-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
Animals switch between periods of behavioral arousal and quiescence in response to environmental, developmental, and circadian cues. Little is known about the circuit mechanisms that produce these behavioral states. During larval molts, C. elegans exhibits a sleep-like state (termed lethargus) that is characterized by the absence of feeding and profound locomotion quiescence. We previously showed that mutants lacking the neuropeptide receptor NPR-1 exhibit increased arousal during larval molts, which is in part mediated by increased secretion of an arousal peptide (PDF-1). Here, we compare the circuits regulating arousal in larval molts and adults. We show that a broad network of sensory neurons arouses locomotion but that the impact of each neuron differs between lethargus and adults. We propose that this broad sensory network allows C. elegans to adapt its behavior across a broad range of developmental and physiological circumstances.
Published in the journal: Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release. PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005359
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005359Summary
Animals switch between periods of behavioral arousal and quiescence in response to environmental, developmental, and circadian cues. Little is known about the circuit mechanisms that produce these behavioral states. During larval molts, C. elegans exhibits a sleep-like state (termed lethargus) that is characterized by the absence of feeding and profound locomotion quiescence. We previously showed that mutants lacking the neuropeptide receptor NPR-1 exhibit increased arousal during larval molts, which is in part mediated by increased secretion of an arousal peptide (PDF-1). Here, we compare the circuits regulating arousal in larval molts and adults. We show that a broad network of sensory neurons arouses locomotion but that the impact of each neuron differs between lethargus and adults. We propose that this broad sensory network allows C. elegans to adapt its behavior across a broad range of developmental and physiological circumstances.
Introduction
Animals undergo periods of behavioral quiescence and arousal in response to changes in their environment and metabolic state. Arousal is defined as a state of heightened responsiveness to external stimuli coupled with increased motor activity whereas quiescence is associated with diminished responsiveness and motor activity [1]. Quiescence and arousal can persist for minutes to hours. Arousal is associated with fear, stress, hunger, and exposure to sexual partners [1], while quiescence is associated with sleep and satiety [2]. Relatively little is known about the specific circuit mechanisms leading to arousal or quiescence. In particular, it is unclear if similar mechanisms mediate quiescence and arousal in response to different cues, or at different times during development. To address this question, we have analyzed arousal and quiescence of C. elegans locomotion.
During each larval molt, C.elegans undergoes a prolonged period of profound behavioral quiescence, termed lethargus behavior, whereby locomotion and feeding behaviors are inactive for approximately 2 hours [3]. Lethargus has properties of a sleep-like state such as reduced sensory responsiveness and homeostatic rebound of quiescence following perturbation [4]. Several genes and molecular pathways involved in lethargus behavior have been identified [4–11]. Multiple sensory responses are diminished during lethargus, including those mediated by a nociceptive neuron (ASH) [12], and by mechanosensory neurons [11,13].
Mutants lacking NPR-1 Neuropeptide Y (NPY) receptors have been utilized as a model for generalized arousal. NPR-1 inhibits the activity of a central sensory circuit that is defined by gap junctions to the RMG interneuron [14]. In npr-1 mutants, responses mediated by the RMG circuit (e.g. pheromone and oxygen avoidance) are exaggerated, and this heightened acuity is associated with exaggerated locomotion (both during lethargus and in adults) [11,14–16]. Mutations that increase (e.g. npr-1) and decrease (e.g. tax-4 CNG and osm-9 TRPV) RMG circuit activity are associated with locomotion arousal and quiescence respectively [11,14,17,18].
We previously showed that locomotion quiescence during lethargus is dramatically reduced in npr-1 mutants and that this effect requires increased RMG sensory activity [11]. Subsequent studies showed that in microfluidic chambers npr-1 mutants have modest defects in lethargus quiescence when sensory cues are minimized but that dramatic quiescence defects are observed following brief stimulation with light or vibration [19,20]. Taken together, these papers suggest that npr-1 mutants exhibit aroused locomotion as a consequence of enhanced sensory activity.
The arousing effects of the RMG circuit are mediated in part by secretion of a neuropeptide, pigment dispersing factor (PDF-1) [11]. Activation of PDF receptors (PDFR-1) in peripheral mechanosensory neurons enhances sensitivity to vibration, thereby accelerating locomotion. Thus, sensory evoked activity in the RMG circuit arouses locomotion during lethargus through changes in PDF-1 and PDFR-1 signaling. These results raise several interesting questions. Which specific sensory neurons are responsible for arousal? Does the RMG circuit regulate arousal via multiple outputs (i.e. in addition to PDF-1)? Does the RMG circuit function similarly during lethargus and in adults? Is diminished sensory acuity during lethargus required for behavioral quiescence?
Here we show that glutamatergic transmission promotes arousal, we identify glutamatergic neurons and glutamate receptors that mediate arousal, and we show that arousal occurs by distinct mechanisms in lethargus and adult animals.
Results
Cholinergic transmission at NMJs is increased in npr-1 adults
Adult npr-1 mutants exhibit accelerated locomotion (Fig 1A–1C), as shown in prior studies [21]. Faster adult locomotion suggests that locomotion circuit activity has been altered. Consistent with this idea, npr-1 mutant adults have enhanced sensitivity to the paralytic effects of a cholinesterase inhibitor (aldicarb) (Fig 1D–1F and S2A Fig) [22], indicating increased excitatory transmission at neuromuscular junctions (NMJs). To more directly assess changes in synaptic transmission, we recorded miniature excitatory post-synaptic currents (mEPSCs) in body muscles, which are evoked by acetylcholine (ACh) release at NMJs. The mEPSC rate observed in npr-1 adults was significantly higher than in wild type controls while mEPSC amplitudes were unaltered (Fig 1G–1I). Faster mEPSC rates suggest that ACh release from motor neurons was increased whereas unaltered mEPSC amplitudes imply that muscle responsiveness to secreted ACh was unaffected. By contrast, neither ACh release evoked by depolarizing motor neurons with a stimulating electrode (evoked EPSCs), nor transmission at GABAergic NMJs (assessed by miniature inhibitory post-synaptic currents, mIPSCs) was altered in npr-1 mutants (S1 Fig). This constellation of electrophysiological defects suggests that tonic ACh release (assessed by mEPSC rate) was enhanced in npr-1 mutants, whereas other forms of neurotransmitter release (evoked ACh release and tonic GABA release) were unaffected. Enhanced tonic ACh release at NMJs could account for the accelerated locomotion rate observed in npr-1 adults.
