-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Fecal Contamination of Drinking-Water in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
Background:
Access to safe drinking-water is a fundamental requirement for good health and is also a human right. Global access to safe drinking-water is monitored by WHO and UNICEF using as an indicator “use of an improved source,” which does not account for water quality measurements. Our objectives were to determine whether water from “improved” sources is less likely to contain fecal contamination than “unimproved” sources and to assess the extent to which contamination varies by source type and setting.Methods and Findings:
Studies in Chinese, English, French, Portuguese, and Spanish were identified from online databases, including PubMed and Web of Science, and grey literature. Studies in low - and middle-income countries published between 1990 and August 2013 that assessed drinking-water for the presence of Escherichia coli or thermotolerant coliforms (TTC) were included provided they associated results with a particular source type. In total 319 studies were included, reporting on 96,737 water samples. The odds of contamination within a given study were considerably lower for “improved” sources than “unimproved” sources (odds ratio [OR] = 0.15 [0.10–0.21], I2 = 80.3% [72.9–85.6]). However over a quarter of samples from improved sources contained fecal contamination in 38% of 191 studies. Water sources in low-income countries (OR = 2.37 [1.52–3.71]; p<0.001) and rural areas (OR = 2.37 [1.47–3.81] p<0.001) were more likely to be contaminated. Studies rarely reported stored water quality or sanitary risks and few achieved robust random selection. Safety may be overestimated due to infrequent water sampling and deterioration in quality prior to consumption.Conclusion:
Access to an “improved source” provides a measure of sanitary protection but does not ensure water is free of fecal contamination nor is it consistent between source types or settings. International estimates therefore greatly overstate use of safe drinking-water and do not fully reflect disparities in access. An enhanced monitoring strategy would combine indicators of sanitary protection with measures of water quality.
Please see later in the article for the Editors' Summary
Published in the journal: Fecal Contamination of Drinking-Water in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. PLoS Med 11(5): e32767. doi:10.1371/journal.pmed.1001644
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001644Summary
Background:
Access to safe drinking-water is a fundamental requirement for good health and is also a human right. Global access to safe drinking-water is monitored by WHO and UNICEF using as an indicator “use of an improved source,” which does not account for water quality measurements. Our objectives were to determine whether water from “improved” sources is less likely to contain fecal contamination than “unimproved” sources and to assess the extent to which contamination varies by source type and setting.Methods and Findings:
Studies in Chinese, English, French, Portuguese, and Spanish were identified from online databases, including PubMed and Web of Science, and grey literature. Studies in low - and middle-income countries published between 1990 and August 2013 that assessed drinking-water for the presence of Escherichia coli or thermotolerant coliforms (TTC) were included provided they associated results with a particular source type. In total 319 studies were included, reporting on 96,737 water samples. The odds of contamination within a given study were considerably lower for “improved” sources than “unimproved” sources (odds ratio [OR] = 0.15 [0.10–0.21], I2 = 80.3% [72.9–85.6]). However over a quarter of samples from improved sources contained fecal contamination in 38% of 191 studies. Water sources in low-income countries (OR = 2.37 [1.52–3.71]; p<0.001) and rural areas (OR = 2.37 [1.47–3.81] p<0.001) were more likely to be contaminated. Studies rarely reported stored water quality or sanitary risks and few achieved robust random selection. Safety may be overestimated due to infrequent water sampling and deterioration in quality prior to consumption.Conclusion:
Access to an “improved source” provides a measure of sanitary protection but does not ensure water is free of fecal contamination nor is it consistent between source types or settings. International estimates therefore greatly overstate use of safe drinking-water and do not fully reflect disparities in access. An enhanced monitoring strategy would combine indicators of sanitary protection with measures of water quality.
Please see later in the article for the Editors' SummaryIntroduction
The importance of water to human health and wellbeing is encapsulated in the Human Right to Water and Sanitation, which entitles everyone to “sufficient, safe, acceptable physically accessible and affordable water for personal and domestic uses” [1], as reaffirmed by the United Nations General Assembly and Human Rights Council in 2010 [2]. Millennium Development Goals (MDGs) Target 7c aims “to halve the proportion of the population without sustainable access to safe drinking-water …” [3], a step towards universal access. “Use of an improved source” was adopted as an indicator for monitoring access to safe drinking-water globally (Table 1) and relies on national censuses and nationally representative household surveys as the primary sources of data.
Tab. 1. Types of improved source and the estimated proportion of the global population using these as their primary source of drinking-water. 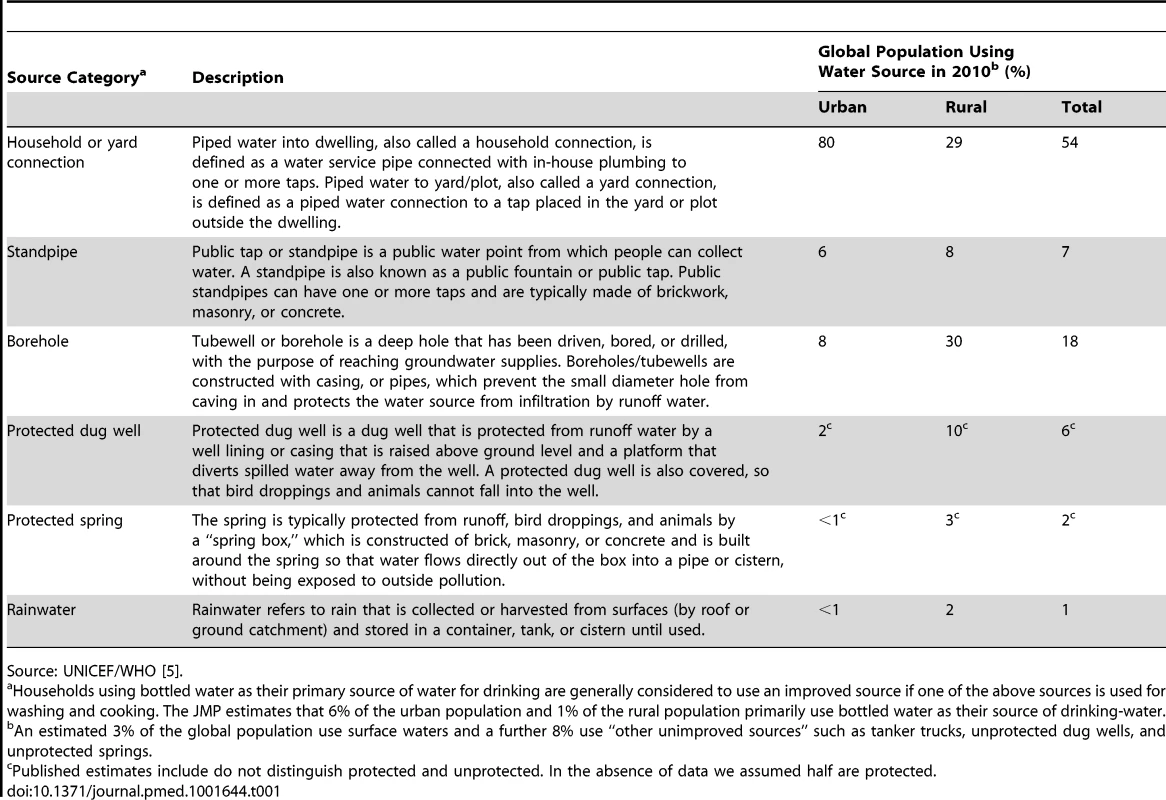
Source: UNICEF/WHO [5]. The Joint Monitoring Programme for Water Supply and Sanitation (JMP) of WHO/UNICEF categorizes a drinking-water source type as improved if “by nature of its construction or through active intervention, [it] is protected from outside contamination, in particular from contamination with faecal matter” [4]. Improved source types include piped water into dwelling, yard, or plot, standpipe, borehole, protected dug well or spring, and rainwater. Unimproved source types are those that do not protect water from outside contamination (unprotected wells, unprotected springs, surface waters, and tanker trucks). While the categorization reflects well-established principles of sanitary protection, on announcing that the target had been met in 2010, the JMP cautioned that the MDG indicator does not take water quality measurements into account [5]. The indicator has been criticized for not adequately reflecting safety [6]–[8], with some estimates suggesting that reported access to safe water might be overestimated by billions of people [9],[10], by not accounting for microbial water safety [8] or more fully accounting for sanitary status [9].
Diseases related to contamination of drinking-water constitute a major burden on public health. The principal risk to health is from ingestion of water contaminated with feces containing pathogens that cause infectious diseases such as cholera and other diarrheal diseases, dysenteries, and enteric fevers [11],[12]. The burden of water-related disease varies according to context and is highest in low-income settings where diarrhea remains a leading cause of child deaths [13]. Systematic reviews of epidemiological evidence from intervention studies [14]–[18], and especially outbreak investigations [19],[20], suggest drinking-water quality plays an important role in fecal-oral transmission, though the magnitude of the effect has been contested owing to a limited number of blinded trials [21]. It is difficult to isolate the effects of one component of the multiple and interrelated fecal-oral pathways, which are highly context-specific.
WHO publishes widely recognized Guidelines for Drinking-water Quality (GDWQ) (4th edition) that include criteria for assessing health risks and setting targets for improving water safety [12]. Direct measurement of pathogens is complex but techniques for assessing fecal contamination using fecal indicator bacteria (FIB) are well established and widely applied. The WHO GDWQ recommend using E. coli, or alternatively thermotolerant coliform (TTC), and new enzymatic methods have made quantification simpler, cheaper, and more robust [22],[23]. The WHO GDWQ recommend that E. coli, or alternatively TTC, be used in assessing fecal contamination of drinking-water [12]. The WHO guideline value for E. coli (“none detected in any 100-ml sample”) [12] is reflected in the standards of most OECD member states and low - and middle-income countries (LMICs). The WHO GDWQ further suggest the use of a risk classification to prioritize interventions as higher levels of indicator organisms are generally indicative of greater levels of fecal contamination. A commonly used risk classification is based on the number of indicator organisms in a 100 ml sample, which includes: <1, “very low risk”; 1–10, “low risk”; 10–100, “medium risk”; >100, “high risk” or “very high risk” [24],[25]. However FIB are imperfect and their level does not necessarily equate to risk [26]; since quality varies both temporally and spatially, occasional sampling may not accurately reflect actual exposure.
A complementary approach in safety assessment is the identification of hazards and preventative risk management measures through “sanitary inspection” of a water source and its surroundings [24],[27]. The improved source indicator is in effect a very simplified form of sanitary inspection. Like FIB, sanitary inspections have long been a tool in assessing drinking-water safety. In 1904, Prescott and Winslow stated, “[t]he first attempt of the expert called in to pronounce upon the character of a potable water should be to make a thorough sanitary inspection…” [28]. Standardized forms can be used to assess sanitary risk and derive a summary measure, the sanitary risk score. These forms typically include questions about the integrity of protective elements, such as fencing or well covers, and the proximity of hazards such as latrines; forms are available for different types of water source. Like water quality, some sanitary risk factors may vary spatially and temporally. The approach can be combined with microbiological analysis, either to yield a risk cross-tabulation [24],[25] or as a part of a more detailed Water Safety Plan [29].
In January 2012, WHO and UNICEF established working groups to develop targets and indicators for enhanced global monitoring of drinking-water, sanitation, and hygiene post-2015. The water working group proposed to continue using the improved water source classification as part of a revised set of indicators for assessing progressive improvements in service [30]. This review was commissioned to assist the group in evaluating the evidence linking improved source types and health-related indicators of water quality. The following specific questions were considered in order to determine the potential and limits of classification by source type in assessing safety in future global reporting: (i) Is water from improved sources less likely to exceed health-based guidelines for microbial water quality than water from unimproved sources? (ii) To what extent does microbial contamination vary between source types, between countries, and between rural and urban areas? (iii) Are some types of water source associated with higher risk scores as assessed by sanitary inspection?
Methods
We conducted a systematic review of studies of fecal contamination of drinking-water in LMICs in adherence with PRISMA guidelines (Text S1) [31]. The protocol for the review is described in Protocol S1.
Search Strategy
Studies were identified from both peer-reviewed and grey literature. To identify peer-reviewed literature, the topic “water quality” was combined with terms to restrict the search to drinking-water and either a measure of microbial water quality (e.g., “coli”) or sanitary risk (e.g., “sanitary inspection”). We further restricted the search to LMICs using a list of country names based on the MDG regions [32]. Online databases were searched including PubMed, Web of Science, and the Global Health Library. Grey literature was sourced from a variety of sites including those used in previous drinking-water–related reviews [33]–[35]. Translated search terms (Chinese, French, Portuguese, and Spanish) were used to identify additional studies. An email requesting submissions of relevant studies was distributed to water sector professional networks. We searched bibliographies of included studies and contacted authors where full texts could not be obtained through other means. Searches were conducted between 7th January and 1st August 2013.
Eligibility and Selection
Studies were included in the review provided they: reported on water quality, at either the point of collection or consumption, from sources used for drinking that would not be classified as surface waters by the JMP; contained extractable data on TTC or E. coli with sample volumes not less than 10 ml; were published between January 1990 (the baseline year for MDG targets) and August 2013; included results from at least ten separate water samples from different water sources of a given type or, in the case of piped systems, individual taps, and in the case of packaged waters, brands; reported data from LMICs as defined by the MDG regions [32] (thereby excluding 55 high-income countries, comprising 18.1% of the global population in 2010 [36]); were published in languages spoken by at least one author (Chinese, English, French, Portuguese, or Spanish); and included sufficient detail about the water sources and associated results with a water source with sufficient detail to be categorized (refer to Figure 1 for details). Other indicators such as coliphage and direct pathogen detection are not as widely used and are not included in this review [37]. We did not include studies that only assessed surface waters as these are generally considered unfit for drinking. We included bottled water and sachet water that do not form part of the JMP improved source classification (which is concerned with the household's primary source of water for drinking, cooking, and personal hygiene [38]) but are nonetheless important sources of drinking-water in many countries.
Fig. 1. Matching drinking-water source types to the classification used by the Joint Monitoring Programme. 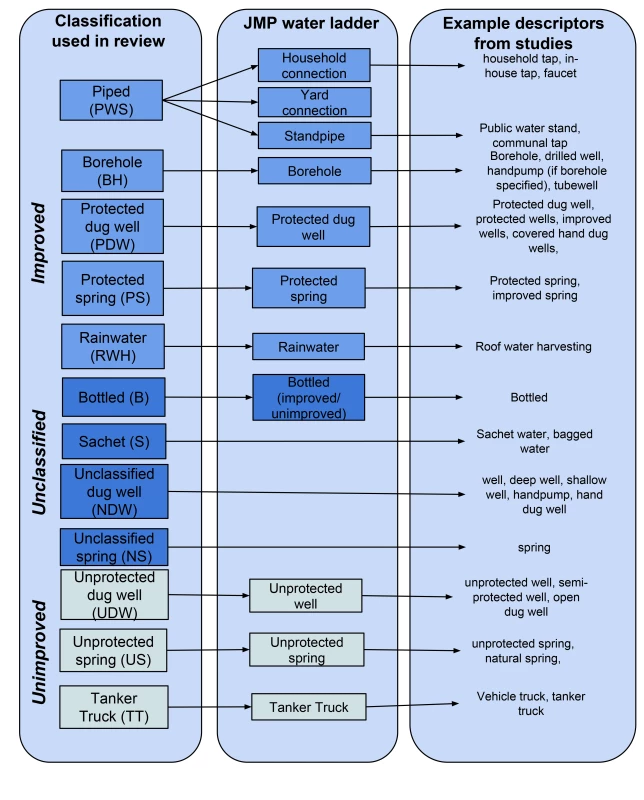
Independent primary screening of English language titles and abstracts for studies was conducted by two authors (RB and RC). If any reviewer selected a study, we referred to the full text. Data from eligible studies were extracted into a standardized spreadsheet and 10% of the English language texts were subjected to independent quality control by a second author (RB and RC). Screening and extraction of data in other languages was conducted by one author (RB or HY).
Data Extraction and Matching
Where possible we extracted or calculated the following information for each type of water source in the studies: (i) total number of samples and proportion containing E. coli or TTC; (ii) proportion of samples within microbial risk categories (<1 or not detected, 1–10, 10–100, and >100 E. coli or TTC per 100 ml); (iii) geometric mean, mean, or median levels of E. coli or TTC; and (iv) risk categories according to the sanitary inspection (“low,” “medium,” “high,” and “very high” risk) as reported in the studies. For intervention studies (other than the provision of an improved source, for example the protection of unprotected springs), estimates could be based on either the baseline or control group; when both were available we used whichever had the largest sample size. For studies reporting both E. coli and TTC, we used only the E. coli results. Where repeated measures were taken at the same source and data permitted we extracted the lowest compliance level (e.g., wet season data) with WHO Guideline values as well as the overall proportion of samples containing FIB. We identified countries as “low,” “lower middle,” “upper middle,” and “high” income using the 2013 World Bank classification [39]. We recorded whether studies took place during or shortly after emergencies or natural disasters and if they were in non-household settings such as schools and health facilities. We identified additional study characteristics expected to influence water quality, including the setting (urban/rural), season (wet/dry or period of sampling), and study design [34].
