-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
article has not abstract
Published in the journal: Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack. PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003815
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003815Summary
article has not abstract
Introduction
The innate immune system employs various defense mechanisms to combat invading microbes. From a pathogen perspective, access to adequate nutrition is one of the fundamental requirements for survival within the host. The ability to counter microbial survival by restricting basic elements of growth, extending from amino acids to sugars and metals, is referred to as nutritional immunity [1]. The mechanisms of Zn acquisition, transport, and storage have been investigated in both prokaryotic and eukaryotic systems. In this review, the total amount of zinc regardless of its chemical form will be referred to as Zn, and the labile fraction as Zn2+. From an immunological perspective, the primary focus has been on the impact of Zn regulation on the numbers and function of lymphocytes and phagocytes and their correlation with susceptibility to infections, but a dissection of the molecular details in these processes has been lacking. More recently, understanding the Zn modulatory mechanisms and how they drive host-pathogen interactions at the molecular level has been a subject of intense scrutiny. This review will accentuate existing and novel insights into the roles of Zn in nutritional immunity and in phagocyte defenses against fungi.
Zinc Takes Center Stage: A Common Requisite in Host-Pathogen Interactions
Regulation of Zn homeostasis is essential for several host functions at multiple levels: i) for cellular processes including, but not limited to, transcription, translation, catalysis, and cell division; ii) for countering Zn2+ deficiency or excess; and iii) for immunomodulatory responses in host-pathogen interactions. An estimated 10% or 2,800 proteins in the human genome are Zn-dependent, implying a critical role for this metal in biological functions [2]. In the immune system, Zn regulation is of paramount importance as the development and function of innate and adaptive arms of immunity are influenced by this metal [3]. Zn homeostasis established by a balance in Zn2+ flux, intracellular distribution, and storage impacts phagocytosis, leukocyte recruitment, cytokine production, glycolysis, and oxidation triggered in response to immune signals. Aberrant Zn regulation in the circulation or in cells mitigates robust immune activation and leads to suboptimal host defenses. For example, Zn deficiency in humans with the genetic disorder acrodermatitis enteropathica is caused by Zn malabsorption and characterized by increased susceptibility to infections. An excess of Zn2+ diminishes T cell mitogenic responses [4]. Thus, an intact immune response requires strict Zn2+ regulation.
The fundamental requirement of Zn2+ for the function of several enzymes, transcription factors, and structural proteins [5] is evident not only in mammals but also in bacteria and fungi [6], in principal, due to the redox-inert property of this metal [7]. Zn2+ enhances the synthesis of toxic secondary metabolites such as Aspergillus flavus mycotoxins that inhibit phagocytosis and cytotoxicity of T cells [8]–[10]. Zn2+-dependent superoxide dismutases (SODs) produced by Cryptococcus neoformans, Histoplasma capsulatum, and Candida albicans are critical for scavenging superoxide radicals produced by phagocytes [11]–[13]. These factors underscore the significance of Zn acquisition and distribution for fungal pathogenesis and survival within the host. Thus, the struggle for Zn2+ between host and pathogen impacts survival of the invader and defense by the immune system.
Zinc Acquisition Strategies: Host versus Fungi
The immune system maintains Zn equilibrium via transporters, storage, and binding mechanisms (Figure 1). While lower eukaryotes such as fungi possess fewer Zn2+ transporters [14], mammals have 24 transporters, called ZIPs (Slc39a, importers) and ZNTs (Slc30a, exporters). Some transporters manifest a ubiquitous expression pattern in several host cells, and others exhibit tissue specificity and function irreplaceably in Zn2+ transport. For example, Slc30a1is widely expressed in >12 organs, while Slc39a4 expression is restricted to the small intestine and kidney and is absolutely essential for dietary Zn absorption [15]. Spatial organization of the transporters regulates Zn2+ in the cytosol and intracellular compartments including Golgi, mitochondria, and zincosomes that are a source of exchangeable metal during deficiency [16]. The remarkable complexity in Zn2+ transporters reflects the need for strict homeostasis and a regulatory system that responds to different biological stimuli in an organelle-, cell-, and tissue-specific manner. For example, interleukin-6 induces Zn2+ import via ZIP14 in hepatocytes [17], while granulocyte macrophage-colony stimulating factor (GM-CSF) triggers Zn2+ uptake via ZIP2 in macrophages [18]. The dependence of mammals on dietary sources for the metal implies the need for mechanisms that efficiently acquire Zn2+ and maintain regulated distribution in organ systems. Metallothioneins (MTs) comprise a class of metal binding proteins that regulate Zn2+ and prevent intoxication. MTs bind Zn2+ with picomolar affinity through seven binding sites, one of which is more readily exchangeable, and interactions with glutathione, ATP, or GTP mediate Zn2+ release [19]. These properties facilitate a controlled exchange mechanism in infected phagocytes, where Zn2+ access to the microorganism needs to be restricted. Thus, phagocytes possess manifold mechanisms to manipulate Zn resources during infection.
