-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
In this study, Santos Susin and colleagues demonstrate that a serum-stable CD47 agonist peptide is highly effective at inducing apoptosis in chronic lymphocytic leukemia B-cells and mice.
Published in the journal: CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans. PLoS Med 12(3): e32767. doi:10.1371/journal.pmed.1001796
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001796Summary
In this study, Santos Susin and colleagues demonstrate that a serum-stable CD47 agonist peptide is highly effective at inducing apoptosis in chronic lymphocytic leukemia B-cells and mice.
Introduction
Chronic lymphocytic leukemia (CLL), a human malignancy caused by an imbalance between proliferation and programmed cell death (PCD) [1], is the most common form of leukemia in adults. CLL is characterized by an accumulation of monoclonal B cells (CD20+, CD5+, and CD23+) in the peripheral blood, bone marrow, and secondary lymphoid organs that leads to the progressive failure of the immune and hematopoietic systems [2]. CLL prognosis is dependent on clinical Rai (United States) or Binet (Europe) staging and biological markers, including IGHV status, cytogenetic abnormalities, NOTCH1 or SF3B1 mutations, and the expression of proteins such as CD38 or ZAP70 [3–5]. Despite intense research and pharmaceutical development, CLL remains an incurable disease. Indeed, 15%–25% of patients remain or become refractory to the current chemotherapeutic regimes [6]. Moreover, patients with a dysfunctional TP53 gene (~7%) require a specific aggressive therapy that often yields negative results [7]. Novel therapies targeting the B cell receptor (BCR)–associated kinases have recently been approved in the United States and Europe for relapsed CLL [8,9]. However, it is still very important to develop alternative PCD approaches that kill specifically the malignant CLL cells, including those in high-risk individuals, while sparing the residual CD5− B lymphocytes and the T cells of the CLL patient.
From this perspective, CD47 appears to be a target of high therapeutic potential in CLL. CD47, a cell surface receptor that binds to signal regulatory protein-α (SIRPα) and thrombospondin-1 (TSP1), serves as a marker of self and participates in the regulation of the cellular responses to stress [10–17]. The binding of CD47 to either SIRPα or TSP1 provides two anticancer strategies. On the one hand, antibodies targeting CD47 or SIRPα, recombinant SIRPα, or TSP1-derived proteins promote phagocytosis and tumor cell elimination by disrupting the CD47–SIRPα interaction and/or inducing Fc-dependent mechanisms or PCD [15,17–28]. On the other hand, the binding of CD47 to 4N1K, a decapeptide derived from the TSP1 C-terminal domain [10,16,29–31], kills tumor cells and inhibits tumor growth in vivo [23,32–37]. Even if peptide-based strategies have so far been underused, a renewed interest in therapeutic peptides has emerged because of advances in the development of novel tools aimed at generating peptides that are resistant to proteolytic degradations (e.g., obtained by introduction of amino acid surrogates or non-natural amino acids) [38]. Of 340 potential therapeutic peptides described to date, 160 (60 in phase I, 80 in phase II, and 20 in phase III) are being clinically investigated. In addition, there are more than 80 approved peptides (58 are drugs and 23 are used as diagnostic agents or vaccines). Note that the overall success rate of peptide drugs from phase I to commercial launch is approximately 25%, which is comparable to the success rate of biologic drugs and twice as high as that of small molecules (~10%–12%) [38]. Moreover, peptides have fewer side effects than biologics and are more cost-effective. Indeed, peptides such as goserelin, octreotide, and lanreotide are commonly used anticancer drugs [38]. Altogether, the success in the development of efficient therapeutic peptides emphasizes the medical and pharmaceutical relevance of a peptide-based approach against CLL [6,7].
In view of the above, here we test the cytotoxic effect of serum-stable CD47 peptide agonists in primary CLL B cells. Then, we use a complementary molecular and cell biology approach to unravel the mechanisms regulating the PCD pathway enabled by these peptides in the malignant B lymphocytes.
Methods
Patients, B Cell Purification, and Culture Conditions
The procedures in our study were in accordance with the Helsinki Declaration and were approved by the ethical committee on human experimentation at Pitié-Salpêtrière Hospital (CPPIDF6, Paris, France). After obtaining written consent, peripheral blood was collected from 80 patients diagnosed with CLL according to classical morphological and immunophenotypic criteria. These criteria include clinical Binet staging and the biological parameters IGHV mutational status and CD38 and ZAP70 levels. Deletions of 17p13, 11q22 and 13q14 and trisomy 12 were detected by fluorescence in situ hybridization (FISH) using the Vysis LSI p53/LSI ATM and LSI D13S319/LSI 13q34/CEP 12 Multi-color Probe Kits (Abbott Molecular). The functional status of TP53 was determined by flow cytometry, as previously described [39]. TP53 mutations were detected using high resolution melting (HRM). The amplicons with an abnormal HRM profile were confirmed by sequencing.
Mononuclear cells were purified from blood samples using a standard Ficoll-Hypaque gradient, and B cells were positively or negatively selected by magnetic microbeads coupled either to an anti-CD19 monoclonal antibody (positive selection) or to anti-CD16,-CD3, and-CD14 monoclonal antibodies (negative depletion) (Miltenyi Biotech). No differences in cell death response were encountered in positively or negatively selected cells. B lymphocytes, MEC-1, and M210B4 bone marrow stromal cells (ATCC) were cultured in complete medium (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin). In selected experiments, CLL cells were cultured in Ca2+-free medium (RPMI 1640 without calcium, supplemented with dialyzed 10% fetal calf serum) prior to the induction of cell death.
Peptide Synthesis
Peptides were synthesized by solid-phase peptide synthesis on preloaded Fmoc-Lys(Boc)-Wang or Fmoc-D-Lys(Boc)-Wang resins (Merck Chemicals). The syntheses were performed on an ABI 433A peptide synthesizer (Applied Biosystems) at the 0.25-mmol scale. Peptides were purified by reverse phase high-performance liquid chromatography (HPLC) on an ACE (C8, 5 μm, 300 Å, 10 mm × 250 mm) column (Waters) with a gradient elution (0.1% [v/v] TFA in acetonitrile) in aqueous 0.1% (v/v) TFA. Homogeneous fractions were pooled and lyophilized after confirming purity greater than 95% by analytical HPLC.
PKHB1, 4N1K, and 4NGG. All Nα-Fmoc amino acids (10 equiv.) were coupled after activation with HBTU (0.45 M in NMP) in the presence of DIEA (6 equiv., 2 M in NMP; 10 equiv., 1 : 1). N-Fmoc deprotection was performed using piperidine (20% in NMP) and was monitored by measuring the UV absorbance of the released N-(9-fluorenylmethyl)-piperidine group at 301 nm. PKHB1, MALDI-TOF MS: k-R-F-Y-V-V-M-W-K-k MH+ calculated: 1,383.8; MH+ actual: 1,385.3. 4N1K, MALDI-TOF MS: K-R-F-Y-V-V-M-W-K-K MH+ calculated: 1,383.8; MH+ actual: 1,385.0. 4NGG, MALDI-TOF MS: K-R-F-Y-G-G-M-W-K-K MH+ calculated: 1,300.7; MH+ actual: 1,300.4.
Peptide Degradation Assays
4N1K or PKHB1 (10 mg/ml), diluted in a 1 : 4 human serum/RPMI 1640 mixture, was incubated at 37°C for different lengths of time, then mixed with ethanol and 5 ml of 1 M NaOH and incubated at 4°C for at least 15 min to precipitate serum proteins. The supernatant was collected and injected in an HPLC system, and the soluble peptide was eluted by a linear gradient of 5% to 50% ACN (0.1% [v/v] TFA in acetonitrile) in aqueous 0.1% (v/v) TFA. The concentration of the peptide was calculated by the integration of the absorbance at 220 nm as a function of the retention time.
Flow Cytometry
Annexin-V-APC (0.1 μg/ml; BD Biosciences) was used for the assessment of phosphatidylserine exposure, propidium iodide (PI, 0.5 μg/ml) for cell viability analysis, and tetramethylrhodamine ethyl ester (TMRE, 20 nM) for mitochondrial transmembrane potential (ΔΨm) quantification. PCD was recorded in a FACSCanto II (BD Biosciences) in the total population (10,000 cells), and data were analyzed using FlowJo software. Chymotrypsin-like serine protease (serpase) or caspase cytofluorometric detection was performed with SerPase or Caspase activity kits from Imgenex (IMI-2301 and IMI-2315). Residual and leukemic B cells from CLL patients were discriminated from the mononuclear blood fraction by double labeling with anti-CD19 PerCP-Cyc5.5 (clone SJ25C1; BD Biosciences) and anti-CD5-PE-Cy7 mAb (clone L17F12; BD Biosciences). T cells from CLL patients were identified by a double anti-CD19 PerCP-Cyc5.5 and anti-CD3-PE-Cy7 (clone SK7; BD Biosciences) staining. Calreticulin cell surface exposure was recorded with anti-calreticulin-PE (clone FMC75; Assay Designs). CD47 analysis was assessed with conjugated anti-CD47-PE (clone B6H12; BD Biosciences), P21 with anti-P21-FITC (clone EA10; Calbiochem), and P53 with anti-P53-PE (clone DO-7; BD Biosciences). A QuantiBRITE flow cytometry system (BD Biosciences) was used to assess the number of CD47 molecules expressed on normal and CLL B lymphocytes.
Cell Death Induction and Inhibition
We mainly used fresh CLL samples presenting low levels of spontaneous apoptosis. To induce PCD, 1 × 106 cells/ml were treated for 2 h with PKHB1 (200 μM), 4N1K (300 μM), 4NGG (300 μM), or an anti-CD47 monoclonal antibody (5 mg/ml, clone B6H12 in soluble or immobilized conditions). To provide pro-survival microenvironment signals, CLL cells were pre-incubated with sCD40L (5 μg/ml) and IL-4 (20 ng/ml) or co-cultured with the M210B4 bone marrow stromal cell line (ATCC) before death was induced. To control for caspase-dependent apoptosis, cells were incubated for 12 h with etoposide (250 μM). For the inhibition assays, BAPTA-AM (20 μM); BAPTA (5 mM); Ru360 (750 nM); dantrolene (40 μM); 2-aminoethoxydiphenyl borate (2-APB, 60 μM); U73122 (200 nM); the broad spectrum caspase inhibitors Q-VD-OPh (QVD, 10 μM) and z-VAD-FMK (50 μM); the specific caspase inhibitors z-DEVD-FMK (50 μM, caspase-3/7), z-LEHD-FMK (50 μM, caspase-8), and z-IETD-FMK (50 μM, caspase-9); the serpase inhibitor TPCK (20 μM); or the fusion protein hSIRPα-Fc (15 μg/ml) [40,41] was added 30 min before inducing PCD.
Protein Extraction and Immunoblotting
Cell fractions were lysed in 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, and 1 mM EDTA supplemented with anti-protease and anti-phosphatase cocktails (Roche). Mitochondrial fraction was obtained with the help of a kit from Pierce. The protein concentration was determined using the Bio-Rad DC kit, and 70 μg of protein was loaded in linear SDS-PAGE gels. After blotting, nitrocellulose filters were probed with primary antibodies against CD47 (clone 2D3; eBioscience), activated caspase-3 (9661; Cell Signaling Technology), PLCγ1 (clone D9H10; Cell Signaling Technology), PLCγ1-Y783 (2821; Cell Signaling Technology), Cox IV (clone 1D6E1A8; Life Technologies), DRP1/DLP1 (clone 8/DLP1; BD Biosciences), and α-tubulin (clone B-5–1–2; Sigma). Immunoreactive proteins were detected using HRP-conjugated secondary antibodies and visualized with the ECL system (Thermo Scientific). Immunoblot images were acquired on a MF-ChemiBIS 4.2 (DNR Bio-Imaging Systems). PLCγ1-Y783 was quantified using Multi Gauge 3.0 software (Fujifilm Life Sciences). The optical density was normalized to the background and was expressed relative to the untreated cells (set at 1.0).
Electron Microscopy
CLL cells were centrifuged for 10 min at 300g. The pellets obtained were fixed at 4°C for 2 h in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.30), postfixed for 1 h in 1% buffered osmium tetroxide, dehydrated through a graded ethanol series, and embedded in Epon 812. Ultrathin sections were counterstained with 2% aqueous uranyl acetate for 30 min, then with lead citrate for 10 min, and viewed under a Philips 100× electron microscope.
Ca2+ Measurement
Intracellular Ca2+ mobilization was assessed as previously described [42,43]. B cells were loaded for 30 min at 37°C with 1 μM Fura2-AM and pluronic acid (both from Life Technologies) in glass bottom dishes (MatTek Corporation) and washed in Ringer’s solution (145 mM NaCl, 5.4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, and 0.1% BSA [pH 7.5] with NaOH) or, in particular cases, with 5 mM BAPTA. Then, the B cells were treated with PKHB1 or ionomycin (maximum response) at the indicated concentrations and were excited by wavelengths of 340 and 380 nm. The fluorescence emissions of several cells were simultaneously recorded at a frequency of 1 Hz using a dual excitation fluorometric imaging system (TILL Photonics) and TILLvisION software. Signals were computed into relative ratio units of the florescence intensity of the different wavelengths (340/380 nm). Fluorescence values were analyzed using Origin software (OriginLab) and were normalized to the first value according to the equation (F/F0) − 1, where F is the fluorescence at a specific time point and F0 is the fluorescence at time 0. The area under the curve, representing the extent of Ca2+ mobilization, was calculated for each cell and graphed.
