-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Convergence of Light and ABA Signaling on the Promoter
Light is one of the most important environmental cues regulating multiple aspects of plant growth and development, and abscisic acid (ABA) is a plant hormone that plays important roles during many phases of the plant life cycle and in plants' responses to various environmental stresses. How plants integrate the external light signal with endogenous ABA pathway for better adaptation and survival remains poorly understood. Here, we show that BBX21 (also known as SALT TOLERANCE HOMOLOG 2), a B-box (BBX) protein previously shown to positively regulate seedling photomorphogenesis, is also involved in ABA signaling. Our genetic data show that BBX21 may act upstream of several ABA INSENSITIVE (ABI) genes and ELONGATED HYPOCOTYL 5 (HY5) in ABA control of seed germination. Previous studies showed that HY5 acts as a direct activator of ABI5 expression, and that BBX21 interacts with HY5. We further demonstrate that BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter. In addition, ABI5 was shown to directly activate its own expression, whereas BBX21 negatively regulates this activity by directly interacting with ABI5. Together, our study indicates that BBX21 coordinates with HY5 and ABI5 on the ABI5 promoter and that these transcriptional regulators work in concert to integrate light and ABA signaling in Arabidopsis thaliana.
Published in the journal: Convergence of Light and ABA Signaling on the Promoter. PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004197
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004197Summary
Light is one of the most important environmental cues regulating multiple aspects of plant growth and development, and abscisic acid (ABA) is a plant hormone that plays important roles during many phases of the plant life cycle and in plants' responses to various environmental stresses. How plants integrate the external light signal with endogenous ABA pathway for better adaptation and survival remains poorly understood. Here, we show that BBX21 (also known as SALT TOLERANCE HOMOLOG 2), a B-box (BBX) protein previously shown to positively regulate seedling photomorphogenesis, is also involved in ABA signaling. Our genetic data show that BBX21 may act upstream of several ABA INSENSITIVE (ABI) genes and ELONGATED HYPOCOTYL 5 (HY5) in ABA control of seed germination. Previous studies showed that HY5 acts as a direct activator of ABI5 expression, and that BBX21 interacts with HY5. We further demonstrate that BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter. In addition, ABI5 was shown to directly activate its own expression, whereas BBX21 negatively regulates this activity by directly interacting with ABI5. Together, our study indicates that BBX21 coordinates with HY5 and ABI5 on the ABI5 promoter and that these transcriptional regulators work in concert to integrate light and ABA signaling in Arabidopsis thaliana.
Introduction
Light is of utmost importance to sessile plants, not only as the primary energy source for photosynthesis, but also as an environmental signal regulating their growth and development throughout their life cycle. Light signals are perceived by several families of photoreceptors in plants, including phytochromes [primarily absorb red (R) and far-red (FR)], cryptochromes and phototropins [absorb blue (B) and ultraviolet-A (UV-A)], and a recently characterized ultraviolet-B (UV-B) photoreceptor UV RESISTANCE LOCUS 8 (UVR8) [1]. Upon light irradiation, multiple photo-activated photoreceptors (except for UVR8) repress the activity of CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), a central repressor of photomorphogenesis induced by visible-light [2]. COP1 is a conserved RING finger E3 ubiquitin ligase targeting several photomorphogenesis-promoting proteins for degradation, including ELONGATED HYPOCOTYL 5 (HY5) [3] and HY5 HOMOLOG (HYH) [4]. HY5, a constitutively-nuclear bZIP protein, is the first known and most extensively studied transcription factor involved in promoting photomorphogenesis under a wide spectrum of wavelengths, including FR, R, B, and UV-B [3], [5], [6], [7]. It was shown that the abundance of HY5 protein is directly correlated with the extent of photomorphogenic development [3]. Recent chromatin immunoprecipitation (ChIP)-chip studies revealed that HY5 binds directly to a large number of genomic sites, mainly at the promoter regions of annotated genes [8]–[9].
Several recent studies demonstrated that B-box (BBX) family proteins act as important transcriptional regulators in response to light and circadian cues. The BBX family represents a subgroup of zinc finger proteins that contain one or more N-terminal zinc binding BBX domains [10]. In Arabidopsis thaliana, the BBX family consists of 32 proteins, and can be divided into five subfamilies according to its protein sequence [10]. CONSTANS (CO), a key player in the regulation of flowering by photoperiod, was the first identified member of BBX family in Arabidopsis [10]–[11]. CO and its five other similar proteins belong to Subfamily I, whose members all contain two BBX domains in the N terminus and a CCT domain in the C terminus [10]. However, for the eight members in Subfamily IV (BBX18 to BBX25), each BBX protein contains two BBX domains in the N terminus but lack the CCT domain [10], [12]. Interestingly, in this subfamily, BBX21/SALT TOLERANCE HOMOLOG 2 (STH2) and BBX22/STH3/LIGHT-REGULATED ZINC FINGER PROTEIN 1 (LZF1) function as positive regulators of photomorphogenesis, whereas BBX18/DBB1a, BBX19/DBB1b, BBX24/SALT TOLERANCE (STO) and BBX25/STH1 act as negative regulators of photomorphogenesis [13]–[22]. Notably, BBX21, BBX22, BBX24 and BBX25 were all shown to physically interact with HY5, and the B-box domains of these BBX proteins and the bZIP domain of HY5 mediate their interactions [13]–[17], [20]. In addition, it was shown that HY5 promotes the expression of BBX22 by directly binding to its promoter [13], whereas BBX24 and BBX25 repress BBX22 expression by interfering with HY5 transcriptional activity [17].
The plant hormone abscisic acid (ABA) regulates several aspects of plant growth and development, plays an essential role in plants' adaptive responses to environmental stress, and is a key regulator of stomatal aperture [23]–[24]. Genetic studies in Arabidopsis have identified several factors that participate in ABA signal transduction, including ABA INSENSITIVE 1 (ABI1) to ABI5. ABI1 and ABI2, group A type protein phosphatases 2C (PP2Cs), negatively regulate SNF1-related protein kinase 2 (SnRK2) proteins which phosphorylate downstream targets such as various AREB/ABFs [25]–[26]. ABI3, ABI4, and ABI5 belong to three distinct classes of transcription factors, i.e. the basic B3, AP2/ERF, and the basic leucine zipper (bZIP) families, respectively, and share overlapping functions in ABA signaling during seed germination and early seedling development [27]–[29]. It was reported that ABI5 acts downstream of ABI3 and is involved in postgerminational developmental arrest and repression of germination [30]–[32]. Interestingly, a recent study showed that HY5 directly binds to the promoter of ABI5 and is required for the expression of ABI5 and ABI5-targeted genes [33]. In addition, HY5 binding to the ABI5 promoter is enhanced by ABA treatment, and overexpression of ABI5 restores ABA sensitivity in hy5 mutants and leads to enhanced light responses in the wild type plants [33]. Thus, HY5 regulation of ABI5 expression represents an integration of light and ABA signaling during seed germination and early seedling development.
Accumulating evidence indicates that BBX proteins work in concert with HY5 in regulating photomorphogenesis [14]–[17], [20]. Since HY5 directly activates ABI5 expression, it is interesting to investigate whether BBX proteins are also involved in ABA signaling. In this paper, we report that bbx21 mutants showed hypersensitive phenotypes to ABA and NaCl treatments, and our genetic data suggest that BBX21 may act upstream of several ABI genes and HY5. Furthermore, we show that BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter. In addition, ABI5 binds to its own promoter and activate its expression, whereas BBX21 negatively regulates this activity. Taken together, our data reveal a complicated but delicate control of crosstalk between light and ABA signaling in Arabidopsis.
