-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Viral Small Interfering RNAs Target Host Genes to Mediate Disease
Symptoms in Plants
The Cucumber mosaic virus (CMV) Y-satellite RNA (Y-Sat) has a
small non-protein-coding RNA genome that induces yellowing symptoms in infected
Nicotiana tabacum (tobacco). How this RNA pathogen induces
such symptoms has been a longstanding question. We show that the yellowing
symptoms are a result of small interfering RNA (siRNA)-directed RNA silencing of
the chlorophyll biosynthetic gene, CHLI. The CHLI mRNA contains a 22-nucleotide
(nt) complementary sequence to the Y-Sat genome, and in Y-Sat-infected plants,
CHLI expression is dramatically down-regulated. Small RNA sequencing and
5′ RACE analyses confirmed that this 22-nt sequence was targeted for mRNA
cleavage by Y-Sat-derived siRNAs. Transformation of tobacco with a RNA
interference (RNAi) vector targeting CHLI induced Y-Sat-like symptoms. In
addition, the symptoms of Y-Sat infection can be completely prevented by
transforming tobacco with a silencing-resistant variant of the CHLI gene. These
results suggest that siRNA-directed silencing of CHLI is solely responsible for
the Y-Sat-induced symptoms. Furthermore, we demonstrate that two
Nicotiana species, which do not develop yellowing symptoms
upon Y-Sat infection, contain a single nucleotide polymorphism within the
siRNA-targeted CHLI sequence. This suggests that the previously observed species
specificity of Y-Sat-induced symptoms is due to natural sequence variation in
the CHLI gene, preventing CHLI silencing in species with a mismatch to the Y-Sat
siRNA. Taken together, these findings provide the first demonstration of small
RNA-mediated viral disease symptom production and offer an explanation of the
species specificity of the viral disease.
Published in the journal: Viral Small Interfering RNAs Target Host Genes to Mediate Disease Symptoms in Plants. PLoS Pathog 7(5): e32767. doi:10.1371/journal.ppat.1002022
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002022Summary
The Cucumber mosaic virus (CMV) Y-satellite RNA (Y-Sat) has a
small non-protein-coding RNA genome that induces yellowing symptoms in infected
Nicotiana tabacum (tobacco). How this RNA pathogen induces
such symptoms has been a longstanding question. We show that the yellowing
symptoms are a result of small interfering RNA (siRNA)-directed RNA silencing of
the chlorophyll biosynthetic gene, CHLI. The CHLI mRNA contains a 22-nucleotide
(nt) complementary sequence to the Y-Sat genome, and in Y-Sat-infected plants,
CHLI expression is dramatically down-regulated. Small RNA sequencing and
5′ RACE analyses confirmed that this 22-nt sequence was targeted for mRNA
cleavage by Y-Sat-derived siRNAs. Transformation of tobacco with a RNA
interference (RNAi) vector targeting CHLI induced Y-Sat-like symptoms. In
addition, the symptoms of Y-Sat infection can be completely prevented by
transforming tobacco with a silencing-resistant variant of the CHLI gene. These
results suggest that siRNA-directed silencing of CHLI is solely responsible for
the Y-Sat-induced symptoms. Furthermore, we demonstrate that two
Nicotiana species, which do not develop yellowing symptoms
upon Y-Sat infection, contain a single nucleotide polymorphism within the
siRNA-targeted CHLI sequence. This suggests that the previously observed species
specificity of Y-Sat-induced symptoms is due to natural sequence variation in
the CHLI gene, preventing CHLI silencing in species with a mismatch to the Y-Sat
siRNA. Taken together, these findings provide the first demonstration of small
RNA-mediated viral disease symptom production and offer an explanation of the
species specificity of the viral disease.Introduction
Plant viruses are often accompanied by small parasitic RNAs termed satellite RNAs. Satellite RNAs range in size from ∼220 to 1400 nucleotides (nt) in length and depend on their associated viruses (known as the helper virus) for replication, encapsidation, movement and transmission, but share little or no sequence homology to the helper virus itself [1]. Most satellite RNAs do not encode functional proteins, yet can induce disease symptoms which range from chlorosis and necrosis, to total death of the infected plant [1], [2]. How such non-protein-coding RNA pathogens induce disease symptoms has been a longstanding question. Early studies showed that the pathogenicity of a satellite RNA is determined at the nucleotide level, with one to several nucleotide changes dramatically altering both the virulence and host specificity of disease induction [2]–[6]. Subsequent studies demonstrated that satellite RNA replication is associated with the accumulation of high levels of satellite RNA-derived small interfering RNAs (siRNA) [7]. This class of small RNA (sRNA) has been shown to direct RNA silencing in plants through sequence-specific mRNA cleavage or DNA methylation [8], [9]. Taken together, these findings led to the suggestion that pathogenic satellite-derived siRNAs might have sequence complementarity to a physiologically important host gene, and that the observed disease symptoms are in fact due to satellite siRNA-directed silencing of the targeted host gene [10]. However, to date, no such host gene has been identified, leaving the satellite RNA-induced disease mechanism unsolved. In this report we explore the sRNA-mediated disease mechanism using the Y-satellite of Cucumber mosaic virus (CMV Y-Sat). The CMV Y-Sat consists of a 369-nt single-stranded RNA genome and induces distinct yellowing symptoms in a number of Nicotiana species including N. tabacum (tobacco) [11]. We show that the Y-Sat-induced yellowing symptoms result from Y-Sat siRNA-directed silencing of the host chlorophyll biosynthetic gene, CHLI. Furthermore, we demonstrate that Y-Sat-induced symptoms can be prevented by transforming tobacco with a silencing-resistant version of CHLI and provide evidence that the observed species specificity of Y-Sat-induced disease symptoms is due to natural sequence variation within the targeted region of the CHLI transcript.
