-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
An elevated triglyceride level is generally considered a risk factor for the development of type-2 diabetes. However, recent studies suggest, somewhat paradoxically, that genetic risk for elevated triglycerides may protect against type-2 diabetes. In this study, we test the relationship of triglyceride-associated genetic variants, collectively and individually, with incident type-2 diabetes across three prospective cohort studies comprised of European - and African-American participants. Our findings across studies, racial groups, and statistical models consistently demonstrate that triglyceride-increasing alleles are associated with decreased type-2 diabetes incidence. These genes therefore appear to both increase triglyceride levels and decrease type-2 diabetes risk. More work is needed to understand the physiological mechanism underlying these findings, and to determine the causal relationship between triglycerides and type-2 diabetes.
Published in the journal: Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes. PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005204
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005204Summary
An elevated triglyceride level is generally considered a risk factor for the development of type-2 diabetes. However, recent studies suggest, somewhat paradoxically, that genetic risk for elevated triglycerides may protect against type-2 diabetes. In this study, we test the relationship of triglyceride-associated genetic variants, collectively and individually, with incident type-2 diabetes across three prospective cohort studies comprised of European - and African-American participants. Our findings across studies, racial groups, and statistical models consistently demonstrate that triglyceride-increasing alleles are associated with decreased type-2 diabetes incidence. These genes therefore appear to both increase triglyceride levels and decrease type-2 diabetes risk. More work is needed to understand the physiological mechanism underlying these findings, and to determine the causal relationship between triglycerides and type-2 diabetes.
Introduction
Along with age, body weight, family history, and other characteristics, triglyceride (TG) level is a major risk factor for cardiovascular disease and type-2 diabetes (T2D). In many prospective studies, TG at baseline is positively associated with T2D incidence, independently of body mass index (BMI) and other risk factors [1], although the direction of causality between TG and T2D is unclear [2], and somewhat ambiguous.
Over thirty single nucleotide polymorphisms (SNPs) have been found to be associated with plasma TG levels through meta-analysis of genome-wide association studies (GWAS) [3,4]. Using a case-control analytical design in over 30,000 individuals, we previously examined the association of 17 genetic risk scores (GRS) for various traits, including lipid traits, with current T2D status (importantly, adjusting only for sex and age), and observed an unexpected, albeit not statistically significant, pattern suggesting that being at high genetic risk for low-density lipoprotein cholesterol, total cholesterol, or TG could be protective against T2D [5]. At the same time, another group found in a cross-sectional study that genetic risk for dyslipidemia is negatively associated with fasting glucose and HbA1c [6] after adjustment for lipid phenotypes. As illustrated and discussed by Li et al. [6], by not adjusting for TG, the negative direct path between TG genes and T2D is weakened by the positive path linking TG genes, TG, and glycemic traits.
Previous studies have shown that GCKR variation is associated with both elevated TG and reduced fasting glucose and T2D risk, [7,8] potentially implicating its role in hepatic de-novo lipogenesis [9], through which it would promote glucose uptake, glycolysis, and subsequently increase plasma TG levels [10]. In a Mendelian randomization study, no evidence to suggest a causal role for TG in T2D or related traits was identified, although the authors did identify a “suggestive protective association” of genetically-raised TG levels with T2D risk [11]. Finally, Qi et al. identified a significant positive association between genetic risk for TG and T2D risk. However, in that analysis there was no adjustment for plasma TG levels which would have biased the results towards the null, according to Li et al. [6]. Likely more important, is their exclusion of SNPs with known pleiotropic effects, such as GCKR [12].
Here, we seek to further examine these seemingly paradoxical findings by drawing from three prospective cohort studies and across two racial groups, and by considering baseline measures of BMI, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), TG, fasting glucose (FG) and fasting insulin (FI) as covariates. Specifically, we hypothesize that baseline TG is associated with incident T2D, that genetic risk for TG is negatively associated with T2D incidence, and that the association between TG and incident T2D is stronger among those individuals with low genetic risk for TG.
Results
Characteristics of the participants in each study are shown in Table 1. In each study, there are more females than males. The number of incident T2D cases ranges from 185 in the Multi-Ethnic Study of Atherosclerosis (MESA) to 789 in the Atherosclerosis Risk in Communities study (ARIC), while the mean follow-up time ranges from 2,844 days in MESA to 9,392 days in the Framingham Heart Study (FHS). Among both EA and AA, we found that the association of the 31-SNP TG GRS with TG was stronger (EA: β = 0.25, p = 7.3 x 10–204; AA: β = 0.058, p = 7.8 x 10–4) than the association of the 40-SNP TG GRS with TG (EA: β = 0.20, p = 4.8 x 10–127; AA: β = 0.042, p = 1.6 x 10–2). Therefore, we proceeded with the 31 SNP TG GRS for all subsequent analyses.