Fibrillar and Globular Actin Assessment
The fibrillar/globular actin ratio was determined by fluorometric assessment. Excitation/emission filters were 485/538 and 544/590 nm for Phalloidin-FITC (F-actin) and DNase-Alexa 594 (G-actin), respectively. One unit equals the basal F-actin/G-actin ratio measured in 106 untreated cells. All reactions were recorded in an Infinite M1000 PRO plate reader (Tecan).
Quantitative Real-Time PCR
Total RNA was extracted from 11 normal and 50 CLL B lymphocyte samples using the Nucleospin RNA II kit (Macherey-Nagel). cDNA was prepared using Superscript II reverse transcriptase (Life Technologies). Quantitative real-time PCR (RT-PCR) was performed using TaqMan Gene Expression Assays (Life Technologies) for CD47, PLCB1 (phospholipase C, beta 1), PLCB2 (phospholipase C, beta 2), PLCB3 (phospholipase C, beta 3), PLCB4 (phospholipase C, beta 4), PLCG1 (phospholipase C, gamma 1), PLCG2 (phospholipase C, gamma 2), ITPR1 (inositol 1,4,5-trisphosphate receptor, type 1), ITPR2 (inositol 1,4,5-trisphosphate receptor, type 2), ITPR3 (inositol 1,4,5-trisphosphate receptor, type 3), RYR1 (ryanodine receptor 1), RYR2 (ryanodine receptor 2), RYR3 (ryanodine receptor 3), STIM1 (stromal interaction molecule 1), STIM2 (stromal interaction molecule 2), ORAI1 (calcium release-activated calcium modulator 1), ORAI2 (calcium release-activated calcium modulator 2), and ORAI3 (calcium release-activated calcium modulator 3). PCR reactions were performed in triplicate using TaqMan Universal PCR Master Mix (Life Technologies). The products were amplified in a ViiA7 Real-Time PCR System (Life Technologies) at 60°C for 40 cycles. Data were analyzed using the comparative threshold cycle method. The expression of GUSB or ABL [44] was utilized to normalize the data.
PLCγ1-Y783 Flow Cytometry Assessment
At various time points after PKHB1 treatment, 5 × 105 cells were fixed in ethanol and permeabilized in Triton X-100. Then cells were saturated in PBS + Triton X-100 + 10% FCS, incubated with anti-PLCγ1-Y783 (Cell Signaling Technology), and detected using an anti-rabbit IgG conjugated with Alexa Fluor 488. The data on the entire cell population were collected on a FACSCanto II flow cytometer. PLCγ1-Y783 was quantified based on the mean fluorescence intensity (MFI) for each sample.
IP3 Quantification
IP3 was measured using IP1 as a surrogate [45] with an HTRF assay (Cisbio). The assays were performed in triplicate in 96-well plates, and the signal was quantified on an Infinite M1000 PRO plate reader (Tecan).
Vectors and Lentiviral Transduction
To down-regulate PLCγ1, we utilized two short hairpin RNAs (shRNAs) (A: CTGAGAAATACGTGAACAA; B: AGTGAATGCTAGACAGAAA) and a control scrambled shRNA (ACGATAGTCGGTCGATAAA). Forward and reverse oligonucleotides (Life Technologies) were annealed and cloned into the pLVTHM lentiviral vector (Addgene 12247). Virus was produced in 293T cells after the CaCl2-mediated transient transfection of the lentiviral constructs and the packaging plasmids pMD2.G and psPAX-2 (Addgene 12259 and 12260, respectively). Forty-eight hours after the transfection, the lentiviral supernatants were harvested, clarified by filtration, and immediately used to transduce 5 × 106 primary CLL or MEC-1 cells. At 72 h post-infection, GFP-positive cells were sorted on a FACSVantage cytofluorimeter (BD Biosciences) to analyze PCD.
Animals
Mice were housed at the Bichat Medical School animal facility in strictly controlled, specific-pathogen-free conditions. All experiments were performed in accordance with ARRIVE ethical guidelines [46] and with the approval of the French national committee on animal experimentation (A74–18–01 and 7413). Unless otherwise specified, eight mice (Charles River Laboratories) were used for each experiment/condition.
In the CLL-xenograft model, MEC-1 cells (3 × 106) were injected subcutaneously into NOD/scid gamma (NSG) mice. When the tumor volume reached 100 mm3, mice received intraperitoneal injections of PBS, PKHB1, or 4N1K (200 μg in 200 μl of PBS) once a week. Tumor size was measured with a caliper, and tumor volume was calculated using the formula (length × width2)/2 and expressed in cubic millimeters. Alternatively, at 28 d after the engraftment, XenoLight RediJect 2-DG 750 Probe (Caliper) was injected into the retro-orbital sinus to visualize tumor glucose uptake, which reflects cell proliferation [47]. Fluorescence was measured using an in vivo imaging system FX Pro (Kodak), and pictures were analyzed with Carestream Molecular Imaging software.
To determine hemoglobin levels, tumors were excised from euthanized mice, weighed, and homogenized in PBS. After centrifugation, formic acid was added to the supernatant, and the hemoglobin concentration was calculated based on the absorbance at 405 nm.
To assess PCD, calreticulin exposure, and PLCγ1-Y783, dissociated tumors were digested in medium containing Liberase-TL (Roche), DNase-I (Calbiochem), and Collagenase-IV (Life Technologies). Single-cell suspension, obtained by filtering through a 70-μM cell strainer, was analyzed by flow cytometry.
In mice toxicity studies, we used the same injection protocol as in xenografted NSG mice. The hematological parameters were monitored in blood drawn from the retro-orbital plexus from either vehicle - (PBS) or PKHB1-treated wild-type mice (C57BL/6) with an MS9–5 analyzer (Melet Schloesing). Kidneys and livers (24 h after the third injection of PBS or PKHB1) were fixed and embedded in paraffin. Paraffin sections (2–3 mm thick) of kidney and liver were deparaffinized and rehydrated before staining with periodic acid—Schiff and hematoxylin-eosin, respectively.
Immunohistochemistry
Tumor vessels were observed on 5-μm cryo-sections by targeting mouse CD31 protein. In order to avoid unspecific background signal, sections were first pretreated with 0.3% H2O2 and Avidin/Biotin (Vector labs). Then, sections were incubated with a rat anti—mouse CD31 antibody (clone MEC 13.3; BD Biosciences) followed by a goat F(ab′)2 anti-rat IgG(H+L)-Biotin (3052–08; Southern Biotech). mCD31 was visualized with the Vectastain Elite ABC Kit (Vector Labs) plus DAB chromogenic substrate (Interchim). Sections were counterstained with hematoxylin and mounted with ImmunoMount (Thermo Scientific).
Statistical Analysis
One-way ANOVA, Mann-Whitney tests, and Student’s t-tests were performed using GraphPad Prism (GraphPad Software). Unless otherwise noted, our assessments included an equal number of Binet Stage A and Binet Stage B/C CLL patients (Tables 1 and 2).
Tab. 1. Features of CLL patients with clinical Binet Stage A. 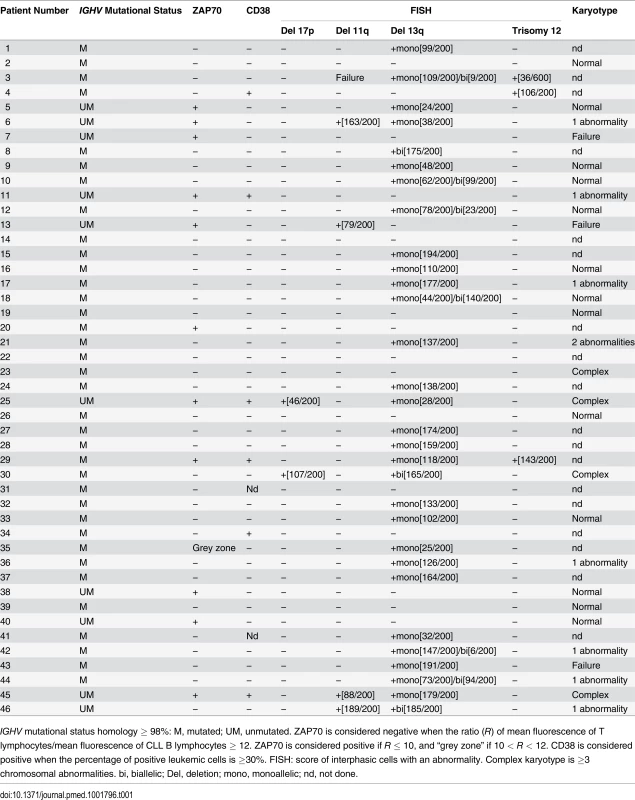
IGHV mutational status homology ≥ 98%: M, mutated; UM, unmutated. ZAP70 is considered negative when the ratio (R) of mean fluorescence of T lymphocytes/mean fluorescence of CLL B lymphocytes ≥ 12. ZAP70 is considered positive if R ≤ 10, and “grey zone” if 10 < R < 12. CD38 is considered positive when the percentage of positive leukemic cells is ≥30%. FISH: score of interphasic cells with an abnormality. Complex karyotype is ≥3 chromosomal abnormalities. bi, biallelic; Del, deletion; mono, monoallelic; nd, not done. Tab. 2. Features of CLL patients with clinical Binet Stage B/C. 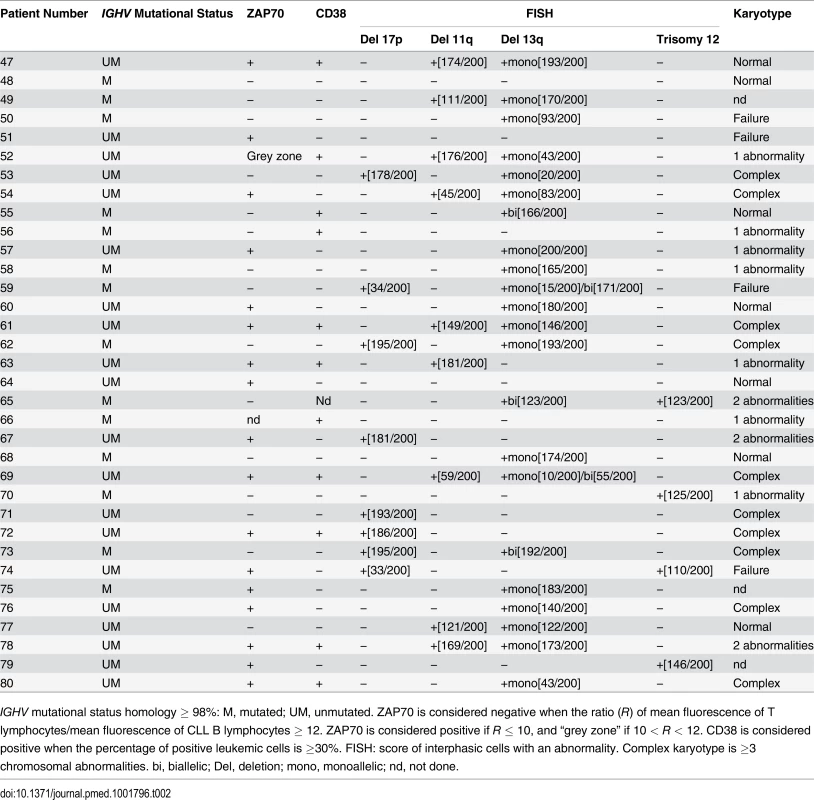
IGHV mutational status homology ≥ 98%: M, mutated; UM, unmutated. ZAP70 is considered negative when the ratio (R) of mean fluorescence of T lymphocytes/mean fluorescence of CLL B lymphocytes ≥ 12. ZAP70 is considered positive if R ≤ 10, and “grey zone” if 10 < R < 12. CD38 is considered positive when the percentage of positive leukemic cells is ≥30%. FISH: score of interphasic cells with an abnormality. Complex karyotype is ≥3 chromosomal abnormalities. bi, biallelic; Del, deletion; mono, monoallelic; nd, not done. Results
Human Samples: Demographic and Clinical Characteristics
Peripheral blood samples from CLL patients were provided by the Hematology Department at Pitié-Salpêtrière Hospital. A total of 80 CLL patients were selected for this study, including 29 women and 51 men, with a mean age of 73 ± 11 y (range: 46–96 y). Of these, 46 patients were Stage A and 34 were Stage B or C according to the Binet classification, and 11 of the patients had a TP53 dysfunction. A more detailed description of the clinical characteristics of the CLL patients is reported in Tables 1–3, including ZAP70 and CD38 expression, cytogenetics, and IGHV mutational status. As a control in our experiments, peripheral blood samples from healthy donors were provided by the French Blood Establishment (Etablissement Français du Sang) transfusion center. In our study we included blood samples from 20 healthy donors (age range: 20–70 y) with a gender ratio similar to that of the CLL panel.