Results
The chlorophyll biosynthetic gene CHLI is silenced by Y-Sat-derived siRNAs
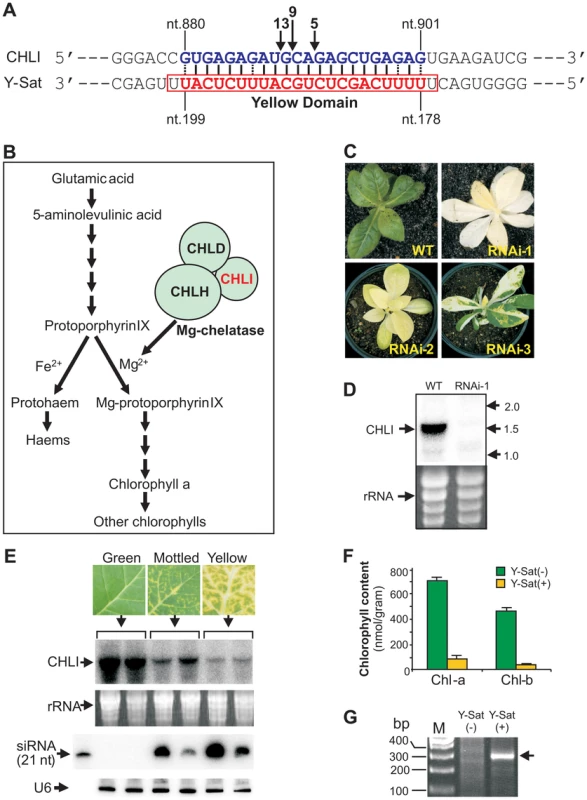
The nucleotide sequence responsible for the yellowing symptoms of the CMV satellite disease has been mapped to a small 24-nt region of the Y-Sat genome (nt. 177–200 and Figure 1A, referred to hereon as the ‘yellow domain’) [11]–[14]. Genetic analysis of progeny plants from a cross between the disease-susceptible species N. bigelovii and the disease-resistant species N. clevelandii, suggested that the yellowing symptoms induced upon CMV Y-Sat infection are associated with a single, incompletely dominant gene in the Nicotiana species [15]. We hypothesized that a siRNA derived from the yellow domain was directing RNA silencing of a tobacco gene, which in turn led to the expression of the observed yellowing symptoms. BLAST searches with the 24-nt yellow domain sequence against tobacco sequences in the NCBI database were performed to identify 21-nt or longer target sequences. No cDNA with perfect 21-nt complementarity was identified, however, accommodating weak G:U base pairings as a match allowed for the identification of a single N. tabacum sequence complementary to 22 nt of the Y-Sat yellow domain (from nt. 178 to nt.199; Figure 1A). The identified sequence is part of the coding region of the magnesium chelatase subunit CHLI, a 426-amino acid protein essential for chlorophyll biosynthesis (Figure 1B; see Text S1 for sequences). Previous studies have shown that Y-Sat-induced yellowing symptoms are associated with reduced chlorophyll content [16], making CHLI a strong target candidate for Y-Sat siRNA-directed silencing. Transformation of N. tabacum with a RNA interference (RNAi) vector targeting CHLI resulted in a dramatic decrease in CHLI expression and severe chlorosis of the transgenic plants, which ranged from yellowing to complete bleaching (Figure 1C–D). The phenotypes expressed by RNAi plants parallels the appearance of Y-Sat-induced symptoms, and are consistent with CHLI silencing being responsible for the disease phenotype.
Northern blot analysis confirmed that CHLI mRNA was dramatically down-regulated upon CMV Y-Sat infection (Figure 1E). CHLI expression was not affected by infection with the CMV helper virus alone (Figure S1). The level of CHLI down-regulation in CMV Y-Sat-infected plants correlated strongly with the severity of chlorosis, and with the accumulation of yellow domain-specific siRNAs (Figure 1E). As expected, CHLI down-regulation was associated with a dramatic reduction in chlorophyll content (Figure 1F). 5′ rapid amplification of cDNA ends (5′ RACE) on RNA samples extracted from CMV Y-Sat-infected and uninfected tobacco plants showed that the down-regulation of CHLI was due to siRNA-directed RNA cleavage at the predicted 22-nt target site. An expected 310-base pair (bp) product was amplified from the CMV Y-Sat-infected plants; however, no such product could be amplified from RNA extracts of uninfected tobacco (Figure 1G). Sequencing of the 5′ RACE product showed that RNA cleavage occurred at three distinct positions within the 22-nt Y-Sat siRNA-targeted CHLI sequence (Figure 1A).