Each type of water source in a given study was classified as improved or unimproved and matched to a specific water source type following the classification used in household surveys including the Demographic and Health Surveys [38]. We recorded whether samples had been taken directly from the water source or after storage, for example in the home. Where the appropriate match could not be determined, our approach differed depending on the type of source. We grouped groundwater sources from studies that did not distinguish between protected and unprotected (unclassified dug well, unclassified spring) and we created groups for studies of other sources such as bottled and sachet water. Further information about the matching is available in Figure 1.
Study Quality and Bias
Studies were rated for quality on the basis of the criteria summarized in Table 2. A quality score between 0 and 13 for each study was determined on the basis of the number of affirmative responses. We also categorized studies on the basis of anticipated susceptibility to bias in estimating the compliance to health-based guidelines and the extent of microbial contamination; our categories were: case-control or cohort, intervention, diagnostic study, cross-sectional survey, and longitudinal survey. Any study of at least 6 months duration and more than two samples at each water point was categorized as longitudinal. We identified studies where authors indicated whether selection was intended to be representative or selection had been randomized.
Tab. 2. Quality criteria used to assess studies of microbial water quality. 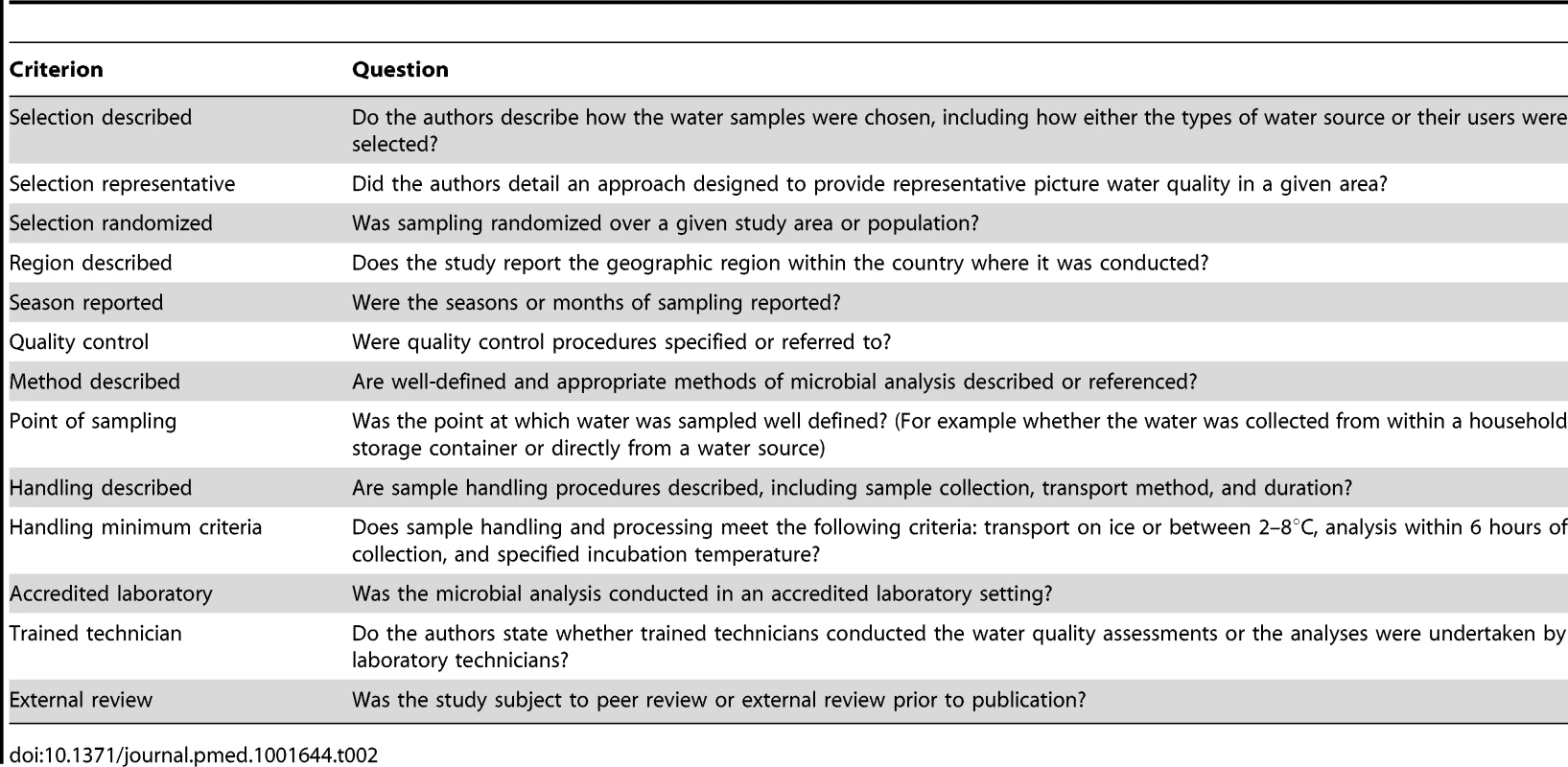
Analysis
Because of the extent of heterogeneity between studies, we chose to plot cumulative density functions (CDFs) of the proportion of samples with detected (>1 per 100 ml) and high (>100 per 100 ml) FIB in each study to compare water source types between studies. This approach has been used in a systematic review of prevalence of schizophrenia [40]. CDFs are used to qualitatively assess the proportion of studies reporting frequent and high levels of microbial contamination. Measures of central tendency from studies were not included in the meta-analysis because of limited reporting of measures of dispersion, inadequate explanation of the handling of censored data, and the difficulty in reconciling diverse reported measures of central tendency (e.g., geometric versus arithmetic mean) [41].
Random effects meta-regression was used to investigate risk factors and settings where fecal contamination is most common and other possible explanations for the observed heterogeneity between studies [42]. A logit transformation is recommended for the analysis of proportions [43] and was applied to both the proportion of samples with detectable (>1 per 100 ml) and high (>100 per 100 ml) levels of FIB. The metareg function in Stata was used after a continuity correction of ±0.5 where the proportion of samples positive was zero or one [44], and we estimated the within study variance for each proportion as the reciprocal of the binomial variance [45]. Subgroup analysis included variables defined a priori (including water source type, rural versus urban, and income-level) and defined a posteriori (for example if piped water had been treated prior to distribution). We separately evaluated piped and other improved sources for those variables reaching significance at the 5% level in bivariate analysis for all source types.
Studies that included both improved and unimproved sources were then combined using meta-analysis with the odds ratio (OR) as the effect measure. We calculated a pooled estimate of the protective effect of an improved source and corresponding confidence intervals using the metan function in Stata. We then assessed the influence of small study bias by the funnel plot method and performed an Egger's test using a normal likelihood approximation. The extent of heterogeneity in protective effect was determined using Higgins I2 and corresponding confidence intervals were calculated [42]. Calculations were performed in Stata 13SE.
Results
Search Results
As shown in Figure 2, in total, 6,586 reports were identified through database searches. A further 1,274 reports were identified from grey literature and correspondence with experts. Most studies were excluded because they did not test water that was clearly used for drinking, did not associate results with a water source type, or did not include enough different water sources or in the case of packaged water, brands. Studies often did not provide an adequate description of the water sources to allow them to be matched to the JMP source categories; this limitation was particularly the case for ground water sources. For example, several studies reported results for “hand pumps” (a description of the technology above ground) but did not provide details about well construction. Although these may often be boreholes, hand pump conversions are also applied to dug wells. Other studies simply described water sources as “wells” or “springs.” Some studies provided details that are not captured in the JMP classification, such as whether water from a piped supply had been treated. Full texts could not be obtained for 99 potentially relevant reports, many of which were conference presentations and most of which were identified from bibliographies. The remaining 310 reports [6],[24],[46]–[353] were incorporated in our review and provide information on 96,737 water samples. The total number of studies is higher (319) due to a small number of multi-country reports. On average each study provides information on 1.7 water source types, resulting in a database with 555 datasets (Dataset S1).
Fig. 2. Flowchart for a review of safety of sources of drinking-water. 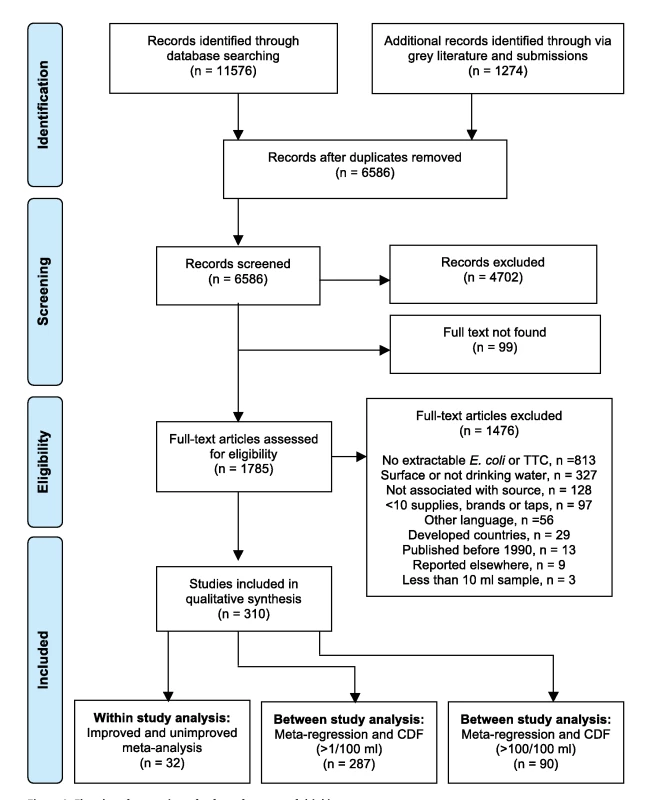
Study Characteristics
Characteristics of included studies are summarized in Table 3. The review is dominated by cross-sectional studies (n = 241, 75%) with fewer longitudinal surveys (n = 39, 12%). Authors report selecting sources or households at random in a minority of studies (n = 68, 21%); most of these studies selected sources randomly within a region or community rather than at national level. The main exceptions were the Rapid Assessment of Drinking-Water Quality (RADWQ) studies commissioned by WHO and UNICEF, of which five have been published [64],[65],[164],[281],[322] and a repeated cross-sectional study in Peru for which only the total coliform results have previously been reported but for which we were able to secure E. coli data [227].
Tab. 3. Characteristics of included studies. 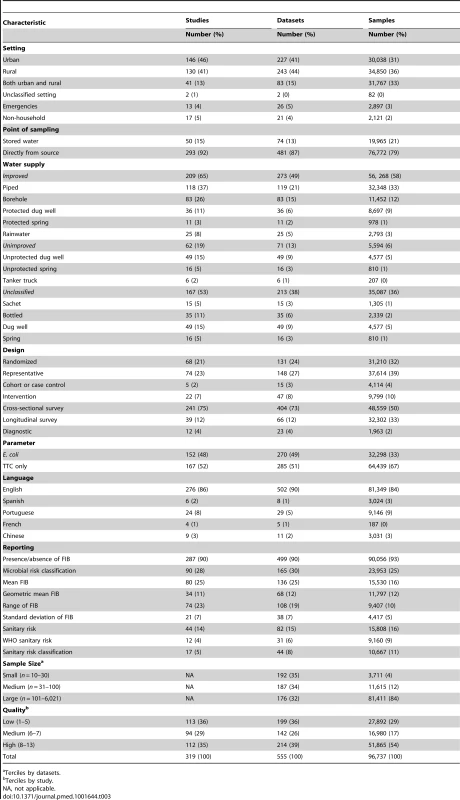
Terciles by datasets. Study quality varied greatly spanning from a quality score of 1 to 12 and with an interquartile range of 5 to 8 (Figure S1). Whereas most studies described the analytical method used to detect E. coli or TTC (80%), how water sources were selected (67%), and the setting in which the study took place (86%), fewer specified quality control procedures (15%), met the basic sample handling criteria (25%), used trained technicians to conduct the water quality tests (15%), or arranged testing in an accredited laboratory (12%) (Figure S2). Most studies were from sub-Saharan Africa, southern Asia, or Latin America and the Caribbean (Figure 3). The majority of included studies investigated water quality at the source. Studies reporting on the quality of water stored in households by provenance were less common (n = 49), and few of these compare quality of stored water with that of the associated source (n = 26). Several studies took place during or after emergencies [97],[201] and natural hazards, including cyclones [235], floods [78],[208], droughts [341], and tsunamis [130],[147],[331]. Non-household settings such as schools and health facilities were addressed in a small number of studies (n = 17). Few studies separately report water quality information from slum or peri-urban settings (n = 7).
Fig. 3. Map of study locations. 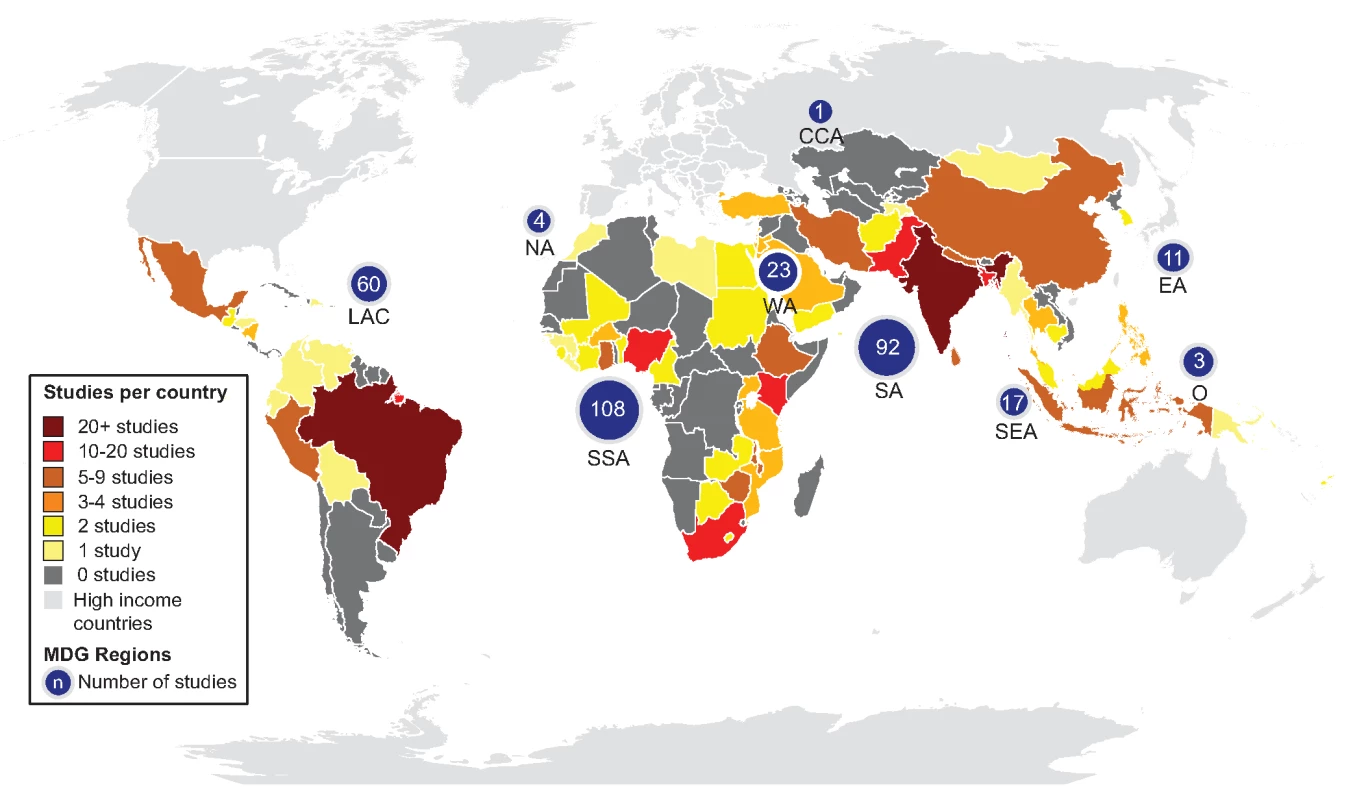
Qualitative Synthesis
In Figures S3 and S4 levels of microbial contamination are shown using the FIB level classification (<1, 1–10, 10–100, and >100 FIB per 100 ml), grouped by type of improved water source. These results are broadly in agreement with a comparison using measures of central tendency (Figure S5) and show great variability in the likelihood and extent of contamination between studies and source types.
Large studies with random sampling demonstrate marked differences in water quality between countries; for example less than 0.01% of samples from utility piped supplies in Jordan [281] were found to contain TTC compared with 9% to 23% of utility piped supplies in the other four RADWQ countries [64],[65],[164],[322]. Only one national randomized study differentiated between rural and urban areas; the proportion of samples from piped supplies containing E. coli was found to be substantially higher in rural (61%, n = 101) than urban (37%, n = 1470) areas in Peru [227].
In comparison to microbial testing, sanitary inspections are less widely practiced or data are rarely published. Sanitary inspection procedures vary considerably and are usually adapted to the local context; of the 44 studies reporting sanitary inspections only 12 used standardized WHO forms. In Figure S6 the sanitary risk levels as reported in nine studies are compared with the proportion of samples containing FIB and suggest that there is no strong association between these two measures.
Between Studies Analysis: CDF and Meta-regression
The number of studies reporting high proportions of samples contaminated or high levels of FIB is lower for improved sources as can be seen in Figure S7. Yet, in 38% of 191 studies reporting the quality of improved sources, at least a quarter of samples exceeded recommended levels of FIB. Figure S8 shows CDFs by source type with similar patterns to those from the FIB level classification.