Fig. 1. Cholinergic transmission at NMJs is enhanced by increased sensory activity in npr-1 adults. 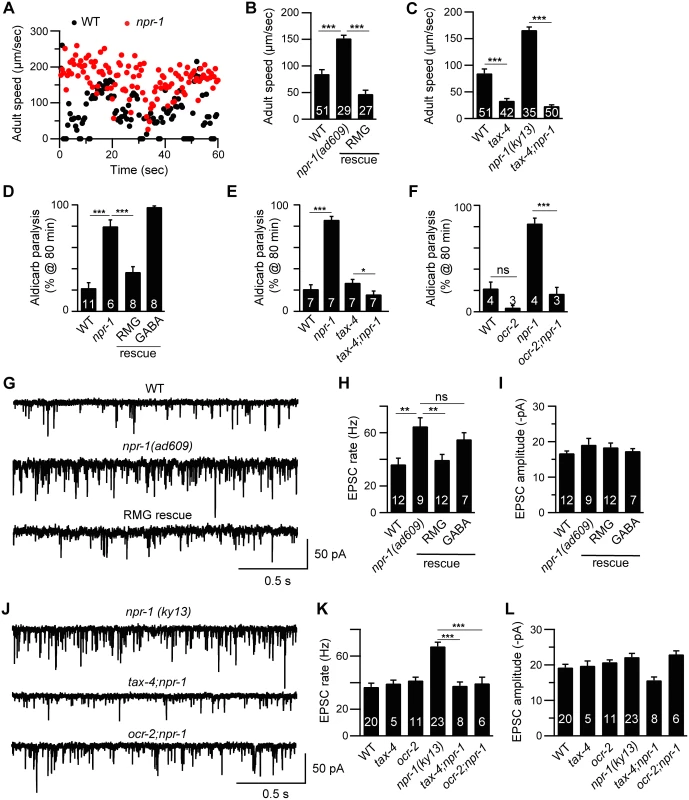
Locomotion behavior of single adult worms was analyzed for the indicated genotypes. Instantaneous locomotion velocity (A) and average locomotion velocity (B-C) are plotted. (A-C) The npr-1 adult locomotion defect was rescued by transgenes expressing NPR-1 in the RMG circuit (RMG rescue, flp-21 promoter), and suppressed in double mutants lacking TAX-4/CNG channels. (D-F) The percentage of animals paralyzed on 1 mM aldicarb at 80 min were plotted for the indicated genotypes. The number of trials is indicated for each genotype. Full time courses of aldicarb-induced paralysis are shown in S2A–S2C Fig. (D) The npr-1 aldicarb hypersensitivity was rescued by transgenes expressing NPR-1 in the RMG circuit (RMG rescue, flp-21 promoter) but not by those expressed in GABAergic neurons (GABA rescue, unc-25 and unc-30 promoters). (E-F) The npr-1 aldicarb hypersensitivity was blocked by mutations inactivating TAX-4/CNG channels or OCR-2/TRPV channels. (G-L) mEPSCs were recorded from body wall muscles of adult worms for the indicated genotypes. Representative traces of mEPSCs (G and J) and summary data are shown (H, I, K, and L). (G-I) The npr-1 cholinergic transmission defect was rescued by transgenes expressing NPR-1 in the RMG circuit (RMG rescue, flp-21 promoter) but not by those expressed in GABAergic neurons (GABA rescue, unc-30 promoter). (J-L) The npr-1 cholinergic transmission defect was abolished by mutations inactivating TAX-4 or OCR-2. The number of animals analyzed is indicated for each genotype. Error bars indicate SEM. Values that differ significantly are indicated (*, p <0.05; **, p <0.01; ***, p <0.001; ns, not significant). Enhanced cholinergic transmission in npr-1 adults is caused by increased sensory activity
Prior studies showed that several behavioral phenotypes exhibited by npr-1 mutants are caused by enhanced sensitivity to environmental cues. In particular, sensory responses mediated by the RMG circuit are enhanced in npr-1 mutants [14,17,18] and this enhanced sensory acuity is required for accelerated locomotion rates during lethargus [11,20]. We did several experiments to determine if enhanced RMG circuit activity is also required for increased cholinergic transmission in npr-1 adults. A transgene restoring npr-1 expression in the RMG circuit (using the flp-21 promoter) rescued the accelerated locomotion (Fig 1B), enhanced aldicarb sensitivity (Fig 1D and S2A Fig), and faster mEPSC rate (Fig 1G–1I) defects of npr-1 adults. By contrast, an npr-1 transgene expressed in GABAergic neurons lacked rescuing activity (Fig 1D–1H). These results indicate that NPR-1 acts in the RMG circuit to slow adult locomotion. Similarly, mutations inactivating ion channels required for sensory transduction (TAX-4/CNG and OCR-2/TRPV) in the RMG circuit suppressed the npr-1 adult locomotion (Fig 1C), aldicarb sensitivity (Fig 1E and 1F and S2B and S2C Fig), and mEPSC rate (Fig 1J and 1K) defects. Collectively, these results suggest that the accelerated adult locomotion exhibited by npr-1 mutants is caused by heightened activity in the RMG sensory circuit and, consequently, corresponds to an aroused state.
Inactivating PDF signaling does not prevent aroused locomotion in npr-1 adults
We previously showed that the lethargus quiescence defects exhibited by npr-1 mutants are caused by increased secretion of Pigment dispersing factor (PDF-1) by cells in the RMG circuit [11]. Because PDF-1 secretion is also increased in npr-1 adults [11], we tested the idea that the hyperactive adult locomotion of npr-1 mutants is also caused by increased PDF signaling. Contrary to this idea, we found that pdf-1 and pdfr-1 (PDF Receptor-1) mutations reduced but did not eliminate the aldicarb hypersensitivity (Fig 2A and 2B and S2D and S2E Fig), the accelerated locomotion (Fig 2C), and increased mEPSC rate (Fig 2D and 2E) defects of npr-1 adults. Collectively, these results suggest that additional excitatory outputs from the RMG circuit (i.e. beyond PDF-1) must contribute to the aroused locomotion of npr-1 adults.
Fig. 2. Inactivating PDF signaling does not prevent aroused locomotion in npr-1 adults. 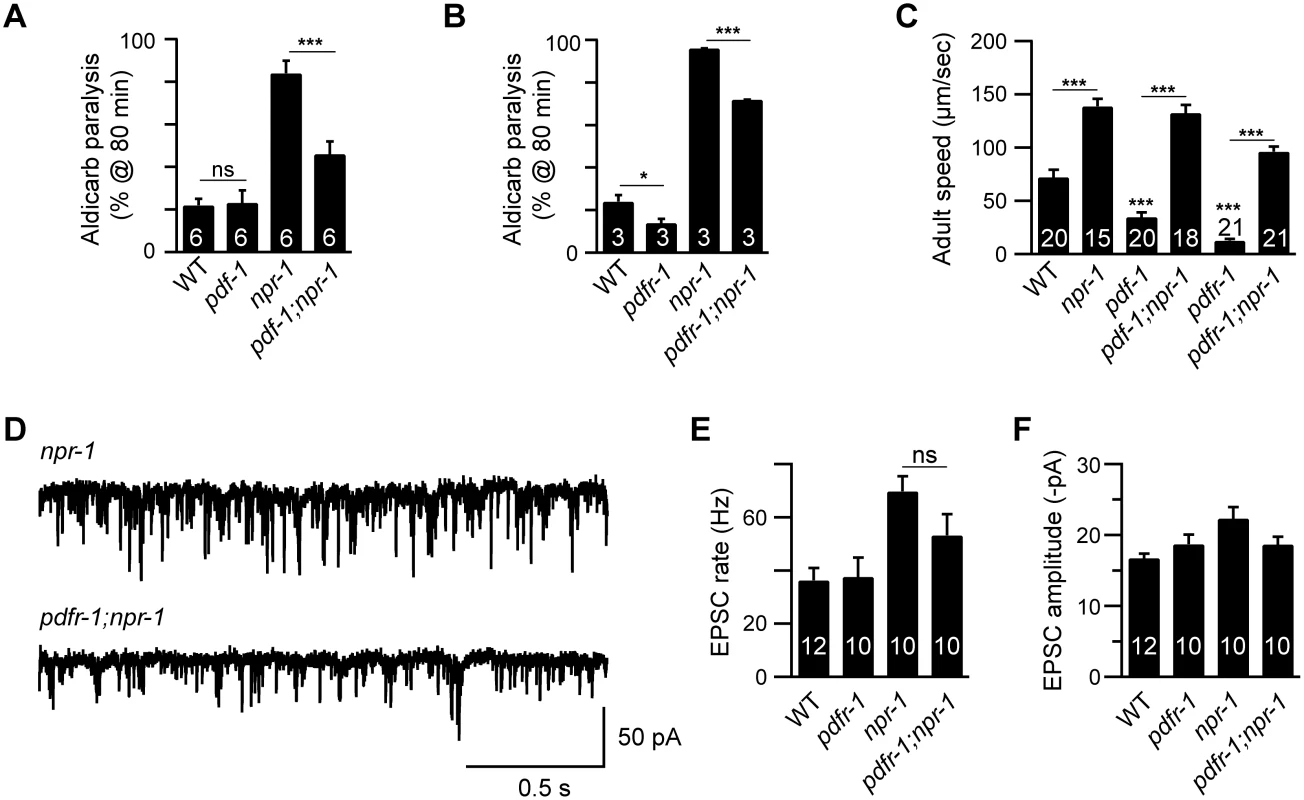
(A-B) The npr-1 aldicarb hypersensitivity was decreased but not abolished by mutations inactivating PDF-1 or PDFR-1. The percentage of animals paralyzed on 1 mM aldicarb at 80 min were plotted for the indicated genotypes. The number of trials is indicated for each genotype. Full time courses of aldicarb-induced paralysis are shown in S2D and S2E Fig. (C) Locomotion behavior of single adult worms was analyzed for the indicated genotypes. The npr-1 adult locomotion defect was not blocked by mutations inactivating PDF-1 or PDFR-1. (D-F) mEPSCs were recorded from body wall muscles of adult worms for the indicated genotypes. Representative traces of mEPSCs (D) and summary data are shown (E-F). The npr-1 cholinergic transmission defect was not suppressed by mutations inactivating PDFR-1. The number of animals analyzed is indicated for each genotype. Error bars indicate SEM. Values that differ significantly are indicated (*, p<0.05;**, p<0.01;***, p <0.001; ns, not significant). Glutamate released by sensory neurons is required for npr-1 locomotion and EPSC defects
Many C. elegans sensory neurons are glutamatergic, including two neurons in the RMG circuit (ASH and ASK) and the body touch neurons [23]. To determine if glutamate release by sensory neurons is required for accelerated locomotion in npr-1 mutants, we analyzed mutations that inactivate the vesicular glutamate transporter (eat-4 VGLUT), which is primarily expressed in sensory neurons [23]. eat-4 VGLUT mutations blocked the increased motile fraction and locomotion speed of npr-1 mutants both during the L4-Adult (L4/A) molt (Fig 3A–3C) and in adults (Fig 3D and 3E). eat-4 mutations also blocked the hypersensitivity to aldicarb (Fig 3F and S2F Fig) and increased mEPSC rate (Fig 3G and 3H) defects of npr-1 adults. Transgenes restoring EAT-4 expression in touch neurons and ASH neurons partially reinstated both lethargus (Fig 3B and 3C) and adult locomotion (Fig 3D and 3E) defects in eat-4; npr-1 double mutants, whereas transgenes expressed in ASK lacked rescuing activity (Fig 3B and 3C). eat-4 transgenes had no effect on lethargus quiescence in wild type animals (S3 Fig). These results suggest that glutamate released by ASH and touch neurons arouses locomotion in L4/A and adult npr-1 mutants.