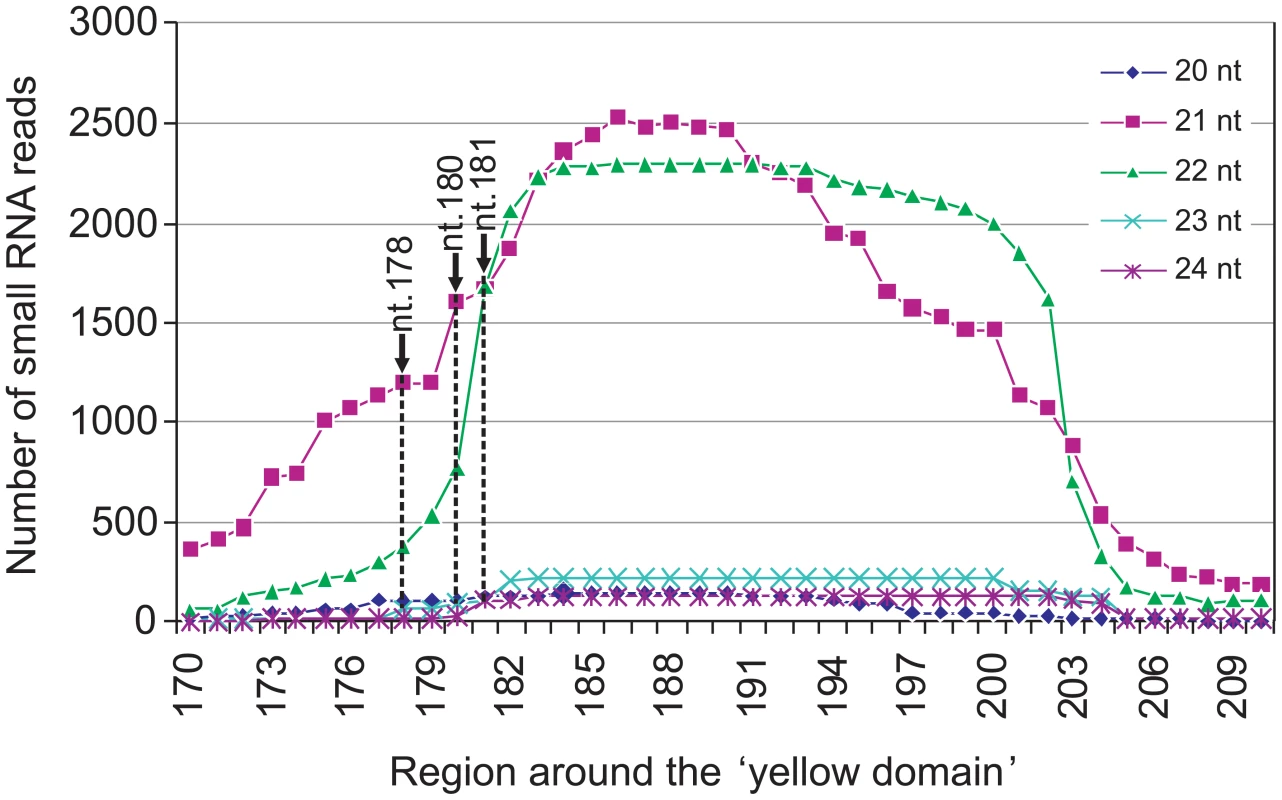
siRNA-directed cleavage of a target RNA normally occurs across nucleotides 10 and 11 of the siRNA [17]. The three cleavage sites detected by the 5′ RACE analyses therefore implied that the silencing of CHLI is directed by three individual Y-Sat siRNA species with their 5′-terminal nucleotides corresponding to nt. 178, 180 and 181 of the Y-Sat RNA genome respectively (Figure 1A). To confirm that these specific siRNAs were present, the total sRNA population of CMV Y-Sat-infected tobacco plants was sequenced using Solexa technology. Approximately 4 million sRNA reads were obtained, of which 1 million were of the 21–22 nt siRNA size class derived from the plus (+) strand of the Y-Sat RNA genome. From this (+) strand-specific pool, siRNAs corresponding to the yellow domain region form part of a siRNA hot spot along the Y-Sat genome (Figure S2). Furthermore, the 21–22-nt siRNAs starting at nt. 178, 180 and 181 accumulated at relatively high frequencies, with 1576, 2368 and 3352 reads detected respectively (Figure 2). The above results correlate with the proposal that the yellowing symptoms associated with CMV Y-Sat infection of tobacco are due to Y-Sat yellow domain-specific siRNA-directed silencing of the CHLI gene.
Transformation of N. tabacum with a silencing-resistant version of CHLI prevents Y-Sat symptoms
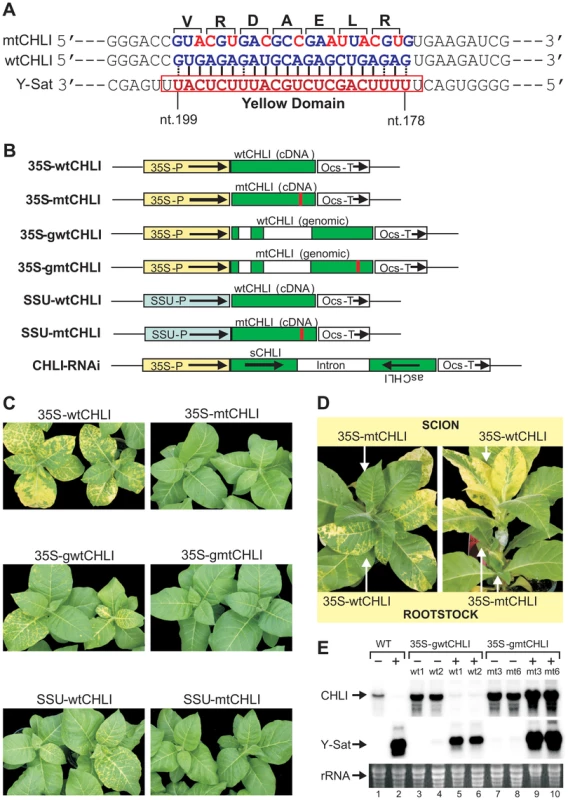
To determine if CHLI silencing alone was responsible for the Y-Sat-induced symptoms, we introduced 10-nt silent mutations into the 22-nt complementary sequence of the wild-type CHLI gene (wtCHLI), and transformed tobacco plants with the mutated version of the CHLI gene (mtCHLI). The modified CHLI transgene contains 10 nucleotide changes within the 22-nt region complementary to Y-Sat yellow domain siRNAs, bringing nine mismatches to this region of complementarity, rending it resistant to cleavage by these Y-Sat siRNAs (Figure 3A–B). Strikingly, the Y-Sat-induced symptoms were completely abolished in tobacco plants transformed with the mtCHLI constructs; none of the 44 independent transgenic lines developed yellowing symptoms upon CMV Y-Sat infection (Figure 3C; Table S1). This is in direct contrast to the population of tobacco plants transformed with the wtCHLI constructs, where 34 of 36 plant lines analysed showed yellowing symptoms upon CMV Y-Sat infection. The absence of symptoms in mtCHLI lines was not due to a lack of Y-Sat accumulation; mtCHLI plants grafted with diseased wtCHLI lines, either as the scion or rootstock, remained symptom free (Figure 3D). Northern blot hybridization analysis confirmed that the Y-Sat RNA accumulated to high levels in both wtCHLI and mtCHLI plants (Figure 3E). The CHLI transcript level was dramatically reduced in infected wtCHLI plants, but remained high in infected mtCHLI plants (Figure 3E, compare lanes 5–6 with lanes 9–10). These analyses indicated that the modified CHLI transcript was resistant to Y-Sat siRNA-directed silencing, allowing for sufficient accumulation of this modified transcript in infected plants for normal chlorophyll biosynthesis. These results also suggest that sRNA-directed silencing of CHLI alone is sufficient for the induction of the disease symptoms associated with CMV Y-Sat infection. Taken together, our findings strongly indicate that sRNA-mediated viral disease symptoms can be prevented through the introduction of a silencing-resistant version of a sRNA targeted host gene(s).
