-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
Plasmodium parasites must control cysteine protease activity that is critical for hepatocyte invasion by sporozoites, liver stage development, host cell survival and merozoite liberation. Here we show that exoerythrocytic P. berghei parasites express a potent cysteine protease inhibitor (PbICP, P. berghei inhibitor of cysteine proteases). We provide evidence that it has an important function in sporozoite invasion and is capable of blocking hepatocyte cell death. Pre-incubation with specific anti-PbICP antiserum significantly decreased the ability of sporozoites to infect hepatocytes and expression of PbICP in mammalian cells protects them against peroxide - and camptothecin-induced cell death. PbICP is secreted by sporozoites prior to and after hepatocyte invasion, localizes to the parasitophorous vacuole as well as to the parasite cytoplasm in the schizont stage and is released into the host cell cytoplasm at the end of the liver stage. Like its homolog falstatin/PfICP in P. falciparum, PbICP consists of a classical N-terminal signal peptide, a long N-terminal extension region and a chagasin-like C-terminal domain. In exoerythrocytic parasites, PbICP is posttranslationally processed, leading to liberation of the C-terminal chagasin-like domain. Biochemical analysis has revealed that both full-length PbICP and the truncated C-terminal domain are very potent inhibitors of cathepsin L-like host and parasite cysteine proteases. The results presented in this study suggest that the inhibitor plays an important role in sporozoite invasion of host cells and in parasite survival during liver stage development by inhibiting host cell proteases involved in programmed cell death.
Published in the journal: Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes. PLoS Pathog 6(3): e32767. doi:10.1371/journal.ppat.1000825
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000825Summary
Plasmodium parasites must control cysteine protease activity that is critical for hepatocyte invasion by sporozoites, liver stage development, host cell survival and merozoite liberation. Here we show that exoerythrocytic P. berghei parasites express a potent cysteine protease inhibitor (PbICP, P. berghei inhibitor of cysteine proteases). We provide evidence that it has an important function in sporozoite invasion and is capable of blocking hepatocyte cell death. Pre-incubation with specific anti-PbICP antiserum significantly decreased the ability of sporozoites to infect hepatocytes and expression of PbICP in mammalian cells protects them against peroxide - and camptothecin-induced cell death. PbICP is secreted by sporozoites prior to and after hepatocyte invasion, localizes to the parasitophorous vacuole as well as to the parasite cytoplasm in the schizont stage and is released into the host cell cytoplasm at the end of the liver stage. Like its homolog falstatin/PfICP in P. falciparum, PbICP consists of a classical N-terminal signal peptide, a long N-terminal extension region and a chagasin-like C-terminal domain. In exoerythrocytic parasites, PbICP is posttranslationally processed, leading to liberation of the C-terminal chagasin-like domain. Biochemical analysis has revealed that both full-length PbICP and the truncated C-terminal domain are very potent inhibitors of cathepsin L-like host and parasite cysteine proteases. The results presented in this study suggest that the inhibitor plays an important role in sporozoite invasion of host cells and in parasite survival during liver stage development by inhibiting host cell proteases involved in programmed cell death.
Introduction
Malaria is caused by apicomplexan parasites of the genus Plasmodium. The infection of the vertebrate host begins with the inoculation of sporozoites into the dermis during blood feeding of an infected Anopheles mosquito [1],[2],[3],[4],[5],[6],[7]. Sporozoites traverse through different cell types [8],[9],[10],[11],[12] until they reach the liver via the bloodstream and finally invade hepatocytes. Here within 2 to 16 days, depending on the Plasmodium species, they develop inside a parasitophorous vacuole to several thousand red blood cell-infective merozoites [13]. Using the rodent malaria model parasite P. berghei, it was shown that infected hepatocytes are protected against apoptosis throughout liver stage development [14],[15],[16]. However, at the end of the liver stage, the parasite induces an unusual form of programmed cell death that facilitates the merozoites leaving the liver and gaining access to the bloodstream [16],[17]. Even during this complex liberation process, which includes parasite-dependent host cell death, classical host cell apoptosis is not induced and cell membrane integrity is maintained [16], suggesting that parasite molecules responsible for inhibition of apoptosis are translocated to the host cell cytoplasm, at least during this last phase of parasite development in hepatocytes.
As in the blood stage and mosquito stage [18],[19], it was shown that cysteine proteases play a crucial role in the infection of the liver by Plasmodium sporozoites [20],[21], in parasite development in liver cells and in the liberation of the fully developed liver merozoites [16]. Therefore, a tight regulation of protease activity is critical for the survival of the parasite throughout its life cycle. Additionally, intra - and extracellular Plasmodium parasites are exposed to host cell proteases and it is likely that they have evolved mechanisms to counteract proteolytic digestion.
Host cell proteases are often involved in pathogen defense mechanisms and a number of other parasites have already been shown to express cysteine protease inhibitors that block these proteases. These include host cell proteases involved in antigen presentation, cytokine responses and host cell apoptosis and proteases that are stored in potentially fusogenic organelles like lysosomes and are liberated upon pathogen recognition [22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34].
Short-term regulation of parasite as well as host proteases can be mediated by specific parasite-derived inhibitors. A prominent example is chagasin, which is expressed by Trypanosoma cruzi and was the first identified member of a new superfamily of reversible, tight-binding cysteine protease inhibitors [35]. Structurally similar inhibitors were found in Trypanosoma brucei, Pseudomonas aeruginosa, Leishmania mexicana, Leishmania major, Entamoeba histolytica, P. falciparum and Toxoplasma gondii [36],[37],[38],[39],[40],[41],[42],[43]. Chagasin-like inhibitors (also termed ICPs for inhibitor of cysteine proteases) are suggested to regulate both endogenous parasite-derived cysteine proteases (T. brucei, T. cruzi, P. falciparum, E. histolytica) and/or host cell proteases (L. mexicana, P. aeruginosa, P. falciparum) [35],[37],[38],[39],[40],[41],[42],[44],[45],[46].
The P. falciparum ICP (PfICP), termed falstatin, has been described previously for the blood stage of the human malaria parasite [40]. Falstatin/PfICP has been characterized as a potent inhibitor of various parasite and host cell cysteine proteases and is expressed by blood schizonts, merozoites and rings but not in trophozoites. Incubation of late schizonts with neutralizing antibodies against falstatin/PfICP partially blocked subsequent invasion of erythrocytes by merozoites, suggesting that regulation of cysteine protease activity is important for this process.
Here we report on the falstatin homolog of the rodent malaria parasite P. berghei, which we name PbICP for Plasmodium berghei inhibitor of cysteine proteases, following the common nomenclature for the entire inhibitor family. PbICP appears to play a critical role at least during the parasite liver and blood stages in the vertebrate host. We analyzed specifically the exoerythrocytic parasite stages and suggest a function of PbICP in sporozoite invasion and host cell survival.
Materials and Methods
Experimental animals
Animals were obtained from Charles River Laboratories. All animal work was conducted in compliance with regulations created and approved by the ethical committee of Hamburg state authorities (Nr. FI 28/06).
Sequence alignments and expression and purification of recombinant PbICP proteins
Homology searches, multiple alignments and secondary structure predictions were performed with public BLAST search tools (PlasmoDB), the Clustal W program (EMBL-EBI) and structure prediction programs (PredictProtein, Jpred).
The pbicp gene (full length, PlasmoDB: PB000502.02.0), pbicp-n and pbicp-c were amplified from cDNA of P. berghei ANKA wildtype mixed blood stage parasites using the following primer pairs: PbICP-fw (5′-GG GAATTCGAAGATAACGACATATACTCTTTTGATATC-3′) / PbICP-rv (5′ - CCCAAGCTT TTATTGGACAGTCACGTATATAAT-3′) to clone MBP-PbICP full length, PbICP-C-fw1 (5′-TTGAATTCGGAGATGAAAAATGTGGTAAATCA-3′) / PbICP-C-rv1 (5′-TTGGATCC TTATTGGACAGTCACGTATATAAT-3′) to clone MBP-PbICP-C, PbICP-C-fw2 (5′ - TTCATATG GGAGATGAAAAATGTGGTAAATCA-3′) / PbICP-C-rv2 (5′-TTGAATTC TTATTGGACAGTCACGTATATAAT-3′) to clone His-PbICP-C and PbICP-C without tag, PbICP-N-fw1 (5′-GGGAATTCGAAGATAACGACATATACTCTTTTGATATC-3′) / PbICP-N-rv1 (5′-TT GGATCC TGGTTAAATGAGTTGTATGAAGTAGTTGGG -3′) to clone MBP-PbICP-N for antibody production, PbICP-N-fw2 (5′-GGGAATTCGAAGATAACGACATATACTCTTTTGATATC-3′) / PbICP-N-rv2 (5′-TTGGATCC AGTCAATTCATATTTACTATCAACTTTACCA -3′) to clone MBP-PbICP-N for protease assays.
The PCR products were cloned into the bacterial expression vectors (pJC25 for untagged PbICP-C, derivative of pJC20 [47]; pJC45 for N-terminal His10-tagged PbICP-C [48]; pMAL-cRI for N-terminal MBP tagged full length PbICP, PbICP-C and PbICP-N) using the appropriate restriction enzymes (New England Biolabs) and clones were analyzed by sequencing. Untagged PbICP-C and His-PbICP-C were expressed in the BL21 (DE3) pAPlacI E. coli strain [48] and purified using a MonoQ column (Pharmacia) or Ni-NTA resin (Qiagen), respectively. MBP-PbICP (full length), MBP-PbICP-C and MBP-PbICP-N were expressed in the BL21 E. coli strain (Stratagene) and purified using amylose resin (New England Biolabs). The MBP-tag of PbICP (full length) was removed by factor Xa (New England Biolabs) and untagged PbICP was purified by using a MonoQ column (Pharmacia).
Protease assays
Cathepsin L [49], papain (Sigma) or falcipain-2 [50] were incubated with 600 µM Z-Phe-Arg-pNA substrate (Bachem) in the presence or absence of indicated amounts of the recombinant purified proteins. Assay buffers were 100 mM acetate buffer, 1 mM DTT, pH 5.0 for cathepsin L and 100 mM acetate buffer, 10 mM DTT, pH 5.5 for papain and falcipain-2. Cathepsin B (Sigma) was incubated with 600 µM Z-Arg-Arg-pNA substrate (Bachem) in the presence or absence of the indicated amounts of the recombinant purified proteins in 100 mM KH2PO4, 2 mM EDTA, 10 mM DTT, pH 6.0. Photometric product formation (E) was measured every 10 seconds and activity was calculated from the linear part of the graph (ΔE/Δt). Protease activity in the presence of the control protein MBP was set to 100% and residual activity in the presence of recombinant PbICP constructs was calculated.
Duplex-PCR
Total RNA was isolated from 106 wildtype oocyst sporozoites, 6×105 wildtype salivary gland sporozoites, infected HepG2 cells at different time points after infection or from saponin-treated (0,05%, Sigma) blood stage parasites using the RNA Extract Kit II (Macherey and Nagel). First strand cDNA synthesis was performed with the Superscript™ First-Strand Synthesis System for RT-PCR (Invitrogen).
Duplex-PCR was performed with the two primer pairs PbICP-fw (5′-ATGCTCCATCCTAGCCCTTT-3′) / PbICP-rev (5′-CCACTTTCATTCATTGTGTTGTT-3′) and Pbtubulin-fw (5′ - TGGAGCAGGAAATAACTGGG-3′) / Pbtubulin-rev (5′-ACCTGACATAGCGGCTGAAA-3′). All RNA preparations were free of genomic DNA (gDNA) contamination as no PCR product was obtained when reverse transcriptase had been omitted from the RT-PCR (negative control).
Generation of antibodies
25 µg of each of the purified fusion proteins His-PbICP-C, MBP-PbICP (full length) and MBP-PbICP-N in PBS buffer were mixed with one volume Freund's adjuvant complete (Sigma) and intraperitoneally injected into BALB/c or NMRI mice. After two weeks, the mice were boosted with the same amount of protein mixed with Freund's adjuvant incomplete (Sigma) followed by a second boost two weeks later. Immunized mice were killed after confirming the antibody response in preliminary experiments, and blood was collected by cardiac puncture. Antisera were obtained after centrifugation and diluted with one volume glycerol (Roth) for long term storage at −20°C.
Rabbit antisera against His-PbICP-C, rabbit antisera against a peptide within the PbICP-N sequence and the appropriate preimmune sera were obtained from Eurogentec S.A.
In vitro infection of HepG2 cells
Human hepatoma cells (HepG2) were obtained from the European cell culture collection. Cells were cultivated at 37°C and 5% CO2 in EMEM (PAA) containing 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (all PAA Laboratories GmbH). For infection, 5×104 cells were seeded per coverslip in a 24-well plate. P. berghei (ANKA) sporozoites were prepared from salivary glands of infected Anopheles stephensi mosquitoes and incubated with HepG2 cells in EMEM (PAA) containing 3% bovine serum albumin (Sigma), 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (all PAA Laboratories GmbH) at 37°C and 5% CO2. After 2 hours, cells were washed and incubated in fresh culture medium for the indicated times.
