-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
There is epidemiological evidence that patients with certain Central Nervous System (CNS) disorders have a lower than expected probability of developing some types of Cancer. We tested here the hypothesis that this inverse comorbidity is driven by molecular processes common to CNS disorders and Cancers, and that are deregulated in opposite directions. We conducted transcriptomic meta-analyses of three CNS disorders (Alzheimer's disease, Parkinson's disease and Schizophrenia) and three Cancer types (Lung, Prostate, Colorectal) previously described with inverse comorbidities. A significant overlap was observed between the genes upregulated in CNS disorders and downregulated in Cancers, as well as between the genes downregulated in CNS disorders and upregulated in Cancers. We also observed expression deregulations in opposite directions at the level of pathways. Our analysis points to specific genes and pathways, the upregulation of which could increase the incidence of CNS disorders and simultaneously lower the risk of developing Cancer, while the downregulation of another set of genes and pathways could contribute to a decrease in the incidence of CNS disorders while increasing the Cancer risk. These results reinforce the previously proposed involvement of the PIN1 gene, Wnt and P53 pathways, and reveal potential new candidates, in particular related with protein degradation processes.
Published in the journal: Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses. PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004173
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004173Summary
There is epidemiological evidence that patients with certain Central Nervous System (CNS) disorders have a lower than expected probability of developing some types of Cancer. We tested here the hypothesis that this inverse comorbidity is driven by molecular processes common to CNS disorders and Cancers, and that are deregulated in opposite directions. We conducted transcriptomic meta-analyses of three CNS disorders (Alzheimer's disease, Parkinson's disease and Schizophrenia) and three Cancer types (Lung, Prostate, Colorectal) previously described with inverse comorbidities. A significant overlap was observed between the genes upregulated in CNS disorders and downregulated in Cancers, as well as between the genes downregulated in CNS disorders and upregulated in Cancers. We also observed expression deregulations in opposite directions at the level of pathways. Our analysis points to specific genes and pathways, the upregulation of which could increase the incidence of CNS disorders and simultaneously lower the risk of developing Cancer, while the downregulation of another set of genes and pathways could contribute to a decrease in the incidence of CNS disorders while increasing the Cancer risk. These results reinforce the previously proposed involvement of the PIN1 gene, Wnt and P53 pathways, and reveal potential new candidates, in particular related with protein degradation processes.
Introduction
Epidemiological evidences point to a lower-than-expected probability of developing some types of Cancer in certain CNS disorders, including Alzheimer's disease (AD), Parkinson's disease (PD) and Schizophrenia (SCZ) [1]–[6]. Our current understanding of such inverse comorbidities suggests that this phenomenon is influenced by environmental factors, drug treatments and other aspects related with disease diagnosis. Genetics can additionally contribute to the inverse comorbidity between complex diseases, together with these external factors (for review, see [3]–[7]). In particular, we propose the deregulation in opposite directions of a common set of genes and pathways as an underlying cause of inverse comorbidities.
To investigate the biological plausibility of this hypothesis, a basic initial step is to establish the existence of inverse gene expression deregulations (i.e., down - versus up-regulations) in CNS disorders and Cancers. Towards this objective, we have performed integrative meta-analyses of collections of gene expression data, publically available for AD, PD and SCZ, and Lung (LC), Colorectal (CRC) and Prostate (PC) Cancers. Clinical and epidemiological data previously reported inverse comorbidities for these complex disorders, according to population studies assessing the Cancer risks among patients with CNS disorders [8]–[17].
Results and Discussion
For each CNS disorder and Cancer type independently, we undertook meta-analyses from a large collection of microarray gene expression datasets to identify the genes that are significantly up - and down-regulated in disease when compared with their corresponding healthy control samples (Differentially Expressed Genes – DEGs –, FDR corrected p-value (q-value)<0.05, see Methods and Table S1). Then, the DEGs of the CNS disorders and Cancer types were compared to each others. There were significant overlaps (Fisher's exact test, corrected p-value (q-value)<0.05, see Methods) between the DEGs upregulated in CNS disorders and those downregulated in Cancers. Similarly, DEGs downregulated in CNS disorders overlapped significantly with DEGs upregulated in Cancers (Figure 1A). Significant overlaps between DEGs deregulated in opposite directions in CNS disorders and Cancers are still observed while setting more stringent cutoffs for the detection of DEGs (qvalues lower than 0.005, 0.0005, 0.00005 and 0.000005, ). A significant overlap between DEGs deregulated in the same direction was only identified in the case of CRC and PD upregulated genes (Figure 1A).