Y-Sat disease-resistant Nicotiana species contain single-nucleotide variation in the siRNA-targeted CHLI sequence
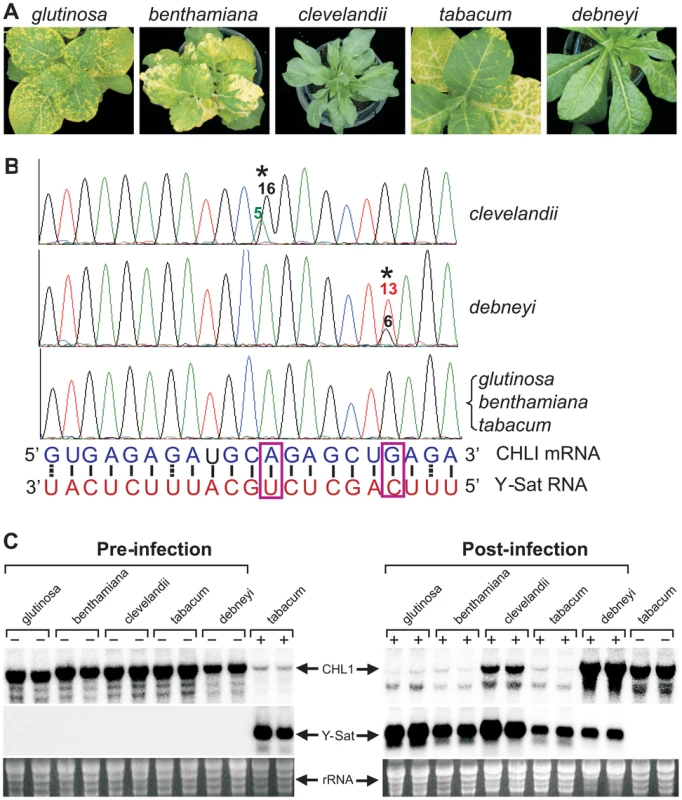
Not all Nicotiana species are susceptible to Y-Sat-induced yellowing symptoms [15]. This suggests that sequence variations might exist in the coding sequence of the CHLI gene in some Nicotiana species, rendering these species ‘resistant’ or free of Y-Sat siRNA-induced CHLI silencing. We sequenced the CHLI transcript from five different Nicotiana species, including three disease-susceptible (N. tabacum, N. glutinosa and N. benthamiana) and two resistant (N. clevelandii and N. debneyi) species (Figure 4A). The three susceptible species which develop yellowing symptoms upon CMV Y-Sat infection had identical target sequences in the CHLI gene (Figure 4B). In contrast, of the two disease-resistant species which do not develop yellowing symptoms upon infection, N. clevelandii expressed two CHLI mRNA species (presumably because it is an allotetraploid containing two different chromosome pairs), with the predominant species containing an A to G change at the targeted CHLI sequence, converting tobacco's A:U base pairing to a weaker G:U wobble pair near the mRNA cleavage site (Figure 4B). Similarly, N. debneyi expressed two CHLI mRNA species with the predominant form containing a G to U conversion compared with the tobacco sequence, changing a strong G:C pairing to a U-C mismatch. Northern blot hybridization of CMV Y-Sat-infected and uninfected plants of these five Nicotiana species showed that CHLI expression was strongly silenced in infected plants of the susceptible species (Figure 4C). In contrast, CHLI mRNA levels remained high in infected N. clevelandii and N. debneyi plants suggesting that the mRNA variants expressed by these two symptomless species are resistant to Y-Sat siRNA-induced silencing. These results suggest that the previously observed host species specificity of satellite RNA-induced diseases [1], [2] results from sequence variation within the viral sRNA-targeted host gene(s). In addition, these results further demonstrate that the disease symptoms associated with CMV Y-Sat infection are solely due to silencing of the CHLI gene.
Discussion
Our results demonstrate that the yellowing symptoms associated with CMV Y-Sat infection in tobacco result from Y-Sat siRNA-directed silencing of the chlorophyll biosynthetic gene CHLI. This finding is consistent with results from previous site-directed mutagenesis studies showing that Y-Sat mutants with nucleotide changes inside the yellow domain either retain or lose the ability to induce yellowing symptoms [13], [14]. In agreement with siRNA-directed silencing of CHLI solely accounting for the yellowing symptoms associated with CMV Y-Sat infection, Y-Sat variants which are capable of inducing such symptoms have higher degrees of sequence complementarity to the CHLI target sequence than those that can no longer induce yellowing upon CMV Y-Sat infection (13,14; Figure S3).