Tab. 3. CLL patients with TP53 abnormalities. 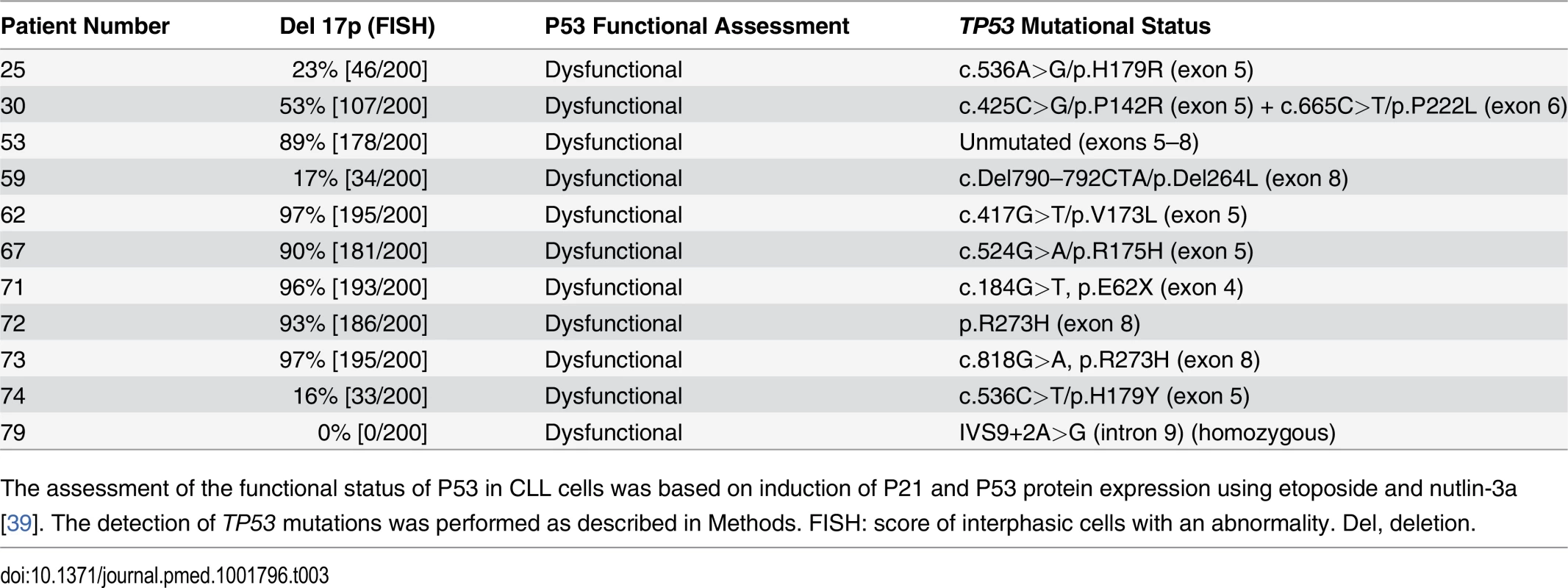
The assessment of the functional status of P53 in CLL cells was based on induction of P21 and P53 protein expression using etoposide and nutlin-3a [39]. The detection of TP53 mutations was performed as described in Methods. FISH: score of interphasic cells with an abnormality. Del, deletion. PKHB1, a Human-Serum-Stable TSP1-Derived Peptide, Selectively Kills Leukemic CD5+ B Lymphocytes, including Those from Individuals with Dysfunctional TP53
To explore the potential of CD47 activation by peptide targeting in CLL, we first analyzed the triggering of CD47 with the decapeptide 4N1K. This ligand of CD47 [10,16,29–31,48] induces PCD in vitro in breast tumors and leukemic cells [23,34–37]. We corroborated that after only 2 h of treatment with soluble 4N1K (300 μM), 46% of the CLL cells obtained from 20 CLL patients were Annexin-V-positive/PI-positive (Fig. 1A and 1B). In contrast to 4N1K, the negative control analogue 4NGG [35] was ineffective at inducing cytotoxicity in CLL cells. Note that, contrary to B6H12, the more commonly used anti-CD47 mAb [18,23] (S1 Fig), 4N1K induces PCD in CLL cells in soluble conditions. Strikingly, 4N1K incubation had no effect on the normal B lymphocytes isolated from eight healthy donors (Fig. 1B). The Mann-Whitney test substantiated the significance of the different response to 4N1K observed in the malignant and the normal B cells (p < 0.001). The specificity of soluble 4N1K to induce PCD in leukemic cells, but not in normal B lymphocytes, led us to further investigate the therapeutic potential of this decapeptide.
Fig. 1. PKHB1 selectively induces cell death in leukemic B cells, including those from patients with dysfunctional TP53. 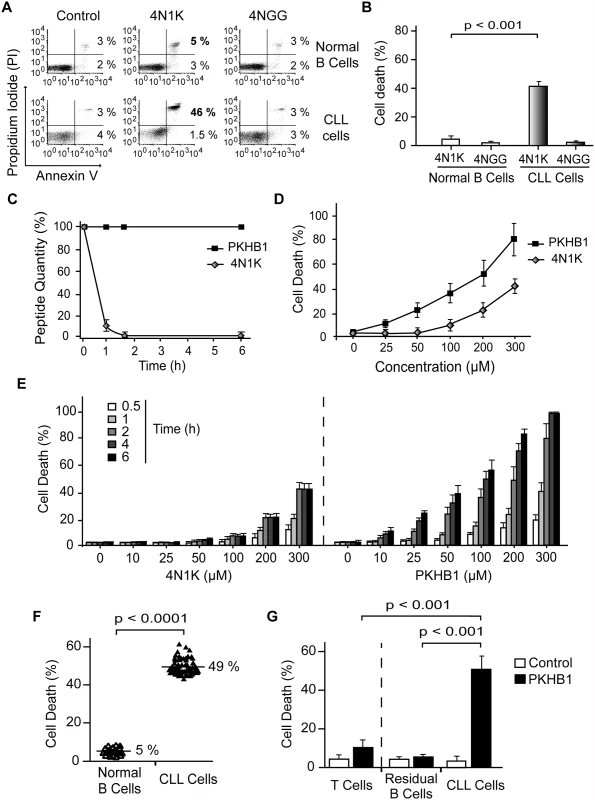
(A) Cell viability measured by Annexin-V and PI staining in normal or CLL B lymphocytes treated with 4N1K (300 μM, 2 h) or the negative control peptide 4NGG (300 μM, 2 h). The percentages refer to Annexin-V-positive or Annexin-V-positive/PI-positive staining. (B) B cells from healthy donors (n = 8) and CLL patients (n = 20) were treated and analyzed as in (A) and graphed. The panel of patients used here included an equal number of CLL patients with Binet Stage A and Binet Stage B/C. Cell death refers to Annexin-V-positive/PI-positive labeling. (C) The proteolytic stabilities of 4N1K and PKHB1 were evaluated in human serum at the indicated times. A mixture of peptide and human plasma was incubated at 37°C, and the kinetics of degradation was followed using HPLC. The relative concentrations of the remaining soluble peptides were analyzed by the integration of the absorbance at 220 nm as a function of retention time. (D) Cell death, measured as Annexin-V and PI co-positivity, was assessed as in (A) in leukemic cells treated with different concentrations of 4N1K or PKHB1. The data in the plot are the mean ± standard deviation (SD) (n = 5). (E) After treatment with the indicated concentration of 4N1K or PKHB1 for the indicated time, cell viability was assessed in the CLL cells using Annexin-V-positive/PI-positive staining. The data are graphed as mean ± SD (n = 6). (F) Cell death induced by PKHB1 (200 μM, 2 h) was measured in B cells from 20 healthy donors and the cohort of 80 CLL patients described in Tables 1–3. The percentages refer to the mean of the Annexin-V-positive/PI-positive staining. Statistical significance was analyzed with the t-test. (G) Cell death was measured as in (F) in PKHB1-treated CD19−/CD3+ T cells (T cells), CD19+/CD5− B cells (residual B cells), and CD19+/CD5+ B lymphocytes (CLL cells) from CLL patients. The data, which refer to Annexin-V and PI co-positivity, are presented as mean ± SD (residual and leukemic B cells, n = 20; T cells, n = 8). Unless otherwise indicated, the statistical analyses included in this figure were performed with the Mann-Whitney test. The major weakness in the use of peptides as therapeutic agents is their short in vivo half-life due to protease degradation. Using an HPLC approach, we observed that a 1-h incubation in human serum resulted in more than 90% of the 4N1K peptide being degraded (Fig. 1C). For that reason, we sought to improve 4N1K stability in human serum by replacing selected natural L amino acids with their D counterparts [49]. The N - and C-terminal lysines of 4N1K, which were introduced to improve solubility, are not related to the CD47 interaction site of the peptide (VVM motif) [50]. Therefore, we replaced these two terminal residues with their D analogues. This novel decapeptide, PKHB1, was not degraded during long-term incubation in human serum (Fig. 1C), but maintained its solubility and the ability to bind CD47 (S2 Fig). This specific binding was validated by the disruption of the PKHB1–CD47 interaction with hSIRPα-Fc (a fusion protein designed to specifically bind CD47 [40,41]), which led to the inhibition of PKHB1-mediated PCD (S3 Fig). Finally, as was expected based on its serum stability and the improved affinity to CD47 (Figs. 1C and S2), PKHB1 induced PCD in the CLL cells more potently than 4N1K did, in terms of both concentration level and incubation times. For example, at 2 h of treatment with 200-μM peptides, PKHB1 induced cytotoxicity in ~49% of the CLL cells, whereas 4N1K induced PCD in only ~25% of the leukemic B cell population (Fig. 1D and 1E).
The PCD response to PKHB1 was verified in 20 B lymphocyte samples from healthy donors and in B cells obtained from the 80 CLL patients described above and in Tables 1 and 2. This CLL cohort incorporated individuals with positive and adverse prognostic features, including those with Binet Stage B and C; unmutated IGHV; positive ZAP70 and CD38 expression; 11q, 13q, or 17p deletion; or trisomy 12. We used PKHB1 at 200 μM, a concentration that resulted in PCD responsiveness in CLL cells similar to that of 300-μM 4N1K at 2 h of treatment. With this length of treatment, the spontaneous apoptosis of the primary normal and CLL B cells is less than 5%. As shown in Fig. 1F, the PKHB1-treated CLL cells underwent a rapid cell viability loss (a mean of 49%). Similar to 4N1K, PKHB1 had no effect on the B lymphocytes from healthy donors (a mean of 5% cytotoxicity). The significance of this difference was verified by t-test (p < 0.001). More interesting from a future therapeutic perspective, 200-μM PKHB1 treatment for 2 h killed the CD5+ tumor B cells (~50% of Annexin-V-positive/PI-positive cells) while sparing the residual CD5− B lymphocytes and T cells of the CLL patients (~5% and ~10% cytotoxicity, respectively; Fig. 1G). Using a Mann-Whitney test, we observed that the difference in PCD response to PKHB1 of the malignant B cells and the residual CD5− B lymphocytes and T cells of the CLL patients is highly significant (p < 0.001). Overall, these findings suggest that PKHB1 selectively kills the leukemic B cells.
In order to better characterize the effect of the CD47 agonist peptide PKHB1 in high-risk CLL patients, we compared the response of cells with functional and dysfunctional TP53 (characterized in S4 Fig and Table 3) to PKHB1 and the P53-dependent PCD inducer etoposide. In cells with functional TP53, a 12-h etoposide treatment was required to provoke PCD comparable to that obtained after 2 h of incubation with PKHB1. B lymphocytes with dysfunctional TP53, which are resistant to etoposide, were killed after 2 h of incubation with PKHB1 (Fig. 2A). Thus, targeting CD47 with PKHB1 efficiently killed CLL cells, including those from individuals with dysfunctional TP53.