Fig. 1. Comparisons of Differentially Expressed Genes (DEGs). 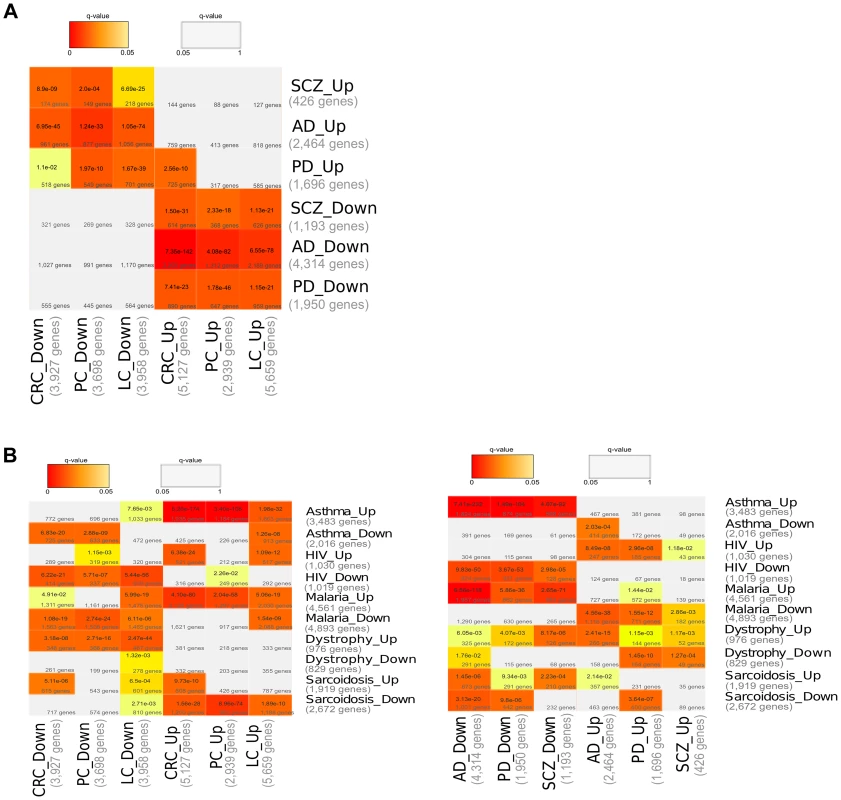
(A) Comparisons of DEGs associated with Central Nervous System (CNS) disorders and Cancers. The DEGs identified as significantly up- and down-regulated (q-value<0.05) after gene expression meta-analysis in each CNS disorder (Alzheimer's Disease, AD; Parkinson's Disease, PD; and Schizophrenia, SCZ) and Cancer type (Lung Cancer, LC; Colorectal Cancer, CRC; and Prostate Cancer, PC) are compared to each others. (B) Comparisons of DEGs between CNS disorders, Cancers and Asthma, HIV, Malaria, Dystrophy, Sarcoidosis. The DEGs identified as significantly up- and down-regulated (q-value<0.05) after gene expression meta-analysis in each CNS disorder (Alzheimer's Disease, AD; Parkinson's Disease, PD; and Schizophrenia, SCZ), Cancer type (Lung Cancer, LC; Colorectal Cancer, CRC; and Prostate Cancer, PC), and in Asthma, HIV, Malaria, Dystrophia and Sarcoidosis, are compared to each others. Cells are coloured according to the significance of the overlaps (Fisher's exact test, Bonferroni correction for multiple testing, see Methods). Grey cells correspond to non-significant overlaps (q-value>0.05). A molecular interpretation of the inverse comorbidity between CNS disorders and Cancers could be that the downregulation of certain genes would at the same time increase the risk of developing CNS disorders, while reducing the risk of developing Cancers. The upregulation of other genes would reduce the risk of developing CNS disorders and increase the risk of developing Cancers.