Tab. 1. Characteristics of the three samples of European-Americans and two samples of African-Americans. 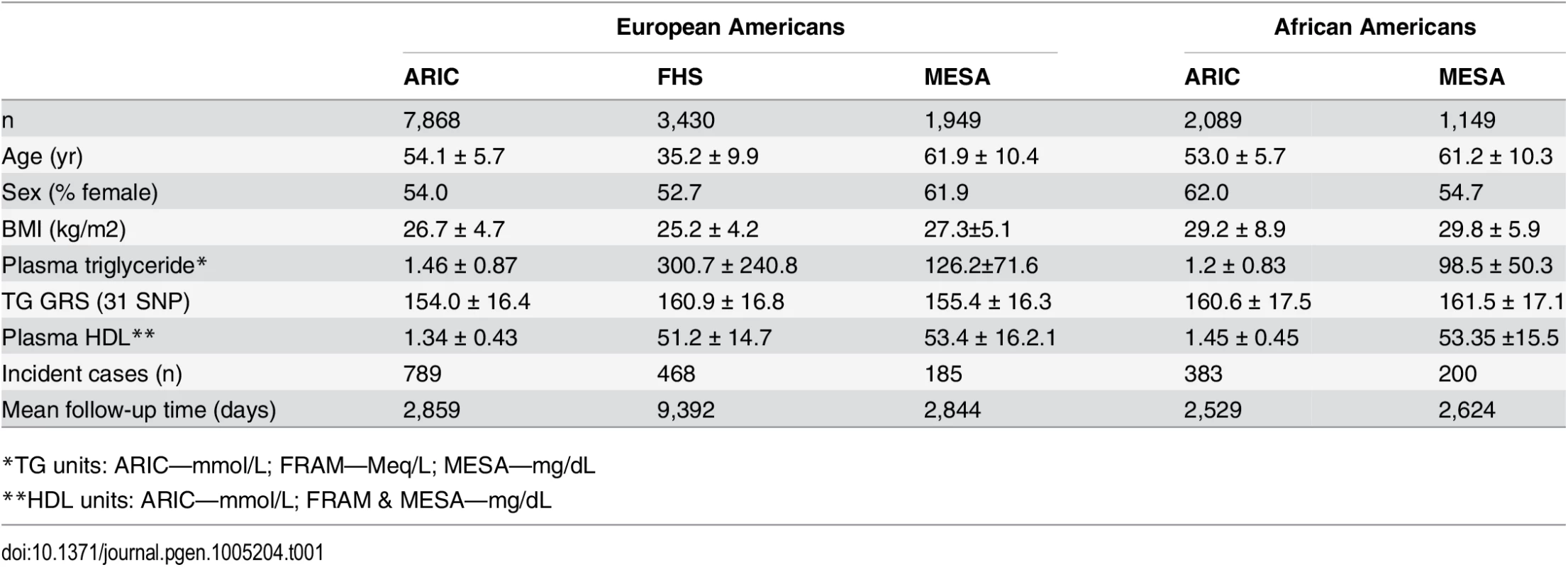
*TG units: ARIC—mmol/L; FRAM—Meq/L; MESA—mg/dL Association of TG, TG GRS, and TG SNPs with T2D incidence
Among EA, TG was strongly positively associated with T2D incidence (hazard ratio (HR) = 1.14, 95% CI [1.09–1.18], p = 8.8 x 10–11), conditioning upon age, sex, BMI, and HDL. The association of the TG GRS with incident T2D, including no other covariates, was not statistically significant, but was in the negative direction (HR = 0.997, 95% CI [0.994–1.000], p = 0.06). Upon conditioning for age, sex, and BMI, the TG GRS was significantly negatively associated with T2D incidence (HR = 0.996, 95% CI [0.993–0.999], p = 0.008). Finally, upon conditioning for age, sex, BMI, TG, and HDL, the TG GRS was significantly negatively associated with T2D incidence (HR = 0.991, 95% CI [0.988–0.995], p = 1.2 x 10–7). We did not include LDL-C as a covariate as it was not significantly associated with T2D incidence in a model which included BMI, TG and HDL-C. Including LDL-C in the full model resulted in a similar association of TG GRS with T2D (HR = 0.991, 95% CI [0.988–0.994], p = 2.6 x 10–8). Including FG and FI as additional covariates resulted in a similar but weaker negative association of the TG GRS with T2D (HR = 0.994, 95% CI [0.990–0.998], p = 3.1 x 10–3).