Fig. 2. PKHB1 induces calreticulin exposure and caspase-independent PCD in CLL cells. 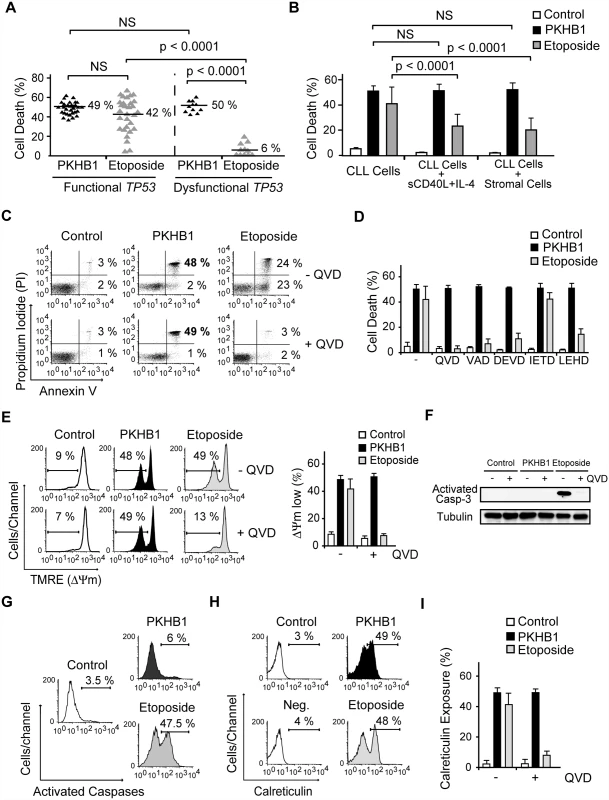
(A) PCD induced by PKHB1 (200 μM, 2 h) or etoposide (250 μM, 12 h) was measured in B cells from 30 CLL patients with functional TP53 and 11 CLL patients with dysfunctional TP53. The percentages refer to Annexin-V-positive/PI-positive staining. (B) Cell death, measured as Annexin-V-positive/PI-positive staining, was detected in CLL cells, CLL cells incubated with sCD40L and IL-4, and CLL cells co-cultured with the bone marrow stromal cell line M210B4 prior to treatment with PKHB1 (200 μM, 2 h) or etoposide (250 μM, 12 h). The data are presented as mean ± SD (n = 8 patients). (C) Cell viability was determined in CLL cells that were pre-incubated with vehicle (−) or QVD (+) and were treated with PKHB1 (200 μM, 2 h) or etoposide (250 μM, 12 h). The percentages refer to Annexin-V-positive or Annexin-V-positive/PI-positive staining. (D) PKHB1- or etoposide-mediated cell death was evaluated by Annexin-V and PI staining in CLL cells pretreated with a panel of broad spectrum or specific caspase inhibitors. The data, which refer to Annexin-V-positive or Annexin-V-positive/PI-positive labeling, are graphed as mean ± SD (n = 7). (E) ΔΨm loss was induced by PKHB1 (200 μM, 2 h) or etoposide (250 μM, 12 h) in CLL cells pretreated with vehicle or QVD. The data are plotted as mean ± SD (n = 7). (F) A representative immunoblot of activated caspase-3 is shown for the untreated (control) and PKHB1- or etoposide-treated CLL cells pre-incubated with vehicle (−) or QVD (+). Equal loading was confirmed by α-tubulin probing. (G) B lymphocytes from a representative CLL donor were left untreated (control) or were incubated with PKHB1 (200 μM, 2 h) or etoposide (250 μM, 12 h) before assessment of caspase activity with a FAM caspase detection kit. The percentages refer to positive staining. (H) Calreticulin exposure was assessed in untreated (control) and PKHB1- or etoposide-treated CLL cells. The percentages refer to calreticulin-positive cells. Note that, in the absence of α-calreticulin, the control isotype antibody yields negative results (“Neg.” in the cytofluorometric plot). (I) Calreticulin exposure was ascertained in untreated (control) and PKHB1- or etoposide-treated CLL cells in the absence (−) or presence (+) of the caspase inhibitor QVD. The data are presented as mean ± SD (n = 9). Statistical analyses in (A) and (B) were performed using one-way ANOVA. NS, not significant. Next, given that the microenvironment plays a critical role in the progression and drug resistance of tumors [51,52], we analyzed whether PKHB1-mediated PCD is modulated by the presence of either bone marrow stromal cells or sCD40L and IL-4, two anti-apoptotic cytokines that are generated by lymphoid tissues. Under these conditions, we observed that the responsiveness of CLL cells to PKHB1 remained unchanged (Fig. 2B). In contrast, as corroborated by one-way ANOVA (p < 0.001), the induction of PCD by etoposide was significantly diminished. These data strongly suggest that, contrary to other forms of cell death, PKHB1-mediated cell death is not down-regulated by the survival stimuli provided by the lymphocyte microenvironment.
PKHB1 Induces Caspase-Independent Programmed Cell Death in Chronic Lymphocytic Leukemia Cells
We next assessed the mechanism regulating PKHB1-induced PCD in CLL cells. In contrast to etoposide-induced caspase-dependent PCD, the features of PKHB1-induced killing—including phosphatidylserine exposure, cell viability loss, and ΔΨm disruption—were not prevented by pre-incubation with broad spectrum or specific caspase inhibitors (Fig. 2C–E). The main effector caspase-3 remained an inactive pro-enzyme after the CLL cells were treated with PKHB1 (Fig. 2F). Thus, it seems that this CD47 peptide agonist induces caspase-independent PCD. This result was substantiated with the help of a FAM-labeled analogue that binds to the active site of the caspases. In contrast to etoposide triggering of cell death (~47% of positive cells), after PKHB1 triggering, the leukemic CLL cells displayed low caspase labeling (6% of positive cells) (Fig. 2G). Finally, as shown in Fig. 2H and 2I, PKHB1 also provoked a caspase-independent exposure of calreticulin, a protein that enables phagocytes to efficiently engulf dead cells [53]. Note that, in contrast to the constitutive expression of calreticulin in acute leukemia cells and solid tumors [54], this protein was not detected on the surface of untreated CLL cells (“control” in Fig. 2H). Thus, PKHB1 activated a caspase-independent PCD path that provoked calreticulin exposure on the surface of the dying cells. In vivo, such exposure would be expected to enable the dying cells to be recognized and engulfed [53].
PKHB1 Provoked Programmed Cell Death in Chronic Lymphocytic Leukemia Cells via Endoplasmic Reticulum Stress, Ca2+ Overload, and Mitochondrial Damage
From the above experiments, we learned that PKHB1 treatment triggers PCD in leukemic but not in normal B lymphocytes. Interestingly, using flow cytometry and immunoblot analyses, we did not see a correlation between PCD response to PKHB1 and the level of CD47 expression on the cell surface of the malignant and normal B cells (Fig. 3A–C). Therefore, we searched for the specific PCD mechanism that could be activated by PKHB1 in CLL cells, but not in normal B lymphocytes.
Fig. 3. PKHB1 generates endoplasmic reticulum stress that provokes Ca2+-mediated PCD in the CLL cells. 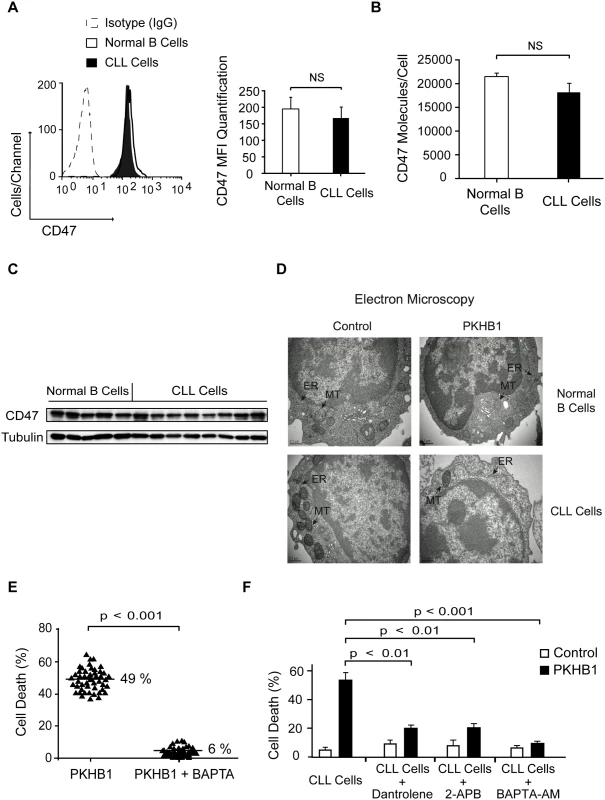
(A) CD47 expression was quantified using flow cytometry in normal and CLL B cells. A representative cytofluorometric plot is presented. In the bar chart, CD47 was quantified based on the MFI in each sample. The data are presented as mean ± SD (normal B cells, n = 20 healthy donors; CLL cells, n = 50 patients). (B) The cell surface expression of CD47 was quantified using a QuantiBRITE flow cytometry system. The number of CD47 molecules/cell in normal (n = 5 healthy donors) and CLL (n = 12 patients) B cells is plotted. (C) CD47 expression in normal and CLL B lymphocytes was determined by immunoblot analysis. Equal loading was confirmed by α-tubulin probing. (D) Representative electron micrographs of untreated (control) or 200-μM PKHB1-treated normal and CLL B cells (2 h of treatment). The black arrows denote the endoplasmic reticulum (ER) and the mitochondria (MT). Bar: 0.5 μm. (E) Cell death was measured in PKHB1-treated B cells (200 μM, 2 h) from 50 CLL patients pre-incubated with vehicle or the external Ca2+ chelator BAPTA. The percentages refer to the mean of the Annexin-V-positive/PI-positive staining. (F) Cell death was measured by Annexin-V-positive/PI-positive labeling in PKHB1-treated CLL cells (200 μM, 2 h) pre-incubated with vehicle, dantrolene, 2-APB, or BAPTA-AM. The data are plotted as mean ± SD (n = 10). Statistical relevance was assessed with the Mann-Whitney test in (A) and (B) and the t-test in (E) and (F).NS, not significant. The absence of caspase activation observed after CD47 peptide triggering of cell death led us to evaluate the potential role of serpases, the F-actin cytoskeleton, and the fission protein DRP1 in PKHB1-mediated PCD in CLL cells. These three elements are key in the regulation of the caspase-independent cell death process induced in CLL cells by the immobilized anti-CD47 mAb B6H12 [24,55,56]. Using a fluorochrome-labeled analogue, we corroborated that, after PKHB1 triggering, the leukemic CLL cells activated the serpase family of proteases (~45% of cells showed positive serpase labeling) (S5A Fig). To analyze the F-actin cytoskeleton, we measured the intracellular F-actin/G-actin ratio (fibrillar actin = polymerized actin; globular actin = depolymerized actin) [57] in PKHB1-treated cells. This approach showed that, similar to the immobilized anti-CD47 mAb B6H12, PKHB1 ligation provoked actin depolymerization. Interestingly, inhibition of the serpases by TPCK controlled actin damage (S5B Fig). These data suggest a hierarchical relationship between serpases and F-actin depolymerization in PKHB1-mediated PCD. Surprisingly, in contrast to the immobilized anti-CD47 mAb [55], the presence of the cell death effector DRP1 was not observed in the mitochondria of PKHB1-treated CLL cells (S5C Fig). Thus, it seems that PKHB1 and the immobilized anti-CD47 mAb B6H12 induce different types of caspase-independent killing in the CLL cells.
Because morphological alterations in intracellular organelles correlate with the different forms of PCD [58], we next performed an ultra-structural analysis of PKHB1-treated CLL cells. We observed that PKHB1 treatment induced a significant dilation of the endoplasmic reticulum (ER) in leukemic CLL cells, but not in normal B lymphocytes. Moreover, in contrast to immobilized anti-CD47 mAb [55], no morphological changes or swelling were detected in the mitochondria of PKHB1-treated CLL cells (Fig. 3D). Since the ER plays a key role in modulating Ca2+ mobilization [59], we considered a potential role of Ca2+ in PKHB1-mediated PCD. This role was confirmed by pretreating CLL cells with the Ca2+ chelator BAPTA prior to incubation with PKHB1. As depicted in Figs. 3E and S5, this pretreatment abolished the features characterizing PKHB1-mediated killing. A similar result was obtained by incubating CLL cells in a Ca2+-free medium prior to treatment with PKHB1 (S5D Fig). Moreover, as corroborated by a t-test, pre-incubation with BAPTA-AM (intracellular Ca2+ chelator; p < 0.001), 2-APB (inositol-1,4,5-triphosphate receptor [IP3R] inhibitor; p < 0.01), or dantrolene (ryanodine receptor inhibitor; p < 0.01) significantly decreased PKHB1-mediated death (Fig. 3F). Altogether, these findings suggest that PKHB1 treatment induces ER stress, which provokes Ca2+ overload and PCD in CLL cells.
Next, we compared Ca2+ mobilization in normal and CLL B lymphocytes using a fluorescence video-microscopy technology that analyzes the Ca2+ signal in single cells. In normal B cells, PKHB1 triggered a classical Ca2+ signal with a rapid transient increase in intracellular Ca2+ that then returned to baseline [59,60] (Fig. 4A, left panel, and S6A Fig, upper panels). In CLL cells, PKHB1 incubation triggered a strong and sustained Ca2+ mobilization that did not return to basal level (Fig. 4A, right panel, and S6A Fig, lower panels). Thus, PKHB1 seems to provoke a different Ca2+ mobilization in normal and leukemic cells. To determine whether the sustained Ca2+ mobilization observed in PKHB1-treated CLL cells was the consequence of a sustained Ca2+ influx or of persistent Ca2+ release from intracellular stores, we analyzed the Ca2+ mobilization generated by PKHB1 in cells incubated in a Ca2+-free medium. In PKHB1-treated normal B lymphocytes, Ca2+ released from internal stores returned to basal levels. However, in CLL cells, PKHB1 incubation triggered a sustained Ca2+ release from internal stores, which did not return to basal level (Figs. 4B and S6B). Overall, these data indicate that the Ca2+ overload observed in PKHB1-treated CLL cells appears to be the consequence of continuous liberation of Ca2+ from the ER. Moreover, Ca2+ mobilization and PCD increased in PKHB1-treated CLL cells in a dose-dependent manner (Figs. 4C and 1D). These results strongly support a direct link between Ca2+ mobilization and induction of PKHB1-mediated PCD in CLL.