We then compared the CNS disorder and Cancer DEGs with DEGs of a number of diseases for which, to our knowledge, inverse comorbidities have not been reported in the literature. These diseases, for which large enough expression datasets were available, included Asthma, HIV, Malaria, Dystrophy and Sarcoidosis (see Methods). Significant overlaps were observed between DEGs of all these diseases and DEGS of CNS disorders or Cancers (Figure 1B). However, patterns of expression deregulation in opposite directions, which were found to be characteristic of the relation between CNS disorders and Cancers, are in most cases not observed with these other genetic or infectious diseases (Figure 1B). Indeed, the overlaps are predominantly significant between DEGs deregulated in the same directions, i.e. between upregulated genes of the different diseases (or conversely between down-regulated genes), and could be a signature of putative positive comorbidities. It is to note that Malaria and CNS disorders DEGs present overlaps between DEGs deregulated in opposite directions, contrarily to what is detected for other diseases. This observation will require additional research.
Overall, these observations support the indication of a signature for inverse comorbidity in gene expression deregulations in opposite directions.
The PIN1 gene has been proposed previously as a putative link between the pathogeneses of AD and Cancer [4]. Through the isomerization of a proline preceded by phosphorylated Ser/Thr residues, the PIN1 protein is known to be a key regulator of cell division [18]. PIN1 gene is typically overexpressed in human Cancers and as such, it has been assessed as a potential target for anticancer drugs [4]. In addition, PIN1 is depleted in AD, it has been shown to restore the function of the phosphorylated tau protein, and mouse models in which this protein is knocked-down present neurodegenerative phenotypes [18]–[19]. Our transcriptomic meta-analyses confirm and extend these observations as the expression of PIN1 is downregulated in AD and PD, and upregulated in CRC (Table S2). Another interesting case is the ATP13A2 gene, involved in the intracellular cation homeostasis. ATP13A2 is part of a list established by Devine et al. of familial PD genes frequently mutated in Cancers [5]. Indeed, loss-of-function mutations of ATP13A2 have been associated with early-onset Parkinsonism, and somatic mutations have been independently observed in Cancer [5]. We identified ATP13A2 as downregulated in AD and PD, and upregulated in the three Cancer types considered (Table S2).
In the light of these findings, our approach appears to be capable of identifying candidate genes potentially associated with inverse comorbidity. In particular, 74 genes may be of interest since they are simultaneously downregulated in the three CNS disorders and upregulated in the three Cancer types examined (Table 1). RNA splicing (four genes: PPIH, LSM4, NUDT21, SRSF2) and aminoacyl t-RNA ligases (three genes: FARSA, IARS, IARS2) represent particularly interesting functions.
Tab. 1. DEGs significantly downregulated in the three CNS disorders and upregulated in the three Cancer types (q-value<0.05). 
We also pinpoint two genes involved in lipid biogenesis (ACLY and MECR), and other two are transcription factors: NME2 and TFCP2, for which a genetic association with AD is debated [20]. Finally, two other genes, OAZ2 and the spermine synthase SMS, are dedicated to polyamine metabolic processes. Interestingly, defects in the spermine synthase gene are associated with the X-linked mental retardation Snyder-Robison syndrom [21], and spermine is often the most abundant polyamine in Cancers [22]. The polyamine metabolic process hence may play a role in the pathological mechanisms of both CNS disorders and Cancers.
Conversely, 19 genes are simultaneously upregulated in the three CNS disorders and downregulated in the three Cancer types examined (Table 2), including for instance six genes involved in signal transduction (TNFRSF1A, CDKN1A, NFKBIA, PTH1R, IL4R, MID1). Particularly, NFKBIA is an interesting candidate because this gene is often deleted in glioblastoma [23], although to our knowledge no mutations or polymorphisms have been described in CNS disorders.
Tab. 2. DEGs significantly upregulated in the three CNS disorders and downregulated in the three Cancer types (q-value<0.05). 
In order to enhance the functional interpretation of the molecular bases of inverse comorbidity, we broaden the comparisons of expression deregulations by considering pathways instead of individual genes [24].