Results
BBX21 is involved in ABA signaling
To investigate whether BBX21 is involved in ABA signaling, we examined the responses of bbx21-1 mutants (Col background) [15] to ABA treatment. We observed that bbx21-1 mutants were significantly more sensitive to 1 µM ABA treatment than was the wild type (Figure 1A, 1B). In addition, bbx21-1 mutants showed more sensitivity to 100 mM NaCl than did the wild type (Figure 1A, 1B). These observations suggest that BBX21 may be involved in ABA responses.
Fig. 1. The bbx21 mutants are hypersensitive to ABA and NaCl during germination. 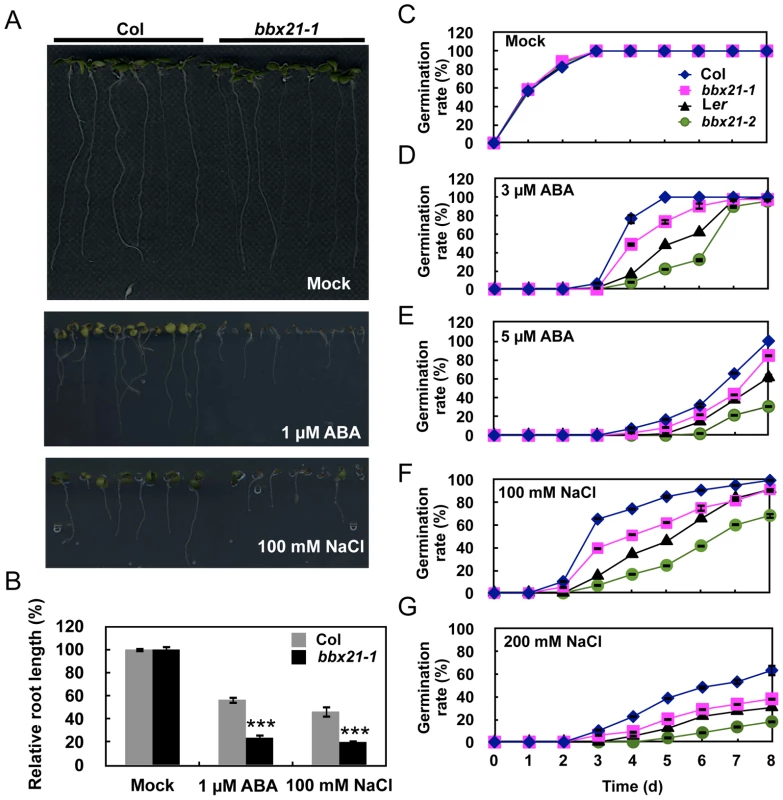
(A) ABA and NaCl hypersensitivity phenotypes of bbx21-1 mutants. All seedlings were grown vertically on germination medium (GM) plates with or without 1 µM ABA or 100 mM NaCl for 7 d after stratification. (B) Root lengths of the wild type (Col) and bbx21-1 grown on GM with or without 1 µM ABA or 100 mM NaCl. Relative root lengths compared with those of Col grown on GM plates are indicated. Values are means ± SD (n = 20). ***P<0.001 (Student's t test) for the differences between bbx21-1 and the wild type. (C–G) Germination rates of the bbx21-1 (Col) and bbx21-2 (Ler) mutants and their corresponding wild type controls under mock (C) and various concentrations of ABA (D and E) or NaCl (F and G) treatments. Germination rate was determined from three replicates (>150 seeds from each genotype), and error bars represent SD. To examine whether the shorter roots observed in bbx21 mutants resulted from delayed germination or the inhibition of primary root growth, we compared the germination rate of wild type and bbx21 mutant seeds grown under various ABA concentrations. As shown in Figure 1C–1E, the germination rate of both bbx21-1 and bbx21-2 (Ler background) [15] mutants was lower than that of the wild type at all tested ABA concentrations (i.e. 1, 3 and 5 µM ABA). Consistently, the germination rate of both bbx21 mutants was also lower than that of the wild type plants under treatments of 100 and 200 mM NaCl (Figure 1F, 1G). However, no difference in root length was observed between the wild type and bbx21 mutants after they were transferred to media containing ABA or NaCl (data not shown), indicating that the short root length of bbx21 mutants shown in Figure 1A and 1B appeared to be predominantly due to delayed germination rather than inhibition of root growth.
Since ABA plays an important role in dehydration tolerance through stomatal closure [23]–[24], we also examined whether BBX21 is involved in ABA-mediated dehydration tolerance. As shown in Figure 2A and 2B, water loss assays showed that the leaves of both bbx21 mutants lost water more slowly than did the wild type plants after detachment, indicating that BBX21 is involved in dehydration tolerance in adult plants. Because water loss primarily depends on stomatal regulation, we then compared the stomatal apertures of 3-w-old plants of bbx21 mutants and their respective wild type. The epidermal peels from rosette leaves at the same developmental stage were observed with a microscope. As shown in Figure 2C, whereas no differences were observed in stomatal apertures between wild type and bbx21 mutant plants under mock treatment, wild type plants displayed wider stomatal apertures than did the bbx21 mutants under the treatment of 0.5 µM ABA. These observations were consistent with the statistical analysis shown in Figure 2D, in which the ratio of stomatal length to width, indicating the degree of stomatal closure, was calculated for each plant. Taken together, our data showed that mutation in BBX21 leads to increased sensitivity to ABA.
Fig. 2. The bbx21 mutants lost water more slowly than did the wild type plants. 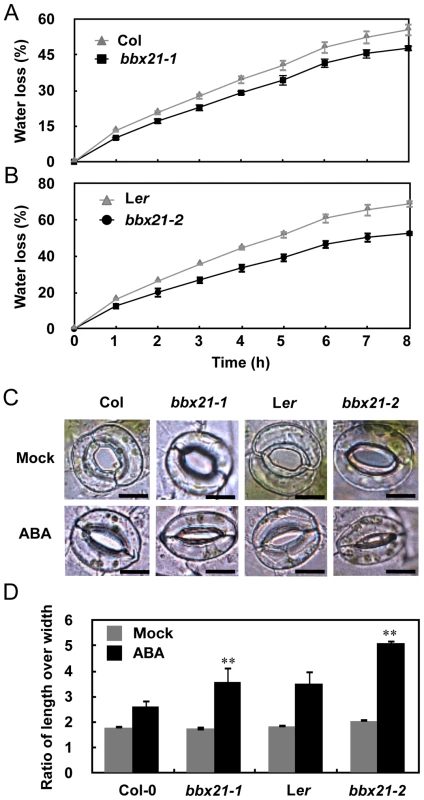
(A–B) Water loss from the detached leaves of the wild type, bbx21-1, and bbx21-2. Results are means of three replicates, and error bars represent SD. (C) Representative images of stomata of wild type and bbx21 mutant plants treated with mock or 0.5 µM ABA. Bar = 10 µm. (D) Ratios of stomatal aperture length to width. Three independent experiments were performed with similar results. Data were from one experiment with 30 stomata cells from leaves of three different plants with triple replicates. Data are means ±SEs. **P<0.01 (Student's t test) for the differences between bbx21 and the wild type. To rule out the possibility that BBX21 may be involved in ABA biosynthesis instead of ABA signaling, we measured the ABA levels in dry seeds and 2-d-old seedlings of wild type and bbx21 mutants using liquid chromatography–mass spectrometry (LC-MS). Our data show that there is no significant difference in ABA levels between wild type and the bbx21 mutants in these two developmental stages, and as expected, ABA levels were dramatically reduced in 2-d-old seedlings relative to dry seeds in both wild type and the bbx21 mutants (Figure S1). We further examined the expression pattern of BBX21 during seed germination. BBX21 is expressed in dry seeds of the wild type, but its expression is repressed in imbibed seeds (Figure 3A). Interestingly, after the seeds were germinated, BBX21 expression was again induced, and reached its peak 2 days after germination (Figure 3A). In addition, BBX21 expression was not regulated by endogenous ABA levels, because in two aba1 alleles, which are impaired in ABA biosynthesis, the expression level of BBX21 was indistinguishable from those of the wild type (Figure 3B). Taken together, our data indicate that BBX21 is involved in ABA signaling in Arabidopsis.
