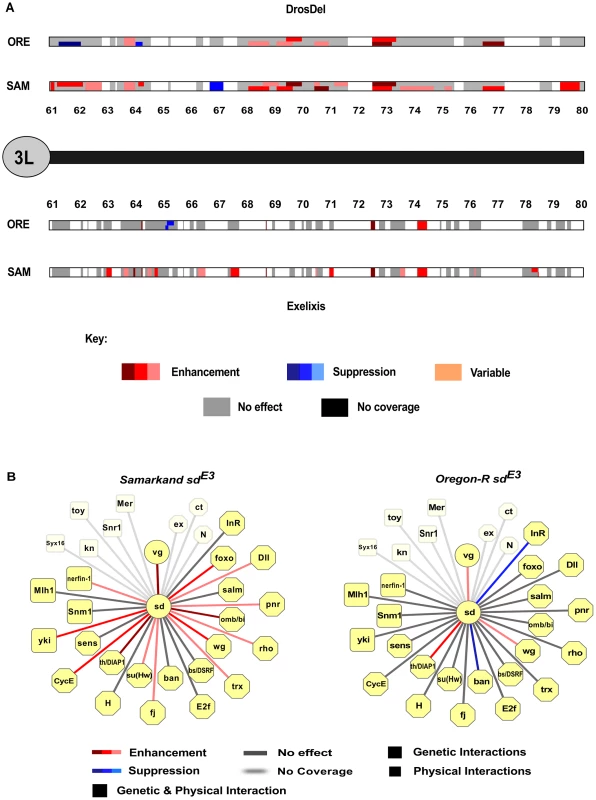
A) Example of the distribution of background-dependent and -independent modifiers of sdE3 on the left arm of chromosome 3 for each deletion collection. The cytological location (61–80) of all deletions on the left arm of chromosome 3 are shown. Regions with no coverage are left blank (white). While there are several locations that show co-enhancement or suppression for Samarkand (SAM) or Oregon-R (ORE), most show an effect in only one background, and occasionally opposite effects (i.e. between 61–62 in DrosDel), consistent with sign epistasis. In a given collection where there were two deletions with overlapping genomic locations (or were nested), the regions in the figure are divided vertically to show the effect of each deletion. The remaining chromosome arms are shown in Figure S4. B) Evidence for background dependent interactions for apriori known interacting loci. For deletions that covered the known interacting factors of sd, we show the background dependent effects [59]. Unlike the finding for the genome as a whole, there appears to be more synthetic enhancers in Samarkand than Oregon-R. Tab. 1. Number of background dependent and independent modifiers recovered by chromosome arm and deletion collection. 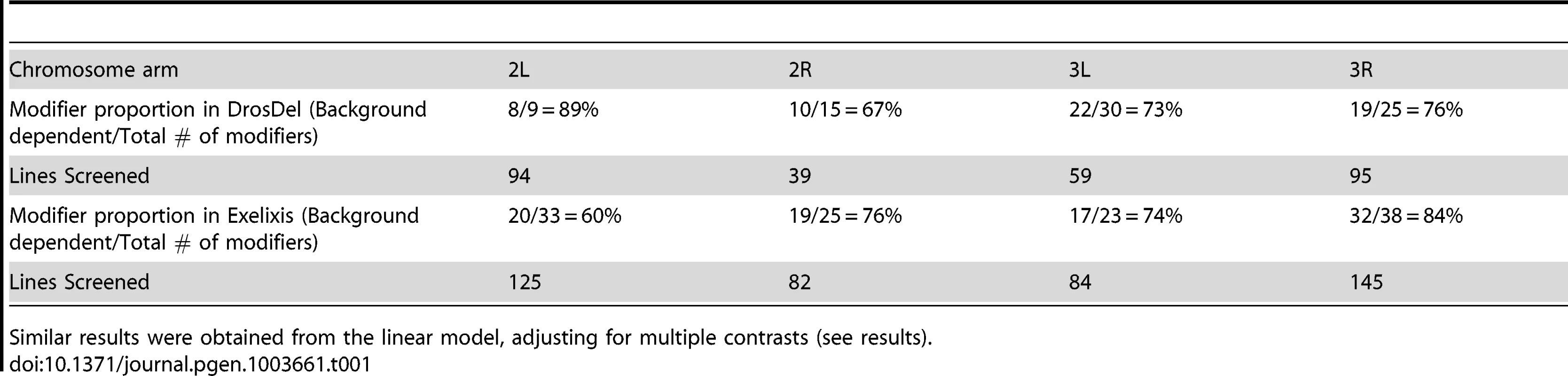
Similar results were obtained from the linear model, adjusting for multiple contrasts (see results). We confirmed these results using a linear model (ANOVA), by asking what proportion of all “significant” modifiers also had a “significant” interaction effect between genetic background and the deletion. Based upon these criteria ∼79% of modifiers demonstrated background dependence. While each cross was carried out independently, there were a large number of crosses performed, and each deletion bearing genotype was compared to a common set of controls from within each block of crosses (see methods). Therefore we utilized several methods that adjust for multiple comparisons. While these methods will decrease the number of deletions deemed modifiers using standard comparisons (i.e. α = 0.05), we are primarily interested in the proportion of such modifiers that are due to background dependent effects. Using False Discovery Rate (FDR) we observed a similar frequency (∼78%) as with unadjusted p-values, while with the sequential Bonferroni (Holm) it was ∼68%. Regardless of the exact approach used, it is clear that the vast majority of modifiers recovered are background dependent.