We identified the pathways that were significantly up - and downregulated (GSEA analyses, q-value<0.05, see Methods and Table S3) in each of the six diseases independently. Among all the KEGG [25] pathways significantly up - and down-regulated in the 6 diseases, 30 are shared by CNS disorders and Cancers (i.e., significantly deregulated in at least 1 CNS disorder and 1 Cancer type). Strikingly, of these 30 shared pathways, 24 (80%) are deregulated in opposite directions in CNS disorders and Cancers (Figure 2, 63% and 86% for the Biocarta (http://www.biocarta.com/) and Reactome [26] databases, respectively, Figure S2).
Fig. 2. KEGG pathways significantly deregulated in Central Nervous System (CNS) disorders and Cancer types. 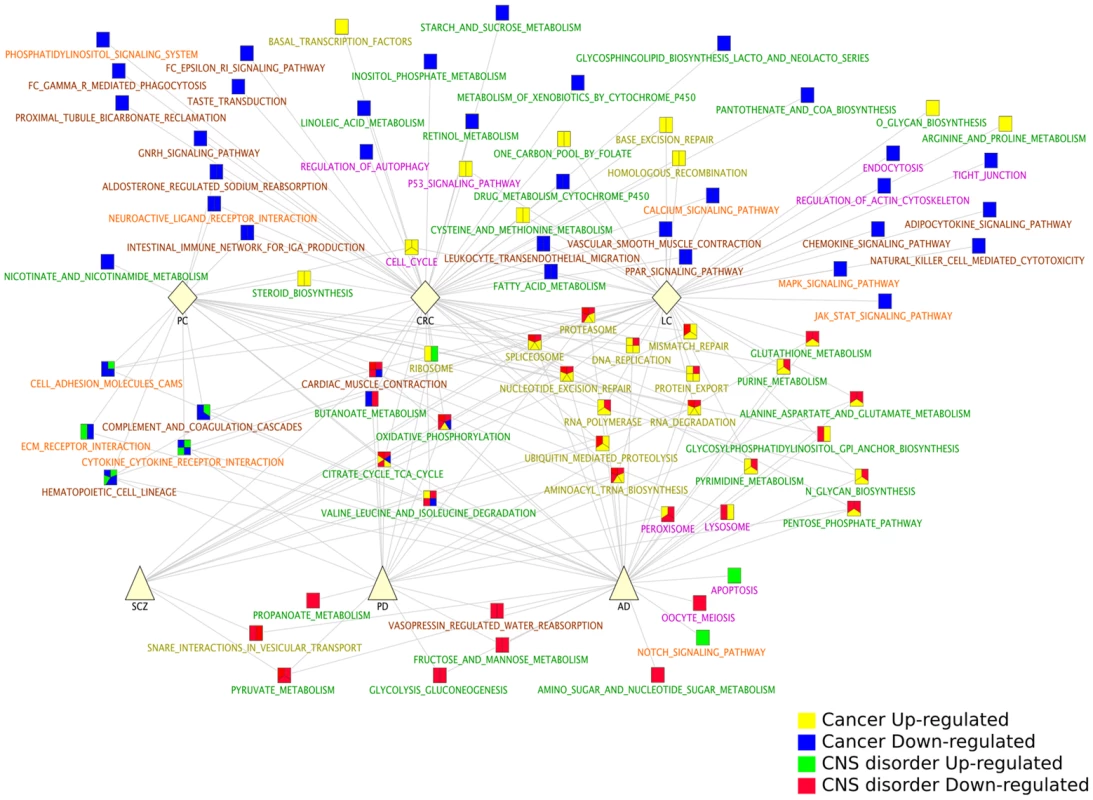
KEGG pathways [24] significantly up- and downregulated in each disease were identified using the GSEA method [34] (q-value<0.05). The significant pathways were compared between the 6 diseases and combined in a network representation. Node pie charts are coloured according to the pathway status as Cancer upregulated (yellow), Cancer downregulated (blue), CNS disorder upregulated (green) and CNS disorder downregulated (red). The green/blue and yellow/red associations thus correspond to pathways deregulated in opposite directions in CNS disorders and Cancers. Pathway labels are coloured according to their classifications provided by KEGG [24], as: Metabolism (green), Genetic Information Processing (yellow), Cellular Process (pink), Environmental Information Processing (red) and Organismal Systems (dark red). All networks are available at bioinfo.cnio.es/people/cboullosa/validation/cytoscape/Ibanezetal.zip, in cytoscape format (http://www.cytoscape.org/). The p53 signalling pathway is an anticipated candidate for deregulations in these diseases and for a role in inverse comorbidity [4]. Indeed, deregulations of the p53 signalling pathway are associated with the initiation and progression of Cancers, while recent studies also point to a role for this pathway in CNS disorders [27]. As such, specific polymorphisms in the TP53 gene are found in SCZ patients [27]. Although the TP53 gene itself does not appear to be differentially regulated in our analysis, the p53 pathway is upregulated in CRC and LC, while it is downregulated in PD, AD and SCZ (Reactome database; Figure S2, Table S3).