Fig. 3. Expression pattern of BBX21. 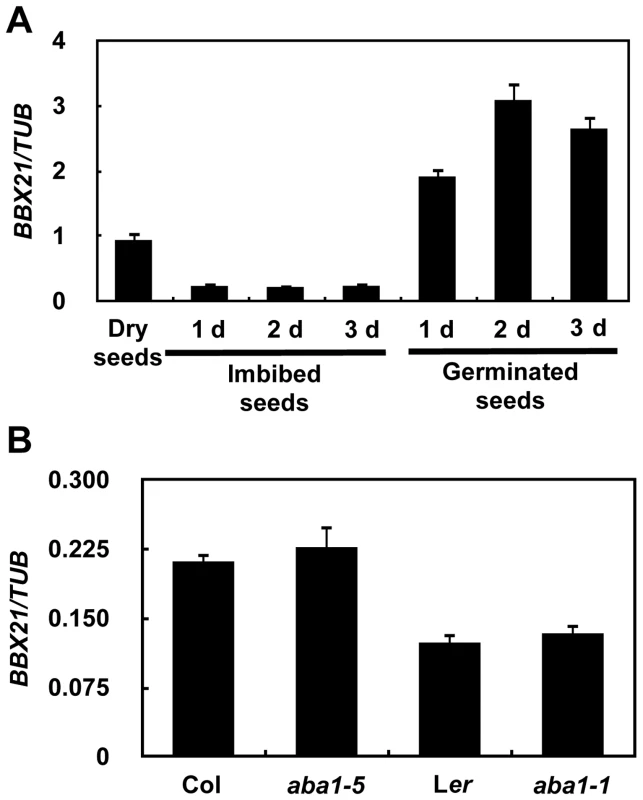
(A) Expression pattern of BBX21 during germination. (B) The expression levels of BBX21 in the wild type and aba1 mutants. Data are means of three independent experiments, and error bars represent SD. BBX21 may act upstream of ABI2, ABI3, ABI4, ABI5 and HY5
To determine the possible roles of BBX21 in ABA signal transduction, we generated double mutants of bbx21 with several ABA insensitive mutants, including abi2-1 [34], abi3-1 [35], abi4-101 [36] and abi5-1 [27]. The abi3-1, abi4-101 and abi5-1 mutants are null alleles of ABI3, ABI4 and ABI5, respectively, whereas abi2-1 contains a dominant mutation (G168D) in the PP2C domain of ABI2. We analyzed the germination rates of the respective homozygous double mutants treated with 5 µM ABA. Surprisingly, we observed that all of the double mutants displayed ABA insensitivity similar as that observed in the respective abi single mutants (Figure 4). These observations suggest that BBX21 may act upstream of ABI2, ABI3, ABI4 and ABI5 in the ABA signaling pathway.
Fig. 4. Germination rates of bbx21, abi and abi bbx21 mutants. 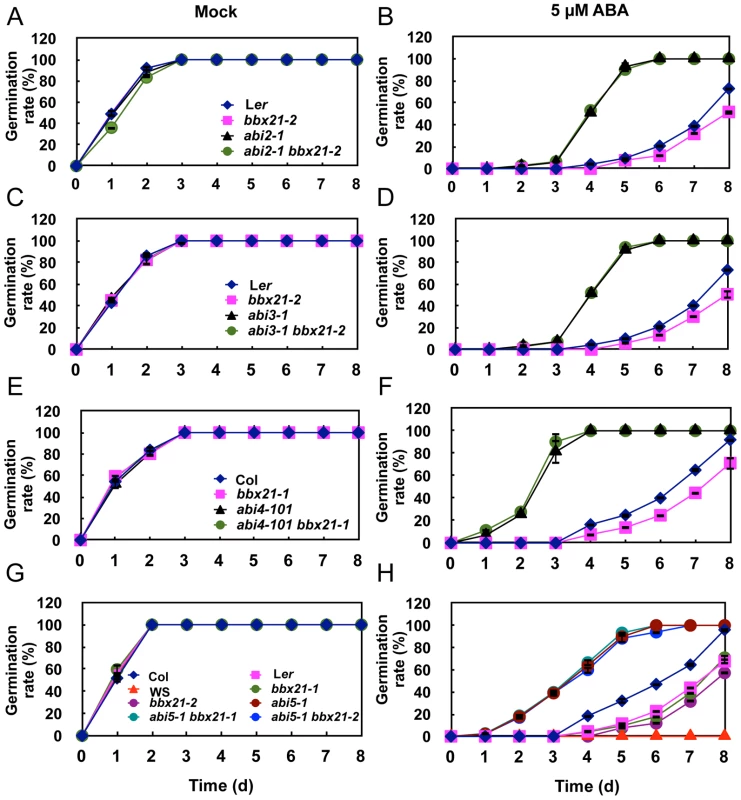
Germination rates of the bbx21, abi2, abi3, abi4, abi5 and abi bbx21 mutants under mock (A, C, E and G) and 5 µM of ABA (B, D, F and H) treatments. Germination rate was determined from three replicates (>150 seeds from each genotype), and error bars represent SD. It was reported that HY5 also mediates ABA responses in seed germination [33]. Therefore, we sought to investigate the genetic relationship between HY5 and BBX21 in ABA control of seed germination. To this end, we first confirmed that all three hy5 alleles, hy5-215 (Col-0), hy5-ks50 (WS) and hy5-1 (Ler), displayed reduced ABA sensitivity in seed germination (Figure S2). Then, we examined the germination rates of hy5-215 bbx21-1 double mutants treated with 5 µM ABA. Our data showed that hy5-215 bbx21-1 exhibited ABA insensitivity during germination, almost indistinguishable from that of hy5-215 (Figure 5). These data indicate that hy5 is epistatic to bbx21, and that the ABA insensitivity of bbx21 during seed germination is dependent on a functional HY5 protein.