Fig. 1. Schematic of Zn regulation in phagocytes. 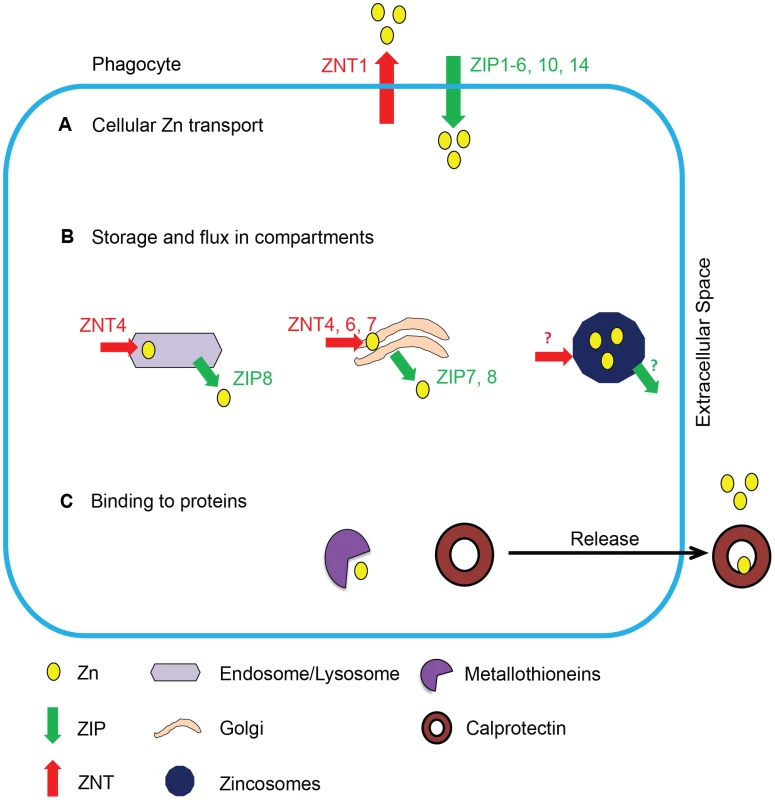
Mechanisms of Zn regulation in phagocytes, grouped into three categories: Zn2+ transport, storage, and binding. (A) Zn2+ transport across the cell membrane is mediated by ZIPs and ZNTs. (B) Intracellular Zn2+ is transported into and stored in organelles such as endosomes, lysosomes, Golgi, and zincosomes by various transporters represented in the figure; the transporters that mediate Zn2+ flux across zincosomes have not been identified. (C) Zn2+ is bound and sequestered by intracellular or secreted metal binding proteins such as MTs and calprotectin. To establish infection, fungi must adapt to limited nutrient availability upon encounter with the host. Upon phagocytosis, C. albicans triggers a transcriptional response signature reflecting a state of nutrient deprivation within macrophages [20]. For pathogenic fungi, gaining entry into the host is associated with a transition from a possibly Zn2+-sufficient external environment [21] to a lower Zn2+-containing milieu. Similarly, for opportunistic fungi such as C. albicans, the shift from a commensal to a pathogenic state may be accompanied by a dramatic paucity in Zn2+, primarily due to Zn2+ restriction in the extracellular environment [22]. Histoplasma capsulatum, Cryptococcus neoformans, and Blastomyces dermatitidis thrive in soil containing 30–350 µM bioavailable Zn2+ [23]. Within macrophages, they are confronted with an environment containing only picomolar quantities of freely exchangeable Zn2+ [7]. To thwart host defenses and establish infection, fungi must exert strategies to sense and respond to metal scarcity caused by sequestration into intracellular niches or binding to host proteins via high affinity interactions. Mechanisms responding to Zn2+ availability in fungi include proteins that are directly affected by the presence or absence of Zn2+. For instance, Zn2+ inhibits DNA-binding activity of the Zn-responsive activator protein, Zap1p; however, a limiting milieu leads to transcription of Zap1p-dependent Zn2+ acquisition machinery. The upregulation of ZRT1 and ZRT2 transporters by Zap1p under a Zn2+-deficient state is critical, as the absence of these importers diminishes fungal pathogenicity [14], [24]. In Cryptococcus gattii, an ortholog of ZAP1 is upregulated by Zn2+ deficiency. Genetic deletion of ZAP1 impairs growth in a Zn2+-limiting environment, and mice infected with ZAP1 mutant yeasts exhibit increased survival [25]. These findings emphasize a role for Zn2+ regulation in fungal virulence.