We performed this screen using two different sets of deletions, each of which varied in the size of the deletion. We observed little association between deletion size and severity of phenotypic modification (Samarkand: correlation-0.09 & -0.08 using Exelixis & DrosDel respectively; Oregon: −0.061 & −0.067 using Exelixis & DrosDel deletions respectively, Figure S5). The lack of association between size of deletion and magnitude of effect suggests that it is unlikely that the observed effects are due to the number of genes perturbed in each deletion.
These key results suggest that at least in sensitization screens, and possibly for many studies of genetic interaction, wild-type genetic background will have profound influences on the range of phenotypes observed and the modifiers that are identified, with only a subset of modifiers being background-independent. Using Flymine and Droid [40], [41] as well as literature mining we examined all of the previously identified genes that act as genetic modifiers, protein-protein interacting partners, or are targets of transcriptional regulation by SD. From these sources we collated evidence for 19 genes that were covered by deletions in this screen (i.e. excluding genes on the X), and all but one (sens) were recovered as genetically interacting with sdE3 (Figure 3B). However, more than 50% of these specific loci demonstrated background-specific interactions with sdE3, including vg, which is known to physically interact with SD to form a heterodimer, and is transcriptionally regulated by this complex. Several well-known genetically or physically interacting genes (such as salm and yki) showed surprisingly mild enhancement of the phenotype, which may be a result of the particular wild-type backgrounds used in this study. These findings suggest that even for well-characterized interacting genes, the influence of genetic background can be substantial, consistent with the flexible nature of genetic interactions. An important caveat to this interpretation is that many of these deletions may contain more than one gene. This could potentially mean that the interaction is due to both the deletion of the focal gene as well as other loci nearby. Yet, as described above, we observed no evidence for a relationship between deletion size and magnitude of effect, suggesting that this may be a minor contributing factor.
Variation in the extent of epistatic effects is in part due to differences among the wild-types in sensitivity to mutational perturbation
To further validate, refine, and extend our analysis we quantified a subset of 44 of the Exelixis deletion lines that spanned the range of modifier phenotypes across both severity and background-dependence. Interestingly (Figure 4), the background-dependent interactions are clearly a result of both specific differences with respect to the nature of sensitizing mutational effects in each background, as well as to the degree of sensitivity to mutational perturbation. Indeed, the sdE3/Y; Deletion/+ combinations in the Oregon-R wild-type background demonstrated considerably more variation between deletion strains, compared to the same genotypes in Samarkand (Figure 4). Despite the fact that the sdE3 mutation in the Oregon-R background had more severe loss of wing tissue (Figure 1, Figure S1), the range of both enhancement and suppression exceed that of the same mutation in the Samarkand background (Figure 4). The between deletion co-efficient of variation (CV) for wing size in the Oregon-R background is approximately double that (0.34) of the Samarkand background (0.15). These results were confirmed using a Levene's test with a non-parametric bootstrap. Despite the differences in both degree and spectrum of sensitivity, there was still a moderate correlation of effects of the sdE3/Y; Deletion/+ combinations (0.66, CI(0.46,0.8)) across the two wild-type backgrounds. These data indicate many of the modifiers are acting in the same direction, although vary for magnitude of effect. Interestingly, even the non-genetic component of phenotypic variation observed for Oregon-R sdE3/Y; +/+ in crosses to the wild-type deletion progenitor shows considerably greater phenotypic variation for wing size compared to Samarkand (Figure 4), although it is unclear if this is related to the changes in within strain variation (robustness).
Fig. 4. Background dependence is partially a consequence of strain specific sensitivity to genetic perturbation. 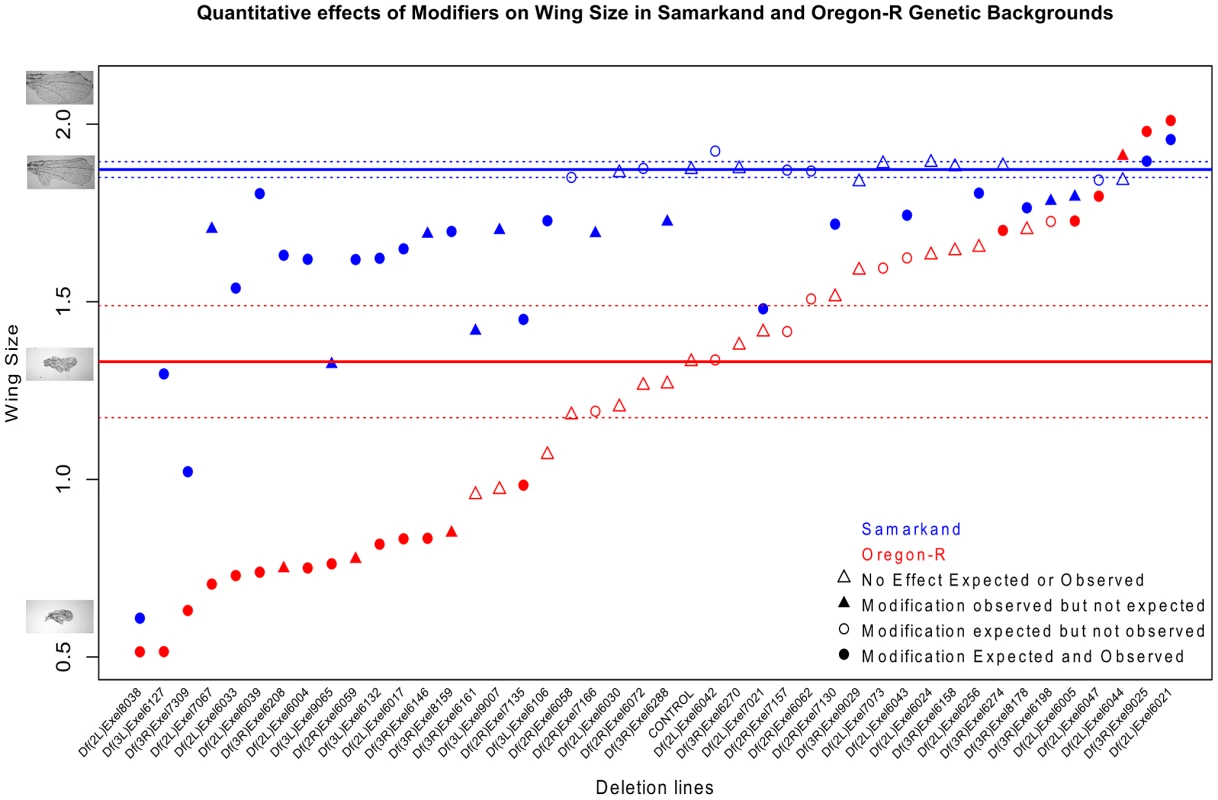