Fig. 5. The hy5 bbx21 mutants are insensitive to ABA during germination. 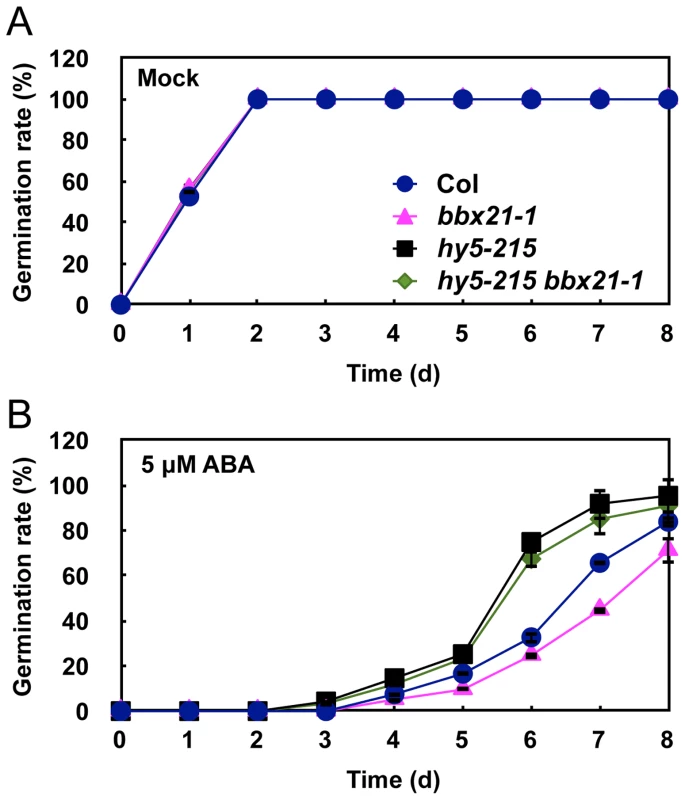
Germination rates of Col, bbx21-1, hy5-215 and hy5-215 bbx21-1 mutants under mock (A) and 5 µM ABA (B) treatments. Error bars represent SD. BBX21 regulates the expression of ABI3 and ABI5
Since BBX21 acts as a transcriptional regulator, we were interested to investigate whether BBX21 could be involved in regulating the expression of ABA signaling intermediates, such as several ABI genes. Therefore, we examined the expression of ABI1 to ABI5 in 2-d-old wild type and bbx21 mutant plants treated with mock or 0.5 µM ABA. Our real time quantitative RT-PCR (qRT-PCR) assays showed that BBX21 negatively regulates the expression of ABI3 and ABI5 under both mock and ABA treatments, but is not involved in regulation of other ABI genes (Figure S3). Interestingly, this regulation mainly occurred in Col background, whereas in Ler background, it was less significant or even absent (Figure S3).
It was previously shown that HY5 directly binds to the promoter of ABI5 and activate its gene expression [33]. To investigate whether HY5 is also involved in regulating ABI3 expression, and to better understand how BBX21 works in concert with HY5 in regulating ABI5, we examined the expression of ABI3 and ABI5 in hy5, bbx21, and hy5/bbx21 mutants treated with mock or ABA. Our qRT-PCR results show that HY5 may not be involved in regulating ABI3 expression (Figure 6A). However, BBX21 is dependent on functional HY5 to negatively regulate the expression of ABI3 and ABI5 (Figure 6), consistent with our genetic evidence that hy5 is epistatic to bbx21 (Figure 5).
Fig. 6. BBX21 regulates the expression of ABI3 and ABI5. 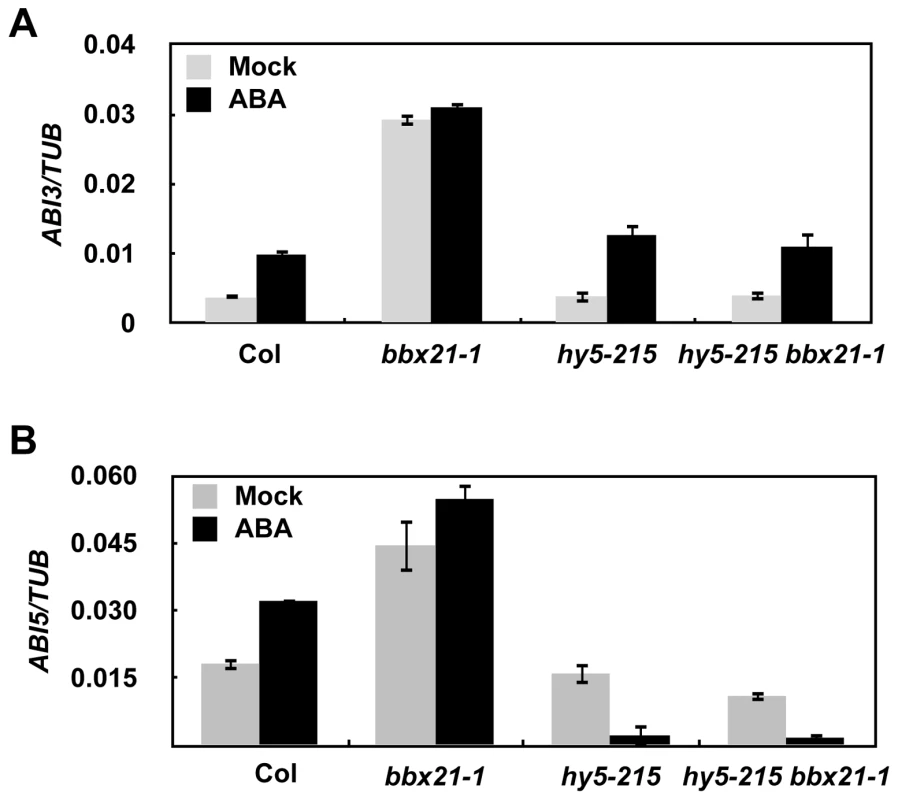
Expression levels of ABI3 (A) and ABI5 (B) in 2-d-old germinating seeds of Col, hy5-215, bbx21-1, and hy5-215 bbx21-1 mutant treated with mock or 0.5 µM ABA. BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter
To unravel the molecular mechanisms by which BBX21 negatively regulates ABI5 expression, we performed transient transfection assays in Arabidopsis protoplasts using a reporter in which the luciferase reporter gene (LUC) was under the control of a 2-kb promoter fragment of ABI5. The ABI5p:LUC reporter was transfected into Arabidopsis mesophyll protoplasts along with 35S:RnLUC (an internal control of transformation efficiency) and the effectors (35S:BBX21, 35S:HY5, or both) (Figure 7A). As shown in Figure 7B, HY5 alone robustly activate the ABI5 promoter, consistent with a previous report that HY5 is a direct activator of ABI5 [33]. BBX21 alone was unable to either activate or repress the ABI5 promoter; however, co-expression of BBX21 with HY5 obviously decreased the ABI5 promoter activity (Figure 7B), implying that BBX21 represses the activation of the ABI5 promoter by interfering with HY5 action.
Fig. 7. BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter. 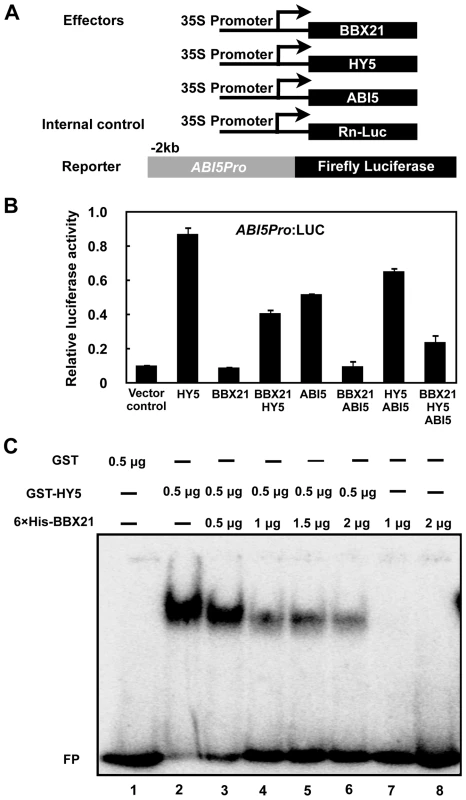
(A) Schematic representation of constructs used in the transient transfection assays in Arabidopsis protoplasts. Arrows after the 35S promoter indicate the transcription start site, and −2 kb indicate the length of the ABI5 promoter that was fused to the firefly luciferase to create the reporter construct. (B) Activation of ABI5p:LUC by indicated combinations of proteins. Error bars represent SE (n = 3). (C) EMSAs showing that increasing amounts of His-BBX21 protein (lanes 2 to 6) decreased the binding of GST-HY5 to the ABI5 promoter. To further investigate how BBX21 negatively regulates ABI5 expression, we expressed GST (glutathione S-transferase), GST-HY5, and 6×His-tagged BBX21 proteins in Escherichia coli, and performed electrophoretic mobility shift assays (EMSAs) to test whether BBX21 could affect HY5 binding to the ABI5 promoter. As shown in Figure 7C, GST-HY5, but not GST alone, bound to the promoter fragment of ABI5. The 6×His-BBX21 protein was unable to bind to the ABI5 promoter. However, increasing amounts of 6×His-BBX21 obviously decreased the GST-HY5 binding to the ABI5 promoter (Figure 7C), indicating that the physical interaction between BBX21 and HY5 prevents HY5 from binding to the ABI5 promoter. Taken together, our data demonstrated that BBX21 negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter.
