-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An Extensive Circuitry for Cell Wall Regulation in
Protein kinases play key roles in signaling and response to changes in the external environment. The ability of Candida albicans to quickly sense and respond to changes in its environment is key to its survival in the human host. Our guiding hypothesis was that creating and screening a set of protein kinase mutant strains would reveal signaling pathways that mediate stress response in C. albicans. A library of protein kinase mutant strains was created and screened for sensitivity to a variety of stresses. For the majority of stresses tested, stress response was largely conserved between C. albicans, Saccharomyces cerevisiae, and Schizosaccharomyces pombe. However, we identified eight protein kinases whose roles in cell wall regulation (CWR) were not expected from functions of their orthologs in the model fungi Saccharomyces cerevisiae and Schizosaccharomyces pombe. Analysis of the conserved roles of these protein kinases indicates that establishment of cell polarity is critical for CWR. In addition, we found that septins, crucial to budding, are both important for surviving and are mislocalized by cell wall stress. Our study shows an expanded role for protein kinase signaling in C. albicans cell wall integrity. Our studies suggest that in some cases, this expansion represents a greater importance for certain pathways in cell wall biogenesis. In other cases, it appears that signaling pathways have been rewired for a cell wall integrity response.
Published in the journal: An Extensive Circuitry for Cell Wall Regulation in. PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000752
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000752Summary
Protein kinases play key roles in signaling and response to changes in the external environment. The ability of Candida albicans to quickly sense and respond to changes in its environment is key to its survival in the human host. Our guiding hypothesis was that creating and screening a set of protein kinase mutant strains would reveal signaling pathways that mediate stress response in C. albicans. A library of protein kinase mutant strains was created and screened for sensitivity to a variety of stresses. For the majority of stresses tested, stress response was largely conserved between C. albicans, Saccharomyces cerevisiae, and Schizosaccharomyces pombe. However, we identified eight protein kinases whose roles in cell wall regulation (CWR) were not expected from functions of their orthologs in the model fungi Saccharomyces cerevisiae and Schizosaccharomyces pombe. Analysis of the conserved roles of these protein kinases indicates that establishment of cell polarity is critical for CWR. In addition, we found that septins, crucial to budding, are both important for surviving and are mislocalized by cell wall stress. Our study shows an expanded role for protein kinase signaling in C. albicans cell wall integrity. Our studies suggest that in some cases, this expansion represents a greater importance for certain pathways in cell wall biogenesis. In other cases, it appears that signaling pathways have been rewired for a cell wall integrity response.
Introduction
The cell surface has two pivotal roles in the survival of microorganisms: protection and interaction. The protective role preserves the integrity of the cell in the face of environmental assaults [1],[2]. The interactive role includes both sensing of external signals and binding to other cells or external surfaces [3],[4]. These two functions necessitate a delicate balance for organisms that can adapt to diverse niches: protection is accomplished by a rigid and impermeable surface; interaction is accomplished by a dynamic one.
The fungus Candida albicans has considerable adaptive ability that is manifested through its impact on humans. It is a commensal microbe that is acquired soon after birth, and occupies both the GI and GU tracts. These colonization sites represent distinct environments in terms of cohabitant microbiota, pH, and nutrients. The adaptive ability of C. albicans is most apparent when circumstances compromise host barriers to infection. Immunological deficiency or presence of an implanted device provides the opportunity for C. albicans to invade and grow in almost any tissue in the body. Hence C. albicans is the major fungal commensal and the major fungal pathogen of humans.
C. albicans is protected by a carbohydrate-based cell wall, whose major constituent is β-glucan, a glucose polymer that imparts shape [5]. Chitin lends the wall rigidity, while mannoproteins that coat the surface serve to interact with the external environment [6]. The cell wall is connected to every known C. albicans biological process, including growth, morphogenesis, mating, and pathogenicity [7]. It is also an inviting therapeutic target, being a source of fungal-specific antigens and essential functions. Thus the mechanisms and regulatory pathways that govern C. albicans cell wall dynamics are a critical area of understanding.
Much of what we know about fungal cell wall integrity and cell wall biogenesis (herein collectively called cell wall regulation, CWR) comes from studies of the model yeast Saccharomyces cerevisiae, where the protein kinase C-MAPK (PKC-MAPK) pathway, largely responsible for the transcriptional response to cell wall disturbance, is a major cell wall-responsive regulatory system (reviewed in [8]). The PKC-MAPK pathway is also vital for cell wall integrity the pathogenic fungus Cryptococcus neoformans [9]. PKC itself has additional targets that impact cell wall structure independently of the MAPK pathway [10]–[13]. PKC-MAPK signaling is activated by cell wall disruption, or by diverse signals that include oxidative stress, hypo-osmotic shock, and mating projection formation. The spectrum of PKC-MAPK signaling inputs reflects the central role of the cell wall in S. cerevisiae biological processes.
The PKC-MAPK pathway is conserved in C. albicans, where it also has a significant role in CWR [14],[15]. However, several observations suggest that C. albicans cell wall dynamics may be under control of a broader signaling network [16],[17]. We have considered the set of C. albicans protein kinases (PKs) to be representatives of the spectrum of signaling pathways, and created a panel of mutant strains defective in PK genes and some PK-related genes. The panel has been used to connect PK function to CWR as well as such features as morphogenesis, biofilm formation, and stress sensitivity. Our findings reveal that the C. albicans cell wall is highly connected to a much broader range of signaling pathways than has been found for S. cerevisiae. The C. albicans cell wall signaling network may reflect a balance of diverse inputs that are poised to promote modification and support adaptation.
Results
Construction of a protein kinase (PK) mutant library
We set out to define functional networks that connect signal transduction pathways to signature C. albicans biological features. We created homozygous insertion or deletion mutations in 67 PK genes and 13 PK-related genes. More detail about these mutants, including insertion sites, can be found in Table S1. Most genes were represented by multiple independent mutant isolates. We were unable to recover homozygous mutations in 41 PK genes.
PK function was surveyed through a screen of the mutants for altered biological properties that included stress or drug sensitivity, filamentation, and biofilm formation (Table S1). Prior studies of Saccharomyces cerevisiae and Schizosaccharomyces pombe [18] provided clear hypotheses for the function of many PKs, and we generally found good correspondence with these predictions (Tables 1 and 2). However, there was a strikingly expanded role for C. albicans PKs in CWR: 24 of the 80 mutants were hypersensitive to the cell wall inhibitor caspofungin, compared to 10 of the mutants that were predicted by model organism studies (Table 1). In addition, 2 of the 10 C. albicans mutants predicted to be sensitive to cell wall stress based on the phenotypes of orthologous S. cerevisiae mutants were not sensitive in our assays.