Among AA, TG was strongly positively associated with T2D incidence (HR = 1.18, 95% CI [1.12–1.23], p = 3.6 x10-11), conditioning on age, sex, BMI, and HDL. The TG GRS was negatively but not significantly associated with T2D incidence (HR = 0.996, 95% CI [0.991–1.001], p = 0.087), conditioning only on age and sex. As in EA, LDL-C was not significantly associated with T2D incidence in any model. Conditioning upon age, sex, BMI, HDL, and TG, the TG GRS association with T2D incidence was negative, but not statistically significant (HR = 0.995, 95% CI [0.991–1.000], p = 0.055). Fig 1 shows the TG GRS association on a study-by-study basis in both EA and AA.
Fig. 1. Association of TG GRS with incident T2D and interaction of TG with TG GRS on incident T2D in each dataset and in EA (top; n = 13,247) and AA (bottom; n = 3,238). 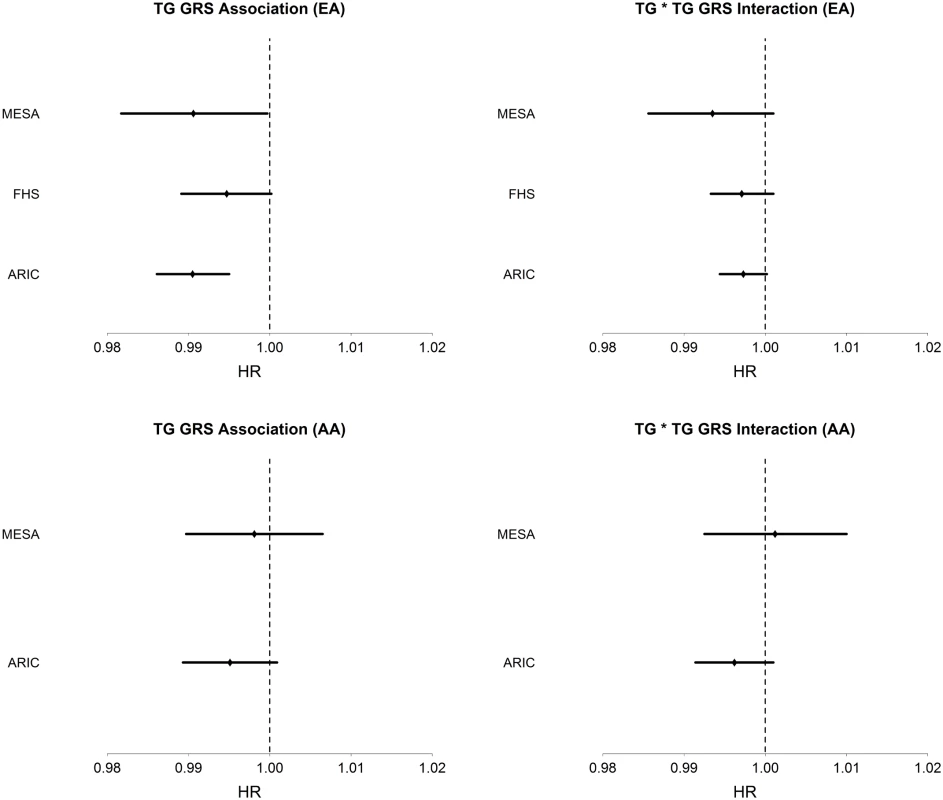
Hazard ratios and 95% CI are shown. Covariates include age, sex, BMI, TG, and HDL. We examined the association of the TG GRS with T2D incidence, conditioning only on race, in a combined dataset of EA and AA. We find that the TG GRS is negatively and significantly associated with T2D incidence (HR = 0.997, 95% CI [0.994–0.999], p = 0.013). Upon conditioning on race as well as age, sex, TG and HDL, we find a very strong negative association of the TG GRS with T2D (HR = 0.993, 95% CI [0.990–0.995], p = 7.0 x 10–8).