As observed for the yellowing symptoms associated with CMV Y-Sat infection, several other disease symptoms induced by viral satellite RNAs have also been associated with a short sequence within the respective satellite RNA genomes. For instance, the chlorotic phenotypes induced by the B2 and WL3 satellite RNAs of CMV in tobacco and tomato are determined by a ∼26-nt region (nt. 141–166) of the satellite genome [2], [5], [6]. Interestingly, BLAST searching with this sequence identifies a 21-nt match to a tobacco cDNA (accession # U62485) encoding a glycolate oxidase, which is involved in plant photorespiration and, when the expression of the gene is down-regulated, plants develop yellowing [18]. Furthermore, the necrotic symptoms induced by CMV Y-Sat infection in tomato are also associated with a short sequence, from nt. 309 to 334, of the Y-Sat genome [4]. Single nucleotide changes to these short sequences have been shown to dramatically affect both the severity and host specificity of these satellite RNA-induced disease symptoms [4]–[6]. Taken together, these observations suggest that siRNA-directed host gene silencing is a common mechanism for satellite RNA-induced symptoms. This mechanism could also be extended to viroids, another group of small non-protein-coding RNA pathogens in plants [19]. The pathogenicity of Potato spindle tuber viroid (PSTVd), a 359-nt non-protein-coding RNA pathogen, has been associated with two ∼20-nt partially complementary regions of the PSTVd RNA genome known as virulence-modulating regions [20]. Furthermore, expression of an hairpin RNA transgene derived from PSTVd in tomato resulted in the expression of symptoms similar to PSTVd infection [10], suggesting that a sRNA-directed RNA silencing mechanism is also responsible for the disease symptoms induced by PSTVd infection.
The siRNA-mediated disease mechanism reported here is consistent with the previous observation that disease induction by satellite RNAs involves the interaction of satellite RNAs, their helper viruses, and the host plant [1], [2]. Diseases caused by satellite-derived siRNA-mediated host gene silencing would require i) the existence of sufficient sequence complementarity between the satellite RNA genome and a host gene mRNA; ii) a helper virus that supports high levels of replication of the satellite; and iii) host RNA silencing machineries for efficient siRNA biogenesis and siRNA-directed mRNA degradation. One of the key helper virus-encoded factors for satellite-induced disease symptoms would be a RNA silencing suppressor protein. Most plant viruses encode silencing suppressor proteins, which inhibit sRNA-mediated silencing in their host [21]. Silencing suppressors from helper viruses could affect satellite siRNA-mediated diseases in two ways. The suppressor protein could inhibit host antiviral silencing to enhance the accumulation of satellite RNAs, or, it could act to inhibit satellite siRNA-induced silencing of complementary host gene sequences to minimize the disease symptoms. Consistent with the latter possibility, transgenic expression of the potyvirus silencing suppressor P1/HC-Pro in tobacco almost completely abolished the yellowing symptoms induced by CMV Y-Sat infection [10]. Also, the chlorotic symptoms induced by the B2 and WL3 satellites of CMV in tobacco are associated specifically with RNA2 of subgroup II CMV: the symptoms are diminished when the satellites are replicated by subgroup I CMV [22]. A recent study demonstrated that subgroup I CMV encodes a stronger silencing suppressor protein (2b, encoded by RNA 2) than subgroup II CMV [23], raising the possibility that the lack of strong B2 and WL3 satellite-induced chlorosis in the presence of subgroup I CMV is due to CMV 2b-mediated suppression of satellite siRNA-induced host gene silencing. Thus, viral-encoded silencing suppressor proteins may have a dual function, helping the virus or subviral agent to evade sRNA-mediated antiviral defence by preventing the silencing of viral RNAs, and minimizing symptom severity by inhibiting the silencing of host genes.
RNA silencing has been suggested to be a driving force for the evolution of subviral RNAs including viral satellite RNAs [1], [10]. These RNA species tend to form stable secondary structures that are resistant to siRNA-mediated degradation [10], [24]. Also, satellite RNAs have little or no sequence homology with their helper virus genome [1], and this is presumably to avoid the silencing of the helper viruses by satellite-derived siRNAs. Our findings have further implications for the involvement of RNA silencing in the evolution of viral satellite RNAs in plants. Satellite RNAs with extensive sequence homologies to host genes would result in silencing of the targeted gene(s), affecting the viability of the host plant. This in turn, would affect the survival of both the helper virus and its satellite RNA. Consistent with this scenario, there has been no report showing extensive sequence homology between satellite RNAs and their host genome.
As discussed for satellites and viroids, infection of plants with both RNA and DNA viruses is also associated with the accumulation of virus-derived siRNAs [21]. Furthermore, more than 100 miRNAs have been identified from animal viruses [25]. Whether sRNA-directed host gene silencing plays a wider role in plant and animal viral disease remains to be investigated. Nevertheless, numerous plant and animal viral sRNAs have been shown to match host gene sequences and therefore have the potential to down-regulate their expression [26]–[30]. This raises the possibility that at least some virus-induced diseases are due to viral sRNA-directed silencing of host genes. Recent advances in high-throughput DNA and RNA sequencing technologies are expected to result in complete genomic sequences, not only for the infecting viruses, but also for their respective hosts, providing new opportunities to identify host genes that are potentially targeted by virus-derived sRNAs.
We have demonstrated here that transformation of tobacco with a silencing-resistant version of the CHLI gene completely prevented the yellowing symptoms associated with Y-Sat infection. This finding offers a potential new strategy for preventing viral siRNA-mediated diseases in plants and animals. However, viral replication has relatively high error rates and viruses often exist as quasispecies (mixtures of minor sequence variants) [31]. Thus, a modified host target gene protected against siRNAs of the original virus could potentially be silenced by siRNAs from a variant virus with an altered sequence. Introducing multiple nucleotide changes into the target sequence of the host gene, as demonstrated for the modified CHLI sequence harbouring 10 single nucleotide modifications, could minimize the recurrence of host gene silencing by siRNAs of variant viruses.