Fig. 3. Glutamate released by sensory neurons is required for the npr-1 locomotion and the cholinergic transmission defects. 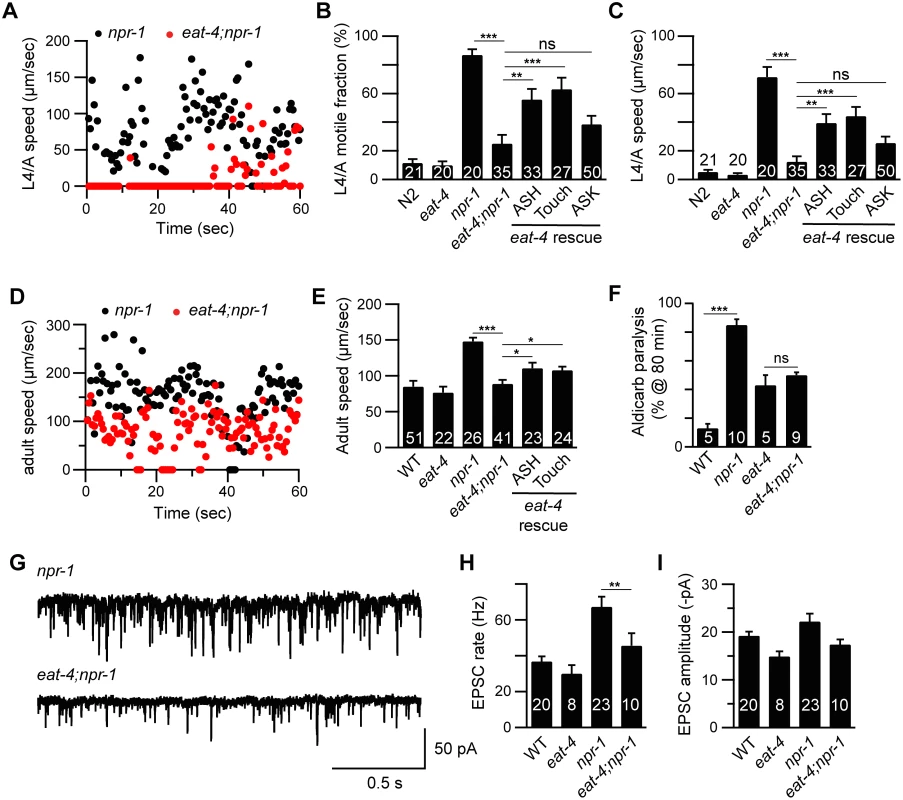
Locomotion behavior of single worms during the L4/A lethargus (A-C) and in adults (D-E) was analyzed in the indicated genotypes. Instantaneous locomotion velocity (A, D), average motile fraction (B), and average locomotion velocity (C, E) are plotted. The npr-1 locomotion defect was suppressed by mutations inactivating EAT-4/VGLUT, and partially reinstated by transgenes expressing EAT-4 in ASH neurons (sra-6 promoter) and touch neurons (mec-4 promoter) in eat-4;npr-1 double mutants using the indicated promoters. An EAT-4 transgene expressed in ASK neurons (sra-9 promoter) lacked rescuing activity. (F) The npr-1 aldicarb hypersensitivity was suppressed by mutations inactivating EAT-4/VGLUT. The percentage of animals paralyzed on 1 mM aldicarb at 80 min were plotted for the indicated genotypes. The number of trials is indicated for each genotype. Full time courses of aldicarb-inuced paralysis are shown in S2F Fig. (G-I) The npr-1 cholinergic transmission defect was abolished by mutations inactivating EAT-4/VGLUT. mEPSCs were recorded from body wall muscles of adult worms for the indicated genotypes. Representative traces of mEPSCs (G) and summary data are shown (H-I). The number of animals analyzed is indicated for each genotype. Error bars indicate SEM. Values that differ significantly are indicated (*, p <0.05; **, p <0.01; ***, p <0.001; ns, not significant). ASH activity is associated with locomotion arousal
The preceding results suggest that ASH synaptic output arouses locomotion in npr-1 mutants. We did several additional experiments to test this idea. If altered ASH output were required for aroused locomotion, we would expect that npr-1 mutants lacking ASH neurons would have increased locomotion quiescence. To test this idea, we induced ASH cell death with a transgene that expresses the pro-apoptotic caspase CED-3. Killing ASH significantly decreased the L4/A motile fraction and locomotion rate in npr-1 mutants (Fig 4A–4C). By contrast, ASH ablation had little effect on the locomotion rate of npr-1 adults (Fig 4D).