Tab. 1. Comparison of C. albicans, S. cerevisiae, and S. pombe mutant PK and PK-related cell wall stress sensitivity phenotypes. 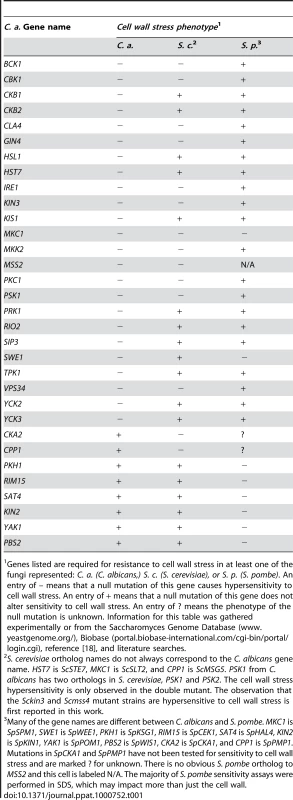
Genes listed are required for resistance to cell wall stress in at least one of the fungi represented: C. a. (C. albicans,) S. c. (S. cerevisiae), or S. p. (S. pombe). An entry of – means that a null mutation of this gene causes hypersensitivity to cell wall stress. An entry of + means that a null mutation of this gene does not alter sensitivity to cell wall stress. An entry of ? means the phenotype of the null mutation is unknown. Information for this table was gathered experimentally or from the Saccharomyces Genome Database (www.yeastgenome.org/), Biobase (portal.biobase-international.com/cgi-bin/portal/login.cgi), reference [18], and literature searches. Tab. 2. A comparison of C. albicans, S. cerevisiae, and S. pombe PK and PK-related mutant phenotypes. 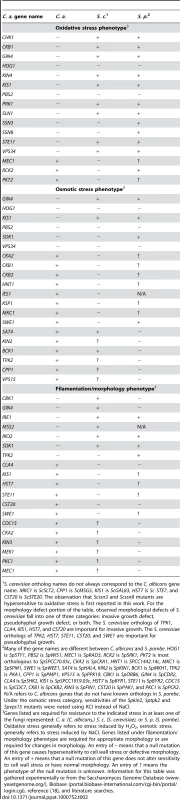
S. cerevisiae ortholog names do not always correspond to the C. albicans gene name. MKC1 is ScSLT2, CPP1 is ScMSG5, KIS1 is ScGAL83, HST7 is Sc STE7, and CST20 is ScSTE20. The observation that Scssn3 and Scssn8 mutants are hypersensitive to oxidative stress is first reported in this work. For the morphology defect portion of the table, observed morphological defects of S. cerevisiae fall into one of three categories: invasive growth defect, pseudohyphal growth defect, or both. The S. cerevisiae orthologs of TPK1, CLA4, KIS1, HST7, and CST20 are important for invasive growth. The S. cerevisiae orthologs of TPK2, HST7, STE11, CST20, and SWE1 are important for pseudohyphal growth. PKs with conserved roles in CWR included members of the PKC MAPK pathway, as well as Cla4, Cbk1, Psk1, Gin4, Ire1, and Vps34 (Fig. 1, Table 1). However, the PKs Hsl1, Kin3, Swe1, Tpk1, Yck3, Prk1, Yck2, Rio2, and Hst7, along with the PK-related proteins Ckb1, Ckb2, Sip3, Mss2, and Kis1, were also required for normal sensitivity to caspofungin (Fig. 1). These genes had not previously been uncovered in large-scale S. cerevisiae screens for CWR, although a role for Hst7 in C. albicans CWR was previously identified [19]. Our own caspofungin-sensitivity tests of S. cerevisiae mutants lacking the respective orthologs revealed new roles in CWR for S. cerevisiae Kin3 and Mss2 (data not shown), but not for the other orthologs. Complementation of each new C. albicans caspofungin-hypersensitive mutant restored normal sensitivity to caspofungin (Fig. 1). Thus the defined mutation causes the CWR defect. Therefore, among the PKs and related proteins surveyed, we found 12 with conserved roles in CWR and 12 with apparent C. albicans-specific roles in CWR.
Fig. 1. PKs play conserved and novel roles in CWR. 
A wild type marker-matched strain (DAY286), a hypersensitive cas5Δ/cas5Δ control, and the indicated prototrophic PK mutant strains and their complements were serially diluted onto YPD (−) or YPD+ 125 ng/ml caspofungin (+) and grown for 2 days at 30°C. Data for PSK1 were published in Rauceo, et al [17]. In most cases, complementation fully restored growth on the caspofungin plates, but it should be noted that complementation of the gin4−/− strain with one copy of GIN4 was not sufficient to restore growth on caspofungin. The cell wall PK network is unique
We also surveyed PK function in a panel of tests for traits related to survival and virulence. The mutants were tested for sensitivity to oxidative, osmotic, and pH stress, as well as for the ability to form filamentous cells and biofilms. The results of these screens are shown in Table 2 and Fig. S1. While these additional screens demonstrated both conserved and novel roles for PKs in these responses, only the oxidative stress assay uncovered a significant expansion of roles among conserved PKs. Five PK genes (PRK1, KIN4, VPS34, STE11, and GIN4) and two PK-related genes (CKB1 and KIS1) had previously undescribed roles in the oxidative stress response (Table 2 and Fig. S1). Analysis of S. cerevisiae mutants defective in their orthologs confirmed that their requirement for oxidative stress survival is unique to C. albicans (data not shown). This expanded role may reflect frequent encounters of C. albicans with host phagocytic cells that use oxidative attack during commensal growth. Rather than developing entirely new signaling pathways to combat this frequent stress, it appears that conserved pathways were enlisted to improve this fungus's chances for survival in vivo. The identification of almost twice the number of PKs with novel roles in CWR suggests that cell wall damage is also an important in vivo stress.
PK mutants evoke a cell wall damage response in the absence of stress
We sought to determine whether the PK and PK-related genes with novel roles in CWR (Table 1) are required specifically for the response to cell wall damage, or have a role in cell wall biogenesis. Indeed, some of the conserved CWR PKs that appeared in our screens such as Cbk1, are important for cell wall biogenesis [20],[21]. We hypothesized that such mutants would exhibit a cell wall damage response even in the absence of exogenous cell wall stress. We therefore monitored the transcription of six cell wall damage response genes in the absence of externally induced cell wall stress in PK mutant and complement strains (Fig. 2 and S2). Two of these cell wall damage response genes, DDR48 and SOD5, exhibit the highest upregulation following cell wall stress in previous microarray studies [17] and are also upregulated in hyphal growth and in the presence of other stresses. The regulation of RTA4 is tied to both cell wall stress and to external pH [16],[22]. ALS1, an adhesin, is upregulated during biofilm formation and cell wall stress while STP4 and ECM331 appear to be more specific for caspofungin response [16],[23],[24].