Quantitative effects of a subset of 44 deletions on the modification of the sdE3 phenotype are shown. The deletions are rank-ordered based on wing size in the Oregon-R background. Enhancement and suppression of the sdE3 phenotype is much greater in the Oregon-R background, relative to Samarkand, in both absolute (shown) and relative terms (not shown). Solid and stippled lines (blue and red) represent the mean and 95% confidence interval, respectively, for wing size in the control sd hemizygous males (sdE3/Y). Circles represent deletions with an a priori expectation of modification based on the initial semi-quantitative screen, while triangles represent deletions with no observed effect in that screen. Filled symbols represent a significant observed effect in the quantitative screen. The Y axis shows a measure of wing size using centroid size (see methods). While the semi-quantitative measure of wing size used for the initial screen, and quantitative measure described above are highly correlated (see methods), a few putative modifier regions failed to replicate in the tertiary validation cross with quantitative measures. Similarly a few deletion lines that were expected to not have an effect (based on the initial screen), did have one with the quantitative measure. However these potential false positives and negatives are few, of similar numbers, and thus are not expected to influence the overall conclusions.
The influence of the deletions for modifying sdE3 is not correlated with their effect on wild-type wing size
One possible explanation for these results would be that the deletions influenced wing size, per se, and the results were not a specific consequence of the interaction between sd and the deletion. To investigate this we quantitatively examined females who were heterozygous for the sdE3 mutation and for the deletions (i.e. sdE3/+ ; Deletion/+) across each genetic background. These females have qualitatively “wild-type” wings, and previous work did not observe an effect of sdE3 on wing size in females as heterozygotes [42] (although it did influence wing shape). Therefore we quantified these females across the same set of deletions as described above. If the deletions were not generally acting as modifiers of the “sensitized” sd mutant phenotype in hemizygous males, but as general modulators of size, then we would expect a strong positive correlation between the effects on size in males and females (sdE3/+ ; Deletion/+ vs. sdE3/Y ; Deletion/+). The correlation between Samarkand and Oregon-R sdE3/+ ; Deletion/+ females was ∼0.8, suggesting that the effects of the deletions on overall wing size is similar across backgrounds. However the correlations within each background (i.e. sdE3/+; Deletion/+ vs. sdE3/Y ; Deletion/+) were 0.22, (CI −0.08, 0.49), and 0.21, (CI −0.08, 0.48) respectively, and neither case was significantly different from 0. The lack of a correlation indicates that the influence of the deletions in sdE3 hemizygous males is largely independent of any effects on overall wing size. More importantly the CV for wing size in females (across deletions) for both backgrounds was ∼0.03, which is 5× and 10× less than that observed for sdE3 hemizygotes in Samarkand and Oregon-R respectively (Figure S6). This suggests that most of the phenotypic variation for wing size due to the deletion is observed when the backgrounds are “sensitized” with the sd mutation, while having relatively little influence on wild-type wing size.
Loci influencing background dependent interactions are linked to those influencing phenotypic expressivity of sd
Are the loci influencing the background-specific genetic interactions the same as those that modulate phenotypic expressivity for wing size of the focal sdE3 mutation? To address this question we generated a set of backcross lines between Oregon-R and Samarkand (both fixed for sdE3), where “long” wings were selected in the backcross to the Oregon-R background, and “short” wings in backcrosses to the Samarkand background (Figure S3). Using ∼30 SNPs polymorphic across backgrounds, we verified that these backcross lineages showed expected genotypes for more than 90% of markers (i.e. phenotypically short wings but with Samarkand genotypes). Among the molecular markers that did introgress, include those tightly linked to the unknown causal loci on 2R near cytological band 48 and at the centromere of 3L [36]. If the loci modulating the magnitude of the genetic interactions were caused by genes other than those influencing the background-specific disruption of wing development, we would predict weak correlations between sdE3/Y; Deletion/+ in Oregon-R and the equivalent genotype from the “short” backcross (with an otherwise Samarkand background). Similar logic prevails for the Samarkand and the “long” phenotype. However, even using semi-quantitative measures, it is clear that these are highly correlated; 0.82 (CI 0.66–0.91) and 0.86 (CI 0.73–0.93) respectively. These results are consistent with the loci influencing the background-dependent genetic interactions being the same as those influencing the background-dependent effects on the phenotypic expressivity of the focal sdE3 mutation.