Fig. 4. ASH activity is associated with locomotion arousal. 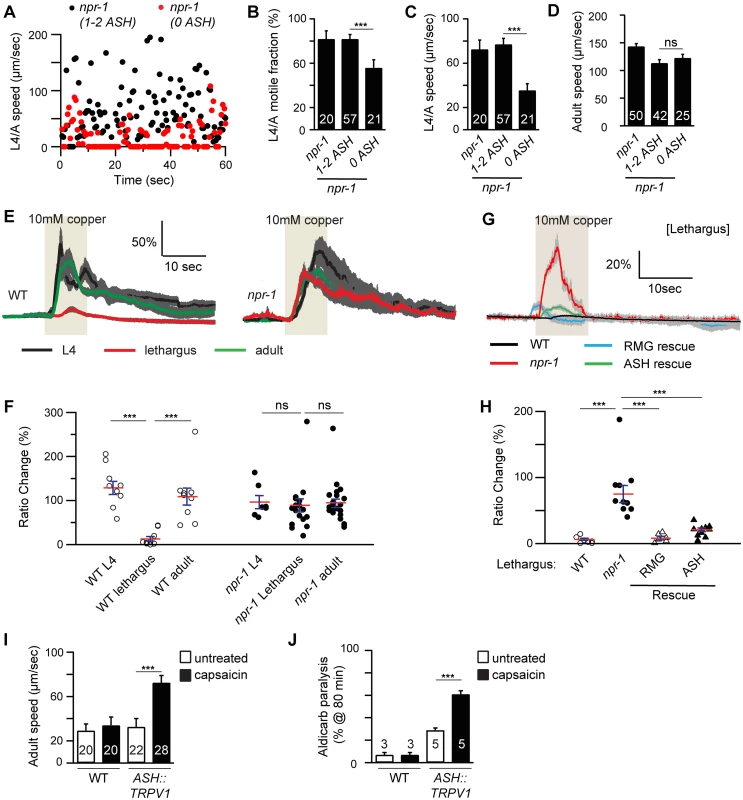
Locomotion behavior during the L4/A lethargus (A-C) and in adults (D) of single worms whose ASH neurons were ablated by transgenic overexpression of CED-3 in ASH neurons (sra-6 promoter) was analyzed in the indicated genotypes. Animals were analyzed by fluorescence microscopy after locomotion recordings to determine if ASH neurons were ablated (1–2 ASH: animals with 1 or 2 ASH intact neurons; 0 ASH: animals lacking viable ASH neurons). Instantaneous locomotion velocity (A), average motile fraction (B), and average locomotion velocity (C-D) are plotted. The npr-1 locomotion defect during the L4/A lethargus, but not in adults, was partially suppressed in the transgenic animals in which both of ASH neurons were ablated (0 ASH). (E-H) Copper-evoked calcium transients in ASH were analyzed in L4, L4/A, and adults of the indicated genotypes using cameleon as a calcium indicator. Averaged responses (E, G), and the amplitudes of individual trials (F, H) are shown for each genotype. Each trace represents the average percentage change in YFP/CFP fluorescence ratio. The light tan rectangle indicates the duration for which 10 mM copper was applied. Dark gray shading of each trace indicates SEM of the mean response. (E-F) Copper-evoked calcium transients in ASH neurons were significantly reduced during L4/A lethargus, and this effect was abolished in npr-1 mutants. (G-H) This defect during L4/A lethargus was rescued by transgenes expressing NPR-1 in the RMG circuit (RMG rescue, flp-21 promoter) or in ASH neurons (ASH rescue, sra-6 promoter). (I-J) Forced depolarization of ASH neurons increased adult locomotion velocity (I) and aldicarb sensitivity (J). Rat TRPV1 was ectopically expressed in ASH neurons (using the sra-6 promoter). (I) Locomotion behavior of adult transgenic worms was analyzed with or without capsaicin treatment (5 hours). Average locomotion velocity (I) is plotted. Capsaicin treatment increased adult locomotion velocity in transgenic animals expressing TRPV1 in ASH neurons, but not in wild type controls. The number of animals analyzed is indicated for each genotype. (J) The percentage of animals paralyzed on 1 mM aldicarb at 80 min with or without capsaicin treatment (2–3 hours pretreatment) were plotted for the indicated genotypes. The number of trials is indicated for each genotype. Full time courses for aldicarb-induced paralysis are shown in S2G Fig. Capsaicin treatment increased aldicarb sensitivity in transgenic animals expressing TRPV1 in ASH neurons, but not in wild type controls. Error bars indicate SEM. Values that differ significantly are indicated (***, p <0.001; ns, not significant). To determine if ASH activity is increased in npr-1 mutants during lethargus, we examined sensory-evoked calcium responses in ASH, using the genetically encoded calcium indicator Cameleon. ASH mediates avoidance responses to copper and hyper-osmotic stimuli. Consistent with a recent study [12], the magnitude of copper (Fig 4E and 4F) and glycerol-evoked (S4A and S4B Fig) calcium transients in ASH was significantly decreased during lethargus in wild-type animals. Decreased ASH responsiveness to copper and glycerol during L4/A lethargus was blocked in npr-1 mutants, whereas ASH responsiveness in adults was unaltered in npr-1 mutants (Fig 4E and 4F and S4A and S4B Fig). Transgenes expressing NPR-1 in the RMG circuit (using the flp-21 promoter) or in ASH (using the sra-6 promoter) reinstated the L4/A decrease in copper and glycerol-evoked ASH calcium transients in npr-1 mutants (Fig 4G and 4H and S4C and S4D Fig). These results suggest that NPR-1 acts in ASH to inhibit sensory responses and that increased ASH activity is required for accelerated locomotion of npr-1 mutants during lethargus but not in adults.
To determine if increased ASH activity is sufficient to arouse locomotion, we analyzed locomotion after artificially depolarizing ASH neurons. For this experiment, we utilized transgenic animals that express rat TRPV1 capsaicin receptors in ASH neurons [24]. In these animals, capsaicin treatment evokes ASH-mediated avoidance behaviors [24]. A 5-hour capsaicin treatment had little effect on L4/A motile fraction and locomotion velocity [11], whereas capsaicin treatment significantly accelerated adult locomotion and increased aldicarb sensitivity (Figs 4I–5J and S2G Fig). These effects were not observed in animals lacking TRPV1 expression in ASH neurons (Fig 4I and 4J). Thus, forced ASH depolarization was sufficient to arouse adult but not lethargus locomotion. Collectively, these results suggest that diminished and heightened ASH activity is associated with locomotion quiescence and arousal respectively; however, the magnitude of ASH’s arousing effects differ between lethargus and adult animals.
Fig. 5. GLR-2 AMPA receptors are required for the npr-1 lethargus defect. 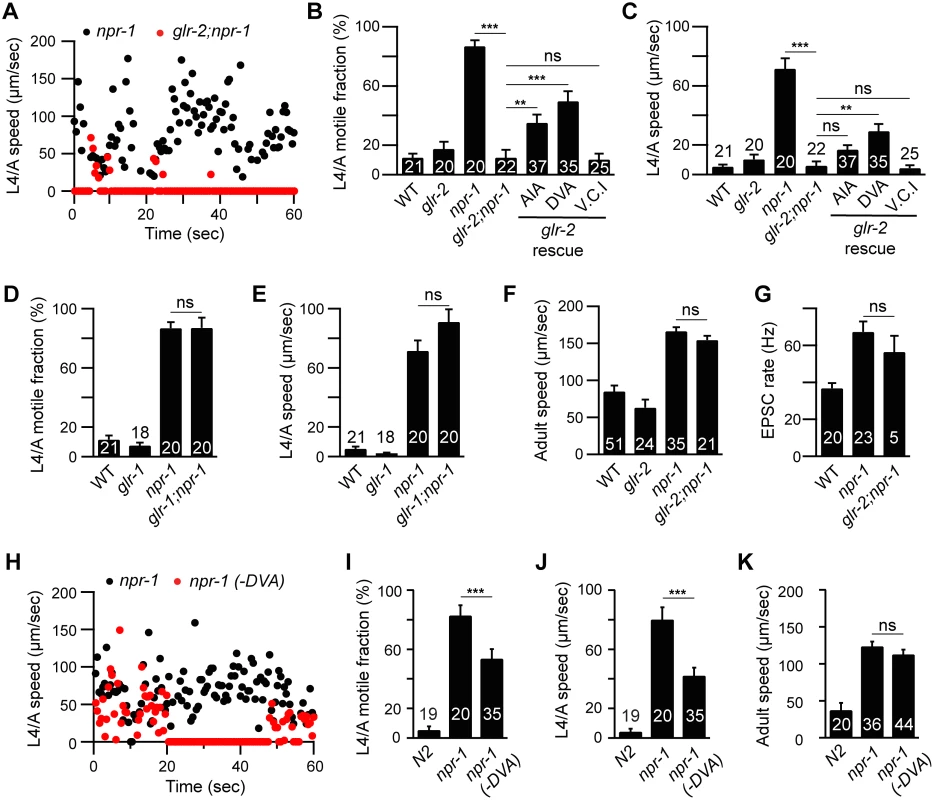
Locomotion behavior of single worms during the L4/A lethargus (A-E and H-J) and in adults (F, K) was analyzed in the indicated genotypes. Instantaneous locomotion velocity (A, H), average motile fraction (B, D, and I), and average locomotion velocity (C, E, F, J and K) are plotted. (A-C) The npr-1 locomotion defect during L4/A lethargus was suppressed by mutations inactivating glr-2 AMPA receptors, and partially reinstated by transgenes expressing GLR-2 in AIA (gcy-28(d) promoter) and DVA (nlp-12 promoter) neurons, but not in Ventral Cord Interneurons (V.C.I., glr-1 promoter) in glr-2;npr-1 double mutants using the indicated promoters. (D-E) glr-1 mutations had no suppressing effect. (F) glr-2 mutations did not block the increased locomotion in npr-1 adults. (G) mEPSCs were recorded from body wall muscles of the adult worms for the indicated genotypes. Summary data are shown. (G) glr-2 mutations did not block the increased mEPSC rate in npr-1 adults. (H-K) Locomotion behavior during the L4/A lethargus (H-J) and in adults (K) of single worms whose DVA neuron is ablated by transgenic overexpression of CED-3 in DVA neuron (nlp-12 promoter) was analyzed in the indicated genotypes. Animals were analyzed by fluorescence microscopy after locomotion recordings to determine if DVA was ablated. The npr-1 locomotion defect during the L4/A lethargus, but not in adults, was partially suppressed in the transgenic animals in which DVA was ablated (-DVA). The number of animals analyzed is indicated for each genotype. Error bars indicate SEM. Values that differ significantly are indicated (**, p <0.01; ***, p <0.001; ns, not significant). GLR-2 AMPA receptors are required for the npr-1 lethargus defect
Which glutamate receptors arouse locomotion in npr-1 mutants? Glutamate-activated cation channels, AMPA (GLR-1 and -2) and NMDA (NMR-1 and -2) receptors, mediate excitatory transmission at ASH-interneuron [25–27]. The npr-1 L4/A quiescence defect was abolished in glr-2; npr-1 double mutants (Fig 5A–5C), while glr-1 mutations had no effect (Fig 5D and 5E). By contrast, glr-1, glr-2, and nmr-1 mutations had little effect on npr-1 adult locomotion (Fig 5F and S5 Fig). Similarly, glr-2 mutations did not block the increased mEPSC rate in npr-1 adults (Fig 5G). These results suggest that GLR-2 AMPA receptors are specifically required for the aroused locomotion during the L4/A lethargus in npr-1 mutants.