Results of the meta-regression are shown in Table 4. We find that country income-level is a significant determinant of water quality and the odds of contamination are 2.37 times (95% CI 1.52–3.72 [p = 0.001]; Table 4) higher in low-income countries compared with wealthier countries. However this result is not significant when separately considering piped and other improved sources (Tables S1 and S2).
Tab. 4. Between studies meta-regression. 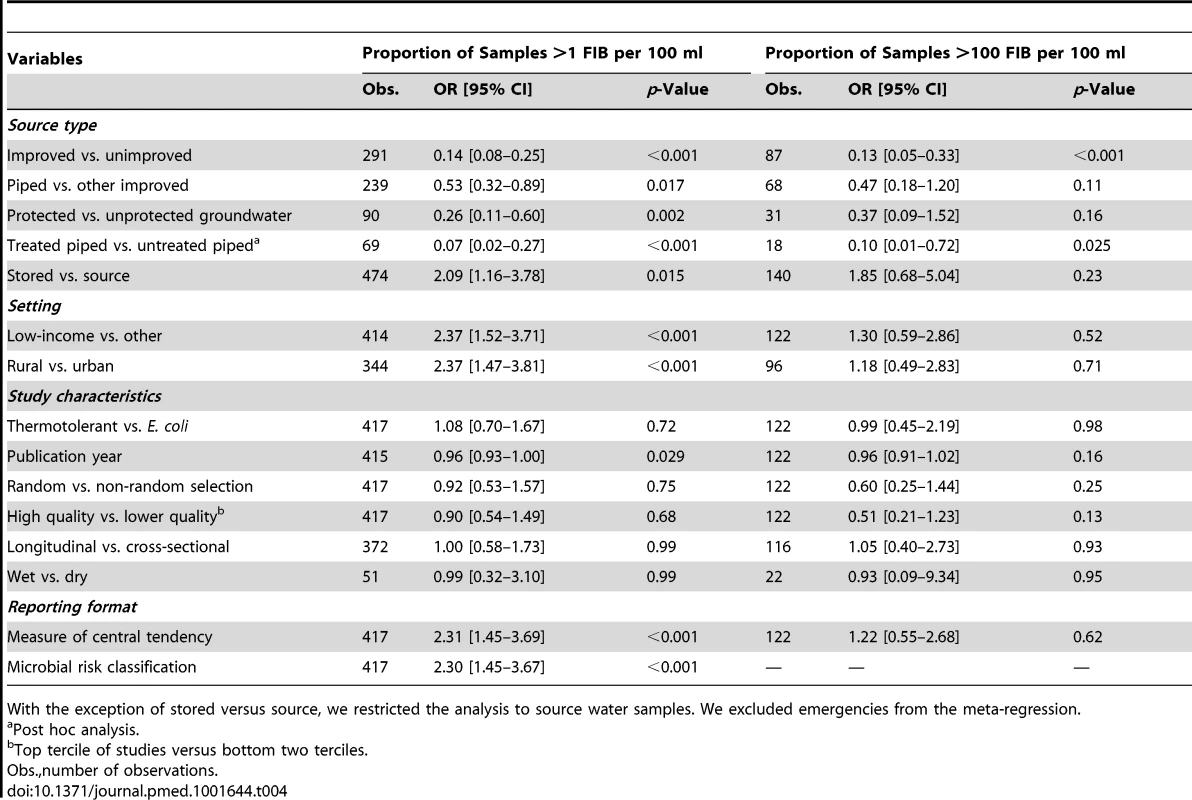
With the exception of stored versus source, we restricted the analysis to source water samples. We excluded emergencies from the meta-regression. Meta-regression showed a substantial difference in the proportion of samples containing FIB between urban and rural areas (OR = 2.37 [95% CI 1.47–3.81], p<0.001). There is weak evidence to suggest that piped supplies are more likely to be contaminated in rural areas (OR = 2.4 [95% CI 0.98–5.92], p = 0.054; Table S1), but no evidence of differences for all other improved source types (OR = 1.19 [95% CI 0.52–2.72], p = 0.67; Table S2).
Protection of groundwater (OR = 0.26 [95% CI 0.11–0.60]; p = 0.002; Table 3) and treatment of piped water (OR = 0.07 [95% CI 0.02–0.27], p<0.001; Table 3) were both strongly related to better water quality. Contamination of stored water was more likely than water at the source (OR = 2.09 [95% CI 1.16–3.78], p = 0.015; Table 3), including for piped supplies (OR = 2.35 [95% CI 1.08–5.12], p = 0.032; Table S1).
Within Studies Analysis: Meta-analysis
Figure 4 is a forest plot showing the ORs of contamination for improved sources compared to unimproved sources from eligible studies. Meta-analysis of randomized and non-randomized studies showed that improved sources are less likely to be contaminated (pooled OR = 0.15 [0.10–0.21]; Figure 4); the protective effect was found to be greater in randomized studies and none of the randomized studies found contamination to be more frequent in improved sources. Heterogeneity was relatively high (I2 = 80.3% [95% CI 72.9–85.6) indicating that the protective effect varies considerably between settings. The OR for a small number of studies was greater than one, suggesting that in some settings improved sources may not always be less likely to contain FIB than the unimproved alternative.
Fig. 4. Forest plot of the odds of fecal contamination for improved and unimproved sources. 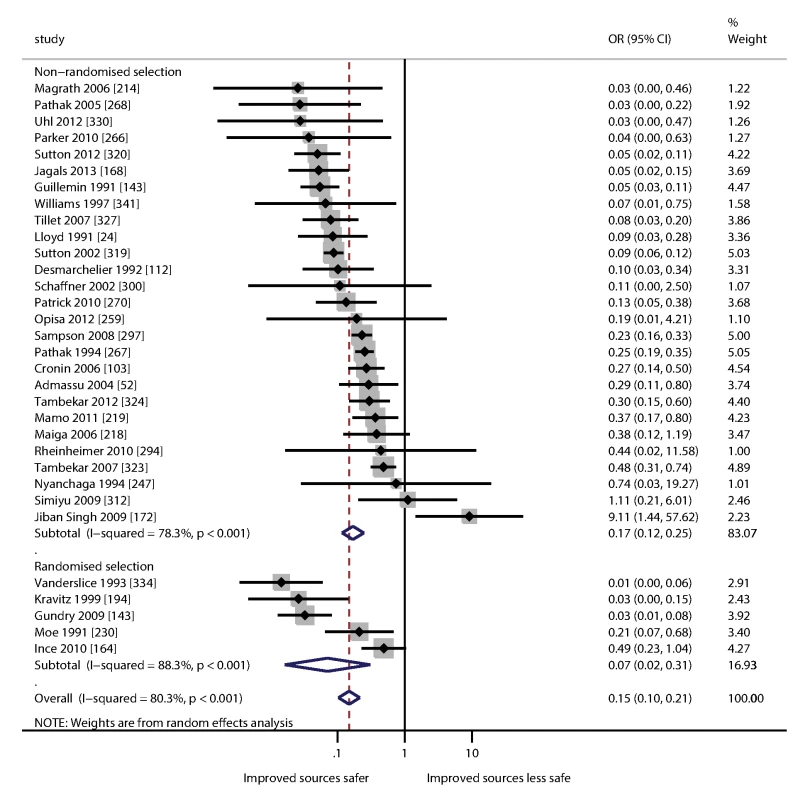
Assessment of Bias
Egger's test found no evidence of small study effects for the meta-analysis of improved versus unimproved sources (p = 0.64; Figure S9). Meta-regression did not detect evidence of bias due to study design, lack of randomization, study quality, or season (“wet” or “dry”). As expected, we find that levels of FIB (risk classification or measures of central tendency) were more likely to be reported in studies where water was more contaminated with FIB (Tables 4, S1, and S2) and therefore may constrain comparisons between studies. Publication year was also related to the proportion of samples containing FIB (OR = 0.96 [95% CI 0.93–1.00]; p = 0.029; Table 4), but this was not significant when separately considering piped and other improved sources (Tables S1 and S2). Studies testing the same sources in different seasons report considerable variation in microbial water quality (Table S3).
Discussion
Safety of Improved Sources
We demonstrate that water from improved sources is less likely to contain FIB than unimproved sources. Using meta-analysis we compare water from unimproved sources and improved sources within the same study and found improved sources were less likely to contain FIB (OR = 0.15 [95% CI 0.10–0.21]) (Figure 4), with the greatest protective effects in studies where selection of water sources was randomized. Comparison between studies of improved versus unimproved sources yielded an OR of 0.14 (95% CI 0.08–0.25) for the presence of FIB and 0.13 (95% CI 0.05–0.34) for samples exceeding 100 FIB per 100 ml (Table 4). Cumulative density plots show markedly lower contamination for improved sources relative to unimproved sources.
While improved sources clearly offer a greater degree of protection compared to unimproved sources, they are not all nor consistently safe [354]. In particular, protected dug wells were rarely free of fecal contamination and it is not uncommon for these sources to contain high levels of FIB. High levels of contamination were occasionally reported for boreholes and piped water, which are typically perceived as high quality and lower risk. Risk factors for microbial contamination of piped supplies include intermittency [198] and inadequate chlorination [20],[355]. For boreholes and dug wells, the reasons for fecal contamination can be more difficult to ascertain owing to the possibility of aquifer contamination and/or inadequate sanitary completion. In many cases contamination is associated with poor hygiene and inadequate sanitation, but specific risks can be readily identified through sanitary inspection of the water source and its surroundings and may in part explain the heterogeneity in FIB concentrations observed for a given source type.
Studies included in this review show great variability in water quality for water sources of a given type, suggesting considerable scope for reducing exposure to fecal contamination through systematic management of water safety. Microbial water quality displays substantial heterogeneity between studies and we found a high I2 when comparing improved and unimproved sources from the same study (I2 = 80.3% [72.9–85.6]; Figure 4).
Across all studies, we find a higher risk of contamination in rural areas compared to urban areas (OR = 2.37 [1.47–3.81], p<0.001; Table 4) and a higher risk of contamination in low-income countries (OR = 2.37 [1.52–3.71], p<0.001; Table 4). Higher risk in rural areas is consistent with a recent multi-country study of over 25,000 hand pumps, which found greater risk of non-functioning water sources in areas distant from district centers [356].
There is some evidence to suggest that overall water quality has gradually improved over time. Publication year was associated with the proportion of samples containing FIB (OR 0.96 [0.93–1.00], p = 0.023; Table 4); this may reflect a progressive trend towards greater use of source types associated with less contamination and potentially a lessening of population-level exposure.
We found only limited published data on sanitary risk, suggesting that sanitary risk inspection techniques are not widely used and/or reported, despite being well-established in national and international drinking-water guidelines [12]. Studies report sanitary risks in some sources that are not found to contain detectable FIB, indicating that infrequent monitoring does not provide assurance of water safety. Conversely, low sanitary risk scores are reported for water sources that contain FIB, indicating that sanitary inspections alone do not capture water safety fully. Although individual studies report different levels of sanitary risk, the data are too few to draw general conclusions. Moreover, the use of different questions for each source type and their equal weighting limits the comparability of sanitary risk scores. While a simple prevalence of factor scoring is unlikely to be appropriate in more complex systems, there has been progress in developing scoring for quality of water safety plans and higher scoring utilities have been linked to both improved water quality and health outcomes [357].
Limitations of the Review
At the outcome level, our principal analyses are based on the proportion of samples detecting FIB rather than compliance to health guidelines over the course of a year. These prevalence measures overestimate compliance to health guidelines and national standards that require minimum sampling frequencies. Furthermore, where the sample volume is less than the recommended 100 ml [12], contamination is less likely to be detected and will be detected less frequently.
At the study level, our review was limited by infrequent reporting of a consistent measure of central tendency (or of individual sample data), sanitary risk inspections, and stored water quality. In analysis, we combine studies that used diverse sample handling and microbiological analytical methods and these factors may account for some of the variability in reported water quality. The majority of the included studies were cross-sectional and do not provide information on temporal variability in water quality. Few studies achieved robust random selection of water sources, and few received high scores for study quality (14% with >9 out of 13; Figure S1) with description of quality control procedures, meeting handling criteria, and statement of season(s) of sampling most frequently omitted quality factors. Many studies, particularly of groundwaters, were excluded as we could not match water source types or determine whether they were “improved.”
At the review level, we may not have identified all studies that meet the inclusion criteria. To capture additional studies would have required the screening of tens of thousands of records, as we were unable to identify more specific search terms. Two sources of water quality information that could be used in future studies and monitoring: regulatory surveillance and utility quality control data are likely to be extensive and not well represented as they may not be published and publicly available. Publicly available data from these sources rarely matched our inclusion criteria, usually because of failure to report sample sizes or associate water quality with source type. We identified few studies in languages other than English despite conducting searches in four other languages, and several regions are underrepresented (Figure 3) including Caucasus and Central Asia and Oceania for which studies may be available in other languages. Since few studies separately report water quality in slums, we combined studies of slum and peri-urban populations with those taking place in formal urban areas and we were therefore unable to investigate intra-urban disparities [7]. There may be a small number of errors in the database; in the 10% of English language studies independent extraction <0.5% errors were identified.
There are two sources of bias that will tend to cause overestimation of the safety of improved sources. Firstly, for many source types, water (including unreliable piped systems, public standpipes, and wells) is collected at the source, carried to, and stored in the household—affording multiple opportunities for contamination—such that final water quality is often worse than in the associated source [34]. Few studies identified the source type of origin of stored water; those that did supported the suggestion that stored water is more frequently contaminated and contaminated at higher levels. Data interpretation is confounded because samples are not paired [204] or are temporally displaced. Despite the potential to improve matters, evidence for the impact of household water treatment on stored water quality is inconsistent [295],[358]. Secondly, most studies were cross-sectional. One-off or infrequent sampling overestimates safety by missing seasonal [359],[360] or sporadic contamination and longitudinal studies suggest seasonal effects can be substantial (Table S3).
Monitoring Implications: Improving on Improved
There is a widely perceived hierarchy for water source desirability, typically with piped-to-household-tap as the ideal. Such general hierarchies combine many aspects of water service and their value, including quantity, affordability, accessibility, and reliability or continuity of service as well as safety [361]. It has been argued that a more graduated approach to monitoring than the “improved”/“unimproved” dichotomy is required [24],[362]. These concepts were reflected in initial JMP working group proposals that called for the monitoring of “basic” and “intermediate” service levels representing improvements in quality, continuity of supply, and accessibility [30]. Such monitoring could contribute to assessing progressive realization of the Human Right to Water and Sanitation [363] and to encourage improvements in service delivery, including improvements within water source-type categories.
A variety of indicators could be used to improve global estimates of water safety. Account of safety could be enhanced by ranking or scoring improved source types according to the proportion of sources showing fecal contamination (in descending order): piped (treated), boreholes, protected springs, rainwater, piped (untreated), and protected dug wells. Although based on a posteriori analysis, we find that untreated piped supplies are much more likely to be contaminated than treated piped supplies (OR = 0.07 [95% CI 0.02–0.27], p<0.001; Table 4) and may usefully be considered separately. This ranking could be refined as more data become available but may have unintended consequences such as disincentivising improvements within each source type and could discourage the use of some source types in regions where these may in fact provide comparatively safe water. We find that bottled and sachet water are typically high quality, but their environmental sustainability has been questioned [318]. They are excluded from global progress efforts not for reasons of quality but because they provide insufficient quantity for domestic uses, such as cooking and hygiene, other than direct consumption.
Adjustment of the improved source indicator for country-specific source-type compliance with microbial water quality guidelines would capture the substantial heterogeneity both between and within countries, highlighting disparities in the use of safe drinking-water [364]. It would provide a more robust and consistent means of assessing safety than the use of source-type classifications alone and would enable improvements in quality to be reflected in monitoring. This review indicates that such an adjustment would have a large (downward) impact on international estimates of the number of people using safe drinking-water, in agreement with previous estimates based on five studies [8],[9].
Prevalent FIB are imperfect indices of water safety [26] and health risk. They are known to be more sensitive to chlorine than some important waterborne pathogens such as cryptosporidium [12], and can persist or multiply in some tropical waters [365]. These factors and the fact of temporal variability in water quality suggest that it would be strongly preferable to combine periodic measurement of water quality with assessment of sanitary status through sanitary inspection or water safety plans [29] in assessing water safety. These approaches can serve to highlight the condition, operation, and maintenance of water sources and provide a more complete picture than access to infrastructure alone. Such approaches would benefit from standardization to ensure comparability. However, adjustment for the proportion of samples containing FIB would not account for all hazards to health, including the two major chemical hazards: fluoride [366],[367] and arsenic [368]. The JMP has outlined an approach that could be taken that combines a hierarchy of measures of water quality with sanitary risk or management data [22].
Cost-effectiveness arguments suggest basing future monitoring efforts on both regulatory and utility data and building monitoring capacity especially in peri-urban and rural areas of low-income countries. Special initiatives such as dedicated water safety surveys (e.g., RADWQ) and integration of water quality testing in household surveys are likely to play an interim role and will assist in filling gaps in the available data [22].
Implications for Public Health Policy
Our review provides strong evidence that by equating “improved” with “safe,” the number of people with access to a safe water source has been greatly overstated, and suggests that a large number and proportion of the world's population use unsafe water. We analyze the implications following a framework of health sector functions in environmental health [369] and highlight key implications for policy in Box 1.
