-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
Influenza viruses infect millions of people annually causing significant morbidity, mortality and socio-economic burdens. Host immune responses against influenza virus are initiated upon virus recognition by specific intracellular receptors. Signals relayed from these receptors trigger various signaling cascades, which induce an antiviral immune response to control infection. Herein, we identified the serine-threonine kinase tumor progression locus 2 (Tpl2) as an essential component of virus sensing pathways, regulating induction of interferons (IFNs) and IFN-induced antiviral genes that restrict virus replication. We also demonstrate that Tpl2 is necessary for generation of effector CD8+ T cells, which are required for viral clearance from infected lungs. Consistent with the impaired antiviral responses, Tpl2-deficient mice are defective in controlling virus replication and succumb to influenza virus infection with a normally low pathogenicity strain. Thus, our study identifies Tpl2 as a host factor that integrates antiviral innate and adaptive responses to restrict morbidity and mortality during influenza virus infection.
Published in the journal: Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus. PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005038
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005038Summary
Influenza viruses infect millions of people annually causing significant morbidity, mortality and socio-economic burdens. Host immune responses against influenza virus are initiated upon virus recognition by specific intracellular receptors. Signals relayed from these receptors trigger various signaling cascades, which induce an antiviral immune response to control infection. Herein, we identified the serine-threonine kinase tumor progression locus 2 (Tpl2) as an essential component of virus sensing pathways, regulating induction of interferons (IFNs) and IFN-induced antiviral genes that restrict virus replication. We also demonstrate that Tpl2 is necessary for generation of effector CD8+ T cells, which are required for viral clearance from infected lungs. Consistent with the impaired antiviral responses, Tpl2-deficient mice are defective in controlling virus replication and succumb to influenza virus infection with a normally low pathogenicity strain. Thus, our study identifies Tpl2 as a host factor that integrates antiviral innate and adaptive responses to restrict morbidity and mortality during influenza virus infection.
Introduction
Mitogen-activated protein kinase (MAP) cascades represent major intracellular signaling pathways activated in response to a variety of external stimuli. Their activation during infection leads to transcriptional induction of immune and inflammatory mediators. Although MAP kinase signaling is important in eliciting host protective responses, many viruses are known to utilize these pathways directly for their replication [1]. Activation of MAP kinases occurs during virus recognition by pattern recognition receptors (PRRs) like toll-like receptors (TLRs) and RIG-I-like RNA helicases (RLH) [2]. Virus sensing by these receptors activates multiple intracellular signaling cascades including NFκB, MAP kinase and IRF pathways that coordinately regulate induction of interferons (IFNs) which are important mediators of antiviral resistance [3]. Among the MAP kinases, tumor progression locus 2 (Tpl2/MAP3K8), a MAP3 kinase, plays an important role in regulating IFN production by promoting the ERK-dependent induction of c-fos, a component of AP-1 heterodimeric transcription factors [4]. While Tpl2 is required for IFNα production by plasmacytoid dendritic cells (pDCs) and IFNγ secretion by CD4+ T cells, it is a potent negative regulator of IFNβ in macrophages and DCs [4, 5]. Despite being identified as a major regulator of both Type I (IFNα/β) and Type II (IFNγ) IFNs, Tpl2 regulation of Type III IFNs (IFNλs) has not been investigated so far.
Tpl2 was initially identified as an oncogene that induces T cell lymphomas in rodents [6], but more recent studies have established its criticality in regulating both innate and adaptive immune responses via its cell type - and stimulus-specific activation of the MEK-ERK MAPK pathway. Tpl2 regulates signal transduction and cellular responses downstream of TLRs, cytokine receptors, antigen receptors and G protein-coupled receptors [4, 7–9]. In addition to IFNs, Tpl2 also regulates the production of other prominent immune mediators like TNFα, IL-1β IL-10, IL-12 and COX-2 [4, 10–12]. Consequently, Tpl2 is essential for mounting effective immune responses during infections, and Tpl2-/- mice are more susceptible to Toxoplasma gondii [5], Listeria monocytogenes [11], Mycobacterium tuberculosis [13] and Group B Streptococcus [14]. Surprisingly, there is still limited and contradictory information about how Tpl2 contributes to host defense against viruses. Early studies reported normal cytotoxic T cell responses against lymphocytic choriomeningitis virus [10] and resistance to mouse cytomegalovirus infection [14]. However, another study delineating the signaling circuitry in virus sensing pathways implicated Tpl2 as a key regulator of both inflammatory and antiviral gene induction in response to model viral ligands [15]. A recent study also reported increased replication of vesicular stomatitis virus in Tpl2-deficient mouse embryonic fibroblasts (MEFs) [16].
We recently demonstrated that among the TLRs implicated in virus sensing (TLRs 3, 7 and 9), Tpl2 plays a prominent role in TLR7 signaling [17]. In this study, we investigated Tpl2’s regulation of antiviral responses using a murine model of influenza virus infection, which relies upon TLR7 for virus sensing [18], ERK MAP kinase for virus replication [19] and where both IFNα/β and IFNλ are host protective [20]. Our experiments demonstrate positive regulation of IFNλ and cell-type specific regulation of IFNα/β production in Tpl2-deficient cells following stimulation with model viral ligands that trigger influenza virus sensing receptors, TLR7 or RIG-I. However, during influenza virus infection, IFNλ uniquely required Tpl2 for its induction. Moreover, Tpl2 is involved in IFN signaling, regulating ERK activation and STAT1ser727 phosphorylation, and is required for proper induction of antiviral IFN-stimulated genes (ISGs). Impaired ISG induction coupled with reduced antigen-specific CD8+ T cells resulted in failure to control virus replication and significant morbidity and mortality of Tpl2-/- mice to an otherwise low pathogenicity strain of influenza virus. Collectively, this study establishes Tpl2 as a host factor that integrates antiviral responses to control influenza virus infection.
Results
Tpl2 ablation enhances virus replication and inflammatory responses during influenza infection
To determine whether Tpl2 regulates influenza virus replication, wild type (WT) and Tpl2-/- mice were infected with 104 plaque forming units (pfu) of mouse-adapted influenza virus A/HK-X31(H3N2) (X31), and viral titers in the lungs were evaluated on days 3, 5 and 7 post infection (pi). The average lung viral titers were significantly higher in Tpl2-/- mice compared to WT mice at all time points examined (Fig 1A). Notably, average viral titers were more than ten-fold higher in Tpl2-/- lungs at day 7 pi. This increase in virus replication was also observed in littermate control mice (S1 Fig). In addition to viral titers, early proinflammatory cytokines, except TNFα were significantly higher in the BALF of Tpl2-/- mice compared to WT mice (Fig 1B). Consistent with increased virus replication, total cellular infiltration was also significantly increased in the lungs of Tpl2-/- mice at day 7 pi (Fig 1C). The increased lung viral titers in Tpl2-/- mice early after infection on day 3 suggest a critical role for Tpl2 in limiting virus replication during influenza virus infection.
Fig. 1. Tpl2 ablation enhances virus replication and inflammatory responses during influenza infection. 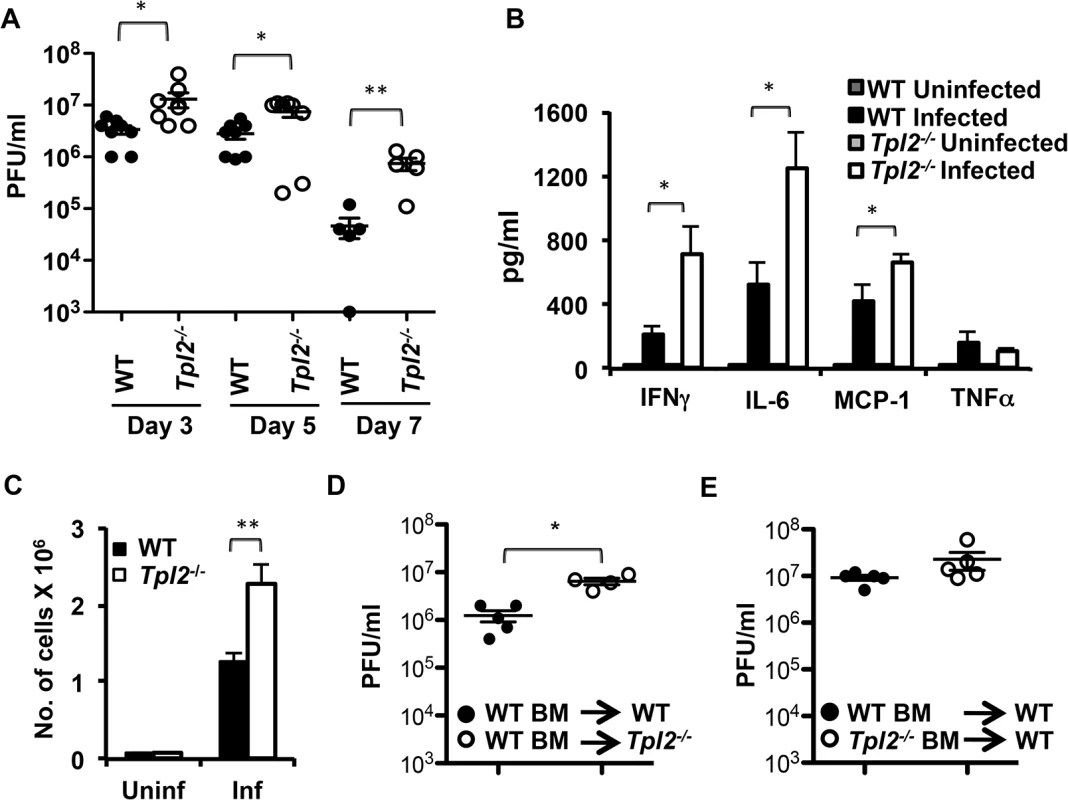
(A) WT and Tpl2-/- mice were intranasally infected with 104 pfu of X31 virus, and lung viral titers were enumerated by plaque assays; n = 8 (D3 and D5) or n = 5 (D7). WT and Tpl2-/- mice were infected with 104 pfu of X31 virus, and the cytokine levels (B) and number of cells recovered (C) in BALF were measured on D7 pi; n = 6 uninfected and 10 (WT) and 8 (Tpl2-/-) infected. (D-E) Chimeric mice were intranasally infected with 104 pfu of X31 virus, and lung viral titers were enumerated by plaque assays D3 pi. * indicates p<0.05, ** indicates p<0.01. Tpl2 signaling in nonhematopoietic cells is necessary for limiting early virus replication
Airway epithelial cells are the primary targets for influenza virus infection. Early studies after the discovery of Tpl2 demonstrated high levels of Tpl2 expression in the lungs [21]. Moreover, similar to hematopoietic cells, Tpl2 regulation of signal transduction and cytokine gene induction was also demonstrated in airway epithelial cells [22]. To elucidate whether Tpl2 functions in hematopoietic or nonhematopoietic cells to limit virus replication, we assessed lung viral titers in chimeric mice in which WT or Tpl2-/- bone marrow cells were transferred into either WT or Tpl2-/- irradiated recipients. At day 3 pi, average lung viral titers were significantly higher in Tpl2-/- mice reconstituted with WT hematopoietic cells (Fig 1D). In contrast, there was no statistically significant increase in viral titers of WT mice that received Tpl2-/- bone marrow (Fig 1E). These data demonstrate that Tpl2 signaling within radioresistant, nonhematopoietic lung cells is necessary for limiting virus replication early after infection.
