-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial
In a randomized controlled trial, Job Godino and colleagues study the effects of providing personalized information about genetic and phenotypic risk of type 2 diabetes as compared with standard lifestyle advice.
Published in the journal: Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial. PLoS Med 13(11): e32767. doi:10.1371/journal.pmed.1002185
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002185Summary
In a randomized controlled trial, Job Godino and colleagues study the effects of providing personalized information about genetic and phenotypic risk of type 2 diabetes as compared with standard lifestyle advice.
Introduction
The prevalence of type 2 diabetes is increasing worldwide, and primary prevention of the disease is a global priority [1]. Evidence from randomized controlled trials shows that positive changes in health behavior can significantly reduce the incidence of type 2 diabetes among those considered high-risk [2,3]. However, translating these findings into preventive strategies has proven difficult, as it requires motivation of individuals to adopt and maintain changes in physical activity and diet [4].
Risk of type 2 diabetes is also influenced by genetics. Despite questions regarding the clinical validity and utility of recently developed predictive genetic tests [5,6], some researchers and direct-to-consumer genetic testing companies are optimistic that the provision of genetic risk information for type 2 diabetes will motivate behavior change more than widely available phenotypic risk information [7–9]. This hypothesis has support from health behavior theory [10,11]. However, there is concern about the potential negative psychological impact of widely available genetic risk information including fatalism, anxiety, and false reassurance [12–14]. Furthermore, there is evidence that risk perceptions and communication of biomarker information have limited influence on behavior [15,16].
There are few clinical studies concerning the impact of genetic risk information [17]. The majority have been nonrandomized or underpowered. Furthermore, interpretation has been limited by risk of bias and additional differences between study groups than merely the provision of DNA-based disease risk information. Two recent trials report no behavioral impact of information about the genetic risk of type 2 diabetes [18,19]. However, neither included precise measures of behavior. In addition, Grant et al. recruited a small sample of individuals willing to participate in an intensive diabetes prevention program [19], and Voils et al. did not include a control group receiving no phenotypic risk information [18].
We examined the effect of communicating genetic or phenotypic risk of type 2 diabetes in combination with standard lifestyle advice on objectively-measured physical activity, self-reported diet, self-reported weight, anxiety, and several cognitive and emotional theory-based antecedents of behavior change in a sample of healthy middle-aged adults.
Methods
Details regarding the trial methods have been reported previously [20]. We obtained ethical approval from the Cambridgeshire 1 Research Ethics Committee (No. 10/H0304/78). Each participant provided written informed consent. The trial is registered with Current Controlled Trials (ISRCTN09650496; Date applied: April 4, 2011; Date assigned: June 10, 2011).
Participant Screening and Recruitment
We recruited participants from the Fenland Study, an ongoing population-based observational study investigating the influence of lifestyle and genetic factors on the development of diabetes, obesity, and related metabolic disorders [21]. Individuals born between 1950 and 1975 registered with participating general practices in Cambridgeshire, UK were invited to take part. General practitioners excluded those with a diagnosis of diabetes, a terminal illness with a prognosis of less than one year, a psychotic illness, being pregnant or lactating, or being unable to walk unaided. Fenland Study participants undergo a health assessment, and blood samples are collected for the genotyping of single nucleotide polymorphisms (SNPs) associated with type 2 diabetes. At the end of the assessment, participants are fitted with a combined heart rate monitor and accelerometer (Actiheart) [22], which they are instructed to wear continuously for six days and nights to measure physical activity.
We sent invitations to take part in the Diabetes Risk Communication Trial (DRCT) to Fenland Study participants who 1) had agreed to be contacted regarding future studies, 2) had sufficient data to calculate their genetic and phenotypic risk of type 2 diabetes, 3) wore the combined heart rate monitor and accelerometer for three or more full days without experiencing a severe skin reaction, and 4) provided at least 36 h of complete physical activity data. Upon response, we excluded those who reported being diagnosed with diabetes or actively participating in another study.
Randomization and Blinding
We randomly allocated eligible participants who completed a baseline questionnaire to one of three groups that received either standard lifestyle advice alone (control group) or in combination with a genetic or a phenotypic risk estimate for type 2 diabetes (intervention groups). A statistician without knowledge of participant characteristics created a computer-generated list comprised of blocks of six that contained two of each of the three study groups per block in a random order. This was incorporated into an automated randomization computer program. Allocation was concealed from the researchers and participants until the interventions were assigned. Researchers assessing the primary outcome remained blinded to the allocation of participants throughout the study.