Box 1. Key Implications for Policy
-
Fecal contamination of drinking-water is widespread globally, especially in low-income countries and rural areas, and affects many improved sources.
-
The Global Burden of Disease 2010 study may greatly underestimate diarrheal disease burden by assuming zero risk from improved sources.
-
Adjustment of safe drinking-water coverage estimates for water quality and ideally sanitary risk would highlight disparities and enable improvements in quality to be reflected in monitoring.
-
Piped water is not a panacea: high levels of contamination have been reported in a range of settings and water stored in the household, often motivated by an intermittent or distant source, is more likely to be contaminated, especially in rural areas.
-
Quality and sanitary risks are heterogeneous indicating that it is possible to substantially enhance safety and reduce exposure through incremental improvements in service.
-
Greater use should be made of sanitary inspections as these provide a complementary means of assessing safety and are able to identify corrective actions to prevent contamination.
-
Studies of microbial contamination and sanitary risk could be improved by adhering to higher standards, including those outlined in our quality criteria.
Policy makers
Health policy makers framing post-2015 goals for achieving universal health coverage and reducing the global burden of disease need to ensure that targets and indicators go beyond health care services and address underlying determinants of health including progressive improvements in access to safe drinking-water, sanitation, and hygiene services. Adequate quantities of safe water at home are essential for good health [370] and, together with improvements in sanitation and hygiene, considered one of the more cost-effective interventions to protect and improve public health [371].
Through the process of implementing the MDGs, great strides have been made in increasing coverage of improved water sources although an estimated 783 million people still lack an improved source, most living in sub-Saharan Africa and Southeast Asia [5]. This review confirms that there are also pronounced disparities in access to “safe” water between and within countries. The Human Right to Water and Sanitation calls for progressive reduction in inequalities, and public health considerations suggest that reducing exposure among the most vulnerable, including the poor, undernourished, and immunocompromised, is a key public health concern. Health policy makers therefore have an important role to play in advocating for health protecting policies in other sectors, including those by actors concerned with water supply services.
Standard setting for water quality
The health sector plays a substantive role in drinking-water quality standard setting in many countries; this is logical since the underlying rationale is based on health concerns. The evidence presented here suggests that failures in water safety are frequent in LMICs, and that effective standard setting would combine outcomes measures (such as the measurement of FIB) with the verification of preventive or protective measures through sanitary inspection, water safety plans, or similar approaches. Future standard setting should take account of inequalities in access so as to direct efforts to those most affected and be informed by the availability of effective interventions.
Surveillance
The health sector also plays an important role in environmental health including drinking-water quality surveillance in many countries. However in many LMICs surveillance of water quality is limited outside large consolidated urban centers and enforcement of guidelines can be weak [372]. Surveillance is typically weakest in rural areas where levels of access to “improved sources” are lowest and the likelihood of contamination is greatest. The lack of disaggregated data for peri-urban and urban slum areas is also a problem in many countries. To-date the focus of public health policy, targets, and monitoring has been a household's primary source of drinking-water [373], but there is growing concern over inadequate water, sanitation, and hygiene services in non-household settings, such as schools and health care facilities [30],[373]. A public health perspective suggests that health sector surveillance should focus particularly on these settings where the risk of exposure is high.
Health care settings
Ensuring adequate environmental health in health care settings is a key responsibility of the health profession [374], but in many countries access to water, sanitation, and hygiene in health care facilities remains inadequate. While data were few, those studies that addressed water safety in health care settings documented water safety deficiencies [142],[235]. Furthermore, nationally representative surveys of health care facilities in Uganda and Rwanda found two-thirds of health care facilities to lack an improved water source [375],[376]. There are also opportunities to better protect and improve health through incorporation of water safety components in health programs such as those focused on specific diseases [377] or sensitive life stages such as maternal and child health [378]. Given the vulnerability of the populations using them and the potential for health facilities to serve as models for the wider community, the health sector has both a duty of care and an opportunity to advance health through better management of water safety in its own facilities.
Outbreak investigation
Despite clear evidence of its prevalence, the global burden of disease attributable to fecal contamination of drinking-water remains poorly understood. Data on outbreaks of waterborne disease [19] and of the impact of interventions to improve water quality on endemic disease [15],[16] provide evidence of the importance of fecal contamination. The associated studies frequently provide only weak insight into causal factors that might otherwise contribute to improved preventive action. There is an opportunity to enhance current outbreak investigation, advancing its role from one of curtailment to general prevention, thereby improving the ability to retrieve information that can be generalized for future prevention.
Waterborne disease burden
Study of the national and global burden of disease provides an opportunity to enhance public health protection and increase cost-effective action by focusing efforts on disease burdens and risk factors of greatest significance. The recent Global Burden of Disease study [379] based its estimates on the assumption of zero risk for those supplied by improved drinking-water sources and no additional benefit of a piped supply on premises [354]. The findings of this review indicate that fecal contamination of drinking-water is widespread, particularly in rural areas and low-income countries. Some improved source types, especially protected dug wells and protected springs, are frequently and sometimes highly contaminated. Contamination reported in piped supplies in especially rural but also urban areas is concerning given that these serve the majority (63%; Table 1) of the world's population and the use of this source type is expanding rapidly in many countries, especially in China [380]. In assuming improved sources are safe [379], current estimates may greatly underestimate waterborne disease burden and this gap would be expected to grow as improved source coverage increases.
Implications for Research
We have applied analytical tools usually associated with the medical sciences (meta-analysis of prevalence) to the study of environmental contaminants. There are differences in the underlying data that merit highlighting and limit the transferability of these techniques. Firstly, robust sampling frames are usually available for the selection of households (e.g., from national statistical offices), but random selection of water sources is more challenging. Studies such as RADWQ that adapted stratified cluster sampling techniques used in household surveys to address this problem have been subject to methodological criticism [381]. Future directions to achieve representative samples could include the use of water point mapping [382] or satellite imagery to create lists of water points but both are relatively complex and approaches based on population may be more feasible. Secondly, whereas epidemiological studies seek to measure outcome variables such as the number of events at a given point in time (“point prevalence”) or the rate at which they occur (“incidence”), environmental studies often seek to determine whether a threshold condition of safety has been exceeded (“compliance”). Even brief failures in safety may negate much of the potential health benefits of otherwise safe water [383],[384]. As a consequence, assessments of safety can be particularly susceptible to the frequency of monitoring and temporal representativity can be as important as spatial or population-based randomization.
In order to assess levels of compliance with regulatory standards or international guidelines based on infrequent surveys or limited data, research is required to understand the effect of repeated sampling of both source and stored water that results from variability over time (e.g., seasonality) and replicate sampling (sequential testing). Given their potential to inform assessments of safety, there is also a need to improve understanding of the relative importance of sanitary risks and their temporal variation for which very limited data are currently available. There is unlikely to be a simple correlation between sanitary risks and microbial contamination [103],[209] but the predictive value of sanitary risks may be much higher when accounting for compliance over time because some infrastructure failures will only lead to contamination in the presence of a co-factor such as rainfall. There is also a need to better understand the role of water collection and storage on microbial contamination and the associated risk to health.
We find strong evidence of differences in water quality between rural and urban areas, including for piped supplies. Further work is needed to characterize intra-urban differences; we encourage randomized water quality surveys to include slum or peri-urban populations as part of the sampling frame. Finally, the approaches taken in this review could be extended to other drinking-water contaminants, such as arsenic and fluoride.
Conclusions
Fecal contamination of drinking-water in LMICs is widespread. We demonstrate that improved sources are in general safer than unimproved sources of drinking-water, but they are not universally nor consistently free of fecal contamination. In 38% of 191 studies at least a quarter of samples from improved sources exceeded WHO recommended levels of FIB. By equating “use of an improved source” with “safe,” international estimates greatly overstate access to safe drinking-water. Substantial differences are observed in the presence and levels of contamination between countries, between urban and rural regions, and between water source types. Infrequent measurements of water quality alone tend to overestimate safety and so an improved future strategy would combine sanitary status with water quality measurements.
Supporting Information
Zdroje
1. Committee on Economic Socal and Community Rights (2002) General comment no. 15, The right to water. UN Doc. E/C.12/2010. New York: United Nations.
2. United Nations (2010) Resolution on Human Right to Water and Sanitation. United Nations General Assembly. A/64/292. New York: United Nations.
3. United Nations (2013) UN Goal 7: Ensure Environmental Sustainability. Available: http://www.un.org/millenniumgoals/environ.shtml. Accessed 1 December 2013.
4. UNICEF/WHO Joint Monitoring Programme: definitions and methods. Available: http://www.wssinfo.org/definitions-methods/. Accessed 1 December 2013.
5. WHO/UNICEF (2013) Progress on Sanitation and Drinking-Water: 2012 Update WHO/UNICEF. Available: http://www.wssinfo.org/definitions-methods. Accessed 1 December 2013.
6. GodfreyS, LabhasetwarP, WateS, PimpalkarS (2011) How safe are the global water coverage figures? Case study from Madhya Pradesh, India. Environ Monit Asses 176 : 561–574.
7. Schäfer D, Werchota R, Dölle K (2007) MDG monitoring for urban water supply and sanitation: catching up with reality in Sub-Saharan Africa. Eschborn: Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ).
8. BainRES, GundrySW, WrightJA, YangH, PedleyS, et al. (2012) Accounting for water quality in monitoring access to safe drinking-water as part of the Millennium Development Goals: lessons from five countries. Bull World Health Organ 90 : 228–235A.
9. OndaK, LoBuglioJ, BartramJ (2012) Global access to safe water: accounting for water quality and the resulting impact on MDG progress. Int J Environ Res Public Health 9 : 880–894.
10. Payen G (2011) Worldwide needs for safe drinking water are underestimated: billions of people are impacted. Paris: AquaFed.
11. White GF, Bradley DJ, White AU (1972) Drawers of water: domestic water use in East Africa. Chicago: The University of Chicago Press.
12. WHO (2011) Guidelines for drinking-water quality. Geneva: World Health Organization.
13. LiuL, JohnsonHL, CousensS, PerinJ, ScottS, et al. (2012) Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379 : 2151–2161.
14. EsreySA, FeachemRG, HughesJM (1985) Interventions for the control of diarrhoeal diseases among young children: improving water supplies and excreta disposal facilities. Bull World Health Organ 63 : 757–772.
15. FewtrellL, ColfordJMJr (2005) Water, sanitation and hygiene in developing countries: interventions and diarrhoea—a review. Water Sci Technol 52 : 133–142.
16. ClasenT, RobertsI, RabieT, SchmidtW, CairncrossS (2006) Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev 19: CD004794.
17. WaddingtonH, SnilstveitB (2009) Effectiveness and sustainability of water, sanitation, and hygiene interventions in combating diarrhoea. Journal of Development Effectiveness 1 : 295–335.
18. CairncrossS, HuntC, BoissonS, BostoenK, CurtisV, et al. (2010) Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol 39: i193–i205.
19. Ligon GC (2012) Waterborne disease outbreaks: a systematic review of the health effects of drinking water system failures, Chapel Hill (North Carolina): University of North Carolina at Chapel Hill.
20. CraunGF, BrunkardJM, YoderJS, RobertsVA, et al. (2010) Causes of Outbreaks Associated with Drinking Water in the United States from 1971 to 2006. Clin Microbiol Rev 23(3): 507–528.
21. SchmidtWP, CairncrossS (2009) Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol 43 : 986–992.
22. WHO/UNICEF (2010) JMP Technical Task Force Meeting on Monitoring Drinking-water Quality. Geneva and New York: WHO/UNICEF Joint Monitoring Programme. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/JMP-Task-Force-Meeting-on-Monitoring-Drinking-water-Quality.pdf. Accessed 1 December 2013.
23. BainRES, BartramJK, ElliottM, MatthewsRL, McMahanL, et al. (2012) A summary catalogue of microbial drinking water tests for low and medium resource settings. Int J Environ Res Public Health 9 : 1609–1625.
24. LloydBJ, BartramJK (1991) Surveillance solutions to microbiological problems in water-quality control in developing-countries. Water Sci Technol 24 : 61–75.
25. WHO (1997) Guidelines for Drinking-Water Quality. Geneva: World Health Organization
26. Gleeson C, Gray NF (1997) The coliform index and waterborne disease: problems of microbial drinking water assessment. London; New York: E & FN SPON. xii, 194 p. p.
27. WHO (2012) Water safety planning for small community water supplies: Step-by-step risk management guidance for drinking-water supplies in small communities. Geneva: World Health Organization.
28. Prescott SC, Winslow CEA (1904) Elements of Water Bacteriology, with special reference to sanitary water analysis (1904). New York: John Wiley & Sons.
29. Davison A, Howard G, Stevens M, Callan P, Fewtrell L, et al.. (2005) Water safety plans: Managing drinking-water quality from catchment to consumer. Geneva: World Health Organization.
30. WHO/UNICEF (2013) Proposal for consolidated drinking water, sanitation and hygiene targets, indicators and definitions. Geneva and New York: WHO/UNICEF. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/A-proposal-for-consolidated-WASH-goal-targets-definitions-and-indicators_version7_Nov22_final.pdf. Accessed 1 December 2013.
31. MoherD, LiberatiA, TetzlaffJ, AltmanDG (2009) Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097.
32. UN (2013) Millennium Development Indicators: World and regional groupings. Available: http://mdgs.un.org/unsd/mdg/Host.aspx?Content=Data/RegionalGroupings. Accessed 1 December 2013.
33. OpryszkoMC, HuangH, SoderlundK, SchwabKJ (2009) Data gaps in evidence-based research on small water enterprises in developing countries. J Water Health 7 : 609–622.
34. WrightJ, GundryS, ConroyR (2004) Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health 9 : 106–117.
35. WrightJA, YangH, WalkerK, PedleyS, ElliottJ, et al. (2012) The H(2)S test versus standard indicator bacteria tests for faecal contamination of water: systematic review and meta-analysis. Trop Med Int Health 17 : 94–105.
36. United Nations Department of Economic and Social Affairs (2012) World Population Prospects: The 2012 Revision. Available: http://esa.un.org/wpp/. Accessed 1 December 2013.
37. RompreA, ServaisP, BaudartJ, de-RoubinMR, LaurentP (2002) Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods 49 : 31–54.
38. WHO/UNICEF (2006) Core questions on drinking-water and sanitation for household surveys WHO/UNICEF. Geneva and New York: WHO/UNICEF. Available: http://www.who.int/water_sanitation_health/monitoring/oms_brochure_core_questionsfinal24608.pdf. Accessed 1 December 2013.
39. World Bank (2013) Country and lending groups. Available: http://data.worldbank.org/about/country-classifications/country-and-lending-groups. Accessed 1 December 2013.
40. SahaS, ChantD, WelhamJ, McGrathJ (2005) A systematic review of the prevalence of schizophrenia. PLoS Med 2: e141.
41. McBride GB (2005) Using statistical methods for water quality management: issues, problems, and solutions. Hoboken (New Jersey): Wiley-Interscience. xxiv, 313 p. p.
42. Borenstein M (2009) Introduction to meta-analysis. Chichester, U.K.: John Wiley & Sons. xxviii, 421 p. p.
43. WartonDI, HuiFK (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92 : 3–10.
44. SweetingM, J. SuttonA, C. LambertP (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine 23 : 1351–1375.
45. Rodríguez G. (2007) Logit Models for Binary Data. Lecture Notes on Generalized Linear Models. Available: http://data.princeton.edu/wws509/notes/c3.pdf. Accessed 30 December 2013.
46. AbdellahAM, Abdel-MagidHM, YahiaNA (2012) Assessment of Drinking Water Microbial Contamination in Al-Butana Region of Sudan. Journal of Applied Sciences (Faisalabad) 12 : 856–862.
47. AberaS, ZeyinudinA, KebedeB, DeribewA, AliS, et al. (2011) Bacteriological analysis of drinking water sources. Afr J Microbiol Res 5 : 2638–2641.
48. Abu AmrSS, YassinMM (2008) Microbial contamination of the drinking water distribution system and its impact on human health in Khan Yunis Governorate, Gaza Strip: seven years of monitoring (2000–2006). Public Health 122 : 1275–1283.
49. Abu MaylaYS, Abu AmrSS (2010) Chemical and microbiological quality of drinking water in Gaza Strip, Palestine. Science Vision 10 : 80–88.
50. AbusafaA, ArafatHA, Abu-BakerM, KhaliliKN (2012) Utilisation of drinking water from rainwater-harvesting cisterns in the Palestinian territories: assessment of contamination risk. Int J Environment and Waste Management 9 : 358–371.
51. Addo KK, Mensah GI, Bekoe M, Bonsu C, Akyeh ML (2009) Bacteriological quality of sachet water produced and sold in Teshie-Nungua suburbs of Accra, Ghana. African Journal of Food Agriculture Nutrition and Development 9.
52. AdmassuM, WubshetM, GelawB (2004) A survey of bacteriological quality of drinking water in North Gondar. Ethiop J Health Dev 18 : 112–115.
53. AgaT, BekaNC, EziashiAC (2010) Spatial variation in groundwater quality of Jos metropolis and environs, north-central Nigeria. Continental J Environmental Sciences 4 : 1–11.
54. AgardL, AlexanderC, GreenS, JacksonM, PatelS, et al. (2002) Microbial quality of water supply to an urban community in Trinidad. Journal of Food Protection 65 : 1297–1303.