Materials and Methods
Plant growth, viral infection, RNA isolation and analysis, BLAST searching, and high-throughput small RNA sequencing
All Nicotiana species were grown in a 25°C glasshouse with natural light. Infection of tobacco plants with Cucumber mosaic virus plus Y-Sat, total RNA extraction and northern blot hybridization were performed as previously described [32]. Solexa sequencing of small RNAs from Y-Sat-infected tobacco was carried out at the Allan Wilson Centre Genome Service (New Zealand). BLAST searching to identify Y-Sat-targeted tobacco genes was performed as follows: 21-nt segments of the Y-Sat yellow domain sequence (e.g. nt.177–197, nt. 178–198, nt. 179–199) were used to BLAST search common tobacco sequences (taxid:4097) of the NCBI database “nucleotide collection (nr/nt)”. This search identified the N. tobacum CHLI cDNA (accessions: U67064 and AF014053) as the “best” match with the Y-Sat sequence.
Plasmid construction
Full-length genomic and cDNA sequences of the CHLI gene were amplified from total DNA and RNA using the NEB Long Amp Taq kit and Qiagen One Step RT-PCR kit respectively according to the manufacturer's instructions. The CHLI forward primer (5′ ATCTGGTACCAAAATGGCTTCACTACTAGGAACTTCC 3′) and reverse primer (5′ TCTAGTCTAGAAGCTTAAAACAGCTTAGGCGAAAACCTC 3′) were used for both the PCR and RT-PCR reactions. PCR products were purified using a QIAquick PCR purification kit (Qiagen), cloned into the pGem-T Easy cloning vector (Promega) and sequenced. The full-length genomic and cDNA sequences (see Text S1) were digested with KpnI/XbaI and cloned into the intermediate vector pBC (Strategene).
To create the modified CHLI sequence (mtCHLI and gmtCHLI), a 630 bp sequence containing the Y-Sat targeted 22-nt sequence was amplified as two halves; i) the 5′ half was amplified with forward (WT-F1, 5′ TGGCACAATCGACATTGAGAAAGC 3′) and reverse (MT-R1, 5′ ACGTAATTCGGCGTCACGTACGGTCCCCACTTGGGGATGC 3′) primers that spanned the 22-nt CHLI target site and allowed for the introduction of modified nucleotides, and ii) the 3′ segment was amplified with a forward primer (MT-F2, 5′ GTACGTGACGCCGAATTACGTGTGAAGATAGTTGAGGAAAGAG 3′) that also contained modified nucleotides spanning the 22-nt CHLI target sequence overlapping with MT-R1 and a reverse primer (WT-R2, 5′ AGCAGTTGGGAATGACAGTGGC3′). The two amplified products were joined together using overlapping PCR with Pfu polymerase (Promega) to generate a 630 bp fragment with a modified Y-satellite target sequence. A 223 bp fragment, containing the modified target sequence, was released by PstI and EcoRI digestion and used to replace the PstI-EcoRI fragment of the wild type sequence in the CHLI cDNA, giving rise to the modified sequence mtCHLI. Digestion of the mtCHLI sequence with PstI and BamHI released the modified region which was used to replace the corresponding region in the wild-type CHLI genomic sequence, giving rise to the modified genomic clone gmtCHLI.
The wild-type and mutated cDNA or genomic sequences were digested with KpnI and XbaI and inserted between the 35S promoter and the Ocs terminator in pART7 [33], and the resulting expression cassettes were cloned into the binary vector pART27 [33] as a NotI fragment. Similarly, the wild-type and mutant CHLI cDNA clones were cloned into a binary vector with the rubisco small sub unit promoter.
To prepare the RNAi construct, CHLI cDNA was digested with PstI and XbaI releasing a 571 bp fragment spanning the Y-Sat target site. This fragment was cloned into the Gateway-based hairpin RNA gene silencing vector Hellsgate 12 which incorporates a spliceable intron for improved silencing efficiency [34], [35].
Tobacco transformation
All plant expression vectors were introduced into Agrobacterium tumefaciens strain LBA4404 by triparental mating in the presence of the helper plasmid pRK2013. Agrobacterium-mediated transformation of tobacco was carried out as described previously [36], using 50 mg/L kanamycin as the selective agent.
5′ RACE
Total RNA (2 µg) was ligated to a 24-nt RNA adaptor (5′ AACAGACGCGUGGUUACAGUCUUG 3′) using T4 RNA ligase (Promega) at room temperature for 2 hours in 50 mM HEPES pH 7.5, 0.1 mg/mL BSA, 8% glycerol, 2 units/µL RNasein RNase inhibitor (Promega) and 0.5 unit/µL T4 RNA ligase (Promega). The ligation was purified by phenol-chloroform extraction and ethanol precipitation. The purified product was reverse-transcribed using a CHLI-specific reverse primer (5′ AGCAGTTGGGAATGACAGTGGC 3′). Primary PCR was then performed using a forward primer matching the RNA adaptor (5′ AACAGACGCGTGGTTACAGTC 3′) and the CHLI reverse primer (as above). The RT-PCR product was then amplified using the same forward primer with a nested CHLI reverse primer (5′ ATATCTTCCGGAGTTACCTTATC 3′). The nested PCR product was separated on a 2% agarose gel, purified with the Ultra Clean-15 DNA purification kit (Mo Bio Laboratories), and ligated into the pGEM-T Easy vector (Promega) for sequencing.