Background dependent interactions are consistent across additional alleles of sd
The results described above demonstrate that the loci that influence the background dependent nature are linked to those influencing phenotypic expressivity of the mutation itself. However, it was unclear if the observations were due to some particular properties of the sdE3 allele, or a more general function of perturbation at the sd locus. To address this, we retested a subset (29) of the deletions spanning the range of phenotypic effects with sdE3, using an additional allele sdETX4, across each genetic background. The phenotypic consequences of sdETX4, while background-dependent, are somewhat weaker than sdE3 (Figure S7A). Despite these phenotypic differences, there was a moderate to high correlation across the modifiers' effects on these two alleles. In the Oregon-R and Samarkand wild-type genetic backgrounds respectively, the correlation between the effects of the deletions on the phenotypes of the sdE3 and sdETX4 allele was 0.66 (CI 0.38–0.82), and 0.76 (CI 0.55–0.88). In addition the general pattern of greater sensitivity to mutational perturbation by modifiers of the sd phenotype appears to be generally maintained (Figure S7B). These results demonstrate that even across multiple alleles, the background dependence of the modifiers is maintained.
vestigial (vg) interacts with sd in a background dependent manner
Although the primary goal of this study was to explore the flexibility in genetic interactions, not to identify candidate genes, for confirmatory purposes, we examined several genomic regions that demonstrated background-dependent or -independent modifiers (Table S2). Interestingly, one region, 49E1, contained vg, which encodes a SD-regulated transcriptional factor that forms a heterodimer with SD. Fine mapping, followed by the use of candidate insertional mutants (co-isogenic to the Exelixis deletions) confirmed that the vgF02736 allele behaved as a background-dependent enhancer with strong enhancement in Samarkand, but very weak enhancement in Oregon-R. We followed this up by introgressing this allele into both the Samarkand and Oregon-R background. Again we observed background-specific enhancement of the sd phenotype. Other fine mapping regions suggest several candidate genes, although for at least one region, no obvious candidate gene could be determined (Table S2).
Discussion
Genetic modifier screens have provided an indispensible tool for identifying interacting sets of genes, providing a glimpse into the underlying genetic network, and a point of entry for further molecular characterization. Much of our knowledge of network topology has depended on the use and interpretation of such genetic interactions [43], and such information is included in many common databases and graphical representations of networks such as in FlyMine and DroID [40], [41] as well as flybase [44]. The importance of modifier screens cannot be over-stated for the identification of interacting genes. Yet the generality of networks defined by these interactions is unclear, given that such screens (and thus the nature of the interactions) are generally performed in isogenic wild-type backgrounds to prevent numerous artefactual findings. In this study, we demonstrate that the majority of such genetic interactions are dependent on wild-type genetic background. Our results suggest that different wild-type strains vary in their general sensitivity to mutational perturbation, as well as having strain specific responses to such modifiers (Figure 3, Figure 4, Figure S4). Both of these factors contribute to both quantitative and qualitative changes in the observed phenotypic effects across the focal sd mutations and the deletions. While the majority of the observed background dependent effects changed the magnitude of the interaction, we did observe several instances of sign epistasis, where the deletion modified the phenotypic expressivity of the sd allele in opposite ways across the different backgrounds. This genotypic conditionality suggests that genetic networks may be quite flexible, with segregating variation in natural populations influencing magnitude and possibly sign of interactions. Indeed, such context dependence in genetic interactions, whether due to genetic background, or other factors needs to be recognized as a likely general phenomenon.
It is probable that the results presented here under-estimate the degree of background dependent genetic interactions. In this study we screened for dominant modifiers of the sd mutations, and only two wild-type strains were used heterozygous against common isogenic tester strains. It is to be expected that double mutant combinations in each homozygous genetic background would demonstrate even more background dependence from the phenotypic expression of recessive alleles, as has been examined for particular pairs of interacting loci in a few model systems [4], [23]. Yet in this relatively simple design, ∼74% of modifiers were background-dependent (Figure 2A, Figure 3A). Even for functionally characterized genes that interact with sd, over 50% demonstrated interactions that were background-dependent (Figure 3B). The results were consistent both across multiple alleles of sd (Figure S7), and across backcross-introgression lines (Figure S3). In addition the results were consistent when we moved from particular deletions to individual mutations. The well-known interacting factor vg demonstrated background-specific interactions from the segmental deletion containing it, to an individual mutation in the gene, with strong enhancement in Samarkand but mild effects in Oregon-R, similar to previous observations between sd and omb [36].
Overall, the observed background-dependence was due to a combination of both sensitivity of the wild-type background to mutational perturbation, as well as specific patterns of interactions between deletions and the sdE3 mutation across backgrounds. Despite the principal effect of sdE3 being more severe in Oregon-R than in Samarkand, both the suppressors and enhancers recovered were also of greater magnitude in the Oregon-R background (Figure 4). The choice of a particular wild-type background for sensitization screens could lead to profoundly different interpretations with respect to the number and nature of modifiers recovered. This is of some concern when it is acknowledged that wild-type strains with the same names may not be genetically identical across different labs due to new mutations, bottlenecks, recombination and contamination. Thus the inferences made from studies of pairwise mutational interactions may be difficult to generalize, and may in part explain why the same allelic combinations can result in different phenotypic outcomes. In this study, it was not just change in magnitude of the genetic interactions, but in some instances the sign (i.e. enhancer vs. suppressor) of the interaction that was contingent on the genetic background. Such findings may explain why attempts to replicate findings of genetic effects (such as GWAS) can be difficult. Despite the obvious complications, the background-dependent nature of these effects has a beneficial aspect; new loci can be identified by performing modifier screens in additional wild-type backgrounds. Indeed with many wild-type strains being sequenced to perform genome wide associations, this may provide an additional tool for rapid identification of new interacting loci. Additionally, the use of RNAi across multiple genetic backgrounds may be able to facilitate such studies [45]. However leveraging such complex genetic interaction data may require a new population level framework to interpret the results.