ABI5 directly activates its own expression, whereas BBX21 negatively regulates this activity by directly interacting with ABI5
We performed yeast one-hybrid assays to test whether other transcription factors may bind to the 2-kb promoter fragment of ABI5. Unexpectedly, it was shown that ABI5 itself binds directly to its promoter (Figure 8A and 8B), suggesting that ABI5 may play a role in regulating its own expression. To further confirm this conclusion, we divided the ABI5 promoter into four overlapping fragments, designated A, B, C, and D, and generated yeast one-hybrid reporter constructs allowing the respective fragments to drive LacZ reporter gene expression (Figure 8A). Interestingly, ABI5 binds only to the C fragment of the ABI5 promoter (Figure 8B), indicating that the C fragment contains the ABI5 binding sites.
Fig. 8. ABI5 directly binds to its own promoter. 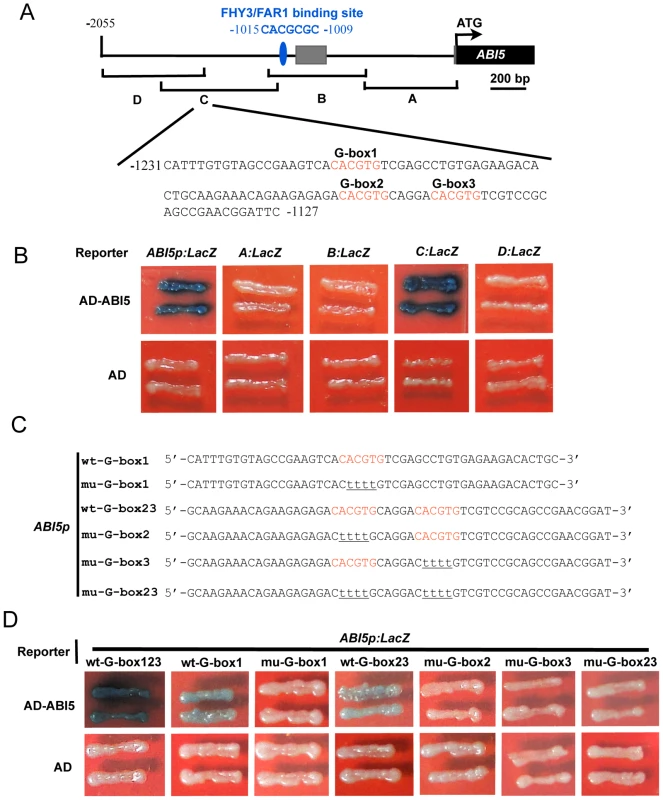
(A) Diagram of the promoter fragments of the ABI5 promoter and the sequence of a subfragment containing three G-box motifs in the C fragment. The adenine residue of the translational start codon (ATG) was assigned position +1, and the numbers flanking the sequences of the subfragment were counted based on this number. A, B, C, and D indicate the corresponding promoter fragments used in yeast one-hybrid assays shown in (B). (B) Yeast one hybrid assays showing that ABI5 binds to the C fragments of its own promoter. Empty vector expressing AD domain alone was used as negative controls. (C) Diagram of the wild type (wt) and mutant (mu) ABI5 subfragments used to drive LacZ reporter gene expression in yeast one-hybrid assays. Wild type G-box elements are shown in red, and nucleotide substitutions in the mutant fragments are underlined. (D) Yeast one-hybrid assays showing that all three G-box motifs mediate ABI5 binding to its promoter. The subfragment of the ABI5 promoter was mutated to abolish G-box1, 2, 3 alone, or both G-box2 and 3, and used to drive LacZ reporter gene expression. In these assays, the respective CACGTG was mutated to CttttG to facilitate mutagenesis reactions. Because ABI5 binds to the class of G-box (CACGTG) motifs known as ABA response elements (ABREs) [37], we then analyzed the distribution of G-box motifs in the C fragment of the ABI5 promoter. Our analysis showed that there are three typical G-box motifs present in the C fragment, which are located ∼200 to 300 bp upstream of the transcription start site and are very close to each other (Figure 8A). To delineate the exact G-box motifs in the ABI5 promoter that are bound by ABI5, we generated several reporter constructs to allow the subfragment 1 (which contains only G-box 1) or subfragment 2 (which contains both G-box 2 and 3) to drive LacZ reporter gene expression, respectively, in yeast cells (Figure 8C). Our results show that for subfragment 1, ABI5 binds to the wild type, but not the mutant (in which CACGTG was mutated to CttttG) fragment, whereas for subfragment 2, mutations of G-box 2 or 3 alone or together all abolished ABI5 binding (Figure 8D). Together, our data demonstrate that all three G-box motifs located in the C fragment mediate ABI5 binding to the ABI5 promoter.
To further explore the biological significance of ABI5 binding to its own promoter, we employed again the transient transfection system using Arabidopsis protoplasts. As shown in Figure 7B, ABI5 alone also activates its own promoter, although not as robustly as HY5. Interestingly, when BBX21 was co-expressed with ABI5, BBX21 was also able to decrease ABI5-activated ABI5 expression (Figure 7B). In addition, when BBX21, ABI5 and HY5 were expressed together, the reporter gene expression was much lower than when ABI5 and HY5 were co-expressed (Figure 7B). These data indicate that BBX21 negatively regulates both HY5 - and ABI5-activated ABI5 expression.
It seems likely that BBX21 may act through a common mechanism (i.e. sequestration of transcription factors) to regulate the transcriptional activities of both HY5 and ABI5. To confirm this hypothesis, we performed yeast two-hybrid and in vitro pull-down assays to investigate whether there is a direct physical interaction between BBX21 and ABI5. Results from these experiments showed that BBX21 indeed interacts directly with ABI5 (Figure 9A, 9B and 9C). Interestingly, individual substitutions of three conserved Asp residues in the B-box domain of BBX21 (i.e. D20A, D75A and D84A), which all abolished the direct interaction between BBX21 and HY5 due to presumable disruption of the structure of the B-box domain [15], did not affect the physical interaction between BBX21 and ABI5 (Figure 9A and 9B). These results suggest that the B-box domain may not mediate the interaction between BBX21 and ABI5. To further investigate which region of BBX21 may be responsible for its interaction with ABI5, we generated the yeast two-hybrid constructs in which the N - (the B-box domain) or C-terminal domain of BBX21 was fused, respectively, with GAL4 activation domain (GAL4-AD). As shown in Figure 9B, our results show that either domain was not able to interact with ABI5 in yeast cells (the interaction of BBX21 and HY5 was included as the positive control), implying that the entire protein of BBX21 is required for interacting with ABI5. Taken together, our data indicate that HY5 and ABI5 are both direct activators of ABI5 expression, whereas BBX21 acts as a negative regulator, possibly by interacting with both transcription factors and interfering with their binding to the ABI5 promoter (Figure 10).
