-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
The mucosa-associated invariant T (MAIT) cells recognize antigens that are byproducts of the riboflavin biosynthesis pathway shared by many microbes. These antigens are presented by the MHC class I-like MR1 molecules and trigger rapid activation of MAIT cells in an innate-like fashion with deployment of effector mechanisms including cytokine production and cytolysis. Here, we investigated the MAIT cell response to bacteria in humans infected with HIV-1, and possible means to restore functionality to these cells. MAIT cell dysfunction in HIV-infected patients included an inability to express components of the cytolytic effector machinery. Impairment of the MAIT cell population involved the loss of expression of the transcription factors T-bet and Eomes. Interestingly, IL-7 had strong effects on MAIT cells, including the antigen-independent arming of cytolytic function and enhanced sensitivity for low levels of bacteria. In HIV-infected patients, plasma IL-7 levels were positively associated with the size of the MAIT cell population, and IL-7 could rescue their function. These findings indicate that MAIT cell impairment in HIV-1 infection is broad-based, includes loss of critical transcription factors, and loss of cytolytic function. Furthermore, the data support the notion that IL-7 is a strong candidate for immunotherapy in diseases associated with MAIT cell loss.
Published in the journal: Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection. PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005072
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005072Summary
The mucosa-associated invariant T (MAIT) cells recognize antigens that are byproducts of the riboflavin biosynthesis pathway shared by many microbes. These antigens are presented by the MHC class I-like MR1 molecules and trigger rapid activation of MAIT cells in an innate-like fashion with deployment of effector mechanisms including cytokine production and cytolysis. Here, we investigated the MAIT cell response to bacteria in humans infected with HIV-1, and possible means to restore functionality to these cells. MAIT cell dysfunction in HIV-infected patients included an inability to express components of the cytolytic effector machinery. Impairment of the MAIT cell population involved the loss of expression of the transcription factors T-bet and Eomes. Interestingly, IL-7 had strong effects on MAIT cells, including the antigen-independent arming of cytolytic function and enhanced sensitivity for low levels of bacteria. In HIV-infected patients, plasma IL-7 levels were positively associated with the size of the MAIT cell population, and IL-7 could rescue their function. These findings indicate that MAIT cell impairment in HIV-1 infection is broad-based, includes loss of critical transcription factors, and loss of cytolytic function. Furthermore, the data support the notion that IL-7 is a strong candidate for immunotherapy in diseases associated with MAIT cell loss.
Introduction
Mucosa-associated invariant T (MAIT) cells are a recently described subset of unconventional, innate-like T cells that are highly abundant in mucosal tissues, liver and circulation of healthy humans [1–4]. MAIT cells express a semi-invariant T cell receptor (TCR), including Vα7.2 coupled with restricted Jα segments (Jα33, Jα12, or Jα20), and limited Vβ repertoires [5, 6]. Together with their semi-invariant TCR, human MAIT cells are also defined by their high expression of CD161, the IL-18 receptor α subunit (IL-18Rα), and the transcription factor ZBTB16 [7], also known as promyelocytic leukemia zinc finger protein (PLZF) [8, 9]. The vast majority of MAIT cells are either CD8αα or CD8αβ, with some CD4/8 double-negative (DN), and minor CD4+ populations [8–11]. Human MAIT cells acquire innate-like antimicrobial activity in the fetal intestinal mucosa pre-natally, prior to the establishment of the commensal microflora [12].
MAIT cells recognize antigens in complex with the MHC-Ib-related protein (MR1) [2, 4], which displays an extraordinary level of evolutionary conservation among placental and marsupial mammals [4, 13, 14]. MR1 presents microbial vitamin B2 (riboflavin) metabolites from a wide range of microbes [15, 16], including unstable intermediates that are formed from non-enzymatic condensation of the early intermediate of riboflavin biosynthesis 5-amino-6-D-ribitylaminouracil (5-A-RU) with host - or microbe-derived glyoxal or methylglyoxal [15, 17]. MR1 captures these otherwise unstable compounds and presents them to MAIT cells [17]. Once activated by antigens, MAIT cells can rapidly kill infected cells [18, 19], inhibit intracellular microbial growth [20], and produce pro-inflammatory cytokines including IFNγ, TNF, and IL-17 [8, 10–12, 21]. Certain innate cytokines, including IL-12 and IL-18, can stimulate MAIT cells to produce IFNγ independently of the MR1-TCR interaction [22, 23]. These findings are strongly supportive of the notion that MAIT cells perform critical functions in the immune system, also beyond their role as antimicrobial T cells, particularly at mucosal sites.
Despite the great advances of antiretroviral therapy (ART) in the management of HIV disease, infected patients are still at an increased risk of microbial co-infections such as Mycobacterium tuberculosis, non-typhoidal Salmonella and Streptococcus pneumoniae [24–27]. Such microbial co-infection burdens are particularly apparent in individuals who are diagnosed at advanced stages, lack access to ART, and those who are non-adherent to therapy and clinical care. Although MAIT cells do not directly recognise viral antigens, indirect involvement of these cells in viral immunopathogenesis can occur, as we originally and others recently described for HIV-1 infection [11, 21, 28–31]. MAIT cell numbers and Th1/17 cytokine production are severely and persistently reduced in chronic HIV-infected patients [11]. The polymicrobial reactivity and breadth of the MAIT cell functional profile most likely contribute to the reported role of MAIT cells in the protection against diverse bacterial and mycobacterial infections in animal models, as well as in severe bacterial infections and pulmonary tuberculosis in humans [8, 20, 32–35]. MAIT cell defects may therefore predispose HIV-infected patients to an increased risk of acquiring microbial co-infections. Furthermore, the loss of MAIT cells is potentially irreversible if the disease is not treated at a very early stage [11, 21, 28–31]. In most settings of HIV-1 infection early diagnosis and treatment is challenging, and this necessitates a strategy where MAIT cells can be rescued through adjunctive immunotherapies.
IL-7 is a pleiotropic cytokine that has strong survival and homeostatic effects towards T cells, particularly the memory T cell populations with which MAIT cells show similarities (reviewed in [36]). IL-7 has attracted interest in the context of HIV-1 disease, and was proposed as a potential cytokine intervention therapy in treatment failure as well as in approaches to purge the viral reservoir (reviewed in [37]). Furthermore, IL-7 was recently shown by Tang et al. to enhance MAIT cell Th1/17 cytokine production in response to polyclonal stimulation [38]. In the present study, we investigate the cytolytic function of MAIT cells in the context of HIV-1 infection, the basis for dysfunction of MAIT cells in this disease, and possible approaches to rescue their function. Our findings indicate that arming of cytolytic capacity in MAIT cells is severely defective in HIV-1 infected patients, and that the broad-based functional defects in these cells are associated with deficiency in critical transcription factors. Furthermore, IL-7 induces the arming of effector functions and enhances the sensitivity of MAIT cells in healthy donors, and can partially reverse the functional and transcription factor defects in MAIT cells from HIV-1 infected patients. Thus, inclusion of IL-7 may be considered in immunotherapeutic approaches to restore MAIT cell numbers and function in conditions associated with loss and dysfunction of these cells.
Results
MAIT cells express cytolytic proteins at steady state and become rapidly armed into full effector T cells following bacterial stimulation
The expression levels of the cytolytic proteins perforin (Prf), granzyme (Grz)A, GrzB, and granulysin (Gnly), as well as the degranulation marker CD107a, were investigated in unstimulated MAIT cells obtained from 20 HIV-uninfected healthy controls (Fig 1A and 1B), and compared with those of non-MAIT, conventional CD4+ and CD8+ T cells (Fig 1B). The majority of MAIT cells expressed Prf and GrzA, whereas Gnly was expressed only by a proportion of MAIT cells and displayed high inter-donor variability (Fig 1B). Interestingly, MAIT cells did not express GrzB at steady state (Fig 1B). This pattern of cytolytic protein expression by MAIT cells was significantly different from non-MAIT, conventional CD4+ and CD8+ T cells (Fig 1B), consistent with recent studies [18, 19]. In addition, GrzA and Gnly in MAIT cells were co-expressed with the pore-forming protein Prf (S1A Fig).