What is the genetic architecture underlying the background dependent interactions?
There are outstanding questions that our study is unable to address. The background dependent nature of the genetic interactions could be the result of a “third-order” effect between the sd mutation, the hemizygous allele uncovered over the deletion and other loci across each wild-type genetic background. An alternative, and perhaps simpler explanation would be of differential quantitative complementation uncovered by the deletion [46]. In such cases, the variation in the degree of the modification of the focal mutation (sd) is a direct result of the alleles that differ across backgrounds uncovered by the deletion. While we expect that our results are a combination of both explanations, it is likely that without very high resolution mapping of the genomic regions, or test of specific polymorphisms will we be able to determine the relative contribution of each type of interaction. However the previous work that motivated this current study, namely the background dependent interaction between sd and Omb was clearly due to a third order effect [36]. Understanding the degree to which increasingly higher order epistasis contributes to phenotypic variation is under-explored but of great importance [47].
One curious finding of our study was that the background (Oregon-R) that demonstrated the higher degree of phenotypic expressivity of the focal sd mutations, showed increased sensitivity to mutational perturbation (both enhancers and suppressors) as well as greater phenotypic variation within strain. Recent work has demonstrated that loci can influence trait variability (“noise”) directly [48]–[50], including naturally occurring variants in the Hsp90 gene of Drosophila [51]. Indeed even cell-to-cell variation, and variation in penetrance appears to have a complex genetic architecture [48] influenced by variability in gene expression [52]. It is unclear whether the loci that contribute to increased phenotypic “noise” also contribute to the amplified sensitivity to mutational perturbation as seen in the Oregon-R vs. Samarkand wild-type backgrounds. In previous work Oregon-R does have higher levels of phenotypic variation in quantitative measures of wing shape, but no increased sensitivity to weak (heterozygous) mutational perturbation [42]. However the focal mutations used in the current study (sdE3 and sdETX4) represented more severe perturbations to wing development, so this may not provide an adequate comparison. Regardless, this remains an unanswered question, and a potential link between so-called variance controlling genes and sensitivity to perturbation would have important implications for the genetic architecture of canalization and robustness [5], [53].
One constraint of the current study is that we utilized a hypomorph of moderate phenotypic effect, as opposed to a null allele. While a formal definition of functional epistasis (sensu [54]) requires the use of null alleles, most interaction screens utilize alleles of comparable (hypomorphic) effect to allow the recovery of both enhancers and suppressors. Nevertheless, previous work has demonstrated that null alleles can also show background-dependence effects in the primary effect of the mutation, including on development, growth and viability [1], [2], and our results demonstrate that these conditional effects are likely to be reflected in the genetic interactions between mutations as well. In addition we demonstrated that the quantitative effects we observed with the interaction between sdE3 and segmental deletions in each wild-type genetic background were correlated when observed across another (weaker) allele, sdETX4, suggesting that such effects are not due to a particular allele. We also demonstrated that the effects of these interactions are tightly linked to the same genomic regions that contribute to the primary background-dependent phenotypic effects of the mutations. Thus for our system, the genetic variants influencing the phenotypic expressivity of the focal mutation appear to be the same as those modulating both the magnitude, and potentially the sign of genetic interactions between mutations.
While the positive and negative implications for modifier (and other genomic) screens is clear, the potential flexibility of genetic networks given segregating variation in a population needs to also be considered. In particular an allele entering a population (either as a new mutation, or as a result of introgression from another population or species) may not have a “fixed” effect on fitness; instead the genetically contingent effects of the allele result in a distribution of phenotypic effects, including a possible change in sign (i.e. from deleterious to beneficial).
Materials and Methods
Data and scripts associated with this manuscript are located on DRYAD: http://dx.doi.org/10.5061/dryad.4dt7c
Fly stocks
The Oregon-R strain was originally obtained from the Bloomington stock center, while Samarkand was obtained from the lab of Dr. Trudy Mackay. For both strains, we further inbred them to near isogenicity, and tested via a panel of 30 polymorphic markers to confirm there was no contamination or residual heterozygosity. A combination of sequencing and PCR-based genotyping suggests that these two strains have an approximately 2% divergence from one another, and that all sequenced regions examined to date are a subset of variation from natural populations. The X-linked sdE3 mutant allele (obtained from the Drosophila stock center, Bloomington IN), used in this study is caused by a P{w[E] ry[1t7.2] = wE} transposon located in the third intron of the sd gene [55]. This mutant allele was introgressed into two lab wild-type strains, Oregon-R and Samarkand, both marked with white (w), by repeated backcrosses involving homozygous mutant female and the wild type male for over 20 generations [36]. These lines have been subjected to extensive genotyping to verify the extent of the introgression, and to avoid contamination. The sdETX4 and vgF02736 alleles were also obtained from the Bloomington stock center, and were introgressed for 20 generations into each wild-type strain.
Deletion lines (obtained from Bloomington stock center)
We utilized the DrosDel [37] and Exelixis/BSC [38] collections of lines that have defined segmental deletions collectively spanning ∼90% of the autosomes, with an average deletion size of 400 kb and 140 kb respectively. Deletion panels were generated in isogenic backgrounds and include overlapping as well as nested deletions within and between each panel. The progenitor wild-type strains (one for DrosDel & one for Exelixis/BSC) were used in crosses to generate background-specific control flies. While spontaneous loss of the tip of chromosome 2L, containing l(2)gl could potentially confound the results of our screen [56], our tests of a subset of these deletions did not demonstrate non-complementation with l(2)gl. Thus it is unlikely that this is a confounding factor in our analysis.