Fig. 9. BBX21 physically interacts with ABI5. 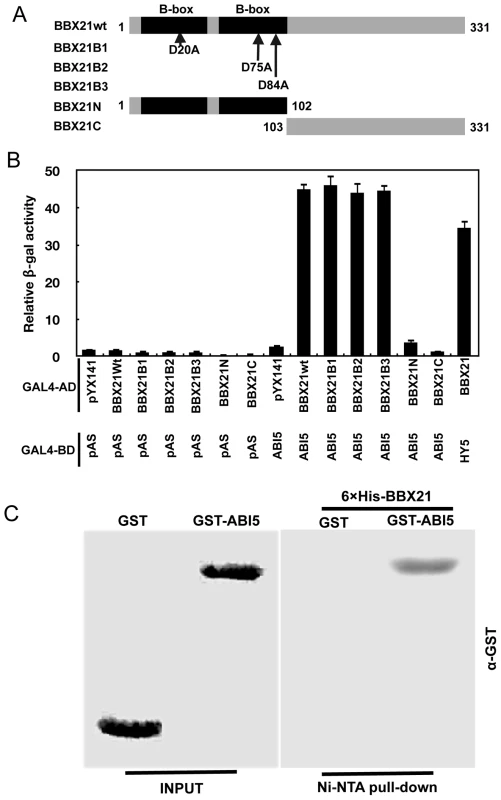
(A) Schematic representation of the domain structures of BBX21 showing mutations in the B-box domain. (B) Yeast two-hybrid interactions between BBX21 and ABI5 proteins. Error bars indicate SE (n = 3). (C) In vitro pull down of ABI5 with BBX21. The GST-ABI5 proteins pulled down with 6×His-BBX21 were detected by anti-GST antibody. Input, 5% of the purified GST-tagged target proteins used in pull-down assays. Fig. 10. A working model depicting how BBX21 works in concert with HY5 and ABI5 on the ABI5 promoter to integrate light and ABA signaling. 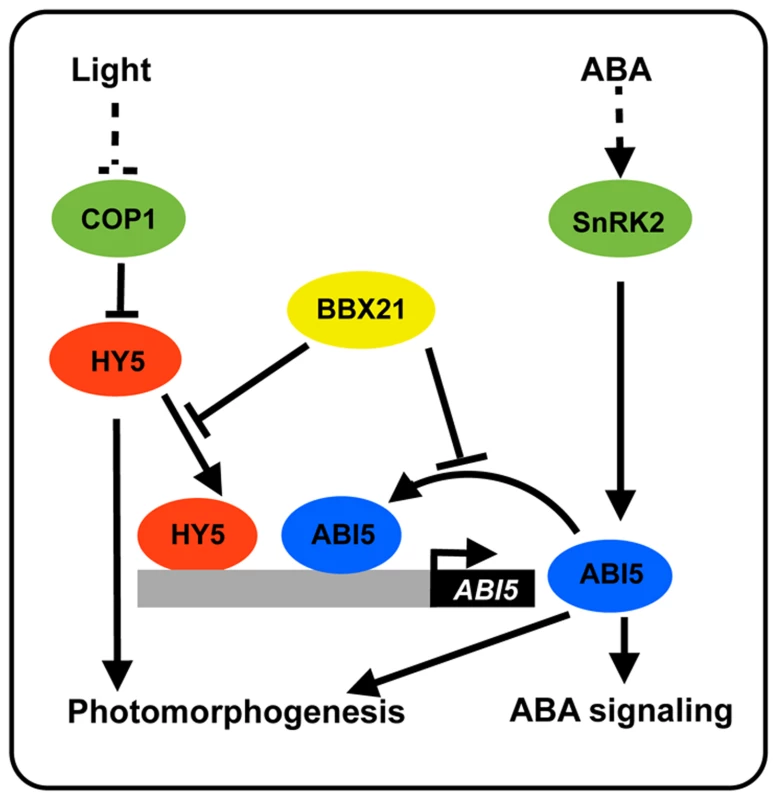
HY5 acts as a transcriptional activator of ABI5 expression [33]. ABI5 directly binds to its own promoter through three typical G-box motifs, and activates the expression of itself. BBX21 acts as a negative regulator of ABI5 expression, possibly by interfering with the binding of HY5 and ABI5 to the ABI5 promoter. ABI5 may play a positive role in photomorphogenesis [33]. Discussion
Light and ABA are the external environmental and the endogenous hormonal cues, respectively, that both play important roles in the control of seed germination and seedling development. The ability of plants to integrate external signals with internal regulatory pathways is vital for their survival [38]–[39]. However, the molecular mechanisms underlying the cross-talk between light and ABA are just starting to be understood. It was reported that HY5, a well-studied transcription factor involved in promoting seedling photomorphogenesis, mediates ABA responses in seed germination, early seedling growth, and root development in Arabidopsis by directly binding to the ABI5 promoter and activating its expression [33]. A recent study reported that FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and FAR-RED IMPAIRED RESPONSE1 (FAR1), two key transcription factors in the phytochrome A pathway, are positive regulators of ABA signaling by directly activating ABI5 expression [40]. In addition, it was shown that ABI5 is also a direct target of PIF1 (also known as PIL5), a phytochrome-interacting bHLH transcription factor [41]. Therefore, it is evident that multiple transcription factors in light signaling pathway may regulate ABA responses by regulating ABI5 expression. In this study, we show that BBX21, another transcriptional regulator implicated in regulating seedling photomorphogenesis, negatively regulates ABI5 expression by interfering with HY5 binding to the ABI5 promoter. Interestingly, ABI5 was also shown to directly activate its own expression, whereas BBX21 negatively regulates this activation by directly interacting with ABI5. Therefore, BBX21 acts as a transcriptional repressor of ABI5, possibly by modulating the binding activities of both HY5 and ABI5 to the ABI5 promoter (Figure 10).
On the other hand, several components of ABA signaling pathway have been shown to be implicated in photomorphogenesis. For example, overexpression of ABI5 in wild type background led to shorter hypocotyls of the transgenic seedlings in FR, R and B light conditions [33]. Consistent with this observation, disruption of RPN10 and KEG, which stabilize the ABI5 protein, both caused decreased hypocotyl growth of the mutants [42]–[43]. Another study proposed that seeds in shallow soil could sense their light environment through phytochrome B, so as to down-regulate ABI3 expression and promote hypocotyl growth [44]. In darkness, the hypocotyl length was slightly longer in abi3 mutants but shorter in ABI3 overexpression plants [44]. Therefore, both ABI3 and ABI5 may play positive roles in photomorphogenesis, and ABA seems to inhibit hypocotyl elongation of seedlings, which may confer better adaptation of seedlings to environmental stresses.