Fig. 1. MAIT cells are rapidly armed into full effector T cells following bacterial stimulation. 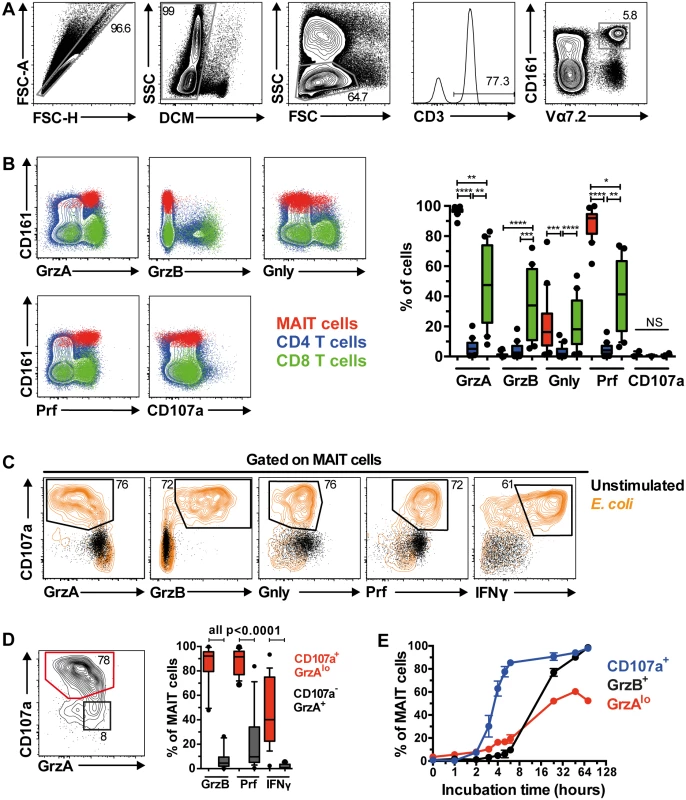
(A) The gating strategy to identify Vα7.2+CD161hi MAIT cells through flow cytometry. (B) The comparison of cytolytic proteins (granzyme A (GrzA), granzyme B (GrzB), granulysin (Gnly)), pore-forming protein perforin (Prf), and degranulation marker (CD107a) expression by MAIT cells (red), and CD4+ (blue) and CD8+ (green) conventional T cells at resting state from 20 HIV-uninfected, healthy controls. (C) Expression of cytolytic proteins by MAIT cells following stimulation with PFA-fixed E. coli (orange contour plot) or left unstimulated for 24 h (black dot plot). (D) Activated CD107a+GrzAlo MAIT cells (red contour plot) following bacterial stimulation expressed high levels of GrzB, Prf, and IFNγ, whereas the non-activated CD107a-GrzA+ MAIT cells (black dot plot) were variable for Prf expression and mostly negative for GrzB and IFNγ (n = 20). (E) The kinetics of MAIT cell expression for CD107a (blue line), GrzAlo (red line), and GrzB (black line) after PFA-fixed E. coli stimulation (n = 3). Error bars represent mean and standard deviation. Representative FACS plots from at least 5 healthy donors are shown. Box and whisker plot shows median, IQR and the 10th to the 90th percentile. The Friedman test followed by Dunn’s post-hoc test was used to detect significant differences across multiple, paired samples (B), and the Wilcoxon signed rank test for paired samples (D). NS: not significant (p≥0.05), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. We next determined MAIT cell cytolytic protein expression profile following overnight stimulation with mildly PFA-fixed E. coli in 20 healthy individuals, as we have previously shown that such stimulation triggers robust expression of pro-inflammatory cytokines in MAIT cells [11, 12]. Following stimulation, MAIT cells expressed high levels of the degranulation marker CD107a, coupled with loss of Gnly and GrzA expression, a concomitant upregulation of GrzB and Prf, as well as IFNγ production (Fig 1C). Furthermore, the high Prf and GrzB expression levels were predominantly found within the CD107a+GrzAlo MAIT cell population (Fig 1D), indicating that MAIT cells exocytose these cytolytic proteins following bacterial stimulation. Interestingly, not all CD107a+GrzAlo MAIT cells produced IFNγ (Fig 1D), suggesting that some functional heterogeneity within the MAIT cell population may exist. MAIT cell degranulation occurred rapidly, with detectable CD107a expression within two hours following bacterial feeding, followed by the appearance of GrzAlo MAIT cells, and then finally GrzB upregulation within 24 h post-stimulation with optimal upregulation at 72 h post-stimulation (Fig 1E). Together, these results indicate that MAIT cells rapidly mobilize their cytolytic granules upon antigen stimulation, and upregulate GrzB expression to become fully armed effector T cells.
MAIT cells express a distinct combination of transcription factors
Recent studies show that MAIT cells express the innate-like T cell transcription factor PLZF and the Th17 master transcription factor RORγt, which are likely to be responsible for the effector memory-like and Th17-like phenotype in MAIT cells, respectively [3, 8, 9]. However, the MAIT cell expression profile of other T cell transcription factors is unknown, including T box transcription factor 21 (TBX21, or T-bet), Eomesodermin (Eomes), and Helios. Therefore, the intracellular expression of PLZF, RORγt, T-bet, Eomes, and Helios was investigated in peripheral blood MAIT cells from 10 healthy donors. As expected, MAIT cells expressed PLZF and RORγt (Fig 2A). PLZF and RORγt co-expression in CD8+ T cells, total T cells, and to a lesser extent, in DN T cell populations accurately identified the MAIT cell population (S1B Fig), whereas far fewer of either PLZF+RORγt- or PLZF-RORγt+ cells from any T cell population were MAIT cells (S1B Fig). In addition, MAIT cells expressed the classical effector T cell transcription factors T-bet and Eomes, as well as Helios, with low expression levels for T-bet, high levels for Eomes, and intermediate levels for Helios (Fig 2A). MAIT cells also appeared to express lower levels of T-bet coupled with higher levels of Eomes (T-betdim Eomeshi) when compared to conventional T cells and CD3- CD161+ lymphocytes (predominantly NK cells) (Fig 2B). The MAIT cell expression level patterns for transcription factors were distinct to those expressed by conventional CD4+ and CD8+ T cells (Fig 2A), and were homogeneous across the predominant DN and CD8+ MAIT cell subsets, although lower expression levels were observed in the minor CD4+ MAIT cell subset (Fig 2C).
Fig. 2. MAIT cells express a distinct transcription factor profile. 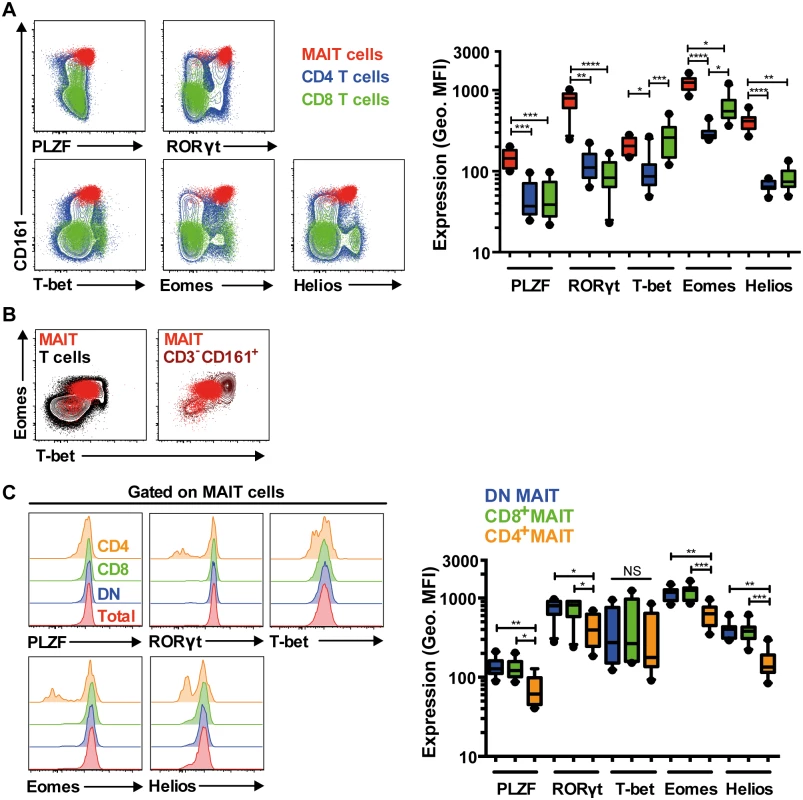
(A) Freshly isolated PBMCs from 10 HIV-uninfected, healthy controls were stained for the transcription factors PLZF, RORγt, T-bet, Eomes, and Helios. The expression levels for these transcription factors were determined and compared in MAIT cells (red), and in conventional CD4+ (blue) and CD8+ (green) T cells. (B) MAIT cells (red) T-bet:Eomes expression ratio when compared to conventional T cells (black) and CD3-CD161+ lymphocytes (brown). MAIT cells express a low T-bet: high Eomes ratio (T-betdimEomeshi). (C) The transcription factor expression levels among the CD4-CD8- DN (blue) and CD8+ MAIT cell populations (green) that comprised the vast majority of total MAIT cells (typically >95%; red) were similar. Variable expression levels within the small minority CD4+ MAIT cells (orange) were observed. Representative FACS plots are shown. Box and whisker plots show median, IQR and the 10th to the 90th percentile. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (the Friedman test followed by Dunn’s post-hoc test). IL-7 arms MAIT cells and potently enhances MAIT cell cytolysis of target cells
A recent study showed that IL-7 can enhance MAIT cell production of Th1/17 cytokines following CD3/CD28 stimulation [38]. Here, we assessed whether IL-7 could enhance MAIT cell antimicrobial cytotoxic potential in healthy individuals. Following a 48 h stimulation with IL-7 alone, the expression levels of Prf and GrzA were elevated and GrzB expression was induced, and this occurred in the absence of concurrent production of Th1/17 cytokines (Fig 3A and 3B). Such induction of cytolytic effector molecules by MAIT cells reached their maxima when incubated with 5–25 ng/ml of IL-7 (S2A Fig). There was no significant effect of IL-7 on Gnly expression in resting MAIT cells (S2B Fig). The induction of cytolytic effector molecule expression in MAIT cells by IL-7 was MR1-independent as determined by MR1 blocking using the 26.5 mAb (S2B Fig). Interestingly, IL-7 enhanced the expression of PLZF, RORγt, T-bet, Eomes, and Helios in MAIT cells examined in eight healthy individuals (Fig 3A and 3B). In contrast, there was no upregulation of Ki67 expression following IL-7 treatment, indicating that IL-7 did not trigger proliferation of MAIT cells (S2C Fig).