Fig. 4. PKHB1 treatment results in sustained Ca2+ mobilization that induces mitochondrial damage and PCD in CLL cells. 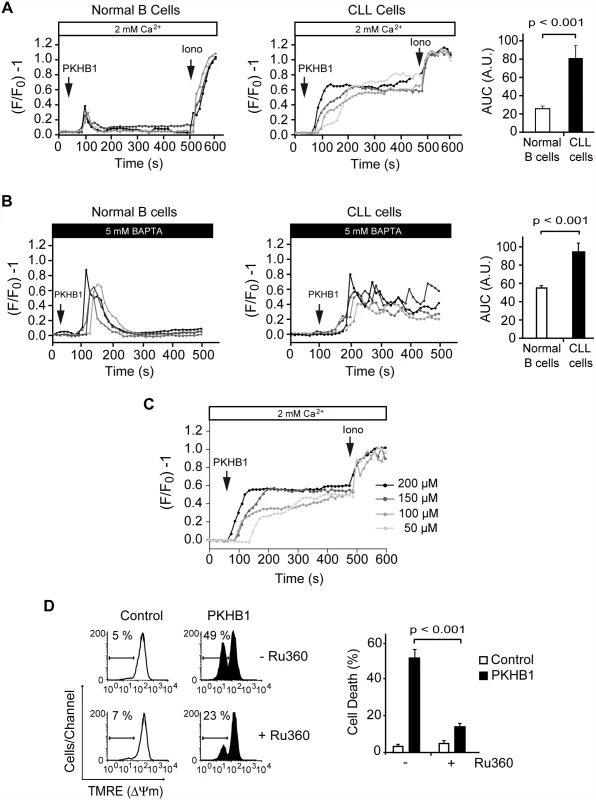
(A) Representative Ca2+ mobilization recorded in 200-μM PKHB1-treated normal (left) and CLL (right) B cells (n = 4 each). Ionomycin (Iono, 1 μM) was utilized as a control to show the maximum response. The histograms show mean ± SD of the area under the curve (AUC) (in arbitrary units [A.U.]) (n = 12). (B) Representative Ca2+ release from internal stores was visualized in PKHB1-treated normal (left) and CLL (right) B cells (n = 4 each) in Ca2+-free medium (plus 5 mM BAPTA). Histograms represent mean ± SD of the area under the curve (n = 13). (C) Representative Ca2+ mobilization curves were recorded in CLL cells after treatment with different concentrations of PKHB1. Ionomycin (1 μM) was used as a control to show the maximum response. (D) The loss of ΔΨm induced by PKHB1 (200 μM, 2 h) was measured in CLL cells pre-incubated with vehicle (−) or the mitochondrial Ca2+ uniporter inhibitor Ru360 (+). Representative cytofluorometric plots are shown. The percentages refer to cells with low ΔΨm. The data from eight patients are presented in a plot as mean ± SD. The Mann-Whitney test was used in (A) and (B) and the t-test in (D). An important question arising from our above data is how the PKHB1-induced sustained Ca2+ mobilization affects CLL cell viability. Because regulated Ca2+ entry into the mitochondria is required to maintain intracellular Ca2+ homeostasis [61], we analyzed whether the sustained Ca2+ mobilization induced by PKHB1 treatment in CLL cells affected the mitochondria. As shown in Fig. 4D, a t-test analysis indicated that the pharmacological blockade of Ca2+ entry into the mitochondria using the mitochondrial Ca2+ uniporter inhibitor Ru360 significantly moderated the ΔΨm loss induced by PKHB1 (49% of cells showed ΔΨm loss in Ru360-untreated cells, whereas only 23% of cells pre-incubated with Ru360 presented ΔΨm loss; p < 0.001), thus preventing PCD. Therefore, it seems that treatment of CLL cells with PKHB1 induces a Ca2+ overload that provokes PCD via mitochondrial damage.
PLCγ1 Is Over-Expressed in Chronic Lymphocytic Leukemia Cells, with Increased Expression Correlated to Disease Progression
In order to unravel the molecular mechanism responsible for the sustained Ca2+ mobilization leading to PCD in CLL cells—but not in normal B lymphocytes—treated with PKHB1, we performed a quantitative RT-PCR analysis in normal (n = 11) and CLL (n = 50) B cells of the major genes involved in the regulation of cellular Ca2+ homeostasis [59]. Out of the 17 different genes tested, only PLCG1 mRNA was found to be over-expressed by more than 3-fold in CLL cells compared to normal B lymphocytes (Fig. 5A; Table 4). This difference in expression is highly significant, as verified by t-test (p < 0.001). The immunoblot assessment depicted in Fig. 5B corroborated the different expression of PLCγ1 in normal and CLL B cells. Because the panel of PLCG1 mRNA expression in CLL cells was quite scattered (Fig. 5A), we wondered whether there was a correlation between the level of PLCG1 mRNA and progression of the disease. The statistical t-test performed in 50 individuals with CLL indicated, with p < 0.001, that more PLCG1 mRNA is expressed in patients with advanced disease (Binet Stage B/C) than in patients with indolent CLL (Binet Stage A) (Fig. 5C, left panel). This finding is further supported by results obtained in B cells from patients with unfavorable CLL evolution. In these patients, PLCG1 mRNA was found to be over-expressed in CLL cells obtained at Binet Stage B/C of diagnosis, compared to CLL B lymphocytes purified when the patient was diagnosed at Binet Stage A (Fig. 5C, right panels). Altogether, these data indicate that the expression of PLCG1 mRNA could be considered a marker of CLL severity.
Fig. 5. PLCγ1 is over-expressed in CLL cells. 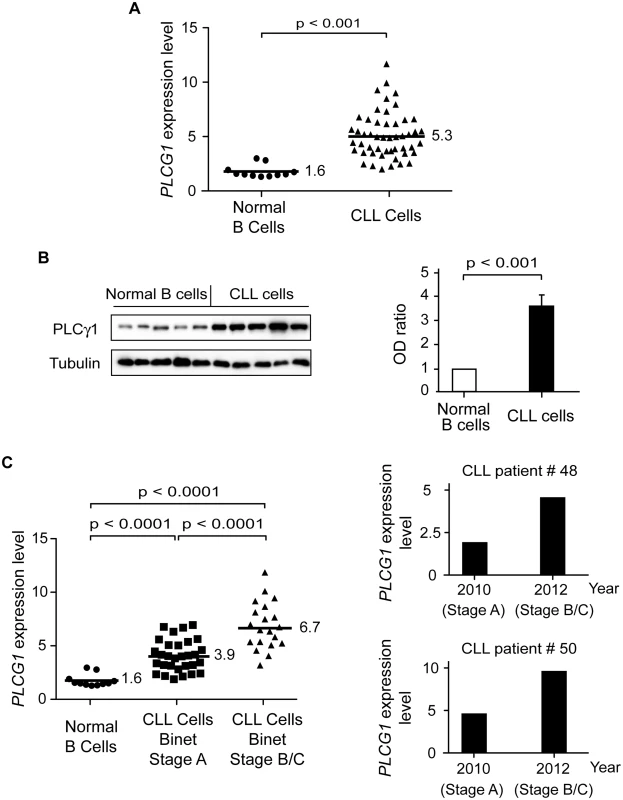
(A) PLCG1 mRNA levels were determined in normal (n = 11) and CLL (n = 50) B cells. The numbers refer to mean PLCG1 transcript expression. GUSB mRNA expression was used to normalize the data. (B) PLCγ1 was detected by immunoblot analysis in normal and CLL B lymphocytes. Equal loading was confirmed by α-tubulin detection. The optical density (OD) ratio represents the difference in protein expression. The plot depicts mean ± SD (n = 3 independent blots). (C) Left: PLCG1 mRNA levels measured in the normal and leukemic B cells used in (A) are shown by the clinical Binet stage of the CLL patients. The numbers refer to mean PLCG1 mRNA expression. Right: PLCG1 mRNA levels measured in patients #48 and #50 at different times. In 2010, both CLL patients were classified as having Binet Stage A, and in 2012 as having Binet Stage B/C. Note that the PLCG1 mRNA levels measured in these patients correlate with CLL progression. GUSB mRNA expression was used to normalize the data. The t-test was used in (A) and (C), and the Mann-Whitney test was used in (B). Tab. 4. Transcript expression levels of Ca2+-related signaling molecules. 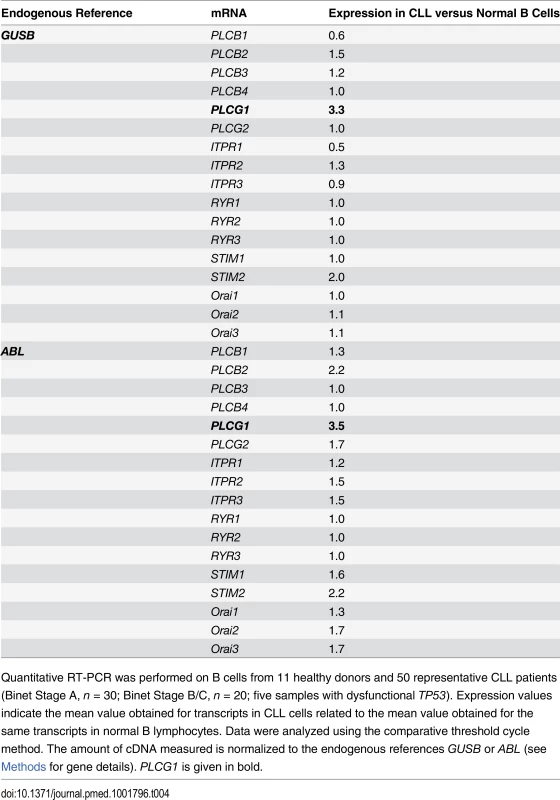
Quantitative RT-PCR was performed on B cells from 11 healthy donors and 50 representative CLL patients (Binet Stage A, n = 30; Binet Stage B/C, n = 20; five samples with dysfunctional TP53). Expression values indicate the mean value obtained for transcripts in CLL cells related to the mean value obtained for the same transcripts in normal B lymphocytes. Data were analyzed using the comparative threshold cycle method. The amount of cDNA measured is normalized to the endogenous references GUSB or ABL (see Methods for gene details). PLCG1 is given in bold. The Sustained Activation of PLCγ1 Controls PKHB1-Mediated Programmed Cell Death in Chronic Lymphocytic Leukemia Cells
PLCγ1 catalyzes the formation of IP3, which binds to its receptors on the ER and, subsequently, triggers store-operated Ca2+ release. PLCγ1 is over-expressed in CLL; therefore, the disparate Ca2+ mobilization recorded in the PKHB1-treated normal and CLL B cells could be related to the differential activation of PLCγ1. Measuring PLCγ1 activation by its phosphorylation at Y783 [62], we observed that PLCγ1 was rapidly phosphorylated before returning to basal levels in the PKHB1-treated normal B cells. However, PLCγ1-Y783 phosphorylation in the PKHB1-treated CLL cells remained high for at least 2 h (Fig. 6A). Note that the kinetics of PLCγ1 phosphorylation matched the kinetics of Ca2+ response measured in the normal and CLL B cells (Fig. 4A and 4B). Next, by measuring IP1 production in the PKHB1-treated CLL cells, we validated that the sustained PLCγ1-Y783 phosphorylation correlated with the increased catalytic activity of the protein in the CLL B cells (Fig. 6B). Moreover, IP1 production following PKHB1 incubation was reduced using the PLC inhibitor U73122 (Fig. 6C), emphasizing the correlation between the over-activation of PLCγ1 and the Ca2+ overload measured in CLL cells. Consequently, as indicated by t-test (p < 0.01), the pre-incubation of CLL cells with U73122 prior to PKHB1 treatment significantly decreased PCD (a mean of ~50% Annexin-V/PI co-positivity was measured in U73122-untreated cells and a mean of ~18% in B lymphocytes pre-incubated with U73122) (Fig. 6D).
Fig. 6. PLCγ1 controls PKHB1-induced PCD. 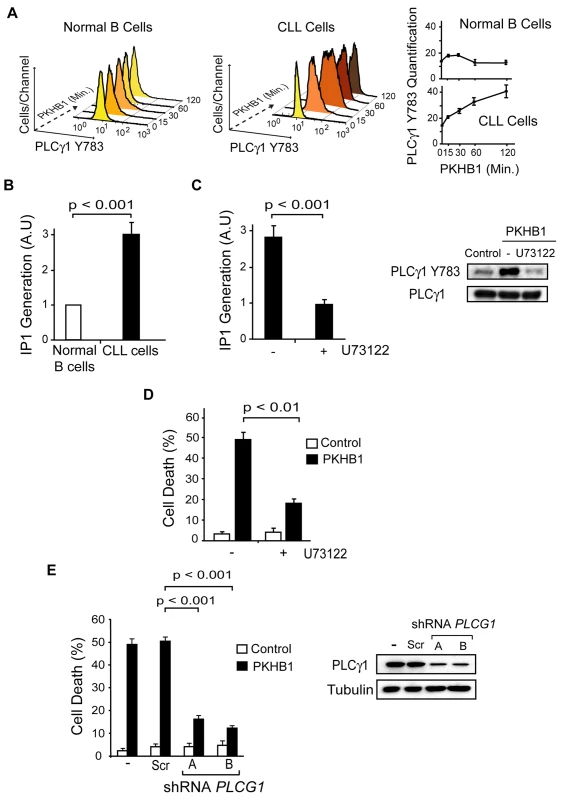
(A) PLCγ1-Y783 phosphorylation was detected at different times in the 200-μM PKHB1-treated normal and CLL B lymphocytes. Representative flow cytometry histograms are shown. In the right panel, the plotted data are presented as MFI ± SD (n = 4). (B) IP1 was quantified in 200-μM PKHB1-treated normal and CLL B cells at 2 h. The histogram depicts mean ± SD (n = 7). (C) IP1 generation was determined in 200-μM PKHB1-treated CLL B lymphocytes pre-incubated with vehicle or the PLC inhibitor U73122. The data in the plot are mean ± SD (n = 5). Immunoblot analysis confirmed that U73122 inhibited PLCγ1-Y783 phosphorylation in PKHB1-treated cells (200 μM, 2 h). Equal loading was confirmed by PLCγ1 probing. (D) Cell death was measured by Annexin-V-positive/PI-positive staining in untreated (control) and PKHB1-treated CLL cells (200 μM, 2 h) pre-incubated with vehicle or U73122. The data are presented as mean ± SD (n = 10). (E) The effects of PLCG1 down-regulation on CD47-mediated PCD were assessed in PKHB1-treated CLL B cells (200 μM, 2 h) transduced with scrambled shRNA (Scr) or two shRNAs targeting PLCG1 (shRNA A and B). Cell death, measured by Annexin-V-positive/PI-positive staining, was plotted as mean ± SD (n = 6). Changes in PLCγ1 expression were observed by immunoblot analysis. Equal loading was confirmed by α-tubulin detection. Statistical analyses were performed with the Mann-Whitney test in (B) and (E) and the t-test in (C) and (D). In addition to the above pharmacological approach, we assessed the role of PLCγ1 in PKHB1-mediated PCD in primary CLL cells using lentiviral down-regulation of PLCG1 with two independent shRNAs. As shown in Fig. 6E, the Mann-Whitney test (p < 0.001) indicated that the down-regulation of PLCG1 significantly diminished PKHB1-induced PCD in the primary CLL lymphocytes (~50% Annexin/PI co-positivity in control or scramble-transducted cells and less than 20% in B lymphocytes transducted with a shRNA PLCG1). Overall, these findings strongly support the key role of PLCγ1 in the PCD mediated by CD47 peptide targeting in CLL cells.
Treatment with PKHB1 Reduced Chronic Lymphocytic Leukemia Tumor Burden In Vivo
Finally, we analyzed the in vivo effect of PKHB1 on the growth of MEC-1 tumors in NSG mice [63]. MEC-1 is an established CLL cell line with dysfunctional TP53 that is resistant to etoposide treatment but responds to PKHB1 in exactly the same way as primary CLL cells (S7 and S8 Figs.). NSG mice were subcutaneously injected with MEC-1 cells, and the tumors were allowed to grow for 14 d until they reached 100 mm3. The mice were then treated intraperitoneally once a week with vehicle or PKHB1. After 2 wk of treatment, the PKHB1-treated, but not the vehicle-treated, mice had a significantly decreased tumor growth rate (~50% tumor volume diminution in PKHB1-treated mice compared to vehicle-treated mice; Fig. 7A, left panel, and Fig. 7B; Mann-Whitney test, p < 0.05). However, as could be expected from the instability of the peptide, 4N1K treatment was ineffective (Fig. 7A, right panel). No blood, liver, or kidney toxicity was found in PKHB1-treated mice (S9 Fig). Analysis of the tumors from the PKHB1 - and vehicle-treated mice shows similar hemoglobin levels within the engrafted tumors, indicating that PKHB1 did not provoke anemia (Fig. 7C). Moreover, anti-CD31 labeling performed in the tumors from the PKHB1 - and vehicle-treated mice suggested that the decreased tumor growth rate induced by PKHB1 was not a consequence of an anti-angiogenic effect (Fig. 7D). Indeed, compared to mice receiving vehicle, the mice receiving PKHB1 showed significantly increased levels of PLCγ1-Y783 phosphorylation (Fig. 7E), as well as enhanced calreticulin exposure (Fig. 7F) and a loss of cell viability (Fig. 7G) within the engrafted tumors (Mann-Whitney test, p < 0.001). Together, these findings strongly suggest that treatment with PKHB1 eliminates CLL cells in vivo by inducing PLCγ1-mediated PCD.
Fig. 7. PKHB1 reduced in vivo CLL tumor burden by inducing PLCγ1 activation and PCD. 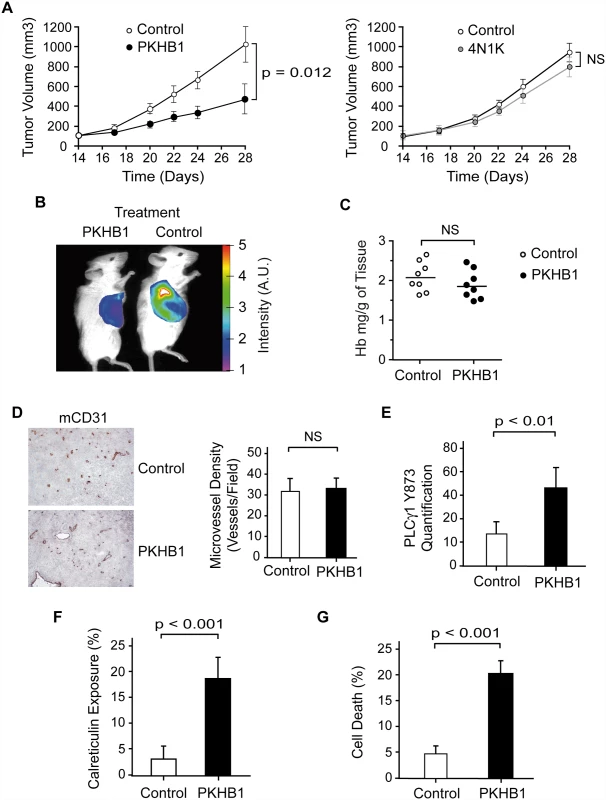
(A) NSG mice were subcutaneously transplanted with MEC-1 cells. Starting 14 d after the engraftment, the mice received a weekly intraperitoneal injection of vehicle (control), PKHB1 (left panel), or 4N1K (right panel). Tumor volume was measured using a caliper and graphed. The data are presented as mean ± SD (n = 8). In contrast to vehicle or 4N1K treatment, PKHB1 treatment significantly reduced the tumor volume. (B) In an experiment similar to (A), tumor growth was visualized by measuring glucose uptake. The color scale indicates the fluorescence intensity. (C) The hemoglobin (Hb) concentration (n = 8 per group) was assessed in vehicle- (control) or PKHB1-treated tumors. The bars indicate the mean of the data obtained. (D) Tumor vascularity was investigated by immunohistochemistry analysis of mCD31. Representative photographs and visual quantification of microvessel density (right panel) confirmed similar vascularity in tumors from vehicle- and PKHB1-treated mice. The data are presented as mean ± SD. (E) PLCγ1-Y783 phosphorylation was assessed in tumors obtained from vehicle- (control) and PKHB1-treated mice. Phospho-PLCγ1 was quantified by flow cytometry using MFI. The data are presented as mean ± SD (n = 6). (F) Calreticulin exposure was assessed in tumors from vehicle- (control) and PKHB1-treated mice and graphed. The data are presented as mean ± SD (n = 6). (G) Percentage of cell death was measured by Annexin-V-positive/PI-positive staining in tumors obtained from vehicle- (control) and PKHB1-treated mice. The data are plotted as mean ± SD (n = 8). The statistical analyses included in this figure were performed with the Mann-Whitney test. A.U., arbitrary units; NS, not significant. Discussion
In this work we describe the targeting of CD47 by serum-stable TSP1-derived peptides as a novel approach that could be used to broadly eliminate malignant CLL B cells. We performed in vitro cellular and molecular biology assessments in primary CD5+ B lymphocytes obtained from a cohort of 80 CLL patients, and we assessed, in a CLL-xenograft mouse model, the in vivo capacity of the CD47 agonist peptides to reduce tumor burden. Our in vitro approach shows that the CD47 peptide agonists enable a Ca2+-mediated, caspase-independent PCD pathway that, sparing the normal T and B lymphocytes, efficiently kills CLL B cells, including those from drug-refractory patients (e.g., with dysfunctional TP53). This PCD pathway, to our knowledge molecularly described here for the first time, involves a sequence of events initiated by the triggering of CD47 by serum-stable peptide agonists and the subsequent activation of the signal transduction protein PLCγ1, an over-expressed protein in CLL. Further, PLCγ1 activation leads, by means of the second messenger IP3, to ER stress, cellular Ca2+ overload, mitochondrial damage, and leukemic B cell death (Fig. 8). The in vivo data obtained in the CLL-xenograft mouse model demonstrated that, by inducing PLCγ1-mediated, caspase-independent PCD, the injection of CD47 agonist peptides significantly reduced tumor burden.
Fig. 8. Schematic representation of CD47-mediated PCD in CLL cells. 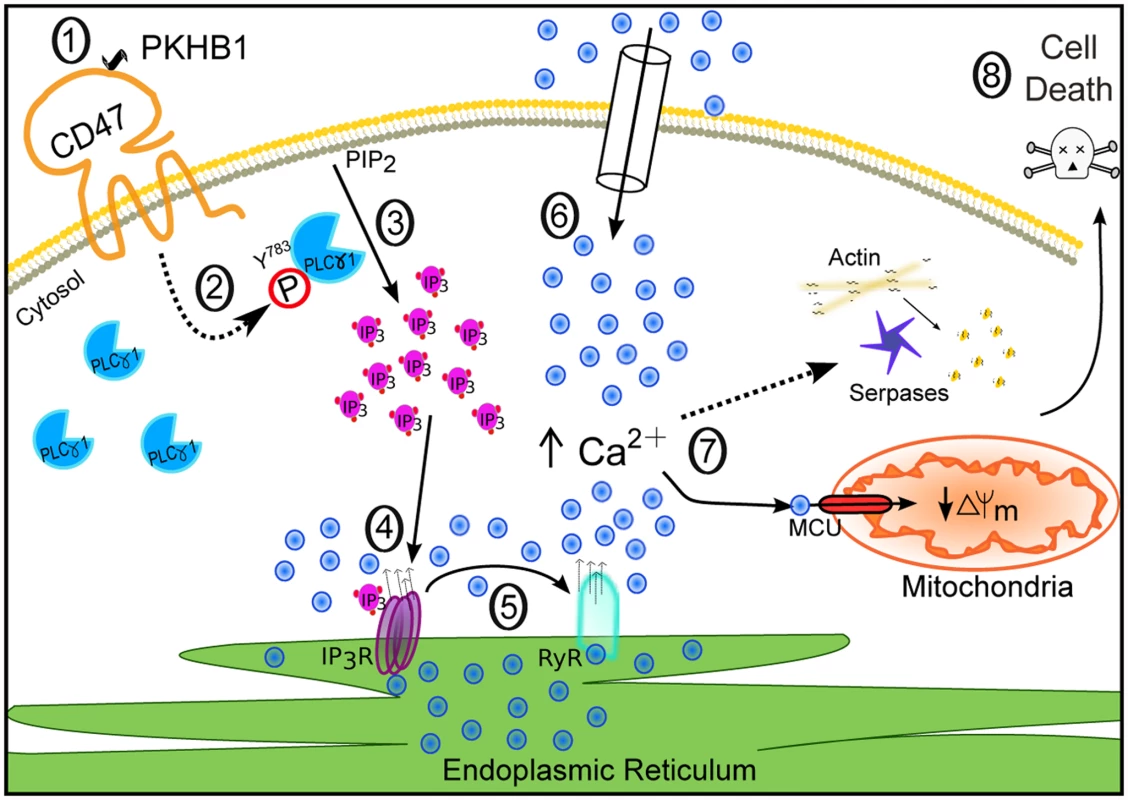
CD47 ligation by the agonist peptide PKHB1 (1) leads to activation of the over-expressed second messenger PLCγ1 by phosphorylation at Y783 (2). PLCγ1-Y783 cleaves phosphatidylinositol 4,5 bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) (3), which binds IP3Rs in the ER to provoke Ca2+ release (4). The increased intracellular Ca2+ concentration activates the ER RYRs (5). The decrease of Ca2+ in the ER results in the opening of store-operated Ca2+ channels in the plasma membrane, provoking substantial calcium entry into the cell (6). This high Ca2+ concentration provokes serpase activation, actin depolymerization, and mitochondrial damage (7), followed by PCD (8). Strategies to Target CD47 for Chronic Lymphocytic Leukemia Treatment
A number of anticancer approaches attempting to kill tumor cells by phagocytosis, antibody-dependent cell-mediated cytotoxicity, or PCD have relied on CD47 targeting by specific mAbs [15,20,64–66]. For CLL, a monoclonal single-chain variable fragment that induces PCD [27] and a therapy that favors the disruption of the CD47–SIRPα link have been proposed [67]. This latter strategy requires the complementary use of rituximab (anti-CD20) to efficiently eliminate tumor cells from a mice xenograft model [67]. In previous research, mainly performed with an immobilized anti-CD47 mAb (B6H12), it has been reported that it is possible to induce PCD in vitro in CLL B cells by CD47 triggering [23]. However, as reported in this previous work and confirmed by our current data, when used in vitro in soluble conditions, B6H12 is unable to directly induce PCD in CLL B lymphocytes [23] (S1 Fig). With this work in mind, we developed PKHB1, a serum-stable CD47 agonist peptide that, in soluble conditions, induces PCD in malignant CLL B cells. Moreover, we demonstrated that the stabilization of TSP1-derived peptides against serum degradation increases both in vitro and in vivo biological PCD activity. Overall, our findings strongly support the idea that the targeting of CD47 with peptide agonists could offer medical advantages, such as specificity in provoking PCD in the leukemic CD5+ B cells, no detected resistance, and a lack of microenvironment down-regulation. Moreover, as opposed to therapeutic antibodies, peptides and small molecules can be synthesized with a better yield and cost, are less likely to induce immune responses, and do not accumulate in the kidney or liver, thus minimizing toxic side effects [68]. All of these characteristics highlight the viability of the proposed peptide-based approach.