Dominant modifier screen
Crosses
To assess the influence of wild-type background on genetic interactions we used a dominant modifier screen, and examined sd mutant hemizygotes who were heterozygous for the deletions. Deletion lines (see above), and their isogenic wild-type progenitor strains were crossed to homozygous sdE3 mutant females (Figure 1). Flies were allowed to mate and lay eggs for 3–4 days and then transferred into fresh vials for a backup. All crosses were performed at 24°C 65% RH on a 12∶12 hr light∶dark cycle in a Percival (Model: I41VLC8) incubator. For each deletion, sdE3/Y; Deletion/+ male progeny were scored in each genetic background (Oregon-R and Samarkand) for enhancement or suppression of the sdE3 phenotype (Figure 1A). Thus we scored flies hemizygous for sd, and heterozygous for the deletions. Deletion crosses were performed in large blocks, involving 25 to 100 deletions per block (paired across backgrounds), and for each block a simultaneous set of control crosses with the progenitor wild-type strains for DrosDel and Exelixis flies was also performed. Nevertheless, there was negligible variation in the wing phenotypes of the flies resulting from the control crosses across all the blocks (not shown). However, for appropriate inferences, phenotypic analysis for all crosses within a block were made with respect to specific sets of control crosses from within that same block. We screened between (5–20) flies for each cross (crosses with fewer than 5 progeny were re-tested), with a mean/median of 8.2/7 flies per cross. Any deletion that showed evidence for modification (see below) of sdE3 was re-tested (new crosses) to verify the phenotypic effects. Crosses performed with DrosDel deletions on chromosome arm 3L showed a marked increase in the number of modifiers relative to other arms (22/59 compared to 37/228 for the rest of the chromosome arms for the DrosDel collection). Thus putative modifiers on 3L were re-tested 3 times each, with consistent results, suggesting that these modifiers are unlikely due to a sampling artefact. In total 723 deletions were tested, with 18,167 flies scored.
Scoring technique
For initial assessment of phenotypic modification we performed a semi-quantitative analysis similar to that used by other investigators [57], grouping the progressive loss of wing tissue based on shape and size (proxy for severity of mutation) into 10 categories from A through J (nominal scores of 1–10) such that, category “A” represented a wild type wing phenotype and “J” represented a severely reduced wing phenotype (Figure S1). Pure Samarkand sdE3 individuals were generally category D while Oregon-R sdE3 individuals were category H, with relatively minor variation in these scores. The rationale for such a semi-quantitative approach was two-fold. First, we wished to mirror the genetic screen approaches used in many functional genetic studies (using qualitative or semi-quantitative measures), and second this allowed us to screen a much larger panel of lines. As discussed below, these semi-quantitative measures correlated well with quantitative measures of wing size.
To mimic a traditional genetic screen we assessed interactions based largely on non-overlap distributions of phenotypes, comparing genotypes bearing deletions to their co-isogenic wild-types. While this likely underestimates the number of true interactions of the deletions with sdE3, it was done so that the observed effects were of an almost qualitative nature (as is often done for visual screens). As discussed above, all putative modifiers were verified at least once with an independent replication cross.
In addition, we also utilized a more quantitative approach, fitting the data to the following linear model:
where Y is the semi-quantitative measure of size (1–10), B is the wild-type genetic background (Oregon-R and Samarkand) and D is the deletion (deletion bearing chromosome, or co-isogenic wild-type). We evaluated the results from the linear model. While each cross was performed independently, given that so many crosses were performed, we examined the results (with respect to significant “hits”), with unadjusted p-values, as well as using several methods to control for multiple comparisons (FDR and Holm/Sequential Bonferroni). The analysis was performed using the lm function and p.adjust in R (V 2.12).Quantification of size and shape
To validate the primary findings of this study, we repeated crosses, and quantified wing size for a subset of 44 deletions, spanning the direction and magnitude of effects (background dependent-independent, suppressor-enhancer, as well as negative controls) observed in the genome-wide screen. A single wing from each of 5 male flies (w sdE3/Y; Deletion/+) was dissected and mounted in glycerol, for both backgrounds. For the isogenic wild-type control strain, 30 individuals were used from each background-specific set of crosses to better ascertain the degree of variability. Images of the wings were captured using an Olympus DP30BW camera mounted on an Olympus BW51 microscope. Six landmarks (Figure S2) were digitized using tpsDIG software [58] and centroid size was used as a measure of wing size. The landmarks were specifically chosen as they could be discerned on all wings (Figure S2). To quantitatively verify the background-dependent effects of a given deletion on wing size (Figure 4) the following model was used:
where Y is the Centroid Size, B is the background and D is the deletion. The analysis was performed using the lm function in R (V 2.12) and 95% confidence intervals were constructed using confint. Significance was determined by non-overlapping confidence intervals with controls.The quantitative measure of wing size used for this analysis, correlates well with the semi-quantitative method and results used for the initial screening (r = 0.82, CI:0.69–0.9 in Oregon-R, r = 0.78, CI:0.63–0.87 in Samarkand). This suggests high repeatability of the initial screen, as well as the semi-quantitative measure of wing size.
To ascertain whether there was a commensurate effect of the genomic deletions in “wild-type” wings (as opposed to the mutant phenotype caused by sd mutants), we quantified wing size in females heterozygous for the focal sdE3 mutation with each deletion (w sdE3/w sd+; Deletion/+) digitizing the same 6 landmarks on the wing.