Fig. 3. IL-7 arms MAIT cell cytotoxic capacity. 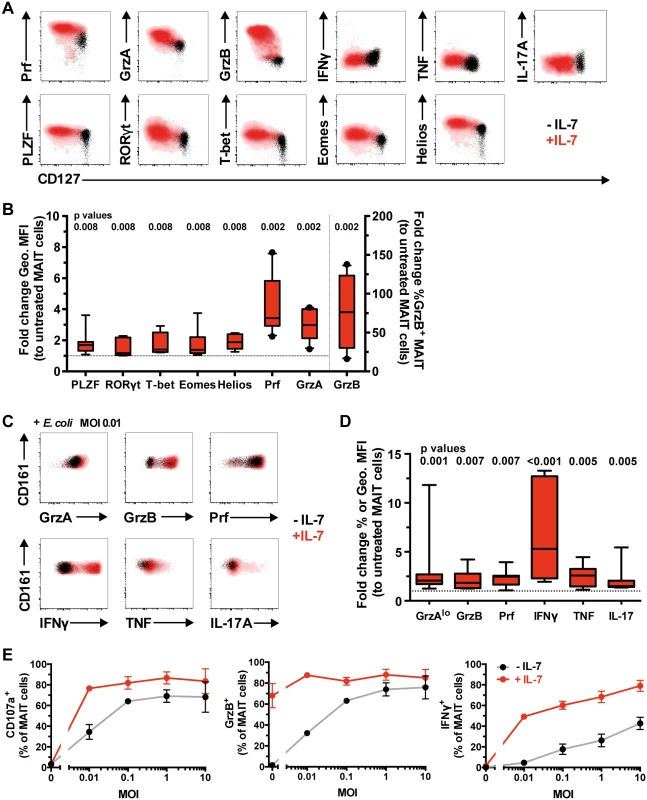
(A) Freshly isolated PBMCs from healthy blood donors were left untreated (black) or treated with IL-7 (red) for 48 h, and the expression levels of cytolytic proteins, pro-inflammatory cytokines, and innate and classical T cell transcription factors were determined in MAIT cells without bacterial stimulation (A), where the levels of cytolytic proteins (n = 10) and transcription factors (n = 8) expressed by IL-7-treated MAIT cells were then compared to those of untreated cells (B), or (C) after stimulation with a low dose of PFA-fixed E. coli (n = 8). (D) Fold-change geometric MFI was measured for Prf levels, and fold-change frequency for the rest of cytolytic proteins and cytokines. (E) Dose-response curve for MAIT cell degranulation (CD107a), cytotoxic capacity (GrzB), and pro-inflammatory cytokine production (IFNγ) after PFA-fixed E. coli stimulation in untreated (black line) or IL-7-treated (red line) MAIT cells (n = 3). Representative FACS plots are shown. Box and whisker plots show median, IQR and the 10th to the 90th percentile. Dotted line indicates the normalised levels of transcription factors or cytolytic proteins expressed by IL-7-untreated MAIT cells. The Wilcoxon signed rank test (B) or paired t-test (D) was used to determine significance between IL-7-untreated and-treated samples. Error bars represent mean and standard deviation. We next examined the effect of IL-7 treatment on MAIT cell effector responses following overnight stimulation with a suboptimal dose of mildly fixed E. coli (MOI 0.01). IL-7 treatment significantly enhanced expression of MAIT cell cytotoxic effector molecules and pro-inflammatory cytokine production as compared to MAIT cells exposed to bacteria alone (Fig 3C and 3D). In addition, IL-7 boosted the sensitivity of MAIT cells and allowed degranulation, GrzB upregulation, and IFNγ production at an antigenic dose up to 1000-fold lower when compared to IL-7-untreated MAIT cells (Fig 3E). The enhancement of MAIT cell effector function in response to E. coli stimulation reached its maximum when treated with 5–25 ng/ml IL-7 (S2D Fig).
IL-18 and IL-12 have been shown to stimulate MAIT cell effector function [19, 22, 23]. We therefore compared these cytokines with IL-7 in their capacity to arm MAIT cell effector function. In three HIV-uninfected donors, IL-7 was similar or superior to IL-18/IL-12 in arming and enhancing MAIT cell effector functions alone or following bacterial stimulation (S3A and S3B Fig). The exception to this pattern was IFNγ, as IL-12/IL-18 triggered direct activation of this effector function both alone and together with E. coli stimulus (S3A and S3B Fig). Furthermore and contrary to IL-7 treatment, IL-18/IL-12 seemed to enhance the decrease in MAIT cell numbers following bacterial stimulation (S3C Fig). This observation is in line with previous studies where IL-18 and IL-12 triggered direct effector responses and were implicated in MAIT cell loss [30, 39].
Because IL-7 treatment armed MAIT cells to become GrzB-expressing effector T cells, we investigated the effect of IL-7 on MAIT cell-mediated killing of bacteria-fed cells. Here, we utilized an in vitro model system where human MR1-expressing 293T (293T-hMR1) cells were fed mildly fixed E. coli and co-cultured with MAIT cells. Untreated MAIT cells were not able to significantly kill bacteria-fed target cells, whereas IL-7 treatment boosted MAIT cell killing of target cells by about 10-fold (Fig 4A and 4B). In resting IL-7 untreated cells, there was a correlation between degranulation and GrzB co-expression (CD107a+ GrzB+) by effector MAIT cells and target cell killing following exposure to E. coli (S3D Fig). This pattern indicated that the lackluster killing of target cells by IL-7 untreated MAIT cells was due to the low levels of degranulation and GrzB (Fig 4). In contrast, MAIT cells that were treated with IL-7 expressed GrzB, readily degranulated and had even higher GrzB levels upon co-culture with bacteria-fed target cells (Fig 4A). Both IL-7-treated and-untreated MAIT cell killing of target cells was ablated by anti-MR1, suggesting that MAIT cell killing is predominantly MR1-dependent (Fig 4A and 4B). Taken together, these results indicate that IL-7 arms MAIT cells to become effector T cells and potently induces MR1-dependent killing capacity through the upregulation of GrzB expression as well as other effector proteins, and this occurs concomitantly with elevated expression of transcription factors including T-bet and Eomes.
Fig. 4. IL-7 potently enhances MAIT cell killing of bacteria-exposed cells. 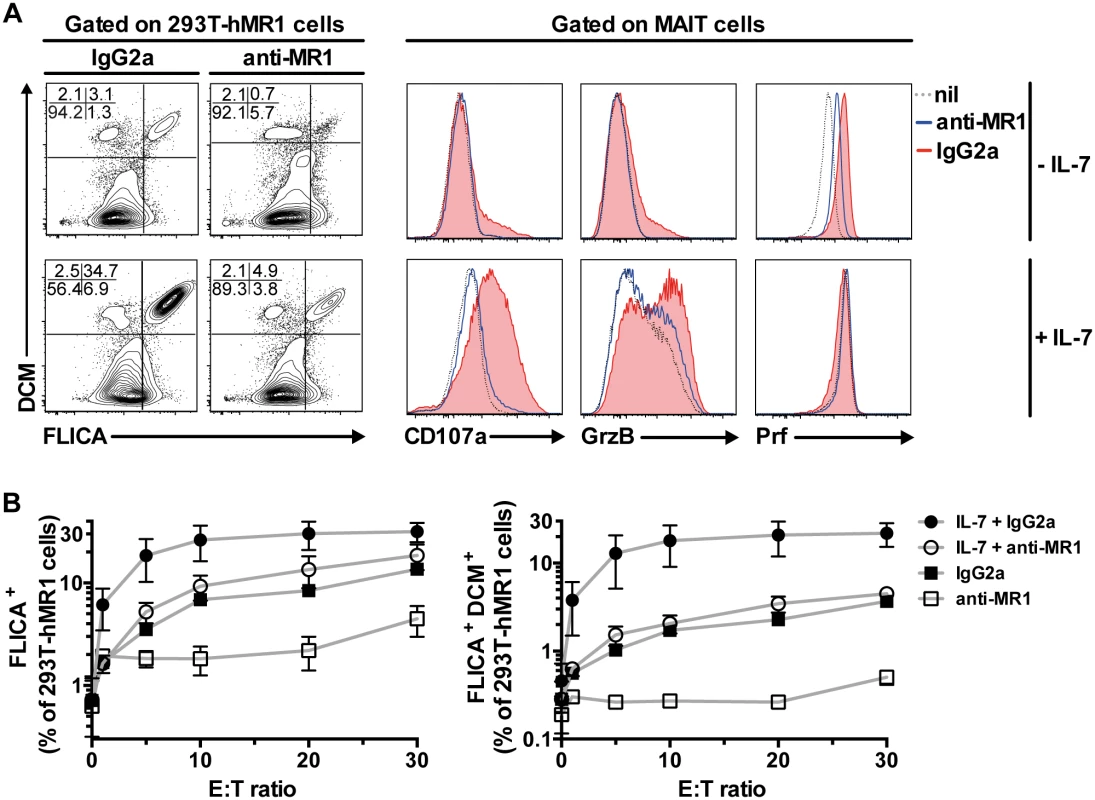
(A) The effect of IL-7 on MAIT cell killing of bacteria-exposed cells was determined using 293T-hMR1 cells that were pulsed with PFA-fixed E. coli (MOI 10) and co-cultured with purified MAIT cells that were previously untreated or treated with IL-7 for 72 h. Anti-MR1 or IgG2a isotype controls were added to determine MR1-dependency of MAIT cell killing of bacteria-exposed cells. 293T-hMR1 cell death was defined as cells that were positive for both poly-caspases activities (FLICA+) and amine-reactive live/dead cell marker (DCM+). MAIT cell degranulation, and expression of GrzB and Prf were simultaneously assessed. (B) The effector to target cells (E:T) ratio curve for MAIT cell killing of PFA-fixed E. coli-pulsed 293T-hMR1 cells was determined in 3 independent donors. FLICA+ cells denote total apoptotic cells (left panel), whereas FLICA+DCM+ cells denote dead cells (right panel). Representative FACS plots are shown. Error bars represent mean and standard deviation. MAIT cell arming and cytotoxic capacity is impaired in chronic HIV-1 infection
The capacity of MAIT cells to produce pro-inflammatory cytokines is severely decreased in untreated chronic HIV-1 infection [11]. However, it remains unknown whether MAIT cell cytotoxic capacity is also impaired in this condition. To address this, we compared the expression of cytolytic proteins in unstimulated MAIT cells from 20 healthy controls and 25 ART untreated HIV-1 infected patients (Table 1). Levels of GrzA and Prf expressed by MAIT cells in HIV-1 infected patients were modestly, but significantly, lower than those in uninfected healthy controls (Fig 5A, p = 0.0007 and p = 0.0008, respectively). Interestingly, the levels of GrzB expression in MAIT cells from infected patients were significantly higher when compared to healthy controls (p = 0.0004), a pattern in line with the notion that these residual MAIT cells are partially activated, consistent with our previously published data [11]. There was no significant difference in the levels of Gnly in MAIT cells between HIV-1 infected patients and healthy controls (median (IQR) = 16.3 (7.3–28.6)% and 14.5 (7.2–26.0)% respectively; p = 0.98). The modest changes in expression of GrzA, Prf, and GrzB in unstimulated MAIT cells from these patients were not significantly restored following long-term ART (n = 18; Fig 5A) (median (IQR) duration of ART = 38.5 (28–49) months).
Tab. 1. Characteristics of HIV-1 infected patients and healthy controls. 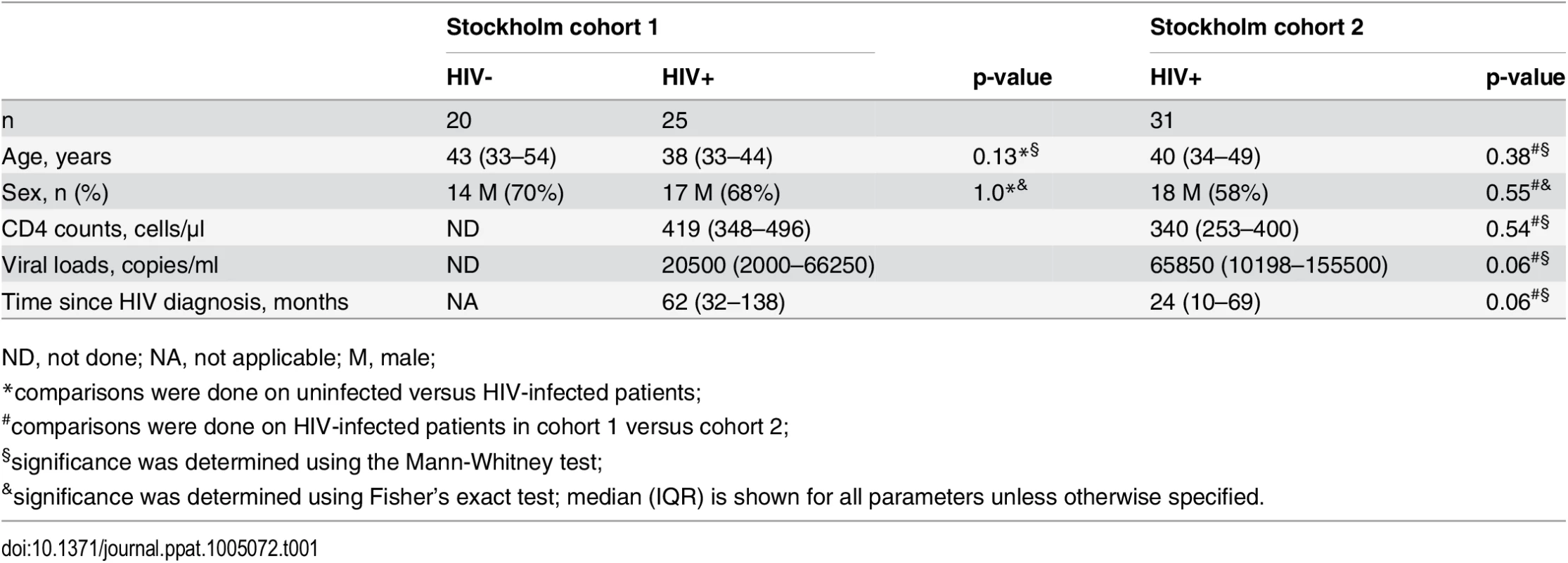
ND, not done; NA, not applicable; M, male; Fig. 5. IL-7 arming of MAIT cells and MAIT cell cytotoxic capacity are impaired in chronic HIV-1 infection. 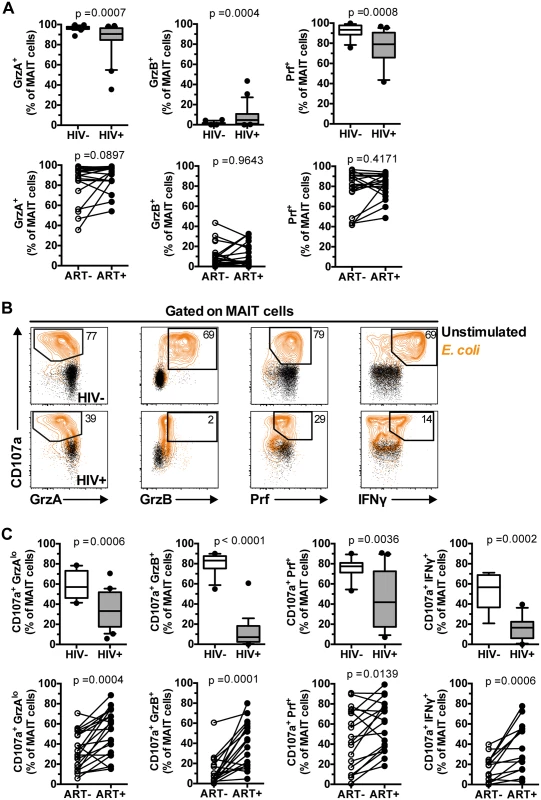
(A) The expression of GrzA, GrzB, and Prf at resting state was determined in 20 healthy controls and 25 HIV-infected, untreated patients, as well as in 18 paired patient samples before and after effective ART. (B,C) MAIT cell cytotoxic capacity and IFNγ production after 24 h of stimulation with PFA-fixed E. coli (MOI 10) was determined in 20 healthy controls and 25 HIV-infected, untreated patients, as well as in 18 paired patient samples before and after effective ART as described in (A). Box and whisker plots show median, IQR and the 10th to the 90th percentile. Representative FACS plots are shown. The Mann-Whitney test was used to determine significance between healthy controls and HIV-infected, untreated patients, and the Wilcoxon test for paired samples. Next, we investigated the responses of MAIT cells in terms of GrzB arming and cytolytic potential following a 24 h bacterial stimulation (Fig 5B). All measured facets of MAIT cell cytotoxic potential examined were impaired in chronic HIV-1 infection, including reduction in the levels of MAIT cells expressing CD107a+GrzAlo (p = 0.0006), CD107a+GrzB+ (p<0.0001), and CD107a+Prf+ (p = 0.0036) phenotypes in response to bacteria (Fig 5B and 5C). The severe deficiency of GrzB-expressing MAIT cells following bacterial stimulation was particularly striking given that this cell population had slightly upregulated GrzB levels at steady state (Fig 5A), indicating that MAIT cells from HIV-1 infected patients have severely compromised ability to upregulate GrzB de novo following new microbial encounters. There were no significant correlations between MAIT cell cytotoxic potential and cytokine production with CD4 counts (CD107a+GrzAlo Spearman’s r = 0.22, p = 0.32; CD107a+GrzB+ r = 0.29, p = 0.20; CD107a+Prf+ r = -0.04, p = 0.88; CD107a+IFNγ+ r = -0.03, p = 0.91), plasma viral loads (CD107a+GrzAlo Spearman’s r = 0.15, p = 0.53; CD107a+GrzB+ r = 0.02, p = 0.92; CD107a+Prf+ r = 0.05, p = 0.83; CD107a+IFNγ+ r = -0.02, p = 0.94), nor with time since HIV diagnosis (CD107a+GrzAlo Spearman’s r = -0.17, p = 0.44; CD107a+GrzB+ r = 0.09, p = 0.69; CD107a+Prf+ r = -0.26, p = 0.26; CD107a+IFNγ+ r = -0.22, p = 0.38). Long-term ART partially restored all evaluated aspects of MAIT cell cytotoxic potential (Fig 5C). However, it is important to note that in treated HIV-infected individuals the levels of GrzB up-regulation were still significantly lower (p<0.0001), and levels of Prf tended to be lower (p = 0.063), when compared to healthy controls. There were no significant correlations between the magnitude of MAIT cell cytotoxic capacity and cytokine production recovery after ART with duration of ART (CD107a+GrzAlo Spearman’s r = 0.20, p = 0.42; CD107a+GrzB+ r = 0.14, p = 0.59; CD107a+Prf+ r = 0.30, p = 0.25; CD107a+IFNγ+ r = -0.01, p = 0.99).
Expansion of aberrant MAIT cells lacking T-bet and Eomes in chronic HIV-1 infection correlates with loss of MAIT cells and impaired effector function
In healthy individuals, the vast majority of MAIT cells express low but detectable levels of T-bet and high levels of Eomes (T-betdimEomeshi; Fig 2B and Fig 6A). However, in HIV-1 infected patients there was a significant expansion of a MAIT cell population expressing neither transcription factors at detectable levels (T-betnegEomesneg), and this was only partially reduced following long-term ART (n = 14) (Fig 6A). Detailed investigation revealed that the T-betnegEomesneg MAIT cell population also expressed significantly lower levels of PLZF, RORγt, and Helios when compared to the T-betdimEomeshi MAIT cell population (all p<0.0001; Fig 6B). More importantly, the expansion of the T-betnegEomesneg MAIT cell population in HIV-infected patients was strongly correlated with MAIT cell depletion in the periphery (Fig 6C), loss of MAIT cell cytotoxic potential (CD107a+GrzAlo and CD107a+GrzB+; Fig 6D and 6E, respectively), and impaired IFNγ production (Fig 6F) in response to bacterial stimulation.
Fig. 6. Aberrant MAIT cell transcription factor expression in chronic HIV-1 infection correlates with MAIT cell effector dysfunction. 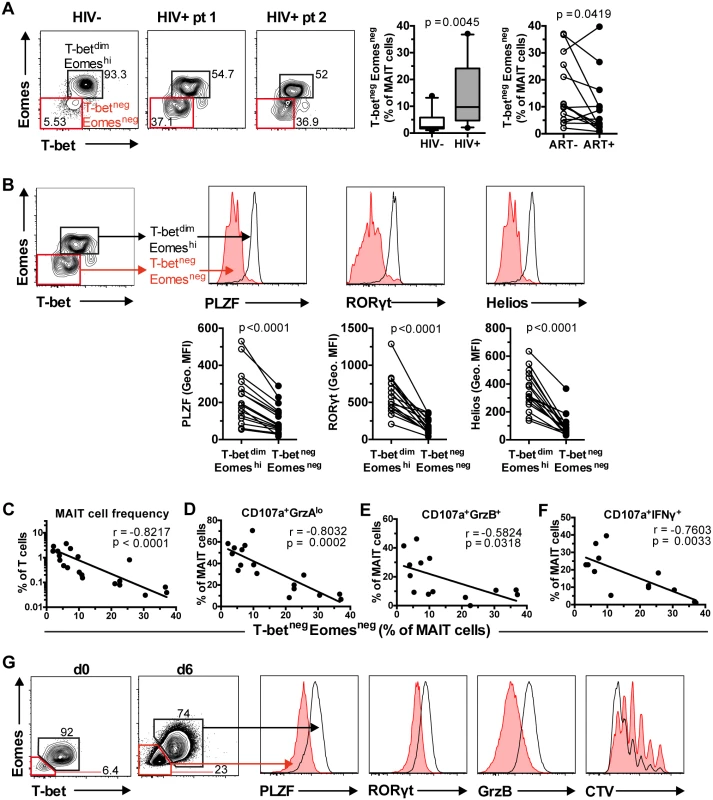
(A) Unstimulated PBMCs from 10 healthy controls and 17 HIV-1 infected, ART-untreated patients were stained for transcription factors, and their MAIT cell T-bet and Eomes expression profile was determined. The effect of long-term ART on MAIT cell T-bet and Eomes expression profile was also determined in paired samples from 14 HIV-infected patients. (B) Comparison of PLZF, RORγt, and Helios levels between the two MAIT cell populations in the same set of HIV-1 infected, ART-untreated patients. (C, D, E, F) Correlations between the levels of T-betnegEomesneg MAIT cells in ART-untreated HIV-1 infected patients with MAIT cell numbers ex vivo and effector functions after E. coli stimulation (MOI 10) were calculated using Spearman’s test. (G) T-betnegEomesneg MAIT cells were generated following a six day incubation of PBMCs from healthy controls with PFA-fixed E. coli (MOI 10). The expression levels of PLZF, RORγt, and GrzB, as well as measurement of proliferation using a Cell Trace Violet (CTV) dilution method, were determined in both T-betdimEomeshi and T-betnegEomesneg MAIT cells (n = 3). Box and whisker plot shows median, IQR and the 10th to the 90th percentile. Representative FACS plots are shown. The Mann-Whitney test was used to determine significance between healthy controls and HIV-1 infected, ART-untreated patients, and the Wilcoxon test for paired samples. Next, we investigated whether this aberrant MAIT cell transcription factor profile could be generated in vitro following a strong chronic antigenic exposure. A six day culture of PBMCs from healthy donors (n = 3) with mildly fixed E. coli increased the frequency of T-betnegEomesneg MAIT cells (Fig 6G). Similar to MAIT cells in HIV-infected patients with this phenotype, these in vitro-generated T-betnegEomesneg MAIT cells also expressed lower levels of PLZF and RORγt (Fig 6G). The T-betnegEomesneg MAIT cells also expressed lower levels of GrzB. Finally, the aberrant T-betnegEomesneg MAIT cells proliferated less when compared to T-betdimEomeshi MAIT cells (Fig 6G).
IL-7 significantly restores the impaired MAIT cell effector function, and correlates with MAIT cell numbers in HIV-1 infected patients
The potential of IL-7 treatment to restore MAIT cell functional defects in HIV-1 infected patients was evaluated in vitro. Interestingly, GrzA and Prf upregulation following IL-7 treatment in samples from healthy controls (n = 8) and HIV-1 infected patients (n = 6) was not significantly different (Fig 7A). However, whereas GrzB upregulation occurred in MAIT cells from both groups, it was weaker in HIV-1 infected patients suggesting that the cytotoxicity arming effect of IL-7 in these patients may be less distinct (p = 0.0293, Fig 7A). In a second round of experiments the ability of IL-7 to support the MAIT cell response in HIV-1 infected patients to a 24 h bacterial stimulation was evaluated. IL-7 treatment significantly improved all measured phenotypic aspects of MAIT cell cytotoxic potential including the generation of CD107a+GrzAlo, CD107a+GrzB+, and CD107a+Prf+ phenotypes, as well as the production of IFNγ by MAIT cells (n = 6) (Fig 7B). Next, we investigated whether the improvement of MAIT cell effector functions by IL-7 treatment might be linked with restoration of the abnormal MAIT cell transcription factor profile in nine ART-untreated HIV-1 infected patients. Importantly there was no difference in CD127 levels by T-betnegEomesneg and T-betdimEomeshi MAIT cells (S4A Fig). Next, PBMCs were cultured with IL-7 for a maximum of 48 h to minimize introducing potential confounders, including global cellular proliferation, activation-induced cell death, and HIV-1 replication that may result from a long-term IL-7 exposure. The frequency of T-betnegEomesneg MAIT cells showed a trend towards a decrease during this culture (n = 9, p = 0.0732; Fig 7C). Short-term IL-7 treatment also reduced the already low frequency of T-betnegEomesneg MAIT cells in seven HIV-uninfected healthy controls (p = 0.0156; S4B Fig).
Fig. 7. IL-7 restores MAIT cell effector dysfunction. 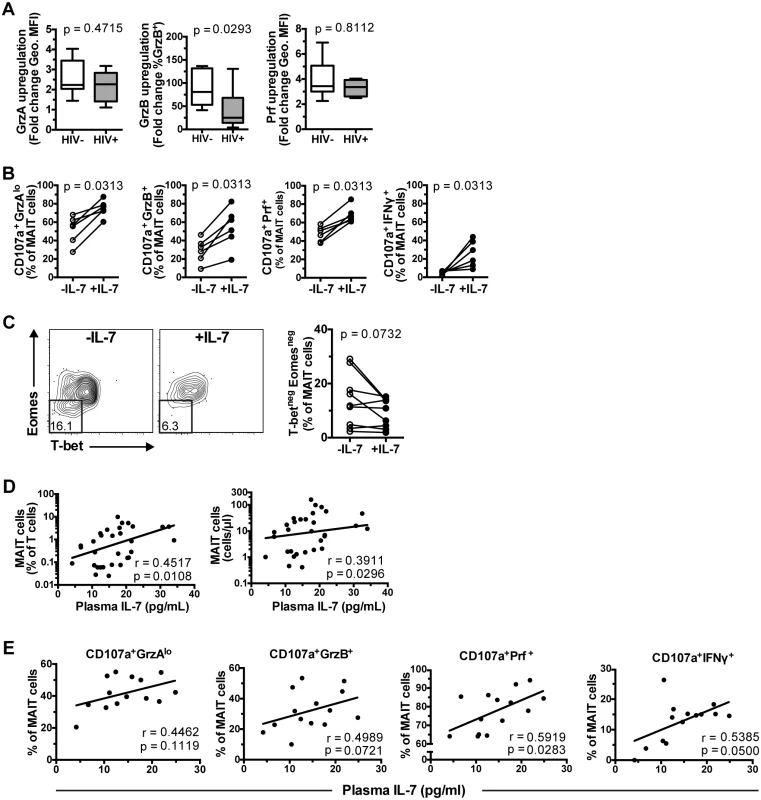
(A) MAIT cell arming following IL-7 treatment in vitro for 48 h in healthy controls (n = 8), and HIV-1 infected ART-untreated patients (n = 6). GrzA and Prf upregulation was determined as fold-change of geometric MFI to that of IL-7-untreated cells, whereas GrzB upregulation was determined as fold change of the percentage GrzB-expressing cells. (B) PBMCs from six HIV-1 infected ART-untreated patients were either left untreated or treated with IL-7 for 24 h, then stimulated with mildly PFA-fixed E. coli (MOI 10) for a further 24 h before measurements of MAIT cell effector functions. (C) PBMCs from nine HIV-1 infected ART-untreated patients were incubated in the presence or absence of 10 ng/ml IL-7 for 48 h and then stained for transcription factor expression as described in Fig 6. Correlation between plasma levels of IL-7 and MAIT cell frequency and numbers from 31 HIV-1 infected ART-untreated patients (D) or MAIT cell effector function from 14 patients (E) were calculated using Spearman’s test. Box and whisker plots show median, IQR and the 10th to the 90th percentile. Representative FACS plots are shown. Significance between independent samples was determined using Mann-Whitney’s test, and paired samples using Wilcoxon’s signed rank test (B) or paired t-test (C). We next evaluated whether plasma levels of IL-7 in vivo were associated with MAIT cell levels in 31 individuals enrolled in a second cohort of HIV-infected patients recruited from the same site (Table 1). The clinical parameters of cohort 2 were not significantly different when compared to those of cohort 1. Furthermore, patients in cohort 2 saw a similarly significant depletion of MAIT cells, and a similarly significant increase in the levels of Vα7.2+CD161- T cells in peripheral blood (S5A Fig) as previously reported [11]. There was also a significant increase in MAIT cell activation as evaluated by expression of CD38, HLA-DR, CD57, and TIM-3 (S5B Fig), and an inverse correlation between the levels of MAIT cells in circulation and MAIT cell CD38 expression (S5C Fig). Consistent with our previous study and other studies [11, 21, 29, 31], there were no significant correlations between MAIT cell levels and either CD4 counts and plasma viral loads (Spearman’s r = 0.074, p = 0.70; and r = 0.22, p = 0.23, respectively). We also did not find any correlation between MAIT cell levels and plasma markers of microbial translocation (LPS; Spearman’s r = 0.018, p = 0.93) and innate immune activation (sCD14; r = -0.14, p = 0.45).
Having thus validated that cohort 2 was comparable to cohort 1, the patients in cohort 2 displayed a positive correlation between the plasma levels of IL-7 and MAIT cell frequency and absolute counts in peripheral blood (r = 0.45, p = 0.011, and r = 0.39, p = 0.030, respectively; Fig 7D). Furthermore, plasma IL-7 levels showed a weak positive correlation with the capacity of MAIT cells to respond to PFA-fixed E. coli stimulation (CD107a+GrzAlo Spearman’s r = 0.45, p = 0.11; CD107a+GrzB+ r = 0.50, p = 0.072; CD107a+Prf+ r = 0.59, p = 0.028; CD107a+IFNγ+ r = 0.54, p = 0.050) (Fig 7E). While there were weak correlations with CD4 counts (Spearman’s r = -0.34, p = 0.017) and plasma viral loads (r = 0.29, p = 0.040), there were no significant relationships between plasma IL-7 levels and CD38, HLA-DR, CD57, and TIM-3 expression in MAIT cells (S6 Fig). Taken together, these results suggest that IL-7 plasma levels may directly influence MAIT cell numbers and their capacity to respond to microbial challenge in HIV-infected patients.
Discussion
MAIT cells are emerging as a significant component of the cellular immune defenses against microbial infection. They are found in circulation and enriched in intestinal mucosa, liver and lung. We and others have shown that patients with HIV-1 infection suffer numerical loss and functional decline of their MAIT cells. This was at first glance unexpected given that MAIT cells are mostly CD8+ or DN T cells with only very few expressing CD4. Furthermore, the antigens recognized by MAIT cells are of bacterial and fungal origin meaning that the exhausted functional phenotype of MAIT cells may be due to engagement not in antiviral immune responses, but rather in a response against microbes at mucosal barriers. Here, we have shown that the functional defect in MAIT cells also includes the cytolytic potential, with low levels of GrzA and Prf expression and a particularly striking defect in GrzB arming. This is likely to have significant consequences for MAIT cell-mediated control of intracellular microbes where direct cytolysis plays a significant role. MAIT cells play a role in immune defense against mycobacteria [8, 33], are involved in tuberculosis [40], as well as sepsis and severe bacterial infections in humans [35]. Even in the current era of effective ART, HIV-1 infected patients have impaired control of mycobacterial and other non-opportunistic pathogens, with increased risk of developing infections from such pathogens [27]. Bacterial sepsis is now the principal cause of intensive care unit admission and death for HIV-1-infected patients who are admitted to hospital even in western countries [41]. Because of MAIT cells’ abundance, strategic locations at the body barrier sites, and potent antimicrobial activities against diverse pathogenic microbes, the broad numerical decline and severe dysfunction of MAIT cells may significantly contribute to morbidities and mortalities from both AIDS-defining infections and non-AIDS-related infections in HIV-1-infected patients. Importantly, long-term effective ART did not rescue the ability of MAIT cells to arm with GrzB in response to bacterial antigen, indicating that this deficiency may be largely irreversible in HIV-infected people.
The development and function of MAIT cells is dependent on expression of the transcription factor PLZF, and we also confirm that they express RORγt consistent with their potential to produce IL-17. In fact, our data suggest that the combination of PLZF and RORγt expression is sufficiently distinctive such that it can be used to identify this innate T cell population in the absence of the Vα7.2 TCR and CD161 combination. In addition, MAIT cells express the two classical effector T cell T-box transcription factors T-bet and Eomes, as well as Helios, with low expression levels for T-bet, high levels for Eomes, and intermediate levels for Helios. This pattern is in keeping with the effector memory-like phenotype of MAIT cells [42, 43]. Helios is a member of the Ikaros family and is predominantly studied in relation to regulatory T (Treg) cell subsets [44, 45]. Recently, however, the role of Helios beyond Treg subsets has been recognized, including as a marker for T cell activation, proliferation, and helper T cell subsets [46, 47]. The role of Helios in MAIT cells is currently unclear and warrants further studies. Nevertheless, this pattern indicates that MAIT cells express a unique combination of these critical transcription factors and may have a unique transcriptional program underlying their distinct phenotypic and functional profile. Interestingly, the exhausted characteristics of MAIT cells in HIV-1 infected patients were paired with the appearance of a dysregulated expression pattern of these critical transcription factors. The occurrence of MAIT cells deficient in both Eomes and T-bet correlates strongly with both the functional impairment and the numerical decline of these cells. Of note, such aberrant MAIT cells also had low levels of RORγt, which is in line with our previous observation that MAIT cells from HIV-infected patients were unable to produce IL-17 following bacterial stimulation [11]. This indicates that the defects of MAIT cells are broad-based and occur at the transcriptional level. It is possible that this effect might be due to continuous antigenic burden relating to loss of control of bacterial infections as well as to microbial translocation in HIV-infected patients. Indeed, chronic stimulation with mildly fixed E. coli generated MAIT cells deficient in both T-bet and Eomes. Importantly, these in vitro-generated MAIT cells lacking T-bet and Eomes also had low GrzB levels, consistent with the partially redundant activities of T-bet and Eomes in inducing GrzB in cytotoxic CD8 T cells [48, 49]. It is however possible that other pathways may be involved in the generation of T-bet and Eomes defective MAIT cells in vivo.