55. AhmadM, BajahlanAS (2009) Quality comparison of tap water vs. bottled water in the industrial city of Yanbu (Saudi Arabia). Environ Monit Asses 159 : 1–14.
56. AhmedK, AhmedM, AhmedJ, KhanA (2012) Risk Assessment by Bacteriological Evaluation of Drinking Water of Gilgit-Baltistan. Pakistan Journal of Zoology 44 : 427–432.
57. Ahmed MF, Shamsuddin SAJ, Mahmud SG, Rashid HU, Deere D, et al.. (2005) Risk Assessment of Arsenic Mitigation Options (RAAMO). Dhaka, Bangladesh: APSU.
58. AhmedSA, HoqueBA, MahmudA (1998) Water management practices in rural and urban homes: a case study from Bangladesh on ingestion of polluted water. Public Health 112 : 317–321.
59. AhoussiKE, KoffiYB, KouassiAM, OsemwegieI, BiémiJ (2013) Influence of Anthropogenic Activities of Groundwater from Hand Dug Wells within the Precarious Settlements of Southern Abidjan, Côte d′Ivoire: Case of the Slums of Anoumabo (Marcory) and Adjouffou (Port-Bouët). Journal of Water Resource and Protection 5 : 427–439.
60. AkoachereJF, OmamLA, MassallaTN (2013) Assessment of the relationship between bacteriological quality of dug-wells, hygiene behaviour and well characteristics in two cholera endemic localities in Douala, Cameroon. BMC Public Health 13 : 692.
61. Al-KhatibIA, ArafatHA (2009) Chemical and microbiological quality of desalinated water, groundwater and rain-fed cisterns in the Gaza strip, Palestine. Desalination 249 : 1165–1170.
62. Al-SalaymehA, Al-KhatibIA, ArafatHA (2011) Towards Sustainable Water Quality: Management of Rainwater Harvesting Cisterns in Southern Palestine. Water Resour Manag 25 : 1721–1736.
63. AlbertJ, LuotoJ, LevineD (2010) End-User Preferences for and Performance of Competing POU Water Treatment Technologies among the Rural Poor of Kenya. Environ Sci Technol 44 : 4426–4432.
64. Aldana JM (2010) Rapid assessment of drinking-water quality in the Republic of Nicaragua: country report of the pilot project implementation in 2004–2005. Geneva: WHO/UNICEF. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/RADWQ_Nicaragua.pdf. Accessed 1 December 2013.
65. Aliev S, Shodmonov P, Babakhanova N, Schmoll O (2010) Rapid assessment of drinking-water quality in the Republic of Tajikistan: country report of the pilot project implementation in 2004–2005. Geneva: WHO/UNICEF. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/RADWQ_Tajikistan.pdf. Accessed 1 December 2013.
66. AlvesNC, OdorizziAC, GoulartFC (2002) Microbiological analysis of mineral water and drinking water of reservoir supplies, Brazil. Rev Saúde Pública 36 : 749–751.
67. AmoueiA, MiranzadehMB, ShahandehZ, TaheriT, AsgharniaHA, et al. (2012) A Study on the Microbial Quality of Drinking Water in Rural Areas of Mazandaran Province in North of Iran (2011). Journal of Environmental Protection 3 : 605–605.
68. AmpofoJA, AndohA, TettehW, BelloM (2007) Microbiological quality and health risks of packaged water produced in southern Ghana. Journal of Applied Science and Technology 12 : 88–97.
69. AnY-J, BreindenbachGP (2005) Monitoring E-coli and total coliforms in natural spring water as related to recreational mountain areas. Environ Monit Asses 102 : 131–137.
70. AndradeCdS, LeiteCC, da SilvaMD, de AssisPN, GuimaraesAG (2004) Quality of water used in the beach huts of Salvador-Bahia seashore microbiological aspects. Revista do Instituto Adolfo Lutz 63 : 215–219.
71. ArnoldM, VandersliceJA, TaylorB, BensonS, AllenS, et al. (2013) Drinking water quality and source reliability in rural Ashanti region, Ghana. J Water Health 11 : 161–172.
72. AugoustinosMT, VenterSN, KfirR (1995) Assessment of water quality problems due to microbial growth in drinking water distribution systems. Environ Toxic Water 10 : 295–299.
73. Austin CJ (1993) Chlorinating household water in The Gambia. 19th WEDC conference. Accra, Ghana: WEDC.
74. AwuahE, NyarkoKB, OwusuPA, Osei-BonsuK (2009) Small town water quality. Desalination 248 : 453–459.
75. AyantoboOO, OluwasanyaGO, IdowuOA, EruolaAO (2012) (2012) Water Quality Evaluation of Hand-dug Wells in Ibadan, Oyo State, Nigeria. Special Publication of the Nigerian Association of Hydrological Sciences 231–239.
76. AydinA (2007) The microbiological and physico-chemical quality of groundwater in West Thrace, Turkey. Pol J Environ Stud 16 : 377–383.
77. BahramA, HamidB, AkramN (2012) Estimation of coliform contamination rate and impact of environmental factor on bacterial quality of tube well water supplies in Khorramdarreh County, Iran. Afr J Biotechnol 11 : 7912–7915.
78. BaigSA, XuX, Navedullah, MuhammadN, KhanZU, et al. (2012) Pakistan's drinking water and environmental sanitation status in post 2010 flood scenario: humanitarian response and community needs. Journal of Applied Sciences in Environmental Sanitation 7 : 49–54.
79. BakerKK, SowS, KotloffKL, NataroJP, FaragTH, et al. (2013) Quality of piped and stored water in households with children under five years of age enrolled in the Mali Site of the Global Enteric Multi-Center Study (GEMS). Am J Trop Med Hyg 89(2): 214–22.
80. BarbosaDA, LageMM, BadaroACL (2009) Microbiological quality of water drinking fountains of a university campus in Ipatinga, Minas Gerais. Nutrir Gerais 3 : 505–517.
81. Barthiban S, Lloyd BJ (2012) Validity of the application of open dug well sanitary survey methodology in the development of a water safety plan in the Maldives islands. Brebbia CA, Zubir SS, editors. Management of natural resources, sustainable development and ecological hazards III. Ashurst, UK: Wit Press. pp. 369–380.
82. BarthibanS, LloydBJ, MaierM (2012) Sanitary hazards and microbial quality of open dug wells in the Maldives Islands. Journal of Water Resource and Protection 4 : 474–474.
83. Bartram JK (1996) Optimizing the monitoring and assessment of rural water supplies. Guildford, UK: University of Surrey.
84. BegumJ, AhmedK, BoraKN (2004) Isolation and identification of coliform bacteria from different sources of drinking water. Nature, Environment and Pollution Technology 3 : 51–53.
85. BesserRE (1995) Prevención de la transmisión del cólera: evaluación rápida de la calidad del agua municipal en Trujillo, Perú. Boletin - Oficina Sanitaria Panamericana 119 : 189–194.
86. BharathJ, MosodeenM, MotilalS, SandyS, SharmaS, et al. (2003) Microbial quality of domestic and imported brands of bottled water in Trinidad. Int J Food Microbiol 81 : 53–62.
87. BordaloAA, Savva-BordaloJ (2007) The quest for safe drinking water: an example from Guinea-Bissau (West Africa). Water Research 41 : 2978–2986.
88. BrachoMG, MoronV, LuzardoM, MontielM, BoteroL (2008) Hepatitis A virus, adenovirus 40–41 and bacteriophages in water for human consumption. Ciencia (Maracaibo) 16 : 271–278.
89. Broshears RE, Amin Akbari M, Chornack MP, Mueller DK, Ruddy BC (2005) Inventory of ground-water resources in the Kabul Basin, Afghanistan. Reston (Virginia): U.S. Geological Survey (USGS).
90. Camara O (2011) Pollution microbiologique des eaux souterraines dans le quartier Tanghin de Ouagadougou: etats des lieux et perspectives. Ouagadougou, Burkina Faso: Foundation 2iE.
91. Cardoso ALSP, Tessari ENC, de Castro AGM, Kanashiro AMI (2001) Avaliação da qualidade microbiológica de águas minerais comercializadas em supermercados da cidade de Alfenas. Higiene Alimentar 15.
92. CarrascoL, MenaKD, MotaLC, OrtizM, BehraveshCB, et al. (2008) Occurrence of faecal contamination in households along the US-Mexico border. Lett Appl Microbiol 46 : 682–687.
93. CaylakE, TokarM (2012) Metallic and microbial contaminants in drinking water of Cankiri, Turkey. E-Journal of Chemistry 9 : 608–614.
94. ChaidezC, SotoM, MartinezC, KeswickB (2008) Drinking water microbiological survey of the Northwestern State of Sinaloa, Mexico. J Water Health 6 : 125–130.
95. ChemulitiJK, GathuraPB, KyuleMM, NjeruhFM (2002) Bacteriological qualities of indoor and out-door drinking water in Kibera sub-location of Nairobi, Kenya. East Afr Med J 79 : 271–273.
96. ChenD, LanL (2011) The analysis of drinking-water sanitation in cities and towns of Nanping in the year 2008-2010. Chinese Journal of Health Laboratory Technology 21 : 2519–2521.
97. Chung C (2011) The challenges of a water system management handover in Eastern Ethiopia - from the United Nations Refugee Agency to a local community. Cambridge: Massachusetts Institute of Technology.
98. ClasenT, SaeedTF, BoissonS, EdmondsonP, ShipinO (2007) Household water treatment using sodium dichloroisocyanurate (NaDCC) tablets: a randomized, controlled trial to assess microbiological effectiveness in Bangladesh. Am J Trop Med Hyg 76 : 187–192.
99. CoelhoDA, Faria e SilvaPM, VeigaSMOM, FioriniJE (2007) Avaliação da qualidade microbiológica de águas minerais comercializadas em supermercados da cidade de Alfenas. Higiene Alimentar 21 : 88–92.
100. Coloru B, Mgaya S, Taubert R (2012) Appropriate technologies for rural water supply: a comparative study between “rope pumps” and conventional piston-pumps on water quality and other sustainability parameters. Milan: ACRA/SHIPO.
101. CopelandCC, BeersBB, ThompsonMR, FitzgeraldRP, BarrettLJ, et al. (2009) Faecal contamination of drinking water in a Brazilian shanty town: importance of household storage and new human faecal marker testing. J Water Health 7 : 324–331.
102. Costello DH (2013) An Evaluation of a Water, Sanitation, and Hygiene (WASH) Program for rural communities in Northern Afghanistan. Corvallis: Oregon State University.
103. CroninAA, BreslinN, GibsonJ, PedleyS (2006) Monitoring source and domestic water quality in parallel with sanitary risk identification in northern Mozambique to prioritise protection interventions. J Water Health 4 : 333–345.
104. CuiB, DengH, JingY, YueF, YuQ, et al. (2012) Investigation on the quality of groundwater in Rural Fengtai District. Journal of Environmental Hygiene 2 : 280–284.
105. da SilvaMEZ, SantanaRG, GuilhermettiM, FilhoIC, EndoEH, et al. (2008) Comparison of the bacteriological quality of tap water and bottled mineral water. Int J Hyg Environ Health 211 : 504–509.
106. DadaAC (2009) Sachet water phenomenon in Nigeria: Assessment of the potential health impacts. Afr J Microbiol Res 3 : 15–21.
107. DaoudAK, SwailehKM, HusseinRM, MataniM (2011) Quality assessment of roof-harvested rainwater in the West Bank, Palestinian Authority. J Water Health 9 : 525–533.
108. de SaLLC, de JesusIM, SantosECO, ValeER, LoureiroECB, et al. (2005) Microbiological quality of drinking water in two areas following sanitation interventions – Belém, Pará State, Brazil. Epidemiol Serv Saude 14 : 171–180.
109. de SiqueiraLP, ShinoharaNK, de LimaRM, de Paiva JdoE, de Lima FilhoJL, et al. (2010) Microbiological evaluation of drinking water used in feeding units. Cien Saude Colet 15 : 63–66.
110. DegbeyC, MakoutodeM, FayomiB, BrouwerCD (2010) La qualité de l ′ eau de boisson en milieu professionnel à Godomey en 2009 au Bénin Afrique de l′Ouest. J Int Santé Trav 1 : 15–22.
111. DegbeyC, MakoutodeM, OuendoEM, FayomiB, De BrouwerC (2008) The quality of well water in the municipality of Abomey-Calavi in Benin. Environnement Risques & Sante 7 : 279–283.
112. DesmarchelierP, LewA, CaiqueW, KnightS, ToodayanW, et al. (1992) An evaluation of the hydrogen sulphide water screening test and coliform counts for water quality assessment in rural Malaysia. Trans R Soc Trop Med Hyg 86 : 448–450.
113. Devoto F, Duflo E, Dupas P, Pariente W, Pons V (2011) Happiness on tap: piped water adoption in Urban Morocco. Cambridge (Massachusetts): National Bureau of Economic Research.
114. DongolB, MerzJ, SchaffnerM, NakarmiG, ShahP, et al. (2005) Shallow groundwater in a middle mountain catchment of Nepal: quantity and quality issues. Environ Geol 49 : 219–229.
115. dos ReisJAP (1998) Perfil Higienico sanitario das aguas de consumo do distrito federal. Revista de Saude do Distrito 9 : 32–35.
116. EgwariL, AboabaOO (2002) Environmental impact on the bacteriological quality of domestic water supplies in Lagos, Nigeria. Revista de Saude Publica 36 : 513–520.
117. EhlersMM, van ZylWB, PavlovDN, MullerEE (2004) Random survey of the microbial quality of bottled water in South Africa. Water SA 30 : 203–210.
118. EjechiBO, OlobaniyiSB, OgbanFE, UgbeFC (2007) Physical and sanitary quality of hand-dug well water from oil-producing area of Nigeria. Environ Monit Assess 128 : 495–501.
119. EjechiEO, EjechiBO (2008) Safe drinking water and satisfaction with environmental quality of life in some oil and gas industry impacted cities of Nigeria. Soc Indic Res 85 : 211–222.
120. El-SalamMM, Al-GhitanyEM, KassemMM (2008) Quality of bottled water brands in Egypt Part II: biological water examination. J Egypt Public Health Assoc 83 : 468–486.
121. EldinMNA, MadanyIM, Al-TayaranA, Al-JubairAH, GomaaA (1993) Quality of water from some wells in Saudi Arabia. Water Air Soil Poll 66 : 135–143.
122. Empereur-BissonnetP, SalzmanV, MonjourL (1992) Evaluation of a new type of transport and storage recipient for improving the quality of drinking-water in rural african areas. Bulletin De La Societe De Pathologie Exotique 85 : 390–394.
123. EscamillaV, KnappettPSK, YunusM, StreatfieldPK, EmchM (2013) Influence of latrine proximity and type on tubewell water quality and diarrheal disease in Bangladesh. Ann Assoc Am Geogr 103 : 299–308.
124. EshcolJ, MahapatraP, KeshapaguS (2009) Is fecal contamination of drinking water after collection associated with household water handling and hygiene practices? A study of urban slum households in Hyderabad, India. J Water Health 7 : 145–154.
125. EsterhuizenL, FosseyA, LuesJFR (2012) Dairy farm borehole water quality in the greater Mangaung region of the Free State Province, South Africa. Water SA 38 : 803–806.
126. Falcone-DiasMF, EmerickGL, Farache-FilhoA (2012) Mineral water: a microbiological approach. Wa Sci Technol 12 : 556–562.
127. Farache FilhoA, DiasMFF (2008) Qualidade microbiológica de águas minerais em galões de 20 litros. Alim Nutr 19 : 243–248.
128. Farache FilhoA, DiasMFF, TaromaruRH, CerqueiraCS, DuqueJD (2008) Qualidade microbiológica de águas minerais não carbonatadas em embalagens de 1,5 litros, comercializadas em araraquara-sp. Alim Nutr 19 : 421–425.
129. FergusonAS, LaytonAC, MaillouxBJ, CulliganPJ, WilliamsDE, et al. (2012) Comparison of fecal indicators with pathogenic bacteria and rotavirus in groundwater. Sci Total Environ 431 : 314–322.
130. FerrettiE, BonadonnaL, LucentiniL, Della LiberaS, SemproniM, et al. (2010) A case study of sanitary survey on community drinking water supplies after a severe (post-Tsunami) flooding event. Annali Dell Istituto Superiore Di Sanita 46 : 236–241.
131. FioreMM, MinningsK, FioreLD (2010) Assessment of biosand filter performance in rural communities in southern coastal Nicaragua: an evaluation of 199 households. Rural Remote Health 10 : 1483.
132. Flores-AbuxapquiJJ, Suarez-HoilGJ, Puc-FrancoMA, Heredia-NavarreteMR, Vivas-RoselMD, et al. (1995) Bacteriological quality of drinking water in the City of Merida, Mexico. Salud Publica Mex 37 : 236–239.
133. Frischknecht DS (2006) Análise de NMP de coliformes em águas minerais comercializadas no Distrito Federal. Brasília: Universidade de Brasília.
134. GarodeAM, NanotyVD, BodhankarMG (1998) Bacteriological status of drinking water in and around Chikhli town of Buldana district, Maharashtra. Pollution Research 17 : 293–294.
135. GaytanM, CastroT, BonillaP, LugoA, VilaclaraG (1997) Preliminary study of selected drinking water samples in Mexico City. Revista Internacional de Contaminacion Ambiental 13 : 73–78.