GLR-2 AMPA receptors act in AIA and DVA to mediate arousal
Which synaptic targets of ASH and touch neurons mediate locomotion arousal? To address this question, we identified the neurons in which GLR-2 function is required. Aroused L4/A locomotion requires GLR-2 but not GLR-1 receptors; consequently, we reasoned that the relevant neurons are likely to express GLR-2 but not GLR-1. GLR-1 and GLR-2 are co-expressed in many neurons; however, a few GLR-2-expressing neurons lack GLR-1, including DVA (a stretch-activated neuron) and AIA (an interneuron in the head ganglia) [25–27]. The L4/A quiescence defect was partially restored in glr-2; npr-1 double mutants by transgenes expressing GLR-2 in DVA and AIA neurons, whereas transgenes expressed in the ventral cord interneurons (using the glr-1 promoter) failed to rescue (Fig 5B and 5C). Transgenic expression of GLR-2 in DVA or AIA had no effect on lethargus quiescence in wild type worms (S3 Sig). These results suggest that GLR-2 AMPA receptors expressed in AIA and DVA neurons arouse L4/A locomotion in npr-1 mutants. DVA receives direct synaptic input from the touch neuron PLM while AIA receives direct input from ASH [28]. Thus, increased transmission at ASH-AIA and PLM-DVA synapses could account for GLR-2’s effects on locomotion rate. Because we only observed partial rescue by glr-2 transgenes expressed in AIA and DVA, it is likely the GLR-2 function is required in additional (as yet unidentified) neurons.
How do AIA and DVA arouse locomotion? AIA neurons provide synaptic input to ASK and ASI, both of which express PDF-1 [11,29]. Thus, heightened AIA activity could arouse locomotion by enhancing PDF-1 secretion. To assess the level of PDF-1 secretion, we analyzed PDF-1::YFP fluorescence in the endolysosomal compartment of coelomocytes, which are specialized scavenger cells that internalize proteins secreted into the body cavity [30,31]. Inactivating GLR-2 did not alter PDF-1::YFP fluorescence in coelomocytes in both adult and L4/A animals (Fig 6). These results suggest that the arousing effects of GLR-2 are not mediated by changes in PDF secretion. DVA neurons receive direct synaptic input from the PLM touch neurons [32], and secrete NLP-12 (a neuropeptide that accelerates locomotion) [33]. Thus, increased DVA activity could contribute to locomotion arousal in npr-1 mutants. Three results support this idea. First, PLM neurons exhibit enhanced touch-evoked calcium responses in adult npr-1 mutants (S6 Fig). Thus, PLM neurons have increased sensory acuity in npr-1 mutants, similar to the effect we previously showed for ALM neurons [11]. Second, inducing DVA cell death (with a CED-3 transgene) significantly reduced npr-1 locomotion rate during L4/A lethargus (Fig 5H–5J), but not in adults (Fig 5K). Third, DVA secretion of NLP-12 is significantly increased in npr-1 mutants [33], indicating increased DVA activity. These results suggest that PLM neurons provide enhanced excitatory input to DVA in npr-1 mutants, which promotes aroused L4/A locomotion.
Fig. 6. PDF-1 secretion is not altered in glr-2 mutants. 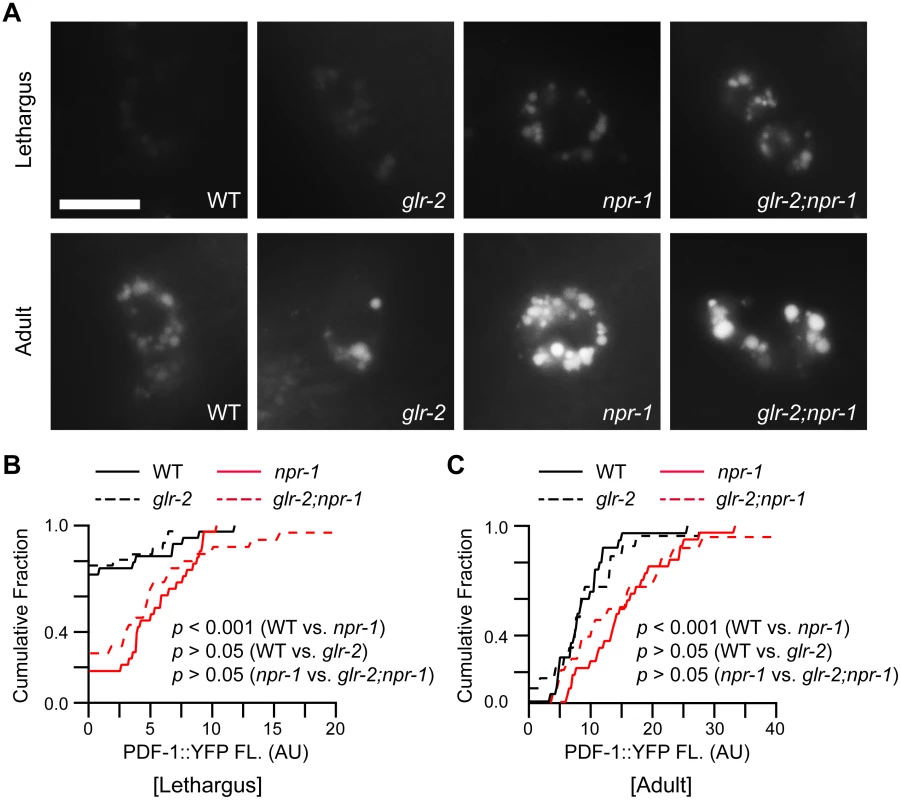
PDF-1 secretion was analyzed in the indicated genotypes. YFP-tagged PDF-1 was expressed with the pdf-1 promoter. Representative images (A) and summary data (cumulative fraction) (B-C) are shown for coelomocyte fluorescence in L4/A lethargus and 1-day old adults of the indicated genotypes. PDF-1::YFP coelomocyte fluorescence was dramatically increased in npr-1 mutants during the L4/A lethargus and in adults as previously reported [11]. Mutations inactivating GLR-2 did not alter PDF-1::YFP coelomocyte fluorescence during L4/A lethargus (B) and in adults (C) in either wild type or npr-1 mutants. Scale bar indicates 10 μm. p values are indicated for each comparison (Kolmogorov-Smirnov test). Discussion
To investigate the circuit mechanisms for arousal, we analyzed the locomotion of npr-1 mutants in awake (adult) and quiescent (lethargus) states. Our results lead to five conclusions. First, multiple classes of sensory neurons contribute to arousal. Second, diminished sensory acuity is a circuit mechanism for promoting behavioral quiescence. Third, glutamate and neuropeptides are utilized as excitatory outputs from sensory neurons to arouse locomotion. Fourth, different mechanisms are utilized to arouse locomotion at different times during development. And fifth, we provide further evidence that arousal mechanisms are conserved across phylogeny.
A broad network of sensory neurons contribute to arousal
Multiple classes of sensory neurons arouse locomotion during lethargus and in adults, including: mechanosensory neurons (ALM and PLM), a nociceptive neuron (ASH), a pheromone sensing neuron (ASK), and a stretch sensing neuron (DVA). Lethargus quiescence is accompanied by diminished sensory-evoked responses in ALM, PLM, and ASH (this study and [11–13]). PDF-1 secretion from ASK neurons is significantly reduced during lethargus, implying that ASK neurons also have diminished activity during lethargus [11]. npr-1 mutations prevent the dampened ALM (mechanosensory) and ASH (nociceptive) responses during lethargus and this was accompanied by decreased locomotion quiescence (this study and [11]). The arousing effects of npr-1 mutations are blocked (or diminished) by mutations that decrease sensory responsiveness (e.g. tax-4 CNG and osm-9 TRPV mutations) [11], or by ablating sensory neurons (e.g. ASH and DVA). Forced activation of ASH neurons arouses adult locomotion. Collectively, these results imply that a broad network of sensory neurons arouses locomotion, which allows C. elegans to adapt its behavior across a broad range of developmental and physiological circumstances.