In contrast to the previously described TSP1-derived peptide 4N1K, which induces cell death only in vitro [23,34–37], PKHB1 reduces in vivo tumor burden in a CLL mouse model. To generate our in vivo data, we used one of the few CLL-xenografted mouse models described to date [63], a model widely used in cancer research. Although it does not recreate the physiopathology of human CLL, this CLL-xenograft mouse model fully supports our in vitro observations, especially because, contrary to 4N1K, PKHB1 is stable and can reach the xenografted tumor. We also observed in this mouse model that PKHB1 reduced tumor burden by inducing PLCγ1-mediated PCD and that PKHB1 did not induce apparent toxicity in the tumor-engrafted mice.
Mechanisms of PKHB1-Mediated Cell Death
The targeting of CD47 by peptide agonists enables a form of B cell death that, following the classification of the Nomenclature Committee on Cell Death [69], could be defined as “type III-PCD” or “programmed necrosis.” More precisely, three main hallmarks allow us to classify PKHB1-mediated PCD into the “programmed necrosis” group. First, the killing induced by PKHB1 in the CLL B lymphocytes can be considered “programmed” because it activates the enzymatic machinery of the cell (e.g., by inducing PLCγ1 sustained phosphorylation). Second, PKHB1 enables in the malignant B cells a caspase-independent form of PCD, and not a typical caspase-dependent apoptotic pathway. Finally, the targeting of CD47 by PKHB1 generates biochemical and morphological necrotic features—such as Annexin-V-positive/PI-positive staining and the swelling of intracellular organelles (e.g., the ER) [23,55,69,70]—in the CLL B lymphocytes. As such, PKHB1-mediated cell death shares common features with the mode of PCD induced by the immobilized anti-CD47 mAb B6H12 [23,24,55,56,71]. Certain biochemical features are conserved, namely double Annexin-V-positive/PI-positive staining, activation of the serpase family of proteases, and F-actin network disruption. In addition, both forms of PCD are marked by a dependence on input from several cellular compartments, including the mitochondrion. However, our data suggest that PKHB1-mediated PCD takes place in CLL cells via the induction of cellular alterations that are regulated by the ER and the signal transduction protein PLCγ1, but are independent from the fission protein DRP1, one of the major effectors of killing mediated by anti-CD47 mAb [55]. It seems that, as a consequence of this difference, in contrast to PCD mediated by anti-CD47 mAb, PKHB1 treatment does not provoke morphological changes or swelling in the mitochondria of CLL cells. Therefore, PKHB1-mediated killing and anti-CD47-mAb-induced cell death could represent alternate outcomes of a similar necrotic PCD pathway. The relationship between PKHB1-mediated PCD and other ways to induce PCD by CD47 triggering [27]—which seem to implicate the activation of BNIP3/Hif or the opening of the mitochondrial permeability transition pore—needs to be evaluated in more specific work.
In deciphering how the CD47 agonist peptide triggers PCD, we found that PKHB1 provoked Ca2+ overload. The regulation of Ca2+ homeostasis within hematopoietic cells is crucial for their function and fate [72]. According to the current model, Ca2+ influx is the result of the membrane-receptor-mediated production of IP3 following the activation of PLC, which binds to specific receptors on the ER membrane and provokes a quick release of internal Ca2+ that is followed by extracellular Ca2+ entry through a process known as store-operated Ca2+ entry. This Ca2+ signaling is transient and controlled, mainly by Ca2+ re-sequestration into the mitochondria and ER [73]. Our assessment of the intracellular Ca2+ influx after CD47 peptide triggering suggested a totally different pattern of Ca2+ mobilization between normal and leukemic B cells. Although the Ca2+ influx was rapidly controlled in normal B cells, we recorded a massive and sustained Ca2+ mobilization in CLL cells. In these malignant B lymphocytes, the Ca2+ overload was due to sustained activation of PLCγ1. This mechanism of PCD induction, described here to our knowledge for the first time in primary B cells, is very similar to that previously reported for Fas-mediated apoptosis in Jurkat T cells [74].
New Roles for PLCγ1 in Programmed Cell Death and Chronic Lymphocytic Leukemia
Together with other characteristic CLL molecules, such as CD5 or ZAP70, the function of PLCγ1 is mainly associated with the T cell lineage. Indeed, PLCγ1 is critical for T cell function, whereas PLCγ2 is more relevant in platelets, natural killer cells, B cells, and mast cells [75]. In T cells, PLCγ1 is activated downstream of the T cell receptor by a large panoply of SRC, SYK, and TEC kinases. Among them, the SYK-related kinase ZAP70 phosphorylates LAT and SLP76, two adaptors that are critical for PLCγ1 activation. In B cells, cross-linking the BCR results in tyrosine phosphorylation and the activation of PLCγ2 rather than PLCγ1. The SRC, SYK, and TEC kinases also play relevant roles in PLCγ2 activation, which is essential for B cell survival [76]. Related to CLL, IgM—BCR engagement triggers the phosphorylation of SYK, the activation of PLCγ2, and intracellular calcium mobilization [77]. Moreover, some reports have found PLCγ2 to be over-expressed in CLL [78]. In spite of that, our shRNA down-regulation approach indicates that, in PKHB1-mediated PCD, PLCγ1 is the key effector. This original result opens the way to further molecular and cell biology studies. As such, it will be interesting to search for the molecular link between CD47 triggering and PLCγ1-Y783 phosphorylation. As CD47 does not possess tyrosine‐based activation motifs, we hypothesize that the phosphorylation of PLCγ1 after CD47 triggering results from the association of CD47 with other counter-receptors. This association would generate a signalization complex able to (i) activate SRC, SYK, and/or other TEC-related tyrosine kinases and (ii) recruit/phosphorylate PLCγ1. Thus, future work should analyze whether PLCγ1 over-expression plays a role in CLL survival; whether ZAP70 or other SRC, SYK, and TEC kinases are implicated in the sustained PLCγ1-Y783 phosphorylation observed after CD47 peptide triggering; and whether a direct relationship exists between CD47 and BCR or, as in other cell types [79,80], between CD47 and integrins.
Our present study not only highlights a novel PCD role for PLCγ1, but also reveals that PLCG1 mRNA expression correlates with CLL progression. Even if additional work correlating PLCG1 mRNA expression level with other CLL prognostic markers (more than 35 have been described to date) is necessary to confirm the clinical value of these findings, our results indicate that PLCG1 mRNA expression could be a suitable candidate for further consideration as a prognostic marker in CLL.
Clinical Potential for CD47 Targeting
Deficiencies in the PCD program are frequently involved in tumor drug resistance [81]. The design of novel strategies that bypass this blockade is a major challenge in PCD research. In recent years, most PCD-based pharmacological therapies have focused on the caspase-dependent mode of PCD, also known as apoptosis. In CLL, malignant B cells exhibit alterations that make them resistant to this form of cell death (e.g., ATM/P53 inactivation or over-expression of the anti-apoptotic proteins MCL1 or BCL2). However, there are alternative caspase-independent forms of PCD that could be used to eliminate CLL cells [23,24,55]. Our experiments indicated that the binding of CD47 by the peptide agonists provokes caspase-independent PCD with a broad efficacy (e.g., it is effective even in cells with caspase-dependent PCD blockade). Therefore, the induction of PCD by CD47 peptide targeting overcomes the apoptotic avoidance that is characteristic of CLL.
Limitations and Strengths
One of the main limitations of our study is the only moderate affinity of the TSP1-derived peptides to CD47. As a consequence, (i) the induction of PCD in primary CLL B lymphocytes was achieved only at a micromolar concentration, still distant from the standard requirements in drug development (nanomolar range), and (ii) the reduction in tumor burden in our CLL-xenograft mouse model was limited. However, based on our original results on peptide serum stability, we may anticipate that a second set of CD47 peptide agonists will be generated with better efficacy and/or bioavailability than PKHB1 (e.g., obtained by introduction of amino acid surrogates). In addition, it is generally agreed that the current CLL animal models do not fully mimic the situation in humans. Our in vivo studies were based on one of the previously described CLL-xenografted mouse models [63]. This model involves xenotransplanting MEC-1 cells as a solid mass in NSG mice. Given that CLL is a disseminated disease, our mouse model does not strictly recapitulate CLL features. Consequently, we cannot rule out the possibility that the CD47 peptide agonists might behave differently in CLL patients. Thus, complementary preclinical and toxicological studies in more appropriate animal models (e.g., novel CLL mouse models or primates) will be necessary before the therapeutic effect of PKHB1 and derivatives can be fully assessed in humans. Finally, although PLCγ1 over-expression was clearly observed in CLL B cells, the mRNA assessment of PLCG1 was done using samples from 50 CLL patients and 11 healthy donors, which is not enough to determine whether this protein could be considered as a prognostic marker in CLL. A more specific study, with a higher number of samples, correlating PLCG1 mRNA expression level with other CLL prognostic markers will be necessary to verify the clinical value of our findings.
Our work presents several strengths. First, it is a translational study with three parts: (i) generation of PKHB1 (a serum-stable CD47 peptide agonist), (ii) analysis of the biological activity of PKHB1 in vitro on a panel of primary B lymphocytes that reproduced the diversity of CLL patients and incorporated the cytogenetic abnormalities that are associated with CLL refractoriness, and (iii) an in vivo approach to assess the effect of PKHB1 on a CLL mouse model, which revealed that this peptide reduces tumor burden without apparent side effects. Second, from a mechanistic point of view, our work reveals the existence of a Ca2+-mediated, caspase-independent PCD signaling pathway in the tumor cells that could be enabled independently from the classical apoptotic path (e.g., in cells with dysfunctional TP53) and that is not down-modulated by the lymphocyte microenvironment. This mode of PCD efficiently and specifically targets malignant cells. Finally, in investigating the molecular determinants regulating PKHB1-mediated killing, we uncovered an unexpected role for PLCγ1 in PCD and revealed a potential link between PLCG1 mRNA expression and CLL severity. Both within the CLL field and in the broader scope of cancer research, our mechanistic results regarding caspase-independent PCD and PLCγ1 pave the way for the development of novel pharmacological tools that could circumvent the chemotherapy resistance characterizing CLL cells.
Conclusions
Patients with heavily treated and refractory CLL face a critical medical need that is still unmet. The standard front-line therapy against this leukemia (fludarabine, cyclophosphamide, and rituximab), as well as the current alternative treatments (e.g., anti-CD52, optimized anti-CD20, and anti-CD23 antibodies), can generate refractoriness or undesirable side effects [6]. Recently approved in the United States and Europe, PI3K delta and Bruton tyrosine kinase (BTK) inhibitors represent novel anti-CLL approaches that yield apparent durable remission in patients with relapsed or refractory CLL. Yet, the establishment of PCD approaches such as our peptide-based cell death strategy remains of great interest. Our strategy (i) is less expensive to produce, (ii) is specific to the tumor cells (sparing the residual CD5- B lymphocytes and T cells of the patient), (iii) could be broadly used in CLL patients with high-risk genetic lesions, (iv) is not down-regulated by the lymphocyte microenvironment, and (v) does not provoke apparent anemia or toxicity in a CLL mouse model. Overall, our work represents a first step toward the development of a new peptide-based treatment for CLL, which still remains an incurable disease.