136. GelinasY, RandallH, RobidouxL, SchmitJ-P (1996) Well water survey in two districts of Conakry (Republic of Guinea), and comparison with the piped city water. Water Research 30 : 2017–2026.
137. Genthe B, Seager J, Vundule C, Strauss N, Maforah F (1996) The effect of water supply, handling and usage on water quality in relation to health indicies in developing communities Stellenbosch, South Africa: Water Research Council.
138. GhengheshKS, BelhajK, AlgauiA, AlturkiE, EltomiA (2004) Blessed water: bacteriological quality of drinking water obtained from mosques in Tripoli-Libya. Int J Antimicrob Ag 24: S150.
139. GonulSA, KarapinarM (1991) The microbiological quality of drinking water supplies of Izmir City: the incidence of Yersinia enterocolitica. Int J Food Microbiol 13 : 69–73.
140. GorterAC, AlbertsJH, GagoJF, SandifordP (1995) A randomized trial of the impact of rope-pumps on water quality. J Trop Med Hyg 98 : 247–255.
141. Grimason AM, Beattie TK, Morse TD, Masangwi SJ, Jabu GC, et al.. (2013) Classication and quality of groundwater supplies in the Lower Shire Valley, Malawi – part 2: classification of borehole water supplies in Chikhwawa, Malawi. Water SA 39.
142. GuedesZBL, OriáHF, BrittoNPB, NetoJWS, Silveira;JWd, et al. (2004) Controle sanitario da agua consumida nas unidades de saude do municipio de Fortaleza, CE. Higiene Alimentar 18 : 28–31.
143. GuilleminF, HenryP, UwechueN, MonjourL (1991) Faecal contamination of rural water supply in the Sahelian area. Water Research 25 : 923–927.
144. Guimaraes APRC, Serafini AB (2005) Avaliação da qualidade microbiológica de amostras de água mineral natural, envasada, comercializadas em Goiânia, Goiás. Goiânia, Brazil: UFG.
145. GundrySW, WrightJA, ConroyRM, Du PreezM, GentheB, et al. (2009) Child dysentery in the Limpopo Valley: a cohort study of water, sanitation and hygiene risk factors. J Water Health 7 : 259–266.
146. GuptaSK, SheikhMA, IslamMS, RahmanKS, JahanN, et al. (2008) Usefulness of the hydrogen sulfide test for assessment of water quality in Bangladesh. J Appl Microbiol 104 : 388–395.
147. GuptaSK, SuantioA, GrayA, WidyastutiE, JainN, et al. (2007) Factors associated with E. coli contamination of household drinking water among tsunami and earthquake survivors, Indonesia. Am J Trop Med Hyg 76 : 1158–1162.
148. GwimbiP (2011) The microbial quality of drinking water in Manonyane community: Maseru District (Lesotho). African Health Sciences 11 : 474–480.
149. Handzel T (1998) The effect of improved drinking water quality on the risk of diarrheal disease in an urban slum of Dhaka, Bangladesh: A home chlorination intervention trial. Chapel Hill: University of North Carolina at Chapel Hill.
150. HarunaR, EjobiF, KabagambeEK (2005) The quality of water from protected springs in Katwe and Kisenyi parishes, Kampala city, Uganda. Afr Health Sci 5 : 14–20.
151. HashmiI, FarooqS, QaiserS (2009) Chlorination and water quality monitoring within a public drinking water supply in Rawalpindi Cantt (Westridge and Tench) area, Pakistan. Environ Monit Asses 158 : 393–403.
152. HashmiI, FarooqS, QaiserS (2009) Incidence of fecal contamination within a public drinking water supply in Ratta Amral, Rawalpindi. Desalination and Water Treatment 11 : 124–131.
153. HenryFJ, RahimZ (1990) Transmission of diarrhoea in two crowded areas with different sanitary facilities in Dhaka, Bangladesh. J Trop Med Hyg 93 : 121–126.
154. HerathAT, AbayasekaraCL, ChandrajithR, AdikaramNKB (2012) Temporal variation of microbiological and chemical quality of noncarbonated bottled drinking water sold in Sri Lanka. J Food Sci 77: M160–M164.
155. Hira-SmithMM, YuanY, SavarimuthuX, LiawJ, HiraA, et al. (2007) Arsenic concentrations and bacterial contamination in a pilot shallow dugwell program in West Bengal, India. J Environ Sci Health A Tox Hazard Subst Environ Eng 42 : 89–95.
156. Holm RH (2012) Survey of water quality in northern Malawi, Africa, and communication of human health risk. Pullman: Washington State University.
157. Holt S (2009) A survey of water storage practices and beliefs in households in Bonao, Dominican Republic in 2005. Atlanta: Georgia State University.
158. HoqueBA, SackRB, SiddiqiM, JahangirAM, HazeraN, et al. (1993) Environmental health and the 1991 Bangladesh cyclone. Disasters 17 : 143–152.
159. HoqueBA, YamauraS, SakaiA, KhanamS, KarimM, et al. (2006) Arsenic mitigation for water supply in bangladesh: appropriate technological and policy perspectives. Water Qual Res J Can 41 : 226–234.
160. HorakHM, ChynowethJS, MyersWP, DavisJ, FendorfS, et al. (2010) Microbial and metal water quality in rain catchments compared with traditional drinking water sources in the East Sepik Province, Papua New Guinea. J Water Health 8 : 126–138.
161. Howard G, Luyima P (1999) Urban and peri-urban water supply surveillance programme report on water supply surveillance in ten selected urban areas of Uganda. Loughborough, UK: Loughborough University.
162. HubbardB, GeltingR, BaffigoV, SariskyJ (2005) Community environmental health assessment strengthens environmental public health services in the Peruvian Amazon. Int J Hyg Envir Heal 208 : 101–107.
163. HussainJ, HussainI, OjhaKG, SharmaKC (2001) Bacteriological pollution of ground water in textile city, Bhilwara, Rajasthan (India). Asian J Chem 13 : 1123–1126.
164. Ince M, Bashire D, Oni OOO, Awe EO, Ogbechie V, et al.. (2010) Rapid assessment of drinking-water quality in the Federal Republic of Nigeria: country report of the pilot project implementation in 2004–2005. Geneva: WHO/UNICEF. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/RADWQ_Nigeria.pdf. Accessed 1 December 2013.
165. Isaac-MarquezAP, Lezama-DavilaCM, Ku-PechPP, Tamay-SegoviaP (1994) Sanitary quality of water supply for human consumption in Campeche. Salud Publica Mex 36 : 655–661.
166. IslamMA, SakakibaraH, KarimMR, SekineM, MahmudZH (2011) Bacteriological assessment of drinking water supply options in coastal areas of Bangladesh. J Water Health 9 : 415–428.
167. ItamaE, OlasehaIO, SridharMKC (2006) Springs as supplementary potable water supplies for inner city populations: a study from Ibadan, Nigeria. Urban Water J 3 : 215–223.
168. JagalsP, BarnardTG, MokoenaMM, AshboltN, RoserDJ (2013) Pathogenic Escherichia coli in rural household container waters. Water Sci Technol 67 : 1230–1237.
169. JagalsP, BokakoTC, GrabowWOK (1999) Changing consumer water-use patterns and their effect on microbiological water quality as a result of an engineering intervention. Water SA 25 : 297–297.
170. JagalsP, GrabowWOK, WilliamsE (1997) The effects of supplied water quality on human health in an urban development with limited basic subsistence facilities. Water SA 23 : 373–378.
171. JayatillekeSK, GunawardanaSS (2009) Microbiological quality of well water in Kalutara District. Ceylon Med J 54 : 44–45.
172. Jiban SinghM, SomashekarRK, PrakashKL, ShivannaK (2009) Bacteriological assessment of groundwater in Arkavathi and Vrishabhavathi basins, Bangalore, Karnataka. Journal of Ecology and The Natural Environment 1 : 156–159.
173. JimmyDH, SundufuAJ, MalanoskiAP, JacobsenKH, AnsumanaR, et al. (2013) Water quality associated public health risk in Bo, Sierra Leone. Environ Monit Assess 185 : 241–251.
174. JoyaSA, MostofaG, YousufJ, IslamA, ElahiA, et al. (2006) One solution to the arsenic problem: a return to surface (improved dug) wells. J Health Popul Nutr 24 : 363–375.
175. JunaiduAU, AdamuRYA, IbrahimMTO (2001) Bacteriological assessment of pipe borne water in Sokoto metropolis, Nigeria. Tropical Veterinarian 19 : 160–162.
176. JungJH, YooCH, KooES, KimHM, NaY, et al. (2011) Occurrence of norovirus and other enteric viruses in untreated groundwaters of Korea. J Water Health 9 : 544–555.
177. KanyerereT, LevyJ, XuY, SakaJ (2012) Assessment of microbial contamination of groundwater in upper Limphasa River catchment, located in a rural area of northern Malawi. Water SA (Pretoria) 38 : 581–595.
178. KarimMR (2010) Microbial contamination and associated health burden of rainwater harvesting in Bangladesh. Water Sci Technol 61 : 2129–2135.
179. KarnchanawongS, KoottatepS, IkeguchiT (1993) Monitoring and evaluation of shallow well water quality near a waste disposal site. Environ Int 19 : 579–587.
180. KassengaGR (2007) The health-related microbiological quality of bottled drinking water solid in Dar es Salaam, Tanzania. J Water Health 5 : 179–185.
181. Kerala State Pollution Control Board (1991) The bacterial quality of water in selected wells in Kerala: an investigation. Trivandrum SEU-Kerala.
182. KhadseGK, KalitaM, PimpalkarSN, LabhsetwarPK (2011) Drinking water quality monitoring and surveillance for safe water supply in Gangtok, India. Environ Monit Asses 178 : 401–414.
183. KhadseGK, KalitaMD, LabhsetwarPK (2012) Change in drinking water quality from source to point-of-use and storage: a case study from Guwahati, India. Environ Monit Asses 184 : 5343–5361.
184. KhadseGK, KalitaMD, PimpalkarSN, LabhasetwarPK (2011) Surveillance of drinking water quality for safe water supply-a case study from Shillong, India. Water Resour Manag 25 : 3321–3342.
185. KhanSU, BangashFK (2001) Drinking water quality forecast of Peshawar valley on the basis of sample data. J Chem Soc Pakistan 23 : 243–252.
186. Khush R, London A, Arnold J, Ramaswamy K (2009) Evaluating the sustainability and impacts of water, sanitation & hygiene interventions. San Francisco: Aquaya.
187. Kilanko-Oluwasanya GO (2009) Better safe than sorry: towards appropriate water safety plans for urban self supply systems. Cranfield, UK: Cranfield University.
188. Kimani-MurageEW, NginduAM (2007) Quality of water the slum dwellers use: the case of a Kenyan slum. J Urban Health 84 : 829–838.
189. KiptumCK, NdambukiJM (2012) Well water contamination by pit latrines: a case study of Langas. International Journal of Water Resources and Environmental Engineering 4 : 35–43.
190. KlasenS, LechtenfeldT, MeierK, RieckmannJ (2012) Benefits trickling away: the health impact of extending access to piped water and sanitation in urban Yemen. Journal of Development Effectiveness 4 : 537–565.
191. KnappettPSK, McKayLD, LaytonA, WilliamsDE, AlamMJ, et al. (2012) Unsealed tubewells lead to increased fecal contamination of drinking water. J Water Health 10 : 565–578.
192. KnightSM, ToodayanW, CaiqueWC, KyiW, BarnesA, et al. (1992) Risk factors for the transmission of diarrhoea in children: a case-control study in rural Malaysia. Int J Epidemiol 21 : 812–818.
193. KorfaliSI, JurdiM (2009) Provision of safe domestic water for the promotion and protection of public health: a case study of the city of Beirut, Lebanon. Environ Geochem Hlth 31 : 283–295.
194. KravitzJD, NyaphisiM, MandelR, PetersenE (1999) Quantitative bacterial examination of domestic water supplies in the Lesotho Highlands: Water quality, sanitation, and village health. Bull World Health Organ 77 : 829–836.
195. KremerM, LeinoJ, MiguelE, ZwaneAP (2011) Spring cleaning: rural water impacts, valuation, and property rights institutions. Q J Econ 126 : 145–205.
196. Kremer M, Null C, Zwane AP (2008) Trickle down: diffusion of chlorine for drinking water treatment in Kenya. Available: http://www.stanford.edu/group/SITE/archive/SITE_2008/segment_1/papers/de_giorgi_SITE_Trickle_Down.pdf. Accessed 1 December 2013.
197. KromoredjoP, FujiokaRS (1991) Evaluating three simple methods to assess the microbial quality of drinking water in indonesia. Environ Toxic Water 6 : 259–270.
198. KumpelE, NelsonKL (2013) Comparing microbial water quality in an intermittent and continuous piped water supply. Water Res 47 : 5176–5188.
199. LaceyS, LopezR, FrangosC, KhodadoustA (2011) Water quality degradation after water storage at household level in a piped water system in rural Guatemala. International Journal for Service Learning in Engineering 6 : 118–129.
200. LalM, KaurH (2006) A microbiological study of bottled mineral water marketed in Ludhiana. Indian J Public Health 50 : 31–32.
201. Lantagne D, Clasen T (2011) Assessing the Implementation of Selected Household Water Treatment and Safe Storage (HWTS) Methods in Emergency Settings. London: London School of Hygiene and Tropical Medicine.
202. LeberJ, RahmanMM, AhmedKM, MaillouxB, van GeenA (2011) Contrasting influence of geology on E. coli and arsenic in aquifers of Bangladesh. Ground Water 49 : 111–123.
203. LeiterM, LevyJ, MutitiS, BoardmanM, WojnarA, et al. (2013) Drinking water quality in the Mount Kasigau region of Kenya: a source to point-of-use assessment. Environmental Earth Sciences 68 : 1–12.
204. LevyK, NelsonKL, HubbardA, EisenbergJNS (2008) Following the water: a controlled study of drinking water storage in northern coastal Ecuador. Environ Health Persp 116 : 1533–1540.
205. LinM-Y, ChenX-L, YouT-Z (2009) Hygienic quality of bottled drinking water in Longyan from 2006 to 2008. Chinese Journal of Public Health Engineering/Zhongguo Weisheng Gongcheng Xue 8 : 168–169.
206. Lira I, Matamoros A, Jacob F, Baltodano F, Harvey R (2008) Measurement of the quality of potable water by detection of H2S-producing bacteria; comparison with detection of fecal coliforms by the Millipore method in rural Nicaragua. Matagalpa/Jinotega, Nicaragua: ENACAL/DAR. Available: http://tascanica.org/images/Pathoscreenreport.pdf. Accessed 1 December 2013.
207. LongWF, YangJJ, XuQJ (2012) Microbial contamination of household drinking water in five Haikou residential groups. Huanjing yu Zhiye Yixue 29 : 566–568.
208. LubyS, IslamMS, JohnstonR (2006) Chlorine spot treatment of flooded tube wells, an efficacy trial. J Appl Microbiol 100 : 1154–1158.
209. LubySP, GuptaSK, SheikhMA, JohnstonRB, RamPK, et al. (2008) Tubewell water quality and predictors of contamination in three flood-prone areas in Bangladesh. J Appl Microbiol 105 : 1002–1008.
210. Lukacs H (2002) From design to implementation: innovative slow sand filtration for use in developing countries. Cambridge: Massachusetts Institute of Technology.
211. MachdarE, van der SteenNP, Raschid-SallyL, LensPN (2013) Application of Quantitative Microbial Risk Assessment to analyze the public health risk from poor drinking water quality in a low income area in Accra, Ghana. Sci Total Environ 449 : 134–142.
212. MacyJT, DunneEF, Angoran-BenieYH, Kamelan-TanoY, KouadioL, et al. (2005) Comparison of two methods for evaluating the quality of stored drinking water in Abidjan, Cote d′lvoire, and review of other comparisons in the literature. J Water Health 3 : 221–228.
213. MaganM, BileKM, KaziBM, GardeziZ (2010) Safe water supply in emergencies and the need for an exit strategy to sustain health gains: lessons learned from the 2005 earthquake in Pakistan. East Mediterr Health J 16 Suppl: S91–97
214. Magrath J (2006) Towards sustainable water-supply solutions in Rural Sierra Leone: a pragmatic approach, using comparisons with Mozambique. Oxford: Oxfam/WaterAid.
215. MahU, HaqT, PerveenF, SultanaL (2007) Bacteriological analysis of groundwater of Karachi. Pakistan J Sci Ind R 50 : 273–277.
216. MahasnehIA (1992) Isolation and characterization of faecal indicator bacteria from urban and rural natural drinking water sources. Biomedical Letters 47 : 347–354.
217. MahfouzAA, Abdel-MoneimM, al-ErianRA, al-AmariOM (1995) Impact of chlorination of water in domestic storage tanks on childhood diarrhoea: a community trial in the rural areas of Saudi Arabia. J Trop Med Hyg 98 : 126–130.
218. MaigaH, MaigaB, SuttonS (2006) Self-supply in Mali. Waterlines 25 : 13–14.
219. Mamo E, Mekonta L, Butterworth J, Sutton S (2011) New insights on the oldest approach: family wells in Ethiopia. 6th Rural Water Supply Network Forum 2011. Uganda. Kampala, Uganda: RWSN.