Although extracellular fungi do not directly compete for the pool of Zn2+ within cells, they must secure the metal from a restricted environment in infected tissue. A. fumigatus possesses ZrfA and ZrfB analogous to Zrt1p and Zrt2p that facilitate Zn2+ uptake in a low-pH environment during deficiency [26]. In a specialized mechanism described as the “zincophore system,” C. albicans hyphae sequester host Zn2+ by secretion of pH-regulated antigen-1, which reassociates with Zrt1p for subsequent import [24]. Thus, multiple Zn2+ acquisition strategies in fungi collectively diminish vulnerability to host immunity. To establish virulence in vivo, these factors must contribute persistently to cope with metal scarcity induced by the immune system.
Host Zinc Pool: Restricted Access
Microbes are extremely sensitive to metal availability, and phagocytes have mastered mechanisms to curtail pathogen access to Zn2+. Despite our knowledge of Zn homeostasis, the manner in which the innate system modulates Zn2+ regulatory proteins in the context of fungal interactions and its influence on survival has been sparingly investigated.
In macrophages, a dual stimulus involving GM-CSF and H. capsulatum infection potently induces Zn2+ influx by ZIP2. The enhanced Zn2+ uptake may reflect at least two possibilities: i) a stress response during infection to support macrophage functions such as increased transcription, and ii) a mechanism to deprive extracellular yeasts of Zn2+ analogous to the induction of hypozincemia during bacterial sepsis. This phenomenon can be viewed as an opportunity for H. capsulatum to exploit Zn2+ elevation to capture more labile Zn2+ within the host. However, despite increased influx, GM-CSF creates a state of “deprived” intracellular Zn2+ by two mechanisms. First, GM-CSF causes Zn2+ localization in the Golgi, a shift associated with expression of exporters ZNT4 and ZNT7 that export cytosolic Zn2+ into the Golgi. Second, signaling via signal transducer and activator of transcription STAT3 and STAT5 triggers the production of MTs that constrict the labile Zn2+ pool by binding the metal [18]. These studies highlight a fundamental Zn2+ sequestration property of transporters and MTs, which starves the pathogen and orchestrates a Zn2+ deprivation mechanism deployed by GM-CSF in macrophages (Figure 2).
Fig. 2. Schematic of Zn regulation in activated macrophages infected with a fungal pathogen. 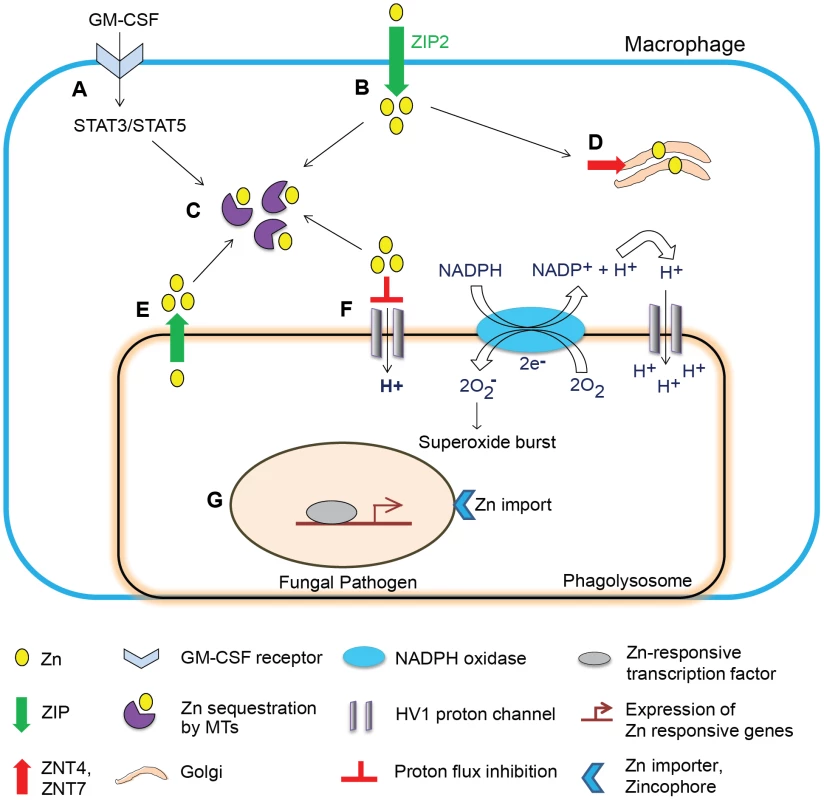
Zn regulation in a GM-CSF–activated macrophage leading to defense against fungal infection. (A) GM-CSF binds to the GM-CSF receptor on infected macrophages, activates STAT3 and STAT5 signaling, and triggers transcriptional activation in the nucleus. (B) Induction of ZIP2 causes increased Zn2+ influx, which may support increased metabolic functions to cope with stress in the infected macrophage. (C) STAT3 and STAT5 induce expression of MTs that sequester labile intracellular Zn2+. (D) Zn2+ is mobilized into the Golgi apparatus, associated with increased expression of Golgi membrane transporters ZNT4 and ZNT7. (E) Speculated lysosomal Zn deprivation by influx into the cytosol by ZIPs; the dotted arrow represents predicted sequestration of Zn2+ from this source by MTs. (F) Zn2+ inhibits proton flux via HV1, but the “Zn2+-deprived” environment lifts the inhibitory action (shown on extreme right of the phagolysosomal membrane) and H+ generated by Nox activity is channeled into phagolysosomes effectively sustaining production of superoxide radicals by the enzyme. (G) The pathogen senses a