Sensory gain control as a mechanism for producing quiescence and arousal
NPR-1 promotes behavioral quiescence by diminishing the sensitivity of many sensory modalities. NPR-1 directly inhibits ASH responses and indirectly inhibits other sensory neurons (ALM, PLM, and DVA) via decreased glutamate and neuropeptide release. Thus, gating of sensory perception by NPR-1 provides a circuit mechanism for producing aroused and quiescent locomotion in C. elegans.
Our results do not exclude the possibility that additional mechanisms (beyond sensory gating by NPR-1) contribute to arousal and quiescence. Both quiescence (during lethargus) and arousal (following molts) persist in microfluidic chambers where many sensory cues are minimized [19]. In particular, oxygen tension is likely to be very low in these chambers, which would greatly diminish NPR-1’s effects on behavior [15,16]. Thus, the quiescence and arousal exhibited in microfluidic chambers implies that additional mechanisms beyond NPR-1 must contribute to expressing these behavioral states. It will be interesting to determine if these NPR-1 independent mechanisms also act by gating sensory activity.
Sensory-evoked glutamate and neuropeptide release arouses locomotion
Sensory neurons release glutamate and/or neuropeptides in response to external cues, which then engage downstream motor circuits in behavioral outputs. Our prior study shows that sensory-evoked PDF-1 secretion promotes locomotion arousal by enhancing touch neuron responsiveness. Neuropeptides also mediate arousal in flies (PDF) [34], fish and mammals (orexin/hypocretin) [35,36].
Here we show that sensory evoked glutamate release also plays a role in arousal. Mutations inactivating the EAT-4/VGLUT decreased locomotion arousal in lethargus and in adults. EAT-4 is almost exclusively expressed in sensory neurons [23] and transgenes restoring EAT-4 in touch neurons and ASH neurons re-instates locomotion arousal in npr-1 mutants. These results suggest that sensory neurons utilize both glutamate and neuropeptides as excitatory outputs to arouse locomotion.
Our results suggest that exaggerated glutamate release at ASH-AIA and PLM-DVA synapses arouses locomotion during lethargus in npr-1 mutants. ASH and PLM neurons have enhanced sensory evoked activity in npr-1 mutants, which is expected to produce enhanced glutamate release at ASH-AIA and PLM-DVA synapses. GLR-2 receptors are expressed in AIA and DVA. glr-2 mutations block the aroused L4/A locomotion of npr-1 mutants and arousal is re-instated by transgenes expressing GLR-2 in AIA and DVA. Finally, calcium responses in AIA [14], and neuropeptide secretion from DVA [33] are both enhanced in npr-1 mutants, indicating that these neurons have increased activity. We observed only partial rescue of aroused locomotion by transgenes restoring EAT-4 expression in ASH and touch neurons or by those expressing GLR-2 in AIA or DVA; consequently, it is likely that glutamate released by other sensory neurons also contributes to the aroused L4/A locomotion in npr-1 mutants.
Much less is known about the role of glutamate in arousal in other systems. Glutamate release has widespread effects throughout the brain in mammals, which complicates the analysis of its effects on arousal. Microinjection of glutamate or AMPA into lateral hypothalamic area increased locomotor activity and duration of waking episodes in rodents [37,38], while microdialysis of CNQX, an AMPA receptor antagonist, into the thalamus promotes sleep in cats [39]. Glutamate also induces fictive locomotion in lamprey [40]. In these cases, however, the circuit mechanisms underlying glutamate’s arousing effects are not known.
Comparing lethargus and adult arousal mechanisms
Mutants lacking NPR-1 exhibit accelerated locomotion in adults and during lethargus [11,18]. Several results suggest that locomotion arousal in adult and lethargus is established by a shared central sensory circuit. First, in both adult and lethargus, enhanced activity in the RMG sensory circuit accelerates locomotion, whereas decreased sensory transduction in the RMG circuit (i.e. by inactivating TAX-4 or OSM-9) abolishes npr-1’s hyperactive locomotion defect [11,14], suggesting that the RMG circuit activity stimulates arousal in both awake and quiescent states. Second, EAT-4 acts in ASH and touch neurons to mediate hyperactive locomotion of npr-1 adult and lethargus stage animals, suggesting that glutamate release from these sensory neurons is required for locomotion arousal in npr-1 mutants.
On the other hand, several results suggest that the mechanisms that arouse locomotion differ between adult and lethargus animals. Inactivating GLR-2 AMPA receptors blocks the hyperactive locomotion of npr-1 mutants during lethargus but not in adults. Aroused locomotion in npr-1 adults persists in glr-1, glr-2, and nmr-1 mutants, indicating that other glutamate receptors are responsible for arousing adult locomotion. Similarly, artificial activation of ASH accelerates adult but not lethargus locomotion. Collectively, our results suggest that multiple sensory circuits govern locomotion arousal throughout development but that the relative contribution of each circuit to arousal differs depending on the developmental stage.
Materials and Methods
Strains
Strain maintenance and genetic manipulation were performed as described [41]. Animals were cultivated at 20°C on agar nematode growth media (NGM) seeded with OP50 (for imaging and behavior) or HB101 E.coli (for electrophysiology). Wild type reference strain was N2 Bristol. Strains used in this study are as follows:
Mutant strains and integrants
KP6048 npr-1(ky13) X
DA609 npr-1(ad609) X
KP6064 npr-1(ok1447) X
PR678 tax-4(p678) III
CX4544 ocr-2(ak47) IV
LSC27 pdf-1(tm1996) III
KP6340 pdfr-1(ok3425) III
MT6308 eat-4(ky5) III
KP0004 glr-1(n2461) III
VM487 nmr-1(ak4) II
KP6057 ocr-2(ak47) IV;npr-1(ok1447) X
KP6058 ocr-2(ak47) IV;npr-1(ky13) X
KP6060 tax-4(p678) III;npr-1(ky13) X
KP6061 tax-4(p678) III;npr-1(ok1447) X
KP6100 pdf-1(tm1996) III;npr-1(ky13) X
KP6410 pdfr-1(ok3425) III;npr-1(ky13) X
KP6349 eat-4(ky5) III; npr-1(ky13) X
CX4978 kyIs200[sra-6p::VR1, elt-2p::NLS-gfp] (Gift from Cori Bargmann)
KP6414 nmr-1(ak4) II; npr-1(ky13) X
KP6415 glr-1(n2461) III;npr-1(ky13) X
VM1123 dpy-19(n1347) glr-2(ak10) III
KP6740 dpy-19(n1347) glr-2(ak10) III; npr-1(ky13) X
KP7362 npr-1(ky13) X; nuIs439[nlp-12p::GFP]; nuIs519[nlp-12p::ced-3::GFP, vha-6::mCherry]
KP6693 nuIs472 [pdf-1p::pdf-1::venus, vha-6p::mCherry]
KP6743 npr-1(ky13) X; nuIs472
KP7194 dpy-19(n1347) glr-2(ak10) III; nuIs472
KP7195 dpy-19(n1347) glr-2(ak10) III; npr-1(ky13) X; nuIs472
AQ906 bzIs17[mec-4p::YC2.12]
KP6681 npr-1(ky13) X; bzIS17
Strains containing extrachromosomal arrays
CX9396 npr-1(ad609) X;kyEx1966[flp-21p::npr-1 SL2 GFP, ofm-1p::dsRed] (Gift from Cori Bargmann)
KP6051 npr-1(ad609) X;nuEx1519[unc-25p::npr-1::gfp, myo-2p::NLS-mCherry]
KP6053 npr-1(ad609) X;nuEx1520[unc-30p::npr-1::gfp, myo-2p::NLS-mCherry]
KP7149, KP7150 eat-4(ky5) III; npr-1(ky13) X; nuEx1613-1614[sra-6p::eat-4, myo-2p::NLS-mCherry]
KP7176, KP7177 eat-4(ky5) III; npr-1(ky13) X; nuEx1615-1616[sra-9p::eat-4, vha-6p::mCherry]
KP7198, KP7199 eat-4(ky5) III; npr-1(ky13) X; nuEx1640-1641[mec-4p::eat-4, vha-6p::mCherry]
KP7442 npr-1(ky13) X; nuEx1684[sra-6p::ced-3::GFP, sra-6p::mCherry, vha-6p::mCherry]
KP7633 nuEx1613[sra-6p::eat-4, myo-2p::NLS-mCherry]
KP7634 nuEx1640[mec-4p::eat-4, vha-6p::mCherry]
AQ3304 ljEx239[sra-6::YC.360]
KP7353 npr-1(ky13) X; ljEx239
KP7443 npr-1(ky13) X; ljEx239; nuEX1607[flp-21p::npr-1, myo-2p::NLS-mCherry]
KP7495 npr-1(ky13) X; ljEx239; nuEX1683[sra-6p::npr-1, vha-6p::mCherry]
KP7191 dpy-19(n1347) glr-2(ak10) III; npr-1(ky13) X; nuEx1637[nlp-12p::glr-2(gDNA),myo-2p::NLS-mCherry]
KP7192 dpy-19(n1347) glr-2(ak10) III; npr-1(ky13) X; nuEx1638[gcy-28(d)p::glr-2(gDNA),vha-6p::mCherry]
KP7354, KP7355, KP7356 dpy-19(n1347) glr-2(ak10) III; npr-1(ky13) X; nuEx1642-1644[glr-1p::glr-2(gDNA), vha-6p::mCherry]
KP7635 nuEx1637[nlp-12p::glr-2(gDNA),myo-2p::NLS-mCherry]
KP7636 nuEx1638[gcy-28(d)p::glr-2(gDNA),vha-6p::mCherry]
Constructs
eat-4 rescue constructs (sra-6p::eat-4 (KP#2204), sra-9p::eat-4 (KP#2205), and mec-4p::eat-4 (KP#2207)
eat-4 cDNA was amplified by PCR and ligated into expression vectors (pPD49.26) containing the sra-6 (~3.8kb 5’ regulatory sequence: ASH expression), sra-9 (~3kb 5’ regulatory sequence: ASK expression), or mec-4 (~1.1kb 5’ regulatory sequence: Touch neuron expression) promoters.