220. ManeVR, ChandorkarAA, KumarR (2005) Prevalence of pollution in surface and ground water sources in the rural areas of Satara Region, Maharashtra, India. Asian Journal of Water, Environment and Pollution 2 : 81–87.
221. MassoudMA, Al-AbadyA, JurdiM, NuwayhidI (2010) The challenges of sustainable access to safe drinking water in rural areas of developing countries: case of Zawtar El-Charkieh, Southern Lebanon. J Environ Health 72 : 24–30.
222. MatsinheNP, JuizoD, RietveldLC, PerssonKM (2008) Water services with independent providers in peri-urban Maputo: challenges and opportunities for long-term development. Water SA 34 : 411–420.
223. MazengiaE, ChidavaenziMT, BradleyM, JereM, NhandaraC, et al. (2002) Effective and culturally acceptable water storage in Zimbabwe: maintaining the quality of water abstracted from upgraded family wells. J Environ Health 64 : 15–18.
224. MertensTE, FernandoMA, MarshallTFDC, KirkwoodBR, CairncrossS, et al. (1990) Determinants of water quality availability and use in Kurunegala Sri Lanka. Trop Med Parasitol 41 : 89–97.
225. MetwaliRM (2003) Water quality of some wells in Taiz City (Yemen Republic) and its surroundings. Folia Microbiol (Praha) 48 : 90–94.
226. MichelinaAF, BronharoaTM, DarebF, PonsanocEHG (2006) Qualidade microbiológica de águas de sistemas de abastecimento público da região de Araçatuba, SP. Higiene Alimentar 20 : 90–95.
227. MirandaM, AramburuA, JuncoJ, CamposM (2010) State of the quality of drinking water in households in children under five years in Peru, 2007–2010. Rev Peru Med Exp Salud Publica 27 : 506–511.
228. MiranzadehMB, HeidariM, MesdaghiniaAR, YounesianM (2011) Survey of microbial quality of drinking water in rural areas of Kashan-Iran in second half of 2008. Pak J Biol Sci 14 : 59–63.
229. MoazeniM, AtefiM, EbrahimiA, RazmjooP, Vahid DastjerdiM (2013) Evaluation of chemical and microbiological quality in 21 brands of Iranian bottled drinking waters in 2012: a comparison study on label and real contents. Journal of Environmental and Public Health 2013 : 4.
230. MoeCL, SobseyMD, SamsaGP, MesoloV (1991) Bacterial indicators of risk of diarrhoeal disease from drinking-water in the Philippines. Bull World Health Organ 69 : 305–317.
231. MombaMNB, ObiCL, ThompsonP (2009) Survey of disinfection efficiency of small drinking water treatment plants: challenges facing small water treatment plants in South Africa. Water Sa 35 : 485–493.
232. Mora-BuenoD, Sanchez-PenaLC, del RazoLM, Gonzalez-AriasCA, Medina-DiazIM, et al. (2012) Presencia de arsénico y coliformes en agua potable del municipio de Tecuala, Nayarit, México. Rev Int Contam Ambie 28 : 127–135.
233. Morgan P (1990) Rural water supplies and sanitation. London: Macmillan. 358 p.
234. MoshtaghiH, BoniadianM (2007) Microbial quality of drinking water in Shahrekord (Iran). Research Journal of Microbiology 2 : 299–302.
235. MosleyLM, SharpDS, SinghS (2004) Effects of a tropical cyclone on the drinking-water quality of a remote Pacific Island. Disasters 28 : 405–417.
236. MoyoS, WrightJ, NdambaJ, GundryS (2004) Realising the maximum health benefits from water quality improvements in the home: a case from Zaka district, Zimbabwe. Phys Chem Earth 29 : 1295–1299.
237. Mpenyana-MonyatsiL, OnyangoMS, MombaMNB (2012) Groundwater quality in a South African rural community: a possible threat to public health. Pol J Environ Stud 21 : 1349–1358.
238. Muzuga JM, Tole MP, Ucakuwun EK (1998) The impact of geology and pit latrines on ground water quality in Kwale District. Dunes, groundwater, mangroves and birdlife in costal Kenya. Nairobi, Kenya: Acts Press.
239. MzugaJM, ToleMP, UcakuwunEK (2001) Contamination of groundwater resources by pit latrines in Kwale District, Kenya. Discov Innovat 13 : 203–212.
240. NascimentoAR, AzevedoTKL, FilhoNEM, RojasMOAI (2000) Qualidade microbiológica das águas minerais consumidas na cidade de Säo Luís - MA. Higiene Alimentar 14 : 69–72.
241. NEERI (2005) Study on surveillance of drinking water quality in selected cities/towns in India. Volume I: surveillance volume II: city appraisal. Nehru Marg, Nagpur: NEERI.
242. NikaeenM, PejhanA, JalaliM (2009) Rapid monitoring of indicator coliforms in drinking water by an enzymatic assay. Iranian Journal of Environmental Health Science and Engineering 6 : 7–10.
243. NogueiraG, NakamuraCV, TognimMCB, Abreu FilhoBA, Dias FilhoBP (2003) Microbiological quality of drinking water of urban and rural communities, Brazil. Revista de Saude Publica 37 : 232–236.
244. NortonDM, RahmanM, ShaneAL, HossainZ, KulickRM, et al. (2009) Flocculant-disinfectant point-of-use water treatment for reducing arsenic exposure in rural Bangladesh. Int J Environ Heal R 19 : 17–29.
245. NunesSM, FuziharaTO (2011) Availacao microbiologica das aguas envadas e commercializadas na regiao do ABC, SP. Higiene Alimentar 25 : 195–199.
246. NwosuJN, OguekeCC (2004) Evaluation of sachet water samples in Owerri metropolis. Nigerian Food Journal 22 : 164–170.
247. NyanchagaEN (1994) Rehabilitation of hand-dug wells and springs. Aqua (Oxford) 43 : 233–237.
248. Obiri-DansoK, Okore-HansonA, JonesK (2003) The microbiological quality of drinking water sold on the streets in Kumasi, Ghana. Lett Appl Microbiol 37 : 334–339.
249. OganMT (1992) Microbiological quality of bottled water sold in retail outlets in Nigeria. J Appl Bacteriol 73 : 175–181.
250. OkagbueRN, DlaminiNR, SiwelaM, MpofuF (2002) Microbiological quality of water processed and bottled in Zimbabwe. Afr J Health Sci 9 : 99–103.
251. Okioga T (2007) Water quality and business aspects of sachet-vended water in Tamale, Ghana [Master of Engineering dissertation]. Cambridge: Massachusetts Institute of Technology.
252. OkuraMH, SiqueiraKB (2005) Enumeracao de coliformes totais e coliformes termotolerantes em agua de abastecimento e de minas. Higiene Alimentar 19 : 86–91.
253. OlaoyeOA, OniludeAA (2009) Assessment of microbiological quality of sachet-packaged drinking water in Western Nigeria and its public health significance. Public Health 123 : 729–734.
254. OloruntobaEO, SridharMK (2007) Bacteriological quality of drinking water from source to household in Ibadan, Nigeria. Afr J Med Med Sci 36 : 169–175.
255. OmarKB, PotgieterN, BarnardTG (2010) Development of a rapid screening method for the detection of pathogenic Escherichia coli using a combination of Colilert Quanti-Trays/2000 and PCR. Wa Sci Technol 10 : 7–13.
256. OmerEOM, SallamT (2012) Bacteriogical investigation of drinking water in Shendi Locality, River Nile State, Sudan. Open Access Scientific Reports 1 : 329.
257. OnifadeAK, MLR (2008) Microbiological analysis of sachet water vended in Ondo State, Nigeria. Environmental Research Journal 2 : 107–110.
258. OoKN, HanAM, HlaingT, AyeT (1991) Bacteriologic studies of food and water consumed by children in Myanmar: 1. The nature of contamination. J Diarrhoeal Dis Res 9 : 87–90.
259. OpisaS, OdiereMR, JuraWG, KaranjaDM, MwinziPN (2012) Faecal contamination of public water sources in informal settlements of Kisumu City, western Kenya. Water Sci Technol 66 : 2674–2681.
260. OpryszkoMC, GuoY, MacdonaldL, MacdonaldL, KiihlS, et al. (2013) Impact of water-vending kiosks and hygiene education on household drinking water quality in rural Ghana. Am J Trop Med Hyg 88 : 651–660.
261. OrdiniohaB (2011) A survey of the community water supply of some rural riverine communities in the Niger Delta region, Nigeria: Health implications and literature search for suitable interventions. Niger Med J 52 : 13–18.
262. OswaldWE, LescanoAG, BernC, CalderonMM, CabreraL, et al. (2007) Fecal contamination of drinking water within peri-urban households, Lima, Peru. Am J Trop Med Hyg 77 : 699–704.
263. OyedejiO, OlutiolaPO, MoninuolaMA (2010) Microbiological quality of packaged drinking water brands marketed in Ibadan metropolis and Ile-Ife city in South Western Nigeria. Afr J Microbiol Res 4 : 96–102.
264. PanF, MengK, ZhaoH (2011) Hygienic investigation on rural drinking water in Daxing District of Beijing. Occupation and Health 27 : 2140–2141.
265. PantH, SarfarazS, IyengarL (2002) Evaluation of L-Cystine-amended H2S test for the detection of faecal pollution in potable water: comparison with standard multiple tube fermentation method. World Journal of Microbiology and Biotechnology 18 : 317–320.
266. ParkerAH, YoultenR, DillonM, NussbaumerT, CarterRC, et al. (2010) An assessment of microbiological water quality of six water source categories in north-east Uganda. J Water Health 8 : 550–560.
267. PathakSP, GopalK (1994) Antibiotic resistance and metal tolerance among Coliform sp. from drinking water in a hilly area. J Environ Biol 15 : 139–139.
268. PathakSP, GopalK (2005) Efficiency of modified H2S test for detection of faecal contamination in water. Environ Monit Assess 108 : 59–65.
269. PathakSP, GopalK (2008) Prevalence of bacterial contamination with antibiotic resistant and enterotoxigenic fecal coliforms in treated drinking water. J Toxicol Env Heal A 71 : 427–433.
270. Patrick JM (2010) Recommendations for at-risk water supplies in Capiz Province, Philippines: using water source and community assessments. Cambridge: Massachusetts Institute of Technology.
271. PhatthararangrongN, ChantratongN, JitsurongS (1998) Bacteriological quality of holywater from Thai temples in Songkhla Province, southern Thailand. J Med Assoc Thailand 81 : 547–550.
272. PickeringAJ, DavisJ, WaltersSP, HorakHM, KeymerDP, et al. (2010) Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol 44 : 3267–3272.
273. PinfoldJV, HoranNJ, WirojanagudW, MaraD (1993) The bacteriological quality of rainjar water in rural northeast Thailand. Water Research 27 : 297–302.
274. PiranhaJM, PachecoA, GambaRC, MehnertDU, GarrafaP, et al. (2006) Faecal contamination (viral and bacteria) detection in groundwater used for drinking purposes in Sao Paulo, Brazil. Geomicrobiol J 23 : 279–283.
275. PlatenburgRJPM, ZakiM (1993) Patterns of water quality in rural areas of Assyut Governorate, Egypt. Water Sci Technol 27 : 55–55.
276. PotgieterN, BeckerPJ, EhlersMM (2009) Evaluation of the CDC safe water-storage intervention to improve the microbiological quality of point-of-use drinking water in rural communities in South Africa. Water SA 35 : 505–516.
277. Potgieter N, Mudau LS, Maluleke FRS (2006) Microbiological quality of groundwater sources used by rural communities in Limpopo Province, South Africa. Kroiss H, Potgieter N, editors. London: IWA Publishing. 371–377 p.
278. Pradhan B (2004) Rural communities' perception on water quality and water borne disease: the case of Bungamati Village Development Committee in Kathmandu Valley, Nepal. Journal of Nepal Health Research Council 2.
279. PritchardM, MkandawireT, O′NeillJG (2007) Biological, chemical and physical drinking water quality from shallow wells in Malawi: case study of Blantyre, Chiradzulu and Mulanje. Phys Chem Earth 32 : 1167–1177.
280. PritchardM, MkandawireT, O′NeillJG (2008) Assessment of groundwater quality in shallow wells within the southern districts of Malawi. Phys Chem Earth 33 : 812–823.
281. Properzi F (2010) Rapid assessment of drinking-water quality in the Hashemite Kingdom of Jordan: country report of the pilot project implementation in 2004–2005. Geneva: WHO/UNICEF. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/RADWQ_Jordan.pdf. Accessed 1 December 2013.
282. PutriLSE, KustantiN, YunitaE (2013) Effect of land use on ground water quality (a casestudy from Ciracas Sub District, East Jakarta, Indonesia). International Journal of Bioscience, Biochemistry and Bioinformatics 3 : 33–36.
283. QiW (2007) Analysis of water quality of 186 samples from homemade wells of Yuci District of Jinzhong City in 2005. Preventive Medicine Tribune 13 : 536–537.
284. QuickRE, KimuraA, ThevosA, TemboM, ShamputaI, et al. (2002) Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am J Trop Med Hyg 66 : 584–589.
285. RadaidehJ, Al-ZboonK, Al-HarahshehA, Al-AdamatR (2009) Quality assessment of harvested rainwater for domestic uses. Jordan Journal of Earth and Environmental Sciences 2 : 26–31.
286. RaineyRC, HardingAK (2005) Drinking water quality and solar disinfection: effectiveness in peri-urbanhouseholds in Nepal. J Water Health 3 : 239–248.
287. RamtekePW (1995) Comparison of standard most probable number method with three alternate tests for detection of bacteriological water quality indicators. Environ Toxic Water 10 : 173–178.
288. RamtekePW, BhattacharjeeJW, PathakSP, KalraN (1992) Evaluation of coliforms as indicators of water-quality in India. J Appl Bacteriol 72 : 352–356.
289. RaoPNS, SureshK, BasavarjappaKG (2006) Bacteriological quallity of potable water in Davangere city. Journal of Communicable Diseases 38 : 381–384.
290. RawatV, JhaSK, BagA, SinghaiM, RawatCMS (2012) The bacteriological quality of drinking water in Haldwani Block of Nainital District, Uttarakhand, India. J Water Health 10 : 465–470.
291. RazzoliniMTP, SantosTFD, BastosVK (2010) Detection of Giardia and Cryptosporidium cysts/oocysts in watersheds and drinking water sources in Brazil urban areas. J Water Health 8 : 399–404.
292. ReddyPS, Ata-Ur-RasheedMD, SharmaS (2000) Microbiological analysis of bottled water. Indian J Med Microbiol 18 : 72–76.
293. RejithPG, JeevaSP, VijithH, SowmyaM, HathaAAM (2009) Determination of groundwater quality index of a Highland Village of Kerala (India) using geographical information system. J Environ Health 71 : 51–58.
294. RheinheimerDS, GonÇalvesCS, BortoluzziEC, PellegrinJBR, da SilvaJLS, et al. (2010) Qualidade de águas subterrâneas captadas em fontes em função da presença de proteção física e de sua posição na paisagem. Eng Agríc, Jaboticabal 30 : 948–957.
295. RosaG, MillerL, ClasenT (2010) Microbiological effectiveness of disinfecting water by boiling in rural Guatemala. Am J Trop Med Hyg 82 : 473–477.
296. RufenerS, MaeusezahlD, MoslerH-J, WeingartnerR (2010) Quality of drinking-water at source and point-of-consumption-drinking cup as a high potential recontamination risk: a field study in Bolivia. J Health Popul Nutr 28 : 34–41.
297. Sampson M (2008) Water quality assessment of hand dug wells. Phnom Penh, Cambodia: Resource Development International.
298. SantanaA, SilvaSCFL, FaraniIO, AmaralCHR, MacedoVF (2003) Qualidade microbiológica de águas minerais. Ciênc Tecnol Aliment 23 : 190–194.
299. SasikaranS, SritharanK, BalakumarS, ArasaratnamV (2012) Physical, chemical and microbial analysis of bottled drinking water. Ceylon Med J 57 : 111–116.
300. Schaffner M (2002) Drinking water quality assessment and improvement in the Jhikhu Khola watershed. Bern: Universität Bern.
301. SemerjianLA (2011) Quality assessment of various bottled waters marketed in Lebanon. Environ Monit Asses 172 : 275–285.
302. ShaheedA, OrgillJ, RatanaC, MontgomeryMA, JeulandA, et al. (2013) Water quality risks of “improved ” water sources: evidence from Cambodia. Trop Med Int Health 19 : 186–194.
303. ShaikhSA, GulN, SultanaL (2008) Surveillance of drinking water of Karachi City: microbiological quality. Pakistan J Sci Ind R 51 : 272–275.
304. Shakya (2004) An integrated drinking water quality assessment of rainwater harvesting jars and the related socio-economic conditions and gender issues of Chappani VDC-1, Palpa District. Available: http://www.aehms.org/pdf/Shakya%20&%20Sharma%20Proceedings%20FE.pdf. Accessed 30 December 2014.
305. SharAH, KaziYF, KanharNA, SoomroIH, ZiaSM, et al. (2010) Drinking water quality in Rohri City, Sindh, Pakistan. Afr J Biotechnol 9 : 7102–7107.
306. SharAH, KaziYF, SoomroIH (2009) Antibiotic susceptibility of thermo-tolerant escherichia coli 2 isolated from drinking water of Khairpur City, Sindh, Pakistan. Pakistan Journal of Biological Sciences 12 : 648–652.