Of 31 TG-associated SNPs, 24 (77.4%), and 15 (48.4%) are negatively associated with T2D incidence in EA and AA, respectively (see Table 2). However, none are significantly associated with T2D incidence. SNPs that are negatively associated with T2D among EA, at a nominal level of significance (p<0.05) are those in/near APOA1, PLA2G6, GCKR, CETP, and CILP2. Among AA, no single SNP was at least nominally significant. However, we did observe the same negative trend of association for all SNPs that are nominally significant in EA, except for PLA2G6.
Tab. 2. List of 31 triglyceride (TG)-associated SNPs with their respective genomic position, risk allele (TG increasing), TG effect size (weight used in GRS), association with T2D incidence, and interaction with TG phenotype in European- and African-Americans (EA, AA). 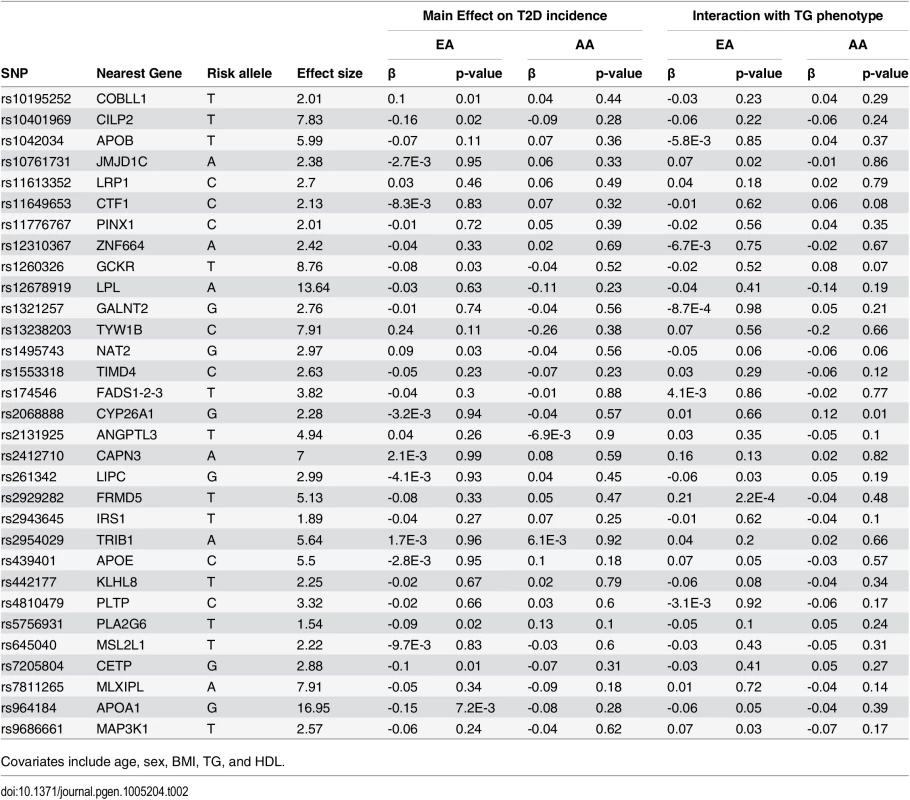
Covariates include age, sex, BMI, TG, and HDL. Interaction of TG GRS with TG on T2D incidence
Among EA, we find a significant interaction of TG and TG GRS (HRinteraction = 0.997, 95% CI [0.995–0.999], p = 1.1 x 10–3), with the inclusion of age, sex, BMI, TG and HDL as covariates. Fig 1 shows the TG x TG GRS interaction HRs on a study-by-study basis in EA and AA. Fig 2 shows the association of TG with incident T2D in each of three tertiles of TG GRS. Among EA, we observe a stronger association of TG with incident T2D among individuals with a low TG GRS (HR = 1.24, 95% CI [1.16–1.32], p = 8.0 x 10–10) compared to those with a high TG GRS (HR = 1.12, 95% CI [1.05–1.19], p = 4.6 x 10–4). Among AA, we do not find a statistically significant interaction of TG and TG GRS (HRinteraction = 0.998, 95% CI [0.993–1.0003], p = 0.39), although the trend is similar as that in EA (see Fig 2). Including all possible TG x covariate and TG GRS x covariate interaction terms in the model results in a TG x TG GRS interaction that has the same direction as the model without all possible interaction terms, but that is not statistically significant (EA: p = 0.15, AA: p = 0.38, combined: p = 0.06). In a combined analysis, conditioning upon race as well as the other covariates, we find a statistically significant interaction of the TG GRS and TG on T2D (HRinteraction = 0.997, 95% CI [0.995–0.999], p = 3.3 x 10–4). Among the 31 individual SNPs, only rs2929282 in FRMD5 shows a significant interaction (p = 2.2 x 10–4) with TG on T2D incidence among EA (see Table 2).