glr-2 rescue constructs (nlp-12p::glr-2 (KP#2211), gcy-28(d)p::glr-2 (KP#2209), and glr-1p::glr-1 (KP#2208)
glr-2 genomic DNA was amplified by PCR and ligated into expression vectors (pPD49.26) containing the nlp-12 (~400 bp 5’ regulatory sequence: DVA expression), gcy-28(d) (~2,9kb 5’ regulatory sequence: AIA expression), or glr-1 (~5.3kb 5’ regulatory sequence: ventral cord interneuron (VCI) expression) promoters.
Cell ablation constructs (sra-6p::ced-3::GFP (KP#2151) and nlp-12p::ced-3::GFP (KP#2302)
ced-3 genomic DNA and GFP were amplified by overlapping PCR and ligated into expression vectors (pPD49.26) (using NheI and SacI restriction sites) containing the sra-6 (~3.8 kb 5’ regulatory sequence: ASH expression) or nlp-12 (~400 bp 5’ regulatory sequence: DVA expression) promoters.
Transgenes and germline transformation
Transgenic strains were generated by microinjection of various plasmids with coinjection markers (myo-2p::NLS-mCherry (KP#1480) and vha-6p::mcherry (KP#1874)). Injection concentration was 40–50 ng/μl for all the expression constructs and 10 ng/μl for coinjection markers. The empty vector pBluescript was used to bring the final DNA concentration to 100 ng/μl. The flp-21 promoter (which is expressed in the RMG, ASH, ADL, ASK, URX, and ASI neurons [14]) was used to express transgenes in the RMG circuit.
Lethargus locomotion and behavior analysis
Lethargus locomotion was analyzed as previously described [11]. Well-fed late L4 animals were transferred to full lawn OP50 bacterial plates. After 1 hour, locomotion of animals in lethargus (determined by absence of pharyngeal pumping) was recorded on a Zeiss Discovery Stereomicroscope using Axiovision software. Locomotion was recorded at 2 Hz for 60 seconds. Centroid velocity of each animal was analyzed at each frame using object-tracking software in Axiovision. Motile fraction of each animal was calculated by dividing the number of frames with positive velocity value with total number of frames. Speed of each animal was calculated by averaging the velocity value at each frame. Quantitative analysis was done using a custom written MATLAB program (Mathworks). Statistical significance was determined using one-way ANOVA with Tukey test for multiple comparisons and two-tailed Student’s t test for pairwise comparison.
Adult locomotion and behavior analysis
Locomotion of adult animals was analyzed with the same setup as lethargus locomotion analysis described above, except that well-fed adult animals were monitored 1–1.5hr after the transfer to full lawn OP50 bacterial plates. For the capsaicin treatment (Fig 4I), 1 day-old animals were transferred to NGM plates containing 50 μM capsaicin (with food), treated with capsaicin for 5 hours, and recorded for their locomotion. Statistical significance was determined using one-way ANOVA with Tukey test for multiple comparisons and two-tailed Student’s t test for pairwise comparison.
Cell ablations
Neurons were ablated in npr-1(ky13) mutant worms by transgenes co-expressing CED-3 and a fluorescent protein (GFP or mCherry) under the sra-6 (ASH ablation) or nlp-12 (DVA ablation) promoters. ASH or DVA ablations were confirmed after locomotion analysis by fluorescence microscopy.
Aldicarb assay
Sensitivity to aldicarb was determined by analyzing the time course of paralysis following treatment with 1 mM aldicarb (Sigma-Aldrich) as previously described [42]. Briefly, movement of animals was assessed by prodding animals with a platinum wire every 10 minute following exposure to aldicarb. 20–30 animals were tested for each trial. For the capsaicin treatment (Fig 4J), adult animals were transferred to NGM plates containing 50 μM capsaicin (with food), treated with capsaicin for 2–3 hours, and assayed for their paralysis on 1 mM aldicarb plates containing 50 μM capsaicin.
Electrophysiology
Electrophysiology was performed on dissected adult worms as previously described [43]. Worms were superfused in an extracellular solution containing 127 mM NaCl, 5 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 20 mM glucose, 1 mM CaCl2, and 4 mM MgCl2, bubbled with 5% CO2, 95% O2 at 20°C. Whole cell recordings were carried out at –60 mV using an internal solution containing 105 mM CsCH3SO3, 10 mM CsCl, 15 mM CsF, 4mM MgCl2, 5mM EGTA, 0.25mM CaCl2, 10mM HEPES, and 4 mM Na2ATP, adjusted to pH 7.2 using CsOH. Under these conditions, we only observed endogenous acetylcholine EPSCs. To record GABAergic postsynaptic currents, the holding potential was 0 mV, at which we only observe mIPSCs. All recording conditions were as described [44]. To record evoked EPSCs, a 0.4 ms, 30 μA square pulse was applied to a motor neuron cell body with a stimulating electrode placed near the ventral nerve cord (one muscle distance from the recording pipette). Statistical significance was determined using one-way ANOVA with Tukey test for multiple comparisons and two-tailed Student’s t test for pairwise comparison.
Fluorescence microscopy and image analysis
Quantitative imaging of coelomocyte fluorescence was performed as previously described [11] using a Zeiss Axioskop equipped with an Olympus PlanAPO 100x (NA = 1.4) objective and a CoolSNAP HQ CCD camera (Photometrics). Worms were immobilized with 30 mg/ml BDM (Sigma). The anterior coelomocytes were imaged in L4/A lethargus (determined by absence of pharyngeal pumping), and 1 day-old adult animals. Image stacks were captured and maximum intensity projections were obtained using Metamorph 7.1 software (Universal Imaging). YFP fluorescence was normalized to the absolute mean fluorescence of 0.5 mm FluoSphere beads (Molecular Probes). Statistical significance was determined using Kolmogorov-Smirnov test.