IL-7 has strong effects on T cell homeostasis and supports the survival of T cells by upregulating Bcl-2. These characteristics have made IL-7 attractive for cytokine immunotherapy in humans. MAIT cells express high levels of the IL-7Rα (CD127) [12, 38], a finding that opened the possibility that IL-7 may have strong effects on MAIT cells. Interestingly, IL-7 alone in the absence of other stimuli was capable of arming resting MAIT cells from healthy donors into cytotoxic GrzB+ effector T cells. The induction of GrzB is profound, as resting MAIT cells essentially lack expression of this protein. Along with this effect, MAIT cells also further upregulated Prf and GrzA, without inducing any detectable spontaneous cytokine production. In the absence of bacterial antigen the effect of IL-7 is thus specifically to induce the cytotoxic arming of MAIT cells. Importantly, this in turn leads to a significantly increased cytolytic activity against MR1-expressing targets pulsed with bacteria. Perhaps an even more striking and potentially physiologically important effect is the radically enhanced sensitivity of MAIT cells to very low bacterial doses after IL-7 treatment. IL-7 supports strong cytokine production and cytolytic responses at antigen doses that are otherwise non-stimulatory for MAIT cells. These effects are consistent with the observations by Tang et al., where IL-7 enhanced expression of the TCR, CD8 chains and components of TCR signaling pathway [38]. Altogether, IL-7 may be suitable for immunotherapy approaches aimed at enhancing MAIT cell function in humans.
MAIT cells from HIV-infected patients significantly restored their effector functions after a short incubation with IL-7 in vitro. This effect was evident both in terms of arming, i.e. upregulation of in particular GrzB in resting non-antigen activated MAIT cells, and in terms of boosting the anti-bacterial degranulation and IFNγ response in MAIT cells. It should however be noted that the low levels of residual MAIT cells did not allow direct assessment of MAIT cell cytolytic capacity in HIV-1 infected patients. Interestingly, IL-7 has received attention as an adjunctive cytokine immunotherapy in HIV-1 infected patients to help restore immune functions that remain impaired even after successful ART. Particularly interesting were the recent observations by Sereti et al., that IL-7 treatment helped replenish the mucosal T cell compartment and supported an improved mucosal barrier function as evidenced by decreases in relevant biomarkers of microbial translocation [50]. Our findings in the present study open the possibility that these effects in patients involve the MAIT cell compartment. In such a model the mucosal T cell subsets including MAIT cells improve their numbers and function to alleviate the gut mucosal abnormalities of chronic HIV-1 infection. This possibility is further supported by the weak positive correlation we observe between plasma IL-7 levels and MAIT cell levels in vivo.
In summary, the findings in this study indicate that arming of cytolytic capacity occurs rapidly upon detection of bacterial antigen in MAIT cells in healthy donors, and this response is severely defective in HIV-1 infected patients. The broad-based functional defects in MAIT cells in chronic HIV-1 infection are associated with deficiency in the critical transcription factors Eomes and T-bet. Furthermore, our data show that IL-7 induces the arming of effector functions and enhances the sensitivity of MAIT cells in healthy donors, and can partially reverse the functional and transcription factor defects in MAIT cells from HIV-1 infected patients. These findings support the inclusion of IL-7 in immunotherapeutic approaches to restore MAIT cell numbers and function in HIV-1 infection as well as other conditions associated with loss and dysfunction of these cells.
Materials and Methods
Ethics statement
Written informed consent was obtained from all study participants in accordance with study protocols conforming to the provisions of the Declaration of Helsinki and approved by the Regional Ethics Review Board in Stockholm (Protocols 2007/772-32, 401/01, and 2009-1485-31-3).
Study participants
HIV-1 infected patients were from the Karolinska University Hospital Huddinge Infectious Diseases Outpatient Clinic (Stockholm, Sweden) (Table 1) [11]. Inclusion criteria were that patients were HIV-1 seropositive and had no history of AIDS-defining illness in the 12 months prior to recruitment. Healthy HIV-uninfected individuals were recruited at the Blood Transfusion Clinic at the Karolinska University Hospital Huddinge.
Peripheral blood processing
PBMCs were isolated from peripheral blood by Ficoll-Hypaque density gradient centrifugation (Pfizer-Pharmacia or Axis-Shield), and either allowed to rest overnight in complete medium, or cryopreserved in liquid nitrogen until required.
Functional assay
PBMCs were cultured in 10 ng/ml recombinant human IL-7 (R&D Systems), a combination of IL-12 and IL-18 (10 ng/ml and 100 ng/ml, respectively; PeproTech) for 24–48 h, as indicated or left untreated at 37°C and 5% CO2 in RPMI medium supplemented with 10% fetal calf serum and 50 μg/ml gentamicin (Gibco) (RF10 medium) prior to functional assay. MAIT cell functions were determined in vitro using a paraformaldehyde (PFA)-fixed E. coli stimulation (D21 strain, MOI as indicated) in the presence of 1.25 μg/ml anti-CD28 mAb (BD Biosciences) [11]. PBMCs were further cultured for 24 h, and in selected experiments, 0.4 μg/ml anti-CD107a PECy7 (BD Biosciences) was added at the start of bacterial stimulation, and monensin (Golgi Stop, BD Biosciences) was added during the last six hours of the stimulation. In selected experiments, cells were stained with Cell Trace Violet (CTV) Cell Proliferation Kit (Life Technologies) as per manufacturer’s instructions and cultured in RF10 medium with fixed E. coli in the presence of anti-CD28 for six days as described [12].
Cytotoxicity assay
Vα7.2+ T cells were purified from freshly isolated PBMCs using Vα7.2-APC antibody, followed by anti-APC microbeads (Miltenyi Biotec) and positive selection using MACS Cell Separation (Miltenyi Biotec). The purity of enriched Vα7.2+ T cells was typically >95%, with minimum MAIT cell purity of 90%. Vα7.2+ T cells were cultured in RF10 medium supplemented with 25 ng/ml recombinant human IL-7 or in RF10 medium alone for 72 h. Human 293T cells stably transfected with human MR1 (293T-hMR1 cells; a kind gift from Dr. Ted Hansen) [4] were incubated with PFA-fixed E. coli at an MOI of 10 for three hours, followed by the addition of anti-MR1 mAb (26.5; Biolegend) or IgG2a isotype control (MOPC-173; Biolegend) for 60 min prior to the addition of Vα7.2+ T effector cells at the indicated effector to target (MAIT:293T-hMR1) ratio. The MAIT:293T-hMR1 ratio was adjusted to take into accounts the overall purity of MAIT cells within the Vα7.2+ T cell population as the contaminating Vα7.2+CD161- T cells did not mediate cytotoxicity. Target cell apoptosis was detected through the fluorescent inhibitor of caspases (FLICA) flow cytometry-based methodology. Briefly, the FLICA reagent (Vybrant FAM Poly Caspases Assay Kit, Life Technologies) was added at a final concentration of 0.2% (v/v) to the MAIT-293T-hMR1 cell culture at the beginning of the assay, and anti-CD107a PECy7 at a total concentration of 0.4 μg/ml was also added to detect MAIT cell degranulation. After 24 h of culture, cells were harvested and stained to simultaneously detect MAIT cell cytotoxicity and 293T-hMR1 cell death.
Antibodies, flow cytometry and IL-7 measurement
Anti-CD3 Alexa Fluor (AF)700 (clone UCHT1), anti-CD4 APC-H7 (clone SK3), anti-CD38 PECy7 (clone HIT2), anti-CD161 PECy5 (clone DX12), anti-GrzB AF700 (clone GB11), anti-HLA-DR APC-H7 (clone L243), anti-RORγt PE (clone Q21-559), anti-TNF PECy7 (clone MAb11) were from BD Biosciences; anti-CD3 Brilliant Violet (BV)510 and BV785 (clone OKT3), anti-CD4 BV711 (clone OKT4), anti-CD8 BV570 (clone RPA-T8), anti-CD57 Pacific Blue, anti-CD107a PECy7 (clone H4A3), anti-CD127 BV650 (clone A019D5), anti-CD161 BV605 (clone HP-3G10), anti-granulysin PE (clone DH2), anti-granzyme (Grz)A AF700 (clone CB9), anti-GrzB FITC (clone GB11), anti-IFNγ BV785 (clone 4S.B3), anti-IL-17A BV421 and BV711 (clone BL168), anti-Ki67 BV421 (clone Ki-67), anti-perforin BV421 (clone B-D48), anti-T-bet BV605 and BV711 (clone 4B10), and anti-Vα7.2 APC, PE, and PECy7 (clone 3C10) were from Biolegend; anti-Eomes FITC (clone WD1928), and anti-Helios eFluor 450 (clone 22F6) were from Ebioscience; anti-CD8 Q-dot 655 (clone 3B5), and live/dead aqua and near infrared fixable cell stain were from Life Technologies; anti-PLZF APC (clone 6318100), and anti-TIM-3 AF488 (clone 344823) were from R&D systems.
Cell surface staining was performed using directly conjugated antibodies and fixed in Cytofix/Cytoperm or in Transcription Factor Fixation/Permeabilization buffer (both from BD Biosciences) as appropriate. Intracellular staining was performed using the relevant mAbs in Perm/Wash or Transcription Factor Perm/Wash buffer as appropriate (both from BD Biosciences). Samples were acquired on an LSRFortessa 18-colour flow cytometer (BD Biosciences) equipped with 405, 488, 561 and 639 nm lasers. Single-stained polystyrene beads (BD Biosciences) were used for compensation purposes. Software-based compensation was performed using the compensation platform in FlowJo software version 9.6 (Tree Star).
Circulating IL-7 was detected in plasma (diluted 1 : 4) using the Quantikine HS Human IL-7 Assay (RnD Systems, Abingdon, UK), according to the manufacturer’s instructions. Each sample was assayed in duplicate.