SDS-PAGE and Western blotting
Parasite proteins were separated on 12% to 14% SDS-PAGE gels under reducing conditions and transferred to nitrocellulose membranes. Membranes were probed with rabbit antisera directed against His-PbICP-C or a peptide of PbICP-N, anti-GFP mouse monoclonal antibodies (Roche) or rabbit antisera (Molecular Probes) and mouse anti-tubulin monoclonal antibody (Sigma). Horseradish peroxidase-conjugated anti-rabbit (Cell Signalling) or anti-mouse IgG (Pierce or Rockland) and enhancing chemiluminescence substrate detection kits (Pierce and Bio-Rad) were used for detection.
Immunofluorescence microscopy
Sporozoite IFA
Sporozoites were isolated from salivary glands of A. stephensi mosquitoes and incubated on glass coverslips with or without HepG2 cells in EMEM (PAA) containing 3% bovine serum albumin (Sigma), 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (all PAA Laboratories GmbH) at 37°C and 5% CO2. After 2 hours, medium was carefully removed and sporozoites were fixed with 4% formaldehyde in PBS (20 min, room temperature), permeabilized with ice-cold methanol (10 min) and incubated with primary antisera (rabbit anti-His-PbICP-C, mouse anti-CSP, mouse anti-TRAP) and subsequently with fluorescently labeled secondary antibodies (Cy2-labeled antibodies, Dianova and Alexa594-labeled antibodies, Molecular Probes). DNA was visualized by staining with 10 µg/ml DAPI (Sigma). Labeled cells were analyzed by widefield or confocal microscopy using the Leica DM RB or Olympus FV1000.
Staining of unfixed sporozoites
Sporozoites expressing cytosolic mCherry [51] were isolated from salivary glands of A. stephensi mosquitoes and incubated for 40 minutes on ice in PBS with a 1∶100 dilution of rabbit antiserum directed against His-PbICP-C, the appropriate preimmune serum or mouse antiserum directed against CSP. Sporozoites were washed twice with cold PBS and subsequently incubated with a 1∶200 dilution of the Cy2-labeled anti-rabbit or anti-mouse secondary antibody (Dianova) and Hoechst 33258 (Molecular Probes) for 30 min on ice. Sporozoites were washed twice, settled on coverslips with DAKO Fluorescent Mounting Medium (DAKO) and immediately analyzed by widefield fluorescence microscopy using the Leica DM RB.
IFA of infected HepG2 cells
HepG2 cells were infected as described above. After the indicated time periods, cells were fixed with 4% formaldehyde in PBS (20 min, room temperature), permeabilized with ice-cold methanol (10 min) and incubated with primary antibody (chicken anti-ExpI, mouse anti-CSP) and subsequently with fluorescently labeled secondary antibodies (Cy2-labeled antibodies, Dianova and Alexa594-labeled antibodies, Molecular Probes). DNA was visualized by staining with 10 µg/ml DAPI (Sigma). Labeled cells were analyzed by widefield or confocal microscopy using the Leica DM RB or Olympus FV1000.
Immunoelectron microscopy
Midguts and salivary glands of P. berghei infected A. stephensi mosquitoes were isolated, fixed in 2% paraformaldehyde and 0.025% glutaraldehyde and slowly embedded in LR WhiteTM Resin (London Resin Company) after dehydration. Briefly, ultrathin sections were incubated with rabbit antiserum directed against His-PbICP-C and subsequently with gold (10 nm)-labeled Protein A.
For double staining, ultrathin sections were first incubated with anti-TRAP mouse antiserum and subsequently with gold (10 nm)-labeled anti-mouse IgG and afterwards with rabbit antiserum directed against His-PbICP-C and with gold (25 nm)-labeled Protein A.
Sections were stained with 2% uranyl acetate and 1∶10 diluted Reynold's lead citrate solution in 0.01 N NaOH. Sections were analyzed using the FEI Tecnai TEM.
Transfection of P. berghei
DNA was amplified from cDNA of P. berghei ANKA wildtype mixed blood stage parasites using the primer pair PbICPpL17-fw (5′-CTGGGATCCATGAAAAGTATAACTTTTTTCGTGTTTAAT-3′) / PbICPpL17-rev (5′-CTGGGATCCTTGGACAGTCACGTATATAATTTTAGTGTT-3′). The resulting fragment was cloned into the P. berghei GFP transfection plasmid pL0017 (MR4) using the BamHI site in front of the gfp gene. The plasmid was linearized for transfection using the restriction sites of SacII and ApaI in the ssu integration site sequence. Schizont-stage parasites were transfected with 2.5 and 7.5 µg purified plasmid DNA as described by Janse et al. [52].
Analysis of the transgenic parasites during the liver stage
To monitor parasite growth over the course of development, parasite size was measured by the density slice module of the OpenLab software version 5.0.2. Briefly, at different time points after infection (24 hpi (hours post infection), 48 hpi, 63 hpi), transgenic parasites over-expressing PbICP-GFP and control parasites were photographed. Images from each time point were merged and OpenLab software version 5.0.2 was used to produce a density slice of the image and to calculate the parasite areas.
An inside/outside assay to investigate infection efficiency was performed as follows: 1×105 HepG2 cells were seeded per coverslip in a 24-well plate. The following day, transgenic PbICP-GFP sporozoites and GFPcon sporozoites as control were prepared from salivary glands of infected A. stephensi mosquitoes and counted in a Neubauer chamber. 2×104 sporozoites per sample were added to the HepG2 cells. After 1 h incubation at 37°C and 5% CO2, cells were fixed for 2 min with 2% formaldehyde in PBS (no permeabilization) and incubated with rabbit or mouse anti-CSP antiserum and subsequently with fluorescently labeled secondary antibody (Alexa594-labeled antibodies, Dianova). DNA was visualized by staining with 10 µg/ml DAPI (Sigma). Sporozoites that have invaded cells are protected against CSP staining and are visualized just by the GFP fluorescence. In contrast, free GFP-positive sporozoites are stained by the CSP antibodies. Free and intracellular sporozoites were counted and their percentages were calculated.
Sporozoites neutralization assays
Pre-incubation in antisera
The neutralization assay was mainly performed as described by Kumar et al. [53]. 1×105 HepG2 cells were seeded per coverslip in a 24-well plate. The following day, P. berghei (ANKA) sporozoites were prepared from salivary glands of infected A. stephensi mosquitoes and counted in a Neubauer chamber. For the inside/outside assay, red fluorescent sporozoites expressing cytosolic mCherry protein were used [51]. 2×104 sporozoites per sample were incubated in 30 µl of the anti-His-PbICP-C rabbit antiserum, the appropriate preimmune serum or anti-CSP rabbit antiserum for 40 min on ice. Afterwards, pre-warmed medium containing 3% bovine serum albumin (Sigma), 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (all PAA Laboratories GmbH) was added to the samples. The sporozoite suspension was then added to HepG2 cells. Analysis of the neutralization assays was performed by different established methods (transmigration assay, inside/outside assay for invading sporozoites or counting of infected hepatocytes 30 hpi (detailed description see below)).
Transmigration assay
The suspension of sporozoites was mixed with 1 mg/ml dextran-fluorescein (10,000 MW, Molecular Probes) prior being added to the HepG2 cells. 1 h after incubation at 37°C and 5% CO2, cells were washed three times in PBS, fixed with 4% formaldehyde in PBS (20 min, room temperature) and permeabilized with ice-cold methanol (10 minutes). To visualize the sporozoites, CSP-staining was performed (mouse anti-CSP, Alexa594-labeled anti-mouse antibody, Molecular Probes). DNA was stained with 10 µg/ml DAPI (Sigma). The number of transmigrated cells was determined by calculating the percentage of dextran-fluorescein-positive cells as a percentage of all cells. HepG2 cells without sporozoites served as control (basal rate of wounded cells).
Inside/outside assay
1 h after incubation at 37°C and 5% CO2, cells were fixed for 2 min with 2% formaldehyde in PBS (no permeabilization) and incubated with rabbit or mouse anti-CSP antiserum and subsequently with fluorescently labeled secondary antibody (Cy2-labeled antibodies, Dianova). DNA was visualized by staining with 10 µg/ml DAPI (Sigma). Sporozoites that have invaded cells are protected against the CSP-staining and were visualized only by the mCherry expression. Free mCherry sporozoites were additionally stained by the CSP antibodies. Sporozoites were counted and the percentage of free and intracellular sporozoites was calculated.
Counting of infected hepatocytes in the schizont stage
After 2 h incubation at 37°C and 5% CO2, cells were washed and fresh culture medium was added. 24 to 30 hours after infection, cells were used for IFA and the numbers of infected HepG2 cells per coverslip were determined. More than 200 infected cells were counted in the preimmune controls and set to 100% for each of the three independent experiments.
Transfection of HepG2 cells and cell death assays
DNA was amplified from cDNA of P. berghei ANKA wildtype mixed blood stage parasites using the primer pair PbICP-CpEGFP-fw (5′-TTGAATTCGGAGATGAAAAATGTGGTAAATCA-3′) / PbICP-C pEGFP -rev (5′-TTGGATCCTTATTGGACAGTCACGTATATAAT-3′).
The resulting fragment was cloned into the pEGFP-C2 vector (Clontech) to generate a GFP-PbICP-C-fusion protein. The vector pEGFP-C2 alone was used as the GFP control.
HepG2 cells were grown in single wells of a 6-well plate at 70–80% confluence and transfected using MATra technology (IBA) as recommended by the manufacturer. Briefly, cells were washed with PBS and reduced-serum OptiMEM media (PAA) was added. 5 µg of the appropriate plasmid DNA was pre-incubated with 5 µl of MATra-A reagent in OptiMEM media for 20 min at room temperature and then added to the cells. The cells were incubated with the DNA-MATra complex on a universal magnetic plate (IBA) for 30 min at 37°C and 5% CO2.
The next day transfected cells were treated with 70 nM tBHP (tert-butylhydroperoxid, Sigma-Aldrich) for 4 hours or 1 µM CAM (camptothecin, Sigma-Aldrich) for 48 hours. Cells were washed with PBS and stained with 25 nM TMRE (tetramethylrhodaminethylesterperchlorat, Molecular Probes) and 16 µM Hoechst 33258 (Molecular Probes) at 37°C and 5% CO2 for 30 min. Live imaging was performed using an inverse microscope (Axiovert 25, Zeiss) and the Openlab 5 software (Improvision).
Results
Sequence peculiarities, expression and biochemical characterization of PbICP
To investigate the regulation of cysteine protease activity by exoerythrocytic P. berghei parasites, we analyzed the structural and biochemical properties of the P. berghei homolog of falstatin/PfICP, PbICP. It belongs to the chagasin-like inhibitor family (MEROPS-family I42, termed ICP for inhibitor of cysteine proteases). ICPs are so far the only cysteine protease inhibitors known to exist in protozoan parasites. Typically, ICPs are small proteins with a low sequence similarity to each other but a characteristic β-sheet-rich secondary structure similar to immunoglobulins [54],[55],[56],[57]. Three loops (L2, L4, L6) form a wedge-like structure that extends into the active site cleft of C1 family cysteine proteases (papain superfamily). The ICPs of the apicomplexan protozoan parasites Plasmodium and Toxoplasma gondii are unusual chagasin family members [40],[43] in that they differ more than other members in sequence and even in length of the wedge-forming loops (Figure S1, S2A). Moreover, the Plasmodium ICPs contain an additional N-terminal extension region resulting in an overall molecular weight of about 40 kDa. PbICP shares approximately 40% amino acid sequence identity with falstatin/PfICP but only about 21% with chagasin of Trypanosoma cruzi, the first described member of the inhibitor family. Secondary-structure prediction programs propose that PbICP contains the characteristic β-sheet-rich structure with two additional β-strands (β5′and β5″) between the elongated L3 and L4 (Figure S2B).
The pbicp gene consists of two exons (exon 1 : 63 bp; exon 2 : 999 bp) and an intron of 519 bp. The first exon codes for a classical signal peptide while the second exon codes for the mature protein.