Similarly, the Wnt pathway may be particularly relevant as mutations in the genes encoding APC and β-catenin, elements of the Wnt pathway, have been described in CRC, while β-amyloid induced neurotoxicity in AD has been associated with impaired Wnt signalling [4], [18]. Furthermore, alterations in the Wnt signalling pathway are known to be involved in SCZ [28]. In our meta-analyses, we found the Wnt pathway to be downregulated in AD and PD, and upregulated in CRC (Reactome database; Figure S2).
Aside the Wnt and p53 pathways, our analysis reveals other pathways related to protein folding and protein degradation displaying patterns of downregulation in CNS disorders and upregulation in Cancers, and that may be relevant for inverse comorbidity. For instance, the Ubiquitin/Proteasome system is consistently downregulated in CNS disorders and upregulated in Cancers according to the three pathway databases analyzed (Figure 2, Figure S2, Table S3). The inverse relationship between the levels of expression deregulations of these pathways possibly suggests opposite roles in CNS disorders and Cancers.
A detailed examination of the KEGG pathways deregulated in opposite directions in CNS disorders and Cancers finally revealed that 89% of the KEGG pathways that were upregulated in Cancers and downregulated in CNS disorders are related to Metabolism and Genetic Information Processing (Figure 2, Figure 3). By contrast, the pathways downregulated in Cancers and upregulated in CNS disorders are related to the cell's communication with its environment (Environmental Information Processing and Organismal System; Figure 2, Figure 3). Hence, global regulations of cellular activity may account for a protective effect between inversely comorbid diseases.
Fig. 3. KEGG pathway classifications. 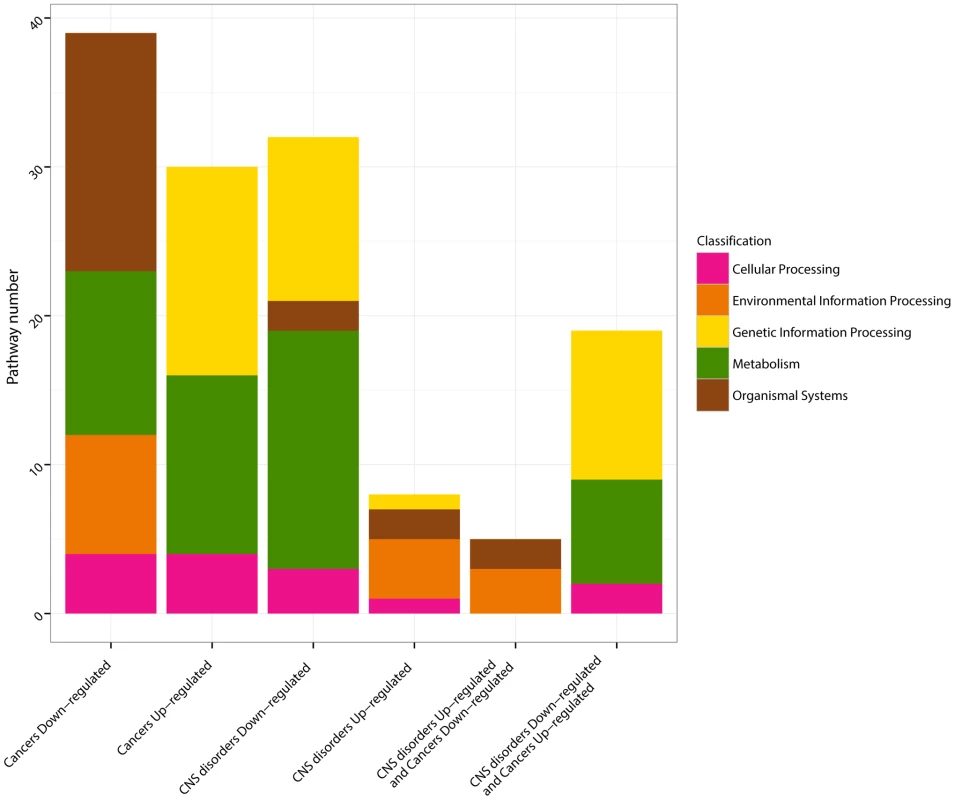
The KEGG pathways [24] identified by the GSEA analysis [34] as significantly up- and down-regulated in CNS disorders, in Cancers, and simultaneously up-regulated in CNS disorders/down-regulated in Cancers, and down-regulated in CNS disorders/up-regulated in Cancers (q-values<0.05, Figure 2) are classified as Metabolism (green), Genetic Information Processing (yellow), Cellular Process (pink), Environmental Information Processing (orange) and Organismal Systems (dark red), according to the classification provided by KEGG. Further analyses will be necessary to conclude to a direct protective effect of gene expression deregulations in cancer-prone tissues of patients suffering from CNS disorders. Indeed, the DEGs analyzed in this study are computed for each disease in the corresponding affected tissues, and cannot be extrapolated to gene expression deregulations in other tissues of the same patients. However, despite these limitations, the identification of antagonistically deregulated genes and pathways in complex diseases that have been previously described as inversely comorbid provides, to our knowledge, the first systematic insights into the possible molecular basis of these associations.