307. SharAH, KaziYF, ZardariM, SoomroIH (2007) Enumeration of total and faecal coliform bacteria in drinking water of Khairpur City, Sindh, Pakistan. Bangladesh J Microbiol 24 : 163–165.
308. SharmaBK, SharmaLL, DurveVS (2008) Assessment of hand pump waters in three tribal dominated districts of southern Rajasthan, India. Journal of Environmental Science & Engineering 50 : 133–136.
309. ShresthaS, MallaSS, AiharaY, KondoN, NishidaK (2013) Water Quality at Supply Source and Point of Use in the Kathmandu Valley. Journal of Water and Environment Technology 11 : 331–340.
310. SilvaEF, SalgueiroAA (2001) Avaliacao da qualidade bacteriologica de agua de pocos na regio metropolitana de Recife - P. Higiene Alimentar. 15 : 73–78.
311. SimangoC, DindiweJ, RukureG (1992) Bacterial-contamination of food and household stored drinking-water in a farmworker community in Zimbabwe. Cent Afr J Med 38 : 143–149.
312. SimiyuS (2009) Assessment of spring water quality and quantity, and health implications in Tongaren division, Nzoia River catchment, Kenya. Afr J Ecol 47 : 99–104.
313. Singh KP, Gaur A, Chandra H, Ray PK (1993) Drinking water quality status of Bankura District (West Bengal) as indicator of environmental pollution. Agrawal VP, Abidi SAH, Verma GP, Singh KP, editors. Muzaffarnagar (India): Society of Bioscience. 71–78 p.
314. SmithPG, SaboneTG (1994) Drinking water quality in the Gantsi District of Botswana. Int J Environ Heal R 4 : 141–147.
315. SotoFRM, FonsecaYSK, AntunesDV, RissetoMR, AmakuM, et al. (2005) Microbiological evaluation of public water supply in schools of Ibiuna-SP: comparative study of water quality in conduit and post conduit. Revista do Instituto Adolfo Lutz 64 : 128–131.
316. SotoFRM, FonsecaYSK, RissetoMR, de AzevedoSS, AriniMdLB, et al. (2006) Monitoring the quality of water from groundwater located in the rural public schools of the Ibiuna municipality/SP: microbiological and physicochemical parameters, and factors for environmental risk. Revista do Instituto Adolfo Lutz 65 : 106–111.
317. Souza CoelhoMI, MendesES, Soares CruzMC, BezerraSS, Pinheiro e SilvaRP (2010) Evaluation of the microbiological quality of mineral water consumed in the metropolitan region of Recife, Pernambuco State. Acta Scientiarum Health Science 32 : 1–8.
318. StolerJ, FinkG, WeeksJR, OtooRA, AmpofoJA, et al. (2012) When urban taps run dry: sachet water consumption and health effects in low income neighborhoods of Accra, Ghana. Health Place 18 : 250–262.
319. Sutton S (2002) Community led improvements of rural drinking water supplies. Shewsbury, UK: SWL Consultants. Available: http://www.rural-water-supply.net/en/resources/details/249. Accessed 1 December 2013.
320. Sutton S, Butterworth J, Mekonta L (2012) A hidden resource: household-led rural water supply in Ethiopia. The Netherlands: IRC International Water and Sanitation Centre. Available: http://www.irc.nl/content/download/185695/850981/file/A%20hidden%20resource%20web_2012.pdf. Accessed 1 December 2013.
321. TaborM, KibretM, AberaB (2011) Bacteriological and physicochemical quality of drinking water and hygiene-sanitation practices of the consumers in bahir dar city, ethiopia. Ethiop J Health Sci 21 : 19–26.
322. Tadesse D, Desta A, Geyid A, Girma W, Fisseha S, et al.. (2010) Rapid assessment of drinking-water quality in the Federal Democratic Republic of Ethiopia: country report of the pilot project implementation in 2004–2005. Geneva: WHO/UNICEF.
323. TambekarDH, HirulkarNB, WerulkarSA (2007) Multidrug resistance in Salmonella typhi isolated from drinking water in Amravati. Nature, Environment and Pollution Technology 6 : 285–288.
324. TambekarDH, NewareBB (2012) Water quality index and multivariate analysis for groundwater quality assessment of villages of rural india. Science Research Reporter 2 : 229–235.
325. Taylor H, Ebdon J, Phillips R, Chavula G, Kapudzama O (2012) Assessment of drinking water quality for low-cost water options in rural Malawi. Brighton, UK: University of Brighton.
326. TewariS, RamtekePW, GargSK (2003) Evaluation of simple microbiol tests for detection of fecal coliforms directly at 44.5 C. Environ Monit Asses 85 : 191–198.
327. Tillet W (2007) An investigation into the impacts and challenges of implementing self supply in Eastern Uganda. Cranfield, UK: Cranfield University.
328. ToleMP (1997) Pollution of groundwater in the coastal Kwale District, Kenya. IAHS Publications Series of the Proceedings and Reports - Intern Assoc Hydrological Sciences 240 : 287–310.
329. TrevettAF, CarterRC, TyrrelSF (2004) Water quality deterioration: a study of household drinking water quality in rural Honduras. Int J Environ Heal R 14 : 273–283.
330. Uhl VW, Daw A, Baron JA (2012) How a city gets its drinking water a case study - capital city of Monrovia, Liberia. Lambertville (New Jersey): UHL & Associates, Inc.
331. VaccariM, CollivignarelliC, TharnpoophasiamP, VitaliF (2010) Wells sanitary inspection and water quality monitoring in Ban Nam Khem (Thailand) 30 months after 2004 Indian Ocean tsunami. Environ Monit Assess 161 : 123–133.
332. ValenteJPS, LopesCAM, CaminhasAMT, HoracioA (1999) Bacteriological evaluation of the water supplies of the Eldorado municipality — Vale do Ribeira (SP). Revista do Instituto Adolfo Lutz 58 : 9–13.
333. van GeenA, AhmedKM, AkitaY, AlamMJ, CulliganPJ, et al. (2011) Fecal contamination of shallow tubewells in Bangladesh inversely related to arsenic. Environ Sci Technol 45 : 1199–1205.
334. VandersliceJ, BriscoeJ (1993) All coliforms are not created equal - a comparison of the effects of water source and in-house water contamination on infantile diarrheal disease. Water Resour Res 29 : 1983–1995.
335. VargheseJ, JayaDS (2008) Drinking water quality assessment of rain water harvested in ferrocement tanks in Alappuzha District, Kerala (India). Journal of Environmental Science & Engineering 50 : 115–120.
336. VidalJ, ConsuegraA, GomescaseresL, MarrugoJ (2009) Assessment of the microbiological quality of water packed in bags manufactured in Sincelejo-Colombia. Revista Mvz Cordoba 14 : 1736–1744.
337. VollaardAM, AliS, SmetJ, van AstenH, WidjajaS, et al. (2005) A survey of the supply and bacteriologic quality of drinking water and sanitation in Jakarta, Indonesia. SE Asian J Trop Med 36 : 1552–1561.
338. WarnerNR, LevyJ, HarppK, FarruggiaF (2008) Drinking water quality in Nepal's Kathmandu Valley: a survey and assessment of selected controlling site characteristics. Hydrogeology Journal 16 : 321–334.
339. WattsR (1992) Low-cost water supplies and their contribution to health. Afr Health 15 : 10–11.
340. WelchP, DavidJ, ClarkeW, TrinidadeA, PennerD, et al. (2000) Microbial quality of water in rural communities of Trinidad. Rev Panam Salud Publica 8 : 172–180.
341. WilliamsMA, MoranP, NhandaraC, HoveI, CharimariL, et al. (1997) Contamination of traditional drinking water sources during a period of extreme drought in the Zvimba communal lands, Zimbabwe. Cent Afr J Med 43 : 316–321.
342. WrightJ, CroninA, Okotto-OkottoJ, YangH, PedleyS, et al. (2013) A spatial analysis of pit latrine density and groundwater source contamination. Environ Monit Asses 185 : 4261–4272.
343. WuZ (2008) Drinking water test of rural centralized water supply in Dianjiang. Prev Med 46 : 123–125.
344. XavierRP, SiqueiraLP, Chaves VitalFA, Soares RochaFJ, IrmaoJI, et al. (2011) Microbiological quality of drinking rainwater in the inland region of Pajeu, Pernambuco, northeast Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo 53 : 121–124.
345. YaoY-B, LiQ-J, LiuX-Z (2012) Health survey of rural drinking water in Jiangxi Province from 2008 to 2010. Journal of Environment and Health 29 : 340–342.
346. YasinN, ShahN, KhanJ, SabaNU, ul IslamZ (2012) Bacteriological status of drinking water in the peri-urban areas of Rawalpindi and Islamabad-Pakistan. Afr J Microbiol Res 6 : 169–175.
347. YassinMM, Abu AmrSS, Al-NajarHM (2006) Assessment of microbiological water quality and its relation to human health in Gaza Governorate, Gaza Strip. Public Health 120 : 1177–1187.
348. YongsiHBN (2010) Suffering for water, suffering from water: access to drinking-water and associated health risks in Cameroon. J Health Popul Nutr 28 : 424–435.
349. ZeenatA, HathaAAM, ViolaL, VipraK (2009) Bacteriological quality and risk assessment of the imported and domestic bottled mineral water sold in Fiji. J Water Health 7 : 642–649.
350. ZeilhoferP, ZeilhoferLV, HardoimEL, LimaZM, OliveiraCS (2007) GIS applications for mapping and spatial modeling of urban-use water quality: a case study in District of Cuiaba, Mato Grosso, Brazil. Cad Saude Publica 23 : 875–884.
351. ZhangXD, GeZX, XiangJH, ZhuMF, ZhangJF, et al. (2012) Water quality in automatic water vending machines in Pudong New Area: an evaluation using drinking water quality index. Huanjing yu Zhiye Yixue 29 : 231–233.
352. ZuinV, OrtolanoL, AlvarinhoM, RusselK, TheboA, et al. (2011) Water supply services for Africa's urban poor: the role of resale. J Water Health 9 : 773–784.
353. Elala D, Labhasetwar P, Tyrrel SF (2011) Deterioration in water quality from supply chain to household and appropriate storage in the context of intermittent water supplies. Wa Sci Technol 11.
354. ShaheedA, OrgillJ, MontgomeryMA, JeulandMA, BrownJ (2014) Why “improved” water sources are not always safe. Bull World Health Organ 92 : 283–289.
355. HrudeySE, HrudeyEJ, PollardSJT (2006) Risk management for assuring safe drinking water. Environ Int 32(8): 948–957.
356. FosterT (2013) Predictors of Sustainability for Community-Managed Handpumps in Sub-Saharan Africa: Evidence from Liberia, Sierra Leone, and Uganda. Environ Sci Technol 47 : 12037–12046.
357. GunnarsdottirMJ, GardarssonSM, ElliottM, SigmundsdottirG, BartramJ (2012) Benefits of water safety plans: microbiology, compliance, and public health. Environ Sci Technol 46 : 7782–7789.
358. PsutkaR, PeletzR, MicheloS, KellyP, ClasenT (2011) Assessing the microbiological performance and potential cost of boiling drinking water in urban Zambia. Environ Sci Technol 45 : 6095–6101.
359. BlumD, HuttlySRA, OkoroJI, AkujobiC, KirkwoodBR (1987) Bacteriological quality of traditional water sources in North-Eastern Imo State, Nigeria. Epidemiol Infect 99 : 429–437.
360. WrightRC (1986) Seasonality of bacterial quality of water in a tropical developing country (Sierra Leone). J Hygiene-Camb 96 : 75–82.
361. Kayser GL, Moriarty P, Fonseca C, Bartram J (2013) Drinking water service delivery frameworks for monitoring evaluation, policy and planning: a review. Int J Environ Res Public Health Vol 10, No 10, p 4812–4835.
362. BartramJ (2008) Improving on haves and have-nots. Nature 452 : 283–284.
363. de Albuquerque C, Roaf V (2012) On the right track: good practices in realising the rights to water and sanitation. Geneva: OHCRH.
364. YangH, BainR, BartramJ, GundryS, PedleyS, et al. (2013) Water safety and inequality in access to drinking-water between rich and poor households. Environ Sci Technol 47 : 1222–1230.
365. RiveraSC, HazenTC, ToranzosGA (1988) Isolation of fecal coliforms from pristine sites in a tropical rain forest. Appl Environ Microbiol 54 : 513–517.
366. AyoobS, GuptaAK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Env Sci Tec 36 : 433–487.
367. AminiM, MuellerK, AbbaspourKC, RosenbergT, AfyuniM, et al. (2008) Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42 : 3662–3668.
368. AminiM, AbbaspourKC, BergM, WinkelL, HugSJ, et al. (2008) Statistical modeling of global geogenic arsenic contamination in groundwater. Environ Sci Technol 42 : 3669–3675.
369. RehfuessEA, BruceN, BartramJK (2009) More health for your buck: health sector functions to secure environmental health. Bull World Health Organ 87 : 880–882.
370. HunterPR, MacDonaldAM, CarterRC (2010) Water Supply and Health. PLoS Med 7: e1000361.
371. Hutton G (2012) Global costs and benefits of drinking-water supply and sanitation interventions to reach the MDG target and universal coverage. Geneva: WHO. WHO/HSE/WSH/12.01.
372. HowardG, BartramJ (2005) Effective water supply surveillance in urban areas of developing countries. J Water Health 3 : 31–43.
373. BradleyDJ, BartramJK (2013) Domestic water and sanitation as water security: monitoring, concepts and strategy. Philos T Roy Soc A 371 : 20120420.
374. WHO (2008) Essential environmental health standards in health care. Geneva: WHO.
375. Ministry of Health Uganda, Macro International Inc. (2008) Uganda Service Provision Assessment Survey 2007. Uganda; Calverton (Maryland): Ministry of Health; Macro International Inc.
376. Ministry of Health Rwanda, Macro International Inc. (2008) Rwanda Service Provision Assessment Survey 2007. Rwanda; Calverton (Maryland): NIS, MOH; Macro International Inc.
377. PeletzR, MahinT, ElliottM, HarrisMS, ChanKS, et al. (2013) Water, sanitation, and hygiene interventions to improve health among people living with HIV/AIDS: a systematic review. AIDS 27 : 2593–2601.
378. BenovaL, CummingO, CampbellOM (2014) Systematic review and meta-analysis: association between water and sanitation environment and maternal mortality. Trop Med Int Health 19 : 368–387.
379. LimSS, VosT, FlaxmanAD, DanaeiG, ShibuyaK, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 : 2224–2260.
380. YangH, WrightJA, GundrySW (2012) Water accessibility: boost water safety in rural China. Nature 484 : 318.
381. WHO/UNICEF (2012) Review: sampling issues in tools for water quality monitoring. Geneva and New York: WHO/UNICEF Joint Monitoring Programme. Available: http://www.wssinfo.org/fileadmin/user_upload/resources/Report-of-Review-WQ-sampling-Geneva-18-19June2012_final.pdf. Accessed 1 December 2013.
382. JimenezA, Perez-FoguetA (2008) Improving water access indicators in developing countries: a proposal using water point mapping methodology. Wa Sci Technol 8 : 279–287.
383. BrownJ, ClasenT (2012) High adherence is necessary to realize health gains from water quality interventions. PLoS One 7: e36735.
384. HunterPR, Zmirou-NavierD, HartemannP (2009) Estimating the impact on health of poor reliability of drinking water interventions in developing countries. Sci Total Environ 407 : 2621–2624.
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2014 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- The Role of Open Access in Reducing Waste in Medical Research
- Provider-Initiated HIV Testing and Counselling for Children
- Fecal Contamination of Drinking-Water in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- Call to Action: Promoting Domestic and Global Tobacco Control by Ratifying the Framework Convention on Tobacco Control in the United States
- Methods for Specifying the Target Difference in a Randomised Controlled Trial: The Difference ELicitation in TriAls (DELTA) Systematic Review
- Achieving the HIV Prevention Impact of Voluntary Medical Male Circumcision: Lessons and Challenges for Managing Programs
- Effectiveness of a Pre-treatment Snack on the Uptake of Mass Treatment for Schistosomiasis in Uganda: A Cluster Randomized Trial
- Communicating and Monitoring Surveillance and Response Activities for Malaria Elimination: China's “1-3-7” Strategy
- Improving the Quality of Adult Mortality Data Collected in Demographic Surveys: Validation Study of a New Siblings' Survival Questionnaire in Niakhar, Senegal
- Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data
- Gene-Lifestyle Interaction and Type 2 Diabetes: The EPIC InterAct Case-Cohort Study
- Barriers to Provider-Initiated Testing and Counselling for Children in a High HIV Prevalence Setting: A Mixed Methods Study
- Maternal Overweight and Obesity and Risks of Severe Birth-Asphyxia-Related Complications in Term Infants: A Population-Based Cohort Study in Sweden
- Ethical Alternatives to Experiments with Novel Potential Pandemic Pathogens
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Provider-Initiated HIV Testing and Counselling for Children
- Fecal Contamination of Drinking-Water in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- Achieving the HIV Prevention Impact of Voluntary Medical Male Circumcision: Lessons and Challenges for Managing Programs
- Effectiveness of a Pre-treatment Snack on the Uptake of Mass Treatment for Schistosomiasis in Uganda: A Cluster Randomized Trial
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