Tpl2 is required for optimal IFNλ production during influenza infection in vivo and in vitro
Interferons are induced early during infection and are key factors initiating host protective antiviral responses [3]. To determine whether the observed increase in viral titers in Tpl2-/- mice is due to defective induction of IFNs, WT and Tpl2-/- mice were infected with 106 pfu X31 virus, and IFNα/β/λ levels in lung homogenate or BALF were measured at day 1 or day 3 pi. Induction of both IFNα and β were comparable between WT and Tpl2-/- lung homogenates and BALF (Fig 2A). Notably, IFNλ secretion was significantly reduced in Tpl2-/- mice following influenza virus infection (Fig 2B). Surprisingly, while IFNλ was induced to a higher level compared to Type I IFNs in WT mice, there was minimal induction in Tpl2-/- mice in response to infection at both time points. Reduced IFNλ production in Tpl2-/- mice was independent of viral titers which were similar between WT and Tpl2-/- mice at day 1 pi (S2 Fig). Despite differences in IFNλ induction, total cellular infiltration and IFNγ levels in BALF were significantly elevated in Tpl2-/- mice compared to WT mice at day 3 pi (S3 Fig).
Fig. 2. Tpl2 is required for optimal IFNλ production during influenza virus infection in vitro and in vivo. 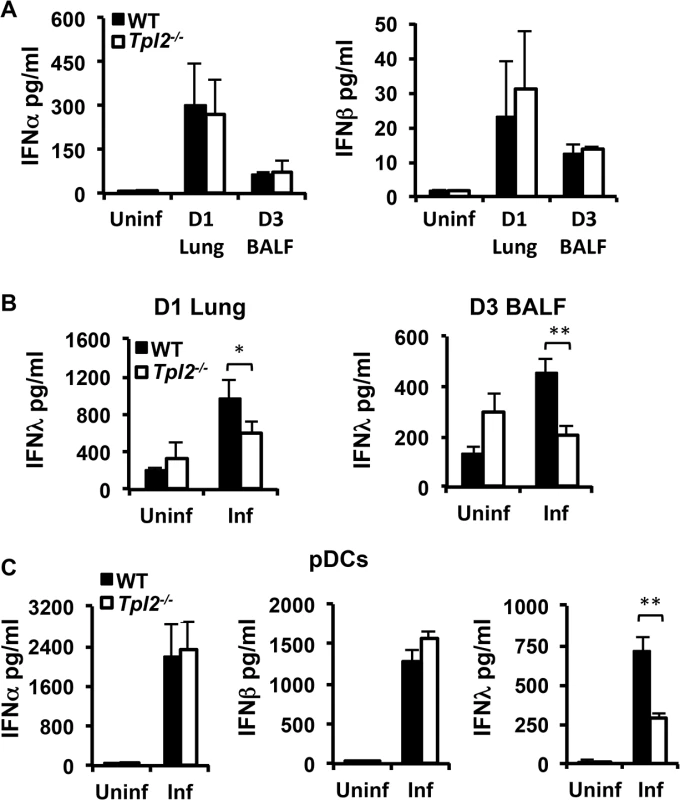
WT and Tpl2-/- mice were infected with 106 pfu of X31 virus, and IFNα, β (A), and λ (B) levels in D1 lung homogenates or D3 BALF were measured by ELISA or bead based assay. For IFNα, n = 4 uninfected and 7 infected mice per group; for IFNβ, n = 2 uninfected and 3 infected per group; for D1 IFNλ n = 5 (WT) and 6 (Tpl2-/-) uninfected and 10 (WT) and 12 (Tpl2-/-) infected; for D3 IFNλ n = 5 uninfected and 14 infected (WT) and 13 (Tpl2-/-) infected. (C) pDCs from WT and Tpl2-/- mice were infected with WSN virus at an MOI of 0.2 for 24 hr, and IFNα, β and λ levels were measured. Data are representative of 3–4 independent experiments. * indicates p<0.05, ** indicates p<0.01. The observation that Tpl2 is uniquely required for IFNλ, but not IFNα or IFNβ, production in influenza-infected lungs is especially significant, because IFNλ is regarded as the principal IFN induced during influenza virus infection. Airway epithelial cells and pDCs are considered the major sources of IFNs during respiratory virus infections, including influenza [20, 23]. Although we observed a decrease in IFNλ levels in Tpl2-/- mice at day 1 pi, a more consistent and significant reduction was observed at day 3 pi, which corresponds to the migration of pDCs to infected lungs [23]. Since Tpl2 is required for macrophage and neutrophil migration during acute inflammation [9, 24], we investigated whether Tpl2 similarly regulates the recruitment of pDCs to the infected lung. The reduction in IFNλ levels in influenza-infected Tpl2-/- mice was not due to impaired recruitment of pDCs (S4 Fig). To investigate whether defective IFN induction by pDCs contributes to the reduced IFNλ in BALF from Tpl2-/- mice during influenza infection, bone marrow-derived pDCs (CD11c+B220+CD11b-) from WT and Tpl2-/- mice were infected with influenza virus A/WSN/1933 (H1N1), and the production of IFNα, β and λ was assessed. Consistent with in vivo infections, the levels of both IFNα and IFNβ were comparable between WT and Tpl2-/- cells, whereas IFNλ secretion was significantly less in Tpl2-/- pDCs infected with influenza virus (Fig 2C). A similar reduction in IFNλ induction was also observed in Tpl2-deficient cells infected with X31 influenza virus strain (S5 Fig). Collectively, these data demonstrate the unique requirement for Tpl2 in IFNλ production during influenza infection in vitro and in vivo.
Tpl2 differentially regulates IFN production in response to model viral ligands in a cell type-specific manner
During influenza virus infection, receptors from both TLR and RLR families recognize viral PAMPs and trigger rapid induction of IFNs. Recognition of viral components by PRRs typically occurs in respiratory epithelial cells, alveolar macrophages, DCs and pDCs in a cell type-specific manner [25]. The major receptors involved in recognition of influenza virus are TLR7, which recognizes single-stranded viral RNA, and RIG-I, which recognizes the 5’-triphosphate of single-stranded RNA genomes (5’ppp-RNA). The single-stranded RNA genome is recognized through endosomal TLR7 in pDCs [18] in contrast to epithelial cells and DCs where virus recognition is mediated primarily by the cytosolic sensor RIG-I [26]. We therefore investigated whether differential regulation of IFN production observed during infection is due to differences in Tpl2-mediated sensing by PRRs. MEFs and bone marrow-derived macrophages (BMDMs) from WT and Tpl2-/- mice were either transfected with the RIG-I ligand 5’ppp-RNA or stimulated with the TLR7 ligand R848 [27], and IFNβ production was measured by ELISA. Consistent with previous studies using the TLR4 ligand LPS [4], IFNβ production was significantly increased in Tpl2-/- cells treated with both 5’ppp-RNA and R848 (Fig 3A–3C). This increase in IFNβ correlated with impaired ERK phosphorylation in Tpl2-deficient cells in response to these ligands (S6 Fig). Unlike epithelial cells and DCs, virus recognition in pDCs is mediated via TLRs rather than RLHs, and Type I IFN production occurred normally in RIG-I-deficient pDCs infected with RNA viruses [18, 26]. To determine whether Tpl2 regulates TLR7-mediated IFN production by pDCs, bone marrow-derived pDCs from WT and Tpl2-/- mice were treated with the TLR7 ligand, R848, and IFN levels were quantitated. Consistent with previous studies using the TLR9 ligand CpG [4], and in contrast to BMDMs, secretion of both IFNα and IFNβ were significantly decreased in culture supernatants from Tpl2-/- pDCs treated with R848 (Fig 3D). Notably, IFNλ secretion was also significantly less in Tpl2-/- pDCs compared to WT cells in response to R848 (Fig 3D). Unlike Ifna but similar to NFκB-regulated Il12p40 and Tnfa [28], IFNλ3 (Il28b) transcription occurred early, by 2 hr of stimulation (S7 Fig). Collectively, these data demonstrate that Tpl2 differentially regulates IFN production downstream of PRRs involved in influenza virus sensing in a cell type-specific manner.