Zn2+-deprived environment and activates Zn-responsive transcription machinery to trigger Zn2+ import via fungal transporters and zincophore systems; ultimately, deficiency of Zn2+ starves the pathogen of this metal and simultaneously enhances superoxide burst in phagocytes, culminating into inhibition of fungal growth. Spatial localization of Zn2+ transporters in the host potentially influences Zn2+ acquisition by intracellular fungi. ZNT4 enhances endosomal Zn2+ [27] that may be advantageous to the pathogen upon phagolysosomal fusion. In contrast, ZIP8 in T cells deprives lysosomal Zn2+ by importing it into the cytosol [28]. Though a role for importers in lysosomal Zn2+ deprivation has not been described in phagocytes, the existence of such a mechanism would starve the pathogen of Zn2+. Subcellular localization of transporters may be sensitive to the cellular microenvironment causing differential transport of Zn2+ in response to varying stimuli.
Apart from transporters and MTs, phagocytes produce calprotectin that binds Zn2+ with nanomolar affinity. Calprotectin in neutrophil extracellular traps (NETs) inhibits growth of C. albicans and also contributes to the fungistatic effect of plasmacytoid dendritic cells in A. fumigatus infection [29], [30]. Conversely, an inability to regulate excess of Zn2+ jeopardizes pathogen survival. In exploiting this to the host's advantage, human macrophages impose Zn2+ intoxication in mycobacteria-laden phagosomes. Mycobacterial P1-type ATPases efflux the metal and alleviate Zn2+ poisoning [31]. A role for heavy metal efflux pumps in fungal resistance to metal poisoning remains to be dissected. Thus, the immune system utilizes divergent Zn2+ restriction or intoxication mechanisms to combat infection. How immune cells preferentially utilize opposing stratagems against different microbial classes and the molecular cues that govern these decisions remain unclear. Collectively, Zn2+ modulation is a compelling arm of the innate system in restraining fungal persistence.
Zinc Regulation: An Impact beyond Nutritional Immunity
Regulation of Zn2+ shapes the functional attributes of innate defense, impacting phagocyte function beyond nutritional immunity. GM-CSF–activated macrophages counter pathogen attack by eliciting a dual defense strategy comprising Zn2+ restriction to H. capsulatum, while concurrently enhancing phagocyte effector function. Zn2+ abates superoxide production by NADPH oxidase (Nox) by inhibiting hydrogen voltage-gated channel HV1. Fungi scavenge superoxide radicals via Zn and Cu or Mn dependent SODs [11], [12]. In activated macrophages, MTs bind Zn2+ and create an environment deficient in Zn2+ ions, in effect, sustaining HV1 and Nox function (Figure 2). In this milieu, H. capsulatum is susceptible to ROS [18], presumably due to an ineffectual Zn and Cu dependent SOD response. The extent of Zn2+ deprivation by MTs results in effective superoxide production, and may simultaneously compromise fungal SOD-mediated defenses.
Collectively, Zn2+ restriction drives antifungal defense through a concurrent twofold effect: first, it induces Zn2+ starvation in the pathogen, and second, it strengthens oxidative burst-mediated defenses of the innate system. Thus, innate immunity is equipped with a variety of Zn2+ restriction strategies that function cooperatively to eliminate pathogens. As novel roles for metals are being described in dictating the outcome of immune regulation, we may be only beginning to appreciate the prominence of trace metals in immune defenses against infection.
Zdroje
1. HoodMI, SkaarEP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10 : 525–537.
2. AndreiniC, BanciL, BertiniI, RosatoA (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5 : 196–201.
3. FrakerPJ, GershwinME, GoodRA, PrasadA (1986) Interrelationships between zinc and immune function. Fed Proc 45 : 1474–1479.
4. RinkL, GabrielP (2000) Zinc and the immune system. Proc Nutr Soc 59 : 541–552.
5. ColemanJE (1992) Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem 61 : 897–946.
6. AndreiniC, BertiniI, RosatoA (2009) Metalloproteomes: a bioinformatic approach. Acc Chem Res 42 : 1471–1479.
7. ColvinRA, HolmesWR, FontaineCP, MaretW (2010) Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2 : 306–317.
8. CueroR, OuelletT (2005) Metal ions modulate gene expression and accumulation of the mycotoxins aflatoxin and zearalenone. J Appl Microbiol 98 : 598–605.
9. YamadaA, KataokaT, NagaiK (2000) The fungal metabolite gliotoxin: immunosuppressive activity on CTL-mediated cytotoxicity. Immunol Lett 71 : 27–32.
10. YoshidaLS, AbeS, TsunawakiS (2000) Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem Biophys Res Commun 268 : 716–723.
11. YouseffBH, HolbrookED, SmolnyckiKA, RappleyeCA (2012) Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PLoS Pathog 8: e1002713 doi:10.1371/journal.ppat.1002713
12. HwangCS, RhieGE, OhJH, HuhWK, YimHS, et al. (2002) Copper - and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 148 : 3705–3713.
13. CoxGM, HarrisonTS, McDadeHC, TabordaCP, HeinrichG, et al. (2003) Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun 71 : 173–180.
14. WilsonD, CitiuloF, HubeB (2012) Zinc exploitation by pathogenic fungi. PLoS Pathog 8: e1003034 doi:10.1371/journal.ppat.1003034
15. LiuzziJP, CousinsRJ (2004) Mammalian zinc transporters. Annu Rev Nutr 24 : 151–172.
16. EideDJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763 : 711–722.
17. LiuzziJP, LichtenLA, RiveraS, BlanchardRK, AydemirTB, et al. (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A 102 : 6843–6848.
18. Subramanian VigneshK, Landero FigueroaJA, PorolloA, CarusoJA, DeepeGSJr (2013) Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39 : 697–710.
19. MaretW (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130 : 1455S–1458S.
20. LorenzMC, BenderJA, FinkGR (2004) Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3 : 1076–1087.
21. RajapakshaRM, Tobor-KaplonMA, BaathE (2004) Metal toxicity affects fungal and bacterial activities in soil differently. Appl Environ Microbiol 70 : 2966–2973.
22. CorbinBD, SeeleyEH, RaabA, FeldmannJ, MillerMR, et al. (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319 : 962–965.
23. Schulte EE (2004) Soil and applied zinc. In: Understanding plant nutrients. Wisconsin Cooperative Extension Publications. Available: http://corn.agronomy.wisc.edu/Management/pdfs/a2528.pdf. Accessed 30 November 2013.
24. CitiuloF, JacobsenID, MiramonP, SchildL, BrunkeS, et al. (2012) Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 8: e1002777 doi:10.1371/journal.ppat.1002777
25. SchneiderRdO, FogaçaNdSS, KmetzschL, SchrankA, VainsteinMH, et al. (2012) Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS ONE 7: e43773 doi:10.1371/journal.pone.0043773
26. VicentefranqueiraR, MorenoMA, LealF, CaleraJA (2005) The zrfA and zrfB genes of Aspergillus fumigatus encode the zinc transporter proteins of a zinc uptake system induced in an acid, zinc-depleted environment. Eukaryot Cell 4 : 837–848.
27. MurgiaC, VespignaniI, CeraseJ, NobiliF, PerozziG (1999) Cloning, expression, and vesicular localization of zinc transporter Dri 27/ZnT4 in intestinal tissue and cells. Am J Physiol 277: G1231–G1239.
28. AydemirTB, LiuzziJP, McClellanS, CousinsRJ (2009) Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol 86 : 337–348.
29. UrbanCF, ErmertD, SchmidM, Abu-AbedU, GoosmannC, et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5: e1000639 doi:10.1371/journal.ppat.1000639
30. Ramirez-OrtizZG, LeeCK, WangJP, BoonL, SpechtCA, et al. (2011) A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 9 : 415–424.
31. BotellaH, PeyronP, LevillainF, PoinclouxR, PoquetY, et al. (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10 : 248–259.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2013 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