It suggests that the upregulation of a set of genes or processes could increase the incidence of CNS disorders and simultaneously lower the chances of developing Cancers, while the downregulation of another set of genes or processes could contribute to a decrease in the incidence of CNS disorders while increasing the Cancer risks.
The individuals delivering post-mortem brain samples in CNS disorders, or tumor tissues in the case of Cancers, are likely to have received drug treatments. Hence, the observed expression deregulations could be the consequence of the drugs administered to the patients. If this is the case, it can be hypothesized that some of the drugs used to treat CNS disorders might be able to revert the expression of a number of Cancer genes. In this context, the repurposing of drugs from the CNS to the Cancer field could open new therapeutic avenues. Indeed some punctual observations have been made. For example, the thioridazine, an anti-psychotic drug antagonizing the dopamine receptor and potentially able to alter physiological states and expression patterns, have been reported to target cancer stem cells selectively [29].
Finally, the analyses of inverse expression deregulations could serve as a new approach to investigate possible relations between complex diseases, of which the ones reported here between CNS disorders and Cancers can be considered as an initial example.
Methods
The analysis pipeline (Text S1, workflow) is available as literate programming file describing the different steps of the analysis, together with the dynamic report (Text S2, knitr (http://yihui.name/knitr/) that can be used directly in R.)
Gene expression data
Gene expression raw data (CEL files) were downloaded from NCBI GEO omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), EBI ArrayExpress (AE, http://www.ebi.ac.uk/arrayexpress/) and Stanley Medical Research Institute, Online Genomics Database (SMRI, https://www.stanleygenomics.org) for Colorectal (CRC), Lung (LC) and Prostate (PC) Cancers, Alzheimer's disease (AD), Parkinson's disease (PD) and Schizophrenia (SZC), and for Asthma, HIV, Malaria, Dystrophy, Sarcoidosis (Text S1). For each disease, studies were filtered to select only the ones profiling at least 9 samples for disease and control cases, with Affymetrix arrays (GeneChip Human Genome U133 Plus 2.0, GeneChip Human Genome U133A and GeneChip Human Genome U133A 2.0 containing 23,945, 14,538 and 14,538 genes, respectively). For CNS disorders, only studies that measure gene expression in brain tissues were selected. For Cancers, only gene expression studies carried out in the LC, CRC and PC tumor tissues were considered.