Fig. 3. Tpl2 differentially regulates IFN production in response to model viral ligands in a cell type-specific manner. 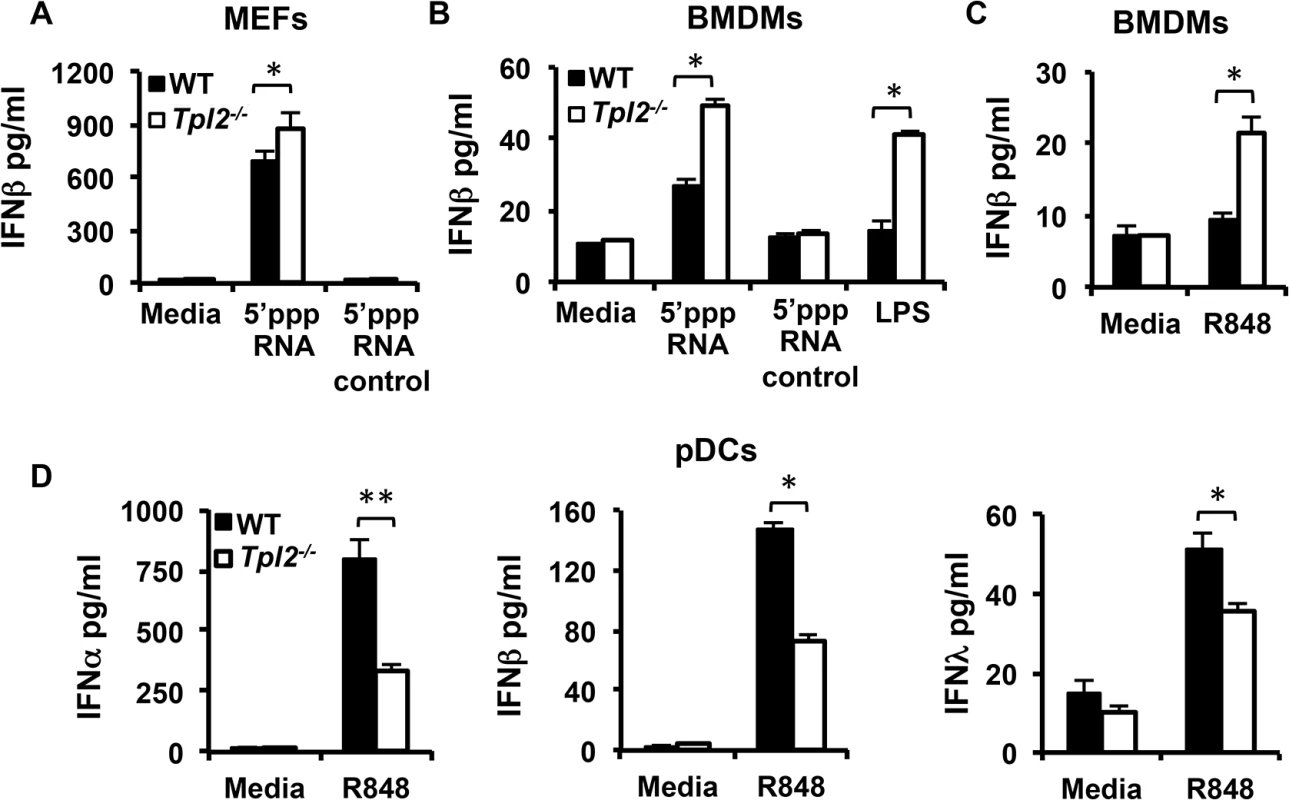
MEFs (A) or BMDMs (B-C) from WT and Tpl2-/- mice were transfected with 5’ppp-RNA or stimulated R848 or LPS for 24 hr, and IFNβ levels were measured by ELISA. (D) Plasmacytoid DCs were stimulated with R848 for 24 hr, and IFNα, β and λ levels were measured. Data are representative of 3–4 independent experiments. Graphs show means±SD. * indicates p<0.05, ** indicates p<0.01. ERK and Akt are involved in Tpl2-dependent IFNλ production in pDCs
The importance of IFNλs in host protection against many viruses is well established, however, the mechanisms that regulate their production are largely unexplored. Common mechanisms have been postulated to regulate Type I and III IFNs during viral infections [29, 30]. Despite their importance in mediating Type I IFN production in pDCs [4, 31], the significance of MAP kinase and PI3 kinase cascades in murine IFNλ production has not been directly investigated. In order to elucidate the potential mechanism by which Tpl2 regulates IFNλ production in pDCs, we evaluated the involvement of ERK and PI3K-mTOR signaling in IFNλ induction. Tpl2 regulation of both ERK and mTOR-Akt signaling in different cell types has been reported previously [8, 32–34]. In addition to the MEK/ERK pathway [4], we demonstrate that Tpl2 also promotes mTOR/Akt signaling in pDCs as determined by a decrease in the proportion of phospho-Akt+ pDCs in the absence of Tpl2 signaling (Fig 4A and 4B). To confirm whether ERK, PI3K or mTOR signaling also contributes to IFNλ production in pDCs, cells were pre-treated with rapamycin (mTOR inhibitor), LY294002 (PI3K inhibitor) or U0126 (MEK inhibitor) 30 min prior to TLR stimulation, and CpG-induced IFNλ secretion was measured by ELISA. CpG was used as the stimulant in these experiments because TLR9 ligation induced higher levels of IFNλ compared to TLR7 stimulation with R848. Pharmacological inhibition of each of these signaling pathways significantly reduced IFNλ secretion to the levels observed in Tpl2-/- cells (Fig 4C). In contrast, only a modest reduction in IFNλ induction was observed in Tpl2-deficient cells treated with rapamycin or U0126 (S8 Fig). These results demonstrate the significance of Tpl2 and both MAPK and PI3 kinase signaling cascades in regulating IFNλ production in pDCs.
Fig. 4. ERK and Akt are involved in Tpl2-dependent IFNλ production in pDCs. 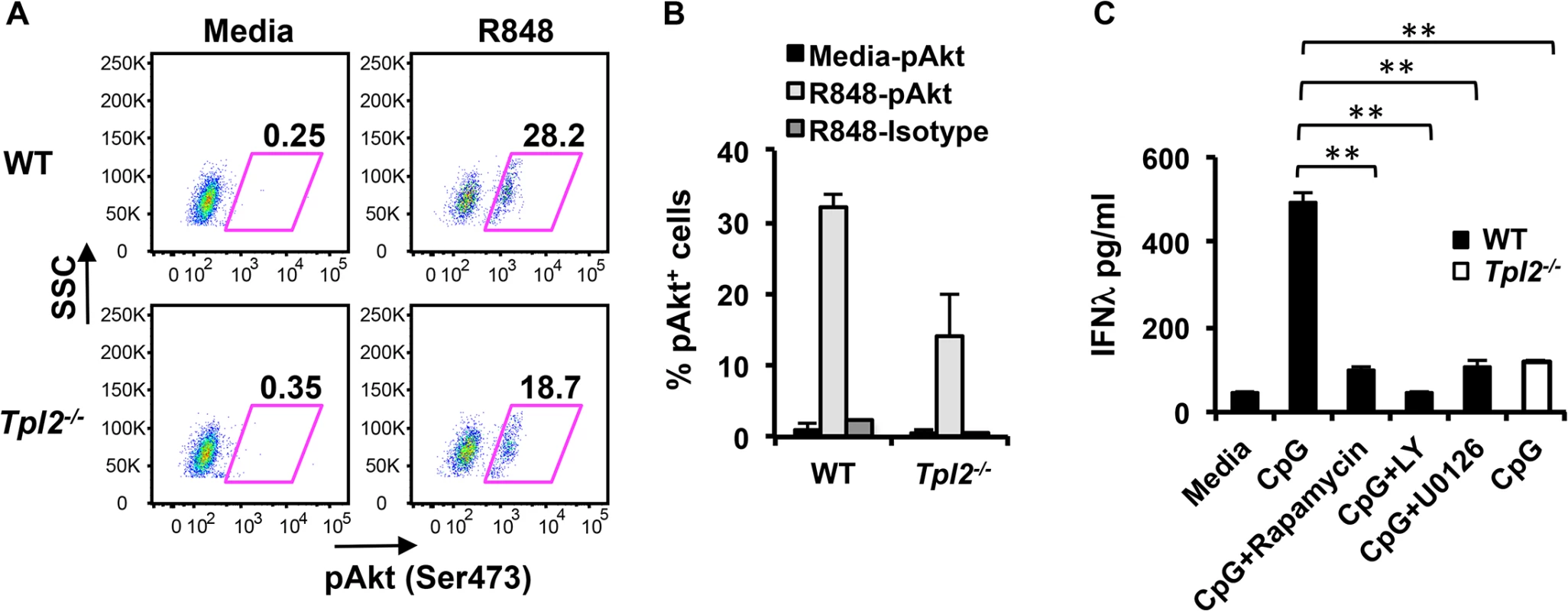
pDCs from WT and Tpl2-/- mice were stimulated with R848 for 18 hr, and analyzed by intracellular staining for pAktSer473. (A) Representative flow cytometry plots showing pAktSer473 staining within pDCs. (B) Proportion of pAkt positive pDCs from 2 independent experiments. (C) pDCs were pretreated with inhibitors for 30 min before stimulation with CpG, and IFNλ levels were measured by ELISA. Data are representative of 2 (A-B) or 3 (C) independent experiments. Graphs show means±SD. * indicates p<0.05, ** indicates p<0.01. Induction of IFNλ in influenza virus-infected lungs occurs independently of Type I IFN signaling
Robust production of Type I IFNs in pDCs is dependent upon IRF7 and autocrine IFN signaling, and consequently IFNα secretion is abrogated in both Irf7-/- and Ifnar1-/- pDCs [35]. Similar to IFNα, and as reported previously [20], IFNλ production was abolished in Ifnar1-/- pDCs infected with influenza virus (Fig 5A) demonstrating the absolute requirement for IFNAR signaling in IFNλ secretion by pDCs. Induction of IFNλ in response to direct IFN stimulation has been reported in hepatocyte carcinoma HepG2 cell lines [36]. Although a high dose of IFNβ could induce modest IFNλ secretion, the levels induced were lower than that induced by TLR-stimulation, demonstrating that IFN/IRF7 signaling alone is not sufficient for driving high levels of IFNλ secretion (Fig 5B). Nevertheless, Tpl2 contributed to IFNAR-induced IFNλ production, since significantly less IFNλ was secreted by Tpl2-/- pDCs directly treated with IFNβ (Fig 5B). In addition to demonstrating the role of Tpl2 in IFNAR-mediated IFNλ production, these data also suggest a role for Tpl2 in directly transducing Type I IFN signals.