Interventions
All participants received standard written lifestyle advice, which included a brief description of type 2 diabetes and an explanation of the risk factors, symptoms, diagnosis, treatment, and consequences of the disease. We informed participants that the disease is preventable and encouraged them to maintain a healthy weight and to adhere to United Kingdom governmental guidelines for physical activity and diet [23,24].
The interventions were designed to incorporate evidence regarding the most effective methods for communicating disease risk estimates [25]. As it remains unclear whether an individual’s understanding of risk is more accurate after the provision of a numerical risk estimate or a verbal risk estimate [26,27], both the genetic and phenotypic risk estimates included estimates of the participant’s lifetime risk of developing type 2 diabetes expressed as a percentage and verbal estimation of risk (i.e., “below average,” “average,” or “above average”). Moreover, research suggests that comparative risk estimates may have a greater influence on behavior than absolute risk estimates [28,29], and that visual representations of risk elicit greater recall and understanding of risk [26,30,31]. Thus, estimates were framed in comparison to the average risk within each participant’s age and sex-specific group, and participants were told what percentage of the study sample had a risk estimate higher, lower, and equal to their own. Each piece of information was represented using a visual scales [27].
Methods for calculating the genetic and phenotypic risk estimates have been described in detail previously [20]. We calculated genetic risk using methods similar to those outlined by several direct-to-consumer genetic testing companies [32–34]. The 23 SNPs utilized in the calculation were identified through adequately powered genome-wide association studies, had associations with type 2 diabetes that reached the genome-wide significance level (p-values for associations less than 5x10−8), and had associations that were replicated in at least one independently published study. We took the odds ratio for each SNP from replication samples and the allele frequency from the HapMap population. We calculated phenotypic risk using the Cambridge Diabetes Risk Score [35,36]. Age, sex, smoking status, family history of diabetes, and prescription of steroid or antihypertensive medication were assessed via questionnaire. We measured height and weight using standardized procedures and calculated body mass index as weight (in kg) divided by the square of height (in m). All data used in the estimation of risk were collected during the participant’s Fenland Study health assessment.
Outcome Measures
The primary outcome was physical activity, defined as physical activity energy expenditure (kJ/kg/d), measured objectively using the Actiheart continuously for six days and night [22]. We used a submaximal exercise test for individual calibration of heart rate response [37] and a branched equation model to estimate physical activity energy expenditure from acceleration and heart rate [38]. This approach has high validity for estimating the intensity of physical activity [39,40] and overcomes some of the key limitations associated with either accelerometers or heart rate monitors alone [22].
Baseline physical activity was measured in the Fenland Study (median 1.76 years prior to enrollment in the DRCT), and follow-up occurred 8 wk postintervention. This relatively short follow-up period was chosen on the basis that it is unlikely that a long-term effect would exist in the absence of an impact in the short term. All prespecified secondary outcomes were measured via questionnaire and included self-reported diet, self-reported weight, self-rated health, worry (measured at baseline and follow-up), anxiety, behavioral intention, perceived risk, self-efficacy, response efficacy, perceived severity, and diabetes risk representations (measured at baseline, immediately post receipt of the intervention, and follow-up; more details are available in S1 Appendix).
Statistical Analysis
All analyses were performed on an intention-to-treat basis (i.e., analysis of data according to randomized study group, regardless of whether or not the intervention was received) using STATA software [41]. We used univariate descriptive statistics (means, standard deviations [SDs], numbers, and percentages) to summarise the characteristics of the study sample at baseline. We used analysis of covariance to assess differences between groups in physical activity at follow-up, adjusted for baseline. Prespecified exploratory analyses were conducted to examine whether sex, age, body mass index, time since the Fenland Study, and baseline measurements of the trial outcomes moderated the intervention effects on physical activity. A further subgroup analysis explored whether a high or low risk estimate moderated the effect of the type of risk estimate (i.e., genetic or phenotypic) on physical activity. The study protocol and statistical analysis plan specified that the analyses should include only participants with complete postintervention or follow-up data (i.e., a complete case analysis). We included participants with missing baseline data in the analyses using the missing-indicator method [42]. Similar regression procedures were used to examine differences in all secondary outcomes. The acceptability of the interventions was assessed by summarizing recruitment rates, loss to follow-up, and reasons for loss to follow-up. Additionally, differences in responses to questions regarding the perceived accuracy of the risk estimates, as well as the retention and discussion of the risk estimates were examined.