Generation and crossing of “large-wing” and “small-wing” backcross lines of sdE3
Potentially the genomic regions (from the wild-type strains) that influence the genetic interaction between the deletions and sdE3 could be independent of those regions that influence the variation for phenotypic expressivity of the sdE3 mutation itself. To test this we generated lines that had “high expressivity” sdE3 phenotypes in an otherwise “low expressivity” background (Figure S3). A backcross-selection procedure was used to introgress the modifiers that contribute to the “large wing” phenotype from the Samarkand background into the “small wing” background of Oregon-R and vice-versa (Figure S3). Upon generation of these lines, we repeated the dominant modifier screen as described above using a subset of 32 of the 44 confirmed modifiers and negative controls. These lines were used in identical crosses to those outlined above, with sdE3/Y; Deletion/+ individuals examined.
Fine scale mapping
To narrow down several genomic regions to a set of a few candidate genes we utilized an additional set of overlapping deletions in DrosDel, Exelixis and BSC strains followed by use of P-element insertional mutations co-isogenic with the Exelixis panel of lines. We utilized this approach for four genomic regions (49E1, 57B3-B5, 63F2-F7, and 86E13-E16) detailed in Table S2.
Supporting Information
Zdroje
1. DowellRD, RyanO, JansenA, CheungD, AgarwalaS, et al. (2010) Genotype to phenotype: a complex problem. Science 328 : 469.
2. ThreadgillDW, DlugoszAA, HansenLA, TennenbaumT, LichtiU, et al. (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269 : 230–234.
3. NadeauJH (2001) Modifier genes in mice and humans. Nat Rev Genet 2 : 165–174.
4. RemoldSK, LenskiRE (2004) Pervasive joint influence of epistasis and plasticity on mutational effects in Escherichia coli. Nat Genet 36 : 423–426.
5. DworkinI (2005) A Study of Canalization and Developmental Stability in the Sternopleural Bristle System of Drosophila melanogaster. Evolution 59 : 1500–1509.
6. LamMY, NadeauJH (2003) Genetic control of susceptibility to spontaneous testicular germ cell tumors in mice. Apmis 111 : 184–190; discussion 191.
7. SangsterTA, SalathiaN, LeeHN, WatanabeE, SchellenbergK, et al. (2008) HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc Natl Acad Sci U S A 105 : 2969–2974.
8. PolaczykPJ, GasperiniR, GibsonG (1998) Naturally occurring genetic variation affects Drosophila photoreceptor determination. Development Genes and Evolution 207 : 462–470.
9. DworkinI, PalssonA, BirdsallK, GibsonG (2003) Evidence that Egfr contributes to cryptic genetic variation for photoreceptor determination in natural populations of Drosophila melanogaster. Current Biology 13 : 1888–1893.
10. ToivonenJM, WalkerGA, Martinez-DiazP, BjedovI, DriegeY, et al. (2007) No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet 3: e95.
11. ClancyDJ (2008) Variation in mitochondrial genotype has substantial lifespan effects which may be modulated by nuclear background. Aging Cell 7 : 795–804.
12. NerettiN, WangPY, BrodskyAS, NyguyenHH, WhiteKP, et al. (2009) Long-lived Indy induces reduced mitochondrial reactive oxygen species production and oxidative damage. Proc Natl Acad Sci U S A 106 : 2277–2282.
13. ToivonenJM, GemsD, PartridgeL (2009) Longevity of Indy mutant Drosophila not attributable to Indy mutation. Proc Natl Acad Sci U S A 106: E53; author reply E54.
14. RoginaB, HelfandSL (2013) Indy mutations and Drosophila longevity. Front Genet 4 : 47.
15. GreenbergAJ, MoranJR, CoyneJA, WuCI (2003) Ecological adaptation during incipient speciation revealed by precise gene replacement. Science 302 : 1754–1757.
16. CoyneJA, ElwynS (2006) Does the desaturase-2 locus in Drosophila melanogaster cause adaptation and sexual isolation? Evolution 60 : 279–291.
17. GreenbergAJ, MoranJR, WuCI (2006) Proper control of genetic background with precise allele substitution: a comment on Coyne and Elwyn. Evolution 60 : 623–625; discussion 626–627.
18. JeongS, RebeizM, AndolfattoP, WernerT, TrueJ, et al. (2008) The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132 : 783–793.
19. MatuteDR, ButlerIA, CoyneJA (2009) Little effect of the tan locus on pigmentation in female hybrids between Drosophila santomea and D. melanogaster. Cell 139 : 1180–1188.
20. RebeizM, Ramos-WomackM, JeongS, AndolfattoP, WernerT, et al. (2009) Evolution of the tan Locus Contributed to Pigment Loss in Drosophila santomea: A Response to Matute et al. Cell 139 : 1189–1196.
21. ChandlerCH, ChariS, DworkinI (2013) Does your gene need a background check? How genetic background impacts the analysis of mutations, genes, and evolution. Trends Genet 29(6): 358–366.
22. KhanAI, DinhDM, SchneiderD, LenskiRE, CooperTF (2011) Negative Epistasis Between Beneficial Mutations in an Evolving Bacterial Population. Science 332 : 1193–1196.
23. WangY, ArenasCD, StoebelDM, CooperTF (2013) Genetic background affects epistatic interactions between two beneficial mutations. Biol Lett 9(1): 20120328.
24. LalićJ, ElenaSF (2013) Epistasis between mutations is host-dependent for an RNA virus. Biol Lett 9(1): 20120396.
25. GersteinAC (2013) Mutational effects depend on ploidy level: all else is not equal. Biology letters 9(1): 20120614.
26. St JohnstonD (2002) The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet 3 : 176–188.
27. KarimFD, ChangHC, TherrienM, WassarmanDA, LavertyT, et al. (1996) A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics 143 : 315–329.