Supporting Information
Zdroje
1. Chiorazzi N, Ferrarini M (2003) B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu Rev Immunol 21 : 841–894. 12615894
2. Dighiero G, Hamblin TJ (2008) Chronic lymphocytic leukaemia. Lancet 371 : 1017–1029. doi: 10.1016/S0140-6736(08)60456-0 18358929
3. Pekarsky Y, Zanesi N, Croce CM (2010) Molecular basis of CLL. Semin Cancer Biol 20 : 370–376. doi: 10.1016/j.semcancer.2010.09.003 20863894
4. Martin-Subero JI, Lopez-Otin C, Campo E (2013) Genetic and epigenetic basis of chronic lymphocytic leukemia. Curr Opin Hematol 20 : 362–368. doi: 10.1097/MOH.0b013e32836235dc 23719185
5. Gaidano G, Foa R, Dalla-Favera R (2012) Molecular pathogenesis of chronic lymphocytic leukemia. J Clin Invest 122 : 3432–3438. doi: 10.1172/JCI64101 23023714
6. Gribben JG (2010) How I treat CLL up front. Blood 115 : 187–197. doi: 10.1182/blood-2009-08-207126 19850738
7. Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, et al. (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343 : 1910–1916. 11136261
8. Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, et al. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369 : 32–42. doi: 10.1056/NEJMoa1215637 23782158
9. Burger JA, Chiorazzi N (2013) B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol 34 : 592–601. doi: 10.1016/j.it.2013.07.002 23928062
10. Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, et al. (1996) Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem 271 : 21–24. 8550562
11. Hatherley D, Graham SC, Turner J, Harlos K, Stuart DI, et al. (2008) Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell 31 : 266–277. doi: 10.1016/j.molcel.2008.05.026 18657508
12. Brown EJ, Frazier WA (2001) Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11 : 130–135. 11306274
13. Sarfati M, Fortin G, Raymond M, Susin S (2008) CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Curr Drug Targets 9 : 842–850. 18855618
14. Oldenborg PA (2013) CD47: A cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol 2013 : 614619. doi: 10.1155/2013/614619 23401787
15. Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, et al. (2009) Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284 : 1116–1125. doi: 10.1074/jbc.M804860200 19004835
16. Floquet N, Dedieu S, Martiny L, Dauchez M, Perahia D (2008) Human thrombospondin’s (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: a molecular modeling study. Arch Biochem Biophys 478 : 103–109. doi: 10.1016/j.abb.2008.07.015 18675774
17. Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, et al. (2013) Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 3 : 1673. doi: 10.1038/srep01673 23591719
18. Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, et al. (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142 : 699–713. doi: 10.1016/j.cell.2010.07.044 20813259
19. Zhao XW, Matlung HL, Kuijpers TW, van den Berg TK (2012) On the mechanism of CD47 targeting in cancer. Proc Natl Acad Sci U S A 109: E2843. doi: 10.1073/pnas.1209265109 22923695
20. Chao MP, Weissman IL, Majeti R (2012) The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol 24 : 225–232. doi: 10.1016/j.coi.2012.01.010 22310103
21. Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD (2013) Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep 3 : 1038. doi: 10.1038/srep01038 23301159
22. Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, et al. (2013) Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341 : 88–91. doi: 10.1126/science.1238856 23722425
23. Mateo V, Lagneaux L, Bron D, Biron G, Armant M, et al. (1999) CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med 5 : 1277–1284. 10545994
24. Merle-Beral H, Barbier S, Roue G, Bras M, Sarfati M, et al. (2009) Caspase-independent type III PCD: a new means to modulate cell death in chronic lymphocytic leukemia. Leukemia 23 : 974–977. doi: 10.1038/leu.2008.321 19005478
25. Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V (2005) Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood 106 : 658–667. 15784731
26. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, et al. (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138 : 286–299. doi: 10.1016/j.cell.2009.05.045 19632179
27. Sagawa M, Shimizu T, Fukushima N, Kinoshita Y, Ohizumi I, et al. (2011) A new disulfide-linked dimer of a single-chain antibody fragment against human CD47 induces apoptosis in lymphoid malignant cells via the hypoxia inducible factor-1α pathway. Cancer Sci 102 : 1208–1215. doi: 10.1111/j.1349-7006.2011.01925.x 21401803
28. Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP (1999) CD47 signals T cell death. J Immunol 162 : 7031–7040. 10358145
29. Kosfeld MD, Frazier WA (1993) Identification of a new cell adhesion motif in two homologous peptides from the COOH-terminal cell binding domain of human thrombospondin. J Biol Chem 268 : 8808–8814. 8473325
30. Gao AG, Frazier WA (1994) Identification of a receptor candidate for the carboxyl-terminal cell binding domain of thrombospondins. J Biol Chem 269 : 29650–29657. 7525586
31. Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R (2012) CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119 : 5512–5521. doi: 10.1182/blood-2011-10-386805 22427202
32. Kalas W, Swiderek E, Switalska M, Wietrzyk J, Rak J, et al. (2013) Thrombospondin-1 receptor mediates autophagy of RAS-expressing cancer cells and triggers tumour growth inhibition. Anticancer Res 33 : 1429–1438. 23564783
33. Miyata Y, Watanabe S, Kanetake H, Sakai H (2012) Thrombospondin-1-derived 4N1K peptide expression is negatively associated with malignant aggressiveness and prognosis in urothelial carcinoma of the upper urinary tract. BMC Cancer 12 : 372. doi: 10.1186/1471-2407-12-372 22928942
34. Manna PP, Frazier WA (2003) The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol 170 : 3544–3553. 12646616
35. Manna PP, Frazier WA (2004) CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res 64 : 1026–1036. 14871834
36. Johansson U, Higginbottom K, Londei M (2004) CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand J Immunol 59 : 40–49. 14723620
37. Johansson U, Londei M (2004) Ligation of CD47 during monocyte differentiation into dendritic cells results in reduced capacity for interleukin-12 production. Scand J Immunol 59 : 50–57. 14723621
38. Sun L (2013) Peptide-based drug development. Mod Chem Appl 1: e103.
39. Le Garff-Tavernier M, Blons H, Nguyen-Khac F, Pannetier M, Brissard M, et al. (2011) Functional assessment of p53 in chronic lymphocytic leukemia. Blood Cancer J 1: e5. doi: 10.1038/bcj.2011.3 22829111
40. Van VQ, Baba N, Rubio M, Wakahara K, Panzini B, et al. (2012) CD47(low) status on CD4 effectors is necessary for the contraction/resolution of the immune response in humans and mice. PLoS ONE 7: e41972. doi: 10.1371/journal.pone.0041972 22870271
41. Raymond M, Rubio M, Fortin G, Shalaby KH, Hammad H, et al. (2009) Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J Allergy Clin Immunol 124 : 1333–1342. doi: 10.1016/j.jaci.2009.07.021 19748659
42. Barbet G, Demion M, Moura IC, Serafini N, Leger T, et al. (2008) The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol 9 : 1148–1156. doi: 10.1038/ni.1648 18758465
43. Serafini N, Dahdah A, Barbet G, Demion M, Attout T, et al. (2012) The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J Immunol 189 : 3689–3699. 22933633
44. Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, et al. (2003) Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe Against Cancer program. Leukemia 17 : 2474–2486. 14562124
45. Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, et al. (2006) D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem 358 : 126–135. 16965760
46. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412. doi: 10.1371/journal.pbio.1000412 20613859
47. O’Neil RG, Wu L, Mullani N (2005) Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol Imaging Biol 7 : 388–392. 16284704
48. Sick E, Niederhoffer N, Takeda K, Landry Y, Gies JP (2009) Activation of CD47 receptors causes histamine secretion from mast cells. Cell Mol Life Sci 66 : 1271–1282. doi: 10.1007/s00018-009-8778-2 19205621
49. Powell MF, Stewart T, Otvos L Jr, Urge L, Gaeta FC, et al. (1993) Peptide stability in drug development. II. Effect of single amino acid substitution and glycosylation on peptide reactivity in human serum. Pharm Res 10 : 1268–1273. 8234161
50. McDonald JF, Dimitry JM, Frazier WA (2003) An amyloid-like C-terminal domain of thrombospondin-1 displays CD47 agonist activity requiring both VVM motifs. Biochemistry 42 : 10001–10011. 12924949
51. Hu M, Polyak K (2008) Microenvironmental regulation of cancer development. Curr Opin Genet Dev 18 : 27–34. doi: 10.1016/j.gde.2007.12.006 18282701
52. Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, et al. (2012) Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol 14 : 276–286. doi: 10.1038/ncb2432 22344033
53. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, et al. (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123 : 321–334. 16239148
54. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, et al. (2010) Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2 : 63ra94. doi: 10.1126/scitranslmed.3001375 21178137
55. Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, et al. (2007) Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol 27 : 7073–7088. 17682056
56. Barbier S, Chatre L, Bras M, Sancho P, Roue G, et al. (2009) Caspase-independent type III programmed cell death in chronic lymphocytic leukemia: the key role of the F-actin cytoskeleton. Haematologica 94 : 507–517. doi: 10.3324/haematol.13690 19278964
57. Hirshman CA, Zhu D, Pertel T, Panettieri RA, Emala CW (2005) Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol 288: L924–L931. 15821021
58. Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116 : 205–219. 14744432
59. Feske S (2007) Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7 : 690–702. 17703229
60. Scharenberg AM, Humphries LA, Rawlings DJ (2007) Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol 7 : 778–789. 17853903
61. Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, et al. (2012) Mitochondrial Ca(2+) and apoptosis. Cell Calcium 52 : 36–43. doi: 10.1016/j.ceca.2012.02.008 22480931
62. Poulin B, Sekiya F, Rhee SG (2005) Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1. Proc Natl Acad Sci U S A 102 : 4276–4281. 15764700
63. Bertilaccio MT, Scielzo C, Simonetti G, Ponzoni M, Apollonio B, et al. (2010) A novel Rag2-/-gammac-/—xenograft model of human CLL. Blood 115 : 1605–1609. doi: 10.1182/blood-2009-05-223586 20018917
64. Soto-Pantoja DR, Stein EV, Rogers NM, Sharifi-Sanjani M, Isenberg JS, et al. (2013) Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther Targets 17 : 89–103. doi: 10.1517/14728222.2013.733699 23101472
65. Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, et al. (2011) CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A 108 : 18342–18347. doi: 10.1073/pnas.1106550108 22042861
66. Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, et al. (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 109 : 6662–6667. doi: 10.1073/pnas.1121623109 22451913
67. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, et al. (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138 : 271–285. doi: 10.1016/j.cell.2009.05.046 19632178
68. Lax R (2010) The future of peptide development in the pharmaceutical industry. Pharmanuf Int Pept Rev 2 : 10–15.
69. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, et al. (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16 : 3–11. doi: 10.1038/cdd.2008.150 18846107
70. Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, et al. (2007) Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol 27 : 4844–4862. 17470554
71. Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, et al. (2002) Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood 100 : 2882–2890. 12351399
72. Hogan PG, Lewis RS, Rao A (2010) Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol 28 : 491–533. doi: 10.1146/annurev.immunol.021908.132550 20307213
73. Parekh AB, Putney JW Jr (2005) Store-operated calcium channels. Physiol Rev 85 : 757–810. 15788710
74. Wozniak AL, Wang X, Stieren ES, Scarbrough SG, Elferink CJ, et al. (2006) Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J Cell Biol 175 : 709–714. 17130290
75. Wilde JI, Watson SP (2001) Regulation of phospholipase C gamma isoforms in haematopoietic cells: why one, not the other? Cell Signal 13 : 691–701. 11602179
76. Wang D, Feng J, Wen R, Marine JC, Sangster MY, et al. (2000) Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 13 : 25–35. 10933392
77. Le Roy C, Deglesne PA, Chevallier N, Beitar T, Eclache V, et al. (2012) The degree of BCR and NFAT activation predicts clinical outcomes in chronic lymphocytic leukemia. Blood 120 : 356–365. doi: 10.1182/blood-2011-12-397158 22613791
78. Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, et al. (2009) Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res 69 : 5424–5432. doi: 10.1158/0008-5472.CAN-08-4252 19549911
79. Fujimoto TT, Katsutani S, Shimomura T, Fujimura K (2003) Thrombospondin-bound integrin-associated protein (CD47) physically and functionally modifies integrin alphaIIbbeta3 by its extracellular domain. J Biol Chem 278 : 26655–26665. 12736272
80. Chung J, Gao AG, Frazier WA (1997) Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J Biol Chem 272 : 14740–14746. 9169439
81. Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267 : 1456–1462. 7878464
82. Parker JL, Newstead S (2014) Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507 : 68–72. doi: 10.1038/nature13116 24572366
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2015 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Testing and Treating the Missing Millions with Tuberculosis
- UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age
- Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study
- Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial
- Strengthening the Detection of and Early Response to Public Health Emergencies: Lessons from the West African Ebola Epidemic
- HIV Treatment-As-Prevention Research: Authors’ Reply
- Role of Acute HIV Infection in Driving HIV Transmission: Implications for HIV Treatment as Prevention
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- HIV Treatment-As-Prevention Research: Taking the Right Road at the Crossroads
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
- A Public Health Approach to Hepatitis C Control in Low- and Middle-Income Countries
- Development and Validation of a Risk Score for Chronic Kidney Disease in HIV Infection Using Prospective Cohort Data from the D:A:D Study
- Reassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts
- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