Fig. 2. Association of TG with T2D in each of three strata of TG GRS, in EA and AA. 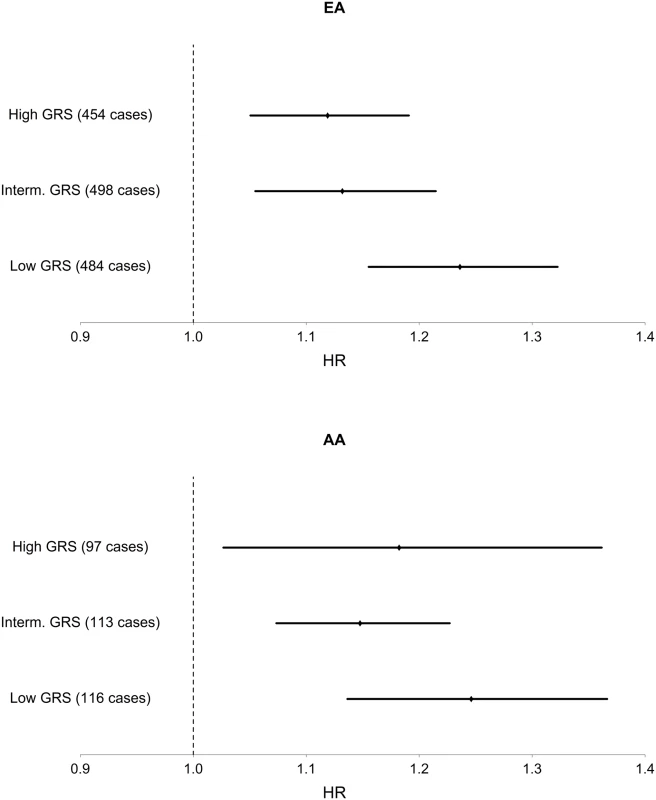
Hazard ratios and 95% CI are shown. Covariates include age, sex, BMI, TG, and HDL. Discussion
Using data from three prospective cohorts, we show that genetic risk for elevated TG is negatively associated with T2D incidence. Furthermore, our findings suggest that the risk conferred by TG is greatest among individuals with low genetic predisposition for TG. We find a similar pattern in both EA and AA, despite 1) the smaller sample size of AA, 2) the fact that TG SNPs were mainly identified in EA, and 3) that the association of the TG GRS with TG among AA in our sample is relatively weak, compared to that in EA.
Our findings are in accord with those of Klimentidis et al. [5] and Li et al. [6]. By adjusting analyses for phenotypic levels of TG and HDL, we were more easily able to identify the negative association of the TG GRS with T2D, and thus confirm the likely pleiotropic nature of TG genes. However, our results differ with respect to the specific SNPs identified. Li et al. identified SNPs in/near CETP, MLXIPL, PLTP, GCKR, APOB, APOE-C1-C2, CYP7A1 and TIMD4 as being most strongly negatively associated with glycemic traits. Among these, we find that only CETP and GCKR SNPs are negatively associated with T2D, albeit at a nominal significance level. All others, except APOE, were directionally consistent. Differences in identified SNPs between our study and the Li et al. study may be attributed to, among other things, a difference in the outcome measured (T2D vs glycemic traits). Indeed, the genetic determinants of glycemic traits (i.e. normal glycemic variation) has been shown to differ somewhat from the genetic determinants of T2D [13].
We find that rs964184 near APOA1 was most strongly negatively associated with T2D, according to the p-value. To our knowledge there is no previous report of an APOA1 variant being negatively associated with T2D or related trait. The variation near the APOA1 gene is thought to increase TG levels through impairment of the capacity of APOA1 to bind with lipoprotein lipase (LPL, the enzyme responsible for lipolysis), resulting in decreased LPL-mediated lipolysis of TG [14,15]. In the DIAGRAM meta-analysis, the G allele (TG increasing allele) at this SNP is non-significantly (and positively), associated with a greater risk of T2D (p = 0.15) [16]. We suspect that this non-significant result (and positive direction) is due to the fact that TG is typically not included as a covariate in GWAS of T2D.
Previous studies have shown that variants in GCKR may have pleiotropic effects of increasing TG and decreasing insulin resistance [7,8,17–19]. We confirm this in our study. However, our results suggest that GCKR is just one of several drivers of the pleiotropic effect of TG genes on T2D risk. A recent functional analysis of GCKR variant rs1260326 found that it is highly expressed in hepatic cells in response to glucokinase (GCK) activity, as compared to pancreatic islet cells, indicating its functional role inside liver cells [10,20]. Furthermore, the researchers found the GCK-inhibiting activity, mediated through fructose-6 phosphate, to be significantly attenuated among those with the rs1260326 variant, thus potentially enhancing glucose uptake in the liver and further increasing levels of TG precursor substrate, malonyl-CoA [10]. This influx of glucose inside the liver and resulting increase in the synthesis of malonyl-CoA may account for low glucose and high TG levels. A previous study has also implicated the CILP2 locus as having opposite putative effects on TG and T2D [21].
The SNP discovery efforts of large GWAS meta-analyses could have down-prioritized SNPs involved in lipid variation among individuals with T2D, if T2D cases were excluded from the analysis. This could potentially have the effect of only discovering SNPs involved in lipid variation among individuals who are non-diabetic. Although T2D cases were excluded from the ARIC study in the Teslovich et al. meta-analysis, for most other studies, T2D cases were included in the analysis, and analyzed separately from controls [3].
These findings have implications for how we understand the causal relationship between TG and T2D. They suggest that additional studies are needed to closely examine the biological and causal links connecting lipid and glycemic phenotypes. For example, several SNPs associated with FI have been found to be associated with higher TG [13,22] providing support for a causal pathway in which a T2D-related phenotype causes elevated TG.
In conclusion, further research is needed to understand the molecular and physiological mechanisms underlying the putative pleiotropic nature of TG-associated genes.
Methods
Studies
We used phenotypic and genotypic data of European - and African-Americans (EA, AA) from three prospective cohort studies conducted in the United States for a combined sample size of 13,285. We used data from 7,868 EA and 2,089 AA participants from the Atherosclerosis Risk in Communities (ARIC) study, which is a multi-center prospective study of men and women between the ages of 45 and 64 to investigate risk factors associated with atherosclerosis [23]. We used data from 3,430 EA participants from the Framingham Heart Study Offspring Study (FHS), which is a prospective cohort study to examine the causes of heart disease [24]. Finally, we used data from 1,949 EA and 1,149 AA participants in the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study of the risk factors for atherosclerosis among middle age men and women aged 45–85 years [25]. This study was approved by the University of Arizona Human Subjects Protection Program (Protocol number: 1300000659R001). No patient consent was given as the data were analyzed anonymously.. Data was obtained from the database of Genotypes and Phenotypes (dbGaP).
Phenotypic measurements
Individuals with prevalent T2D at the baseline examination were excluded from this study. Prevalent T2D was defined as having a FG level > 125 mg/dL, a report of taking T2D medication, or a physician diagnosis. We also excluded individuals who reported using cholesterol medications, including statins, as these medications can influence blood lipids levels and other blood constituents, which may artificially reduce associations between TG and incident T2D. We also excluded individuals who had not fasted (< 8 hours) at the baseline exam. The samples drawn at the first visit of each study were processed and analyzed using standardized procedures and protocols, the details of which are described elsewhere [26–28]. We used baseline measurements of age, TG, HDL-C, LDL-C, BMI, FG and FI as covariates in the analysis. Height and weight were also measured at baseline, and body mass index (BMI; kg/m2) was calculated. Incident T2D cases were identified at one of three follow-up visits in ARIC, seven follow-up visits in FHS, and four follow-up visits in MESA, based on FG, medication criteria, or physician diagnosis. In ARIC, time to incident type 2 diabetes was extrapolated based upon glucose values at the ascertaining visit and the previous visit, as previously described [29].
Genotypes and genetic risk score
FHS participants were genotyped with the Affymetrix 500K SNP Array (Affymetrix, Santa Clara, CA, USA). ARIC and MESA participants were genotyped with the Affymetrix Genome-Wide Human SNP Array 6.0. Standard quality-control measures were employed prior to imputation. We used IMPUTE2 with the 1,000 Genomes data as a reference, to impute millions of genotypes in each study [30]. To assess genetic risk for elevated TG, we calculated two GRS. The first GRS was based on 31 single nucleotide polymorphisms (SNPs) identified in a large-scale meta-analysis of lipid levels [3]. The second GRS was based on 40 SNPs identified in a more recent meta-analysis of lipid levels [4]. All SNPs in the three studies had imputation quality scores (‘info’) > 0.6. Weighted genetic risk scores were calculated by taking the sum of risk alleles for all SNPs and weighting each risk allele by its respective effect size. Risk alleles and effect sizes were defined according to the findings in the respective meta-analysis.
Statistical analyses
Since TG, HDL-C, LDL-C, FG and FI levels were measured using different units in each study, we standardized them to a mean of 0 and standard deviation of 1 in each study so that we could combine the studies. Hazard ratios (HR), defined as the ratio of hazard rates corresponding to different levels of TG genetic risk, were estimated using Cox proportional hazards regression models in each of the three studies and in the combined data. We considered the association of TG genes with incident T2D by conditioning on variables including age, sex, BMI, HDL-C, LDL-C, FG, FI, and TG. As mentioned above, phenotypic covariates were measured at the baseline examination in each respective study. Interactions were modeled as the product of TG and the TG GRS (or TG SNP) and included as covariates in the Cox proportional hazards regression model. We also tested a model in which additional interaction terms of both the TG and TG GRS with all other covariates were added to the model, based upon the recommendation of Keller [31]. We combined the studies into one (one for EA and one for AA) to conduct analyses, and accounted for the potential within-study dependence for the subjects from the same study by including a ‘frailty’ term for the study in the model. We also tested proportionality of hazard over time by including a time-dependent covariate consisting of the interaction of the logarithm of the time to event with TG and the TG GRS, and their interaction term. Among EA, there was not sufficient evidence (p>0.05) to reject the null hypothesis of hazard proportionality over time in the TG GRS association or the TG-by-GRS interactions. Among AA, we found the same, except for the TG variable (p = 0.04). However, upon visual inspection and comparison of the Kaplan-Meier survival curves, we observe that the TG >-0.22 (median) and < = -0.22 curves look proportional. We suspect that the p-value<0.05 is attributed to the large sample size. For the SNP analysis, we considered a Bonferroni correction for 31 tests performed, resulting in an alpha of 0.0016. To test the association of each TG GRS with TG, we first natural-log (ln) transformed TG separately in each study, then standardized it as described above. We also standardized each TG GRS in order to compare the association of each with TG. We used linear regression to test the association of each TG GRS with standardized ln(TG) in the set of combined studies, including age, sex, BMI and the random effect of study as covariates. All analyses were conducted with R Statistical Software [32] and SAS Software (Cary, NC).
Accession numbers
Data for this studies was obtained from dbGaP through accession numbers: phs000007.v23.p8, phs000280.v2.p1, phs000209.v10.p2.
Zdroje
1. Noble D, Mathur R, Dent T, Meads C, Greenhalgh T (2011) Risk models and scores for type 2 diabetes: systematic review. Bmj Clinical Research Ed 343: d7163–d7163. doi: 10.1136/bmj.d7163 22123912
2. Li N, Fu J, Koonen DP, Kuivenhoven JA, Snieder H, et al. (2014) Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis 233 : 130–138. doi: 10.1016/j.atherosclerosis.2013.12.013 24529133
3. Teslovich TM, Musunuru K, Smith A V, Edmondson AC, Stylianou IM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 : 707–713. doi: 10.1038/nature09270 20686565
4. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, et al. (2013) Discovery and refinement of loci associated with lipid levels. Nature genetics 45 : 1274–1283. doi: 10.1038/ng.2797 24097068
5. Klimentidis YC, Wineinger NE, Vazquez AI, De los Campos G (2014) Multiple Metabolic Genetic Risk Scores and Type 2 Diabetes Risk in Three Racial/Ethnic Groups. Journal of Clinical Endocrinology & Metabolism 99: E1814–E1818.