28. TherrienM, MorrisonDK, WongAM, RubinGM (2000) A genetic screen for modifiers of a kinase suppressor of Ras-dependent rough eye phenotype in Drosophila. Genetics 156 : 1231–1242.
29. SimonMA (1994) Signal transduction during the development of the Drosophila R7 photoreceptor. Dev Biol 166 : 431–442.
30. DicksonBJ, van der StratenA, DominguezM, HafenE (1996) Mutations Modulating Raf signaling in Drosophila eye development. Genetics 142 : 163–171.
31. DagaA, BanerjeeU (1994) Resolving the sevenless pathway using sensitized genetic backgrounds. Cell Mol Biol Res 40 : 245–251.
32. NadeauJH (2003) Genetics. Modifying the message. Science 301 : 927–928.
33. RutherfordSL (2000) From genotype to phenotype: buffering mechanisms and the storage of genetic information. Bioessays 22 : 1095–1105.
34. KeriRA, LozadaKL, Abdul-KarimFW, NadeauJH, NilsonJH (2000) Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. Proc Natl Acad Sci U S A 97 : 383–387.
35. NadeauJH (2003) Modifier genes and protective alleles in humans and mice. Curr Opin Genet Dev 13 : 290–295.
36. DworkinI, KennerlyE, TackD, HutchinsonJ, BrownJ, et al. (2009) Genomic consequences of background effects on scalloped mutant expressivity in the wing of Drosophila melanogaster. Genetics 181 : 1065–1076.
37. RyderE, BlowsF, AshburnerM, Bautista-LlacerR, CoulsonD, et al. (2004) The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167 : 797–813.
38. ThibaultST, SingerMA, MiyazakiWY, MilashB, DompeNA, et al. (2004) A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet 36 : 283–287.
39. ParksAL, CookKR, BelvinM, DompeNA, FawcettR, et al. (2004) Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36 : 288–292.
40. MuraliT, PacificoS, YuJ, GuestS, RobertsGG3rd, et al. (2011) DroID 2011: a comprehensive, integrated resource for protein, transcription factor, RNA and gene interactions for Drosophila. Nucleic Acids Res 39: D736–743.
41. LyneR, SmithR, RutherfordK, WakelingM, VarleyA, et al. (2007) FlyMine: an integrated database for Drosophila and Anopheles genomics. Genome Biol 8: R129.
42. DworkinI, GibsonG (2006) Epidermal growth factor receptor and transforming growth factor-beta signaling contributes to variation for wing shape in Drosophila melanogaster. Genetics 173 : 1417–1431.
43. HartwellL (2004) Genetics. Robust interactions. Science 303 : 774–775.
44. TweedieS, AshburnerM, FallsK, LeylandP, McQuiltonP, et al. (2009) FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res 37: D555–559.
45. KitzmannP, SchwirzJ, Schmitt-EngelC, BucherG (2013) RNAi phenotypes are influenced by the genetic background of the injected strain. BMC Genomics 14 : 5.
46. MackayTFC (2004) Complementing complexity. Nat Genet 36 : 1145–1147.
47. WhitlockMC, BourguetD (2000) Factors affecting the genetic load in Drosophila: synergistic epistasis and correlations among fitness components. Evolution 54 : 1654–1660.
48. AnselJ, BottinH, Rodriguez-BeltranC, DamonC, NagarajanM, et al. (2008) Cell-to-cell stochastic variation in gene expression is a complex genetic trait. PLoS Genet 4: e1000049.
49. HallMC, DworkinI, UngererMC, PuruggananM (2007) Genetics of microenvironmental canalization in Arabidopsis thaliana. Proc Natl Acad Sci U S A 104 : 13717–13722.
50. ShenX, PetterssonM, RonnegardL, CarlborgO (2012) Inheritance beyond plain heritability: variance-controlling genes in Arabidopsis thaliana. PLoS Genet 8: e1002839.
51. SgroCM, WegenerB, HoffmannAA (2010) A naturally occurring variant of Hsp90 that is associated with decanalization. P R Soc B 277 : 2049–2057.
52. RajA, RifkinSA, AndersenE, van OudenaardenA (2010) Variability in gene expression underlies incomplete penetrance. Nature 463 : 913–918.
53. LevyS, SiegalM (2008) Network Hubs Buffer Environmental Variation in Saccharomyces cerevisiae. PLoS Biol 6: e264.
54. Hawley RS, Walker MY (2003) Advanced Genetic Analysis: Finding meaning in a genome. Oxford: Blackwell Publishing.
55. InamdarM, VijayRaghavanK, RodriguesV (1993) The Drosophila homolog of the human transcription factor TEF-1, scalloped, is essential for normal taste behavior. Journal of Neurogenetics 9 : 123–139.
56. RoegiersF, KavalerJ, TolwinskiN, ChouYT, DuanH, et al. (2009) Frequent unanticipated alleles of lethal giant larvae in Drosophila second chromosome stocks. Genetics 182 : 407–410.
57. TanakaE (1960) A study on the difference of temperature responses in several vestigial strains of Drosophila melanogaster. Japanese Journal of Genetics 35 : 222–227.
58. RohlfFJ (2003) tpsDig. 1.39 ed: Department of Ecology and Evolution. SUNY Stony Brook
59. SmootME, OnoK, RuscheinskiJ, WangPL, IdekerT (2011) Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27 : 431–432.
Štítky
Genetika Reprodukčná medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy





