-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
Embryonic development necessitates a delicate balancing act. On one hand, precise regulation of the expression of developmental genes is crucial for the maintenance of morphology and function. On the other hand, these same regulatory networks must allow normal development to proceed through genetic variation and environmental changes. To learn how regulatory circuits operate robustly within natural variation, we study the temporal expression profiles of key regulatory genes in the Mediterranean sea urchin, Paracentrotus lividus, and compare them to those of its Pacific Ocean relative, Strongylocentrotus purpuratus. These species shared a common ancestor about 40 million years ago and show highly similar embryonic morphologies. Our studies reveal highly reproducible gene initiation times that show lower variations than the variations in maximal mRNA levels within the species (Pl). We observe high interspecies conservation of the temporal order of gene activation within regulatory circuits and some cases of divergence. This conservation was even more profound when expression levels were normalized and scaled to the different developmental rates between the species. Our findings highlight that, despite genetic variations and different growth conditions, expression dynamics in developmental gene regulatory networks are extremely conserved over 40 million years of evolution.
Published in the journal: Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics. PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005435
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005435Summary
Embryonic development necessitates a delicate balancing act. On one hand, precise regulation of the expression of developmental genes is crucial for the maintenance of morphology and function. On the other hand, these same regulatory networks must allow normal development to proceed through genetic variation and environmental changes. To learn how regulatory circuits operate robustly within natural variation, we study the temporal expression profiles of key regulatory genes in the Mediterranean sea urchin, Paracentrotus lividus, and compare them to those of its Pacific Ocean relative, Strongylocentrotus purpuratus. These species shared a common ancestor about 40 million years ago and show highly similar embryonic morphologies. Our studies reveal highly reproducible gene initiation times that show lower variations than the variations in maximal mRNA levels within the species (Pl). We observe high interspecies conservation of the temporal order of gene activation within regulatory circuits and some cases of divergence. This conservation was even more profound when expression levels were normalized and scaled to the different developmental rates between the species. Our findings highlight that, despite genetic variations and different growth conditions, expression dynamics in developmental gene regulatory networks are extremely conserved over 40 million years of evolution.
Introduction
Normal development requires precise temporal control of differential gene expression, yet development must be robust to natural genetic variation and environmental changes. [1–3]. This resilience of developmental systems is important for keeping a wide genotypic pool adaptable in changing environmental conditions and thus, for the survival of the species [4,5]. Identifying how the control systems overcome genetic and environmental changes is important to the mechanistic understanding of developmental processes and their evolution [1,3,4]. Specifically, comparing different aspects of expression dynamics between individuals within the species and between closely related species can illuminate the range of variation in temporal expression that can still produce similar embryonic structures [1,6–8].
Comparative studies of interspecies differences in the kinetics of gene regulatory circuits can provide predictions for trans and cis evolutionary changes in circuit connectivity. The timing of gene expression depends on the temporal expression profiles of the inputs (trans) and the logic applied on the inputs by the cis-regulatory modules [9,10] (S1A–S1C Fig). For example, if two inputs are activated sequentially and the target cis-regulatory element requires both of them (necessary inputs, AND logic), the target gene will turn on only after the activation of the later input gene (S1B Fig) [9]. If the two inputs are additive (OR logic), the target gene will turn on immediately after the activation of the earlier input gene [11] (S1C Fig). Thus, evolutionary changes in cis-regulatory logic, e.g. from AND to OR, could result in changes in gene expression timing. Comparing the expression profiles of both input and target genes between two species can provide predictions for changes in input dynamics and in the target's cis-regulatory logic.
Comparative studies of temporal variation of gene regulatory circuits between related species must rely on detailed experimentally-based models of the gene regulatory networks in these organisms. The current models of the gene regulatory networks that drive ectoderm, endoderm and mesoderm specification in the sea urchin embryo are among the most comprehensive of their kind and are based on experimental studies in a few main species. [12–16]. The purple sea urchin, Strongylocentrotus purpuratus (Sp) inhabits the Pacific coasts of North America while the sea urchin Paracentrotus lividus (Pl) inhabits the eastern Atlantic Ocean and the Mediterranean Sea. These species shared a common ancestor about 40 million years ago and the average similarity in their coding sequences is about 85%, which is similar to that found between human and mouse. The growth temperature of these two species is different, reflecting their different environments; Pl embryos will successfully develop over a temperature range that is higher than Sp (standard lab temperatures 18°C versus 15°C, respectively). These species show apparent similarities in size, morphology, spatial gene expression patterns and gene regulatory networks, despite their genomic divergence and geographic distance (Fig 1A) [14–25]. High resolution studies of the temporal expression profiles of more than a hundred regulatory and differentiation genes that operate at different embryonic territories were performed for Sp [13,26], but equivalent information for Pl is still limited [18].
Fig. 1. Sp and Pl development. 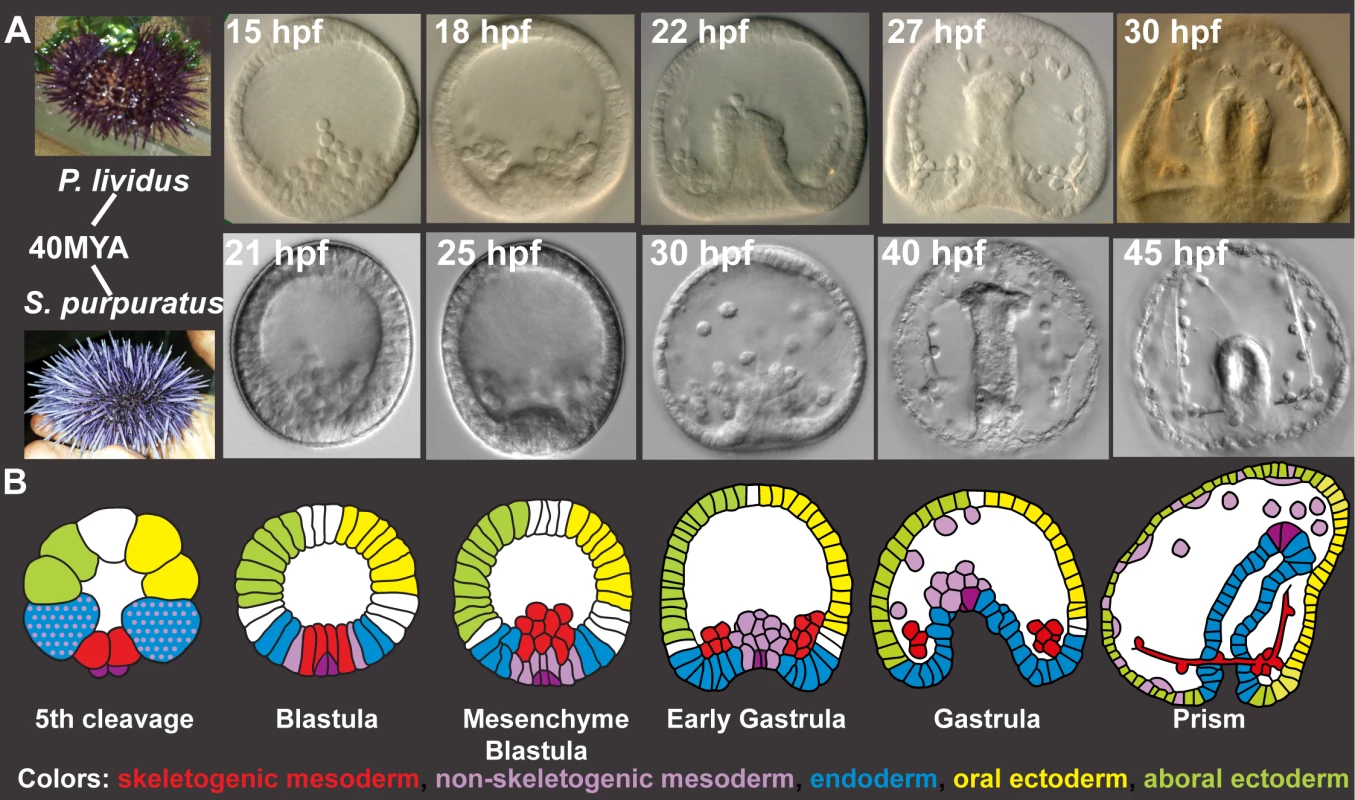
A, Comparison of Pl (top) and Sp (bottom) embryo development up to prism stage. B, Schematic diagrams of sea urchin cell lineages [28]. Red–skeletogenic mesoderm, light purple–non skeletogenic mesoderm, blue–endoderm, yellow–oral ectoderm, green–aboral ectoderm, dark purple–small micromeres. Here, we perform high-resolution quantitative analysis of the transcriptional expression profiles of key regulatory genes in Pl, asses the temporal expression variation within the species and compare gene expression dynamics to those measured in Sp [26]. For these studies, we selected regulatory circuits that operate in five embryonic territories and contain common network motifs found in many other gene regulatory networks, such as positive feedback and feedforward structures. The positive feedback circuitry locks down a specification state within a cell (intracellular, S1D Fig) or within an embryonic territory (intercellular, S1E Fig) and is important for cell fate decision [15,27–29]. Coherent and incoherent feedforward motifs are used for the sequential activation of genes in a cell (S1F Fig) [30–32]. Our results portray a tight control of timing of gene activation that is highly conserved between the species despite their genetic and geographic distance. The developmental rates of the two species scale linearly, in agreement with the species’ different growth temperatures. When we scale the developmental rates of the two species, we reveal a remarkable conservation of relative expression dynamics. Thus our study illuminates the dynamic properties of biological regulatory systems and their ability to control relative dynamics accurately despite genetic and growth condition differences.
Results
High resolution temporal profiles of Paracentrotus lividus regulatory genes reveal tight control of initiation timing
Generation of temporal expression profiles of 25 developmental genes in early development of Paracentrotus lividus
The models of the sea urchin embryo gene regulatory networks describe cell fate specification and differentiation up to gastrulation [12,15,16,33,34]. During this time interval, multiple developmental programs are executed in different embryonic territories (Fig 1A and 1B). Relevant to our studies are the large micromeres that differentiate to skeletogenic mesoderm and generate the larval skeleton (Fig 1B). The ring of cells adjacent to the micromeres at early blastula stage is the non-skeletogenic mesoderm that gives rise to several cell lineages, including pigment cells. Gastrulation begins with the invagination of the endodermal cells and the formation of a gut. In the ectoderm, we focus on the oral ectoderm where the mouth forms and the aboral ectoderm that differentiates into squamous epithelium. The region between the oral and aboral ectoderm is the ciliary band and the apical domain is located at its most animal region.
To generate temporal expression profiles of Pl embryogenesis from the fertilized egg to prism stage, we pooled thousands of Pl embryos in 1–2 hour intervals up to 30 hours post fertilization (hpf), and measured gene expression levels by quantitative PCR (QPCR, see experimental methods for details). We studied 25 regulatory and differentiation genes that initiate the specification of the skeletogenic mesoderm, the aboral non-skeletogenic mesoderm that generates pigment cells, the endoderm, the oral ectoderm and the aboral ectoderm. We repeated the measurements for three pairs of parents to study the natural variation of mRNA level and dynamics between different individuals from the same species. Thus, offspring from each set of parents are considered here as a different individual/biological replicate. The temporal profiles of all genes at the three biological repeats, their averages and the standard deviations are provided in the supporting S1 Dataset.
Highly repeatable gene initiation timing and mild variations in maximal mRNA levels in Paracentrotus lividus expression profiles
Our results reveal highly reproducible initiation time of gene activation between different biological replicates while the mRNA levels demonstrate higher variation for most genes. An example of our measurements for the gene blimp1b is presented in Fig 2A and 2B. The variations in blimp1b expression between the three biological repeats are the least pronounced during the initial phase of its activation (Fig 2A). This is quite surprising as the initiation time is the most dynamic phase in gene expression when the expression increases from basal to maximal levels. Therefore high variations between biological replicates could be expected at this highly dynamic period. Yet, highly repeatable gene initiation timing is observed for many of the genes (S2 Fig).
Fig. 2. Mild variations of mRNA maximal levels and highly repeatable gene initiation times in Pl expression profiles. 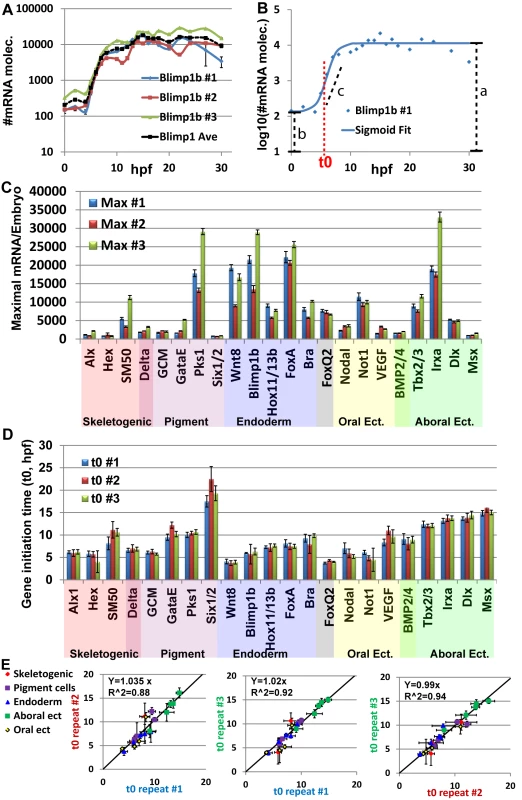
A, blimp1b mRNA level in three biological repeats (blue, red and green lines) and average of the three replicates (black dashed line). Expression is presented in Log10 scale. B, Example for the use of the sigmoid fit to measure the initiation time, t0 (See text). Blue dots are the measured expression of blimp1b at #1 biological repeat, blue line is the sigmoidal function using the fit parameters obtained from Matlab. Red dashed line indicates the time of half rise, t0, which is one of the parameters of the sigmoid function. Black dashed lines indicate the parameters a, b, c of the sigmoid function. C, Maximal mRNA level at the three biological repeats. Error bars represent technical standard error. D, Gene initiation time of the zygotic genes in Pl extracted using the sigmoid fit. Error bars represent 95% confidence bounds reported by Matlab. E, The ratio between estimated initiation times in pairs of biological repeats, interception was set at zero. Linear relations with slopes of ~1 indicate highly reproducible developmental rates within the species. To estimate the variation in mRNA expression we measured the maximal mRNA level in each biological repeat (Fig 2C). An average of 1.5-fold difference between the maximal mRNA levels of different biological repeats was detected. We divide the standard deviation with the average maximal level of the three biological replicates to normalize the standard deviation with respect to gene expression level. The normalized standard deviation varies from 7% (dlx) to 64% (gatae) with average of 29% over all genes (Fig 2C). For most genes, the biological variations were larger than the technical variation in the measurements, indicating an actual difference in expression level between individuals. However, a 1.5-fold difference is only 0.6 cycle difference in QPCR, which is close to the QPCR resolution limit. Additionally, the Pearson correlation of maximal mRNA levels between the biological repeats is strong (0.93–0.94). Thus, these are relatively mild differences between individuals that indicate that the order of magnitude of mRNA level is well controlled for the studied regulatory and developmental genes.
To estimate gene initiation time and quantify the biological variations in this parameter, we used the sigmoid function: log(mRNA(t)) = a − b/(1 + exp(c(t − t0)) (Fig 2B) as was performed in Yanai et el, 2011 [35]. The function is a good fit for log10(mRNA(t)) where mRNA(t) is the mRNA level at time t and the parameters correspond to time of half-rise, t0, log10 of the initial expression level (b), log10 of the average maximal expression (a) and expression generation slope (c) as demonstrated for blimp1b in Fig 2B (see materials and methods for details). We consider t0 as a good estimate for gene initiation time. The normalized standard deviation of t0 varies from 1.6% (tbx2/3) to 19% (hex) and has average of 8% over all genes (Fig 2D). Strong Pearson correlations between the initiation times of different biological repeats (0.95–0.97) indicate a linear relation between the developmental rates of different individuals. We used linear regression to calculate the ratio between gene initiation times of different biological repeats and obtained factors of ~1-fold in the three pairwise comparisons (Fig 2E). This illustrates highly repeatable molecular developmental rates between different biological repeats that are largely uniform across different embryonic territories.
The observed difference in the variation between initiation time and mRNA level is not due to the different estimation method (maximal level measured directly from our experimental results and initiation time estimated computationally). Calculating the maximal level using the fit parameter, a, which gives an estimate of log10 of the maximal level, results in a similar variation in mRNA levels to those measured directly, with an average standard deviation of 35%. There are low correlations between the variation of mRNA levels and variation of gene initiation time (0.13). Thus, our measurements reveal mild variations of mRNA levels and highly reproducible initiation timing and molecular developmental rates between different individuals within the species. The observed variations in mRNA levels and in initiation timing are not dependent on each other and could indicate differences in the molecular control mechanisms of these two properties.
Comparing Pl and Sp temporal expression profiles illuminates similarities and changes in circuits’ connectivity
Comparing gene expression profiles between Pl and Sp can identify both conserved and diverged expression patterns and suggest similarity and changes in circuits’ connectivity. High resolution time courses in Sp were measured by nanostring up to 48 hpf in this species [26], which includes the time interval 0–30 hpf in Pl (Fig 1A). While comparing actual mRNA levels between species is difficult due to the different methods used [26], comparison of initiation times and relative gene expression levels is possible. In Fig 3, we present comparative expression profiles of the studied genes separated into five regulatory circuits that initiate the specification of the skeletogenic mesoderm (Fig 3A–3C), the aboral non-skeletogenic mesoderm that form pigment cells (Fig 3D–3F), the endoderm (Fig 3G–3I), the aboral ectoderm (Fig 3J–3L) and the oral ectoderm (Fig 3M–3O). Sp expression profiles are taken from Materna et al, 2010, [26] (running averages of two biological replicates measured by nanostring technique, the data is available at http://vanbeneden.caltech.edu/~m/cgi-bin/hd-tc/plot.cgi). The circuit diagrams are based on experimental validations that include perturbation and cis-regulatory analysis in Sp [13,15,27,33,34,36]. Below we discuss the level of conservation of each circuit between the species in the light of our temporal expression comparison and previous studies.
Fig. 3. Comparison of transcriptional expression profiles of five regulatory circuits that operate at different embryonic territories. 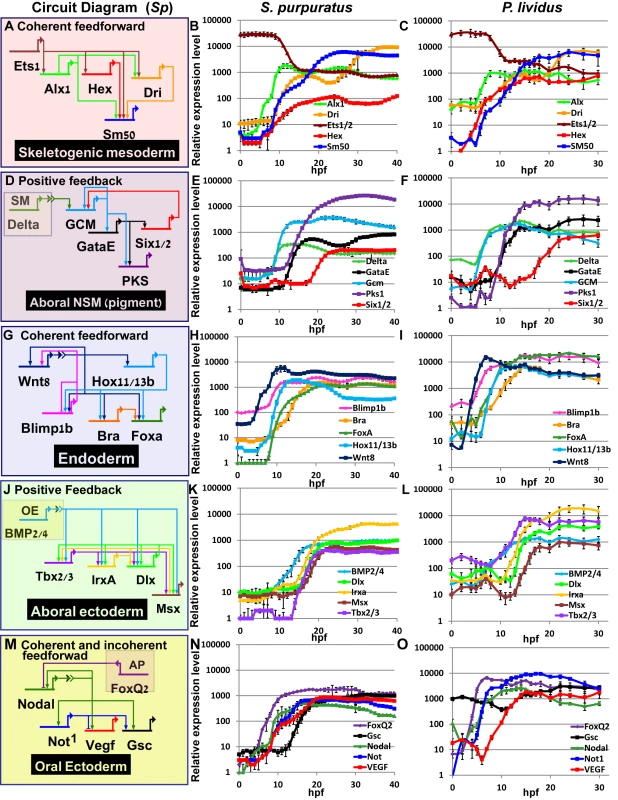
Circuit diagrams are based on experimental studies in Sp. A-C, Skeletogenic circuit: A, schematic diagram; B, time course in Sp; C, time course in Pl. The color code of the genes matches throughout A-C and the same is for the rest of the circuits. D-F, aboral non-skeletogenic mesoderm circuit (pigment cell specification), G-I, Endoderm specification circuit, J-L, aboral ectoderm circuit. M-O, oral ectoderm circuit. Each point in Pl time courses is an average of three biological repeats, see material and methods for experimental details. Interspecies conservation and change of temporal profiles in the mesodermal circuits
In the skeletogenic lineage, the transcription factor Ets1 is maternal in both organisms and according to studies in Sp it activates the genes that encode transcription factors Alx1 and Hex. Ets1 and Alx1 are activating inputs of dri; and Ets1, Alx1, Hex and Dri activate the expression of the spicule matric gene SM50 in a coherent feedforward structure (Fig 3A) [13,37,38]. In both Sp and Pl, alx1 turns on first, and dri and SM50 turn on later at a similar time (Fig 3A–3C). The similar initiation times of dri and SM50 indicates that Dri is an additive but not necessary input to the activation of SM50 (Fig 3B and 3C). The gene that encodes the transcription factor Hex turns on at the same time as Alx1 in Pl but together with dri and SM50 in Sp which might indicate a change in this gene’s regulation between the two species.
The specification of the non-skeletogenic mesoderm is initiated by the activation of the gene encoding the transcription factor GCM by Delta signaling received from the skeletogenic mesoderm (Fig 3D) [34,39–41]. According to studies in Sp, GCM activates gataE and pks1 and feeds back to its own gene activation. GataE activates pks1 and six1/2; then Six1/2 feeds back to activate GCM [27,34,39,42,43].The activation of GCM is quite rapid and GCM turns on immediately after delta expression commences in both species (Fig 3E). In both species, gataE and pks1 turn on at about the same time and six1/2 turns on later and is delayed in Pl compared to Sp (Fig 3E and 3F). Further studies are required to identify whether the observed shift in six1/2 expression are due to cis-regulatory modifications or due to changes in upstream input dynamics.
Interspecies conservation of temporal profiles in the endodermal circuit
The endodermal circuit includes genes that have strong endodermal phenotypes, yet their spatial expression patterns are not excluded to the endoderm at all times [12,44,45]. The spatial expression of the early genes, wnt8, and blimp1b starts in the skeletogenic mesoderm and then expands to the endomesoderm cells where they are co-expressed with hox11/13b, foxa and later bra [12,44]. Only later (18hpf in Sp) the genes clear from the mesoderm and are expressed only in the endoderm [12,46,47]. After 24hpf wnt8 is expressed in the ectoderm, bra and hox11/13b in the anterior endoderm and foxa and blimp1b in the posterior endoderm [12,44]. The links in the endoderm circuit diagram, Fig 3G, are based on extensive studies in Sp, executed from the onset of these genes activation and up 27hpf [12,36,44,48–51]. According to our studies, the temporal order and timing of these genes is conserved between the two species and the genes turn on at equivalent developmental times; wnt8 turns on first and then blimp1b and hox11/13b and then foxa and finaly bra (Fig 3H and 3I). The activation of foxa precedes the activation of bra in both species, and Spbra is a direct input that is additive but not necessary to Spfoxa expression as shown in Spfoxa cis-regulatory analysis [36]. Our findings support a high conservation of regulatory links and cis-regulatory logic in the endodermal circuit between the species.
Interspecies variations in temporal activation in the ectoderm circuits implies cis-regulatory changes
Gene expression in the aboral ectoderm is boosted by the reception of BMP signaling (Fig 3J) [15,33,52,53]. BMP2/4 is expressed at the oral ectoderm but its inhibition by Chordin prevents its reception there so the activity of BMP is restricted to the aboral side of the embryo. BMP2/4 expression is earlier in Pl compared to its expression in Sp and the expression of its target gene, tbx2/3, precedes the expression of its other target genes, irxa, dlx and msx in Pl while in Sp the four genes turn on at about the same time (Fig 3K and 3L). The prominent role of Tbx2/3 in activating the aboral ectoderm regulatory genes was demonstrated in both species [15,16]. However, in Pl, Tbx2/3 is necessary for the activation of msx, irxa and dlx, implying AND logic [16] (S1B Fig), while in Sp Tbx2/3 and BMP activate their downstream genes in an additive manner as demonstrated by a cis-regulatory analysis of dlx [15] (OR logic, S2C Fig). Therefore, the temporal expression differences between the two species could be explained by a change in the cis-regulatory logic of the genes: in Pl msx, dlx and irxa require both Tbx2/3 and BMP2/4 inputs and thus are activated only after Tbx2/3 is on, while in Sp BMP2/4 is sufficient so the genes are activated earlier (see Figs 3K and 3L and S1B and S1C).
The temporal profiles of the oral ectoderm circuit show a few differences between the two species. Nodal signaling controls oral ectoderm specification [15,33,52,53] and it activates the genes that encode the transcription factors Not1 and Gsc and the ligand VEGF [14,16,20,33,53]. Nodal is restricted from the apical domain by the repressor FoxQ2 [54]. According to studies in Sp, Not1 activates the expression of gsc and represses the expression of VEGF in the oral ectoderm and restricts VEGF expression into two lateral domains [16,53]. According to our studies, in the two species foxQ2 is the earliest gene and then nodal turns on and then Nodal’s target gene not1 (Fig 3N and 3O). gsc expression is maternal and zygotic in Pl (Fig 3N in agreement with [16]) while in Sp it has only a zygotic phase (Fig 3O), [14,26]. The difference in gsc expression pattern could explain the weaker effect of Gsc perturbation in Sp [14] compared to the effect in Pl [16]. VEGF expression initiates together with not1 expression in Sp, but only 4h after not1 initiation in Pl (Fig 3N and 3O). This could indicate that VEGF is directly regulated by Nodal in Sp, but indirectly in Pl, possibly due to cis-regulatory changes in this gene. The delayed expression of VEGF in Pl means that Not1 is already active at the time of VEGF initiation and could be repressing VEGF expression in the oral ectoderm. Indeed, spatial studies of VEGF expression in Sp detect an early broad oral expression of VEGF [20] while at the earliest time of VEGF expression in Pl VEGF is restricted to two lateral domains and absent from oral ectoderm, possibly due to its repression by Not1 [55]. Thus, our high-resolution comparison of ectodermal gene expression reveals a few modifications in the timing of gene activation that could be explained in cis-regulatory module changes in a few of the downstream ectodermal genes.
Temporal and level scaling of Pl and Sp expression profiles reveals strong conservation of relative expression dynamics
The developmental rate of the Pl embryo is about ×1.3 times that of Sp.
We wanted to learn how the molecular developmental rates scale between the two species, across the embryo and over developmental time. Comparison of embryo morphology between the species gives a crude factor of about ×1.3–1.4 between Pl and Sp developmental rates (Fig 1A), however we wanted to use our data to improve this estimation and base it on measured molecular progression. Since the developmental rates and initiation times are quite repeatable between individuals (Fig 2D and 2E) we used the initiation time to assess the ratio between the species’ developmental rates. We used the sigmoid fit described above log(mRNA(t)) = a − b/(1 + exp(c(t − t0)) (Fig 2B) to estimate the initiation time, t0, of each gene, using the average expression profiles presented in Fig 3 [35]. We plotted the estimated initiation times of all Sp genes against the initiation times of their orthologues in Pl (Fig 4). Gene initiation times in the two species show a high Pearson correlation (0.95) which suggests a linear relationship between the developmental rates of the two species in the developmental window we study. We used linear regression to calculate the ratio between Sp and Pl developmental rates and obtained factor of ×1.30 (R2 = 0.84) between Pl and Sp developmental rates. This ratio is consistent with the enhanced rate of Pl embryo development observed by morphological comparison (Fig 1A) and is similar to the increase in Sp developmental rate when cultured at 18°C [3]. The linear relationship between the developmental rates of the two species indicates that despite the observed delays in a few genes in either Pl or Sp, the developmental progression is quite uniform throughout the embryonic territories and developmental window we study.
Fig. 4. Linear relationship between gene initiation times in Sp and Pl. 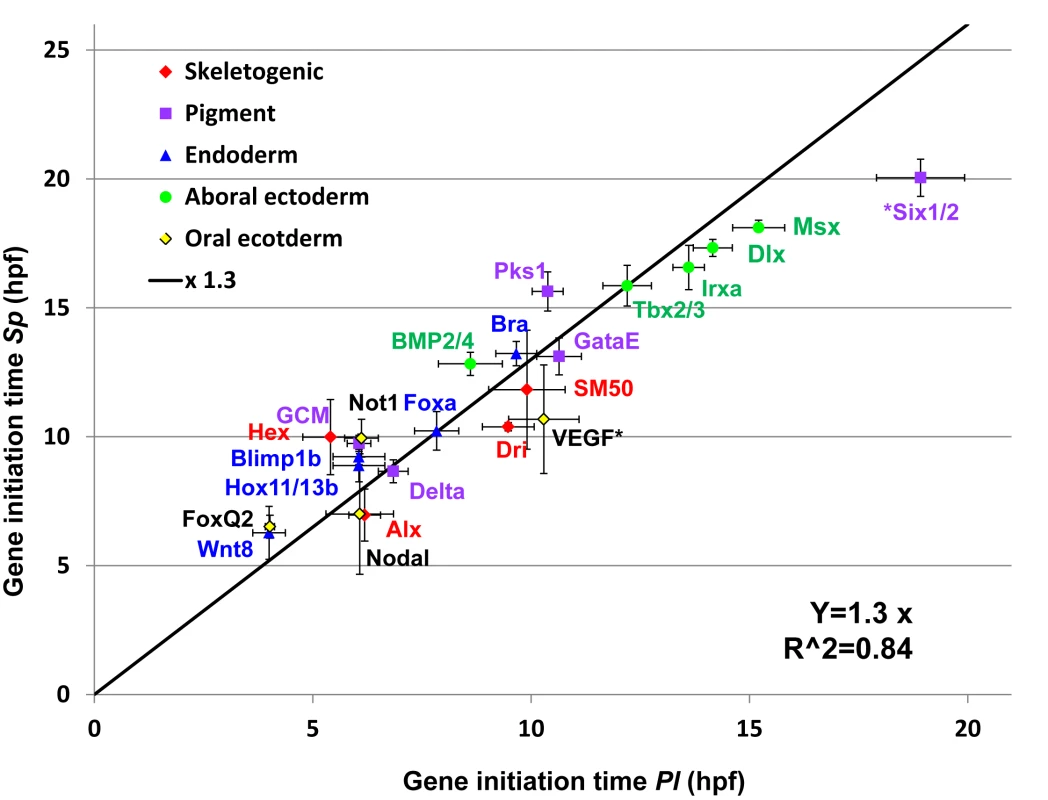
Gene initiation times for each species was calculated using the sigmoid fit. Black line is the result of a linear regression of the Sp-Pl slope (slope of ×1.3, R2 = 0.84, interception was set to zero). The genes six1/2 and VEGF were excluded from the linear regression as their temporal expression profiles indicate changes in their regulation. Temporal scaling and level normalization of Pl and Sp expression profiles reveals strong interspecies conservation of relative dynamics
The measured expression profiles in both species are highly dynamic within the developmental window we study. Many of the genes have complex spatial expression patterns, and the changes in expression levels correspond to different spatial expression phases. For example, the first peak in wnt8 expression corresponds to its expression in the skeletogenic mesoderm while the second peak is due to its expansion to the next tier of cells. Thus, the observed changes in expression levels though development reflect the genes’ developmental function through time. If the gene’s developmental function is conserved, we expect to see high conservation of the expression dynamics.
To quantify the similarity between the temporal dynamics of each gene throughout the developmental window, we scaled the developmental rates of the two species and normalized gene expression levels. We used the estimated scaling factor of ×1.3 (Fig 4) to align Pl time points with those of Sp. We normalized the expression level of each gene by dividing the level at each time point in the maximal mRNA level measured so 100% is the maximal expression in this time interval. In Fig 5, we present the results of this scaling for all the genes studied at the time interval 0–30 hpf in Pl and 0–39 hpf in Sp (exact time points in each species are provided in S1 Table). The degree of interspecies conservation of relative dynamics is quite striking and indicates tight and highly conserved regulation of the entire kinetic profile throughout development. This alignment allows us to calculate the correlation between gene expression profiles in the two species throughout this developmental window and the resulting Sp-Pl correlations are very strong, averaging 0.90 (S3 Fig). In comparison, when we calculate Pearson correlation between random pairs of genes the average correlation decreases to 0.49. Together, these results indicate that the relative changes in expression levels are highly conserved between the species and most likely reflect a highly conserved developmental role.
Fig. 5. High similarities between Sp and Pl normalized expression profiles scaled according to developmental rates. 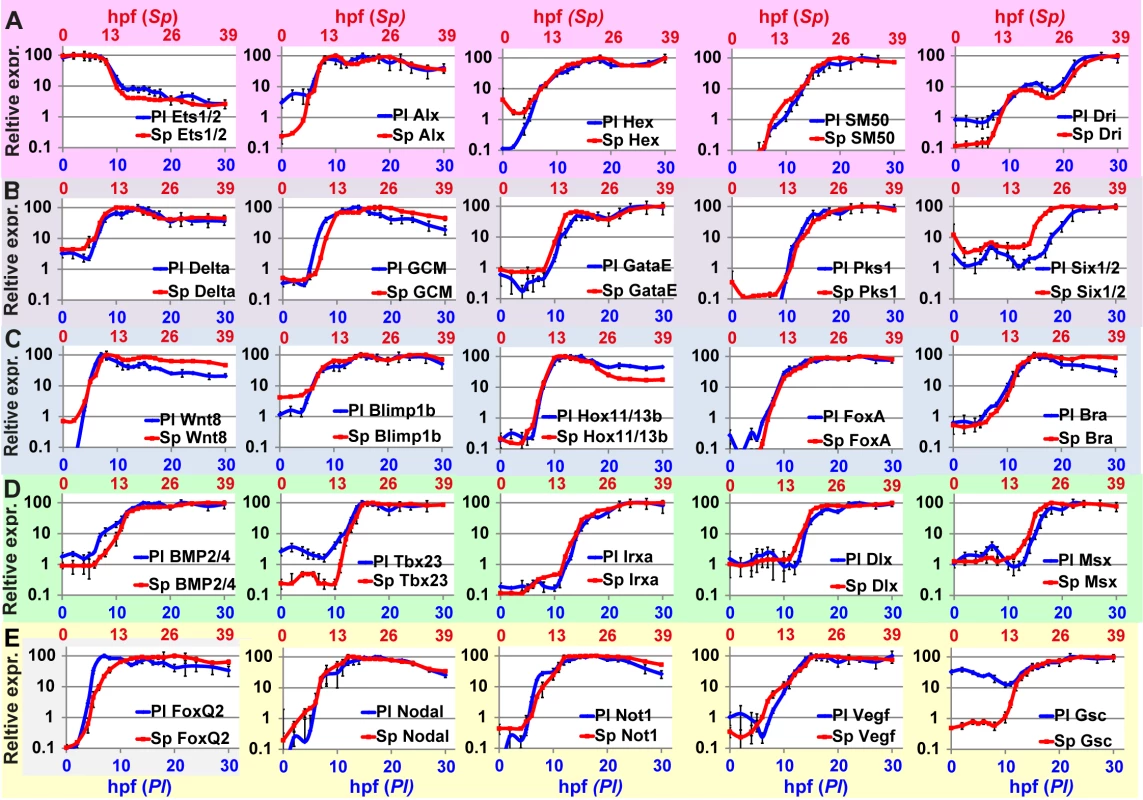
Corresponding developmental time points in the two species are provided in S1 Table. A, Skeletogenic mesoderm circuit; B, aboral non-skeletogenic mesoderm (pigment) circuit; C, endoderm circuit; D, Aboral ectoderm circuit; E, oral ectoderm circuit. Discussion
Embryo development generates similar morphologies despite natural genetic variation and within broad environmental conditions. This flexibility of the developmental program is essential for the survival of the species and keeping a wide genotypic pool adaptable in a changing environment. Understanding the properties of the regulatory control system that underlie cell fate specification is a key to the mechanistic understanding of this developmental stability. Here we studied the reproducibility and conservation of expression dynamics of regulatory circuits in two sea urchin species that shared common ancestor about 40 million years ago and inhabit distinct geographic habitats. Embryo size, cell types and morphologies of these two species are highly similar despite their genomic and geographic distance (Fig 1). Our studies illuminate tight control of gene activation timing within the species (Fig 2) and a striking similarity of relative dynamics revealed by scaling the developmental rates of the two species and normalizing gene expression levels (Fig 5). The regulatory systems that enable this reproducibility and conservation are the underlying mechanisms of morphological similarity amidst genetic and environmental variation.
The high resolution of our studies reveals tight control of initiation times that show lower variation than the variations in maximal mRNA levels between different individuals in the same species (Fig 2). Interestingly, lower variations of initiation time compared to the variation of expression levels were also detected in a comparative study of the developmental transcriptomes of two Xenopus species [35]. Previous studies in yeast provide a possible mechanistic explanation of these findings [56,57]. These studies show explicitly that the initiation of gene activation is highly similar for different levels of the activating input once the input level is above a certain threshold for long enough time [56]. On the other hand, once the gene is on, the level of gene expression is highly dependent of the level of the activating input. The molecular explanation for the different behavior of initiation timing and expression level was suggested by the same group several years before [57]. Their measurements and modeling of expression kinetics indicated that the timing of gene initiation is controlled by the slow rate of nucleosome removal from the DNA. Once the nucleosomes are removed, the level of gene expression depends on the affinity of the transcription factor binding sites and the concentration of the activating transcription factor that define the binding site occupancy and the rate of mRNA generation. Thus, the ability to buffer variations in expression level and still tightly control the timing of gene activation, possibly by using nucleosomal positioning as a threshold mechanism, could be a general property of eukaryote gene regulatory networks.
Our interspecies comparison of temporal expression profiles of key regulatory circuits revealed a high conservation of the temporal order of gene activation within the circuits but also some cases of divergence (Fig 3). Integrating the differences in temporal profiles with available perturbation and spatial expression data provides predictions for specific cis-regulatory changes within the ectodermal circuits. The highest interspecies conservation of temporal ordering and the timing of gene activation are observed in the endoderm circuit (Figs 2G–2I and 5C). This degree of conservation supports the conservation of both the architecture and the cis-regulatory logic of this circuit. The endodermal circuit is one of the most conserved circuits within echinoderms, with a similar architecture detected in the sea star that shared a common ancestor with the sea urchin ~500 mya [58,59]. The mesodermal and ectodermal networks show higher variation of circuit connectivity between the sea urchin and sea star [60–62], emphasizing the strong developmental constraints on the endoderm circuit. The constraints that define this high degree of temporal conservation could be the requirement to initiate gastrulation and the invagination of the gut at the right developmental time. Thus, high-resolution comparison of circuits’ dynamics is a good tool for the prediction of conservation and changes in circuit connectivity when the general circuit structure is known at least in one of the species.
We used gene initiation times measured in the two species to estimate a ×1.3 ratio between the molecular developmental rates in Pl and Sp (Fig 4). Apparently, a major contribution to the accelerated developmental rate in Pl is its higher culture temperature compared to the culture temperature of Sp (18°C in Pl vs. 15°C in Sp). A recent study had shown that when Sp embryos are cultured in 18°C their developmental rate increases by about ×1.24 fold based on morphological comparison, close to the ratio we obtained [3]. This is in agreement with recent studies in invertebrate and vertebrate embryos that show morphological and molecular scaling with temperature of diverse species [35,63]. A recent morphological comparison of ten Drosophila species shows that the rate of embryogenesis scales with temperature within a wide range of temperature (17.5°C-32°C) [63]. A comparative study of two Xenopus species grown in different culture temperature (28°C vs. 22°C) shows that the rate of embryogenesis scales with temperature based on morphology and on the timing of gene activation for most studied genes [35]. Thus, the ability to adapt to different temperatures by scaling the developmental rates without distinct morphological phenotypes is a common property to both vertebrate and invertebrate species.
Our studies reveal remarkable interspecies conservation of expression dynamics when the developmental rates of the two species are scaled and gene expression levels are normalized (Fig 5). This demonstrates an impressive ability of biological developmental systems to tightly control gene activation timing and relative expression dynamics despite genetic and growth conditions differences. This raises the question: Is the observed conservation an outcome of a strong negative selection against genetic changes of regulatory circuits or due to the structure of regulatory circuits that buffers genetic and environmental changes? We tend to support the second option and the ability of the regulatory system to overcome expression noise. This could be achieved by noise filtration mechanisms, e.g., the threshold activation suggested above, or by the use of network motifs that define different levels of sensitivity to upstream variation. For example, computational studies show that positive feedback circuitry is more efficient than other architectures in buffering noise in the inducing signal while keeping high responsivity to the level of the signal [64,65]. On the other hand, incoherent feedforward motifs can generate consistent response to activating input that depends mostly on fold changes in input and not on noisy absolute protein levels [64,66–68]. Apparently, this flexible design of gene regulatory circuits enables them to conserve similar expression dynamics and specify similar cell types while allowing the species to keep a broad genotypic variance and survive through changing environmental conditions.
Materials and Methods
Sea urchin embryo cultures and RNA extraction
Adult sea urchins were supplied from a mariculture facility of the Israel Oceanographic and Limnological research in Eilat. Sea urchin eggs and sperm were obtained by injecting adult sea urchins with 0.5M KCl. Embryos were cultured at 18°C in artificial sea water. Total RNA was extracted using Qiagen mini RNeasy kit from embryos at indicated time points. 1 μg of total RNA from each time point of each three independent biological replicates was used to generate cDNA using BioRad i-script kit and subsequently used for QPCR.
QPCR
Protocols
QPCR reactions were executed in 384-well plates using 384CFX-real time machine (BioRad). Each reaction was run in experimental triplicate and biological triplicate, hence leading to at least nine measurements per gene for each time point. Every reaction contained 10 μl SYBR Green mix from BioRad including 3 μM forward and reverse gene pecific primers and 2.5 μl of cDNA (diluted 1 : 100 for each assay). Thermal cycling parameters were 95°C for 3 min (one cycle) and then 95°C for 10 s, 55°C for 10 s, and 72°C for 30 s (40 cycles), followed by a denaturation step to verify the amplification of a single product.
Primer design and efficiency
Sequences of Pl genes were retrieved by blastn searches of the transcriptome databases available by permission on the Octupus web portal (http://octopus.obs-vlfr.fr/) using as templates the annotated sea urchin Sp mRNA sequences retrieved from the sea urchin transcriptome web page (http://www.spbase.org:3838/quantdev/). Based on these sequences we designed QPCR primers for each gene using Primer3 web site (http://primer3.ut.ee/). We wanted to capture transcription initiation time and therefore the primers were designed for the most 5' end 500 bp of each gene, within the ORF. The size of the amplicons was 120–150 bp long. Primer sequences are available in supporting S2 Table. All primer pairs were initially validated by regular PCR amplification and their amplification efficiencies were subsequently determined by standard curve analyses carried out using 4-fold serial dilutions of appropriate cDNA samples. Only primers with an amplification efficiency ranging from 1.85 to 2.03 (hence 89–103%) were used for further analyses.
Quantification of gene expression
To quantify the relative levels of mRNA per sample in Pl we inserted a known number of GFP cDNA molecules to each sample that includes cDNA transcribed from 1.25 ng of extracted total RNA of Pl at different time points. The calculation of gene prevalence compared to GFP was performed using the formula, GFP × 1.9(CtGFP−Ctgene), with a constant coefficient efficiency factor, 1.9, corresponding to the average value of all primers set. In our experiments we used either 2.6×108 or 1.1×107 GFP molecules and obtained similar results. The measured value of total RNA of Sp embryo is about 2.8 ng. Considering the efficiency of the extraction procedure and RNA instability we estimated that about 1.25 ng of total RNA extracted by RNeasy kit is equivalent to RNA of roughly one embryo, so the mRNA levels we present are a good estimate for number of mRNA copies per embryo. Technical error was measured as SEMGFP2+SEMGene2, where SEM is the standard error of the QPCR measurement. Percent of standard deviation is calculated as standard deviation of a quantity (either maximal mRNA level or initiation time) measured at the three biological repeats divided by the average quantity measured. To generate Fig 5, we normalized each averaged time course by dividing the measured level at each time point by the maximum level of this gene during the time window of the experiment.
Data analysis
Initiation times, t0, for both Fig 2D and Fig 4 were estimated by the use of the sigmoid function: Log(mRNA(t)) = a − b/(1 + exp(c(t − t0)) as in Yanai et el, 2011 [35]. The sigmoid was fit using Matlab’s Curve Fitting Toolbox, using the nonlinear least-squares method. For all genes R2>0.94, except from PlDlx repeat #3 that had R2 = 0.88. To calculate Pearson correlation between Sp and Pl time course, we first scaled the developmental rates in the two species using a factor of ×1.3 between Pl and Sp. The exact time points we compared are given in S1 Table. Then we calculated Pearson correlation between the averaged expression levels in Sp and Pl using the CORREL function in excel.
Supporting Information
Zdroje
1. Garfield DA, Runcie DE, Babbitt CC, Haygood R, Nielsen WJ, et al. (2013) The impact of gene expression variation on the robustness and evolvability of a developmental gene regulatory network. PLoS Biol 11: e1001696. doi: 10.1371/journal.pbio.1001696 24204211
2. Levin M, Hashimshony T, Wagner F, Yanai I (2012) Developmental milestones punctuate gene expression in the Caenorhabditis embryo. Dev Cell 22 : 1101–1108. doi: 10.1016/j.devcel.2012.04.004 22560298
3. Runcie DE, Garfield DA, Babbitt CC, Wygoda JA, Mukherjee S, et al. (2012) Genetics of gene expression responses to temperature stress in a sea urchin gene network. Mol Ecol 21 : 4547–4562. doi: 10.1111/j.1365-294X.2012.05717.x 22856327
4. Payne JL, Wagner A (2014) The robustness and evolvability of transcription factor binding sites. Science 343 : 875–877. doi: 10.1126/science.1249046 24558158
5. Albergante L, Blow JJ, Newman TJ (2014) Buffered Qualitative Stability explains the robustness and evolvability of transcriptional networks. Elife 3: e02863. doi: 10.7554/eLife.02863 25182846
6. Peter IS, Davidson EH (2011) Evolution of gene regulatory networks controlling body plan development. Cell 144 : 970–985. doi: 10.1016/j.cell.2011.02.017 21414487
7. Carroll SB (2011) Evolution. How great wings can look alike. Science 333 : 1100–1101. doi: 10.1126/science.1211025 21868661
8. Shubin N, Tabin C, Carroll S (2009) Deep homology and the origins of evolutionary novelty. Nature 457 : 818–823. doi: 10.1038/nature07891 19212399
9. Ben-Tabou de-Leon S, Davidson EH (2009) Modeling the dynamics of transcriptional gene regulatory networks for animal development. Dev Biol 325 : 317–328. doi: 10.1016/j.ydbio.2008.10.043 19028486
10. Istrail S, Ben-Tabou de-Leon S, Davidson EH (2007) The regulatory genome and the computer. Dev Biol 310 : 187–195. 17822690
11. Ben-Tabou de-Leon S (2010) Perturbation analysis analyzed—mathematical modeling of intact and perturbed gene regulatory circuits for animal development. Dev Biol 344 : 1110–1118. doi: 10.1016/j.ydbio.2010.06.020 20599898
12. Peter IS, Davidson EH (2011) A gene regulatory network controlling the embryonic specification of endoderm. Nature 474 : 635–639. doi: 10.1038/nature10100 21623371
13. Oliveri P, Tu Q, Davidson EH (2008) Global regulatory logic for specification of an embryonic cell lineage. Proc Natl Acad Sci U S A 105 : 5955–5962. doi: 10.1073/pnas.0711220105 18413610
14. Li E, Materna SC, Davidson EH (2013) New regulatory circuit controlling spatial and temporal gene expression in the sea urchin embryo oral ectoderm GRN. Dev Biol 382 : 268–279. doi: 10.1016/j.ydbio.2013.07.027 23933172
15. Ben-Tabou de-Leon S, Su YH, Lin KT, Li E, Davidson EH (2013) Gene regulatory control in the sea urchin aboral ectoderm: spatial initiation, signaling inputs, and cell fate lockdown. Dev Biol 374 : 245–254. doi: 10.1016/j.ydbio.2012.11.013 23211652
16. Saudemont A, Haillot E, Mekpoh F, Bessodes N, Quirin M, et al. (2010) Ancestral regulatory circuits governing ectoderm patterning downstream of Nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet 6: e1001259. doi: 10.1371/journal.pgen.1001259 21203442
17. Lapraz F, Besnardeau L, Lepage T (2009) Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol 7: e1000248. doi: 10.1371/journal.pbio.1000248 19956794
18. Robert N, Lhomond G, Schubert M, Croce JC (2014) A comprehensive survey of wnt and frizzled expression in the sea urchin Paracentrotus lividus. Genesis 52 : 235–250. doi: 10.1002/dvg.22754 24550167
19. Materna SC, Davidson EH (2012) A comprehensive analysis of Delta signaling in pre-gastrular sea urchin embryos. Dev Biol 364 : 77–87. doi: 10.1016/j.ydbio.2012.01.017 22306924
20. Li E, Materna SC, Davidson EH (2012) Direct and indirect control of oral ectoderm regulatory gene expression by Nodal signaling in the sea urchin embryo. Dev Biol 369 : 377–385. doi: 10.1016/j.ydbio.2012.06.022 22771578
21. Materna SC, Howard-Ashby M, Gray RF, Davidson EH (2006) The C2H2 zinc finger genes of Strongylocentrotus purpuratus and their expression in embryonic development. Dev Biol 300 : 108–120. 16997293
22. Howard-Ashby M, Materna SC, Brown CT, Chen L, Cameron RA, et al. (2006) Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev Biol 300 : 90–107. 17054934
23. Howard-Ashby M, Materna SC, Brown CT, Chen L, Cameron RA, et al. (2006) Identification and characterization of homeobox transcription factor genes in Strongylocentrotus purpuratus, and their expression in embryonic development. Dev Biol 300 : 74–89. 17055477
24. Range R, Lapraz F, Quirin M, Marro S, Besnardeau L, et al. (2007) Cis-regulatory analysis of nodal and maternal control of dorsal-ventral axis formation by Univin, a TGF-beta related to Vg1. Development 134 : 3649–3664. 17855430
25. Nam J, Su YH, Lee PY, Robertson AJ, Coffman JA, et al. (2007) Cis-regulatory control of the nodal gene, initiator of the sea urchin oral ectoderm gene network. Dev Biol 306 : 860–869. 17451671
26. Materna SC, Nam J, Davidson EH (2010) High accuracy, high-resolution prevalence measurement for the majority of locally expressed regulatory genes in early sea urchin development. Gene Expr Patterns 10 : 177–184. doi: 10.1016/j.gep.2010.04.002 20398801
27. Ransick A, Davidson EH (2012) Cis-regulatory logic driving glial cells missing: self-sustaining circuitry in later embryogenesis. Dev Biol 364 : 259–267. 22509525
28. Davidson EH (2006) The regulatory genome: gene regulatory networks in development and evolution. San-Diego: Academic press.
29. Bolouri H, Davidson EH (2010) The gene regulatory network basis of the "community effect," and analysis of a sea urchin embryo example. Dev Biol 340 : 170–178. doi: 10.1016/j.ydbio.2009.06.007 19523466
30. Mangan S, Alon U (2003) Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A 100 : 11980–11985. 14530388
31. Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, et al. (2002) Network motifs: simple building blocks of complex networks. Science 298 : 824–827. 12399590
32. Alon U (2007) Network motifs: theory and experimental approaches. Nat Rev Genet 8 : 450–461. 17510665
33. Li E, Cui M, Peter IS, Davidson EH (2014) Encoding regulatory state boundaries in the pregastrular oral ectoderm of the sea urchin embryo. Proc Natl Acad Sci U S A 111: E906–913. doi: 10.1073/pnas.1323105111 24556994
34. Materna SC, Ransick A, Li E, Davidson EH (2013) Diversification of oral and aboral mesodermal regulatory states in pregastrular sea urchin embryos. Dev Biol 375 : 92–104. doi: 10.1016/j.ydbio.2012.11.033 23261933
35. Yanai I, Peshkin L, Jorgensen P, Kirschner MW (2011) Mapping gene expression in two Xenopus species: evolutionary constraints and developmental flexibility. Dev Cell 20 : 483–496. doi: 10.1016/j.devcel.2011.03.015 21497761
36. Ben-Tabou de Leon S, Davidson EH (2010) Information processing at the foxa node of the sea urchin endomesoderm specification network. Proc Natl Acad Sci U S A 107 : 10103–10108. doi: 10.1073/pnas.1004824107 20479235
37. Damle S, Davidson EH (2011) Precise cis-regulatory control of spatial and temporal expression of the alx-1 gene in the skeletogenic lineage of s. purpuratus. Dev Biol 357 : 505–517. doi: 10.1016/j.ydbio.2011.06.016 21723273
38. Revilla-i-Domingo R, Minokawa T, Davidson EH (2004) R11: a cis-regulatory node of the sea urchin embryo gene network that controls early expression of SpDelta in micromeres. Dev Biol 274 : 438–451. 15385170
39. Ransick A, Davidson EH (2006) cis-regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev Biol 297 : 587–602. 16925988
40. Croce JC, McClay DR (2010) Dynamics of Delta/Notch signaling on endomesoderm segregation in the sea urchin embryo. Development 137 : 83–91. doi: 10.1242/dev.044149 20023163
41. Peterson RE, McClay DR (2005) A Fringe-modified Notch signal affects specification of mesoderm and endoderm in the sea urchin embryo. Dev Biol 282 : 126–137. 15936334
42. Lee PY, Davidson EH (2004) Expression of Spgatae, the Strongylocentrotus purpuratus ortholog of vertebrate GATA4/5/6 factors. Gene Expr Patterns 5 : 161–165. 15567710
43. Lee PY, Nam J, Davidson EH (2007) Exclusive developmental functions of gatae cis-regulatory modules in the Strongylocentrorus purpuratus embryo. Dev Biol 307 : 434–445. 17570356
44. Peter IS, Davidson EH (2010) The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev Biol 340 : 188–199. doi: 10.1016/j.ydbio.2009.10.037 19895806
45. Wikramanayake AH, Peterson R, Chen J, Huang L, Bince JM, et al. (2004) Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis 39 : 194–205. 15282746
46. Galvani E, Alfieri R, Giovannetti E, Cavazzoni A, La Monica S, et al. (2013) Epidermal growth factor receptor tyrosine kinase inhibitors: current status and future perspectives in the development of novel irreversible inhibitors for the treatment of mutant non-small cell lung cancer. Curr Pharm Des 19 : 818–832. 22973953
47. Peter I, Davidson EH (2010) Genomic programs for endoderm specification in sea urchin embryos. Developmental Biology 344 : 469–469.
48. Minokawa T, Wikramanayake AH, Davidson EH (2005) cis-Regulatory inputs of the wnt8 gene in the sea urchin endomesoderm network. Dev Biol 288 : 545–558. 16289024
49. Smith J, Theodoris C, Davidson EH (2007) A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science 318 : 794–797. 17975065
50. Smith J, Davidson EH (2008) Gene regulatory network subcircuit controlling a dynamic spatial pattern of signaling in the sea urchin embryo. Proc Natl Acad Sci U S A 105 : 20089–20094. doi: 10.1073/pnas.0806442105 19104065
51. Smith J, Kraemer E, Liu H, Theodoris C, Davidson E (2008) A spatially dynamic cohort of regulatory genes in the endomesodermal gene network of the sea urchin embryo. Dev Biol 313 : 863–875. 18061160
52. Duboc V, Rottinger E, Besnardeau L, Lepage T (2004) Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev Cell 6 : 397–410. 15030762
53. Duboc V, Lapraz F, Saudemont A, Bessodes N, Mekpoh F, et al. (2010) Nodal and BMP2/4 pattern the mesoderm and endoderm during development of the sea urchin embryo. Development 137 : 223–235. doi: 10.1242/dev.042531 20040489
54. Yaguchi S, Yaguchi J, Angerer RC, Angerer LM (2008) A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev Cell 14 : 97–107. doi: 10.1016/j.devcel.2007.10.012 18194656
55. Duloquin L, Lhomond G, Gache C (2007) Localized VEGF signaling from ectoderm to mesenchyme cells controls morphogenesis of the sea urchin embryo skeleton. Development 134 : 2293–2302. 17507391
56. Hansen AS, O'Shea EK (2013) Promoter decoding of transcription factor dynamics involves a trade-off between noise and control of gene expression. Mol Syst Biol 9 : 704. doi: 10.1038/msb.2013.56 24189399
57. Raser JM, O'Shea EK (2004) Control of stochasticity in eukaryotic gene expression. Science 304 : 1811–1814. 15166317
58. Hinman VF, Yankura KA, McCauley BS (2009) Evolution of gene regulatory network architectures: examples of subcircuit conservation and plasticity between classes of echinoderms. Biochim Biophys Acta 1789 : 326–332. doi: 10.1016/j.bbagrm.2009.01.004 19284985
59. Hinman VF, Nguyen AT, Cameron RA, Davidson EH (2003) Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc Natl Acad Sci U S A 100 : 13356–13361. 14595011
60. McCauley BS, Weideman EP, Hinman VF (2010) A conserved gene regulatory network subcircuit drives different developmental fates in the vegetal pole of highly divergent echinoderm embryos. Dev Biol 340 : 200–208. doi: 10.1016/j.ydbio.2009.11.020 19941847
61. McCauley BS, Wright EP, Exner C, Kitazawa C, Hinman VF (2012) Development of an embryonic skeletogenic mesenchyme lineage in a sea cucumber reveals the trajectory of change for the evolution of novel structures in echinoderms. Evodevo 3 : 17. doi: 10.1186/2041-9139-3-17 22877149
62. Yankura KA, Koechlein CS, Cryan AF, Cheatle A, Hinman VF (2013) Gene regulatory network for neurogenesis in a sea star embryo connects broad neural specification and localized patterning. Proc Natl Acad Sci U S A 110 : 8591–8596. doi: 10.1073/pnas.1220903110 23650356
63. Kuntz SG, Eisen MB (2014) Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species. PLoS Genet 10: e1004293. doi: 10.1371/journal.pgen.1004293 24762628
64. Hornung G, Barkai N (2008) Noise propagation and signaling sensitivity in biological networks: a role for positive feedback. PLoS Comput Biol 4: e8. doi: 10.1371/journal.pcbi.0040008 18179281
65. Zhang H, Chen Y (2012) Noise propagation in gene regulation networks involving interlinked positive and negative feedback loops. PLoS One 7: e51840. doi: 10.1371/journal.pone.0051840 23284787
66. Adler M, Mayo A, Alon U (2014) Logarithmic and power law input-output relations in sensory systems with fold-change detection. PLoS Comput Biol 10: e1003781. doi: 10.1371/journal.pcbi.1003781 25121598
67. Shoval O, Goentoro L, Hart Y, Mayo A, Sontag E, et al. (2010) Fold-change detection and scalar symmetry of sensory input fields. Proc Natl Acad Sci U S A 107 : 15995–16000. doi: 10.1073/pnas.1002352107 20729472
68. Goentoro L, Kirschner MW (2009) Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell 36 : 872–884. doi: 10.1016/j.molcel.2009.11.017 20005849
Štítky
Genetika Reprodukčná medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