Estimates used in the sample size calculation were taken from the Feedback, Awareness and Behavior (FAB) study, which had a similar sample population and the same primary outcome as proposed here (i.e., physical activity energy expenditure) [43]. The mean (SD) physical activity energy expenditure at follow-up in the FAB study was 46.2 (15.4) kJ/kg/d, and the correlation between baseline and follow-up was high (0.69). After making a Bonferroni adjustment for multiple comparisons in a three-group trial, we determined that in order to detect a between-group difference of 4.1 kJ/kg/d at follow-up (which equates to approximately 20 to 25 min of walking per day), with 98.3% significance and 80% power, approximately 465 participants would need to complete the trial [44,45]. We conservatively allowed for an attrition rate of 20% and targeted the recruitment of 580 participants.
Results
Participant Characteristics
Between February 11, 2011 and September 5, 2011, we sent invitations to take part in the DRCT to 1150 Fenland Study volunteers and 635 (55%) replied positively and were assessed for eligibility. Between March 8, 2011 and September 14, 2011, 569 (49%) participants were randomized (Fig 1). Reasons for exclusion included not responding after an initial positive reply (68%), responding after enrollment closed (12%), being unavailable prior to the expected trial completion date (8%), reporting a rash while wearing the Actiheart during the Fenland Study (1%), participating in another study (6%), and being diagnosed with diabetes (5%). Those who did not reply and those who were excluded from participation did not differ from those randomized according to sex, body mass index, glycated haemoglobin (HbA1c) or phenotypic risk. However, they were slightly younger than participants at the time of their Fenland Study health assessment (mean [SD] age 45.0 [6.9] y versus 47.2 [7.4] y) and had a higher genetic lifetime risk estimate (mean [SD] of 18.8% [8.2%] versus 17.8% [8.1%]).
Fig. 1. Flow of participants through the DRCT. 
Baseline characteristics were similar among the three study groups (Table 1). There were slightly more female (52.9%) than male participants. The mean (SD) age at which participants finished full-time education was 19.4 (4.4) y and most were employed full-time (68.0%). Overall, 10.6% were current smokers, and 26.8% consumed more than 11 units of alcohol per wk. Few participants were prescribed steroid or antihypertensive medication (5.8%) or had a positive family history for diabetes (23.0%). On average, participants were overweight (mean [SD] body mass index of 26.1 [4.2] kg/m2), but their HbA1c level was in the normal range (mean [SD] of 36.3 [4.4] mmol/mol). Tab. 1. Baseline characteristics of participants. 
*Plus–minus values are means ±SD. After randomization, 12 (2.1%) participants were lost to follow-up, and we excluded 7 (1.2%) from the primary analysis because they had insufficient physical activity data (the monitor did not record more than three days of data) (Fig 1). The complete case sample comprised 550 participants: 184 received standard lifestyle advice alone, 184 received standard lifestyle advice and a genetic risk estimate, and 182 received standard lifestyle advice and a phenotypic risk estimate.
Outcomes
There were no significant between-group differences in objectively measured physical activity at the 8-wk follow-up (Fig 2). Prespecified exploratory analyses showed that the interventions had no effect on physical activity within subgroups defined by age, body mass index, physical activity, self-reported diet, self-reported weight, self-rated health, behavioral intention, perceived risk, anxiety, worry, time since participation in the Fenland Study, or receipt of a high or low risk estimate (Table A in S1 Appendix). However, when compared to the control group, the genetic risk estimate was associated with a greater increase in physical activity among women than among men (women: β = 4.29, 95% CI = 0.27 to 8.33, p = 0.037; men: β = −2.69, 95% CI = −6.92 to 1.55, p = 0.213). The phenotypic risk estimate did not have a differential effect by sex (women: β = 1.70, 95% CI = −2.45 to 5.86, p = 0.421; men: β = 1.33, 95% CI = −2.75 to 5.41, p = 0.523).
Fig. 2. Intervention effects on the primary outcome: physical activity. 
Physical activity was defined as physical activity energy expenditure (kJ/kg/d) measured with a combined heart rate monitor and accelerometer. Plus–minus values are means ± standard error (SE). Analysis of covariance was used to assess differences between groups at follow-up, adjusted for baseline. No significant differences were observed between trial groups in self-reported diet, self-reported weight, self-rated health, and worry at follow-up, nor were there any significant differences in behavioral intention and anxiety immediately postintervention or at follow-up (Tables 2 and 3). Participants who received a risk estimate had a lower perceived risk immediately postintervention than those who did not receive an estimate. These effects were attenuated at follow-up, but remained statistically significant, and did not differ by the type of risk estimate received (Tables 2 and 3). Additionally, immediately postintervention, both diet response efficacy and illness understanding were significantly lower in the phenotypic risk group compared to the control group, and perceived severity was significantly lower in the genetic risk group compared to the control group (Tables 2 and 3).