It is interesting that several families of transcription factors directly bind to the ABI5 promoter and regulate its expression. FHY3/FAR1 bind to the FHY3/FAR1-binding site (FBS; CACGCGC) [40], whereas ABI5 binds to the G-box motifs which are ∼130 bp upstream of FBS in the ABI5 promoter (Figure 8). In addition, HY5 presumably binds to a typical G-box motif in the ABI5 promoter [33] which is ∼500 bp upstream of the ABI5-binding sites. Although both HY5 and ABI5 belong to the bZIP transcription factor family and preferentially bind to G-box motif, they apparently bind to different G-box motifs in the ABI5 promoter, suggesting that additional sequences around the core G-box motif may be important for selective binding of these bZIP transcription factors. The binding site of PIF1 (which also binds to G-box motif) in the ABI5 promoter remains to be determined. Moreover, ABI4, an AP2/ERF family transcription factor, was shown to bind to CE1-like element (CACCG) in the 5′-untranslated region of ABI5 [45]. Therefore, at least FHY3/FAR1, ABI5, HY5 and ABI4 directly bind to the ABI5 promoter, and they seem to occupy different regions of the ABI5 promoter. Interestingly, based on our data and those reported in previous studies, all of these transcription factors function as activators of ABI5. How their activities are coordinated and whether they could regulate each other on the ABI5 promoter remain to be elucidated. Since BBX21 is the only negative regulator identified so far on the ABI5 promoter, it remains unknown whether BBX21 could also regulate the binding activities of other transcription factors, such as FHY3/FAR1 and ABI4. In addition, it was shown that ABI3, a B3-domain containing transcription factor, functions as essential regulator upstream of ABI5 [31]. ABI5 expression is greatly reduced in the abi3 mutants, and 35S:ABI5 could rescue ABA insensitivity of abi3 [31]. Whether ABI3 is also a direct regulator of ABI5 awaits further investigation. Together, the ABI5 promoter may represent a converging point on which the transcriptional regulators of light and ABA signaling pathways integrate the signals of both pathways by fine-tuning ABI5 expression.
In addition, it was documented that light could affect ABA metabolism in germinating seeds, and that PIF1 represses seed germination by regulating the expression of ABA biosynthetic and catabolic genes [46]–[48]. Moreover, PIF1 interacts with ABI3 to activate the expression of SOMNUS (SOM) in imbibed seeds [49], suggesting that light and ABA signals also converge on other gene promoters. Together, it is evident that light and ABA integrate at multiple layers to fine-tune seed germination and seedling development, which may ensure the young seedlings to better adapt to their environmental stresses and dynamic light conditions.
Materials and Methods
Plant materials and growth conditions
The bbx21-1 [15], hy5-215 [6], aba1-5 [50] and abi4-101 [36] mutants are of the Columbia (Col) ecotype, and the bbx21-2 [15], aba1-1 [51], abi2-1 [34] and abi3-1 [35] mutants are of the Landsberg (Ler) ecotype, and the abi5-1 [27] mutant is of the Wassilewskija (Ws) ecotype and have been described previously. Seeds were surface-sterilized with 30% commercial Clorox bleach and 0.02% Triton X-100 for 15 min and washed three times with sterile water, then plated on GM medium supplemented with 0.8% Bacto-agar (Difco) and 1% sucrose. The plates were kept at 4°C for 3 days for stratification and then transferred to light chambers maintained at 22°C.
Analyses of ABA responses and quantification of endogenous ABA levels
For root growth measurement shown in Figures 1A and 1B, all seedlings were grown vertically on GM plates with or without 1 µM ABA (Sigma-Aldrich) or 100 mM NaCl for 7 d after stratification under long-day conditions (16 h light/8 h dark).
For the germination assay, at least 150 seeds for each genotype were sterilized and plated on MS medium supplemented with or without various concentration of ABA or NaCl. Germination was defined as the first sign of radicle tip emergence and scored daily until the 7th day of the incubation, and the germination results were calculated based on at least three independent experiments.
Stomatal closing assays were conducted as previously described [52]. Rosette leaves were floated in KCl-Tris solution (50 mM KCl, 10 mM Mes-Tris, pH 6.15), and exposed to light (150 µmol m−2 s−1) for 3 h. Subsequently, rosette leaves were incubated in KCl-Tris solution with or without 0.5 µM ABA assay under the light condition for an additional 3 h.
Water loss was measured in ∼1 g detached rosette leaves from 3-week-old wild type and mutant plants grown in long-day condition. The leaves were weighed immediately after detaching, kept on the laboratory bench and weighted at the indicated times. Water loss shows the percentage of weight loss at the indicated time versus initial fresh weight. To minimize variation, three independent experiments were performed, and similar results were obtained.
Endogenous ABA was quantified in germinated seeds by liquid chromatography coupled to electrospray tandem mass spectrometry as described previously [53].
Measurement of hypocotyl length
To measure the hypocotyl length of seedlings, seeds were cold-treated at 4°C for 3 days and then transferred to continuous white light for 8 h to induce uniform germination. Then, the seeds were transferred to monochromatic light conditions (B, R, and FR light) and incubated at 22°C for 4 d. The hypocotyl length of seedlings was measured using ImageJ software.
Real-Time qRT-PCR
Total RNA was extracted from Arabidopsis seedlings using the RNeasy plant mini kit (Qiagen). Then, cDNAs were synthesized from 2 µg total RNA using SuperScript II first-strand cDNA synthesis system (Invitrogen) according to the manufacturer's instructions. Quantitative PCR was performed using the CFX96 real-time PCR detection system (Bio-Rad) and SYBR Green PCR Master Mix (Applied Biosystems). PCR reactions were performed in triplicate for each sample, and the expression levels were normalized to that of a ubiquitin gene. All primers used for this assay are listed in Supplemental Table S1.
EMSA
EMSAs were performed using biotin-labeled probes and the Light Shift Chemiluminescent EMSA kit (Pierce) as described previously [54]. Briefly, 0.5 µg of GST or GST fusion proteins were incubated together with biotin-labeled probes in 20 µl reaction mixtures containing 10 mM Tris-HCl, 150 mM KCl, 1 mM DTT, 50 ng/ml poly (dI-dC), 2.5% glycerol, 0.05% Nonidet P-40, 100 mM ZnCl2, and 0.5 µg/ml BSA for 20 min at room temperature and separated on 6% native polyacrylamide gels in Tris-glycine buffer. The labeled probes were detected according to the instructions provided with the EMSA kit. The sequences of the complementary oligonucleotides used to generate the biotin-labeled probes are shown in Supplemental Table S1.
Protoplast assay
Arabidopsis mesophyll cell protoplasts were prepared and transfected as described previously [55]. The promoter-reporter used was the 2-kb ABI5 promoter driving firefly luciferase (pPCV814-ABI5p-LUC). The full-length HY5, BBX21, and ABI5 driven by the cauliflower mosaic virus 35S promoter (pRLT2-HY5, pRTL2-BBX21 and pRTL2-ABI5) were used as the effectors. The dual luciferase kit (Promega) was used for detection of reporter activity. Renilla luciferase driven by a full-length cauliflower mosaic virus 35S promoter (pRNL) was used as an internal control.
Yeast assays
Yeast assays were performed as previously described [54]. Transformants were grown on proper drop-out plates containing X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) for blue color development. Yeast transformation and liquid assay were conducted as described in the Yeast Protocols Handbook (Clontech).
In vitro pull-down assay
For in vitro pull-down assays, 2 µg purified 6×His-BBX21 proteins were bound to Ni-NTA magnetic beads (Qiagen) for 2 hours at 4°C, followed by overnight incubation at 4°C with 2 µg purified GST or GST-ABI5 proteins, then washed by washing buffer (10 mM Tris pH 8.0, 100 mM NaCl, and 0.1% Triton X-100, and 5 mM imidazol). Pulled-down proteins mixed with Ni-NTA magnetic beads was spun down and boiled for 10 minutes prior to being loaded to 12% SDS polyacrylamide gels and were detected by Western blot using anti-GST antibody (Sigma-Aldrich).