Fig. 2. PK and PK-related mutant strains show a damage response in the absence of cell wall stress. 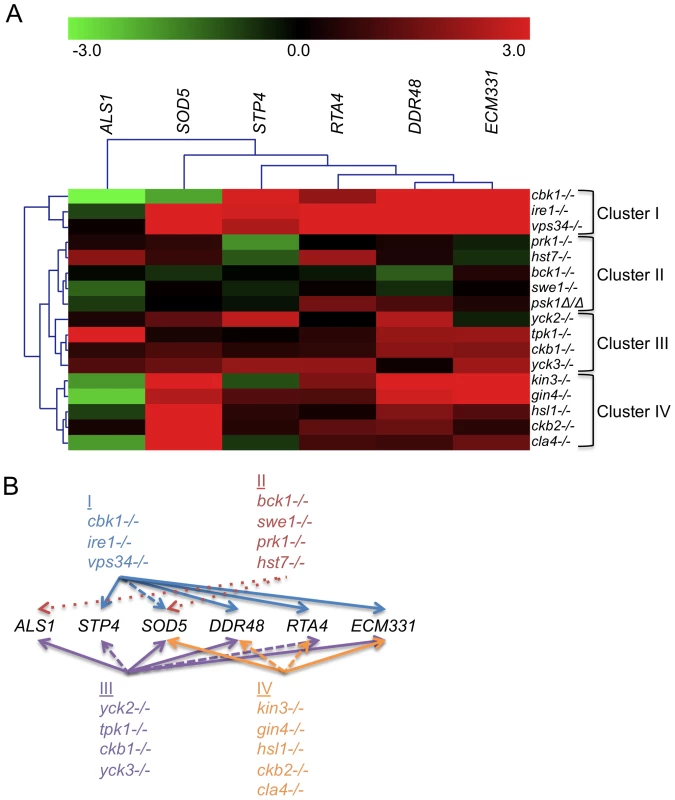
(A) The expression of six genes upregulated by caspofungin treatment, DDR48, SOD5, STP4, ALS1, RTA4, and ECM331, was analyzed in PK and PK-related mutant strains in the absence of cell well stress. The expression of TDH3, a gene involved in glycolysis, was used to normalize expression between strains and expression values were further normalized to wild type (DAY185) expression for comparison between experiments. Resultant values were log base 2 transformed (wild type expression for all six genes is therefore at 0). (B) A graphical representation of the expression data. Arrows point to targets upregulated in all (solid arrows), most (dashed lines), or half (dotted lines) of the mutants indicated in the clusters. Our first hypothesis, that strains mutant for genes involved in cell wall biogenesis will exhibit a damage profile in the absence of stress, is supported by the transcription profile from the cbk1−/− mutant (Fig. 2 and S2). We therefore tested the rest of the caspofungin-sensitive PK mutants for a damage response in the absence of cell wall stress by quantitative rtPCR (QrtPCR). Many of the PK mutant strains tested did display distinctive damage profiles compared to wild type and their respective complemented strains (data not shown). Hierarchical clustering of the QrtPCR data produced groupings of strains that may give us insight into shared functions between the PK and PK-related genes within the clusters. Cbk1 is important for cell wall biogenesis [20],[21] and the cbk1−/− mutant is in Cluster I. This cluster also contains the ire1−/− mutant, which had an even more pronounced damage profile than did the cbk1−/− mutant, and the vps34−/− mutant (Fig. 2). The magnitude of the damage response in Cluster I was significantly higher than other clusters (Fig. S2). We hypothesize that Cluster I represents PKs with intrinsic roles in cell wall biogenesis. Cluster II, representing mutant strains with very little difference in expression compared to the wild type strain, included the bck1−/− mutant. Bck1 is a member of the Pkc1 MAP kinase cascade, which is involved in sensing cell wall stress and we hypothesize that other members of this cluster may also play roles in cell wall stress response. These PKs may govern induction of caspofungin-inducible genes that we have yet to test, or may have posttranslational roles in CWR. This QrtPCR analysis included only a small subset of genes downstream of cell wall stress, and more detailed analysis will likely be required to tease out their potential roles in cell wall stress response. Thus, our gene expression analysis not only suggests that many of the PK and PK-related genes identified in our study have some role in cell wall biogenesis, but also has separated these genes into clusters that may reflect function.
Relationship between septins and CWR
Cluster IV, identified in the previous analysis, contains several PKs with known connections to polar septin localization, including Cla4, Gin4, and Hsl1 [25],[26]. We hypothesized that septin function itself may be related to CWR. Two further observations support this hypothesis. First, we found that mutants defective in the septin genes cdc10 and cdc11 demonstrated increased sensitivity to caspofungin (Fig. 3A). These two septins are important for septin ring formation at high temperatures and chitin deposition at all temperatures [27]. In contrast, a sep7 deletion mutant, which causes no apparent defect in yeast-form septin ring formation or chitin deposition, does not have heightened sensitivity to caspofungin. Severity of sensitivity thus correlated with the severity of the septation defects (Fig. 3A), an indication that septin function is vital for C. albicans survival during cell wall stress. Second, we asked whether cell wall disruption might alter septin localization. A Sep7-GFP fusion protein is concentrated at mother-bud necks in untreated budded cells, with two rings visible in separating mother-daughter cells (Fig. 3B), as previously reported [28]. Caspofungin treatment affected this localization dramatically: mislocalization was observed on one side of the mother-bud neck or in other regions of 46.7% of cells, while there were partial septa in 3.3% of budded cells (N = 92) (Fig. 3B). Caspofungin treatment of CDC10-GFP, CDC12-GFP, and CDC3-GFP strains caused similar abnormalities (data not shown). Therefore, septin localization depends upon normal CWR.
Fig. 3. Septins play an integral role in CWR. 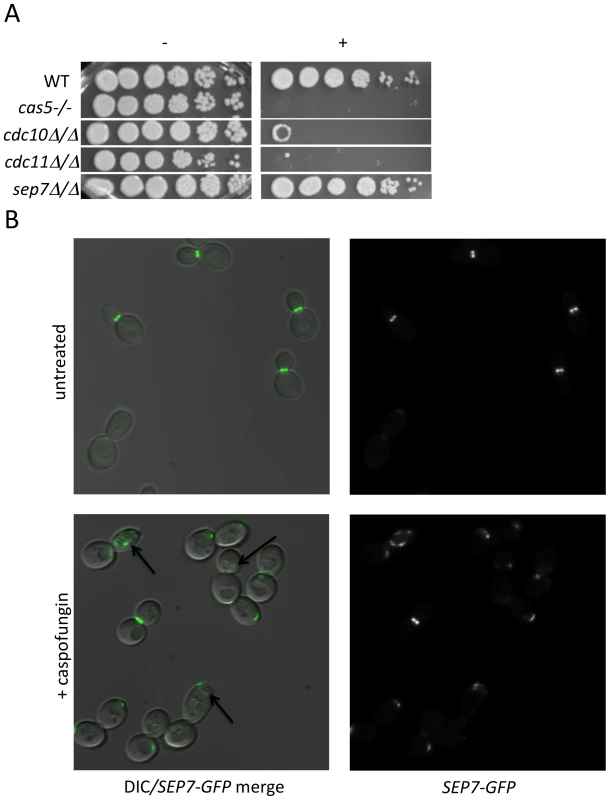
(A) A wild type marker-matched strain (DAY185), a cas5Δ/cas5Δ negative control, a cdc10Δ/cdc10Δ (YAW7), a cdc11Δcdc11/Δ (YAW11), and a sep7Δ/sep7Δ (YAW41) mutant strain were serially diluted onto YPD (−) or YPD+ 125 ng/ml caspofungin (+) and grown for 3 days at 30°C. (B) SEP7-GFP cells (JRB217) were grown to log phase and either treated with 125 ng/ml caspofungin (+ caspofungin) for 30 minutes or left untreated. The cells were then visualized on glass slides. Arrows point to aberrant septin localization. We were led to the discovery of a role for septins in CWR by the sensitive phenotype of PK mutants with known roles in septin localization. We hypothesized that these PK mutants might display aberrant septin localization even in the absence of stress. Mutations causing a complete delocalization of septins at the mother-bud neck would probably be lethal to the cell and thus would not have been recovered in our library. To determine whether any of the caspofungin-sensitive PK and PK-related mutant strains displayed aberrant septin localization, a GFP tag was inserted into the 3′ end of the SEP7 gene at the endogenous SEP7 locus. We were able to insert the SEP7-GFP tagged allele into all but two of the caspofungin-sensitive PK and PK-related mutant strains (hst7−/− and psk1−/−). Cluster IV mutant strains, particularly gin4−/− and cla4−/− are the clearest candidates for delocalization based on phenotypes of the corresponding S. cerevisiae mutants [25],[29]. Indeed, septin localization was disrupted in the gin4−/− mutant strain (Fig. 4 and S3). Two cell phenotypes were observed: cells with normal buds had normal septin localization, but cells growing as pseudohyphae were either lacking septin rings or had septin rings that did not completely span the neck. Neither the cla4−/− nor the hsl1−/− had delocalized septins in the absence of stress (data not shown). This is consistent with the known roles of Cla4 in localization of septins in hyphal cells [30] and Hsl1 in signaling downstream of septin localization. However, one member of Cluster IV with no previous links to septin organization, Kin3, did demonstrate a septin organization defect, suggesting that this PK is linked to septin localization as predicted by its inclusion in this cluster. Septins in this strain appear normal in buds until cytokinesis, where separation of septin rings is aberrant. In addition to these Cluster IV mutants, we also observed partial septin mislocalization in the vps34−/− and cbk1−/− mutant strains (Fig. 4 and S3). Whether septin disorganization contributes to the caspofungin sensitivity of these strains or it is a symptom of an intrinsic cell wall defect has not yet been determined. However, the disorganization of septins in these strains lends further support to the hypothesis that septin localization or regulation is linked to cell wall integrity.