Microarray gene expression preprocessing and meta-analyses
The collected microarray data from the different studies were normalized with frozen Robust Multiarray Analysis (fRMA) [30] from the R Affy package [31]. Then, microarray meta-analyses were undertaken for each disease independently using the R MetaDE package [32]. MetaDE implements meta-analysis methods for differential expression analysis, and we used the Fixed Effects Model (FEM) [33]. This model assumes that the standardized effect sizes can be combined between the different studies, and that the variations in observed effects are only due to random error [34]–[35].
Similar results were obtained with the Random Effects Model (REM) approach that allows heterogeneity in the effect sizes between the different datasets (unpublished observations).
The meta-analyses led to the identification of genes up - and down-regulated in each disease, and significant differentially expressed genes (DEGs) were selected as those displaying a FDR corrected p-value (q-value)<0.05. Four other q-value cutoffs (0.005, 0.0005, 0.00005 and 0.000005) were selected to validate our results on more stringent DEGs sets (Figure S1).
Comparisons of DEGs between the different diseases
Each CNS disorder DEGs were compared to each Cancer type DEGs, and the significances of the overlaps between the DEGs were assessed by a one-tailed Fisher's exact test, corrected for multiple testing by the Bonferroni approach (Figure 1A, Figure S1). The background number of genes necessary for the Fisher's test was set to 14,538.
The same procedure was applied for Cancers, CNS disorders and Asthma, HIV, Malaria, Dystrophy and Sarcoidosis (Figure 1B).
GSEA analyses
For each CNS disorder and Cancer type independently, a gene set enrichment analysis was undertaken using GSEA [36] on the output of the meta-analyses, and focusing on KEGG [24], Biocarta (http://www.biocarta.com/) and Reactome [26] pathway databases. Significant pathways were selected as those with q-value (FDR)<0.05. Significant pathways in each disease were then compared to each others, and a network of pathways was built (Figure 2, Figure S2).
For the KEGG pathways, further classification of the pathways in Metabolism, Genetic Information Processing, Cellular Processes, Environmental Processes and Organismal Processes, as provided by KEGG, was done (Figure 2, Table S2). Pathways corresponding to Human Diseases were discarded.
Supporting Information
Zdroje
1. Tabarés-SeisdedosR, DumontN, BaudotA, ValderasJM, ClimentJ, et al. (2011) No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol 12 : 604–608.
2. BehrensMI, SilvaM, SalechF, PonceDP, MerinoD, et al. (2012) Inverse susceptibility to oxidative death of lymphocytes obtained from Alzheimer's patients and skin cancer survivors: increased apoptosis in Alzheimer's and reduced necrosis in cancer. Series A: J Gerontol 67 : 1036–1040.
3. Tabarés-SeisdedosR, RubensteinJL (2013) Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat Rev Neurosci 14 : 293–304.
4. BehrensMI, LendonC, RoeCM (2009) A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res 6 : 196–204.
5. DevineMJ, Plun-FavreauH, WoodNW (2011) Parkinson's disease and cancer: two wars, one front. Nat Rev Cancer 11 : 812–823.
6. Catalá-LópezF, Crespo-FacorroB, VietaE, ValderasJM, ValenciaA, et al. (2014) Alzheimer's Disease and Cancer: Current Epidemiological Evidence for a Mutual Protection. Neuroepidemiology 42 : 121–122.
7. WestAB, DawsonVL, DawsonTM (2005) To die or grow: Parkinson's disease and cancer. Trends Neurosci 28 : 348–352.
8. MusiccoM, AdorniF, Di SantoS, PrinelliF, PettenatiC, et al. (2013) Inverse occurrence of cancer and Alzheimer disease: A population-based incidence study. Neurology 81 : 322–328.
9. FerreiraJJ, NeutelD, MestreT, CoelhoM, RosaMM, et al. (2010) Skin cancer and Parkinson's disease. Mov Disord 25 : 139–148.
10. BajajA, DriverJA, SchernhammerES (2010) Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control 21 : 697–707.
11. KimJS, YimSV, KohIS, ChoiJS, YooJY, et al. (2009) Single-nucleotide polymorphisms (SNPs) and haplotype analysis in vascular endothelial growth factor (VEGF) gene in the patients with Parkinson disease and lung cancer. Arch Gerontol Geriatr 48 : 287–290.