Fig. 5. IFNλ production is IFNAR-independent in influenza virus-infected lungs. 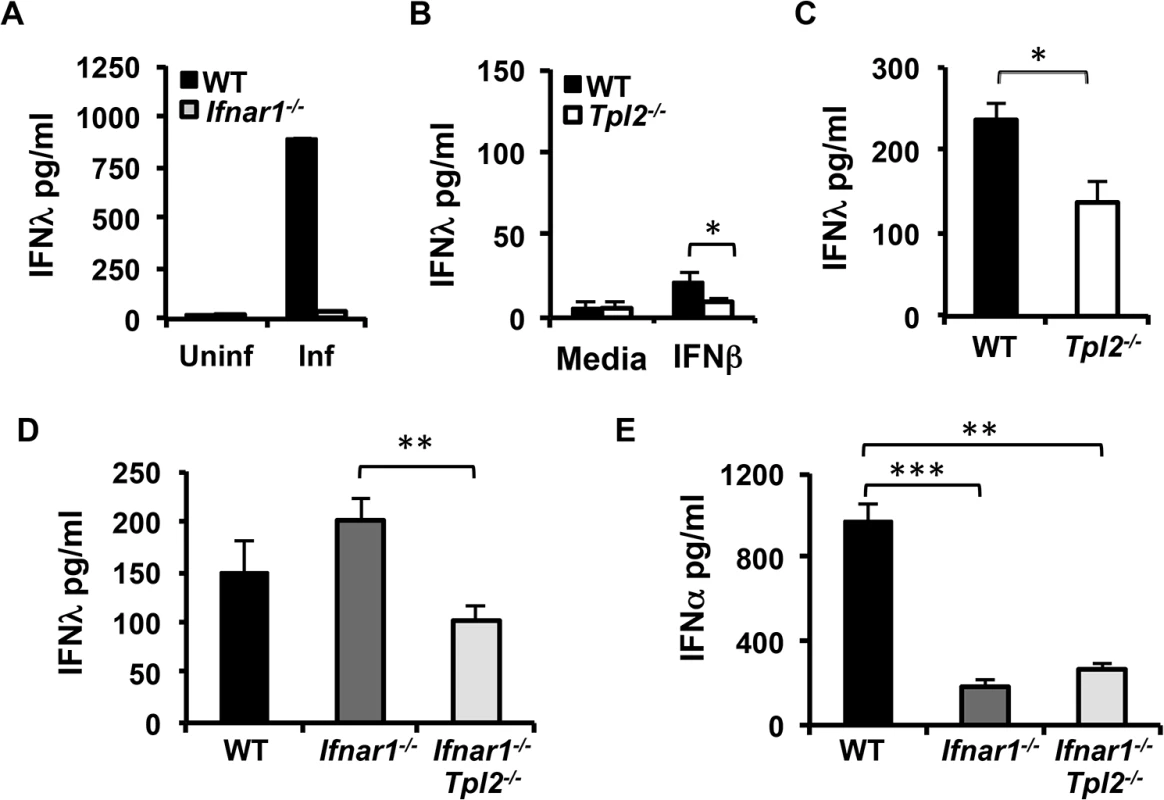
(A) Flt3 ligand-derived DCs from WT and Ifnar1-/- mice were infected with WSN virus for 24 hr, and IFNλ secretion was measured by ELISA. (B) Flt3 ligand-derived DCs from WT and Tpl2-/- mice were treated with IFNβ for 24 hr, and IFNλ secretion was quantitated by ELISA. Data are representative of 2 (A) or 3 (B) independent experiments. Graphs show means±SD. (C) WT and Tpl2-/- mice were infected with 104 pfu of X31 virus, and IFNλ levels in lung homogenates were measured by ELISA on D3 pi; n = 5 WT and 5 Tpl2-/- mice. WT, Ifnar1-/- and Ifnar1-/-Tpl2-/- mice were infected with 104 pfu of X31 virus, and IFNλ (D) and IFNα (E) levels in lung homogenates were measured by ELISA on D3 pi; n = 4 WT, 5 Ifnar1-/- and 9 Ifnar1-/-Tpl2-/- mice. Graphs show means±SEM. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. To determine whether Tpl2 regulates IFNλ production in influenza virus-infected lungs directly via virus sensing pathways or indirectly via IFNAR feedback signaling, we assessed IFNλ levels in lung homogenates from mice that are deficient in both Tpl2 and IFNAR1. Consistent with reduced IFNλ levels in BALF from Tpl2-/- mice day 3 pi (Fig 2B), IFNλ levels were similarly reduced in day 3 lung homogenates (Fig 5C). IFNλ levels were significantly decreased in Ifnar1-/-Tpl2-/- compared to Ifnar1-/- mice, demonstrating that Tpl2 promotes early IFNλ induction independent of Type I IFN signaling (Fig 5D). Notably, the level of IFNλ induction was similar in Tpl2-/- and Ifnar1-/-Tpl2-/- mice (Fig 5C and 5D). In striking contrast to the abrogation of IFNλ production in Ifnar1-/- pDCs (Fig 5A), IFNλ production occurred normally in Ifnar1-/- mice (Fig 5D). Consistent with the critical role of IFNAR signaling in IFNα induction, IFNα levels were significantly diminished in both Ifnar1-/- and Ifnar1-/-Tpl2-/- mice (Fig 5E). These data demonstrate that Tpl2-dependent IFNλ production during influenza virus infection is IFNAR-independent.
Tpl2 mediates IFN signaling and induction of IFN-stimulated genes (ISGs)
Both IFNα/β and IFNλ are known to induce expression of ISGs that establish an antiviral state in infected tissue to prevent virus replication and spread [3, 37]. Because of the observed increase in early virus replication in Tpl2-/- mice (Fig 1A), we questioned whether Tpl2 regulates the induction of ISGs. We first addressed whether Tpl2 regulates IFN signaling. BMDMs from WT and Tpl2-/- mice were stimulated with IFNα or IFNβ, and activation of downstream cascades, especially STAT1, which is the principle regulator of IFN responses, was evaluated by immunoblotting. BMDMs were used in these experiments due to limited availability of pDCs. The phosphorylation of STAT1 Tyr701 and Ser727, which is necessary for maximal STAT1 transcriptional activation, were examined [38]. While phosphorylation of Tyr701 occurred normally in Tpl2-deficient cells in response to stimulation with Type I IFNs, a consistent reduction in Ser727 phosphorylation was observed in Tpl2-/- cells compared to WT cells (Fig 6A–6C). In addition to the classical JAK-STAT pathway, signaling via the Type I IFN receptor also activates other downstream cascades including MAP kinases [39]. Despite the existence of multiple MAP3 kinases, Tpl2 has an essential, non-redundant role in transducing ERK activation signals during TLR, TNF - and IL-1-receptor signaling [7, 8]. We therefore investigated whether Tpl2 is similarly required for ERK activation during Type I IFN signaling, or whether other MAP3Ks could fulfill this role. ERK phosphorylation was strongly induced by both IFNα and IFNβ. Importantly, ERK phosphorylation was absent in Tpl2-/- BMDMs stimulated with IFNα/β demonstrating an absolute requirement for Tpl2 in transducing ERK activation signals in response to Type I IFNs (Fig 6A). Of note, unlike LPS - and TNFα-treated BMDMs and similar to poly I:C-, CpG-, and IL-1β-treated BMDMs [17, 40], no mobility shift (indicative of phosphorylation) or degradation of the p58 isoform of Tpl2 was detected following stimulation with Type I IFNs (Fig 6A). Consistent with our previous studies [41], both Tpl2 protein and mRNA expression were induced upon either Type I IFN stimulation or influenza virus infection (Fig 6A and S9 Fig). Overall, these data demonstrate that Tpl2 contributes to Type I IFN signaling.
Fig. 6. Tpl2 mediates IFN signaling and induction of ISGs. 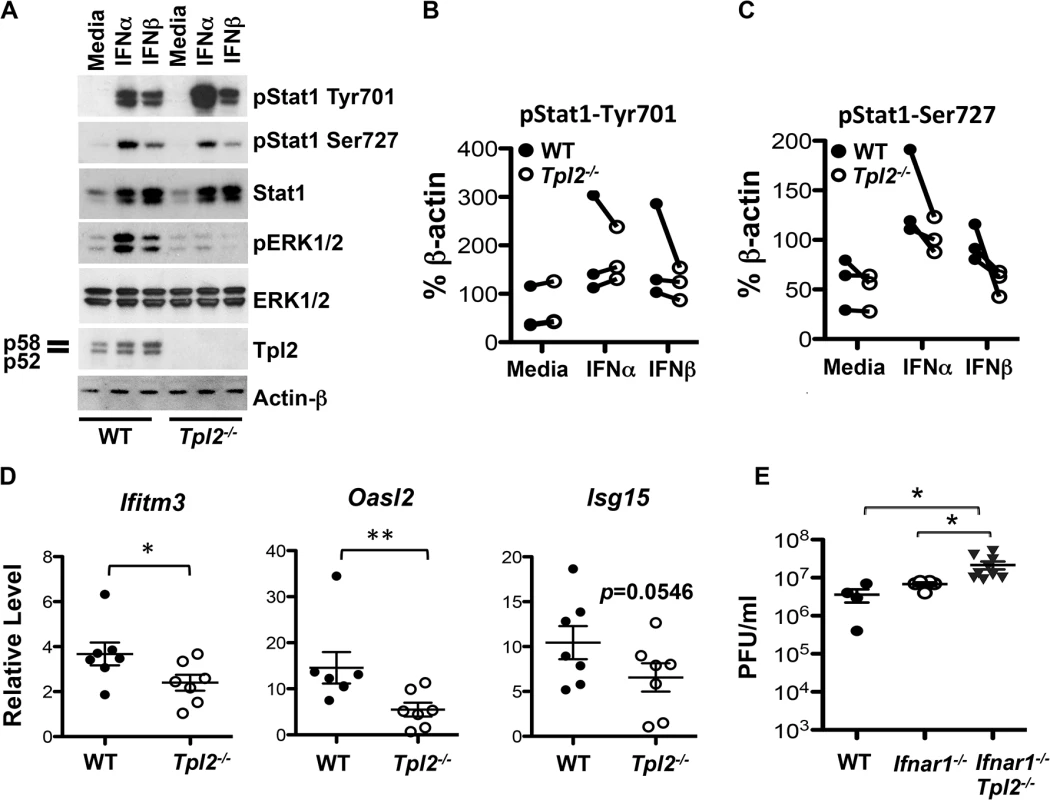
(A) BMDMs from WT and Tpl2-/- mice were stimulated with IFNα or IFNβ for 1 hr, and STAT1 and ERK phosphorylation were assessed by immunoblotting. Data are representative of 3 independent experiments. Average intensities of pSTAT1Tyr701 (B) and pSTAT1Ser727 (C) bands normalized to actin bands by densitometric analysis. Data collected on the same day are connected by lines. (D) WT and Tpl2-/- mice were infected with 106 pfu of X31 virus, and the expression of Ifitm3, Isg15 and Oasl2 in lung tissue D1 pi was measured by RT-PCR with normalization to actin mRNA and WT uninfected sample (n = 7). (E) WT, Ifnar1-/- and Ifnar1-/-Tpl2-/- mice were intranasally infected with 104 pfu of X31 virus, and lung viral titers on D3 pi were enumerated by plaque assays; n = 4 WT, 5 Ifnar1-/- and 9 Ifnar1-/-Tpl2-/- mice. * indicates p<0.05, ** indicates p<0.01. Since Tpl2 is known to modulate the antiviral transcriptome [16], we next investigated whether the induction of ISGs in infected lungs is impaired in the absence of Tpl2. The induction of Ifitm3, Isg15 and Oasl2, ISGs known to be important in limiting influenza virus infection [25], were measured. We observed a modest, but statistically significant decrease in Ifitm3 and Oasl2 expression in Tpl2-/- compared to WT mice infected with influenza virus (Fig 6D). A trend towards reduction in Isg15 expression was also noted in Tpl2-/- mice (Fig 6D). In addition to infected lungs, the induction of Oasl2 was significantly reduced in Tpl2-/- BMDMs, while the expression of Ifitm3 and Isg15 was largely unaffected by Tpl2 ablation (S10 Fig). These data demonstrate that Tpl2 promotes the induction of ISGs in influenza-infected lungs to limit virus replication.
Although Tpl2 is important in transducing Type I IFN signals, this would not alone account for the increase in viral titers or reduction in ISGs observed in Tpl2-/- mice, since either Type I or Type III IFN is sufficient for protection during influenza virus infection. This is because both types of IFNs drive redundant amplification loops inducing the expression of similar antiviral genes [42]. To investigate whether IFNAR signaling contributes to the observed increase in virus replication, we next assessed lung viral titers in mice deficient in both Tpl2 and IFNAR1. Consistent with previous studies [20], viral titers were comparable between WT and Ifnar1-/- mice (Fig 6E). Although average lung viral titers were significantly higher in Ifnar1-/-Tpl2-/- mice compared to both WT and Ifnar1-/- mice (Fig 6E), the titers were similar to those observed in Tpl2-/- mice (Fig 1A). These data demonstrate that Tpl2 restricts early virus replication in an IFNAR-independent manner.
Tpl2 ablation limits the induction of antigen-specific CD8+ T cells and enhances susceptibility to influenza infection
Even though the observed reduction in ISGs helps to explain the early increase in viral titers, a more pronounced and biologically significant increase in viral titers was observed at day 7 pi which correlates with the recruitment of influenza-specific CD8+ T cells to the lungs [43]. Since many seminal studies have identified CD8+ T cells as the major mediators of influenza virus clearance from infected lungs [44, 45], we investigated whether virus-specific CD8+ T cell responses are impaired in Tpl2-/- mice. Consistent with defective viral clearance observed in Tpl2-/- mice, induction of protective nucleoprotein (NP)-specific CD8+ T cells [46] was significantly reduced in BAL cells from Tpl2-/- mice compared to WT animals (Fig 7A and 7B). In addition, antigen-specific secretion of IFNγ was also decreased in BAL cells from Tpl2-/- mice (Fig 7C). During the course of this experiment, we unexpectedly observed severe clinical signs in Tpl2-/- mice despite the fact that the mice were infected with the low pathogenicity A/HK-X31(H3N2) (X31) influenza virus. To confirm whether Tpl2 ablation alters the susceptibility to influenza virus infection, WT and Tpl2-/- mice were infected with 104 pfu of X31 virus, and weight loss and clinical symptoms were monitored over a period of 14 days. All Tpl2-/- mice exhibited severe clinical signs and succumbed to infection by day 10 pi, whereas all WT animals survived and returned to pre-infection body weights by day 14 pi (Fig 7D and 7E). Similar to infection with X31 virus, Tpl2-/- mice infected with the virulent PR8 [A/Puerto Rico/8/34 (PR8; H1N1)] strain showed increased disease severity compared to WT mice, although not to the same extent seen with the low pathogenicity virus (S11 Fig). Body weights were collected to day 10 pi, at which time the Tpl2-/- mice met the humane endpoints of the study. At this time point, the body weights were just beginning to show the characteristic switch between the WT and Tpl2-/- mice, such that the Tpl2-/- mice were showing more severe clinical signs of disease. Accordingly, systemic pro-inflammatory cytokine levels were also increased in the Tpl2-/- mice at day 10 pi. Analysis of BAL cells also showed decreased antigen-specific CD8+ T cell responses in Tpl2-/- mice compared to WT mice at this late time point, consistent with the observations with X31 infections. Collectively, these data demonstrate the critical role of Tpl2 in promoting viral clearance and restricting morbidity and mortality associated with influenza virus infection.
Fig. 7. Tpl2 ablation limits antigen-specific CD8+ T cell responses and enhances susceptibility to influenza infection. 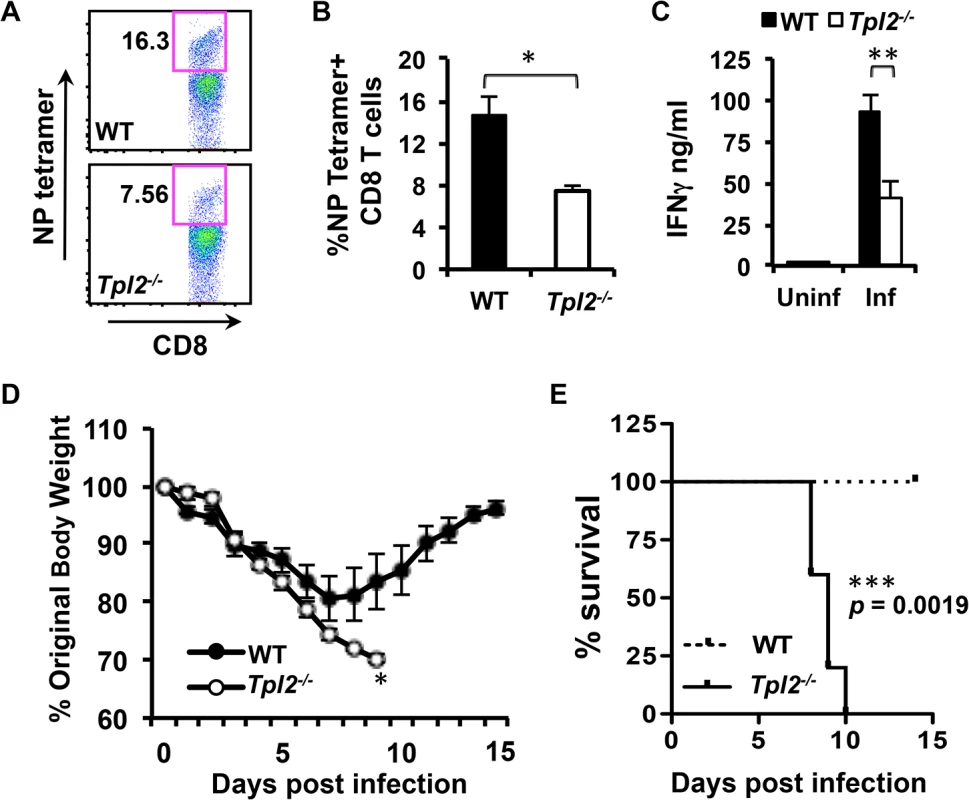
(A-B) WT and Tpl2-/- mice were infected with 104 pfu of X31 virus, and the proportion of NP366–374 tetramer positive CD8+ T cells in BAL were assessed. (C) BAL cells were stimulated with a cocktail of influenza immunodominant peptides for 24 hr, and secretion of IFNγ was measured by ELISA; n = 5. (D-E) WT and Tpl2-/- mice were infected with 104 pfu of X31 virus, body weights were recorded daily for 14 days, and mice exhibiting severe signs of disease, including more than 30% weight loss were euthanized. Data are representative of 3 independent experiments; n = 5. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.005. Discussion
Tpl2 is now appreciated to regulate the induction of Type I and Type II IFNs as well as other cytokines that may contribute to antiviral responses. However, there is very limited information on how Tpl2 coordinates antiviral immune responses in vivo. In this study, we demonstrate Tpl2’s obligate role in promoting antiviral responses and viral clearance during influenza virus infection. These findings are important because influenza virus is a ubiquitous seasonal virus that afflicts millions of people annually, causing significant morbidity, mortality and socio-economic burdens [47]. Therefore, understanding the role of host factors like Tpl2 in restricting morbidity and mortality associated with influenza virus infection is critical for developing disease intervention strategies. Mechanistically, Tpl2 promotes the induction of ISGs and virus-specific CD8+ T cells that facilitate viral clearance as shown in the proposed model (Fig 8). Thus, the findings reported here establish an essential role for Tpl2 in host protective innate and adaptive antiviral responses.
Fig. 8. Model of Tpl2 regulation of antiviral immune responses. 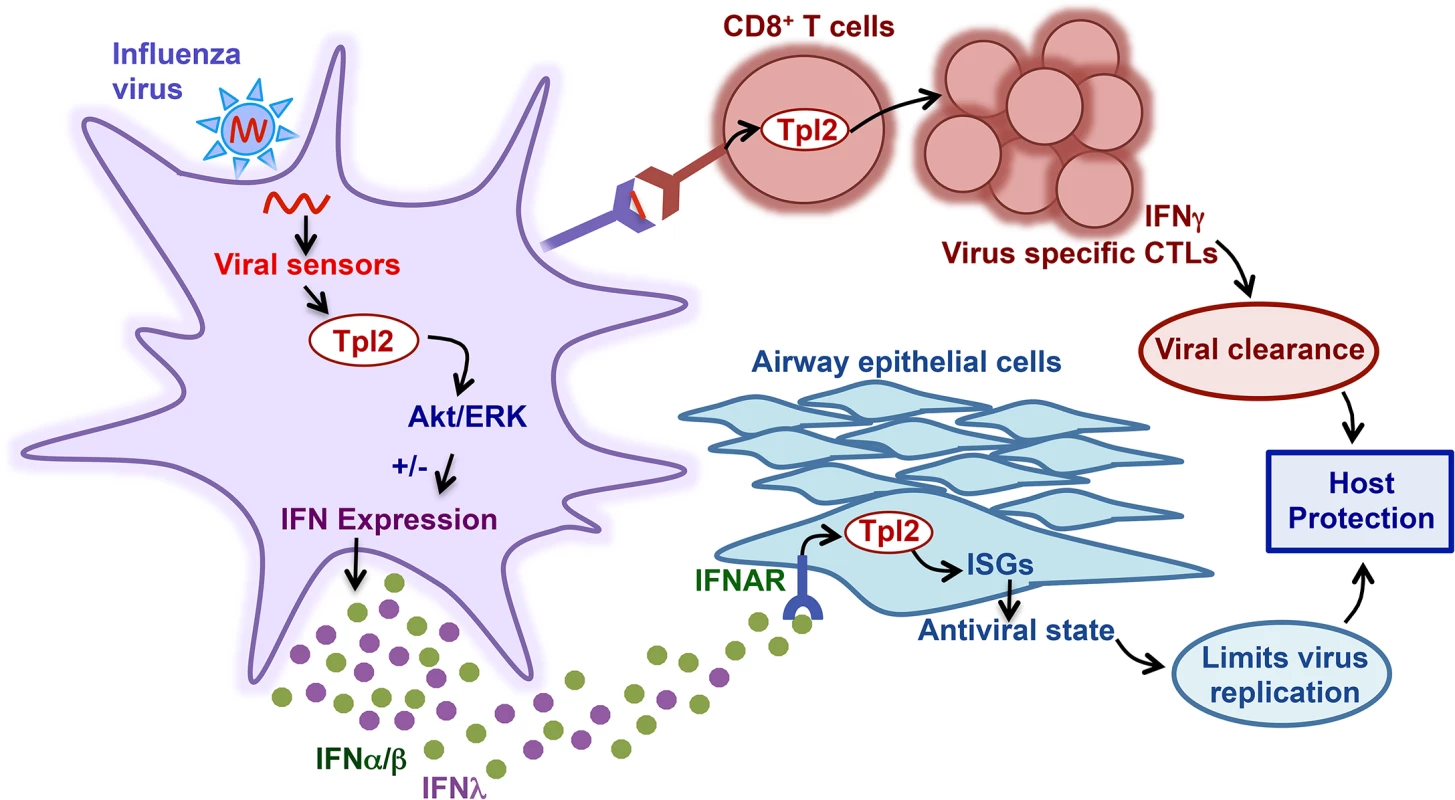
During influenza virus infection, the viral RNA is recognized by TLR7 (in pDCs) or RIG-I (in other cell types). Virus recognition by these receptors activates various downstream signaling cascades, including Tpl2-ERK signaling, which either positively or negatively regulates secretion of IFNα/β or IFNλ in a cell-type specific manner. Specifically, Tpl2 inhibits TLR- and RLR-induced IFNα/β production in macrophages, but promotes IFNα/β and IFNλ in TLR-stimulated pDCs. Tpl2 is also involved in transducing Type I IFN signals. Moreover, Tpl2 regulates induction of ISGs, which are important in limiting virus replication. In addition to early innate responses, Tpl2 promotes expansion of virus-specific CD8+ T cells that facilitate viral clearance from infected lungs. Therefore, by integrating both innate and adaptive antiviral responses, Tpl2 promotes host protection during influenza virus infection. Tpl2 deficiency led to cell-type specific alterations in the regulation of Type I IFN production. Specifically, IFNβ production was increased in response to LPS, R848 and the RIG-I ligand, 5’-triphosphate RNA in Tpl2-/- MEFs and BMDMs. In contrast, Type I IFN was significantly reduced in pDCs in response to TLR7 stimulation with R848. This differential regulation of Type I IFN production by Tpl2 in different cell types in response to TLR ligands is consistent with a previous report by O’Garra and colleagues [4]. Importantly, we also demonstrated that Tpl2 similarly functions as a negative regulator of Type I IFN production upon activation of the RIG-I cytosolic sensor with 5’-triphosphate RNA.
One striking observation was the absolute requirement for Tpl2 in the TLR-dependent induction of both Type I (IFNα/β) and Type III IFNs (IFNλ) by pDCs. The fact that pDCs uniquely require Tpl2 for production of both Type I and Type III IFNs suggests that pDCs differ fundamentally from BMDMs and MEFs in their signaling pathways. Indeed, impaired IFN production correlated with reduced activation of the PI3K/Akt signaling pathway in Tpl2-/- pDCs. This finding is also consistent with the observation that the PI3K/Akt pathway appears to be especially important in driving TLR-dependent IFN expression by pDCs [31].
In addition to cell-type specific regulation, Tpl2 also differentially regulates the production of Type I and Type III IFNs during viral infection. Importantly, influenza virus has been reported to utilize the Raf pathway to activate ERK, which explains why Type I IFN induction occurs in a Tpl2-independent manner in mice and pDCs infected with influenza virus [48]. On the contrary, IFNλ production was uniquely dependent upon Tpl2 during the course of influenza infection in vitro and in vivo. Although Type I and Type III IFNs have common regulatory elements in their promoters and are usually co-expressed in response to viruses and TLR ligands [36], selective induction of IFNλ by transcription factors NFκB and IRF1 has been reported [49, 50]. The distinct requirement for Tpl2 in IFNλ induction in virus-infected pDCs likely represents the unique requirement of the IFNλ promoter for an early NFκB-dependent priming event. In support of this, our own data demonstrate that IFNλ induction is rapid and parallels the regulation of NFκB-dependent genes more closely than IFNα (S7 Fig).
With the exception of a recent study reporting that p38, but not ERK, is required for Ifnl1 expression in human cells [49], the roles of MAPK or PI3K pathways in the regulation of IFNλs have not been evaluated. Although the regulation of IFNλ1 by PI3K-mTOR is still unexplored, our data demonstrate a different mechanism of IFNλ3 regulation that relies on the Tpl2-ERK pathway in contrast to the p38-dependent regulation described for IFNλ1. Therefore, in addition to transcription factors [30], diverse signaling cascades also specify induction of different IFNs.
The complexity of the IFN response is not completely understood, since multiple signaling cascades and transcription factors activated during IFN signaling can independently or cooperatively regulate the transcriptional response to IFNs [39]. Importantly, our data demonstrate the involvement of Tpl2 in IFN signaling leading to the phosphorylation of ERK and STAT1Ser727. Previous studies have demonstrated the significance of STAT1Ser727 phosphorylation for full transcriptional activation and induction of ISGs [38, 51]. Conflicting reports exist regarding the identity of the serine kinase responsible for STAT1Ser727 phosphorylation; different kinases including p38, ERK and PKC-δ have been implicated [52–54]. Importantly, an association of ERK with STAT1 and a requirement of ERK activity for expression of ISGs have been demonstrated [55]. Tpl2 regulation of STAT1Ser727 phosphorylation and induction of ISGs might be indirect via its regulation of ERK phosphorylation during IFN signaling. In addition to regulating ISG transcription, Tpl2-ERK signaling also regulates the phosphorylation and dissociation of the translation initiation factor 4E-Bp-eIF4E complex, which is involved in cap-dependent translation of many genes, including ISG15 [34, 56]. Therefore, the Tpl2-ERK pathway regulates the biological effects of IFNs at the transcriptional level and possibly also at the posttranscriptional level.
Although MAP kinase pathways are known to be activated in response to IFNs, the importance of Tpl2 in regulating IFN-inducible effectors has not yet been described. The induction of ISGs is mainly attributed to IFN-stimulated gene factor-3 (ISGF3; consists of STAT1, STAT2 and IRF9). In addition to ISGF3, IRF7 can also act independently to regulate transcription of antiviral genes, and Tpl2 has been shown to promote IRF7-dependent transcription [16]. However, normal induction of IFNα/β during influenza virus infection argues against a major role for IRF7 in the observed phenotype, since IRF7 is regarded as the ‘master regulator’ of Type I IFN induction [35].
To understand the mechanism by which Tpl2 exerts its antiviral effect, we examined the contribution of Tpl2 to virus replication in different cellular compartments and in the context of IFNAR deficiency. Using bone marrow chimeras, we demonstrated that Tpl2 was required within the nonhematopoietic compartment to restrict early virus replication. This likely reflects Tpl2 functions in airway epithelial cells, the primary target of influenza virus. In this regard, Tpl2 is known to be expressed and to regulate inflammation within airway epithelial cells [22]. Studies using Ifnar1-/-Il28ra-/- mice have also demonstrated that interferon responsiveness of these cells is critical for restricting early viral replication [42]. It is well known that abrogation of Type I IFN signaling does not increase influenza virus replication due to the presence of compensatory Type III IFNs [57]. Consistent with this, we observed that Tpl2 ablation promoted virus replication to the same extent on both Ifnar1+/+ and Ifnar1-/- genetic backgrounds. The 50% reduction in IFNλ levels that we observed in Tpl2-/- mice on day 3 pi is unable to explain the increase in virus replication, because compensatory Type I IFNs are induced to normal levels. Furthermore, the presence of IFNs, rather than quantity, is important in driving antiviral responses [42]. One possible explanation for the increased viral replication in Tpl2-/- mice is that Type III IFN signaling is also Tpl2-dependent, like we have demonstrated for Type I IFNs. Additional studies using Il28ra-/- mice are needed to determine the contribution of Tpl2 to Type III IFN signaling.
In addition to antiviral innate responses, we also identified a critical role for Tpl2 in the induction of antigen-specific CD8+ T cell responses. This is in contrast to a recent study reporting a major role for Tpl2 in human, but not murine, CD8+ T cell responses [58]. The impaired induction of virus-specific CD8+ T cells resulting in defective viral clearance and increased mortality in Tpl2-/- mice clearly warrants detailed studies on Tpl2 regulation of effector CD8+ T cell responses.
The increased mortality observed in Tpl2-/- mice infected with X31 virus was surprising because infection with this low pathogenicity virus does not typically cause severe clinical signs or mortality in mice. Even though IFNλ production was impaired in Tpl2-/- mice, this defect is not sufficient to explain their increased morbidity and mortality, because several studies have shown that either Type I or Type III IFN alone is sufficient to limit influenza virus infection [20, 42, 59]. In addition to impaired CD8+ T cell responses [45], the reduction in expression of some ISGs may also contribute to the enhanced pathogenesis, since defective expression of individual antiviral factors, like IFITM3, can alter the course of infection [60]. Early increases in virus replication in Tpl2-deficient lung stromal cells, demonstrated by bone marrow chimera experiments, coupled with defective viral clearance by CD8+ T cells likely potentiate the inflammatory response, which is considered a major factor contributing to morbidity and mortality during pathogenic influenza infection [61].
Overall, our study establishes Tpl2 as a host factor with intrinsic ability to restrict influenza virus replication and also demonstrates immune regulatory functions of Tpl2 within the lungs. The involvement of Tpl2 in major virus sensing pathways as well as antiviral signaling cascades suggests a key role for Tpl2 in integrating antiviral responses. These results are especially significant considering a very recent study demonstrating the requirement of IRF7 as well as Type I and Type III IFNs, all regulated by Tpl2, in protecting humans from life-threatening influenza virus infection [62]. Whether Tpl2 similarly restricts the replication of other classes of viruses requires further investigation. The findings reported here also suggest that therapeutic inhibition of Tpl2 during chronic inflammatory diseases might predispose patients to viral infections.
Materials and Methods
Ethics statement
All animal experiments were performed in accordance to the national guidelines provided by “The Guide for Care and Use of Laboratory Animals” and The University of Georgia Institutional Animal Care and Use Committee (IACUC). The Institutional Animal Care and Use Committee (IACUC) of the University of Georgia approved all animal experiments (Assurance Number A3437-01).
Mice and viruses
Wild type (WT) C57BL/6J (CD45.2+) mice were purchased from The Jackson Laboratory. Tpl2-/- mice backcrossed to C57B6/J were kindly provided by Dr. Philip Tsichlis (Tufts University) and Thomas Jefferson University. For some experiments, littermate control WT and Tpl2-/- mice were obtained by interbreeding Tpl2+/- mice. Ifnar1-/- mice were kindly provided by Dr. Biao He (University of Georgia). Mice deficient in both IFNAR1 and Tpl2 were generated by interbreeding single knockout animals. To generate chimeric mice, WT or Tpl2-/- recipient mice (both CD45.2+) were lethally irradiated with 1100 rad and reconstituted with donor B6.SJL-PtprcaPepcb/BoyJ (WT CD45.1+ congenic) or Tpl2-/- bone marrow cells. Chimeric mice were maintained for 8 weeks. Animals were housed in sterile microisolator cages in the Central Animal Facility of the College of Veterinary Medicine. Embryonated specific pathogen free (SPF) chicken eggs were purchased from Sunrise Farms, New York. Influenza viruses A/HKX31 (H3N2), A/Puerto Rico/8/34 (PR8; H1N1) and A/WSN/1933 (H1N1) stocks were propagated in the allantoic cavity of 9 - to 11-day-old embryonated SPF chicken eggs at 37°C for 72 hr, and viral titers were enumerated by plaque assays [63].
In vivo infections
Age-matched, 6 - to 8-week-old WT, Tpl2-/-, Ifnar1-/-, Ifnar1-/-Tpl2-/- or chimeric mice were anesthetized with 250 mg/kg Avertin (2,2,2-tribromoethanol) followed by intranasal infection with influenza A/HK-X31 (H3N2) in 50 μl PBS. Control mice were mock-infected with a similar dilution of allantoic fluid. To determine lung viral titers, whole lungs from WT and Tpl2-/- mice infected with 104 pfu of X31 virus were harvested on days 3, 5 and 7 pi. Lungs were placed in 1 ml PBS and dissociated with a bead mill homogenizer (Qiagen), and virus titers were enumerated by plaque assays. To assess susceptibility to influenza infection, WT and Tpl2-/- mice infected with 104 pfu of X31 virus were observed over a period of 14 days. Body weights were recorded daily, and mice exhibiting severe signs of disease or more than 30% weight loss were euthanized. To measure IFN and cytokine secretion, mice infected with 106 or 104 pfu of X31 virus were euthanized 3 or 7 days pi, and bronchoalveolar lavage fluid (BALF) or BAL cells were obtained by washing the lungs twice with 1 mL PBS. Cells were recovered by centrifugation of the lavage fluid for 10 min at 250xg. BALF from the first wash was used for quantitation of cytokine secretion. Cellular recruitment was assessed by quantifying total leukocyte recovery from both washes.
Measurement of antigen-specific CD8+ T cell responses
Mice infected with 104 pfu of X31 virus were euthanized on day 10 pi, and cells were obtained by washing the lungs twice with 1 mL PBS. BAL cells were stained with anti-CD4, anti-CD8 (eBiosciences), and H2DbNP366–374 tetramer (NIH Tetramer Core Facility, Emory University, Atlanta, GA) for 30 min at 4°C and fixed in 1% formaldehyde. Cells were acquired on a BD LSRII flow cytometer and analyzed using FlowJo software (Tree Star, Inc.). For IFNγ measurement, BAL cells were stimulated with a cocktail of influenza immunodominant peptides (NP366–374, PA224–233, PB1703–711) (1 μg/mL) for 24 hr at 37°C, and IFNγ levels in culture supernatant was measured by ELISA (eBiosciences).
Cell culture
Bone marrow derived macrophages (BMDMs), pDCs and mouse embryonic fibroblasts (MEFs) were generated from age - and sex-matched mice as described previously [17, 64]. CD11c+CD11b-B220+ pDCs were sorted using a Beckman Coulter MoFlo XDP cell sorter. In some experiments, cells were used on day 10 without sorting (referred as Flt3 ligand-derived DCs). Triggering of RIG-I was accomplished by directly delivering 5’-triphosphate RNA (5’ppp-RNA; 0.5 μg/mL) or a control RNA to the cytosol of BMDMs or MEFs using LyoVec transfection reagent (InvivoGen). 20 μL 5’ppp RNA or control RNA (100 μg/mL) was incubated with 200 μL LyoVec (62.5 μg/mL) at room temperature for 15 min to form complexes. Twenty-five microliters of the complexes were used to stimulate 2.5x105 BMDMs or 0.5x105 MEFs per well for 24 hr. BMDMs at 1x106/mL were also treated with R848 (InvivoGen) (1 μg/mL) for 24 hr. To investigate IFN signaling, BMDMs at 1x106/mL were treated with rmIFNα (2000 IU/mL; R&D Systems), or rhIFNβ (10 ng/mL; Peprotech) for 1–4 hr.
Plasmacytoid DCs at a concentration of 0.5-1x106/mL were left untreated or stimulated with R848 (1 μg/mL), CpG ODN2395 (10 μg/mL) (InvivoGen), 50 ng/mL rhIFNβ (PeproTech) or infected with WSN virus at a MOI of 0.2 for 24 hr. In some experiments, cells were pretreated with LY294002 hydrochloride (20 μM), rapamycin (30 nM) or U0126 (20 μM) (Sigma) for 30 min before stimulating with CpG.
Cytokine measurements
Cytokine levels were measured by ELISA (IFNα, IFNλ and IFNγ, eBioscience; IFNβ, PBL Interferon Source) or bead-based detection assays (Mouse IFNα Flowcytomix simplex, eBioscience; Mouse inflammation cytokine bead array, BD Biosciences).
Analysis of mRNA expression
Cells stimulated with R848 or IFNs were lysed using TRK lysis buffer (Omega Bio-Tek). For in vivo infections, RNA lysates were prepared from tissue after homogenizing whole lungs. RNA was extracted using a Total RNA Kit (Omega Bio-Tek). Real-time PCR was performed after synthesizing cDNA using a High capacity cDNA Reverse Transcription kit (Applied Biosystems). The expression of Irf7 (Mm00516791_g1), Il28b (ifnl3) (Mm00663660_g1), Ifitm3 (Mm00847057_s1), Isg15 (Mm01705338_s1), Oasl2 (Mm00496187-m1), Il12b (Mm00434174_m1), Il6 (Mm00446190_m1), Tnfa (Mm00443258_m1), Ifna (Mm03030145-gH), Ccl5 (Mm01302427-m1) and Actinb (4352341E-1112017) were determined by RT-PCR (Applied Biosystems). RT-PCR reactions were performed in microAmp Fast plates (Applied Biosystems) using SensiFAST Probe Hi-ROX kit (Bioline) and a StepOnePlus RT-PCR machine (Applied Biosystems). Relative gene expression levels were calculated by normalizing the Ct levels of the target gene to both endogenous actin levels and an unstimulated WT control using the ΔΔCt method.
Protein analysis
Cell lysates were separated on 4–12% gradient gels (Invitrogen) and were transferred to PVDF membranes using the iBlot Gel Transfer system (Invitrogen). Membranes were probed with various antibodies followed by horseradish peroxidase (HRP)-labeled secondary antibodies. Protein bands were visualized by enhanced chemiluminescent reagent (Lumigen) and Amersham Hyperfilm ECL (GE Healthcare). The following antibodies were used for immunoblotting: Tpl2 (Cot M-20), ERK1, ERK2 and β-actin (Santa Cruz Biotechnology), p-ERK1/2 (Thr202/Tyr204), p-STAT1 (Tyr701), p-STAT1 (Ser727) and STAT1 (Cell Signaling Technology).
Intracellular staining
Cells harvested after overnight stimulation were fixed, permeabilized with triton buffer (PBS+0.5%triton+0.1%BSA) and stained for p-Akt (Ser473) according to manufacturers’ protocol (Cell Signaling Technology). Samples were acquired on a BD LSRII flow cytometer and analyzed using FlowJo software (Tree Star, Inc.).