Fig. 4. Septins are mislocalized in some PK mutant strains. 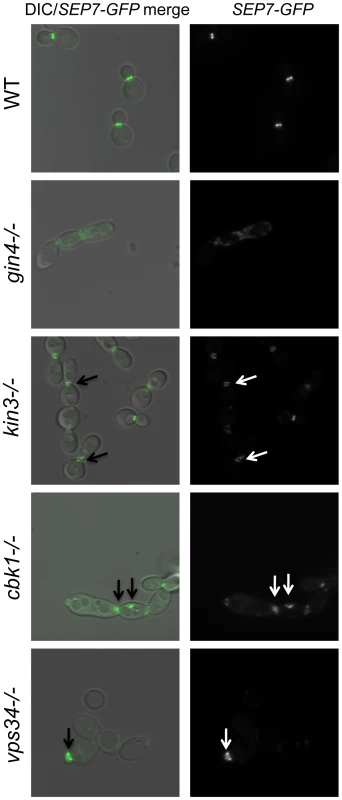
SEP7-GFP tagged wild type (JRB217), gin4−/− (JRB221), kin3−/− (JRB193), cbk1−/− (JRB224), and vps34−/− (JRB216) strains were grown to log phase and imaged at 100× on glass slides. An overlay of DIC and GFP is on the left and GFP alone is on the right. Arrows point to aberrant septin localization. Discussion
PKs occupy key positions between environmental sensors and responses. Our study of C. albicans PKs emphasizes their pivotal roles: almost half of the PK mutants display a prominent phenotype. In contrast, only ∼5% of viable C. albicans transcription factor mutants have comparable phenotypes [16],[17],[31],[32]. We observed that C. albicans PK biological function is conserved with their S. cerevisiae and S. pombe orthologs in many cases (Tables 1 and 2). We also detected novel roles related to C. albicans filamentation and biofilm formation, traits not examined systematically with model organism mutant panels. However, our most striking result is that C. albicans CWR relies upon conserved PKs with novel functions compared to their orthologs, representing an expansion of the circuitry involved in this process (Fig. 5). A few of these PKs have been linked to cell wall integrity previously, most notably Prk1 and Hst7 [33]–[35], but their importance in CWR seems much greater in C. albicans than in S. cerevisiae, given that the orthologous S. cerevisiae mutants do not demonstrate heightened caspofungin sensitivity. CWR depends upon eight PKs with roles in this process that were not predicted from model yeast studies, plus a PK gene and a PK-related gene whose conserved CWR function is newly described in this report.
Fig. 5. A model of the role of PKs in septin morphology and cell wall biogenesis. 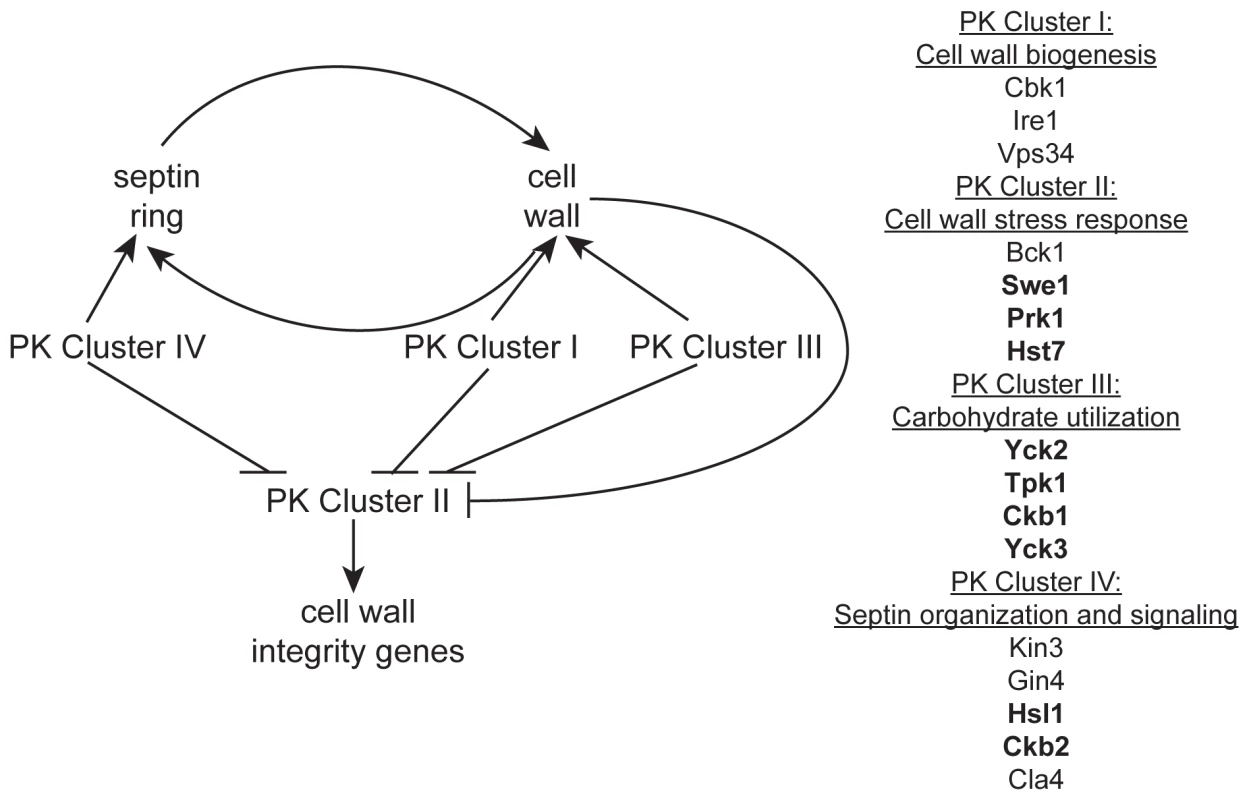
Based on our observations, an intact septin ring is required for normal cell wall production and a normal cell wall is required for the formation of a septin ring. PK Cluster IV genes have known and predicted roles in septin morphology and signaling and may impact cell wall biogenesis indirectly via this role. We hypothesize PK Cluster I genes have direct roles in biogenesis of the cell wall, while PK Cluster III may indirectly effect cell wall biogenesis by regulating the flow of carbohydrates into cell wall biosynthesis pathways. We hypothesize that the genes in PK Cluster II are involved in cell wall stress response and the upregulation of cell wall integrity genes. PK Clusters I, III, and IV negatively regulate PK Cluster II, either directly or indirectly, based on the observation that cell wall integrity genes are upregulated in the absence of these PKs. Two simple scenarios can account for the functional roles of the eight unpredicted CWR PKs: they may have a role in ongoing cell wall biogenesis, or they may only be required for response to cell wall stress. These possibilities are not mutually exclusive: a CWR PK may function in both capacities. Our analysis of PK mutant transcriptional responses strongly supports a role for Ire1 in normal cell wall biogenesis and suggests that several of the other novel PKs identified in this study may also have roles in cell wall biogenesis. In contrast, transcriptional responses of Cluster II PK mutants are not very distinct from wt and we suspect that these PKs may be important for cell wall stress response. Indeed, BCK1, a member of the cell wall stress response PKC1 MAPK pathway, and PSK1, which is also thought to have a role in cell wall stress response [17] are both in Cluster II. Microarray analysis of the other PKs in this cluster, Swe1 and Prk1, may identify a role for these PKs in stress response as well.