Calcium imaging and analysis
Using Dermabond topical skin adhesive, individual worms were glued to 2% agarose pads in extracellular saline (145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 20 mM D-glucose, and 10 mM HEPES buffer [pH7.2]). To image copper and glycerol responses, single animals were placed in a perfusion chamber (RC-26GLP,Warner Instruments) under a constant flow rate (0.4 ml min-1) of buffer using a perfusion pencil (AutoMate). Outflow was regulated using a peristaltic pump (Econo Pump, Bio-Rad). 10mM CuCl2 (copper(II)chloride dihydrate, Sigma) or 500mM glycerol (Fisher) were delivered using the perfusion, pencil and switch between control and stimulus solutions was done using manually controlled valves. Solutions contained either 10mM CuCl2 in M13 buffer or 500mM glycerol in 40mM NaCl, 1 mM MgSO4, 1 mM CaCl2 and 5 mM KPO4. The stimulus was delivered for 10 seconds starting on the 10th second from the beginning of the movie. Optical recordings were performed on a Zeiss Axioskop 2 upright compound microscope equipped with a Dual View beam splitter and a Uniblitz Shutter. Images were recorded at 10 Hz using an iXon EM camera (Andor Technology) and captured using IQ1.9 software (Andor Technology). For ratiometric imaging, ROIY tracked the neuron in the yellow channel, and in the cyan channel, ROIC moved at a fixed offset from ROIY. F was computed as FY/FC following a correction for bleed through. No correction for bleaching was required. Ratio changes were detected and parametrized using scripts for MATLAB (The Mathworks). Briefly, the scripts average the F value for 5 preceding and including the marked start stimulus frame (F0) and the 5 frames centered on the marked peak frame (F1). ΔF was equal to (F1—F0) / F0 x 100. Touch-evoked calcium responses in PLM neurons were analyzed as previously described [11]. Statistical significance was determined using one-way ANOVA with Tukey test for multiple comparisons.
Supporting Information
Zdroje
1. Pfaff D, Ribeiro A, Matthews J, Kow LM (2008) Concepts and mechanisms of generalized central nervous system arousal. Annals of the New York Academy of Sciences 1129 : 11–25. doi: 10.1196/annals.1417.019 18591465
2. Cirelli C (2009) The genetic and molecular regulation of sleep: from fruit flies to humans. Nature reviews Neuroscience 10 : 549–560. doi: 10.1038/nrn2683 19617891
3. Cassada RC, Russell RL (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Developmental biology 46 : 326–342. 1183723
4. Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, et al. (2008) Lethargus is a Caenorhabditis elegans sleep-like state. Nature 451 : 569–572. doi: 10.1038/nature06535 18185515
5. Van Buskirk C, Sternberg PW (2007) Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nature neuroscience 10 : 1300–1307. 17891142
6. Singh K, Chao MY, Somers GA, Komatsu H, Corkins ME, et al. (2011) C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Current biology: CB 21 : 825–834. doi: 10.1016/j.cub.2011.04.010 21549604
7. Monsalve GC, Van Buskirk C, Frand AR (2011) LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Current biology: CB 21 : 2033–2045. doi: 10.1016/j.cub.2011.10.054 22137474
8. Turek M, Lewandrowski I, Bringmann H (2013) An AP2 transcription factor is required for a sleep-active neuron to induce sleep-like quiescence in C. elegans. Curr Biol 23 : 2215–2223. doi: 10.1016/j.cub.2013.09.028 24184105
9. Nagy S, Wright C, Tramm N, Labello N, Burov S, et al. (2013) A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by Galphas signaling. Elife 2: e00782. doi: 10.7554/eLife.00782 23840929
10. Nelson MD, Trojanowski NF, George-Raizen JB, Smith CJ, Yu CC, et al. (2013) The neuropeptide NLP-22 regulates a sleep-like state in Caenorhabditis elegans. Nat Commun 4 : 2846. doi: 10.1038/ncomms3846 24301180
11. Choi S, Chatzigeorgiou M, Taylor KP, Schafer WR, Kaplan JM (2013) Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C. elegans. Neuron 78 : 869–880. doi: 10.1016/j.neuron.2013.04.002 23764289
12. Cho JY, Sternberg PW (2014) Multilevel modulation of a sensory motor circuit during C. elegans sleep and arousal. Cell 156 : 249–260. doi: 10.1016/j.cell.2013.11.036 24439380
13. Schwarz J, Lewandrowski I, Bringmann H (2011) Reduced activity of a sensory neuron during a sleep-like state in Caenorhabditis elegans. Current biology: CB 21: R983–984. doi: 10.1016/j.cub.2011.10.046 22192827
14. Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, et al. (2009) A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458 : 1171–1175. doi: 10.1038/nature07886 19349961
15. Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, et al. (2004) Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430 : 317–322. 15220933
16. Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M (2005) Experience-dependent modulation of C. elegans behavior by ambient oxygen. Current biology: CB 15 : 905–917. 15916947
17. Coates JC, de Bono M (2002) Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature 419 : 925–929. 12410311
18. de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI (2002) Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419 : 899–903. 12410303
19. Nagy S, Raizen DM, Biron D (2014) Measurements of behavioral quiescence in Caenorhabditis elegans. Methods 68 : 500–507. doi: 10.1016/j.ymeth.2014.03.009 24642199
20. Nagy S, Tramm N, Sanders J, Iwanir S, Shirley IA, et al. (2014) Homeostasis in C. elegans sleep is characterized by two behaviorally and genetically distinct mechanisms. Elife 3: e04380. doi: 10.7554/eLife.04380 25474127
21. de Bono M, Bargmann CI (1998) Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94 : 679–689. 9741632
22. Vashlishan AB, Madison JM, Dybbs M, Bai J, Sieburth D, et al. (2008) An RNAi screen identifies genes that regulate GABA synapses. Neuron 58 : 346–361. doi: 10.1016/j.neuron.2008.02.019 18466746
23. Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L (1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in caenorhabditis elegans. The Journal of neuroscience: the official journal of the Society for Neuroscience 19 : 159–167.
24. Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, et al. (2002) Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35 : 307–318. 12160748
25. Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV (2001) Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. The Journal of neuroscience: the official journal of the Society for Neuroscience 21 : 1510–1522.
26. Hart AC, Sims S, Kaplan JM (1995) Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378 : 82–85. 7477294
27. Maricq AV, Peckol E, Driscoll M, Bargmann CI (1995) Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 378 : 78–81. 7477293
28. White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of Caenorhabditis elegans. Philos Trans R Soc Lond 314 : 1–340.
29. Janssen T, Husson SJ, Meelkop E, Temmerman L, Lindemans M, et al. (2009) Discovery and characterization of a conserved pigment dispersing factor-like neuropeptide pathway in Caenorhabditis elegans. Journal of neurochemistry 111 : 228–241. doi: 10.1111/j.1471-4159.2009.06323.x 19686386
30. Fares H, Greenwald I (2001) Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 159 : 133–145. 11560892
31. Sieburth D, Madison JM, Kaplan JM (2007) PKC-1 regulates secretion of neuropeptides. Nature neuroscience 10 : 49–57. 17128266
32. White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical transactions of the Royal Society of London Series B, Biological sciences 314 : 1–340. 22462104
33. Hu Z, Pym EC, Babu K, Vashlishan Murray AB, Kaplan JM (2011) A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron 71 : 92–102. doi: 10.1016/j.neuron.2011.04.021 21745640
34. Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, et al. (2008) PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60 : 672–682. doi: 10.1016/j.neuron.2008.10.042 19038223
35. Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF (2006) Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci 26 : 13400–13410. 17182791
36. Sutcliffe JG, de Lecea L (2002) The hypocretins: setting the arousal threshold. Nature reviews Neuroscience 3 : 339–349. 11988773
37. Alam MA, Mallick BN (2008) Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci Lett 439 : 281–286. doi: 10.1016/j.neulet.2008.05.042 18534750
38. Li FW, Deurveilher S, Semba K (2011) Behavioural and neuronal activation after microinjections of AMPA and NMDA into the perifornical lateral hypothalamus in rats. Behav Brain Res 224 : 376–386. doi: 10.1016/j.bbr.2011.06.021 21723327
39. Juhasz G, Kekesi K, Emri Z, Soltesz I, Crunelli V (1990) Sleep-promoting action of excitatory amino acid antagonists: a different role for thalamic NMDA and non-NMDA receptors. Neurosci Lett 114 : 333–338. 1976237
40. Brodin L, Grillner S, Rovainen CM (1985) N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res 325 : 302–306. 2858251
41. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94. 4366476
42. Nurrish S, Segalat L, Kaplan JM (1999) Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 24 : 231–242. 10677040
43. Richmond JE, Davis WS, Jorgensen EM (1999) UNC-13 is required for synaptic vesicle fusion in C. elegans. Nature neuroscience 2 : 959–964. 10526333
44. McEwen JM, Madison JM, Dybbs M, Kaplan JM (2006) Antagonistic regulation of synaptic vesicle priming by Tomosyn and UNC-13. Neuron 51 : 303–315. 16880125
Štítky
Genetika Reprodukčná medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