12. CattsVS, CattsSV, O'TooleBI, FrostAD (2008) Cancer incidence in patients with schizophrenia and their first-degree relatives - a meta-analysis. Acta Psychiatr Scand 117 : 323–336.
13. LinC-Y, LaneH-Y, ChenT-T, WuY-H, WuC-Y, et al. (2013) Inverse association between cancer risks and age in schizophrenic patients: A 12-year nationwide cohort study. Cancer sci 104 : 383–390.
14. LinG-M, ChenY-J, KuoD-J, JaitehLES, WuY-C, et al. (2013) Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in taiwan, 1997–2009. Schizophr Bull 39 : 407–416.
15. DriverJA, BeiserA, AuR, KregerBE, SplanskyGL, et al. (2012) Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ 344: e1442–e1442.
16. RoeCM, BehrensMI, XiongC, MillerJP, MorrisJC (2005) Alzheimer disease and cancer. Neurology 64 : 895–898.
17. RoeCM, FitzpatrickAL, XiongC, SiehW, KullerL, et al. (2010) Cancer linked to Alzheimer disease but not vascular dementia. Neurology 74 : 106–12.
18. LuKP (2004) Pinning down cell signaling, cancer and Alzheimer's disease. Trends Biochem Sci 29 : 200–209.
19. LiouY-C, ZhouXZ, LuKP (2011) Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci 36 : 501–514.
20. CousinE, MacéS, RocherC, DibC, MuzardG, et al. (2011) No replication of genetic association between candidate polymorphisms and Alzheimer's disease. Neurobiol Aging 32 : 1443–1451.
21. CasonAL, IkeguchiY, SkinnerC, WoodTC, HoldenKR, et al. (2003) X-linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur J Hum Genet 11 : 937–944.
22. HuberM, PoulinR (1995) Antiproliferative effect of spermine depletion by N-cyclohexyl-1,3-diaminopropane in human breast cancer cells. Cancer Res 55 : 934–943.
23. BredelM, ScholtensDM, YadavAK, AlvarezAA, RenfrowJJ, et al. (2011) NFKBIA deletion in glioblastomas. N Engl J Med 364 : 627–637.
24. RamananVK, ShenL, MooreJH, SaykinAJ (2012) Pathway analysis of genomic data: concepts, methods, and prospects for future development. Trends Genet 28 : 323–332.
25. KanehisaM, ArakiM, GotoS, HattoriM, HirakawaM, et al. (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36: D480–484.
26. MatthewsL, GopinathG, GillespieM, CaudyM, CroftD, et al. (2009) Reactome knowledgebase of human biological pathways and processes. Nucleic Acids Res 37: D619–22.
27. Tabarés-SeisdedosR, RubensteinJL (2009) Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry 14 : 563–589.
28. OkerlundND, CheyetteBNR (2011) Synaptic Wnt signaling-a contributor to major psychiatric disorders? J Neurodev Disord 3 : 162–174.
29. SachlosE, RisueñoRM, LarondeS, ShapovalovaZ, LeeJ-H, et al. (2012) Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell 149 : 1284–1297.
30. McCallMN, JaffeeHA, IrizarryRA (2012) fRMA ST: frozen robust multiarray analysis for Affymetrix Exon and Gene ST arrays. Bioinformatics 28 : 3153–3154.
31. GautierL, CopeL, BolstadBM, IrizarryRA (2004) affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20 : 307–315.
32. WangX, KangDD, ShenK, SongC, LuS, et al. (2012) An R package suite for microarray meta-analysis in quality control, differentially expressed gene analysis and pathway enrichment detection. Bioinformatics 28 : 2534–2536.
33. ChoiJK, YuU, KimS, YooOJ (2003) Combining multiple microarray studies and modeling interstudy variation. Bioinformatics 19 Suppl 1: i84–90.
34. TsengGC, GhoshD, FeingoldE (2012) Comprehensive literature review and statistical considerations for microarray meta-analysis. Nucleic Acids Res 40 : 3785–3799.
35. Borenstein M, Rothstein H (2009) Introduction to Meta-Analysis. 1st ed. John Wiley & Sons, New York.
36. SubramanianA, TamayoP, MoothaVK, MukherjeeS, EbertBL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102 : 15545–15550.
Štítky
Genetika Reprodukčná medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