6. Li N, Van der Sijde MR, Study LC, Bakker SJL, Dullaart RPF, et al. (2014) Pleiotropic Effects of Lipid Genes on Plasma Glucose, HbA1c, and HOMA-IR Levels. Diabetes 63 : 3149–3158. doi: 10.2337/db13-1800 24722249
7. Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, et al. (2008) Common Missense Variant in the Glucokinase Regulatory Protein Gene Is Associated With Increased Plasma Triglyceride and C-Reactive Protein but Lower Fasting Glucose Concentrations. Diabetes 57 : 3112–3121. doi: 10.2337/db08-0516 18678614
8. Sparsø T, Andersen G, Nielsen T, Burgdorf KS, Gjesing AP, et al. (2008) The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia 51 : 70–75. 18008060
9. Sanders FWB, Griffin JL (2015) De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biological Reviews: n/a–n/a.
10. Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR V, et al. (2009) The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Human Molecular Genetics 18 : 4081–4088. doi: 10.1093/hmg/ddp357 19643913
11. De Silva NMG, Freathy RM, Palmer TM, Donnelly LA, Luan J, et al. (2011) Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes 60 : 1008–1018. doi: 10.2337/db10-1317 21282362
12. Qi Q, Liang L, Doria A, Hu FB, Qi L (2012) Genetic Predisposition to Dyslipidemia and Type 2 Diabetes Risk in Two Prospective Cohorts. Diabetes 61 : 745–752. doi: 10.2337/db11-1254 22315312
13. Scott R, Lagou V, Welch RP, Wheeler E, Montasser ME, et al. (2012) Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nature genetics 44 : 991–1005. doi: 10.1038/ng.2385 22885924
14. Souverein OW, Jukema JW, Boekholdt SM, Zwinderman AH, Tanck MWT (2005) Polymorphisms in APOA1 and LPL genes are statistically independently associated with fasting TG in men with CAD. European journal of human genetics : EJHG 13 : 445–451. 15657615
15. Yamamoto M, Morita SY, Kumon M, Kawabe M, Nishitsuji K, et al. (2003) Effects of plasma apolipoproteins on lipoprotein lipase-mediated lipolysis of small and large lipid emulsions. Biochimica et Biophysica Acta—Molecular and Cell Biology of Lipids 1632 : 31–39.
16. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè A V, et al. (2012) Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nature genetics 44 : 981–990. doi: 10.1038/ng.2383 22885922
17. Onuma H, Tabara Y, Kawamoto R, Shimizu I, Kawamura R, et al. (2010) The GCKR rs780094 polymorphism is associated with susceptibility of type 2 diabetes, reduced fasting plasma glucose levels, increased triglycerides levels and lower HOMA-IR in Japanese population. Journal of human genetics 55 : 600–604. doi: 10.1038/jhg.2010.75 20574426
18. Saxena R, Voight BF, Lyssenko V, Burtt NP, De Bakker PI, et al. (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 : 1331–1336. 17463246
19. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, et al. (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 : 105–116. doi: 10.1038/ng.520 20081858
20. Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, et al. (2006) The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 55 : 1–12. 16380470
21. Saxena R, Elbers C, Guo Y, Peter I, Gaunt T, et al. (2012) Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. The American Journal of Human Genetics 90 : 1–16.
22. Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, et al. (2014) Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independently of obesity. Diabetes: 1–37.
23. The Aric Investigators (1989) The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American Journal of Epidemiology 129 : 687–702. 2646917
24. DAWBER TR, MEADORS GF, MOORE FE Jr. (1951) Epidemiological approaches to heart disease: the Framingham Study. AmJ Public HealthNationsHealth 41 : 279–281. 14819398
25. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux A V, et al. (2002) Multi-ethnic study of atherosclerosis: objectives and design. AmJ Epidemiol 156 : 871–881. 12397006
26. Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, et al. (2001) Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104 : 1108–1113. 11535564
27. Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, et al. (2007) LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study-Implications for LDL management. Journal of Clinical Lipidology 1 : 583–592. doi: 10.1016/j.jacl.2007.10.001 19657464
28. Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR (2007) The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 194 : 245–252. 16982059
29. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, et al. (2003) Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52 : 1799–1805. 12829649
30. Howie B, Marchini J, Stephens M, Chakravarti A (2011) Genotype Imputation with Thousands of Genomes. G3 GenesGenomesGenetics 1 : 457–470. doi: 10.1534/g3.111.001198 22384356
31. Keller MC (2014) Gene × environment interaction studies have not properly controlled for potential confounders: The problem and the (simple) solution. Biological Psychiatry 75 : 18–24. doi: 10.1016/j.biopsych.2013.09.006 24135711
32. R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.Rproject.org.
Štítky
Genetika Reprodukčná medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 5- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