Tab. 2. Baseline and follow-up values for secondary outcomes by study group. 
Plus–minus values are means ± SE. Diet was defined as self-reported fruit and vegetable consumption measured via the Food Frequency Questionnaire. Self-rated health was measured on a scale (from 1 to 4), with higher values indicating a poorer rating of health. Worry was measured using an adapted version of the Cancer Worry Scale (from 6 to 24), with higher values indicating greater type 2 diabetes related worry. Anxiety was measured using the short-form of the state scale of the Spielberger State Trait Anxiety Inventory (from 20 to 80), with higher values indicating greater anxiety. Intention was measured on a scale (from 1 to 5), with higher values indicating greater intention to change behavior. Perceived risk was measured on a scale (from 0 to 100), with higher values indicating a greater perceived risk of developing type 2 diabetes. Self-efficacy, response efficacy, and perceived severity were each measured on a scale (from 1 to 5), with higher values indicating a greater likelihood of behavior change. Diabetes risk representations were measured using an adapted Brief Illness Perceptions Questionnaire (from 0 to 10), with higher values indicating that the representation is more important in the participant’s construction of perceived risk. Tab. 3. Intervention effects on secondary outcomes. 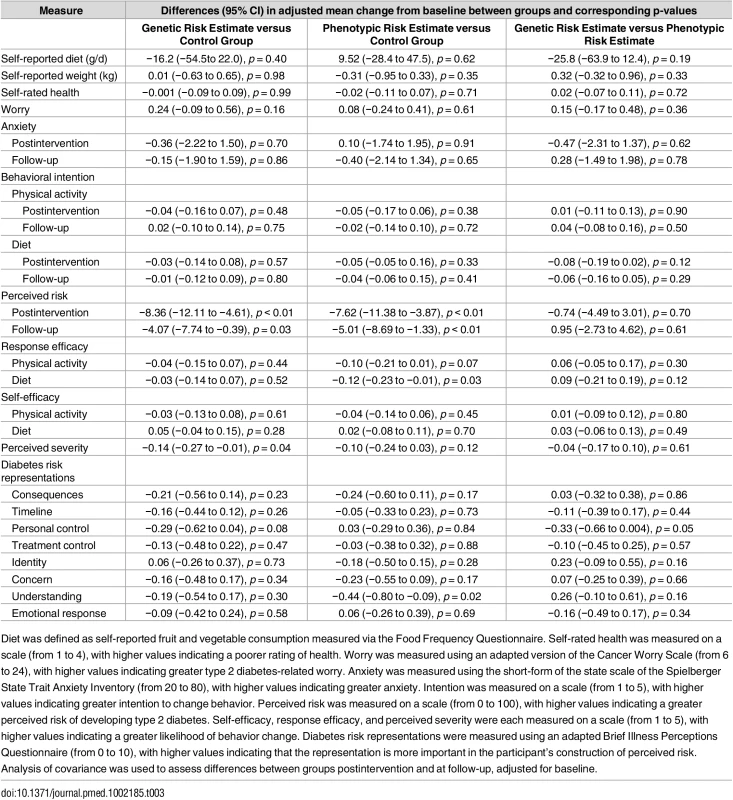
Diet was defined as self-reported fruit and vegetable consumption measured via the Food Frequency Questionnaire. Self-rated health was measured on a scale (from 1 to 4), with higher values indicating a poorer rating of health. Worry was measured using an adapted version of the Cancer Worry Scale (from 6 to 24), with higher values indicating greater type 2 diabetes-related worry. Anxiety was measured using the short-form of the state scale of the Spielberger State Trait Anxiety Inventory (from 20 to 80), with higher values indicating greater anxiety. Intention was measured on a scale (from 1 to 5), with higher values indicating greater intention to change behavior. Perceived risk was measured on a scale (from 0 to 100), with higher values indicating a greater perceived risk of developing type 2 diabetes. Self-efficacy, response efficacy, and perceived severity were each measured on a scale (from 1 to 5), with higher values indicating a greater likelihood of behavior change. Diabetes risk representations were measured using an adapted Brief Illness Perceptions Questionnaire (from 0 to 10), with higher values indicating that the representation is more important in the participant’s construction of perceived risk. Analysis of covariance was used to assess differences between groups postintervention and at follow-up, adjusted for baseline. Among those who received a risk estimate, the majority (93.0%) reported that they believed their risk estimate to be either fairly or very accurate. Most participants (90.5%) stated that they had kept their risk estimate, and many (63.7%) reported discussing it with others (for example family members, friends, or health professionals).