To confirm that PbICP acts as a cysteine protease inhibitor, we produced recombinant PbICP as an MBP (maltose binding protein) fusion protein in E. coli, purified the protein by affinity chromatography and removed the MBP tag by factor Xa digestion and subsequent ion exchange chromatography (Figure 1A). PAGE analysis of the purified proteins revealed MBP-PbICP to be 97 kDa in size (55 kDa without tag), which is considerably larger than the size calculated from the amino acid sequence (82 kDa and 40 kDa with and without tag, respectively). We tested the purified MBP-tagged and untagged PbICP in protease assays using cysteine proteases of the C1 family that are the known targets of chagasin-like inhibitors. 1 µM and 100 nM of both tagged and untagged PbICP strongly inhibited papain, the P. falciparum cysteine protease falcipain-2 and human cathepsin-L (Figure 1A, Table 1). Like falstatin [40], PbICP did not inhibit cathepsin B. This is in contrast to chagasin, the ICP of L. mexicana (LmICP) and the ICP of T. brucei (TbICP) [37],[58], which are all reported to inhibit cathepsin B, to various extents. We compared the ICP1 of E. histolytica (EhICP1) and PbICP in their capacity to inhibit cathepsin B and found that EhICP1 blocked this protease but PbICP did not (data not shown).
Fig. 1. PbICP is a potent inhibitor of C1 cysteine proteases and the pbicp gene is constitutively transcribed. 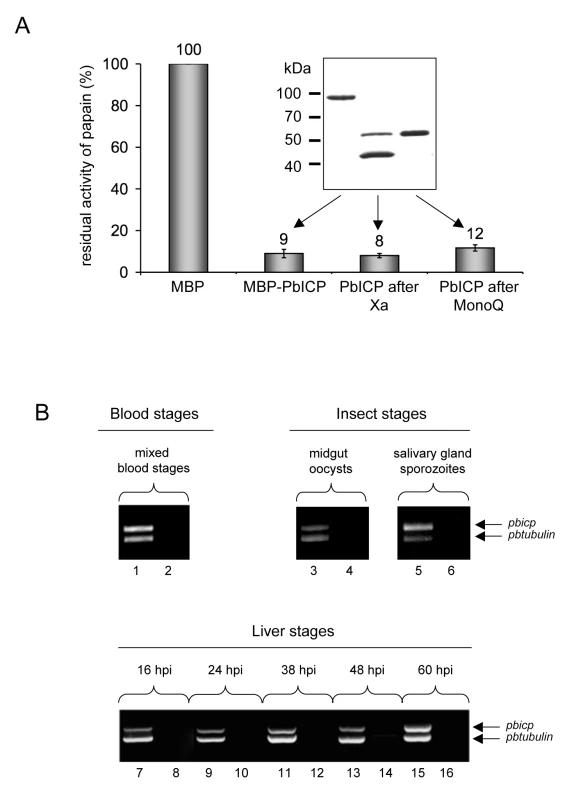
(A) Recombinant PbICP inhibits papain activity. Recombinant PbICP was produced in E. coli as an MBP-tagged soluble protein and purified from the bacterial lysate by amylose-bead affinity chromatography (insert, lane 1). The MBP tag was cleaved off by factor Xa digestion (insert, lane 2) and PbICP was purified using a MonoQ column (insert, lane 3). Proteins were resolved by SDS-PAGE and stained with Coomassie Blue. Hydrolysis of the substrate Z-Phe-Arg-pNA by papain was measured in the presence of 100 nM of the control protein MBP or 100 nM of MBP-PbICP, factor Xa-cleaved MBP-PbICP or PbICP after tag cleavage and MonoQ purification. Protease activity in presence of 100 nM MBP was considered 100% and the percentage of residual protease activity was calculated from that. MBP: maltose binding protein. (B) Constitutive transcription of the pbicp gene. RNA was isolated from P. berghei-infected mouse blood, mosquito midgut and salivary glands and from infected HepG2 cells. The purified RNA was then used for a duplex RT-PCR with specific primer pairs for the amplification of pbicp cDNA and the tubulin cDNA of P. berghei. In samples with odd numbers, cDNA was used as a template. In samples with even numbers, reverse transcriptase was omitted from the cDNA preparation reaction to control for the presence of genomic DNA. Tab. 1. Recombinant PbICP is a potent inhibitor of cysteine proteases but not of cathepsin B. 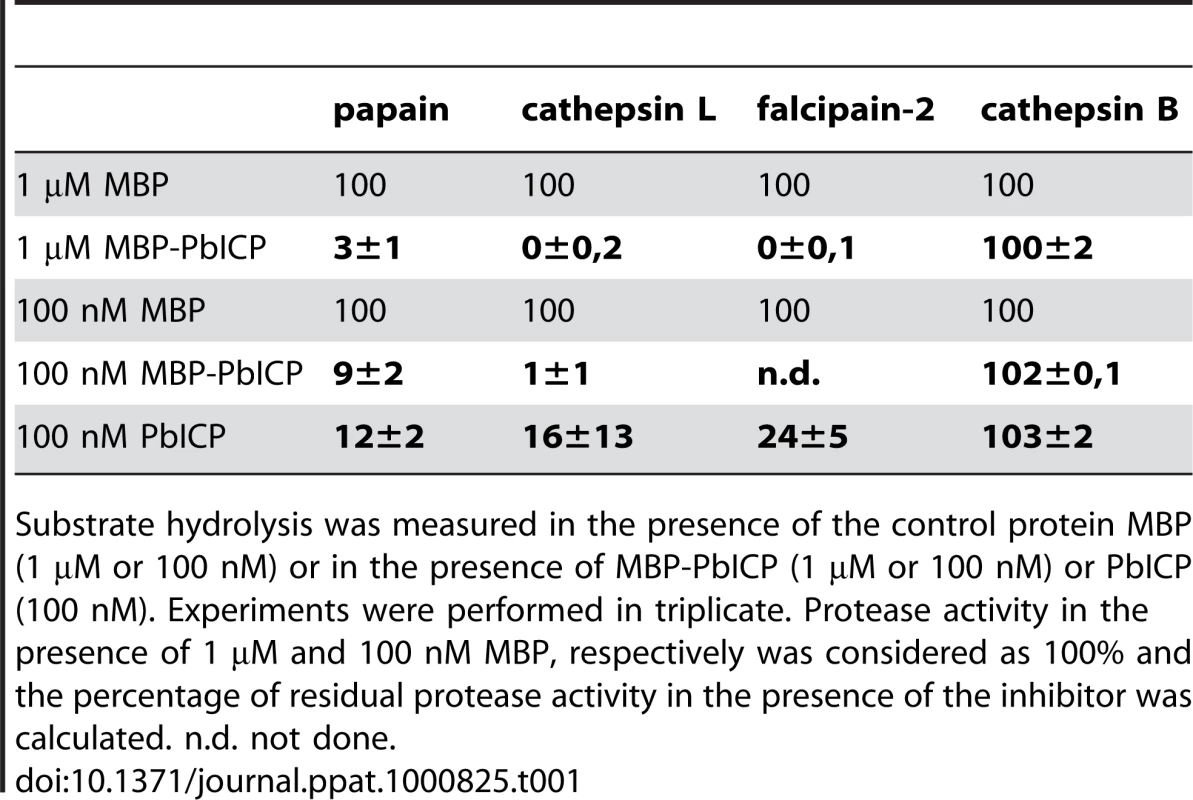
Substrate hydrolysis was measured in the presence of the control protein MBP (1 µM or 100 nM) or in the presence of MBP-PbICP (1 µM or 100 nM) or PbICP (100 nM). Experiments were performed in triplicate. Protease activity in the presence of 1 µM and 100 nM MBP, respectively was considered as 100% and the percentage of residual protease activity in the presence of the inhibitor was calculated. n.d. not done. PbICP is expressed throughout the life cycle of P. berghei
Our next aim was to analyze expression of PbICP in various life cycle stages of P. berghei. First, we investigated pbicp mRNA expression. RNA was isolated from P. berghei insect stages (oocyst and salivary gland sporozoites), red blood cells from infected NMRI mice and infected hepatoma cells. Duplex RT-PCR assays suggested that pbicp, like tubulin, is constitutively transcribed throughout the life cycle stages analyzed (Figure 1B).
To determine PbICP expression in the exoerythrocytic parasite stages at the protein level, we produced specific antisera against recombinant PbICP in rabbits as well as mice and used them in indirect immunofluorescence analysis (IFA), immunoelectron microscopy (IEM) and western blot analysis. An overview is provided of the different antisera generated against the full-length PbICP, PbICP domains (PbICP-N and PbICP-C) and PbICP peptides (Figure S3), as well as of representative experiments demonstrating the specificity of the antisera (Figure S4 and S5).
In agreement with the transcription profile of the pbicp gene, the PbICP protein could be detected in all exoerythrocytic parasite stages (Figures 2–4). PbICP expression and localization in sporozoites, liver schizonts and detached cells/merosomes will now be explained in detail.
Fig. 2. PbICP is secreted by salivary gland sporozoites. 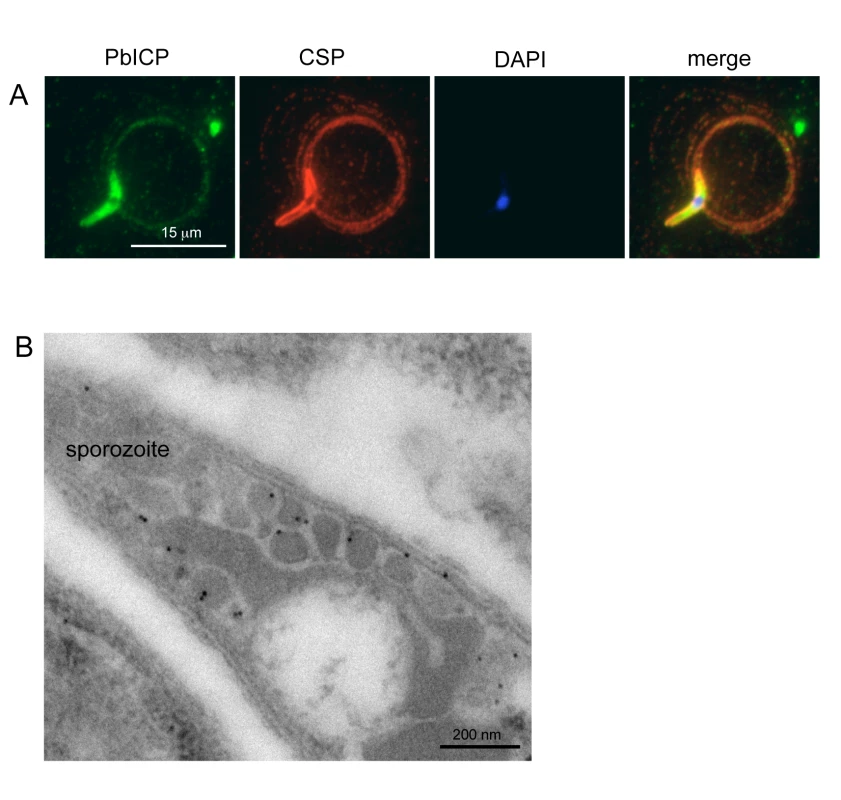
(A) Widefield IFA of P. berghei sporozoites that have been allowed to glide on glass coverslips. Sporozoites were fixed after 1 h and subsequently stained with anti-CSP antiserum (mouse, red) and polyclonal antiserum against PbICP-C (rabbit, green). DNA was stained with DAPI (blue). Like the shedded surface protein CSP, PbICP was detected in a patchy pattern in the trails of the circling sporozoites. (B) IEM confirms PbICP localization in vesicles of a midgut sporozoite. P. berghei infected A. stephensi midguts were fixed 20 days after infection and prepared for IEM. Ultrathin sections were stained with polyclonal antiserum directed against PbICP-C (rabbit) and subsequently with gold-labeled Protein A (10 nm gold particles). The sections were contrasted with uranyl acetate and lead citrate. Fig. 3. PbICP partially colocalizes with TRAP at the apical pole and in vesicles of sporozoites. 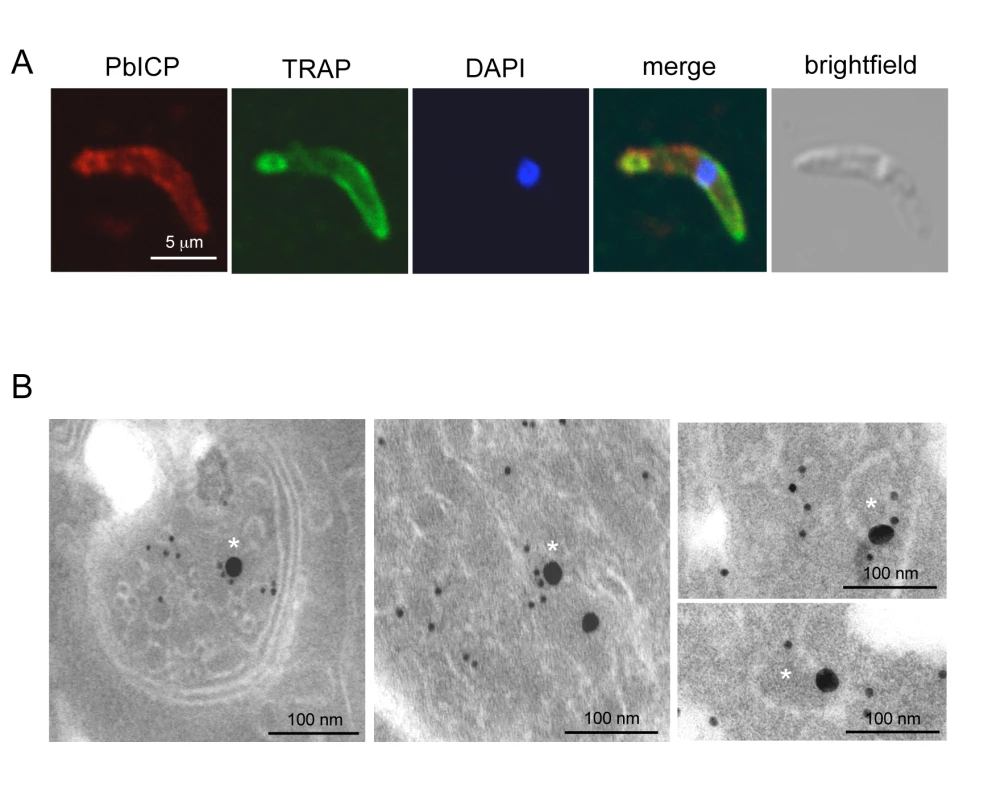
(A) Confocal IFA of an extracellular P. berghei sporozoite in the presence of HepG2 cells. HepG2 cells were co-cultivated for 1 h with sporozoites, fixed and stained with an anti-TRAP antiserum (mouse, green) and a polyclonal antiserum against PbICP-C (rabbit, red). DNA was stained with DAPI (blue). (B) Double-stained IEM of a salivary gland sporozoite shows partial co-localization of TRAP and PbICP in vesicles (marked with asterisk). P. berghei-infected A. stephensi salivary glands were fixed 25 days after infection and prepared for IEM. Ultrathin sections were stained with PbICP-C (rabbit, 25 nm gold particles) and anti-TRAP (mouse, 10 nm gold particles). The sections were contrasted with uranyl acetate and lead citrate. Fig. 4. PbICP is expressed throughout the liver stage and localizes to different microenvironments. 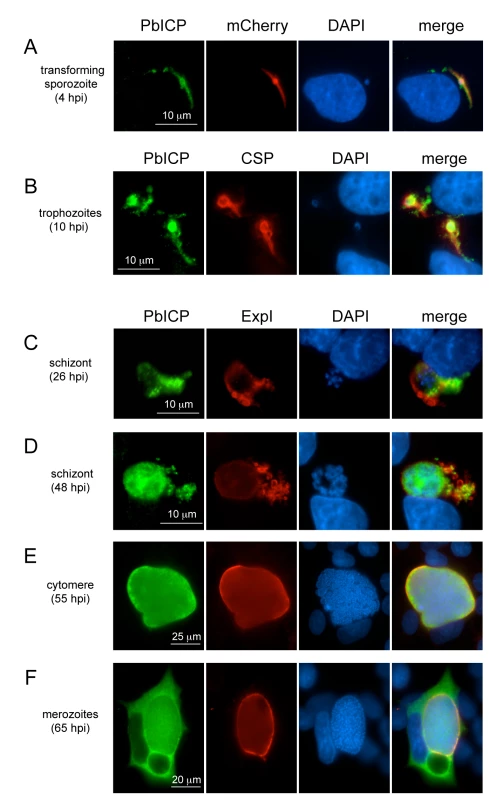
Wide-field IFA of HepG2 cells infected with P. berghei-expressing mCherry in the cytoplasm (A) or wildtype P. berghei (B-F) at different time points after infection (hpi, hours post infection). Infected cells were fixed, incubated with anti-CSP antiserum (mouse, red) (B) or with anti-ExpI antiserum (chicken, red) (C-F) and antiserum against PbICP-C (rabbit, green) (A-F). DNA was stained with DAPI (blue). Sporozoites secrete PbICP
Extracellular gliding sporozoites were fixed and stained with an antiserum directed against the C-terminal domain of PbICP, revealing that sporozoites secrete the inhibitor (Figure 2A). PbICP was detected in distinct areas of the parasite and in protein trails left behind by gliding sporozoites. These trails also contain the major sporozoite surface protein CSP (circumsporozoite protein) that is shed during gliding motility., In permeabilized sporozoites, PbICP staining showed a patchy pattern suggesting a localization in secretory vesicles, which was confirmed by IEM (Figure 2B). TRAP (Thrombospondin-related anonymous protein) is a micronemal protein and, in the presence of host hepatocytes, accumulates at the apical end of the sporozoite [59],[60]. IFA revealed a similar staining pattern for PbICP, suggesting that an exocytosis-like secretion of the inhibitor occurs as for TRAP (Figure 3A). In addition, staining of unfixed sporozoites with an anti-PbICP-C antiserum confirmed the localization at the apical pole of the sporozoites (Figure S5).
To investigate whether the PbICP-containing vesicles are micronemes, we performed double staining IEM analysis of sporozoites using TRAP as a micronemal marker protein (Figure 3B). In agreement with the confocal IFA, the IEM analysis showed a partial colocalization of TRAP and PbICP in secretory vesicles. Thus, we conclude that at least a portion of PbICP is translocated to the micronemes.