Chlorophyll assay
Approximately 0.5 grams fresh weight of plant material was ground into fine powder in liquid nitrogen, mixed with 15 mL methanol and filtered through filter paper. Chlorophyll a and chlorophyll b were measured in a spectrophotometer (Biochrom WPA light wave II) at a wavelength of 663 nm and 645 nm respectively. Chlorophyll concentration was measured as nmol per gram of fresh weight.
Supporting Information
Zdroje
1. HuCCHsuYHLinNS
2009
Satellite RNAs and satellite viruses of plants.
Viruses
1
1325
1350
2. Garcia-ArenalFPalukaitisP
1999
Structure and functional relationship of satellite RNAs of
cucumber mosaic virus.
Curr Top Microbiol Immunol
239
37
63
3. PalukaitisPRoossinckMJ
1996
Spontaneous change of a benign satellite RNA of cucumber mosaic
virus to a pathogenic variant.
Nat Biotechnol
14
1264
1268
4. DevicMJaegleMBaulcombeD
1990
Cucumber mosaic virus satellite RNA (strain Y): analysis of
sequences which affect systemic necrosis on tomato.
J Gen Virol
71
1443
1449
5. SleatDEPalukaitisP
1992
A single nucleotide change within a plant virus satellite RNA
alters the host specificity of disease induction.
Plant J
2
43
49
6. ZhangLKimCHPalukaitisP
1994
The chlorosis-induction domain of the satellite RNA of cucumber
mosaic virus: identifying sequences that affect accumulation and the degree
of chlorosis.
Mol Plant Microbe Interact
7
208
213
7. WangMBWesleySVFinneganEJSmithNAWaterhousePM
2001
Replicating satellite RNA induces sequence-specific DNA
methylation and truncated transcripts in plants.
RNA
7
16
28
8. GhildiyalMZamorePD
2009
Small silencing RNAs: an expanding universe.
Nat Rev Genet
10
94
108
9. WirthSCrespiM
2009
Non-protein coding RNAs, a diverse class of gene regulators, and
their action in plants.
RNA Biol
6
161
164
10. WangMBBianXYWuLMLiuLXSmithNA
2004
On the role of RNA silencing in the pathogenicity and evolution
of viroids and viral satellites.
Proc Natl Acad Sci U S A
101
3275
3280
11. MasutaCTakanamiY
1989
Determination of sequence and structural requirements for
pathogenicity of a cucumber mosaic virus satellite RNA
(Y-satRNA).
Plant Cell
1
1165
1173
12. DevicMJaegleMBaulcombeD
1989
Symptom production on tobacco and tomato is determined by two
distinct domains of the satellite RNA of cucumber mosaic virus (strain
Y).
J Gen Virol
70
2765
2774
13. JaegleMDevicMLongstaffMBaulcombeD
1990
Cucumber mosaic virus satellite RNA (Y strain): analysis of
sequences which affect yellow mosaic symptoms on tobacco.
J Gen Virol
71
1905
1912
14. KuwataSMasutaCTakanamiY
1991
Reciprocal phenotype alterations between two satellite RNAs of
cucumber mosaic virus.
J Gen Virol
72
2385
2389
15. MasutaCSuzukiMKuwataSTakanamiYKoiwaiA
1993
Yellow mosaic induced by Y satellite RNA of cucumber mosaic virus
is regulated by a single incompletely dominant gene in wild
Nicotiana species.
Genetics
83
411
413
16. MasutaCSuzukiMMatsuzakiTHondaIKuwataS
1993
Bright yellow chlorosis by cucumber mosaic virus Y satellite RNA
is specifically induced without severe chloroplast damage.
Physiol Mol Plant Pathol
42
267
278
17. HaleyBZamorePD
2004
Kinetic analysis of the RNAi enzyme complex.
Nat Struct Mol Biol
11
599
606
18. YamaguchiKNishimuraM
2000
Reduction to below threshold levels of glycolate oxidase
activities in transgenic tobacco enhances photoinhibition during
irradiation.
Plant Cell Physiol
41
1397
1406
19. TsagrisEMMartínez de AlbaAEGozmanovaMKalantidisK
2008
Viroids.
Cell Microbiol
10
2168
2179
20. SchmitzARiesnerD
1998
Correlation between bending of the VM region and pathogenicity of
different Potato Spindle Tuber Viroid strains.
RNA
4
1295
1303
21. DingSWVoinnetO
2007
Antiviral immunity directed by small RNAs.
Cell
130
413
426
22. SleatDEPalukaitisP
1990
Induction of tobacco chlorosis by certain cucumber mosaic virus
satellite RNAs is specific to subgroup II helper strains.
Virol
176
292
295
23. YeJQuJZhangJFGengYFFangRX
2009
A critical domain of the Cucumber mosaic virus 2b protein for RNA
silencing suppressor activity.
FEBS Lett
583
101
6
24. ItayaAZhongXBundschuhRQiYWangY
2007
A structured viroid RNA serves as a substrate for dicer-like
cleavage to produce biologically active small RNAs but is resistant to
RNA-induced silencing complex-mediated degradation.
J Virol
81
2980
2984
25. GottweinECullenBR
2008
Viral and cellular microRNAs as determinants of viral
pathogenesis and immunity.
Cell Host Microbe
3
375
387
26. MoissiardGVoinnetO
2006
RNA silencing of host transcripts by cauliflower mosaic virus
requires coordinated action of the four Arabidopsis Dicer-like
proteins.
Proc Natl Acad Sci U S A
103
19593
19598
27. GreyFHookLNelsonJ
2008
The functions of herpesvirus-encoded microRNAs.
Med Microbiol Immunol
197
261
267
28. QiXBaoFSXieZ
2009
Small RNA deep sequencing reveals role for Arabidopsis thaliana
RNA-dependent RNA polymerases in viral siRNA biogenesis.
PLoS One
4
e4971
29. GreyFTirabassiRMeyersHWuGMcWeeneyS
2010
A viral microRNA down-regulates multiple cell cycle genes through
mRNA 5′ UTRs.