Discussion
In a sample of healthy, middle-aged men and women who were given information about type 2 diabetes and standard lifestyle advice, there was no effect of communicating a genetic or phenotypic estimate of the risk of developing type 2 diabetes on objectively measured physical activity. We did not observe significant intervention effects on self-reported diet and weight, self-rated health, behavioral intentions, anxiety, or worry. This is an important observation, given the expectations that such communications might facilitate behavior change and the concerns about the potential adverse psychological consequences of predictive genetic testing. We also did not observe significant intervention effects on a range of other cognitive and emotional theory-based antecedents to behavior change. We examined several potential moderators, and only sex was found to interact with the intervention effect on physical activity, raising the possibility that genetic risk information may be more influential among women than among men. More research is needed to explore whether women and men respond differently to genetic risk information.
Risk information was received and understood, and had a sustained effect on participants’ perceptions of their risk. However, the volunteers tended to overestimate their risk at baseline and may therefore have been somewhat reassured by the information that they received, albeit not to the extent that they adopted unhealthy behaviors [46]. Nevertheless, we cannot exclude the possibility that provision of risk estimates that exceed participants’ perceived risk might influence behavior, although previous trials have not reported effects among high-risk subgroups [18,19]. Furthermore, it is possible that information concerning the genetic risk of diseases other than diabetes, such as various cancers or chronic neurodegenerative diseases, might elicit a greater response, although this has not been the finding of published trials [47].
This trial provides much needed robust evidence on the behavioral impact of communicating genetic risk information. A systematic review identified only two clinical trials that assessed physical activity and diet as outcomes [47]. The authors concluded that given the limited number of low quality studies, strong conclusions could not be drawn and larger, higher quality studies were needed. The findings of this study suggest that the provision of a genetic risk information, which reduced perceived risk in the majority of participants, did not motivate healthy changes in behavior over and above phenotypic risk information or standard lifestyle advice alone. Findings are consistent with those of a cohort study of the impact of direct-to-consumer genome-wide testing [48] and recent trials of type 2 diabetes genetic risk information [18,19] and add to existing evidence showing that those who undergo testing seldom experience psychological harm [13,49]. While risk information appears not to motivate changes in health behaviors, there is some evidence that it may influence decisions about use of medication [50,51].
Previous research indicates that the effect of communicating genetic risk information on perceived risk, a central construct in many health behavior theories, is unclear. Most studies report that perceived risk decreases after receipt of risk information, usually towards a more accurate perception of risk [52]. We found that the provision of a genetic risk estimate was associated with lower and more accurate perceived risk both immediately and after eight weeks. However, there were no differences in risk perception between those receiving genetic information and a phenotypic risk estimate that can be calculated using routinely collected clinical data. Small but statistically significant immediate effects of risk information on diet response efficacy, illness understanding, and perceived severity may have arisen through multiple testing.
The strengths of this trial include the recruitment of a relatively large sample, a randomized design with sufficient power to assess clinically important impacts on objectively measured behavior, and a high rate of study completion (97%). We presented risk information in a manner similar to that of several direct-to-consumer genetic testing companies. Potential limitations are that participants were recruited from one location in the UK, were well-educated, physically and psychologically healthy, and exhibited limited socioeconomic and ethnic diversity. Consequently, the results might not generalize to other settings or groups. Other limitations include the use of a baseline measure of physical activity that occurred prior to enrollment in the study, self-report questionnaires to measure all secondary outcomes, and a relatively short time to follow-up. However, it is widely assumed that the continuous measure of physical activity for three or more days accurately captures habitual physical activity levels. To the extent that this is true and baseline differences were equally distributed across study groups, the time between the baseline measure of physical activity and enrolment should not have influenced the results. Furthermore, it is unlikely that communication of risk information would have a long-term effect in the absence of an impact in the short term. We did not attempt to assess the clinical validity and utility of the genetic or phenotypic risk estimates as the objective of the trial was to determine the effect of the reported estimates on behavior, regardless of the accuracy of the estimate. However, the risk estimates have been validated in other studies. Although nearly all participants who received a risk estimate believed it to be accurate, the extent to which they were aware of the uncertain clinical validity and utility of predictive genetic tests may have influenced the results of the trial.