Following invasion of hepatocytes, intracellular parasites continue secreting PbICP, which was detected in the host cell cytoplasm (Figure 4A,B, Figure S6, Figure S7). To ensure we were monitoring invading and not transmigrating parasites, we fixed cells at 4 hpi (hours post infection), when sporozoites are no longer motile. At this stage, some sporozoites had already begun transformation into early trophozoites (Figure S6A). To confirm export of PbICP into the host cytoplasm, we compared the localization of PbICP with that of CSP, a molecule known to be secreted by young trophozoites. Although we did not detect co-localization of these proteins, the distribution of CSP and PbICP in the infected hepatocyte was similar for the majority of parasites, indicating PbICP secretion (Figure S6B).
In later liver stages, PbICP is translocated into the parasitophorous vacuole and is released into the hepatocyte cytoplasm upon merozoite formation
The PVM of early schizont stages can be stained with an antiserum against the PVM-marker protein Exp1 of P. berghei. PbICP co-localized partly with Exp1 but was additionally clearly seen outside of the ring-shaped Exp1 staining, strongly suggesting that the inhibitor is in contact with the host cell cytoplasm (Figure 4C). In later schizont and cytomere stages, PbICP localized mainly to the PV (Figure 4D, E, Figure S4B, Figure S8, Figure S9), but was also found in the parasite cytosol. For these stages we could not detect PbICP in the host cytoplasm. However, PbICP was frequently found in close proximity to Exp1-positive structures that appear to bud off or be released from the PVM (Figure 4D, Figure S4B, Figure S8 and Figure S9).
After completion of daughter parasite development, the PVM starts to disintegrate and this clearly correlates with marked PbICP release into the hepatocyte cytoplasm (Figure 4F, Figure S10) suggesting that PbICP could have an important function in the regulation of host cell proteases and the regulated host cell death induced at this time. Upon PVM rupture and PbICP release, merozoites are liberated into the host cell cytoplasm and thus are in direct contact with PbICP, which might protect them from proteolytic damage.
PbICP is posttranslationally processed
Western blot analysis using anti-PbICP antisera revealed that PbICP is subject to posttranslational processing during the sporozoite stage and frequently also during the blood stage of the parasite (Figure 5A). Antisera directed against the C-terminal chagasin-like domain of PbICP not only detected a protein that corresponds to the full-length PbICP but also a 23 kDa protein that corresponds to the chagasin-like C-terminal domain of PbICP. An antiserum directed against the N-terminal extension region of PbICP only detected the full-length inhibitor, indicating that the N-terminal region is rapidly degraded after the proteolytic cleavage. While in sporozoites processing of PbICP was always detected, the situation was less clear for blood stage parasites. In protein extracts of this stage we sometimes exclusively found unprocessed PbICP (Figure 5A), similarly to what has been published for falstatin/PfICP of P. falciparum [40] but in other preperations processed PbICP was also detected. As both non-processing and partial processing were seen in the schizont stage, the degree of processing does not seem to relate to the developmental stage of the parasite. Since the focus of this study was on exoerythrocytic parasites, we did not follow up this interesting phenomenon in detail. Blood stage protein extracts were nevertheless used as an easily accessible source of protein for western blotting to address questions unrelated to processing. Importantly, the size of the full-length inhibitor detected by western blotting corresponds to the size of recombinant PbICP, suggesting that parasite-derived PbICP is not posttranslationally modified other than by processing (data not shown). To analyze processing of PbICP during liver stage development by western blotting, we generated a P. berghei strain expressing a PbICP-GFP fusion protein under the control of a strong constitutive promotor (eef1aa) (Figure 5C). PbICP overexpression was necessary because infection rates of HepG2 cells are in general very low (2–10%) and do not allow detection of endogenous proteins by western blotting. Using this PbICP-overexpressing transgenic strain for HepG2 cell infection, it was then possible to demonstrate PbICP processing before and after the detachment of infected hepatocytes at the end of the liver stage (Figure 5D). Overexpression of the PbICP-GFP fusion protein had no negative effects on parasite development suggesting that the presence of the fusion protein is not toxic for exoerythrocytic parasites (Figure S11A-E). Interestingly, transgenic parasites constitutively expressing PbICP-GFP showed a slightly but significantly increased level of HepG2 cell invasion compared to wildtype parasites (Figure S12). To prove PbICP processing also occurs in wildtype parasites, we employed immunofluorescence analysis using antisera against both PbICP-C and PbICP-N. We reasoned that co-localization of these domains would indicate unprocessed PbICP whereas staining exclusively with the PbICP-C antiserum would indicate PbICP processing. The result of the immunofluorescence analysis confirmed a proteolytic cleavage of endogenous PbICP during the schizont stage of the parasite (Figure 5B).
Fig. 5. PbICP is posttranslationally processed in the blood stage and exoerythrocytic stages. 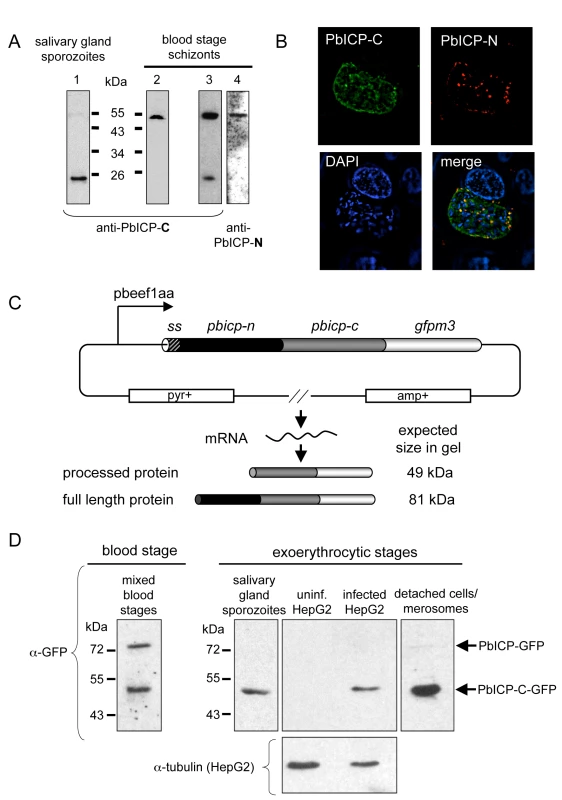
(A) Domain-specific western blot analysis of protein extracts prepared from P. berghei salivary gland sporozoites and blood stage schizonts. Protein extracts of blood stage schizonts (two independent samples: one in lane 2 and a second one in lane 3+4) and sporozoites were separated by SDS-PAGE and blotted on nitrocellulose filters. Filters were then probed with antisera against the chagasin-like domain of PbICP (anti-PbICP-C, rabbit, lane 1–3) or the N-terminal domain (anti-PbICP-N, lane 4). Anti-PbICP-C antiserum detected the full-length inhibitor (55 kDa) as well as the processed form after cleavage of the N-terminal extension domain (23 kDa), whereas anti-PbICP-N antiserum detected only the full-length protein of 55 kDa. (B) IFA of a P. berghei-infected HepG2 cell 48 hpi (widefield deconvolution). Infected cells were fixed and stained with anti-PbICP-N antiserum (mouse, red) and antiserum against PbICP-C (rabbit, green). DNA was stained with DAPI (blue). The images were deconvoluted to remove out-of-focus signals. (C) Schematic illustration of the transfection plasmid pL0017-pbicp-gfp for constitutive strong expression of PbICP. The pbicp-gfpm3 coding region (ss, signal sequence of pbicp) is under the control of the strong constitutive pbeef1aa promotor. The vector confers ampicillin resistance (amp+) in E. coli and pyrimethamine resistance (pyr+) in transfected P. berghei parasites. Following transfection, P. berghei parasites constitutively express the fusion protein. The expected in gel sizes of the GFP-tagged full-length PbICP and the GFP-tagged processed form of the inhibitor were calculated and are presented. (D) Anti-GFP western blot analysis of GFP-PbICP-expressing P. berghei blood stage extracts, sporozoite total lysates, infected HepG2 cell total lysates (60 hours after infection) and detached infected HepG2 cells/merosomes. Uninfected HepG2 cells served as a specificity control and reprobing with anti-tubulin antiserum served as a loading control for protein extracts of infected and uninfected HepG2 cells. The anti-GFP antiserum detected proteins that correspond to the size of the full-length GFP-tagged inhibitor (81 kDa) as well as the processed form after cleavage of the N-terminal extension domain (49 kDa). The C-terminal domain is necessary and sufficient for the inhibitory function of PbICP
We then investigated whether the C-terminal domain of PbICP on its own has inhibitory potential. PbICP-C, the region homologous to chagasin, was expressed as an MBP fusion protein (Figure 6A) and used in protease assays (Figure 6B, Table 2). Full-length MBP-PbICP served as a positive control, MBP without any fusion as a negative control. Additionally, PbICP-C was expressed and purified without a tag and included in the analysis. Both MBP-PbICP-C and PbICP-C blocked papain, cathepsin L and falcipain-2 activity to the same extent as full-length MBP-PbICP, whereas the tagged N-terminal domain (MBP-PbICP-N) did not show any inhibitory effect on protease activity (Figure 6B, Table 2). None of the recombinant proteins blocked the activity of cathepsin B. Since the full-length inhibitor and PbICP-C did not differ in inhibitory strength and specificity for the analyzed proteases, the N-terminal domain does not appear to have a modulatory effect on the inhibitor function at least in a non-cellular environment.