Supporting Information
Zdroje
1. RizziniL, FavoryJJ, CloixC, FaggionatoD, O'HaraA, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332 : 103–106.
2. YiC, DengXW (2005) COP1-from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol 15 : 618–625.
3. OsterlundMT, HardtkeCS, WeiN, DengXW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405 : 462–466.
4. HolmM, MaLG, QuLJ, DengXW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16 : 1247–1259.
5. KoornneefM, RolffE, SpruitCJP (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.). Heynh Z Pflanzenphysiol 100 : 147–160.
6. OyamaT, ShimuraY, OkadaK (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11 : 2983–2995.
7. UlmR, BaumannA, OraveczA, MateZ, AdamE, et al. (2004) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci U S A 101 : 1397–1402.
8. LeeJ, HeK, StolcV, LeeH, FigueroaP, et al. (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19 : 731–749.
9. ZhangH, HeH, WangX, WangX, YangX, et al. (2011) Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J 65 : 346–358.
10. KhannaR, KronmillerB, MaszleDR, CouplandG, HolmM, et al. (2009) The Arabidopsis B-box zinc finger family. Plant Cell 21 : 3416–3420.
11. PutterillJ, RobsonF, LeeK, SimonR, CouplandG (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80 : 847–857.
12. SarmientoF (2013) The BBX subfamily IV: Additional cogs and sprockets to fine-tune light-dependent development. Plant Signal Behav 8: e23831.
13. ChangCS, MaloofJN, WuSH (2011) COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol 156 : 228–239.
14. ChangCS, LiYH, ChenLT, ChenWC, HsiehWP, et al. (2008) LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J 54 : 205–219.
15. DattaS, HettiarachchiC, JohanssonH, HolmM (2007) SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 19 : 3242–3255.
16. DattaS, JohanssonH, HettiarachchiC, IrigoyenML, DesaiM, et al. (2008) LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 20 : 2324–2338.
17. GangappaSN, CroccoCD, JohanssonH, DattaS, HettiarachchiC, et al. (2013) The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell 25 : 1243–1257.
18. HolmM, HardtkeCS, GaudetR, DengXW (2001) Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 20 : 118–127.
19. IndorfM, CorderoJ, NeuhausG, Rodriguez-FrancoM (2007) Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J 51 : 563–574.
20. JiangL, WangY, LiQF, BjornLO, HeJX, et al. (2012) Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res 22 : 1046–1057.
21. KumagaiT, ItoS, NakamichiN, NiwaY, MurakamiM, et al. (2008) The common function of a novel subfamily of B-Box zinc finger proteins with reference to circadian-associated events in Arabidopsis thaliana. Biosci Biotechnol Biochem 72 : 1539–1549.
22. YanH, MarquardtK, IndorfM, JuttD, KircherS, et al. (2011) Nuclear localization and interaction with COP1 are required for STO/BBX24 function during photomorphogenesis. Plant Physiol 156 : 1772–1782.
23. CutlerSR, RodriguezPL, FinkelsteinRR, AbramsSR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61 : 651–679.
24. NakashimaK, Yamaguchi-ShinozakiK (2013) ABA signaling in stress-response and seed development. Plant Cell Rep 32 : 959–970.
25. KobayashiY, MurataM, MinamiH, YamamotoS, KagayaY, et al. (2005) Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J 44 : 939–949.
26. UmezawaT, SugiyamaN, MizoguchiM, HayashiS, MyougaF, et al. (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci U S A 106 : 17588–17593.
27. FinkelsteinRR, LynchTJ (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12 : 599–609.
28. FinkelsteinRR, WangML, LynchTJ, RaoS, GoodmanHM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10 : 1043–1054.
29. ParcyF, ValonC, RaynalM, Gaubier-ComellaP, DelsenyM, et al. (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6 : 1567–1582.
30. Lopez-MolinaL, MongrandS, ChuaNH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci U S A 98 : 4782–4787.
31. Lopez-MolinaL, MongrandS, McLachlinDT, ChaitBT, ChuaNH (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32 : 317–328.
32. PiskurewiczU, JikumaruY, KinoshitaN, NambaraE, KamiyaY, et al. (2008) The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20 : 2729–2745.
33. ChenH, ZhangJ, NeffMM, HongSW, ZhangH, et al. (2008) Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci U S A 105 : 4495–4500.
34. LeungJ, MerlotS, GiraudatJ (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9 : 759–771.
35. ParcyF, ValonC, KoharaA, MiseraS, GiraudatJ (1997) The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 9 : 1265–1277.
36. LabyRJ, KincaidMS, KimD, GibsonSI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response.. Plant J 23 : 587–596.
37. ReevesWM, LynchTJ, MobinR, FinkelsteinRR (2011) Direct targets of the transcription factors ABA-Insensitive(ABI)4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol 75 : 347–363.
38. AchardP, ChengH, De GrauweL, DecatJ, SchouttetenH, et al. (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311 : 91–94.
39. CasalJJ, FankhauserC, CouplandG, BlazquezMA (2004) Signalling for developmental plasticity. Trends Plant Sci 9 : 309–314.
40. TangW, JiQ, HuangY, JiangZ, BaoM, et al. (2013) FAR-RED ELONGATED HYPOCOTYL3 and FAR-RED IMPAIRED RESPONSE1 transcription factors integrate light and abscisic acid signaling in Arabidopsis. Plant Physiol 163 : 857–866.
41. OhE, KangH, YamaguchiS, ParkJ, LeeD, et al. (2009) Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21 : 403–419.
42. SmalleJ, KurepaJ, YangP, EmborgTJ, BabiychukE, et al. (2003) The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. Plant Cell 15 : 965–980.
43. StoneSL, WilliamsLA, FarmerLM, VierstraRD, CallisJ (2006) KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 18 : 3415–3428.
44. MazzellaMA, AranaMV, StaneloniRJ, PerelmanS, Rodriguez BatillerMJ, et al. (2005) Phytochrome control of the Arabidopsis transcriptome anticipates seedling exposure to light. Plant Cell 17 : 2507–2516.
45. BossiF, CordobaE, DupréP, MendozaMS, RománCS, et al. (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59 : 359–374.
46. OhE, KimJ, ParkE, KimJI, KangC, et al. (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16 : 3045–3058.
47. OhE, YamaguchiS, HuJ, YusukeJ, JungB, et al. (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19 : 1192–1208.
48. SeoM, HanadaA, KuwaharaA, EndoA, OkamotoM, et al. (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48 : 354–366.
49. ParkJ, LeeN, KimW, LimS, ChoiG (2011) ABI3 and PIL5 collaboratively activate the expression of SOMNUS by directly binding to its promoter in imbibed Arabidopsis seeds. Plant Cell 23 : 1404–1415.
50. Leon-KloosterzielKM, GilMA, RuijsGJ, JacobsenSE, OlszewskiNE, et al. (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10 : 655–661.
51. KoornneefJM, Brinkhorst-Van der SwanDLC, KarssenCM (1982) The isolation of abscisic acid (ABA)-deficient mutants by selection of induced revertants in non-germinating gibberellin-sensitive lines of Arabidopsis thaliana. Theor Appl Genet 61 : 385–393.
52. RenX, ChenZ, LiuY, ZhangH, ZhangM, et al. (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J 63 : 417–429.
53. PanX, WeltiR, WangX (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5 : 986–992.
54. LiJ, LiG, GaoS, MartinezC, HeG, et al. (2010) Plant Cell 22 : 3634–3649.
55. YooSD, ChoYH, SheenJ (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2 : 1565–1572.
Štítky
Genetika Reprodukčná medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