Our survey of gene expression alterations, though limited, revealed some qualitative differences among the mutants. Such distinctions may be useful in assembling the PKs into existing or novel pathways, a task that has generally proved challenging in organisms with limited genetic tractability. We were able to organize the data into distinct clusters that may reveal functional relationships. Indeed, mutants in GIN4, CLA4, and HSL1, genes important for the localization of septins (GIN4 and CLA4) or signaling downstream of septin localization (HSL1), fell into a cluster (Cluster IV, Fig. 3). The kin3−/− and ckb2−/− mutants were also in this cluster. Although only tentatively linked, close association of the kin3−/− and ckb2−/− strains with mutants in genes involved in septin localization and signaling suggests Kin3 and Ckb2 may also play a role in this process (Fig. 5). Interestingly, the cbk1−/− and ire1−/− strains also clustered together (Cluster I, Fig. 2), lending further support to the hypothesis that IRE1 has a role in cell wall biogenesis. Cbk1 is upstream of cell wall biogenesis genes, such as the chitin synthases [20], and along with the roles of Vps34 and Ire1 in cellular transport, this cluster seems to represent the production and delivery of cell wall building enzymes to the cell periphery (Fig. 5). Mutations in SWE1, PRK1, PSK1, and HST7, which fall into a cluster with the bck1−/− mutant (Cluster II, Fig. 2), clustered together because the genes tested in the QrtPCR analysis were not differentially regulated compared to wild type. Perhaps, like bck1−/−, they also have some role in responding to cell wall stress (Fig. 5). The third cluster includes both of the casein kinase I mutants (yck2−/− and yck3−/−), tpk1−/−, and the casein kinase II regulatory subunit mutant ckb1−/− (Fig. 2). The genes included in this cluster have a variety of roles within cells, but one intriguing correlation between their functions is carbohydrate sensing (Yck2/3) and signaling (Tpk1). The cAMP pathway, of which Tpk1 is a key member, has a conserved role in glucose sensing (reviewed in [36]) and Yck1 and 2 have been shown to have a role in regulation of glucose transporters [37]. Glucose is the major building block of the β-glucans in the cell wall as well as an important source of energy for cells and perhaps these genes help cells balance the need for food versus protection (Fig. 5). While such inferences are clearly tentative, they make numerous testable predictions that will be useful in unraveling the complex relationships among cell wall regulators.
Ire1 showed the largest damage profiles in all three cell wall damage response genes. In all organisms where it has been studied, Ire1 is a component of the conserved unfolded protein response (UPR), which is activated by accumulation of unfolded proteins in the ER [38]. S. cerevisiae ire1 mutants do exhibit sensitivity to cell wall damage under certain conditions [39], but the C. albicans ire1−/− mutant seems much more sensitive to cell wall damage, suggesting perhaps a more central role in this process. A simple model is that the UPR is critical for C. albicans cell wall damage response, perhaps because of the large number of cell wall protein genes that are induced by caspofungin [16]. We note that a relatively distal 3′ homozygous insertion mutation was created for this study (Table S1; insertion at bp 3520 of the 3675 bp ORF), and we have been unable to generate homozygous 5′ insertions or a deletion of this gene in C. albicans. Thus the UPR may be essential in C. albicans even in the absence of exogenous stresses. We suggest that the UPR has a critical role in cell wall biogenesis and may be partially activated under our growth conditions.
Our findings also reveal a clear relationship between septin function and CWR. The PKs that govern CWR include Yck2, Cla4, and Gin4, which are involved in the assembly of septins at the mother-bud interface, and Swe1 and Hsl1, which are important for signaling that septins are appropriately localized [25],[40]. Furthermore, we observed that septins are required for normal sensitivity to caspofungin, and that septins are delocalized in response to caspofungin treatment. This phenomenon appears to be conserved in S. cerevisiae (Briggs, Mitchell, and Blankenship unpublished results), suggesting a conserved role for septin delocalization in cell wall integrity. Septins are connected to cell wall biogenesis through their known roles in determining sites of secretion and in directing the localization of chitin synthase. Septin delocalization may act positively in the cell wall damage response to permit delocalized secretion and cell wall synthesis when glucan synthase inhibition makes the bud neck an unsuitable site. A second possibility is that septin delocalization may function passively to prevent unbalanced production of cell wall components when glucan synthesis is blocked by caspofungin. A third possibility is that septin delocalization may protect cells by blocking cell cycle progression through Swe1 and Hsl1, thus reducing the need for new cell wall material. Thus, the analysis of C. albicans PKs has pointed toward a novel cell biological phenomenon, and several testable hypotheses may explain its functional consequences.