Statistical analysis
Data represent means ± SEM, except where indicated. P-values were determined by Students t-test, and significance was assigned for p-values <0.05. Kaplan-Meier analysis using PRISM software was performed to estimate percentage survival of WT and Tpl2-/- groups infected with influenza virus, and p value was determined using a Mantel-Cox test.
Supporting Information
Zdroje
1. Pleschka S. RNA viruses and the mitogenic Raf/MEK/ERK signal transduction cascade. Biol Chem. 2008;389(10):1273–82. doi: 10.1515/BC.2008.145 18713014
2. Pichlmair A, Sousa CRE. Innate recognition of viruses. Immunity. 2007;27(3):370–83. 17892846
3. Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–81. 16979569
4. Kaiser F, Cook D, Papoutsopoulou S, Rajsbaum R, Wu X, Yang HT, et al. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med. 2009;206(9):1863–71. doi: 10.1084/jem.20091059 19667062
5. Watford WT, Hissong BD, Durant LR, Yamane H, Muul LM, Kanno Y, et al. Tpl2 kinase regulates T cell interferon-gamma production and host resistance to Toxoplasma gondii. J Exp Med. 2008;205(12):2803–12. doi: 10.1084/jem.20081461 19001140
6. Makris A, Patriotis C, Bear SE, Tsichlis PN. Genomic organization and expression of Tpl-2 in normal cells and Moloney murine leukemia virus-induced rat T-cell lymphomas: activation by provirus insertion. J Virol. 1993;67(7):4283–9. 8510223
7. Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci U S A. 2006;103(9):3274–9. 16484370
8. Das S, Cho J, Lambertz I, Kelliher MA, Eliopoulos AG, Du KY, et al. Tpl2/Cot signals activate ERK, JNK, and NF-kappa B in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280(25):23748–57. 15833743
9. Rowley SM, Kuriakose T, Dockery LM, Tran-Ngyuen T, Gingerich AD, Wei L, et al. Tumor Progression Locus 2 (Tpl2) Kinase Promotes Chemokine Receptor Expression and Macrophage Migration during Acute Inflammation. J Biol Chem. 2014;289(22):15788–97. doi: 10.1074/jbc.M114.559344 24713702
10. Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–83. 11163183
11. Mielke LA, Elkins KL, Wei L, Starr R, Tsichlis PN, O'Shea JJ, et al. Tumor Progression Locus 2 (Map3k8) Is Critical for Host Defense against Listeria monocytogenes and IL-1 beta Production. J Immunol. 2009;183(12):7984–93. doi: 10.4049/jimmunol.0901336 19933865
12. Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21(18):4831–40. 12234923
13. McNab FW, Ewbank J, Rajsbaum R, Stavropoulos E, Martirosyan A, Redford PS, et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J Immunol. 2013;191(4):1732–43. doi: 10.4049/jimmunol.1300146 23842752
14. Xiao N, Eidenschenk C, Krebs P, Brandl K, Blasius AL, Xia Y, et al. The Tpl2 mutation Sluggish impairs type I IFN production and increases susceptibility to group B streptococcal disease. J Immunol. 2009;183(12):7975–83. doi: 10.4049/jimmunol.0902718 19923465
15. Chevrier N, Mertins P, Artyomov MN, Shalek AK, Iannacone M, Ciaccio MF, et al. Systematic discovery of TLR signaling components delineates viral-sensing circuits. Cell. 2011;147(4):853–67. doi: 10.1016/j.cell.2011.10.022 22078882
16. Schmid S, Sachs D, Tenoever BR. Mitogen-activated Protein Kinase-mediated Licensing of Interferon Regulatory Factor 3/7 Reinforces the Cell Response to Virus. J Biol Chem. 2014;289(1):299–311. doi: 10.1074/jbc.M113.519934 24275658
17. Kuriakose T, Rada B, Watford WT. Tumor Progression Locus 2-dependent oxidative burst drives phosphorylation of Extracellular Signal-regulated Kinase during TLR3 and 9 signaling. J Biol Chem. 2014; 289(52): 36089–100. doi: 10.1074/jbc.M114.587121 25378393
18. Diebold SS, Kaisho T, Hemmi H, Akira S, Sousa CRE. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–31. 14976261
19. Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, et al. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol. 2001;3(3):301–5. 11231581
20. Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, et al. Lambda Interferon Is the Predominant Interferon Induced by Influenza A Virus Infection In Vivo. J Virol. 2010;84(21):11515–22. doi: 10.1128/JVI.01703-09 20739515
21. Patriotis C, Makris A, Bear SE, Tsichlis PN. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci U S A. 1993;90(6):2251–5. 7681591
22. Martel G, Berube J, Rousseau S. The protein kinase TPL2 is essential for ERK1/ERK2 activation and cytokine gene expression in airway epithelial cells exposed to pathogen-associated molecular patterns (PAMPs). PLoS One. 2013;8(3):e59116. doi: 10.1371/journal.pone.0059116 23527104
23. Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Bakaletz LO, et al. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J Virol. 2007;81(18):9790–800. 17626092
24. Soria-Castro I, Krzyzanowska A, Pelaez ML, Regadera J, Ferrer G, Montoliu L, et al. Cot/tpl2 (MAP3K8) mediates myeloperoxidase activity and hypernociception following peripheral inflammation. J Biol Chem. 2010;285(44):33805–15. doi: 10.1074/jbc.M110.169409 20736176
25. Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–28. doi: 10.1038/nri3665 24762827
26. Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23(1):19–28. 16039576
27. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. 11812998
28. Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329(5998):1530–4. doi: 10.1126/science.1187029 20847273
29. Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, et al. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282(10):7576–81. 17204473
30. Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179(6):3434–42. 17785777
31. Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9(10):1157–64. doi: 10.1038/ni.1645 18758466
32. Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol Cell Biol. 2002;22(16):5962–74. 12138205
33. Lopez-Pelaez M, Soria-Castro I, Bosca L, Fernandez M, Alemany S. Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression. Eur J Immunol. 2011;41(6):1733–41. doi: 10.1002/eji.201041101 21469113
34. Lopez-Pelaez M, Fumagalli S, Sanz C, Herrero C, Guerra S, Fernandez M, et al. Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages. Mol Biol Cell. 2012;23(15):2982–92. doi: 10.1091/mbc.E12-02-0135 22675026
35. Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–7. 15800576
36. Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80(9):4501–9. 16611910
37. Kotenko SV. IFN-lambdas. Curr Opin Immunol. 2011;23(5):583–90. doi: 10.1016/j.coi.2011.07.007 21840693
38. Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82(2):241–50. 7543024
39. Platanias LC. Mechanisms of type-I - and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. 15864272
40. Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol. 2004;24(21):9658–67. 15485931
41. Gil MP, Ploquin MJ, Watford WT, Lee SH, Kim K, Wang X, et al. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood. 2012;120(18):3718–28. doi: 10.1182/blood-2012-05-428672 22968462
42. Crotta S, Davidson S, Mahlakoiv T, Desmet CJ, Buckwalter MR, Albert ML, et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 2013;9(11):e1003773. doi: 10.1371/journal.ppat.1003773 24278020
43. Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144(10):3980–6. 1692070
44. Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273(5659):238–9. 306072
45. Bender BS, Croghan T, Zhang L, Small PA Jr. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175(4):1143–5. 1552285
46. Taylor PM, Askonas BA. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58(3):417–20. 2426185
47. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. 12517228
48. Olschlager V, Pleschka S, Fischer T, Rziha HJ, Wurzer W, Stitz L, et al. Lung-specific expression of active Raf kinase results in increased mortality of influenza A virus-infected mice. Oncogene. 2004;23(39):6639–46. 15235583
49. Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, et al. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat Immunol. 2014.
50. Thomson SJ, Goh FG, Banks H, Krausgruber T, Kotenko SV, Foxwell BM, et al. The role of transposable elements in the regulation of IFN-lambda1 gene expression. Proc Natl Acad Sci U S A. 2009;106(28):11564–9. doi: 10.1073/pnas.0904477106 19570999
51. Varinou L, Ramsauer K, Karaghiosoff M, Kolbe T, Pfeffer K, Muller M, et al. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity. 2003;19(6):793–802. 14670297
52. Li N, McLaren JE, Michael DR, Clement M, Fielding CA, Ramji DP. ERK is integral to the IFN-gamma-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol. 2010;185(5):3041–8. doi: 10.4049/jimmunol.1000993 20675591
53. Ramsauer K, Sadzak I, Porras A, Pilz A, Nebreda AR, Decker T, et al. p38 MAPK enhances STAT1-dependent transcription independently of Ser-727 phosphorylation. Proc Natl Acad Sci U S A. 2002;99(20):12859–64. 12232043
54. Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, et al. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem. 2002;277(17):14408–16. 11839738
55. David M, Petricoin E 3rd, Benjamin C, Pine R, Weber MJ, Larner AC. Requirement for MAP kinase (ERK2) activity in interferon alpha - and interferon beta-stimulated gene expression through STAT proteins. Science. 1995;269(5231):1721–3. 7569900
56. Joshi S, Kaur S, Redig AJ, Goldsborough K, David K, Ueda T, et al. Type I interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc Natl Acad Sci U S A. 2009;106(29):12097–102. doi: 10.1073/pnas.0900562106 19574459
57. Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74(9):3996–4003. 10756011
58. Chowdhury FZ, Estrada LD, Murray S, Forman J, Farrar JD. Pharmacological inhibition of TPL2/MAP3K8 blocks human cytotoxic T lymphocyte effector functions. PLoS One. 2014;9(3):e92187. doi: 10.1371/journal.pone.0092187 24642963
59. Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4(9):e1000151. doi: 10.1371/journal.ppat.1000151 18787692
60. Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484(7395):519–23. doi: 10.1038/nature10921 22446628
61. de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–7. 16964257
62. Ciancanelli MJ, Huang SX, Luthra P, Garner H, Itan Y, Volpi S, et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348(6233):448–53. doi: 10.1126/science.aaa1578 25814066
63. Matrosovich M, Matrosovich T, Garten W, Klenk HD. New low-viscosity overlay medium for viral plaque assays. Virol J. 2006;3 : 63. 16945126
64. Garfield AS. Derivation of primary mouse embryonic fibroblast (PMEF) cultures. Methods Mol Biol. 2010;633 : 19–27. doi: 10.1007/978-1-59745-019-5_2 20204617
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