In conclusion, we found that communicating an estimate of the risk of type 2 diabetes, either based on genotype or phenotype, did not motivate changes in behavior in the short term, but neither did it cause an increase in worry or anxiety. These findings are consistent with systematic review evidence and should inform the ongoing debate regarding the regulatory response to the proliferation of direct-to-consumer genetic testing companies. Additional research is needed to investigate the conditions under which risk information might enhance preventive strategies. Approaches targeting individual behavior change, such as communicating genetic risk, are unlikely to be successful in isolation in an environment in which there are many impediments to being physically active and eating a healthy diet. The results of the current study thus provide further evidence for a shift in focus for promoting healthy changes in habitual, environmentally patterned behaviors, such as physical activity and diet, away from interventions solely based on provision of information and advice to individuals towards interventions that target the wider collective determinants of disease [53].
Supporting Information
Zdroje
1. Hu FB. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care. 2011;34 : 1249–57. doi: 10.2337/dc11-0442 21617109
2. Simmons RK, Unwin N, Griffin SJ. International Diabetes Federation: An update of the evidence concerning the prevention of type 2 diabetes. Diabetes Res Clin Pract. 2010;87 : 143–149. doi: 10.1016/j.diabres.2009.10.003 19913319
3. Ahmad LA, Crandall JP. Type 2 diabetes prevention: A review. Clin Diabetes. 2010;28 : 53–59. doi: 10.2337/diaclin.28.2.53
4. Yates T, Davies M, Khunti K. Preventing type 2 diabetes: can we make the evidence work? Postgrad Med J. 2009;85 : 475–80. doi: 10.1136/pgmj.2008.076166 19734515
5. Edelman E, Eng C. A practical guide to interpretation and clinical application of personal genomic screening. BMJ. 2009;339: b4253. doi: 10.1136/bmj.b4253 19875427
6. Mihaescu R, Meigs J, Sijbrands E, Janssens AC. Genetic risk profiling for prediction of type 2 diabetes. PLOS Curr. 2011;3: RRN1208. doi: 10.1371/currents.RRN1208 21278902
7. McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: Will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;31 : 89–103. doi: 10.1146/annurev.publhealth.012809.103532 20070198
8. Wang C, Bowen DJ, Kardia SLR. Research and practice opportunities at the intersection of health education, health behavior, and genomics. Heal Educ Behav. 2005;32 : 686–701. doi: 10.1177/1090198105278827 16148214
9. Loos RJF. Genetics: Genome-wide risk profiles—will they change your life(style)? Nat Rev Endocrinol. Nature Publishing Group; 2011;7 : 252–4. doi: 10.1038/nrendo.2011.41 21403666
10. Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research. Soc Sci Med. 2006;62 : 1360–8. doi: 10.1016/j.socscimed.2005.08.005 16162383
11. Marteau TM. Genetic risk and behavioural change. BMJ. 2001;322 : 1056–1059. doi: 10.1136/bmj.322.7293.1056 11325776
12. Bensend TA, Veach PMC, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23 : 48–63. doi: 10.1007/s10897-013-9605-3 23754506
13. Collins RE, Wright AJ, Marteau TM. Impact of communicating personalized genetic risk information on perceived control over the risk: A systematic review. Genet Med. 2011;13 : 273–7. doi: 10.1097/GIM.0b013e3181f710ca 20921892
14. Cameron LD, Sherman KA, Marteau TM, Brown PM. Impact of genetic risk information and type of disease on perceived risk, anticipated affect, and expected consequences of genetic tests. Heal Psychol. 2009;28 : 307–16. doi: 10.1037/a0013947 19450036
15. Milne S, Sheeran P, Orbell S. Prediction and Intervention in Health-Related Behavior: A Meta-Analytic Review of Protection Motivation Theory. J Appl Soc Psychol. 2000;30 : 106–143. doi: 10.1111/j.1559-1816.2000.tb02308.x
16. McClure J. Are biomarkers useful treatment aids for promoting health behavior change? An empirical review. Am J Prev Med. 2002;22 : 200–207. doi: 10.1016/S0749-3797(01)00425-1 11897465
17. Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. Bmj. 2016;352: i1102. doi: 10.1136/bmj.i1102 26979548