In some of our PK mutant strains, septins are mislocalized even in the absence of caspofungin treatment. This was expected for the gin4−/− mutant [29] and may contribute to the caspofungin sensitivity of this mutant strain, but we also identified several PKs with previously uncharacterized roles in septin organization. The kin3−/− strain clustered with mutants involved in septin organization and signaling (Class IV), and the septation defect of this mutant (Fig. 4) served as a validation of the clustering. Ckb2 was also a member of this cluster and although it did not have a septation defect, it may, like Hsl1, have a role in signaling downstream of septin organization. Septin mislocalization defects were also noted in the cbk1−/− and vps34−/− mutant strains. The cbk1−/− strain had septa at the mother-bud neck but also had aberrant septa in punctae throughout cells. Septa in vps34−/− mutant cells appeared normal in mature cells, but were wide in young buds, extending well into the bud, suggesting that septa in this mutant are disorganized at early stages of bud formation. Both of these mutants are in Cluster I, and perhaps the mislocalization of septins in these strains is due to the damaged walls present in these mutants. The cbk1−/− and vps34−/− phenotypes are different and the ire1−/− mutant does not have a septin localization defect at all, suggesting that the nature of the cell wall defect in these strains are distinct. We propose that Class I PKs are important for cell wall biogenesis and that defects in biogenesis or defects due to external stress inactivate Class IV PKs (perhaps via Class II PKs), which leads to septin mislocalization.
Several studies have pointed toward extensive rewiring of C. albicans transcriptional regulatory pathways. There are two main kinds of phenomena. First, for many orthologous C. albicans-S. cerevisiae transcription factors, both the nature of target genes and the signals to which they respond are distinct in each organism [41]–[44]. Second, C. albicans has some functionally significant transcriptional regulators, such as Mtla2 or Cas5, that do not have clear S. cerevisiae orthologs [16],[45]. In contrast, many of the relationships among C. albicans PKs and the cell wall seem to reflect not “rewiring” but perhaps a greater amount of “current” in C. albicans. That is, one can develop plausible mechanistic explanations for the C. albicans PKs implicated in CWR, based upon the known functions of their orthologs in S. cerevisiae and the assumption that there is greater flux through, or stress upon, the C. albicans secretory system. Ire1 and the septin regulatory network are cases in point. However, we also find indications of true PK rewiring. The MAPKKK Hst7, which functions in the C. albicans mating response-filamentous growth pathway similarly to its S. cerevisiae ortholog Ste7 [46]–[49], seems to have acquired an adjunct function in cell wall biosynthesis or integrity. Similarly, the connection of CWR to Kis1 and Sip3, two proteins whose S. cerevisiae orthologs interact with the glucose repression regulatory PK Snf1 [50],[51], suggests that the C. albicans glucose repression system may be connected to cell wall biogenesis or integrity. Although a S. cerevisiae snf1 mutant shows synthetic lethality with the β-1,3-glucan synthase FKS1 [52] we propose that this PK has a more prominent role in CWR in C. albicans and may explain why it is essential for viability in this fungus [53],[54]. Caspofungin hypersensitivity of mutants defective in Rio2, Tpk1, and casein kinase 2 regulatory subunits Ckb1 and Ckb2 also suggests that PK rewiring has occurred during divergence of S. cerevisiae and C. albicans. These cases provide a foundation for further mechanistic inquiry into cell wall regulatory responses of this highly successful commensal and pathogen.
Materials and Methods
Strains and media
Strains were grown on yeast extract-peptone-dextrose (YPD) rich medium, defined synthetic dextrose medium, prepared as previously described [55], spider medium (10 g D-mannitol (Sigma), 10 g nutrient broth (BD Difco), 2 g K2HPO4 (Sigma) in 1 l of H2O), and M199 cell culture medium (Roche) buffered with 150 mM Hepes (Gibco). NaCl (Sigma), H2O2 (Sigma), Caspofungin (Merck), and sorbitol (Sigma) were added to media at the concentrations described. M199 medium was adjusted to the appropriate pH using NaOH and HCl, and pH was measured on a Corning pH Meter 240.
All PK mutants tested were made in the BWP17 [56] background. Construction of the PK mutant library followed the methods established in Davis et al [57]. Briefly, clones bearing genomic DNA from C. albicans strain CAI4 with an identified insertion of Tn7-UAU1 cassette were excised from the plasmid backbone by digestion with NotI. These linearized constructs were then transformed into BWP17. Putative heterozygote (Arg+) transformants were selected on SC-Arg+Uridine plates, and twelve independent colonies were grown in YPD liquid media overnight and patched onto SC-Arg-Ura plates. The Arg+ Ura+ putative homozygotes were screened by colony PCR using primers ∼500 bp up and downstream of the insertion and within the transformant cassette (Arg4detect [32]) to ensure absence of the wild type allele and presence of the transformed alleles. A BWP17 colony was amplified with the same primer set as a control. These strains have been deposited and are available at the Fungal Genetics Stock Center http://www.fgsc.net/candida/FGSCcandidaresources.htm (Kansas City, MO).
To complement specific deletions, a fragment of DNA∼800 bp upstream and 200 bp downstream of the open reading frame was amplified from BWP17 genomic DNA. Primers for the complementation were roughly 60–70 bp in length and the 3′ 20–30 bp was gene specific. A 40mer sequence (upstream primer-TTCACACAGGAAACAGCTATGACCATGATTACGCCAAGCT, downstream primer TCGACCATATGGGAGAGCTCCCAACGCGTTGGATGCATAG) was tacked onto the 5′ end of each primer sequence to guide in vivo recombination into the pDDB78 plasmid [58]. The amplified complementation fragment was co-transformed into the S. cerevisiae BY4741 Δtrp1 strain with an EcoRI/NotI linearized pDDB78 plasmid, and the resulting complementation clone was amplified in E. coli. Each complement clone was digested with NruI to target insertion to the HIS1 locus and transformed into the appropriate mutant strains, complementing the mutation and rendering the strains His+ as well. Presence of a wild-type band was assayed by colony PCR using the complement primers. pDDB78, digested with NruI, was transformed into the same mutant strains to generate prototrophic, marker-matched strains for comparison with the complemented strains.
To insert GFP into the SEP7 locus in his1−/− PK mutant and wild type (DAY286) his1−/− strains, a fragment of the SEP7 gene was amplified from the YSM26-1 strain [28] containing the 3′ end of the SEP7 gene and the N-terminal GFP tag. The same strategy used for creating the complementation clones was utilized to make a HIS1-tagged SEP7-GFP integrating plasmid, generating pJRB103. This plasmid was digested with BclI (New England Biolabs), which cuts within the SEP7 sequence to direct integration at the native SEP7 locus. Integration of the construct into the native SEP7 locus was assayed by PCR using primers within the GFP sequence and upstream of the SEP7 integration.
Identification of PK genes
A total of 108 PK were identified by scanning C. albicans genome databases (CGD [59] and CandidaDB [60]) for genes annotated as PKs as well as searching for S. cerevisiae and S. pombe PK homologs by blastp analysis (Table S1). Identities between C. albicans PKs and putative S. cerevisiae orthologs ranged from 23%–83% and putative S. pombe orthologs from 22%–78% (although in four instances, the best matches with S. pombe were not PKs). An additional 13 genes, identified as PK-related, were also included in subsequent analyses.
Sensitivity assays
Osmotic, oxidative, pH, caspofungin sensitivity. Strains were grown overnight in YPD media at 30°C. Cell density was measured at OD600 for each strain, which were then diluted to an OD600 of 3 in H2O. Five-fold dilutions were made of the OD600 3 stock and these were plated on the indicated media. Plates were incubated at 30°C for 24–48 hours, and digitally photographed. When sensitivity was identified in C. albicans, orthologous a and α S. cerevisiae mutants from the S. cerevisiae mutant library [61] were also assayed for sensitivity in a similar manner.
Filamentation and biofilm assays
For filamentation assays, overnight cultures of strains were diluted to an OD600 of 0.3 in M199 liquid medium. Cultures were incubated at 37°C with shaking for 90 minutes and cells were examined under the microscope (see below).
Biofilm assays were performed as previously described [31]. Briefly, silicon disks were incubated at 37°C overnight in a 12-well plate with bovine serum (Sigma) and washed with 2 ml PBS. 2 ml of Spider medium was added to each well. Aliquots of overnight cultures were added to an OD600 of 0.5 to each well, and incubated with the pre-treated silicon disks for 90 minutes at 37°C with light shaking. Following this adherence step, the silicon disks were carefully transferred to a new 12-well plate with 2 ml of PBS in each well and then to another 12-well plate with fresh spider medium. Cultures were incubated at 37°C with light shaking for 60 hours and then digitally imaged.
RNA collection and quantitative rtPCR
Overnight cultures of cells were diluted to an OD600 of 0.2 in 50 ml fresh YPD medium. Cultures were allowed to grow at 30°C with shaking until culture density reached an OD600 of ∼1. Cells for the untreated assay were then harvested by vacuum filtration and flash frozen in a dry ice/EtOH bath. Cultures for the 30 minute caspofungin treatment assay were split into two cultures after reaching an OD600 of ∼1. 125 ng/ml caspofungin was added to one culture, an equal volume of water was added to the other, and the cultures were allowed to incubate for 30 minutes before harvesting. Strains carrying homozygous mutants of YCK2, YCK3, RIO2, and PRK1 were generated after the completion of the treatment experiment and were thus not included in the final assay. Cells were kept frozen on filters at −80°C until RNA extraction. RNA was extracted using a RiboPure-Yeast kit (Ambion) following manufacturers instructions with the following modifications. Cells were resuspended from filters with 1.5 ml ice-cold dH2O followed by 10–15 seconds of vigorous vortexing. Resuspended cells were transferred to a 1.5 ml tube and spun down following the manufacturers protocol. Furthermore, during the cell disruption step, cells were beaten with a Next Advance Bullet Blender for 3 min at 4°C to maximize cell lysis.
10 µg of the resulting total RNA was treated with the DNA-free kit (Ambion) followed by first-strand cDNA synthesis from half of the DNA-free RNA using the AffinityScript multiple temperature cDNA synthesis kit (Stratagene). Absence of DNA contamination was confirmed using control sets for which reverse transcriptase was omitted from the cDNA reaction.
Primer3 software (http://frodo.wi.mit.edu/) was used to design primers for four genes, TDH3, DDR48, SOD5, and STP4. The primers were as follows: for TDH3, TDH3FOR, 5′-ATCCCACAAGGACTGGAGA-3′, and TDH3REV, 5′-GCAGAAGCTTTAGCAACGTG-3′; for DDR48, JRB212, 5′-TTTCGGTTTCGGTAAAGACG-3′, and JRB213, 5′-CTGTTGGAGGAACCGTAGGA-3′; for SOD5, JRB249, 5′-TCCTGCTGCTCATGAAGTTG-3′, and JRB250, 5′-TTGTGTTAGCATTGCCGTGT-3′; and for STP4, JRB244, 5′-TCCTTTCAAGAACATCGATTCA-3′, and JRB245, 5′-TTATGCATCCAATCATCGACA-3′. 2× iQ SYBR Green Supermix (Biorad), 1 µl of first-strand cDNA reaction mixture, and 0.1 µM of primers were mixed in a total volume of 50 µl per reaction, and real-time PCR was performed in triplicate for each sample on an iCycler iQ real-time PCR detection system (Bio-Rad). The program for amplification had an initial denaturation step at 95°C for 5 min, followed by 40 cycles of 95°C for 45 s and 58°C for 30 s. Product amplification was detected using SYBR Green fluorescence during the 58°C step, and specificity of the reaction was monitored by melt-curve analysis following the real-time program. TDH3 was used as a reference gene for normalization of gene expression, which was determined using Bio-Rad iQ5 software (ΔΔCT method). Cluster analysis was performed with the Multiexperiment Viewer (MeV 4.3) from TIGR. Hierarchical clustering was performed with average linkage and a Manhattan distance metric.
Septin morphology and microscopy
SEP7-GFP cells were diluted and grown overnight to an OD600 of ∼1 in YPD. 200 µl of this culture was pipeted onto glass bottom dishes (MatTek Corporation, Ashalnd, MA) coated with concanavalin A or poly-D-lysine (for caspofungin treatment). The dishes were washed twice with PBS and resuspended with PBS for immediate visualization (untreated cells) or incubated with 125 ng/ml caspofungin in YPD for 30 minutes (treated cells). Following caspofungin treatment, the dishes were washed again and PBS was added for visualization. Cells visualized with a Zeiss Axio Observer Z.1 fluorescence microscope and a 100× NA 1.4 objective. Fluorescent images were acquired with an exposure time of 1 s on a Coolsnap HQ2 (Photometrics) camera using Axiovision (Zeiss) software.
Supporting Information
Zdroje
1. WalkerLA
MunroCA
de BruijnI
LenardonMD
McKinnonA
2008 Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4 e1000040 doi:10.1371/journal.ppat.1000040
2. WheelerRT
FinkGR
2006 A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathogens 2 e35 doi:10.1371/journal.ppat.0020035
3. KumamotoCA
2008 Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol 6 667 673
4. SundstromP
2002 Adhesion in Candida spp. Cell Microbiol 4 461 469
5. ChattawayFW
HolmesMR
BarlowAJ
1968 Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol 51 367 376
6. ChauhanN
LiD
SinghP
CalderoneR
KruppaM
2002 The cell wall of Candida spp.
CalderoneR
Candida and candidiasis Washington, D. C. ASM Press 159 175
7. ChaffinWL
Lopez-RibotJL
CasanovaM
GozalboD
MartinezJP
1998 Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiology & Molecular Biology Reviews 62 130 180
8. LevinDE
2005 Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiology & Molecular Biology Reviews 69 262 291
9. GerikKJ
DonlinMJ
SotoCE
BanksAM
BanksIR
2005 Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol Microbiol 58 393 408
10. DelleyPA
HallMN
1999 Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol 147 163 174
11. LiY
MoirRD
Sethy-CoraciIK
WarnerJR
WillisIM
2000 Repression of ribosome and tRNA synthesis in secretion-defective cells is signaled by a novel branch of the cell integrity pathway. Mol Cell Biol 20 3843 3851
12. NanduriJ
TartakoffAM
2001 Perturbation of the nucleus: a novel Hog1p-independent, Pkc1p-dependent consequence of hypertonic shock in yeast. Mol Biol Cell 12 1835 1841
13. ImazuH
SakuraiH
2005 Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock. Eukaryot Cell 4 1050 1056
14. ParaviciniG
MendozaA
AntonssonB
CooperM
LosbergerC
1996 The Candida albicans PKC1 gene encodes a protein kinase C homolog necessary for cellular integrity but not dimorphism. Yeast 12 741 756
15. Navarro-GarciaF
SanchezM
PlaJ
NombelaC
1995 Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol Cell Biol 15 2197 2206
16. BrunoVM
KalachikovS
SubaranR
NobileCJ
KyratsousC
2006 Control of the C. albicans cell wall damage response by transcriptional regulator Cas5. PLoS Pathog 2 e21 doi:10.1371/journal.ppat.0020021
17. RauceoJM
BlankenshipJR
FanningS
HamakerJJ
DeneaultJS
2008 Regulation of the Candida albicans cell wall damage response by transcription factor Sko1 and PAS kinase Psk1. Mol Biol Cell 19 2741 2751
18. BimboA
JiaY
PohSL
KaruturiRKM
den ElzenN
2005 Systematic deletion analysis of fission yeast protein kinases. Eukaryot Cell 4 799 813
19. EismanB
Alonso-MongeR
RomanE
AranaD
NombelaC
2006 The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryot Cell 5 347 358
20. BidlingmaierS
WeissEL
SeidelC
DrubinDG
SnyderM
2001 The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol Cell Biol 21 2449 2462
21. SongY
CheonSA
LeeKE
LeeSY
LeeBK
2008 Role of the RAM network in cell polarity and hyphal morphogenesis in Candida albicans. Mol Biol Cell 19 5456 5477
22. BensenES
MartinSJ
LiM
BermanJ
DavisDA
2004 Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol 54 1335 1351
23. O'ConnorL
LahiffS
CaseyF
GlennonM
CormicanM
2005 Quantification of ALS1 gene expression in Candida albicans biofilms by RT-PCR using hybridisation probes on the LightCycler. Mol Cell Probes 19 153 162
24. LiuTT
LeeRE
BarkerKS
LeeRE
WeiL
2005 Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrobial Agents & Chemotherapy 49 2226 2236
25. LongtineMS
TheesfeldCL
McMillanJN
WeaverE
PringleJR
2000 Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Molecular & Cellular Biology 20 4049 4061
26. KeatonMA
LewDJ
2006 Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Current Opinion in Microbiology 9 540 546
27. WarendaA
KonopkaJ
2002 Septin function in Candida albicans morphogenesis. Mol Biol Cell 13 2732 2746
28. MartinS
KonopkaJ
2004 SUMO modification of septin-interacting proteins in Candida albicans. J Biol Chem 279 40861 40867
29. WightmanR
BatesS
AmornrrattanapanP
SudberyP
2004 In Candida albicans, the Nim1 kinases Gin4 and Hsl1 negatively regulate pseudohypha formation and Gin4 also controls septin organization. Journal of Cell Biology 164 581 591
30. LebererE
ZiegelbauerK
SchmidtA
HarcusD
DignardD
1997 Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr Biol 7 539 546
31. NobileCJ
MitchellAP
2005 Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1. Curr Biol 15 1150 1155
32. NoriceCT
SmithFJ
SolisN
FillerSG
MitchellAP
2007 Requirement for Candida albicans Sun41 in biofilm formation and virulence. Eukaryot Cell 6 2046 2055
33. ZengG
YuX
CaiM
2001 Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol Biol Cell 12 3759 3772
34. LeeBN
ElionEA
1999 The MAPKKK Ste11 regulates vegetative growth through a kinase cascade of shared signaling components. Proc Natl Acad Sci U S A 96 12679 12684
35. CullenPJ
SchultzJ
HoreckaJ
StevensonBJ
JigamiY
2000 Defects in protein glycosylation cause SHO1-dependent activation of a STE12 signaling pathway in yeast. Genetics 155 1005 1018
36. SabinaJ
BrownV
2009 Glucose sensing network in Candida albicans: a sweet spot for fungal morphogenesis. Eukaryot Cell 8 1314 1320
37. MoriyaH
JohnstonM
2004 Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc Natl Acad Sci U S A 101 1572 1577
38. PatilC
WalterP
2001 Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Bio 13 349 356
39. ScrimaleT
DidoneL
de Mesy BentleyKL
KrysanDJ
2009 The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol Biol Cell 20 164 175
40. RobinsonLC
BradleyC
BryanJD
JeromeA
KweonY
1999 The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Molecular Biology of the Cell 10 1077 1092
41. IhmelsJ
BergmannS
Gerami-NejadM
YanaiI
McClellanM
2005 Rewiring of the yeast transcriptional network through the evolution of motif usage. Science 309 938 940
42. TanayA
RegevA
ShamirR
2005 Conservation and evolvability in regulatory networks: the evolution of ribosomal regulation in yeast. Proc Natl Acad Sci U S A 102 7203 7208
43. TsongAE
TuchBB
LiH
JohnsonAD
2006 Evolution of alternative transcriptional circuits with identical logic. Nature 443 415 420
44. MartchenkoM
LevitinA
HoguesH
NantelA
WhitewayM
2007 Transcriptional rewiring of fungal galactose-metabolism circuitry. Curr Biol 17 1007 1013
45. TsongAE
MillerMG
RaisnerRM
JohnsonAD
2003 Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell 115 389 399
46. LebererE
HarcusD
BroadbentID
ClarkKL
DignardD
1996 Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci U S A 93 13217 13222
47. KohlerJR
FinkGR
1996 Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci U S A 93 13223 13228
48. ChenJ
ChenJ
LaneS
LiuH
2002 A conserved mitogen-activated protein kinase pathway is required for mating in Candida albicans. Mol Microbiol 46
49. MageeBB
LegrandM
AlarcoAM
RaymondM
MageePT
2002 Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol Microbiol 46 1345 1351
50. YangX
JiangR
CarlsonM
1994 A family of proteins containing a conserved domain that mediates interaction with the yeast SNF1 protein kinase complex. Embo J 13 5878 5886
51. LesageP
YangX
CarlsonM
1994 Analysis of the SIP3 protein identified in a two-hybrid screen for interaction with the SNF1 protein kinase. Nucleic Acids Res 22 597 603
52. TongAH
LesageG
BaderGD
DingH
XuH
2004 Global mapping of the yeast genetic interaction network. Science 303 808 813
53. PetterR
ChangYC
Kwon-ChungKJ
1997 A gene homologous to Saccharomyces cerevisiae SNF1 appears to be essential for the viability of Candida albicans. Infect Immun 65 4909 4917
54. EnloeB
DiamondA
MitchellAP
2000 A single-transformation gene function test in diploid Candida albicans. J Bacteriol 182 5730 5736
55. ShermanF
1991 Getting started with yeast.
GuthrieC
FinkGR
Methods in Enzymology San Diego, CA Academic Press, Inc 3 21
56. WilsonRB
DavisD
MitchellAP
1999 Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181 1868 1874
57. DavisDA
BrunoVM
LozaL
FillerSG
MitchellAP
2002 Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162 1573 1581
58. MaH
KunesS
SchatzPJ
BotsteinD
1987 Plasmid construction by homologous recombination in yeast. Gene 58 201 216
59. ArnaudMB
CostanzoMC
SkrzypekMS
ShahP
BinkleyG
2007 Sequence resources at the Candida Genome Database (CGD). Nucleic Acids Res 35 D452 456
60. RossignolT
LechatP
CuomoC
ZengQ
MoszerI
2008 CandidaDB: a multi-genome database for Candida species and related Saccharomycotina. Nucleic Acids Res 36 D557 561
61. GiaeverG
ChuAM
NiL
ConnellyC
RilesL
2002 Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 387 391
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