Statistical analysis
Significant differences in independent samples were assessed using Fisher’s exact test for categorical variables. Continuous variables were first assessed for normality, and differences in independent samples were assessed using t-test or Mann-Whitney test for continuous variables as appropriate. The Wilcoxon signed rank test or paired t-test as appropriate was used to determine significance between paired samples. The Friedman test followed by Dunn’s post-hoc test was used to detect differences across multiple, paired samples. Correlations were evaluated using Spearman’s rank correlation. Statistical analyses were performed on raw data using Prism version 6.0f (GraphPad), and two-sided p-values < 0.05 were considered significant.
Supporting Information
Zdroje
1. Le Bourhis L, Mburu YK, Lantz O. MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol. 2013;25(2):174–80. Epub 2013/02/21. doi: S0952-7915(13)00006-X [pii] doi: 10.1016/j.coi.2013.01.005 23422835.
2. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1 (vol 422, pg 164, 2003). Nature. 2003;423(6943):1018-. doi: 10.1038/Nature01700 ISI:000183753900057.
3. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–9. Epub 2010/11/19. doi: blood-2010-08-303339 [pii] doi: 10.1182/blood-2010-08-303339 21084709.
4. Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, et al. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A. 2009;106(20):8290–5. Epub 2009/05/07. doi: 0903196106 [pii] doi: 10.1073/pnas.0903196106 19416870; PubMed Central PMCID: PMC2688861.
5. Reantragoon R, Kjer-Nielsen L, Patel O, Chen Z, Illing PT, Bhati M, et al. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J Exp Med. 2012;209(4):761–74. Epub 2012/03/14. doi: jem.20112095 [pii] doi: 10.1084/jem.20112095 22412157; PubMed Central PMCID: PMC3328369.
6. Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nature communications. 2014;5 : 3866. doi: 10.1038/ncomms4866 24832684.
7. Beaulieu AM, Sant'Angelo DB. The BTB-ZF family of transcription factors: key regulators of lineage commitment and effector function development in the immune system. J Immunol. 2011;187(6):2841–7. Epub 2011/09/09. doi: 187/6/2841 [pii] doi: 10.4049/jimmunol.1004006 21900183; PubMed Central PMCID: PMC3170133.
8. Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–8. Epub 2010/06/29. doi: ni.1890 [pii] doi: 10.1038/ni.1890 20581831.
9. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7(3):e54. Epub 2009/03/13. doi: 08-PLBI-RA-3994 [pii] doi: 10.1371/journal.pbio.1000054 19278296; PubMed Central PMCID: PMC2653554.
10. Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, et al. Human MAIT and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119(2):422–33. Epub 2011/11/17. doi: blood-2011-05-353789 [pii] doi: 10.1182/blood-2011-05-353789 22086415; PubMed Central PMCID: PMC3257008.
11. Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121(7):1124–35. Epub 2012/12/18. doi: blood-2012-07-445429 [pii] doi: 10.1182/blood-2012-07-445429 23243281.
12. Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nature communications. 2014;5 : 3143. doi: 10.1038/ncomms4143 24452018; PubMed Central PMCID: PMC3916833.
13. Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998;161(8):4066–77. Epub 1998/10/21. 9780177.
14. Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2013;65(2):115–24. Epub 2012/12/12. doi: 10.1007/s00251-012-0666-5 23229473.
15. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–23. Epub 2012/10/12. doi: nature11605 [pii] doi: 10.1038/nature11605 23051753.
16. Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nature communications. 2013;4 : 2142. doi: 10.1038/ncomms3142 23846752.
17. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–5. doi: 10.1038/nature13160 24695216.
18. Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9(10):e1003681. doi: 10.1371/journal.ppat.1003681 24130485; PubMed Central PMCID: PMC3795036.
19. Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2014. doi: 10.1038/mi.2014.81 25269706.
20. Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci U S A. 2013. doi: 10.1073/pnas.1302799110 23898209.
21. Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2013;121(6):951–61. Epub 2012/12/21. doi: blood-2012-06-436436 [pii] doi: 10.1182/blood-2012-06-436436 23255555; PubMed Central PMCID: PMC3567342.
22. Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161 CD8 T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2013. doi: 10.1002/eji.201343509 24019201.
23. Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10(6):e1004210. doi: 10.1371/journal.ppat.1004210 24967632; PubMed Central PMCID: PMC4072808.
24. Buchacz K, Baker RK, Palella FJ Jr., Chmiel JS, Lichtenstein KA, Novak RM, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24(10):1549–59. doi: 10.1097/QAD.0b013e32833a3967 20502317.
25. Perbost I, Malafronte B, Pradier C, Santo LD, Dunais B, Counillon E, et al. In the era of highly active antiretroviral therapy, why are HIV-infected patients still admitted to hospital for an inaugural opportunistic infection? HIV medicine. 2005;6(4):232–9. doi: 10.1111/j.1468-1293.2005.00282.x 16011527.
26. Sabin CA, Smith CJ, Gumley H, Murphy G, Lampe FC, Phillips AN, et al. Late presenters in the era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS. 2004;18(16):2145–51. 15577647.
27. Huson MA, Grobusch MP, van der Poll T. The effect of HIV infection on the host response to bacterial sepsis. Lancet Infect Dis. 2014. doi: 10.1016/S1473-3099(14)70917-X 25459220.
28. Sandberg JK, Dias J, Shacklett BL, Leeansyah E. Will loss of your MAITs weaken your HAART? AIDS. 2013. Epub 2013/04/19. doi: 10.1097/QAD.0b013e3283620726 23595154.
29. Wong EB, Akilimali NA, Govender P, Sullivan ZA, Cosgrove C, Pillay M, et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS One. 2013;8(12):e83474. doi: 10.1371/journal.pone.0083474 24391773; PubMed Central PMCID: PMC3877057.
30. Eberhard JM, Hartjen P, Kummer S, Schmidt RE, Bockhorn M, Lehmann C, et al. CD161+ MAIT Cells Are Severely Reduced in Peripheral Blood and Lymph Nodes of HIV-Infected Individuals Independently of Disease Progression. PLoS One. 2014;9(11):e111323. doi: 10.1371/journal.pone.0111323 25369333; PubMed Central PMCID: PMC4219715.
31. Fernandez CS, Amarasena T, Kelleher AD, Rossjohn J, McCluskey J, Godfrey DI, et al. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol. 2014. doi: 10.1038/icb.2014.91 25348935.
32. Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80(9):3256–67. Epub 2012/07/11. doi: IAI.00279-12 [pii] doi: 10.1128/IAI.00279-12 22778103; PubMed Central PMCID: PMC3418730.
33. Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. Epub 2010/07/09. doi: 10.1371/journal.pbio.1000407 20613858; PubMed Central PMCID: PMC2893946.
34. Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48(5):769–75. Epub 2010/12/31. doi: S0161-5890(10)00659-0 [pii] doi: 10.1016/j.molimm.2010.12.002 21190736.
35. Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive care medicine. 2014;40(2):192–201. doi: 10.1007/s00134-013-3163-x 24322275.
36. Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24(3):209–17. doi: 10.1016/j.smim.2012.04.010 22551764; PubMed Central PMCID: PMC3367861.
37. Carcelain G, Autran B. Immune interventions in HIV infection. Immunol Rev. 2013;254(1):355–71. doi: 10.1111/imr.12083 23772631.
38. Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142–52. Epub 2013/03/01. doi: jimmunol.1203218 [pii] doi: 10.4049/jimmunol.1203218 23447689.
39. Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, et al. CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol. 2014;44(10):3119–28. doi: 10.1002/eji.201344160 25043505.
40. Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. American journal of respiratory and critical care medicine. 2014;190(3):329–39. doi: 10.1164/rccm.201401-0106OC 24977786.
41. Kim JH, Psevdos G Jr., Gonzalez E, Singh S, Kilayko MC, Sharp V. All-cause mortality in hospitalized HIV-infected patients at an acute tertiary care hospital with a comprehensive outpatient HIV care program in New York City in the era of highly active antiretroviral therapy (HAART). Infection. 2013;41(2):545–51. doi: 10.1007/s15010-012-0386-7 23264096.
42. Blom K, Braun M, Pakalniene J, Dailidyte L, Beziat V, Lampen MH, et al. Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection. PLoS Pathog. 2015;11(1):e1004622. doi: 10.1371/journal.ppat.1004622 25611738.
43. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–61. doi: 10.1038/nri3307 23080391; PubMed Central PMCID: PMC4137483.
44. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–41. doi: 10.4049/jimmunol.0904028 20181882; PubMed Central PMCID: PMC3725574.
45. Verhagen J, Wraith DC. Comment on "Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells". J Immunol. 2010;185(12):7129; author reply 30. doi: 10.4049/jimmunol.1090105 21127313.
46. Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6(8):e24226. doi: 10.1371/journal.pone.0024226 21918685; PubMed Central PMCID: PMC3168881.
47. Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R, et al. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One. 2011;6(6):e20731. doi: 10.1371/journal.pone.0020731 21677778; PubMed Central PMCID: PMC3108993.
48. Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. doi: 10.1016/j.immuni.2009.11.012 20096607; PubMed Central PMCID: PMC2906224.
49. Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–44. doi: 10.1038/ni1268 16273099.
50. Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10(1):e1003890. doi: 10.1371/journal.ppat.1003890 24497828; PubMed Central PMCID: PMC3907377.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