18. Voils CI, Coffman CJ, Grubber JM, Edelman D, Sadeghpour A, Maciejewski ML, et al. Does Type 2 Diabetes Genetic Testing and Counseling Reduce Modifiable Risk Factors? A Randomized Controlled Trial of Veterans. J Gen Intern Med. 2015;30 : 1591–8. doi: 10.1007/s11606-015-3315-5 25876740
19. Grant RW, O’Brien KE, Waxler JL, Vassy JL, Delahanty LM, Bissett LG, et al. Personalized genetic risk counseling to motivate diabetes prevention: a randomized trial. Diabetes Care. 2013;36 : 13–9. doi: 10.2337/dc12-0884 22933432
20. Godino JG, van Sluijs EMF, Marteau TM, Sutton S, Sharp SJ, Griffin SJ. Effect of communicating genetic and phenotypic risk for type 2 diabetes in combination with lifestyle advice on objectively measured physical activity: protocol of a randomised controlled trial. BMC Public Health. BMC Public Health; 2012;12 : 444. doi: 10.1186/1471-2458-12-444 22708638
21. MRC Epidemiology Unit. The Fenland Study. In: 2007 [Internet]. [cited 1 Nov 2011]. Available: http://www.mrc-epid.cam.ac.uk/research/studies/fenland-study/
22. Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59 : 561–70. doi: 10.1038/sj.ejcn.1602118 15714212
23. Cardiovascular Review Group Committee on Medical Aspects of Food Policy. Nutritional aspects of cardiovascular disease. Report on health and social subjects 46. London; 1994.
24. Department of Health Physical Activity Health Improvement and Prevention. At least five a week: Evidence on the impact of physical activity and its relationship to health. A report from the Chief Medical Officer. London; 2004 Dec.
25. Price H. Risk communication: Why, what and how? Diabetes Prim Care. 2010;12 : 100–104.
26. Gigerenzer G, Edwards A. Simple tools for understanding risks: From innumeracy to insight. BMJ. 2003;327 : 741–744. doi: 10.1136/bmj.327.7417.741 14512488
27. Edwards A, Gray J, Clarke A, Dundon J, Elwyn G, Gaff C, et al. Interventions to improve risk communication in clinical genetics: Systematic review. Patient Educ Couns. 2008;71 : 4–25. doi: 10.1016/j.pec.2007.11.026 18207694
28. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. “If I’m better than average, then I’m ok?”: Comparative information influences beliefs about risk and benefits. Patient Educ Couns. 2007;69 : 140–144. doi: 10.1016/j.pec.2007.08.008 17942271
29. Mason D, Prevost AT, Sutton S. Perceptions of absolute versus relative differences between personal and comparison health risk. Heal Psychol. 2008;27 : 87–92. doi: 10.1037/0278-6133.27.1.87 18230018
30. Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324 : 827–30. doi: 10.1136/bmj.324.7341.827 11934777
31. Cameron L. Can our health behaviour models handle imagery-based processes and communications? Eur Heal Psychol. 2009;11 : 56–58.
32. DeCODEme. Risk calculations. Reykjavík, Iceland; 2007.
33. Navigenics. The science behind the Navigenics service. Forster City, California; 2007.
34. andMe. (White Paper 23–01) Estimating genotype-specific incidence for one or several loci. Mountain View, California; 2007.
35. Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: Towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev. 2000;16 : 164–71. 10867715
36. Rahman M, Simmons RK, Harding A-H, Wareham NJ, Griffin SJ. A simple risk score identifies individuals at high risk of developing type 2 diabetes: A prospective cohort study. Fam Pract. 2008;25 : 191–6. doi: 10.1093/fampra/cmn024 18515811
37. Brage S, Ekelund U, Brage N, Hennings M a, Froberg K, Franks PW, et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol. 2007;103 : 682–92. doi: 10.1152/japplphysiol.00092.2006 17463305
38. Brage S, Brage N, Franks PW, Ekelund U, Wong M-Y, Andersen LB, et al. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol. 2004;96 : 343–51. doi: 10.1152/japplphysiol.00703.2003 12972441
39. Thompson D, Batterham AM, Bock S, Robson C, Stokes K. Assessment of low-to-moderate intensity physical activity thermogenesis in young adults using synchronized heart rate and accelerometry with branched-equation modeling. J Nutr. 2006;136 : 1037–42. 16549471
40. Strath SJ, Brage S, Ekelund U. Integration of physiological and accelerometer data to improve physical activity assessment. Med Sci Sport Exerc. 2005;37: S563–S571. doi: 10.1249/01.mss.0000185650.68232.3f
41. StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011.
42. White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med. 2005;24 : 993–1007. doi: 10.1002/sim.1981 15570623
43. Godino JG, Watkinson C, Corder K, Marteau TM, Sutton S, Sharp SJ, et al. Impact of personalised feedback about physical activity on change in objectively measured physical activity (the FAB Study): A randomised controlled trial. Baradaran HR, editor. PLoS One. Public Library of Science; 2013;8: e75398. doi: 10.1371/journal.pone.0075398 24066178
44. Borm GF, Fransen J, Lemmens W a JG. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60 : 1234–8. doi: 10.1016/j.jclinepi.2007.02.006 17998077
45. Zlowodzki M, Bhandari M. Outcome measures and implications for sample-size calculations. J Bone Jt Surg. 2009;91 Suppl 3 : 35–40. doi: 10.2106/JBJS.H.01602 19411498
46. Godino JG, van Sluijs EMF, Sutton S, Griffin SJ. Understanding perceived risk of type 2 diabetes in healthy middle-aged adults: A cross-sectional study of associations with modelled risk, clinical risk factors, and psychological factors. Diabetes Res Clin Pract. 2014;106 : 412–419. doi: 10.1016/j.diabres.2014.10.004 25467619
47. Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010;10: CD007275. doi: 10.1002/14651858.CD007275.pub2 20927756
48. Bloss CS, Schork NJ, Topol EJ. Effect of Direct-to-Consumer Genomewide Profiling to Assess Disease Risk. N Engl J Med. 2011;364 : 524–534. doi: 10.1056/NEJMoa1011893 21226570
49. Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10 : 19–32. doi: 10.1097/GIM.0b013e31815f524f 18197053
50. Usher-Smith JA, Silarova B, Schuit E, Moons KGM, Griffin SJ. Impact of provision of cardiovascular disease risk estimates to healthcare professionals and patients: a systematic review. BMJ Open. 2015;5: e008717. doi: 10.1136/bmjopen-2015-008717 26503388
51. Kullo IJ, Jouni H, Austin EE, Brown S-A, Kruisselbrink TM, Isseh IN, et al. Incorporating a Genetic Risk Score Into Coronary Heart Disease Risk Estimates: Effect on Low-Density Lipoprotein Cholesterol Levels (the MI-GENES Clinical Trial). Circulation. 2016;133 : 1181–8. doi: 10.1161/CIRCULATIONAHA.115.020109 26915630
52. Sivell S, Elwyn G, Gaff CL, Clarke AJ, Iredale R, Shaw C, et al. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: Systematic review. J Genet Couns. 2008;17 : 30–63. doi: 10.1007/s10897-007-9132-1 17968638
53. Frieden TR. A framework for public health action: The health impact pyramid. Am J Public Health. 2010;100 : 590–5. doi: 10.2105/AJPH.2009.185652 20167880
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Effectiveness of Seasonal Malaria Chemoprevention in Children under Ten Years of Age in Senegal: A Stepped-Wedge Cluster-Randomised Trial
- Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial
- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Projected Impact of Mexico’s Sugar-Sweetened Beverage Tax Policy on Diabetes and Cardiovascular Disease: A Modeling Study
- Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?
- Educational Outreach with an Integrated Clinical Tool for Nurse-Led Non-communicable Chronic Disease Management in Primary Care in South Africa: A Pragmatic Cluster Randomised Controlled Trial
- Measures of Malaria Burden after Long-Lasting Insecticidal Net Distribution and Indoor Residual Spraying at Three Sites in Uganda: A Prospective Observational Study
- Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults
- Under-prescribing of Prevention Drugs and Primary Prevention of Stroke and Transient Ischaemic Attack in UK General Practice: A Retrospective Analysis
- The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
- Improving the Pipeline for Developing and Testing Pharmacological Treatments in Pregnancy
- Seasonal Malaria Chemoprevention: An Evolving Research Paradigm
- Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study
- Minimally Invasive Autopsy: A New Paradigm for Understanding Global Health?
- Towards Equity in Health: Researchers Take Stock
- Willingness to Know the Cause of Death and Hypothetical Acceptability of the Minimally Invasive Autopsy in Six Diverse African and Asian Settings: A Mixed Methods Socio-Behavioural Study
- Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels
- Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis
- Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study
- The Dengue Vaccine Dilemma: Balancing the Individual and Population Risks and Benefits
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