Plos Pathog
6
e1000967
30. LinKYChengCPChangBCWangWCHuangYW
2010
Global analyses of small interfering RNAs derived from Bamboo
mosaic virus and its associated satellite RNAs in different
plants.
PLoS One
5
e11928
31. SteinhauerDAHollandJJ
1987
Rapid evolution of RNA viruses.
Ann Rev Microbiol
41
409
433
32. SmithNAEamensALWangMB
2010
The presence of high-molecular-weight viral RNAs interferes with
the detection of viral small RNAs.
RNA
16
1062
1067
33. GleaveAP
1992
A versatile binary vector system with a T-DNA organisational
structure conducive to efficient integration of cloned DNA into the plant
genome.
Plant Mol Biol
20
1203
1207
34. HelliwellCAWaterhousePM
2005
Constructs and methods for hairpin RNA-mediated gene silencing in
plants.
Methods Enzymol
392
24
35
35. SmithNASinghSPWangMBStoutjesdijkPAGreenAG
2000
Total silencing by intron-spliced hairpin RNAs.
Nature
407
319
20
36. EllisJGLlewellynDJDennisESPeacockWJ
1987
Maize Adh1 promoter sequences control anaerobic regulation:
Addition of upstream promoter elements from constitutive genes is necessary
for expression in tobacco.
EMBO J
6
11
16
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus SpeciesČlánek SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion ReleaseČlánek Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T CellsČlánek A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the CytosolČlánek Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- Infections Are Virulent and Inhibit the Human Malaria Parasite in
- A Gamma Interferon Independent Mechanism of CD4 T Cell Mediated Control of Infection
- MDA5 and TLR3 Initiate Pro-Inflammatory Signaling Pathways Leading to Rhinovirus-Induced Airways Inflammation and Hyperresponsiveness
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- An E2F1-Mediated DNA Damage Response Contributes to the Replication of Human Cytomegalovirus
- Quantitative Subcellular Proteome and Secretome Profiling of Influenza A Virus-Infected Human Primary Macrophages
- Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus Species
- Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA
- A Viral Satellite RNA Induces Yellow Symptoms on Tobacco by Targeting a Gene Involved in Chlorophyll Biosynthesis using the RNA Silencing Machinery
- Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Investigating the Host Binding Signature on the PfEMP1 Protein Family
- Human Neutrophil Clearance of Bacterial Pathogens Triggers Anti-Microbial γδ T Cell Responses in Early Infection
- Septation of Infectious Hyphae Is Critical for Appressoria Formation and Virulence in the Smut Fungus
- A Family of Helminth Molecules that Modulate Innate Cell Responses via Molecular Mimicry of Host Antimicrobial Peptides
- Phospholipids Trigger Capsular Enlargement during Interactions with Amoebae and Macrophages
- CTL Escape Mediated by Proteasomal Destruction of an HIV-1 Cryptic Epitope
- Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens
- Extensive Genome-Wide Variability of Human Cytomegalovirus in Congenitally Infected Infants
- The Antiviral Efficacy of HIV-Specific CD8 T-Cells to a Conserved Epitope Is Heavily Dependent on the Infecting HIV-1 Isolate
- Epstein-Barr Virus Infection of Polarized Epithelial Cells via the Basolateral Surface by Memory B Cell-Mediated Transfer Infection
- Reactive Oxygen Species Hydrogen Peroxide Mediates Kaposi's Sarcoma-Associated Herpesvirus Reactivation from Latency
- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- The Dot/Icm System Delivers a Unique Repertoire of Type IV Effectors into Host Cells and Is Required for Intracellular Replication
- AAV Exploits Subcellular Stress Associated with Inflammation, Endoplasmic Reticulum Expansion, and Misfolded Proteins in Models of Cystic Fibrosis
- Suboptimal Activation of Antigen-Specific CD4 Effector Cells Enables Persistence of In Vivo
- SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion Release
- Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T Cells
- Transition of Sporozoites into Liver Stage-Like Forms Is Regulated by the RNA Binding Protein Pumilio
- A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the Cytosol
- Transcriptome Analysis of in Human Whole Blood and Mutagenesis Studies Identify Virulence Factors Involved in Blood Survival
- Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
- Structural Insights into Viral Determinants of Nematode Mediated Transmission
- Protective Efficacy of Serially Up-Ranked Subdominant CD8 T Cell Epitopes against Virus Challenges
- Viral CTL Escape Mutants Are Generated in Lymph Nodes and Subsequently Become Fixed in Plasma and Rectal Mucosa during Acute SIV Infection of Macaques
- Taking Some of the Mystery out of Host∶Virus Interactions
- Viral Small Interfering RNAs Target Host Genes to Mediate Disease Symptoms in Plants
- : An Emerging Cause of Sexually Transmitted Disease in Women
- Mitochondrial Ubiquitin Ligase MARCH5 Promotes TLR7 Signaling by Attenuating TANK Action
- The Hexamer Structure of the Rift Valley Fever Virus Nucleoprotein Suggests a Mechanism for its Assembly into Ribonucleoprotein Complexes
- Acquisition of Human-Type Receptor Binding Specificity by New H5N1 Influenza Virus Sublineages during Their Emergence in Birds in Egypt
- Stromal Down-Regulation of Macrophage CD4/CCR5 Expression and NF-κB Activation Mediates HIV-1 Non-Permissiveness in Intestinal Macrophages
- A Component of the Xanthomonadaceae Type IV Secretion System Combines a VirB7 Motif with a N0 Domain Found in Outer Membrane Transport Proteins
- Perturbs Iron Trafficking in the Epithelium to Grow on the Cell Surface
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- : An Emerging Cause of Sexually Transmitted Disease in Women
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy