-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
article has not abstract
Published in the journal: Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions. PLoS Pathog 10(7): e32767. doi:10.1371/journal.ppat.1004093
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1004093Summary
article has not abstract
The term holobiont (Greek, from holos, whole; bios, life; -ont, to be; whole unit of life) describes a long-term physical association between different living organisms [1]. Theoretically, this definition encompasses all symbiotic associations (along the mutualism–parasitism continuum) spanning all taxa. However, in most cases, the term holobiont is restricted to the host and its associated mutualistic symbionts. The hologenome theory of evolution considers that the holobiont is the unit under natural selection in evolution [2], [3]. I argue that this opens new perspectives on the study of host–parasite interactions. Evidence suggests that all of the diverse microorganisms associated with the host and parasite play a part in the coevolution. This new paradigm has the potential to impact our comprehension of the development and evolution of disease.
It has been established in different model species that immune system maturation requires the presence of mutualistic bacteria [4]–[6]. The tsetse fly Glossina moritans carries an obligate mutualist, the bacteria Wigglesworthia glossinidia, which is necessary for maturation of the immune system during development [6], [7]. In vertebrates, species-specific gut bacteria are necessary for the maturation and the maintenance of a healthy immune system [4], [8]–[13]. Organisms are associated with a great variety of microorganisms, including viruses and unicellular eukaryotes, and we are starting to realize that they also play an important role in shaping a healthy immune system [14]–[16].
Thus, symbionts indirectly protect the host against various pathogens via immune activation (Figure 1A, 1B). In some cases, even parasites improve the fitness of their host; this process is called conditional mutualism [17]. For example, the hepatitis G virus limits the progression of HIV to AIDS [18], [19], the hepatitis A virus suppresses infection by the hepatitis C virus [20], and the murine cytomegalovirus protects mice against infection by Listeria monocytgenes and Yersinia pestis [21].
Fig. 1. Role of microorganisms associated with the host or the parasite in the host–parasite interaction. 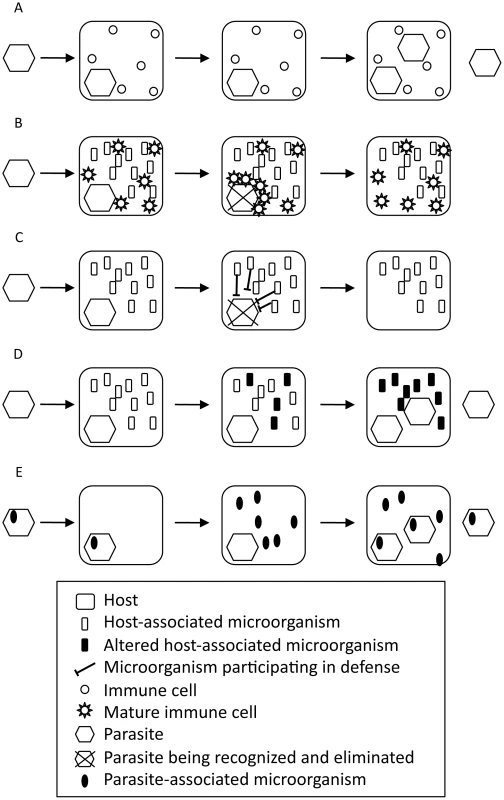
(A) Host–parasite interaction without associated microorganisms. (B) Host-associated microorganisms participate indirectly in the immune defense by promoting immune system maturation. (C) Host-associated microorganisms participate directly in the immune defense. (D) Parasite interferes with host-associated microorganisms. (E) Parasite-associated microorganisms participate in the disease. Host-associated microorganisms also contribute directly to the defense against pathogens (Figure 1C). The bacteriophage carried by the bacteria Halmitonella defensa, Acyrthosiphon pisum secondary endosymbiont (APSE) is a conditional mutualist of the pea aphid A. pisum [22]–[24]. It encodes toxins targeting the developing larva of the parasitic wasp Aphidius ervi [25], [26]. Human gut bacteria directly antagonize bacterial pathogens by producing antibacterial factors, by competing for elements necessary for pathogen growth (competitive exclusion), and by limiting their adhesion to host cells [9]. In addition, mucus-associated bacteriophages participate in the first line of defense against bacteria in various species, from cnidarians to mammals [27]. Thus, the “holo-immunome” must be studied for a comprehensive understanding of host resistance to infections.
Host-associated microorganisms are also affected by parasitosis (Figure 1D). In the coral Oculina patagonica, infection by Vibrio shiloi induces coral bleaching by directly attacking the photosynthetic microalgal endosymbionts [28], [29]. Symbiotic bacterial communities associated with the lichen Solorina crocea are also affected by the fungal parasite Rhagadostoma lichenicola [30]. HIV and SIV infections are frequently associated with gastrointestinal disorders that can be explained by an alteration of the gut microbial community [31]–[33]. As discussed above, such disruptions of host–symbiont interactions favor pathogenesis, therefore indirectly participating in the disease.
Finally, parasites are also associated with microorganisms that will directly benefit from an improved fitness of their parasitic host. These symbionts can directly participate in the disease caused by the parasite (Figure 1E). For instance, parasitoid wasps of the Ichneumonidae and Braconidae families have independently evolved mutual associations with DNA or RNA viruses (unpublished work) and play an essential role in the parasite's success and evolution [34]–[35]. Entomopathogenic nematodes are associated with bacteria that produce toxins that help degrade tissues for the nematode to feed on [36], [37]. Similarly, the plant-pathogenic fungi Rhizopus sp. has an endosymbiotic bacteria that produces toxins that have a key role in the disease [38].
Until recently, the role of parasite-associated microorganisms in human diseases had been underestimated, but examples are now starting to emerge. The Leishmania RNA virus promotes the persistence of Leishmania vienna parasites by inducing a TLR3-mediated inflammatory response that renders the host more susceptible to infection [39]. Similarly, Trichomonasvirus, an endosymbiotic of the protozoan parasite Trichomonas vaginalis is responsible for the strong proinflammatory response that causes preterm birth [40]. Microorganisms associated with such medically important parasites can now be targeted to limit the impact or development of the disease.
The theoretical framework provided by considering not only the host but also the parasite as a holobiont revealed that some interactions have been underestimated and others have not yet been explored. For example, can microorganisms associated with the host directly interact with microorganisms associated with the parasite? Can the host defend itself against infection by recognizing the microorganisms associated with the parasite? Can parasite-associated microorganisms indirectly promote the disease (by increasing its fecundity, for example)? Parasitologists, microbiologists, and immunologists have the monumental task of revealing the myriad interactions occurring between holobiont hosts and holobiont parasites. This knowledge promises to greatly impact our ability to develop new treatments and therapies.
These interactions within interactions have major implications for ecologists and evolutionary biologists, because any host–parasite interaction will be dependent on all other interactions in the system [41], [42]. The short generation time of microorganisms, along with the genetic diversity and novelty they provide [43], [44], can play an important role in the adaptation and evolution of hosts and parasites in their evolutionary arms race [45]. This coevolution may also be driven by fluctuating selection [46], in which hosts and parasites interact with different microorganisms over thousands of years, constantly evolving to favor the most advantageous symbiont at the time. In addition, associated microorganisms may be pathogenic to non-adapted individuals and drive speciation [35], [47], [48]. Thus, the study of microorganisms associated with hosts and parasites is no longer optional; it is, rather, an obligatory path that must be taken for a comprehensive understanding of the ecology and evolution of hosts and parasites. It is a necessary step for the prevention and prediction of disease outbreaks.
Zdroje
1. Margulis L (1991) Symbiogenesis and symbionticism. In: L. Margulis RFE, editor. Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. Cambridge: MIT press. pp 1–14.
2. Zilber-RosenbergI, RosenbergE (2008) Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev 32 : 723–735.
3. BruckerRM, BordensteinSR (2013) The capacious hologenome. Zoology 116 : 260–261.
4. ChungH, PampSnJ, HillJA, SuranaNK, EdelmanSM, et al. (2012) Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149 : 1578–1593.
5. SchnupfP, Gaboriau-RouthiauVr, Cerf-BensussanN (2013) Host interactions with Segmented Filamentous Bacteria: An unusual trade-off that drives the post-natal maturation of the gut immune system. Sem Immunol 25 : 342–351.
6. WeissBL, WangJ, AksoyS (2011) Tsetse immune system Maturation requires the presence of obligate symbionts in larvae. PLoS Biol 9: e1000619.
7. AksoyS (2000) Tsetse—A haven for microorganisms. Parasitol Today 16 : 114–118.
8. AbtMC, OsborneLC, MonticelliLA, DoeringTA, AlenghatT, et al. (2012) Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37 : 158–170.
9. BuffieCG, PamerEG (2013) Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13 : 790–801.
10. GanalSC, SanosSL, KallfassC, OberleK, JohnerC, et al. (2012) Priming of Natural Killer Cells by Nonmucosal Mononuclear Phagocytes Requires Instructive Signals from Commensal Microbiota. Immunity 37 : 171–186.
11. MazmanianSK, LiuCH, TzianabosAO, KasperDL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122 : 107–118.
12. MazmanianSK, RoundJL, KasperDL (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453 : 620–625.
13. RoundJL, MazmanianSK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9 : 313–323.
14. RookGAW (2009) Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunol 126 : 3–11.
15. VirginHW, WherryEJ, AhmedR (2009) Redefining chronic viral infection. Cell 138 : 30–50.
16. DuerkopBA, HooperLV (2013) Resident viruses and their interactions with the immune system. Nat Immunol 14 : 654–659.
17. HerreEA, KnowltonN, MuellerUG, RehnerSA (1999) The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol 14 : 49–53.
18. TillmannHL, HeikenH, Knapik-BotorA, HeringlakeS, OckengaJ, et al. (2001) Infection with GB Virus C and reduced mortality among HIV-infected patients. N Engl J Med 345 : 715–724.
19. XiangJ, WünschmannS, DiekemaDJ, KlinzmanD, PatrickKD, et al. (2001) Effect of coinfection with GB Virus C on survival among patients with HIV infection. N Engl J Med 345 : 707–714.
20. DeterdingK, TegtmeyerBr, CornbergM, HademJ, PotthoffA, et al. (2006) Hepatitis A virus infection suppresses hepatitis C virus replication and may lead to clearance of HCV. J Hepatol 45 : 770–778.
21. BartonES, WhiteDW, CathelynJS, Brett-McClellanKA, EngleM, et al. (2007) Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447 : 326–329.
22. PolinS, SimonJ-C, OutremanY (2014) An ecological cost associated with protective symbionts of aphids. Ecol Evol 4 : 826–830.
23. WeldonSR, StrandMR, OliverKM (2013) Phage loss and the breakdown of a defensive symbiosis in aphids. Proc Roy Soc B Biol Sci 280 : 20122103.
24. DionE, PolinSE, SimonJ-C, OutremanY (2011) Symbiont infection affects aphid defensive behaviours. Biol Lett 7 : 743–746.
25. OliverKM, DegnanPH, HunterMS, MoranNA (2009) Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325 : 992–994.
26. DegnanPH, MoranNA (2008) Diverse phage-encoded toxins in a protective insect endosymbiont. App Environ Microbiol 74 : 6782–6791.
27. BarrJJ, AuroR, FurlanM, WhitesonKL, ErbML, et al. (2013) Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Nat Acad Sci U S A 110 : 10771–10776.
28. BaninE, IsraelyT, KushmaroA, LoyaY, OrrE, et al. (2000) Penetration of the coral-bleaching bacterium Vibrio shiloi into Oculina patagonica. App Environ Microbiol 66 : 3031–3036.
29. BaninE, IsraelyT, FineM, LoyaY, RosenbergE (2001) Role of endosymbiotic zooxanthellae and coral mucus in the adhesion of the coral-bleaching pathogen Vibrio shiloi to its host. FEMS Microbiol Lett 199 : 33–37.
30. GrubeM, KöberlM, LacknerS, BergC, BergG (2012) Host–parasite interaction and microbiome response: effects of fungal infections on the bacterial community of the Alpine lichen Solorina crocea. FEMS Microbiol Ecol 82 : 472–481.
31. McKennaP, HoffmannC, MinkahN, AyePP, LacknerA, et al. (2008) The macaque gut microbiome in health, lentiviral infection, and chronic Enterocolitis. PLoS Pathog 4: e20.
32. KnoxTA, SpiegelmanD, SkinnerSC, GorbachS (2000) Diarrhea and abnormalities of gastrointestinal function in a cohort of men and women with HIV infection. Am J Gastroenterol 95 : 3482–3489.
33. GoriA, TincatiC, RizzardiniG, TortiC, QuirinoT, et al. (2008) Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in Human Immunodeficiency Virus pathogenesis. J Clinic Microbiol 46 : 757–758.
34. Beckage NE, Drezen J-M (2012) Parasitoid viruses: symbionts and pathogens. Waltham: Academic Press. 312 p.
35. JancekS, BézierA, GayralP, PaillussonC, KaiserL, et al. (2013) Adaptive selection on bracovirus genomes drives the specialization of Cotesia parasitoid wasps. PLoS ONE 8: e64432.
36. AnR, SreevatsanS, GrewalP (2009) Comparative in vivo gene expression of the closely related bacteria Photorhabdus temperata and Xenorhabdus koppenhoeferi upon infection of the same insect host, Rhizotrogus majalis. BMC Genomics 10 : 433.
37. AdamsBJ, FodorA, KoppenhöferHS, StackebrandtE, Patricia StockS, et al. (2006) Biodiversity and systematics of nematode-bacterium entomopathogens. Biol Control 37 : 32–49.
38. Partida-MartinezLP, HertweckC (2005) Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 437 : 884–888.
39. IvesA, RonetC, PrevelF, RuzzanteG, Fuertes-MarracoS, et al. (2011) Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331 : 775–778.
40. FichorovaRN, LeeY, YamamotoHS, TakagiY, HayesGR, et al. (2012) Endobiont viruses sensed by the Human host ‚ beyond conventional antiparasitic therapy. PLoS ONE 7: e48418.
41. MøllerAP (2008) Interactions between interactions: predator-prey, parasite-host, and mutualistic interactions. Ann N Y Acad Sci 1133 : 180–186.
42. OliverK, NogeK, HuangE, CamposJ, BecerraJ, et al. (2012) Parasitic wasp responses to symbiont-based defense in aphids. BMC Biol 10 : 11.
43. TaylorFJR (1979) Symbionticism revisited: A discussion of the evolutionary impact of intracellular symbioses. Proc Roy Soc Lond B Biol Sci 204 : 267–286.
44. Margulis L, Fester R (1991) Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. L. Margulis RFE, editor. Cambridge: MIT press.
45. FerrariJ, VavreF (2011) Bacterial symbionts in insects or the story of communities affecting communities. Phil Trans Roy Soc B Biol Sci 366 : 1389–1400.
46. ThompsonJN (1999) The evolution of species interactions. Science 284 : 2116–2118.
47. BruckerRM, BordensteinSR (2013) The Hologenomic Basis of Speciation: Gut Bacteria Cause Hybrid Lethality in the Genus Nasonia. Science 341 : 667–669.
48. Le Clec'hW, Braquart-VarnierC, RaimondM, FerdyJ-B, BouchonD, et al. (2012) High Virulence of Wolbachia after Host Switching: When Autophagy Hurts. PLoS Pathog 8: e1002844.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDSČlánek The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV InfectionČlánek Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Bacteriophages as Vehicles for Antibiotic Resistance Genes in the Environment
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
- Defensins and Viral Infection: Dispelling Common Misconceptions
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- The Wide World of Ribosomally Encoded Bacterial Peptides
- Microbial Egress: A Hitchhiker's Guide to Freedom
- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- HIV-1 Capture and Transmission by Dendritic Cells: The Role of Viral Glycolipids and the Cellular Receptor Siglec-1
- Tetherin Can Restrict Cell-Free and Cell-Cell Transmission of HIV from Primary Macrophages to T Cells
- The Frustrated Host Response to Is Bypassed by MyD88-Dependent Translation of Pro-inflammatory Cytokines
- Larger Mammalian Body Size Leads to Lower Retroviral Activity
- The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV Infection
- Lytic Gene Expression Is Frequent in HSV-1 Latent Infection and Correlates with the Engagement of a Cell-Intrinsic Transcriptional Response
- Phase Variation of Poly-N-Acetylglucosamine Expression in
- A Screen of Mutants Reveals Important Roles for Dot/Icm Effectors and Host Autophagy in Vacuole Biogenesis
- Structure of the Trehalose-6-phosphate Phosphatase from Reveals Key Design Principles for Anthelmintic Drugs
- The Impact of Juvenile Coxsackievirus Infection on Cardiac Progenitor Cells and Postnatal Heart Development
- Vertical Transmission Selects for Reduced Virulence in a Plant Virus and for Increased Resistance in the Host
- Characterization of the Largest Effector Gene Cluster of
- Novel Drosophila Viruses Encode Host-Specific Suppressors of RNAi
- Pto Kinase Binds Two Domains of AvrPtoB and Its Proximity to the Effector E3 Ligase Determines if It Evades Degradation and Activates Plant Immunity
- Genetic Analysis of Tropism Using a Naturally Attenuated Cutaneous Strain
- Plasmacytoid Dendritic Cells Suppress HIV-1 Replication but Contribute to HIV-1 Induced Immunopathogenesis in Humanized Mice
- A Novel Mouse Model of Gastroenteritis Reveals Key Pro-inflammatory and Tissue Protective Roles for Toll-like Receptor Signaling during Infection
- Pathogenicity of Is Expressed by Regulating Metabolic Thresholds of the Host Macrophage
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Independent Bottlenecks Characterize Colonization of Systemic Compartments and Gut Lymphoid Tissue by
- Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
- G3BP1, G3BP2 and CAPRIN1 Are Required for Translation of Interferon Stimulated mRNAs and Are Targeted by a Dengue Virus Non-coding RNA
- Cytolethal Distending Toxins Require Components of the ER-Associated Degradation Pathway for Host Cell Entry
- The Machinery at Endoplasmic Reticulum-Plasma Membrane Contact Sites Contributes to Spatial Regulation of Multiple Effector Proteins
- Arabidopsis LIP5, a Positive Regulator of Multivesicular Body Biogenesis, Is a Critical Target of Pathogen-Responsive MAPK Cascade in Plant Basal Defense
- Plant Surface Cues Prime for Biotrophic Development
- Real-Time Imaging Reveals the Dynamics of Leukocyte Behaviour during Experimental Cerebral Malaria Pathogenesis
- The CD27L and CTP1L Endolysins Targeting Contain a Built-in Trigger and Release Factor
- cGMP and NHR Signaling Co-regulate Expression of Insulin-Like Peptides and Developmental Activation of Infective Larvae in
- Systemic Hematogenous Maintenance of Memory Inflation by MCMV Infection
- Strain-Specific Variation of the Decorin-Binding Adhesin DbpA Influences the Tissue Tropism of the Lyme Disease Spirochete
- Distinct Lipid A Moieties Contribute to Pathogen-Induced Site-Specific Vascular Inflammation
- Serovar Typhi Conceals the Invasion-Associated Type Three Secretion System from the Innate Immune System by Gene Regulation
- LANA Binds to Multiple Active Viral and Cellular Promoters and Associates with the H3K4Methyltransferase hSET1 Complex
- A Molecularly Cloned, Live-Attenuated Japanese Encephalitis Vaccine SA-14-2 Virus: A Conserved Single Amino Acid in the Hairpin of the Viral E Glycoprotein Determines Neurovirulence in Mice
- Illuminating Fungal Infections with Bioluminescence
- Comparative Genomics of Plant Fungal Pathogens: The - Paradigm
- Motility and Chemotaxis Mediate the Preferential Colonization of Gastric Injury Sites by
- Widespread Sequence Variations in VAMP1 across Vertebrates Suggest a Potential Selective Pressure from Botulinum Neurotoxins
- An Immunity-Triggering Effector from the Barley Smut Fungus Resides in an Ustilaginaceae-Specific Cluster Bearing Signs of Transposable Element-Assisted Evolution
- Establishment of Murine Gammaherpesvirus Latency in B Cells Is Not a Stochastic Event
- Oncogenic Herpesvirus KSHV Hijacks BMP-Smad1-Id Signaling to Promote Tumorigenesis
- Human APOBEC3 Induced Mutation of Human Immunodeficiency Virus Type-1 Contributes to Adaptation and Evolution in Natural Infection
- Innate Immune Responses and Rapid Control of Inflammation in African Green Monkeys Treated or Not with Interferon-Alpha during Primary SIVagm Infection
- Chitin-Degrading Protein CBP49 Is a Key Virulence Factor in American Foulbrood of Honey Bees
- Influenza A Virus Host Shutoff Disables Antiviral Stress-Induced Translation Arrest
- Nsp9 and Nsp10 Contribute to the Fatal Virulence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Emerging in China
- Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis
- Syk Signaling in Dendritic Cells Orchestrates Innate Resistance to Systemic Fungal Infection
- A Repetitive DNA Element Regulates Expression of the Sialic Acid Binding Adhesin by a Rheostat-like Mechanism
- T-bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection
- Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health
- Influence of ND10 Components on Epigenetic Determinants of Early KSHV Latency Establishment
- Antibody to gp41 MPER Alters Functional Properties of HIV-1 Env without Complete Neutralization
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