Fig. 6. The C-terminal domain of PbICP is necessary and sufficient for the inhibitor function. 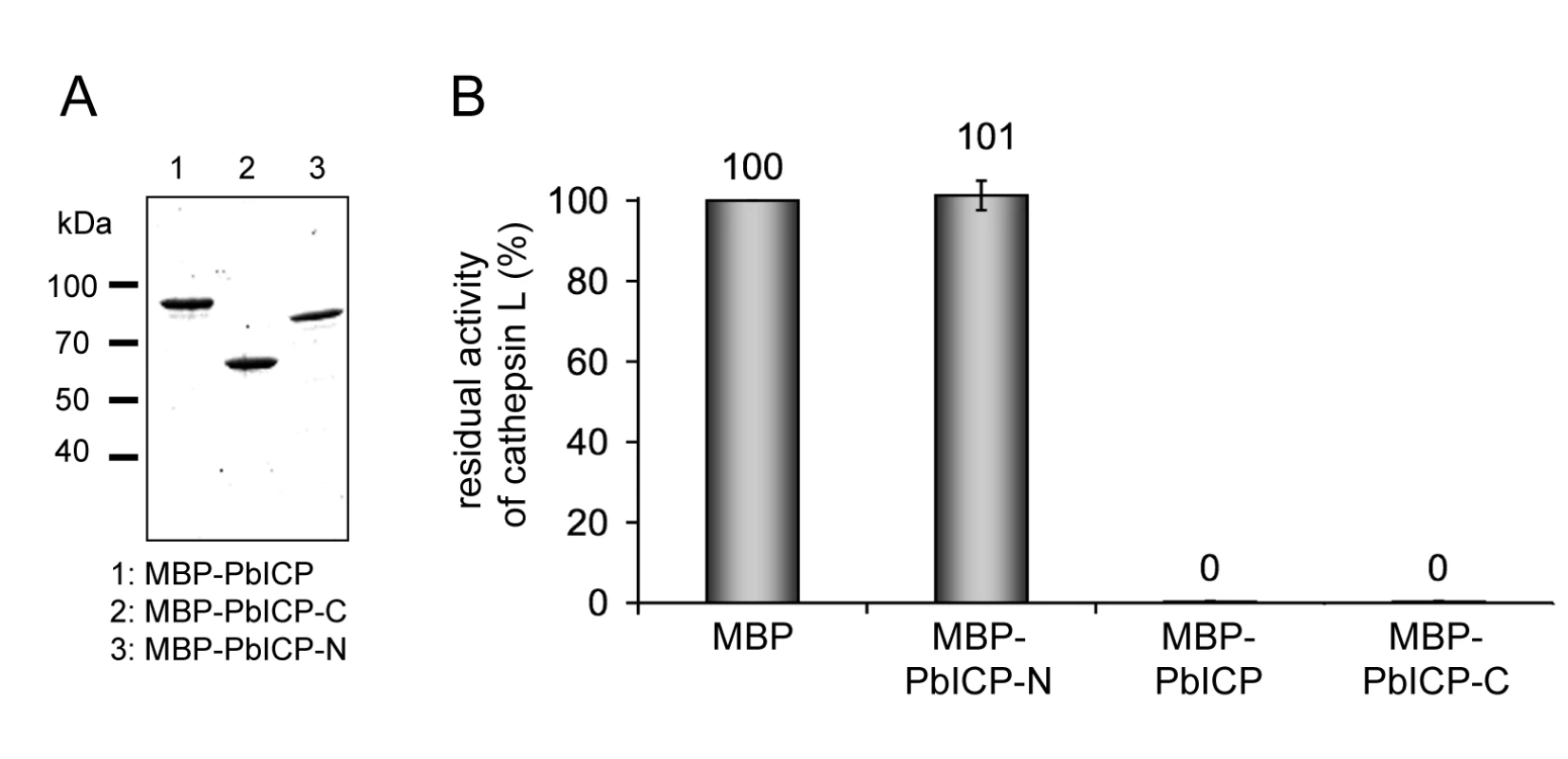
(A) Purification of recombinant PbICP, PbICP-C and -N. Like the full-length protein, recombinant PbICP-C and -N were expressed in E. coli as MBP-tagged soluble proteins and purified from the bacterial total lysate by amylose-bead affinity chromatography (lane 1, 2, 3). The proteins were resolved by SDS-PAGE and stained with Coomassie Blue. (B) Recombinant PbICP-C inhibits the cysteine protease cathepsin L to a similar extent as full-length PbICP, whereas PbICP-N does not block cathepsin L activity. Hydrolysis of the substrate Z-FR-pNA by cathepsin L was measured in the presence of 1 µM MBP-PbICP-N, MBP-PbICP or MBP-PbICP-C or the control protein MBP. Tab. 2. The C-terminal domain but not the N-terminal domain of PbICP is a potent inhibitor of cysteine proteases except cathepsin B. 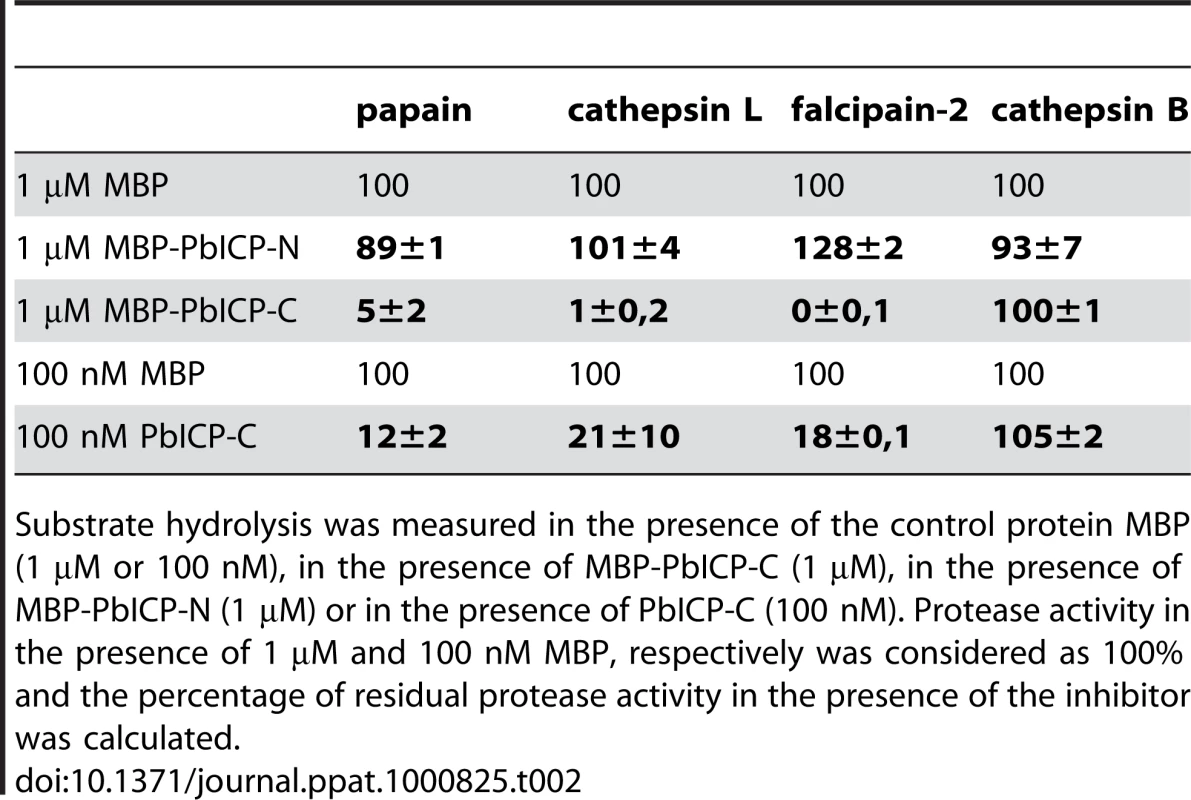
Substrate hydrolysis was measured in the presence of the control protein MBP (1 µM or 100 nM), in the presence of MBP-PbICP-C (1 µM), in the presence of MBP-PbICP-N (1 µM) or in the presence of PbICP-C (100 nM). Protease activity in the presence of 1 µM and 100 nM MBP, respectively was considered as 100% and the percentage of residual protease activity in the presence of the inhibitor was calculated. PbICP is involved in sporozoite invasion of hepatocytes
Since PbICP is secreted by sporozoites, we tested whether the protein plays a role during sporozoite transmigration through cells and invasion of HepG2 cells in vitro (Figure 7). Sporozoites were pre-incubated in anti-PbICP antiserum and then used for the different assays. Interestingly, the number of transmigrated cells was higher for sporozoites pre-incubated with anti-PbICP antiserum compared to sporozoites pre-incubated with anti-CSP antiserum or preimmune serum (Figure 7A). However, since this effect was not significant, we did not follow up this observation. The most important conclusion from the transmigration assay was that neutralization of PbICP does not interfere with the traversal capability of sporozoites. Following transmigration, sporozoites finally invade cells and reside in a PV. To investigate the effect of PbICP blockage on the invasion process, we combined the invasion assay with an inside/outside assay that allowed us to distinguish between extracellular and intracelluar parasites [5] (Figure 7B). Preincubation of sporozoites with antiserum against PbICP-C significantly reduced the level of HepG2 cell infection by P. berghei sporozoites by about 40% (from 37.6% to 22.8% in Figure 7B). The inhibitory effect on sporozoite invasion by blocking PbICP activity fits well with the observation that transgenic P. berghei parasites over-expressing a GFP-PbICP fusion protein exhibited an improved invasion rate (Figure S12).
Fig. 7. Antibody-mediated neutralization of PbICP reduces infectivity of sporozoites in vitro but does not inhibit cell traversal of sporozoites. 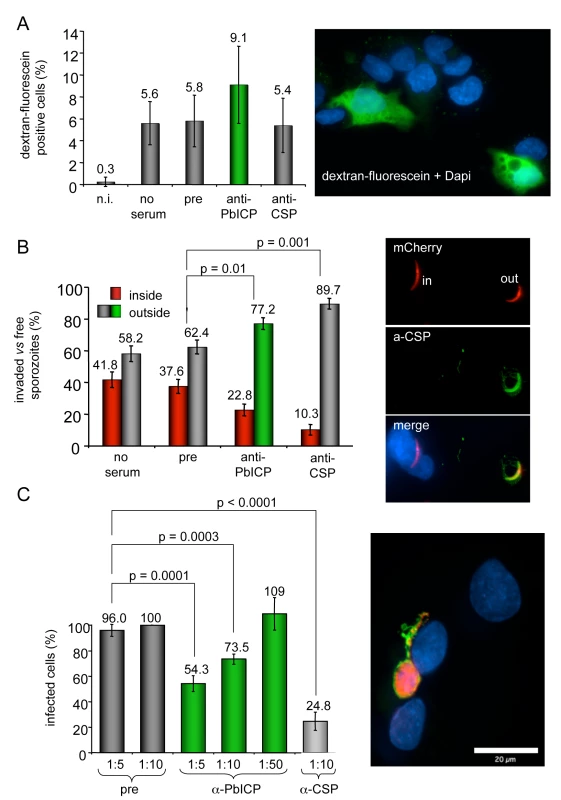
(A) Transmigration assay: P. berghei sporozoites were pre-incubated with medium (no serum) preimmune serum (1∶5), anti-PbICP-C antiserum (1∶5) or anti-CSP-antiserum (1∶5) and subsequently added to HepG2 cells in the presence of 1 mM dextran-fluorescein for 1 h to stain wounded cells. Non-infected HepG2 cells (n.i.) cultivated in the presence of 1 mM dextran-fluorescein for 1 h served as a negative control for cell wounding. After this incubation period cells were washed, fixed and the DNA was stained with DAPI to allow visualization of both intact and wounded cells. The total cell number compared to dextran-fluorescein positive cells was determined using an immunofluorescence microscope and the percentage of dextran-fluorescein positive cells was calculated. Presented are the means and standard deviations of three independent experiments. The image presented shows a number of cells, whose nuclei are stained with DAPI. Transmigrated cells are additionally stained with dextran-fluorescein (green). (B) Invasion assay: P. berghei sporozoites expressing mCherry were pre-incubated with preimmune serum (1∶5), anti-PbICP-C antiserum (1∶5) or anti-CSP-antiserum (1∶5) and subsequently added to HepG2 cells. After 1 h, cells were fixed but not permeabilized and stained with an anti-CSP antiserum (inside/outside assay). All sporozoites can be detected by their mCherry expression but only extracellular sporozoites are additionally stained by the anti-CSP antiserum. DNA was stained with DAPI (blue). Free and intracellular sporozoites were counted and the percentage was calculated. Presented are the means and standard deviations of three independent experiments. Typical examples of intracellular and extracellular parasites are presented in the image next to the graph. mCherry expression can be seen in all sporozoites but additional CSP staining (green) is visible only in extracellualr parasites. (C) Parasite development following PbICP neutralization of sporozoites: HepG2 cell infection was performed as described in (B). Serum dilutions (1∶5 to 1∶50) used to pre-incubate sporozoites are indicated. Infected cells were fixed 30 hpi and stained with anti-Exp1 antiserum (chicken, green) and anti-PbICP-C antiserum (rabbit, red) (image shows a typical example of a infected cell 30 hpi stained as described). Infected cells in the individual wells were counted and the percentage of infected cells after treatment was calculated from three independent experiments. The number of infected cells in wells infected with sporozoites that had been exposed to a 1∶10 dilution of the preimmune serum, was considered as 100%. As in the CSP-control, pre-incubation with anti-PbICP-antiserum significantly reduced the infectivity of sporozoites. To exclude an effect of PbICP neutralization during invasion on parasite development, we additionally analyzed infected cells 30 hpi. The number of parasites that developed to the schizont stage (see image in Figure 7C) was significantly reduced by 46% or 26% when sporozoites were pre-treated with 1∶5 or 1∶10 dilutions, respectively, of the anti-PbICP antiserum in comparison to the pre-immune control serum (Figure 7C). The reduction of developing parasites by 46% after pretreatment of sporozoites with 1∶5 dilution of the serum reflects the result of the inside/outside assay, where the number of invaded sporozoites was reduced by almost the same extent (40%). In conclusion, the blocking capacity of the antiserum is restricted to sporozoite neutralization and does not affect later parasite development. As expected, preincubation of sporozoites in 1∶10 diluted anti-CSP antiserum resulted in a strong (75%) reduction of the number of infected HepG2 cells 30 hpi.
PbICP blocks host cell death
Since PbICP is released into the host cell cytoplasm both by sporozoites and at the end of the liver stage, the question is raised whether host cell cysteine proteases are target molecules of the parasite inhibitor. Cysteine proteases such as caspases, calpains and cathepsins are often key enzymes in programmed cell death execution. Interestingly, when host hepatocytes undergo their unusual cell death upon merozoite release from the PVM, caspases are not activated and other classical signs of programmed host cell death are also absent, including DNA fragmentation and phosphatidylserine switching to the outer plasma membrane leaflet [16]. We hypothesized that PbICP may modulate activation of programmed host cell death by inhibiting host cell cysteine proteases. HepG2 cells were transfected with a plasmid coding for N-terminally GFP-tagged PbICP-C or with a control vector and subsequently cell death was induced by treatment with tert-butyl hydroperoxide (tBHP). Viability of cells was analyzed by staining with TMRE, a dye which only labels mitochondria with intact membrane potentials and thus indicates viable cells. Additionally, cells were stained with the DNA dye Hoechst 33258 to visualize chromatin condensation of dying cells (Figure 8A). In contrast to the control cells expressing GFP, of which only 26% were alive, after 7 h of tBHP treatment, 80% of the GFP-PbICP-C - expressing HepG2 cells were still viable (Figure 8B). Similar results were achieved when infected cells were treated with the apoptosis-inducing agent camptothecin (CAM) for 48 h (Figure S14). From these experiments we conclude that PbICP indeed has the capacity to suppress or interfere with the cell death machinery of hepatocytes by blocking host cell cysteine proteases involved in cell death execution. The possible function of PbICP during the entire exoerythrocytic development in vitro is summarized in Figure 9.
Fig. 8. PbICP-C expression protects HepG2 cells against host cell death. 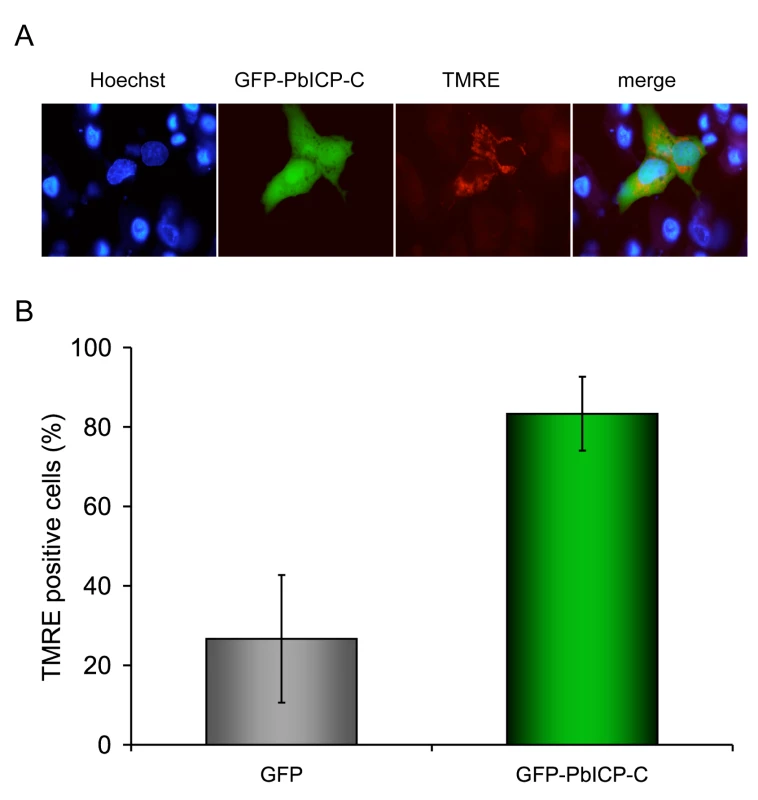
(A) HepG2 cells were transiently transfected with plasmids leading to either cytosolic expression of GFP-tagged PbICP-C or GFP as a control. The transfection efficiency of HepG2 cells with the control plasmid and the GFP-PbICP-C plasmid (absolute numbers of GFP-positive cells/cover) were similar. Host cell death was induced by tBHP treatment for 4 h and analyzed by live imaging using TMRE staining of intact mitochondria (red). DNA condensation was visualized by Hoechst 33258 staining (blue). Dying cells exhibited condensed chromatin in the nucleus and a loss of the mitochondrial membrane potential. (B) Fluorescent cells were counted and the percentage of dead and viable cells was calculated. Presented are the means and standard deviations of three independent experiments. Cells expressing GFP-PbICP-C showed a significantly better survival following tBHP treatment in comparison to GFP-expressing cells. Fig. 9. Possible roles of PbICP during the exoerythrocytic development of P. berghei in vitro. 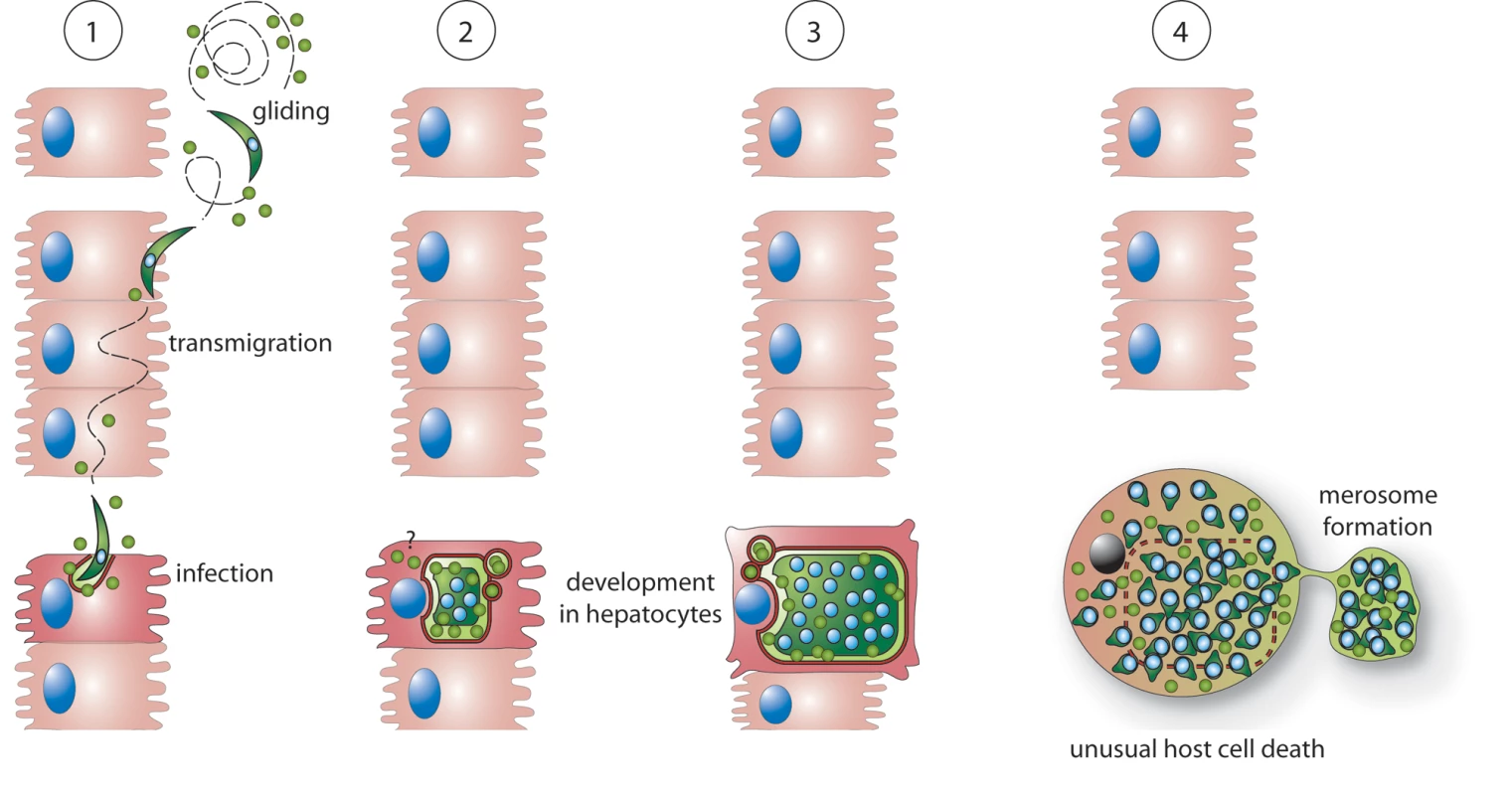
1: PbICP (green circles) is secreted by free gliding sporozoites and supports invasion of HepG2 cells. Intracellular sporozoites and trophozoites continue to express and partially secrete PbICP. Thus PbICP can potentially control parasite-derived cysteine proteases as well as host cell cysteine proteases. 2+3: During schizogony, PbICP is predominately located in the PV and the parasite cytosol, suggesting that during this developmental phase, parasite cysteine proteases are the main target of the inhibitor. However, it cannot be excluded that small portions of the inhibitor, which are beyond the detection level for IFA, are still exported into the host cell cytoplasm. 4: At the end of the liver stage, upon disruption of the PVM, a substantial amount of PbICP is passively released into the host cell cytoplasm. At this stage, the main function of PbICP might be inhibition of host cell cysteine proteases to allow a slow and ordered host cell death and merosome formation. Discussion
Parasite and host cell cysteine proteases play an important role during the entire life cycle of the Plasmodium parasite. Cysteine protease activity is essential for sporozoite egress from oocysts in the mosquito [19], sporozoite invasion of hepatocytes in the vertebrate host [20],[21], liver stage development and liberation of exo-erythrocytic merozoites from hepatocytes [16] as well as for invasion, nutrition and egress in the blood stage [18]. All these protease activities must be strictly regulated to prevent undesired and harmful proteolytic activity. In addition to controlling its own proteases, the malaria parasite is exposed to proteases of the host, such as those from inflammatory cells that are secreted to eliminate pathogens and perhaps also intra-hepatocyte proteases encountered by the parasite during liver stage development.
In this study, we report the identification of the potent cysteine protease inhibitor PbICP, the falstatin/PfICP homolog of the rodent malaria parasite P. berghei. Like falstatin/PfICP [40], PbICP is an unusual member of the chagasin inhibitor family with obvious structural peculiarities due to (1) the long N-terminal extension domain, (2) sequence insertions in the chagasin-like C-terminal domain and (3) amino acid exchanges in conserved motifs. The inhibitor is expressed in all analyzed stages of P. berghei (blood stage, sporozoites, liver stages) and according to its different localizations it can potentially control parasite as well as host cell-derived proteases.
We provide several lines of evidence that PbICP is secreted by P. berghei sporozoites to support host cell invasion. First, the inhibitor was detected in typical CSP-positive sporozoite trails [61] by IFA of fixed parasites using a specific anti-PbICP antiserum. Second, IEM analysis revealed the presence of PbICP in vesicles within sporozoites. Third, some of the PbICP-positive vesicles appear to be secretory micronemes since they were found by IEM analysis to be positive for both PbICP and the micronemal marker protein TRAP [62],[63],[64],[65]. Fourth, IFA of live sporozoites revealed PbICP to be in a characteristic protein cap formation at the apical pole, as has been seen for the TRAP protein after regulated exocytosis [59],[60]. Finally, pre-incubation of viable sporozoites in anti-PbICP immune serum significantly reduced the number of sporozoites invading HepG2 cells.
PbICP does not contain an obvious micronemal targeting motif but since these motifs are rather heterogenous (as in TRAP and EBL proteins [62],[66]), empirical studies will be necessary to determine the motif that targets PbICP to the micronemes of the sporozoite. As a soluble protein, PbICP may even lack such a motif but might be sorted by formation of complexes with escorter proteins, as is known for soluble micronemal MIC proteins of Toxoplasma [67].
Our results are supported by a previous study on the Plasmodium gallinaceum ICP [68], which was termed PgSES for P. gallineceum sporozoite and erythrocyte stage protein. In this study, PgSES/PgICP was not identified as a cysteine protease inhibitor but was characterized as a secreted sporozoite protein with a characteristic localization pattern. Similar to what we describe for P. berghei sporozoites, LaCrue et al. found a patchy intracellular distribution of PgSES/PgICP in the sporozoite and an extracellular staining pattern distinct from CSP, mainly on salivary gland sporozoites [68].
The target protease of sporozoite-secreted PbICP remains to be identified, but interestingly it was reported that a cysteine protease-dependent CSP cleavage is essential for the invasion process [20],[21]. The responsible protease has not yet been identified but is of parasite origin and becomes activated upon interaction of the parasite with highly sulfated heparan sulfate proteoglycans (HSPGs) on hepatocytes. Since PbICP is capable of regulating cysteine proteases, and since both molecules act outside of the sporozoite, it is possible that PbICP controls this parasite protease in a timely and well-orchestrated manner to avoid premature CSP proteolysis.
When sporozoites were pre-incubated with anti-PbICP antiserum, we not only observed a reduced invasion rate but also a tendency of enhanced transmigration. It has been suggested earlier that sporozoites which are blocked from invasion persist in the migratory state [21] and it might well be that we provoked a similar effect by neutralizing the protease inhibitor.
In agreement with our findings that PbICP contributes to hepatocyte infection by P. berghei sporozoites, many of the so far characterized chagasin-like inhibitors of other intracellular protozoan parasites also play an important role during host cell invasion processes. Falstatin/PfICP is released together with P. falciparum merozoites upon rupture of infected red blood cells. Treatment with antibodies directed against falstatin/PfICP decreased the subsequent invasion of erythrocytes in a dose-dependent manner, suggesting a role in limiting unwanted proteolysis during erythrocyte invasion [40]. The ICPs of Trypanosoma are predicted to regulate endogenous proteases of the parasite [45],[46]. Amongst other effects on differentiation and protein turnover, overexpression of chagasin in T. cruzi resulted in a reduced infection rate in vitro, while T. brucei ICP null mutants reached higher parasitemia levels in mice [45],[46]. Endogenous L. mexicana ICP had no impact on the infectivity of the parasites in vitro. However, when LmICP null mutants and overexpressors were analysed in vivo in the mouse model system, both showed a reduced virulence and infectivity [38]. It is suggested therefore, that LmICP controls host-derived rather than parasite-derived proteases.
Upon sporozoite invasion of hepatocytes and transformation into trophozoites, PbICP is still secreted by the parasite, entering the PV and apparently reaching the host cell cytoplasm. Since a PEXEL export motif is absent in PbICP, it is so far unknown how the inhibitor crosses the PVM. It has been shown previously that P. berghei infection protects host cells from apoptosis [14],[15] and PbICP translocated to the host cell cytoplasm might be involved in the inhibition of caspases, which are cysteine proteases involved in programmed cell death execution. Indeed, we provide the first evidence that PbICP expression in the host cell cytoplasm is sufficient to inhibit parasite-independent host cell death. Interestingly, Pandey et al. have shown that falstatin/PfICP efficiently blocks proteolytic activity of caspases in nano - to micromolar concentrations [40]. It remains to be shown how PbICP could act on enzymes as different as cathepsin-L-like proteases and caspases. For C1 family members such as cathepsins, it is known that ICPs bind to the active site cleft between the R and the L domain, but caspases do not contain such a cleft [69]. A possible explanation is that Plasmodium ICPs act similarly to members of the serpin family due to the sequence insertions, which lead to elongated loops. Serpins inhibit serine and cysteine proteases by using a flexible loop (reactive site loop) as a bait to trap structurally different proteases [70]. If Plasmodium ICPs indeed function similarly to serpins, this would explain why falstatin/PfICP is an efficient inhibitor of structurally different proteases.
At the end of the liver stage, the situation changes completely and an ordered parasite-mediated cell death is induced. This form of cell death clearly differs from apoptosis since caspases are not involved, there is no switch in phosphatidylserine residues to the outer leaflet of the host cell membrane and the dying cell does not shrink or form apoptotic bodies but rather expands in size [16]. Since this parasite-induced cell death can be inhibited by treatment with the cysteine protease inhibitor E64, cysteine proteases appear to be key players in this process. We found considerable amounts of PbICP in the host cell upon PVM breakdown. Thus, it can be predicted that the cysteine proteases responsible for cell death induction belong to the cathepsin-B type that is not targeted by PbICP. Potential candidates are SERA proteases, a family of parasite-derived putative cysteine proteases. They are released into the host cell cytoplasm at the same time as PbICP. Unfortunately, experimental proof of the hypothesis that PbICP does not inhibit SERA protease activity is not possible because SERA proteases cannot currently be recombinantly produced in their enzymatically active state. Why would the parasite not simply allow the host cell to undergo apoptosis but instead use its own proteases to induce host cell death? The answer might be that activation of apoptosis-related proteases such as caspases and calpain-1 induces a rapid destruction of the cytoskeleton and a breakdown of the cell into apoptotic bodies or rupture of the host cell membrane. A rapid destruction of the host cell would result in a premature release of merozoites before they have been transported inside merosomes safely into the blood vessels. Merosome formation and merozoite transport can last for several hours and thus the parasite needs to maintain control of host cell proteases. On the other hand, the parasite requires cysteine proteases that induce an ordered but slow host cell death and allow the formation of merosomes.
To further analyze the function of PbICP translocated into the host cell cytoplasm, it will be necessary to identify its target protease. We suggest that PbICP acts on both parasite and host cell proteases. An effect on host cell proteases has already been shown for a number of ICPs of other pathogens. A good example is the prokaryote P. aeruginosa, which expresses a functional chagasin homolog but no potential target proteases of the C1 protease family [37], suggesting that chagasin-like inhibitors in general may have evolved to inhibit foreign proteases.
All so-far characterized chagasin-like inhibitors have shown a significantly lower affinity to cathepsin B than to cathepsin L-like C1 cysteine proteases [37],[58]. For chagasin, it was shown that this decreased cathepsin B affinity is due to the occluding loop that is in close proximity to the active site of the protease [58] [71]. In contrast to the so-far characterized chagasin members of other organisms, the Plasmodium ICPs show a complete loss of cathepsin B inhibition [40]. Plasmodium ICPs contain sequence insertions in the chagasin-like C-terminal domain, which might, in principle, interfere with cathepsin B binding. A recent publication reports that T. gondii-derived toxostatins, two other members of the chagasin family, contain similar extensions as found in the chagasin-like domain of PbICP. Remarkably, toxostatin-1 was already shown to retain the capability of inhibiting cathepsin B [43]. Together with our own data, it can be concluded that the lack of cathepsin B inhibition by Plasmodium ICPs is not caused by an interference of the extension loops with the occluding loop of the protease but must be due to other structural motifs.
To characterize the function of PbICP, we generated transgenic P. berghei parasites constitutively over-expressing a GFP-tagged version of the inhibitor. Although these parasites were very helpful for analyzing PbICP localization and processing during the liver stage, the genetic manipulation did not provoke a pronounced phenotype apart from a slightly better invasion rate. The best genetic manipulation to analyze the biological function of PbICP would be to knock out the pbicp gene. This approach has been tried several times without success, strongly suggesting that PbICP expression is essential during the blood stage because transfection and the selection of transgenic parasites is performed at this stage.
In conclusion, we identified a potent cysteine protease inhibitor of P. berghei that seems to play different roles during the life cycle of the malaria parasite. In the exo-erythrocytic stage, PbICP is important for the invasion of sporozoites and is able to protect the host cell from apoptosis, which is essential for the completion of liver stage development.
Supporting Information
Zdroje
1. SidjanskiS
VanderbergJP
1997 Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am J Trop Med Hyg 57 426 429
2. MatsuokaH
YoshidaS
HiraiM
IshiiA
2002 A rodent malaria, Plasmodium berghei, is experimentally transmitted to mice by merely probing of infective mosquito, Anopheles stephensi. Parasitol Int 51 17 23
3. VanderbergJP
FrevertU
2004 Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol 34 991 996
4. MedicaDL
SinnisP
2005 Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun 73 4363 4369
5. AminoR
ThibergeS
MartinB
CelliS
ShorteS
2006 Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med 12 220 224
6. JinY
KebaierC
VanderbergJ
2007 Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect Immun 75 5532 5539
7. AminoR
ThibergeS
BlazquezS
BaldacciP
RenaudO
2007 Imaging malaria sporozoites in the dermis of the mammalian host. Nat Protoc 2 1705 1712
8. MotaMM
PradelG
VanderbergJP
HafallaJC
FrevertU
2001 Migration of Plasmodium sporozoites through cells before infection. Science 291 141 144
9. PradelG
FrevertU
2001 Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 33 1154 1165
10. IshinoT
YanoK
ChinzeiY
YudaM
2004 Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol 2 e4 doi:10.1371/journal.pbio.0020004
11. BaerK
RooseveltM
ClarksonABJr
van RooijenN
SchniederT
2007 Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol 9 397 412
12. AminoR
GiovanniniD
ThibergeS
GueirardP
BoissonB
2008 Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3 88 96
13. PrudencioM
RodriguezA
MotaMM
2006 The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol 4 849 856
14. LeiriaoP
AlbuquerqueSS
CorsoS
van GemertGJ
SauerweinRW
2005 HGF/MET signalling protects Plasmodium-infected host cells from apoptosis. Cell Microbiol 7 603 609
15. van de SandC
HorstmannS
SchmidtA
SturmA
BolteS
2005 The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol Microbiol 58 731 742
16. SturmA
AminoR
van de SandC
RegenT
RetzlaffS
2006 Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313 1287 1290
17. BaerK
KlotzC
KappeSH
SchniederT
FrevertU
2007 Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog 3 e171 doi:10.1371/journal.ppat.0030171
18. RosenthalPJ
2004 Cysteine proteases of malaria parasites. Int J Parasitol 34 1489 1499
19. AlyAS
MatuschewskiK
2005 A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. J Exp Med 202 225 230
20. CoppiA
Pinzon-OrtizC
HutterC
SinnisP
2005 The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med 201 27 33
21. CoppiA
TewariR
BishopJR
BennettBL
LawrenceR
2007 Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2 316 327
22. HartmannS
KyewskiB
SonnenburgB
LuciusR
1997 A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol 27 2253 2260
23. ManouryB
GregoryWF
MaizelsRM
WattsC
2001 Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr Biol 11 447 451
24. HartmannS
LuciusR
2003 Modulation of host immune responses by nematode cystatins. Int J Parasitol 33 1291 1302
25. SchierackP
LuciusR
SonnenburgB
SchillingK
HartmannS
2003 Parasite-specific immunomodulatory functions of filarial cystatin. Infect Immun 71 2422 2429
26. MurrayJ
ManouryB
BalicA
WattsC
MaizelsRM
2005 Bm-CPI-2, a cystatin from Brugia malayi nematode parasites, differs from Caenorhabditis elegans cystatins in a specific site mediating inhibition of the antigen-processing enzyme AEP. Mol Biochem Parasitol 139 197 203
27. KotsyfakisM
Sa-NunesA
FrancischettiIM
MatherTN
AndersenJF
2006 Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem 281 26298 26307
28. SchnoellerC
RauschS
PillaiS
AvagyanA
WittigBM
2008 A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol 180 4265 4272
29. Sa-NunesA
BaficaA
AntonelliLR
ChoiEY
FrancischettiIM
2009 The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J Immunol 182 7422 7429
30. HackerG
HawkinsCJ
SmithKG
VauxDL
1996 Effects of viral inhibitors of apoptosis in models of mammalian cell death. Behring Inst Mitt 118 126
31. ZhouQ
SalvesenGS
2000 Viral caspase inhibitors CrmA and p35. Methods Enzymol 322 143 154
32. BlottEJ
GriffithsGM
2002 Secretory lysosomes. Nat Rev Mol Cell Biol 3 122 131
33. LuzioJP
PryorPR
BrightNA
2007 Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8 622 632
34. Jose CazzuloJ
StokaV
TurkV
2001 The major cysteine proteinase of Trypanosoma cruzi: a valid target for chemotherapy of Chagas disease. Curr Pharm Des 7 1143 1156
35. MonteiroAC
AbrahamsonM
LimaAP
Vannier-SantosMA
ScharfsteinJ
2001 Identification, characterization and localization of chagasin, a tight-binding cysteine protease inhibitor in Trypanosoma cruzi. J Cell Sci 114 3933 3942
36. RigdenDJ
MosolovVV
GalperinMY
2002 Sequence conservation in the chagasin family suggests a common trend in cysteine proteinase binding by unrelated protein inhibitors. Protein Sci 11 1971 1977
37. SandersonSJ
WestropGD
ScharfsteinJ
MottramJC
CoombsGH
2003 Functional conservation of a natural cysteine peptidase inhibitor in protozoan and bacterial pathogens. FEBS Lett 542 12 16
38. BesteiroS
CoombsGH
MottramJC
2004 A potential role for ICP, a Leishmanial inhibitor of cysteine peptidases, in the interaction between host and parasite. Mol Microbiol 54 1224 1236
39. RiekenbergS
WitjesB
SaricM
BruchhausI
ScholzeH
2005 Identification of EhICP1, a chagasin-like cysteine protease inhibitor of Entamoeba histolytica. FEBS Lett 579 1573 1578
40. PandeyKC
SinghN
Arastu-KapurS
BogyoM
RosenthalPJ
2006 Falstatin, a cysteine protease inhibitor of Plasmodium falciparum, facilitates erythrocyte invasion. PLoS Pathog 2 e117 doi:10.1371/journal.ppat.0020117
41. SaricM
VahrmannA
BruchhausI
Bakker-GrunwaldT
ScholzeH
2006 The second cysteine protease inhibitor, EhICP2, has a different localization in trophozoites of Entamoeba histolytica than EhICP1. Parasitol Res 100 171 174
42. SatoD
Nakada-TsukuiK
OkadaM
NozakiT
2006 Two cysteine protease inhibitors, EhICP1 and 2, localized in distinct compartments, negatively regulate secretion in Entamoeba histolytica. FEBS Lett 580 5306 5312
43. HuangR
QueX
HirataK
BrinenLS
LeeJH
2009 The cathepsin L of Toxoplasma gondii (TgCPL) and its endogenous macromolecular inhibitor, toxostatin. Mol Biochem Parasitol 164 86 94
44. SantosCC
ScharfsteinJ
LimaAP
2006 Role of chagasin-like inhibitors as endogenous regulators of cysteine proteases in parasitic protozoa. Parasitol Res 99 323 324
45. SantosCC
Sant'annaC
TerresA
Cunha-e-SilvaNL
ScharfsteinJ
2005 Chagasin, the endogenous cysteine-protease inhibitor of Trypanosoma cruzi, modulates parasite differentiation and invasion of mammalian cells. J Cell Sci 118 901 915
46. SantosCC
CoombsGH
LimaAP
MottramJC
2007 Role of the Trypanosoma brucei natural cysteine peptidase inhibitor ICP in differentiation and virulence. Mol Microbiol 66 991 1002
47. ClosJ
BrandauS
1994 pJC20 and pJC40–two high-copy-number vectors for T7 RNA polymerase-dependent expression of recombinant genes in Escherichia coli. Protein Expr Purif 5 133 137
48. SchluterA
WiesgiglM
HoyerC
FleischerS
KlaholzL
2000 Expression and subcellular localization of cpn60 protein family members in Leishmania donovani. Biochim Biophys Acta 1491 65 74
49. TurkB
DolencI
TurkV
BiethJG
1993 Kinetics of the pH-induced inactivation of human cathepsin L. Biochemistry 32 375 380
50. HoggT
NagarajanK
HerzbergS
ChenL
ShenX
2006 Structural and functional characterization of Falcipain-2, a hemoglobinase from the malarial parasite Plasmodium falciparum. J Biol Chem 281 25425 25437
51. GraeweS
RetzlaffS
StruckN
JanseCJ
HeusslerVT
2009 Going live: A comparative analysis of the suitability of the RFP derivatives RedStar, mCherry and tdTomato for intravital and in vitro live imaging of Plasmodium parasites. Biotechnol J
52. JanseCJ
RamesarJ
WatersAP
2006 High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1 346 356
53. KumarKA
OliveiraGA
EdelmanR
NardinE
NussenzweigV
2004 Quantitative Plasmodium sporozoite neutralization assay (TSNA). J Immunol Methods 292 157 164
54. RigdenDJ
MonteiroAC
Grossi de SaMF
2001 The protease inhibitor chagasin of Trypanosoma cruzi adopts an immunoglobulin-type fold and may have arisen by horizontal gene transfer. FEBS Lett 504 41 44
55. SmithBO
PickenNC
WestropGD
BromekK
MottramJC
2006 The structure of Leishmania mexicana ICP provides evidence for convergent evolution of cysteine peptidase inhibitors. J Biol Chem 281 5821 5828
56. SalmonD
do Aido-MachadoR
DiehlA
LeidertM
SchmetzerO
2006 Solution structure and backbone dynamics of the Trypanosoma cruzi cysteine protease inhibitor chagasin. J Mol Biol 357 1511 1521
57. Figueiredo da SilvaAA
de Carvalho VieiraL
KriegerMA
GoldenbergS
ZanchinNI
2007 Crystal structure of chagasin, the endogenous cysteine-protease inhibitor from Trypanosoma cruzi. J Struct Biol 157 416 423
58. RedzyniaI
LjunggrenA
AbrahamsonM
MortJS
KrupaJC
2008 Displacement of the occluding loop by the parasite protein, chagasin, results in efficient inhibition of human cathepsin B. J Biol Chem 283 22815 22825
59. MotaMM
HafallaJC
RodriguezA
2002 Migration through host cells activates Plasmodium sporozoites for infection. Nat Med 8 1318 1322
60. OnoT
Cabrita-SantosL
LeitaoR
BettiolE
PurcellLA
2008 Adenylyl cyclase alpha and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathog 4 e1000008 doi:10.1371/journal.ppat.1000008
61. StewartMJ
VanderbergJP
1991 Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J Protozool 38 411 421
62. BhanotP
FrevertU
NussenzweigV
PerssonC
2003 Defective sorting of the thrombospondin-related anonymous protein (TRAP) inhibits Plasmodium infectivity. Mol Biochem Parasitol 126 263 273
63. GanttS
PerssonC
RoseK
BirkettAJ
AbagyanR
2000 Antibodies against thrombospondin-related anonymous protein do not inhibit Plasmodium sporozoite infectivity in vivo. Infect Immun 68 3667 3673
64. RobsonKJ
FrevertU
ReckmannI
CowanG
BeierJ
1995 Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. Embo J 14 3883 3894
65. SultanAA
ThathyV
FrevertU
RobsonKJ
CrisantiA
1997 TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90 511 522
66. TreeckM
StruckNS
HaaseS
LangerC
HerrmannS
2006 A conserved region in the EBL proteins is implicated in microneme targeting of the malaria parasite Plasmodium falciparum. J Biol Chem 281 31995 32003
67. ReissM
ViebigN
BrechtS
FourmauxMN
SoeteM
2001 Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. J Cell Biol 152 563 578
68. LaCrueAN
SivaguruM
WalterMF
FidockDA
JamesAA
2006 A ubiquitous Plasmodium protein displays a unique surface labeling pattern in sporozoites. Mol Biochem Parasitol 148 199 209
69. RawlingsND
MortonFR
BarrettAJ
2006 MEROPS: the peptidase database. Nucleic Acids Res 34 D270 272
70. SilvermanGA
BirdPI
CarrellRW
ChurchFC
CoughlinPB
2001 The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276 33293 33296
71. MusilD
ZucicD
TurkD
EnghRA
MayrI
1991 The refined 2.15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. Embo J 10 2321 2330
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens
- Tsetse EP Protein Protects the Fly Midgut from Trypanosome Establishment
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- N-Acetylglucosamine Induces White to Opaque Switching, a Mating Prerequisite in
- Origin and Evolution of Sulfadoxine Resistant
- Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage
- Natural Strain Variation and Antibody Neutralization of Dengue Serotype 3 Viruses
- Fine-Tuning Translation Kinetics Selection as the Driving Force of Codon Usage Bias in the Hepatitis A Virus Capsid
- Structural Basis of Cell Wall Cleavage by a Staphylococcal Autolysin
- Direct Visualization by Cryo-EM of the Mycobacterial Capsular Layer: A Labile Structure Containing ESX-1-Secreted Proteins
- Lipopolysaccharide Is Synthesized via a Novel Pathway with an Evolutionary Connection to Protein -Glycosylation
- MicroRNA Antagonism of the Picornaviral Life Cycle: Alternative Mechanisms of Interference
- Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response
- Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles
- RNAIII Binds to Two Distant Regions of mRNA to Arrest Translation and Promote mRNA Degradation
- Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection
- The Essentials of Protein Import in the Degenerate Mitochondrion of
- Dynamic Imaging of Experimental Induced Hepatic Granulomas Detects Kupffer Cell-Restricted Antigen Presentation to Antigen-Specific CD8 T Cells
- An Accessory to the ‘Trinity’: SR-As Are Essential Pathogen Sensors of Extracellular dsRNA, Mediating Entry and Leading to Subsequent Type I IFN Responses
- Innate Killing of by Macrophages of the Splenic Marginal Zone Requires IRF-7
- Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
- Membrane Damage Elicits an Immunomodulatory Program in
- Fatal Transmissible Amyloid Encephalopathy: A New Type of Prion Disease Associated with Lack of Prion Protein Membrane Anchoring
- Nucleophosmin Phosphorylation by v-Cyclin-CDK6 Controls KSHV Latency
- A Combination of Independent Transcriptional Regulators Shapes Bacterial Virulence Gene Expression during Infection
- Inhibition of Host Vacuolar H-ATPase Activity by a Effector
- Human Cytomegalovirus Protein pUL117 Targets the Mini-Chromosome Maintenance Complex and Suppresses Cellular DNA Synthesis
- Dispersion as an Important Step in the Biofilm Developmental Cycle
- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Differential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
- The Human Polyoma JC Virus Agnoprotein Acts as a Viroporin
- Expansion, Maintenance, and Memory in NK and T Cells during Viral Infections: Responding to Pressures for Defense and Regulation
- T Cell-Dependence of Lassa Fever Pathogenesis
- HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse?
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
- Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence
- Serological Profiling of a Protein Microarray Reveals Permanent Host-Pathogen Interplay and Stage-Specific Responses during Candidemia
- YfiBNR Mediates Cyclic di-GMP Dependent Small Colony Variant Formation and Persistence in
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



